User login
Don’t Forget the Pulses! Aortoiliac Peripheral Artery Disease Masquerading as Lumbar Radiculopathy—A Report of 3 Cases
Lumbar radiculopathy is a common problem encountered by orthopedic surgeons, and typically presents with lower back or buttock pain radiating down the leg.1 While the most common causes of lumbar radiculopathy are lumbar disc herniation and spinal stenosis, the differential diagnosis for lower extremity pain is broad and can be musculoskeletal, vascular, neurologic, or inflammatory in nature.1,2 Differentiating between orthopedic, neurologic, and vascular causes of leg pain, such as peripheral artery disease (PAD), can sometimes be challenging. This is especially true in aortoiliac PAD, which can present with hip, buttock, and thigh pain. Dorsalis pedis pulses can be palpable due to collateral circulation. A careful history and physical examination is crucial to the correct diagnosis. The history should clearly document the nature of the pain, details of walking impairment, and the alleviating effects of standing still or positional changes. A complete neurovascular examination should include observations regarding the skin, hair, and nails, examination of dorsal pedis, popliteal, and femoral pulses in comparison to the contralateral side, and documentation of dural tension signs. Misdiagnoses can send the patient down a path of unnecessary tests, unindicated procedures, and ultimately, a delay in definitive diagnosis and treatment.1
To our knowledge, this is the first report on a series of patients with thigh pain initially diagnosed as radiculopathy who underwent unproductive diagnostic tests and procedures, and ultimately were given delayed diagnoses of aortoiliac PAD. The patients provided written informed consent for print and electronic publication of these case reports.
Case 1
An 81-year-old woman with a medical history notable for hypertension, hyperlipidemia, and stroke initially presented to an outside orthopedic institution with complaints of several months of lower back and right hip, thigh, and leg pain when walking. She did not report any history of night pain, weakness, or numbness. Examination at the time was notable for painful back extension, 4/5 hip flexion strength on the right compared to 5/5 on the left, but symmetric reflexes and negative dural tension signs. X-rays showed multilevel degenerative disc disease of the lumbar spine, and magnetic resonance imaging (MRI) showed a small L3/4 disc protrusion causing impingement of the L4 nerve root.
A transforaminal epidural steroid injection at the L4 level was performed with minimal resolution of symptoms. Several months later, right-sided intra-articular facet injections were performed at the L4/5 and L5/S1 levels, again with minimal relief of symptoms. At this point, the patient was sent for further physical therapy.
Over a year after symptom onset, the patient presented to our institution and was evaluated by a vascular surgeon. Physical examination was notable for 1+ femoral artery and dorsal pedis pulses on the right side, compared to 2+ on the left. An aortoiliac duplex ultrasound showed severe significant stenosis of the right common iliac artery (>75%).
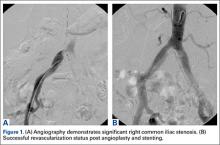
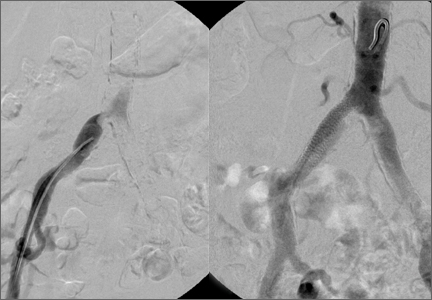
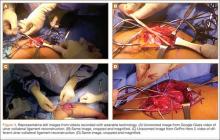
The patient underwent a right common iliac artery angioplasty and stenting (Figures 1A, 1B), which resolved her symptoms.
Case 2
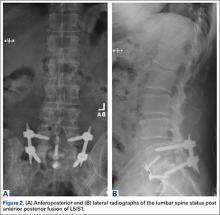
A 65-year-old man, who is a former smoker with a medical history notable for hyperlipidemia and coronary artery disease status post myocardial infarction, presented with a long history of right leg pain. He underwent a L5/S1 anterior posterior fusion at an outside institution and did well for about 5 years after the procedure (Figures 2A, 2B). The pain returned and he underwent several years of physical therapy, epidural steroid injections, and implantation of a spinal cord stimulator with no improvement. He reported right leg pain with minimal back pain, primarily in the thigh and not radiating to the feet and toes. The pain limited him from walking more than 1 block. On examination, strength was 5/5 bilaterally. Pulse examination was notable for lack of dorsalis pedis/posterior tibial pulses bilaterally. He had no bowel or bladder dysfunction.
Computed tomography myelogram showed a moderate amount of stenosis at L3/4 and L4/5. He was sent for evaluation by a vascular surgeon. Arterial duplex ultrasound showed significant stenosis of the right common iliac artery.
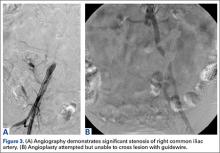
Angioplasty was attempted but vascular surgery was unable to cross the lesion (Figures 3A, 3B), and the patient ultimately had a femoral-femoral bypass, which resolved his leg pain.
Case 3
A 78-year-old woman, nonsmoker, presented with a 1-year history of left buttock and thigh pain exacerbated by ambulation. Ambulation was limited to 2 blocks. The patient was being worked up for spinal and hip etiologies of pain at an outside hospital. MRI revealed a mild posterior disc herniation at L3/4 and L4/5 and moderate narrowing of the spinal canal. She underwent 2 epidural steroid injections with no improvement. The patient’s relative, a physician, suggested that the patient receive a vascular surgery consultation, and the patient ultimately presented to our institution for evaluation by vascular surgery.
The physical examination was significant for a 1+ dorsal pedis pulse on the left compared to 2+ on the right. Moreover, the patient only demonstrated trace L femoral pulse compared to the right. Strength was 5/5 bilaterally.
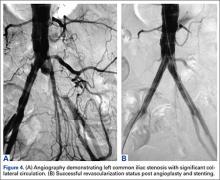
The patient was taken to the operating room for angioplasty and stenting of the left common iliac artery (Figures 4A, 4B). This provided immediate symptom relief, and she has remained asymptomatic.
Discussion
Lumbar radiculopathy is a common diagnosis encountered by orthopedic surgeons. Although the diagnosis can appear to be straightforward in a patient presenting with lower back and leg pain, the etiology of lower back and leg pain can be extremely varied, and can be musculoskeletal, neurologic, vascular, rheumatologic, or oncologic in origin.1 In particular, differentiating between radiculopathy and vascular claudication can sometimes be challenging.
The 2 most common causes of lumbar radiculopathy are lumbar disc herniation and spinal stenosis.1 Lumbar disc herniation results from tear in the annulus of the intervertebral disc, resulting in herniation of disc material into the spinal canal causing compression and irritation of spinal nerve roots.1 Spinal stenosis is narrowing of the spinal canal that produces compression of neural elements before they exit the neural foramen.3 Adult degenerative spinal stenosis is most often caused by osteophytes from the facet joints or hypertrophy of the ligamentum flavum, and can be broadly categorized into central spinal stenosis or lateral spinal stenosis.
PAD is defined as progressive stenosis or occlusion, or aneurysmal dilation of noncoronary arteries.2 When PAD affects the vessels of the lower extremities, the symptoms typically manifest as intermittent claudication, which is exercise-induced ischemic pain in the lower extremity that is relieved by rest.2 As the disease progresses, symptoms can progress to rest pain, ulceration, and, eventually, gangrene. The most common cause of PAD is atherosclerosis, and the risk factors include smoking, hypertension, diabetes, and hyperlipidemia. The prevalence of PAD rises sharply with age, starting from <3% in ages less than 60 years to >20% in ages 75 years and older.4
A detailed and pertinent history from the patient provides important information for differentiating radiculopathy and neurogenic claudication from vascular claudication. Patients with lumbar radiculopathy typically report pain in the lower back radiating down the leg past the knee in a dermatomal distribution. The pain often begins soon if not immediately after activity, but often takes time for relief onset after rest. Positional changes in the back such as flexion can provide relief.2 Patients with neurogenic claudication from central spinal stenosis can present with bilateral thigh pain from prolonged standing and activity that is alleviated with flexion or stooping.3 Patients may admit to a positive “shopping cart sign,” with increased walking comfort stooped forward with hands on a shopping cart.
In contrast, patients with vascular claudication often report pain in the calf, thigh, or hip, but rarely in the foot. The location of pain varies with area of stenosis; generally, patients with superficial femoral artery occlusion present with calf claudication, while patients with aortoiliac disease present with buttock and thigh pain. The pain typically occurs after a very reproducible length of walking, and is relieved by cessation of walking, often even if the patient remains standing. Back positioning should have no effect on the pain.2-5
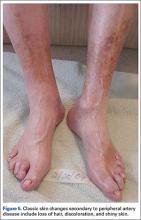
Physical examination should begin with observation of the patient’s gait and posture, which may be hunched over in the setting of spinal stenosis. Examination of the patient’s skin may show loss of hair, shiny skin, or atrophic changes suggestive of vascular disease (Figure 5).1 Prior to proceeding to a spine examination, palpating the trochanteric bursa and testing for hip range of motion is important to rule out intra-articular hip pathology and trochanteric bursitis as common causes of pain in the area. Patients with radiculopathy may show sensory disturbances in a dermatomal distribution, muscular weakness at the corresponding spinal level, and decreased deep tendon reflexes. The straight leg raise test can elicit signs of nerve root tension. A careful examination of bilateral lower extremity pulses at the dorsal pedis, popliteal, and femoral levels can help identify any asymmetric or decreased pulses that would indicate peripheral vascular disease. With chronic aortoiliac disease, it is important to check for femoral pulses, given the dorsal pedis pulse can be present due to collateral circulation. And finally, the ankle brachial index (ABI), measured as the ratio of the systolic pressure at the ankle divided by the systolic pressure at the arm, is a good screening test for PAD.6 A normal ABI is >1.
A thorough history and physical examination can elicit important information that is helpful in evaluating orthopedic patients, especially to differentiate between spinal and vascular causes of leg pain. This can help avoid misdiagnoses, which result in unnecessary tests, procedures, and wasted time. Don’t forget the pulses!
1. Grimm BD, Blessinger BJ, Darden BV, Brigham CD, Kneisl JS, Laxer EB. Mimickers of lumbar radiculopathy. J Am Acad Orthop Surg. 2015;23(1):7-17.
2. Hirsch AT, Haskal ZJ, Hertzer NR, et al. ACC/AHA Guidelines for the Management of Patients with Peripheral Arterial Disease (lower extremity, renal, mesenteric, and abdominal aortic): a collaborative report from the American Associations for Vascular Surgery/Society for Vascular Surgery, Society for Cardiovascular Angiography and Interventions, Society for Vascular Medicine and Biology, Society of Interventional Radiology, and the ACC/AHA Task Force on Practice Guidelines (writing committee to develop guidelines for the management of patients with peripheral arterial disease)--summary of recommendations. J Vasc Interv Radiol. 2006;17(9):1383-1397.
3. Spivak JM. Degenerative lumbar spinal stenosis. J Bone Joint Surg Am. 1998;80(7):1053-1066.
4. Criqui MH, Fronek A, Barrett-Connor E, Klauber MR, Gabriel S, Goodman D. The prevalence of peripheral arterial disease in a defined population. Circulation. 1985;71(3):510-515.
5. Ouriel K. Peripheral arterial disease. Lancet. 2001;358(9289):1257-1264.
6. Jeon CH, Han SH, Chung NS, Hyun HS. The validity of ankle-brachial index for the differential diagnosis of peripheral arterial disease and lumbar spinal stenosis in patients with atypical claudication. Eur Spine J. 2012;21(6):1165-1170.
Lumbar radiculopathy is a common problem encountered by orthopedic surgeons, and typically presents with lower back or buttock pain radiating down the leg.1 While the most common causes of lumbar radiculopathy are lumbar disc herniation and spinal stenosis, the differential diagnosis for lower extremity pain is broad and can be musculoskeletal, vascular, neurologic, or inflammatory in nature.1,2 Differentiating between orthopedic, neurologic, and vascular causes of leg pain, such as peripheral artery disease (PAD), can sometimes be challenging. This is especially true in aortoiliac PAD, which can present with hip, buttock, and thigh pain. Dorsalis pedis pulses can be palpable due to collateral circulation. A careful history and physical examination is crucial to the correct diagnosis. The history should clearly document the nature of the pain, details of walking impairment, and the alleviating effects of standing still or positional changes. A complete neurovascular examination should include observations regarding the skin, hair, and nails, examination of dorsal pedis, popliteal, and femoral pulses in comparison to the contralateral side, and documentation of dural tension signs. Misdiagnoses can send the patient down a path of unnecessary tests, unindicated procedures, and ultimately, a delay in definitive diagnosis and treatment.1
To our knowledge, this is the first report on a series of patients with thigh pain initially diagnosed as radiculopathy who underwent unproductive diagnostic tests and procedures, and ultimately were given delayed diagnoses of aortoiliac PAD. The patients provided written informed consent for print and electronic publication of these case reports.
Case 1
An 81-year-old woman with a medical history notable for hypertension, hyperlipidemia, and stroke initially presented to an outside orthopedic institution with complaints of several months of lower back and right hip, thigh, and leg pain when walking. She did not report any history of night pain, weakness, or numbness. Examination at the time was notable for painful back extension, 4/5 hip flexion strength on the right compared to 5/5 on the left, but symmetric reflexes and negative dural tension signs. X-rays showed multilevel degenerative disc disease of the lumbar spine, and magnetic resonance imaging (MRI) showed a small L3/4 disc protrusion causing impingement of the L4 nerve root.
A transforaminal epidural steroid injection at the L4 level was performed with minimal resolution of symptoms. Several months later, right-sided intra-articular facet injections were performed at the L4/5 and L5/S1 levels, again with minimal relief of symptoms. At this point, the patient was sent for further physical therapy.
Over a year after symptom onset, the patient presented to our institution and was evaluated by a vascular surgeon. Physical examination was notable for 1+ femoral artery and dorsal pedis pulses on the right side, compared to 2+ on the left. An aortoiliac duplex ultrasound showed severe significant stenosis of the right common iliac artery (>75%).
The patient underwent a right common iliac artery angioplasty and stenting (Figures 1A, 1B), which resolved her symptoms.
Case 2
A 65-year-old man, who is a former smoker with a medical history notable for hyperlipidemia and coronary artery disease status post myocardial infarction, presented with a long history of right leg pain. He underwent a L5/S1 anterior posterior fusion at an outside institution and did well for about 5 years after the procedure (Figures 2A, 2B). The pain returned and he underwent several years of physical therapy, epidural steroid injections, and implantation of a spinal cord stimulator with no improvement. He reported right leg pain with minimal back pain, primarily in the thigh and not radiating to the feet and toes. The pain limited him from walking more than 1 block. On examination, strength was 5/5 bilaterally. Pulse examination was notable for lack of dorsalis pedis/posterior tibial pulses bilaterally. He had no bowel or bladder dysfunction.
Computed tomography myelogram showed a moderate amount of stenosis at L3/4 and L4/5. He was sent for evaluation by a vascular surgeon. Arterial duplex ultrasound showed significant stenosis of the right common iliac artery.
Angioplasty was attempted but vascular surgery was unable to cross the lesion (Figures 3A, 3B), and the patient ultimately had a femoral-femoral bypass, which resolved his leg pain.
Case 3
A 78-year-old woman, nonsmoker, presented with a 1-year history of left buttock and thigh pain exacerbated by ambulation. Ambulation was limited to 2 blocks. The patient was being worked up for spinal and hip etiologies of pain at an outside hospital. MRI revealed a mild posterior disc herniation at L3/4 and L4/5 and moderate narrowing of the spinal canal. She underwent 2 epidural steroid injections with no improvement. The patient’s relative, a physician, suggested that the patient receive a vascular surgery consultation, and the patient ultimately presented to our institution for evaluation by vascular surgery.
The physical examination was significant for a 1+ dorsal pedis pulse on the left compared to 2+ on the right. Moreover, the patient only demonstrated trace L femoral pulse compared to the right. Strength was 5/5 bilaterally.
The patient was taken to the operating room for angioplasty and stenting of the left common iliac artery (Figures 4A, 4B). This provided immediate symptom relief, and she has remained asymptomatic.
Discussion
Lumbar radiculopathy is a common diagnosis encountered by orthopedic surgeons. Although the diagnosis can appear to be straightforward in a patient presenting with lower back and leg pain, the etiology of lower back and leg pain can be extremely varied, and can be musculoskeletal, neurologic, vascular, rheumatologic, or oncologic in origin.1 In particular, differentiating between radiculopathy and vascular claudication can sometimes be challenging.
The 2 most common causes of lumbar radiculopathy are lumbar disc herniation and spinal stenosis.1 Lumbar disc herniation results from tear in the annulus of the intervertebral disc, resulting in herniation of disc material into the spinal canal causing compression and irritation of spinal nerve roots.1 Spinal stenosis is narrowing of the spinal canal that produces compression of neural elements before they exit the neural foramen.3 Adult degenerative spinal stenosis is most often caused by osteophytes from the facet joints or hypertrophy of the ligamentum flavum, and can be broadly categorized into central spinal stenosis or lateral spinal stenosis.
PAD is defined as progressive stenosis or occlusion, or aneurysmal dilation of noncoronary arteries.2 When PAD affects the vessels of the lower extremities, the symptoms typically manifest as intermittent claudication, which is exercise-induced ischemic pain in the lower extremity that is relieved by rest.2 As the disease progresses, symptoms can progress to rest pain, ulceration, and, eventually, gangrene. The most common cause of PAD is atherosclerosis, and the risk factors include smoking, hypertension, diabetes, and hyperlipidemia. The prevalence of PAD rises sharply with age, starting from <3% in ages less than 60 years to >20% in ages 75 years and older.4
A detailed and pertinent history from the patient provides important information for differentiating radiculopathy and neurogenic claudication from vascular claudication. Patients with lumbar radiculopathy typically report pain in the lower back radiating down the leg past the knee in a dermatomal distribution. The pain often begins soon if not immediately after activity, but often takes time for relief onset after rest. Positional changes in the back such as flexion can provide relief.2 Patients with neurogenic claudication from central spinal stenosis can present with bilateral thigh pain from prolonged standing and activity that is alleviated with flexion or stooping.3 Patients may admit to a positive “shopping cart sign,” with increased walking comfort stooped forward with hands on a shopping cart.
In contrast, patients with vascular claudication often report pain in the calf, thigh, or hip, but rarely in the foot. The location of pain varies with area of stenosis; generally, patients with superficial femoral artery occlusion present with calf claudication, while patients with aortoiliac disease present with buttock and thigh pain. The pain typically occurs after a very reproducible length of walking, and is relieved by cessation of walking, often even if the patient remains standing. Back positioning should have no effect on the pain.2-5
Physical examination should begin with observation of the patient’s gait and posture, which may be hunched over in the setting of spinal stenosis. Examination of the patient’s skin may show loss of hair, shiny skin, or atrophic changes suggestive of vascular disease (Figure 5).1 Prior to proceeding to a spine examination, palpating the trochanteric bursa and testing for hip range of motion is important to rule out intra-articular hip pathology and trochanteric bursitis as common causes of pain in the area. Patients with radiculopathy may show sensory disturbances in a dermatomal distribution, muscular weakness at the corresponding spinal level, and decreased deep tendon reflexes. The straight leg raise test can elicit signs of nerve root tension. A careful examination of bilateral lower extremity pulses at the dorsal pedis, popliteal, and femoral levels can help identify any asymmetric or decreased pulses that would indicate peripheral vascular disease. With chronic aortoiliac disease, it is important to check for femoral pulses, given the dorsal pedis pulse can be present due to collateral circulation. And finally, the ankle brachial index (ABI), measured as the ratio of the systolic pressure at the ankle divided by the systolic pressure at the arm, is a good screening test for PAD.6 A normal ABI is >1.
