User login
Time to rethink MCL treatment, trial design
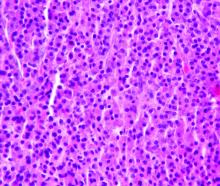
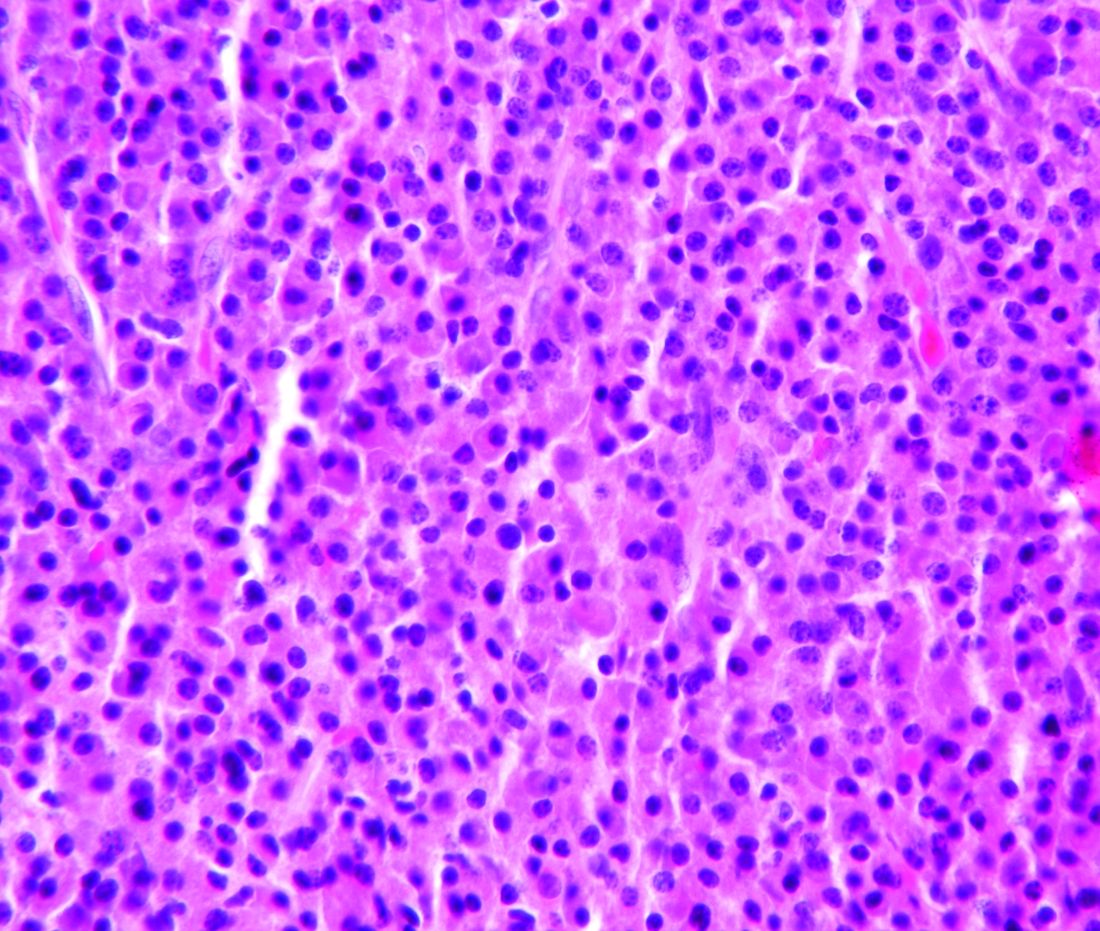
Classic mantle cell lymphoma (cMCL) has long been treated as a uniformly aggressive disease in need of similarly aggressive treatment, but that approach may be leading to overtreatment, according to one expert.
“The cMCL encompasses a broad category of lymphomas with highly variable clinical behaviors. A contemporary categorization of cMCL as a predominantly aggressive entity is misleading, as only an estimated 20% to 25% of patients with cMCL present with a symptomatic or aggressively behaving disease,” Leonid L. Yavorkovsky, MD, PhD, of the Kaiser Permanente San Jose Medical Center (Calif.), wrote in a commentary in JAMA Oncology.
