User login
Botanical Briefs: Neem Oil (Azadirachta indica)
Commonly known as neem or nimba, Azadirachta indica traditionally has been used as an oil or poultice to lighten skin pigment and reduce joint inflammation. Neem is a drought-resistant evergreen tree with thin serrated leaves, white fragrant flowers, and olivelike fruit (Figure 1). This plant is indigenous to India but also is readily found within tropical and semitropical environments throughout the Middle East, Southeast Asia, North Africa, and Australia.

Traditional Uses
For more than 4000 years, neem leaves, bark, fruit, and seeds have been used in food, insecticide, and herbal medicine cross-culturally in Indian Ayurvedic medicine and across Southeast Asia, particularly in Cambodia, Laos, Thailand, Myanmar, and Vietnam.1-3 Because of its many essential nutrients—oleic acid, palmitic acid, stearic acid, linoleic acid, behenic acid, arachidic acid, and palmitoleic acid—and readily available nature, some ethnic groups include neem in their diet.4 Neem commonly is used as a seasoning in soups and rice, eaten as a cooked vegetable, infused into teas and tonics, and pickled with other spices.5
All parts of the neem tree—both externally and internally—have been utilized in traditional medicine for the treatment of various diseases and ailments. The flowers have been used to treat eye diseases and dyspepsia, the fruit has been employed as an anthelmintic, the seeds and leaves have been used for malaria treatment and insecticide, the stem bark has been used for the treatment of diarrhea, and the root bark has been used for skin diseases and inflammation.6 Neem oil is a yellow-brown bitter substance that often is utilized to treat skin diseases such as psoriasis, eczema, fungal infections, and abscesses.
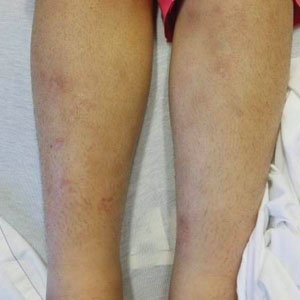
Case Report—A 77-year-old man presented with a diffuse rash across the lower back. He reported that he had been using topical neem oil to alleviate lower back pain and arthritis for the last 6 months with noted relief and improvement of back pain. After roughly 3 to 4 months of using neem oil, he noted a rash on the lower back, bilateral flanks, and buttocks (Figure 2). The rash was asymptomatic, and he denied any pruritus, scaling, pain, or burning. The patient was referred to dermatology and received a diagnosis of chemical leukoderma secondary to contact with A indica. The patient was advised to stop using the topical neem oil, and the rash was simply monitored, as it was asymptomatic.
Bioactivity
Research has elucidated multiple bioactivity mechanisms of neem, including melanogenesis-inhibitory activity, toxicity against pests, antimalarial activity, and antioxidant activity.1,7-9 Literature on the diverse phytochemical components of A indica indicate high levels of limonoids, flavonoids, and triterpenoids that are responsible for much of its antioxidant, anti-inflammatory, and insecticide properties.1,10
Melanogenesis-Inhibitory Activity—To date, neem has been added to a number of cosmetic products used in Ayurvedic medicine. One study of isolated compounds in A indica showed superior inhibitory activities against melanogenesis with minimal toxicity to cells (86.5%–105.1% cell viability). Western blot analysis of samples extracted and isolated from neem root and bark showed melanogenesis-inhibitory activities in B16 melanoma cells through the inhibition of microphthalmia-associated transcription factor expression and decreased expression of tyrosinase, as well as tyrosinase-related proteins 1 and 2, which are largely responsible for melanin synthesis.11 In another study, A indica flowers and their extracted constituents—6-deacetylnimbin and kaempferide—suggest melanogenesis-inhibitory activities in B16 melanoma cells with little to no toxicity to the cells (81.0%–111.7% cell viability).1 In an evaluationof A indica seed extracts, some of the isolated limonoids and diterpenoids exhibited a marked melanogenesis-inhibitory effect (74%–91% reduction of melanin content) with no toxicity to the cell.5 All of these studies indicate that active compounds in neem root, bark, flowers, and seeds may be potential skin-lightening agents.
Toxicity Against Pests—Neem seeds have phytochemicals that convey some insecticidal properties. The seeds often are ground into a powder, combined with water, and sprayed onto crops to act as an insecticide. As a natural method of nonpesticidal management, A indica acts as an antifeedant, insect repellent, and egg-laying deterrent that protects crops from damage. Studies of A indica have noted effective nonpesticidal management against arthropod pests such as armyworm, termites, and the oriental fruit fly.7,12,13
Antimalarial Activity—One study indicated that nimbolide, a limonoid from the neem plant, demonstrated antimalarial activity against Plasmodium falciparum. In separate cultures of asexual parasites and mature gametocytes, parasite numbers were less than 50% of the number in control cultures (8.0% vs 8.5% parasitemia, respectively).14 Thus, the lower parasite numbers indicated by this study highlight the antimalarial utility of nimbolide and neem oil.
Antioxidant and Anti-inflammatory Activity—Neem bark has been reported to have considerable antioxidant activity due to its high phenolic content.1,15 One study showed that azadirachtin and nimbolide in neem exhibited concentration-dependent antiradical scavenging activity and antioxidant properties.16
The anti-inflammatory potential for neem may occur via the inhibition of the nuclear factor-κB signaling pathway, which is linked to cancer, inflammation, and apoptosis.17 It also has been observed that nimbidin within neem extracts—such as leaves, bark, and seed extract—suppresses the function of macrophages and neutrophils relevant to inflammation.16 Another study indicated neem’s anti-inflammatory activity due to the regulation of proinflammatory enzymes such as cyclooxygenase and lipoxygenase.18
Safety, Toxicity, and Risks
Ingestion—Although neem is safe to use in the general population, neem oil poisoning has been reported, particularly in young children. Ingesting large quantities of neem has resulted in vomiting, hepatic toxicity, metabolic acidosis, late neurologic sequelae, and encephalopathy in young children.19 The diagnosis of neem oil poisoning is based on patient history, clinical examination, and imaging findings. Poisoning can manifest as drowsiness, tachypnea, and generalized seizures.20
Topical Application—Topical use of neem appears to be safe if the substance is diluted with other ingredients. However, direct application to the skin is not advised, as it may cause leukoderma and could induce allergic contact dermatitis and other allergic reactions.4
Final Thoughts
The use of neem extract for disease prevention and treatment has been prevalent around the world since ancient times. Neem has been documented to possess melanogenesis-inhibitory activity, toxicity against pests, antimalarial activity, and antioxidant activity by means of tyrosinase inhibition, phytochemical production, limonoid expression, and nuclear factor-κB regulation, respectively. However, topical use of neem may trigger a cutaneous response, highlighting the importance of considering a diagnosis of neem oil–induced chemical leukoderma when patients present with a hypopigmented rash and relevant history.
- Kitdamrongtham W, Ishii K, Ebina K, et al. Limonoids and flavonoids from the flowers of Azadirachta indica var. siamensis, and their melanogenesis-inhibitory and cytotoxic activities. Chem Biodivers. 2014;11:73-84. doi:10.1002/cbdv.201300266
- Singh A, Srivastava PS, Lakshmikumaran M. Comparison of AFLP and SAMPL markers for assessment of intra-population genetic variation in Azadirachta indica A. Juss. Plant Sci. 2002;162:17-25. doi:10.1016/S0168-9452(01)00503-9
- Pandey G, Verma K, Singh M. Evaluation of phytochemical, antibacterial and free radical scavenging properties of Azadirachta Indica (neem) leaves. Int J Pharm Pharmaceut Sci. 2014;6:444-447.
- Romita P, Calogiuri G, Bellino M, et al. Allergic contact dermatitis caused by neem oil: an underrated allergen. Contact Dermatitis. 2019;81:133-134. doi:10.1111/cod. 13256
- Akihisa T, Noto T, Takahashi A, et al. Melanogenesis inhibitory, anti-inflammatory, and chemopreventive effects of limonoids from the seeds of Azadirachta indica A. Juss. (neem). J Oleo Sci. 2009;58:581-594.
- Subapriya R, Nagini S. Medicinal properties of neem leaves: a review. Curr Med Chem Anticancer Agents. 2005;5:149-156. doi:10.2174/1568011053174828
- Areekul S, Sinchaisri P, Tigvatananon S. Effect of Thai plant extracts on the Oriental fruit fly. I: toxicity test. Agriculture and Natural Resources. 1987;21:395-407.
- Rochanakij S, Thebtaranonth Y, Yenjai C, et al. Nimbolide, a constituent of Azadirachta indica, inhibits Plasmodium falciparum in culture. Southeast Asian J Trop Med Public Health. 1985;16:66-72.
- Sithisarn P, Supabphol R, Gritsanapan W. Antioxidant activity of Siamese neem tree (VP1209). J Ethnopharmacol. 2005;99:109-112. doi:10.1016/j.jep.2005.02.008
- Yin F, Lei XX, Cheng L, et al. Isolation and structure identification of the compounds from the seeds and leaves of Azadirachta indica A. Juss. J China Pharmaceut University. 2005;36:10-12.
- Su S, Cheng J, Zhang C, et al. Melanogenesis-inhibitory activities of limonoids and tricyclic diterpenoids from Azadirachta indica. Bioorganic Chemistry. 2020;100:103941. doi:j.bioorg.2020.103941
- Tulashie SK, Adjei F, Abraham J, et al. Potential of neem extracts as natural insecticide against fall armyworm (Spodoptera frugiperda (JE Smith)(Lepidoptera: Noctuidae). Case Stud Chem Environ Eng. 2021;4:100130. doi:10.1016/j.cscee.2021.100130
- Yashroy RC, Gupta PK. Neem-seed oil inhibits growth of termite surface-tunnels. Indian J Toxicol. 2000;7:49-50.
- Udeinya JI, Shu EN, Quakyi I, et al. An antimalarial neem leaf extract has both schizonticidal and gametocytocidal activities. Am J Therapeutics. 2008;15:108-110. doi:10.1097/MJT.0b013e31804c6d1d
- Bindurani R, Kumar K. Evaluation of antioxidant activity of hydro distilled extracts of leaf, heart wood and flower of Azadirachta indica. Int J Pharm Sci Rev Res. 2013;20:222.
