User login
Advances in the treatment of dyslipidemia
The 2013 joint guidelines of the American College of Cardiology and American Heart Association (ACC/AHA)1 on the treatment of blood cholesterol to reduce cardiovascular risk recommend high-intensity statin therapy for secondary prevention of cardiovascular events. The question of primary prevention is not so straightforward, and the recommended strategy has come under fire. In addition, the guidelines focus on statins and not on LDL-C levels, and the role of nonstatin lipid-lowering drugs and the value of reducing LDL-C levels to well below levels currently regarded as “normal” remain unclear.
This article comments on the 2013 ACC/AHA guidelines, reviews the data on optimal LDL-C levels, and discusses new nonstatin agents.
ACC/AHA GUIDELINES: A MIXED MESSAGE
The 2013 ACC/AHA cholesterol guidelines1 can be characterized by the title from the famous Western film “The Good, the Bad, and the Ugly.”
The good: A clear message to treat
The guidelines deliver an unambiguous message to treat patients at high risk with high-intensity statin therapy. This mandate is very helpful as it should reduce the undertreatment of patients.
The seemingly bad
Two common misconceptions regarding the guidelines:
They abandon LDL-C targets. Actually, the guidelines do not argue for or against targets; they simply remain silent, citing that randomized trials have not been conducted with LDL-C targets as specific goals. Technically, this statement is true. However, it seems contrived to argue, for example, that the benefit of atorvastatin 80 mg over 10 mg in the Treating to New Targets trial could not be reliably ascribed to the lower LDL-C achieved with the higher dose, but rather to some undefined benefit of high-intensity statin therapy, especially as the guidelines define the intensity of statins by the degree of LDL-C lowering. In fact, by correlating the incidence of coronary heart disease events with the levels of LDL-C achieved in those trials, conclusions can reasonably be drawn from such data (Figure 1).2
The guidelines do not recommend nonstatin drugs. Actually, the guidelines note that clinicians are free to consider other therapies, especially those proven to reduce the risk of cardiovascular events, a central principle of medicine. Since the guidelines were published, data have emerged indicating that the role of nonstatin drugs also needs consideration.
The ugly: Risk calculator untested
The guidelines promote the use of a risk calculator developed by the ACC/AHA to estimate the 10-year risk of an atherosclerotic event for people whose LDL-C levels are between 70 and 189 mg/dL to help decide whether to initiate statin therapy for primary prevention of atherosclerotic cardiovascular disease. Such an approach is reasonable, although the risk score was promulgated without evidence to support its utility.
Media coverage of the risk calculator was fierce. Some physicians found imperfections in the risk score (as is true for all risk scores), resulting in public mistrust of the guidelines and of the medical community as a whole. This needless controversy may have compromised the main message—that LDL-C should be lowered in many people—a message backed by strong evidence.
Alternative strategies proposed
Ridker et al3 have proposed a hybrid strategy to guide statin use for apparently healthy people that combines the ACC/AHA guideline approach with entry criteria for randomized clinical trials that showed statin efficacy for primary prevention.
Genetic analysis may offer another approach. Mega et al4 stratified more than 48,000 people by a genetic risk score based on 27 genetic variants and found a significant association with risk of coronary events. Targeting therapy to people found to be at higher risk on this basis offers greater risk reduction than expected for the general population. Biomarkers and imaging tests are other potentially useful risk determinants.
LDL-C: LOWER IS BETTER
Although no clinical trial has yet targeted specific LDL-C levels, there is plenty of evidence that lower LDL-C levels offer greater benefit (Figure 1).2
In 1994, the Scandinavian Simvastatin Survival Study5 established the benefit of statins in patients with known vascular disease. The mean LDL-C level achieved in the active treatment group was 120 mg/dL. More trials followed supporting the benefits of statins and of reducing LDL-C from average levels in the 120s down to 100 mg/dL.
In 2004, the Pravastatin or Atorvastatin Evaluation and Infection Therapy–Thrombolysis in Myocardial Infarction 22 trial6 observed an even greater risk reduction in patients with known risk by treating with statins; the mean LDL-C level achieved in the group randomized to an intensive regimen of atorvastatin 80 mg per day was 62 mg/dL. The same year, the Adult Treatment Panel III of the National Cholesterol Education Program7 issued updated guidelines including an optional goal of LDL-C less than 70 mg/dL for patients at very high risk.
In 2008, the Justification for the Use of Statins in Prevention: an Intervention Trial Evaluating Rosuvastatin (JUPITER)8 found a significantly lower incidence of major cardiovascular events at 2 years in apparently healthy men and women with baseline LDL-C levels of less than 130 mg/dL after treatment with rosuvastatin 20 mg daily, with an achieved median LDL-C of 55 mg/dL.
How low should LDL-C go?
Evidence from clinical trials indicates a 20% to 25% reduction in the risk of cardiovascular events for every 39-mg/dL decrease in LDL-C. Extrapolating the data, cardiovascular disease risk would be reduced to zero if LDL-C were brought down below 40 mg/dL.
Brown and Goldstein,9 who won the 1985 Nobel Prize in medicine for their work in cholesterol metabolism, estimated that a plasma level of LDL-C of only 25 mg/dL would be sufficient to nourish cells with cholesterol. Cells can synthesize all the cholesterol they need, underscoring that LDL-C is simply the final end-product that the liver removes from circulation.
Other evidence that lower LDL-C does not have adverse effects comes from non-Western populations as well as from other mammals. Total cholesterol levels range in the low 100s mg/dL in Native American and African tribal populations, with LDL-C estimated to be about 50 to 75 mg/dL. Elephants, baboons, and foxes have even lower levels.10
Clinical trial data also support that LDL-C levels below the current “normal” are better. The Cholesterol Treatment Trialists’ Collaboration11 analyzed data from more than 160,000 patients in 26 trials that evaluated either more- vs less-intensive statin regimens or statin treatment vs control. No baseline level below which lowering LDL-C further was not beneficial was found. Patients who started out with an LDL-C level of less than 77 mg/dL had the same risk reduction of major vascular events when the level was dropped to 50 mg/dL as those who started at higher levels and reduced their LDL-C by the same amount. In the JUPITER trial, even those with a baseline LDL-C of less than 60 mg/dL benefited from statin therapy.12
BEYOND STATINS
Ezetimibe further lowers risk
Ezetimibe is a nonstatin drug that reduces LDL-C by about 15% to 20%. The Improved Reduction of Outcomes: Vytorin Efficacy International Trial13 registered more than 18,000 patients with a baseline LDL-C level of less than 125 mg/dL (or 100 mg/dL if already on lipid-lowering therapy) who had been stabilized shortly after an acute cardiovascular event. They were randomized to receive either simvastatin 40 mg or combined simvastatin 40 mg and ezetimibe 10 mg. The study intended to determine two things: whether ezetimibe could further lower LDL-C when combined with a statin, and whether risk could be reduced further by driving the LDL-C below 70 mg/dL and down to the mid-50s.
After 1 year, the average LDL-C level was 70 mg/dL in the simvastatin group and 53 mg/dL in the combined simvastatin and ezetimibe group. At 7 years, for the primary end point (cardiovascular death, myocardial infarction, unstable angina requiring hospitalization, coronary revascularization, or stroke), there was a 6% reduction of events in the combined drug treatment group, with the number of people needed to treat being 50 to prevent one event. For the narrower end point of cardiovascular death, nonfatal myocardial infarction, and nonfatal stroke, there was a 10% risk reduction in the combined drug treatment arm.14
The amount of risk reduction is exactly what was predicted by the Cholesterol Treatment Trialists’ Collaboration’s plot of reduction in events vs reduction in LDL-C based on the analysis of 26 trials, adding further evidence that it is the LDL-C reduction itself, rather than the means by which LDL-C is reduced, that is critical for benefit.
PCSK9 inhibitors: A new approach
Mutations in the gene for proprotein convertase subtilisin kexin type 9 (PCSK9) have become a new focus of interest for reducing LDL-C and cardiovascular risk.15 PCSK9 binds to the LDL-C receptor on the surface of hepatocytes and escorts it to its destruction in the lysosomes, rather than allowing it to return to the cell surface to take more LDL-C out of circulation.
People with a gain-of-function mutation (conferring too much PCSK9, resulting in fewer LDL-C receptors and more LDL-C in circulation) are a more recently recognized subset of those with autosomal-dominant familial hypercholesterolemia. They have total cholesterol levels in the 90th percentile, tendon xanthomas, and a high risk of myocardial infarction and stroke at a young age.
Conversely, those with a nonsense mutation in PCSK9—leading to loss of function—have a 28% reduction in mean LDL-C and 88% reduction in risk of coronary heart disease compared with those without the mutation.16 Two women (ages 32 and 21, fertile) have been found who have inactivating mutations in both PCSK9 alleles, and both are in apparent good health, with LDL-C levels of 14 mg/dL and 15 mg/dL, respectively.17,18
Dramatic reduction in LDL-C
Monoclonal antibodies have been developed that bind PCSK9 and block its action with the goal of developing new LDL-C–lowering treatments. Phase 2 clinical trials of varying doses of evolocumab (Repatha), a drug in this class, combined with standard therapy (a statin with or without ezetimibe), found a 66% reduction of LDL-C at high doses at 12 weeks compared with standard therapy alone, with concomitant reductions in other atherogenic lipoproteins.19 Patients who could not tolerate statins because of myalgia responded well to evolocumab.20
Patients with heterozygous familial hypercholesterolemia also had a substantial reduction in LDL-C (55% at the highest dosage), even though they have fewer LDL-C receptors for the drug to act upon.21 People with homozygous familial hypercholesterolemia and no LDL-C receptors had a lesser relative reduction in LDL-C that depended on the type of mutations they had. Nonetheless, given how high LDL-C levels are in this population, the absolute decreases in LDL-C level were quite impressive.
Cardiovascular risk reduced
Data at nearly 1 year showed continued reduction of LDL-C by about 60% (absolute reduction: 73 mg/dL), as well as a lower incidence of cardiovascular events starting at just 3 months, much sooner than observed in some statin trials.22 Benefits were found regardless of subgroup (sex, age, statin use, baseline LDL-C level, or known vascular disease). No difference was found in the safety profile between the evolocumab and control arms. Only 2.4% of participants discontinued evolocumab because of adverse events, and the incidence of adverse effects did not correlate with LDL-C level achieved.
Neurocognitive effects occurred in 0.9% of the evolocumab arm vs 0.3% in the control arm. This difference has not been explained: although there is cholesterol in the central nervous system, it is generated locally, and lipoproteins—and evolocumab—are not thought to cross the blood-brain barrier.
Long-term trials of evolocumab are currently under way for patients with cardiovascular disease, as are trials of two other PCSK9 inhibitors, alirocumab and bococizumab, in addition to standard statin therapy.
On July 24, 2015, the US Food and Drug Administration (FDA) approved the first PCSK9 inhibitor, alirocumab (Praluent) for patients with heterozygous familial hypercholesterolemia or those with clinical atherosclerotic cardiovascular disease who require additional lowering of LDL-C. The starting dosage is 75 mg subcutaneously every 2 weeks, which can be increased up to 150 mg every 2 weeks.
Evolocumab was approved by the FDA on August 27, 2015, for the same indications. The dosage is 140 mg subcutaneously every 2 weeks or 420 mg every month.
- Stone NJ, Robinson JG, Lichtenstein AH, et al; American College of Cardiology/American Heart Association Task Force on Practice Guidelines. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation 2014; 129:S1-S45. Erratum in: Circulation 2014; 129:S46–S48.
- Raymond C, Cho L, Rocco M, Hazen SL. New cholesterol guidelines: worth the wait? Cleve Clin J Med 2014; 81:11–19.
- Ridker PM, Rose L, Cook NR. A proposal to incorporate trial data into a hybrid ACC/AHA algorithm for the allocation of statin therapy in primary prevention. J Am Coll Cardiol 2015; 65:942–948.
- Mega JL, Stitziel NO, Smith JG, et al. Genetic risk, coronary heart disease events, and the clinical benefit of statin therapy: an analysis of primary and secondary prevention trials. Lancet 2015; 385:2264–2271.
- Randomised trial of cholesterol lowering in 4444 patients with coronary heart disease: the Scandinavian Simvastatin Survival Study (4S). Lancet 1994; 344:1383–1389.
- Cannon CP, Braunwald E, McCabe CH, et al; Pravastatin or Atorvastatin Evaluation and Infection Therapy-Thrombolysis in Myocardial Infarction 22 Investigators. Intensive versus moderate lipid lowering with statins after acute coronary syndromes. N Engl J Med 2004; 350:1495–1504.
- Grundy SM, Cleeman JI, Merz CN, et al; National Heart, Lung, and Blood Institute; American College of Cardiology Foundation; American Heart Association. Implications of recent clinical trials for the National Cholesterol Education Program Adult Treatment Panel III guidelines. Circulation 2004; 110:227–239. Erratum in Circulation 2004; 110:763.
- Ridker PM, Danielson E, Fonseca FAH, et al; JUPITER Study Group. Rosuvastatin to prevent vascular events in men and women with elevated C-reactive protein. N Engl J Med 2008; 359:2195–2207.
- Brown MS, Goldstein JL. A receptor-mediated pathway for cholesterol homeostasis. Science 1986; 232:34–47.
- Hochholzer W, Giugliano RP. Lipid lowering goals: back to nature? Ther Adv Cardiovasc Dis 2010; 4:185–191.
- Cholesterol Treatment Trialists’ (CTT) Collaboration, Baigent C, Blackwell L, Emberson J, et al. Efficacy and safety of more intensive lowering of LDL cholesterol: a meta-analysis of data from 170,000 participants in 26 randomised trials. Lancet 2010; 376:1670–1681.
- Hsia J, MacFadyen JG, Monyak J, Ridker PM. Cardiovascular event reduction and adverse events among subjects attaining low-density lipoprotein cholesterol <50 mg/dl with rosuvastatin. The JUPITER trial (Justification for the Use of Statins in Prevention: an Intervention Trial Evaluating Rosuvastatin). J Am Coll Cardiol 2011; 57:1666–1675.
- Cannon CP, Giugliano RP, Blazing MA, et al; IMPROVE-IT Investigators. Rationale and design of IMPROVE-IT (IMProved Reduction of Outcomes: Vytorin Efficacy International Trial): comparison of ezetimibe/simvastatin versus simvastatin monotherapy on cardiovascular outcomes in patients with acute coronary syndromes. Am Heart J 2008; 156:826–832.
- Cannon CP, Blazing MA, Giugliano RP, et al for the IMPROVE-IT Investigators. Ezetimibe added to statin therapy after acute coronary syndromes. N Engl J Med 2015; 372:2387–2397.
- Giugliano RP, Sabatine MS. Are PCSK9 Inhibitors the next breakthrough in the cardiovascular field? J Am Coll Cardiol 2015; 65:2638–2651.
- Cohen JC, Boerwinkle E, Mosley TH Jr, Hobbs HH. Sequence variations in PCSK9, low LDL, and protection against coronary heart disease. N Engl J Med 2006; 354:1264–1272.
- Zhao Z, Tuakli-Wosornu Y, Lagace TA, et al. Molecular characterization of loss-of-function mutations in PCSK9 and identification of a compound heterozygote. Am J Hum Genet 2006; 79:514-523.
- Hooper AJ, Marais AD, Tanyanyiwa DM, Burnett JR. The C679X mutation in PCSK9 is present and lowers blood cholesterol in a Southern African population. Atherosclerosis 2007; 193:445–448.
- Giugliano RP, Desai NR, Kohli P, et al; LAPLACE-TIMI 57 Investigators. Efficacy, safety, and tolerability of a monoclonal antibody to proprotein convertase subtilisin/kexin type 9 in combination with a statin in patients with hypercholesterolaemia (LAPLACE-TIMI 57): a randomised, placebo-controlled, dose-ranging, phase 2 study. Lancet 2012; 380:2007–2017.
- Sullivan D, Olsson AG, Scott R, et al. Effect of a monoclonal antibody to PCSK9 on low-density lipoprotein cholesterol levels in statin-intolerant patients: the GAUSS randomized trial. JAMA 2012; 308:2497–2506.
- Raal F, Scott R, Somaratne R, et al. Low-density lipoprotein cholesterol-lowering effects of AMG 145, a monoclonal antibody to proprotein convertase subtilisin/kexin type 9 serine protease in patients with heterozygous familial hypercholesterolemia: the Reduction of LDL-C with PCSK9 Inhibition in Heterozygous Familial Hypercholesterolemia Disorder (RUTHERFORD) randomized trial. Circulation 2012; 126:2408–2417.
- Sabatine MS, Giugliano RP, Wiviott SD, et al; Open-Label Study of Long-Term Evaluation against LDL Cholesterol (OSLER) Investigators. Efficacy and safety of evolocumab in reducing lipids and cardiovascular events. N Engl J Med 2015; 372:1500–1509.
The 2013 joint guidelines of the American College of Cardiology and American Heart Association (ACC/AHA)1 on the treatment of blood cholesterol to reduce cardiovascular risk recommend high-intensity statin therapy for secondary prevention of cardiovascular events. The question of primary prevention is not so straightforward, and the recommended strategy has come under fire. In addition, the guidelines focus on statins and not on LDL-C levels, and the role of nonstatin lipid-lowering drugs and the value of reducing LDL-C levels to well below levels currently regarded as “normal” remain unclear.
This article comments on the 2013 ACC/AHA guidelines, reviews the data on optimal LDL-C levels, and discusses new nonstatin agents.
ACC/AHA GUIDELINES: A MIXED MESSAGE
The 2013 ACC/AHA cholesterol guidelines1 can be characterized by the title from the famous Western film “The Good, the Bad, and the Ugly.”
The good: A clear message to treat
The guidelines deliver an unambiguous message to treat patients at high risk with high-intensity statin therapy. This mandate is very helpful as it should reduce the undertreatment of patients.
The seemingly bad
Two common misconceptions regarding the guidelines:
They abandon LDL-C targets. Actually, the guidelines do not argue for or against targets; they simply remain silent, citing that randomized trials have not been conducted with LDL-C targets as specific goals. Technically, this statement is true. However, it seems contrived to argue, for example, that the benefit of atorvastatin 80 mg over 10 mg in the Treating to New Targets trial could not be reliably ascribed to the lower LDL-C achieved with the higher dose, but rather to some undefined benefit of high-intensity statin therapy, especially as the guidelines define the intensity of statins by the degree of LDL-C lowering. In fact, by correlating the incidence of coronary heart disease events with the levels of LDL-C achieved in those trials, conclusions can reasonably be drawn from such data (Figure 1).2
The guidelines do not recommend nonstatin drugs. Actually, the guidelines note that clinicians are free to consider other therapies, especially those proven to reduce the risk of cardiovascular events, a central principle of medicine. Since the guidelines were published, data have emerged indicating that the role of nonstatin drugs also needs consideration.
The ugly: Risk calculator untested
The guidelines promote the use of a risk calculator developed by the ACC/AHA to estimate the 10-year risk of an atherosclerotic event for people whose LDL-C levels are between 70 and 189 mg/dL to help decide whether to initiate statin therapy for primary prevention of atherosclerotic cardiovascular disease. Such an approach is reasonable, although the risk score was promulgated without evidence to support its utility.
Media coverage of the risk calculator was fierce. Some physicians found imperfections in the risk score (as is true for all risk scores), resulting in public mistrust of the guidelines and of the medical community as a whole. This needless controversy may have compromised the main message—that LDL-C should be lowered in many people—a message backed by strong evidence.
Alternative strategies proposed
Ridker et al3 have proposed a hybrid strategy to guide statin use for apparently healthy people that combines the ACC/AHA guideline approach with entry criteria for randomized clinical trials that showed statin efficacy for primary prevention.
Genetic analysis may offer another approach. Mega et al4 stratified more than 48,000 people by a genetic risk score based on 27 genetic variants and found a significant association with risk of coronary events. Targeting therapy to people found to be at higher risk on this basis offers greater risk reduction than expected for the general population. Biomarkers and imaging tests are other potentially useful risk determinants.
LDL-C: LOWER IS BETTER
Although no clinical trial has yet targeted specific LDL-C levels, there is plenty of evidence that lower LDL-C levels offer greater benefit (Figure 1).2
In 1994, the Scandinavian Simvastatin Survival Study5 established the benefit of statins in patients with known vascular disease. The mean LDL-C level achieved in the active treatment group was 120 mg/dL. More trials followed supporting the benefits of statins and of reducing LDL-C from average levels in the 120s down to 100 mg/dL.
In 2004, the Pravastatin or Atorvastatin Evaluation and Infection Therapy–Thrombolysis in Myocardial Infarction 22 trial6 observed an even greater risk reduction in patients with known risk by treating with statins; the mean LDL-C level achieved in the group randomized to an intensive regimen of atorvastatin 80 mg per day was 62 mg/dL. The same year, the Adult Treatment Panel III of the National Cholesterol Education Program7 issued updated guidelines including an optional goal of LDL-C less than 70 mg/dL for patients at very high risk.
In 2008, the Justification for the Use of Statins in Prevention: an Intervention Trial Evaluating Rosuvastatin (JUPITER)8 found a significantly lower incidence of major cardiovascular events at 2 years in apparently healthy men and women with baseline LDL-C levels of less than 130 mg/dL after treatment with rosuvastatin 20 mg daily, with an achieved median LDL-C of 55 mg/dL.
How low should LDL-C go?
Evidence from clinical trials indicates a 20% to 25% reduction in the risk of cardiovascular events for every 39-mg/dL decrease in LDL-C. Extrapolating the data, cardiovascular disease risk would be reduced to zero if LDL-C were brought down below 40 mg/dL.
Brown and Goldstein,9 who won the 1985 Nobel Prize in medicine for their work in cholesterol metabolism, estimated that a plasma level of LDL-C of only 25 mg/dL would be sufficient to nourish cells with cholesterol. Cells can synthesize all the cholesterol they need, underscoring that LDL-C is simply the final end-product that the liver removes from circulation.
Other evidence that lower LDL-C does not have adverse effects comes from non-Western populations as well as from other mammals. Total cholesterol levels range in the low 100s mg/dL in Native American and African tribal populations, with LDL-C estimated to be about 50 to 75 mg/dL. Elephants, baboons, and foxes have even lower levels.10
Clinical trial data also support that LDL-C levels below the current “normal” are better. The Cholesterol Treatment Trialists’ Collaboration11 analyzed data from more than 160,000 patients in 26 trials that evaluated either more- vs less-intensive statin regimens or statin treatment vs control. No baseline level below which lowering LDL-C further was not beneficial was found. Patients who started out with an LDL-C level of less than 77 mg/dL had the same risk reduction of major vascular events when the level was dropped to 50 mg/dL as those who started at higher levels and reduced their LDL-C by the same amount. In the JUPITER trial, even those with a baseline LDL-C of less than 60 mg/dL benefited from statin therapy.12
BEYOND STATINS
Ezetimibe further lowers risk
Ezetimibe is a nonstatin drug that reduces LDL-C by about 15% to 20%. The Improved Reduction of Outcomes: Vytorin Efficacy International Trial13 registered more than 18,000 patients with a baseline LDL-C level of less than 125 mg/dL (or 100 mg/dL if already on lipid-lowering therapy) who had been stabilized shortly after an acute cardiovascular event. They were randomized to receive either simvastatin 40 mg or combined simvastatin 40 mg and ezetimibe 10 mg. The study intended to determine two things: whether ezetimibe could further lower LDL-C when combined with a statin, and whether risk could be reduced further by driving the LDL-C below 70 mg/dL and down to the mid-50s.
After 1 year, the average LDL-C level was 70 mg/dL in the simvastatin group and 53 mg/dL in the combined simvastatin and ezetimibe group. At 7 years, for the primary end point (cardiovascular death, myocardial infarction, unstable angina requiring hospitalization, coronary revascularization, or stroke), there was a 6% reduction of events in the combined drug treatment group, with the number of people needed to treat being 50 to prevent one event. For the narrower end point of cardiovascular death, nonfatal myocardial infarction, and nonfatal stroke, there was a 10% risk reduction in the combined drug treatment arm.14
The amount of risk reduction is exactly what was predicted by the Cholesterol Treatment Trialists’ Collaboration’s plot of reduction in events vs reduction in LDL-C based on the analysis of 26 trials, adding further evidence that it is the LDL-C reduction itself, rather than the means by which LDL-C is reduced, that is critical for benefit.
PCSK9 inhibitors: A new approach
Mutations in the gene for proprotein convertase subtilisin kexin type 9 (PCSK9) have become a new focus of interest for reducing LDL-C and cardiovascular risk.15 PCSK9 binds to the LDL-C receptor on the surface of hepatocytes and escorts it to its destruction in the lysosomes, rather than allowing it to return to the cell surface to take more LDL-C out of circulation.
People with a gain-of-function mutation (conferring too much PCSK9, resulting in fewer LDL-C receptors and more LDL-C in circulation) are a more recently recognized subset of those with autosomal-dominant familial hypercholesterolemia. They have total cholesterol levels in the 90th percentile, tendon xanthomas, and a high risk of myocardial infarction and stroke at a young age.
Conversely, those with a nonsense mutation in PCSK9—leading to loss of function—have a 28% reduction in mean LDL-C and 88% reduction in risk of coronary heart disease compared with those without the mutation.16 Two women (ages 32 and 21, fertile) have been found who have inactivating mutations in both PCSK9 alleles, and both are in apparent good health, with LDL-C levels of 14 mg/dL and 15 mg/dL, respectively.17,18
Dramatic reduction in LDL-C
Monoclonal antibodies have been developed that bind PCSK9 and block its action with the goal of developing new LDL-C–lowering treatments. Phase 2 clinical trials of varying doses of evolocumab (Repatha), a drug in this class, combined with standard therapy (a statin with or without ezetimibe), found a 66% reduction of LDL-C at high doses at 12 weeks compared with standard therapy alone, with concomitant reductions in other atherogenic lipoproteins.19 Patients who could not tolerate statins because of myalgia responded well to evolocumab.20
Patients with heterozygous familial hypercholesterolemia also had a substantial reduction in LDL-C (55% at the highest dosage), even though they have fewer LDL-C receptors for the drug to act upon.21 People with homozygous familial hypercholesterolemia and no LDL-C receptors had a lesser relative reduction in LDL-C that depended on the type of mutations they had. Nonetheless, given how high LDL-C levels are in this population, the absolute decreases in LDL-C level were quite impressive.
Cardiovascular risk reduced
Data at nearly 1 year showed continued reduction of LDL-C by about 60% (absolute reduction: 73 mg/dL), as well as a lower incidence of cardiovascular events starting at just 3 months, much sooner than observed in some statin trials.22 Benefits were found regardless of subgroup (sex, age, statin use, baseline LDL-C level, or known vascular disease). No difference was found in the safety profile between the evolocumab and control arms. Only 2.4% of participants discontinued evolocumab because of adverse events, and the incidence of adverse effects did not correlate with LDL-C level achieved.
Neurocognitive effects occurred in 0.9% of the evolocumab arm vs 0.3% in the control arm. This difference has not been explained: although there is cholesterol in the central nervous system, it is generated locally, and lipoproteins—and evolocumab—are not thought to cross the blood-brain barrier.
Long-term trials of evolocumab are currently under way for patients with cardiovascular disease, as are trials of two other PCSK9 inhibitors, alirocumab and bococizumab, in addition to standard statin therapy.
On July 24, 2015, the US Food and Drug Administration (FDA) approved the first PCSK9 inhibitor, alirocumab (Praluent) for patients with heterozygous familial hypercholesterolemia or those with clinical atherosclerotic cardiovascular disease who require additional lowering of LDL-C. The starting dosage is 75 mg subcutaneously every 2 weeks, which can be increased up to 150 mg every 2 weeks.
Evolocumab was approved by the FDA on August 27, 2015, for the same indications. The dosage is 140 mg subcutaneously every 2 weeks or 420 mg every month.
The 2013 joint guidelines of the American College of Cardiology and American Heart Association (ACC/AHA)1 on the treatment of blood cholesterol to reduce cardiovascular risk recommend high-intensity statin therapy for secondary prevention of cardiovascular events. The question of primary prevention is not so straightforward, and the recommended strategy has come under fire. In addition, the guidelines focus on statins and not on LDL-C levels, and the role of nonstatin lipid-lowering drugs and the value of reducing LDL-C levels to well below levels currently regarded as “normal” remain unclear.
This article comments on the 2013 ACC/AHA guidelines, reviews the data on optimal LDL-C levels, and discusses new nonstatin agents.
ACC/AHA GUIDELINES: A MIXED MESSAGE
The 2013 ACC/AHA cholesterol guidelines1 can be characterized by the title from the famous Western film “The Good, the Bad, and the Ugly.”
The good: A clear message to treat
The guidelines deliver an unambiguous message to treat patients at high risk with high-intensity statin therapy. This mandate is very helpful as it should reduce the undertreatment of patients.
The seemingly bad
Two common misconceptions regarding the guidelines:
They abandon LDL-C targets. Actually, the guidelines do not argue for or against targets; they simply remain silent, citing that randomized trials have not been conducted with LDL-C targets as specific goals. Technically, this statement is true. However, it seems contrived to argue, for example, that the benefit of atorvastatin 80 mg over 10 mg in the Treating to New Targets trial could not be reliably ascribed to the lower LDL-C achieved with the higher dose, but rather to some undefined benefit of high-intensity statin therapy, especially as the guidelines define the intensity of statins by the degree of LDL-C lowering. In fact, by correlating the incidence of coronary heart disease events with the levels of LDL-C achieved in those trials, conclusions can reasonably be drawn from such data (Figure 1).2
The guidelines do not recommend nonstatin drugs. Actually, the guidelines note that clinicians are free to consider other therapies, especially those proven to reduce the risk of cardiovascular events, a central principle of medicine. Since the guidelines were published, data have emerged indicating that the role of nonstatin drugs also needs consideration.
The ugly: Risk calculator untested
The guidelines promote the use of a risk calculator developed by the ACC/AHA to estimate the 10-year risk of an atherosclerotic event for people whose LDL-C levels are between 70 and 189 mg/dL to help decide whether to initiate statin therapy for primary prevention of atherosclerotic cardiovascular disease. Such an approach is reasonable, although the risk score was promulgated without evidence to support its utility.
Media coverage of the risk calculator was fierce. Some physicians found imperfections in the risk score (as is true for all risk scores), resulting in public mistrust of the guidelines and of the medical community as a whole. This needless controversy may have compromised the main message—that LDL-C should be lowered in many people—a message backed by strong evidence.
Alternative strategies proposed
Ridker et al3 have proposed a hybrid strategy to guide statin use for apparently healthy people that combines the ACC/AHA guideline approach with entry criteria for randomized clinical trials that showed statin efficacy for primary prevention.
Genetic analysis may offer another approach. Mega et al4 stratified more than 48,000 people by a genetic risk score based on 27 genetic variants and found a significant association with risk of coronary events. Targeting therapy to people found to be at higher risk on this basis offers greater risk reduction than expected for the general population. Biomarkers and imaging tests are other potentially useful risk determinants.
LDL-C: LOWER IS BETTER
Although no clinical trial has yet targeted specific LDL-C levels, there is plenty of evidence that lower LDL-C levels offer greater benefit (Figure 1).2
In 1994, the Scandinavian Simvastatin Survival Study5 established the benefit of statins in patients with known vascular disease. The mean LDL-C level achieved in the active treatment group was 120 mg/dL. More trials followed supporting the benefits of statins and of reducing LDL-C from average levels in the 120s down to 100 mg/dL.
In 2004, the Pravastatin or Atorvastatin Evaluation and Infection Therapy–Thrombolysis in Myocardial Infarction 22 trial6 observed an even greater risk reduction in patients with known risk by treating with statins; the mean LDL-C level achieved in the group randomized to an intensive regimen of atorvastatin 80 mg per day was 62 mg/dL. The same year, the Adult Treatment Panel III of the National Cholesterol Education Program7 issued updated guidelines including an optional goal of LDL-C less than 70 mg/dL for patients at very high risk.
