User login
Predictors of Long-Term Opioid Use After Opioid Initiation at Discharge From Medical and Surgical Hospitalizations
While patients may be newly exposed to opioids during medical and surgical hospitalization and the prescription of opioids at discharge is common,1-5 prescribers of opioids at discharge may not intend to initiate long-term opioid (LTO) use. By understanding the frequency of progression to LTO use, hospitalists can better balance postdischarge pain treatment and the risk for unintended LTO initiation.
Estimates of LTO use rates following hospital discharge in selected populations1,2,4-6 have varied depending on the population studied and the method of defining LTO use.7 Rates of LTO use following incident opioid prescription have not been directly compared at medical versus surgical discharge or compared with initiation in the ambulatory setting. We present the rates of LTO use following incident opioid exposure at surgical discharge and medical discharge and identify the factors associated with LTO use following surgical and medical discharge.
METHODS
Data Sources
Veterans Health Administration (VHA) data were obtained through the Austin Information Technology Center for fiscal years (FYs) 2003 through 2012 (Austin, Texas). Decision support system national data extracts were used to identify prescription-dispensing events, and inpatient and outpatient medical SAS data sets were used to identify diagnostic codes. The study was approved by the University of Iowa Institutional Review Board and the Iowa City Veterans Affairs (VA) Health Care System Research and Development Committee.
Patients
We included all patients with an outpatient opioid prescription during FY 2011 that was preceded by a 1-year opioid-free period.7 Patients with broadly accepted indications for LTO use (eg, metastatic cancer, palliative care, or opioid-dependence treatment) were excluded.7
Opioid Exposure
We included all outpatient prescription fills for noninjectable dosage forms of butorphanol, fentanyl, hydrocodone, hydromorphone, levorphanol, meperidine, methadone, morphine, oxycodone, oxymorphone, pentazocine, and tramadol. Consistent with the Centers for Disease Control and Prevention and VA/Department of Defense guidelines, LTO use was defined conceptually as regular use for >90 days. Operationalizing this definition to pharmacy refill data was established by using a cabinet supply methodology,7 which allows for the construction of episodes of continuous medication therapy by estimating the medication supply available to a patient for each day during a defined period based on the pattern of observed refills. LTO use was defined as an episode of continuous opioid supply for >90 days and beginning within 30 days of the initial prescription. While some studies have defined LTO use based on onset within 1 year following surgery,5 the requirement for onset within 30 days of initiation was applied to more strongly tie the association of developing LTO use with the discharge event and minimize various forms of bias that are introduced with extended follow-up periods.
Clinical Characteristics
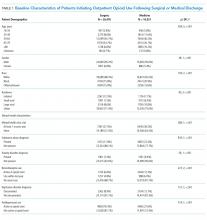
Patients were classified as being medical discharges, surgical discharges, or outpatient initiators. Patients with an opioid index date within 2 days following discharge were designated based on discharge bed section; additionally, if patients had a surgical bed section during hospitalization, they were assigned as surgical discharges. Demographic, diagnosis, and medication exposure variables that were previously associated with LTO use were selected.8,9 Substance use disorder, chronic pain, anxiety disorder, and depressive disorder were based on International Classification of Diseases, 9th Revision (ICD-9) codes in the preceding year. The use of concurrent benzodiazepines, skeletal muscle relaxants, and antidepressants were determined at opioid initiation.10 Rural or urban residence was assigned by using the Rural-Urban Commuting Area Codes system and mapped with the zip code of a veteran’s residence.11
Analysis
Bivariate and multivariable relationships were determined by using logistic regression. The multivariable model considered all pairwise interaction terms between inpatient service (surgery versus medicine) and each of the variables in the model. Statistically significant interaction terms (P < .05) were retained, and all others were omitted from the final model. The main effects for variables that were involved in a significant interaction term were not reported in the final multivariable model; instead, we created fully specified multivariable models for surgery service and medicine service and reported odds ratios (ORs) for the main effects. All analyses were conducted by using SAS version 9.4 (SAS Institute Inc, Cary, North Carolina).
RESULTS
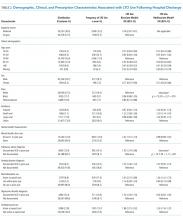
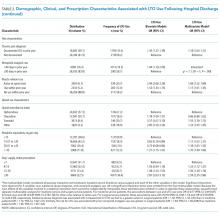
Days’ supply was associated with LTO use in a dose-dependent fashion relative to the reference category of ≤7 days: OR of 1.24 (95% CI, 1.12-1.37) for 8 to 14 days; OR of 1.56 (95% CI, 1.39-1.76) for 15 to 29 days; and OR of 2.59 (95% CI, 2.35-2.86) for 30 days (Table 2). LTO risk was higher among patients with an estimated dose of ≥15 morphine equivalents per day (MED) compared with those with doses of <15 equivalents (OR = 1.11; 95% CI, 1.02-1.21); patients who received >45 MED were at the greatest risk (OR = 1.70; 95% CI, 1.49-1.94).
DISCUSSION
The observation that subsequent LTO use occurs more frequently in discharged medical patients than surgical patients is consistent with the findings of Calcaterra et al.1 that among patients with no surgery versus surgery during hospitalization, opioid receipt at discharge resulted in a higher adjusted OR (7.24 for no surgery versus 3.40 for surgery) for chronic opioid use at 1 year. One explanation for this finding may be an artifact of cohort selection in the study design: patients with prior opioid use are excluded from the cohort, and prior use may be more common among surgical patients presenting for elective inpatient surgery for painful conditions. Previous work suggests that opioid use preoperatively is a robust predictor of postoperative use, and rates of LTO use are low among patients without preoperative opioid exposure.6
Demographic characteristics associated with persistent opioid receipt were similar to those previously reported.5,8,9 The inclusion of medication classes indicated in the treatment of mental health or pain conditions (ie, antidepressants, benzodiazepines, muscle relaxants, and nonopioid analgesics) resulted in diagnoses based on ICD-9 codes being no longer associated with LTO use. Severity or activity of illness, preferences regarding pharmacologic or nonpharmacologic treatment and undiagnosed or undocumented pain-comorbid conditions may all contribute to this finding. Future work studying opioid-related outcomes should include variables that reflect pharmacologic management of comorbid diagnoses in the cohort development or analytic design.
The strongest risk factors were potentially modifiable: days’ supply, dose, and concurrent medications. The measures of opioid quantity supplied are associated with subsequent ongoing use and are consistent with recent work based on prescription drug–monitoring data in a single state14 and in a nationally representative sample.15 That this relationship persists following hospital discharge, a scenario in which LTO use is unlikely to be initiated by a provider (who would be expected to subsequently titrate or monitor therapy), further supports the potential to curtail unintended LTO use through judicious early prescribing decisions.
We assessed only opioids that were supplied through a VA pharmacy, which may lead to the misclassification of patients as opioid naive for inclusion and an underestimation of the rate of opioid use following discharge. It is possible that differences in the rates of non-VA pharmacy use differ in medical and surgical populations in a nonrandom way. This study was performed in a large, integrated health system and may not be generalizable outside the VA system, where more discontinuities between hospital and ambulatory care may exist.
CONCLUSION
The initiation of LTO use at discharge is more common in veterans who are discharged from medical than surgical hospitalizations, likely reflecting differences in the patient population, pain conditions, and discharge prescribing decisions. While patient characteristics are associated with LTO use, the strongest associations are with increasing index dose and days’ supply; both represent potentially modifiable prescriber behaviors. These findings support policy changes and other efforts to minimize dose and days supplied when short-term use is intended as a means to address the current opioid epidemic.
Acknowledgments
The work reported here was supported by the Department of Veterans Affairs Office of Academic Affiliations and Office of Research and Development (Dr. Mosher and Dr. Hofmeyer), and Health Services Research and Development Service (HSR&D) through the Comprehensive Access and Delivery Research and Evaluation Center (CIN 13-412) and a Career Development Award (CDA 10-017; Dr. Lund).
Disclosures
The authors report no conflict of interest in regard to this study. The authors had full access to and take full responsibility for the integrity of the data. All analyses were conducted by using SAS version 9.2 (SAS Institute Inc, Cary, NC). This manuscript is not under review elsewhere, and there is no prior publication of the manuscript contents. The views expressed in this article are those of the authors and do not necessarily represent the views of the Department of Veterans Affairs. The study was approved by the University of Iowa Institutional Review Board and the Iowa City Healthcare System Research and Development Committee.
1. Calcaterra SL, Yamashita TE, Min SJ, Keniston A, Frank JW, Binswanger IA. Opioid Prescribing at Hospital Discharge Contributes to Chronic Opioid Use. J Gen Intern Med. 2016;31(5):478-485. PubMed
2. Raebel MA, Newcomer SR, Reifler LM, et al. Chronic use of opioid medications before and after bariatric surgery. JAMA. 2013;310(13):1369-1376. PubMed
3. Mosher HJ, Jiang L, Vaughan Sarrazin MS, Cram P, Kaboli PJ, Vander Weg MW. Prevalence and characteristics of hospitalized adults on chronic opioid therapy. J Hosp Med. 2014;9(2):82-87. PubMed
4. Holman JE, Stoddard GJ, Higgins TF. Rates of prescription opiate use before and after injury in patients with orthopaedic trauma and the risk factors for prolonged opiate use. J Bone Joint Surg Am. 2013;95(12):1075-1080.
5. Sun EC, Darnall BD, Baker LC, Mackey S. Incidence of and Risk Factors for Chronic Opioid Use Among Opioid-Naive Patients in the Postoperative Period. JAMA Intern Med. 2016;176(9):1286-1293. PubMed
6. Goesling J, Moser SE, Zaidi B, et al. Trends and predictors of opioid use after total knee and total hip arthroplasty. Pain. 2016;157(6):1259-1265. PubMed
7. Mosher HJ, Richardson KK, Lund BC. The 1-Year Treatment Course of New Opioid Recipients in Veterans Health Administration. Pain Med. 2016. [Epub ahead of print]. PubMed
8. Sullivan MD, Edlund MJ, Fan MY, Devries A, Brennan Braden J, Martin BC. Risks for possible and probable opioid misuse among recipients of chronic opioid therapy in commercial and medicaid insurance plans: The TROUP Study. Pain. 2010;150(2):332-339. PubMed
9. Seal KH, Shi Y, Cohen G, et al. Association of mental health disorders with prescription opioids and high-risk opioid use in US veterans of Iraq and Afghanistan. JAMA. 2012;307(9):940-947. PubMed
10. Mosher HJ, Richardson KK, Lund BC. Sedative Prescriptions Are Common at Opioid Initiation: An Observational Study in the Veterans Health Administration. Pain Med. 2017. [Epub ahead of print]. PubMed
11. Lund BC, Abrams TE, Bernardy NC, Alexander B, Friedman MJ. Benzodiazepine prescribing variation and clinical uncertainty in treating posttraumatic stress disorder. Psychiatr Serv. 2013;64(1):21-27. PubMed
12. Brummett CM, Waljee JF, Goesling J, et al. New Persistent Opioid Use After Minor and Major Surgical Procedures in US Adults. JAMA Surg. 2017;152(6):e170504. PubMed
13. Mellbye A, Karlstad O, Skurtveit S, Borchgrevink PC, Fredheim OM. The duration and course of opioid therapy in patients with chronic non-malignant pain. Acta Anaesthesiol Scand. 2016;60(1):128-137. PubMed
14. Deyo RA, Hallvik SE, Hildebran C, et al. Association Between Initial Opioid Prescribing Patterns and Subsequent Long-Term Use Among Opioid-Naive Patients: A Statewide Retrospective Cohort Study. J Gen Intern Med. 2017;32(1):21-27. PubMed
15. Shah A, Hayes CJ, Martin BC. Factors Influencing Long-Term Opioid Use Among Opioid Naive Patients: An Examination of Initial Prescription Characteristics and Pain Etiologies. J Pain. 2017;18(11):1374-1383. PubMed
While patients may be newly exposed to opioids during medical and surgical hospitalization and the prescription of opioids at discharge is common,1-5 prescribers of opioids at discharge may not intend to initiate long-term opioid (LTO) use. By understanding the frequency of progression to LTO use, hospitalists can better balance postdischarge pain treatment and the risk for unintended LTO initiation.
Estimates of LTO use rates following hospital discharge in selected populations1,2,4-6 have varied depending on the population studied and the method of defining LTO use.7 Rates of LTO use following incident opioid prescription have not been directly compared at medical versus surgical discharge or compared with initiation in the ambulatory setting. We present the rates of LTO use following incident opioid exposure at surgical discharge and medical discharge and identify the factors associated with LTO use following surgical and medical discharge.
METHODS
Data Sources
Veterans Health Administration (VHA) data were obtained through the Austin Information Technology Center for fiscal years (FYs) 2003 through 2012 (Austin, Texas). Decision support system national data extracts were used to identify prescription-dispensing events, and inpatient and outpatient medical SAS data sets were used to identify diagnostic codes. The study was approved by the University of Iowa Institutional Review Board and the Iowa City Veterans Affairs (VA) Health Care System Research and Development Committee.
Patients
We included all patients with an outpatient opioid prescription during FY 2011 that was preceded by a 1-year opioid-free period.7 Patients with broadly accepted indications for LTO use (eg, metastatic cancer, palliative care, or opioid-dependence treatment) were excluded.7
Opioid Exposure
We included all outpatient prescription fills for noninjectable dosage forms of butorphanol, fentanyl, hydrocodone, hydromorphone, levorphanol, meperidine, methadone, morphine, oxycodone, oxymorphone, pentazocine, and tramadol. Consistent with the Centers for Disease Control and Prevention and VA/Department of Defense guidelines, LTO use was defined conceptually as regular use for >90 days. Operationalizing this definition to pharmacy refill data was established by using a cabinet supply methodology,7 which allows for the construction of episodes of continuous medication therapy by estimating the medication supply available to a patient for each day during a defined period based on the pattern of observed refills. LTO use was defined as an episode of continuous opioid supply for >90 days and beginning within 30 days of the initial prescription. While some studies have defined LTO use based on onset within 1 year following surgery,5 the requirement for onset within 30 days of initiation was applied to more strongly tie the association of developing LTO use with the discharge event and minimize various forms of bias that are introduced with extended follow-up periods.
Clinical Characteristics
Patients were classified as being medical discharges, surgical discharges, or outpatient initiators. Patients with an opioid index date within 2 days following discharge were designated based on discharge bed section; additionally, if patients had a surgical bed section during hospitalization, they were assigned as surgical discharges. Demographic, diagnosis, and medication exposure variables that were previously associated with LTO use were selected.8,9 Substance use disorder, chronic pain, anxiety disorder, and depressive disorder were based on International Classification of Diseases, 9th Revision (ICD-9) codes in the preceding year. The use of concurrent benzodiazepines, skeletal muscle relaxants, and antidepressants were determined at opioid initiation.10 Rural or urban residence was assigned by using the Rural-Urban Commuting Area Codes system and mapped with the zip code of a veteran’s residence.11
Analysis
Bivariate and multivariable relationships were determined by using logistic regression. The multivariable model considered all pairwise interaction terms between inpatient service (surgery versus medicine) and each of the variables in the model. Statistically significant interaction terms (P < .05) were retained, and all others were omitted from the final model. The main effects for variables that were involved in a significant interaction term were not reported in the final multivariable model; instead, we created fully specified multivariable models for surgery service and medicine service and reported odds ratios (ORs) for the main effects. All analyses were conducted by using SAS version 9.4 (SAS Institute Inc, Cary, North Carolina).
RESULTS
Days’ supply was associated with LTO use in a dose-dependent fashion relative to the reference category of ≤7 days: OR of 1.24 (95% CI, 1.12-1.37) for 8 to 14 days; OR of 1.56 (95% CI, 1.39-1.76) for 15 to 29 days; and OR of 2.59 (95% CI, 2.35-2.86) for 30 days (Table 2). LTO risk was higher among patients with an estimated dose of ≥15 morphine equivalents per day (MED) compared with those with doses of <15 equivalents (OR = 1.11; 95% CI, 1.02-1.21); patients who received >45 MED were at the greatest risk (OR = 1.70; 95% CI, 1.49-1.94).
DISCUSSION
The observation that subsequent LTO use occurs more frequently in discharged medical patients than surgical patients is consistent with the findings of Calcaterra et al.1 that among patients with no surgery versus surgery during hospitalization, opioid receipt at discharge resulted in a higher adjusted OR (7.24 for no surgery versus 3.40 for surgery) for chronic opioid use at 1 year. One explanation for this finding may be an artifact of cohort selection in the study design: patients with prior opioid use are excluded from the cohort, and prior use may be more common among surgical patients presenting for elective inpatient surgery for painful conditions. Previous work suggests that opioid use preoperatively is a robust predictor of postoperative use, and rates of LTO use are low among patients without preoperative opioid exposure.6
Demographic characteristics associated with persistent opioid receipt were similar to those previously reported.5,8,9 The inclusion of medication classes indicated in the treatment of mental health or pain conditions (ie, antidepressants, benzodiazepines, muscle relaxants, and nonopioid analgesics) resulted in diagnoses based on ICD-9 codes being no longer associated with LTO use. Severity or activity of illness, preferences regarding pharmacologic or nonpharmacologic treatment and undiagnosed or undocumented pain-comorbid conditions may all contribute to this finding. Future work studying opioid-related outcomes should include variables that reflect pharmacologic management of comorbid diagnoses in the cohort development or analytic design.
The strongest risk factors were potentially modifiable: days’ supply, dose, and concurrent medications. The measures of opioid quantity supplied are associated with subsequent ongoing use and are consistent with recent work based on prescription drug–monitoring data in a single state14 and in a nationally representative sample.15 That this relationship persists following hospital discharge, a scenario in which LTO use is unlikely to be initiated by a provider (who would be expected to subsequently titrate or monitor therapy), further supports the potential to curtail unintended LTO use through judicious early prescribing decisions.
We assessed only opioids that were supplied through a VA pharmacy, which may lead to the misclassification of patients as opioid naive for inclusion and an underestimation of the rate of opioid use following discharge. It is possible that differences in the rates of non-VA pharmacy use differ in medical and surgical populations in a nonrandom way. This study was performed in a large, integrated health system and may not be generalizable outside the VA system, where more discontinuities between hospital and ambulatory care may exist.
