User login
Journal of Hospital Medicine – Jan. 2018
BACKGROUND: Hospital charges and lengths of stay may be greater when adults with chronic conditions are admitted to children’s hospitals. Despite multiple efforts to improve pediatric-adult health care transitions, little guidance exists for transitioning inpatient care.
OBJECTIVE: This study sought to characterize pediatric-adult inpatient care transitions across general pediatric services at U.S. children’s hospitals.
DESIGN and SETTING: National survey of inpatient general pediatric service leaders at U.S. children’s hospitals from January 2016 to July 2016.
MEASUREMENT: Questionnaires assessed institutional characteristics, presence of inpatient transition initiatives (having a specific process and/or leader), and 22 inpatient transition activities. Scales of highly correlated activities were created using exploratory factor analysis. Logistic regression identified associations among institutional characteristics, transition activities, and presence of an inpatient transition initiative.
RESULTS: Of 195 children’s hospitals, 96 responded (49.2% response rate). Transition initiatives were present at 38% of children’s hospitals, more often where there were providers who were trained in both internal medicine and pediatrics or where there were outpatient transition processes. Specific activities were infrequent and varied widely from 2.1% (systems to track youth in transition) to 40.5% (addressing potential insurance problems). Institutions with initiatives more often consistently performed the majority of activities, including using checklists and creating patient-centered transition care plans. Of remaining activities, half involved transition planning, the essential step between readiness and transfer.
CONCLUSION: Relatively few inpatient general pediatric services at U.S. children’s hospitals have leaders or dedicated processes to shepherd transitions to adult-oriented inpatient care. Across institutions, there is wide variability in performance of activities to facilitate this transition. Feasible process and outcome measures are needed.
Also in JHM this month
Characterizing hospitalist practice and perceptions of critical care delivery
AUTHORS: Joseph R. Sweigart, MD, FACP, FHM; David Aymond, MD; Alfred Burger, MD, FACP, SFHM; Andy Kelly, MAS, MS; Nick Marzano, Med; Thomas McIlraith, MD, SFHM; Peter Morris, MD; Mark V. Williams, MD, FACP, MHM; and Eric M. Siegal, MD, SFHM, FCCM
Clinical decision making: Observing the smartphone user an observational study in predicting acute surgical patients’ suitability for discharge
AUTHORS: Richard Hoffmann, MBBS; Simon Harley, MBBS; Samuel Ellison, MBBS; and Peter G. Devitt, MBBS, FRACS
BACKGROUND: Hospital charges and lengths of stay may be greater when adults with chronic conditions are admitted to children’s hospitals. Despite multiple efforts to improve pediatric-adult health care transitions, little guidance exists for transitioning inpatient care.
OBJECTIVE: This study sought to characterize pediatric-adult inpatient care transitions across general pediatric services at U.S. children’s hospitals.
DESIGN and SETTING: National survey of inpatient general pediatric service leaders at U.S. children’s hospitals from January 2016 to July 2016.
MEASUREMENT: Questionnaires assessed institutional characteristics, presence of inpatient transition initiatives (having a specific process and/or leader), and 22 inpatient transition activities. Scales of highly correlated activities were created using exploratory factor analysis. Logistic regression identified associations among institutional characteristics, transition activities, and presence of an inpatient transition initiative.
RESULTS: Of 195 children’s hospitals, 96 responded (49.2% response rate). Transition initiatives were present at 38% of children’s hospitals, more often where there were providers who were trained in both internal medicine and pediatrics or where there were outpatient transition processes. Specific activities were infrequent and varied widely from 2.1% (systems to track youth in transition) to 40.5% (addressing potential insurance problems). Institutions with initiatives more often consistently performed the majority of activities, including using checklists and creating patient-centered transition care plans. Of remaining activities, half involved transition planning, the essential step between readiness and transfer.
CONCLUSION: Relatively few inpatient general pediatric services at U.S. children’s hospitals have leaders or dedicated processes to shepherd transitions to adult-oriented inpatient care. Across institutions, there is wide variability in performance of activities to facilitate this transition. Feasible process and outcome measures are needed.
Also in JHM this month
Characterizing hospitalist practice and perceptions of critical care delivery
AUTHORS: Joseph R. Sweigart, MD, FACP, FHM; David Aymond, MD; Alfred Burger, MD, FACP, SFHM; Andy Kelly, MAS, MS; Nick Marzano, Med; Thomas McIlraith, MD, SFHM; Peter Morris, MD; Mark V. Williams, MD, FACP, MHM; and Eric M. Siegal, MD, SFHM, FCCM
Clinical decision making: Observing the smartphone user an observational study in predicting acute surgical patients’ suitability for discharge
AUTHORS: Richard Hoffmann, MBBS; Simon Harley, MBBS; Samuel Ellison, MBBS; and Peter G. Devitt, MBBS, FRACS
BACKGROUND: Hospital charges and lengths of stay may be greater when adults with chronic conditions are admitted to children’s hospitals. Despite multiple efforts to improve pediatric-adult health care transitions, little guidance exists for transitioning inpatient care.
OBJECTIVE: This study sought to characterize pediatric-adult inpatient care transitions across general pediatric services at U.S. children’s hospitals.
DESIGN and SETTING: National survey of inpatient general pediatric service leaders at U.S. children’s hospitals from January 2016 to July 2016.
MEASUREMENT: Questionnaires assessed institutional characteristics, presence of inpatient transition initiatives (having a specific process and/or leader), and 22 inpatient transition activities. Scales of highly correlated activities were created using exploratory factor analysis. Logistic regression identified associations among institutional characteristics, transition activities, and presence of an inpatient transition initiative.
RESULTS: Of 195 children’s hospitals, 96 responded (49.2% response rate). Transition initiatives were present at 38% of children’s hospitals, more often where there were providers who were trained in both internal medicine and pediatrics or where there were outpatient transition processes. Specific activities were infrequent and varied widely from 2.1% (systems to track youth in transition) to 40.5% (addressing potential insurance problems). Institutions with initiatives more often consistently performed the majority of activities, including using checklists and creating patient-centered transition care plans. Of remaining activities, half involved transition planning, the essential step between readiness and transfer.
CONCLUSION: Relatively few inpatient general pediatric services at U.S. children’s hospitals have leaders or dedicated processes to shepherd transitions to adult-oriented inpatient care. Across institutions, there is wide variability in performance of activities to facilitate this transition. Feasible process and outcome measures are needed.
Also in JHM this month
Characterizing hospitalist practice and perceptions of critical care delivery
AUTHORS: Joseph R. Sweigart, MD, FACP, FHM; David Aymond, MD; Alfred Burger, MD, FACP, SFHM; Andy Kelly, MAS, MS; Nick Marzano, Med; Thomas McIlraith, MD, SFHM; Peter Morris, MD; Mark V. Williams, MD, FACP, MHM; and Eric M. Siegal, MD, SFHM, FCCM
Clinical decision making: Observing the smartphone user an observational study in predicting acute surgical patients’ suitability for discharge
AUTHORS: Richard Hoffmann, MBBS; Simon Harley, MBBS; Samuel Ellison, MBBS; and Peter G. Devitt, MBBS, FRACS
Transitioning from General Pediatric to Adult-Oriented Inpatient Care: National Survey of US Children’s Hospitals
Over 90% of children with chronic diseases now survive into adulthood.1,2 Clinical advances overcoming diseases previously fatal in childhood create new challenges for health systems with limited capacity to manage young adults with complicated and unfamiliar childhood-onset conditions. Consequently, improving the transition from pediatric to adult-oriented care has become a national priority.
Although major pediatric-adult transition initiatives—such as the Six Core Elements Framework,3 a technical brief from the Agency for Healthcare Research and Quality,4 and joint statements from major medical societies5,6—outline key transition recommendations generally and for outpatients, they contain limited or no guidance specifically devoted to transitioning inpatient hospital care from pediatric to adult-oriented settings. Key unknowns include whether, when, and how to transition inpatient care from children’s to nonchildren’s hospitals and how this can be integrated into comprehensive youth-adult transition care.
Nevertheless, the number of discharges of 18- to 21-year-old patients with chronic conditions admitted to children’s hospitals is increasing at a faster rate than discharges of other age groups,7 suggesting both that the population is growing in size and that there are important barriers to transitioning these patients into nonchildren’s hospital settings. Spending on adult patients 18 years or older admitted to children’s hospitals has grown to $1 billion annually.8 Hospitalizations are a commonly proposed outcome measure of pediatric-adult transition work.1,9,10 For example, higher rates of avoidable hospitalizations during early adulthood have been observed for 15- to 22-year-olds with kidney failure cared for exclusively in adult-oriented facilities and during the years immediately after transfer to adult care.11
While research is beginning to describe outcomes of adult-aged patients with childhood-onset chronic conditions admitted to children’s hospitals,7,12,13 there has been no comprehensive description of efforts within children’s hospitals to transition such patients into adult-oriented inpatient settings. This information is necessary to outline institutional needs, delineate opportunities for improvement, and help clinicians strategically organize services for patients requiring this transition.
We sought to characterize the current state of the transition from pediatric- to adult-oriented inpatient care across general pediatric inpatient services at US children’s hospitals. We hypothesized that only a limited and inconsistent set of activities would be practiced. We also hypothesized that institutions having formal outpatient transition processes or providers with specialization to care for this age group, such as dual-trained internal medicine–pediatrics (med–peds) physicians, would report performing more activities.
METHODS
Study Design, Setting, Participants
We conducted a national survey of leaders of inpatient general pediatrics services at US children’s hospitals from January 2016 to July 2016. Hospitals were identified using the online Children’s Hospital Association directory. Hospitals without inpatient general pediatrics services (eg, rehabilitation or subspecialty-only facilities) were excluded.
We identified a single respondent from each of the 195 remaining children’s hospitals using a structured protocol. Phone numbers and e-mail addresses of potential respondents were gathered from hospital or medical school directories. Following a standard script, study team members contacted potential respondents to describe the purpose of the study and to confirm their contact information. Hospitals were also allowed to designate a different individual with more specific expertise to participate, when relevant (eg, specific faculty member leading a related quality improvement initiative). The goal was to identify a leader of inpatient care with the most knowledge of institutional practices related to the transition to adult inpatient care. Examples of respondent roles included director of inpatient pediatrics, chief of hospital medicine or general pediatrics, medical director, and similar titles.
Survey Elements
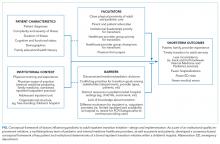
As part of a larger quality improvement initiative at our institution, a multidisciplinary team of pediatric and internal medicine healthcare providers (physicians, nurse practitioners, nurses, case managers, social workers, child life specialists), as well as parents and patients, developed an “ideal state” with this transition and a consensus-based conceptual framework of key patient and institutional determinants of a formal inpatient transition initiative for children with chronic conditions within a children’s hospital (Figure).
Institutional Context and Factors Influencing Inpatient Transitions
The following hospital characteristics were assessed: administrative structure (free-standing, hospital-within-hospital, or “free-leaning,” ie, separate physical structure but same administrative structure as a general hospital), urban versus rural, academic versus nonacademic, presence of an inpatient adolescent unit, presence of subspecialty admitting services, and providers with med–peds or family medicine training. The following provider group characteristics were assessed: number of full-time equivalents (FTEs), scope of practice (inpatient only, combination inpatient/outpatient), proportion of providers at a “senior” level (ie, at least 7 years posttraining or at an associate professor rank), estimated number of discharges per week, and proportion of patients cared for without resident physicians.
Inpatient Transition Initiative
Each institution was categorized as having or not having an inpatient transition initiative by whether they indicated having either (1) an institutional leader of the transition from pediatric to adult-oriented inpatient settings or (2) an inpatient transition process, for which “process” was defined as “a standard, organized, and predictable set of transition activities that may or may not be documented, but the steps are generally agreed upon.”
Specific Inpatient Transition Activities
Respondents indicated whether 22 activities occurred consistently, defined as at least 50% of the time. To facilitate description, activities were grouped into categories using the labels from the Six Core Elements framework3 (Table 1): Policy, Tracking and Monitoring, Readiness, Planning, Transfer of Care, and Transfer Completion. Respondents were also asked whether outpatient pediatric-adult transition activities existed at their institution and whether they were linked to inpatient transition activities.
Data Collection
After verifying contact information, respondents received an advanced priming phone call followed by a mailed request to participate with a printed uniform resource locator (URL) to the web survey. Two email reminders containing the URL were sent to nonresponders at 5 and 10 days after the initial mailing. Remaining nonresponders then received a reminder phone call, followed by a mailed paper copy of the survey questionnaire to be completed by hand approximately 2 weeks after the last emailed request. The survey was administered using the Qualtrics web survey platform (www.qualtrics.com). Data collection occurred between January 2016 and July 2016. Participants received a $20 incentive.
Statistical Analysis
Descriptive statistics summarized the current state of inpatient transition at general pediatrics services across US children’s hospitals. Exploratory factor analysis assessed whether individual activities were sufficiently correlated to allow grouping items and constructing scales. Differences in institutional or respondent characteristics between hospitals that did and did not report having an inpatient initiative were compared using t tests for continuous data. Fisher’s exact test was used for categorical data because some cell sizes were ≤5. Bivariate logistic regression quantified associations between presence versus absence of specific transition activities and presence versus absence of an inpatient transition initiative. Analyses were completed in STATA (SE version 14.0; StataCorp, College Station, Texas). The institutional review board at our institution approved this study.
RESULTS
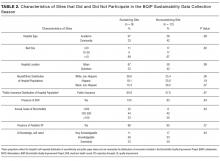
Responses were received from 96 of 195 children’s hospitals (49.2% response rate). Responding institution characteristics are summarized in Table 2. Free-standing children’s hospitals made up just over one-third of the sample (36%), while the remaining were free-leaning (22%) or hospital-within-hospital (43%). Most children’s hospitals (58%) did not have a specific adult-oriented hospital identified to receive transitioning patients. Slightly more than 10% had an inpatient adolescent unit. The majority of institutions were academic medical centers (78%) in urban locations (88%). Respondents represented small (<5 FTE, 21%), medium (6-10 FTE, 36%), and large provider groups (11+ FTE, 44%). Although 70% of respondents described their groups as “hospitalist only,” meaning providers only practiced inpatient general pediatrics, nearly 30% had providers practicing inpatient and outpatient general pediatrics. Just over 40% of respondents reported having med–peds providers. Pediatric-adult transition processes for outpatient care were present at 45% of institutions.
Transition Activities
Thirty-eight percent of children’s hospitals had an inpatient transition initiative using our study definition—31% by having a set of generally agreed upon activities, 19% by having a leader, and 11% having both. Inpatient transition leaders included pediatric hospitalists (43%), pediatric subspecialists and primary care providers (14% each), med–peds providers (11%), or case managers (7%). Respondent and institutional characteristics were similar at institutions that did and did not have an inpatient transition initiative (Table 2); however, children’s hospitals with inpatient transition initiatives more often had med–peds providers (P = .04). Institutions with pediatric-adult outpatient care transition processes more often had an inpatient initiative (71% and 29%, respectively; P = .001).
Exploratory factor analysis identified 2 groups of well-correlated items, which we grouped into “preparation” and “transfer initiation” scales (supplementary Appendix). The preparation scale was composed of the following 5 items (Cronbach α = 0.84): proactive identification of patients anticipated to need transition, proactive identification of patients overdue for transition, readiness formally assessed, timing discussed with family, and patient and/or family informed that the next stay would be at the adult facility. The transfer initiation scale comprised the following 6 items (Cronbach α = 0.72): transition education provided to families, primary care–subspecialist agreement on timing, subspecialist–subspecialist agreement on timing, patient decision-making ability established, adult facility tour, and standardized handoff communication between healthcare providers. While these items were analyzed only in this scale, other activities were analyzed as independent variables. In this analysis, 40.9% of institutions had a preparation scale score of 0 (no items performed), while 13% had all 5 items performed. Transfer initiation scale scores ranged from 0 (47%) to 6 (2%).
Specific activities varied widely across institutions, and none of the activities occurred at a majority of children’s hospitals (Table 3). Only 11% of children’s hospital transition policies referenced transitions of inpatient care. The activity most commonly reported across children’s hospitals was addressing potential insurance problems (41%). The least common inpatient transition activities were having child life consult during the first adult hospital stay (6%) or having a system to track and monitor youth in the inpatient transition process (2%). Transition processes and policies were relatively new among institutions that had them—average years an inpatient transition process had been in place was 1.2 (SD 0.4), and average years with a transition policy, including inpatient care, was 1.3 (SD 0.4).