- Alzohairy MA. Therapeutics role of Azadirachta indica (Neem) and their active constituents in diseases prevention and treatment [published online March 1, 2016]. Evid Based Complement Alternat Med. doi:10.1155/2016/7382506
- Schumacher M, Cerella C, Reuter S, et al. Anti-inflammatory, pro-apoptotic, and anti-proliferative effects of a methanolic neem (Azadirachta indica) leaf extract are mediated via modulation of the nuclear factor-κB pathway. Genes Nutr. 2011;6:149-160. doi:10.1007/s12263-010-0194-6
- Kaur G, Sarwar Alam M, Athar M. Nimbidin suppresses functions of macrophages and neutrophils: relevance to its anti-inflammatory mechanisms. Phytotherapy Res. 2004;18:419-424. doi:10.1002/ptr.1474
- Dhongade RK, Kavade SG, Damle RS. Neem oil poisoning. Indian Pediatr. 2008;45:56-57.
- Bhaskar MV, Pramod SJ, Jeevika MU, et al. MR imaging findings of neem oil poisoning. Am J Neuroradiol. 2010;31:E60-E61. doi:10.3174/ajnr.A2146
Commonly known as neem or nimba, Azadirachta indica traditionally has been used as an oil or poultice to lighten skin pigment and reduce joint inflammation. Neem is a drought-resistant evergreen tree with thin serrated leaves, white fragrant flowers, and olivelike fruit (Figure 1). This plant is indigenous to India but also is readily found within tropical and semitropical environments throughout the Middle East, Southeast Asia, North Africa, and Australia.

Traditional Uses
For more than 4000 years, neem leaves, bark, fruit, and seeds have been used in food, insecticide, and herbal medicine cross-culturally in Indian Ayurvedic medicine and across Southeast Asia, particularly in Cambodia, Laos, Thailand, Myanmar, and Vietnam.1-3 Because of its many essential nutrients—oleic acid, palmitic acid, stearic acid, linoleic acid, behenic acid, arachidic acid, and palmitoleic acid—and readily available nature, some ethnic groups include neem in their diet.4 Neem commonly is used as a seasoning in soups and rice, eaten as a cooked vegetable, infused into teas and tonics, and pickled with other spices.5
All parts of the neem tree—both externally and internally—have been utilized in traditional medicine for the treatment of various diseases and ailments. The flowers have been used to treat eye diseases and dyspepsia, the fruit has been employed as an anthelmintic, the seeds and leaves have been used for malaria treatment and insecticide, the stem bark has been used for the treatment of diarrhea, and the root bark has been used for skin diseases and inflammation.6 Neem oil is a yellow-brown bitter substance that often is utilized to treat skin diseases such as psoriasis, eczema, fungal infections, and abscesses.
Case Report—A 77-year-old man presented with a diffuse rash across the lower back. He reported that he had been using topical neem oil to alleviate lower back pain and arthritis for the last 6 months with noted relief and improvement of back pain. After roughly 3 to 4 months of using neem oil, he noted a rash on the lower back, bilateral flanks, and buttocks (Figure 2). The rash was asymptomatic, and he denied any pruritus, scaling, pain, or burning. The patient was referred to dermatology and received a diagnosis of chemical leukoderma secondary to contact with A indica. The patient was advised to stop using the topical neem oil, and the rash was simply monitored, as it was asymptomatic.

Bioactivity
Research has elucidated multiple bioactivity mechanisms of neem, including melanogenesis-inhibitory activity, toxicity against pests, antimalarial activity, and antioxidant activity.1,7-9 Literature on the diverse phytochemical components of A indica indicate high levels of limonoids, flavonoids, and triterpenoids that are responsible for much of its antioxidant, anti-inflammatory, and insecticide properties.1,10
Melanogenesis-Inhibitory Activity—To date, neem has been added to a number of cosmetic products used in Ayurvedic medicine. One study of isolated compounds in A indica showed superior inhibitory activities against melanogenesis with minimal toxicity to cells (86.5%–105.1% cell viability). Western blot analysis of samples extracted and isolated from neem root and bark showed melanogenesis-inhibitory activities in B16 melanoma cells through the inhibition of microphthalmia-associated transcription factor expression and decreased expression of tyrosinase, as well as tyrosinase-related proteins 1 and 2, which are largely responsible for melanin synthesis.11 In another study, A indica flowers and their extracted constituents—6-deacetylnimbin and kaempferide—suggest melanogenesis-inhibitory activities in B16 melanoma cells with little to no toxicity to the cells (81.0%–111.7% cell viability).1 In an evaluationof A indica seed extracts, some of the isolated limonoids and diterpenoids exhibited a marked melanogenesis-inhibitory effect (74%–91% reduction of melanin content) with no toxicity to the cell.5 All of these studies indicate that active compounds in neem root, bark, flowers, and seeds may be potential skin-lightening agents.
Toxicity Against Pests—Neem seeds have phytochemicals that convey some insecticidal properties. The seeds often are ground into a powder, combined with water, and sprayed onto crops to act as an insecticide. As a natural method of nonpesticidal management, A indica acts as an antifeedant, insect repellent, and egg-laying deterrent that protects crops from damage. Studies of A indica have noted effective nonpesticidal management against arthropod pests such as armyworm, termites, and the oriental fruit fly.7,12,13
Antimalarial Activity—One study indicated that nimbolide, a limonoid from the neem plant, demonstrated antimalarial activity against Plasmodium falciparum. In separate cultures of asexual parasites and mature gametocytes, parasite numbers were less than 50% of the number in control cultures (8.0% vs 8.5% parasitemia, respectively).14 Thus, the lower parasite numbers indicated by this study highlight the antimalarial utility of nimbolide and neem oil.
Antioxidant and Anti-inflammatory Activity—Neem bark has been reported to have considerable antioxidant activity due to its high phenolic content.1,15 One study showed that azadirachtin and nimbolide in neem exhibited concentration-dependent antiradical scavenging activity and antioxidant properties.16
The anti-inflammatory potential for neem may occur via the inhibition of the nuclear factor-κB signaling pathway, which is linked to cancer, inflammation, and apoptosis.17 It also has been observed that nimbidin within neem extracts—such as leaves, bark, and seed extract—suppresses the function of macrophages and neutrophils relevant to inflammation.16 Another study indicated neem’s anti-inflammatory activity due to the regulation of proinflammatory enzymes such as cyclooxygenase and lipoxygenase.18
Safety, Toxicity, and Risks
Ingestion—Although neem is safe to use in the general population, neem oil poisoning has been reported, particularly in young children. Ingesting large quantities of neem has resulted in vomiting, hepatic toxicity, metabolic acidosis, late neurologic sequelae, and encephalopathy in young children.19 The diagnosis of neem oil poisoning is based on patient history, clinical examination, and imaging findings. Poisoning can manifest as drowsiness, tachypnea, and generalized seizures.20
Topical Application—Topical use of neem appears to be safe if the substance is diluted with other ingredients. However, direct application to the skin is not advised, as it may cause leukoderma and could induce allergic contact dermatitis and other allergic reactions.4
Final Thoughts
The use of neem extract for disease prevention and treatment has been prevalent around the world since ancient times. Neem has been documented to possess melanogenesis-inhibitory activity, toxicity against pests, antimalarial activity, and antioxidant activity by means of tyrosinase inhibition, phytochemical production, limonoid expression, and nuclear factor-κB regulation, respectively. However, topical use of neem may trigger a cutaneous response, highlighting the importance of considering a diagnosis of neem oil–induced chemical leukoderma when patients present with a hypopigmented rash and relevant history.
Commonly known as neem or nimba, Azadirachta indica traditionally has been used as an oil or poultice to lighten skin pigment and reduce joint inflammation. Neem is a drought-resistant evergreen tree with thin serrated leaves, white fragrant flowers, and olivelike fruit (Figure 1). This plant is indigenous to India but also is readily found within tropical and semitropical environments throughout the Middle East, Southeast Asia, North Africa, and Australia.

Traditional Uses
For more than 4000 years, neem leaves, bark, fruit, and seeds have been used in food, insecticide, and herbal medicine cross-culturally in Indian Ayurvedic medicine and across Southeast Asia, particularly in Cambodia, Laos, Thailand, Myanmar, and Vietnam.1-3 Because of its many essential nutrients—oleic acid, palmitic acid, stearic acid, linoleic acid, behenic acid, arachidic acid, and palmitoleic acid—and readily available nature, some ethnic groups include neem in their diet.4 Neem commonly is used as a seasoning in soups and rice, eaten as a cooked vegetable, infused into teas and tonics, and pickled with other spices.5
All parts of the neem tree—both externally and internally—have been utilized in traditional medicine for the treatment of various diseases and ailments. The flowers have been used to treat eye diseases and dyspepsia, the fruit has been employed as an anthelmintic, the seeds and leaves have been used for malaria treatment and insecticide, the stem bark has been used for the treatment of diarrhea, and the root bark has been used for skin diseases and inflammation.6 Neem oil is a yellow-brown bitter substance that often is utilized to treat skin diseases such as psoriasis, eczema, fungal infections, and abscesses.
Case Report—A 77-year-old man presented with a diffuse rash across the lower back. He reported that he had been using topical neem oil to alleviate lower back pain and arthritis for the last 6 months with noted relief and improvement of back pain. After roughly 3 to 4 months of using neem oil, he noted a rash on the lower back, bilateral flanks, and buttocks (Figure 2). The rash was asymptomatic, and he denied any pruritus, scaling, pain, or burning. The patient was referred to dermatology and received a diagnosis of chemical leukoderma secondary to contact with A indica. The patient was advised to stop using the topical neem oil, and the rash was simply monitored, as it was asymptomatic.

