User login
Make a Move
As elderly patients suffering functional decline and immobility face prolonged hospital stays, placement in nursing homes, and increased risk of mortality, hospitalists must focus on one mission: Keep them moving.#1
With today’s emphasis on cost containment and quality assurance, keeping patients moving is one small step toward improving the quality of a patient’s hospitalization.
At the core of elders’ quality of life is independent mobility. When mobility is lost, a patient’s ability to socialize with peers and family, perform activities of daily living (ADLs), and participate in decisions regarding their well-being is severely compromised.
Most hospital admissions begin with the assignment of a patient to a bed. Although acute illness, medications, and a new environment all take their toll on patient functionality, simply putting a patient in recline has a significant impact.
If a young healthy person is ordered to rest for more than 72 hours, muscle mass and strength decrease, gait speed slows, and coordination becomes impaired.#2 If that patient is put to bed for more than 72 hours, organs other than the musculoskeletal system become compromised. Cardiovascular deconditioning with resting tachycardia and orthostatic hypotension, glucose intolerance, venous thromboembolism, hypercalcemia and osteoporosis, constipation and fecal impaction, pressure ulcers, and even depression can occur.3# Imagine then how elderly patients would fare.
Unfortunately, the independence of elderly patients is continually undermined by the same environment that offers treatment and care—the inpatient ward. And staff and patient family members are at fault.#4
Establish a Baseline
Teaching patients to move autonomously requires extra nursing time. Most on the nursing staff find it easier to provide a bedpan rather than assist a patient to the bathroom. When assisted ambulation is offered, patients may resist.
Families may hinder the resumption of mobility by performing tasks for patients instead of encouraging them to do them themselves. Further, changes in mobility are difficult to quantify and communicate due to limited mobility terminology in nursing practice and limited physician time. When a pre-admission functional status is not clearly documented, hospital staff often assumes that the patient’s compromised state is little changed from its baseline.
With so many barriers to patient mobility, obtaining an accurate assessment of a patient’s functional status two weeks prior to admission is key in establishing a plan for helping elderly patients regain mobility.
Ideally, one should speak to the patient as well as someone closely involved with the patient’s care who can verify or clarify the patient’s description of his or her prior activities. Significant information to obtain includes which ADLs the patient can independently perform, how far the patient can ambulate and with what assistive devices, and whether glasses, hearing aids, specially fitted shoes and orthotics, and knee braces are normally required for ambulation.#5
Though no screening tool has been validated as an absolute predictor of inpatient functional decline, lower functional status before admission, cognitive impairment, depression, advanced age, and prolonged length of hospital stay have been associated with loss of independence. Their presence may warrant a more aggressive regimen for regaining mobility.
Set the Stage
Before calling in a transfer to inpatient rehab, there are several steps one can take to maximize the return of function. By optimizing a patient’s functional capabilities during the admission, you enable them to integrate necessary skills into a daily routine—something they’re unlikely to learn at a rehabilitation center. Take these steps:6
- Control pain. Adequate analgesia is imperative for regaining mobility. Opiates and opiate agonists may be necessary for optimal control. Constipation should be expected and treated, and patients should be closely monitored for orthostasis, confusion, and urinary retention;
- Get the patient to a chair with assistive devices nearby. This includes canes, braces, walkers, orthopedic shoes, glasses, and hearing aids;
- Minimize IVs, catheters, and drains. Those that cannot be removed can be taped to minimize their interference with ambulation. Regular clothes, particularly jogging suits, promote ambulation and comfort;
- Coordinate with nursing department so the patient has periods of activity and rest. A walk down the corridor should be followed by a commensurate period of minimal activity, not a two-hour nap;
- Encourage sleep hygiene. Daytime activities can be maximized only when preceded by a restful night’s sleep. Limiting caffeinated beverages, restricting television time, and encouraging relaxing evening activities like reading may be necessary to ensure adequate sleep. Well-rested patients are better equipped to challenge themselves physically during the day and are less at risk for the side effects associated with sleeping medications;
- Give early referral to physical and occupational therapies. Even if the patient can barely tolerate sitting in a chair, a passive range of motion exercises for all joints should be undertaken daily. Additionally, active resistance exercises may be feasible for even debilitated patients if they receive daily assistance and continual encouragement. With persistence, skeletal muscles and the cardiovascular and pulmonary systems will show more endurance.#
Follow Progress
Accurately following a patient’s progress in regaining mobility requires the use of an assessment tool. The Elderly Mobility Scale (EMS) is useful for assessing improvements in mobility of elderly patients receiving physical therapy.
Balance, range of motion, and ambulation are scored initially, and the scores are updated during daily physical therapy. A review of this assessment tool was published in the Journal of Ageing this year, with the authors concluding that the EMS is a valid, reliable scale that can be readily applied during daily clinical work.7# Further, a review in Clinical Rehabilitation found the EMS to be a reliable test of motor function in elderly patients with a range of functional levels.#8 This assessment falls short in its lack of predictive validity in terms of falls or discharge destination.
Elderly patients suffer more hospital-associated falls than those younger than 65. According to a 2000 article from the British Medical Journal, patients older than 65 were seven times as likely to experience a preventable fall while in the hospital compared with younger age groups.#9
Patient factors that contribute to falls include age-related changes in postural control, impaired gait, decreased visual acuity, medications, the presence of acute and chronic diseases that affect sensory input, the central nervous system, and coordination. Osteoporosis is also an important factor—pathologic fractures often precede a fall. Environmental factors include poor lighting, obtrusive furniture, slippery floors, loose floor coverings, and bathrooms without handrails or grab bars.
The items most commonly included in fall risk-assessment tools include:10
- Comorbid patient characteristics or conditions associated with falling, such as cognitive impairment;
- History of a fall;
- Mobility impairment;
- Incontinence;
- Medications affecting balance/cognition and polypharmacy;
- Sensory deficits; and
- Advanced age.
The presence of more than three of these items identifies a patient at high risk for falling. But calculating a fall assessment includes not only identifying relevant risk factors, but also performing a focused physical exam. In ambulatory patients, the timed “get up and go” test is a useful predictor of falls. The patient is observed as she rises from a chair, walks 10 feet, then returns to the chair. If the patient requires more than 16 seconds to complete the task, he or she is at greater risk for a fall.
Early Intervention
While management depends on the underlying etiology of the fall, some generally acceptable practices are effective:
- Maintain a safe physical environment. Making sure spills are cleaned up quickly and walkways are kept free of obstruction is as important as maintaining adequate lighting in all areas where older adults will walk;
- Avoid use of restraints. Though restraints are often employed to prevent falls, they have not proved effective in medical trials. It has been demonstrated that their use increases the injury associated with falls, and several restraint-reduction projects have demonstrated that restraints can be removed without a significant increase in falls or injuries;
- Deal with medication side effects. The side effects of CNS altering drugs, and drugs affecting postural blood pressure, balance, and gait should be expected and addressed. Polypharmacy should be minimized;
- Watch patients closely. High risk patients should be positioned by the nursing station so that their visibility to the staff is maximized; and
- Promote mobility. There has been considerable research demonstrating the positive effect of exercise on reducing fall risk among community-residing older adults. While no study to date has addressed the impact of exercise in the hospital-based community, improved balance, mobility, and flexibility have been documented in nursing home residents receiving aggressive physical therapy. TH
Dr. Landis is a frequent contributor to The Hospitalist.
References
- Hoogerduijn JG, Schuurmans MJ, Duijnstee MS, et al. A systematic review of predictors and screening instruments to identify older hospitalized patients at risk for functional decline. J Clin Nurs. 2007 Jan;16(1):46-57.
- Callen BL, Mahoney JE, Wells TJ, et al. Admission and discharge mobility of frail hospitalized older adults. MedSurg Nursing 2004:13(3):156-163.
- Resnick NM, Marcantonio ER. How should clinical care of the aged differ? Lancet 2002; 350:1157-1167.
- Watters JM, McClaran JC, Man-Son-Hing M. The elderly surgical patient. ACS surgery: principles and practice. Medscape 2005.
- Miller KE, Zylstra RG, Standridge JB. The geriatric patient: a systematic approach to maintaining health. Am Fam Physician. 2000;61(4):1080-1098.
- Rydwik E, Frändin K, Akner G. Effects of physical training on physical performance in institutionalized elderly patients with multiple diagnoses. Age Ageing. 2004 Jan;33(1):13-23.
- Kuys SS , Brauer SG. Validation and reliability of the Modified Elderly Mobility Scale. Australas J Ageing 2006; 25(3):140-144.
- Spilg EG, Martin BJ, Mitchell SL, et al. A comparison of mobility assessments in a geriatric day hospital. Clin Rehabil. 2001 Jun;15(3):296-300.
- Thomas EJ, Brennan TA. Incidence and types of preventable adverse events in elderly patients: population based review of medical records. BMJ 2000;320(7237):741-744
- Gray-Miceli DL, Capezuti E. A nursing guide to the prevention and management of falls in geriatric patients in long-term care settings. Medscape; May 19, 2005.
As elderly patients suffering functional decline and immobility face prolonged hospital stays, placement in nursing homes, and increased risk of mortality, hospitalists must focus on one mission: Keep them moving.#1
With today’s emphasis on cost containment and quality assurance, keeping patients moving is one small step toward improving the quality of a patient’s hospitalization.
At the core of elders’ quality of life is independent mobility. When mobility is lost, a patient’s ability to socialize with peers and family, perform activities of daily living (ADLs), and participate in decisions regarding their well-being is severely compromised.
Most hospital admissions begin with the assignment of a patient to a bed. Although acute illness, medications, and a new environment all take their toll on patient functionality, simply putting a patient in recline has a significant impact.
If a young healthy person is ordered to rest for more than 72 hours, muscle mass and strength decrease, gait speed slows, and coordination becomes impaired.#2 If that patient is put to bed for more than 72 hours, organs other than the musculoskeletal system become compromised. Cardiovascular deconditioning with resting tachycardia and orthostatic hypotension, glucose intolerance, venous thromboembolism, hypercalcemia and osteoporosis, constipation and fecal impaction, pressure ulcers, and even depression can occur.3# Imagine then how elderly patients would fare.
Unfortunately, the independence of elderly patients is continually undermined by the same environment that offers treatment and care—the inpatient ward. And staff and patient family members are at fault.#4
Establish a Baseline
Teaching patients to move autonomously requires extra nursing time. Most on the nursing staff find it easier to provide a bedpan rather than assist a patient to the bathroom. When assisted ambulation is offered, patients may resist.
Families may hinder the resumption of mobility by performing tasks for patients instead of encouraging them to do them themselves. Further, changes in mobility are difficult to quantify and communicate due to limited mobility terminology in nursing practice and limited physician time. When a pre-admission functional status is not clearly documented, hospital staff often assumes that the patient’s compromised state is little changed from its baseline.
With so many barriers to patient mobility, obtaining an accurate assessment of a patient’s functional status two weeks prior to admission is key in establishing a plan for helping elderly patients regain mobility.
Ideally, one should speak to the patient as well as someone closely involved with the patient’s care who can verify or clarify the patient’s description of his or her prior activities. Significant information to obtain includes which ADLs the patient can independently perform, how far the patient can ambulate and with what assistive devices, and whether glasses, hearing aids, specially fitted shoes and orthotics, and knee braces are normally required for ambulation.#5
Though no screening tool has been validated as an absolute predictor of inpatient functional decline, lower functional status before admission, cognitive impairment, depression, advanced age, and prolonged length of hospital stay have been associated with loss of independence. Their presence may warrant a more aggressive regimen for regaining mobility.
Set the Stage
Before calling in a transfer to inpatient rehab, there are several steps one can take to maximize the return of function. By optimizing a patient’s functional capabilities during the admission, you enable them to integrate necessary skills into a daily routine—something they’re unlikely to learn at a rehabilitation center. Take these steps:6
- Control pain. Adequate analgesia is imperative for regaining mobility. Opiates and opiate agonists may be necessary for optimal control. Constipation should be expected and treated, and patients should be closely monitored for orthostasis, confusion, and urinary retention;
- Get the patient to a chair with assistive devices nearby. This includes canes, braces, walkers, orthopedic shoes, glasses, and hearing aids;
- Minimize IVs, catheters, and drains. Those that cannot be removed can be taped to minimize their interference with ambulation. Regular clothes, particularly jogging suits, promote ambulation and comfort;
- Coordinate with nursing department so the patient has periods of activity and rest. A walk down the corridor should be followed by a commensurate period of minimal activity, not a two-hour nap;
- Encourage sleep hygiene. Daytime activities can be maximized only when preceded by a restful night’s sleep. Limiting caffeinated beverages, restricting television time, and encouraging relaxing evening activities like reading may be necessary to ensure adequate sleep. Well-rested patients are better equipped to challenge themselves physically during the day and are less at risk for the side effects associated with sleeping medications;
- Give early referral to physical and occupational therapies. Even if the patient can barely tolerate sitting in a chair, a passive range of motion exercises for all joints should be undertaken daily. Additionally, active resistance exercises may be feasible for even debilitated patients if they receive daily assistance and continual encouragement. With persistence, skeletal muscles and the cardiovascular and pulmonary systems will show more endurance.#
Follow Progress
Accurately following a patient’s progress in regaining mobility requires the use of an assessment tool. The Elderly Mobility Scale (EMS) is useful for assessing improvements in mobility of elderly patients receiving physical therapy.
Balance, range of motion, and ambulation are scored initially, and the scores are updated during daily physical therapy. A review of this assessment tool was published in the Journal of Ageing this year, with the authors concluding that the EMS is a valid, reliable scale that can be readily applied during daily clinical work.7# Further, a review in Clinical Rehabilitation found the EMS to be a reliable test of motor function in elderly patients with a range of functional levels.#8 This assessment falls short in its lack of predictive validity in terms of falls or discharge destination.
Elderly patients suffer more hospital-associated falls than those younger than 65. According to a 2000 article from the British Medical Journal, patients older than 65 were seven times as likely to experience a preventable fall while in the hospital compared with younger age groups.#9
Patient factors that contribute to falls include age-related changes in postural control, impaired gait, decreased visual acuity, medications, the presence of acute and chronic diseases that affect sensory input, the central nervous system, and coordination. Osteoporosis is also an important factor—pathologic fractures often precede a fall. Environmental factors include poor lighting, obtrusive furniture, slippery floors, loose floor coverings, and bathrooms without handrails or grab bars.
The items most commonly included in fall risk-assessment tools include:10
- Comorbid patient characteristics or conditions associated with falling, such as cognitive impairment;
- History of a fall;
- Mobility impairment;
- Incontinence;
- Medications affecting balance/cognition and polypharmacy;
- Sensory deficits; and
- Advanced age.
The presence of more than three of these items identifies a patient at high risk for falling. But calculating a fall assessment includes not only identifying relevant risk factors, but also performing a focused physical exam. In ambulatory patients, the timed “get up and go” test is a useful predictor of falls. The patient is observed as she rises from a chair, walks 10 feet, then returns to the chair. If the patient requires more than 16 seconds to complete the task, he or she is at greater risk for a fall.
Early Intervention
While management depends on the underlying etiology of the fall, some generally acceptable practices are effective:
- Maintain a safe physical environment. Making sure spills are cleaned up quickly and walkways are kept free of obstruction is as important as maintaining adequate lighting in all areas where older adults will walk;
- Avoid use of restraints. Though restraints are often employed to prevent falls, they have not proved effective in medical trials. It has been demonstrated that their use increases the injury associated with falls, and several restraint-reduction projects have demonstrated that restraints can be removed without a significant increase in falls or injuries;
- Deal with medication side effects. The side effects of CNS altering drugs, and drugs affecting postural blood pressure, balance, and gait should be expected and addressed. Polypharmacy should be minimized;
- Watch patients closely. High risk patients should be positioned by the nursing station so that their visibility to the staff is maximized; and
- Promote mobility. There has been considerable research demonstrating the positive effect of exercise on reducing fall risk among community-residing older adults. While no study to date has addressed the impact of exercise in the hospital-based community, improved balance, mobility, and flexibility have been documented in nursing home residents receiving aggressive physical therapy. TH
Dr. Landis is a frequent contributor to The Hospitalist.
References
- Hoogerduijn JG, Schuurmans MJ, Duijnstee MS, et al. A systematic review of predictors and screening instruments to identify older hospitalized patients at risk for functional decline. J Clin Nurs. 2007 Jan;16(1):46-57.
- Callen BL, Mahoney JE, Wells TJ, et al. Admission and discharge mobility of frail hospitalized older adults. MedSurg Nursing 2004:13(3):156-163.
- Resnick NM, Marcantonio ER. How should clinical care of the aged differ? Lancet 2002; 350:1157-1167.
- Watters JM, McClaran JC, Man-Son-Hing M. The elderly surgical patient. ACS surgery: principles and practice. Medscape 2005.
- Miller KE, Zylstra RG, Standridge JB. The geriatric patient: a systematic approach to maintaining health. Am Fam Physician. 2000;61(4):1080-1098.
- Rydwik E, Frändin K, Akner G. Effects of physical training on physical performance in institutionalized elderly patients with multiple diagnoses. Age Ageing. 2004 Jan;33(1):13-23.
- Kuys SS , Brauer SG. Validation and reliability of the Modified Elderly Mobility Scale. Australas J Ageing 2006; 25(3):140-144.
- Spilg EG, Martin BJ, Mitchell SL, et al. A comparison of mobility assessments in a geriatric day hospital. Clin Rehabil. 2001 Jun;15(3):296-300.
- Thomas EJ, Brennan TA. Incidence and types of preventable adverse events in elderly patients: population based review of medical records. BMJ 2000;320(7237):741-744
- Gray-Miceli DL, Capezuti E. A nursing guide to the prevention and management of falls in geriatric patients in long-term care settings. Medscape; May 19, 2005.
As elderly patients suffering functional decline and immobility face prolonged hospital stays, placement in nursing homes, and increased risk of mortality, hospitalists must focus on one mission: Keep them moving.#1
With today’s emphasis on cost containment and quality assurance, keeping patients moving is one small step toward improving the quality of a patient’s hospitalization.