In 2008, the Justification for the Use of Statins in Prevention: an Intervention Trial Evaluating Rosuvastatin (JUPITER)8 found a significantly lower incidence of major cardiovascular events at 2 years in apparently healthy men and women with baseline LDL-C levels of less than 130 mg/dL after treatment with rosuvastatin 20 mg daily, with an achieved median LDL-C of 55 mg/dL.
How low should LDL-C go?
Evidence from clinical trials indicates a 20% to 25% reduction in the risk of cardiovascular events for every 39-mg/dL decrease in LDL-C. Extrapolating the data, cardiovascular disease risk would be reduced to zero if LDL-C were brought down below 40 mg/dL.
Brown and Goldstein,9 who won the 1985 Nobel Prize in medicine for their work in cholesterol metabolism, estimated that a plasma level of LDL-C of only 25 mg/dL would be sufficient to nourish cells with cholesterol. Cells can synthesize all the cholesterol they need, underscoring that LDL-C is simply the final end-product that the liver removes from circulation.
Other evidence that lower LDL-C does not have adverse effects comes from non-Western populations as well as from other mammals. Total cholesterol levels range in the low 100s mg/dL in Native American and African tribal populations, with LDL-C estimated to be about 50 to 75 mg/dL. Elephants, baboons, and foxes have even lower levels.10
Clinical trial data also support that LDL-C levels below the current “normal” are better. The Cholesterol Treatment Trialists’ Collaboration11 analyzed data from more than 160,000 patients in 26 trials that evaluated either more- vs less-intensive statin regimens or statin treatment vs control. No baseline level below which lowering LDL-C further was not beneficial was found. Patients who started out with an LDL-C level of less than 77 mg/dL had the same risk reduction of major vascular events when the level was dropped to 50 mg/dL as those who started at higher levels and reduced their LDL-C by the same amount. In the JUPITER trial, even those with a baseline LDL-C of less than 60 mg/dL benefited from statin therapy.12
BEYOND STATINS
Ezetimibe further lowers risk
Ezetimibe is a nonstatin drug that reduces LDL-C by about 15% to 20%. The Improved Reduction of Outcomes: Vytorin Efficacy International Trial13 registered more than 18,000 patients with a baseline LDL-C level of less than 125 mg/dL (or 100 mg/dL if already on lipid-lowering therapy) who had been stabilized shortly after an acute cardiovascular event. They were randomized to receive either simvastatin 40 mg or combined simvastatin 40 mg and ezetimibe 10 mg. The study intended to determine two things: whether ezetimibe could further lower LDL-C when combined with a statin, and whether risk could be reduced further by driving the LDL-C below 70 mg/dL and down to the mid-50s.
After 1 year, the average LDL-C level was 70 mg/dL in the simvastatin group and 53 mg/dL in the combined simvastatin and ezetimibe group. At 7 years, for the primary end point (cardiovascular death, myocardial infarction, unstable angina requiring hospitalization, coronary revascularization, or stroke), there was a 6% reduction of events in the combined drug treatment group, with the number of people needed to treat being 50 to prevent one event. For the narrower end point of cardiovascular death, nonfatal myocardial infarction, and nonfatal stroke, there was a 10% risk reduction in the combined drug treatment arm.14
The amount of risk reduction is exactly what was predicted by the Cholesterol Treatment Trialists’ Collaboration’s plot of reduction in events vs reduction in LDL-C based on the analysis of 26 trials, adding further evidence that it is the LDL-C reduction itself, rather than the means by which LDL-C is reduced, that is critical for benefit.
PCSK9 inhibitors: A new approach
Mutations in the gene for proprotein convertase subtilisin kexin type 9 (PCSK9) have become a new focus of interest for reducing LDL-C and cardiovascular risk.15 PCSK9 binds to the LDL-C receptor on the surface of hepatocytes and escorts it to its destruction in the lysosomes, rather than allowing it to return to the cell surface to take more LDL-C out of circulation.
People with a gain-of-function mutation (conferring too much PCSK9, resulting in fewer LDL-C receptors and more LDL-C in circulation) are a more recently recognized subset of those with autosomal-dominant familial hypercholesterolemia. They have total cholesterol levels in the 90th percentile, tendon xanthomas, and a high risk of myocardial infarction and stroke at a young age.
Conversely, those with a nonsense mutation in PCSK9—leading to loss of function—have a 28% reduction in mean LDL-C and 88% reduction in risk of coronary heart disease compared with those without the mutation.16 Two women (ages 32 and 21, fertile) have been found who have inactivating mutations in both PCSK9 alleles, and both are in apparent good health, with LDL-C levels of 14 mg/dL and 15 mg/dL, respectively.17,18
Dramatic reduction in LDL-C
Monoclonal antibodies have been developed that bind PCSK9 and block its action with the goal of developing new LDL-C–lowering treatments. Phase 2 clinical trials of varying doses of evolocumab (Repatha), a drug in this class, combined with standard therapy (a statin with or without ezetimibe), found a 66% reduction of LDL-C at high doses at 12 weeks compared with standard therapy alone, with concomitant reductions in other atherogenic lipoproteins.19 Patients who could not tolerate statins because of myalgia responded well to evolocumab.20
Patients with heterozygous familial hypercholesterolemia also had a substantial reduction in LDL-C (55% at the highest dosage), even though they have fewer LDL-C receptors for the drug to act upon.21 People with homozygous familial hypercholesterolemia and no LDL-C receptors had a lesser relative reduction in LDL-C that depended on the type of mutations they had. Nonetheless, given how high LDL-C levels are in this population, the absolute decreases in LDL-C level were quite impressive.
Cardiovascular risk reduced
Data at nearly 1 year showed continued reduction of LDL-C by about 60% (absolute reduction: 73 mg/dL), as well as a lower incidence of cardiovascular events starting at just 3 months, much sooner than observed in some statin trials.22 Benefits were found regardless of subgroup (sex, age, statin use, baseline LDL-C level, or known vascular disease). No difference was found in the safety profile between the evolocumab and control arms. Only 2.4% of participants discontinued evolocumab because of adverse events, and the incidence of adverse effects did not correlate with LDL-C level achieved.
Neurocognitive effects occurred in 0.9% of the evolocumab arm vs 0.3% in the control arm. This difference has not been explained: although there is cholesterol in the central nervous system, it is generated locally, and lipoproteins—and evolocumab—are not thought to cross the blood-brain barrier.
Long-term trials of evolocumab are currently under way for patients with cardiovascular disease, as are trials of two other PCSK9 inhibitors, alirocumab and bococizumab, in addition to standard statin therapy.
On July 24, 2015, the US Food and Drug Administration (FDA) approved the first PCSK9 inhibitor, alirocumab (Praluent) for patients with heterozygous familial hypercholesterolemia or those with clinical atherosclerotic cardiovascular disease who require additional lowering of LDL-C. The starting dosage is 75 mg subcutaneously every 2 weeks, which can be increased up to 150 mg every 2 weeks.
Evolocumab was approved by the FDA on August 27, 2015, for the same indications. The dosage is 140 mg subcutaneously every 2 weeks or 420 mg every month.
- Stone NJ, Robinson JG, Lichtenstein AH, et al; American College of Cardiology/American Heart Association Task Force on Practice Guidelines. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation 2014; 129:S1-S45. Erratum in: Circulation 2014; 129:S46–S48.
- Raymond C, Cho L, Rocco M, Hazen SL. New cholesterol guidelines: worth the wait? Cleve Clin J Med 2014; 81:11–19.
- Ridker PM, Rose L, Cook NR. A proposal to incorporate trial data into a hybrid ACC/AHA algorithm for the allocation of statin therapy in primary prevention. J Am Coll Cardiol 2015; 65:942–948.
- Mega JL, Stitziel NO, Smith JG, et al. Genetic risk, coronary heart disease events, and the clinical benefit of statin therapy: an analysis of primary and secondary prevention trials. Lancet 2015; 385:2264–2271.
- Randomised trial of cholesterol lowering in 4444 patients with coronary heart disease: the Scandinavian Simvastatin Survival Study (4S). Lancet 1994; 344:1383–1389.
- Cannon CP, Braunwald E, McCabe CH, et al; Pravastatin or Atorvastatin Evaluation and Infection Therapy-Thrombolysis in Myocardial Infarction 22 Investigators. Intensive versus moderate lipid lowering with statins after acute coronary syndromes. N Engl J Med 2004; 350:1495–1504.
- Grundy SM, Cleeman JI, Merz CN, et al; National Heart, Lung, and Blood Institute; American College of Cardiology Foundation; American Heart Association. Implications of recent clinical trials for the National Cholesterol Education Program Adult Treatment Panel III guidelines. Circulation 2004; 110:227–239. Erratum in Circulation 2004; 110:763.
- Ridker PM, Danielson E, Fonseca FAH, et al; JUPITER Study Group. Rosuvastatin to prevent vascular events in men and women with elevated C-reactive protein. N Engl J Med 2008; 359:2195–2207.
- Brown MS, Goldstein JL. A receptor-mediated pathway for cholesterol homeostasis. Science 1986; 232:34–47.
- Hochholzer W, Giugliano RP. Lipid lowering goals: back to nature? Ther Adv Cardiovasc Dis 2010; 4:185–191.
- Cholesterol Treatment Trialists’ (CTT) Collaboration, Baigent C, Blackwell L, Emberson J, et al. Efficacy and safety of more intensive lowering of LDL cholesterol: a meta-analysis of data from 170,000 participants in 26 randomised trials. Lancet 2010; 376:1670–1681.
- Hsia J, MacFadyen JG, Monyak J, Ridker PM. Cardiovascular event reduction and adverse events among subjects attaining low-density lipoprotein cholesterol <50 mg/dl with rosuvastatin. The JUPITER trial (Justification for the Use of Statins in Prevention: an Intervention Trial Evaluating Rosuvastatin). J Am Coll Cardiol 2011; 57:1666–1675.
- Cannon CP, Giugliano RP, Blazing MA, et al; IMPROVE-IT Investigators. Rationale and design of IMPROVE-IT (IMProved Reduction of Outcomes: Vytorin Efficacy International Trial): comparison of ezetimibe/simvastatin versus simvastatin monotherapy on cardiovascular outcomes in patients with acute coronary syndromes. Am Heart J 2008; 156:826–832.
- Cannon CP, Blazing MA, Giugliano RP, et al for the IMPROVE-IT Investigators. Ezetimibe added to statin therapy after acute coronary syndromes. N Engl J Med 2015; 372:2387–2397.
- Giugliano RP, Sabatine MS. Are PCSK9 Inhibitors the next breakthrough in the cardiovascular field? J Am Coll Cardiol 2015; 65:2638–2651.
- Cohen JC, Boerwinkle E, Mosley TH Jr, Hobbs HH. Sequence variations in PCSK9, low LDL, and protection against coronary heart disease. N Engl J Med 2006; 354:1264–1272.
- Zhao Z, Tuakli-Wosornu Y, Lagace TA, et al. Molecular characterization of loss-of-function mutations in PCSK9 and identification of a compound heterozygote. Am J Hum Genet 2006; 79:514-523.
- Hooper AJ, Marais AD, Tanyanyiwa DM, Burnett JR. The C679X mutation in PCSK9 is present and lowers blood cholesterol in a Southern African population. Atherosclerosis 2007; 193:445–448.
- Giugliano RP, Desai NR, Kohli P, et al; LAPLACE-TIMI 57 Investigators. Efficacy, safety, and tolerability of a monoclonal antibody to proprotein convertase subtilisin/kexin type 9 in combination with a statin in patients with hypercholesterolaemia (LAPLACE-TIMI 57): a randomised, placebo-controlled, dose-ranging, phase 2 study. Lancet 2012; 380:2007–2017.
- Sullivan D, Olsson AG, Scott R, et al. Effect of a monoclonal antibody to PCSK9 on low-density lipoprotein cholesterol levels in statin-intolerant patients: the GAUSS randomized trial. JAMA 2012; 308:2497–2506.
- Raal F, Scott R, Somaratne R, et al. Low-density lipoprotein cholesterol-lowering effects of AMG 145, a monoclonal antibody to proprotein convertase subtilisin/kexin type 9 serine protease in patients with heterozygous familial hypercholesterolemia: the Reduction of LDL-C with PCSK9 Inhibition in Heterozygous Familial Hypercholesterolemia Disorder (RUTHERFORD) randomized trial. Circulation 2012; 126:2408–2417.
- Sabatine MS, Giugliano RP, Wiviott SD, et al; Open-Label Study of Long-Term Evaluation against LDL Cholesterol (OSLER) Investigators. Efficacy and safety of evolocumab in reducing lipids and cardiovascular events. N Engl J Med 2015; 372:1500–1509.
- Stone NJ, Robinson JG, Lichtenstein AH, et al; American College of Cardiology/American Heart Association Task Force on Practice Guidelines. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation 2014; 129:S1-S45. Erratum in: Circulation 2014; 129:S46–S48.
- Raymond C, Cho L, Rocco M, Hazen SL. New cholesterol guidelines: worth the wait? Cleve Clin J Med 2014; 81:11–19.
- Ridker PM, Rose L, Cook NR. A proposal to incorporate trial data into a hybrid ACC/AHA algorithm for the allocation of statin therapy in primary prevention. J Am Coll Cardiol 2015; 65:942–948.
- Mega JL, Stitziel NO, Smith JG, et al. Genetic risk, coronary heart disease events, and the clinical benefit of statin therapy: an analysis of primary and secondary prevention trials. Lancet 2015; 385:2264–2271.
- Randomised trial of cholesterol lowering in 4444 patients with coronary heart disease: the Scandinavian Simvastatin Survival Study (4S). Lancet 1994; 344:1383–1389.
- Cannon CP, Braunwald E, McCabe CH, et al; Pravastatin or Atorvastatin Evaluation and Infection Therapy-Thrombolysis in Myocardial Infarction 22 Investigators. Intensive versus moderate lipid lowering with statins after acute coronary syndromes. N Engl J Med 2004; 350:1495–1504.
- Grundy SM, Cleeman JI, Merz CN, et al; National Heart, Lung, and Blood Institute; American College of Cardiology Foundation; American Heart Association. Implications of recent clinical trials for the National Cholesterol Education Program Adult Treatment Panel III guidelines. Circulation 2004; 110:227–239. Erratum in Circulation 2004; 110:763.
- Ridker PM, Danielson E, Fonseca FAH, et al; JUPITER Study Group. Rosuvastatin to prevent vascular events in men and women with elevated C-reactive protein. N Engl J Med 2008; 359:2195–2207.
- Brown MS, Goldstein JL. A receptor-mediated pathway for cholesterol homeostasis. Science 1986; 232:34–47.
- Hochholzer W, Giugliano RP. Lipid lowering goals: back to nature? Ther Adv Cardiovasc Dis 2010; 4:185–191.
- Cholesterol Treatment Trialists’ (CTT) Collaboration, Baigent C, Blackwell L, Emberson J, et al. Efficacy and safety of more intensive lowering of LDL cholesterol: a meta-analysis of data from 170,000 participants in 26 randomised trials. Lancet 2010; 376:1670–1681.
- Hsia J, MacFadyen JG, Monyak J, Ridker PM. Cardiovascular event reduction and adverse events among subjects attaining low-density lipoprotein cholesterol <50 mg/dl with rosuvastatin. The JUPITER trial (Justification for the Use of Statins in Prevention: an Intervention Trial Evaluating Rosuvastatin). J Am Coll Cardiol 2011; 57:1666–1675.
- Cannon CP, Giugliano RP, Blazing MA, et al; IMPROVE-IT Investigators. Rationale and design of IMPROVE-IT (IMProved Reduction of Outcomes: Vytorin Efficacy International Trial): comparison of ezetimibe/simvastatin versus simvastatin monotherapy on cardiovascular outcomes in patients with acute coronary syndromes. Am Heart J 2008; 156:826–832.
- Cannon CP, Blazing MA, Giugliano RP, et al for the IMPROVE-IT Investigators. Ezetimibe added to statin therapy after acute coronary syndromes. N Engl J Med 2015; 372:2387–2397.
- Giugliano RP, Sabatine MS. Are PCSK9 Inhibitors the next breakthrough in the cardiovascular field? J Am Coll Cardiol 2015; 65:2638–2651.
- Cohen JC, Boerwinkle E, Mosley TH Jr, Hobbs HH. Sequence variations in PCSK9, low LDL, and protection against coronary heart disease. N Engl J Med 2006; 354:1264–1272.
- Zhao Z, Tuakli-Wosornu Y, Lagace TA, et al. Molecular characterization of loss-of-function mutations in PCSK9 and identification of a compound heterozygote. Am J Hum Genet 2006; 79:514-523.
- Hooper AJ, Marais AD, Tanyanyiwa DM, Burnett JR. The C679X mutation in PCSK9 is present and lowers blood cholesterol in a Southern African population. Atherosclerosis 2007; 193:445–448.
- Giugliano RP, Desai NR, Kohli P, et al; LAPLACE-TIMI 57 Investigators. Efficacy, safety, and tolerability of a monoclonal antibody to proprotein convertase subtilisin/kexin type 9 in combination with a statin in patients with hypercholesterolaemia (LAPLACE-TIMI 57): a randomised, placebo-controlled, dose-ranging, phase 2 study. Lancet 2012; 380:2007–2017.
- Sullivan D, Olsson AG, Scott R, et al. Effect of a monoclonal antibody to PCSK9 on low-density lipoprotein cholesterol levels in statin-intolerant patients: the GAUSS randomized trial. JAMA 2012; 308:2497–2506.
- Raal F, Scott R, Somaratne R, et al. Low-density lipoprotein cholesterol-lowering effects of AMG 145, a monoclonal antibody to proprotein convertase subtilisin/kexin type 9 serine protease in patients with heterozygous familial hypercholesterolemia: the Reduction of LDL-C with PCSK9 Inhibition in Heterozygous Familial Hypercholesterolemia Disorder (RUTHERFORD) randomized trial. Circulation 2012; 126:2408–2417.
- Sabatine MS, Giugliano RP, Wiviott SD, et al; Open-Label Study of Long-Term Evaluation against LDL Cholesterol (OSLER) Investigators. Efficacy and safety of evolocumab in reducing lipids and cardiovascular events. N Engl J Med 2015; 372:1500–1509.
KEY POINTS
- Patients at high risk of atherosclerotic cardiovascular disease should be treated with high-intensity statin therapy.
- To date, no baseline level has been identified beneath which lowering LDL-C does not provide clinical benefit.
- The benefits of lower LDL-C are seen with a variety of pharmacologic interventions and in people who have naturally low levels due to genetic variants.
- Clinical trial evidence supports that ezetimibe reduces the risk of cardiovascular events.
- Proprotein convertase subtilisin kexin type 9 (PCSK9) inhibitors reduce LDL-C by approximately 60%, and preliminary data show that they reduce the risk of cardiovascular events.
Novel antiplatelet strategies in acute coronary syndromes
An enhanced understanding of platelet biology, as reviewed in the previous article in this supplement, has made it possible to identify a wide variety of platelet agonists. This knowledge has fostered the development of a host of pharmacologic strategies to block agonists such as cyclooxygenase, thromboxane, adenosine diphosphate (ADP), and thrombin, among others. This article will discuss the pharmacologic properties of novel antiplatelet agents, as well as alternative dosing of the established antiplatelet agent clopidogrel, and will review data from available comparative and placebo-controlled trials of these agents. The article concludes with comparative perspectives on the potential roles and relative advantages of these agents in the evolving management of patients with acute coronary syndromes (ACS).
CLOPIDOGREL AND THE CHALLENGE OF VARIABLE RESPONSE
Clopidogrel, a member of the thienopyridine class of ADP receptor inhibitors, is well established for use in patients with ACS at a loading dose of 300 mg followed by a maintenance dose of 75 mg/day. At this loading dose, inhibition of platelet aggregation to ADP is approximately 30%, and the time to peak effect is approximately 4 to 6 hours.1
As with most other drugs, the response to clopidogrel is variable. However, in contrast to the accepted measures of response to antihypertensive or lipid-lowering drugs, there are no routinely used tests for measuring response to antiplatelet therapies. As a result, a “one size fits all” strategy in the dosing of clopidogrel has prevailed.
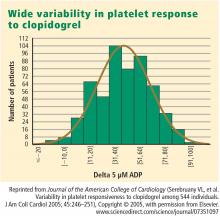
This variability in response is clinically relevant. In a study assessing clopidogrel responsiveness by ADP-induced platelet aggregation in 60 patients who experienced ST-segment-elevation myocardial infarction (MI), Matetzky et al found that the lowest levels of clopidogrel responsiveness were associated with a significantly elevated rate (P = .007) of recurrent cardiovascular events 6 months after the MI.3 Gurbel et al found a similar association between clopidogrel responsiveness and subacute stent thrombosis in a study of 120 patients using two different methods—light transmission aggregotomy to 5 μmol/L of ADP, and the ratio of vasodilator-stimulated phosphoprotein reactivity—to assess clopidogrel responsiveness.4
Increasing the loading dose raises response rates
One proposed method for boosting responsiveness to clopidogrel in suboptimal responders is the use of a higher dose. In a study of 190 patients undergoing coronary stenting, increasing the loading dose from 300 mg to 600 mg reduced the rate of clopidogrel resistance (defined as a < 10% absolute change in aggregation to 5 μM of ADP at 24 hours) from 28% to 8% (P < .001),5 a finding that supports the notion of enhanced response at doses up to 600 mg. Single loading doses in excess of 600 mg yield diminishing returns in terms of platelet inhibition, most likely as a result of clopidogrel pharmacokinetics.6
Compared with 300 mg of clopidogrel, the more potent platelet inhibitory effect of a 600-mg dose translated to a two-thirds reduction (P = .041) in the composite end point of death, MI, or target vessel revascularization at 30 days in a study of 255 patients with stable coronary artery disease undergoing percutaneous coronary intervention (PCI).7 The reduction in this composite end point with high-dose clopidogrel was driven by a reduction in the incidence of periprocedural MI.
In a separate study of 292 patients with non‑ST-segment-elevation ACS who were scheduled for PCI, the superior platelet response to 600 mg versus 300 mg of clopidogrel translated to a 60% reduction in adverse thrombotic events (P = .02), and this benefit extended beyond rates of periprocedural MI.8
Similar results with increased maintenance dose
Similarly, emerging data suggest that raising the maintenance dose of clopidogrel can also raise response rates. In a study of 60 patients, doubling the maintenance dose of clopidogrel after PCI from 75 mg/day to 150 mg/day resulted in improved platelet inhibition as assessed by rapid platelet function analysis.9 Likewise, a 150-mg/day maintenance dose of clopidogrel was associated with a superior antiplatelet effect compared with 75 mg/day in a study of 40 patients with type 2 diabetes.10
Large definitive trial is under way
In the wake of these smaller trials, a large randomized trial known as CURRENT is comparing a strategy of high-dose clopidogrel with standard-dose clopidogrel in patients with ACS for whom an early invasive management strategy is planned.11 The high-dose regimen involves a 600-mg loading dose followed by 150 mg/day for 1 week and then 75 mg/day for 3 weeks, whereas the standard-dose regimen involves a 300-mg loading dose followed by 75 mg/day for 4 weeks. Both groups are being further randomized to low-dose aspirin (75 to 100 mg/day) or high-dose aspirin (300 to 325 mg/day) for 30 days after PCI. With a target enrollment well beyond 10,000 patients, CURRENT should definitively clarify the relative efficacy and safety of high-dose clopidogrel in this setting.
Tailoring clopidogrel therapy
Investigators have explored tailoring the dosing of clopidogrel around the time of PCI based on the degree of platelet inhibition. In one study, administering additional loading doses of clopidogrel, up to a total of 2,400 mg, before PCI in patients with a suboptimal degree of platelet inhibition resulted in a lower rate of ischemic complications following PCI.12
PRASUGREL, A NOVEL THIENOPYRIDINE
Prasugrel is an investigational third-generation thienopyridine currently under US Food and Drug Administration (FDA) review for use in patients with ACS being managed with PCI. Like clopidogrel, prasugrel is a prodrug that requires conversion to an active metabolite prior to binding to the platelet P2Y12 receptor for ADP to confer antiplatelet activity. Prasugrel is metabolized more efficiently than clopidogrel, allowing for faster activation and superior bioavailability to produce a greater and more consistent antiplatelet effect.1,13
The active metabolites of clopidogrel and prasugrel are no different in their ability to inhibit platelet aggregation, but approximately 85% of clopidogrel is inactivated by esterases, with the remaining 15% being converted to the active metabolite using the cytochrome P450 pathway via two successive oxidative steps in the liver.14 In contrast, esterases facilitate the transformation of prasugrel to its active metabolite.14 This activation requires only one oxidative step that can occur in either the liver or the gut through cytochrome P450.
Both prasugrel and clopidogrel are irreversible P2Y12 receptor blockers. For this reason, one must wait approximately 5 days after the last dose of either medication for generation of a sufficient number of new platelets to allow restoration of normal platelet-mediated hemostasis.
Inhibition of platelet aggregation relative to clopidogrel
In a study among healthy volunteers, inhibition of platelet aggregation was significantly higher after a 60-mg loading dose of prasugrel compared with a 300-mg loading dose of clopidogrel.13 Further, suboptimal responders to clopidogrel who crossed over to prasugrel had levels of platelet inhibition as high as 80% following prasugrel administration. The time to peak effect of prasugrel was about 1 hour. Inhibition of platelet aggregation was more consistent following dosing of prasugrel compared with clopidogrel.13
In a study of 201 patients undergoing cardiac catheterization with planned PCI, Wiviott et al demonstrated better levels of inhibition of platelet aggregation at 6 hours after a 60-mg loading dose of prasugrel than after a 600-mg loading dose of clopidogrel (P < .0001).1
Clinical effects relative to clopidogrel: TRITON-TIMI 38
A large phase 3 clinical trial—the Trial to Assess Improvement in Therapeutic Outcomes by Optimizing Platelet Inhibition with Prasugrel–Thrombolysis in Myocardial Infarction (TRITON-TIMI) 38—was conducted to compare the effects of prasugrel and standard-dose clopidogrel on death and ischemic end points in 13,608 patients with ACS scheduled to undergo PCI.15 Patients randomized to clopidogrel were given the standard regimen of a 300-mg loading dose followed by a 75-mg daily maintenance dose; those randomized to prasugrel were given a 60-mg loading dose followed by a 10-mg daily maintenance dose. The study drug was typically given immediately before PCI, a time frame that may mimic real-life use but that favored the faster-onset prasugrel over the slower-onset clopidogrel. Both groups also received low-dose aspirin. Approximately half of the patients in each group were treated with a glycoprotein IIb/IIIa inhibitor. The median duration of therapy was approximately 15 months.
Efficacy. The primary end point—a composite of cardiovascular death, MI, or stroke—occurred in 9.9% of patients randomized to prasugrel compared with 12.1% of those randomized to clopidogrel, corresponding to a 19% relative risk reduction (P = .0004) with prasugrel. Based on these results, 46 patients would need to be treated with prasugrel rather than with clopidogrel to prevent 1 additional cardiovascular death, MI, or stroke.15
The reduction in the primary end point with prasugrel was driven primarily by a reduction in nonfatal MI; nonsignificant trends favored prasugrel over clopidogrel on rates of cardiovascular death and all-cause mortality, but there was no difference in stroke rates. Prasugrel’s effect was consistent across subgroups based on MI type, sex, age, the type of stent used, adjunctive antithrombotic therapy, and renal function.15
In the subgroup of patients with diabetes, the relative reduction in the primary end point with prasugrel compared with clopidogrel was 30% (P < .001), and the respective relative reduction among patients with diabetes who required insulin was 37%.16
Safety. Higher antiplatelet potency carries the trade-off of increased bleeding, and this trade-off was apparent with prasugrel in TRITON-TIMI 38.15 TIMI major bleeding (not counting bleeding related to coronary artery bypass graft surgery [CABG]) occurred significantly more often in prasugrel-treated subjects than in those receiving clopidogrel (2.4% vs 1.8%; P = .03), as did life-threatening bleeds (1.4% vs 0.9%; P = .01). Because absolute rates of major bleeding were low in each treatment group, based on these results, 167 patients would need to be treated with prasugrel rather than clopidogrel to result in 1 excess non-CABG-related major bleeding episode. Rates of intracranial hemorrhage were identical in the two treatment groups.15
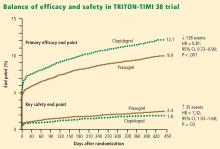
Net clinical outcome and therapeutic considerations. Overall analysis of the balance of efficacy and safety in TRITON-TIMI 38 revealed that 138 events were prevented with randomization to prasugrel instead of clopidogrel, at a cost of 35 additional TIMI major bleeds (Figure 2).15
In a post hoc analysis of net clinical outcome, in which major bleeding events were added to the primary composite efficacy end point, prasugrel was associated with a 13% relative risk reduction (P = .004).15 Twenty-three MIs were prevented per 1,000 treated patients with the use of prasugrel instead of clopidogrel, at a cost of 6 excess non-CABG-related major bleeds.15
Another post hoc assessment identified three subgroups who had a significantly increased risk of TIMI major bleeds with randomization to prasugrel15:
- Patients aged 75 years or older
- Patients with a body weight less than 60 kg
- Patients with a history of stroke or transient ischemic attack (TIA).
In these three subgroups, the net clinical effect either was neutral (for those aged ≥ 75years and for those weighing < 60 kg) or favored clopidogrel (for those with a history of stroke or TIA). The group with a history of stroke or TIA represented 4% of the entire cohort, and the TRITON-TIMI 38 investigators recommended avoiding prasugrel in patients with a history of these events. The other two subgroups with a significantly increased bleeding risk with prasugrel represented 16% of the entire cohort, and in these two groups the investigators suggested a pharmacokinetics-guided reduction in the maintenance dose of prasugrel, although a recommendation for such dosing is based on modeling and not actual outcomes data.15
Stent thrombosis. A subanalysis of TRITON-TIMI 38 examined the risk of stent thrombosis in the 12,844 patients enrolled in the trial who had stents implanted.17 Stent thrombosis was assessed using the Academic Research Consortium definitions of definite, probable, and possible stent thrombosis.18 The risk of definite or probable stent thrombosis was halved (hazard ratio = 0.48; P < .0001) with the use of prasugrel compared with clopidogrel, and the reduction was highly significant regardless of the type of stent implanted or the way stent thrombosis was defined. Significant reductions in both early (within the first 30 days) stent thrombosis (P < .0001) and late (beyond 30 days) stent thrombosis (P = .03) were observed in the prasugrel arm compared with the clopidogrel arm.17
AZD6140, A REVERSIBLE P2Y12 RECEPTOR ANTAGONIST
AZD6140, another investigational antiplatelet agent, is an orally active reversible P2Y12 receptor antagonist, in contrast to the thienopyridines, which are irreversible inhibitors. A member of the cyclo-pentyl-triazolo-pyrimidine (CPTP) class, AZD6140 has a rapid onset of action (≤ 2 hours) and does not require metabolic activation. Its plasma half-life is approximately 12 hours, which translates to twice-daily dosing.
Inhibition of platelet aggregation relative to clopidogrel
In a study of clopidogrel-naïve patients with ACS, inhibition of platelet aggregation 12 hours after administration of AZD6140 was approximately 75% with 90-mg, 180-mg, and 270-mg doses, significantly greater than the 30% inhibition achieved after administration of 300 mg of clopidogrel (P < .0002 for all doses of AZD6140 vs clopidogrel).19 Whereas steady state was achieved in approximately 4 to 6 hours with clopidogrel, it was achieved in approximately 2 hours or less with AZD6140.
