User login
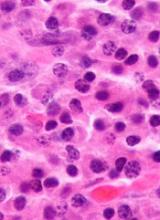
FDA grants priority review to daratumumab sBLA
The US Food and Drug Administration (FDA) has granted priority review to a supplemental biologics license application (sBLA) for daratumumab (Darzalex®).
This sBLA is for daratumumab (D) to be used in combination with bortezomib, melphalan, and prednisone (VMP) for the treatment of patients with newly diagnosed multiple myeloma (MM) who are ineligible for autologous stem cell transplant.
The FDA expects to make a decision on the sBLA by May 21, 2018.
The agency’s goal is to take action on a priority review application within 6 months of receiving it, rather than the standard 10 months.
The FDA grants priority review to applications for products that may provide significant improvements in the treatment, diagnosis, or prevention of serious conditions.
The priority review for this sBLA is based on data from the phase 3 ALCYONE study, which were presented at the 2017 ASH Annual Meeting and simultaneously published in NEJM.
In this study, researchers compared VMP to D-VMP in 706 patients with newly diagnosed MM who were not eligible for high-dose chemotherapy with autologous stem cell transplant.
D-VMP produced deeper responses than VMP. The overall response rate was 74% in the VMP arm and 91% in the D-VMP arm (P<0.0001). The rate of complete response was 24% and 43%, respectively (P<0.0001).
D-VMP also prolonged progression-free survival (PFS) compared to VMP.
The median PFS was 18.1 months in the VMP arm and was not reached in the D-VMP arm. The 12-month PFS was 76% and 87%, respectively. And the 18-month PFS was 50% and 72%, respectively.
The median overall survival was not reached in either treatment arm.
The most common grade 3/4 treatment-emergent adverse events (in the D-VMP and VMP arms, respectively) were neutropenia (40% and 39%), thrombocytopenia (34% and 38%), and anemia (16% and 20%).
The rate of grade 3/4 infections was higher in the D-VMP arm than the VMP arm—23% and 15%, respectively. The most common of these was pneumonia, with rates of 11% and 4%, respectively.
There were 6 deaths due to treatment-emergent adverse events in the D-VMP arm and 5 in the VMP arm.
About daratumumab
Daratumumab is a CD38-directed cytolytic antibody that is FDA approved for the following indications:
- In combination with lenalidomide and dexamethasone, or bortezomib and dexamethasone, for the treatment of MM patients who have received at least 1 prior therapy
- In combination with pomalidomide and dexamethasone for the treatment of MM patients who have received at least 2 prior therapies, including lenalidomide and a proteasome inhibitor (PI)
- As monotherapy for MM patients who have received at least 3 prior lines of therapy, including a PI and an immunomodulatory agent, or who are double-refractory to a PI and an immunomodulatory agent.

The US Food and Drug Administration (FDA) has granted priority review to a supplemental biologics license application (sBLA) for daratumumab (Darzalex®).
This sBLA is for daratumumab (D) to be used in combination with bortezomib, melphalan, and prednisone (VMP) for the treatment of patients with newly diagnosed multiple myeloma (MM) who are ineligible for autologous stem cell transplant.
The FDA expects to make a decision on the sBLA by May 21, 2018.
The agency’s goal is to take action on a priority review application within 6 months of receiving it, rather than the standard 10 months.
The FDA grants priority review to applications for products that may provide significant improvements in the treatment, diagnosis, or prevention of serious conditions.
The priority review for this sBLA is based on data from the phase 3 ALCYONE study, which were presented at the 2017 ASH Annual Meeting and simultaneously published in NEJM.
In this study, researchers compared VMP to D-VMP in 706 patients with newly diagnosed MM who were not eligible for high-dose chemotherapy with autologous stem cell transplant.
D-VMP produced deeper responses than VMP. The overall response rate was 74% in the VMP arm and 91% in the D-VMP arm (P<0.0001). The rate of complete response was 24% and 43%, respectively (P<0.0001).
D-VMP also prolonged progression-free survival (PFS) compared to VMP.
The median PFS was 18.1 months in the VMP arm and was not reached in the D-VMP arm. The 12-month PFS was 76% and 87%, respectively. And the 18-month PFS was 50% and 72%, respectively.
The median overall survival was not reached in either treatment arm.
The most common grade 3/4 treatment-emergent adverse events (in the D-VMP and VMP arms, respectively) were neutropenia (40% and 39%), thrombocytopenia (34% and 38%), and anemia (16% and 20%).
The rate of grade 3/4 infections was higher in the D-VMP arm than the VMP arm—23% and 15%, respectively. The most common of these was pneumonia, with rates of 11% and 4%, respectively.
There were 6 deaths due to treatment-emergent adverse events in the D-VMP arm and 5 in the VMP arm.
About daratumumab
Daratumumab is a CD38-directed cytolytic antibody that is FDA approved for the following indications:
- In combination with lenalidomide and dexamethasone, or bortezomib and dexamethasone, for the treatment of MM patients who have received at least 1 prior therapy
- In combination with pomalidomide and dexamethasone for the treatment of MM patients who have received at least 2 prior therapies, including lenalidomide and a proteasome inhibitor (PI)
- As monotherapy for MM patients who have received at least 3 prior lines of therapy, including a PI and an immunomodulatory agent, or who are double-refractory to a PI and an immunomodulatory agent.

The US Food and Drug Administration (FDA) has granted priority review to a supplemental biologics license application (sBLA) for daratumumab (Darzalex®).
This sBLA is for daratumumab (D) to be used in combination with bortezomib, melphalan, and prednisone (VMP) for the treatment of patients with newly diagnosed multiple myeloma (MM) who are ineligible for autologous stem cell transplant.
The FDA expects to make a decision on the sBLA by May 21, 2018.
The agency’s goal is to take action on a priority review application within 6 months of receiving it, rather than the standard 10 months.
The FDA grants priority review to applications for products that may provide significant improvements in the treatment, diagnosis, or prevention of serious conditions.
The priority review for this sBLA is based on data from the phase 3 ALCYONE study, which were presented at the 2017 ASH Annual Meeting and simultaneously published in NEJM.
In this study, researchers compared VMP to D-VMP in 706 patients with newly diagnosed MM who were not eligible for high-dose chemotherapy with autologous stem cell transplant.
D-VMP produced deeper responses than VMP. The overall response rate was 74% in the VMP arm and 91% in the D-VMP arm (P<0.0001). The rate of complete response was 24% and 43%, respectively (P<0.0001).
D-VMP also prolonged progression-free survival (PFS) compared to VMP.
The median PFS was 18.1 months in the VMP arm and was not reached in the D-VMP arm. The 12-month PFS was 76% and 87%, respectively. And the 18-month PFS was 50% and 72%, respectively.
The median overall survival was not reached in either treatment arm.
The most common grade 3/4 treatment-emergent adverse events (in the D-VMP and VMP arms, respectively) were neutropenia (40% and 39%), thrombocytopenia (34% and 38%), and anemia (16% and 20%).
The rate of grade 3/4 infections was higher in the D-VMP arm than the VMP arm—23% and 15%, respectively. The most common of these was pneumonia, with rates of 11% and 4%, respectively.
There were 6 deaths due to treatment-emergent adverse events in the D-VMP arm and 5 in the VMP arm.
About daratumumab
Daratumumab is a CD38-directed cytolytic antibody that is FDA approved for the following indications:
- In combination with lenalidomide and dexamethasone, or bortezomib and dexamethasone, for the treatment of MM patients who have received at least 1 prior therapy
- In combination with pomalidomide and dexamethasone for the treatment of MM patients who have received at least 2 prior therapies, including lenalidomide and a proteasome inhibitor (PI)
- As monotherapy for MM patients who have received at least 3 prior lines of therapy, including a PI and an immunomodulatory agent, or who are double-refractory to a PI and an immunomodulatory agent.

Predicting response to AZA in MDS, CMML
Researchers have developed a technique that may help predict whether patients with myelodysplastic syndromes (MDS) or chronic myelomonocytic leukemia (CMML) will respond to treatment with azacytidine (AZA).
“The new method, called AZA-MS, utilizes a cutting-edge technique known as mass spectrometry to measure the different forms of AZA inside blood cells of patients—such as the AZA molecules that are incorporated into the DNA or RNA,” said Ashwin Unnikrishnan, PhD, of the University of New South Wales in Sydney, Australia.
With this method, Dr Unnikrishnan and his colleagues found that patients who do not respond to AZA may incorporate fewer AZA molecules in their DNA and have lower DNA demethylation than responders. However, this is not always the case.
The researchers reported these findings in Leukemia.
The team initially tested AZA-MS in AZA-treated RKO cells and found that AZA-MS could quantify the ribonucleoside (5-AZA-cR) and deoxyribonucleoside (5-AZA-CdR) forms of AZA in RNA, DNA, and the cytoplasm—all in the same sample.
The researchers also found that AZA induced dose-dependent DNA demethylation but did not have an effect on RNA methylation.
The team then used AZA-MS to analyze bone marrow samples from patients with MDS (n=4) or CMML (n=4) who were undergoing treatment with AZA. All of the patients had received at least 6 cycles of the drug.
Each patient had 3 bone marrow samples collected—one immediately before starting treatment; one on day 8 of cycle 1 (C1d8); and one on day 28 of cycle 1 (C1d28), when they had spent 20 days off the drug.
Four of the patients were complete responders, and 4 were nonresponders. In each group, 2 patients had MDS, and 2 had CMML.
At C1d8, DNA-5-AZA-CdR was significantly greater in responders than nonresponders. And, overall, responders had increased DNA demethylation compared to nonresponders.
However, the researchers also observed differences among the nonresponders. Two nonresponders had very low levels of DNA-5-AZA-CdR at C1d8 and no demethylation. The other 2 nonresponders had much higher DNA-5-AZA-CdR and DNA demethylation levels, which were comparable to levels in responders.
The researchers said they could detect AZA and DNA-5-AZA-CdR intracellularly, as well as RNA-AZA, in the nonresponders with minimal DNA-5-AZA-CdR and DNA demethylation.
