User login
Penile Paraffinoma: Dramatic Recurrence After Surgical Resection
To the Editor:
The term paraffinoma refers to a chronic granulomatous response to injection of paraffin, silicone, or other mineral oils into skin and soft tissue. Paraffinomas develop when the material is injected into the skin for cosmetic purposes to augment or enhance one’s appearance. Although they may occur in any location, the most common sites include the breasts and buttocks. The penis is a rare but emerging site for paraffinomas.1-3 We present a rare case of recurrence of a penile paraffinoma following surgical resection.
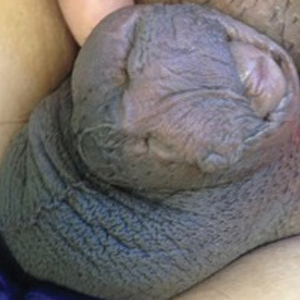
A 26-year-old uncircumcised Trinidadian man presented with a 5-cm, exquisitely tender tumor involving the penile shaft and median raphe that rapidly evolved over the course of 3 weeks (Figure 1). He presented with inability to urinate, attain an erection, or ambulate without notable tenderness. Additionally, he developed swelling of the penis and surrounding tissue. He had no other medical comorbidities; however, 1 year prior he presented to a urologist with a 1-cm nodule involving the median raphe that was surgically resected and required circumcision. Biopsy at the time of his surgical procedure revealed an exuberant foreign body giant cell reaction with surrounding empty spaces in the dermis resembling Swiss cheese, consistent with a paraffinoma (Figure 2). The recurrent tumor, which was 5 times the size of the initial nodule, was biopsied. Again, histopathologic findings were consistent with a paraffinoma with extensive dermal fibrosis and absence of polarizable material.
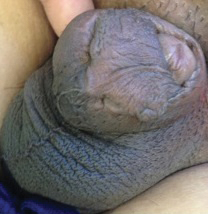
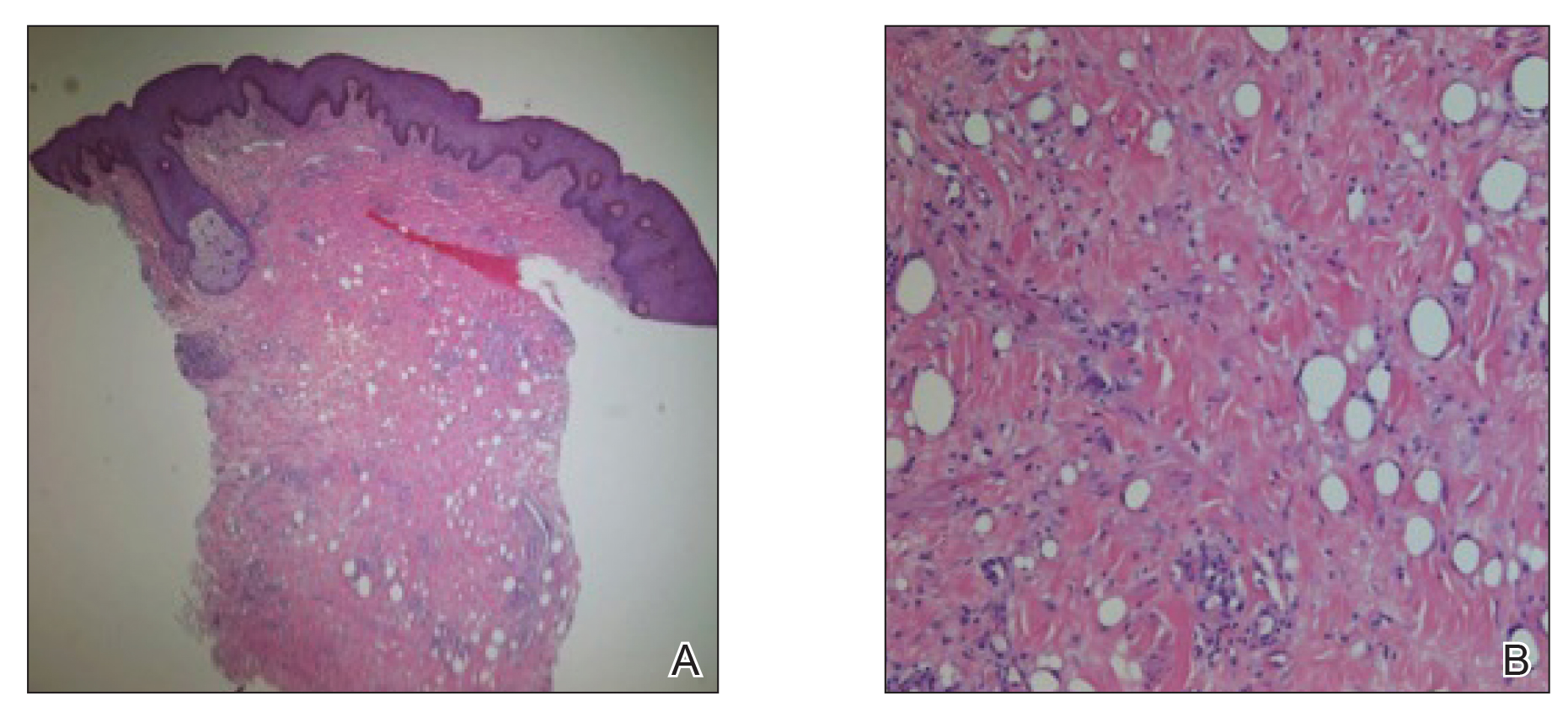
The patient underwent extensive reconstructive surgery requiring skin grafting to the penile shaft. Given the size and location of this recurrent tumor with the ability to destroy vital urologic and reproductive function, consideration for prevention of recurrent episodes included novel therapeutic treatment options to suppress inflammation and fibrosis with doxycycline and nicotinamide.
Paraffin injections are used for cosmetic enhancement and most often occur in a nonclinical setting without medical supervision, as they are not US Food and Drug Administration–approved medical injectable materials. Examples of oils injected include paraffin, camphorated oil, cottonseed or sesame oil, mineral oil, petroleum jelly, and beeswax. These oils are not hydrolyzed by tissue lipases but are instead treated as a foreign body substance with subsequent granuloma formation (also known as sclerosing lipogranuloma), which can occur many years after injection.4 The granulomatous response may be observed months to years after injection. The paraffinoma normally affects the injection site; however, regional lymphadenopathy and systemic disease has been reported.2 Histopathologic findings are characteristic and consist of a foreign body giant cell reaction, variably sized round to oval cavities within the dermis, and varying degrees of dermal fibrosis.5
In 1899, mineral oil was first injected into male genitalia to restore architecture in a patient’s testicles following bilateral orchiectomy. After the success of this endeavor, mineral oil injections were used as filler for other defects.3 However, by 1906 the complications of these injections became public knowledge when 2 patients developed subcutaneous nodules after receiving injections for facial wrinkles.2 Despite public knowledge of these complications, penile paraffin injections continued to occur both in medical and eventually nonmedical settings.
In 1947, Quérnu and Pérol6 described 6 penile paraffinoma cases outside the United States. Patients had petroleum jelly injections that eventuated in penile paraffinomas, and all of them lost the ability to attain an erection.6 Four years later, Bradley and Ehrgott7 described a case of penile paraffinoma likely caused by application of paraffin in association with occupational exposure. In 1956, May and Pickering8 cited a case of penile paraffinoma affecting the entire penile shaft in which the patient had undergone paraffin injection 7 years prior to treat premature ejaculation. Unfortunately, the injection resulted in a painful and unsatisfactory erection without resolution of premature ejaculation.8 Lee et al9 analyzed 26 cases of penile paraffinomas that occurred from 1981 to 1993. They found that all patients underwent injections of paraffin or petroleum jelly performed by nonmedical personnel with the predominant goal of enhancing penis size. Within 18.5 months of injection, 19 patients already experienced tenderness at the injection site. The remaining 7 patients experienced penile skin discoloration and abnormal contouring of the penis. Biopsy specimens revealed hyaline necrosis of subcutaneous adipose septa, cystlike spaces throughout involved tissue, and macrophages engulfing adipose tissue were found near blood vessels.9 In 2007, Eandi et al4 reported a case of penile paraffinoma with a 40-year delay of onset. Four years later, Manny et al10 reported penile paraffinomas in 3 Laotian men who injected a mineral oil.
