User login
Emergency Imaging: Abdominal Pain 6 Months After Cesarean Delivery
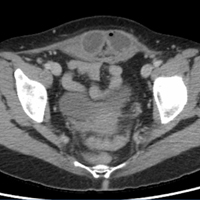
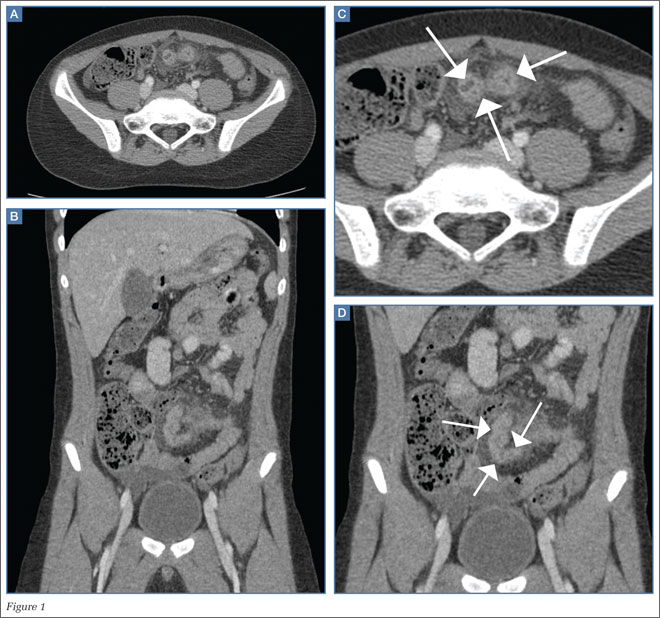
A 45-year-old woman with a history of polycystic ovary syndrome presented to the ED for evaluation of acute abdominal pain. The patient’s surgical history was significant for a cesarean delivery 6 months prior to presentation. Abdominal examination revealed a well-healed suprapubic cesarean incision scar, which was tender upon palpation. A computed tomography (CT) scan of the abdomen and pelvis with contrast were ordered; representative images are shown above (Figure 1a-1d).
What is the diagnosis? What are the associated complications and preferred management for this entity?
Answer
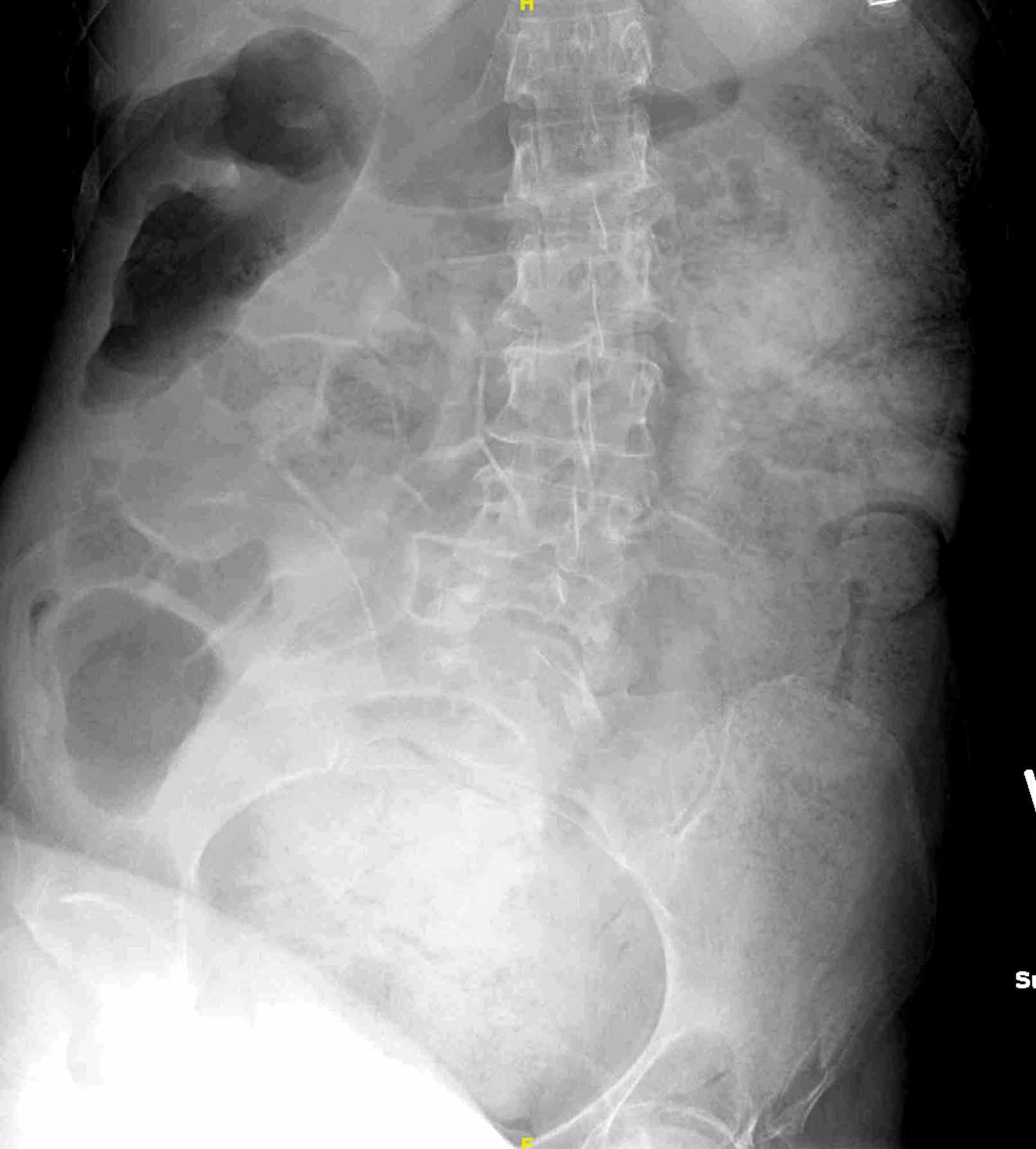
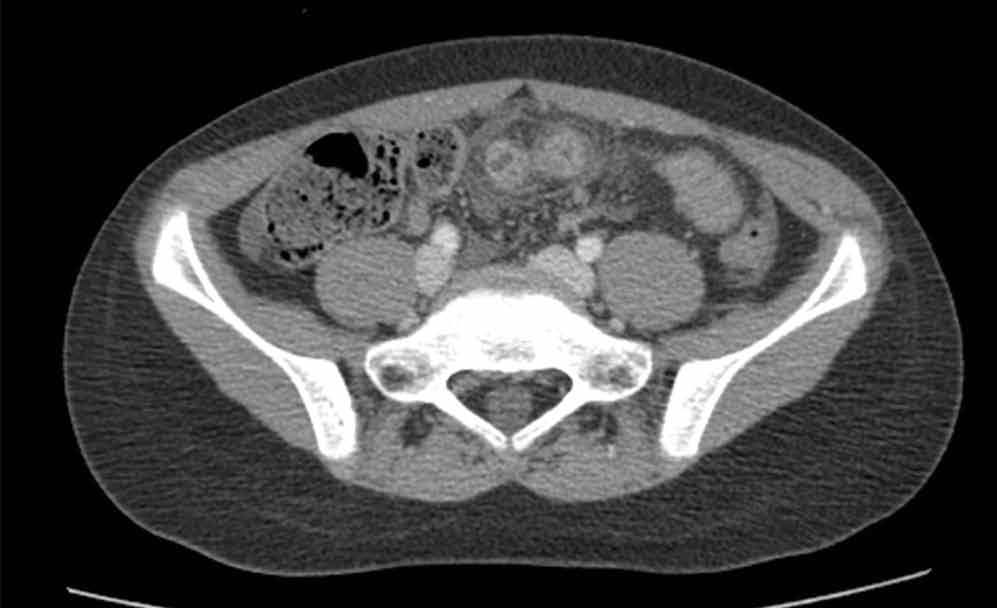
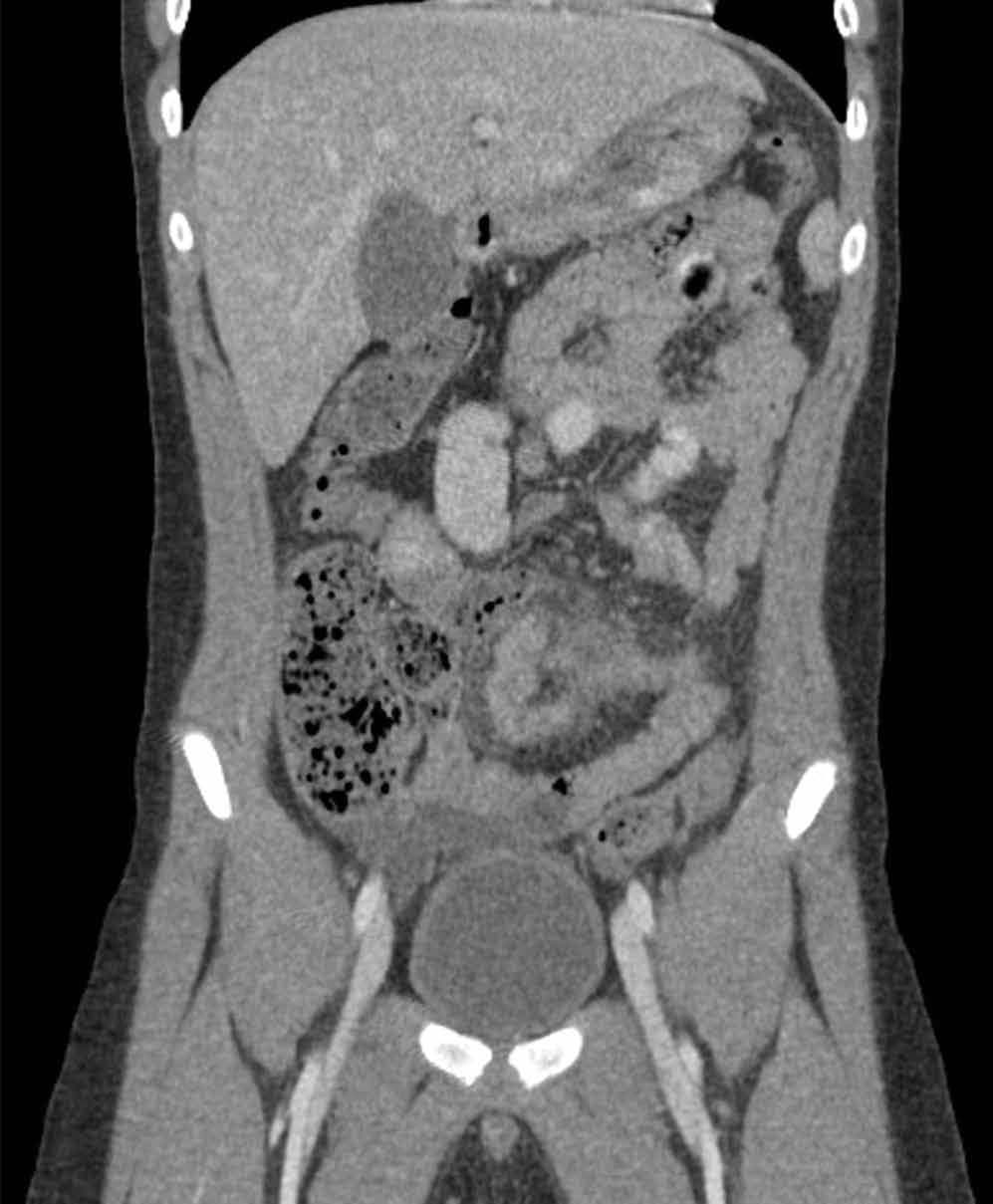
