User login
Tweeting Into the Void: Effective Use of Social Media for Healthcare Professionals
Communication has always played a central role in facilitating technological advances and social progress. The printing press, mail, telegraph, radio, television, electronic mail, and social media have all allowed for the exchange of ideas that led to progress, and have done so with increasing speed. But some people are beginning to question whether we are experiencing diminishing returns from making such communication easier, faster, and more widespread. Disinformation, conspiracies, inappropriate messages, and personal attacks are just as easy to communicate as truth, good ideas, and empathy. In many cases, truth and falsehood are nearly indistinguishable. Raw, nasty emotions contained in personal attacks are often provocative, thus generating even more engagement, which many people view as the purpose of social media. In this context, it is more important than ever for trusted voices, such as those of scientists and physicians, to play a role in the public sphere.
In this essay, we offer our personal recommendations on how healthcare professionals, who in our view have outsized authority and responsibility on healthcare topics, might improve communication on social media. We focus particularly on Twitter given its prominent role in the public exchange of ideas and its recognized benefits (and challenges) for scientific communication.1 We make these recommendations with some trepidation because we are sure readers will be able to find times when we have not followed our own advice. And we are sure many will disagree or feel that our advice raises the bar too high. We divide our recommendations into lists of Do’s and Don’ts. Let’s start with the Do’s.
DO
DO separate facts from inferences, ideally labeling them as such. For example, you can report that public health has found five cases of the delta variant in people in a specific nursing home as a fact. You might then infer that the variant is widespread in that facility, and that community spread in the region is likely. Stating the source of your facts helps the reader evaluate their reliability and precision.
DO state when you are quoting preliminary evidence. If posting a preprint, press release, or other non-peer-reviewed paper (even if it is your own!), make its preliminary status clear to the reader (Figure, part A).
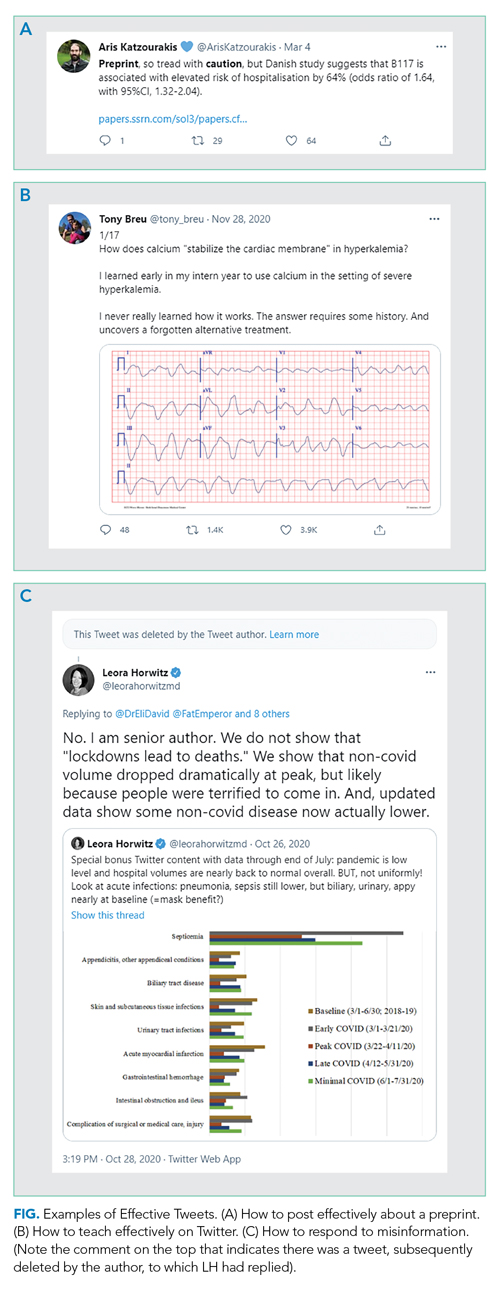
DO read the full article before posting. If you are posting an article, make sure you understand the whole context of any results you are highlighting. Avoid exaggerating, fear-mongering, or selectively picking facts or results to bolster your opinion.DO seek to add value to the public discourse. Rather than simply retweeting popular posts, consider taking the time to collate evidence (including contrary evidence) into a thread if seeking to prove a point or to teach, especially when it relates to something in your field. You likely are more knowledgeable about topics in your field than 99% of readers; use Twitter to spread your expertise. Clinical “tweetorials,” such as those popularized by @tony_breu, can be very effective teaching tools (Figure, part B).
DO make recommendations as specific as possible. If your goal is to improve adherence to evidence-based medicine or support disadvantaged people, be explicit about how you would achieve these goals. Tell readers exactly what you have in mind so that individuals and leaders can operationalize the recommendations. Use threads to expand on your advice and its rationale.
DO consider engaging with misinformation. We suggest doing so if the misinformation is posted by someone prominent who is likely to have broad reach, but only once per post and in a factual manner. You are unlikely to convince persons who post disinformation that they are wrong; extended arguments are unhelpful. Your role here is simply to inform readers of the post, who may be more open to reason. Occasionally, you may even convince the initial poster, as seen in Figure, part C, to delete certain misinformation. But don’t count on it.
DO consider your obligation to the general public. It is fine to engage explicitly with the medical community (ie, through tweetchats),2 but also consider that your comments will be accessible to everyone. Now more than ever the public is looking to healthcare professionals for clarity, reassurance, and evidence about medical matters.
DO acknowledge when you were wrong. Update your opinions as facts change or when you realize you made a mistake. The COVID-19 pandemic has brought home the rapidity with which we can gain scientific knowledge. Many of the things we thought were right early on—and posted about on Twitter—we now know to be wrong. Be forthright about this, while making it clear that the fact that we know more now doesn’t mean no information can be trusted. (A corollary: Don’t overstate what we know to be true at any time, so that it does not feel as much of a surprise if we later learn more and need to revise an opinion or a statement.)
DO be kind. This is perhaps the most important thing. We are all experiencing stress as physicians, parents, children, and colleagues. Spend your time focusing on people’s actions rather than impugning their motives or intelligence. Most of the time you don’t really know what their motives are. We recognize that kindness may not generate the same amount of engagement as sarcasm, but at least take time to consider whether you want to be seen as mean-spirited forever.
DO pause before sending. Twitter creates a false perception of the need for speed (and doesn’t really lend itself to revising drafts). But in reality, there is no rush. The torrent of Twitter posts means that people typically only see what has been posted around the time they log in; an early post is not necessarily more likely to be noticed. So, take your time and avoid falling into a trap of writing something you will regret, or, in extreme cases, that will get you fired or otherwise ruin your career. There is no rush to be first; Twitter will still be there tomorrow.
DON’T
For some time, mentors have warned physicians (and others whose careers depend on their reputation) to be careful in their use of social media. Electronic dissemination of inappropriate words or images can come back to haunt people—sometimes immediately, sometimes many years later.3 Physicians are also at risk of falling into some pitfalls specific to the profession. That said, here are some Don’ts to avoid or be cautious about.
DON’T reveal information about patients in a recognizable fashion. Journals ask for written consent from patients when authors submit a manuscript about individual cases so readers can be sure consent has been obtained. The same standard should apply to social media; if not, you are clearly violating a professional standard. Yet, on Twitter, people may assume you have not obtained consent, conveying a false sense of invasion of privacy and undermining confidence in the profession. The safest thing to do is not tell stories about patients, or to completely disguise the story so even the patients can’t recognize themselves. If you do choose to post about a patient, obtain written permission that you save, and clearly indicate that you have that permission in the Tweet.
DON’T claim to have expertise in areas where you have little training or education. For example, just because you are an expert in critical care and have seen the ravages of COVID-19 on your patients doesn’t mean you are an expert in how to stop a pandemic, though your observations may be helpful to those who are. This does not mean you shouldn’t speak out on important moral issues like climate change, nuclear war, or injustices, which clearly reflect personal opinion and values. Rather, be cautious about commenting authoritatively on areas in which the lay reader might mistakenly think you have specific expertise.
DON’T make yourself the hero of every story. Implicitly seeking praise for doing your job (Look at me, I’m working on Christmas!) may breed resentment and undercut professionalism. Rather, state what it is about your job that works well and what doesn’t (for example, teaching tips, wellness advice, and organizational strategies) in a way that helps others emulate your successes.
DON’T let emotions get the better of you. This past year has been full of outrageous and appalling events and behavior. We do not suggest that you ignore these. Rather, make sure that if you are blaming an individual for something that it really was their fault, because they had control of the factors that led to the disastrous outcome. Consider focusing on systemic and structural explanations for unacceptable phenomena to minimize defensiveness and maximize the potential for identifying solutions. And yes, sometimes you just have to let it rip, but be selective—maybe show your post to someone else and sleep on it before you send it.
CONCLUSION
We hope that you will find these suggestions helpful in both creating and reading social media posts on important topics. We recognize that some people like the spontaneity of the social media platform and will thus find our suggestions stunting. But at least everyone ought to consider what they are trying to achieve when they make public statements. The exchange of ideas has always been a key ingredient in creating progress. Let’s optimize the usefulness of those exchanges for that purpose, and to promote knowledge and science in a way that helps us all live healthier and happier lives.
1. Choo EK, Ranney ML, Chan TM, et al. Twitter as a tool for communication and knowledge exchange in academic medicine: a guide for skeptics and novices. Med Teach. 2015;37(5):411-416. https://doi.org/10.3109/0142159X.2014.993371
2. Admon AJ, Kaul V, Cribbs SK, Guzman E, Jimenez O, Richards JB. Twelve tips for developing and implementing a medical education Twitter chat. Med Teach. 2020;42(5):500-506. https://doi.org/10.1080/0142159X.2019.1598553
3. Langenfeld SJ, Batra R. How can social media get us in trouble? Clin Colon Rectal Surg. 2017;30(4):264-269. https://doi.org/10.1055/s-0037-1604255
Communication has always played a central role in facilitating technological advances and social progress. The printing press, mail, telegraph, radio, television, electronic mail, and social media have all allowed for the exchange of ideas that led to progress, and have done so with increasing speed. But some people are beginning to question whether we are experiencing diminishing returns from making such communication easier, faster, and more widespread. Disinformation, conspiracies, inappropriate messages, and personal attacks are just as easy to communicate as truth, good ideas, and empathy. In many cases, truth and falsehood are nearly indistinguishable. Raw, nasty emotions contained in personal attacks are often provocative, thus generating even more engagement, which many people view as the purpose of social media. In this context, it is more important than ever for trusted voices, such as those of scientists and physicians, to play a role in the public sphere.
In this essay, we offer our personal recommendations on how healthcare professionals, who in our view have outsized authority and responsibility on healthcare topics, might improve communication on social media. We focus particularly on Twitter given its prominent role in the public exchange of ideas and its recognized benefits (and challenges) for scientific communication.1 We make these recommendations with some trepidation because we are sure readers will be able to find times when we have not followed our own advice. And we are sure many will disagree or feel that our advice raises the bar too high. We divide our recommendations into lists of Do’s and Don’ts. Let’s start with the Do’s.
DO
DO separate facts from inferences, ideally labeling them as such. For example, you can report that public health has found five cases of the delta variant in people in a specific nursing home as a fact. You might then infer that the variant is widespread in that facility, and that community spread in the region is likely. Stating the source of your facts helps the reader evaluate their reliability and precision.
DO state when you are quoting preliminary evidence. If posting a preprint, press release, or other non-peer-reviewed paper (even if it is your own!), make its preliminary status clear to the reader (Figure, part A).