Transition Activities at Hospitals With and Without an Inpatient Transition Initiative
Most activities assessed in this study (both scales plus 5 of 11 individual activities) were significantly more common in children’s hospitals with an inpatient transition initiative (Table 3). The most common activity was addressing potential insurance problems (46%), and the least common activity was having a system to track and monitor youth in the inpatient transition process (3%). The majority of institutions without an inpatient transition initiative (53%) performed 0 transfer initiation scale items. Large effect sizes between hospitals with and without a transition initiative were observed for use of a checklist to complete tasks (odds ratio [OR] 9.6, P = .04) and creation of a transition care plan (OR 9.0, P = .008). Of the 6 activities performed at similarly low frequencies at institutions with and without an initiative, half involved transition planning, the essential step after readiness but before actual transfer of care.
DISCUSSION
We conducted the first national survey describing the policies and procedures of the transition of general inpatient care from children’s to adult-oriented hospitals for youth and young adults with chronic conditions. Our main findings demonstrate that a relatively small number of general inpatient services at children’s hospitals have leaders or dedicated processes to shepherd this transition, and a minority have a specific adult hospital identified to receive their patients. Even among institutions with inpatient transition initiatives, there is wide variability in the performance of activities to facilitate transitioning out of US children’s hospitals. In these institutions, performance seems to be more lacking in later links of the transition chain. Results from this work can serve as a baseline and identify organizational needs and opportunities for future work.
Children’s hospital general services with and without an inpatient pediatric-adult transition initiative had largely similar characteristics; however, the limited sample size may lack power to detect some differences. Perhaps not surprisingly, having med–peds providers and outpatient transition processes were the characteristics most associated with having an inpatient pediatric-adult transition initiative. The observation that over 70% of hospitals with an outpatient process had an inpatient transition leader or dedicated process makes us optimistic that as general transition efforts expand, more robust inpatient transition activities may be achievable.
We appreciate that the most appropriate location to care for hospitalized young adults with childhood-onset chronic conditions is neither known nor answered with this study. Both options face challenges—adult-oriented hospitals may not be equipped to care for adult manifestations of childhood-onset conditions,14,15 while children’s hospitals may lack the resources and expertise to provide comprehensive care to adults.7 Although hospital charges and lengths of stay may be greater when adults with childhood-onset chronic conditions are admitted to children’s compared with adult hospitals,12,13,16 important confounders such as severity of illness could explain why adult-aged patients may both remain in children’s hospitals at older ages and simultaneously have worse outcomes than peers. Regardless, at some point, transitioning care into an adult-oriented hospital may be in patients’ best interests. If so, families and providers need guidance on (1) the important aspects of this transition and (2) how to effectively implement the transition.
Because the most important inpatient transition care activities are not empirically known, we designed our survey to assess a broad set of desirable activities emerging from our multidisciplinary quality improvement work. We mapped these activities to the categories used by the Six Core Elements framework.3 Addressing insurance issues was one of the most commonly reported activities, although still fewer than 50% of hospitals reported addressing these problems. It was notable that the majority of institutions without a transition initiative performed none of the transfer initiation scale items. In addition, 2 features of transition efforts highlighted by advocates nationally—use of a checklist and creation of a transition care plan— were 9 times more likely when sites had transition initiatives. Such findings may be motivating for institutions that are considering establishing a transition initiative. Overall, we were not surprised with hospitals’ relatively low performance across most transition activities because only about 40% of US families of children with special healthcare needs report receiving the general services they need to transition to adult healthcare.17
We suspect that a number of the studied inpatient transition activities may be uncommon for structural reasons. For example, having child life consultation during an initial adult stay was rare. In fact, we observed post hoc that it occurred only in hospital-within-hospital systems, an expected finding because adult-only facilities are unlikely to have child life personnel. Other barriers, however, are less obviously structural. Almost no respondents indicated providing a tour of an adult facility, which was true whether the children’s hospital was free-standing or hospital-within-hospital. Given that hospitals with med–peds providers more often had inpatient transition initiatives, it would be interesting to examine whether institutions with med–peds training programs are able to overcome more of these barriers because of the bridges inherently created between departments even when at physically separated sites.
Having a system to track and/or monitor youth going through the transition process was also uncommon. This presumably valuable activity is one of the Six Core Elements3 and is reminiscent of population management strategies increasingly common in primary care.18 Pediatric hospitalists might benefit from adopting a similar philosophy for certain patient populations. Determining whether this activity would be most appropriately managed by inpatient providers versus being integrated into a comprehensive tracking and/or monitoring strategy (ie, inpatient care plus primary care, subspecialty care, school, employment, insurance, etc.) is worth continued consideration.
Although the activities we studied spanned many important dimensions, the most important transition activities in any given context may differ based on institutional resources and those of nearby adult healthcare providers.16 For example, an activity may be absent at a children’s hospital because it is already readily handled in primary care within that health system. Understanding how local resources and patient needs influence the relationship between transition activities and outcomes is an important next step in this line of work. Such research could inform how institutions adapt effective transition activities (eg, developing care plans) to most efficiently meet the needs of their patients and families.
Our findings align with and advance the limited work published on this aspect of transition. A systematic literature review of general healthcare transition interventions found that meeting adult providers prior to transitioning out of the pediatric system was associated with less concern about admission to the adult hospital floor.9 Formally recognizing inpatient care as a part of a comprehensive approach to transition may help adults with childhood-onset chronic conditions progress into adult-oriented hospitals. Inpatient and outpatient providers can educate one another on critical aspects of transition that span across settings. The Cystic Fibrosis (CF) Foundation has established a set of processes to facilitate the transition to adult care and specifically articulates the transfer to adult inpatient settings.19,20 Perhaps as a result, CF is also one of few conditions with fewer adult patients being admitted to children’s hospitals7 despite the increasing number of adults living with the condition.19 Adapting the CF Foundation approach to other chronic conditions may be an effective approach.
Our study has important limitations. Most pertinently, the list of transition activities was developed at a single institution. Although drawing on accepted national guidelines and a diverse local quality improvement group, our listed activities could not be exhaustive. Care plan development and posttransition follow-up activities may benefit from ongoing development in subsequent work. Continuing to identify and integrate approaches taken at other children’s hospitals will also be informative. For example, some children’s hospitals have introduced adult medicine consultative services to focus on transition, attending children’s hospital safety rounds, and sharing standard care protocols for adult patients still cared for in pediatric settings (eg, stroke and myocardial infarction).16
In addition, our findings are limited to generalist teams at children’s hospitals and may not be applicable to inpatient subspecialty services. We could not compare differences in respondents versus nonrespondents to determine whether important selection bias exists. Respondent answers could not be verified. Despite our attempt to identify the most informed respondent at each hospital, responses may have differed with other hospital respondents. We used a novel instrument with unknown psychometric properties. Our data provide only the children’s hospital perspective, and perspectives of others (eg, families, primary care pediatricians or internists, subspecialists, etc.) will be valuable to explore in subsequent research. Subsequent research should investigate the relative importance and feasibility of specific inpatient transition activities, ideal timing, as well as the expected outcomes of high-quality inpatient transition. An important question for future work is to identify which patients are most likely to benefit by having inpatient care as part of their transition plan.
CONCLUSIONS
Nevertheless, the clinical and health services implications of this facet of transition appear to be substantial.16 To meet the Maternal and Child Health Bureau (MCHB) core outcome for children with special healthcare needs to receive “the services necessary to make transitions to adult healthcare,”21 development, validation, and implementation of effective inpatient-specific transition activities and a set of measurable processes and outcomes are needed. A key direction for the healthcare transitions field, with respect to inpatient care, is to determine the activities most effective at improving relevant patient and family outcomes. Ultimately, we advocate that the transition of inpatient care be integrated into comprehensive approaches to transitional care.
Disclosure: The project described was supported in part by the Clinical and Translational Science Award (CTSA) program, through the National Institutes of Health (NIH) National Center for Advancing Translational Sciences (NCATS), grant UL1TR000427. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. The project was also supported by the University of Wisconsin Departments of Pediatrics and Medicine. The authors have no financial or other relationships relevant to this article to disclose.
1. Vaks Y, Bensen R, Steidtmann D, et al. Better health, less spending: Redesigning the transition from pediatric to adult healthcare for youth with chronic illness. Healthc (Amst). 2016;4(1):57-68.
2. Bensen R, Steidtmann D, Vaks Y. A Triple Aim Approach to Transition from Pediatric to Adult Health Care for Youth with Special Health Care Needs. Palo Alto, CA: Lucile Packard Foundation for Children’s Health; 2014.
3. Got Transition. Center for Health Care Transition Improvement 2016; http://www.gottransition.org/. Accessed April 4, 2016.
4. McPheeters M, Davis AM, Taylor JL, Brown RF, Potter SA, Epstein RA. Transition Care for Children with Special Health Needs. Technical Brief No. 15. Rockville, MD: Agency for Healthcare Research and Quality; 2014.
5. American Academy of Pediatrics, American Academy of Family Physicians, American College of Physicians, Transitions Clinical Report Authoring Group, Cooley WC, Sagerman PJ. Supporting the health care transition from adolescence to adulthood in the medical home. Pediatrics. 2011;128(1):182-200.
6. American Academy of Pediatrics, American Academy of Family Physicians, American College of Physicians-American Society of Internal Medicine. A consensus statement on health care transitions for young adults with special health care needs. Pediatrics. 2002;110(6 Pt 2):1304-1306.
7. Goodman DM, Hall M, Levin A, et al. Adults with chronic health conditions originating in childhood: inpatient experience in children’s hospitals. Pediatrics. 2011;128(1):5-13.
8. Goodman DM, Mendez E, Throop C, Ogata ES. Adult survivors of pediatric illness: the impact on pediatric hospitals. Pediatrics. 2002;110(3):583-589.
9. Bloom SR, Kuhlthau K, Van Cleave J, Knapp AA, Newacheck P, Perrin JM. Health care transition for youth with special health care needs. J Adolesc Health. 2012;51(3):213-219.
10. Fair C, Cuttance J, Sharma N, et al. International and Interdisciplinary Identification of Health Care Transition Outcomes. JAMA Pediatr. 2016;170(3):205-211.
11. Samuel SM, Nettel-Aguirre A, Soo A, Hemmelgarn B, Tonelli M, Foster B. Avoidable hospitalizations in youth with kidney failure after transfer to or with only adult care. Pediatrics. 2014;133(4):e993-e1000.
12. Okumura MJ, Campbell AD, Nasr SZ, Davis MM. Inpatient health care use among adult survivors of chronic childhood illnesses in the United States. Arch Pediatr Adolesc Med. 2006;160(10):1054-1060.
13. Edwards JD, Houtrow AJ, Vasilevskis EE, Dudley RA, Okumura MJ. Multi-institutional profile of adults admitted to pediatric intensive care units. JAMA Pediatr. 2013;167(5):436-443.
14. Peter NG, Forke CM, Ginsburg KR, Schwarz DF. Transition from pediatric to adult care: internists’ perspectives. Pediatrics. 2009;123(2):417-423.
15. Okumura MJ, Heisler M, Davis MM, Cabana MD, Demonner S, Kerr EA. Comfort of general internists and general pediatricians in providing care for young adults with chronic illnesses of childhood. J Gen Intern Med. 2008;23(10):1621-1627.
16. Kinnear B, O’Toole JK. Care of Adults in Children’s Hospitals: Acknowledging the Aging Elephant in the Room. JAMA Pediatr. 2015;169(12):1081-1082.
17. McManus MA, Pollack LR, Cooley WC, et al. Current status of transition preparation among youth with special needs in the United States. Pediatrics. 2013;131(6):1090-1097.
18. Kelleher KJ, Cooper J, Deans K, et al. Cost saving and quality of care in a pediatric accountable care organization. Pediatrics. 2015;135(3):e582-e589.
19. Tuchman LK, Schwartz LA, Sawicki GS, Britto MT. Cystic fibrosis and transition to adult medical care. Pediatrics. 2010;125(3):566-573.
20. Yankaskas JR, Marshall BC, Sufian B, Simon RH, Rodman D. Cystic fibrosis adult care: consensus conference report. Chest. 2004;125(1 Suppl):1S-39S.
21. CSHCN Core System Outcomes: Goals for a System of Care. The National Survey of Children with Special Health Care Needs Chartbook 2009-2010. http://mchb.hrsa.gov/cshcn0910/core/co.html Accessed November 30, 2016.
Over 90% of children with chronic diseases now survive into adulthood.1,2 Clinical advances overcoming diseases previously fatal in childhood create new challenges for health systems with limited capacity to manage young adults with complicated and unfamiliar childhood-onset conditions. Consequently, improving the transition from pediatric to adult-oriented care has become a national priority.
Although major pediatric-adult transition initiatives—such as the Six Core Elements Framework,3 a technical brief from the Agency for Healthcare Research and Quality,4 and joint statements from major medical societies5,6—outline key transition recommendations generally and for outpatients, they contain limited or no guidance specifically devoted to transitioning inpatient hospital care from pediatric to adult-oriented settings. Key unknowns include whether, when, and how to transition inpatient care from children’s to nonchildren’s hospitals and how this can be integrated into comprehensive youth-adult transition care.
Nevertheless, the number of discharges of 18- to 21-year-old patients with chronic conditions admitted to children’s hospitals is increasing at a faster rate than discharges of other age groups,7 suggesting both that the population is growing in size and that there are important barriers to transitioning these patients into nonchildren’s hospital settings. Spending on adult patients 18 years or older admitted to children’s hospitals has grown to $1 billion annually.8 Hospitalizations are a commonly proposed outcome measure of pediatric-adult transition work.1,9,10 For example, higher rates of avoidable hospitalizations during early adulthood have been observed for 15- to 22-year-olds with kidney failure cared for exclusively in adult-oriented facilities and during the years immediately after transfer to adult care.11
While research is beginning to describe outcomes of adult-aged patients with childhood-onset chronic conditions admitted to children’s hospitals,7,12,13 there has been no comprehensive description of efforts within children’s hospitals to transition such patients into adult-oriented inpatient settings. This information is necessary to outline institutional needs, delineate opportunities for improvement, and help clinicians strategically organize services for patients requiring this transition.
We sought to characterize the current state of the transition from pediatric- to adult-oriented inpatient care across general pediatric inpatient services at US children’s hospitals. We hypothesized that only a limited and inconsistent set of activities would be practiced. We also hypothesized that institutions having formal outpatient transition processes or providers with specialization to care for this age group, such as dual-trained internal medicine–pediatrics (med–peds) physicians, would report performing more activities.
METHODS
Study Design, Setting, Participants
We conducted a national survey of leaders of inpatient general pediatrics services at US children’s hospitals from January 2016 to July 2016. Hospitals were identified using the online Children’s Hospital Association directory. Hospitals without inpatient general pediatrics services (eg, rehabilitation or subspecialty-only facilities) were excluded.
We identified a single respondent from each of the 195 remaining children’s hospitals using a structured protocol. Phone numbers and e-mail addresses of potential respondents were gathered from hospital or medical school directories. Following a standard script, study team members contacted potential respondents to describe the purpose of the study and to confirm their contact information. Hospitals were also allowed to designate a different individual with more specific expertise to participate, when relevant (eg, specific faculty member leading a related quality improvement initiative). The goal was to identify a leader of inpatient care with the most knowledge of institutional practices related to the transition to adult inpatient care. Examples of respondent roles included director of inpatient pediatrics, chief of hospital medicine or general pediatrics, medical director, and similar titles.
Survey Elements
As part of a larger quality improvement initiative at our institution, a multidisciplinary team of pediatric and internal medicine healthcare providers (physicians, nurse practitioners, nurses, case managers, social workers, child life specialists), as well as parents and patients, developed an “ideal state” with this transition and a consensus-based conceptual framework of key patient and institutional determinants of a formal inpatient transition initiative for children with chronic conditions within a children’s hospital (Figure).
Institutional Context and Factors Influencing Inpatient Transitions
The following hospital characteristics were assessed: administrative structure (free-standing, hospital-within-hospital, or “free-leaning,” ie, separate physical structure but same administrative structure as a general hospital), urban versus rural, academic versus nonacademic, presence of an inpatient adolescent unit, presence of subspecialty admitting services, and providers with med–peds or family medicine training. The following provider group characteristics were assessed: number of full-time equivalents (FTEs), scope of practice (inpatient only, combination inpatient/outpatient), proportion of providers at a “senior” level (ie, at least 7 years posttraining or at an associate professor rank), estimated number of discharges per week, and proportion of patients cared for without resident physicians.
