User login
When are vasoactive agents indicated in acute heart failure?
Case
A 72-year-old retired nurse with known nonischemic dilated cardiomyopathy with an ejection fraction of approximately 20% and status-post cardiac resynchronization therapy presents to the emergency department with dyspnea with minimal activity, three-pillow orthopnea, and paroxysmal nocturnal dyspnea.
She had been hospitalized twice during the past 60 days for similar symptoms. Her medications included losartan (20 mg po q daily), carvedilol (3.125 mg twice daily), spironolactone (25 mg daily), digoxin (0.125 mg daily), and furosemide (80 mg twice daily). Vital signs are notable for a blood pressure of 90/50 mmHg and an irregular pulse of 90 beats per minute. Physical examination is notable for marked jugular venous distension, lungs clear to auscultation bilaterally, biventricular heaves, a markedly displaced left ventricular point of maximal impulse, and a prominent S3 gallop.
Despite treatment with intravenous furosemide and temporary withdrawal of carvedilol, the patient remains symptomatic with persistent jugular venous distension.
Should she be given a vasoactive agent?
Overview
Acute heart failure syndrome (AHFS), defined as a gradual or rapid change in heart failure signs and symptoms, is the most common cause of hospitalization in the United States1. It is associated with an average in-hospital mortality of 4% to 5%, a 30-day mortality of 7% to11%, and a one-year mortality of 33%2.
In patients with previously established myocardial dysfunction, AHFS commonly reflects exacerbation of symptoms after a period of stability. The clinical presentation and severity of AHFS may range from mild volume overload to life-threatening cardiogenic shock and multi-organ failure unresponsive to pharmacologic therapy.2
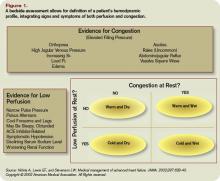
Initial management of AHFS depends on definition of the patient’s hemodynamic profile. To guide initial therapy, classify patients into one of four hemodynamic profiles during a brief bedside assessment that relies on evaluation of filling pressures (wet or dry) and adequacy of perfusion (hot or cold) (see figure 1).3
Treating volume overload or elevated filling pressures generally begins with diuretics. Diuretics have been shown to provide symptomatic relief, though they have not yet been proven safe.4 Initial treatment can include a loop diuretic at a dose higher than the patient’s chronic dose, with intravenous dosing offering greater bio-absorption and rapidity in onset of action.5 If perfusion is inadequate, escalate therapy beyond diuretics to include vasoactive agents.
Review of the Data
The use of vasoactive medications is largely based on anecdotal experiences and physiologic assumptions rather than on adequately powered prospective randomized controlled trials.6 Vasoactive therapy includes vasodilator and inotropic support and is generally limited for use in patients with advanced disease not responding to standard medical treatment and diuresis. The physiologic premise rests in the expected improvement in ventricular filling pressures and cardiac output with reduction in afterload and/or preload. Vasodilators counteract vascular constriction, reducing both preload and afterload. Positive inotropic agents amplify cardiac output by increasing the strength of myocardial contraction.
Vasodilators
The Heart Failure Society of America (HFSA) 2006 Comprehensive Heart Failure Practice Guidelines state, “In the absence of symptomatic hypotension, intravenous vasodilators (nitroglycerin, nitroprusside, or nesiritide) may be considered as an addition to diuretic therapy for rapid improvement of congestive symptoms.”7 The clinical utility of nesiritide remains in question with clinical and hemodynamic improvement demonstrated in three randomized trials 8-10; but tempered against meta-analyses 11-12 of selected trials, demonstrating a non-significant trend toward increased kidney dysfunction and death within 30 days (35/485 [7.2%] vs. 15/377 [4.0%] patients; risk ratio from meta-analyses, 1.74; 95% confidence interval, 0.97-3.12; p=0.059). In a randomized trial of 489 in-patients with dyspnea at rest from AHFS, treatment with three hours of intravenous nesiritide resulted in a significant improvement in dyspnea compared with placebo (p=0.03). Similar improvement was observed with intravenous nitroglycerin and did not differ statistically from that observed with nesiritide.8 Nitroprusside, an attractive option among those with hypertension and cardiogenic pulmonary edema, is limited by the need for invasive hemodynamic monitoring and potential for either cyanide toxicity or worsening myocardial ischemia.
Inotropes
Again, there is little evidence from adequately powered randomized controlled trials guiding the use of inotropes. Their use is generally limited to the following indications (see figure 2): (1) Short-term treatment for AHFS that is unresponsive to adequate doses of diuretics and especially when associated with systemic hypotension, (2) Bridge to recovery (as following myocarditis) or to definitive treatment (as with transplant), or (3) For palliation when symptomatic relief is the agreed upon goal.13 The HFSA 2006 guideline states: “Intravenous inotropes may be considered to relieve symptoms and improve end-organ function in patients with advanced HF characterized by left ventricle dilation, reduced left ventricular ejection fraction, and diminished peripheral perfusion or end-organ dysfunction, particularly if these patients have marginal systolic blood pressure, have symptomatic hypotension despite adequate filling pressures, or are unresponsive to, or intolerant of, intravenous vasodilators.”7
Dobutamine and milrinone are the most commonly used IV inotropes for the treatment of AHFS and increase contractility by increasing intracellular levels of cyclic adenylate monophosphate (cAMP). Dobutamine is a catechlamine agonist that increases cAMP production through stimulation of adenylate cyclase. Milrinone selectively inhibits phosphodiesterase III, which catalyzes the breakdown of cAMP.