At the core of elders’ quality of life is independent mobility. When mobility is lost, a patient’s ability to socialize with peers and family, perform activities of daily living (ADLs), and participate in decisions regarding their well-being is severely compromised.
Most hospital admissions begin with the assignment of a patient to a bed. Although acute illness, medications, and a new environment all take their toll on patient functionality, simply putting a patient in recline has a significant impact.
If a young healthy person is ordered to rest for more than 72 hours, muscle mass and strength decrease, gait speed slows, and coordination becomes impaired.#2 If that patient is put to bed for more than 72 hours, organs other than the musculoskeletal system become compromised. Cardiovascular deconditioning with resting tachycardia and orthostatic hypotension, glucose intolerance, venous thromboembolism, hypercalcemia and osteoporosis, constipation and fecal impaction, pressure ulcers, and even depression can occur.3# Imagine then how elderly patients would fare.
Unfortunately, the independence of elderly patients is continually undermined by the same environment that offers treatment and care—the inpatient ward. And staff and patient family members are at fault.#4
Establish a Baseline
Teaching patients to move autonomously requires extra nursing time. Most on the nursing staff find it easier to provide a bedpan rather than assist a patient to the bathroom. When assisted ambulation is offered, patients may resist.
Families may hinder the resumption of mobility by performing tasks for patients instead of encouraging them to do them themselves. Further, changes in mobility are difficult to quantify and communicate due to limited mobility terminology in nursing practice and limited physician time. When a pre-admission functional status is not clearly documented, hospital staff often assumes that the patient’s compromised state is little changed from its baseline.
With so many barriers to patient mobility, obtaining an accurate assessment of a patient’s functional status two weeks prior to admission is key in establishing a plan for helping elderly patients regain mobility.
Ideally, one should speak to the patient as well as someone closely involved with the patient’s care who can verify or clarify the patient’s description of his or her prior activities. Significant information to obtain includes which ADLs the patient can independently perform, how far the patient can ambulate and with what assistive devices, and whether glasses, hearing aids, specially fitted shoes and orthotics, and knee braces are normally required for ambulation.#5
Though no screening tool has been validated as an absolute predictor of inpatient functional decline, lower functional status before admission, cognitive impairment, depression, advanced age, and prolonged length of hospital stay have been associated with loss of independence. Their presence may warrant a more aggressive regimen for regaining mobility.
Set the Stage
Before calling in a transfer to inpatient rehab, there are several steps one can take to maximize the return of function. By optimizing a patient’s functional capabilities during the admission, you enable them to integrate necessary skills into a daily routine—something they’re unlikely to learn at a rehabilitation center. Take these steps:6
- Control pain. Adequate analgesia is imperative for regaining mobility. Opiates and opiate agonists may be necessary for optimal control. Constipation should be expected and treated, and patients should be closely monitored for orthostasis, confusion, and urinary retention;
- Get the patient to a chair with assistive devices nearby. This includes canes, braces, walkers, orthopedic shoes, glasses, and hearing aids;
- Minimize IVs, catheters, and drains. Those that cannot be removed can be taped to minimize their interference with ambulation. Regular clothes, particularly jogging suits, promote ambulation and comfort;
- Coordinate with nursing department so the patient has periods of activity and rest. A walk down the corridor should be followed by a commensurate period of minimal activity, not a two-hour nap;
- Encourage sleep hygiene. Daytime activities can be maximized only when preceded by a restful night’s sleep. Limiting caffeinated beverages, restricting television time, and encouraging relaxing evening activities like reading may be necessary to ensure adequate sleep. Well-rested patients are better equipped to challenge themselves physically during the day and are less at risk for the side effects associated with sleeping medications;
- Give early referral to physical and occupational therapies. Even if the patient can barely tolerate sitting in a chair, a passive range of motion exercises for all joints should be undertaken daily. Additionally, active resistance exercises may be feasible for even debilitated patients if they receive daily assistance and continual encouragement. With persistence, skeletal muscles and the cardiovascular and pulmonary systems will show more endurance.#
Follow Progress
Accurately following a patient’s progress in regaining mobility requires the use of an assessment tool. The Elderly Mobility Scale (EMS) is useful for assessing improvements in mobility of elderly patients receiving physical therapy.
Balance, range of motion, and ambulation are scored initially, and the scores are updated during daily physical therapy. A review of this assessment tool was published in the Journal of Ageing this year, with the authors concluding that the EMS is a valid, reliable scale that can be readily applied during daily clinical work.7# Further, a review in Clinical Rehabilitation found the EMS to be a reliable test of motor function in elderly patients with a range of functional levels.#8 This assessment falls short in its lack of predictive validity in terms of falls or discharge destination.
Elderly patients suffer more hospital-associated falls than those younger than 65. According to a 2000 article from the British Medical Journal, patients older than 65 were seven times as likely to experience a preventable fall while in the hospital compared with younger age groups.#9
Patient factors that contribute to falls include age-related changes in postural control, impaired gait, decreased visual acuity, medications, the presence of acute and chronic diseases that affect sensory input, the central nervous system, and coordination. Osteoporosis is also an important factor—pathologic fractures often precede a fall. Environmental factors include poor lighting, obtrusive furniture, slippery floors, loose floor coverings, and bathrooms without handrails or grab bars.
The items most commonly included in fall risk-assessment tools include:10
- Comorbid patient characteristics or conditions associated with falling, such as cognitive impairment;
- History of a fall;
- Mobility impairment;
- Incontinence;
- Medications affecting balance/cognition and polypharmacy;
- Sensory deficits; and
- Advanced age.
The presence of more than three of these items identifies a patient at high risk for falling. But calculating a fall assessment includes not only identifying relevant risk factors, but also performing a focused physical exam. In ambulatory patients, the timed “get up and go” test is a useful predictor of falls. The patient is observed as she rises from a chair, walks 10 feet, then returns to the chair. If the patient requires more than 16 seconds to complete the task, he or she is at greater risk for a fall.
Early Intervention
While management depends on the underlying etiology of the fall, some generally acceptable practices are effective:
- Maintain a safe physical environment. Making sure spills are cleaned up quickly and walkways are kept free of obstruction is as important as maintaining adequate lighting in all areas where older adults will walk;
- Avoid use of restraints. Though restraints are often employed to prevent falls, they have not proved effective in medical trials. It has been demonstrated that their use increases the injury associated with falls, and several restraint-reduction projects have demonstrated that restraints can be removed without a significant increase in falls or injuries;
- Deal with medication side effects. The side effects of CNS altering drugs, and drugs affecting postural blood pressure, balance, and gait should be expected and addressed. Polypharmacy should be minimized;
- Watch patients closely. High risk patients should be positioned by the nursing station so that their visibility to the staff is maximized; and
- Promote mobility. There has been considerable research demonstrating the positive effect of exercise on reducing fall risk among community-residing older adults. While no study to date has addressed the impact of exercise in the hospital-based community, improved balance, mobility, and flexibility have been documented in nursing home residents receiving aggressive physical therapy. TH
Dr. Landis is a frequent contributor to The Hospitalist.
References
- Hoogerduijn JG, Schuurmans MJ, Duijnstee MS, et al. A systematic review of predictors and screening instruments to identify older hospitalized patients at risk for functional decline. J Clin Nurs. 2007 Jan;16(1):46-57.
- Callen BL, Mahoney JE, Wells TJ, et al. Admission and discharge mobility of frail hospitalized older adults. MedSurg Nursing 2004:13(3):156-163.
- Resnick NM, Marcantonio ER. How should clinical care of the aged differ? Lancet 2002; 350:1157-1167.
- Watters JM, McClaran JC, Man-Son-Hing M. The elderly surgical patient. ACS surgery: principles and practice. Medscape 2005.
- Miller KE, Zylstra RG, Standridge JB. The geriatric patient: a systematic approach to maintaining health. Am Fam Physician. 2000;61(4):1080-1098.
- Rydwik E, Frändin K, Akner G. Effects of physical training on physical performance in institutionalized elderly patients with multiple diagnoses. Age Ageing. 2004 Jan;33(1):13-23.
- Kuys SS , Brauer SG. Validation and reliability of the Modified Elderly Mobility Scale. Australas J Ageing 2006; 25(3):140-144.
- Spilg EG, Martin BJ, Mitchell SL, et al. A comparison of mobility assessments in a geriatric day hospital. Clin Rehabil. 2001 Jun;15(3):296-300.
- Thomas EJ, Brennan TA. Incidence and types of preventable adverse events in elderly patients: population based review of medical records. BMJ 2000;320(7237):741-744
- Gray-Miceli DL, Capezuti E. A nursing guide to the prevention and management of falls in geriatric patients in long-term care settings. Medscape; May 19, 2005.
Nutrition Mission
Despite a general understanding among hospitalists that malnutrition has severe negative effects on hospitalized patients, preventive or corrective measures often aren’t taken.
The ill effects of nutritional deficiency are particularly profound in elderly inpatients. Estimates of protein-energy malnutrition vary between 20%-78% of elderly medical patients, who are uniquely disposed to the cognitive, metabolic, and immune-mediating consequences of malnutrition.1
Most hospitalists know when to request a nutritionist consultation or order extra mealtime cans of Ensure. But many do not realize these efforts often do little to alter patients’ descent into nutritional deficiency.
Define the Problem
Four patterns of problematic eating have been described in elderly inpatients.
The first and most common is the patient who is permitted nothing by mouth and is not provided an alternate route of nutrition. Data show 44% of elderly malnourished inpatients fall into this category.2
Other abnormal feeding subgroups include patients who need to be fed but have no other eating problem, patients who refuse food but can swallow with difficulty, and those who aspirate liquid or solid food. In a study of 73 institutionalized patients with Alzheimer’s dementia, the latter subgroup accounted for 34% of the patients assessed.
Poor diet is the main source of protein-energy deficiency in elderly inpatients. Occult malabsorption secondary to bacterial overgrowth in the small intestine may also be an important factor, as is the increased catabolic state associated with acute illness.
Though the most at-risk patients have severe mental and physical incapacities, other problems including respiratory disease, gastrointestinal disease, and stroke are associated with a malnourished state.3
Though hospitalists generally acknowledge the potential seriousness of a patient developing nutritional deficits, the attending healthcare team may be slow to diagnose or manage this problem because:
- Elderly patients can be malnourished on admission, but classic signs of protein-energy deficiency are mistaken for normal signs of aging;
- Nutritional problems are observed by the medical staff, but aggressive treatment is deferred in light of seemingly more pressing medical issues;
- Many physicians take action to prevent nutritional deficiencies, but these interventions are often insufficient or ineffective in preventing the spiral into malnutrition; and
- Physicians may assume a nutritionist is working to prevent and treat nutritional deficiencies, while the nutritionist is waiting for the medical staff to address the problem with a feeding tube.
Clinical Outcomes
Most physicians have observed the declining physical and cognitive capabilities of a nutritionally deprived elderly inpatient.
Although a causal relationship between malnutrition and adverse events has not been established, this is most likely because an older person’s clinical course affects and is affected by his nutritional status. Further, frequently compromised homeostatic mechanisms make the risk of complications related to malnutrition potentially more severe.
Though researchers are studying how inadequate nutritional intake contributes to the risk of adverse outcomes in elderly inpatients, numerous studies have identified strong correlations between the severity of the nutritional deficit and the risk of subsequent morbid events.
Sullivan, et al., found in their 1999 study of protein-energy undernutrition among elderly hospitalized patients that those maintained on nutrient intakes far less than their estimated energy requirements were at more risk of in-hospital mortality.
Other studies have shown that the risk of in-hospital starvation correlates strongly with polypharmacy and long stay. The severity of the nutritional deficiency correlates not only with weight loss and secretory protein loss, but also the risk of in-hospital and long-term complications.
Who Needs Help
Basic nutritional requirements vary much less than might be expected among younger and older patients. However, while a malnourished 20-year-old can be easily identified, the classic signs of malnutrition (wasting, brittle hair, dry skin, fissured mucus membranes) are less easily detected in elderly patients. They are often mistaken for signs of normal aging. Questions that can elicit evidence of a protein-energy deficiency include:
- Has food intake decreased recently?
- Are there physical difficulties with eating?
- Is the patient confused or depressed?
- Has there been diarrhea or vomiting?
- Has the patient been able to shop and manage food preparation?
- Has the patient or family noticed weight loss?
- What does the patient regard as a normal weight?
However, because the clinical signs of malnutrition-weight loss, muscle wasting, and fatigue can be difficult to detect from history alone, use of an assessment tool is often necessary.
There is no simple diagnostic test for undernutrition. Measurements of albumin, prealbumin, body-mass index (BMI), and weight loss have been used, albeit problematically, for this purpose. More useful for the quantification of nutritional status in elderly outpatients, these measurements are difficult to use in the hospital because albumin levels are frequently affected by disease processes involving the liver, kidney, and immune system, and correct baseline weights and heights are notoriously difficult to obtain from elderly patients.4
The Nutritional Risk Index was developed in response to these difficulties, but it was originally calibrated for young, post-surgical patients. Because of the problematic nature of obtaining the accurate heights and weights needed for the NRI in elderly patients, Bouillanne, et al,. developed the Geriatric Nutritional Risk Index (GNRI) in 2005 based on albumin levels, hospital recorded weights, and weight loss (see Table 1, above).
The GNRI’s creators classified patients according to their level of malnutrition and calculated their risk of related comorbidities. They found that 44% of their study population had major or moderate nutrition-related risk and recommended nutritional supplementation.
Path to Recovery
Refeeding regimens for elderly patients diagnosed as undernourished are best started with meals of personally chosen foods, timed for when patients are hungry.
Many elderly patients do not eat on a strict schedule at home and have difficulty complying with such a schedule. If oral intake remains inadequate, offer nutritional supplements. Because low-energy diets are usually low in vitamins and minerals, supplementation with a multivitamin, thiamine, folic acid, and zinc can be beneficial.
A common approach to problems with nutritional intake in elderly patients, particularly those with altered cognition or fluctuating consciousness, is insertion of a feeding tube.5 According to Medicare data from the 1990s, in individuals older than 85, one in 131 whites, and one in 58 African-Americans had a gastrostomy.
Despite the large numbers of feeding-tube placements in elderly patients, proof of their effectiveness is scant. No randomized trials have been performed to determine whether this practice improves survival, and observational data reveal that the one-year survival for these patients is less than 40%.
Feeding tubes are often placed to reverse the clinical sequelae of malnutrition—to heal pressure ulcers, prevent infection, and improve the patient’s functional status. But prospective observational studies do not support this methodology, and some contradictory evidence in the form of worsening pressure ulcers has been observed.
Aspiration pneumonia is the most serious infection for which tube feeding is considered a preventive measure. The condition results from the misdirection of pharyngeal contents and is believed to develop when nonpathologic oral secretions are deposited in dependent areas of the lung. The bacterial inoculum is high enough to overcome local defenses, which results in an infectious, febrile illness, usually involving mixed gram-negative rods and anaerobes.
Summaries of current data show that tube feeding may increase the risk of aspiration pneumonia. Further support for this hypothesis comes from the observation that jejunostomy feeding also does not appear to reduce the risk of aspiration pneumonia. If a patient is unable to protect his airway during mealtimes, he will also be at risk of aspiration and pneumonia between meals, regardless of how nutrition is delivered. TH
Dr. Landis is a rheumatologist and freelance writer.
References
- Sullivan DH, Sun S, Walls RC. Protein-energy undernutrition among elderly hospitalized patients. JAMA. 1999;281(21):2013-2019.
- Incalzi RA, Gemma A, Capparella O, et al. Energy intake and in-hospital starvation: a clinically relevant relationship. Arch Intern Med. 1996;156(4):425-429.
- Tierney A. Undernutrition and elderly patients. J Adv Nurs. 1995;23(2):228-236.
- Bouillanne O, Morineau G, Dupont C, et al. Geriatric nutritional risk index: a new index for evaluating at risk elderly medical patients. Am J Clin Nutr. 2005;82(4):777-783.
- Finucane T, Williams M. Tube feeding the demented patient: a review of the evidence. American Geriatrics Society Annual Scientific Meeting 2001.
Despite a general understanding among hospitalists that malnutrition has severe negative effects on hospitalized patients, preventive or corrective measures often aren’t taken.
The ill effects of nutritional deficiency are particularly profound in elderly inpatients. Estimates of protein-energy malnutrition vary between 20%-78% of elderly medical patients, who are uniquely disposed to the cognitive, metabolic, and immune-mediating consequences of malnutrition.1
Most hospitalists know when to request a nutritionist consultation or order extra mealtime cans of Ensure. But many do not realize these efforts often do little to alter patients’ descent into nutritional deficiency.
Define the Problem
Four patterns of problematic eating have been described in elderly inpatients.
The first and most common is the patient who is permitted nothing by mouth and is not provided an alternate route of nutrition. Data show 44% of elderly malnourished inpatients fall into this category.2
Other abnormal feeding subgroups include patients who need to be fed but have no other eating problem, patients who refuse food but can swallow with difficulty, and those who aspirate liquid or solid food. In a study of 73 institutionalized patients with Alzheimer’s dementia, the latter subgroup accounted for 34% of the patients assessed.
Poor diet is the main source of protein-energy deficiency in elderly inpatients. Occult malabsorption secondary to bacterial overgrowth in the small intestine may also be an important factor, as is the increased catabolic state associated with acute illness.
Though the most at-risk patients have severe mental and physical incapacities, other problems including respiratory disease, gastrointestinal disease, and stroke are associated with a malnourished state.3
Though hospitalists generally acknowledge the potential seriousness of a patient developing nutritional deficits, the attending healthcare team may be slow to diagnose or manage this problem because:
- Elderly patients can be malnourished on admission, but classic signs of protein-energy deficiency are mistaken for normal signs of aging;
- Nutritional problems are observed by the medical staff, but aggressive treatment is deferred in light of seemingly more pressing medical issues;
- Many physicians take action to prevent nutritional deficiencies, but these interventions are often insufficient or ineffective in preventing the spiral into malnutrition; and
- Physicians may assume a nutritionist is working to prevent and treat nutritional deficiencies, while the nutritionist is waiting for the medical staff to address the problem with a feeding tube.