A thorough history and physical examination can elicit important information that is helpful in evaluating orthopedic patients, especially to differentiate between spinal and vascular causes of leg pain. This can help avoid misdiagnoses, which result in unnecessary tests, procedures, and wasted time. Don’t forget the pulses!
Lumbar radiculopathy is a common problem encountered by orthopedic surgeons, and typically presents with lower back or buttock pain radiating down the leg.1 While the most common causes of lumbar radiculopathy are lumbar disc herniation and spinal stenosis, the differential diagnosis for lower extremity pain is broad and can be musculoskeletal, vascular, neurologic, or inflammatory in nature.1,2 Differentiating between orthopedic, neurologic, and vascular causes of leg pain, such as peripheral artery disease (PAD), can sometimes be challenging. This is especially true in aortoiliac PAD, which can present with hip, buttock, and thigh pain. Dorsalis pedis pulses can be palpable due to collateral circulation. A careful history and physical examination is crucial to the correct diagnosis. The history should clearly document the nature of the pain, details of walking impairment, and the alleviating effects of standing still or positional changes. A complete neurovascular examination should include observations regarding the skin, hair, and nails, examination of dorsal pedis, popliteal, and femoral pulses in comparison to the contralateral side, and documentation of dural tension signs. Misdiagnoses can send the patient down a path of unnecessary tests, unindicated procedures, and ultimately, a delay in definitive diagnosis and treatment.1
To our knowledge, this is the first report on a series of patients with thigh pain initially diagnosed as radiculopathy who underwent unproductive diagnostic tests and procedures, and ultimately were given delayed diagnoses of aortoiliac PAD. The patients provided written informed consent for print and electronic publication of these case reports.
Case 1
An 81-year-old woman with a medical history notable for hypertension, hyperlipidemia, and stroke initially presented to an outside orthopedic institution with complaints of several months of lower back and right hip, thigh, and leg pain when walking. She did not report any history of night pain, weakness, or numbness. Examination at the time was notable for painful back extension, 4/5 hip flexion strength on the right compared to 5/5 on the left, but symmetric reflexes and negative dural tension signs. X-rays showed multilevel degenerative disc disease of the lumbar spine, and magnetic resonance imaging (MRI) showed a small L3/4 disc protrusion causing impingement of the L4 nerve root.
A transforaminal epidural steroid injection at the L4 level was performed with minimal resolution of symptoms. Several months later, right-sided intra-articular facet injections were performed at the L4/5 and L5/S1 levels, again with minimal relief of symptoms. At this point, the patient was sent for further physical therapy.
Over a year after symptom onset, the patient presented to our institution and was evaluated by a vascular surgeon. Physical examination was notable for 1+ femoral artery and dorsal pedis pulses on the right side, compared to 2+ on the left. An aortoiliac duplex ultrasound showed severe significant stenosis of the right common iliac artery (>75%).
The patient underwent a right common iliac artery angioplasty and stenting (Figures 1A, 1B), which resolved her symptoms.
Case 2
A 65-year-old man, who is a former smoker with a medical history notable for hyperlipidemia and coronary artery disease status post myocardial infarction, presented with a long history of right leg pain. He underwent a L5/S1 anterior posterior fusion at an outside institution and did well for about 5 years after the procedure (Figures 2A, 2B). The pain returned and he underwent several years of physical therapy, epidural steroid injections, and implantation of a spinal cord stimulator with no improvement. He reported right leg pain with minimal back pain, primarily in the thigh and not radiating to the feet and toes. The pain limited him from walking more than 1 block. On examination, strength was 5/5 bilaterally. Pulse examination was notable for lack of dorsalis pedis/posterior tibial pulses bilaterally. He had no bowel or bladder dysfunction.
Computed tomography myelogram showed a moderate amount of stenosis at L3/4 and L4/5. He was sent for evaluation by a vascular surgeon. Arterial duplex ultrasound showed significant stenosis of the right common iliac artery.
Angioplasty was attempted but vascular surgery was unable to cross the lesion (Figures 3A, 3B), and the patient ultimately had a femoral-femoral bypass, which resolved his leg pain.
Case 3
A 78-year-old woman, nonsmoker, presented with a 1-year history of left buttock and thigh pain exacerbated by ambulation. Ambulation was limited to 2 blocks. The patient was being worked up for spinal and hip etiologies of pain at an outside hospital. MRI revealed a mild posterior disc herniation at L3/4 and L4/5 and moderate narrowing of the spinal canal. She underwent 2 epidural steroid injections with no improvement. The patient’s relative, a physician, suggested that the patient receive a vascular surgery consultation, and the patient ultimately presented to our institution for evaluation by vascular surgery.
The physical examination was significant for a 1+ dorsal pedis pulse on the left compared to 2+ on the right. Moreover, the patient only demonstrated trace L femoral pulse compared to the right. Strength was 5/5 bilaterally.
The patient was taken to the operating room for angioplasty and stenting of the left common iliac artery (Figures 4A, 4B). This provided immediate symptom relief, and she has remained asymptomatic.
Discussion
Lumbar radiculopathy is a common diagnosis encountered by orthopedic surgeons. Although the diagnosis can appear to be straightforward in a patient presenting with lower back and leg pain, the etiology of lower back and leg pain can be extremely varied, and can be musculoskeletal, neurologic, vascular, rheumatologic, or oncologic in origin.1 In particular, differentiating between radiculopathy and vascular claudication can sometimes be challenging.
The 2 most common causes of lumbar radiculopathy are lumbar disc herniation and spinal stenosis.1 Lumbar disc herniation results from tear in the annulus of the intervertebral disc, resulting in herniation of disc material into the spinal canal causing compression and irritation of spinal nerve roots.1 Spinal stenosis is narrowing of the spinal canal that produces compression of neural elements before they exit the neural foramen.3 Adult degenerative spinal stenosis is most often caused by osteophytes from the facet joints or hypertrophy of the ligamentum flavum, and can be broadly categorized into central spinal stenosis or lateral spinal stenosis.
PAD is defined as progressive stenosis or occlusion, or aneurysmal dilation of noncoronary arteries.2 When PAD affects the vessels of the lower extremities, the symptoms typically manifest as intermittent claudication, which is exercise-induced ischemic pain in the lower extremity that is relieved by rest.2 As the disease progresses, symptoms can progress to rest pain, ulceration, and, eventually, gangrene. The most common cause of PAD is atherosclerosis, and the risk factors include smoking, hypertension, diabetes, and hyperlipidemia. The prevalence of PAD rises sharply with age, starting from <3% in ages less than 60 years to >20% in ages 75 years and older.4
A detailed and pertinent history from the patient provides important information for differentiating radiculopathy and neurogenic claudication from vascular claudication. Patients with lumbar radiculopathy typically report pain in the lower back radiating down the leg past the knee in a dermatomal distribution. The pain often begins soon if not immediately after activity, but often takes time for relief onset after rest. Positional changes in the back such as flexion can provide relief.2 Patients with neurogenic claudication from central spinal stenosis can present with bilateral thigh pain from prolonged standing and activity that is alleviated with flexion or stooping.3 Patients may admit to a positive “shopping cart sign,” with increased walking comfort stooped forward with hands on a shopping cart.
In contrast, patients with vascular claudication often report pain in the calf, thigh, or hip, but rarely in the foot. The location of pain varies with area of stenosis; generally, patients with superficial femoral artery occlusion present with calf claudication, while patients with aortoiliac disease present with buttock and thigh pain. The pain typically occurs after a very reproducible length of walking, and is relieved by cessation of walking, often even if the patient remains standing. Back positioning should have no effect on the pain.2-5
Physical examination should begin with observation of the patient’s gait and posture, which may be hunched over in the setting of spinal stenosis. Examination of the patient’s skin may show loss of hair, shiny skin, or atrophic changes suggestive of vascular disease (Figure 5).1 Prior to proceeding to a spine examination, palpating the trochanteric bursa and testing for hip range of motion is important to rule out intra-articular hip pathology and trochanteric bursitis as common causes of pain in the area. Patients with radiculopathy may show sensory disturbances in a dermatomal distribution, muscular weakness at the corresponding spinal level, and decreased deep tendon reflexes. The straight leg raise test can elicit signs of nerve root tension. A careful examination of bilateral lower extremity pulses at the dorsal pedis, popliteal, and femoral levels can help identify any asymmetric or decreased pulses that would indicate peripheral vascular disease. With chronic aortoiliac disease, it is important to check for femoral pulses, given the dorsal pedis pulse can be present due to collateral circulation. And finally, the ankle brachial index (ABI), measured as the ratio of the systolic pressure at the ankle divided by the systolic pressure at the arm, is a good screening test for PAD.6 A normal ABI is >1.
A thorough history and physical examination can elicit important information that is helpful in evaluating orthopedic patients, especially to differentiate between spinal and vascular causes of leg pain. This can help avoid misdiagnoses, which result in unnecessary tests, procedures, and wasted time. Don’t forget the pulses!
1. Grimm BD, Blessinger BJ, Darden BV, Brigham CD, Kneisl JS, Laxer EB. Mimickers of lumbar radiculopathy. J Am Acad Orthop Surg. 2015;23(1):7-17.
2. Hirsch AT, Haskal ZJ, Hertzer NR, et al. ACC/AHA Guidelines for the Management of Patients with Peripheral Arterial Disease (lower extremity, renal, mesenteric, and abdominal aortic): a collaborative report from the American Associations for Vascular Surgery/Society for Vascular Surgery, Society for Cardiovascular Angiography and Interventions, Society for Vascular Medicine and Biology, Society of Interventional Radiology, and the ACC/AHA Task Force on Practice Guidelines (writing committee to develop guidelines for the management of patients with peripheral arterial disease)--summary of recommendations. J Vasc Interv Radiol. 2006;17(9):1383-1397.
3. Spivak JM. Degenerative lumbar spinal stenosis. J Bone Joint Surg Am. 1998;80(7):1053-1066.
4. Criqui MH, Fronek A, Barrett-Connor E, Klauber MR, Gabriel S, Goodman D. The prevalence of peripheral arterial disease in a defined population. Circulation. 1985;71(3):510-515.
5. Ouriel K. Peripheral arterial disease. Lancet. 2001;358(9289):1257-1264.
6. Jeon CH, Han SH, Chung NS, Hyun HS. The validity of ankle-brachial index for the differential diagnosis of peripheral arterial disease and lumbar spinal stenosis in patients with atypical claudication. Eur Spine J. 2012;21(6):1165-1170.
1. Grimm BD, Blessinger BJ, Darden BV, Brigham CD, Kneisl JS, Laxer EB. Mimickers of lumbar radiculopathy. J Am Acad Orthop Surg. 2015;23(1):7-17.
2. Hirsch AT, Haskal ZJ, Hertzer NR, et al. ACC/AHA Guidelines for the Management of Patients with Peripheral Arterial Disease (lower extremity, renal, mesenteric, and abdominal aortic): a collaborative report from the American Associations for Vascular Surgery/Society for Vascular Surgery, Society for Cardiovascular Angiography and Interventions, Society for Vascular Medicine and Biology, Society of Interventional Radiology, and the ACC/AHA Task Force on Practice Guidelines (writing committee to develop guidelines for the management of patients with peripheral arterial disease)--summary of recommendations. J Vasc Interv Radiol. 2006;17(9):1383-1397.
3. Spivak JM. Degenerative lumbar spinal stenosis. J Bone Joint Surg Am. 1998;80(7):1053-1066.
4. Criqui MH, Fronek A, Barrett-Connor E, Klauber MR, Gabriel S, Goodman D. The prevalence of peripheral arterial disease in a defined population. Circulation. 1985;71(3):510-515.
5. Ouriel K. Peripheral arterial disease. Lancet. 2001;358(9289):1257-1264.
6. Jeon CH, Han SH, Chung NS, Hyun HS. The validity of ankle-brachial index for the differential diagnosis of peripheral arterial disease and lumbar spinal stenosis in patients with atypical claudication. Eur Spine J. 2012;21(6):1165-1170.
Lateral Ulnar Collateral Ligament Reconstruction: An Analysis of Ulnar Tunnel Locations
Posterolateral rotatory instability (PLRI) of the elbow is well recognized1 and is the most common type of chronic elbow instability. PLRI is often an end result of traumatic elbow dislocation.2 The “essential lesion” in patients with PLRI of the elbow is injury to the lateral ulnar collateral ligament (LUCL).1 However, more recent research has emphasized the importance of other ligaments in the lateral ligament complex (radial collateral and annular ligaments) in preventing PLRI.3-5 Nevertheless, when conservative treatment fails, the most commonly used surgical treatment involves LUCL reconstruction.1,6-11
Numerous techniques for LUCL reconstruction have been described.1,7-9,11-13 The chosen technique ideally restores normal anatomy. Therefore, the isometric point of origin at the lateral epicondyle and insertion at the supinator tubercle are important landmarks for creating tunnels that reproduce isometry, function, and normal anatomy. Most often, 2 tunnels are created in the ulna to secure the graft. It has been our experience that ulnar tunnel creation can affect the length of the bony bridge and the orientation of the graft.
We conducted a study to identify the precise proximal ulna tunnel location—anterior to posterior, with the distal tunnel at the supinator tubercle on the crest—that allows for the largest bony bridge and most geometrically favorable construct. We hypothesized that a most posteriorly placed proximal tunnel would increase bony bridge size and allow for a more isosceles graft configuration. An isosceles configuration with the humerus tunnel at the isometric location would allow for anterior and posterior bands of the same length with theoretically equal force distribution.
Methods
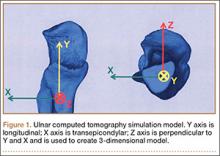
After obtaining institutional review board approval, we retrospectively reviewed the cases of 17 adults with elbow computed tomography (CT) scans for inclusion in this study. The scans were previously performed for diagnostic workup of several pathologies, including valgus instability, olecranon stress fracture, and valgus extension overload. The scan protocol involved 0.5-mm axial cuts with inclusion of the distal humerus through the proximal radius and ulna in the DICOM (Digital Imaging and Communications in Medicine) format. Exclusion criteria included poor CT quality, inadequate visualization of the entire supinator crest, and age under 18 years. Fifteen patients with adequate CT scans met the inclusion criteria. MIMICS (Materialise’s Interactive Medical Image Control System) software was used to convert scans into patient-specific 3-dimensional (3-D) computer models. (Use of this software to produce anatomically accurate models has been verified in shoulder14 and elbow15 models.) These models were uploaded into Magics rapid prototyping software (Materialise) and manipulated for simulated tunnel drilling by precise bone subtraction methods. This software was used to define an ulnar Cartesian coordinate system with anatomical landmarks as reference points in order to standardize the position of each model (Figure 1).16 The y-axis was defined by the longitudinal axis of the ulna, and the x-axis was the transepicondylar axis, defined as the perpendicular line connecting the y-axis with the supinator crest. The z-axis was then established as the line perpendicular to the x- and y-axes—yielding a 3-D coordinate system that allowed us to manipulate the models in standardized fashion, maintaining the exact positions of the ulna while making measurements.
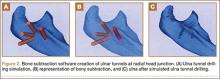
Surgical simulations were performed in the rapid prototyping software by creating a cylinder and placing it at the desired location of each tunnel. Cylinder diameter was 4 mm, matching the diameter of the drill we use to create each tunnel in our practice. The cylinder was inserted into the bone, perpendicular to the surface of the ulna at the point of insertion, so the cylinder’s deepest point entered the medullary canal of the ulna. Using a Boolean operation in the rapid prototyping software, we subtracted cylinder from bone to create a tunnel (Figure 2).15
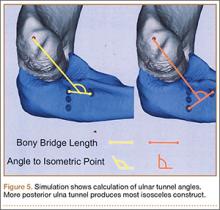
In a previous study,17 we determined that the radial head junction is reproducibly about 15 mm proximal to the distinct supinator tubercle, which may be absent or not readily appreciated in up to 50% of cases. Therefore, proximal ulnar tunnels were placed 0, 5, and 10 mm posterior to the supinator crest at the radial head junction. Distal tunnels were placed 15 mm anterior to the radial head junction on the supinator crest (Figure 2). The bony bridges created by these tunnels were measured, as was the distance between the distal tunnel and the supinator tubercle.
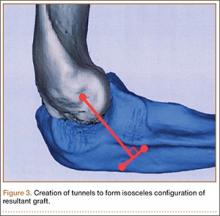
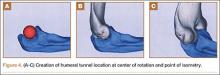
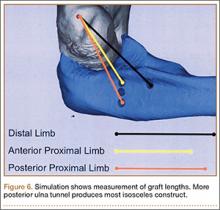
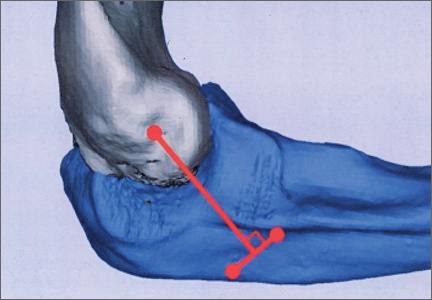
Ideal graft configuration was described as an isosceles triangle with ulna tunnels perpendicular to the humeral tunnel (Figure 3).11 Location of the humeral origin in the sagittal plane was determined by finding the isometric point of the lateral humerus using only bony landmarks. Similar techniques have been used to find the isometric point on the medial epicondyle for medial ulnar collateral ligament reconstruction.15,18 With a circle fit into the trochlear notch of the ulna, the isometric point can be determined by the center of the circle. This point was then superimposed on the humerus to identify the starting point (Figure 4). In our simulation, we measured the isosceles configuration by drawing a line between the proximal and distal tunnels, and then another line connecting the bisecting point of the first line with the isometric point on the humerus from which the graft would originate. The angle between the 2 lines was measured; if isosceles, the angle was 90° (Figure 5). Length of the more proximal limb of the graft and the more distal limb of the graft was determined by measuring the distance from the isometric point to the proximal and distal tunnels, respectively (Figure 6).
One-way analysis of variance was used to compare all the tunnels’ bony bridge sizes, graft lengths, and angles to the isometric point. For all comparisons, statistical significance was set at P < .05. As no other studies have compared bony bridges by varying tunnel creation parameters, and as the present study is observational and not comparative, no power analysis was performed.
Results
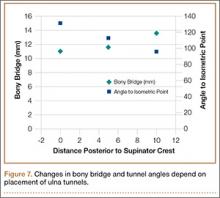
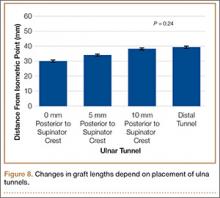
Bony bridges were significantly longer, and angles more perpendicular, with increasing distance from the proximal tunnel to the supinator crest (Table 1, Figure 5, Figure 7). The bony bridge 0 mm posterior to the supinator crest yielded a mean (SE) bony bridge length of 11.0 (0.2) mm. This proximal tunnel also yielded the smallest mean (SE) perpendicular angle to the isometric point, 131.2° (1.9°). The tunnel most posterior to the supinator crest yielded the longest mean (SE) bony bridge, 13.7 (0.2) mm, and the largest mean (SE) degree of perpendicularity, 95.8° (1.4°). The differences between all tunnels’ bony bridges and isometric angles were statistically significant (P < .00001). The difference between the more distal limb and the more proximal limb of the graft was smallest in the more posteriorly placed proximal tunnel (Table 2, Figure 8). In fact, there was no statistical difference between the proximal and distal limbs of the graft when the proximal tunnel was placed 10 mm posterior to the supinator crest: Mean (SE) was 9.4 (0.5) mm at 0 mm (P < .00001) and 1.1 (0.6) mm at 10 mm (P = .24).
