User login
Assessing Treatment Delays for Vitiligo Patients: A Retrospective Chart Review
Similar to other dermatologic conditions, barriers to early care in patients with vitiligo can exacerbate health disparities.1 Delayed treatment of vitiligo is known to hamper successful disease stabilization and repigmentation, as therapies tend to work more effectively in early stages of the disease.2
To investigate the factors associated with treatment delays for patients with vitiligo, we conducted a retrospective chart review of 102 consecutive patients with vitiligo attending an academic outpatient clinic in Austin, Texas, over 36 months.
Methods
Our sample included 102 consecutive patients with vitiligo who attended an academic outpatient clinic in Austin, Texas, from January 2017 to January 2020. Demographic information, clinical characteristics of vitiligo, and treatment data were self-reported via a standardized questionnaire given to all patients with vitiligo and gathered from medical chart review. Patient characteristics are outlined in the Table. The delay to treatment was the time (in months) from the date the patient first noticed the lesion to the start date of first treatment. This retrospective chart review was reviewed by the University of Texas at Austin institutional review board and was determined to be exempt.
Statistical Analysis—The data were analyzed descriptively with a Wilcoxon rank sum test (type I error rate of .05).
Results
Of the 102 charts that were analyzed, 45 were females and 57 were males. More than half of the patients (54.9% [56/102]) were White. Sixteen were Asian, 13 were Hispanic non-White, 11 were Black/African American, and 4 were American Indian/Alaska Native. The median age of disease onset was 21 years, minimum age was 1 year, and maximum age was 83 years. The diagnosis of vitiligo was made by a dermatologist for 72 patients and by a physician of another specialty for 20 patients. Ten patients did not declare the specialty of the diagnosing physician.
Individuals older than 21 years when their disease started had a shorter delay to treatment than individuals who noticed their first lesion at an age younger than 21 years (median, 75 months vs 13 months; P<.01). Individuals diagnosed by a dermatologist had a shorter delay to treatment than individuals diagnosed by a physician of another specialty (median, 13 months vs 58 months; P<.05). White individuals had a shorter delay to treatment than individuals with skin of color (median, 13 months vs 31 months; P=.08), though this trend did not reach statistical significance. Individuals with 1% to 25% of body surface area (BSA) affected at time of presentation to clinic also had a shorter delay to treatment than those with a greater BSA affected (median, 13 months vs 74 months; P<.06), though this trend did not reach statistical significance. Type of vitiligo (P<.8), Fitzpatrick skin type (P<.6), and smoking status (P<.7) were not associated with differential delays.
Comment
Impact of Age on Vitiligo Treatment—Our data suggest that individuals who develop vitiligo at a younger age experience longer treatment delays compared to older individuals. Reasons for this are uncertain but could include access issues, medical decision-making agency, and younger patients not remembering being treated during their youth. Our data also could be influenced by some of the adult patients in our study first noticing their lesions many years ago when treatments for vitiligo were more limited. Nevertheless, detrimental effects on quality of life in children and adolescents with vitiligo suggest that motivating younger individuals with vitiligo to seek treatment or proactively making them aware of treatment opportunities may be beneficial.3
Diagnosis of Vitiligo by Nondermatologists—The increase in delay to treatment when a nondermatologist diagnoses vitiligo suggests that prompt initiation of treatment or referrals to dermatology by primary care providers may not routinely be occurring.4 Our data indicate the need to educate primary care providers on treatment opportunities for individuals with vitiligo and that early treatment generally is more effective.5
Impact of Race/Ethnicity on Vitiligo Treatment—Our data also show trends for longer treatment delays for individuals with skin of color. Although this did not reach statistical significance, we hope future studies will investigate this issue, especially because patients with skin of color experience more stigmatization and quality-of-life impacts by vitiligo than White patients.5
Impact of BSA on Vitiligo Treatment—Our data show that patients with a smaller BSA had a shorter delay to treatment than those with a greater BSA affected. This was a unique finding given it initially was hypothesized that patients with greater BSA would seek treatment earlier because of the associated increase in quality of life impact. This trend was not statistically significant, but further investigation would be helpful to analyze the reason behind treatment delays in patients with greater BSA affected.
Conclusion
The delay to treatment in our study population was correlated with the diagnosing physician’s specialty and patient age at disease onset, with trends also observed for race and BSA affected. These findings emphasize the need to investigate specific causes of barriers to early care to promote health equity among individuals with vitiligo.
- Tripathi R, Archibald LK, Mazmudar RS, et al. Racial differences in time to treatment for melanoma. J Am Acad Dermatol. 2020;83:854-859.
- Boniface K, Seneschal J. Vitiligo as a skin memory disease: the need for early intervention with immunomodulating agents and a maintenance therapy to target resident memory T cells. Exp Dermatol. 2019;28:656-661.
- Silverberg JI, Silverberg NB. Quality of life impairment in children and adolescents with vitiligo. Pediatr Dermatol. 2014;31:309-318.
- Amer AA, Gao XH. Quality of life in patients with vitiligo: an analysis of the dermatology life quality index outcome over the past two decades. Int J Dermatol. 2016;55:608-614.
- Weibel L, Laguda B, Atherton D, et al. Misdiagnosis and delay in referral of children with localized scleroderma. Br J Dermatol. 2011;165:1308-1313.
Similar to other dermatologic conditions, barriers to early care in patients with vitiligo can exacerbate health disparities.1 Delayed treatment of vitiligo is known to hamper successful disease stabilization and repigmentation, as therapies tend to work more effectively in early stages of the disease.2
To investigate the factors associated with treatment delays for patients with vitiligo, we conducted a retrospective chart review of 102 consecutive patients with vitiligo attending an academic outpatient clinic in Austin, Texas, over 36 months.
Methods
Our sample included 102 consecutive patients with vitiligo who attended an academic outpatient clinic in Austin, Texas, from January 2017 to January 2020. Demographic information, clinical characteristics of vitiligo, and treatment data were self-reported via a standardized questionnaire given to all patients with vitiligo and gathered from medical chart review. Patient characteristics are outlined in the Table. The delay to treatment was the time (in months) from the date the patient first noticed the lesion to the start date of first treatment. This retrospective chart review was reviewed by the University of Texas at Austin institutional review board and was determined to be exempt.
Statistical Analysis—The data were analyzed descriptively with a Wilcoxon rank sum test (type I error rate of .05).
Results
Of the 102 charts that were analyzed, 45 were females and 57 were males. More than half of the patients (54.9% [56/102]) were White. Sixteen were Asian, 13 were Hispanic non-White, 11 were Black/African American, and 4 were American Indian/Alaska Native. The median age of disease onset was 21 years, minimum age was 1 year, and maximum age was 83 years. The diagnosis of vitiligo was made by a dermatologist for 72 patients and by a physician of another specialty for 20 patients. Ten patients did not declare the specialty of the diagnosing physician.
Individuals older than 21 years when their disease started had a shorter delay to treatment than individuals who noticed their first lesion at an age younger than 21 years (median, 75 months vs 13 months; P<.01). Individuals diagnosed by a dermatologist had a shorter delay to treatment than individuals diagnosed by a physician of another specialty (median, 13 months vs 58 months; P<.05). White individuals had a shorter delay to treatment than individuals with skin of color (median, 13 months vs 31 months; P=.08), though this trend did not reach statistical significance. Individuals with 1% to 25% of body surface area (BSA) affected at time of presentation to clinic also had a shorter delay to treatment than those with a greater BSA affected (median, 13 months vs 74 months; P<.06), though this trend did not reach statistical significance. Type of vitiligo (P<.8), Fitzpatrick skin type (P<.6), and smoking status (P<.7) were not associated with differential delays.
Comment
Impact of Age on Vitiligo Treatment—Our data suggest that individuals who develop vitiligo at a younger age experience longer treatment delays compared to older individuals. Reasons for this are uncertain but could include access issues, medical decision-making agency, and younger patients not remembering being treated during their youth. Our data also could be influenced by some of the adult patients in our study first noticing their lesions many years ago when treatments for vitiligo were more limited. Nevertheless, detrimental effects on quality of life in children and adolescents with vitiligo suggest that motivating younger individuals with vitiligo to seek treatment or proactively making them aware of treatment opportunities may be beneficial.3
Diagnosis of Vitiligo by Nondermatologists—The increase in delay to treatment when a nondermatologist diagnoses vitiligo suggests that prompt initiation of treatment or referrals to dermatology by primary care providers may not routinely be occurring.4 Our data indicate the need to educate primary care providers on treatment opportunities for individuals with vitiligo and that early treatment generally is more effective.5
Impact of Race/Ethnicity on Vitiligo Treatment—Our data also show trends for longer treatment delays for individuals with skin of color. Although this did not reach statistical significance, we hope future studies will investigate this issue, especially because patients with skin of color experience more stigmatization and quality-of-life impacts by vitiligo than White patients.5
Impact of BSA on Vitiligo Treatment—Our data show that patients with a smaller BSA had a shorter delay to treatment than those with a greater BSA affected. This was a unique finding given it initially was hypothesized that patients with greater BSA would seek treatment earlier because of the associated increase in quality of life impact. This trend was not statistically significant, but further investigation would be helpful to analyze the reason behind treatment delays in patients with greater BSA affected.