CONCLUSION
The initiation of LTO use at discharge is more common in veterans who are discharged from medical than surgical hospitalizations, likely reflecting differences in the patient population, pain conditions, and discharge prescribing decisions. While patient characteristics are associated with LTO use, the strongest associations are with increasing index dose and days’ supply; both represent potentially modifiable prescriber behaviors. These findings support policy changes and other efforts to minimize dose and days supplied when short-term use is intended as a means to address the current opioid epidemic.
Acknowledgments
The work reported here was supported by the Department of Veterans Affairs Office of Academic Affiliations and Office of Research and Development (Dr. Mosher and Dr. Hofmeyer), and Health Services Research and Development Service (HSR&D) through the Comprehensive Access and Delivery Research and Evaluation Center (CIN 13-412) and a Career Development Award (CDA 10-017; Dr. Lund).
Disclosures
The authors report no conflict of interest in regard to this study. The authors had full access to and take full responsibility for the integrity of the data. All analyses were conducted by using SAS version 9.2 (SAS Institute Inc, Cary, NC). This manuscript is not under review elsewhere, and there is no prior publication of the manuscript contents. The views expressed in this article are those of the authors and do not necessarily represent the views of the Department of Veterans Affairs. The study was approved by the University of Iowa Institutional Review Board and the Iowa City Healthcare System Research and Development Committee.
While patients may be newly exposed to opioids during medical and surgical hospitalization and the prescription of opioids at discharge is common,1-5 prescribers of opioids at discharge may not intend to initiate long-term opioid (LTO) use. By understanding the frequency of progression to LTO use, hospitalists can better balance postdischarge pain treatment and the risk for unintended LTO initiation.
Estimates of LTO use rates following hospital discharge in selected populations1,2,4-6 have varied depending on the population studied and the method of defining LTO use.7 Rates of LTO use following incident opioid prescription have not been directly compared at medical versus surgical discharge or compared with initiation in the ambulatory setting. We present the rates of LTO use following incident opioid exposure at surgical discharge and medical discharge and identify the factors associated with LTO use following surgical and medical discharge.
METHODS
Data Sources
Veterans Health Administration (VHA) data were obtained through the Austin Information Technology Center for fiscal years (FYs) 2003 through 2012 (Austin, Texas). Decision support system national data extracts were used to identify prescription-dispensing events, and inpatient and outpatient medical SAS data sets were used to identify diagnostic codes. The study was approved by the University of Iowa Institutional Review Board and the Iowa City Veterans Affairs (VA) Health Care System Research and Development Committee.
Patients
We included all patients with an outpatient opioid prescription during FY 2011 that was preceded by a 1-year opioid-free period.7 Patients with broadly accepted indications for LTO use (eg, metastatic cancer, palliative care, or opioid-dependence treatment) were excluded.7
Opioid Exposure
We included all outpatient prescription fills for noninjectable dosage forms of butorphanol, fentanyl, hydrocodone, hydromorphone, levorphanol, meperidine, methadone, morphine, oxycodone, oxymorphone, pentazocine, and tramadol. Consistent with the Centers for Disease Control and Prevention and VA/Department of Defense guidelines, LTO use was defined conceptually as regular use for >90 days. Operationalizing this definition to pharmacy refill data was established by using a cabinet supply methodology,7 which allows for the construction of episodes of continuous medication therapy by estimating the medication supply available to a patient for each day during a defined period based on the pattern of observed refills. LTO use was defined as an episode of continuous opioid supply for >90 days and beginning within 30 days of the initial prescription. While some studies have defined LTO use based on onset within 1 year following surgery,5 the requirement for onset within 30 days of initiation was applied to more strongly tie the association of developing LTO use with the discharge event and minimize various forms of bias that are introduced with extended follow-up periods.
Clinical Characteristics
Patients were classified as being medical discharges, surgical discharges, or outpatient initiators. Patients with an opioid index date within 2 days following discharge were designated based on discharge bed section; additionally, if patients had a surgical bed section during hospitalization, they were assigned as surgical discharges. Demographic, diagnosis, and medication exposure variables that were previously associated with LTO use were selected.8,9 Substance use disorder, chronic pain, anxiety disorder, and depressive disorder were based on International Classification of Diseases, 9th Revision (ICD-9) codes in the preceding year. The use of concurrent benzodiazepines, skeletal muscle relaxants, and antidepressants were determined at opioid initiation.10 Rural or urban residence was assigned by using the Rural-Urban Commuting Area Codes system and mapped with the zip code of a veteran’s residence.11
Analysis
Bivariate and multivariable relationships were determined by using logistic regression. The multivariable model considered all pairwise interaction terms between inpatient service (surgery versus medicine) and each of the variables in the model. Statistically significant interaction terms (P < .05) were retained, and all others were omitted from the final model. The main effects for variables that were involved in a significant interaction term were not reported in the final multivariable model; instead, we created fully specified multivariable models for surgery service and medicine service and reported odds ratios (ORs) for the main effects. All analyses were conducted by using SAS version 9.4 (SAS Institute Inc, Cary, North Carolina).
RESULTS
Days’ supply was associated with LTO use in a dose-dependent fashion relative to the reference category of ≤7 days: OR of 1.24 (95% CI, 1.12-1.37) for 8 to 14 days; OR of 1.56 (95% CI, 1.39-1.76) for 15 to 29 days; and OR of 2.59 (95% CI, 2.35-2.86) for 30 days (Table 2). LTO risk was higher among patients with an estimated dose of ≥15 morphine equivalents per day (MED) compared with those with doses of <15 equivalents (OR = 1.11; 95% CI, 1.02-1.21); patients who received >45 MED were at the greatest risk (OR = 1.70; 95% CI, 1.49-1.94).
DISCUSSION
The observation that subsequent LTO use occurs more frequently in discharged medical patients than surgical patients is consistent with the findings of Calcaterra et al.1 that among patients with no surgery versus surgery during hospitalization, opioid receipt at discharge resulted in a higher adjusted OR (7.24 for no surgery versus 3.40 for surgery) for chronic opioid use at 1 year. One explanation for this finding may be an artifact of cohort selection in the study design: patients with prior opioid use are excluded from the cohort, and prior use may be more common among surgical patients presenting for elective inpatient surgery for painful conditions. Previous work suggests that opioid use preoperatively is a robust predictor of postoperative use, and rates of LTO use are low among patients without preoperative opioid exposure.6
Demographic characteristics associated with persistent opioid receipt were similar to those previously reported.5,8,9 The inclusion of medication classes indicated in the treatment of mental health or pain conditions (ie, antidepressants, benzodiazepines, muscle relaxants, and nonopioid analgesics) resulted in diagnoses based on ICD-9 codes being no longer associated with LTO use. Severity or activity of illness, preferences regarding pharmacologic or nonpharmacologic treatment and undiagnosed or undocumented pain-comorbid conditions may all contribute to this finding. Future work studying opioid-related outcomes should include variables that reflect pharmacologic management of comorbid diagnoses in the cohort development or analytic design.
The strongest risk factors were potentially modifiable: days’ supply, dose, and concurrent medications. The measures of opioid quantity supplied are associated with subsequent ongoing use and are consistent with recent work based on prescription drug–monitoring data in a single state14 and in a nationally representative sample.15 That this relationship persists following hospital discharge, a scenario in which LTO use is unlikely to be initiated by a provider (who would be expected to subsequently titrate or monitor therapy), further supports the potential to curtail unintended LTO use through judicious early prescribing decisions.
We assessed only opioids that were supplied through a VA pharmacy, which may lead to the misclassification of patients as opioid naive for inclusion and an underestimation of the rate of opioid use following discharge. It is possible that differences in the rates of non-VA pharmacy use differ in medical and surgical populations in a nonrandom way. This study was performed in a large, integrated health system and may not be generalizable outside the VA system, where more discontinuities between hospital and ambulatory care may exist.
CONCLUSION
The initiation of LTO use at discharge is more common in veterans who are discharged from medical than surgical hospitalizations, likely reflecting differences in the patient population, pain conditions, and discharge prescribing decisions. While patient characteristics are associated with LTO use, the strongest associations are with increasing index dose and days’ supply; both represent potentially modifiable prescriber behaviors. These findings support policy changes and other efforts to minimize dose and days supplied when short-term use is intended as a means to address the current opioid epidemic.
Acknowledgments
The work reported here was supported by the Department of Veterans Affairs Office of Academic Affiliations and Office of Research and Development (Dr. Mosher and Dr. Hofmeyer), and Health Services Research and Development Service (HSR&D) through the Comprehensive Access and Delivery Research and Evaluation Center (CIN 13-412) and a Career Development Award (CDA 10-017; Dr. Lund).
Disclosures
The authors report no conflict of interest in regard to this study. The authors had full access to and take full responsibility for the integrity of the data. All analyses were conducted by using SAS version 9.2 (SAS Institute Inc, Cary, NC). This manuscript is not under review elsewhere, and there is no prior publication of the manuscript contents. The views expressed in this article are those of the authors and do not necessarily represent the views of the Department of Veterans Affairs. The study was approved by the University of Iowa Institutional Review Board and the Iowa City Healthcare System Research and Development Committee.
1. Calcaterra SL, Yamashita TE, Min SJ, Keniston A, Frank JW, Binswanger IA. Opioid Prescribing at Hospital Discharge Contributes to Chronic Opioid Use. J Gen Intern Med. 2016;31(5):478-485. PubMed
2. Raebel MA, Newcomer SR, Reifler LM, et al. Chronic use of opioid medications before and after bariatric surgery. JAMA. 2013;310(13):1369-1376. PubMed
3. Mosher HJ, Jiang L, Vaughan Sarrazin MS, Cram P, Kaboli PJ, Vander Weg MW. Prevalence and characteristics of hospitalized adults on chronic opioid therapy. J Hosp Med. 2014;9(2):82-87. PubMed
4. Holman JE, Stoddard GJ, Higgins TF. Rates of prescription opiate use before and after injury in patients with orthopaedic trauma and the risk factors for prolonged opiate use. J Bone Joint Surg Am. 2013;95(12):1075-1080.
5. Sun EC, Darnall BD, Baker LC, Mackey S. Incidence of and Risk Factors for Chronic Opioid Use Among Opioid-Naive Patients in the Postoperative Period. JAMA Intern Med. 2016;176(9):1286-1293. PubMed
6. Goesling J, Moser SE, Zaidi B, et al. Trends and predictors of opioid use after total knee and total hip arthroplasty. Pain. 2016;157(6):1259-1265. PubMed
7. Mosher HJ, Richardson KK, Lund BC. The 1-Year Treatment Course of New Opioid Recipients in Veterans Health Administration. Pain Med. 2016. [Epub ahead of print]. PubMed
8. Sullivan MD, Edlund MJ, Fan MY, Devries A, Brennan Braden J, Martin BC. Risks for possible and probable opioid misuse among recipients of chronic opioid therapy in commercial and medicaid insurance plans: The TROUP Study. Pain. 2010;150(2):332-339. PubMed
9. Seal KH, Shi Y, Cohen G, et al. Association of mental health disorders with prescription opioids and high-risk opioid use in US veterans of Iraq and Afghanistan. JAMA. 2012;307(9):940-947. PubMed
10. Mosher HJ, Richardson KK, Lund BC. Sedative Prescriptions Are Common at Opioid Initiation: An Observational Study in the Veterans Health Administration. Pain Med. 2017. [Epub ahead of print]. PubMed
11. Lund BC, Abrams TE, Bernardy NC, Alexander B, Friedman MJ. Benzodiazepine prescribing variation and clinical uncertainty in treating posttraumatic stress disorder. Psychiatr Serv. 2013;64(1):21-27. PubMed
12. Brummett CM, Waljee JF, Goesling J, et al. New Persistent Opioid Use After Minor and Major Surgical Procedures in US Adults. JAMA Surg. 2017;152(6):e170504. PubMed
13. Mellbye A, Karlstad O, Skurtveit S, Borchgrevink PC, Fredheim OM. The duration and course of opioid therapy in patients with chronic non-malignant pain. Acta Anaesthesiol Scand. 2016;60(1):128-137. PubMed
14. Deyo RA, Hallvik SE, Hildebran C, et al. Association Between Initial Opioid Prescribing Patterns and Subsequent Long-Term Use Among Opioid-Naive Patients: A Statewide Retrospective Cohort Study. J Gen Intern Med. 2017;32(1):21-27. PubMed
15. Shah A, Hayes CJ, Martin BC. Factors Influencing Long-Term Opioid Use Among Opioid Naive Patients: An Examination of Initial Prescription Characteristics and Pain Etiologies. J Pain. 2017;18(11):1374-1383. PubMed
1. Calcaterra SL, Yamashita TE, Min SJ, Keniston A, Frank JW, Binswanger IA. Opioid Prescribing at Hospital Discharge Contributes to Chronic Opioid Use. J Gen Intern Med. 2016;31(5):478-485. PubMed
2. Raebel MA, Newcomer SR, Reifler LM, et al. Chronic use of opioid medications before and after bariatric surgery. JAMA. 2013;310(13):1369-1376. PubMed
3. Mosher HJ, Jiang L, Vaughan Sarrazin MS, Cram P, Kaboli PJ, Vander Weg MW. Prevalence and characteristics of hospitalized adults on chronic opioid therapy. J Hosp Med. 2014;9(2):82-87. PubMed
4. Holman JE, Stoddard GJ, Higgins TF. Rates of prescription opiate use before and after injury in patients with orthopaedic trauma and the risk factors for prolonged opiate use. J Bone Joint Surg Am. 2013;95(12):1075-1080.
5. Sun EC, Darnall BD, Baker LC, Mackey S. Incidence of and Risk Factors for Chronic Opioid Use Among Opioid-Naive Patients in the Postoperative Period. JAMA Intern Med. 2016;176(9):1286-1293. PubMed
6. Goesling J, Moser SE, Zaidi B, et al. Trends and predictors of opioid use after total knee and total hip arthroplasty. Pain. 2016;157(6):1259-1265. PubMed
7. Mosher HJ, Richardson KK, Lund BC. The 1-Year Treatment Course of New Opioid Recipients in Veterans Health Administration. Pain Med. 2016. [Epub ahead of print]. PubMed
8. Sullivan MD, Edlund MJ, Fan MY, Devries A, Brennan Braden J, Martin BC. Risks for possible and probable opioid misuse among recipients of chronic opioid therapy in commercial and medicaid insurance plans: The TROUP Study. Pain. 2010;150(2):332-339. PubMed
9. Seal KH, Shi Y, Cohen G, et al. Association of mental health disorders with prescription opioids and high-risk opioid use in US veterans of Iraq and Afghanistan. JAMA. 2012;307(9):940-947. PubMed
10. Mosher HJ, Richardson KK, Lund BC. Sedative Prescriptions Are Common at Opioid Initiation: An Observational Study in the Veterans Health Administration. Pain Med. 2017. [Epub ahead of print]. PubMed
11. Lund BC, Abrams TE, Bernardy NC, Alexander B, Friedman MJ. Benzodiazepine prescribing variation and clinical uncertainty in treating posttraumatic stress disorder. Psychiatr Serv. 2013;64(1):21-27. PubMed
12. Brummett CM, Waljee JF, Goesling J, et al. New Persistent Opioid Use After Minor and Major Surgical Procedures in US Adults. JAMA Surg. 2017;152(6):e170504. PubMed
13. Mellbye A, Karlstad O, Skurtveit S, Borchgrevink PC, Fredheim OM. The duration and course of opioid therapy in patients with chronic non-malignant pain. Acta Anaesthesiol Scand. 2016;60(1):128-137. PubMed
14. Deyo RA, Hallvik SE, Hildebran C, et al. Association Between Initial Opioid Prescribing Patterns and Subsequent Long-Term Use Among Opioid-Naive Patients: A Statewide Retrospective Cohort Study. J Gen Intern Med. 2017;32(1):21-27. PubMed
15. Shah A, Hayes CJ, Martin BC. Factors Influencing Long-Term Opioid Use Among Opioid Naive Patients: An Examination of Initial Prescription Characteristics and Pain Etiologies. J Pain. 2017;18(11):1374-1383. PubMed
A Problem of Capacity
For a number of years, those challenged with improving discharge transitions and preventing readmissions have suggested more—more case managers, more checklists and systems, more discharge pharmacists; and better—better communication, better medication reconciliation, better discharge documentation, better follow-up. In a study by Chan Carusone et al.,1 high-need, high-complexity patients receiving treatment at Casey House, a specialized urban hospital providing inpatient and community programs, were afforded a full complement of discharge planning and posthospitalization services. Despite these services, the patients achieved little success in maintaining their health and following their discharge plans after hospitalization.
This longitudinal qualitative study detailing the lived experience of discharge extends our knowledge of challenges faced by patients during the posthospital transition,2 and further elucidates the differences between patients’ expectations and assessments of their resources and goals, and their actual abilities and priorities on discharge. Despite substantial assistance, including housing, food assistance, and case management, Chan Carusone et al. found that the exigencies of day-to-day existence exceeded the patients’ capacities to sustain themselves outside the hospital. This failure implies a question: If the interventions alluded to in this study were not enough, then how much more, and how much better, is needed?
Attention to this question of how to best serve high-need patients continues to increase,3 and success in intervening to improve care transitions for this population is limited,4 in part because providing more care and more coordination requires more resources. Observing the challenges that remain for patients treated in the highly-resourced setting that is Casey House, the authors propose a previously described theoretical construct, minimally disruptive medicine (MDM),5 as a framework to guide patients and providers in creating a discharge plan that relies on the patient’s capacity to integrate disease self-management into his or her daily circumstances. MDM hinges on the concept of balancing workload and capacity: the burden of managing disease with the resources and abilities to do so. On first consideration, this seems an attractive approach to operationalizing patient-centered care by tailoring a discharge plan to a patient’s goals and capacities. On closer examination, however, MDM, applied to a single transition episode, raises some important concerns.
As Chan Carusone et al. describe, patients may poorly judge their future resources and capacity when making decisions in the hospital setting. Likewise, physicians and other team members may lack insight, perspective, and detailed knowledge of resources and barriers in the outpatient setting. From their vantage point, they may not see the fragile contingencies of the discharge plan that is reflected in the patients’ spoken words. At any moment, a well-meant, seemingly well-crafted discharge plan could fall apart.
Within the walls of the hospital, we tend to perform what might be termed maximally disruptive medicine—the treatments provided are exactly those that can’t be delivered in a nonhospital setting. For many patients, these interventions are not curative, but rather stabilizing;6 we assuage chronic conditions that had become exacerbated by new illness, disease progression, or conditions outside the hospital. To return the patient to his or her home situation, especially one that is under-resourced, with minimized workload can feel counterproductive and demoralizing at best. What prevents one from worrying that, where capacity can’t be improved, planning for MDM is, in essence, planning for minimal care?
