User login
Emergency Imaging
Case
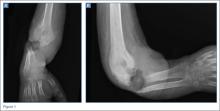
A 32-year-old woman presented to the ED with a 6-month history of worsening skin erosion along her right elbow and forearm. The patient described unremitting pain and progressive shortening of her forearm. Radiographs were obtained; representative images are shown above (Figures 1a and 1b).
What is the diagnosis?
***BREAKING***
Answer
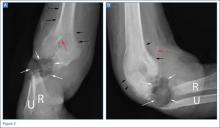
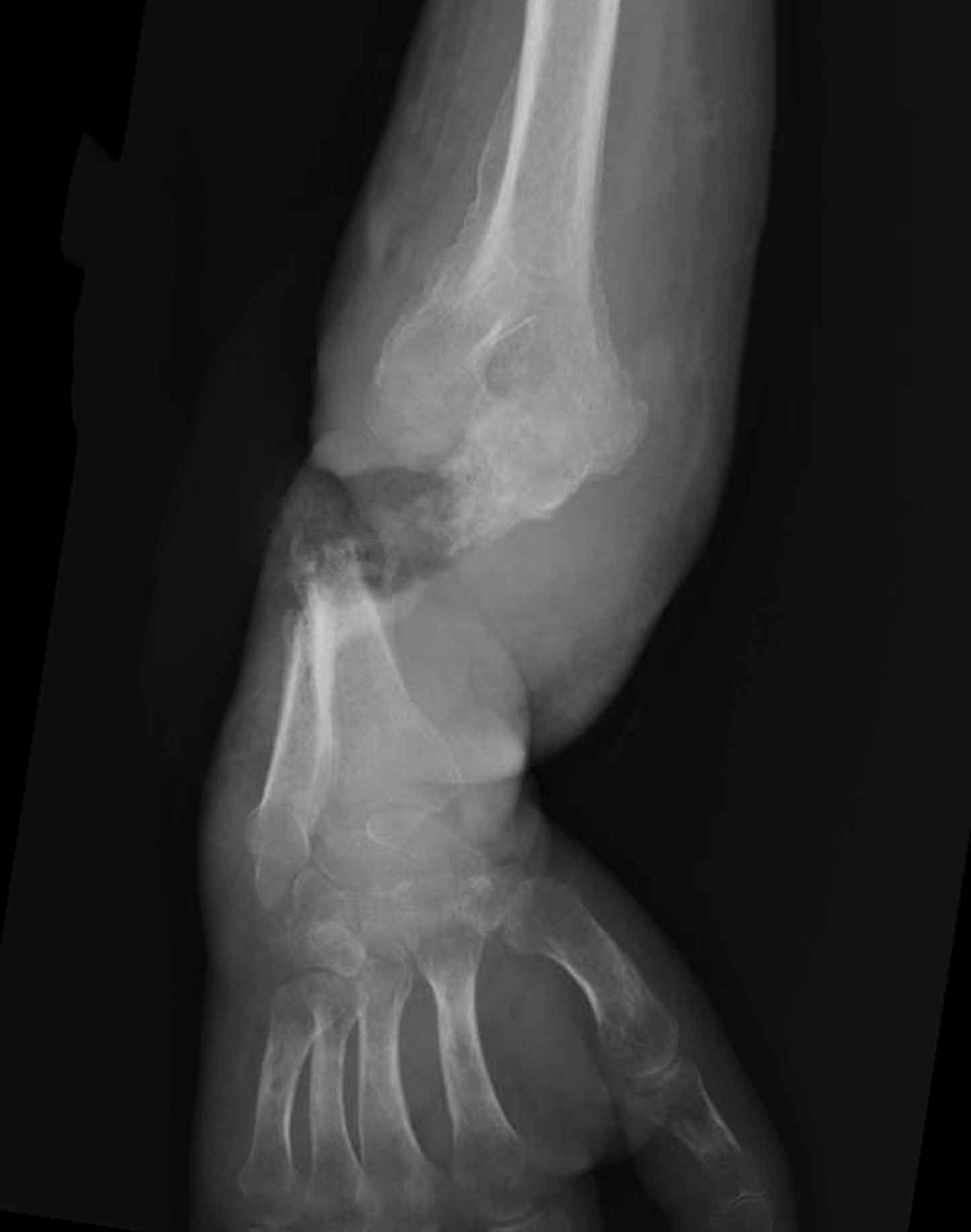
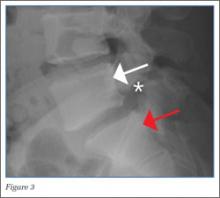
Anteroposterior and lateral radiographs of the right elbow demonstrated a large soft tissue defect involving the proximal forearm (white arrows, Figures 2a and 2b) with extensive osseous destruction of the proximal radius (R) and ulna (U). Deformity of the forearm was apparent. There was marked periosteal reaction involving the distal humerus (black arrows, Figures 2a and 2b). A needle fragment projected over the antecubital space on both radiographs (red arrow, Figures 2a and 2b). These findings indicated the presence of osteomyelitis and septic arthritis. The needle fragment suggested drug abuse as the likely etiology.
This patient had a 2-year history of intravenous (IV) drug abuse. She initially noticed an open wound on her elbow about 6 months prior to presentation. As the wound worsened, she developed increasing pain, numbness, and weakness in her forearm and hand. Physical examination revealed a markedly contracted forearm. There was a large, deep ulcer at the lateral aspect of the elbow and proximal forearm, with exposed bone and surrounding necrotic tissue. Motor function was limited to minimal movement of the thumb and index finger, and there was complete loss of sensation in an ulnar distribution. A deep tissue culture grew methicillin-resistant Staphylococcus aureus, and a bone biopsy confirmed the diagnosis of osteomyelitis. The patient was treated with antibiotics but ultimately required transhumeral amputation.
Intravenous drug users are at risk for soft tissue and bone complications, including ulcer, abscess, septic bursitis, tenosynovitis, cellulitis, necrotizing fasciitis, septic arthritis, and osteomyelitis.1 Osteomyelitis can arise from direct inoculation, hematogenous spread, or, as was the case in this patient, secondary to seeding from a contiguous soft tissue infection.2 Unlike hematogenous osteomyelitis, contiguous focus osteomyelitis is often polymicrobial.
Radiographs are not sensitive for osteomyelitis, particularly early in the process, but may reveal loss of soft tissue planes prior to the development of focal osteopenia and osseous destruction.3 In this case, the diagnosis could be made through radiography given the marked osseous destruction, periosteal reaction, and soft tissue ulceration. However, in presentations in which there is a continued clinical suspicion for osteomyelitis despite normal or inconclusive radiographs, magnetic resonance imaging (MRI) with contrast is the preferred examination for further evaluation. While both contrast and noncontrast-enhanced MRI may show the bone marrow edema related to osteomyelitis, contrast is helpful in evaluating for soft tissue abscesses and in determining if there is an adequate blood supply for IV treatment to be effective. An alternative exam to MRI would be nuclear scintigraphy (a tagged, white blood cell scan and/or a three-phase bone scan).
Later in the disease course, periosteal bone reaction and sclerotic reactive bone formation can be seen. A sequestrum forms when there is complete resorption of the bone adjacent to a devitalized segment of infected bone, leaving the isolated devitalized segment to function as a nidus for continued infection.3
Dr Spivey is a resident in the department of radiology at New York Presbyterian Hospital/Weill Cornell Medical College in New York City. Dr Bartolotta is an assistant professor of radiology at Weill Cornell Medical College in New York City and assistant attending radiologist at New York-Presbyterian Hospital/Weill Cornell Medical Center. Dr Hentel is an associate professor of clinical radiology at Weill Cornell Medical College in New York City. He is also chief of emergency/musculoskeletal imaging and executive vice-chairman for the department of radiology at New York-Presbyterian Hospital/Weill Cornell Medical Center; and associate editor, imaging, of the EMERGENCY MEDICINE editorial board.
- Theodorou SJ, Theodorou DJ, Resnick D. Imaging findings of complications affecting the upper extremity in intravenous drug users: featured cases. Emerg Radiol. 2008;15(4):227-239.
- Sia IG, Berbari, EF. Infection and musculoskeletal conditions: Osteomyelitis. Best Pract Res Clin Rheumatol. 2006;20(6):1065-1081.
- Gold RH, Hawkins RA, Katz RD. Bacterial osteomyelitis: findings on plain radiography, CT, MR, and scintigraphy. AJR Am J Roentgenol. 1991;157(2):365-370.
Case
A 32-year-old woman presented to the ED with a 6-month history of worsening skin erosion along her right elbow and forearm. The patient described unremitting pain and progressive shortening of her forearm. Radiographs were obtained; representative images are shown above (Figures 1a and 1b).
What is the diagnosis?
***BREAKING***
Answer
Anteroposterior and lateral radiographs of the right elbow demonstrated a large soft tissue defect involving the proximal forearm (white arrows, Figures 2a and 2b) with extensive osseous destruction of the proximal radius (R) and ulna (U). Deformity of the forearm was apparent. There was marked periosteal reaction involving the distal humerus (black arrows, Figures 2a and 2b). A needle fragment projected over the antecubital space on both radiographs (red arrow, Figures 2a and 2b). These findings indicated the presence of osteomyelitis and septic arthritis. The needle fragment suggested drug abuse as the likely etiology.
This patient had a 2-year history of intravenous (IV) drug abuse. She initially noticed an open wound on her elbow about 6 months prior to presentation. As the wound worsened, she developed increasing pain, numbness, and weakness in her forearm and hand. Physical examination revealed a markedly contracted forearm. There was a large, deep ulcer at the lateral aspect of the elbow and proximal forearm, with exposed bone and surrounding necrotic tissue. Motor function was limited to minimal movement of the thumb and index finger, and there was complete loss of sensation in an ulnar distribution. A deep tissue culture grew methicillin-resistant Staphylococcus aureus, and a bone biopsy confirmed the diagnosis of osteomyelitis. The patient was treated with antibiotics but ultimately required transhumeral amputation.
Intravenous drug users are at risk for soft tissue and bone complications, including ulcer, abscess, septic bursitis, tenosynovitis, cellulitis, necrotizing fasciitis, septic arthritis, and osteomyelitis.1 Osteomyelitis can arise from direct inoculation, hematogenous spread, or, as was the case in this patient, secondary to seeding from a contiguous soft tissue infection.2 Unlike hematogenous osteomyelitis, contiguous focus osteomyelitis is often polymicrobial.
Radiographs are not sensitive for osteomyelitis, particularly early in the process, but may reveal loss of soft tissue planes prior to the development of focal osteopenia and osseous destruction.3 In this case, the diagnosis could be made through radiography given the marked osseous destruction, periosteal reaction, and soft tissue ulceration. However, in presentations in which there is a continued clinical suspicion for osteomyelitis despite normal or inconclusive radiographs, magnetic resonance imaging (MRI) with contrast is the preferred examination for further evaluation. While both contrast and noncontrast-enhanced MRI may show the bone marrow edema related to osteomyelitis, contrast is helpful in evaluating for soft tissue abscesses and in determining if there is an adequate blood supply for IV treatment to be effective. An alternative exam to MRI would be nuclear scintigraphy (a tagged, white blood cell scan and/or a three-phase bone scan).
Later in the disease course, periosteal bone reaction and sclerotic reactive bone formation can be seen. A sequestrum forms when there is complete resorption of the bone adjacent to a devitalized segment of infected bone, leaving the isolated devitalized segment to function as a nidus for continued infection.3
Dr Spivey is a resident in the department of radiology at New York Presbyterian Hospital/Weill Cornell Medical College in New York City. Dr Bartolotta is an assistant professor of radiology at Weill Cornell Medical College in New York City and assistant attending radiologist at New York-Presbyterian Hospital/Weill Cornell Medical Center. Dr Hentel is an associate professor of clinical radiology at Weill Cornell Medical College in New York City. He is also chief of emergency/musculoskeletal imaging and executive vice-chairman for the department of radiology at New York-Presbyterian Hospital/Weill Cornell Medical Center; and associate editor, imaging, of the EMERGENCY MEDICINE editorial board.
Case
A 32-year-old woman presented to the ED with a 6-month history of worsening skin erosion along her right elbow and forearm. The patient described unremitting pain and progressive shortening of her forearm. Radiographs were obtained; representative images are shown above (Figures 1a and 1b).
What is the diagnosis?
***BREAKING***
Answer
Anteroposterior and lateral radiographs of the right elbow demonstrated a large soft tissue defect involving the proximal forearm (white arrows, Figures 2a and 2b) with extensive osseous destruction of the proximal radius (R) and ulna (U). Deformity of the forearm was apparent. There was marked periosteal reaction involving the distal humerus (black arrows, Figures 2a and 2b). A needle fragment projected over the antecubital space on both radiographs (red arrow, Figures 2a and 2b). These findings indicated the presence of osteomyelitis and septic arthritis. The needle fragment suggested drug abuse as the likely etiology.