Inpatient Transition Initiative
Each institution was categorized as having or not having an inpatient transition initiative by whether they indicated having either (1) an institutional leader of the transition from pediatric to adult-oriented inpatient settings or (2) an inpatient transition process, for which “process” was defined as “a standard, organized, and predictable set of transition activities that may or may not be documented, but the steps are generally agreed upon.”
Specific Inpatient Transition Activities
Respondents indicated whether 22 activities occurred consistently, defined as at least 50% of the time. To facilitate description, activities were grouped into categories using the labels from the Six Core Elements framework3 (Table 1): Policy, Tracking and Monitoring, Readiness, Planning, Transfer of Care, and Transfer Completion. Respondents were also asked whether outpatient pediatric-adult transition activities existed at their institution and whether they were linked to inpatient transition activities.
Data Collection
After verifying contact information, respondents received an advanced priming phone call followed by a mailed request to participate with a printed uniform resource locator (URL) to the web survey. Two email reminders containing the URL were sent to nonresponders at 5 and 10 days after the initial mailing. Remaining nonresponders then received a reminder phone call, followed by a mailed paper copy of the survey questionnaire to be completed by hand approximately 2 weeks after the last emailed request. The survey was administered using the Qualtrics web survey platform (www.qualtrics.com). Data collection occurred between January 2016 and July 2016. Participants received a $20 incentive.
Statistical Analysis
Descriptive statistics summarized the current state of inpatient transition at general pediatrics services across US children’s hospitals. Exploratory factor analysis assessed whether individual activities were sufficiently correlated to allow grouping items and constructing scales. Differences in institutional or respondent characteristics between hospitals that did and did not report having an inpatient initiative were compared using t tests for continuous data. Fisher’s exact test was used for categorical data because some cell sizes were ≤5. Bivariate logistic regression quantified associations between presence versus absence of specific transition activities and presence versus absence of an inpatient transition initiative. Analyses were completed in STATA (SE version 14.0; StataCorp, College Station, Texas). The institutional review board at our institution approved this study.
RESULTS
Responses were received from 96 of 195 children’s hospitals (49.2% response rate). Responding institution characteristics are summarized in Table 2. Free-standing children’s hospitals made up just over one-third of the sample (36%), while the remaining were free-leaning (22%) or hospital-within-hospital (43%). Most children’s hospitals (58%) did not have a specific adult-oriented hospital identified to receive transitioning patients. Slightly more than 10% had an inpatient adolescent unit. The majority of institutions were academic medical centers (78%) in urban locations (88%). Respondents represented small (<5 FTE, 21%), medium (6-10 FTE, 36%), and large provider groups (11+ FTE, 44%). Although 70% of respondents described their groups as “hospitalist only,” meaning providers only practiced inpatient general pediatrics, nearly 30% had providers practicing inpatient and outpatient general pediatrics. Just over 40% of respondents reported having med–peds providers. Pediatric-adult transition processes for outpatient care were present at 45% of institutions.
Transition Activities
Thirty-eight percent of children’s hospitals had an inpatient transition initiative using our study definition—31% by having a set of generally agreed upon activities, 19% by having a leader, and 11% having both. Inpatient transition leaders included pediatric hospitalists (43%), pediatric subspecialists and primary care providers (14% each), med–peds providers (11%), or case managers (7%). Respondent and institutional characteristics were similar at institutions that did and did not have an inpatient transition initiative (Table 2); however, children’s hospitals with inpatient transition initiatives more often had med–peds providers (P = .04). Institutions with pediatric-adult outpatient care transition processes more often had an inpatient initiative (71% and 29%, respectively; P = .001).
Exploratory factor analysis identified 2 groups of well-correlated items, which we grouped into “preparation” and “transfer initiation” scales (supplementary Appendix). The preparation scale was composed of the following 5 items (Cronbach α = 0.84): proactive identification of patients anticipated to need transition, proactive identification of patients overdue for transition, readiness formally assessed, timing discussed with family, and patient and/or family informed that the next stay would be at the adult facility. The transfer initiation scale comprised the following 6 items (Cronbach α = 0.72): transition education provided to families, primary care–subspecialist agreement on timing, subspecialist–subspecialist agreement on timing, patient decision-making ability established, adult facility tour, and standardized handoff communication between healthcare providers. While these items were analyzed only in this scale, other activities were analyzed as independent variables. In this analysis, 40.9% of institutions had a preparation scale score of 0 (no items performed), while 13% had all 5 items performed. Transfer initiation scale scores ranged from 0 (47%) to 6 (2%).
Specific activities varied widely across institutions, and none of the activities occurred at a majority of children’s hospitals (Table 3). Only 11% of children’s hospital transition policies referenced transitions of inpatient care. The activity most commonly reported across children’s hospitals was addressing potential insurance problems (41%). The least common inpatient transition activities were having child life consult during the first adult hospital stay (6%) or having a system to track and monitor youth in the inpatient transition process (2%). Transition processes and policies were relatively new among institutions that had them—average years an inpatient transition process had been in place was 1.2 (SD 0.4), and average years with a transition policy, including inpatient care, was 1.3 (SD 0.4).
Transition Activities at Hospitals With and Without an Inpatient Transition Initiative
Most activities assessed in this study (both scales plus 5 of 11 individual activities) were significantly more common in children’s hospitals with an inpatient transition initiative (Table 3). The most common activity was addressing potential insurance problems (46%), and the least common activity was having a system to track and monitor youth in the inpatient transition process (3%). The majority of institutions without an inpatient transition initiative (53%) performed 0 transfer initiation scale items. Large effect sizes between hospitals with and without a transition initiative were observed for use of a checklist to complete tasks (odds ratio [OR] 9.6, P = .04) and creation of a transition care plan (OR 9.0, P = .008). Of the 6 activities performed at similarly low frequencies at institutions with and without an initiative, half involved transition planning, the essential step after readiness but before actual transfer of care.
DISCUSSION
We conducted the first national survey describing the policies and procedures of the transition of general inpatient care from children’s to adult-oriented hospitals for youth and young adults with chronic conditions. Our main findings demonstrate that a relatively small number of general inpatient services at children’s hospitals have leaders or dedicated processes to shepherd this transition, and a minority have a specific adult hospital identified to receive their patients. Even among institutions with inpatient transition initiatives, there is wide variability in the performance of activities to facilitate transitioning out of US children’s hospitals. In these institutions, performance seems to be more lacking in later links of the transition chain. Results from this work can serve as a baseline and identify organizational needs and opportunities for future work.
Children’s hospital general services with and without an inpatient pediatric-adult transition initiative had largely similar characteristics; however, the limited sample size may lack power to detect some differences. Perhaps not surprisingly, having med–peds providers and outpatient transition processes were the characteristics most associated with having an inpatient pediatric-adult transition initiative. The observation that over 70% of hospitals with an outpatient process had an inpatient transition leader or dedicated process makes us optimistic that as general transition efforts expand, more robust inpatient transition activities may be achievable.
We appreciate that the most appropriate location to care for hospitalized young adults with childhood-onset chronic conditions is neither known nor answered with this study. Both options face challenges—adult-oriented hospitals may not be equipped to care for adult manifestations of childhood-onset conditions,14,15 while children’s hospitals may lack the resources and expertise to provide comprehensive care to adults.7 Although hospital charges and lengths of stay may be greater when adults with childhood-onset chronic conditions are admitted to children’s compared with adult hospitals,12,13,16 important confounders such as severity of illness could explain why adult-aged patients may both remain in children’s hospitals at older ages and simultaneously have worse outcomes than peers. Regardless, at some point, transitioning care into an adult-oriented hospital may be in patients’ best interests. If so, families and providers need guidance on (1) the important aspects of this transition and (2) how to effectively implement the transition.
Because the most important inpatient transition care activities are not empirically known, we designed our survey to assess a broad set of desirable activities emerging from our multidisciplinary quality improvement work. We mapped these activities to the categories used by the Six Core Elements framework.3 Addressing insurance issues was one of the most commonly reported activities, although still fewer than 50% of hospitals reported addressing these problems. It was notable that the majority of institutions without a transition initiative performed none of the transfer initiation scale items. In addition, 2 features of transition efforts highlighted by advocates nationally—use of a checklist and creation of a transition care plan— were 9 times more likely when sites had transition initiatives. Such findings may be motivating for institutions that are considering establishing a transition initiative. Overall, we were not surprised with hospitals’ relatively low performance across most transition activities because only about 40% of US families of children with special healthcare needs report receiving the general services they need to transition to adult healthcare.17
We suspect that a number of the studied inpatient transition activities may be uncommon for structural reasons. For example, having child life consultation during an initial adult stay was rare. In fact, we observed post hoc that it occurred only in hospital-within-hospital systems, an expected finding because adult-only facilities are unlikely to have child life personnel. Other barriers, however, are less obviously structural. Almost no respondents indicated providing a tour of an adult facility, which was true whether the children’s hospital was free-standing or hospital-within-hospital. Given that hospitals with med–peds providers more often had inpatient transition initiatives, it would be interesting to examine whether institutions with med–peds training programs are able to overcome more of these barriers because of the bridges inherently created between departments even when at physically separated sites.
Having a system to track and/or monitor youth going through the transition process was also uncommon. This presumably valuable activity is one of the Six Core Elements3 and is reminiscent of population management strategies increasingly common in primary care.18 Pediatric hospitalists might benefit from adopting a similar philosophy for certain patient populations. Determining whether this activity would be most appropriately managed by inpatient providers versus being integrated into a comprehensive tracking and/or monitoring strategy (ie, inpatient care plus primary care, subspecialty care, school, employment, insurance, etc.) is worth continued consideration.
Although the activities we studied spanned many important dimensions, the most important transition activities in any given context may differ based on institutional resources and those of nearby adult healthcare providers.16 For example, an activity may be absent at a children’s hospital because it is already readily handled in primary care within that health system. Understanding how local resources and patient needs influence the relationship between transition activities and outcomes is an important next step in this line of work. Such research could inform how institutions adapt effective transition activities (eg, developing care plans) to most efficiently meet the needs of their patients and families.
Our findings align with and advance the limited work published on this aspect of transition. A systematic literature review of general healthcare transition interventions found that meeting adult providers prior to transitioning out of the pediatric system was associated with less concern about admission to the adult hospital floor.9 Formally recognizing inpatient care as a part of a comprehensive approach to transition may help adults with childhood-onset chronic conditions progress into adult-oriented hospitals. Inpatient and outpatient providers can educate one another on critical aspects of transition that span across settings. The Cystic Fibrosis (CF) Foundation has established a set of processes to facilitate the transition to adult care and specifically articulates the transfer to adult inpatient settings.19,20 Perhaps as a result, CF is also one of few conditions with fewer adult patients being admitted to children’s hospitals7 despite the increasing number of adults living with the condition.19 Adapting the CF Foundation approach to other chronic conditions may be an effective approach.
Our study has important limitations. Most pertinently, the list of transition activities was developed at a single institution. Although drawing on accepted national guidelines and a diverse local quality improvement group, our listed activities could not be exhaustive. Care plan development and posttransition follow-up activities may benefit from ongoing development in subsequent work. Continuing to identify and integrate approaches taken at other children’s hospitals will also be informative. For example, some children’s hospitals have introduced adult medicine consultative services to focus on transition, attending children’s hospital safety rounds, and sharing standard care protocols for adult patients still cared for in pediatric settings (eg, stroke and myocardial infarction).16
In addition, our findings are limited to generalist teams at children’s hospitals and may not be applicable to inpatient subspecialty services. We could not compare differences in respondents versus nonrespondents to determine whether important selection bias exists. Respondent answers could not be verified. Despite our attempt to identify the most informed respondent at each hospital, responses may have differed with other hospital respondents. We used a novel instrument with unknown psychometric properties. Our data provide only the children’s hospital perspective, and perspectives of others (eg, families, primary care pediatricians or internists, subspecialists, etc.) will be valuable to explore in subsequent research. Subsequent research should investigate the relative importance and feasibility of specific inpatient transition activities, ideal timing, as well as the expected outcomes of high-quality inpatient transition. An important question for future work is to identify which patients are most likely to benefit by having inpatient care as part of their transition plan.
CONCLUSIONS
Nevertheless, the clinical and health services implications of this facet of transition appear to be substantial.16 To meet the Maternal and Child Health Bureau (MCHB) core outcome for children with special healthcare needs to receive “the services necessary to make transitions to adult healthcare,”21 development, validation, and implementation of effective inpatient-specific transition activities and a set of measurable processes and outcomes are needed. A key direction for the healthcare transitions field, with respect to inpatient care, is to determine the activities most effective at improving relevant patient and family outcomes. Ultimately, we advocate that the transition of inpatient care be integrated into comprehensive approaches to transitional care.
Disclosure: The project described was supported in part by the Clinical and Translational Science Award (CTSA) program, through the National Institutes of Health (NIH) National Center for Advancing Translational Sciences (NCATS), grant UL1TR000427. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. The project was also supported by the University of Wisconsin Departments of Pediatrics and Medicine. The authors have no financial or other relationships relevant to this article to disclose.
Over 90% of children with chronic diseases now survive into adulthood.1,2 Clinical advances overcoming diseases previously fatal in childhood create new challenges for health systems with limited capacity to manage young adults with complicated and unfamiliar childhood-onset conditions. Consequently, improving the transition from pediatric to adult-oriented care has become a national priority.
Although major pediatric-adult transition initiatives—such as the Six Core Elements Framework,3 a technical brief from the Agency for Healthcare Research and Quality,4 and joint statements from major medical societies5,6—outline key transition recommendations generally and for outpatients, they contain limited or no guidance specifically devoted to transitioning inpatient hospital care from pediatric to adult-oriented settings. Key unknowns include whether, when, and how to transition inpatient care from children’s to nonchildren’s hospitals and how this can be integrated into comprehensive youth-adult transition care.
Nevertheless, the number of discharges of 18- to 21-year-old patients with chronic conditions admitted to children’s hospitals is increasing at a faster rate than discharges of other age groups,7 suggesting both that the population is growing in size and that there are important barriers to transitioning these patients into nonchildren’s hospital settings. Spending on adult patients 18 years or older admitted to children’s hospitals has grown to $1 billion annually.8 Hospitalizations are a commonly proposed outcome measure of pediatric-adult transition work.1,9,10 For example, higher rates of avoidable hospitalizations during early adulthood have been observed for 15- to 22-year-olds with kidney failure cared for exclusively in adult-oriented facilities and during the years immediately after transfer to adult care.11
While research is beginning to describe outcomes of adult-aged patients with childhood-onset chronic conditions admitted to children’s hospitals,7,12,13 there has been no comprehensive description of efforts within children’s hospitals to transition such patients into adult-oriented inpatient settings. This information is necessary to outline institutional needs, delineate opportunities for improvement, and help clinicians strategically organize services for patients requiring this transition.
We sought to characterize the current state of the transition from pediatric- to adult-oriented inpatient care across general pediatric inpatient services at US children’s hospitals. We hypothesized that only a limited and inconsistent set of activities would be practiced. We also hypothesized that institutions having formal outpatient transition processes or providers with specialization to care for this age group, such as dual-trained internal medicine–pediatrics (med–peds) physicians, would report performing more activities.
METHODS
Study Design, Setting, Participants
We conducted a national survey of leaders of inpatient general pediatrics services at US children’s hospitals from January 2016 to July 2016. Hospitals were identified using the online Children’s Hospital Association directory. Hospitals without inpatient general pediatrics services (eg, rehabilitation or subspecialty-only facilities) were excluded.
We identified a single respondent from each of the 195 remaining children’s hospitals using a structured protocol. Phone numbers and e-mail addresses of potential respondents were gathered from hospital or medical school directories. Following a standard script, study team members contacted potential respondents to describe the purpose of the study and to confirm their contact information. Hospitals were also allowed to designate a different individual with more specific expertise to participate, when relevant (eg, specific faculty member leading a related quality improvement initiative). The goal was to identify a leader of inpatient care with the most knowledge of institutional practices related to the transition to adult inpatient care. Examples of respondent roles included director of inpatient pediatrics, chief of hospital medicine or general pediatrics, medical director, and similar titles.
Survey Elements
As part of a larger quality improvement initiative at our institution, a multidisciplinary team of pediatric and internal medicine healthcare providers (physicians, nurse practitioners, nurses, case managers, social workers, child life specialists), as well as parents and patients, developed an “ideal state” with this transition and a consensus-based conceptual framework of key patient and institutional determinants of a formal inpatient transition initiative for children with chronic conditions within a children’s hospital (Figure).