Conclusion
The delay to treatment in our study population was correlated with the diagnosing physician’s specialty and patient age at disease onset, with trends also observed for race and BSA affected. These findings emphasize the need to investigate specific causes of barriers to early care to promote health equity among individuals with vitiligo.
Similar to other dermatologic conditions, barriers to early care in patients with vitiligo can exacerbate health disparities.1 Delayed treatment of vitiligo is known to hamper successful disease stabilization and repigmentation, as therapies tend to work more effectively in early stages of the disease.2
To investigate the factors associated with treatment delays for patients with vitiligo, we conducted a retrospective chart review of 102 consecutive patients with vitiligo attending an academic outpatient clinic in Austin, Texas, over 36 months.
Methods
Our sample included 102 consecutive patients with vitiligo who attended an academic outpatient clinic in Austin, Texas, from January 2017 to January 2020. Demographic information, clinical characteristics of vitiligo, and treatment data were self-reported via a standardized questionnaire given to all patients with vitiligo and gathered from medical chart review. Patient characteristics are outlined in the Table. The delay to treatment was the time (in months) from the date the patient first noticed the lesion to the start date of first treatment. This retrospective chart review was reviewed by the University of Texas at Austin institutional review board and was determined to be exempt.
Statistical Analysis—The data were analyzed descriptively with a Wilcoxon rank sum test (type I error rate of .05).
Results
Of the 102 charts that were analyzed, 45 were females and 57 were males. More than half of the patients (54.9% [56/102]) were White. Sixteen were Asian, 13 were Hispanic non-White, 11 were Black/African American, and 4 were American Indian/Alaska Native. The median age of disease onset was 21 years, minimum age was 1 year, and maximum age was 83 years. The diagnosis of vitiligo was made by a dermatologist for 72 patients and by a physician of another specialty for 20 patients. Ten patients did not declare the specialty of the diagnosing physician.
Individuals older than 21 years when their disease started had a shorter delay to treatment than individuals who noticed their first lesion at an age younger than 21 years (median, 75 months vs 13 months; P<.01). Individuals diagnosed by a dermatologist had a shorter delay to treatment than individuals diagnosed by a physician of another specialty (median, 13 months vs 58 months; P<.05). White individuals had a shorter delay to treatment than individuals with skin of color (median, 13 months vs 31 months; P=.08), though this trend did not reach statistical significance. Individuals with 1% to 25% of body surface area (BSA) affected at time of presentation to clinic also had a shorter delay to treatment than those with a greater BSA affected (median, 13 months vs 74 months; P<.06), though this trend did not reach statistical significance. Type of vitiligo (P<.8), Fitzpatrick skin type (P<.6), and smoking status (P<.7) were not associated with differential delays.
Comment
Impact of Age on Vitiligo Treatment—Our data suggest that individuals who develop vitiligo at a younger age experience longer treatment delays compared to older individuals. Reasons for this are uncertain but could include access issues, medical decision-making agency, and younger patients not remembering being treated during their youth. Our data also could be influenced by some of the adult patients in our study first noticing their lesions many years ago when treatments for vitiligo were more limited. Nevertheless, detrimental effects on quality of life in children and adolescents with vitiligo suggest that motivating younger individuals with vitiligo to seek treatment or proactively making them aware of treatment opportunities may be beneficial.3
Diagnosis of Vitiligo by Nondermatologists—The increase in delay to treatment when a nondermatologist diagnoses vitiligo suggests that prompt initiation of treatment or referrals to dermatology by primary care providers may not routinely be occurring.4 Our data indicate the need to educate primary care providers on treatment opportunities for individuals with vitiligo and that early treatment generally is more effective.5
Impact of Race/Ethnicity on Vitiligo Treatment—Our data also show trends for longer treatment delays for individuals with skin of color. Although this did not reach statistical significance, we hope future studies will investigate this issue, especially because patients with skin of color experience more stigmatization and quality-of-life impacts by vitiligo than White patients.5
Impact of BSA on Vitiligo Treatment—Our data show that patients with a smaller BSA had a shorter delay to treatment than those with a greater BSA affected. This was a unique finding given it initially was hypothesized that patients with greater BSA would seek treatment earlier because of the associated increase in quality of life impact. This trend was not statistically significant, but further investigation would be helpful to analyze the reason behind treatment delays in patients with greater BSA affected.
Conclusion
The delay to treatment in our study population was correlated with the diagnosing physician’s specialty and patient age at disease onset, with trends also observed for race and BSA affected. These findings emphasize the need to investigate specific causes of barriers to early care to promote health equity among individuals with vitiligo.
- Tripathi R, Archibald LK, Mazmudar RS, et al. Racial differences in time to treatment for melanoma. J Am Acad Dermatol. 2020;83:854-859.
- Boniface K, Seneschal J. Vitiligo as a skin memory disease: the need for early intervention with immunomodulating agents and a maintenance therapy to target resident memory T cells. Exp Dermatol. 2019;28:656-661.
- Silverberg JI, Silverberg NB. Quality of life impairment in children and adolescents with vitiligo. Pediatr Dermatol. 2014;31:309-318.
- Amer AA, Gao XH. Quality of life in patients with vitiligo: an analysis of the dermatology life quality index outcome over the past two decades. Int J Dermatol. 2016;55:608-614.
- Weibel L, Laguda B, Atherton D, et al. Misdiagnosis and delay in referral of children with localized scleroderma. Br J Dermatol. 2011;165:1308-1313.
- Tripathi R, Archibald LK, Mazmudar RS, et al. Racial differences in time to treatment for melanoma. J Am Acad Dermatol. 2020;83:854-859.
- Boniface K, Seneschal J. Vitiligo as a skin memory disease: the need for early intervention with immunomodulating agents and a maintenance therapy to target resident memory T cells. Exp Dermatol. 2019;28:656-661.
- Silverberg JI, Silverberg NB. Quality of life impairment in children and adolescents with vitiligo. Pediatr Dermatol. 2014;31:309-318.
- Amer AA, Gao XH. Quality of life in patients with vitiligo: an analysis of the dermatology life quality index outcome over the past two decades. Int J Dermatol. 2016;55:608-614.
- Weibel L, Laguda B, Atherton D, et al. Misdiagnosis and delay in referral of children with localized scleroderma. Br J Dermatol. 2011;165:1308-1313.
Practice Points
- The medical community should be aware of factors associated with delay to treatment in patients with vitiligo, such as the diagnosing physician’s specialty and patient age at disease onset.
- Race and percentage of body surface area affected at time of presentation also demonstrate trends regarding treatment delays in patients with vitiligo.
A Fixed Drug Eruption to Medroxyprogesterone Acetate Injectable Suspension
To the Editor:
A fixed drug eruption (FDE) is a well-documented form of cutaneous hypersensitivity that typically manifests as a sharply demarcated, dusky, round to oval, edematous, red-violaceous macule or patch on the skin and mucous membranes. The lesion often resolves with residual postinflammatory hyperpigmentation, most commonly as a reaction to ingested drugs or drug components.1 Lesions generally occur at the same anatomic site with repeated exposure to the offending drug. Typically, a single site is affected, but additional sites with more generalized involvement have been reported to occur with subsequent exposure to the offending medication. The diagnosis usually is clinical, but histopathologic findings can help confirm the diagnosis in unusual presentations. We present a novel case of a patient with an FDE from medroxyprogesterone acetate, a contraceptive injection that contains the hormone progestin.