This patient had a 2-year history of intravenous (IV) drug abuse. She initially noticed an open wound on her elbow about 6 months prior to presentation. As the wound worsened, she developed increasing pain, numbness, and weakness in her forearm and hand. Physical examination revealed a markedly contracted forearm. There was a large, deep ulcer at the lateral aspect of the elbow and proximal forearm, with exposed bone and surrounding necrotic tissue. Motor function was limited to minimal movement of the thumb and index finger, and there was complete loss of sensation in an ulnar distribution. A deep tissue culture grew methicillin-resistant Staphylococcus aureus, and a bone biopsy confirmed the diagnosis of osteomyelitis. The patient was treated with antibiotics but ultimately required transhumeral amputation.
Intravenous drug users are at risk for soft tissue and bone complications, including ulcer, abscess, septic bursitis, tenosynovitis, cellulitis, necrotizing fasciitis, septic arthritis, and osteomyelitis.1 Osteomyelitis can arise from direct inoculation, hematogenous spread, or, as was the case in this patient, secondary to seeding from a contiguous soft tissue infection.2 Unlike hematogenous osteomyelitis, contiguous focus osteomyelitis is often polymicrobial.
Radiographs are not sensitive for osteomyelitis, particularly early in the process, but may reveal loss of soft tissue planes prior to the development of focal osteopenia and osseous destruction.3 In this case, the diagnosis could be made through radiography given the marked osseous destruction, periosteal reaction, and soft tissue ulceration. However, in presentations in which there is a continued clinical suspicion for osteomyelitis despite normal or inconclusive radiographs, magnetic resonance imaging (MRI) with contrast is the preferred examination for further evaluation. While both contrast and noncontrast-enhanced MRI may show the bone marrow edema related to osteomyelitis, contrast is helpful in evaluating for soft tissue abscesses and in determining if there is an adequate blood supply for IV treatment to be effective. An alternative exam to MRI would be nuclear scintigraphy (a tagged, white blood cell scan and/or a three-phase bone scan).
Later in the disease course, periosteal bone reaction and sclerotic reactive bone formation can be seen. A sequestrum forms when there is complete resorption of the bone adjacent to a devitalized segment of infected bone, leaving the isolated devitalized segment to function as a nidus for continued infection.3
Dr Spivey is a resident in the department of radiology at New York Presbyterian Hospital/Weill Cornell Medical College in New York City. Dr Bartolotta is an assistant professor of radiology at Weill Cornell Medical College in New York City and assistant attending radiologist at New York-Presbyterian Hospital/Weill Cornell Medical Center. Dr Hentel is an associate professor of clinical radiology at Weill Cornell Medical College in New York City. He is also chief of emergency/musculoskeletal imaging and executive vice-chairman for the department of radiology at New York-Presbyterian Hospital/Weill Cornell Medical Center; and associate editor, imaging, of the EMERGENCY MEDICINE editorial board.
- Theodorou SJ, Theodorou DJ, Resnick D. Imaging findings of complications affecting the upper extremity in intravenous drug users: featured cases. Emerg Radiol. 2008;15(4):227-239.
- Sia IG, Berbari, EF. Infection and musculoskeletal conditions: Osteomyelitis. Best Pract Res Clin Rheumatol. 2006;20(6):1065-1081.
- Gold RH, Hawkins RA, Katz RD. Bacterial osteomyelitis: findings on plain radiography, CT, MR, and scintigraphy. AJR Am J Roentgenol. 1991;157(2):365-370.
- Theodorou SJ, Theodorou DJ, Resnick D. Imaging findings of complications affecting the upper extremity in intravenous drug users: featured cases. Emerg Radiol. 2008;15(4):227-239.
- Sia IG, Berbari, EF. Infection and musculoskeletal conditions: Osteomyelitis. Best Pract Res Clin Rheumatol. 2006;20(6):1065-1081.
- Gold RH, Hawkins RA, Katz RD. Bacterial osteomyelitis: findings on plain radiography, CT, MR, and scintigraphy. AJR Am J Roentgenol. 1991;157(2):365-370.
Emergency Imaging
Case
A 41-year-old woman presented to the ED with a 3-day history of posterior neck pain that radiated to the occipital region. The patient stated that the day prior to presentation, the pain had worsened to what she rated as a “9” on a pain scale of 0 to 10. The patient’s surgical history included a remote prior cervical fusion at C5-C6. A lateral radiograph of the cervical spine is shown above (Figure 1a).
What is the diagnosis? Is additional imaging necessary? If so, why?
Answer
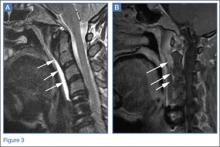
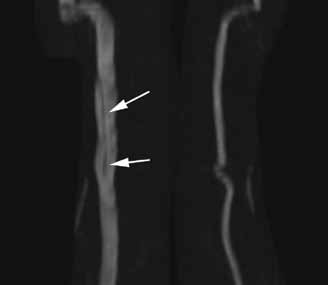
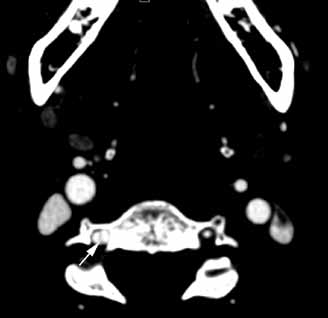
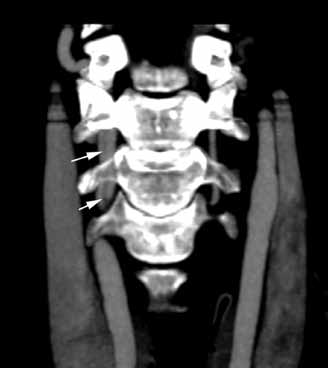
The lateral view of the cervical spine demonstrated soft tissue swelling in the upper prevertebral region (red arrowheads, Figure 1b). (The soft tissues at this level in an adult normally measure less than 3 mm between the airway and the vertebral body.) The radiograph also showed an area of calcification within the prevertebral soft tissues (white arrow, Figure 1b).
The patient denied fevers, chills, dysphagia, visual changes, and nasal congestion. Physical examination demonstrated tenderness to palpation of the posterior neck, but there was no evidence of palpable mass or lymphadenopathy. Neck extension and lateral movement to the left were severely limited due to pain; neck flexion and lateral movement range of motion to the right were mildly decreased due to pain. The physical examination was otherwise normal.
Longus colli (prevertebral) calcific tendinitis is a self-limiting condition that typically lasts 1 to 3 weeks, though it can be very painful during the acute phase.1,2 Treatment is typically conservative, and the patient in this case received a course of oral corticosteroids and nonsteroidal anti-inflammatory drugs to help alleviate the associated inflammation.
Dr Bartolotta is an assistant professor of radiology at Weill Cornell Medical College in New York City and assistant attending radiologist at New York-Presbyterian Hospital/Weill Cornell Medical Center. Dr Baradaran is a resident in the department of radiology at Weill Cornell Medical College in New York City. Dr Hentel is an associate professor of clinical radiology at Weill Cornell Medical College, New York. He is also chief of emergency/musculoskeletal imaging and executive vice-chairman for the department of radiology at New York-Presbyterian Hospital/Weill Cornell Medical Center; and associate editor, imaging, of the EMERGENCY MEDICINE editorial board.
- Coulier B, Macsim M, Desgain O. Retropharyngeal calcific tendinitis--longus colli tendinitis--an unusual cause of acute dysphagia. Emerg Radiol. 2011;18(5):449-451.
- Silva CF, Soffia PS, Pruzzo E. Acute prevertebral calcific tendinitis: a source of non-surgical acute cervical pain. Acta Radiologica. 2014;55(1):91-94.
- Zibis AH, Giannis D, Malizos KN, Kitsioulis P, Arvanitis DL. Acute calcific tendinitis of the longus colli muscle: case report and review of the literature. Eur Spine J. 2013;22(Suppl 3):S434-S438.
Case
A 41-year-old woman presented to the ED with a 3-day history of posterior neck pain that radiated to the occipital region. The patient stated that the day prior to presentation, the pain had worsened to what she rated as a “9” on a pain scale of 0 to 10. The patient’s surgical history included a remote prior cervical fusion at C5-C6. A lateral radiograph of the cervical spine is shown above (Figure 1a).
What is the diagnosis? Is additional imaging necessary? If so, why?
Answer
The lateral view of the cervical spine demonstrated soft tissue swelling in the upper prevertebral region (red arrowheads, Figure 1b). (The soft tissues at this level in an adult normally measure less than 3 mm between the airway and the vertebral body.) The radiograph also showed an area of calcification within the prevertebral soft tissues (white arrow, Figure 1b).
The patient denied fevers, chills, dysphagia, visual changes, and nasal congestion. Physical examination demonstrated tenderness to palpation of the posterior neck, but there was no evidence of palpable mass or lymphadenopathy. Neck extension and lateral movement to the left were severely limited due to pain; neck flexion and lateral movement range of motion to the right were mildly decreased due to pain. The physical examination was otherwise normal.
Longus colli (prevertebral) calcific tendinitis is a self-limiting condition that typically lasts 1 to 3 weeks, though it can be very painful during the acute phase.1,2 Treatment is typically conservative, and the patient in this case received a course of oral corticosteroids and nonsteroidal anti-inflammatory drugs to help alleviate the associated inflammation.
Dr Bartolotta is an assistant professor of radiology at Weill Cornell Medical College in New York City and assistant attending radiologist at New York-Presbyterian Hospital/Weill Cornell Medical Center. Dr Baradaran is a resident in the department of radiology at Weill Cornell Medical College in New York City. Dr Hentel is an associate professor of clinical radiology at Weill Cornell Medical College, New York. He is also chief of emergency/musculoskeletal imaging and executive vice-chairman for the department of radiology at New York-Presbyterian Hospital/Weill Cornell Medical Center; and associate editor, imaging, of the EMERGENCY MEDICINE editorial board.
Case
A 41-year-old woman presented to the ED with a 3-day history of posterior neck pain that radiated to the occipital region. The patient stated that the day prior to presentation, the pain had worsened to what she rated as a “9” on a pain scale of 0 to 10. The patient’s surgical history included a remote prior cervical fusion at C5-C6. A lateral radiograph of the cervical spine is shown above (Figure 1a).
What is the diagnosis? Is additional imaging necessary? If so, why?
Answer
The lateral view of the cervical spine demonstrated soft tissue swelling in the upper prevertebral region (red arrowheads, Figure 1b). (The soft tissues at this level in an adult normally measure less than 3 mm between the airway and the vertebral body.) The radiograph also showed an area of calcification within the prevertebral soft tissues (white arrow, Figure 1b).
The patient denied fevers, chills, dysphagia, visual changes, and nasal congestion. Physical examination demonstrated tenderness to palpation of the posterior neck, but there was no evidence of palpable mass or lymphadenopathy. Neck extension and lateral movement to the left were severely limited due to pain; neck flexion and lateral movement range of motion to the right were mildly decreased due to pain. The physical examination was otherwise normal.
Longus colli (prevertebral) calcific tendinitis is a self-limiting condition that typically lasts 1 to 3 weeks, though it can be very painful during the acute phase.1,2 Treatment is typically conservative, and the patient in this case received a course of oral corticosteroids and nonsteroidal anti-inflammatory drugs to help alleviate the associated inflammation.
Dr Bartolotta is an assistant professor of radiology at Weill Cornell Medical College in New York City and assistant attending radiologist at New York-Presbyterian Hospital/Weill Cornell Medical Center. Dr Baradaran is a resident in the department of radiology at Weill Cornell Medical College in New York City. Dr Hentel is an associate professor of clinical radiology at Weill Cornell Medical College, New York. He is also chief of emergency/musculoskeletal imaging and executive vice-chairman for the department of radiology at New York-Presbyterian Hospital/Weill Cornell Medical Center; and associate editor, imaging, of the EMERGENCY MEDICINE editorial board.
- Coulier B, Macsim M, Desgain O. Retropharyngeal calcific tendinitis--longus colli tendinitis--an unusual cause of acute dysphagia. Emerg Radiol. 2011;18(5):449-451.
- Silva CF, Soffia PS, Pruzzo E. Acute prevertebral calcific tendinitis: a source of non-surgical acute cervical pain. Acta Radiologica. 2014;55(1):91-94.
- Zibis AH, Giannis D, Malizos KN, Kitsioulis P, Arvanitis DL. Acute calcific tendinitis of the longus colli muscle: case report and review of the literature. Eur Spine J. 2013;22(Suppl 3):S434-S438.
- Coulier B, Macsim M, Desgain O. Retropharyngeal calcific tendinitis--longus colli tendinitis--an unusual cause of acute dysphagia. Emerg Radiol. 2011;18(5):449-451.
- Silva CF, Soffia PS, Pruzzo E. Acute prevertebral calcific tendinitis: a source of non-surgical acute cervical pain. Acta Radiologica. 2014;55(1):91-94.
- Zibis AH, Giannis D, Malizos KN, Kitsioulis P, Arvanitis DL. Acute calcific tendinitis of the longus colli muscle: case report and review of the literature. Eur Spine J. 2013;22(Suppl 3):S434-S438.
Emergency Imaging
Case
A 49-year-old man presented to the ED with low-back pain. Radiographs of the lumbosacral spine were obtained; a coned-down frontal representative radiograph of the lower lumbar spine and sacrum is presented (Figure 1a).