Currently, paraffin injections are uncommon but still are being performed in some countries in Eastern Europe and the Far East11; they rarely are reported in the United States. Injections can occur in unusual sites such as the knee, and paraffinomas can develop many years after the procedure.12 Additionally, paraffinomas can obscure proper diagnosis of carcinomas, as described by Lee et al13 in a case in which a cervical paraffin injection confounded the diagnosis of a thyroid tumor. Furthermore, these injections usually are performed by nonmedical personnel and typically are repeated multiple times to reach cosmetic goals, rendering the patient vulnerable to early complications including allergic reactions, paraphimosis, infection, and inflammation.3
The clinical presentation of a penile paraffinoma may be a mimicker of several different entities, which are important to consider in the evaluation of a presenting patient. Infectious etiologies must be considered including lymphogranuloma venereum, granuloma inguinale, atypical mycobacteria, lupus vulgaris, and sexually transmitted infections. Importantly, neoplasms must be ruled out including squamous cell carcinoma, soft tissue sarcomas, melanoma, adenocarcinoma, or metastasis. Lymphedema, prior surgical procedures, trauma, and inflammatory etiologies also are in the differential diagnosis.14 Nonetheless, physicians must have a high clinical suspicion in the evaluation of a possible paraffinoma, as patients may not be forthcoming with relevant clinical history regarding a prior injection to the affected site, particularly if the injection occurred many years ago. As such, the patient may not consider this history relevant or may not even remember the event occurred, as was observed in our case. Furthermore, embarrassment, social taboo, and stigma may be associated with the behavior of undergoing injections in nonclinical settings without medical supervision.15
Patients may be motivated to undergo dangerous procedures to potentially alter their appearance due to perceived enhanced sexual ability, influence by loved ones, cultural rituals, and societal pressure.15,16 Furthermore, patients may not be aware of the material being injected or the volume. Given that these injections often are used with the goal of cosmetic enhancement, biopsies in cosmetically sensitive areas must be given careful consideration, and a thorough clinical history must support the decision to pursue a biopsy to obtain a definitive diagnosis.
The definitive diagnosis of a paraffinoma is determined by histopathology. However, the use of imaging modalities such as magnetic resonance imaging and computed tomography have been employed to delineate the extent of involvement. Imaging studies allow for surgical planning and may assist in narrowing a differential diagnosis.17 Currently, wide and complete surgical resection is the only definitive treatment of paraffinomas, including penile paraffinomas, as there is no evidence of spontaneous regression.3 A report of a reconstructive surgery involving penile resurfacing without T-style anastomosis has been found effective at preventing necrosis of the ventral penile skin. Not all paraffinomas behave similarly, and there is no reliable method to determine which paraffinoma may possess a more aggressive clinical course compared to those which have a more indolent course.18 As such, early detection is critical in the management of paraffinomas, especially in anatomic locations where tissue preservation is of utmost importance. In the case of a large penile paraffinoma with the ability to destroy vital urologic and reproductive function, physicians must consider prevention of recurrent episodes through suppression of inflammation and fibrosis with doxycycline and nicotinamide.19 Other medical treatments reported with varying success include corticosteroids, imiquimod, and isotretinoin.19-24 Employing adjunctive medical treatment may decrease the size of the mass, reducing the surgical defect size and preserving tissue vitality. Ultimately, the most crucial aspect in treatment is prevention, as injection of foreign materials elicits a foreign body response and can lead to notable morbidity.
- De Siati M, Selvaggio O, Di Fino G, et al. An unusual delayed complication of paraffin self-injection for penile girth augmentation. BMC Urol. 2013;13:66.
- Sejben I, Rácz A, Svébis M, et al. Petroleum jelly-induced penile paraffinoma with inguinal lymphadenitis mimicking incarcerated inguinal hernia. Can Urol Assoc J. 2012;6:E137-E139.
- Bayraktar N, Basar I. Penile paraffinoma [published online September 17, 2012]. Case Rep Urol. 2012;2012:202840.
- Eandi JA, Yao AP, Javidan J. Penile paraffinoma: the delayed presentation. Int Urol Nephrol. 2007;29:553-555.
- HirshBCJohnsonWC. Pathology of granulomatous diseases. foreign body granulomas. Int J Dermatol. 1984;23:531-538.
- Quérnu J, Pérol E. Paraffinomas of the penis. J Chir Par. 1947;63:345.
- Bradley, RH, Ehrgott WA. Paraffinoma of the penis: case report. J Urol. 1951;65:453.
- May JA, Pickering PP. Paraffinoma of the penis. Calif Med. 1956;85:42-44.
Yonsei Med J. 1994;35:344-348. - Lee T, Choi HR, Lee YT, et al. Paraffinoma of the penis.
- Manny T, Pettus J, Hemal A, et al. Penile sclerosing lipogranulomas and disfigurement from use of “1Super Extenze” among Laotian immigrants. J Sex Med. 2011;8:3505-3510.
- Akkus E Paraffinoma and ulcer of the external genitalia after self-injection of vaseline. J Sex Med. 2006;3:170-172.
- Grassetti L, Lazzeri D, Torresetti M, et al. Paraffinoma of the knee 60 years after primary infection. Arch Plast Surg. 2013;40:789-790.
- Lee YS, Son EJ, Kim BW, et al. Difficult evaluation of thyroid cancer due to cervical paraffin injection. J Korean Surg Soc. 2011;81(suppl 1):S17-S20.
- Gómez-Armayones S, Penín R, Marcoval J. Penile paraffinoma [in Spanish]. Actas Dermosifiliogr. 2014;105:957-959.
- Moon DG, Yoo JW, Bae JH, et al. Sexual function and psychological characteristics of penile paraffinoma. Asian J Androl. 2003;5:191-194.
- Pehlivanov G, Kavaklieva S, Kazandjieva J, et al. Foreign-body granuloma of the penis in sexually active individuals (penile paraffinoma). J Eur Acad Dermatol Venereol. 2008;22:845-851.
- Cormio L, Di Fino G, Scavone C, et al. Magnetic resonance imaging of penile paraffinoma: case report. BMC Med Imaging. 2014;14:39.
- Shin YS, Zhao C, Park JK. New reconstructive surgery for penile paraffinoma to prevent necrosis of ventral penile skin. Urology. 2013;81:437-441.
- Feldmann R, Harms M, Chavaz P, et al. Orbital and palpebral paraffinoma. J Am Acad Dermatol. 1992;26:833-835.
- MastruserioDNPesqueiraMJCobbMW. Severe granulomatous reaction and facial ulceration occurring after subcutaneous silicone injection. J Am Acad Dermatol. 1996;34:849-852.
- HoWS Management of paraffinoma of the breast. Br J Plast Surg. 2001;54:232-234.
- LloretPSuccessful treatment of granulomatous reactions secondary to injection of esthetic implants. Dermatol Surg. 2005;31:486-490.
- RosenbergEThree cases of penile paraffinoma. Urology. 2007;70:372.
- Baumann LS, Halem ML. Lip silicone granulomatous foreign body reaction treated with Aldara (imiquimod 5%). Dermatol Surg. 2003;29:429-432.
To the Editor:
The term paraffinoma refers to a chronic granulomatous response to injection of paraffin, silicone, or other mineral oils into skin and soft tissue. Paraffinomas develop when the material is injected into the skin for cosmetic purposes to augment or enhance one’s appearance. Although they may occur in any location, the most common sites include the breasts and buttocks. The penis is a rare but emerging site for paraffinomas.1-3 We present a rare case of recurrence of a penile paraffinoma following surgical resection.
A 26-year-old uncircumcised Trinidadian man presented with a 5-cm, exquisitely tender tumor involving the penile shaft and median raphe that rapidly evolved over the course of 3 weeks (Figure 1). He presented with inability to urinate, attain an erection, or ambulate without notable tenderness. Additionally, he developed swelling of the penis and surrounding tissue. He had no other medical comorbidities; however, 1 year prior he presented to a urologist with a 1-cm nodule involving the median raphe that was surgically resected and required circumcision. Biopsy at the time of his surgical procedure revealed an exuberant foreign body giant cell reaction with surrounding empty spaces in the dermis resembling Swiss cheese, consistent with a paraffinoma (Figure 2). The recurrent tumor, which was 5 times the size of the initial nodule, was biopsied. Again, histopathologic findings were consistent with a paraffinoma with extensive dermal fibrosis and absence of polarizable material.