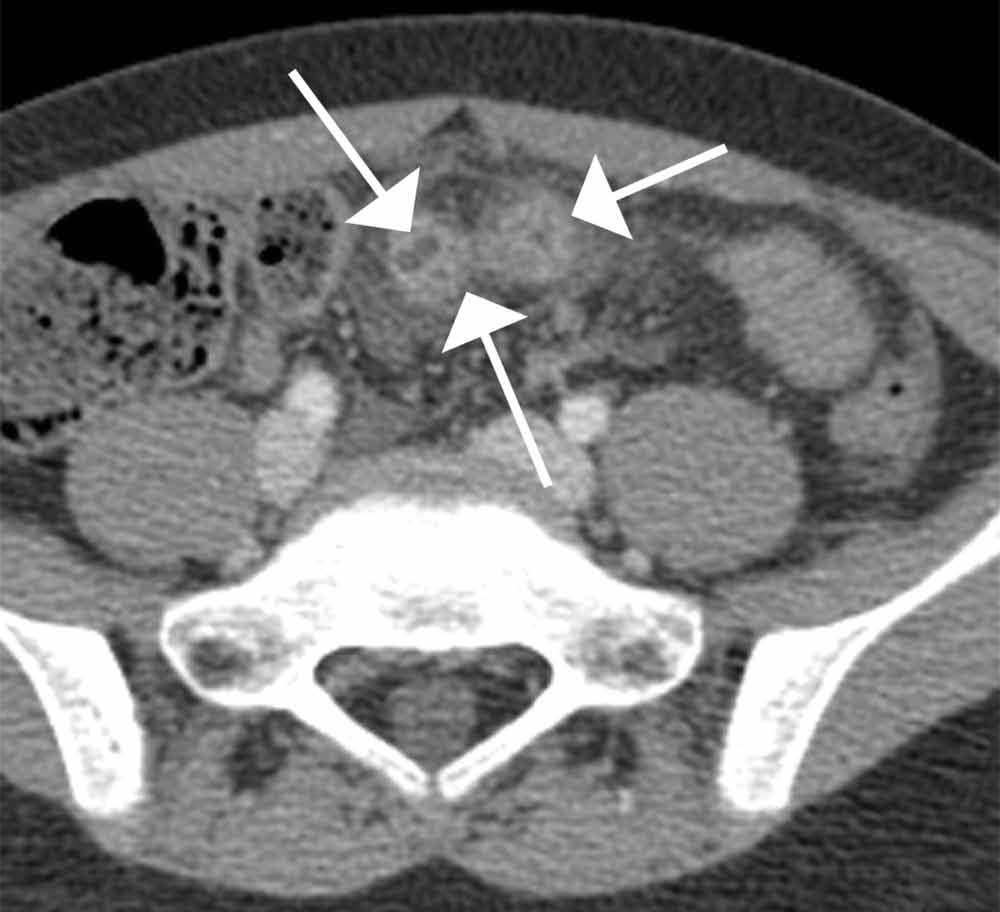
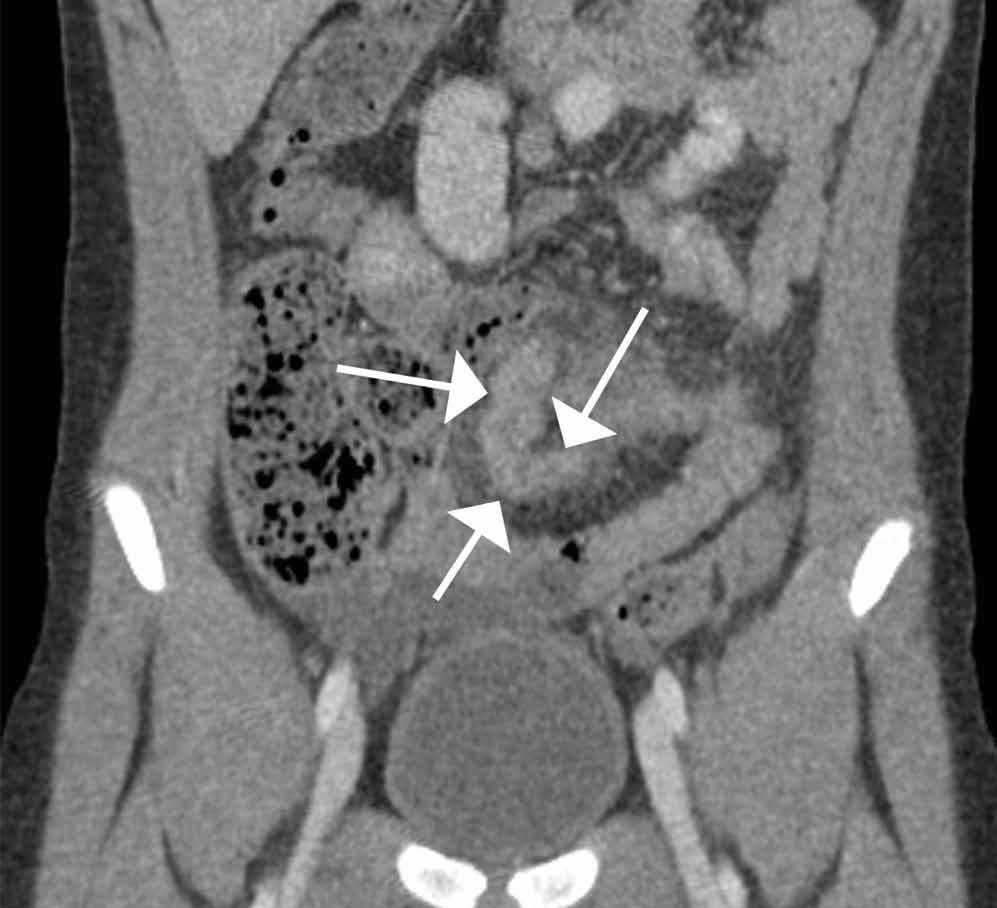
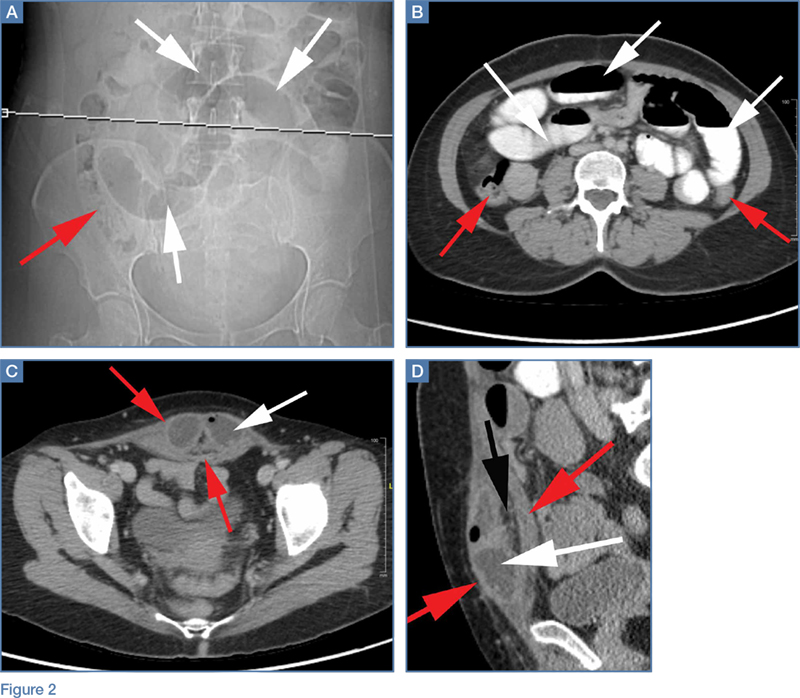
The scout image from the CT scan shows multiple dilated loops of small bowel (white arrows, Figure 2a) and only a small amount of air within a decompressed colon (red arrow, Figure 2a). The multiplanar CT image confirmed multiple dilated small bowel loops (white arrows, Figure 2b) and the decompressed large bowel (red arrows, Figure 2b), indicating the presence of a small bowel obstruction. A distal small bowel loop (white arrows, Figure 2c and 2d) was identified in a hernia sac within the walls of the rectus abdominis muscle (red arrows, Figure 2c and 2d). Mesenteric stranding within the hernia sac was suggestive of incarceration (black arrow, Figure 2d). No signs of intestinal ischemia, such as pneumatosis or wall thickening, were present.