Clinical safety and efficacy relative to clopidogrel
In a dose-ranging study of AZD6140, adjudicated bleeding rates were similar among two different doses of AZD6140 (90 mg twice daily and 180 mg twice daily) and clopidogrel 75 mg once daily, with no evidence of a dose effect for major bleeding with AZD6140.20 Although this study, conducted in 990 patients with ACS, was underpowered for efficacy end points, rates of adjudicated MI were numerically lower in each of the AZD6140 groups than in the clopidogrel group.
A more definitive evaluation of the relative effcicacy and safety of AZD6140 is expected from the ongoing PLATO trial, which is comparing 90 mg of AZD6140 twice daily with clopidogrel 75 mg/day among 18,000 patients randomized to one of the two treatments within 24 hours of an index ACS event.21
CANGRELOR, A RAPID PARENTERAL P2Y12 RECEPTOR ANTAGONIST
Cangrelor (formerly known as AR-C69931MX) is an intravenously (IV) administered P2Y12 receptor antagonist under investigation for treatment of ACS and use during PCI and other coronary procedures. The compound is an adenosine triphosphate analogue with a plasma half-life of 5 to 9 minutes. Cangrelor is highly reversible, as platelet function returns to normal within 20 minutes of dosing. Within 15 minutes of initiation, cangrelor produces profound platelet inhibition and rapidly achieves steady state; peak effect occurs within minutes.22 The response to cangrelor is highly consistent, with virtually all recipients achieving the same degree of platelet inhibition. Platelet response approaches baseline 15 minutes after termination.22
If approved by the FDA, cangrelor would be administered similar to the way that glycoprotein IIb/IIIa inhibitors are, as it would be used primarily in the catheterization laboratory and then discontinued after the procedure, at which point transition to a long-term oral therapy would be necessary.
Clinical effects relative to abciximab
Cangrelor has been compared with the glycoprotein IIb/IIIa inhibitor abciximab and placebo in 249 patients undergoing elective or urgent PCI.22 Rates of the combined end point of death, MI, or need for repeat revascularization at 30 days were similar with cangrelor and abciximab (5.7% vs 5.4%, respectively; P = NS), both of which were lower than the rate with placebo (10.0%). Major or minor bleeding through 7 days occurred in numerically fewer cangrelor recipients compared with abciximab recipients (7.0% vs 9.0%), although the small sample size precluded evaluation for statistical significance.
Clinical effects relative to clopidogrel—the CHAMPION trials
A phase 3 trial program consisting of two multinational studies of cangrelor—the Cangrelor Versus Standard Therapy to Achieve Optimal Management of Platelet Inhibition (CHAMPION) program—is currently under way.
CHAMPION-PCI is enrolling 9,000 patients presenting with ACS who are being randomized in a double-blind fashion at the start of PCI to a 600-mg loading dose of clopidogrel or to cangrelor given as an IV bolus of 30 μg/kg followed by an IV infusion of 4 μg/kg/min. The primary end point is a composite of all-cause mortality, MI, or ischemia-driven revascularization in the 48 hours following randomization. Secondary end points include rates of all-cause mortality and MI at 48 hours.23
CHAMPION-PLATFORM is enrolling 4,400 patients scheduled for PCI as a result of ACS who are being randomized in a double-blind, double-dummy manner to (1) cangrelor bolus and infusion plus oral placebo or (2) oral clopidogrel plus placebo bolus and infusion before their index procedures. Dosages of the two agents are the same as in CHAMPION-PCI. The primary end point is a composite of death, MI, or urgent target vessel revascularization at 48 hours. Secondary end points include 30-day and 1-year clinical outcomes.23
The rationale for the CHAMPION investigations stems from the need to initiate clopidogrel before a patient is taken to the catheterization laboratory, owing to the inability to achieve a high degree of platelet inhibition until 4 to 6 hours after clopidogrel administration. Although this strategy can be undertaken without complication for most patients, a subset of patients with three-vessel disease or left-main disease will require CABG, which then must be delayed several days until clopidogrel’s platelet-inhibiting effect diminishes. A rapid-acting IV inhibitor of the P2Y12 receptor such as cangrelor would obviate this concern.
THROMBIN INHIBITORS
Thrombin plays an important role in platelet activation, and thrombin receptor antagonists may represent a safer means of inhibiting platelet activation relative to traditional antiplatelet agents. This theoretical safety advantage stems from the notion that blocking the action of platelets at the thrombin receptor would preserve platelets’ function as mediators of primary hemostasis. Because thrombin’s activation of platelets should occur only during clot formation, blocking platelet activation at the thrombin receptor would interrupt thrombin’s ability to propagate platelet activation during formation of coronary artery clots.
One agent in this class that is being studied extensively is SCH 530348, an oral thrombin receptor antagonist with potent antiplatelet activity. Its peak antiplatelet potency is achieved within hours when a loading dose is given, and within days without a loading dose. Wearing-off of the action of SCH 530348 takes weeks.24
Inhibition of platelet aggregation with thrombin receptor antagonists is measured in response to the thrombin receptor antagonist peptide (TRAP), not ADP. The proportion of subjects treated with SCH 530348 who achieve greater than 80% inhibition of platelet aggregation to 15 μM of TRAP ranges from 91% (with 0.5 mg of SCH 530348) to 100% (with 1.0 mg and 2.5 mg) at both 30 days and 60 days.25
Clinical effects in placebo-controlled trials
SCH 530348 was studied in the Thrombin Receptor Antagonist (TRA)–PCI trial, a dose-ranging study in which patients were randomized to one of three oral loading doses of the study drug (10 mg, 20 mg, or 40 mg) on top of a clopidogrel loading dose before undergoing cardiac catheterization for planned PCI; patients were then randomized to one of three maintenance doses of SCH 530348 (0.5 mg, 1.0 mg, or 2.5 mg) or placebo (depending on loading therapy) for 60 days.25
Among the 573 patients undergoing PCI , the rate of TIMI major or minor bleeding was not significantly higher with any dose of SCH 530348 compared with placebo,25 supporting the hypothesis that thrombin receptor antagonism inhibits platelet aggregation without a significant increase in bleeding.
Although the TRA-PCI study was not powered to detect differences in clinical event rates, a reduction in the rate of major adverse cardiovascular events was observed in a dose-dependent manner with SCH 530348 compared with placebo in the PCI cohort.25
On the basis of the TRA-PCI trial, a pair of phase 3 trials of SCH 530348 have been launched—the Thrombin Receptor Antagonist in Secondary Prevention of Atherothrombotic Ischemic Events (TRA 2°P-TIMI 50) study and the Thrombin Receptor Antagonist for Clinical Event Reduction in ACS (TRA-CER) study.
TRA 2°P-TIMI 50 is a multinational double-blind study enrolling 19,500 patients with prior MI or stroke or with existing peripheral arterial disease. Patients are being randomized to placebo plus standard medical care (including aspirin and clopidogrel) or to 2.5 mg of SCH 530348 once daily plus standard medical care. The primary end point is the composite of cardiovascular death, MI, urgent coronary revascularization, or stroke.26
TRA-CER is a multinational double-blind study with planned enrollment of 10,000 patients with non-ST-segment-elevation MI. Patients are being randomized to placebo plus standard medical care (including aspirin or clopidogrel) or to SCH 530348 (using the oral 40-mg loading dose and a maintenance dose of 2.5 mg once daily) plus standard medical care. The primary end point is the composite of cardiovascular death, MI, rehospitalization for ACS, urgent coronary revascularization, or stroke. The key secondary end point is the composite of cardiovascular death, MI, or stroke.27
COMPARATIVE CONSIDERATIONS
Inhibition of platelet aggregation
Clopidogrel achieves about 30% inhibition of platelet aggregation to ADP at its current FDA-approved loading dose of 300 mg and about 40% inhibition when its dose is doubled to 600 mg. These levels of inhibition are increased to 75% to 80% by clopidogrel’s fellow thienopyridine prasugrel, and this increase is attributable to prasugrel’s more efficient metabolism from prodrug to active metabolite. The reversible P2Y12 receptor antagonist AZD6140 achieves a comparable 75% to 80% inhibition of platelet aggregation. The parenterally administered P2Y12 receptor antagonist cangrelor achieves greater than 90% inhibition, as does the oral thrombin receptor antagonist SCH 530348, although the latter agent’s inhibition is to the agonist TRAP rather than ADP.
Time to peak effect
The time to peak effect with clopidogrel is approximately 4 hours regardless of the loading dose used (300 mg or 600 mg); this is substantially reduced with all of the investigational agents except SCH 530348. The novel agents’ reduced time to peak effect can offer advantages in speeding patients’ readiness to undergo catheterization procedures. This is particularly true for the IV agent cangrelor, which achieves its peak effect within minutes, although the 1-hour to 2-hour time frame with oral agents prasugrel and AZD6140 also would usually obviate any need to delay catheterization.
Consistency of platelet response
Standard-dose clopidogrel has the least consistency of platelet response among the therapies reviewed. Although increasing the clopidogrel dose yields somewhat greater consistency in response, it is still lower than the very high degrees of consistency observed with all of the novel compounds, each of which appears to achieve the same degree of inhibition of aggregation in virtually all patients.
Offset of effect
Both of the thienopyridines—clopidogrel and prasugrel—have an offset of effect of about 5 days, which requires delay of surgery, if possible, for several days in patients taking these agents. This is not an issue for the reversible oral agent AZD6140, whose offset of action takes just 1 to 2 days. While this rapid wearing-off of effect translates to a potential advantage for AZD6140, it also poses the potential drawback that a missed dose or two may leave the patient exposed to the risk of a thrombotic event. Cangrelor’s rapid offset of 20 minutes promotes its envisioned use as a catheterization lab–based medication like the glycoprotein IIb/IIIa inhibitors that can be started right before a PCI procedure and stopped immediately afterward. Because SCH 530348 has a very long half-life and thus a weeks-long washout period, the practicality of its use may depend on the hypothesis that thrombin receptor antagonists do not interfere with primary hemostasis, which is supported by data to date but remains to be definitively confirmed.
CONCLUSIONS
Clopidogrel achieves modest platelet inhibition with wide variability in response. Higher doses of clopidogrel achieve modestly greater degrees of inhibition than standard doses, and appear to result in a decreased rate of ischemic events. Although higher doses of clopidogrel have been embraced by some clinicians, we await definitive phase 3 trial evidence of net benefit before making high-dose clopidogrel the new standard of care.
Compared with clopidogrel, the investigational thienopyridine prasugrel is a more potent and consistent blocker of the ADP receptor. It results in a decreased rate of ischemic events relative to clopidogrel, including a 50% reduction in the rate of stent thrombosis, but is associated with an increased rate of bleeding. If prasugrel is approved for marketing, its use should be avoided in patients with a history of stroke or TIA, and avoidance or dose adjustment may be necessary in patients aged 75 years or older and in patients weighing less than 60 kg.
Other novel antiplatelet agents being evaluated for use in patients with ACS—the reversible oral ADP receptor blocker AZD6140, the rapid-acting IV ADP receptor blocker cangrelor, and oral thrombin receptor antagonists—offer potential advantages that need to be examined in the context of large-scale clinical trials.
- Wiviott SD, Trenk D, Frelinger AL, et al. Prasugrel compared with high loading- and maintenance-dose clopidogrel in patients with planned percutaneous coronary intervention: the Prasugrel in Comparison to Clopidogrel for Inhibition of Platelet Activation and Aggregation–Thrombolysis in Myocardial Infarction 44 trial. Circulation 2007; 116:2923–2932.
- Serebruany VL, Steinhubl SR, Berger PB, Malinin AI, Bhatt DL, Topol EJ. Variability in platelet responsiveness to clopidogrel among 544 individuals. J Am Coll Cardiol 2005; 45:246–251.
- Matetzky S, Shenkman B, Guetta V, et al. Clopidogrel resistance is associated with increased risk of recurrent atherothrombotic events in patients with acute myocardial infarction. Circulation 2004; 109:3171–3175.
- Gurbel PA, Bliden KP, Samara W, et al. Clopidogrel effect on platelet reactivity in patients with stent thrombosis: results of the CREST Study. J Am Coll Cardiol 2005; 46:1827–1832.
- Gurbel PA, Bliden KP, Hayes KM, Yoho JA, Herzog WR, Tantry US. The relation of dosing to clopidogrel responsiveness and the incidence of high post-treatment platelet aggregation in patients undergoing coronary stenting. J Am Coll Cardiol 2005; 45:1392–1396.
- von Beckerath N, Taubert D, Pogatsa-Murray G, Schömig E, Kastrati A, Schömig A. Absorption, metabolization, and antiplatelet effects of 300-, 600-, and 900-mg loading doses of clopidogrel: results of the ISAR-CHOICE (Intracoronary Stenting and Antithrombotic Regimen: Choose Between 3 High Oral Doses for Immediate Clopidogrel Effect) Trial. Circulation 2005; 112:2946–2950.
- Patti G, Colonna G, Pasceri V, Pepe LL, Montinaro A, Di Sciascio G. Randomized trial of high loading dose of clopidogrel for reduction of periprocedural myocardial infarction in patients undergoing coronary intervention: results from the ARMYDA-2 (Antiplatelet therapy for Reduction of MYocardial Damage during Angioplasty) study. Circulation 2005; 111:2099–2106.
- Cuisset T, Frere C, Quilici J, et al. Benefit of a 600-mg loading dose of clopidogrel on platelet reactivity and clinical outcomes in patients with non-ST-segment elevation acute coronary syndrome undergoing coronary stenting. J Am Coll Cardiol 2006; 48:1339–1345.
- von Beckerath N, Kastrati A, Wieczorek A, et al. A double-blind, randomized study on platelet aggregation in patients treated with a daily dose of 150 or 75 mg of clopidogrel for 30 days. Eur Heart J 2007; 28:1814–1819.
- Angiolillo DJ, Shoemaker SB, Desai B, et al. Randomized comparison of a high clopidogrel maintenance dose in patients with diabetes mellitus and coronary artery disease: results of the Optimizing Antiplatelet Therapy in Diabetes Mellitus (OPTIMUS) study. Circulation 2007; 115:708–716.
- Clopidogrel optimal loading dose usage to reduce recurrent events/optimal antiplatelet strategy for interventions (CURRENT/OASIS7). Clinical Trials.gov Web site. http://clinicaltrials.gov/ct2/show/NCT00335452. Updated September 1, 2008. Accessed December 16, 2008.
- Bonello L, Camoin-Jau L, Arques S, et al. Adjusted clopidogrel loading doses according to vasodilator-stimulated phosphoprotein phosphorylation index decrease rate of major adverse cardiovascular events in patients with clopidogrel resistance: a multicenter randomized prospective study. J Am Coll Cardiol 2008; 51:1404–1411.
- Brandt JT, Payne CD, Wiviott SD, et al. A comparison of prasugrel and clopidogrel loading doses on platelet function: magnitude of platelet inhibition is related to active metabolic formation. Am Heart J 2007; 153:66.e9–e16.
- Herbert JM, Savi P. P2Y12, a new platelet ADP receptor, target of clopidogrel. Semin Vasc Med 2003; 3:113–122.
- Wiviott SD, Braunwald E, McCabe CH, et al. Prasugrel versus clopidogrel in patients with acute coronary syndromes. N Engl J Med 2007; 357:2001–2015.
- Wiviott SD, Braunwald E, Angiolillo DJ, et al. Greater clinical benefit of more intensive oral antiplatelet therapy with prasugrel in patients with diabetes mellitus in the Trial to Assess Improvement in Therapeutic Outcomes by Optimizing Platelet Inhibition with Prasugrel–Thrombolysis in Myocardial Infarction 38. Circulation 2008; 118:1626–1636.
- Wiviott SD, Braunwald E, McCabe CH, et al. Intensive oral antiplatelet therapy for reduction of ischaemic events including stent thrombosis in patients with acute coronary syndromes treated with percutaneous coronary intervention and stenting in the TRITON-TIMI 38 trial: a subanalysis of a randomised trial. Lancet 2008; 371:1353–1363.
- Mauri L, Hsieh WH, Massaro JM, Ho KK, D’Agostino R, Cutlip DE. Stent thrombosis in randomized clinical trials of drug-eluting stents. N Engl J Med 2007; 356:1020–1029.
- Storey RF, Husted S, Harrington RA, et al. Inhibition of platelet aggregation by AZD6140, a reversible oral P2Y12 receptor antagonist, compared with clopidogrel in patients with acute coronary syndromes. J Am Coll Cardiol 2007; 50:1852–1856.
- Cannon CP, Husted S, Harrington RA, et al. Safety, tolerability, and initial efficacy of AZD6140, the first reversible oral adenosine diphosphate receptor antagonist, compared with clopidogrel, in patients with non-ST-segment elevation acute coronary syndrome: primary results of the DISPERSE-2 trial. J Am Coll Cardiol 2007; 50:1844–1851.
- A comparison of AZD6140 and clopidogrel in patients with acute coronary syndrome (PLATO). Clinical Trials.gov Web site. http://clinicaltrials.gov/ct2/show/NCT00391872. Updated December 3, 2008. Accessed December 5, 2008.
- Greenbaum AB, Grines CL, Bittl JA, et al. Initial experience with an intravenous P2Y12 platelet receptor antagonist in patients undergoing percutaneous coronary intervention: results from a 2-part, phase II, multicenter, randomized, placebo- and active-controlled trial. Am Heart J 2006; 151:689.e1–689.e10.
- A clinical trial to demonstrate the efficacy of cangrelor (PCI). Clinical Trials.gov Web site. http://www.clinicaltrials.gov/ct/show/nct00305162. Updated December 3, 2008. Accessed December 5, 2008.
- Bhatt DL, Topol EJ. Scientific and therapeutic advances in antiplatelet therapy. Nat Rev Drug Discov 2003; 2:15–28.
- Moliterno DJ, Becker RC, Jennings LK, et al; TRA-PCI Study Investigators. Results of a multinational randomized, double-blind, placebo-controlled study of a novel thrombin receptor antagonist (SCH 530348) in percutaneous coronary intervention. Presented at: 56th Annual Scientific Session of the American College of Cardiology; March 24–27, 2007; New Orleans, LA.
- Trial to assess the effects of SCH 530348 in preventing heart attack and stroke in patients with atherosclerosis (TRA 2°P-TIMI 50). Clinical Trials.gov Web site. http://clinicaltrials.gov/ct2/show/NCT00526474. Updated November 13, 2008. Accessed December 16, 2008.
- Trial to assess the effects of SCH 530348 in preventing heart attack and stroke in patients with acute coronary syndrome (TRA-CER). Clinical Trials.gov Web site. http://clinicaltrials.gov/ct2/show/NCT00527943. Updated November 12, 2008. Accessed December 16, 2008.
An enhanced understanding of platelet biology, as reviewed in the previous article in this supplement, has made it possible to identify a wide variety of platelet agonists. This knowledge has fostered the development of a host of pharmacologic strategies to block agonists such as cyclooxygenase, thromboxane, adenosine diphosphate (ADP), and thrombin, among others. This article will discuss the pharmacologic properties of novel antiplatelet agents, as well as alternative dosing of the established antiplatelet agent clopidogrel, and will review data from available comparative and placebo-controlled trials of these agents. The article concludes with comparative perspectives on the potential roles and relative advantages of these agents in the evolving management of patients with acute coronary syndromes (ACS).
CLOPIDOGREL AND THE CHALLENGE OF VARIABLE RESPONSE
Clopidogrel, a member of the thienopyridine class of ADP receptor inhibitors, is well established for use in patients with ACS at a loading dose of 300 mg followed by a maintenance dose of 75 mg/day. At this loading dose, inhibition of platelet aggregation to ADP is approximately 30%, and the time to peak effect is approximately 4 to 6 hours.1
As with most other drugs, the response to clopidogrel is variable. However, in contrast to the accepted measures of response to antihypertensive or lipid-lowering drugs, there are no routinely used tests for measuring response to antiplatelet therapies. As a result, a “one size fits all” strategy in the dosing of clopidogrel has prevailed.
This variability in response is clinically relevant. In a study assessing clopidogrel responsiveness by ADP-induced platelet aggregation in 60 patients who experienced ST-segment-elevation myocardial infarction (MI), Matetzky et al found that the lowest levels of clopidogrel responsiveness were associated with a significantly elevated rate (P = .007) of recurrent cardiovascular events 6 months after the MI.3 Gurbel et al found a similar association between clopidogrel responsiveness and subacute stent thrombosis in a study of 120 patients using two different methods—light transmission aggregotomy to 5 μmol/L of ADP, and the ratio of vasodilator-stimulated phosphoprotein reactivity—to assess clopidogrel responsiveness.4
Increasing the loading dose raises response rates
One proposed method for boosting responsiveness to clopidogrel in suboptimal responders is the use of a higher dose. In a study of 190 patients undergoing coronary stenting, increasing the loading dose from 300 mg to 600 mg reduced the rate of clopidogrel resistance (defined as a < 10% absolute change in aggregation to 5 μM of ADP at 24 hours) from 28% to 8% (P < .001),5 a finding that supports the notion of enhanced response at doses up to 600 mg. Single loading doses in excess of 600 mg yield diminishing returns in terms of platelet inhibition, most likely as a result of clopidogrel pharmacokinetics.6
Compared with 300 mg of clopidogrel, the more potent platelet inhibitory effect of a 600-mg dose translated to a two-thirds reduction (P = .041) in the composite end point of death, MI, or target vessel revascularization at 30 days in a study of 255 patients with stable coronary artery disease undergoing percutaneous coronary intervention (PCI).7 The reduction in this composite end point with high-dose clopidogrel was driven by a reduction in the incidence of periprocedural MI.
In a separate study of 292 patients with non‑ST-segment-elevation ACS who were scheduled for PCI, the superior platelet response to 600 mg versus 300 mg of clopidogrel translated to a 60% reduction in adverse thrombotic events (P = .02), and this benefit extended beyond rates of periprocedural MI.8
Similar results with increased maintenance dose
Similarly, emerging data suggest that raising the maintenance dose of clopidogrel can also raise response rates. In a study of 60 patients, doubling the maintenance dose of clopidogrel after PCI from 75 mg/day to 150 mg/day resulted in improved platelet inhibition as assessed by rapid platelet function analysis.9 Likewise, a 150-mg/day maintenance dose of clopidogrel was associated with a superior antiplatelet effect compared with 75 mg/day in a study of 40 patients with type 2 diabetes.10
Large definitive trial is under way
In the wake of these smaller trials, a large randomized trial known as CURRENT is comparing a strategy of high-dose clopidogrel with standard-dose clopidogrel in patients with ACS for whom an early invasive management strategy is planned.11 The high-dose regimen involves a 600-mg loading dose followed by 150 mg/day for 1 week and then 75 mg/day for 3 weeks, whereas the standard-dose regimen involves a 300-mg loading dose followed by 75 mg/day for 4 weeks. Both groups are being further randomized to low-dose aspirin (75 to 100 mg/day) or high-dose aspirin (300 to 325 mg/day) for 30 days after PCI. With a target enrollment well beyond 10,000 patients, CURRENT should definitively clarify the relative efficacy and safety of high-dose clopidogrel in this setting.
Tailoring clopidogrel therapy
Investigators have explored tailoring the dosing of clopidogrel around the time of PCI based on the degree of platelet inhibition. In one study, administering additional loading doses of clopidogrel, up to a total of 2,400 mg, before PCI in patients with a suboptimal degree of platelet inhibition resulted in a lower rate of ischemic complications following PCI.12
PRASUGREL, A NOVEL THIENOPYRIDINE
Prasugrel is an investigational third-generation thienopyridine currently under US Food and Drug Administration (FDA) review for use in patients with ACS being managed with PCI. Like clopidogrel, prasugrel is a prodrug that requires conversion to an active metabolite prior to binding to the platelet P2Y12 receptor for ADP to confer antiplatelet activity. Prasugrel is metabolized more efficiently than clopidogrel, allowing for faster activation and superior bioavailability to produce a greater and more consistent antiplatelet effect.1,13
The active metabolites of clopidogrel and prasugrel are no different in their ability to inhibit platelet aggregation, but approximately 85% of clopidogrel is inactivated by esterases, with the remaining 15% being converted to the active metabolite using the cytochrome P450 pathway via two successive oxidative steps in the liver.14 In contrast, esterases facilitate the transformation of prasugrel to its active metabolite.14 This activation requires only one oxidative step that can occur in either the liver or the gut through cytochrome P450.
Both prasugrel and clopidogrel are irreversible P2Y12 receptor blockers. For this reason, one must wait approximately 5 days after the last dose of either medication for generation of a sufficient number of new platelets to allow restoration of normal platelet-mediated hemostasis.
Inhibition of platelet aggregation relative to clopidogrel
In a study among healthy volunteers, inhibition of platelet aggregation was significantly higher after a 60-mg loading dose of prasugrel compared with a 300-mg loading dose of clopidogrel.13 Further, suboptimal responders to clopidogrel who crossed over to prasugrel had levels of platelet inhibition as high as 80% following prasugrel administration. The time to peak effect of prasugrel was about 1 hour. Inhibition of platelet aggregation was more consistent following dosing of prasugrel compared with clopidogrel.13
In a study of 201 patients undergoing cardiac catheterization with planned PCI, Wiviott et al demonstrated better levels of inhibition of platelet aggregation at 6 hours after a 60-mg loading dose of prasugrel than after a 600-mg loading dose of clopidogrel (P < .0001).1
Clinical effects relative to clopidogrel: TRITON-TIMI 38
A large phase 3 clinical trial—the Trial to Assess Improvement in Therapeutic Outcomes by Optimizing Platelet Inhibition with Prasugrel–Thrombolysis in Myocardial Infarction (TRITON-TIMI) 38—was conducted to compare the effects of prasugrel and standard-dose clopidogrel on death and ischemic end points in 13,608 patients with ACS scheduled to undergo PCI.15 Patients randomized to clopidogrel were given the standard regimen of a 300-mg loading dose followed by a 75-mg daily maintenance dose; those randomized to prasugrel were given a 60-mg loading dose followed by a 10-mg daily maintenance dose. The study drug was typically given immediately before PCI, a time frame that may mimic real-life use but that favored the faster-onset prasugrel over the slower-onset clopidogrel. Both groups also received low-dose aspirin. Approximately half of the patients in each group were treated with a glycoprotein IIb/IIIa inhibitor. The median duration of therapy was approximately 15 months.
Efficacy. The primary end point—a composite of cardiovascular death, MI, or stroke—occurred in 9.9% of patients randomized to prasugrel compared with 12.1% of those randomized to clopidogrel, corresponding to a 19% relative risk reduction (P = .0004) with prasugrel. Based on these results, 46 patients would need to be treated with prasugrel rather than with clopidogrel to prevent 1 additional cardiovascular death, MI, or stroke.15
The reduction in the primary end point with prasugrel was driven primarily by a reduction in nonfatal MI; nonsignificant trends favored prasugrel over clopidogrel on rates of cardiovascular death and all-cause mortality, but there was no difference in stroke rates. Prasugrel’s effect was consistent across subgroups based on MI type, sex, age, the type of stent used, adjunctive antithrombotic therapy, and renal function.15
In the subgroup of patients with diabetes, the relative reduction in the primary end point with prasugrel compared with clopidogrel was 30% (P < .001), and the respective relative reduction among patients with diabetes who required insulin was 37%.16
Safety. Higher antiplatelet potency carries the trade-off of increased bleeding, and this trade-off was apparent with prasugrel in TRITON-TIMI 38.15 TIMI major bleeding (not counting bleeding related to coronary artery bypass graft surgery [CABG]) occurred significantly more often in prasugrel-treated subjects than in those receiving clopidogrel (2.4% vs 1.8%; P = .03), as did life-threatening bleeds (1.4% vs 0.9%; P = .01). Because absolute rates of major bleeding were low in each treatment group, based on these results, 167 patients would need to be treated with prasugrel rather than clopidogrel to result in 1 excess non-CABG-related major bleeding episode. Rates of intracranial hemorrhage were identical in the two treatment groups.15
Net clinical outcome and therapeutic considerations. Overall analysis of the balance of efficacy and safety in TRITON-TIMI 38 revealed that 138 events were prevented with randomization to prasugrel instead of clopidogrel, at a cost of 35 additional TIMI major bleeds (Figure 2).15
In a post hoc analysis of net clinical outcome, in which major bleeding events were added to the primary composite efficacy end point, prasugrel was associated with a 13% relative risk reduction (P = .004).15 Twenty-three MIs were prevented per 1,000 treated patients with the use of prasugrel instead of clopidogrel, at a cost of 6 excess non-CABG-related major bleeds.15
Another post hoc assessment identified three subgroups who had a significantly increased risk of TIMI major bleeds with randomization to prasugrel15:
- Patients aged 75 years or older
- Patients with a body weight less than 60 kg
- Patients with a history of stroke or transient ischemic attack (TIA).
In these three subgroups, the net clinical effect either was neutral (for those aged ≥ 75years and for those weighing < 60 kg) or favored clopidogrel (for those with a history of stroke or TIA). The group with a history of stroke or TIA represented 4% of the entire cohort, and the TRITON-TIMI 38 investigators recommended avoiding prasugrel in patients with a history of these events. The other two subgroups with a significantly increased bleeding risk with prasugrel represented 16% of the entire cohort, and in these two groups the investigators suggested a pharmacokinetics-guided reduction in the maintenance dose of prasugrel, although a recommendation for such dosing is based on modeling and not actual outcomes data.15
Stent thrombosis. A subanalysis of TRITON-TIMI 38 examined the risk of stent thrombosis in the 12,844 patients enrolled in the trial who had stents implanted.17 Stent thrombosis was assessed using the Academic Research Consortium definitions of definite, probable, and possible stent thrombosis.18 The risk of definite or probable stent thrombosis was halved (hazard ratio = 0.48; P < .0001) with the use of prasugrel compared with clopidogrel, and the reduction was highly significant regardless of the type of stent implanted or the way stent thrombosis was defined. Significant reductions in both early (within the first 30 days) stent thrombosis (P < .0001) and late (beyond 30 days) stent thrombosis (P = .03) were observed in the prasugrel arm compared with the clopidogrel arm.17
AZD6140, A REVERSIBLE P2Y12 RECEPTOR ANTAGONIST
AZD6140, another investigational antiplatelet agent, is an orally active reversible P2Y12 receptor antagonist, in contrast to the thienopyridines, which are irreversible inhibitors. A member of the cyclo-pentyl-triazolo-pyrimidine (CPTP) class, AZD6140 has a rapid onset of action (≤ 2 hours) and does not require metabolic activation. Its plasma half-life is approximately 12 hours, which translates to twice-daily dosing.
Inhibition of platelet aggregation relative to clopidogrel
In a study of clopidogrel-naïve patients with ACS, inhibition of platelet aggregation 12 hours after administration of AZD6140 was approximately 75% with 90-mg, 180-mg, and 270-mg doses, significantly greater than the 30% inhibition achieved after administration of 300 mg of clopidogrel (P < .0002 for all doses of AZD6140 vs clopidogrel).19 Whereas steady state was achieved in approximately 4 to 6 hours with clopidogrel, it was achieved in approximately 2 hours or less with AZD6140.
Clinical safety and efficacy relative to clopidogrel
In a dose-ranging study of AZD6140, adjudicated bleeding rates were similar among two different doses of AZD6140 (90 mg twice daily and 180 mg twice daily) and clopidogrel 75 mg once daily, with no evidence of a dose effect for major bleeding with AZD6140.20 Although this study, conducted in 990 patients with ACS, was underpowered for efficacy end points, rates of adjudicated MI were numerically lower in each of the AZD6140 groups than in the clopidogrel group.