The patient underwent extensive reconstructive surgery requiring skin grafting to the penile shaft. Given the size and location of this recurrent tumor with the ability to destroy vital urologic and reproductive function, consideration for prevention of recurrent episodes included novel therapeutic treatment options to suppress inflammation and fibrosis with doxycycline and nicotinamide.
Paraffin injections are used for cosmetic enhancement and most often occur in a nonclinical setting without medical supervision, as they are not US Food and Drug Administration–approved medical injectable materials. Examples of oils injected include paraffin, camphorated oil, cottonseed or sesame oil, mineral oil, petroleum jelly, and beeswax. These oils are not hydrolyzed by tissue lipases but are instead treated as a foreign body substance with subsequent granuloma formation (also known as sclerosing lipogranuloma), which can occur many years after injection.4 The granulomatous response may be observed months to years after injection. The paraffinoma normally affects the injection site; however, regional lymphadenopathy and systemic disease has been reported.2 Histopathologic findings are characteristic and consist of a foreign body giant cell reaction, variably sized round to oval cavities within the dermis, and varying degrees of dermal fibrosis.5
In 1899, mineral oil was first injected into male genitalia to restore architecture in a patient’s testicles following bilateral orchiectomy. After the success of this endeavor, mineral oil injections were used as filler for other defects.3 However, by 1906 the complications of these injections became public knowledge when 2 patients developed subcutaneous nodules after receiving injections for facial wrinkles.2 Despite public knowledge of these complications, penile paraffin injections continued to occur both in medical and eventually nonmedical settings.
In 1947, Quérnu and Pérol6 described 6 penile paraffinoma cases outside the United States. Patients had petroleum jelly injections that eventuated in penile paraffinomas, and all of them lost the ability to attain an erection.6 Four years later, Bradley and Ehrgott7 described a case of penile paraffinoma likely caused by application of paraffin in association with occupational exposure. In 1956, May and Pickering8 cited a case of penile paraffinoma affecting the entire penile shaft in which the patient had undergone paraffin injection 7 years prior to treat premature ejaculation. Unfortunately, the injection resulted in a painful and unsatisfactory erection without resolution of premature ejaculation.8 Lee et al9 analyzed 26 cases of penile paraffinomas that occurred from 1981 to 1993. They found that all patients underwent injections of paraffin or petroleum jelly performed by nonmedical personnel with the predominant goal of enhancing penis size. Within 18.5 months of injection, 19 patients already experienced tenderness at the injection site. The remaining 7 patients experienced penile skin discoloration and abnormal contouring of the penis. Biopsy specimens revealed hyaline necrosis of subcutaneous adipose septa, cystlike spaces throughout involved tissue, and macrophages engulfing adipose tissue were found near blood vessels.9 In 2007, Eandi et al4 reported a case of penile paraffinoma with a 40-year delay of onset. Four years later, Manny et al10 reported penile paraffinomas in 3 Laotian men who injected a mineral oil.
Currently, paraffin injections are uncommon but still are being performed in some countries in Eastern Europe and the Far East11; they rarely are reported in the United States. Injections can occur in unusual sites such as the knee, and paraffinomas can develop many years after the procedure.12 Additionally, paraffinomas can obscure proper diagnosis of carcinomas, as described by Lee et al13 in a case in which a cervical paraffin injection confounded the diagnosis of a thyroid tumor. Furthermore, these injections usually are performed by nonmedical personnel and typically are repeated multiple times to reach cosmetic goals, rendering the patient vulnerable to early complications including allergic reactions, paraphimosis, infection, and inflammation.3
The clinical presentation of a penile paraffinoma may be a mimicker of several different entities, which are important to consider in the evaluation of a presenting patient. Infectious etiologies must be considered including lymphogranuloma venereum, granuloma inguinale, atypical mycobacteria, lupus vulgaris, and sexually transmitted infections. Importantly, neoplasms must be ruled out including squamous cell carcinoma, soft tissue sarcomas, melanoma, adenocarcinoma, or metastasis. Lymphedema, prior surgical procedures, trauma, and inflammatory etiologies also are in the differential diagnosis.14 Nonetheless, physicians must have a high clinical suspicion in the evaluation of a possible paraffinoma, as patients may not be forthcoming with relevant clinical history regarding a prior injection to the affected site, particularly if the injection occurred many years ago. As such, the patient may not consider this history relevant or may not even remember the event occurred, as was observed in our case. Furthermore, embarrassment, social taboo, and stigma may be associated with the behavior of undergoing injections in nonclinical settings without medical supervision.15
Patients may be motivated to undergo dangerous procedures to potentially alter their appearance due to perceived enhanced sexual ability, influence by loved ones, cultural rituals, and societal pressure.15,16 Furthermore, patients may not be aware of the material being injected or the volume. Given that these injections often are used with the goal of cosmetic enhancement, biopsies in cosmetically sensitive areas must be given careful consideration, and a thorough clinical history must support the decision to pursue a biopsy to obtain a definitive diagnosis.
The definitive diagnosis of a paraffinoma is determined by histopathology. However, the use of imaging modalities such as magnetic resonance imaging and computed tomography have been employed to delineate the extent of involvement. Imaging studies allow for surgical planning and may assist in narrowing a differential diagnosis.17 Currently, wide and complete surgical resection is the only definitive treatment of paraffinomas, including penile paraffinomas, as there is no evidence of spontaneous regression.3 A report of a reconstructive surgery involving penile resurfacing without T-style anastomosis has been found effective at preventing necrosis of the ventral penile skin. Not all paraffinomas behave similarly, and there is no reliable method to determine which paraffinoma may possess a more aggressive clinical course compared to those which have a more indolent course.18 As such, early detection is critical in the management of paraffinomas, especially in anatomic locations where tissue preservation is of utmost importance. In the case of a large penile paraffinoma with the ability to destroy vital urologic and reproductive function, physicians must consider prevention of recurrent episodes through suppression of inflammation and fibrosis with doxycycline and nicotinamide.19 Other medical treatments reported with varying success include corticosteroids, imiquimod, and isotretinoin.19-24 Employing adjunctive medical treatment may decrease the size of the mass, reducing the surgical defect size and preserving tissue vitality. Ultimately, the most crucial aspect in treatment is prevention, as injection of foreign materials elicits a foreign body response and can lead to notable morbidity.
To the Editor:
The term paraffinoma refers to a chronic granulomatous response to injection of paraffin, silicone, or other mineral oils into skin and soft tissue. Paraffinomas develop when the material is injected into the skin for cosmetic purposes to augment or enhance one’s appearance. Although they may occur in any location, the most common sites include the breasts and buttocks. The penis is a rare but emerging site for paraffinomas.1-3 We present a rare case of recurrence of a penile paraffinoma following surgical resection.
A 26-year-old uncircumcised Trinidadian man presented with a 5-cm, exquisitely tender tumor involving the penile shaft and median raphe that rapidly evolved over the course of 3 weeks (Figure 1). He presented with inability to urinate, attain an erection, or ambulate without notable tenderness. Additionally, he developed swelling of the penis and surrounding tissue. He had no other medical comorbidities; however, 1 year prior he presented to a urologist with a 1-cm nodule involving the median raphe that was surgically resected and required circumcision. Biopsy at the time of his surgical procedure revealed an exuberant foreign body giant cell reaction with surrounding empty spaces in the dermis resembling Swiss cheese, consistent with a paraffinoma (Figure 2). The recurrent tumor, which was 5 times the size of the initial nodule, was biopsied. Again, histopathologic findings were consistent with a paraffinoma with extensive dermal fibrosis and absence of polarizable material.


The patient underwent extensive reconstructive surgery requiring skin grafting to the penile shaft. Given the size and location of this recurrent tumor with the ability to destroy vital urologic and reproductive function, consideration for prevention of recurrent episodes included novel therapeutic treatment options to suppress inflammation and fibrosis with doxycycline and nicotinamide.
Paraffin injections are used for cosmetic enhancement and most often occur in a nonclinical setting without medical supervision, as they are not US Food and Drug Administration–approved medical injectable materials. Examples of oils injected include paraffin, camphorated oil, cottonseed or sesame oil, mineral oil, petroleum jelly, and beeswax. These oils are not hydrolyzed by tissue lipases but are instead treated as a foreign body substance with subsequent granuloma formation (also known as sclerosing lipogranuloma), which can occur many years after injection.4 The granulomatous response may be observed months to years after injection. The paraffinoma normally affects the injection site; however, regional lymphadenopathy and systemic disease has been reported.2 Histopathologic findings are characteristic and consist of a foreign body giant cell reaction, variably sized round to oval cavities within the dermis, and varying degrees of dermal fibrosis.5
In 1899, mineral oil was first injected into male genitalia to restore architecture in a patient’s testicles following bilateral orchiectomy. After the success of this endeavor, mineral oil injections were used as filler for other defects.3 However, by 1906 the complications of these injections became public knowledge when 2 patients developed subcutaneous nodules after receiving injections for facial wrinkles.2 Despite public knowledge of these complications, penile paraffin injections continued to occur both in medical and eventually nonmedical settings.
