User login
IVC and Mortality in ADHF
Heart failure costs the United States an excess of $30 billion annually, and costs are projected to increase to nearly $70 billion by 2030.[1] Heart failure accounts for over 1 million hospitalizations and is the leading cause of hospitalization in patients >65 years of age.[2] After hospitalization, approximately 50% of patients are readmitted within 6 months of hospital discharge.[3] Mortality rates from heart failure have improved but remain high.[4] Approximately 50% of patients diagnosed with heart failure die within 5 years, and the overall 1‐year mortality rate is 30%.[1]
Prognostic markers and scoring systems for acute decompensated heart failure (ADHF) continue to emerge, but few bedside tools are available to clinicians. Age, brain natriuretic peptide, and N‐terminal pro‐brain natriuretic peptide (NT‐proBNP) levels have been shown to correlate with postdischarge rates of readmission and mortality.[5] A study evaluating the prognostic value of a bedside inferior vena cava (IVC) ultrasound exam demonstrated that lack of improvement in IVC distention from admission to discharge was associated with higher 30‐day readmission rates.[6] Two studies using data from comprehensive transthoracic echocardiograms in heart failure patients demonstrated that a dilated, noncollapsible IVC is associated with higher risk of mortality; however, it is well recognized that obtaining comprehensive transthoracic echocardiograms in all patients hospitalized with heart failure is neither cost‐effective nor practical.[7]
In recent years, multiple studies have emerged demonstrating that noncardiologists can perform focused cardiac ultrasound exams with high reproducibility and accuracy to guide management of patients with ADHF.[8, 9, 10, 11, 12, 13, 14] However, it is unknown whether IVC characteristics from a focused cardiac ultrasound exam performed by a noncardiologist can predict mortality of patients hospitalized with ADHF. The aim of this study was to assess whether a hospitalist‐performed focused ultrasound exam to measure the IVC diameter at admission and discharge can predict mortality in a general medicine ward population hospitalized with ADHF.
METHODS
Study Design
A prospective, observational study of patients admitted to a general medicine ward with ADHF between January 2012 and March 2013 was performed using convenience sampling. The setting was a 247‐bed, university‐affiliated hospital in Madrid, Spain. Inclusion criteria were adult patients admitted with a primary diagnosis of ADHF per the European Society of Cardiology (ESC) criteria.[15] Exclusion criteria were admission to the intensive care unit for mechanical ventilation, need for chronic hemodialysis, or a noncardiac terminal illness with a life expectancy of less than 3 months. All patients provided written informed consent prior to enrollment. This study complies with the Declaration of Helsinki and was approved by the local ethics committee.
The primary outcome was all‐cause mortality at 90 days after hospitalization. The secondary outcomes were hospital readmission at 90 and 180 days, and mortality at 180 days. Patients were prospectively followed up at 30, 60, 90, and 180 days after discharge by telephone interview or by review of the patient's electronic health record. Patients who died within 90 days of discharge were categorized as nonsurvivors, whereas those alive at 90 days were categorized as survivors.
The following data were recorded on admission: age, gender, blood pressure, heart rate, functional class per New York Heart Association (NYHA) classification, comorbidities (hypertension, diabetes mellitus, atrial fibrillation, chronic obstructive pulmonary disease), primary etiology of heart failure, medications, electrocardiogram, NT‐terminal pro‐BNP, hemoglobin, albumin, creatinine, sodium, measurement of performance of activities of daily living (modified Barthel index), and comorbidity score (age‐adjusted Charlson score). A research coordinator interviewed subjects to gather data to calculate a modified Barthel index.[16] Age‐adjusted Charlson comorbidity scores were calculated using age and diagnoses per International Classification of Diseases, Ninth Revision coding.[17]
IVC Measurement
An internal medicine hospitalist with expertise in point‐of‐care ultrasonography (G.G.C.) performed all focused cardiac ultrasound exams to measure the IVC diameter and collapsibility at the time of admission and discharge. This physician was not involved in the inpatient medical management of study subjects. A second physician (N.J.S.) randomly reviewed 10% of the IVC images for quality assurance. Admission IVC measurements were acquired within 24 hours of arrival to the emergency department after the on‐call medical team was contacted to admit the patient. Measurement of the IVC maximum (IVCmax) and IVC minimum (IVCmin) diameters was obtained just distal to the hepatic veinIVC junction, or 2 cm from the IVCright atrial junction using a long‐axis view of the IVC. Measurement of the IVC diameter was consistent with the technique recommended by the American Society of Echocardiography and European Society of Echocardiography guidelines.[18, 19] The IVC collapsibility index (IVCCI) was calculated as (IVCmaxIVCmin)/IVCmax per guidelines.[18] Focused cardiac ultrasound exams were performed using a General Electric Logiq E device (GE Healthcare, Little Chalfont, United Kingdom) with a 3.5 MHz curvilinear transducer. Inpatient medical management by the primary medical team was guided by protocols from the ESC guidelines on the treatment of ADHF.[15] A comprehensive transthoracic echocardiogram (TTE) was performed on all study subjects by the echocardiography laboratory within 24 hours of hospitalization as part of the study protocol. One of 3 senior cardiologists read all comprehensive TTEs. NT‐proBNP was measured on admission and discharge by electrochemiluminescence.
Statistical Analysis
We calculated the required sample size based on published mortality and readmission rates. For our primary outcome of 90‐day mortality, we calculated a required sample size of 64 to achieve 80% power based on 90‐day and 1‐year mortality rates of 21% and 33%, respectively, among Spanish elderly patients (age 70 years) hospitalized with ADHF.[20] For our secondary outcome of 90‐day readmissions, we calculated a sample size of 28 based on a 41% readmission rate.[21] Therefore, our target subject enrollment was at least 70 patients to achieve a power of 80%.
Statistical analyses were performed using SPSS 17.0 statistical package (SPSS Inc., Chicago, IL). Subject characteristics that were categorical variables (demographics and comorbidities) were summarized as counts and percentages. Continuous variables, including IVC measurements, were summarized as means with standard deviations. Differences between categorical variables were analyzed using the Fisher exact test. Survival curves with log‐rank statistics were used to perform survival analysis. The nonparametric Mann‐Whitney U test was used to assess associations between the change in IVCCI, and readmissions and mortality at 90 and 180 days. Predictors of readmission and death were evaluated using a multivariate Cox proportional hazards regression analysis. Given the limited number of primary outcome events, we used age, IVC diameter, and log NT‐proBNP in the multivariate regression analysis based on past studies showing prognostic significance of these variables.[6, 22, 23, 24, 25, 26, 27, 28] Optimal cutoff values for IVC diameter for death and readmission prediction were determined by constructing receiver operating characteristic (ROC) curves and calculating the area under the curve (AUC) for different IVC diameters. NT‐proBNP values were log‐transformed to minimize skewing as reported in previous studies.[29]
RESULTS
Patient Characteristics
Ninety‐seven patients admitted with ADHF were recruited for the study. Optimal acoustic windows to measure the IVC diameter were acquired in 90 patients (93%). Because measurement of discharge IVC diameter was required to calculate the change from admission to discharge, 8 patients who died during initial hospitalization were excluded from the final data analysis. An additional two patients were excluded due to missing discharge NT‐proBNP measurement or missing comprehensive echocardiogram data. The study cohort from whom data were analyzed included 80 of 97 total patients (82%).
Baseline demographic, clinical, laboratory, and comprehensive echocardiographic characteristics of nonsurvivors and survivors at 90 days are demonstrated in Table 1. Eleven patients (13.7%) died during the first 90 days postdischarge, and all deaths were due to cardiovascular complications. Nonsurvivors were older (86 vs 76 years; P = 0.02), less independent in performance of their activities of daily living (Barthel index of 58.1 vs 81.9; P = 0.01), and were more likely to have advanced heart failure with an NYHA functional class of III or IV (72% vs 33%; P = 0.016). Atrial fibrillation (90% vs 55%; P = 0.008) and lower systolic blood pressure (127 mm Hg vs 147 mm Hg; P = 0.01) were more common in nonsurvivors than survivors, and fewer nonsurvivors were taking a ‐blocker (18% vs 59%; P = 0.01). Baseline comprehensive echocardiographic findings were similar between the survivors and nonsurvivors, except left atrial diameter was larger in nonsurvivors versus survivors (54 mm vs 49 mm; P = 0.04).
| Total Cohort, n = 80 | Nonsurvivors, n = 11 | Survivors, n = 69 | P Value | |
|---|---|---|---|---|
| ||||
| Demographics | ||||
| Age, y* | 78 (13) | 86 (7) | 76 (14) | 0.02 |
| Men, n (%) | 34 (42) | 3 (27) | 26 (38) | 0.3 |
| Vital signs* | ||||
| Heart rate, beats/min | 94 (23) | 99 (26) | 95 (23) | 0.5 |
| SBP, mm Hg | 141 (27) | 127 (22) | 147 (25) | 0.01 |
| Comorbidities, n (%) | ||||
| Hypertension | 72 (90) | 10 (91) | 54 (78) | 0.3 |
| Diabetes mellitus | 35 (44) | 3 (27) | 26 (38) | 0.3 |
| Atrial fibrillation | 48 (60) | 10 (90) | 38 (55) | 0.008 |
| COPD | 22 (27) | 3 (27) | 16 (23) | 0.5 |
| Etiology of heart failure | ||||
| Ischemic | 20 (25) | 1 (9) | 16 (23) | 0.1 |
| Hypertensive | 22 (27) | 2 (18) | 18 (26) | 0.4 |
| Valvulopathy | 29 (36) | 7 (64) | 19 (27) | 0.07 |
| Other | 18 (22) | 1 (9) | 16 (23) | 0.09 |
| NYHA IIIIV | 38 (47) | 8 (72) | 23 (33) | 0.016 |
| Charlson score* | 7.5 (2) | 9.0 (3) | 7.1 (2) | 0.02 |
| Barthel index* | 76 (31) | 58 (37) | 81.9 (28) | 0.01 |
| Medications | ||||
| ‐blocker | 44 (55) | 2 (18) | 41 (59) | 0.01 |
| ACE inhibitor/ARB | 48 (60) | 3 (27) | 35 (51) | 0.1 |
| Loop diuretic | 78 (97) | 10 (91) | 67 (97) | 0.9 |
| Aldosterone antagonist | 31 (39) | 4 (36) | 21 (30) | 0.4 |
| Lab results* | ||||
| Sodium, mmol/L | 137 (4.8) | 138 (6) | 139 (4) | 0.6 |
| Creatinine, umol/L | 1.24 (0.4) | 1.40 (0.5) | 1.17 (0.4) | 0.1 |
| eGFR, mL/min | 57.8 (20) | 51.2 (20) | 60.2 (19) | 0.1 |
| Albumin, g/L | 3.4 (0.4) | 3.3 (0.38) | 3.5 (0.41) | 0.1 |
| Hemoglobin, g/dL | 12.0 (2) | 10.9 (1.8) | 12.5 (2.0) | 0.01 |
| Echo parameters* | ||||
| LVEF, % | 52.1 (15) | 51.9 (17) | 51.6 (15) | 0.9 |
| LA diameter, mm | 50.1 (10) | 54 (11) | 49 (11) | 0.04 |
| RVDD, mm | 32.0 (11) | 34 (10) | 31 (11) | 0.2 |
| TAPSE, mm | 18.5 (7) | 17.4 (4) | 18.8 (7) | 0.6 |
| PASP, mm Hg | 51.2 (16) | 53.9 (17) | 50.2 (17) | 0.2 |
| Admission* | ||||
| NT‐proBNP, pg/mL | 8,816 (14,260) | 9,413 (5,703) | 8,762 (15,368) | 0.81 |
| Log NT‐proBNP | 3.66 (0.50) | 3.88 (0.31 | 3.62 (0.52) | 0.11 |
| IVCmax, cm | 2.12 (0.59) | 2.39 (0.37) | 2.06 (0.59) | 0.02 |
| IVCmin, cm | 1.63 (0.69) | 1.82 (0.66) | 1.56 (0.67) | 0.25 |
| IVCCI, % | 25.7 (0.16) | 25.9 (17.0) | 26.2 (16.0) | 0.95 |
| Discharge* | ||||
| NT‐proBNP, pg/mL | 3,132 (3,093) | 4,693 (4,383) | 2,909 (2,847) | 0.08 |
| Log NT‐proBNP | 3.27 (0.49) | 3.51 (0.37) | 3.23 (0.50) | 0.08 |
| IVCmax, cm | 1.87 (0.68) | 1.97 (0.54) | 1.81 (0.66) | 0.45 |
| IVCmin, cm | 1.33 (0.75) | 1.40 (0.65) | 1.27 (0.71) | 0.56 |
| IVCCI, % | 33.1 (0.20) | 32.0 (21.0) | 34.2 (19.0) | 0.74 |
From admission to discharge, the total study cohort demonstrated a highly statistically significant reduction in NT‐proBNP (8816 vs 3093; P < 0.001), log NT‐proBNP (3.66 vs 3.27; P < 0.001), IVCmax (2.12 vs 1.87; P < 0.001), IVCmin (1.63 vs 1.33; P < 0.001), and IVCCI (25.7% vs 33.1%; P < 0.001). The admission and discharge NT‐proBNP and IVC characteristics of the survivors and nonsurvivors are displayed in Table 2. The only statistically significant difference between nonsurvivors and survivors was the admission IVCmax (2.39 vs 2.06; P = 0.02). There was not a statistically significant difference in the discharge IVCmax between nonsurvivors and survivors.
| Admission | Discharge | Difference (DischargeAdmission) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Nonsurvivors | Survivors | P Value | Nonsurvivors | Survivors | P Value | Nonsurvivors | Survivors | P Value | |
| |||||||||
| NT‐proBNP, pg/mL | 9,413 (5,703) | 8,762 (15,368) | 0.81 | 4,693 (4,383) | 2,909 (2,847) | 0.08 | 3,717 5,043 | 5,026 11,507 | 0.7 |
| Log NT‐proBNP | 3.88 0.31 | 3.62 0.52 | 0.11 | 3.51 0.37 | 3.23 0.50 | 0.08 | 0.29 0.36 | 0.38 0.37 | 0.4 |
| IVCmax, cm | 2.39 0.37 | 2.06 0.59 | 0.02 | 1.97 0.54 | 1.81 0.66 | 0.45 | 0.39 0.56 | 0.25 0.51 | 0.4 |
| IVCmin, cm | 1.82 0.66 | 1.56 0.67 | 0.25 | 1.40 0.65 | 1.27 0.71 | 0.56 | 0.37 0.52 | 0.30 0.64 | 0.7 |
| IVCCI, % | 25.9 17.0 | 26.2 16.0 | 0.95 | 32.0 21.0 | 34.2 19.0 | 0.74 | 3.7 7.9 | 8.3 22 | 0.5 |
Outcomes
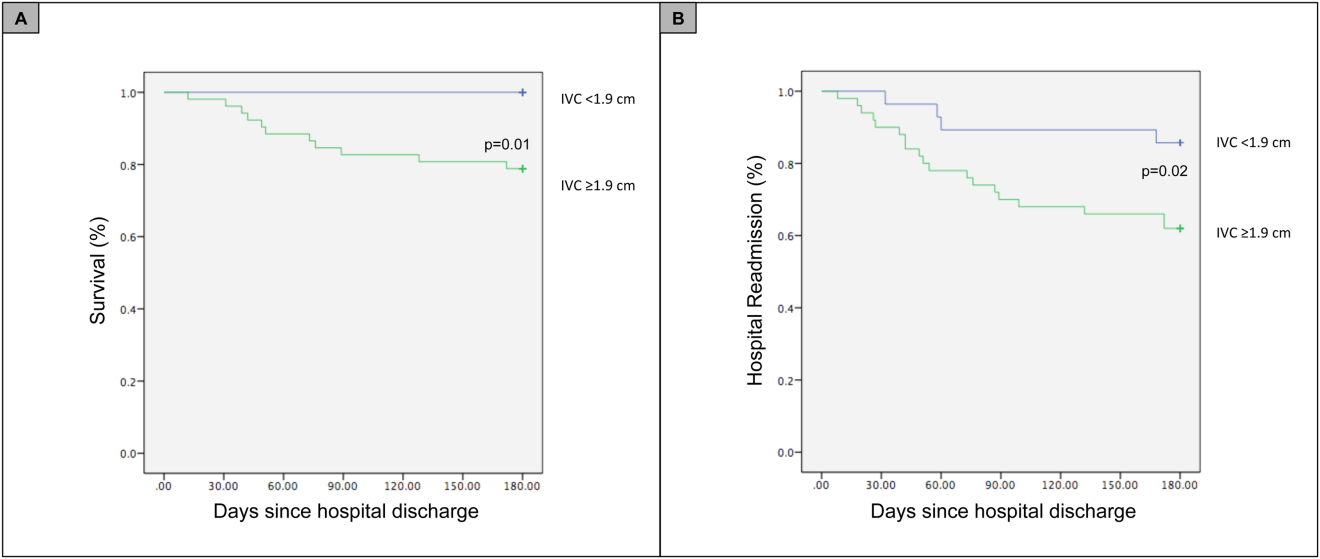
For the primary outcome of 90‐day mortality, the ROC curves showed a similar AUC for the admission IVCmax diameter (AUC: 0.69; 95% confidence interval [CI]: 0.53‐0.85), log NT‐proBNP at discharge (AUC: 0.67; 95% CI: 0.49‐0.85), and log NT‐proBNP at admission (AUC: 0.69; 95% CI: 0.52‐0.85). The optimal cutoff value for the admission IVCmax diameter to predict mortality was 1.9 cm (sensitivity 100%, specificity 38%) based on the ROC curves (see Supporting Information, Appendices 1 and 2, in the online version of this article). An admission IVCmax diameter 1.9 cm was associated with a higher mortality rate at 90 days (25.4% vs 3.4%; P = 0.009) and 180 days (29.3% vs 3.4%; P = 0.003). The Cox survival curves showed significantly lower survival rates in patients with an admission IVCmax diameter 1.9 cm (74.1 vs 96.7%; P = 0.012) (Figures 1 and 2). Based on the multivariate Cox proportional hazards regression analysis with age, IVCmax diameter, and log NT‐proBNP at admission, the admission IVCmax diameter and age were independent predictors of 90‐ and 180‐day mortality. The hazard ratios for death by age, admission IVCmax diameter, and log NT‐proBNP are shown in Table 3.
| Endpoint | Variable | HR (95% CI) | P Value |
|---|---|---|---|
| |||
| 90‐day mortality | Age | 1.14 (1.031.26) | 0.009 |
| IVC diameter at admission | 5.88 (1.2128.1) | 0.025 | |
| Log NT‐proBNP at admission | 1.00 (1.001.00) | 0.910 | |
| 90‐day readmission | Age | 1.06 (1.001.12) | 0.025 |
| IVC diameter at admission | 3.20 (1.248.21) | 0.016 | |
| Log NT‐proBNP at discharge | 1.00 (1.001.00) | 0.910 | |
| 180‐day mortality | Age | 1.12 (1.031.22) | 0.007 |
| IVC diameter at admission | 4.77 (1.2118.7) | 0.025 | |
| Log NT‐proBNP at admission | 1.00 (1.001.00) | 0.610 | |
| 180‐day readmission | Age | 1.06 (1.011.11) | 0.009 |
| IVC diameter at admission | 2.56 (1.145.74) | 0.022 | |
| Log NT‐proBNP at discharge | 1.00 (1.001.00) | 0.610 | |
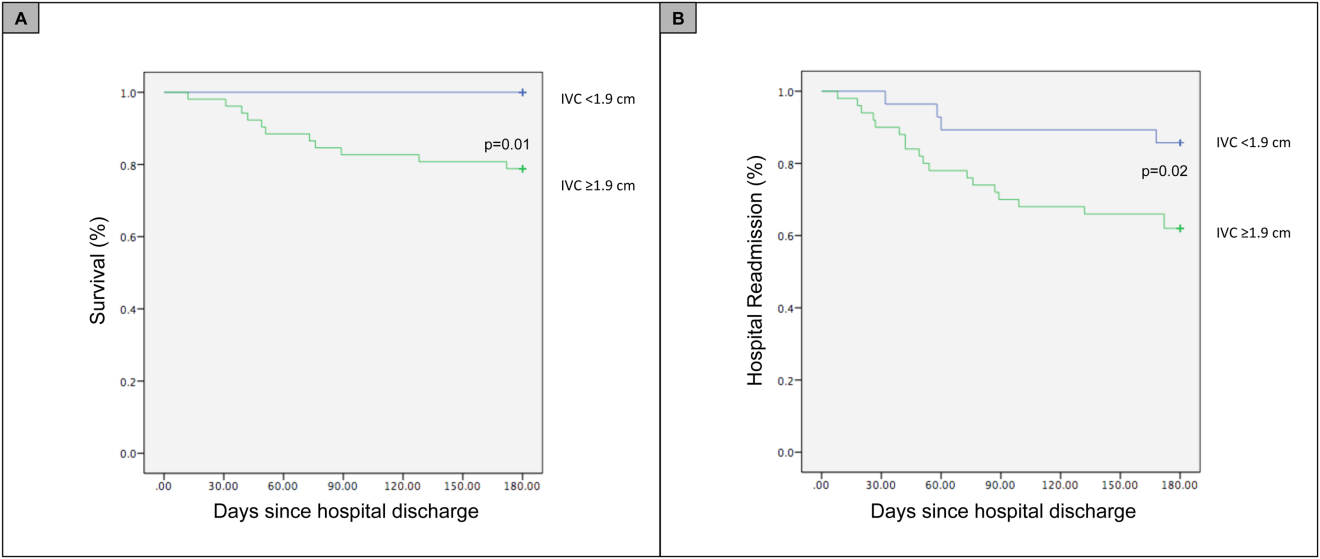
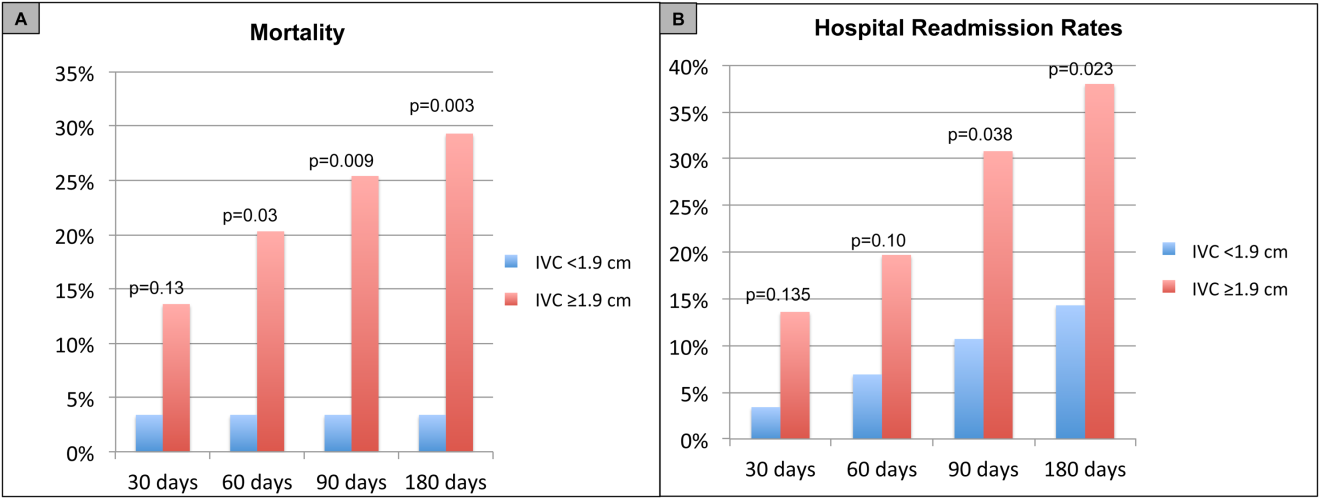
For the secondary outcome of 90‐day readmissions, 19 patients (24%) were readmitted, and the mean index admission IVCmax diameter was significantly greater in patients who were readmitted (2.36 vs 1.98 cm; P = 0.04). The ROC curves for readmission at 90 days showed that an index admission IVCmax diameter of 1.9 cm had the greatest AUC (0.61; 95% CI: 0.49‐0.74). The optimal cutoff value of an index admission IVCmax to predict readmission was also 1.9 cm (sensitivity 94%, specificity 42%) (see Supporting Information, Appendices 1 and 2, in the online version of this article). The Cox survival analysis showed that patients with an index admission IVCmax diameter 1.9 cm had a higher readmission rate at 90 days (30.8% vs 10.7%; P = 0.04) and 180 days (38.0 vs 14.3%; P = 0.02) (Figures 1 and 2). Using a multivariate Cox proportional regression analysis, the hazard ratios for the variables of age, admission IVCmax diameter, and log NT‐proBNP are shown in Table 3.