DO read the full article before posting. If you are posting an article, make sure you understand the whole context of any results you are highlighting. Avoid exaggerating, fear-mongering, or selectively picking facts or results to bolster your opinion.DO seek to add value to the public discourse. Rather than simply retweeting popular posts, consider taking the time to collate evidence (including contrary evidence) into a thread if seeking to prove a point or to teach, especially when it relates to something in your field. You likely are more knowledgeable about topics in your field than 99% of readers; use Twitter to spread your expertise. Clinical “tweetorials,” such as those popularized by @tony_breu, can be very effective teaching tools (Figure, part B).
DO make recommendations as specific as possible. If your goal is to improve adherence to evidence-based medicine or support disadvantaged people, be explicit about how you would achieve these goals. Tell readers exactly what you have in mind so that individuals and leaders can operationalize the recommendations. Use threads to expand on your advice and its rationale.
DO consider engaging with misinformation. We suggest doing so if the misinformation is posted by someone prominent who is likely to have broad reach, but only once per post and in a factual manner. You are unlikely to convince persons who post disinformation that they are wrong; extended arguments are unhelpful. Your role here is simply to inform readers of the post, who may be more open to reason. Occasionally, you may even convince the initial poster, as seen in Figure, part C, to delete certain misinformation. But don’t count on it.
DO consider your obligation to the general public. It is fine to engage explicitly with the medical community (ie, through tweetchats),2 but also consider that your comments will be accessible to everyone. Now more than ever the public is looking to healthcare professionals for clarity, reassurance, and evidence about medical matters.
DO acknowledge when you were wrong. Update your opinions as facts change or when you realize you made a mistake. The COVID-19 pandemic has brought home the rapidity with which we can gain scientific knowledge. Many of the things we thought were right early on—and posted about on Twitter—we now know to be wrong. Be forthright about this, while making it clear that the fact that we know more now doesn’t mean no information can be trusted. (A corollary: Don’t overstate what we know to be true at any time, so that it does not feel as much of a surprise if we later learn more and need to revise an opinion or a statement.)
DO be kind. This is perhaps the most important thing. We are all experiencing stress as physicians, parents, children, and colleagues. Spend your time focusing on people’s actions rather than impugning their motives or intelligence. Most of the time you don’t really know what their motives are. We recognize that kindness may not generate the same amount of engagement as sarcasm, but at least take time to consider whether you want to be seen as mean-spirited forever.
DO pause before sending. Twitter creates a false perception of the need for speed (and doesn’t really lend itself to revising drafts). But in reality, there is no rush. The torrent of Twitter posts means that people typically only see what has been posted around the time they log in; an early post is not necessarily more likely to be noticed. So, take your time and avoid falling into a trap of writing something you will regret, or, in extreme cases, that will get you fired or otherwise ruin your career. There is no rush to be first; Twitter will still be there tomorrow.
DON’T
For some time, mentors have warned physicians (and others whose careers depend on their reputation) to be careful in their use of social media. Electronic dissemination of inappropriate words or images can come back to haunt people—sometimes immediately, sometimes many years later.3 Physicians are also at risk of falling into some pitfalls specific to the profession. That said, here are some Don’ts to avoid or be cautious about.
DON’T reveal information about patients in a recognizable fashion. Journals ask for written consent from patients when authors submit a manuscript about individual cases so readers can be sure consent has been obtained. The same standard should apply to social media; if not, you are clearly violating a professional standard. Yet, on Twitter, people may assume you have not obtained consent, conveying a false sense of invasion of privacy and undermining confidence in the profession. The safest thing to do is not tell stories about patients, or to completely disguise the story so even the patients can’t recognize themselves. If you do choose to post about a patient, obtain written permission that you save, and clearly indicate that you have that permission in the Tweet.
DON’T claim to have expertise in areas where you have little training or education. For example, just because you are an expert in critical care and have seen the ravages of COVID-19 on your patients doesn’t mean you are an expert in how to stop a pandemic, though your observations may be helpful to those who are. This does not mean you shouldn’t speak out on important moral issues like climate change, nuclear war, or injustices, which clearly reflect personal opinion and values. Rather, be cautious about commenting authoritatively on areas in which the lay reader might mistakenly think you have specific expertise.
DON’T make yourself the hero of every story. Implicitly seeking praise for doing your job (Look at me, I’m working on Christmas!) may breed resentment and undercut professionalism. Rather, state what it is about your job that works well and what doesn’t (for example, teaching tips, wellness advice, and organizational strategies) in a way that helps others emulate your successes.
DON’T let emotions get the better of you. This past year has been full of outrageous and appalling events and behavior. We do not suggest that you ignore these. Rather, make sure that if you are blaming an individual for something that it really was their fault, because they had control of the factors that led to the disastrous outcome. Consider focusing on systemic and structural explanations for unacceptable phenomena to minimize defensiveness and maximize the potential for identifying solutions. And yes, sometimes you just have to let it rip, but be selective—maybe show your post to someone else and sleep on it before you send it.
CONCLUSION
We hope that you will find these suggestions helpful in both creating and reading social media posts on important topics. We recognize that some people like the spontaneity of the social media platform and will thus find our suggestions stunting. But at least everyone ought to consider what they are trying to achieve when they make public statements. The exchange of ideas has always been a key ingredient in creating progress. Let’s optimize the usefulness of those exchanges for that purpose, and to promote knowledge and science in a way that helps us all live healthier and happier lives.
Communication has always played a central role in facilitating technological advances and social progress. The printing press, mail, telegraph, radio, television, electronic mail, and social media have all allowed for the exchange of ideas that led to progress, and have done so with increasing speed. But some people are beginning to question whether we are experiencing diminishing returns from making such communication easier, faster, and more widespread. Disinformation, conspiracies, inappropriate messages, and personal attacks are just as easy to communicate as truth, good ideas, and empathy. In many cases, truth and falsehood are nearly indistinguishable. Raw, nasty emotions contained in personal attacks are often provocative, thus generating even more engagement, which many people view as the purpose of social media. In this context, it is more important than ever for trusted voices, such as those of scientists and physicians, to play a role in the public sphere.
In this essay, we offer our personal recommendations on how healthcare professionals, who in our view have outsized authority and responsibility on healthcare topics, might improve communication on social media. We focus particularly on Twitter given its prominent role in the public exchange of ideas and its recognized benefits (and challenges) for scientific communication.1 We make these recommendations with some trepidation because we are sure readers will be able to find times when we have not followed our own advice. And we are sure many will disagree or feel that our advice raises the bar too high. We divide our recommendations into lists of Do’s and Don’ts. Let’s start with the Do’s.
DO
DO separate facts from inferences, ideally labeling them as such. For example, you can report that public health has found five cases of the delta variant in people in a specific nursing home as a fact. You might then infer that the variant is widespread in that facility, and that community spread in the region is likely. Stating the source of your facts helps the reader evaluate their reliability and precision.
DO state when you are quoting preliminary evidence. If posting a preprint, press release, or other non-peer-reviewed paper (even if it is your own!), make its preliminary status clear to the reader (Figure, part A).

DO read the full article before posting. If you are posting an article, make sure you understand the whole context of any results you are highlighting. Avoid exaggerating, fear-mongering, or selectively picking facts or results to bolster your opinion.DO seek to add value to the public discourse. Rather than simply retweeting popular posts, consider taking the time to collate evidence (including contrary evidence) into a thread if seeking to prove a point or to teach, especially when it relates to something in your field. You likely are more knowledgeable about topics in your field than 99% of readers; use Twitter to spread your expertise. Clinical “tweetorials,” such as those popularized by @tony_breu, can be very effective teaching tools (Figure, part B).
DO make recommendations as specific as possible. If your goal is to improve adherence to evidence-based medicine or support disadvantaged people, be explicit about how you would achieve these goals. Tell readers exactly what you have in mind so that individuals and leaders can operationalize the recommendations. Use threads to expand on your advice and its rationale.
DO consider engaging with misinformation. We suggest doing so if the misinformation is posted by someone prominent who is likely to have broad reach, but only once per post and in a factual manner. You are unlikely to convince persons who post disinformation that they are wrong; extended arguments are unhelpful. Your role here is simply to inform readers of the post, who may be more open to reason. Occasionally, you may even convince the initial poster, as seen in Figure, part C, to delete certain misinformation. But don’t count on it.
DO consider your obligation to the general public. It is fine to engage explicitly with the medical community (ie, through tweetchats),2 but also consider that your comments will be accessible to everyone. Now more than ever the public is looking to healthcare professionals for clarity, reassurance, and evidence about medical matters.
DO acknowledge when you were wrong. Update your opinions as facts change or when you realize you made a mistake. The COVID-19 pandemic has brought home the rapidity with which we can gain scientific knowledge. Many of the things we thought were right early on—and posted about on Twitter—we now know to be wrong. Be forthright about this, while making it clear that the fact that we know more now doesn’t mean no information can be trusted. (A corollary: Don’t overstate what we know to be true at any time, so that it does not feel as much of a surprise if we later learn more and need to revise an opinion or a statement.)
DO be kind. This is perhaps the most important thing. We are all experiencing stress as physicians, parents, children, and colleagues. Spend your time focusing on people’s actions rather than impugning their motives or intelligence. Most of the time you don’t really know what their motives are. We recognize that kindness may not generate the same amount of engagement as sarcasm, but at least take time to consider whether you want to be seen as mean-spirited forever.
DO pause before sending. Twitter creates a false perception of the need for speed (and doesn’t really lend itself to revising drafts). But in reality, there is no rush. The torrent of Twitter posts means that people typically only see what has been posted around the time they log in; an early post is not necessarily more likely to be noticed. So, take your time and avoid falling into a trap of writing something you will regret, or, in extreme cases, that will get you fired or otherwise ruin your career. There is no rush to be first; Twitter will still be there tomorrow.
DON’T
For some time, mentors have warned physicians (and others whose careers depend on their reputation) to be careful in their use of social media. Electronic dissemination of inappropriate words or images can come back to haunt people—sometimes immediately, sometimes many years later.3 Physicians are also at risk of falling into some pitfalls specific to the profession. That said, here are some Don’ts to avoid or be cautious about.
DON’T reveal information about patients in a recognizable fashion. Journals ask for written consent from patients when authors submit a manuscript about individual cases so readers can be sure consent has been obtained. The same standard should apply to social media; if not, you are clearly violating a professional standard. Yet, on Twitter, people may assume you have not obtained consent, conveying a false sense of invasion of privacy and undermining confidence in the profession. The safest thing to do is not tell stories about patients, or to completely disguise the story so even the patients can’t recognize themselves. If you do choose to post about a patient, obtain written permission that you save, and clearly indicate that you have that permission in the Tweet.
DON’T claim to have expertise in areas where you have little training or education. For example, just because you are an expert in critical care and have seen the ravages of COVID-19 on your patients doesn’t mean you are an expert in how to stop a pandemic, though your observations may be helpful to those who are. This does not mean you shouldn’t speak out on important moral issues like climate change, nuclear war, or injustices, which clearly reflect personal opinion and values. Rather, be cautious about commenting authoritatively on areas in which the lay reader might mistakenly think you have specific expertise.
DON’T make yourself the hero of every story. Implicitly seeking praise for doing your job (Look at me, I’m working on Christmas!) may breed resentment and undercut professionalism. Rather, state what it is about your job that works well and what doesn’t (for example, teaching tips, wellness advice, and organizational strategies) in a way that helps others emulate your successes.
DON’T let emotions get the better of you. This past year has been full of outrageous and appalling events and behavior. We do not suggest that you ignore these. Rather, make sure that if you are blaming an individual for something that it really was their fault, because they had control of the factors that led to the disastrous outcome. Consider focusing on systemic and structural explanations for unacceptable phenomena to minimize defensiveness and maximize the potential for identifying solutions. And yes, sometimes you just have to let it rip, but be selective—maybe show your post to someone else and sleep on it before you send it.