While groups like the National Comprehensive Cancer Network and the European Society for Medical Oncology recommend that only older patients and those with significant comorbidities should be exempted from intensive therapy, Dr. Yavorkovsky said to identify asymptomatic patients who might be able to safely delay aggressive treatment. He also called for more risk-adapted patient assignment to cMCL clinical trials.
“The failure to recognize the erratic nature of cMCL in clinical studies may confound the outcome gains and, ultimately, undermine the ensuing treatment recommendations,” Dr. Yavorkovsky wrote.
Read his full commentary in JAMA Oncology.
mschneider@frontlinemedcom.com
SOURCE: Yavorkovsky L, JAMA Oncology. 2018 Mar 1. doi: 10.1001/jamaoncol.2017.5685.
Classic mantle cell lymphoma (cMCL) has long been treated as a uniformly aggressive disease in need of similarly aggressive treatment, but that approach may be leading to overtreatment, according to one expert.
“The cMCL encompasses a broad category of lymphomas with highly variable clinical behaviors. A contemporary categorization of cMCL as a predominantly aggressive entity is misleading, as only an estimated 20% to 25% of patients with cMCL present with a symptomatic or aggressively behaving disease,” Leonid L. Yavorkovsky, MD, PhD, of the Kaiser Permanente San Jose Medical Center (Calif.), wrote in a commentary in JAMA Oncology.
While groups like the National Comprehensive Cancer Network and the European Society for Medical Oncology recommend that only older patients and those with significant comorbidities should be exempted from intensive therapy, Dr. Yavorkovsky said to identify asymptomatic patients who might be able to safely delay aggressive treatment. He also called for more risk-adapted patient assignment to cMCL clinical trials.
“The failure to recognize the erratic nature of cMCL in clinical studies may confound the outcome gains and, ultimately, undermine the ensuing treatment recommendations,” Dr. Yavorkovsky wrote.
Read his full commentary in JAMA Oncology.
mschneider@frontlinemedcom.com
SOURCE: Yavorkovsky L, JAMA Oncology. 2018 Mar 1. doi: 10.1001/jamaoncol.2017.5685.
Classic mantle cell lymphoma (cMCL) has long been treated as a uniformly aggressive disease in need of similarly aggressive treatment, but that approach may be leading to overtreatment, according to one expert.
“The cMCL encompasses a broad category of lymphomas with highly variable clinical behaviors. A contemporary categorization of cMCL as a predominantly aggressive entity is misleading, as only an estimated 20% to 25% of patients with cMCL present with a symptomatic or aggressively behaving disease,” Leonid L. Yavorkovsky, MD, PhD, of the Kaiser Permanente San Jose Medical Center (Calif.), wrote in a commentary in JAMA Oncology.
While groups like the National Comprehensive Cancer Network and the European Society for Medical Oncology recommend that only older patients and those with significant comorbidities should be exempted from intensive therapy, Dr. Yavorkovsky said to identify asymptomatic patients who might be able to safely delay aggressive treatment. He also called for more risk-adapted patient assignment to cMCL clinical trials.
“The failure to recognize the erratic nature of cMCL in clinical studies may confound the outcome gains and, ultimately, undermine the ensuing treatment recommendations,” Dr. Yavorkovsky wrote.
Read his full commentary in JAMA Oncology.
mschneider@frontlinemedcom.com
SOURCE: Yavorkovsky L, JAMA Oncology. 2018 Mar 1. doi: 10.1001/jamaoncol.2017.5685.
FROM JAMA ONCOLOGY
Consider steroid-induced hypertension when treating pediatric ALL
Nearly 15% of children undergoing induction therapy for acute lymphoblastic leukemia (ALL) developed and were treated for steroid-induced hypertension, according to a study conducted by researchers at the Ohio State University in Columbus.
Ian Bakk and his associates performed a retrospective review of data from the Pediatric Health Information System, a database of the Child Health Corporation of America consisting of inpatient information from 40 free-standing children’s hospitals in the United States. They looked at new cases of ALL from the period of 2009-2013 and analyzed data from 5,578 children who received induction chemotherapy for ALL. In all, .