DISCUSSION
Our study found that a dilated IVC at admission is associated with a poor prognosis after hospitalization for ADHF. Patients with a dilated IVC 1.9 cm at admission had higher mortality and readmission rates at 90 and 180 days postdischarge.
The effect of a dilated IVC on mortality may be mediated through unrecognized right ventricular disease with or without significant pulmonary hypertension, supporting the notion that right heart function is an important determinant of prognosis in patients with ADHF.[30, 31] Similar to elevated jugular venous distension, bedside ultrasound examination of the IVC diameter can serve as a rapid and noninvasive measurement of right atrial pressure.[32] Elevated right atrial pressure is most often due to elevated left ventricular filling pressure transmitted via the pulmonary vasculature, but it is important to note that right‐ and left‐sided cardiac pressures are often discordant in heart failure patients.[33, 34]
Few studies have evaluated the prognostic value of IVC diameter and collapsibility in patients with heart failure. Nath et al.[24] evaluated the prognostic value of IVC diameter in stable veterans referred for outpatient echocardiography. Patients with a dilated IVC >2 cm that did not collapse with inspiration had higher 90‐day and 1‐year mortality rates. A subsequent study by Pellicori et al.[22] investigated the relationship between IVC diameter and other prognostic markers in stable cardiac patients. Pellicori et al. demonstrated that IVC diameter and serum NT‐proBNP levels were independent predictors of a composite endpoint of cardiovascular death or heart failure hospitalization at 1 year.[22] Most recently, Lee et al.[23] evaluated whether a dilated IVC in patients with a history of advanced systolic heart failure with a reduced ejection fraction of 30% and repeated hospitalizations (2) predicted worsening renal failure and adverse cardiovascular outcomes (death or hospitalization for ADHF). The study concluded that age, IVC diameter >2.1 cm, and worsening renal failure predicted cardiovascular death or hospitalization for ADHF.[23]
Our study demonstrated that an admission IVCmax 1.9 cm in hospitalized ADHF patients predicted higher postdischarge mortality at 90 and 180 days. Our findings are consistent with the above‐mentioned studies with a few important differences. First, all of our patients were hospitalized with acute decompensated heart failure. Nath et al. and Pellicori et al. evaluated stable ambulatory patients seen in an echocardiography lab and cardiology clinic, respectively. Only 12.1% of patients in the Nath study had a history of heart failure, and none were reported to have ADHF. More importantly, our study improves our understanding of patients with heart failure with a preserved ejection fraction, an important gap in the literature. The mean ejection fraction of patients in our study was 52% consistent with heart failure with preserved ejection fraction, whereas patients in the Pellicori et al. and Lee et al. studies had heart failure with reduced (42%) or severely reduced (30%) ejection fraction, respectively. We did not anticipate finding heart failure with preserved ejection fraction in the majority of patients, but our study's findings will add to our understanding of this increasingly common type of heart failure.
Compared to previous studies that utilized a registered diagnostic cardiac sonographer to obtain a comprehensive TTE to prognosticate patients, our study utilized point‐of‐care ultrasonography. Nath et al. commented that obtaining a comprehensive echocardiogram on every patient with ADHF is unlikely to be cost‐effective or feasible. Our study utilized a more realistic approach with a frontline internal medicinetrained hospitalist acquiring and interpreting images of the IVC at the bedside using a basic portable ultrasound machine.
Our study did not show that plasma natriuretic peptides levels are predictive of death or readmission after hospitalization for ADHF as shown in previous studies.[22, 35, 36] The small sample size, relatively low event rate, or predominance of heart failure with preserved ejection fraction may explain this inconsistency with prior studies.
Previous studies have reported hospital readmission rates for ADHF of 30% to 44% after 1 to 6 months.[6, 37] Goonewardena et al. showed a 41.3% readmission rate at 30 days in patients with severely reduced left ventricular ejection fraction (mean 29%), and readmitted patients had an IVCmax diameter >2 cm and an IVC collapsibility <50% on admission and discharge.[6] Carbone et al. demonstrated absence of improvement in the minimum IVC diameter from admission to discharge using hand‐carried ultrasound in patients with ischemic heart disease (ejection fraction 33%) predicted readmission at 60 days.[38] Hospital readmission rates in our study are consistent with these previously published studies. We found readmission rates for patients with ADHF and an admission IVCmax 1.9 cm to be 30.8% and 38.0% after 90 and 180 days, respectively.
Important limitations of our study are the small sample size and single institution setting. A larger sample size may have demonstrated that change in IVC diameter and NT‐proBNP levels from admission to discharge to be predictive of mortality or readmission. Further, we found an IVCmax diameter 1.9 cm to be the optimal cutoff to predict mortality, which is less than an IVCmax diameter >2.0 cm reported in other studies. The relatively smaller IVC diameter in Spanish heart failure patients may be explained by the lower body mass index of this population. An IVCmax diameter 1.9 cm was found to be the optimal cutoff to predict an elevated right atrial pressure >10 mm Hg in a study of Japanese cardiac patients with a relatively lower body mass index.[39] Another limitation is the timing of the admission IVC measurement within the first 24 hours of arrival to the hospital rather than immediately upon arrival to the emergency department. We were not able to control for interventions given in the emergency department prior to the measurement of the admission IVC, including doses of diuretics. Further, unlike the comprehensive TTEs in the United States, TTEs in Spain do not routinely include an assessment of the IVC. Therefore, we were not able to compare our bedside IVC measurements to those from a comprehensive TTE. An important limitation of our regression analysis is the inclusion of only 3 variables. The selection of variables (age, NT‐proBNP, and IVC diameter) was based on prior studies demonstrating their prognostic value.[6, 22, 25] Due to the low event rate (n = 11), we could not include in the regression model other variables that differed significantly between nonsurvivors and survivors, including NYHA class, presence of atrial fibrillation, and use of ‐blockers.
Perhaps in a larger study population the admission IVCmax diameter may not be as predictive of 90‐day mortality as other variables. The findings of our exploratory analysis should be confirmed in a future study with a larger sample size.
The clinical implications of our study are 3‐fold. First, our study demonstrates that IVC images acquired by a hospitalist at the bedside using a portable ultrasound machine can be used to predict postdischarge mortality and readmission of patients with ADHF. Second, the predominant type of heart failure in our study was heart failure with preserved ejection fraction. Currently, approximately 50% of patients hospitalized with ADHF have heart failure with preserved ejection fraction.[40] Our study adds to the understanding of prognosis of these patients whose heart failure pathophysiology is not well understood. Finally, palliative care services are underutilized in patients with advanced heart failure.[41, 42] IVC measurements and other prognostic markers in heart failure may guide discussions about goals of care with patients and families, and facilitate timely referrals for palliative care services.
CONCLUSIONS
Point‐of‐care ultrasound evaluation of IVC diameter at the time of admission can be used to prognosticate patients hospitalized with acute decompensated heart failure. An admission IVCmax diameter 1.9 cm is associated with a higher rate of 90‐day and 180‐day readmission and mortality after hospitalization. Future studies should evaluate the combination of IVC characteristics with other markers of severity of illness to prognosticate patients with heart failure.
Disclosures
This study was supported by a grant from the Madrid‐Castilla la Mancha Society of Internal Medicine. Dr. Restrepo is partially supported by award number K23HL096054 from the National Heart, Lung, and Blood Institute. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Heart, Lung, and Blood Institute or the National Institutes of Health. The authors report no conflicts of interest.
- , , , et al. Heart disease and stroke statistics—2015 update: a report from the American Heart Association. Circulation. 2015;131(4):e29–e322.
- , , . Hospitalization for congestive heart failure: United States, 2000–2010. NCHS Data Brief. 2012(108):1–8.
- , . Rehospitalization for heart failure: predict or prevent? Circulation. 2012;126(4):501–506.
- , , , et al. Generalizability and longitudinal outcomes of a national heart failure clinical registry: Comparison of Acute Decompensated Heart Failure National Registry (ADHERE) and non‐ADHERE Medicare beneficiaries. Am Heart J. 2010;160(5):885–892.
- , , , et al. Prognostic markers of acute decompensated heart failure: the emerging roles of cardiac biomarkers and prognostic scores. Arch Cardiovasc Dis. 2015;108(1):64–74.
- , , , et al. Comparison of hand‐carried ultrasound assessment of the inferior vena cava and N‐terminal pro‐brain natriuretic peptide for predicting readmission after hospitalization for acute decompensated heart failure. JACC Cardiovasc Imaging. 2008;1(5):595–601.
- , , , , . Echocardiography in acute heart failure: current perspectives. J Card Fail. 2016;22(1):82–94.
- , , , , . Usefulness of a hand‐held ultrasound device for bedside examination of left ventricular function. Am J Cardiol. 2002;90(9):1038–1039.
- , , , , , . Feasibility of point‐of‐care echocardiography by internal medicine house staff. Am Heart J. 2004;147(3):476–481.
- , , , , , . The use of small personal ultrasound devices by internists without formal training in echocardiography. Eur J Echocardiogr. 2003;4(2):141–147.
- , , , et al. Diagnostic accuracy of hospitalist‐performed hand‐carried ultrasound echocardiography after a brief training program. J Hosp Med. 2009;4(6):340–349.
- , , , . Point‐of‐care multi‐organ ultrasound improves diagnostic accuracy in adults presenting to the emergency department with acute dyspnea. West J Emerg Med. 2016;17(1):46–53.
- , , , , , . Acute heart failure: the role of focused emergency cardiopulmonary ultrasound in identification and early management. Eur J Heart Fail. 2015;17(12):1223–1227.
- , , , et al. Hand‐carried echocardiography by hospitalists: a randomized trial. Am J Med. 2011;124(8):766–774.
- , , , et al. ESC guidelines for the diagnosis and treatment of acute and chronic heart failure 2008: the Task Force for the diagnosis and treatment of acute and chronic heart failure 2008 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association of the ESC (HFA) and endorsed by the European Society of Intensive Care Medicine (ESICM). Eur J Heart Fail. 2008;10(10):933–989.
- , , , , , . Adaptation of the modified Barthel Index for use in physical medicine and rehabilitation in Turkey. Scand J Rehabil Med. 2000;32(2):87–92.
- , , , . Complications, comorbidities, and mortality: improving classification and prediction. Health Serv Res. 1997;32(2):229–238; discussion 239–242.
- , , , et al. Guidelines for the echocardiographic assessment of the right heart in adults: a report from the American Society of Echocardiography endorsed by the European Association of Echocardiography, a registered branch of the European Society of Cardiology, and the Canadian Society of Echocardiography. J Am Soc Echocardiogr. 2010;23(7):685–713; quiz 786–688.
- , , , et al. Recommendations for chamber quantification: a report from the American Society of Echocardiography's Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr. 2005;18(12):1440–1463.
- , , , , . Mortality and functional evolution at one year after hospital admission due to heart failure (HF) in elderly patients. Arch Gerontol Geriatr. 2012;54(1):261–265.
- , , , et al. Early and long‐term outcomes of heart failure in elderly persons, 2001–2005. Arch Intern Med. 2008;168(22):2481–2488.
- , , , et al. IVC diameter in patients with chronic heart failure: relationships and prognostic significance. JACC Cardiovasc Imaging. 2013;6(1):16–28.
- , , , et al. Prognostic significance of dilated inferior vena cava in advanced decompensated heart failure. Int J Cardiovasc Imaging. 2014;30(7):1289–1295.
- , , . A dilated inferior vena cava is a marker of poor survival. Am Heart J. 2006;151(3):730–735.
- , , , et al. Predischarge B‐type natriuretic peptide assay for identifying patients at high risk of re‐admission after decompensated heart failure. J Am Coll Cardiol. 2004;43(4):635–641.
- , , , et al. A rapid bedside test for B‐type peptide predicts treatment outcomes in patients admitted for decompensated heart failure: a pilot study. J Am Coll Cardiol. 2001;37(2):386–391.
- , , , , , . N‐terminal‐pro‐brain natriuretic peptide predicts outcome after hospital discharge in heart failure patients. Circulation. 2004;110(15):2168–2174.
- , , , , , . Lowered B‐type natriuretic peptide in response to levosimendan or dobutamine treatment is associated with improved survival in patients with severe acutely decompensated heart failure. J Am Coll Cardiol. 2009;53(25):2343–2348.
- , , , . Long‐term clinical variation of NT‐proBNP in stable chronic heart failure patients. Eur Heart J. 2007;28(2):177–182.
- , , , et al. Right atrial volume index in chronic systolic heart failure and prognosis. JACC Cardiovasc Imaging. 2009;2(5):527–534.
- , , , et al. Pulmonary pressures and death in heart failure: a community study. J Am Coll Cardiol. 2012;59(3):222–231.
- , , , et al. A comparison by medicine residents of physical examination versus hand‐carried ultrasound for estimation of right atrial pressure. Am J Cardiol. 2007;99(11):1614–1616.
- , , . Noninvasive estimation of right atrial pressure from the inspiratory collapse of the inferior vena cava. Am J Cardiol. 1990;66(4):493–496.
- , , , , , . Relationship between right and left‐sided filling pressures in 1000 patients with advanced heart failure. J Heart Lung Transplant. 1999;18(11):1126–1132.
- , , , et al. Admission B‐type natriuretic peptide levels and in‐hospital mortality in acute decompensated heart failure. J Am Coll Cardiol. 2007;49(19):1943–1950.
- , , , et al. State of the art: using natriuretic peptide levels in clinical practice. Eur J Heart Fail. 2008;10(9):824–839.
- , , , et al. Readmission after hospitalization for congestive heart failure among Medicare beneficiaries. Arch Intern Med. 1997;157(1):99–104.
- , , , et al. Inferior vena cava parameters predict re‐admission in ischaemic heart failure. Eur J Clin Invest. 2014;44(4):341–349.
- , , , et al. Estimation of right atrial pressure on inferior vena cava ultrasound in Asian patients. Circ J. 2014;78(4):962–966.
- , , , , . Clinical presentation, management, and in‐hospital outcomes of patients admitted with acute decompensated heart failure with preserved systolic function: a report from the Acute Decompensated Heart Failure National Registry (ADHERE) Database. J Am Coll Cardiol. 2006;47(1):76–84.
- , , , , . Palliative care referral among patients hospitalized with advanced heart failure. J Palliat Med. 2014;17(10):1115–1120.
- , , . Engaging heart failure clinicians to increase palliative care referrals: overcoming barriers, improving techniques. J Palliat Med. 2014;17(7):753–760.
Heart failure costs the United States an excess of $30 billion annually, and costs are projected to increase to nearly $70 billion by 2030.[1] Heart failure accounts for over 1 million hospitalizations and is the leading cause of hospitalization in patients >65 years of age.[2] After hospitalization, approximately 50% of patients are readmitted within 6 months of hospital discharge.[3] Mortality rates from heart failure have improved but remain high.[4] Approximately 50% of patients diagnosed with heart failure die within 5 years, and the overall 1‐year mortality rate is 30%.[1]
Prognostic markers and scoring systems for acute decompensated heart failure (ADHF) continue to emerge, but few bedside tools are available to clinicians. Age, brain natriuretic peptide, and N‐terminal pro‐brain natriuretic peptide (NT‐proBNP) levels have been shown to correlate with postdischarge rates of readmission and mortality.[5] A study evaluating the prognostic value of a bedside inferior vena cava (IVC) ultrasound exam demonstrated that lack of improvement in IVC distention from admission to discharge was associated with higher 30‐day readmission rates.[6] Two studies using data from comprehensive transthoracic echocardiograms in heart failure patients demonstrated that a dilated, noncollapsible IVC is associated with higher risk of mortality; however, it is well recognized that obtaining comprehensive transthoracic echocardiograms in all patients hospitalized with heart failure is neither cost‐effective nor practical.[7]
In recent years, multiple studies have emerged demonstrating that noncardiologists can perform focused cardiac ultrasound exams with high reproducibility and accuracy to guide management of patients with ADHF.[8, 9, 10, 11, 12, 13, 14] However, it is unknown whether IVC characteristics from a focused cardiac ultrasound exam performed by a noncardiologist can predict mortality of patients hospitalized with ADHF. The aim of this study was to assess whether a hospitalist‐performed focused ultrasound exam to measure the IVC diameter at admission and discharge can predict mortality in a general medicine ward population hospitalized with ADHF.
METHODS
Study Design
A prospective, observational study of patients admitted to a general medicine ward with ADHF between January 2012 and March 2013 was performed using convenience sampling. The setting was a 247‐bed, university‐affiliated hospital in Madrid, Spain. Inclusion criteria were adult patients admitted with a primary diagnosis of ADHF per the European Society of Cardiology (ESC) criteria.[15] Exclusion criteria were admission to the intensive care unit for mechanical ventilation, need for chronic hemodialysis, or a noncardiac terminal illness with a life expectancy of less than 3 months. All patients provided written informed consent prior to enrollment. This study complies with the Declaration of Helsinki and was approved by the local ethics committee.
The primary outcome was all‐cause mortality at 90 days after hospitalization. The secondary outcomes were hospital readmission at 90 and 180 days, and mortality at 180 days. Patients were prospectively followed up at 30, 60, 90, and 180 days after discharge by telephone interview or by review of the patient's electronic health record. Patients who died within 90 days of discharge were categorized as nonsurvivors, whereas those alive at 90 days were categorized as survivors.
The following data were recorded on admission: age, gender, blood pressure, heart rate, functional class per New York Heart Association (NYHA) classification, comorbidities (hypertension, diabetes mellitus, atrial fibrillation, chronic obstructive pulmonary disease), primary etiology of heart failure, medications, electrocardiogram, NT‐terminal pro‐BNP, hemoglobin, albumin, creatinine, sodium, measurement of performance of activities of daily living (modified Barthel index), and comorbidity score (age‐adjusted Charlson score). A research coordinator interviewed subjects to gather data to calculate a modified Barthel index.[16] Age‐adjusted Charlson comorbidity scores were calculated using age and diagnoses per International Classification of Diseases, Ninth Revision coding.[17]
IVC Measurement
An internal medicine hospitalist with expertise in point‐of‐care ultrasonography (G.G.C.) performed all focused cardiac ultrasound exams to measure the IVC diameter and collapsibility at the time of admission and discharge. This physician was not involved in the inpatient medical management of study subjects. A second physician (N.J.S.) randomly reviewed 10% of the IVC images for quality assurance. Admission IVC measurements were acquired within 24 hours of arrival to the emergency department after the on‐call medical team was contacted to admit the patient. Measurement of the IVC maximum (IVCmax) and IVC minimum (IVCmin) diameters was obtained just distal to the hepatic veinIVC junction, or 2 cm from the IVCright atrial junction using a long‐axis view of the IVC. Measurement of the IVC diameter was consistent with the technique recommended by the American Society of Echocardiography and European Society of Echocardiography guidelines.[18, 19] The IVC collapsibility index (IVCCI) was calculated as (IVCmaxIVCmin)/IVCmax per guidelines.[18] Focused cardiac ultrasound exams were performed using a General Electric Logiq E device (GE Healthcare, Little Chalfont, United Kingdom) with a 3.5 MHz curvilinear transducer. Inpatient medical management by the primary medical team was guided by protocols from the ESC guidelines on the treatment of ADHF.[15] A comprehensive transthoracic echocardiogram (TTE) was performed on all study subjects by the echocardiography laboratory within 24 hours of hospitalization as part of the study protocol. One of 3 senior cardiologists read all comprehensive TTEs. NT‐proBNP was measured on admission and discharge by electrochemiluminescence.