In 1947, Quérnu and Pérol6 described 6 penile paraffinoma cases outside the United States. Patients had petroleum jelly injections that eventuated in penile paraffinomas, and all of them lost the ability to attain an erection.6 Four years later, Bradley and Ehrgott7 described a case of penile paraffinoma likely caused by application of paraffin in association with occupational exposure. In 1956, May and Pickering8 cited a case of penile paraffinoma affecting the entire penile shaft in which the patient had undergone paraffin injection 7 years prior to treat premature ejaculation. Unfortunately, the injection resulted in a painful and unsatisfactory erection without resolution of premature ejaculation.8 Lee et al9 analyzed 26 cases of penile paraffinomas that occurred from 1981 to 1993. They found that all patients underwent injections of paraffin or petroleum jelly performed by nonmedical personnel with the predominant goal of enhancing penis size. Within 18.5 months of injection, 19 patients already experienced tenderness at the injection site. The remaining 7 patients experienced penile skin discoloration and abnormal contouring of the penis. Biopsy specimens revealed hyaline necrosis of subcutaneous adipose septa, cystlike spaces throughout involved tissue, and macrophages engulfing adipose tissue were found near blood vessels.9 In 2007, Eandi et al4 reported a case of penile paraffinoma with a 40-year delay of onset. Four years later, Manny et al10 reported penile paraffinomas in 3 Laotian men who injected a mineral oil.
Currently, paraffin injections are uncommon but still are being performed in some countries in Eastern Europe and the Far East11; they rarely are reported in the United States. Injections can occur in unusual sites such as the knee, and paraffinomas can develop many years after the procedure.12 Additionally, paraffinomas can obscure proper diagnosis of carcinomas, as described by Lee et al13 in a case in which a cervical paraffin injection confounded the diagnosis of a thyroid tumor. Furthermore, these injections usually are performed by nonmedical personnel and typically are repeated multiple times to reach cosmetic goals, rendering the patient vulnerable to early complications including allergic reactions, paraphimosis, infection, and inflammation.3
The clinical presentation of a penile paraffinoma may be a mimicker of several different entities, which are important to consider in the evaluation of a presenting patient. Infectious etiologies must be considered including lymphogranuloma venereum, granuloma inguinale, atypical mycobacteria, lupus vulgaris, and sexually transmitted infections. Importantly, neoplasms must be ruled out including squamous cell carcinoma, soft tissue sarcomas, melanoma, adenocarcinoma, or metastasis. Lymphedema, prior surgical procedures, trauma, and inflammatory etiologies also are in the differential diagnosis.14 Nonetheless, physicians must have a high clinical suspicion in the evaluation of a possible paraffinoma, as patients may not be forthcoming with relevant clinical history regarding a prior injection to the affected site, particularly if the injection occurred many years ago. As such, the patient may not consider this history relevant or may not even remember the event occurred, as was observed in our case. Furthermore, embarrassment, social taboo, and stigma may be associated with the behavior of undergoing injections in nonclinical settings without medical supervision.15
Patients may be motivated to undergo dangerous procedures to potentially alter their appearance due to perceived enhanced sexual ability, influence by loved ones, cultural rituals, and societal pressure.15,16 Furthermore, patients may not be aware of the material being injected or the volume. Given that these injections often are used with the goal of cosmetic enhancement, biopsies in cosmetically sensitive areas must be given careful consideration, and a thorough clinical history must support the decision to pursue a biopsy to obtain a definitive diagnosis.
The definitive diagnosis of a paraffinoma is determined by histopathology. However, the use of imaging modalities such as magnetic resonance imaging and computed tomography have been employed to delineate the extent of involvement. Imaging studies allow for surgical planning and may assist in narrowing a differential diagnosis.17 Currently, wide and complete surgical resection is the only definitive treatment of paraffinomas, including penile paraffinomas, as there is no evidence of spontaneous regression.3 A report of a reconstructive surgery involving penile resurfacing without T-style anastomosis has been found effective at preventing necrosis of the ventral penile skin. Not all paraffinomas behave similarly, and there is no reliable method to determine which paraffinoma may possess a more aggressive clinical course compared to those which have a more indolent course.18 As such, early detection is critical in the management of paraffinomas, especially in anatomic locations where tissue preservation is of utmost importance. In the case of a large penile paraffinoma with the ability to destroy vital urologic and reproductive function, physicians must consider prevention of recurrent episodes through suppression of inflammation and fibrosis with doxycycline and nicotinamide.19 Other medical treatments reported with varying success include corticosteroids, imiquimod, and isotretinoin.19-24 Employing adjunctive medical treatment may decrease the size of the mass, reducing the surgical defect size and preserving tissue vitality. Ultimately, the most crucial aspect in treatment is prevention, as injection of foreign materials elicits a foreign body response and can lead to notable morbidity.
- De Siati M, Selvaggio O, Di Fino G, et al. An unusual delayed complication of paraffin self-injection for penile girth augmentation. BMC Urol. 2013;13:66.
- Sejben I, Rácz A, Svébis M, et al. Petroleum jelly-induced penile paraffinoma with inguinal lymphadenitis mimicking incarcerated inguinal hernia. Can Urol Assoc J. 2012;6:E137-E139.
- Bayraktar N, Basar I. Penile paraffinoma [published online September 17, 2012]. Case Rep Urol. 2012;2012:202840.
- Eandi JA, Yao AP, Javidan J. Penile paraffinoma: the delayed presentation. Int Urol Nephrol. 2007;29:553-555.
- HirshBCJohnsonWC. Pathology of granulomatous diseases. foreign body granulomas. Int J Dermatol. 1984;23:531-538.
- Quérnu J, Pérol E. Paraffinomas of the penis. J Chir Par. 1947;63:345.
- Bradley, RH, Ehrgott WA. Paraffinoma of the penis: case report. J Urol. 1951;65:453.
- May JA, Pickering PP. Paraffinoma of the penis. Calif Med. 1956;85:42-44.
Yonsei Med J. 1994;35:344-348. - Lee T, Choi HR, Lee YT, et al. Paraffinoma of the penis.
- Manny T, Pettus J, Hemal A, et al. Penile sclerosing lipogranulomas and disfigurement from use of “1Super Extenze” among Laotian immigrants. J Sex Med. 2011;8:3505-3510.
- Akkus E Paraffinoma and ulcer of the external genitalia after self-injection of vaseline. J Sex Med. 2006;3:170-172.
- Grassetti L, Lazzeri D, Torresetti M, et al. Paraffinoma of the knee 60 years after primary infection. Arch Plast Surg. 2013;40:789-790.
- Lee YS, Son EJ, Kim BW, et al. Difficult evaluation of thyroid cancer due to cervical paraffin injection. J Korean Surg Soc. 2011;81(suppl 1):S17-S20.
- Gómez-Armayones S, Penín R, Marcoval J. Penile paraffinoma [in Spanish]. Actas Dermosifiliogr. 2014;105:957-959.
- Moon DG, Yoo JW, Bae JH, et al. Sexual function and psychological characteristics of penile paraffinoma. Asian J Androl. 2003;5:191-194.
- Pehlivanov G, Kavaklieva S, Kazandjieva J, et al. Foreign-body granuloma of the penis in sexually active individuals (penile paraffinoma). J Eur Acad Dermatol Venereol. 2008;22:845-851.
- Cormio L, Di Fino G, Scavone C, et al. Magnetic resonance imaging of penile paraffinoma: case report. BMC Med Imaging. 2014;14:39.
- Shin YS, Zhao C, Park JK. New reconstructive surgery for penile paraffinoma to prevent necrosis of ventral penile skin. Urology. 2013;81:437-441.
- Feldmann R, Harms M, Chavaz P, et al. Orbital and palpebral paraffinoma. J Am Acad Dermatol. 1992;26:833-835.
- MastruserioDNPesqueiraMJCobbMW. Severe granulomatous reaction and facial ulceration occurring after subcutaneous silicone injection. J Am Acad Dermatol. 1996;34:849-852.