Institutional Context and Factors Influencing Inpatient Transitions
The following hospital characteristics were assessed: administrative structure (free-standing, hospital-within-hospital, or “free-leaning,” ie, separate physical structure but same administrative structure as a general hospital), urban versus rural, academic versus nonacademic, presence of an inpatient adolescent unit, presence of subspecialty admitting services, and providers with med–peds or family medicine training. The following provider group characteristics were assessed: number of full-time equivalents (FTEs), scope of practice (inpatient only, combination inpatient/outpatient), proportion of providers at a “senior” level (ie, at least 7 years posttraining or at an associate professor rank), estimated number of discharges per week, and proportion of patients cared for without resident physicians.
Inpatient Transition Initiative
Each institution was categorized as having or not having an inpatient transition initiative by whether they indicated having either (1) an institutional leader of the transition from pediatric to adult-oriented inpatient settings or (2) an inpatient transition process, for which “process” was defined as “a standard, organized, and predictable set of transition activities that may or may not be documented, but the steps are generally agreed upon.”
Specific Inpatient Transition Activities
Respondents indicated whether 22 activities occurred consistently, defined as at least 50% of the time. To facilitate description, activities were grouped into categories using the labels from the Six Core Elements framework3 (Table 1): Policy, Tracking and Monitoring, Readiness, Planning, Transfer of Care, and Transfer Completion. Respondents were also asked whether outpatient pediatric-adult transition activities existed at their institution and whether they were linked to inpatient transition activities.
Data Collection
After verifying contact information, respondents received an advanced priming phone call followed by a mailed request to participate with a printed uniform resource locator (URL) to the web survey. Two email reminders containing the URL were sent to nonresponders at 5 and 10 days after the initial mailing. Remaining nonresponders then received a reminder phone call, followed by a mailed paper copy of the survey questionnaire to be completed by hand approximately 2 weeks after the last emailed request. The survey was administered using the Qualtrics web survey platform (www.qualtrics.com). Data collection occurred between January 2016 and July 2016. Participants received a $20 incentive.
Statistical Analysis
Descriptive statistics summarized the current state of inpatient transition at general pediatrics services across US children’s hospitals. Exploratory factor analysis assessed whether individual activities were sufficiently correlated to allow grouping items and constructing scales. Differences in institutional or respondent characteristics between hospitals that did and did not report having an inpatient initiative were compared using t tests for continuous data. Fisher’s exact test was used for categorical data because some cell sizes were ≤5. Bivariate logistic regression quantified associations between presence versus absence of specific transition activities and presence versus absence of an inpatient transition initiative. Analyses were completed in STATA (SE version 14.0; StataCorp, College Station, Texas). The institutional review board at our institution approved this study.
RESULTS
Responses were received from 96 of 195 children’s hospitals (49.2% response rate). Responding institution characteristics are summarized in Table 2. Free-standing children’s hospitals made up just over one-third of the sample (36%), while the remaining were free-leaning (22%) or hospital-within-hospital (43%). Most children’s hospitals (58%) did not have a specific adult-oriented hospital identified to receive transitioning patients. Slightly more than 10% had an inpatient adolescent unit. The majority of institutions were academic medical centers (78%) in urban locations (88%). Respondents represented small (<5 FTE, 21%), medium (6-10 FTE, 36%), and large provider groups (11+ FTE, 44%). Although 70% of respondents described their groups as “hospitalist only,” meaning providers only practiced inpatient general pediatrics, nearly 30% had providers practicing inpatient and outpatient general pediatrics. Just over 40% of respondents reported having med–peds providers. Pediatric-adult transition processes for outpatient care were present at 45% of institutions.
Transition Activities
Thirty-eight percent of children’s hospitals had an inpatient transition initiative using our study definition—31% by having a set of generally agreed upon activities, 19% by having a leader, and 11% having both. Inpatient transition leaders included pediatric hospitalists (43%), pediatric subspecialists and primary care providers (14% each), med–peds providers (11%), or case managers (7%). Respondent and institutional characteristics were similar at institutions that did and did not have an inpatient transition initiative (Table 2); however, children’s hospitals with inpatient transition initiatives more often had med–peds providers (P = .04). Institutions with pediatric-adult outpatient care transition processes more often had an inpatient initiative (71% and 29%, respectively; P = .001).
Exploratory factor analysis identified 2 groups of well-correlated items, which we grouped into “preparation” and “transfer initiation” scales (supplementary Appendix). The preparation scale was composed of the following 5 items (Cronbach α = 0.84): proactive identification of patients anticipated to need transition, proactive identification of patients overdue for transition, readiness formally assessed, timing discussed with family, and patient and/or family informed that the next stay would be at the adult facility. The transfer initiation scale comprised the following 6 items (Cronbach α = 0.72): transition education provided to families, primary care–subspecialist agreement on timing, subspecialist–subspecialist agreement on timing, patient decision-making ability established, adult facility tour, and standardized handoff communication between healthcare providers. While these items were analyzed only in this scale, other activities were analyzed as independent variables. In this analysis, 40.9% of institutions had a preparation scale score of 0 (no items performed), while 13% had all 5 items performed. Transfer initiation scale scores ranged from 0 (47%) to 6 (2%).
Specific activities varied widely across institutions, and none of the activities occurred at a majority of children’s hospitals (Table 3). Only 11% of children’s hospital transition policies referenced transitions of inpatient care. The activity most commonly reported across children’s hospitals was addressing potential insurance problems (41%). The least common inpatient transition activities were having child life consult during the first adult hospital stay (6%) or having a system to track and monitor youth in the inpatient transition process (2%). Transition processes and policies were relatively new among institutions that had them—average years an inpatient transition process had been in place was 1.2 (SD 0.4), and average years with a transition policy, including inpatient care, was 1.3 (SD 0.4).
Transition Activities at Hospitals With and Without an Inpatient Transition Initiative
Most activities assessed in this study (both scales plus 5 of 11 individual activities) were significantly more common in children’s hospitals with an inpatient transition initiative (Table 3). The most common activity was addressing potential insurance problems (46%), and the least common activity was having a system to track and monitor youth in the inpatient transition process (3%). The majority of institutions without an inpatient transition initiative (53%) performed 0 transfer initiation scale items. Large effect sizes between hospitals with and without a transition initiative were observed for use of a checklist to complete tasks (odds ratio [OR] 9.6, P = .04) and creation of a transition care plan (OR 9.0, P = .008). Of the 6 activities performed at similarly low frequencies at institutions with and without an initiative, half involved transition planning, the essential step after readiness but before actual transfer of care.
DISCUSSION
We conducted the first national survey describing the policies and procedures of the transition of general inpatient care from children’s to adult-oriented hospitals for youth and young adults with chronic conditions. Our main findings demonstrate that a relatively small number of general inpatient services at children’s hospitals have leaders or dedicated processes to shepherd this transition, and a minority have a specific adult hospital identified to receive their patients. Even among institutions with inpatient transition initiatives, there is wide variability in the performance of activities to facilitate transitioning out of US children’s hospitals. In these institutions, performance seems to be more lacking in later links of the transition chain. Results from this work can serve as a baseline and identify organizational needs and opportunities for future work.
Children’s hospital general services with and without an inpatient pediatric-adult transition initiative had largely similar characteristics; however, the limited sample size may lack power to detect some differences. Perhaps not surprisingly, having med–peds providers and outpatient transition processes were the characteristics most associated with having an inpatient pediatric-adult transition initiative. The observation that over 70% of hospitals with an outpatient process had an inpatient transition leader or dedicated process makes us optimistic that as general transition efforts expand, more robust inpatient transition activities may be achievable.
We appreciate that the most appropriate location to care for hospitalized young adults with childhood-onset chronic conditions is neither known nor answered with this study. Both options face challenges—adult-oriented hospitals may not be equipped to care for adult manifestations of childhood-onset conditions,14,15 while children’s hospitals may lack the resources and expertise to provide comprehensive care to adults.7 Although hospital charges and lengths of stay may be greater when adults with childhood-onset chronic conditions are admitted to children’s compared with adult hospitals,12,13,16 important confounders such as severity of illness could explain why adult-aged patients may both remain in children’s hospitals at older ages and simultaneously have worse outcomes than peers. Regardless, at some point, transitioning care into an adult-oriented hospital may be in patients’ best interests. If so, families and providers need guidance on (1) the important aspects of this transition and (2) how to effectively implement the transition.
Because the most important inpatient transition care activities are not empirically known, we designed our survey to assess a broad set of desirable activities emerging from our multidisciplinary quality improvement work. We mapped these activities to the categories used by the Six Core Elements framework.3 Addressing insurance issues was one of the most commonly reported activities, although still fewer than 50% of hospitals reported addressing these problems. It was notable that the majority of institutions without a transition initiative performed none of the transfer initiation scale items. In addition, 2 features of transition efforts highlighted by advocates nationally—use of a checklist and creation of a transition care plan— were 9 times more likely when sites had transition initiatives. Such findings may be motivating for institutions that are considering establishing a transition initiative. Overall, we were not surprised with hospitals’ relatively low performance across most transition activities because only about 40% of US families of children with special healthcare needs report receiving the general services they need to transition to adult healthcare.17
We suspect that a number of the studied inpatient transition activities may be uncommon for structural reasons. For example, having child life consultation during an initial adult stay was rare. In fact, we observed post hoc that it occurred only in hospital-within-hospital systems, an expected finding because adult-only facilities are unlikely to have child life personnel. Other barriers, however, are less obviously structural. Almost no respondents indicated providing a tour of an adult facility, which was true whether the children’s hospital was free-standing or hospital-within-hospital. Given that hospitals with med–peds providers more often had inpatient transition initiatives, it would be interesting to examine whether institutions with med–peds training programs are able to overcome more of these barriers because of the bridges inherently created between departments even when at physically separated sites.
Having a system to track and/or monitor youth going through the transition process was also uncommon. This presumably valuable activity is one of the Six Core Elements3 and is reminiscent of population management strategies increasingly common in primary care.18 Pediatric hospitalists might benefit from adopting a similar philosophy for certain patient populations. Determining whether this activity would be most appropriately managed by inpatient providers versus being integrated into a comprehensive tracking and/or monitoring strategy (ie, inpatient care plus primary care, subspecialty care, school, employment, insurance, etc.) is worth continued consideration.
Although the activities we studied spanned many important dimensions, the most important transition activities in any given context may differ based on institutional resources and those of nearby adult healthcare providers.16 For example, an activity may be absent at a children’s hospital because it is already readily handled in primary care within that health system. Understanding how local resources and patient needs influence the relationship between transition activities and outcomes is an important next step in this line of work. Such research could inform how institutions adapt effective transition activities (eg, developing care plans) to most efficiently meet the needs of their patients and families.
Our findings align with and advance the limited work published on this aspect of transition. A systematic literature review of general healthcare transition interventions found that meeting adult providers prior to transitioning out of the pediatric system was associated with less concern about admission to the adult hospital floor.9 Formally recognizing inpatient care as a part of a comprehensive approach to transition may help adults with childhood-onset chronic conditions progress into adult-oriented hospitals. Inpatient and outpatient providers can educate one another on critical aspects of transition that span across settings. The Cystic Fibrosis (CF) Foundation has established a set of processes to facilitate the transition to adult care and specifically articulates the transfer to adult inpatient settings.19,20 Perhaps as a result, CF is also one of few conditions with fewer adult patients being admitted to children’s hospitals7 despite the increasing number of adults living with the condition.19 Adapting the CF Foundation approach to other chronic conditions may be an effective approach.
Our study has important limitations. Most pertinently, the list of transition activities was developed at a single institution. Although drawing on accepted national guidelines and a diverse local quality improvement group, our listed activities could not be exhaustive. Care plan development and posttransition follow-up activities may benefit from ongoing development in subsequent work. Continuing to identify and integrate approaches taken at other children’s hospitals will also be informative. For example, some children’s hospitals have introduced adult medicine consultative services to focus on transition, attending children’s hospital safety rounds, and sharing standard care protocols for adult patients still cared for in pediatric settings (eg, stroke and myocardial infarction).16
In addition, our findings are limited to generalist teams at children’s hospitals and may not be applicable to inpatient subspecialty services. We could not compare differences in respondents versus nonrespondents to determine whether important selection bias exists. Respondent answers could not be verified. Despite our attempt to identify the most informed respondent at each hospital, responses may have differed with other hospital respondents. We used a novel instrument with unknown psychometric properties. Our data provide only the children’s hospital perspective, and perspectives of others (eg, families, primary care pediatricians or internists, subspecialists, etc.) will be valuable to explore in subsequent research. Subsequent research should investigate the relative importance and feasibility of specific inpatient transition activities, ideal timing, as well as the expected outcomes of high-quality inpatient transition. An important question for future work is to identify which patients are most likely to benefit by having inpatient care as part of their transition plan.
CONCLUSIONS
Nevertheless, the clinical and health services implications of this facet of transition appear to be substantial.16 To meet the Maternal and Child Health Bureau (MCHB) core outcome for children with special healthcare needs to receive “the services necessary to make transitions to adult healthcare,”21 development, validation, and implementation of effective inpatient-specific transition activities and a set of measurable processes and outcomes are needed. A key direction for the healthcare transitions field, with respect to inpatient care, is to determine the activities most effective at improving relevant patient and family outcomes. Ultimately, we advocate that the transition of inpatient care be integrated into comprehensive approaches to transitional care.
Disclosure: The project described was supported in part by the Clinical and Translational Science Award (CTSA) program, through the National Institutes of Health (NIH) National Center for Advancing Translational Sciences (NCATS), grant UL1TR000427. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. The project was also supported by the University of Wisconsin Departments of Pediatrics and Medicine. The authors have no financial or other relationships relevant to this article to disclose.
1. Vaks Y, Bensen R, Steidtmann D, et al. Better health, less spending: Redesigning the transition from pediatric to adult healthcare for youth with chronic illness. Healthc (Amst). 2016;4(1):57-68.
2. Bensen R, Steidtmann D, Vaks Y. A Triple Aim Approach to Transition from Pediatric to Adult Health Care for Youth with Special Health Care Needs. Palo Alto, CA: Lucile Packard Foundation for Children’s Health; 2014.
3. Got Transition. Center for Health Care Transition Improvement 2016; http://www.gottransition.org/. Accessed April 4, 2016.
4. McPheeters M, Davis AM, Taylor JL, Brown RF, Potter SA, Epstein RA. Transition Care for Children with Special Health Needs. Technical Brief No. 15. Rockville, MD: Agency for Healthcare Research and Quality; 2014.
5. American Academy of Pediatrics, American Academy of Family Physicians, American College of Physicians, Transitions Clinical Report Authoring Group, Cooley WC, Sagerman PJ. Supporting the health care transition from adolescence to adulthood in the medical home. Pediatrics. 2011;128(1):182-200.
6. American Academy of Pediatrics, American Academy of Family Physicians, American College of Physicians-American Society of Internal Medicine. A consensus statement on health care transitions for young adults with special health care needs. Pediatrics. 2002;110(6 Pt 2):1304-1306.
7. Goodman DM, Hall M, Levin A, et al. Adults with chronic health conditions originating in childhood: inpatient experience in children’s hospitals. Pediatrics. 2011;128(1):5-13.
8. Goodman DM, Mendez E, Throop C, Ogata ES. Adult survivors of pediatric illness: the impact on pediatric hospitals. Pediatrics. 2002;110(3):583-589.
9. Bloom SR, Kuhlthau K, Van Cleave J, Knapp AA, Newacheck P, Perrin JM. Health care transition for youth with special health care needs. J Adolesc Health. 2012;51(3):213-219.
10. Fair C, Cuttance J, Sharma N, et al. International and Interdisciplinary Identification of Health Care Transition Outcomes. JAMA Pediatr. 2016;170(3):205-211.
11. Samuel SM, Nettel-Aguirre A, Soo A, Hemmelgarn B, Tonelli M, Foster B. Avoidable hospitalizations in youth with kidney failure after transfer to or with only adult care. Pediatrics. 2014;133(4):e993-e1000.
12. Okumura MJ, Campbell AD, Nasr SZ, Davis MM. Inpatient health care use among adult survivors of chronic childhood illnesses in the United States. Arch Pediatr Adolesc Med. 2006;160(10):1054-1060.
13. Edwards JD, Houtrow AJ, Vasilevskis EE, Dudley RA, Okumura MJ. Multi-institutional profile of adults admitted to pediatric intensive care units. JAMA Pediatr. 2013;167(5):436-443.
