User login
Positively False
The approach to clinical conundrums by an expert clinician is revealed through presentation of an actual patient's case in an approach typical of morning report. Similar to patient care, sequential pieces of information are provided to the clinician who is unfamiliar with the case. The focus is on the thought processes of both the clinical team caring for the patient and the discussant.
A51‐year‐old woman presented after 5 days of fever, rigors, anorexia, right‐sided abdominal pain, nausea, and dizziness. She had 2 loose stools the day before admission, without blood or mucus, but otherwise had recently been constipated. She denied cough, shortness of breath, chest pain, headache, sore throat, rash, arthritis, or dysuria.
In a 51‐year‐old woman with right‐sided abdominal pain and systemic symptoms, major concerns include biliary disease, liver abscess, or appendicitis. Right‐sided diverticulitis would be more unusual. Pyelonephritis infrequently presents with epigastric and lower quadrant pain, instead of flank pain. Basilar pneumonia may present with abdominal pain, but this is less likely in the absence of respiratory symptoms.
The patient used an albuterol inhaler for mild asthma and had experienced an episode of herpes zoster 7 years prior, but was otherwise well. Her surgical history was notable for a remote appendectomy. She was a native of the Dominican Republic who had lived in the United States for the past 20 years. She visited the Dominican Republic for 3 weeks every year, with her last visit occurring about 10 months before. She was a cleaning and maintenance worker. She had 2 adult children in good health, was divorced from her husband, and had not been sexually active for the past 8 years. The patient had no pets or other animal exposures. She did not smoke, drink alcohol, or use intravenous drugs.
The remote episode of shingles makes me a bit worried about chronic human immunodeficiency virus (HIV) infection. As a native of and traveler to the Dominican Republic, she is at risk for a variety of tropical pathogens. Hyperinfection syndrome from strongyloides can cause fever and bacteremia, but this is almost always associated with significant immunosuppression. Dengue fever has become very common in the Caribbean, but should occur within 2 weeks of travel. Her work in cleaning and maintenance might bring her into contact with rats and mice, putting her at risk for leptospirosis. This can present as a fairly nonspecific febrile syndrome, but this is unlikely without a major complaint of headache.
The patient appeared fatigued. Her temperature was 39.7C, her heart rate was 110 beats per minute, and her blood pressure 80/62 mm Hg. The oropharynx was normal. Mild cervical lymphadenopathy was present (less than 1 cm in diameter). The chest was clear and the cardiac examination unremarkable. Bowel sounds were present. Moderate right‐sided abdominal tenderness was noted, somewhat more marked in the right lower quadrant, without guarding or rebound. There was no hepatosplenomegaly. There was no rash. A bedside right upper quadrant ultrasound was negative for gallstones.
Her low blood pressure is concerning for bacterial sepsis. The negative right upper quadrant ultrasound makes cholecystitis or cholangitis less likely, but does not exclude diverticulitis or pelvic inflammatory disease. She lacks peritoneal signs, but they may be absent in these conditions. Another worrisome finding on her physical examination is cervical lymphadenopathy. In an older patient, this raises the specter of malignancy. In a younger patient, it could suggest a mononucleosis syndrome from Epstein‐Barr virus (EBV) or cytomegalovirus (CMV). In addition, HIV must be considered in any patient with unexplained lymphadenopathy.
She received intravenous levofloxacin and 1 L of intravenous normal saline, with a rise in her blood pressure to 100/59 mm Hg. Her white blood cell count was 5.0, with 71% polys and 9% bands. The hematocrit was 32%, with a normal mean corpuscular volume. The erythrocyte sedimentation rate (ESR) was 109 mm/hour. The platelet count, serum electrolytes, creatinine, aminotransferases, alkaline phosphatase, bilirubin, amylase, and lipase were normal. The serum level of lactate dehydrogenase (LDH) was 498 unit/L (normal range, 107231 units/L). Computed tomography (CT) scan of the abdomen showed multiple enlarged lymph nodes up to 1.2 cm in size along the gastrohepatic ligament and the mesentery, with mild associated fat stranding, consistent with mesenteric lymphadenitis.
Her blood pressure has responded to fluids; perhaps she was just volume‐depleted from not eating for several days. She has bandemia, which is consistent with acute bacterial infection, but might also signify a stress response. The low hematocrit and high ESR raise the possibility of anemia of chronic disease; perhaps her illness is more longstanding than her presentation suggests. Mesenteric lymphadenitis may be related to EBV and HIV, but in a patient originally from the Caribbean, it raises the possibility of gastrointestinal tuberculosis or histoplasmosis. Human T‐cell lymphotropic virus type 1 (HTLV‐1) is also endemic in the Caribbean, and may cause adult T‐cell leukemia/lymphoma (ATLL), which could explain her lymphadenopathy and elevated LDH. Mesenteric lymphadenitis is also characteristic of several bacterial infections, especially Yersinia, Salmonella, and Bartonella. The lack of diarrhea makes yersiniosis doubtful, and the absence of cat exposure makes bartonellosis unlikely. Salmonella infection is also associated with diarrhea, except for typhoid fever, in which patients have diarrhea, constipation, or normal stools.
A urine culture and 3 sets of blood cultures obtained prior to the initiation of antibiotics were negative. A blood smear showed no malaria or babesia parasites. The patient's fever continued for the first 2 days of her hospitalization, but subsequently abated. The patient had no loose stools during her hospitalization. Serologies for EBV, hepatitis A, CMV, and toxoplasmosis were indicative of remote infection. Serum rapid plasma reagin (RPR), Bartonella antibodies, antinuclear antibodies, hepatitis B surface antigen, and hepatitis C antibody were negative. Tuberculin skin testing and urinary histoplasma antigen were negative. By the fifth hospital day, she had been afebrile for over 48 hours, and her abdominal pain had improved, though it had not completely resolved. She was discharged to complete a 10‐day course of levofloxacin.
It is not clear to me whether she has just experienced a spontaneous remission in her illness, or whether her disease course has truly been modified with antibiotics. Could she have typhoid fever or another salmonellosis? The patient has not traveled abroad in several months. If she had typhoid fever, the source of infection would have to be imported food, or exposure to a family member who was a carrier. The negative tuberculin skin test makes tuberculosis less likely, but does not exclude it entirely. Similarly, while a negative histoplasma urinary antigen essentially rules out acute disseminated histoplasmosis, as would be seen in acquired immune deficiency syndrome (AIDS), it is less sensitive for more chronic forms of disseminated histoplasmosis, including gastrointestinal involvement.
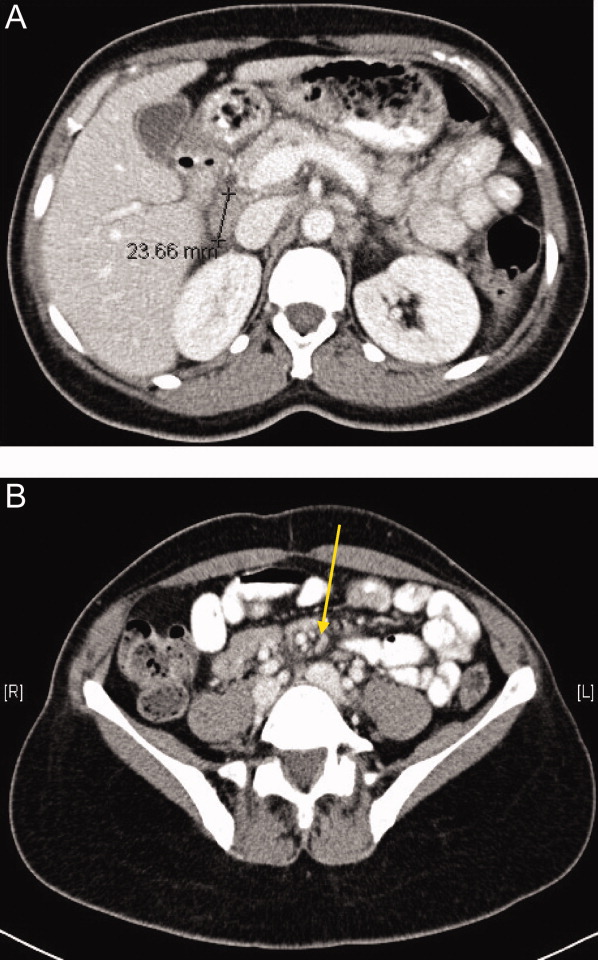
One week later, the patient returned to the emergency department with recurrent abdominal pain, anorexia, fever, night sweats, and increased swelling of the lymph nodes in her neck. She had lost a total of 4.4 kg (10 lb) since the onset of her illness. Physical examination revealed moderate (up to 2 cm), slightly tender cervical and inguinal lymphadenopathy, and continued moderate right‐sided abdominal tenderness. Mesenteric, retroperitoneal, and inguinal lymphadenopathy was more prominent than on the prior CT scan (Figure 1).She was told by the emergency room physicians that the HIV test obtained during the prior hospitalization was positive. Further questioning elicited that her estranged husband had been promiscuous prior to their separation. She was transferred to a second hospital for further care. Blood cultures were sent for fungi and acid‐fast bacilli.
Everyone with fever of unknown origin (FUO) deserves an HIV test. Is this just lymphadenopathy from HIV, or is she suffering from an opportunistic infection? Mycobacterium avium infection is an attractive explanation for her fever, abdominal pain, and lymphadenopathy, but I would hold off on empiric treatment until the results of a CD4+ cell count were available. Could she have a secondary, HIV‐related lymphoma?
Full review of records from the outside hospital showed that the patient had a positive HIV enzyme immunoassay, with an indeterminate HIV Western blot. The patient's CD4 cell count was 313/cm3, with a CD4/CD8 ratio within the normal range. Her HIV enzyme immunoassay was repeated and found to be negative, and the HIV viral load was undetectable.
The HIV enzyme immunoassay is only a screening test, and must be confirmed with a positive HIV Western blot. (In most clinical laboratories, this is done automatically before the test is reported.) Indeterminate HIV Western blots are common in acute HIV infection, but outside of this setting, most patients with indeterminate HIV Western blots turn out not to have HIV infection. I am still very concerned about lymphoma, and would pursue a lymph node biopsy. Disseminated tuberculosis is still possible.
Biopsy of a right inguinal lymph node was performed on the third day of the second hospitalization. The patient was persistently febrile, developed swelling of the knees, ankles, and interphalangeal joints of the second and third digits of the left hand, and complained of pruritus. Anti‐double‐stranded DNA antibody, antineutrophilic cytoplasmic antibody, and Brucella antibody were negative. Antibody against cyclic citrullinated peptide (CCP) was weakly positive.
Now she has a more florid syndrome, with arthritic symptoms. Sarcoidosis is an attractive explanation for her fever, lymphadenopathy, and arthritis. Lupus could explain some features of her presentation, but the negative serology makes it unlikely. Antibody to cyclic citrullinated peptide is a newer diagnostic test for rheumatoid arthritis. Although anti‐CCP is more specific than rheumatoid factor for the diagnosis of rheumatoid arthritis, this result could still be a false positive, particularly given the low titer. As well, the patient has more impressive lymphadenopathy than is usual for rheumatoid arthritis. Reactive arthritis can follow enteric infection with Campylobacter, Salmonella, Yersinia, and Shigella, but lymphadenopathy is not a feature. Arthritis may be prominent in parvovirus B19, rubella, disseminated gonococcal infection, and Lyme disease, but mesenteric lymphadenitis and a prolonged, waxing and waning course would not be expected with any of these. I worry that the arthritis and pruritus are paraneoplastic manifestations of lymphoma.