A more definitive evaluation of the relative effcicacy and safety of AZD6140 is expected from the ongoing PLATO trial, which is comparing 90 mg of AZD6140 twice daily with clopidogrel 75 mg/day among 18,000 patients randomized to one of the two treatments within 24 hours of an index ACS event.21
CANGRELOR, A RAPID PARENTERAL P2Y12 RECEPTOR ANTAGONIST
Cangrelor (formerly known as AR-C69931MX) is an intravenously (IV) administered P2Y12 receptor antagonist under investigation for treatment of ACS and use during PCI and other coronary procedures. The compound is an adenosine triphosphate analogue with a plasma half-life of 5 to 9 minutes. Cangrelor is highly reversible, as platelet function returns to normal within 20 minutes of dosing. Within 15 minutes of initiation, cangrelor produces profound platelet inhibition and rapidly achieves steady state; peak effect occurs within minutes.22 The response to cangrelor is highly consistent, with virtually all recipients achieving the same degree of platelet inhibition. Platelet response approaches baseline 15 minutes after termination.22
If approved by the FDA, cangrelor would be administered similar to the way that glycoprotein IIb/IIIa inhibitors are, as it would be used primarily in the catheterization laboratory and then discontinued after the procedure, at which point transition to a long-term oral therapy would be necessary.
Clinical effects relative to abciximab
Cangrelor has been compared with the glycoprotein IIb/IIIa inhibitor abciximab and placebo in 249 patients undergoing elective or urgent PCI.22 Rates of the combined end point of death, MI, or need for repeat revascularization at 30 days were similar with cangrelor and abciximab (5.7% vs 5.4%, respectively; P = NS), both of which were lower than the rate with placebo (10.0%). Major or minor bleeding through 7 days occurred in numerically fewer cangrelor recipients compared with abciximab recipients (7.0% vs 9.0%), although the small sample size precluded evaluation for statistical significance.
Clinical effects relative to clopidogrel—the CHAMPION trials
A phase 3 trial program consisting of two multinational studies of cangrelor—the Cangrelor Versus Standard Therapy to Achieve Optimal Management of Platelet Inhibition (CHAMPION) program—is currently under way.
CHAMPION-PCI is enrolling 9,000 patients presenting with ACS who are being randomized in a double-blind fashion at the start of PCI to a 600-mg loading dose of clopidogrel or to cangrelor given as an IV bolus of 30 μg/kg followed by an IV infusion of 4 μg/kg/min. The primary end point is a composite of all-cause mortality, MI, or ischemia-driven revascularization in the 48 hours following randomization. Secondary end points include rates of all-cause mortality and MI at 48 hours.23
CHAMPION-PLATFORM is enrolling 4,400 patients scheduled for PCI as a result of ACS who are being randomized in a double-blind, double-dummy manner to (1) cangrelor bolus and infusion plus oral placebo or (2) oral clopidogrel plus placebo bolus and infusion before their index procedures. Dosages of the two agents are the same as in CHAMPION-PCI. The primary end point is a composite of death, MI, or urgent target vessel revascularization at 48 hours. Secondary end points include 30-day and 1-year clinical outcomes.23
The rationale for the CHAMPION investigations stems from the need to initiate clopidogrel before a patient is taken to the catheterization laboratory, owing to the inability to achieve a high degree of platelet inhibition until 4 to 6 hours after clopidogrel administration. Although this strategy can be undertaken without complication for most patients, a subset of patients with three-vessel disease or left-main disease will require CABG, which then must be delayed several days until clopidogrel’s platelet-inhibiting effect diminishes. A rapid-acting IV inhibitor of the P2Y12 receptor such as cangrelor would obviate this concern.
THROMBIN INHIBITORS
Thrombin plays an important role in platelet activation, and thrombin receptor antagonists may represent a safer means of inhibiting platelet activation relative to traditional antiplatelet agents. This theoretical safety advantage stems from the notion that blocking the action of platelets at the thrombin receptor would preserve platelets’ function as mediators of primary hemostasis. Because thrombin’s activation of platelets should occur only during clot formation, blocking platelet activation at the thrombin receptor would interrupt thrombin’s ability to propagate platelet activation during formation of coronary artery clots.
One agent in this class that is being studied extensively is SCH 530348, an oral thrombin receptor antagonist with potent antiplatelet activity. Its peak antiplatelet potency is achieved within hours when a loading dose is given, and within days without a loading dose. Wearing-off of the action of SCH 530348 takes weeks.24
Inhibition of platelet aggregation with thrombin receptor antagonists is measured in response to the thrombin receptor antagonist peptide (TRAP), not ADP. The proportion of subjects treated with SCH 530348 who achieve greater than 80% inhibition of platelet aggregation to 15 μM of TRAP ranges from 91% (with 0.5 mg of SCH 530348) to 100% (with 1.0 mg and 2.5 mg) at both 30 days and 60 days.25
Clinical effects in placebo-controlled trials
SCH 530348 was studied in the Thrombin Receptor Antagonist (TRA)–PCI trial, a dose-ranging study in which patients were randomized to one of three oral loading doses of the study drug (10 mg, 20 mg, or 40 mg) on top of a clopidogrel loading dose before undergoing cardiac catheterization for planned PCI; patients were then randomized to one of three maintenance doses of SCH 530348 (0.5 mg, 1.0 mg, or 2.5 mg) or placebo (depending on loading therapy) for 60 days.25
Among the 573 patients undergoing PCI , the rate of TIMI major or minor bleeding was not significantly higher with any dose of SCH 530348 compared with placebo,25 supporting the hypothesis that thrombin receptor antagonism inhibits platelet aggregation without a significant increase in bleeding.
Although the TRA-PCI study was not powered to detect differences in clinical event rates, a reduction in the rate of major adverse cardiovascular events was observed in a dose-dependent manner with SCH 530348 compared with placebo in the PCI cohort.25
On the basis of the TRA-PCI trial, a pair of phase 3 trials of SCH 530348 have been launched—the Thrombin Receptor Antagonist in Secondary Prevention of Atherothrombotic Ischemic Events (TRA 2°P-TIMI 50) study and the Thrombin Receptor Antagonist for Clinical Event Reduction in ACS (TRA-CER) study.
TRA 2°P-TIMI 50 is a multinational double-blind study enrolling 19,500 patients with prior MI or stroke or with existing peripheral arterial disease. Patients are being randomized to placebo plus standard medical care (including aspirin and clopidogrel) or to 2.5 mg of SCH 530348 once daily plus standard medical care. The primary end point is the composite of cardiovascular death, MI, urgent coronary revascularization, or stroke.26
TRA-CER is a multinational double-blind study with planned enrollment of 10,000 patients with non-ST-segment-elevation MI. Patients are being randomized to placebo plus standard medical care (including aspirin or clopidogrel) or to SCH 530348 (using the oral 40-mg loading dose and a maintenance dose of 2.5 mg once daily) plus standard medical care. The primary end point is the composite of cardiovascular death, MI, rehospitalization for ACS, urgent coronary revascularization, or stroke. The key secondary end point is the composite of cardiovascular death, MI, or stroke.27
COMPARATIVE CONSIDERATIONS
Inhibition of platelet aggregation
Clopidogrel achieves about 30% inhibition of platelet aggregation to ADP at its current FDA-approved loading dose of 300 mg and about 40% inhibition when its dose is doubled to 600 mg. These levels of inhibition are increased to 75% to 80% by clopidogrel’s fellow thienopyridine prasugrel, and this increase is attributable to prasugrel’s more efficient metabolism from prodrug to active metabolite. The reversible P2Y12 receptor antagonist AZD6140 achieves a comparable 75% to 80% inhibition of platelet aggregation. The parenterally administered P2Y12 receptor antagonist cangrelor achieves greater than 90% inhibition, as does the oral thrombin receptor antagonist SCH 530348, although the latter agent’s inhibition is to the agonist TRAP rather than ADP.
Time to peak effect
The time to peak effect with clopidogrel is approximately 4 hours regardless of the loading dose used (300 mg or 600 mg); this is substantially reduced with all of the investigational agents except SCH 530348. The novel agents’ reduced time to peak effect can offer advantages in speeding patients’ readiness to undergo catheterization procedures. This is particularly true for the IV agent cangrelor, which achieves its peak effect within minutes, although the 1-hour to 2-hour time frame with oral agents prasugrel and AZD6140 also would usually obviate any need to delay catheterization.
Consistency of platelet response
Standard-dose clopidogrel has the least consistency of platelet response among the therapies reviewed. Although increasing the clopidogrel dose yields somewhat greater consistency in response, it is still lower than the very high degrees of consistency observed with all of the novel compounds, each of which appears to achieve the same degree of inhibition of aggregation in virtually all patients.
Offset of effect
Both of the thienopyridines—clopidogrel and prasugrel—have an offset of effect of about 5 days, which requires delay of surgery, if possible, for several days in patients taking these agents. This is not an issue for the reversible oral agent AZD6140, whose offset of action takes just 1 to 2 days. While this rapid wearing-off of effect translates to a potential advantage for AZD6140, it also poses the potential drawback that a missed dose or two may leave the patient exposed to the risk of a thrombotic event. Cangrelor’s rapid offset of 20 minutes promotes its envisioned use as a catheterization lab–based medication like the glycoprotein IIb/IIIa inhibitors that can be started right before a PCI procedure and stopped immediately afterward. Because SCH 530348 has a very long half-life and thus a weeks-long washout period, the practicality of its use may depend on the hypothesis that thrombin receptor antagonists do not interfere with primary hemostasis, which is supported by data to date but remains to be definitively confirmed.
CONCLUSIONS
Clopidogrel achieves modest platelet inhibition with wide variability in response. Higher doses of clopidogrel achieve modestly greater degrees of inhibition than standard doses, and appear to result in a decreased rate of ischemic events. Although higher doses of clopidogrel have been embraced by some clinicians, we await definitive phase 3 trial evidence of net benefit before making high-dose clopidogrel the new standard of care.
Compared with clopidogrel, the investigational thienopyridine prasugrel is a more potent and consistent blocker of the ADP receptor. It results in a decreased rate of ischemic events relative to clopidogrel, including a 50% reduction in the rate of stent thrombosis, but is associated with an increased rate of bleeding. If prasugrel is approved for marketing, its use should be avoided in patients with a history of stroke or TIA, and avoidance or dose adjustment may be necessary in patients aged 75 years or older and in patients weighing less than 60 kg.
Other novel antiplatelet agents being evaluated for use in patients with ACS—the reversible oral ADP receptor blocker AZD6140, the rapid-acting IV ADP receptor blocker cangrelor, and oral thrombin receptor antagonists—offer potential advantages that need to be examined in the context of large-scale clinical trials.
An enhanced understanding of platelet biology, as reviewed in the previous article in this supplement, has made it possible to identify a wide variety of platelet agonists. This knowledge has fostered the development of a host of pharmacologic strategies to block agonists such as cyclooxygenase, thromboxane, adenosine diphosphate (ADP), and thrombin, among others. This article will discuss the pharmacologic properties of novel antiplatelet agents, as well as alternative dosing of the established antiplatelet agent clopidogrel, and will review data from available comparative and placebo-controlled trials of these agents. The article concludes with comparative perspectives on the potential roles and relative advantages of these agents in the evolving management of patients with acute coronary syndromes (ACS).
CLOPIDOGREL AND THE CHALLENGE OF VARIABLE RESPONSE
Clopidogrel, a member of the thienopyridine class of ADP receptor inhibitors, is well established for use in patients with ACS at a loading dose of 300 mg followed by a maintenance dose of 75 mg/day. At this loading dose, inhibition of platelet aggregation to ADP is approximately 30%, and the time to peak effect is approximately 4 to 6 hours.1
As with most other drugs, the response to clopidogrel is variable. However, in contrast to the accepted measures of response to antihypertensive or lipid-lowering drugs, there are no routinely used tests for measuring response to antiplatelet therapies. As a result, a “one size fits all” strategy in the dosing of clopidogrel has prevailed.
This variability in response is clinically relevant. In a study assessing clopidogrel responsiveness by ADP-induced platelet aggregation in 60 patients who experienced ST-segment-elevation myocardial infarction (MI), Matetzky et al found that the lowest levels of clopidogrel responsiveness were associated with a significantly elevated rate (P = .007) of recurrent cardiovascular events 6 months after the MI.3 Gurbel et al found a similar association between clopidogrel responsiveness and subacute stent thrombosis in a study of 120 patients using two different methods—light transmission aggregotomy to 5 μmol/L of ADP, and the ratio of vasodilator-stimulated phosphoprotein reactivity—to assess clopidogrel responsiveness.4
Increasing the loading dose raises response rates
One proposed method for boosting responsiveness to clopidogrel in suboptimal responders is the use of a higher dose. In a study of 190 patients undergoing coronary stenting, increasing the loading dose from 300 mg to 600 mg reduced the rate of clopidogrel resistance (defined as a < 10% absolute change in aggregation to 5 μM of ADP at 24 hours) from 28% to 8% (P < .001),5 a finding that supports the notion of enhanced response at doses up to 600 mg. Single loading doses in excess of 600 mg yield diminishing returns in terms of platelet inhibition, most likely as a result of clopidogrel pharmacokinetics.6
Compared with 300 mg of clopidogrel, the more potent platelet inhibitory effect of a 600-mg dose translated to a two-thirds reduction (P = .041) in the composite end point of death, MI, or target vessel revascularization at 30 days in a study of 255 patients with stable coronary artery disease undergoing percutaneous coronary intervention (PCI).7 The reduction in this composite end point with high-dose clopidogrel was driven by a reduction in the incidence of periprocedural MI.
In a separate study of 292 patients with non‑ST-segment-elevation ACS who were scheduled for PCI, the superior platelet response to 600 mg versus 300 mg of clopidogrel translated to a 60% reduction in adverse thrombotic events (P = .02), and this benefit extended beyond rates of periprocedural MI.8
Similar results with increased maintenance dose
Similarly, emerging data suggest that raising the maintenance dose of clopidogrel can also raise response rates. In a study of 60 patients, doubling the maintenance dose of clopidogrel after PCI from 75 mg/day to 150 mg/day resulted in improved platelet inhibition as assessed by rapid platelet function analysis.9 Likewise, a 150-mg/day maintenance dose of clopidogrel was associated with a superior antiplatelet effect compared with 75 mg/day in a study of 40 patients with type 2 diabetes.10
Large definitive trial is under way
In the wake of these smaller trials, a large randomized trial known as CURRENT is comparing a strategy of high-dose clopidogrel with standard-dose clopidogrel in patients with ACS for whom an early invasive management strategy is planned.11 The high-dose regimen involves a 600-mg loading dose followed by 150 mg/day for 1 week and then 75 mg/day for 3 weeks, whereas the standard-dose regimen involves a 300-mg loading dose followed by 75 mg/day for 4 weeks. Both groups are being further randomized to low-dose aspirin (75 to 100 mg/day) or high-dose aspirin (300 to 325 mg/day) for 30 days after PCI. With a target enrollment well beyond 10,000 patients, CURRENT should definitively clarify the relative efficacy and safety of high-dose clopidogrel in this setting.
Tailoring clopidogrel therapy
Investigators have explored tailoring the dosing of clopidogrel around the time of PCI based on the degree of platelet inhibition. In one study, administering additional loading doses of clopidogrel, up to a total of 2,400 mg, before PCI in patients with a suboptimal degree of platelet inhibition resulted in a lower rate of ischemic complications following PCI.12
PRASUGREL, A NOVEL THIENOPYRIDINE
Prasugrel is an investigational third-generation thienopyridine currently under US Food and Drug Administration (FDA) review for use in patients with ACS being managed with PCI. Like clopidogrel, prasugrel is a prodrug that requires conversion to an active metabolite prior to binding to the platelet P2Y12 receptor for ADP to confer antiplatelet activity. Prasugrel is metabolized more efficiently than clopidogrel, allowing for faster activation and superior bioavailability to produce a greater and more consistent antiplatelet effect.1,13
The active metabolites of clopidogrel and prasugrel are no different in their ability to inhibit platelet aggregation, but approximately 85% of clopidogrel is inactivated by esterases, with the remaining 15% being converted to the active metabolite using the cytochrome P450 pathway via two successive oxidative steps in the liver.14 In contrast, esterases facilitate the transformation of prasugrel to its active metabolite.14 This activation requires only one oxidative step that can occur in either the liver or the gut through cytochrome P450.
Both prasugrel and clopidogrel are irreversible P2Y12 receptor blockers. For this reason, one must wait approximately 5 days after the last dose of either medication for generation of a sufficient number of new platelets to allow restoration of normal platelet-mediated hemostasis.
Inhibition of platelet aggregation relative to clopidogrel
In a study among healthy volunteers, inhibition of platelet aggregation was significantly higher after a 60-mg loading dose of prasugrel compared with a 300-mg loading dose of clopidogrel.13 Further, suboptimal responders to clopidogrel who crossed over to prasugrel had levels of platelet inhibition as high as 80% following prasugrel administration. The time to peak effect of prasugrel was about 1 hour. Inhibition of platelet aggregation was more consistent following dosing of prasugrel compared with clopidogrel.13
In a study of 201 patients undergoing cardiac catheterization with planned PCI, Wiviott et al demonstrated better levels of inhibition of platelet aggregation at 6 hours after a 60-mg loading dose of prasugrel than after a 600-mg loading dose of clopidogrel (P < .0001).1
Clinical effects relative to clopidogrel: TRITON-TIMI 38
A large phase 3 clinical trial—the Trial to Assess Improvement in Therapeutic Outcomes by Optimizing Platelet Inhibition with Prasugrel–Thrombolysis in Myocardial Infarction (TRITON-TIMI) 38—was conducted to compare the effects of prasugrel and standard-dose clopidogrel on death and ischemic end points in 13,608 patients with ACS scheduled to undergo PCI.15 Patients randomized to clopidogrel were given the standard regimen of a 300-mg loading dose followed by a 75-mg daily maintenance dose; those randomized to prasugrel were given a 60-mg loading dose followed by a 10-mg daily maintenance dose. The study drug was typically given immediately before PCI, a time frame that may mimic real-life use but that favored the faster-onset prasugrel over the slower-onset clopidogrel. Both groups also received low-dose aspirin. Approximately half of the patients in each group were treated with a glycoprotein IIb/IIIa inhibitor. The median duration of therapy was approximately 15 months.
Efficacy. The primary end point—a composite of cardiovascular death, MI, or stroke—occurred in 9.9% of patients randomized to prasugrel compared with 12.1% of those randomized to clopidogrel, corresponding to a 19% relative risk reduction (P = .0004) with prasugrel. Based on these results, 46 patients would need to be treated with prasugrel rather than with clopidogrel to prevent 1 additional cardiovascular death, MI, or stroke.15
The reduction in the primary end point with prasugrel was driven primarily by a reduction in nonfatal MI; nonsignificant trends favored prasugrel over clopidogrel on rates of cardiovascular death and all-cause mortality, but there was no difference in stroke rates. Prasugrel’s effect was consistent across subgroups based on MI type, sex, age, the type of stent used, adjunctive antithrombotic therapy, and renal function.15
In the subgroup of patients with diabetes, the relative reduction in the primary end point with prasugrel compared with clopidogrel was 30% (P < .001), and the respective relative reduction among patients with diabetes who required insulin was 37%.16
Safety. Higher antiplatelet potency carries the trade-off of increased bleeding, and this trade-off was apparent with prasugrel in TRITON-TIMI 38.15 TIMI major bleeding (not counting bleeding related to coronary artery bypass graft surgery [CABG]) occurred significantly more often in prasugrel-treated subjects than in those receiving clopidogrel (2.4% vs 1.8%; P = .03), as did life-threatening bleeds (1.4% vs 0.9%; P = .01). Because absolute rates of major bleeding were low in each treatment group, based on these results, 167 patients would need to be treated with prasugrel rather than clopidogrel to result in 1 excess non-CABG-related major bleeding episode. Rates of intracranial hemorrhage were identical in the two treatment groups.15
Net clinical outcome and therapeutic considerations. Overall analysis of the balance of efficacy and safety in TRITON-TIMI 38 revealed that 138 events were prevented with randomization to prasugrel instead of clopidogrel, at a cost of 35 additional TIMI major bleeds (Figure 2).15
In a post hoc analysis of net clinical outcome, in which major bleeding events were added to the primary composite efficacy end point, prasugrel was associated with a 13% relative risk reduction (P = .004).15 Twenty-three MIs were prevented per 1,000 treated patients with the use of prasugrel instead of clopidogrel, at a cost of 6 excess non-CABG-related major bleeds.15
Another post hoc assessment identified three subgroups who had a significantly increased risk of TIMI major bleeds with randomization to prasugrel15:
- Patients aged 75 years or older
- Patients with a body weight less than 60 kg
- Patients with a history of stroke or transient ischemic attack (TIA).
In these three subgroups, the net clinical effect either was neutral (for those aged ≥ 75years and for those weighing < 60 kg) or favored clopidogrel (for those with a history of stroke or TIA). The group with a history of stroke or TIA represented 4% of the entire cohort, and the TRITON-TIMI 38 investigators recommended avoiding prasugrel in patients with a history of these events. The other two subgroups with a significantly increased bleeding risk with prasugrel represented 16% of the entire cohort, and in these two groups the investigators suggested a pharmacokinetics-guided reduction in the maintenance dose of prasugrel, although a recommendation for such dosing is based on modeling and not actual outcomes data.15
Stent thrombosis. A subanalysis of TRITON-TIMI 38 examined the risk of stent thrombosis in the 12,844 patients enrolled in the trial who had stents implanted.17 Stent thrombosis was assessed using the Academic Research Consortium definitions of definite, probable, and possible stent thrombosis.18 The risk of definite or probable stent thrombosis was halved (hazard ratio = 0.48; P < .0001) with the use of prasugrel compared with clopidogrel, and the reduction was highly significant regardless of the type of stent implanted or the way stent thrombosis was defined. Significant reductions in both early (within the first 30 days) stent thrombosis (P < .0001) and late (beyond 30 days) stent thrombosis (P = .03) were observed in the prasugrel arm compared with the clopidogrel arm.17
AZD6140, A REVERSIBLE P2Y12 RECEPTOR ANTAGONIST
AZD6140, another investigational antiplatelet agent, is an orally active reversible P2Y12 receptor antagonist, in contrast to the thienopyridines, which are irreversible inhibitors. A member of the cyclo-pentyl-triazolo-pyrimidine (CPTP) class, AZD6140 has a rapid onset of action (≤ 2 hours) and does not require metabolic activation. Its plasma half-life is approximately 12 hours, which translates to twice-daily dosing.
Inhibition of platelet aggregation relative to clopidogrel
In a study of clopidogrel-naïve patients with ACS, inhibition of platelet aggregation 12 hours after administration of AZD6140 was approximately 75% with 90-mg, 180-mg, and 270-mg doses, significantly greater than the 30% inhibition achieved after administration of 300 mg of clopidogrel (P < .0002 for all doses of AZD6140 vs clopidogrel).19 Whereas steady state was achieved in approximately 4 to 6 hours with clopidogrel, it was achieved in approximately 2 hours or less with AZD6140.
Clinical safety and efficacy relative to clopidogrel
In a dose-ranging study of AZD6140, adjudicated bleeding rates were similar among two different doses of AZD6140 (90 mg twice daily and 180 mg twice daily) and clopidogrel 75 mg once daily, with no evidence of a dose effect for major bleeding with AZD6140.20 Although this study, conducted in 990 patients with ACS, was underpowered for efficacy end points, rates of adjudicated MI were numerically lower in each of the AZD6140 groups than in the clopidogrel group.
A more definitive evaluation of the relative effcicacy and safety of AZD6140 is expected from the ongoing PLATO trial, which is comparing 90 mg of AZD6140 twice daily with clopidogrel 75 mg/day among 18,000 patients randomized to one of the two treatments within 24 hours of an index ACS event.21
CANGRELOR, A RAPID PARENTERAL P2Y12 RECEPTOR ANTAGONIST
Cangrelor (formerly known as AR-C69931MX) is an intravenously (IV) administered P2Y12 receptor antagonist under investigation for treatment of ACS and use during PCI and other coronary procedures. The compound is an adenosine triphosphate analogue with a plasma half-life of 5 to 9 minutes. Cangrelor is highly reversible, as platelet function returns to normal within 20 minutes of dosing. Within 15 minutes of initiation, cangrelor produces profound platelet inhibition and rapidly achieves steady state; peak effect occurs within minutes.22 The response to cangrelor is highly consistent, with virtually all recipients achieving the same degree of platelet inhibition. Platelet response approaches baseline 15 minutes after termination.22
If approved by the FDA, cangrelor would be administered similar to the way that glycoprotein IIb/IIIa inhibitors are, as it would be used primarily in the catheterization laboratory and then discontinued after the procedure, at which point transition to a long-term oral therapy would be necessary.
Clinical effects relative to abciximab
Cangrelor has been compared with the glycoprotein IIb/IIIa inhibitor abciximab and placebo in 249 patients undergoing elective or urgent PCI.22 Rates of the combined end point of death, MI, or need for repeat revascularization at 30 days were similar with cangrelor and abciximab (5.7% vs 5.4%, respectively; P = NS), both of which were lower than the rate with placebo (10.0%). Major or minor bleeding through 7 days occurred in numerically fewer cangrelor recipients compared with abciximab recipients (7.0% vs 9.0%), although the small sample size precluded evaluation for statistical significance.
Clinical effects relative to clopidogrel—the CHAMPION trials
A phase 3 trial program consisting of two multinational studies of cangrelor—the Cangrelor Versus Standard Therapy to Achieve Optimal Management of Platelet Inhibition (CHAMPION) program—is currently under way.
CHAMPION-PCI is enrolling 9,000 patients presenting with ACS who are being randomized in a double-blind fashion at the start of PCI to a 600-mg loading dose of clopidogrel or to cangrelor given as an IV bolus of 30 μg/kg followed by an IV infusion of 4 μg/kg/min. The primary end point is a composite of all-cause mortality, MI, or ischemia-driven revascularization in the 48 hours following randomization. Secondary end points include rates of all-cause mortality and MI at 48 hours.23
CHAMPION-PLATFORM is enrolling 4,400 patients scheduled for PCI as a result of ACS who are being randomized in a double-blind, double-dummy manner to (1) cangrelor bolus and infusion plus oral placebo or (2) oral clopidogrel plus placebo bolus and infusion before their index procedures. Dosages of the two agents are the same as in CHAMPION-PCI. The primary end point is a composite of death, MI, or urgent target vessel revascularization at 48 hours. Secondary end points include 30-day and 1-year clinical outcomes.23
The rationale for the CHAMPION investigations stems from the need to initiate clopidogrel before a patient is taken to the catheterization laboratory, owing to the inability to achieve a high degree of platelet inhibition until 4 to 6 hours after clopidogrel administration. Although this strategy can be undertaken without complication for most patients, a subset of patients with three-vessel disease or left-main disease will require CABG, which then must be delayed several days until clopidogrel’s platelet-inhibiting effect diminishes. A rapid-acting IV inhibitor of the P2Y12 receptor such as cangrelor would obviate this concern.
THROMBIN INHIBITORS
Thrombin plays an important role in platelet activation, and thrombin receptor antagonists may represent a safer means of inhibiting platelet activation relative to traditional antiplatelet agents. This theoretical safety advantage stems from the notion that blocking the action of platelets at the thrombin receptor would preserve platelets’ function as mediators of primary hemostasis. Because thrombin’s activation of platelets should occur only during clot formation, blocking platelet activation at the thrombin receptor would interrupt thrombin’s ability to propagate platelet activation during formation of coronary artery clots.
One agent in this class that is being studied extensively is SCH 530348, an oral thrombin receptor antagonist with potent antiplatelet activity. Its peak antiplatelet potency is achieved within hours when a loading dose is given, and within days without a loading dose. Wearing-off of the action of SCH 530348 takes weeks.24
Inhibition of platelet aggregation with thrombin receptor antagonists is measured in response to the thrombin receptor antagonist peptide (TRAP), not ADP. The proportion of subjects treated with SCH 530348 who achieve greater than 80% inhibition of platelet aggregation to 15 μM of TRAP ranges from 91% (with 0.5 mg of SCH 530348) to 100% (with 1.0 mg and 2.5 mg) at both 30 days and 60 days.25
Clinical effects in placebo-controlled trials
SCH 530348 was studied in the Thrombin Receptor Antagonist (TRA)–PCI trial, a dose-ranging study in which patients were randomized to one of three oral loading doses of the study drug (10 mg, 20 mg, or 40 mg) on top of a clopidogrel loading dose before undergoing cardiac catheterization for planned PCI; patients were then randomized to one of three maintenance doses of SCH 530348 (0.5 mg, 1.0 mg, or 2.5 mg) or placebo (depending on loading therapy) for 60 days.25
Among the 573 patients undergoing PCI , the rate of TIMI major or minor bleeding was not significantly higher with any dose of SCH 530348 compared with placebo,25 supporting the hypothesis that thrombin receptor antagonism inhibits platelet aggregation without a significant increase in bleeding.
Although the TRA-PCI study was not powered to detect differences in clinical event rates, a reduction in the rate of major adverse cardiovascular events was observed in a dose-dependent manner with SCH 530348 compared with placebo in the PCI cohort.25
On the basis of the TRA-PCI trial, a pair of phase 3 trials of SCH 530348 have been launched—the Thrombin Receptor Antagonist in Secondary Prevention of Atherothrombotic Ischemic Events (TRA 2°P-TIMI 50) study and the Thrombin Receptor Antagonist for Clinical Event Reduction in ACS (TRA-CER) study.
TRA 2°P-TIMI 50 is a multinational double-blind study enrolling 19,500 patients with prior MI or stroke or with existing peripheral arterial disease. Patients are being randomized to placebo plus standard medical care (including aspirin and clopidogrel) or to 2.5 mg of SCH 530348 once daily plus standard medical care. The primary end point is the composite of cardiovascular death, MI, urgent coronary revascularization, or stroke.26
TRA-CER is a multinational double-blind study with planned enrollment of 10,000 patients with non-ST-segment-elevation MI. Patients are being randomized to placebo plus standard medical care (including aspirin or clopidogrel) or to SCH 530348 (using the oral 40-mg loading dose and a maintenance dose of 2.5 mg once daily) plus standard medical care. The primary end point is the composite of cardiovascular death, MI, rehospitalization for ACS, urgent coronary revascularization, or stroke. The key secondary end point is the composite of cardiovascular death, MI, or stroke.27
COMPARATIVE CONSIDERATIONS
Inhibition of platelet aggregation
Clopidogrel achieves about 30% inhibition of platelet aggregation to ADP at its current FDA-approved loading dose of 300 mg and about 40% inhibition when its dose is doubled to 600 mg. These levels of inhibition are increased to 75% to 80% by clopidogrel’s fellow thienopyridine prasugrel, and this increase is attributable to prasugrel’s more efficient metabolism from prodrug to active metabolite. The reversible P2Y12 receptor antagonist AZD6140 achieves a comparable 75% to 80% inhibition of platelet aggregation. The parenterally administered P2Y12 receptor antagonist cangrelor achieves greater than 90% inhibition, as does the oral thrombin receptor antagonist SCH 530348, although the latter agent’s inhibition is to the agonist TRAP rather than ADP.
Time to peak effect
The time to peak effect with clopidogrel is approximately 4 hours regardless of the loading dose used (300 mg or 600 mg); this is substantially reduced with all of the investigational agents except SCH 530348. The novel agents’ reduced time to peak effect can offer advantages in speeding patients’ readiness to undergo catheterization procedures. This is particularly true for the IV agent cangrelor, which achieves its peak effect within minutes, although the 1-hour to 2-hour time frame with oral agents prasugrel and AZD6140 also would usually obviate any need to delay catheterization.
Consistency of platelet response
Standard-dose clopidogrel has the least consistency of platelet response among the therapies reviewed. Although increasing the clopidogrel dose yields somewhat greater consistency in response, it is still lower than the very high degrees of consistency observed with all of the novel compounds, each of which appears to achieve the same degree of inhibition of aggregation in virtually all patients.