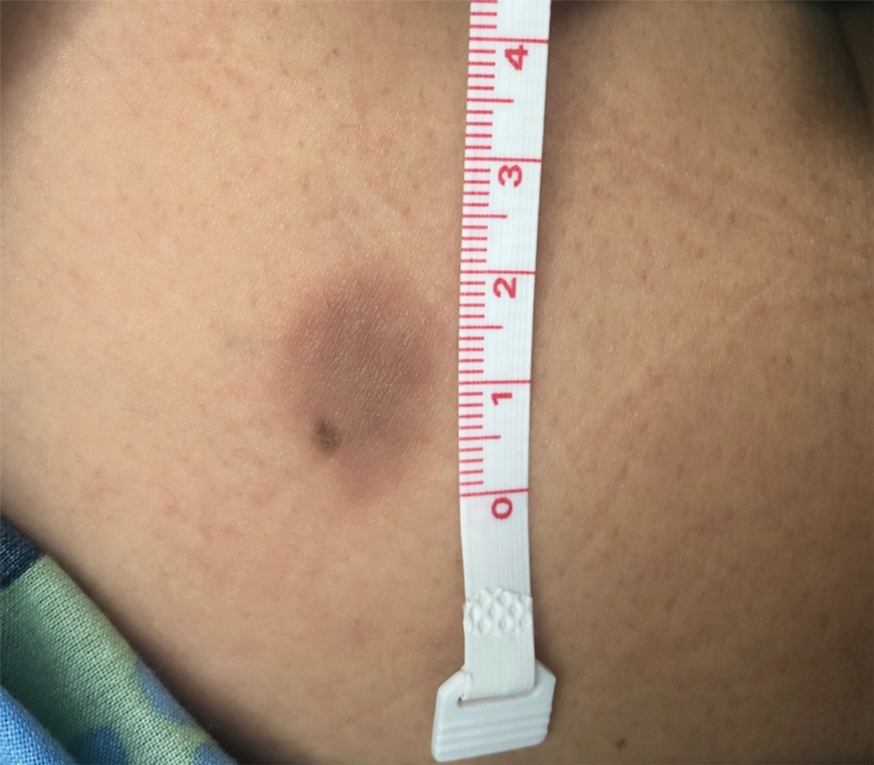
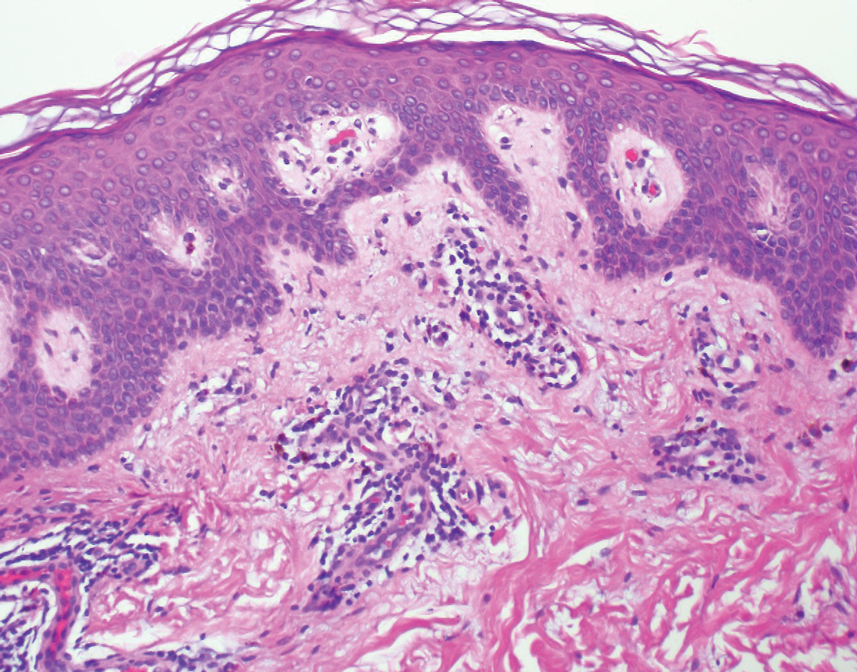
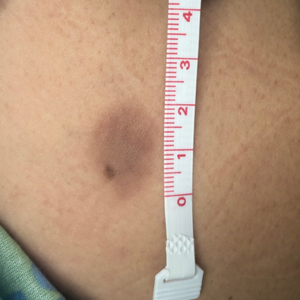
A 35-year-old woman presented to the dermatology clinic for evaluation of a lesion on the left lower buttock of 1 year’s duration. She reported periodic swelling and associated pruritus of the lesion. She denied any growth in size, and no other similar lesions were present. The patient reported a medication history of medroxyprogesterone acetate for birth control, but she denied any other prescription or over-the-counter medication, oral supplements, or recreational drug use. Upon further inquiry, she reported that the recurrence of symptoms appeared to coincide with each administration of medroxyprogesterone acetate, which occurred approximately every 3 months. The eruption cleared between injections and recurred in the same location following subsequent injections. The lesion appeared approximately 2 weeks after the first injection (approximately 1 year prior to presentation to dermatology) and within 2 to 3 days after each subsequent injection. Physical examination revealed a 2×2-cm, circular, slightly violaceous patch on the left buttock (Figure 1). A biopsy was recommended to aid in diagnosis, and the patient was offered a topical steroid for symptomatic relief. A punch biopsy revealed subtle interface dermatitis with superficial perivascular lymphoid infiltrate and marked pigmentary incontinence consistent with an FDE (Figure 2).
An FDE was first reported in 1889 by Bourns,2 and over time more implicated agents and varying clinical presentations have been linked to the disease. The FDE can be accompanied by symptoms of pruritus or paresthesia. Most cases are devoid of systemic symptoms. An FDE can be located anywhere on the body, but it most frequently manifests on the lips, face, hands, feet, and genitalia. Although the eruption often heals with residual postinflammatory hyperpigmentation, a nonpigmenting FDE due to pseudoephedrine has been reported.3
Common culprits include antibiotics (eg, sulfonamides, trimethoprim, fluoroquinolones, tetracyclines), nonsteroidal anti-inflammatory medications (eg, naproxen sodium, ibuprofen, celecoxib), barbiturates, antimalarials, and anticonvulsants. Rare cases of FDE induced by foods and food additives also have been reported.4 Oral fluconazole, levocetirizine dihydrochloride, loperamide, and multivitamin-mineral preparations are other rare inducers of FDE.5-8 In 2004, Ritter and Meffert9 described an FDE to the green dye used in inactive oral contraceptive pills. A similar case was reported by Rea et al10 that described an FDE from the inactive sugar pills in ethinyl estradiol and levonorgestrel, which is another combined oral contraceptive.
The time between ingestion of the offending agent and the manifestation of the disease usually is 1 to 2 weeks; however, upon subsequent exposure, the disease has been reported to manifest within hours.1 CD8+ memory T cells have been shown to be major players in the development of FDE and can be found along the dermoepidermal junction as part of a delayed type IV hypersensitivity reaction.11 Histopathology reveals superficial and deep interstitial and perivascular infiltrates consisting of lymphocytes with admixed eosinophils and possibly neutrophils in the dermis. In the epidermis, necrotic keratinocytes can be present. In rare cases, FDE may have atypical features, such as in generalized bullous FDE and nonpigmenting FDE, the latter of which more commonly is associated with pseudoephedrine.1
The differential diagnosis for FDE includes erythema multiforme, Stevens-Johnson syndrome/toxic epidermal necrolysis, autoimmune progesterone dermatitis, and large plaque parapsoriasis. The number and morphology of lesions in erythema multiforme help differentiate it from FDE, as erythema multiforme presents with multiple targetoid lesions. The lesions of generalized bullous FDE can be similar to those of Stevens-Johnson syndrome/toxic epidermal necrolysis, and the pigmented patches of FDE can resemble large plaque parapsoriasis.
It is important to consider any medication ingested in the 1- to 2-week period before FDE onset, including over-the-counter medications, health food supplements, and prescription medications. Discontinuation of the implicated medication or any medication potentially cross-reacting with another medication is the most important step in management. Wound care may be needed for any bullous or eroded lesions. Lesions typically resolve within a few days to weeks of stopping the offending agent. Importantly, patients should be counseled on the secondary pigment alterations that may be persistent for several months. Other treatment for FDEs is aimed at symptomatic relief and may include topical corticosteroids and oral antihistamines.1
Medroxyprogesterone acetate is a highly effective contraceptive drug with low rates of failure.12 It is a weak androgenic progestin that is administered as a single 150-mg intramuscular injection every 3 months and inhibits gonadotropins. Common side effects include local injection-site reactions, unscheduled bleeding, amenorrhea, weight gain, headache, and mood changes. However, FDE has not been reported as an adverse effect to medroxyprogesterone acetate, both in official US Food and Drug Administration information and in the current literature.12
Autoimmune progesterone dermatitis (also known as progestin hypersensitivity) is a well-characterized cyclic hypersensitivity reaction to the hormone progesterone that occurs during the luteal phase of the menstrual cycle. It is known to have a variable clinical presentation including urticaria, erythema multiforme, eczema, and angioedema.13 Autoimmune progesterone dermatitis also has been reported to present as an FDE.14-16 The onset of the cutaneous manifestation often starts a few days before the onset of menses, with spontaneous resolution occurring after the onset of menstruation. The mechanism by which endogenous progesterone or other secretory products become antigenic is unknown. It has been suggested that there is an alteration in the properties of the hormone that would predispose it to be antigenic as it would not be considered self. In 2001, Warin17 proposed the following diagnostic criteria for autoimmune progesterone dermatitis: (1) skin lesions associated with menstrual cycle (premenstrual flare); (2) a positive response to the progesterone intradermal or intramuscular test; and (3) symptomatic improvement after inhibiting progesterone secretion by suppressing ovulation.17 The treatment includes antiallergy medications, progesterone desensitization, omalizumab injection, and leuprolide acetate injection.
Our case represents FDE from medroxyprogesterone acetate. Although we did not formally investigate the antigenicity of the exogenous progesterone, we postulate that the pathophysiology likely is similar to an FDE associated with endogenous progesterone. This reasoning is supported by the time course of the patient’s lesion as well as the worsening of symptoms in the days following the administration of the medication. Additionally, the patient had no history of skin lesions prior to the initiation of medroxyprogesterone acetate or similar lesions associated with her menstrual cycles.
A careful and detailed review of medication history is necessary to evaluate FDEs. Our case emphasizes that not only endogenous but also exogenous forms of progesterone may cause hypersensitivity, leading to an FDE. With more than 2 million prescriptions of medroxyprogesterone acetate written every year, dermatologists should be aware of the rare but potential risk for an FDE in patients using this medication.18
- Bolognia J, Jorizzo JL, Rapini RP. Dermatology. 2nd ed. Mosby; 2008.
- Bourns DCG. Unusual effects of antipyrine. Br Med J. 1889;2:818-820.