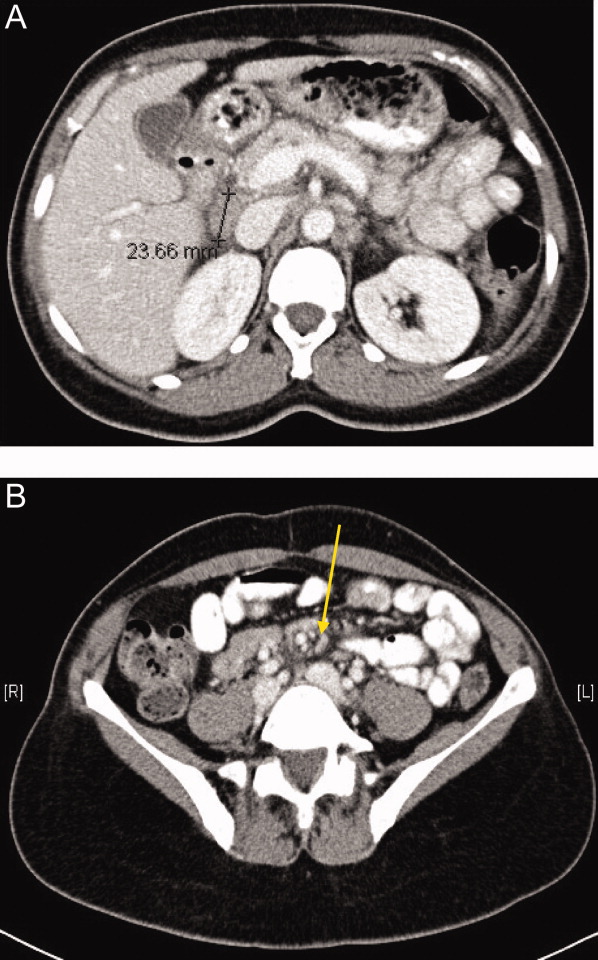
The inguinal lymph node biopsy showed markedly distorted nodal architecture with an atypical proliferation of small‐sized to large‐sized lymphoid cells, with predominantly round nuclei, vesicular chromatin, small nucleoli, and variable amounts of clear to eosinophilic cytoplasm (Figure 2). Residual follicles and occasional apoptotic bodies and mitoses were seen. Large B‐cells were frequently present, which stained positive for EBV‐associated mRNA by in situ hybridization studies. Immunoperoxidase staining revealed that the atypical cell population was largely composed of CD4+ T‐cells. T‐cell receptor gene rearrangement studies demonstrated that the T‐cell population was monoclonal. These results were consistent with angioimmunoblastic T‐cell lymphoma (AITL). Positron emission tomography (PET) scanning was performed, showing diffuse fluorodeoxyglucose (FDG)‐avid lymphadenopathy involving cervical, axillary, mediastinal, retroperitoneal, mesenteric, and inguinal lymph nodes, up to 2 cm in diameter. The patient completed 6 cycles of cyclophosphamide, doxorubicin, vincristine, and prednisone (CHOP), as well as experimental treatment with denileukin diftitox. Her fever and arthritis subsided quickly, and she was clinically well and free of disease by CT scans and PET scans 1 year after diagnosis.
COMMENTARY
The differential diagnosis of FUO is one of the largest in medicine, encompassing a dizzying range of infectious, inflammatory, and neoplastic conditions. This has made the development of standardized diagnostic algorithms difficult.1 The workup of patients with FUO begins with a detailed history and physical examination, followed by a core set of microbiology cultures, imaging studies, and blood tests on all patients. Further testing is individualized, based on key clinical findings, also known as pivot points.2 Unfortunately, many of the diseases presenting as FUO have overlapping symptoms and signs, somewhat limiting the utility of pivot points. In a prospective study, 81% of these potentially diagnostic clues were misleading, although 19% of them contributed to the final diagnosis.3 Key clinical findings in patients with FUO may trigger a barrage of diagnostic tests, often leading to a large number of false‐positive results.4 Clinicians who investigate patients with FUO must remember that many clues are diagnostic dead ends, and should be wary of drawing positively‐false conclusions.
Fever pattern is usually not helpful in the diagnosis of FUO, with occasional exceptions, such as the tertian and quartan fevers in some forms of malaria. A minority of patients with Hodgkin's disease have Pel‐Ebstein fevers, in which 1 to 2 weeks of fever alternate with an afebrile period of similar or longer duration. More often, fever in lymphoma waxes and wanes unpredictably,5 a circumstance that may lead to the mistaken impression of response to antibiotics, as in this case.
Tissue biopsies are helpful in FUO when suggestive findings are present on physical examination or imaging studies. In patients with lymphadenopathy and FUO, lymph node biopsy is a high‐yield procedure, contributing to the final diagnosis 46% of the time. This is exceeded only by biopsies of skin lesions, which have a 63% diagnostic yield in FUO.3 In older patients with FUO, temporal artery biopsies have a significant diagnostic yield, in the range of 16% to 17%. Liver biopsies have a similar yield (14‐17%), but a higher risk of complications. Bone marrow biopsies have a low yield in most FUO patients.1
AITL makes up 1% of all non‐Hodgkin's lymphomas. AITL was once known as angioimmunoblastic lymphadenopathy, and was thought to be either a disorder of immune regulation or a premalignant lymphoid disease. However, molecular diagnostic techniques have established that monoclonal T‐cell populations and cytogenetic abnormalities are usually present at the time of diagnosis.6, 7 The prognosis in AITL is unfavorable. Disease is usually widespread at the time of diagnosis. Most patients achieve complete remission with anthracycline‐based chemotherapy, such as CHOP, but the duration of remission is often brief, and median survival after diagnosis is only 3 years.6 Novel treatments under investigation include denileukin diftitox,8 a fusion protein of interleukin‐2 (IL‐2) conjugated to diphtheria toxin, which leads to apoptosis of cells expressing the IL‐2 receptor; rituximab, which targets the reactive population of B‐cells in AITL, rather than the malignant clone of T‐cells; and antiangiogenic therapy, such as thalidomide.9
The diagnosis of AITL is usually elusive, and the average patient has symptoms for 4 months prior to diagnosis.6 This patient's clinical presentation, while certainly not specific, was typical of AITL. AITL usually presents as fever of unknown origin with generalized, nonbulky lymphadenopathy. Fever is present in 57% of patients with AITL, and up to 2% of FUO is caused by AITL.6, 10 Other features of this patient's illness, such as night sweats, weight loss, pruritus, and arthritis, are common in AITL.
T‐cell depletion and immune dysregulation are frequent in AITL, explaining why AITL shares a number of clinical features with HIV disease. These include a high incidence of drug rashes, immune thrombocytopenic purpura, polyclonal hypergammaglobulinemia, and autoantibodies.6, 7 As with HIV patients, death in AITL is often due to opportunistic infections or diffuse large B‐cell lymphomas.7 Over 95% of patients with AITL display a proliferation of EBV‐infected B cells, presumably from immune dysregulation, and the B‐cell lymphomas in these patients are usually EBV‐positive.11
Because AITL is a proinflammatory state, false‐positive antibody tests are fairly common. The occasional occurrence of positive HIV enzyme immunoassays, with indeterminate Western blot results, may be a particular source of diagnostic confusion.12, 13 The significance of indeterminate Western blots is often erroneously communicated to patients, as happened here. Indeterminate HIV Western blots may be seen in HIV seroconversion, HIV‐2 infection, or advanced HIV disease with loss of core antibody. They may also result from laboratory error, multiparity, syphilis, malaria, or cross‐reacting antibodies in autoimmune diseases. Indeterminate HIV Western blots in low‐risk patients usually do not represent true HIV infection.14
KEY POINTS FOR HOSPITALISTS
-
FUO is one of the most challenging diagnoses faced by hospitalists. Biopsies of new skin lesions and enlarged lymph nodes are particularly high‐yield diagnostic procedures in FUO. Fever pattern is generally not helpful in the diagnosis of FUO, with few exceptions, such as the tertian fevers of malaria.
-
Misleading and false‐positive tests results often occur in the course of FUO evaluation, due to the sheer number of tests ordered, and the higher likelihood of false‐positive serologic tests in the setting of inflammatory states.
-
AITL may be an elusive diagnosis in FUO with diverse clinical features, including weight loss, night sweats, rashes, arthritis, autoantibodies, immune dysregulation, and opportunistic infections. The prognosis traditionally has been guarded, but may be more hopeful in an era of emerging molecular therapies.
- ,,.A comprehensive evidence‐based approach to fever of unknown origin.Arch Intern Med.2003;163:545–551.
- ,.The art of diagnosis: solving the clinicopathological exercise.N Engl J Med.1982;306:1263–1268.
- ,,, et al.A prospective multicenter study on fever of unknown origin: the yield of a structured diagnostic protocol.Medicine (Baltimore).2007;86:26–38.
- ,,.Fever of unknown origin (FUO). II. Diagnostic procedures in a prospective multicenter study of 167 patients. The Netherlands FUO Study Group.Medicine (Baltimore).1997;76:401–414.
- ,.Neoplastic diseases. In:Murray HW, ed.FUO: Fever of Undetermined Origin.New York, NY:Futura Publishing;1983:39–48.
- ,,, et al.Angioimmunoblastic T‐cell lymphoma: clinical and laboratory features at diagnosis in 77 patients.Medicine (Baltimore).2007;86:282–292.
- ,,.Angioimmunoblastic T‐cell lymphoma.Br J Haematol.2003;121:681–691.
- ,,, et al.Phase II trial of denileukin diftitox for relapsed/refractory T‐cell non‐Hodgkin lymphoma.Br J Haematol.2007;136:439–447.
- ,.Angioimmunoblastic T‐cell lymphoma: still a dismal prognosis with current treatment approaches.Leuk Lymphoma.2007;48:645–646.
- ,,.Fever of unknown origin (FUO). I. A prospective multicenter study of 167 patients with FUO, using fixed epidemiologic entry criteria. The Netherlands FUO Study Group.Medicine (Baltimore).1997;76:392–400.
- ,,, et al.Histologic evolution of angioimmunoblastic T‐cell lymphoma in consecutive biopsies: clinical correlation and insights into natural history and disease progression.Am J Surg Pathol.2007;31:1077–1088.
- ,,,.Angioimmunoblastic lymphadenopathy, immunoblastic lymphoma, and false‐positive seroconversion for human immunodeficiency virus.Ann Intern Med.1987;107:114.
- ,.Angioimmunoblastic T‐cell lymphoma associated with an antibody to human immunodeficiency virus protein.Int J Hematol.2003;78:160–162.
- ,,.Communicating indeterminate HIV Western blot test results to clients: an observational study of three community testing sites.AIDS Patient Care STDS.2006;20:620–627.
The approach to clinical conundrums by an expert clinician is revealed through presentation of an actual patient's case in an approach typical of morning report. Similar to patient care, sequential pieces of information are provided to the clinician who is unfamiliar with the case. The focus is on the thought processes of both the clinical team caring for the patient and the discussant.
A51‐year‐old woman presented after 5 days of fever, rigors, anorexia, right‐sided abdominal pain, nausea, and dizziness. She had 2 loose stools the day before admission, without blood or mucus, but otherwise had recently been constipated. She denied cough, shortness of breath, chest pain, headache, sore throat, rash, arthritis, or dysuria.