Offset of effect
Both of the thienopyridines—clopidogrel and prasugrel—have an offset of effect of about 5 days, which requires delay of surgery, if possible, for several days in patients taking these agents. This is not an issue for the reversible oral agent AZD6140, whose offset of action takes just 1 to 2 days. While this rapid wearing-off of effect translates to a potential advantage for AZD6140, it also poses the potential drawback that a missed dose or two may leave the patient exposed to the risk of a thrombotic event. Cangrelor’s rapid offset of 20 minutes promotes its envisioned use as a catheterization lab–based medication like the glycoprotein IIb/IIIa inhibitors that can be started right before a PCI procedure and stopped immediately afterward. Because SCH 530348 has a very long half-life and thus a weeks-long washout period, the practicality of its use may depend on the hypothesis that thrombin receptor antagonists do not interfere with primary hemostasis, which is supported by data to date but remains to be definitively confirmed.
CONCLUSIONS
Clopidogrel achieves modest platelet inhibition with wide variability in response. Higher doses of clopidogrel achieve modestly greater degrees of inhibition than standard doses, and appear to result in a decreased rate of ischemic events. Although higher doses of clopidogrel have been embraced by some clinicians, we await definitive phase 3 trial evidence of net benefit before making high-dose clopidogrel the new standard of care.
Compared with clopidogrel, the investigational thienopyridine prasugrel is a more potent and consistent blocker of the ADP receptor. It results in a decreased rate of ischemic events relative to clopidogrel, including a 50% reduction in the rate of stent thrombosis, but is associated with an increased rate of bleeding. If prasugrel is approved for marketing, its use should be avoided in patients with a history of stroke or TIA, and avoidance or dose adjustment may be necessary in patients aged 75 years or older and in patients weighing less than 60 kg.
Other novel antiplatelet agents being evaluated for use in patients with ACS—the reversible oral ADP receptor blocker AZD6140, the rapid-acting IV ADP receptor blocker cangrelor, and oral thrombin receptor antagonists—offer potential advantages that need to be examined in the context of large-scale clinical trials.
- Wiviott SD, Trenk D, Frelinger AL, et al. Prasugrel compared with high loading- and maintenance-dose clopidogrel in patients with planned percutaneous coronary intervention: the Prasugrel in Comparison to Clopidogrel for Inhibition of Platelet Activation and Aggregation–Thrombolysis in Myocardial Infarction 44 trial. Circulation 2007; 116:2923–2932.
- Serebruany VL, Steinhubl SR, Berger PB, Malinin AI, Bhatt DL, Topol EJ. Variability in platelet responsiveness to clopidogrel among 544 individuals. J Am Coll Cardiol 2005; 45:246–251.
- Matetzky S, Shenkman B, Guetta V, et al. Clopidogrel resistance is associated with increased risk of recurrent atherothrombotic events in patients with acute myocardial infarction. Circulation 2004; 109:3171–3175.
- Gurbel PA, Bliden KP, Samara W, et al. Clopidogrel effect on platelet reactivity in patients with stent thrombosis: results of the CREST Study. J Am Coll Cardiol 2005; 46:1827–1832.
- Gurbel PA, Bliden KP, Hayes KM, Yoho JA, Herzog WR, Tantry US. The relation of dosing to clopidogrel responsiveness and the incidence of high post-treatment platelet aggregation in patients undergoing coronary stenting. J Am Coll Cardiol 2005; 45:1392–1396.
- von Beckerath N, Taubert D, Pogatsa-Murray G, Schömig E, Kastrati A, Schömig A. Absorption, metabolization, and antiplatelet effects of 300-, 600-, and 900-mg loading doses of clopidogrel: results of the ISAR-CHOICE (Intracoronary Stenting and Antithrombotic Regimen: Choose Between 3 High Oral Doses for Immediate Clopidogrel Effect) Trial. Circulation 2005; 112:2946–2950.
- Patti G, Colonna G, Pasceri V, Pepe LL, Montinaro A, Di Sciascio G. Randomized trial of high loading dose of clopidogrel for reduction of periprocedural myocardial infarction in patients undergoing coronary intervention: results from the ARMYDA-2 (Antiplatelet therapy for Reduction of MYocardial Damage during Angioplasty) study. Circulation 2005; 111:2099–2106.
- Cuisset T, Frere C, Quilici J, et al. Benefit of a 600-mg loading dose of clopidogrel on platelet reactivity and clinical outcomes in patients with non-ST-segment elevation acute coronary syndrome undergoing coronary stenting. J Am Coll Cardiol 2006; 48:1339–1345.
- von Beckerath N, Kastrati A, Wieczorek A, et al. A double-blind, randomized study on platelet aggregation in patients treated with a daily dose of 150 or 75 mg of clopidogrel for 30 days. Eur Heart J 2007; 28:1814–1819.
- Angiolillo DJ, Shoemaker SB, Desai B, et al. Randomized comparison of a high clopidogrel maintenance dose in patients with diabetes mellitus and coronary artery disease: results of the Optimizing Antiplatelet Therapy in Diabetes Mellitus (OPTIMUS) study. Circulation 2007; 115:708–716.
- Clopidogrel optimal loading dose usage to reduce recurrent events/optimal antiplatelet strategy for interventions (CURRENT/OASIS7). Clinical Trials.gov Web site. http://clinicaltrials.gov/ct2/show/NCT00335452. Updated September 1, 2008. Accessed December 16, 2008.
- Bonello L, Camoin-Jau L, Arques S, et al. Adjusted clopidogrel loading doses according to vasodilator-stimulated phosphoprotein phosphorylation index decrease rate of major adverse cardiovascular events in patients with clopidogrel resistance: a multicenter randomized prospective study. J Am Coll Cardiol 2008; 51:1404–1411.
- Brandt JT, Payne CD, Wiviott SD, et al. A comparison of prasugrel and clopidogrel loading doses on platelet function: magnitude of platelet inhibition is related to active metabolic formation. Am Heart J 2007; 153:66.e9–e16.
- Herbert JM, Savi P. P2Y12, a new platelet ADP receptor, target of clopidogrel. Semin Vasc Med 2003; 3:113–122.
- Wiviott SD, Braunwald E, McCabe CH, et al. Prasugrel versus clopidogrel in patients with acute coronary syndromes. N Engl J Med 2007; 357:2001–2015.
- Wiviott SD, Braunwald E, Angiolillo DJ, et al. Greater clinical benefit of more intensive oral antiplatelet therapy with prasugrel in patients with diabetes mellitus in the Trial to Assess Improvement in Therapeutic Outcomes by Optimizing Platelet Inhibition with Prasugrel–Thrombolysis in Myocardial Infarction 38. Circulation 2008; 118:1626–1636.
- Wiviott SD, Braunwald E, McCabe CH, et al. Intensive oral antiplatelet therapy for reduction of ischaemic events including stent thrombosis in patients with acute coronary syndromes treated with percutaneous coronary intervention and stenting in the TRITON-TIMI 38 trial: a subanalysis of a randomised trial. Lancet 2008; 371:1353–1363.
- Mauri L, Hsieh WH, Massaro JM, Ho KK, D’Agostino R, Cutlip DE. Stent thrombosis in randomized clinical trials of drug-eluting stents. N Engl J Med 2007; 356:1020–1029.
- Storey RF, Husted S, Harrington RA, et al. Inhibition of platelet aggregation by AZD6140, a reversible oral P2Y12 receptor antagonist, compared with clopidogrel in patients with acute coronary syndromes. J Am Coll Cardiol 2007; 50:1852–1856.
- Cannon CP, Husted S, Harrington RA, et al. Safety, tolerability, and initial efficacy of AZD6140, the first reversible oral adenosine diphosphate receptor antagonist, compared with clopidogrel, in patients with non-ST-segment elevation acute coronary syndrome: primary results of the DISPERSE-2 trial. J Am Coll Cardiol 2007; 50:1844–1851.
- A comparison of AZD6140 and clopidogrel in patients with acute coronary syndrome (PLATO). Clinical Trials.gov Web site. http://clinicaltrials.gov/ct2/show/NCT00391872. Updated December 3, 2008. Accessed December 5, 2008.
- Greenbaum AB, Grines CL, Bittl JA, et al. Initial experience with an intravenous P2Y12 platelet receptor antagonist in patients undergoing percutaneous coronary intervention: results from a 2-part, phase II, multicenter, randomized, placebo- and active-controlled trial. Am Heart J 2006; 151:689.e1–689.e10.
- A clinical trial to demonstrate the efficacy of cangrelor (PCI). Clinical Trials.gov Web site. http://www.clinicaltrials.gov/ct/show/nct00305162. Updated December 3, 2008. Accessed December 5, 2008.
- Bhatt DL, Topol EJ. Scientific and therapeutic advances in antiplatelet therapy. Nat Rev Drug Discov 2003; 2:15–28.
- Moliterno DJ, Becker RC, Jennings LK, et al; TRA-PCI Study Investigators. Results of a multinational randomized, double-blind, placebo-controlled study of a novel thrombin receptor antagonist (SCH 530348) in percutaneous coronary intervention. Presented at: 56th Annual Scientific Session of the American College of Cardiology; March 24–27, 2007; New Orleans, LA.
- Trial to assess the effects of SCH 530348 in preventing heart attack and stroke in patients with atherosclerosis (TRA 2°P-TIMI 50). Clinical Trials.gov Web site. http://clinicaltrials.gov/ct2/show/NCT00526474. Updated November 13, 2008. Accessed December 16, 2008.
- Trial to assess the effects of SCH 530348 in preventing heart attack and stroke in patients with acute coronary syndrome (TRA-CER). Clinical Trials.gov Web site. http://clinicaltrials.gov/ct2/show/NCT00527943. Updated November 12, 2008. Accessed December 16, 2008.
- Wiviott SD, Trenk D, Frelinger AL, et al. Prasugrel compared with high loading- and maintenance-dose clopidogrel in patients with planned percutaneous coronary intervention: the Prasugrel in Comparison to Clopidogrel for Inhibition of Platelet Activation and Aggregation–Thrombolysis in Myocardial Infarction 44 trial. Circulation 2007; 116:2923–2932.
- Serebruany VL, Steinhubl SR, Berger PB, Malinin AI, Bhatt DL, Topol EJ. Variability in platelet responsiveness to clopidogrel among 544 individuals. J Am Coll Cardiol 2005; 45:246–251.
- Matetzky S, Shenkman B, Guetta V, et al. Clopidogrel resistance is associated with increased risk of recurrent atherothrombotic events in patients with acute myocardial infarction. Circulation 2004; 109:3171–3175.
- Gurbel PA, Bliden KP, Samara W, et al. Clopidogrel effect on platelet reactivity in patients with stent thrombosis: results of the CREST Study. J Am Coll Cardiol 2005; 46:1827–1832.
- Gurbel PA, Bliden KP, Hayes KM, Yoho JA, Herzog WR, Tantry US. The relation of dosing to clopidogrel responsiveness and the incidence of high post-treatment platelet aggregation in patients undergoing coronary stenting. J Am Coll Cardiol 2005; 45:1392–1396.
- von Beckerath N, Taubert D, Pogatsa-Murray G, Schömig E, Kastrati A, Schömig A. Absorption, metabolization, and antiplatelet effects of 300-, 600-, and 900-mg loading doses of clopidogrel: results of the ISAR-CHOICE (Intracoronary Stenting and Antithrombotic Regimen: Choose Between 3 High Oral Doses for Immediate Clopidogrel Effect) Trial. Circulation 2005; 112:2946–2950.
- Patti G, Colonna G, Pasceri V, Pepe LL, Montinaro A, Di Sciascio G. Randomized trial of high loading dose of clopidogrel for reduction of periprocedural myocardial infarction in patients undergoing coronary intervention: results from the ARMYDA-2 (Antiplatelet therapy for Reduction of MYocardial Damage during Angioplasty) study. Circulation 2005; 111:2099–2106.
- Cuisset T, Frere C, Quilici J, et al. Benefit of a 600-mg loading dose of clopidogrel on platelet reactivity and clinical outcomes in patients with non-ST-segment elevation acute coronary syndrome undergoing coronary stenting. J Am Coll Cardiol 2006; 48:1339–1345.
- von Beckerath N, Kastrati A, Wieczorek A, et al. A double-blind, randomized study on platelet aggregation in patients treated with a daily dose of 150 or 75 mg of clopidogrel for 30 days. Eur Heart J 2007; 28:1814–1819.
- Angiolillo DJ, Shoemaker SB, Desai B, et al. Randomized comparison of a high clopidogrel maintenance dose in patients with diabetes mellitus and coronary artery disease: results of the Optimizing Antiplatelet Therapy in Diabetes Mellitus (OPTIMUS) study. Circulation 2007; 115:708–716.
- Clopidogrel optimal loading dose usage to reduce recurrent events/optimal antiplatelet strategy for interventions (CURRENT/OASIS7). Clinical Trials.gov Web site. http://clinicaltrials.gov/ct2/show/NCT00335452. Updated September 1, 2008. Accessed December 16, 2008.
- Bonello L, Camoin-Jau L, Arques S, et al. Adjusted clopidogrel loading doses according to vasodilator-stimulated phosphoprotein phosphorylation index decrease rate of major adverse cardiovascular events in patients with clopidogrel resistance: a multicenter randomized prospective study. J Am Coll Cardiol 2008; 51:1404–1411.
- Brandt JT, Payne CD, Wiviott SD, et al. A comparison of prasugrel and clopidogrel loading doses on platelet function: magnitude of platelet inhibition is related to active metabolic formation. Am Heart J 2007; 153:66.e9–e16.
- Herbert JM, Savi P. P2Y12, a new platelet ADP receptor, target of clopidogrel. Semin Vasc Med 2003; 3:113–122.
- Wiviott SD, Braunwald E, McCabe CH, et al. Prasugrel versus clopidogrel in patients with acute coronary syndromes. N Engl J Med 2007; 357:2001–2015.
- Wiviott SD, Braunwald E, Angiolillo DJ, et al. Greater clinical benefit of more intensive oral antiplatelet therapy with prasugrel in patients with diabetes mellitus in the Trial to Assess Improvement in Therapeutic Outcomes by Optimizing Platelet Inhibition with Prasugrel–Thrombolysis in Myocardial Infarction 38. Circulation 2008; 118:1626–1636.
- Wiviott SD, Braunwald E, McCabe CH, et al. Intensive oral antiplatelet therapy for reduction of ischaemic events including stent thrombosis in patients with acute coronary syndromes treated with percutaneous coronary intervention and stenting in the TRITON-TIMI 38 trial: a subanalysis of a randomised trial. Lancet 2008; 371:1353–1363.
- Mauri L, Hsieh WH, Massaro JM, Ho KK, D’Agostino R, Cutlip DE. Stent thrombosis in randomized clinical trials of drug-eluting stents. N Engl J Med 2007; 356:1020–1029.
- Storey RF, Husted S, Harrington RA, et al. Inhibition of platelet aggregation by AZD6140, a reversible oral P2Y12 receptor antagonist, compared with clopidogrel in patients with acute coronary syndromes. J Am Coll Cardiol 2007; 50:1852–1856.
- Cannon CP, Husted S, Harrington RA, et al. Safety, tolerability, and initial efficacy of AZD6140, the first reversible oral adenosine diphosphate receptor antagonist, compared with clopidogrel, in patients with non-ST-segment elevation acute coronary syndrome: primary results of the DISPERSE-2 trial. J Am Coll Cardiol 2007; 50:1844–1851.
- A comparison of AZD6140 and clopidogrel in patients with acute coronary syndrome (PLATO). Clinical Trials.gov Web site. http://clinicaltrials.gov/ct2/show/NCT00391872. Updated December 3, 2008. Accessed December 5, 2008.
- Greenbaum AB, Grines CL, Bittl JA, et al. Initial experience with an intravenous P2Y12 platelet receptor antagonist in patients undergoing percutaneous coronary intervention: results from a 2-part, phase II, multicenter, randomized, placebo- and active-controlled trial. Am Heart J 2006; 151:689.e1–689.e10.
- A clinical trial to demonstrate the efficacy of cangrelor (PCI). Clinical Trials.gov Web site. http://www.clinicaltrials.gov/ct/show/nct00305162. Updated December 3, 2008. Accessed December 5, 2008.
- Bhatt DL, Topol EJ. Scientific and therapeutic advances in antiplatelet therapy. Nat Rev Drug Discov 2003; 2:15–28.
- Moliterno DJ, Becker RC, Jennings LK, et al; TRA-PCI Study Investigators. Results of a multinational randomized, double-blind, placebo-controlled study of a novel thrombin receptor antagonist (SCH 530348) in percutaneous coronary intervention. Presented at: 56th Annual Scientific Session of the American College of Cardiology; March 24–27, 2007; New Orleans, LA.
- Trial to assess the effects of SCH 530348 in preventing heart attack and stroke in patients with atherosclerosis (TRA 2°P-TIMI 50). Clinical Trials.gov Web site. http://clinicaltrials.gov/ct2/show/NCT00526474. Updated November 13, 2008. Accessed December 16, 2008.
- Trial to assess the effects of SCH 530348 in preventing heart attack and stroke in patients with acute coronary syndrome (TRA-CER). Clinical Trials.gov Web site. http://clinicaltrials.gov/ct2/show/NCT00527943. Updated November 12, 2008. Accessed December 16, 2008.
KEY POINTS
- There is substantial interpatient variability in the response to clopidogrel.
- In the large TRITON-TIMI 38 trial, the composite rate of death, myocardial infarction, or stroke was reduced by 19% and the rate of stent thrombosis was halved in patients receiving prasugrel compared with standard-dose clopidogrel.
- The risk of major bleeding with prasugrel is highest in patients aged 75 or older, those weighing less than 60 kg, and those with a history of stroke or transient ischemic attack.
- Thrombin receptor antagonists are being studied to see if their use can reduce ischemic events without increasing bleeding.
Platelet response in practice: Applying new insights and tools for testing and treatment
CASE STUDY: THROMBOSIS AFTER STENTING DESPITE ANTIPLATELET THERAPY
Dr. Deepak Bhatt: We have taken in a wealth of terrific information from the three preceding talks in this symposium. Let’s now share some questions from the audience and explore some of the points raised in the preceding talks in a bit more practical detail for clinicians. Our three prior speakers are joined in this panel discussion by Cleveland Clinic’s Dr. Frank Peacock, who brings an emergency medicine perspective.
Let’s begin with a case-based question supplied from the audience. The patient is a 42-year-old morbidly obese man without diabetes who had a non-ST-elevation myocardial infarction (MI) less than 1 year ago. A drug-eluting stent was placed at the time of his MI, and now restenosis has occurred. He is on aspirin and clopidogrel 75 mg/day. Do you recommend running a vasodilator-stimulated phosphoprotein (VASP) test and possibly increasing the clopidogrel dose to 150 mg/ day, or should the patient just be switched to prasugrel (assuming it is commercially available) without running the VASP test?
I’ll take a quick initial stab at this question. Studies of antiplatelet therapies to prevent instent restenosis have been a mixed bag. Some of the trials with glycoprotein IIb/IIIa inhibitors have shown an effect on restenosis, but most have not. Similarly, some of the analyses of the thienopyridines ticlopodine and clopidogrel have shown an effect on restenosis, but most have not.
For the most part, restenosis does not appear to be heavily mediated by platelets, at least not in a way that we can influence by therapy. On the other hand, stent thrombosis is highly platelet mediated, so I would alter the case to one in which stent thrombosis is the clinical problem. Assuming that the patient has been adherent to his antiplatelet regimen, which tests would you perform, and how would you act on the information from those tests?
Dr. Kandice Kottke-Marchant: The 2007 guidelines on acute coronary syndrome (ACS) management from the American College of Cardiology and American Heart Association (ACC/AHA)1 do not address platelet function testing, and almost none of the clinical trials of antiplatelet agents had an arm that included testing and dose adjustment based on platelet function studies. Platelet testing is available at some centers; at Cleveland Clinic, we use platelet aggregation testing. One can do platelet aggregation testing on a patient-by-patient basis; if inhibition appears to be suboptimal, a treatment decision should be made, but there is little guidance from the literature to steer that decision. I have seen clinicians increase the dose of clopidogrel or aspirin in response to platelet function tests, which occasionally triggers a confirmatory call from the pharmacy department.
Dr. Bhatt: When I was still at Cleveland Clinic, our chief medical resident did an analysis of platelet function testing, and it was remarkable how much testing was performed and how often it changed management, largely in the absence of any outcomes data, as Dr. Kottke-Marchant pointed out. Dr. Alexander, what are your recommendations with respect to platelet function testing today?
Dr. John Alexander: The case you describe is one in which applying evidence is not easy. There are no trials to supply any evidence to change therapy in this patient, a morbidly obese man receiving 75 mg/day of clopidogrel. There is certainly a rationale, however, to believe that a standard “one size fits all” 75-mg daily dose of clopidogrel may not be enough for him. The trade-off with a higher dosage is a higher risk of bleeding, however, so I would first be sure that he has been adherent to his current regimen of clopidogrel and aspirin.
Dr. Bhatt: Is there a role for point-of-care testing to determine whether he is adherent to the medicines?
Dr. Kottke-Marchant: Several of the point-of-care tests, such as the VerifyNow rapid platelet function analyzer, have specific cartridges for aspirin and for clopidogrel. If platelets were not being inhibited, it would suggest that the doses were too low, given the patient’s weight, but you probably would not be able to determine whether he was resistant to clopidogrel.
Dr. W. Frank Peacock: One way to verify that patients are taking their aspirin is to take a small urine sample and squirt in 2 mL of ferric chloride. If the sample turns purple, it means they are taking their aspirin. Once that is established, you can try to determine whether the drug is working on their platelets.
Dr. Alexander: Another potential explanation for stent thrombosis is faulty stent placement. In this case I would consider asking an interventional colleague to perform intravascular ultrasonography to make sure the stent was implanted properly before I changed the patient’s antithrombotic therapy.
Dr. Bhatt: That’s a great technical point. We always want to make sure that a case of stent thrombosis is not due to a mechanical problem. We should be asking: Is the stent properly sized and well opposed? Is there a distal dissection or any other issue that could predispose to stent thrombosis?
Dr. Alexander: This case illustrates a host of other challenges that underscore how much more work we need to do to define optimal antiplatelet therapy. Suppose we perform platelet function testing and find a low level of platelet inhibition in this patient with stent thrombosis, and we change his antiplatelet regimen. When should we test him again? If we retest in 3 months and find that he has a higher than expected level of platelet inhibition on the new antiplatelet regimen, do we dial down the intensity? Once again, there is no evidence to guide these decisions, and levels of platelet inhibition are driven not just by the medications but also by what is going on in the patient’s platelets—it is quite multifactorial.
POINT-OF-CARE PLATELET FUNCTION TESTING: CURRENT LIMITS, FUTURE ROLES
Dr. Bhatt: While we’re discussing platelet function testing, I found it interesting, Dr. Kottke-Marchant, that you said the use of bleeding time as a platelet test is finally going away. Testing of bleeding time has been around forever, but I agree that it doesn’t have much value in clinical practice. Do you think bleeding time will continue to have any role in drug development? Most phase 2 trials, and certainly phase 1 trials, still capture bleeding time to assess whether or not a drug is working. Should that, too, be jettisoned, or does bleeding time still have some merit in this context?
Dr. Kottke-Marchant: I would jettison it in drug development as well because of the considerable variability in bleeding time. It is not a test that can be standardized, and no quality control can be done. The results depend on skin turgor, age, and many other variables.
We need a global assay that will pick up multiple aspects of platelet function, such as flow-based adhesion, aggregation, and granule release. The bleeding time is a shear-dependent test, whereas the platelet aggregation test that is used in most drug trials is an artificial assay that measures only aggregation, but not under shear. The VerifyNow rapid platelet function analyzer does not measure platelets under shear and is not a global assay.
Dr. Marc Sabatine: I would underscore the need for a reliable point-of-care test of platelet function. When we prescribe a statin or an antihypertensive drug, we don’t just send the patient out the door and hope that everything will be okay. We measure the response, knowing that genotype, environmental factors, or medication factors can affect the response. When we prescribe an antiplatelet drug, we need a reliable point-of-care device to make certain that the patient is getting appropriate platelet inhibition.
I am reminded of a recent study of point-of-care measurement of platelet inhibition in patients receiving clopidogrel prior to nonemergent percutaneous coronary intervention (PCI).2 Rather than just treating patients with PCI and sending them out the door, the investigators kept giving patients clopidogrel and measuring their platelet inhibition until they achieved an appropriate degree of inhibition, after which PCI was performed. Event rates were significantly reduced in the patient group treated this way, which suggests a need to individualize therapy and move away from the “one size fits all” mindset.
Dr. Bhatt: Dr. Peacock, you’ve led a study of point-of-care assays in the emergency department. What might ultimately be the role of point-of-care testing in emergency medicine, and might it influence drug selection?
Dr. Peacock: My short answer is that I think there will be a role for point-of-care testing, with all the caveats that have been discussed. There may even be a day when we do genetic testing and look for DNA. Honestly, though, I’m somewhat of a skeptic because I’m not looking at the genetics. I see many patients who do crack cocaine who come to the emergency room with chest pain and have risk factors, but I send these patients home because they are not having an event. The real question is, “Is it an event?” If a patient is having an event and he or she has platelet resistance or hyperreactivity—whatever we term it—then you have to decide the next step.
As you mentioned, we just completed a study that evaluated a couple hundred patients for platelet inhibition resistance to aspirin, and one finding was that the incidence of platelet resistance to aspirin was much lower than we had anticipated. Studies from the literature suggest that the prevalence of resistance is around 30%, but in our study it was 6.5%.3
Dr. Kottke-Marchant: It depends on how and in whom you measure resistance. Different tests will give you different numbers. Even among studies using the same measurement techniques, the results depend on the patient population. If it’s a fairly stable cardiac population, you may see aspirin resistance rates of 4% or 5%. If it’s a population of patients who have had multiple MIs, the rate may be higher.
Dr. Peacock: That’s exactly my point. In the emergency department we see a mixed bag. We see many people who have had no prior events and have no acute event occurring. So in that setting you are going to get results that suggest that no intervention is required, whereas in that small percentage of patients in whom something is happening, your drug choice may be different.
Dr. Alexander: We are still talking about resistance to antiplatelet drugs as though it were a patient-level variable, but it’s my impression that it changes over time and within a patient.
Dr. Kottke-Marchant: It can change over time. There aren’t many good longitudinal studies. Most of the studies of “aspirin resistance” are really snapshot studies with measurements taken at one point in time. A term I prefer is “platelet reactivity.” To really assess treatment efficacy, we are going to have to look at the basal level of platelet reactivity.
WHAT ROLE FOR GENOTYPING IN GUIDING ANTIPLATELET THERAPY?
Dr. Bhatt: Dr. Peacock alluded to a potential role for genetic testing. Dr. Sabatine, you have done a lot of interesting work with genotyping in the TRITON-TIMI 38 study of prasugrel and clopidogrel. What is the future role of genotyping in determining which antiplatelet therapy is best for which patient?
Dr. Sabatine: As I mentioned, cytochrome P450 enzymes play a critical role in the metabolism of clopidogrel. These enzymes are fairly polymorphic—mutations in their encoding genes are responsible for subtle changes in effect, unlike the traditional mutations that we think about for sickle cell disease, for example. A wealth of data has been published showing that genetic variants are associated with decreased functional activity of cytochrome P450 enzymes, demonstrating the pharmacologic importance of these variants.
Individuals who carry variant alleles appear to respond differently to clopidogrel. A variety of small studies show that those who carry specific variants—particularly in the CYP2C19 enzyme, but in other enzymes as well—appear to have a diminished response to clopidogrel. There are also data showing that individuals with a diminished response to clopidogrel have worse outcomes.4 Our group is studying the impact of genetic variants that decrease the functional activity of cytochrome P450 enzymes on clinical outcomes. (Editor’s note: This study has since been published by Mega et al.5)
The practical implication may lie in point-of-care genotyping, which appears possible and will be clinically useful if a strong link can be demonstrated between genotype and outcomes. If point-of-care genotyping becomes practical, it will raise the question of whether both genotyping and platelet aggregation testing are needed. I think they might indeed be complementary in risk prediction, as is the case with genetic variants that affect low-density lipoprotein cholesterol (LDL-C) levels. In the lipid arena, we have seen that genetic effects and lipid levels provide independent incremental information about risk. That’s because of the high degree of variation in LDL-C levels: an LDL-C measurement is a snapshot in time, yet a variety of factors can influence LDL-C levels. In contrast, genotype is an invariant factor. Similarly, in the platelet arena, platelet aggregation studies and genotyping may be synergistic in predicting an individual’s predisposition to events and response to medications.
Dr. Bhatt: While we’re discussing pathways of metabolism, the literature, though scant, suggests a potential interaction between proton pump inhibitors and clopidogrel. I was co-chair of a recent American College of Cardiology/ American Heart Association/American College of Gastro-enterology consensus document that endorsed liberal use of proton pump inhibitors in patients who are at gastrointestinal risk, including those on antiplatelet therapy.6 The gastroenterologists believed strongly that proton pump inhibitors were safe and in fact underused in these patients. What do you think about the clopidogrel–proton pump inhibitor interaction? Should we be concerned?
Dr. Sabatine: Proton pump inhibitors are not only substrates for, but also inhibitors of, CYP2C19, a key enzyme that helps transform clopidogrel into an active metabolite. For this reason, there has been interest in whether concomitant use of proton pump inhibitors would blunt the efficacy of clopidogrel. The same concern was raised about giving clopidogrel with certain statin drugs that are also metabolized by the cytochrome P450 system, and several studies have shown an effect of these statins on clopidogrel’s platelet inhibition. However, there is no evidence that coadministration of these statins has affected clinical outcomes with clopidogrel in clinical trials. So it may be that while competition for the cytochrome P450 system is one factor, it’s not enough of a factor to tip the scale and result in a clinical event. The same may be true of coadministration of proton pump inhibitors; meanwhile, we await definitive data that concomitant use with clopidogrel leads to higher rates of ischemic events.
DIAGNOSTIC UNCERTAINTY IN THE EMERGENCY SETTING
Dr. Bhatt: We heard about quite a few new antiplatelet drugs in Dr. Sabatine’s presentation, some of which will likely be taken up in clinical practice. Dr. Peacock, from an emergency department perspective, how will you integrate all these new agents with the numerous therapies already available? What should emergency departments do to come to grips with and ultimately take advantage of these different forms of therapy as well as emerging platelet function tests?
Dr. Peacock: The piece that’s unique or especially pertinent to the emergency department is diagnostic uncertainty. Diagnosis and management are easy when a patient has an ST-elevation MI because we all know what that looks like and we know what to do in response. To some extent non-ST-elevation MI is fairly simple too. ACS is a lot more difficult because we don’t have a good definition for unstable angina, and that’s where diagnosis and management become problematic. And with high-sensitivity troponins coming out now, the question of non-ST-elevation MI is going to get more and more confusing because we will have a lot more patients who meet criteria without having an acute coronary artery event.
So it is going to be important that studies be designed correctly. A lot of the studies reviewed today were efficacy studies, showing that a particular drug works well in a carefully defined population, but they were not efficiency studies: they did not take into account the real-world diagnostic uncertainty—and inevitable misdiagnoses—that emergency departments encounter before starting therapy.
Take the CURE trial, for example. It was a great study, showing that clopidogrel reduced the hazard ratio for major coronary events by 20% in patients with unstable angina,7 and the message was that everybody should be using clopidogrel. A close look at the study, however, reveals that about half the patients did not receive clopidogrel in the emergency department. When patients did receive it early, it was driven by the cardiologist, who was absolutely certain of the diagnosis. But if the study was not designed to test early use, then it is a big leap to extrapolate its findings to this circumstance.
Many of the patients in the CURE trial were enrolled the day after presentation, when their diagnosis was certain—ie, they had a rise in troponin after their symptoms. But when a patient first arrives in the emergency department, we are not certain of the diagnosis. And if we use a drug such as clopidogrel, with a duration of action as long as 5 days, we have committed the entire medical system to a certain course of management for that period of time. If we get the diagnosis wrong, this commitment could restrict management options for up to 5 days.
