User login
Fall risk and anticoagulation for atrial fibrillation in the elderly: A delicate balance
An 86-year-old woman with hypertension, osteoporosis, and mild cognitive impairment presents with episodes of palpitations and heart “fluttering.” These episodes occur 1 to 2 times per week, last for up to several hours, and are associated with mild shortness of breath and reduced activity tolerance. She is widowed and lives in a retirement facility, but she is independent in activities of daily living. She has fallen twice in the past year without significant injury.
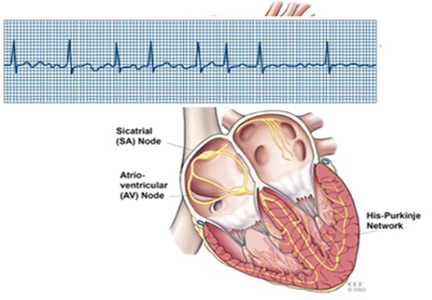
Physical examination is unremarkable. An electrocardiogram demonstrates sinus rhythm with left ventricular hypertrophy. A 30-day event monitor reveals several episodes of paroxysmal atrial fibrillation that correspond with her symptoms. A subsequent echocardiogram shows normal left ventricular systolic function, mild diastolic dysfunction, and no significant valvular abnormalities. Laboratory studies, including thyroid-stimulating hormone, are normal.
What is this patient’s risk of stroke? What is her risk of major bleeding from anticoagulation? How should fall risk be addressed in the decision-making process? What other factors should be considered?
AGE, ATRIAL FIBRILLATION, AND STROKE RISK
The prevalence of atrial fibrillation increases with age, and nearly half of patients with atrial fibrillation in the United States are 75 or older.1 In addition, older age is an independent risk factor for stroke in patients with atrial fibrillation, and the proportion of strokes attributable to atrial fibrillation increases exponentially with age:
- 1.5% at age 50 to 59
- 2.8% at age 60 to 69
- 9.9% at age 70 to 79
- 23.5% at age 80 to 89.2
Numerous large randomized trials have shown that anticoagulation with warfarin reduces the risk of stroke by about two-thirds in patients with atrial fibrillation, and that this benefit extends to the elderly.
In the Birmingham Atrial Fibrillation Treatment of the Aged trial,3 973 patients at least 75 years old (mean age 81.5, 55% male) were randomized to receive either warfarin with a target international normalized ratio of 2.0 to 3.0 or aspirin 75 mg/day. Over an average follow-up of 2.7 years, the composite outcome of fatal or disabling stroke, arterial embolism, or intracranial hemorrhage occurred in 24 (4.9%) of the 488 patients in the warfarin group and 48 (9.9%) of the 485 patients in the aspirin group (absolute yearly risk reduction 2%, 95% confidence interval 0.7–3.2, number needed to treat 50 for 1 year). Importantly, the benefit of warfarin was similar in men and women, and in patients ages 75 to 79, 80 to 84, and 85 and older.
More recently, the oral anticoagulants dabigatran, rivaroxaban, apixaban, and edoxaban have been shown to be at least as effective as warfarin with respect to both stroke prevention and major bleeding complications, and subgroup analyses have confirmed similar outcomes in older and younger patients.4,5
But despite the proven value of anticoagulation for stroke prevention in older adults, only 40% to 60% of older patients who are suitable candidates for anticoagulation actually receive it.6 Moreover, the proportion of patients who are treated declines progressively with age. The most frequently cited reason for nontreatment is perception of a high risk of falls and associated concerns about bleeding, especially intracranial hemorrhage.7–10
BALANCING STROKE RISK VS BLEEDING RISK
Balancing the risk of stroke against the risk of bleeding related to falls is a commonly encountered conundrum in older patients with atrial fibrillation.