In a 51‐year‐old woman with right‐sided abdominal pain and systemic symptoms, major concerns include biliary disease, liver abscess, or appendicitis. Right‐sided diverticulitis would be more unusual. Pyelonephritis infrequently presents with epigastric and lower quadrant pain, instead of flank pain. Basilar pneumonia may present with abdominal pain, but this is less likely in the absence of respiratory symptoms.
The patient used an albuterol inhaler for mild asthma and had experienced an episode of herpes zoster 7 years prior, but was otherwise well. Her surgical history was notable for a remote appendectomy. She was a native of the Dominican Republic who had lived in the United States for the past 20 years. She visited the Dominican Republic for 3 weeks every year, with her last visit occurring about 10 months before. She was a cleaning and maintenance worker. She had 2 adult children in good health, was divorced from her husband, and had not been sexually active for the past 8 years. The patient had no pets or other animal exposures. She did not smoke, drink alcohol, or use intravenous drugs.
The remote episode of shingles makes me a bit worried about chronic human immunodeficiency virus (HIV) infection. As a native of and traveler to the Dominican Republic, she is at risk for a variety of tropical pathogens. Hyperinfection syndrome from strongyloides can cause fever and bacteremia, but this is almost always associated with significant immunosuppression. Dengue fever has become very common in the Caribbean, but should occur within 2 weeks of travel. Her work in cleaning and maintenance might bring her into contact with rats and mice, putting her at risk for leptospirosis. This can present as a fairly nonspecific febrile syndrome, but this is unlikely without a major complaint of headache.
The patient appeared fatigued. Her temperature was 39.7C, her heart rate was 110 beats per minute, and her blood pressure 80/62 mm Hg. The oropharynx was normal. Mild cervical lymphadenopathy was present (less than 1 cm in diameter). The chest was clear and the cardiac examination unremarkable. Bowel sounds were present. Moderate right‐sided abdominal tenderness was noted, somewhat more marked in the right lower quadrant, without guarding or rebound. There was no hepatosplenomegaly. There was no rash. A bedside right upper quadrant ultrasound was negative for gallstones.
Her low blood pressure is concerning for bacterial sepsis. The negative right upper quadrant ultrasound makes cholecystitis or cholangitis less likely, but does not exclude diverticulitis or pelvic inflammatory disease. She lacks peritoneal signs, but they may be absent in these conditions. Another worrisome finding on her physical examination is cervical lymphadenopathy. In an older patient, this raises the specter of malignancy. In a younger patient, it could suggest a mononucleosis syndrome from Epstein‐Barr virus (EBV) or cytomegalovirus (CMV). In addition, HIV must be considered in any patient with unexplained lymphadenopathy.
She received intravenous levofloxacin and 1 L of intravenous normal saline, with a rise in her blood pressure to 100/59 mm Hg. Her white blood cell count was 5.0, with 71% polys and 9% bands. The hematocrit was 32%, with a normal mean corpuscular volume. The erythrocyte sedimentation rate (ESR) was 109 mm/hour. The platelet count, serum electrolytes, creatinine, aminotransferases, alkaline phosphatase, bilirubin, amylase, and lipase were normal. The serum level of lactate dehydrogenase (LDH) was 498 unit/L (normal range, 107231 units/L). Computed tomography (CT) scan of the abdomen showed multiple enlarged lymph nodes up to 1.2 cm in size along the gastrohepatic ligament and the mesentery, with mild associated fat stranding, consistent with mesenteric lymphadenitis.
Her blood pressure has responded to fluids; perhaps she was just volume‐depleted from not eating for several days. She has bandemia, which is consistent with acute bacterial infection, but might also signify a stress response. The low hematocrit and high ESR raise the possibility of anemia of chronic disease; perhaps her illness is more longstanding than her presentation suggests. Mesenteric lymphadenitis may be related to EBV and HIV, but in a patient originally from the Caribbean, it raises the possibility of gastrointestinal tuberculosis or histoplasmosis. Human T‐cell lymphotropic virus type 1 (HTLV‐1) is also endemic in the Caribbean, and may cause adult T‐cell leukemia/lymphoma (ATLL), which could explain her lymphadenopathy and elevated LDH. Mesenteric lymphadenitis is also characteristic of several bacterial infections, especially Yersinia, Salmonella, and Bartonella. The lack of diarrhea makes yersiniosis doubtful, and the absence of cat exposure makes bartonellosis unlikely. Salmonella infection is also associated with diarrhea, except for typhoid fever, in which patients have diarrhea, constipation, or normal stools.
A urine culture and 3 sets of blood cultures obtained prior to the initiation of antibiotics were negative. A blood smear showed no malaria or babesia parasites. The patient's fever continued for the first 2 days of her hospitalization, but subsequently abated. The patient had no loose stools during her hospitalization. Serologies for EBV, hepatitis A, CMV, and toxoplasmosis were indicative of remote infection. Serum rapid plasma reagin (RPR), Bartonella antibodies, antinuclear antibodies, hepatitis B surface antigen, and hepatitis C antibody were negative. Tuberculin skin testing and urinary histoplasma antigen were negative. By the fifth hospital day, she had been afebrile for over 48 hours, and her abdominal pain had improved, though it had not completely resolved. She was discharged to complete a 10‐day course of levofloxacin.
It is not clear to me whether she has just experienced a spontaneous remission in her illness, or whether her disease course has truly been modified with antibiotics. Could she have typhoid fever or another salmonellosis? The patient has not traveled abroad in several months. If she had typhoid fever, the source of infection would have to be imported food, or exposure to a family member who was a carrier. The negative tuberculin skin test makes tuberculosis less likely, but does not exclude it entirely. Similarly, while a negative histoplasma urinary antigen essentially rules out acute disseminated histoplasmosis, as would be seen in acquired immune deficiency syndrome (AIDS), it is less sensitive for more chronic forms of disseminated histoplasmosis, including gastrointestinal involvement.
One week later, the patient returned to the emergency department with recurrent abdominal pain, anorexia, fever, night sweats, and increased swelling of the lymph nodes in her neck. She had lost a total of 4.4 kg (10 lb) since the onset of her illness. Physical examination revealed moderate (up to 2 cm), slightly tender cervical and inguinal lymphadenopathy, and continued moderate right‐sided abdominal tenderness. Mesenteric, retroperitoneal, and inguinal lymphadenopathy was more prominent than on the prior CT scan (Figure 1).She was told by the emergency room physicians that the HIV test obtained during the prior hospitalization was positive. Further questioning elicited that her estranged husband had been promiscuous prior to their separation. She was transferred to a second hospital for further care. Blood cultures were sent for fungi and acid‐fast bacilli.

Everyone with fever of unknown origin (FUO) deserves an HIV test. Is this just lymphadenopathy from HIV, or is she suffering from an opportunistic infection? Mycobacterium avium infection is an attractive explanation for her fever, abdominal pain, and lymphadenopathy, but I would hold off on empiric treatment until the results of a CD4+ cell count were available. Could she have a secondary, HIV‐related lymphoma?
Full review of records from the outside hospital showed that the patient had a positive HIV enzyme immunoassay, with an indeterminate HIV Western blot. The patient's CD4 cell count was 313/cm3, with a CD4/CD8 ratio within the normal range. Her HIV enzyme immunoassay was repeated and found to be negative, and the HIV viral load was undetectable.
The HIV enzyme immunoassay is only a screening test, and must be confirmed with a positive HIV Western blot. (In most clinical laboratories, this is done automatically before the test is reported.) Indeterminate HIV Western blots are common in acute HIV infection, but outside of this setting, most patients with indeterminate HIV Western blots turn out not to have HIV infection. I am still very concerned about lymphoma, and would pursue a lymph node biopsy. Disseminated tuberculosis is still possible.
Biopsy of a right inguinal lymph node was performed on the third day of the second hospitalization. The patient was persistently febrile, developed swelling of the knees, ankles, and interphalangeal joints of the second and third digits of the left hand, and complained of pruritus. Anti‐double‐stranded DNA antibody, antineutrophilic cytoplasmic antibody, and Brucella antibody were negative. Antibody against cyclic citrullinated peptide (CCP) was weakly positive.
Now she has a more florid syndrome, with arthritic symptoms. Sarcoidosis is an attractive explanation for her fever, lymphadenopathy, and arthritis. Lupus could explain some features of her presentation, but the negative serology makes it unlikely. Antibody to cyclic citrullinated peptide is a newer diagnostic test for rheumatoid arthritis. Although anti‐CCP is more specific than rheumatoid factor for the diagnosis of rheumatoid arthritis, this result could still be a false positive, particularly given the low titer. As well, the patient has more impressive lymphadenopathy than is usual for rheumatoid arthritis. Reactive arthritis can follow enteric infection with Campylobacter, Salmonella, Yersinia, and Shigella, but lymphadenopathy is not a feature. Arthritis may be prominent in parvovirus B19, rubella, disseminated gonococcal infection, and Lyme disease, but mesenteric lymphadenitis and a prolonged, waxing and waning course would not be expected with any of these. I worry that the arthritis and pruritus are paraneoplastic manifestations of lymphoma.
The inguinal lymph node biopsy showed markedly distorted nodal architecture with an atypical proliferation of small‐sized to large‐sized lymphoid cells, with predominantly round nuclei, vesicular chromatin, small nucleoli, and variable amounts of clear to eosinophilic cytoplasm (Figure 2). Residual follicles and occasional apoptotic bodies and mitoses were seen. Large B‐cells were frequently present, which stained positive for EBV‐associated mRNA by in situ hybridization studies. Immunoperoxidase staining revealed that the atypical cell population was largely composed of CD4+ T‐cells. T‐cell receptor gene rearrangement studies demonstrated that the T‐cell population was monoclonal. These results were consistent with angioimmunoblastic T‐cell lymphoma (AITL). Positron emission tomography (PET) scanning was performed, showing diffuse fluorodeoxyglucose (FDG)‐avid lymphadenopathy involving cervical, axillary, mediastinal, retroperitoneal, mesenteric, and inguinal lymph nodes, up to 2 cm in diameter. The patient completed 6 cycles of cyclophosphamide, doxorubicin, vincristine, and prednisone (CHOP), as well as experimental treatment with denileukin diftitox. Her fever and arthritis subsided quickly, and she was clinically well and free of disease by CT scans and PET scans 1 year after diagnosis.

COMMENTARY
The differential diagnosis of FUO is one of the largest in medicine, encompassing a dizzying range of infectious, inflammatory, and neoplastic conditions. This has made the development of standardized diagnostic algorithms difficult.1 The workup of patients with FUO begins with a detailed history and physical examination, followed by a core set of microbiology cultures, imaging studies, and blood tests on all patients. Further testing is individualized, based on key clinical findings, also known as pivot points.2 Unfortunately, many of the diseases presenting as FUO have overlapping symptoms and signs, somewhat limiting the utility of pivot points. In a prospective study, 81% of these potentially diagnostic clues were misleading, although 19% of them contributed to the final diagnosis.3 Key clinical findings in patients with FUO may trigger a barrage of diagnostic tests, often leading to a large number of false‐positive results.4 Clinicians who investigate patients with FUO must remember that many clues are diagnostic dead ends, and should be wary of drawing positively‐false conclusions.