Statistical Analysis
We calculated the required sample size based on published mortality and readmission rates. For our primary outcome of 90‐day mortality, we calculated a required sample size of 64 to achieve 80% power based on 90‐day and 1‐year mortality rates of 21% and 33%, respectively, among Spanish elderly patients (age 70 years) hospitalized with ADHF.[20] For our secondary outcome of 90‐day readmissions, we calculated a sample size of 28 based on a 41% readmission rate.[21] Therefore, our target subject enrollment was at least 70 patients to achieve a power of 80%.
Statistical analyses were performed using SPSS 17.0 statistical package (SPSS Inc., Chicago, IL). Subject characteristics that were categorical variables (demographics and comorbidities) were summarized as counts and percentages. Continuous variables, including IVC measurements, were summarized as means with standard deviations. Differences between categorical variables were analyzed using the Fisher exact test. Survival curves with log‐rank statistics were used to perform survival analysis. The nonparametric Mann‐Whitney U test was used to assess associations between the change in IVCCI, and readmissions and mortality at 90 and 180 days. Predictors of readmission and death were evaluated using a multivariate Cox proportional hazards regression analysis. Given the limited number of primary outcome events, we used age, IVC diameter, and log NT‐proBNP in the multivariate regression analysis based on past studies showing prognostic significance of these variables.[6, 22, 23, 24, 25, 26, 27, 28] Optimal cutoff values for IVC diameter for death and readmission prediction were determined by constructing receiver operating characteristic (ROC) curves and calculating the area under the curve (AUC) for different IVC diameters. NT‐proBNP values were log‐transformed to minimize skewing as reported in previous studies.[29]
RESULTS
Patient Characteristics
Ninety‐seven patients admitted with ADHF were recruited for the study. Optimal acoustic windows to measure the IVC diameter were acquired in 90 patients (93%). Because measurement of discharge IVC diameter was required to calculate the change from admission to discharge, 8 patients who died during initial hospitalization were excluded from the final data analysis. An additional two patients were excluded due to missing discharge NT‐proBNP measurement or missing comprehensive echocardiogram data. The study cohort from whom data were analyzed included 80 of 97 total patients (82%).
Baseline demographic, clinical, laboratory, and comprehensive echocardiographic characteristics of nonsurvivors and survivors at 90 days are demonstrated in Table 1. Eleven patients (13.7%) died during the first 90 days postdischarge, and all deaths were due to cardiovascular complications. Nonsurvivors were older (86 vs 76 years; P = 0.02), less independent in performance of their activities of daily living (Barthel index of 58.1 vs 81.9; P = 0.01), and were more likely to have advanced heart failure with an NYHA functional class of III or IV (72% vs 33%; P = 0.016). Atrial fibrillation (90% vs 55%; P = 0.008) and lower systolic blood pressure (127 mm Hg vs 147 mm Hg; P = 0.01) were more common in nonsurvivors than survivors, and fewer nonsurvivors were taking a ‐blocker (18% vs 59%; P = 0.01). Baseline comprehensive echocardiographic findings were similar between the survivors and nonsurvivors, except left atrial diameter was larger in nonsurvivors versus survivors (54 mm vs 49 mm; P = 0.04).
| Total Cohort, n = 80 | Nonsurvivors, n = 11 | Survivors, n = 69 | P Value | |
|---|---|---|---|---|
| ||||
| Demographics | ||||
| Age, y* | 78 (13) | 86 (7) | 76 (14) | 0.02 |
| Men, n (%) | 34 (42) | 3 (27) | 26 (38) | 0.3 |
| Vital signs* | ||||
| Heart rate, beats/min | 94 (23) | 99 (26) | 95 (23) | 0.5 |
| SBP, mm Hg | 141 (27) | 127 (22) | 147 (25) | 0.01 |
| Comorbidities, n (%) | ||||
| Hypertension | 72 (90) | 10 (91) | 54 (78) | 0.3 |
| Diabetes mellitus | 35 (44) | 3 (27) | 26 (38) | 0.3 |
| Atrial fibrillation | 48 (60) | 10 (90) | 38 (55) | 0.008 |
| COPD | 22 (27) | 3 (27) | 16 (23) | 0.5 |
| Etiology of heart failure | ||||
| Ischemic | 20 (25) | 1 (9) | 16 (23) | 0.1 |
| Hypertensive | 22 (27) | 2 (18) | 18 (26) | 0.4 |
| Valvulopathy | 29 (36) | 7 (64) | 19 (27) | 0.07 |
| Other | 18 (22) | 1 (9) | 16 (23) | 0.09 |
| NYHA IIIIV | 38 (47) | 8 (72) | 23 (33) | 0.016 |
| Charlson score* | 7.5 (2) | 9.0 (3) | 7.1 (2) | 0.02 |
| Barthel index* | 76 (31) | 58 (37) | 81.9 (28) | 0.01 |
| Medications | ||||
| ‐blocker | 44 (55) | 2 (18) | 41 (59) | 0.01 |
| ACE inhibitor/ARB | 48 (60) | 3 (27) | 35 (51) | 0.1 |
| Loop diuretic | 78 (97) | 10 (91) | 67 (97) | 0.9 |
| Aldosterone antagonist | 31 (39) | 4 (36) | 21 (30) | 0.4 |
| Lab results* | ||||
| Sodium, mmol/L | 137 (4.8) | 138 (6) | 139 (4) | 0.6 |
| Creatinine, umol/L | 1.24 (0.4) | 1.40 (0.5) | 1.17 (0.4) | 0.1 |
| eGFR, mL/min | 57.8 (20) | 51.2 (20) | 60.2 (19) | 0.1 |
| Albumin, g/L | 3.4 (0.4) | 3.3 (0.38) | 3.5 (0.41) | 0.1 |
| Hemoglobin, g/dL | 12.0 (2) | 10.9 (1.8) | 12.5 (2.0) | 0.01 |
| Echo parameters* | ||||
| LVEF, % | 52.1 (15) | 51.9 (17) | 51.6 (15) | 0.9 |
| LA diameter, mm | 50.1 (10) | 54 (11) | 49 (11) | 0.04 |
| RVDD, mm | 32.0 (11) | 34 (10) | 31 (11) | 0.2 |
| TAPSE, mm | 18.5 (7) | 17.4 (4) | 18.8 (7) | 0.6 |
| PASP, mm Hg | 51.2 (16) | 53.9 (17) | 50.2 (17) | 0.2 |
| Admission* | ||||
| NT‐proBNP, pg/mL | 8,816 (14,260) | 9,413 (5,703) | 8,762 (15,368) | 0.81 |
| Log NT‐proBNP | 3.66 (0.50) | 3.88 (0.31 | 3.62 (0.52) | 0.11 |
| IVCmax, cm | 2.12 (0.59) | 2.39 (0.37) | 2.06 (0.59) | 0.02 |
| IVCmin, cm | 1.63 (0.69) | 1.82 (0.66) | 1.56 (0.67) | 0.25 |
| IVCCI, % | 25.7 (0.16) | 25.9 (17.0) | 26.2 (16.0) | 0.95 |
| Discharge* | ||||
| NT‐proBNP, pg/mL | 3,132 (3,093) | 4,693 (4,383) | 2,909 (2,847) | 0.08 |
| Log NT‐proBNP | 3.27 (0.49) | 3.51 (0.37) | 3.23 (0.50) | 0.08 |
| IVCmax, cm | 1.87 (0.68) | 1.97 (0.54) | 1.81 (0.66) | 0.45 |
| IVCmin, cm | 1.33 (0.75) | 1.40 (0.65) | 1.27 (0.71) | 0.56 |
| IVCCI, % | 33.1 (0.20) | 32.0 (21.0) | 34.2 (19.0) | 0.74 |
From admission to discharge, the total study cohort demonstrated a highly statistically significant reduction in NT‐proBNP (8816 vs 3093; P < 0.001), log NT‐proBNP (3.66 vs 3.27; P < 0.001), IVCmax (2.12 vs 1.87; P < 0.001), IVCmin (1.63 vs 1.33; P < 0.001), and IVCCI (25.7% vs 33.1%; P < 0.001). The admission and discharge NT‐proBNP and IVC characteristics of the survivors and nonsurvivors are displayed in Table 2. The only statistically significant difference between nonsurvivors and survivors was the admission IVCmax (2.39 vs 2.06; P = 0.02). There was not a statistically significant difference in the discharge IVCmax between nonsurvivors and survivors.
| Admission | Discharge | Difference (DischargeAdmission) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Nonsurvivors | Survivors | P Value | Nonsurvivors | Survivors | P Value | Nonsurvivors | Survivors | P Value | |
| |||||||||
| NT‐proBNP, pg/mL | 9,413 (5,703) | 8,762 (15,368) | 0.81 | 4,693 (4,383) | 2,909 (2,847) | 0.08 | 3,717 5,043 | 5,026 11,507 | 0.7 |
| Log NT‐proBNP | 3.88 0.31 | 3.62 0.52 | 0.11 | 3.51 0.37 | 3.23 0.50 | 0.08 | 0.29 0.36 | 0.38 0.37 | 0.4 |
| IVCmax, cm | 2.39 0.37 | 2.06 0.59 | 0.02 | 1.97 0.54 | 1.81 0.66 | 0.45 | 0.39 0.56 | 0.25 0.51 | 0.4 |
| IVCmin, cm | 1.82 0.66 | 1.56 0.67 | 0.25 | 1.40 0.65 | 1.27 0.71 | 0.56 | 0.37 0.52 | 0.30 0.64 | 0.7 |
| IVCCI, % | 25.9 17.0 | 26.2 16.0 | 0.95 | 32.0 21.0 | 34.2 19.0 | 0.74 | 3.7 7.9 | 8.3 22 | 0.5 |
Outcomes
For the primary outcome of 90‐day mortality, the ROC curves showed a similar AUC for the admission IVCmax diameter (AUC: 0.69; 95% confidence interval [CI]: 0.53‐0.85), log NT‐proBNP at discharge (AUC: 0.67; 95% CI: 0.49‐0.85), and log NT‐proBNP at admission (AUC: 0.69; 95% CI: 0.52‐0.85). The optimal cutoff value for the admission IVCmax diameter to predict mortality was 1.9 cm (sensitivity 100%, specificity 38%) based on the ROC curves (see Supporting Information, Appendices 1 and 2, in the online version of this article). An admission IVCmax diameter 1.9 cm was associated with a higher mortality rate at 90 days (25.4% vs 3.4%; P = 0.009) and 180 days (29.3% vs 3.4%; P = 0.003). The Cox survival curves showed significantly lower survival rates in patients with an admission IVCmax diameter 1.9 cm (74.1 vs 96.7%; P = 0.012) (Figures 1 and 2). Based on the multivariate Cox proportional hazards regression analysis with age, IVCmax diameter, and log NT‐proBNP at admission, the admission IVCmax diameter and age were independent predictors of 90‐ and 180‐day mortality. The hazard ratios for death by age, admission IVCmax diameter, and log NT‐proBNP are shown in Table 3.
| Endpoint | Variable | HR (95% CI) | P Value |
|---|---|---|---|
| |||
| 90‐day mortality | Age | 1.14 (1.031.26) | 0.009 |
| IVC diameter at admission | 5.88 (1.2128.1) | 0.025 | |
| Log NT‐proBNP at admission | 1.00 (1.001.00) | 0.910 | |
| 90‐day readmission | Age | 1.06 (1.001.12) | 0.025 |
| IVC diameter at admission | 3.20 (1.248.21) | 0.016 | |
| Log NT‐proBNP at discharge | 1.00 (1.001.00) | 0.910 | |
| 180‐day mortality | Age | 1.12 (1.031.22) | 0.007 |
| IVC diameter at admission | 4.77 (1.2118.7) | 0.025 | |
| Log NT‐proBNP at admission | 1.00 (1.001.00) | 0.610 | |
| 180‐day readmission | Age | 1.06 (1.011.11) | 0.009 |
| IVC diameter at admission | 2.56 (1.145.74) | 0.022 | |
| Log NT‐proBNP at discharge | 1.00 (1.001.00) | 0.610 | |


For the secondary outcome of 90‐day readmissions, 19 patients (24%) were readmitted, and the mean index admission IVCmax diameter was significantly greater in patients who were readmitted (2.36 vs 1.98 cm; P = 0.04). The ROC curves for readmission at 90 days showed that an index admission IVCmax diameter of 1.9 cm had the greatest AUC (0.61; 95% CI: 0.49‐0.74). The optimal cutoff value of an index admission IVCmax to predict readmission was also 1.9 cm (sensitivity 94%, specificity 42%) (see Supporting Information, Appendices 1 and 2, in the online version of this article). The Cox survival analysis showed that patients with an index admission IVCmax diameter 1.9 cm had a higher readmission rate at 90 days (30.8% vs 10.7%; P = 0.04) and 180 days (38.0 vs 14.3%; P = 0.02) (Figures 1 and 2). Using a multivariate Cox proportional regression analysis, the hazard ratios for the variables of age, admission IVCmax diameter, and log NT‐proBNP are shown in Table 3.
DISCUSSION
Our study found that a dilated IVC at admission is associated with a poor prognosis after hospitalization for ADHF. Patients with a dilated IVC 1.9 cm at admission had higher mortality and readmission rates at 90 and 180 days postdischarge.
The effect of a dilated IVC on mortality may be mediated through unrecognized right ventricular disease with or without significant pulmonary hypertension, supporting the notion that right heart function is an important determinant of prognosis in patients with ADHF.[30, 31] Similar to elevated jugular venous distension, bedside ultrasound examination of the IVC diameter can serve as a rapid and noninvasive measurement of right atrial pressure.[32] Elevated right atrial pressure is most often due to elevated left ventricular filling pressure transmitted via the pulmonary vasculature, but it is important to note that right‐ and left‐sided cardiac pressures are often discordant in heart failure patients.[33, 34]
Few studies have evaluated the prognostic value of IVC diameter and collapsibility in patients with heart failure. Nath et al.[24] evaluated the prognostic value of IVC diameter in stable veterans referred for outpatient echocardiography. Patients with a dilated IVC >2 cm that did not collapse with inspiration had higher 90‐day and 1‐year mortality rates. A subsequent study by Pellicori et al.[22] investigated the relationship between IVC diameter and other prognostic markers in stable cardiac patients. Pellicori et al. demonstrated that IVC diameter and serum NT‐proBNP levels were independent predictors of a composite endpoint of cardiovascular death or heart failure hospitalization at 1 year.[22] Most recently, Lee et al.[23] evaluated whether a dilated IVC in patients with a history of advanced systolic heart failure with a reduced ejection fraction of 30% and repeated hospitalizations (2) predicted worsening renal failure and adverse cardiovascular outcomes (death or hospitalization for ADHF). The study concluded that age, IVC diameter >2.1 cm, and worsening renal failure predicted cardiovascular death or hospitalization for ADHF.[23]
Our study demonstrated that an admission IVCmax 1.9 cm in hospitalized ADHF patients predicted higher postdischarge mortality at 90 and 180 days. Our findings are consistent with the above‐mentioned studies with a few important differences. First, all of our patients were hospitalized with acute decompensated heart failure. Nath et al. and Pellicori et al. evaluated stable ambulatory patients seen in an echocardiography lab and cardiology clinic, respectively. Only 12.1% of patients in the Nath study had a history of heart failure, and none were reported to have ADHF. More importantly, our study improves our understanding of patients with heart failure with a preserved ejection fraction, an important gap in the literature. The mean ejection fraction of patients in our study was 52% consistent with heart failure with preserved ejection fraction, whereas patients in the Pellicori et al. and Lee et al. studies had heart failure with reduced (42%) or severely reduced (30%) ejection fraction, respectively. We did not anticipate finding heart failure with preserved ejection fraction in the majority of patients, but our study's findings will add to our understanding of this increasingly common type of heart failure.
Compared to previous studies that utilized a registered diagnostic cardiac sonographer to obtain a comprehensive TTE to prognosticate patients, our study utilized point‐of‐care ultrasonography. Nath et al. commented that obtaining a comprehensive echocardiogram on every patient with ADHF is unlikely to be cost‐effective or feasible. Our study utilized a more realistic approach with a frontline internal medicinetrained hospitalist acquiring and interpreting images of the IVC at the bedside using a basic portable ultrasound machine.
Our study did not show that plasma natriuretic peptides levels are predictive of death or readmission after hospitalization for ADHF as shown in previous studies.[22, 35, 36] The small sample size, relatively low event rate, or predominance of heart failure with preserved ejection fraction may explain this inconsistency with prior studies.
Previous studies have reported hospital readmission rates for ADHF of 30% to 44% after 1 to 6 months.[6, 37] Goonewardena et al. showed a 41.3% readmission rate at 30 days in patients with severely reduced left ventricular ejection fraction (mean 29%), and readmitted patients had an IVCmax diameter >2 cm and an IVC collapsibility <50% on admission and discharge.[6] Carbone et al. demonstrated absence of improvement in the minimum IVC diameter from admission to discharge using hand‐carried ultrasound in patients with ischemic heart disease (ejection fraction 33%) predicted readmission at 60 days.[38] Hospital readmission rates in our study are consistent with these previously published studies. We found readmission rates for patients with ADHF and an admission IVCmax 1.9 cm to be 30.8% and 38.0% after 90 and 180 days, respectively.
Important limitations of our study are the small sample size and single institution setting. A larger sample size may have demonstrated that change in IVC diameter and NT‐proBNP levels from admission to discharge to be predictive of mortality or readmission. Further, we found an IVCmax diameter 1.9 cm to be the optimal cutoff to predict mortality, which is less than an IVCmax diameter >2.0 cm reported in other studies. The relatively smaller IVC diameter in Spanish heart failure patients may be explained by the lower body mass index of this population. An IVCmax diameter 1.9 cm was found to be the optimal cutoff to predict an elevated right atrial pressure >10 mm Hg in a study of Japanese cardiac patients with a relatively lower body mass index.[39] Another limitation is the timing of the admission IVC measurement within the first 24 hours of arrival to the hospital rather than immediately upon arrival to the emergency department. We were not able to control for interventions given in the emergency department prior to the measurement of the admission IVC, including doses of diuretics. Further, unlike the comprehensive TTEs in the United States, TTEs in Spain do not routinely include an assessment of the IVC. Therefore, we were not able to compare our bedside IVC measurements to those from a comprehensive TTE. An important limitation of our regression analysis is the inclusion of only 3 variables. The selection of variables (age, NT‐proBNP, and IVC diameter) was based on prior studies demonstrating their prognostic value.[6, 22, 25] Due to the low event rate (n = 11), we could not include in the regression model other variables that differed significantly between nonsurvivors and survivors, including NYHA class, presence of atrial fibrillation, and use of ‐blockers.
Perhaps in a larger study population the admission IVCmax diameter may not be as predictive of 90‐day mortality as other variables. The findings of our exploratory analysis should be confirmed in a future study with a larger sample size.
The clinical implications of our study are 3‐fold. First, our study demonstrates that IVC images acquired by a hospitalist at the bedside using a portable ultrasound machine can be used to predict postdischarge mortality and readmission of patients with ADHF. Second, the predominant type of heart failure in our study was heart failure with preserved ejection fraction. Currently, approximately 50% of patients hospitalized with ADHF have heart failure with preserved ejection fraction.[40] Our study adds to the understanding of prognosis of these patients whose heart failure pathophysiology is not well understood. Finally, palliative care services are underutilized in patients with advanced heart failure.[41, 42] IVC measurements and other prognostic markers in heart failure may guide discussions about goals of care with patients and families, and facilitate timely referrals for palliative care services.
CONCLUSIONS
Point‐of‐care ultrasound evaluation of IVC diameter at the time of admission can be used to prognosticate patients hospitalized with acute decompensated heart failure. An admission IVCmax diameter 1.9 cm is associated with a higher rate of 90‐day and 180‐day readmission and mortality after hospitalization. Future studies should evaluate the combination of IVC characteristics with other markers of severity of illness to prognosticate patients with heart failure.
Disclosures
This study was supported by a grant from the Madrid‐Castilla la Mancha Society of Internal Medicine. Dr. Restrepo is partially supported by award number K23HL096054 from the National Heart, Lung, and Blood Institute. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Heart, Lung, and Blood Institute or the National Institutes of Health. The authors report no conflicts of interest.
Heart failure costs the United States an excess of $30 billion annually, and costs are projected to increase to nearly $70 billion by 2030.[1] Heart failure accounts for over 1 million hospitalizations and is the leading cause of hospitalization in patients >65 years of age.[2] After hospitalization, approximately 50% of patients are readmitted within 6 months of hospital discharge.[3] Mortality rates from heart failure have improved but remain high.[4] Approximately 50% of patients diagnosed with heart failure die within 5 years, and the overall 1‐year mortality rate is 30%.[1]
Prognostic markers and scoring systems for acute decompensated heart failure (ADHF) continue to emerge, but few bedside tools are available to clinicians. Age, brain natriuretic peptide, and N‐terminal pro‐brain natriuretic peptide (NT‐proBNP) levels have been shown to correlate with postdischarge rates of readmission and mortality.[5] A study evaluating the prognostic value of a bedside inferior vena cava (IVC) ultrasound exam demonstrated that lack of improvement in IVC distention from admission to discharge was associated with higher 30‐day readmission rates.[6] Two studies using data from comprehensive transthoracic echocardiograms in heart failure patients demonstrated that a dilated, noncollapsible IVC is associated with higher risk of mortality; however, it is well recognized that obtaining comprehensive transthoracic echocardiograms in all patients hospitalized with heart failure is neither cost‐effective nor practical.[7]
In recent years, multiple studies have emerged demonstrating that noncardiologists can perform focused cardiac ultrasound exams with high reproducibility and accuracy to guide management of patients with ADHF.[8, 9, 10, 11, 12, 13, 14] However, it is unknown whether IVC characteristics from a focused cardiac ultrasound exam performed by a noncardiologist can predict mortality of patients hospitalized with ADHF. The aim of this study was to assess whether a hospitalist‐performed focused ultrasound exam to measure the IVC diameter at admission and discharge can predict mortality in a general medicine ward population hospitalized with ADHF.