14. Peter NG, Forke CM, Ginsburg KR, Schwarz DF. Transition from pediatric to adult care: internists’ perspectives. Pediatrics. 2009;123(2):417-423.
15. Okumura MJ, Heisler M, Davis MM, Cabana MD, Demonner S, Kerr EA. Comfort of general internists and general pediatricians in providing care for young adults with chronic illnesses of childhood. J Gen Intern Med. 2008;23(10):1621-1627.
16. Kinnear B, O’Toole JK. Care of Adults in Children’s Hospitals: Acknowledging the Aging Elephant in the Room. JAMA Pediatr. 2015;169(12):1081-1082.
17. McManus MA, Pollack LR, Cooley WC, et al. Current status of transition preparation among youth with special needs in the United States. Pediatrics. 2013;131(6):1090-1097.
18. Kelleher KJ, Cooper J, Deans K, et al. Cost saving and quality of care in a pediatric accountable care organization. Pediatrics. 2015;135(3):e582-e589.
19. Tuchman LK, Schwartz LA, Sawicki GS, Britto MT. Cystic fibrosis and transition to adult medical care. Pediatrics. 2010;125(3):566-573.
20. Yankaskas JR, Marshall BC, Sufian B, Simon RH, Rodman D. Cystic fibrosis adult care: consensus conference report. Chest. 2004;125(1 Suppl):1S-39S.
21. CSHCN Core System Outcomes: Goals for a System of Care. The National Survey of Children with Special Health Care Needs Chartbook 2009-2010. http://mchb.hrsa.gov/cshcn0910/core/co.html Accessed November 30, 2016.
1. Vaks Y, Bensen R, Steidtmann D, et al. Better health, less spending: Redesigning the transition from pediatric to adult healthcare for youth with chronic illness. Healthc (Amst). 2016;4(1):57-68.
2. Bensen R, Steidtmann D, Vaks Y. A Triple Aim Approach to Transition from Pediatric to Adult Health Care for Youth with Special Health Care Needs. Palo Alto, CA: Lucile Packard Foundation for Children’s Health; 2014.
3. Got Transition. Center for Health Care Transition Improvement 2016; http://www.gottransition.org/. Accessed April 4, 2016.
4. McPheeters M, Davis AM, Taylor JL, Brown RF, Potter SA, Epstein RA. Transition Care for Children with Special Health Needs. Technical Brief No. 15. Rockville, MD: Agency for Healthcare Research and Quality; 2014.
5. American Academy of Pediatrics, American Academy of Family Physicians, American College of Physicians, Transitions Clinical Report Authoring Group, Cooley WC, Sagerman PJ. Supporting the health care transition from adolescence to adulthood in the medical home. Pediatrics. 2011;128(1):182-200.
6. American Academy of Pediatrics, American Academy of Family Physicians, American College of Physicians-American Society of Internal Medicine. A consensus statement on health care transitions for young adults with special health care needs. Pediatrics. 2002;110(6 Pt 2):1304-1306.
7. Goodman DM, Hall M, Levin A, et al. Adults with chronic health conditions originating in childhood: inpatient experience in children’s hospitals. Pediatrics. 2011;128(1):5-13.
8. Goodman DM, Mendez E, Throop C, Ogata ES. Adult survivors of pediatric illness: the impact on pediatric hospitals. Pediatrics. 2002;110(3):583-589.
9. Bloom SR, Kuhlthau K, Van Cleave J, Knapp AA, Newacheck P, Perrin JM. Health care transition for youth with special health care needs. J Adolesc Health. 2012;51(3):213-219.
10. Fair C, Cuttance J, Sharma N, et al. International and Interdisciplinary Identification of Health Care Transition Outcomes. JAMA Pediatr. 2016;170(3):205-211.
11. Samuel SM, Nettel-Aguirre A, Soo A, Hemmelgarn B, Tonelli M, Foster B. Avoidable hospitalizations in youth with kidney failure after transfer to or with only adult care. Pediatrics. 2014;133(4):e993-e1000.
12. Okumura MJ, Campbell AD, Nasr SZ, Davis MM. Inpatient health care use among adult survivors of chronic childhood illnesses in the United States. Arch Pediatr Adolesc Med. 2006;160(10):1054-1060.
13. Edwards JD, Houtrow AJ, Vasilevskis EE, Dudley RA, Okumura MJ. Multi-institutional profile of adults admitted to pediatric intensive care units. JAMA Pediatr. 2013;167(5):436-443.
14. Peter NG, Forke CM, Ginsburg KR, Schwarz DF. Transition from pediatric to adult care: internists’ perspectives. Pediatrics. 2009;123(2):417-423.
15. Okumura MJ, Heisler M, Davis MM, Cabana MD, Demonner S, Kerr EA. Comfort of general internists and general pediatricians in providing care for young adults with chronic illnesses of childhood. J Gen Intern Med. 2008;23(10):1621-1627.
16. Kinnear B, O’Toole JK. Care of Adults in Children’s Hospitals: Acknowledging the Aging Elephant in the Room. JAMA Pediatr. 2015;169(12):1081-1082.
17. McManus MA, Pollack LR, Cooley WC, et al. Current status of transition preparation among youth with special needs in the United States. Pediatrics. 2013;131(6):1090-1097.
18. Kelleher KJ, Cooper J, Deans K, et al. Cost saving and quality of care in a pediatric accountable care organization. Pediatrics. 2015;135(3):e582-e589.
19. Tuchman LK, Schwartz LA, Sawicki GS, Britto MT. Cystic fibrosis and transition to adult medical care. Pediatrics. 2010;125(3):566-573.
20. Yankaskas JR, Marshall BC, Sufian B, Simon RH, Rodman D. Cystic fibrosis adult care: consensus conference report. Chest. 2004;125(1 Suppl):1S-39S.
21. CSHCN Core System Outcomes: Goals for a System of Care. The National Survey of Children with Special Health Care Needs Chartbook 2009-2010. http://mchb.hrsa.gov/cshcn0910/core/co.html Accessed November 30, 2016.
© 2018 Society of Hospital Medicine
Journal of Hospital Medicine – Nov. 2017
BACKGROUND AND OBJECTIVES: Adherence to American Academy of Pediatrics (AAP) bronchiolitis clinical practice guideline recommendations improved significantly through the AAP’s multi-institutional collaborative the Bronchiolitis Quality Improvement Project (BQIP). We assessed sustainability of improvements at participating institutions for 1 year following completion of the collaborative.
METHODS: Twenty-one multidisciplinary hospital-based teams provided monthly data for key inpatient bronchiolitis measures during baseline and intervention bronchiolitis seasons. Nine sites provided data in the season following completion of the collaborative. Encounters included children younger than 24 months who were hospitalized for bronchiolitis without comorbid chronic illness, prematurity, or intensive care. Changes between baseline-, intervention-, and sustainability-season data were assessed using generalized linear mixed-effects models with site-specific random effects. Differences between hospital characteristics, baseline performance, and initial improvement among sites that did and did not participate in the sustainability season were compared.
RESULTS: A total of 2,275 discharges were reviewed, comprising 995 baseline, 877 intervention, and 403 sustainability-season encounters. Improvements in all key bronchiolitis quality measures achieved during the intervention season were maintained during the sustainability season, and orders for intermittent pulse oximetry increased from 40.6% (95% confidence interval, 22.8-61.1) to 79.2% (95% CI, 58.0-91.3). Sites that did and did not participate in the sustainability season had similar characteristics.
DISCUSSION: BQIP participating sites maintained improvements in key bronchiolitis quality measures for 1 year following the project’s completion. This approach, which provided an evidence-based best-practice toolkit while building the quality-improvement capacity of local interdisciplinary teams, may support performance gains that persist beyond the active phase of the collaborative.
Also in JHM this month
The effect of an inpatient smoking cessation treatment program on hospital readmissions and length of stayAUTHORS: Eline M. van den Broek-Altenburg, MS, MA, Adam J. Atherly, PhD
Treatment trends and outcomes in healthcare-associated pneumoniaAUTHORS: Sarah Haessler, MD; Tara Lagu, MD, MPH; Peter K. Lindenauer, MD, MSc; Daniel J. Skiest, MD; Aruna Priya, MA, MSc; Penelope S. Pekow, PhD; Marya D. Zilberberg, MD, MPH; Thomas L. Higgins, MD, MBA; Michael B. Rothberg, MD, MPH
What’s the purpose of rounds? A qualitative study examining the perceptions of faculty and studentsAUTHORS: Oliver Hulland; Jeanne Farnan, MD, MHPE; Raphael Rabinowitz; Lisa Kearns, MD, MS; Michele Long, MD; Bradley Monash, MD; Priti Bhansali, MD; H. Barrett Fromme, MD, MHPE
Association between anemia and fatigue in hospitalized patients: does the measure of anemia matter?AUTHORS: Micah T. Prochaska, MD, MS; Richard Newcomb, BA; Graham Block, BA; Brian Park, BA; David O. Meltzer MD, PhD
Helping seniors plan for posthospital discharge needs before a hospitalization occurs: Results from the randomized control trial of planyourlifespan.orgAUTHORS: Lee A. Lindquist, MD, MPH, MBA; Vanessa Ramirez-Zohfeld, MPH; Priya D. Sunkara, MA; Chris Forcucci, RN, BSN; Dianne S. Campbell, BS; Phyllis Mitzen, MA; Jody D. Ciolino, PhD; Gayle Kricke, MSW; Anne Seltzer, LSW; Ana V. Ramirez, BA; Kenzie A. Cameron, PhD, MPH
BACKGROUND AND OBJECTIVES: Adherence to American Academy of Pediatrics (AAP) bronchiolitis clinical practice guideline recommendations improved significantly through the AAP’s multi-institutional collaborative the Bronchiolitis Quality Improvement Project (BQIP). We assessed sustainability of improvements at participating institutions for 1 year following completion of the collaborative.
METHODS: Twenty-one multidisciplinary hospital-based teams provided monthly data for key inpatient bronchiolitis measures during baseline and intervention bronchiolitis seasons. Nine sites provided data in the season following completion of the collaborative. Encounters included children younger than 24 months who were hospitalized for bronchiolitis without comorbid chronic illness, prematurity, or intensive care. Changes between baseline-, intervention-, and sustainability-season data were assessed using generalized linear mixed-effects models with site-specific random effects. Differences between hospital characteristics, baseline performance, and initial improvement among sites that did and did not participate in the sustainability season were compared.
RESULTS: A total of 2,275 discharges were reviewed, comprising 995 baseline, 877 intervention, and 403 sustainability-season encounters. Improvements in all key bronchiolitis quality measures achieved during the intervention season were maintained during the sustainability season, and orders for intermittent pulse oximetry increased from 40.6% (95% confidence interval, 22.8-61.1) to 79.2% (95% CI, 58.0-91.3). Sites that did and did not participate in the sustainability season had similar characteristics.
DISCUSSION: BQIP participating sites maintained improvements in key bronchiolitis quality measures for 1 year following the project’s completion. This approach, which provided an evidence-based best-practice toolkit while building the quality-improvement capacity of local interdisciplinary teams, may support performance gains that persist beyond the active phase of the collaborative.
Also in JHM this month
The effect of an inpatient smoking cessation treatment program on hospital readmissions and length of stayAUTHORS: Eline M. van den Broek-Altenburg, MS, MA, Adam J. Atherly, PhD
Treatment trends and outcomes in healthcare-associated pneumoniaAUTHORS: Sarah Haessler, MD; Tara Lagu, MD, MPH; Peter K. Lindenauer, MD, MSc; Daniel J. Skiest, MD; Aruna Priya, MA, MSc; Penelope S. Pekow, PhD; Marya D. Zilberberg, MD, MPH; Thomas L. Higgins, MD, MBA; Michael B. Rothberg, MD, MPH
What’s the purpose of rounds? A qualitative study examining the perceptions of faculty and studentsAUTHORS: Oliver Hulland; Jeanne Farnan, MD, MHPE; Raphael Rabinowitz; Lisa Kearns, MD, MS; Michele Long, MD; Bradley Monash, MD; Priti Bhansali, MD; H. Barrett Fromme, MD, MHPE
Association between anemia and fatigue in hospitalized patients: does the measure of anemia matter?AUTHORS: Micah T. Prochaska, MD, MS; Richard Newcomb, BA; Graham Block, BA; Brian Park, BA; David O. Meltzer MD, PhD
Helping seniors plan for posthospital discharge needs before a hospitalization occurs: Results from the randomized control trial of planyourlifespan.orgAUTHORS: Lee A. Lindquist, MD, MPH, MBA; Vanessa Ramirez-Zohfeld, MPH; Priya D. Sunkara, MA; Chris Forcucci, RN, BSN; Dianne S. Campbell, BS; Phyllis Mitzen, MA; Jody D. Ciolino, PhD; Gayle Kricke, MSW; Anne Seltzer, LSW; Ana V. Ramirez, BA; Kenzie A. Cameron, PhD, MPH
BACKGROUND AND OBJECTIVES: Adherence to American Academy of Pediatrics (AAP) bronchiolitis clinical practice guideline recommendations improved significantly through the AAP’s multi-institutional collaborative the Bronchiolitis Quality Improvement Project (BQIP). We assessed sustainability of improvements at participating institutions for 1 year following completion of the collaborative.
METHODS: Twenty-one multidisciplinary hospital-based teams provided monthly data for key inpatient bronchiolitis measures during baseline and intervention bronchiolitis seasons. Nine sites provided data in the season following completion of the collaborative. Encounters included children younger than 24 months who were hospitalized for bronchiolitis without comorbid chronic illness, prematurity, or intensive care. Changes between baseline-, intervention-, and sustainability-season data were assessed using generalized linear mixed-effects models with site-specific random effects. Differences between hospital characteristics, baseline performance, and initial improvement among sites that did and did not participate in the sustainability season were compared.
RESULTS: A total of 2,275 discharges were reviewed, comprising 995 baseline, 877 intervention, and 403 sustainability-season encounters. Improvements in all key bronchiolitis quality measures achieved during the intervention season were maintained during the sustainability season, and orders for intermittent pulse oximetry increased from 40.6% (95% confidence interval, 22.8-61.1) to 79.2% (95% CI, 58.0-91.3). Sites that did and did not participate in the sustainability season had similar characteristics.
DISCUSSION: BQIP participating sites maintained improvements in key bronchiolitis quality measures for 1 year following the project’s completion. This approach, which provided an evidence-based best-practice toolkit while building the quality-improvement capacity of local interdisciplinary teams, may support performance gains that persist beyond the active phase of the collaborative.
Also in JHM this month
The effect of an inpatient smoking cessation treatment program on hospital readmissions and length of stayAUTHORS: Eline M. van den Broek-Altenburg, MS, MA, Adam J. Atherly, PhD
Treatment trends and outcomes in healthcare-associated pneumoniaAUTHORS: Sarah Haessler, MD; Tara Lagu, MD, MPH; Peter K. Lindenauer, MD, MSc; Daniel J. Skiest, MD; Aruna Priya, MA, MSc; Penelope S. Pekow, PhD; Marya D. Zilberberg, MD, MPH; Thomas L. Higgins, MD, MBA; Michael B. Rothberg, MD, MPH
What’s the purpose of rounds? A qualitative study examining the perceptions of faculty and studentsAUTHORS: Oliver Hulland; Jeanne Farnan, MD, MHPE; Raphael Rabinowitz; Lisa Kearns, MD, MS; Michele Long, MD; Bradley Monash, MD; Priti Bhansali, MD; H. Barrett Fromme, MD, MHPE
Association between anemia and fatigue in hospitalized patients: does the measure of anemia matter?AUTHORS: Micah T. Prochaska, MD, MS; Richard Newcomb, BA; Graham Block, BA; Brian Park, BA; David O. Meltzer MD, PhD
Helping seniors plan for posthospital discharge needs before a hospitalization occurs: Results from the randomized control trial of planyourlifespan.orgAUTHORS: Lee A. Lindquist, MD, MPH, MBA; Vanessa Ramirez-Zohfeld, MPH; Priya D. Sunkara, MA; Chris Forcucci, RN, BSN; Dianne S. Campbell, BS; Phyllis Mitzen, MA; Jody D. Ciolino, PhD; Gayle Kricke, MSW; Anne Seltzer, LSW; Ana V. Ramirez, BA; Kenzie A. Cameron, PhD, MPH
Sustainability in the AAP Bronchiolitis Quality Improvement Project
Acute viral bronchiolitis is the most common cause of hospitalization for children less than 1 year of age.1 Overuse of ineffective therapies has persisted despite the existence of the evidence-based American Academy of Pediatrics (AAP) clinical practice guideline (CPG), which recommends primarily supportive care.2-8 Adherence to the AAP CPG recommendations for management of bronchiolitis improved significantly through the AAP’s Bronchiolitis Quality Improvement Project (BQIP), a 12-month, multiinstitutional collaborative of community and free-standing children’s hospitals.9 This subsequent study investigates if these improvements were sustained after completion of the formal 12-month project.
