User login
Letter to the Editor
We greatly appreciate the thoughtful points made by Dr. Kerman regarding our recently published study evaluating the association of hospitalist continuity on adverse events (AEs).[1] We agree that a 7‐on/7‐off staffing model may limit discontinuity relative to models using shorter rotations lengths. Many hospital medicine programs use a 7‐on/7‐off model to optimize continuity. Longer rotation lengths are uncommon, as they may lead to fatigue and negatively affect physician work‐life balance. Shorter rotation lengths do exist, and we acknowledge that a study in a setting with greater fragmentation may have detected an effect.
We respectfully disagree with Dr. Kerman's concern that our methods for AE detection and confirmation may have been insensitive. We did not rely on incident reports, as these systems suffer from under‐reporting and often represent only a fraction of true AEs. We used a modified version of the classic 2‐stage method to identify and confirm AEs.[2] In the first stage, we used computerized screens, based on criteria from the Harvard Medical Practice Study and Institute for Healthcare Improvement global trigger tool, to identify potential AEs.[3, 4, 5] A research nurse created narrative summaries of potential AEs. A physician researcher then reviewed the narrative summaries to confirm whether an AE was truly present. This time‐consuming method is much more sensitive and specific than other options for patient safety measurement, including administrative data analyses and incident reporting systems.[6, 7]
With respect to other outcomes that may be affected by hospitalist continuity, we recently published a separate study showing that lower inpatient physician continuity was significantly associated with modest increases in hospital costs.[8] We found no association between continuity and patient satisfaction, but were likely underpowered to detect one. Interestingly, some of the models in our study suggested a slightly reduced risk of readmission with lower continuity. We were surprised by this finding and hypothesized that countervailing forces may be at play during handoffs of care from 1 hospitalist to another. Transitions of care introduce the opportunity for critical information to be lost, but they also introduce the potential for patient reassessment. A hospitalist newly taking over care from another may not be anchored to the initial diagnostic impressions and management plan established by the first. Of course, the potential benefit of a reassessment could only occur if the new hospitalist has time to perform one. At extremely high patient volumes, this theoretical benefit is unlikely to exist.
We did not include length of stay (LOS) as an outcome because hospitalist continuity and LOS are interdependent. Although discontinuity may lead to longer LOS, longer LOS definitely increases the probability of discontinuity. Thus, we controlled for LOS in our statistical models to isolate the effect of continuity. The study by Epstein and colleagues did not take into account the interdependence between LOS and hospitalist continuity.[9] Observational studies are not ideal for determining the effect of continuity on LOS. The Combing Incentives and Continuity Leading to Efficiency (CICLE) study by Chandra and colleagues was a pre‐post evaluation of a hospitalist staffing model specifically designed to improve continuity.[10] In the CICLE model, physicians work in a 4‐day rotation. On day 1, physicians exclusively admit patients. On day 2, physicians care for patients admitted on day 1 and accept patients admitted overnight. On days 3 and 4, physicians continue to care for patients received on days 1 and 2, but receive no additional patients. The remaining patients are transitioned to the next physician entering the cycle at the end of day 4. Chandra and colleagues found a 7.5% reduction in LOS and an 8.5% reduction in charges. Interestingly, they also found a 13.5% increase in readmissions that did not achieve statistical significance (P=0.08). The CICLE study suggests continuity does affect LOS, but is limited in that it did not account for a potential preexisting trend toward lower LOS.
Dr. Kerman presents data showing that it takes longer for a physician to care for a patient who is new to him or her than for a patient who is previously known. This finding has face validity. However, as we have suggested, the extra time spent by the oncoming physician may have both advantages and disadvantages. The disadvantages include time‐consuming cognitive work for the physician and the potential for information loss affecting patient care. The potential advantage is a second physician reassessing the diagnosis and management decisions established by the first, potentially correcting errors and optimizing care.
Ultimately, more research is needed to illuminate the effect of hospitalist continuity on patient outcomes. For now, we feel that hospital medicine group leaders need not institute lengthy rotations or staffing models that prioritize continuity above all other factors, as continuity appears to have little impact on patient outcomes.
- , , , et al. The effect of hospitalist discontinuity on adverse events. J Hosp Med. 2015;10(3):147–151.
- , , , et al. Comparison of manual abstraction to data warehouse facilitated abstraction to identify hosptial adverse events. BMJ Qual Saf. 2013;22(2):130–138.
- , . IHI global trigger tool for measuring adverse events: IHI innovation series white paper. Cambridge, MA: Institute for Healthcare Improvement; 2007.
- , BA, , et al. A study of medical injury and medical malpractice. N Engl J Med. 1989;321(7):480–484.
- , , , et al. Incidence and types of adverse events and negligent care in Utah and Colorado. Med Care. 2000;38(3):261–271.
- . The elephant of patient safety: what you see depends on how you look. Jt Comm J Qual Patient Saf. 2010;36(9):399–401.
- , . Measuring errors and adverse events in health care. J Gen Intern Med. 2003;18(1):61–67.
- , , , et al. The impact of hospitalist discontinuity on hospital cost, readmissions, and patient satisfaction. J Gen Intern Med. 2014;29(7):1004–1008.
- , , , , . The impact of fragmentation of hospitalist care on length of stay. J Hosp Med. 2010;5(6):335–338.
- , , . The Creating Incentives and Continuity Leading to Efficiency staffing model: a quality improvement initiative in hospital medicine. Mayo Clin Proc. 2012;87(4):364–371.
We greatly appreciate the thoughtful points made by Dr. Kerman regarding our recently published study evaluating the association of hospitalist continuity on adverse events (AEs).[1] We agree that a 7‐on/7‐off staffing model may limit discontinuity relative to models using shorter rotations lengths. Many hospital medicine programs use a 7‐on/7‐off model to optimize continuity. Longer rotation lengths are uncommon, as they may lead to fatigue and negatively affect physician work‐life balance. Shorter rotation lengths do exist, and we acknowledge that a study in a setting with greater fragmentation may have detected an effect.
We respectfully disagree with Dr. Kerman's concern that our methods for AE detection and confirmation may have been insensitive. We did not rely on incident reports, as these systems suffer from under‐reporting and often represent only a fraction of true AEs. We used a modified version of the classic 2‐stage method to identify and confirm AEs.[2] In the first stage, we used computerized screens, based on criteria from the Harvard Medical Practice Study and Institute for Healthcare Improvement global trigger tool, to identify potential AEs.[3, 4, 5] A research nurse created narrative summaries of potential AEs. A physician researcher then reviewed the narrative summaries to confirm whether an AE was truly present. This time‐consuming method is much more sensitive and specific than other options for patient safety measurement, including administrative data analyses and incident reporting systems.[6, 7]
With respect to other outcomes that may be affected by hospitalist continuity, we recently published a separate study showing that lower inpatient physician continuity was significantly associated with modest increases in hospital costs.[8] We found no association between continuity and patient satisfaction, but were likely underpowered to detect one. Interestingly, some of the models in our study suggested a slightly reduced risk of readmission with lower continuity. We were surprised by this finding and hypothesized that countervailing forces may be at play during handoffs of care from 1 hospitalist to another. Transitions of care introduce the opportunity for critical information to be lost, but they also introduce the potential for patient reassessment. A hospitalist newly taking over care from another may not be anchored to the initial diagnostic impressions and management plan established by the first. Of course, the potential benefit of a reassessment could only occur if the new hospitalist has time to perform one. At extremely high patient volumes, this theoretical benefit is unlikely to exist.
We did not include length of stay (LOS) as an outcome because hospitalist continuity and LOS are interdependent. Although discontinuity may lead to longer LOS, longer LOS definitely increases the probability of discontinuity. Thus, we controlled for LOS in our statistical models to isolate the effect of continuity. The study by Epstein and colleagues did not take into account the interdependence between LOS and hospitalist continuity.[9] Observational studies are not ideal for determining the effect of continuity on LOS. The Combing Incentives and Continuity Leading to Efficiency (CICLE) study by Chandra and colleagues was a pre‐post evaluation of a hospitalist staffing model specifically designed to improve continuity.[10] In the CICLE model, physicians work in a 4‐day rotation. On day 1, physicians exclusively admit patients. On day 2, physicians care for patients admitted on day 1 and accept patients admitted overnight. On days 3 and 4, physicians continue to care for patients received on days 1 and 2, but receive no additional patients. The remaining patients are transitioned to the next physician entering the cycle at the end of day 4. Chandra and colleagues found a 7.5% reduction in LOS and an 8.5% reduction in charges. Interestingly, they also found a 13.5% increase in readmissions that did not achieve statistical significance (P=0.08). The CICLE study suggests continuity does affect LOS, but is limited in that it did not account for a potential preexisting trend toward lower LOS.
Dr. Kerman presents data showing that it takes longer for a physician to care for a patient who is new to him or her than for a patient who is previously known. This finding has face validity. However, as we have suggested, the extra time spent by the oncoming physician may have both advantages and disadvantages. The disadvantages include time‐consuming cognitive work for the physician and the potential for information loss affecting patient care. The potential advantage is a second physician reassessing the diagnosis and management decisions established by the first, potentially correcting errors and optimizing care.
Ultimately, more research is needed to illuminate the effect of hospitalist continuity on patient outcomes. For now, we feel that hospital medicine group leaders need not institute lengthy rotations or staffing models that prioritize continuity above all other factors, as continuity appears to have little impact on patient outcomes.
We greatly appreciate the thoughtful points made by Dr. Kerman regarding our recently published study evaluating the association of hospitalist continuity on adverse events (AEs).[1] We agree that a 7‐on/7‐off staffing model may limit discontinuity relative to models using shorter rotations lengths. Many hospital medicine programs use a 7‐on/7‐off model to optimize continuity. Longer rotation lengths are uncommon, as they may lead to fatigue and negatively affect physician work‐life balance. Shorter rotation lengths do exist, and we acknowledge that a study in a setting with greater fragmentation may have detected an effect.
We respectfully disagree with Dr. Kerman's concern that our methods for AE detection and confirmation may have been insensitive. We did not rely on incident reports, as these systems suffer from under‐reporting and often represent only a fraction of true AEs. We used a modified version of the classic 2‐stage method to identify and confirm AEs.[2] In the first stage, we used computerized screens, based on criteria from the Harvard Medical Practice Study and Institute for Healthcare Improvement global trigger tool, to identify potential AEs.[3, 4, 5] A research nurse created narrative summaries of potential AEs. A physician researcher then reviewed the narrative summaries to confirm whether an AE was truly present. This time‐consuming method is much more sensitive and specific than other options for patient safety measurement, including administrative data analyses and incident reporting systems.[6, 7]
With respect to other outcomes that may be affected by hospitalist continuity, we recently published a separate study showing that lower inpatient physician continuity was significantly associated with modest increases in hospital costs.[8] We found no association between continuity and patient satisfaction, but were likely underpowered to detect one. Interestingly, some of the models in our study suggested a slightly reduced risk of readmission with lower continuity. We were surprised by this finding and hypothesized that countervailing forces may be at play during handoffs of care from 1 hospitalist to another. Transitions of care introduce the opportunity for critical information to be lost, but they also introduce the potential for patient reassessment. A hospitalist newly taking over care from another may not be anchored to the initial diagnostic impressions and management plan established by the first. Of course, the potential benefit of a reassessment could only occur if the new hospitalist has time to perform one. At extremely high patient volumes, this theoretical benefit is unlikely to exist.
We did not include length of stay (LOS) as an outcome because hospitalist continuity and LOS are interdependent. Although discontinuity may lead to longer LOS, longer LOS definitely increases the probability of discontinuity. Thus, we controlled for LOS in our statistical models to isolate the effect of continuity. The study by Epstein and colleagues did not take into account the interdependence between LOS and hospitalist continuity.[9] Observational studies are not ideal for determining the effect of continuity on LOS. The Combing Incentives and Continuity Leading to Efficiency (CICLE) study by Chandra and colleagues was a pre‐post evaluation of a hospitalist staffing model specifically designed to improve continuity.[10] In the CICLE model, physicians work in a 4‐day rotation. On day 1, physicians exclusively admit patients. On day 2, physicians care for patients admitted on day 1 and accept patients admitted overnight. On days 3 and 4, physicians continue to care for patients received on days 1 and 2, but receive no additional patients. The remaining patients are transitioned to the next physician entering the cycle at the end of day 4. Chandra and colleagues found a 7.5% reduction in LOS and an 8.5% reduction in charges. Interestingly, they also found a 13.5% increase in readmissions that did not achieve statistical significance (P=0.08). The CICLE study suggests continuity does affect LOS, but is limited in that it did not account for a potential preexisting trend toward lower LOS.
Dr. Kerman presents data showing that it takes longer for a physician to care for a patient who is new to him or her than for a patient who is previously known. This finding has face validity. However, as we have suggested, the extra time spent by the oncoming physician may have both advantages and disadvantages. The disadvantages include time‐consuming cognitive work for the physician and the potential for information loss affecting patient care. The potential advantage is a second physician reassessing the diagnosis and management decisions established by the first, potentially correcting errors and optimizing care.
Ultimately, more research is needed to illuminate the effect of hospitalist continuity on patient outcomes. For now, we feel that hospital medicine group leaders need not institute lengthy rotations or staffing models that prioritize continuity above all other factors, as continuity appears to have little impact on patient outcomes.
- , , , et al. The effect of hospitalist discontinuity on adverse events. J Hosp Med. 2015;10(3):147–151.
- , , , et al. Comparison of manual abstraction to data warehouse facilitated abstraction to identify hosptial adverse events. BMJ Qual Saf. 2013;22(2):130–138.
- , . IHI global trigger tool for measuring adverse events: IHI innovation series white paper. Cambridge, MA: Institute for Healthcare Improvement; 2007.
- , BA, , et al. A study of medical injury and medical malpractice. N Engl J Med. 1989;321(7):480–484.
- , , , et al. Incidence and types of adverse events and negligent care in Utah and Colorado. Med Care. 2000;38(3):261–271.
- . The elephant of patient safety: what you see depends on how you look. Jt Comm J Qual Patient Saf. 2010;36(9):399–401.
- , . Measuring errors and adverse events in health care. J Gen Intern Med. 2003;18(1):61–67.
- , , , et al. The impact of hospitalist discontinuity on hospital cost, readmissions, and patient satisfaction. J Gen Intern Med. 2014;29(7):1004–1008.
- , , , , . The impact of fragmentation of hospitalist care on length of stay. J Hosp Med. 2010;5(6):335–338.
- , , . The Creating Incentives and Continuity Leading to Efficiency staffing model: a quality improvement initiative in hospital medicine. Mayo Clin Proc. 2012;87(4):364–371.
- , , , et al. The effect of hospitalist discontinuity on adverse events. J Hosp Med. 2015;10(3):147–151.
- , , , et al. Comparison of manual abstraction to data warehouse facilitated abstraction to identify hosptial adverse events. BMJ Qual Saf. 2013;22(2):130–138.
- , . IHI global trigger tool for measuring adverse events: IHI innovation series white paper. Cambridge, MA: Institute for Healthcare Improvement; 2007.
- , BA, , et al. A study of medical injury and medical malpractice. N Engl J Med. 1989;321(7):480–484.
- , , , et al. Incidence and types of adverse events and negligent care in Utah and Colorado. Med Care. 2000;38(3):261–271.
- . The elephant of patient safety: what you see depends on how you look. Jt Comm J Qual Patient Saf. 2010;36(9):399–401.
- , . Measuring errors and adverse events in health care. J Gen Intern Med. 2003;18(1):61–67.
- , , , et al. The impact of hospitalist discontinuity on hospital cost, readmissions, and patient satisfaction. J Gen Intern Med. 2014;29(7):1004–1008.
- , , , , . The impact of fragmentation of hospitalist care on length of stay. J Hosp Med. 2010;5(6):335–338.
- , , . The Creating Incentives and Continuity Leading to Efficiency staffing model: a quality improvement initiative in hospital medicine. Mayo Clin Proc. 2012;87(4):364–371.
Effect of Hospitalist Discontinuity on AE
Although definitions vary, continuity of care can be thought of as the patient's experience of a continuous caring relationship with an identified healthcare professional.[1] Research in ambulatory settings has found that patients who see their primary care physician for a higher proportion of office visits have higher patient satisfaction, better hypertensive control, lower risk of hospitalization, and fewer emergency department visits.[2, 3, 4, 5] Continuity with a single hospital‐based physician is difficult to achieve because of the need to provide care 24 hours a day, 7 days a week. Key clinical information may be lost during physician‐to‐physician handoffs (eg, at admission, at the end of rotations on service) during hospitalization. Our research group recently found that lower hospital physician continuity was associated with modestly increased hospital costs, but also a trend toward lower readmissions.[6] We speculated that physicians newly taking over patient care from colleagues reassess diagnoses and treatment plans. This reassessment may identify errors missed by the previous hospital physician. Thus, discontinuity may theoretically help or hinder the provision of safe hospital care.