Clinical Outcomes
Most physicians have observed the declining physical and cognitive capabilities of a nutritionally deprived elderly inpatient.
Although a causal relationship between malnutrition and adverse events has not been established, this is most likely because an older person’s clinical course affects and is affected by his nutritional status. Further, frequently compromised homeostatic mechanisms make the risk of complications related to malnutrition potentially more severe.
Though researchers are studying how inadequate nutritional intake contributes to the risk of adverse outcomes in elderly inpatients, numerous studies have identified strong correlations between the severity of the nutritional deficit and the risk of subsequent morbid events.
Sullivan, et al., found in their 1999 study of protein-energy undernutrition among elderly hospitalized patients that those maintained on nutrient intakes far less than their estimated energy requirements were at more risk of in-hospital mortality.
Other studies have shown that the risk of in-hospital starvation correlates strongly with polypharmacy and long stay. The severity of the nutritional deficiency correlates not only with weight loss and secretory protein loss, but also the risk of in-hospital and long-term complications.
Who Needs Help
Basic nutritional requirements vary much less than might be expected among younger and older patients. However, while a malnourished 20-year-old can be easily identified, the classic signs of malnutrition (wasting, brittle hair, dry skin, fissured mucus membranes) are less easily detected in elderly patients. They are often mistaken for signs of normal aging. Questions that can elicit evidence of a protein-energy deficiency include:
- Has food intake decreased recently?
- Are there physical difficulties with eating?
- Is the patient confused or depressed?
- Has there been diarrhea or vomiting?
- Has the patient been able to shop and manage food preparation?
- Has the patient or family noticed weight loss?
- What does the patient regard as a normal weight?
However, because the clinical signs of malnutrition-weight loss, muscle wasting, and fatigue can be difficult to detect from history alone, use of an assessment tool is often necessary.
There is no simple diagnostic test for undernutrition. Measurements of albumin, prealbumin, body-mass index (BMI), and weight loss have been used, albeit problematically, for this purpose. More useful for the quantification of nutritional status in elderly outpatients, these measurements are difficult to use in the hospital because albumin levels are frequently affected by disease processes involving the liver, kidney, and immune system, and correct baseline weights and heights are notoriously difficult to obtain from elderly patients.4
The Nutritional Risk Index was developed in response to these difficulties, but it was originally calibrated for young, post-surgical patients. Because of the problematic nature of obtaining the accurate heights and weights needed for the NRI in elderly patients, Bouillanne, et al,. developed the Geriatric Nutritional Risk Index (GNRI) in 2005 based on albumin levels, hospital recorded weights, and weight loss (see Table 1, above).
The GNRI’s creators classified patients according to their level of malnutrition and calculated their risk of related comorbidities. They found that 44% of their study population had major or moderate nutrition-related risk and recommended nutritional supplementation.
Path to Recovery
Refeeding regimens for elderly patients diagnosed as undernourished are best started with meals of personally chosen foods, timed for when patients are hungry.
Many elderly patients do not eat on a strict schedule at home and have difficulty complying with such a schedule. If oral intake remains inadequate, offer nutritional supplements. Because low-energy diets are usually low in vitamins and minerals, supplementation with a multivitamin, thiamine, folic acid, and zinc can be beneficial.
A common approach to problems with nutritional intake in elderly patients, particularly those with altered cognition or fluctuating consciousness, is insertion of a feeding tube.5 According to Medicare data from the 1990s, in individuals older than 85, one in 131 whites, and one in 58 African-Americans had a gastrostomy.
Despite the large numbers of feeding-tube placements in elderly patients, proof of their effectiveness is scant. No randomized trials have been performed to determine whether this practice improves survival, and observational data reveal that the one-year survival for these patients is less than 40%.
Feeding tubes are often placed to reverse the clinical sequelae of malnutrition—to heal pressure ulcers, prevent infection, and improve the patient’s functional status. But prospective observational studies do not support this methodology, and some contradictory evidence in the form of worsening pressure ulcers has been observed.
Aspiration pneumonia is the most serious infection for which tube feeding is considered a preventive measure. The condition results from the misdirection of pharyngeal contents and is believed to develop when nonpathologic oral secretions are deposited in dependent areas of the lung. The bacterial inoculum is high enough to overcome local defenses, which results in an infectious, febrile illness, usually involving mixed gram-negative rods and anaerobes.
Summaries of current data show that tube feeding may increase the risk of aspiration pneumonia. Further support for this hypothesis comes from the observation that jejunostomy feeding also does not appear to reduce the risk of aspiration pneumonia. If a patient is unable to protect his airway during mealtimes, he will also be at risk of aspiration and pneumonia between meals, regardless of how nutrition is delivered. TH
Dr. Landis is a rheumatologist and freelance writer.
References
- Sullivan DH, Sun S, Walls RC. Protein-energy undernutrition among elderly hospitalized patients. JAMA. 1999;281(21):2013-2019.
- Incalzi RA, Gemma A, Capparella O, et al. Energy intake and in-hospital starvation: a clinically relevant relationship. Arch Intern Med. 1996;156(4):425-429.
- Tierney A. Undernutrition and elderly patients. J Adv Nurs. 1995;23(2):228-236.
- Bouillanne O, Morineau G, Dupont C, et al. Geriatric nutritional risk index: a new index for evaluating at risk elderly medical patients. Am J Clin Nutr. 2005;82(4):777-783.
- Finucane T, Williams M. Tube feeding the demented patient: a review of the evidence. American Geriatrics Society Annual Scientific Meeting 2001.
Despite a general understanding among hospitalists that malnutrition has severe negative effects on hospitalized patients, preventive or corrective measures often aren’t taken.
The ill effects of nutritional deficiency are particularly profound in elderly inpatients. Estimates of protein-energy malnutrition vary between 20%-78% of elderly medical patients, who are uniquely disposed to the cognitive, metabolic, and immune-mediating consequences of malnutrition.1
Most hospitalists know when to request a nutritionist consultation or order extra mealtime cans of Ensure. But many do not realize these efforts often do little to alter patients’ descent into nutritional deficiency.
Define the Problem
Four patterns of problematic eating have been described in elderly inpatients.
The first and most common is the patient who is permitted nothing by mouth and is not provided an alternate route of nutrition. Data show 44% of elderly malnourished inpatients fall into this category.2
Other abnormal feeding subgroups include patients who need to be fed but have no other eating problem, patients who refuse food but can swallow with difficulty, and those who aspirate liquid or solid food. In a study of 73 institutionalized patients with Alzheimer’s dementia, the latter subgroup accounted for 34% of the patients assessed.
Poor diet is the main source of protein-energy deficiency in elderly inpatients. Occult malabsorption secondary to bacterial overgrowth in the small intestine may also be an important factor, as is the increased catabolic state associated with acute illness.
Though the most at-risk patients have severe mental and physical incapacities, other problems including respiratory disease, gastrointestinal disease, and stroke are associated with a malnourished state.3
Though hospitalists generally acknowledge the potential seriousness of a patient developing nutritional deficits, the attending healthcare team may be slow to diagnose or manage this problem because:
- Elderly patients can be malnourished on admission, but classic signs of protein-energy deficiency are mistaken for normal signs of aging;
- Nutritional problems are observed by the medical staff, but aggressive treatment is deferred in light of seemingly more pressing medical issues;
- Many physicians take action to prevent nutritional deficiencies, but these interventions are often insufficient or ineffective in preventing the spiral into malnutrition; and
- Physicians may assume a nutritionist is working to prevent and treat nutritional deficiencies, while the nutritionist is waiting for the medical staff to address the problem with a feeding tube.
Clinical Outcomes
Most physicians have observed the declining physical and cognitive capabilities of a nutritionally deprived elderly inpatient.
Although a causal relationship between malnutrition and adverse events has not been established, this is most likely because an older person’s clinical course affects and is affected by his nutritional status. Further, frequently compromised homeostatic mechanisms make the risk of complications related to malnutrition potentially more severe.
Though researchers are studying how inadequate nutritional intake contributes to the risk of adverse outcomes in elderly inpatients, numerous studies have identified strong correlations between the severity of the nutritional deficit and the risk of subsequent morbid events.
Sullivan, et al., found in their 1999 study of protein-energy undernutrition among elderly hospitalized patients that those maintained on nutrient intakes far less than their estimated energy requirements were at more risk of in-hospital mortality.
Other studies have shown that the risk of in-hospital starvation correlates strongly with polypharmacy and long stay. The severity of the nutritional deficiency correlates not only with weight loss and secretory protein loss, but also the risk of in-hospital and long-term complications.
Who Needs Help
Basic nutritional requirements vary much less than might be expected among younger and older patients. However, while a malnourished 20-year-old can be easily identified, the classic signs of malnutrition (wasting, brittle hair, dry skin, fissured mucus membranes) are less easily detected in elderly patients. They are often mistaken for signs of normal aging. Questions that can elicit evidence of a protein-energy deficiency include:
- Has food intake decreased recently?
- Are there physical difficulties with eating?
- Is the patient confused or depressed?
- Has there been diarrhea or vomiting?
- Has the patient been able to shop and manage food preparation?
- Has the patient or family noticed weight loss?
- What does the patient regard as a normal weight?
However, because the clinical signs of malnutrition-weight loss, muscle wasting, and fatigue can be difficult to detect from history alone, use of an assessment tool is often necessary.
There is no simple diagnostic test for undernutrition. Measurements of albumin, prealbumin, body-mass index (BMI), and weight loss have been used, albeit problematically, for this purpose. More useful for the quantification of nutritional status in elderly outpatients, these measurements are difficult to use in the hospital because albumin levels are frequently affected by disease processes involving the liver, kidney, and immune system, and correct baseline weights and heights are notoriously difficult to obtain from elderly patients.4
The Nutritional Risk Index was developed in response to these difficulties, but it was originally calibrated for young, post-surgical patients. Because of the problematic nature of obtaining the accurate heights and weights needed for the NRI in elderly patients, Bouillanne, et al,. developed the Geriatric Nutritional Risk Index (GNRI) in 2005 based on albumin levels, hospital recorded weights, and weight loss (see Table 1, above).
The GNRI’s creators classified patients according to their level of malnutrition and calculated their risk of related comorbidities. They found that 44% of their study population had major or moderate nutrition-related risk and recommended nutritional supplementation.
Path to Recovery
Refeeding regimens for elderly patients diagnosed as undernourished are best started with meals of personally chosen foods, timed for when patients are hungry.
Many elderly patients do not eat on a strict schedule at home and have difficulty complying with such a schedule. If oral intake remains inadequate, offer nutritional supplements. Because low-energy diets are usually low in vitamins and minerals, supplementation with a multivitamin, thiamine, folic acid, and zinc can be beneficial.
A common approach to problems with nutritional intake in elderly patients, particularly those with altered cognition or fluctuating consciousness, is insertion of a feeding tube.5 According to Medicare data from the 1990s, in individuals older than 85, one in 131 whites, and one in 58 African-Americans had a gastrostomy.
Despite the large numbers of feeding-tube placements in elderly patients, proof of their effectiveness is scant. No randomized trials have been performed to determine whether this practice improves survival, and observational data reveal that the one-year survival for these patients is less than 40%.
Feeding tubes are often placed to reverse the clinical sequelae of malnutrition—to heal pressure ulcers, prevent infection, and improve the patient’s functional status. But prospective observational studies do not support this methodology, and some contradictory evidence in the form of worsening pressure ulcers has been observed.
Aspiration pneumonia is the most serious infection for which tube feeding is considered a preventive measure. The condition results from the misdirection of pharyngeal contents and is believed to develop when nonpathologic oral secretions are deposited in dependent areas of the lung. The bacterial inoculum is high enough to overcome local defenses, which results in an infectious, febrile illness, usually involving mixed gram-negative rods and anaerobes.
Summaries of current data show that tube feeding may increase the risk of aspiration pneumonia. Further support for this hypothesis comes from the observation that jejunostomy feeding also does not appear to reduce the risk of aspiration pneumonia. If a patient is unable to protect his airway during mealtimes, he will also be at risk of aspiration and pneumonia between meals, regardless of how nutrition is delivered. TH
Dr. Landis is a rheumatologist and freelance writer.
References
- Sullivan DH, Sun S, Walls RC. Protein-energy undernutrition among elderly hospitalized patients. JAMA. 1999;281(21):2013-2019.
- Incalzi RA, Gemma A, Capparella O, et al. Energy intake and in-hospital starvation: a clinically relevant relationship. Arch Intern Med. 1996;156(4):425-429.
- Tierney A. Undernutrition and elderly patients. J Adv Nurs. 1995;23(2):228-236.
- Bouillanne O, Morineau G, Dupont C, et al. Geriatric nutritional risk index: a new index for evaluating at risk elderly medical patients. Am J Clin Nutr. 2005;82(4):777-783.
- Finucane T, Williams M. Tube feeding the demented patient: a review of the evidence. American Geriatrics Society Annual Scientific Meeting 2001.
Drugs and the Elderly
Never before have doctors had such an abundance of therapeutic options. And—not surprisingly—elderly patients are taking more medications than ever.
A national survey from 1998 revealed that more than 40% of elderly American adults take five or more medications a day—and that’s at home. Meantime, drug-related complications have risen steadily.
In 2005, the United States spent $177 billion in the management of drug-related problems—$34 billion more than was spent on the drugs themselves.1 Because up to a third of adverse medication effects warrant a hospital admission, hospitalists are the front line in the diagnosis and treatment of these syndromes.
Additionally, medication-related consequences can complicate hospitalizations required for other reasons. They can be observed as frequently as weekly according to hospitalist Balazs Zsenits, MD, FACP, of Rochester (N.Y.) General Hospital—and they’re often serious. In fact, medication reactions are so frequently fatal they represent the fifth-leading cause of death in the United States.
As one might expect, the elderly are disproportionately affected by the potentially toxic consequences of medication. In fact, a 2005 study published in Pharmacotherapy revealed that more than two-thirds of hospitalized elderly adults had an adverse drug effect over a four-year period.2 Among the more common outcomes were constipation, falls, immobility, confusion, hip fractures, and a decline in functional status requiring nursing home placement. Moreover, the authors noted that drug side effects frequently mimicked other geriatric syndromes, prompting physicians to prescribe additional medication.
While multiple medications may be necessary to prevent the progression of disease in older people, the overuse and misuse of drugs has been linked to serious health problems, including hospitalizations and death.
Polypharmacy
Patients at greatest risk for a polypharmacy-associated medical complication are those taking five or more concurrent drugs, those with multiple physicians, patients with significant medical comorbidities or impairments in vision or dexterity, and individuals who have recently been hospitalized.4-5 At least 25% of elderly Americans fall into at least one of these categories
But polypharmacy is not the only reason elderly patients experience a disproportionately high rate of adverse medication effects. Age-related altered drug metabolism is also responsible for unexpected drug consequences in this age group.
Aging influences every aspect of physiologic drug processing. While the absorption of oral medications from the GI tract remains relatively constant in the absence of disease states and gastric pH altering medications, bioavailability and clearance dramatically change with aging. These changes become the most pronounced after age 75, when kidney and liver function become limited.
As people age, their total body water decreases, their lean body mass is reduced, and their percentage of body fat increases. This increase in body fat expands the volume of distribution for lipophilic drugs and also decreases the volume of distribution for hydrophilic drugs.6 The result is that water-soluble medications have an elevated active serum concentration, and lipid-soluble agents, while they may have a decreased serum concentration, have a prolonged half-life.
These effects are best exemplified by examining what happens after a geriatric patient takes diazepam. A lipid-soluble drug, diazepam and its metabolites will be stored in an increasingly large body compartment. This will temporarily decrease the serum level of the drug, but will prolong the half-life from an average of 20 hours to greater than 50 hours. Repeated dosing will quickly result in toxic serum levels, at which point the patient is at risk for CNS side effects as well as falls and fractures.
The aging process also affects the role of drug-binding serum proteins. The total serum protein level is usually maintained (while albumin levels may diminish slightly, increasing levels of alpha 1 antitrypsin keeps the total protein level normal). More significantly, the affinity of the serum proteins for protein-bound drugs lessens as patients age. The degree of plasma protein binding has a significant impact on the pharmacologic activity of the drug, because it is the free drug that is physiologically active and exerts the pharmacologic effect.
In treating patients with highly protein-bound drugs, like phenytoin, one should expect toxic reactions at a normal serum level because more of the drug is unbound, and, hence, active. Elderly patients with low albumin levels secondary to malnutrition or liver disease will have an even more pronounced effect.
Effects of Metabolism
Many drugs undergo hepatic metabolism to produce more soluble forms for subsequent elimination through renal excretion. Though hepatic metabolism is affected by multiple variables including genotype, lifestyle, hepatic blood flow, hepatic diseases, and interactions with other medications, aging also plays a significant role.7
Of the two biotransformation systems through which hepatic metabolism occurs, it is the cytochrome P450 system (Phase I) most affected by increasing years. For most drugs, this leads to increased serum levels of the unmetabolized entity, leading to a greater potential for toxicity. Disease states that reduce blood flow to the liver, like congestive heart failure and cirrhosis, further inhibit this process. For drugs whose pharmacological activity requires biotransformation from a pro-drug form, inhibition can lead to decreased efficacy.
In contrast, Phase II metabolism, including acetylation, sulfonation, conjugation, and glucuronidation, is little influenced by advanced age.
Drug Elimination
The renal elimination of drugs is altered by aging, although there is significant variation between individuals for any given decade.8 Drug excretion does correlate with creatinine clearance, which declines by 50% by age 75. However, because lean body mass decreases with aging, the serum creatinine level tends to overestimate the creatinine clearance of older adults.
Utilization of the Cockroft-Gault formula (Figure 1, above) allows for an accurate estimation of the creatinine clearance in these patients.9 For example, a 25-year-old man and an 85-year-old man, each weighing 158 pounds and having a serum creatinine value of 1 mg per dL, would have different estimated creatinine clearance even though their serum creatinine value is the same. The younger man would have an estimated creatinine clearance of 115 mL per minute, while the older man’s would be 55 mL per minute.