Discussion
PLRI of the elbow is best initially managed nonoperatively. However, when nonoperative management fails, the LUCL is often surgically reconstructed. Reconstruction methods vary by fixation method, graft choice, and bone tunnels.1,7-9,11-13 In 1991, O’Driscoll and colleagues1 described a “yoke” technique for LUCL reconstruction. Since then, the docking technique7 and other techniques have been developed. All these techniques emphasize maximizing anatomical precision and isometry with careful placement of tunnels or fixation devices. The humeral fixation site, at the anterior inferior aspect of the lateral epicondyle at the point of isometry, can be accessed relatively reproducibly. By contrast, the ulnar points of fixation are more variable, because of increased bone stock and overlying soft-tissue and bony anatomy.
Among the challenges in determining the points of ulnar fixation is the bony anatomy that is often used for landmarks. In the literature, the supinator crest or the supintor tubercle is the landmark for placing the distal tunnel.1,7-9,11-13 This is a problem for 2 reasons. First, the supintor crest, a longitudinal structure on the lateral aspect of the ulna, originates from the radial head junction and extends tens of millimeters distally; further specification is needed to guide these ulnar tunnels. The second reason is that use of the supinator tubercle, a prominence on the supinator crest, adds specificity to the location of the ulnar tunnels. During surgery, however, the supinator tubercle may not be a reliable, independently prominent structure; instead, it may be indistinguishable from the supinator crest, on which it rests. One study determined that only about 50% of computer models of patient ulnas had a distinct prominence that could be classified as the supinator tubercle.17 The percentage presumably is lower during surgery, with limited exposure and overlying soft tissues.
In a study of patients with a prominent tubercle, mean (SE) distance from radial head junction to tubercle was 15 (2) mm.17 This finding led us to use the radial head junction as the primary bony landmark in determining the location of the proximal tunnel and placing the distal tunnel 15 mm distally—achieving the same fixation described in the literature but using more distinct landmarks. Our study thus provided a reliable, verified approach to locating the ulnar tunnels in the proximal-distal axis.
We also explored the anterior-posterior orientation of the proximal ulnar tunnel. The 2 primary considerations surrounding the varied proximal tunnel placements were the bony bridge formed between the proximal and distal tunnels and the perpendicularity of the triangle formed by the fixation points. Maximizing the bony bridge is obviously ideal in securing and preventing fixation blowout. Achieving an isoceles reconstruction has been reported in the literature on the various fixation techniques for LUCL reconstruction.11 Although the biomechanical advantage of this fixation type is not fully clear, we assume the construct produces graft stands of equal length, tension, and stability. In addition, the larger footprint created by an isoceles reconstructed ligament increases the stability of the radial head.
Results of the present study showed that the more posterior the proximal ulnar tunnel, the longer the bony bridge and the more isoceles the reconstruction. The difference in bony bridge distance from the most anterior to the most posterior tunnel was about 2 mm, or 18%. For every 1 mm of posteriorization, the bony bridge was 0.2 mm longer. The line from the isometric point of humeral fixation bisecting the proximal and distal tunnels was also more perpendicular with the most posterior tunnel, by about 40°. The resulting proximal and distal limbs of the reconstruction were equal in length, as demonstrated by the smaller difference between the limbs. We assume this isoceles reconstruction more likely applies uniform restraint on the radial head. Thus, an effort should be made to posteriorize the proximal ulnar tunnel during reconstruction.
The study was limited by the number of patient-specific elbow models used. However, given the statistical consistency of measurements, sample size was sufficient. Another limitation, inherent to the model, was that only bony anatomy was incorporated. However, the overlying muscles, tendons, and ligaments can significantly alter tunnel placement, and this study provided other means and cues using more reliable landmarks to adequately place the tunnels. As this was a simulation study, we cannot confirm whether these results would make a difference clinically. The strengths of this study include development and verification of reliable landmarks that can be used to guide ulnar tunnel locations during LUCL reconstruction; these landmarks have been used for medial ulnar collateral ligament reconstruction.15 Other strengths include precise and accurate placement of tunnels and measurement of resulting bony bridges—accomplished independently and without compromising specimen quality.
Conclusion
We recommend drilling the proximal ulnar tunnel posterior to the supinator crest at the level of the radial head junction. A reasonable goal is 10 mm posterior to the crest, though the overlying soft tissue must be considered, and care should be taken to aim the drill anteriorly, toward the ulna’s intramedullary canal, to avoid posterior cortical breach. The distal ulnar tunnel should be drilled just posterior to the supinator crest, 15 mm distal to the radial head junction.
1. O’Driscoll SW, Bell DF, Morrey BF. Posterolateral rotatory instability of the elbow. J Bone Joint Surg Am. 1991;73(3):440-446.
2. O’Driscoll SW. Classification and evaluation of recurrent instability of the elbow. Clin Orthop Relat Res. 2000;370:34-43.
3. Takigawa N, Ryu J, Kish VL, Kinoshita M, Abe M. Functional anatomy of the lateral collateral ligament complex of the elbow: morphology and strain. J Hand Surg Br. 2005;30(2):143-147.
4. McAdams TR, Masters GW, Srivastava S. The effect of arthroscopic sectioning of the lateral ligament complex of the elbow on posterolateral rotatory stability. J Shoulder Elbow Surg. 2005;14(3):298-301.
5. Dunning CE, Zarzour ZD, Patterson SD, Johnson JA, King GJ. Ligamentous stabilizers against posterolateral rotatory instability of the elbow. J Bone Joint Surg Am. 2001;83(12):1823-1828.
6. Eygendaal D. Ligamentous reconstruction around the elbow using triceps tendon. Acta Orthop Scand. 2004;75(5):516-523.
7. Jones KJ, Dodson CC, Osbahr DC, et al. The docking technique for lateral ulnar collateral ligament reconstruction: surgical technique and clinical outcomes. J Shoulder Elbow Surg. 2012;21(3):389-395.
8. Lee BP, Teo LH. Surgical reconstruction for posterolateral rotatory instability of the elbow. J Shoulder Elbow Surg. 2003;12(5):476-479.
9. Lin KY, Shen PH, Lee CH, Pan RY, Lin LC, Shen HC. Functional outcomes of surgical reconstruction for posterolateral rotatory instability of the elbow. Injury. 2012;43(10):1657-1661.
10. Olsen BS, Søjbjerg JO. The treatment of recurrent posterolateral instability of the elbow. J Bone Joint Surg Br. 2003;85(3):342-346.
11. Sanchez-Sotelo J, Morrey BF, O’Driscoll SW. Ligamentous repair and reconstruction for posterolateral rotatory instability of the elbow. J Bone Joint Surg Br. 2005;87(1):54-61.
12. Savoie FH 3rd, Field LD, Gurley DJ. Arthroscopic and open radial ulnohumeral ligament reconstruction for posterolateral rotatory instability of the elbow. Hand Clin. 2009;25(3):323-329.
13. Savoie FH 3rd, O’Brien MJ, Field LD, Gurley DJ. Arthroscopic and open radial ulnohumeral ligament reconstruction for posterolateral rotatory instability of the elbow. Clin Sports Med. 2010;29(4):611-618.
14. Bryce CD, Pennypacker JL, Kulkarni N, et al. Validation of three-dimensional models of in situ scapulae. J Shoulder Elbow Surg. 2008;17(5):825-832.
15. Byram IR, Khanna K, Gardner TR, Ahmad CS. Characterizing bone tunnel placement in medial ulnar collateral ligament reconstruction using patient-specific 3-dimensional computed tomography modeling. Am J Sports Med. 2013;41(4):894-902.
16. Shiba R, Sorbie C, Siu DW, Bryant JT, Cooke TD, Wevers HW. Geometry of the humeroulnar joint. J Orthop Res. 1988;6(6):897-906.
17. Anakwenze OA, Khanna K, Levine WN, Ahmad CS. Characterization of the supinator tubercle for lateral ulnar collateral ligament reconstruction. Orthop J Sports Med. 2014;2(4):2325967114530969. doi:10.1177/2325967114530969.
18. Sasashige Y, Ochi M, Ikuta Y. Optimal attachment site for reconstruction of the ulnar collateral ligament. A cadaver study. Arch Orthop Trauma Surg. 1994;113(5):265-270.
Posterolateral rotatory instability (PLRI) of the elbow is well recognized1 and is the most common type of chronic elbow instability. PLRI is often an end result of traumatic elbow dislocation.2 The “essential lesion” in patients with PLRI of the elbow is injury to the lateral ulnar collateral ligament (LUCL).1 However, more recent research has emphasized the importance of other ligaments in the lateral ligament complex (radial collateral and annular ligaments) in preventing PLRI.3-5 Nevertheless, when conservative treatment fails, the most commonly used surgical treatment involves LUCL reconstruction.1,6-11
Numerous techniques for LUCL reconstruction have been described.1,7-9,11-13 The chosen technique ideally restores normal anatomy. Therefore, the isometric point of origin at the lateral epicondyle and insertion at the supinator tubercle are important landmarks for creating tunnels that reproduce isometry, function, and normal anatomy. Most often, 2 tunnels are created in the ulna to secure the graft. It has been our experience that ulnar tunnel creation can affect the length of the bony bridge and the orientation of the graft.
We conducted a study to identify the precise proximal ulna tunnel location—anterior to posterior, with the distal tunnel at the supinator tubercle on the crest—that allows for the largest bony bridge and most geometrically favorable construct. We hypothesized that a most posteriorly placed proximal tunnel would increase bony bridge size and allow for a more isosceles graft configuration. An isosceles configuration with the humerus tunnel at the isometric location would allow for anterior and posterior bands of the same length with theoretically equal force distribution.
Methods
After obtaining institutional review board approval, we retrospectively reviewed the cases of 17 adults with elbow computed tomography (CT) scans for inclusion in this study. The scans were previously performed for diagnostic workup of several pathologies, including valgus instability, olecranon stress fracture, and valgus extension overload. The scan protocol involved 0.5-mm axial cuts with inclusion of the distal humerus through the proximal radius and ulna in the DICOM (Digital Imaging and Communications in Medicine) format. Exclusion criteria included poor CT quality, inadequate visualization of the entire supinator crest, and age under 18 years. Fifteen patients with adequate CT scans met the inclusion criteria. MIMICS (Materialise’s Interactive Medical Image Control System) software was used to convert scans into patient-specific 3-dimensional (3-D) computer models. (Use of this software to produce anatomically accurate models has been verified in shoulder14 and elbow15 models.) These models were uploaded into Magics rapid prototyping software (Materialise) and manipulated for simulated tunnel drilling by precise bone subtraction methods. This software was used to define an ulnar Cartesian coordinate system with anatomical landmarks as reference points in order to standardize the position of each model (Figure 1).16 The y-axis was defined by the longitudinal axis of the ulna, and the x-axis was the transepicondylar axis, defined as the perpendicular line connecting the y-axis with the supinator crest. The z-axis was then established as the line perpendicular to the x- and y-axes—yielding a 3-D coordinate system that allowed us to manipulate the models in standardized fashion, maintaining the exact positions of the ulna while making measurements.
Surgical simulations were performed in the rapid prototyping software by creating a cylinder and placing it at the desired location of each tunnel. Cylinder diameter was 4 mm, matching the diameter of the drill we use to create each tunnel in our practice. The cylinder was inserted into the bone, perpendicular to the surface of the ulna at the point of insertion, so the cylinder’s deepest point entered the medullary canal of the ulna. Using a Boolean operation in the rapid prototyping software, we subtracted cylinder from bone to create a tunnel (Figure 2).15
In a previous study,17 we determined that the radial head junction is reproducibly about 15 mm proximal to the distinct supinator tubercle, which may be absent or not readily appreciated in up to 50% of cases. Therefore, proximal ulnar tunnels were placed 0, 5, and 10 mm posterior to the supinator crest at the radial head junction. Distal tunnels were placed 15 mm anterior to the radial head junction on the supinator crest (Figure 2). The bony bridges created by these tunnels were measured, as was the distance between the distal tunnel and the supinator tubercle.
Ideal graft configuration was described as an isosceles triangle with ulna tunnels perpendicular to the humeral tunnel (Figure 3).11 Location of the humeral origin in the sagittal plane was determined by finding the isometric point of the lateral humerus using only bony landmarks. Similar techniques have been used to find the isometric point on the medial epicondyle for medial ulnar collateral ligament reconstruction.15,18 With a circle fit into the trochlear notch of the ulna, the isometric point can be determined by the center of the circle. This point was then superimposed on the humerus to identify the starting point (Figure 4). In our simulation, we measured the isosceles configuration by drawing a line between the proximal and distal tunnels, and then another line connecting the bisecting point of the first line with the isometric point on the humerus from which the graft would originate. The angle between the 2 lines was measured; if isosceles, the angle was 90° (Figure 5). Length of the more proximal limb of the graft and the more distal limb of the graft was determined by measuring the distance from the isometric point to the proximal and distal tunnels, respectively (Figure 6).
One-way analysis of variance was used to compare all the tunnels’ bony bridge sizes, graft lengths, and angles to the isometric point. For all comparisons, statistical significance was set at P < .05. As no other studies have compared bony bridges by varying tunnel creation parameters, and as the present study is observational and not comparative, no power analysis was performed.
Results
Bony bridges were significantly longer, and angles more perpendicular, with increasing distance from the proximal tunnel to the supinator crest (Table 1, Figure 5, Figure 7). The bony bridge 0 mm posterior to the supinator crest yielded a mean (SE) bony bridge length of 11.0 (0.2) mm. This proximal tunnel also yielded the smallest mean (SE) perpendicular angle to the isometric point, 131.2° (1.9°). The tunnel most posterior to the supinator crest yielded the longest mean (SE) bony bridge, 13.7 (0.2) mm, and the largest mean (SE) degree of perpendicularity, 95.8° (1.4°). The differences between all tunnels’ bony bridges and isometric angles were statistically significant (P < .00001). The difference between the more distal limb and the more proximal limb of the graft was smallest in the more posteriorly placed proximal tunnel (Table 2, Figure 8). In fact, there was no statistical difference between the proximal and distal limbs of the graft when the proximal tunnel was placed 10 mm posterior to the supinator crest: Mean (SE) was 9.4 (0.5) mm at 0 mm (P < .00001) and 1.1 (0.6) mm at 10 mm (P = .24).
Discussion
PLRI of the elbow is best initially managed nonoperatively. However, when nonoperative management fails, the LUCL is often surgically reconstructed. Reconstruction methods vary by fixation method, graft choice, and bone tunnels.1,7-9,11-13 In 1991, O’Driscoll and colleagues1 described a “yoke” technique for LUCL reconstruction. Since then, the docking technique7 and other techniques have been developed. All these techniques emphasize maximizing anatomical precision and isometry with careful placement of tunnels or fixation devices. The humeral fixation site, at the anterior inferior aspect of the lateral epicondyle at the point of isometry, can be accessed relatively reproducibly. By contrast, the ulnar points of fixation are more variable, because of increased bone stock and overlying soft-tissue and bony anatomy.
Among the challenges in determining the points of ulnar fixation is the bony anatomy that is often used for landmarks. In the literature, the supinator crest or the supintor tubercle is the landmark for placing the distal tunnel.1,7-9,11-13 This is a problem for 2 reasons. First, the supintor crest, a longitudinal structure on the lateral aspect of the ulna, originates from the radial head junction and extends tens of millimeters distally; further specification is needed to guide these ulnar tunnels. The second reason is that use of the supinator tubercle, a prominence on the supinator crest, adds specificity to the location of the ulnar tunnels. During surgery, however, the supinator tubercle may not be a reliable, independently prominent structure; instead, it may be indistinguishable from the supinator crest, on which it rests. One study determined that only about 50% of computer models of patient ulnas had a distinct prominence that could be classified as the supinator tubercle.17 The percentage presumably is lower during surgery, with limited exposure and overlying soft tissues.
In a study of patients with a prominent tubercle, mean (SE) distance from radial head junction to tubercle was 15 (2) mm.17 This finding led us to use the radial head junction as the primary bony landmark in determining the location of the proximal tunnel and placing the distal tunnel 15 mm distally—achieving the same fixation described in the literature but using more distinct landmarks. Our study thus provided a reliable, verified approach to locating the ulnar tunnels in the proximal-distal axis.
We also explored the anterior-posterior orientation of the proximal ulnar tunnel. The 2 primary considerations surrounding the varied proximal tunnel placements were the bony bridge formed between the proximal and distal tunnels and the perpendicularity of the triangle formed by the fixation points. Maximizing the bony bridge is obviously ideal in securing and preventing fixation blowout. Achieving an isoceles reconstruction has been reported in the literature on the various fixation techniques for LUCL reconstruction.11 Although the biomechanical advantage of this fixation type is not fully clear, we assume the construct produces graft stands of equal length, tension, and stability. In addition, the larger footprint created by an isoceles reconstructed ligament increases the stability of the radial head.
Results of the present study showed that the more posterior the proximal ulnar tunnel, the longer the bony bridge and the more isoceles the reconstruction. The difference in bony bridge distance from the most anterior to the most posterior tunnel was about 2 mm, or 18%. For every 1 mm of posteriorization, the bony bridge was 0.2 mm longer. The line from the isometric point of humeral fixation bisecting the proximal and distal tunnels was also more perpendicular with the most posterior tunnel, by about 40°. The resulting proximal and distal limbs of the reconstruction were equal in length, as demonstrated by the smaller difference between the limbs. We assume this isoceles reconstruction more likely applies uniform restraint on the radial head. Thus, an effort should be made to posteriorize the proximal ulnar tunnel during reconstruction.
The study was limited by the number of patient-specific elbow models used. However, given the statistical consistency of measurements, sample size was sufficient. Another limitation, inherent to the model, was that only bony anatomy was incorporated. However, the overlying muscles, tendons, and ligaments can significantly alter tunnel placement, and this study provided other means and cues using more reliable landmarks to adequately place the tunnels. As this was a simulation study, we cannot confirm whether these results would make a difference clinically. The strengths of this study include development and verification of reliable landmarks that can be used to guide ulnar tunnel locations during LUCL reconstruction; these landmarks have been used for medial ulnar collateral ligament reconstruction.15 Other strengths include precise and accurate placement of tunnels and measurement of resulting bony bridges—accomplished independently and without compromising specimen quality.
Conclusion
We recommend drilling the proximal ulnar tunnel posterior to the supinator crest at the level of the radial head junction. A reasonable goal is 10 mm posterior to the crest, though the overlying soft tissue must be considered, and care should be taken to aim the drill anteriorly, toward the ulna’s intramedullary canal, to avoid posterior cortical breach. The distal ulnar tunnel should be drilled just posterior to the supinator crest, 15 mm distal to the radial head junction.
Posterolateral rotatory instability (PLRI) of the elbow is well recognized1 and is the most common type of chronic elbow instability. PLRI is often an end result of traumatic elbow dislocation.2 The “essential lesion” in patients with PLRI of the elbow is injury to the lateral ulnar collateral ligament (LUCL).1 However, more recent research has emphasized the importance of other ligaments in the lateral ligament complex (radial collateral and annular ligaments) in preventing PLRI.3-5 Nevertheless, when conservative treatment fails, the most commonly used surgical treatment involves LUCL reconstruction.1,6-11
Numerous techniques for LUCL reconstruction have been described.1,7-9,11-13 The chosen technique ideally restores normal anatomy. Therefore, the isometric point of origin at the lateral epicondyle and insertion at the supinator tubercle are important landmarks for creating tunnels that reproduce isometry, function, and normal anatomy. Most often, 2 tunnels are created in the ulna to secure the graft. It has been our experience that ulnar tunnel creation can affect the length of the bony bridge and the orientation of the graft.
We conducted a study to identify the precise proximal ulna tunnel location—anterior to posterior, with the distal tunnel at the supinator tubercle on the crest—that allows for the largest bony bridge and most geometrically favorable construct. We hypothesized that a most posteriorly placed proximal tunnel would increase bony bridge size and allow for a more isosceles graft configuration. An isosceles configuration with the humerus tunnel at the isometric location would allow for anterior and posterior bands of the same length with theoretically equal force distribution.
