User login
Things We Do For No Reason: Prealbumin Testing to Diagnose Malnutrition in the Hospitalized Patient
The “Things We Do for No Reason” series reviews practices which have become common parts of hospital care but which may provide little value to our patients. Practices reviewed in the TWDFNR series do not represent “black and white” conclusions or clinical practice standards, but are meant as a starting place for research and active discussions among hospitalists and patients. We invite you to be part of that discussion. https://www.choosingwisely.org/

CASE PRESENTATION
A 34-year-old man is admitted for a complicated urinary tract infection related to a chronic in-dwelling Foley catheter. The patient suffered a spinal cord injury at the C4/C5 level as a result of a motor vehicle accident 10 years ago and is confined to a motorized wheelchair. He is an engineer and lives independently but has caregivers. His body mass index (BMI) is 18.5 kg/m2, and he reports his weight has been stable. He has slight muscle atrophy of the biceps, triceps, interosseous muscles, and quadriceps. The patient reports that he eats well, has no chronic conditions, and has not had any gastrointestinal symptoms (eg, anorexia, nausea, diarrhea) over the last six months. You consider whether to order a serum prealbumin test to assess for possible malnutrition.
BACKGROUND
The presence of malnutrition in hospitalized patients is widely recognized as an independent predictor of hospital mortality.1 According to the American Society for Parenteral and Enteral Nutrition (ASPEN), malnutrition is defined as “an acute, subacute or chronic state of nutrition, in which varying degrees of overnutrition or undernutrition with or without inflammatory activity have led to a change in body composition and diminished function.”2 In one large European study, patients screening positive for being at risk of malnutrition had a 12-fold increase in hospital mortality.1
Inpatient malnutrition is remarkably underdocumented. Studies using chart reviews have found a prevalence of malnutrition in hospitalized patients of between 20% and 50%, and only 3% of hospital discharges are associated with a diagnostic code for malnutrition.3–5 Appropriate diagnosis and documentation of malnutrition is important given the profound prognostic and management implications of a malnutrition diagnosis. Appropriate documentation benefits health systems as malnutrition documentation increases expected mortality, thereby improving the observed-to-expected mortality ratio.
Serum prealbumin testing is widely available and frequently ordered in the inpatient setting. In a query we performed of the large aggregate Cerner Electronic Health Record database, HealthFacts, which includes data from inpatient encounters for approximately 700 United States hospitals, prealbumin tests were ordered 129,152 times in 2015. This activity corresponds to estimated total charges of $2,562,375 based on the 2015 clinical laboratory fee schedule.6
WHY YOU MIGHT THINK PREALBUMIN DIAGNOSES MALNUTRITION
Prealbumin is synthesized in the liver and released into circulation prior to excretion by the kidneys and gastrointestinal tract. Prealbumin transports thyroxine, triiodothyronine, and holo-retinol binding protein and, as a result, is also known as transthyretin.7 It was first proposed as a nutritional marker in 1972 with the publication of a study that showed low levels of prealbumin in 40 children with kwashiorkor that improved with intensive dietary supplementation.8 The shorter half-life of prealbumin (2.5 days) as compared with other identified nutritional markers, such as albumin, indicate that it would be suitable for detecting rapid changes in nutritional status.
WHY PREALBUMIN IS NOT HELPFUL FOR DIAGNOSING MALNUTRITION
Prealbumin Is Not Specific
An ideal nutritional marker should be specific enough that changes in this marker reflect changes in nutritional status.9 While there are many systemic factors that affect nutritional markers, such as prealbumin (Table 1), the acute phase response triggered by inflammation is the most significant confounder in the acutely ill hospitalized patient.9 This response to infection, stress, and malignancy leads to an increase in proinflammatory cytokines, increased liver synthesis of inflammatory proteins, such as C-reactive protein (CRP), and increased vascular permeability. Prealbumin is a negative acute phase reactant that decreases in concentration during the stress response due to slowed synthesis and extravasation.9 In a study of 24 patients with severe sepsis and trauma, levels of prealbumin inversely correlated with CRP, a reflection of the stress response, and returned to normal when CRP levels normalized. Neither prealbumin nor CRP, however, correlated with total body protein changes.10 Unfortunately, many studies supporting the use of prealbumin as a nutritional marker do not address the role of the acute phase response in their results. These studies include the original report on prealbumin in kwashiorkor, a condition known to be associated with a high rate of infectious diseases that can trigger the acute phase response.9 A consensus statement from the Academy of Nutrition and Dietetics (AND) and ASPEN noted that prealbumin is an indicator of inflammation and lacks the specificity to diagnose malnutrition.11
Prealbumin Is Not Sensitive
A sensitive laboratory test for malnutrition should allow for detection of malnutrition at an early stage.9 However, patients who demonstrate severe malnutrition without a coexisting inflammatory state do not consistently show low levels of prealbumin. In a systematic review of 20 studies in nondiseased malnourished patients, only two studies, both of which assessed patients with anorexia nervosa, had a mean prealbumin below normal (<20 mg/dL), and this finding corresponded to patient populations with mean BMIs less than 12 kg/m2. More importantly, normal prealbumin levels were seen in groups of patients with a mean BMI as low as 12.9 kg/m2.12 Analysis by AND found insufficient evidence to support a correlation between prealbumin and weight loss in anorexia nervosa, calorie restricted diets, or starvation.13 The data suggest that prealbumin lacks sufficient sensitivity to consistently detect cases of malnutrition easily diagnosed by history and/or physical exam.
Prealbumin Is Not Consistently Responsive to Nutritional Interventions
An accurate marker for malnutrition should improve when nutritional intervention results in adequate nutritional intake.9 While some studies have shown improvements in prealbumin in the setting of a nutritional intervention, many of these works are subject to the same limitations related to specificity and lack of control for concurrent inflammatory processes. In a retrospective study, prealbumin increased significantly in 102 patients receiving TPN for one week. Unfortunately, patients with renal or hepatic disease were excluded, and the role of inflammation was not assessed.14 Institutionalized patients with Alzheimer’s disease and normal CRP levels showed a statistically significant increase in weight gain, arm muscle circumference, and triceps skin-fold thickness following a nutritional program without a notable change in prealbumin.15 In a study assessing the relationship of prealbumin, CRP, and nutritional intake, critically ill populations receiving less than or greater than 60% of their estimated caloric needs showed no significant difference in prealbumin. In fact, prealbumin levels were only correlated with CRP levels.16 This finding argues against the routine use of prealbumin for nutrition monitoring in the acutely ill hospitalized patient.
Prealbumin Is Not Consistently Correlated with Health Outcomes
Even if prealbumin increased consistently in response to nutritional intervention, whether this change corresponds to an improvement in clinical outcomes has yet to be demonstrated.9 In 2005, Koretz reviewed 99 clinical trials and concluded that even when changes in nutritional markers are seen with nutritional support, the “changes in nutritional markers do not predict clinical outcomes.”17
WHAT YOU SHOULD DO INSTEAD: USE NONBIOLOGIC METHODS FOR SCREENING AND DIAGNOSING MALNUTRITION
Given the lack of a suitable biologic assay to identify malnutrition, dieticians and clinicians must rely on other means to assess malnutrition. Professional societies, including ASPEN and the European Society for Clinical Nutrition and Metabolism, have proposed different guidelines for the screening and assessment of malnutrition (Table 2).11,18 In 2016, these organizations, along with the Latin American Federation of Nutritional Therapy, Clinical Nutrition, and Metabolism and the Parenteral and Enteral Nutrition Society of Asia, formed The Global Leadership Initiative on Malnutrition (GLIM). In 2017, the GLIM taskforce agreed on clinically relevant diagnostic variables for the screening and assessment of malnutrition, including reduced food intake (anorexia), nonvolitional weight loss, (reduced) lean mass, status of disease burden and inflammation, and low body mass index or underweight status.19
RECOMMENDATIONS
- Do not use prealbumin to screen for or diagnose malnutrition.
- Consult with local dietitians to ensure that your institutional approach is in agreement with consensus recommendations.
CONCLUSION
In revisiting the case above, the patient does not have clear evidence of malnutrition based on his history (stable weight and good reported nutritional intake), although he does have a low BMI of 18.5 kg/m2. Rather than prealbumin testing, which would likely be low secondary to the acute phase response, he would better benefit from a nutrition-focused history and physical exam.
The uncertainties faced by clinicians in diagnosing malnutrition cannot readily be resolved by relying on a solitary laboratory marker (eg, prealbumin) or a stand-alone assessment protocol. The data obtained reflect the need for multidisciplinary teams of dieticians and clinicians to contextualize each patient’s medical history and ensure that the selected metrics are used appropriately to aid in diagnosis and documentation. We advocate that clinicians not routinely use prealbumin to screen for, confirm the diagnosis of, or assess the severity of malnutrition in the hospitalized patient.
Do you think this is a low-value practice? Is this truly a “Thing We Do for No Reason?” Share what you do in your practice and join in the conversation online by retweeting it on Twitter (#TWDFNR) and liking it on Facebook. We invite you to propose ideas for other “Things We Do for No Reason” topics by emailing TWDFNR@hospitalmedicine.org.
Disclosures
The authors have nothing to disclose.
1. Sorensen J, Kondrup J, Prokopowicz J, et al. EuroOOPS: an international, multicentre study to implement nutritional risk screening and evaluate clinical outcome. Clin Nutr Edinb Scotl. 2008;27(3):340-349. PubMed
2. Mueller C, Compher C, Ellen DM, American Society for Parenteral and Enteral Nutrition (A.S.P.E.N.) Board of Directors. A.S.P.E.N. clinical guidelines: nutrition screening, assessment, and intervention in adults. JPEN J Parenter Enteral Nutr. 2011;35(1):16-24. PubMed
3. Kaiser MJ, Bauer JM, Rämsch C, et al. Frequency of malnutrition in older adults: a multinational perspective using the mini nutritional assessment. J Am Geriatr Soc. 2010;58(9):1734-1738. PubMed
4. Robinson MK, Trujillo EB, Mogensen KM, Rounds J, McManus K, Jacobs DO. Improving nutritional screening of hospitalized patients: the role of prealbumin. JPEN J Parenter Enteral Nutr. 2003;27(6):389-395; quiz 439. PubMed
5. Corkins MR, Guenter P, DiMaria-Ghalili RA, et al. Malnutrition diagnoses in hospitalized patients: United States, 2010. JPEN J Parenter Enteral Nutr. 2014;38(2):186-195. PubMed
6. Clinical Laboratory Fee Schedule Files. cms.org. https://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/ClinicalLabFeeSched/Clinical-Laboratory-Fee-Schedule-Files.html. Published September 29, 2016. Accessed January 5, 2018.
7. Myron Johnson A, Merlini G, Sheldon J, Ichihara K, Scientific Division Committee on Plasma Proteins (C-PP), International Federation of Clinical Chemistry and Laboratory Medicine (IFCC). Clinical indications for plasma protein assays: transthyretin (prealbumin) in inflammation and malnutrition. Clin Chem Lab Med. 2007;45(3):419-426. PubMed
8. Ingenbleek Y, De Visscher M, De Nayer P. Measurement of prealbumin as index of protein-calorie malnutrition. Lancet. 1972;2(7768):106-109. PubMed
9. Barbosa-Silva MCG. Subjective and objective nutritional assessment methods: what do they really assess? Curr Opin Clin Nutr Metab Care. 2008;11(3):248-254. PubMed
10. Clark MA, Hentzen BTH, Plank LD, Hill GL. Sequential changes in insulin-like growth factor 1, plasma proteins, and total body protein in severe sepsis and multiple injury. J Parenter Enter Nutr. 1996;20(5):363-370. PubMed
11. White JV, Guenter P, Jensen G, et al. Consensus statement of the Academy of Nutrition and Dietetics/American Society for Parenteral and Enteral Nutrition: characteristics recommended for the identification and documentation of adult malnutrition (undernutrition). J Acad Nutr Diet. 2012;112(5):730-738. PubMed
12. Lee JL, Oh ES, Lee RW, Finucane TE. Serum albumin and prealbumin in calorically restricted, nondiseased individuals: a systematic review. Am J Med. 2015;128(9):1023.e1-22. PubMed
13. Academy of Nutrition and Dietetics Evidence Analysis Library. Nutrition Screening (NSCR) Systematic Review (2009-2010). https://www.andeal.org/tmp/pdf-print-919C51237950859AE3E15F978CEF49D8.pdf. Accessed August 23, 2017.