METHODS
Study Design
A prospective, observational study of patients admitted to a general medicine ward with ADHF between January 2012 and March 2013 was performed using convenience sampling. The setting was a 247‐bed, university‐affiliated hospital in Madrid, Spain. Inclusion criteria were adult patients admitted with a primary diagnosis of ADHF per the European Society of Cardiology (ESC) criteria.[15] Exclusion criteria were admission to the intensive care unit for mechanical ventilation, need for chronic hemodialysis, or a noncardiac terminal illness with a life expectancy of less than 3 months. All patients provided written informed consent prior to enrollment. This study complies with the Declaration of Helsinki and was approved by the local ethics committee.
The primary outcome was all‐cause mortality at 90 days after hospitalization. The secondary outcomes were hospital readmission at 90 and 180 days, and mortality at 180 days. Patients were prospectively followed up at 30, 60, 90, and 180 days after discharge by telephone interview or by review of the patient's electronic health record. Patients who died within 90 days of discharge were categorized as nonsurvivors, whereas those alive at 90 days were categorized as survivors.
The following data were recorded on admission: age, gender, blood pressure, heart rate, functional class per New York Heart Association (NYHA) classification, comorbidities (hypertension, diabetes mellitus, atrial fibrillation, chronic obstructive pulmonary disease), primary etiology of heart failure, medications, electrocardiogram, NT‐terminal pro‐BNP, hemoglobin, albumin, creatinine, sodium, measurement of performance of activities of daily living (modified Barthel index), and comorbidity score (age‐adjusted Charlson score). A research coordinator interviewed subjects to gather data to calculate a modified Barthel index.[16] Age‐adjusted Charlson comorbidity scores were calculated using age and diagnoses per International Classification of Diseases, Ninth Revision coding.[17]
IVC Measurement
An internal medicine hospitalist with expertise in point‐of‐care ultrasonography (G.G.C.) performed all focused cardiac ultrasound exams to measure the IVC diameter and collapsibility at the time of admission and discharge. This physician was not involved in the inpatient medical management of study subjects. A second physician (N.J.S.) randomly reviewed 10% of the IVC images for quality assurance. Admission IVC measurements were acquired within 24 hours of arrival to the emergency department after the on‐call medical team was contacted to admit the patient. Measurement of the IVC maximum (IVCmax) and IVC minimum (IVCmin) diameters was obtained just distal to the hepatic veinIVC junction, or 2 cm from the IVCright atrial junction using a long‐axis view of the IVC. Measurement of the IVC diameter was consistent with the technique recommended by the American Society of Echocardiography and European Society of Echocardiography guidelines.[18, 19] The IVC collapsibility index (IVCCI) was calculated as (IVCmaxIVCmin)/IVCmax per guidelines.[18] Focused cardiac ultrasound exams were performed using a General Electric Logiq E device (GE Healthcare, Little Chalfont, United Kingdom) with a 3.5 MHz curvilinear transducer. Inpatient medical management by the primary medical team was guided by protocols from the ESC guidelines on the treatment of ADHF.[15] A comprehensive transthoracic echocardiogram (TTE) was performed on all study subjects by the echocardiography laboratory within 24 hours of hospitalization as part of the study protocol. One of 3 senior cardiologists read all comprehensive TTEs. NT‐proBNP was measured on admission and discharge by electrochemiluminescence.
Statistical Analysis
We calculated the required sample size based on published mortality and readmission rates. For our primary outcome of 90‐day mortality, we calculated a required sample size of 64 to achieve 80% power based on 90‐day and 1‐year mortality rates of 21% and 33%, respectively, among Spanish elderly patients (age 70 years) hospitalized with ADHF.[20] For our secondary outcome of 90‐day readmissions, we calculated a sample size of 28 based on a 41% readmission rate.[21] Therefore, our target subject enrollment was at least 70 patients to achieve a power of 80%.
Statistical analyses were performed using SPSS 17.0 statistical package (SPSS Inc., Chicago, IL). Subject characteristics that were categorical variables (demographics and comorbidities) were summarized as counts and percentages. Continuous variables, including IVC measurements, were summarized as means with standard deviations. Differences between categorical variables were analyzed using the Fisher exact test. Survival curves with log‐rank statistics were used to perform survival analysis. The nonparametric Mann‐Whitney U test was used to assess associations between the change in IVCCI, and readmissions and mortality at 90 and 180 days. Predictors of readmission and death were evaluated using a multivariate Cox proportional hazards regression analysis. Given the limited number of primary outcome events, we used age, IVC diameter, and log NT‐proBNP in the multivariate regression analysis based on past studies showing prognostic significance of these variables.[6, 22, 23, 24, 25, 26, 27, 28] Optimal cutoff values for IVC diameter for death and readmission prediction were determined by constructing receiver operating characteristic (ROC) curves and calculating the area under the curve (AUC) for different IVC diameters. NT‐proBNP values were log‐transformed to minimize skewing as reported in previous studies.[29]
RESULTS
Patient Characteristics
Ninety‐seven patients admitted with ADHF were recruited for the study. Optimal acoustic windows to measure the IVC diameter were acquired in 90 patients (93%). Because measurement of discharge IVC diameter was required to calculate the change from admission to discharge, 8 patients who died during initial hospitalization were excluded from the final data analysis. An additional two patients were excluded due to missing discharge NT‐proBNP measurement or missing comprehensive echocardiogram data. The study cohort from whom data were analyzed included 80 of 97 total patients (82%).
Baseline demographic, clinical, laboratory, and comprehensive echocardiographic characteristics of nonsurvivors and survivors at 90 days are demonstrated in Table 1. Eleven patients (13.7%) died during the first 90 days postdischarge, and all deaths were due to cardiovascular complications. Nonsurvivors were older (86 vs 76 years; P = 0.02), less independent in performance of their activities of daily living (Barthel index of 58.1 vs 81.9; P = 0.01), and were more likely to have advanced heart failure with an NYHA functional class of III or IV (72% vs 33%; P = 0.016). Atrial fibrillation (90% vs 55%; P = 0.008) and lower systolic blood pressure (127 mm Hg vs 147 mm Hg; P = 0.01) were more common in nonsurvivors than survivors, and fewer nonsurvivors were taking a ‐blocker (18% vs 59%; P = 0.01). Baseline comprehensive echocardiographic findings were similar between the survivors and nonsurvivors, except left atrial diameter was larger in nonsurvivors versus survivors (54 mm vs 49 mm; P = 0.04).
| Total Cohort, n = 80 | Nonsurvivors, n = 11 | Survivors, n = 69 | P Value | |
|---|---|---|---|---|
| ||||
| Demographics | ||||
| Age, y* | 78 (13) | 86 (7) | 76 (14) | 0.02 |
| Men, n (%) | 34 (42) | 3 (27) | 26 (38) | 0.3 |
| Vital signs* | ||||
| Heart rate, beats/min | 94 (23) | 99 (26) | 95 (23) | 0.5 |
| SBP, mm Hg | 141 (27) | 127 (22) | 147 (25) | 0.01 |
| Comorbidities, n (%) | ||||
| Hypertension | 72 (90) | 10 (91) | 54 (78) | 0.3 |
| Diabetes mellitus | 35 (44) | 3 (27) | 26 (38) | 0.3 |
| Atrial fibrillation | 48 (60) | 10 (90) | 38 (55) | 0.008 |
| COPD | 22 (27) | 3 (27) | 16 (23) | 0.5 |
| Etiology of heart failure | ||||
| Ischemic | 20 (25) | 1 (9) | 16 (23) | 0.1 |
| Hypertensive | 22 (27) | 2 (18) | 18 (26) | 0.4 |
| Valvulopathy | 29 (36) | 7 (64) | 19 (27) | 0.07 |
| Other | 18 (22) | 1 (9) | 16 (23) | 0.09 |
| NYHA IIIIV | 38 (47) | 8 (72) | 23 (33) | 0.016 |
| Charlson score* | 7.5 (2) | 9.0 (3) | 7.1 (2) | 0.02 |
| Barthel index* | 76 (31) | 58 (37) | 81.9 (28) | 0.01 |
| Medications | ||||
| ‐blocker | 44 (55) | 2 (18) | 41 (59) | 0.01 |
| ACE inhibitor/ARB | 48 (60) | 3 (27) | 35 (51) | 0.1 |
| Loop diuretic | 78 (97) | 10 (91) | 67 (97) | 0.9 |
| Aldosterone antagonist | 31 (39) | 4 (36) | 21 (30) | 0.4 |
| Lab results* | ||||
| Sodium, mmol/L | 137 (4.8) | 138 (6) | 139 (4) | 0.6 |
| Creatinine, umol/L | 1.24 (0.4) | 1.40 (0.5) | 1.17 (0.4) | 0.1 |
| eGFR, mL/min | 57.8 (20) | 51.2 (20) | 60.2 (19) | 0.1 |
| Albumin, g/L | 3.4 (0.4) | 3.3 (0.38) | 3.5 (0.41) | 0.1 |
| Hemoglobin, g/dL | 12.0 (2) | 10.9 (1.8) | 12.5 (2.0) | 0.01 |
| Echo parameters* | ||||
| LVEF, % | 52.1 (15) | 51.9 (17) | 51.6 (15) | 0.9 |
| LA diameter, mm | 50.1 (10) | 54 (11) | 49 (11) | 0.04 |
| RVDD, mm | 32.0 (11) | 34 (10) | 31 (11) | 0.2 |
| TAPSE, mm | 18.5 (7) | 17.4 (4) | 18.8 (7) | 0.6 |
| PASP, mm Hg | 51.2 (16) | 53.9 (17) | 50.2 (17) | 0.2 |
| Admission* | ||||
| NT‐proBNP, pg/mL | 8,816 (14,260) | 9,413 (5,703) | 8,762 (15,368) | 0.81 |
| Log NT‐proBNP | 3.66 (0.50) | 3.88 (0.31 | 3.62 (0.52) | 0.11 |
| IVCmax, cm | 2.12 (0.59) | 2.39 (0.37) | 2.06 (0.59) | 0.02 |
| IVCmin, cm | 1.63 (0.69) | 1.82 (0.66) | 1.56 (0.67) | 0.25 |
| IVCCI, % | 25.7 (0.16) | 25.9 (17.0) | 26.2 (16.0) | 0.95 |
| Discharge* | ||||
| NT‐proBNP, pg/mL | 3,132 (3,093) | 4,693 (4,383) | 2,909 (2,847) | 0.08 |
| Log NT‐proBNP | 3.27 (0.49) | 3.51 (0.37) | 3.23 (0.50) | 0.08 |
| IVCmax, cm | 1.87 (0.68) | 1.97 (0.54) | 1.81 (0.66) | 0.45 |
| IVCmin, cm | 1.33 (0.75) | 1.40 (0.65) | 1.27 (0.71) | 0.56 |
| IVCCI, % | 33.1 (0.20) | 32.0 (21.0) | 34.2 (19.0) | 0.74 |
From admission to discharge, the total study cohort demonstrated a highly statistically significant reduction in NT‐proBNP (8816 vs 3093; P < 0.001), log NT‐proBNP (3.66 vs 3.27; P < 0.001), IVCmax (2.12 vs 1.87; P < 0.001), IVCmin (1.63 vs 1.33; P < 0.001), and IVCCI (25.7% vs 33.1%; P < 0.001). The admission and discharge NT‐proBNP and IVC characteristics of the survivors and nonsurvivors are displayed in Table 2. The only statistically significant difference between nonsurvivors and survivors was the admission IVCmax (2.39 vs 2.06; P = 0.02). There was not a statistically significant difference in the discharge IVCmax between nonsurvivors and survivors.
| Admission | Discharge | Difference (DischargeAdmission) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Nonsurvivors | Survivors | P Value | Nonsurvivors | Survivors | P Value | Nonsurvivors | Survivors | P Value | |
| |||||||||
| NT‐proBNP, pg/mL | 9,413 (5,703) | 8,762 (15,368) | 0.81 | 4,693 (4,383) | 2,909 (2,847) | 0.08 | 3,717 5,043 | 5,026 11,507 | 0.7 |
| Log NT‐proBNP | 3.88 0.31 | 3.62 0.52 | 0.11 | 3.51 0.37 | 3.23 0.50 | 0.08 | 0.29 0.36 | 0.38 0.37 | 0.4 |
| IVCmax, cm | 2.39 0.37 | 2.06 0.59 | 0.02 | 1.97 0.54 | 1.81 0.66 | 0.45 | 0.39 0.56 | 0.25 0.51 | 0.4 |
| IVCmin, cm | 1.82 0.66 | 1.56 0.67 | 0.25 | 1.40 0.65 | 1.27 0.71 | 0.56 | 0.37 0.52 | 0.30 0.64 | 0.7 |
| IVCCI, % | 25.9 17.0 | 26.2 16.0 | 0.95 | 32.0 21.0 | 34.2 19.0 | 0.74 | 3.7 7.9 | 8.3 22 | 0.5 |
Outcomes
For the primary outcome of 90‐day mortality, the ROC curves showed a similar AUC for the admission IVCmax diameter (AUC: 0.69; 95% confidence interval [CI]: 0.53‐0.85), log NT‐proBNP at discharge (AUC: 0.67; 95% CI: 0.49‐0.85), and log NT‐proBNP at admission (AUC: 0.69; 95% CI: 0.52‐0.85). The optimal cutoff value for the admission IVCmax diameter to predict mortality was 1.9 cm (sensitivity 100%, specificity 38%) based on the ROC curves (see Supporting Information, Appendices 1 and 2, in the online version of this article). An admission IVCmax diameter 1.9 cm was associated with a higher mortality rate at 90 days (25.4% vs 3.4%; P = 0.009) and 180 days (29.3% vs 3.4%; P = 0.003). The Cox survival curves showed significantly lower survival rates in patients with an admission IVCmax diameter 1.9 cm (74.1 vs 96.7%; P = 0.012) (Figures 1 and 2). Based on the multivariate Cox proportional hazards regression analysis with age, IVCmax diameter, and log NT‐proBNP at admission, the admission IVCmax diameter and age were independent predictors of 90‐ and 180‐day mortality. The hazard ratios for death by age, admission IVCmax diameter, and log NT‐proBNP are shown in Table 3.
| Endpoint | Variable | HR (95% CI) | P Value |
|---|---|---|---|
| |||
| 90‐day mortality | Age | 1.14 (1.031.26) | 0.009 |
| IVC diameter at admission | 5.88 (1.2128.1) | 0.025 | |
| Log NT‐proBNP at admission | 1.00 (1.001.00) | 0.910 | |
| 90‐day readmission | Age | 1.06 (1.001.12) | 0.025 |
| IVC diameter at admission | 3.20 (1.248.21) | 0.016 | |
| Log NT‐proBNP at discharge | 1.00 (1.001.00) | 0.910 | |
| 180‐day mortality | Age | 1.12 (1.031.22) | 0.007 |
| IVC diameter at admission | 4.77 (1.2118.7) | 0.025 | |
| Log NT‐proBNP at admission | 1.00 (1.001.00) | 0.610 | |
| 180‐day readmission | Age | 1.06 (1.011.11) | 0.009 |
| IVC diameter at admission | 2.56 (1.145.74) | 0.022 | |
| Log NT‐proBNP at discharge | 1.00 (1.001.00) | 0.610 | |


For the secondary outcome of 90‐day readmissions, 19 patients (24%) were readmitted, and the mean index admission IVCmax diameter was significantly greater in patients who were readmitted (2.36 vs 1.98 cm; P = 0.04). The ROC curves for readmission at 90 days showed that an index admission IVCmax diameter of 1.9 cm had the greatest AUC (0.61; 95% CI: 0.49‐0.74). The optimal cutoff value of an index admission IVCmax to predict readmission was also 1.9 cm (sensitivity 94%, specificity 42%) (see Supporting Information, Appendices 1 and 2, in the online version of this article). The Cox survival analysis showed that patients with an index admission IVCmax diameter 1.9 cm had a higher readmission rate at 90 days (30.8% vs 10.7%; P = 0.04) and 180 days (38.0 vs 14.3%; P = 0.02) (Figures 1 and 2). Using a multivariate Cox proportional regression analysis, the hazard ratios for the variables of age, admission IVCmax diameter, and log NT‐proBNP are shown in Table 3.
DISCUSSION
Our study found that a dilated IVC at admission is associated with a poor prognosis after hospitalization for ADHF. Patients with a dilated IVC 1.9 cm at admission had higher mortality and readmission rates at 90 and 180 days postdischarge.
The effect of a dilated IVC on mortality may be mediated through unrecognized right ventricular disease with or without significant pulmonary hypertension, supporting the notion that right heart function is an important determinant of prognosis in patients with ADHF.[30, 31] Similar to elevated jugular venous distension, bedside ultrasound examination of the IVC diameter can serve as a rapid and noninvasive measurement of right atrial pressure.[32] Elevated right atrial pressure is most often due to elevated left ventricular filling pressure transmitted via the pulmonary vasculature, but it is important to note that right‐ and left‐sided cardiac pressures are often discordant in heart failure patients.[33, 34]
Few studies have evaluated the prognostic value of IVC diameter and collapsibility in patients with heart failure. Nath et al.[24] evaluated the prognostic value of IVC diameter in stable veterans referred for outpatient echocardiography. Patients with a dilated IVC >2 cm that did not collapse with inspiration had higher 90‐day and 1‐year mortality rates. A subsequent study by Pellicori et al.[22] investigated the relationship between IVC diameter and other prognostic markers in stable cardiac patients. Pellicori et al. demonstrated that IVC diameter and serum NT‐proBNP levels were independent predictors of a composite endpoint of cardiovascular death or heart failure hospitalization at 1 year.[22] Most recently, Lee et al.[23] evaluated whether a dilated IVC in patients with a history of advanced systolic heart failure with a reduced ejection fraction of 30% and repeated hospitalizations (2) predicted worsening renal failure and adverse cardiovascular outcomes (death or hospitalization for ADHF). The study concluded that age, IVC diameter >2.1 cm, and worsening renal failure predicted cardiovascular death or hospitalization for ADHF.[23]
Our study demonstrated that an admission IVCmax 1.9 cm in hospitalized ADHF patients predicted higher postdischarge mortality at 90 and 180 days. Our findings are consistent with the above‐mentioned studies with a few important differences. First, all of our patients were hospitalized with acute decompensated heart failure. Nath et al. and Pellicori et al. evaluated stable ambulatory patients seen in an echocardiography lab and cardiology clinic, respectively. Only 12.1% of patients in the Nath study had a history of heart failure, and none were reported to have ADHF. More importantly, our study improves our understanding of patients with heart failure with a preserved ejection fraction, an important gap in the literature. The mean ejection fraction of patients in our study was 52% consistent with heart failure with preserved ejection fraction, whereas patients in the Pellicori et al. and Lee et al. studies had heart failure with reduced (42%) or severely reduced (30%) ejection fraction, respectively. We did not anticipate finding heart failure with preserved ejection fraction in the majority of patients, but our study's findings will add to our understanding of this increasingly common type of heart failure.
Compared to previous studies that utilized a registered diagnostic cardiac sonographer to obtain a comprehensive TTE to prognosticate patients, our study utilized point‐of‐care ultrasonography. Nath et al. commented that obtaining a comprehensive echocardiogram on every patient with ADHF is unlikely to be cost‐effective or feasible. Our study utilized a more realistic approach with a frontline internal medicinetrained hospitalist acquiring and interpreting images of the IVC at the bedside using a basic portable ultrasound machine.
Our study did not show that plasma natriuretic peptides levels are predictive of death or readmission after hospitalization for ADHF as shown in previous studies.[22, 35, 36] The small sample size, relatively low event rate, or predominance of heart failure with preserved ejection fraction may explain this inconsistency with prior studies.
Previous studies have reported hospital readmission rates for ADHF of 30% to 44% after 1 to 6 months.[6, 37] Goonewardena et al. showed a 41.3% readmission rate at 30 days in patients with severely reduced left ventricular ejection fraction (mean 29%), and readmitted patients had an IVCmax diameter >2 cm and an IVC collapsibility <50% on admission and discharge.[6] Carbone et al. demonstrated absence of improvement in the minimum IVC diameter from admission to discharge using hand‐carried ultrasound in patients with ischemic heart disease (ejection fraction 33%) predicted readmission at 60 days.[38] Hospital readmission rates in our study are consistent with these previously published studies. We found readmission rates for patients with ADHF and an admission IVCmax 1.9 cm to be 30.8% and 38.0% after 90 and 180 days, respectively.
Important limitations of our study are the small sample size and single institution setting. A larger sample size may have demonstrated that change in IVC diameter and NT‐proBNP levels from admission to discharge to be predictive of mortality or readmission. Further, we found an IVCmax diameter 1.9 cm to be the optimal cutoff to predict mortality, which is less than an IVCmax diameter >2.0 cm reported in other studies. The relatively smaller IVC diameter in Spanish heart failure patients may be explained by the lower body mass index of this population. An IVCmax diameter 1.9 cm was found to be the optimal cutoff to predict an elevated right atrial pressure >10 mm Hg in a study of Japanese cardiac patients with a relatively lower body mass index.[39] Another limitation is the timing of the admission IVC measurement within the first 24 hours of arrival to the hospital rather than immediately upon arrival to the emergency department. We were not able to control for interventions given in the emergency department prior to the measurement of the admission IVC, including doses of diuretics. Further, unlike the comprehensive TTEs in the United States, TTEs in Spain do not routinely include an assessment of the IVC. Therefore, we were not able to compare our bedside IVC measurements to those from a comprehensive TTE. An important limitation of our regression analysis is the inclusion of only 3 variables. The selection of variables (age, NT‐proBNP, and IVC diameter) was based on prior studies demonstrating their prognostic value.[6, 22, 25] Due to the low event rate (n = 11), we could not include in the regression model other variables that differed significantly between nonsurvivors and survivors, including NYHA class, presence of atrial fibrillation, and use of ‐blockers.