Methods
After obtaining institutional review board approval, we retrospectively reviewed the cases of 17 adults with elbow computed tomography (CT) scans for inclusion in this study. The scans were previously performed for diagnostic workup of several pathologies, including valgus instability, olecranon stress fracture, and valgus extension overload. The scan protocol involved 0.5-mm axial cuts with inclusion of the distal humerus through the proximal radius and ulna in the DICOM (Digital Imaging and Communications in Medicine) format. Exclusion criteria included poor CT quality, inadequate visualization of the entire supinator crest, and age under 18 years. Fifteen patients with adequate CT scans met the inclusion criteria. MIMICS (Materialise’s Interactive Medical Image Control System) software was used to convert scans into patient-specific 3-dimensional (3-D) computer models. (Use of this software to produce anatomically accurate models has been verified in shoulder14 and elbow15 models.) These models were uploaded into Magics rapid prototyping software (Materialise) and manipulated for simulated tunnel drilling by precise bone subtraction methods. This software was used to define an ulnar Cartesian coordinate system with anatomical landmarks as reference points in order to standardize the position of each model (Figure 1).16 The y-axis was defined by the longitudinal axis of the ulna, and the x-axis was the transepicondylar axis, defined as the perpendicular line connecting the y-axis with the supinator crest. The z-axis was then established as the line perpendicular to the x- and y-axes—yielding a 3-D coordinate system that allowed us to manipulate the models in standardized fashion, maintaining the exact positions of the ulna while making measurements.
Surgical simulations were performed in the rapid prototyping software by creating a cylinder and placing it at the desired location of each tunnel. Cylinder diameter was 4 mm, matching the diameter of the drill we use to create each tunnel in our practice. The cylinder was inserted into the bone, perpendicular to the surface of the ulna at the point of insertion, so the cylinder’s deepest point entered the medullary canal of the ulna. Using a Boolean operation in the rapid prototyping software, we subtracted cylinder from bone to create a tunnel (Figure 2).15
In a previous study,17 we determined that the radial head junction is reproducibly about 15 mm proximal to the distinct supinator tubercle, which may be absent or not readily appreciated in up to 50% of cases. Therefore, proximal ulnar tunnels were placed 0, 5, and 10 mm posterior to the supinator crest at the radial head junction. Distal tunnels were placed 15 mm anterior to the radial head junction on the supinator crest (Figure 2). The bony bridges created by these tunnels were measured, as was the distance between the distal tunnel and the supinator tubercle.
Ideal graft configuration was described as an isosceles triangle with ulna tunnels perpendicular to the humeral tunnel (Figure 3).11 Location of the humeral origin in the sagittal plane was determined by finding the isometric point of the lateral humerus using only bony landmarks. Similar techniques have been used to find the isometric point on the medial epicondyle for medial ulnar collateral ligament reconstruction.15,18 With a circle fit into the trochlear notch of the ulna, the isometric point can be determined by the center of the circle. This point was then superimposed on the humerus to identify the starting point (Figure 4). In our simulation, we measured the isosceles configuration by drawing a line between the proximal and distal tunnels, and then another line connecting the bisecting point of the first line with the isometric point on the humerus from which the graft would originate. The angle between the 2 lines was measured; if isosceles, the angle was 90° (Figure 5). Length of the more proximal limb of the graft and the more distal limb of the graft was determined by measuring the distance from the isometric point to the proximal and distal tunnels, respectively (Figure 6).
One-way analysis of variance was used to compare all the tunnels’ bony bridge sizes, graft lengths, and angles to the isometric point. For all comparisons, statistical significance was set at P < .05. As no other studies have compared bony bridges by varying tunnel creation parameters, and as the present study is observational and not comparative, no power analysis was performed.
Results
Bony bridges were significantly longer, and angles more perpendicular, with increasing distance from the proximal tunnel to the supinator crest (Table 1, Figure 5, Figure 7). The bony bridge 0 mm posterior to the supinator crest yielded a mean (SE) bony bridge length of 11.0 (0.2) mm. This proximal tunnel also yielded the smallest mean (SE) perpendicular angle to the isometric point, 131.2° (1.9°). The tunnel most posterior to the supinator crest yielded the longest mean (SE) bony bridge, 13.7 (0.2) mm, and the largest mean (SE) degree of perpendicularity, 95.8° (1.4°). The differences between all tunnels’ bony bridges and isometric angles were statistically significant (P < .00001). The difference between the more distal limb and the more proximal limb of the graft was smallest in the more posteriorly placed proximal tunnel (Table 2, Figure 8). In fact, there was no statistical difference between the proximal and distal limbs of the graft when the proximal tunnel was placed 10 mm posterior to the supinator crest: Mean (SE) was 9.4 (0.5) mm at 0 mm (P < .00001) and 1.1 (0.6) mm at 10 mm (P = .24).
Discussion
PLRI of the elbow is best initially managed nonoperatively. However, when nonoperative management fails, the LUCL is often surgically reconstructed. Reconstruction methods vary by fixation method, graft choice, and bone tunnels.1,7-9,11-13 In 1991, O’Driscoll and colleagues1 described a “yoke” technique for LUCL reconstruction. Since then, the docking technique7 and other techniques have been developed. All these techniques emphasize maximizing anatomical precision and isometry with careful placement of tunnels or fixation devices. The humeral fixation site, at the anterior inferior aspect of the lateral epicondyle at the point of isometry, can be accessed relatively reproducibly. By contrast, the ulnar points of fixation are more variable, because of increased bone stock and overlying soft-tissue and bony anatomy.
Among the challenges in determining the points of ulnar fixation is the bony anatomy that is often used for landmarks. In the literature, the supinator crest or the supintor tubercle is the landmark for placing the distal tunnel.1,7-9,11-13 This is a problem for 2 reasons. First, the supintor crest, a longitudinal structure on the lateral aspect of the ulna, originates from the radial head junction and extends tens of millimeters distally; further specification is needed to guide these ulnar tunnels. The second reason is that use of the supinator tubercle, a prominence on the supinator crest, adds specificity to the location of the ulnar tunnels. During surgery, however, the supinator tubercle may not be a reliable, independently prominent structure; instead, it may be indistinguishable from the supinator crest, on which it rests. One study determined that only about 50% of computer models of patient ulnas had a distinct prominence that could be classified as the supinator tubercle.17 The percentage presumably is lower during surgery, with limited exposure and overlying soft tissues.
In a study of patients with a prominent tubercle, mean (SE) distance from radial head junction to tubercle was 15 (2) mm.17 This finding led us to use the radial head junction as the primary bony landmark in determining the location of the proximal tunnel and placing the distal tunnel 15 mm distally—achieving the same fixation described in the literature but using more distinct landmarks. Our study thus provided a reliable, verified approach to locating the ulnar tunnels in the proximal-distal axis.
We also explored the anterior-posterior orientation of the proximal ulnar tunnel. The 2 primary considerations surrounding the varied proximal tunnel placements were the bony bridge formed between the proximal and distal tunnels and the perpendicularity of the triangle formed by the fixation points. Maximizing the bony bridge is obviously ideal in securing and preventing fixation blowout. Achieving an isoceles reconstruction has been reported in the literature on the various fixation techniques for LUCL reconstruction.11 Although the biomechanical advantage of this fixation type is not fully clear, we assume the construct produces graft stands of equal length, tension, and stability. In addition, the larger footprint created by an isoceles reconstructed ligament increases the stability of the radial head.
Results of the present study showed that the more posterior the proximal ulnar tunnel, the longer the bony bridge and the more isoceles the reconstruction. The difference in bony bridge distance from the most anterior to the most posterior tunnel was about 2 mm, or 18%. For every 1 mm of posteriorization, the bony bridge was 0.2 mm longer. The line from the isometric point of humeral fixation bisecting the proximal and distal tunnels was also more perpendicular with the most posterior tunnel, by about 40°. The resulting proximal and distal limbs of the reconstruction were equal in length, as demonstrated by the smaller difference between the limbs. We assume this isoceles reconstruction more likely applies uniform restraint on the radial head. Thus, an effort should be made to posteriorize the proximal ulnar tunnel during reconstruction.
The study was limited by the number of patient-specific elbow models used. However, given the statistical consistency of measurements, sample size was sufficient. Another limitation, inherent to the model, was that only bony anatomy was incorporated. However, the overlying muscles, tendons, and ligaments can significantly alter tunnel placement, and this study provided other means and cues using more reliable landmarks to adequately place the tunnels. As this was a simulation study, we cannot confirm whether these results would make a difference clinically. The strengths of this study include development and verification of reliable landmarks that can be used to guide ulnar tunnel locations during LUCL reconstruction; these landmarks have been used for medial ulnar collateral ligament reconstruction.15 Other strengths include precise and accurate placement of tunnels and measurement of resulting bony bridges—accomplished independently and without compromising specimen quality.
Conclusion
We recommend drilling the proximal ulnar tunnel posterior to the supinator crest at the level of the radial head junction. A reasonable goal is 10 mm posterior to the crest, though the overlying soft tissue must be considered, and care should be taken to aim the drill anteriorly, toward the ulna’s intramedullary canal, to avoid posterior cortical breach. The distal ulnar tunnel should be drilled just posterior to the supinator crest, 15 mm distal to the radial head junction.
1. O’Driscoll SW, Bell DF, Morrey BF. Posterolateral rotatory instability of the elbow. J Bone Joint Surg Am. 1991;73(3):440-446.
2. O’Driscoll SW. Classification and evaluation of recurrent instability of the elbow. Clin Orthop Relat Res. 2000;370:34-43.
3. Takigawa N, Ryu J, Kish VL, Kinoshita M, Abe M. Functional anatomy of the lateral collateral ligament complex of the elbow: morphology and strain. J Hand Surg Br. 2005;30(2):143-147.
4. McAdams TR, Masters GW, Srivastava S. The effect of arthroscopic sectioning of the lateral ligament complex of the elbow on posterolateral rotatory stability. J Shoulder Elbow Surg. 2005;14(3):298-301.
5. Dunning CE, Zarzour ZD, Patterson SD, Johnson JA, King GJ. Ligamentous stabilizers against posterolateral rotatory instability of the elbow. J Bone Joint Surg Am. 2001;83(12):1823-1828.
6. Eygendaal D. Ligamentous reconstruction around the elbow using triceps tendon. Acta Orthop Scand. 2004;75(5):516-523.
7. Jones KJ, Dodson CC, Osbahr DC, et al. The docking technique for lateral ulnar collateral ligament reconstruction: surgical technique and clinical outcomes. J Shoulder Elbow Surg. 2012;21(3):389-395.
8. Lee BP, Teo LH. Surgical reconstruction for posterolateral rotatory instability of the elbow. J Shoulder Elbow Surg. 2003;12(5):476-479.
9. Lin KY, Shen PH, Lee CH, Pan RY, Lin LC, Shen HC. Functional outcomes of surgical reconstruction for posterolateral rotatory instability of the elbow. Injury. 2012;43(10):1657-1661.
10. Olsen BS, Søjbjerg JO. The treatment of recurrent posterolateral instability of the elbow. J Bone Joint Surg Br. 2003;85(3):342-346.
11. Sanchez-Sotelo J, Morrey BF, O’Driscoll SW. Ligamentous repair and reconstruction for posterolateral rotatory instability of the elbow. J Bone Joint Surg Br. 2005;87(1):54-61.
12. Savoie FH 3rd, Field LD, Gurley DJ. Arthroscopic and open radial ulnohumeral ligament reconstruction for posterolateral rotatory instability of the elbow. Hand Clin. 2009;25(3):323-329.
13. Savoie FH 3rd, O’Brien MJ, Field LD, Gurley DJ. Arthroscopic and open radial ulnohumeral ligament reconstruction for posterolateral rotatory instability of the elbow. Clin Sports Med. 2010;29(4):611-618.
14. Bryce CD, Pennypacker JL, Kulkarni N, et al. Validation of three-dimensional models of in situ scapulae. J Shoulder Elbow Surg. 2008;17(5):825-832.
15. Byram IR, Khanna K, Gardner TR, Ahmad CS. Characterizing bone tunnel placement in medial ulnar collateral ligament reconstruction using patient-specific 3-dimensional computed tomography modeling. Am J Sports Med. 2013;41(4):894-902.
16. Shiba R, Sorbie C, Siu DW, Bryant JT, Cooke TD, Wevers HW. Geometry of the humeroulnar joint. J Orthop Res. 1988;6(6):897-906.
17. Anakwenze OA, Khanna K, Levine WN, Ahmad CS. Characterization of the supinator tubercle for lateral ulnar collateral ligament reconstruction. Orthop J Sports Med. 2014;2(4):2325967114530969. doi:10.1177/2325967114530969.
18. Sasashige Y, Ochi M, Ikuta Y. Optimal attachment site for reconstruction of the ulnar collateral ligament. A cadaver study. Arch Orthop Trauma Surg. 1994;113(5):265-270.
1. O’Driscoll SW, Bell DF, Morrey BF. Posterolateral rotatory instability of the elbow. J Bone Joint Surg Am. 1991;73(3):440-446.
2. O’Driscoll SW. Classification and evaluation of recurrent instability of the elbow. Clin Orthop Relat Res. 2000;370:34-43.
3. Takigawa N, Ryu J, Kish VL, Kinoshita M, Abe M. Functional anatomy of the lateral collateral ligament complex of the elbow: morphology and strain. J Hand Surg Br. 2005;30(2):143-147.
4. McAdams TR, Masters GW, Srivastava S. The effect of arthroscopic sectioning of the lateral ligament complex of the elbow on posterolateral rotatory stability. J Shoulder Elbow Surg. 2005;14(3):298-301.
5. Dunning CE, Zarzour ZD, Patterson SD, Johnson JA, King GJ. Ligamentous stabilizers against posterolateral rotatory instability of the elbow. J Bone Joint Surg Am. 2001;83(12):1823-1828.
6. Eygendaal D. Ligamentous reconstruction around the elbow using triceps tendon. Acta Orthop Scand. 2004;75(5):516-523.
7. Jones KJ, Dodson CC, Osbahr DC, et al. The docking technique for lateral ulnar collateral ligament reconstruction: surgical technique and clinical outcomes. J Shoulder Elbow Surg. 2012;21(3):389-395.
8. Lee BP, Teo LH. Surgical reconstruction for posterolateral rotatory instability of the elbow. J Shoulder Elbow Surg. 2003;12(5):476-479.
9. Lin KY, Shen PH, Lee CH, Pan RY, Lin LC, Shen HC. Functional outcomes of surgical reconstruction for posterolateral rotatory instability of the elbow. Injury. 2012;43(10):1657-1661.
10. Olsen BS, Søjbjerg JO. The treatment of recurrent posterolateral instability of the elbow. J Bone Joint Surg Br. 2003;85(3):342-346.
11. Sanchez-Sotelo J, Morrey BF, O’Driscoll SW. Ligamentous repair and reconstruction for posterolateral rotatory instability of the elbow. J Bone Joint Surg Br. 2005;87(1):54-61.
12. Savoie FH 3rd, Field LD, Gurley DJ. Arthroscopic and open radial ulnohumeral ligament reconstruction for posterolateral rotatory instability of the elbow. Hand Clin. 2009;25(3):323-329.
13. Savoie FH 3rd, O’Brien MJ, Field LD, Gurley DJ. Arthroscopic and open radial ulnohumeral ligament reconstruction for posterolateral rotatory instability of the elbow. Clin Sports Med. 2010;29(4):611-618.
14. Bryce CD, Pennypacker JL, Kulkarni N, et al. Validation of three-dimensional models of in situ scapulae. J Shoulder Elbow Surg. 2008;17(5):825-832.
15. Byram IR, Khanna K, Gardner TR, Ahmad CS. Characterizing bone tunnel placement in medial ulnar collateral ligament reconstruction using patient-specific 3-dimensional computed tomography modeling. Am J Sports Med. 2013;41(4):894-902.
16. Shiba R, Sorbie C, Siu DW, Bryant JT, Cooke TD, Wevers HW. Geometry of the humeroulnar joint. J Orthop Res. 1988;6(6):897-906.
17. Anakwenze OA, Khanna K, Levine WN, Ahmad CS. Characterization of the supinator tubercle for lateral ulnar collateral ligament reconstruction. Orthop J Sports Med. 2014;2(4):2325967114530969. doi:10.1177/2325967114530969.
18. Sasashige Y, Ochi M, Ikuta Y. Optimal attachment site for reconstruction of the ulnar collateral ligament. A cadaver study. Arch Orthop Trauma Surg. 1994;113(5):265-270.
Google Glass Distal Biceps Repair
To read the authors' full article "5 Points on Using Wearable Technology to Record Surgical Videos," click here.
To read the authors' full article "5 Points on Using Wearable Technology to Record Surgical Videos," click here.
To read the authors' full article "5 Points on Using Wearable Technology to Record Surgical Videos," click here.
GoPro Hero 3 Latarjet Procedure
Coracoid harvest for transfer during Latarjet procedure performed and filmed by Dr. Jobin using GoPro Hero 3.
To read the authors' full article "5 Points on Using Wearable Technology to Record Surgical Videos," click here.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Coracoid harvest for transfer during Latarjet procedure performed and filmed by Dr. Jobin using GoPro Hero 3.
To read the authors' full article "5 Points on Using Wearable Technology to Record Surgical Videos," click here.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Coracoid harvest for transfer during Latarjet procedure performed and filmed by Dr. Jobin using GoPro Hero 3.
To read the authors' full article "5 Points on Using Wearable Technology to Record Surgical Videos," click here.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Using Wearable Technology to Record Surgical Videos
Safe and efficient advanced surgical skill training is of tremendous importance. With the recent increase in Internet use for medical education, there has been a concomitant increase in video recording of surgical procedures and techniques. Surgical recordings have been used in a variety of ways—as live webcasts for remote participants, as “coaching” opportunities for surgeons evaluating their own performance in the operating room, and even as informational resources for patients about to undergo the same surgery.
Surgical multimedia is being delivered through several different outlets. Many academic conferences and meetings showcase videos of different procedures, and several subspecialty societies (eg, Arthroscopy Association of North America) house archives of technical videos for viewing by members. In addition, the VuMedi website offers videos and allows members to comment on them and interact with the videographers. Surgeons are even posting technique videos on YouTube and other public websites.
A large proportion of surgical multimedia is recorded with conventional high-definition video cameras.1 Besides being able to experience a case at any time and from outside the operating room, the audience can watch from numerous vantage points, angles, and zoom levels. Also, surgeons’ narration can be valuable in helping the audience follow along with the case.
Recording surgical multimedia historically required tight coordination and precise planning by surgeon and videographer. However, innovations in wearable technology now allow surgeons to literally wear video cameras and record procedures as they perform them, in real time—to act as both surgeon and videographer.
Two such products are Google Glass (Google, Mountain View, California) and GoPro Hero (GoPro, San Mateo, California), both of which allow surgeons to record exactly what they see during procedures (Figure 1). Using a wearable technology for surgical multimedia creation requires a deep familiarity with its capabilities and limitations. In this article, we summarize these products’ similarities and differences and provide a technical overview for using wearable technologies in surgical multimedia creation.
1. Choosing a device
When purchasing either wearable device, several factors must be considered, including budget, possible uses outside the operating room, and possible limitations of the technology (Table 1). At this time, Google Glass is significantly more expensive than GoPro Hero. The Google Glass base unit costs $1500, and the GoPro Hero 3 model costs approximately $200 (higher-priced Hero models are available). Both devices require accessories (eg, portable battery unit, dedicated hard drive).