Bioactivity
Research has elucidated multiple bioactivity mechanisms of neem, including melanogenesis-inhibitory activity, toxicity against pests, antimalarial activity, and antioxidant activity.1,7-9 Literature on the diverse phytochemical components of A indica indicate high levels of limonoids, flavonoids, and triterpenoids that are responsible for much of its antioxidant, anti-inflammatory, and insecticide properties.1,10
Melanogenesis-Inhibitory Activity—To date, neem has been added to a number of cosmetic products used in Ayurvedic medicine. One study of isolated compounds in A indica showed superior inhibitory activities against melanogenesis with minimal toxicity to cells (86.5%–105.1% cell viability). Western blot analysis of samples extracted and isolated from neem root and bark showed melanogenesis-inhibitory activities in B16 melanoma cells through the inhibition of microphthalmia-associated transcription factor expression and decreased expression of tyrosinase, as well as tyrosinase-related proteins 1 and 2, which are largely responsible for melanin synthesis.11 In another study, A indica flowers and their extracted constituents—6-deacetylnimbin and kaempferide—suggest melanogenesis-inhibitory activities in B16 melanoma cells with little to no toxicity to the cells (81.0%–111.7% cell viability).1 In an evaluationof A indica seed extracts, some of the isolated limonoids and diterpenoids exhibited a marked melanogenesis-inhibitory effect (74%–91% reduction of melanin content) with no toxicity to the cell.5 All of these studies indicate that active compounds in neem root, bark, flowers, and seeds may be potential skin-lightening agents.
Toxicity Against Pests—Neem seeds have phytochemicals that convey some insecticidal properties. The seeds often are ground into a powder, combined with water, and sprayed onto crops to act as an insecticide. As a natural method of nonpesticidal management, A indica acts as an antifeedant, insect repellent, and egg-laying deterrent that protects crops from damage. Studies of A indica have noted effective nonpesticidal management against arthropod pests such as armyworm, termites, and the oriental fruit fly.7,12,13
Antimalarial Activity—One study indicated that nimbolide, a limonoid from the neem plant, demonstrated antimalarial activity against Plasmodium falciparum. In separate cultures of asexual parasites and mature gametocytes, parasite numbers were less than 50% of the number in control cultures (8.0% vs 8.5% parasitemia, respectively).14 Thus, the lower parasite numbers indicated by this study highlight the antimalarial utility of nimbolide and neem oil.
Antioxidant and Anti-inflammatory Activity—Neem bark has been reported to have considerable antioxidant activity due to its high phenolic content.1,15 One study showed that azadirachtin and nimbolide in neem exhibited concentration-dependent antiradical scavenging activity and antioxidant properties.16
The anti-inflammatory potential for neem may occur via the inhibition of the nuclear factor-κB signaling pathway, which is linked to cancer, inflammation, and apoptosis.17 It also has been observed that nimbidin within neem extracts—such as leaves, bark, and seed extract—suppresses the function of macrophages and neutrophils relevant to inflammation.16 Another study indicated neem’s anti-inflammatory activity due to the regulation of proinflammatory enzymes such as cyclooxygenase and lipoxygenase.18
Safety, Toxicity, and Risks
Ingestion—Although neem is safe to use in the general population, neem oil poisoning has been reported, particularly in young children. Ingesting large quantities of neem has resulted in vomiting, hepatic toxicity, metabolic acidosis, late neurologic sequelae, and encephalopathy in young children.19 The diagnosis of neem oil poisoning is based on patient history, clinical examination, and imaging findings. Poisoning can manifest as drowsiness, tachypnea, and generalized seizures.20
Topical Application—Topical use of neem appears to be safe if the substance is diluted with other ingredients. However, direct application to the skin is not advised, as it may cause leukoderma and could induce allergic contact dermatitis and other allergic reactions.4
Final Thoughts
The use of neem extract for disease prevention and treatment has been prevalent around the world since ancient times. Neem has been documented to possess melanogenesis-inhibitory activity, toxicity against pests, antimalarial activity, and antioxidant activity by means of tyrosinase inhibition, phytochemical production, limonoid expression, and nuclear factor-κB regulation, respectively. However, topical use of neem may trigger a cutaneous response, highlighting the importance of considering a diagnosis of neem oil–induced chemical leukoderma when patients present with a hypopigmented rash and relevant history.
- Kitdamrongtham W, Ishii K, Ebina K, et al. Limonoids and flavonoids from the flowers of Azadirachta indica var. siamensis, and their melanogenesis-inhibitory and cytotoxic activities. Chem Biodivers. 2014;11:73-84. doi:10.1002/cbdv.201300266
- Singh A, Srivastava PS, Lakshmikumaran M. Comparison of AFLP and SAMPL markers for assessment of intra-population genetic variation in Azadirachta indica A. Juss. Plant Sci. 2002;162:17-25. doi:10.1016/S0168-9452(01)00503-9
- Pandey G, Verma K, Singh M. Evaluation of phytochemical, antibacterial and free radical scavenging properties of Azadirachta Indica (neem) leaves. Int J Pharm Pharmaceut Sci. 2014;6:444-447.
- Romita P, Calogiuri G, Bellino M, et al. Allergic contact dermatitis caused by neem oil: an underrated allergen. Contact Dermatitis. 2019;81:133-134. doi:10.1111/cod. 13256
- Akihisa T, Noto T, Takahashi A, et al. Melanogenesis inhibitory, anti-inflammatory, and chemopreventive effects of limonoids from the seeds of Azadirachta indica A. Juss. (neem). J Oleo Sci. 2009;58:581-594.
- Subapriya R, Nagini S. Medicinal properties of neem leaves: a review. Curr Med Chem Anticancer Agents. 2005;5:149-156. doi:10.2174/1568011053174828
- Areekul S, Sinchaisri P, Tigvatananon S. Effect of Thai plant extracts on the Oriental fruit fly. I: toxicity test. Agriculture and Natural Resources. 1987;21:395-407.
- Rochanakij S, Thebtaranonth Y, Yenjai C, et al. Nimbolide, a constituent of Azadirachta indica, inhibits Plasmodium falciparum in culture. Southeast Asian J Trop Med Public Health. 1985;16:66-72.
- Sithisarn P, Supabphol R, Gritsanapan W. Antioxidant activity of Siamese neem tree (VP1209). J Ethnopharmacol. 2005;99:109-112. doi:10.1016/j.jep.2005.02.008
- Yin F, Lei XX, Cheng L, et al. Isolation and structure identification of the compounds from the seeds and leaves of Azadirachta indica A. Juss. J China Pharmaceut University. 2005;36:10-12.
- Su S, Cheng J, Zhang C, et al. Melanogenesis-inhibitory activities of limonoids and tricyclic diterpenoids from Azadirachta indica. Bioorganic Chemistry. 2020;100:103941. doi:j.bioorg.2020.103941
- Tulashie SK, Adjei F, Abraham J, et al. Potential of neem extracts as natural insecticide against fall armyworm (Spodoptera frugiperda (JE Smith)(Lepidoptera: Noctuidae). Case Stud Chem Environ Eng. 2021;4:100130. doi:10.1016/j.cscee.2021.100130
- Yashroy RC, Gupta PK. Neem-seed oil inhibits growth of termite surface-tunnels. Indian J Toxicol. 2000;7:49-50.
- Udeinya JI, Shu EN, Quakyi I, et al. An antimalarial neem leaf extract has both schizonticidal and gametocytocidal activities. Am J Therapeutics. 2008;15:108-110. doi:10.1097/MJT.0b013e31804c6d1d
- Bindurani R, Kumar K. Evaluation of antioxidant activity of hydro distilled extracts of leaf, heart wood and flower of Azadirachta indica. Int J Pharm Sci Rev Res. 2013;20:222.
- Alzohairy MA. Therapeutics role of Azadirachta indica (Neem) and their active constituents in diseases prevention and treatment [published online March 1, 2016]. Evid Based Complement Alternat Med. doi:10.1155/2016/7382506
- Schumacher M, Cerella C, Reuter S, et al. Anti-inflammatory, pro-apoptotic, and anti-proliferative effects of a methanolic neem (Azadirachta indica) leaf extract are mediated via modulation of the nuclear factor-κB pathway. Genes Nutr. 2011;6:149-160. doi:10.1007/s12263-010-0194-6
- Kaur G, Sarwar Alam M, Athar M. Nimbidin suppresses functions of macrophages and neutrophils: relevance to its anti-inflammatory mechanisms. Phytotherapy Res. 2004;18:419-424. doi:10.1002/ptr.1474
- Dhongade RK, Kavade SG, Damle RS. Neem oil poisoning. Indian Pediatr. 2008;45:56-57.
- Bhaskar MV, Pramod SJ, Jeevika MU, et al. MR imaging findings of neem oil poisoning. Am J Neuroradiol. 2010;31:E60-E61. doi:10.3174/ajnr.A2146
- Kitdamrongtham W, Ishii K, Ebina K, et al. Limonoids and flavonoids from the flowers of Azadirachta indica var. siamensis, and their melanogenesis-inhibitory and cytotoxic activities. Chem Biodivers. 2014;11:73-84. doi:10.1002/cbdv.201300266
- Singh A, Srivastava PS, Lakshmikumaran M. Comparison of AFLP and SAMPL markers for assessment of intra-population genetic variation in Azadirachta indica A. Juss. Plant Sci. 2002;162:17-25. doi:10.1016/S0168-9452(01)00503-9
- Pandey G, Verma K, Singh M. Evaluation of phytochemical, antibacterial and free radical scavenging properties of Azadirachta Indica (neem) leaves. Int J Pharm Pharmaceut Sci. 2014;6:444-447.
- Romita P, Calogiuri G, Bellino M, et al. Allergic contact dermatitis caused by neem oil: an underrated allergen. Contact Dermatitis. 2019;81:133-134. doi:10.1111/cod. 13256
- Akihisa T, Noto T, Takahashi A, et al. Melanogenesis inhibitory, anti-inflammatory, and chemopreventive effects of limonoids from the seeds of Azadirachta indica A. Juss. (neem). J Oleo Sci. 2009;58:581-594.
- Subapriya R, Nagini S. Medicinal properties of neem leaves: a review. Curr Med Chem Anticancer Agents. 2005;5:149-156. doi:10.2174/1568011053174828
- Areekul S, Sinchaisri P, Tigvatananon S. Effect of Thai plant extracts on the Oriental fruit fly. I: toxicity test. Agriculture and Natural Resources. 1987;21:395-407.
- Rochanakij S, Thebtaranonth Y, Yenjai C, et al. Nimbolide, a constituent of Azadirachta indica, inhibits Plasmodium falciparum in culture. Southeast Asian J Trop Med Public Health. 1985;16:66-72.