Viewed in the broader context of a life course health development framework,7 which integrates biological, psychological, cultural, and historical experience to explain the development of health trajectories over an individual’s lifetime, a minimally disruptive approach might be viewed as amplifying disparities. The patients contributing to the study by Chan Carusone et al. may have arrived in their respective situations through a life course marked by poverty, violence, inadequate housing, poor nutrition, discrimination, and other disadvantages that may have resulted from accident, malfeasance, or choice. Their limited personal capacity and the ongoing chaos that is reflected in many of their comments requires that discharge planning uses imagination and dialogue, with careful, compassionate listening by providers, and close partnering and decision-making by patient and providers. Approaches to building the capacity for such compassion, as well as structural interventions to provide care that is necessary and just for these most vulnerable patients by considering their experiences and beliefs,8 remain to be articulated.
In a sense, the narrative unfolded by Chan Carusone et al. appropriately emphasizes that care transitions contain both complex problems and “wicked” problems.9 While aspects of transitions are complex and can be reasonably addressed with complex solutions, these same complex solutions are inadequate to mitigate the seemingly intractable socioeconomic challenges that drive hospital dependence for many high-need patients. Addressing these likely requires a reexamination of what we expect from hospitals, what systems we are able to design and are willing to support to keep people from returning to them, and what it means that for some people returning is the best, and sometimes only, thing to do.
As we continue to seek new models for healthcare in high-need, high-risk populations, we may do well to focus further longitudinal qualitative study on building a deep understanding of when and how patients achieve success following discharge. What characterizes patients, caregivers, service networks, and communities in healthcare settings with the highest rates of effective transitions? Maintaining equilibrium outside an institutional setting is convoluted, time-consuming, nuanced, and taxing; that those who have not experienced doing so as a patient or caregiver might struggle to help others should not surprise us. The concepts of capacity and workload lend themselves to structuring discovery of the resources that patients, not providers and policy-makers, have found through their lived experience to be most crucial to their enduring well-being. Learning from these experiences may shift the balance by increasing our own capacity to understand what constitutes success.
Disclosures
The views expressed in this article are those of the authors and do not necessarily represent the views of the Department of Veterans Affairs. The authors report no conflicts of interest.
References
1. Chan Carusone S, O’Leary B, McWatt S, Stewart S, Craig S, Brennan D. The lived experience of the hospital discharge “plan”: a longitudinal qualitative study of complex patients. J Hosp Med. 2017;12(1):5-10. PubMed
2. Kangovi S, Barg FK, Carter T, et al. Challenges faced by patients with low socioeconomic status during the post-hospital transition. J Gen Intern Med. 2014;29:283-289. PubMed
3. Blumenthal D, Chernof B, Fulmer T, Lumpkin J, Selberg J. Caring for high-need, high-cost patients - an urgent priority. N Engl J Med. 2016;375:909-911. PubMed
4. Powers BW, Milstein A, Jain SH. Delivery models for high-risk older patients: back to the future? JAMA. 2016;315:23-24. PubMed
5. Abu Dabrh AM, Gallacher K, Boehmer KR, Hargraves IG, Mair FS. Minimally disruptive medicine: the evidence and conceptual progress supporting a new era of healthcare. J R Coll Physicians Edinb. 2015;45:114-117. PubMed
6. Pannick S, Wachter RM, Vincent C, Sevdalis N. Rethinking medical ward quality. BMJ. 2016;355:i5417. PubMed
7. Kressin NR, Chapman SE, Magnani JW. A tale of two patients: patient-centered approaches to adherence as a gateway to reducing disparities. Circulation. 2016;133:2583-2592. PubMed
8. Thiel de Bocanegra H, Gany F. Good provider, good patient: changing behaviors to eliminate disparities in healthcare. Am J Manag Care. 2004;10:SP20-28. PubMed
9. Churchman CW. Wicked problems. Manage Sci. 1967;14(4):B141-B142.
For a number of years, those challenged with improving discharge transitions and preventing readmissions have suggested more—more case managers, more checklists and systems, more discharge pharmacists; and better—better communication, better medication reconciliation, better discharge documentation, better follow-up. In a study by Chan Carusone et al.,1 high-need, high-complexity patients receiving treatment at Casey House, a specialized urban hospital providing inpatient and community programs, were afforded a full complement of discharge planning and posthospitalization services. Despite these services, the patients achieved little success in maintaining their health and following their discharge plans after hospitalization.
This longitudinal qualitative study detailing the lived experience of discharge extends our knowledge of challenges faced by patients during the posthospital transition,2 and further elucidates the differences between patients’ expectations and assessments of their resources and goals, and their actual abilities and priorities on discharge. Despite substantial assistance, including housing, food assistance, and case management, Chan Carusone et al. found that the exigencies of day-to-day existence exceeded the patients’ capacities to sustain themselves outside the hospital. This failure implies a question: If the interventions alluded to in this study were not enough, then how much more, and how much better, is needed?
Attention to this question of how to best serve high-need patients continues to increase,3 and success in intervening to improve care transitions for this population is limited,4 in part because providing more care and more coordination requires more resources. Observing the challenges that remain for patients treated in the highly-resourced setting that is Casey House, the authors propose a previously described theoretical construct, minimally disruptive medicine (MDM),5 as a framework to guide patients and providers in creating a discharge plan that relies on the patient’s capacity to integrate disease self-management into his or her daily circumstances. MDM hinges on the concept of balancing workload and capacity: the burden of managing disease with the resources and abilities to do so. On first consideration, this seems an attractive approach to operationalizing patient-centered care by tailoring a discharge plan to a patient’s goals and capacities. On closer examination, however, MDM, applied to a single transition episode, raises some important concerns.
As Chan Carusone et al. describe, patients may poorly judge their future resources and capacity when making decisions in the hospital setting. Likewise, physicians and other team members may lack insight, perspective, and detailed knowledge of resources and barriers in the outpatient setting. From their vantage point, they may not see the fragile contingencies of the discharge plan that is reflected in the patients’ spoken words. At any moment, a well-meant, seemingly well-crafted discharge plan could fall apart.
Within the walls of the hospital, we tend to perform what might be termed maximally disruptive medicine—the treatments provided are exactly those that can’t be delivered in a nonhospital setting. For many patients, these interventions are not curative, but rather stabilizing;6 we assuage chronic conditions that had become exacerbated by new illness, disease progression, or conditions outside the hospital. To return the patient to his or her home situation, especially one that is under-resourced, with minimized workload can feel counterproductive and demoralizing at best. What prevents one from worrying that, where capacity can’t be improved, planning for MDM is, in essence, planning for minimal care?
Viewed in the broader context of a life course health development framework,7 which integrates biological, psychological, cultural, and historical experience to explain the development of health trajectories over an individual’s lifetime, a minimally disruptive approach might be viewed as amplifying disparities. The patients contributing to the study by Chan Carusone et al. may have arrived in their respective situations through a life course marked by poverty, violence, inadequate housing, poor nutrition, discrimination, and other disadvantages that may have resulted from accident, malfeasance, or choice. Their limited personal capacity and the ongoing chaos that is reflected in many of their comments requires that discharge planning uses imagination and dialogue, with careful, compassionate listening by providers, and close partnering and decision-making by patient and providers. Approaches to building the capacity for such compassion, as well as structural interventions to provide care that is necessary and just for these most vulnerable patients by considering their experiences and beliefs,8 remain to be articulated.
In a sense, the narrative unfolded by Chan Carusone et al. appropriately emphasizes that care transitions contain both complex problems and “wicked” problems.9 While aspects of transitions are complex and can be reasonably addressed with complex solutions, these same complex solutions are inadequate to mitigate the seemingly intractable socioeconomic challenges that drive hospital dependence for many high-need patients. Addressing these likely requires a reexamination of what we expect from hospitals, what systems we are able to design and are willing to support to keep people from returning to them, and what it means that for some people returning is the best, and sometimes only, thing to do.
As we continue to seek new models for healthcare in high-need, high-risk populations, we may do well to focus further longitudinal qualitative study on building a deep understanding of when and how patients achieve success following discharge. What characterizes patients, caregivers, service networks, and communities in healthcare settings with the highest rates of effective transitions? Maintaining equilibrium outside an institutional setting is convoluted, time-consuming, nuanced, and taxing; that those who have not experienced doing so as a patient or caregiver might struggle to help others should not surprise us. The concepts of capacity and workload lend themselves to structuring discovery of the resources that patients, not providers and policy-makers, have found through their lived experience to be most crucial to their enduring well-being. Learning from these experiences may shift the balance by increasing our own capacity to understand what constitutes success.
Disclosures
The views expressed in this article are those of the authors and do not necessarily represent the views of the Department of Veterans Affairs. The authors report no conflicts of interest.
For a number of years, those challenged with improving discharge transitions and preventing readmissions have suggested more—more case managers, more checklists and systems, more discharge pharmacists; and better—better communication, better medication reconciliation, better discharge documentation, better follow-up. In a study by Chan Carusone et al.,1 high-need, high-complexity patients receiving treatment at Casey House, a specialized urban hospital providing inpatient and community programs, were afforded a full complement of discharge planning and posthospitalization services. Despite these services, the patients achieved little success in maintaining their health and following their discharge plans after hospitalization.
This longitudinal qualitative study detailing the lived experience of discharge extends our knowledge of challenges faced by patients during the posthospital transition,2 and further elucidates the differences between patients’ expectations and assessments of their resources and goals, and their actual abilities and priorities on discharge. Despite substantial assistance, including housing, food assistance, and case management, Chan Carusone et al. found that the exigencies of day-to-day existence exceeded the patients’ capacities to sustain themselves outside the hospital. This failure implies a question: If the interventions alluded to in this study were not enough, then how much more, and how much better, is needed?
Attention to this question of how to best serve high-need patients continues to increase,3 and success in intervening to improve care transitions for this population is limited,4 in part because providing more care and more coordination requires more resources. Observing the challenges that remain for patients treated in the highly-resourced setting that is Casey House, the authors propose a previously described theoretical construct, minimally disruptive medicine (MDM),5 as a framework to guide patients and providers in creating a discharge plan that relies on the patient’s capacity to integrate disease self-management into his or her daily circumstances. MDM hinges on the concept of balancing workload and capacity: the burden of managing disease with the resources and abilities to do so. On first consideration, this seems an attractive approach to operationalizing patient-centered care by tailoring a discharge plan to a patient’s goals and capacities. On closer examination, however, MDM, applied to a single transition episode, raises some important concerns.
As Chan Carusone et al. describe, patients may poorly judge their future resources and capacity when making decisions in the hospital setting. Likewise, physicians and other team members may lack insight, perspective, and detailed knowledge of resources and barriers in the outpatient setting. From their vantage point, they may not see the fragile contingencies of the discharge plan that is reflected in the patients’ spoken words. At any moment, a well-meant, seemingly well-crafted discharge plan could fall apart.
Within the walls of the hospital, we tend to perform what might be termed maximally disruptive medicine—the treatments provided are exactly those that can’t be delivered in a nonhospital setting. For many patients, these interventions are not curative, but rather stabilizing;6 we assuage chronic conditions that had become exacerbated by new illness, disease progression, or conditions outside the hospital. To return the patient to his or her home situation, especially one that is under-resourced, with minimized workload can feel counterproductive and demoralizing at best. What prevents one from worrying that, where capacity can’t be improved, planning for MDM is, in essence, planning for minimal care?
Viewed in the broader context of a life course health development framework,7 which integrates biological, psychological, cultural, and historical experience to explain the development of health trajectories over an individual’s lifetime, a minimally disruptive approach might be viewed as amplifying disparities. The patients contributing to the study by Chan Carusone et al. may have arrived in their respective situations through a life course marked by poverty, violence, inadequate housing, poor nutrition, discrimination, and other disadvantages that may have resulted from accident, malfeasance, or choice. Their limited personal capacity and the ongoing chaos that is reflected in many of their comments requires that discharge planning uses imagination and dialogue, with careful, compassionate listening by providers, and close partnering and decision-making by patient and providers. Approaches to building the capacity for such compassion, as well as structural interventions to provide care that is necessary and just for these most vulnerable patients by considering their experiences and beliefs,8 remain to be articulated.
In a sense, the narrative unfolded by Chan Carusone et al. appropriately emphasizes that care transitions contain both complex problems and “wicked” problems.9 While aspects of transitions are complex and can be reasonably addressed with complex solutions, these same complex solutions are inadequate to mitigate the seemingly intractable socioeconomic challenges that drive hospital dependence for many high-need patients. Addressing these likely requires a reexamination of what we expect from hospitals, what systems we are able to design and are willing to support to keep people from returning to them, and what it means that for some people returning is the best, and sometimes only, thing to do.
As we continue to seek new models for healthcare in high-need, high-risk populations, we may do well to focus further longitudinal qualitative study on building a deep understanding of when and how patients achieve success following discharge. What characterizes patients, caregivers, service networks, and communities in healthcare settings with the highest rates of effective transitions? Maintaining equilibrium outside an institutional setting is convoluted, time-consuming, nuanced, and taxing; that those who have not experienced doing so as a patient or caregiver might struggle to help others should not surprise us. The concepts of capacity and workload lend themselves to structuring discovery of the resources that patients, not providers and policy-makers, have found through their lived experience to be most crucial to their enduring well-being. Learning from these experiences may shift the balance by increasing our own capacity to understand what constitutes success.
Disclosures
The views expressed in this article are those of the authors and do not necessarily represent the views of the Department of Veterans Affairs. The authors report no conflicts of interest.
References
1. Chan Carusone S, O’Leary B, McWatt S, Stewart S, Craig S, Brennan D. The lived experience of the hospital discharge “plan”: a longitudinal qualitative study of complex patients. J Hosp Med. 2017;12(1):5-10. PubMed
2. Kangovi S, Barg FK, Carter T, et al. Challenges faced by patients with low socioeconomic status during the post-hospital transition. J Gen Intern Med. 2014;29:283-289. PubMed
3. Blumenthal D, Chernof B, Fulmer T, Lumpkin J, Selberg J. Caring for high-need, high-cost patients - an urgent priority. N Engl J Med. 2016;375:909-911. PubMed
4. Powers BW, Milstein A, Jain SH. Delivery models for high-risk older patients: back to the future? JAMA. 2016;315:23-24. PubMed
5. Abu Dabrh AM, Gallacher K, Boehmer KR, Hargraves IG, Mair FS. Minimally disruptive medicine: the evidence and conceptual progress supporting a new era of healthcare. J R Coll Physicians Edinb. 2015;45:114-117. PubMed
6. Pannick S, Wachter RM, Vincent C, Sevdalis N. Rethinking medical ward quality. BMJ. 2016;355:i5417. PubMed
7. Kressin NR, Chapman SE, Magnani JW. A tale of two patients: patient-centered approaches to adherence as a gateway to reducing disparities. Circulation. 2016;133:2583-2592. PubMed
8. Thiel de Bocanegra H, Gany F. Good provider, good patient: changing behaviors to eliminate disparities in healthcare. Am J Manag Care. 2004;10:SP20-28. PubMed
9. Churchman CW. Wicked problems. Manage Sci. 1967;14(4):B141-B142.
References
1. Chan Carusone S, O’Leary B, McWatt S, Stewart S, Craig S, Brennan D. The lived experience of the hospital discharge “plan”: a longitudinal qualitative study of complex patients. J Hosp Med. 2017;12(1):5-10. PubMed
2. Kangovi S, Barg FK, Carter T, et al. Challenges faced by patients with low socioeconomic status during the post-hospital transition. J Gen Intern Med. 2014;29:283-289. PubMed
3. Blumenthal D, Chernof B, Fulmer T, Lumpkin J, Selberg J. Caring for high-need, high-cost patients - an urgent priority. N Engl J Med. 2016;375:909-911. PubMed
4. Powers BW, Milstein A, Jain SH. Delivery models for high-risk older patients: back to the future? JAMA. 2016;315:23-24. PubMed
5. Abu Dabrh AM, Gallacher K, Boehmer KR, Hargraves IG, Mair FS. Minimally disruptive medicine: the evidence and conceptual progress supporting a new era of healthcare. J R Coll Physicians Edinb. 2015;45:114-117. PubMed
6. Pannick S, Wachter RM, Vincent C, Sevdalis N. Rethinking medical ward quality. BMJ. 2016;355:i5417. PubMed
7. Kressin NR, Chapman SE, Magnani JW. A tale of two patients: patient-centered approaches to adherence as a gateway to reducing disparities. Circulation. 2016;133:2583-2592. PubMed
8. Thiel de Bocanegra H, Gany F. Good provider, good patient: changing behaviors to eliminate disparities in healthcare. Am J Manag Care. 2004;10:SP20-28. PubMed
9. Churchman CW. Wicked problems. Manage Sci. 1967;14(4):B141-B142.
© 2017 Society of Hospital Medicine
The Goals of Goals
In their study of goals of care (GOC) discussions and documentation, Wong et al. add to already robust evidence that communication, in this case from physicians caring for hospitalized patients back to long‐term care facilities, has room for improvement. They highlight that 37.5% of patients had documented discussions, and for cases in which these discussions resulted in changes to a patient's advance directive, only 1 in 4 were relayed in the discharge summary.[1]
As physicians caring for hospitalized patients and concerned with improving care quality and efficiency, many of us are familiar with potential systems solutions to augmenting communication: reminders in the electronic health record, checklists, multidisciplinary teams, scripts, and posthospitalization follow‐up phone calls. However, important as they are, these solutions often elide the underlying cognitive elements related to how we, as physicians, think about and engage in the diversity of cases presented to us, and to how we prioritize communication work.
Wong et al. looked at patient characteristics associated with performance of GOC discussions to understand when and why physicians might engage in GOC conversations in the hospital and to generate insights into potential targets for improvement. They found that characteristics of patients prior to hospital admission were not associated with GOC discussions; signs of acuity of illness were.[1] In other words, physicians in the hospital are pretty good at recognizing patients in extremis, and prioritize GOC discussions with these patients. What we are not good at, or might not be considering, is assessing the broader context of a patient's health.
Whether we interpret these results as appropriate prioritization, or as a sign that we are waiting too long to broach the subject of care goals, depends on how we conceptualize the hospital stay in the context of a patient's health story, and, by extension, the role of the hospitalist in this story. For some patients, an acute illness requiring hospitalization is unexpected and readily treated, and the patient rapidly returns to a prior level of health and function. The need for hospitalization represents an outlier state.