- Shelley WB, Shelley ED. Nonpigmenting fixed drug eruption as a distinctive reaction pattern: examples caused by sensitivity to pseudoephedrine hydrochloride and tetrahydrozoline. J Am Acad Dermatol. 1987;17:403-407.
- Sohn KH, Kim BK, Kim JY, et al. Fixed food eruption caused by Actinidia arguta (hardy kiwi): a case report and literature review. Allergy Asthma Immunol Res. 2017;9:182-184.
- Nakai N, Katoh N. Fixed drug eruption caused by fluconazole: a case report and mini-review of the literature. Allergol Int. 2013;6:139-141.
- An I, Demir V, Ibiloglu I, et al. Fixed drug eruption induced by levocetirizine. Indian Dermatol Online J. 2017;8:276-278.
- Matarredona J, Borrás Blasco J, Navarro-Ruiz A, et al. Fixed drug eruption associated to loperamide [in Spanish]. Med Clin (Barc). 2005;124:198-199.
- Gohel D. Fixed drug eruption due to multi-vitamin multi-mineral preparation. J Assoc Physicians India. 2000;48:268.
- Ritter SE, Meffert J. A refractory fixed drug reaction to a dye used in an oral contraceptive. Cutis. 2004;74:243-244.
- Rea S, McMeniman E, Darch K, et al. A fixed drug eruption to the sugar pills of a combined oral contraceptive. Poster presented at: The Australasian College of Dermatologists 51st Annual Scientific Meeting; May 22, 2018; Queensland, Australia.
- Shiohara T, Mizukawa Y. Fixed drug eruption: a disease mediated by self-inflicted responses of intraepidermal T cells. Eur J Dermatol. 2007;17:201-208.
- Depo-Provera CI. Prescribing information. Pfizer; 2020. Accessed March 10, 2022. https://labeling.pfizer.com/ShowLabeling.aspx?format=PDF&id=522
- George R, Badawy SZ. Autoimmune progesterone dermatitis: a case report. Case Rep Obstet Gynecol. 2012;2012:757854.
- Mokhtari R, Sepaskhah M, Aslani FS, et al. Autoimmune progesterone dermatitis presenting as fixed drug eruption: a case report. Dermatol Online J. 2017;23:13030/qt685685p4.
- Asai J, Katoh N, Nakano M, et al. Case of autoimmune progesterone dermatitis presenting as fixed drug eruption. J Dermatol. 2009;36:643-645.
- Bhardwaj N, Jindal R, Chauhan P. Autoimmune progesterone dermatitis presenting as fixed drug eruption. BMJ Case Rep. 2019;12:E231873.
- Warin AP. Case 2. diagnosis: erythema multiforme as a presentation of autoimmune progesterone dermatitis. Clin Exp Dermatol. 2001;26:107-108.
- Medroxyprogesterone Drug Usage Statistics, United States, 2013-2019. ClinCalc website. Updated September 15, 2021. Accessed March 17, 2022. https://clincalc.com/DrugStats/Drugs/Medroxyprogesterone
To the Editor:
A fixed drug eruption (FDE) is a well-documented form of cutaneous hypersensitivity that typically manifests as a sharply demarcated, dusky, round to oval, edematous, red-violaceous macule or patch on the skin and mucous membranes. The lesion often resolves with residual postinflammatory hyperpigmentation, most commonly as a reaction to ingested drugs or drug components.1 Lesions generally occur at the same anatomic site with repeated exposure to the offending drug. Typically, a single site is affected, but additional sites with more generalized involvement have been reported to occur with subsequent exposure to the offending medication. The diagnosis usually is clinical, but histopathologic findings can help confirm the diagnosis in unusual presentations. We present a novel case of a patient with an FDE from medroxyprogesterone acetate, a contraceptive injection that contains the hormone progestin.
A 35-year-old woman presented to the dermatology clinic for evaluation of a lesion on the left lower buttock of 1 year’s duration. She reported periodic swelling and associated pruritus of the lesion. She denied any growth in size, and no other similar lesions were present. The patient reported a medication history of medroxyprogesterone acetate for birth control, but she denied any other prescription or over-the-counter medication, oral supplements, or recreational drug use. Upon further inquiry, she reported that the recurrence of symptoms appeared to coincide with each administration of medroxyprogesterone acetate, which occurred approximately every 3 months. The eruption cleared between injections and recurred in the same location following subsequent injections. The lesion appeared approximately 2 weeks after the first injection (approximately 1 year prior to presentation to dermatology) and within 2 to 3 days after each subsequent injection. Physical examination revealed a 2×2-cm, circular, slightly violaceous patch on the left buttock (Figure 1). A biopsy was recommended to aid in diagnosis, and the patient was offered a topical steroid for symptomatic relief. A punch biopsy revealed subtle interface dermatitis with superficial perivascular lymphoid infiltrate and marked pigmentary incontinence consistent with an FDE (Figure 2).
An FDE was first reported in 1889 by Bourns,2 and over time more implicated agents and varying clinical presentations have been linked to the disease. The FDE can be accompanied by symptoms of pruritus or paresthesia. Most cases are devoid of systemic symptoms. An FDE can be located anywhere on the body, but it most frequently manifests on the lips, face, hands, feet, and genitalia. Although the eruption often heals with residual postinflammatory hyperpigmentation, a nonpigmenting FDE due to pseudoephedrine has been reported.3
Common culprits include antibiotics (eg, sulfonamides, trimethoprim, fluoroquinolones, tetracyclines), nonsteroidal anti-inflammatory medications (eg, naproxen sodium, ibuprofen, celecoxib), barbiturates, antimalarials, and anticonvulsants. Rare cases of FDE induced by foods and food additives also have been reported.4 Oral fluconazole, levocetirizine dihydrochloride, loperamide, and multivitamin-mineral preparations are other rare inducers of FDE.5-8 In 2004, Ritter and Meffert9 described an FDE to the green dye used in inactive oral contraceptive pills. A similar case was reported by Rea et al10 that described an FDE from the inactive sugar pills in ethinyl estradiol and levonorgestrel, which is another combined oral contraceptive.
The time between ingestion of the offending agent and the manifestation of the disease usually is 1 to 2 weeks; however, upon subsequent exposure, the disease has been reported to manifest within hours.1 CD8+ memory T cells have been shown to be major players in the development of FDE and can be found along the dermoepidermal junction as part of a delayed type IV hypersensitivity reaction.11 Histopathology reveals superficial and deep interstitial and perivascular infiltrates consisting of lymphocytes with admixed eosinophils and possibly neutrophils in the dermis. In the epidermis, necrotic keratinocytes can be present. In rare cases, FDE may have atypical features, such as in generalized bullous FDE and nonpigmenting FDE, the latter of which more commonly is associated with pseudoephedrine.1
The differential diagnosis for FDE includes erythema multiforme, Stevens-Johnson syndrome/toxic epidermal necrolysis, autoimmune progesterone dermatitis, and large plaque parapsoriasis. The number and morphology of lesions in erythema multiforme help differentiate it from FDE, as erythema multiforme presents with multiple targetoid lesions. The lesions of generalized bullous FDE can be similar to those of Stevens-Johnson syndrome/toxic epidermal necrolysis, and the pigmented patches of FDE can resemble large plaque parapsoriasis.
It is important to consider any medication ingested in the 1- to 2-week period before FDE onset, including over-the-counter medications, health food supplements, and prescription medications. Discontinuation of the implicated medication or any medication potentially cross-reacting with another medication is the most important step in management. Wound care may be needed for any bullous or eroded lesions. Lesions typically resolve within a few days to weeks of stopping the offending agent. Importantly, patients should be counseled on the secondary pigment alterations that may be persistent for several months. Other treatment for FDEs is aimed at symptomatic relief and may include topical corticosteroids and oral antihistamines.1
Medroxyprogesterone acetate is a highly effective contraceptive drug with low rates of failure.12 It is a weak androgenic progestin that is administered as a single 150-mg intramuscular injection every 3 months and inhibits gonadotropins. Common side effects include local injection-site reactions, unscheduled bleeding, amenorrhea, weight gain, headache, and mood changes. However, FDE has not been reported as an adverse effect to medroxyprogesterone acetate, both in official US Food and Drug Administration information and in the current literature.12
Autoimmune progesterone dermatitis (also known as progestin hypersensitivity) is a well-characterized cyclic hypersensitivity reaction to the hormone progesterone that occurs during the luteal phase of the menstrual cycle. It is known to have a variable clinical presentation including urticaria, erythema multiforme, eczema, and angioedema.13 Autoimmune progesterone dermatitis also has been reported to present as an FDE.14-16 The onset of the cutaneous manifestation often starts a few days before the onset of menses, with spontaneous resolution occurring after the onset of menstruation. The mechanism by which endogenous progesterone or other secretory products become antigenic is unknown. It has been suggested that there is an alteration in the properties of the hormone that would predispose it to be antigenic as it would not be considered self. In 2001, Warin17 proposed the following diagnostic criteria for autoimmune progesterone dermatitis: (1) skin lesions associated with menstrual cycle (premenstrual flare); (2) a positive response to the progesterone intradermal or intramuscular test; and (3) symptomatic improvement after inhibiting progesterone secretion by suppressing ovulation.17 The treatment includes antiallergy medications, progesterone desensitization, omalizumab injection, and leuprolide acetate injection.