What is the diagnosis? Is additional imaging necessary? If so, why?
Answer
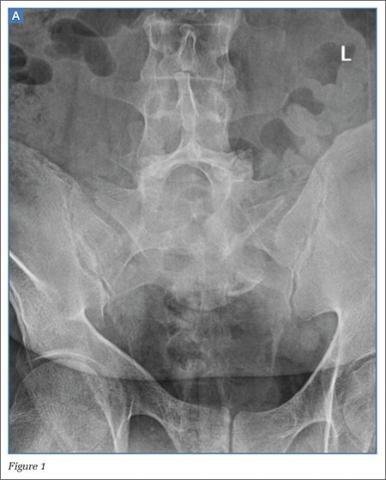
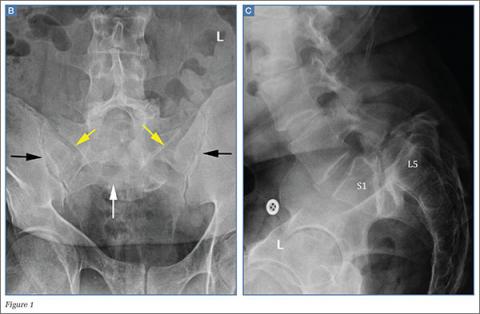
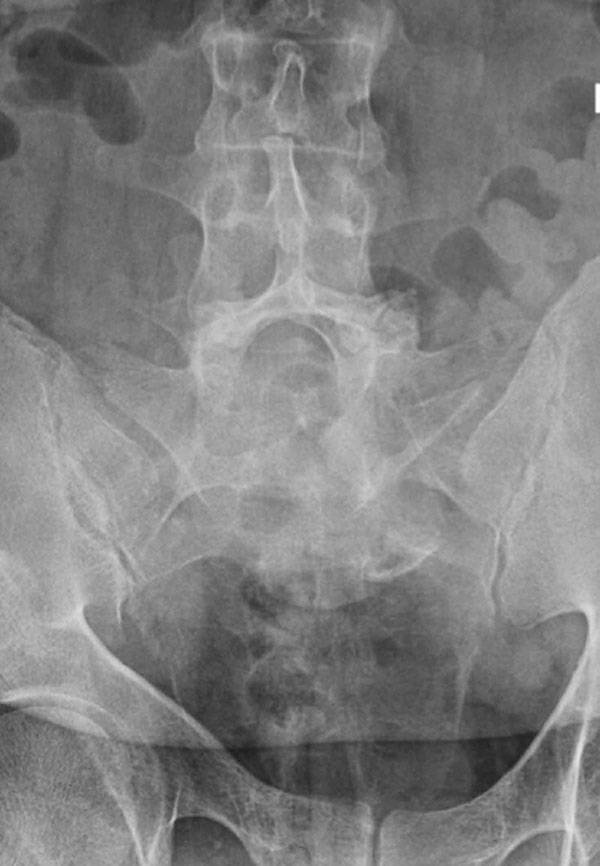
The frontal radiograph (Figure 1b) shows an “inverted Napoleon hat” sign,1 indicating high-grade anterolisthesis of L5 on S1 (less frequently, this sign also may be indicative of severe lumbar lordosis at the lumbosacral junction).
Anterolisthesis refers to anterior displacement of a vertebral body with respect to the vertebral body immediately below it. The dome of the “inverted hat” is formed by the anteroinferior endplate of the L5 vertebral body (white arrow, Figure 1b), and the tapered edges of the inverted hat are formed by the anteroinferiorly displaced/rotated L5 transverse processes (yellow arrows, Figure 1b). The contours of the L5 transverse processes project medial to the normal sacroiliac joints (black arrows, Figure 1b).
If the inverted Napoleon hat sign is identified on a single frontal view (eg, anteroposterior abdomen or pelvis radiograph), a lateral radiograph of the lumbosacral spine should be obtained to further evaluate the degree of L5-S1 anterolisthesis.
There are five grades of anterolisthesis, each based on quartiles of anterior displacement. Grade 1 anterolisthesis refers to less than 25% anterior displacement; grade 2 refers to 25% to 50% anterior displacement; grade 3 refers to 50% to 75% anterior displacement; and grade 4 refers to greater than 75% anterior displacement. In grade 5 anterolisthesis (also referred to as spondyloptosis), there is 100% anterior displacement with resultant anteroinferior slippage of the displaced vertebral body, which consequently lies anterior to the vertebral body below it. In this patient, the lateral radiograph of the lumbosacral spine (Figure 1c) demonstrates 100% anterior displacement of the L5 vertebral body with respect to S1, compatible with grade 5 anterolisthesis (spondyloptosis).
Spondylolisthesis is the more generalized term that includes all sagittal misalignments of the spine—more specifically, anterolisthesis for anterior displacement or retrolisthesis for posterior displacement. By convention, the displacement is named by the directional displacement of the more superior vertebral body in relation to the vertebral body immediately below it. Spondylolisthesis can be further delineated into one of six etiologic categories according to the modified Newman classification: congenital/dysplastic, spondylolytic, degenerative, traumatic, pathologic, or postsurgical.1,2 Of these six etiologies, spondylolysis (ie, defect of the pars interarticularis) and degenerative change (ie, disc degeneration and facet arthrosis) are the two most common causes of spondylolisthesis. High-grade spondylolisthesis (grade 3 or 4) typically requires bilateral spondylolysis (pars defects), while lower grade spondylolisthesis (particularly grade 1) can occur with any of the above etiologies. As seen in this case, anterolisthesis of L5 on S1 has the greatest effect on the exiting L5 nerve roots, and patients may present with low-back pain and/or hamstring tightness.1
With respect to imaging modalities, noncontrast computed tomography (CT) is of low utility in the workup of high-grade anterolisthesis, since it only serves to demonstrate the bilateral pars defects that are already presumed to be present—even if not seen radiographically. Conversely, magnetic resonance imaging (MRI), or CT myelography in patients in whom MRI is contraindicated, allows evaluation of the exiting nerve roots at the level of the spondylolisthesis. In addition to the spondylolisthesis, MRI can also reveal nerve impingement at other levels that might not be radiographically apparent (eg, neural foraminal stenosis that occurs due to disc herniation, facet arthrosis, and/or ligamentum flavum hypertrophy).
Management of high-grade spondylolisthesis remains controversial.3,4 Surgical treatment options include instrumented fusion, noninstrumented fusion, and L5 corpectomy with L4-S1 fusion. Fusion is generally recommended for patients with radicular symptoms, chronic incapacitating low-back pain, or risk of progression to spondyloptosis.4
Dr Bartolotta is an assistant professor of radiology at Weill Cornell Medical College in New York City, and an assistant attending radiologist at New York-Presbyterian Hospital/Weill Cornell Medical Center. Dr Hentel is an associate professor of clinical radiology at Weill Cornell Medical College, New York. He is also chief of emergency/musculoskeletal imaging and executive vice-chairman for the department of radiology at New York-Presbyterian Hospital/Weill Cornell Medical Center; and associate editor, imaging, of the EMERGENCY MEDICINE editorial board.
- Talangbayan LE. The inverted Napoleon’s hat sign. Radiology. 2007;243(2): 603-604.
- Wiltse LL, Newman PH, Macnab I. Classification of spondylolisis and spondylolisthesis. Clin Orthop Relat Res. 1976;117:23–29.
- Hart RA, Domes CM, Goodwin B, et al. High-grade spondylolisthesis treated using a modified Bohlman technique: results among multiple surgeons. J Neurosurg Spine. 2014;20(5):523-530.
- Lengert R, Charles YP, Walter A, Schuller S, Godet J, Steib JP. Posterior surgery in high-grade spondylolisthesis. Orthop Traumatol Surg Res. 2014;100(5):481-484.
Case
A 49-year-old man presented to the ED with low-back pain. Radiographs of the lumbosacral spine were obtained; a coned-down frontal representative radiograph of the lower lumbar spine and sacrum is presented (Figure 1a).
What is the diagnosis? Is additional imaging necessary? If so, why?
Answer
The frontal radiograph (Figure 1b) shows an “inverted Napoleon hat” sign,1 indicating high-grade anterolisthesis of L5 on S1 (less frequently, this sign also may be indicative of severe lumbar lordosis at the lumbosacral junction).
Anterolisthesis refers to anterior displacement of a vertebral body with respect to the vertebral body immediately below it. The dome of the “inverted hat” is formed by the anteroinferior endplate of the L5 vertebral body (white arrow, Figure 1b), and the tapered edges of the inverted hat are formed by the anteroinferiorly displaced/rotated L5 transverse processes (yellow arrows, Figure 1b). The contours of the L5 transverse processes project medial to the normal sacroiliac joints (black arrows, Figure 1b).
If the inverted Napoleon hat sign is identified on a single frontal view (eg, anteroposterior abdomen or pelvis radiograph), a lateral radiograph of the lumbosacral spine should be obtained to further evaluate the degree of L5-S1 anterolisthesis.
There are five grades of anterolisthesis, each based on quartiles of anterior displacement. Grade 1 anterolisthesis refers to less than 25% anterior displacement; grade 2 refers to 25% to 50% anterior displacement; grade 3 refers to 50% to 75% anterior displacement; and grade 4 refers to greater than 75% anterior displacement. In grade 5 anterolisthesis (also referred to as spondyloptosis), there is 100% anterior displacement with resultant anteroinferior slippage of the displaced vertebral body, which consequently lies anterior to the vertebral body below it. In this patient, the lateral radiograph of the lumbosacral spine (Figure 1c) demonstrates 100% anterior displacement of the L5 vertebral body with respect to S1, compatible with grade 5 anterolisthesis (spondyloptosis).
Spondylolisthesis is the more generalized term that includes all sagittal misalignments of the spine—more specifically, anterolisthesis for anterior displacement or retrolisthesis for posterior displacement. By convention, the displacement is named by the directional displacement of the more superior vertebral body in relation to the vertebral body immediately below it. Spondylolisthesis can be further delineated into one of six etiologic categories according to the modified Newman classification: congenital/dysplastic, spondylolytic, degenerative, traumatic, pathologic, or postsurgical.1,2 Of these six etiologies, spondylolysis (ie, defect of the pars interarticularis) and degenerative change (ie, disc degeneration and facet arthrosis) are the two most common causes of spondylolisthesis. High-grade spondylolisthesis (grade 3 or 4) typically requires bilateral spondylolysis (pars defects), while lower grade spondylolisthesis (particularly grade 1) can occur with any of the above etiologies. As seen in this case, anterolisthesis of L5 on S1 has the greatest effect on the exiting L5 nerve roots, and patients may present with low-back pain and/or hamstring tightness.1
With respect to imaging modalities, noncontrast computed tomography (CT) is of low utility in the workup of high-grade anterolisthesis, since it only serves to demonstrate the bilateral pars defects that are already presumed to be present—even if not seen radiographically. Conversely, magnetic resonance imaging (MRI), or CT myelography in patients in whom MRI is contraindicated, allows evaluation of the exiting nerve roots at the level of the spondylolisthesis. In addition to the spondylolisthesis, MRI can also reveal nerve impingement at other levels that might not be radiographically apparent (eg, neural foraminal stenosis that occurs due to disc herniation, facet arthrosis, and/or ligamentum flavum hypertrophy).
Management of high-grade spondylolisthesis remains controversial.3,4 Surgical treatment options include instrumented fusion, noninstrumented fusion, and L5 corpectomy with L4-S1 fusion. Fusion is generally recommended for patients with radicular symptoms, chronic incapacitating low-back pain, or risk of progression to spondyloptosis.4
Dr Bartolotta is an assistant professor of radiology at Weill Cornell Medical College in New York City, and an assistant attending radiologist at New York-Presbyterian Hospital/Weill Cornell Medical Center. Dr Hentel is an associate professor of clinical radiology at Weill Cornell Medical College, New York. He is also chief of emergency/musculoskeletal imaging and executive vice-chairman for the department of radiology at New York-Presbyterian Hospital/Weill Cornell Medical Center; and associate editor, imaging, of the EMERGENCY MEDICINE editorial board.
Case
A 49-year-old man presented to the ED with low-back pain. Radiographs of the lumbosacral spine were obtained; a coned-down frontal representative radiograph of the lower lumbar spine and sacrum is presented (Figure 1a).
What is the diagnosis? Is additional imaging necessary? If so, why?
Answer
The frontal radiograph (Figure 1b) shows an “inverted Napoleon hat” sign,1 indicating high-grade anterolisthesis of L5 on S1 (less frequently, this sign also may be indicative of severe lumbar lordosis at the lumbosacral junction).
Anterolisthesis refers to anterior displacement of a vertebral body with respect to the vertebral body immediately below it. The dome of the “inverted hat” is formed by the anteroinferior endplate of the L5 vertebral body (white arrow, Figure 1b), and the tapered edges of the inverted hat are formed by the anteroinferiorly displaced/rotated L5 transverse processes (yellow arrows, Figure 1b). The contours of the L5 transverse processes project medial to the normal sacroiliac joints (black arrows, Figure 1b).