- Sithisarn P, Supabphol R, Gritsanapan W. Antioxidant activity of Siamese neem tree (VP1209). J Ethnopharmacol. 2005;99:109-112. doi:10.1016/j.jep.2005.02.008
- Yin F, Lei XX, Cheng L, et al. Isolation and structure identification of the compounds from the seeds and leaves of Azadirachta indica A. Juss. J China Pharmaceut University. 2005;36:10-12.
- Su S, Cheng J, Zhang C, et al. Melanogenesis-inhibitory activities of limonoids and tricyclic diterpenoids from Azadirachta indica. Bioorganic Chemistry. 2020;100:103941. doi:j.bioorg.2020.103941
- Tulashie SK, Adjei F, Abraham J, et al. Potential of neem extracts as natural insecticide against fall armyworm (Spodoptera frugiperda (JE Smith)(Lepidoptera: Noctuidae). Case Stud Chem Environ Eng. 2021;4:100130. doi:10.1016/j.cscee.2021.100130
- Yashroy RC, Gupta PK. Neem-seed oil inhibits growth of termite surface-tunnels. Indian J Toxicol. 2000;7:49-50.
- Udeinya JI, Shu EN, Quakyi I, et al. An antimalarial neem leaf extract has both schizonticidal and gametocytocidal activities. Am J Therapeutics. 2008;15:108-110. doi:10.1097/MJT.0b013e31804c6d1d
- Bindurani R, Kumar K. Evaluation of antioxidant activity of hydro distilled extracts of leaf, heart wood and flower of Azadirachta indica. Int J Pharm Sci Rev Res. 2013;20:222.
- Alzohairy MA. Therapeutics role of Azadirachta indica (Neem) and their active constituents in diseases prevention and treatment [published online March 1, 2016]. Evid Based Complement Alternat Med. doi:10.1155/2016/7382506
- Schumacher M, Cerella C, Reuter S, et al. Anti-inflammatory, pro-apoptotic, and anti-proliferative effects of a methanolic neem (Azadirachta indica) leaf extract are mediated via modulation of the nuclear factor-κB pathway. Genes Nutr. 2011;6:149-160. doi:10.1007/s12263-010-0194-6
- Kaur G, Sarwar Alam M, Athar M. Nimbidin suppresses functions of macrophages and neutrophils: relevance to its anti-inflammatory mechanisms. Phytotherapy Res. 2004;18:419-424. doi:10.1002/ptr.1474
- Dhongade RK, Kavade SG, Damle RS. Neem oil poisoning. Indian Pediatr. 2008;45:56-57.
- Bhaskar MV, Pramod SJ, Jeevika MU, et al. MR imaging findings of neem oil poisoning. Am J Neuroradiol. 2010;31:E60-E61. doi:10.3174/ajnr.A2146
Practice Points
- Neem is a traditional herb with various bioactivities, such as melanogenesis-inhibitory activity, toxicity against pests, antimalarial activity, and antioxidant activity.
- Neem should be used with caution as a remedy because of its skin-lightening properties, which are attributed to melanogenesis-inhibitory activity via tyrosinase inhibition.
- Chemical leukoderma should be included in the differential diagnosis when a patient presents with a hypopigmented rash after topical use of neem products.
Juvenile Dermatomyositis–Associated Panniculitis
To the Editor:
Juvenile dermatomyositis (JDM) is an autoimmune disorder with childhood onset that predominantly affects the muscles and skin, among other organs. Since the recognition of dermatomyositis (DM) more than 100 years ago, a variety of clinical diagnostic criteria have been utilized. Classically, DM presents with muscle weakness and a pathognomonic cutaneous macular, violaceous, erythematous eruption. The juvenile variant is defined by onset prior to 16 years of age. Histologically, these entities are indistinguishable and demonstrate an interface dermatitis with epidermal atrophy. Clinically, JDM has a higher incidence of calcinosis cutis and is not associated with an increased risk for malignancy in contrast to the adult-onset variant.1 Panniculitis is a rare but serious complication in a subset of patients with DM and may represent a precursor to calcinosis cutis.2 We describe a case of JDM-associated panniculitis that was difficult to control with prednisone and rituximab.
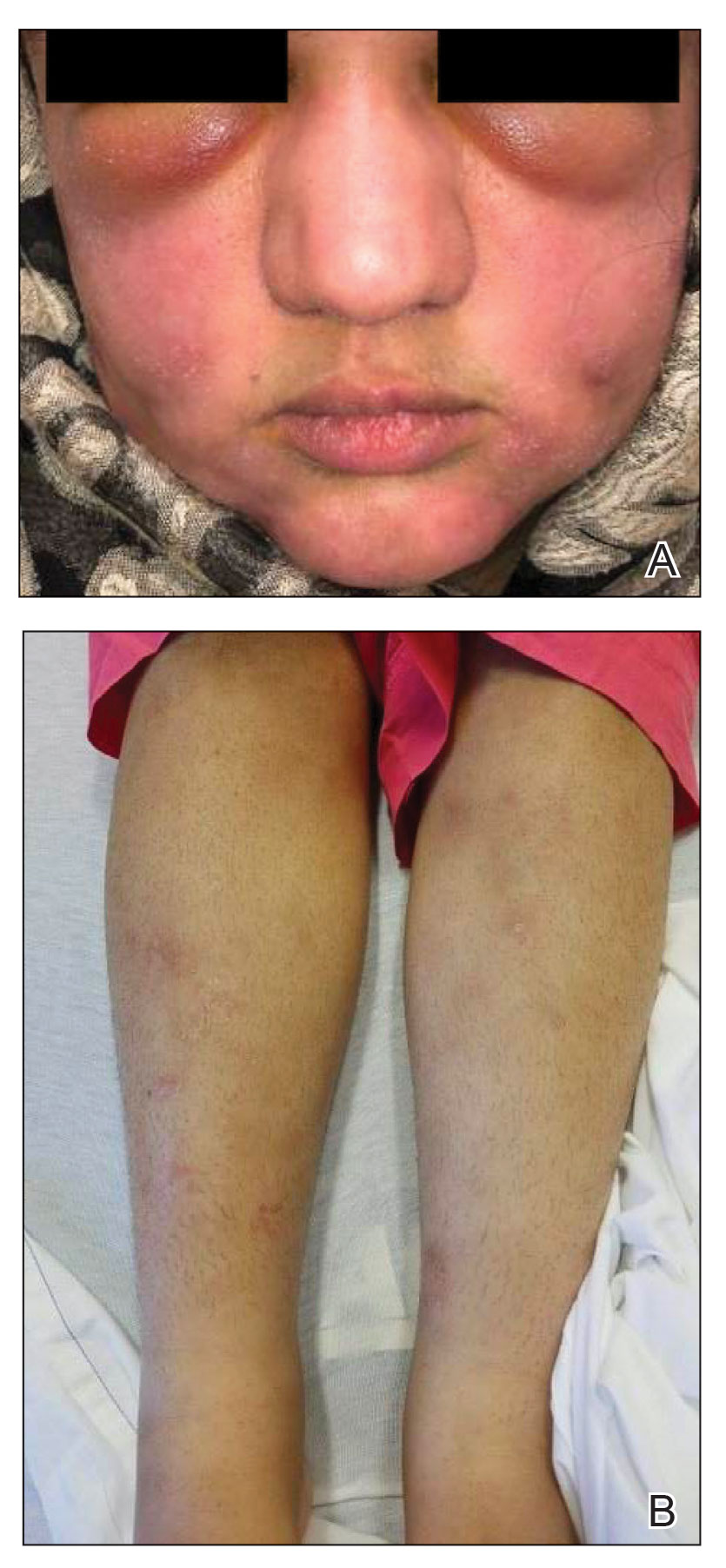
A 21-year-old woman with fever, fatigue, muscle pain, and new-onset swelling of 2 weeks’ duration was admitted to the hospital. She had a 5-year history of intermittent muscle weakness and concomitant rash. Prior to presentation, she had been hospitalized twice for fever of unknown origin, and the source remained undetermined. Physical examination revealed prominent facial and periorbital edema. There was tender nonpitting edema present on all 4 extremities and hyperpigmented indurated nodules on the shins (Figure 1). A full laboratory and imaging workup was performed for autoantibodies and infectious etiologies. The complete blood cell count was notable for pancytopenia, and a thorough infectious workup was negative. Creatine kinase level was within reference range. A biopsy of the right shin was performed, and histopathology revealed a lobular panniculitis with fat necrosis and mixed inflammation with neutrophils with perieccrine involvement as well as an interface dermatitis (Figure 2). Periodic acid–Schiff, Grocott methenamine-silver, and Gram stains were negative. Myositis-specific antibody testing revealed anti-p155/140 autoantibodies, and magnetic resonance imaging did not reveal active myositis within the visualized muscles, consistent with stable nonprogressing DM. A diagnosis of JDM with panniculitis was made. The patient was started on oral prednisone. Subsequently, a trial of rituximab was initiated. Although the patient’s symptoms initially improved, the response was not sustained on rituximab, and the patient was continued on systemic steroids with initiation of cyclosporine.