An exploratory laparotomy was emergently performed, which confirmed the presence of incarcerated small bowel within the posterior rectus sheath defect without evidence of strangulation. Reduction of small bowel and primary closure of the hernia defect was subsequently performed without complication.
Abdominal Wall Hernias
Abdominal wall hernias are common in the United States, with more than 1 million abdominal wall hernia repairs performed annually.1 A posterior rectus sheath hernia is a rare type of abdominal wall hernia; the majority are postsurgical (as seen in this patient) or posttraumatic, with only a few reported congenital cases.2
Anatomy
The rectus sheath encloses the rectus abdominis muscle and is composed of the aponeuroses of the transversus abdominis, external oblique, and internal oblique muscles. The aponeuroses form an anterior and posterior sheath, which together serve as a strong barrier against the herniation of abdominal contents, accounting for the rarity of a spontaneous rectus sheath hernia. However, inferior to the umbilicus (below the arcuate line), the posterior rectus sheath is composed primarily of transversalis fascia, which may make this region more susceptible to herniation.3 Additional predisposing factors to herniation include increased muscle weakness and elevated intra-abdominal pressure, such as that which occurs during pregnancy or from ascites.4
Clinical Presentation
Like other abdominal wall hernias, the clinical presentation of posterior rectus sheath hernias is nonspecific. Patients may be asymptomatic or may develop abdominal pain, distension, and vomiting as a result of acute complications that necessitate emergent surgery. During history-taking, inquiry into a patient’s surgical history is crucial because it may raise clinical suspicion for an abdominal wall hernia, as was the case in our patient, who recently had a cesarean delivery.
Diagnosis
Because prompt and accurate diagnosis of acute complications of abdominal wall hernias is essential, imaging studies are typically required for diagnosis. Computed tomography is the modality of choice based on its ability to provide superior anatomic detail of the abdominal wall, permitting identification of hernias and differentiating them from other abdominal masses, such as hematomas, abscesses, or tumors. Additionally, CT is able to detect early signs of hernia sac complications, including bowel obstruction, incarceration, and strangulation.5
Treatment
Treatment for a posterior rectus sheath hernia is surgical with primary closure being the preferred method. Prosthetic repair may also be performed, particularly when the hernia defect is large, but it has been shown to be associated with an increased risk of intestinal strangulation.3
1. Rutkow IM. Demographic and socioeconomic aspects of hernia repair in the United States in 2003. Surg Clin North Am. 2003;83(5):1045-1051, v-vi. doi:10.1016/S0039-6109(03)00132-4.
2. Lenobel S, Lenobel R, Yu J. Posterior rectus sheath hernia causing intermittent small bowel obstruction. J Radiol Case Rep J. 2014;8(9):25-29. doi:10.3941/jrcr.v8i9.2081.
3. Losanoff JE, Basson MD, Gruber SA. Spontaneous hernia through the posterior rectus abdominis sheath: case report and review of the published literature 1937-2008. Hernia. 2009;13(5):555-558. doi:10.1007/s10029-009-0481-6.
4. Bentzon N, Adamsen S. Hernia of the posterior rectus sheath: a new entity? Eur J Surg. 1995;161(3):215-216.
5. Aguirre DA, Santosa AC, Casola G, Sirlin CB. Abdominal wall hernias: imaging features, complications, and diagnostic pitfalls at mutli-detector row CT. Radiographics. 2005;25(6):1501-1520. doi:10.1148/rg.256055018.
A 45-year-old woman with a history of polycystic ovary syndrome presented to the ED for evaluation of acute abdominal pain. The patient’s surgical history was significant for a cesarean delivery 6 months prior to presentation. Abdominal examination revealed a well-healed suprapubic cesarean incision scar, which was tender upon palpation. A computed tomography (CT) scan of the abdomen and pelvis with contrast were ordered; representative images are shown above (Figure 1a-1d).

What is the diagnosis? What are the associated complications and preferred management for this entity?
Answer
The scout image from the CT scan shows multiple dilated loops of small bowel (white arrows, Figure 2a) and only a small amount of air within a decompressed colon (red arrow, Figure 2a). The multiplanar CT image confirmed multiple dilated small bowel loops (white arrows, Figure 2b) and the decompressed large bowel (red arrows, Figure 2b), indicating the presence of a small bowel obstruction. A distal small bowel loop (white arrows, Figure 2c and 2d) was identified in a hernia sac within the walls of the rectus abdominis muscle (red arrows, Figure 2c and 2d). Mesenteric stranding within the hernia sac was suggestive of incarceration (black arrow, Figure 2d). No signs of intestinal ischemia, such as pneumatosis or wall thickening, were present.

An exploratory laparotomy was emergently performed, which confirmed the presence of incarcerated small bowel within the posterior rectus sheath defect without evidence of strangulation. Reduction of small bowel and primary closure of the hernia defect was subsequently performed without complication.
Abdominal Wall Hernias
Abdominal wall hernias are common in the United States, with more than 1 million abdominal wall hernia repairs performed annually.1 A posterior rectus sheath hernia is a rare type of abdominal wall hernia; the majority are postsurgical (as seen in this patient) or posttraumatic, with only a few reported congenital cases.2
Anatomy
The rectus sheath encloses the rectus abdominis muscle and is composed of the aponeuroses of the transversus abdominis, external oblique, and internal oblique muscles. The aponeuroses form an anterior and posterior sheath, which together serve as a strong barrier against the herniation of abdominal contents, accounting for the rarity of a spontaneous rectus sheath hernia. However, inferior to the umbilicus (below the arcuate line), the posterior rectus sheath is composed primarily of transversalis fascia, which may make this region more susceptible to herniation.3 Additional predisposing factors to herniation include increased muscle weakness and elevated intra-abdominal pressure, such as that which occurs during pregnancy or from ascites.4
Clinical Presentation
Like other abdominal wall hernias, the clinical presentation of posterior rectus sheath hernias is nonspecific. Patients may be asymptomatic or may develop abdominal pain, distension, and vomiting as a result of acute complications that necessitate emergent surgery. During history-taking, inquiry into a patient’s surgical history is crucial because it may raise clinical suspicion for an abdominal wall hernia, as was the case in our patient, who recently had a cesarean delivery.