- HoWS Management of paraffinoma of the breast. Br J Plast Surg. 2001;54:232-234.
- LloretPSuccessful treatment of granulomatous reactions secondary to injection of esthetic implants. Dermatol Surg. 2005;31:486-490.
- RosenbergEThree cases of penile paraffinoma. Urology. 2007;70:372.
- Baumann LS, Halem ML. Lip silicone granulomatous foreign body reaction treated with Aldara (imiquimod 5%). Dermatol Surg. 2003;29:429-432.
- De Siati M, Selvaggio O, Di Fino G, et al. An unusual delayed complication of paraffin self-injection for penile girth augmentation. BMC Urol. 2013;13:66.
- Sejben I, Rácz A, Svébis M, et al. Petroleum jelly-induced penile paraffinoma with inguinal lymphadenitis mimicking incarcerated inguinal hernia. Can Urol Assoc J. 2012;6:E137-E139.
- Bayraktar N, Basar I. Penile paraffinoma [published online September 17, 2012]. Case Rep Urol. 2012;2012:202840.
- Eandi JA, Yao AP, Javidan J. Penile paraffinoma: the delayed presentation. Int Urol Nephrol. 2007;29:553-555.
- HirshBCJohnsonWC. Pathology of granulomatous diseases. foreign body granulomas. Int J Dermatol. 1984;23:531-538.
- Quérnu J, Pérol E. Paraffinomas of the penis. J Chir Par. 1947;63:345.
- Bradley, RH, Ehrgott WA. Paraffinoma of the penis: case report. J Urol. 1951;65:453.
- May JA, Pickering PP. Paraffinoma of the penis. Calif Med. 1956;85:42-44.
Yonsei Med J. 1994;35:344-348. - Lee T, Choi HR, Lee YT, et al. Paraffinoma of the penis.
- Manny T, Pettus J, Hemal A, et al. Penile sclerosing lipogranulomas and disfigurement from use of “1Super Extenze” among Laotian immigrants. J Sex Med. 2011;8:3505-3510.
- Akkus E Paraffinoma and ulcer of the external genitalia after self-injection of vaseline. J Sex Med. 2006;3:170-172.
- Grassetti L, Lazzeri D, Torresetti M, et al. Paraffinoma of the knee 60 years after primary infection. Arch Plast Surg. 2013;40:789-790.
- Lee YS, Son EJ, Kim BW, et al. Difficult evaluation of thyroid cancer due to cervical paraffin injection. J Korean Surg Soc. 2011;81(suppl 1):S17-S20.
- Gómez-Armayones S, Penín R, Marcoval J. Penile paraffinoma [in Spanish]. Actas Dermosifiliogr. 2014;105:957-959.
- Moon DG, Yoo JW, Bae JH, et al. Sexual function and psychological characteristics of penile paraffinoma. Asian J Androl. 2003;5:191-194.
- Pehlivanov G, Kavaklieva S, Kazandjieva J, et al. Foreign-body granuloma of the penis in sexually active individuals (penile paraffinoma). J Eur Acad Dermatol Venereol. 2008;22:845-851.
- Cormio L, Di Fino G, Scavone C, et al. Magnetic resonance imaging of penile paraffinoma: case report. BMC Med Imaging. 2014;14:39.
- Shin YS, Zhao C, Park JK. New reconstructive surgery for penile paraffinoma to prevent necrosis of ventral penile skin. Urology. 2013;81:437-441.
- Feldmann R, Harms M, Chavaz P, et al. Orbital and palpebral paraffinoma. J Am Acad Dermatol. 1992;26:833-835.
- MastruserioDNPesqueiraMJCobbMW. Severe granulomatous reaction and facial ulceration occurring after subcutaneous silicone injection. J Am Acad Dermatol. 1996;34:849-852.
- HoWS Management of paraffinoma of the breast. Br J Plast Surg. 2001;54:232-234.
- LloretPSuccessful treatment of granulomatous reactions secondary to injection of esthetic implants. Dermatol Surg. 2005;31:486-490.
- RosenbergEThree cases of penile paraffinoma. Urology. 2007;70:372.
- Baumann LS, Halem ML. Lip silicone granulomatous foreign body reaction treated with Aldara (imiquimod 5%). Dermatol Surg. 2003;29:429-432.
Practice Points
- Taking a thorough history in patients with possible paraffinomas is vital, including a history of injectables even in the genital region.
- Biopsies in cosmetically sensitive areas must be given careful consideration. Clinical history must support the decision to pursue a definitive diagnosis.
- Early detection is critical in the management of paraffinomas, especially in anatomic locations where tissue preservation is of utmost importance.
Pustular Tinea Id Reaction
To the Editor:
A 17-year-old adolescent girl presented to the dermatology clinic with a tender pruritic rash on the left wrist that was spreading to the bilateral arms and legs of several years’ duration. An area of a prior biopsy on the left wrist was healing well with use of petroleum jelly and halcinonide cream. The patient denied any constitutional symptoms.
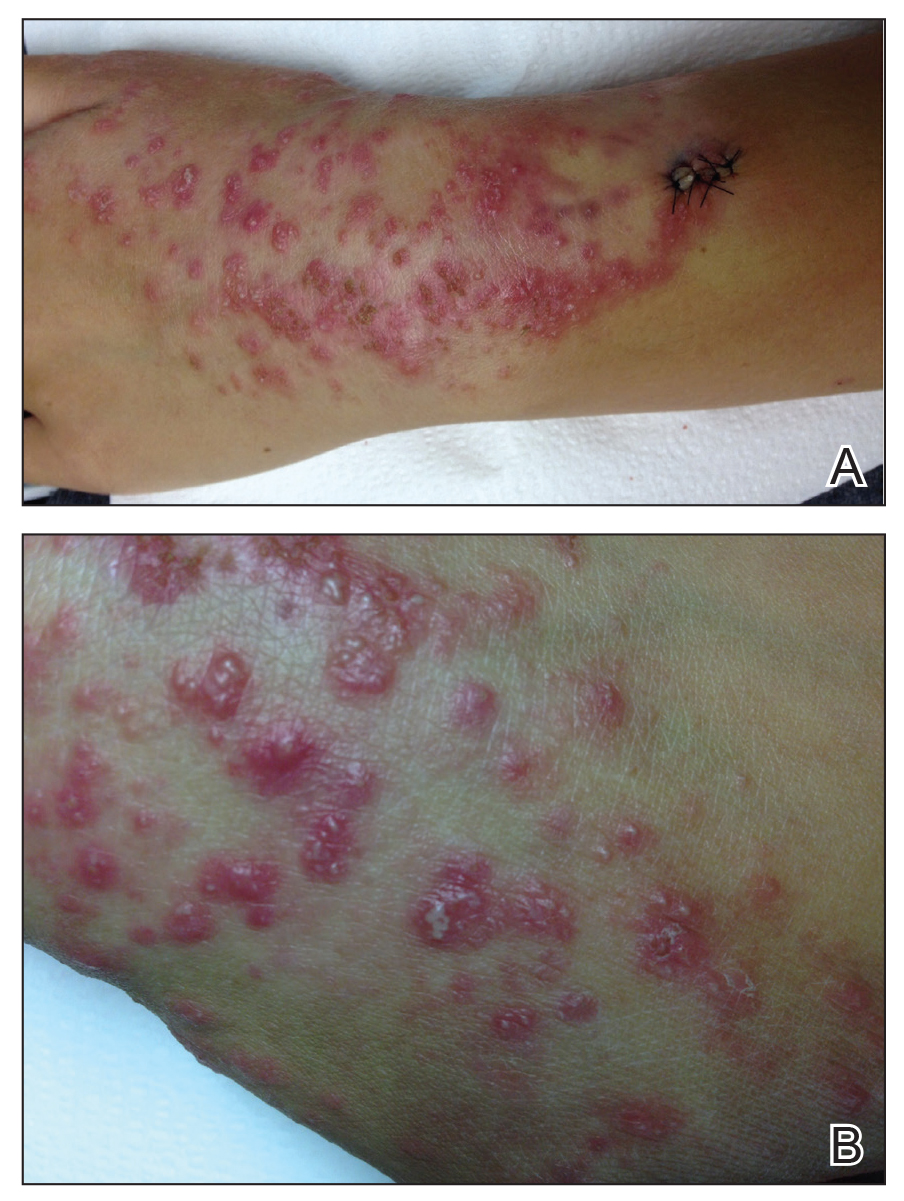
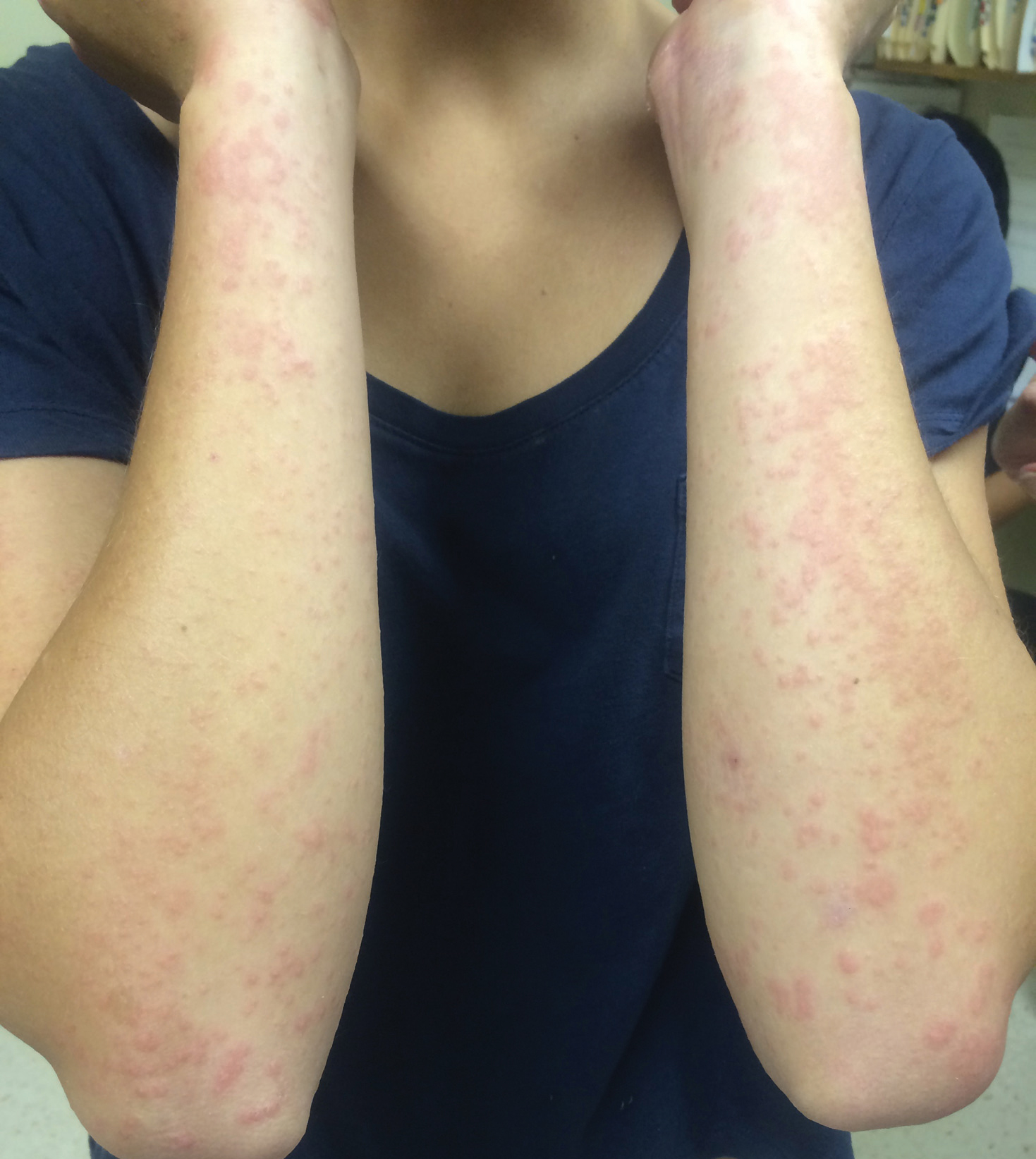
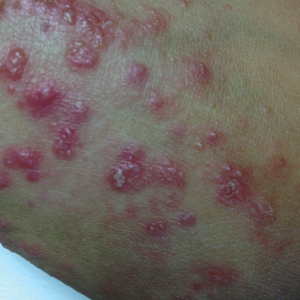