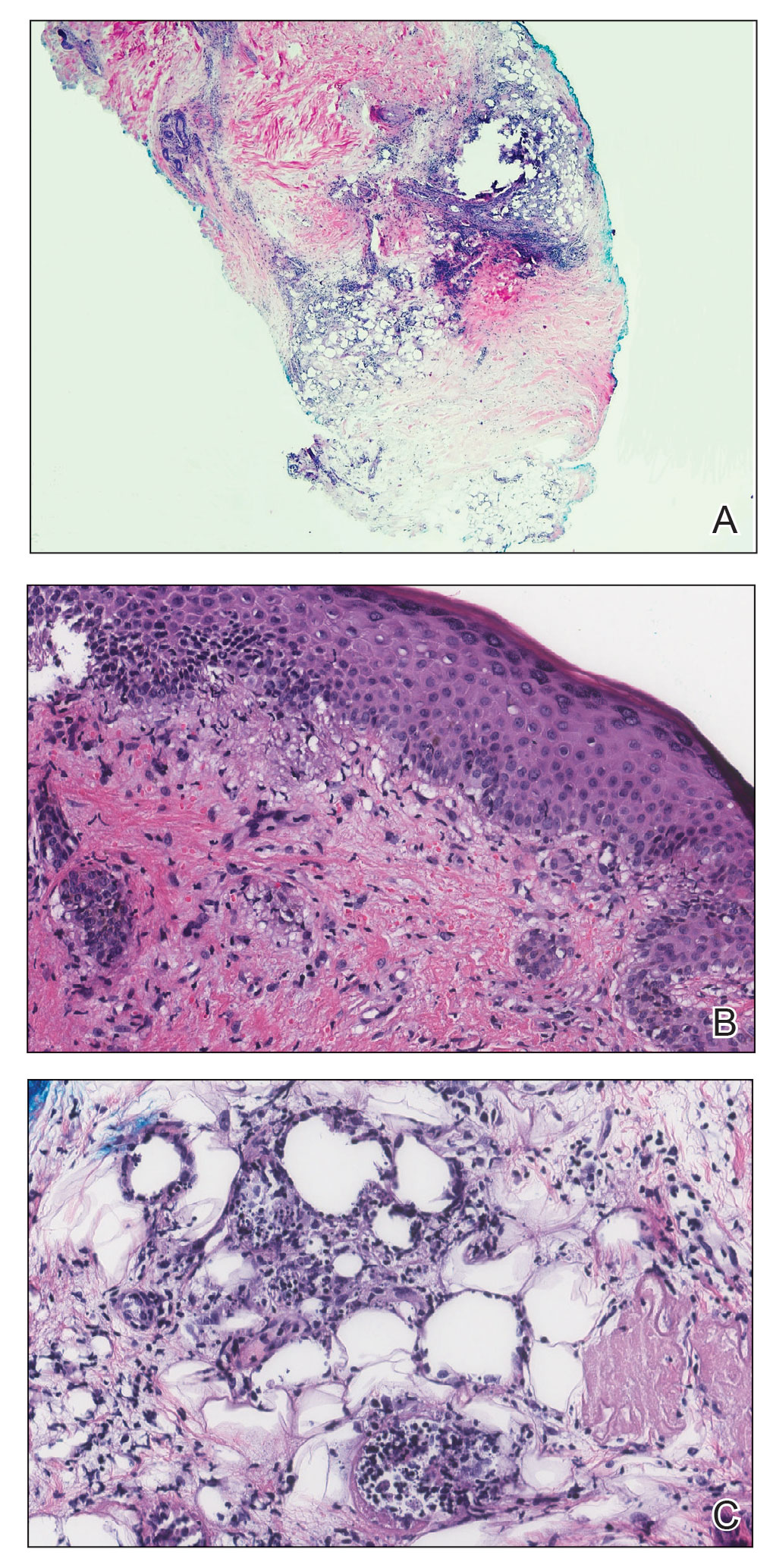
Juvenile dermatomyositis is an autoimmune disorder with childhood onset that involves systemic inflammation of the muscles, skin, and internal organs. It often can present diagnostic and therapeutic challenges.2,3 Bohan and Peter4,5 clinical criteria may help identify potential patients with JDM, but magnetic resonance imaging, electromyography, and muscle biopsy often are required to confirm the diagnosis.6 Skin manifestations include heliotrope rash; V sign; shawl sign; Gottron papules; periorbital edema; and infrequently panniculitis, the subcutaneous inflammation of adipose tissue.3,7
Although panniculitis is found in approximately 10% of skin biopsies in patients with DM, our patient presented with anti-p155/140 antibodies.8-10 Fat involvement in these patients traditionally manifests as lipodystrophy. Panniculitis also may precede calcinosis cutis, a debilitating skin change that may occur in approximately 46% of patients with JDM and can cause severe morbidity.2,6,9
Subcutaneous edema rarely is described in DM-panniculitis, present in only 6% of 86 DM patients in one study.7 The pathophysiology of DM may be due to antibodies that target endothelial cells and activate complement, resulting in the membranolytic attack complex. This leads to microischemia, and microinfarction of the muscle fibers has been suggested to result in edema of the subcutaneous tissue in severe cases.7,11 Microinfarction has been found to be present 2.3 times more often in edematous DM compared with nonedematous DM.7 Subcutaneous edema may be an isolated presentation of DM that arises more quickly with severe disease activity. As such, recommendations have been made to consider edema in future classification schemes.7
Because of the severity of edematous and/or subcutaneous DM, aggressive therapy may be required. First-line therapy consists of corticosteroids with additional immunosuppressants and immunomodulatory agents if adequate response is not achieved.3,12 The effectiveness of rituximab in DM has been suggested.2,12,13 The Rituximab in Myositis (RIM) trial (N=200) was the first double-blind, placebo-controlled, phase 3 clinical trial to assess rituximab’s efficacy in refractory compared with early-onset inflammatory myopathies. Although outcomes were similar in both groups, 83% of patients overall, including the JDM subset, met the definition of improvement.12 In re-examining the RIM trial data and other cases using rituximab to treat inflammatory myopathies, an overall response rate of 78.3% was observed, with 52.1% of patients with DM reporting improvement in skin lesions (N=458, pooled from 48 studies).13 Further analysis of the RIM data revealed that panniculitis affected 10.4% of patients with JDM at baseline, which decreased to 6.8% at 36 weeks of rituximab therapy (N=48).12
As exhibited in our patient, subcutaneous tissue involvement, including calcinosis cutis and panniculitis, is seen more often in JDM than adult DM.2,6 However, panniculitis in anti-p155/140 patients is rare. Our patient also had antibody positivity, which likely predisposed her to a more severe course. Despite not having sustained improvement on rituximab, initiating aggressive therapy earlier in the disease course may be beneficial, and our patient continues with alternative therapies.
- Jorizzo JL, Vleugels RA. Dermatomyositis. In: Bolognia J, Schaffer J, Cerroni L. Dermatology. 4th ed. Elsevier; 2019:681-687.
- Aggarwal R, Loganathan P, Koontz D, et al. Cutaneous improvement in refractory adult and juvenile dermatomyositis after treatment with rituximab. Rheumatology. 2016;56:247-254.
- Santos-Briz A, Calle A, Linos K, et al. Dermatomyositis panniculitis: a clinicopathological and immunohistochemical study of 18 cases. J Eur Acad Dermatol Venereol. 2018;32:1352-1359.
- Bohan A, Peter JB. Polymyositis and dermatomyositis (first of two parts). N Engl J Med. 1975;292:344-347.
- Bohan A, Peter JB. Polymyositis and dermatomyositis (second of two parts). N Engl J Med. 1975;292:403-407.
- Sakurai N, Hino-Shishikura A, Nozawa T, et al. Clinical significance of subcutaneous fat and fascial involvement in juvenile dermatomyositis. Mod Rheumatol. 2019;29:808-813.
- Milisenda JC, Doti PI, Prieto-Gonzalez S, et al. Dermatomyositis presenting with severe subcutaneous edema: five additional cases and review of the literature. Semin Arthritis Rheum. 2014;44:228-233.
- Janis JF, Winkelmann RK. Histopathology of the skin in dermatomyositis: a histopathologic study of 55 cases. Arch Dermatol. 1968;97:640-650.
- van Dongen HM, van Vugt RM, Stoof TJ. Extensive persistent panniculitis in the context of dermatomyositis. J Clin Rheumatol. 2020;26:e187-e188.
- Gunawardena H, Wedderburn LR, North J, et al. Clinical associations of autoantibodies to a p155/140 kDa doublet protein in juvenile dermatomyositis. Rheumatology. 2008;47:324-328.
- Dalakas MC, Hohlfeld R. Polymyositis and dermatomyositis. Lancet. 2003;362:971-982.
- Oddis CV, Reed AM, Aggarwal R, et al. Rituximab in the treatment of refractory adult and juvenile dermatomyositis and adult polymyositis: a randomized, placebo-phase trial. Arthritis Rheum. 2013;65:314-324.
- Fasano S, Gordon P, Hajji R, et al. Rituximab in the treatment of inflammatory myopathies: a review. Rheumatology. 2016;56:26-36.
To the Editor:
Juvenile dermatomyositis (JDM) is an autoimmune disorder with childhood onset that predominantly affects the muscles and skin, among other organs. Since the recognition of dermatomyositis (DM) more than 100 years ago, a variety of clinical diagnostic criteria have been utilized. Classically, DM presents with muscle weakness and a pathognomonic cutaneous macular, violaceous, erythematous eruption. The juvenile variant is defined by onset prior to 16 years of age. Histologically, these entities are indistinguishable and demonstrate an interface dermatitis with epidermal atrophy. Clinically, JDM has a higher incidence of calcinosis cutis and is not associated with an increased risk for malignancy in contrast to the adult-onset variant.1 Panniculitis is a rare but serious complication in a subset of patients with DM and may represent a precursor to calcinosis cutis.2 We describe a case of JDM-associated panniculitis that was difficult to control with prednisone and rituximab.

A 21-year-old woman with fever, fatigue, muscle pain, and new-onset swelling of 2 weeks’ duration was admitted to the hospital. She had a 5-year history of intermittent muscle weakness and concomitant rash. Prior to presentation, she had been hospitalized twice for fever of unknown origin, and the source remained undetermined. Physical examination revealed prominent facial and periorbital edema. There was tender nonpitting edema present on all 4 extremities and hyperpigmented indurated nodules on the shins (Figure 1). A full laboratory and imaging workup was performed for autoantibodies and infectious etiologies. The complete blood cell count was notable for pancytopenia, and a thorough infectious workup was negative. Creatine kinase level was within reference range. A biopsy of the right shin was performed, and histopathology revealed a lobular panniculitis with fat necrosis and mixed inflammation with neutrophils with perieccrine involvement as well as an interface dermatitis (Figure 2). Periodic acid–Schiff, Grocott methenamine-silver, and Gram stains were negative. Myositis-specific antibody testing revealed anti-p155/140 autoantibodies, and magnetic resonance imaging did not reveal active myositis within the visualized muscles, consistent with stable nonprogressing DM. A diagnosis of JDM with panniculitis was made. The patient was started on oral prednisone. Subsequently, a trial of rituximab was initiated. Although the patient’s symptoms initially improved, the response was not sustained on rituximab, and the patient was continued on systemic steroids with initiation of cyclosporine.