An adjusted regression analysis showed that infants less than 1 year of age had the highest odds of developing steroid-induced hypertension (adjusted odds ratio, 4.05), followed by children with abnormal glucose (aOR, 2.09), those with secondary diabetes mellitus (aOR, 1.67), and obese patients (aOR, 1.63).
“These findings can help physicians identify patients at high risk for [hypertension] at the time of ALL diagnosis,” the researchers wrote.
mschneider@frontlinemedcom.com
SOURCE: Bakk, I et al. Am J Pediatr Hematol Oncol. 2018;40:27-30.
Nearly 15% of children undergoing induction therapy for acute lymphoblastic leukemia (ALL) developed and were treated for steroid-induced hypertension, according to a study conducted by researchers at the Ohio State University in Columbus.
Ian Bakk and his associates performed a retrospective review of data from the Pediatric Health Information System, a database of the Child Health Corporation of America consisting of inpatient information from 40 free-standing children’s hospitals in the United States. They looked at new cases of ALL from the period of 2009-2013 and analyzed data from 5,578 children who received induction chemotherapy for ALL. In all, .
An adjusted regression analysis showed that infants less than 1 year of age had the highest odds of developing steroid-induced hypertension (adjusted odds ratio, 4.05), followed by children with abnormal glucose (aOR, 2.09), those with secondary diabetes mellitus (aOR, 1.67), and obese patients (aOR, 1.63).
“These findings can help physicians identify patients at high risk for [hypertension] at the time of ALL diagnosis,” the researchers wrote.
mschneider@frontlinemedcom.com
SOURCE: Bakk, I et al. Am J Pediatr Hematol Oncol. 2018;40:27-30.
Nearly 15% of children undergoing induction therapy for acute lymphoblastic leukemia (ALL) developed and were treated for steroid-induced hypertension, according to a study conducted by researchers at the Ohio State University in Columbus.
Ian Bakk and his associates performed a retrospective review of data from the Pediatric Health Information System, a database of the Child Health Corporation of America consisting of inpatient information from 40 free-standing children’s hospitals in the United States. They looked at new cases of ALL from the period of 2009-2013 and analyzed data from 5,578 children who received induction chemotherapy for ALL. In all, .
An adjusted regression analysis showed that infants less than 1 year of age had the highest odds of developing steroid-induced hypertension (adjusted odds ratio, 4.05), followed by children with abnormal glucose (aOR, 2.09), those with secondary diabetes mellitus (aOR, 1.67), and obese patients (aOR, 1.63).
“These findings can help physicians identify patients at high risk for [hypertension] at the time of ALL diagnosis,” the researchers wrote.
mschneider@frontlinemedcom.com
SOURCE: Bakk, I et al. Am J Pediatr Hematol Oncol. 2018;40:27-30.
Hemophilia A treatment gains approval in Europe
The European Commission has approved emicizumab for routine prophylaxis of bleeding episodes in adults and children with hemophilia A with factor VIII inhibitors.
The European Medicines Agency’s Committee for Medicinal Products for Human Use recommended granting marketing authorization to emicizumab (Hemlibra) in January 2018 and it was approved by the Food and Drug Administration in November 2017.
The approvals are based on findings from the HAVEN 1 trial (in 109 adults and adolescents) and the HAVEN 2 trial (in children younger than age 12 years), according to the drug’s maker, Roche. In HAVEN 1 (NCT02622321), emicizumab showed a significant reduction in treatment bleeds (87%), compared with no prophylaxis. And the drug reduced treated bleeds by 79%, compared with previous treatment with bypassing agent prophylaxis. An interim analysis of HAVEN 2 (NCT02795767) of 23 children, showed that 87% of children who received emicizumab prophylaxis had zero treated bleeds, according to Roche.
The European Commission has approved emicizumab for routine prophylaxis of bleeding episodes in adults and children with hemophilia A with factor VIII inhibitors.
The European Medicines Agency’s Committee for Medicinal Products for Human Use recommended granting marketing authorization to emicizumab (Hemlibra) in January 2018 and it was approved by the Food and Drug Administration in November 2017.