Despite their frequent use when traditional treatments have failed, the data supporting the use of dobutamine and milrinone is limited. The largest registry of patients with AHFS to date associated excess mortality with intravenous inotrope use compared to nitroglycerin or nesiritide.14 In a study population of 255 patients randomized to receive either intravenous nesiritide or intravenous dobutamine, Burger et al.15 demonstrated that dobutamine significantly increased the mean number of ventricular tachycardia events per 24 hours (p=0.001), suggesting increased arrhythmogenicity associated with inotrope use. Nonetheless, in a randomized trial of 15 patients admitted with AHFS, functional class improved in six of eight dobutamine-treated patients, but in only two of seven patients treated with placebo, suggesting clinical improvement as a consequence of inotropic stimulation.16 Unverferth et al. demonstrated a similar sustained functional improvement up to 10 weeks following a 72-hour infusion of intravenous dobutamine. 17
The Outcomes of a Prospective Trial of Intravenous Milrinone for Exacerbations of Chronic Heart Failure trial (OPTIME-CHF), randomized 951 patients with AHFS to receive either intravenous milrinone or placebo within 48 hours of hospitalization.18 Compared to placebo, milrinone was associated with a significant increase in sustained hypotension requiring intervention (10.7% vs. 3.2%; p<.001) and new atrial arrhythmias (4.6% vs. 1.5%; p=0.004), along with a non-significant trend toward increased mortality (3.8% vs. 2.3%; p=0.19). However, as measured by a visual analog scale, milrinone-treated patients reported feeling better than those treated with placebo at 30 days post-randomization (p=0.02).
Although there are not randomized data comparing the efficacy of milrinone and dobutamine in AHFS, a retrospective analysis of 329 patients compared the hemodynamic and clinical effects of these two inotropes.19 Milrinone consistently was associated with a more favorable hemodynamic response, including lower systemic vascular resistance (p=0.01); lower pulmonary artery wedge pressure (p<0.001); larger percentage increase in cardiac index (p=0.03); and larger percentage decrease in pulmonary vascular resistance (p=0.0001). In-hospital mortality (dobutamine 7.8% vs. milrinone 10%) was not significantly different.
Conclusion
Clearly, vasoactive and inotropic agents are available when AHFS is refractory to traditional diuresis and may offer short-term symptomatic relief, palliation in the context of end-of-life care, or bridge to recovery or more definitive treatment. Unfortunately, sufficient and robust evidence that supports the safety and efficacy of such agents is lacking and their use is largely guided by historical practices, clinical experience, and anticipation of theoretic physiologic changes. While adequately powered prospective randomized data emerge, newer agents such as vasopressin receptor antagonists, cardiac myosin activators, calcium sensitizers, and adenosine-receptor antagonists will offer additional pharmacologic options.20 When continued pharmacologic support becomes ineffective, device therapy is available to aid in the treatment of AHFS and includes ultrafiltration to reduce filling pressures and intra-aortic balloon pump counterpulsation or left ventricular assist device placement for pharmacologically resistant cardiogenic shock.21
Back to the Case
Despite maximal medical therapy for her chronic heart failure and biventricular pacing, the patient continued to have markedly limited functional status and repeated hospitalizations for AHFS. Given her advanced age and poor nutritional status, she was not a candidate for cardiac transplantation or placement of a left ventricular assist device. To allow for palliative tailored therapy, right heart catheterization was performed. Right heart catheterization revealed elevated filling pressures, as follows: right atrium, 20 mmHg; pulmonary artery, 63/34 mmHg (mean 47 mmHg); and pulmonary capillary wedge, 29 mmHg. Her mixed venous oxygen saturation was only 41% with a calculated cardiac output of 2.9 liters per minute and cardiac index of 2 liters per minute per meter squared.
As she expressed symptomatic relief as her goal, she was started on intravenous milrinone at 0.2 micrograms per kilogram per minute. This was done with the understanding her symptoms would likely would improve, at the expense of worsening longevity and prognosis. With uptitration of her intravenous milrinone and a continuous infusion of furosemide, she demonstrated the following filling pressures within 24 hours: right atrium, 18 mmHg; pulmonary artery, 63/33 mmHg (mean 43 mmHg); and pulmonary capillary wedge, 24. Importantly, her mixed venous oxygen saturation improved to 68% with a calculated cardiac output of 3.4 liters per minute and cardiac index of 2.4 liters per minute per meter squared. These favorable hemodynamic changes were accompanied by modest improvement in symptoms. After continued intravenous diuresis, she was transitioned back to an oral diuretic regimen and was ultimately discharged to home with a continuous infusion of milrinone for palliation. TH
Drs. Vaishnava, McKean, Nohria, and Baughman are from Brigham and Women’s Hospital and Harvard Medical School in Boston, Mass.
REFERENCES:
- Thom T, Haase N, Rosamond W, et al. Heart disease and stroke statistics – 2006 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2006;113:85-151.
- Iong P, Vowinckel E, Liu PP, Gong Y, Tu JV. Prognosis and determinants of survival in patients newly hospitalized for heart failure: a population-based study. Arch Intern Med. 2002;162:1689-94.
- Nohria A, Lewis EF, Stevenson LW. Medical management of advanced heart failure. JAMA. 2002;287;628-40.