Device capabilities must also be considered (Table 2). Google Glass consists of both hardware and software. Users can record what is seen and heard through the lens and then use apps to create text and e-mail portals to online gaming, social media, and even golf-course GPS. The app market for Google Glass is nascent but undoubtedly will increase in volume and scope as more users adopt the technology (Google Glass comes with both Bluetooth and Wi-Fi and can function tethered through a smartphone). GoPro is mainly a hardware unit that can record in various settings (it is popular with athletes who want to capture and broadcast their participation in action sports). Newer GoPro Hero versions offer Wi-Fi, which allows streaming of video content to a smartphone or tablet through an app. Having clearly defined goals for a device—as they pertain to use outside the operating room, such as outdoor activities and underwater recording—may help the surgeon decide which product is more suitable. Last, it is important to consider limitations. Google Glass resolution is 720p (1280×720) for video and 5 MP for still images, and GoPro resolution can reach 1080p (1920×1080) for video and 5 MP for stills.
Both devices require purchase of accessories. An external USB battery pack is useful for both devices, as is a password-encrypted hard drive for media storage. Lenswear does not come with the base version of Google Glass and is purchased separately from the company. GoPro users buy micro SD cards (~$50 per 64-GB high-speed transfer card) for storage on the device and may buy lithium-ion batteries as an alternative to the external USB battery pack.
Author Update
In January 2015, Google announced that it was temporarily suspending its “Explorer” program, which allowed individual users to buy and test the device for personal use. However, Google is continuing its development of Glass with health care technology, among other areas of growth and development.2,3
2. Recording a successful surgical video
Unlike a camcorder, which typically is set on a tripod for conventional video recording of surgery, Google Glass and GoPro are intricately linked to the operator. Surgeons must be constantly aware of where they are during surgery and try not to let anything obstruct the camera’s view.
Before starting a case, the surgeon using either device must ensure that its battery is fully charged, as a full charge typically supports 1 hour of continuous recording (the Google Glass battery is a lithium-ion 670-mAh internal unit). A full charge should be enough to capture a short case. Newer GoPro models, with a battery listed at 1050 mAh, provide 1 to 2 hours of recording. When more than 1 hour is needed, an external USB battery pack can be used. This pack allows the device to remain plugged in throughout the case (the pack is kept in the surgeon’s back pocket). We recommend having an external battery pack that is at least 10,000 mAh (~$30 online retail), which easily provides 3+ hours of recording. Unfortunately, this arrangement can be cumbersome. Alternatively, with GoPro, additional batteries may be purchased, but the user needs to dismount the device in order to swap them in (may be difficult during surgery). With both units, partitioning a video into shorter segments conserves battery power and minimizes the risk of file corruption, which may occur if the battery dies or the device overheats.
Google Glass users can bypass manual operation of the device by giving it voice commands (eg, start video, take still image). The exception is for recording video for more than 10 seconds (current default setting). Unfortunately, the surgeon must touch the device to start this recording, which means using extra gloves to preserve operating field sterility. Still images can be made through a combination of voice and head gestures and without manual intervention (Figure 2). Last, users must ensure that the device is not actively connected through Bluetooth to a mobile phone, as incoming calls, text messages, and e-mails may disrupt active recording and become a distraction. The connection can be deactivated by disabling Bluetooth on the host smartphone or by placing the phone into airplane mode and turning off Wi-Fi.
Google Glass users can see what is being recorded through the viewfinder prism, whereas GoPro requires precise framing of the video before recording. Framing is done by grossly aiming the device in the desired direction. However, there is no way to ensure exact aim during recording. If at any point during a case there is slight repositioning of the GoPro, there is a risk of recording the case out of the center of view. An important advantage to newer GoPro versions is the ability to control the device through a wireless remote that can be placed under the surgeon’s gown. The remote can be used to pause and resume recording, without changing gloves, as is done with Google Glass. Last, because the minimum viewing distance from the surgical field is usually 18 inches or more, typically there is no loss of focus or blurring of the image from short-distance recording on either device.
3. File management and playback
Before using wearable technology in the operating room, surgeons must become aware of its limitations with respect to file storage and playback. Google Glass has a usable memory of about 12 GB (1 hour of video may require 1.5-2.0 GB). Conversely, GoPro’s capacity is defined by the micro SD card used. Therefore, the Google Glass hard drive must be regularly maintained well before being brought into the operating room, whereas recording can be extended (with respect to memory) for the GoPro if the media card is large enough.
Both devices allow for wired file transfer, which may be done with Windows Explorer (PC) or iPhoto (Macintosh). However, Google Glass also allows for wireless transfer, through portable storage supported by Google. Although this type of file transfer may be convenient for short, everyday clips made outside the operating room, it is prohibitive for surgical media, mainly because of patient privacy concerns. With wireless transfer to a nonsecure cloud platform, there is a risk of breach of patient confidentiality. We therefore recommend against using wireless upload when producing surgical multimedia, as patient identifiers are likely to be included in the recorded audio or video contents. Conversely, with GoPro, the micro SD card can be used as a portable hard drive to transfer files to a laptop or media reader, obviating the need for wired or wireless transmission. Last, when using traditional wire transfer or memory card to upload to a hard drive, users must ensure that the drive complies with patient privacy laws and regulations.
4. Privacy and patient consent
As mentioned, great care must be taken to ensure that patient privacy laws are followed. This is especially relevant with content uploaded to online cloud storage, as with Google Glass. The upload may occur automatically if the unit is connected to a Wi-Fi hotspot. In addition, when using surgical media for a real-time webcast for education or demonstration purposes, surgeons must ensure that no protected health information is broadcast and that the patient and the surgical team are aware of the webcast and its purposes.
Before using wearable technology during patient care, patient consent must be obtained. Surgeons should ask the patient to consent to video recording of surgery or an encounter (eg, clinic visit) for education purposes. Our institution’s consent form includes a section for this particular type of consent. If an institution’s form lacks such a section, surgeons should consult their risk management department to ensure there is a proper avenue for obtaining patient consent to record the procedure or encounter. A separate, dedicated media consent form may be required. Last, whoever operates a wearable device should be careful to use the device only during encounters that have received explicit recording consent—as opposed to wearing the device in the hallways or elsewhere in the hospital, where protected health information might be inadvertently recorded.4
5. Putting it all to use
After successful recording of surgery, an effort should be made to produce a high-quality video for education or demonstration purposes. Unfortunately, there is no built-in optical zooming with Google Glass or GoPro, and recording segments in which surgeons focus on detailed anatomy (with high-quality zoom) may prove difficult. Online descriptions of do-it-yourself modifications to place zoom capability on GoPro devices may be useful in surgical video recording, particularly for small surgical fields (hand or foot surgery). In addition, footage may be zoomed in on during postprocessing (Figure 3), though some resolution will be lost in the editing.
There is no practical way to incorporate Google Glass or GoPro while using surgical loupes or a surgical microscope. As a result, videos recorded with wearable technology may not reach the minimum resolution needed for useful surgical technique videos, as these traditionally are produced on high-definition camcorders with optical zoom, allowing detailed viewing of anatomical structures without resolution loss through digital zoom or postprocessing editing.
There has been tremendous benefit in incorporating wearable technology into our practice. Videos made with Google Glass and GoPro have been successfully used for surgical preparation and training, allowing orthopedic surgical residents to rehearse surgery before participating in it. Alternatively, having used Google Glass or GoPro to record a case, residents have then been able to review each surgical step on video—thereby reinforcing their knowledge of the steps, techniques, pearls, and pitfalls before performing the surgery again. Footage from surgeries recorded with Google Glass and GoPro has also been shown at weekly technique-focused conferences, allowing surgeons to analyze particular steps and highlight applicable learning points. Last, attending surgeons in our practice have used wearable technology in “coaching” mode, either reviewing case footage to identify areas for improvement or sharing footage with senior surgeons in order to elicit feedback and suggestions for possible improvement.
As new iterations of wearable video technology come to market, with advancements in both hardware and software, surgeons may be able to enhance education and teaching through seamless recording of surgical procedures. Use of wearable technology may also begin to extend beyond the operating room—to outpatient settings, such as preoperative and postoperative physical examinations. The latest versions of Google Glass and GoPro Hero allow surgeons to record surgical procedures with relative ease, without the personnel, equipment, and coordination required for traditional surgical videography.
Video 1. Coracoid harvest for transfer during Latarjet procedure performed and filmed by Dr. Jobin using GoPro Hero 3.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Video 2. Distal biceps repair performed by Dr. Makhni and Dr. Jobin, filmed by Dr. Makhni using Google Glass.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
1. Leahy M. Creating a good surgical technique video. AAOS Now. 2010;4(11). http://www.aaos.org/news/aaosnow/nov10/clinical4.asp. Accessed February 15, 2015.
2. Google Glass sales halted but firm says kit is not dead. BBC News website. http://www.bbc.com/news/technology-30831128. Published January 15, 2015. Accessed February 18, 2015.
3. Metz C. Sorry, but Google Glass isn’t anywhere close to dead. Wired website. http://www.wired.com/2015/02/sorry-google-glass-isnt-anywhere-close-dead/. Published February 8, 2015. Accessed February 18, 2015.
4. Peregrin T. Surgeons see future applications for Google Glass. Bull Am Coll Surg. 2014;99(7):9-16. http://bulletin.facs.org/2014/07/surgeons-see-future-applications-for-google-glass/#.U8SLKZaJAyZ.twitter. Accessed February 15, 2015.
Safe and efficient advanced surgical skill training is of tremendous importance. With the recent increase in Internet use for medical education, there has been a concomitant increase in video recording of surgical procedures and techniques. Surgical recordings have been used in a variety of ways—as live webcasts for remote participants, as “coaching” opportunities for surgeons evaluating their own performance in the operating room, and even as informational resources for patients about to undergo the same surgery.
Surgical multimedia is being delivered through several different outlets. Many academic conferences and meetings showcase videos of different procedures, and several subspecialty societies (eg, Arthroscopy Association of North America) house archives of technical videos for viewing by members. In addition, the VuMedi website offers videos and allows members to comment on them and interact with the videographers. Surgeons are even posting technique videos on YouTube and other public websites.
A large proportion of surgical multimedia is recorded with conventional high-definition video cameras.1 Besides being able to experience a case at any time and from outside the operating room, the audience can watch from numerous vantage points, angles, and zoom levels. Also, surgeons’ narration can be valuable in helping the audience follow along with the case.
Recording surgical multimedia historically required tight coordination and precise planning by surgeon and videographer. However, innovations in wearable technology now allow surgeons to literally wear video cameras and record procedures as they perform them, in real time—to act as both surgeon and videographer.
Two such products are Google Glass (Google, Mountain View, California) and GoPro Hero (GoPro, San Mateo, California), both of which allow surgeons to record exactly what they see during procedures (Figure 1). Using a wearable technology for surgical multimedia creation requires a deep familiarity with its capabilities and limitations. In this article, we summarize these products’ similarities and differences and provide a technical overview for using wearable technologies in surgical multimedia creation.
1. Choosing a device
When purchasing either wearable device, several factors must be considered, including budget, possible uses outside the operating room, and possible limitations of the technology (Table 1). At this time, Google Glass is significantly more expensive than GoPro Hero. The Google Glass base unit costs $1500, and the GoPro Hero 3 model costs approximately $200 (higher-priced Hero models are available). Both devices require accessories (eg, portable battery unit, dedicated hard drive).
Device capabilities must also be considered (Table 2). Google Glass consists of both hardware and software. Users can record what is seen and heard through the lens and then use apps to create text and e-mail portals to online gaming, social media, and even golf-course GPS. The app market for Google Glass is nascent but undoubtedly will increase in volume and scope as more users adopt the technology (Google Glass comes with both Bluetooth and Wi-Fi and can function tethered through a smartphone). GoPro is mainly a hardware unit that can record in various settings (it is popular with athletes who want to capture and broadcast their participation in action sports). Newer GoPro Hero versions offer Wi-Fi, which allows streaming of video content to a smartphone or tablet through an app. Having clearly defined goals for a device—as they pertain to use outside the operating room, such as outdoor activities and underwater recording—may help the surgeon decide which product is more suitable. Last, it is important to consider limitations. Google Glass resolution is 720p (1280×720) for video and 5 MP for still images, and GoPro resolution can reach 1080p (1920×1080) for video and 5 MP for stills.
Both devices require purchase of accessories. An external USB battery pack is useful for both devices, as is a password-encrypted hard drive for media storage. Lenswear does not come with the base version of Google Glass and is purchased separately from the company. GoPro users buy micro SD cards (~$50 per 64-GB high-speed transfer card) for storage on the device and may buy lithium-ion batteries as an alternative to the external USB battery pack.
Author Update
In January 2015, Google announced that it was temporarily suspending its “Explorer” program, which allowed individual users to buy and test the device for personal use. However, Google is continuing its development of Glass with health care technology, among other areas of growth and development.2,3
2. Recording a successful surgical video
Unlike a camcorder, which typically is set on a tripod for conventional video recording of surgery, Google Glass and GoPro are intricately linked to the operator. Surgeons must be constantly aware of where they are during surgery and try not to let anything obstruct the camera’s view.
Before starting a case, the surgeon using either device must ensure that its battery is fully charged, as a full charge typically supports 1 hour of continuous recording (the Google Glass battery is a lithium-ion 670-mAh internal unit). A full charge should be enough to capture a short case. Newer GoPro models, with a battery listed at 1050 mAh, provide 1 to 2 hours of recording. When more than 1 hour is needed, an external USB battery pack can be used. This pack allows the device to remain plugged in throughout the case (the pack is kept in the surgeon’s back pocket). We recommend having an external battery pack that is at least 10,000 mAh (~$30 online retail), which easily provides 3+ hours of recording. Unfortunately, this arrangement can be cumbersome. Alternatively, with GoPro, additional batteries may be purchased, but the user needs to dismount the device in order to swap them in (may be difficult during surgery). With both units, partitioning a video into shorter segments conserves battery power and minimizes the risk of file corruption, which may occur if the battery dies or the device overheats.
Google Glass users can bypass manual operation of the device by giving it voice commands (eg, start video, take still image). The exception is for recording video for more than 10 seconds (current default setting). Unfortunately, the surgeon must touch the device to start this recording, which means using extra gloves to preserve operating field sterility. Still images can be made through a combination of voice and head gestures and without manual intervention (Figure 2). Last, users must ensure that the device is not actively connected through Bluetooth to a mobile phone, as incoming calls, text messages, and e-mails may disrupt active recording and become a distraction. The connection can be deactivated by disabling Bluetooth on the host smartphone or by placing the phone into airplane mode and turning off Wi-Fi.
Google Glass users can see what is being recorded through the viewfinder prism, whereas GoPro requires precise framing of the video before recording. Framing is done by grossly aiming the device in the desired direction. However, there is no way to ensure exact aim during recording. If at any point during a case there is slight repositioning of the GoPro, there is a risk of recording the case out of the center of view. An important advantage to newer GoPro versions is the ability to control the device through a wireless remote that can be placed under the surgeon’s gown. The remote can be used to pause and resume recording, without changing gloves, as is done with Google Glass. Last, because the minimum viewing distance from the surgical field is usually 18 inches or more, typically there is no loss of focus or blurring of the image from short-distance recording on either device.
3. File management and playback
Before using wearable technology in the operating room, surgeons must become aware of its limitations with respect to file storage and playback. Google Glass has a usable memory of about 12 GB (1 hour of video may require 1.5-2.0 GB). Conversely, GoPro’s capacity is defined by the micro SD card used. Therefore, the Google Glass hard drive must be regularly maintained well before being brought into the operating room, whereas recording can be extended (with respect to memory) for the GoPro if the media card is large enough.
Both devices allow for wired file transfer, which may be done with Windows Explorer (PC) or iPhoto (Macintosh). However, Google Glass also allows for wireless transfer, through portable storage supported by Google. Although this type of file transfer may be convenient for short, everyday clips made outside the operating room, it is prohibitive for surgical media, mainly because of patient privacy concerns. With wireless transfer to a nonsecure cloud platform, there is a risk of breach of patient confidentiality. We therefore recommend against using wireless upload when producing surgical multimedia, as patient identifiers are likely to be included in the recorded audio or video contents. Conversely, with GoPro, the micro SD card can be used as a portable hard drive to transfer files to a laptop or media reader, obviating the need for wired or wireless transmission. Last, when using traditional wire transfer or memory card to upload to a hard drive, users must ensure that the drive complies with patient privacy laws and regulations.
4. Privacy and patient consent
As mentioned, great care must be taken to ensure that patient privacy laws are followed. This is especially relevant with content uploaded to online cloud storage, as with Google Glass. The upload may occur automatically if the unit is connected to a Wi-Fi hotspot. In addition, when using surgical media for a real-time webcast for education or demonstration purposes, surgeons must ensure that no protected health information is broadcast and that the patient and the surgical team are aware of the webcast and its purposes.
Before using wearable technology during patient care, patient consent must be obtained. Surgeons should ask the patient to consent to video recording of surgery or an encounter (eg, clinic visit) for education purposes. Our institution’s consent form includes a section for this particular type of consent. If an institution’s form lacks such a section, surgeons should consult their risk management department to ensure there is a proper avenue for obtaining patient consent to record the procedure or encounter. A separate, dedicated media consent form may be required. Last, whoever operates a wearable device should be careful to use the device only during encounters that have received explicit recording consent—as opposed to wearing the device in the hallways or elsewhere in the hospital, where protected health information might be inadvertently recorded.4
5. Putting it all to use
After successful recording of surgery, an effort should be made to produce a high-quality video for education or demonstration purposes. Unfortunately, there is no built-in optical zooming with Google Glass or GoPro, and recording segments in which surgeons focus on detailed anatomy (with high-quality zoom) may prove difficult. Online descriptions of do-it-yourself modifications to place zoom capability on GoPro devices may be useful in surgical video recording, particularly for small surgical fields (hand or foot surgery). In addition, footage may be zoomed in on during postprocessing (Figure 3), though some resolution will be lost in the editing.
There is no practical way to incorporate Google Glass or GoPro while using surgical loupes or a surgical microscope. As a result, videos recorded with wearable technology may not reach the minimum resolution needed for useful surgical technique videos, as these traditionally are produced on high-definition camcorders with optical zoom, allowing detailed viewing of anatomical structures without resolution loss through digital zoom or postprocessing editing.
There has been tremendous benefit in incorporating wearable technology into our practice. Videos made with Google Glass and GoPro have been successfully used for surgical preparation and training, allowing orthopedic surgical residents to rehearse surgery before participating in it. Alternatively, having used Google Glass or GoPro to record a case, residents have then been able to review each surgical step on video—thereby reinforcing their knowledge of the steps, techniques, pearls, and pitfalls before performing the surgery again. Footage from surgeries recorded with Google Glass and GoPro has also been shown at weekly technique-focused conferences, allowing surgeons to analyze particular steps and highlight applicable learning points. Last, attending surgeons in our practice have used wearable technology in “coaching” mode, either reviewing case footage to identify areas for improvement or sharing footage with senior surgeons in order to elicit feedback and suggestions for possible improvement.
As new iterations of wearable video technology come to market, with advancements in both hardware and software, surgeons may be able to enhance education and teaching through seamless recording of surgical procedures. Use of wearable technology may also begin to extend beyond the operating room—to outpatient settings, such as preoperative and postoperative physical examinations. The latest versions of Google Glass and GoPro Hero allow surgeons to record surgical procedures with relative ease, without the personnel, equipment, and coordination required for traditional surgical videography.
Video 1. Coracoid harvest for transfer during Latarjet procedure performed and filmed by Dr. Jobin using GoPro Hero 3.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Video 2. Distal biceps repair performed by Dr. Makhni and Dr. Jobin, filmed by Dr. Makhni using Google Glass.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Safe and efficient advanced surgical skill training is of tremendous importance. With the recent increase in Internet use for medical education, there has been a concomitant increase in video recording of surgical procedures and techniques. Surgical recordings have been used in a variety of ways—as live webcasts for remote participants, as “coaching” opportunities for surgeons evaluating their own performance in the operating room, and even as informational resources for patients about to undergo the same surgery.