Diagnosis
Because prompt and accurate diagnosis of acute complications of abdominal wall hernias is essential, imaging studies are typically required for diagnosis. Computed tomography is the modality of choice based on its ability to provide superior anatomic detail of the abdominal wall, permitting identification of hernias and differentiating them from other abdominal masses, such as hematomas, abscesses, or tumors. Additionally, CT is able to detect early signs of hernia sac complications, including bowel obstruction, incarceration, and strangulation.5
Treatment
Treatment for a posterior rectus sheath hernia is surgical with primary closure being the preferred method. Prosthetic repair may also be performed, particularly when the hernia defect is large, but it has been shown to be associated with an increased risk of intestinal strangulation.3
A 45-year-old woman with a history of polycystic ovary syndrome presented to the ED for evaluation of acute abdominal pain. The patient’s surgical history was significant for a cesarean delivery 6 months prior to presentation. Abdominal examination revealed a well-healed suprapubic cesarean incision scar, which was tender upon palpation. A computed tomography (CT) scan of the abdomen and pelvis with contrast were ordered; representative images are shown above (Figure 1a-1d).

What is the diagnosis? What are the associated complications and preferred management for this entity?
Answer
The scout image from the CT scan shows multiple dilated loops of small bowel (white arrows, Figure 2a) and only a small amount of air within a decompressed colon (red arrow, Figure 2a). The multiplanar CT image confirmed multiple dilated small bowel loops (white arrows, Figure 2b) and the decompressed large bowel (red arrows, Figure 2b), indicating the presence of a small bowel obstruction. A distal small bowel loop (white arrows, Figure 2c and 2d) was identified in a hernia sac within the walls of the rectus abdominis muscle (red arrows, Figure 2c and 2d). Mesenteric stranding within the hernia sac was suggestive of incarceration (black arrow, Figure 2d). No signs of intestinal ischemia, such as pneumatosis or wall thickening, were present.

An exploratory laparotomy was emergently performed, which confirmed the presence of incarcerated small bowel within the posterior rectus sheath defect without evidence of strangulation. Reduction of small bowel and primary closure of the hernia defect was subsequently performed without complication.
Abdominal Wall Hernias
Abdominal wall hernias are common in the United States, with more than 1 million abdominal wall hernia repairs performed annually.1 A posterior rectus sheath hernia is a rare type of abdominal wall hernia; the majority are postsurgical (as seen in this patient) or posttraumatic, with only a few reported congenital cases.2
Anatomy
The rectus sheath encloses the rectus abdominis muscle and is composed of the aponeuroses of the transversus abdominis, external oblique, and internal oblique muscles. The aponeuroses form an anterior and posterior sheath, which together serve as a strong barrier against the herniation of abdominal contents, accounting for the rarity of a spontaneous rectus sheath hernia. However, inferior to the umbilicus (below the arcuate line), the posterior rectus sheath is composed primarily of transversalis fascia, which may make this region more susceptible to herniation.3 Additional predisposing factors to herniation include increased muscle weakness and elevated intra-abdominal pressure, such as that which occurs during pregnancy or from ascites.4
Clinical Presentation
Like other abdominal wall hernias, the clinical presentation of posterior rectus sheath hernias is nonspecific. Patients may be asymptomatic or may develop abdominal pain, distension, and vomiting as a result of acute complications that necessitate emergent surgery. During history-taking, inquiry into a patient’s surgical history is crucial because it may raise clinical suspicion for an abdominal wall hernia, as was the case in our patient, who recently had a cesarean delivery.
Diagnosis
Because prompt and accurate diagnosis of acute complications of abdominal wall hernias is essential, imaging studies are typically required for diagnosis. Computed tomography is the modality of choice based on its ability to provide superior anatomic detail of the abdominal wall, permitting identification of hernias and differentiating them from other abdominal masses, such as hematomas, abscesses, or tumors. Additionally, CT is able to detect early signs of hernia sac complications, including bowel obstruction, incarceration, and strangulation.5
Treatment
Treatment for a posterior rectus sheath hernia is surgical with primary closure being the preferred method. Prosthetic repair may also be performed, particularly when the hernia defect is large, but it has been shown to be associated with an increased risk of intestinal strangulation.3
1. Rutkow IM. Demographic and socioeconomic aspects of hernia repair in the United States in 2003. Surg Clin North Am. 2003;83(5):1045-1051, v-vi. doi:10.1016/S0039-6109(03)00132-4.
2. Lenobel S, Lenobel R, Yu J. Posterior rectus sheath hernia causing intermittent small bowel obstruction. J Radiol Case Rep J. 2014;8(9):25-29. doi:10.3941/jrcr.v8i9.2081.
3. Losanoff JE, Basson MD, Gruber SA. Spontaneous hernia through the posterior rectus abdominis sheath: case report and review of the published literature 1937-2008. Hernia. 2009;13(5):555-558. doi:10.1007/s10029-009-0481-6.
4. Bentzon N, Adamsen S. Hernia of the posterior rectus sheath: a new entity? Eur J Surg. 1995;161(3):215-216.
5. Aguirre DA, Santosa AC, Casola G, Sirlin CB. Abdominal wall hernias: imaging features, complications, and diagnostic pitfalls at mutli-detector row CT. Radiographics. 2005;25(6):1501-1520. doi:10.1148/rg.256055018.
1. Rutkow IM. Demographic and socioeconomic aspects of hernia repair in the United States in 2003. Surg Clin North Am. 2003;83(5):1045-1051, v-vi. doi:10.1016/S0039-6109(03)00132-4.
2. Lenobel S, Lenobel R, Yu J. Posterior rectus sheath hernia causing intermittent small bowel obstruction. J Radiol Case Rep J. 2014;8(9):25-29. doi:10.3941/jrcr.v8i9.2081.
3. Losanoff JE, Basson MD, Gruber SA. Spontaneous hernia through the posterior rectus abdominis sheath: case report and review of the published literature 1937-2008. Hernia. 2009;13(5):555-558. doi:10.1007/s10029-009-0481-6.
4. Bentzon N, Adamsen S. Hernia of the posterior rectus sheath: a new entity? Eur J Surg. 1995;161(3):215-216.
5. Aguirre DA, Santosa AC, Casola G, Sirlin CB. Abdominal wall hernias: imaging features, complications, and diagnostic pitfalls at mutli-detector row CT. Radiographics. 2005;25(6):1501-1520. doi:10.1148/rg.256055018.
Emergency Imaging
Case
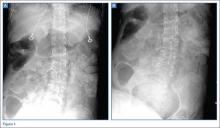
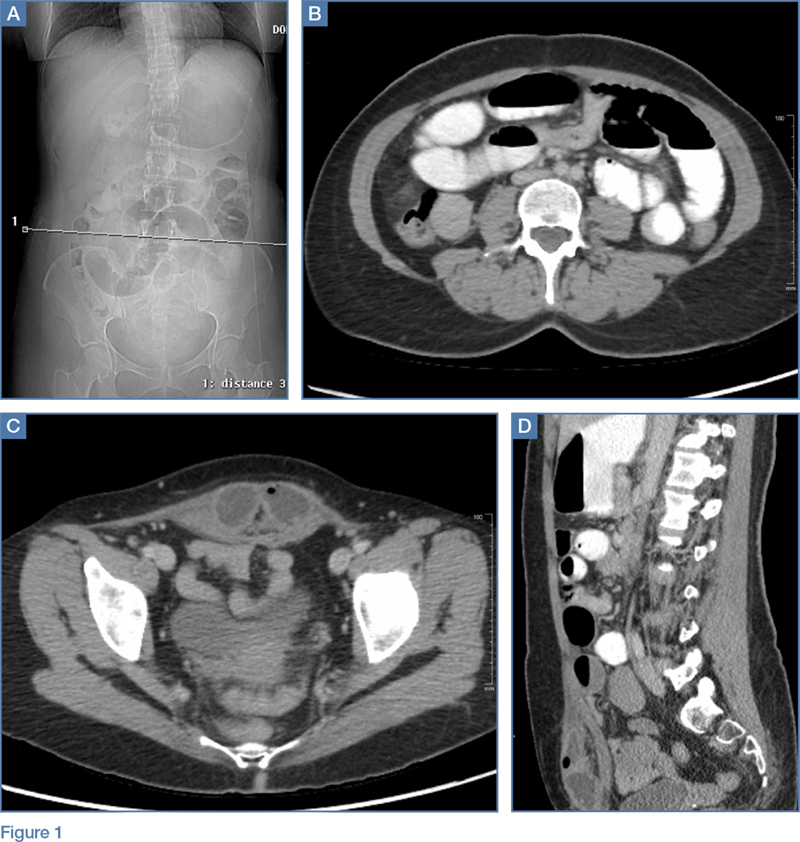
A 63-year-old woman with multiple medical conditions presented to the ED with abdominal distention, pain, and 4-day history of fever. Physical examination revealed a distended but nontender abdomen. Her vital signs included a fever of 103.1°F, tachycardia, tachypnea, and a blood pressure of 101/69. To further evaluate the abdominal distention, supine abdominal radiographs were obtained (Figure 1a and 1b).
What is the differential diagnosis?
What would be the most appropriate next imaging test?
Answer
The abdominal radiographs demonstrated a nonobstructed bowel gas pattern—ie, there were no dilated loops of small or large bowel (>3 cm or >7 cm, respectively). Although limited by supine position, there was no evidence of perforation as no signs of free air were visualized. There was a large amount of stool within the large bowel, appearing on radiographic images as the mottled air and soft-tissue density (white asterisks, Figure 2a and 2b).