The team said this suggests that neither cellular uptake nor intracellular metabolism explain the low DNA-5-AZA-CdR in these patients. Instead, the researchers believe these patients may have a greater proportion of bone marrow cells that are quiescent and not undergoing DNA replication.
The researchers also believe the nonresponders with higher DNA-5-AZA-CdR may be explained by a failure to induce an interferon response, which is necessary for a clinical response.
On the other hand, these nonresponders could have defective immune cell-mediated clearance of dysplastic cells or increased tolerance to this clearance, the researchers said.
The team also noted that, at C1d28, DNA-5-AZA-CdR levels dropped (but were still detectable) in all 8 patients, and DNA methylation had nearly returned to pretreatment levels in all patients. ![]()
Researchers have developed a technique that may help predict whether patients with myelodysplastic syndromes (MDS) or chronic myelomonocytic leukemia (CMML) will respond to treatment with azacytidine (AZA).
“The new method, called AZA-MS, utilizes a cutting-edge technique known as mass spectrometry to measure the different forms of AZA inside blood cells of patients—such as the AZA molecules that are incorporated into the DNA or RNA,” said Ashwin Unnikrishnan, PhD, of the University of New South Wales in Sydney, Australia.
With this method, Dr Unnikrishnan and his colleagues found that patients who do not respond to AZA may incorporate fewer AZA molecules in their DNA and have lower DNA demethylation than responders. However, this is not always the case.
The researchers reported these findings in Leukemia.
The team initially tested AZA-MS in AZA-treated RKO cells and found that AZA-MS could quantify the ribonucleoside (5-AZA-cR) and deoxyribonucleoside (5-AZA-CdR) forms of AZA in RNA, DNA, and the cytoplasm—all in the same sample.
The researchers also found that AZA induced dose-dependent DNA demethylation but did not have an effect on RNA methylation.
The team then used AZA-MS to analyze bone marrow samples from patients with MDS (n=4) or CMML (n=4) who were undergoing treatment with AZA. All of the patients had received at least 6 cycles of the drug.
Each patient had 3 bone marrow samples collected—one immediately before starting treatment; one on day 8 of cycle 1 (C1d8); and one on day 28 of cycle 1 (C1d28), when they had spent 20 days off the drug.
Four of the patients were complete responders, and 4 were nonresponders. In each group, 2 patients had MDS, and 2 had CMML.
At C1d8, DNA-5-AZA-CdR was significantly greater in responders than nonresponders. And, overall, responders had increased DNA demethylation compared to nonresponders.
However, the researchers also observed differences among the nonresponders. Two nonresponders had very low levels of DNA-5-AZA-CdR at C1d8 and no demethylation. The other 2 nonresponders had much higher DNA-5-AZA-CdR and DNA demethylation levels, which were comparable to levels in responders.
The researchers said they could detect AZA and DNA-5-AZA-CdR intracellularly, as well as RNA-AZA, in the nonresponders with minimal DNA-5-AZA-CdR and DNA demethylation.
The team said this suggests that neither cellular uptake nor intracellular metabolism explain the low DNA-5-AZA-CdR in these patients. Instead, the researchers believe these patients may have a greater proportion of bone marrow cells that are quiescent and not undergoing DNA replication.
The researchers also believe the nonresponders with higher DNA-5-AZA-CdR may be explained by a failure to induce an interferon response, which is necessary for a clinical response.
On the other hand, these nonresponders could have defective immune cell-mediated clearance of dysplastic cells or increased tolerance to this clearance, the researchers said.
The team also noted that, at C1d28, DNA-5-AZA-CdR levels dropped (but were still detectable) in all 8 patients, and DNA methylation had nearly returned to pretreatment levels in all patients. ![]()
Researchers have developed a technique that may help predict whether patients with myelodysplastic syndromes (MDS) or chronic myelomonocytic leukemia (CMML) will respond to treatment with azacytidine (AZA).
“The new method, called AZA-MS, utilizes a cutting-edge technique known as mass spectrometry to measure the different forms of AZA inside blood cells of patients—such as the AZA molecules that are incorporated into the DNA or RNA,” said Ashwin Unnikrishnan, PhD, of the University of New South Wales in Sydney, Australia.
With this method, Dr Unnikrishnan and his colleagues found that patients who do not respond to AZA may incorporate fewer AZA molecules in their DNA and have lower DNA demethylation than responders. However, this is not always the case.
The researchers reported these findings in Leukemia.
The team initially tested AZA-MS in AZA-treated RKO cells and found that AZA-MS could quantify the ribonucleoside (5-AZA-cR) and deoxyribonucleoside (5-AZA-CdR) forms of AZA in RNA, DNA, and the cytoplasm—all in the same sample.
The researchers also found that AZA induced dose-dependent DNA demethylation but did not have an effect on RNA methylation.
The team then used AZA-MS to analyze bone marrow samples from patients with MDS (n=4) or CMML (n=4) who were undergoing treatment with AZA. All of the patients had received at least 6 cycles of the drug.
Each patient had 3 bone marrow samples collected—one immediately before starting treatment; one on day 8 of cycle 1 (C1d8); and one on day 28 of cycle 1 (C1d28), when they had spent 20 days off the drug.
Four of the patients were complete responders, and 4 were nonresponders. In each group, 2 patients had MDS, and 2 had CMML.
At C1d8, DNA-5-AZA-CdR was significantly greater in responders than nonresponders. And, overall, responders had increased DNA demethylation compared to nonresponders.
However, the researchers also observed differences among the nonresponders. Two nonresponders had very low levels of DNA-5-AZA-CdR at C1d8 and no demethylation. The other 2 nonresponders had much higher DNA-5-AZA-CdR and DNA demethylation levels, which were comparable to levels in responders.
The researchers said they could detect AZA and DNA-5-AZA-CdR intracellularly, as well as RNA-AZA, in the nonresponders with minimal DNA-5-AZA-CdR and DNA demethylation.
The team said this suggests that neither cellular uptake nor intracellular metabolism explain the low DNA-5-AZA-CdR in these patients. Instead, the researchers believe these patients may have a greater proportion of bone marrow cells that are quiescent and not undergoing DNA replication.
The researchers also believe the nonresponders with higher DNA-5-AZA-CdR may be explained by a failure to induce an interferon response, which is necessary for a clinical response.
On the other hand, these nonresponders could have defective immune cell-mediated clearance of dysplastic cells or increased tolerance to this clearance, the researchers said.
The team also noted that, at C1d28, DNA-5-AZA-CdR levels dropped (but were still detectable) in all 8 patients, and DNA methylation had nearly returned to pretreatment levels in all patients. ![]()
Bright light therapy improves sleep in cancer survivors
Results of a pilot study suggest that systematic bright light exposure can improve sleep in fatigued cancer survivors.
Subjects who were exposed to bright light every morning for 4 weeks had a significantly greater improvement in sleep efficiency than those who were exposed to dim light over the same period.
In fact, subjects in the bright light group were able to achieve clinically normal levels of sleep efficiency, and subjects in the dim light group were not.
Sleep efficiency is the percentage of time in bed that subjects spent sleeping.
Lisa M. Wu, PhD, of Northwestern University in Chicago, Illinois, and her colleagues reported these results in the Journal of Clinical Sleep Medicine.
The team noted that cancer patients report sleep disturbances at a significantly higher rate than the general population. Between 23% and 44% of cancer patients experience insomnia symptoms even years after treatment.
With this in mind, the researchers studied 44 individuals who had completed cancer treatment and met criteria for clinically significant fatigue at screening.
The subjects had an average age of 53.6, and 75% percent were female. Roughly 55% (n=24) had been diagnosed with a hematologic malignancy.
The subjects were randomized to a bright white light intervention or a dim red light intervention. Subjects in both treatment arms were provided with a light box and instructed to use it every morning for 30 minutes for 4 weeks. Sleep was evaluated using wrist actigraphy and the Pittsburgh Sleep Quality Index.
At baseline, 52.6% of subjects in the dim light group and 60% in the bright light group exceeded the clinical cutoff for poor sleep efficiency (≤ 85%). The mean sleep efficiency was 81.8% and 82.8%, respectively.
During the study period, sleep efficiency improved significantly more among subjects exposed to the bright light than those exposed to the dim light (P=0.003).
The mean sleep efficiency was in the clinically normal range for subjects in the bright light group at the end of the intervention (86.06%) and 3 weeks after (85.77%).
However, the cutoff for poor sleep efficiency was not reached in the dim light group, either at the end of the intervention (mean=79.35%) or 3 weeks after (mean=80.88%).
Total sleep time tended to increase over the study period for subjects in the bright light group, but there was no significant difference in total sleep time between the bright light and dim light groups.
Likewise, there was no significant between-group difference in waking after sleep onset, although this outcome tended to decrease over the study period for subjects in the bright light group.
“Systematic light exposure using bright white light is a low-cost and easily disseminated intervention that offers a feasible and potentially effective alternative to improve sleep in cancer survivors,” Dr Wu said.
However, she and her colleagues noted that larger-scale studies are needed. ![]()
Results of a pilot study suggest that systematic bright light exposure can improve sleep in fatigued cancer survivors.
Subjects who were exposed to bright light every morning for 4 weeks had a significantly greater improvement in sleep efficiency than those who were exposed to dim light over the same period.
In fact, subjects in the bright light group were able to achieve clinically normal levels of sleep efficiency, and subjects in the dim light group were not.
Sleep efficiency is the percentage of time in bed that subjects spent sleeping.
Lisa M. Wu, PhD, of Northwestern University in Chicago, Illinois, and her colleagues reported these results in the Journal of Clinical Sleep Medicine.
The team noted that cancer patients report sleep disturbances at a significantly higher rate than the general population. Between 23% and 44% of cancer patients experience insomnia symptoms even years after treatment.
With this in mind, the researchers studied 44 individuals who had completed cancer treatment and met criteria for clinically significant fatigue at screening.
The subjects had an average age of 53.6, and 75% percent were female. Roughly 55% (n=24) had been diagnosed with a hematologic malignancy.
The subjects were randomized to a bright white light intervention or a dim red light intervention. Subjects in both treatment arms were provided with a light box and instructed to use it every morning for 30 minutes for 4 weeks. Sleep was evaluated using wrist actigraphy and the Pittsburgh Sleep Quality Index.