Approximating creatinine clearance is particularly important when prescribing medications that have a narrow therapeutic index (aminoglycosides, lithium, digoxin, procainamide, vancomycin). Even minimally excessive doses of these drugs will result in a prolonged the half-life, and an increased potential for toxic effects.
Expect and account for these alterations in drug metabolism in elderly patients. Typical changes result in increased active serum concentrations of the drug and extended half-life. Elevated drug concentrations result in more adverse drug events, and these include not only known complications, but also uncommon problems such as blood dyscrasias. If a rare adverse drug reaction does occur, it is most likely to happen in an elderly person.
The Acute Care Setting
In light of the physiologic changes associated with aging, as well as the problems posed by taking multiple medications, it is clear that active intervention is required to prevent adverse drug reactions in geriatric patients.
A large cohort study of Medicare enrollees with more than 30,000 patient-years of observation found that 28% of adverse drug reactions were potentially avoidable. Most errors occurred during prescribing and monitoring. A number of strategies have been proposed for reducing these unwanted medication consequences in the hospital setting, including:
- Avoid inappropriate drug prescribing;
- Avoid overprescribing;
- Implement age-appropriate dosing; and
- Encourage a multidisciplinary ap-proach.
Drugs to Avoid
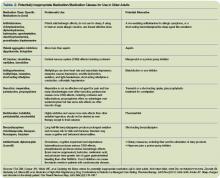
Though precise clinical data regarding which medications are harmful to elderly patients in the acute care setting is lacking, multiple expert panels have attempted to delineate which drugs should be generally avoided in this population (Table 1, above).
The most notable of these evaluations is the Beers criteria, a frequently updated set of medications deemed inappropriate for use in geriatric patients. Most recently amended in 2003, this list is formulated by experts in pharmacology and geriatrics, and has been validated in large studies as a useful tool for decreasing medication-related problems in the nursing home setting.10
Though a 2006 study of hospital morbidity found that adverse drug reactions in the acute care setting often occur from drugs not listed in the Beers criteria, avoiding medications like those listed above is still a useful tool in preventing side effects.11-12
Avoid Overprescribing
To prevent a polypharmacy-induced iatrogenic illness, it is important to consider any new signs and symptoms to be a possible consequence of current drug therapy. Steps for reducing polypharmacy include:
- Get into the habit of identifying all drugs by generic name and drug class;
- Make certain the drug being prescribed has a clinical indication;
- Know the side-effect profile of the drugs being prescribed;
- Understand how changes in drug distribution, metabolism, and elimination associated with aging increase the risk of adverse drug events;
- Stop any drug without known benefit;
- Stop any drug without a clinical indication;
- Attempt to substitute a less-toxic drug; and
- Be aware of the prescribing-cascade treating an adverse drug reaction as an illness with another drug.
Age-Appropriate Dosing
When starting a new drug, start with a low dose and titrate slowly to the desired clinical effect. While the manufacturers of many commonly used medications do not delineate the lower-dosage recommendations necessary for elderly patients, you can bypass this problem by starting with one-third to half the recommended dosage.
After observing that the patient tolerates the new drug, slowly increase the dose until the desired result is obtained. This approach is particularly important in minimizing potential harmful drug effects in patients with severely reduced renal function.14
Multidisciplinary Approach
In its 2001 report “Crossing the Quality Chasm: A New Health System of the 21st Century,” the U.S. Institute of Medicine declared: “The current care systems cannot do the job. Trying harder will not work. If we want safer, higher-quality care, we will need to have redesigned systems of care, including the use of information technology to support clinical and administrative processes.”
While hospitalists are on the front line for preventing adverse drug reactions, they can’t do it by themselves. Here are a few tips for making your job easier:
- Request that medications inappropriate for geriatric patients (based on the Beers criteria) be notated as such by the pharmacist;
- Ask for a geriatric dosing option in the computer-based medication ordering system;
- Flag charts of patients with previous adverse drug effects with the name of the offending drug;
- Warn nurses and other caregivers to monitor for specific side effects; and
- Advocate that midlevel providers receive hospital-based training in the prevention of medication-related adverse events.
The elderly portion of the population is expanding more rapidly than the population as a whole, and the recognition and prevention of medication side effects in this group is one of the most critical safety and economic issues facing the healthcare system today. While the magnitude of this problem demands multidisciplinary involvement, hospitalists can be key players in making a difference. TH
Dr. Landis is a rheumatologist and a freelance writer
References
- Bates DW, Spell N, Cullen DJ, et al. The costs of adverse drug events in hospitalized patients. Adverse Drug Events Prevention Study Group. JAMA. 1997 Jan 22-29;277(4):307-311. Comment in: JAMA. 1997 Jan 22-29;277(4):341-3422: JAMA. 1997 May 7;277(17):1351-1352; author reply 1353-1354.
- Zarowitz BJ, Stebelsky LA, Muma BK, et al. Reduction of high-risk polypharmacy drug combinations in patients in a managed care setting. Pharmacotherapy. 2005;25(11):1636-1645. Comment in: Pharmacotherapy. 2006 Jun;26(6):886-887; discussion 887.
- Byron C, Hochberg MC. Changing the patterns of Coxibs/NSAIDs prescribing: balancing CV and GI risks. Medscape. Available at www.medscape.com/viewprogram/5060. Last accessed May 2, 2007.
- Shapiro K. The Complexities of Geriatric Pain Management. 20th Annual Meeting of the American Pain Society. Medscape CME. Available at www.medscape.com/viewarticle/416593. Last accessed May 2, 2007.
- Lau DT, Kasper JD, Potter DE, et al. Potentially inappropriate medication prescriptions among elderly nursing home residents: their scope and associated resident and facility characteristics. Health Serv Res. 2004 Oct; 39(5):1257-1276.
- Longa GJ, Cross RE. Laboratory Monitoring of Drug Therapy. Part II: Variable Protein Binding and Free (Unbound) Drug Concentration. Bull Lab Me. 1984;80:1-6. 7. Chutka DS, Evans JM, Fleming KC, et al. Symposium on geriatrics—Part I: Drug prescribing for elderly patients. Mayo Clin Proc. 1995 Jul;70(7):685-693.
- Feely J, Coakley D. Altered pharmacodynamics in the elderly. Clin Geriatr Med. 1990 May; 6(2): 269-283.
- Williams CM. Using medications appropriately in older adults. Am Fam Phys. 2002 Nov 15;66(10):1917-1924.
- Fick DN, Cooper JW, Wade WE. Updating the Beers criteria for potentially inappropriate medication use in older adults. Arch Intern Med. 2003 Dec 8-22;163(22):2716-2724. Erratum in: Arch Intern Med. 2004 Feb 9;164(3):298. Comment in: Arch Intern Med. 2004 Aug 9-23;164(15):1701.
- Johnston PE, France DJ, Byrne DW, et al. Assessment of adverse drug events among patients in a tertiary care medical center. Am J Health Syst Pharm., 2006;63(22):2218-2227.
- Page RL, Ruscin JM. The risk of adverse drug events and hospital related morbidity and mortality among older adults with potentially inappropriate medication use. Am J Geriatr Pharmacother. 2006 Dec;4(4):297-305.
- Avidan AY. Sleep changes and disorders in the elderly patient. Curr Neurol Neurosci Rep. 2002 Mar;2(2):178-185.
- Pugh MJV, Fincke G, Bierman AS, et al. Potentially inappropriate prescribing in elderly veterans: Are we using the wrong drug, wrong dose, or wrong duration? J Am Geriatr Soc. 2005 Aug;53(8):1282-1289.
Never before have doctors had such an abundance of therapeutic options. And—not surprisingly—elderly patients are taking more medications than ever.
A national survey from 1998 revealed that more than 40% of elderly American adults take five or more medications a day—and that’s at home. Meantime, drug-related complications have risen steadily.
In 2005, the United States spent $177 billion in the management of drug-related problems—$34 billion more than was spent on the drugs themselves.1 Because up to a third of adverse medication effects warrant a hospital admission, hospitalists are the front line in the diagnosis and treatment of these syndromes.
Additionally, medication-related consequences can complicate hospitalizations required for other reasons. They can be observed as frequently as weekly according to hospitalist Balazs Zsenits, MD, FACP, of Rochester (N.Y.) General Hospital—and they’re often serious. In fact, medication reactions are so frequently fatal they represent the fifth-leading cause of death in the United States.
As one might expect, the elderly are disproportionately affected by the potentially toxic consequences of medication. In fact, a 2005 study published in Pharmacotherapy revealed that more than two-thirds of hospitalized elderly adults had an adverse drug effect over a four-year period.2 Among the more common outcomes were constipation, falls, immobility, confusion, hip fractures, and a decline in functional status requiring nursing home placement. Moreover, the authors noted that drug side effects frequently mimicked other geriatric syndromes, prompting physicians to prescribe additional medication.
While multiple medications may be necessary to prevent the progression of disease in older people, the overuse and misuse of drugs has been linked to serious health problems, including hospitalizations and death.
Polypharmacy
Patients at greatest risk for a polypharmacy-associated medical complication are those taking five or more concurrent drugs, those with multiple physicians, patients with significant medical comorbidities or impairments in vision or dexterity, and individuals who have recently been hospitalized.4-5 At least 25% of elderly Americans fall into at least one of these categories
But polypharmacy is not the only reason elderly patients experience a disproportionately high rate of adverse medication effects. Age-related altered drug metabolism is also responsible for unexpected drug consequences in this age group.
Aging influences every aspect of physiologic drug processing. While the absorption of oral medications from the GI tract remains relatively constant in the absence of disease states and gastric pH altering medications, bioavailability and clearance dramatically change with aging. These changes become the most pronounced after age 75, when kidney and liver function become limited.
As people age, their total body water decreases, their lean body mass is reduced, and their percentage of body fat increases. This increase in body fat expands the volume of distribution for lipophilic drugs and also decreases the volume of distribution for hydrophilic drugs.6 The result is that water-soluble medications have an elevated active serum concentration, and lipid-soluble agents, while they may have a decreased serum concentration, have a prolonged half-life.
These effects are best exemplified by examining what happens after a geriatric patient takes diazepam. A lipid-soluble drug, diazepam and its metabolites will be stored in an increasingly large body compartment. This will temporarily decrease the serum level of the drug, but will prolong the half-life from an average of 20 hours to greater than 50 hours. Repeated dosing will quickly result in toxic serum levels, at which point the patient is at risk for CNS side effects as well as falls and fractures.
The aging process also affects the role of drug-binding serum proteins. The total serum protein level is usually maintained (while albumin levels may diminish slightly, increasing levels of alpha 1 antitrypsin keeps the total protein level normal). More significantly, the affinity of the serum proteins for protein-bound drugs lessens as patients age. The degree of plasma protein binding has a significant impact on the pharmacologic activity of the drug, because it is the free drug that is physiologically active and exerts the pharmacologic effect.
In treating patients with highly protein-bound drugs, like phenytoin, one should expect toxic reactions at a normal serum level because more of the drug is unbound, and, hence, active. Elderly patients with low albumin levels secondary to malnutrition or liver disease will have an even more pronounced effect.
Effects of Metabolism
Many drugs undergo hepatic metabolism to produce more soluble forms for subsequent elimination through renal excretion. Though hepatic metabolism is affected by multiple variables including genotype, lifestyle, hepatic blood flow, hepatic diseases, and interactions with other medications, aging also plays a significant role.7
Of the two biotransformation systems through which hepatic metabolism occurs, it is the cytochrome P450 system (Phase I) most affected by increasing years. For most drugs, this leads to increased serum levels of the unmetabolized entity, leading to a greater potential for toxicity. Disease states that reduce blood flow to the liver, like congestive heart failure and cirrhosis, further inhibit this process. For drugs whose pharmacological activity requires biotransformation from a pro-drug form, inhibition can lead to decreased efficacy.
In contrast, Phase II metabolism, including acetylation, sulfonation, conjugation, and glucuronidation, is little influenced by advanced age.
Drug Elimination
The renal elimination of drugs is altered by aging, although there is significant variation between individuals for any given decade.8 Drug excretion does correlate with creatinine clearance, which declines by 50% by age 75. However, because lean body mass decreases with aging, the serum creatinine level tends to overestimate the creatinine clearance of older adults.
Utilization of the Cockroft-Gault formula (Figure 1, above) allows for an accurate estimation of the creatinine clearance in these patients.9 For example, a 25-year-old man and an 85-year-old man, each weighing 158 pounds and having a serum creatinine value of 1 mg per dL, would have different estimated creatinine clearance even though their serum creatinine value is the same. The younger man would have an estimated creatinine clearance of 115 mL per minute, while the older man’s would be 55 mL per minute.
Approximating creatinine clearance is particularly important when prescribing medications that have a narrow therapeutic index (aminoglycosides, lithium, digoxin, procainamide, vancomycin). Even minimally excessive doses of these drugs will result in a prolonged the half-life, and an increased potential for toxic effects.
Expect and account for these alterations in drug metabolism in elderly patients. Typical changes result in increased active serum concentrations of the drug and extended half-life. Elevated drug concentrations result in more adverse drug events, and these include not only known complications, but also uncommon problems such as blood dyscrasias. If a rare adverse drug reaction does occur, it is most likely to happen in an elderly person.
The Acute Care Setting
In light of the physiologic changes associated with aging, as well as the problems posed by taking multiple medications, it is clear that active intervention is required to prevent adverse drug reactions in geriatric patients.
A large cohort study of Medicare enrollees with more than 30,000 patient-years of observation found that 28% of adverse drug reactions were potentially avoidable. Most errors occurred during prescribing and monitoring. A number of strategies have been proposed for reducing these unwanted medication consequences in the hospital setting, including:
- Avoid inappropriate drug prescribing;
- Avoid overprescribing;
- Implement age-appropriate dosing; and
- Encourage a multidisciplinary ap-proach.
Drugs to Avoid
Though precise clinical data regarding which medications are harmful to elderly patients in the acute care setting is lacking, multiple expert panels have attempted to delineate which drugs should be generally avoided in this population (Table 1, above).
The most notable of these evaluations is the Beers criteria, a frequently updated set of medications deemed inappropriate for use in geriatric patients. Most recently amended in 2003, this list is formulated by experts in pharmacology and geriatrics, and has been validated in large studies as a useful tool for decreasing medication-related problems in the nursing home setting.10
Though a 2006 study of hospital morbidity found that adverse drug reactions in the acute care setting often occur from drugs not listed in the Beers criteria, avoiding medications like those listed above is still a useful tool in preventing side effects.11-12
Avoid Overprescribing
To prevent a polypharmacy-induced iatrogenic illness, it is important to consider any new signs and symptoms to be a possible consequence of current drug therapy. Steps for reducing polypharmacy include:
- Get into the habit of identifying all drugs by generic name and drug class;
- Make certain the drug being prescribed has a clinical indication;
- Know the side-effect profile of the drugs being prescribed;
- Understand how changes in drug distribution, metabolism, and elimination associated with aging increase the risk of adverse drug events;
- Stop any drug without known benefit;
- Stop any drug without a clinical indication;
- Attempt to substitute a less-toxic drug; and
- Be aware of the prescribing-cascade treating an adverse drug reaction as an illness with another drug.
Age-Appropriate Dosing
When starting a new drug, start with a low dose and titrate slowly to the desired clinical effect. While the manufacturers of many commonly used medications do not delineate the lower-dosage recommendations necessary for elderly patients, you can bypass this problem by starting with one-third to half the recommended dosage.
After observing that the patient tolerates the new drug, slowly increase the dose until the desired result is obtained. This approach is particularly important in minimizing potential harmful drug effects in patients with severely reduced renal function.14
Multidisciplinary Approach
In its 2001 report “Crossing the Quality Chasm: A New Health System of the 21st Century,” the U.S. Institute of Medicine declared: “The current care systems cannot do the job. Trying harder will not work. If we want safer, higher-quality care, we will need to have redesigned systems of care, including the use of information technology to support clinical and administrative processes.”
While hospitalists are on the front line for preventing adverse drug reactions, they can’t do it by themselves. Here are a few tips for making your job easier:
- Request that medications inappropriate for geriatric patients (based on the Beers criteria) be notated as such by the pharmacist;
- Ask for a geriatric dosing option in the computer-based medication ordering system;
- Flag charts of patients with previous adverse drug effects with the name of the offending drug;
- Warn nurses and other caregivers to monitor for specific side effects; and
- Advocate that midlevel providers receive hospital-based training in the prevention of medication-related adverse events.
The elderly portion of the population is expanding more rapidly than the population as a whole, and the recognition and prevention of medication side effects in this group is one of the most critical safety and economic issues facing the healthcare system today. While the magnitude of this problem demands multidisciplinary involvement, hospitalists can be key players in making a difference. TH
Dr. Landis is a rheumatologist and a freelance writer
References
- Bates DW, Spell N, Cullen DJ, et al. The costs of adverse drug events in hospitalized patients. Adverse Drug Events Prevention Study Group. JAMA. 1997 Jan 22-29;277(4):307-311. Comment in: JAMA. 1997 Jan 22-29;277(4):341-3422: JAMA. 1997 May 7;277(17):1351-1352; author reply 1353-1354.
- Zarowitz BJ, Stebelsky LA, Muma BK, et al. Reduction of high-risk polypharmacy drug combinations in patients in a managed care setting. Pharmacotherapy. 2005;25(11):1636-1645. Comment in: Pharmacotherapy. 2006 Jun;26(6):886-887; discussion 887.
- Byron C, Hochberg MC. Changing the patterns of Coxibs/NSAIDs prescribing: balancing CV and GI risks. Medscape. Available at www.medscape.com/viewprogram/5060. Last accessed May 2, 2007.
- Shapiro K. The Complexities of Geriatric Pain Management. 20th Annual Meeting of the American Pain Society. Medscape CME. Available at www.medscape.com/viewarticle/416593. Last accessed May 2, 2007.
- Lau DT, Kasper JD, Potter DE, et al. Potentially inappropriate medication prescriptions among elderly nursing home residents: their scope and associated resident and facility characteristics. Health Serv Res. 2004 Oct; 39(5):1257-1276.