Although bowel obstruction had been ruled out in this patient, the differential for abdominal pain with fever remained wide, predominately for infectious and inflammatory conditions (eg, appendicitis, diverticulitis, inflammatory bowel disease, other forms of colitis/enteritis, pancreatitis, abscess, mesenteric).In cases such as the one presented, the most appropriate next imaging examination would be a computed tomography (CT) scan with both oral and intravenous (IV) contrast. Oral contrast is useful since several of the conditions in the differential diagnosis require evaluation of the bowel wall and, in cases of suspected abscess, it assists in differentiating a collection from adjacent loops of bowel. Intravenous contrast is useful to evaluate for inflammation and assessing the bowel.
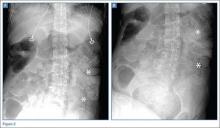
In this case, an abdominal CT was performed, using both oral and IV contrast. Axial and sagittal images demonstrated a large amount of stool in a distended rectum (white asterisks, Figures 3a and 3b), thickening of the rectal wall (white arrows, Figures 3a and 3b), and stranding of the perirectal fat indicative of perirectal inflammation (red arrows, Figures 3a and 3b). Based on these findings, the patient was diagnosed with stercoral colitis.
Stercoral colitis is inflammation of the colonic or rectal wall secondary to increased intraluminal pressure caused by impacted fecal material. This condition is most common in elderly patients and bedridden patients with chronic constipation. As this case illustrates, the clinical presentation of stercoral colitis is nonspecific, with wide range of symptoms including constipation, abdominal distention, vomiting, abdominal tenderness, peritonitis, fever, and sepsis.1
Computed tomography of the abdomen and pelvis is the most reliable test for detecting stercoral colitis and its associated complications. Characteristic findings, as seen in the presented patient, include a large dense mass of fecal material, focal or diffuse thickening of the colonic wall, and pericolonic fat-stranding that affects the impacted region. When ulceration or perforation occurs, CT will reveal and extra luminal gas and/or abscess.1,3
Since stercoral colitis is associated with a reported mortality rate of 32% to 57%, with death often occurring within the first 24 hours from presentation, rapid diagnosis is essential.1 Once diagnosed, stercoral colitis is treated with aggressive fecal disimpaction, hyperosmolar enema, and, when indicated, surgery. The patient in this case was admitted for treatment. Unfortunately, despite all appropriate therapy efforts, she succumbed due to medical complications of her underlying illnesses.
Dr Belfi is an assistant professor of radiology at Weill Cornell Medical College in New York City, and an assistant attending radiologist at New York-Presbyterian Hospital/Weill Cornell Medical Center. Dr Hildick-Smith is a medical student at Weill Cornell Medical College in New York City. Dr Hentel is an associate professor of clinical radiology at Weill Cornell Medical College in New York City. He is also chief of emergency/musculoskeletal imaging and executive vice-chairman for the department of radiology at New York-Presbyterian Hospital/Weill Cornell Medical Center; and associate editor, imaging, of the EMERGENCY MEDICINE editorial board.
- Saksonov M, Bachar GN, Morgenstern S. Stercoral colitis: a lethal disease-computed tomographic findings and clinical characteristic. J Comput Assist Tomogr. 2014;38(5):721-726.
- Gans SL, Stoker J, Boermeester MA. Plain abdominal radiography in acute abdominal pain; past, present, and future. Int J Gen Med. 2012;5:525-533.
- Heffernan C, Pachter HL, Megibow AJ, Macari M. Stercoral colitis leading to fatal peritonitis: CT findings. AJR Am J Roentgenol. 2005;184(4):1189-1193.
Case
A 63-year-old woman with multiple medical conditions presented to the ED with abdominal distention, pain, and 4-day history of fever. Physical examination revealed a distended but nontender abdomen. Her vital signs included a fever of 103.1°F, tachycardia, tachypnea, and a blood pressure of 101/69. To further evaluate the abdominal distention, supine abdominal radiographs were obtained (Figure 1a and 1b).
What is the differential diagnosis?
What would be the most appropriate next imaging test?
Answer
The abdominal radiographs demonstrated a nonobstructed bowel gas pattern—ie, there were no dilated loops of small or large bowel (>3 cm or >7 cm, respectively). Although limited by supine position, there was no evidence of perforation as no signs of free air were visualized. There was a large amount of stool within the large bowel, appearing on radiographic images as the mottled air and soft-tissue density (white asterisks, Figure 2a and 2b).
Although bowel obstruction had been ruled out in this patient, the differential for abdominal pain with fever remained wide, predominately for infectious and inflammatory conditions (eg, appendicitis, diverticulitis, inflammatory bowel disease, other forms of colitis/enteritis, pancreatitis, abscess, mesenteric).In cases such as the one presented, the most appropriate next imaging examination would be a computed tomography (CT) scan with both oral and intravenous (IV) contrast. Oral contrast is useful since several of the conditions in the differential diagnosis require evaluation of the bowel wall and, in cases of suspected abscess, it assists in differentiating a collection from adjacent loops of bowel. Intravenous contrast is useful to evaluate for inflammation and assessing the bowel.
In this case, an abdominal CT was performed, using both oral and IV contrast. Axial and sagittal images demonstrated a large amount of stool in a distended rectum (white asterisks, Figures 3a and 3b), thickening of the rectal wall (white arrows, Figures 3a and 3b), and stranding of the perirectal fat indicative of perirectal inflammation (red arrows, Figures 3a and 3b). Based on these findings, the patient was diagnosed with stercoral colitis.
Stercoral colitis is inflammation of the colonic or rectal wall secondary to increased intraluminal pressure caused by impacted fecal material. This condition is most common in elderly patients and bedridden patients with chronic constipation. As this case illustrates, the clinical presentation of stercoral colitis is nonspecific, with wide range of symptoms including constipation, abdominal distention, vomiting, abdominal tenderness, peritonitis, fever, and sepsis.1
Computed tomography of the abdomen and pelvis is the most reliable test for detecting stercoral colitis and its associated complications. Characteristic findings, as seen in the presented patient, include a large dense mass of fecal material, focal or diffuse thickening of the colonic wall, and pericolonic fat-stranding that affects the impacted region. When ulceration or perforation occurs, CT will reveal and extra luminal gas and/or abscess.1,3
Since stercoral colitis is associated with a reported mortality rate of 32% to 57%, with death often occurring within the first 24 hours from presentation, rapid diagnosis is essential.1 Once diagnosed, stercoral colitis is treated with aggressive fecal disimpaction, hyperosmolar enema, and, when indicated, surgery. The patient in this case was admitted for treatment. Unfortunately, despite all appropriate therapy efforts, she succumbed due to medical complications of her underlying illnesses.
Dr Belfi is an assistant professor of radiology at Weill Cornell Medical College in New York City, and an assistant attending radiologist at New York-Presbyterian Hospital/Weill Cornell Medical Center. Dr Hildick-Smith is a medical student at Weill Cornell Medical College in New York City. Dr Hentel is an associate professor of clinical radiology at Weill Cornell Medical College in New York City. He is also chief of emergency/musculoskeletal imaging and executive vice-chairman for the department of radiology at New York-Presbyterian Hospital/Weill Cornell Medical Center; and associate editor, imaging, of the EMERGENCY MEDICINE editorial board.
Case
A 63-year-old woman with multiple medical conditions presented to the ED with abdominal distention, pain, and 4-day history of fever. Physical examination revealed a distended but nontender abdomen. Her vital signs included a fever of 103.1°F, tachycardia, tachypnea, and a blood pressure of 101/69. To further evaluate the abdominal distention, supine abdominal radiographs were obtained (Figure 1a and 1b).
What is the differential diagnosis?
What would be the most appropriate next imaging test?
Answer
The abdominal radiographs demonstrated a nonobstructed bowel gas pattern—ie, there were no dilated loops of small or large bowel (>3 cm or >7 cm, respectively). Although limited by supine position, there was no evidence of perforation as no signs of free air were visualized. There was a large amount of stool within the large bowel, appearing on radiographic images as the mottled air and soft-tissue density (white asterisks, Figure 2a and 2b).
Although bowel obstruction had been ruled out in this patient, the differential for abdominal pain with fever remained wide, predominately for infectious and inflammatory conditions (eg, appendicitis, diverticulitis, inflammatory bowel disease, other forms of colitis/enteritis, pancreatitis, abscess, mesenteric).In cases such as the one presented, the most appropriate next imaging examination would be a computed tomography (CT) scan with both oral and intravenous (IV) contrast. Oral contrast is useful since several of the conditions in the differential diagnosis require evaluation of the bowel wall and, in cases of suspected abscess, it assists in differentiating a collection from adjacent loops of bowel. Intravenous contrast is useful to evaluate for inflammation and assessing the bowel.