Perhaps in a larger study population the admission IVCmax diameter may not be as predictive of 90‐day mortality as other variables. The findings of our exploratory analysis should be confirmed in a future study with a larger sample size.
The clinical implications of our study are 3‐fold. First, our study demonstrates that IVC images acquired by a hospitalist at the bedside using a portable ultrasound machine can be used to predict postdischarge mortality and readmission of patients with ADHF. Second, the predominant type of heart failure in our study was heart failure with preserved ejection fraction. Currently, approximately 50% of patients hospitalized with ADHF have heart failure with preserved ejection fraction.[40] Our study adds to the understanding of prognosis of these patients whose heart failure pathophysiology is not well understood. Finally, palliative care services are underutilized in patients with advanced heart failure.[41, 42] IVC measurements and other prognostic markers in heart failure may guide discussions about goals of care with patients and families, and facilitate timely referrals for palliative care services.
CONCLUSIONS
Point‐of‐care ultrasound evaluation of IVC diameter at the time of admission can be used to prognosticate patients hospitalized with acute decompensated heart failure. An admission IVCmax diameter 1.9 cm is associated with a higher rate of 90‐day and 180‐day readmission and mortality after hospitalization. Future studies should evaluate the combination of IVC characteristics with other markers of severity of illness to prognosticate patients with heart failure.
Disclosures
This study was supported by a grant from the Madrid‐Castilla la Mancha Society of Internal Medicine. Dr. Restrepo is partially supported by award number K23HL096054 from the National Heart, Lung, and Blood Institute. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Heart, Lung, and Blood Institute or the National Institutes of Health. The authors report no conflicts of interest.
- , , , et al. Heart disease and stroke statistics—2015 update: a report from the American Heart Association. Circulation. 2015;131(4):e29–e322.
- , , . Hospitalization for congestive heart failure: United States, 2000–2010. NCHS Data Brief. 2012(108):1–8.
- , . Rehospitalization for heart failure: predict or prevent? Circulation. 2012;126(4):501–506.
- , , , et al. Generalizability and longitudinal outcomes of a national heart failure clinical registry: Comparison of Acute Decompensated Heart Failure National Registry (ADHERE) and non‐ADHERE Medicare beneficiaries. Am Heart J. 2010;160(5):885–892.
- , , , et al. Prognostic markers of acute decompensated heart failure: the emerging roles of cardiac biomarkers and prognostic scores. Arch Cardiovasc Dis. 2015;108(1):64–74.
- , , , et al. Comparison of hand‐carried ultrasound assessment of the inferior vena cava and N‐terminal pro‐brain natriuretic peptide for predicting readmission after hospitalization for acute decompensated heart failure. JACC Cardiovasc Imaging. 2008;1(5):595–601.
- , , , , . Echocardiography in acute heart failure: current perspectives. J Card Fail. 2016;22(1):82–94.
- , , , , . Usefulness of a hand‐held ultrasound device for bedside examination of left ventricular function. Am J Cardiol. 2002;90(9):1038–1039.
- , , , , , . Feasibility of point‐of‐care echocardiography by internal medicine house staff. Am Heart J. 2004;147(3):476–481.
- , , , , , . The use of small personal ultrasound devices by internists without formal training in echocardiography. Eur J Echocardiogr. 2003;4(2):141–147.
- , , , et al. Diagnostic accuracy of hospitalist‐performed hand‐carried ultrasound echocardiography after a brief training program. J Hosp Med. 2009;4(6):340–349.
- , , , . Point‐of‐care multi‐organ ultrasound improves diagnostic accuracy in adults presenting to the emergency department with acute dyspnea. West J Emerg Med. 2016;17(1):46–53.
- , , , , , . Acute heart failure: the role of focused emergency cardiopulmonary ultrasound in identification and early management. Eur J Heart Fail. 2015;17(12):1223–1227.
- , , , et al. Hand‐carried echocardiography by hospitalists: a randomized trial. Am J Med. 2011;124(8):766–774.
- , , , et al. ESC guidelines for the diagnosis and treatment of acute and chronic heart failure 2008: the Task Force for the diagnosis and treatment of acute and chronic heart failure 2008 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association of the ESC (HFA) and endorsed by the European Society of Intensive Care Medicine (ESICM). Eur J Heart Fail. 2008;10(10):933–989.
- , , , , , . Adaptation of the modified Barthel Index for use in physical medicine and rehabilitation in Turkey. Scand J Rehabil Med. 2000;32(2):87–92.
- , , , . Complications, comorbidities, and mortality: improving classification and prediction. Health Serv Res. 1997;32(2):229–238; discussion 239–242.
- , , , et al. Guidelines for the echocardiographic assessment of the right heart in adults: a report from the American Society of Echocardiography endorsed by the European Association of Echocardiography, a registered branch of the European Society of Cardiology, and the Canadian Society of Echocardiography. J Am Soc Echocardiogr. 2010;23(7):685–713; quiz 786–688.
- , , , et al. Recommendations for chamber quantification: a report from the American Society of Echocardiography's Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr. 2005;18(12):1440–1463.
- , , , , . Mortality and functional evolution at one year after hospital admission due to heart failure (HF) in elderly patients. Arch Gerontol Geriatr. 2012;54(1):261–265.
- , , , et al. Early and long‐term outcomes of heart failure in elderly persons, 2001–2005. Arch Intern Med. 2008;168(22):2481–2488.
- , , , et al. IVC diameter in patients with chronic heart failure: relationships and prognostic significance. JACC Cardiovasc Imaging. 2013;6(1):16–28.
- , , , et al. Prognostic significance of dilated inferior vena cava in advanced decompensated heart failure. Int J Cardiovasc Imaging. 2014;30(7):1289–1295.
- , , . A dilated inferior vena cava is a marker of poor survival. Am Heart J. 2006;151(3):730–735.
- , , , et al. Predischarge B‐type natriuretic peptide assay for identifying patients at high risk of re‐admission after decompensated heart failure. J Am Coll Cardiol. 2004;43(4):635–641.
- , , , et al. A rapid bedside test for B‐type peptide predicts treatment outcomes in patients admitted for decompensated heart failure: a pilot study. J Am Coll Cardiol. 2001;37(2):386–391.
- , , , , , . N‐terminal‐pro‐brain natriuretic peptide predicts outcome after hospital discharge in heart failure patients. Circulation. 2004;110(15):2168–2174.
- , , , , , . Lowered B‐type natriuretic peptide in response to levosimendan or dobutamine treatment is associated with improved survival in patients with severe acutely decompensated heart failure. J Am Coll Cardiol. 2009;53(25):2343–2348.
- , , , . Long‐term clinical variation of NT‐proBNP in stable chronic heart failure patients. Eur Heart J. 2007;28(2):177–182.
- , , , et al. Right atrial volume index in chronic systolic heart failure and prognosis. JACC Cardiovasc Imaging. 2009;2(5):527–534.
- , , , et al. Pulmonary pressures and death in heart failure: a community study. J Am Coll Cardiol. 2012;59(3):222–231.
- , , , et al. A comparison by medicine residents of physical examination versus hand‐carried ultrasound for estimation of right atrial pressure. Am J Cardiol. 2007;99(11):1614–1616.
- , , . Noninvasive estimation of right atrial pressure from the inspiratory collapse of the inferior vena cava. Am J Cardiol. 1990;66(4):493–496.
- , , , , , . Relationship between right and left‐sided filling pressures in 1000 patients with advanced heart failure. J Heart Lung Transplant. 1999;18(11):1126–1132.
- , , , et al. Admission B‐type natriuretic peptide levels and in‐hospital mortality in acute decompensated heart failure. J Am Coll Cardiol. 2007;49(19):1943–1950.
- , , , et al. State of the art: using natriuretic peptide levels in clinical practice. Eur J Heart Fail. 2008;10(9):824–839.
- , , , et al. Readmission after hospitalization for congestive heart failure among Medicare beneficiaries. Arch Intern Med. 1997;157(1):99–104.
- , , , et al. Inferior vena cava parameters predict re‐admission in ischaemic heart failure. Eur J Clin Invest. 2014;44(4):341–349.
- , , , et al. Estimation of right atrial pressure on inferior vena cava ultrasound in Asian patients. Circ J. 2014;78(4):962–966.
- , , , , . Clinical presentation, management, and in‐hospital outcomes of patients admitted with acute decompensated heart failure with preserved systolic function: a report from the Acute Decompensated Heart Failure National Registry (ADHERE) Database. J Am Coll Cardiol. 2006;47(1):76–84.
- , , , , . Palliative care referral among patients hospitalized with advanced heart failure. J Palliat Med. 2014;17(10):1115–1120.
- , , . Engaging heart failure clinicians to increase palliative care referrals: overcoming barriers, improving techniques. J Palliat Med. 2014;17(7):753–760.
- , , , et al. Heart disease and stroke statistics—2015 update: a report from the American Heart Association. Circulation. 2015;131(4):e29–e322.
- , , . Hospitalization for congestive heart failure: United States, 2000–2010. NCHS Data Brief. 2012(108):1–8.
- , . Rehospitalization for heart failure: predict or prevent? Circulation. 2012;126(4):501–506.
- , , , et al. Generalizability and longitudinal outcomes of a national heart failure clinical registry: Comparison of Acute Decompensated Heart Failure National Registry (ADHERE) and non‐ADHERE Medicare beneficiaries. Am Heart J. 2010;160(5):885–892.
- , , , et al. Prognostic markers of acute decompensated heart failure: the emerging roles of cardiac biomarkers and prognostic scores. Arch Cardiovasc Dis. 2015;108(1):64–74.
- , , , et al. Comparison of hand‐carried ultrasound assessment of the inferior vena cava and N‐terminal pro‐brain natriuretic peptide for predicting readmission after hospitalization for acute decompensated heart failure. JACC Cardiovasc Imaging. 2008;1(5):595–601.
- , , , , . Echocardiography in acute heart failure: current perspectives. J Card Fail. 2016;22(1):82–94.
- , , , , . Usefulness of a hand‐held ultrasound device for bedside examination of left ventricular function. Am J Cardiol. 2002;90(9):1038–1039.
- , , , , , . Feasibility of point‐of‐care echocardiography by internal medicine house staff. Am Heart J. 2004;147(3):476–481.
- , , , , , . The use of small personal ultrasound devices by internists without formal training in echocardiography. Eur J Echocardiogr. 2003;4(2):141–147.
- , , , et al. Diagnostic accuracy of hospitalist‐performed hand‐carried ultrasound echocardiography after a brief training program. J Hosp Med. 2009;4(6):340–349.
- , , , . Point‐of‐care multi‐organ ultrasound improves diagnostic accuracy in adults presenting to the emergency department with acute dyspnea. West J Emerg Med. 2016;17(1):46–53.
- , , , , , . Acute heart failure: the role of focused emergency cardiopulmonary ultrasound in identification and early management. Eur J Heart Fail. 2015;17(12):1223–1227.
- , , , et al. Hand‐carried echocardiography by hospitalists: a randomized trial. Am J Med. 2011;124(8):766–774.
- , , , et al. ESC guidelines for the diagnosis and treatment of acute and chronic heart failure 2008: the Task Force for the diagnosis and treatment of acute and chronic heart failure 2008 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association of the ESC (HFA) and endorsed by the European Society of Intensive Care Medicine (ESICM). Eur J Heart Fail. 2008;10(10):933–989.
- , , , , , . Adaptation of the modified Barthel Index for use in physical medicine and rehabilitation in Turkey. Scand J Rehabil Med. 2000;32(2):87–92.
- , , , . Complications, comorbidities, and mortality: improving classification and prediction. Health Serv Res. 1997;32(2):229–238; discussion 239–242.
- , , , et al. Guidelines for the echocardiographic assessment of the right heart in adults: a report from the American Society of Echocardiography endorsed by the European Association of Echocardiography, a registered branch of the European Society of Cardiology, and the Canadian Society of Echocardiography. J Am Soc Echocardiogr. 2010;23(7):685–713; quiz 786–688.
- , , , et al. Recommendations for chamber quantification: a report from the American Society of Echocardiography's Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr. 2005;18(12):1440–1463.
- , , , , . Mortality and functional evolution at one year after hospital admission due to heart failure (HF) in elderly patients. Arch Gerontol Geriatr. 2012;54(1):261–265.
- , , , et al. Early and long‐term outcomes of heart failure in elderly persons, 2001–2005. Arch Intern Med. 2008;168(22):2481–2488.
- , , , et al. IVC diameter in patients with chronic heart failure: relationships and prognostic significance. JACC Cardiovasc Imaging. 2013;6(1):16–28.
- , , , et al. Prognostic significance of dilated inferior vena cava in advanced decompensated heart failure. Int J Cardiovasc Imaging. 2014;30(7):1289–1295.
- , , . A dilated inferior vena cava is a marker of poor survival. Am Heart J. 2006;151(3):730–735.
- , , , et al. Predischarge B‐type natriuretic peptide assay for identifying patients at high risk of re‐admission after decompensated heart failure. J Am Coll Cardiol. 2004;43(4):635–641.
- , , , et al. A rapid bedside test for B‐type peptide predicts treatment outcomes in patients admitted for decompensated heart failure: a pilot study. J Am Coll Cardiol. 2001;37(2):386–391.
- , , , , , . N‐terminal‐pro‐brain natriuretic peptide predicts outcome after hospital discharge in heart failure patients. Circulation. 2004;110(15):2168–2174.
- , , , , , . Lowered B‐type natriuretic peptide in response to levosimendan or dobutamine treatment is associated with improved survival in patients with severe acutely decompensated heart failure. J Am Coll Cardiol. 2009;53(25):2343–2348.
- , , , . Long‐term clinical variation of NT‐proBNP in stable chronic heart failure patients. Eur Heart J. 2007;28(2):177–182.
- , , , et al. Right atrial volume index in chronic systolic heart failure and prognosis. JACC Cardiovasc Imaging. 2009;2(5):527–534.
- , , , et al. Pulmonary pressures and death in heart failure: a community study. J Am Coll Cardiol. 2012;59(3):222–231.
- , , , et al. A comparison by medicine residents of physical examination versus hand‐carried ultrasound for estimation of right atrial pressure. Am J Cardiol. 2007;99(11):1614–1616.
- , , . Noninvasive estimation of right atrial pressure from the inspiratory collapse of the inferior vena cava. Am J Cardiol. 1990;66(4):493–496.
- , , , , , . Relationship between right and left‐sided filling pressures in 1000 patients with advanced heart failure. J Heart Lung Transplant. 1999;18(11):1126–1132.
- , , , et al. Admission B‐type natriuretic peptide levels and in‐hospital mortality in acute decompensated heart failure. J Am Coll Cardiol. 2007;49(19):1943–1950.
- , , , et al. State of the art: using natriuretic peptide levels in clinical practice. Eur J Heart Fail. 2008;10(9):824–839.
- , , , et al. Readmission after hospitalization for congestive heart failure among Medicare beneficiaries. Arch Intern Med. 1997;157(1):99–104.
- , , , et al. Inferior vena cava parameters predict re‐admission in ischaemic heart failure. Eur J Clin Invest. 2014;44(4):341–349.
- , , , et al. Estimation of right atrial pressure on inferior vena cava ultrasound in Asian patients. Circ J. 2014;78(4):962–966.
- , , , , . Clinical presentation, management, and in‐hospital outcomes of patients admitted with acute decompensated heart failure with preserved systolic function: a report from the Acute Decompensated Heart Failure National Registry (ADHERE) Database. J Am Coll Cardiol. 2006;47(1):76–84.
- , , , , . Palliative care referral among patients hospitalized with advanced heart failure. J Palliat Med. 2014;17(10):1115–1120.
- , , . Engaging heart failure clinicians to increase palliative care referrals: overcoming barriers, improving techniques. J Palliat Med. 2014;17(7):753–760.
Ultrasound and Pleural Effusions
Hospitalists commonly encounter pleural effusions, and their detection and characterization by point‐of‐care ultrasound can guide management. Approximately 44% to 57% of hospitalized patients with bacterial pneumonia,[1, 2] and up to 62% of intensive care unit (ICU) patients[3] have a pleural effusion. For patients with a parapneumonic effusion, hospitalists can use ultrasound to quantify and characterize pleural fluid to determine whether diagnostic or therapeutic drainage is indicated, as well as guide performance of thoracentesis. For patients with lung cancer, detection of a malignant pleural effusion changes staging to stage IV, regardless of tumor size or lymph node involvement, and hospitalists may discuss more appropriate treatment options with patients and consultants.
Routine use of pleural ultrasonography may help hospitalists provide high‐value care by reducing ancillary testing, including computerized tomography (CT) scans that expose patients to ionizing radiation, and reducing complications of thoracentesis. However, many hospitalists may not be familiar with the use of point‐of‐care ultrasound. A national survey in 2012 revealed only 25% of internal medicine residencies have formal curricula to teach point‐of‐care ultrasonography.[4] The purpose of this review is to provide an overview of how point‐of‐care ultrasound can be utilized by hospitalists in the care of patients with pleural effusions. We review the literature on the diagnosis and evaluation of pleural effusions with ultrasound, as well as techniques to examine and drain the pleural space.
DIAGNOSIS OF PLEURAL EFFUSION
History and Physical Exam
Pleural effusions are most commonly associated with heart failure, pneumonia, cancer, pulmonary embolism, viral disease, coronary artery bypass surgery, and cirrhosis with ascites. The most common symptoms related to pleural effusion are nonspecific and often indistinguishable from those of the underlying disease process, including cough, dyspnea, and pleuritic chest pain.[5]
Diagnostic accuracy of a physical examination to detect pleural fluid is highly dependent on the size of the effusion and is unlikely to detect effusions <300 mL. A systematic review found the most accurate physical exam findings to rule in a pleural effusion were dullness to percussion (positive likelihood ratio [LR]: 8.7; 95% CI: 2.2‐33.8) and asymmetric chest expansion (positive LR: 8.1; 95% CI: 5.2‐12.7). Normal tactile vocal fremitus was the most accurate physical exam finding to rule out a pleural effusion (negative LR: 0.21; 95% CI: 0.12‐0.37).[6] A major limitation of all these studies is that physical exam was compared to chest radiography as the reference standard, and posterior‐anterior chest radiographs are not sensitive for detection of pleural effusions until 200 mL of fluid has accumulated.[7] Further, chest percussion penetrates to a maximum depth of 6 cm, and its utility is limited in obese patients.[8] Characteristics of pleural fluid that can change management, such as loculations, cannot be detected by physical exam.
Chest Radiography
Chest radiography has traditionally been used to diagnose pleural effusions. Free‐flowing pleural fluid collects in the most dependent portions of the thorax, initially in the subpulmonic space followed by the costophrenic recesses. Pleural fluid is detectable in the costophrenic recesses on lateral upright chest radiograph after 50 mL has accumulated. On standard posterior‐anterior chest radiograph, blunting of the costophrenic recesses and obliteration of the hemidiaphragm are seen when >200 mL and >500 mL of pleural fluid have accumulated, respectively.[7] However, upright chest radiographs can miss a considerable number of effusions, including as many as 10% of parapneumonic effusions large enough to indicate need for drainage.[9] Supine anterior‐posterior chest radiographs can miss a significant proportion of large effusions seen on chest CT,[10] ultrasound,[11] and lateral decubitus radiographs.[12] Pleural effusions are frequently mistaken for parenchymal opacities on portable anterior‐posterior chest radiographs.[10]
Computerized Tomography
Chest CT serves as the reference standard in most modern diagnostic accuracy studies. Limitations of chest CT include difficulty distinguishing small effusions from pleural thickening, dependent atelectasis, or tumor; lower sensitivity for detecting pleural fluid septations compared to ultrasound[13]; exposure of patients to approximately 7 mSv of ionizing radiation (the equivalent radiation dose of 350 chest radiographs)[14]; high cost; and need to transport patients to radiology departments where CT scanners are located.