For other patients, often older, more debilitated, or with multiple and chronic medical conditions, minor changes in health or declines in mental, social, or physical function precipitate the need for hospitalization. Likewise, iatrogenic harms of hospitalizationsleeplessness, fasting, delirium, immobilitycan contribute to enduring decline.[2, 3] For these patients, the need for hospitalization is not so far from, or may be, their norm.[4]
I suspect that Wong et al.'s findings reflect a collective response to the uncertainties of prognostication, and the resultant discomfort in raising questions that are difficult to answer. How do we know it is time to start talking about the right amount of care? Some might answer, I think rightly, that it is rarely if ever too early, yet robust discussions are challenging if we are not sure of the relevance or the immediate goal. In the case of the patient who is ill, declining, yet not in extremis, many of us might conclude that raising the question would not produce actionable information; it would not change immediate in‐hospital management.
This common conclusion leads to a significant missed opportunity, both on an individual level for physicians and patients, and for hospital medicine as a specialty. Health, and the losses that come with declining health, are wrapped up with fundamental aspects of our identities, and take time and consideration to change and evolve. Decisions about our healthcare are statements about who we have been, who we are, and who we will no longer be. Especially for the second group of patients described above, each hospital stay affords a chance to assess, counsel, educate, support, and empower patients to move in the direction of their values, and to ready them for that eventuality when they or their loved ones are faced with decisions about how, and where, they will die. As specialists in hospital‐based healthcare, hospitalists have the privilege and professional duty to facilitate this journey.
However, as hospitalists, we are often meeting patients for the first time; how do we assimilate an understanding of that point in time within the context of a patient's life with enough confidence to engage discussions? As Wong et al. show, it appears that in regard to very ill patients, respiratory rate and Glasgow Coma Scale inform action.[1] What signs or observations help inform action earlier in the trajectory of decline, to allow for anticipatory guidance and discussion? Increasingly, we see evidence that measures of frailty and functional status, applied in the hospital, are associated with hospital outcomes including readmission and death.[5, 6, 7] Future work might explore if training physicians to systematically assess frailty and functional status leads to greater frequency of, and comfort with, initiating GOC discussions during hospitalization.
Moreover, an emphasis on evaluating frailty and function, and explicitly including this assessment in our clinical decision‐making might help shift our thinking toward valuing each hospitalization as an opportunity to both intervene to improve function[8, 9] and to support, educate, and prepare patients under our care for the journey aheadin other words, to fully engage with our role as specialists in the comprehensive and coordinated treatment of patients who require hospitalization.
- , , , . Goals of care discussions among hospitalized long‐term care residents: predictors and associated outcomes of care. J Hosp Med. 2016;11(12):824–831.
- . Hazards of hospitalization of the elderly. Ann Intern Med. 1993;118(3):219–223.
- , , . Hospitalization‐associated disability: “she was probably able to ambulate, but I'm not sure.” JAMA. 2011;306(16):1782–1793.
- , . The hospital‐dependent patient. N Engl J Med. 2014;370(8):694–697.
- , , , et al. Functional status outperforms comorbidities in predicting acute care readmissions in medically complex patients. J Gen Intern Med. 2015;30(11):1688–1695.
- , , , et al. Comparing three different measures of frailty in medical inpatients: multicenter prospective cohort study examining 30‐day risk of readmission or death [published online May 17, 2016]. J Hosp Med. doi: 10.1002/jhm.2607.
- , , , et al. Association between frailty and 30‐day outcomes after discharge from hospital. CMAJ. 2015;187(11):799–804.
- , , , et al. Comparison of posthospitalization function and community mobility in hospital mobility program and usual care patients: a randomized clinical trial [published online May 31, 2016]. JAMA Intern Med. doi: 10.1001/jamainternmed.2016.1870.
- . Activating hospitalized older patients to confront the epidemic of low mobility [published online May 31 2016]. JAMA Intern Med. doi: 10.1001/jamainternmed.2016.1874.
In their study of goals of care (GOC) discussions and documentation, Wong et al. add to already robust evidence that communication, in this case from physicians caring for hospitalized patients back to long‐term care facilities, has room for improvement. They highlight that 37.5% of patients had documented discussions, and for cases in which these discussions resulted in changes to a patient's advance directive, only 1 in 4 were relayed in the discharge summary.[1]
As physicians caring for hospitalized patients and concerned with improving care quality and efficiency, many of us are familiar with potential systems solutions to augmenting communication: reminders in the electronic health record, checklists, multidisciplinary teams, scripts, and posthospitalization follow‐up phone calls. However, important as they are, these solutions often elide the underlying cognitive elements related to how we, as physicians, think about and engage in the diversity of cases presented to us, and to how we prioritize communication work.
Wong et al. looked at patient characteristics associated with performance of GOC discussions to understand when and why physicians might engage in GOC conversations in the hospital and to generate insights into potential targets for improvement. They found that characteristics of patients prior to hospital admission were not associated with GOC discussions; signs of acuity of illness were.[1] In other words, physicians in the hospital are pretty good at recognizing patients in extremis, and prioritize GOC discussions with these patients. What we are not good at, or might not be considering, is assessing the broader context of a patient's health.
Whether we interpret these results as appropriate prioritization, or as a sign that we are waiting too long to broach the subject of care goals, depends on how we conceptualize the hospital stay in the context of a patient's health story, and, by extension, the role of the hospitalist in this story. For some patients, an acute illness requiring hospitalization is unexpected and readily treated, and the patient rapidly returns to a prior level of health and function. The need for hospitalization represents an outlier state.
For other patients, often older, more debilitated, or with multiple and chronic medical conditions, minor changes in health or declines in mental, social, or physical function precipitate the need for hospitalization. Likewise, iatrogenic harms of hospitalizationsleeplessness, fasting, delirium, immobilitycan contribute to enduring decline.[2, 3] For these patients, the need for hospitalization is not so far from, or may be, their norm.[4]
I suspect that Wong et al.'s findings reflect a collective response to the uncertainties of prognostication, and the resultant discomfort in raising questions that are difficult to answer. How do we know it is time to start talking about the right amount of care? Some might answer, I think rightly, that it is rarely if ever too early, yet robust discussions are challenging if we are not sure of the relevance or the immediate goal. In the case of the patient who is ill, declining, yet not in extremis, many of us might conclude that raising the question would not produce actionable information; it would not change immediate in‐hospital management.
This common conclusion leads to a significant missed opportunity, both on an individual level for physicians and patients, and for hospital medicine as a specialty. Health, and the losses that come with declining health, are wrapped up with fundamental aspects of our identities, and take time and consideration to change and evolve. Decisions about our healthcare are statements about who we have been, who we are, and who we will no longer be. Especially for the second group of patients described above, each hospital stay affords a chance to assess, counsel, educate, support, and empower patients to move in the direction of their values, and to ready them for that eventuality when they or their loved ones are faced with decisions about how, and where, they will die. As specialists in hospital‐based healthcare, hospitalists have the privilege and professional duty to facilitate this journey.
However, as hospitalists, we are often meeting patients for the first time; how do we assimilate an understanding of that point in time within the context of a patient's life with enough confidence to engage discussions? As Wong et al. show, it appears that in regard to very ill patients, respiratory rate and Glasgow Coma Scale inform action.[1] What signs or observations help inform action earlier in the trajectory of decline, to allow for anticipatory guidance and discussion? Increasingly, we see evidence that measures of frailty and functional status, applied in the hospital, are associated with hospital outcomes including readmission and death.[5, 6, 7] Future work might explore if training physicians to systematically assess frailty and functional status leads to greater frequency of, and comfort with, initiating GOC discussions during hospitalization.
Moreover, an emphasis on evaluating frailty and function, and explicitly including this assessment in our clinical decision‐making might help shift our thinking toward valuing each hospitalization as an opportunity to both intervene to improve function[8, 9] and to support, educate, and prepare patients under our care for the journey aheadin other words, to fully engage with our role as specialists in the comprehensive and coordinated treatment of patients who require hospitalization.
In their study of goals of care (GOC) discussions and documentation, Wong et al. add to already robust evidence that communication, in this case from physicians caring for hospitalized patients back to long‐term care facilities, has room for improvement. They highlight that 37.5% of patients had documented discussions, and for cases in which these discussions resulted in changes to a patient's advance directive, only 1 in 4 were relayed in the discharge summary.[1]
As physicians caring for hospitalized patients and concerned with improving care quality and efficiency, many of us are familiar with potential systems solutions to augmenting communication: reminders in the electronic health record, checklists, multidisciplinary teams, scripts, and posthospitalization follow‐up phone calls. However, important as they are, these solutions often elide the underlying cognitive elements related to how we, as physicians, think about and engage in the diversity of cases presented to us, and to how we prioritize communication work.
Wong et al. looked at patient characteristics associated with performance of GOC discussions to understand when and why physicians might engage in GOC conversations in the hospital and to generate insights into potential targets for improvement. They found that characteristics of patients prior to hospital admission were not associated with GOC discussions; signs of acuity of illness were.[1] In other words, physicians in the hospital are pretty good at recognizing patients in extremis, and prioritize GOC discussions with these patients. What we are not good at, or might not be considering, is assessing the broader context of a patient's health.
Whether we interpret these results as appropriate prioritization, or as a sign that we are waiting too long to broach the subject of care goals, depends on how we conceptualize the hospital stay in the context of a patient's health story, and, by extension, the role of the hospitalist in this story. For some patients, an acute illness requiring hospitalization is unexpected and readily treated, and the patient rapidly returns to a prior level of health and function. The need for hospitalization represents an outlier state.
For other patients, often older, more debilitated, or with multiple and chronic medical conditions, minor changes in health or declines in mental, social, or physical function precipitate the need for hospitalization. Likewise, iatrogenic harms of hospitalizationsleeplessness, fasting, delirium, immobilitycan contribute to enduring decline.[2, 3] For these patients, the need for hospitalization is not so far from, or may be, their norm.[4]
I suspect that Wong et al.'s findings reflect a collective response to the uncertainties of prognostication, and the resultant discomfort in raising questions that are difficult to answer. How do we know it is time to start talking about the right amount of care? Some might answer, I think rightly, that it is rarely if ever too early, yet robust discussions are challenging if we are not sure of the relevance or the immediate goal. In the case of the patient who is ill, declining, yet not in extremis, many of us might conclude that raising the question would not produce actionable information; it would not change immediate in‐hospital management.
This common conclusion leads to a significant missed opportunity, both on an individual level for physicians and patients, and for hospital medicine as a specialty. Health, and the losses that come with declining health, are wrapped up with fundamental aspects of our identities, and take time and consideration to change and evolve. Decisions about our healthcare are statements about who we have been, who we are, and who we will no longer be. Especially for the second group of patients described above, each hospital stay affords a chance to assess, counsel, educate, support, and empower patients to move in the direction of their values, and to ready them for that eventuality when they or their loved ones are faced with decisions about how, and where, they will die. As specialists in hospital‐based healthcare, hospitalists have the privilege and professional duty to facilitate this journey.
However, as hospitalists, we are often meeting patients for the first time; how do we assimilate an understanding of that point in time within the context of a patient's life with enough confidence to engage discussions? As Wong et al. show, it appears that in regard to very ill patients, respiratory rate and Glasgow Coma Scale inform action.[1] What signs or observations help inform action earlier in the trajectory of decline, to allow for anticipatory guidance and discussion? Increasingly, we see evidence that measures of frailty and functional status, applied in the hospital, are associated with hospital outcomes including readmission and death.[5, 6, 7] Future work might explore if training physicians to systematically assess frailty and functional status leads to greater frequency of, and comfort with, initiating GOC discussions during hospitalization.
Moreover, an emphasis on evaluating frailty and function, and explicitly including this assessment in our clinical decision‐making might help shift our thinking toward valuing each hospitalization as an opportunity to both intervene to improve function[8, 9] and to support, educate, and prepare patients under our care for the journey aheadin other words, to fully engage with our role as specialists in the comprehensive and coordinated treatment of patients who require hospitalization.
- , , , . Goals of care discussions among hospitalized long‐term care residents: predictors and associated outcomes of care. J Hosp Med. 2016;11(12):824–831.
- . Hazards of hospitalization of the elderly. Ann Intern Med. 1993;118(3):219–223.
- , , . Hospitalization‐associated disability: “she was probably able to ambulate, but I'm not sure.” JAMA. 2011;306(16):1782–1793.
- , . The hospital‐dependent patient. N Engl J Med. 2014;370(8):694–697.
- , , , et al. Functional status outperforms comorbidities in predicting acute care readmissions in medically complex patients. J Gen Intern Med. 2015;30(11):1688–1695.
- , , , et al. Comparing three different measures of frailty in medical inpatients: multicenter prospective cohort study examining 30‐day risk of readmission or death [published online May 17, 2016]. J Hosp Med. doi: 10.1002/jhm.2607.
- , , , et al. Association between frailty and 30‐day outcomes after discharge from hospital. CMAJ. 2015;187(11):799–804.
- , , , et al. Comparison of posthospitalization function and community mobility in hospital mobility program and usual care patients: a randomized clinical trial [published online May 31, 2016]. JAMA Intern Med. doi: 10.1001/jamainternmed.2016.1870.
- . Activating hospitalized older patients to confront the epidemic of low mobility [published online May 31 2016]. JAMA Intern Med. doi: 10.1001/jamainternmed.2016.1874.
- , , , . Goals of care discussions among hospitalized long‐term care residents: predictors and associated outcomes of care. J Hosp Med. 2016;11(12):824–831.
- . Hazards of hospitalization of the elderly. Ann Intern Med. 1993;118(3):219–223.
- , , . Hospitalization‐associated disability: “she was probably able to ambulate, but I'm not sure.” JAMA. 2011;306(16):1782–1793.
- , . The hospital‐dependent patient. N Engl J Med. 2014;370(8):694–697.
- , , , et al. Functional status outperforms comorbidities in predicting acute care readmissions in medically complex patients. J Gen Intern Med. 2015;30(11):1688–1695.
- , , , et al. Comparing three different measures of frailty in medical inpatients: multicenter prospective cohort study examining 30‐day risk of readmission or death [published online May 17, 2016]. J Hosp Med. doi: 10.1002/jhm.2607.
- , , , et al. Association between frailty and 30‐day outcomes after discharge from hospital. CMAJ. 2015;187(11):799–804.
- , , , et al. Comparison of posthospitalization function and community mobility in hospital mobility program and usual care patients: a randomized clinical trial [published online May 31, 2016]. JAMA Intern Med. doi: 10.1001/jamainternmed.2016.1870.
- . Activating hospitalized older patients to confront the epidemic of low mobility [published online May 31 2016]. JAMA Intern Med. doi: 10.1001/jamainternmed.2016.1874.
Prior Opioid use Among Veterans
Recent trends show a marked increase in outpatient use of chronic opioid therapy (COT) for chronic noncancer pain (CNCP)[1, 2] without decreases in reported CNCP,[3] raising concerns about the efficacy and risk‐to‐benefit ratio of opioids in this population.[4, 5, 6, 7, 8] Increasing rates of outpatient use likely are accompanied by increasing rates of opioid exposure among patients admitted to the hospital. To our knowledge there are no published data regarding the prevalence of COT during the months preceding hospitalization.
Opioid use has been linked to increased emergency room utilization[9, 10] and emergency hospitalization,[11] but associations between opioid use and inpatient metrics (eg, mortality, readmission) have not been explored. Furthermore, lack of knowledge about the prevalence of opioid use prior to hospitalization may impede efforts to improve inpatient pain management and satisfaction with care. Although there is reason to expect that strategies to safely and effectively treat acute pain during the inpatient stay differ between opioid‐nave patients and opioid‐exposed patients, evidence regarding treatment strategies is limited.[12, 13, 14] Opioid pain medications are associated with hospital adverse events, with both prior opioid exposure and lack of opioid use as proposed risk factors.[15] A better understanding of the prevalence and characteristics of hospitalized COT patients is fundamental to future work to achieve safer and more effective inpatient pain management.
The primary purpose of this study was to determine the prevalence of prior COT among hospitalized medical patients. Additionally, we aimed to characterize inpatients with occasional and chronic opioid therapy prior to admission in comparison to opioid‐nave inpatients, as differences between these groups may suggest directions for further investigation into the distinct needs or challenges of hospitalized opioid‐exposed patients.
METHODS
We used inpatient and outpatient administrative data from the Department of Veterans Affairs (VA) Healthcare System. The primary data source to identify acute medical admissions was the VA Patient Treatment File, a national administrative database of all inpatient admissions, including patient demographic characteristics, primary and secondary diagnoses (using International Classification of Diseases, 9th Revision, Clinical Modification [ICD‐9‐CM], codes), and hospitalization characteristics. Outpatient pharmacy data were from the VA Pharmacy Prescription Data Files. The VA Vital Status Files provided dates of death.
We identified all first acute medical admissions to 129 VA hospitals during fiscal years (FYs) 2009 to 2011 (October 2009September 2011). We defined first admissions as the initial medical hospitalization occurring following a minimum 365‐day hospitalization‐free period. Patients were required to demonstrate pharmacy use by receipt of any outpatient medication from the VA on 2 separate occasions within 270 days preceding the first admission, to avoid misclassification of patients who routinely obtained medications only from a non‐VA provider. Patients admitted from extended care facilities were excluded.
We grouped patients by opioid‐use status based on outpatient prescription records: (1) no opioid use, defined as no opioid prescriptions in the 6 months prior to hospitalization; (2) occasional opioid use, defined as patients who received any opioid prescription during the 6 months prior but did not meet definition of chronic use; and (3) chronic opioid therapy, defined as 90 or more days' supply of opioids received within 6 months preceding hospitalization. We did not specify continuous prescribing. Opioids included in the definition were codeine, dihydrocodeine, fentanyl (mucosal and topical), hydrocodone, hydromorphone, meperidine, methadone, morphine, oxycodone, oxymorphone, pentazocine, propoxyphene, tapentadol, and tramadol.[16, 17]
We compared groups by demographic variables including age, sex, race, income, rural vs urban residence (determined from Rural‐Urban Commuting Area codes), region based on hospital location; overall comorbidity using the Charlson Comorbidity Index (CCI);[18] and 10 selected conditions to characterize comorbidity (see Supporting Information, Appendix A, in the online version of this article). These 10 conditions were chosen based on probable associations with chronic opioid use or high prevalence among hospitalized veterans.[9, 19, 20]
We used a CNCP definition based on ICD‐9‐CM codes.[9] This definition did not include episodic conditions such as migraine[2] or a measure of pain intensity.[21] All conditions were determined from diagnoses coded during any encounter in the year prior to hospitalization, exclusive of the first (ie, index) admission. We also determined the frequency of palliative care use, defined as presence of ICD‐9‐CM code V667 during index hospitalization or within the past year. Patients with palliative care use (n=3070) were excluded from further analyses.