At baseline, 52.6% of subjects in the dim light group and 60% in the bright light group exceeded the clinical cutoff for poor sleep efficiency (≤ 85%). The mean sleep efficiency was 81.8% and 82.8%, respectively.
During the study period, sleep efficiency improved significantly more among subjects exposed to the bright light than those exposed to the dim light (P=0.003).
The mean sleep efficiency was in the clinically normal range for subjects in the bright light group at the end of the intervention (86.06%) and 3 weeks after (85.77%).
However, the cutoff for poor sleep efficiency was not reached in the dim light group, either at the end of the intervention (mean=79.35%) or 3 weeks after (mean=80.88%).
Total sleep time tended to increase over the study period for subjects in the bright light group, but there was no significant difference in total sleep time between the bright light and dim light groups.
Likewise, there was no significant between-group difference in waking after sleep onset, although this outcome tended to decrease over the study period for subjects in the bright light group.
“Systematic light exposure using bright white light is a low-cost and easily disseminated intervention that offers a feasible and potentially effective alternative to improve sleep in cancer survivors,” Dr Wu said.
However, she and her colleagues noted that larger-scale studies are needed. ![]()
Results of a pilot study suggest that systematic bright light exposure can improve sleep in fatigued cancer survivors.
Subjects who were exposed to bright light every morning for 4 weeks had a significantly greater improvement in sleep efficiency than those who were exposed to dim light over the same period.
In fact, subjects in the bright light group were able to achieve clinically normal levels of sleep efficiency, and subjects in the dim light group were not.
Sleep efficiency is the percentage of time in bed that subjects spent sleeping.
Lisa M. Wu, PhD, of Northwestern University in Chicago, Illinois, and her colleagues reported these results in the Journal of Clinical Sleep Medicine.
The team noted that cancer patients report sleep disturbances at a significantly higher rate than the general population. Between 23% and 44% of cancer patients experience insomnia symptoms even years after treatment.
With this in mind, the researchers studied 44 individuals who had completed cancer treatment and met criteria for clinically significant fatigue at screening.
The subjects had an average age of 53.6, and 75% percent were female. Roughly 55% (n=24) had been diagnosed with a hematologic malignancy.
The subjects were randomized to a bright white light intervention or a dim red light intervention. Subjects in both treatment arms were provided with a light box and instructed to use it every morning for 30 minutes for 4 weeks. Sleep was evaluated using wrist actigraphy and the Pittsburgh Sleep Quality Index.
At baseline, 52.6% of subjects in the dim light group and 60% in the bright light group exceeded the clinical cutoff for poor sleep efficiency (≤ 85%). The mean sleep efficiency was 81.8% and 82.8%, respectively.
During the study period, sleep efficiency improved significantly more among subjects exposed to the bright light than those exposed to the dim light (P=0.003).
The mean sleep efficiency was in the clinically normal range for subjects in the bright light group at the end of the intervention (86.06%) and 3 weeks after (85.77%).
However, the cutoff for poor sleep efficiency was not reached in the dim light group, either at the end of the intervention (mean=79.35%) or 3 weeks after (mean=80.88%).
Total sleep time tended to increase over the study period for subjects in the bright light group, but there was no significant difference in total sleep time between the bright light and dim light groups.
Likewise, there was no significant between-group difference in waking after sleep onset, although this outcome tended to decrease over the study period for subjects in the bright light group.
“Systematic light exposure using bright white light is a low-cost and easily disseminated intervention that offers a feasible and potentially effective alternative to improve sleep in cancer survivors,” Dr Wu said.
However, she and her colleagues noted that larger-scale studies are needed. ![]()
KRd improves OS in relapsed/refractory MM
Adding carfilzomib (K) to treatment with lenalidomide and dexamethasone (Rd) can improve overall survival (OS) in patients with relapsed or refractory multiple myeloma (MM), according to research published in the Journal of Clinical Oncology.
Final results from the phase 3 ASPIRE trial showed that KRd reduced the risk of death by 21% and extended OS by 7.9 months, when compared to Rd.
In patients at first relapse, KRd was associated with an OS improvement of 11.4 months.
“Results from the final analysis of the phase 3 ASPIRE trial . . . are significant, as they further validate carfilzomib, lenalidomide, and dexamethasone as a standard-of-care regimen for patients with relapsed or refractory multiple myeloma,” said study author Keith Stewart, MBChB, of Mayo Clinic in Scottsdale, Arizona.
“Furthermore, these data showed that early use of carfilzomib, lenalidomide, and dexamethasone at first relapse provided nearly 1 additional year of survival for patients, regardless of prior treatment with bortezomib or transplant.”
ASPIRE enrolled 792 MM patients who had received a median of 2 prior therapies (range, 1-3). They were randomized to receive KRd (n=396) or Rd (n=396). Baseline characteristics were well balanced between the treatment arms.
Details on patients and treatment, as well as interim results from ASPIRE, were reported at the 2014 ASH Annual Meeting and published in NEJM in January 2015.
Treatment update
In the final analysis, there were 340 patients in the KRd arm and 358 in the Rd arm who stopped study treatment.
Reasons for discontinuation (in the KRd and Rd arms, respectively) were disease progression (n=188 and 224), adverse events (AEs, n=79 and 85), other reasons (n=61 and 35), withdrawn consent (n=10 and 12), and noncompliance (n=2 and 1).
A total of 182 patients in the KRd arm and 211 in the Rd arm received subsequent treatment for MM. These treatments were generally balanced between the KRd and Rd arms.
The median time to next treatment from the time of randomization was 39.0 months in the KRd arm and 24.4 months in the Rd arm (hazard ratio [HR]=0.65 P<0.001).
Survival
Interim ASPIRE data had shown a significant improvement in progression-free survival (PFS) and a trend toward improved OS in patients who received KRd. Now, researchers have observed a significant improvement in both endpoints with KRd.
The data cutoff for the final analysis was April 28, 2017. For PFS, the median follow-up was 48.8 months in the KRd arm and 48.0 months in the Rd arm.
The median PFS was 26.1 months in the KRd arm and 16.6 months in the Rd arm (9.5-month improvement, HR=0.66; P<0.001). The 3-year PFS rates were 38.2% and 28.4%, respectively. And the 5-year PFS rates were 25.6% and 17.3%, respectively.
The median follow-up for OS was 67.1 months. The median OS was 48.3 months in the KRd arm and 40.4 months in the Rd arm (7.9-month improvement, HR=0.79, P=0.0045).
The researchers also performed subgroup analyses according to prior lines of therapy, prior bortezomib exposure at first relapse, and prior transplant at first relapse.
In patients who had received 1 prior line of therapy, the median OS was 47.3 months in the KRd arm and 35.9 months in the Rd arm (11.4-month improvement, HR=0.81). For patients with 2 or more prior lines of therapy, the median OS was 48.8 months and 42.3 months, respectively (6.5-month improvement, HR=0.79).
Among patients with prior bortezomib exposure at first relapse, the median OS was 45.9 months in the KRd arm and 33.9 months in the Rd arm (12-month improvement, HR=0.82). Among patients without prior bortezomib exposure at first relapse, the median OS was 48.3 months and 40.4 months, respectively (7.9-month improvement, HR=0.80).
Among patients with prior transplant at first relapse, the median OS was 57.2 months in the KRd arm and 38.6 months in the Rd arm (18.6-month improvement, HR=0.71).
Safety
The incidence of treatment-emergent AEs was 98% in the KRd arm and 97.9% in the Rd arm. The incidence of grade 3 or higher AEs was 87% and 83.3%, respectively. The incidence of serious AEs was 65.3% and 56.8%, respectively.
Treatment discontinuation due to an AE occurred in 19.9% of patients in the KRd arm and 21.5% of patients in the Rd arm.
AEs of interest (in the KRd and Rd arms, respectively) included acute renal failure (9.2% and 7.7%), cardiac failure (7.1% and 4.1%), ischemic heart disease (6.9% and 4.6%), hypertension (17.1% and 8.7%), hematopoietic thrombocytopenia (32.7% and 26.2%), and peripheral neuropathy (18.9% and 17.2%).
Fatal AEs were reported in 11.5% of patients in the KRd arm and 10.8% of those in the Rd arm.
Fatal AEs reported in at least 2 patients in the KRd arm included (in the KRd and Rd arms, respectively) cardiac disorders (2.6% and 2.3%), pneumonia (1.5% and 0.8%), sepsis (0.8% for both), myocardial infarction (0.8% and 0.5%), acute respiratory distress syndrome (0.8% and 0%), death (0.5% for both), and cardiac arrest (0.5% and 0.3%).
This trial was funded by Onyx Pharmaceuticals, Inc. ![]()
Adding carfilzomib (K) to treatment with lenalidomide and dexamethasone (Rd) can improve overall survival (OS) in patients with relapsed or refractory multiple myeloma (MM), according to research published in the Journal of Clinical Oncology.
Final results from the phase 3 ASPIRE trial showed that KRd reduced the risk of death by 21% and extended OS by 7.9 months, when compared to Rd.
In patients at first relapse, KRd was associated with an OS improvement of 11.4 months.
“Results from the final analysis of the phase 3 ASPIRE trial . . . are significant, as they further validate carfilzomib, lenalidomide, and dexamethasone as a standard-of-care regimen for patients with relapsed or refractory multiple myeloma,” said study author Keith Stewart, MBChB, of Mayo Clinic in Scottsdale, Arizona.
“Furthermore, these data showed that early use of carfilzomib, lenalidomide, and dexamethasone at first relapse provided nearly 1 additional year of survival for patients, regardless of prior treatment with bortezomib or transplant.”
ASPIRE enrolled 792 MM patients who had received a median of 2 prior therapies (range, 1-3). They were randomized to receive KRd (n=396) or Rd (n=396). Baseline characteristics were well balanced between the treatment arms.
Details on patients and treatment, as well as interim results from ASPIRE, were reported at the 2014 ASH Annual Meeting and published in NEJM in January 2015.
Treatment update
In the final analysis, there were 340 patients in the KRd arm and 358 in the Rd arm who stopped study treatment.