- Faris R, Flather MD, Purcell H, et al. Diuretics for heart failure. Cochrane Database Syst Rev. 2006;1;CD003838.
- Wang DJ and Gottlieb SS. Diuretics: Still the mainstay of treatment. Crit Care Med. 2008;36(Suppl.):S89-S94.
- Fares WH. Management of acute decompensated heart failure in an evidence-based era: What is the evidence behind the current standard of care? Heart & Lung. 2008;37(3):173-8.
- Adams KF, Lindenfield J, Arnold J, et al. Executive summary: HFSA 2006 comprehensive heart failure practice guidelines. J Card Fail. 2006;12:10-38.
- Publication Committee for the VMAC Investigators (Vasodilatation in the Management of Acute CHF). Intravenous nesiritide vs nitroglycerin for treatment of decompensated congestive heart failure: a randomized controlled trial. JAMA. 2002;297:1531-40.
- Peacock WF, Enerman CL, Silver MA, on behalf of the PROACTION Study Group. Am J Emerg Med. 2005;23:327-31.
- Cotter G, Metzkor E, Kaluski E, et al. Randomized trial of high-dose isosorbide dinitrate plus low-dose furosemide versus high-dose furosemide plus low-dose isosorbide dinitrate in severe pulmonary edema. Lancet. 1998;351:389-93.
- Sackner-Bernstein JD, Kowalski M, Fox M, Aaronson K. Short-term risk of death after treatment with nesiritide for decompensated heart failure: a pooled analysis of randomized controlled trials. JAMA. 2005;293:1900-5.
- Sackner-Bernstein JD, Skopicki HA, Aaronson K. Risk of worsening renal function with nesiritide in patients with acutely decompensated heart failure. Circulation. 2005;111:1487-91.
- Felker GM and O’Connor CM. Inotropic therapy for heart failure: An evidence-based approach. American Heart Journal. 2001; 142:393-401.
- Abrahm WT, Adams KF, Fonarow GC, et al. In-hospital mortality in patients with acute decompensated heart failure requiring intravenous vasoactive medications: an analysis from the Acute Decompensated Heart Failure National Registry (ADHERE). J Am Coll Cardiol. 2005;46:57-64.
- Burger AJ, Houton DP, LeJemtel T, et al. Effect of nesiritide and dobutamine on ventricular arrhythmias in the treatment of patients with acutely decompensated congestive heart failure: the PRECEDENT study. American Heart Journal. 2002;144:1102-8.
- Liang CS, Sherman LG, Doherty JU, et al. Sustained improvement of cardiac function in patients with congestive heart failure after short-term infusion of dobutamine. Circulation. 1984;69:113-9.
- Unverferth DV, Magorien RD, Lewis RP, et al. Long-term benefit of dobutamine in patients with congestive cardiomyopathy. American Heart Journal. 1980;100:622-30.
- Cuffe MS, Califf RM, Adams KF Jr, et al. Outcomes of a Prospective Trial of Intravenous Milrinone for Exacerbations of Chronic Heart Failure (OPTIME-CHF) Investigators. Short-term intravenous milrinone for acute exacerbation of chronic heart failure: a randomized controlled trial. JAMA. 2002;287:1541-7.
- Yamani MH, Haji SA, Starling RC, et al. Comparison of dobutamine-based and milrinone-based therapy for advanced decompensated congestive heart failure: Hemodynamic efficacy, clinical outcome, and economic impact. American Heart Journal. 2001;142:998-1002.
- Shin, DD, Brandimarte F, De Luca L, et al. Review of current and investigational pharmacologic agents for acute heart failure syndromes. Am J Cardiol. 2007;99(suppl):4A-23A.
- Kale P and Fang JC. Devices in acute heart failure. Crit Care Med. 2008;36(Suppl.):S121-128.
Case
A 72-year-old retired nurse with known nonischemic dilated cardiomyopathy with an ejection fraction of approximately 20% and status-post cardiac resynchronization therapy presents to the emergency department with dyspnea with minimal activity, three-pillow orthopnea, and paroxysmal nocturnal dyspnea.
She had been hospitalized twice during the past 60 days for similar symptoms. Her medications included losartan (20 mg po q daily), carvedilol (3.125 mg twice daily), spironolactone (25 mg daily), digoxin (0.125 mg daily), and furosemide (80 mg twice daily). Vital signs are notable for a blood pressure of 90/50 mmHg and an irregular pulse of 90 beats per minute. Physical examination is notable for marked jugular venous distension, lungs clear to auscultation bilaterally, biventricular heaves, a markedly displaced left ventricular point of maximal impulse, and a prominent S3 gallop.
Despite treatment with intravenous furosemide and temporary withdrawal of carvedilol, the patient remains symptomatic with persistent jugular venous distension.
Should she be given a vasoactive agent?
Overview
Acute heart failure syndrome (AHFS), defined as a gradual or rapid change in heart failure signs and symptoms, is the most common cause of hospitalization in the United States1. It is associated with an average in-hospital mortality of 4% to 5%, a 30-day mortality of 7% to11%, and a one-year mortality of 33%2.