Physical examination revealed numerous erythematous papules coalescing into plaques on the bilateral anterior and posterior arms and legs, including some erythematous macules and papules on the palms and soles. The original area of involvement on the left dorsal medial wrist demonstrated a background of erythema with overlying peripheral scaling and resolving violaceous to erythematous papules with signs of serosanguineous crusting (Figure 1). Scattered perifollicular erythema was present on the posterior aspects of the bilateral thighs and arms (Figure 2). Baseline complete blood cell count and complete metabolic panel were within reference range.
Clinical histopathology showed evidence of a pustular superficial dermatophyte infection, and Grocott-Gomori methenamine-silver stain demonstrated numerous fungal hyphae within subcorneal pustules, indicating pustular tinea. Based on the clinicopathologic correlation, the initial presentation was diagnosed as pustular tinea of the entire left wrist, followed by a generalized id reaction 1 week later.
The patient was prescribed oral terbinafine 250 mg once daily to treat the diffuse involvement of the pustular tinea as well as once-daily oral cetirizine, once-daily oral diphenhydramine, a topical emollient, and a topical nonsteroidal antipruritic gel.
Tinea is a superficial fungal infection commonly caused by the dermatophytes Epidermophyton, Trichophyton, and Microsporum. It has a variety of clinical presentations based on the anatomic location, including tinea capitis (hair/scalp), tinea pedis (feet), tinea corporis (face/trunk/extremities), tinea cruris (groin), and tinea unguium (nails).1 Tinea infections occur in the stratum corneum, hair, and nails, thriving on dead keratin in these areas.2 Tinea corporis usually appears as an erythematous ring-shaped lesion with a scaly border, but atypical cases presenting with vesicles, pustules, and bullae also have been reported.3 Additionally, secondary eruptions called id reactions, or autoeczematization, can present in the setting of dermatophyte infections. Such outbreaks may be due to a delayed hypersensitivity reaction to the fungal antigens. Id reactions can manifest in many forms of tinea with patients generally exhibiting pruritic papulovesicular lesions that can present far from the site of origin.4
Patients with id reactions can have atypical and varied presentations. In a case of id reaction due to tinea corporis, a patient presented with vesicles and pustules that grew in number and coalesced to form annular lesions.5 A case of an id reaction caused by tinea pedis also noted the presence of pustules, which are atypical in this form of tinea.6 In another case of tinea pedis, a generalized id reaction was noted, illustrating that such eruptions do not necessarily appear at the original site of infection.7 Additionally, in a rare presentation of tinea invading the nares, a patient developed an erythema multiforme id reaction.8 Id reactions also were noted in 14 patients with refractory otitis externa, illustrating the ability of this fungal infection to persist and infect distant locations.9
Because the differential diagnoses for tinea infection are extensive, pathology or laboratory confirmation is necessary for diagnosis, and potassium hydroxide preparation often is used to diagnose dermatophyte infections.1,2 Additionally, the possibility of a hypersensitivity drug rash should remain in the differential if the patient received allergy-inducing medications prior to the outbreak, which may in turn complicate the diagnosis.
Tinea infections typically can be treated with topical antifungals such as terbinafine, butenafine,1 and luliconazole10; however, more involved cases may require oral antifungal treatment.1 Systemic treatment of tinea corporis includes itraconazole, terbinafine, and fluconazole,11 all of which exhibit fewer side effects and greater efficacy when compared to griseofulvin.12-15
Treatment of id reactions centers on the proper clearance of the dermatophyte infection, and treatment with oral antifungals generally is sufficient. In the cases of id reaction in patients with refractory otitis, some success was achieved with treatment involving immunotherapy with dermatophyte and dust mite allergen extracts coupled with a yeast elimination diet.9 In acute id reactions, topical corticosteroids and antipruritic agents can be applied.4 Rarely, systemic glucocorticoids are required, such as in cases in which the id reaction persists despite proper treatment of the primary infection.16
- Ely JW, Rosenfeld S, Seabury Stone M. Diagnosis and management of tinea infections. Am Fam Physician. 2014;90:702-710.
- Habif TP. Clinical Dermatology: A Color Guide to Diagnosis and Therapy. 5th ed. Hanover, NH: Elsevier, Inc; 2010.
- Ziemer M, Seyfarth F, Elsner P, et al. Atypical manifestations of tinea corporis. Mycoses. 2007;50(suppl 2):31-35.