Reasons for discontinuation (in the KRd and Rd arms, respectively) were disease progression (n=188 and 224), adverse events (AEs, n=79 and 85), other reasons (n=61 and 35), withdrawn consent (n=10 and 12), and noncompliance (n=2 and 1).
A total of 182 patients in the KRd arm and 211 in the Rd arm received subsequent treatment for MM. These treatments were generally balanced between the KRd and Rd arms.
The median time to next treatment from the time of randomization was 39.0 months in the KRd arm and 24.4 months in the Rd arm (hazard ratio [HR]=0.65 P<0.001).
Survival
Interim ASPIRE data had shown a significant improvement in progression-free survival (PFS) and a trend toward improved OS in patients who received KRd. Now, researchers have observed a significant improvement in both endpoints with KRd.
The data cutoff for the final analysis was April 28, 2017. For PFS, the median follow-up was 48.8 months in the KRd arm and 48.0 months in the Rd arm.
The median PFS was 26.1 months in the KRd arm and 16.6 months in the Rd arm (9.5-month improvement, HR=0.66; P<0.001). The 3-year PFS rates were 38.2% and 28.4%, respectively. And the 5-year PFS rates were 25.6% and 17.3%, respectively.
The median follow-up for OS was 67.1 months. The median OS was 48.3 months in the KRd arm and 40.4 months in the Rd arm (7.9-month improvement, HR=0.79, P=0.0045).
The researchers also performed subgroup analyses according to prior lines of therapy, prior bortezomib exposure at first relapse, and prior transplant at first relapse.
In patients who had received 1 prior line of therapy, the median OS was 47.3 months in the KRd arm and 35.9 months in the Rd arm (11.4-month improvement, HR=0.81). For patients with 2 or more prior lines of therapy, the median OS was 48.8 months and 42.3 months, respectively (6.5-month improvement, HR=0.79).
Among patients with prior bortezomib exposure at first relapse, the median OS was 45.9 months in the KRd arm and 33.9 months in the Rd arm (12-month improvement, HR=0.82). Among patients without prior bortezomib exposure at first relapse, the median OS was 48.3 months and 40.4 months, respectively (7.9-month improvement, HR=0.80).
Among patients with prior transplant at first relapse, the median OS was 57.2 months in the KRd arm and 38.6 months in the Rd arm (18.6-month improvement, HR=0.71).
Safety
The incidence of treatment-emergent AEs was 98% in the KRd arm and 97.9% in the Rd arm. The incidence of grade 3 or higher AEs was 87% and 83.3%, respectively. The incidence of serious AEs was 65.3% and 56.8%, respectively.
Treatment discontinuation due to an AE occurred in 19.9% of patients in the KRd arm and 21.5% of patients in the Rd arm.
AEs of interest (in the KRd and Rd arms, respectively) included acute renal failure (9.2% and 7.7%), cardiac failure (7.1% and 4.1%), ischemic heart disease (6.9% and 4.6%), hypertension (17.1% and 8.7%), hematopoietic thrombocytopenia (32.7% and 26.2%), and peripheral neuropathy (18.9% and 17.2%).
Fatal AEs were reported in 11.5% of patients in the KRd arm and 10.8% of those in the Rd arm.
Fatal AEs reported in at least 2 patients in the KRd arm included (in the KRd and Rd arms, respectively) cardiac disorders (2.6% and 2.3%), pneumonia (1.5% and 0.8%), sepsis (0.8% for both), myocardial infarction (0.8% and 0.5%), acute respiratory distress syndrome (0.8% and 0%), death (0.5% for both), and cardiac arrest (0.5% and 0.3%).
This trial was funded by Onyx Pharmaceuticals, Inc. ![]()
Adding carfilzomib (K) to treatment with lenalidomide and dexamethasone (Rd) can improve overall survival (OS) in patients with relapsed or refractory multiple myeloma (MM), according to research published in the Journal of Clinical Oncology.
Final results from the phase 3 ASPIRE trial showed that KRd reduced the risk of death by 21% and extended OS by 7.9 months, when compared to Rd.
In patients at first relapse, KRd was associated with an OS improvement of 11.4 months.
“Results from the final analysis of the phase 3 ASPIRE trial . . . are significant, as they further validate carfilzomib, lenalidomide, and dexamethasone as a standard-of-care regimen for patients with relapsed or refractory multiple myeloma,” said study author Keith Stewart, MBChB, of Mayo Clinic in Scottsdale, Arizona.
“Furthermore, these data showed that early use of carfilzomib, lenalidomide, and dexamethasone at first relapse provided nearly 1 additional year of survival for patients, regardless of prior treatment with bortezomib or transplant.”
ASPIRE enrolled 792 MM patients who had received a median of 2 prior therapies (range, 1-3). They were randomized to receive KRd (n=396) or Rd (n=396). Baseline characteristics were well balanced between the treatment arms.
Details on patients and treatment, as well as interim results from ASPIRE, were reported at the 2014 ASH Annual Meeting and published in NEJM in January 2015.
Treatment update
In the final analysis, there were 340 patients in the KRd arm and 358 in the Rd arm who stopped study treatment.
Reasons for discontinuation (in the KRd and Rd arms, respectively) were disease progression (n=188 and 224), adverse events (AEs, n=79 and 85), other reasons (n=61 and 35), withdrawn consent (n=10 and 12), and noncompliance (n=2 and 1).
A total of 182 patients in the KRd arm and 211 in the Rd arm received subsequent treatment for MM. These treatments were generally balanced between the KRd and Rd arms.
The median time to next treatment from the time of randomization was 39.0 months in the KRd arm and 24.4 months in the Rd arm (hazard ratio [HR]=0.65 P<0.001).
Survival
Interim ASPIRE data had shown a significant improvement in progression-free survival (PFS) and a trend toward improved OS in patients who received KRd. Now, researchers have observed a significant improvement in both endpoints with KRd.
The data cutoff for the final analysis was April 28, 2017. For PFS, the median follow-up was 48.8 months in the KRd arm and 48.0 months in the Rd arm.
The median PFS was 26.1 months in the KRd arm and 16.6 months in the Rd arm (9.5-month improvement, HR=0.66; P<0.001). The 3-year PFS rates were 38.2% and 28.4%, respectively. And the 5-year PFS rates were 25.6% and 17.3%, respectively.
The median follow-up for OS was 67.1 months. The median OS was 48.3 months in the KRd arm and 40.4 months in the Rd arm (7.9-month improvement, HR=0.79, P=0.0045).
The researchers also performed subgroup analyses according to prior lines of therapy, prior bortezomib exposure at first relapse, and prior transplant at first relapse.
In patients who had received 1 prior line of therapy, the median OS was 47.3 months in the KRd arm and 35.9 months in the Rd arm (11.4-month improvement, HR=0.81). For patients with 2 or more prior lines of therapy, the median OS was 48.8 months and 42.3 months, respectively (6.5-month improvement, HR=0.79).
Among patients with prior bortezomib exposure at first relapse, the median OS was 45.9 months in the KRd arm and 33.9 months in the Rd arm (12-month improvement, HR=0.82). Among patients without prior bortezomib exposure at first relapse, the median OS was 48.3 months and 40.4 months, respectively (7.9-month improvement, HR=0.80).
Among patients with prior transplant at first relapse, the median OS was 57.2 months in the KRd arm and 38.6 months in the Rd arm (18.6-month improvement, HR=0.71).
Safety
The incidence of treatment-emergent AEs was 98% in the KRd arm and 97.9% in the Rd arm. The incidence of grade 3 or higher AEs was 87% and 83.3%, respectively. The incidence of serious AEs was 65.3% and 56.8%, respectively.
Treatment discontinuation due to an AE occurred in 19.9% of patients in the KRd arm and 21.5% of patients in the Rd arm.
AEs of interest (in the KRd and Rd arms, respectively) included acute renal failure (9.2% and 7.7%), cardiac failure (7.1% and 4.1%), ischemic heart disease (6.9% and 4.6%), hypertension (17.1% and 8.7%), hematopoietic thrombocytopenia (32.7% and 26.2%), and peripheral neuropathy (18.9% and 17.2%).
Fatal AEs were reported in 11.5% of patients in the KRd arm and 10.8% of those in the Rd arm.
Fatal AEs reported in at least 2 patients in the KRd arm included (in the KRd and Rd arms, respectively) cardiac disorders (2.6% and 2.3%), pneumonia (1.5% and 0.8%), sepsis (0.8% for both), myocardial infarction (0.8% and 0.5%), acute respiratory distress syndrome (0.8% and 0%), death (0.5% for both), and cardiac arrest (0.5% and 0.3%).
This trial was funded by Onyx Pharmaceuticals, Inc. ![]()
FDA surveillance shows how rivaroxaban compares to warfarin
The US Food and Drug Administration’s (FDA) Mini-Sentinel surveillance system has revealed no new safety concerns associated with rivaroxaban use in patients with nonvalvular atrial fibrillation.
Results from this surveillance showed the risk of ischemic stroke was lower in patients who received rivaroxaban than in those who received warfarin.
Rivaroxaban was also associated with a lower risk of intracranial hemorrhage but a higher risk of gastrointestinal bleeding compared to warfarin.
These findings were published in Pharmacoepidemiology & Drug Safety.
About the surveillance
The FDA’s Sentinel Initiative began in 2008 as a multi-year effort to create a national electronic system for monitoring the safety of approved and FDA-regulated medical products using existing electronic healthcare data from multiple sources.
The initiative is the FDA’s response to the Food and Drug Administration Amendments Act (FDAAA), and it includes Mini-Sentinel, a working pilot project to develop an active surveillance system and complement existing methods of safety surveillance.
Rivaroxaban results
Researchers analyzed Mini-Sentinel data for patients with nonvalvular atrial fibrillation who initiated treatment with rivaroxaban or warfarin from November 2011 to April 2015.
To examine the safety of both products, methodologies included assessing ICD-9-CM codes from inpatient claims and evaluating rates of gastrointestinal bleeding, ischemic stroke, and intracranial hemorrhage.*
The incidence of ischemic stroke was higher among patients on warfarin (268/80,180) than among those on rivaroxaban (82/36,512). The adjusted incidence rate per 1000 person-years was 9.57 among rivaroxaban users and 17.10 among warfarin users. The adjusted hazard ratio was 0.61 (95% CI, 0.47-0.79).