Our case represents FDE from medroxyprogesterone acetate. Although we did not formally investigate the antigenicity of the exogenous progesterone, we postulate that the pathophysiology likely is similar to an FDE associated with endogenous progesterone. This reasoning is supported by the time course of the patient’s lesion as well as the worsening of symptoms in the days following the administration of the medication. Additionally, the patient had no history of skin lesions prior to the initiation of medroxyprogesterone acetate or similar lesions associated with her menstrual cycles.
A careful and detailed review of medication history is necessary to evaluate FDEs. Our case emphasizes that not only endogenous but also exogenous forms of progesterone may cause hypersensitivity, leading to an FDE. With more than 2 million prescriptions of medroxyprogesterone acetate written every year, dermatologists should be aware of the rare but potential risk for an FDE in patients using this medication.18
To the Editor:
A fixed drug eruption (FDE) is a well-documented form of cutaneous hypersensitivity that typically manifests as a sharply demarcated, dusky, round to oval, edematous, red-violaceous macule or patch on the skin and mucous membranes. The lesion often resolves with residual postinflammatory hyperpigmentation, most commonly as a reaction to ingested drugs or drug components.1 Lesions generally occur at the same anatomic site with repeated exposure to the offending drug. Typically, a single site is affected, but additional sites with more generalized involvement have been reported to occur with subsequent exposure to the offending medication. The diagnosis usually is clinical, but histopathologic findings can help confirm the diagnosis in unusual presentations. We present a novel case of a patient with an FDE from medroxyprogesterone acetate, a contraceptive injection that contains the hormone progestin.
A 35-year-old woman presented to the dermatology clinic for evaluation of a lesion on the left lower buttock of 1 year’s duration. She reported periodic swelling and associated pruritus of the lesion. She denied any growth in size, and no other similar lesions were present. The patient reported a medication history of medroxyprogesterone acetate for birth control, but she denied any other prescription or over-the-counter medication, oral supplements, or recreational drug use. Upon further inquiry, she reported that the recurrence of symptoms appeared to coincide with each administration of medroxyprogesterone acetate, which occurred approximately every 3 months. The eruption cleared between injections and recurred in the same location following subsequent injections. The lesion appeared approximately 2 weeks after the first injection (approximately 1 year prior to presentation to dermatology) and within 2 to 3 days after each subsequent injection. Physical examination revealed a 2×2-cm, circular, slightly violaceous patch on the left buttock (Figure 1). A biopsy was recommended to aid in diagnosis, and the patient was offered a topical steroid for symptomatic relief. A punch biopsy revealed subtle interface dermatitis with superficial perivascular lymphoid infiltrate and marked pigmentary incontinence consistent with an FDE (Figure 2).
An FDE was first reported in 1889 by Bourns,2 and over time more implicated agents and varying clinical presentations have been linked to the disease. The FDE can be accompanied by symptoms of pruritus or paresthesia. Most cases are devoid of systemic symptoms. An FDE can be located anywhere on the body, but it most frequently manifests on the lips, face, hands, feet, and genitalia. Although the eruption often heals with residual postinflammatory hyperpigmentation, a nonpigmenting FDE due to pseudoephedrine has been reported.3
Common culprits include antibiotics (eg, sulfonamides, trimethoprim, fluoroquinolones, tetracyclines), nonsteroidal anti-inflammatory medications (eg, naproxen sodium, ibuprofen, celecoxib), barbiturates, antimalarials, and anticonvulsants. Rare cases of FDE induced by foods and food additives also have been reported.4 Oral fluconazole, levocetirizine dihydrochloride, loperamide, and multivitamin-mineral preparations are other rare inducers of FDE.5-8 In 2004, Ritter and Meffert9 described an FDE to the green dye used in inactive oral contraceptive pills. A similar case was reported by Rea et al10 that described an FDE from the inactive sugar pills in ethinyl estradiol and levonorgestrel, which is another combined oral contraceptive.
The time between ingestion of the offending agent and the manifestation of the disease usually is 1 to 2 weeks; however, upon subsequent exposure, the disease has been reported to manifest within hours.1 CD8+ memory T cells have been shown to be major players in the development of FDE and can be found along the dermoepidermal junction as part of a delayed type IV hypersensitivity reaction.11 Histopathology reveals superficial and deep interstitial and perivascular infiltrates consisting of lymphocytes with admixed eosinophils and possibly neutrophils in the dermis. In the epidermis, necrotic keratinocytes can be present. In rare cases, FDE may have atypical features, such as in generalized bullous FDE and nonpigmenting FDE, the latter of which more commonly is associated with pseudoephedrine.1
The differential diagnosis for FDE includes erythema multiforme, Stevens-Johnson syndrome/toxic epidermal necrolysis, autoimmune progesterone dermatitis, and large plaque parapsoriasis. The number and morphology of lesions in erythema multiforme help differentiate it from FDE, as erythema multiforme presents with multiple targetoid lesions. The lesions of generalized bullous FDE can be similar to those of Stevens-Johnson syndrome/toxic epidermal necrolysis, and the pigmented patches of FDE can resemble large plaque parapsoriasis.
It is important to consider any medication ingested in the 1- to 2-week period before FDE onset, including over-the-counter medications, health food supplements, and prescription medications. Discontinuation of the implicated medication or any medication potentially cross-reacting with another medication is the most important step in management. Wound care may be needed for any bullous or eroded lesions. Lesions typically resolve within a few days to weeks of stopping the offending agent. Importantly, patients should be counseled on the secondary pigment alterations that may be persistent for several months. Other treatment for FDEs is aimed at symptomatic relief and may include topical corticosteroids and oral antihistamines.1
Medroxyprogesterone acetate is a highly effective contraceptive drug with low rates of failure.12 It is a weak androgenic progestin that is administered as a single 150-mg intramuscular injection every 3 months and inhibits gonadotropins. Common side effects include local injection-site reactions, unscheduled bleeding, amenorrhea, weight gain, headache, and mood changes. However, FDE has not been reported as an adverse effect to medroxyprogesterone acetate, both in official US Food and Drug Administration information and in the current literature.12
Autoimmune progesterone dermatitis (also known as progestin hypersensitivity) is a well-characterized cyclic hypersensitivity reaction to the hormone progesterone that occurs during the luteal phase of the menstrual cycle. It is known to have a variable clinical presentation including urticaria, erythema multiforme, eczema, and angioedema.13 Autoimmune progesterone dermatitis also has been reported to present as an FDE.14-16 The onset of the cutaneous manifestation often starts a few days before the onset of menses, with spontaneous resolution occurring after the onset of menstruation. The mechanism by which endogenous progesterone or other secretory products become antigenic is unknown. It has been suggested that there is an alteration in the properties of the hormone that would predispose it to be antigenic as it would not be considered self. In 2001, Warin17 proposed the following diagnostic criteria for autoimmune progesterone dermatitis: (1) skin lesions associated with menstrual cycle (premenstrual flare); (2) a positive response to the progesterone intradermal or intramuscular test; and (3) symptomatic improvement after inhibiting progesterone secretion by suppressing ovulation.17 The treatment includes antiallergy medications, progesterone desensitization, omalizumab injection, and leuprolide acetate injection.
Our case represents FDE from medroxyprogesterone acetate. Although we did not formally investigate the antigenicity of the exogenous progesterone, we postulate that the pathophysiology likely is similar to an FDE associated with endogenous progesterone. This reasoning is supported by the time course of the patient’s lesion as well as the worsening of symptoms in the days following the administration of the medication. Additionally, the patient had no history of skin lesions prior to the initiation of medroxyprogesterone acetate or similar lesions associated with her menstrual cycles.
A careful and detailed review of medication history is necessary to evaluate FDEs. Our case emphasizes that not only endogenous but also exogenous forms of progesterone may cause hypersensitivity, leading to an FDE. With more than 2 million prescriptions of medroxyprogesterone acetate written every year, dermatologists should be aware of the rare but potential risk for an FDE in patients using this medication.18
- Bolognia J, Jorizzo JL, Rapini RP. Dermatology. 2nd ed. Mosby; 2008.
- Bourns DCG. Unusual effects of antipyrine. Br Med J. 1889;2:818-820.
- Shelley WB, Shelley ED. Nonpigmenting fixed drug eruption as a distinctive reaction pattern: examples caused by sensitivity to pseudoephedrine hydrochloride and tetrahydrozoline. J Am Acad Dermatol. 1987;17:403-407.
- Sohn KH, Kim BK, Kim JY, et al. Fixed food eruption caused by Actinidia arguta (hardy kiwi): a case report and literature review. Allergy Asthma Immunol Res. 2017;9:182-184.
- Nakai N, Katoh N. Fixed drug eruption caused by fluconazole: a case report and mini-review of the literature. Allergol Int. 2013;6:139-141.
- An I, Demir V, Ibiloglu I, et al. Fixed drug eruption induced by levocetirizine. Indian Dermatol Online J. 2017;8:276-278.
- Matarredona J, Borrás Blasco J, Navarro-Ruiz A, et al. Fixed drug eruption associated to loperamide [in Spanish]. Med Clin (Barc). 2005;124:198-199.