Published multiinstitutional bronchiolitis quality improvement (QI) work is limited to 1 study5 that describes the results of a single intervention season at academic medical centers. Multiyear bronchiolitis QI projects are limited to single-center studies, and results have been mixed.5,6,8,10-13 One study11 observed continued improvement in bronchodilator use in subsequent seasons, whereas a second study10 observed a return to baseline bronchodilator use in the following season. Mittal6 observed inconsistent improvements in key bronchiolitis measures during postintervention seasons.
Our specific aim was to assess the sustainability of improvements in bronchiolitis management at participating institutions 1 year following completion of the AAP BQIP collaborative.9 Because no studies demonstrate the most effective way to support long-term improvement through a QI collaborative, we hypothesized that the initial collaborative activities, which were designed to build the capacity of local interdisciplinary teams while providing standardized evidence-based care pathways, would lead to performance in the subsequent season at levels similar to or better than those observed during the active phase of the collaborative, without additional project interventions.
METHODS
Study Design and Setting
This was a follow-up study of the AAP Quality Improvement Innovation Networks project entitled “A Quality Collaborative for Improving Hospital Compliance with the AAP Bronchiolitis Guideline” (BQIP).9 The AAP Institutional Review Board approved this project.
Twenty-one multidisciplinary, hospital-based teams participated in the BQIP collaborative and provided monthly data during the January through March bronchiolitis season. Teams submitted 2013 baseline data and 2014 intervention data. Nine sites provided 2015 sustainability data following the completion of the collaborative.
Participants
Hospital encounters with a primary diagnosis of acute viral bronchiolitis were eligible for inclusion among patients from 1 month to 2 years of age. Encounters were excluded for prematurity (<35 weeks gestational age), congenital heart disease, bronchopulmonary dysplasia, genetic, congenital or neuromuscular abnormalities, and pediatric intensive-care admission.
Data Collection
Hospital characteristics were collected, including hospital type (academic, community), bed size, location (urban, rural), hospital distributions of race/ethnicity and public payer, cases of bronchiolitis per year, presence of an electronic medical record and a pediatric respiratory therapist, and self-rated QI knowledge of the multidisciplinary team (very knowledgeable, knowledgeable, and somewhat knowledgeable). A trained member at each site collected data through structured chart review in baseline, intervention, and sustainability bronchiolitis seasons for January, February, and March. Site members reviewed the first 20 charts per month that met the inclusion criteria or all charts if there were fewer than 20 eligible encounters. Sites input data about key quality measures into the AAP’s Quality Improvement Data Aggregator, a web-based data repository.
Intervention
The BQIP project was designed as a virtual collaborative consisting of monthly education webinars about QI methods and bronchiolitis management, opportunities for collaboration via teleconference and e-mail listserv, and individual site-coaching by e-mail or telephone.9 A change package was shared with sites that included examples of evidence-based pathways, ordersets, a respiratory scoring tool, communication tools for parents and referring physicians, and slide sets for individual site education efforts. Following completion of the collaborative, written resources remained available to participants, although virtual collaboration ceased and no additional project interventions to promote sustainability were introduced.
Bronchiolitis Process and Outcome Measures
Process measures following admission included the following: severity assessment using a respiratory score, respiratory score use to assess response to bronchodilators, bronchodilator use, bronchodilator doses, steroid doses per patient encounter, chest radiographs per encounter, and presence of an order to transition to intermittent pulse oximetry monitoring. Outcome measures included length of stay and readmissions within 72 hours.
Analysis
Changes among baseline-, intervention-, and sustainability-season data were assessed using generalized linear mixed-effects models with random effect for study sites. Negative binomial models were used for count variables to allow for overdispersion. Length of stay was log-transformed to achieve a normal distribution. We also analyzed each site individually to assess whether sustained improvements were the result of broad sustainability across all sites or whether they represented an aggregation of some sites that continued to improve while other sites actually worsened.
To address any bias introduced by the voluntary and incomplete participation of sites in the sustainability season, we planned a priori to conduct 3 additional analyses. First, we compared the characteristics of sites that did participate in the sustainability season with those that did not participate by using Chi-squared tests for differences in proportions and t tests for differences in means. Second, we determined whether the baseline-season process and outcome measures were different between sites that did and did not participate using descriptive statistics. Third, we assessed whether improvements between the baseline and intervention seasons were different between sites that did and did not participate using a linear mixed-effects model for normally distributed outcomes and generalized linear mixed-effects model with site-specific random effects for nonnormally distributed outcomes. All study outcomes were summarized in terms of model-adjusted means along with the corresponding 95% confidence intervals. All P values are 2-sided, and P < 0.05 was used to define statistical significance. Data analyses were conducted using SAS software (SAS Institute Inc., Cary, North Carolina) version 9.4.
RESULTS
Differences in baseline bronchiolitis quality measures between sites that did and did not participate in the sustainability season are displayed in Table 3. Sustainability sites had significantly lower baseline use of a respiratory score, both to assess severity of illness at any point after hospitalization as well as to assess responsiveness following bronchodilator treatments (P < 0.001). At baseline they also had fewer orders for intermittent pulse oximetry use (P = 0.01) and fewer doses of bronchodilators per encounter (P = 0.04). Sites were not significantly different in their baseline use of bronchodilators, oral steroid doses, or chest radiographs. Sites that participated in the sustainability season demonstated larger magnitude improvement between baseline and intervention seasons for respiratory score use (P < 0.001 for any use and P = 0.02 to assess bronchodilator responsiveness; Appendix 1b).
DISCUSSION
To our knowledge, this is the first report of sustained improvements in care achieved through a multiinstitutional QI collaborative of community and academic hospitals focused on bronchiolitis care. We found that overall sites participating in a national bronchiolitis QI project sustained improvements in key bronchiolitis quality measures for 1 year following the project’s completion. For the aggregate group no measures worsened, and one measure, orders for intermittent pulse oximetry monitoring, continued to increase during the sustainability season. Furthermore, the sustained improvements were primarily the result of consistent sustained performance of each individual site, as opposed to averages wherein some sites worsened while others improved (Appendix 1a). These findings suggest that designing a collaborative approach, which provides an evidence-based best-practice toolkit while building the QI capacity of local interdisciplinary teams, can support performance gains that persist beyond the project’s active phase.
There are a number of possible reasons why improvements were sustained following the collaborative. The BQIP requirement for institutional leadership buy-in may have motivated accountability to local leaders in subsequent bronchiolitis seasons at each site. We suspect that culture change such as flattened hierarchies through multidisciplinary teams,14 which empowered nurse and respiratory therapy staff, may have facilitated consistent use of tools created locally. The synergy of interdisciplinary teams composed of physician, nurse, and respiratory therapy champions may have created accountability to perpetuate the previous year’s efforts.15 In addition, the sites adopted elements of the evidence-based toolkit, such as pathways,16,17 forcing function tools13,18 and order sets that limited management decision options and bronchodilator use contingent on respiratory scores,9,19 which may have driven desired behaviors.
Moreover, the 2014 AAP CPG for the management of bronchiolitis,20 released prior to the sustainability bronchiolitis season, may have underscored the key concepts of the collaborative. Similarly, national exposure of best practices for bronchiolitis management, including the 3 widespread Choosing Wisely recommendations related to bronchiolitis,21 might have been a compelling reason for sites to maintain their improvement efforts and contribute to secular trends toward decreasing interventions in bronchiolitis management nationally.3 Lastly, the mechanisms developed for local data collection may have created opportunities at each site to conduct ongoing evaluation of performance on key bronchiolitis quality measures through data-driven feedback systems.22 Our study highlights the need for additional research in order to understand why improvements are or are not sustained.
Even with substantial, sustained improvements in this initiative, further reduction in unnecessary care may be possible. Findings from previous studies suggest that even multifaceted QI interventions, including provider education, guidelines and use of respiratory scores, may only modestly reduce bronchodilators, steroids, and chest radiograph use.8,13 To achieve continued improvements in bronchiolitis care, additional active efforts may be needed to develop new interventions that target root causes for areas of overuse at individual sites.
Future multiinstitutional collaboratives might benefit their participants if they include a focus on helping sites develop skills to ensure that local improvement activities continue after the collaborative phases are completed. Proactively scheduling intermittent check-ins with collaborative members to discuss experiences with both sustainability and ongoing improvement may be valuable and likely needs to be incorporated into the initial collaborative planning.
As these sustainability data represent a subset of 9 of the original 21 BQIP sites, there is concern for potential selection bias related to factors that could have motivated sites to participate in the sustainability season’s data collection and simultaneously influenced their performance. These concerns were mitigated to some extent through 3 specific analyses: finding limited differences in hospital characteristics, baseline performance in key bronchiolitis measures, and performance change from baseline to intervention seasons between sites that did and did not participate in the sustainability season.
Notably, sites that participated in the sustainability phase actually had lower baseline respiratory score use and fewer orders for intermittent pulse oximetry at baseline. Theoretically, if participation in the collaborative highlighted this disparity for these sites, it could have been a motivating factor for their continued participation and sustained performance across these measures. Similarly, sites that recognized their higher baseline performance through participation in the collaborative might have felt less motivation to participate in ongoing data collection during the sustainability season. Whether they might have also sustained, declined, or continued improving is not known. Additionally, the magnitude of improvement in the collaborative period might have also motivated ongoing participation during the sustainability phase. For example, although all sites improved in score use during the collaborative, sites participating in the sustainability season demonstrated significantly more improvement in these measures. Sites with a higher magnitude of improvement in collaborative measures might have more enthusiasm about the project, more commitment to the project activities, or feel a sense of obligation to respond to requests for additional data collection.
This work has several limitations. Selection bias may limit generalizability of the results, as sites that did not participate in the sustainability season may have had different results than those that did participate. It is unknown whether sites that regressed toward their baseline were deterred from participating in the sustainability season. The analyses that we were able to preform, however, suggest that the 2 groups were similar in their characteristics as well as in their baseline and improvement performance.
We have limited knowledge of the local improvement work that sites conducted between the completion of the collaborative and the sustainability season. Site-specific factors may have influenced improvement sustainability. For example, qualitative research with the original group found that team engagement had a quantitative association with better performance, but only for the bronchodilator use measure.23 Sites were responsible for their own data collection, and despite attempts to centralize and standardize the process, data collection inconsistencies may have occurred. For instance, it is unknown how closely that orders for intermittent pulse oximetry correlate with intermittent use at the bedside. Lastly, the absence of a control group limits examination of the causal relationships of interventions and the influence of secular trends.
CONCLUSIONS
Improvements gained during the BQIP collaborative were sustained at 1 year following completion of the collaborative. These findings are encouraging, as national QI collaborative efforts are increasingly common. Our findings suggest that opportunities exist to even further reduce unnecessary care in the management of bronchiolitis. Such opportunities highlight the importance of integrating strategies to both measure sustainability and plan for ongoing independent local activities after completion of the collaborative. Future efforts should focus on supporting local sites to continue individual practice-improvement as they transition from collaborative to independent quality initiatives.
Acknowledgments
The authors thank the 21 hospitals that participated in the BQIP collaborative, and in particular the 9 hospital teams that contributed sustainability data for their ongoing dedication. There was no external funding for this manuscript.
Disclosure
The authors report no financial conflicts of interest.
1. Healthcare Cost and Utilization Project (HCUP) KID Trends Supplemental File. Agency for Healthcare Research and Quality website. http://hcupnet.ahrq.gov/HCUPnet.jsp?Id=2C331B13FB40957D&Form=DispTab&JS=Y&Action=Accept. 2012. Accessed July 21, 2016.
2. Ralston S, Parikh K, Goodman D. Benchmarking overuse of medical interventions for bronchiolitis. JAMA Pediatr. 2015;169:805-806. PubMed
3. Parikh K, Hall M, Teach SJ. Bronchiolitis management before and after the AAP guidelines. Pediatrics. 2014;133:e1-e7. PubMed
4. Johnson LW, Robles J, Hudgins A, Osburn S, Martin D, Thompson A. Management of bronchiolitis in the emergency department: impact of evidence-based guidelines? Pediatrics. 2013;131 Suppl 1:S103-S109. PubMed
5. Kotagal UR, Robbins JM, Kini NM, Schoettker PJ, Atherton HD, Kirschbaum MS. Impact of a bronchiolitis guideline: a multisite demonstration project. Chest. 2002;121:1789-1797. PubMed
6. Mittal V, Darnell C, Walsh B, et al. Inpatient bronchiolitis guideline implementation and resource utilization. Pediatrics. 2014;133:e730-e737. PubMed
7. Mittal V, Hall M, Morse R, et al. Impact of inpatient bronchiolitis clinical practice guideline implementation on testing and treatment. J Pediatr. 2014;165:570.e3-576.e3. PubMed
8. Ralston S, Garber M, Narang S, et al. Decreasing unnecessary utilization in acute bronchiolitis care: results from the value in inpatient pediatrics network. J Hosp Med. 2013;8:25-30. PubMed
9. Ralston SL, Garber MD, Rice-Conboy E, et al. A multicenter collaborative to reduce unnecessary care in inpatient bronchiolitis. Pediatrics. 2016;137. PubMed
10. Perlstein PH, Kotagal UR, Schoettker PJ, et al. Sustaining the implementation of an evidence-based guideline for bronchiolitis. Arch Pediatr Adolesc Med. 2000;154:1001-1007. PubMed
11. Walker C, Danby S, Turner S. Impact of a bronchiolitis clinical care pathway on treatment and hospital stay. Eur J Pediatr. 2012;171:827-832. PubMed
12. Cheney J, Barber S, Altamirano L, et al. A clinical pathway for bronchiolitis is effective in reducing readmission rates. J Pediatr. 2005;147:622-626. PubMed
13. Ralston S, Comick A, Nichols E, Parker D, Lanter P. Effectiveness of quality improvement in hospitalization for bronchiolitis: a systematic review. Pediatrics. 2014;134:571-581. PubMed
14. Schwartz RW, Tumblin TF. The power of servant leadership to transform health care organizations for the 21st-century economy. Arch Surg. 2002;137:1419-1427; discussion 27. PubMed
15. Schalock RL, Verdugo M, Lee T. A systematic approach to an organization’s sustainability. Eval Program Plann. 2016;56:56-63. PubMed
16. Wilson SD, Dahl BB, Wells RD. An evidence-based clinical pathway for bronchiolitis safely reduces antibiotic overuse. Am J Med Qual. 2002;17:195-199. PubMed
17. Muething S, Schoettker PJ, Gerhardt WE, Atherton HD, Britto MT, Kotagal UR. Decreasing overuse of therapies in the treatment of bronchiolitis by incorporating evidence at the point of care. J Pediatr. 2004;144:703-710. PubMed
18. Streiff MB, Carolan HT, Hobson DB, et al. Lessons from the Johns Hopkins multi-disciplinary venous thromboembolism (VTE) prevention collaborative. BMJ. 2012;344:e3935. PubMed
19. Todd J, Bertoch D, Dolan S. Use of a large national database for comparative evaluation of the effect of a bronchiolitis/viral pneumonia clinical care guideline on patient outcome and resource utilization. Arch Pediatr Adolesc Med. 2002;156:1086-1090. PubMed
20. Ralston SL, Lieberthal AS, Meissner HC, et al. Clinical practice guideline: the diagnosis, management, and prevention of bronchiolitis. Pediatrics. 2014;134:e1474-e1502. PubMed
21. Quinonez RA, Garber MD, Schroeder AR, et al. Choosing wisely in pediatric hospital medicine: five opportunities for improved healthcare value. J Hosp Med. 2013;8:479-485. PubMed
22. Stone S, Lee HC, Sharek PJ. Perceived factors associated with sustained improvement following participation in a multicenter quality improvement collaborative. Jt Comm J Qual Patient Saf. 2016;42:309-315. PubMed
23. Ralston SL, Atwood EC, Garber MD, Holmes AV. What works to reduce unnecessary care for bronchiolitis? A qualitative analysis of a national collaborative. Acad Pediatr. 2017;17(2):198-204. PubMed
Acute viral bronchiolitis is the most common cause of hospitalization for children less than 1 year of age.1 Overuse of ineffective therapies has persisted despite the existence of the evidence-based American Academy of Pediatrics (AAP) clinical practice guideline (CPG), which recommends primarily supportive care.2-8 Adherence to the AAP CPG recommendations for management of bronchiolitis improved significantly through the AAP’s Bronchiolitis Quality Improvement Project (BQIP), a 12-month, multiinstitutional collaborative of community and free-standing children’s hospitals.9 This subsequent study investigates if these improvements were sustained after completion of the formal 12-month project.