CONCLUSION
We hope that you will find these suggestions helpful in both creating and reading social media posts on important topics. We recognize that some people like the spontaneity of the social media platform and will thus find our suggestions stunting. But at least everyone ought to consider what they are trying to achieve when they make public statements. The exchange of ideas has always been a key ingredient in creating progress. Let’s optimize the usefulness of those exchanges for that purpose, and to promote knowledge and science in a way that helps us all live healthier and happier lives.
1. Choo EK, Ranney ML, Chan TM, et al. Twitter as a tool for communication and knowledge exchange in academic medicine: a guide for skeptics and novices. Med Teach. 2015;37(5):411-416. https://doi.org/10.3109/0142159X.2014.993371
2. Admon AJ, Kaul V, Cribbs SK, Guzman E, Jimenez O, Richards JB. Twelve tips for developing and implementing a medical education Twitter chat. Med Teach. 2020;42(5):500-506. https://doi.org/10.1080/0142159X.2019.1598553
3. Langenfeld SJ, Batra R. How can social media get us in trouble? Clin Colon Rectal Surg. 2017;30(4):264-269. https://doi.org/10.1055/s-0037-1604255
1. Choo EK, Ranney ML, Chan TM, et al. Twitter as a tool for communication and knowledge exchange in academic medicine: a guide for skeptics and novices. Med Teach. 2015;37(5):411-416. https://doi.org/10.3109/0142159X.2014.993371
2. Admon AJ, Kaul V, Cribbs SK, Guzman E, Jimenez O, Richards JB. Twelve tips for developing and implementing a medical education Twitter chat. Med Teach. 2020;42(5):500-506. https://doi.org/10.1080/0142159X.2019.1598553
3. Langenfeld SJ, Batra R. How can social media get us in trouble? Clin Colon Rectal Surg. 2017;30(4):264-269. https://doi.org/10.1055/s-0037-1604255
© 2021 Society of Hospital Medicine
Trends in Risk-Adjusted 28-Day Mortality Rates for Patients Hospitalized with COVID-19 in England
The early phase of the coronavirus disease 2019 (COVID-19) pandemic in the United Kingdom (UK) was characterized by uncertainty as clinicians grappled to understand and manage an unfamiliar disease that affected very high numbers of patients amid radically evolving working environments, with little evidence to support their efforts. Early reports indicated high mortality in patients hospitalized with COVID-19.
As the disease became better understood, treatment evolved and the mortality appears to have decreased. For example, two recent papers, a national study of critical care patients in the UK and a single-site study from New York, have demonstrated a significant reduction in adjusted mortality between the pre- and post-peak periods.1,2 However, the UK study was restricted to patients receiving critical care, potentially introducing bias due to varying critical care admission thresholds over time, while the single-site US study may not be generalizable. Moreover, both studies measured only in-hospital mortality. It remains uncertain therefore whether overall mortality has decreased on a broad scale after accounting for changes in patient characteristics.
The aim of this study was to use a national dataset to assess the
METHODS
We conducted a retrospective, secondary analysis of English National Health Services (NHS) hospitals’ admissions of patients at least 18 years of age between March 1 and July 31, 2020. Data were obtained from the Hospital Episode Statistics (HES) admitted patient care dataset.3 This is an administrative dataset that contains data on diagnoses and procedures as well as organizational characteristics and patient demographics for all NHS activity in England. We included all patients with an International Statistical Classification of Diseases, Tenth Revision (ICD-10) diagnosis of U07.1 (COVID-19, virus identified) and U07.2 (COVID-19, virus not identified).
The primary outcome of death within 28 days of admission was obtained by linking to the Civil Registrations (Deaths) - Secondary Care Cut - Information dataset, which includes the date, place, and cause of death from the Office for National Statistics4 and which was complete through September 31, 2020. The time horizon of 28 days from admission was chosen to approximate the Public Health England definition of a death from COVID-19 as being within 28 days of testing positive.5 We restricted our analysis to emergency admissions of persons age >18 years. If a patient had multiple emergency admissions, we restricted our analysis to the first admission to ensure comparability across hospitalizations and to best represent outcomes from the earliest onset of COVID-19.
We estimated a modified Poisson regression6 to predict death at 28 days, with month of admission, region, source of admission, age, deprivation, gender, ethnic group, and the 29 comorbidities in the Elixhauser comorbidity measure as variables in the regression.7 The derivation of each of these variables from the HES dataset is shown in Appendix Table 1.
Deprivation was measured by the Index of Multiple Deprivation, a methodology used widely within the UK to classify relative deprivation.8 To control for clustering, hospital system (known as Trust) was added as a random effect. Robust errors were estimated using the sandwich package.9 Modified Poisson regression was chosen in preference to the more common logistic regression because the coefficients can be interpreted as relative risks and not odds ratios. The model was fitted using R, version 4.0.3, geepack library.10 We carried out three sensitivity analyses, restricting to laboratory-confirmed COVID-19, length of stay ≥3 days, and primary respiratory disease.
For each month, we obtained a standardized mortality ratio (SMR) by fixing the month to the reference month of March 2020 and repredicting the outcome using the existing model. We calculated the ratio of the sum of observed and expected deaths (obtained from the model) in each month, comparing observed deaths to the number we would have expected had those patients been hospitalized in March. We then multiplied each period’s SMR by the March crude mortality to generate monthly adjusted mortality rates. We calculated Poisson confidence intervals around the SMR and used these to obtain confidence intervals for the adjusted rate. The binomial exact method was used to obtain confidence intervals for the crude rate. Multicollinearity was assessed using both the variance inflation factor (VIF) and the condition number test.7 All analyses used two-sided statistical tests, and we considered a P value < .05 to be statistically significant without adjustment for multiple testing. The study was exempt from UK National Research Ethics Committee approval because it involved secondary analysis of anonymized data.
RESULTS
The dataset included 115,643 emergency admissions from 179 healthcare systems, of which 103,202 were first admissions eligible for inclusion. A total of 592 patients were excluded due to missing demographic data (0.5%), resulting in 102,610 admissions included in the analysis. Peak hospitalizations occurred in late March to mid April, accounting for 44% of the hospitalizations (Table). Median length of stay for patients who died was 7 days (interquartile range, 3-12). The median age and number of Elixhauser comorbidities decreased in July. The proportion of men decreased between May and July. 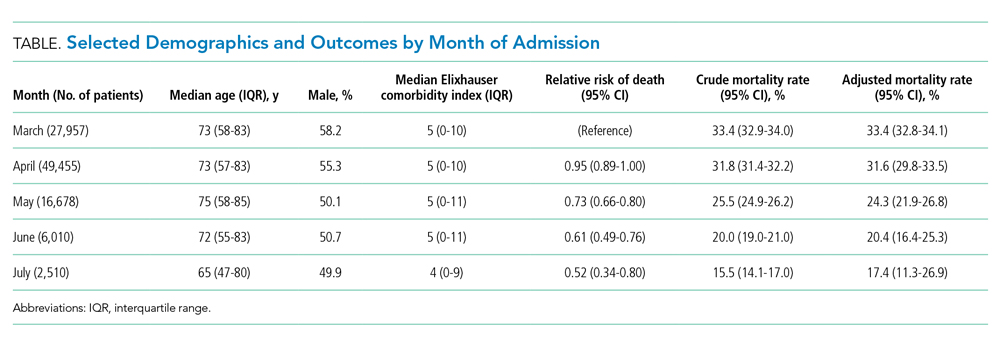
The modified Poisson regression had a C statistic of 0.743 (95% CI, 0.740-0.746) (Appendix Table 4). The VIF and condition number test found no evidence of multicollinearity.11
Adjusted mortality decreased each month, from 33.4% in March to 17.4% in July (Figure). The relative risk of death declined progressively to a minimum of 0.52 (95% CI, 0.34-0.80) in July, compared to March.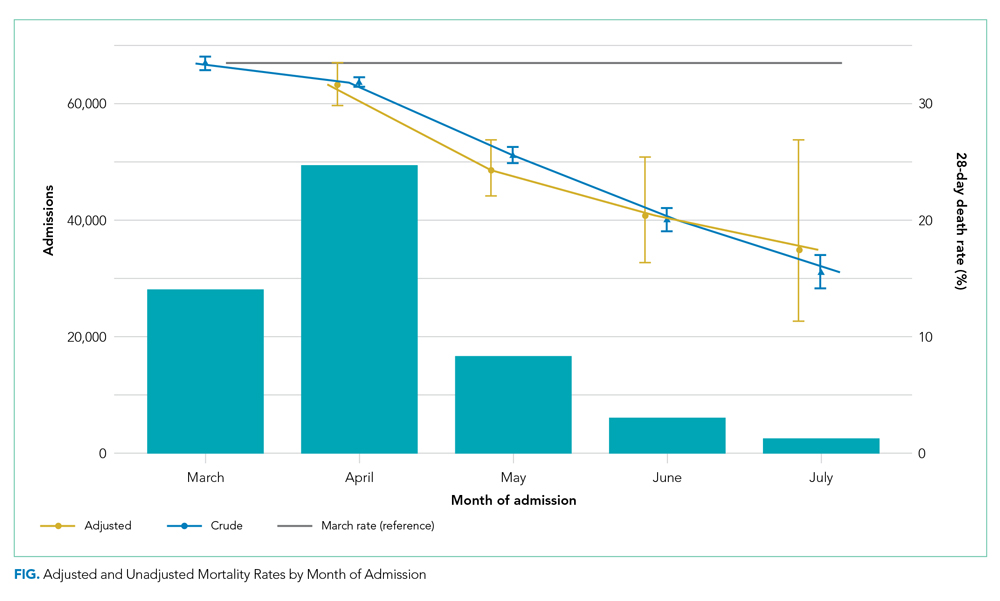
Admission from another hospital and being female were associated with reduced risk of death. Admission from a skilled nursing facility and being >75 years were associated with increased risk of death. Ten of the 29 Elixhauser comorbidities were associated with increased risk of mortality (cardiac arrhythmia, peripheral vascular disease, other neurologic disorders, renal failure, lymphoma, metastatic cancer, solid tumor without metastasis, coagulopathy, fluid and electrolyte disorders, and anemia). Deprivation and ethnic group were not associated with death among hospitalized patients.
DISCUSSION
Our study of all emergency hospital admissions in England during the first wave of the COVID-19 pandemic demonstrated that, even after adjusting for patient comorbidity and risk factors, the mortality rate decreased by approximately half over the first 5 months. Although the demographics of hospitalized patients changed over that period (with both the median age and the number of comorbidities decreasing), this does not fully explain the decrease in mortality. It is therefore likely that the decrease is due, at least in part, to an improvement in treatment and/or a reduction in hospital strain.
For example, initially the use of corticosteroids was controversial, in part due to previous experience with severe acute respiratory syndrome and Middle East respiratory syndrome (in which a Cochrane review demonstrated no benefit but potential harm). However, this changed as a result of the Randomized Evaluation of Covid-19 Therapy (RECOVERY) trial,12 which showed a significant survival benefit.One of the positive defining characteristics of the COVID-19 pandemic has been the intensive collaborative research effort combined with the rapid dissemination and discussion of new management protocols. The RECOVERY trial randomly assigned >11,000 participants in just 3 months, amounting to approximately 15% of all patients hospitalized with COVID-19 in the UK. Its results were widely publicized via professional networks and rapidly adopted into widespread clinical practice.
Examples of other changes include a higher threshold for mechanical ventilation (and a lower threshold for noninvasive ventilation), increased clinician experience, and, potentially, a reduced viral load arising from increased social distancing and mask wearing. Finally, the hospitals and staff themselves were under enormous physical and mental strain in the early months from multiple factors, including unfamiliar working environments, the large-scale redeployment of inexperienced staff, and very high numbers of patients with an unfamiliar disease. These factors all lessened as the initial peak passed. It is therefore likely that the reduction in adjusted mortality we observed arises from a combination of all these factors, as well as other incremental benefits.