- Longa GJ, Cross RE. Laboratory Monitoring of Drug Therapy. Part II: Variable Protein Binding and Free (Unbound) Drug Concentration. Bull Lab Me. 1984;80:1-6. 7. Chutka DS, Evans JM, Fleming KC, et al. Symposium on geriatrics—Part I: Drug prescribing for elderly patients. Mayo Clin Proc. 1995 Jul;70(7):685-693.
- Feely J, Coakley D. Altered pharmacodynamics in the elderly. Clin Geriatr Med. 1990 May; 6(2): 269-283.
- Williams CM. Using medications appropriately in older adults. Am Fam Phys. 2002 Nov 15;66(10):1917-1924.
- Fick DN, Cooper JW, Wade WE. Updating the Beers criteria for potentially inappropriate medication use in older adults. Arch Intern Med. 2003 Dec 8-22;163(22):2716-2724. Erratum in: Arch Intern Med. 2004 Feb 9;164(3):298. Comment in: Arch Intern Med. 2004 Aug 9-23;164(15):1701.
- Johnston PE, France DJ, Byrne DW, et al. Assessment of adverse drug events among patients in a tertiary care medical center. Am J Health Syst Pharm., 2006;63(22):2218-2227.
- Page RL, Ruscin JM. The risk of adverse drug events and hospital related morbidity and mortality among older adults with potentially inappropriate medication use. Am J Geriatr Pharmacother. 2006 Dec;4(4):297-305.
- Avidan AY. Sleep changes and disorders in the elderly patient. Curr Neurol Neurosci Rep. 2002 Mar;2(2):178-185.
- Pugh MJV, Fincke G, Bierman AS, et al. Potentially inappropriate prescribing in elderly veterans: Are we using the wrong drug, wrong dose, or wrong duration? J Am Geriatr Soc. 2005 Aug;53(8):1282-1289.
Never before have doctors had such an abundance of therapeutic options. And—not surprisingly—elderly patients are taking more medications than ever.
A national survey from 1998 revealed that more than 40% of elderly American adults take five or more medications a day—and that’s at home. Meantime, drug-related complications have risen steadily.
In 2005, the United States spent $177 billion in the management of drug-related problems—$34 billion more than was spent on the drugs themselves.1 Because up to a third of adverse medication effects warrant a hospital admission, hospitalists are the front line in the diagnosis and treatment of these syndromes.
Additionally, medication-related consequences can complicate hospitalizations required for other reasons. They can be observed as frequently as weekly according to hospitalist Balazs Zsenits, MD, FACP, of Rochester (N.Y.) General Hospital—and they’re often serious. In fact, medication reactions are so frequently fatal they represent the fifth-leading cause of death in the United States.
As one might expect, the elderly are disproportionately affected by the potentially toxic consequences of medication. In fact, a 2005 study published in Pharmacotherapy revealed that more than two-thirds of hospitalized elderly adults had an adverse drug effect over a four-year period.2 Among the more common outcomes were constipation, falls, immobility, confusion, hip fractures, and a decline in functional status requiring nursing home placement. Moreover, the authors noted that drug side effects frequently mimicked other geriatric syndromes, prompting physicians to prescribe additional medication.
While multiple medications may be necessary to prevent the progression of disease in older people, the overuse and misuse of drugs has been linked to serious health problems, including hospitalizations and death.
Polypharmacy
Patients at greatest risk for a polypharmacy-associated medical complication are those taking five or more concurrent drugs, those with multiple physicians, patients with significant medical comorbidities or impairments in vision or dexterity, and individuals who have recently been hospitalized.4-5 At least 25% of elderly Americans fall into at least one of these categories
But polypharmacy is not the only reason elderly patients experience a disproportionately high rate of adverse medication effects. Age-related altered drug metabolism is also responsible for unexpected drug consequences in this age group.
Aging influences every aspect of physiologic drug processing. While the absorption of oral medications from the GI tract remains relatively constant in the absence of disease states and gastric pH altering medications, bioavailability and clearance dramatically change with aging. These changes become the most pronounced after age 75, when kidney and liver function become limited.
As people age, their total body water decreases, their lean body mass is reduced, and their percentage of body fat increases. This increase in body fat expands the volume of distribution for lipophilic drugs and also decreases the volume of distribution for hydrophilic drugs.6 The result is that water-soluble medications have an elevated active serum concentration, and lipid-soluble agents, while they may have a decreased serum concentration, have a prolonged half-life.
These effects are best exemplified by examining what happens after a geriatric patient takes diazepam. A lipid-soluble drug, diazepam and its metabolites will be stored in an increasingly large body compartment. This will temporarily decrease the serum level of the drug, but will prolong the half-life from an average of 20 hours to greater than 50 hours. Repeated dosing will quickly result in toxic serum levels, at which point the patient is at risk for CNS side effects as well as falls and fractures.
The aging process also affects the role of drug-binding serum proteins. The total serum protein level is usually maintained (while albumin levels may diminish slightly, increasing levels of alpha 1 antitrypsin keeps the total protein level normal). More significantly, the affinity of the serum proteins for protein-bound drugs lessens as patients age. The degree of plasma protein binding has a significant impact on the pharmacologic activity of the drug, because it is the free drug that is physiologically active and exerts the pharmacologic effect.
In treating patients with highly protein-bound drugs, like phenytoin, one should expect toxic reactions at a normal serum level because more of the drug is unbound, and, hence, active. Elderly patients with low albumin levels secondary to malnutrition or liver disease will have an even more pronounced effect.
Effects of Metabolism
Many drugs undergo hepatic metabolism to produce more soluble forms for subsequent elimination through renal excretion. Though hepatic metabolism is affected by multiple variables including genotype, lifestyle, hepatic blood flow, hepatic diseases, and interactions with other medications, aging also plays a significant role.7
Of the two biotransformation systems through which hepatic metabolism occurs, it is the cytochrome P450 system (Phase I) most affected by increasing years. For most drugs, this leads to increased serum levels of the unmetabolized entity, leading to a greater potential for toxicity. Disease states that reduce blood flow to the liver, like congestive heart failure and cirrhosis, further inhibit this process. For drugs whose pharmacological activity requires biotransformation from a pro-drug form, inhibition can lead to decreased efficacy.
In contrast, Phase II metabolism, including acetylation, sulfonation, conjugation, and glucuronidation, is little influenced by advanced age.
Drug Elimination
The renal elimination of drugs is altered by aging, although there is significant variation between individuals for any given decade.8 Drug excretion does correlate with creatinine clearance, which declines by 50% by age 75. However, because lean body mass decreases with aging, the serum creatinine level tends to overestimate the creatinine clearance of older adults.
Utilization of the Cockroft-Gault formula (Figure 1, above) allows for an accurate estimation of the creatinine clearance in these patients.9 For example, a 25-year-old man and an 85-year-old man, each weighing 158 pounds and having a serum creatinine value of 1 mg per dL, would have different estimated creatinine clearance even though their serum creatinine value is the same. The younger man would have an estimated creatinine clearance of 115 mL per minute, while the older man’s would be 55 mL per minute.
Approximating creatinine clearance is particularly important when prescribing medications that have a narrow therapeutic index (aminoglycosides, lithium, digoxin, procainamide, vancomycin). Even minimally excessive doses of these drugs will result in a prolonged the half-life, and an increased potential for toxic effects.
Expect and account for these alterations in drug metabolism in elderly patients. Typical changes result in increased active serum concentrations of the drug and extended half-life. Elevated drug concentrations result in more adverse drug events, and these include not only known complications, but also uncommon problems such as blood dyscrasias. If a rare adverse drug reaction does occur, it is most likely to happen in an elderly person.
The Acute Care Setting
In light of the physiologic changes associated with aging, as well as the problems posed by taking multiple medications, it is clear that active intervention is required to prevent adverse drug reactions in geriatric patients.
A large cohort study of Medicare enrollees with more than 30,000 patient-years of observation found that 28% of adverse drug reactions were potentially avoidable. Most errors occurred during prescribing and monitoring. A number of strategies have been proposed for reducing these unwanted medication consequences in the hospital setting, including:
- Avoid inappropriate drug prescribing;
- Avoid overprescribing;
- Implement age-appropriate dosing; and
- Encourage a multidisciplinary ap-proach.
Drugs to Avoid
Though precise clinical data regarding which medications are harmful to elderly patients in the acute care setting is lacking, multiple expert panels have attempted to delineate which drugs should be generally avoided in this population (Table 1, above).
The most notable of these evaluations is the Beers criteria, a frequently updated set of medications deemed inappropriate for use in geriatric patients. Most recently amended in 2003, this list is formulated by experts in pharmacology and geriatrics, and has been validated in large studies as a useful tool for decreasing medication-related problems in the nursing home setting.10
Though a 2006 study of hospital morbidity found that adverse drug reactions in the acute care setting often occur from drugs not listed in the Beers criteria, avoiding medications like those listed above is still a useful tool in preventing side effects.11-12
Avoid Overprescribing
To prevent a polypharmacy-induced iatrogenic illness, it is important to consider any new signs and symptoms to be a possible consequence of current drug therapy. Steps for reducing polypharmacy include:
- Get into the habit of identifying all drugs by generic name and drug class;
- Make certain the drug being prescribed has a clinical indication;
- Know the side-effect profile of the drugs being prescribed;
- Understand how changes in drug distribution, metabolism, and elimination associated with aging increase the risk of adverse drug events;
- Stop any drug without known benefit;
- Stop any drug without a clinical indication;
- Attempt to substitute a less-toxic drug; and
- Be aware of the prescribing-cascade treating an adverse drug reaction as an illness with another drug.
Age-Appropriate Dosing
When starting a new drug, start with a low dose and titrate slowly to the desired clinical effect. While the manufacturers of many commonly used medications do not delineate the lower-dosage recommendations necessary for elderly patients, you can bypass this problem by starting with one-third to half the recommended dosage.
After observing that the patient tolerates the new drug, slowly increase the dose until the desired result is obtained. This approach is particularly important in minimizing potential harmful drug effects in patients with severely reduced renal function.14
Multidisciplinary Approach
In its 2001 report “Crossing the Quality Chasm: A New Health System of the 21st Century,” the U.S. Institute of Medicine declared: “The current care systems cannot do the job. Trying harder will not work. If we want safer, higher-quality care, we will need to have redesigned systems of care, including the use of information technology to support clinical and administrative processes.”
While hospitalists are on the front line for preventing adverse drug reactions, they can’t do it by themselves. Here are a few tips for making your job easier:
- Request that medications inappropriate for geriatric patients (based on the Beers criteria) be notated as such by the pharmacist;
- Ask for a geriatric dosing option in the computer-based medication ordering system;
- Flag charts of patients with previous adverse drug effects with the name of the offending drug;
- Warn nurses and other caregivers to monitor for specific side effects; and
- Advocate that midlevel providers receive hospital-based training in the prevention of medication-related adverse events.
The elderly portion of the population is expanding more rapidly than the population as a whole, and the recognition and prevention of medication side effects in this group is one of the most critical safety and economic issues facing the healthcare system today. While the magnitude of this problem demands multidisciplinary involvement, hospitalists can be key players in making a difference. TH
Dr. Landis is a rheumatologist and a freelance writer
References
- Bates DW, Spell N, Cullen DJ, et al. The costs of adverse drug events in hospitalized patients. Adverse Drug Events Prevention Study Group. JAMA. 1997 Jan 22-29;277(4):307-311. Comment in: JAMA. 1997 Jan 22-29;277(4):341-3422: JAMA. 1997 May 7;277(17):1351-1352; author reply 1353-1354.
- Zarowitz BJ, Stebelsky LA, Muma BK, et al. Reduction of high-risk polypharmacy drug combinations in patients in a managed care setting. Pharmacotherapy. 2005;25(11):1636-1645. Comment in: Pharmacotherapy. 2006 Jun;26(6):886-887; discussion 887.
- Byron C, Hochberg MC. Changing the patterns of Coxibs/NSAIDs prescribing: balancing CV and GI risks. Medscape. Available at www.medscape.com/viewprogram/5060. Last accessed May 2, 2007.
- Shapiro K. The Complexities of Geriatric Pain Management. 20th Annual Meeting of the American Pain Society. Medscape CME. Available at www.medscape.com/viewarticle/416593. Last accessed May 2, 2007.
- Lau DT, Kasper JD, Potter DE, et al. Potentially inappropriate medication prescriptions among elderly nursing home residents: their scope and associated resident and facility characteristics. Health Serv Res. 2004 Oct; 39(5):1257-1276.
- Longa GJ, Cross RE. Laboratory Monitoring of Drug Therapy. Part II: Variable Protein Binding and Free (Unbound) Drug Concentration. Bull Lab Me. 1984;80:1-6. 7. Chutka DS, Evans JM, Fleming KC, et al. Symposium on geriatrics—Part I: Drug prescribing for elderly patients. Mayo Clin Proc. 1995 Jul;70(7):685-693.
- Feely J, Coakley D. Altered pharmacodynamics in the elderly. Clin Geriatr Med. 1990 May; 6(2): 269-283.
- Williams CM. Using medications appropriately in older adults. Am Fam Phys. 2002 Nov 15;66(10):1917-1924.
- Fick DN, Cooper JW, Wade WE. Updating the Beers criteria for potentially inappropriate medication use in older adults. Arch Intern Med. 2003 Dec 8-22;163(22):2716-2724. Erratum in: Arch Intern Med. 2004 Feb 9;164(3):298. Comment in: Arch Intern Med. 2004 Aug 9-23;164(15):1701.
- Johnston PE, France DJ, Byrne DW, et al. Assessment of adverse drug events among patients in a tertiary care medical center. Am J Health Syst Pharm., 2006;63(22):2218-2227.
- Page RL, Ruscin JM. The risk of adverse drug events and hospital related morbidity and mortality among older adults with potentially inappropriate medication use. Am J Geriatr Pharmacother. 2006 Dec;4(4):297-305.
- Avidan AY. Sleep changes and disorders in the elderly patient. Curr Neurol Neurosci Rep. 2002 Mar;2(2):178-185.
- Pugh MJV, Fincke G, Bierman AS, et al. Potentially inappropriate prescribing in elderly veterans: Are we using the wrong drug, wrong dose, or wrong duration? J Am Geriatr Soc. 2005 Aug;53(8):1282-1289.
Inflammatory Findings
Mrs. K, an 81-year-old golf-enthusiast admitted with congestive heart failure, now refuses to walk and complains of ankle pain. When you see her, she refuses to let even a bedsheet near her left ankle, and she claims that you did this to her.
Unfortunately, she’s probably right. Mrs. K also has a history of podagra, and she developed an acute gouty monoarthritis after receiving treatment with diuretics and aspirin. Gout—along with the other causes of inpatient acute monoarthritis (pseudogout, septic arthritis, and trauma)—are increasingly common diagnoses in the geriatric patient population. Because the elderly are uniquely predisposed to losing functional independence following an acute attack, making a timely diagnosis is particularly important in this age group. And though the patient’s clinical features may point toward an etiology, making the correct diagnosis ultimately depends on the results of the joint tap.
Gout
Gout occurs in patients with high serum levels of uric acid, though not all hyperuricemic patients develop gout. Among elderly hospitalized patients with hyperuricemia, approximately 65% have significant renal impairment, and others have advanced hypertension, coronary artery disease, and congestive heart failure. Over time, high serum uric acid levels may lead to the deposition of monosodium urate crystals in the joints; these lesions are the precursors of a gouty attack.
Gouty attacks occur when crystal deposits become inflamed. The inflammation may be triggered by a medication-induced change in uric acid concentration or by a medical condition, including acute illness, trauma, surgery, and dehydration. (See Table 1, p.18.) Though some elderly patients with acute gouty arthritis will manifest confusion or a sudden change in ambulatory status, most will present with a monoarthritis and a rise in temperature. Gout is suspected clinically when the first metatarsal phalangeal joint is involved (podagra), but other commonly involved joints include the ankle and the knee. Uric acid levels in the blood are usually elevated but can also temporarily normalize or even dip low during attacks. An X-ray of the involved joint may be normal or may already show the characteristic erosive lesion of gout, the “overhanging edge.”
Making the correct diagnosis is dependent upon visualizing intracellular, needle-shaped, negatively birefringent crystals in the synovial fluid. After the fluid is removed from the affected joint, it should be examined under polarized light within two hours; if more time is needed, the fluid can be refrigerated for up to 12 hours. Co-infection of a gouty joint has been described in the elderly population, and the synovial fluid should be sent to the laboratory for a cell count, Gram’s stain, and culture, as well as glucose and lactate levels. (See Table 2, below.)
Direct in-hospital treatment of gout toward alleviating the current attack, preventing future attacks, and providing appropriate antibiotic coverage in suspected co-infected cases until joint culture results are finalized. Treatment options for the acute attack include NSAIDs and corticosteroids—either oral or intra-articular. Hourly oral colchicine is not a good option for an elderly patient because the diarrhea that ensues is particularly disruptive. Nor is IV colchicine a good option thanks to its side-effect profile that renders it unusable in patients with reduced renal function. The treatment of hyperuricemia with allopurinol should not be undertaken during the acute attack because any change in the serum uric acid concentration will serve only to exacerbate the current inflammation.
Anti-inflammatory doses of NSAIDs are effective and will shorten the duration of symptoms substantially. Seven to 10 days of indomethacin at a dose of 50 mg taken orally three times daily is the traditional choice, and though it is generally conceded that ibuprofen and naproxen also work well, no comparative trials have been performed. Elderly patients are at increased risk for adverse effects from NSAIDs, particularly those patients with severely reduced renal function, gastropathy, asthma, congestive heart failure, or other intravascularly depleted states. Gastric mucosal protection, using proton-pump inhibitors, and careful monitoring of fluid status, renal function, and mental status are of particular concern in this population.
Because recent research indicates that COX-2 inhibitors have thrombotic potential and are contraindicated in patients at high risk for cardiovascular events or stroke, the extent to which they can be used in an elderly patient with an acute gouty attack is limited. A traditional NSAID in combination with a proton-pump inhibitor may be as effective as a COX-2 inhibitor in reducing the risk of gastroduodenal toxicity, however.