Stroke risk
The CHADS2 score was, until recently, the most widely used method for assessing stroke risk in patients with nonvalvular atrial fibrillation. CHADS2 assigns 1 point each for congestive heart failure, hypertension, age ≥ 75, and diabetes, and 2 points for prior stroke or transient ischemic attack (range 0–6 points). Annual stroke risk based on the CHADS2 score ranges from about 2% to about 18%
(Table 1).11
The CHA2DS2-VASc score,12 a modification of CHADS2, appears to assess the risk of stroke more accurately, especially at the lower end of the scale, and recent guidelines for managing atrial fibrillation recommend using the CHA2DS2-VASc algorithm.13 CHA2DS2-VASc is similar to CHADS2, except that it assigns 1 point for ages 65 to 74, 2 points for ages 75 and older, 1 point for vascular disease (coronary artery disease, peripheral arterial disease, aortic aneurysm), and 1 point for female sex (Table 1).11,12
For both CHADS2 and CHA2DS2-VASc, systemic anticoagulation is recommended for patients who have a score of 2 or higher. Our patient’s CHADS2 score is 2, and her CHA2DS2-VASc score is 4, corresponding to an annual estimated stroke risk of 4% with both scores (Table 1). Note, however, that the CHA2DS2-VASc score provides more information at the lower end of the spectrum.
Bleeding risk
Several scoring systems for assessing bleeding risk have also been developed (Table 2).14–16 Of these, the HAS-BLED score has come to be used more widely in recent years.
Perhaps not surprisingly, some of the same factors associated with risk of stroke also predict increased risk of bleeding (eg, older age, hypertension, prior stroke).14 Note, however, that history of falling or high risk of falling is only included in one of the bleeding risk models (HEMORR2HAGES).15
These tools are somewhat limited by their lack of consideration of concomitant antiplatelet therapy (only included in HAS-BLED) or history of bleeding (only ATRIA16 considers major and minor bleeding, HEMORR2HAGES does not specify bleeding severity, and HAS-BLED only considers major bleeding). The models also fail to include medications such as antibiotics or antiarrhythmic agents, which are commonly used by older patients with atrial fibrillation and may increase bleeding risk. In addition, all bleeding risk scores were developed for warfarin, and their applicability to patients treated with the newer oral anticoagulants has not been established.
At the time of presentation, our patient has a HAS-BLED score of 2 (1 point each for age and hypertension), placing her at intermediate risk of bleeding.14
Fear the clot, not the bleed
So how does one balance the risk of stroke vs the risk of bleeding? An adage from the early days of thrombolytic therapy for acute myocardial infarction was “fear the clot, not the bleed.” In other words, in the present context the consequences of a thrombus embolizing from the heart to the brain are likely to be more devastating and more permanent than the consequences of anticoagulation-associated hemorrhage.
Support for this view is underscored by a 2015 study by Lip et al,17 who examined stroke and bleeding risks and outcomes in a large real-world population of patients age 75 and older. The analysis included 819 patients ages 85 to 89 and 386 patients age 90 and older. The key finding was that the oldest patients derived the greatest net benefit from anticoagulation.
Moreover, the Canadian stroke registry of 3,197 patients, mean age 79, showed that advanced age was a more potent risk factor for ischemic stroke than it was for hemorrhagic stroke.18
Thus, the benefit from anticoagulation in patients with atrial fibrillation does not appear to have an upper age limit.
FALLS AND ANTICOAGULATION
Falls are an important source of morbidity, disability, and activity curtailment in older adults and, like atrial fibrillation, the incidence and prevalence of falls increase with age. In community-dwelling adults age 65 and older, the overall proportion with at least 1 fall in the preceding year ranges from about 30% to 40%.19 However, the rate increases with age and exceeds 50% in nursing home residents.20
Although anticoagulation is associated with a higher risk of bleeding in patients who fall, the absolute risk is small.
In a study of older adults with nonvalvular atrial fibrillation, a history of falls or documented high risk of falling was associated with a risk of intracranial hemorrhage during follow-up that was 1.9 times higher.21 Importantly, however, this risk did not differ among patients treated with warfarin, aspirin, or no antithrombotic therapy. In this analysis, patients with a CHADS2 score of 2 or higher benefited from anticoagulation, whether or not they were considered to be at risk for falls.