We compared opioid use groups by baseline characteristics using the [2] statistic to determine if the distribution was nonrandom. We used analysis of variance to compare hospital length of stay between groups. We used the [2] statistic to compare rates of 4 outcomes of interest: intensive care unit (ICU) admission during the index hospitalization, discharge disposition other than home, 30‐day readmission rate, and in‐hospital or 30‐day mortality.
To assess the association between opioid‐use status and the 4 outcomes of interest, we constructed 2 multivariable regression models; the first was adjusted only for admission diagnosis using the Clinical Classification Software (CCS),[22] and the second was adjusted for demographics, CCI, and the 10 selected comorbidities in addition to admission diagnosis.
The authors had full access to and take full responsibility for the integrity of the data. All analyses were conducted using SAS statistical software version 9.2 (SAS Institute, Cary, NC). The study was approved by the University of Iowa institutional review board and the Iowa City VA Health Care System Research and Development Committee.
RESULTS
Patient Demographics
Demographic characteristics of patients differed by opioid‐use group (Table 1). Hospitalized patients who received COT in the 6 months prior to admission tended to be younger than their comparators, more often female, white, have a rural residence, and live in the South or West.
| Variables | No Opioids, n=66,899 (54.5%) | Occasional Opioids, n=24,093 (19.6%) | Chronic Opioids, n=31,802 (25.9%) |
|---|---|---|---|
| |||
| Age, y, mean (SD) | 68.7 (12.8) | 66.5 (12.7) | 64.5 (11.5) |
| Age, n (%) | |||
| 59 (reference) | 15,170 (22.7) | 6,703 (27.8) | 10,334 (32.5) |
| 6065 | 15,076 (22.5) | 5,973 (24.8) | 8,983 (28.3) |
| 6677 | 17,226 (25.8) | 5,871 (24.4) | 7,453 (23.4) |
| 78 | 19,427 (29.0) | 5,546 (23.0) | 5,032 (15.8) |
| Male, n (%) | 64,673 (96.7) | 22,964 (95.3) | 30,200 (95.0) |
| Race, n (%) | |||
| White | 48,888 (73.1) | 17,358 (72.1) | 25,087 (78.9) |
| Black | 14,480 (21.6) | 5,553 (23.1) | 5,089 (16.0) |
| Other | 1,172 (1.8) | 450 (1.9) | 645 (2.0) |
| Unknown | 2,359 (3.5) | 732 (3.0) | 981 (3.1) |
| Income $20,000, n (%) | 40,414 (60.4) | 14,105 (58.5) | 18,945 (59.6) |
| Rural residence, n (%) | 16,697 (25.0) | 6,277 (26.1) | 9,356 (29.4) |
| Region, n (%) | |||
| Northeast | 15,053 (22.5) | 4,437 (18.4) | 5,231 (16.5) |
| South | 24,083 (36.0) | 9,390 (39.0) | 12,720 (40.0) |
| Midwest | 16,000 (23.9) | 5,714 (23.7) | 7,762 (24.4) |
| West | 11,763 (17.6) | 4,552 (18.9) | 6,089 (19.2) |
| Charlson Comorbidity Index, mean (SD) | 2.3 (2.0) | 2.6 (2.3) | 2.7 (2.3) |
| Comorbidities, n (%) | |||
| Cancer (not metastatic) | 11,818 (17.7) | 5,549 (23.0) | 6,874 (21.6) |
| Metastatic cancer | 866 (1.3) | 733 (3.0) | 1,104 (3.5) |
| Chronic pain | 25,748 (38.5) | 14,811 (61.5) | 23,894 (75.1) |
| COPD | 20,750 (31.0) | 7,876 (32.7) | 12,117 (38.1) |
| Diabetes, complicated | 10,917 (16.3) | 4,620 (19.2) | 6,304 (19.8) |
| Heart failure | 14,267 (21.3) | 5,035 (20.9) | 6,501 (20.4) |
| Renal disease | 11,311 (16.9) | 4,586 (19.0) | 4,981 (15.7) |
| Dementia | 2,180 (3.3) | 459 (1.9) | 453 (1.4) |
| Mental health other than PTSD | 33,390 (49.9) | 13,657 (56.7) | 20,726 (65.2) |
| PTSD | 7,216 (10.8) | 3,607 (15.0) | 5,938 (18.7) |
| Palliative care use, n (%) | 1,407 (2.1) | 639 (2.7) | 1,024 (3.2) |
Prevalence of Opioid Use
Among the cohort (N=122,794) of hospitalized veterans, 66,899 (54.5%) received no opioids from the VA during the 6‐month period prior to hospitalization; 31,802 (25.9%) received COT in the 6 months prior to admission. An additional 24,093 (19.6%) had occasional opioid therapy (Table 1). A total of 257,623 opioid prescriptions were provided to patients in the 6‐month period prior to their index hospitalization. Of these, 100,379 (39.0%) were for hydrocodone, 48,584 (18.9%) for oxycodone, 36,658 (14.2%) for tramadol, and 35,471 (13.8%) for morphine. These 4 medications accounted for 85.8% of total opioid prescriptions (see Supporting Information, Appendix B, in the online version of this article).
Among the COT group, 3610 (11.4%) received opioids 90 days, 10,110 (31.8%) received opioids between 91 and 179 days, and 18,082 (56.9%) patients received opioids 180 days in the prior 6 months (see Supporting Information, Appendix C, in the online version of this article).
Among the subset of patients with cancer (metastatic and nonmetastatic, n=26,944), 29.6% were prescribed COT, and 23.3% had occasional opioid use. Among the subset of patients with CNCP (n=64,453), 37.1% were prescribed COT, and 23.0% had occasional opioid use.
Comorbid Conditions
Compared to patients not receiving opioids, a larger proportion of patients receiving both occasional and chronic opioids had diagnoses of cancer and of CNCP. Diagnoses more common in COT patients included chronic obstructive pulmonary disease (COPD), complicated diabetes, post‐traumatic stress disorder (PTSD), and other mental health disorders. In contrast, COT patients were less likely than no‐opioid and occasional opioid patients to have heart failure (HF), renal disease, and dementia. Palliative care was used by 2.1% of patients in the no‐opioid group, and 3.2% of patients in the COT group (Table 1). Renal disease was most common among the occasional‐use group.
Unadjusted Hospitalization Outcomes
Unadjusted hospitalization outcomes differed between opioid‐exposure groups (Table 2). Patients receiving occasional or chronic opioids had shorter length of stay and lower rates of non‐home discharge than did patients without any opioid use. The rate of death during hospitalization or within 30 days did not differ between groups. The occasional‐use and COT groups had higher 30‐day readmission rates than did the no‐use group.
| No Opioids, n=65,492 | Occasional Opioids, n=23,454 | Chronic Opioids, n=30,778 | P | |
|---|---|---|---|---|
| ||||
| Hospital length of stay, d, mean (SD) | 4.7 (5.1) | 4.5 (4.8) | 4.5 (4.8) | 0.0003 |
| ICU stay, n (%) | 10,281 (15.7) | 3,299 (14.1) | 4,570 (14.9) | <0.0001 |
| Non‐home discharge, n (%) | 2,944 (4.5) | 997 (4.3) | 1,233 (4.0) | 0.0020 |
| 30‐day readmission, n (%) | 9,023 (13.8) | 3,629 (15.5) | 4,773 (15.5) | <0.0001 |
| Death during hospitalization or within 30 days, n (%) | 2,532 (3.9) | 863 (3.7) | 1,191 (3.9) | 0.4057 |
Multivariable Models
In the fully adjusted multivariable models, opioid exposure (in the form of either chronic or occasional use) had no significant association with ICU stay during index admission or non‐home discharge (Table 3). Both the occasional‐opioid use and COT groups were more likely to experience 30‐day hospital readmission, a relationship that remained consistent across the partially and fully adjusted models. The occasional‐opioid use group saw no increased mortality risk. In the model adjusted only for admission diagnosis, COT was not associated with increased mortality risk. When additionally adjusted for demographic variables, CCI, and selected comorbidities, however, COT was associated with increased risk of death during hospitalization or within 30 days (odds ratio: 1.19, 90% confidence interval: 1.10‐1.29).
| Occasional Opioid Use | Chronic Opioid Therapy | |||
|---|---|---|---|---|
| Model 1, OR (95% CI) | Model 2, OR (95% CI) | Model 1, OR (95% CI) | Model 2, OR (95% CI) | |
| ||||
| ICU stay | 0.94 (0.90‐0.99) | 0.95 (0.91‐1.00) | 1.00 (0.96‐1.04) | 1.01 (0.97‐1.05) |
| Non‐home discharge | 0.92 (0.85‐0.99) | 0.97 (0.90‐1.05) | 0.85 (0.80‐0.92) | 0.95 (0.88‐1.03) |
| 30‐day readmission | 1.14 (1.09‐1.19) | 1.14 (1.09‐1.19) | 1.14 (1.10‐1.19) | 1.15 (1.10‐1.20) |
| Death during hospitalization or within 30 days | 0.96 (0.88‐1.04) | 1.04 (0.95‐1.13) | 0.96 (0.90‐1.04) | 1.19 (1.10‐1.29) |
DISCUSSION
This observational study is, to our knowledge, the first to report prevalence of and characteristics associated with prior opioid use among hospitalized medical patients. The prevalence of any opioid use and of COT was substantially higher in this hospitalized cohort than reported in outpatient settings. The prevalence of any opioid use during 1 year (FY 2009) among all veterans with VA primary care use was 26.1%.[23] A study of incident prescribing rates among veterans with new diagnoses of noncancer‐related pain demonstrated 11% received an opioid prescription within 1 year.[24] Using a definition of 90 consecutive prescription days to define COT, Dobscha et al.[25] found that 5% of veterans with persistent elevated pain intensity and no previous opioid prescriptions subsequently received COT within 12 months. The high prevalence we found likely reflects cumulative effects of incident use as well as an increased symptom burden in a population defined by need for medical hospitalization.
Although a veteran population may not be generalizable to a nonveteran setting, we do note prior studies reporting prevalence of any opioid use in outpatient cohorts (in 2000 and 2005) of between 18% and 30%, with higher rates among women and patients over 65 years of age.[1, 2]
Our work was purposefully inclusive of cancer patients so that we might assess the degree to which cancer diagnoses accounted for prior opioid use in hospitalized patients. Surprisingly, the rate of COT for patients with cancer was lower than that for patients with CNCP, perhaps reflecting that a cancer condition defined in administrative data may not constitute a pain‐causing disease.
Recognition of the prevalence of opioid therapy is important as we work to understand and improve safety, satisfaction, utilization, and long‐term health outcomes associated with hospitalization. Our finding that over half of medical inpatients have preexisting CNCP diagnoses, and a not entirely overlapping proportion has prior opioid exposure, implies a need for future work to refine expectations and strategies for inpatient management, potentially tailored to prior opioid use and presence of CNCP.
A recent Joint Commission sentinel event alert[26] highlights opioid adverse events in the hospital and identifies both lack of previous opioid therapy and prior opioid therapy as factors increasing risk. ICU admission during the hospital stay may reflect adverse events such as opioid‐induced respiratory depression; in our study, patients with no opioid use prior to admission were more likely to have an ICU stay, although the effect was small. One might speculate that clinicians, accustomed to treating pain in opioid‐exposed patients, are using inappropriately large starting dosages of narcotics for inpatients without first assessing prior opioid exposure. Another possible explanation is that patients on COT are admitted to the hospital with less severe illness, potentially reflecting functional, social, or access limitations that compromise ability to manage illness in the outpatient setting. More detailed comparison of illness severity is beyond the scope of the present work.
Patient satisfaction with pain management is reflected in 2 of the Hospital Consumer Assessment of Healthcare Providers and Systems (HCAHPS) questions, and is publically reported.[27] HCAHPS results also figure in the formula for the Centers for Medicare and Medicaid Services value‐based purchasing.[28] Preadmission pain is predictive of postoperative pain[29, 30] and may shape patient expectations; how preadmission opioid use modulates nonsurgical pain and satisfaction with management in the medical inpatient remains to be studied. The high prevalence of prior COT underscores the importance of understanding characteristics of patients on COT, and potential differences and disparities in pain management, when designing interventions to augment patient satisfaction with pain management.
Although the age distribution and patterns of comorbidities differed between the opioid‐use groups, opioid therapy remained a small but significant predictor of hospital readmission; this association was independent of CNCP diagnosis. Functional outcomes are recognized as important measures of efficacy of outpatient pain management strategies,[31] with some evidence that opioids are associated with worse functioning.[32, 33] Functional limitations, as well as inadequately or inappropriately treated pain, may drive both admissions and readmissions. Alternately, COT may be a marker for unmeasured factors that increase a patient's risk of returning to the hospital. Further work is needed to elucidate the relationship between COT and healthcare utilization associated with the inpatient stay.
Our finding that patients on COT have an increased mortality risk is concerning, given the rapid expansion in use of these medications. Although pain is increasingly prevalent toward end of life,[34] we did not observe an association between either CNCP (data not shown) or occasional opioid use and mortality. COT may complicate chronic disease through adverse drug effects including respiratory depression, apnea, or endocrine or immune alteration. Complex chronically ill patients with conditions such as COPD, HF, or diabetes may be particularly susceptible to these effects. Incident use of morphine is associated with increased mortality in acute coronary syndrome and HF[35, 36]: we are not aware of any work describing the relationship between prior opioid use and incident use during hospitalization in medical patients.
Limitations
Our work focuses on hospitalized veterans, a population that remains predominately male, limiting generalizability of the findings. Rates of mental health diagnoses and PTSD, associated with CNCP and COT,[24, 37] are higher in this population than would be expected in a general hospitalized population. Because our outcomes included readmission, and our definition of opioid exposure was designed to reflect outpatient prescribing, we included only patients without recent hospitalization. Therefore, our results may not be generalizable to patients with frequent and recurring hospitalization.
Our definition of opioid exposure depended on pharmacy dispensing records; we are not able to confirm if veterans were taking the medications as prescribed. Further, we were not able to capture data on opioids prescribed by non‐VA providers, which may have led to underestimation of prevalence.
Our definitions of COT and CNCP are imperfect, and should be noted when comparing to other studies. Because we did not specify continuous 90‐day prescribing, we may have misclassified occasional opioid therapy as COT in comparison to other authors. That continuous prescribing is equivalent to continuous use assumes that patients take medications exactly as prescribed. We used occasional opioid therapy as a comparison group, and detailed the distribution of days prescribed among the COT group (see Supporting Information, Appendix C, in the online version of this article), to augment interpretability of these results. Our CNCP diagnosis was less inclusive than others,[2] as we omitted episodic pain (eg, migraine and sprains) and human immunodeficiency virus‐related pain. As COT for CNCP conditions lacks a robust evidence base,[38] defining pain diagnoses using administrative data to reflect conditions for which COT is used in a guideline‐concordant way remains difficult.
Last, differences observed between opioid‐use groups may be due to an unmeasured confounder not captured by the variables we included. Specifically, we did not include other long‐term outpatient medications in our models. It is possible that COT is part of a larger context of inappropriate prescribing, rather than a single‐medication effect on outcomes studied.
CONCLUSION
Nearly 1 in 4 hospitalized veterans has current or recent COT at the time of hospital admission for nonsurgical conditions; nearly half have been prescribed any opioids. Practitioners designing interventions to improve pain management in the inpatient setting should account for prior opioid use. Patients who are on COT prior to hospitalization differ in age and comorbidities from their counterparts who are not on COT. Further elucidation of differences between opioid‐use groups may help providers address care needs during the transition to posthospitalization care. CNCP diagnoses and chronic opioid exposure are different entities and cannot serve as proxies in administrative data. Additional work on utilization and outcomes in specific patient populations may improve our understanding of the long‐term health effects of chronic opioid therapy.
Disclosures: Dr. Mosher is supported by the Veterans Administration (VA) Quality Scholars Fellowship, Office of Academic Affiliations, Department of Veterans Affairs. Dr. Cram is supported by a K24 award from NIAMS (AR062133) at the National Institutes of Health. The preliminary results of this article were presented at the Society of General Internal Medicine Annual Meeting in Denver, Colordao, April 2013. The views expressed in this article are those of the authors and do not necessarily represent the views of the Department of Veterans Affairs. Data are available to researchers with VA accreditation, the statistical code and the protocol are available to interested readers by contacting Dr. Mosher. The authors report no conflict of interest in regard to this study.
- , , , et al. Age and gender trends in long‐term opioid analgesic use for noncancer pain. Am J Public Health. 2010;100:2541–2547.
- , , , , , . Trends in use of opioids for non‐cancer pain conditions 2000–2005 in commercial and Medicaid insurance plans: the TROUP study. Pain. 2008;138:440–449.
- Institute of Medicine (US) Committee on Advancing Pain Research, Care, and Education. Relieving pain in America: a blueprint for transforming prevention, care, education, and research. Washington, DC: National Academies Press; 2011.
- , , , . Long‐term opioid therapy reconsidered. Ann Intern Med. 2011;155:325–328.
- , . What are we treating with long‐term opioid therapy? Arch Intern Med. 2012;172:433–434.
- , , , . Opioids for chronic noncancer pain: a meta‐analysis of effectiveness and side effects. CMAJ. 2006;174:1589–1594.
- , , , . Opioids in chronic non‐cancer pain: systematic review of efficacy and safety. Pain. 2004;112:372–380.
- , , , et al. A systematic review of randomized trials of long‐term opioid management for chronic non‐cancer pain. Pain Physician. 2011;14:91–121.
- , , , , , . Rates of adverse events of long‐acting opioids in a state Medicaid program. Ann Pharmacother. 2007;41:921–928.
- , , , et al. Emergency department visits among recipients of chronic opioid therapy. Arch Intern Med. 2010;170:1425–1432.
- , , , . Emergency hospitalizations for adverse drug events in older Americans. N Engl J Med. 2011;365:2002–2012.
- , . Assessment and management of acute pain in adult medical inpatients: a systematic review. Pain Med. 2009;10:1183–1199.