If the inverted Napoleon hat sign is identified on a single frontal view (eg, anteroposterior abdomen or pelvis radiograph), a lateral radiograph of the lumbosacral spine should be obtained to further evaluate the degree of L5-S1 anterolisthesis.
There are five grades of anterolisthesis, each based on quartiles of anterior displacement. Grade 1 anterolisthesis refers to less than 25% anterior displacement; grade 2 refers to 25% to 50% anterior displacement; grade 3 refers to 50% to 75% anterior displacement; and grade 4 refers to greater than 75% anterior displacement. In grade 5 anterolisthesis (also referred to as spondyloptosis), there is 100% anterior displacement with resultant anteroinferior slippage of the displaced vertebral body, which consequently lies anterior to the vertebral body below it. In this patient, the lateral radiograph of the lumbosacral spine (Figure 1c) demonstrates 100% anterior displacement of the L5 vertebral body with respect to S1, compatible with grade 5 anterolisthesis (spondyloptosis).
Spondylolisthesis is the more generalized term that includes all sagittal misalignments of the spine—more specifically, anterolisthesis for anterior displacement or retrolisthesis for posterior displacement. By convention, the displacement is named by the directional displacement of the more superior vertebral body in relation to the vertebral body immediately below it. Spondylolisthesis can be further delineated into one of six etiologic categories according to the modified Newman classification: congenital/dysplastic, spondylolytic, degenerative, traumatic, pathologic, or postsurgical.1,2 Of these six etiologies, spondylolysis (ie, defect of the pars interarticularis) and degenerative change (ie, disc degeneration and facet arthrosis) are the two most common causes of spondylolisthesis. High-grade spondylolisthesis (grade 3 or 4) typically requires bilateral spondylolysis (pars defects), while lower grade spondylolisthesis (particularly grade 1) can occur with any of the above etiologies. As seen in this case, anterolisthesis of L5 on S1 has the greatest effect on the exiting L5 nerve roots, and patients may present with low-back pain and/or hamstring tightness.1
With respect to imaging modalities, noncontrast computed tomography (CT) is of low utility in the workup of high-grade anterolisthesis, since it only serves to demonstrate the bilateral pars defects that are already presumed to be present—even if not seen radiographically. Conversely, magnetic resonance imaging (MRI), or CT myelography in patients in whom MRI is contraindicated, allows evaluation of the exiting nerve roots at the level of the spondylolisthesis. In addition to the spondylolisthesis, MRI can also reveal nerve impingement at other levels that might not be radiographically apparent (eg, neural foraminal stenosis that occurs due to disc herniation, facet arthrosis, and/or ligamentum flavum hypertrophy).
Management of high-grade spondylolisthesis remains controversial.3,4 Surgical treatment options include instrumented fusion, noninstrumented fusion, and L5 corpectomy with L4-S1 fusion. Fusion is generally recommended for patients with radicular symptoms, chronic incapacitating low-back pain, or risk of progression to spondyloptosis.4
Dr Bartolotta is an assistant professor of radiology at Weill Cornell Medical College in New York City, and an assistant attending radiologist at New York-Presbyterian Hospital/Weill Cornell Medical Center. Dr Hentel is an associate professor of clinical radiology at Weill Cornell Medical College, New York. He is also chief of emergency/musculoskeletal imaging and executive vice-chairman for the department of radiology at New York-Presbyterian Hospital/Weill Cornell Medical Center; and associate editor, imaging, of the EMERGENCY MEDICINE editorial board.
- Talangbayan LE. The inverted Napoleon’s hat sign. Radiology. 2007;243(2): 603-604.
- Wiltse LL, Newman PH, Macnab I. Classification of spondylolisis and spondylolisthesis. Clin Orthop Relat Res. 1976;117:23–29.
- Hart RA, Domes CM, Goodwin B, et al. High-grade spondylolisthesis treated using a modified Bohlman technique: results among multiple surgeons. J Neurosurg Spine. 2014;20(5):523-530.
- Lengert R, Charles YP, Walter A, Schuller S, Godet J, Steib JP. Posterior surgery in high-grade spondylolisthesis. Orthop Traumatol Surg Res. 2014;100(5):481-484.
- Talangbayan LE. The inverted Napoleon’s hat sign. Radiology. 2007;243(2): 603-604.
- Wiltse LL, Newman PH, Macnab I. Classification of spondylolisis and spondylolisthesis. Clin Orthop Relat Res. 1976;117:23–29.
- Hart RA, Domes CM, Goodwin B, et al. High-grade spondylolisthesis treated using a modified Bohlman technique: results among multiple surgeons. J Neurosurg Spine. 2014;20(5):523-530.
- Lengert R, Charles YP, Walter A, Schuller S, Godet J, Steib JP. Posterior surgery in high-grade spondylolisthesis. Orthop Traumatol Surg Res. 2014;100(5):481-484.
Emergency Imaging
A 48-year-old man presented to the ED via emergency medical services after experiencing two episodes of syncope following the acute onset of right lower quadrant pain. He was unresponsive at the time of presentation. His vital signs were notable for tachycardia with a heart rate of 130 beats/minute. Although normotensive at initial presentation, the patient’s blood pressure began to fall rapidly. Laboratory values revealed a decreased hemoglobin and hematocrit of 5 g/dL and 18.1%, respectively.
Following his stabilization, a computed tomography (CT) scan of the abdomen and pelvis was performed. Figures 1a and 1b represent selected noncontrast and postcontrast axial images obtained through the lower abdomen/upper pelvis.
What is the diagnosis?
Answer
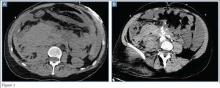
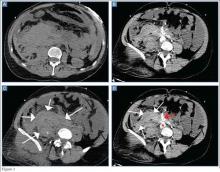
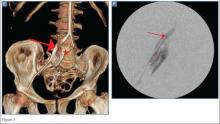
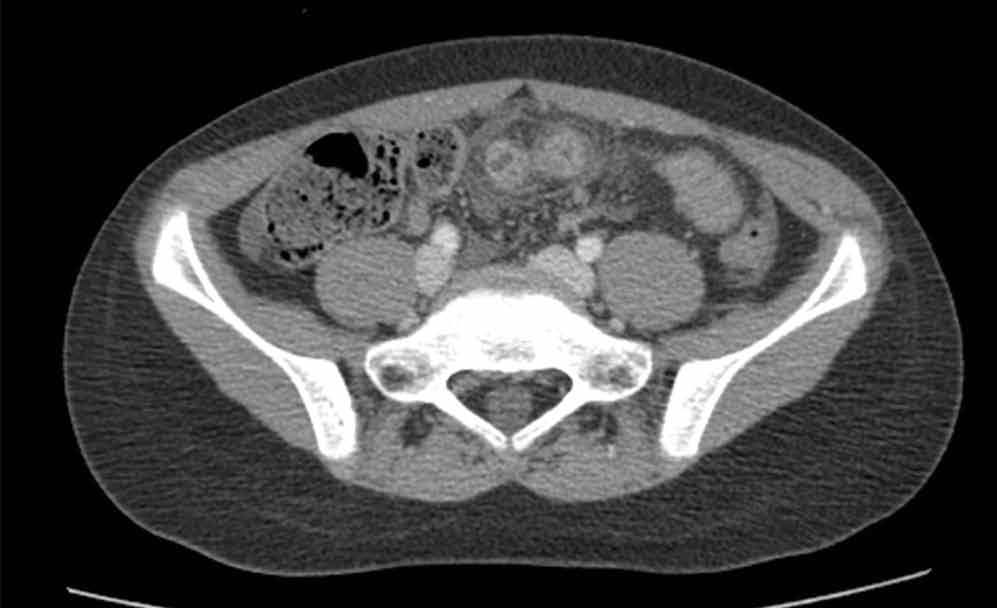
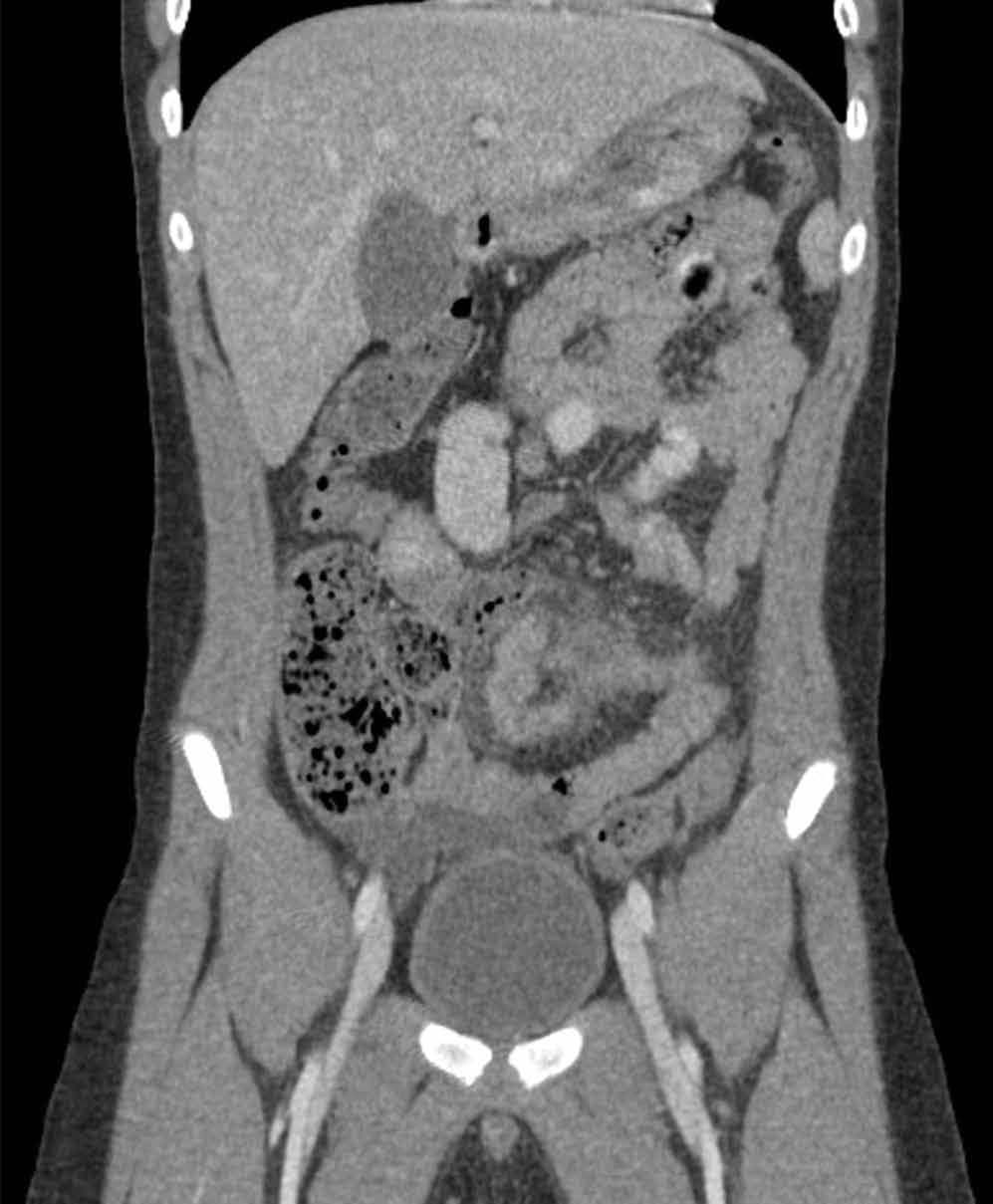
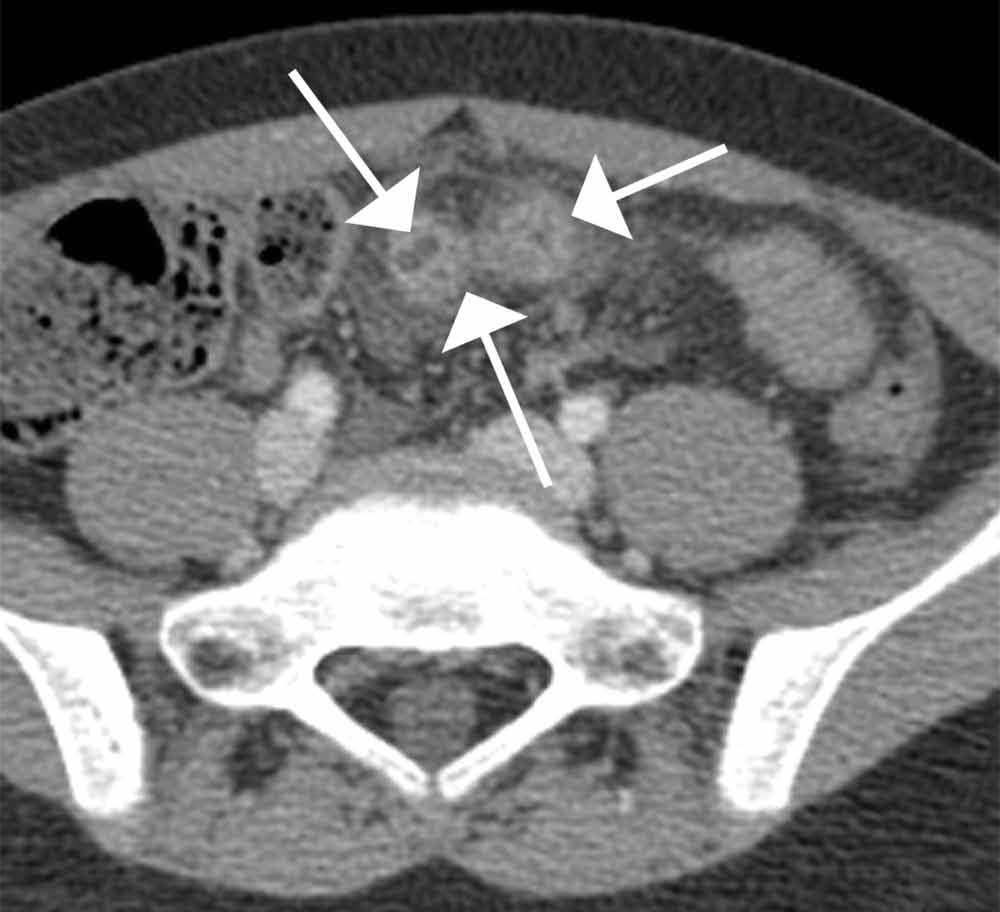
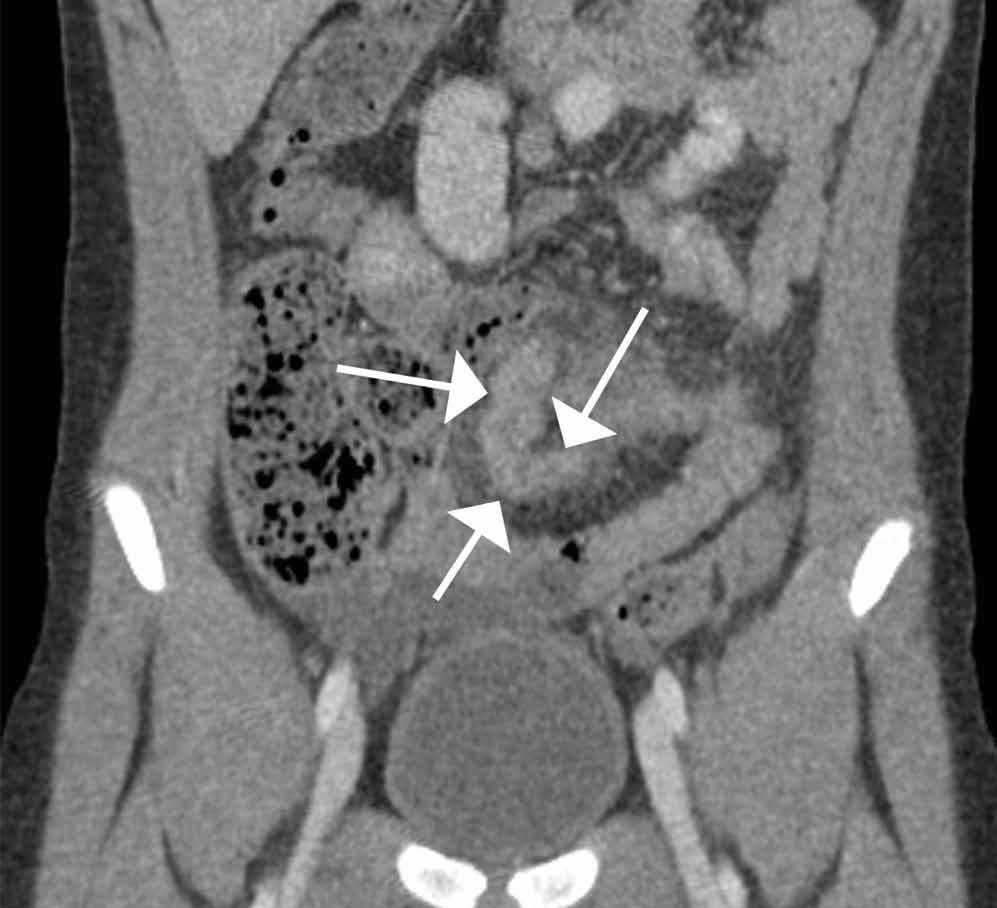
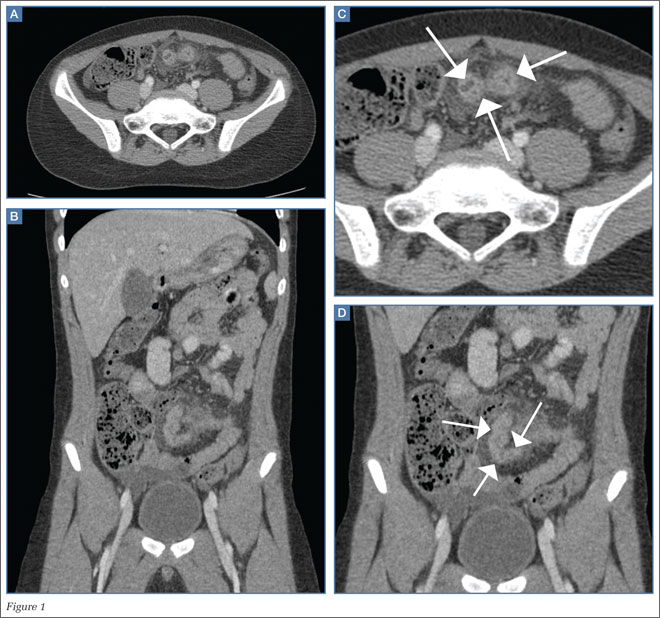
The precontrast image (Figure 1c) demonstrates a large and irregular high-density collection within the right upper pelvis (white arrows) and high density free fluid along the left lateral abdominal wall (black arrows). The right psoas muscle is obscured (white asterisk, Figure 1c) when compared to the normal psoas muscle on the contralateral side (black asterisk, Figure 1c). The postcontrast image (Figure 1d) shows the same findings and also reveals an enlarged right common iliac artery (red asterisk) and contrast actively extravasating from the artery (red arrow). These findings indicate the presence of a ruptured common iliac artery aneurysm. Both the enlarged common iliac artery aneurysm (red arrow, Figure 1d) and the extravasated contrast (red asterisk, Figure 1d) were confirmed on the three-dimensional reformats of the CT scan (Figure 1e).
An isolated aneurysm of the common iliac artery is uncommon, occurring in only 1% to 2% of the population—the same frequency as aortic aneurysm.1 However, the risk of rupture of this type of aneurysm is high.2 Patients with common iliac artery aneurysm are typically asymptomatic prior to rupture. However, some patients have reportedly presented with claudication, tenesmus/constipation, sciatica, and lower extremity paresis due to nerve compression, as well as urinary obstruction caused by ureteral obstruction.1,3 Once the iliac artery aneurysm has ruptured, patients typically present with acute abdominal, groin, and/or thigh pain, although isolated testicular pain has been described in the literature.1,4