In another study,22 it was estimated that an individual would have to fall 295 times in 1 year for the risk of fall-related major bleeding to outweigh the benefit of warfarin in reducing the risk of stroke.
Thus, based on available evidence, perception of a high risk of falling should not be construed as justification for withholding anticoagulation in older patients who are otherwise suitable candidates for such therapy.
AT WHAT POINT DOES BLEEDING RISK OUTWEIGH ANTICOAGULATION BENEFIT?
Absolute contraindications to anticoagulation include an intracranial hemorrhage or neurosurgical procedure with high risk for bleeding within the past 30 days, an intracranial neoplasm or vascular abnormality with high risk of bleeding, recurrent life-threatening gastrointestinal or other bleeding events, and severe bleeding disorders, including severe thrombocytopenia.
In patients with atrial fibrillation at high risk of bleeding as assessed by one of the bleeding risk scores and relatively low risk of ischemic stroke, the risk of anticoagulation may outweigh the benefit, although no studies have specifically addressed this issue.
In patients with frequent falls, including injurious falls, the benefits of anticoagulation usually outweigh the risks of bleeding, but management should incorporate interventions designed to mitigate fall risk.
Finally, in patients with a poor prognosis approaching the end of life, the risks and burdens of anticoagulation may exceed the perceived benefits, in which case discontinuation of anticoagulation may be appropriate.
SHOULD OUR PATIENT RECEIVE ANTICOAGULATION?
As noted above, our patient has a high risk of stroke and a moderate risk of bleeding, and multiple lines of evidence indicate that the benefits of anticoagulation (ie, prevention of stroke and systemic embolization) substantially outweigh the risks of bleeding. Although she has a history of falls, which may seem to muddy the waters, this factor should not play a major role in decision-making. Moreover, her advanced age should, if anything, be considered a point in favor of anticoagulation. So from the scientific standpoint, anticoagulation is the clear winner.
A shared decision
But that is not the end of the story. Since there is tension between benefits and risks with either approach (ie, anticoagulation or no anticoagulation), it is important to discuss the issues and options with the patient and relevant caregivers. Most older adults have witnessed the ravages of stroke in a friend or relative, and a recent study showed that most would be willing to accept a modest risk of bleeding to prevent a stroke.23
However, this is ultimately a personal decision for each patient, and in accordance with the principle of patient autonomy, the patient’s expressed wishes should be honored by using a process of shared decision-making.
Which anticoagulant?
Finally, what about the choice of anticoagulation? The complexities of using warfarin, including its narrow therapeutic range and myriad interactions with other medications and foods, can make it a less appealing option for both patient and provider.
We recommend a novel oral anticoagulant as first-line therapy in the absence of contraindications such as severe renal insufficiency, and prefer apixaban because it is the only agent shown to be superior to warfarin with respect to both stroke prevention and bleeding risk.24
Important disadvantages of the novel oral anticoagulants include their higher cost and lack of an effective antidote in the event of clinically significant bleeding (with the exception of idarucizumab, which was recently approved for reversal of serious bleeding associated with dabigatran), issues that may be of particular concern to older adults. While there is no therapeutic range to monitor for the newer agents, more frequent monitoring for occult anemia may be needed.
Thus, selection of an anticoagulant should also be individualized through shared decision-making.
Is aspirin alone an alternative?
And what if the patient chooses to forgo anticoagulation? In that case, aspirin 75 to 325 mg/day may seem reasonable, but there is scant evidence that aspirin is beneficial for stroke prevention in patients with atrial fibrillation in this age group, and aspirin, too, is associated with an increased risk of bleeding.25
As a result, current US and European guidelines recommend a very limited role for aspirin as a single agent in the management of atrial fibrillation.26 The joint 2014 guidelines of the American Heart Association, American College of Cardiology, and Heart Rhythm Society give aspirin a class IIB recommendation (ie, it “may” be considered), level of evidence C (ie, very limited) for use as an alternative to no antithrombotic therapy or systemic anticoagulation only in patients with a CHA2DS2-VASc score of 1, thereby excluding all patients age 75 and older.13
In most cases, aspirin as sole prophylaxis against stroke in atrial fibrillation should be avoided in the absence of another indication for its use, such as coexisting coronary artery disease or peripheral arterial disease.