Corticosteroids—given either orally or intra-articularly—are an appropriate treatment for patients who can’t tolerate an NSAID. As long as a septic joint has been excluded, an intra-articular injection of 40–80 mg triamcinolone acetonide or 40 mg of methylprednisolone acetate will result in major improvement within 24 hours for most patients. Another option is a seven- to 10-day course of oral prednisone, starting with 40 mg on day one and reducing the dosage by 5 mg/day. Elderly patients taking oral prednisone should also receive adequate calcium, vitamin D, and a proton pump inhibitor for gastrointestinal protection, as well as close monitoring of blood pressure, glucose, and mental status.
If a patient has a history of frequent attacks or tophi, has a serum uric acid level higher than 12 mg/dl, or is consistently receiving high doses of diuretics, that person is at high risk for subsequent attacks and should receive prophylactic treatment with either a low-dose daily NSAID or a renally dosed oral colchicine.
Pseudogout
Pseudogout is the articular manifestation of calcium pyrophosphate dihydrate (CPPD) deposition, and this process is associated with aging as well as with various endocrinopathies, the most common of which is hyperparathyroidism. (See Table 3, below.) The shedding of CPPD crystals initiates an inflammatory process, and these crystals invoke an inflammatory response in much the same manner as uric acid crystals.
While the precipitants of a pseudogout attack are less well defined than those of gout, dehydration and joint surgery have both been identified as predisposing factors. The acute monoarticular pain and swelling (the knee is most common, followed by the ankle and then any other synovial joint) that ensues usually has a more insidious onset, and an X-ray may show chondrocalcinosis within the joint space. The diagnosis is confirmed by the demonstration of intracellular CPPD crystals in the aspirated joint fluid. Though less easily seen than monosodium urate crystals, rhomboidal crystals that display weakly positive birefringence under polarized light will be revealed with careful observation. Vitally important to the diagnosis of any crystal-associated arthritis is the exclusion of septic arthritis. To this end, conduct synovial fluid and blood cultures even if the suspicion of sepsis is low.
Treatment goals for pseudogout center on the abatement of the current arthritis and the exclusion of an infected joint or a concurrent metabolic syndrome. NSAIDs are the mainstay of therapy for the management of pseudogout; they are prescribed in anti-inflammatory doses similar to those used in the treatment of gout. Corticosteroids can also be used, particularly an intra-articular injection, as long as infection has been excluded. As with any crystal arthropathy, a septic joint should be considered and treated in high-risk patients even before the results of the joint fluid cultures are available. (See Table 4, above.)
Septic Arthritis
Even with timely antibacterial treatment, an elderly patient with a septic joint has a 7% to 32% mortality rate. Staphylococcus and Streptococcus are the most commonly cultured pathogens, but consider E. coli, Pseudomonas, and Klebsiella species in patients with diabetes mellitus, malignancy, or other debilitating chronic syndromes; less common agents include tuberculosis and gonococcus. Fever may be present, but a recent study revealed that fewer than 60% of geriatric patients with septic arthritis presented with a febrile illness. Thus, systemic features are not reliable enough to warrant making or excluding the diagnosis of septic arthritis without examination of the synovial fluid. (See Table 5, below.)
Send synovial fluid for leukocyte count, Gram’s stain, and culture in all suspected cases, and several studies suggest that the diagnostic yield may be improved with direct inoculation of fluid into blood culture vials or isolator tubes. Synovial fluid will also show very low glucose (less than 25% of simultaneous plasma glucose) and very high lactate (greater than 10 mm/l) in the untreated bacterial septic joint.
Treatment of a septic joint includes both appropriate antimicrobial therapy and joint drainage. Three weeks of parenteral antimicrobial therapy directed against the isolated pathogen is usually sufficient once the affected joint has been drained. Surgical drainage is indicated in joints—like the hip—that are difficult to aspirate or monitor. Other indications include pus in the synovial fluid, spread of infection to the soft tissues, or an inadequate clinical response to appropriate antibiotics after five to seven days. Otherwise, daily aspiration is the treatment of choice for an uncomplicated infected joint. Additionally, as is true in any acute monoarthritis, bed rest and optimal joint positioning are required to prevent the occurrence of joint deformation and harmful contractures. TH
Dr. Landis is a rheumatologist and a freelance writer.
Special thanks to Bradley Flansbaum, MD, for his assistance with this article.
References
- Tenenbaum J. Inflammatory musculoskeletal conditions in older adults. Geriatr Aging. 2005;8(3):14-17.
- Bieber JD, Terkeltaub RA. Gout: on the brink of novel therapeutic options for an ancient disease. Arthritis Rheum. 2004 Aug;50(8):2400-2414.
- Terkeltaub RA. Clinical practice. Gout. N Engl J Med. 2003 Oct 23;349(17):1647-1655.
- Leirisalo-Repo M. Early arthritis and infection. Curr Opin Rheumatol. 2005 Jul;17(4):433-439.
- Siva C, Velazquez C, Mody A, et al. Diagnosing acute monoarthritis in adults: a practical approach for the family physician. Am Fam Physician. 2003 Jul 1;68(1):83-90.
Mrs. K, an 81-year-old golf-enthusiast admitted with congestive heart failure, now refuses to walk and complains of ankle pain. When you see her, she refuses to let even a bedsheet near her left ankle, and she claims that you did this to her.
Unfortunately, she’s probably right. Mrs. K also has a history of podagra, and she developed an acute gouty monoarthritis after receiving treatment with diuretics and aspirin. Gout—along with the other causes of inpatient acute monoarthritis (pseudogout, septic arthritis, and trauma)—are increasingly common diagnoses in the geriatric patient population. Because the elderly are uniquely predisposed to losing functional independence following an acute attack, making a timely diagnosis is particularly important in this age group. And though the patient’s clinical features may point toward an etiology, making the correct diagnosis ultimately depends on the results of the joint tap.
Gout
Gout occurs in patients with high serum levels of uric acid, though not all hyperuricemic patients develop gout. Among elderly hospitalized patients with hyperuricemia, approximately 65% have significant renal impairment, and others have advanced hypertension, coronary artery disease, and congestive heart failure. Over time, high serum uric acid levels may lead to the deposition of monosodium urate crystals in the joints; these lesions are the precursors of a gouty attack.
Gouty attacks occur when crystal deposits become inflamed. The inflammation may be triggered by a medication-induced change in uric acid concentration or by a medical condition, including acute illness, trauma, surgery, and dehydration. (See Table 1, p.18.) Though some elderly patients with acute gouty arthritis will manifest confusion or a sudden change in ambulatory status, most will present with a monoarthritis and a rise in temperature. Gout is suspected clinically when the first metatarsal phalangeal joint is involved (podagra), but other commonly involved joints include the ankle and the knee. Uric acid levels in the blood are usually elevated but can also temporarily normalize or even dip low during attacks. An X-ray of the involved joint may be normal or may already show the characteristic erosive lesion of gout, the “overhanging edge.”
Making the correct diagnosis is dependent upon visualizing intracellular, needle-shaped, negatively birefringent crystals in the synovial fluid. After the fluid is removed from the affected joint, it should be examined under polarized light within two hours; if more time is needed, the fluid can be refrigerated for up to 12 hours. Co-infection of a gouty joint has been described in the elderly population, and the synovial fluid should be sent to the laboratory for a cell count, Gram’s stain, and culture, as well as glucose and lactate levels. (See Table 2, below.)
Direct in-hospital treatment of gout toward alleviating the current attack, preventing future attacks, and providing appropriate antibiotic coverage in suspected co-infected cases until joint culture results are finalized. Treatment options for the acute attack include NSAIDs and corticosteroids—either oral or intra-articular. Hourly oral colchicine is not a good option for an elderly patient because the diarrhea that ensues is particularly disruptive. Nor is IV colchicine a good option thanks to its side-effect profile that renders it unusable in patients with reduced renal function. The treatment of hyperuricemia with allopurinol should not be undertaken during the acute attack because any change in the serum uric acid concentration will serve only to exacerbate the current inflammation.
Anti-inflammatory doses of NSAIDs are effective and will shorten the duration of symptoms substantially. Seven to 10 days of indomethacin at a dose of 50 mg taken orally three times daily is the traditional choice, and though it is generally conceded that ibuprofen and naproxen also work well, no comparative trials have been performed. Elderly patients are at increased risk for adverse effects from NSAIDs, particularly those patients with severely reduced renal function, gastropathy, asthma, congestive heart failure, or other intravascularly depleted states. Gastric mucosal protection, using proton-pump inhibitors, and careful monitoring of fluid status, renal function, and mental status are of particular concern in this population.
Because recent research indicates that COX-2 inhibitors have thrombotic potential and are contraindicated in patients at high risk for cardiovascular events or stroke, the extent to which they can be used in an elderly patient with an acute gouty attack is limited. A traditional NSAID in combination with a proton-pump inhibitor may be as effective as a COX-2 inhibitor in reducing the risk of gastroduodenal toxicity, however.
Corticosteroids—given either orally or intra-articularly—are an appropriate treatment for patients who can’t tolerate an NSAID. As long as a septic joint has been excluded, an intra-articular injection of 40–80 mg triamcinolone acetonide or 40 mg of methylprednisolone acetate will result in major improvement within 24 hours for most patients. Another option is a seven- to 10-day course of oral prednisone, starting with 40 mg on day one and reducing the dosage by 5 mg/day. Elderly patients taking oral prednisone should also receive adequate calcium, vitamin D, and a proton pump inhibitor for gastrointestinal protection, as well as close monitoring of blood pressure, glucose, and mental status.
If a patient has a history of frequent attacks or tophi, has a serum uric acid level higher than 12 mg/dl, or is consistently receiving high doses of diuretics, that person is at high risk for subsequent attacks and should receive prophylactic treatment with either a low-dose daily NSAID or a renally dosed oral colchicine.
Pseudogout
Pseudogout is the articular manifestation of calcium pyrophosphate dihydrate (CPPD) deposition, and this process is associated with aging as well as with various endocrinopathies, the most common of which is hyperparathyroidism. (See Table 3, below.) The shedding of CPPD crystals initiates an inflammatory process, and these crystals invoke an inflammatory response in much the same manner as uric acid crystals.
While the precipitants of a pseudogout attack are less well defined than those of gout, dehydration and joint surgery have both been identified as predisposing factors. The acute monoarticular pain and swelling (the knee is most common, followed by the ankle and then any other synovial joint) that ensues usually has a more insidious onset, and an X-ray may show chondrocalcinosis within the joint space. The diagnosis is confirmed by the demonstration of intracellular CPPD crystals in the aspirated joint fluid. Though less easily seen than monosodium urate crystals, rhomboidal crystals that display weakly positive birefringence under polarized light will be revealed with careful observation. Vitally important to the diagnosis of any crystal-associated arthritis is the exclusion of septic arthritis. To this end, conduct synovial fluid and blood cultures even if the suspicion of sepsis is low.
Treatment goals for pseudogout center on the abatement of the current arthritis and the exclusion of an infected joint or a concurrent metabolic syndrome. NSAIDs are the mainstay of therapy for the management of pseudogout; they are prescribed in anti-inflammatory doses similar to those used in the treatment of gout. Corticosteroids can also be used, particularly an intra-articular injection, as long as infection has been excluded. As with any crystal arthropathy, a septic joint should be considered and treated in high-risk patients even before the results of the joint fluid cultures are available. (See Table 4, above.)
Septic Arthritis
Even with timely antibacterial treatment, an elderly patient with a septic joint has a 7% to 32% mortality rate. Staphylococcus and Streptococcus are the most commonly cultured pathogens, but consider E. coli, Pseudomonas, and Klebsiella species in patients with diabetes mellitus, malignancy, or other debilitating chronic syndromes; less common agents include tuberculosis and gonococcus. Fever may be present, but a recent study revealed that fewer than 60% of geriatric patients with septic arthritis presented with a febrile illness. Thus, systemic features are not reliable enough to warrant making or excluding the diagnosis of septic arthritis without examination of the synovial fluid. (See Table 5, below.)
Send synovial fluid for leukocyte count, Gram’s stain, and culture in all suspected cases, and several studies suggest that the diagnostic yield may be improved with direct inoculation of fluid into blood culture vials or isolator tubes. Synovial fluid will also show very low glucose (less than 25% of simultaneous plasma glucose) and very high lactate (greater than 10 mm/l) in the untreated bacterial septic joint.
Treatment of a septic joint includes both appropriate antimicrobial therapy and joint drainage. Three weeks of parenteral antimicrobial therapy directed against the isolated pathogen is usually sufficient once the affected joint has been drained. Surgical drainage is indicated in joints—like the hip—that are difficult to aspirate or monitor. Other indications include pus in the synovial fluid, spread of infection to the soft tissues, or an inadequate clinical response to appropriate antibiotics after five to seven days. Otherwise, daily aspiration is the treatment of choice for an uncomplicated infected joint. Additionally, as is true in any acute monoarthritis, bed rest and optimal joint positioning are required to prevent the occurrence of joint deformation and harmful contractures. TH
Dr. Landis is a rheumatologist and a freelance writer.
Special thanks to Bradley Flansbaum, MD, for his assistance with this article.
References
- Tenenbaum J. Inflammatory musculoskeletal conditions in older adults. Geriatr Aging. 2005;8(3):14-17.
- Bieber JD, Terkeltaub RA. Gout: on the brink of novel therapeutic options for an ancient disease. Arthritis Rheum. 2004 Aug;50(8):2400-2414.
- Terkeltaub RA. Clinical practice. Gout. N Engl J Med. 2003 Oct 23;349(17):1647-1655.
- Leirisalo-Repo M. Early arthritis and infection. Curr Opin Rheumatol. 2005 Jul;17(4):433-439.
- Siva C, Velazquez C, Mody A, et al. Diagnosing acute monoarthritis in adults: a practical approach for the family physician. Am Fam Physician. 2003 Jul 1;68(1):83-90.
Mrs. K, an 81-year-old golf-enthusiast admitted with congestive heart failure, now refuses to walk and complains of ankle pain. When you see her, she refuses to let even a bedsheet near her left ankle, and she claims that you did this to her.
Unfortunately, she’s probably right. Mrs. K also has a history of podagra, and she developed an acute gouty monoarthritis after receiving treatment with diuretics and aspirin. Gout—along with the other causes of inpatient acute monoarthritis (pseudogout, septic arthritis, and trauma)—are increasingly common diagnoses in the geriatric patient population. Because the elderly are uniquely predisposed to losing functional independence following an acute attack, making a timely diagnosis is particularly important in this age group. And though the patient’s clinical features may point toward an etiology, making the correct diagnosis ultimately depends on the results of the joint tap.
Gout
Gout occurs in patients with high serum levels of uric acid, though not all hyperuricemic patients develop gout. Among elderly hospitalized patients with hyperuricemia, approximately 65% have significant renal impairment, and others have advanced hypertension, coronary artery disease, and congestive heart failure. Over time, high serum uric acid levels may lead to the deposition of monosodium urate crystals in the joints; these lesions are the precursors of a gouty attack.
Gouty attacks occur when crystal deposits become inflamed. The inflammation may be triggered by a medication-induced change in uric acid concentration or by a medical condition, including acute illness, trauma, surgery, and dehydration. (See Table 1, p.18.) Though some elderly patients with acute gouty arthritis will manifest confusion or a sudden change in ambulatory status, most will present with a monoarthritis and a rise in temperature. Gout is suspected clinically when the first metatarsal phalangeal joint is involved (podagra), but other commonly involved joints include the ankle and the knee. Uric acid levels in the blood are usually elevated but can also temporarily normalize or even dip low during attacks. An X-ray of the involved joint may be normal or may already show the characteristic erosive lesion of gout, the “overhanging edge.”
Making the correct diagnosis is dependent upon visualizing intracellular, needle-shaped, negatively birefringent crystals in the synovial fluid. After the fluid is removed from the affected joint, it should be examined under polarized light within two hours; if more time is needed, the fluid can be refrigerated for up to 12 hours. Co-infection of a gouty joint has been described in the elderly population, and the synovial fluid should be sent to the laboratory for a cell count, Gram’s stain, and culture, as well as glucose and lactate levels. (See Table 2, below.)
Direct in-hospital treatment of gout toward alleviating the current attack, preventing future attacks, and providing appropriate antibiotic coverage in suspected co-infected cases until joint culture results are finalized. Treatment options for the acute attack include NSAIDs and corticosteroids—either oral or intra-articular. Hourly oral colchicine is not a good option for an elderly patient because the diarrhea that ensues is particularly disruptive. Nor is IV colchicine a good option thanks to its side-effect profile that renders it unusable in patients with reduced renal function. The treatment of hyperuricemia with allopurinol should not be undertaken during the acute attack because any change in the serum uric acid concentration will serve only to exacerbate the current inflammation.
Anti-inflammatory doses of NSAIDs are effective and will shorten the duration of symptoms substantially. Seven to 10 days of indomethacin at a dose of 50 mg taken orally three times daily is the traditional choice, and though it is generally conceded that ibuprofen and naproxen also work well, no comparative trials have been performed. Elderly patients are at increased risk for adverse effects from NSAIDs, particularly those patients with severely reduced renal function, gastropathy, asthma, congestive heart failure, or other intravascularly depleted states. Gastric mucosal protection, using proton-pump inhibitors, and careful monitoring of fluid status, renal function, and mental status are of particular concern in this population.
Because recent research indicates that COX-2 inhibitors have thrombotic potential and are contraindicated in patients at high risk for cardiovascular events or stroke, the extent to which they can be used in an elderly patient with an acute gouty attack is limited. A traditional NSAID in combination with a proton-pump inhibitor may be as effective as a COX-2 inhibitor in reducing the risk of gastroduodenal toxicity, however.
Corticosteroids—given either orally or intra-articularly—are an appropriate treatment for patients who can’t tolerate an NSAID. As long as a septic joint has been excluded, an intra-articular injection of 40–80 mg triamcinolone acetonide or 40 mg of methylprednisolone acetate will result in major improvement within 24 hours for most patients. Another option is a seven- to 10-day course of oral prednisone, starting with 40 mg on day one and reducing the dosage by 5 mg/day. Elderly patients taking oral prednisone should also receive adequate calcium, vitamin D, and a proton pump inhibitor for gastrointestinal protection, as well as close monitoring of blood pressure, glucose, and mental status.