- Gohel D. Fixed drug eruption due to multi-vitamin multi-mineral preparation. J Assoc Physicians India. 2000;48:268.
- Ritter SE, Meffert J. A refractory fixed drug reaction to a dye used in an oral contraceptive. Cutis. 2004;74:243-244.
- Rea S, McMeniman E, Darch K, et al. A fixed drug eruption to the sugar pills of a combined oral contraceptive. Poster presented at: The Australasian College of Dermatologists 51st Annual Scientific Meeting; May 22, 2018; Queensland, Australia.
- Shiohara T, Mizukawa Y. Fixed drug eruption: a disease mediated by self-inflicted responses of intraepidermal T cells. Eur J Dermatol. 2007;17:201-208.
- Depo-Provera CI. Prescribing information. Pfizer; 2020. Accessed March 10, 2022. https://labeling.pfizer.com/ShowLabeling.aspx?format=PDF&id=522
- George R, Badawy SZ. Autoimmune progesterone dermatitis: a case report. Case Rep Obstet Gynecol. 2012;2012:757854.
- Mokhtari R, Sepaskhah M, Aslani FS, et al. Autoimmune progesterone dermatitis presenting as fixed drug eruption: a case report. Dermatol Online J. 2017;23:13030/qt685685p4.
- Asai J, Katoh N, Nakano M, et al. Case of autoimmune progesterone dermatitis presenting as fixed drug eruption. J Dermatol. 2009;36:643-645.
- Bhardwaj N, Jindal R, Chauhan P. Autoimmune progesterone dermatitis presenting as fixed drug eruption. BMJ Case Rep. 2019;12:E231873.
- Warin AP. Case 2. diagnosis: erythema multiforme as a presentation of autoimmune progesterone dermatitis. Clin Exp Dermatol. 2001;26:107-108.
- Medroxyprogesterone Drug Usage Statistics, United States, 2013-2019. ClinCalc website. Updated September 15, 2021. Accessed March 17, 2022. https://clincalc.com/DrugStats/Drugs/Medroxyprogesterone
- Bolognia J, Jorizzo JL, Rapini RP. Dermatology. 2nd ed. Mosby; 2008.
- Bourns DCG. Unusual effects of antipyrine. Br Med J. 1889;2:818-820.
- Shelley WB, Shelley ED. Nonpigmenting fixed drug eruption as a distinctive reaction pattern: examples caused by sensitivity to pseudoephedrine hydrochloride and tetrahydrozoline. J Am Acad Dermatol. 1987;17:403-407.
- Sohn KH, Kim BK, Kim JY, et al. Fixed food eruption caused by Actinidia arguta (hardy kiwi): a case report and literature review. Allergy Asthma Immunol Res. 2017;9:182-184.
- Nakai N, Katoh N. Fixed drug eruption caused by fluconazole: a case report and mini-review of the literature. Allergol Int. 2013;6:139-141.
- An I, Demir V, Ibiloglu I, et al. Fixed drug eruption induced by levocetirizine. Indian Dermatol Online J. 2017;8:276-278.
- Matarredona J, Borrás Blasco J, Navarro-Ruiz A, et al. Fixed drug eruption associated to loperamide [in Spanish]. Med Clin (Barc). 2005;124:198-199.
- Gohel D. Fixed drug eruption due to multi-vitamin multi-mineral preparation. J Assoc Physicians India. 2000;48:268.
- Ritter SE, Meffert J. A refractory fixed drug reaction to a dye used in an oral contraceptive. Cutis. 2004;74:243-244.
- Rea S, McMeniman E, Darch K, et al. A fixed drug eruption to the sugar pills of a combined oral contraceptive. Poster presented at: The Australasian College of Dermatologists 51st Annual Scientific Meeting; May 22, 2018; Queensland, Australia.
- Shiohara T, Mizukawa Y. Fixed drug eruption: a disease mediated by self-inflicted responses of intraepidermal T cells. Eur J Dermatol. 2007;17:201-208.
- Depo-Provera CI. Prescribing information. Pfizer; 2020. Accessed March 10, 2022. https://labeling.pfizer.com/ShowLabeling.aspx?format=PDF&id=522
- George R, Badawy SZ. Autoimmune progesterone dermatitis: a case report. Case Rep Obstet Gynecol. 2012;2012:757854.
- Mokhtari R, Sepaskhah M, Aslani FS, et al. Autoimmune progesterone dermatitis presenting as fixed drug eruption: a case report. Dermatol Online J. 2017;23:13030/qt685685p4.
- Asai J, Katoh N, Nakano M, et al. Case of autoimmune progesterone dermatitis presenting as fixed drug eruption. J Dermatol. 2009;36:643-645.
- Bhardwaj N, Jindal R, Chauhan P. Autoimmune progesterone dermatitis presenting as fixed drug eruption. BMJ Case Rep. 2019;12:E231873.
- Warin AP. Case 2. diagnosis: erythema multiforme as a presentation of autoimmune progesterone dermatitis. Clin Exp Dermatol. 2001;26:107-108.
- Medroxyprogesterone Drug Usage Statistics, United States, 2013-2019. ClinCalc website. Updated September 15, 2021. Accessed March 17, 2022. https://clincalc.com/DrugStats/Drugs/Medroxyprogesterone
Practice Points
- Exogenous progesterone from the administration of the contraceptive injectable medroxyprogesterone acetate has the potential to cause a cutaneous hypersensitivity reaction in the form of a fixed drug eruption (FDE).
- Dermatologists should perform a careful and detailed review of medication history to evaluate drug eruptions.
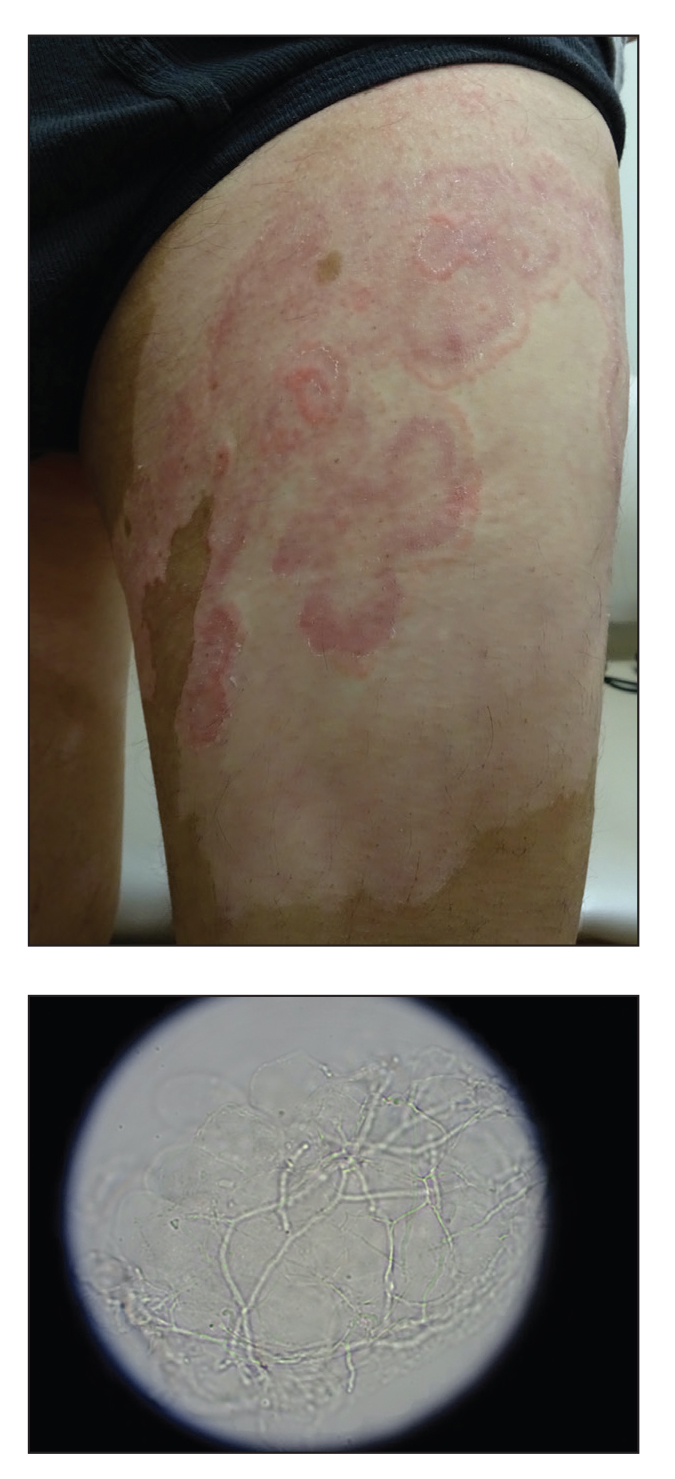
Pink, Scaly, Annular Plaques in Concentric Rings Localized to Vitiliginous Patches
The Diagnosis: Tinea Pseudoimbricata
Tinea pseudoimbricata and tinea indecisiva are synonyms describing cases of tinea corporis that manifest in scaly plaques in concentric rings evocative of those present in tinea imbricata. However, in contrast to tinea imbricata, cases of tinea pseudoimbricata are caused by dermatophytes other than Trichophyton concentricum. 1 Tinea pseudoimbricata usually presents in association with immunosuppression, either systemic or local, and can be produced by application of topical medications such as corticosteroids.2 Mask-Bull et al3 reported the case of a 21-year-old man in the United States with no history of immunosuppressive conditions who presented with scaly erythematous annular plaques on the lateral neck that resolved with 2 pulsed doses of terbinafine. Potassium hydroxide preparation and fungal culture were both consistent with Trichophyton tonsurans.3
Trichophyton concentricum is an anthropophilic species of dermatophyte endemic to areas within the South Pacific, Southeast Asia, and Central and South America. Infection with T concentricum produces tinea imbricata, which presents with concentric, scaly, annular rings. Cutaneous lesions of tinea imbricata caused by T concentricum have a more generalized distribution and more densely grouped, concentric circles than the cutaneous findings seen in patients with tinea pseudoimbricata.4 Affected patients typically demonstrate negative delayed-type hypersensitivity to T concentricum cytoplasmic antigen and T-lymphocyte hyporeactivity, which may contribute to the development of sequential waves of scaling observed in tinea imbricata.5
Trichophyton rubrum, the most common cause of tinea corporis, has been reported to cause some cases of tinea pseudoimbricata (indecisiva).1,2 It utilizes keratinases such as subtilisins (Sub3 and Sub4), leucine aminopeptidases (Lap1 and Lap2), and dipeptidyl peptidases (DppIV and DppV) to invade the skin. Once inside, mannans, glycoprotein constituents of the cell wall, are released and bind to the cell surface of mononuclear phagocytes, subsequently moving into the cell by phagocytosis, thereafter interfering with RNA synthesis that is necessary for presentation of antigens to appropriate T cells and allowing for initiation of chronic infection.6,7 The cytotoxic response to superficial dermatophyte infection is triggered by major histocompatibility complex class I molecule activation of CD8+ cells.6,8
Our case is of interest given the localization of the superficial dermatophyte infection to only vitiliginous skin. This distribution and appearance while undergoing narrowband UVB (NB-UVB) treatment is rare. We postulate that our patient likely represents a case of locus minoris resistentiae, a phenomenon in which an area of skin exhibits a compromised immune microenvironment that predisposes it to disease.9