A COMPLEX DECISION
In summary, the decisions surrounding anticoagulation of elderly patients with nonvalvular atrial fibrillation are complex. Accurate assessment of stroke risk is key, and although bleeding risk is also an essential consideration, it is important not to overemphasize bleeding and fall risks in the decision-making process.
- Go AS, Hylek EM, Phillips KA, et al. Prevalence of diagnosed atrial fibrillation in adults: national implications for rhythm management and stroke prevention: the AnTicoagulation and Risk Factors in Atrial Fibrillation (ATRIA) Study. JAMA 2001; 285:2370–2375.
- Wolf PA, Abbott RD, Kannel WB. Atrial fibrillation as an independent risk factor for stroke: the Framingham Study. Stroke 1991; 22:983–988.
- Mant J, Hobbs FD, Fletcher K, et al; BAFTA investigators; Midland Research Practices Network (MidReC). Warfarin versus aspirin for stroke prevention in an elderly community population with atrial fibrillation (the Birmingham Atrial Fibrillation Treatment of the Aged Study, BAFTA): a randomised controlled trial. Lancet 2007; 370:493–503.
- Chatterjee S, Sardar P, Biondi-Zoccai G, Kumbhani DJ. New oral anticoagulants and the risk of intracranial hemorrhage: traditional and Bayesian meta-analysis and mixed treatment comparison of randomized trials of new oral anticoagulants in atrial fibrillation. JAMA Neurology 2013; 70:1486–1490.
- Sardar P, Chatterjee S, Chaudhari S, Lip GY. New oral anticoagulants in elderly adults: evidence from a meta-analysis of randomized trials. J Am Geriatr Soc 2014; 62:857–864.
- Rich MW. Atrial fibrillation in long term care. J Am Med Dir Assoc 2012; 13:688–691.
- McCrory DC, Matchar DB, Samsa G, Sanders LL, Pritchett EL. Physician attitudes about anticoagulation for nonvalvular atrial fibrillation in the elderly. Arch Intern Med 1995; 155:277–281.
- Pugh D, Pugh J, Mead GE. Attitudes of physicians regarding anticoagulation for atrial fibrillation: a systematic review. Age Ageing 2011; 40:675–683.
- Sellers MB, Newby LK. Atrial fibrillation, anticoagulation, fall risk, and outcomes in elderly patients. Am Heart J 2011; 161:241–246.
- Bahri O, Roca F, Lechani T, et al. Underuse of oral anticoagulation for individuals with atrial fibrillation in a nursing home setting in France: comparisons of resident characteristics and physician attitude. J Am Geriatr Soc 2015; 63:71–76.
- Gage BF, Waterman AD, Shannon W, Boechler M, Rich MW, Radford MJ. Validation of clinical classification schemes for predicting stroke: results from the National Registry of Atrial Fibrillation. JAMA 2001; 285:2864–2870.
- Lip GY, Nieuwlaat R, Pisters R, Lane DA, Crijns HJ. Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor-based approach: the Euro Heart Survey on Atrial Fibrillation. Chest 2010; 137:263–272.
- January CT, Wann LS, Alpert JS, et al; American College of Cardiology/American Heart Association Task Force on Practice Guidelines. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol 2014; 64:e1–e76.
- Pisters R, Lane DA, Nieuwlaat R, de Vos CB, Crijns HJ, Lip GY. A novel user-friendly score (HAS-BLED) to assess 1-year risk of major bleeding in patients with atrial fibrillation: the Euro Heart Survey. Chest 2010; 138:1093–1100.
- Gage BF, Yan Y, Milligan PE, et al. Clinical classification schemes for predicting hemorrhage: results from the National Registry of Atrial Fibrillation (NRAF). Am Heart J 2006; 151:713–719.