We sought to examine the relationship between hospital physician continuity and the incidence of adverse events (AEs). We combined data from 2 previously published studies by our research group; one investigated the relationship between hospital physician continuity and costs and 30‐day readmissions, the other assessed the impact of unit‐based interventions on AEs.[6, 7]
METHODS
Setting and Study Design
This retrospective, observational study was conducted at Northwestern Memorial Hospital, an 876‐bed tertiary care teaching hospital in Chicago, Illinois, and was approved by the institutional review board of Northwestern University. Subjects included patients admitted to an adult nonteaching hospitalist service between March 1, 2009 and December 31, 2011. Hospitalists on this service worked without resident physicians in rotations usually lasting 7 consecutive days beginning on Mondays and ending on Sundays. Hospitalists were allowed to switch portions of their schedule with one another, creating the possibility that certain rotations may have been slightly shorter or longer than 7 days. Hospitalists gave verbal sign‐out via telephone to the hospitalist taking over their service on the afternoon of the last day of their rotation. These handoffs customarily involved both hospitalists viewing the electronic health record during the discussion but were not standardized. Night hospitalists performed admissions and cross‐coverage each night from 7 pm to 7 am. Night hospitalists printed history and physicals for day hospitalists, but typically did not give verbal sign‐out on new admissions.
Acquisition of Study Population Data
We identified all patients admitted to the nonteaching hospitalist service using the Northwestern Medicine Enterprise Data Warehouse (EDW), an integrated repository of all clinical and research data sources on the campus. We excluded patients admitted under observation status, those initially admitted to other services (eg, intensive care, general surgery), those discharged from other services, and those cared for by advanced practice providers (ie, nurse practitioners and physician assistants).
Predictor Variables
We identified physicians completing the primary service history and physicals (H&P) and progress notes throughout patients' hospitalizations to calculate 2 measures of continuity: the Number of Physicians Index (NPI), and the Usual Provider of Continuity (UPC) Index.[8, 9] The NPI represented the total number of unique hospitalists completing H&Ps and/or progress notes for a patient. The UPC was calculated as the largest number of notes signed by a single hospitalist divided by the total number of hospitalist notes for a patient. For example, if Dr. John Smith wrote notes on the first 4 days of a patient's hospital stay, and Dr. Mary Jones wrote notes on the following 2 days (total stay=6 days), the NPI would be 2 and the UPC would be 0.67. Therefore, higher NPI and lower UPC designate lower continuity. Significant events occurring during the nighttime were documented in separate notes titled cross‐cover notes. These cross‐cover notes were not included in the calculation of NPI or UPC. In the rare event that 2 or more progress notes were written on the same day, we selected the one used for billing to calculate UPC and NPI.
Outcome Variables
We used AE data from a study we conducted to assess the impact of unit‐based interventions to improve teamwork and patient safety, the methods of which have been previously described.[7] Briefly, we used a 2‐stage medical record review similar to that performed in prior studies.[10, 11, 12, 13] In the first stage, we identified potential AEs using automated queries of the Northwestern Medicine EDW. These queries were based on screening criteria used in the Harvard Medical Practice Study and the Institute for Healthcare Improvement (IHI) Global Trigger Tool.[12, 13] Examples of queries included abnormal laboratory values (eg, international normalized ratio [INR] >6 after hospital day 2 and excluding patients with INR >4 on day 1), administration of rescue medications (eg, naloxone), certain types of incident reports (eg, pressure ulcer), International Classification of Diseases, Ninth Revision (ICD‐9) codes indicating hospital‐acquired conditions (eg, venous thromboembolism), and text searches of progress notes and discharge summaries using natural language processing.[14] Prior research by our group confirmed these automated screens identify a similar number of AEs as manual medical record screening.[14] For each patient with 1 or more potential AE, a research nurse performed a medical record abstraction and created a description of each potential AE.
In the second stage, 2 physician researchers independently reviewed each potential AE in a blinded fashion to determine whether or not an AE was present. An AE was defined as injury due to medical management rather than the natural history of the illness,[15] and included injuries that prolonged the hospital stay or produced disability as well as those resulting in transient disability or abnormal lab values.[16] After independent review, physician reviewers discussed discrepancies in their ratings to achieve consensus.
We tested the reliability of medical record abstractions in our prior study by conducting duplicate abstractions and consensus ratings for a randomly selected sample of 294 patients.[7] The inter‐rater reliability was good for determining the presence of AEs (=0.63).
Statistical Analyses
We calculated descriptive statistics for patient characteristics. Primary discharge diagnosis ICD‐9 codes were categorized using the Healthcare Cost and Utilization Project Clinical Classification Software.[17] We created multivariable logistic regression models with the independent variable being the measure of continuity (NPI or UPC) and the dependent variable being experiencing 1 or more AEs. Covariates included patient age, sex, race, payer, night admission, weekend admission, intensive care unit stay, Medicare Severity Diagnosis Related Group (MS‐DRG) weight, and total number of Elixhauser comorbidities.[18] The length of stay (LOS) was also included as a covariate, as longer LOS increases the probability of discontinuity and may increase the risk for AEs. Because MS‐DRG weight and LOS were highly correlated, we created several models; the first including both as continuous variables, the second including both categorized into quartiles, and a third excluding MS‐DRG weight and including LOS as a continuous variable. Our prior study assessing the impact of unit‐based interventions did not show a statistically significant difference in the pre‐ versus postintervention period, thus we did not include study period as a covariate.
RESULTS
Patient Characteristics
Our analyses included data from 474 hospitalizations. Patient characteristics are shown in Table 1. Patients were a mean 51.118.8 years of age, hospitalized for a mean 3.43.1 days, included 241 (50.8%) women, and 233 (49.2%) persons of nonwhite race. The mean and standard deviation of NPI and UPC were 2.51.0 and 0.60.2. Overall, 47 patients (9.9%) experienced 55 total AEs. AEs included 31 adverse drug events, 6 falls, 5 procedural injuries, 4 manifestations of poor glycemic control, 3 hospital‐acquired infections, 2 episodes of acute renal failure, 1 episode of delirium, 1 pressure ulcer, and 2 categorized as other.
| Characteristic | Value |
|---|---|
| |
| Mean age (SD), y | 55.1 (18.8) |
| Mean length of stay (SD), d | 3.4 (3.1) |
| Women, n (%) | 241 (50.8) |
| Nonwhite race, n (%) | 233 (49.2) |
| Payer, n (%) | |
| Private | 180 (38) |
| Medicare | 165 (34.8) |
| Medicaid | 47 (9.9) |
| Self‐pay/other | 82 (17.3) |
| Night admission, n (%) | 245 (51.7) |
| Weekend admission, n (%) | 135 (28.5) |
| Intensive care unit stay, n (%) | 18 (3.8) |
| Diagnosis, n (%) | |
| Diseases of the circulatory system | 95 (20.0) |
| Diseases of the digestive system | 65 (13.7) |
| Diseases of the respiratory system | 49 (10.3) |
| Injury and poisoning | 41 (8.7) |
| Diseases of the skin and soft tissue | 31 (6.5) |
| Symptoms, signs, and ill‐defined conditions and factors influencing health status | 28 (5.9) |
| Endocrine, nutritional, and metabolic diseases and immunity disorders | 25 (5.3) |
| Diseases of the genitourinary system | 24 (5.1) |
| Diseases of the musculoskeletal system and connective tissue | 23 (4.9) |
| Diseases of the nervous system | 23 (4.9) |
| Other | 70 (14.8) |
| Mean no. of Elixhauser comorbidities (SD) | 2.3 (1.7) |
| Mean MS‐DRG weight (SD) | 1.0 (1.0) |
| Mean NPI (SD) | 2.5 (1.0) |
| Mean UPC (SD) | 0.6 (0.2) |
Association Between Continuity and Adverse Events
In unadjusted models, each 1‐unit increase in the NPI (ie, less continuity) was significantly associated with the incidence of 1 or more AEs (odds ratio [OR]=1.75; P<0.001). However, UPC was not associated with incidence of AEs (OR=1.03; P=0.68) (Table 2). Across all adjusted models, neither NPI nor UPC was significantly associated with the incidence of AEs. The direction of the effect of discontinuity on AEs was inconsistent across models. Though all 3 adjusted models using NPI as the independent variable showed a trend toward increased odds of experiencing 1 or more AE with discontinuity, 2 of the 3 models using UPC showed trends in the opposite direction.
| NPI OR (95% CI)* | P Value | UPC OR (95% CI)* | P Value | ||
|---|---|---|---|---|---|
| |||||
| Unadjusted model | 1.75 (1.332.29) | <0.0001 | 1.03 (0.89‐1.21) | 0.68 | |
| Adjusted models | |||||
| Model 1 | MS‐DRG and LOS continuous | 1.16 (0.781.72) | 0.47 | 0.96 (0.791.14) | 0.60 |
| Model 2 | MS‐DRG and LOS in quartiles | 1.38 (0.981.94) | 0.07 | 1.05 (0.881.26) | 0.59 |
| Model 3 | MS‐DRG dropped, LOS continuous | 1.14 (0.771.70) | 0.51 | 0.95 (0.791.14) | 0.56 |
DISCUSSION
We found that hospitalist physician continuity was not associated with the incidence of AEs. Our findings are somewhat surprising because of the high value placed on continuity of care and patient safety concerns related to handoffs. Key clinical information may be lost when patient care is transitioned to a new hospitalist shortly after admission (eg, from a night hospitalist) or at the end of a rotation. Thus, it is logical to assume that discontinuity inherently increases the risk for harm. On the other hand, a physician newly taking over patient care from another may not be anchored to the initial diagnosis and treatment plan established by the first. This second look could potentially prevent missed/delayed diagnoses and optimize the plan of care.[19] These countervailing forces may explain our findings.
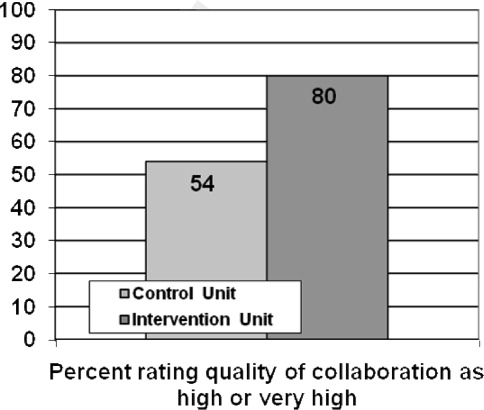
Several other potential explanations for our findings should be considered. First, the quality of handoffs may have been sufficient to overcome the potential for information loss. We feel this is unlikely given that little attention had been dedicated to improving the quality of patient handoffs among hospitalists in our institution. Notably, though a number of studies have evaluated resident physician handoffs, most of the work has focused on night coverage, and little is known about the quality of attending handoffs.[20] Second, access to a fully integrated electronic health record may have assisted hospitalists in complementing information received during handoffs. For example, a hospitalist about to start his or her rotation may have remotely accessed and reviewed patient medical records prior to receiving the phone handoff from the outgoing hospitalist. Third, other efforts to improve patient safety may have reduced the overall risk and provided some resilience in the system. Unit‐based interventions, including structured interdisciplinary rounds and nurse‐physician coleadership, improved teamwork climate and reduced AEs in the study hospital over time.[7]
Another factor to consider relates to the fact that hospital care is provided by teams of clinicians (eg, nurses, specialist physicians, therapists, social workers). Hospital teams are often large and have dynamic team membership. Similar to hospitalists, nurses, physician specialists, and other team members handoff care throughout the course of a patient's hospital stay. Yet, discontinuity for each professional type may occur at different times and frequencies. For example, a patient may be handed off from one hospitalist to another, yet the care continues with the same cardiologist or nurse. Future research should better characterize hospital team complexity (eg, size, relationships among members) and dynamics (eg, continuity for various professional types) and the impact of these factors on patient outcomes.
Our findings are important because hospitalist physician discontinuity is common during hospital stays. Hospital medicine groups vary in their staffing and scheduling models. Policies related to admission distribution and rotation length (consecutive days worked) systematically impact physician continuity. Few studies have evaluated the effect on continuity on hospitalized patient outcomes, and no prior research, to our knowledge, has explored the association of continuity on measures of patient safety.[6, 21, 22] Though our study might suggest that staffing models have little impact on patient safety, as previously mentioned, other team factors may influence patient outcomes.
Our study has several limitations. First, we assessed the impact of continuity on AEs in a single site. Although the 7 days on/7 days off model is the most common scheduling pattern used by adult hospital medicine groups,[23] staffing models and patient safety practices vary across hospitals, potentially limiting the generalizability of our study. Second, continuity can be defined and measured in a variety of ways. We used 2 different measures of physician continuity. As previously mentioned, assessing continuity of other clinicians may allow for a more complete understanding of the potential problems related to fragmentation of care. Third, this study excluded patients who experienced care transitions from other hospitals or other units within the hospital. Patients transferred from other hospitals are often complex, severely ill, and may be at higher risk for loss of key clinical information. Fourth, we used automated screens of an EDW to identify potential AEs. Although our prior research found that this method identified a similar number of AEs as manual medical record review screening, there was poor agreement between the 2 methods. Unfortunately, there is no gold standard to identify AEs. The EDW‐facilitated method allowed us to feasibly screen a larger number of charts, increasing statistical power, and minimized any potential bias that might occur during a manual review to identify potential AEs. Finally, we used data available from 2 prior studies and may have been underpowered to detect a significant association between continuity and AEs due to the relatively low percentage of patients experiencing an AE. In a post hoc power calculation, we estimated that we had 70% power to detect a 33% change in the proportion of patients with 1 or more AE for each 1‐unit increase in NPI, and 80% power to detect a 20% change for each 0.1‐unit decrease in UPC.
CONCLUSION
In conclusion, we found that hospitalist physician continuity was not associated with the incidence of AEs. We speculate that hospitalist continuity is only 1 of many team factors that may influence patient safety, and that prior efforts within our institution may have reduced our ability to detect an association. Future research should better characterize hospital team complexity and dynamics and the impact of these factors on patient outcomes.
Disclosures
This project was supported by a grant from the Agency for Healthcare Research and Quality and an Excellence in Academic Medicine Award, administered by Northwestern Memorial Hospital. The authors report no conflicts of interest.
- , , . What is “continuity of care”? J Health Serv Res Policy. 2006;11:248–250.
- , . Interpersonal continuity of care and care outcomes: a critical review. Ann Fam Med. 2005;3:159–166.
- , , , . The association between continuity of care and outcomes: a systematic and critical review. J Eval Clin Pract. 2010;16:947–956.
- , . Interpersonal continuity of care and patient satisfaction: a critical review. Ann Fam Med. 2004;2:445–451.
- , , , . Continuity of care in a family practice residency program. Impact on physician satisfaction. J Fam Pract. 1990;31:69–73.
- , , , et al. The impact of hospitalist discontinuity on hospital cost, readmissions, and patient satisfaction. J Gen Intern Med. 2014;29:1004–1008.
- , , , et al. Implementation of unit‐based interventions to improve teamwork and patient safety on a medical service [published online ahead of print June 11, 2014]. Am J Med Qual. doi: 10.1177/1062860614538093.
- . Measuring provider continuity in ambulatory care: an assessment of alternative approaches. Med Care. 1979;17:551–565.
- . Defining and measuring interpersonal continuity of care. Ann Fam Med. 2003;1:134–143.
- U.S. Department of Health and Human Services. Agency for Healthcare Research and Quality. Adverse events in hospitals: national incidence among medical beneficiaries. Available at: http://psnet.ahrq.gov/resource.aspx?resourceID=19811. Published November 2010. Accessed on December 15, 2014.
- , , , et al. “Global trigger tool” shows that adverse events in hospitals may be ten times greater than previously measured. Health Aff (Millwood). 2011;30:581–589.
- , , , et al. A study of medical injury and medical malpractice. N Engl J Med. 1989;321:480–484.
- , , , et al. Incidence and types of adverse events and negligent care in Utah and Colorado. Med Care. 2000;38:261–271.
- , , , et al. Comparison of traditional trigger tool to data warehouse based screening for identifying hospital adverse events. BMJ Qual Saf. 2013;22:130–138.
- , , , et al. Incidence of adverse events and negligence in hospitalized patients. Results of the Harvard Medical Practice Study I. N Engl J Med. 1991;324:370–376.
- , , . Safety of patients isolated for infection control. JAMA. 2003;290:1899–1905.
- HCUP Clinical Classification Software. Agency for Healthcare Research and Quality, Rockville, MD. http://www.hcup-us.ahrq.gov/toolssoftware/ccs/ccs.jsp. Accessed on December 15, 2014.
- , , , . Comorbidity measures for use with administrative data. Med Care. 1998;36:8–27.
- . Does continuity of care matter? No: discontinuity can improve patient care. West J Med. 2001;175:5.
- , , , , , . Hospitalist handoffs: a systematic review and task force recommendations. J Hosp Med. 2009;4:433–440.
- , , , , . The impact of fragmentation of hospitalist care on length of stay. J Hosp Med. 2010;5:335–338.
- , , . The Creating Incentives and Continuity Leading to Efficiency staffing model: a quality improvement initiative in hospital medicine. Mayo Clin Proc. 2012;87:364–371.