In vitiligo, NB-UVB modulates the immune response by increasing IL-10, thereby promoting regulatory T-cell differentiation with suppression of autoreactive T cells and induction of direct T-lymphocyte apoptosis.10,11 Although the mechanism accounting for our patient’s presentation is unknown, we suspect NB-UVB–induced immunosuppression enabled persistence of the dermatophyte infection. The localization of the infection to the vitiliginous patches may result from the greater penetration of the UV light relative to the surrounding, normally pigmented skin. This relative difference in UV penetration would be expected to result in increased immunosuppression in the vitiliginous lesions and enhanced susceptibility to the fungal organisms.
Erythema annulare centrifugum is characterized by annular lesions with a trailing scale instead of the concentric rings seen in tinea pseudoimbricata. Erythema marginatum is seen in acute rheumatic fever and presents with a transient nonpruritic rash, usually on the trunk or extremities. Erythema migrans presents with fewer lesions that are less circinate in shape, and the patient often has a history of a tick bite. Tinea imbricata is caused by T concentricum, while tinea pseudoimbricata is caused by T tonsurans and other dermatophytes.
With the increasing use of immunosuppressant drugs, the prevalence of tinea pseudoimbricata is hypothesized to increase.1 The presence of tinea pseudoimbricata should alert dermatologists to the possible overuse of topical corticosteroids, and other forms of immunosuppression also should be considered.
- Lim SP, Smith AG. “Tinea pseudoimbricata”: tinea corporis in a renal transplant recipient mimicking the concentric rings of tinea imbricata. Clin Exp Dermatol. 2003;28:332-333.
- Batta K, Ramlogan D, Smith AG, et al. ‘Tinea indecisiva’ may mimic the concentric rings of tinea imbricata. Br J Dermatol. 2002;147:384.
- Mask-Bull L, Patel R, Tarbox MB. America’s first case of tinea pseudoimbricata. Am J Dermatol Venereol. 2015;4:15-17.
- Meena M, Mittal A. Tinea pseudo-imbricata. J Assoc Physicians India. 2018;66:79.
- Hay RJ, Reid S, Talwat E, et al. Immune responses of patients with tinea imbricata. Br J Dermatol. 1983;108:581-586.
- Dahl MV. Suppression of immunity and inflammation by products produced by dermatophytes. J Am Acad Dermatol. 1993;28(5 pt 1):S19-S23.
- Blutfield MS, Lohre JM, Pawich DA, et al. The immunologic response to Trichophyton rubrum in lower extremity fungal infections. J Fungi (Basel). 2015;1:130-137.
- De Hoog S, Monod M, Dawson T, et al. Skin fungi from colonization to infection [published online July 2017]. Microbiol Spectr. doi:10.1128/ microbiolspec.FUNK-0049-2016
- Lo Schiavo A, Ruocco E, Russo T, et al. Locus minoris resistentiae: an old but still valid way of thinking in medicine. Clin Dermatol. 2014;32:553-556.
- Ponsonby AL, Lucas RM, van der Mei IA. UVR, vitamin D and three autoimmune diseases—multiple sclerosis, type 1 diabetes, rheumatoid arthritis. Photochem Photobiol. 2005;81:1267-1275.
- Yazdani Abyaneh M, Griffith RD, Falto-Aizpurua L, et al. Narrowband ultraviolet B phototherapy in combination with other therapies for vitiligo: mechanisms and efficacies. J Eur Acad Dermatol Venereol. 2014;28:1610-1622.
The Diagnosis: Tinea Pseudoimbricata
Tinea pseudoimbricata and tinea indecisiva are synonyms describing cases of tinea corporis that manifest in scaly plaques in concentric rings evocative of those present in tinea imbricata. However, in contrast to tinea imbricata, cases of tinea pseudoimbricata are caused by dermatophytes other than Trichophyton concentricum. 1 Tinea pseudoimbricata usually presents in association with immunosuppression, either systemic or local, and can be produced by application of topical medications such as corticosteroids.2 Mask-Bull et al3 reported the case of a 21-year-old man in the United States with no history of immunosuppressive conditions who presented with scaly erythematous annular plaques on the lateral neck that resolved with 2 pulsed doses of terbinafine. Potassium hydroxide preparation and fungal culture were both consistent with Trichophyton tonsurans.3
Trichophyton concentricum is an anthropophilic species of dermatophyte endemic to areas within the South Pacific, Southeast Asia, and Central and South America. Infection with T concentricum produces tinea imbricata, which presents with concentric, scaly, annular rings. Cutaneous lesions of tinea imbricata caused by T concentricum have a more generalized distribution and more densely grouped, concentric circles than the cutaneous findings seen in patients with tinea pseudoimbricata.4 Affected patients typically demonstrate negative delayed-type hypersensitivity to T concentricum cytoplasmic antigen and T-lymphocyte hyporeactivity, which may contribute to the development of sequential waves of scaling observed in tinea imbricata.5
Trichophyton rubrum, the most common cause of tinea corporis, has been reported to cause some cases of tinea pseudoimbricata (indecisiva).1,2 It utilizes keratinases such as subtilisins (Sub3 and Sub4), leucine aminopeptidases (Lap1 and Lap2), and dipeptidyl peptidases (DppIV and DppV) to invade the skin. Once inside, mannans, glycoprotein constituents of the cell wall, are released and bind to the cell surface of mononuclear phagocytes, subsequently moving into the cell by phagocytosis, thereafter interfering with RNA synthesis that is necessary for presentation of antigens to appropriate T cells and allowing for initiation of chronic infection.6,7 The cytotoxic response to superficial dermatophyte infection is triggered by major histocompatibility complex class I molecule activation of CD8+ cells.6,8
Our case is of interest given the localization of the superficial dermatophyte infection to only vitiliginous skin. This distribution and appearance while undergoing narrowband UVB (NB-UVB) treatment is rare. We postulate that our patient likely represents a case of locus minoris resistentiae, a phenomenon in which an area of skin exhibits a compromised immune microenvironment that predisposes it to disease.9
In vitiligo, NB-UVB modulates the immune response by increasing IL-10, thereby promoting regulatory T-cell differentiation with suppression of autoreactive T cells and induction of direct T-lymphocyte apoptosis.10,11 Although the mechanism accounting for our patient’s presentation is unknown, we suspect NB-UVB–induced immunosuppression enabled persistence of the dermatophyte infection. The localization of the infection to the vitiliginous patches may result from the greater penetration of the UV light relative to the surrounding, normally pigmented skin. This relative difference in UV penetration would be expected to result in increased immunosuppression in the vitiliginous lesions and enhanced susceptibility to the fungal organisms.
Erythema annulare centrifugum is characterized by annular lesions with a trailing scale instead of the concentric rings seen in tinea pseudoimbricata. Erythema marginatum is seen in acute rheumatic fever and presents with a transient nonpruritic rash, usually on the trunk or extremities. Erythema migrans presents with fewer lesions that are less circinate in shape, and the patient often has a history of a tick bite. Tinea imbricata is caused by T concentricum, while tinea pseudoimbricata is caused by T tonsurans and other dermatophytes.