In this case, an abdominal CT was performed, using both oral and IV contrast. Axial and sagittal images demonstrated a large amount of stool in a distended rectum (white asterisks, Figures 3a and 3b), thickening of the rectal wall (white arrows, Figures 3a and 3b), and stranding of the perirectal fat indicative of perirectal inflammation (red arrows, Figures 3a and 3b). Based on these findings, the patient was diagnosed with stercoral colitis.
Stercoral colitis is inflammation of the colonic or rectal wall secondary to increased intraluminal pressure caused by impacted fecal material. This condition is most common in elderly patients and bedridden patients with chronic constipation. As this case illustrates, the clinical presentation of stercoral colitis is nonspecific, with wide range of symptoms including constipation, abdominal distention, vomiting, abdominal tenderness, peritonitis, fever, and sepsis.1
Computed tomography of the abdomen and pelvis is the most reliable test for detecting stercoral colitis and its associated complications. Characteristic findings, as seen in the presented patient, include a large dense mass of fecal material, focal or diffuse thickening of the colonic wall, and pericolonic fat-stranding that affects the impacted region. When ulceration or perforation occurs, CT will reveal and extra luminal gas and/or abscess.1,3
Since stercoral colitis is associated with a reported mortality rate of 32% to 57%, with death often occurring within the first 24 hours from presentation, rapid diagnosis is essential.1 Once diagnosed, stercoral colitis is treated with aggressive fecal disimpaction, hyperosmolar enema, and, when indicated, surgery. The patient in this case was admitted for treatment. Unfortunately, despite all appropriate therapy efforts, she succumbed due to medical complications of her underlying illnesses.
Dr Belfi is an assistant professor of radiology at Weill Cornell Medical College in New York City, and an assistant attending radiologist at New York-Presbyterian Hospital/Weill Cornell Medical Center. Dr Hildick-Smith is a medical student at Weill Cornell Medical College in New York City. Dr Hentel is an associate professor of clinical radiology at Weill Cornell Medical College in New York City. He is also chief of emergency/musculoskeletal imaging and executive vice-chairman for the department of radiology at New York-Presbyterian Hospital/Weill Cornell Medical Center; and associate editor, imaging, of the EMERGENCY MEDICINE editorial board.
- Saksonov M, Bachar GN, Morgenstern S. Stercoral colitis: a lethal disease-computed tomographic findings and clinical characteristic. J Comput Assist Tomogr. 2014;38(5):721-726.
- Gans SL, Stoker J, Boermeester MA. Plain abdominal radiography in acute abdominal pain; past, present, and future. Int J Gen Med. 2012;5:525-533.
- Heffernan C, Pachter HL, Megibow AJ, Macari M. Stercoral colitis leading to fatal peritonitis: CT findings. AJR Am J Roentgenol. 2005;184(4):1189-1193.
- Saksonov M, Bachar GN, Morgenstern S. Stercoral colitis: a lethal disease-computed tomographic findings and clinical characteristic. J Comput Assist Tomogr. 2014;38(5):721-726.
- Gans SL, Stoker J, Boermeester MA. Plain abdominal radiography in acute abdominal pain; past, present, and future. Int J Gen Med. 2012;5:525-533.
- Heffernan C, Pachter HL, Megibow AJ, Macari M. Stercoral colitis leading to fatal peritonitis: CT findings. AJR Am J Roentgenol. 2005;184(4):1189-1193.
Emergency Imaging
A 48-year-old man presented to the ED via emergency medical services after experiencing two episodes of syncope following the acute onset of right lower quadrant pain. He was unresponsive at the time of presentation. His vital signs were notable for tachycardia with a heart rate of 130 beats/minute. Although normotensive at initial presentation, the patient’s blood pressure began to fall rapidly. Laboratory values revealed a decreased hemoglobin and hematocrit of 5 g/dL and 18.1%, respectively.
Following his stabilization, a computed tomography (CT) scan of the abdomen and pelvis was performed. Figures 1a and 1b represent selected noncontrast and postcontrast axial images obtained through the lower abdomen/upper pelvis.
What is the diagnosis?
Answer
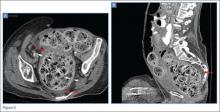
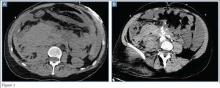
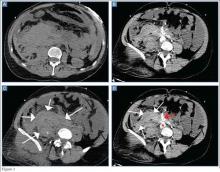
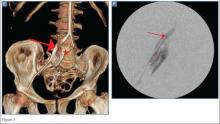
The precontrast image (Figure 1c) demonstrates a large and irregular high-density collection within the right upper pelvis (white arrows) and high density free fluid along the left lateral abdominal wall (black arrows). The right psoas muscle is obscured (white asterisk, Figure 1c) when compared to the normal psoas muscle on the contralateral side (black asterisk, Figure 1c). The postcontrast image (Figure 1d) shows the same findings and also reveals an enlarged right common iliac artery (red asterisk) and contrast actively extravasating from the artery (red arrow). These findings indicate the presence of a ruptured common iliac artery aneurysm. Both the enlarged common iliac artery aneurysm (red arrow, Figure 1d) and the extravasated contrast (red asterisk, Figure 1d) were confirmed on the three-dimensional reformats of the CT scan (Figure 1e).
An isolated aneurysm of the common iliac artery is uncommon, occurring in only 1% to 2% of the population—the same frequency as aortic aneurysm.1 However, the risk of rupture of this type of aneurysm is high.2 Patients with common iliac artery aneurysm are typically asymptomatic prior to rupture. However, some patients have reportedly presented with claudication, tenesmus/constipation, sciatica, and lower extremity paresis due to nerve compression, as well as urinary obstruction caused by ureteral obstruction.1,3 Once the iliac artery aneurysm has ruptured, patients typically present with acute abdominal, groin, and/or thigh pain, although isolated testicular pain has been described in the literature.1,4
Treatment for iliac artery aneurysm includes open and endovascular repair, depending on patient presentation and the adjacent structures involved. While mortality is low for patients with a nonruptured common iliac aneurysm, acute rupture is present in approximately one out of three cases, and the surgical mortality rate is as high as 55%.3
The patient presented in this case was taken to the operating room where an angiogram of the right common iliac artery (red arrow, Figure 1f) revealed continued acute extravasation of contrast (red asterisk, Figure 1f). Surgical repair was attempted but the patient did not survive due to complications of hypotension and cardiac arrest.
2. Reber PU, Brunner K, Hakki H, Stirnemann P, Kniemeyer HW. Incidence, classification and therapy of isolated pelvic artery aneurysm. Chirurg. 2001;72(4):419-424.
3. Bacharach JM, Slovut DP. State of the art: management of iliac artery aneurysmal disease. Catheter Cardiovasc Interv. 2008;71(5):708-714.
4. Dolan RD, Zino S. A ruptured left common iliac aneurysm presenting as testicular pain in a 56-year-old man. BMJ Case Rep. 2014. doi:10.1136/bcr-2012-006568.
A 48-year-old man presented to the ED via emergency medical services after experiencing two episodes of syncope following the acute onset of right lower quadrant pain. He was unresponsive at the time of presentation. His vital signs were notable for tachycardia with a heart rate of 130 beats/minute. Although normotensive at initial presentation, the patient’s blood pressure began to fall rapidly. Laboratory values revealed a decreased hemoglobin and hematocrit of 5 g/dL and 18.1%, respectively.
Following his stabilization, a computed tomography (CT) scan of the abdomen and pelvis was performed. Figures 1a and 1b represent selected noncontrast and postcontrast axial images obtained through the lower abdomen/upper pelvis.
What is the diagnosis?
Answer
The precontrast image (Figure 1c) demonstrates a large and irregular high-density collection within the right upper pelvis (white arrows) and high density free fluid along the left lateral abdominal wall (black arrows). The right psoas muscle is obscured (white asterisk, Figure 1c) when compared to the normal psoas muscle on the contralateral side (black asterisk, Figure 1c). The postcontrast image (Figure 1d) shows the same findings and also reveals an enlarged right common iliac artery (red asterisk) and contrast actively extravasating from the artery (red arrow). These findings indicate the presence of a ruptured common iliac artery aneurysm. Both the enlarged common iliac artery aneurysm (red arrow, Figure 1d) and the extravasated contrast (red asterisk, Figure 1d) were confirmed on the three-dimensional reformats of the CT scan (Figure 1e).
An isolated aneurysm of the common iliac artery is uncommon, occurring in only 1% to 2% of the population—the same frequency as aortic aneurysm.1 However, the risk of rupture of this type of aneurysm is high.2 Patients with common iliac artery aneurysm are typically asymptomatic prior to rupture. However, some patients have reportedly presented with claudication, tenesmus/constipation, sciatica, and lower extremity paresis due to nerve compression, as well as urinary obstruction caused by ureteral obstruction.1,3 Once the iliac artery aneurysm has ruptured, patients typically present with acute abdominal, groin, and/or thigh pain, although isolated testicular pain has been described in the literature.1,4
Treatment for iliac artery aneurysm includes open and endovascular repair, depending on patient presentation and the adjacent structures involved. While mortality is low for patients with a nonruptured common iliac aneurysm, acute rupture is present in approximately one out of three cases, and the surgical mortality rate is as high as 55%.3
The patient presented in this case was taken to the operating room where an angiogram of the right common iliac artery (red arrow, Figure 1f) revealed continued acute extravasation of contrast (red asterisk, Figure 1f). Surgical repair was attempted but the patient did not survive due to complications of hypotension and cardiac arrest.