Surgical multimedia is being delivered through several different outlets. Many academic conferences and meetings showcase videos of different procedures, and several subspecialty societies (eg, Arthroscopy Association of North America) house archives of technical videos for viewing by members. In addition, the VuMedi website offers videos and allows members to comment on them and interact with the videographers. Surgeons are even posting technique videos on YouTube and other public websites.
A large proportion of surgical multimedia is recorded with conventional high-definition video cameras.1 Besides being able to experience a case at any time and from outside the operating room, the audience can watch from numerous vantage points, angles, and zoom levels. Also, surgeons’ narration can be valuable in helping the audience follow along with the case.
Recording surgical multimedia historically required tight coordination and precise planning by surgeon and videographer. However, innovations in wearable technology now allow surgeons to literally wear video cameras and record procedures as they perform them, in real time—to act as both surgeon and videographer.
Two such products are Google Glass (Google, Mountain View, California) and GoPro Hero (GoPro, San Mateo, California), both of which allow surgeons to record exactly what they see during procedures (Figure 1). Using a wearable technology for surgical multimedia creation requires a deep familiarity with its capabilities and limitations. In this article, we summarize these products’ similarities and differences and provide a technical overview for using wearable technologies in surgical multimedia creation.
1. Choosing a device
When purchasing either wearable device, several factors must be considered, including budget, possible uses outside the operating room, and possible limitations of the technology (Table 1). At this time, Google Glass is significantly more expensive than GoPro Hero. The Google Glass base unit costs $1500, and the GoPro Hero 3 model costs approximately $200 (higher-priced Hero models are available). Both devices require accessories (eg, portable battery unit, dedicated hard drive).
Device capabilities must also be considered (Table 2). Google Glass consists of both hardware and software. Users can record what is seen and heard through the lens and then use apps to create text and e-mail portals to online gaming, social media, and even golf-course GPS. The app market for Google Glass is nascent but undoubtedly will increase in volume and scope as more users adopt the technology (Google Glass comes with both Bluetooth and Wi-Fi and can function tethered through a smartphone). GoPro is mainly a hardware unit that can record in various settings (it is popular with athletes who want to capture and broadcast their participation in action sports). Newer GoPro Hero versions offer Wi-Fi, which allows streaming of video content to a smartphone or tablet through an app. Having clearly defined goals for a device—as they pertain to use outside the operating room, such as outdoor activities and underwater recording—may help the surgeon decide which product is more suitable. Last, it is important to consider limitations. Google Glass resolution is 720p (1280×720) for video and 5 MP for still images, and GoPro resolution can reach 1080p (1920×1080) for video and 5 MP for stills.
Both devices require purchase of accessories. An external USB battery pack is useful for both devices, as is a password-encrypted hard drive for media storage. Lenswear does not come with the base version of Google Glass and is purchased separately from the company. GoPro users buy micro SD cards (~$50 per 64-GB high-speed transfer card) for storage on the device and may buy lithium-ion batteries as an alternative to the external USB battery pack.
Author Update
In January 2015, Google announced that it was temporarily suspending its “Explorer” program, which allowed individual users to buy and test the device for personal use. However, Google is continuing its development of Glass with health care technology, among other areas of growth and development.2,3
2. Recording a successful surgical video
Unlike a camcorder, which typically is set on a tripod for conventional video recording of surgery, Google Glass and GoPro are intricately linked to the operator. Surgeons must be constantly aware of where they are during surgery and try not to let anything obstruct the camera’s view.
Before starting a case, the surgeon using either device must ensure that its battery is fully charged, as a full charge typically supports 1 hour of continuous recording (the Google Glass battery is a lithium-ion 670-mAh internal unit). A full charge should be enough to capture a short case. Newer GoPro models, with a battery listed at 1050 mAh, provide 1 to 2 hours of recording. When more than 1 hour is needed, an external USB battery pack can be used. This pack allows the device to remain plugged in throughout the case (the pack is kept in the surgeon’s back pocket). We recommend having an external battery pack that is at least 10,000 mAh (~$30 online retail), which easily provides 3+ hours of recording. Unfortunately, this arrangement can be cumbersome. Alternatively, with GoPro, additional batteries may be purchased, but the user needs to dismount the device in order to swap them in (may be difficult during surgery). With both units, partitioning a video into shorter segments conserves battery power and minimizes the risk of file corruption, which may occur if the battery dies or the device overheats.
Google Glass users can bypass manual operation of the device by giving it voice commands (eg, start video, take still image). The exception is for recording video for more than 10 seconds (current default setting). Unfortunately, the surgeon must touch the device to start this recording, which means using extra gloves to preserve operating field sterility. Still images can be made through a combination of voice and head gestures and without manual intervention (Figure 2). Last, users must ensure that the device is not actively connected through Bluetooth to a mobile phone, as incoming calls, text messages, and e-mails may disrupt active recording and become a distraction. The connection can be deactivated by disabling Bluetooth on the host smartphone or by placing the phone into airplane mode and turning off Wi-Fi.
Google Glass users can see what is being recorded through the viewfinder prism, whereas GoPro requires precise framing of the video before recording. Framing is done by grossly aiming the device in the desired direction. However, there is no way to ensure exact aim during recording. If at any point during a case there is slight repositioning of the GoPro, there is a risk of recording the case out of the center of view. An important advantage to newer GoPro versions is the ability to control the device through a wireless remote that can be placed under the surgeon’s gown. The remote can be used to pause and resume recording, without changing gloves, as is done with Google Glass. Last, because the minimum viewing distance from the surgical field is usually 18 inches or more, typically there is no loss of focus or blurring of the image from short-distance recording on either device.
3. File management and playback
Before using wearable technology in the operating room, surgeons must become aware of its limitations with respect to file storage and playback. Google Glass has a usable memory of about 12 GB (1 hour of video may require 1.5-2.0 GB). Conversely, GoPro’s capacity is defined by the micro SD card used. Therefore, the Google Glass hard drive must be regularly maintained well before being brought into the operating room, whereas recording can be extended (with respect to memory) for the GoPro if the media card is large enough.
Both devices allow for wired file transfer, which may be done with Windows Explorer (PC) or iPhoto (Macintosh). However, Google Glass also allows for wireless transfer, through portable storage supported by Google. Although this type of file transfer may be convenient for short, everyday clips made outside the operating room, it is prohibitive for surgical media, mainly because of patient privacy concerns. With wireless transfer to a nonsecure cloud platform, there is a risk of breach of patient confidentiality. We therefore recommend against using wireless upload when producing surgical multimedia, as patient identifiers are likely to be included in the recorded audio or video contents. Conversely, with GoPro, the micro SD card can be used as a portable hard drive to transfer files to a laptop or media reader, obviating the need for wired or wireless transmission. Last, when using traditional wire transfer or memory card to upload to a hard drive, users must ensure that the drive complies with patient privacy laws and regulations.
4. Privacy and patient consent
As mentioned, great care must be taken to ensure that patient privacy laws are followed. This is especially relevant with content uploaded to online cloud storage, as with Google Glass. The upload may occur automatically if the unit is connected to a Wi-Fi hotspot. In addition, when using surgical media for a real-time webcast for education or demonstration purposes, surgeons must ensure that no protected health information is broadcast and that the patient and the surgical team are aware of the webcast and its purposes.
Before using wearable technology during patient care, patient consent must be obtained. Surgeons should ask the patient to consent to video recording of surgery or an encounter (eg, clinic visit) for education purposes. Our institution’s consent form includes a section for this particular type of consent. If an institution’s form lacks such a section, surgeons should consult their risk management department to ensure there is a proper avenue for obtaining patient consent to record the procedure or encounter. A separate, dedicated media consent form may be required. Last, whoever operates a wearable device should be careful to use the device only during encounters that have received explicit recording consent—as opposed to wearing the device in the hallways or elsewhere in the hospital, where protected health information might be inadvertently recorded.4
5. Putting it all to use
After successful recording of surgery, an effort should be made to produce a high-quality video for education or demonstration purposes. Unfortunately, there is no built-in optical zooming with Google Glass or GoPro, and recording segments in which surgeons focus on detailed anatomy (with high-quality zoom) may prove difficult. Online descriptions of do-it-yourself modifications to place zoom capability on GoPro devices may be useful in surgical video recording, particularly for small surgical fields (hand or foot surgery). In addition, footage may be zoomed in on during postprocessing (Figure 3), though some resolution will be lost in the editing.
There is no practical way to incorporate Google Glass or GoPro while using surgical loupes or a surgical microscope. As a result, videos recorded with wearable technology may not reach the minimum resolution needed for useful surgical technique videos, as these traditionally are produced on high-definition camcorders with optical zoom, allowing detailed viewing of anatomical structures without resolution loss through digital zoom or postprocessing editing.
There has been tremendous benefit in incorporating wearable technology into our practice. Videos made with Google Glass and GoPro have been successfully used for surgical preparation and training, allowing orthopedic surgical residents to rehearse surgery before participating in it. Alternatively, having used Google Glass or GoPro to record a case, residents have then been able to review each surgical step on video—thereby reinforcing their knowledge of the steps, techniques, pearls, and pitfalls before performing the surgery again. Footage from surgeries recorded with Google Glass and GoPro has also been shown at weekly technique-focused conferences, allowing surgeons to analyze particular steps and highlight applicable learning points. Last, attending surgeons in our practice have used wearable technology in “coaching” mode, either reviewing case footage to identify areas for improvement or sharing footage with senior surgeons in order to elicit feedback and suggestions for possible improvement.
As new iterations of wearable video technology come to market, with advancements in both hardware and software, surgeons may be able to enhance education and teaching through seamless recording of surgical procedures. Use of wearable technology may also begin to extend beyond the operating room—to outpatient settings, such as preoperative and postoperative physical examinations. The latest versions of Google Glass and GoPro Hero allow surgeons to record surgical procedures with relative ease, without the personnel, equipment, and coordination required for traditional surgical videography.
Video 1. Coracoid harvest for transfer during Latarjet procedure performed and filmed by Dr. Jobin using GoPro Hero 3.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Video 2. Distal biceps repair performed by Dr. Makhni and Dr. Jobin, filmed by Dr. Makhni using Google Glass.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
1. Leahy M. Creating a good surgical technique video. AAOS Now. 2010;4(11). http://www.aaos.org/news/aaosnow/nov10/clinical4.asp. Accessed February 15, 2015.
2. Google Glass sales halted but firm says kit is not dead. BBC News website. http://www.bbc.com/news/technology-30831128. Published January 15, 2015. Accessed February 18, 2015.
3. Metz C. Sorry, but Google Glass isn’t anywhere close to dead. Wired website. http://www.wired.com/2015/02/sorry-google-glass-isnt-anywhere-close-dead/. Published February 8, 2015. Accessed February 18, 2015.
4. Peregrin T. Surgeons see future applications for Google Glass. Bull Am Coll Surg. 2014;99(7):9-16. http://bulletin.facs.org/2014/07/surgeons-see-future-applications-for-google-glass/#.U8SLKZaJAyZ.twitter. Accessed February 15, 2015.
1. Leahy M. Creating a good surgical technique video. AAOS Now. 2010;4(11). http://www.aaos.org/news/aaosnow/nov10/clinical4.asp. Accessed February 15, 2015.
2. Google Glass sales halted but firm says kit is not dead. BBC News website. http://www.bbc.com/news/technology-30831128. Published January 15, 2015. Accessed February 18, 2015.
3. Metz C. Sorry, but Google Glass isn’t anywhere close to dead. Wired website. http://www.wired.com/2015/02/sorry-google-glass-isnt-anywhere-close-dead/. Published February 8, 2015. Accessed February 18, 2015.
4. Peregrin T. Surgeons see future applications for Google Glass. Bull Am Coll Surg. 2014;99(7):9-16. http://bulletin.facs.org/2014/07/surgeons-see-future-applications-for-google-glass/#.U8SLKZaJAyZ.twitter. Accessed February 15, 2015.
Sports Activity After Reverse Total Shoulder Arthroplasty With Minimum 2-Year Follow-Up
The treatment of patients with severe shoulder pain and disability combined with a nonfunctional rotator cuff was a clinical challenge until the development of the reverse total shoulder arthroplasty (RTSA).1-3 Massive rotator cuff tears can leave patients with a pseudoparalytic upper extremity and may result in advanced arthritis of the joint because of altered mechanical and nutritional factors.4 In this setting, simply replacing the arthritic joint with standard total shoulder arthroplasty (TSA) is not recommended because it does not address the functional deficits, and it has poor long-term outcomes.3,5 RTSA works by changing the center of rotation of the shoulder joint so that the deltoid muscle can be used to elevate the arm.6,7 The 4 rotator cuff muscles are not required for forward elevation or stability of this constrained implant.6,8
Current indications for RTSA are cuff tear arthropathy, complex proximal humerus fractures, and revision from hemiarthroplasty or TSA with rotator cuff dysfunction. Patients with advanced cuff tear arthropathy have minimal forward elevation and pseudoparalysis. Previous studies have shown mean preoperative forward flexion of 55º and mean ASES (American Shoulder and Elbow Surgeons) Standardized Shoulder Assessment Form score of 34.3.9 Thus, minimal overhead activity is possible without RTSA. Advances in the RTSA technique have led to promising results (excellent functional improvement), but there is limited information regarding the activity levels patients can achieve after surgery.7,9-11
We conducted a study of the types of sporting activities in which patients with RTSA could participate. We hypothesized that, relative to historic controls, patients with RTSA could return to low-intensity sporting activities with improvement in motion and ASES scores.
Materials and Methods
After this study received institutional review board approval, patients who had undergone RTSA at our institution between January 1, 2004 and December 31, 2010 were identified by the billing codes used for the procedure. Each patient who had RTSA performed during the study period was included in the study. Charts were then reviewed to extract demographic data, preoperative diagnosis, surgery date, operative side, dominant side, type of implant used, operative complications, and subsequent revisions. A questionnaire (Appendix) was designed and used to assess activity, functional status, pain, and satisfaction levels after RTSA. Patients had to be willing and able to complete this questionnaire in order to be included in the study.
The questionnaire included demographic questions; a list of 42 activities patients could choose from to describe their current activity level, activities they were able to perform before the surgery, and activities they wish they could perform; a list of reasons for any limitations; and questions about overall pain, strength, and satisfaction with the procedure. In addition, there was an open-ended question for activities that may not have been listed. The questionnaire also included a validated method for assessing shoulder range of motion (ROM) at home, where patients rated their overhead motion according to standardized physical landmarks, including the level of the shoulder, chin, eyebrows, top of head, and above head.12-14 Also provided was the ASES Standardized Shoulder Assessment Form, which features a 100-point visual analog scale for pain plus functional ability questions, with higher scores indicating less pain and better function.15,16 The minimal clinical significance in the ASES score is 6.4 points.17,18 Scores were recorded and analyzed. Student t test was used to calculate statistical differences between patients who had primary RTSA performed and patients who underwent revision RTSA.
Study personnel contacted patients by telephone and direct mailing. Patients who could not be reached initially were called at least 4 more times: twice during the weekday, once during the evening, and once on the weekend. Patients who could not be contacted by telephone were then cross-referenced with the Social Security database to see if any were deceased. Response data were tabulated, and patients were stratified into high-, moderate-, and low-intensity activity.
One of the 3 senior authors (Dr. Ahmad, Dr. Bigliani, Dr. Levine) performed the 95 RTSAs: 84 Zimmer (Warsaw, Indiana), 7 DePuy (Warsaw, Indiana), 4 Tornier (Minneapolis, Minnesota). The DePuy and Tornier implants were used when a 30-mm glenoid peg was required (before Zimmer offered this length in its system). The procedure was done with a deltopectoral approach with 20° of retroversion. In revision cases, the same approach was used, the hardware or implants were removed, and the position of the humeral component was determined based on the pectoralis major insertion and the deltoid tension. In 80% of cases, the subscapularis was not repaired; in the other 20%, it was. Whether it was repaired depended on tendon viability and surgeon preference, as subscapularis repair status has been shown not to affect functional outcome.19-21 No combined latissimus transfers were performed. Patients wore a sling the first 4 weeks after surgery (only wrist and elbow motion allowed) and then advanced to active shoulder ROM. Eight weeks after surgery, they began gentle shoulder strengthening.
Results
One hundred nine consecutive patients underwent RTSA at a single institution. Fifteen patients subsequently died, 14 could not be contacted, and 2 declined, leaving 78 patients available for clinical follow-up. Mean follow-up was 4.8 years (range 2-9 years). Mean (SD) age at surgery was 75.3 (7.5) years. Seventy-five percent of the patients were women. Sixty-one percent underwent surgery for cuff tear arthropathy, 31% for revision of previous arthroplasty or internal fixation, 7% for complex fractures, and 1% for tumor. Of the 24 revisions, 15 were for failed hemiarthroplasty, 3 were for failed TSA with rotator cuff dysfunction, 4 were for fracture with failed internal fixation, and 2 were for failed RTSA referred from other institutions. The dominant shoulder was involved 62% of the time. Preoperative active forward shoulder elevation was less than 90° in all patients. There were 10 complications: 2 dislocations that were closed-reduced and remained stable, 1 dislocation that required revision of the liner, 1 aseptic loosening in a patient who has declined revision, 2 dissociated glenosphere baseplates, 2 deep infections that required 2-stage exchanges, 1 deep infection that required a 2-stage exchange that was then complicated by dissociation of the glenosphere baseplate requiring revision, and 1 superficial infection that resolved with oral antibiotics.
After surgery, mean active forward elevation was 140°, mean active external rotation was 48°, and mean active internal rotation was to S1. Mean (SD) postoperative ASES score was 77.5 (23.4). Satisfaction level was high (mean, 8.3/10), and mean pain levels were low: 2.3 out of 10 on the visual analog scale and 44.0 (SD, 11.7) on the ASES pain component. Strength was rated a mean of good. Table 1 lists the clinical data for the primary and revision surgery patients.
Eighteen patients (23.1%) returned to 24 different high-intensity activities, such as hunting, golf, and skiing; 38 patients (48.7%) returned to moderate-intensity activities, such as swimming, bowling, and raking leaves; and 22 patients (28.2%) returned to low-intensity activities, such as riding a stationary bike, playing a musical instrument, and walking (Table 2). Four patients played golf before and after RTSA, but neither of the 2 patients who played tennis before RTSA were able to do so after. Patients reported they engaged in their favorite leisure activity a mean of 4.8 times per week and a mean of 1.5 hours each time.
A medical problem was cited by 58% of patients as the reason for limited activity. These patients reported physical decline resulting from cardiac disease, diabetes, asthma/chronic obstructive pulmonary disease, or arthritis in other joints. Reasons for activity limitation are listed in Table 3. Post-RTSA activities that patients could not do for any reason are listed in Table 4. Activity limitations that patients attributed to the RTSA are listed in Table 5.
The majority of patients (57.7%) reported no change, from before RTSA to after RTSA, in being unable to do certain desired activities (eg, softball, target shooting, horseback riding, running, traveling). Sixteen patients (20.8%) reported being unable to return to an activity (eg, tennis, swimming, baseball, kayaking) they had been able to do before surgery. Most (69%) of those patients reported being unable to return to a moderate- or high-intensity activity after RTSA, but 81.8% were able to return to different moderate- or high-intensity activities.
Revision patients, who reported lower overhead activity levels, constituted 73% of the patients who felt their shoulder mechanically limited their activity, despite the fact that revisions constituted only 25% of the cases overall. Mean active ROM was statistically lower for revision patients than for primary patients (P < .05). Mean ASES score was statistically lower for the revision group (P < .001) and represented a clinically significant difference. Mean pain level was low (3.3) and satisfaction still generally high (7.4), but pain, satisfaction, and strength were about 1 point worse on average in the revision group than in the primary group.