The factors associated with increased mortality risk in our study (increasing age, male gender, certain comorbidities, and frailty [with care home residency acting as a proxy in our study]) are consistent with multiple previous reports. Although not the focus of our analysis, we found no effect of ethnicity or deprivation on mortality. This is consistent with many US studies that demonstrate that the widely reported effect of these factors is likely due to differences in exposure to the disease. Once patients are hospitalized, adjusted mortality risks are similar across ethnic groups and deprivation levels.
The strengths of this study include complete capture of hospitalizations across all hospitals and areas in England. Likewise, linking the hospital data to death data from the Office for National Statistics allows complete capture of outcomes, irrespective of where the patient died. This is a significant strength compared to prior studies, which only included in-hospital mortality. Our results are therefore likely robust and a true observation of the mortality trend.
Limitations include the lack of physiologic and laboratory data; having these would have allowed us to adjust for disease severity on admission and strengthened the risk stratification. Likewise, although the complete national coverage is overall a significant strength, aggregating data from numerous areas that might be at different stages of local outbreaks, have different management strategies, and have differing data quality introduces its own biases.
Furthermore, these results predate the second wave in the UK, so we cannot distinguish whether the reduced mortality is due to improved treatment, a seasonal effect, evolution of the virus itself, or a reduction in the strain on hospitals.
CONCLUSION
This nationwide study indicates that, even after accounting for changing patient characteristics, the mortality of patients hospitalized with COVID-19 in England decreased significantly as the outbreak progressed. This is likely due to a combination of incremental treatment improvements.
1. Horwitz LI, Jones SA, Cerfolio RJ, et al. Trends in COVID-19 risk-adjusted mortality rates. J Hosp Med. 2020;16(2):90-92. https://doi.org/10.12788/jhm.3552
2. Dennis JM, McGovern AP, Vollmer SJ, Mateen BA. Improving survival of critical care patients with coronavirus disease 2019 in England: a national cohort study, March to June 2020. Crit Care Med. 2021;49(2):209-214. https://doi.org/10.1097/CCM.0000000000004747
3. NHS Digital. Hospital Episode Statistics Data Dictionary. Published March 2018. Accessed October 15, 2020. https://digital.nhs.uk/data-and-information/data-tools-and-services/data-services/hospital-episode-statistics/hospital-episode-statistics-data-dictionary
4. NHS Digital. HES-ONS Linked Mortality Data Dictionary. Accessed October 15, 2020. https://digital.nhs.uk/binaries/content/assets/legacy/word/i/p/hes-ons_linked_mortality_data_dictionary_-_mar_20181.docx
5. Public Health England. Technical summary: Public Health England data series on deaths in people with COVID-19. Accessed November 11, 2020. https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/916035/RA_Technical_Summary_-_PHE_Data_Series_COVID_19_Deaths_20200812.pdf
6. Zou G. A modified Poisson regression approach to prospective studies with binary data. Am J Epidemiol. 2004;159(7):702-706. https://doi.org/10.1093/aje/kwh090
7. van Walraven C, Austin PC, Jennings A, et al. A modification of the Elixhauser comorbidity measures into a point system for hospital death using administrative data. Med Care. 2009;47(6):626-633. https://doi.org /10.1097/MLR.0b013e31819432e5
8. Ministry of Housing Communities & Local Government. The English Indices of Deprivation 2019 (IoD2019). Published September 26, 2020. Accessed January 15, 2021. https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/835115/IoD2019_Statistical_Release.pdf
9. Zeileis A. Object-oriented computation of sandwich estimators. J Stat Software. 2006;16:1-16. https://doi.org/10.18637/jss.v016.i09
10. Højsgaard S, Halekoh U, Yan J. The R package geepack for generalized estimating equations. J Stat Software. 2006;15:1-11. https://doi.org/10.18637/jss.v015.i02
11. Belsley DA, Kuh E, Welsch RE. Diagnostics: Identifying Influential Data and Sources of Collinearity. John Wiley & Sons; 1980.
12. RECOVERY Collaborative Group, Horby P, Lim WS, Emberson JR, et al. Dexamethasone in hospitalized patients with covid-19 - preliminary report. N Engl J Med. 2020:NEJMoa2021436. https://doi.org/10.1056/NEJMoa2021436
The early phase of the coronavirus disease 2019 (COVID-19) pandemic in the United Kingdom (UK) was characterized by uncertainty as clinicians grappled to understand and manage an unfamiliar disease that affected very high numbers of patients amid radically evolving working environments, with little evidence to support their efforts. Early reports indicated high mortality in patients hospitalized with COVID-19.
As the disease became better understood, treatment evolved and the mortality appears to have decreased. For example, two recent papers, a national study of critical care patients in the UK and a single-site study from New York, have demonstrated a significant reduction in adjusted mortality between the pre- and post-peak periods.1,2 However, the UK study was restricted to patients receiving critical care, potentially introducing bias due to varying critical care admission thresholds over time, while the single-site US study may not be generalizable. Moreover, both studies measured only in-hospital mortality. It remains uncertain therefore whether overall mortality has decreased on a broad scale after accounting for changes in patient characteristics.
The aim of this study was to use a national dataset to assess the
METHODS
We conducted a retrospective, secondary analysis of English National Health Services (NHS) hospitals’ admissions of patients at least 18 years of age between March 1 and July 31, 2020. Data were obtained from the Hospital Episode Statistics (HES) admitted patient care dataset.3 This is an administrative dataset that contains data on diagnoses and procedures as well as organizational characteristics and patient demographics for all NHS activity in England. We included all patients with an International Statistical Classification of Diseases, Tenth Revision (ICD-10) diagnosis of U07.1 (COVID-19, virus identified) and U07.2 (COVID-19, virus not identified).
The primary outcome of death within 28 days of admission was obtained by linking to the Civil Registrations (Deaths) - Secondary Care Cut - Information dataset, which includes the date, place, and cause of death from the Office for National Statistics4 and which was complete through September 31, 2020. The time horizon of 28 days from admission was chosen to approximate the Public Health England definition of a death from COVID-19 as being within 28 days of testing positive.5 We restricted our analysis to emergency admissions of persons age >18 years. If a patient had multiple emergency admissions, we restricted our analysis to the first admission to ensure comparability across hospitalizations and to best represent outcomes from the earliest onset of COVID-19.
We estimated a modified Poisson regression6 to predict death at 28 days, with month of admission, region, source of admission, age, deprivation, gender, ethnic group, and the 29 comorbidities in the Elixhauser comorbidity measure as variables in the regression.7 The derivation of each of these variables from the HES dataset is shown in Appendix Table 1.
Deprivation was measured by the Index of Multiple Deprivation, a methodology used widely within the UK to classify relative deprivation.8 To control for clustering, hospital system (known as Trust) was added as a random effect. Robust errors were estimated using the sandwich package.9 Modified Poisson regression was chosen in preference to the more common logistic regression because the coefficients can be interpreted as relative risks and not odds ratios. The model was fitted using R, version 4.0.3, geepack library.10 We carried out three sensitivity analyses, restricting to laboratory-confirmed COVID-19, length of stay ≥3 days, and primary respiratory disease.
For each month, we obtained a standardized mortality ratio (SMR) by fixing the month to the reference month of March 2020 and repredicting the outcome using the existing model. We calculated the ratio of the sum of observed and expected deaths (obtained from the model) in each month, comparing observed deaths to the number we would have expected had those patients been hospitalized in March. We then multiplied each period’s SMR by the March crude mortality to generate monthly adjusted mortality rates. We calculated Poisson confidence intervals around the SMR and used these to obtain confidence intervals for the adjusted rate. The binomial exact method was used to obtain confidence intervals for the crude rate. Multicollinearity was assessed using both the variance inflation factor (VIF) and the condition number test.7 All analyses used two-sided statistical tests, and we considered a P value < .05 to be statistically significant without adjustment for multiple testing. The study was exempt from UK National Research Ethics Committee approval because it involved secondary analysis of anonymized data.
RESULTS
The dataset included 115,643 emergency admissions from 179 healthcare systems, of which 103,202 were first admissions eligible for inclusion. A total of 592 patients were excluded due to missing demographic data (0.5%), resulting in 102,610 admissions included in the analysis. Peak hospitalizations occurred in late March to mid April, accounting for 44% of the hospitalizations (Table). Median length of stay for patients who died was 7 days (interquartile range, 3-12). The median age and number of Elixhauser comorbidities decreased in July. The proportion of men decreased between May and July. 
The modified Poisson regression had a C statistic of 0.743 (95% CI, 0.740-0.746) (Appendix Table 4). The VIF and condition number test found no evidence of multicollinearity.11
Adjusted mortality decreased each month, from 33.4% in March to 17.4% in July (Figure). The relative risk of death declined progressively to a minimum of 0.52 (95% CI, 0.34-0.80) in July, compared to March.
Admission from another hospital and being female were associated with reduced risk of death. Admission from a skilled nursing facility and being >75 years were associated with increased risk of death. Ten of the 29 Elixhauser comorbidities were associated with increased risk of mortality (cardiac arrhythmia, peripheral vascular disease, other neurologic disorders, renal failure, lymphoma, metastatic cancer, solid tumor without metastasis, coagulopathy, fluid and electrolyte disorders, and anemia). Deprivation and ethnic group were not associated with death among hospitalized patients.
DISCUSSION
Our study of all emergency hospital admissions in England during the first wave of the COVID-19 pandemic demonstrated that, even after adjusting for patient comorbidity and risk factors, the mortality rate decreased by approximately half over the first 5 months. Although the demographics of hospitalized patients changed over that period (with both the median age and the number of comorbidities decreasing), this does not fully explain the decrease in mortality. It is therefore likely that the decrease is due, at least in part, to an improvement in treatment and/or a reduction in hospital strain.
For example, initially the use of corticosteroids was controversial, in part due to previous experience with severe acute respiratory syndrome and Middle East respiratory syndrome (in which a Cochrane review demonstrated no benefit but potential harm). However, this changed as a result of the Randomized Evaluation of Covid-19 Therapy (RECOVERY) trial,12 which showed a significant survival benefit.One of the positive defining characteristics of the COVID-19 pandemic has been the intensive collaborative research effort combined with the rapid dissemination and discussion of new management protocols. The RECOVERY trial randomly assigned >11,000 participants in just 3 months, amounting to approximately 15% of all patients hospitalized with COVID-19 in the UK. Its results were widely publicized via professional networks and rapidly adopted into widespread clinical practice.
Examples of other changes include a higher threshold for mechanical ventilation (and a lower threshold for noninvasive ventilation), increased clinician experience, and, potentially, a reduced viral load arising from increased social distancing and mask wearing. Finally, the hospitals and staff themselves were under enormous physical and mental strain in the early months from multiple factors, including unfamiliar working environments, the large-scale redeployment of inexperienced staff, and very high numbers of patients with an unfamiliar disease. These factors all lessened as the initial peak passed. It is therefore likely that the reduction in adjusted mortality we observed arises from a combination of all these factors, as well as other incremental benefits.
The factors associated with increased mortality risk in our study (increasing age, male gender, certain comorbidities, and frailty [with care home residency acting as a proxy in our study]) are consistent with multiple previous reports. Although not the focus of our analysis, we found no effect of ethnicity or deprivation on mortality. This is consistent with many US studies that demonstrate that the widely reported effect of these factors is likely due to differences in exposure to the disease. Once patients are hospitalized, adjusted mortality risks are similar across ethnic groups and deprivation levels.
The strengths of this study include complete capture of hospitalizations across all hospitals and areas in England. Likewise, linking the hospital data to death data from the Office for National Statistics allows complete capture of outcomes, irrespective of where the patient died. This is a significant strength compared to prior studies, which only included in-hospital mortality. Our results are therefore likely robust and a true observation of the mortality trend.