If a patient has a history of frequent attacks or tophi, has a serum uric acid level higher than 12 mg/dl, or is consistently receiving high doses of diuretics, that person is at high risk for subsequent attacks and should receive prophylactic treatment with either a low-dose daily NSAID or a renally dosed oral colchicine.
Pseudogout
Pseudogout is the articular manifestation of calcium pyrophosphate dihydrate (CPPD) deposition, and this process is associated with aging as well as with various endocrinopathies, the most common of which is hyperparathyroidism. (See Table 3, below.) The shedding of CPPD crystals initiates an inflammatory process, and these crystals invoke an inflammatory response in much the same manner as uric acid crystals.
While the precipitants of a pseudogout attack are less well defined than those of gout, dehydration and joint surgery have both been identified as predisposing factors. The acute monoarticular pain and swelling (the knee is most common, followed by the ankle and then any other synovial joint) that ensues usually has a more insidious onset, and an X-ray may show chondrocalcinosis within the joint space. The diagnosis is confirmed by the demonstration of intracellular CPPD crystals in the aspirated joint fluid. Though less easily seen than monosodium urate crystals, rhomboidal crystals that display weakly positive birefringence under polarized light will be revealed with careful observation. Vitally important to the diagnosis of any crystal-associated arthritis is the exclusion of septic arthritis. To this end, conduct synovial fluid and blood cultures even if the suspicion of sepsis is low.
Treatment goals for pseudogout center on the abatement of the current arthritis and the exclusion of an infected joint or a concurrent metabolic syndrome. NSAIDs are the mainstay of therapy for the management of pseudogout; they are prescribed in anti-inflammatory doses similar to those used in the treatment of gout. Corticosteroids can also be used, particularly an intra-articular injection, as long as infection has been excluded. As with any crystal arthropathy, a septic joint should be considered and treated in high-risk patients even before the results of the joint fluid cultures are available. (See Table 4, above.)
Septic Arthritis
Even with timely antibacterial treatment, an elderly patient with a septic joint has a 7% to 32% mortality rate. Staphylococcus and Streptococcus are the most commonly cultured pathogens, but consider E. coli, Pseudomonas, and Klebsiella species in patients with diabetes mellitus, malignancy, or other debilitating chronic syndromes; less common agents include tuberculosis and gonococcus. Fever may be present, but a recent study revealed that fewer than 60% of geriatric patients with septic arthritis presented with a febrile illness. Thus, systemic features are not reliable enough to warrant making or excluding the diagnosis of septic arthritis without examination of the synovial fluid. (See Table 5, below.)
Send synovial fluid for leukocyte count, Gram’s stain, and culture in all suspected cases, and several studies suggest that the diagnostic yield may be improved with direct inoculation of fluid into blood culture vials or isolator tubes. Synovial fluid will also show very low glucose (less than 25% of simultaneous plasma glucose) and very high lactate (greater than 10 mm/l) in the untreated bacterial septic joint.
Treatment of a septic joint includes both appropriate antimicrobial therapy and joint drainage. Three weeks of parenteral antimicrobial therapy directed against the isolated pathogen is usually sufficient once the affected joint has been drained. Surgical drainage is indicated in joints—like the hip—that are difficult to aspirate or monitor. Other indications include pus in the synovial fluid, spread of infection to the soft tissues, or an inadequate clinical response to appropriate antibiotics after five to seven days. Otherwise, daily aspiration is the treatment of choice for an uncomplicated infected joint. Additionally, as is true in any acute monoarthritis, bed rest and optimal joint positioning are required to prevent the occurrence of joint deformation and harmful contractures. TH
Dr. Landis is a rheumatologist and a freelance writer.
Special thanks to Bradley Flansbaum, MD, for his assistance with this article.
References
- Tenenbaum J. Inflammatory musculoskeletal conditions in older adults. Geriatr Aging. 2005;8(3):14-17.
- Bieber JD, Terkeltaub RA. Gout: on the brink of novel therapeutic options for an ancient disease. Arthritis Rheum. 2004 Aug;50(8):2400-2414.
- Terkeltaub RA. Clinical practice. Gout. N Engl J Med. 2003 Oct 23;349(17):1647-1655.
- Leirisalo-Repo M. Early arthritis and infection. Curr Opin Rheumatol. 2005 Jul;17(4):433-439.
- Siva C, Velazquez C, Mody A, et al. Diagnosing acute monoarthritis in adults: a practical approach for the family physician. Am Fam Physician. 2003 Jul 1;68(1):83-90.
Safety after Surgery
An 86-year-old female with Alzheimer’s dementia, hypertension, type 2 diabetes, and chronic obstructive pulmonary disease was admitted with lethargy, fever, and vomiting. After she was diagnosed with necrotizing cholecystitis, she underwent an emergent cholecystectomy. Three days later the patient was short of breath, confused, and hadn’t urinated since the indwelling catheter was removed.
Sound familiar? If this scenario doesn’t ring a bell now, then it soon will. The 65-and-up age group is the fastest growing section of the United States population. A recent poll found that elderly patients now account for more than 60% of most general surgeons’ practices. Additionally, the use of minimally invasive surgical techniques and advanced perioperative monitoring has permitted elderly patients who were previously considered too debilitated to now become surgical candidates.
Though patients and their families most often worry about events in the operating room, the vast majority of complications occur in the postoperative period. Morbidity and mortality rates double during the first 24 hours after surgery and are tenfold higher over the remainder of the first postoperative week. In a recent study of more than 500 elderly general surgery patients, 21% experienced complications during this period.
The most common postoperative complications in the geriatric population include delirium, ileus, nutritional deficiencies, respiratory complications—including pulmonary embolism—and urinary retention. The goal in managing any elderly patient is to preserve cognitive and physical function. Maintaining this goal in the postoperative setting requires the early implementation of preventive measures, as well as an understanding of when age-appropriate intervention is necessary.
Hospitalists are often the first line of defense for postoperative situations in medically ill patients, and an amplification of issues unique to the geriatric patient follows.
Delirium
Postoperative delirium occurs in 10%-15% of older general surgery patients and in 30%-60% of older patients who undergo orthopedic procedures. The most common presentation of delirium in the elderly postoperative patient is a “quiet confusion” that is more pronounced in the evening—otherwise known as sundowning. An acute change in mental status, manifested as a fluctuating level of consciousness or a cognitive deficit, is also common. Though delirium may result solely from the acute stress of the operation, other medically relevant causes include metabolic abnormalities, abnormal respiratory parameters, infections, and medications, and these causes should be aggressively investigated and treated.
After potential medical etiologies have been addressed, focus the treatment of delirium in the elderly postoperative patient on interventions to restore mental and physical function as well as pharmacotherapy. Measures to restore function, such as early mobilization and ambulation, sleep hygiene, volume repletion, and restoration of vision and hearing with appropriate devices, have been shown to decrease the duration of the delirium episode. Other non-pharmacologic interventions, including placing a patient near the nurses’ station, encouraging social visits with caregivers, and avoiding the use of physical restraints (which can aggravate agitation) may also prove helpful.
Avoid the use of psychoactive medications (e.g., antiarrhythmic agents, tricyclic antidepressants, neuroleptics, gastrointestinal medications, antihistamines, ciprofloxacin, nonsteroidal anti-inflammatory drugs, meperidine, and cimetidine) as much as possible during the acute confusional state.
Pharmacologic treatment of delirium may be warranted in patients experiencing symptoms of psychosis or in those exhibiting signs of physical aggression or severe personal distress. Haloperidol and risperidol are the medications of choice, though the FDA has approved neither drug specifically for this indication. High doses of these medications are associated with extrapyramidal effects, dystonic reactions, and torsade de pointe. Once the delirium begins to resolve, doses should be tapered gradually over several days.
Ileus
Postsurgical ileus can cause profound clinical consequences in elderly patients. This complication is associated with delayed enteral feeding and malnutrition, increased length of hospital stay, and increased risk of pulmonary complications. Patients present with abdominal distension, nausea and vomiting, limited flatus, and a decreased presence of bowel sounds on auscultation. In cases of prolonged postsurgical ileus, consider pseudo-obstruction (Ogilvie’s syndrome) and mechanical obstruction.
Intravenous hydration and nutrition (in prolonged cases), assisted ambulation, and the avoidance of opiates remain the mainstays of treatment. Nasogastric tubes may provide symptomatic relief in patients with nausea and vomiting, but studies don’t support the use of this intervention to enhance resolution of the ileus. Many prokinetic agents have been examined for this use, including neostigmine and cisapride, but the results have been mixed, and the side effect profiles are generally unacceptable for elderly patients. Delay oral feeding until satisfactory bowel function has been restored.
Nutritional Care
An estimated 12%-50% of geriatric patients are found to be malnourished in the acute hospital setting. The adverse effects of malnutrition include delayed wound healing, greater risk of sepsis and wound infections, deterioration of functional status secondary to muscle wasting, and increased mortality.
Early identification of the patient’s feeding limitations is the key to preventing adverse outcomes. If a patient is restricted from oral or enteral feeding, parenteral nutrition should be started within 48 hours. When volitional food intake is permitted, the addition of canned nutritional supplements, fortified meals, and between-meal snacks may improve elderly patients’ energy and protein intake.
Initiate enteral feeding in patients for whom voluntary food intake is decreased. Parenteral nutrition may still be required until enteral feeding is established, however, and prescribed nutrients can be administered enterally. Because glucose tolerance diminishes with normal aging and may be further reduced in a state of acute illness, initiation of insulin therapy may be necessary in patients receiving either enteral or parenteral supplementation. Additionally, supplementation with a zinc-containing daily multivitamin has been shown to enhance immune function and prevent infections.
Respiratory Care
Respiratory function may be diminished in elderly patients due to age-related changes in the upper and lower respiratory tracts. Factors that contribute to an increased rate of pulmonary postoperative complications include diminished protective mechanisms like coughing and swallowing, decreased compliance of the chest wall and lung tissue, inadequate mucociliary transport, and a blunted ventilatory response to hypoxia and hypercapnia. Postoperative respiratory complications, including pneumonia, hypoxemia, hypoventilation, and atelectasis, occur in 2.1%-10.2% of elderly patients. These complications are associated with increased length of stay and a higher risk of long-term mortality.
Respiratory function may be preserved in the postoperative geriatric patient using a variety of measures. Effective pain control is essential in maintaining adequate lung volumes, and regional analgesia is associated with less-severe postoperative decreases in vital capacity and functional residual capacity (FRC). Once postoperative pain has been controlled, encourage the early resumption of physical activity (with appropriate assistance). Positioning patients in a seated position increases FRC and improves gas exchange in those recovering from abdominal procedures. Additionally, incentive spirometers, breathing exercises, and intermittent positive-pressure breathing may reduce the incidence of pulmonary complications after upper-abdominal operations, shortening the length of hospital stay.
Thromboembolic Disease
Fatal pulmonary embolism accounts for a large proportion of postoperative deaths in the elderly population. Between 20%-30% of patients undergoing general surgery without prophylaxis develop deep vein thrombosis, and the incidence is as high as 40% in those undergoing orthopedic surgeries, gynecologic cancer operations, and major neurosurgical procedures.
The Fifth American College of Chest Physicians Consensus Conference on Antithrombotic Therapy recommends the following postoperative interventions for older surgical patients:
- General surgery without clinical risk factors for thrombosis: Give low-dose unfractionated heparin two hours before and every 12 hours after the operation;
- General surgery with any clinical risk factors such as prolonged immobilization or paralysis, obesity, varicose veins, congestive heart failure, or pelvic or leg fractures: Administer low molecular weight heparin (LMWH) or low-dose unfractionated heparin every eight hours. If the patient is also prone to bleeding or infection, intermittent pneumatic compression (IPC) can be used instead;
- General surgery with multiple clinical risk factors or with a history of previous deep vein thrombosis, malignancy, stroke, spinal cord injury, or hip fracture: Use low dose unfractionated heparin or LMWH combined with intermittent pneumatic compression; for very high risk patients, perioperative warfarin is an alternative;
- Total hip replacement: Give postoperative LMWH every 12 hours; initiate low-intensity warfarin therapy—to keep International Normalized Ratio of 2-3—preoperatively or immediately postoperatively;
- Total knee replacement: Administer postoperative LMWH every 12 hours. IPC is the most effective non-pharmacologic regimen and is comparable to LMWH. Low-intensity warfarin can also be used; and
- Hip fracture repair: Start preoperative fixed-dose LMWH or low-intensity warfarin.
Urinary Retention
The incidence of postoperative urinary retention in elderly patients has been reported to be as high as 87%. Factors contributing to the development of this complication include immobility, analgesics and opiates, intravenous hydration, and general anesthesia. Urinary retention can lead to overflow incontinence and urinary tract infection and is associated with a decline in function and nursing home placement. The first indication of urinary retention may be a diminished urinary output after removal of an indwelling catheter, overflow incontinence, or the frequent voiding of small amounts of urine.
Urinary retention is treated with catheterization. This prevents bladder distension, which leads to reduced detrusor contractile function, and helps restore preoperative bladder function.
Recent studies have found that normal voiding resumes earlier with the use of intermittent catheterization (if begun at the onset of urinary retention and repeated every six to eight hours) than with the use of an indwelling catheter. Additionally, the use of indwelling catheters in the elderly after the immediate perioperative period is associated with an increased risk of urosepsis and a more dependent postoperative functional status.
Conclusion
The 65-and-up age group is the fastest growing section of the United States population. The vast majority of complications for this age group occur in the postoperative period. It’s important for hospitalists to remain involved in key areas of postoperative complications in the geriatric population—specifically, delirium, ileus, nutritional deficiencies, respiratory complications—including pulmonary embolism—and urinary retention. TH
Jill Landis is a frequent contributor to The Hospitalist.
References
- Souders JE, Rooke GA. Perioperative care for geriatric patients. Ann Long Term Care. 2005;13(6):17-29.
- Williams SL, Jones PB, Pofahl WE. Preoperative management of the older patient—a surgeon’s perspective: part I. Ann Long Term Care. 2006;14(6):24-30.
- Palmer RM. Management of common clinical disorders in geriatric patients: delirium. ACP Medicine Online. June 7, 2006. Available at: www.medscape.com/viewarticle/534766. Last accessed January 11, 2007.
- Manku K, Bacchetti P, Leung JM. Prognostic significance of postoperative in-hospital complications in elderly patients. I. Long-term survival. Anesth Analg. 2003 Feb;96(2):583-589.
- Watters JM, McClaran JC, Man-Son-Hing M. The elderly surgical patient: introduction. ACS Surgery Online. June 7, 2006. Available at: www.medscape.com/viewarticle/535461?rss. Last accessed January 11, 2006.
- Skelly JM, Guyatt GH, Kalbfleisch R, et al. Management of urinary retention after surgical repair of hip fracture. CMAJ. 1992 Apr 1;146(7):1185-1189.
- Wittbrodt E. The impact of postoperative ileus and emerging therapies. Pharm Treatment. 2006 Jan;31(1):39-59.
An 86-year-old female with Alzheimer’s dementia, hypertension, type 2 diabetes, and chronic obstructive pulmonary disease was admitted with lethargy, fever, and vomiting. After she was diagnosed with necrotizing cholecystitis, she underwent an emergent cholecystectomy. Three days later the patient was short of breath, confused, and hadn’t urinated since the indwelling catheter was removed.
Sound familiar? If this scenario doesn’t ring a bell now, then it soon will. The 65-and-up age group is the fastest growing section of the United States population. A recent poll found that elderly patients now account for more than 60% of most general surgeons’ practices. Additionally, the use of minimally invasive surgical techniques and advanced perioperative monitoring has permitted elderly patients who were previously considered too debilitated to now become surgical candidates.
Though patients and their families most often worry about events in the operating room, the vast majority of complications occur in the postoperative period. Morbidity and mortality rates double during the first 24 hours after surgery and are tenfold higher over the remainder of the first postoperative week. In a recent study of more than 500 elderly general surgery patients, 21% experienced complications during this period.
The most common postoperative complications in the geriatric population include delirium, ileus, nutritional deficiencies, respiratory complications—including pulmonary embolism—and urinary retention. The goal in managing any elderly patient is to preserve cognitive and physical function. Maintaining this goal in the postoperative setting requires the early implementation of preventive measures, as well as an understanding of when age-appropriate intervention is necessary.
Hospitalists are often the first line of defense for postoperative situations in medically ill patients, and an amplification of issues unique to the geriatric patient follows.
Delirium
Postoperative delirium occurs in 10%-15% of older general surgery patients and in 30%-60% of older patients who undergo orthopedic procedures. The most common presentation of delirium in the elderly postoperative patient is a “quiet confusion” that is more pronounced in the evening—otherwise known as sundowning. An acute change in mental status, manifested as a fluctuating level of consciousness or a cognitive deficit, is also common. Though delirium may result solely from the acute stress of the operation, other medically relevant causes include metabolic abnormalities, abnormal respiratory parameters, infections, and medications, and these causes should be aggressively investigated and treated.
After potential medical etiologies have been addressed, focus the treatment of delirium in the elderly postoperative patient on interventions to restore mental and physical function as well as pharmacotherapy. Measures to restore function, such as early mobilization and ambulation, sleep hygiene, volume repletion, and restoration of vision and hearing with appropriate devices, have been shown to decrease the duration of the delirium episode. Other non-pharmacologic interventions, including placing a patient near the nurses’ station, encouraging social visits with caregivers, and avoiding the use of physical restraints (which can aggravate agitation) may also prove helpful.
Avoid the use of psychoactive medications (e.g., antiarrhythmic agents, tricyclic antidepressants, neuroleptics, gastrointestinal medications, antihistamines, ciprofloxacin, nonsteroidal anti-inflammatory drugs, meperidine, and cimetidine) as much as possible during the acute confusional state.