The approvals are based on findings from the HAVEN 1 trial (in 109 adults and adolescents) and the HAVEN 2 trial (in children younger than age 12 years), according to the drug’s maker, Roche. In HAVEN 1 (NCT02622321), emicizumab showed a significant reduction in treatment bleeds (87%), compared with no prophylaxis. And the drug reduced treated bleeds by 79%, compared with previous treatment with bypassing agent prophylaxis. An interim analysis of HAVEN 2 (NCT02795767) of 23 children, showed that 87% of children who received emicizumab prophylaxis had zero treated bleeds, according to Roche.
The European Commission has approved emicizumab for routine prophylaxis of bleeding episodes in adults and children with hemophilia A with factor VIII inhibitors.
The European Medicines Agency’s Committee for Medicinal Products for Human Use recommended granting marketing authorization to emicizumab (Hemlibra) in January 2018 and it was approved by the Food and Drug Administration in November 2017.
The approvals are based on findings from the HAVEN 1 trial (in 109 adults and adolescents) and the HAVEN 2 trial (in children younger than age 12 years), according to the drug’s maker, Roche. In HAVEN 1 (NCT02622321), emicizumab showed a significant reduction in treatment bleeds (87%), compared with no prophylaxis. And the drug reduced treated bleeds by 79%, compared with previous treatment with bypassing agent prophylaxis. An interim analysis of HAVEN 2 (NCT02795767) of 23 children, showed that 87% of children who received emicizumab prophylaxis had zero treated bleeds, according to Roche.
FDA approves tests to screen for tickborne parasite in blood supply
The Food and Drug Administration has approved two new tests to screen whole blood and plasma supplies for the tickborne parasite Babesia microti.
The Imugen Babesia microti Arrayed Fluorescent Immunoassay (AFIA) detects antibodies to B. microti in human plasma samples and the Imugen Babesia microti Nucleic Acid Test (NAT) detects B. microti DNA in human whole blood samples. The tests can be used as donor screening tests on individual samples from donors of whole blood and blood components, as well as living organ and tissue donors, according to the FDA. The tests are not intended as a way to diagnose babesiosis infections.
The new screening tests are the first approvals for B. microti detection. The tests have been available under investigational use since 2012. The tests are manufactured by Oxford Immunotec and are currently only available as in-house tests at the company’s Norwood, Mass., facility.
There is no FDA guidance for testing blood and plasma samples for B. microti, but the agency is planning to issue draft guidance later in 2018.
The Food and Drug Administration has approved two new tests to screen whole blood and plasma supplies for the tickborne parasite Babesia microti.
The Imugen Babesia microti Arrayed Fluorescent Immunoassay (AFIA) detects antibodies to B. microti in human plasma samples and the Imugen Babesia microti Nucleic Acid Test (NAT) detects B. microti DNA in human whole blood samples. The tests can be used as donor screening tests on individual samples from donors of whole blood and blood components, as well as living organ and tissue donors, according to the FDA. The tests are not intended as a way to diagnose babesiosis infections.
The new screening tests are the first approvals for B. microti detection. The tests have been available under investigational use since 2012. The tests are manufactured by Oxford Immunotec and are currently only available as in-house tests at the company’s Norwood, Mass., facility.
There is no FDA guidance for testing blood and plasma samples for B. microti, but the agency is planning to issue draft guidance later in 2018.
The Food and Drug Administration has approved two new tests to screen whole blood and plasma supplies for the tickborne parasite Babesia microti.
The Imugen Babesia microti Arrayed Fluorescent Immunoassay (AFIA) detects antibodies to B. microti in human plasma samples and the Imugen Babesia microti Nucleic Acid Test (NAT) detects B. microti DNA in human whole blood samples. The tests can be used as donor screening tests on individual samples from donors of whole blood and blood components, as well as living organ and tissue donors, according to the FDA. The tests are not intended as a way to diagnose babesiosis infections.
The new screening tests are the first approvals for B. microti detection. The tests have been available under investigational use since 2012. The tests are manufactured by Oxford Immunotec and are currently only available as in-house tests at the company’s Norwood, Mass., facility.