The incidence of gastrointestinal bleeding was higher among patients on rivaroxaban (423/36,173) than among those on warfarin (651/79,520). The incidence rate was 50.20 among rivaroxaban users and 34.82 among warfarin users. The hazard ratio was 1.47 (95% CI, 1.29-1.67).
The incidence of intracranial hemorrhage was higher among patients on warfarin (143/79,529) than among those on rivaroxaban (46/36,171).The incidence rate was 5.41 among rivaroxaban users and 7.49 among warfarin users. The hazard ratio was 0.71 (95% CI, 0.50-1.01). ![]()
*Total patient numbers differed in the analyses for each endpoint. For example, there were 80,180 warfarin users in the ischemic stroke analysis and 79,520 warfarin users in the analysis of gastrointestinal bleeding.
The US Food and Drug Administration’s (FDA) Mini-Sentinel surveillance system has revealed no new safety concerns associated with rivaroxaban use in patients with nonvalvular atrial fibrillation.
Results from this surveillance showed the risk of ischemic stroke was lower in patients who received rivaroxaban than in those who received warfarin.
Rivaroxaban was also associated with a lower risk of intracranial hemorrhage but a higher risk of gastrointestinal bleeding compared to warfarin.
These findings were published in Pharmacoepidemiology & Drug Safety.
About the surveillance
The FDA’s Sentinel Initiative began in 2008 as a multi-year effort to create a national electronic system for monitoring the safety of approved and FDA-regulated medical products using existing electronic healthcare data from multiple sources.
The initiative is the FDA’s response to the Food and Drug Administration Amendments Act (FDAAA), and it includes Mini-Sentinel, a working pilot project to develop an active surveillance system and complement existing methods of safety surveillance.
Rivaroxaban results
Researchers analyzed Mini-Sentinel data for patients with nonvalvular atrial fibrillation who initiated treatment with rivaroxaban or warfarin from November 2011 to April 2015.
To examine the safety of both products, methodologies included assessing ICD-9-CM codes from inpatient claims and evaluating rates of gastrointestinal bleeding, ischemic stroke, and intracranial hemorrhage.*
The incidence of ischemic stroke was higher among patients on warfarin (268/80,180) than among those on rivaroxaban (82/36,512). The adjusted incidence rate per 1000 person-years was 9.57 among rivaroxaban users and 17.10 among warfarin users. The adjusted hazard ratio was 0.61 (95% CI, 0.47-0.79).
The incidence of gastrointestinal bleeding was higher among patients on rivaroxaban (423/36,173) than among those on warfarin (651/79,520). The incidence rate was 50.20 among rivaroxaban users and 34.82 among warfarin users. The hazard ratio was 1.47 (95% CI, 1.29-1.67).
The incidence of intracranial hemorrhage was higher among patients on warfarin (143/79,529) than among those on rivaroxaban (46/36,171).The incidence rate was 5.41 among rivaroxaban users and 7.49 among warfarin users. The hazard ratio was 0.71 (95% CI, 0.50-1.01). ![]()
*Total patient numbers differed in the analyses for each endpoint. For example, there were 80,180 warfarin users in the ischemic stroke analysis and 79,520 warfarin users in the analysis of gastrointestinal bleeding.
The US Food and Drug Administration’s (FDA) Mini-Sentinel surveillance system has revealed no new safety concerns associated with rivaroxaban use in patients with nonvalvular atrial fibrillation.
Results from this surveillance showed the risk of ischemic stroke was lower in patients who received rivaroxaban than in those who received warfarin.
Rivaroxaban was also associated with a lower risk of intracranial hemorrhage but a higher risk of gastrointestinal bleeding compared to warfarin.
These findings were published in Pharmacoepidemiology & Drug Safety.
About the surveillance
The FDA’s Sentinel Initiative began in 2008 as a multi-year effort to create a national electronic system for monitoring the safety of approved and FDA-regulated medical products using existing electronic healthcare data from multiple sources.
The initiative is the FDA’s response to the Food and Drug Administration Amendments Act (FDAAA), and it includes Mini-Sentinel, a working pilot project to develop an active surveillance system and complement existing methods of safety surveillance.
Rivaroxaban results
Researchers analyzed Mini-Sentinel data for patients with nonvalvular atrial fibrillation who initiated treatment with rivaroxaban or warfarin from November 2011 to April 2015.
To examine the safety of both products, methodologies included assessing ICD-9-CM codes from inpatient claims and evaluating rates of gastrointestinal bleeding, ischemic stroke, and intracranial hemorrhage.*
The incidence of ischemic stroke was higher among patients on warfarin (268/80,180) than among those on rivaroxaban (82/36,512). The adjusted incidence rate per 1000 person-years was 9.57 among rivaroxaban users and 17.10 among warfarin users. The adjusted hazard ratio was 0.61 (95% CI, 0.47-0.79).
The incidence of gastrointestinal bleeding was higher among patients on rivaroxaban (423/36,173) than among those on warfarin (651/79,520). The incidence rate was 50.20 among rivaroxaban users and 34.82 among warfarin users. The hazard ratio was 1.47 (95% CI, 1.29-1.67).
The incidence of intracranial hemorrhage was higher among patients on warfarin (143/79,529) than among those on rivaroxaban (46/36,171).The incidence rate was 5.41 among rivaroxaban users and 7.49 among warfarin users. The hazard ratio was 0.71 (95% CI, 0.50-1.01). ![]()
*Total patient numbers differed in the analyses for each endpoint. For example, there were 80,180 warfarin users in the ischemic stroke analysis and 79,520 warfarin users in the analysis of gastrointestinal bleeding.
Compound may treat drug-resistant malaria
The antimicrobial agent triclosan may be able to fight drug-resistant malaria, according to research published in Scientific Reports.
For this work, researchers used an artificially intelligent “robot scientist” named Eve to perform a high-throughput screen of potential antimalarial compounds.
The screen revealed that triclosan is effective against Plasmodium parasites that have grown resistant to the antimalarial drug pyrimethamine.
Researchers have known for some time that triclosan inhibits in vitro growth of Plasmodium parasites. They assumed this was because triclosan inhibits the enzyme enoyl reductase (ENR).
However, subsequent work showed that improving triclosan’s ability to target ENR had no effect on parasite growth in the blood.
With the current study, researchers discovered that triclosan also affects parasite growth by inhibiting an enzyme called dihydrofolate reductase (DHFR), which is the target of the antimalarial drug pyrimethamine.
The researchers conducted growth competition experiments with 3 yeast strains dependent on a DHFR enzyme from Plasmodium falciparum, a DHFR enzyme from Plasmodium vivax, and human DHFR.
The team used Eve to screen compounds and identify drugs that inhibit the parasite targets but not the human counterpart.
The researchers found that triclosan was able to target and act on DHFR in both wild-type and pyrimethamine-resistant P falciparum and P vivax parasites.
Because triclosan inhibits both ENR and DHFR, the researchers think it may be possible to target Plasmodium parasites at both the liver and blood stages.
“The discovery by our robot ‘colleague’ Eve that triclosan is effective against malaria targets offers hope that we may be able to use it to develop a new drug,” said study author Elizabeth Bilsland, PhD, of the University of Campinas in Brazil.
“We know it is a safe compound, and its ability to target 2 points in the malaria parasite’s life-cycle means the parasite will find it difficult to evolve resistance.”
Eve was developed to speed up the drug discovery process by automatically developing and testing hypotheses to explain observations, run experiments using laboratory robotics, interpret the results to amend hypotheses, and then repeat the cycle.
“Artificial intelligence and machine-learning enables us to create automated scientists that do not just take a ‘brute force’ approach but, rather, take an intelligent approach to science,” said Ross King, PhD, a professor at the University of Manchester in the UK who led the development of Eve.
“This could greatly speed up the drug discovery process and potentially reap huge rewards.” ![]()
The antimicrobial agent triclosan may be able to fight drug-resistant malaria, according to research published in Scientific Reports.
For this work, researchers used an artificially intelligent “robot scientist” named Eve to perform a high-throughput screen of potential antimalarial compounds.
The screen revealed that triclosan is effective against Plasmodium parasites that have grown resistant to the antimalarial drug pyrimethamine.
Researchers have known for some time that triclosan inhibits in vitro growth of Plasmodium parasites. They assumed this was because triclosan inhibits the enzyme enoyl reductase (ENR).
However, subsequent work showed that improving triclosan’s ability to target ENR had no effect on parasite growth in the blood.
With the current study, researchers discovered that triclosan also affects parasite growth by inhibiting an enzyme called dihydrofolate reductase (DHFR), which is the target of the antimalarial drug pyrimethamine.
The researchers conducted growth competition experiments with 3 yeast strains dependent on a DHFR enzyme from Plasmodium falciparum, a DHFR enzyme from Plasmodium vivax, and human DHFR.
The team used Eve to screen compounds and identify drugs that inhibit the parasite targets but not the human counterpart.
The researchers found that triclosan was able to target and act on DHFR in both wild-type and pyrimethamine-resistant P falciparum and P vivax parasites.
Because triclosan inhibits both ENR and DHFR, the researchers think it may be possible to target Plasmodium parasites at both the liver and blood stages.
“The discovery by our robot ‘colleague’ Eve that triclosan is effective against malaria targets offers hope that we may be able to use it to develop a new drug,” said study author Elizabeth Bilsland, PhD, of the University of Campinas in Brazil.
“We know it is a safe compound, and its ability to target 2 points in the malaria parasite’s life-cycle means the parasite will find it difficult to evolve resistance.”
Eve was developed to speed up the drug discovery process by automatically developing and testing hypotheses to explain observations, run experiments using laboratory robotics, interpret the results to amend hypotheses, and then repeat the cycle.
“Artificial intelligence and machine-learning enables us to create automated scientists that do not just take a ‘brute force’ approach but, rather, take an intelligent approach to science,” said Ross King, PhD, a professor at the University of Manchester in the UK who led the development of Eve.