- Fang MC, Go AS, Chang Y, et al. A new risk scheme to predict warfarin-associated hemorrhage: The ATRIA (Anticoagulation and Risk Factors in Atrial Fibrillation) Study. J Am Coll Cardiol 2011; 58:395–401.
- Lip GY, Clementy N, Pericart L, Banerjee A, Fauchier L. Stroke and major bleeding risk in elderly patients aged ≥ 75 years with atrial fibrillation: the Loire Valley atrial fibrillation project. Stroke 2015; 46:143–150.
- McGrath ER, Kapral MK, Fang J, et al; Investigators of the Registry of the Canadian Stroke Network. Which risk factors are more associated with ischemic stroke than intracerebral hemorrhage in patients with atrial fibrillation? Stroke 2012; 43:2048–2054.
- Phelan EA, Mahoney JE, Voit JC, Stevens JA. Assessment and management of fall risk in primary care settings. Med Clin North Am 2015; 99:281–293.
- Deandrea S, Bravi F, Turati F, Lucenteforte E, La Vecchia C, Negri E. Risk factors for falls in older people in nursing homes and hospitals. A systematic review and meta-analysis. Arch Gerontol Geriatr 2013; 56:407–415.
- Gage BF, Birman-Deych E, Kerzner R, Radford MJ, Nilasena DS, Rich MW. Incidence of intracranial hemorrhage in patients with atrial fibrillation who are prone to fall. Am J Med 2005; 118:612–617.
- Man-Son-Hing M, Nichol G, Lau A, Laupacis A. Choosing antithrombotic therapy for elderly patients with atrial fibrillation who are at risk for falls. Arch Intern Med 1999; 159:677–685.
- Riva N, Smith DE, Lip GY, Lane DA. Advancing age and bleeding risk are the strongest barriers to anticoagulant prescription in atrial fibrillation. Age Ageing 2011; 40:653–655.
- De Caterina R, Andersson U, Alexander JH, et al; ARISTOTLE Investigators. History of bleeding and outcomes with apixaban versus warfarin in patients with atrial fibrillation in the Apixaban for Reduction in Stroke and Other Thromboembolic Events in Atrial Fibrillation trial. Am Heart J 2016; 175:175–183.
- Ben Freedman S, Gersh BJ, Lip GY. Misperceptions of aspirin efficacy and safety may perpetuate anticoagulant underutilization in atrial fibrillation. Eur Heart J 2015; 36:653–656.
- Camm AJ, Lip GY, De Caterina R, et al; ESC Committee for Practice Guidelines (CPG). 2012 focused update of the ESC Guidelines for the management of atrial fibrillation: an update of the 2010 ESC Guidelines for the management of atrial fibrillation. Developed with the special contribution of the European Heart Rhythm Association. Eur Heart J 2012; 33:2719–2747.
An 86-year-old woman with hypertension, osteoporosis, and mild cognitive impairment presents with episodes of palpitations and heart “fluttering.” These episodes occur 1 to 2 times per week, last for up to several hours, and are associated with mild shortness of breath and reduced activity tolerance. She is widowed and lives in a retirement facility, but she is independent in activities of daily living. She has fallen twice in the past year without significant injury.
Physical examination is unremarkable. An electrocardiogram demonstrates sinus rhythm with left ventricular hypertrophy. A 30-day event monitor reveals several episodes of paroxysmal atrial fibrillation that correspond with her symptoms. A subsequent echocardiogram shows normal left ventricular systolic function, mild diastolic dysfunction, and no significant valvular abnormalities. Laboratory studies, including thyroid-stimulating hormone, are normal.
What is this patient’s risk of stroke? What is her risk of major bleeding from anticoagulation? How should fall risk be addressed in the decision-making process? What other factors should be considered?
AGE, ATRIAL FIBRILLATION, AND STROKE RISK
The prevalence of atrial fibrillation increases with age, and nearly half of patients with atrial fibrillation in the United States are 75 or older.1 In addition, older age is an independent risk factor for stroke in patients with atrial fibrillation, and the proportion of strokes attributable to atrial fibrillation increases exponentially with age:
- 1.5% at age 50 to 59
- 2.8% at age 60 to 69
- 9.9% at age 70 to 79
- 23.5% at age 80 to 89.2
Numerous large randomized trials have shown that anticoagulation with warfarin reduces the risk of stroke by about two-thirds in patients with atrial fibrillation, and that this benefit extends to the elderly.