Limitations include the lack of physiologic and laboratory data; having these would have allowed us to adjust for disease severity on admission and strengthened the risk stratification. Likewise, although the complete national coverage is overall a significant strength, aggregating data from numerous areas that might be at different stages of local outbreaks, have different management strategies, and have differing data quality introduces its own biases.
Furthermore, these results predate the second wave in the UK, so we cannot distinguish whether the reduced mortality is due to improved treatment, a seasonal effect, evolution of the virus itself, or a reduction in the strain on hospitals.
CONCLUSION
This nationwide study indicates that, even after accounting for changing patient characteristics, the mortality of patients hospitalized with COVID-19 in England decreased significantly as the outbreak progressed. This is likely due to a combination of incremental treatment improvements.
The early phase of the coronavirus disease 2019 (COVID-19) pandemic in the United Kingdom (UK) was characterized by uncertainty as clinicians grappled to understand and manage an unfamiliar disease that affected very high numbers of patients amid radically evolving working environments, with little evidence to support their efforts. Early reports indicated high mortality in patients hospitalized with COVID-19.
As the disease became better understood, treatment evolved and the mortality appears to have decreased. For example, two recent papers, a national study of critical care patients in the UK and a single-site study from New York, have demonstrated a significant reduction in adjusted mortality between the pre- and post-peak periods.1,2 However, the UK study was restricted to patients receiving critical care, potentially introducing bias due to varying critical care admission thresholds over time, while the single-site US study may not be generalizable. Moreover, both studies measured only in-hospital mortality. It remains uncertain therefore whether overall mortality has decreased on a broad scale after accounting for changes in patient characteristics.
The aim of this study was to use a national dataset to assess the
METHODS
We conducted a retrospective, secondary analysis of English National Health Services (NHS) hospitals’ admissions of patients at least 18 years of age between March 1 and July 31, 2020. Data were obtained from the Hospital Episode Statistics (HES) admitted patient care dataset.3 This is an administrative dataset that contains data on diagnoses and procedures as well as organizational characteristics and patient demographics for all NHS activity in England. We included all patients with an International Statistical Classification of Diseases, Tenth Revision (ICD-10) diagnosis of U07.1 (COVID-19, virus identified) and U07.2 (COVID-19, virus not identified).
The primary outcome of death within 28 days of admission was obtained by linking to the Civil Registrations (Deaths) - Secondary Care Cut - Information dataset, which includes the date, place, and cause of death from the Office for National Statistics4 and which was complete through September 31, 2020. The time horizon of 28 days from admission was chosen to approximate the Public Health England definition of a death from COVID-19 as being within 28 days of testing positive.5 We restricted our analysis to emergency admissions of persons age >18 years. If a patient had multiple emergency admissions, we restricted our analysis to the first admission to ensure comparability across hospitalizations and to best represent outcomes from the earliest onset of COVID-19.
We estimated a modified Poisson regression6 to predict death at 28 days, with month of admission, region, source of admission, age, deprivation, gender, ethnic group, and the 29 comorbidities in the Elixhauser comorbidity measure as variables in the regression.7 The derivation of each of these variables from the HES dataset is shown in Appendix Table 1.
Deprivation was measured by the Index of Multiple Deprivation, a methodology used widely within the UK to classify relative deprivation.8 To control for clustering, hospital system (known as Trust) was added as a random effect. Robust errors were estimated using the sandwich package.9 Modified Poisson regression was chosen in preference to the more common logistic regression because the coefficients can be interpreted as relative risks and not odds ratios. The model was fitted using R, version 4.0.3, geepack library.10 We carried out three sensitivity analyses, restricting to laboratory-confirmed COVID-19, length of stay ≥3 days, and primary respiratory disease.
For each month, we obtained a standardized mortality ratio (SMR) by fixing the month to the reference month of March 2020 and repredicting the outcome using the existing model. We calculated the ratio of the sum of observed and expected deaths (obtained from the model) in each month, comparing observed deaths to the number we would have expected had those patients been hospitalized in March. We then multiplied each period’s SMR by the March crude mortality to generate monthly adjusted mortality rates. We calculated Poisson confidence intervals around the SMR and used these to obtain confidence intervals for the adjusted rate. The binomial exact method was used to obtain confidence intervals for the crude rate. Multicollinearity was assessed using both the variance inflation factor (VIF) and the condition number test.7 All analyses used two-sided statistical tests, and we considered a P value < .05 to be statistically significant without adjustment for multiple testing. The study was exempt from UK National Research Ethics Committee approval because it involved secondary analysis of anonymized data.
RESULTS
The dataset included 115,643 emergency admissions from 179 healthcare systems, of which 103,202 were first admissions eligible for inclusion. A total of 592 patients were excluded due to missing demographic data (0.5%), resulting in 102,610 admissions included in the analysis. Peak hospitalizations occurred in late March to mid April, accounting for 44% of the hospitalizations (Table). Median length of stay for patients who died was 7 days (interquartile range, 3-12). The median age and number of Elixhauser comorbidities decreased in July. The proportion of men decreased between May and July. 
The modified Poisson regression had a C statistic of 0.743 (95% CI, 0.740-0.746) (Appendix Table 4). The VIF and condition number test found no evidence of multicollinearity.11
Adjusted mortality decreased each month, from 33.4% in March to 17.4% in July (Figure). The relative risk of death declined progressively to a minimum of 0.52 (95% CI, 0.34-0.80) in July, compared to March.
Admission from another hospital and being female were associated with reduced risk of death. Admission from a skilled nursing facility and being >75 years were associated with increased risk of death. Ten of the 29 Elixhauser comorbidities were associated with increased risk of mortality (cardiac arrhythmia, peripheral vascular disease, other neurologic disorders, renal failure, lymphoma, metastatic cancer, solid tumor without metastasis, coagulopathy, fluid and electrolyte disorders, and anemia). Deprivation and ethnic group were not associated with death among hospitalized patients.
DISCUSSION
Our study of all emergency hospital admissions in England during the first wave of the COVID-19 pandemic demonstrated that, even after adjusting for patient comorbidity and risk factors, the mortality rate decreased by approximately half over the first 5 months. Although the demographics of hospitalized patients changed over that period (with both the median age and the number of comorbidities decreasing), this does not fully explain the decrease in mortality. It is therefore likely that the decrease is due, at least in part, to an improvement in treatment and/or a reduction in hospital strain.
For example, initially the use of corticosteroids was controversial, in part due to previous experience with severe acute respiratory syndrome and Middle East respiratory syndrome (in which a Cochrane review demonstrated no benefit but potential harm). However, this changed as a result of the Randomized Evaluation of Covid-19 Therapy (RECOVERY) trial,12 which showed a significant survival benefit.One of the positive defining characteristics of the COVID-19 pandemic has been the intensive collaborative research effort combined with the rapid dissemination and discussion of new management protocols. The RECOVERY trial randomly assigned >11,000 participants in just 3 months, amounting to approximately 15% of all patients hospitalized with COVID-19 in the UK. Its results were widely publicized via professional networks and rapidly adopted into widespread clinical practice.
Examples of other changes include a higher threshold for mechanical ventilation (and a lower threshold for noninvasive ventilation), increased clinician experience, and, potentially, a reduced viral load arising from increased social distancing and mask wearing. Finally, the hospitals and staff themselves were under enormous physical and mental strain in the early months from multiple factors, including unfamiliar working environments, the large-scale redeployment of inexperienced staff, and very high numbers of patients with an unfamiliar disease. These factors all lessened as the initial peak passed. It is therefore likely that the reduction in adjusted mortality we observed arises from a combination of all these factors, as well as other incremental benefits.
The factors associated with increased mortality risk in our study (increasing age, male gender, certain comorbidities, and frailty [with care home residency acting as a proxy in our study]) are consistent with multiple previous reports. Although not the focus of our analysis, we found no effect of ethnicity or deprivation on mortality. This is consistent with many US studies that demonstrate that the widely reported effect of these factors is likely due to differences in exposure to the disease. Once patients are hospitalized, adjusted mortality risks are similar across ethnic groups and deprivation levels.
The strengths of this study include complete capture of hospitalizations across all hospitals and areas in England. Likewise, linking the hospital data to death data from the Office for National Statistics allows complete capture of outcomes, irrespective of where the patient died. This is a significant strength compared to prior studies, which only included in-hospital mortality. Our results are therefore likely robust and a true observation of the mortality trend.
Limitations include the lack of physiologic and laboratory data; having these would have allowed us to adjust for disease severity on admission and strengthened the risk stratification. Likewise, although the complete national coverage is overall a significant strength, aggregating data from numerous areas that might be at different stages of local outbreaks, have different management strategies, and have differing data quality introduces its own biases.
Furthermore, these results predate the second wave in the UK, so we cannot distinguish whether the reduced mortality is due to improved treatment, a seasonal effect, evolution of the virus itself, or a reduction in the strain on hospitals.
CONCLUSION
This nationwide study indicates that, even after accounting for changing patient characteristics, the mortality of patients hospitalized with COVID-19 in England decreased significantly as the outbreak progressed. This is likely due to a combination of incremental treatment improvements.
1. Horwitz LI, Jones SA, Cerfolio RJ, et al. Trends in COVID-19 risk-adjusted mortality rates. J Hosp Med. 2020;16(2):90-92. https://doi.org/10.12788/jhm.3552
2. Dennis JM, McGovern AP, Vollmer SJ, Mateen BA. Improving survival of critical care patients with coronavirus disease 2019 in England: a national cohort study, March to June 2020. Crit Care Med. 2021;49(2):209-214. https://doi.org/10.1097/CCM.0000000000004747
3. NHS Digital. Hospital Episode Statistics Data Dictionary. Published March 2018. Accessed October 15, 2020. https://digital.nhs.uk/data-and-information/data-tools-and-services/data-services/hospital-episode-statistics/hospital-episode-statistics-data-dictionary
4. NHS Digital. HES-ONS Linked Mortality Data Dictionary. Accessed October 15, 2020. https://digital.nhs.uk/binaries/content/assets/legacy/word/i/p/hes-ons_linked_mortality_data_dictionary_-_mar_20181.docx
5. Public Health England. Technical summary: Public Health England data series on deaths in people with COVID-19. Accessed November 11, 2020. https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/916035/RA_Technical_Summary_-_PHE_Data_Series_COVID_19_Deaths_20200812.pdf
6. Zou G. A modified Poisson regression approach to prospective studies with binary data. Am J Epidemiol. 2004;159(7):702-706. https://doi.org/10.1093/aje/kwh090
7. van Walraven C, Austin PC, Jennings A, et al. A modification of the Elixhauser comorbidity measures into a point system for hospital death using administrative data. Med Care. 2009;47(6):626-633. https://doi.org /10.1097/MLR.0b013e31819432e5
8. Ministry of Housing Communities & Local Government. The English Indices of Deprivation 2019 (IoD2019). Published September 26, 2020. Accessed January 15, 2021. https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/835115/IoD2019_Statistical_Release.pdf
9. Zeileis A. Object-oriented computation of sandwich estimators. J Stat Software. 2006;16:1-16. https://doi.org/10.18637/jss.v016.i09
10. Højsgaard S, Halekoh U, Yan J. The R package geepack for generalized estimating equations. J Stat Software. 2006;15:1-11. https://doi.org/10.18637/jss.v015.i02
11. Belsley DA, Kuh E, Welsch RE. Diagnostics: Identifying Influential Data and Sources of Collinearity. John Wiley & Sons; 1980.