“This could greatly speed up the drug discovery process and potentially reap huge rewards.” ![]()
The antimicrobial agent triclosan may be able to fight drug-resistant malaria, according to research published in Scientific Reports.
For this work, researchers used an artificially intelligent “robot scientist” named Eve to perform a high-throughput screen of potential antimalarial compounds.
The screen revealed that triclosan is effective against Plasmodium parasites that have grown resistant to the antimalarial drug pyrimethamine.
Researchers have known for some time that triclosan inhibits in vitro growth of Plasmodium parasites. They assumed this was because triclosan inhibits the enzyme enoyl reductase (ENR).
However, subsequent work showed that improving triclosan’s ability to target ENR had no effect on parasite growth in the blood.
With the current study, researchers discovered that triclosan also affects parasite growth by inhibiting an enzyme called dihydrofolate reductase (DHFR), which is the target of the antimalarial drug pyrimethamine.
The researchers conducted growth competition experiments with 3 yeast strains dependent on a DHFR enzyme from Plasmodium falciparum, a DHFR enzyme from Plasmodium vivax, and human DHFR.
The team used Eve to screen compounds and identify drugs that inhibit the parasite targets but not the human counterpart.
The researchers found that triclosan was able to target and act on DHFR in both wild-type and pyrimethamine-resistant P falciparum and P vivax parasites.
Because triclosan inhibits both ENR and DHFR, the researchers think it may be possible to target Plasmodium parasites at both the liver and blood stages.
“The discovery by our robot ‘colleague’ Eve that triclosan is effective against malaria targets offers hope that we may be able to use it to develop a new drug,” said study author Elizabeth Bilsland, PhD, of the University of Campinas in Brazil.
“We know it is a safe compound, and its ability to target 2 points in the malaria parasite’s life-cycle means the parasite will find it difficult to evolve resistance.”
Eve was developed to speed up the drug discovery process by automatically developing and testing hypotheses to explain observations, run experiments using laboratory robotics, interpret the results to amend hypotheses, and then repeat the cycle.
“Artificial intelligence and machine-learning enables us to create automated scientists that do not just take a ‘brute force’ approach but, rather, take an intelligent approach to science,” said Ross King, PhD, a professor at the University of Manchester in the UK who led the development of Eve.
“This could greatly speed up the drug discovery process and potentially reap huge rewards.” ![]()
Risks of MGUS persist beyond 30 years
A long-term study showed that patients with monoclonal gammopathy of undetermined significance (MGUS) were still at risk of progressing to other plasma-cell or lymphoid disorders after more than 30 years of follow-up.
The risk of developing such disorders was nearly 7 times higher in MGUS patients than in matched control subjects.
Patients with MGUS also had a significantly shorter median survival than controls.
Researchers reported these findings in NEJM.
“Monoclonal gammopathy of undetermined significance is present in more than 3% of the general population age 50 and older,” said study author S. Vincent Rajkumar, MD, of the Mayo Clinic in Rochester, Minnesota.
“In some cases, people with monoclonal gammopathy of undetermined significance go on to develop multiple myeloma.”
With this in mind, Dr Rajkumar and his colleagues studied 1384 patients—210 with IgM MGUS and 1129 with non-IgM MGUS. Patients were diagnosed with MGUS from 1960 through 1994, and their median age at diagnosis was 72.
The median follow-up was 34.1 years (range, 0.0 to 43.6), so there were 14,130 person-years of follow-up.
During that time, 147 patients progressed to another disorder, including:
- 97 to multiple myeloma
- 19 to non-Hodgkin lymphoma
- 14 to AL amyloidosis
- 13 to Waldenstrom’s macroglobulinemia
- 3 to chronic lymphocytic leukemia
- 1 to plasmacytoma.
The rate of progression in MGUS patients—11%—represented a risk of these disorders that was 6.5 times higher than the risk observed in an age- and sex-matched control population.
The risk of progression also increased over time for MGUS patients. Without accounting for death due to competing causes, the risk of progression was 10% at 10 years, 18% at 20 years, 28% at 30 years, and 36% at both 35 and 40 years.
“We also found that patients with monoclonal gammopathy of undetermined significance had shorter survival than comparable people without the condition, which raises the possibility there may be other disorders associated with monoclonal gammopathy of undetermined significance that still need further study,” Dr Rajkumar said.
The median survival was 8.1 years in MGUS patients and 12.4 years in controls (P<0.001).
Overall, 1300 MGUS patients (94%) had died at last follow-up. Of the 84 patients who were still alive, 5 had progressed. ![]()
A long-term study showed that patients with monoclonal gammopathy of undetermined significance (MGUS) were still at risk of progressing to other plasma-cell or lymphoid disorders after more than 30 years of follow-up.
The risk of developing such disorders was nearly 7 times higher in MGUS patients than in matched control subjects.
Patients with MGUS also had a significantly shorter median survival than controls.
Researchers reported these findings in NEJM.
“Monoclonal gammopathy of undetermined significance is present in more than 3% of the general population age 50 and older,” said study author S. Vincent Rajkumar, MD, of the Mayo Clinic in Rochester, Minnesota.
“In some cases, people with monoclonal gammopathy of undetermined significance go on to develop multiple myeloma.”
With this in mind, Dr Rajkumar and his colleagues studied 1384 patients—210 with IgM MGUS and 1129 with non-IgM MGUS. Patients were diagnosed with MGUS from 1960 through 1994, and their median age at diagnosis was 72.
The median follow-up was 34.1 years (range, 0.0 to 43.6), so there were 14,130 person-years of follow-up.
During that time, 147 patients progressed to another disorder, including:
- 97 to multiple myeloma
- 19 to non-Hodgkin lymphoma
- 14 to AL amyloidosis
- 13 to Waldenstrom’s macroglobulinemia
- 3 to chronic lymphocytic leukemia
- 1 to plasmacytoma.
The rate of progression in MGUS patients—11%—represented a risk of these disorders that was 6.5 times higher than the risk observed in an age- and sex-matched control population.
The risk of progression also increased over time for MGUS patients. Without accounting for death due to competing causes, the risk of progression was 10% at 10 years, 18% at 20 years, 28% at 30 years, and 36% at both 35 and 40 years.
“We also found that patients with monoclonal gammopathy of undetermined significance had shorter survival than comparable people without the condition, which raises the possibility there may be other disorders associated with monoclonal gammopathy of undetermined significance that still need further study,” Dr Rajkumar said.
The median survival was 8.1 years in MGUS patients and 12.4 years in controls (P<0.001).
Overall, 1300 MGUS patients (94%) had died at last follow-up. Of the 84 patients who were still alive, 5 had progressed. ![]()
A long-term study showed that patients with monoclonal gammopathy of undetermined significance (MGUS) were still at risk of progressing to other plasma-cell or lymphoid disorders after more than 30 years of follow-up.
The risk of developing such disorders was nearly 7 times higher in MGUS patients than in matched control subjects.
Patients with MGUS also had a significantly shorter median survival than controls.
Researchers reported these findings in NEJM.
“Monoclonal gammopathy of undetermined significance is present in more than 3% of the general population age 50 and older,” said study author S. Vincent Rajkumar, MD, of the Mayo Clinic in Rochester, Minnesota.
“In some cases, people with monoclonal gammopathy of undetermined significance go on to develop multiple myeloma.”
With this in mind, Dr Rajkumar and his colleagues studied 1384 patients—210 with IgM MGUS and 1129 with non-IgM MGUS. Patients were diagnosed with MGUS from 1960 through 1994, and their median age at diagnosis was 72.
The median follow-up was 34.1 years (range, 0.0 to 43.6), so there were 14,130 person-years of follow-up.
During that time, 147 patients progressed to another disorder, including:
- 97 to multiple myeloma
- 19 to non-Hodgkin lymphoma
- 14 to AL amyloidosis
- 13 to Waldenstrom’s macroglobulinemia
- 3 to chronic lymphocytic leukemia
- 1 to plasmacytoma.
The rate of progression in MGUS patients—11%—represented a risk of these disorders that was 6.5 times higher than the risk observed in an age- and sex-matched control population.
The risk of progression also increased over time for MGUS patients. Without accounting for death due to competing causes, the risk of progression was 10% at 10 years, 18% at 20 years, 28% at 30 years, and 36% at both 35 and 40 years.
“We also found that patients with monoclonal gammopathy of undetermined significance had shorter survival than comparable people without the condition, which raises the possibility there may be other disorders associated with monoclonal gammopathy of undetermined significance that still need further study,” Dr Rajkumar said.
The median survival was 8.1 years in MGUS patients and 12.4 years in controls (P<0.001).
Overall, 1300 MGUS patients (94%) had died at last follow-up. Of the 84 patients who were still alive, 5 had progressed.
CAR T-cell therapy on fast track in US, EU
The chimeric antigen receptor (CAR) T-cell therapy tisagenlecleucel (Kymriah, formerly CTL019) is getting fast-tracked in the United States (US) and European Union (EU).
The US Food and Drug Administration (FDA) has accepted for priority review the supplemental biologics license application (sBLA) for tisagenlecleucel for the treatment of adults with relapsed or refractory (R/R) diffuse large B-cell lymphoma (DLBCL) who are ineligible for, or relapse after, autologous hematopoietic stem cell transplant (auto-HSCT).
Meanwhile, the European Medicines Agency (EMA) has granted accelerated assessment to the marketing authorization application (MAA) for tisagenlecleucel for the treatment of children and young adults with R/R B-cell acute lymphoblastic leukemia (ALL) and for adults with R/R DLBCL who are ineligible for auto-HSCT.
If the sBLA and MAA are approved, tisagenlecleucel will be the first CAR T-cell therapy available for 2 distinct indications in non-Hodgkin lymphoma and B-cell ALL.
Tisagenlecleucel became the first CAR T-cell therapy to receive regulatory approval when it was approved by the FDA in August 2017 for use in patients up to 25 years of age who have B-cell precursor ALL that is refractory or in second or later relapse.
Supporting data
The regulatory applications for tisagenlecleucel in the US and EU are supported by data from the Novartis-sponsored global clinical trial program in children and young adults with R/R B-cell ALL and adults with R/R DLBCL.