The question for emergency physicians becomes, “How long is long enough to know whether I can pull the trigger on a therapy and be correct?” With all the new drugs coming along, the way to answer this is to do efficiency studies in a real-world environment in addition to efficacy studies.
Dr. Alexander: Yes, one of the biggest limitations of antiplatelet drug studies to date is that they usually haven’t really addressed the timing of drug initiation. We often assume that if a drug is shown to be beneficial, then it should be started as soon as possible. As we just heard, that may have been an unfounded extrapolation from the CURE trial. The same sort of thing happened with the ISIS trial of aspirin in patients with ST-elevation MI.8 In response to the ISIS results, clinicians rushed to give patients aspirin right away even though many of the patients in the trial may have received their aspirin the day after presentation. For these reasons, the EARLY-ACS study,9 which is addressing a very simple question—whether early upstream use of glycoprotein IIb/IIIa inhibitors is beneficial—has been a challenging trial to complete.
WHAT ROLE FOR THIENOPYRIDINE PRETREATMENT?
Dr. Bhatt: Dr. Sabatine, you presented data from the large TRITON-TIMI 38 trial comparing prasugrel with clopidogrel. I’m interested in how you would use prasugrel in practice, assuming it receives marketing approval, especially in light of its bleeding risk, particularly in patients in whom coronary artery bypass graft surgery (CABG) is planned. Many hospitals pretreat patients with clopidogrel in the emergency department. How would you manage a patient who shows up in the emergency room with ACS? Would you give clopidogrel, would you wait and give prasugrel, or would you do something else? If you gave clopidogrel, what loading dose would you use—300 mg, 600 mg, or, as some have suggested, 900 or 1,200 mg?
Dr. Sabatine: I am a strong proponent of pretreatment. Data from multiple studies show a benefit to this strategy, and even the original CURE trial showed a roughly 30% reduction in ischemic events within the first 24 hours of clopidogrel initiation.7
I think the dosing strategy depends on how the patient is going to be managed. If management is going to be conservative, then I would start the patient on 300 mg of clopidogrel when he or she came in. If the patient is going to the cardiac catheterization laboratory in a few hours, I would pretreat with 600 mg of clopidogrel. For prasugrel, the need for pretreatment is less clear, given the drug’s faster onset of action and greater degree of platelet inhibition. In the TRITON-TIMI 38 study,10 prasugrel was given, by and large, after diagnostic angiography, and thus one could use that approach in practice.
In terms of clopidogrel versus prasugrel, I would embrace prasugrel for the large majority of my patients, being mindful of the risk of bleeding. I would not hesitate to give the medication to diabetics or to younger, more robust patients. The 50% reduction in stent thrombosis with prasugrel versus clopidogrel in TRITON-TIMI 38 is huge,11 given that the risk of death with stent thrombosis is probably 25% or greater. So I would want to have prasugrel on board to reduce the risk of stent thrombosis, especially if a drug-eluting stent were being implanted.
Dr. Bhatt: Dr. Alexander, let’s get your take on a similar scenario. Assuming that prasugrel gains marketing approval, how would you manage patients with non-ST-elevation MI who present to the emergency department? Would you pretreat with clopidogrel? Would you wait until angiography and then, depending on the anatomy, treat with prasugrel? Or would you potentially pretreat with prasugrel, which has not been studied and would not be a labeled indication? How would you reconcile the data?
Dr. Alexander: At Duke, I expect that prasugrel will not be used prior to the catheterization laboratory in patients with non-ST-elevation ACS due to concerns about whether the patients will undergo PCI or be managed medically or with CABG.
Dr. Bhatt: That makes sense, since there was a fair amount of bleeding with prasugrel in those patients in TRITON-TIMI 38.
Dr. Alexander: Correct. Moreover, at Duke we don’t use as much upstream clopidogrel as we would, based on the evidence, if I were managing all the patients. There is still a lot of pushback about upstream clopidogrel from our surgeons because patients are going to surgery quickly these days, sometimes just a day after catheterization, and that’s when a loading dose of clopidogrel can be problematic. We are also still fairly heavy users of glycoprotein IIb/IIIa inhibitors.
Where I can see prasugrel being used prior to the cath lab at Duke is in ST-elevation MI, where the rate of PCI is very high. In primary angioplasty for ST-elevation MI, it would likely be given upstream. The bigger issue for us will be that we serve as a referral base for a lot of regional hospitals, and thus have some influence on their practices.
Dr. Bhatt: In that case, what would you advise those regional hospitals to do for non-ST-elevation MI?
Dr. Alexander: For the time being, we would advise continuing with our current practice, which is to load clopidogrel in patients in whom there is a reasonable certainty that CABG will not be performed, and to use glycoprotein IIb/IIIa inhibitors in high-risk patients. As we get more experience with prasugrel or with additional trial results, however, that practice could easily change.
Dr. Bhatt: So you would still use glycoprotein IIb/IIIa inhibitors?
Dr. Alexander: Yes, I advocate upstream clopidogrel use, but not all my colleagues do. Based on the guidelines, I’d use one or the other—either clopidogrel or a glycoprotein IIb/IIIa inhibitor. As I mentioned in my talk, if a patient is at high risk for bleeding, I am more inclined to use clopidogrel, although patients at higher risk of bleeding are often at higher risk for ischemic events as well.
WHAT’S DRIVEN THE DROPOFF IN GLYCOPROTEIN IIb/IIIa INHIBITOR USE?
Dr. Bhatt: While we’re on the topic of glycoprotein IIb/IIIa inhibitors, a question card from the audience asks why there has been a decrease in glycoprotein IIb/ IIIa inhibitor use and whether this decline is appropriate or inappropriate. Have clopidogrel pretreatment, higher loading doses of clopidogrel, and use of the direct thrombin inhibitor bivalirudin contributed to the decrease in glycoprotein IIb/IIIa inhibitor use?
Dr. Alexander: I do think that the decline has been driven by the changing environment, with greater use of other antithrombotic strategies that include clopidogrel and bivalirudin, as you suggest, as well as an increased attention to bleeding. From an evidence-based standpoint, we don’t know whether the decrease in glycoprotein IIb/IIIa use is appropriate or not because the studies of these agents were conducted before the widespread upstream use of clopidogrel and bivalirudin. Clopidogrel is attractive because it’s a pill given as one dose in the emergency department, the wards, or the catheterization laboratory, rather than a much more complicated infusion with weight-based dosing and dosage adjustments based on creatinine clearance. It is possible that we should perhaps be dosing clopidogrel the same way, but we don’t know that yet.
PRASUGREL IN PRACTICE: HOW LOW CAN THE DOSE GO, AND IS THERE A GENDER EFFECT?
Dr. Bhatt: Let’s stick with this focus on dosing but turn back to discussion of prasugrel. In your presentation of the TRITON-TIMI 38 data, Dr. Sabatine, you proposed a potential prasugrel dosage modification, down to a 5-mg loading dose, in subgroups that were identified as being at high bleeding risk—namely, elderly patients and patients with low body weight. However, no outcomes data with 5 mg of prasugrel came out of TRITON-TIMI 38.10 Is this proposed modification based on pharmacokinetic extrapolation? Could clinicians be comfortable using 5 mg of prasugrel, assuming the drug receives regulatory approval and a 5-mg tablet would be available?
Dr. Sabatine: Of course, evidence at the grade A level would consist of a trial showing that patients who received a lower dose enjoyed the same benefit as those who got standard dosing in TRITON-TIMI 38—a 60-mg loading dose followed by 10 mg/day—with an acceptable risk profile. However, such a trial would be difficult and costly to conduct, and would take roughly half a decade to pull off. It is only through large trials like TRITON-TIMI 38 that you identify subgroups that respond differently, and then to go back and do a separate trial for those subgroups takes a great deal of time. It may not be practical.
I think the Food and Drug Administration is moving toward embracing careful pharmacokinetic/pharmacodynamic substudies within trials, with these substudies having adequate numbers of subjects to provide a sense for the ideal target dose and what an acceptable dose range would be, without limiting approval to a single dose. The analogy would be warfarin dosing, with the aim being to figure out an acceptable dose range, discover which patients fall outside that range, and then model the effect of a lower dose in those patients. Thus, approving a 5-mg dose of prasugrel based on TRITON-TIMI 38 would be a reasonable approach if this dose passed muster under pharmacokinetic/pharmacodynamic modeling. If this approach were taken, there would clearly be a need for postmarketing surveillance to confirm whether the modeling on the effects of the lower dose was borne out by actual outcomes.
Dr. Bhatt: The audience has posed another interesting question raised by TRITON-TIMI 38: Can you comment on the lesser effect of prasugrel in women?
Dr. Sabatine: It is true that there was not a statistically significant effect of prasugrel among women in TRITON-TIMI 38, but statistical tests among subgroups found no significant heterogeneity for the effect between men and women, and that is the relevant measure to determine any gender effect. Keep in mind that not all subgroups represent a univariate slice of the population. For example, women generally have lower body weight than men, and since prasugrel’s net clinical benefit was reduced in patients with lower body weight, that may explain some of the differing extent of effect between men and women.
Dr. Bhatt: That’s a good point about the lack of heterogeneity between men and women. In fact, a meta-analysis of clopidogrel data conducted by one of the fellows I work with revealed that men and women appear to benefit similarly from clopidogrel.12 There was a slight signal of excess bleeding in women, but there were more elderly women in the pooled population, which may have been a confounding factor. As best as anyone can tell, antiplate-let therapy works well in both men and women.
NAVIGATING MANAGEMENT ACROSS THE SPECTRUM OF CARE
Dr. Bhatt: I would like to explore a bit further how all of these issues translate across the spectrum of care, beginning in the emergency department, which we know is a key component of the entire ACS management strategy for a health care system. What should emergency medicine doctors do, given all of the potential options—clopidogrel, different loading doses of clopidogrel, prasugrel, glycoprotein IIb/IIIa inhibitors, even bivalirudin?
Dr. Peacock: It depends on the practice setting. Some emergency physicians work at community hospitals with no backup. They must have relationships with the larger centers to which they’ll be transferring patients, because ACS patients should not be staying at community hospitals. These emergency physicians must have close relationships with the physicians who will be receiving their patients, and they know the potential head-butting with surgeons surrounding early clopidogrel use better than anybody does. If they treat with clopidogrel in the emergency room, and it turns out that the patient needs to go to the catheterization laboratory, can the receiving hospital use platelet testing to shorten the standard 5-day interval from treatment to catheterization?
Dr. Bhatt: Yes, that’s a rather useful, although not completely validated, way of using point-of-care platelet testing—to potentially reduce the time to surgery.
Dr. Peacock: Right. So if the policies for handling these types of transfer-related issues are worked out in advance, all players have a pathway to follow, which can allow quick action when necessary. If you don’t have these issues worked out in advance, you either lose many opportunities to act quickly in the emergency room or you risk taking actions that will cause problems later in the course of management.
Dr. Alexander: I totally agree. The key is to sit down with all the players involved—the surgeons, the interventional cardiologists, the intensivists, the emergency room personnel—and come up with strategies for different populations of patients. Write down the collective strategy and hang it on the wall so that everybody can be comfortable with it. The strategy can be reevaluated when prasugrel or other new antithrombotic drugs come on the market.
Dr. Peacock: The other environment is the academic center, which is even more challenging, but for different reasons. At a large academic center like the Cleveland Clinic, any of 25 different cardiologists may be taking call and receiving patients from the emergency department on a particular night. A lot of phone interaction is required to elicit the planned management strategy, including if and when the patient will be going to the cath lab. Individualizing care to a particular cardiologist then becomes a time-consuming challenge, especially in clinical situations where outcomes are time-dependent.
Dr. Alexander: Agreed. Management needs to be integrated across the entire spectrum of care. The anticoagulants that we plan to use in the cath lab will affect the antithrombotic regimen used upstream.
Dr. Kottke-Marchant: One circumstance where platelet function testing has been helpful is in determining the washout of the clopidogrel effect before surgery. At Cleveland Clinic, we have implemented platelet function testing in this circumstance instead of waiting a blanket 5 days after clopidogrel administration to go to surgery. A return to normal platelet function on platelet aggregation testing, depending on the cutoff value used, is an indicator that the patient can proceed to surgery.
Dr. Bhatt: That’s a logical approach. How should we be using antiplatelet therapy in the medically managed patient, Dr. Alexander?
Dr. Alexander: When I think of medical management, I include patients who don’t go to the cath lab, but also those who do, with regards to their management prior to and following their time in the cath lab.
In patients who don’t go to the cath lab for angiography, the ACC/AHA guidelines recommend aspirin and either clopidogrel, a glycoprotein IIb/IIIa inhibitor, or both.1 In making this choice, I consider the patient’s risk of bleeding and the dosing complexity of the regimen, especially with the use of glycoprotein IIb/IIIa inhibitors in a patient with renal insufficiency. In a patient at relatively low risk for bleeding, I often use both clopidogrel and a glycoprotein IIb/IIIa inhibitor, although this strategy does not have a lot of data to support it.
The more challenging population consists of patients who go to the cath lab but do not undergo PCI; this population is managed medically too. We often drop the ball with clopidogrel in this population. Many patients in whom PCI is not performed do not receive clopidogrel upstream, for all of the reasons we’ve discussed, and there is pretty good evidence that if clopidogrel is not instituted before hospital discharge, the patient is not likely to be receiving it at 30 days either. We have an obligation to treat these patients.
Treatment following bypass surgery is much murkier, and I don’t really know what we should be doing. The ACC/AHA guidelines suggest that clopidogrel be started in a patient with non-ST-elevation ACS after bypass surgery,1 but I believe the evidence to support that recommendation is pretty weak.
Dr. Bhatt: Well, the CURE trial did contain a sizeable group that underwent bypass surgery,7 and although this group was underpowered in some respects, it was still a very large group, so I personally favor treatment in those patients. We should mention that an ongoing trial called TRILOGY ACS is comparing clopidogrel and prasugrel specifically in patients who are being managed medically,13 so more data on this strategy will be emerging.
ARE GUIDELINES DESTINED TO BECOME EVER MORE COMPLEX?
Dr. Bhatt: Here’s a comment and question from the audience that pulls together a lot of what we’ve discussed while also looking forward: The antiplatelet therapy guidelines are already complicated. If the ongoing studies of emerging antiplatelet drugs all have results that are qualitatively similar to those of the TRITON-TIMI 38 study of prasugrel—ie, better efficacy with more potent therapy but more bleeding—how do you foresee these antiplatelet drugs being used in clinical practice?
Dr. Sabatine: The contrast between the US guidelines and the European guidelines for ACS management is stark. The US guidelines—from the ACC and AHA1—are essentially an encyclopedia that includes nearly every trial of anti-platelet therapy in ACS along with complicated algorithms; they do a wonderful job of being complete. The European guidelines14 are probably one tenth the size of their US counterpart document, and they suggest treatments for various patient types; they are very simple.
In a sense, the US guidelines lay out the data and force practitioners to evaluate the trials and consider how our patients fit into the study populations. In this way they are analogous to current guidelines for anticoagulant therapy. Several anticoagulants have been compared with heparin in clinical trials. These newer anticoagulants appear to reduce the risk of ischemic events compared with heparin; some have lower rates of bleeding, while others have higher rates of bleeding. There have been few head-to-head studies of these agents, however, so we wind up with guidelines that are not definitive but rather suggest agents to “consider” in various settings.
It’s unlikely that a head-to-head trial will be conducted comparing prasugrel with the reversible P2Y12 antagonist AZD6140, assuming that both are approved for marketing. If the drugs appear equally efficacious in placebo-controlled trials, it will take consensus to determine the appropriate choice at your hospital, factoring in your patient profile, the cost of the drugs, and other variables. It’s more complicated when one agent is slightly more efficacious but causes more bleeding or, conversely, a little less efficacious but less apt to cause bleeding. In such cases, you may need to tailor therapy to the patient, trying to gauge bleeding risk. All of the emerging data appear to point to the importance of bleeding on outcomes: patients who bleed fare poorly, in part due to the bleeding itself and in part perhaps because they have a proclivity for bleeding.
THE FUTURE: MONITORING-BASED DOSING AND NICHE ANTIPLATELETS?
Dr. Bhatt: That’s a good observation. Let’s wrap up by having the other panelists share any final thoughts you may have.
Dr. Alexander: I’d like to return to the issue of measuring antiplatelet response and using it to guide therapy. Earlier we cited the examples of antihypertensive therapy and lipid-lowering therapy to support this model of monitoring-based treatment. Guidelines for dyslipidemia treatment recommend using LDL-C levels to guide therapy, but this practice is difficult to study in a randomized trial. In fact, none of the randomized trials of statins used LDL-C levels to guide therapy. They all studied fixed doses of statins versus placebo or fixed doses of another statin. Higher doses of statins were always beneficial compared with lower doses, and this finding was extrapolated into the guidelines as a justification to treat to target LDL-C levels.
Dr. Bhatt: It’s not even necessarily clear that LDL-C level is the best target, if you consider the JUPITER trial, in which patients received statin therapy based on their baseline level of high-sensitivity C-reactive protein, not their LDL-C level.15 It goes to show how incomplete our knowledge of a class of drugs may be, even decades after the drugs are introduced.
Dr. Kottke-Marchant: To speak to Dr. Alexander’s point, dose adjustment guided by platelet monitoring is a bit more problematic for antiplatelet drugs that are irreversible inhibitors, such as clopidogrel and aspirin, than for those that are reversible inhibitors, which are being developed and may eventually make more sense to use. From a drug development standpoint, a drug that requires monitoring and dose adjustment will not gain wide acceptance because it will increase medical costs and morbidity.
Dr. Bhatt: Yes, we know from experience with warfarin that doctors and patients don’t like the ongoing need for monitoring and testing.
Dr. Peacock: The drugs that are going to be adopted by the emergency department are those with the shortest half-lives, for several reasons: (1) using a drug with a short half-life won’t commit us to a particular course of action; (2) the potential for drug interactions is lower; and (3) in the event of an erroneous diagnosis, the consequence of misapplication may be mitigated by early recognition and termination of the drug. If we later decide that we’ve gone down the wrong therapeutic road or reached a wrong diagnosis, or if a complication occurs, we can turn off the therapy quickly. That level of flexibility is needed.
Dr. Kottke-Marchant: I think we are moving into an era of niche antiplatelet drugs. One might be used in a patient going to surgery, for example, and another for long-term therapy.
Dr. Peacock: One thing that I don’t have a feel for is how to transition from one drug to another. When you change drugs for a patient, it so often seems like it goes badly. If we’re eventually going to use drugs with ultra-short half-lives in the in the emergency department for the first day or two, and then switch patients to a pill for a week, a lot more platelet function testing may be needed.
- Anderson JL, Adams CD, Antman EM, et al. ACC/AHA 2007 guidelines for the management of patients with unstable angina/ non-ST-elevation myocardial infarction: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2007; 50:e1–e157.
- Bonello L, Camoin-Jau L, Arques S, et al. Adjusted clopidogrel loading doses according to vasodilator-stimulated phosphoprotein phosphorylation index decrease rate of major adverse cardiovascular events in patients with clopidogrel resistance: a multicenter randomized prospective study. J Am Coll Cardiol 2008; 51:1404–1411.
- Glauser J, Emerman CL, Bhatt DL, Peacock WF. Platelet aspirin resistance in emergency department patients with suspected acute coronary syndrome. Am J Emerg Med. In press
- Patti G, Nusca A, Mangiacapra F, Gatto L, D’Ambrosio A, Di Sciascio G. Point-of-care measurement of clopidogrel responsiveness predicts clinical outcome in patients undergoing percutaneous coronary intervention. J Am Coll Cardiol 2008; 52:1128–1133.
- Mega JL, Close SL, Wiviott SD, et al. Cytochrome P-450 polymorphisms and response to clopidogrel. N Engl J Med 2009; 360:354–362.
- Bhatt DL, Scheiman J, Abraham NS, et al. ACCF/ACG/AHA 2008 expert consensus document on reducing the gastrointestinal risks of antiplatelet therapy and NSAID use. J Am Coll Cardiol 2008; 52:1502–1517.
- Fox KA, Mehta SR, Peters R, et al. Benefits and risks of the combination of clopidogrel and aspirin in patients undergoing surgical revascularization for non-ST-elevation acute coronary syndrome: the Clopidogrel in Unstable angina to prevent Recurrent ischemic Events (CURE) Trial. Circulation 2004; 110:1202–1208.
- ISIS-2 Collaborative Group. Randomised trial of intravenous streptokinase, oral aspirin, both, or neither among 17,187 cases of suspected acute myocardial infarction: ISIS-2. Lancet 1988; 2:349–360.
- EARLY-ACS: Glycoprotein IIb/IIIa inhibition in patients with non-ST-segment elevation acute coronary syndrome. Clinical Trials.gov Web site. http://clinicaltrials.gov/ct2/show/NCT00089895. Updated December 17, 2008. Accessed December 18, 2008.
- Wiviott SD, Braunwald E, McCabe CH, et al; TRITON-TIMI 38 Investigators. Prasugrel versus clopidogrel in patients with acute coronary syndromes. N Engl J Med 2007; 357:2001–2015.
- Wiviott SD, Braunwald E, McCabe CH, et al. Intensive oral anti-platelet therapy for reduction of ischaemic events including stent thrombosis in patients with acute coronary dyndromes treated with percutaneous coronary intervention and stenting in the TRITON-TIMI 38 trial: a subanalysis of a randomised trial. Lancet 2008; 371:1353–1363.
- Berger JS, Bhatt DL, Chen Z, et al. The relationship between sex, mortality and cardiovascular events among patients with established cardiovascular disease: a meta-analysis [ACC abstract 1012-149]. J Am Coll Cardiol 2008; 51 10 suppl A:A247.
- TRILOGY ACS: A comparison of prasugrel and clopidogrel in acute coronary syndrome subjects. ClinicalTrials.gov Web site. http://clinicaltrials.gov/ct2/show/NCT00699998. Updated December 15, 2008. Accessed January 2, 2009.
- Guidelines for the diagnosis and treatment of non-ST-segment elevation acute coronary syndromes. Eur Heart J 2007; 28:1598–1660.
- Ridker PM, Danielson E, Fonseca FA, et al. Rosuvastatin to prevent vascular events in men and women with elevated C-reactive protein. N Engl J Med 2008; 359:2195–2207.
CASE STUDY: THROMBOSIS AFTER STENTING DESPITE ANTIPLATELET THERAPY
Dr. Deepak Bhatt: We have taken in a wealth of terrific information from the three preceding talks in this symposium. Let’s now share some questions from the audience and explore some of the points raised in the preceding talks in a bit more practical detail for clinicians. Our three prior speakers are joined in this panel discussion by Cleveland Clinic’s Dr. Frank Peacock, who brings an emergency medicine perspective.
Let’s begin with a case-based question supplied from the audience. The patient is a 42-year-old morbidly obese man without diabetes who had a non-ST-elevation myocardial infarction (MI) less than 1 year ago. A drug-eluting stent was placed at the time of his MI, and now restenosis has occurred. He is on aspirin and clopidogrel 75 mg/day. Do you recommend running a vasodilator-stimulated phosphoprotein (VASP) test and possibly increasing the clopidogrel dose to 150 mg/ day, or should the patient just be switched to prasugrel (assuming it is commercially available) without running the VASP test?
I’ll take a quick initial stab at this question. Studies of antiplatelet therapies to prevent instent restenosis have been a mixed bag. Some of the trials with glycoprotein IIb/IIIa inhibitors have shown an effect on restenosis, but most have not. Similarly, some of the analyses of the thienopyridines ticlopodine and clopidogrel have shown an effect on restenosis, but most have not.
For the most part, restenosis does not appear to be heavily mediated by platelets, at least not in a way that we can influence by therapy. On the other hand, stent thrombosis is highly platelet mediated, so I would alter the case to one in which stent thrombosis is the clinical problem. Assuming that the patient has been adherent to his antiplatelet regimen, which tests would you perform, and how would you act on the information from those tests?
Dr. Kandice Kottke-Marchant: The 2007 guidelines on acute coronary syndrome (ACS) management from the American College of Cardiology and American Heart Association (ACC/AHA)1 do not address platelet function testing, and almost none of the clinical trials of antiplatelet agents had an arm that included testing and dose adjustment based on platelet function studies. Platelet testing is available at some centers; at Cleveland Clinic, we use platelet aggregation testing. One can do platelet aggregation testing on a patient-by-patient basis; if inhibition appears to be suboptimal, a treatment decision should be made, but there is little guidance from the literature to steer that decision. I have seen clinicians increase the dose of clopidogrel or aspirin in response to platelet function tests, which occasionally triggers a confirmatory call from the pharmacy department.
Dr. Bhatt: When I was still at Cleveland Clinic, our chief medical resident did an analysis of platelet function testing, and it was remarkable how much testing was performed and how often it changed management, largely in the absence of any outcomes data, as Dr. Kottke-Marchant pointed out. Dr. Alexander, what are your recommendations with respect to platelet function testing today?
Dr. John Alexander: The case you describe is one in which applying evidence is not easy. There are no trials to supply any evidence to change therapy in this patient, a morbidly obese man receiving 75 mg/day of clopidogrel. There is certainly a rationale, however, to believe that a standard “one size fits all” 75-mg daily dose of clopidogrel may not be enough for him. The trade-off with a higher dosage is a higher risk of bleeding, however, so I would first be sure that he has been adherent to his current regimen of clopidogrel and aspirin.
Dr. Bhatt: Is there a role for point-of-care testing to determine whether he is adherent to the medicines?
Dr. Kottke-Marchant: Several of the point-of-care tests, such as the VerifyNow rapid platelet function analyzer, have specific cartridges for aspirin and for clopidogrel. If platelets were not being inhibited, it would suggest that the doses were too low, given the patient’s weight, but you probably would not be able to determine whether he was resistant to clopidogrel.
Dr. W. Frank Peacock: One way to verify that patients are taking their aspirin is to take a small urine sample and squirt in 2 mL of ferric chloride. If the sample turns purple, it means they are taking their aspirin. Once that is established, you can try to determine whether the drug is working on their platelets.
Dr. Alexander: Another potential explanation for stent thrombosis is faulty stent placement. In this case I would consider asking an interventional colleague to perform intravascular ultrasonography to make sure the stent was implanted properly before I changed the patient’s antithrombotic therapy.
Dr. Bhatt: That’s a great technical point. We always want to make sure that a case of stent thrombosis is not due to a mechanical problem. We should be asking: Is the stent properly sized and well opposed? Is there a distal dissection or any other issue that could predispose to stent thrombosis?
Dr. Alexander: This case illustrates a host of other challenges that underscore how much more work we need to do to define optimal antiplatelet therapy. Suppose we perform platelet function testing and find a low level of platelet inhibition in this patient with stent thrombosis, and we change his antiplatelet regimen. When should we test him again? If we retest in 3 months and find that he has a higher than expected level of platelet inhibition on the new antiplatelet regimen, do we dial down the intensity? Once again, there is no evidence to guide these decisions, and levels of platelet inhibition are driven not just by the medications but also by what is going on in the patient’s platelets—it is quite multifactorial.
POINT-OF-CARE PLATELET FUNCTION TESTING: CURRENT LIMITS, FUTURE ROLES
Dr. Bhatt: While we’re discussing platelet function testing, I found it interesting, Dr. Kottke-Marchant, that you said the use of bleeding time as a platelet test is finally going away. Testing of bleeding time has been around forever, but I agree that it doesn’t have much value in clinical practice. Do you think bleeding time will continue to have any role in drug development? Most phase 2 trials, and certainly phase 1 trials, still capture bleeding time to assess whether or not a drug is working. Should that, too, be jettisoned, or does bleeding time still have some merit in this context?
Dr. Kottke-Marchant: I would jettison it in drug development as well because of the considerable variability in bleeding time. It is not a test that can be standardized, and no quality control can be done. The results depend on skin turgor, age, and many other variables.
We need a global assay that will pick up multiple aspects of platelet function, such as flow-based adhesion, aggregation, and granule release. The bleeding time is a shear-dependent test, whereas the platelet aggregation test that is used in most drug trials is an artificial assay that measures only aggregation, but not under shear. The VerifyNow rapid platelet function analyzer does not measure platelets under shear and is not a global assay.
Dr. Marc Sabatine: I would underscore the need for a reliable point-of-care test of platelet function. When we prescribe a statin or an antihypertensive drug, we don’t just send the patient out the door and hope that everything will be okay. We measure the response, knowing that genotype, environmental factors, or medication factors can affect the response. When we prescribe an antiplatelet drug, we need a reliable point-of-care device to make certain that the patient is getting appropriate platelet inhibition.
I am reminded of a recent study of point-of-care measurement of platelet inhibition in patients receiving clopidogrel prior to nonemergent percutaneous coronary intervention (PCI).2 Rather than just treating patients with PCI and sending them out the door, the investigators kept giving patients clopidogrel and measuring their platelet inhibition until they achieved an appropriate degree of inhibition, after which PCI was performed. Event rates were significantly reduced in the patient group treated this way, which suggests a need to individualize therapy and move away from the “one size fits all” mindset.
Dr. Bhatt: Dr. Peacock, you’ve led a study of point-of-care assays in the emergency department. What might ultimately be the role of point-of-care testing in emergency medicine, and might it influence drug selection?
Dr. Peacock: My short answer is that I think there will be a role for point-of-care testing, with all the caveats that have been discussed. There may even be a day when we do genetic testing and look for DNA. Honestly, though, I’m somewhat of a skeptic because I’m not looking at the genetics. I see many patients who do crack cocaine who come to the emergency room with chest pain and have risk factors, but I send these patients home because they are not having an event. The real question is, “Is it an event?” If a patient is having an event and he or she has platelet resistance or hyperreactivity—whatever we term it—then you have to decide the next step.
As you mentioned, we just completed a study that evaluated a couple hundred patients for platelet inhibition resistance to aspirin, and one finding was that the incidence of platelet resistance to aspirin was much lower than we had anticipated. Studies from the literature suggest that the prevalence of resistance is around 30%, but in our study it was 6.5%.3
Dr. Kottke-Marchant: It depends on how and in whom you measure resistance. Different tests will give you different numbers. Even among studies using the same measurement techniques, the results depend on the patient population. If it’s a fairly stable cardiac population, you may see aspirin resistance rates of 4% or 5%. If it’s a population of patients who have had multiple MIs, the rate may be higher.
Dr. Peacock: That’s exactly my point. In the emergency department we see a mixed bag. We see many people who have had no prior events and have no acute event occurring. So in that setting you are going to get results that suggest that no intervention is required, whereas in that small percentage of patients in whom something is happening, your drug choice may be different.
Dr. Alexander: We are still talking about resistance to antiplatelet drugs as though it were a patient-level variable, but it’s my impression that it changes over time and within a patient.
Dr. Kottke-Marchant: It can change over time. There aren’t many good longitudinal studies. Most of the studies of “aspirin resistance” are really snapshot studies with measurements taken at one point in time. A term I prefer is “platelet reactivity.” To really assess treatment efficacy, we are going to have to look at the basal level of platelet reactivity.
WHAT ROLE FOR GENOTYPING IN GUIDING ANTIPLATELET THERAPY?
Dr. Bhatt: Dr. Peacock alluded to a potential role for genetic testing. Dr. Sabatine, you have done a lot of interesting work with genotyping in the TRITON-TIMI 38 study of prasugrel and clopidogrel. What is the future role of genotyping in determining which antiplatelet therapy is best for which patient?
Dr. Sabatine: As I mentioned, cytochrome P450 enzymes play a critical role in the metabolism of clopidogrel. These enzymes are fairly polymorphic—mutations in their encoding genes are responsible for subtle changes in effect, unlike the traditional mutations that we think about for sickle cell disease, for example. A wealth of data has been published showing that genetic variants are associated with decreased functional activity of cytochrome P450 enzymes, demonstrating the pharmacologic importance of these variants.