Published multiinstitutional bronchiolitis quality improvement (QI) work is limited to 1 study5 that describes the results of a single intervention season at academic medical centers. Multiyear bronchiolitis QI projects are limited to single-center studies, and results have been mixed.5,6,8,10-13 One study11 observed continued improvement in bronchodilator use in subsequent seasons, whereas a second study10 observed a return to baseline bronchodilator use in the following season. Mittal6 observed inconsistent improvements in key bronchiolitis measures during postintervention seasons.
Our specific aim was to assess the sustainability of improvements in bronchiolitis management at participating institutions 1 year following completion of the AAP BQIP collaborative.9 Because no studies demonstrate the most effective way to support long-term improvement through a QI collaborative, we hypothesized that the initial collaborative activities, which were designed to build the capacity of local interdisciplinary teams while providing standardized evidence-based care pathways, would lead to performance in the subsequent season at levels similar to or better than those observed during the active phase of the collaborative, without additional project interventions.
METHODS
Study Design and Setting
This was a follow-up study of the AAP Quality Improvement Innovation Networks project entitled “A Quality Collaborative for Improving Hospital Compliance with the AAP Bronchiolitis Guideline” (BQIP).9 The AAP Institutional Review Board approved this project.
Twenty-one multidisciplinary, hospital-based teams participated in the BQIP collaborative and provided monthly data during the January through March bronchiolitis season. Teams submitted 2013 baseline data and 2014 intervention data. Nine sites provided 2015 sustainability data following the completion of the collaborative.
Participants
Hospital encounters with a primary diagnosis of acute viral bronchiolitis were eligible for inclusion among patients from 1 month to 2 years of age. Encounters were excluded for prematurity (<35 weeks gestational age), congenital heart disease, bronchopulmonary dysplasia, genetic, congenital or neuromuscular abnormalities, and pediatric intensive-care admission.
Data Collection
Hospital characteristics were collected, including hospital type (academic, community), bed size, location (urban, rural), hospital distributions of race/ethnicity and public payer, cases of bronchiolitis per year, presence of an electronic medical record and a pediatric respiratory therapist, and self-rated QI knowledge of the multidisciplinary team (very knowledgeable, knowledgeable, and somewhat knowledgeable). A trained member at each site collected data through structured chart review in baseline, intervention, and sustainability bronchiolitis seasons for January, February, and March. Site members reviewed the first 20 charts per month that met the inclusion criteria or all charts if there were fewer than 20 eligible encounters. Sites input data about key quality measures into the AAP’s Quality Improvement Data Aggregator, a web-based data repository.
Intervention
The BQIP project was designed as a virtual collaborative consisting of monthly education webinars about QI methods and bronchiolitis management, opportunities for collaboration via teleconference and e-mail listserv, and individual site-coaching by e-mail or telephone.9 A change package was shared with sites that included examples of evidence-based pathways, ordersets, a respiratory scoring tool, communication tools for parents and referring physicians, and slide sets for individual site education efforts. Following completion of the collaborative, written resources remained available to participants, although virtual collaboration ceased and no additional project interventions to promote sustainability were introduced.
Bronchiolitis Process and Outcome Measures
Process measures following admission included the following: severity assessment using a respiratory score, respiratory score use to assess response to bronchodilators, bronchodilator use, bronchodilator doses, steroid doses per patient encounter, chest radiographs per encounter, and presence of an order to transition to intermittent pulse oximetry monitoring. Outcome measures included length of stay and readmissions within 72 hours.
Analysis
Changes among baseline-, intervention-, and sustainability-season data were assessed using generalized linear mixed-effects models with random effect for study sites. Negative binomial models were used for count variables to allow for overdispersion. Length of stay was log-transformed to achieve a normal distribution. We also analyzed each site individually to assess whether sustained improvements were the result of broad sustainability across all sites or whether they represented an aggregation of some sites that continued to improve while other sites actually worsened.
To address any bias introduced by the voluntary and incomplete participation of sites in the sustainability season, we planned a priori to conduct 3 additional analyses. First, we compared the characteristics of sites that did participate in the sustainability season with those that did not participate by using Chi-squared tests for differences in proportions and t tests for differences in means. Second, we determined whether the baseline-season process and outcome measures were different between sites that did and did not participate using descriptive statistics. Third, we assessed whether improvements between the baseline and intervention seasons were different between sites that did and did not participate using a linear mixed-effects model for normally distributed outcomes and generalized linear mixed-effects model with site-specific random effects for nonnormally distributed outcomes. All study outcomes were summarized in terms of model-adjusted means along with the corresponding 95% confidence intervals. All P values are 2-sided, and P < 0.05 was used to define statistical significance. Data analyses were conducted using SAS software (SAS Institute Inc., Cary, North Carolina) version 9.4.
RESULTS
Differences in baseline bronchiolitis quality measures between sites that did and did not participate in the sustainability season are displayed in Table 3. Sustainability sites had significantly lower baseline use of a respiratory score, both to assess severity of illness at any point after hospitalization as well as to assess responsiveness following bronchodilator treatments (P < 0.001). At baseline they also had fewer orders for intermittent pulse oximetry use (P = 0.01) and fewer doses of bronchodilators per encounter (P = 0.04). Sites were not significantly different in their baseline use of bronchodilators, oral steroid doses, or chest radiographs. Sites that participated in the sustainability season demonstated larger magnitude improvement between baseline and intervention seasons for respiratory score use (P < 0.001 for any use and P = 0.02 to assess bronchodilator responsiveness; Appendix 1b).
DISCUSSION
To our knowledge, this is the first report of sustained improvements in care achieved through a multiinstitutional QI collaborative of community and academic hospitals focused on bronchiolitis care. We found that overall sites participating in a national bronchiolitis QI project sustained improvements in key bronchiolitis quality measures for 1 year following the project’s completion. For the aggregate group no measures worsened, and one measure, orders for intermittent pulse oximetry monitoring, continued to increase during the sustainability season. Furthermore, the sustained improvements were primarily the result of consistent sustained performance of each individual site, as opposed to averages wherein some sites worsened while others improved (Appendix 1a). These findings suggest that designing a collaborative approach, which provides an evidence-based best-practice toolkit while building the QI capacity of local interdisciplinary teams, can support performance gains that persist beyond the project’s active phase.
There are a number of possible reasons why improvements were sustained following the collaborative. The BQIP requirement for institutional leadership buy-in may have motivated accountability to local leaders in subsequent bronchiolitis seasons at each site. We suspect that culture change such as flattened hierarchies through multidisciplinary teams,14 which empowered nurse and respiratory therapy staff, may have facilitated consistent use of tools created locally. The synergy of interdisciplinary teams composed of physician, nurse, and respiratory therapy champions may have created accountability to perpetuate the previous year’s efforts.15 In addition, the sites adopted elements of the evidence-based toolkit, such as pathways,16,17 forcing function tools13,18 and order sets that limited management decision options and bronchodilator use contingent on respiratory scores,9,19 which may have driven desired behaviors.
Moreover, the 2014 AAP CPG for the management of bronchiolitis,20 released prior to the sustainability bronchiolitis season, may have underscored the key concepts of the collaborative. Similarly, national exposure of best practices for bronchiolitis management, including the 3 widespread Choosing Wisely recommendations related to bronchiolitis,21 might have been a compelling reason for sites to maintain their improvement efforts and contribute to secular trends toward decreasing interventions in bronchiolitis management nationally.3 Lastly, the mechanisms developed for local data collection may have created opportunities at each site to conduct ongoing evaluation of performance on key bronchiolitis quality measures through data-driven feedback systems.22 Our study highlights the need for additional research in order to understand why improvements are or are not sustained.
Even with substantial, sustained improvements in this initiative, further reduction in unnecessary care may be possible. Findings from previous studies suggest that even multifaceted QI interventions, including provider education, guidelines and use of respiratory scores, may only modestly reduce bronchodilators, steroids, and chest radiograph use.8,13 To achieve continued improvements in bronchiolitis care, additional active efforts may be needed to develop new interventions that target root causes for areas of overuse at individual sites.
Future multiinstitutional collaboratives might benefit their participants if they include a focus on helping sites develop skills to ensure that local improvement activities continue after the collaborative phases are completed. Proactively scheduling intermittent check-ins with collaborative members to discuss experiences with both sustainability and ongoing improvement may be valuable and likely needs to be incorporated into the initial collaborative planning.
As these sustainability data represent a subset of 9 of the original 21 BQIP sites, there is concern for potential selection bias related to factors that could have motivated sites to participate in the sustainability season’s data collection and simultaneously influenced their performance. These concerns were mitigated to some extent through 3 specific analyses: finding limited differences in hospital characteristics, baseline performance in key bronchiolitis measures, and performance change from baseline to intervention seasons between sites that did and did not participate in the sustainability season.
Notably, sites that participated in the sustainability phase actually had lower baseline respiratory score use and fewer orders for intermittent pulse oximetry at baseline. Theoretically, if participation in the collaborative highlighted this disparity for these sites, it could have been a motivating factor for their continued participation and sustained performance across these measures. Similarly, sites that recognized their higher baseline performance through participation in the collaborative might have felt less motivation to participate in ongoing data collection during the sustainability season. Whether they might have also sustained, declined, or continued improving is not known. Additionally, the magnitude of improvement in the collaborative period might have also motivated ongoing participation during the sustainability phase. For example, although all sites improved in score use during the collaborative, sites participating in the sustainability season demonstrated significantly more improvement in these measures. Sites with a higher magnitude of improvement in collaborative measures might have more enthusiasm about the project, more commitment to the project activities, or feel a sense of obligation to respond to requests for additional data collection.
This work has several limitations. Selection bias may limit generalizability of the results, as sites that did not participate in the sustainability season may have had different results than those that did participate. It is unknown whether sites that regressed toward their baseline were deterred from participating in the sustainability season. The analyses that we were able to preform, however, suggest that the 2 groups were similar in their characteristics as well as in their baseline and improvement performance.
We have limited knowledge of the local improvement work that sites conducted between the completion of the collaborative and the sustainability season. Site-specific factors may have influenced improvement sustainability. For example, qualitative research with the original group found that team engagement had a quantitative association with better performance, but only for the bronchodilator use measure.23 Sites were responsible for their own data collection, and despite attempts to centralize and standardize the process, data collection inconsistencies may have occurred. For instance, it is unknown how closely that orders for intermittent pulse oximetry correlate with intermittent use at the bedside. Lastly, the absence of a control group limits examination of the causal relationships of interventions and the influence of secular trends.
CONCLUSIONS
Improvements gained during the BQIP collaborative were sustained at 1 year following completion of the collaborative. These findings are encouraging, as national QI collaborative efforts are increasingly common. Our findings suggest that opportunities exist to even further reduce unnecessary care in the management of bronchiolitis. Such opportunities highlight the importance of integrating strategies to both measure sustainability and plan for ongoing independent local activities after completion of the collaborative. Future efforts should focus on supporting local sites to continue individual practice-improvement as they transition from collaborative to independent quality initiatives.
Acknowledgments
The authors thank the 21 hospitals that participated in the BQIP collaborative, and in particular the 9 hospital teams that contributed sustainability data for their ongoing dedication. There was no external funding for this manuscript.
Disclosure
The authors report no financial conflicts of interest.
Acute viral bronchiolitis is the most common cause of hospitalization for children less than 1 year of age.1 Overuse of ineffective therapies has persisted despite the existence of the evidence-based American Academy of Pediatrics (AAP) clinical practice guideline (CPG), which recommends primarily supportive care.2-8 Adherence to the AAP CPG recommendations for management of bronchiolitis improved significantly through the AAP’s Bronchiolitis Quality Improvement Project (BQIP), a 12-month, multiinstitutional collaborative of community and free-standing children’s hospitals.9 This subsequent study investigates if these improvements were sustained after completion of the formal 12-month project.
Published multiinstitutional bronchiolitis quality improvement (QI) work is limited to 1 study5 that describes the results of a single intervention season at academic medical centers. Multiyear bronchiolitis QI projects are limited to single-center studies, and results have been mixed.5,6,8,10-13 One study11 observed continued improvement in bronchodilator use in subsequent seasons, whereas a second study10 observed a return to baseline bronchodilator use in the following season. Mittal6 observed inconsistent improvements in key bronchiolitis measures during postintervention seasons.
Our specific aim was to assess the sustainability of improvements in bronchiolitis management at participating institutions 1 year following completion of the AAP BQIP collaborative.9 Because no studies demonstrate the most effective way to support long-term improvement through a QI collaborative, we hypothesized that the initial collaborative activities, which were designed to build the capacity of local interdisciplinary teams while providing standardized evidence-based care pathways, would lead to performance in the subsequent season at levels similar to or better than those observed during the active phase of the collaborative, without additional project interventions.
METHODS
Study Design and Setting
This was a follow-up study of the AAP Quality Improvement Innovation Networks project entitled “A Quality Collaborative for Improving Hospital Compliance with the AAP Bronchiolitis Guideline” (BQIP).9 The AAP Institutional Review Board approved this project.
Twenty-one multidisciplinary, hospital-based teams participated in the BQIP collaborative and provided monthly data during the January through March bronchiolitis season. Teams submitted 2013 baseline data and 2014 intervention data. Nine sites provided 2015 sustainability data following the completion of the collaborative.
Participants
Hospital encounters with a primary diagnosis of acute viral bronchiolitis were eligible for inclusion among patients from 1 month to 2 years of age. Encounters were excluded for prematurity (<35 weeks gestational age), congenital heart disease, bronchopulmonary dysplasia, genetic, congenital or neuromuscular abnormalities, and pediatric intensive-care admission.
Data Collection
Hospital characteristics were collected, including hospital type (academic, community), bed size, location (urban, rural), hospital distributions of race/ethnicity and public payer, cases of bronchiolitis per year, presence of an electronic medical record and a pediatric respiratory therapist, and self-rated QI knowledge of the multidisciplinary team (very knowledgeable, knowledgeable, and somewhat knowledgeable). A trained member at each site collected data through structured chart review in baseline, intervention, and sustainability bronchiolitis seasons for January, February, and March. Site members reviewed the first 20 charts per month that met the inclusion criteria or all charts if there were fewer than 20 eligible encounters. Sites input data about key quality measures into the AAP’s Quality Improvement Data Aggregator, a web-based data repository.
Intervention
The BQIP project was designed as a virtual collaborative consisting of monthly education webinars about QI methods and bronchiolitis management, opportunities for collaboration via teleconference and e-mail listserv, and individual site-coaching by e-mail or telephone.9 A change package was shared with sites that included examples of evidence-based pathways, ordersets, a respiratory scoring tool, communication tools for parents and referring physicians, and slide sets for individual site education efforts. Following completion of the collaborative, written resources remained available to participants, although virtual collaboration ceased and no additional project interventions to promote sustainability were introduced.
Bronchiolitis Process and Outcome Measures
Process measures following admission included the following: severity assessment using a respiratory score, respiratory score use to assess response to bronchodilators, bronchodilator use, bronchodilator doses, steroid doses per patient encounter, chest radiographs per encounter, and presence of an order to transition to intermittent pulse oximetry monitoring. Outcome measures included length of stay and readmissions within 72 hours.
Analysis
Changes among baseline-, intervention-, and sustainability-season data were assessed using generalized linear mixed-effects models with random effect for study sites. Negative binomial models were used for count variables to allow for overdispersion. Length of stay was log-transformed to achieve a normal distribution. We also analyzed each site individually to assess whether sustained improvements were the result of broad sustainability across all sites or whether they represented an aggregation of some sites that continued to improve while other sites actually worsened.
To address any bias introduced by the voluntary and incomplete participation of sites in the sustainability season, we planned a priori to conduct 3 additional analyses. First, we compared the characteristics of sites that did participate in the sustainability season with those that did not participate by using Chi-squared tests for differences in proportions and t tests for differences in means. Second, we determined whether the baseline-season process and outcome measures were different between sites that did and did not participate using descriptive statistics. Third, we assessed whether improvements between the baseline and intervention seasons were different between sites that did and did not participate using a linear mixed-effects model for normally distributed outcomes and generalized linear mixed-effects model with site-specific random effects for nonnormally distributed outcomes. All study outcomes were summarized in terms of model-adjusted means along with the corresponding 95% confidence intervals. All P values are 2-sided, and P < 0.05 was used to define statistical significance. Data analyses were conducted using SAS software (SAS Institute Inc., Cary, North Carolina) version 9.4.