Results from the phase 2 JULIET trial served as the basis of the sBLA and MAA for tisagenlecleucel in adults with R/R DLCBL. Data from this trial were presented at the 2017 ASH Annual Meeting in December.
Results from the phase 2 ELIANA study were submitted as part of the MAA for tisagenlecleucel in children and young adults with R/R B-cell ALL. Data from this trial were presented at the 2017 EHA Congress last June.
About priority review, accelerated assessment
The FDA grants priority review to applications for products that may provide significant improvements in the treatment, diagnosis, or prevention of serious conditions.
The FDA’s goal is to take action on a priority review application within 6 months of receiving it, rather than the standard 10 months.
The EMA grants accelerated assessment when a product is expected to be of major public health interest, particularly from the point of view of therapeutic innovation.
Accelerated assessment shortens the review period from 210 days to 150 days.
The chimeric antigen receptor (CAR) T-cell therapy tisagenlecleucel (Kymriah, formerly CTL019) is getting fast-tracked in the United States (US) and European Union (EU).
The US Food and Drug Administration (FDA) has accepted for priority review the supplemental biologics license application (sBLA) for tisagenlecleucel for the treatment of adults with relapsed or refractory (R/R) diffuse large B-cell lymphoma (DLBCL) who are ineligible for, or relapse after, autologous hematopoietic stem cell transplant (auto-HSCT).
Meanwhile, the European Medicines Agency (EMA) has granted accelerated assessment to the marketing authorization application (MAA) for tisagenlecleucel for the treatment of children and young adults with R/R B-cell acute lymphoblastic leukemia (ALL) and for adults with R/R DLBCL who are ineligible for auto-HSCT.
If the sBLA and MAA are approved, tisagenlecleucel will be the first CAR T-cell therapy available for 2 distinct indications in non-Hodgkin lymphoma and B-cell ALL.
Tisagenlecleucel became the first CAR T-cell therapy to receive regulatory approval when it was approved by the FDA in August 2017 for use in patients up to 25 years of age who have B-cell precursor ALL that is refractory or in second or later relapse.
Supporting data
The regulatory applications for tisagenlecleucel in the US and EU are supported by data from the Novartis-sponsored global clinical trial program in children and young adults with R/R B-cell ALL and adults with R/R DLBCL.
Results from the phase 2 JULIET trial served as the basis of the sBLA and MAA for tisagenlecleucel in adults with R/R DLCBL. Data from this trial were presented at the 2017 ASH Annual Meeting in December.
Results from the phase 2 ELIANA study were submitted as part of the MAA for tisagenlecleucel in children and young adults with R/R B-cell ALL. Data from this trial were presented at the 2017 EHA Congress last June.
About priority review, accelerated assessment
The FDA grants priority review to applications for products that may provide significant improvements in the treatment, diagnosis, or prevention of serious conditions.
The FDA’s goal is to take action on a priority review application within 6 months of receiving it, rather than the standard 10 months.
The EMA grants accelerated assessment when a product is expected to be of major public health interest, particularly from the point of view of therapeutic innovation.
Accelerated assessment shortens the review period from 210 days to 150 days.
The chimeric antigen receptor (CAR) T-cell therapy tisagenlecleucel (Kymriah, formerly CTL019) is getting fast-tracked in the United States (US) and European Union (EU).
The US Food and Drug Administration (FDA) has accepted for priority review the supplemental biologics license application (sBLA) for tisagenlecleucel for the treatment of adults with relapsed or refractory (R/R) diffuse large B-cell lymphoma (DLBCL) who are ineligible for, or relapse after, autologous hematopoietic stem cell transplant (auto-HSCT).
Meanwhile, the European Medicines Agency (EMA) has granted accelerated assessment to the marketing authorization application (MAA) for tisagenlecleucel for the treatment of children and young adults with R/R B-cell acute lymphoblastic leukemia (ALL) and for adults with R/R DLBCL who are ineligible for auto-HSCT.
If the sBLA and MAA are approved, tisagenlecleucel will be the first CAR T-cell therapy available for 2 distinct indications in non-Hodgkin lymphoma and B-cell ALL.
Tisagenlecleucel became the first CAR T-cell therapy to receive regulatory approval when it was approved by the FDA in August 2017 for use in patients up to 25 years of age who have B-cell precursor ALL that is refractory or in second or later relapse.
Supporting data
The regulatory applications for tisagenlecleucel in the US and EU are supported by data from the Novartis-sponsored global clinical trial program in children and young adults with R/R B-cell ALL and adults with R/R DLBCL.
Results from the phase 2 JULIET trial served as the basis of the sBLA and MAA for tisagenlecleucel in adults with R/R DLCBL. Data from this trial were presented at the 2017 ASH Annual Meeting in December.
Results from the phase 2 ELIANA study were submitted as part of the MAA for tisagenlecleucel in children and young adults with R/R B-cell ALL. Data from this trial were presented at the 2017 EHA Congress last June.
About priority review, accelerated assessment
The FDA grants priority review to applications for products that may provide significant improvements in the treatment, diagnosis, or prevention of serious conditions.
The FDA’s goal is to take action on a priority review application within 6 months of receiving it, rather than the standard 10 months.
The EMA grants accelerated assessment when a product is expected to be of major public health interest, particularly from the point of view of therapeutic innovation.
Accelerated assessment shortens the review period from 210 days to 150 days.
HLF proves ‘critical’ for HSC quiescence
Preclinical research suggests hepatic leukemia factor (HLF) protects hematopoietic stem cells (HSCs) by helping them maintain quiescence.
Researchers found that HLF-deficient HSCs were more sensitive than wild-type HSCs to chemotherapy and irradiation.
After transplantation in mice, HLF-deficient HSCs were less able than wild-type HSCs to reconstitute hematopoiesis.
These findings were published in Cell Reports.
“The study confirms several previous studies that show the HLF gene’s significance in blood formation,” said study author Mattias Magnusson, PhD, of Lund University in Sweden.
“Identifying the factors that control blood stem cells provides knowledge needed to be able to propagate the stem cells outside the body. This has long been one of the major goals in the blood stem cell field, as it would increase possibilities for blood stem cell transplantation when, for example, there is a shortage of stem cells or donors. In addition, we will increase our understanding of how leukemia arises.”
Previous research by Dr Magnusson and his colleagues suggested that HLF may regulate HSCs in both normal and malignant hematopoiesis.
With the current study, the researchers found that HLF was “dispensable for steady-state hematopoiesis.” In fact, HLF-knockout mice had “essentially normal hematopoietic parameters” in steady-state conditions.
However, when HLF-deficient HSCs were serially transplanted in mice, the cells showed a reduction in regenerative potential, when compared to wild-type HSCs.
Additionally, mice with HLF-deficient HSCs exhibited increased sensitivity to the myeloablative agent 5-fluorouracil and reduced survival after sublethal irradiation, as compared to control mice.
“It’s surprising that the mice [initially] lived a normal life without the HLF gene, but when they had an acute need for new blood after an external damage such as cytostatic treatment, the mice did not survive,” Dr Magnusson said.
“All the blood stem cells were eliminated by the treatment, as they were active rather than in a resting state. Without the HLF gene, the blood stem cells were no longer protected against cytostatic treatment or other types of stress such as transplantation.”
Taking their findings together, Dr Magnusson and his colleagues concluded that HLF is a “critical” regulator of HSC quiescence and “essential” for maintaining HSCs’ ability to produce new blood.
Preclinical research suggests hepatic leukemia factor (HLF) protects hematopoietic stem cells (HSCs) by helping them maintain quiescence.
Researchers found that HLF-deficient HSCs were more sensitive than wild-type HSCs to chemotherapy and irradiation.
After transplantation in mice, HLF-deficient HSCs were less able than wild-type HSCs to reconstitute hematopoiesis.
These findings were published in Cell Reports.
“The study confirms several previous studies that show the HLF gene’s significance in blood formation,” said study author Mattias Magnusson, PhD, of Lund University in Sweden.
“Identifying the factors that control blood stem cells provides knowledge needed to be able to propagate the stem cells outside the body. This has long been one of the major goals in the blood stem cell field, as it would increase possibilities for blood stem cell transplantation when, for example, there is a shortage of stem cells or donors. In addition, we will increase our understanding of how leukemia arises.”
Previous research by Dr Magnusson and his colleagues suggested that HLF may regulate HSCs in both normal and malignant hematopoiesis.
With the current study, the researchers found that HLF was “dispensable for steady-state hematopoiesis.” In fact, HLF-knockout mice had “essentially normal hematopoietic parameters” in steady-state conditions.
However, when HLF-deficient HSCs were serially transplanted in mice, the cells showed a reduction in regenerative potential, when compared to wild-type HSCs.
Additionally, mice with HLF-deficient HSCs exhibited increased sensitivity to the myeloablative agent 5-fluorouracil and reduced survival after sublethal irradiation, as compared to control mice.
“It’s surprising that the mice [initially] lived a normal life without the HLF gene, but when they had an acute need for new blood after an external damage such as cytostatic treatment, the mice did not survive,” Dr Magnusson said.
“All the blood stem cells were eliminated by the treatment, as they were active rather than in a resting state. Without the HLF gene, the blood stem cells were no longer protected against cytostatic treatment or other types of stress such as transplantation.”
Taking their findings together, Dr Magnusson and his colleagues concluded that HLF is a “critical” regulator of HSC quiescence and “essential” for maintaining HSCs’ ability to produce new blood.
Preclinical research suggests hepatic leukemia factor (HLF) protects hematopoietic stem cells (HSCs) by helping them maintain quiescence.
Researchers found that HLF-deficient HSCs were more sensitive than wild-type HSCs to chemotherapy and irradiation.
After transplantation in mice, HLF-deficient HSCs were less able than wild-type HSCs to reconstitute hematopoiesis.
These findings were published in Cell Reports.
“The study confirms several previous studies that show the HLF gene’s significance in blood formation,” said study author Mattias Magnusson, PhD, of Lund University in Sweden.