12. RECOVERY Collaborative Group, Horby P, Lim WS, Emberson JR, et al. Dexamethasone in hospitalized patients with covid-19 - preliminary report. N Engl J Med. 2020:NEJMoa2021436. https://doi.org/10.1056/NEJMoa2021436
1. Horwitz LI, Jones SA, Cerfolio RJ, et al. Trends in COVID-19 risk-adjusted mortality rates. J Hosp Med. 2020;16(2):90-92. https://doi.org/10.12788/jhm.3552
2. Dennis JM, McGovern AP, Vollmer SJ, Mateen BA. Improving survival of critical care patients with coronavirus disease 2019 in England: a national cohort study, March to June 2020. Crit Care Med. 2021;49(2):209-214. https://doi.org/10.1097/CCM.0000000000004747
3. NHS Digital. Hospital Episode Statistics Data Dictionary. Published March 2018. Accessed October 15, 2020. https://digital.nhs.uk/data-and-information/data-tools-and-services/data-services/hospital-episode-statistics/hospital-episode-statistics-data-dictionary
4. NHS Digital. HES-ONS Linked Mortality Data Dictionary. Accessed October 15, 2020. https://digital.nhs.uk/binaries/content/assets/legacy/word/i/p/hes-ons_linked_mortality_data_dictionary_-_mar_20181.docx
5. Public Health England. Technical summary: Public Health England data series on deaths in people with COVID-19. Accessed November 11, 2020. https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/916035/RA_Technical_Summary_-_PHE_Data_Series_COVID_19_Deaths_20200812.pdf
6. Zou G. A modified Poisson regression approach to prospective studies with binary data. Am J Epidemiol. 2004;159(7):702-706. https://doi.org/10.1093/aje/kwh090
7. van Walraven C, Austin PC, Jennings A, et al. A modification of the Elixhauser comorbidity measures into a point system for hospital death using administrative data. Med Care. 2009;47(6):626-633. https://doi.org /10.1097/MLR.0b013e31819432e5
8. Ministry of Housing Communities & Local Government. The English Indices of Deprivation 2019 (IoD2019). Published September 26, 2020. Accessed January 15, 2021. https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/835115/IoD2019_Statistical_Release.pdf
9. Zeileis A. Object-oriented computation of sandwich estimators. J Stat Software. 2006;16:1-16. https://doi.org/10.18637/jss.v016.i09
10. Højsgaard S, Halekoh U, Yan J. The R package geepack for generalized estimating equations. J Stat Software. 2006;15:1-11. https://doi.org/10.18637/jss.v015.i02
11. Belsley DA, Kuh E, Welsch RE. Diagnostics: Identifying Influential Data and Sources of Collinearity. John Wiley & Sons; 1980.
12. RECOVERY Collaborative Group, Horby P, Lim WS, Emberson JR, et al. Dexamethasone in hospitalized patients with covid-19 - preliminary report. N Engl J Med. 2020:NEJMoa2021436. https://doi.org/10.1056/NEJMoa2021436
© 2021 Society of Hospital Medicine
Trends in COVID-19 Risk-Adjusted Mortality Rates
Early reports showed high mortality from coronavirus disease 2019 (COVID-19), while current United States data mortality rates are lower, raising hope that new treatments and management strategies have improved outcomes. For instance, Centers for Disease Control and Prevention data show that 6.7% of cases resulted in death in April, compared with 1.9% in September.1 However, the demographics of those infected have also changed, and more available testing may mean more comprehensive identification and earlier treatment. Nationally, for instance, the median age of confirmed cases was 38 years at the end of August, down from 46 years at the start of May.2 Therefore, whether decreasing COVID-19 mortality rates simply reflect changing demographics or represent actual improvements in clinical care is unknown. The objective of this analysis was to assess outcomes over time in a single health system, accounting for changes in demographics, clinical factors, and severity of disease at presentation.
METHODS
We analyzed monthly mortality rates for admissions between March 1 and August 31, 2020, in a single health system in New York City. Outcomes were obtained as of October 8, 2020. We included all hospitalizations of people 18 years and older with laboratory-confirmed severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection identified during the hospitalization or in the prior 2 weeks, excluding those admitted to hospice care. Patients with multiple hospitalizations (N=208 patients, 229 hospitalizations, 4.4%) were included repeatedly if they continued to have laboratory-confirmed disease. Patients without admission vital signs (N=28) were excluded. Mortality was defined as in-hospital death or discharge to hospice care. In-house laboratory testing began March 16 and all inpatients were tested for SARS-CoV-2 by April 1; elective surgeries resumed May 4-11 and were only conducted on confirmed SARS-CoV-2–negative patients.
All data were obtained from the electronic health record (Epic Systems, Verona, Wisconsin). Diagnosis codes were obtained from the problem list, past medical history, and billing codes. In addition, we used objective data such as hemoglobin A1c, ejection fraction, outpatient creatinine, and outpatient blood pressure to augment problem list diagnoses where relevant.
Based on prior literature, we constructed multivariable logistic regression models for mortality adjusting for age; sex; self-reported race and ethnicity; body mass index; smoking history; presence of hypertension, heart failure, hyperlipidemia, coronary artery disease, diabetes, cancer, chronic kidney disease, dementia, or pulmonary disease individually as dummy variables; and admission oxygen saturation, D-dimer, ferritin, and C-reactive protein.3-6 In the first model (C statistic 0.82), we did not include month of admission as a covariate and calculated the ratio of the sum of observed and expected deaths (obtained from the model) in each month to obtain the standardized mortality ratio (SMR) for each month. We then multiplied each period’s SMR by the overall average crude mortality to generate monthly adjusted mortality rates. We calculated Poisson control limits and indicated points outside the control limits as significantly different.
In a second model (C statistic 0.84), we included month as a covariate and calculated average marginal effects (AME) for each time period by using the margins library in R,7 which uses a discrete first-difference in predicted outcomes to obtain the AME. The average marginal effect represents the percentage point difference between the reference period (March) and a subsequent time period in probability of death or discharge to hospice, for equivalent patients. We obtained lower and upper confidence intervals for the AME using a bootstrapping approach described in Green.8 Finally, we conducted two sensitivity analyses: one, restricting the analysis to only those patients with principal diagnosis of COVID-19, sepsis, or respiratory disease (see Appendix A for complete list of codes) and one restricting the analysis to only those with length of stay of at least 3 days.
All statistical analyses were conducted with R, version 4.0.2. All analyses used 2-sided statistical tests, and we considered a P value < .05 to be statistically significant without adjustment for multiple testing. The NYU institutional review board approved the study and granted a waiver of consent and a waiver of the Health Information Portability and Accountability Act.
RESULTS
We included 5,121 hospitalizations, of which 5,118 (99.94%) had known outcomes (death or hospital discharge). Peak hospitalizations occurred in late March to mid-April, which accounted for 53% of the hospitalizations. Median length of stay for patients who died or were discharged to hospice was 8 days (interquartile range, 4-15; max 140 days). The median age and the proportion male or with any comorbidity decreased over time (Table). For instance, the proportion with any chronic condition decreased from 81% in March to 72% in August.
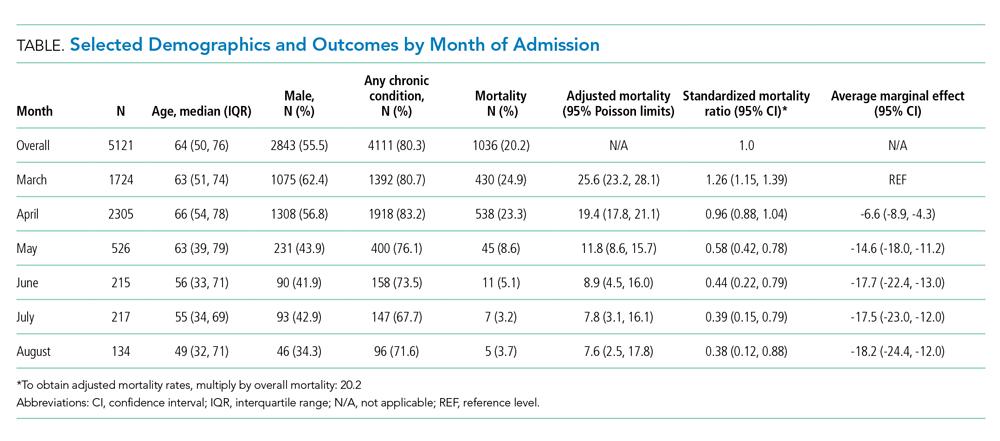
Adjusted mortality dropped each month, from 25.6% in March to 7.6% in August (Table and Figure). The SMR declined progressively over time, from 1.26 (95% CI, 1.15-1.39) in March to 0.38 (95% CI, 0.12-0.88) in August (Table). The adjusted average marginal effect was also significantly lower than in March in every subsequent month, reaching a maximum of an average 18.2 (95% CI, 12.0-24.4) percentage point decrease in probability of death in August, accounting for changes in demographics and clinical severity (Table and Appendix B). The decrease in unadjusted mortality over time was observed across age groups (Appendix C).
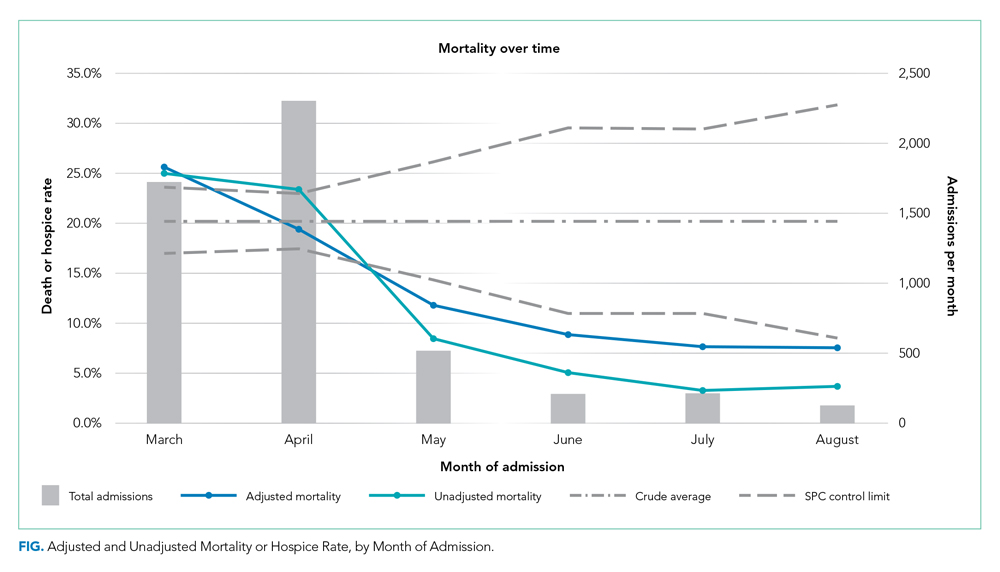
Results of the two sensitivity analyses were similar (Appendices D and E), though attenuated in the case of the sepsis/respiratory cohort, with adjusted mortality falling from 31.4% to 14.4%, SMR decreasing from 1.28 (95% CI, 1.16-1.41) to 0.59 (95% CI, 0.16-1.50), and AME in August 17.0 percentage points (95% CI, 6.0-28.1).
DISCUSSION
In this study of COVID-19 mortality over 6 months at a single health system, we found that changes in demographics and severity of illness at presentation did not fully explain decreases in mortality seen over time. Even after risk adjustment for a variety of clinical and demographic factors, including severity of illness at presentation, mortality was significantly and progressively lower over the course of the study period.