14. Sawicky CP, Nippo J, Winkler MF, Albina JE. Adequate energy intake and improved prealbumin concentration as indicators of the response to total parenteral nutrition. J Am Diet Assoc. 1992;92(10):1266-1268. PubMed
15. Van Wymelbeke V, Guédon A, Maniere D, Manckoundia P, Pfitzenmeyer P. A 6-month follow-up of nutritional status in institutionalized patients with Alzheimer’s disease. J Nutr Health Aging. 2004;8(6):505-508. PubMed
16. Davis CJ, Sowa D, Keim KS, Kinnare K, Peterson S. The use of prealbumin and C-reactive protein for monitoring nutrition support in adult patients receiving enteral nutrition in an urban medical center. JPEN J Parenter Enteral Nutr. 2012;36(2):197-204. PubMed
17. Koretz RL. Death, morbidity and economics are the only end points for trials. Proc Nutr Soc. 2005;64(3):277-284. PubMed
18. Cederholm T, Bosaeus I, Barazzoni R, et al. Diagnostic criteria for malnutrition - an ESPEN consensus statement. Clin Nutr Edinb Scotl. 2015;34(3):335-340. PubMed
19. Jensen GL, Cederholm T. Global leadership initiative on malnutrition: progress report from ASPEN clinical nutrition week 2017. JPEN J Parenter Enteral Nutr. April 2017:148607117707761. PubMed
The “Things We Do for No Reason” series reviews practices which have become common parts of hospital care but which may provide little value to our patients. Practices reviewed in the TWDFNR series do not represent “black and white” conclusions or clinical practice standards, but are meant as a starting place for research and active discussions among hospitalists and patients. We invite you to be part of that discussion. https://www.choosingwisely.org/

CASE PRESENTATION
A 34-year-old man is admitted for a complicated urinary tract infection related to a chronic in-dwelling Foley catheter. The patient suffered a spinal cord injury at the C4/C5 level as a result of a motor vehicle accident 10 years ago and is confined to a motorized wheelchair. He is an engineer and lives independently but has caregivers. His body mass index (BMI) is 18.5 kg/m2, and he reports his weight has been stable. He has slight muscle atrophy of the biceps, triceps, interosseous muscles, and quadriceps. The patient reports that he eats well, has no chronic conditions, and has not had any gastrointestinal symptoms (eg, anorexia, nausea, diarrhea) over the last six months. You consider whether to order a serum prealbumin test to assess for possible malnutrition.
BACKGROUND
The presence of malnutrition in hospitalized patients is widely recognized as an independent predictor of hospital mortality.1 According to the American Society for Parenteral and Enteral Nutrition (ASPEN), malnutrition is defined as “an acute, subacute or chronic state of nutrition, in which varying degrees of overnutrition or undernutrition with or without inflammatory activity have led to a change in body composition and diminished function.”2 In one large European study, patients screening positive for being at risk of malnutrition had a 12-fold increase in hospital mortality.1
Inpatient malnutrition is remarkably underdocumented. Studies using chart reviews have found a prevalence of malnutrition in hospitalized patients of between 20% and 50%, and only 3% of hospital discharges are associated with a diagnostic code for malnutrition.3–5 Appropriate diagnosis and documentation of malnutrition is important given the profound prognostic and management implications of a malnutrition diagnosis. Appropriate documentation benefits health systems as malnutrition documentation increases expected mortality, thereby improving the observed-to-expected mortality ratio.
Serum prealbumin testing is widely available and frequently ordered in the inpatient setting. In a query we performed of the large aggregate Cerner Electronic Health Record database, HealthFacts, which includes data from inpatient encounters for approximately 700 United States hospitals, prealbumin tests were ordered 129,152 times in 2015. This activity corresponds to estimated total charges of $2,562,375 based on the 2015 clinical laboratory fee schedule.6
WHY YOU MIGHT THINK PREALBUMIN DIAGNOSES MALNUTRITION
Prealbumin is synthesized in the liver and released into circulation prior to excretion by the kidneys and gastrointestinal tract. Prealbumin transports thyroxine, triiodothyronine, and holo-retinol binding protein and, as a result, is also known as transthyretin.7 It was first proposed as a nutritional marker in 1972 with the publication of a study that showed low levels of prealbumin in 40 children with kwashiorkor that improved with intensive dietary supplementation.8 The shorter half-life of prealbumin (2.5 days) as compared with other identified nutritional markers, such as albumin, indicate that it would be suitable for detecting rapid changes in nutritional status.
WHY PREALBUMIN IS NOT HELPFUL FOR DIAGNOSING MALNUTRITION
Prealbumin Is Not Specific
An ideal nutritional marker should be specific enough that changes in this marker reflect changes in nutritional status.9 While there are many systemic factors that affect nutritional markers, such as prealbumin (Table 1), the acute phase response triggered by inflammation is the most significant confounder in the acutely ill hospitalized patient.9 This response to infection, stress, and malignancy leads to an increase in proinflammatory cytokines, increased liver synthesis of inflammatory proteins, such as C-reactive protein (CRP), and increased vascular permeability. Prealbumin is a negative acute phase reactant that decreases in concentration during the stress response due to slowed synthesis and extravasation.9 In a study of 24 patients with severe sepsis and trauma, levels of prealbumin inversely correlated with CRP, a reflection of the stress response, and returned to normal when CRP levels normalized. Neither prealbumin nor CRP, however, correlated with total body protein changes.10 Unfortunately, many studies supporting the use of prealbumin as a nutritional marker do not address the role of the acute phase response in their results. These studies include the original report on prealbumin in kwashiorkor, a condition known to be associated with a high rate of infectious diseases that can trigger the acute phase response.9 A consensus statement from the Academy of Nutrition and Dietetics (AND) and ASPEN noted that prealbumin is an indicator of inflammation and lacks the specificity to diagnose malnutrition.11
Prealbumin Is Not Sensitive
A sensitive laboratory test for malnutrition should allow for detection of malnutrition at an early stage.9 However, patients who demonstrate severe malnutrition without a coexisting inflammatory state do not consistently show low levels of prealbumin. In a systematic review of 20 studies in nondiseased malnourished patients, only two studies, both of which assessed patients with anorexia nervosa, had a mean prealbumin below normal (<20 mg/dL), and this finding corresponded to patient populations with mean BMIs less than 12 kg/m2. More importantly, normal prealbumin levels were seen in groups of patients with a mean BMI as low as 12.9 kg/m2.12 Analysis by AND found insufficient evidence to support a correlation between prealbumin and weight loss in anorexia nervosa, calorie restricted diets, or starvation.13 The data suggest that prealbumin lacks sufficient sensitivity to consistently detect cases of malnutrition easily diagnosed by history and/or physical exam.
Prealbumin Is Not Consistently Responsive to Nutritional Interventions
An accurate marker for malnutrition should improve when nutritional intervention results in adequate nutritional intake.9 While some studies have shown improvements in prealbumin in the setting of a nutritional intervention, many of these works are subject to the same limitations related to specificity and lack of control for concurrent inflammatory processes. In a retrospective study, prealbumin increased significantly in 102 patients receiving TPN for one week. Unfortunately, patients with renal or hepatic disease were excluded, and the role of inflammation was not assessed.14 Institutionalized patients with Alzheimer’s disease and normal CRP levels showed a statistically significant increase in weight gain, arm muscle circumference, and triceps skin-fold thickness following a nutritional program without a notable change in prealbumin.15 In a study assessing the relationship of prealbumin, CRP, and nutritional intake, critically ill populations receiving less than or greater than 60% of their estimated caloric needs showed no significant difference in prealbumin. In fact, prealbumin levels were only correlated with CRP levels.16 This finding argues against the routine use of prealbumin for nutrition monitoring in the acutely ill hospitalized patient.
Prealbumin Is Not Consistently Correlated with Health Outcomes
Even if prealbumin increased consistently in response to nutritional intervention, whether this change corresponds to an improvement in clinical outcomes has yet to be demonstrated.9 In 2005, Koretz reviewed 99 clinical trials and concluded that even when changes in nutritional markers are seen with nutritional support, the “changes in nutritional markers do not predict clinical outcomes.”17
WHAT YOU SHOULD DO INSTEAD: USE NONBIOLOGIC METHODS FOR SCREENING AND DIAGNOSING MALNUTRITION
Given the lack of a suitable biologic assay to identify malnutrition, dieticians and clinicians must rely on other means to assess malnutrition. Professional societies, including ASPEN and the European Society for Clinical Nutrition and Metabolism, have proposed different guidelines for the screening and assessment of malnutrition (Table 2).11,18 In 2016, these organizations, along with the Latin American Federation of Nutritional Therapy, Clinical Nutrition, and Metabolism and the Parenteral and Enteral Nutrition Society of Asia, formed The Global Leadership Initiative on Malnutrition (GLIM). In 2017, the GLIM taskforce agreed on clinically relevant diagnostic variables for the screening and assessment of malnutrition, including reduced food intake (anorexia), nonvolitional weight loss, (reduced) lean mass, status of disease burden and inflammation, and low body mass index or underweight status.19
RECOMMENDATIONS
- Do not use prealbumin to screen for or diagnose malnutrition.
- Consult with local dietitians to ensure that your institutional approach is in agreement with consensus recommendations.
CONCLUSION
In revisiting the case above, the patient does not have clear evidence of malnutrition based on his history (stable weight and good reported nutritional intake), although he does have a low BMI of 18.5 kg/m2. Rather than prealbumin testing, which would likely be low secondary to the acute phase response, he would better benefit from a nutrition-focused history and physical exam.
The uncertainties faced by clinicians in diagnosing malnutrition cannot readily be resolved by relying on a solitary laboratory marker (eg, prealbumin) or a stand-alone assessment protocol. The data obtained reflect the need for multidisciplinary teams of dieticians and clinicians to contextualize each patient’s medical history and ensure that the selected metrics are used appropriately to aid in diagnosis and documentation. We advocate that clinicians not routinely use prealbumin to screen for, confirm the diagnosis of, or assess the severity of malnutrition in the hospitalized patient.
Do you think this is a low-value practice? Is this truly a “Thing We Do for No Reason?” Share what you do in your practice and join in the conversation online by retweeting it on Twitter (#TWDFNR) and liking it on Facebook. We invite you to propose ideas for other “Things We Do for No Reason” topics by emailing TWDFNR@hospitalmedicine.org.
Disclosures
The authors have nothing to disclose.
The “Things We Do for No Reason” series reviews practices which have become common parts of hospital care but which may provide little value to our patients. Practices reviewed in the TWDFNR series do not represent “black and white” conclusions or clinical practice standards, but are meant as a starting place for research and active discussions among hospitalists and patients. We invite you to be part of that discussion. https://www.choosingwisely.org/

CASE PRESENTATION
A 34-year-old man is admitted for a complicated urinary tract infection related to a chronic in-dwelling Foley catheter. The patient suffered a spinal cord injury at the C4/C5 level as a result of a motor vehicle accident 10 years ago and is confined to a motorized wheelchair. He is an engineer and lives independently but has caregivers. His body mass index (BMI) is 18.5 kg/m2, and he reports his weight has been stable. He has slight muscle atrophy of the biceps, triceps, interosseous muscles, and quadriceps. The patient reports that he eats well, has no chronic conditions, and has not had any gastrointestinal symptoms (eg, anorexia, nausea, diarrhea) over the last six months. You consider whether to order a serum prealbumin test to assess for possible malnutrition.
BACKGROUND
The presence of malnutrition in hospitalized patients is widely recognized as an independent predictor of hospital mortality.1 According to the American Society for Parenteral and Enteral Nutrition (ASPEN), malnutrition is defined as “an acute, subacute or chronic state of nutrition, in which varying degrees of overnutrition or undernutrition with or without inflammatory activity have led to a change in body composition and diminished function.”2 In one large European study, patients screening positive for being at risk of malnutrition had a 12-fold increase in hospital mortality.1
Inpatient malnutrition is remarkably underdocumented. Studies using chart reviews have found a prevalence of malnutrition in hospitalized patients of between 20% and 50%, and only 3% of hospital discharges are associated with a diagnostic code for malnutrition.3–5 Appropriate diagnosis and documentation of malnutrition is important given the profound prognostic and management implications of a malnutrition diagnosis. Appropriate documentation benefits health systems as malnutrition documentation increases expected mortality, thereby improving the observed-to-expected mortality ratio.
Serum prealbumin testing is widely available and frequently ordered in the inpatient setting. In a query we performed of the large aggregate Cerner Electronic Health Record database, HealthFacts, which includes data from inpatient encounters for approximately 700 United States hospitals, prealbumin tests were ordered 129,152 times in 2015. This activity corresponds to estimated total charges of $2,562,375 based on the 2015 clinical laboratory fee schedule.6
WHY YOU MIGHT THINK PREALBUMIN DIAGNOSES MALNUTRITION
Prealbumin is synthesized in the liver and released into circulation prior to excretion by the kidneys and gastrointestinal tract. Prealbumin transports thyroxine, triiodothyronine, and holo-retinol binding protein and, as a result, is also known as transthyretin.7 It was first proposed as a nutritional marker in 1972 with the publication of a study that showed low levels of prealbumin in 40 children with kwashiorkor that improved with intensive dietary supplementation.8 The shorter half-life of prealbumin (2.5 days) as compared with other identified nutritional markers, such as albumin, indicate that it would be suitable for detecting rapid changes in nutritional status.