Fever pattern is usually not helpful in the diagnosis of FUO, with occasional exceptions, such as the tertian and quartan fevers in some forms of malaria. A minority of patients with Hodgkin's disease have Pel‐Ebstein fevers, in which 1 to 2 weeks of fever alternate with an afebrile period of similar or longer duration. More often, fever in lymphoma waxes and wanes unpredictably,5 a circumstance that may lead to the mistaken impression of response to antibiotics, as in this case.
Tissue biopsies are helpful in FUO when suggestive findings are present on physical examination or imaging studies. In patients with lymphadenopathy and FUO, lymph node biopsy is a high‐yield procedure, contributing to the final diagnosis 46% of the time. This is exceeded only by biopsies of skin lesions, which have a 63% diagnostic yield in FUO.3 In older patients with FUO, temporal artery biopsies have a significant diagnostic yield, in the range of 16% to 17%. Liver biopsies have a similar yield (14‐17%), but a higher risk of complications. Bone marrow biopsies have a low yield in most FUO patients.1
AITL makes up 1% of all non‐Hodgkin's lymphomas. AITL was once known as angioimmunoblastic lymphadenopathy, and was thought to be either a disorder of immune regulation or a premalignant lymphoid disease. However, molecular diagnostic techniques have established that monoclonal T‐cell populations and cytogenetic abnormalities are usually present at the time of diagnosis.6, 7 The prognosis in AITL is unfavorable. Disease is usually widespread at the time of diagnosis. Most patients achieve complete remission with anthracycline‐based chemotherapy, such as CHOP, but the duration of remission is often brief, and median survival after diagnosis is only 3 years.6 Novel treatments under investigation include denileukin diftitox,8 a fusion protein of interleukin‐2 (IL‐2) conjugated to diphtheria toxin, which leads to apoptosis of cells expressing the IL‐2 receptor; rituximab, which targets the reactive population of B‐cells in AITL, rather than the malignant clone of T‐cells; and antiangiogenic therapy, such as thalidomide.9
The diagnosis of AITL is usually elusive, and the average patient has symptoms for 4 months prior to diagnosis.6 This patient's clinical presentation, while certainly not specific, was typical of AITL. AITL usually presents as fever of unknown origin with generalized, nonbulky lymphadenopathy. Fever is present in 57% of patients with AITL, and up to 2% of FUO is caused by AITL.6, 10 Other features of this patient's illness, such as night sweats, weight loss, pruritus, and arthritis, are common in AITL.
T‐cell depletion and immune dysregulation are frequent in AITL, explaining why AITL shares a number of clinical features with HIV disease. These include a high incidence of drug rashes, immune thrombocytopenic purpura, polyclonal hypergammaglobulinemia, and autoantibodies.6, 7 As with HIV patients, death in AITL is often due to opportunistic infections or diffuse large B‐cell lymphomas.7 Over 95% of patients with AITL display a proliferation of EBV‐infected B cells, presumably from immune dysregulation, and the B‐cell lymphomas in these patients are usually EBV‐positive.11
Because AITL is a proinflammatory state, false‐positive antibody tests are fairly common. The occasional occurrence of positive HIV enzyme immunoassays, with indeterminate Western blot results, may be a particular source of diagnostic confusion.12, 13 The significance of indeterminate Western blots is often erroneously communicated to patients, as happened here. Indeterminate HIV Western blots may be seen in HIV seroconversion, HIV‐2 infection, or advanced HIV disease with loss of core antibody. They may also result from laboratory error, multiparity, syphilis, malaria, or cross‐reacting antibodies in autoimmune diseases. Indeterminate HIV Western blots in low‐risk patients usually do not represent true HIV infection.14
KEY POINTS FOR HOSPITALISTS
-
FUO is one of the most challenging diagnoses faced by hospitalists. Biopsies of new skin lesions and enlarged lymph nodes are particularly high‐yield diagnostic procedures in FUO. Fever pattern is generally not helpful in the diagnosis of FUO, with few exceptions, such as the tertian fevers of malaria.
-
Misleading and false‐positive tests results often occur in the course of FUO evaluation, due to the sheer number of tests ordered, and the higher likelihood of false‐positive serologic tests in the setting of inflammatory states.
-
AITL may be an elusive diagnosis in FUO with diverse clinical features, including weight loss, night sweats, rashes, arthritis, autoantibodies, immune dysregulation, and opportunistic infections. The prognosis traditionally has been guarded, but may be more hopeful in an era of emerging molecular therapies.
The approach to clinical conundrums by an expert clinician is revealed through presentation of an actual patient's case in an approach typical of morning report. Similar to patient care, sequential pieces of information are provided to the clinician who is unfamiliar with the case. The focus is on the thought processes of both the clinical team caring for the patient and the discussant.
A51‐year‐old woman presented after 5 days of fever, rigors, anorexia, right‐sided abdominal pain, nausea, and dizziness. She had 2 loose stools the day before admission, without blood or mucus, but otherwise had recently been constipated. She denied cough, shortness of breath, chest pain, headache, sore throat, rash, arthritis, or dysuria.
In a 51‐year‐old woman with right‐sided abdominal pain and systemic symptoms, major concerns include biliary disease, liver abscess, or appendicitis. Right‐sided diverticulitis would be more unusual. Pyelonephritis infrequently presents with epigastric and lower quadrant pain, instead of flank pain. Basilar pneumonia may present with abdominal pain, but this is less likely in the absence of respiratory symptoms.
The patient used an albuterol inhaler for mild asthma and had experienced an episode of herpes zoster 7 years prior, but was otherwise well. Her surgical history was notable for a remote appendectomy. She was a native of the Dominican Republic who had lived in the United States for the past 20 years. She visited the Dominican Republic for 3 weeks every year, with her last visit occurring about 10 months before. She was a cleaning and maintenance worker. She had 2 adult children in good health, was divorced from her husband, and had not been sexually active for the past 8 years. The patient had no pets or other animal exposures. She did not smoke, drink alcohol, or use intravenous drugs.
The remote episode of shingles makes me a bit worried about chronic human immunodeficiency virus (HIV) infection. As a native of and traveler to the Dominican Republic, she is at risk for a variety of tropical pathogens. Hyperinfection syndrome from strongyloides can cause fever and bacteremia, but this is almost always associated with significant immunosuppression. Dengue fever has become very common in the Caribbean, but should occur within 2 weeks of travel. Her work in cleaning and maintenance might bring her into contact with rats and mice, putting her at risk for leptospirosis. This can present as a fairly nonspecific febrile syndrome, but this is unlikely without a major complaint of headache.
The patient appeared fatigued. Her temperature was 39.7C, her heart rate was 110 beats per minute, and her blood pressure 80/62 mm Hg. The oropharynx was normal. Mild cervical lymphadenopathy was present (less than 1 cm in diameter). The chest was clear and the cardiac examination unremarkable. Bowel sounds were present. Moderate right‐sided abdominal tenderness was noted, somewhat more marked in the right lower quadrant, without guarding or rebound. There was no hepatosplenomegaly. There was no rash. A bedside right upper quadrant ultrasound was negative for gallstones.
Her low blood pressure is concerning for bacterial sepsis. The negative right upper quadrant ultrasound makes cholecystitis or cholangitis less likely, but does not exclude diverticulitis or pelvic inflammatory disease. She lacks peritoneal signs, but they may be absent in these conditions. Another worrisome finding on her physical examination is cervical lymphadenopathy. In an older patient, this raises the specter of malignancy. In a younger patient, it could suggest a mononucleosis syndrome from Epstein‐Barr virus (EBV) or cytomegalovirus (CMV). In addition, HIV must be considered in any patient with unexplained lymphadenopathy.
She received intravenous levofloxacin and 1 L of intravenous normal saline, with a rise in her blood pressure to 100/59 mm Hg. Her white blood cell count was 5.0, with 71% polys and 9% bands. The hematocrit was 32%, with a normal mean corpuscular volume. The erythrocyte sedimentation rate (ESR) was 109 mm/hour. The platelet count, serum electrolytes, creatinine, aminotransferases, alkaline phosphatase, bilirubin, amylase, and lipase were normal. The serum level of lactate dehydrogenase (LDH) was 498 unit/L (normal range, 107231 units/L). Computed tomography (CT) scan of the abdomen showed multiple enlarged lymph nodes up to 1.2 cm in size along the gastrohepatic ligament and the mesentery, with mild associated fat stranding, consistent with mesenteric lymphadenitis.
Her blood pressure has responded to fluids; perhaps she was just volume‐depleted from not eating for several days. She has bandemia, which is consistent with acute bacterial infection, but might also signify a stress response. The low hematocrit and high ESR raise the possibility of anemia of chronic disease; perhaps her illness is more longstanding than her presentation suggests. Mesenteric lymphadenitis may be related to EBV and HIV, but in a patient originally from the Caribbean, it raises the possibility of gastrointestinal tuberculosis or histoplasmosis. Human T‐cell lymphotropic virus type 1 (HTLV‐1) is also endemic in the Caribbean, and may cause adult T‐cell leukemia/lymphoma (ATLL), which could explain her lymphadenopathy and elevated LDH. Mesenteric lymphadenitis is also characteristic of several bacterial infections, especially Yersinia, Salmonella, and Bartonella. The lack of diarrhea makes yersiniosis doubtful, and the absence of cat exposure makes bartonellosis unlikely. Salmonella infection is also associated with diarrhea, except for typhoid fever, in which patients have diarrhea, constipation, or normal stools.
A urine culture and 3 sets of blood cultures obtained prior to the initiation of antibiotics were negative. A blood smear showed no malaria or babesia parasites. The patient's fever continued for the first 2 days of her hospitalization, but subsequently abated. The patient had no loose stools during her hospitalization. Serologies for EBV, hepatitis A, CMV, and toxoplasmosis were indicative of remote infection. Serum rapid plasma reagin (RPR), Bartonella antibodies, antinuclear antibodies, hepatitis B surface antigen, and hepatitis C antibody were negative. Tuberculin skin testing and urinary histoplasma antigen were negative. By the fifth hospital day, she had been afebrile for over 48 hours, and her abdominal pain had improved, though it had not completely resolved. She was discharged to complete a 10‐day course of levofloxacin.
It is not clear to me whether she has just experienced a spontaneous remission in her illness, or whether her disease course has truly been modified with antibiotics. Could she have typhoid fever or another salmonellosis? The patient has not traveled abroad in several months. If she had typhoid fever, the source of infection would have to be imported food, or exposure to a family member who was a carrier. The negative tuberculin skin test makes tuberculosis less likely, but does not exclude it entirely. Similarly, while a negative histoplasma urinary antigen essentially rules out acute disseminated histoplasmosis, as would be seen in acquired immune deficiency syndrome (AIDS), it is less sensitive for more chronic forms of disseminated histoplasmosis, including gastrointestinal involvement.
One week later, the patient returned to the emergency department with recurrent abdominal pain, anorexia, fever, night sweats, and increased swelling of the lymph nodes in her neck. She had lost a total of 4.4 kg (10 lb) since the onset of her illness. Physical examination revealed moderate (up to 2 cm), slightly tender cervical and inguinal lymphadenopathy, and continued moderate right‐sided abdominal tenderness. Mesenteric, retroperitoneal, and inguinal lymphadenopathy was more prominent than on the prior CT scan (Figure 1).She was told by the emergency room physicians that the HIV test obtained during the prior hospitalization was positive. Further questioning elicited that her estranged husband had been promiscuous prior to their separation. She was transferred to a second hospital for further care. Blood cultures were sent for fungi and acid‐fast bacilli.