Pleural Ultrasonography
Ultrasound can rapidly differentiate conditions that demonstrate a nonspecific, radiopaque appearance of lower lung fields on chest radiographs, including pleural effusions, pneumonia, atelectasis, elevated hemidiaphragm, and lung or pleural masses. Advantages of point‐of‐care ultrasound include the ability of hospitalists to acquire and interpret images at the bedside and integrate findings into clinical decision making immediately. Multiple studies have demonstrated superior diagnostic accuracy of ultrasound compared to chest radiography for detection of pleural effusions. Pleural ultrasound can detect physiologic amounts of pleural fluid (5 mL),[15] but a minimal volume of 20 mL is more reliably detected,[16] and ultrasound is 100% sensitive for effusions >100 mL.[17] In a prospective study of critically ill patients with acute respiratory distress syndrome, the diagnostic accuracy of ultrasound for pleural effusions was superior (93%) compared to auscultation (61%) and anterior‐posterior chest radiograph (47%), using chest CT as the reference standard.[18] A meta‐analysis of 4 studies calculated a pooled sensitivity and specificity of ultrasound for detection of pleural effusions as 93% (95% CI: 89%‐96%) and 96% (95% CI: 95%‐98%), respectively.[18, 19, 20, 21, 22] Ultrasound has the additional benefit of characterizing underlying lung parenchyma, which is well described in the literature but beyond the scope of this review.[23]
Sensitivity and specificity of chest radiography and ultrasonography to detect a pleural effusion are displayed in Table 1.[9, 10, 11, 12, 18, 20, 21, 22, 24, 25, 26]
| Exam | Reference Standard | Sensitivity | Specificity | Study | |
|---|---|---|---|---|---|
| |||||
| Chest radiograph | Supine AP | Upright PA/lateral | 92% | Woodring[24] | |
| Lateral decubitus XR | 67% | 70% | Ruskin[12] | ||
| Ultrasound | 82% | 82% | Emamian[11] | ||
| Ultrasound or thoracentesis | 33% | Kocijancic[25] | |||
| CT | 39% | 85% | Lichtenstein[18] | ||
| CT | 66% | 89% | Kitazano[10] | ||
| CT | 65% | 81% | Xirouchaki[26] | ||
| CT | 78% | 76% | Brixey[9] | ||
| Lateral decubitus | Ultrasound or thoracentesis | 94% | 100% | Kocijancic[25] | |
| Upright PA | CT | 82% | 81% | Brixey[9] | |
| Lateral upright | CT | 86% | 88% | Brixey[9] | |
| Ultrasound | Cardiology | CT | 93% | 88% | Kataoka[20] |
| Point of care | CT or tube thoracostomy | 96% | 100% | Ma[21] | |
| CT | 92% | 93% | Lichtenstein[18] | ||
| CT | 94% | 99% | Rocco[22] | ||
| CT | 100% | 100% | Xirouchaki[26] | ||
PLEURAL ULTRASOUND EXAMINATION
A pleural ultrasound exam may be performed as part of a complete lung ultrasound exam, such as the BLUE (Bedside Lung Ultrasound in Emergency) protocol,[27] or a focused exam to evaluate a suspected or known pleural effusion seen on chest radiograph or CT scan.[27] Free‐flowing pleural effusions accumulate in the most dependent portions of the thorax, most commonly, the posterolateral costophrenic recesses in semirecumbent or seated patients, but anteriorly in mechanically ventilated patients in a prone position.
A low‐frequency (25 MHz) phased‐array transducer is generally preferred for imaging in between the ribs. High‐frequency linear transducers do not provide adequate penetration to visualize deep structures, but do provide superior visualization of the pleural line to assess pleural thickness, measure pleural depth, and evaluate for pneumothorax.
Pleural effusions are best evaluated starting at the level of the diaphragm. Place the transducer in a longitudinal plane on the posterior axillary line at the level of the diaphragm with the transducer orientation marker (notch) pointed cephalad (Figure 1). Five structures must be definitively identified to diagnose a pleural effusion: liver/spleen, diaphragm, pleural fluid, lung, and chest wall (Figure 2A). Large pleural effusions compress the adjacent lung causing atelectasis, which gives the lung a tissue‐like echogenicity similar to the liver (Figure 2B). Static air bronchograms are commonly seen in atelectatic lung bases with pleural effusions.[28]
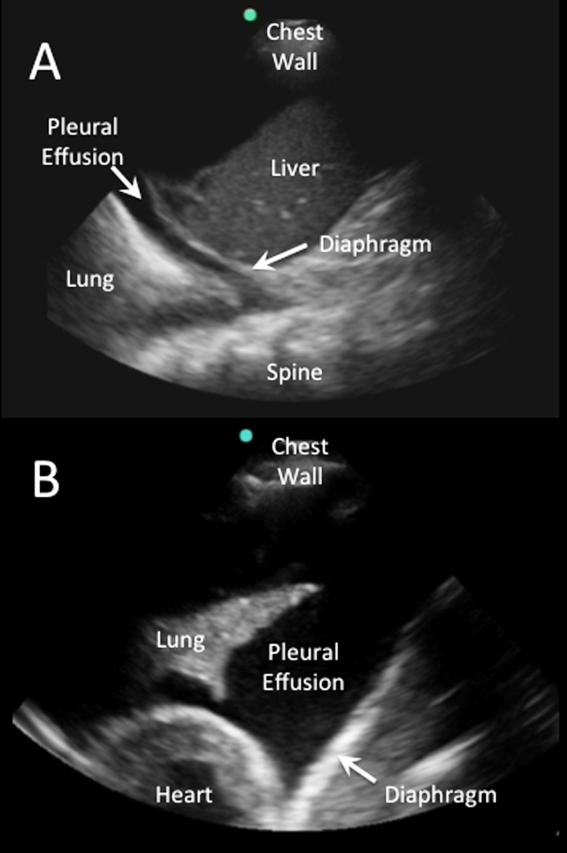
Color flow Doppler and M‐mode ultrasound may be utilized as adjuncts to routine 2‐dimensional ultrasonography. Free‐flowing pleural effusions will demonstrate flow by color Doppler (Figure 3A). Using M‐mode ultrasound, the lung can been seen moving within a pleural effusion to and from the chest wall (sinusoid sign).[29] Absence of flow or movement is seen with dense pleural loculations, pleural thickening, and peripheral lung or pleural masses (Figure 3B).
CHARACTERIZATION OF PLEURAL EFFUSION
Pleural Fluid Volume
Quantification of pleural fluid volume has been proposed using formulas with sonographic measurements.[30, 31, 32] These formulas are most accurate for moderate‐sized effusions but have not been validated beyond individual study cohorts. The largest study (n = 150) found a strong correlation between calculated and actual volumes drained by thoracentesis (r2 = 0.79; P < 0.001) using the formula (Volume [mL] = 16 parietal to visceral pleura distance (mm) at the mid‐diaphragm).[31] Although an accurate quantitative pleural fluid volume assessment may be possible, these formulas are not commonly used in clinical practice. A qualitative assessment is adequate for most clinical decision making using categories of minimal, small, moderate, or large volume.
Simple Versus Complex Effusions
Based on its sonographic appearance, pleural effusions are categorized as simple or complex. Simple pleural effusions are anechoic and usually transudative. Complex pleural effusions are subcategorized as homogeneously or heterogeneously echogenic, with or without septations, and are more often exudative.[33]
Effusions with heterogeneous echogenicity with swirling echoes suggest high cellular content that is often associated with malignancy.[34] Fibrinous stranding, septations, and loculations also suggest an exudative effusion (Figure 4A), and are more readily identified and characterized on lung ultrasound than CT scan.[35]
Homogenously echogenic effusions are most often due to hemothorax or empyema.[36] The high cell count of a hemothorax creates a layering effect in costophrenic recesses (hematocrit sign). Empyemas develop from complex effusions that organize into collections of pus and usually have a homogeneously echogenic, speckled appearance (Figure 4B). Sonographic evidence of septations in the presence of empyema predicts the need for intrapleural fibrinolytic therapy, longer duration of drainage, and possible surgical intervention.[37]
Isolated dense loculations may be challenging to differentiate from peripheral lung or pleural lesions, such as abscess or tumor.
Pleural Thickness
Normal visceral and parietal pleura are apposed and 0.2 to 0.3 mm thick.[38] Pleural effusions with parietal pleural thickness >10 mm, pleural nodularity, and diaphragmatic thickness >7 mm predicted underlying malignancy with high specificity and positive predictive value in 1 study.[39] As many as 20% of anechoic lesions of the pleura are solid rather than fluid. Color flow Doppler ultrasound can differentiate small pleural effusions from solid pleural abnormalities with sensitivity and specificity of 89% and 100%, respectively.[40]
PLEURAL FLUID DRAINAGE
Since its first description in 1967, use of ultrasound guidance for thoracentesis has evolved to become the standard of care in many hospitals in the United States.[41] The British Thoracic Society guidelines recommend that all thoracenteses be performed with ultrasound guidance.[42] The American College of Graduate Medical Education now requires proficiency in the use of ultrasound for thoracentesis and pleural catheter insertion by pulmonary and critical care fellows.[43]
The impetus for these recommendations stems from increased procedural success and decreased complications associated with ultrasound‐guided drainage of pleural effusions. A study evaluating thoracentesis site selection based on physical exam and chest radiographs demonstrated inaccurate site selection in 15% of patients, and use of ultrasound for site selection prevented possible accidental organ puncture in 10% of all cases.[44] The success rate of thoracentesis for small pleural effusions has been shown to increase from 66% to 90% with ultrasound guidance.[42] Using ultrasound, the distance from the skin to parietal and visceral pleura can be measured to determine whether thoracentesis can be safely performed, and to guide selection of an adequate length needle (Figure 5). A minimum pleural effusion depth of 1.5 cm between the visceral and parietal pleura has been recommended to perform diagnostic thoracentesis.[28] Diagnostic thoracentesis of complex septated pleural effusions or empyemas may be performed with a straight needle, but therapeutic drainage often requires temporary insertion of a catheter. Traditionally, large‐bore chest tubes (>24 F) had been advocated to drain viscid pus, but recent evidence suggests that small‐bore catheters (1014 F) with instillation of thrombolytics may be as effective and performed with less discomfort.[45] Video‐assisted thoracoscopy to lyse septations and evacuate infected materials is indicated when chest tube drainage has failed.
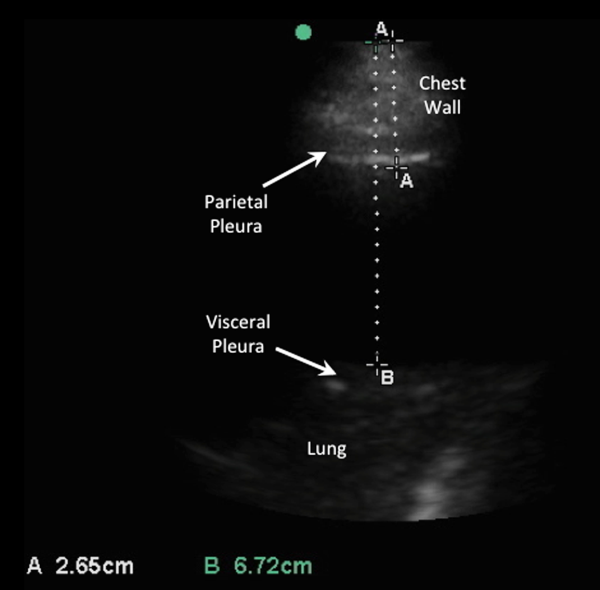
The most common complication of pleural drainage is pneumothorax. A meta‐analysis demonstrated a reduction in post‐thoracentesis pneumothorax rates from 9% to 4% with use of ultrasound.[46] Transporting patients to radiology for ultrasound marking has not been shown to decrease pneumothorax rates compared to thoracentesis without ultrasound guidance, likely due to changes in patient position and prolonged delays between marking and drainage.[47] Postprocedure pneumothorax can be ruled out if lung sliding is visualized. A meta‐analysis demonstrated superior sensitivity and similar specificity of pleural ultrasonography versus chest radiography to detect pneumothorax (sensitivity 91% vs 50% and specificity 98% vs 99%, respectively).[48] Real‐time ultrasound guidance for thoracentesis, or use of ultrasound to track the needle tip, has not been well studied but may be performed by experienced proceduralists to drain small effusions.
FUTURE RESEARCH
Future research should focus on the clinical effectiveness of point‐of‐care pleural ultrasonography when integrated with other diagnostic tools, and application of new ultrasound technologies to evaluate pleural diseases. Routine use of point‐of‐care ultrasound as the primary imaging modality in a medical ICU demonstrated a highly statistically significant reduction in chest x‐rays (3.75 vs 0.82, P < 0.0001) and chest CT scans (0.10 vs 0.04, P = 0.0007).[49] Similar studies have yet to be performed with the use of ultrasound specifically in the management of pleural diseases. Thus, clinical effectiveness studies are needed to assess the impact of routine use of pleural ultrasound on the initiation of appropriate therapies, length of stay, and costs in the management of pleural disease.
Point‐of‐care pleural ultrasound findings need to be evaluated in the context of other clinical findings and diagnostic tests. Certain ultrasound findings have been associated with exudative pleural effusions, but whether exudative and transudative effusions can be differentiated noninvasively using ultrasound findings alone, or in combination with other clinical data, warrants investigation. Similar to severity of illness scores, models that incorporate clinical, laboratory, and ultrasound findings need to be developed to guide treatment decisions, such as type of drainage procedure, as well as prognostication.
Finally, new technologies may advance the utility of point‐of‐care pleural ultrasonography. Even though 3‐dimensional ultrasonography has been available for over 2 decades, comparative studies of conventional 2‐dimensional versus 3‐dimensional ultrasonography to characterize pleural effusions have yet to be performed. Furthermore, computer‐aided detection has been shown to facilitate interpretation of ultrasound images, but this technology has yet to be applied to pleural ultrasonography.
CONCLUSIONS
Point‐of‐care pleural ultrasound is a powerful bedside tool in the hospitalist's armamentarium that is superior to physical examination and chest radiographs in the detection and characterization of pleural effusions. Furthermore, ultrasound performs similarly when compared to CT scans but offers the advantages of decreased cost, avoidance of ionizing radiation, and availability at the bedside. Ultrasound guidance reduces complications and increases the success rate of pleural drainage procedures, leading to improved patient safety. As clinical effectiveness studies emerge revealing its true value, point‐of‐care pleural ultrasonography is likely to become the standard diagnostic tool to evaluate and manage patients with pleural effusions.
Disclosures: Dr. Restrepo is partially supported by award number K23HL096054 from the National Heart, Lung, and Blood Institute. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Heart, Lung, and Blood Institute or the National Institutes of Health. The authors report no conflicts of interest.
- , , , . Parapneumonic effusions. Am J Med. 1980;69(4):507–512.
- , , . The incidence and clinical correlates of parapneumonic effusions in pneumococcal pneumonia. Chest. 1978;74(2):170–173.
- , , , , . Pleural effusions in the medical ICU: prevalence, causes, and clinical implications. Chest. 1997;111(4):1018–1023.
- , , , . Point‐of‐care ultrasound in internal medicine: a national survey of educational leadership. J Grad Med Educ. 2013;5(3):498–502.
- . Pleural Diseases. Philadelphia, PA: Lippincott Williams 2007.
- , , . Does this patient have a pleural effusion? JAMA. 2009;301(3):309–317.
- , , , . Pleural fluid volume estimation: a chest radiograph prediction rule. Acad Radiol. 1996;3(2):103–109.
- , . Accuracy of the physical examination in evaluating pleural effusion. Cleve Clin J Med. 2008;75(4):297–303.
- , , , , . The efficacy of chest radiographs in detecting parapneumonic effusions. Respirology. 2011;16(6):1000–1004.
- , , , , . Differentiation of pleural effusions from parenchymal opacities: accuracy of bedside chest radiography. AJR Am J Roentgenol. 2010;194(2):407–412.
- , , , . Accuracy of the diagnosis of pleural effusion on supine chest X‐ray. Eur Radiol. 1997;7(1):57–60.
- , , , . Detection of pleural effusions on supine chest radiographs. AJR Am J Roentgenol. 1987;148(4):681–683.
- , , , , . Multiloculated pleural effusion detected by ultrasound only in a critically‐ill patient. Am J Case Rep. 2013;14:63–66.
- , , , et al. Exposure to low‐dose ionizing radiation from medical imaging procedures. N Engl J Med. 2009;361(9):849–857.
- , , . The diagnosis of pleural effusion by ultrasonic and radiologic techniques. Chest. 1976;70(1):33–37.
- , , , , , . Ultrasound in blunt abdominal and thoracic trauma. J Trauma. 1993;34(4):488–495.
- , , , et al. Application of color Doppler ultrasound in the study of small pleural effusion. Med Ultrason. 2010;12(1):12–16.
- , , , , , . Comparative diagnostic performances of auscultation, chest radiography, and lung ultrasonography in acute respiratory distress syndrome. Anesthesiology. 2004;100(1):9–15.
- , , , , . Diagnostic accuracy of sonography for pleural effusion: systematic review. Sao Paulo Med J. 2010;128(2):90–95.
- , . The role of thoracic ultrasonography for evaluation of patients with decompensated chronic heart failure. J Am Coll Cardiol. 2000;35(6):1638–1646.
- , . Trauma ultrasound examination versus chest radiography in the detection of hemothorax. Ann Emerg Med. 1997;29(3):312–315; discussion 315–316.
- , , , et al. Diagnostic accuracy of bedside ultrasonography in the ICU: feasibility of detecting pulmonary effusion and lung contusion in patients on respiratory support after severe blunt thoracic trauma. Acta Anaesthesiol Scand. 2008;52(6):776–784.
- . Lung ultrasound in the critically ill. Ann Intensive Care. 2014;4(1):1.
- . Recognition of pleural effusion on supine radiographs: how much fluid is required? AJR. Am J Roentgenol. 1984;142(1):59–64.
- , , . Chest sonography versus lateral decubitus radiography in the diagnosis of small pleural effusions. J Clin Ultrasound. 2003;31(2):69–74.
- , , , , . Impact of lung ultrasound on clinical decision making in critically ill patients. Intensive Care Med. 2014;40(1):57–65.
- . Lung ultrasound in acute respiratory failure an introduction to the BLUE‐protocol. Minerva Anestesiol. 2009;75(5):313–317.
- , , . Point‐of‐Care Ultrasound. 1st ed. Philadelphia, PA: Saunders; 2014.
- , , , et al. International evidence‐based recommendations for point‐of‐care lung ultrasound. Intensive Care Med. 2012;38(4):577–591.
- , , , et al. Ultrasound estimation of volume of pleural fluid in mechanically ventilated patients. Intensive Care Med. 2006;32(2):318–321.
- , , . Ultrasound estimation of volume of postoperative pleural effusion in cardiac surgery patients. Interact Cardiovasc Thorac Surg. 2010;10(2):204–207.
- , , , et al. Multiplane ultrasound approach to quantify pleural effusion at the bedside. Intensive Care Med. 2010;36(4):656–664.
- , , , , , . Value of sonography in determining the nature of pleural effusion: analysis of 320 cases. AJR Am J Roentgenol. 1992;159(1):29–33.
- , , , , , . Echogenic swirling pattern as a predictor of malignant pleural effusions in patients with malignancies. Chest. 2004;126(1):129–134.
- , . Imaging the pleura: sonography, CT, and MR imaging. AJR Am J Roentgenol. 1991;156(6):1145–1153.
- , , , et al. Pleural effusions in febrile medical ICU patients: chest ultrasound study. Chest. 2004;126(4):1274–1280.
- , , , , . Sonographic septation: a useful prognostic indicator of acute thoracic empyema. J Ultrasound Med. 2000;19(12):837–843.
- . Sonography of the pleura [in German]. Ultraschall Med. 2010;31(1):8–22, quiz 23–25.
- , , . Thoracic ultrasound in the diagnosis of malignant pleural effusion. Thorax. 2009;64(2):139–143.
- , , , . “Fluid color” sign: a useful indicator for discrimination between pleural thickening and pleural effusion. J Ultrasound Med. 1995;14(10):767–769.
- , , . Reflected ultrasound in the detection and localization of pleural effusion. JAMA. 1967;200(5):399–402.
- , , , . Pleural procedures and thoracic ultrasound: British Thoracic Society Pleural Disease Guideline 2010. Thorax. 2010;65(suppl 2):ii61–ii76.
- Accreditation Council for Graduate Medical Education. http://www.acgme.org/acgmeweb. Accessed January 15, 2015.
- , , . Accuracy of pleural puncture sites: a prospective comparison of clinical examination with ultrasound. Chest. 2003;123(2):436–441.
- , , , , , . A double blind randomized cross over trial comparing rate of decortication and efficacy of intrapleural instillation of alteplase vs placebo in patients with empyemas and complicated parapneumonic effusions. Respir Med. 2012;106(5):716–723.
- , , , . Pneumothorax following thoracentesis: a systematic review and meta‐analysis. Arch Intern Med. 2010;170(4):332–339.
- , , , , , . Factors affecting the development of pneumothorax associated with thoracentesis. AJR Am J Roentgenol. 1991;156(5):917–920.
- , , . Test characteristics of ultrasonography for the detection of pneumothorax: a systematic review and meta‐analysis. Chest. 2012;141(3):703–708.
- , , , et al. The effect of point‐of‐care ultrasonography on imaging studies in the medical ICU: a comparative study. Chest. 2014;146(6):1574–1577.
Hospitalists commonly encounter pleural effusions, and their detection and characterization by point‐of‐care ultrasound can guide management. Approximately 44% to 57% of hospitalized patients with bacterial pneumonia,[1, 2] and up to 62% of intensive care unit (ICU) patients[3] have a pleural effusion. For patients with a parapneumonic effusion, hospitalists can use ultrasound to quantify and characterize pleural fluid to determine whether diagnostic or therapeutic drainage is indicated, as well as guide performance of thoracentesis. For patients with lung cancer, detection of a malignant pleural effusion changes staging to stage IV, regardless of tumor size or lymph node involvement, and hospitalists may discuss more appropriate treatment options with patients and consultants.
Routine use of pleural ultrasonography may help hospitalists provide high‐value care by reducing ancillary testing, including computerized tomography (CT) scans that expose patients to ionizing radiation, and reducing complications of thoracentesis. However, many hospitalists may not be familiar with the use of point‐of‐care ultrasound. A national survey in 2012 revealed only 25% of internal medicine residencies have formal curricula to teach point‐of‐care ultrasonography.[4] The purpose of this review is to provide an overview of how point‐of‐care ultrasound can be utilized by hospitalists in the care of patients with pleural effusions. We review the literature on the diagnosis and evaluation of pleural effusions with ultrasound, as well as techniques to examine and drain the pleural space.