In the Birmingham Atrial Fibrillation Treatment of the Aged trial,3 973 patients at least 75 years old (mean age 81.5, 55% male) were randomized to receive either warfarin with a target international normalized ratio of 2.0 to 3.0 or aspirin 75 mg/day. Over an average follow-up of 2.7 years, the composite outcome of fatal or disabling stroke, arterial embolism, or intracranial hemorrhage occurred in 24 (4.9%) of the 488 patients in the warfarin group and 48 (9.9%) of the 485 patients in the aspirin group (absolute yearly risk reduction 2%, 95% confidence interval 0.7–3.2, number needed to treat 50 for 1 year). Importantly, the benefit of warfarin was similar in men and women, and in patients ages 75 to 79, 80 to 84, and 85 and older.
More recently, the oral anticoagulants dabigatran, rivaroxaban, apixaban, and edoxaban have been shown to be at least as effective as warfarin with respect to both stroke prevention and major bleeding complications, and subgroup analyses have confirmed similar outcomes in older and younger patients.4,5
But despite the proven value of anticoagulation for stroke prevention in older adults, only 40% to 60% of older patients who are suitable candidates for anticoagulation actually receive it.6 Moreover, the proportion of patients who are treated declines progressively with age. The most frequently cited reason for nontreatment is perception of a high risk of falls and associated concerns about bleeding, especially intracranial hemorrhage.7–10
BALANCING STROKE RISK VS BLEEDING RISK
Balancing the risk of stroke against the risk of bleeding related to falls is a commonly encountered conundrum in older patients with atrial fibrillation.
Stroke risk
The CHADS2 score was, until recently, the most widely used method for assessing stroke risk in patients with nonvalvular atrial fibrillation. CHADS2 assigns 1 point each for congestive heart failure, hypertension, age ≥ 75, and diabetes, and 2 points for prior stroke or transient ischemic attack (range 0–6 points). Annual stroke risk based on the CHADS2 score ranges from about 2% to about 18%
(Table 1).11
The CHA2DS2-VASc score,12 a modification of CHADS2, appears to assess the risk of stroke more accurately, especially at the lower end of the scale, and recent guidelines for managing atrial fibrillation recommend using the CHA2DS2-VASc algorithm.13 CHA2DS2-VASc is similar to CHADS2, except that it assigns 1 point for ages 65 to 74, 2 points for ages 75 and older, 1 point for vascular disease (coronary artery disease, peripheral arterial disease, aortic aneurysm), and 1 point for female sex (Table 1).11,12
For both CHADS2 and CHA2DS2-VASc, systemic anticoagulation is recommended for patients who have a score of 2 or higher. Our patient’s CHADS2 score is 2, and her CHA2DS2-VASc score is 4, corresponding to an annual estimated stroke risk of 4% with both scores (Table 1). Note, however, that the CHA2DS2-VASc score provides more information at the lower end of the spectrum.
Bleeding risk
Several scoring systems for assessing bleeding risk have also been developed (Table 2).14–16 Of these, the HAS-BLED score has come to be used more widely in recent years.
Perhaps not surprisingly, some of the same factors associated with risk of stroke also predict increased risk of bleeding (eg, older age, hypertension, prior stroke).14 Note, however, that history of falling or high risk of falling is only included in one of the bleeding risk models (HEMORR2HAGES).15
These tools are somewhat limited by their lack of consideration of concomitant antiplatelet therapy (only included in HAS-BLED) or history of bleeding (only ATRIA16 considers major and minor bleeding, HEMORR2HAGES does not specify bleeding severity, and HAS-BLED only considers major bleeding). The models also fail to include medications such as antibiotics or antiarrhythmic agents, which are commonly used by older patients with atrial fibrillation and may increase bleeding risk. In addition, all bleeding risk scores were developed for warfarin, and their applicability to patients treated with the newer oral anticoagulants has not been established.