- Society of Hospital Medicine. 2014 state of hospital medicine report. Philadelphia, PA: Society of Hospital Medicine; 2014.
Although definitions vary, continuity of care can be thought of as the patient's experience of a continuous caring relationship with an identified healthcare professional.[1] Research in ambulatory settings has found that patients who see their primary care physician for a higher proportion of office visits have higher patient satisfaction, better hypertensive control, lower risk of hospitalization, and fewer emergency department visits.[2, 3, 4, 5] Continuity with a single hospital‐based physician is difficult to achieve because of the need to provide care 24 hours a day, 7 days a week. Key clinical information may be lost during physician‐to‐physician handoffs (eg, at admission, at the end of rotations on service) during hospitalization. Our research group recently found that lower hospital physician continuity was associated with modestly increased hospital costs, but also a trend toward lower readmissions.[6] We speculated that physicians newly taking over patient care from colleagues reassess diagnoses and treatment plans. This reassessment may identify errors missed by the previous hospital physician. Thus, discontinuity may theoretically help or hinder the provision of safe hospital care.
We sought to examine the relationship between hospital physician continuity and the incidence of adverse events (AEs). We combined data from 2 previously published studies by our research group; one investigated the relationship between hospital physician continuity and costs and 30‐day readmissions, the other assessed the impact of unit‐based interventions on AEs.[6, 7]
METHODS
Setting and Study Design
This retrospective, observational study was conducted at Northwestern Memorial Hospital, an 876‐bed tertiary care teaching hospital in Chicago, Illinois, and was approved by the institutional review board of Northwestern University. Subjects included patients admitted to an adult nonteaching hospitalist service between March 1, 2009 and December 31, 2011. Hospitalists on this service worked without resident physicians in rotations usually lasting 7 consecutive days beginning on Mondays and ending on Sundays. Hospitalists were allowed to switch portions of their schedule with one another, creating the possibility that certain rotations may have been slightly shorter or longer than 7 days. Hospitalists gave verbal sign‐out via telephone to the hospitalist taking over their service on the afternoon of the last day of their rotation. These handoffs customarily involved both hospitalists viewing the electronic health record during the discussion but were not standardized. Night hospitalists performed admissions and cross‐coverage each night from 7 pm to 7 am. Night hospitalists printed history and physicals for day hospitalists, but typically did not give verbal sign‐out on new admissions.
Acquisition of Study Population Data
We identified all patients admitted to the nonteaching hospitalist service using the Northwestern Medicine Enterprise Data Warehouse (EDW), an integrated repository of all clinical and research data sources on the campus. We excluded patients admitted under observation status, those initially admitted to other services (eg, intensive care, general surgery), those discharged from other services, and those cared for by advanced practice providers (ie, nurse practitioners and physician assistants).
Predictor Variables
We identified physicians completing the primary service history and physicals (H&P) and progress notes throughout patients' hospitalizations to calculate 2 measures of continuity: the Number of Physicians Index (NPI), and the Usual Provider of Continuity (UPC) Index.[8, 9] The NPI represented the total number of unique hospitalists completing H&Ps and/or progress notes for a patient. The UPC was calculated as the largest number of notes signed by a single hospitalist divided by the total number of hospitalist notes for a patient. For example, if Dr. John Smith wrote notes on the first 4 days of a patient's hospital stay, and Dr. Mary Jones wrote notes on the following 2 days (total stay=6 days), the NPI would be 2 and the UPC would be 0.67. Therefore, higher NPI and lower UPC designate lower continuity. Significant events occurring during the nighttime were documented in separate notes titled cross‐cover notes. These cross‐cover notes were not included in the calculation of NPI or UPC. In the rare event that 2 or more progress notes were written on the same day, we selected the one used for billing to calculate UPC and NPI.
Outcome Variables
We used AE data from a study we conducted to assess the impact of unit‐based interventions to improve teamwork and patient safety, the methods of which have been previously described.[7] Briefly, we used a 2‐stage medical record review similar to that performed in prior studies.[10, 11, 12, 13] In the first stage, we identified potential AEs using automated queries of the Northwestern Medicine EDW. These queries were based on screening criteria used in the Harvard Medical Practice Study and the Institute for Healthcare Improvement (IHI) Global Trigger Tool.[12, 13] Examples of queries included abnormal laboratory values (eg, international normalized ratio [INR] >6 after hospital day 2 and excluding patients with INR >4 on day 1), administration of rescue medications (eg, naloxone), certain types of incident reports (eg, pressure ulcer), International Classification of Diseases, Ninth Revision (ICD‐9) codes indicating hospital‐acquired conditions (eg, venous thromboembolism), and text searches of progress notes and discharge summaries using natural language processing.[14] Prior research by our group confirmed these automated screens identify a similar number of AEs as manual medical record screening.[14] For each patient with 1 or more potential AE, a research nurse performed a medical record abstraction and created a description of each potential AE.
In the second stage, 2 physician researchers independently reviewed each potential AE in a blinded fashion to determine whether or not an AE was present. An AE was defined as injury due to medical management rather than the natural history of the illness,[15] and included injuries that prolonged the hospital stay or produced disability as well as those resulting in transient disability or abnormal lab values.[16] After independent review, physician reviewers discussed discrepancies in their ratings to achieve consensus.
We tested the reliability of medical record abstractions in our prior study by conducting duplicate abstractions and consensus ratings for a randomly selected sample of 294 patients.[7] The inter‐rater reliability was good for determining the presence of AEs (=0.63).
Statistical Analyses
We calculated descriptive statistics for patient characteristics. Primary discharge diagnosis ICD‐9 codes were categorized using the Healthcare Cost and Utilization Project Clinical Classification Software.[17] We created multivariable logistic regression models with the independent variable being the measure of continuity (NPI or UPC) and the dependent variable being experiencing 1 or more AEs. Covariates included patient age, sex, race, payer, night admission, weekend admission, intensive care unit stay, Medicare Severity Diagnosis Related Group (MS‐DRG) weight, and total number of Elixhauser comorbidities.[18] The length of stay (LOS) was also included as a covariate, as longer LOS increases the probability of discontinuity and may increase the risk for AEs. Because MS‐DRG weight and LOS were highly correlated, we created several models; the first including both as continuous variables, the second including both categorized into quartiles, and a third excluding MS‐DRG weight and including LOS as a continuous variable. Our prior study assessing the impact of unit‐based interventions did not show a statistically significant difference in the pre‐ versus postintervention period, thus we did not include study period as a covariate.
RESULTS
Patient Characteristics
Our analyses included data from 474 hospitalizations. Patient characteristics are shown in Table 1. Patients were a mean 51.118.8 years of age, hospitalized for a mean 3.43.1 days, included 241 (50.8%) women, and 233 (49.2%) persons of nonwhite race. The mean and standard deviation of NPI and UPC were 2.51.0 and 0.60.2. Overall, 47 patients (9.9%) experienced 55 total AEs. AEs included 31 adverse drug events, 6 falls, 5 procedural injuries, 4 manifestations of poor glycemic control, 3 hospital‐acquired infections, 2 episodes of acute renal failure, 1 episode of delirium, 1 pressure ulcer, and 2 categorized as other.
| Characteristic | Value |
|---|---|
| |
| Mean age (SD), y | 55.1 (18.8) |
| Mean length of stay (SD), d | 3.4 (3.1) |
| Women, n (%) | 241 (50.8) |
| Nonwhite race, n (%) | 233 (49.2) |
| Payer, n (%) | |
| Private | 180 (38) |
| Medicare | 165 (34.8) |
| Medicaid | 47 (9.9) |
| Self‐pay/other | 82 (17.3) |
| Night admission, n (%) | 245 (51.7) |
| Weekend admission, n (%) | 135 (28.5) |
| Intensive care unit stay, n (%) | 18 (3.8) |
| Diagnosis, n (%) | |
| Diseases of the circulatory system | 95 (20.0) |
| Diseases of the digestive system | 65 (13.7) |
| Diseases of the respiratory system | 49 (10.3) |
| Injury and poisoning | 41 (8.7) |
| Diseases of the skin and soft tissue | 31 (6.5) |
| Symptoms, signs, and ill‐defined conditions and factors influencing health status | 28 (5.9) |
| Endocrine, nutritional, and metabolic diseases and immunity disorders | 25 (5.3) |
| Diseases of the genitourinary system | 24 (5.1) |
| Diseases of the musculoskeletal system and connective tissue | 23 (4.9) |
| Diseases of the nervous system | 23 (4.9) |
| Other | 70 (14.8) |
| Mean no. of Elixhauser comorbidities (SD) | 2.3 (1.7) |
| Mean MS‐DRG weight (SD) | 1.0 (1.0) |
| Mean NPI (SD) | 2.5 (1.0) |
| Mean UPC (SD) | 0.6 (0.2) |
Association Between Continuity and Adverse Events
In unadjusted models, each 1‐unit increase in the NPI (ie, less continuity) was significantly associated with the incidence of 1 or more AEs (odds ratio [OR]=1.75; P<0.001). However, UPC was not associated with incidence of AEs (OR=1.03; P=0.68) (Table 2). Across all adjusted models, neither NPI nor UPC was significantly associated with the incidence of AEs. The direction of the effect of discontinuity on AEs was inconsistent across models. Though all 3 adjusted models using NPI as the independent variable showed a trend toward increased odds of experiencing 1 or more AE with discontinuity, 2 of the 3 models using UPC showed trends in the opposite direction.
| NPI OR (95% CI)* | P Value | UPC OR (95% CI)* | P Value | ||
|---|---|---|---|---|---|
| |||||
| Unadjusted model | 1.75 (1.332.29) | <0.0001 | 1.03 (0.89‐1.21) | 0.68 | |
| Adjusted models | |||||
| Model 1 | MS‐DRG and LOS continuous | 1.16 (0.781.72) | 0.47 | 0.96 (0.791.14) | 0.60 |
| Model 2 | MS‐DRG and LOS in quartiles | 1.38 (0.981.94) | 0.07 | 1.05 (0.881.26) | 0.59 |
| Model 3 | MS‐DRG dropped, LOS continuous | 1.14 (0.771.70) | 0.51 | 0.95 (0.791.14) | 0.56 |
DISCUSSION
We found that hospitalist physician continuity was not associated with the incidence of AEs. Our findings are somewhat surprising because of the high value placed on continuity of care and patient safety concerns related to handoffs. Key clinical information may be lost when patient care is transitioned to a new hospitalist shortly after admission (eg, from a night hospitalist) or at the end of a rotation. Thus, it is logical to assume that discontinuity inherently increases the risk for harm. On the other hand, a physician newly taking over patient care from another may not be anchored to the initial diagnosis and treatment plan established by the first. This second look could potentially prevent missed/delayed diagnoses and optimize the plan of care.[19] These countervailing forces may explain our findings.
Several other potential explanations for our findings should be considered. First, the quality of handoffs may have been sufficient to overcome the potential for information loss. We feel this is unlikely given that little attention had been dedicated to improving the quality of patient handoffs among hospitalists in our institution. Notably, though a number of studies have evaluated resident physician handoffs, most of the work has focused on night coverage, and little is known about the quality of attending handoffs.[20] Second, access to a fully integrated electronic health record may have assisted hospitalists in complementing information received during handoffs. For example, a hospitalist about to start his or her rotation may have remotely accessed and reviewed patient medical records prior to receiving the phone handoff from the outgoing hospitalist. Third, other efforts to improve patient safety may have reduced the overall risk and provided some resilience in the system. Unit‐based interventions, including structured interdisciplinary rounds and nurse‐physician coleadership, improved teamwork climate and reduced AEs in the study hospital over time.[7]
Another factor to consider relates to the fact that hospital care is provided by teams of clinicians (eg, nurses, specialist physicians, therapists, social workers). Hospital teams are often large and have dynamic team membership. Similar to hospitalists, nurses, physician specialists, and other team members handoff care throughout the course of a patient's hospital stay. Yet, discontinuity for each professional type may occur at different times and frequencies. For example, a patient may be handed off from one hospitalist to another, yet the care continues with the same cardiologist or nurse. Future research should better characterize hospital team complexity (eg, size, relationships among members) and dynamics (eg, continuity for various professional types) and the impact of these factors on patient outcomes.
Our findings are important because hospitalist physician discontinuity is common during hospital stays. Hospital medicine groups vary in their staffing and scheduling models. Policies related to admission distribution and rotation length (consecutive days worked) systematically impact physician continuity. Few studies have evaluated the effect on continuity on hospitalized patient outcomes, and no prior research, to our knowledge, has explored the association of continuity on measures of patient safety.[6, 21, 22] Though our study might suggest that staffing models have little impact on patient safety, as previously mentioned, other team factors may influence patient outcomes.
Our study has several limitations. First, we assessed the impact of continuity on AEs in a single site. Although the 7 days on/7 days off model is the most common scheduling pattern used by adult hospital medicine groups,[23] staffing models and patient safety practices vary across hospitals, potentially limiting the generalizability of our study. Second, continuity can be defined and measured in a variety of ways. We used 2 different measures of physician continuity. As previously mentioned, assessing continuity of other clinicians may allow for a more complete understanding of the potential problems related to fragmentation of care. Third, this study excluded patients who experienced care transitions from other hospitals or other units within the hospital. Patients transferred from other hospitals are often complex, severely ill, and may be at higher risk for loss of key clinical information. Fourth, we used automated screens of an EDW to identify potential AEs. Although our prior research found that this method identified a similar number of AEs as manual medical record review screening, there was poor agreement between the 2 methods. Unfortunately, there is no gold standard to identify AEs. The EDW‐facilitated method allowed us to feasibly screen a larger number of charts, increasing statistical power, and minimized any potential bias that might occur during a manual review to identify potential AEs. Finally, we used data available from 2 prior studies and may have been underpowered to detect a significant association between continuity and AEs due to the relatively low percentage of patients experiencing an AE. In a post hoc power calculation, we estimated that we had 70% power to detect a 33% change in the proportion of patients with 1 or more AE for each 1‐unit increase in NPI, and 80% power to detect a 20% change for each 0.1‐unit decrease in UPC.
CONCLUSION
In conclusion, we found that hospitalist physician continuity was not associated with the incidence of AEs. We speculate that hospitalist continuity is only 1 of many team factors that may influence patient safety, and that prior efforts within our institution may have reduced our ability to detect an association. Future research should better characterize hospital team complexity and dynamics and the impact of these factors on patient outcomes.
Disclosures
This project was supported by a grant from the Agency for Healthcare Research and Quality and an Excellence in Academic Medicine Award, administered by Northwestern Memorial Hospital. The authors report no conflicts of interest.
Although definitions vary, continuity of care can be thought of as the patient's experience of a continuous caring relationship with an identified healthcare professional.[1] Research in ambulatory settings has found that patients who see their primary care physician for a higher proportion of office visits have higher patient satisfaction, better hypertensive control, lower risk of hospitalization, and fewer emergency department visits.[2, 3, 4, 5] Continuity with a single hospital‐based physician is difficult to achieve because of the need to provide care 24 hours a day, 7 days a week. Key clinical information may be lost during physician‐to‐physician handoffs (eg, at admission, at the end of rotations on service) during hospitalization. Our research group recently found that lower hospital physician continuity was associated with modestly increased hospital costs, but also a trend toward lower readmissions.[6] We speculated that physicians newly taking over patient care from colleagues reassess diagnoses and treatment plans. This reassessment may identify errors missed by the previous hospital physician. Thus, discontinuity may theoretically help or hinder the provision of safe hospital care.
We sought to examine the relationship between hospital physician continuity and the incidence of adverse events (AEs). We combined data from 2 previously published studies by our research group; one investigated the relationship between hospital physician continuity and costs and 30‐day readmissions, the other assessed the impact of unit‐based interventions on AEs.[6, 7]
METHODS
Setting and Study Design
This retrospective, observational study was conducted at Northwestern Memorial Hospital, an 876‐bed tertiary care teaching hospital in Chicago, Illinois, and was approved by the institutional review board of Northwestern University. Subjects included patients admitted to an adult nonteaching hospitalist service between March 1, 2009 and December 31, 2011. Hospitalists on this service worked without resident physicians in rotations usually lasting 7 consecutive days beginning on Mondays and ending on Sundays. Hospitalists were allowed to switch portions of their schedule with one another, creating the possibility that certain rotations may have been slightly shorter or longer than 7 days. Hospitalists gave verbal sign‐out via telephone to the hospitalist taking over their service on the afternoon of the last day of their rotation. These handoffs customarily involved both hospitalists viewing the electronic health record during the discussion but were not standardized. Night hospitalists performed admissions and cross‐coverage each night from 7 pm to 7 am. Night hospitalists printed history and physicals for day hospitalists, but typically did not give verbal sign‐out on new admissions.
Acquisition of Study Population Data
We identified all patients admitted to the nonteaching hospitalist service using the Northwestern Medicine Enterprise Data Warehouse (EDW), an integrated repository of all clinical and research data sources on the campus. We excluded patients admitted under observation status, those initially admitted to other services (eg, intensive care, general surgery), those discharged from other services, and those cared for by advanced practice providers (ie, nurse practitioners and physician assistants).