Everyone with fever of unknown origin (FUO) deserves an HIV test. Is this just lymphadenopathy from HIV, or is she suffering from an opportunistic infection? Mycobacterium avium infection is an attractive explanation for her fever, abdominal pain, and lymphadenopathy, but I would hold off on empiric treatment until the results of a CD4+ cell count were available. Could she have a secondary, HIV‐related lymphoma?
Full review of records from the outside hospital showed that the patient had a positive HIV enzyme immunoassay, with an indeterminate HIV Western blot. The patient's CD4 cell count was 313/cm3, with a CD4/CD8 ratio within the normal range. Her HIV enzyme immunoassay was repeated and found to be negative, and the HIV viral load was undetectable.
The HIV enzyme immunoassay is only a screening test, and must be confirmed with a positive HIV Western blot. (In most clinical laboratories, this is done automatically before the test is reported.) Indeterminate HIV Western blots are common in acute HIV infection, but outside of this setting, most patients with indeterminate HIV Western blots turn out not to have HIV infection. I am still very concerned about lymphoma, and would pursue a lymph node biopsy. Disseminated tuberculosis is still possible.
Biopsy of a right inguinal lymph node was performed on the third day of the second hospitalization. The patient was persistently febrile, developed swelling of the knees, ankles, and interphalangeal joints of the second and third digits of the left hand, and complained of pruritus. Anti‐double‐stranded DNA antibody, antineutrophilic cytoplasmic antibody, and Brucella antibody were negative. Antibody against cyclic citrullinated peptide (CCP) was weakly positive.
Now she has a more florid syndrome, with arthritic symptoms. Sarcoidosis is an attractive explanation for her fever, lymphadenopathy, and arthritis. Lupus could explain some features of her presentation, but the negative serology makes it unlikely. Antibody to cyclic citrullinated peptide is a newer diagnostic test for rheumatoid arthritis. Although anti‐CCP is more specific than rheumatoid factor for the diagnosis of rheumatoid arthritis, this result could still be a false positive, particularly given the low titer. As well, the patient has more impressive lymphadenopathy than is usual for rheumatoid arthritis. Reactive arthritis can follow enteric infection with Campylobacter, Salmonella, Yersinia, and Shigella, but lymphadenopathy is not a feature. Arthritis may be prominent in parvovirus B19, rubella, disseminated gonococcal infection, and Lyme disease, but mesenteric lymphadenitis and a prolonged, waxing and waning course would not be expected with any of these. I worry that the arthritis and pruritus are paraneoplastic manifestations of lymphoma.
The inguinal lymph node biopsy showed markedly distorted nodal architecture with an atypical proliferation of small‐sized to large‐sized lymphoid cells, with predominantly round nuclei, vesicular chromatin, small nucleoli, and variable amounts of clear to eosinophilic cytoplasm (Figure 2). Residual follicles and occasional apoptotic bodies and mitoses were seen. Large B‐cells were frequently present, which stained positive for EBV‐associated mRNA by in situ hybridization studies. Immunoperoxidase staining revealed that the atypical cell population was largely composed of CD4+ T‐cells. T‐cell receptor gene rearrangement studies demonstrated that the T‐cell population was monoclonal. These results were consistent with angioimmunoblastic T‐cell lymphoma (AITL). Positron emission tomography (PET) scanning was performed, showing diffuse fluorodeoxyglucose (FDG)‐avid lymphadenopathy involving cervical, axillary, mediastinal, retroperitoneal, mesenteric, and inguinal lymph nodes, up to 2 cm in diameter. The patient completed 6 cycles of cyclophosphamide, doxorubicin, vincristine, and prednisone (CHOP), as well as experimental treatment with denileukin diftitox. Her fever and arthritis subsided quickly, and she was clinically well and free of disease by CT scans and PET scans 1 year after diagnosis.

COMMENTARY
The differential diagnosis of FUO is one of the largest in medicine, encompassing a dizzying range of infectious, inflammatory, and neoplastic conditions. This has made the development of standardized diagnostic algorithms difficult.1 The workup of patients with FUO begins with a detailed history and physical examination, followed by a core set of microbiology cultures, imaging studies, and blood tests on all patients. Further testing is individualized, based on key clinical findings, also known as pivot points.2 Unfortunately, many of the diseases presenting as FUO have overlapping symptoms and signs, somewhat limiting the utility of pivot points. In a prospective study, 81% of these potentially diagnostic clues were misleading, although 19% of them contributed to the final diagnosis.3 Key clinical findings in patients with FUO may trigger a barrage of diagnostic tests, often leading to a large number of false‐positive results.4 Clinicians who investigate patients with FUO must remember that many clues are diagnostic dead ends, and should be wary of drawing positively‐false conclusions.
Fever pattern is usually not helpful in the diagnosis of FUO, with occasional exceptions, such as the tertian and quartan fevers in some forms of malaria. A minority of patients with Hodgkin's disease have Pel‐Ebstein fevers, in which 1 to 2 weeks of fever alternate with an afebrile period of similar or longer duration. More often, fever in lymphoma waxes and wanes unpredictably,5 a circumstance that may lead to the mistaken impression of response to antibiotics, as in this case.
Tissue biopsies are helpful in FUO when suggestive findings are present on physical examination or imaging studies. In patients with lymphadenopathy and FUO, lymph node biopsy is a high‐yield procedure, contributing to the final diagnosis 46% of the time. This is exceeded only by biopsies of skin lesions, which have a 63% diagnostic yield in FUO.3 In older patients with FUO, temporal artery biopsies have a significant diagnostic yield, in the range of 16% to 17%. Liver biopsies have a similar yield (14‐17%), but a higher risk of complications. Bone marrow biopsies have a low yield in most FUO patients.1
AITL makes up 1% of all non‐Hodgkin's lymphomas. AITL was once known as angioimmunoblastic lymphadenopathy, and was thought to be either a disorder of immune regulation or a premalignant lymphoid disease. However, molecular diagnostic techniques have established that monoclonal T‐cell populations and cytogenetic abnormalities are usually present at the time of diagnosis.6, 7 The prognosis in AITL is unfavorable. Disease is usually widespread at the time of diagnosis. Most patients achieve complete remission with anthracycline‐based chemotherapy, such as CHOP, but the duration of remission is often brief, and median survival after diagnosis is only 3 years.6 Novel treatments under investigation include denileukin diftitox,8 a fusion protein of interleukin‐2 (IL‐2) conjugated to diphtheria toxin, which leads to apoptosis of cells expressing the IL‐2 receptor; rituximab, which targets the reactive population of B‐cells in AITL, rather than the malignant clone of T‐cells; and antiangiogenic therapy, such as thalidomide.9
The diagnosis of AITL is usually elusive, and the average patient has symptoms for 4 months prior to diagnosis.6 This patient's clinical presentation, while certainly not specific, was typical of AITL. AITL usually presents as fever of unknown origin with generalized, nonbulky lymphadenopathy. Fever is present in 57% of patients with AITL, and up to 2% of FUO is caused by AITL.6, 10 Other features of this patient's illness, such as night sweats, weight loss, pruritus, and arthritis, are common in AITL.
T‐cell depletion and immune dysregulation are frequent in AITL, explaining why AITL shares a number of clinical features with HIV disease. These include a high incidence of drug rashes, immune thrombocytopenic purpura, polyclonal hypergammaglobulinemia, and autoantibodies.6, 7 As with HIV patients, death in AITL is often due to opportunistic infections or diffuse large B‐cell lymphomas.7 Over 95% of patients with AITL display a proliferation of EBV‐infected B cells, presumably from immune dysregulation, and the B‐cell lymphomas in these patients are usually EBV‐positive.11
Because AITL is a proinflammatory state, false‐positive antibody tests are fairly common. The occasional occurrence of positive HIV enzyme immunoassays, with indeterminate Western blot results, may be a particular source of diagnostic confusion.12, 13 The significance of indeterminate Western blots is often erroneously communicated to patients, as happened here. Indeterminate HIV Western blots may be seen in HIV seroconversion, HIV‐2 infection, or advanced HIV disease with loss of core antibody. They may also result from laboratory error, multiparity, syphilis, malaria, or cross‐reacting antibodies in autoimmune diseases. Indeterminate HIV Western blots in low‐risk patients usually do not represent true HIV infection.14
KEY POINTS FOR HOSPITALISTS
-
FUO is one of the most challenging diagnoses faced by hospitalists. Biopsies of new skin lesions and enlarged lymph nodes are particularly high‐yield diagnostic procedures in FUO. Fever pattern is generally not helpful in the diagnosis of FUO, with few exceptions, such as the tertian fevers of malaria.
-
Misleading and false‐positive tests results often occur in the course of FUO evaluation, due to the sheer number of tests ordered, and the higher likelihood of false‐positive serologic tests in the setting of inflammatory states.
-
AITL may be an elusive diagnosis in FUO with diverse clinical features, including weight loss, night sweats, rashes, arthritis, autoantibodies, immune dysregulation, and opportunistic infections. The prognosis traditionally has been guarded, but may be more hopeful in an era of emerging molecular therapies.
- ,,.A comprehensive evidence‐based approach to fever of unknown origin.Arch Intern Med.2003;163:545–551.
- ,.The art of diagnosis: solving the clinicopathological exercise.N Engl J Med.1982;306:1263–1268.
- ,,, et al.A prospective multicenter study on fever of unknown origin: the yield of a structured diagnostic protocol.Medicine (Baltimore).2007;86:26–38.
- ,,.Fever of unknown origin (FUO). II. Diagnostic procedures in a prospective multicenter study of 167 patients. The Netherlands FUO Study Group.Medicine (Baltimore).1997;76:401–414.
- ,.Neoplastic diseases. In:Murray HW, ed.FUO: Fever of Undetermined Origin.New York, NY:Futura Publishing;1983:39–48.
- ,,, et al.Angioimmunoblastic T‐cell lymphoma: clinical and laboratory features at diagnosis in 77 patients.Medicine (Baltimore).2007;86:282–292.
- ,,.Angioimmunoblastic T‐cell lymphoma.Br J Haematol.2003;121:681–691.
- ,,, et al.Phase II trial of denileukin diftitox for relapsed/refractory T‐cell non‐Hodgkin lymphoma.Br J Haematol.2007;136:439–447.
- ,.Angioimmunoblastic T‐cell lymphoma: still a dismal prognosis with current treatment approaches.Leuk Lymphoma.2007;48:645–646.
- ,,.Fever of unknown origin (FUO). I. A prospective multicenter study of 167 patients with FUO, using fixed epidemiologic entry criteria. The Netherlands FUO Study Group.Medicine (Baltimore).1997;76:392–400.
- ,,, et al.Histologic evolution of angioimmunoblastic T‐cell lymphoma in consecutive biopsies: clinical correlation and insights into natural history and disease progression.Am J Surg Pathol.2007;31:1077–1088.
- ,,,.Angioimmunoblastic lymphadenopathy, immunoblastic lymphoma, and false‐positive seroconversion for human immunodeficiency virus.Ann Intern Med.1987;107:114.
- ,.Angioimmunoblastic T‐cell lymphoma associated with an antibody to human immunodeficiency virus protein.Int J Hematol.2003;78:160–162.
- ,,.Communicating indeterminate HIV Western blot test results to clients: an observational study of three community testing sites.AIDS Patient Care STDS.2006;20:620–627.
- ,,.A comprehensive evidence‐based approach to fever of unknown origin.Arch Intern Med.2003;163:545–551.