WHY PREALBUMIN IS NOT HELPFUL FOR DIAGNOSING MALNUTRITION
Prealbumin Is Not Specific
An ideal nutritional marker should be specific enough that changes in this marker reflect changes in nutritional status.9 While there are many systemic factors that affect nutritional markers, such as prealbumin (Table 1), the acute phase response triggered by inflammation is the most significant confounder in the acutely ill hospitalized patient.9 This response to infection, stress, and malignancy leads to an increase in proinflammatory cytokines, increased liver synthesis of inflammatory proteins, such as C-reactive protein (CRP), and increased vascular permeability. Prealbumin is a negative acute phase reactant that decreases in concentration during the stress response due to slowed synthesis and extravasation.9 In a study of 24 patients with severe sepsis and trauma, levels of prealbumin inversely correlated with CRP, a reflection of the stress response, and returned to normal when CRP levels normalized. Neither prealbumin nor CRP, however, correlated with total body protein changes.10 Unfortunately, many studies supporting the use of prealbumin as a nutritional marker do not address the role of the acute phase response in their results. These studies include the original report on prealbumin in kwashiorkor, a condition known to be associated with a high rate of infectious diseases that can trigger the acute phase response.9 A consensus statement from the Academy of Nutrition and Dietetics (AND) and ASPEN noted that prealbumin is an indicator of inflammation and lacks the specificity to diagnose malnutrition.11
Prealbumin Is Not Sensitive
A sensitive laboratory test for malnutrition should allow for detection of malnutrition at an early stage.9 However, patients who demonstrate severe malnutrition without a coexisting inflammatory state do not consistently show low levels of prealbumin. In a systematic review of 20 studies in nondiseased malnourished patients, only two studies, both of which assessed patients with anorexia nervosa, had a mean prealbumin below normal (<20 mg/dL), and this finding corresponded to patient populations with mean BMIs less than 12 kg/m2. More importantly, normal prealbumin levels were seen in groups of patients with a mean BMI as low as 12.9 kg/m2.12 Analysis by AND found insufficient evidence to support a correlation between prealbumin and weight loss in anorexia nervosa, calorie restricted diets, or starvation.13 The data suggest that prealbumin lacks sufficient sensitivity to consistently detect cases of malnutrition easily diagnosed by history and/or physical exam.
Prealbumin Is Not Consistently Responsive to Nutritional Interventions
An accurate marker for malnutrition should improve when nutritional intervention results in adequate nutritional intake.9 While some studies have shown improvements in prealbumin in the setting of a nutritional intervention, many of these works are subject to the same limitations related to specificity and lack of control for concurrent inflammatory processes. In a retrospective study, prealbumin increased significantly in 102 patients receiving TPN for one week. Unfortunately, patients with renal or hepatic disease were excluded, and the role of inflammation was not assessed.14 Institutionalized patients with Alzheimer’s disease and normal CRP levels showed a statistically significant increase in weight gain, arm muscle circumference, and triceps skin-fold thickness following a nutritional program without a notable change in prealbumin.15 In a study assessing the relationship of prealbumin, CRP, and nutritional intake, critically ill populations receiving less than or greater than 60% of their estimated caloric needs showed no significant difference in prealbumin. In fact, prealbumin levels were only correlated with CRP levels.16 This finding argues against the routine use of prealbumin for nutrition monitoring in the acutely ill hospitalized patient.
Prealbumin Is Not Consistently Correlated with Health Outcomes
Even if prealbumin increased consistently in response to nutritional intervention, whether this change corresponds to an improvement in clinical outcomes has yet to be demonstrated.9 In 2005, Koretz reviewed 99 clinical trials and concluded that even when changes in nutritional markers are seen with nutritional support, the “changes in nutritional markers do not predict clinical outcomes.”17
WHAT YOU SHOULD DO INSTEAD: USE NONBIOLOGIC METHODS FOR SCREENING AND DIAGNOSING MALNUTRITION
Given the lack of a suitable biologic assay to identify malnutrition, dieticians and clinicians must rely on other means to assess malnutrition. Professional societies, including ASPEN and the European Society for Clinical Nutrition and Metabolism, have proposed different guidelines for the screening and assessment of malnutrition (Table 2).11,18 In 2016, these organizations, along with the Latin American Federation of Nutritional Therapy, Clinical Nutrition, and Metabolism and the Parenteral and Enteral Nutrition Society of Asia, formed The Global Leadership Initiative on Malnutrition (GLIM). In 2017, the GLIM taskforce agreed on clinically relevant diagnostic variables for the screening and assessment of malnutrition, including reduced food intake (anorexia), nonvolitional weight loss, (reduced) lean mass, status of disease burden and inflammation, and low body mass index or underweight status.19
RECOMMENDATIONS
- Do not use prealbumin to screen for or diagnose malnutrition.
- Consult with local dietitians to ensure that your institutional approach is in agreement with consensus recommendations.
CONCLUSION
In revisiting the case above, the patient does not have clear evidence of malnutrition based on his history (stable weight and good reported nutritional intake), although he does have a low BMI of 18.5 kg/m2. Rather than prealbumin testing, which would likely be low secondary to the acute phase response, he would better benefit from a nutrition-focused history and physical exam.
The uncertainties faced by clinicians in diagnosing malnutrition cannot readily be resolved by relying on a solitary laboratory marker (eg, prealbumin) or a stand-alone assessment protocol. The data obtained reflect the need for multidisciplinary teams of dieticians and clinicians to contextualize each patient’s medical history and ensure that the selected metrics are used appropriately to aid in diagnosis and documentation. We advocate that clinicians not routinely use prealbumin to screen for, confirm the diagnosis of, or assess the severity of malnutrition in the hospitalized patient.
Do you think this is a low-value practice? Is this truly a “Thing We Do for No Reason?” Share what you do in your practice and join in the conversation online by retweeting it on Twitter (#TWDFNR) and liking it on Facebook. We invite you to propose ideas for other “Things We Do for No Reason” topics by emailing TWDFNR@hospitalmedicine.org.
Disclosures
The authors have nothing to disclose.
1. Sorensen J, Kondrup J, Prokopowicz J, et al. EuroOOPS: an international, multicentre study to implement nutritional risk screening and evaluate clinical outcome. Clin Nutr Edinb Scotl. 2008;27(3):340-349. PubMed
2. Mueller C, Compher C, Ellen DM, American Society for Parenteral and Enteral Nutrition (A.S.P.E.N.) Board of Directors. A.S.P.E.N. clinical guidelines: nutrition screening, assessment, and intervention in adults. JPEN J Parenter Enteral Nutr. 2011;35(1):16-24. PubMed
3. Kaiser MJ, Bauer JM, Rämsch C, et al. Frequency of malnutrition in older adults: a multinational perspective using the mini nutritional assessment. J Am Geriatr Soc. 2010;58(9):1734-1738. PubMed
4. Robinson MK, Trujillo EB, Mogensen KM, Rounds J, McManus K, Jacobs DO. Improving nutritional screening of hospitalized patients: the role of prealbumin. JPEN J Parenter Enteral Nutr. 2003;27(6):389-395; quiz 439. PubMed
5. Corkins MR, Guenter P, DiMaria-Ghalili RA, et al. Malnutrition diagnoses in hospitalized patients: United States, 2010. JPEN J Parenter Enteral Nutr. 2014;38(2):186-195. PubMed
6. Clinical Laboratory Fee Schedule Files. cms.org. https://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/ClinicalLabFeeSched/Clinical-Laboratory-Fee-Schedule-Files.html. Published September 29, 2016. Accessed January 5, 2018.
7. Myron Johnson A, Merlini G, Sheldon J, Ichihara K, Scientific Division Committee on Plasma Proteins (C-PP), International Federation of Clinical Chemistry and Laboratory Medicine (IFCC). Clinical indications for plasma protein assays: transthyretin (prealbumin) in inflammation and malnutrition. Clin Chem Lab Med. 2007;45(3):419-426. PubMed
8. Ingenbleek Y, De Visscher M, De Nayer P. Measurement of prealbumin as index of protein-calorie malnutrition. Lancet. 1972;2(7768):106-109. PubMed
9. Barbosa-Silva MCG. Subjective and objective nutritional assessment methods: what do they really assess? Curr Opin Clin Nutr Metab Care. 2008;11(3):248-254. PubMed
10. Clark MA, Hentzen BTH, Plank LD, Hill GL. Sequential changes in insulin-like growth factor 1, plasma proteins, and total body protein in severe sepsis and multiple injury. J Parenter Enter Nutr. 1996;20(5):363-370. PubMed
11. White JV, Guenter P, Jensen G, et al. Consensus statement of the Academy of Nutrition and Dietetics/American Society for Parenteral and Enteral Nutrition: characteristics recommended for the identification and documentation of adult malnutrition (undernutrition). J Acad Nutr Diet. 2012;112(5):730-738. PubMed
12. Lee JL, Oh ES, Lee RW, Finucane TE. Serum albumin and prealbumin in calorically restricted, nondiseased individuals: a systematic review. Am J Med. 2015;128(9):1023.e1-22. PubMed
13. Academy of Nutrition and Dietetics Evidence Analysis Library. Nutrition Screening (NSCR) Systematic Review (2009-2010). https://www.andeal.org/tmp/pdf-print-919C51237950859AE3E15F978CEF49D8.pdf. Accessed August 23, 2017.
14. Sawicky CP, Nippo J, Winkler MF, Albina JE. Adequate energy intake and improved prealbumin concentration as indicators of the response to total parenteral nutrition. J Am Diet Assoc. 1992;92(10):1266-1268. PubMed
15. Van Wymelbeke V, Guédon A, Maniere D, Manckoundia P, Pfitzenmeyer P. A 6-month follow-up of nutritional status in institutionalized patients with Alzheimer’s disease. J Nutr Health Aging. 2004;8(6):505-508. PubMed
16. Davis CJ, Sowa D, Keim KS, Kinnare K, Peterson S. The use of prealbumin and C-reactive protein for monitoring nutrition support in adult patients receiving enteral nutrition in an urban medical center. JPEN J Parenter Enteral Nutr. 2012;36(2):197-204. PubMed
17. Koretz RL. Death, morbidity and economics are the only end points for trials. Proc Nutr Soc. 2005;64(3):277-284. PubMed
18. Cederholm T, Bosaeus I, Barazzoni R, et al. Diagnostic criteria for malnutrition - an ESPEN consensus statement. Clin Nutr Edinb Scotl. 2015;34(3):335-340. PubMed
19. Jensen GL, Cederholm T. Global leadership initiative on malnutrition: progress report from ASPEN clinical nutrition week 2017. JPEN J Parenter Enteral Nutr. April 2017:148607117707761. PubMed
1. Sorensen J, Kondrup J, Prokopowicz J, et al. EuroOOPS: an international, multicentre study to implement nutritional risk screening and evaluate clinical outcome. Clin Nutr Edinb Scotl. 2008;27(3):340-349. PubMed
2. Mueller C, Compher C, Ellen DM, American Society for Parenteral and Enteral Nutrition (A.S.P.E.N.) Board of Directors. A.S.P.E.N. clinical guidelines: nutrition screening, assessment, and intervention in adults. JPEN J Parenter Enteral Nutr. 2011;35(1):16-24. PubMed
3. Kaiser MJ, Bauer JM, Rämsch C, et al. Frequency of malnutrition in older adults: a multinational perspective using the mini nutritional assessment. J Am Geriatr Soc. 2010;58(9):1734-1738. PubMed
4. Robinson MK, Trujillo EB, Mogensen KM, Rounds J, McManus K, Jacobs DO. Improving nutritional screening of hospitalized patients: the role of prealbumin. JPEN J Parenter Enteral Nutr. 2003;27(6):389-395; quiz 439. PubMed
5. Corkins MR, Guenter P, DiMaria-Ghalili RA, et al. Malnutrition diagnoses in hospitalized patients: United States, 2010. JPEN J Parenter Enteral Nutr. 2014;38(2):186-195. PubMed
6. Clinical Laboratory Fee Schedule Files. cms.org. https://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/ClinicalLabFeeSched/Clinical-Laboratory-Fee-Schedule-Files.html. Published September 29, 2016. Accessed January 5, 2018.