Predictor Variables
We identified physicians completing the primary service history and physicals (H&P) and progress notes throughout patients' hospitalizations to calculate 2 measures of continuity: the Number of Physicians Index (NPI), and the Usual Provider of Continuity (UPC) Index.[8, 9] The NPI represented the total number of unique hospitalists completing H&Ps and/or progress notes for a patient. The UPC was calculated as the largest number of notes signed by a single hospitalist divided by the total number of hospitalist notes for a patient. For example, if Dr. John Smith wrote notes on the first 4 days of a patient's hospital stay, and Dr. Mary Jones wrote notes on the following 2 days (total stay=6 days), the NPI would be 2 and the UPC would be 0.67. Therefore, higher NPI and lower UPC designate lower continuity. Significant events occurring during the nighttime were documented in separate notes titled cross‐cover notes. These cross‐cover notes were not included in the calculation of NPI or UPC. In the rare event that 2 or more progress notes were written on the same day, we selected the one used for billing to calculate UPC and NPI.
Outcome Variables
We used AE data from a study we conducted to assess the impact of unit‐based interventions to improve teamwork and patient safety, the methods of which have been previously described.[7] Briefly, we used a 2‐stage medical record review similar to that performed in prior studies.[10, 11, 12, 13] In the first stage, we identified potential AEs using automated queries of the Northwestern Medicine EDW. These queries were based on screening criteria used in the Harvard Medical Practice Study and the Institute for Healthcare Improvement (IHI) Global Trigger Tool.[12, 13] Examples of queries included abnormal laboratory values (eg, international normalized ratio [INR] >6 after hospital day 2 and excluding patients with INR >4 on day 1), administration of rescue medications (eg, naloxone), certain types of incident reports (eg, pressure ulcer), International Classification of Diseases, Ninth Revision (ICD‐9) codes indicating hospital‐acquired conditions (eg, venous thromboembolism), and text searches of progress notes and discharge summaries using natural language processing.[14] Prior research by our group confirmed these automated screens identify a similar number of AEs as manual medical record screening.[14] For each patient with 1 or more potential AE, a research nurse performed a medical record abstraction and created a description of each potential AE.
In the second stage, 2 physician researchers independently reviewed each potential AE in a blinded fashion to determine whether or not an AE was present. An AE was defined as injury due to medical management rather than the natural history of the illness,[15] and included injuries that prolonged the hospital stay or produced disability as well as those resulting in transient disability or abnormal lab values.[16] After independent review, physician reviewers discussed discrepancies in their ratings to achieve consensus.
We tested the reliability of medical record abstractions in our prior study by conducting duplicate abstractions and consensus ratings for a randomly selected sample of 294 patients.[7] The inter‐rater reliability was good for determining the presence of AEs (=0.63).
Statistical Analyses
We calculated descriptive statistics for patient characteristics. Primary discharge diagnosis ICD‐9 codes were categorized using the Healthcare Cost and Utilization Project Clinical Classification Software.[17] We created multivariable logistic regression models with the independent variable being the measure of continuity (NPI or UPC) and the dependent variable being experiencing 1 or more AEs. Covariates included patient age, sex, race, payer, night admission, weekend admission, intensive care unit stay, Medicare Severity Diagnosis Related Group (MS‐DRG) weight, and total number of Elixhauser comorbidities.[18] The length of stay (LOS) was also included as a covariate, as longer LOS increases the probability of discontinuity and may increase the risk for AEs. Because MS‐DRG weight and LOS were highly correlated, we created several models; the first including both as continuous variables, the second including both categorized into quartiles, and a third excluding MS‐DRG weight and including LOS as a continuous variable. Our prior study assessing the impact of unit‐based interventions did not show a statistically significant difference in the pre‐ versus postintervention period, thus we did not include study period as a covariate.
RESULTS
Patient Characteristics
Our analyses included data from 474 hospitalizations. Patient characteristics are shown in Table 1. Patients were a mean 51.118.8 years of age, hospitalized for a mean 3.43.1 days, included 241 (50.8%) women, and 233 (49.2%) persons of nonwhite race. The mean and standard deviation of NPI and UPC were 2.51.0 and 0.60.2. Overall, 47 patients (9.9%) experienced 55 total AEs. AEs included 31 adverse drug events, 6 falls, 5 procedural injuries, 4 manifestations of poor glycemic control, 3 hospital‐acquired infections, 2 episodes of acute renal failure, 1 episode of delirium, 1 pressure ulcer, and 2 categorized as other.
| Characteristic | Value |
|---|---|
| |
| Mean age (SD), y | 55.1 (18.8) |
| Mean length of stay (SD), d | 3.4 (3.1) |
| Women, n (%) | 241 (50.8) |
| Nonwhite race, n (%) | 233 (49.2) |
| Payer, n (%) | |
| Private | 180 (38) |
| Medicare | 165 (34.8) |
| Medicaid | 47 (9.9) |
| Self‐pay/other | 82 (17.3) |
| Night admission, n (%) | 245 (51.7) |
| Weekend admission, n (%) | 135 (28.5) |
| Intensive care unit stay, n (%) | 18 (3.8) |
| Diagnosis, n (%) | |
| Diseases of the circulatory system | 95 (20.0) |
| Diseases of the digestive system | 65 (13.7) |
| Diseases of the respiratory system | 49 (10.3) |
| Injury and poisoning | 41 (8.7) |
| Diseases of the skin and soft tissue | 31 (6.5) |
| Symptoms, signs, and ill‐defined conditions and factors influencing health status | 28 (5.9) |
| Endocrine, nutritional, and metabolic diseases and immunity disorders | 25 (5.3) |
| Diseases of the genitourinary system | 24 (5.1) |
| Diseases of the musculoskeletal system and connective tissue | 23 (4.9) |
| Diseases of the nervous system | 23 (4.9) |
| Other | 70 (14.8) |
| Mean no. of Elixhauser comorbidities (SD) | 2.3 (1.7) |
| Mean MS‐DRG weight (SD) | 1.0 (1.0) |
| Mean NPI (SD) | 2.5 (1.0) |
| Mean UPC (SD) | 0.6 (0.2) |
Association Between Continuity and Adverse Events
In unadjusted models, each 1‐unit increase in the NPI (ie, less continuity) was significantly associated with the incidence of 1 or more AEs (odds ratio [OR]=1.75; P<0.001). However, UPC was not associated with incidence of AEs (OR=1.03; P=0.68) (Table 2). Across all adjusted models, neither NPI nor UPC was significantly associated with the incidence of AEs. The direction of the effect of discontinuity on AEs was inconsistent across models. Though all 3 adjusted models using NPI as the independent variable showed a trend toward increased odds of experiencing 1 or more AE with discontinuity, 2 of the 3 models using UPC showed trends in the opposite direction.
| NPI OR (95% CI)* | P Value | UPC OR (95% CI)* | P Value | ||
|---|---|---|---|---|---|
| |||||
| Unadjusted model | 1.75 (1.332.29) | <0.0001 | 1.03 (0.89‐1.21) | 0.68 | |
| Adjusted models | |||||
| Model 1 | MS‐DRG and LOS continuous | 1.16 (0.781.72) | 0.47 | 0.96 (0.791.14) | 0.60 |
| Model 2 | MS‐DRG and LOS in quartiles | 1.38 (0.981.94) | 0.07 | 1.05 (0.881.26) | 0.59 |
| Model 3 | MS‐DRG dropped, LOS continuous | 1.14 (0.771.70) | 0.51 | 0.95 (0.791.14) | 0.56 |
DISCUSSION
We found that hospitalist physician continuity was not associated with the incidence of AEs. Our findings are somewhat surprising because of the high value placed on continuity of care and patient safety concerns related to handoffs. Key clinical information may be lost when patient care is transitioned to a new hospitalist shortly after admission (eg, from a night hospitalist) or at the end of a rotation. Thus, it is logical to assume that discontinuity inherently increases the risk for harm. On the other hand, a physician newly taking over patient care from another may not be anchored to the initial diagnosis and treatment plan established by the first. This second look could potentially prevent missed/delayed diagnoses and optimize the plan of care.[19] These countervailing forces may explain our findings.
Several other potential explanations for our findings should be considered. First, the quality of handoffs may have been sufficient to overcome the potential for information loss. We feel this is unlikely given that little attention had been dedicated to improving the quality of patient handoffs among hospitalists in our institution. Notably, though a number of studies have evaluated resident physician handoffs, most of the work has focused on night coverage, and little is known about the quality of attending handoffs.[20] Second, access to a fully integrated electronic health record may have assisted hospitalists in complementing information received during handoffs. For example, a hospitalist about to start his or her rotation may have remotely accessed and reviewed patient medical records prior to receiving the phone handoff from the outgoing hospitalist. Third, other efforts to improve patient safety may have reduced the overall risk and provided some resilience in the system. Unit‐based interventions, including structured interdisciplinary rounds and nurse‐physician coleadership, improved teamwork climate and reduced AEs in the study hospital over time.[7]
Another factor to consider relates to the fact that hospital care is provided by teams of clinicians (eg, nurses, specialist physicians, therapists, social workers). Hospital teams are often large and have dynamic team membership. Similar to hospitalists, nurses, physician specialists, and other team members handoff care throughout the course of a patient's hospital stay. Yet, discontinuity for each professional type may occur at different times and frequencies. For example, a patient may be handed off from one hospitalist to another, yet the care continues with the same cardiologist or nurse. Future research should better characterize hospital team complexity (eg, size, relationships among members) and dynamics (eg, continuity for various professional types) and the impact of these factors on patient outcomes.
Our findings are important because hospitalist physician discontinuity is common during hospital stays. Hospital medicine groups vary in their staffing and scheduling models. Policies related to admission distribution and rotation length (consecutive days worked) systematically impact physician continuity. Few studies have evaluated the effect on continuity on hospitalized patient outcomes, and no prior research, to our knowledge, has explored the association of continuity on measures of patient safety.[6, 21, 22] Though our study might suggest that staffing models have little impact on patient safety, as previously mentioned, other team factors may influence patient outcomes.
Our study has several limitations. First, we assessed the impact of continuity on AEs in a single site. Although the 7 days on/7 days off model is the most common scheduling pattern used by adult hospital medicine groups,[23] staffing models and patient safety practices vary across hospitals, potentially limiting the generalizability of our study. Second, continuity can be defined and measured in a variety of ways. We used 2 different measures of physician continuity. As previously mentioned, assessing continuity of other clinicians may allow for a more complete understanding of the potential problems related to fragmentation of care. Third, this study excluded patients who experienced care transitions from other hospitals or other units within the hospital. Patients transferred from other hospitals are often complex, severely ill, and may be at higher risk for loss of key clinical information. Fourth, we used automated screens of an EDW to identify potential AEs. Although our prior research found that this method identified a similar number of AEs as manual medical record review screening, there was poor agreement between the 2 methods. Unfortunately, there is no gold standard to identify AEs. The EDW‐facilitated method allowed us to feasibly screen a larger number of charts, increasing statistical power, and minimized any potential bias that might occur during a manual review to identify potential AEs. Finally, we used data available from 2 prior studies and may have been underpowered to detect a significant association between continuity and AEs due to the relatively low percentage of patients experiencing an AE. In a post hoc power calculation, we estimated that we had 70% power to detect a 33% change in the proportion of patients with 1 or more AE for each 1‐unit increase in NPI, and 80% power to detect a 20% change for each 0.1‐unit decrease in UPC.
CONCLUSION
In conclusion, we found that hospitalist physician continuity was not associated with the incidence of AEs. We speculate that hospitalist continuity is only 1 of many team factors that may influence patient safety, and that prior efforts within our institution may have reduced our ability to detect an association. Future research should better characterize hospital team complexity and dynamics and the impact of these factors on patient outcomes.
Disclosures
This project was supported by a grant from the Agency for Healthcare Research and Quality and an Excellence in Academic Medicine Award, administered by Northwestern Memorial Hospital. The authors report no conflicts of interest.
- , , . What is “continuity of care”? J Health Serv Res Policy. 2006;11:248–250.
- , . Interpersonal continuity of care and care outcomes: a critical review. Ann Fam Med. 2005;3:159–166.
- , , , . The association between continuity of care and outcomes: a systematic and critical review. J Eval Clin Pract. 2010;16:947–956.
- , . Interpersonal continuity of care and patient satisfaction: a critical review. Ann Fam Med. 2004;2:445–451.
- , , , . Continuity of care in a family practice residency program. Impact on physician satisfaction. J Fam Pract. 1990;31:69–73.
- , , , et al. The impact of hospitalist discontinuity on hospital cost, readmissions, and patient satisfaction. J Gen Intern Med. 2014;29:1004–1008.
- , , , et al. Implementation of unit‐based interventions to improve teamwork and patient safety on a medical service [published online ahead of print June 11, 2014]. Am J Med Qual. doi: 10.1177/1062860614538093.
- . Measuring provider continuity in ambulatory care: an assessment of alternative approaches. Med Care. 1979;17:551–565.
- . Defining and measuring interpersonal continuity of care. Ann Fam Med. 2003;1:134–143.
- U.S. Department of Health and Human Services. Agency for Healthcare Research and Quality. Adverse events in hospitals: national incidence among medical beneficiaries. Available at: http://psnet.ahrq.gov/resource.aspx?resourceID=19811. Published November 2010. Accessed on December 15, 2014.
- , , , et al. “Global trigger tool” shows that adverse events in hospitals may be ten times greater than previously measured. Health Aff (Millwood). 2011;30:581–589.
- , , , et al. A study of medical injury and medical malpractice. N Engl J Med. 1989;321:480–484.
- , , , et al. Incidence and types of adverse events and negligent care in Utah and Colorado. Med Care. 2000;38:261–271.
- , , , et al. Comparison of traditional trigger tool to data warehouse based screening for identifying hospital adverse events. BMJ Qual Saf. 2013;22:130–138.
- , , , et al. Incidence of adverse events and negligence in hospitalized patients. Results of the Harvard Medical Practice Study I. N Engl J Med. 1991;324:370–376.
- , , . Safety of patients isolated for infection control. JAMA. 2003;290:1899–1905.
- HCUP Clinical Classification Software. Agency for Healthcare Research and Quality, Rockville, MD. http://www.hcup-us.ahrq.gov/toolssoftware/ccs/ccs.jsp. Accessed on December 15, 2014.
- , , , . Comorbidity measures for use with administrative data. Med Care. 1998;36:8–27.
- . Does continuity of care matter? No: discontinuity can improve patient care. West J Med. 2001;175:5.
- , , , , , . Hospitalist handoffs: a systematic review and task force recommendations. J Hosp Med. 2009;4:433–440.
- , , , , . The impact of fragmentation of hospitalist care on length of stay. J Hosp Med. 2010;5:335–338.
- , , . The Creating Incentives and Continuity Leading to Efficiency staffing model: a quality improvement initiative in hospital medicine. Mayo Clin Proc. 2012;87:364–371.
- Society of Hospital Medicine. 2014 state of hospital medicine report. Philadelphia, PA: Society of Hospital Medicine; 2014.
- , , . What is “continuity of care”? J Health Serv Res Policy. 2006;11:248–250.
- , . Interpersonal continuity of care and care outcomes: a critical review. Ann Fam Med. 2005;3:159–166.
- , , , . The association between continuity of care and outcomes: a systematic and critical review. J Eval Clin Pract. 2010;16:947–956.
- , . Interpersonal continuity of care and patient satisfaction: a critical review. Ann Fam Med. 2004;2:445–451.
- , , , . Continuity of care in a family practice residency program. Impact on physician satisfaction. J Fam Pract. 1990;31:69–73.
- , , , et al. The impact of hospitalist discontinuity on hospital cost, readmissions, and patient satisfaction. J Gen Intern Med. 2014;29:1004–1008.
- , , , et al. Implementation of unit‐based interventions to improve teamwork and patient safety on a medical service [published online ahead of print June 11, 2014]. Am J Med Qual. doi: 10.1177/1062860614538093.
- . Measuring provider continuity in ambulatory care: an assessment of alternative approaches. Med Care. 1979;17:551–565.
- . Defining and measuring interpersonal continuity of care. Ann Fam Med. 2003;1:134–143.
- U.S. Department of Health and Human Services. Agency for Healthcare Research and Quality. Adverse events in hospitals: national incidence among medical beneficiaries. Available at: http://psnet.ahrq.gov/resource.aspx?resourceID=19811. Published November 2010. Accessed on December 15, 2014.
- , , , et al. “Global trigger tool” shows that adverse events in hospitals may be ten times greater than previously measured. Health Aff (Millwood). 2011;30:581–589.
- , , , et al. A study of medical injury and medical malpractice. N Engl J Med. 1989;321:480–484.
- , , , et al. Incidence and types of adverse events and negligent care in Utah and Colorado. Med Care. 2000;38:261–271.
- , , , et al. Comparison of traditional trigger tool to data warehouse based screening for identifying hospital adverse events. BMJ Qual Saf. 2013;22:130–138.
- , , , et al. Incidence of adverse events and negligence in hospitalized patients. Results of the Harvard Medical Practice Study I. N Engl J Med. 1991;324:370–376.