- ,.The art of diagnosis: solving the clinicopathological exercise.N Engl J Med.1982;306:1263–1268.
- ,,, et al.A prospective multicenter study on fever of unknown origin: the yield of a structured diagnostic protocol.Medicine (Baltimore).2007;86:26–38.
- ,,.Fever of unknown origin (FUO). II. Diagnostic procedures in a prospective multicenter study of 167 patients. The Netherlands FUO Study Group.Medicine (Baltimore).1997;76:401–414.
- ,.Neoplastic diseases. In:Murray HW, ed.FUO: Fever of Undetermined Origin.New York, NY:Futura Publishing;1983:39–48.
- ,,, et al.Angioimmunoblastic T‐cell lymphoma: clinical and laboratory features at diagnosis in 77 patients.Medicine (Baltimore).2007;86:282–292.
- ,,.Angioimmunoblastic T‐cell lymphoma.Br J Haematol.2003;121:681–691.
- ,,, et al.Phase II trial of denileukin diftitox for relapsed/refractory T‐cell non‐Hodgkin lymphoma.Br J Haematol.2007;136:439–447.
- ,.Angioimmunoblastic T‐cell lymphoma: still a dismal prognosis with current treatment approaches.Leuk Lymphoma.2007;48:645–646.
- ,,.Fever of unknown origin (FUO). I. A prospective multicenter study of 167 patients with FUO, using fixed epidemiologic entry criteria. The Netherlands FUO Study Group.Medicine (Baltimore).1997;76:392–400.
- ,,, et al.Histologic evolution of angioimmunoblastic T‐cell lymphoma in consecutive biopsies: clinical correlation and insights into natural history and disease progression.Am J Surg Pathol.2007;31:1077–1088.
- ,,,.Angioimmunoblastic lymphadenopathy, immunoblastic lymphoma, and false‐positive seroconversion for human immunodeficiency virus.Ann Intern Med.1987;107:114.
- ,.Angioimmunoblastic T‐cell lymphoma associated with an antibody to human immunodeficiency virus protein.Int J Hematol.2003;78:160–162.
- ,,.Communicating indeterminate HIV Western blot test results to clients: an observational study of three community testing sites.AIDS Patient Care STDS.2006;20:620–627.
Diabetic Dilemma
Diabetic foot infections are common, costly, and potentially catastrophic for patients. The average patient with a diabetic foot infection undergoes three surgical procedures, including toe amputation in 19% and leg amputation in 14%.1 The annual cost of diabetic foot osteomyelitis in the United States is $2.8 billion.2
Diabetics are uniquely predisposed to foot infections. Because of neuropathy, minor repetitive injury leads to large foot ulcers, especially over the metatarsal heads. Foot deformities result in soft tissue injury from poorly fitting shoes. Because of autonomic neuropathy, the diabetic foot sweats less, leading to dry, cracked skin for bacteria to invade. Resistance to infection is lower because of neutrophil dysfunction in hyperglycemia. Vascular insufficiency impairs both wound healing and the immune response.3
The major principles of therapy are as follows:
1. Use an empiric antibiotic regimen with broad coverage against gram-positive, gram-negative, and anaerobic organisms. Diabetic foot infections are usually polymicrobial. Except for mild infections, in which therapy directed at gram-positive organisms may suffice, initial therapy should cover streptococci, methicillin-sensitive Staphylococcus aureus, E. coli, Proteus, and anaerobes.
Many studies of antibiotic therapy in diabetic foot infections have been performed, without demonstrating a clear superiority for any one regimen. The 2004 guidelines of the Infectious Diseases Society of America list 13 acceptable regimens for moderate diabetic foot infections.4 Useful single drug regimens include ampicillin-sulbactam, piperacillin-tazobactam, levofloxacin, and cefoxitin. For the penicillin-allergic patient, clindamycin and ciprofloxacin is a useful combination. Empiric vancomycin should be reserved for patients with a history of MRSA, treatment failure, or severe infection. (I would also reserve carbapenems, such as imipenem-cilastatin, for more severe infections, to prevent antibiotic resistance against a class of drugs representing our last defense against highly resistant gram-negative organisms.)
Once the patient is clinically improved and results of adequate cultures are available, consideration can be given to narrowing the course of therapy. Most diabetic foot infections can be treated with some combination of intravenous and oral therapy.
2. Have a high clinical suspicion for osteomyelitis. Osteomyelitis is extremely common in diabetic foot infections due to plantar ulceration and poor soft tissue coverage. Cure rates are reduced in osteomyelitis because dead bone acts as a nidus for persistent infection. The most useful diagnostic maneuver for osteomyelitis is deep probing of the wound with a sterile swab at the bedside. If a gritty sensation is felt, osteomyelitis is likely, and further diagnostic testing is probably unnecessary.
If the physical examination is equivocal, plain radiographs should be obtained to look for bony erosions. If these are negative, MRI is the next most useful diagnostic step. Bone scans are of limited value due to their low specificity. (Charcot arthropathy is a common cause of false-positive bone scans in this setting.)
Because of the high prevalence of osteomyelitis in diabetic foot infections, my own practice is to err on the side of longer, rather than shorter, antibiotic therapy.
3. Surgical debridement is required for many diabetic foot infections. Develop a collaborative relationship with a vascular or orthopedic surgeon with interest and expertise in the management of diabetic foot infections. Necrotic and gangrenous material should be removed. Ideally, dead bone should be debrided, both therapeutically and to help establish a bacteriologic diagnosis. (When removal of dead bone would result in loss of function or might create a non-healing wound, the option of long-term antibiotic suppression could be explored with an infectious disease specialist.)
4. When osteomyelitis is present, bone cultures help to define the optimal antibiotic therapy. Recent studies have confirmed older data regarding the poor correlation between surface cultures and bone cultures. The latter are preferred, when feasible.
5. Consider revascularization. Most diabetic foot infections arise in the setting of vascular insufficiency. At a minimum, patients with diabetic foot infections should have ankle-brachial indices performed for screening. Because diabetics may have falsely elevated ankle pressures due to calcified and non-compressible arteries, additional diagnostic studies may be useful, such as segmental pressures and Doppler pulse volume recordings.
Measurement of the transcutaneous oxygen concentration (TcP02) has been recommended, particularly in assessing which patients may benefit from hyperbaric oxygen. However, the TcP02 is not widely available, and the benefit of hyperbaric oxygen in this setting remains controversial.
6. Patients should be educated in meticulous foot care to prevent recurrences and reinfections. A diabetic foot infection may indicate that the patient lacks the knowledge, resources, or motivation for proper foot care. It also suggests that something is seriously awry with the patient’s diabetic regimen, compliance, or both. Hospital admissions for diabetic foot infections provide an opportunity to revise the patient’s diabetic medications; to educate the patient regarding wound care, skin care, and daily foot self-examination; to provide additional resources such as visiting nurses; and to refer patients for podiatric care, including tailored shoes, orthotics, and, if necessary, casting to off-load ulcers. TH
Dr. Ross is an instructor in medicine at Harvard Medical School.
References
- Hill SL, Holtzman GI, Buse R. The effects of peripheral vascular disease with osteomyelitis in the diabetic foot. Am J Surg. 1999 Apr;177(4):282-286.
- Gordois A, Scuffham P, Shearer A, et al. The health care costs of diabetic peripheral neuropathy in the United States. Diabetes Care. 2003;26(6):1790-1795.
- Lazzarini L, Mader JT, Calhoun JH. Diabetic foot infection. In: Calhoun JH, Mader JT, eds. Musculoskeletal Infections. New York, NY. Marcel Dekker. 2003.
- Lipsky BA, Berendt AR, Deery HG, et al. Diagnosis and treatment of diabetic foot infections. Clin Infect Dis. 2004 Oct;39(7):885-910.
Diabetic foot infections are common, costly, and potentially catastrophic for patients. The average patient with a diabetic foot infection undergoes three surgical procedures, including toe amputation in 19% and leg amputation in 14%.1 The annual cost of diabetic foot osteomyelitis in the United States is $2.8 billion.2
Diabetics are uniquely predisposed to foot infections. Because of neuropathy, minor repetitive injury leads to large foot ulcers, especially over the metatarsal heads. Foot deformities result in soft tissue injury from poorly fitting shoes. Because of autonomic neuropathy, the diabetic foot sweats less, leading to dry, cracked skin for bacteria to invade. Resistance to infection is lower because of neutrophil dysfunction in hyperglycemia. Vascular insufficiency impairs both wound healing and the immune response.3
The major principles of therapy are as follows:
1. Use an empiric antibiotic regimen with broad coverage against gram-positive, gram-negative, and anaerobic organisms. Diabetic foot infections are usually polymicrobial. Except for mild infections, in which therapy directed at gram-positive organisms may suffice, initial therapy should cover streptococci, methicillin-sensitive Staphylococcus aureus, E. coli, Proteus, and anaerobes.
Many studies of antibiotic therapy in diabetic foot infections have been performed, without demonstrating a clear superiority for any one regimen. The 2004 guidelines of the Infectious Diseases Society of America list 13 acceptable regimens for moderate diabetic foot infections.4 Useful single drug regimens include ampicillin-sulbactam, piperacillin-tazobactam, levofloxacin, and cefoxitin. For the penicillin-allergic patient, clindamycin and ciprofloxacin is a useful combination. Empiric vancomycin should be reserved for patients with a history of MRSA, treatment failure, or severe infection. (I would also reserve carbapenems, such as imipenem-cilastatin, for more severe infections, to prevent antibiotic resistance against a class of drugs representing our last defense against highly resistant gram-negative organisms.)
Once the patient is clinically improved and results of adequate cultures are available, consideration can be given to narrowing the course of therapy. Most diabetic foot infections can be treated with some combination of intravenous and oral therapy.
2. Have a high clinical suspicion for osteomyelitis. Osteomyelitis is extremely common in diabetic foot infections due to plantar ulceration and poor soft tissue coverage. Cure rates are reduced in osteomyelitis because dead bone acts as a nidus for persistent infection. The most useful diagnostic maneuver for osteomyelitis is deep probing of the wound with a sterile swab at the bedside. If a gritty sensation is felt, osteomyelitis is likely, and further diagnostic testing is probably unnecessary.
If the physical examination is equivocal, plain radiographs should be obtained to look for bony erosions. If these are negative, MRI is the next most useful diagnostic step. Bone scans are of limited value due to their low specificity. (Charcot arthropathy is a common cause of false-positive bone scans in this setting.)
Because of the high prevalence of osteomyelitis in diabetic foot infections, my own practice is to err on the side of longer, rather than shorter, antibiotic therapy.
3. Surgical debridement is required for many diabetic foot infections. Develop a collaborative relationship with a vascular or orthopedic surgeon with interest and expertise in the management of diabetic foot infections. Necrotic and gangrenous material should be removed. Ideally, dead bone should be debrided, both therapeutically and to help establish a bacteriologic diagnosis. (When removal of dead bone would result in loss of function or might create a non-healing wound, the option of long-term antibiotic suppression could be explored with an infectious disease specialist.)
4. When osteomyelitis is present, bone cultures help to define the optimal antibiotic therapy. Recent studies have confirmed older data regarding the poor correlation between surface cultures and bone cultures. The latter are preferred, when feasible.