7. Myron Johnson A, Merlini G, Sheldon J, Ichihara K, Scientific Division Committee on Plasma Proteins (C-PP), International Federation of Clinical Chemistry and Laboratory Medicine (IFCC). Clinical indications for plasma protein assays: transthyretin (prealbumin) in inflammation and malnutrition. Clin Chem Lab Med. 2007;45(3):419-426. PubMed
8. Ingenbleek Y, De Visscher M, De Nayer P. Measurement of prealbumin as index of protein-calorie malnutrition. Lancet. 1972;2(7768):106-109. PubMed
9. Barbosa-Silva MCG. Subjective and objective nutritional assessment methods: what do they really assess? Curr Opin Clin Nutr Metab Care. 2008;11(3):248-254. PubMed
10. Clark MA, Hentzen BTH, Plank LD, Hill GL. Sequential changes in insulin-like growth factor 1, plasma proteins, and total body protein in severe sepsis and multiple injury. J Parenter Enter Nutr. 1996;20(5):363-370. PubMed
11. White JV, Guenter P, Jensen G, et al. Consensus statement of the Academy of Nutrition and Dietetics/American Society for Parenteral and Enteral Nutrition: characteristics recommended for the identification and documentation of adult malnutrition (undernutrition). J Acad Nutr Diet. 2012;112(5):730-738. PubMed
12. Lee JL, Oh ES, Lee RW, Finucane TE. Serum albumin and prealbumin in calorically restricted, nondiseased individuals: a systematic review. Am J Med. 2015;128(9):1023.e1-22. PubMed
13. Academy of Nutrition and Dietetics Evidence Analysis Library. Nutrition Screening (NSCR) Systematic Review (2009-2010). https://www.andeal.org/tmp/pdf-print-919C51237950859AE3E15F978CEF49D8.pdf. Accessed August 23, 2017.
14. Sawicky CP, Nippo J, Winkler MF, Albina JE. Adequate energy intake and improved prealbumin concentration as indicators of the response to total parenteral nutrition. J Am Diet Assoc. 1992;92(10):1266-1268. PubMed
15. Van Wymelbeke V, Guédon A, Maniere D, Manckoundia P, Pfitzenmeyer P. A 6-month follow-up of nutritional status in institutionalized patients with Alzheimer’s disease. J Nutr Health Aging. 2004;8(6):505-508. PubMed
16. Davis CJ, Sowa D, Keim KS, Kinnare K, Peterson S. The use of prealbumin and C-reactive protein for monitoring nutrition support in adult patients receiving enteral nutrition in an urban medical center. JPEN J Parenter Enteral Nutr. 2012;36(2):197-204. PubMed
17. Koretz RL. Death, morbidity and economics are the only end points for trials. Proc Nutr Soc. 2005;64(3):277-284. PubMed
18. Cederholm T, Bosaeus I, Barazzoni R, et al. Diagnostic criteria for malnutrition - an ESPEN consensus statement. Clin Nutr Edinb Scotl. 2015;34(3):335-340. PubMed
19. Jensen GL, Cederholm T. Global leadership initiative on malnutrition: progress report from ASPEN clinical nutrition week 2017. JPEN J Parenter Enteral Nutr. April 2017:148607117707761. PubMed
© 2018 Society of Hospital Medicine
10 Strategies for Delivering a Great Presentation
It’s noon on Tuesday, and James, a new PGY-2 resident, begins his presentation on COPD. After five minutes, you notice half of the residents playing Words with Friends, the “ortho-bound” medical student talking with a buddy in the back, and the attendings looking on with innate skepticism.
Your talk on atrial fibrillation is next month, and just watching James brings on palpitations of your own. So what do you do?
Introduction
Public speaking is a near certainty for most of us regardless of training stage. A well-executed presentation establishes the clinician as an institutional authority, adroitly educating anyone around you.
So how can you deliver that killer update on atrial fibrillation? Here, we provide you with 10 tips for preparing and delivering a great presentation.
Preparation
1. Consider the audience and what they already know. No matter how interesting we think we are, if we don’t present with the audience’s needs in mind, we might as well be talking to an empty room. Consider what the audience may or may not know about the topic; this allows you to decide whether to give a comprehensive didactic on atrial fibrillation for trainees or an anticoagulation update for cardiologists. Great presenters survey their audience early on with a question such as, “How many of you here know the results of the AFFIRM trial?” This allows you to make small alterations to meet the needs of your audience.
2. Visualize the stage and setting. Understanding the stage helps you anticipate and address barriers to learning. Imagine for a moment the difference in these two scenarios: a discussion of hyponatremia with a group of medical students at 4 p.m. in a dark room versus a discussion on principles of atrial fibrillation management at 11 a.m. in an auditorium. Both require interaction, although an auditorium-based presentation requires testing your audio-visual equipment in advance.
3. Determine your objectives. To determine your objectives, begin with the end in mind. If you were to visualize your audience members at the end of the talk, what would they know (knowledge), be able to do (behavior), or have a new outlook on (attitude)? The objectives will determine the content you deliver and the activities for learning. For a one-hour presentation, identifying three to five objectives is a good rule of thumb.
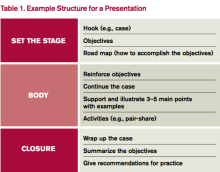
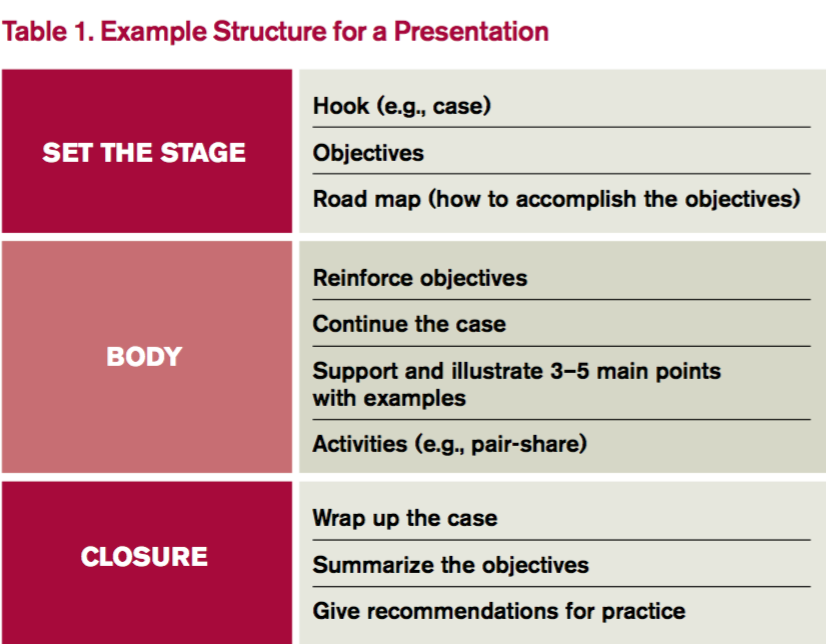
4. Build your presentation. Whether using PowerPoint, Prezi, or a white board, “build” the presentation from the objectives. Table 1 outlines one example format; Figure 1 outlines some best practices of PowerPoint.
Humans evolved to interpret visual imagery, not read text, so try to use pictures instead of bullet points. Consider first building slides with text and then using an internet search engine to convert words to pictures. For example, “atrial rate 200 bpm” is better displayed with an actual ECG.
5. Practice. Practicing helps you become more comfortable with the content itself as well as how to present that content. If you can, practice with a colleague and receive feedback to sharpen your material. No time to spare? Practice the introduction and any major point that you want to get across. Audiences decide within the first five minutes whether your talk is worth listening to before pulling out their cellphones to open up Facebook.
Delivery
1. Confront nervousness. Many of us become nervous when speaking in front of an audience. To address this, it’s perfectly reasonable to rehearse a presentation at home or in a quiet call room ahead of time. If you feel extremely nervous, breathe deeply for five- to 10-second intervals. During the presentation itself, find friendly or familiar faces in the audience and look them in the eyes as you speak. This eases nerves and improves your technique.
2. Hook your audience. The purpose of the hook is to “grab” the attention of the audience. The best presenters intrigue the audience with a story or problem at the outset and use the presentation to address that problem. Consider the differences in these openings:
- “As of 2010, atrial fibrillation has affected 33.5 million Americans each year, with a reported prevalence of stroke of 2.8% to 24.2%.”
- “Sarah is a 67-year-old woman with a history of atrial fibrillation who loved to play the piano until she experienced a stroke, paralysis of her left arm, and the end of her career as a pianist. Today, I’m going to teach you how to reduce the risk of stroke in your patients with atrial fibrillation.”
Then as the presentation proceeds, develop the case to keep the audience thinking about their differential diagnosis or management strategy.
3. Speak clearly. We all use fillers such as “uh” and “um” without noticing. To learn to speak well, practice as much as possible and ask for feedback on your diction. Consider watching TED Talks, short clips of fascinating stories whose presenters are highly coached in public speaking. Use specific statements to key in the audience on important points, such as, “If you remember anything from this talk, I want you to remember …”
Remember, too, that public speaking requires enthusiasm. There’s nothing worse than beginning with, “I know that you all have heard about atrial fibrillation 500 times, so let’s just get through this.” The energy of the audience reflects the energy of the speaker.
4. Facilitate learning. Don’t do all of the talking; in fact, let the audience talk for you. For audience members to learn, they must engage with the material. Use a question/answer forum such as polleverywhere.com, where the audience responds in real time. Alternatively, pose a scenario to discuss using a pair-share technique. For a talk on atrial fibrillation, give direction to “turn to a neighbor and discuss anticoagulation for Mr. Jones, a 66-year-old man with cirrhosis, CVA, and hypertension admitted with atrial fibrillation.” Debrief this activity to solicit thoughts from the audience and then address the scenario.
5. Break the glass. Don’t hide behind the podium! “Breaking the glass” means stepping away from the podium to create an experience more akin to a dialogue. Remember, the audience is interested in hearing what you have to say—otherwise, they would have read about atrial fibrillation from UpToDate. Stepping away from the podium breaks the expected monotony and can help burn nervous energy.
Bottom Line
A fantastic presentation requires preparation and a thoughtful delivery. Spend the time to prepare. After all, that upcoming presentation on atrial fibrillation is only one month away. It will arrive sooner than you think. TH
Dr. Rendon is associate program director and Dr. Roesch is an assistant professor in the Division of Hospital Medicine at the University of New Mexico Hospital in Albuquerque. They are co-directors of the medical student clinical reasoning course. Both are members of SHM’s Physicians in Training Committee.
References
- Anderson C. How to give a killer presentation. Harvard Business Review. June 2013.
- Covey C. The 7 Habits of Highly Effective People. Franklin Covey Co.; 2004.
- Ganz L. Epidemiology of and risk factors for atrial fibrillation. Updated October 15, 2015.
- Sharpe B. How to give a great talk. Presented as part of SHM national conference; 2014; Las Vegas.
- Skeff K, Stratos G. Methods for Teaching Medicine. Philadelphia: ACP Press; 2010.
It’s noon on Tuesday, and James, a new PGY-2 resident, begins his presentation on COPD. After five minutes, you notice half of the residents playing Words with Friends, the “ortho-bound” medical student talking with a buddy in the back, and the attendings looking on with innate skepticism.
Your talk on atrial fibrillation is next month, and just watching James brings on palpitations of your own. So what do you do?
Introduction
Public speaking is a near certainty for most of us regardless of training stage. A well-executed presentation establishes the clinician as an institutional authority, adroitly educating anyone around you.
So how can you deliver that killer update on atrial fibrillation? Here, we provide you with 10 tips for preparing and delivering a great presentation.
Preparation
1. Consider the audience and what they already know. No matter how interesting we think we are, if we don’t present with the audience’s needs in mind, we might as well be talking to an empty room. Consider what the audience may or may not know about the topic; this allows you to decide whether to give a comprehensive didactic on atrial fibrillation for trainees or an anticoagulation update for cardiologists. Great presenters survey their audience early on with a question such as, “How many of you here know the results of the AFFIRM trial?” This allows you to make small alterations to meet the needs of your audience.
2. Visualize the stage and setting. Understanding the stage helps you anticipate and address barriers to learning. Imagine for a moment the difference in these two scenarios: a discussion of hyponatremia with a group of medical students at 4 p.m. in a dark room versus a discussion on principles of atrial fibrillation management at 11 a.m. in an auditorium. Both require interaction, although an auditorium-based presentation requires testing your audio-visual equipment in advance.
3. Determine your objectives. To determine your objectives, begin with the end in mind. If you were to visualize your audience members at the end of the talk, what would they know (knowledge), be able to do (behavior), or have a new outlook on (attitude)? The objectives will determine the content you deliver and the activities for learning. For a one-hour presentation, identifying three to five objectives is a good rule of thumb.