There is no FDA guidance for testing blood and plasma samples for B. microti, but the agency is planning to issue draft guidance later in 2018.
Phase 2 study tests low-dose maintenance therapy for ALL
Researchers at MD Anderson Cancer Center in Houston are studying the safety and clinical effectiveness of low-dose inotuzumab ozogamicin in controlling acute lymphocytic leukemia (ALL).
The phase 2 study (NCT03441061), which was launched on Feb. 15, will include up to 40 adult patients with B-cell ALL who are in complete remission with molecular failure or molecular relapse at any point after 3 months of frontline therapy. The study is looking first at relapse-free survival, and secondarily at overall survival and minimal residual disease negativity rate overall and after the first cycle. In addition, the researchers will consider the safety of the drug in this setting.
Inotuzumab ozogamicin (Besponsa) was approved by the Food and Drug Administration in August 2017 for the treatment of adults with relapsed or refractory B-cell precursor ALL.
During the open-label, single-arm study, patients can receive up to six cycles of the drug and each cycle is 28 (plus or minus 7) days. Eligible patients will receive inotuzumab ozogamicin on days 1,8, and 15 of cycle 1 and days 1 and 8 of cycles 2-6.
If a patient chooses to undergo a stem cell transplant from a donor, that patient will receive only two to three cycles of the drug. Study participants may also be taken off treatment after cycle 2 if the disease has had no response.
The study is being conducted at MD Anderson Cancer Center and is expected to be completed in February 2023. The study is sponsored by Pfizer.
Researchers at MD Anderson Cancer Center in Houston are studying the safety and clinical effectiveness of low-dose inotuzumab ozogamicin in controlling acute lymphocytic leukemia (ALL).
The phase 2 study (NCT03441061), which was launched on Feb. 15, will include up to 40 adult patients with B-cell ALL who are in complete remission with molecular failure or molecular relapse at any point after 3 months of frontline therapy. The study is looking first at relapse-free survival, and secondarily at overall survival and minimal residual disease negativity rate overall and after the first cycle. In addition, the researchers will consider the safety of the drug in this setting.
Inotuzumab ozogamicin (Besponsa) was approved by the Food and Drug Administration in August 2017 for the treatment of adults with relapsed or refractory B-cell precursor ALL.
During the open-label, single-arm study, patients can receive up to six cycles of the drug and each cycle is 28 (plus or minus 7) days. Eligible patients will receive inotuzumab ozogamicin on days 1,8, and 15 of cycle 1 and days 1 and 8 of cycles 2-6.
If a patient chooses to undergo a stem cell transplant from a donor, that patient will receive only two to three cycles of the drug. Study participants may also be taken off treatment after cycle 2 if the disease has had no response.
The study is being conducted at MD Anderson Cancer Center and is expected to be completed in February 2023. The study is sponsored by Pfizer.
Researchers at MD Anderson Cancer Center in Houston are studying the safety and clinical effectiveness of low-dose inotuzumab ozogamicin in controlling acute lymphocytic leukemia (ALL).
The phase 2 study (NCT03441061), which was launched on Feb. 15, will include up to 40 adult patients with B-cell ALL who are in complete remission with molecular failure or molecular relapse at any point after 3 months of frontline therapy. The study is looking first at relapse-free survival, and secondarily at overall survival and minimal residual disease negativity rate overall and after the first cycle. In addition, the researchers will consider the safety of the drug in this setting.
Inotuzumab ozogamicin (Besponsa) was approved by the Food and Drug Administration in August 2017 for the treatment of adults with relapsed or refractory B-cell precursor ALL.
During the open-label, single-arm study, patients can receive up to six cycles of the drug and each cycle is 28 (plus or minus 7) days. Eligible patients will receive inotuzumab ozogamicin on days 1,8, and 15 of cycle 1 and days 1 and 8 of cycles 2-6.
If a patient chooses to undergo a stem cell transplant from a donor, that patient will receive only two to three cycles of the drug. Study participants may also be taken off treatment after cycle 2 if the disease has had no response.
The study is being conducted at MD Anderson Cancer Center and is expected to be completed in February 2023. The study is sponsored by Pfizer.