Individuals who carry variant alleles appear to respond differently to clopidogrel. A variety of small studies show that those who carry specific variants—particularly in the CYP2C19 enzyme, but in other enzymes as well—appear to have a diminished response to clopidogrel. There are also data showing that individuals with a diminished response to clopidogrel have worse outcomes.4 Our group is studying the impact of genetic variants that decrease the functional activity of cytochrome P450 enzymes on clinical outcomes. (Editor’s note: This study has since been published by Mega et al.5)
The practical implication may lie in point-of-care genotyping, which appears possible and will be clinically useful if a strong link can be demonstrated between genotype and outcomes. If point-of-care genotyping becomes practical, it will raise the question of whether both genotyping and platelet aggregation testing are needed. I think they might indeed be complementary in risk prediction, as is the case with genetic variants that affect low-density lipoprotein cholesterol (LDL-C) levels. In the lipid arena, we have seen that genetic effects and lipid levels provide independent incremental information about risk. That’s because of the high degree of variation in LDL-C levels: an LDL-C measurement is a snapshot in time, yet a variety of factors can influence LDL-C levels. In contrast, genotype is an invariant factor. Similarly, in the platelet arena, platelet aggregation studies and genotyping may be synergistic in predicting an individual’s predisposition to events and response to medications.
Dr. Bhatt: While we’re discussing pathways of metabolism, the literature, though scant, suggests a potential interaction between proton pump inhibitors and clopidogrel. I was co-chair of a recent American College of Cardiology/ American Heart Association/American College of Gastro-enterology consensus document that endorsed liberal use of proton pump inhibitors in patients who are at gastrointestinal risk, including those on antiplatelet therapy.6 The gastroenterologists believed strongly that proton pump inhibitors were safe and in fact underused in these patients. What do you think about the clopidogrel–proton pump inhibitor interaction? Should we be concerned?
Dr. Sabatine: Proton pump inhibitors are not only substrates for, but also inhibitors of, CYP2C19, a key enzyme that helps transform clopidogrel into an active metabolite. For this reason, there has been interest in whether concomitant use of proton pump inhibitors would blunt the efficacy of clopidogrel. The same concern was raised about giving clopidogrel with certain statin drugs that are also metabolized by the cytochrome P450 system, and several studies have shown an effect of these statins on clopidogrel’s platelet inhibition. However, there is no evidence that coadministration of these statins has affected clinical outcomes with clopidogrel in clinical trials. So it may be that while competition for the cytochrome P450 system is one factor, it’s not enough of a factor to tip the scale and result in a clinical event. The same may be true of coadministration of proton pump inhibitors; meanwhile, we await definitive data that concomitant use with clopidogrel leads to higher rates of ischemic events.
DIAGNOSTIC UNCERTAINTY IN THE EMERGENCY SETTING
Dr. Bhatt: We heard about quite a few new antiplatelet drugs in Dr. Sabatine’s presentation, some of which will likely be taken up in clinical practice. Dr. Peacock, from an emergency department perspective, how will you integrate all these new agents with the numerous therapies already available? What should emergency departments do to come to grips with and ultimately take advantage of these different forms of therapy as well as emerging platelet function tests?
Dr. Peacock: The piece that’s unique or especially pertinent to the emergency department is diagnostic uncertainty. Diagnosis and management are easy when a patient has an ST-elevation MI because we all know what that looks like and we know what to do in response. To some extent non-ST-elevation MI is fairly simple too. ACS is a lot more difficult because we don’t have a good definition for unstable angina, and that’s where diagnosis and management become problematic. And with high-sensitivity troponins coming out now, the question of non-ST-elevation MI is going to get more and more confusing because we will have a lot more patients who meet criteria without having an acute coronary artery event.
So it is going to be important that studies be designed correctly. A lot of the studies reviewed today were efficacy studies, showing that a particular drug works well in a carefully defined population, but they were not efficiency studies: they did not take into account the real-world diagnostic uncertainty—and inevitable misdiagnoses—that emergency departments encounter before starting therapy.
Take the CURE trial, for example. It was a great study, showing that clopidogrel reduced the hazard ratio for major coronary events by 20% in patients with unstable angina,7 and the message was that everybody should be using clopidogrel. A close look at the study, however, reveals that about half the patients did not receive clopidogrel in the emergency department. When patients did receive it early, it was driven by the cardiologist, who was absolutely certain of the diagnosis. But if the study was not designed to test early use, then it is a big leap to extrapolate its findings to this circumstance.
Many of the patients in the CURE trial were enrolled the day after presentation, when their diagnosis was certain—ie, they had a rise in troponin after their symptoms. But when a patient first arrives in the emergency department, we are not certain of the diagnosis. And if we use a drug such as clopidogrel, with a duration of action as long as 5 days, we have committed the entire medical system to a certain course of management for that period of time. If we get the diagnosis wrong, this commitment could restrict management options for up to 5 days.
The question for emergency physicians becomes, “How long is long enough to know whether I can pull the trigger on a therapy and be correct?” With all the new drugs coming along, the way to answer this is to do efficiency studies in a real-world environment in addition to efficacy studies.
Dr. Alexander: Yes, one of the biggest limitations of antiplatelet drug studies to date is that they usually haven’t really addressed the timing of drug initiation. We often assume that if a drug is shown to be beneficial, then it should be started as soon as possible. As we just heard, that may have been an unfounded extrapolation from the CURE trial. The same sort of thing happened with the ISIS trial of aspirin in patients with ST-elevation MI.8 In response to the ISIS results, clinicians rushed to give patients aspirin right away even though many of the patients in the trial may have received their aspirin the day after presentation. For these reasons, the EARLY-ACS study,9 which is addressing a very simple question—whether early upstream use of glycoprotein IIb/IIIa inhibitors is beneficial—has been a challenging trial to complete.
WHAT ROLE FOR THIENOPYRIDINE PRETREATMENT?
Dr. Bhatt: Dr. Sabatine, you presented data from the large TRITON-TIMI 38 trial comparing prasugrel with clopidogrel. I’m interested in how you would use prasugrel in practice, assuming it receives marketing approval, especially in light of its bleeding risk, particularly in patients in whom coronary artery bypass graft surgery (CABG) is planned. Many hospitals pretreat patients with clopidogrel in the emergency department. How would you manage a patient who shows up in the emergency room with ACS? Would you give clopidogrel, would you wait and give prasugrel, or would you do something else? If you gave clopidogrel, what loading dose would you use—300 mg, 600 mg, or, as some have suggested, 900 or 1,200 mg?
Dr. Sabatine: I am a strong proponent of pretreatment. Data from multiple studies show a benefit to this strategy, and even the original CURE trial showed a roughly 30% reduction in ischemic events within the first 24 hours of clopidogrel initiation.7
I think the dosing strategy depends on how the patient is going to be managed. If management is going to be conservative, then I would start the patient on 300 mg of clopidogrel when he or she came in. If the patient is going to the cardiac catheterization laboratory in a few hours, I would pretreat with 600 mg of clopidogrel. For prasugrel, the need for pretreatment is less clear, given the drug’s faster onset of action and greater degree of platelet inhibition. In the TRITON-TIMI 38 study,10 prasugrel was given, by and large, after diagnostic angiography, and thus one could use that approach in practice.
In terms of clopidogrel versus prasugrel, I would embrace prasugrel for the large majority of my patients, being mindful of the risk of bleeding. I would not hesitate to give the medication to diabetics or to younger, more robust patients. The 50% reduction in stent thrombosis with prasugrel versus clopidogrel in TRITON-TIMI 38 is huge,11 given that the risk of death with stent thrombosis is probably 25% or greater. So I would want to have prasugrel on board to reduce the risk of stent thrombosis, especially if a drug-eluting stent were being implanted.
Dr. Bhatt: Dr. Alexander, let’s get your take on a similar scenario. Assuming that prasugrel gains marketing approval, how would you manage patients with non-ST-elevation MI who present to the emergency department? Would you pretreat with clopidogrel? Would you wait until angiography and then, depending on the anatomy, treat with prasugrel? Or would you potentially pretreat with prasugrel, which has not been studied and would not be a labeled indication? How would you reconcile the data?
Dr. Alexander: At Duke, I expect that prasugrel will not be used prior to the catheterization laboratory in patients with non-ST-elevation ACS due to concerns about whether the patients will undergo PCI or be managed medically or with CABG.
Dr. Bhatt: That makes sense, since there was a fair amount of bleeding with prasugrel in those patients in TRITON-TIMI 38.
Dr. Alexander: Correct. Moreover, at Duke we don’t use as much upstream clopidogrel as we would, based on the evidence, if I were managing all the patients. There is still a lot of pushback about upstream clopidogrel from our surgeons because patients are going to surgery quickly these days, sometimes just a day after catheterization, and that’s when a loading dose of clopidogrel can be problematic. We are also still fairly heavy users of glycoprotein IIb/IIIa inhibitors.
Where I can see prasugrel being used prior to the cath lab at Duke is in ST-elevation MI, where the rate of PCI is very high. In primary angioplasty for ST-elevation MI, it would likely be given upstream. The bigger issue for us will be that we serve as a referral base for a lot of regional hospitals, and thus have some influence on their practices.
Dr. Bhatt: In that case, what would you advise those regional hospitals to do for non-ST-elevation MI?
Dr. Alexander: For the time being, we would advise continuing with our current practice, which is to load clopidogrel in patients in whom there is a reasonable certainty that CABG will not be performed, and to use glycoprotein IIb/IIIa inhibitors in high-risk patients. As we get more experience with prasugrel or with additional trial results, however, that practice could easily change.
Dr. Bhatt: So you would still use glycoprotein IIb/IIIa inhibitors?
Dr. Alexander: Yes, I advocate upstream clopidogrel use, but not all my colleagues do. Based on the guidelines, I’d use one or the other—either clopidogrel or a glycoprotein IIb/IIIa inhibitor. As I mentioned in my talk, if a patient is at high risk for bleeding, I am more inclined to use clopidogrel, although patients at higher risk of bleeding are often at higher risk for ischemic events as well.
WHAT’S DRIVEN THE DROPOFF IN GLYCOPROTEIN IIb/IIIa INHIBITOR USE?
Dr. Bhatt: While we’re on the topic of glycoprotein IIb/IIIa inhibitors, a question card from the audience asks why there has been a decrease in glycoprotein IIb/ IIIa inhibitor use and whether this decline is appropriate or inappropriate. Have clopidogrel pretreatment, higher loading doses of clopidogrel, and use of the direct thrombin inhibitor bivalirudin contributed to the decrease in glycoprotein IIb/IIIa inhibitor use?
Dr. Alexander: I do think that the decline has been driven by the changing environment, with greater use of other antithrombotic strategies that include clopidogrel and bivalirudin, as you suggest, as well as an increased attention to bleeding. From an evidence-based standpoint, we don’t know whether the decrease in glycoprotein IIb/IIIa use is appropriate or not because the studies of these agents were conducted before the widespread upstream use of clopidogrel and bivalirudin. Clopidogrel is attractive because it’s a pill given as one dose in the emergency department, the wards, or the catheterization laboratory, rather than a much more complicated infusion with weight-based dosing and dosage adjustments based on creatinine clearance. It is possible that we should perhaps be dosing clopidogrel the same way, but we don’t know that yet.
PRASUGREL IN PRACTICE: HOW LOW CAN THE DOSE GO, AND IS THERE A GENDER EFFECT?
Dr. Bhatt: Let’s stick with this focus on dosing but turn back to discussion of prasugrel. In your presentation of the TRITON-TIMI 38 data, Dr. Sabatine, you proposed a potential prasugrel dosage modification, down to a 5-mg loading dose, in subgroups that were identified as being at high bleeding risk—namely, elderly patients and patients with low body weight. However, no outcomes data with 5 mg of prasugrel came out of TRITON-TIMI 38.10 Is this proposed modification based on pharmacokinetic extrapolation? Could clinicians be comfortable using 5 mg of prasugrel, assuming the drug receives regulatory approval and a 5-mg tablet would be available?
Dr. Sabatine: Of course, evidence at the grade A level would consist of a trial showing that patients who received a lower dose enjoyed the same benefit as those who got standard dosing in TRITON-TIMI 38—a 60-mg loading dose followed by 10 mg/day—with an acceptable risk profile. However, such a trial would be difficult and costly to conduct, and would take roughly half a decade to pull off. It is only through large trials like TRITON-TIMI 38 that you identify subgroups that respond differently, and then to go back and do a separate trial for those subgroups takes a great deal of time. It may not be practical.
I think the Food and Drug Administration is moving toward embracing careful pharmacokinetic/pharmacodynamic substudies within trials, with these substudies having adequate numbers of subjects to provide a sense for the ideal target dose and what an acceptable dose range would be, without limiting approval to a single dose. The analogy would be warfarin dosing, with the aim being to figure out an acceptable dose range, discover which patients fall outside that range, and then model the effect of a lower dose in those patients. Thus, approving a 5-mg dose of prasugrel based on TRITON-TIMI 38 would be a reasonable approach if this dose passed muster under pharmacokinetic/pharmacodynamic modeling. If this approach were taken, there would clearly be a need for postmarketing surveillance to confirm whether the modeling on the effects of the lower dose was borne out by actual outcomes.
Dr. Bhatt: The audience has posed another interesting question raised by TRITON-TIMI 38: Can you comment on the lesser effect of prasugrel in women?
Dr. Sabatine: It is true that there was not a statistically significant effect of prasugrel among women in TRITON-TIMI 38, but statistical tests among subgroups found no significant heterogeneity for the effect between men and women, and that is the relevant measure to determine any gender effect. Keep in mind that not all subgroups represent a univariate slice of the population. For example, women generally have lower body weight than men, and since prasugrel’s net clinical benefit was reduced in patients with lower body weight, that may explain some of the differing extent of effect between men and women.
Dr. Bhatt: That’s a good point about the lack of heterogeneity between men and women. In fact, a meta-analysis of clopidogrel data conducted by one of the fellows I work with revealed that men and women appear to benefit similarly from clopidogrel.12 There was a slight signal of excess bleeding in women, but there were more elderly women in the pooled population, which may have been a confounding factor. As best as anyone can tell, antiplate-let therapy works well in both men and women.
NAVIGATING MANAGEMENT ACROSS THE SPECTRUM OF CARE
Dr. Bhatt: I would like to explore a bit further how all of these issues translate across the spectrum of care, beginning in the emergency department, which we know is a key component of the entire ACS management strategy for a health care system. What should emergency medicine doctors do, given all of the potential options—clopidogrel, different loading doses of clopidogrel, prasugrel, glycoprotein IIb/IIIa inhibitors, even bivalirudin?
Dr. Peacock: It depends on the practice setting. Some emergency physicians work at community hospitals with no backup. They must have relationships with the larger centers to which they’ll be transferring patients, because ACS patients should not be staying at community hospitals. These emergency physicians must have close relationships with the physicians who will be receiving their patients, and they know the potential head-butting with surgeons surrounding early clopidogrel use better than anybody does. If they treat with clopidogrel in the emergency room, and it turns out that the patient needs to go to the catheterization laboratory, can the receiving hospital use platelet testing to shorten the standard 5-day interval from treatment to catheterization?
Dr. Bhatt: Yes, that’s a rather useful, although not completely validated, way of using point-of-care platelet testing—to potentially reduce the time to surgery.
Dr. Peacock: Right. So if the policies for handling these types of transfer-related issues are worked out in advance, all players have a pathway to follow, which can allow quick action when necessary. If you don’t have these issues worked out in advance, you either lose many opportunities to act quickly in the emergency room or you risk taking actions that will cause problems later in the course of management.
Dr. Alexander: I totally agree. The key is to sit down with all the players involved—the surgeons, the interventional cardiologists, the intensivists, the emergency room personnel—and come up with strategies for different populations of patients. Write down the collective strategy and hang it on the wall so that everybody can be comfortable with it. The strategy can be reevaluated when prasugrel or other new antithrombotic drugs come on the market.
Dr. Peacock: The other environment is the academic center, which is even more challenging, but for different reasons. At a large academic center like the Cleveland Clinic, any of 25 different cardiologists may be taking call and receiving patients from the emergency department on a particular night. A lot of phone interaction is required to elicit the planned management strategy, including if and when the patient will be going to the cath lab. Individualizing care to a particular cardiologist then becomes a time-consuming challenge, especially in clinical situations where outcomes are time-dependent.
Dr. Alexander: Agreed. Management needs to be integrated across the entire spectrum of care. The anticoagulants that we plan to use in the cath lab will affect the antithrombotic regimen used upstream.
Dr. Kottke-Marchant: One circumstance where platelet function testing has been helpful is in determining the washout of the clopidogrel effect before surgery. At Cleveland Clinic, we have implemented platelet function testing in this circumstance instead of waiting a blanket 5 days after clopidogrel administration to go to surgery. A return to normal platelet function on platelet aggregation testing, depending on the cutoff value used, is an indicator that the patient can proceed to surgery.
Dr. Bhatt: That’s a logical approach. How should we be using antiplatelet therapy in the medically managed patient, Dr. Alexander?
Dr. Alexander: When I think of medical management, I include patients who don’t go to the cath lab, but also those who do, with regards to their management prior to and following their time in the cath lab.
In patients who don’t go to the cath lab for angiography, the ACC/AHA guidelines recommend aspirin and either clopidogrel, a glycoprotein IIb/IIIa inhibitor, or both.1 In making this choice, I consider the patient’s risk of bleeding and the dosing complexity of the regimen, especially with the use of glycoprotein IIb/IIIa inhibitors in a patient with renal insufficiency. In a patient at relatively low risk for bleeding, I often use both clopidogrel and a glycoprotein IIb/IIIa inhibitor, although this strategy does not have a lot of data to support it.
The more challenging population consists of patients who go to the cath lab but do not undergo PCI; this population is managed medically too. We often drop the ball with clopidogrel in this population. Many patients in whom PCI is not performed do not receive clopidogrel upstream, for all of the reasons we’ve discussed, and there is pretty good evidence that if clopidogrel is not instituted before hospital discharge, the patient is not likely to be receiving it at 30 days either. We have an obligation to treat these patients.
Treatment following bypass surgery is much murkier, and I don’t really know what we should be doing. The ACC/AHA guidelines suggest that clopidogrel be started in a patient with non-ST-elevation ACS after bypass surgery,1 but I believe the evidence to support that recommendation is pretty weak.
Dr. Bhatt: Well, the CURE trial did contain a sizeable group that underwent bypass surgery,7 and although this group was underpowered in some respects, it was still a very large group, so I personally favor treatment in those patients. We should mention that an ongoing trial called TRILOGY ACS is comparing clopidogrel and prasugrel specifically in patients who are being managed medically,13 so more data on this strategy will be emerging.
ARE GUIDELINES DESTINED TO BECOME EVER MORE COMPLEX?
Dr. Bhatt: Here’s a comment and question from the audience that pulls together a lot of what we’ve discussed while also looking forward: The antiplatelet therapy guidelines are already complicated. If the ongoing studies of emerging antiplatelet drugs all have results that are qualitatively similar to those of the TRITON-TIMI 38 study of prasugrel—ie, better efficacy with more potent therapy but more bleeding—how do you foresee these antiplatelet drugs being used in clinical practice?
Dr. Sabatine: The contrast between the US guidelines and the European guidelines for ACS management is stark. The US guidelines—from the ACC and AHA1—are essentially an encyclopedia that includes nearly every trial of anti-platelet therapy in ACS along with complicated algorithms; they do a wonderful job of being complete. The European guidelines14 are probably one tenth the size of their US counterpart document, and they suggest treatments for various patient types; they are very simple.
In a sense, the US guidelines lay out the data and force practitioners to evaluate the trials and consider how our patients fit into the study populations. In this way they are analogous to current guidelines for anticoagulant therapy. Several anticoagulants have been compared with heparin in clinical trials. These newer anticoagulants appear to reduce the risk of ischemic events compared with heparin; some have lower rates of bleeding, while others have higher rates of bleeding. There have been few head-to-head studies of these agents, however, so we wind up with guidelines that are not definitive but rather suggest agents to “consider” in various settings.
It’s unlikely that a head-to-head trial will be conducted comparing prasugrel with the reversible P2Y12 antagonist AZD6140, assuming that both are approved for marketing. If the drugs appear equally efficacious in placebo-controlled trials, it will take consensus to determine the appropriate choice at your hospital, factoring in your patient profile, the cost of the drugs, and other variables. It’s more complicated when one agent is slightly more efficacious but causes more bleeding or, conversely, a little less efficacious but less apt to cause bleeding. In such cases, you may need to tailor therapy to the patient, trying to gauge bleeding risk. All of the emerging data appear to point to the importance of bleeding on outcomes: patients who bleed fare poorly, in part due to the bleeding itself and in part perhaps because they have a proclivity for bleeding.
THE FUTURE: MONITORING-BASED DOSING AND NICHE ANTIPLATELETS?
Dr. Bhatt: That’s a good observation. Let’s wrap up by having the other panelists share any final thoughts you may have.
Dr. Alexander: I’d like to return to the issue of measuring antiplatelet response and using it to guide therapy. Earlier we cited the examples of antihypertensive therapy and lipid-lowering therapy to support this model of monitoring-based treatment. Guidelines for dyslipidemia treatment recommend using LDL-C levels to guide therapy, but this practice is difficult to study in a randomized trial. In fact, none of the randomized trials of statins used LDL-C levels to guide therapy. They all studied fixed doses of statins versus placebo or fixed doses of another statin. Higher doses of statins were always beneficial compared with lower doses, and this finding was extrapolated into the guidelines as a justification to treat to target LDL-C levels.
Dr. Bhatt: It’s not even necessarily clear that LDL-C level is the best target, if you consider the JUPITER trial, in which patients received statin therapy based on their baseline level of high-sensitivity C-reactive protein, not their LDL-C level.15 It goes to show how incomplete our knowledge of a class of drugs may be, even decades after the drugs are introduced.
Dr. Kottke-Marchant: To speak to Dr. Alexander’s point, dose adjustment guided by platelet monitoring is a bit more problematic for antiplatelet drugs that are irreversible inhibitors, such as clopidogrel and aspirin, than for those that are reversible inhibitors, which are being developed and may eventually make more sense to use. From a drug development standpoint, a drug that requires monitoring and dose adjustment will not gain wide acceptance because it will increase medical costs and morbidity.
Dr. Bhatt: Yes, we know from experience with warfarin that doctors and patients don’t like the ongoing need for monitoring and testing.
Dr. Peacock: The drugs that are going to be adopted by the emergency department are those with the shortest half-lives, for several reasons: (1) using a drug with a short half-life won’t commit us to a particular course of action; (2) the potential for drug interactions is lower; and (3) in the event of an erroneous diagnosis, the consequence of misapplication may be mitigated by early recognition and termination of the drug. If we later decide that we’ve gone down the wrong therapeutic road or reached a wrong diagnosis, or if a complication occurs, we can turn off the therapy quickly. That level of flexibility is needed.
Dr. Kottke-Marchant: I think we are moving into an era of niche antiplatelet drugs. One might be used in a patient going to surgery, for example, and another for long-term therapy.
Dr. Peacock: One thing that I don’t have a feel for is how to transition from one drug to another. When you change drugs for a patient, it so often seems like it goes badly. If we’re eventually going to use drugs with ultra-short half-lives in the in the emergency department for the first day or two, and then switch patients to a pill for a week, a lot more platelet function testing may be needed.
CASE STUDY: THROMBOSIS AFTER STENTING DESPITE ANTIPLATELET THERAPY
Dr. Deepak Bhatt: We have taken in a wealth of terrific information from the three preceding talks in this symposium. Let’s now share some questions from the audience and explore some of the points raised in the preceding talks in a bit more practical detail for clinicians. Our three prior speakers are joined in this panel discussion by Cleveland Clinic’s Dr. Frank Peacock, who brings an emergency medicine perspective.
Let’s begin with a case-based question supplied from the audience. The patient is a 42-year-old morbidly obese man without diabetes who had a non-ST-elevation myocardial infarction (MI) less than 1 year ago. A drug-eluting stent was placed at the time of his MI, and now restenosis has occurred. He is on aspirin and clopidogrel 75 mg/day. Do you recommend running a vasodilator-stimulated phosphoprotein (VASP) test and possibly increasing the clopidogrel dose to 150 mg/ day, or should the patient just be switched to prasugrel (assuming it is commercially available) without running the VASP test?
I’ll take a quick initial stab at this question. Studies of antiplatelet therapies to prevent instent restenosis have been a mixed bag. Some of the trials with glycoprotein IIb/IIIa inhibitors have shown an effect on restenosis, but most have not. Similarly, some of the analyses of the thienopyridines ticlopodine and clopidogrel have shown an effect on restenosis, but most have not.
For the most part, restenosis does not appear to be heavily mediated by platelets, at least not in a way that we can influence by therapy. On the other hand, stent thrombosis is highly platelet mediated, so I would alter the case to one in which stent thrombosis is the clinical problem. Assuming that the patient has been adherent to his antiplatelet regimen, which tests would you perform, and how would you act on the information from those tests?
Dr. Kandice Kottke-Marchant: The 2007 guidelines on acute coronary syndrome (ACS) management from the American College of Cardiology and American Heart Association (ACC/AHA)1 do not address platelet function testing, and almost none of the clinical trials of antiplatelet agents had an arm that included testing and dose adjustment based on platelet function studies. Platelet testing is available at some centers; at Cleveland Clinic, we use platelet aggregation testing. One can do platelet aggregation testing on a patient-by-patient basis; if inhibition appears to be suboptimal, a treatment decision should be made, but there is little guidance from the literature to steer that decision. I have seen clinicians increase the dose of clopidogrel or aspirin in response to platelet function tests, which occasionally triggers a confirmatory call from the pharmacy department.
Dr. Bhatt: When I was still at Cleveland Clinic, our chief medical resident did an analysis of platelet function testing, and it was remarkable how much testing was performed and how often it changed management, largely in the absence of any outcomes data, as Dr. Kottke-Marchant pointed out. Dr. Alexander, what are your recommendations with respect to platelet function testing today?
Dr. John Alexander: The case you describe is one in which applying evidence is not easy. There are no trials to supply any evidence to change therapy in this patient, a morbidly obese man receiving 75 mg/day of clopidogrel. There is certainly a rationale, however, to believe that a standard “one size fits all” 75-mg daily dose of clopidogrel may not be enough for him. The trade-off with a higher dosage is a higher risk of bleeding, however, so I would first be sure that he has been adherent to his current regimen of clopidogrel and aspirin.
Dr. Bhatt: Is there a role for point-of-care testing to determine whether he is adherent to the medicines?
Dr. Kottke-Marchant: Several of the point-of-care tests, such as the VerifyNow rapid platelet function analyzer, have specific cartridges for aspirin and for clopidogrel. If platelets were not being inhibited, it would suggest that the doses were too low, given the patient’s weight, but you probably would not be able to determine whether he was resistant to clopidogrel.
Dr. W. Frank Peacock: One way to verify that patients are taking their aspirin is to take a small urine sample and squirt in 2 mL of ferric chloride. If the sample turns purple, it means they are taking their aspirin. Once that is established, you can try to determine whether the drug is working on their platelets.
Dr. Alexander: Another potential explanation for stent thrombosis is faulty stent placement. In this case I would consider asking an interventional colleague to perform intravascular ultrasonography to make sure the stent was implanted properly before I changed the patient’s antithrombotic therapy.
Dr. Bhatt: That’s a great technical point. We always want to make sure that a case of stent thrombosis is not due to a mechanical problem. We should be asking: Is the stent properly sized and well opposed? Is there a distal dissection or any other issue that could predispose to stent thrombosis?
Dr. Alexander: This case illustrates a host of other challenges that underscore how much more work we need to do to define optimal antiplatelet therapy. Suppose we perform platelet function testing and find a low level of platelet inhibition in this patient with stent thrombosis, and we change his antiplatelet regimen. When should we test him again? If we retest in 3 months and find that he has a higher than expected level of platelet inhibition on the new antiplatelet regimen, do we dial down the intensity? Once again, there is no evidence to guide these decisions, and levels of platelet inhibition are driven not just by the medications but also by what is going on in the patient’s platelets—it is quite multifactorial.
POINT-OF-CARE PLATELET FUNCTION TESTING: CURRENT LIMITS, FUTURE ROLES
Dr. Bhatt: While we’re discussing platelet function testing, I found it interesting, Dr. Kottke-Marchant, that you said the use of bleeding time as a platelet test is finally going away. Testing of bleeding time has been around forever, but I agree that it doesn’t have much value in clinical practice. Do you think bleeding time will continue to have any role in drug development? Most phase 2 trials, and certainly phase 1 trials, still capture bleeding time to assess whether or not a drug is working. Should that, too, be jettisoned, or does bleeding time still have some merit in this context?
Dr. Kottke-Marchant: I would jettison it in drug development as well because of the considerable variability in bleeding time. It is not a test that can be standardized, and no quality control can be done. The results depend on skin turgor, age, and many other variables.
We need a global assay that will pick up multiple aspects of platelet function, such as flow-based adhesion, aggregation, and granule release. The bleeding time is a shear-dependent test, whereas the platelet aggregation test that is used in most drug trials is an artificial assay that measures only aggregation, but not under shear. The VerifyNow rapid platelet function analyzer does not measure platelets under shear and is not a global assay.
Dr. Marc Sabatine: I would underscore the need for a reliable point-of-care test of platelet function. When we prescribe a statin or an antihypertensive drug, we don’t just send the patient out the door and hope that everything will be okay. We measure the response, knowing that genotype, environmental factors, or medication factors can affect the response. When we prescribe an antiplatelet drug, we need a reliable point-of-care device to make certain that the patient is getting appropriate platelet inhibition.
I am reminded of a recent study of point-of-care measurement of platelet inhibition in patients receiving clopidogrel prior to nonemergent percutaneous coronary intervention (PCI).2 Rather than just treating patients with PCI and sending them out the door, the investigators kept giving patients clopidogrel and measuring their platelet inhibition until they achieved an appropriate degree of inhibition, after which PCI was performed. Event rates were significantly reduced in the patient group treated this way, which suggests a need to individualize therapy and move away from the “one size fits all” mindset.
Dr. Bhatt: Dr. Peacock, you’ve led a study of point-of-care assays in the emergency department. What might ultimately be the role of point-of-care testing in emergency medicine, and might it influence drug selection?
Dr. Peacock: My short answer is that I think there will be a role for point-of-care testing, with all the caveats that have been discussed. There may even be a day when we do genetic testing and look for DNA. Honestly, though, I’m somewhat of a skeptic because I’m not looking at the genetics. I see many patients who do crack cocaine who come to the emergency room with chest pain and have risk factors, but I send these patients home because they are not having an event. The real question is, “Is it an event?” If a patient is having an event and he or she has platelet resistance or hyperreactivity—whatever we term it—then you have to decide the next step.
As you mentioned, we just completed a study that evaluated a couple hundred patients for platelet inhibition resistance to aspirin, and one finding was that the incidence of platelet resistance to aspirin was much lower than we had anticipated. Studies from the literature suggest that the prevalence of resistance is around 30%, but in our study it was 6.5%.3
Dr. Kottke-Marchant: It depends on how and in whom you measure resistance. Different tests will give you different numbers. Even among studies using the same measurement techniques, the results depend on the patient population. If it’s a fairly stable cardiac population, you may see aspirin resistance rates of 4% or 5%. If it’s a population of patients who have had multiple MIs, the rate may be higher.
Dr. Peacock: That’s exactly my point. In the emergency department we see a mixed bag. We see many people who have had no prior events and have no acute event occurring. So in that setting you are going to get results that suggest that no intervention is required, whereas in that small percentage of patients in whom something is happening, your drug choice may be different.