4. Build your presentation. Whether using PowerPoint, Prezi, or a white board, “build” the presentation from the objectives. Table 1 outlines one example format; Figure 1 outlines some best practices of PowerPoint.
Humans evolved to interpret visual imagery, not read text, so try to use pictures instead of bullet points. Consider first building slides with text and then using an internet search engine to convert words to pictures. For example, “atrial rate 200 bpm” is better displayed with an actual ECG.
5. Practice. Practicing helps you become more comfortable with the content itself as well as how to present that content. If you can, practice with a colleague and receive feedback to sharpen your material. No time to spare? Practice the introduction and any major point that you want to get across. Audiences decide within the first five minutes whether your talk is worth listening to before pulling out their cellphones to open up Facebook.
Delivery
1. Confront nervousness. Many of us become nervous when speaking in front of an audience. To address this, it’s perfectly reasonable to rehearse a presentation at home or in a quiet call room ahead of time. If you feel extremely nervous, breathe deeply for five- to 10-second intervals. During the presentation itself, find friendly or familiar faces in the audience and look them in the eyes as you speak. This eases nerves and improves your technique.
2. Hook your audience. The purpose of the hook is to “grab” the attention of the audience. The best presenters intrigue the audience with a story or problem at the outset and use the presentation to address that problem. Consider the differences in these openings:
- “As of 2010, atrial fibrillation has affected 33.5 million Americans each year, with a reported prevalence of stroke of 2.8% to 24.2%.”
- “Sarah is a 67-year-old woman with a history of atrial fibrillation who loved to play the piano until she experienced a stroke, paralysis of her left arm, and the end of her career as a pianist. Today, I’m going to teach you how to reduce the risk of stroke in your patients with atrial fibrillation.”
Then as the presentation proceeds, develop the case to keep the audience thinking about their differential diagnosis or management strategy.
3. Speak clearly. We all use fillers such as “uh” and “um” without noticing. To learn to speak well, practice as much as possible and ask for feedback on your diction. Consider watching TED Talks, short clips of fascinating stories whose presenters are highly coached in public speaking. Use specific statements to key in the audience on important points, such as, “If you remember anything from this talk, I want you to remember …”
Remember, too, that public speaking requires enthusiasm. There’s nothing worse than beginning with, “I know that you all have heard about atrial fibrillation 500 times, so let’s just get through this.” The energy of the audience reflects the energy of the speaker.
4. Facilitate learning. Don’t do all of the talking; in fact, let the audience talk for you. For audience members to learn, they must engage with the material. Use a question/answer forum such as polleverywhere.com, where the audience responds in real time. Alternatively, pose a scenario to discuss using a pair-share technique. For a talk on atrial fibrillation, give direction to “turn to a neighbor and discuss anticoagulation for Mr. Jones, a 66-year-old man with cirrhosis, CVA, and hypertension admitted with atrial fibrillation.” Debrief this activity to solicit thoughts from the audience and then address the scenario.
5. Break the glass. Don’t hide behind the podium! “Breaking the glass” means stepping away from the podium to create an experience more akin to a dialogue. Remember, the audience is interested in hearing what you have to say—otherwise, they would have read about atrial fibrillation from UpToDate. Stepping away from the podium breaks the expected monotony and can help burn nervous energy.
Bottom Line
A fantastic presentation requires preparation and a thoughtful delivery. Spend the time to prepare. After all, that upcoming presentation on atrial fibrillation is only one month away. It will arrive sooner than you think. TH
Dr. Rendon is associate program director and Dr. Roesch is an assistant professor in the Division of Hospital Medicine at the University of New Mexico Hospital in Albuquerque. They are co-directors of the medical student clinical reasoning course. Both are members of SHM’s Physicians in Training Committee.
References
- Anderson C. How to give a killer presentation. Harvard Business Review. June 2013.
- Covey C. The 7 Habits of Highly Effective People. Franklin Covey Co.; 2004.
- Ganz L. Epidemiology of and risk factors for atrial fibrillation. Updated October 15, 2015.
- Sharpe B. How to give a great talk. Presented as part of SHM national conference; 2014; Las Vegas.
- Skeff K, Stratos G. Methods for Teaching Medicine. Philadelphia: ACP Press; 2010.
It’s noon on Tuesday, and James, a new PGY-2 resident, begins his presentation on COPD. After five minutes, you notice half of the residents playing Words with Friends, the “ortho-bound” medical student talking with a buddy in the back, and the attendings looking on with innate skepticism.
Your talk on atrial fibrillation is next month, and just watching James brings on palpitations of your own. So what do you do?
Introduction
Public speaking is a near certainty for most of us regardless of training stage. A well-executed presentation establishes the clinician as an institutional authority, adroitly educating anyone around you.
So how can you deliver that killer update on atrial fibrillation? Here, we provide you with 10 tips for preparing and delivering a great presentation.
Preparation
1. Consider the audience and what they already know. No matter how interesting we think we are, if we don’t present with the audience’s needs in mind, we might as well be talking to an empty room. Consider what the audience may or may not know about the topic; this allows you to decide whether to give a comprehensive didactic on atrial fibrillation for trainees or an anticoagulation update for cardiologists. Great presenters survey their audience early on with a question such as, “How many of you here know the results of the AFFIRM trial?” This allows you to make small alterations to meet the needs of your audience.
2. Visualize the stage and setting. Understanding the stage helps you anticipate and address barriers to learning. Imagine for a moment the difference in these two scenarios: a discussion of hyponatremia with a group of medical students at 4 p.m. in a dark room versus a discussion on principles of atrial fibrillation management at 11 a.m. in an auditorium. Both require interaction, although an auditorium-based presentation requires testing your audio-visual equipment in advance.
3. Determine your objectives. To determine your objectives, begin with the end in mind. If you were to visualize your audience members at the end of the talk, what would they know (knowledge), be able to do (behavior), or have a new outlook on (attitude)? The objectives will determine the content you deliver and the activities for learning. For a one-hour presentation, identifying three to five objectives is a good rule of thumb.
4. Build your presentation. Whether using PowerPoint, Prezi, or a white board, “build” the presentation from the objectives. Table 1 outlines one example format; Figure 1 outlines some best practices of PowerPoint.
Humans evolved to interpret visual imagery, not read text, so try to use pictures instead of bullet points. Consider first building slides with text and then using an internet search engine to convert words to pictures. For example, “atrial rate 200 bpm” is better displayed with an actual ECG.
5. Practice. Practicing helps you become more comfortable with the content itself as well as how to present that content. If you can, practice with a colleague and receive feedback to sharpen your material. No time to spare? Practice the introduction and any major point that you want to get across. Audiences decide within the first five minutes whether your talk is worth listening to before pulling out their cellphones to open up Facebook.
Delivery
1. Confront nervousness. Many of us become nervous when speaking in front of an audience. To address this, it’s perfectly reasonable to rehearse a presentation at home or in a quiet call room ahead of time. If you feel extremely nervous, breathe deeply for five- to 10-second intervals. During the presentation itself, find friendly or familiar faces in the audience and look them in the eyes as you speak. This eases nerves and improves your technique.
2. Hook your audience. The purpose of the hook is to “grab” the attention of the audience. The best presenters intrigue the audience with a story or problem at the outset and use the presentation to address that problem. Consider the differences in these openings:
- “As of 2010, atrial fibrillation has affected 33.5 million Americans each year, with a reported prevalence of stroke of 2.8% to 24.2%.”
- “Sarah is a 67-year-old woman with a history of atrial fibrillation who loved to play the piano until she experienced a stroke, paralysis of her left arm, and the end of her career as a pianist. Today, I’m going to teach you how to reduce the risk of stroke in your patients with atrial fibrillation.”
Then as the presentation proceeds, develop the case to keep the audience thinking about their differential diagnosis or management strategy.
3. Speak clearly. We all use fillers such as “uh” and “um” without noticing. To learn to speak well, practice as much as possible and ask for feedback on your diction. Consider watching TED Talks, short clips of fascinating stories whose presenters are highly coached in public speaking. Use specific statements to key in the audience on important points, such as, “If you remember anything from this talk, I want you to remember …”
Remember, too, that public speaking requires enthusiasm. There’s nothing worse than beginning with, “I know that you all have heard about atrial fibrillation 500 times, so let’s just get through this.” The energy of the audience reflects the energy of the speaker.
4. Facilitate learning. Don’t do all of the talking; in fact, let the audience talk for you. For audience members to learn, they must engage with the material. Use a question/answer forum such as polleverywhere.com, where the audience responds in real time. Alternatively, pose a scenario to discuss using a pair-share technique. For a talk on atrial fibrillation, give direction to “turn to a neighbor and discuss anticoagulation for Mr. Jones, a 66-year-old man with cirrhosis, CVA, and hypertension admitted with atrial fibrillation.” Debrief this activity to solicit thoughts from the audience and then address the scenario.
5. Break the glass. Don’t hide behind the podium! “Breaking the glass” means stepping away from the podium to create an experience more akin to a dialogue. Remember, the audience is interested in hearing what you have to say—otherwise, they would have read about atrial fibrillation from UpToDate. Stepping away from the podium breaks the expected monotony and can help burn nervous energy.
Bottom Line
A fantastic presentation requires preparation and a thoughtful delivery. Spend the time to prepare. After all, that upcoming presentation on atrial fibrillation is only one month away. It will arrive sooner than you think. TH
Dr. Rendon is associate program director and Dr. Roesch is an assistant professor in the Division of Hospital Medicine at the University of New Mexico Hospital in Albuquerque. They are co-directors of the medical student clinical reasoning course. Both are members of SHM’s Physicians in Training Committee.
References
- Anderson C. How to give a killer presentation. Harvard Business Review. June 2013.
- Covey C. The 7 Habits of Highly Effective People. Franklin Covey Co.; 2004.
- Ganz L. Epidemiology of and risk factors for atrial fibrillation. Updated October 15, 2015.
- Sharpe B. How to give a great talk. Presented as part of SHM national conference; 2014; Las Vegas.
- Skeff K, Stratos G. Methods for Teaching Medicine. Philadelphia: ACP Press; 2010.
Tips for Hospitalists on Improving Diagnostic Skills
Case
A 67-year-old man presents to the hospital with persistent, subjective fevers and malaise for one month, subacute onset of dyspnea, and nonproductive cough for the preceding six days. The patient is a nonsmoker, denies sick contacts, and has had no foreign travel. What would be the best approach to making the diagnosis while working to enhance diagnostic skills?
Diagnostic Reasoning
With clinical experience, making a diagnosis can become so routine that physicians might not contemplate their problem-solving strategies. Diagnostic reasoning is the process of thinking about a clinical problem to form a diagnosis. Experienced clinicians typically rely upon nonanalytic reasoning (i.e., pattern recognition) for straightforward problems, reverting to analytic reasoning if a pattern is not recognized.
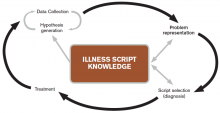
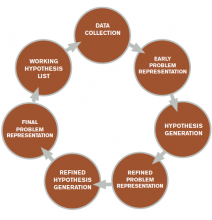
The literature describes five steps in the reasoning process (see Figure 1). In the early stages of data collection, hypotheses emerge that feed back into data collection behaviors as the clinician seeks confirmatory evidence. This complex interplay between data collection and hypothesis generation/elimination leads to a more clearly defined understanding of the patient’s presentation. The synthesis of the patient’s presentation, including epidemiologic risk factors, symptoms, signs, and laboratory and radiologic studies, is called the “problem representation.” After a clinician conceives the problem representation, he or she reviews the mental representations of diseases (i.e., illness scripts) to determine hypotheses by finding disease presentations that best match the formulated problem representation (see Figure 2).
Analytic and nonanalytic reasoning. In what is known as the dual process theory, diagnostic reasoning is believed to occur both analytically and nonanalytically.1 Nonanalytic reasoning is often exemplified by rapid, subconscious “pattern recognition” and is developed through clinical experience and other nonclinical learning experiences (e.g. reading).
Conversely, analytic reasoning, the “slow,” conscious, cognitive processing, is typically utilized when a patient presentation is complicated or does not fit a known disease pattern. Clinicians apply both strategies to make diagnoses in evaluating complex cases.
In the outlined case, while the symptoms of fever and cough might lead to the diagnosis of community-acquired pneumonia (CAP), the time course seems unusually long. This atypical pattern for CAP could trigger analytic reasoning, leading to new considerations such as tuberculosis (TB).