FROM CLINICALTRIALS.GOV
Gene therapy for hemophilia A gets fast-track review
The investigational gene therapy SPK-8011 for the treatment of hemophilia A is getting an accelerated review at the Food and Drug Administration, according to Spark Therapeutics.
The FDA granted breakthrough therapy designation to the gene therapy product in February and orphan drug status in January.
The investigational gene therapy SPK-8011 for the treatment of hemophilia A is getting an accelerated review at the Food and Drug Administration, according to Spark Therapeutics.
The FDA granted breakthrough therapy designation to the gene therapy product in February and orphan drug status in January.
The investigational gene therapy SPK-8011 for the treatment of hemophilia A is getting an accelerated review at the Food and Drug Administration, according to Spark Therapeutics.
The FDA granted breakthrough therapy designation to the gene therapy product in February and orphan drug status in January.
FDA grants priority review for AML drug
The Food and Drug Administration has granted priority review status to ivosidenib for the treatment of patients with relapsed or refractory acute myeloid leukemia with an isocitrate dehydrogenase 1 mutation.
The drug, marketed by Agios Pharmaceuticals, was given a Prescription Drug User Free Act action date of Aug. 21, 2018.
Results from a phase 1 dose-escalation and expansion study (AG120-C-001) presented at the annual meeting of the American Society of Hematology showed a complete response and complete response with partial hematologic recovery rate of 30.4% in 125 patients with relapsed/refractory AML who received the drug, according to Agios.
The Food and Drug Administration has granted priority review status to ivosidenib for the treatment of patients with relapsed or refractory acute myeloid leukemia with an isocitrate dehydrogenase 1 mutation.
The drug, marketed by Agios Pharmaceuticals, was given a Prescription Drug User Free Act action date of Aug. 21, 2018.
Results from a phase 1 dose-escalation and expansion study (AG120-C-001) presented at the annual meeting of the American Society of Hematology showed a complete response and complete response with partial hematologic recovery rate of 30.4% in 125 patients with relapsed/refractory AML who received the drug, according to Agios.
The Food and Drug Administration has granted priority review status to ivosidenib for the treatment of patients with relapsed or refractory acute myeloid leukemia with an isocitrate dehydrogenase 1 mutation.
The drug, marketed by Agios Pharmaceuticals, was given a Prescription Drug User Free Act action date of Aug. 21, 2018.
Results from a phase 1 dose-escalation and expansion study (AG120-C-001) presented at the annual meeting of the American Society of Hematology showed a complete response and complete response with partial hematologic recovery rate of 30.4% in 125 patients with relapsed/refractory AML who received the drug, according to Agios.
Three-drug combo delivers PFS for myeloma in OPTIMISMM trial
The addition of pomalidomide to bortezomib and low-dose dexamethasone showed a statistically significant improvement in progression-free survival for patients with relapsed/refractory multiple myeloma, compared with just the two agents, according to Celgene.
Celgene, which markets pomalidomide, announced the results from the phase 3 OPTIMISMM trial (NCT01734928) on Feb. 6. The company expects the results to be presented at future medical meetings, they said.
OPTIMISMM is the first phase 3 trial to examine a triple-drug combination for multiple myeloma patients who have all received prior lenalidomide, Celgene noted.
The pomalidomide/bortezomib/low-dose dexamethasone combination is not currently approved, but pomalidomide plus dexamethasone is approved for multiple myeloma patients who have received at least two prior therapies, including lenalidomide and a proteasome inhibitor, and have shown disease progression within 60 days of last therapy.
The addition of pomalidomide to bortezomib and low-dose dexamethasone showed a statistically significant improvement in progression-free survival for patients with relapsed/refractory multiple myeloma, compared with just the two agents, according to Celgene.
Celgene, which markets pomalidomide, announced the results from the phase 3 OPTIMISMM trial (NCT01734928) on Feb. 6. The company expects the results to be presented at future medical meetings, they said.
OPTIMISMM is the first phase 3 trial to examine a triple-drug combination for multiple myeloma patients who have all received prior lenalidomide, Celgene noted.