DIAGNOSIS OF PLEURAL EFFUSION
History and Physical Exam
Pleural effusions are most commonly associated with heart failure, pneumonia, cancer, pulmonary embolism, viral disease, coronary artery bypass surgery, and cirrhosis with ascites. The most common symptoms related to pleural effusion are nonspecific and often indistinguishable from those of the underlying disease process, including cough, dyspnea, and pleuritic chest pain.[5]
Diagnostic accuracy of a physical examination to detect pleural fluid is highly dependent on the size of the effusion and is unlikely to detect effusions <300 mL. A systematic review found the most accurate physical exam findings to rule in a pleural effusion were dullness to percussion (positive likelihood ratio [LR]: 8.7; 95% CI: 2.2‐33.8) and asymmetric chest expansion (positive LR: 8.1; 95% CI: 5.2‐12.7). Normal tactile vocal fremitus was the most accurate physical exam finding to rule out a pleural effusion (negative LR: 0.21; 95% CI: 0.12‐0.37).[6] A major limitation of all these studies is that physical exam was compared to chest radiography as the reference standard, and posterior‐anterior chest radiographs are not sensitive for detection of pleural effusions until 200 mL of fluid has accumulated.[7] Further, chest percussion penetrates to a maximum depth of 6 cm, and its utility is limited in obese patients.[8] Characteristics of pleural fluid that can change management, such as loculations, cannot be detected by physical exam.
Chest Radiography
Chest radiography has traditionally been used to diagnose pleural effusions. Free‐flowing pleural fluid collects in the most dependent portions of the thorax, initially in the subpulmonic space followed by the costophrenic recesses. Pleural fluid is detectable in the costophrenic recesses on lateral upright chest radiograph after 50 mL has accumulated. On standard posterior‐anterior chest radiograph, blunting of the costophrenic recesses and obliteration of the hemidiaphragm are seen when >200 mL and >500 mL of pleural fluid have accumulated, respectively.[7] However, upright chest radiographs can miss a considerable number of effusions, including as many as 10% of parapneumonic effusions large enough to indicate need for drainage.[9] Supine anterior‐posterior chest radiographs can miss a significant proportion of large effusions seen on chest CT,[10] ultrasound,[11] and lateral decubitus radiographs.[12] Pleural effusions are frequently mistaken for parenchymal opacities on portable anterior‐posterior chest radiographs.[10]
Computerized Tomography
Chest CT serves as the reference standard in most modern diagnostic accuracy studies. Limitations of chest CT include difficulty distinguishing small effusions from pleural thickening, dependent atelectasis, or tumor; lower sensitivity for detecting pleural fluid septations compared to ultrasound[13]; exposure of patients to approximately 7 mSv of ionizing radiation (the equivalent radiation dose of 350 chest radiographs)[14]; high cost; and need to transport patients to radiology departments where CT scanners are located.
Pleural Ultrasonography
Ultrasound can rapidly differentiate conditions that demonstrate a nonspecific, radiopaque appearance of lower lung fields on chest radiographs, including pleural effusions, pneumonia, atelectasis, elevated hemidiaphragm, and lung or pleural masses. Advantages of point‐of‐care ultrasound include the ability of hospitalists to acquire and interpret images at the bedside and integrate findings into clinical decision making immediately. Multiple studies have demonstrated superior diagnostic accuracy of ultrasound compared to chest radiography for detection of pleural effusions. Pleural ultrasound can detect physiologic amounts of pleural fluid (5 mL),[15] but a minimal volume of 20 mL is more reliably detected,[16] and ultrasound is 100% sensitive for effusions >100 mL.[17] In a prospective study of critically ill patients with acute respiratory distress syndrome, the diagnostic accuracy of ultrasound for pleural effusions was superior (93%) compared to auscultation (61%) and anterior‐posterior chest radiograph (47%), using chest CT as the reference standard.[18] A meta‐analysis of 4 studies calculated a pooled sensitivity and specificity of ultrasound for detection of pleural effusions as 93% (95% CI: 89%‐96%) and 96% (95% CI: 95%‐98%), respectively.[18, 19, 20, 21, 22] Ultrasound has the additional benefit of characterizing underlying lung parenchyma, which is well described in the literature but beyond the scope of this review.[23]
Sensitivity and specificity of chest radiography and ultrasonography to detect a pleural effusion are displayed in Table 1.[9, 10, 11, 12, 18, 20, 21, 22, 24, 25, 26]
| Exam | Reference Standard | Sensitivity | Specificity | Study | |
|---|---|---|---|---|---|
| |||||
| Chest radiograph | Supine AP | Upright PA/lateral | 92% | Woodring[24] | |
| Lateral decubitus XR | 67% | 70% | Ruskin[12] | ||
| Ultrasound | 82% | 82% | Emamian[11] | ||
| Ultrasound or thoracentesis | 33% | Kocijancic[25] | |||
| CT | 39% | 85% | Lichtenstein[18] | ||
| CT | 66% | 89% | Kitazano[10] | ||
| CT | 65% | 81% | Xirouchaki[26] | ||
| CT | 78% | 76% | Brixey[9] | ||
| Lateral decubitus | Ultrasound or thoracentesis | 94% | 100% | Kocijancic[25] | |
| Upright PA | CT | 82% | 81% | Brixey[9] | |
| Lateral upright | CT | 86% | 88% | Brixey[9] | |
| Ultrasound | Cardiology | CT | 93% | 88% | Kataoka[20] |
| Point of care | CT or tube thoracostomy | 96% | 100% | Ma[21] | |
| CT | 92% | 93% | Lichtenstein[18] | ||
| CT | 94% | 99% | Rocco[22] | ||
| CT | 100% | 100% | Xirouchaki[26] | ||
PLEURAL ULTRASOUND EXAMINATION
A pleural ultrasound exam may be performed as part of a complete lung ultrasound exam, such as the BLUE (Bedside Lung Ultrasound in Emergency) protocol,[27] or a focused exam to evaluate a suspected or known pleural effusion seen on chest radiograph or CT scan.[27] Free‐flowing pleural effusions accumulate in the most dependent portions of the thorax, most commonly, the posterolateral costophrenic recesses in semirecumbent or seated patients, but anteriorly in mechanically ventilated patients in a prone position.
A low‐frequency (25 MHz) phased‐array transducer is generally preferred for imaging in between the ribs. High‐frequency linear transducers do not provide adequate penetration to visualize deep structures, but do provide superior visualization of the pleural line to assess pleural thickness, measure pleural depth, and evaluate for pneumothorax.
Pleural effusions are best evaluated starting at the level of the diaphragm. Place the transducer in a longitudinal plane on the posterior axillary line at the level of the diaphragm with the transducer orientation marker (notch) pointed cephalad (Figure 1). Five structures must be definitively identified to diagnose a pleural effusion: liver/spleen, diaphragm, pleural fluid, lung, and chest wall (Figure 2A). Large pleural effusions compress the adjacent lung causing atelectasis, which gives the lung a tissue‐like echogenicity similar to the liver (Figure 2B). Static air bronchograms are commonly seen in atelectatic lung bases with pleural effusions.[28]


Color flow Doppler and M‐mode ultrasound may be utilized as adjuncts to routine 2‐dimensional ultrasonography. Free‐flowing pleural effusions will demonstrate flow by color Doppler (Figure 3A). Using M‐mode ultrasound, the lung can been seen moving within a pleural effusion to and from the chest wall (sinusoid sign).[29] Absence of flow or movement is seen with dense pleural loculations, pleural thickening, and peripheral lung or pleural masses (Figure 3B).

CHARACTERIZATION OF PLEURAL EFFUSION
Pleural Fluid Volume
Quantification of pleural fluid volume has been proposed using formulas with sonographic measurements.[30, 31, 32] These formulas are most accurate for moderate‐sized effusions but have not been validated beyond individual study cohorts. The largest study (n = 150) found a strong correlation between calculated and actual volumes drained by thoracentesis (r2 = 0.79; P < 0.001) using the formula (Volume [mL] = 16 parietal to visceral pleura distance (mm) at the mid‐diaphragm).[31] Although an accurate quantitative pleural fluid volume assessment may be possible, these formulas are not commonly used in clinical practice. A qualitative assessment is adequate for most clinical decision making using categories of minimal, small, moderate, or large volume.
Simple Versus Complex Effusions
Based on its sonographic appearance, pleural effusions are categorized as simple or complex. Simple pleural effusions are anechoic and usually transudative. Complex pleural effusions are subcategorized as homogeneously or heterogeneously echogenic, with or without septations, and are more often exudative.[33]
Effusions with heterogeneous echogenicity with swirling echoes suggest high cellular content that is often associated with malignancy.[34] Fibrinous stranding, septations, and loculations also suggest an exudative effusion (Figure 4A), and are more readily identified and characterized on lung ultrasound than CT scan.[35]

Homogenously echogenic effusions are most often due to hemothorax or empyema.[36] The high cell count of a hemothorax creates a layering effect in costophrenic recesses (hematocrit sign). Empyemas develop from complex effusions that organize into collections of pus and usually have a homogeneously echogenic, speckled appearance (Figure 4B). Sonographic evidence of septations in the presence of empyema predicts the need for intrapleural fibrinolytic therapy, longer duration of drainage, and possible surgical intervention.[37]
Isolated dense loculations may be challenging to differentiate from peripheral lung or pleural lesions, such as abscess or tumor.
Pleural Thickness
Normal visceral and parietal pleura are apposed and 0.2 to 0.3 mm thick.[38] Pleural effusions with parietal pleural thickness >10 mm, pleural nodularity, and diaphragmatic thickness >7 mm predicted underlying malignancy with high specificity and positive predictive value in 1 study.[39] As many as 20% of anechoic lesions of the pleura are solid rather than fluid. Color flow Doppler ultrasound can differentiate small pleural effusions from solid pleural abnormalities with sensitivity and specificity of 89% and 100%, respectively.[40]
PLEURAL FLUID DRAINAGE
Since its first description in 1967, use of ultrasound guidance for thoracentesis has evolved to become the standard of care in many hospitals in the United States.[41] The British Thoracic Society guidelines recommend that all thoracenteses be performed with ultrasound guidance.[42] The American College of Graduate Medical Education now requires proficiency in the use of ultrasound for thoracentesis and pleural catheter insertion by pulmonary and critical care fellows.[43]
The impetus for these recommendations stems from increased procedural success and decreased complications associated with ultrasound‐guided drainage of pleural effusions. A study evaluating thoracentesis site selection based on physical exam and chest radiographs demonstrated inaccurate site selection in 15% of patients, and use of ultrasound for site selection prevented possible accidental organ puncture in 10% of all cases.[44] The success rate of thoracentesis for small pleural effusions has been shown to increase from 66% to 90% with ultrasound guidance.[42] Using ultrasound, the distance from the skin to parietal and visceral pleura can be measured to determine whether thoracentesis can be safely performed, and to guide selection of an adequate length needle (Figure 5). A minimum pleural effusion depth of 1.5 cm between the visceral and parietal pleura has been recommended to perform diagnostic thoracentesis.[28] Diagnostic thoracentesis of complex septated pleural effusions or empyemas may be performed with a straight needle, but therapeutic drainage often requires temporary insertion of a catheter. Traditionally, large‐bore chest tubes (>24 F) had been advocated to drain viscid pus, but recent evidence suggests that small‐bore catheters (1014 F) with instillation of thrombolytics may be as effective and performed with less discomfort.[45] Video‐assisted thoracoscopy to lyse septations and evacuate infected materials is indicated when chest tube drainage has failed.

The most common complication of pleural drainage is pneumothorax. A meta‐analysis demonstrated a reduction in post‐thoracentesis pneumothorax rates from 9% to 4% with use of ultrasound.[46] Transporting patients to radiology for ultrasound marking has not been shown to decrease pneumothorax rates compared to thoracentesis without ultrasound guidance, likely due to changes in patient position and prolonged delays between marking and drainage.[47] Postprocedure pneumothorax can be ruled out if lung sliding is visualized. A meta‐analysis demonstrated superior sensitivity and similar specificity of pleural ultrasonography versus chest radiography to detect pneumothorax (sensitivity 91% vs 50% and specificity 98% vs 99%, respectively).[48] Real‐time ultrasound guidance for thoracentesis, or use of ultrasound to track the needle tip, has not been well studied but may be performed by experienced proceduralists to drain small effusions.
FUTURE RESEARCH
Future research should focus on the clinical effectiveness of point‐of‐care pleural ultrasonography when integrated with other diagnostic tools, and application of new ultrasound technologies to evaluate pleural diseases. Routine use of point‐of‐care ultrasound as the primary imaging modality in a medical ICU demonstrated a highly statistically significant reduction in chest x‐rays (3.75 vs 0.82, P < 0.0001) and chest CT scans (0.10 vs 0.04, P = 0.0007).[49] Similar studies have yet to be performed with the use of ultrasound specifically in the management of pleural diseases. Thus, clinical effectiveness studies are needed to assess the impact of routine use of pleural ultrasound on the initiation of appropriate therapies, length of stay, and costs in the management of pleural disease.
Point‐of‐care pleural ultrasound findings need to be evaluated in the context of other clinical findings and diagnostic tests. Certain ultrasound findings have been associated with exudative pleural effusions, but whether exudative and transudative effusions can be differentiated noninvasively using ultrasound findings alone, or in combination with other clinical data, warrants investigation. Similar to severity of illness scores, models that incorporate clinical, laboratory, and ultrasound findings need to be developed to guide treatment decisions, such as type of drainage procedure, as well as prognostication.
Finally, new technologies may advance the utility of point‐of‐care pleural ultrasonography. Even though 3‐dimensional ultrasonography has been available for over 2 decades, comparative studies of conventional 2‐dimensional versus 3‐dimensional ultrasonography to characterize pleural effusions have yet to be performed. Furthermore, computer‐aided detection has been shown to facilitate interpretation of ultrasound images, but this technology has yet to be applied to pleural ultrasonography.
CONCLUSIONS
Point‐of‐care pleural ultrasound is a powerful bedside tool in the hospitalist's armamentarium that is superior to physical examination and chest radiographs in the detection and characterization of pleural effusions. Furthermore, ultrasound performs similarly when compared to CT scans but offers the advantages of decreased cost, avoidance of ionizing radiation, and availability at the bedside. Ultrasound guidance reduces complications and increases the success rate of pleural drainage procedures, leading to improved patient safety. As clinical effectiveness studies emerge revealing its true value, point‐of‐care pleural ultrasonography is likely to become the standard diagnostic tool to evaluate and manage patients with pleural effusions.
Disclosures: Dr. Restrepo is partially supported by award number K23HL096054 from the National Heart, Lung, and Blood Institute. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Heart, Lung, and Blood Institute or the National Institutes of Health. The authors report no conflicts of interest.
Hospitalists commonly encounter pleural effusions, and their detection and characterization by point‐of‐care ultrasound can guide management. Approximately 44% to 57% of hospitalized patients with bacterial pneumonia,[1, 2] and up to 62% of intensive care unit (ICU) patients[3] have a pleural effusion. For patients with a parapneumonic effusion, hospitalists can use ultrasound to quantify and characterize pleural fluid to determine whether diagnostic or therapeutic drainage is indicated, as well as guide performance of thoracentesis. For patients with lung cancer, detection of a malignant pleural effusion changes staging to stage IV, regardless of tumor size or lymph node involvement, and hospitalists may discuss more appropriate treatment options with patients and consultants.
Routine use of pleural ultrasonography may help hospitalists provide high‐value care by reducing ancillary testing, including computerized tomography (CT) scans that expose patients to ionizing radiation, and reducing complications of thoracentesis. However, many hospitalists may not be familiar with the use of point‐of‐care ultrasound. A national survey in 2012 revealed only 25% of internal medicine residencies have formal curricula to teach point‐of‐care ultrasonography.[4] The purpose of this review is to provide an overview of how point‐of‐care ultrasound can be utilized by hospitalists in the care of patients with pleural effusions. We review the literature on the diagnosis and evaluation of pleural effusions with ultrasound, as well as techniques to examine and drain the pleural space.
DIAGNOSIS OF PLEURAL EFFUSION
History and Physical Exam
Pleural effusions are most commonly associated with heart failure, pneumonia, cancer, pulmonary embolism, viral disease, coronary artery bypass surgery, and cirrhosis with ascites. The most common symptoms related to pleural effusion are nonspecific and often indistinguishable from those of the underlying disease process, including cough, dyspnea, and pleuritic chest pain.[5]
Diagnostic accuracy of a physical examination to detect pleural fluid is highly dependent on the size of the effusion and is unlikely to detect effusions <300 mL. A systematic review found the most accurate physical exam findings to rule in a pleural effusion were dullness to percussion (positive likelihood ratio [LR]: 8.7; 95% CI: 2.2‐33.8) and asymmetric chest expansion (positive LR: 8.1; 95% CI: 5.2‐12.7). Normal tactile vocal fremitus was the most accurate physical exam finding to rule out a pleural effusion (negative LR: 0.21; 95% CI: 0.12‐0.37).[6] A major limitation of all these studies is that physical exam was compared to chest radiography as the reference standard, and posterior‐anterior chest radiographs are not sensitive for detection of pleural effusions until 200 mL of fluid has accumulated.[7] Further, chest percussion penetrates to a maximum depth of 6 cm, and its utility is limited in obese patients.[8] Characteristics of pleural fluid that can change management, such as loculations, cannot be detected by physical exam.
Chest Radiography
Chest radiography has traditionally been used to diagnose pleural effusions. Free‐flowing pleural fluid collects in the most dependent portions of the thorax, initially in the subpulmonic space followed by the costophrenic recesses. Pleural fluid is detectable in the costophrenic recesses on lateral upright chest radiograph after 50 mL has accumulated. On standard posterior‐anterior chest radiograph, blunting of the costophrenic recesses and obliteration of the hemidiaphragm are seen when >200 mL and >500 mL of pleural fluid have accumulated, respectively.[7] However, upright chest radiographs can miss a considerable number of effusions, including as many as 10% of parapneumonic effusions large enough to indicate need for drainage.[9] Supine anterior‐posterior chest radiographs can miss a significant proportion of large effusions seen on chest CT,[10] ultrasound,[11] and lateral decubitus radiographs.[12] Pleural effusions are frequently mistaken for parenchymal opacities on portable anterior‐posterior chest radiographs.[10]
Computerized Tomography
Chest CT serves as the reference standard in most modern diagnostic accuracy studies. Limitations of chest CT include difficulty distinguishing small effusions from pleural thickening, dependent atelectasis, or tumor; lower sensitivity for detecting pleural fluid septations compared to ultrasound[13]; exposure of patients to approximately 7 mSv of ionizing radiation (the equivalent radiation dose of 350 chest radiographs)[14]; high cost; and need to transport patients to radiology departments where CT scanners are located.
Pleural Ultrasonography
Ultrasound can rapidly differentiate conditions that demonstrate a nonspecific, radiopaque appearance of lower lung fields on chest radiographs, including pleural effusions, pneumonia, atelectasis, elevated hemidiaphragm, and lung or pleural masses. Advantages of point‐of‐care ultrasound include the ability of hospitalists to acquire and interpret images at the bedside and integrate findings into clinical decision making immediately. Multiple studies have demonstrated superior diagnostic accuracy of ultrasound compared to chest radiography for detection of pleural effusions. Pleural ultrasound can detect physiologic amounts of pleural fluid (5 mL),[15] but a minimal volume of 20 mL is more reliably detected,[16] and ultrasound is 100% sensitive for effusions >100 mL.[17] In a prospective study of critically ill patients with acute respiratory distress syndrome, the diagnostic accuracy of ultrasound for pleural effusions was superior (93%) compared to auscultation (61%) and anterior‐posterior chest radiograph (47%), using chest CT as the reference standard.[18] A meta‐analysis of 4 studies calculated a pooled sensitivity and specificity of ultrasound for detection of pleural effusions as 93% (95% CI: 89%‐96%) and 96% (95% CI: 95%‐98%), respectively.[18, 19, 20, 21, 22] Ultrasound has the additional benefit of characterizing underlying lung parenchyma, which is well described in the literature but beyond the scope of this review.[23]
Sensitivity and specificity of chest radiography and ultrasonography to detect a pleural effusion are displayed in Table 1.[9, 10, 11, 12, 18, 20, 21, 22, 24, 25, 26]
| Exam | Reference Standard | Sensitivity | Specificity | Study | |
|---|---|---|---|---|---|
| |||||
| Chest radiograph | Supine AP | Upright PA/lateral | 92% | Woodring[24] | |
| Lateral decubitus XR | 67% | 70% | Ruskin[12] | ||
| Ultrasound | 82% | 82% | Emamian[11] | ||
| Ultrasound or thoracentesis | 33% | Kocijancic[25] | |||
| CT | 39% | 85% | Lichtenstein[18] | ||
| CT | 66% | 89% | Kitazano[10] | ||
| CT | 65% | 81% | Xirouchaki[26] | ||
| CT | 78% | 76% | Brixey[9] | ||
| Lateral decubitus | Ultrasound or thoracentesis | 94% | 100% | Kocijancic[25] | |
| Upright PA | CT | 82% | 81% | Brixey[9] | |
| Lateral upright | CT | 86% | 88% | Brixey[9] | |
| Ultrasound | Cardiology | CT | 93% | 88% | Kataoka[20] |
| Point of care | CT or tube thoracostomy | 96% | 100% | Ma[21] | |
| CT | 92% | 93% | Lichtenstein[18] | ||
| CT | 94% | 99% | Rocco[22] | ||
| CT | 100% | 100% | Xirouchaki[26] | ||
PLEURAL ULTRASOUND EXAMINATION
A pleural ultrasound exam may be performed as part of a complete lung ultrasound exam, such as the BLUE (Bedside Lung Ultrasound in Emergency) protocol,[27] or a focused exam to evaluate a suspected or known pleural effusion seen on chest radiograph or CT scan.[27] Free‐flowing pleural effusions accumulate in the most dependent portions of the thorax, most commonly, the posterolateral costophrenic recesses in semirecumbent or seated patients, but anteriorly in mechanically ventilated patients in a prone position.
A low‐frequency (25 MHz) phased‐array transducer is generally preferred for imaging in between the ribs. High‐frequency linear transducers do not provide adequate penetration to visualize deep structures, but do provide superior visualization of the pleural line to assess pleural thickness, measure pleural depth, and evaluate for pneumothorax.