- , , . Safety of patients isolated for infection control. JAMA. 2003;290:1899–1905.
- HCUP Clinical Classification Software. Agency for Healthcare Research and Quality, Rockville, MD. http://www.hcup-us.ahrq.gov/toolssoftware/ccs/ccs.jsp. Accessed on December 15, 2014.
- , , , . Comorbidity measures for use with administrative data. Med Care. 1998;36:8–27.
- . Does continuity of care matter? No: discontinuity can improve patient care. West J Med. 2001;175:5.
- , , , , , . Hospitalist handoffs: a systematic review and task force recommendations. J Hosp Med. 2009;4:433–440.
- , , , , . The impact of fragmentation of hospitalist care on length of stay. J Hosp Med. 2010;5:335–338.
- , , . The Creating Incentives and Continuity Leading to Efficiency staffing model: a quality improvement initiative in hospital medicine. Mayo Clin Proc. 2012;87:364–371.
- Society of Hospital Medicine. 2014 state of hospital medicine report. Philadelphia, PA: Society of Hospital Medicine; 2014.
© 2014 Society of Hospital Medicine
Improving Teamwork with SIDR
Communication among hospital care providers is critically important to provide safe and effective care.15 Yet, studies in operating rooms, intensive care units (ICUs), and general medical units have revealed widely discrepant views on the quality of collaboration and communication between physicians and nurses.68 Although physicians consistently gave high ratings to the quality of collaboration with nurses, nurses rated the quality of collaboration with physicians relatively poorly.
A significant barrier to communication among providers on patient care units is the fluidity and geographic dispersion of team members.8 Physicians, nurses, and other hospital care providers have difficulty finding a way to discuss the care of their patients in person. Research has shown that nurses and physicians on patient care units do not communicate consistently and frequently are not in agreement about their patients' plans of care9, 10
Interdisciplinary Rounds (IDR) have been used as a means to assemble patient care unit team members and improve collaboration on the plan of care.1114 Prior research has demonstrated improved ratings of collaboration on the part of physicians,13, 14 but the effect of IDR on nurses' ratings of collaboration and teamwork has not been adequately assessed. One IDR study did not assess nurses' perceptions,13 while others used instruments not previously described and/or validated in the literature.12, 14 Regarding more concrete outcomes, research indicates variable effects of IDR on length of stay (LOS) and cost. Although 2 studies documented a reduction in LOS and cost with the use of IDR,12, 13 another study showed no effect.15 Furthermore, prior studies evaluated the use of IDR on resident‐covered teaching services. The effect IDR has on collaboration, LOS, and cost in a nonteaching hospitalist service setting is not known.
This study had 3 aims. The first was to assess the impact of an intervention, Structured Inter‐Disciplinary Rounds (SIDR), on nurses' ratings of collaboration and teamwork. The second was to assess the feasibility and sustainability of the intervention. The third was to assess the impact of the intervention on hospital LOS and cost.
Methods
Setting and Study Design
The study was conducted at Northwestern Memorial Hospital (NMH), an 897‐bed tertiary care teaching hospital in Chicago, IL, and was approved by the Institutional Review Board of Northwestern University. The study was a controlled trial of an intervention, SIDR, on collaboration and teamwork on patient care units. One of 2 similar hospitalist service units was randomly selected for the intervention, while the other served as a control unit. SIDR was implemented in August 2008 and data were collected over a 24 week study period.
Each hospitalist service unit consisted of 30 beds and was equipped with continuous cardiac telemetry monitoring. Units were also identical in structure and staffing of nonphysician personnel. The intervention unit included a heart failure‐hospitalist comanagement service. Patients followed at the Center for Heart Failure in the Bluhm Cardiovascular Institute of Northwestern were preferentially admitted to this service. All other patients were admitted to units based on bed availability in a quasi‐randomized fashion. Hospitalists worked 7 consecutive days while on service and cared for patients primarily on the units involved in this study. Therefore, hospitalists cared for patients on both the intervention and control units during their weeks on service. Hospitalists cared for patients independently without the assistance of resident physicians or mid‐level providers (ie, physician assistants or nurse practitioners).
Intervention
SIDR combined a structured format for communication with a forum for regular interdisciplinary meetings. A working group, consisting of nurses, hospitalists, and the unit pharmacist, social worker, and case manager, met weekly for 12 weeks prior to implementation. The working group determined the optimal timing, frequency, and location for SIDR. Additionally, the working group finalized the content of a structured communication tool (Supporting Information) to be used during SIDR. The structured communication tool was modeled after prior research demonstrating the benefit of daily goals of care forms16, 17 and ensured that important elements of the daily plan of care were discussed. Based on the working group's recommendation, SIDR took place each weekday at 11:00 AM in the unit conference room and lasted approximately 30 minutes. The nurse manager and a unit medical director co‐led rounds each day. SIDR was attended by all nurses and hospitalists caring for patients on the unit, as well as the pharmacist, social worker, and case manager assigned to the unit.
Provider Survey
Nurses working on the intervention and control units during the study period were administered a survey 16 weeks to 20 weeks after implementation of SIDR to assess ratings of collaboration and teamwork. The first portion of the survey was based on previously published surveys assessing teamwork attitudes among providers.6, 7 We asked nurses to rate the quality of communication and collaboration they had experienced with hospitalists using a 5‐point ordinal scale (1 = very low, 2 = low, 3 = adequate, 4 = high, 5 = very high). The second portion of the survey assessed teamwork and safety climate using the teamwork and safety domains of the Safety Attitudes Questionnaire (SAQ) developed by Sexton et al.18 The SAQ is based on previous research in aviation and medicine and has been validated in clinical settings.19, 20 Because hospitalists worked with nurses on both units, and in light of our prior research demonstrating that hospitalists rate the quality of collaboration with nurses highly,8 we did not assess hospitalists' ratings of collaboration. A final portion of the survey assessed nurses' perceptions of whether SIDR improved efficiency of communication, collaboration among team members, and patient care using a 5‐point Likert scale (1 = strongly disagree; 2 = disagree; 3 = neutral; 4 = agree; 5 = strongly agree). Hospitalists also received this portion of the survey at the completion of each clinical rotation. All surveys were administered in a web‐based format using an internet link (
SIDR Characteristics and Attendance
The unit medical director recorded the duration of SIDR, the number of patients on the unit, and the number of patients discussed each day. Attendance for each discipline was also recorded each day during the study period.
Data Analysis
Provider demographic data were obtained from completed surveys and group comparisons were done using chi‐square and t tests. The percentage of nurses on each unit rating of the quality of communication and collaboration with hospitalist physicians as high or very high was compared using chi‐square. Teamwork and safety climate scores were compared using the Mann Whitney U test.
Patient data were obtained from administrative databases for both the control and intervention unit during the study period as well as for the intervention unit in the 24 weeks preceding the study period. Demographic data were compared using chi‐square and t tests. Primary discharge diagnosis ICD‐9 codes were grouped into diagnosis clusters using the Healthcare Cost and Utilization Project system of the Agency for Healthcare Research and Quality.21 Diagnosis clusters were then analyzed using the chi‐square test. Because of case mix differences between patients on the intervention and control units, we analyzed LOS and cost using a concurrent control as well as an historic control. Unadjusted LOS and costs were compared using the Mann Whitney U test. We then conducted multivariable linear regression analyses to assess the impact of SIDR on LOS and cost. To satisfy normality requirements and distribution of residuals, we explored 2 methods of transforming skewed data on LOS and cost: logarithmic conversion and truncation at the mean LOS + 3 standard deviations (SDs). Since both techniques yielded similar results, we chose to present results by using truncation. Covariates for multivariable analyses included age, gender, race, payor, admission source, case‐mix, discharge disposition, presence of ICU stay during hospitalization, and Medicare Severity‐Diagnosis Related Group (MS‐DRG) weight. We used standard errors robust to the clustering of patients within each physician. All analyses were conducted using Stata version 10.0 (College Station, TX).
Results
Characteristics of Providers, Patients, and SIDR
Forty‐nine of 58 (84%) nurses completed the survey. Eighty‐eight of 96 (92%) surveys were completed by hospitalists at the end of their week on service. Hospitalist surveys represented 33 different hospitalists because individuals may have worked on study units more than once during the study period. Nurses were a mean 35.0 10.4 years of age and had been working at the hospital for a mean 5.0 6.3 years. Hospitalists were a mean 32.8 2.8 years of age and had been working at the hospital for a mean 2.6 1.9 years.
Patient characteristics are shown in Table 1. Intervention unit patients were admitted from the Emergency Department slightly more often in the postSIDR period. Patient case mix differed between the control and intervention unit, but was similar when comparing the intervention unit preSIDR and postSIDR. Intervention unit MS‐DRG weight was lower in the postSIDR period.
| Control Unit (n = 815) | Intervention Unit Pre‐SIDR (n = 722) | Intervention Unit Post‐SIDR (n = 684) | P Value for Comparison of Intervention Unit Post‐SIDR vs. Control | P Value for Comparison of Intervention Unit Post‐ vs. Pre‐SIDR | |
|---|---|---|---|---|---|
| |||||
| Mean age, years (SD) | 63.8 (16.0) | 64.2 (16.3) | 64.1 (17.2) | 0.74 | 0.92 |
| Women, n (%) | 403 (49) | 347 (48) | 336 (49) | 0.90 | 0.69 |
| Ethnicity, n (%) | 0.22 | 0.71 | |||
| White | 438 (54) | 350 (48) | 334 (49) | ||
| Black | 269 (33) | 266 (37) | 264 (39) | ||
| Hispanic | 48 (6) | 40 (6) | 34 (5) | ||
| Asian | 6 (1) | 8 (1) | 4 (1) | ||
| Other | 54 (7) | 58 (8) | 48 (7) | ||
| Payor, n (%) | 0.07 | 0.67 | |||
| Medicare | 456 (56) | 436 (60) | 399 (58) | ||
| Private | 261 (32) | 176 (24) | 182 (27) | ||
| Medicaid | 67 (8) | 75 (10) | 65 (10) | ||
| Self pay | 31 (4) | 35 (5) | 38 (6) | ||
| Admission source, n (%) | 0.51 | 0.03 | |||
| Emergency department | 695 (85) | 590 (82) | 593 (87) | ||
| Direct admission | 92 (11) | 99 (14) | 65 (10) | ||
| Transfer | 28 (3) | 33 (5) | 26 (4) | ||
| Case mix, n (%) | |||||
| Congestive heart failure | 78 (10) | 164 (23) | 144 (21) | <0.01 | 0.45 |
| Cardiac dysrhythmia | 167 (20) | 69 (10) | 81 (12) | <0.01 | 0.17 |
| Chest pain | 100 (12) | 47 (7) | 59 (9) | 0.02 | 0.13 |
| Coronary atherosclerosis | 52 (6) | 19 (3) | 19 (3) | <0.01 | 0.87 |
| Hypertension | 24 (3) | 38 (5) | 24 (4) | 0.54 | 0.11 |
| Syncope | 27 (3) | 23 (3) | 26 (4) | 0.61 | 0.53 |
| Fluid or electrolyte disorder | 11 (1) | 25 (3) | 23 (3) | 0.01 | 0.92 |
| Pneumonia | 14 (2) | 13 (2) | 22 (3) | 0.06 | 0.09 |
| Pulmonary heart disease | 16 (2) | 13 (2) | 14 (2) | 0.91 | 0.74 |
| Intervertebral disc or other back problem | 32 (4) | 3 (0) | 6 (1) | <0.01 | 0.28 |
| Other diagnosis | 294 (36) | 308 (43) | 266 (39) | 0.26 | 0.15 |
| Cardiovascular procedure during admission | 151 (19) | 95 (13) | 86 (13) | <0.01 | 0.74 |
| Intensive care unit stay during admission, n (%) | 39 (5) | 44 (6) | 27 (4) | 0.43 | 0.07 |
| Discharge disposition, n (%) | |||||
| Home | 736 (90) | 646 (89) | 610 (89) | 0.88 | 0.82 |
| Skilled nursing facility or rehabilitation | 66 (8) | 61 (8) | 63 (9) | ||
| Other facility | 9 (1) | 11 (2) | 7 (1) | ||
| Expired | 4 (0) | 4 (1) | 4 (1) | ||
| Mean Medicare severity ‐diagnosis related group weight (SD) | 1.08 (0.73) | 1.14 (0.76) | 1.06 (0.72) | 0.61 | 0.04 |
SIDR occurred each weekday (with the exception of holidays) on the intervention unit and lasted a mean 27.7 4.6 minutes. The unit had a mean 27 patients per day and 86% of patients on the unit were discussed each day. Attendance exceeded 85% for each discipline (hospitalists, nurses, and the unit pharmacist, social worker, and case manager).
Ratings of Teamwork and Perceptions of SIDR
As shown in Figure 1, a larger percentage of nurses rated the quality of communication and collaboration with hospitalists as high or very high on the intervention unit compared to the control unit (80% vs. 54%; P = 0.05).
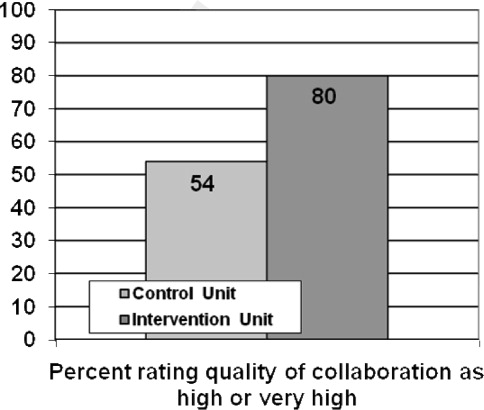
Nurses' ratings of the teamwork and safety climate are summarized in Table 2. The median teamwork climate score was 85.7 (interquartile range [IQR], 75.092.9) for the intervention unit as compared to 61.6 (IQR, 48.283.9) for the control unit (P = 0.008). The median safety climate score was 75.0 (IQR, 70.581.3) for the intervention unit as compared to 61.1 (IQR, 30.281.3) for the control unit (P = 0.03).
| Control Unit, n = 24 | Intervention Unit, n = 25 | P Value | |
|---|---|---|---|
| |||
| Median Teamwork Climate Score (IQR) | 75.0 (70.581.3) | 61.6 (48.283.9) | 0.008 |
| Median Safety Climate Score (IQR) | 85.7 (75.092.9) | 61.1 (30.281.3) | 0.03 |
Sixty‐five of 88 (74%) hospitalists and 18 of 24 (75%) nurses agreed that SIDR improved the efficiency of their work day. Eighty of 88 (91%) hospitalists and 18 of 24 (75%) nurses agreed that SIDR improved team collaboration. Seventy‐six of 88 (86%) hospitalists and 18 of 24 (75%) nurses agreed that SIDR improved patient care. Sixty‐seven of 88 (76%) hospitalists and 22 of 25 (88%) nurses indicated that they wanted SIDR to continue indefinitely.
SIDR Impact on LOS and Cost
The unadjusted mean LOS was significantly higher for the intervention unit postSIDR as compared to the control unit (4.0 3.4 vs. 3.7 3.3 days; P = 0.03). However, the unadjusted mean LOS was not significantly different for the intervention unit postSIDR as compared to the intervention unit preSIDR (4.0 3.4 vs. 4.26 3.5 days; P = 0.10). The unadjusted cost was lower for the intervention unit postSIDR as compared to the control unit ($7,513.23 7,085.10 vs. $8,588.66 7,381.03; P < 0.001). The unadjusted mean cost was not significantly different for the invention unit postSIDR as compared to the intervention unit preSIDR ($7,513.23 7,085.10 vs. $7,937.00 7,512.23; P = 0.19).
Multivariable analyses of LOS and cost are summarized in Table 3. The adjusted LOS was not significantly different when comparing the intervention unit postSIDR to either the control unit or the intervention unit preSIDR. The adjusted cost for the intervention unit postSIDR was $739.55 less than the control unit (P = 0.02). The adjusted cost was not significantly different when comparing the intervention unit postSIDR to the intervention unit preSIDR.
| Adjusted Difference for Intervention Unit Post‐SIDR vs. Control | P Value for Adjusted Difference for Intervention Unit Post‐SIDR vs. Control | Adjusted Difference for Intervention Unit Post‐ vs. Pre‐SIDR | P Value for Adjusted Difference for Intervention Unit Post‐ vs. Pre‐SIDR | |
|---|---|---|---|---|
| ||||
| Length of stay | 0.05 | 0.75 | 0.04 | 0.83 |
| Cost | 739.55 | 0.02 | 302.94 | 0.34 |
Discussion
We found that nurses working on a unit using SIDR rated the quality of communication and collaboration with hospitalists significantly higher as compared to a control unit. Notably, because hospitalists worked on both the intervention and control unit during their weeks on service, nurses on each unit were rating the quality of collaboration with the same hospitalists. Nurses also rated the teamwork and safety climate higher on the intervention unit. These findings are important because prior research has shown that nurses are often dissatisfied with the quality of collaboration and teamwork with physicians.68 Potential explanations include fundamental differences between nurses and physicians with regard to status/authority, gender, training, and patient care responsibilities.6 Unfortunately, a culture of poor teamwork may lead to a workplace in which team members feel unable to approach certain individuals and uncomfortable raising concerns. Not surprisingly, higher ratings of teamwork culture have been associated with nurse retention.22, 23 SIDR provided a facilitated forum for interdisciplinary discussion, exchange of critical clinical information, and collaboration on the plan of care.