- , , , . Acute pain management in opioid‐tolerant patients: a growing challenge. Anaesth Intensive Care. 2011;39:804–823.
- , , , . Acute pain management of the chronic pain patient on opiates: a survey of caregivers at University of Washington Medical Center. Clin J Pain. 1994;10:133–138.
- The Joint Commission and the FDA take steps to curb adverse events related to the use and misuse of opioid drugs. ED Manag. 2012;24:112–116.
- , . Tramadol. CMAJ. 2013;185:E352.
- , , , , . Prescription opioid abuse in the United Kingdom. Br J Clin Pharmacol. 2013;76:823–824.
- , , , . Validation of a combined comorbidity index. J Clin Epidemiol. 1994;47:1245–1251.
- , , , , . Bringing the war back home: mental health disorders among 103,788 US veterans returning from Iraq and Afghanistan seen at Department of Veterans Affairs facilities. Arch Intern Med. 2007;167:476–482.
- , , , et al. Coding algorithms for defining comorbidities in ICD‐9‐CM and ICD‐10 administrative data. Med Care. 2005;43:1130–1139.
- , , , , , . Sex Differences in the medical care of VA patients with chronic non‐cancer pain [published online ahead of print June 26, 2013]. Pain Med. doi: 10.1111/pme.12177.
- Agency for Healthcare Research and Quality. Clinical Classifications Software (CCS) for ICD‐9‐CM. Available at: http://www.hcup‐us.ahrq.gov/toolssoftware/ccs/ccs.jsp. Accessed October 17, 2013.
- , , , et al. Predicting risk of hospitalization or death among patients receiving primary care in the Veterans Health Administration. Med Care. 2013;51:368–373.
- , , , et al. Association of mental health disorders with prescription opioids and high‐risk opioid use in US veterans of Iraq and Afghanistan. JAMA. 2012;307:940–947.
- , , , , . Correlates of prescription opioid initiation and long‐term opioid use in veterans with persistent pain. Clin J Pain. 2013;29:102–108.
- Safe use of opioids in hospitals. Sentinel Event Alert. 2012;49:1–5.
- Centers for Medicare (2):2–9.
- , , , , , . The risk of severe postoperative pain: modification and validation of a clinical prediction rule. Anesth Analg. 2008;107:1330–1339.
- , , , et al. Preoperative predictors of moderate to intense acute postoperative pain in patients undergoing abdominal surgery. Acta Anaesthesiol Scand. 2002;46:1265–1271.
- , , , , , . Successful and unsuccessful outcomes with long‐term opioid therapy: a survey of physicians' opinions. J Palliat Med. 2006;9:50–56.
- , , , . Opioid use among low back pain patients in primary care: is opioid prescription associated with disability at 6‐month follow‐up? Pain. 2013;154:1038–1044.
- , , , , ; Disability Risk Identification Study Cohort. Early opioid prescription and subsequent disability among workers with back injuries: the Disability Risk Identification Study Cohort. Spine (Phila Pa 1976). 2008;33:199–204.
- , , , et al. The epidemiology of pain during the last 2 years of life. Ann Intern Med. 2010;153:563–569.
- , , , et al. Association of intravenous morphine use and outcomes in acute coronary syndromes: results from the CRUSADE Quality Improvement Initiative. Am Heart J. 2005;149:1043–1049.
- , , , et al. Use of intravenous morphine for acute decompensated heart failure in patients with and without acute coronary syndromes. Acute Card Care. 2011;13:76–80.
- , , , et al. VA mental health services utilization in Iraq and Afghanistan veterans in the first year of receiving new mental health diagnoses. J Trauma Stress. 2010;23:5–16.
- , , , et al. Long‐term opioid management for chronic noncancer pain. Cochrane Database Syst Rev. 2010;(1):CD006605.
Recent trends show a marked increase in outpatient use of chronic opioid therapy (COT) for chronic noncancer pain (CNCP)[1, 2] without decreases in reported CNCP,[3] raising concerns about the efficacy and risk‐to‐benefit ratio of opioids in this population.[4, 5, 6, 7, 8] Increasing rates of outpatient use likely are accompanied by increasing rates of opioid exposure among patients admitted to the hospital. To our knowledge there are no published data regarding the prevalence of COT during the months preceding hospitalization.
Opioid use has been linked to increased emergency room utilization[9, 10] and emergency hospitalization,[11] but associations between opioid use and inpatient metrics (eg, mortality, readmission) have not been explored. Furthermore, lack of knowledge about the prevalence of opioid use prior to hospitalization may impede efforts to improve inpatient pain management and satisfaction with care. Although there is reason to expect that strategies to safely and effectively treat acute pain during the inpatient stay differ between opioid‐nave patients and opioid‐exposed patients, evidence regarding treatment strategies is limited.[12, 13, 14] Opioid pain medications are associated with hospital adverse events, with both prior opioid exposure and lack of opioid use as proposed risk factors.[15] A better understanding of the prevalence and characteristics of hospitalized COT patients is fundamental to future work to achieve safer and more effective inpatient pain management.
The primary purpose of this study was to determine the prevalence of prior COT among hospitalized medical patients. Additionally, we aimed to characterize inpatients with occasional and chronic opioid therapy prior to admission in comparison to opioid‐nave inpatients, as differences between these groups may suggest directions for further investigation into the distinct needs or challenges of hospitalized opioid‐exposed patients.
METHODS
We used inpatient and outpatient administrative data from the Department of Veterans Affairs (VA) Healthcare System. The primary data source to identify acute medical admissions was the VA Patient Treatment File, a national administrative database of all inpatient admissions, including patient demographic characteristics, primary and secondary diagnoses (using International Classification of Diseases, 9th Revision, Clinical Modification [ICD‐9‐CM], codes), and hospitalization characteristics. Outpatient pharmacy data were from the VA Pharmacy Prescription Data Files. The VA Vital Status Files provided dates of death.
We identified all first acute medical admissions to 129 VA hospitals during fiscal years (FYs) 2009 to 2011 (October 2009September 2011). We defined first admissions as the initial medical hospitalization occurring following a minimum 365‐day hospitalization‐free period. Patients were required to demonstrate pharmacy use by receipt of any outpatient medication from the VA on 2 separate occasions within 270 days preceding the first admission, to avoid misclassification of patients who routinely obtained medications only from a non‐VA provider. Patients admitted from extended care facilities were excluded.
We grouped patients by opioid‐use status based on outpatient prescription records: (1) no opioid use, defined as no opioid prescriptions in the 6 months prior to hospitalization; (2) occasional opioid use, defined as patients who received any opioid prescription during the 6 months prior but did not meet definition of chronic use; and (3) chronic opioid therapy, defined as 90 or more days' supply of opioids received within 6 months preceding hospitalization. We did not specify continuous prescribing. Opioids included in the definition were codeine, dihydrocodeine, fentanyl (mucosal and topical), hydrocodone, hydromorphone, meperidine, methadone, morphine, oxycodone, oxymorphone, pentazocine, propoxyphene, tapentadol, and tramadol.[16, 17]
We compared groups by demographic variables including age, sex, race, income, rural vs urban residence (determined from Rural‐Urban Commuting Area codes), region based on hospital location; overall comorbidity using the Charlson Comorbidity Index (CCI);[18] and 10 selected conditions to characterize comorbidity (see Supporting Information, Appendix A, in the online version of this article). These 10 conditions were chosen based on probable associations with chronic opioid use or high prevalence among hospitalized veterans.[9, 19, 20]
We used a CNCP definition based on ICD‐9‐CM codes.[9] This definition did not include episodic conditions such as migraine[2] or a measure of pain intensity.[21] All conditions were determined from diagnoses coded during any encounter in the year prior to hospitalization, exclusive of the first (ie, index) admission. We also determined the frequency of palliative care use, defined as presence of ICD‐9‐CM code V667 during index hospitalization or within the past year. Patients with palliative care use (n=3070) were excluded from further analyses.
We compared opioid use groups by baseline characteristics using the [2] statistic to determine if the distribution was nonrandom. We used analysis of variance to compare hospital length of stay between groups. We used the [2] statistic to compare rates of 4 outcomes of interest: intensive care unit (ICU) admission during the index hospitalization, discharge disposition other than home, 30‐day readmission rate, and in‐hospital or 30‐day mortality.
To assess the association between opioid‐use status and the 4 outcomes of interest, we constructed 2 multivariable regression models; the first was adjusted only for admission diagnosis using the Clinical Classification Software (CCS),[22] and the second was adjusted for demographics, CCI, and the 10 selected comorbidities in addition to admission diagnosis.
The authors had full access to and take full responsibility for the integrity of the data. All analyses were conducted using SAS statistical software version 9.2 (SAS Institute, Cary, NC). The study was approved by the University of Iowa institutional review board and the Iowa City VA Health Care System Research and Development Committee.
RESULTS
Patient Demographics
Demographic characteristics of patients differed by opioid‐use group (Table 1). Hospitalized patients who received COT in the 6 months prior to admission tended to be younger than their comparators, more often female, white, have a rural residence, and live in the South or West.
| Variables | No Opioids, n=66,899 (54.5%) | Occasional Opioids, n=24,093 (19.6%) | Chronic Opioids, n=31,802 (25.9%) |
|---|---|---|---|
| |||
| Age, y, mean (SD) | 68.7 (12.8) | 66.5 (12.7) | 64.5 (11.5) |
| Age, n (%) | |||
| 59 (reference) | 15,170 (22.7) | 6,703 (27.8) | 10,334 (32.5) |
| 6065 | 15,076 (22.5) | 5,973 (24.8) | 8,983 (28.3) |
| 6677 | 17,226 (25.8) | 5,871 (24.4) | 7,453 (23.4) |
| 78 | 19,427 (29.0) | 5,546 (23.0) | 5,032 (15.8) |
| Male, n (%) | 64,673 (96.7) | 22,964 (95.3) | 30,200 (95.0) |
| Race, n (%) | |||
| White | 48,888 (73.1) | 17,358 (72.1) | 25,087 (78.9) |
| Black | 14,480 (21.6) | 5,553 (23.1) | 5,089 (16.0) |
| Other | 1,172 (1.8) | 450 (1.9) | 645 (2.0) |
| Unknown | 2,359 (3.5) | 732 (3.0) | 981 (3.1) |
| Income $20,000, n (%) | 40,414 (60.4) | 14,105 (58.5) | 18,945 (59.6) |
| Rural residence, n (%) | 16,697 (25.0) | 6,277 (26.1) | 9,356 (29.4) |
| Region, n (%) | |||
| Northeast | 15,053 (22.5) | 4,437 (18.4) | 5,231 (16.5) |
| South | 24,083 (36.0) | 9,390 (39.0) | 12,720 (40.0) |
| Midwest | 16,000 (23.9) | 5,714 (23.7) | 7,762 (24.4) |
| West | 11,763 (17.6) | 4,552 (18.9) | 6,089 (19.2) |
| Charlson Comorbidity Index, mean (SD) | 2.3 (2.0) | 2.6 (2.3) | 2.7 (2.3) |
| Comorbidities, n (%) | |||
| Cancer (not metastatic) | 11,818 (17.7) | 5,549 (23.0) | 6,874 (21.6) |
| Metastatic cancer | 866 (1.3) | 733 (3.0) | 1,104 (3.5) |
| Chronic pain | 25,748 (38.5) | 14,811 (61.5) | 23,894 (75.1) |
| COPD | 20,750 (31.0) | 7,876 (32.7) | 12,117 (38.1) |
| Diabetes, complicated | 10,917 (16.3) | 4,620 (19.2) | 6,304 (19.8) |
| Heart failure | 14,267 (21.3) | 5,035 (20.9) | 6,501 (20.4) |
| Renal disease | 11,311 (16.9) | 4,586 (19.0) | 4,981 (15.7) |
| Dementia | 2,180 (3.3) | 459 (1.9) | 453 (1.4) |
| Mental health other than PTSD | 33,390 (49.9) | 13,657 (56.7) | 20,726 (65.2) |
| PTSD | 7,216 (10.8) | 3,607 (15.0) | 5,938 (18.7) |
| Palliative care use, n (%) | 1,407 (2.1) | 639 (2.7) | 1,024 (3.2) |
Prevalence of Opioid Use
Among the cohort (N=122,794) of hospitalized veterans, 66,899 (54.5%) received no opioids from the VA during the 6‐month period prior to hospitalization; 31,802 (25.9%) received COT in the 6 months prior to admission. An additional 24,093 (19.6%) had occasional opioid therapy (Table 1). A total of 257,623 opioid prescriptions were provided to patients in the 6‐month period prior to their index hospitalization. Of these, 100,379 (39.0%) were for hydrocodone, 48,584 (18.9%) for oxycodone, 36,658 (14.2%) for tramadol, and 35,471 (13.8%) for morphine. These 4 medications accounted for 85.8% of total opioid prescriptions (see Supporting Information, Appendix B, in the online version of this article).
Among the COT group, 3610 (11.4%) received opioids 90 days, 10,110 (31.8%) received opioids between 91 and 179 days, and 18,082 (56.9%) patients received opioids 180 days in the prior 6 months (see Supporting Information, Appendix C, in the online version of this article).
Among the subset of patients with cancer (metastatic and nonmetastatic, n=26,944), 29.6% were prescribed COT, and 23.3% had occasional opioid use. Among the subset of patients with CNCP (n=64,453), 37.1% were prescribed COT, and 23.0% had occasional opioid use.
Comorbid Conditions
Compared to patients not receiving opioids, a larger proportion of patients receiving both occasional and chronic opioids had diagnoses of cancer and of CNCP. Diagnoses more common in COT patients included chronic obstructive pulmonary disease (COPD), complicated diabetes, post‐traumatic stress disorder (PTSD), and other mental health disorders. In contrast, COT patients were less likely than no‐opioid and occasional opioid patients to have heart failure (HF), renal disease, and dementia. Palliative care was used by 2.1% of patients in the no‐opioid group, and 3.2% of patients in the COT group (Table 1). Renal disease was most common among the occasional‐use group.
Unadjusted Hospitalization Outcomes
Unadjusted hospitalization outcomes differed between opioid‐exposure groups (Table 2). Patients receiving occasional or chronic opioids had shorter length of stay and lower rates of non‐home discharge than did patients without any opioid use. The rate of death during hospitalization or within 30 days did not differ between groups. The occasional‐use and COT groups had higher 30‐day readmission rates than did the no‐use group.
| No Opioids, n=65,492 | Occasional Opioids, n=23,454 | Chronic Opioids, n=30,778 | P | |
|---|---|---|---|---|
| ||||
| Hospital length of stay, d, mean (SD) | 4.7 (5.1) | 4.5 (4.8) | 4.5 (4.8) | 0.0003 |
| ICU stay, n (%) | 10,281 (15.7) | 3,299 (14.1) | 4,570 (14.9) | <0.0001 |
| Non‐home discharge, n (%) | 2,944 (4.5) | 997 (4.3) | 1,233 (4.0) | 0.0020 |
| 30‐day readmission, n (%) | 9,023 (13.8) | 3,629 (15.5) | 4,773 (15.5) | <0.0001 |
| Death during hospitalization or within 30 days, n (%) | 2,532 (3.9) | 863 (3.7) | 1,191 (3.9) | 0.4057 |
Multivariable Models
In the fully adjusted multivariable models, opioid exposure (in the form of either chronic or occasional use) had no significant association with ICU stay during index admission or non‐home discharge (Table 3). Both the occasional‐opioid use and COT groups were more likely to experience 30‐day hospital readmission, a relationship that remained consistent across the partially and fully adjusted models. The occasional‐opioid use group saw no increased mortality risk. In the model adjusted only for admission diagnosis, COT was not associated with increased mortality risk. When additionally adjusted for demographic variables, CCI, and selected comorbidities, however, COT was associated with increased risk of death during hospitalization or within 30 days (odds ratio: 1.19, 90% confidence interval: 1.10‐1.29).
| Occasional Opioid Use | Chronic Opioid Therapy | |||
|---|---|---|---|---|
| Model 1, OR (95% CI) | Model 2, OR (95% CI) | Model 1, OR (95% CI) | Model 2, OR (95% CI) | |
| ||||
| ICU stay | 0.94 (0.90‐0.99) | 0.95 (0.91‐1.00) | 1.00 (0.96‐1.04) | 1.01 (0.97‐1.05) |
| Non‐home discharge | 0.92 (0.85‐0.99) | 0.97 (0.90‐1.05) | 0.85 (0.80‐0.92) | 0.95 (0.88‐1.03) |
| 30‐day readmission | 1.14 (1.09‐1.19) | 1.14 (1.09‐1.19) | 1.14 (1.10‐1.19) | 1.15 (1.10‐1.20) |
| Death during hospitalization or within 30 days | 0.96 (0.88‐1.04) | 1.04 (0.95‐1.13) | 0.96 (0.90‐1.04) | 1.19 (1.10‐1.29) |
DISCUSSION
This observational study is, to our knowledge, the first to report prevalence of and characteristics associated with prior opioid use among hospitalized medical patients. The prevalence of any opioid use and of COT was substantially higher in this hospitalized cohort than reported in outpatient settings. The prevalence of any opioid use during 1 year (FY 2009) among all veterans with VA primary care use was 26.1%.[23] A study of incident prescribing rates among veterans with new diagnoses of noncancer‐related pain demonstrated 11% received an opioid prescription within 1 year.[24] Using a definition of 90 consecutive prescription days to define COT, Dobscha et al.[25] found that 5% of veterans with persistent elevated pain intensity and no previous opioid prescriptions subsequently received COT within 12 months. The high prevalence we found likely reflects cumulative effects of incident use as well as an increased symptom burden in a population defined by need for medical hospitalization.
Although a veteran population may not be generalizable to a nonveteran setting, we do note prior studies reporting prevalence of any opioid use in outpatient cohorts (in 2000 and 2005) of between 18% and 30%, with higher rates among women and patients over 65 years of age.[1, 2]
Our work was purposefully inclusive of cancer patients so that we might assess the degree to which cancer diagnoses accounted for prior opioid use in hospitalized patients. Surprisingly, the rate of COT for patients with cancer was lower than that for patients with CNCP, perhaps reflecting that a cancer condition defined in administrative data may not constitute a pain‐causing disease.