Treatment for iliac artery aneurysm includes open and endovascular repair, depending on patient presentation and the adjacent structures involved. While mortality is low for patients with a nonruptured common iliac aneurysm, acute rupture is present in approximately one out of three cases, and the surgical mortality rate is as high as 55%.3
The patient presented in this case was taken to the operating room where an angiogram of the right common iliac artery (red arrow, Figure 1f) revealed continued acute extravasation of contrast (red asterisk, Figure 1f). Surgical repair was attempted but the patient did not survive due to complications of hypotension and cardiac arrest.
2. Reber PU, Brunner K, Hakki H, Stirnemann P, Kniemeyer HW. Incidence, classification and therapy of isolated pelvic artery aneurysm. Chirurg. 2001;72(4):419-424.
3. Bacharach JM, Slovut DP. State of the art: management of iliac artery aneurysmal disease. Catheter Cardiovasc Interv. 2008;71(5):708-714.
4. Dolan RD, Zino S. A ruptured left common iliac aneurysm presenting as testicular pain in a 56-year-old man. BMJ Case Rep. 2014. doi:10.1136/bcr-2012-006568.
A 48-year-old man presented to the ED via emergency medical services after experiencing two episodes of syncope following the acute onset of right lower quadrant pain. He was unresponsive at the time of presentation. His vital signs were notable for tachycardia with a heart rate of 130 beats/minute. Although normotensive at initial presentation, the patient’s blood pressure began to fall rapidly. Laboratory values revealed a decreased hemoglobin and hematocrit of 5 g/dL and 18.1%, respectively.
Following his stabilization, a computed tomography (CT) scan of the abdomen and pelvis was performed. Figures 1a and 1b represent selected noncontrast and postcontrast axial images obtained through the lower abdomen/upper pelvis.
What is the diagnosis?
Answer
The precontrast image (Figure 1c) demonstrates a large and irregular high-density collection within the right upper pelvis (white arrows) and high density free fluid along the left lateral abdominal wall (black arrows). The right psoas muscle is obscured (white asterisk, Figure 1c) when compared to the normal psoas muscle on the contralateral side (black asterisk, Figure 1c). The postcontrast image (Figure 1d) shows the same findings and also reveals an enlarged right common iliac artery (red asterisk) and contrast actively extravasating from the artery (red arrow). These findings indicate the presence of a ruptured common iliac artery aneurysm. Both the enlarged common iliac artery aneurysm (red arrow, Figure 1d) and the extravasated contrast (red asterisk, Figure 1d) were confirmed on the three-dimensional reformats of the CT scan (Figure 1e).
An isolated aneurysm of the common iliac artery is uncommon, occurring in only 1% to 2% of the population—the same frequency as aortic aneurysm.1 However, the risk of rupture of this type of aneurysm is high.2 Patients with common iliac artery aneurysm are typically asymptomatic prior to rupture. However, some patients have reportedly presented with claudication, tenesmus/constipation, sciatica, and lower extremity paresis due to nerve compression, as well as urinary obstruction caused by ureteral obstruction.1,3 Once the iliac artery aneurysm has ruptured, patients typically present with acute abdominal, groin, and/or thigh pain, although isolated testicular pain has been described in the literature.1,4
Treatment for iliac artery aneurysm includes open and endovascular repair, depending on patient presentation and the adjacent structures involved. While mortality is low for patients with a nonruptured common iliac aneurysm, acute rupture is present in approximately one out of three cases, and the surgical mortality rate is as high as 55%.3
The patient presented in this case was taken to the operating room where an angiogram of the right common iliac artery (red arrow, Figure 1f) revealed continued acute extravasation of contrast (red asterisk, Figure 1f). Surgical repair was attempted but the patient did not survive due to complications of hypotension and cardiac arrest.
A 48-year-old man presented to the ED via emergency medical services after experiencing two episodes of syncope following the acute onset of right lower quadrant pain. He was unresponsive at the time of presentation. His vital signs were notable for tachycardia with a heart rate of 130 beats/minute. Although normotensive at initial presentation, the patient’s blood pressure began to fall rapidly. Laboratory values revealed a decreased hemoglobin and hematocrit of 5 g/dL and 18.1%, respectively.
Following his stabilization, a computed tomography (CT) scan of the abdomen and pelvis was performed. Figures 1a and 1b represent selected noncontrast and postcontrast axial images obtained through the lower abdomen/upper pelvis.
What is the diagnosis?
Answer
The precontrast image (Figure 1c) demonstrates a large and irregular high-density collection within the right upper pelvis (white arrows) and high density free fluid along the left lateral abdominal wall (black arrows). The right psoas muscle is obscured (white asterisk, Figure 1c) when compared to the normal psoas muscle on the contralateral side (black asterisk, Figure 1c). The postcontrast image (Figure 1d) shows the same findings and also reveals an enlarged right common iliac artery (red asterisk) and contrast actively extravasating from the artery (red arrow). These findings indicate the presence of a ruptured common iliac artery aneurysm. Both the enlarged common iliac artery aneurysm (red arrow, Figure 1d) and the extravasated contrast (red asterisk, Figure 1d) were confirmed on the three-dimensional reformats of the CT scan (Figure 1e).
An isolated aneurysm of the common iliac artery is uncommon, occurring in only 1% to 2% of the population—the same frequency as aortic aneurysm.1 However, the risk of rupture of this type of aneurysm is high.2 Patients with common iliac artery aneurysm are typically asymptomatic prior to rupture. However, some patients have reportedly presented with claudication, tenesmus/constipation, sciatica, and lower extremity paresis due to nerve compression, as well as urinary obstruction caused by ureteral obstruction.1,3 Once the iliac artery aneurysm has ruptured, patients typically present with acute abdominal, groin, and/or thigh pain, although isolated testicular pain has been described in the literature.1,4
Treatment for iliac artery aneurysm includes open and endovascular repair, depending on patient presentation and the adjacent structures involved. While mortality is low for patients with a nonruptured common iliac aneurysm, acute rupture is present in approximately one out of three cases, and the surgical mortality rate is as high as 55%.3
The patient presented in this case was taken to the operating room where an angiogram of the right common iliac artery (red arrow, Figure 1f) revealed continued acute extravasation of contrast (red asterisk, Figure 1f). Surgical repair was attempted but the patient did not survive due to complications of hypotension and cardiac arrest.