Our findings are also important because poor communication represents a major etiology of preventable adverse events in hospitals.15 Higher ratings of collaboration and teamwork have been associated with better patient outcomes in observational studies.2426 Further research should evaluate the impact of improved interdisciplinary collaboration as a result of SIDR on the safety of care delivered on inpatient medical units.
The majority of providers agreed that SIDR improved patient care and that SIDR should continue indefinitely. Importantly, providers also felt that SIDR improved the efficiency of their workday and attendance was high among all disciplines. Prior studies on IDR either did not report attendance or struggled with attendance.11 Incorporating the input of frontline providers into the design of SIDR allowed us to create a sustainable intervention which fit into daily workflow.
Our bivariate analyses found significant patient case‐mix differences between the intervention and control unit, limiting our ability to perform direct comparisons in LOS and cost. Pre‐post analyses of LOS and cost may be affected by cyclical or secular trends. Because each approach has its own limitations, we felt that analyses using both an historic as well as a concurrent control would provide a more complete assessment of the effect of the intervention. We included case mix, among other variables, in out multivariable regression analyses and found no benefit to SIDR with regard to LOS and cost. Two prior studies have shown a reduction in LOS and cost with the use of IDR.12, 13 However, one study was conducted approximately 15 years ago and included patients with a longer mean LOS.12 The second study used a pre‐post study design which may not have accounted for unmeasured confounders affecting LOS and cost.13 A third, smaller study showed no effect on LOS and cost with the use of IDR.15 No prior study has evaluated the effect of IDR on LOS and cost in a nonteaching hospitalist service setting.
Our study has several limitations. First, our study reflects the experience of an intervention unit compared to a control unit in a single hospital. Larger studies will be required to test the reproducibility and generalizability of our findings. Second, we did not conduct preintervention provider surveys for comparison ratings of collaboration and teamwork. A prior study, conducted by our research group, found that nurses gave low ratings to the teamwork climate and the quality of collaboration with hospitalists.8 Because this baseline study showed consistently low nurse ratings of collaboration and teamwork across all medical units, and because the units in the current study were identical in size, structure, and staffing of nonphysician personnel, we did not repeat nurse surveys prior to the intervention. Third, as previously mentioned, our study did not directly assess the effect of improved teamwork and collaboration on patient safety. Further study is needed to evaluate this. Although we are not aware of any other interventions to improve interdisciplinary communication on the intervention unit, it is possible that other unknown factors contributed to our findings. We believe this is unlikely due to the magnitude of the improvement in collaboration and the high ratings of SIDR by nurses and physicians on the intervention unit.
In summary, SIDR had a positive effect on nurses' ratings of collaboration and teamwork on a nonteaching hospitalist unit. Future research efforts should assess whether improved teamwork as a result of SIDR also translates into safer patient care.
- Joint Commission on Accreditation of Healthcare Organizations. Sentinel Event Statistics. Available at: http://www.jointcommission.org/SentinelEvents/Statistics. Accessed March2010.
- ,,, et al.A look into the nature and causes of human errors in the intensive care unit.Crit Care Med.1995;23(2):294–300.
- ,,, et al.The nature of adverse events in hospitalized patients. Results of the Harvard Medical Practice Study II.N Engl J Med.1991;324(6):377–384.
- ,,.Communication failures: an insidious contributor to medical mishaps.Acad Med.2004;79(2):186–194.
- ,,,,,.The quality in Australian Health Care Study.Med J Aust.1995;163(9):458–471.
- ,,, et al.Operating room teamwork among physicians and nurses: teamwork in the eye of the beholder.J Am Coll Surg.2006;202(5):746–752.
- ,,.Discrepant attitudes about teamwork among critical care nurses and physicians.Crit Care Med.2003;31(3):956–959.
- ,,,,,.Teamwork on inpatient medical units: assessing attitudes and barriers.Qual Saf Health Care2010;19(2):117–121.
- ,,,,,.Can we talk? Priorities for patient care differed among health care providers:AHRQ;2005.
- ,,, et al.Patterns of nurse—physicians communication and agreement on the plan of care.Qual Saf Health Care. In press.
- ,,, et al.The effect of a multidisciplinary hospitalist/physician and advanced practice nurse collaboration on hospital costs.J Nurs Adm.2006;36(2):79–85.
- ,,.A firm trial of interdisciplinary rounds on the inpatient medical wards: an intervention designed using continuous quality improvement.Med Care.1998;36(8 Suppl):AS4–A12.
- ,,,,.Use of multidisciplinary rounds to simultaneously improve quality outcomes, enhance resident education, and shorten length of stay.J Gen Intern Med.2007;22(8):1073–1079.
- ,,,.Effect of a multidisciplinary intervention on communication and collaboration among physicians and nurses.Am J Crit Care.2005;14(1):71–77.
- ,,,.Effects of interdisciplinary rounds on length of stay in a telemetry unit.J Public Health Manag Pract.2004;10(1):63–69.
- ,,,,.Improving nurse‐physician communication and satisfaction in the intensive care unit with a daily goals worksheet.Am J Crit Care.2006;15(2):217–222.
- ,,,,,.Improving communication in the ICU using daily goals.J Crit Care.2003;18(2):71–75.
- ,,, et al.The Safety Attitudes Questionnaire: psychometric properties, benchmarking data, and emerging research.BMC Health Serv Res.2006;6:44.
- ,,,.Safety Climate Survey: reliability of results from a multicenter ICU survey.Qual Saf Health Care.2005;14(4):273–278.
- ,,, et al.Teamwork in the operating room: frontline perspectives among hospitals and operating room personnel.Anesthesiology.2006;105(5):877–884.
- HCUP Clinical Classification Software [computer program]. Version: Agency for Healthcare Research and Quality, Rockville, MD. Available at: http://www.hcup‐us.ahrq.gov/toolssoftware/ccs/ccs.jsp. Accessed March2010.
- ,,.The influence of teamwork culture on physician and nurse resignation rates in hospitals.Health Serv Manage Res.2008;21(1):23–31.
- .Original research: nurse‐physician relationships: impact on nurse satisfaction and retention.Am J Nurs.2002;102(6):26–34.
- ,,, et al.Association between nurse‐physician collaboration and patient outcomes in three intensive care units.Crit Care Med.1999;27(9):1991–1998.
- ,,,,.Risk‐adjusted morbidity in teaching hospitals correlates with reported levels of communication and collaboration on surgical teams but not with scale measures of teamwork climate, safety climate, or working conditions.J Am Coll Surg.2007;205(6):778–784.
- ,,.The link between teamwork and patients' outcomes in intensive care units.Am J Crit Care.2003;12(6):527–534.
Communication among hospital care providers is critically important to provide safe and effective care.15 Yet, studies in operating rooms, intensive care units (ICUs), and general medical units have revealed widely discrepant views on the quality of collaboration and communication between physicians and nurses.68 Although physicians consistently gave high ratings to the quality of collaboration with nurses, nurses rated the quality of collaboration with physicians relatively poorly.
A significant barrier to communication among providers on patient care units is the fluidity and geographic dispersion of team members.8 Physicians, nurses, and other hospital care providers have difficulty finding a way to discuss the care of their patients in person. Research has shown that nurses and physicians on patient care units do not communicate consistently and frequently are not in agreement about their patients' plans of care9, 10
Interdisciplinary Rounds (IDR) have been used as a means to assemble patient care unit team members and improve collaboration on the plan of care.1114 Prior research has demonstrated improved ratings of collaboration on the part of physicians,13, 14 but the effect of IDR on nurses' ratings of collaboration and teamwork has not been adequately assessed. One IDR study did not assess nurses' perceptions,13 while others used instruments not previously described and/or validated in the literature.12, 14 Regarding more concrete outcomes, research indicates variable effects of IDR on length of stay (LOS) and cost. Although 2 studies documented a reduction in LOS and cost with the use of IDR,12, 13 another study showed no effect.15 Furthermore, prior studies evaluated the use of IDR on resident‐covered teaching services. The effect IDR has on collaboration, LOS, and cost in a nonteaching hospitalist service setting is not known.
This study had 3 aims. The first was to assess the impact of an intervention, Structured Inter‐Disciplinary Rounds (SIDR), on nurses' ratings of collaboration and teamwork. The second was to assess the feasibility and sustainability of the intervention. The third was to assess the impact of the intervention on hospital LOS and cost.
Methods
Setting and Study Design
The study was conducted at Northwestern Memorial Hospital (NMH), an 897‐bed tertiary care teaching hospital in Chicago, IL, and was approved by the Institutional Review Board of Northwestern University. The study was a controlled trial of an intervention, SIDR, on collaboration and teamwork on patient care units. One of 2 similar hospitalist service units was randomly selected for the intervention, while the other served as a control unit. SIDR was implemented in August 2008 and data were collected over a 24 week study period.
Each hospitalist service unit consisted of 30 beds and was equipped with continuous cardiac telemetry monitoring. Units were also identical in structure and staffing of nonphysician personnel. The intervention unit included a heart failure‐hospitalist comanagement service. Patients followed at the Center for Heart Failure in the Bluhm Cardiovascular Institute of Northwestern were preferentially admitted to this service. All other patients were admitted to units based on bed availability in a quasi‐randomized fashion. Hospitalists worked 7 consecutive days while on service and cared for patients primarily on the units involved in this study. Therefore, hospitalists cared for patients on both the intervention and control units during their weeks on service. Hospitalists cared for patients independently without the assistance of resident physicians or mid‐level providers (ie, physician assistants or nurse practitioners).
Intervention
SIDR combined a structured format for communication with a forum for regular interdisciplinary meetings. A working group, consisting of nurses, hospitalists, and the unit pharmacist, social worker, and case manager, met weekly for 12 weeks prior to implementation. The working group determined the optimal timing, frequency, and location for SIDR. Additionally, the working group finalized the content of a structured communication tool (Supporting Information) to be used during SIDR. The structured communication tool was modeled after prior research demonstrating the benefit of daily goals of care forms16, 17 and ensured that important elements of the daily plan of care were discussed. Based on the working group's recommendation, SIDR took place each weekday at 11:00 AM in the unit conference room and lasted approximately 30 minutes. The nurse manager and a unit medical director co‐led rounds each day. SIDR was attended by all nurses and hospitalists caring for patients on the unit, as well as the pharmacist, social worker, and case manager assigned to the unit.
Provider Survey
Nurses working on the intervention and control units during the study period were administered a survey 16 weeks to 20 weeks after implementation of SIDR to assess ratings of collaboration and teamwork. The first portion of the survey was based on previously published surveys assessing teamwork attitudes among providers.6, 7 We asked nurses to rate the quality of communication and collaboration they had experienced with hospitalists using a 5‐point ordinal scale (1 = very low, 2 = low, 3 = adequate, 4 = high, 5 = very high). The second portion of the survey assessed teamwork and safety climate using the teamwork and safety domains of the Safety Attitudes Questionnaire (SAQ) developed by Sexton et al.18 The SAQ is based on previous research in aviation and medicine and has been validated in clinical settings.19, 20 Because hospitalists worked with nurses on both units, and in light of our prior research demonstrating that hospitalists rate the quality of collaboration with nurses highly,8 we did not assess hospitalists' ratings of collaboration. A final portion of the survey assessed nurses' perceptions of whether SIDR improved efficiency of communication, collaboration among team members, and patient care using a 5‐point Likert scale (1 = strongly disagree; 2 = disagree; 3 = neutral; 4 = agree; 5 = strongly agree). Hospitalists also received this portion of the survey at the completion of each clinical rotation. All surveys were administered in a web‐based format using an internet link (
SIDR Characteristics and Attendance
The unit medical director recorded the duration of SIDR, the number of patients on the unit, and the number of patients discussed each day. Attendance for each discipline was also recorded each day during the study period.
Data Analysis
Provider demographic data were obtained from completed surveys and group comparisons were done using chi‐square and t tests. The percentage of nurses on each unit rating of the quality of communication and collaboration with hospitalist physicians as high or very high was compared using chi‐square. Teamwork and safety climate scores were compared using the Mann Whitney U test.
Patient data were obtained from administrative databases for both the control and intervention unit during the study period as well as for the intervention unit in the 24 weeks preceding the study period. Demographic data were compared using chi‐square and t tests. Primary discharge diagnosis ICD‐9 codes were grouped into diagnosis clusters using the Healthcare Cost and Utilization Project system of the Agency for Healthcare Research and Quality.21 Diagnosis clusters were then analyzed using the chi‐square test. Because of case mix differences between patients on the intervention and control units, we analyzed LOS and cost using a concurrent control as well as an historic control. Unadjusted LOS and costs were compared using the Mann Whitney U test. We then conducted multivariable linear regression analyses to assess the impact of SIDR on LOS and cost. To satisfy normality requirements and distribution of residuals, we explored 2 methods of transforming skewed data on LOS and cost: logarithmic conversion and truncation at the mean LOS + 3 standard deviations (SDs). Since both techniques yielded similar results, we chose to present results by using truncation. Covariates for multivariable analyses included age, gender, race, payor, admission source, case‐mix, discharge disposition, presence of ICU stay during hospitalization, and Medicare Severity‐Diagnosis Related Group (MS‐DRG) weight. We used standard errors robust to the clustering of patients within each physician. All analyses were conducted using Stata version 10.0 (College Station, TX).
Results
Characteristics of Providers, Patients, and SIDR
Forty‐nine of 58 (84%) nurses completed the survey. Eighty‐eight of 96 (92%) surveys were completed by hospitalists at the end of their week on service. Hospitalist surveys represented 33 different hospitalists because individuals may have worked on study units more than once during the study period. Nurses were a mean 35.0 10.4 years of age and had been working at the hospital for a mean 5.0 6.3 years. Hospitalists were a mean 32.8 2.8 years of age and had been working at the hospital for a mean 2.6 1.9 years.
Patient characteristics are shown in Table 1. Intervention unit patients were admitted from the Emergency Department slightly more often in the postSIDR period. Patient case mix differed between the control and intervention unit, but was similar when comparing the intervention unit preSIDR and postSIDR. Intervention unit MS‐DRG weight was lower in the postSIDR period.
| Control Unit (n = 815) | Intervention Unit Pre‐SIDR (n = 722) | Intervention Unit Post‐SIDR (n = 684) | P Value for Comparison of Intervention Unit Post‐SIDR vs. Control | P Value for Comparison of Intervention Unit Post‐ vs. Pre‐SIDR | |
|---|---|---|---|---|---|
| |||||
| Mean age, years (SD) | 63.8 (16.0) | 64.2 (16.3) | 64.1 (17.2) | 0.74 | 0.92 |
| Women, n (%) | 403 (49) | 347 (48) | 336 (49) | 0.90 | 0.69 |
| Ethnicity, n (%) | 0.22 | 0.71 | |||
| White | 438 (54) | 350 (48) | 334 (49) | ||
| Black | 269 (33) | 266 (37) | 264 (39) | ||
| Hispanic | 48 (6) | 40 (6) | 34 (5) | ||
| Asian | 6 (1) | 8 (1) | 4 (1) | ||
| Other | 54 (7) | 58 (8) | 48 (7) | ||
| Payor, n (%) | 0.07 | 0.67 | |||
| Medicare | 456 (56) | 436 (60) | 399 (58) | ||
| Private | 261 (32) | 176 (24) | 182 (27) | ||
| Medicaid | 67 (8) | 75 (10) | 65 (10) | ||
| Self pay | 31 (4) | 35 (5) | 38 (6) | ||
| Admission source, n (%) | 0.51 | 0.03 | |||
| Emergency department | 695 (85) | 590 (82) | 593 (87) | ||
| Direct admission | 92 (11) | 99 (14) | 65 (10) | ||
| Transfer | 28 (3) | 33 (5) | 26 (4) | ||
| Case mix, n (%) | |||||
| Congestive heart failure | 78 (10) | 164 (23) | 144 (21) | <0.01 | 0.45 |
| Cardiac dysrhythmia | 167 (20) | 69 (10) | 81 (12) | <0.01 | 0.17 |
| Chest pain | 100 (12) | 47 (7) | 59 (9) | 0.02 | 0.13 |
| Coronary atherosclerosis | 52 (6) | 19 (3) | 19 (3) | <0.01 | 0.87 |
| Hypertension | 24 (3) | 38 (5) | 24 (4) | 0.54 | 0.11 |
| Syncope | 27 (3) | 23 (3) | 26 (4) | 0.61 | 0.53 |
| Fluid or electrolyte disorder | 11 (1) | 25 (3) | 23 (3) | 0.01 | 0.92 |
| Pneumonia | 14 (2) | 13 (2) | 22 (3) | 0.06 | 0.09 |
| Pulmonary heart disease | 16 (2) | 13 (2) | 14 (2) | 0.91 | 0.74 |
| Intervertebral disc or other back problem | 32 (4) | 3 (0) | 6 (1) | <0.01 | 0.28 |
| Other diagnosis | 294 (36) | 308 (43) | 266 (39) | 0.26 | 0.15 |
| Cardiovascular procedure during admission | 151 (19) | 95 (13) | 86 (13) | <0.01 | 0.74 |
| Intensive care unit stay during admission, n (%) | 39 (5) | 44 (6) | 27 (4) | 0.43 | 0.07 |
| Discharge disposition, n (%) | |||||
| Home | 736 (90) | 646 (89) | 610 (89) | 0.88 | 0.82 |
| Skilled nursing facility or rehabilitation | 66 (8) | 61 (8) | 63 (9) | ||
| Other facility | 9 (1) | 11 (2) | 7 (1) | ||
| Expired | 4 (0) | 4 (1) | 4 (1) | ||
| Mean Medicare severity ‐diagnosis related group weight (SD) | 1.08 (0.73) | 1.14 (0.76) | 1.06 (0.72) | 0.61 | 0.04 |
SIDR occurred each weekday (with the exception of holidays) on the intervention unit and lasted a mean 27.7 4.6 minutes. The unit had a mean 27 patients per day and 86% of patients on the unit were discussed each day. Attendance exceeded 85% for each discipline (hospitalists, nurses, and the unit pharmacist, social worker, and case manager).