A 48-year-old man presented to the ED via emergency medical services after experiencing two episodes of syncope following the acute onset of right lower quadrant pain. He was unresponsive at the time of presentation. His vital signs were notable for tachycardia with a heart rate of 130 beats/minute. Although normotensive at initial presentation, the patient’s blood pressure began to fall rapidly. Laboratory values revealed a decreased hemoglobin and hematocrit of 5 g/dL and 18.1%, respectively.
Following his stabilization, a computed tomography (CT) scan of the abdomen and pelvis was performed. Figures 1a and 1b represent selected noncontrast and postcontrast axial images obtained through the lower abdomen/upper pelvis.
What is the diagnosis?
Answer
The precontrast image (Figure 1c) demonstrates a large and irregular high-density collection within the right upper pelvis (white arrows) and high density free fluid along the left lateral abdominal wall (black arrows). The right psoas muscle is obscured (white asterisk, Figure 1c) when compared to the normal psoas muscle on the contralateral side (black asterisk, Figure 1c). The postcontrast image (Figure 1d) shows the same findings and also reveals an enlarged right common iliac artery (red asterisk) and contrast actively extravasating from the artery (red arrow). These findings indicate the presence of a ruptured common iliac artery aneurysm. Both the enlarged common iliac artery aneurysm (red arrow, Figure 1d) and the extravasated contrast (red asterisk, Figure 1d) were confirmed on the three-dimensional reformats of the CT scan (Figure 1e).
An isolated aneurysm of the common iliac artery is uncommon, occurring in only 1% to 2% of the population—the same frequency as aortic aneurysm.1 However, the risk of rupture of this type of aneurysm is high.2 Patients with common iliac artery aneurysm are typically asymptomatic prior to rupture. However, some patients have reportedly presented with claudication, tenesmus/constipation, sciatica, and lower extremity paresis due to nerve compression, as well as urinary obstruction caused by ureteral obstruction.1,3 Once the iliac artery aneurysm has ruptured, patients typically present with acute abdominal, groin, and/or thigh pain, although isolated testicular pain has been described in the literature.1,4
Treatment for iliac artery aneurysm includes open and endovascular repair, depending on patient presentation and the adjacent structures involved. While mortality is low for patients with a nonruptured common iliac aneurysm, acute rupture is present in approximately one out of three cases, and the surgical mortality rate is as high as 55%.3
The patient presented in this case was taken to the operating room where an angiogram of the right common iliac artery (red arrow, Figure 1f) revealed continued acute extravasation of contrast (red asterisk, Figure 1f). Surgical repair was attempted but the patient did not survive due to complications of hypotension and cardiac arrest.
2. Reber PU, Brunner K, Hakki H, Stirnemann P, Kniemeyer HW. Incidence, classification and therapy of isolated pelvic artery aneurysm. Chirurg. 2001;72(4):419-424.
3. Bacharach JM, Slovut DP. State of the art: management of iliac artery aneurysmal disease. Catheter Cardiovasc Interv. 2008;71(5):708-714.
4. Dolan RD, Zino S. A ruptured left common iliac aneurysm presenting as testicular pain in a 56-year-old man. BMJ Case Rep. 2014. doi:10.1136/bcr-2012-006568.
2. Reber PU, Brunner K, Hakki H, Stirnemann P, Kniemeyer HW. Incidence, classification and therapy of isolated pelvic artery aneurysm. Chirurg. 2001;72(4):419-424.
3. Bacharach JM, Slovut DP. State of the art: management of iliac artery aneurysmal disease. Catheter Cardiovasc Interv. 2008;71(5):708-714.
4. Dolan RD, Zino S. A ruptured left common iliac aneurysm presenting as testicular pain in a 56-year-old man. BMJ Case Rep. 2014. doi:10.1136/bcr-2012-006568.
Emergency Imaging
An 11-year-old boy is brought to the ED with a 1-week of history of increasing crampy lower-quadrant abdominal pain. His vital signs were only significant for mild tachycardia. On physical examination, the child’s abdomen was tender to palpation in the bilateral lower abdominal quadrants with guarding. Laboratory evaluations were unremarkable.
An abdominal radiograph did not reveal any abnormality, and targeted ultrasound did not reveal a dilated appendix. Computed tomography (CT) of the abdomen and pelvis with oral and intravenous contrast were ordered and representative images are provided (Figures 1a and 1b). Note that additional images from the CT demonstrate the abnormality depicted in these figures was not a loop of small bowel (although it appeared to originate from a loop of distal small bowel) and that the appendix was normal.
|
|
|
What is the diagnosis?
Answer
Computed tomography revealed a blind-ending tubular structure (white arrows, Figure 1c) deep to the umbilicus arising inferiorly from a loop of distal ileum with surrounding fat stranding (Figure 1d). The fluid-containing tubular structure demonstrates marked enhancement of the mucosa. These findings are most consistent with Meckel’s diverticulitis.
Meckel’s diverticulum is the most common anomaly of the gastrointestinal (GI) tract and results from incomplete obliteration of the vitelline duct. As per the rule of “twos,” Meckel’s diverticulum usually occurs 2 feet (40-60 cm) proximal to the ileocecal valve; is 2 cm wide (and 3 cm long); is found in 2% of the population; typically presents before age 2 years; is twice as likely to be symptomatic in boys; and contains ectopic gastric mucosa in approximately half of the cases.1
|
|
|
As many patients are asymptomatic, Meckel’s diverticulum is diagnosed as an incidental finding after a barium study or abdominal surgery is performed for other GI conditions. Symptoms occur as a result of ectopic gastric tissue, obstruction, and/or inflammation. Painless lower GI bleeding, the most common presentation, is reported in up to 50% of patients with symptomatic Meckel’s diverticulosis.2 Hemorrhage results from ulceration caused by secreted acid and enzymes from ectopic digestive mucosa. Intestinal obstruction is another common complication usually seen in children, which can be caused by volvulus of the small bowel around a diverticulum, intussusception, incarceration within a hernia, and internal herniation. Inflammation of the Meckel’s diverticulum, or Meckel’s diverticulitis, is more common in older patients and presents similarly to acute appendicitis.2
After removal of a complicated Meckel’s diverticulitis, postoperative morbidity and mortality rates have been reported to be 12% and 2%, respectively. In contrast, postoperative complications after resection of incidental diverticula are fewer, and morbidity and mortality rates are as low as 2% and 1%, respectively.3-5 Meckel’s diverticulitis should be included as a differential diagnosis when appendicitis or medically managed abdominopelvic inflammatory processes are suspected, as delayed diagnosis can lead to perforation, abscess formation, peritonitis, sepsis, bowel obstruction, and death.
The patient presented in this case was taken to the operating room, and the Meckel’s diverticula confirmed and removed. He experienced an uneventful postoperative course and was discharged a few days later.
Dr Rotman is a radiology resident at Weill Cornell Medical College in New York City. Dr Belfi is an assistant professor of radiology at Weill Cornell Medical College in New York City and an assistant attending radiologist at New York-Presbyterian Hospital/Weill Cornell Medical Center. Dr Hentel is an associate professor of clinical radiology, Weill Cornell Medical College, New York. He is also chief of emergency/musculoskeletal imaging and executive vice-chairman for the department of radiology, New York-Presbyterian Hospital/Weill Cornell Medical Center. He is associate editor, imaging, of the EMERGENCY MEDICINE editorial board.
- Anderson DJ. Carcinoid tumor in Meckel’s diverticulum: laparoscopic treatment and review of the literature. J Am Osteopath Assoc. 2000;100(7):432-434.
- Malik AA, Shams-ul-Bari, Wani KA, Khaja AR. Meckel’s diverticulum-Revisited. Saudi J Gastroenterol. 2010;16(1):3-7.
- Altinli E, Pekmezci S, Gorgun E, Sirin F. Laparoscopy-assisted resection of complicated Meckel’s diverticulum in adults. Surg Laparosc Endosc Percutan Tech. 2002;12(3):190-194.
- Nath, DS, Morris TA. Small bowel obstruction in an adolescent: a case of Meckel’s diverticulum. Minn Med. 2004;87(11):46-48.
- Cullen, JJ, Kelly KA, Moir CR, et al. Surgical management of Meckel’s diverticulum. An epidemiologic, population-based study. Ann Surg. 1994;220(4):564-568; discussion 568,569.
An 11-year-old boy is brought to the ED with a 1-week of history of increasing crampy lower-quadrant abdominal pain. His vital signs were only significant for mild tachycardia. On physical examination, the child’s abdomen was tender to palpation in the bilateral lower abdominal quadrants with guarding. Laboratory evaluations were unremarkable.