Discussion
In the United States and other countries, RTSA implant survivorship is good.9,22 In this article, we have reported on post-RTSA activity levels, on the significant impact of comorbidities on this group, and on the negative effect of revisions on postoperative activity. Patients in this population reported that concomitant medical problems were the most important factor limiting their post-RTSA activity levels. Understanding and interpreting quality-of-life or functional scores in this elderly group must take into account the impact of comorbidities.23
Patients should have realistic postoperative expectations.24 In this study, some patients engaged in high-intensity overhead activities, such as golf, chopping wood, and shooting. However, the most difficulty was encountered trying to return to activities (eg, tennis, kayaking, archery, combing hair) that required external rotation in abduction.
Patients who had a previous implant (eg, hemiarthroplasty, TSA, failed internal fixation) revised to RTSA had lower activity levels and were 9 times more likely than primary patients to report having a mechanical shoulder limitation affecting their activity. Revision patients also had worse forward elevation, external rotation, pain, and satisfaction.
This study is limited in that it is retrospective. Subsequent prospective studies focused on younger patients who undergo primary RTSA may be useful if indications expand. In addition, subscapularis status and especially infraspinatus status may affect activity levels and could be analyzed in a study. Another limitation is that we did not specifically record detailed preoperative data, though all patients were known to have preoperative forward elevation of less than 90°.
In general, the primary measure of success for RTSA has been pain relief. Some studies have also reported on strength and ROM.2,20,25,26 A recent study using similar methodology demonstrated comparable ROM and low pain after RTSA, though revisions were not included in that study.26 In contrast to the present study, no patient in that study was able to play tennis or golf, but the reasons for the limited activity were not explored. In both studies, post-RTSA sports were generally of lower intensity than those played by patients after anatomical TSA.27
Overall, the majority of patients were very satisfied with their low pain level after RTSA. In addition, many patients not limited by other medical conditions were able to return to their pre-RTSA moderate-intensity recreational activities.
1. Baulot E, Chabernaud D, Grammont PM. Results of Grammont’s inverted prosthesis in omarthritis associated with major cuff destruction. Apropos of 16 cases [in French]. Acta Orthop Belg. 1995;61(suppl 1):112-119.
2. Sirveaux F, Favard L, Oudet D, Huquet D, Walch G, Molé D. Grammont inverted total shoulder arthroplasty in the treatment of glenohumeral osteoarthritis with massive rupture of the cuff. Results of a multicentre study of 80 shoulders. J Bone Joint Surg Br. 2004;86(3):388-395.
3. Franklin JL, Barrett WP, Jackins SE, Matsen FA 3rd. Glenoid loosening in total shoulder arthroplasty. Association with rotator cuff deficiency. J Arthroplasty. 1988;3(1):39-46.
4. Neer CS 2nd, Craig EV, Fukuda H. Cuff-tear arthropathy. J Bone Joint Surg Am. 1983;65(9):1232-1244.
5. Edwards TB, Boulahia A, Kempf JF, Boileau P, Nemoz C, Walch G. The influence of rotator cuff disease on the results of shoulder arthroplasty for primary osteoarthritis: results of a multicenter study. J Bone Joint Surg Am. 2002;84(12):2240-2248.
6. Boileau P, Watkinson DJ, Hatzidakis AM, Balg F. Grammont reverse prosthesis: design, rationale, and biomechanics. J Shoulder Elbow Surg. 2005;14(1 suppl S):147S-161S.
7. Nam D, Kepler CK, Neviaser AS, et al. Reverse total shoulder arthroplasty: current concepts, results, and component wear analysis. J Bone Joint Surg Am. 2010;92(suppl 2):23-35.
8. Ackland DC, Roshan-Zamir S, Richardson M, Pandy MG. Moment arms of the shoulder musculature after reverse total shoulder arthroplasty. J Bone Joint Surg Am. 2010;92(5):1221-1230.
9. Frankle M, Siegal S, Pupello D, Saleem A, Mighell M, Vasey M. The reverse shoulder prosthesis for glenohumeral arthritis associated with severe rotator cuff deficiency. A minimum two-year follow-up study of sixty patients. J Bone Joint Surg Am. 2005;87(8):1697-1705.
10. Cazeneuve JF, Cristofari DJ. Long term functional outcome following reverse shoulder arthroplasty in the elderly. Orthop Traumatol Surg Res. 2011;97(6):583-589.
11. Gerber C, Pennington, SD, Nyffeler RW. Reverse total shoulder arthroplasty. J Am Acad Orthop Surg. 2009;17(5):284-295.
12. Brophy RH, Beauvais RL, Jones EC, Cordasco FA, Marx RG. Measurement of shoulder activity level. Clin Orthop. 2005;(439):101-108.
13. Smith AM, Barnes SA, Sperling JW, Farrell CM, Cummings JD, Cofield RH. Patient and physician-assessed shoulder function after arthroplasty. J Bone Joint Surg Am. 2006;88(3):508-513.
14. Zarkadas PC, Throckmorton TQ, Dahm DL, Sperling J, Schleck CD, Cofield R. Patient reported activities after shoulder replacement: total and hemiarthroplasty. J Shoulder Elbow Surg. 2011;20(2):273-280.
15. Kocher, MS, Horan MP, Briggs KK, Richardson TR, O’Holleran J, Hawkins RJ. Reliability, validity, and responsiveness of the American Shoulder and Elbow Surgeons subjective shoulder scale in patients with shoulder instability, rotator cuff disease, and glenohumeral arthritis. J Bone Joint Surg Am. 2005;87(9):2006-2011.
16. Richards RR, An KN, Bigliani LU, et al. A standardized method for the assessment of shoulder function. J Shoulder Elbow Surg. 1994;3(6):347-352.
17. Michener LA, McClure PW, Sennett BJ. American Shoulder and Elbow Surgeons Standardized Shoulder Assessment Form, patient self-report section: reliability, validity, and responsiveness. J Shoulder Elbow Surg. 2002;11(6):587-594.
18. Hunsaker FG, Cioffi DA, Amadio PC, Wright JG, Caughlin B. The American Academy of Orthopaedic Surgeons outcomes instruments: normative values from the general population. J Bone Joint Surg Am. 2002;84(2):208-215.
19. Molé D, Favard L. Excentered scapulohumeral osteoarthritis [in French]. Rev Chir Orthop Reparatrice Appar Mot. 2007;93(6 suppl):37-94.
20. Clark JC, Ritchie J, Song FS, et al. Complication rates, dislocation, pain, and postoperative range of motion after reverse shoulder arthroplasty in patients with and without repair of the subscapularis. J Shoulder Elbow Surg. 2012;21(1):36-41.
21. Boulahia A, Edwards TB, Walch G, Baratta RV. Early results of a reverse design prosthesis in the treatment of arthritis of the shoulder in elderly patients with a large rotator cuff tear. Orthopedics. 2002;25(2):129-133.
22. Guery J, Favard L, Sirveaux F, Oudet D, Mole D, Walch G. Reverse total shoulder arthroplasty. Survivorship analysis of eighty replacements followed for five to ten years. J Bone Joint Surg Am. 2006;88(8):1742-1747.
23. Antuña SA, Sperling JW, Sánchez-Sotelo J, Cofield RH. Shoulder arthroplasty for proximal humeral nonunions. J Shoulder Elbow Surg. 2002;11(2):114-121.
24. Cheung E, Willis M, Walker M, Clark R, Frankle MA. Complications in reverse total shoulder arthroplasty. J Am Acad Orthop Surg. 2011;19(7):439-449.
25. Nolan BM, Ankerson E, Wiater JM. Reverse total shoulder arthroplasty improves function in cuff tear arthropathy. Clin Orthop. 2011;469(9):2476-2482.
26. Lawrence TM, Ahmadi S, Sanchez-Sotelo J, Sperling JW, Cofield RH. Patient reported activities after reverse shoulder arthroplasty: part II. J Shoulder Elbow Surg. 2012;21(11):1464-1469.
27. Schumann K, Flury MP, Schwyzer HK, Simmen BR, Drerup S, Goldhahn J. Sports activity after anatomical total shoulder arthroplasty. Am J Sports Med. 2010;38(10):2097-2105.
The treatment of patients with severe shoulder pain and disability combined with a nonfunctional rotator cuff was a clinical challenge until the development of the reverse total shoulder arthroplasty (RTSA).1-3 Massive rotator cuff tears can leave patients with a pseudoparalytic upper extremity and may result in advanced arthritis of the joint because of altered mechanical and nutritional factors.4 In this setting, simply replacing the arthritic joint with standard total shoulder arthroplasty (TSA) is not recommended because it does not address the functional deficits, and it has poor long-term outcomes.3,5 RTSA works by changing the center of rotation of the shoulder joint so that the deltoid muscle can be used to elevate the arm.6,7 The 4 rotator cuff muscles are not required for forward elevation or stability of this constrained implant.6,8
Current indications for RTSA are cuff tear arthropathy, complex proximal humerus fractures, and revision from hemiarthroplasty or TSA with rotator cuff dysfunction. Patients with advanced cuff tear arthropathy have minimal forward elevation and pseudoparalysis. Previous studies have shown mean preoperative forward flexion of 55º and mean ASES (American Shoulder and Elbow Surgeons) Standardized Shoulder Assessment Form score of 34.3.9 Thus, minimal overhead activity is possible without RTSA. Advances in the RTSA technique have led to promising results (excellent functional improvement), but there is limited information regarding the activity levels patients can achieve after surgery.7,9-11
We conducted a study of the types of sporting activities in which patients with RTSA could participate. We hypothesized that, relative to historic controls, patients with RTSA could return to low-intensity sporting activities with improvement in motion and ASES scores.
Materials and Methods
After this study received institutional review board approval, patients who had undergone RTSA at our institution between January 1, 2004 and December 31, 2010 were identified by the billing codes used for the procedure. Each patient who had RTSA performed during the study period was included in the study. Charts were then reviewed to extract demographic data, preoperative diagnosis, surgery date, operative side, dominant side, type of implant used, operative complications, and subsequent revisions. A questionnaire (Appendix) was designed and used to assess activity, functional status, pain, and satisfaction levels after RTSA. Patients had to be willing and able to complete this questionnaire in order to be included in the study.
The questionnaire included demographic questions; a list of 42 activities patients could choose from to describe their current activity level, activities they were able to perform before the surgery, and activities they wish they could perform; a list of reasons for any limitations; and questions about overall pain, strength, and satisfaction with the procedure. In addition, there was an open-ended question for activities that may not have been listed. The questionnaire also included a validated method for assessing shoulder range of motion (ROM) at home, where patients rated their overhead motion according to standardized physical landmarks, including the level of the shoulder, chin, eyebrows, top of head, and above head.12-14 Also provided was the ASES Standardized Shoulder Assessment Form, which features a 100-point visual analog scale for pain plus functional ability questions, with higher scores indicating less pain and better function.15,16 The minimal clinical significance in the ASES score is 6.4 points.17,18 Scores were recorded and analyzed. Student t test was used to calculate statistical differences between patients who had primary RTSA performed and patients who underwent revision RTSA.
Study personnel contacted patients by telephone and direct mailing. Patients who could not be reached initially were called at least 4 more times: twice during the weekday, once during the evening, and once on the weekend. Patients who could not be contacted by telephone were then cross-referenced with the Social Security database to see if any were deceased. Response data were tabulated, and patients were stratified into high-, moderate-, and low-intensity activity.
One of the 3 senior authors (Dr. Ahmad, Dr. Bigliani, Dr. Levine) performed the 95 RTSAs: 84 Zimmer (Warsaw, Indiana), 7 DePuy (Warsaw, Indiana), 4 Tornier (Minneapolis, Minnesota). The DePuy and Tornier implants were used when a 30-mm glenoid peg was required (before Zimmer offered this length in its system). The procedure was done with a deltopectoral approach with 20° of retroversion. In revision cases, the same approach was used, the hardware or implants were removed, and the position of the humeral component was determined based on the pectoralis major insertion and the deltoid tension. In 80% of cases, the subscapularis was not repaired; in the other 20%, it was. Whether it was repaired depended on tendon viability and surgeon preference, as subscapularis repair status has been shown not to affect functional outcome.19-21 No combined latissimus transfers were performed. Patients wore a sling the first 4 weeks after surgery (only wrist and elbow motion allowed) and then advanced to active shoulder ROM. Eight weeks after surgery, they began gentle shoulder strengthening.
Results
One hundred nine consecutive patients underwent RTSA at a single institution. Fifteen patients subsequently died, 14 could not be contacted, and 2 declined, leaving 78 patients available for clinical follow-up. Mean follow-up was 4.8 years (range 2-9 years). Mean (SD) age at surgery was 75.3 (7.5) years. Seventy-five percent of the patients were women. Sixty-one percent underwent surgery for cuff tear arthropathy, 31% for revision of previous arthroplasty or internal fixation, 7% for complex fractures, and 1% for tumor. Of the 24 revisions, 15 were for failed hemiarthroplasty, 3 were for failed TSA with rotator cuff dysfunction, 4 were for fracture with failed internal fixation, and 2 were for failed RTSA referred from other institutions. The dominant shoulder was involved 62% of the time. Preoperative active forward shoulder elevation was less than 90° in all patients. There were 10 complications: 2 dislocations that were closed-reduced and remained stable, 1 dislocation that required revision of the liner, 1 aseptic loosening in a patient who has declined revision, 2 dissociated glenosphere baseplates, 2 deep infections that required 2-stage exchanges, 1 deep infection that required a 2-stage exchange that was then complicated by dissociation of the glenosphere baseplate requiring revision, and 1 superficial infection that resolved with oral antibiotics.
After surgery, mean active forward elevation was 140°, mean active external rotation was 48°, and mean active internal rotation was to S1. Mean (SD) postoperative ASES score was 77.5 (23.4). Satisfaction level was high (mean, 8.3/10), and mean pain levels were low: 2.3 out of 10 on the visual analog scale and 44.0 (SD, 11.7) on the ASES pain component. Strength was rated a mean of good. Table 1 lists the clinical data for the primary and revision surgery patients.
Eighteen patients (23.1%) returned to 24 different high-intensity activities, such as hunting, golf, and skiing; 38 patients (48.7%) returned to moderate-intensity activities, such as swimming, bowling, and raking leaves; and 22 patients (28.2%) returned to low-intensity activities, such as riding a stationary bike, playing a musical instrument, and walking (Table 2). Four patients played golf before and after RTSA, but neither of the 2 patients who played tennis before RTSA were able to do so after. Patients reported they engaged in their favorite leisure activity a mean of 4.8 times per week and a mean of 1.5 hours each time.
A medical problem was cited by 58% of patients as the reason for limited activity. These patients reported physical decline resulting from cardiac disease, diabetes, asthma/chronic obstructive pulmonary disease, or arthritis in other joints. Reasons for activity limitation are listed in Table 3. Post-RTSA activities that patients could not do for any reason are listed in Table 4. Activity limitations that patients attributed to the RTSA are listed in Table 5.
The majority of patients (57.7%) reported no change, from before RTSA to after RTSA, in being unable to do certain desired activities (eg, softball, target shooting, horseback riding, running, traveling). Sixteen patients (20.8%) reported being unable to return to an activity (eg, tennis, swimming, baseball, kayaking) they had been able to do before surgery. Most (69%) of those patients reported being unable to return to a moderate- or high-intensity activity after RTSA, but 81.8% were able to return to different moderate- or high-intensity activities.
Revision patients, who reported lower overhead activity levels, constituted 73% of the patients who felt their shoulder mechanically limited their activity, despite the fact that revisions constituted only 25% of the cases overall. Mean active ROM was statistically lower for revision patients than for primary patients (P < .05). Mean ASES score was statistically lower for the revision group (P < .001) and represented a clinically significant difference. Mean pain level was low (3.3) and satisfaction still generally high (7.4), but pain, satisfaction, and strength were about 1 point worse on average in the revision group than in the primary group.
Discussion
In the United States and other countries, RTSA implant survivorship is good.9,22 In this article, we have reported on post-RTSA activity levels, on the significant impact of comorbidities on this group, and on the negative effect of revisions on postoperative activity. Patients in this population reported that concomitant medical problems were the most important factor limiting their post-RTSA activity levels. Understanding and interpreting quality-of-life or functional scores in this elderly group must take into account the impact of comorbidities.23
Patients should have realistic postoperative expectations.24 In this study, some patients engaged in high-intensity overhead activities, such as golf, chopping wood, and shooting. However, the most difficulty was encountered trying to return to activities (eg, tennis, kayaking, archery, combing hair) that required external rotation in abduction.
Patients who had a previous implant (eg, hemiarthroplasty, TSA, failed internal fixation) revised to RTSA had lower activity levels and were 9 times more likely than primary patients to report having a mechanical shoulder limitation affecting their activity. Revision patients also had worse forward elevation, external rotation, pain, and satisfaction.
This study is limited in that it is retrospective. Subsequent prospective studies focused on younger patients who undergo primary RTSA may be useful if indications expand. In addition, subscapularis status and especially infraspinatus status may affect activity levels and could be analyzed in a study. Another limitation is that we did not specifically record detailed preoperative data, though all patients were known to have preoperative forward elevation of less than 90°.
In general, the primary measure of success for RTSA has been pain relief. Some studies have also reported on strength and ROM.2,20,25,26 A recent study using similar methodology demonstrated comparable ROM and low pain after RTSA, though revisions were not included in that study.26 In contrast to the present study, no patient in that study was able to play tennis or golf, but the reasons for the limited activity were not explored. In both studies, post-RTSA sports were generally of lower intensity than those played by patients after anatomical TSA.27
Overall, the majority of patients were very satisfied with their low pain level after RTSA. In addition, many patients not limited by other medical conditions were able to return to their pre-RTSA moderate-intensity recreational activities.
The treatment of patients with severe shoulder pain and disability combined with a nonfunctional rotator cuff was a clinical challenge until the development of the reverse total shoulder arthroplasty (RTSA).1-3 Massive rotator cuff tears can leave patients with a pseudoparalytic upper extremity and may result in advanced arthritis of the joint because of altered mechanical and nutritional factors.4 In this setting, simply replacing the arthritic joint with standard total shoulder arthroplasty (TSA) is not recommended because it does not address the functional deficits, and it has poor long-term outcomes.3,5 RTSA works by changing the center of rotation of the shoulder joint so that the deltoid muscle can be used to elevate the arm.6,7 The 4 rotator cuff muscles are not required for forward elevation or stability of this constrained implant.6,8
Current indications for RTSA are cuff tear arthropathy, complex proximal humerus fractures, and revision from hemiarthroplasty or TSA with rotator cuff dysfunction. Patients with advanced cuff tear arthropathy have minimal forward elevation and pseudoparalysis. Previous studies have shown mean preoperative forward flexion of 55º and mean ASES (American Shoulder and Elbow Surgeons) Standardized Shoulder Assessment Form score of 34.3.9 Thus, minimal overhead activity is possible without RTSA. Advances in the RTSA technique have led to promising results (excellent functional improvement), but there is limited information regarding the activity levels patients can achieve after surgery.7,9-11
We conducted a study of the types of sporting activities in which patients with RTSA could participate. We hypothesized that, relative to historic controls, patients with RTSA could return to low-intensity sporting activities with improvement in motion and ASES scores.
Materials and Methods
After this study received institutional review board approval, patients who had undergone RTSA at our institution between January 1, 2004 and December 31, 2010 were identified by the billing codes used for the procedure. Each patient who had RTSA performed during the study period was included in the study. Charts were then reviewed to extract demographic data, preoperative diagnosis, surgery date, operative side, dominant side, type of implant used, operative complications, and subsequent revisions. A questionnaire (Appendix) was designed and used to assess activity, functional status, pain, and satisfaction levels after RTSA. Patients had to be willing and able to complete this questionnaire in order to be included in the study.