Ratings of Teamwork and Perceptions of SIDR
As shown in Figure 1, a larger percentage of nurses rated the quality of communication and collaboration with hospitalists as high or very high on the intervention unit compared to the control unit (80% vs. 54%; P = 0.05).

Nurses' ratings of the teamwork and safety climate are summarized in Table 2. The median teamwork climate score was 85.7 (interquartile range [IQR], 75.092.9) for the intervention unit as compared to 61.6 (IQR, 48.283.9) for the control unit (P = 0.008). The median safety climate score was 75.0 (IQR, 70.581.3) for the intervention unit as compared to 61.1 (IQR, 30.281.3) for the control unit (P = 0.03).
| Control Unit, n = 24 | Intervention Unit, n = 25 | P Value | |
|---|---|---|---|
| |||
| Median Teamwork Climate Score (IQR) | 75.0 (70.581.3) | 61.6 (48.283.9) | 0.008 |
| Median Safety Climate Score (IQR) | 85.7 (75.092.9) | 61.1 (30.281.3) | 0.03 |
Sixty‐five of 88 (74%) hospitalists and 18 of 24 (75%) nurses agreed that SIDR improved the efficiency of their work day. Eighty of 88 (91%) hospitalists and 18 of 24 (75%) nurses agreed that SIDR improved team collaboration. Seventy‐six of 88 (86%) hospitalists and 18 of 24 (75%) nurses agreed that SIDR improved patient care. Sixty‐seven of 88 (76%) hospitalists and 22 of 25 (88%) nurses indicated that they wanted SIDR to continue indefinitely.
SIDR Impact on LOS and Cost
The unadjusted mean LOS was significantly higher for the intervention unit postSIDR as compared to the control unit (4.0 3.4 vs. 3.7 3.3 days; P = 0.03). However, the unadjusted mean LOS was not significantly different for the intervention unit postSIDR as compared to the intervention unit preSIDR (4.0 3.4 vs. 4.26 3.5 days; P = 0.10). The unadjusted cost was lower for the intervention unit postSIDR as compared to the control unit ($7,513.23 7,085.10 vs. $8,588.66 7,381.03; P < 0.001). The unadjusted mean cost was not significantly different for the invention unit postSIDR as compared to the intervention unit preSIDR ($7,513.23 7,085.10 vs. $7,937.00 7,512.23; P = 0.19).
Multivariable analyses of LOS and cost are summarized in Table 3. The adjusted LOS was not significantly different when comparing the intervention unit postSIDR to either the control unit or the intervention unit preSIDR. The adjusted cost for the intervention unit postSIDR was $739.55 less than the control unit (P = 0.02). The adjusted cost was not significantly different when comparing the intervention unit postSIDR to the intervention unit preSIDR.
| Adjusted Difference for Intervention Unit Post‐SIDR vs. Control | P Value for Adjusted Difference for Intervention Unit Post‐SIDR vs. Control | Adjusted Difference for Intervention Unit Post‐ vs. Pre‐SIDR | P Value for Adjusted Difference for Intervention Unit Post‐ vs. Pre‐SIDR | |
|---|---|---|---|---|
| ||||
| Length of stay | 0.05 | 0.75 | 0.04 | 0.83 |
| Cost | 739.55 | 0.02 | 302.94 | 0.34 |
Discussion
We found that nurses working on a unit using SIDR rated the quality of communication and collaboration with hospitalists significantly higher as compared to a control unit. Notably, because hospitalists worked on both the intervention and control unit during their weeks on service, nurses on each unit were rating the quality of collaboration with the same hospitalists. Nurses also rated the teamwork and safety climate higher on the intervention unit. These findings are important because prior research has shown that nurses are often dissatisfied with the quality of collaboration and teamwork with physicians.68 Potential explanations include fundamental differences between nurses and physicians with regard to status/authority, gender, training, and patient care responsibilities.6 Unfortunately, a culture of poor teamwork may lead to a workplace in which team members feel unable to approach certain individuals and uncomfortable raising concerns. Not surprisingly, higher ratings of teamwork culture have been associated with nurse retention.22, 23 SIDR provided a facilitated forum for interdisciplinary discussion, exchange of critical clinical information, and collaboration on the plan of care.
Our findings are also important because poor communication represents a major etiology of preventable adverse events in hospitals.15 Higher ratings of collaboration and teamwork have been associated with better patient outcomes in observational studies.2426 Further research should evaluate the impact of improved interdisciplinary collaboration as a result of SIDR on the safety of care delivered on inpatient medical units.
The majority of providers agreed that SIDR improved patient care and that SIDR should continue indefinitely. Importantly, providers also felt that SIDR improved the efficiency of their workday and attendance was high among all disciplines. Prior studies on IDR either did not report attendance or struggled with attendance.11 Incorporating the input of frontline providers into the design of SIDR allowed us to create a sustainable intervention which fit into daily workflow.
Our bivariate analyses found significant patient case‐mix differences between the intervention and control unit, limiting our ability to perform direct comparisons in LOS and cost. Pre‐post analyses of LOS and cost may be affected by cyclical or secular trends. Because each approach has its own limitations, we felt that analyses using both an historic as well as a concurrent control would provide a more complete assessment of the effect of the intervention. We included case mix, among other variables, in out multivariable regression analyses and found no benefit to SIDR with regard to LOS and cost. Two prior studies have shown a reduction in LOS and cost with the use of IDR.12, 13 However, one study was conducted approximately 15 years ago and included patients with a longer mean LOS.12 The second study used a pre‐post study design which may not have accounted for unmeasured confounders affecting LOS and cost.13 A third, smaller study showed no effect on LOS and cost with the use of IDR.15 No prior study has evaluated the effect of IDR on LOS and cost in a nonteaching hospitalist service setting.
Our study has several limitations. First, our study reflects the experience of an intervention unit compared to a control unit in a single hospital. Larger studies will be required to test the reproducibility and generalizability of our findings. Second, we did not conduct preintervention provider surveys for comparison ratings of collaboration and teamwork. A prior study, conducted by our research group, found that nurses gave low ratings to the teamwork climate and the quality of collaboration with hospitalists.8 Because this baseline study showed consistently low nurse ratings of collaboration and teamwork across all medical units, and because the units in the current study were identical in size, structure, and staffing of nonphysician personnel, we did not repeat nurse surveys prior to the intervention. Third, as previously mentioned, our study did not directly assess the effect of improved teamwork and collaboration on patient safety. Further study is needed to evaluate this. Although we are not aware of any other interventions to improve interdisciplinary communication on the intervention unit, it is possible that other unknown factors contributed to our findings. We believe this is unlikely due to the magnitude of the improvement in collaboration and the high ratings of SIDR by nurses and physicians on the intervention unit.
In summary, SIDR had a positive effect on nurses' ratings of collaboration and teamwork on a nonteaching hospitalist unit. Future research efforts should assess whether improved teamwork as a result of SIDR also translates into safer patient care.
Communication among hospital care providers is critically important to provide safe and effective care.15 Yet, studies in operating rooms, intensive care units (ICUs), and general medical units have revealed widely discrepant views on the quality of collaboration and communication between physicians and nurses.68 Although physicians consistently gave high ratings to the quality of collaboration with nurses, nurses rated the quality of collaboration with physicians relatively poorly.
A significant barrier to communication among providers on patient care units is the fluidity and geographic dispersion of team members.8 Physicians, nurses, and other hospital care providers have difficulty finding a way to discuss the care of their patients in person. Research has shown that nurses and physicians on patient care units do not communicate consistently and frequently are not in agreement about their patients' plans of care9, 10
Interdisciplinary Rounds (IDR) have been used as a means to assemble patient care unit team members and improve collaboration on the plan of care.1114 Prior research has demonstrated improved ratings of collaboration on the part of physicians,13, 14 but the effect of IDR on nurses' ratings of collaboration and teamwork has not been adequately assessed. One IDR study did not assess nurses' perceptions,13 while others used instruments not previously described and/or validated in the literature.12, 14 Regarding more concrete outcomes, research indicates variable effects of IDR on length of stay (LOS) and cost. Although 2 studies documented a reduction in LOS and cost with the use of IDR,12, 13 another study showed no effect.15 Furthermore, prior studies evaluated the use of IDR on resident‐covered teaching services. The effect IDR has on collaboration, LOS, and cost in a nonteaching hospitalist service setting is not known.
This study had 3 aims. The first was to assess the impact of an intervention, Structured Inter‐Disciplinary Rounds (SIDR), on nurses' ratings of collaboration and teamwork. The second was to assess the feasibility and sustainability of the intervention. The third was to assess the impact of the intervention on hospital LOS and cost.
Methods
Setting and Study Design
The study was conducted at Northwestern Memorial Hospital (NMH), an 897‐bed tertiary care teaching hospital in Chicago, IL, and was approved by the Institutional Review Board of Northwestern University. The study was a controlled trial of an intervention, SIDR, on collaboration and teamwork on patient care units. One of 2 similar hospitalist service units was randomly selected for the intervention, while the other served as a control unit. SIDR was implemented in August 2008 and data were collected over a 24 week study period.
Each hospitalist service unit consisted of 30 beds and was equipped with continuous cardiac telemetry monitoring. Units were also identical in structure and staffing of nonphysician personnel. The intervention unit included a heart failure‐hospitalist comanagement service. Patients followed at the Center for Heart Failure in the Bluhm Cardiovascular Institute of Northwestern were preferentially admitted to this service. All other patients were admitted to units based on bed availability in a quasi‐randomized fashion. Hospitalists worked 7 consecutive days while on service and cared for patients primarily on the units involved in this study. Therefore, hospitalists cared for patients on both the intervention and control units during their weeks on service. Hospitalists cared for patients independently without the assistance of resident physicians or mid‐level providers (ie, physician assistants or nurse practitioners).
Intervention
SIDR combined a structured format for communication with a forum for regular interdisciplinary meetings. A working group, consisting of nurses, hospitalists, and the unit pharmacist, social worker, and case manager, met weekly for 12 weeks prior to implementation. The working group determined the optimal timing, frequency, and location for SIDR. Additionally, the working group finalized the content of a structured communication tool (Supporting Information) to be used during SIDR. The structured communication tool was modeled after prior research demonstrating the benefit of daily goals of care forms16, 17 and ensured that important elements of the daily plan of care were discussed. Based on the working group's recommendation, SIDR took place each weekday at 11:00 AM in the unit conference room and lasted approximately 30 minutes. The nurse manager and a unit medical director co‐led rounds each day. SIDR was attended by all nurses and hospitalists caring for patients on the unit, as well as the pharmacist, social worker, and case manager assigned to the unit.
Provider Survey
Nurses working on the intervention and control units during the study period were administered a survey 16 weeks to 20 weeks after implementation of SIDR to assess ratings of collaboration and teamwork. The first portion of the survey was based on previously published surveys assessing teamwork attitudes among providers.6, 7 We asked nurses to rate the quality of communication and collaboration they had experienced with hospitalists using a 5‐point ordinal scale (1 = very low, 2 = low, 3 = adequate, 4 = high, 5 = very high). The second portion of the survey assessed teamwork and safety climate using the teamwork and safety domains of the Safety Attitudes Questionnaire (SAQ) developed by Sexton et al.18 The SAQ is based on previous research in aviation and medicine and has been validated in clinical settings.19, 20 Because hospitalists worked with nurses on both units, and in light of our prior research demonstrating that hospitalists rate the quality of collaboration with nurses highly,8 we did not assess hospitalists' ratings of collaboration. A final portion of the survey assessed nurses' perceptions of whether SIDR improved efficiency of communication, collaboration among team members, and patient care using a 5‐point Likert scale (1 = strongly disagree; 2 = disagree; 3 = neutral; 4 = agree; 5 = strongly agree). Hospitalists also received this portion of the survey at the completion of each clinical rotation. All surveys were administered in a web‐based format using an internet link (
SIDR Characteristics and Attendance
The unit medical director recorded the duration of SIDR, the number of patients on the unit, and the number of patients discussed each day. Attendance for each discipline was also recorded each day during the study period.
Data Analysis
Provider demographic data were obtained from completed surveys and group comparisons were done using chi‐square and t tests. The percentage of nurses on each unit rating of the quality of communication and collaboration with hospitalist physicians as high or very high was compared using chi‐square. Teamwork and safety climate scores were compared using the Mann Whitney U test.
Patient data were obtained from administrative databases for both the control and intervention unit during the study period as well as for the intervention unit in the 24 weeks preceding the study period. Demographic data were compared using chi‐square and t tests. Primary discharge diagnosis ICD‐9 codes were grouped into diagnosis clusters using the Healthcare Cost and Utilization Project system of the Agency for Healthcare Research and Quality.21 Diagnosis clusters were then analyzed using the chi‐square test. Because of case mix differences between patients on the intervention and control units, we analyzed LOS and cost using a concurrent control as well as an historic control. Unadjusted LOS and costs were compared using the Mann Whitney U test. We then conducted multivariable linear regression analyses to assess the impact of SIDR on LOS and cost. To satisfy normality requirements and distribution of residuals, we explored 2 methods of transforming skewed data on LOS and cost: logarithmic conversion and truncation at the mean LOS + 3 standard deviations (SDs). Since both techniques yielded similar results, we chose to present results by using truncation. Covariates for multivariable analyses included age, gender, race, payor, admission source, case‐mix, discharge disposition, presence of ICU stay during hospitalization, and Medicare Severity‐Diagnosis Related Group (MS‐DRG) weight. We used standard errors robust to the clustering of patients within each physician. All analyses were conducted using Stata version 10.0 (College Station, TX).
Results
Characteristics of Providers, Patients, and SIDR
Forty‐nine of 58 (84%) nurses completed the survey. Eighty‐eight of 96 (92%) surveys were completed by hospitalists at the end of their week on service. Hospitalist surveys represented 33 different hospitalists because individuals may have worked on study units more than once during the study period. Nurses were a mean 35.0 10.4 years of age and had been working at the hospital for a mean 5.0 6.3 years. Hospitalists were a mean 32.8 2.8 years of age and had been working at the hospital for a mean 2.6 1.9 years.