“Identifying the factors that control blood stem cells provides knowledge needed to be able to propagate the stem cells outside the body. This has long been one of the major goals in the blood stem cell field, as it would increase possibilities for blood stem cell transplantation when, for example, there is a shortage of stem cells or donors. In addition, we will increase our understanding of how leukemia arises.”
Previous research by Dr Magnusson and his colleagues suggested that HLF may regulate HSCs in both normal and malignant hematopoiesis.
With the current study, the researchers found that HLF was “dispensable for steady-state hematopoiesis.” In fact, HLF-knockout mice had “essentially normal hematopoietic parameters” in steady-state conditions.
However, when HLF-deficient HSCs were serially transplanted in mice, the cells showed a reduction in regenerative potential, when compared to wild-type HSCs.
Additionally, mice with HLF-deficient HSCs exhibited increased sensitivity to the myeloablative agent 5-fluorouracil and reduced survival after sublethal irradiation, as compared to control mice.
“It’s surprising that the mice [initially] lived a normal life without the HLF gene, but when they had an acute need for new blood after an external damage such as cytostatic treatment, the mice did not survive,” Dr Magnusson said.
“All the blood stem cells were eliminated by the treatment, as they were active rather than in a resting state. Without the HLF gene, the blood stem cells were no longer protected against cytostatic treatment or other types of stress such as transplantation.”
Taking their findings together, Dr Magnusson and his colleagues concluded that HLF is a “critical” regulator of HSC quiescence and “essential” for maintaining HSCs’ ability to produce new blood.
EC approves product to treat hemophilia A
The European Commission (EC) has granted marketing authorization for rurioctocog alfa pegol (Adynovi).
Rurioctocog alfa pegol (formerly BAX 855) is a pegylated, full-length, recombinant factor VIII product built on a previously licensed recombinant factor VIII product (Advate).
Rurioctocog alfa pegol is now EC-approved for on-demand and prophylactic use in patients age 12 and older with hemophilia A.
With the EC’s approval, Shire is authorized to market rurioctocog alfa pegol in all member states of the European Union as well as in Iceland, Liechtenstein, and Norway.
The marketing authorization for rurioctocog alfa pegol is based on outcomes from three phase 3 trials—a study of adolescents and adults, a trial of patients undergoing surgical procedures, and a study of pediatric patients.
Trial of adolescents/adults
This study included 137 patients, ages 12 to 58, with previously treated hemophilia A. They were assigned to either twice-weekly prophylaxis (40-50 IU/kg, n=120) or on-demand treatment (10-60 IU/kg, n=17) with rurioctocog alfa pegol. One hundred and twenty-six patients completed the study.
Patients who received prophylaxis had a 90% reduction in annualized bleeding rate (ABR) compared to those who received on-demand treatment. The median ABR was 1.9 in the prophylactic arm and 41.5 in the on-demand arm.
There were 7 adverse events (occurring in 6 patients) that were considered possibly related to rurioctocog alfa pegol. These included diarrhea, nausea, headache, and flushing.
These results were published in Blood in July 2015.
Perioperative study
This study included 15 patients with severe hemophilia A who were undergoing surgical procedures (11 of them major and 4 minor).
The patients received rurioctocog alfa pegol at varying doses and schedules, depending on each patient’s pharmacokinetic profile for major procedures or rurioctocog alfa pegol incremental recovery for minor procedures.
Intra-operative and peri-operative hemostatic efficacy of rurioctocog alfa pegol was deemed “excellent” for all 15 patients. The “excellent” rating meant that blood loss was less than or equal to that expected for the type of procedure performed in a non-hemophilic population.
Post-operatively (day 1 after the procedure), hemostatic efficacy was rated “good” for 1 procedure and “excellent” for the rest. The “good” rating meant that post-operative blood loss was up to 50% more than expected for the type of procedure performed in a non-hemophilic population.
There were no treatment-related adverse events or signs of immunogenicity in this trial.
Results were published in Haemophilia in June 2016.
Pediatric trial
This study included 66 patients, ages 1 to 11, who had previously treated hemophilia A. They received twice-weekly prophylaxis with rurioctocog alfa pegol (50 ± 10 IU/kg) for at least 6 months or 50 exposure days, whichever occurred last.
The median ABR was 2.0, and 38% of patients did not have any bleeding episodes.
None of the patients developed inhibitory antibodies, and there were no treatment-related adverse events.
Results from this trial were presented at the World Federation of Hemophilia 2016 World Congress in July 2016 and published in Haemophilia in November 2016.
The European Commission (EC) has granted marketing authorization for rurioctocog alfa pegol (Adynovi).
Rurioctocog alfa pegol (formerly BAX 855) is a pegylated, full-length, recombinant factor VIII product built on a previously licensed recombinant factor VIII product (Advate).
Rurioctocog alfa pegol is now EC-approved for on-demand and prophylactic use in patients age 12 and older with hemophilia A.
With the EC’s approval, Shire is authorized to market rurioctocog alfa pegol in all member states of the European Union as well as in Iceland, Liechtenstein, and Norway.
The marketing authorization for rurioctocog alfa pegol is based on outcomes from three phase 3 trials—a study of adolescents and adults, a trial of patients undergoing surgical procedures, and a study of pediatric patients.
Trial of adolescents/adults
This study included 137 patients, ages 12 to 58, with previously treated hemophilia A. They were assigned to either twice-weekly prophylaxis (40-50 IU/kg, n=120) or on-demand treatment (10-60 IU/kg, n=17) with rurioctocog alfa pegol. One hundred and twenty-six patients completed the study.
Patients who received prophylaxis had a 90% reduction in annualized bleeding rate (ABR) compared to those who received on-demand treatment. The median ABR was 1.9 in the prophylactic arm and 41.5 in the on-demand arm.
There were 7 adverse events (occurring in 6 patients) that were considered possibly related to rurioctocog alfa pegol. These included diarrhea, nausea, headache, and flushing.
These results were published in Blood in July 2015.
Perioperative study
This study included 15 patients with severe hemophilia A who were undergoing surgical procedures (11 of them major and 4 minor).
The patients received rurioctocog alfa pegol at varying doses and schedules, depending on each patient’s pharmacokinetic profile for major procedures or rurioctocog alfa pegol incremental recovery for minor procedures.
Intra-operative and peri-operative hemostatic efficacy of rurioctocog alfa pegol was deemed “excellent” for all 15 patients. The “excellent” rating meant that blood loss was less than or equal to that expected for the type of procedure performed in a non-hemophilic population.
Post-operatively (day 1 after the procedure), hemostatic efficacy was rated “good” for 1 procedure and “excellent” for the rest. The “good” rating meant that post-operative blood loss was up to 50% more than expected for the type of procedure performed in a non-hemophilic population.
There were no treatment-related adverse events or signs of immunogenicity in this trial.
Results were published in Haemophilia in June 2016.
Pediatric trial
This study included 66 patients, ages 1 to 11, who had previously treated hemophilia A. They received twice-weekly prophylaxis with rurioctocog alfa pegol (50 ± 10 IU/kg) for at least 6 months or 50 exposure days, whichever occurred last.
The median ABR was 2.0, and 38% of patients did not have any bleeding episodes.
None of the patients developed inhibitory antibodies, and there were no treatment-related adverse events.
Results from this trial were presented at the World Federation of Hemophilia 2016 World Congress in July 2016 and published in Haemophilia in November 2016.
The European Commission (EC) has granted marketing authorization for rurioctocog alfa pegol (Adynovi).
Rurioctocog alfa pegol (formerly BAX 855) is a pegylated, full-length, recombinant factor VIII product built on a previously licensed recombinant factor VIII product (Advate).
Rurioctocog alfa pegol is now EC-approved for on-demand and prophylactic use in patients age 12 and older with hemophilia A.
With the EC’s approval, Shire is authorized to market rurioctocog alfa pegol in all member states of the European Union as well as in Iceland, Liechtenstein, and Norway.
The marketing authorization for rurioctocog alfa pegol is based on outcomes from three phase 3 trials—a study of adolescents and adults, a trial of patients undergoing surgical procedures, and a study of pediatric patients.
Trial of adolescents/adults
This study included 137 patients, ages 12 to 58, with previously treated hemophilia A. They were assigned to either twice-weekly prophylaxis (40-50 IU/kg, n=120) or on-demand treatment (10-60 IU/kg, n=17) with rurioctocog alfa pegol. One hundred and twenty-six patients completed the study.
Patients who received prophylaxis had a 90% reduction in annualized bleeding rate (ABR) compared to those who received on-demand treatment. The median ABR was 1.9 in the prophylactic arm and 41.5 in the on-demand arm.
There were 7 adverse events (occurring in 6 patients) that were considered possibly related to rurioctocog alfa pegol. These included diarrhea, nausea, headache, and flushing.
These results were published in Blood in July 2015.
Perioperative study
This study included 15 patients with severe hemophilia A who were undergoing surgical procedures (11 of them major and 4 minor).
The patients received rurioctocog alfa pegol at varying doses and schedules, depending on each patient’s pharmacokinetic profile for major procedures or rurioctocog alfa pegol incremental recovery for minor procedures.
Intra-operative and peri-operative hemostatic efficacy of rurioctocog alfa pegol was deemed “excellent” for all 15 patients. The “excellent” rating meant that blood loss was less than or equal to that expected for the type of procedure performed in a non-hemophilic population.
Post-operatively (day 1 after the procedure), hemostatic efficacy was rated “good” for 1 procedure and “excellent” for the rest. The “good” rating meant that post-operative blood loss was up to 50% more than expected for the type of procedure performed in a non-hemophilic population.
There were no treatment-related adverse events or signs of immunogenicity in this trial.
Results were published in Haemophilia in June 2016.
Pediatric trial
This study included 66 patients, ages 1 to 11, who had previously treated hemophilia A. They received twice-weekly prophylaxis with rurioctocog alfa pegol (50 ± 10 IU/kg) for at least 6 months or 50 exposure days, whichever occurred last.
The median ABR was 2.0, and 38% of patients did not have any bleeding episodes.
None of the patients developed inhibitory antibodies, and there were no treatment-related adverse events.
Results from this trial were presented at the World Federation of Hemophilia 2016 World Congress in July 2016 and published in Haemophilia in November 2016.