At the time of presentation, our patient has a HAS-BLED score of 2 (1 point each for age and hypertension), placing her at intermediate risk of bleeding.14
Fear the clot, not the bleed
So how does one balance the risk of stroke vs the risk of bleeding? An adage from the early days of thrombolytic therapy for acute myocardial infarction was “fear the clot, not the bleed.” In other words, in the present context the consequences of a thrombus embolizing from the heart to the brain are likely to be more devastating and more permanent than the consequences of anticoagulation-associated hemorrhage.
Support for this view is underscored by a 2015 study by Lip et al,17 who examined stroke and bleeding risks and outcomes in a large real-world population of patients age 75 and older. The analysis included 819 patients ages 85 to 89 and 386 patients age 90 and older. The key finding was that the oldest patients derived the greatest net benefit from anticoagulation.
Moreover, the Canadian stroke registry of 3,197 patients, mean age 79, showed that advanced age was a more potent risk factor for ischemic stroke than it was for hemorrhagic stroke.18
Thus, the benefit from anticoagulation in patients with atrial fibrillation does not appear to have an upper age limit.
FALLS AND ANTICOAGULATION
Falls are an important source of morbidity, disability, and activity curtailment in older adults and, like atrial fibrillation, the incidence and prevalence of falls increase with age. In community-dwelling adults age 65 and older, the overall proportion with at least 1 fall in the preceding year ranges from about 30% to 40%.19 However, the rate increases with age and exceeds 50% in nursing home residents.20
Although anticoagulation is associated with a higher risk of bleeding in patients who fall, the absolute risk is small.
In a study of older adults with nonvalvular atrial fibrillation, a history of falls or documented high risk of falling was associated with a risk of intracranial hemorrhage during follow-up that was 1.9 times higher.21 Importantly, however, this risk did not differ among patients treated with warfarin, aspirin, or no antithrombotic therapy. In this analysis, patients with a CHADS2 score of 2 or higher benefited from anticoagulation, whether or not they were considered to be at risk for falls.
In another study,22 it was estimated that an individual would have to fall 295 times in 1 year for the risk of fall-related major bleeding to outweigh the benefit of warfarin in reducing the risk of stroke.
Thus, based on available evidence, perception of a high risk of falling should not be construed as justification for withholding anticoagulation in older patients who are otherwise suitable candidates for such therapy.
AT WHAT POINT DOES BLEEDING RISK OUTWEIGH ANTICOAGULATION BENEFIT?
Absolute contraindications to anticoagulation include an intracranial hemorrhage or neurosurgical procedure with high risk for bleeding within the past 30 days, an intracranial neoplasm or vascular abnormality with high risk of bleeding, recurrent life-threatening gastrointestinal or other bleeding events, and severe bleeding disorders, including severe thrombocytopenia.
In patients with atrial fibrillation at high risk of bleeding as assessed by one of the bleeding risk scores and relatively low risk of ischemic stroke, the risk of anticoagulation may outweigh the benefit, although no studies have specifically addressed this issue.
In patients with frequent falls, including injurious falls, the benefits of anticoagulation usually outweigh the risks of bleeding, but management should incorporate interventions designed to mitigate fall risk.
Finally, in patients with a poor prognosis approaching the end of life, the risks and burdens of anticoagulation may exceed the perceived benefits, in which case discontinuation of anticoagulation may be appropriate.
SHOULD OUR PATIENT RECEIVE ANTICOAGULATION?
As noted above, our patient has a high risk of stroke and a moderate risk of bleeding, and multiple lines of evidence indicate that the benefits of anticoagulation (ie, prevention of stroke and systemic embolization) substantially outweigh the risks of bleeding. Although she has a history of falls, which may seem to muddy the waters, this factor should not play a major role in decision-making. Moreover, her advanced age should, if anything, be considered a point in favor of anticoagulation. So from the scientific standpoint, anticoagulation is the clear winner.