In patients with previously established myocardial dysfunction, AHFS commonly reflects exacerbation of symptoms after a period of stability. The clinical presentation and severity of AHFS may range from mild volume overload to life-threatening cardiogenic shock and multi-organ failure unresponsive to pharmacologic therapy.2
Initial management of AHFS depends on definition of the patient’s hemodynamic profile. To guide initial therapy, classify patients into one of four hemodynamic profiles during a brief bedside assessment that relies on evaluation of filling pressures (wet or dry) and adequacy of perfusion (hot or cold) (see figure 1).3
Treating volume overload or elevated filling pressures generally begins with diuretics. Diuretics have been shown to provide symptomatic relief, though they have not yet been proven safe.4 Initial treatment can include a loop diuretic at a dose higher than the patient’s chronic dose, with intravenous dosing offering greater bio-absorption and rapidity in onset of action.5 If perfusion is inadequate, escalate therapy beyond diuretics to include vasoactive agents.
Review of the Data
The use of vasoactive medications is largely based on anecdotal experiences and physiologic assumptions rather than on adequately powered prospective randomized controlled trials.6 Vasoactive therapy includes vasodilator and inotropic support and is generally limited for use in patients with advanced disease not responding to standard medical treatment and diuresis. The physiologic premise rests in the expected improvement in ventricular filling pressures and cardiac output with reduction in afterload and/or preload. Vasodilators counteract vascular constriction, reducing both preload and afterload. Positive inotropic agents amplify cardiac output by increasing the strength of myocardial contraction.
Vasodilators
The Heart Failure Society of America (HFSA) 2006 Comprehensive Heart Failure Practice Guidelines state, “In the absence of symptomatic hypotension, intravenous vasodilators (nitroglycerin, nitroprusside, or nesiritide) may be considered as an addition to diuretic therapy for rapid improvement of congestive symptoms.”7 The clinical utility of nesiritide remains in question with clinical and hemodynamic improvement demonstrated in three randomized trials 8-10; but tempered against meta-analyses 11-12 of selected trials, demonstrating a non-significant trend toward increased kidney dysfunction and death within 30 days (35/485 [7.2%] vs. 15/377 [4.0%] patients; risk ratio from meta-analyses, 1.74; 95% confidence interval, 0.97-3.12; p=0.059). In a randomized trial of 489 in-patients with dyspnea at rest from AHFS, treatment with three hours of intravenous nesiritide resulted in a significant improvement in dyspnea compared with placebo (p=0.03). Similar improvement was observed with intravenous nitroglycerin and did not differ statistically from that observed with nesiritide.8 Nitroprusside, an attractive option among those with hypertension and cardiogenic pulmonary edema, is limited by the need for invasive hemodynamic monitoring and potential for either cyanide toxicity or worsening myocardial ischemia.
Inotropes
Again, there is little evidence from adequately powered randomized controlled trials guiding the use of inotropes. Their use is generally limited to the following indications (see figure 2): (1) Short-term treatment for AHFS that is unresponsive to adequate doses of diuretics and especially when associated with systemic hypotension, (2) Bridge to recovery (as following myocarditis) or to definitive treatment (as with transplant), or (3) For palliation when symptomatic relief is the agreed upon goal.13 The HFSA 2006 guideline states: “Intravenous inotropes may be considered to relieve symptoms and improve end-organ function in patients with advanced HF characterized by left ventricle dilation, reduced left ventricular ejection fraction, and diminished peripheral perfusion or end-organ dysfunction, particularly if these patients have marginal systolic blood pressure, have symptomatic hypotension despite adequate filling pressures, or are unresponsive to, or intolerant of, intravenous vasodilators.”7
Dobutamine and milrinone are the most commonly used IV inotropes for the treatment of AHFS and increase contractility by increasing intracellular levels of cyclic adenylate monophosphate (cAMP). Dobutamine is a catechlamine agonist that increases cAMP production through stimulation of adenylate cyclase. Milrinone selectively inhibits phosphodiesterase III, which catalyzes the breakdown of cAMP.
Despite their frequent use when traditional treatments have failed, the data supporting the use of dobutamine and milrinone is limited. The largest registry of patients with AHFS to date associated excess mortality with intravenous inotrope use compared to nitroglycerin or nesiritide.14 In a study population of 255 patients randomized to receive either intravenous nesiritide or intravenous dobutamine, Burger et al.15 demonstrated that dobutamine significantly increased the mean number of ventricular tachycardia events per 24 hours (p=0.001), suggesting increased arrhythmogenicity associated with inotrope use. Nonetheless, in a randomized trial of 15 patients admitted with AHFS, functional class improved in six of eight dobutamine-treated patients, but in only two of seven patients treated with placebo, suggesting clinical improvement as a consequence of inotropic stimulation.16 Unverferth et al. demonstrated a similar sustained functional improvement up to 10 weeks following a 72-hour infusion of intravenous dobutamine. 17
The Outcomes of a Prospective Trial of Intravenous Milrinone for Exacerbations of Chronic Heart Failure trial (OPTIME-CHF), randomized 951 patients with AHFS to receive either intravenous milrinone or placebo within 48 hours of hospitalization.18 Compared to placebo, milrinone was associated with a significant increase in sustained hypotension requiring intervention (10.7% vs. 3.2%; p<.001) and new atrial arrhythmias (4.6% vs. 1.5%; p=0.004), along with a non-significant trend toward increased mortality (3.8% vs. 2.3%; p=0.19). However, as measured by a visual analog scale, milrinone-treated patients reported feeling better than those treated with placebo at 30 days post-randomization (p=0.02).