- Cheng N, Rucker Wright D, Cohen BA. Dermatophytid in tinea capitis: rarely reported common phenomenon with clinical implications [published online July 4, 2011]. Pediatrics. 2011;128:e453-e457.
- Ohno S, Tanabe H, Kawasaki M, et al. Tinea corporis with acute inflammation caused by Trichophyton tonsurans. J Dermatol. 2008;35:590-593.
- Hirschmann JV, Raugi GJ. Pustular tinea pedis. J Am Acad Dermatol. 2000;42:132-133.
- Iglesias ME, España A, Idoate MA, et al. Generalized skin reaction following tinea pedis (dermatophytids). J Dermatol. 1994;21:31-34.
- Atzori L, Pau M, Aste M. Erythema multiforme ID reaction in atypical dermatophytosis: a case report. J Eur Acad Dermatol Venereol. 2003;17:699-701.
- Derebery J, Berliner KI. Foot and ear disease—the dermatophytid reaction in otology. Laryngoscope. 1996;106(2 Pt 1):181-186.
- Khanna D, Bharti S. Luliconazole for the treatment of fungal infections: an evidence-based review. Core Evid. 2014;9:113-124.
- Korting HC, Schöllmann C. The significance of itraconazole for treatment of fungal infections of skin, nails and mucous membranes. J Dtsch Dermatol Ges. 2009;7:11-20.
- Goldstein AO, Goldstein BG. Dermatophyte (tinea) infections. UpToDate website. https://www.uptodate.com/contents/dermatophyte-tinea-infections. Updated December 28, 2018. Accessed April 24, 2019.
- Cole GW, Stricklin G. A comparison of a new oral antifungal, terbinafine, with griseofulvin as therapy for tinea corporis. Arch Dermatol. 1989;125:1537.
- Panagiotidou D, Kousidou T, Chaidemenos G, et al. A comparison of itraconazole and griseofulvin in the treatment of tinea corporis and tinea cruris: a double-blind study. J Int Med Res. 1992;20:392-400.
- Faergemann J, Mörk NJ, Haglund A, et al. A multicentre (double-blind) comparative study to assess the safety and efficacy of fluconazole and griseofulvin in the treatment of tinea corporis and tinea cruris. Br J Dermatol. 1997;136:575-577.
- Ilkit M, Durdu M, Karakas M. Cutaneous id reactions: a comprehensive review of clinical manifestations, epidemiology, etiology, and management. Crit Rev Microbiol. 2012;38:191-202.
To the Editor:
A 17-year-old adolescent girl presented to the dermatology clinic with a tender pruritic rash on the left wrist that was spreading to the bilateral arms and legs of several years’ duration. An area of a prior biopsy on the left wrist was healing well with use of petroleum jelly and halcinonide cream. The patient denied any constitutional symptoms.
Physical examination revealed numerous erythematous papules coalescing into plaques on the bilateral anterior and posterior arms and legs, including some erythematous macules and papules on the palms and soles. The original area of involvement on the left dorsal medial wrist demonstrated a background of erythema with overlying peripheral scaling and resolving violaceous to erythematous papules with signs of serosanguineous crusting (Figure 1). Scattered perifollicular erythema was present on the posterior aspects of the bilateral thighs and arms (Figure 2). Baseline complete blood cell count and complete metabolic panel were within reference range.
Clinical histopathology showed evidence of a pustular superficial dermatophyte infection, and Grocott-Gomori methenamine-silver stain demonstrated numerous fungal hyphae within subcorneal pustules, indicating pustular tinea. Based on the clinicopathologic correlation, the initial presentation was diagnosed as pustular tinea of the entire left wrist, followed by a generalized id reaction 1 week later.
The patient was prescribed oral terbinafine 250 mg once daily to treat the diffuse involvement of the pustular tinea as well as once-daily oral cetirizine, once-daily oral diphenhydramine, a topical emollient, and a topical nonsteroidal antipruritic gel.
Tinea is a superficial fungal infection commonly caused by the dermatophytes Epidermophyton, Trichophyton, and Microsporum. It has a variety of clinical presentations based on the anatomic location, including tinea capitis (hair/scalp), tinea pedis (feet), tinea corporis (face/trunk/extremities), tinea cruris (groin), and tinea unguium (nails).1 Tinea infections occur in the stratum corneum, hair, and nails, thriving on dead keratin in these areas.2 Tinea corporis usually appears as an erythematous ring-shaped lesion with a scaly border, but atypical cases presenting with vesicles, pustules, and bullae also have been reported.3 Additionally, secondary eruptions called id reactions, or autoeczematization, can present in the setting of dermatophyte infections. Such outbreaks may be due to a delayed hypersensitivity reaction to the fungal antigens. Id reactions can manifest in many forms of tinea with patients generally exhibiting pruritic papulovesicular lesions that can present far from the site of origin.4
Patients with id reactions can have atypical and varied presentations. In a case of id reaction due to tinea corporis, a patient presented with vesicles and pustules that grew in number and coalesced to form annular lesions.5 A case of an id reaction caused by tinea pedis also noted the presence of pustules, which are atypical in this form of tinea.6 In another case of tinea pedis, a generalized id reaction was noted, illustrating that such eruptions do not necessarily appear at the original site of infection.7 Additionally, in a rare presentation of tinea invading the nares, a patient developed an erythema multiforme id reaction.8 Id reactions also were noted in 14 patients with refractory otitis externa, illustrating the ability of this fungal infection to persist and infect distant locations.9
Because the differential diagnoses for tinea infection are extensive, pathology or laboratory confirmation is necessary for diagnosis, and potassium hydroxide preparation often is used to diagnose dermatophyte infections.1,2 Additionally, the possibility of a hypersensitivity drug rash should remain in the differential if the patient received allergy-inducing medications prior to the outbreak, which may in turn complicate the diagnosis.
Tinea infections typically can be treated with topical antifungals such as terbinafine, butenafine,1 and luliconazole10; however, more involved cases may require oral antifungal treatment.1 Systemic treatment of tinea corporis includes itraconazole, terbinafine, and fluconazole,11 all of which exhibit fewer side effects and greater efficacy when compared to griseofulvin.12-15
Treatment of id reactions centers on the proper clearance of the dermatophyte infection, and treatment with oral antifungals generally is sufficient. In the cases of id reaction in patients with refractory otitis, some success was achieved with treatment involving immunotherapy with dermatophyte and dust mite allergen extracts coupled with a yeast elimination diet.9 In acute id reactions, topical corticosteroids and antipruritic agents can be applied.4 Rarely, systemic glucocorticoids are required, such as in cases in which the id reaction persists despite proper treatment of the primary infection.16
To the Editor:
A 17-year-old adolescent girl presented to the dermatology clinic with a tender pruritic rash on the left wrist that was spreading to the bilateral arms and legs of several years’ duration. An area of a prior biopsy on the left wrist was healing well with use of petroleum jelly and halcinonide cream. The patient denied any constitutional symptoms.
Physical examination revealed numerous erythematous papules coalescing into plaques on the bilateral anterior and posterior arms and legs, including some erythematous macules and papules on the palms and soles. The original area of involvement on the left dorsal medial wrist demonstrated a background of erythema with overlying peripheral scaling and resolving violaceous to erythematous papules with signs of serosanguineous crusting (Figure 1). Scattered perifollicular erythema was present on the posterior aspects of the bilateral thighs and arms (Figure 2). Baseline complete blood cell count and complete metabolic panel were within reference range.
Clinical histopathology showed evidence of a pustular superficial dermatophyte infection, and Grocott-Gomori methenamine-silver stain demonstrated numerous fungal hyphae within subcorneal pustules, indicating pustular tinea. Based on the clinicopathologic correlation, the initial presentation was diagnosed as pustular tinea of the entire left wrist, followed by a generalized id reaction 1 week later.
The patient was prescribed oral terbinafine 250 mg once daily to treat the diffuse involvement of the pustular tinea as well as once-daily oral cetirizine, once-daily oral diphenhydramine, a topical emollient, and a topical nonsteroidal antipruritic gel.