Pharmacologic treatment of delirium may be warranted in patients experiencing symptoms of psychosis or in those exhibiting signs of physical aggression or severe personal distress. Haloperidol and risperidol are the medications of choice, though the FDA has approved neither drug specifically for this indication. High doses of these medications are associated with extrapyramidal effects, dystonic reactions, and torsade de pointe. Once the delirium begins to resolve, doses should be tapered gradually over several days.
Ileus
Postsurgical ileus can cause profound clinical consequences in elderly patients. This complication is associated with delayed enteral feeding and malnutrition, increased length of hospital stay, and increased risk of pulmonary complications. Patients present with abdominal distension, nausea and vomiting, limited flatus, and a decreased presence of bowel sounds on auscultation. In cases of prolonged postsurgical ileus, consider pseudo-obstruction (Ogilvie’s syndrome) and mechanical obstruction.
Intravenous hydration and nutrition (in prolonged cases), assisted ambulation, and the avoidance of opiates remain the mainstays of treatment. Nasogastric tubes may provide symptomatic relief in patients with nausea and vomiting, but studies don’t support the use of this intervention to enhance resolution of the ileus. Many prokinetic agents have been examined for this use, including neostigmine and cisapride, but the results have been mixed, and the side effect profiles are generally unacceptable for elderly patients. Delay oral feeding until satisfactory bowel function has been restored.
Nutritional Care
An estimated 12%-50% of geriatric patients are found to be malnourished in the acute hospital setting. The adverse effects of malnutrition include delayed wound healing, greater risk of sepsis and wound infections, deterioration of functional status secondary to muscle wasting, and increased mortality.
Early identification of the patient’s feeding limitations is the key to preventing adverse outcomes. If a patient is restricted from oral or enteral feeding, parenteral nutrition should be started within 48 hours. When volitional food intake is permitted, the addition of canned nutritional supplements, fortified meals, and between-meal snacks may improve elderly patients’ energy and protein intake.
Initiate enteral feeding in patients for whom voluntary food intake is decreased. Parenteral nutrition may still be required until enteral feeding is established, however, and prescribed nutrients can be administered enterally. Because glucose tolerance diminishes with normal aging and may be further reduced in a state of acute illness, initiation of insulin therapy may be necessary in patients receiving either enteral or parenteral supplementation. Additionally, supplementation with a zinc-containing daily multivitamin has been shown to enhance immune function and prevent infections.
Respiratory Care
Respiratory function may be diminished in elderly patients due to age-related changes in the upper and lower respiratory tracts. Factors that contribute to an increased rate of pulmonary postoperative complications include diminished protective mechanisms like coughing and swallowing, decreased compliance of the chest wall and lung tissue, inadequate mucociliary transport, and a blunted ventilatory response to hypoxia and hypercapnia. Postoperative respiratory complications, including pneumonia, hypoxemia, hypoventilation, and atelectasis, occur in 2.1%-10.2% of elderly patients. These complications are associated with increased length of stay and a higher risk of long-term mortality.
Respiratory function may be preserved in the postoperative geriatric patient using a variety of measures. Effective pain control is essential in maintaining adequate lung volumes, and regional analgesia is associated with less-severe postoperative decreases in vital capacity and functional residual capacity (FRC). Once postoperative pain has been controlled, encourage the early resumption of physical activity (with appropriate assistance). Positioning patients in a seated position increases FRC and improves gas exchange in those recovering from abdominal procedures. Additionally, incentive spirometers, breathing exercises, and intermittent positive-pressure breathing may reduce the incidence of pulmonary complications after upper-abdominal operations, shortening the length of hospital stay.
Thromboembolic Disease
Fatal pulmonary embolism accounts for a large proportion of postoperative deaths in the elderly population. Between 20%-30% of patients undergoing general surgery without prophylaxis develop deep vein thrombosis, and the incidence is as high as 40% in those undergoing orthopedic surgeries, gynecologic cancer operations, and major neurosurgical procedures.
The Fifth American College of Chest Physicians Consensus Conference on Antithrombotic Therapy recommends the following postoperative interventions for older surgical patients:
- General surgery without clinical risk factors for thrombosis: Give low-dose unfractionated heparin two hours before and every 12 hours after the operation;
- General surgery with any clinical risk factors such as prolonged immobilization or paralysis, obesity, varicose veins, congestive heart failure, or pelvic or leg fractures: Administer low molecular weight heparin (LMWH) or low-dose unfractionated heparin every eight hours. If the patient is also prone to bleeding or infection, intermittent pneumatic compression (IPC) can be used instead;
- General surgery with multiple clinical risk factors or with a history of previous deep vein thrombosis, malignancy, stroke, spinal cord injury, or hip fracture: Use low dose unfractionated heparin or LMWH combined with intermittent pneumatic compression; for very high risk patients, perioperative warfarin is an alternative;
- Total hip replacement: Give postoperative LMWH every 12 hours; initiate low-intensity warfarin therapy—to keep International Normalized Ratio of 2-3—preoperatively or immediately postoperatively;
- Total knee replacement: Administer postoperative LMWH every 12 hours. IPC is the most effective non-pharmacologic regimen and is comparable to LMWH. Low-intensity warfarin can also be used; and
- Hip fracture repair: Start preoperative fixed-dose LMWH or low-intensity warfarin.
Urinary Retention
The incidence of postoperative urinary retention in elderly patients has been reported to be as high as 87%. Factors contributing to the development of this complication include immobility, analgesics and opiates, intravenous hydration, and general anesthesia. Urinary retention can lead to overflow incontinence and urinary tract infection and is associated with a decline in function and nursing home placement. The first indication of urinary retention may be a diminished urinary output after removal of an indwelling catheter, overflow incontinence, or the frequent voiding of small amounts of urine.
Urinary retention is treated with catheterization. This prevents bladder distension, which leads to reduced detrusor contractile function, and helps restore preoperative bladder function.
Recent studies have found that normal voiding resumes earlier with the use of intermittent catheterization (if begun at the onset of urinary retention and repeated every six to eight hours) than with the use of an indwelling catheter. Additionally, the use of indwelling catheters in the elderly after the immediate perioperative period is associated with an increased risk of urosepsis and a more dependent postoperative functional status.
Conclusion
The 65-and-up age group is the fastest growing section of the United States population. The vast majority of complications for this age group occur in the postoperative period. It’s important for hospitalists to remain involved in key areas of postoperative complications in the geriatric population—specifically, delirium, ileus, nutritional deficiencies, respiratory complications—including pulmonary embolism—and urinary retention. TH
Jill Landis is a frequent contributor to The Hospitalist.
References
- Souders JE, Rooke GA. Perioperative care for geriatric patients. Ann Long Term Care. 2005;13(6):17-29.
- Williams SL, Jones PB, Pofahl WE. Preoperative management of the older patient—a surgeon’s perspective: part I. Ann Long Term Care. 2006;14(6):24-30.
- Palmer RM. Management of common clinical disorders in geriatric patients: delirium. ACP Medicine Online. June 7, 2006. Available at: www.medscape.com/viewarticle/534766. Last accessed January 11, 2007.
- Manku K, Bacchetti P, Leung JM. Prognostic significance of postoperative in-hospital complications in elderly patients. I. Long-term survival. Anesth Analg. 2003 Feb;96(2):583-589.
- Watters JM, McClaran JC, Man-Son-Hing M. The elderly surgical patient: introduction. ACS Surgery Online. June 7, 2006. Available at: www.medscape.com/viewarticle/535461?rss. Last accessed January 11, 2006.
- Skelly JM, Guyatt GH, Kalbfleisch R, et al. Management of urinary retention after surgical repair of hip fracture. CMAJ. 1992 Apr 1;146(7):1185-1189.
- Wittbrodt E. The impact of postoperative ileus and emerging therapies. Pharm Treatment. 2006 Jan;31(1):39-59.
An 86-year-old female with Alzheimer’s dementia, hypertension, type 2 diabetes, and chronic obstructive pulmonary disease was admitted with lethargy, fever, and vomiting. After she was diagnosed with necrotizing cholecystitis, she underwent an emergent cholecystectomy. Three days later the patient was short of breath, confused, and hadn’t urinated since the indwelling catheter was removed.
Sound familiar? If this scenario doesn’t ring a bell now, then it soon will. The 65-and-up age group is the fastest growing section of the United States population. A recent poll found that elderly patients now account for more than 60% of most general surgeons’ practices. Additionally, the use of minimally invasive surgical techniques and advanced perioperative monitoring has permitted elderly patients who were previously considered too debilitated to now become surgical candidates.
Though patients and their families most often worry about events in the operating room, the vast majority of complications occur in the postoperative period. Morbidity and mortality rates double during the first 24 hours after surgery and are tenfold higher over the remainder of the first postoperative week. In a recent study of more than 500 elderly general surgery patients, 21% experienced complications during this period.
The most common postoperative complications in the geriatric population include delirium, ileus, nutritional deficiencies, respiratory complications—including pulmonary embolism—and urinary retention. The goal in managing any elderly patient is to preserve cognitive and physical function. Maintaining this goal in the postoperative setting requires the early implementation of preventive measures, as well as an understanding of when age-appropriate intervention is necessary.
Hospitalists are often the first line of defense for postoperative situations in medically ill patients, and an amplification of issues unique to the geriatric patient follows.
Delirium
Postoperative delirium occurs in 10%-15% of older general surgery patients and in 30%-60% of older patients who undergo orthopedic procedures. The most common presentation of delirium in the elderly postoperative patient is a “quiet confusion” that is more pronounced in the evening—otherwise known as sundowning. An acute change in mental status, manifested as a fluctuating level of consciousness or a cognitive deficit, is also common. Though delirium may result solely from the acute stress of the operation, other medically relevant causes include metabolic abnormalities, abnormal respiratory parameters, infections, and medications, and these causes should be aggressively investigated and treated.
After potential medical etiologies have been addressed, focus the treatment of delirium in the elderly postoperative patient on interventions to restore mental and physical function as well as pharmacotherapy. Measures to restore function, such as early mobilization and ambulation, sleep hygiene, volume repletion, and restoration of vision and hearing with appropriate devices, have been shown to decrease the duration of the delirium episode. Other non-pharmacologic interventions, including placing a patient near the nurses’ station, encouraging social visits with caregivers, and avoiding the use of physical restraints (which can aggravate agitation) may also prove helpful.
Avoid the use of psychoactive medications (e.g., antiarrhythmic agents, tricyclic antidepressants, neuroleptics, gastrointestinal medications, antihistamines, ciprofloxacin, nonsteroidal anti-inflammatory drugs, meperidine, and cimetidine) as much as possible during the acute confusional state.
Pharmacologic treatment of delirium may be warranted in patients experiencing symptoms of psychosis or in those exhibiting signs of physical aggression or severe personal distress. Haloperidol and risperidol are the medications of choice, though the FDA has approved neither drug specifically for this indication. High doses of these medications are associated with extrapyramidal effects, dystonic reactions, and torsade de pointe. Once the delirium begins to resolve, doses should be tapered gradually over several days.
Ileus
Postsurgical ileus can cause profound clinical consequences in elderly patients. This complication is associated with delayed enteral feeding and malnutrition, increased length of hospital stay, and increased risk of pulmonary complications. Patients present with abdominal distension, nausea and vomiting, limited flatus, and a decreased presence of bowel sounds on auscultation. In cases of prolonged postsurgical ileus, consider pseudo-obstruction (Ogilvie’s syndrome) and mechanical obstruction.
Intravenous hydration and nutrition (in prolonged cases), assisted ambulation, and the avoidance of opiates remain the mainstays of treatment. Nasogastric tubes may provide symptomatic relief in patients with nausea and vomiting, but studies don’t support the use of this intervention to enhance resolution of the ileus. Many prokinetic agents have been examined for this use, including neostigmine and cisapride, but the results have been mixed, and the side effect profiles are generally unacceptable for elderly patients. Delay oral feeding until satisfactory bowel function has been restored.
Nutritional Care
An estimated 12%-50% of geriatric patients are found to be malnourished in the acute hospital setting. The adverse effects of malnutrition include delayed wound healing, greater risk of sepsis and wound infections, deterioration of functional status secondary to muscle wasting, and increased mortality.
Early identification of the patient’s feeding limitations is the key to preventing adverse outcomes. If a patient is restricted from oral or enteral feeding, parenteral nutrition should be started within 48 hours. When volitional food intake is permitted, the addition of canned nutritional supplements, fortified meals, and between-meal snacks may improve elderly patients’ energy and protein intake.
Initiate enteral feeding in patients for whom voluntary food intake is decreased. Parenteral nutrition may still be required until enteral feeding is established, however, and prescribed nutrients can be administered enterally. Because glucose tolerance diminishes with normal aging and may be further reduced in a state of acute illness, initiation of insulin therapy may be necessary in patients receiving either enteral or parenteral supplementation. Additionally, supplementation with a zinc-containing daily multivitamin has been shown to enhance immune function and prevent infections.
Respiratory Care
Respiratory function may be diminished in elderly patients due to age-related changes in the upper and lower respiratory tracts. Factors that contribute to an increased rate of pulmonary postoperative complications include diminished protective mechanisms like coughing and swallowing, decreased compliance of the chest wall and lung tissue, inadequate mucociliary transport, and a blunted ventilatory response to hypoxia and hypercapnia. Postoperative respiratory complications, including pneumonia, hypoxemia, hypoventilation, and atelectasis, occur in 2.1%-10.2% of elderly patients. These complications are associated with increased length of stay and a higher risk of long-term mortality.
Respiratory function may be preserved in the postoperative geriatric patient using a variety of measures. Effective pain control is essential in maintaining adequate lung volumes, and regional analgesia is associated with less-severe postoperative decreases in vital capacity and functional residual capacity (FRC). Once postoperative pain has been controlled, encourage the early resumption of physical activity (with appropriate assistance). Positioning patients in a seated position increases FRC and improves gas exchange in those recovering from abdominal procedures. Additionally, incentive spirometers, breathing exercises, and intermittent positive-pressure breathing may reduce the incidence of pulmonary complications after upper-abdominal operations, shortening the length of hospital stay.
Thromboembolic Disease
Fatal pulmonary embolism accounts for a large proportion of postoperative deaths in the elderly population. Between 20%-30% of patients undergoing general surgery without prophylaxis develop deep vein thrombosis, and the incidence is as high as 40% in those undergoing orthopedic surgeries, gynecologic cancer operations, and major neurosurgical procedures.
The Fifth American College of Chest Physicians Consensus Conference on Antithrombotic Therapy recommends the following postoperative interventions for older surgical patients:
- General surgery without clinical risk factors for thrombosis: Give low-dose unfractionated heparin two hours before and every 12 hours after the operation;
- General surgery with any clinical risk factors such as prolonged immobilization or paralysis, obesity, varicose veins, congestive heart failure, or pelvic or leg fractures: Administer low molecular weight heparin (LMWH) or low-dose unfractionated heparin every eight hours. If the patient is also prone to bleeding or infection, intermittent pneumatic compression (IPC) can be used instead;
- General surgery with multiple clinical risk factors or with a history of previous deep vein thrombosis, malignancy, stroke, spinal cord injury, or hip fracture: Use low dose unfractionated heparin or LMWH combined with intermittent pneumatic compression; for very high risk patients, perioperative warfarin is an alternative;
- Total hip replacement: Give postoperative LMWH every 12 hours; initiate low-intensity warfarin therapy—to keep International Normalized Ratio of 2-3—preoperatively or immediately postoperatively;
- Total knee replacement: Administer postoperative LMWH every 12 hours. IPC is the most effective non-pharmacologic regimen and is comparable to LMWH. Low-intensity warfarin can also be used; and
- Hip fracture repair: Start preoperative fixed-dose LMWH or low-intensity warfarin.
Urinary Retention
The incidence of postoperative urinary retention in elderly patients has been reported to be as high as 87%. Factors contributing to the development of this complication include immobility, analgesics and opiates, intravenous hydration, and general anesthesia. Urinary retention can lead to overflow incontinence and urinary tract infection and is associated with a decline in function and nursing home placement. The first indication of urinary retention may be a diminished urinary output after removal of an indwelling catheter, overflow incontinence, or the frequent voiding of small amounts of urine.
Urinary retention is treated with catheterization. This prevents bladder distension, which leads to reduced detrusor contractile function, and helps restore preoperative bladder function.
Recent studies have found that normal voiding resumes earlier with the use of intermittent catheterization (if begun at the onset of urinary retention and repeated every six to eight hours) than with the use of an indwelling catheter. Additionally, the use of indwelling catheters in the elderly after the immediate perioperative period is associated with an increased risk of urosepsis and a more dependent postoperative functional status.
Conclusion
The 65-and-up age group is the fastest growing section of the United States population. The vast majority of complications for this age group occur in the postoperative period. It’s important for hospitalists to remain involved in key areas of postoperative complications in the geriatric population—specifically, delirium, ileus, nutritional deficiencies, respiratory complications—including pulmonary embolism—and urinary retention. TH
Jill Landis is a frequent contributor to The Hospitalist.
References
- Souders JE, Rooke GA. Perioperative care for geriatric patients. Ann Long Term Care. 2005;13(6):17-29.
- Williams SL, Jones PB, Pofahl WE. Preoperative management of the older patient—a surgeon’s perspective: part I. Ann Long Term Care. 2006;14(6):24-30.
- Palmer RM. Management of common clinical disorders in geriatric patients: delirium. ACP Medicine Online. June 7, 2006. Available at: www.medscape.com/viewarticle/534766. Last accessed January 11, 2007.
- Manku K, Bacchetti P, Leung JM. Prognostic significance of postoperative in-hospital complications in elderly patients. I. Long-term survival. Anesth Analg. 2003 Feb;96(2):583-589.
- Watters JM, McClaran JC, Man-Son-Hing M. The elderly surgical patient: introduction. ACS Surgery Online. June 7, 2006. Available at: www.medscape.com/viewarticle/535461?rss. Last accessed January 11, 2006.
- Skelly JM, Guyatt GH, Kalbfleisch R, et al. Management of urinary retention after surgical repair of hip fracture. CMAJ. 1992 Apr 1;146(7):1185-1189.
- Wittbrodt E. The impact of postoperative ileus and emerging therapies. Pharm Treatment. 2006 Jan;31(1):39-59.