RESULTS
Differences in baseline bronchiolitis quality measures between sites that did and did not participate in the sustainability season are displayed in Table 3. Sustainability sites had significantly lower baseline use of a respiratory score, both to assess severity of illness at any point after hospitalization as well as to assess responsiveness following bronchodilator treatments (P < 0.001). At baseline they also had fewer orders for intermittent pulse oximetry use (P = 0.01) and fewer doses of bronchodilators per encounter (P = 0.04). Sites were not significantly different in their baseline use of bronchodilators, oral steroid doses, or chest radiographs. Sites that participated in the sustainability season demonstated larger magnitude improvement between baseline and intervention seasons for respiratory score use (P < 0.001 for any use and P = 0.02 to assess bronchodilator responsiveness; Appendix 1b).
DISCUSSION
To our knowledge, this is the first report of sustained improvements in care achieved through a multiinstitutional QI collaborative of community and academic hospitals focused on bronchiolitis care. We found that overall sites participating in a national bronchiolitis QI project sustained improvements in key bronchiolitis quality measures for 1 year following the project’s completion. For the aggregate group no measures worsened, and one measure, orders for intermittent pulse oximetry monitoring, continued to increase during the sustainability season. Furthermore, the sustained improvements were primarily the result of consistent sustained performance of each individual site, as opposed to averages wherein some sites worsened while others improved (Appendix 1a). These findings suggest that designing a collaborative approach, which provides an evidence-based best-practice toolkit while building the QI capacity of local interdisciplinary teams, can support performance gains that persist beyond the project’s active phase.
There are a number of possible reasons why improvements were sustained following the collaborative. The BQIP requirement for institutional leadership buy-in may have motivated accountability to local leaders in subsequent bronchiolitis seasons at each site. We suspect that culture change such as flattened hierarchies through multidisciplinary teams,14 which empowered nurse and respiratory therapy staff, may have facilitated consistent use of tools created locally. The synergy of interdisciplinary teams composed of physician, nurse, and respiratory therapy champions may have created accountability to perpetuate the previous year’s efforts.15 In addition, the sites adopted elements of the evidence-based toolkit, such as pathways,16,17 forcing function tools13,18 and order sets that limited management decision options and bronchodilator use contingent on respiratory scores,9,19 which may have driven desired behaviors.
Moreover, the 2014 AAP CPG for the management of bronchiolitis,20 released prior to the sustainability bronchiolitis season, may have underscored the key concepts of the collaborative. Similarly, national exposure of best practices for bronchiolitis management, including the 3 widespread Choosing Wisely recommendations related to bronchiolitis,21 might have been a compelling reason for sites to maintain their improvement efforts and contribute to secular trends toward decreasing interventions in bronchiolitis management nationally.3 Lastly, the mechanisms developed for local data collection may have created opportunities at each site to conduct ongoing evaluation of performance on key bronchiolitis quality measures through data-driven feedback systems.22 Our study highlights the need for additional research in order to understand why improvements are or are not sustained.
Even with substantial, sustained improvements in this initiative, further reduction in unnecessary care may be possible. Findings from previous studies suggest that even multifaceted QI interventions, including provider education, guidelines and use of respiratory scores, may only modestly reduce bronchodilators, steroids, and chest radiograph use.8,13 To achieve continued improvements in bronchiolitis care, additional active efforts may be needed to develop new interventions that target root causes for areas of overuse at individual sites.
Future multiinstitutional collaboratives might benefit their participants if they include a focus on helping sites develop skills to ensure that local improvement activities continue after the collaborative phases are completed. Proactively scheduling intermittent check-ins with collaborative members to discuss experiences with both sustainability and ongoing improvement may be valuable and likely needs to be incorporated into the initial collaborative planning.
As these sustainability data represent a subset of 9 of the original 21 BQIP sites, there is concern for potential selection bias related to factors that could have motivated sites to participate in the sustainability season’s data collection and simultaneously influenced their performance. These concerns were mitigated to some extent through 3 specific analyses: finding limited differences in hospital characteristics, baseline performance in key bronchiolitis measures, and performance change from baseline to intervention seasons between sites that did and did not participate in the sustainability season.
Notably, sites that participated in the sustainability phase actually had lower baseline respiratory score use and fewer orders for intermittent pulse oximetry at baseline. Theoretically, if participation in the collaborative highlighted this disparity for these sites, it could have been a motivating factor for their continued participation and sustained performance across these measures. Similarly, sites that recognized their higher baseline performance through participation in the collaborative might have felt less motivation to participate in ongoing data collection during the sustainability season. Whether they might have also sustained, declined, or continued improving is not known. Additionally, the magnitude of improvement in the collaborative period might have also motivated ongoing participation during the sustainability phase. For example, although all sites improved in score use during the collaborative, sites participating in the sustainability season demonstrated significantly more improvement in these measures. Sites with a higher magnitude of improvement in collaborative measures might have more enthusiasm about the project, more commitment to the project activities, or feel a sense of obligation to respond to requests for additional data collection.
This work has several limitations. Selection bias may limit generalizability of the results, as sites that did not participate in the sustainability season may have had different results than those that did participate. It is unknown whether sites that regressed toward their baseline were deterred from participating in the sustainability season. The analyses that we were able to preform, however, suggest that the 2 groups were similar in their characteristics as well as in their baseline and improvement performance.
We have limited knowledge of the local improvement work that sites conducted between the completion of the collaborative and the sustainability season. Site-specific factors may have influenced improvement sustainability. For example, qualitative research with the original group found that team engagement had a quantitative association with better performance, but only for the bronchodilator use measure.23 Sites were responsible for their own data collection, and despite attempts to centralize and standardize the process, data collection inconsistencies may have occurred. For instance, it is unknown how closely that orders for intermittent pulse oximetry correlate with intermittent use at the bedside. Lastly, the absence of a control group limits examination of the causal relationships of interventions and the influence of secular trends.
CONCLUSIONS
Improvements gained during the BQIP collaborative were sustained at 1 year following completion of the collaborative. These findings are encouraging, as national QI collaborative efforts are increasingly common. Our findings suggest that opportunities exist to even further reduce unnecessary care in the management of bronchiolitis. Such opportunities highlight the importance of integrating strategies to both measure sustainability and plan for ongoing independent local activities after completion of the collaborative. Future efforts should focus on supporting local sites to continue individual practice-improvement as they transition from collaborative to independent quality initiatives.
Acknowledgments
The authors thank the 21 hospitals that participated in the BQIP collaborative, and in particular the 9 hospital teams that contributed sustainability data for their ongoing dedication. There was no external funding for this manuscript.
Disclosure
The authors report no financial conflicts of interest.
1. Healthcare Cost and Utilization Project (HCUP) KID Trends Supplemental File. Agency for Healthcare Research and Quality website. http://hcupnet.ahrq.gov/HCUPnet.jsp?Id=2C331B13FB40957D&Form=DispTab&JS=Y&Action=Accept. 2012. Accessed July 21, 2016.
2. Ralston S, Parikh K, Goodman D. Benchmarking overuse of medical interventions for bronchiolitis. JAMA Pediatr. 2015;169:805-806. PubMed
3. Parikh K, Hall M, Teach SJ. Bronchiolitis management before and after the AAP guidelines. Pediatrics. 2014;133:e1-e7. PubMed
4. Johnson LW, Robles J, Hudgins A, Osburn S, Martin D, Thompson A. Management of bronchiolitis in the emergency department: impact of evidence-based guidelines? Pediatrics. 2013;131 Suppl 1:S103-S109. PubMed
5. Kotagal UR, Robbins JM, Kini NM, Schoettker PJ, Atherton HD, Kirschbaum MS. Impact of a bronchiolitis guideline: a multisite demonstration project. Chest. 2002;121:1789-1797. PubMed
6. Mittal V, Darnell C, Walsh B, et al. Inpatient bronchiolitis guideline implementation and resource utilization. Pediatrics. 2014;133:e730-e737. PubMed
7. Mittal V, Hall M, Morse R, et al. Impact of inpatient bronchiolitis clinical practice guideline implementation on testing and treatment. J Pediatr. 2014;165:570.e3-576.e3. PubMed
8. Ralston S, Garber M, Narang S, et al. Decreasing unnecessary utilization in acute bronchiolitis care: results from the value in inpatient pediatrics network. J Hosp Med. 2013;8:25-30. PubMed
9. Ralston SL, Garber MD, Rice-Conboy E, et al. A multicenter collaborative to reduce unnecessary care in inpatient bronchiolitis. Pediatrics. 2016;137. PubMed
10. Perlstein PH, Kotagal UR, Schoettker PJ, et al. Sustaining the implementation of an evidence-based guideline for bronchiolitis. Arch Pediatr Adolesc Med. 2000;154:1001-1007. PubMed
11. Walker C, Danby S, Turner S. Impact of a bronchiolitis clinical care pathway on treatment and hospital stay. Eur J Pediatr. 2012;171:827-832. PubMed
12. Cheney J, Barber S, Altamirano L, et al. A clinical pathway for bronchiolitis is effective in reducing readmission rates. J Pediatr. 2005;147:622-626. PubMed
13. Ralston S, Comick A, Nichols E, Parker D, Lanter P. Effectiveness of quality improvement in hospitalization for bronchiolitis: a systematic review. Pediatrics. 2014;134:571-581. PubMed
14. Schwartz RW, Tumblin TF. The power of servant leadership to transform health care organizations for the 21st-century economy. Arch Surg. 2002;137:1419-1427; discussion 27. PubMed
15. Schalock RL, Verdugo M, Lee T. A systematic approach to an organization’s sustainability. Eval Program Plann. 2016;56:56-63. PubMed
16. Wilson SD, Dahl BB, Wells RD. An evidence-based clinical pathway for bronchiolitis safely reduces antibiotic overuse. Am J Med Qual. 2002;17:195-199. PubMed
17. Muething S, Schoettker PJ, Gerhardt WE, Atherton HD, Britto MT, Kotagal UR. Decreasing overuse of therapies in the treatment of bronchiolitis by incorporating evidence at the point of care. J Pediatr. 2004;144:703-710. PubMed
18. Streiff MB, Carolan HT, Hobson DB, et al. Lessons from the Johns Hopkins multi-disciplinary venous thromboembolism (VTE) prevention collaborative. BMJ. 2012;344:e3935. PubMed
19. Todd J, Bertoch D, Dolan S. Use of a large national database for comparative evaluation of the effect of a bronchiolitis/viral pneumonia clinical care guideline on patient outcome and resource utilization. Arch Pediatr Adolesc Med. 2002;156:1086-1090. PubMed
20. Ralston SL, Lieberthal AS, Meissner HC, et al. Clinical practice guideline: the diagnosis, management, and prevention of bronchiolitis. Pediatrics. 2014;134:e1474-e1502. PubMed
21. Quinonez RA, Garber MD, Schroeder AR, et al. Choosing wisely in pediatric hospital medicine: five opportunities for improved healthcare value. J Hosp Med. 2013;8:479-485. PubMed
22. Stone S, Lee HC, Sharek PJ. Perceived factors associated with sustained improvement following participation in a multicenter quality improvement collaborative. Jt Comm J Qual Patient Saf. 2016;42:309-315. PubMed
23. Ralston SL, Atwood EC, Garber MD, Holmes AV. What works to reduce unnecessary care for bronchiolitis? A qualitative analysis of a national collaborative. Acad Pediatr. 2017;17(2):198-204. PubMed
1. Healthcare Cost and Utilization Project (HCUP) KID Trends Supplemental File. Agency for Healthcare Research and Quality website. http://hcupnet.ahrq.gov/HCUPnet.jsp?Id=2C331B13FB40957D&Form=DispTab&JS=Y&Action=Accept. 2012. Accessed July 21, 2016.
2. Ralston S, Parikh K, Goodman D. Benchmarking overuse of medical interventions for bronchiolitis. JAMA Pediatr. 2015;169:805-806. PubMed
3. Parikh K, Hall M, Teach SJ. Bronchiolitis management before and after the AAP guidelines. Pediatrics. 2014;133:e1-e7. PubMed
4. Johnson LW, Robles J, Hudgins A, Osburn S, Martin D, Thompson A. Management of bronchiolitis in the emergency department: impact of evidence-based guidelines? Pediatrics. 2013;131 Suppl 1:S103-S109. PubMed
5. Kotagal UR, Robbins JM, Kini NM, Schoettker PJ, Atherton HD, Kirschbaum MS. Impact of a bronchiolitis guideline: a multisite demonstration project. Chest. 2002;121:1789-1797. PubMed
6. Mittal V, Darnell C, Walsh B, et al. Inpatient bronchiolitis guideline implementation and resource utilization. Pediatrics. 2014;133:e730-e737. PubMed
7. Mittal V, Hall M, Morse R, et al. Impact of inpatient bronchiolitis clinical practice guideline implementation on testing and treatment. J Pediatr. 2014;165:570.e3-576.e3. PubMed
8. Ralston S, Garber M, Narang S, et al. Decreasing unnecessary utilization in acute bronchiolitis care: results from the value in inpatient pediatrics network. J Hosp Med. 2013;8:25-30. PubMed
9. Ralston SL, Garber MD, Rice-Conboy E, et al. A multicenter collaborative to reduce unnecessary care in inpatient bronchiolitis. Pediatrics. 2016;137. PubMed
10. Perlstein PH, Kotagal UR, Schoettker PJ, et al. Sustaining the implementation of an evidence-based guideline for bronchiolitis. Arch Pediatr Adolesc Med. 2000;154:1001-1007. PubMed
11. Walker C, Danby S, Turner S. Impact of a bronchiolitis clinical care pathway on treatment and hospital stay. Eur J Pediatr. 2012;171:827-832. PubMed
12. Cheney J, Barber S, Altamirano L, et al. A clinical pathway for bronchiolitis is effective in reducing readmission rates. J Pediatr. 2005;147:622-626. PubMed
13. Ralston S, Comick A, Nichols E, Parker D, Lanter P. Effectiveness of quality improvement in hospitalization for bronchiolitis: a systematic review. Pediatrics. 2014;134:571-581. PubMed
14. Schwartz RW, Tumblin TF. The power of servant leadership to transform health care organizations for the 21st-century economy. Arch Surg. 2002;137:1419-1427; discussion 27. PubMed
15. Schalock RL, Verdugo M, Lee T. A systematic approach to an organization’s sustainability. Eval Program Plann. 2016;56:56-63. PubMed
16. Wilson SD, Dahl BB, Wells RD. An evidence-based clinical pathway for bronchiolitis safely reduces antibiotic overuse. Am J Med Qual. 2002;17:195-199. PubMed
17. Muething S, Schoettker PJ, Gerhardt WE, Atherton HD, Britto MT, Kotagal UR. Decreasing overuse of therapies in the treatment of bronchiolitis by incorporating evidence at the point of care. J Pediatr. 2004;144:703-710. PubMed
18. Streiff MB, Carolan HT, Hobson DB, et al. Lessons from the Johns Hopkins multi-disciplinary venous thromboembolism (VTE) prevention collaborative. BMJ. 2012;344:e3935. PubMed
19. Todd J, Bertoch D, Dolan S. Use of a large national database for comparative evaluation of the effect of a bronchiolitis/viral pneumonia clinical care guideline on patient outcome and resource utilization. Arch Pediatr Adolesc Med. 2002;156:1086-1090. PubMed
20. Ralston SL, Lieberthal AS, Meissner HC, et al. Clinical practice guideline: the diagnosis, management, and prevention of bronchiolitis. Pediatrics. 2014;134:e1474-e1502. PubMed
21. Quinonez RA, Garber MD, Schroeder AR, et al. Choosing wisely in pediatric hospital medicine: five opportunities for improved healthcare value. J Hosp Med. 2013;8:479-485. PubMed
22. Stone S, Lee HC, Sharek PJ. Perceived factors associated with sustained improvement following participation in a multicenter quality improvement collaborative. Jt Comm J Qual Patient Saf. 2016;42:309-315. PubMed
23. Ralston SL, Atwood EC, Garber MD, Holmes AV. What works to reduce unnecessary care for bronchiolitis? A qualitative analysis of a national collaborative. Acad Pediatr. 2017;17(2):198-204. PubMed
© 2017 Society of Hospital Medicine