Case Continued
On examination, the patient has severe rigors and diaphoresis, as well as a fever of 39.4°C and a heart rate of 102 bpm. Full examination discloses mild end-expiratory wheezes and bronchial breath sounds in the right lower lobe. The remainder of his examination is normal. Labs reveal WBC 8.5x103, hemoglobin 11g/dL, MCV of 92 fL, and platelet count 22,000 mm3. Blood cultures, sputum cultures, and respiratory virus microarray are normal. The chest X-ray (CXR) is unremarkable.
Further history reveals that the patient is a sheepherder living in a primitive earthen structure in the rural mountains of western New Mexico.
Problem representation revisited. With additional historical, laboratory, and radiological data collected, further interpretation and synthesis occur. Salient elements are highlighted and prioritized, irrelevant details are discarded, and data of uncertain relevance are reevaluated as additional data are gathered. The problem representation—an interpreted, subjective mental model of a patient’s clinical presentation—is updated and reformulated. The verbal expression of the problem representation is variously called the assessment, summary statement, or “one-liner.” Within this summary statement, and fundamental to the creation of a strong problem representation, is the incorporation of “semantic qualifiers.”
“Semantic qualifiers” (e.g. acute vs. chronic or unremitting vs. relapsing) are paired, opposing descriptive adjectives that can be used to compare and contrast diagnostic considerations.² Clinicians distinguish between diseases using key signs and symptoms and use these descriptors to assist with this discrimination in hypothesis generation. An example for this patient would be: A 67-year-old sheepherder living in rural New Mexico presents with persistent fevers and malaise for one month, along with subacute development of nonproductive cough and dyspnea, sepsis, anemia, and thrombocytopenia.
Note how the incorporation of epidemiologic information (sheepherder living in an earthen structure in rural New Mexico) creates a context in which the additional problems can be framed (persistent malaise, subacute cough). In this case, the persistent fevers help the clinician to narrow possibilities in the differential diagnosis and create focused hypotheses.
Although the benefit of teaching accurate and thorough problem representation seems self-evident, studies have not demonstrated that improved problem representation enhances diagnostic accuracy; however, we believe that there is still value in adapting and teaching this skill.3
Hypothesis refinement and the differential diagnosis. Initial hypotheses occur early in data collection, as the patient’s history and physical examination findings trigger connections to clinicians’ bank of known diseases (e.g. orthopnea triggers congestive heart failure). As the clinician collects additional data, he or she refines these hypotheses, changing the likelihood based on “fit” of the problem representation with known diseases or illness scripts.
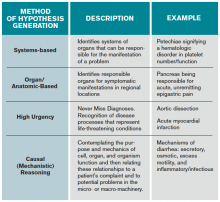
When employing analytic reasoning processes, clinicians may benefit from using organizational frameworks to assist with hypothesis generation (see Table 1). For this patient, possible hypotheses could include CAP, TB, lymphoma, lung neoplasm, or other indolent pulmonary infection.
Illness scripts. Once discrete hypotheses (e.g. CAP, pulmonary embolism) have been generated, clinicians need a method to accurately compare disease processes. This can be done through the use of an illness script. Illness scripts are mental representations of diseases and are likely to include epidemiology, typical and atypical patterns of presentation, and distinguishing features.
For example, a clinician’s illness script for a typical presentation of bacterial CAP likely includes fever, productive cough, pleuritic chest discomfort, and infiltrate on CXR. Clinician educators who teach illness scripts should ensure that students understand that diseases have atypical presentations, even though they may only teach them the prototypical one. Conceptualizing diseases in this fashion allows clinicians to seek the disease with the “script” that best matches the patient’s story (i.e., clinical presentation).
In this case, the clinician is now thinking of causes of persistent fever + nonproductive cough + dyspnea + anemia + thrombocytopenia; possibilities include lymphoma or unusual infection (e.g. tick-borne relapsing fever, or TBRF).
Case Resolution and Script Selection
As the clinician processes the case, a known illness script of TBRF matches the patient’s clinical presentation, and a peripheral smear is ordered. The smear reveals presence of spirochetal organisms, later confirmed by PCR to be Borrelia hermsii, confirming the diagnosis of TBRF.
Errors in Clinical Reasoning
Although most clinicians are quite accurate in typical presentations of common diseases, they are more likely to commit diagnostic errors when faced with uncommon diseases, atypical presentations, and/or challenging contexts. The following sections categorize a selection of some common errors and offer some expert opinion from the literature on avoiding them.
Common diagnostic errors. Clinicians use heuristics, or mental shortcuts, which can occasionally induce diagnostic errors. By definition, the fundamental problem in all diagnostic error is premature closure, or acceptance of a diagnosis before it is fully verified. In the case presented, the clinician may have accepted the diagnosis of CAP without recognizing other possible diagnoses.
Two common heuristics/biases that can sometimes lead to premature closure are the availability and anchoring biases. Availability bias means that the diagnoses easily thought of—and often most recent in the memory—are more likely to be assigned to a patient problem. The diagnosis of pulmonary embolism would be more “available” in a patient with fever, dyspnea, and normal CXR, especially if the clinician recently had seen a patient with PE. Anchoring bias occurs when early information is relied upon to make clinical judgments and the clinician fixates on a diagnosis despite acquiring additional or contrary information. For example, a clinician may rely upon a diagnosis of CAP based on the sign-out from a colleague, despite the one-month history of symptoms, rather than broadening the differential.
Clinician-focused methods to reduce diagnostic errors. Multiple methods exist that may mitigate diagnostic errors, although definitive proof of their value is still lacking, owing to the difficulty involved in studying such errors due to the multitude of causes.4 In our opinion, building a mental database of illness scripts by reading and seeing patients, as well as being metacognitive, are the best methods for individual clinicians to use to reduce their errors (see “deliberate practice” below).
Metacognition, or thinking about one’s thinking, is another method of reducing errors and can be characterized by “reflection in action” (reflection in real time) and “reflection on action” (reflection after an event).5 For example, taking a few moments at the end of a week on clinical service to reflect on the hospital course and diagnostic paths of the most complex patient presentations (reflection on action) is an exercise used to reduce errors.
For reflection in action, a clinician may pause when confronted with paradoxical findings for a current patient’s presentation (e.g. elevated jugular veinous pressure and crackles on exam but normal b-type natriuretic peptide), and “think aloud” (see below) to ensure he or she is processing all of the appropriate elements of the case.5
In the case presented above, the time course might have initiated reflection into erroneous decision-making at the moment the clinician thought that CAP was a possibility (reflection in action). Although direct evidence is inconclusive as to whether these techniques improve diagnostic accuracy, engaging in metacognitive exercises remains a cornerstone of seasoned clinical reasoning experts.6
Teaching and Learning Principles
Making a commitment. During a patient presentation, it is often helpful to ask a learner to develop a two- to four-item prioritized differential diagnosis list based on likelihood and/or lethality. Have the learner describe which diagnosis is most likely (i.e., the working diagnosis), in addition to the reasons “for” or “against” certain hypotheses. Once the diagnosis has been determined, combine commitment with an exercise in metacognition by asking the learner, “Why do you think that your initial diagnosis of Q-fever was incorrect?” Clinical educators may then follow up with teaching pearls and their approach to this type of case (see Table 1).
Think aloud: In this method, an instructor expresses his or her thoughts aloud in real time.7 By modeling this technique, attending physicians allow learners to observe the process of developing a differential diagnosis and plan. For example, during the admission process, instructors could verbalize their approach to fever in a systematic fashion (see Table 1) after the trainee has completed the presentation: “At this point I am considering an infectious cause such as pneumonia, given the respiratory symptoms, although the one-month history of fever and malaise makes me think that I should keep neoplasm and an unusual infection on my list of possibilities.”
Conversely, instructors can ask trainees to voice their thoughts aloud to better understand their reasoning processes. By using this method, instructors can also support, correct, or reinforce the trainees’ appropriate use of knowledge in the clinical reasoning process.
Deliberate practice. To improve diagnostic skills, trainees must engage in deliberate practice, defined as intentional, repetitious practice aimed at improving performance.8 To facilitate this, a trainee should evaluate as many patients as possible and present to an experienced clinician with subsequent feedback. Trainees are likely to miss subtle historical or examination points (e.g. the history of sheepherding) because their illness scripts are limited or incompletely developed. Teachers should emphasize the importance of developing broad and deep illness scripts, so learners will, hopefully, become more aware of their limitations and recognize what they do not know.
Key Takeaways
Clinicians solve diagnostic problems using both nonanalytic and analytic reasoning processes. Although evidence is inconclusive, some clinical reasoning experts suggest the use of reflective strategies to enhance diagnostic accuracy, especially in complicated cases.9 To prevent premature closure, we encourage hospitalists to perform an analytic “double-check” before determining their final diagnosis.
Furthermore, the clinical reasoning literature suggests that knowledge and its organization are key to expert performance.10 In diagnostic reasoning, this key knowledge has been termed “illness scripts.” Thus, the task of the aspiring expert diagnostician is to learn the key features of diseases and focus on discriminating features, starting with typical presentations of common diseases and working up to atypical presentations of uncommon diseases.
Engaging in deliberate practice, seeking feedback on diagnostic accuracy, and reflecting upon your own reasoning process can provide valuable information for improving future diagnostic reasoning. The ultimate goal of these practices is to enhance diagnostic skills in order to avoid errors and improve patient care.
Bottom Line
Diagnosis is a challenging task. Diagnostic accuracy may be enhanced by expanding the learner’s knowledge of illness scripts and using an analytic double-check to confirm initial diagnoses determined by nonanalytic reasoning.
Drs. Rendon, Roesch, and Rao are hospitalists in the department of internal medicine at the University of New Mexico School of Medicine in Albuquerque. Dr. Rencic is a hospitalist in the department of internal medicine at Tufts University School of Medicine in Boston.
References
- Eva KW. What every teacher needs to know about clinical reasoning. Med Educ. 2005;39(1):98-106.
- Bowen JL. Educational strategies to promote clinical diagnostic reasoning. N Engl J Med. 2006;355(21):2217-2225.
- Nendaz MR, Bordage G. Promoting diagnostic problem representation. Med Educ. 2002;36(8):760-766.
- Norman GR, Eva KW. Diagnostic error and clinical reasoning. Med Educ. 2010;44(1):94-100.
- Schön DA. The Reflective Practitioner: How Professionals Think in Action. London: Temple Smith; 1983.
- Croskerry P. Achieving quality in clinical decision making: cognitive strategies and detection of bias. Acad Emerg Med. 2002;9(11):1184-1204.
- Van Someren MW, Burnard YF, Sandberg JAC. The Think Aloud Method: A Practical Guide to Modelling Cognitive Processes. London: Academic Press; 1994.
- Ericsson KA, Krampe RT, Tesch-Romer C. The role of deliberate practice in the acquisition of expert performance. Psychol Rev. 1993;100(3):363-406.
- Mamede S, Schmidt HG, Penaforte JC. Effects of reflective practice on the accuracy of medical diagnoses. Med Educ. 2008;42(5):468-475.
- Elstein, AS, Shulman LS, Sprafka SA. Medical Problem Solving: An Analysis of Clinical Reasoning. Cambridge, Mass.: Harvard University Press; 1978.
- Kassirer JP. Teaching clinical reasoning: case-based and coached. Acad Med. 2010;85(7):1118-1124.
- Rencic J. Twelve tips for teaching expertise in clinical reasoning. Med Teach. 2011;33(11):887-892.
Case
A 67-year-old man presents to the hospital with persistent, subjective fevers and malaise for one month, subacute onset of dyspnea, and nonproductive cough for the preceding six days. The patient is a nonsmoker, denies sick contacts, and has had no foreign travel. What would be the best approach to making the diagnosis while working to enhance diagnostic skills?
Diagnostic Reasoning
With clinical experience, making a diagnosis can become so routine that physicians might not contemplate their problem-solving strategies. Diagnostic reasoning is the process of thinking about a clinical problem to form a diagnosis. Experienced clinicians typically rely upon nonanalytic reasoning (i.e., pattern recognition) for straightforward problems, reverting to analytic reasoning if a pattern is not recognized.
The literature describes five steps in the reasoning process (see Figure 1). In the early stages of data collection, hypotheses emerge that feed back into data collection behaviors as the clinician seeks confirmatory evidence. This complex interplay between data collection and hypothesis generation/elimination leads to a more clearly defined understanding of the patient’s presentation. The synthesis of the patient’s presentation, including epidemiologic risk factors, symptoms, signs, and laboratory and radiologic studies, is called the “problem representation.” After a clinician conceives the problem representation, he or she reviews the mental representations of diseases (i.e., illness scripts) to determine hypotheses by finding disease presentations that best match the formulated problem representation (see Figure 2).