Recognition of the prevalence of opioid therapy is important as we work to understand and improve safety, satisfaction, utilization, and long‐term health outcomes associated with hospitalization. Our finding that over half of medical inpatients have preexisting CNCP diagnoses, and a not entirely overlapping proportion has prior opioid exposure, implies a need for future work to refine expectations and strategies for inpatient management, potentially tailored to prior opioid use and presence of CNCP.
A recent Joint Commission sentinel event alert[26] highlights opioid adverse events in the hospital and identifies both lack of previous opioid therapy and prior opioid therapy as factors increasing risk. ICU admission during the hospital stay may reflect adverse events such as opioid‐induced respiratory depression; in our study, patients with no opioid use prior to admission were more likely to have an ICU stay, although the effect was small. One might speculate that clinicians, accustomed to treating pain in opioid‐exposed patients, are using inappropriately large starting dosages of narcotics for inpatients without first assessing prior opioid exposure. Another possible explanation is that patients on COT are admitted to the hospital with less severe illness, potentially reflecting functional, social, or access limitations that compromise ability to manage illness in the outpatient setting. More detailed comparison of illness severity is beyond the scope of the present work.
Patient satisfaction with pain management is reflected in 2 of the Hospital Consumer Assessment of Healthcare Providers and Systems (HCAHPS) questions, and is publically reported.[27] HCAHPS results also figure in the formula for the Centers for Medicare and Medicaid Services value‐based purchasing.[28] Preadmission pain is predictive of postoperative pain[29, 30] and may shape patient expectations; how preadmission opioid use modulates nonsurgical pain and satisfaction with management in the medical inpatient remains to be studied. The high prevalence of prior COT underscores the importance of understanding characteristics of patients on COT, and potential differences and disparities in pain management, when designing interventions to augment patient satisfaction with pain management.
Although the age distribution and patterns of comorbidities differed between the opioid‐use groups, opioid therapy remained a small but significant predictor of hospital readmission; this association was independent of CNCP diagnosis. Functional outcomes are recognized as important measures of efficacy of outpatient pain management strategies,[31] with some evidence that opioids are associated with worse functioning.[32, 33] Functional limitations, as well as inadequately or inappropriately treated pain, may drive both admissions and readmissions. Alternately, COT may be a marker for unmeasured factors that increase a patient's risk of returning to the hospital. Further work is needed to elucidate the relationship between COT and healthcare utilization associated with the inpatient stay.
Our finding that patients on COT have an increased mortality risk is concerning, given the rapid expansion in use of these medications. Although pain is increasingly prevalent toward end of life,[34] we did not observe an association between either CNCP (data not shown) or occasional opioid use and mortality. COT may complicate chronic disease through adverse drug effects including respiratory depression, apnea, or endocrine or immune alteration. Complex chronically ill patients with conditions such as COPD, HF, or diabetes may be particularly susceptible to these effects. Incident use of morphine is associated with increased mortality in acute coronary syndrome and HF[35, 36]: we are not aware of any work describing the relationship between prior opioid use and incident use during hospitalization in medical patients.
Limitations
Our work focuses on hospitalized veterans, a population that remains predominately male, limiting generalizability of the findings. Rates of mental health diagnoses and PTSD, associated with CNCP and COT,[24, 37] are higher in this population than would be expected in a general hospitalized population. Because our outcomes included readmission, and our definition of opioid exposure was designed to reflect outpatient prescribing, we included only patients without recent hospitalization. Therefore, our results may not be generalizable to patients with frequent and recurring hospitalization.
Our definition of opioid exposure depended on pharmacy dispensing records; we are not able to confirm if veterans were taking the medications as prescribed. Further, we were not able to capture data on opioids prescribed by non‐VA providers, which may have led to underestimation of prevalence.
Our definitions of COT and CNCP are imperfect, and should be noted when comparing to other studies. Because we did not specify continuous 90‐day prescribing, we may have misclassified occasional opioid therapy as COT in comparison to other authors. That continuous prescribing is equivalent to continuous use assumes that patients take medications exactly as prescribed. We used occasional opioid therapy as a comparison group, and detailed the distribution of days prescribed among the COT group (see Supporting Information, Appendix C, in the online version of this article), to augment interpretability of these results. Our CNCP diagnosis was less inclusive than others,[2] as we omitted episodic pain (eg, migraine and sprains) and human immunodeficiency virus‐related pain. As COT for CNCP conditions lacks a robust evidence base,[38] defining pain diagnoses using administrative data to reflect conditions for which COT is used in a guideline‐concordant way remains difficult.
Last, differences observed between opioid‐use groups may be due to an unmeasured confounder not captured by the variables we included. Specifically, we did not include other long‐term outpatient medications in our models. It is possible that COT is part of a larger context of inappropriate prescribing, rather than a single‐medication effect on outcomes studied.
CONCLUSION
Nearly 1 in 4 hospitalized veterans has current or recent COT at the time of hospital admission for nonsurgical conditions; nearly half have been prescribed any opioids. Practitioners designing interventions to improve pain management in the inpatient setting should account for prior opioid use. Patients who are on COT prior to hospitalization differ in age and comorbidities from their counterparts who are not on COT. Further elucidation of differences between opioid‐use groups may help providers address care needs during the transition to posthospitalization care. CNCP diagnoses and chronic opioid exposure are different entities and cannot serve as proxies in administrative data. Additional work on utilization and outcomes in specific patient populations may improve our understanding of the long‐term health effects of chronic opioid therapy.
Disclosures: Dr. Mosher is supported by the Veterans Administration (VA) Quality Scholars Fellowship, Office of Academic Affiliations, Department of Veterans Affairs. Dr. Cram is supported by a K24 award from NIAMS (AR062133) at the National Institutes of Health. The preliminary results of this article were presented at the Society of General Internal Medicine Annual Meeting in Denver, Colordao, April 2013. The views expressed in this article are those of the authors and do not necessarily represent the views of the Department of Veterans Affairs. Data are available to researchers with VA accreditation, the statistical code and the protocol are available to interested readers by contacting Dr. Mosher. The authors report no conflict of interest in regard to this study.
Recent trends show a marked increase in outpatient use of chronic opioid therapy (COT) for chronic noncancer pain (CNCP)[1, 2] without decreases in reported CNCP,[3] raising concerns about the efficacy and risk‐to‐benefit ratio of opioids in this population.[4, 5, 6, 7, 8] Increasing rates of outpatient use likely are accompanied by increasing rates of opioid exposure among patients admitted to the hospital. To our knowledge there are no published data regarding the prevalence of COT during the months preceding hospitalization.
Opioid use has been linked to increased emergency room utilization[9, 10] and emergency hospitalization,[11] but associations between opioid use and inpatient metrics (eg, mortality, readmission) have not been explored. Furthermore, lack of knowledge about the prevalence of opioid use prior to hospitalization may impede efforts to improve inpatient pain management and satisfaction with care. Although there is reason to expect that strategies to safely and effectively treat acute pain during the inpatient stay differ between opioid‐nave patients and opioid‐exposed patients, evidence regarding treatment strategies is limited.[12, 13, 14] Opioid pain medications are associated with hospital adverse events, with both prior opioid exposure and lack of opioid use as proposed risk factors.[15] A better understanding of the prevalence and characteristics of hospitalized COT patients is fundamental to future work to achieve safer and more effective inpatient pain management.
The primary purpose of this study was to determine the prevalence of prior COT among hospitalized medical patients. Additionally, we aimed to characterize inpatients with occasional and chronic opioid therapy prior to admission in comparison to opioid‐nave inpatients, as differences between these groups may suggest directions for further investigation into the distinct needs or challenges of hospitalized opioid‐exposed patients.
METHODS
We used inpatient and outpatient administrative data from the Department of Veterans Affairs (VA) Healthcare System. The primary data source to identify acute medical admissions was the VA Patient Treatment File, a national administrative database of all inpatient admissions, including patient demographic characteristics, primary and secondary diagnoses (using International Classification of Diseases, 9th Revision, Clinical Modification [ICD‐9‐CM], codes), and hospitalization characteristics. Outpatient pharmacy data were from the VA Pharmacy Prescription Data Files. The VA Vital Status Files provided dates of death.
We identified all first acute medical admissions to 129 VA hospitals during fiscal years (FYs) 2009 to 2011 (October 2009September 2011). We defined first admissions as the initial medical hospitalization occurring following a minimum 365‐day hospitalization‐free period. Patients were required to demonstrate pharmacy use by receipt of any outpatient medication from the VA on 2 separate occasions within 270 days preceding the first admission, to avoid misclassification of patients who routinely obtained medications only from a non‐VA provider. Patients admitted from extended care facilities were excluded.
We grouped patients by opioid‐use status based on outpatient prescription records: (1) no opioid use, defined as no opioid prescriptions in the 6 months prior to hospitalization; (2) occasional opioid use, defined as patients who received any opioid prescription during the 6 months prior but did not meet definition of chronic use; and (3) chronic opioid therapy, defined as 90 or more days' supply of opioids received within 6 months preceding hospitalization. We did not specify continuous prescribing. Opioids included in the definition were codeine, dihydrocodeine, fentanyl (mucosal and topical), hydrocodone, hydromorphone, meperidine, methadone, morphine, oxycodone, oxymorphone, pentazocine, propoxyphene, tapentadol, and tramadol.[16, 17]
We compared groups by demographic variables including age, sex, race, income, rural vs urban residence (determined from Rural‐Urban Commuting Area codes), region based on hospital location; overall comorbidity using the Charlson Comorbidity Index (CCI);[18] and 10 selected conditions to characterize comorbidity (see Supporting Information, Appendix A, in the online version of this article). These 10 conditions were chosen based on probable associations with chronic opioid use or high prevalence among hospitalized veterans.[9, 19, 20]
We used a CNCP definition based on ICD‐9‐CM codes.[9] This definition did not include episodic conditions such as migraine[2] or a measure of pain intensity.[21] All conditions were determined from diagnoses coded during any encounter in the year prior to hospitalization, exclusive of the first (ie, index) admission. We also determined the frequency of palliative care use, defined as presence of ICD‐9‐CM code V667 during index hospitalization or within the past year. Patients with palliative care use (n=3070) were excluded from further analyses.
We compared opioid use groups by baseline characteristics using the [2] statistic to determine if the distribution was nonrandom. We used analysis of variance to compare hospital length of stay between groups. We used the [2] statistic to compare rates of 4 outcomes of interest: intensive care unit (ICU) admission during the index hospitalization, discharge disposition other than home, 30‐day readmission rate, and in‐hospital or 30‐day mortality.
To assess the association between opioid‐use status and the 4 outcomes of interest, we constructed 2 multivariable regression models; the first was adjusted only for admission diagnosis using the Clinical Classification Software (CCS),[22] and the second was adjusted for demographics, CCI, and the 10 selected comorbidities in addition to admission diagnosis.
The authors had full access to and take full responsibility for the integrity of the data. All analyses were conducted using SAS statistical software version 9.2 (SAS Institute, Cary, NC). The study was approved by the University of Iowa institutional review board and the Iowa City VA Health Care System Research and Development Committee.
RESULTS
Patient Demographics
Demographic characteristics of patients differed by opioid‐use group (Table 1). Hospitalized patients who received COT in the 6 months prior to admission tended to be younger than their comparators, more often female, white, have a rural residence, and live in the South or West.
| Variables | No Opioids, n=66,899 (54.5%) | Occasional Opioids, n=24,093 (19.6%) | Chronic Opioids, n=31,802 (25.9%) |
|---|---|---|---|
| |||
| Age, y, mean (SD) | 68.7 (12.8) | 66.5 (12.7) | 64.5 (11.5) |
| Age, n (%) | |||
| 59 (reference) | 15,170 (22.7) | 6,703 (27.8) | 10,334 (32.5) |
| 6065 | 15,076 (22.5) | 5,973 (24.8) | 8,983 (28.3) |
| 6677 | 17,226 (25.8) | 5,871 (24.4) | 7,453 (23.4) |
| 78 | 19,427 (29.0) | 5,546 (23.0) | 5,032 (15.8) |
| Male, n (%) | 64,673 (96.7) | 22,964 (95.3) | 30,200 (95.0) |
| Race, n (%) | |||
| White | 48,888 (73.1) | 17,358 (72.1) | 25,087 (78.9) |
| Black | 14,480 (21.6) | 5,553 (23.1) | 5,089 (16.0) |
| Other | 1,172 (1.8) | 450 (1.9) | 645 (2.0) |
| Unknown | 2,359 (3.5) | 732 (3.0) | 981 (3.1) |
| Income $20,000, n (%) | 40,414 (60.4) | 14,105 (58.5) | 18,945 (59.6) |
| Rural residence, n (%) | 16,697 (25.0) | 6,277 (26.1) | 9,356 (29.4) |
| Region, n (%) | |||
| Northeast | 15,053 (22.5) | 4,437 (18.4) | 5,231 (16.5) |
| South | 24,083 (36.0) | 9,390 (39.0) | 12,720 (40.0) |
| Midwest | 16,000 (23.9) | 5,714 (23.7) | 7,762 (24.4) |
| West | 11,763 (17.6) | 4,552 (18.9) | 6,089 (19.2) |
| Charlson Comorbidity Index, mean (SD) | 2.3 (2.0) | 2.6 (2.3) | 2.7 (2.3) |
| Comorbidities, n (%) | |||
| Cancer (not metastatic) | 11,818 (17.7) | 5,549 (23.0) | 6,874 (21.6) |
| Metastatic cancer | 866 (1.3) | 733 (3.0) | 1,104 (3.5) |
| Chronic pain | 25,748 (38.5) | 14,811 (61.5) | 23,894 (75.1) |
| COPD | 20,750 (31.0) | 7,876 (32.7) | 12,117 (38.1) |
| Diabetes, complicated | 10,917 (16.3) | 4,620 (19.2) | 6,304 (19.8) |
| Heart failure | 14,267 (21.3) | 5,035 (20.9) | 6,501 (20.4) |
| Renal disease | 11,311 (16.9) | 4,586 (19.0) | 4,981 (15.7) |
| Dementia | 2,180 (3.3) | 459 (1.9) | 453 (1.4) |
| Mental health other than PTSD | 33,390 (49.9) | 13,657 (56.7) | 20,726 (65.2) |
| PTSD | 7,216 (10.8) | 3,607 (15.0) | 5,938 (18.7) |
| Palliative care use, n (%) | 1,407 (2.1) | 639 (2.7) | 1,024 (3.2) |
Prevalence of Opioid Use
Among the cohort (N=122,794) of hospitalized veterans, 66,899 (54.5%) received no opioids from the VA during the 6‐month period prior to hospitalization; 31,802 (25.9%) received COT in the 6 months prior to admission. An additional 24,093 (19.6%) had occasional opioid therapy (Table 1). A total of 257,623 opioid prescriptions were provided to patients in the 6‐month period prior to their index hospitalization. Of these, 100,379 (39.0%) were for hydrocodone, 48,584 (18.9%) for oxycodone, 36,658 (14.2%) for tramadol, and 35,471 (13.8%) for morphine. These 4 medications accounted for 85.8% of total opioid prescriptions (see Supporting Information, Appendix B, in the online version of this article).
Among the COT group, 3610 (11.4%) received opioids 90 days, 10,110 (31.8%) received opioids between 91 and 179 days, and 18,082 (56.9%) patients received opioids 180 days in the prior 6 months (see Supporting Information, Appendix C, in the online version of this article).
Among the subset of patients with cancer (metastatic and nonmetastatic, n=26,944), 29.6% were prescribed COT, and 23.3% had occasional opioid use. Among the subset of patients with CNCP (n=64,453), 37.1% were prescribed COT, and 23.0% had occasional opioid use.
Comorbid Conditions
Compared to patients not receiving opioids, a larger proportion of patients receiving both occasional and chronic opioids had diagnoses of cancer and of CNCP. Diagnoses more common in COT patients included chronic obstructive pulmonary disease (COPD), complicated diabetes, post‐traumatic stress disorder (PTSD), and other mental health disorders. In contrast, COT patients were less likely than no‐opioid and occasional opioid patients to have heart failure (HF), renal disease, and dementia. Palliative care was used by 2.1% of patients in the no‐opioid group, and 3.2% of patients in the COT group (Table 1). Renal disease was most common among the occasional‐use group.
Unadjusted Hospitalization Outcomes
Unadjusted hospitalization outcomes differed between opioid‐exposure groups (Table 2). Patients receiving occasional or chronic opioids had shorter length of stay and lower rates of non‐home discharge than did patients without any opioid use. The rate of death during hospitalization or within 30 days did not differ between groups. The occasional‐use and COT groups had higher 30‐day readmission rates than did the no‐use group.
| No Opioids, n=65,492 | Occasional Opioids, n=23,454 | Chronic Opioids, n=30,778 | P | |
|---|---|---|---|---|
| ||||
| Hospital length of stay, d, mean (SD) | 4.7 (5.1) | 4.5 (4.8) | 4.5 (4.8) | 0.0003 |
| ICU stay, n (%) | 10,281 (15.7) | 3,299 (14.1) | 4,570 (14.9) | <0.0001 |
| Non‐home discharge, n (%) | 2,944 (4.5) | 997 (4.3) | 1,233 (4.0) | 0.0020 |
| 30‐day readmission, n (%) | 9,023 (13.8) | 3,629 (15.5) | 4,773 (15.5) | <0.0001 |
| Death during hospitalization or within 30 days, n (%) | 2,532 (3.9) | 863 (3.7) | 1,191 (3.9) | 0.4057 |
Multivariable Models
In the fully adjusted multivariable models, opioid exposure (in the form of either chronic or occasional use) had no significant association with ICU stay during index admission or non‐home discharge (Table 3). Both the occasional‐opioid use and COT groups were more likely to experience 30‐day hospital readmission, a relationship that remained consistent across the partially and fully adjusted models. The occasional‐opioid use group saw no increased mortality risk. In the model adjusted only for admission diagnosis, COT was not associated with increased mortality risk. When additionally adjusted for demographic variables, CCI, and selected comorbidities, however, COT was associated with increased risk of death during hospitalization or within 30 days (odds ratio: 1.19, 90% confidence interval: 1.10‐1.29).
| Occasional Opioid Use | Chronic Opioid Therapy | |||
|---|---|---|---|---|
| Model 1, OR (95% CI) | Model 2, OR (95% CI) | Model 1, OR (95% CI) | Model 2, OR (95% CI) | |
| ||||
| ICU stay | 0.94 (0.90‐0.99) | 0.95 (0.91‐1.00) | 1.00 (0.96‐1.04) | 1.01 (0.97‐1.05) |
| Non‐home discharge | 0.92 (0.85‐0.99) | 0.97 (0.90‐1.05) | 0.85 (0.80‐0.92) | 0.95 (0.88‐1.03) |
| 30‐day readmission | 1.14 (1.09‐1.19) | 1.14 (1.09‐1.19) | 1.14 (1.10‐1.19) | 1.15 (1.10‐1.20) |
| Death during hospitalization or within 30 days | 0.96 (0.88‐1.04) | 1.04 (0.95‐1.13) | 0.96 (0.90‐1.04) | 1.19 (1.10‐1.29) |
DISCUSSION
This observational study is, to our knowledge, the first to report prevalence of and characteristics associated with prior opioid use among hospitalized medical patients. The prevalence of any opioid use and of COT was substantially higher in this hospitalized cohort than reported in outpatient settings. The prevalence of any opioid use during 1 year (FY 2009) among all veterans with VA primary care use was 26.1%.[23] A study of incident prescribing rates among veterans with new diagnoses of noncancer‐related pain demonstrated 11% received an opioid prescription within 1 year.[24] Using a definition of 90 consecutive prescription days to define COT, Dobscha et al.[25] found that 5% of veterans with persistent elevated pain intensity and no previous opioid prescriptions subsequently received COT within 12 months. The high prevalence we found likely reflects cumulative effects of incident use as well as an increased symptom burden in a population defined by need for medical hospitalization.