The pomalidomide/bortezomib/low-dose dexamethasone combination is not currently approved, but pomalidomide plus dexamethasone is approved for multiple myeloma patients who have received at least two prior therapies, including lenalidomide and a proteasome inhibitor, and have shown disease progression within 60 days of last therapy.
The addition of pomalidomide to bortezomib and low-dose dexamethasone showed a statistically significant improvement in progression-free survival for patients with relapsed/refractory multiple myeloma, compared with just the two agents, according to Celgene.
Celgene, which markets pomalidomide, announced the results from the phase 3 OPTIMISMM trial (NCT01734928) on Feb. 6. The company expects the results to be presented at future medical meetings, they said.
OPTIMISMM is the first phase 3 trial to examine a triple-drug combination for multiple myeloma patients who have all received prior lenalidomide, Celgene noted.
The pomalidomide/bortezomib/low-dose dexamethasone combination is not currently approved, but pomalidomide plus dexamethasone is approved for multiple myeloma patients who have received at least two prior therapies, including lenalidomide and a proteasome inhibitor, and have shown disease progression within 60 days of last therapy.
Hemophilia A drug heads toward approval in Europe
The European Medicines Agency’s Committee for Medicinal Products for Human Use recommended granting marketing authorization to the drug in January 2018, according to a statement. The recommendation will now be considered by the European Commission.
Emicizumab, which is marketed in the United States as Hemlibra, was approved by the Food and Drug Administration for adult and pediatric patients with hemophilia A with Factor VIII inhibitors in November. It is the first monoclonal antibody to be recommended for use in patients with hemophilia A with inhibitors, says the statement.
The European Medicines Agency’s Committee for Medicinal Products for Human Use recommended granting marketing authorization to the drug in January 2018, according to a statement. The recommendation will now be considered by the European Commission.
Emicizumab, which is marketed in the United States as Hemlibra, was approved by the Food and Drug Administration for adult and pediatric patients with hemophilia A with Factor VIII inhibitors in November. It is the first monoclonal antibody to be recommended for use in patients with hemophilia A with inhibitors, says the statement.
The European Medicines Agency’s Committee for Medicinal Products for Human Use recommended granting marketing authorization to the drug in January 2018, according to a statement. The recommendation will now be considered by the European Commission.
Emicizumab, which is marketed in the United States as Hemlibra, was approved by the Food and Drug Administration for adult and pediatric patients with hemophilia A with Factor VIII inhibitors in November. It is the first monoclonal antibody to be recommended for use in patients with hemophilia A with inhibitors, says the statement.
Real-world study makes case for continuous treatment in multiple myeloma
Longer duration of second-line therapy is strongly linked to improved 1-year overall survival in patients with relapsed and refractory multiple myeloma, according to findings from a real-world analysis.
The retrospective, observational study evaluated outcomes in 628 patients with multiple myeloma who relapsed after initial treatment. Parameswaran Hari, MD, of the Medical College of Wisconsin in Milwaukee and his colleagues sought to test the growing consensus that prolonged therapy is the best approach in a cohort of patients who received care in a routine setting and were older and had more comorbidities than patients in clinical trials.
Median overall survival was 41 months (95% confidence interval, 32.1-59.5) among the 628 relapsed/refractory patients who started second-line therapy. Each extra month of second-line therapy was associated with a reduced risk of death at 1 year (adjusted odds ratio, 0.78; 95% CI, 0.77-0.83; P less than .001). the median duration of second-line therapy for patients in the study was 6.9 months. When researchers extended the duration to 11 months, it was correlated with a 12.7% higher 1-year overall survival probability.
Age was an important factor in survival. The 1-year mortality was significantly lower in patients under 75 years (OR, 0.37; 95% CI, 0.20-0.68).
“Despite substantial heterogeneity in patient and disease characteristics and treatment patterns, the clinical benefit of continued longer-term therapy at relapse appears to be generalizable to patients receiving care in the routine care settings,” the researchers wrote.
Takeda funded the study and four of the authors are Takeda employees. Dr. Hari reported fees from Takeda and several other pharmaceutical companies.
mschneider@frontlinemedcom.com
SOURCE: Hari P et al. Clin Lymphoma Myeloma Leuk. 2018 Jan 5. doi: 10.1016/j.clml.2017.12.012.