Analytic and nonanalytic reasoning. In what is known as the dual process theory, diagnostic reasoning is believed to occur both analytically and nonanalytically.1 Nonanalytic reasoning is often exemplified by rapid, subconscious “pattern recognition” and is developed through clinical experience and other nonclinical learning experiences (e.g. reading).
Conversely, analytic reasoning, the “slow,” conscious, cognitive processing, is typically utilized when a patient presentation is complicated or does not fit a known disease pattern. Clinicians apply both strategies to make diagnoses in evaluating complex cases.
In the outlined case, while the symptoms of fever and cough might lead to the diagnosis of community-acquired pneumonia (CAP), the time course seems unusually long. This atypical pattern for CAP could trigger analytic reasoning, leading to new considerations such as tuberculosis (TB).
Case Continued
On examination, the patient has severe rigors and diaphoresis, as well as a fever of 39.4°C and a heart rate of 102 bpm. Full examination discloses mild end-expiratory wheezes and bronchial breath sounds in the right lower lobe. The remainder of his examination is normal. Labs reveal WBC 8.5x103, hemoglobin 11g/dL, MCV of 92 fL, and platelet count 22,000 mm3. Blood cultures, sputum cultures, and respiratory virus microarray are normal. The chest X-ray (CXR) is unremarkable.
Further history reveals that the patient is a sheepherder living in a primitive earthen structure in the rural mountains of western New Mexico.
Problem representation revisited. With additional historical, laboratory, and radiological data collected, further interpretation and synthesis occur. Salient elements are highlighted and prioritized, irrelevant details are discarded, and data of uncertain relevance are reevaluated as additional data are gathered. The problem representation—an interpreted, subjective mental model of a patient’s clinical presentation—is updated and reformulated. The verbal expression of the problem representation is variously called the assessment, summary statement, or “one-liner.” Within this summary statement, and fundamental to the creation of a strong problem representation, is the incorporation of “semantic qualifiers.”
“Semantic qualifiers” (e.g. acute vs. chronic or unremitting vs. relapsing) are paired, opposing descriptive adjectives that can be used to compare and contrast diagnostic considerations.² Clinicians distinguish between diseases using key signs and symptoms and use these descriptors to assist with this discrimination in hypothesis generation. An example for this patient would be: A 67-year-old sheepherder living in rural New Mexico presents with persistent fevers and malaise for one month, along with subacute development of nonproductive cough and dyspnea, sepsis, anemia, and thrombocytopenia.
Note how the incorporation of epidemiologic information (sheepherder living in an earthen structure in rural New Mexico) creates a context in which the additional problems can be framed (persistent malaise, subacute cough). In this case, the persistent fevers help the clinician to narrow possibilities in the differential diagnosis and create focused hypotheses.
Although the benefit of teaching accurate and thorough problem representation seems self-evident, studies have not demonstrated that improved problem representation enhances diagnostic accuracy; however, we believe that there is still value in adapting and teaching this skill.3
Hypothesis refinement and the differential diagnosis. Initial hypotheses occur early in data collection, as the patient’s history and physical examination findings trigger connections to clinicians’ bank of known diseases (e.g. orthopnea triggers congestive heart failure). As the clinician collects additional data, he or she refines these hypotheses, changing the likelihood based on “fit” of the problem representation with known diseases or illness scripts.
When employing analytic reasoning processes, clinicians may benefit from using organizational frameworks to assist with hypothesis generation (see Table 1). For this patient, possible hypotheses could include CAP, TB, lymphoma, lung neoplasm, or other indolent pulmonary infection.
Illness scripts. Once discrete hypotheses (e.g. CAP, pulmonary embolism) have been generated, clinicians need a method to accurately compare disease processes. This can be done through the use of an illness script. Illness scripts are mental representations of diseases and are likely to include epidemiology, typical and atypical patterns of presentation, and distinguishing features.
For example, a clinician’s illness script for a typical presentation of bacterial CAP likely includes fever, productive cough, pleuritic chest discomfort, and infiltrate on CXR. Clinician educators who teach illness scripts should ensure that students understand that diseases have atypical presentations, even though they may only teach them the prototypical one. Conceptualizing diseases in this fashion allows clinicians to seek the disease with the “script” that best matches the patient’s story (i.e., clinical presentation).
In this case, the clinician is now thinking of causes of persistent fever + nonproductive cough + dyspnea + anemia + thrombocytopenia; possibilities include lymphoma or unusual infection (e.g. tick-borne relapsing fever, or TBRF).
Case Resolution and Script Selection
As the clinician processes the case, a known illness script of TBRF matches the patient’s clinical presentation, and a peripheral smear is ordered. The smear reveals presence of spirochetal organisms, later confirmed by PCR to be Borrelia hermsii, confirming the diagnosis of TBRF.
Errors in Clinical Reasoning
Although most clinicians are quite accurate in typical presentations of common diseases, they are more likely to commit diagnostic errors when faced with uncommon diseases, atypical presentations, and/or challenging contexts. The following sections categorize a selection of some common errors and offer some expert opinion from the literature on avoiding them.
Common diagnostic errors. Clinicians use heuristics, or mental shortcuts, which can occasionally induce diagnostic errors. By definition, the fundamental problem in all diagnostic error is premature closure, or acceptance of a diagnosis before it is fully verified. In the case presented, the clinician may have accepted the diagnosis of CAP without recognizing other possible diagnoses.
Two common heuristics/biases that can sometimes lead to premature closure are the availability and anchoring biases. Availability bias means that the diagnoses easily thought of—and often most recent in the memory—are more likely to be assigned to a patient problem. The diagnosis of pulmonary embolism would be more “available” in a patient with fever, dyspnea, and normal CXR, especially if the clinician recently had seen a patient with PE. Anchoring bias occurs when early information is relied upon to make clinical judgments and the clinician fixates on a diagnosis despite acquiring additional or contrary information. For example, a clinician may rely upon a diagnosis of CAP based on the sign-out from a colleague, despite the one-month history of symptoms, rather than broadening the differential.
Clinician-focused methods to reduce diagnostic errors. Multiple methods exist that may mitigate diagnostic errors, although definitive proof of their value is still lacking, owing to the difficulty involved in studying such errors due to the multitude of causes.4 In our opinion, building a mental database of illness scripts by reading and seeing patients, as well as being metacognitive, are the best methods for individual clinicians to use to reduce their errors (see “deliberate practice” below).
Metacognition, or thinking about one’s thinking, is another method of reducing errors and can be characterized by “reflection in action” (reflection in real time) and “reflection on action” (reflection after an event).5 For example, taking a few moments at the end of a week on clinical service to reflect on the hospital course and diagnostic paths of the most complex patient presentations (reflection on action) is an exercise used to reduce errors.
For reflection in action, a clinician may pause when confronted with paradoxical findings for a current patient’s presentation (e.g. elevated jugular veinous pressure and crackles on exam but normal b-type natriuretic peptide), and “think aloud” (see below) to ensure he or she is processing all of the appropriate elements of the case.5
In the case presented above, the time course might have initiated reflection into erroneous decision-making at the moment the clinician thought that CAP was a possibility (reflection in action). Although direct evidence is inconclusive as to whether these techniques improve diagnostic accuracy, engaging in metacognitive exercises remains a cornerstone of seasoned clinical reasoning experts.6
Teaching and Learning Principles
Making a commitment. During a patient presentation, it is often helpful to ask a learner to develop a two- to four-item prioritized differential diagnosis list based on likelihood and/or lethality. Have the learner describe which diagnosis is most likely (i.e., the working diagnosis), in addition to the reasons “for” or “against” certain hypotheses. Once the diagnosis has been determined, combine commitment with an exercise in metacognition by asking the learner, “Why do you think that your initial diagnosis of Q-fever was incorrect?” Clinical educators may then follow up with teaching pearls and their approach to this type of case (see Table 1).
Think aloud: In this method, an instructor expresses his or her thoughts aloud in real time.7 By modeling this technique, attending physicians allow learners to observe the process of developing a differential diagnosis and plan. For example, during the admission process, instructors could verbalize their approach to fever in a systematic fashion (see Table 1) after the trainee has completed the presentation: “At this point I am considering an infectious cause such as pneumonia, given the respiratory symptoms, although the one-month history of fever and malaise makes me think that I should keep neoplasm and an unusual infection on my list of possibilities.”
Conversely, instructors can ask trainees to voice their thoughts aloud to better understand their reasoning processes. By using this method, instructors can also support, correct, or reinforce the trainees’ appropriate use of knowledge in the clinical reasoning process.
Deliberate practice. To improve diagnostic skills, trainees must engage in deliberate practice, defined as intentional, repetitious practice aimed at improving performance.8 To facilitate this, a trainee should evaluate as many patients as possible and present to an experienced clinician with subsequent feedback. Trainees are likely to miss subtle historical or examination points (e.g. the history of sheepherding) because their illness scripts are limited or incompletely developed. Teachers should emphasize the importance of developing broad and deep illness scripts, so learners will, hopefully, become more aware of their limitations and recognize what they do not know.
Key Takeaways
Clinicians solve diagnostic problems using both nonanalytic and analytic reasoning processes. Although evidence is inconclusive, some clinical reasoning experts suggest the use of reflective strategies to enhance diagnostic accuracy, especially in complicated cases.9 To prevent premature closure, we encourage hospitalists to perform an analytic “double-check” before determining their final diagnosis.
Furthermore, the clinical reasoning literature suggests that knowledge and its organization are key to expert performance.10 In diagnostic reasoning, this key knowledge has been termed “illness scripts.” Thus, the task of the aspiring expert diagnostician is to learn the key features of diseases and focus on discriminating features, starting with typical presentations of common diseases and working up to atypical presentations of uncommon diseases.
Engaging in deliberate practice, seeking feedback on diagnostic accuracy, and reflecting upon your own reasoning process can provide valuable information for improving future diagnostic reasoning. The ultimate goal of these practices is to enhance diagnostic skills in order to avoid errors and improve patient care.
Bottom Line
Diagnosis is a challenging task. Diagnostic accuracy may be enhanced by expanding the learner’s knowledge of illness scripts and using an analytic double-check to confirm initial diagnoses determined by nonanalytic reasoning.
Drs. Rendon, Roesch, and Rao are hospitalists in the department of internal medicine at the University of New Mexico School of Medicine in Albuquerque. Dr. Rencic is a hospitalist in the department of internal medicine at Tufts University School of Medicine in Boston.
References
- Eva KW. What every teacher needs to know about clinical reasoning. Med Educ. 2005;39(1):98-106.
- Bowen JL. Educational strategies to promote clinical diagnostic reasoning. N Engl J Med. 2006;355(21):2217-2225.
- Nendaz MR, Bordage G. Promoting diagnostic problem representation. Med Educ. 2002;36(8):760-766.
- Norman GR, Eva KW. Diagnostic error and clinical reasoning. Med Educ. 2010;44(1):94-100.
- Schön DA. The Reflective Practitioner: How Professionals Think in Action. London: Temple Smith; 1983.
- Croskerry P. Achieving quality in clinical decision making: cognitive strategies and detection of bias. Acad Emerg Med. 2002;9(11):1184-1204.
- Van Someren MW, Burnard YF, Sandberg JAC. The Think Aloud Method: A Practical Guide to Modelling Cognitive Processes. London: Academic Press; 1994.
- Ericsson KA, Krampe RT, Tesch-Romer C. The role of deliberate practice in the acquisition of expert performance. Psychol Rev. 1993;100(3):363-406.
- Mamede S, Schmidt HG, Penaforte JC. Effects of reflective practice on the accuracy of medical diagnoses. Med Educ. 2008;42(5):468-475.
- Elstein, AS, Shulman LS, Sprafka SA. Medical Problem Solving: An Analysis of Clinical Reasoning. Cambridge, Mass.: Harvard University Press; 1978.
- Kassirer JP. Teaching clinical reasoning: case-based and coached. Acad Med. 2010;85(7):1118-1124.
- Rencic J. Twelve tips for teaching expertise in clinical reasoning. Med Teach. 2011;33(11):887-892.
Case
A 67-year-old man presents to the hospital with persistent, subjective fevers and malaise for one month, subacute onset of dyspnea, and nonproductive cough for the preceding six days. The patient is a nonsmoker, denies sick contacts, and has had no foreign travel. What would be the best approach to making the diagnosis while working to enhance diagnostic skills?
Diagnostic Reasoning
With clinical experience, making a diagnosis can become so routine that physicians might not contemplate their problem-solving strategies. Diagnostic reasoning is the process of thinking about a clinical problem to form a diagnosis. Experienced clinicians typically rely upon nonanalytic reasoning (i.e., pattern recognition) for straightforward problems, reverting to analytic reasoning if a pattern is not recognized.