Patient characteristics are shown in Table 1. Intervention unit patients were admitted from the Emergency Department slightly more often in the postSIDR period. Patient case mix differed between the control and intervention unit, but was similar when comparing the intervention unit preSIDR and postSIDR. Intervention unit MS‐DRG weight was lower in the postSIDR period.
| Control Unit (n = 815) | Intervention Unit Pre‐SIDR (n = 722) | Intervention Unit Post‐SIDR (n = 684) | P Value for Comparison of Intervention Unit Post‐SIDR vs. Control | P Value for Comparison of Intervention Unit Post‐ vs. Pre‐SIDR | |
|---|---|---|---|---|---|
| |||||
| Mean age, years (SD) | 63.8 (16.0) | 64.2 (16.3) | 64.1 (17.2) | 0.74 | 0.92 |
| Women, n (%) | 403 (49) | 347 (48) | 336 (49) | 0.90 | 0.69 |
| Ethnicity, n (%) | 0.22 | 0.71 | |||
| White | 438 (54) | 350 (48) | 334 (49) | ||
| Black | 269 (33) | 266 (37) | 264 (39) | ||
| Hispanic | 48 (6) | 40 (6) | 34 (5) | ||
| Asian | 6 (1) | 8 (1) | 4 (1) | ||
| Other | 54 (7) | 58 (8) | 48 (7) | ||
| Payor, n (%) | 0.07 | 0.67 | |||
| Medicare | 456 (56) | 436 (60) | 399 (58) | ||
| Private | 261 (32) | 176 (24) | 182 (27) | ||
| Medicaid | 67 (8) | 75 (10) | 65 (10) | ||
| Self pay | 31 (4) | 35 (5) | 38 (6) | ||
| Admission source, n (%) | 0.51 | 0.03 | |||
| Emergency department | 695 (85) | 590 (82) | 593 (87) | ||
| Direct admission | 92 (11) | 99 (14) | 65 (10) | ||
| Transfer | 28 (3) | 33 (5) | 26 (4) | ||
| Case mix, n (%) | |||||
| Congestive heart failure | 78 (10) | 164 (23) | 144 (21) | <0.01 | 0.45 |
| Cardiac dysrhythmia | 167 (20) | 69 (10) | 81 (12) | <0.01 | 0.17 |
| Chest pain | 100 (12) | 47 (7) | 59 (9) | 0.02 | 0.13 |
| Coronary atherosclerosis | 52 (6) | 19 (3) | 19 (3) | <0.01 | 0.87 |
| Hypertension | 24 (3) | 38 (5) | 24 (4) | 0.54 | 0.11 |
| Syncope | 27 (3) | 23 (3) | 26 (4) | 0.61 | 0.53 |
| Fluid or electrolyte disorder | 11 (1) | 25 (3) | 23 (3) | 0.01 | 0.92 |
| Pneumonia | 14 (2) | 13 (2) | 22 (3) | 0.06 | 0.09 |
| Pulmonary heart disease | 16 (2) | 13 (2) | 14 (2) | 0.91 | 0.74 |
| Intervertebral disc or other back problem | 32 (4) | 3 (0) | 6 (1) | <0.01 | 0.28 |
| Other diagnosis | 294 (36) | 308 (43) | 266 (39) | 0.26 | 0.15 |
| Cardiovascular procedure during admission | 151 (19) | 95 (13) | 86 (13) | <0.01 | 0.74 |
| Intensive care unit stay during admission, n (%) | 39 (5) | 44 (6) | 27 (4) | 0.43 | 0.07 |
| Discharge disposition, n (%) | |||||
| Home | 736 (90) | 646 (89) | 610 (89) | 0.88 | 0.82 |
| Skilled nursing facility or rehabilitation | 66 (8) | 61 (8) | 63 (9) | ||
| Other facility | 9 (1) | 11 (2) | 7 (1) | ||
| Expired | 4 (0) | 4 (1) | 4 (1) | ||
| Mean Medicare severity ‐diagnosis related group weight (SD) | 1.08 (0.73) | 1.14 (0.76) | 1.06 (0.72) | 0.61 | 0.04 |
SIDR occurred each weekday (with the exception of holidays) on the intervention unit and lasted a mean 27.7 4.6 minutes. The unit had a mean 27 patients per day and 86% of patients on the unit were discussed each day. Attendance exceeded 85% for each discipline (hospitalists, nurses, and the unit pharmacist, social worker, and case manager).
Ratings of Teamwork and Perceptions of SIDR
As shown in Figure 1, a larger percentage of nurses rated the quality of communication and collaboration with hospitalists as high or very high on the intervention unit compared to the control unit (80% vs. 54%; P = 0.05).

Nurses' ratings of the teamwork and safety climate are summarized in Table 2. The median teamwork climate score was 85.7 (interquartile range [IQR], 75.092.9) for the intervention unit as compared to 61.6 (IQR, 48.283.9) for the control unit (P = 0.008). The median safety climate score was 75.0 (IQR, 70.581.3) for the intervention unit as compared to 61.1 (IQR, 30.281.3) for the control unit (P = 0.03).
| Control Unit, n = 24 | Intervention Unit, n = 25 | P Value | |
|---|---|---|---|
| |||
| Median Teamwork Climate Score (IQR) | 75.0 (70.581.3) | 61.6 (48.283.9) | 0.008 |
| Median Safety Climate Score (IQR) | 85.7 (75.092.9) | 61.1 (30.281.3) | 0.03 |
Sixty‐five of 88 (74%) hospitalists and 18 of 24 (75%) nurses agreed that SIDR improved the efficiency of their work day. Eighty of 88 (91%) hospitalists and 18 of 24 (75%) nurses agreed that SIDR improved team collaboration. Seventy‐six of 88 (86%) hospitalists and 18 of 24 (75%) nurses agreed that SIDR improved patient care. Sixty‐seven of 88 (76%) hospitalists and 22 of 25 (88%) nurses indicated that they wanted SIDR to continue indefinitely.
SIDR Impact on LOS and Cost
The unadjusted mean LOS was significantly higher for the intervention unit postSIDR as compared to the control unit (4.0 3.4 vs. 3.7 3.3 days; P = 0.03). However, the unadjusted mean LOS was not significantly different for the intervention unit postSIDR as compared to the intervention unit preSIDR (4.0 3.4 vs. 4.26 3.5 days; P = 0.10). The unadjusted cost was lower for the intervention unit postSIDR as compared to the control unit ($7,513.23 7,085.10 vs. $8,588.66 7,381.03; P < 0.001). The unadjusted mean cost was not significantly different for the invention unit postSIDR as compared to the intervention unit preSIDR ($7,513.23 7,085.10 vs. $7,937.00 7,512.23; P = 0.19).
Multivariable analyses of LOS and cost are summarized in Table 3. The adjusted LOS was not significantly different when comparing the intervention unit postSIDR to either the control unit or the intervention unit preSIDR. The adjusted cost for the intervention unit postSIDR was $739.55 less than the control unit (P = 0.02). The adjusted cost was not significantly different when comparing the intervention unit postSIDR to the intervention unit preSIDR.
| Adjusted Difference for Intervention Unit Post‐SIDR vs. Control | P Value for Adjusted Difference for Intervention Unit Post‐SIDR vs. Control | Adjusted Difference for Intervention Unit Post‐ vs. Pre‐SIDR | P Value for Adjusted Difference for Intervention Unit Post‐ vs. Pre‐SIDR | |
|---|---|---|---|---|
| ||||
| Length of stay | 0.05 | 0.75 | 0.04 | 0.83 |
| Cost | 739.55 | 0.02 | 302.94 | 0.34 |
Discussion
We found that nurses working on a unit using SIDR rated the quality of communication and collaboration with hospitalists significantly higher as compared to a control unit. Notably, because hospitalists worked on both the intervention and control unit during their weeks on service, nurses on each unit were rating the quality of collaboration with the same hospitalists. Nurses also rated the teamwork and safety climate higher on the intervention unit. These findings are important because prior research has shown that nurses are often dissatisfied with the quality of collaboration and teamwork with physicians.68 Potential explanations include fundamental differences between nurses and physicians with regard to status/authority, gender, training, and patient care responsibilities.6 Unfortunately, a culture of poor teamwork may lead to a workplace in which team members feel unable to approach certain individuals and uncomfortable raising concerns. Not surprisingly, higher ratings of teamwork culture have been associated with nurse retention.22, 23 SIDR provided a facilitated forum for interdisciplinary discussion, exchange of critical clinical information, and collaboration on the plan of care.
Our findings are also important because poor communication represents a major etiology of preventable adverse events in hospitals.15 Higher ratings of collaboration and teamwork have been associated with better patient outcomes in observational studies.2426 Further research should evaluate the impact of improved interdisciplinary collaboration as a result of SIDR on the safety of care delivered on inpatient medical units.
The majority of providers agreed that SIDR improved patient care and that SIDR should continue indefinitely. Importantly, providers also felt that SIDR improved the efficiency of their workday and attendance was high among all disciplines. Prior studies on IDR either did not report attendance or struggled with attendance.11 Incorporating the input of frontline providers into the design of SIDR allowed us to create a sustainable intervention which fit into daily workflow.
Our bivariate analyses found significant patient case‐mix differences between the intervention and control unit, limiting our ability to perform direct comparisons in LOS and cost. Pre‐post analyses of LOS and cost may be affected by cyclical or secular trends. Because each approach has its own limitations, we felt that analyses using both an historic as well as a concurrent control would provide a more complete assessment of the effect of the intervention. We included case mix, among other variables, in out multivariable regression analyses and found no benefit to SIDR with regard to LOS and cost. Two prior studies have shown a reduction in LOS and cost with the use of IDR.12, 13 However, one study was conducted approximately 15 years ago and included patients with a longer mean LOS.12 The second study used a pre‐post study design which may not have accounted for unmeasured confounders affecting LOS and cost.13 A third, smaller study showed no effect on LOS and cost with the use of IDR.15 No prior study has evaluated the effect of IDR on LOS and cost in a nonteaching hospitalist service setting.
Our study has several limitations. First, our study reflects the experience of an intervention unit compared to a control unit in a single hospital. Larger studies will be required to test the reproducibility and generalizability of our findings. Second, we did not conduct preintervention provider surveys for comparison ratings of collaboration and teamwork. A prior study, conducted by our research group, found that nurses gave low ratings to the teamwork climate and the quality of collaboration with hospitalists.8 Because this baseline study showed consistently low nurse ratings of collaboration and teamwork across all medical units, and because the units in the current study were identical in size, structure, and staffing of nonphysician personnel, we did not repeat nurse surveys prior to the intervention. Third, as previously mentioned, our study did not directly assess the effect of improved teamwork and collaboration on patient safety. Further study is needed to evaluate this. Although we are not aware of any other interventions to improve interdisciplinary communication on the intervention unit, it is possible that other unknown factors contributed to our findings. We believe this is unlikely due to the magnitude of the improvement in collaboration and the high ratings of SIDR by nurses and physicians on the intervention unit.
In summary, SIDR had a positive effect on nurses' ratings of collaboration and teamwork on a nonteaching hospitalist unit. Future research efforts should assess whether improved teamwork as a result of SIDR also translates into safer patient care.
- Joint Commission on Accreditation of Healthcare Organizations. Sentinel Event Statistics. Available at: http://www.jointcommission.org/SentinelEvents/Statistics. Accessed March2010.
- ,,, et al.A look into the nature and causes of human errors in the intensive care unit.Crit Care Med.1995;23(2):294–300.
- ,,, et al.The nature of adverse events in hospitalized patients. Results of the Harvard Medical Practice Study II.N Engl J Med.1991;324(6):377–384.
- ,,.Communication failures: an insidious contributor to medical mishaps.Acad Med.2004;79(2):186–194.
- ,,,,,.The quality in Australian Health Care Study.Med J Aust.1995;163(9):458–471.
- ,,, et al.Operating room teamwork among physicians and nurses: teamwork in the eye of the beholder.J Am Coll Surg.2006;202(5):746–752.
- ,,.Discrepant attitudes about teamwork among critical care nurses and physicians.Crit Care Med.2003;31(3):956–959.
- ,,,,,.Teamwork on inpatient medical units: assessing attitudes and barriers.Qual Saf Health Care2010;19(2):117–121.
- ,,,,,.Can we talk? Priorities for patient care differed among health care providers:AHRQ;2005.
- ,,, et al.Patterns of nurse—physicians communication and agreement on the plan of care.Qual Saf Health Care. In press.
- ,,, et al.The effect of a multidisciplinary hospitalist/physician and advanced practice nurse collaboration on hospital costs.J Nurs Adm.2006;36(2):79–85.
- ,,.A firm trial of interdisciplinary rounds on the inpatient medical wards: an intervention designed using continuous quality improvement.Med Care.1998;36(8 Suppl):AS4–A12.
- ,,,,.Use of multidisciplinary rounds to simultaneously improve quality outcomes, enhance resident education, and shorten length of stay.J Gen Intern Med.2007;22(8):1073–1079.
- ,,,.Effect of a multidisciplinary intervention on communication and collaboration among physicians and nurses.Am J Crit Care.2005;14(1):71–77.
- ,,,.Effects of interdisciplinary rounds on length of stay in a telemetry unit.J Public Health Manag Pract.2004;10(1):63–69.
- ,,,,.Improving nurse‐physician communication and satisfaction in the intensive care unit with a daily goals worksheet.Am J Crit Care.2006;15(2):217–222.
- ,,,,,.Improving communication in the ICU using daily goals.J Crit Care.2003;18(2):71–75.
- ,,, et al.The Safety Attitudes Questionnaire: psychometric properties, benchmarking data, and emerging research.BMC Health Serv Res.2006;6:44.
- ,,,.Safety Climate Survey: reliability of results from a multicenter ICU survey.Qual Saf Health Care.2005;14(4):273–278.
- ,,, et al.Teamwork in the operating room: frontline perspectives among hospitals and operating room personnel.Anesthesiology.2006;105(5):877–884.
- HCUP Clinical Classification Software [computer program]. Version: Agency for Healthcare Research and Quality, Rockville, MD. Available at: http://www.hcup‐us.ahrq.gov/toolssoftware/ccs/ccs.jsp. Accessed March2010.
- ,,.The influence of teamwork culture on physician and nurse resignation rates in hospitals.Health Serv Manage Res.2008;21(1):23–31.
- .Original research: nurse‐physician relationships: impact on nurse satisfaction and retention.Am J Nurs.2002;102(6):26–34.
- ,,, et al.Association between nurse‐physician collaboration and patient outcomes in three intensive care units.Crit Care Med.1999;27(9):1991–1998.
- ,,,,.Risk‐adjusted morbidity in teaching hospitals correlates with reported levels of communication and collaboration on surgical teams but not with scale measures of teamwork climate, safety climate, or working conditions.J Am Coll Surg.2007;205(6):778–784.
- ,,.The link between teamwork and patients' outcomes in intensive care units.Am J Crit Care.2003;12(6):527–534.
- Joint Commission on Accreditation of Healthcare Organizations. Sentinel Event Statistics. Available at: http://www.jointcommission.org/SentinelEvents/Statistics. Accessed March2010.
- ,,, et al.A look into the nature and causes of human errors in the intensive care unit.Crit Care Med.1995;23(2):294–300.
- ,,, et al.The nature of adverse events in hospitalized patients. Results of the Harvard Medical Practice Study II.N Engl J Med.1991;324(6):377–384.
- ,,.Communication failures: an insidious contributor to medical mishaps.Acad Med.2004;79(2):186–194.
- ,,,,,.The quality in Australian Health Care Study.Med J Aust.1995;163(9):458–471.
- ,,, et al.Operating room teamwork among physicians and nurses: teamwork in the eye of the beholder.J Am Coll Surg.2006;202(5):746–752.
- ,,.Discrepant attitudes about teamwork among critical care nurses and physicians.Crit Care Med.2003;31(3):956–959.
- ,,,,,.Teamwork on inpatient medical units: assessing attitudes and barriers.Qual Saf Health Care2010;19(2):117–121.
- ,,,,,.Can we talk? Priorities for patient care differed among health care providers:AHRQ;2005.
- ,,, et al.Patterns of nurse—physicians communication and agreement on the plan of care.Qual Saf Health Care. In press.
- ,,, et al.The effect of a multidisciplinary hospitalist/physician and advanced practice nurse collaboration on hospital costs.J Nurs Adm.2006;36(2):79–85.
- ,,.A firm trial of interdisciplinary rounds on the inpatient medical wards: an intervention designed using continuous quality improvement.Med Care.1998;36(8 Suppl):AS4–A12.
- ,,,,.Use of multidisciplinary rounds to simultaneously improve quality outcomes, enhance resident education, and shorten length of stay.J Gen Intern Med.2007;22(8):1073–1079.
- ,,,.Effect of a multidisciplinary intervention on communication and collaboration among physicians and nurses.Am J Crit Care.2005;14(1):71–77.
- ,,,.Effects of interdisciplinary rounds on length of stay in a telemetry unit.J Public Health Manag Pract.2004;10(1):63–69.
- ,,,,.Improving nurse‐physician communication and satisfaction in the intensive care unit with a daily goals worksheet.Am J Crit Care.2006;15(2):217–222.
- ,,,,,.Improving communication in the ICU using daily goals.J Crit Care.2003;18(2):71–75.
- ,,, et al.The Safety Attitudes Questionnaire: psychometric properties, benchmarking data, and emerging research.BMC Health Serv Res.2006;6:44.
- ,,,.Safety Climate Survey: reliability of results from a multicenter ICU survey.Qual Saf Health Care.2005;14(4):273–278.
- ,,, et al.Teamwork in the operating room: frontline perspectives among hospitals and operating room personnel.Anesthesiology.2006;105(5):877–884.
- HCUP Clinical Classification Software [computer program]. Version: Agency for Healthcare Research and Quality, Rockville, MD. Available at: http://www.hcup‐us.ahrq.gov/toolssoftware/ccs/ccs.jsp. Accessed March2010.
- ,,.The influence of teamwork culture on physician and nurse resignation rates in hospitals.Health Serv Manage Res.2008;21(1):23–31.
- .Original research: nurse‐physician relationships: impact on nurse satisfaction and retention.Am J Nurs.2002;102(6):26–34.
- ,,, et al.Association between nurse‐physician collaboration and patient outcomes in three intensive care units.Crit Care Med.1999;27(9):1991–1998.
- ,,,,.Risk‐adjusted morbidity in teaching hospitals correlates with reported levels of communication and collaboration on surgical teams but not with scale measures of teamwork climate, safety climate, or working conditions.J Am Coll Surg.2007;205(6):778–784.
- ,,.The link between teamwork and patients' outcomes in intensive care units.Am J Crit Care.2003;12(6):527–534.
Copyright © 2010 Society of Hospital Medicine