Although there are not randomized data comparing the efficacy of milrinone and dobutamine in AHFS, a retrospective analysis of 329 patients compared the hemodynamic and clinical effects of these two inotropes.19 Milrinone consistently was associated with a more favorable hemodynamic response, including lower systemic vascular resistance (p=0.01); lower pulmonary artery wedge pressure (p<0.001); larger percentage increase in cardiac index (p=0.03); and larger percentage decrease in pulmonary vascular resistance (p=0.0001). In-hospital mortality (dobutamine 7.8% vs. milrinone 10%) was not significantly different.
Conclusion
Clearly, vasoactive and inotropic agents are available when AHFS is refractory to traditional diuresis and may offer short-term symptomatic relief, palliation in the context of end-of-life care, or bridge to recovery or more definitive treatment. Unfortunately, sufficient and robust evidence that supports the safety and efficacy of such agents is lacking and their use is largely guided by historical practices, clinical experience, and anticipation of theoretic physiologic changes. While adequately powered prospective randomized data emerge, newer agents such as vasopressin receptor antagonists, cardiac myosin activators, calcium sensitizers, and adenosine-receptor antagonists will offer additional pharmacologic options.20 When continued pharmacologic support becomes ineffective, device therapy is available to aid in the treatment of AHFS and includes ultrafiltration to reduce filling pressures and intra-aortic balloon pump counterpulsation or left ventricular assist device placement for pharmacologically resistant cardiogenic shock.21
Back to the Case
Despite maximal medical therapy for her chronic heart failure and biventricular pacing, the patient continued to have markedly limited functional status and repeated hospitalizations for AHFS. Given her advanced age and poor nutritional status, she was not a candidate for cardiac transplantation or placement of a left ventricular assist device. To allow for palliative tailored therapy, right heart catheterization was performed. Right heart catheterization revealed elevated filling pressures, as follows: right atrium, 20 mmHg; pulmonary artery, 63/34 mmHg (mean 47 mmHg); and pulmonary capillary wedge, 29 mmHg. Her mixed venous oxygen saturation was only 41% with a calculated cardiac output of 2.9 liters per minute and cardiac index of 2 liters per minute per meter squared.
As she expressed symptomatic relief as her goal, she was started on intravenous milrinone at 0.2 micrograms per kilogram per minute. This was done with the understanding her symptoms would likely would improve, at the expense of worsening longevity and prognosis. With uptitration of her intravenous milrinone and a continuous infusion of furosemide, she demonstrated the following filling pressures within 24 hours: right atrium, 18 mmHg; pulmonary artery, 63/33 mmHg (mean 43 mmHg); and pulmonary capillary wedge, 24. Importantly, her mixed venous oxygen saturation improved to 68% with a calculated cardiac output of 3.4 liters per minute and cardiac index of 2.4 liters per minute per meter squared. These favorable hemodynamic changes were accompanied by modest improvement in symptoms. After continued intravenous diuresis, she was transitioned back to an oral diuretic regimen and was ultimately discharged to home with a continuous infusion of milrinone for palliation. TH
Drs. Vaishnava, McKean, Nohria, and Baughman are from Brigham and Women’s Hospital and Harvard Medical School in Boston, Mass.
REFERENCES:
- Thom T, Haase N, Rosamond W, et al. Heart disease and stroke statistics – 2006 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2006;113:85-151.
- Iong P, Vowinckel E, Liu PP, Gong Y, Tu JV. Prognosis and determinants of survival in patients newly hospitalized for heart failure: a population-based study. Arch Intern Med. 2002;162:1689-94.
- Nohria A, Lewis EF, Stevenson LW. Medical management of advanced heart failure. JAMA. 2002;287;628-40.
- Faris R, Flather MD, Purcell H, et al. Diuretics for heart failure. Cochrane Database Syst Rev. 2006;1;CD003838.
- Wang DJ and Gottlieb SS. Diuretics: Still the mainstay of treatment. Crit Care Med. 2008;36(Suppl.):S89-S94.
- Fares WH. Management of acute decompensated heart failure in an evidence-based era: What is the evidence behind the current standard of care? Heart & Lung. 2008;37(3):173-8.
- Adams KF, Lindenfield J, Arnold J, et al. Executive summary: HFSA 2006 comprehensive heart failure practice guidelines. J Card Fail. 2006;12:10-38.
- Publication Committee for the VMAC Investigators (Vasodilatation in the Management of Acute CHF). Intravenous nesiritide vs nitroglycerin for treatment of decompensated congestive heart failure: a randomized controlled trial. JAMA. 2002;297:1531-40.
- Peacock WF, Enerman CL, Silver MA, on behalf of the PROACTION Study Group. Am J Emerg Med. 2005;23:327-31.
- Cotter G, Metzkor E, Kaluski E, et al. Randomized trial of high-dose isosorbide dinitrate plus low-dose furosemide versus high-dose furosemide plus low-dose isosorbide dinitrate in severe pulmonary edema. Lancet. 1998;351:389-93.
- Sackner-Bernstein JD, Kowalski M, Fox M, Aaronson K. Short-term risk of death after treatment with nesiritide for decompensated heart failure: a pooled analysis of randomized controlled trials. JAMA. 2005;293:1900-5.
- Sackner-Bernstein JD, Skopicki HA, Aaronson K. Risk of worsening renal function with nesiritide in patients with acutely decompensated heart failure. Circulation. 2005;111:1487-91.
- Felker GM and O’Connor CM. Inotropic therapy for heart failure: An evidence-based approach. American Heart Journal. 2001; 142:393-401.