Dr. Alexander: We are still talking about resistance to antiplatelet drugs as though it were a patient-level variable, but it’s my impression that it changes over time and within a patient.
Dr. Kottke-Marchant: It can change over time. There aren’t many good longitudinal studies. Most of the studies of “aspirin resistance” are really snapshot studies with measurements taken at one point in time. A term I prefer is “platelet reactivity.” To really assess treatment efficacy, we are going to have to look at the basal level of platelet reactivity.
WHAT ROLE FOR GENOTYPING IN GUIDING ANTIPLATELET THERAPY?
Dr. Bhatt: Dr. Peacock alluded to a potential role for genetic testing. Dr. Sabatine, you have done a lot of interesting work with genotyping in the TRITON-TIMI 38 study of prasugrel and clopidogrel. What is the future role of genotyping in determining which antiplatelet therapy is best for which patient?
Dr. Sabatine: As I mentioned, cytochrome P450 enzymes play a critical role in the metabolism of clopidogrel. These enzymes are fairly polymorphic—mutations in their encoding genes are responsible for subtle changes in effect, unlike the traditional mutations that we think about for sickle cell disease, for example. A wealth of data has been published showing that genetic variants are associated with decreased functional activity of cytochrome P450 enzymes, demonstrating the pharmacologic importance of these variants.
Individuals who carry variant alleles appear to respond differently to clopidogrel. A variety of small studies show that those who carry specific variants—particularly in the CYP2C19 enzyme, but in other enzymes as well—appear to have a diminished response to clopidogrel. There are also data showing that individuals with a diminished response to clopidogrel have worse outcomes.4 Our group is studying the impact of genetic variants that decrease the functional activity of cytochrome P450 enzymes on clinical outcomes. (Editor’s note: This study has since been published by Mega et al.5)
The practical implication may lie in point-of-care genotyping, which appears possible and will be clinically useful if a strong link can be demonstrated between genotype and outcomes. If point-of-care genotyping becomes practical, it will raise the question of whether both genotyping and platelet aggregation testing are needed. I think they might indeed be complementary in risk prediction, as is the case with genetic variants that affect low-density lipoprotein cholesterol (LDL-C) levels. In the lipid arena, we have seen that genetic effects and lipid levels provide independent incremental information about risk. That’s because of the high degree of variation in LDL-C levels: an LDL-C measurement is a snapshot in time, yet a variety of factors can influence LDL-C levels. In contrast, genotype is an invariant factor. Similarly, in the platelet arena, platelet aggregation studies and genotyping may be synergistic in predicting an individual’s predisposition to events and response to medications.
Dr. Bhatt: While we’re discussing pathways of metabolism, the literature, though scant, suggests a potential interaction between proton pump inhibitors and clopidogrel. I was co-chair of a recent American College of Cardiology/ American Heart Association/American College of Gastro-enterology consensus document that endorsed liberal use of proton pump inhibitors in patients who are at gastrointestinal risk, including those on antiplatelet therapy.6 The gastroenterologists believed strongly that proton pump inhibitors were safe and in fact underused in these patients. What do you think about the clopidogrel–proton pump inhibitor interaction? Should we be concerned?
Dr. Sabatine: Proton pump inhibitors are not only substrates for, but also inhibitors of, CYP2C19, a key enzyme that helps transform clopidogrel into an active metabolite. For this reason, there has been interest in whether concomitant use of proton pump inhibitors would blunt the efficacy of clopidogrel. The same concern was raised about giving clopidogrel with certain statin drugs that are also metabolized by the cytochrome P450 system, and several studies have shown an effect of these statins on clopidogrel’s platelet inhibition. However, there is no evidence that coadministration of these statins has affected clinical outcomes with clopidogrel in clinical trials. So it may be that while competition for the cytochrome P450 system is one factor, it’s not enough of a factor to tip the scale and result in a clinical event. The same may be true of coadministration of proton pump inhibitors; meanwhile, we await definitive data that concomitant use with clopidogrel leads to higher rates of ischemic events.
DIAGNOSTIC UNCERTAINTY IN THE EMERGENCY SETTING
Dr. Bhatt: We heard about quite a few new antiplatelet drugs in Dr. Sabatine’s presentation, some of which will likely be taken up in clinical practice. Dr. Peacock, from an emergency department perspective, how will you integrate all these new agents with the numerous therapies already available? What should emergency departments do to come to grips with and ultimately take advantage of these different forms of therapy as well as emerging platelet function tests?
Dr. Peacock: The piece that’s unique or especially pertinent to the emergency department is diagnostic uncertainty. Diagnosis and management are easy when a patient has an ST-elevation MI because we all know what that looks like and we know what to do in response. To some extent non-ST-elevation MI is fairly simple too. ACS is a lot more difficult because we don’t have a good definition for unstable angina, and that’s where diagnosis and management become problematic. And with high-sensitivity troponins coming out now, the question of non-ST-elevation MI is going to get more and more confusing because we will have a lot more patients who meet criteria without having an acute coronary artery event.
So it is going to be important that studies be designed correctly. A lot of the studies reviewed today were efficacy studies, showing that a particular drug works well in a carefully defined population, but they were not efficiency studies: they did not take into account the real-world diagnostic uncertainty—and inevitable misdiagnoses—that emergency departments encounter before starting therapy.
Take the CURE trial, for example. It was a great study, showing that clopidogrel reduced the hazard ratio for major coronary events by 20% in patients with unstable angina,7 and the message was that everybody should be using clopidogrel. A close look at the study, however, reveals that about half the patients did not receive clopidogrel in the emergency department. When patients did receive it early, it was driven by the cardiologist, who was absolutely certain of the diagnosis. But if the study was not designed to test early use, then it is a big leap to extrapolate its findings to this circumstance.
Many of the patients in the CURE trial were enrolled the day after presentation, when their diagnosis was certain—ie, they had a rise in troponin after their symptoms. But when a patient first arrives in the emergency department, we are not certain of the diagnosis. And if we use a drug such as clopidogrel, with a duration of action as long as 5 days, we have committed the entire medical system to a certain course of management for that period of time. If we get the diagnosis wrong, this commitment could restrict management options for up to 5 days.
The question for emergency physicians becomes, “How long is long enough to know whether I can pull the trigger on a therapy and be correct?” With all the new drugs coming along, the way to answer this is to do efficiency studies in a real-world environment in addition to efficacy studies.
Dr. Alexander: Yes, one of the biggest limitations of antiplatelet drug studies to date is that they usually haven’t really addressed the timing of drug initiation. We often assume that if a drug is shown to be beneficial, then it should be started as soon as possible. As we just heard, that may have been an unfounded extrapolation from the CURE trial. The same sort of thing happened with the ISIS trial of aspirin in patients with ST-elevation MI.8 In response to the ISIS results, clinicians rushed to give patients aspirin right away even though many of the patients in the trial may have received their aspirin the day after presentation. For these reasons, the EARLY-ACS study,9 which is addressing a very simple question—whether early upstream use of glycoprotein IIb/IIIa inhibitors is beneficial—has been a challenging trial to complete.
WHAT ROLE FOR THIENOPYRIDINE PRETREATMENT?
Dr. Bhatt: Dr. Sabatine, you presented data from the large TRITON-TIMI 38 trial comparing prasugrel with clopidogrel. I’m interested in how you would use prasugrel in practice, assuming it receives marketing approval, especially in light of its bleeding risk, particularly in patients in whom coronary artery bypass graft surgery (CABG) is planned. Many hospitals pretreat patients with clopidogrel in the emergency department. How would you manage a patient who shows up in the emergency room with ACS? Would you give clopidogrel, would you wait and give prasugrel, or would you do something else? If you gave clopidogrel, what loading dose would you use—300 mg, 600 mg, or, as some have suggested, 900 or 1,200 mg?
Dr. Sabatine: I am a strong proponent of pretreatment. Data from multiple studies show a benefit to this strategy, and even the original CURE trial showed a roughly 30% reduction in ischemic events within the first 24 hours of clopidogrel initiation.7
I think the dosing strategy depends on how the patient is going to be managed. If management is going to be conservative, then I would start the patient on 300 mg of clopidogrel when he or she came in. If the patient is going to the cardiac catheterization laboratory in a few hours, I would pretreat with 600 mg of clopidogrel. For prasugrel, the need for pretreatment is less clear, given the drug’s faster onset of action and greater degree of platelet inhibition. In the TRITON-TIMI 38 study,10 prasugrel was given, by and large, after diagnostic angiography, and thus one could use that approach in practice.
In terms of clopidogrel versus prasugrel, I would embrace prasugrel for the large majority of my patients, being mindful of the risk of bleeding. I would not hesitate to give the medication to diabetics or to younger, more robust patients. The 50% reduction in stent thrombosis with prasugrel versus clopidogrel in TRITON-TIMI 38 is huge,11 given that the risk of death with stent thrombosis is probably 25% or greater. So I would want to have prasugrel on board to reduce the risk of stent thrombosis, especially if a drug-eluting stent were being implanted.
Dr. Bhatt: Dr. Alexander, let’s get your take on a similar scenario. Assuming that prasugrel gains marketing approval, how would you manage patients with non-ST-elevation MI who present to the emergency department? Would you pretreat with clopidogrel? Would you wait until angiography and then, depending on the anatomy, treat with prasugrel? Or would you potentially pretreat with prasugrel, which has not been studied and would not be a labeled indication? How would you reconcile the data?
Dr. Alexander: At Duke, I expect that prasugrel will not be used prior to the catheterization laboratory in patients with non-ST-elevation ACS due to concerns about whether the patients will undergo PCI or be managed medically or with CABG.
Dr. Bhatt: That makes sense, since there was a fair amount of bleeding with prasugrel in those patients in TRITON-TIMI 38.
Dr. Alexander: Correct. Moreover, at Duke we don’t use as much upstream clopidogrel as we would, based on the evidence, if I were managing all the patients. There is still a lot of pushback about upstream clopidogrel from our surgeons because patients are going to surgery quickly these days, sometimes just a day after catheterization, and that’s when a loading dose of clopidogrel can be problematic. We are also still fairly heavy users of glycoprotein IIb/IIIa inhibitors.
Where I can see prasugrel being used prior to the cath lab at Duke is in ST-elevation MI, where the rate of PCI is very high. In primary angioplasty for ST-elevation MI, it would likely be given upstream. The bigger issue for us will be that we serve as a referral base for a lot of regional hospitals, and thus have some influence on their practices.
Dr. Bhatt: In that case, what would you advise those regional hospitals to do for non-ST-elevation MI?
Dr. Alexander: For the time being, we would advise continuing with our current practice, which is to load clopidogrel in patients in whom there is a reasonable certainty that CABG will not be performed, and to use glycoprotein IIb/IIIa inhibitors in high-risk patients. As we get more experience with prasugrel or with additional trial results, however, that practice could easily change.
Dr. Bhatt: So you would still use glycoprotein IIb/IIIa inhibitors?
Dr. Alexander: Yes, I advocate upstream clopidogrel use, but not all my colleagues do. Based on the guidelines, I’d use one or the other—either clopidogrel or a glycoprotein IIb/IIIa inhibitor. As I mentioned in my talk, if a patient is at high risk for bleeding, I am more inclined to use clopidogrel, although patients at higher risk of bleeding are often at higher risk for ischemic events as well.
WHAT’S DRIVEN THE DROPOFF IN GLYCOPROTEIN IIb/IIIa INHIBITOR USE?
Dr. Bhatt: While we’re on the topic of glycoprotein IIb/IIIa inhibitors, a question card from the audience asks why there has been a decrease in glycoprotein IIb/ IIIa inhibitor use and whether this decline is appropriate or inappropriate. Have clopidogrel pretreatment, higher loading doses of clopidogrel, and use of the direct thrombin inhibitor bivalirudin contributed to the decrease in glycoprotein IIb/IIIa inhibitor use?
Dr. Alexander: I do think that the decline has been driven by the changing environment, with greater use of other antithrombotic strategies that include clopidogrel and bivalirudin, as you suggest, as well as an increased attention to bleeding. From an evidence-based standpoint, we don’t know whether the decrease in glycoprotein IIb/IIIa use is appropriate or not because the studies of these agents were conducted before the widespread upstream use of clopidogrel and bivalirudin. Clopidogrel is attractive because it’s a pill given as one dose in the emergency department, the wards, or the catheterization laboratory, rather than a much more complicated infusion with weight-based dosing and dosage adjustments based on creatinine clearance. It is possible that we should perhaps be dosing clopidogrel the same way, but we don’t know that yet.
PRASUGREL IN PRACTICE: HOW LOW CAN THE DOSE GO, AND IS THERE A GENDER EFFECT?
Dr. Bhatt: Let’s stick with this focus on dosing but turn back to discussion of prasugrel. In your presentation of the TRITON-TIMI 38 data, Dr. Sabatine, you proposed a potential prasugrel dosage modification, down to a 5-mg loading dose, in subgroups that were identified as being at high bleeding risk—namely, elderly patients and patients with low body weight. However, no outcomes data with 5 mg of prasugrel came out of TRITON-TIMI 38.10 Is this proposed modification based on pharmacokinetic extrapolation? Could clinicians be comfortable using 5 mg of prasugrel, assuming the drug receives regulatory approval and a 5-mg tablet would be available?
Dr. Sabatine: Of course, evidence at the grade A level would consist of a trial showing that patients who received a lower dose enjoyed the same benefit as those who got standard dosing in TRITON-TIMI 38—a 60-mg loading dose followed by 10 mg/day—with an acceptable risk profile. However, such a trial would be difficult and costly to conduct, and would take roughly half a decade to pull off. It is only through large trials like TRITON-TIMI 38 that you identify subgroups that respond differently, and then to go back and do a separate trial for those subgroups takes a great deal of time. It may not be practical.
I think the Food and Drug Administration is moving toward embracing careful pharmacokinetic/pharmacodynamic substudies within trials, with these substudies having adequate numbers of subjects to provide a sense for the ideal target dose and what an acceptable dose range would be, without limiting approval to a single dose. The analogy would be warfarin dosing, with the aim being to figure out an acceptable dose range, discover which patients fall outside that range, and then model the effect of a lower dose in those patients. Thus, approving a 5-mg dose of prasugrel based on TRITON-TIMI 38 would be a reasonable approach if this dose passed muster under pharmacokinetic/pharmacodynamic modeling. If this approach were taken, there would clearly be a need for postmarketing surveillance to confirm whether the modeling on the effects of the lower dose was borne out by actual outcomes.
Dr. Bhatt: The audience has posed another interesting question raised by TRITON-TIMI 38: Can you comment on the lesser effect of prasugrel in women?
Dr. Sabatine: It is true that there was not a statistically significant effect of prasugrel among women in TRITON-TIMI 38, but statistical tests among subgroups found no significant heterogeneity for the effect between men and women, and that is the relevant measure to determine any gender effect. Keep in mind that not all subgroups represent a univariate slice of the population. For example, women generally have lower body weight than men, and since prasugrel’s net clinical benefit was reduced in patients with lower body weight, that may explain some of the differing extent of effect between men and women.
Dr. Bhatt: That’s a good point about the lack of heterogeneity between men and women. In fact, a meta-analysis of clopidogrel data conducted by one of the fellows I work with revealed that men and women appear to benefit similarly from clopidogrel.12 There was a slight signal of excess bleeding in women, but there were more elderly women in the pooled population, which may have been a confounding factor. As best as anyone can tell, antiplate-let therapy works well in both men and women.
NAVIGATING MANAGEMENT ACROSS THE SPECTRUM OF CARE
Dr. Bhatt: I would like to explore a bit further how all of these issues translate across the spectrum of care, beginning in the emergency department, which we know is a key component of the entire ACS management strategy for a health care system. What should emergency medicine doctors do, given all of the potential options—clopidogrel, different loading doses of clopidogrel, prasugrel, glycoprotein IIb/IIIa inhibitors, even bivalirudin?
Dr. Peacock: It depends on the practice setting. Some emergency physicians work at community hospitals with no backup. They must have relationships with the larger centers to which they’ll be transferring patients, because ACS patients should not be staying at community hospitals. These emergency physicians must have close relationships with the physicians who will be receiving their patients, and they know the potential head-butting with surgeons surrounding early clopidogrel use better than anybody does. If they treat with clopidogrel in the emergency room, and it turns out that the patient needs to go to the catheterization laboratory, can the receiving hospital use platelet testing to shorten the standard 5-day interval from treatment to catheterization?
Dr. Bhatt: Yes, that’s a rather useful, although not completely validated, way of using point-of-care platelet testing—to potentially reduce the time to surgery.
Dr. Peacock: Right. So if the policies for handling these types of transfer-related issues are worked out in advance, all players have a pathway to follow, which can allow quick action when necessary. If you don’t have these issues worked out in advance, you either lose many opportunities to act quickly in the emergency room or you risk taking actions that will cause problems later in the course of management.
Dr. Alexander: I totally agree. The key is to sit down with all the players involved—the surgeons, the interventional cardiologists, the intensivists, the emergency room personnel—and come up with strategies for different populations of patients. Write down the collective strategy and hang it on the wall so that everybody can be comfortable with it. The strategy can be reevaluated when prasugrel or other new antithrombotic drugs come on the market.
Dr. Peacock: The other environment is the academic center, which is even more challenging, but for different reasons. At a large academic center like the Cleveland Clinic, any of 25 different cardiologists may be taking call and receiving patients from the emergency department on a particular night. A lot of phone interaction is required to elicit the planned management strategy, including if and when the patient will be going to the cath lab. Individualizing care to a particular cardiologist then becomes a time-consuming challenge, especially in clinical situations where outcomes are time-dependent.
Dr. Alexander: Agreed. Management needs to be integrated across the entire spectrum of care. The anticoagulants that we plan to use in the cath lab will affect the antithrombotic regimen used upstream.
Dr. Kottke-Marchant: One circumstance where platelet function testing has been helpful is in determining the washout of the clopidogrel effect before surgery. At Cleveland Clinic, we have implemented platelet function testing in this circumstance instead of waiting a blanket 5 days after clopidogrel administration to go to surgery. A return to normal platelet function on platelet aggregation testing, depending on the cutoff value used, is an indicator that the patient can proceed to surgery.
Dr. Bhatt: That’s a logical approach. How should we be using antiplatelet therapy in the medically managed patient, Dr. Alexander?
Dr. Alexander: When I think of medical management, I include patients who don’t go to the cath lab, but also those who do, with regards to their management prior to and following their time in the cath lab.
In patients who don’t go to the cath lab for angiography, the ACC/AHA guidelines recommend aspirin and either clopidogrel, a glycoprotein IIb/IIIa inhibitor, or both.1 In making this choice, I consider the patient’s risk of bleeding and the dosing complexity of the regimen, especially with the use of glycoprotein IIb/IIIa inhibitors in a patient with renal insufficiency. In a patient at relatively low risk for bleeding, I often use both clopidogrel and a glycoprotein IIb/IIIa inhibitor, although this strategy does not have a lot of data to support it.
The more challenging population consists of patients who go to the cath lab but do not undergo PCI; this population is managed medically too. We often drop the ball with clopidogrel in this population. Many patients in whom PCI is not performed do not receive clopidogrel upstream, for all of the reasons we’ve discussed, and there is pretty good evidence that if clopidogrel is not instituted before hospital discharge, the patient is not likely to be receiving it at 30 days either. We have an obligation to treat these patients.
Treatment following bypass surgery is much murkier, and I don’t really know what we should be doing. The ACC/AHA guidelines suggest that clopidogrel be started in a patient with non-ST-elevation ACS after bypass surgery,1 but I believe the evidence to support that recommendation is pretty weak.
Dr. Bhatt: Well, the CURE trial did contain a sizeable group that underwent bypass surgery,7 and although this group was underpowered in some respects, it was still a very large group, so I personally favor treatment in those patients. We should mention that an ongoing trial called TRILOGY ACS is comparing clopidogrel and prasugrel specifically in patients who are being managed medically,13 so more data on this strategy will be emerging.
ARE GUIDELINES DESTINED TO BECOME EVER MORE COMPLEX?
Dr. Bhatt: Here’s a comment and question from the audience that pulls together a lot of what we’ve discussed while also looking forward: The antiplatelet therapy guidelines are already complicated. If the ongoing studies of emerging antiplatelet drugs all have results that are qualitatively similar to those of the TRITON-TIMI 38 study of prasugrel—ie, better efficacy with more potent therapy but more bleeding—how do you foresee these antiplatelet drugs being used in clinical practice?
Dr. Sabatine: The contrast between the US guidelines and the European guidelines for ACS management is stark. The US guidelines—from the ACC and AHA1—are essentially an encyclopedia that includes nearly every trial of anti-platelet therapy in ACS along with complicated algorithms; they do a wonderful job of being complete. The European guidelines14 are probably one tenth the size of their US counterpart document, and they suggest treatments for various patient types; they are very simple.
In a sense, the US guidelines lay out the data and force practitioners to evaluate the trials and consider how our patients fit into the study populations. In this way they are analogous to current guidelines for anticoagulant therapy. Several anticoagulants have been compared with heparin in clinical trials. These newer anticoagulants appear to reduce the risk of ischemic events compared with heparin; some have lower rates of bleeding, while others have higher rates of bleeding. There have been few head-to-head studies of these agents, however, so we wind up with guidelines that are not definitive but rather suggest agents to “consider” in various settings.
It’s unlikely that a head-to-head trial will be conducted comparing prasugrel with the reversible P2Y12 antagonist AZD6140, assuming that both are approved for marketing. If the drugs appear equally efficacious in placebo-controlled trials, it will take consensus to determine the appropriate choice at your hospital, factoring in your patient profile, the cost of the drugs, and other variables. It’s more complicated when one agent is slightly more efficacious but causes more bleeding or, conversely, a little less efficacious but less apt to cause bleeding. In such cases, you may need to tailor therapy to the patient, trying to gauge bleeding risk. All of the emerging data appear to point to the importance of bleeding on outcomes: patients who bleed fare poorly, in part due to the bleeding itself and in part perhaps because they have a proclivity for bleeding.
THE FUTURE: MONITORING-BASED DOSING AND NICHE ANTIPLATELETS?
Dr. Bhatt: That’s a good observation. Let’s wrap up by having the other panelists share any final thoughts you may have.
Dr. Alexander: I’d like to return to the issue of measuring antiplatelet response and using it to guide therapy. Earlier we cited the examples of antihypertensive therapy and lipid-lowering therapy to support this model of monitoring-based treatment. Guidelines for dyslipidemia treatment recommend using LDL-C levels to guide therapy, but this practice is difficult to study in a randomized trial. In fact, none of the randomized trials of statins used LDL-C levels to guide therapy. They all studied fixed doses of statins versus placebo or fixed doses of another statin. Higher doses of statins were always beneficial compared with lower doses, and this finding was extrapolated into the guidelines as a justification to treat to target LDL-C levels.
Dr. Bhatt: It’s not even necessarily clear that LDL-C level is the best target, if you consider the JUPITER trial, in which patients received statin therapy based on their baseline level of high-sensitivity C-reactive protein, not their LDL-C level.15 It goes to show how incomplete our knowledge of a class of drugs may be, even decades after the drugs are introduced.
Dr. Kottke-Marchant: To speak to Dr. Alexander’s point, dose adjustment guided by platelet monitoring is a bit more problematic for antiplatelet drugs that are irreversible inhibitors, such as clopidogrel and aspirin, than for those that are reversible inhibitors, which are being developed and may eventually make more sense to use. From a drug development standpoint, a drug that requires monitoring and dose adjustment will not gain wide acceptance because it will increase medical costs and morbidity.
Dr. Bhatt: Yes, we know from experience with warfarin that doctors and patients don’t like the ongoing need for monitoring and testing.
Dr. Peacock: The drugs that are going to be adopted by the emergency department are those with the shortest half-lives, for several reasons: (1) using a drug with a short half-life won’t commit us to a particular course of action; (2) the potential for drug interactions is lower; and (3) in the event of an erroneous diagnosis, the consequence of misapplication may be mitigated by early recognition and termination of the drug. If we later decide that we’ve gone down the wrong therapeutic road or reached a wrong diagnosis, or if a complication occurs, we can turn off the therapy quickly. That level of flexibility is needed.
Dr. Kottke-Marchant: I think we are moving into an era of niche antiplatelet drugs. One might be used in a patient going to surgery, for example, and another for long-term therapy.
Dr. Peacock: One thing that I don’t have a feel for is how to transition from one drug to another. When you change drugs for a patient, it so often seems like it goes badly. If we’re eventually going to use drugs with ultra-short half-lives in the in the emergency department for the first day or two, and then switch patients to a pill for a week, a lot more platelet function testing may be needed.
- Anderson JL, Adams CD, Antman EM, et al. ACC/AHA 2007 guidelines for the management of patients with unstable angina/ non-ST-elevation myocardial infarction: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2007; 50:e1–e157.
- Bonello L, Camoin-Jau L, Arques S, et al. Adjusted clopidogrel loading doses according to vasodilator-stimulated phosphoprotein phosphorylation index decrease rate of major adverse cardiovascular events in patients with clopidogrel resistance: a multicenter randomized prospective study. J Am Coll Cardiol 2008; 51:1404–1411.
- Glauser J, Emerman CL, Bhatt DL, Peacock WF. Platelet aspirin resistance in emergency department patients with suspected acute coronary syndrome. Am J Emerg Med. In press
- Patti G, Nusca A, Mangiacapra F, Gatto L, D’Ambrosio A, Di Sciascio G. Point-of-care measurement of clopidogrel responsiveness predicts clinical outcome in patients undergoing percutaneous coronary intervention. J Am Coll Cardiol 2008; 52:1128–1133.
- Mega JL, Close SL, Wiviott SD, et al. Cytochrome P-450 polymorphisms and response to clopidogrel. N Engl J Med 2009; 360:354–362.
- Bhatt DL, Scheiman J, Abraham NS, et al. ACCF/ACG/AHA 2008 expert consensus document on reducing the gastrointestinal risks of antiplatelet therapy and NSAID use. J Am Coll Cardiol 2008; 52:1502–1517.
- Fox KA, Mehta SR, Peters R, et al. Benefits and risks of the combination of clopidogrel and aspirin in patients undergoing surgical revascularization for non-ST-elevation acute coronary syndrome: the Clopidogrel in Unstable angina to prevent Recurrent ischemic Events (CURE) Trial. Circulation 2004; 110:1202–1208.
- ISIS-2 Collaborative Group. Randomised trial of intravenous streptokinase, oral aspirin, both, or neither among 17,187 cases of suspected acute myocardial infarction: ISIS-2. Lancet 1988; 2:349–360.
- EARLY-ACS: Glycoprotein IIb/IIIa inhibition in patients with non-ST-segment elevation acute coronary syndrome. Clinical Trials.gov Web site. http://clinicaltrials.gov/ct2/show/NCT00089895. Updated December 17, 2008. Accessed December 18, 2008.
- Wiviott SD, Braunwald E, McCabe CH, et al; TRITON-TIMI 38 Investigators. Prasugrel versus clopidogrel in patients with acute coronary syndromes. N Engl J Med 2007; 357:2001–2015.
- Wiviott SD, Braunwald E, McCabe CH, et al. Intensive oral anti-platelet therapy for reduction of ischaemic events including stent thrombosis in patients with acute coronary dyndromes treated with percutaneous coronary intervention and stenting in the TRITON-TIMI 38 trial: a subanalysis of a randomised trial. Lancet 2008; 371:1353–1363.
- Berger JS, Bhatt DL, Chen Z, et al. The relationship between sex, mortality and cardiovascular events among patients with established cardiovascular disease: a meta-analysis [ACC abstract 1012-149]. J Am Coll Cardiol 2008; 51 10 suppl A:A247.
- TRILOGY ACS: A comparison of prasugrel and clopidogrel in acute coronary syndrome subjects. ClinicalTrials.gov Web site. http://clinicaltrials.gov/ct2/show/NCT00699998. Updated December 15, 2008. Accessed January 2, 2009.
- Guidelines for the diagnosis and treatment of non-ST-segment elevation acute coronary syndromes. Eur Heart J 2007; 28:1598–1660.
- Ridker PM, Danielson E, Fonseca FA, et al. Rosuvastatin to prevent vascular events in men and women with elevated C-reactive protein. N Engl J Med 2008; 359:2195–2207.
- Anderson JL, Adams CD, Antman EM, et al. ACC/AHA 2007 guidelines for the management of patients with unstable angina/ non-ST-elevation myocardial infarction: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2007; 50:e1–e157.
- Bonello L, Camoin-Jau L, Arques S, et al. Adjusted clopidogrel loading doses according to vasodilator-stimulated phosphoprotein phosphorylation index decrease rate of major adverse cardiovascular events in patients with clopidogrel resistance: a multicenter randomized prospective study. J Am Coll Cardiol 2008; 51:1404–1411.
- Glauser J, Emerman CL, Bhatt DL, Peacock WF. Platelet aspirin resistance in emergency department patients with suspected acute coronary syndrome. Am J Emerg Med. In press
- Patti G, Nusca A, Mangiacapra F, Gatto L, D’Ambrosio A, Di Sciascio G. Point-of-care measurement of clopidogrel responsiveness predicts clinical outcome in patients undergoing percutaneous coronary intervention. J Am Coll Cardiol 2008; 52:1128–1133.
- Mega JL, Close SL, Wiviott SD, et al. Cytochrome P-450 polymorphisms and response to clopidogrel. N Engl J Med 2009; 360:354–362.
- Bhatt DL, Scheiman J, Abraham NS, et al. ACCF/ACG/AHA 2008 expert consensus document on reducing the gastrointestinal risks of antiplatelet therapy and NSAID use. J Am Coll Cardiol 2008; 52:1502–1517.
- Fox KA, Mehta SR, Peters R, et al. Benefits and risks of the combination of clopidogrel and aspirin in patients undergoing surgical revascularization for non-ST-elevation acute coronary syndrome: the Clopidogrel in Unstable angina to prevent Recurrent ischemic Events (CURE) Trial. Circulation 2004; 110:1202–1208.
- ISIS-2 Collaborative Group. Randomised trial of intravenous streptokinase, oral aspirin, both, or neither among 17,187 cases of suspected acute myocardial infarction: ISIS-2. Lancet 1988; 2:349–360.
- EARLY-ACS: Glycoprotein IIb/IIIa inhibition in patients with non-ST-segment elevation acute coronary syndrome. Clinical Trials.gov Web site. http://clinicaltrials.gov/ct2/show/NCT00089895. Updated December 17, 2008. Accessed December 18, 2008.
- Wiviott SD, Braunwald E, McCabe CH, et al; TRITON-TIMI 38 Investigators. Prasugrel versus clopidogrel in patients with acute coronary syndromes. N Engl J Med 2007; 357:2001–2015.
- Wiviott SD, Braunwald E, McCabe CH, et al. Intensive oral anti-platelet therapy for reduction of ischaemic events including stent thrombosis in patients with acute coronary dyndromes treated with percutaneous coronary intervention and stenting in the TRITON-TIMI 38 trial: a subanalysis of a randomised trial. Lancet 2008; 371:1353–1363.
- Berger JS, Bhatt DL, Chen Z, et al. The relationship between sex, mortality and cardiovascular events among patients with established cardiovascular disease: a meta-analysis [ACC abstract 1012-149]. J Am Coll Cardiol 2008; 51 10 suppl A:A247.
- TRILOGY ACS: A comparison of prasugrel and clopidogrel in acute coronary syndrome subjects. ClinicalTrials.gov Web site. http://clinicaltrials.gov/ct2/show/NCT00699998. Updated December 15, 2008. Accessed January 2, 2009.
- Guidelines for the diagnosis and treatment of non-ST-segment elevation acute coronary syndromes. Eur Heart J 2007; 28:1598–1660.
- Ridker PM, Danielson E, Fonseca FA, et al. Rosuvastatin to prevent vascular events in men and women with elevated C-reactive protein. N Engl J Med 2008; 359:2195–2207.