An abdominal radiograph did not reveal any abnormality, and targeted ultrasound did not reveal a dilated appendix. Computed tomography (CT) of the abdomen and pelvis with oral and intravenous contrast were ordered and representative images are provided (Figures 1a and 1b). Note that additional images from the CT demonstrate the abnormality depicted in these figures was not a loop of small bowel (although it appeared to originate from a loop of distal small bowel) and that the appendix was normal.
|
|
|
What is the diagnosis?
Answer
Computed tomography revealed a blind-ending tubular structure (white arrows, Figure 1c) deep to the umbilicus arising inferiorly from a loop of distal ileum with surrounding fat stranding (Figure 1d). The fluid-containing tubular structure demonstrates marked enhancement of the mucosa. These findings are most consistent with Meckel’s diverticulitis.
Meckel’s diverticulum is the most common anomaly of the gastrointestinal (GI) tract and results from incomplete obliteration of the vitelline duct. As per the rule of “twos,” Meckel’s diverticulum usually occurs 2 feet (40-60 cm) proximal to the ileocecal valve; is 2 cm wide (and 3 cm long); is found in 2% of the population; typically presents before age 2 years; is twice as likely to be symptomatic in boys; and contains ectopic gastric mucosa in approximately half of the cases.1
|
|
|
As many patients are asymptomatic, Meckel’s diverticulum is diagnosed as an incidental finding after a barium study or abdominal surgery is performed for other GI conditions. Symptoms occur as a result of ectopic gastric tissue, obstruction, and/or inflammation. Painless lower GI bleeding, the most common presentation, is reported in up to 50% of patients with symptomatic Meckel’s diverticulosis.2 Hemorrhage results from ulceration caused by secreted acid and enzymes from ectopic digestive mucosa. Intestinal obstruction is another common complication usually seen in children, which can be caused by volvulus of the small bowel around a diverticulum, intussusception, incarceration within a hernia, and internal herniation. Inflammation of the Meckel’s diverticulum, or Meckel’s diverticulitis, is more common in older patients and presents similarly to acute appendicitis.2
After removal of a complicated Meckel’s diverticulitis, postoperative morbidity and mortality rates have been reported to be 12% and 2%, respectively. In contrast, postoperative complications after resection of incidental diverticula are fewer, and morbidity and mortality rates are as low as 2% and 1%, respectively.3-5 Meckel’s diverticulitis should be included as a differential diagnosis when appendicitis or medically managed abdominopelvic inflammatory processes are suspected, as delayed diagnosis can lead to perforation, abscess formation, peritonitis, sepsis, bowel obstruction, and death.
The patient presented in this case was taken to the operating room, and the Meckel’s diverticula confirmed and removed. He experienced an uneventful postoperative course and was discharged a few days later.
Dr Rotman is a radiology resident at Weill Cornell Medical College in New York City. Dr Belfi is an assistant professor of radiology at Weill Cornell Medical College in New York City and an assistant attending radiologist at New York-Presbyterian Hospital/Weill Cornell Medical Center. Dr Hentel is an associate professor of clinical radiology, Weill Cornell Medical College, New York. He is also chief of emergency/musculoskeletal imaging and executive vice-chairman for the department of radiology, New York-Presbyterian Hospital/Weill Cornell Medical Center. He is associate editor, imaging, of the EMERGENCY MEDICINE editorial board.
An 11-year-old boy is brought to the ED with a 1-week of history of increasing crampy lower-quadrant abdominal pain. His vital signs were only significant for mild tachycardia. On physical examination, the child’s abdomen was tender to palpation in the bilateral lower abdominal quadrants with guarding. Laboratory evaluations were unremarkable.
An abdominal radiograph did not reveal any abnormality, and targeted ultrasound did not reveal a dilated appendix. Computed tomography (CT) of the abdomen and pelvis with oral and intravenous contrast were ordered and representative images are provided (Figures 1a and 1b). Note that additional images from the CT demonstrate the abnormality depicted in these figures was not a loop of small bowel (although it appeared to originate from a loop of distal small bowel) and that the appendix was normal.
|
|
|
What is the diagnosis?
Answer
Computed tomography revealed a blind-ending tubular structure (white arrows, Figure 1c) deep to the umbilicus arising inferiorly from a loop of distal ileum with surrounding fat stranding (Figure 1d). The fluid-containing tubular structure demonstrates marked enhancement of the mucosa. These findings are most consistent with Meckel’s diverticulitis.
Meckel’s diverticulum is the most common anomaly of the gastrointestinal (GI) tract and results from incomplete obliteration of the vitelline duct. As per the rule of “twos,” Meckel’s diverticulum usually occurs 2 feet (40-60 cm) proximal to the ileocecal valve; is 2 cm wide (and 3 cm long); is found in 2% of the population; typically presents before age 2 years; is twice as likely to be symptomatic in boys; and contains ectopic gastric mucosa in approximately half of the cases.1
|
|
|
As many patients are asymptomatic, Meckel’s diverticulum is diagnosed as an incidental finding after a barium study or abdominal surgery is performed for other GI conditions. Symptoms occur as a result of ectopic gastric tissue, obstruction, and/or inflammation. Painless lower GI bleeding, the most common presentation, is reported in up to 50% of patients with symptomatic Meckel’s diverticulosis.2 Hemorrhage results from ulceration caused by secreted acid and enzymes from ectopic digestive mucosa. Intestinal obstruction is another common complication usually seen in children, which can be caused by volvulus of the small bowel around a diverticulum, intussusception, incarceration within a hernia, and internal herniation. Inflammation of the Meckel’s diverticulum, or Meckel’s diverticulitis, is more common in older patients and presents similarly to acute appendicitis.2
After removal of a complicated Meckel’s diverticulitis, postoperative morbidity and mortality rates have been reported to be 12% and 2%, respectively. In contrast, postoperative complications after resection of incidental diverticula are fewer, and morbidity and mortality rates are as low as 2% and 1%, respectively.3-5 Meckel’s diverticulitis should be included as a differential diagnosis when appendicitis or medically managed abdominopelvic inflammatory processes are suspected, as delayed diagnosis can lead to perforation, abscess formation, peritonitis, sepsis, bowel obstruction, and death.
The patient presented in this case was taken to the operating room, and the Meckel’s diverticula confirmed and removed. He experienced an uneventful postoperative course and was discharged a few days later.
Dr Rotman is a radiology resident at Weill Cornell Medical College in New York City. Dr Belfi is an assistant professor of radiology at Weill Cornell Medical College in New York City and an assistant attending radiologist at New York-Presbyterian Hospital/Weill Cornell Medical Center. Dr Hentel is an associate professor of clinical radiology, Weill Cornell Medical College, New York. He is also chief of emergency/musculoskeletal imaging and executive vice-chairman for the department of radiology, New York-Presbyterian Hospital/Weill Cornell Medical Center. He is associate editor, imaging, of the EMERGENCY MEDICINE editorial board.
- Anderson DJ. Carcinoid tumor in Meckel’s diverticulum: laparoscopic treatment and review of the literature. J Am Osteopath Assoc. 2000;100(7):432-434.
- Malik AA, Shams-ul-Bari, Wani KA, Khaja AR. Meckel’s diverticulum-Revisited. Saudi J Gastroenterol. 2010;16(1):3-7.
- Altinli E, Pekmezci S, Gorgun E, Sirin F. Laparoscopy-assisted resection of complicated Meckel’s diverticulum in adults. Surg Laparosc Endosc Percutan Tech. 2002;12(3):190-194.
- Nath, DS, Morris TA. Small bowel obstruction in an adolescent: a case of Meckel’s diverticulum. Minn Med. 2004;87(11):46-48.
- Cullen, JJ, Kelly KA, Moir CR, et al. Surgical management of Meckel’s diverticulum. An epidemiologic, population-based study. Ann Surg. 1994;220(4):564-568; discussion 568,569.
- Anderson DJ. Carcinoid tumor in Meckel’s diverticulum: laparoscopic treatment and review of the literature. J Am Osteopath Assoc. 2000;100(7):432-434.
- Malik AA, Shams-ul-Bari, Wani KA, Khaja AR. Meckel’s diverticulum-Revisited. Saudi J Gastroenterol. 2010;16(1):3-7.
- Altinli E, Pekmezci S, Gorgun E, Sirin F. Laparoscopy-assisted resection of complicated Meckel’s diverticulum in adults. Surg Laparosc Endosc Percutan Tech. 2002;12(3):190-194.
- Nath, DS, Morris TA. Small bowel obstruction in an adolescent: a case of Meckel’s diverticulum. Minn Med. 2004;87(11):46-48.
- Cullen, JJ, Kelly KA, Moir CR, et al. Surgical management of Meckel’s diverticulum. An epidemiologic, population-based study. Ann Surg. 1994;220(4):564-568; discussion 568,569.