The questionnaire included demographic questions; a list of 42 activities patients could choose from to describe their current activity level, activities they were able to perform before the surgery, and activities they wish they could perform; a list of reasons for any limitations; and questions about overall pain, strength, and satisfaction with the procedure. In addition, there was an open-ended question for activities that may not have been listed. The questionnaire also included a validated method for assessing shoulder range of motion (ROM) at home, where patients rated their overhead motion according to standardized physical landmarks, including the level of the shoulder, chin, eyebrows, top of head, and above head.12-14 Also provided was the ASES Standardized Shoulder Assessment Form, which features a 100-point visual analog scale for pain plus functional ability questions, with higher scores indicating less pain and better function.15,16 The minimal clinical significance in the ASES score is 6.4 points.17,18 Scores were recorded and analyzed. Student t test was used to calculate statistical differences between patients who had primary RTSA performed and patients who underwent revision RTSA.
Study personnel contacted patients by telephone and direct mailing. Patients who could not be reached initially were called at least 4 more times: twice during the weekday, once during the evening, and once on the weekend. Patients who could not be contacted by telephone were then cross-referenced with the Social Security database to see if any were deceased. Response data were tabulated, and patients were stratified into high-, moderate-, and low-intensity activity.
One of the 3 senior authors (Dr. Ahmad, Dr. Bigliani, Dr. Levine) performed the 95 RTSAs: 84 Zimmer (Warsaw, Indiana), 7 DePuy (Warsaw, Indiana), 4 Tornier (Minneapolis, Minnesota). The DePuy and Tornier implants were used when a 30-mm glenoid peg was required (before Zimmer offered this length in its system). The procedure was done with a deltopectoral approach with 20° of retroversion. In revision cases, the same approach was used, the hardware or implants were removed, and the position of the humeral component was determined based on the pectoralis major insertion and the deltoid tension. In 80% of cases, the subscapularis was not repaired; in the other 20%, it was. Whether it was repaired depended on tendon viability and surgeon preference, as subscapularis repair status has been shown not to affect functional outcome.19-21 No combined latissimus transfers were performed. Patients wore a sling the first 4 weeks after surgery (only wrist and elbow motion allowed) and then advanced to active shoulder ROM. Eight weeks after surgery, they began gentle shoulder strengthening.
Results
One hundred nine consecutive patients underwent RTSA at a single institution. Fifteen patients subsequently died, 14 could not be contacted, and 2 declined, leaving 78 patients available for clinical follow-up. Mean follow-up was 4.8 years (range 2-9 years). Mean (SD) age at surgery was 75.3 (7.5) years. Seventy-five percent of the patients were women. Sixty-one percent underwent surgery for cuff tear arthropathy, 31% for revision of previous arthroplasty or internal fixation, 7% for complex fractures, and 1% for tumor. Of the 24 revisions, 15 were for failed hemiarthroplasty, 3 were for failed TSA with rotator cuff dysfunction, 4 were for fracture with failed internal fixation, and 2 were for failed RTSA referred from other institutions. The dominant shoulder was involved 62% of the time. Preoperative active forward shoulder elevation was less than 90° in all patients. There were 10 complications: 2 dislocations that were closed-reduced and remained stable, 1 dislocation that required revision of the liner, 1 aseptic loosening in a patient who has declined revision, 2 dissociated glenosphere baseplates, 2 deep infections that required 2-stage exchanges, 1 deep infection that required a 2-stage exchange that was then complicated by dissociation of the glenosphere baseplate requiring revision, and 1 superficial infection that resolved with oral antibiotics.
After surgery, mean active forward elevation was 140°, mean active external rotation was 48°, and mean active internal rotation was to S1. Mean (SD) postoperative ASES score was 77.5 (23.4). Satisfaction level was high (mean, 8.3/10), and mean pain levels were low: 2.3 out of 10 on the visual analog scale and 44.0 (SD, 11.7) on the ASES pain component. Strength was rated a mean of good. Table 1 lists the clinical data for the primary and revision surgery patients.
Eighteen patients (23.1%) returned to 24 different high-intensity activities, such as hunting, golf, and skiing; 38 patients (48.7%) returned to moderate-intensity activities, such as swimming, bowling, and raking leaves; and 22 patients (28.2%) returned to low-intensity activities, such as riding a stationary bike, playing a musical instrument, and walking (Table 2). Four patients played golf before and after RTSA, but neither of the 2 patients who played tennis before RTSA were able to do so after. Patients reported they engaged in their favorite leisure activity a mean of 4.8 times per week and a mean of 1.5 hours each time.
A medical problem was cited by 58% of patients as the reason for limited activity. These patients reported physical decline resulting from cardiac disease, diabetes, asthma/chronic obstructive pulmonary disease, or arthritis in other joints. Reasons for activity limitation are listed in Table 3. Post-RTSA activities that patients could not do for any reason are listed in Table 4. Activity limitations that patients attributed to the RTSA are listed in Table 5.
The majority of patients (57.7%) reported no change, from before RTSA to after RTSA, in being unable to do certain desired activities (eg, softball, target shooting, horseback riding, running, traveling). Sixteen patients (20.8%) reported being unable to return to an activity (eg, tennis, swimming, baseball, kayaking) they had been able to do before surgery. Most (69%) of those patients reported being unable to return to a moderate- or high-intensity activity after RTSA, but 81.8% were able to return to different moderate- or high-intensity activities.
Revision patients, who reported lower overhead activity levels, constituted 73% of the patients who felt their shoulder mechanically limited their activity, despite the fact that revisions constituted only 25% of the cases overall. Mean active ROM was statistically lower for revision patients than for primary patients (P < .05). Mean ASES score was statistically lower for the revision group (P < .001) and represented a clinically significant difference. Mean pain level was low (3.3) and satisfaction still generally high (7.4), but pain, satisfaction, and strength were about 1 point worse on average in the revision group than in the primary group.
Discussion
In the United States and other countries, RTSA implant survivorship is good.9,22 In this article, we have reported on post-RTSA activity levels, on the significant impact of comorbidities on this group, and on the negative effect of revisions on postoperative activity. Patients in this population reported that concomitant medical problems were the most important factor limiting their post-RTSA activity levels. Understanding and interpreting quality-of-life or functional scores in this elderly group must take into account the impact of comorbidities.23
Patients should have realistic postoperative expectations.24 In this study, some patients engaged in high-intensity overhead activities, such as golf, chopping wood, and shooting. However, the most difficulty was encountered trying to return to activities (eg, tennis, kayaking, archery, combing hair) that required external rotation in abduction.
Patients who had a previous implant (eg, hemiarthroplasty, TSA, failed internal fixation) revised to RTSA had lower activity levels and were 9 times more likely than primary patients to report having a mechanical shoulder limitation affecting their activity. Revision patients also had worse forward elevation, external rotation, pain, and satisfaction.
This study is limited in that it is retrospective. Subsequent prospective studies focused on younger patients who undergo primary RTSA may be useful if indications expand. In addition, subscapularis status and especially infraspinatus status may affect activity levels and could be analyzed in a study. Another limitation is that we did not specifically record detailed preoperative data, though all patients were known to have preoperative forward elevation of less than 90°.
In general, the primary measure of success for RTSA has been pain relief. Some studies have also reported on strength and ROM.2,20,25,26 A recent study using similar methodology demonstrated comparable ROM and low pain after RTSA, though revisions were not included in that study.26 In contrast to the present study, no patient in that study was able to play tennis or golf, but the reasons for the limited activity were not explored. In both studies, post-RTSA sports were generally of lower intensity than those played by patients after anatomical TSA.27
Overall, the majority of patients were very satisfied with their low pain level after RTSA. In addition, many patients not limited by other medical conditions were able to return to their pre-RTSA moderate-intensity recreational activities.
1. Baulot E, Chabernaud D, Grammont PM. Results of Grammont’s inverted prosthesis in omarthritis associated with major cuff destruction. Apropos of 16 cases [in French]. Acta Orthop Belg. 1995;61(suppl 1):112-119.
2. Sirveaux F, Favard L, Oudet D, Huquet D, Walch G, Molé D. Grammont inverted total shoulder arthroplasty in the treatment of glenohumeral osteoarthritis with massive rupture of the cuff. Results of a multicentre study of 80 shoulders. J Bone Joint Surg Br. 2004;86(3):388-395.
3. Franklin JL, Barrett WP, Jackins SE, Matsen FA 3rd. Glenoid loosening in total shoulder arthroplasty. Association with rotator cuff deficiency. J Arthroplasty. 1988;3(1):39-46.
4. Neer CS 2nd, Craig EV, Fukuda H. Cuff-tear arthropathy. J Bone Joint Surg Am. 1983;65(9):1232-1244.
5. Edwards TB, Boulahia A, Kempf JF, Boileau P, Nemoz C, Walch G. The influence of rotator cuff disease on the results of shoulder arthroplasty for primary osteoarthritis: results of a multicenter study. J Bone Joint Surg Am. 2002;84(12):2240-2248.
6. Boileau P, Watkinson DJ, Hatzidakis AM, Balg F. Grammont reverse prosthesis: design, rationale, and biomechanics. J Shoulder Elbow Surg. 2005;14(1 suppl S):147S-161S.
7. Nam D, Kepler CK, Neviaser AS, et al. Reverse total shoulder arthroplasty: current concepts, results, and component wear analysis. J Bone Joint Surg Am. 2010;92(suppl 2):23-35.
8. Ackland DC, Roshan-Zamir S, Richardson M, Pandy MG. Moment arms of the shoulder musculature after reverse total shoulder arthroplasty. J Bone Joint Surg Am. 2010;92(5):1221-1230.
9. Frankle M, Siegal S, Pupello D, Saleem A, Mighell M, Vasey M. The reverse shoulder prosthesis for glenohumeral arthritis associated with severe rotator cuff deficiency. A minimum two-year follow-up study of sixty patients. J Bone Joint Surg Am. 2005;87(8):1697-1705.
10. Cazeneuve JF, Cristofari DJ. Long term functional outcome following reverse shoulder arthroplasty in the elderly. Orthop Traumatol Surg Res. 2011;97(6):583-589.
11. Gerber C, Pennington, SD, Nyffeler RW. Reverse total shoulder arthroplasty. J Am Acad Orthop Surg. 2009;17(5):284-295.
12. Brophy RH, Beauvais RL, Jones EC, Cordasco FA, Marx RG. Measurement of shoulder activity level. Clin Orthop. 2005;(439):101-108.
13. Smith AM, Barnes SA, Sperling JW, Farrell CM, Cummings JD, Cofield RH. Patient and physician-assessed shoulder function after arthroplasty. J Bone Joint Surg Am. 2006;88(3):508-513.
14. Zarkadas PC, Throckmorton TQ, Dahm DL, Sperling J, Schleck CD, Cofield R. Patient reported activities after shoulder replacement: total and hemiarthroplasty. J Shoulder Elbow Surg. 2011;20(2):273-280.
15. Kocher, MS, Horan MP, Briggs KK, Richardson TR, O’Holleran J, Hawkins RJ. Reliability, validity, and responsiveness of the American Shoulder and Elbow Surgeons subjective shoulder scale in patients with shoulder instability, rotator cuff disease, and glenohumeral arthritis. J Bone Joint Surg Am. 2005;87(9):2006-2011.
16. Richards RR, An KN, Bigliani LU, et al. A standardized method for the assessment of shoulder function. J Shoulder Elbow Surg. 1994;3(6):347-352.
17. Michener LA, McClure PW, Sennett BJ. American Shoulder and Elbow Surgeons Standardized Shoulder Assessment Form, patient self-report section: reliability, validity, and responsiveness. J Shoulder Elbow Surg. 2002;11(6):587-594.
18. Hunsaker FG, Cioffi DA, Amadio PC, Wright JG, Caughlin B. The American Academy of Orthopaedic Surgeons outcomes instruments: normative values from the general population. J Bone Joint Surg Am. 2002;84(2):208-215.
19. Molé D, Favard L. Excentered scapulohumeral osteoarthritis [in French]. Rev Chir Orthop Reparatrice Appar Mot. 2007;93(6 suppl):37-94.
20. Clark JC, Ritchie J, Song FS, et al. Complication rates, dislocation, pain, and postoperative range of motion after reverse shoulder arthroplasty in patients with and without repair of the subscapularis. J Shoulder Elbow Surg. 2012;21(1):36-41.
21. Boulahia A, Edwards TB, Walch G, Baratta RV. Early results of a reverse design prosthesis in the treatment of arthritis of the shoulder in elderly patients with a large rotator cuff tear. Orthopedics. 2002;25(2):129-133.
22. Guery J, Favard L, Sirveaux F, Oudet D, Mole D, Walch G. Reverse total shoulder arthroplasty. Survivorship analysis of eighty replacements followed for five to ten years. J Bone Joint Surg Am. 2006;88(8):1742-1747.
23. Antuña SA, Sperling JW, Sánchez-Sotelo J, Cofield RH. Shoulder arthroplasty for proximal humeral nonunions. J Shoulder Elbow Surg. 2002;11(2):114-121.
24. Cheung E, Willis M, Walker M, Clark R, Frankle MA. Complications in reverse total shoulder arthroplasty. J Am Acad Orthop Surg. 2011;19(7):439-449.
25. Nolan BM, Ankerson E, Wiater JM. Reverse total shoulder arthroplasty improves function in cuff tear arthropathy. Clin Orthop. 2011;469(9):2476-2482.
26. Lawrence TM, Ahmadi S, Sanchez-Sotelo J, Sperling JW, Cofield RH. Patient reported activities after reverse shoulder arthroplasty: part II. J Shoulder Elbow Surg. 2012;21(11):1464-1469.
27. Schumann K, Flury MP, Schwyzer HK, Simmen BR, Drerup S, Goldhahn J. Sports activity after anatomical total shoulder arthroplasty. Am J Sports Med. 2010;38(10):2097-2105.
1. Baulot E, Chabernaud D, Grammont PM. Results of Grammont’s inverted prosthesis in omarthritis associated with major cuff destruction. Apropos of 16 cases [in French]. Acta Orthop Belg. 1995;61(suppl 1):112-119.
2. Sirveaux F, Favard L, Oudet D, Huquet D, Walch G, Molé D. Grammont inverted total shoulder arthroplasty in the treatment of glenohumeral osteoarthritis with massive rupture of the cuff. Results of a multicentre study of 80 shoulders. J Bone Joint Surg Br. 2004;86(3):388-395.
3. Franklin JL, Barrett WP, Jackins SE, Matsen FA 3rd. Glenoid loosening in total shoulder arthroplasty. Association with rotator cuff deficiency. J Arthroplasty. 1988;3(1):39-46.
4. Neer CS 2nd, Craig EV, Fukuda H. Cuff-tear arthropathy. J Bone Joint Surg Am. 1983;65(9):1232-1244.
5. Edwards TB, Boulahia A, Kempf JF, Boileau P, Nemoz C, Walch G. The influence of rotator cuff disease on the results of shoulder arthroplasty for primary osteoarthritis: results of a multicenter study. J Bone Joint Surg Am. 2002;84(12):2240-2248.
6. Boileau P, Watkinson DJ, Hatzidakis AM, Balg F. Grammont reverse prosthesis: design, rationale, and biomechanics. J Shoulder Elbow Surg. 2005;14(1 suppl S):147S-161S.
7. Nam D, Kepler CK, Neviaser AS, et al. Reverse total shoulder arthroplasty: current concepts, results, and component wear analysis. J Bone Joint Surg Am. 2010;92(suppl 2):23-35.
8. Ackland DC, Roshan-Zamir S, Richardson M, Pandy MG. Moment arms of the shoulder musculature after reverse total shoulder arthroplasty. J Bone Joint Surg Am. 2010;92(5):1221-1230.
9. Frankle M, Siegal S, Pupello D, Saleem A, Mighell M, Vasey M. The reverse shoulder prosthesis for glenohumeral arthritis associated with severe rotator cuff deficiency. A minimum two-year follow-up study of sixty patients. J Bone Joint Surg Am. 2005;87(8):1697-1705.
10. Cazeneuve JF, Cristofari DJ. Long term functional outcome following reverse shoulder arthroplasty in the elderly. Orthop Traumatol Surg Res. 2011;97(6):583-589.
11. Gerber C, Pennington, SD, Nyffeler RW. Reverse total shoulder arthroplasty. J Am Acad Orthop Surg. 2009;17(5):284-295.
12. Brophy RH, Beauvais RL, Jones EC, Cordasco FA, Marx RG. Measurement of shoulder activity level. Clin Orthop. 2005;(439):101-108.
13. Smith AM, Barnes SA, Sperling JW, Farrell CM, Cummings JD, Cofield RH. Patient and physician-assessed shoulder function after arthroplasty. J Bone Joint Surg Am. 2006;88(3):508-513.
14. Zarkadas PC, Throckmorton TQ, Dahm DL, Sperling J, Schleck CD, Cofield R. Patient reported activities after shoulder replacement: total and hemiarthroplasty. J Shoulder Elbow Surg. 2011;20(2):273-280.
15. Kocher, MS, Horan MP, Briggs KK, Richardson TR, O’Holleran J, Hawkins RJ. Reliability, validity, and responsiveness of the American Shoulder and Elbow Surgeons subjective shoulder scale in patients with shoulder instability, rotator cuff disease, and glenohumeral arthritis. J Bone Joint Surg Am. 2005;87(9):2006-2011.
16. Richards RR, An KN, Bigliani LU, et al. A standardized method for the assessment of shoulder function. J Shoulder Elbow Surg. 1994;3(6):347-352.
17. Michener LA, McClure PW, Sennett BJ. American Shoulder and Elbow Surgeons Standardized Shoulder Assessment Form, patient self-report section: reliability, validity, and responsiveness. J Shoulder Elbow Surg. 2002;11(6):587-594.
18. Hunsaker FG, Cioffi DA, Amadio PC, Wright JG, Caughlin B. The American Academy of Orthopaedic Surgeons outcomes instruments: normative values from the general population. J Bone Joint Surg Am. 2002;84(2):208-215.
19. Molé D, Favard L. Excentered scapulohumeral osteoarthritis [in French]. Rev Chir Orthop Reparatrice Appar Mot. 2007;93(6 suppl):37-94.
20. Clark JC, Ritchie J, Song FS, et al. Complication rates, dislocation, pain, and postoperative range of motion after reverse shoulder arthroplasty in patients with and without repair of the subscapularis. J Shoulder Elbow Surg. 2012;21(1):36-41.
21. Boulahia A, Edwards TB, Walch G, Baratta RV. Early results of a reverse design prosthesis in the treatment of arthritis of the shoulder in elderly patients with a large rotator cuff tear. Orthopedics. 2002;25(2):129-133.
22. Guery J, Favard L, Sirveaux F, Oudet D, Mole D, Walch G. Reverse total shoulder arthroplasty. Survivorship analysis of eighty replacements followed for five to ten years. J Bone Joint Surg Am. 2006;88(8):1742-1747.
23. Antuña SA, Sperling JW, Sánchez-Sotelo J, Cofield RH. Shoulder arthroplasty for proximal humeral nonunions. J Shoulder Elbow Surg. 2002;11(2):114-121.
24. Cheung E, Willis M, Walker M, Clark R, Frankle MA. Complications in reverse total shoulder arthroplasty. J Am Acad Orthop Surg. 2011;19(7):439-449.
25. Nolan BM, Ankerson E, Wiater JM. Reverse total shoulder arthroplasty improves function in cuff tear arthropathy. Clin Orthop. 2011;469(9):2476-2482.
26. Lawrence TM, Ahmadi S, Sanchez-Sotelo J, Sperling JW, Cofield RH. Patient reported activities after reverse shoulder arthroplasty: part II. J Shoulder Elbow Surg. 2012;21(11):1464-1469.
27. Schumann K, Flury MP, Schwyzer HK, Simmen BR, Drerup S, Goldhahn J. Sports activity after anatomical total shoulder arthroplasty. Am J Sports Med. 2010;38(10):2097-2105.