- Abrahm WT, Adams KF, Fonarow GC, et al. In-hospital mortality in patients with acute decompensated heart failure requiring intravenous vasoactive medications: an analysis from the Acute Decompensated Heart Failure National Registry (ADHERE). J Am Coll Cardiol. 2005;46:57-64.
- Burger AJ, Houton DP, LeJemtel T, et al. Effect of nesiritide and dobutamine on ventricular arrhythmias in the treatment of patients with acutely decompensated congestive heart failure: the PRECEDENT study. American Heart Journal. 2002;144:1102-8.
- Liang CS, Sherman LG, Doherty JU, et al. Sustained improvement of cardiac function in patients with congestive heart failure after short-term infusion of dobutamine. Circulation. 1984;69:113-9.
- Unverferth DV, Magorien RD, Lewis RP, et al. Long-term benefit of dobutamine in patients with congestive cardiomyopathy. American Heart Journal. 1980;100:622-30.
- Cuffe MS, Califf RM, Adams KF Jr, et al. Outcomes of a Prospective Trial of Intravenous Milrinone for Exacerbations of Chronic Heart Failure (OPTIME-CHF) Investigators. Short-term intravenous milrinone for acute exacerbation of chronic heart failure: a randomized controlled trial. JAMA. 2002;287:1541-7.
- Yamani MH, Haji SA, Starling RC, et al. Comparison of dobutamine-based and milrinone-based therapy for advanced decompensated congestive heart failure: Hemodynamic efficacy, clinical outcome, and economic impact. American Heart Journal. 2001;142:998-1002.
- Shin, DD, Brandimarte F, De Luca L, et al. Review of current and investigational pharmacologic agents for acute heart failure syndromes. Am J Cardiol. 2007;99(suppl):4A-23A.
- Kale P and Fang JC. Devices in acute heart failure. Crit Care Med. 2008;36(Suppl.):S121-128.
Case
A 72-year-old retired nurse with known nonischemic dilated cardiomyopathy with an ejection fraction of approximately 20% and status-post cardiac resynchronization therapy presents to the emergency department with dyspnea with minimal activity, three-pillow orthopnea, and paroxysmal nocturnal dyspnea.
She had been hospitalized twice during the past 60 days for similar symptoms. Her medications included losartan (20 mg po q daily), carvedilol (3.125 mg twice daily), spironolactone (25 mg daily), digoxin (0.125 mg daily), and furosemide (80 mg twice daily). Vital signs are notable for a blood pressure of 90/50 mmHg and an irregular pulse of 90 beats per minute. Physical examination is notable for marked jugular venous distension, lungs clear to auscultation bilaterally, biventricular heaves, a markedly displaced left ventricular point of maximal impulse, and a prominent S3 gallop.
Despite treatment with intravenous furosemide and temporary withdrawal of carvedilol, the patient remains symptomatic with persistent jugular venous distension.
Should she be given a vasoactive agent?
Overview
Acute heart failure syndrome (AHFS), defined as a gradual or rapid change in heart failure signs and symptoms, is the most common cause of hospitalization in the United States1. It is associated with an average in-hospital mortality of 4% to 5%, a 30-day mortality of 7% to11%, and a one-year mortality of 33%2.
In patients with previously established myocardial dysfunction, AHFS commonly reflects exacerbation of symptoms after a period of stability. The clinical presentation and severity of AHFS may range from mild volume overload to life-threatening cardiogenic shock and multi-organ failure unresponsive to pharmacologic therapy.2
Initial management of AHFS depends on definition of the patient’s hemodynamic profile. To guide initial therapy, classify patients into one of four hemodynamic profiles during a brief bedside assessment that relies on evaluation of filling pressures (wet or dry) and adequacy of perfusion (hot or cold) (see figure 1).3
Treating volume overload or elevated filling pressures generally begins with diuretics. Diuretics have been shown to provide symptomatic relief, though they have not yet been proven safe.4 Initial treatment can include a loop diuretic at a dose higher than the patient’s chronic dose, with intravenous dosing offering greater bio-absorption and rapidity in onset of action.5 If perfusion is inadequate, escalate therapy beyond diuretics to include vasoactive agents.
Review of the Data
The use of vasoactive medications is largely based on anecdotal experiences and physiologic assumptions rather than on adequately powered prospective randomized controlled trials.6 Vasoactive therapy includes vasodilator and inotropic support and is generally limited for use in patients with advanced disease not responding to standard medical treatment and diuresis. The physiologic premise rests in the expected improvement in ventricular filling pressures and cardiac output with reduction in afterload and/or preload. Vasodilators counteract vascular constriction, reducing both preload and afterload. Positive inotropic agents amplify cardiac output by increasing the strength of myocardial contraction.
Vasodilators
The Heart Failure Society of America (HFSA) 2006 Comprehensive Heart Failure Practice Guidelines state, “In the absence of symptomatic hypotension, intravenous vasodilators (nitroglycerin, nitroprusside, or nesiritide) may be considered as an addition to diuretic therapy for rapid improvement of congestive symptoms.”7 The clinical utility of nesiritide remains in question with clinical and hemodynamic improvement demonstrated in three randomized trials 8-10; but tempered against meta-analyses 11-12 of selected trials, demonstrating a non-significant trend toward increased kidney dysfunction and death within 30 days (35/485 [7.2%] vs. 15/377 [4.0%] patients; risk ratio from meta-analyses, 1.74; 95% confidence interval, 0.97-3.12; p=0.059). In a randomized trial of 489 in-patients with dyspnea at rest from AHFS, treatment with three hours of intravenous nesiritide resulted in a significant improvement in dyspnea compared with placebo (p=0.03). Similar improvement was observed with intravenous nitroglycerin and did not differ statistically from that observed with nesiritide.8 Nitroprusside, an attractive option among those with hypertension and cardiogenic pulmonary edema, is limited by the need for invasive hemodynamic monitoring and potential for either cyanide toxicity or worsening myocardial ischemia.