Similar risk-adjusted results have been preliminarily reported among intensive care unit patients in a preprint from the United Kingdom.9 Incremental improvements in outcomes are likely a combination of increasing clinical experience, decreasing hospital volume, growing use of new pharmacologic treatments (such as systemic corticosteroids,10 remdesivir,11 and anticytokine treatments), nonpharmacologic treatments (such as placing the patient in the prone position, or proning, rather than on their back), earlier intervention, community awareness, and, potentially, lower viral load exposure from increased mask wearing and social distancing.12
Strengths of this study include highly detailed electronic health record data on hospitalizations at three different hospitals, a diverse patient population,6 near-complete study outcomes, and a lengthy period of investigation of 6 months. However, this study does have limitations. All patients were from a single geographic region and treated within a single health system, though restricting data to one system reduces institution-level variability and allows us to assess how care may have evolved with growing experience. Aggregating data from numerous health systems that might be at different stages of local outbreaks, provide different quality of care, and contribute different numbers of patients in each period introduces its own biases. We were also unable to disentangle different potential explanatory factors given the observational nature of the study. Residual confounding, such as a higher proportion of particularly frail patients admitted in earlier periods, is also a possibility, though the fact that we observed declines across all age groups mitigates this concern. Thresholds for hospital admission may also have changed over time with less severely ill patients being admitted in the later time periods. While changing admission thresholds could have contributed to higher survival rates in the latter portions of the study, our inclusion of several highly predictive clinical and laboratory results likely captured many aspects of disease severity.
CONCLUSION
In summary, data from one health system suggest that COVID-19 remains a serious disease for high-risk patients, but that mortality rates are improving over time.
1. CDC COVID Data Tracker. 2020. Centers for Disease Control and Prevention. Accessed October 14, 2020. https://covid.cdc.gov/covid-data-tracker/#trends_dailytrendscases
2. Boehmer TK, DeVies J, Caruso E, et al. Changing age distribution of the COVID-19 pandemic - United States, May-August 2020. MMWR Morb Mortal Wkly Rep. 2020;69(39):1404-1409 http://dx.doi.org/0.15585/mmwr.mm6939e1
3. Lu L, Zhong W, Bian Z, et al. A comparison of mortality-related risk factors of COVID-19, SARS, and MERS: A systematic review and meta-analysis. J Infect. 2020;81(4):318-e25. https://doi.org/10.1016/j.jinf.2020.07.002
4. Parohan M, Yaghoubi S, Seraji A, Javanbakht MH, Sarraf P, Djalali M. Risk factors for mortality in patients with coronavirus disease 2019 (COVID-19) infection: a systematic review and meta-analysis of observational studies. Aging Male. 2020;Jun8:1-9. https://doi.org/10.1080/13685538.2020.1774748
5. Zheng Z, Peng F, Xu B, et al. Risk factors of critical & mortal COVID-19 cases: a systematic literature review and meta-analysis. J Infect. 2020;81(2):e16-e25. https://doi.org/10.1016/j.jinf.2020.04.021
6. Petrilli CM, Jones SA, Yang J, et al. Factors associated with hospital admission and critical illness among 5279 people with coronavirus disease 2019 in New York City: prospective cohort study. BMJ. 2020;369:m1966. https://doi.org/10.1136/bmj.m1966
7. margins: Marginal Effects for Model Objects [computer program]. Version R package version 0.3.232018. Accessed October 1, 2020. https://rdrr.io/cran/margins/
8. Greene WH. Econometric Analysis. 7th ed. Pearson; 2012.
9. Doidge JC, Mouncey PR, Thomas K, et al. Trends in intensive care for patients with COVID-19 in England, Wales and Northern Ireland. Preprints 2020. Preprint posted online August 11, 2020. https://doi.org/10.20944/preprints202008.0267.v1
10. Recovery Collaborative Group, Horby P, Lim WS, et al. Dexamethasone in hospitalized patients with Covid-19 - preliminary report. N Engl J Med. 2020. Online first July 17, 2020. https://doi.org/10.1056/NEJMoa2021436
11. Beigel JH, Tomashek KM, Dodd LE, et al. Remdesivir for the treatment of Covid-19 – final report. N Enl J Med. 2020. Online first October 8, 2020. https://doi.org/10.1056/NEJMoa2007764
12. Gandhi M, Rutherford GW. Facial masking for Covid-19 - potential for “variolation” as we await a vaccine. N Engl J Med. 2020. Online first September 8, 2020. https://doi.org/10.1056/NEJMp2026913
Early reports showed high mortality from coronavirus disease 2019 (COVID-19), while current United States data mortality rates are lower, raising hope that new treatments and management strategies have improved outcomes. For instance, Centers for Disease Control and Prevention data show that 6.7% of cases resulted in death in April, compared with 1.9% in September.1 However, the demographics of those infected have also changed, and more available testing may mean more comprehensive identification and earlier treatment. Nationally, for instance, the median age of confirmed cases was 38 years at the end of August, down from 46 years at the start of May.2 Therefore, whether decreasing COVID-19 mortality rates simply reflect changing demographics or represent actual improvements in clinical care is unknown. The objective of this analysis was to assess outcomes over time in a single health system, accounting for changes in demographics, clinical factors, and severity of disease at presentation.
METHODS
We analyzed monthly mortality rates for admissions between March 1 and August 31, 2020, in a single health system in New York City. Outcomes were obtained as of October 8, 2020. We included all hospitalizations of people 18 years and older with laboratory-confirmed severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection identified during the hospitalization or in the prior 2 weeks, excluding those admitted to hospice care. Patients with multiple hospitalizations (N=208 patients, 229 hospitalizations, 4.4%) were included repeatedly if they continued to have laboratory-confirmed disease. Patients without admission vital signs (N=28) were excluded. Mortality was defined as in-hospital death or discharge to hospice care. In-house laboratory testing began March 16 and all inpatients were tested for SARS-CoV-2 by April 1; elective surgeries resumed May 4-11 and were only conducted on confirmed SARS-CoV-2–negative patients.
All data were obtained from the electronic health record (Epic Systems, Verona, Wisconsin). Diagnosis codes were obtained from the problem list, past medical history, and billing codes. In addition, we used objective data such as hemoglobin A1c, ejection fraction, outpatient creatinine, and outpatient blood pressure to augment problem list diagnoses where relevant.
Based on prior literature, we constructed multivariable logistic regression models for mortality adjusting for age; sex; self-reported race and ethnicity; body mass index; smoking history; presence of hypertension, heart failure, hyperlipidemia, coronary artery disease, diabetes, cancer, chronic kidney disease, dementia, or pulmonary disease individually as dummy variables; and admission oxygen saturation, D-dimer, ferritin, and C-reactive protein.3-6 In the first model (C statistic 0.82), we did not include month of admission as a covariate and calculated the ratio of the sum of observed and expected deaths (obtained from the model) in each month to obtain the standardized mortality ratio (SMR) for each month. We then multiplied each period’s SMR by the overall average crude mortality to generate monthly adjusted mortality rates. We calculated Poisson control limits and indicated points outside the control limits as significantly different.
In a second model (C statistic 0.84), we included month as a covariate and calculated average marginal effects (AME) for each time period by using the margins library in R,7 which uses a discrete first-difference in predicted outcomes to obtain the AME. The average marginal effect represents the percentage point difference between the reference period (March) and a subsequent time period in probability of death or discharge to hospice, for equivalent patients. We obtained lower and upper confidence intervals for the AME using a bootstrapping approach described in Green.8 Finally, we conducted two sensitivity analyses: one, restricting the analysis to only those patients with principal diagnosis of COVID-19, sepsis, or respiratory disease (see Appendix A for complete list of codes) and one restricting the analysis to only those with length of stay of at least 3 days.
All statistical analyses were conducted with R, version 4.0.2. All analyses used 2-sided statistical tests, and we considered a P value < .05 to be statistically significant without adjustment for multiple testing. The NYU institutional review board approved the study and granted a waiver of consent and a waiver of the Health Information Portability and Accountability Act.
RESULTS
We included 5,121 hospitalizations, of which 5,118 (99.94%) had known outcomes (death or hospital discharge). Peak hospitalizations occurred in late March to mid-April, which accounted for 53% of the hospitalizations. Median length of stay for patients who died or were discharged to hospice was 8 days (interquartile range, 4-15; max 140 days). The median age and the proportion male or with any comorbidity decreased over time (Table). For instance, the proportion with any chronic condition decreased from 81% in March to 72% in August.

Adjusted mortality dropped each month, from 25.6% in March to 7.6% in August (Table and Figure). The SMR declined progressively over time, from 1.26 (95% CI, 1.15-1.39) in March to 0.38 (95% CI, 0.12-0.88) in August (Table). The adjusted average marginal effect was also significantly lower than in March in every subsequent month, reaching a maximum of an average 18.2 (95% CI, 12.0-24.4) percentage point decrease in probability of death in August, accounting for changes in demographics and clinical severity (Table and Appendix B). The decrease in unadjusted mortality over time was observed across age groups (Appendix C).

Results of the two sensitivity analyses were similar (Appendices D and E), though attenuated in the case of the sepsis/respiratory cohort, with adjusted mortality falling from 31.4% to 14.4%, SMR decreasing from 1.28 (95% CI, 1.16-1.41) to 0.59 (95% CI, 0.16-1.50), and AME in August 17.0 percentage points (95% CI, 6.0-28.1).
DISCUSSION
In this study of COVID-19 mortality over 6 months at a single health system, we found that changes in demographics and severity of illness at presentation did not fully explain decreases in mortality seen over time. Even after risk adjustment for a variety of clinical and demographic factors, including severity of illness at presentation, mortality was significantly and progressively lower over the course of the study period.
Similar risk-adjusted results have been preliminarily reported among intensive care unit patients in a preprint from the United Kingdom.9 Incremental improvements in outcomes are likely a combination of increasing clinical experience, decreasing hospital volume, growing use of new pharmacologic treatments (such as systemic corticosteroids,10 remdesivir,11 and anticytokine treatments), nonpharmacologic treatments (such as placing the patient in the prone position, or proning, rather than on their back), earlier intervention, community awareness, and, potentially, lower viral load exposure from increased mask wearing and social distancing.12
Strengths of this study include highly detailed electronic health record data on hospitalizations at three different hospitals, a diverse patient population,6 near-complete study outcomes, and a lengthy period of investigation of 6 months. However, this study does have limitations. All patients were from a single geographic region and treated within a single health system, though restricting data to one system reduces institution-level variability and allows us to assess how care may have evolved with growing experience. Aggregating data from numerous health systems that might be at different stages of local outbreaks, provide different quality of care, and contribute different numbers of patients in each period introduces its own biases. We were also unable to disentangle different potential explanatory factors given the observational nature of the study. Residual confounding, such as a higher proportion of particularly frail patients admitted in earlier periods, is also a possibility, though the fact that we observed declines across all age groups mitigates this concern. Thresholds for hospital admission may also have changed over time with less severely ill patients being admitted in the later time periods. While changing admission thresholds could have contributed to higher survival rates in the latter portions of the study, our inclusion of several highly predictive clinical and laboratory results likely captured many aspects of disease severity.
CONCLUSION
In summary, data from one health system suggest that COVID-19 remains a serious disease for high-risk patients, but that mortality rates are improving over time.
Early reports showed high mortality from coronavirus disease 2019 (COVID-19), while current United States data mortality rates are lower, raising hope that new treatments and management strategies have improved outcomes. For instance, Centers for Disease Control and Prevention data show that 6.7% of cases resulted in death in April, compared with 1.9% in September.1 However, the demographics of those infected have also changed, and more available testing may mean more comprehensive identification and earlier treatment. Nationally, for instance, the median age of confirmed cases was 38 years at the end of August, down from 46 years at the start of May.2 Therefore, whether decreasing COVID-19 mortality rates simply reflect changing demographics or represent actual improvements in clinical care is unknown. The objective of this analysis was to assess outcomes over time in a single health system, accounting for changes in demographics, clinical factors, and severity of disease at presentation.