Longer duration of second-line therapy is strongly linked to improved 1-year overall survival in patients with relapsed and refractory multiple myeloma, according to findings from a real-world analysis.
The retrospective, observational study evaluated outcomes in 628 patients with multiple myeloma who relapsed after initial treatment. Parameswaran Hari, MD, of the Medical College of Wisconsin in Milwaukee and his colleagues sought to test the growing consensus that prolonged therapy is the best approach in a cohort of patients who received care in a routine setting and were older and had more comorbidities than patients in clinical trials.
Median overall survival was 41 months (95% confidence interval, 32.1-59.5) among the 628 relapsed/refractory patients who started second-line therapy. Each extra month of second-line therapy was associated with a reduced risk of death at 1 year (adjusted odds ratio, 0.78; 95% CI, 0.77-0.83; P less than .001). the median duration of second-line therapy for patients in the study was 6.9 months. When researchers extended the duration to 11 months, it was correlated with a 12.7% higher 1-year overall survival probability.
Age was an important factor in survival. The 1-year mortality was significantly lower in patients under 75 years (OR, 0.37; 95% CI, 0.20-0.68).
“Despite substantial heterogeneity in patient and disease characteristics and treatment patterns, the clinical benefit of continued longer-term therapy at relapse appears to be generalizable to patients receiving care in the routine care settings,” the researchers wrote.
Takeda funded the study and four of the authors are Takeda employees. Dr. Hari reported fees from Takeda and several other pharmaceutical companies.
mschneider@frontlinemedcom.com
SOURCE: Hari P et al. Clin Lymphoma Myeloma Leuk. 2018 Jan 5. doi: 10.1016/j.clml.2017.12.012.
Longer duration of second-line therapy is strongly linked to improved 1-year overall survival in patients with relapsed and refractory multiple myeloma, according to findings from a real-world analysis.
The retrospective, observational study evaluated outcomes in 628 patients with multiple myeloma who relapsed after initial treatment. Parameswaran Hari, MD, of the Medical College of Wisconsin in Milwaukee and his colleagues sought to test the growing consensus that prolonged therapy is the best approach in a cohort of patients who received care in a routine setting and were older and had more comorbidities than patients in clinical trials.
Median overall survival was 41 months (95% confidence interval, 32.1-59.5) among the 628 relapsed/refractory patients who started second-line therapy. Each extra month of second-line therapy was associated with a reduced risk of death at 1 year (adjusted odds ratio, 0.78; 95% CI, 0.77-0.83; P less than .001). the median duration of second-line therapy for patients in the study was 6.9 months. When researchers extended the duration to 11 months, it was correlated with a 12.7% higher 1-year overall survival probability.
Age was an important factor in survival. The 1-year mortality was significantly lower in patients under 75 years (OR, 0.37; 95% CI, 0.20-0.68).
“Despite substantial heterogeneity in patient and disease characteristics and treatment patterns, the clinical benefit of continued longer-term therapy at relapse appears to be generalizable to patients receiving care in the routine care settings,” the researchers wrote.
Takeda funded the study and four of the authors are Takeda employees. Dr. Hari reported fees from Takeda and several other pharmaceutical companies.
mschneider@frontlinemedcom.com
SOURCE: Hari P et al. Clin Lymphoma Myeloma Leuk. 2018 Jan 5. doi: 10.1016/j.clml.2017.12.012.
FROM CLINICAL LYMPHOMA, MYELOMA & LEUKEMIA
Key clinical point:
Major finding: Each additional month of second-line therapy was associated with a reduced risk of death at 1 year (aOR, 0.78).
Study details: A retrospective study of 628 newly diagnosed multiple myeloma patients between January 2008 and June 2015.
Disclosures: Takeda funded the study and four of the authors are Takeda employees. Dr. Hari reported fees from Takeda and several other pharmaceutical companies.
Source: Hari P et al. Clin Lymphoma Myeloma Leuk. 2018 Jan 5. doi: 10.1016/j.clml.2017.12.012.