The literature describes five steps in the reasoning process (see Figure 1). In the early stages of data collection, hypotheses emerge that feed back into data collection behaviors as the clinician seeks confirmatory evidence. This complex interplay between data collection and hypothesis generation/elimination leads to a more clearly defined understanding of the patient’s presentation. The synthesis of the patient’s presentation, including epidemiologic risk factors, symptoms, signs, and laboratory and radiologic studies, is called the “problem representation.” After a clinician conceives the problem representation, he or she reviews the mental representations of diseases (i.e., illness scripts) to determine hypotheses by finding disease presentations that best match the formulated problem representation (see Figure 2).
Analytic and nonanalytic reasoning. In what is known as the dual process theory, diagnostic reasoning is believed to occur both analytically and nonanalytically.1 Nonanalytic reasoning is often exemplified by rapid, subconscious “pattern recognition” and is developed through clinical experience and other nonclinical learning experiences (e.g. reading).
Conversely, analytic reasoning, the “slow,” conscious, cognitive processing, is typically utilized when a patient presentation is complicated or does not fit a known disease pattern. Clinicians apply both strategies to make diagnoses in evaluating complex cases.
In the outlined case, while the symptoms of fever and cough might lead to the diagnosis of community-acquired pneumonia (CAP), the time course seems unusually long. This atypical pattern for CAP could trigger analytic reasoning, leading to new considerations such as tuberculosis (TB).
Case Continued
On examination, the patient has severe rigors and diaphoresis, as well as a fever of 39.4°C and a heart rate of 102 bpm. Full examination discloses mild end-expiratory wheezes and bronchial breath sounds in the right lower lobe. The remainder of his examination is normal. Labs reveal WBC 8.5x103, hemoglobin 11g/dL, MCV of 92 fL, and platelet count 22,000 mm3. Blood cultures, sputum cultures, and respiratory virus microarray are normal. The chest X-ray (CXR) is unremarkable.
Further history reveals that the patient is a sheepherder living in a primitive earthen structure in the rural mountains of western New Mexico.
Problem representation revisited. With additional historical, laboratory, and radiological data collected, further interpretation and synthesis occur. Salient elements are highlighted and prioritized, irrelevant details are discarded, and data of uncertain relevance are reevaluated as additional data are gathered. The problem representation—an interpreted, subjective mental model of a patient’s clinical presentation—is updated and reformulated. The verbal expression of the problem representation is variously called the assessment, summary statement, or “one-liner.” Within this summary statement, and fundamental to the creation of a strong problem representation, is the incorporation of “semantic qualifiers.”
“Semantic qualifiers” (e.g. acute vs. chronic or unremitting vs. relapsing) are paired, opposing descriptive adjectives that can be used to compare and contrast diagnostic considerations.² Clinicians distinguish between diseases using key signs and symptoms and use these descriptors to assist with this discrimination in hypothesis generation. An example for this patient would be: A 67-year-old sheepherder living in rural New Mexico presents with persistent fevers and malaise for one month, along with subacute development of nonproductive cough and dyspnea, sepsis, anemia, and thrombocytopenia.
Note how the incorporation of epidemiologic information (sheepherder living in an earthen structure in rural New Mexico) creates a context in which the additional problems can be framed (persistent malaise, subacute cough). In this case, the persistent fevers help the clinician to narrow possibilities in the differential diagnosis and create focused hypotheses.
Although the benefit of teaching accurate and thorough problem representation seems self-evident, studies have not demonstrated that improved problem representation enhances diagnostic accuracy; however, we believe that there is still value in adapting and teaching this skill.3
Hypothesis refinement and the differential diagnosis. Initial hypotheses occur early in data collection, as the patient’s history and physical examination findings trigger connections to clinicians’ bank of known diseases (e.g. orthopnea triggers congestive heart failure). As the clinician collects additional data, he or she refines these hypotheses, changing the likelihood based on “fit” of the problem representation with known diseases or illness scripts.
When employing analytic reasoning processes, clinicians may benefit from using organizational frameworks to assist with hypothesis generation (see Table 1). For this patient, possible hypotheses could include CAP, TB, lymphoma, lung neoplasm, or other indolent pulmonary infection.
Illness scripts. Once discrete hypotheses (e.g. CAP, pulmonary embolism) have been generated, clinicians need a method to accurately compare disease processes. This can be done through the use of an illness script. Illness scripts are mental representations of diseases and are likely to include epidemiology, typical and atypical patterns of presentation, and distinguishing features.
For example, a clinician’s illness script for a typical presentation of bacterial CAP likely includes fever, productive cough, pleuritic chest discomfort, and infiltrate on CXR. Clinician educators who teach illness scripts should ensure that students understand that diseases have atypical presentations, even though they may only teach them the prototypical one. Conceptualizing diseases in this fashion allows clinicians to seek the disease with the “script” that best matches the patient’s story (i.e., clinical presentation).
In this case, the clinician is now thinking of causes of persistent fever + nonproductive cough + dyspnea + anemia + thrombocytopenia; possibilities include lymphoma or unusual infection (e.g. tick-borne relapsing fever, or TBRF).
Case Resolution and Script Selection
As the clinician processes the case, a known illness script of TBRF matches the patient’s clinical presentation, and a peripheral smear is ordered. The smear reveals presence of spirochetal organisms, later confirmed by PCR to be Borrelia hermsii, confirming the diagnosis of TBRF.
Errors in Clinical Reasoning
Although most clinicians are quite accurate in typical presentations of common diseases, they are more likely to commit diagnostic errors when faced with uncommon diseases, atypical presentations, and/or challenging contexts. The following sections categorize a selection of some common errors and offer some expert opinion from the literature on avoiding them.
Common diagnostic errors. Clinicians use heuristics, or mental shortcuts, which can occasionally induce diagnostic errors. By definition, the fundamental problem in all diagnostic error is premature closure, or acceptance of a diagnosis before it is fully verified. In the case presented, the clinician may have accepted the diagnosis of CAP without recognizing other possible diagnoses.
Two common heuristics/biases that can sometimes lead to premature closure are the availability and anchoring biases. Availability bias means that the diagnoses easily thought of—and often most recent in the memory—are more likely to be assigned to a patient problem. The diagnosis of pulmonary embolism would be more “available” in a patient with fever, dyspnea, and normal CXR, especially if the clinician recently had seen a patient with PE. Anchoring bias occurs when early information is relied upon to make clinical judgments and the clinician fixates on a diagnosis despite acquiring additional or contrary information. For example, a clinician may rely upon a diagnosis of CAP based on the sign-out from a colleague, despite the one-month history of symptoms, rather than broadening the differential.
Clinician-focused methods to reduce diagnostic errors. Multiple methods exist that may mitigate diagnostic errors, although definitive proof of their value is still lacking, owing to the difficulty involved in studying such errors due to the multitude of causes.4 In our opinion, building a mental database of illness scripts by reading and seeing patients, as well as being metacognitive, are the best methods for individual clinicians to use to reduce their errors (see “deliberate practice” below).
Metacognition, or thinking about one’s thinking, is another method of reducing errors and can be characterized by “reflection in action” (reflection in real time) and “reflection on action” (reflection after an event).5 For example, taking a few moments at the end of a week on clinical service to reflect on the hospital course and diagnostic paths of the most complex patient presentations (reflection on action) is an exercise used to reduce errors.
For reflection in action, a clinician may pause when confronted with paradoxical findings for a current patient’s presentation (e.g. elevated jugular veinous pressure and crackles on exam but normal b-type natriuretic peptide), and “think aloud” (see below) to ensure he or she is processing all of the appropriate elements of the case.5
In the case presented above, the time course might have initiated reflection into erroneous decision-making at the moment the clinician thought that CAP was a possibility (reflection in action). Although direct evidence is inconclusive as to whether these techniques improve diagnostic accuracy, engaging in metacognitive exercises remains a cornerstone of seasoned clinical reasoning experts.6
Teaching and Learning Principles
Making a commitment. During a patient presentation, it is often helpful to ask a learner to develop a two- to four-item prioritized differential diagnosis list based on likelihood and/or lethality. Have the learner describe which diagnosis is most likely (i.e., the working diagnosis), in addition to the reasons “for” or “against” certain hypotheses. Once the diagnosis has been determined, combine commitment with an exercise in metacognition by asking the learner, “Why do you think that your initial diagnosis of Q-fever was incorrect?” Clinical educators may then follow up with teaching pearls and their approach to this type of case (see Table 1).
Think aloud: In this method, an instructor expresses his or her thoughts aloud in real time.7 By modeling this technique, attending physicians allow learners to observe the process of developing a differential diagnosis and plan. For example, during the admission process, instructors could verbalize their approach to fever in a systematic fashion (see Table 1) after the trainee has completed the presentation: “At this point I am considering an infectious cause such as pneumonia, given the respiratory symptoms, although the one-month history of fever and malaise makes me think that I should keep neoplasm and an unusual infection on my list of possibilities.”
Conversely, instructors can ask trainees to voice their thoughts aloud to better understand their reasoning processes. By using this method, instructors can also support, correct, or reinforce the trainees’ appropriate use of knowledge in the clinical reasoning process.
Deliberate practice. To improve diagnostic skills, trainees must engage in deliberate practice, defined as intentional, repetitious practice aimed at improving performance.8 To facilitate this, a trainee should evaluate as many patients as possible and present to an experienced clinician with subsequent feedback. Trainees are likely to miss subtle historical or examination points (e.g. the history of sheepherding) because their illness scripts are limited or incompletely developed. Teachers should emphasize the importance of developing broad and deep illness scripts, so learners will, hopefully, become more aware of their limitations and recognize what they do not know.
Key Takeaways
Clinicians solve diagnostic problems using both nonanalytic and analytic reasoning processes. Although evidence is inconclusive, some clinical reasoning experts suggest the use of reflective strategies to enhance diagnostic accuracy, especially in complicated cases.9 To prevent premature closure, we encourage hospitalists to perform an analytic “double-check” before determining their final diagnosis.
Furthermore, the clinical reasoning literature suggests that knowledge and its organization are key to expert performance.10 In diagnostic reasoning, this key knowledge has been termed “illness scripts.” Thus, the task of the aspiring expert diagnostician is to learn the key features of diseases and focus on discriminating features, starting with typical presentations of common diseases and working up to atypical presentations of uncommon diseases.
Engaging in deliberate practice, seeking feedback on diagnostic accuracy, and reflecting upon your own reasoning process can provide valuable information for improving future diagnostic reasoning. The ultimate goal of these practices is to enhance diagnostic skills in order to avoid errors and improve patient care.
Bottom Line
Diagnosis is a challenging task. Diagnostic accuracy may be enhanced by expanding the learner’s knowledge of illness scripts and using an analytic double-check to confirm initial diagnoses determined by nonanalytic reasoning.
Drs. Rendon, Roesch, and Rao are hospitalists in the department of internal medicine at the University of New Mexico School of Medicine in Albuquerque. Dr. Rencic is a hospitalist in the department of internal medicine at Tufts University School of Medicine in Boston.
References
- Eva KW. What every teacher needs to know about clinical reasoning. Med Educ. 2005;39(1):98-106.
- Bowen JL. Educational strategies to promote clinical diagnostic reasoning. N Engl J Med. 2006;355(21):2217-2225.
- Nendaz MR, Bordage G. Promoting diagnostic problem representation. Med Educ. 2002;36(8):760-766.
- Norman GR, Eva KW. Diagnostic error and clinical reasoning. Med Educ. 2010;44(1):94-100.
- Schön DA. The Reflective Practitioner: How Professionals Think in Action. London: Temple Smith; 1983.
- Croskerry P. Achieving quality in clinical decision making: cognitive strategies and detection of bias. Acad Emerg Med. 2002;9(11):1184-1204.
- Van Someren MW, Burnard YF, Sandberg JAC. The Think Aloud Method: A Practical Guide to Modelling Cognitive Processes. London: Academic Press; 1994.
- Ericsson KA, Krampe RT, Tesch-Romer C. The role of deliberate practice in the acquisition of expert performance. Psychol Rev. 1993;100(3):363-406.
- Mamede S, Schmidt HG, Penaforte JC. Effects of reflective practice on the accuracy of medical diagnoses. Med Educ. 2008;42(5):468-475.
- Elstein, AS, Shulman LS, Sprafka SA. Medical Problem Solving: An Analysis of Clinical Reasoning. Cambridge, Mass.: Harvard University Press; 1978.
- Kassirer JP. Teaching clinical reasoning: case-based and coached. Acad Med. 2010;85(7):1118-1124.
- Rencic J. Twelve tips for teaching expertise in clinical reasoning. Med Teach. 2011;33(11):887-892.