Juvenile dermatomyositis is an autoimmune disorder with childhood onset that involves systemic inflammation of the muscles, skin, and internal organs. It often can present diagnostic and therapeutic challenges.2,3 Bohan and Peter4,5 clinical criteria may help identify potential patients with JDM, but magnetic resonance imaging, electromyography, and muscle biopsy often are required to confirm the diagnosis.6 Skin manifestations include heliotrope rash; V sign; shawl sign; Gottron papules; periorbital edema; and infrequently panniculitis, the subcutaneous inflammation of adipose tissue.3,7
Although panniculitis is found in approximately 10% of skin biopsies in patients with DM, our patient presented with anti-p155/140 antibodies.8-10 Fat involvement in these patients traditionally manifests as lipodystrophy. Panniculitis also may precede calcinosis cutis, a debilitating skin change that may occur in approximately 46% of patients with JDM and can cause severe morbidity.2,6,9
Subcutaneous edema rarely is described in DM-panniculitis, present in only 6% of 86 DM patients in one study.7 The pathophysiology of DM may be due to antibodies that target endothelial cells and activate complement, resulting in the membranolytic attack complex. This leads to microischemia, and microinfarction of the muscle fibers has been suggested to result in edema of the subcutaneous tissue in severe cases.7,11 Microinfarction has been found to be present 2.3 times more often in edematous DM compared with nonedematous DM.7 Subcutaneous edema may be an isolated presentation of DM that arises more quickly with severe disease activity. As such, recommendations have been made to consider edema in future classification schemes.7
Because of the severity of edematous and/or subcutaneous DM, aggressive therapy may be required. First-line therapy consists of corticosteroids with additional immunosuppressants and immunomodulatory agents if adequate response is not achieved.3,12 The effectiveness of rituximab in DM has been suggested.2,12,13 The Rituximab in Myositis (RIM) trial (N=200) was the first double-blind, placebo-controlled, phase 3 clinical trial to assess rituximab’s efficacy in refractory compared with early-onset inflammatory myopathies. Although outcomes were similar in both groups, 83% of patients overall, including the JDM subset, met the definition of improvement.12 In re-examining the RIM trial data and other cases using rituximab to treat inflammatory myopathies, an overall response rate of 78.3% was observed, with 52.1% of patients with DM reporting improvement in skin lesions (N=458, pooled from 48 studies).13 Further analysis of the RIM data revealed that panniculitis affected 10.4% of patients with JDM at baseline, which decreased to 6.8% at 36 weeks of rituximab therapy (N=48).12
As exhibited in our patient, subcutaneous tissue involvement, including calcinosis cutis and panniculitis, is seen more often in JDM than adult DM.2,6 However, panniculitis in anti-p155/140 patients is rare. Our patient also had antibody positivity, which likely predisposed her to a more severe course. Despite not having sustained improvement on rituximab, initiating aggressive therapy earlier in the disease course may be beneficial, and our patient continues with alternative therapies.
To the Editor:
Juvenile dermatomyositis (JDM) is an autoimmune disorder with childhood onset that predominantly affects the muscles and skin, among other organs. Since the recognition of dermatomyositis (DM) more than 100 years ago, a variety of clinical diagnostic criteria have been utilized. Classically, DM presents with muscle weakness and a pathognomonic cutaneous macular, violaceous, erythematous eruption. The juvenile variant is defined by onset prior to 16 years of age. Histologically, these entities are indistinguishable and demonstrate an interface dermatitis with epidermal atrophy. Clinically, JDM has a higher incidence of calcinosis cutis and is not associated with an increased risk for malignancy in contrast to the adult-onset variant.1 Panniculitis is a rare but serious complication in a subset of patients with DM and may represent a precursor to calcinosis cutis.2 We describe a case of JDM-associated panniculitis that was difficult to control with prednisone and rituximab.

A 21-year-old woman with fever, fatigue, muscle pain, and new-onset swelling of 2 weeks’ duration was admitted to the hospital. She had a 5-year history of intermittent muscle weakness and concomitant rash. Prior to presentation, she had been hospitalized twice for fever of unknown origin, and the source remained undetermined. Physical examination revealed prominent facial and periorbital edema. There was tender nonpitting edema present on all 4 extremities and hyperpigmented indurated nodules on the shins (Figure 1). A full laboratory and imaging workup was performed for autoantibodies and infectious etiologies. The complete blood cell count was notable for pancytopenia, and a thorough infectious workup was negative. Creatine kinase level was within reference range. A biopsy of the right shin was performed, and histopathology revealed a lobular panniculitis with fat necrosis and mixed inflammation with neutrophils with perieccrine involvement as well as an interface dermatitis (Figure 2). Periodic acid–Schiff, Grocott methenamine-silver, and Gram stains were negative. Myositis-specific antibody testing revealed anti-p155/140 autoantibodies, and magnetic resonance imaging did not reveal active myositis within the visualized muscles, consistent with stable nonprogressing DM. A diagnosis of JDM with panniculitis was made. The patient was started on oral prednisone. Subsequently, a trial of rituximab was initiated. Although the patient’s symptoms initially improved, the response was not sustained on rituximab, and the patient was continued on systemic steroids with initiation of cyclosporine.

Juvenile dermatomyositis is an autoimmune disorder with childhood onset that involves systemic inflammation of the muscles, skin, and internal organs. It often can present diagnostic and therapeutic challenges.2,3 Bohan and Peter4,5 clinical criteria may help identify potential patients with JDM, but magnetic resonance imaging, electromyography, and muscle biopsy often are required to confirm the diagnosis.6 Skin manifestations include heliotrope rash; V sign; shawl sign; Gottron papules; periorbital edema; and infrequently panniculitis, the subcutaneous inflammation of adipose tissue.3,7
Although panniculitis is found in approximately 10% of skin biopsies in patients with DM, our patient presented with anti-p155/140 antibodies.8-10 Fat involvement in these patients traditionally manifests as lipodystrophy. Panniculitis also may precede calcinosis cutis, a debilitating skin change that may occur in approximately 46% of patients with JDM and can cause severe morbidity.2,6,9
Subcutaneous edema rarely is described in DM-panniculitis, present in only 6% of 86 DM patients in one study.7 The pathophysiology of DM may be due to antibodies that target endothelial cells and activate complement, resulting in the membranolytic attack complex. This leads to microischemia, and microinfarction of the muscle fibers has been suggested to result in edema of the subcutaneous tissue in severe cases.7,11 Microinfarction has been found to be present 2.3 times more often in edematous DM compared with nonedematous DM.7 Subcutaneous edema may be an isolated presentation of DM that arises more quickly with severe disease activity. As such, recommendations have been made to consider edema in future classification schemes.7
Because of the severity of edematous and/or subcutaneous DM, aggressive therapy may be required. First-line therapy consists of corticosteroids with additional immunosuppressants and immunomodulatory agents if adequate response is not achieved.3,12 The effectiveness of rituximab in DM has been suggested.2,12,13 The Rituximab in Myositis (RIM) trial (N=200) was the first double-blind, placebo-controlled, phase 3 clinical trial to assess rituximab’s efficacy in refractory compared with early-onset inflammatory myopathies. Although outcomes were similar in both groups, 83% of patients overall, including the JDM subset, met the definition of improvement.12 In re-examining the RIM trial data and other cases using rituximab to treat inflammatory myopathies, an overall response rate of 78.3% was observed, with 52.1% of patients with DM reporting improvement in skin lesions (N=458, pooled from 48 studies).13 Further analysis of the RIM data revealed that panniculitis affected 10.4% of patients with JDM at baseline, which decreased to 6.8% at 36 weeks of rituximab therapy (N=48).12
As exhibited in our patient, subcutaneous tissue involvement, including calcinosis cutis and panniculitis, is seen more often in JDM than adult DM.2,6 However, panniculitis in anti-p155/140 patients is rare. Our patient also had antibody positivity, which likely predisposed her to a more severe course. Despite not having sustained improvement on rituximab, initiating aggressive therapy earlier in the disease course may be beneficial, and our patient continues with alternative therapies.
- Jorizzo JL, Vleugels RA. Dermatomyositis. In: Bolognia J, Schaffer J, Cerroni L. Dermatology. 4th ed. Elsevier; 2019:681-687.
- Aggarwal R, Loganathan P, Koontz D, et al. Cutaneous improvement in refractory adult and juvenile dermatomyositis after treatment with rituximab. Rheumatology. 2016;56:247-254.
- Santos-Briz A, Calle A, Linos K, et al. Dermatomyositis panniculitis: a clinicopathological and immunohistochemical study of 18 cases. J Eur Acad Dermatol Venereol. 2018;32:1352-1359.
- Bohan A, Peter JB. Polymyositis and dermatomyositis (first of two parts). N Engl J Med. 1975;292:344-347.
- Bohan A, Peter JB. Polymyositis and dermatomyositis (second of two parts). N Engl J Med. 1975;292:403-407.
- Sakurai N, Hino-Shishikura A, Nozawa T, et al. Clinical significance of subcutaneous fat and fascial involvement in juvenile dermatomyositis. Mod Rheumatol. 2019;29:808-813.
- Milisenda JC, Doti PI, Prieto-Gonzalez S, et al. Dermatomyositis presenting with severe subcutaneous edema: five additional cases and review of the literature. Semin Arthritis Rheum. 2014;44:228-233.
- Janis JF, Winkelmann RK. Histopathology of the skin in dermatomyositis: a histopathologic study of 55 cases. Arch Dermatol. 1968;97:640-650.
- van Dongen HM, van Vugt RM, Stoof TJ. Extensive persistent panniculitis in the context of dermatomyositis. J Clin Rheumatol. 2020;26:e187-e188.
- Gunawardena H, Wedderburn LR, North J, et al. Clinical associations of autoantibodies to a p155/140 kDa doublet protein in juvenile dermatomyositis. Rheumatology. 2008;47:324-328.
- Dalakas MC, Hohlfeld R. Polymyositis and dermatomyositis. Lancet. 2003;362:971-982.