Although a veteran population may not be generalizable to a nonveteran setting, we do note prior studies reporting prevalence of any opioid use in outpatient cohorts (in 2000 and 2005) of between 18% and 30%, with higher rates among women and patients over 65 years of age.[1, 2]
Our work was purposefully inclusive of cancer patients so that we might assess the degree to which cancer diagnoses accounted for prior opioid use in hospitalized patients. Surprisingly, the rate of COT for patients with cancer was lower than that for patients with CNCP, perhaps reflecting that a cancer condition defined in administrative data may not constitute a pain‐causing disease.
Recognition of the prevalence of opioid therapy is important as we work to understand and improve safety, satisfaction, utilization, and long‐term health outcomes associated with hospitalization. Our finding that over half of medical inpatients have preexisting CNCP diagnoses, and a not entirely overlapping proportion has prior opioid exposure, implies a need for future work to refine expectations and strategies for inpatient management, potentially tailored to prior opioid use and presence of CNCP.
A recent Joint Commission sentinel event alert[26] highlights opioid adverse events in the hospital and identifies both lack of previous opioid therapy and prior opioid therapy as factors increasing risk. ICU admission during the hospital stay may reflect adverse events such as opioid‐induced respiratory depression; in our study, patients with no opioid use prior to admission were more likely to have an ICU stay, although the effect was small. One might speculate that clinicians, accustomed to treating pain in opioid‐exposed patients, are using inappropriately large starting dosages of narcotics for inpatients without first assessing prior opioid exposure. Another possible explanation is that patients on COT are admitted to the hospital with less severe illness, potentially reflecting functional, social, or access limitations that compromise ability to manage illness in the outpatient setting. More detailed comparison of illness severity is beyond the scope of the present work.
Patient satisfaction with pain management is reflected in 2 of the Hospital Consumer Assessment of Healthcare Providers and Systems (HCAHPS) questions, and is publically reported.[27] HCAHPS results also figure in the formula for the Centers for Medicare and Medicaid Services value‐based purchasing.[28] Preadmission pain is predictive of postoperative pain[29, 30] and may shape patient expectations; how preadmission opioid use modulates nonsurgical pain and satisfaction with management in the medical inpatient remains to be studied. The high prevalence of prior COT underscores the importance of understanding characteristics of patients on COT, and potential differences and disparities in pain management, when designing interventions to augment patient satisfaction with pain management.
Although the age distribution and patterns of comorbidities differed between the opioid‐use groups, opioid therapy remained a small but significant predictor of hospital readmission; this association was independent of CNCP diagnosis. Functional outcomes are recognized as important measures of efficacy of outpatient pain management strategies,[31] with some evidence that opioids are associated with worse functioning.[32, 33] Functional limitations, as well as inadequately or inappropriately treated pain, may drive both admissions and readmissions. Alternately, COT may be a marker for unmeasured factors that increase a patient's risk of returning to the hospital. Further work is needed to elucidate the relationship between COT and healthcare utilization associated with the inpatient stay.
Our finding that patients on COT have an increased mortality risk is concerning, given the rapid expansion in use of these medications. Although pain is increasingly prevalent toward end of life,[34] we did not observe an association between either CNCP (data not shown) or occasional opioid use and mortality. COT may complicate chronic disease through adverse drug effects including respiratory depression, apnea, or endocrine or immune alteration. Complex chronically ill patients with conditions such as COPD, HF, or diabetes may be particularly susceptible to these effects. Incident use of morphine is associated with increased mortality in acute coronary syndrome and HF[35, 36]: we are not aware of any work describing the relationship between prior opioid use and incident use during hospitalization in medical patients.
Limitations
Our work focuses on hospitalized veterans, a population that remains predominately male, limiting generalizability of the findings. Rates of mental health diagnoses and PTSD, associated with CNCP and COT,[24, 37] are higher in this population than would be expected in a general hospitalized population. Because our outcomes included readmission, and our definition of opioid exposure was designed to reflect outpatient prescribing, we included only patients without recent hospitalization. Therefore, our results may not be generalizable to patients with frequent and recurring hospitalization.
Our definition of opioid exposure depended on pharmacy dispensing records; we are not able to confirm if veterans were taking the medications as prescribed. Further, we were not able to capture data on opioids prescribed by non‐VA providers, which may have led to underestimation of prevalence.
Our definitions of COT and CNCP are imperfect, and should be noted when comparing to other studies. Because we did not specify continuous 90‐day prescribing, we may have misclassified occasional opioid therapy as COT in comparison to other authors. That continuous prescribing is equivalent to continuous use assumes that patients take medications exactly as prescribed. We used occasional opioid therapy as a comparison group, and detailed the distribution of days prescribed among the COT group (see Supporting Information, Appendix C, in the online version of this article), to augment interpretability of these results. Our CNCP diagnosis was less inclusive than others,[2] as we omitted episodic pain (eg, migraine and sprains) and human immunodeficiency virus‐related pain. As COT for CNCP conditions lacks a robust evidence base,[38] defining pain diagnoses using administrative data to reflect conditions for which COT is used in a guideline‐concordant way remains difficult.
Last, differences observed between opioid‐use groups may be due to an unmeasured confounder not captured by the variables we included. Specifically, we did not include other long‐term outpatient medications in our models. It is possible that COT is part of a larger context of inappropriate prescribing, rather than a single‐medication effect on outcomes studied.
CONCLUSION
Nearly 1 in 4 hospitalized veterans has current or recent COT at the time of hospital admission for nonsurgical conditions; nearly half have been prescribed any opioids. Practitioners designing interventions to improve pain management in the inpatient setting should account for prior opioid use. Patients who are on COT prior to hospitalization differ in age and comorbidities from their counterparts who are not on COT. Further elucidation of differences between opioid‐use groups may help providers address care needs during the transition to posthospitalization care. CNCP diagnoses and chronic opioid exposure are different entities and cannot serve as proxies in administrative data. Additional work on utilization and outcomes in specific patient populations may improve our understanding of the long‐term health effects of chronic opioid therapy.
Disclosures: Dr. Mosher is supported by the Veterans Administration (VA) Quality Scholars Fellowship, Office of Academic Affiliations, Department of Veterans Affairs. Dr. Cram is supported by a K24 award from NIAMS (AR062133) at the National Institutes of Health. The preliminary results of this article were presented at the Society of General Internal Medicine Annual Meeting in Denver, Colordao, April 2013. The views expressed in this article are those of the authors and do not necessarily represent the views of the Department of Veterans Affairs. Data are available to researchers with VA accreditation, the statistical code and the protocol are available to interested readers by contacting Dr. Mosher. The authors report no conflict of interest in regard to this study.
- , , , et al. Age and gender trends in long‐term opioid analgesic use for noncancer pain. Am J Public Health. 2010;100:2541–2547.
- , , , , , . Trends in use of opioids for non‐cancer pain conditions 2000–2005 in commercial and Medicaid insurance plans: the TROUP study. Pain. 2008;138:440–449.
- Institute of Medicine (US) Committee on Advancing Pain Research, Care, and Education. Relieving pain in America: a blueprint for transforming prevention, care, education, and research. Washington, DC: National Academies Press; 2011.
- , , , . Long‐term opioid therapy reconsidered. Ann Intern Med. 2011;155:325–328.
- , . What are we treating with long‐term opioid therapy? Arch Intern Med. 2012;172:433–434.
- , , , . Opioids for chronic noncancer pain: a meta‐analysis of effectiveness and side effects. CMAJ. 2006;174:1589–1594.
- , , , . Opioids in chronic non‐cancer pain: systematic review of efficacy and safety. Pain. 2004;112:372–380.
- , , , et al. A systematic review of randomized trials of long‐term opioid management for chronic non‐cancer pain. Pain Physician. 2011;14:91–121.
- , , , , , . Rates of adverse events of long‐acting opioids in a state Medicaid program. Ann Pharmacother. 2007;41:921–928.
- , , , et al. Emergency department visits among recipients of chronic opioid therapy. Arch Intern Med. 2010;170:1425–1432.
- , , , . Emergency hospitalizations for adverse drug events in older Americans. N Engl J Med. 2011;365:2002–2012.
- , . Assessment and management of acute pain in adult medical inpatients: a systematic review. Pain Med. 2009;10:1183–1199.
- , , , . Acute pain management in opioid‐tolerant patients: a growing challenge. Anaesth Intensive Care. 2011;39:804–823.
- , , , . Acute pain management of the chronic pain patient on opiates: a survey of caregivers at University of Washington Medical Center. Clin J Pain. 1994;10:133–138.
- The Joint Commission and the FDA take steps to curb adverse events related to the use and misuse of opioid drugs. ED Manag. 2012;24:112–116.
- , . Tramadol. CMAJ. 2013;185:E352.
- , , , , . Prescription opioid abuse in the United Kingdom. Br J Clin Pharmacol. 2013;76:823–824.
- , , , . Validation of a combined comorbidity index. J Clin Epidemiol. 1994;47:1245–1251.
- , , , , . Bringing the war back home: mental health disorders among 103,788 US veterans returning from Iraq and Afghanistan seen at Department of Veterans Affairs facilities. Arch Intern Med. 2007;167:476–482.
- , , , et al. Coding algorithms for defining comorbidities in ICD‐9‐CM and ICD‐10 administrative data. Med Care. 2005;43:1130–1139.
- , , , , , . Sex Differences in the medical care of VA patients with chronic non‐cancer pain [published online ahead of print June 26, 2013]. Pain Med. doi: 10.1111/pme.12177.
- Agency for Healthcare Research and Quality. Clinical Classifications Software (CCS) for ICD‐9‐CM. Available at: http://www.hcup‐us.ahrq.gov/toolssoftware/ccs/ccs.jsp. Accessed October 17, 2013.
- , , , et al. Predicting risk of hospitalization or death among patients receiving primary care in the Veterans Health Administration. Med Care. 2013;51:368–373.
- , , , et al. Association of mental health disorders with prescription opioids and high‐risk opioid use in US veterans of Iraq and Afghanistan. JAMA. 2012;307:940–947.
- , , , , . Correlates of prescription opioid initiation and long‐term opioid use in veterans with persistent pain. Clin J Pain. 2013;29:102–108.
- Safe use of opioids in hospitals. Sentinel Event Alert. 2012;49:1–5.
- Centers for Medicare (2):2–9.
- , , , , , . The risk of severe postoperative pain: modification and validation of a clinical prediction rule. Anesth Analg. 2008;107:1330–1339.
- , , , et al. Preoperative predictors of moderate to intense acute postoperative pain in patients undergoing abdominal surgery. Acta Anaesthesiol Scand. 2002;46:1265–1271.
- , , , , , . Successful and unsuccessful outcomes with long‐term opioid therapy: a survey of physicians' opinions. J Palliat Med. 2006;9:50–56.
- , , , . Opioid use among low back pain patients in primary care: is opioid prescription associated with disability at 6‐month follow‐up? Pain. 2013;154:1038–1044.
- , , , , ; Disability Risk Identification Study Cohort. Early opioid prescription and subsequent disability among workers with back injuries: the Disability Risk Identification Study Cohort. Spine (Phila Pa 1976). 2008;33:199–204.
- , , , et al. The epidemiology of pain during the last 2 years of life. Ann Intern Med. 2010;153:563–569.
- , , , et al. Association of intravenous morphine use and outcomes in acute coronary syndromes: results from the CRUSADE Quality Improvement Initiative. Am Heart J. 2005;149:1043–1049.
- , , , et al. Use of intravenous morphine for acute decompensated heart failure in patients with and without acute coronary syndromes. Acute Card Care. 2011;13:76–80.
- , , , et al. VA mental health services utilization in Iraq and Afghanistan veterans in the first year of receiving new mental health diagnoses. J Trauma Stress. 2010;23:5–16.
- , , , et al. Long‐term opioid management for chronic noncancer pain. Cochrane Database Syst Rev. 2010;(1):CD006605.
- , , , et al. Age and gender trends in long‐term opioid analgesic use for noncancer pain. Am J Public Health. 2010;100:2541–2547.
- , , , , , . Trends in use of opioids for non‐cancer pain conditions 2000–2005 in commercial and Medicaid insurance plans: the TROUP study. Pain. 2008;138:440–449.
- Institute of Medicine (US) Committee on Advancing Pain Research, Care, and Education. Relieving pain in America: a blueprint for transforming prevention, care, education, and research. Washington, DC: National Academies Press; 2011.
- , , , . Long‐term opioid therapy reconsidered. Ann Intern Med. 2011;155:325–328.
- , . What are we treating with long‐term opioid therapy? Arch Intern Med. 2012;172:433–434.
- , , , . Opioids for chronic noncancer pain: a meta‐analysis of effectiveness and side effects. CMAJ. 2006;174:1589–1594.
- , , , . Opioids in chronic non‐cancer pain: systematic review of efficacy and safety. Pain. 2004;112:372–380.
- , , , et al. A systematic review of randomized trials of long‐term opioid management for chronic non‐cancer pain. Pain Physician. 2011;14:91–121.
- , , , , , . Rates of adverse events of long‐acting opioids in a state Medicaid program. Ann Pharmacother. 2007;41:921–928.
- , , , et al. Emergency department visits among recipients of chronic opioid therapy. Arch Intern Med. 2010;170:1425–1432.
- , , , . Emergency hospitalizations for adverse drug events in older Americans. N Engl J Med. 2011;365:2002–2012.
- , . Assessment and management of acute pain in adult medical inpatients: a systematic review. Pain Med. 2009;10:1183–1199.
- , , , . Acute pain management in opioid‐tolerant patients: a growing challenge. Anaesth Intensive Care. 2011;39:804–823.
- , , , . Acute pain management of the chronic pain patient on opiates: a survey of caregivers at University of Washington Medical Center. Clin J Pain. 1994;10:133–138.
- The Joint Commission and the FDA take steps to curb adverse events related to the use and misuse of opioid drugs. ED Manag. 2012;24:112–116.
- , . Tramadol. CMAJ. 2013;185:E352.
- , , , , . Prescription opioid abuse in the United Kingdom. Br J Clin Pharmacol. 2013;76:823–824.
- , , , . Validation of a combined comorbidity index. J Clin Epidemiol. 1994;47:1245–1251.
- , , , , . Bringing the war back home: mental health disorders among 103,788 US veterans returning from Iraq and Afghanistan seen at Department of Veterans Affairs facilities. Arch Intern Med. 2007;167:476–482.
- , , , et al. Coding algorithms for defining comorbidities in ICD‐9‐CM and ICD‐10 administrative data. Med Care. 2005;43:1130–1139.
- , , , , , . Sex Differences in the medical care of VA patients with chronic non‐cancer pain [published online ahead of print June 26, 2013]. Pain Med. doi: 10.1111/pme.12177.
- Agency for Healthcare Research and Quality. Clinical Classifications Software (CCS) for ICD‐9‐CM. Available at: http://www.hcup‐us.ahrq.gov/toolssoftware/ccs/ccs.jsp. Accessed October 17, 2013.
- , , , et al. Predicting risk of hospitalization or death among patients receiving primary care in the Veterans Health Administration. Med Care. 2013;51:368–373.
- , , , et al. Association of mental health disorders with prescription opioids and high‐risk opioid use in US veterans of Iraq and Afghanistan. JAMA. 2012;307:940–947.
- , , , , . Correlates of prescription opioid initiation and long‐term opioid use in veterans with persistent pain. Clin J Pain. 2013;29:102–108.
- Safe use of opioids in hospitals. Sentinel Event Alert. 2012;49:1–5.
- Centers for Medicare (2):2–9.
- , , , , , . The risk of severe postoperative pain: modification and validation of a clinical prediction rule. Anesth Analg. 2008;107:1330–1339.
- , , , et al. Preoperative predictors of moderate to intense acute postoperative pain in patients undergoing abdominal surgery. Acta Anaesthesiol Scand. 2002;46:1265–1271.
- , , , , , . Successful and unsuccessful outcomes with long‐term opioid therapy: a survey of physicians' opinions. J Palliat Med. 2006;9:50–56.
- , , , . Opioid use among low back pain patients in primary care: is opioid prescription associated with disability at 6‐month follow‐up? Pain. 2013;154:1038–1044.
- , , , , ; Disability Risk Identification Study Cohort. Early opioid prescription and subsequent disability among workers with back injuries: the Disability Risk Identification Study Cohort. Spine (Phila Pa 1976). 2008;33:199–204.
- , , , et al. The epidemiology of pain during the last 2 years of life. Ann Intern Med. 2010;153:563–569.
- , , , et al. Association of intravenous morphine use and outcomes in acute coronary syndromes: results from the CRUSADE Quality Improvement Initiative. Am Heart J. 2005;149:1043–1049.
- , , , et al. Use of intravenous morphine for acute decompensated heart failure in patients with and without acute coronary syndromes. Acute Card Care. 2011;13:76–80.
- , , , et al. VA mental health services utilization in Iraq and Afghanistan veterans in the first year of receiving new mental health diagnoses. J Trauma Stress. 2010;23:5–16.
- , , , et al. Long‐term opioid management for chronic noncancer pain. Cochrane Database Syst Rev. 2010;(1):CD006605.
© 2013 Society of Hospital Medicine