Tinea is a superficial fungal infection commonly caused by the dermatophytes Epidermophyton, Trichophyton, and Microsporum. It has a variety of clinical presentations based on the anatomic location, including tinea capitis (hair/scalp), tinea pedis (feet), tinea corporis (face/trunk/extremities), tinea cruris (groin), and tinea unguium (nails).1 Tinea infections occur in the stratum corneum, hair, and nails, thriving on dead keratin in these areas.2 Tinea corporis usually appears as an erythematous ring-shaped lesion with a scaly border, but atypical cases presenting with vesicles, pustules, and bullae also have been reported.3 Additionally, secondary eruptions called id reactions, or autoeczematization, can present in the setting of dermatophyte infections. Such outbreaks may be due to a delayed hypersensitivity reaction to the fungal antigens. Id reactions can manifest in many forms of tinea with patients generally exhibiting pruritic papulovesicular lesions that can present far from the site of origin.4
Patients with id reactions can have atypical and varied presentations. In a case of id reaction due to tinea corporis, a patient presented with vesicles and pustules that grew in number and coalesced to form annular lesions.5 A case of an id reaction caused by tinea pedis also noted the presence of pustules, which are atypical in this form of tinea.6 In another case of tinea pedis, a generalized id reaction was noted, illustrating that such eruptions do not necessarily appear at the original site of infection.7 Additionally, in a rare presentation of tinea invading the nares, a patient developed an erythema multiforme id reaction.8 Id reactions also were noted in 14 patients with refractory otitis externa, illustrating the ability of this fungal infection to persist and infect distant locations.9
Because the differential diagnoses for tinea infection are extensive, pathology or laboratory confirmation is necessary for diagnosis, and potassium hydroxide preparation often is used to diagnose dermatophyte infections.1,2 Additionally, the possibility of a hypersensitivity drug rash should remain in the differential if the patient received allergy-inducing medications prior to the outbreak, which may in turn complicate the diagnosis.
Tinea infections typically can be treated with topical antifungals such as terbinafine, butenafine,1 and luliconazole10; however, more involved cases may require oral antifungal treatment.1 Systemic treatment of tinea corporis includes itraconazole, terbinafine, and fluconazole,11 all of which exhibit fewer side effects and greater efficacy when compared to griseofulvin.12-15
Treatment of id reactions centers on the proper clearance of the dermatophyte infection, and treatment with oral antifungals generally is sufficient. In the cases of id reaction in patients with refractory otitis, some success was achieved with treatment involving immunotherapy with dermatophyte and dust mite allergen extracts coupled with a yeast elimination diet.9 In acute id reactions, topical corticosteroids and antipruritic agents can be applied.4 Rarely, systemic glucocorticoids are required, such as in cases in which the id reaction persists despite proper treatment of the primary infection.16
- Ely JW, Rosenfeld S, Seabury Stone M. Diagnosis and management of tinea infections. Am Fam Physician. 2014;90:702-710.
- Habif TP. Clinical Dermatology: A Color Guide to Diagnosis and Therapy. 5th ed. Hanover, NH: Elsevier, Inc; 2010.
- Ziemer M, Seyfarth F, Elsner P, et al. Atypical manifestations of tinea corporis. Mycoses. 2007;50(suppl 2):31-35.
- Cheng N, Rucker Wright D, Cohen BA. Dermatophytid in tinea capitis: rarely reported common phenomenon with clinical implications [published online July 4, 2011]. Pediatrics. 2011;128:e453-e457.
- Ohno S, Tanabe H, Kawasaki M, et al. Tinea corporis with acute inflammation caused by Trichophyton tonsurans. J Dermatol. 2008;35:590-593.
- Hirschmann JV, Raugi GJ. Pustular tinea pedis. J Am Acad Dermatol. 2000;42:132-133.
- Iglesias ME, España A, Idoate MA, et al. Generalized skin reaction following tinea pedis (dermatophytids). J Dermatol. 1994;21:31-34.
- Atzori L, Pau M, Aste M. Erythema multiforme ID reaction in atypical dermatophytosis: a case report. J Eur Acad Dermatol Venereol. 2003;17:699-701.
- Derebery J, Berliner KI. Foot and ear disease—the dermatophytid reaction in otology. Laryngoscope. 1996;106(2 Pt 1):181-186.
- Khanna D, Bharti S. Luliconazole for the treatment of fungal infections: an evidence-based review. Core Evid. 2014;9:113-124.
- Korting HC, Schöllmann C. The significance of itraconazole for treatment of fungal infections of skin, nails and mucous membranes. J Dtsch Dermatol Ges. 2009;7:11-20.
- Goldstein AO, Goldstein BG. Dermatophyte (tinea) infections. UpToDate website. https://www.uptodate.com/contents/dermatophyte-tinea-infections. Updated December 28, 2018. Accessed April 24, 2019.
- Cole GW, Stricklin G. A comparison of a new oral antifungal, terbinafine, with griseofulvin as therapy for tinea corporis. Arch Dermatol. 1989;125:1537.
- Panagiotidou D, Kousidou T, Chaidemenos G, et al. A comparison of itraconazole and griseofulvin in the treatment of tinea corporis and tinea cruris: a double-blind study. J Int Med Res. 1992;20:392-400.
- Faergemann J, Mörk NJ, Haglund A, et al. A multicentre (double-blind) comparative study to assess the safety and efficacy of fluconazole and griseofulvin in the treatment of tinea corporis and tinea cruris. Br J Dermatol. 1997;136:575-577.
- Ilkit M, Durdu M, Karakas M. Cutaneous id reactions: a comprehensive review of clinical manifestations, epidemiology, etiology, and management. Crit Rev Microbiol. 2012;38:191-202.
- Ely JW, Rosenfeld S, Seabury Stone M. Diagnosis and management of tinea infections. Am Fam Physician. 2014;90:702-710.
- Habif TP. Clinical Dermatology: A Color Guide to Diagnosis and Therapy. 5th ed. Hanover, NH: Elsevier, Inc; 2010.
- Ziemer M, Seyfarth F, Elsner P, et al. Atypical manifestations of tinea corporis. Mycoses. 2007;50(suppl 2):31-35.
- Cheng N, Rucker Wright D, Cohen BA. Dermatophytid in tinea capitis: rarely reported common phenomenon with clinical implications [published online July 4, 2011]. Pediatrics. 2011;128:e453-e457.
- Ohno S, Tanabe H, Kawasaki M, et al. Tinea corporis with acute inflammation caused by Trichophyton tonsurans. J Dermatol. 2008;35:590-593.
- Hirschmann JV, Raugi GJ. Pustular tinea pedis. J Am Acad Dermatol. 2000;42:132-133.
- Iglesias ME, España A, Idoate MA, et al. Generalized skin reaction following tinea pedis (dermatophytids). J Dermatol. 1994;21:31-34.
- Atzori L, Pau M, Aste M. Erythema multiforme ID reaction in atypical dermatophytosis: a case report. J Eur Acad Dermatol Venereol. 2003;17:699-701.
- Derebery J, Berliner KI. Foot and ear disease—the dermatophytid reaction in otology. Laryngoscope. 1996;106(2 Pt 1):181-186.
- Khanna D, Bharti S. Luliconazole for the treatment of fungal infections: an evidence-based review. Core Evid. 2014;9:113-124.
- Korting HC, Schöllmann C. The significance of itraconazole for treatment of fungal infections of skin, nails and mucous membranes. J Dtsch Dermatol Ges. 2009;7:11-20.
- Goldstein AO, Goldstein BG. Dermatophyte (tinea) infections. UpToDate website. https://www.uptodate.com/contents/dermatophyte-tinea-infections. Updated December 28, 2018. Accessed April 24, 2019.
- Cole GW, Stricklin G. A comparison of a new oral antifungal, terbinafine, with griseofulvin as therapy for tinea corporis. Arch Dermatol. 1989;125:1537.
- Panagiotidou D, Kousidou T, Chaidemenos G, et al. A comparison of itraconazole and griseofulvin in the treatment of tinea corporis and tinea cruris: a double-blind study. J Int Med Res. 1992;20:392-400.
- Faergemann J, Mörk NJ, Haglund A, et al. A multicentre (double-blind) comparative study to assess the safety and efficacy of fluconazole and griseofulvin in the treatment of tinea corporis and tinea cruris. Br J Dermatol. 1997;136:575-577.
- Ilkit M, Durdu M, Karakas M. Cutaneous id reactions: a comprehensive review of clinical manifestations, epidemiology, etiology, and management. Crit Rev Microbiol. 2012;38:191-202.
Practice Points
• Id reactions, or autoeczematization, can occur secondary to dermatophyte infections, possibly due to a hypersensitivity reaction to the fungus. These eruptions can occur in many forms of tinea and in a variety of clinical presentations.
• Treatment is based on clearance of the original dermatophyte infection.