Pleural effusions are best evaluated starting at the level of the diaphragm. Place the transducer in a longitudinal plane on the posterior axillary line at the level of the diaphragm with the transducer orientation marker (notch) pointed cephalad (Figure 1). Five structures must be definitively identified to diagnose a pleural effusion: liver/spleen, diaphragm, pleural fluid, lung, and chest wall (Figure 2A). Large pleural effusions compress the adjacent lung causing atelectasis, which gives the lung a tissue‐like echogenicity similar to the liver (Figure 2B). Static air bronchograms are commonly seen in atelectatic lung bases with pleural effusions.[28]


Color flow Doppler and M‐mode ultrasound may be utilized as adjuncts to routine 2‐dimensional ultrasonography. Free‐flowing pleural effusions will demonstrate flow by color Doppler (Figure 3A). Using M‐mode ultrasound, the lung can been seen moving within a pleural effusion to and from the chest wall (sinusoid sign).[29] Absence of flow or movement is seen with dense pleural loculations, pleural thickening, and peripheral lung or pleural masses (Figure 3B).

CHARACTERIZATION OF PLEURAL EFFUSION
Pleural Fluid Volume
Quantification of pleural fluid volume has been proposed using formulas with sonographic measurements.[30, 31, 32] These formulas are most accurate for moderate‐sized effusions but have not been validated beyond individual study cohorts. The largest study (n = 150) found a strong correlation between calculated and actual volumes drained by thoracentesis (r2 = 0.79; P < 0.001) using the formula (Volume [mL] = 16 parietal to visceral pleura distance (mm) at the mid‐diaphragm).[31] Although an accurate quantitative pleural fluid volume assessment may be possible, these formulas are not commonly used in clinical practice. A qualitative assessment is adequate for most clinical decision making using categories of minimal, small, moderate, or large volume.
Simple Versus Complex Effusions
Based on its sonographic appearance, pleural effusions are categorized as simple or complex. Simple pleural effusions are anechoic and usually transudative. Complex pleural effusions are subcategorized as homogeneously or heterogeneously echogenic, with or without septations, and are more often exudative.[33]
Effusions with heterogeneous echogenicity with swirling echoes suggest high cellular content that is often associated with malignancy.[34] Fibrinous stranding, septations, and loculations also suggest an exudative effusion (Figure 4A), and are more readily identified and characterized on lung ultrasound than CT scan.[35]

Homogenously echogenic effusions are most often due to hemothorax or empyema.[36] The high cell count of a hemothorax creates a layering effect in costophrenic recesses (hematocrit sign). Empyemas develop from complex effusions that organize into collections of pus and usually have a homogeneously echogenic, speckled appearance (Figure 4B). Sonographic evidence of septations in the presence of empyema predicts the need for intrapleural fibrinolytic therapy, longer duration of drainage, and possible surgical intervention.[37]
Isolated dense loculations may be challenging to differentiate from peripheral lung or pleural lesions, such as abscess or tumor.
Pleural Thickness
Normal visceral and parietal pleura are apposed and 0.2 to 0.3 mm thick.[38] Pleural effusions with parietal pleural thickness >10 mm, pleural nodularity, and diaphragmatic thickness >7 mm predicted underlying malignancy with high specificity and positive predictive value in 1 study.[39] As many as 20% of anechoic lesions of the pleura are solid rather than fluid. Color flow Doppler ultrasound can differentiate small pleural effusions from solid pleural abnormalities with sensitivity and specificity of 89% and 100%, respectively.[40]
PLEURAL FLUID DRAINAGE
Since its first description in 1967, use of ultrasound guidance for thoracentesis has evolved to become the standard of care in many hospitals in the United States.[41] The British Thoracic Society guidelines recommend that all thoracenteses be performed with ultrasound guidance.[42] The American College of Graduate Medical Education now requires proficiency in the use of ultrasound for thoracentesis and pleural catheter insertion by pulmonary and critical care fellows.[43]
The impetus for these recommendations stems from increased procedural success and decreased complications associated with ultrasound‐guided drainage of pleural effusions. A study evaluating thoracentesis site selection based on physical exam and chest radiographs demonstrated inaccurate site selection in 15% of patients, and use of ultrasound for site selection prevented possible accidental organ puncture in 10% of all cases.[44] The success rate of thoracentesis for small pleural effusions has been shown to increase from 66% to 90% with ultrasound guidance.[42] Using ultrasound, the distance from the skin to parietal and visceral pleura can be measured to determine whether thoracentesis can be safely performed, and to guide selection of an adequate length needle (Figure 5). A minimum pleural effusion depth of 1.5 cm between the visceral and parietal pleura has been recommended to perform diagnostic thoracentesis.[28] Diagnostic thoracentesis of complex septated pleural effusions or empyemas may be performed with a straight needle, but therapeutic drainage often requires temporary insertion of a catheter. Traditionally, large‐bore chest tubes (>24 F) had been advocated to drain viscid pus, but recent evidence suggests that small‐bore catheters (1014 F) with instillation of thrombolytics may be as effective and performed with less discomfort.[45] Video‐assisted thoracoscopy to lyse septations and evacuate infected materials is indicated when chest tube drainage has failed.

The most common complication of pleural drainage is pneumothorax. A meta‐analysis demonstrated a reduction in post‐thoracentesis pneumothorax rates from 9% to 4% with use of ultrasound.[46] Transporting patients to radiology for ultrasound marking has not been shown to decrease pneumothorax rates compared to thoracentesis without ultrasound guidance, likely due to changes in patient position and prolonged delays between marking and drainage.[47] Postprocedure pneumothorax can be ruled out if lung sliding is visualized. A meta‐analysis demonstrated superior sensitivity and similar specificity of pleural ultrasonography versus chest radiography to detect pneumothorax (sensitivity 91% vs 50% and specificity 98% vs 99%, respectively).[48] Real‐time ultrasound guidance for thoracentesis, or use of ultrasound to track the needle tip, has not been well studied but may be performed by experienced proceduralists to drain small effusions.
FUTURE RESEARCH
Future research should focus on the clinical effectiveness of point‐of‐care pleural ultrasonography when integrated with other diagnostic tools, and application of new ultrasound technologies to evaluate pleural diseases. Routine use of point‐of‐care ultrasound as the primary imaging modality in a medical ICU demonstrated a highly statistically significant reduction in chest x‐rays (3.75 vs 0.82, P < 0.0001) and chest CT scans (0.10 vs 0.04, P = 0.0007).[49] Similar studies have yet to be performed with the use of ultrasound specifically in the management of pleural diseases. Thus, clinical effectiveness studies are needed to assess the impact of routine use of pleural ultrasound on the initiation of appropriate therapies, length of stay, and costs in the management of pleural disease.
Point‐of‐care pleural ultrasound findings need to be evaluated in the context of other clinical findings and diagnostic tests. Certain ultrasound findings have been associated with exudative pleural effusions, but whether exudative and transudative effusions can be differentiated noninvasively using ultrasound findings alone, or in combination with other clinical data, warrants investigation. Similar to severity of illness scores, models that incorporate clinical, laboratory, and ultrasound findings need to be developed to guide treatment decisions, such as type of drainage procedure, as well as prognostication.
Finally, new technologies may advance the utility of point‐of‐care pleural ultrasonography. Even though 3‐dimensional ultrasonography has been available for over 2 decades, comparative studies of conventional 2‐dimensional versus 3‐dimensional ultrasonography to characterize pleural effusions have yet to be performed. Furthermore, computer‐aided detection has been shown to facilitate interpretation of ultrasound images, but this technology has yet to be applied to pleural ultrasonography.
CONCLUSIONS
Point‐of‐care pleural ultrasound is a powerful bedside tool in the hospitalist's armamentarium that is superior to physical examination and chest radiographs in the detection and characterization of pleural effusions. Furthermore, ultrasound performs similarly when compared to CT scans but offers the advantages of decreased cost, avoidance of ionizing radiation, and availability at the bedside. Ultrasound guidance reduces complications and increases the success rate of pleural drainage procedures, leading to improved patient safety. As clinical effectiveness studies emerge revealing its true value, point‐of‐care pleural ultrasonography is likely to become the standard diagnostic tool to evaluate and manage patients with pleural effusions.
Disclosures: Dr. Restrepo is partially supported by award number K23HL096054 from the National Heart, Lung, and Blood Institute. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Heart, Lung, and Blood Institute or the National Institutes of Health. The authors report no conflicts of interest.
- , , , . Parapneumonic effusions. Am J Med. 1980;69(4):507–512.
- , , . The incidence and clinical correlates of parapneumonic effusions in pneumococcal pneumonia. Chest. 1978;74(2):170–173.
- , , , , . Pleural effusions in the medical ICU: prevalence, causes, and clinical implications. Chest. 1997;111(4):1018–1023.
- , , , . Point‐of‐care ultrasound in internal medicine: a national survey of educational leadership. J Grad Med Educ. 2013;5(3):498–502.
- . Pleural Diseases. Philadelphia, PA: Lippincott Williams 2007.
- , , . Does this patient have a pleural effusion? JAMA. 2009;301(3):309–317.
- , , , . Pleural fluid volume estimation: a chest radiograph prediction rule. Acad Radiol. 1996;3(2):103–109.
- , . Accuracy of the physical examination in evaluating pleural effusion. Cleve Clin J Med. 2008;75(4):297–303.
- , , , , . The efficacy of chest radiographs in detecting parapneumonic effusions. Respirology. 2011;16(6):1000–1004.
- , , , , . Differentiation of pleural effusions from parenchymal opacities: accuracy of bedside chest radiography. AJR Am J Roentgenol. 2010;194(2):407–412.
- , , , . Accuracy of the diagnosis of pleural effusion on supine chest X‐ray. Eur Radiol. 1997;7(1):57–60.
- , , , . Detection of pleural effusions on supine chest radiographs. AJR Am J Roentgenol. 1987;148(4):681–683.
- , , , , . Multiloculated pleural effusion detected by ultrasound only in a critically‐ill patient. Am J Case Rep. 2013;14:63–66.
- , , , et al. Exposure to low‐dose ionizing radiation from medical imaging procedures. N Engl J Med. 2009;361(9):849–857.
- , , . The diagnosis of pleural effusion by ultrasonic and radiologic techniques. Chest. 1976;70(1):33–37.
- , , , , , . Ultrasound in blunt abdominal and thoracic trauma. J Trauma. 1993;34(4):488–495.
- , , , et al. Application of color Doppler ultrasound in the study of small pleural effusion. Med Ultrason. 2010;12(1):12–16.
- , , , , , . Comparative diagnostic performances of auscultation, chest radiography, and lung ultrasonography in acute respiratory distress syndrome. Anesthesiology. 2004;100(1):9–15.
- , , , , . Diagnostic accuracy of sonography for pleural effusion: systematic review. Sao Paulo Med J. 2010;128(2):90–95.
- , . The role of thoracic ultrasonography for evaluation of patients with decompensated chronic heart failure. J Am Coll Cardiol. 2000;35(6):1638–1646.
- , . Trauma ultrasound examination versus chest radiography in the detection of hemothorax. Ann Emerg Med. 1997;29(3):312–315; discussion 315–316.
- , , , et al. Diagnostic accuracy of bedside ultrasonography in the ICU: feasibility of detecting pulmonary effusion and lung contusion in patients on respiratory support after severe blunt thoracic trauma. Acta Anaesthesiol Scand. 2008;52(6):776–784.
- . Lung ultrasound in the critically ill. Ann Intensive Care. 2014;4(1):1.
- . Recognition of pleural effusion on supine radiographs: how much fluid is required? AJR. Am J Roentgenol. 1984;142(1):59–64.
- , , . Chest sonography versus lateral decubitus radiography in the diagnosis of small pleural effusions. J Clin Ultrasound. 2003;31(2):69–74.
- , , , , . Impact of lung ultrasound on clinical decision making in critically ill patients. Intensive Care Med. 2014;40(1):57–65.
- . Lung ultrasound in acute respiratory failure an introduction to the BLUE‐protocol. Minerva Anestesiol. 2009;75(5):313–317.
- , , . Point‐of‐Care Ultrasound. 1st ed. Philadelphia, PA: Saunders; 2014.
- , , , et al. International evidence‐based recommendations for point‐of‐care lung ultrasound. Intensive Care Med. 2012;38(4):577–591.
- , , , et al. Ultrasound estimation of volume of pleural fluid in mechanically ventilated patients. Intensive Care Med. 2006;32(2):318–321.
- , , . Ultrasound estimation of volume of postoperative pleural effusion in cardiac surgery patients. Interact Cardiovasc Thorac Surg. 2010;10(2):204–207.
- , , , et al. Multiplane ultrasound approach to quantify pleural effusion at the bedside. Intensive Care Med. 2010;36(4):656–664.
- , , , , , . Value of sonography in determining the nature of pleural effusion: analysis of 320 cases. AJR Am J Roentgenol. 1992;159(1):29–33.
- , , , , , . Echogenic swirling pattern as a predictor of malignant pleural effusions in patients with malignancies. Chest. 2004;126(1):129–134.
- , . Imaging the pleura: sonography, CT, and MR imaging. AJR Am J Roentgenol. 1991;156(6):1145–1153.
- , , , et al. Pleural effusions in febrile medical ICU patients: chest ultrasound study. Chest. 2004;126(4):1274–1280.
- , , , , . Sonographic septation: a useful prognostic indicator of acute thoracic empyema. J Ultrasound Med. 2000;19(12):837–843.
- . Sonography of the pleura [in German]. Ultraschall Med. 2010;31(1):8–22, quiz 23–25.
- , , . Thoracic ultrasound in the diagnosis of malignant pleural effusion. Thorax. 2009;64(2):139–143.
- , , , . “Fluid color” sign: a useful indicator for discrimination between pleural thickening and pleural effusion. J Ultrasound Med. 1995;14(10):767–769.
- , , . Reflected ultrasound in the detection and localization of pleural effusion. JAMA. 1967;200(5):399–402.
- , , , . Pleural procedures and thoracic ultrasound: British Thoracic Society Pleural Disease Guideline 2010. Thorax. 2010;65(suppl 2):ii61–ii76.
- Accreditation Council for Graduate Medical Education. http://www.acgme.org/acgmeweb. Accessed January 15, 2015.
- , , . Accuracy of pleural puncture sites: a prospective comparison of clinical examination with ultrasound. Chest. 2003;123(2):436–441.
- , , , , , . A double blind randomized cross over trial comparing rate of decortication and efficacy of intrapleural instillation of alteplase vs placebo in patients with empyemas and complicated parapneumonic effusions. Respir Med. 2012;106(5):716–723.
- , , , . Pneumothorax following thoracentesis: a systematic review and meta‐analysis. Arch Intern Med. 2010;170(4):332–339.
- , , , , , . Factors affecting the development of pneumothorax associated with thoracentesis. AJR Am J Roentgenol. 1991;156(5):917–920.
- , , . Test characteristics of ultrasonography for the detection of pneumothorax: a systematic review and meta‐analysis. Chest. 2012;141(3):703–708.
- , , , et al. The effect of point‐of‐care ultrasonography on imaging studies in the medical ICU: a comparative study. Chest. 2014;146(6):1574–1577.
- , , , . Parapneumonic effusions. Am J Med. 1980;69(4):507–512.
- , , . The incidence and clinical correlates of parapneumonic effusions in pneumococcal pneumonia. Chest. 1978;74(2):170–173.
- , , , , . Pleural effusions in the medical ICU: prevalence, causes, and clinical implications. Chest. 1997;111(4):1018–1023.
- , , , . Point‐of‐care ultrasound in internal medicine: a national survey of educational leadership. J Grad Med Educ. 2013;5(3):498–502.
- . Pleural Diseases. Philadelphia, PA: Lippincott Williams 2007.
- , , . Does this patient have a pleural effusion? JAMA. 2009;301(3):309–317.
- , , , . Pleural fluid volume estimation: a chest radiograph prediction rule. Acad Radiol. 1996;3(2):103–109.
- , . Accuracy of the physical examination in evaluating pleural effusion. Cleve Clin J Med. 2008;75(4):297–303.
- , , , , . The efficacy of chest radiographs in detecting parapneumonic effusions. Respirology. 2011;16(6):1000–1004.
- , , , , . Differentiation of pleural effusions from parenchymal opacities: accuracy of bedside chest radiography. AJR Am J Roentgenol. 2010;194(2):407–412.
- , , , . Accuracy of the diagnosis of pleural effusion on supine chest X‐ray. Eur Radiol. 1997;7(1):57–60.
- , , , . Detection of pleural effusions on supine chest radiographs. AJR Am J Roentgenol. 1987;148(4):681–683.
- , , , , . Multiloculated pleural effusion detected by ultrasound only in a critically‐ill patient. Am J Case Rep. 2013;14:63–66.
- , , , et al. Exposure to low‐dose ionizing radiation from medical imaging procedures. N Engl J Med. 2009;361(9):849–857.
- , , . The diagnosis of pleural effusion by ultrasonic and radiologic techniques. Chest. 1976;70(1):33–37.
- , , , , , . Ultrasound in blunt abdominal and thoracic trauma. J Trauma. 1993;34(4):488–495.
- , , , et al. Application of color Doppler ultrasound in the study of small pleural effusion. Med Ultrason. 2010;12(1):12–16.
- , , , , , . Comparative diagnostic performances of auscultation, chest radiography, and lung ultrasonography in acute respiratory distress syndrome. Anesthesiology. 2004;100(1):9–15.
- , , , , . Diagnostic accuracy of sonography for pleural effusion: systematic review. Sao Paulo Med J. 2010;128(2):90–95.
- , . The role of thoracic ultrasonography for evaluation of patients with decompensated chronic heart failure. J Am Coll Cardiol. 2000;35(6):1638–1646.
- , . Trauma ultrasound examination versus chest radiography in the detection of hemothorax. Ann Emerg Med. 1997;29(3):312–315; discussion 315–316.
- , , , et al. Diagnostic accuracy of bedside ultrasonography in the ICU: feasibility of detecting pulmonary effusion and lung contusion in patients on respiratory support after severe blunt thoracic trauma. Acta Anaesthesiol Scand. 2008;52(6):776–784.
- . Lung ultrasound in the critically ill. Ann Intensive Care. 2014;4(1):1.
- . Recognition of pleural effusion on supine radiographs: how much fluid is required? AJR. Am J Roentgenol. 1984;142(1):59–64.
- , , . Chest sonography versus lateral decubitus radiography in the diagnosis of small pleural effusions. J Clin Ultrasound. 2003;31(2):69–74.
- , , , , . Impact of lung ultrasound on clinical decision making in critically ill patients. Intensive Care Med. 2014;40(1):57–65.
- . Lung ultrasound in acute respiratory failure an introduction to the BLUE‐protocol. Minerva Anestesiol. 2009;75(5):313–317.
- , , . Point‐of‐Care Ultrasound. 1st ed. Philadelphia, PA: Saunders; 2014.
- , , , et al. International evidence‐based recommendations for point‐of‐care lung ultrasound. Intensive Care Med. 2012;38(4):577–591.
- , , , et al. Ultrasound estimation of volume of pleural fluid in mechanically ventilated patients. Intensive Care Med. 2006;32(2):318–321.
- , , . Ultrasound estimation of volume of postoperative pleural effusion in cardiac surgery patients. Interact Cardiovasc Thorac Surg. 2010;10(2):204–207.
- , , , et al. Multiplane ultrasound approach to quantify pleural effusion at the bedside. Intensive Care Med. 2010;36(4):656–664.
- , , , , , . Value of sonography in determining the nature of pleural effusion: analysis of 320 cases. AJR Am J Roentgenol. 1992;159(1):29–33.
- , , , , , . Echogenic swirling pattern as a predictor of malignant pleural effusions in patients with malignancies. Chest. 2004;126(1):129–134.
- , . Imaging the pleura: sonography, CT, and MR imaging. AJR Am J Roentgenol. 1991;156(6):1145–1153.
- , , , et al. Pleural effusions in febrile medical ICU patients: chest ultrasound study. Chest. 2004;126(4):1274–1280.
- , , , , . Sonographic septation: a useful prognostic indicator of acute thoracic empyema. J Ultrasound Med. 2000;19(12):837–843.
- . Sonography of the pleura [in German]. Ultraschall Med. 2010;31(1):8–22, quiz 23–25.
- , , . Thoracic ultrasound in the diagnosis of malignant pleural effusion. Thorax. 2009;64(2):139–143.
- , , , . “Fluid color” sign: a useful indicator for discrimination between pleural thickening and pleural effusion. J Ultrasound Med. 1995;14(10):767–769.
- , , . Reflected ultrasound in the detection and localization of pleural effusion. JAMA. 1967;200(5):399–402.
- , , , . Pleural procedures and thoracic ultrasound: British Thoracic Society Pleural Disease Guideline 2010. Thorax. 2010;65(suppl 2):ii61–ii76.
- Accreditation Council for Graduate Medical Education. http://www.acgme.org/acgmeweb. Accessed January 15, 2015.
- , , . Accuracy of pleural puncture sites: a prospective comparison of clinical examination with ultrasound. Chest. 2003;123(2):436–441.
- , , , , , . A double blind randomized cross over trial comparing rate of decortication and efficacy of intrapleural instillation of alteplase vs placebo in patients with empyemas and complicated parapneumonic effusions. Respir Med. 2012;106(5):716–723.
- , , , . Pneumothorax following thoracentesis: a systematic review and meta‐analysis. Arch Intern Med. 2010;170(4):332–339.
- , , , , , . Factors affecting the development of pneumothorax associated with thoracentesis. AJR Am J Roentgenol. 1991;156(5):917–920.
- , , . Test characteristics of ultrasonography for the detection of pneumothorax: a systematic review and meta‐analysis. Chest. 2012;141(3):703–708.
- , , , et al. The effect of point‐of‐care ultrasonography on imaging studies in the medical ICU: a comparative study. Chest. 2014;146(6):1574–1577.