Inotropes
Again, there is little evidence from adequately powered randomized controlled trials guiding the use of inotropes. Their use is generally limited to the following indications (see figure 2): (1) Short-term treatment for AHFS that is unresponsive to adequate doses of diuretics and especially when associated with systemic hypotension, (2) Bridge to recovery (as following myocarditis) or to definitive treatment (as with transplant), or (3) For palliation when symptomatic relief is the agreed upon goal.13 The HFSA 2006 guideline states: “Intravenous inotropes may be considered to relieve symptoms and improve end-organ function in patients with advanced HF characterized by left ventricle dilation, reduced left ventricular ejection fraction, and diminished peripheral perfusion or end-organ dysfunction, particularly if these patients have marginal systolic blood pressure, have symptomatic hypotension despite adequate filling pressures, or are unresponsive to, or intolerant of, intravenous vasodilators.”7
Dobutamine and milrinone are the most commonly used IV inotropes for the treatment of AHFS and increase contractility by increasing intracellular levels of cyclic adenylate monophosphate (cAMP). Dobutamine is a catechlamine agonist that increases cAMP production through stimulation of adenylate cyclase. Milrinone selectively inhibits phosphodiesterase III, which catalyzes the breakdown of cAMP.
Despite their frequent use when traditional treatments have failed, the data supporting the use of dobutamine and milrinone is limited. The largest registry of patients with AHFS to date associated excess mortality with intravenous inotrope use compared to nitroglycerin or nesiritide.14 In a study population of 255 patients randomized to receive either intravenous nesiritide or intravenous dobutamine, Burger et al.15 demonstrated that dobutamine significantly increased the mean number of ventricular tachycardia events per 24 hours (p=0.001), suggesting increased arrhythmogenicity associated with inotrope use. Nonetheless, in a randomized trial of 15 patients admitted with AHFS, functional class improved in six of eight dobutamine-treated patients, but in only two of seven patients treated with placebo, suggesting clinical improvement as a consequence of inotropic stimulation.16 Unverferth et al. demonstrated a similar sustained functional improvement up to 10 weeks following a 72-hour infusion of intravenous dobutamine. 17
The Outcomes of a Prospective Trial of Intravenous Milrinone for Exacerbations of Chronic Heart Failure trial (OPTIME-CHF), randomized 951 patients with AHFS to receive either intravenous milrinone or placebo within 48 hours of hospitalization.18 Compared to placebo, milrinone was associated with a significant increase in sustained hypotension requiring intervention (10.7% vs. 3.2%; p<.001) and new atrial arrhythmias (4.6% vs. 1.5%; p=0.004), along with a non-significant trend toward increased mortality (3.8% vs. 2.3%; p=0.19). However, as measured by a visual analog scale, milrinone-treated patients reported feeling better than those treated with placebo at 30 days post-randomization (p=0.02).
Although there are not randomized data comparing the efficacy of milrinone and dobutamine in AHFS, a retrospective analysis of 329 patients compared the hemodynamic and clinical effects of these two inotropes.19 Milrinone consistently was associated with a more favorable hemodynamic response, including lower systemic vascular resistance (p=0.01); lower pulmonary artery wedge pressure (p<0.001); larger percentage increase in cardiac index (p=0.03); and larger percentage decrease in pulmonary vascular resistance (p=0.0001). In-hospital mortality (dobutamine 7.8% vs. milrinone 10%) was not significantly different.
Conclusion
Clearly, vasoactive and inotropic agents are available when AHFS is refractory to traditional diuresis and may offer short-term symptomatic relief, palliation in the context of end-of-life care, or bridge to recovery or more definitive treatment. Unfortunately, sufficient and robust evidence that supports the safety and efficacy of such agents is lacking and their use is largely guided by historical practices, clinical experience, and anticipation of theoretic physiologic changes. While adequately powered prospective randomized data emerge, newer agents such as vasopressin receptor antagonists, cardiac myosin activators, calcium sensitizers, and adenosine-receptor antagonists will offer additional pharmacologic options.20 When continued pharmacologic support becomes ineffective, device therapy is available to aid in the treatment of AHFS and includes ultrafiltration to reduce filling pressures and intra-aortic balloon pump counterpulsation or left ventricular assist device placement for pharmacologically resistant cardiogenic shock.21
Back to the Case
Despite maximal medical therapy for her chronic heart failure and biventricular pacing, the patient continued to have markedly limited functional status and repeated hospitalizations for AHFS. Given her advanced age and poor nutritional status, she was not a candidate for cardiac transplantation or placement of a left ventricular assist device. To allow for palliative tailored therapy, right heart catheterization was performed. Right heart catheterization revealed elevated filling pressures, as follows: right atrium, 20 mmHg; pulmonary artery, 63/34 mmHg (mean 47 mmHg); and pulmonary capillary wedge, 29 mmHg. Her mixed venous oxygen saturation was only 41% with a calculated cardiac output of 2.9 liters per minute and cardiac index of 2 liters per minute per meter squared.