2. Reber PU, Brunner K, Hakki H, Stirnemann P, Kniemeyer HW. Incidence, classification and therapy of isolated pelvic artery aneurysm. Chirurg. 2001;72(4):419-424.
3. Bacharach JM, Slovut DP. State of the art: management of iliac artery aneurysmal disease. Catheter Cardiovasc Interv. 2008;71(5):708-714.
4. Dolan RD, Zino S. A ruptured left common iliac aneurysm presenting as testicular pain in a 56-year-old man. BMJ Case Rep. 2014. doi:10.1136/bcr-2012-006568.
2. Reber PU, Brunner K, Hakki H, Stirnemann P, Kniemeyer HW. Incidence, classification and therapy of isolated pelvic artery aneurysm. Chirurg. 2001;72(4):419-424.
3. Bacharach JM, Slovut DP. State of the art: management of iliac artery aneurysmal disease. Catheter Cardiovasc Interv. 2008;71(5):708-714.
4. Dolan RD, Zino S. A ruptured left common iliac aneurysm presenting as testicular pain in a 56-year-old man. BMJ Case Rep. 2014. doi:10.1136/bcr-2012-006568.
Emergency Imaging
An 11-year-old boy is brought to the ED with a 1-week of history of increasing crampy lower-quadrant abdominal pain. His vital signs were only significant for mild tachycardia. On physical examination, the child’s abdomen was tender to palpation in the bilateral lower abdominal quadrants with guarding. Laboratory evaluations were unremarkable.
An abdominal radiograph did not reveal any abnormality, and targeted ultrasound did not reveal a dilated appendix. Computed tomography (CT) of the abdomen and pelvis with oral and intravenous contrast were ordered and representative images are provided (Figures 1a and 1b). Note that additional images from the CT demonstrate the abnormality depicted in these figures was not a loop of small bowel (although it appeared to originate from a loop of distal small bowel) and that the appendix was normal.
|
|
|
What is the diagnosis?
Answer
Computed tomography revealed a blind-ending tubular structure (white arrows, Figure 1c) deep to the umbilicus arising inferiorly from a loop of distal ileum with surrounding fat stranding (Figure 1d). The fluid-containing tubular structure demonstrates marked enhancement of the mucosa. These findings are most consistent with Meckel’s diverticulitis.
Meckel’s diverticulum is the most common anomaly of the gastrointestinal (GI) tract and results from incomplete obliteration of the vitelline duct. As per the rule of “twos,” Meckel’s diverticulum usually occurs 2 feet (40-60 cm) proximal to the ileocecal valve; is 2 cm wide (and 3 cm long); is found in 2% of the population; typically presents before age 2 years; is twice as likely to be symptomatic in boys; and contains ectopic gastric mucosa in approximately half of the cases.1
|
|
|
As many patients are asymptomatic, Meckel’s diverticulum is diagnosed as an incidental finding after a barium study or abdominal surgery is performed for other GI conditions. Symptoms occur as a result of ectopic gastric tissue, obstruction, and/or inflammation. Painless lower GI bleeding, the most common presentation, is reported in up to 50% of patients with symptomatic Meckel’s diverticulosis.2 Hemorrhage results from ulceration caused by secreted acid and enzymes from ectopic digestive mucosa. Intestinal obstruction is another common complication usually seen in children, which can be caused by volvulus of the small bowel around a diverticulum, intussusception, incarceration within a hernia, and internal herniation. Inflammation of the Meckel’s diverticulum, or Meckel’s diverticulitis, is more common in older patients and presents similarly to acute appendicitis.2
After removal of a complicated Meckel’s diverticulitis, postoperative morbidity and mortality rates have been reported to be 12% and 2%, respectively. In contrast, postoperative complications after resection of incidental diverticula are fewer, and morbidity and mortality rates are as low as 2% and 1%, respectively.3-5 Meckel’s diverticulitis should be included as a differential diagnosis when appendicitis or medically managed abdominopelvic inflammatory processes are suspected, as delayed diagnosis can lead to perforation, abscess formation, peritonitis, sepsis, bowel obstruction, and death.
The patient presented in this case was taken to the operating room, and the Meckel’s diverticula confirmed and removed. He experienced an uneventful postoperative course and was discharged a few days later.
Dr Rotman is a radiology resident at Weill Cornell Medical College in New York City. Dr Belfi is an assistant professor of radiology at Weill Cornell Medical College in New York City and an assistant attending radiologist at New York-Presbyterian Hospital/Weill Cornell Medical Center. Dr Hentel is an associate professor of clinical radiology, Weill Cornell Medical College, New York. He is also chief of emergency/musculoskeletal imaging and executive vice-chairman for the department of radiology, New York-Presbyterian Hospital/Weill Cornell Medical Center. He is associate editor, imaging, of the EMERGENCY MEDICINE editorial board.
- Anderson DJ. Carcinoid tumor in Meckel’s diverticulum: laparoscopic treatment and review of the literature. J Am Osteopath Assoc. 2000;100(7):432-434.
- Malik AA, Shams-ul-Bari, Wani KA, Khaja AR. Meckel’s diverticulum-Revisited. Saudi J Gastroenterol. 2010;16(1):3-7.
- Altinli E, Pekmezci S, Gorgun E, Sirin F. Laparoscopy-assisted resection of complicated Meckel’s diverticulum in adults. Surg Laparosc Endosc Percutan Tech. 2002;12(3):190-194.
- Nath, DS, Morris TA. Small bowel obstruction in an adolescent: a case of Meckel’s diverticulum. Minn Med. 2004;87(11):46-48.
- Cullen, JJ, Kelly KA, Moir CR, et al. Surgical management of Meckel’s diverticulum. An epidemiologic, population-based study. Ann Surg. 1994;220(4):564-568; discussion 568,569.
An 11-year-old boy is brought to the ED with a 1-week of history of increasing crampy lower-quadrant abdominal pain. His vital signs were only significant for mild tachycardia. On physical examination, the child’s abdomen was tender to palpation in the bilateral lower abdominal quadrants with guarding. Laboratory evaluations were unremarkable.
An abdominal radiograph did not reveal any abnormality, and targeted ultrasound did not reveal a dilated appendix. Computed tomography (CT) of the abdomen and pelvis with oral and intravenous contrast were ordered and representative images are provided (Figures 1a and 1b). Note that additional images from the CT demonstrate the abnormality depicted in these figures was not a loop of small bowel (although it appeared to originate from a loop of distal small bowel) and that the appendix was normal.
|
|
|
What is the diagnosis?
Answer
Computed tomography revealed a blind-ending tubular structure (white arrows, Figure 1c) deep to the umbilicus arising inferiorly from a loop of distal ileum with surrounding fat stranding (Figure 1d). The fluid-containing tubular structure demonstrates marked enhancement of the mucosa. These findings are most consistent with Meckel’s diverticulitis.
Meckel’s diverticulum is the most common anomaly of the gastrointestinal (GI) tract and results from incomplete obliteration of the vitelline duct. As per the rule of “twos,” Meckel’s diverticulum usually occurs 2 feet (40-60 cm) proximal to the ileocecal valve; is 2 cm wide (and 3 cm long); is found in 2% of the population; typically presents before age 2 years; is twice as likely to be symptomatic in boys; and contains ectopic gastric mucosa in approximately half of the cases.1
|
|
|
As many patients are asymptomatic, Meckel’s diverticulum is diagnosed as an incidental finding after a barium study or abdominal surgery is performed for other GI conditions. Symptoms occur as a result of ectopic gastric tissue, obstruction, and/or inflammation. Painless lower GI bleeding, the most common presentation, is reported in up to 50% of patients with symptomatic Meckel’s diverticulosis.2 Hemorrhage results from ulceration caused by secreted acid and enzymes from ectopic digestive mucosa. Intestinal obstruction is another common complication usually seen in children, which can be caused by volvulus of the small bowel around a diverticulum, intussusception, incarceration within a hernia, and internal herniation. Inflammation of the Meckel’s diverticulum, or Meckel’s diverticulitis, is more common in older patients and presents similarly to acute appendicitis.2
After removal of a complicated Meckel’s diverticulitis, postoperative morbidity and mortality rates have been reported to be 12% and 2%, respectively. In contrast, postoperative complications after resection of incidental diverticula are fewer, and morbidity and mortality rates are as low as 2% and 1%, respectively.3-5 Meckel’s diverticulitis should be included as a differential diagnosis when appendicitis or medically managed abdominopelvic inflammatory processes are suspected, as delayed diagnosis can lead to perforation, abscess formation, peritonitis, sepsis, bowel obstruction, and death.
The patient presented in this case was taken to the operating room, and the Meckel’s diverticula confirmed and removed. He experienced an uneventful postoperative course and was discharged a few days later.
Dr Rotman is a radiology resident at Weill Cornell Medical College in New York City. Dr Belfi is an assistant professor of radiology at Weill Cornell Medical College in New York City and an assistant attending radiologist at New York-Presbyterian Hospital/Weill Cornell Medical Center. Dr Hentel is an associate professor of clinical radiology, Weill Cornell Medical College, New York. He is also chief of emergency/musculoskeletal imaging and executive vice-chairman for the department of radiology, New York-Presbyterian Hospital/Weill Cornell Medical Center. He is associate editor, imaging, of the EMERGENCY MEDICINE editorial board.
An 11-year-old boy is brought to the ED with a 1-week of history of increasing crampy lower-quadrant abdominal pain. His vital signs were only significant for mild tachycardia. On physical examination, the child’s abdomen was tender to palpation in the bilateral lower abdominal quadrants with guarding. Laboratory evaluations were unremarkable.
An abdominal radiograph did not reveal any abnormality, and targeted ultrasound did not reveal a dilated appendix. Computed tomography (CT) of the abdomen and pelvis with oral and intravenous contrast were ordered and representative images are provided (Figures 1a and 1b). Note that additional images from the CT demonstrate the abnormality depicted in these figures was not a loop of small bowel (although it appeared to originate from a loop of distal small bowel) and that the appendix was normal.
|
|
|
What is the diagnosis?
Answer
Computed tomography revealed a blind-ending tubular structure (white arrows, Figure 1c) deep to the umbilicus arising inferiorly from a loop of distal ileum with surrounding fat stranding (Figure 1d). The fluid-containing tubular structure demonstrates marked enhancement of the mucosa. These findings are most consistent with Meckel’s diverticulitis.
Meckel’s diverticulum is the most common anomaly of the gastrointestinal (GI) tract and results from incomplete obliteration of the vitelline duct. As per the rule of “twos,” Meckel’s diverticulum usually occurs 2 feet (40-60 cm) proximal to the ileocecal valve; is 2 cm wide (and 3 cm long); is found in 2% of the population; typically presents before age 2 years; is twice as likely to be symptomatic in boys; and contains ectopic gastric mucosa in approximately half of the cases.1
|
|
|
As many patients are asymptomatic, Meckel’s diverticulum is diagnosed as an incidental finding after a barium study or abdominal surgery is performed for other GI conditions. Symptoms occur as a result of ectopic gastric tissue, obstruction, and/or inflammation. Painless lower GI bleeding, the most common presentation, is reported in up to 50% of patients with symptomatic Meckel’s diverticulosis.2 Hemorrhage results from ulceration caused by secreted acid and enzymes from ectopic digestive mucosa. Intestinal obstruction is another common complication usually seen in children, which can be caused by volvulus of the small bowel around a diverticulum, intussusception, incarceration within a hernia, and internal herniation. Inflammation of the Meckel’s diverticulum, or Meckel’s diverticulitis, is more common in older patients and presents similarly to acute appendicitis.2
After removal of a complicated Meckel’s diverticulitis, postoperative morbidity and mortality rates have been reported to be 12% and 2%, respectively. In contrast, postoperative complications after resection of incidental diverticula are fewer, and morbidity and mortality rates are as low as 2% and 1%, respectively.3-5 Meckel’s diverticulitis should be included as a differential diagnosis when appendicitis or medically managed abdominopelvic inflammatory processes are suspected, as delayed diagnosis can lead to perforation, abscess formation, peritonitis, sepsis, bowel obstruction, and death.
The patient presented in this case was taken to the operating room, and the Meckel’s diverticula confirmed and removed. He experienced an uneventful postoperative course and was discharged a few days later.
Dr Rotman is a radiology resident at Weill Cornell Medical College in New York City. Dr Belfi is an assistant professor of radiology at Weill Cornell Medical College in New York City and an assistant attending radiologist at New York-Presbyterian Hospital/Weill Cornell Medical Center. Dr Hentel is an associate professor of clinical radiology, Weill Cornell Medical College, New York. He is also chief of emergency/musculoskeletal imaging and executive vice-chairman for the department of radiology, New York-Presbyterian Hospital/Weill Cornell Medical Center. He is associate editor, imaging, of the EMERGENCY MEDICINE editorial board.
- Anderson DJ. Carcinoid tumor in Meckel’s diverticulum: laparoscopic treatment and review of the literature. J Am Osteopath Assoc. 2000;100(7):432-434.
- Malik AA, Shams-ul-Bari, Wani KA, Khaja AR. Meckel’s diverticulum-Revisited. Saudi J Gastroenterol. 2010;16(1):3-7.