5. Consider revascularization. Most diabetic foot infections arise in the setting of vascular insufficiency. At a minimum, patients with diabetic foot infections should have ankle-brachial indices performed for screening. Because diabetics may have falsely elevated ankle pressures due to calcified and non-compressible arteries, additional diagnostic studies may be useful, such as segmental pressures and Doppler pulse volume recordings.
Measurement of the transcutaneous oxygen concentration (TcP02) has been recommended, particularly in assessing which patients may benefit from hyperbaric oxygen. However, the TcP02 is not widely available, and the benefit of hyperbaric oxygen in this setting remains controversial.
6. Patients should be educated in meticulous foot care to prevent recurrences and reinfections. A diabetic foot infection may indicate that the patient lacks the knowledge, resources, or motivation for proper foot care. It also suggests that something is seriously awry with the patient’s diabetic regimen, compliance, or both. Hospital admissions for diabetic foot infections provide an opportunity to revise the patient’s diabetic medications; to educate the patient regarding wound care, skin care, and daily foot self-examination; to provide additional resources such as visiting nurses; and to refer patients for podiatric care, including tailored shoes, orthotics, and, if necessary, casting to off-load ulcers. TH
Dr. Ross is an instructor in medicine at Harvard Medical School.
References
- Hill SL, Holtzman GI, Buse R. The effects of peripheral vascular disease with osteomyelitis in the diabetic foot. Am J Surg. 1999 Apr;177(4):282-286.
- Gordois A, Scuffham P, Shearer A, et al. The health care costs of diabetic peripheral neuropathy in the United States. Diabetes Care. 2003;26(6):1790-1795.
- Lazzarini L, Mader JT, Calhoun JH. Diabetic foot infection. In: Calhoun JH, Mader JT, eds. Musculoskeletal Infections. New York, NY. Marcel Dekker. 2003.
- Lipsky BA, Berendt AR, Deery HG, et al. Diagnosis and treatment of diabetic foot infections. Clin Infect Dis. 2004 Oct;39(7):885-910.
Diabetic foot infections are common, costly, and potentially catastrophic for patients. The average patient with a diabetic foot infection undergoes three surgical procedures, including toe amputation in 19% and leg amputation in 14%.1 The annual cost of diabetic foot osteomyelitis in the United States is $2.8 billion.2
Diabetics are uniquely predisposed to foot infections. Because of neuropathy, minor repetitive injury leads to large foot ulcers, especially over the metatarsal heads. Foot deformities result in soft tissue injury from poorly fitting shoes. Because of autonomic neuropathy, the diabetic foot sweats less, leading to dry, cracked skin for bacteria to invade. Resistance to infection is lower because of neutrophil dysfunction in hyperglycemia. Vascular insufficiency impairs both wound healing and the immune response.3
The major principles of therapy are as follows:
1. Use an empiric antibiotic regimen with broad coverage against gram-positive, gram-negative, and anaerobic organisms. Diabetic foot infections are usually polymicrobial. Except for mild infections, in which therapy directed at gram-positive organisms may suffice, initial therapy should cover streptococci, methicillin-sensitive Staphylococcus aureus, E. coli, Proteus, and anaerobes.
Many studies of antibiotic therapy in diabetic foot infections have been performed, without demonstrating a clear superiority for any one regimen. The 2004 guidelines of the Infectious Diseases Society of America list 13 acceptable regimens for moderate diabetic foot infections.4 Useful single drug regimens include ampicillin-sulbactam, piperacillin-tazobactam, levofloxacin, and cefoxitin. For the penicillin-allergic patient, clindamycin and ciprofloxacin is a useful combination. Empiric vancomycin should be reserved for patients with a history of MRSA, treatment failure, or severe infection. (I would also reserve carbapenems, such as imipenem-cilastatin, for more severe infections, to prevent antibiotic resistance against a class of drugs representing our last defense against highly resistant gram-negative organisms.)
Once the patient is clinically improved and results of adequate cultures are available, consideration can be given to narrowing the course of therapy. Most diabetic foot infections can be treated with some combination of intravenous and oral therapy.
2. Have a high clinical suspicion for osteomyelitis. Osteomyelitis is extremely common in diabetic foot infections due to plantar ulceration and poor soft tissue coverage. Cure rates are reduced in osteomyelitis because dead bone acts as a nidus for persistent infection. The most useful diagnostic maneuver for osteomyelitis is deep probing of the wound with a sterile swab at the bedside. If a gritty sensation is felt, osteomyelitis is likely, and further diagnostic testing is probably unnecessary.
If the physical examination is equivocal, plain radiographs should be obtained to look for bony erosions. If these are negative, MRI is the next most useful diagnostic step. Bone scans are of limited value due to their low specificity. (Charcot arthropathy is a common cause of false-positive bone scans in this setting.)
Because of the high prevalence of osteomyelitis in diabetic foot infections, my own practice is to err on the side of longer, rather than shorter, antibiotic therapy.
3. Surgical debridement is required for many diabetic foot infections. Develop a collaborative relationship with a vascular or orthopedic surgeon with interest and expertise in the management of diabetic foot infections. Necrotic and gangrenous material should be removed. Ideally, dead bone should be debrided, both therapeutically and to help establish a bacteriologic diagnosis. (When removal of dead bone would result in loss of function or might create a non-healing wound, the option of long-term antibiotic suppression could be explored with an infectious disease specialist.)
4. When osteomyelitis is present, bone cultures help to define the optimal antibiotic therapy. Recent studies have confirmed older data regarding the poor correlation between surface cultures and bone cultures. The latter are preferred, when feasible.
5. Consider revascularization. Most diabetic foot infections arise in the setting of vascular insufficiency. At a minimum, patients with diabetic foot infections should have ankle-brachial indices performed for screening. Because diabetics may have falsely elevated ankle pressures due to calcified and non-compressible arteries, additional diagnostic studies may be useful, such as segmental pressures and Doppler pulse volume recordings.
Measurement of the transcutaneous oxygen concentration (TcP02) has been recommended, particularly in assessing which patients may benefit from hyperbaric oxygen. However, the TcP02 is not widely available, and the benefit of hyperbaric oxygen in this setting remains controversial.
6. Patients should be educated in meticulous foot care to prevent recurrences and reinfections. A diabetic foot infection may indicate that the patient lacks the knowledge, resources, or motivation for proper foot care. It also suggests that something is seriously awry with the patient’s diabetic regimen, compliance, or both. Hospital admissions for diabetic foot infections provide an opportunity to revise the patient’s diabetic medications; to educate the patient regarding wound care, skin care, and daily foot self-examination; to provide additional resources such as visiting nurses; and to refer patients for podiatric care, including tailored shoes, orthotics, and, if necessary, casting to off-load ulcers. TH
Dr. Ross is an instructor in medicine at Harvard Medical School.
References
- Hill SL, Holtzman GI, Buse R. The effects of peripheral vascular disease with osteomyelitis in the diabetic foot. Am J Surg. 1999 Apr;177(4):282-286.
- Gordois A, Scuffham P, Shearer A, et al. The health care costs of diabetic peripheral neuropathy in the United States. Diabetes Care. 2003;26(6):1790-1795.
- Lazzarini L, Mader JT, Calhoun JH. Diabetic foot infection. In: Calhoun JH, Mader JT, eds. Musculoskeletal Infections. New York, NY. Marcel Dekker. 2003.
- Lipsky BA, Berendt AR, Deery HG, et al. Diagnosis and treatment of diabetic foot infections. Clin Infect Dis. 2004 Oct;39(7):885-910.
Bacterial Meningitis
Perhaps no admission causes so much consternation and dread amongst caregivers and families as a case of suspected bacterial meningitis. Will the patient live? What infection control precautions are necessary? And, perhaps most urgently, do I need antibiotic prophylaxis? In this article I answer the questions hospitalists most often need to address in such circumstances.
1. Who should have a head CT prior to lumbar puncture (LP) for suspected meningitis? Patients with immunocompromise, papilledema, preexisting CNS disease, new onset seizures, altered level of consciousness, and focal neurological findings should have a head CT prior to LP.1 While herniation is rare after LP for purulent meningitis, patients with increased intracranial pressure at risk for herniation often have normal head CT scans. Therefore, herniation may be an uncommon but unpredictable complication of LP in this setting. The cause-and-effect relationship of herniation and LP has also been questioned.
2. Are there any cerebrospinal fluid (CSF) findings that exclude bacterial meningitis? A number of CSF findings make bacterial meningitis quite likely, including total leukocyte counts of more than 2,000/mm3, a positive gram stain, or very low CSF glucose. It is difficult, if not impossible, however, to exclude bacterial meningitis in patients with any degree of CSF pleocytosis. For example, 10% of patients with bacterial meningitis have less than 100 WBCs/mm3 in CSF, and 10% have lymphocyte predominance at presentation. Therefore, the safest course of action when bacterial meningitis is suspected on clinical grounds and CSF pleocytosis is present is to continue antibiotics until results of CSF cultures are available.
3. Which patients with suspected or proven meningitis should receive steroids? Steroids reduce neurologic damage from the inflammatory surge provoked by antibiotic-induced pneumococcal lysis. In a large European trial, dexamethasone given in 10-mg doses every six hours for four days (before or with the first dose of antibiotics) reduced mortality in pneumococcal meningitis.2 Benefits were not seen in patients with bacterial meningitis from other pathogens. Dexamethasone can be safely stopped as soon as pneumococcal meningitis is excluded.
4. How soon should patients receive antibiotics? When bacterial meningitis is likely, antibiotics should be given immediately, prior to imaging studies and lumbar puncture. In patients with a lower clinical likelihood of bacterial meningitis, antibiotics can be deferred, awaiting the results of diagnostic studies.
5. What empiric antibiotic therapy is appropriate? Adults 18-50 with suspected bacterial meningitis should receive therapy directed against Streptococcus pneumoniae and Neisseria meningitidis. Vancomycin should be dosed to achieve a relatively high trough level of 15-20 mcg/mL. For a 70-kg adult male with normal renal function, doses of vancomycin given at the rate of 1.5 gm IV every 12 hours and ceftriaxone at 2 gm IV every 12 hours are appropriate. Adults over 50, alcoholics, and immunocompromised adults of any age should also receive ampicillin doses of 2 gm IV every four hours to cover Listeria, in addition to vancomycin and ceftriaxone.3,4
6. What infection control precautions are required? Meningococcal meningitis patients should be placed on droplet precautions (private room, mask for all entering the room) until they have completed 24 hours of appropriate antibiotic therapy. Negative pressure ventilation is not required. Patients with pneumococcal or viral meningitis do not require isolation.
7. Who needs antibiotic prophylaxis after patient exposure? Chemoprophylaxis is overprescribed after exposures to patients with meningococcal meningitis. The only social contacts who should receive prophylaxis are household contacts, childcare contacts, and people who have had direct exposure to the patient’s oral secretions through actions such as kissing or sharing utensils or toothbrushes. The only healthcare workers requiring chemoprophylaxis are those who performed mouth-to-mouth resuscitation or any staff who were unmasked during intubation or suctioning of a patient. Regimens for chemoprophylaxis in adults include ciprofloxacin, 500 mg taken orally as a single dose, rifampin taken in doses of 600 mg twice daily for two days, or 250 mg of ceftriaxone, given intramuscularly. Ceftriaxone is preferred for pregnant women. Chemoprophylaxis is unnecessary after exposure to patients with pneumococcal or viral meningitis.