- Oddis CV, Reed AM, Aggarwal R, et al. Rituximab in the treatment of refractory adult and juvenile dermatomyositis and adult polymyositis: a randomized, placebo-phase trial. Arthritis Rheum. 2013;65:314-324.
- Fasano S, Gordon P, Hajji R, et al. Rituximab in the treatment of inflammatory myopathies: a review. Rheumatology. 2016;56:26-36.
- Jorizzo JL, Vleugels RA. Dermatomyositis. In: Bolognia J, Schaffer J, Cerroni L. Dermatology. 4th ed. Elsevier; 2019:681-687.
- Aggarwal R, Loganathan P, Koontz D, et al. Cutaneous improvement in refractory adult and juvenile dermatomyositis after treatment with rituximab. Rheumatology. 2016;56:247-254.
- Santos-Briz A, Calle A, Linos K, et al. Dermatomyositis panniculitis: a clinicopathological and immunohistochemical study of 18 cases. J Eur Acad Dermatol Venereol. 2018;32:1352-1359.
- Bohan A, Peter JB. Polymyositis and dermatomyositis (first of two parts). N Engl J Med. 1975;292:344-347.
- Bohan A, Peter JB. Polymyositis and dermatomyositis (second of two parts). N Engl J Med. 1975;292:403-407.
- Sakurai N, Hino-Shishikura A, Nozawa T, et al. Clinical significance of subcutaneous fat and fascial involvement in juvenile dermatomyositis. Mod Rheumatol. 2019;29:808-813.
- Milisenda JC, Doti PI, Prieto-Gonzalez S, et al. Dermatomyositis presenting with severe subcutaneous edema: five additional cases and review of the literature. Semin Arthritis Rheum. 2014;44:228-233.
- Janis JF, Winkelmann RK. Histopathology of the skin in dermatomyositis: a histopathologic study of 55 cases. Arch Dermatol. 1968;97:640-650.
- van Dongen HM, van Vugt RM, Stoof TJ. Extensive persistent panniculitis in the context of dermatomyositis. J Clin Rheumatol. 2020;26:e187-e188.
- Gunawardena H, Wedderburn LR, North J, et al. Clinical associations of autoantibodies to a p155/140 kDa doublet protein in juvenile dermatomyositis. Rheumatology. 2008;47:324-328.
- Dalakas MC, Hohlfeld R. Polymyositis and dermatomyositis. Lancet. 2003;362:971-982.
- Oddis CV, Reed AM, Aggarwal R, et al. Rituximab in the treatment of refractory adult and juvenile dermatomyositis and adult polymyositis: a randomized, placebo-phase trial. Arthritis Rheum. 2013;65:314-324.
- Fasano S, Gordon P, Hajji R, et al. Rituximab in the treatment of inflammatory myopathies: a review. Rheumatology. 2016;56:26-36.
Practice Points
- Juvenile dermatomyositis is an autoimmune disorder with childhood onset that predominantly affects the muscles and skin.
- Juvenile dermatomyositis has a higher incidence of calcinosis cutis and is not associated with an increased risk for malignancy in contrast to the adult-onset variant, dermatomyositis (DM).
- Panniculitis is a rare but severe complication of DM, and this subset of DM may be challenging to treat, requiring aggressive therapy.
Cutaneous Complications Associated With Intraosseous Access Placement
Intraosseous (IO) access can afford a lifesaving means of vascular access in emergency settings, as it allows for the administration of large volumes of fluids, blood products, and medications at high flow rates directly into the highly vascularized osseous medullary cavity.1 Fortunately, the complication rate with this resuscitative effort is low, with many reports demonstrating complication rates of less than 1%.2 The most commonly reported complications include fluid extravasation, osteomyelitis, traumatic bone fracture, and epiphyseal plate damage.1-3 Although compartment syndrome and skin necrosis have been reported,4,5 there is no comprehensive list of sequelae resulting from fluid extravasation in the literature, and there are no known studies examining the incidence and types of cutaneous complications. In this study, we sought to evaluate the dermatologic impacts of this procedure.
Methods
We performed a retrospective chart review approved by the institutional review board at a large metropolitan level I trauma center in the Midwestern United States spanning 18 consecutive months to identify all patients who underwent IO line placement, either en route to or upon arrival at the trauma center. The electronic medical records of 113 patients (age range, 10 days–94 years) were identified using either an automated natural language look-up program with keywords including intraosseous access and IO or a Current Procedural Terminology code 36680. Data including patient age, reason for IO insertion, anatomic location of the IO, and complications secondary to IO line placement were recorded.
Results
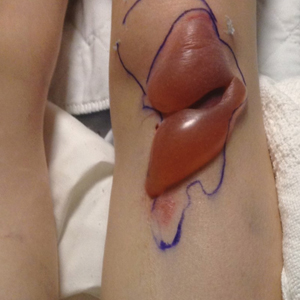
We identified an overall complication rate of 2.7% (3/113), with only 1 patient showing isolated cutaneous complications from IO line placement. The complications in the first 2 patients included compartment syndrome following IO line placement in the right tibia and needle breakage during IO line placement. The third patient, a 30-year-old heart transplant recipient, developed tense bullae on the left leg 5 days after a resuscitative effort required IO access through the bilateral tibiae. The patient had received vasopressors as well as 750 mL of normal saline through these access points. Two days after resuscitation, she developed an enlarg
At a scheduled 7-month dermatology follow-up, the wound bed appeared to be healing well with surrounding scarring with no residual bleeding or drainage (Figure 2) despite the patient reporting a protracted course of wound healing requiring debridement due to eschar formation and multiple follow-up appointments with the wound care service.
Comment
The most commonly reported complications with IO line placement result from fluid infiltration of the subcutaneous tissue secondary to catheter misplacement.1,3 Extravasated fluid may lead to tissue damage, compartment syndrome, and even tissue necrosis in some cases.1,4,5 Localized cellulitis and the formation of subcutaneous abscesses also have been reported, albeit rarely.3,5
In our retrospective cohort review, we identified an additional potential complication of IO line placement that has not been widely reported—development of large traumatic bullae. It is most likely that this patient’s IO catheter became dislodged, resulting in extravasation of fluids into the dermal and subcutaneous tissues.
Our findings support the previously noted complication rate of less than 1% following IO line placement, with an overall complication rate of 2.7% that included only 1 patient with a cutaneous complication.2 Given this low incidence, providers may not be used to recognizing such complications, leading to delayed or incorrect diagnosis of these entities. While there are certain conditions in which IO insertion is contraindicated, including severe bone diseases (eg, osteogenesis imperfecta, osteomyelitis), overlying cellulitis, and bone fracture, these conditions are rare and can be avoided in most cases by use of an alternative site for needle insertion.2 Due to the widespread utility of this tool and its few contraindications, its use in hospitalized patients is rapidly increasing, necessitating a need for quick recognition of potential complications.
From previous data on the incidence of traumatic blisters with underlying bone fractures, there are several identifiable risk factors that could be extended to patients at high risk for developing cutaneous IO complications secondary to the trauma associated with needle insertion,6 including wound-healing impairments in patients with fragile lymphatics, peripheral vascular disease, diabetes, or collagen vascular diseases (eg, lupus, rheumatoid arthritis, Sjögren syndrome). Patients with these conditions should be closely monitored for the development of bullae.6 While the patient we highlighted in our study did not have a history of such conditions, her history of cardiac disease, recent resuscitation attempts, and immunosuppression certainly could have contributed to suboptimal tissue agility and repair after IO line placement.
Conclusion
Intraosseous access is a safe, effective, and reliable option for vascular access in both pediatric and adult populations that is widely used in both prehospital (ie, paramedic administered) and hospital settings, including intensive care units, emergency departments, and any acute situation where rapid vascular access is necessary. This retrospective chart review examining the incidence and types of cutaneous complications associated with IO line placement at a level I trauma center revealed a total complication rate similar to those reported in previous studies and also highlighted a unique postprocedural cutaneous finding of traumatic bullae. Although no unified management recommendations currently exist, providers should consider this complication in the differential for hospitalized patients with large, atypical, asymmetric bullae in the absence of an alternative explanation for such skin findings.
- Day MW. Intraosseous devices for intravascular access in adult trauma patients. Crit Care Nurse. 2011;31:76-90. doi:10.4037/ccn2011615
- Petitpas F, Guenezan J, Vendeuvre T, et al. Use of intra-osseous access in adults: a systematic review. Crit Care. 2016;20:102. doi:10.1186/s13054-016-1277-6
- Desforges JF, Fiser DH. Intraosseous infusion. N Engl J Med. 1990;322:1579-1581. doi:10.1056/NEJM199005313222206
- Simmons CM, Johnson NE, Perkin RM, et al. Intraosseous extravasation complication reports. Ann Emerg Med. 1994;23:363-366. doi:10.1016/S0196-0644(94)70053-2
- Paxton JH. Intraosseous vascular access: a review. Trauma. 2012;14:195-232. doi:10.1177/1460408611430175
- Uebbing CM, Walsh M, Miller JB, et al. Fracture blisters. West J Emerg Med. 2011;12:131-133. doi:10.1016/S0190-9622(09)80152-7
Intraosseous (IO) access can afford a lifesaving means of vascular access in emergency settings, as it allows for the administration of large volumes of fluids, blood products, and medications at high flow rates directly into the highly vascularized osseous medullary cavity.1 Fortunately, the complication rate with this resuscitative effort is low, with many reports demonstrating complication rates of less than 1%.2 The most commonly reported complications include fluid extravasation, osteomyelitis, traumatic bone fracture, and epiphyseal plate damage.1-3 Although compartment syndrome and skin necrosis have been reported,4,5 there is no comprehensive list of sequelae resulting from fluid extravasation in the literature, and there are no known studies examining the incidence and types of cutaneous complications. In this study, we sought to evaluate the dermatologic impacts of this procedure.
Methods
We performed a retrospective chart review approved by the institutional review board at a large metropolitan level I trauma center in the Midwestern United States spanning 18 consecutive months to identify all patients who underwent IO line placement, either en route to or upon arrival at the trauma center. The electronic medical records of 113 patients (age range, 10 days–94 years) were identified using either an automated natural language look-up program with keywords including intraosseous access and IO or a Current Procedural Terminology code 36680. Data including patient age, reason for IO insertion, anatomic location of the IO, and complications secondary to IO line placement were recorded.