- Altinli E, Pekmezci S, Gorgun E, Sirin F. Laparoscopy-assisted resection of complicated Meckel’s diverticulum in adults. Surg Laparosc Endosc Percutan Tech. 2002;12(3):190-194.
- Nath, DS, Morris TA. Small bowel obstruction in an adolescent: a case of Meckel’s diverticulum. Minn Med. 2004;87(11):46-48.
- Cullen, JJ, Kelly KA, Moir CR, et al. Surgical management of Meckel’s diverticulum. An epidemiologic, population-based study. Ann Surg. 1994;220(4):564-568; discussion 568,569.
- Anderson DJ. Carcinoid tumor in Meckel’s diverticulum: laparoscopic treatment and review of the literature. J Am Osteopath Assoc. 2000;100(7):432-434.
- Malik AA, Shams-ul-Bari, Wani KA, Khaja AR. Meckel’s diverticulum-Revisited. Saudi J Gastroenterol. 2010;16(1):3-7.
- Altinli E, Pekmezci S, Gorgun E, Sirin F. Laparoscopy-assisted resection of complicated Meckel’s diverticulum in adults. Surg Laparosc Endosc Percutan Tech. 2002;12(3):190-194.
- Nath, DS, Morris TA. Small bowel obstruction in an adolescent: a case of Meckel’s diverticulum. Minn Med. 2004;87(11):46-48.
- Cullen, JJ, Kelly KA, Moir CR, et al. Surgical management of Meckel’s diverticulum. An epidemiologic, population-based study. Ann Surg. 1994;220(4):564-568; discussion 568,569.
Emergency Imaging: What is the suspected diagnosis? Is additional imaging necessary, and if so, why?
A 25-year-old man with no significant past medical history presented with low-back pain that radiated down into his right thigh. The patient stated the pain began 1 week earlier when he was lifting weights and had increased in severity to the point where he was no longer able to walk or stand up straight. He had taken nonprescription nonsteroidal anti-inflammatory drugs but received no significant relief.
Radiographs of the lumbosacral spine were obtained; representative anteroposterior (AP) and lateral images are shown above (Figures 1 and 2).
The lateral view of the lumbar spine demonstrates mild anterolisthesis of L5 on S1 with the posterior cortex of L5 (white arrow, Figure 3) anterior to the posterior cortex of S1 (red arrow, Figure 3). Normally, the posterior cortices of the adjacent vertebral bodies should align. Lucency is also noted in the region of the pars interarticularis (white asterisk, Figure 3). The combination of anterolisthesis and this lucency in a young patient suggests the diagnosis of spondylolysis (pars defect).
The pathophysiology of spondylolysis is still uncertain. Two theories have been proposed—underlying dysplastic pars interarticularis versus repetitive microtrauma resulting in stress factors are the two proposed underlying mechanism. If patients are genetically predisposed, underlying dysplasia probably contributes to the pathology, while microtrauma triggers the actual defect.2 Most patients respond well with conservative management.
When evaluating for spondylolysis, AP, lateral, 45-degree right and left oblique views, and collimated lateral views of the lumbosacral spine should be obtained. With this five-view study, up to 96.5% of pars defect can be identified.
In the general population, if spondylolysis is suspected and radiographs are negative, magnetic resonance imaging, computed tomography, and/or single-photon emission computed tomography bone scintigraphy can be used for further evaluation.4,5 In this case, the diagnosis was made based on radiographic imaging, and the patient was discharged with a scheduled follow-up with an orthopedic surgeon.
Dr Salama is a resident of radiology, resident of radiology, New York-Presbyterian Hospital/Weill Cornell Medical Center, New York. Dr Belfi is an assistant professor of radiology, Weill Cornell Medical College New York; and an assistant attending radiologist, New York-Presbyterian Hospital/Weill Cornell Medical Center, New York. Dr Hentel is an associate professor of clinical radiology, Weill Cornell Medical College, New York. He is also chief of emergency/musculoskeletal imaging and executive vice-chairman for the department of radiology, New York-Presbyterian Hospital/Weill Cornell Medical Center. He is associate editor, imaging, of the EMERGENCY MEDICINE editorial board.
- Belfi LM, Ortiz AO, Katz DS. Computed tomography evaluation of spondylolysis and spondylolisthesis in asymptomatic patients. Spine (Phila Pa 1976). 2006;31(24):E907-E910. doi:10.1097/01.brs.0000245947.31473.0a.
- Foreman P, Griessenauer CJ, Watanabe K, et al. L5 spondylolysis/spondylolisthesis: a comprehensive review with an anatomic focus. Childs Nerv Syst. 2013;29(2):209-216. doi:10.1007/s00381-012-1942-2.
- Amato M, Totty WG, Gilula LA. Spondylolysis of the lumbar spine: demonstration of defects and laminal fragmentation. Radiology. 1984;153(3):627-629.
- Saraste H, Nilsson B, Broström LA, et al. Relationship between radiological and clinical variables in spondylolysis. Int Orthop. 1984;8(3):163-174. doi:10.1007/BF00269912.
- Lee JH, Ehara S, Tamakawa Y, Shimamura T. Spondylolysis of the upper lumbar spine: Radiological features. Clin Imaging. 1999;23(6):389-393. doi:10.1016/S0899-7071(99)00158-8.
A 25-year-old man with no significant past medical history presented with low-back pain that radiated down into his right thigh. The patient stated the pain began 1 week earlier when he was lifting weights and had increased in severity to the point where he was no longer able to walk or stand up straight. He had taken nonprescription nonsteroidal anti-inflammatory drugs but received no significant relief.
Radiographs of the lumbosacral spine were obtained; representative anteroposterior (AP) and lateral images are shown above (Figures 1 and 2).
The lateral view of the lumbar spine demonstrates mild anterolisthesis of L5 on S1 with the posterior cortex of L5 (white arrow, Figure 3) anterior to the posterior cortex of S1 (red arrow, Figure 3). Normally, the posterior cortices of the adjacent vertebral bodies should align. Lucency is also noted in the region of the pars interarticularis (white asterisk, Figure 3). The combination of anterolisthesis and this lucency in a young patient suggests the diagnosis of spondylolysis (pars defect).
The pathophysiology of spondylolysis is still uncertain. Two theories have been proposed—underlying dysplastic pars interarticularis versus repetitive microtrauma resulting in stress factors are the two proposed underlying mechanism. If patients are genetically predisposed, underlying dysplasia probably contributes to the pathology, while microtrauma triggers the actual defect.2 Most patients respond well with conservative management.
When evaluating for spondylolysis, AP, lateral, 45-degree right and left oblique views, and collimated lateral views of the lumbosacral spine should be obtained. With this five-view study, up to 96.5% of pars defect can be identified.
In the general population, if spondylolysis is suspected and radiographs are negative, magnetic resonance imaging, computed tomography, and/or single-photon emission computed tomography bone scintigraphy can be used for further evaluation.4,5 In this case, the diagnosis was made based on radiographic imaging, and the patient was discharged with a scheduled follow-up with an orthopedic surgeon.
Dr Salama is a resident of radiology, resident of radiology, New York-Presbyterian Hospital/Weill Cornell Medical Center, New York. Dr Belfi is an assistant professor of radiology, Weill Cornell Medical College New York; and an assistant attending radiologist, New York-Presbyterian Hospital/Weill Cornell Medical Center, New York. Dr Hentel is an associate professor of clinical radiology, Weill Cornell Medical College, New York. He is also chief of emergency/musculoskeletal imaging and executive vice-chairman for the department of radiology, New York-Presbyterian Hospital/Weill Cornell Medical Center. He is associate editor, imaging, of the EMERGENCY MEDICINE editorial board.
A 25-year-old man with no significant past medical history presented with low-back pain that radiated down into his right thigh. The patient stated the pain began 1 week earlier when he was lifting weights and had increased in severity to the point where he was no longer able to walk or stand up straight. He had taken nonprescription nonsteroidal anti-inflammatory drugs but received no significant relief.
Radiographs of the lumbosacral spine were obtained; representative anteroposterior (AP) and lateral images are shown above (Figures 1 and 2).
The lateral view of the lumbar spine demonstrates mild anterolisthesis of L5 on S1 with the posterior cortex of L5 (white arrow, Figure 3) anterior to the posterior cortex of S1 (red arrow, Figure 3). Normally, the posterior cortices of the adjacent vertebral bodies should align. Lucency is also noted in the region of the pars interarticularis (white asterisk, Figure 3). The combination of anterolisthesis and this lucency in a young patient suggests the diagnosis of spondylolysis (pars defect).
The pathophysiology of spondylolysis is still uncertain. Two theories have been proposed—underlying dysplastic pars interarticularis versus repetitive microtrauma resulting in stress factors are the two proposed underlying mechanism. If patients are genetically predisposed, underlying dysplasia probably contributes to the pathology, while microtrauma triggers the actual defect.2 Most patients respond well with conservative management.
When evaluating for spondylolysis, AP, lateral, 45-degree right and left oblique views, and collimated lateral views of the lumbosacral spine should be obtained. With this five-view study, up to 96.5% of pars defect can be identified.
In the general population, if spondylolysis is suspected and radiographs are negative, magnetic resonance imaging, computed tomography, and/or single-photon emission computed tomography bone scintigraphy can be used for further evaluation.4,5 In this case, the diagnosis was made based on radiographic imaging, and the patient was discharged with a scheduled follow-up with an orthopedic surgeon.
Dr Salama is a resident of radiology, resident of radiology, New York-Presbyterian Hospital/Weill Cornell Medical Center, New York. Dr Belfi is an assistant professor of radiology, Weill Cornell Medical College New York; and an assistant attending radiologist, New York-Presbyterian Hospital/Weill Cornell Medical Center, New York. Dr Hentel is an associate professor of clinical radiology, Weill Cornell Medical College, New York. He is also chief of emergency/musculoskeletal imaging and executive vice-chairman for the department of radiology, New York-Presbyterian Hospital/Weill Cornell Medical Center. He is associate editor, imaging, of the EMERGENCY MEDICINE editorial board.
- Belfi LM, Ortiz AO, Katz DS. Computed tomography evaluation of spondylolysis and spondylolisthesis in asymptomatic patients. Spine (Phila Pa 1976). 2006;31(24):E907-E910. doi:10.1097/01.brs.0000245947.31473.0a.
- Foreman P, Griessenauer CJ, Watanabe K, et al. L5 spondylolysis/spondylolisthesis: a comprehensive review with an anatomic focus. Childs Nerv Syst. 2013;29(2):209-216. doi:10.1007/s00381-012-1942-2.
- Amato M, Totty WG, Gilula LA. Spondylolysis of the lumbar spine: demonstration of defects and laminal fragmentation. Radiology. 1984;153(3):627-629.
- Saraste H, Nilsson B, Broström LA, et al. Relationship between radiological and clinical variables in spondylolysis. Int Orthop. 1984;8(3):163-174. doi:10.1007/BF00269912.
- Lee JH, Ehara S, Tamakawa Y, Shimamura T. Spondylolysis of the upper lumbar spine: Radiological features. Clin Imaging. 1999;23(6):389-393. doi:10.1016/S0899-7071(99)00158-8.
- Belfi LM, Ortiz AO, Katz DS. Computed tomography evaluation of spondylolysis and spondylolisthesis in asymptomatic patients. Spine (Phila Pa 1976). 2006;31(24):E907-E910. doi:10.1097/01.brs.0000245947.31473.0a.
- Foreman P, Griessenauer CJ, Watanabe K, et al. L5 spondylolysis/spondylolisthesis: a comprehensive review with an anatomic focus. Childs Nerv Syst. 2013;29(2):209-216. doi:10.1007/s00381-012-1942-2.
- Amato M, Totty WG, Gilula LA. Spondylolysis of the lumbar spine: demonstration of defects and laminal fragmentation. Radiology. 1984;153(3):627-629.
- Saraste H, Nilsson B, Broström LA, et al. Relationship between radiological and clinical variables in spondylolysis. Int Orthop. 1984;8(3):163-174. doi:10.1007/BF00269912.
- Lee JH, Ehara S, Tamakawa Y, Shimamura T. Spondylolysis of the upper lumbar spine: Radiological features. Clin Imaging. 1999;23(6):389-393. doi:10.1016/S0899-7071(99)00158-8.
Occipital headache and unstable gait
A 41-year-old-man with a history of hypertension presented to the ED with a sudden onset of severe occipital headache and unstable gait. On physical examination, right-sided ptosis and miosis (Horner’s syndrome) were noted. An emergent head computed tomography (CT) scan was performed and showed no evidence of hemorrhage or infraction. Magnetic resonance imaging (MRI) of the brain and magnetic resonance angiography (MRA) of the head and neck were also ordered. Noncontrast MRA images of the neck are shown above (Figures 1 and 2).
|
|
Figure 1 | Figure 2 |
What is the diagnosis?
What other imaging modalities may be useful for evaluation?