8. What is the significance of arthritis after meningococcal meningitis? A significant number of patients with meningococcal disease develop inflammatory polyarthritis about a week after the onset of infection. In most cases, this is a sterile, immune complex phenomenon that responds to anti-inflammatory therapy. If joint effusions are present, they should be aspirated to exclude septic arthritis and crystalline arthritis. TH
Dr. Ross is a hospitalist at Brigham and Women’s Hospital (Boston) and a fellow of the Infectious Diseases Society of America.
References
- Hasbun R, Abrahams J, Jekel J, et al. Computed tomography of the head before lumbar puncture in adults with suspected meningitis. N Engl J Med. 2001 Dec 13;345(24):1727-1733.
- de Gans J, van de Beek D. Dexamethasone in adults with bacterial meningitis. European dexamethasone in adulthood bacterial meningitis. N Engl J Med. 2002;347(20):1549-156.
- Tunkel AR, Hartman BJ, Kaplan SL, et al. Practice guidelines for the management of bacterial meningitis. Clin Infect Dis. 2004 Nov 1;39(9):1267-1284.
- van de Beek D, de Gans J, Tunkel AR, et al. Community-acquired bacterial meningitis in adults. N Engl J Med. 2006 Jan 5;354(1):44-53.
Perhaps no admission causes so much consternation and dread amongst caregivers and families as a case of suspected bacterial meningitis. Will the patient live? What infection control precautions are necessary? And, perhaps most urgently, do I need antibiotic prophylaxis? In this article I answer the questions hospitalists most often need to address in such circumstances.
1. Who should have a head CT prior to lumbar puncture (LP) for suspected meningitis? Patients with immunocompromise, papilledema, preexisting CNS disease, new onset seizures, altered level of consciousness, and focal neurological findings should have a head CT prior to LP.1 While herniation is rare after LP for purulent meningitis, patients with increased intracranial pressure at risk for herniation often have normal head CT scans. Therefore, herniation may be an uncommon but unpredictable complication of LP in this setting. The cause-and-effect relationship of herniation and LP has also been questioned.
2. Are there any cerebrospinal fluid (CSF) findings that exclude bacterial meningitis? A number of CSF findings make bacterial meningitis quite likely, including total leukocyte counts of more than 2,000/mm3, a positive gram stain, or very low CSF glucose. It is difficult, if not impossible, however, to exclude bacterial meningitis in patients with any degree of CSF pleocytosis. For example, 10% of patients with bacterial meningitis have less than 100 WBCs/mm3 in CSF, and 10% have lymphocyte predominance at presentation. Therefore, the safest course of action when bacterial meningitis is suspected on clinical grounds and CSF pleocytosis is present is to continue antibiotics until results of CSF cultures are available.
3. Which patients with suspected or proven meningitis should receive steroids? Steroids reduce neurologic damage from the inflammatory surge provoked by antibiotic-induced pneumococcal lysis. In a large European trial, dexamethasone given in 10-mg doses every six hours for four days (before or with the first dose of antibiotics) reduced mortality in pneumococcal meningitis.2 Benefits were not seen in patients with bacterial meningitis from other pathogens. Dexamethasone can be safely stopped as soon as pneumococcal meningitis is excluded.
4. How soon should patients receive antibiotics? When bacterial meningitis is likely, antibiotics should be given immediately, prior to imaging studies and lumbar puncture. In patients with a lower clinical likelihood of bacterial meningitis, antibiotics can be deferred, awaiting the results of diagnostic studies.
5. What empiric antibiotic therapy is appropriate? Adults 18-50 with suspected bacterial meningitis should receive therapy directed against Streptococcus pneumoniae and Neisseria meningitidis. Vancomycin should be dosed to achieve a relatively high trough level of 15-20 mcg/mL. For a 70-kg adult male with normal renal function, doses of vancomycin given at the rate of 1.5 gm IV every 12 hours and ceftriaxone at 2 gm IV every 12 hours are appropriate. Adults over 50, alcoholics, and immunocompromised adults of any age should also receive ampicillin doses of 2 gm IV every four hours to cover Listeria, in addition to vancomycin and ceftriaxone.3,4
6. What infection control precautions are required? Meningococcal meningitis patients should be placed on droplet precautions (private room, mask for all entering the room) until they have completed 24 hours of appropriate antibiotic therapy. Negative pressure ventilation is not required. Patients with pneumococcal or viral meningitis do not require isolation.
7. Who needs antibiotic prophylaxis after patient exposure? Chemoprophylaxis is overprescribed after exposures to patients with meningococcal meningitis. The only social contacts who should receive prophylaxis are household contacts, childcare contacts, and people who have had direct exposure to the patient’s oral secretions through actions such as kissing or sharing utensils or toothbrushes. The only healthcare workers requiring chemoprophylaxis are those who performed mouth-to-mouth resuscitation or any staff who were unmasked during intubation or suctioning of a patient. Regimens for chemoprophylaxis in adults include ciprofloxacin, 500 mg taken orally as a single dose, rifampin taken in doses of 600 mg twice daily for two days, or 250 mg of ceftriaxone, given intramuscularly. Ceftriaxone is preferred for pregnant women. Chemoprophylaxis is unnecessary after exposure to patients with pneumococcal or viral meningitis.
8. What is the significance of arthritis after meningococcal meningitis? A significant number of patients with meningococcal disease develop inflammatory polyarthritis about a week after the onset of infection. In most cases, this is a sterile, immune complex phenomenon that responds to anti-inflammatory therapy. If joint effusions are present, they should be aspirated to exclude septic arthritis and crystalline arthritis. TH
Dr. Ross is a hospitalist at Brigham and Women’s Hospital (Boston) and a fellow of the Infectious Diseases Society of America.
References
- Hasbun R, Abrahams J, Jekel J, et al. Computed tomography of the head before lumbar puncture in adults with suspected meningitis. N Engl J Med. 2001 Dec 13;345(24):1727-1733.
- de Gans J, van de Beek D. Dexamethasone in adults with bacterial meningitis. European dexamethasone in adulthood bacterial meningitis. N Engl J Med. 2002;347(20):1549-156.
- Tunkel AR, Hartman BJ, Kaplan SL, et al. Practice guidelines for the management of bacterial meningitis. Clin Infect Dis. 2004 Nov 1;39(9):1267-1284.
- van de Beek D, de Gans J, Tunkel AR, et al. Community-acquired bacterial meningitis in adults. N Engl J Med. 2006 Jan 5;354(1):44-53.
Perhaps no admission causes so much consternation and dread amongst caregivers and families as a case of suspected bacterial meningitis. Will the patient live? What infection control precautions are necessary? And, perhaps most urgently, do I need antibiotic prophylaxis? In this article I answer the questions hospitalists most often need to address in such circumstances.
1. Who should have a head CT prior to lumbar puncture (LP) for suspected meningitis? Patients with immunocompromise, papilledema, preexisting CNS disease, new onset seizures, altered level of consciousness, and focal neurological findings should have a head CT prior to LP.1 While herniation is rare after LP for purulent meningitis, patients with increased intracranial pressure at risk for herniation often have normal head CT scans. Therefore, herniation may be an uncommon but unpredictable complication of LP in this setting. The cause-and-effect relationship of herniation and LP has also been questioned.
2. Are there any cerebrospinal fluid (CSF) findings that exclude bacterial meningitis? A number of CSF findings make bacterial meningitis quite likely, including total leukocyte counts of more than 2,000/mm3, a positive gram stain, or very low CSF glucose. It is difficult, if not impossible, however, to exclude bacterial meningitis in patients with any degree of CSF pleocytosis. For example, 10% of patients with bacterial meningitis have less than 100 WBCs/mm3 in CSF, and 10% have lymphocyte predominance at presentation. Therefore, the safest course of action when bacterial meningitis is suspected on clinical grounds and CSF pleocytosis is present is to continue antibiotics until results of CSF cultures are available.
3. Which patients with suspected or proven meningitis should receive steroids? Steroids reduce neurologic damage from the inflammatory surge provoked by antibiotic-induced pneumococcal lysis. In a large European trial, dexamethasone given in 10-mg doses every six hours for four days (before or with the first dose of antibiotics) reduced mortality in pneumococcal meningitis.2 Benefits were not seen in patients with bacterial meningitis from other pathogens. Dexamethasone can be safely stopped as soon as pneumococcal meningitis is excluded.
4. How soon should patients receive antibiotics? When bacterial meningitis is likely, antibiotics should be given immediately, prior to imaging studies and lumbar puncture. In patients with a lower clinical likelihood of bacterial meningitis, antibiotics can be deferred, awaiting the results of diagnostic studies.
5. What empiric antibiotic therapy is appropriate? Adults 18-50 with suspected bacterial meningitis should receive therapy directed against Streptococcus pneumoniae and Neisseria meningitidis. Vancomycin should be dosed to achieve a relatively high trough level of 15-20 mcg/mL. For a 70-kg adult male with normal renal function, doses of vancomycin given at the rate of 1.5 gm IV every 12 hours and ceftriaxone at 2 gm IV every 12 hours are appropriate. Adults over 50, alcoholics, and immunocompromised adults of any age should also receive ampicillin doses of 2 gm IV every four hours to cover Listeria, in addition to vancomycin and ceftriaxone.3,4
6. What infection control precautions are required? Meningococcal meningitis patients should be placed on droplet precautions (private room, mask for all entering the room) until they have completed 24 hours of appropriate antibiotic therapy. Negative pressure ventilation is not required. Patients with pneumococcal or viral meningitis do not require isolation.
7. Who needs antibiotic prophylaxis after patient exposure? Chemoprophylaxis is overprescribed after exposures to patients with meningococcal meningitis. The only social contacts who should receive prophylaxis are household contacts, childcare contacts, and people who have had direct exposure to the patient’s oral secretions through actions such as kissing or sharing utensils or toothbrushes. The only healthcare workers requiring chemoprophylaxis are those who performed mouth-to-mouth resuscitation or any staff who were unmasked during intubation or suctioning of a patient. Regimens for chemoprophylaxis in adults include ciprofloxacin, 500 mg taken orally as a single dose, rifampin taken in doses of 600 mg twice daily for two days, or 250 mg of ceftriaxone, given intramuscularly. Ceftriaxone is preferred for pregnant women. Chemoprophylaxis is unnecessary after exposure to patients with pneumococcal or viral meningitis.
8. What is the significance of arthritis after meningococcal meningitis? A significant number of patients with meningococcal disease develop inflammatory polyarthritis about a week after the onset of infection. In most cases, this is a sterile, immune complex phenomenon that responds to anti-inflammatory therapy. If joint effusions are present, they should be aspirated to exclude septic arthritis and crystalline arthritis. TH
Dr. Ross is a hospitalist at Brigham and Women’s Hospital (Boston) and a fellow of the Infectious Diseases Society of America.
References
- Hasbun R, Abrahams J, Jekel J, et al. Computed tomography of the head before lumbar puncture in adults with suspected meningitis. N Engl J Med. 2001 Dec 13;345(24):1727-1733.
- de Gans J, van de Beek D. Dexamethasone in adults with bacterial meningitis. European dexamethasone in adulthood bacterial meningitis. N Engl J Med. 2002;347(20):1549-156.
- Tunkel AR, Hartman BJ, Kaplan SL, et al. Practice guidelines for the management of bacterial meningitis. Clin Infect Dis. 2004 Nov 1;39(9):1267-1284.
- van de Beek D, de Gans J, Tunkel AR, et al. Community-acquired bacterial meningitis in adults. N Engl J Med. 2006 Jan 5;354(1):44-53.