METHODS
We analyzed monthly mortality rates for admissions between March 1 and August 31, 2020, in a single health system in New York City. Outcomes were obtained as of October 8, 2020. We included all hospitalizations of people 18 years and older with laboratory-confirmed severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection identified during the hospitalization or in the prior 2 weeks, excluding those admitted to hospice care. Patients with multiple hospitalizations (N=208 patients, 229 hospitalizations, 4.4%) were included repeatedly if they continued to have laboratory-confirmed disease. Patients without admission vital signs (N=28) were excluded. Mortality was defined as in-hospital death or discharge to hospice care. In-house laboratory testing began March 16 and all inpatients were tested for SARS-CoV-2 by April 1; elective surgeries resumed May 4-11 and were only conducted on confirmed SARS-CoV-2–negative patients.
All data were obtained from the electronic health record (Epic Systems, Verona, Wisconsin). Diagnosis codes were obtained from the problem list, past medical history, and billing codes. In addition, we used objective data such as hemoglobin A1c, ejection fraction, outpatient creatinine, and outpatient blood pressure to augment problem list diagnoses where relevant.
Based on prior literature, we constructed multivariable logistic regression models for mortality adjusting for age; sex; self-reported race and ethnicity; body mass index; smoking history; presence of hypertension, heart failure, hyperlipidemia, coronary artery disease, diabetes, cancer, chronic kidney disease, dementia, or pulmonary disease individually as dummy variables; and admission oxygen saturation, D-dimer, ferritin, and C-reactive protein.3-6 In the first model (C statistic 0.82), we did not include month of admission as a covariate and calculated the ratio of the sum of observed and expected deaths (obtained from the model) in each month to obtain the standardized mortality ratio (SMR) for each month. We then multiplied each period’s SMR by the overall average crude mortality to generate monthly adjusted mortality rates. We calculated Poisson control limits and indicated points outside the control limits as significantly different.
In a second model (C statistic 0.84), we included month as a covariate and calculated average marginal effects (AME) for each time period by using the margins library in R,7 which uses a discrete first-difference in predicted outcomes to obtain the AME. The average marginal effect represents the percentage point difference between the reference period (March) and a subsequent time period in probability of death or discharge to hospice, for equivalent patients. We obtained lower and upper confidence intervals for the AME using a bootstrapping approach described in Green.8 Finally, we conducted two sensitivity analyses: one, restricting the analysis to only those patients with principal diagnosis of COVID-19, sepsis, or respiratory disease (see Appendix A for complete list of codes) and one restricting the analysis to only those with length of stay of at least 3 days.
All statistical analyses were conducted with R, version 4.0.2. All analyses used 2-sided statistical tests, and we considered a P value < .05 to be statistically significant without adjustment for multiple testing. The NYU institutional review board approved the study and granted a waiver of consent and a waiver of the Health Information Portability and Accountability Act.
RESULTS
We included 5,121 hospitalizations, of which 5,118 (99.94%) had known outcomes (death or hospital discharge). Peak hospitalizations occurred in late March to mid-April, which accounted for 53% of the hospitalizations. Median length of stay for patients who died or were discharged to hospice was 8 days (interquartile range, 4-15; max 140 days). The median age and the proportion male or with any comorbidity decreased over time (Table). For instance, the proportion with any chronic condition decreased from 81% in March to 72% in August.

Adjusted mortality dropped each month, from 25.6% in March to 7.6% in August (Table and Figure). The SMR declined progressively over time, from 1.26 (95% CI, 1.15-1.39) in March to 0.38 (95% CI, 0.12-0.88) in August (Table). The adjusted average marginal effect was also significantly lower than in March in every subsequent month, reaching a maximum of an average 18.2 (95% CI, 12.0-24.4) percentage point decrease in probability of death in August, accounting for changes in demographics and clinical severity (Table and Appendix B). The decrease in unadjusted mortality over time was observed across age groups (Appendix C).

Results of the two sensitivity analyses were similar (Appendices D and E), though attenuated in the case of the sepsis/respiratory cohort, with adjusted mortality falling from 31.4% to 14.4%, SMR decreasing from 1.28 (95% CI, 1.16-1.41) to 0.59 (95% CI, 0.16-1.50), and AME in August 17.0 percentage points (95% CI, 6.0-28.1).
DISCUSSION
In this study of COVID-19 mortality over 6 months at a single health system, we found that changes in demographics and severity of illness at presentation did not fully explain decreases in mortality seen over time. Even after risk adjustment for a variety of clinical and demographic factors, including severity of illness at presentation, mortality was significantly and progressively lower over the course of the study period.
Similar risk-adjusted results have been preliminarily reported among intensive care unit patients in a preprint from the United Kingdom.9 Incremental improvements in outcomes are likely a combination of increasing clinical experience, decreasing hospital volume, growing use of new pharmacologic treatments (such as systemic corticosteroids,10 remdesivir,11 and anticytokine treatments), nonpharmacologic treatments (such as placing the patient in the prone position, or proning, rather than on their back), earlier intervention, community awareness, and, potentially, lower viral load exposure from increased mask wearing and social distancing.12
Strengths of this study include highly detailed electronic health record data on hospitalizations at three different hospitals, a diverse patient population,6 near-complete study outcomes, and a lengthy period of investigation of 6 months. However, this study does have limitations. All patients were from a single geographic region and treated within a single health system, though restricting data to one system reduces institution-level variability and allows us to assess how care may have evolved with growing experience. Aggregating data from numerous health systems that might be at different stages of local outbreaks, provide different quality of care, and contribute different numbers of patients in each period introduces its own biases. We were also unable to disentangle different potential explanatory factors given the observational nature of the study. Residual confounding, such as a higher proportion of particularly frail patients admitted in earlier periods, is also a possibility, though the fact that we observed declines across all age groups mitigates this concern. Thresholds for hospital admission may also have changed over time with less severely ill patients being admitted in the later time periods. While changing admission thresholds could have contributed to higher survival rates in the latter portions of the study, our inclusion of several highly predictive clinical and laboratory results likely captured many aspects of disease severity.
CONCLUSION
In summary, data from one health system suggest that COVID-19 remains a serious disease for high-risk patients, but that mortality rates are improving over time.
1. CDC COVID Data Tracker. 2020. Centers for Disease Control and Prevention. Accessed October 14, 2020. https://covid.cdc.gov/covid-data-tracker/#trends_dailytrendscases
2. Boehmer TK, DeVies J, Caruso E, et al. Changing age distribution of the COVID-19 pandemic - United States, May-August 2020. MMWR Morb Mortal Wkly Rep. 2020;69(39):1404-1409 http://dx.doi.org/0.15585/mmwr.mm6939e1
3. Lu L, Zhong W, Bian Z, et al. A comparison of mortality-related risk factors of COVID-19, SARS, and MERS: A systematic review and meta-analysis. J Infect. 2020;81(4):318-e25. https://doi.org/10.1016/j.jinf.2020.07.002
4. Parohan M, Yaghoubi S, Seraji A, Javanbakht MH, Sarraf P, Djalali M. Risk factors for mortality in patients with coronavirus disease 2019 (COVID-19) infection: a systematic review and meta-analysis of observational studies. Aging Male. 2020;Jun8:1-9. https://doi.org/10.1080/13685538.2020.1774748
5. Zheng Z, Peng F, Xu B, et al. Risk factors of critical & mortal COVID-19 cases: a systematic literature review and meta-analysis. J Infect. 2020;81(2):e16-e25. https://doi.org/10.1016/j.jinf.2020.04.021
6. Petrilli CM, Jones SA, Yang J, et al. Factors associated with hospital admission and critical illness among 5279 people with coronavirus disease 2019 in New York City: prospective cohort study. BMJ. 2020;369:m1966. https://doi.org/10.1136/bmj.m1966
7. margins: Marginal Effects for Model Objects [computer program]. Version R package version 0.3.232018. Accessed October 1, 2020. https://rdrr.io/cran/margins/
8. Greene WH. Econometric Analysis. 7th ed. Pearson; 2012.
9. Doidge JC, Mouncey PR, Thomas K, et al. Trends in intensive care for patients with COVID-19 in England, Wales and Northern Ireland. Preprints 2020. Preprint posted online August 11, 2020. https://doi.org/10.20944/preprints202008.0267.v1
10. Recovery Collaborative Group, Horby P, Lim WS, et al. Dexamethasone in hospitalized patients with Covid-19 - preliminary report. N Engl J Med. 2020. Online first July 17, 2020. https://doi.org/10.1056/NEJMoa2021436
11. Beigel JH, Tomashek KM, Dodd LE, et al. Remdesivir for the treatment of Covid-19 – final report. N Enl J Med. 2020. Online first October 8, 2020. https://doi.org/10.1056/NEJMoa2007764
12. Gandhi M, Rutherford GW. Facial masking for Covid-19 - potential for “variolation” as we await a vaccine. N Engl J Med. 2020. Online first September 8, 2020. https://doi.org/10.1056/NEJMp2026913
1. CDC COVID Data Tracker. 2020. Centers for Disease Control and Prevention. Accessed October 14, 2020. https://covid.cdc.gov/covid-data-tracker/#trends_dailytrendscases
2. Boehmer TK, DeVies J, Caruso E, et al. Changing age distribution of the COVID-19 pandemic - United States, May-August 2020. MMWR Morb Mortal Wkly Rep. 2020;69(39):1404-1409 http://dx.doi.org/0.15585/mmwr.mm6939e1
3. Lu L, Zhong W, Bian Z, et al. A comparison of mortality-related risk factors of COVID-19, SARS, and MERS: A systematic review and meta-analysis. J Infect. 2020;81(4):318-e25. https://doi.org/10.1016/j.jinf.2020.07.002
4. Parohan M, Yaghoubi S, Seraji A, Javanbakht MH, Sarraf P, Djalali M. Risk factors for mortality in patients with coronavirus disease 2019 (COVID-19) infection: a systematic review and meta-analysis of observational studies. Aging Male. 2020;Jun8:1-9. https://doi.org/10.1080/13685538.2020.1774748
5. Zheng Z, Peng F, Xu B, et al. Risk factors of critical & mortal COVID-19 cases: a systematic literature review and meta-analysis. J Infect. 2020;81(2):e16-e25. https://doi.org/10.1016/j.jinf.2020.04.021
6. Petrilli CM, Jones SA, Yang J, et al. Factors associated with hospital admission and critical illness among 5279 people with coronavirus disease 2019 in New York City: prospective cohort study. BMJ. 2020;369:m1966. https://doi.org/10.1136/bmj.m1966
7. margins: Marginal Effects for Model Objects [computer program]. Version R package version 0.3.232018. Accessed October 1, 2020. https://rdrr.io/cran/margins/
8. Greene WH. Econometric Analysis. 7th ed. Pearson; 2012.
9. Doidge JC, Mouncey PR, Thomas K, et al. Trends in intensive care for patients with COVID-19 in England, Wales and Northern Ireland. Preprints 2020. Preprint posted online August 11, 2020. https://doi.org/10.20944/preprints202008.0267.v1
10. Recovery Collaborative Group, Horby P, Lim WS, et al. Dexamethasone in hospitalized patients with Covid-19 - preliminary report. N Engl J Med. 2020. Online first July 17, 2020. https://doi.org/10.1056/NEJMoa2021436
11. Beigel JH, Tomashek KM, Dodd LE, et al. Remdesivir for the treatment of Covid-19 – final report. N Enl J Med. 2020. Online first October 8, 2020. https://doi.org/10.1056/NEJMoa2007764
12. Gandhi M, Rutherford GW. Facial masking for Covid-19 - potential for “variolation” as we await a vaccine. N Engl J Med. 2020. Online first September 8, 2020. https://doi.org/10.1056/NEJMp2026913
© 2020 Society of Hospital Medicine