Dr Wladyka is an assistant professor of radiology at Weill Cornell Medical College in New York City and an assistant attending radiologist at New York-Presbyterian Hospital/Weill Cornell Medical Center. Dr Hentel is an associate professor of clinical radiology at Weill Cornell Medical College in New York City. He is also chief of emergency/musculoskeletal imaging and the executive vice-chairman for the department of radiology at New York-Presbyterian Hospital/Weill Cornell Medical Center. He is associate editor, imaging, of the EMERGENCY MEDICINE editorial board.
Answer
|
|
Figure 3 | Figure 4 |
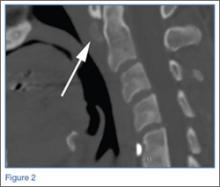
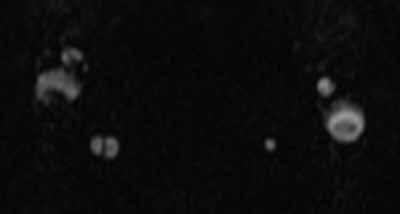
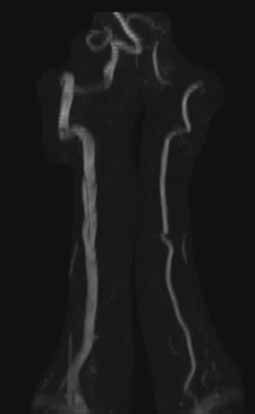
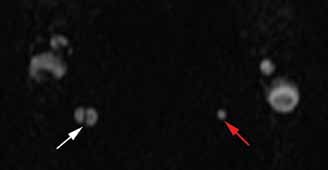
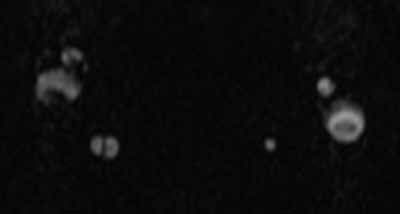
The axial MRA image demonstrates flow in two separate lumens of the right vertebral artery (white arrow, Figure 3), indicating the presence of a dissection. A normal single lumen vertebral artery can be seen on the left (red arrow, Figure 3). This dissection is confirmed on the coronal MRA image (white arrows, Figure 4).
Vertebral artery dissection (VAD) is a common cause of stroke and, less commonly, of transient ischemic attack in patients aged 18 to 45 years. Patients typically present with headache, vertigo, dizziness, and neck pain.1 Additional neurologic signs that may be related to compromise of the posterior cerebral circulation include lateral medullary syndrome (eg, dysphagia, slurred speech, ataxia, facial pain, nystagmus, diplopia, dysphonia) and Horner’s syndrome (eg, ptosis, miosis, anhidrosis). In a small percentage of patients, VAD may present as intracranial subarachnoid hemorrhage.1
VAD can occur spontaneously or following high-energy trauma or minor trauma (eg, resulting from coughing, vomiting, cervical spine manipulation, or sports injury). Approximately 15% of patients have an underlying connective tissue disorder, such as fibromuscular dysplasia.2
The diagnosis of VAD can be made with MRI/MRA or with computed tomography angiography (CTA). A recent review of the literature shows no clear advantage to using either modality; therefore, the choice CTA or MRA should be based on urgency, availability of the imaging modality, and the preferences/expertise of the radiologists.3
|
|
Figure 5 | Figure 6 |
Advantages of CTA include its widespread availability, short examination time, and ability to evaluate for concurrent injuries in a trauma patient. With respect to MRA, in addition the lack of ionizing radiation, images may be performed without intravenous contrast and completed at the same time as MRI, which is useful in detecting alternative or concurrent intracranial abnormalities such as stroke, mass, or demyelination.
In this case, axial and coronal images from follow-up CTA illustrate its ability to depict the dissection of the right vertebral artery (white arrows, Figures 5 and 6). Typical treatment for VAD includes anticoagulation and antiplatelet therapy, though there have also been reports of successful treatment with endovascular stenting and endovascular thrombolysis.4 Since timely and proper treatment decreases the risk of stroke and long-term disabilities, emergent imaging with CTA or MRA should be performed in cases of suspected VAD.
- Gottesman RF, Sharma P, Robinson KA, et al. Clinical characteristics of symptomatic vertebral artery dissection: a systematic review. Neurologist. 2012;18(5):245-254.
- Rodallec MH, Marteau V, Gerber S, Desmottes L, Zins M. Craniocervical arterial dissection: spectrum of imaging findings and differential diagnosis. Radiographics. 2008;28(6):1711-1728.
- Provenzale JM, Sarikaya B. Comparison of test performance characteristics of MRI, MR angiography, and CT angiography in the diagnosis of carotid and vertebral artery dissection: a review of the medical literature. AJR Am J Roentgenol. 2009;193(4):1167-1174.
- Menon R, Kerry S, Norris JW, Markus HS. Treatment of cervical artery dissection: a systematic review and meta-analysis. J Neurol Neurosurg Psychiatry. 2008;79(10):1122-1127.
A 41-year-old-man with a history of hypertension presented to the ED with a sudden onset of severe occipital headache and unstable gait. On physical examination, right-sided ptosis and miosis (Horner’s syndrome) were noted. An emergent head computed tomography (CT) scan was performed and showed no evidence of hemorrhage or infraction. Magnetic resonance imaging (MRI) of the brain and magnetic resonance angiography (MRA) of the head and neck were also ordered. Noncontrast MRA images of the neck are shown above (Figures 1 and 2).
|
|
Figure 1 | Figure 2 |
What is the diagnosis?
What other imaging modalities may be useful for evaluation?
Dr Wladyka is an assistant professor of radiology at Weill Cornell Medical College in New York City and an assistant attending radiologist at New York-Presbyterian Hospital/Weill Cornell Medical Center. Dr Hentel is an associate professor of clinical radiology at Weill Cornell Medical College in New York City. He is also chief of emergency/musculoskeletal imaging and the executive vice-chairman for the department of radiology at New York-Presbyterian Hospital/Weill Cornell Medical Center. He is associate editor, imaging, of the EMERGENCY MEDICINE editorial board.
Answer
|
|
Figure 3 | Figure 4 |
The axial MRA image demonstrates flow in two separate lumens of the right vertebral artery (white arrow, Figure 3), indicating the presence of a dissection. A normal single lumen vertebral artery can be seen on the left (red arrow, Figure 3). This dissection is confirmed on the coronal MRA image (white arrows, Figure 4).
Vertebral artery dissection (VAD) is a common cause of stroke and, less commonly, of transient ischemic attack in patients aged 18 to 45 years. Patients typically present with headache, vertigo, dizziness, and neck pain.1 Additional neurologic signs that may be related to compromise of the posterior cerebral circulation include lateral medullary syndrome (eg, dysphagia, slurred speech, ataxia, facial pain, nystagmus, diplopia, dysphonia) and Horner’s syndrome (eg, ptosis, miosis, anhidrosis). In a small percentage of patients, VAD may present as intracranial subarachnoid hemorrhage.1
VAD can occur spontaneously or following high-energy trauma or minor trauma (eg, resulting from coughing, vomiting, cervical spine manipulation, or sports injury). Approximately 15% of patients have an underlying connective tissue disorder, such as fibromuscular dysplasia.2
The diagnosis of VAD can be made with MRI/MRA or with computed tomography angiography (CTA). A recent review of the literature shows no clear advantage to using either modality; therefore, the choice CTA or MRA should be based on urgency, availability of the imaging modality, and the preferences/expertise of the radiologists.3
|
|
Figure 5 | Figure 6 |
Advantages of CTA include its widespread availability, short examination time, and ability to evaluate for concurrent injuries in a trauma patient. With respect to MRA, in addition the lack of ionizing radiation, images may be performed without intravenous contrast and completed at the same time as MRI, which is useful in detecting alternative or concurrent intracranial abnormalities such as stroke, mass, or demyelination.
In this case, axial and coronal images from follow-up CTA illustrate its ability to depict the dissection of the right vertebral artery (white arrows, Figures 5 and 6). Typical treatment for VAD includes anticoagulation and antiplatelet therapy, though there have also been reports of successful treatment with endovascular stenting and endovascular thrombolysis.4 Since timely and proper treatment decreases the risk of stroke and long-term disabilities, emergent imaging with CTA or MRA should be performed in cases of suspected VAD.
A 41-year-old-man with a history of hypertension presented to the ED with a sudden onset of severe occipital headache and unstable gait. On physical examination, right-sided ptosis and miosis (Horner’s syndrome) were noted. An emergent head computed tomography (CT) scan was performed and showed no evidence of hemorrhage or infraction. Magnetic resonance imaging (MRI) of the brain and magnetic resonance angiography (MRA) of the head and neck were also ordered. Noncontrast MRA images of the neck are shown above (Figures 1 and 2).
|
|
Figure 1 | Figure 2 |
What is the diagnosis?
What other imaging modalities may be useful for evaluation?
Dr Wladyka is an assistant professor of radiology at Weill Cornell Medical College in New York City and an assistant attending radiologist at New York-Presbyterian Hospital/Weill Cornell Medical Center. Dr Hentel is an associate professor of clinical radiology at Weill Cornell Medical College in New York City. He is also chief of emergency/musculoskeletal imaging and the executive vice-chairman for the department of radiology at New York-Presbyterian Hospital/Weill Cornell Medical Center. He is associate editor, imaging, of the EMERGENCY MEDICINE editorial board.
Answer
|
|
Figure 3 | Figure 4 |
The axial MRA image demonstrates flow in two separate lumens of the right vertebral artery (white arrow, Figure 3), indicating the presence of a dissection. A normal single lumen vertebral artery can be seen on the left (red arrow, Figure 3). This dissection is confirmed on the coronal MRA image (white arrows, Figure 4).
Vertebral artery dissection (VAD) is a common cause of stroke and, less commonly, of transient ischemic attack in patients aged 18 to 45 years. Patients typically present with headache, vertigo, dizziness, and neck pain.1 Additional neurologic signs that may be related to compromise of the posterior cerebral circulation include lateral medullary syndrome (eg, dysphagia, slurred speech, ataxia, facial pain, nystagmus, diplopia, dysphonia) and Horner’s syndrome (eg, ptosis, miosis, anhidrosis). In a small percentage of patients, VAD may present as intracranial subarachnoid hemorrhage.1
VAD can occur spontaneously or following high-energy trauma or minor trauma (eg, resulting from coughing, vomiting, cervical spine manipulation, or sports injury). Approximately 15% of patients have an underlying connective tissue disorder, such as fibromuscular dysplasia.2
The diagnosis of VAD can be made with MRI/MRA or with computed tomography angiography (CTA). A recent review of the literature shows no clear advantage to using either modality; therefore, the choice CTA or MRA should be based on urgency, availability of the imaging modality, and the preferences/expertise of the radiologists.3
|
|
Figure 5 | Figure 6 |
Advantages of CTA include its widespread availability, short examination time, and ability to evaluate for concurrent injuries in a trauma patient. With respect to MRA, in addition the lack of ionizing radiation, images may be performed without intravenous contrast and completed at the same time as MRI, which is useful in detecting alternative or concurrent intracranial abnormalities such as stroke, mass, or demyelination.
In this case, axial and coronal images from follow-up CTA illustrate its ability to depict the dissection of the right vertebral artery (white arrows, Figures 5 and 6). Typical treatment for VAD includes anticoagulation and antiplatelet therapy, though there have also been reports of successful treatment with endovascular stenting and endovascular thrombolysis.4 Since timely and proper treatment decreases the risk of stroke and long-term disabilities, emergent imaging with CTA or MRA should be performed in cases of suspected VAD.
- Gottesman RF, Sharma P, Robinson KA, et al. Clinical characteristics of symptomatic vertebral artery dissection: a systematic review. Neurologist. 2012;18(5):245-254.
- Rodallec MH, Marteau V, Gerber S, Desmottes L, Zins M. Craniocervical arterial dissection: spectrum of imaging findings and differential diagnosis. Radiographics. 2008;28(6):1711-1728.
- Provenzale JM, Sarikaya B. Comparison of test performance characteristics of MRI, MR angiography, and CT angiography in the diagnosis of carotid and vertebral artery dissection: a review of the medical literature. AJR Am J Roentgenol. 2009;193(4):1167-1174.
- Menon R, Kerry S, Norris JW, Markus HS. Treatment of cervical artery dissection: a systematic review and meta-analysis. J Neurol Neurosurg Psychiatry. 2008;79(10):1122-1127.
- Gottesman RF, Sharma P, Robinson KA, et al. Clinical characteristics of symptomatic vertebral artery dissection: a systematic review. Neurologist. 2012;18(5):245-254.
- Rodallec MH, Marteau V, Gerber S, Desmottes L, Zins M. Craniocervical arterial dissection: spectrum of imaging findings and differential diagnosis. Radiographics. 2008;28(6):1711-1728.
- Provenzale JM, Sarikaya B. Comparison of test performance characteristics of MRI, MR angiography, and CT angiography in the diagnosis of carotid and vertebral artery dissection: a review of the medical literature. AJR Am J Roentgenol. 2009;193(4):1167-1174.
- Menon R, Kerry S, Norris JW, Markus HS. Treatment of cervical artery dissection: a systematic review and meta-analysis. J Neurol Neurosurg Psychiatry. 2008;79(10):1122-1127.