As she expressed symptomatic relief as her goal, she was started on intravenous milrinone at 0.2 micrograms per kilogram per minute. This was done with the understanding her symptoms would likely would improve, at the expense of worsening longevity and prognosis. With uptitration of her intravenous milrinone and a continuous infusion of furosemide, she demonstrated the following filling pressures within 24 hours: right atrium, 18 mmHg; pulmonary artery, 63/33 mmHg (mean 43 mmHg); and pulmonary capillary wedge, 24. Importantly, her mixed venous oxygen saturation improved to 68% with a calculated cardiac output of 3.4 liters per minute and cardiac index of 2.4 liters per minute per meter squared. These favorable hemodynamic changes were accompanied by modest improvement in symptoms. After continued intravenous diuresis, she was transitioned back to an oral diuretic regimen and was ultimately discharged to home with a continuous infusion of milrinone for palliation. TH
Drs. Vaishnava, McKean, Nohria, and Baughman are from Brigham and Women’s Hospital and Harvard Medical School in Boston, Mass.
REFERENCES:
- Thom T, Haase N, Rosamond W, et al. Heart disease and stroke statistics – 2006 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2006;113:85-151.
- Iong P, Vowinckel E, Liu PP, Gong Y, Tu JV. Prognosis and determinants of survival in patients newly hospitalized for heart failure: a population-based study. Arch Intern Med. 2002;162:1689-94.
- Nohria A, Lewis EF, Stevenson LW. Medical management of advanced heart failure. JAMA. 2002;287;628-40.
- Faris R, Flather MD, Purcell H, et al. Diuretics for heart failure. Cochrane Database Syst Rev. 2006;1;CD003838.
- Wang DJ and Gottlieb SS. Diuretics: Still the mainstay of treatment. Crit Care Med. 2008;36(Suppl.):S89-S94.
- Fares WH. Management of acute decompensated heart failure in an evidence-based era: What is the evidence behind the current standard of care? Heart & Lung. 2008;37(3):173-8.
- Adams KF, Lindenfield J, Arnold J, et al. Executive summary: HFSA 2006 comprehensive heart failure practice guidelines. J Card Fail. 2006;12:10-38.
- Publication Committee for the VMAC Investigators (Vasodilatation in the Management of Acute CHF). Intravenous nesiritide vs nitroglycerin for treatment of decompensated congestive heart failure: a randomized controlled trial. JAMA. 2002;297:1531-40.
- Peacock WF, Enerman CL, Silver MA, on behalf of the PROACTION Study Group. Am J Emerg Med. 2005;23:327-31.
- Cotter G, Metzkor E, Kaluski E, et al. Randomized trial of high-dose isosorbide dinitrate plus low-dose furosemide versus high-dose furosemide plus low-dose isosorbide dinitrate in severe pulmonary edema. Lancet. 1998;351:389-93.
- Sackner-Bernstein JD, Kowalski M, Fox M, Aaronson K. Short-term risk of death after treatment with nesiritide for decompensated heart failure: a pooled analysis of randomized controlled trials. JAMA. 2005;293:1900-5.
- Sackner-Bernstein JD, Skopicki HA, Aaronson K. Risk of worsening renal function with nesiritide in patients with acutely decompensated heart failure. Circulation. 2005;111:1487-91.
- Felker GM and O’Connor CM. Inotropic therapy for heart failure: An evidence-based approach. American Heart Journal. 2001; 142:393-401.
- Abrahm WT, Adams KF, Fonarow GC, et al. In-hospital mortality in patients with acute decompensated heart failure requiring intravenous vasoactive medications: an analysis from the Acute Decompensated Heart Failure National Registry (ADHERE). J Am Coll Cardiol. 2005;46:57-64.
- Burger AJ, Houton DP, LeJemtel T, et al. Effect of nesiritide and dobutamine on ventricular arrhythmias in the treatment of patients with acutely decompensated congestive heart failure: the PRECEDENT study. American Heart Journal. 2002;144:1102-8.
- Liang CS, Sherman LG, Doherty JU, et al. Sustained improvement of cardiac function in patients with congestive heart failure after short-term infusion of dobutamine. Circulation. 1984;69:113-9.
- Unverferth DV, Magorien RD, Lewis RP, et al. Long-term benefit of dobutamine in patients with congestive cardiomyopathy. American Heart Journal. 1980;100:622-30.
- Cuffe MS, Califf RM, Adams KF Jr, et al. Outcomes of a Prospective Trial of Intravenous Milrinone for Exacerbations of Chronic Heart Failure (OPTIME-CHF) Investigators. Short-term intravenous milrinone for acute exacerbation of chronic heart failure: a randomized controlled trial. JAMA. 2002;287:1541-7.
- Yamani MH, Haji SA, Starling RC, et al. Comparison of dobutamine-based and milrinone-based therapy for advanced decompensated congestive heart failure: Hemodynamic efficacy, clinical outcome, and economic impact. American Heart Journal. 2001;142:998-1002.
- Shin, DD, Brandimarte F, De Luca L, et al. Review of current and investigational pharmacologic agents for acute heart failure syndromes. Am J Cardiol. 2007;99(suppl):4A-23A.
- Kale P and Fang JC. Devices in acute heart failure. Crit Care Med. 2008;36(Suppl.):S121-128.