Results
We identified an overall complication rate of 2.7% (3/113), with only 1 patient showing isolated cutaneous complications from IO line placement. The complications in the first 2 patients included compartment syndrome following IO line placement in the right tibia and needle breakage during IO line placement. The third patient, a 30-year-old heart transplant recipient, developed tense bullae on the left leg 5 days after a resuscitative effort required IO access through the bilateral tibiae. The patient had received vasopressors as well as 750 mL of normal saline through these access points. Two days after resuscitation, she developed an enlarg
At a scheduled 7-month dermatology follow-up, the wound bed appeared to be healing well with surrounding scarring with no residual bleeding or drainage (Figure 2) despite the patient reporting a protracted course of wound healing requiring debridement due to eschar formation and multiple follow-up appointments with the wound care service.
Comment
The most commonly reported complications with IO line placement result from fluid infiltration of the subcutaneous tissue secondary to catheter misplacement.1,3 Extravasated fluid may lead to tissue damage, compartment syndrome, and even tissue necrosis in some cases.1,4,5 Localized cellulitis and the formation of subcutaneous abscesses also have been reported, albeit rarely.3,5
In our retrospective cohort review, we identified an additional potential complication of IO line placement that has not been widely reported—development of large traumatic bullae. It is most likely that this patient’s IO catheter became dislodged, resulting in extravasation of fluids into the dermal and subcutaneous tissues.
Our findings support the previously noted complication rate of less than 1% following IO line placement, with an overall complication rate of 2.7% that included only 1 patient with a cutaneous complication.2 Given this low incidence, providers may not be used to recognizing such complications, leading to delayed or incorrect diagnosis of these entities. While there are certain conditions in which IO insertion is contraindicated, including severe bone diseases (eg, osteogenesis imperfecta, osteomyelitis), overlying cellulitis, and bone fracture, these conditions are rare and can be avoided in most cases by use of an alternative site for needle insertion.2 Due to the widespread utility of this tool and its few contraindications, its use in hospitalized patients is rapidly increasing, necessitating a need for quick recognition of potential complications.
From previous data on the incidence of traumatic blisters with underlying bone fractures, there are several identifiable risk factors that could be extended to patients at high risk for developing cutaneous IO complications secondary to the trauma associated with needle insertion,6 including wound-healing impairments in patients with fragile lymphatics, peripheral vascular disease, diabetes, or collagen vascular diseases (eg, lupus, rheumatoid arthritis, Sjögren syndrome). Patients with these conditions should be closely monitored for the development of bullae.6 While the patient we highlighted in our study did not have a history of such conditions, her history of cardiac disease, recent resuscitation attempts, and immunosuppression certainly could have contributed to suboptimal tissue agility and repair after IO line placement.
Conclusion
Intraosseous access is a safe, effective, and reliable option for vascular access in both pediatric and adult populations that is widely used in both prehospital (ie, paramedic administered) and hospital settings, including intensive care units, emergency departments, and any acute situation where rapid vascular access is necessary. This retrospective chart review examining the incidence and types of cutaneous complications associated with IO line placement at a level I trauma center revealed a total complication rate similar to those reported in previous studies and also highlighted a unique postprocedural cutaneous finding of traumatic bullae. Although no unified management recommendations currently exist, providers should consider this complication in the differential for hospitalized patients with large, atypical, asymmetric bullae in the absence of an alternative explanation for such skin findings.
Intraosseous (IO) access can afford a lifesaving means of vascular access in emergency settings, as it allows for the administration of large volumes of fluids, blood products, and medications at high flow rates directly into the highly vascularized osseous medullary cavity.1 Fortunately, the complication rate with this resuscitative effort is low, with many reports demonstrating complication rates of less than 1%.2 The most commonly reported complications include fluid extravasation, osteomyelitis, traumatic bone fracture, and epiphyseal plate damage.1-3 Although compartment syndrome and skin necrosis have been reported,4,5 there is no comprehensive list of sequelae resulting from fluid extravasation in the literature, and there are no known studies examining the incidence and types of cutaneous complications. In this study, we sought to evaluate the dermatologic impacts of this procedure.
Methods
We performed a retrospective chart review approved by the institutional review board at a large metropolitan level I trauma center in the Midwestern United States spanning 18 consecutive months to identify all patients who underwent IO line placement, either en route to or upon arrival at the trauma center. The electronic medical records of 113 patients (age range, 10 days–94 years) were identified using either an automated natural language look-up program with keywords including intraosseous access and IO or a Current Procedural Terminology code 36680. Data including patient age, reason for IO insertion, anatomic location of the IO, and complications secondary to IO line placement were recorded.
Results
We identified an overall complication rate of 2.7% (3/113), with only 1 patient showing isolated cutaneous complications from IO line placement. The complications in the first 2 patients included compartment syndrome following IO line placement in the right tibia and needle breakage during IO line placement. The third patient, a 30-year-old heart transplant recipient, developed tense bullae on the left leg 5 days after a resuscitative effort required IO access through the bilateral tibiae. The patient had received vasopressors as well as 750 mL of normal saline through these access points. Two days after resuscitation, she developed an enlarg
At a scheduled 7-month dermatology follow-up, the wound bed appeared to be healing well with surrounding scarring with no residual bleeding or drainage (Figure 2) despite the patient reporting a protracted course of wound healing requiring debridement due to eschar formation and multiple follow-up appointments with the wound care service.
Comment
The most commonly reported complications with IO line placement result from fluid infiltration of the subcutaneous tissue secondary to catheter misplacement.1,3 Extravasated fluid may lead to tissue damage, compartment syndrome, and even tissue necrosis in some cases.1,4,5 Localized cellulitis and the formation of subcutaneous abscesses also have been reported, albeit rarely.3,5
In our retrospective cohort review, we identified an additional potential complication of IO line placement that has not been widely reported—development of large traumatic bullae. It is most likely that this patient’s IO catheter became dislodged, resulting in extravasation of fluids into the dermal and subcutaneous tissues.
Our findings support the previously noted complication rate of less than 1% following IO line placement, with an overall complication rate of 2.7% that included only 1 patient with a cutaneous complication.2 Given this low incidence, providers may not be used to recognizing such complications, leading to delayed or incorrect diagnosis of these entities. While there are certain conditions in which IO insertion is contraindicated, including severe bone diseases (eg, osteogenesis imperfecta, osteomyelitis), overlying cellulitis, and bone fracture, these conditions are rare and can be avoided in most cases by use of an alternative site for needle insertion.2 Due to the widespread utility of this tool and its few contraindications, its use in hospitalized patients is rapidly increasing, necessitating a need for quick recognition of potential complications.
From previous data on the incidence of traumatic blisters with underlying bone fractures, there are several identifiable risk factors that could be extended to patients at high risk for developing cutaneous IO complications secondary to the trauma associated with needle insertion,6 including wound-healing impairments in patients with fragile lymphatics, peripheral vascular disease, diabetes, or collagen vascular diseases (eg, lupus, rheumatoid arthritis, Sjögren syndrome). Patients with these conditions should be closely monitored for the development of bullae.6 While the patient we highlighted in our study did not have a history of such conditions, her history of cardiac disease, recent resuscitation attempts, and immunosuppression certainly could have contributed to suboptimal tissue agility and repair after IO line placement.
Conclusion
Intraosseous access is a safe, effective, and reliable option for vascular access in both pediatric and adult populations that is widely used in both prehospital (ie, paramedic administered) and hospital settings, including intensive care units, emergency departments, and any acute situation where rapid vascular access is necessary. This retrospective chart review examining the incidence and types of cutaneous complications associated with IO line placement at a level I trauma center revealed a total complication rate similar to those reported in previous studies and also highlighted a unique postprocedural cutaneous finding of traumatic bullae. Although no unified management recommendations currently exist, providers should consider this complication in the differential for hospitalized patients with large, atypical, asymmetric bullae in the absence of an alternative explanation for such skin findings.
- Day MW. Intraosseous devices for intravascular access in adult trauma patients. Crit Care Nurse. 2011;31:76-90. doi:10.4037/ccn2011615
- Petitpas F, Guenezan J, Vendeuvre T, et al. Use of intra-osseous access in adults: a systematic review. Crit Care. 2016;20:102. doi:10.1186/s13054-016-1277-6
- Desforges JF, Fiser DH. Intraosseous infusion. N Engl J Med. 1990;322:1579-1581. doi:10.1056/NEJM199005313222206
- Simmons CM, Johnson NE, Perkin RM, et al. Intraosseous extravasation complication reports. Ann Emerg Med. 1994;23:363-366. doi:10.1016/S0196-0644(94)70053-2
- Paxton JH. Intraosseous vascular access: a review. Trauma. 2012;14:195-232. doi:10.1177/1460408611430175
- Uebbing CM, Walsh M, Miller JB, et al. Fracture blisters. West J Emerg Med. 2011;12:131-133. doi:10.1016/S0190-9622(09)80152-7
- Day MW. Intraosseous devices for intravascular access in adult trauma patients. Crit Care Nurse. 2011;31:76-90. doi:10.4037/ccn2011615
- Petitpas F, Guenezan J, Vendeuvre T, et al. Use of intra-osseous access in adults: a systematic review. Crit Care. 2016;20:102. doi:10.1186/s13054-016-1277-6
- Desforges JF, Fiser DH. Intraosseous infusion. N Engl J Med. 1990;322:1579-1581. doi:10.1056/NEJM199005313222206
- Simmons CM, Johnson NE, Perkin RM, et al. Intraosseous extravasation complication reports. Ann Emerg Med. 1994;23:363-366. doi:10.1016/S0196-0644(94)70053-2
- Paxton JH. Intraosseous vascular access: a review. Trauma. 2012;14:195-232. doi:10.1177/1460408611430175
- Uebbing CM, Walsh M, Miller JB, et al. Fracture blisters. West J Emerg Med. 2011;12:131-133. doi:10.1016/S0190-9622(09)80152-7
Practice Points
- Intraosseous (IO) access provides rapid vascular access for the delivery of fluids, drugs, and blood products in emergent situations.
- Bullae are potential complications from IO line placement.