With the increasing use of immunosuppressant drugs, the prevalence of tinea pseudoimbricata is hypothesized to increase.1 The presence of tinea pseudoimbricata should alert dermatologists to the possible overuse of topical corticosteroids, and other forms of immunosuppression also should be considered.
The Diagnosis: Tinea Pseudoimbricata
Tinea pseudoimbricata and tinea indecisiva are synonyms describing cases of tinea corporis that manifest in scaly plaques in concentric rings evocative of those present in tinea imbricata. However, in contrast to tinea imbricata, cases of tinea pseudoimbricata are caused by dermatophytes other than Trichophyton concentricum. 1 Tinea pseudoimbricata usually presents in association with immunosuppression, either systemic or local, and can be produced by application of topical medications such as corticosteroids.2 Mask-Bull et al3 reported the case of a 21-year-old man in the United States with no history of immunosuppressive conditions who presented with scaly erythematous annular plaques on the lateral neck that resolved with 2 pulsed doses of terbinafine. Potassium hydroxide preparation and fungal culture were both consistent with Trichophyton tonsurans.3
Trichophyton concentricum is an anthropophilic species of dermatophyte endemic to areas within the South Pacific, Southeast Asia, and Central and South America. Infection with T concentricum produces tinea imbricata, which presents with concentric, scaly, annular rings. Cutaneous lesions of tinea imbricata caused by T concentricum have a more generalized distribution and more densely grouped, concentric circles than the cutaneous findings seen in patients with tinea pseudoimbricata.4 Affected patients typically demonstrate negative delayed-type hypersensitivity to T concentricum cytoplasmic antigen and T-lymphocyte hyporeactivity, which may contribute to the development of sequential waves of scaling observed in tinea imbricata.5
Trichophyton rubrum, the most common cause of tinea corporis, has been reported to cause some cases of tinea pseudoimbricata (indecisiva).1,2 It utilizes keratinases such as subtilisins (Sub3 and Sub4), leucine aminopeptidases (Lap1 and Lap2), and dipeptidyl peptidases (DppIV and DppV) to invade the skin. Once inside, mannans, glycoprotein constituents of the cell wall, are released and bind to the cell surface of mononuclear phagocytes, subsequently moving into the cell by phagocytosis, thereafter interfering with RNA synthesis that is necessary for presentation of antigens to appropriate T cells and allowing for initiation of chronic infection.6,7 The cytotoxic response to superficial dermatophyte infection is triggered by major histocompatibility complex class I molecule activation of CD8+ cells.6,8
Our case is of interest given the localization of the superficial dermatophyte infection to only vitiliginous skin. This distribution and appearance while undergoing narrowband UVB (NB-UVB) treatment is rare. We postulate that our patient likely represents a case of locus minoris resistentiae, a phenomenon in which an area of skin exhibits a compromised immune microenvironment that predisposes it to disease.9
In vitiligo, NB-UVB modulates the immune response by increasing IL-10, thereby promoting regulatory T-cell differentiation with suppression of autoreactive T cells and induction of direct T-lymphocyte apoptosis.10,11 Although the mechanism accounting for our patient’s presentation is unknown, we suspect NB-UVB–induced immunosuppression enabled persistence of the dermatophyte infection. The localization of the infection to the vitiliginous patches may result from the greater penetration of the UV light relative to the surrounding, normally pigmented skin. This relative difference in UV penetration would be expected to result in increased immunosuppression in the vitiliginous lesions and enhanced susceptibility to the fungal organisms.
Erythema annulare centrifugum is characterized by annular lesions with a trailing scale instead of the concentric rings seen in tinea pseudoimbricata. Erythema marginatum is seen in acute rheumatic fever and presents with a transient nonpruritic rash, usually on the trunk or extremities. Erythema migrans presents with fewer lesions that are less circinate in shape, and the patient often has a history of a tick bite. Tinea imbricata is caused by T concentricum, while tinea pseudoimbricata is caused by T tonsurans and other dermatophytes.
With the increasing use of immunosuppressant drugs, the prevalence of tinea pseudoimbricata is hypothesized to increase.1 The presence of tinea pseudoimbricata should alert dermatologists to the possible overuse of topical corticosteroids, and other forms of immunosuppression also should be considered.
- Lim SP, Smith AG. “Tinea pseudoimbricata”: tinea corporis in a renal transplant recipient mimicking the concentric rings of tinea imbricata. Clin Exp Dermatol. 2003;28:332-333.
- Batta K, Ramlogan D, Smith AG, et al. ‘Tinea indecisiva’ may mimic the concentric rings of tinea imbricata. Br J Dermatol. 2002;147:384.
- Mask-Bull L, Patel R, Tarbox MB. America’s first case of tinea pseudoimbricata. Am J Dermatol Venereol. 2015;4:15-17.
- Meena M, Mittal A. Tinea pseudo-imbricata. J Assoc Physicians India. 2018;66:79.
- Hay RJ, Reid S, Talwat E, et al. Immune responses of patients with tinea imbricata. Br J Dermatol. 1983;108:581-586.
- Dahl MV. Suppression of immunity and inflammation by products produced by dermatophytes. J Am Acad Dermatol. 1993;28(5 pt 1):S19-S23.
- Blutfield MS, Lohre JM, Pawich DA, et al. The immunologic response to Trichophyton rubrum in lower extremity fungal infections. J Fungi (Basel). 2015;1:130-137.
- De Hoog S, Monod M, Dawson T, et al. Skin fungi from colonization to infection [published online July 2017]. Microbiol Spectr. doi:10.1128/ microbiolspec.FUNK-0049-2016
- Lo Schiavo A, Ruocco E, Russo T, et al. Locus minoris resistentiae: an old but still valid way of thinking in medicine. Clin Dermatol. 2014;32:553-556.
- Ponsonby AL, Lucas RM, van der Mei IA. UVR, vitamin D and three autoimmune diseases—multiple sclerosis, type 1 diabetes, rheumatoid arthritis. Photochem Photobiol. 2005;81:1267-1275.
- Yazdani Abyaneh M, Griffith RD, Falto-Aizpurua L, et al. Narrowband ultraviolet B phototherapy in combination with other therapies for vitiligo: mechanisms and efficacies. J Eur Acad Dermatol Venereol. 2014;28:1610-1622.
- Lim SP, Smith AG. “Tinea pseudoimbricata”: tinea corporis in a renal transplant recipient mimicking the concentric rings of tinea imbricata. Clin Exp Dermatol. 2003;28:332-333.
- Batta K, Ramlogan D, Smith AG, et al. ‘Tinea indecisiva’ may mimic the concentric rings of tinea imbricata. Br J Dermatol. 2002;147:384.
- Mask-Bull L, Patel R, Tarbox MB. America’s first case of tinea pseudoimbricata. Am J Dermatol Venereol. 2015;4:15-17.
- Meena M, Mittal A. Tinea pseudo-imbricata. J Assoc Physicians India. 2018;66:79.
- Hay RJ, Reid S, Talwat E, et al. Immune responses of patients with tinea imbricata. Br J Dermatol. 1983;108:581-586.
- Dahl MV. Suppression of immunity and inflammation by products produced by dermatophytes. J Am Acad Dermatol. 1993;28(5 pt 1):S19-S23.
- Blutfield MS, Lohre JM, Pawich DA, et al. The immunologic response to Trichophyton rubrum in lower extremity fungal infections. J Fungi (Basel). 2015;1:130-137.
- De Hoog S, Monod M, Dawson T, et al. Skin fungi from colonization to infection [published online July 2017]. Microbiol Spectr. doi:10.1128/ microbiolspec.FUNK-0049-2016
- Lo Schiavo A, Ruocco E, Russo T, et al. Locus minoris resistentiae: an old but still valid way of thinking in medicine. Clin Dermatol. 2014;32:553-556.
- Ponsonby AL, Lucas RM, van der Mei IA. UVR, vitamin D and three autoimmune diseases—multiple sclerosis, type 1 diabetes, rheumatoid arthritis. Photochem Photobiol. 2005;81:1267-1275.
- Yazdani Abyaneh M, Griffith RD, Falto-Aizpurua L, et al. Narrowband ultraviolet B phototherapy in combination with other therapies for vitiligo: mechanisms and efficacies. J Eur Acad Dermatol Venereol. 2014;28:1610-1622.
A 64-year-old man presented with generalized vitiligo. In addition to extensive depigmented macules, physical examination revealed the presence of onychomycosis and tinea corporis confirmed by microscopic examination of potassium hydroxide–treated superficial skin scrapings. Vitiligo treatment was postponed, and a 3-month course of oral terbinafine and naftifine cream was undertaken for the dermatophyte infections. Subsequent examination revealed that the patient’s tinea corporis had improved, though there were localized areas of persistence. Given the patient’s eagerness to treat his vitiligo, narrowband UVB phototherapy was started along with tolnaftate cream 1% for treatment of the residual tinea corporis. After 2 months of narrowband UVB, partial repigmentation of the vitiligo was observed; however, he had developed extensive pink, scaly, annular plaques in concentric rings within residual vitiliginous patches on the lower extremities (top). Repeat examination of potassium hydroxide–treated skin scrapings revealed numerous hyphae (bottom). A fungal culture identified Trichophyton rubrum.