A shared decision
But that is not the end of the story. Since there is tension between benefits and risks with either approach (ie, anticoagulation or no anticoagulation), it is important to discuss the issues and options with the patient and relevant caregivers. Most older adults have witnessed the ravages of stroke in a friend or relative, and a recent study showed that most would be willing to accept a modest risk of bleeding to prevent a stroke.23
However, this is ultimately a personal decision for each patient, and in accordance with the principle of patient autonomy, the patient’s expressed wishes should be honored by using a process of shared decision-making.
Which anticoagulant?
Finally, what about the choice of anticoagulation? The complexities of using warfarin, including its narrow therapeutic range and myriad interactions with other medications and foods, can make it a less appealing option for both patient and provider.
We recommend a novel oral anticoagulant as first-line therapy in the absence of contraindications such as severe renal insufficiency, and prefer apixaban because it is the only agent shown to be superior to warfarin with respect to both stroke prevention and bleeding risk.24
Important disadvantages of the novel oral anticoagulants include their higher cost and lack of an effective antidote in the event of clinically significant bleeding (with the exception of idarucizumab, which was recently approved for reversal of serious bleeding associated with dabigatran), issues that may be of particular concern to older adults. While there is no therapeutic range to monitor for the newer agents, more frequent monitoring for occult anemia may be needed.
Thus, selection of an anticoagulant should also be individualized through shared decision-making.
Is aspirin alone an alternative?
And what if the patient chooses to forgo anticoagulation? In that case, aspirin 75 to 325 mg/day may seem reasonable, but there is scant evidence that aspirin is beneficial for stroke prevention in patients with atrial fibrillation in this age group, and aspirin, too, is associated with an increased risk of bleeding.25
As a result, current US and European guidelines recommend a very limited role for aspirin as a single agent in the management of atrial fibrillation.26 The joint 2014 guidelines of the American Heart Association, American College of Cardiology, and Heart Rhythm Society give aspirin a class IIB recommendation (ie, it “may” be considered), level of evidence C (ie, very limited) for use as an alternative to no antithrombotic therapy or systemic anticoagulation only in patients with a CHA2DS2-VASc score of 1, thereby excluding all patients age 75 and older.13
In most cases, aspirin as sole prophylaxis against stroke in atrial fibrillation should be avoided in the absence of another indication for its use, such as coexisting coronary artery disease or peripheral arterial disease.
A COMPLEX DECISION
In summary, the decisions surrounding anticoagulation of elderly patients with nonvalvular atrial fibrillation are complex. Accurate assessment of stroke risk is key, and although bleeding risk is also an essential consideration, it is important not to overemphasize bleeding and fall risks in the decision-making process.
An 86-year-old woman with hypertension, osteoporosis, and mild cognitive impairment presents with episodes of palpitations and heart “fluttering.” These episodes occur 1 to 2 times per week, last for up to several hours, and are associated with mild shortness of breath and reduced activity tolerance. She is widowed and lives in a retirement facility, but she is independent in activities of daily living. She has fallen twice in the past year without significant injury.
Physical examination is unremarkable. An electrocardiogram demonstrates sinus rhythm with left ventricular hypertrophy. A 30-day event monitor reveals several episodes of paroxysmal atrial fibrillation that correspond with her symptoms. A subsequent echocardiogram shows normal left ventricular systolic function, mild diastolic dysfunction, and no significant valvular abnormalities. Laboratory studies, including thyroid-stimulating hormone, are normal.
What is this patient’s risk of stroke? What is her risk of major bleeding from anticoagulation? How should fall risk be addressed in the decision-making process? What other factors should be considered?
AGE, ATRIAL FIBRILLATION, AND STROKE RISK
The prevalence of atrial fibrillation increases with age, and nearly half of patients with atrial fibrillation in the United States are 75 or older.1 In addition, older age is an independent risk factor for stroke in patients with atrial fibrillation, and the proportion of strokes attributable to atrial fibrillation increases exponentially with age:
- 1.5% at age 50 to 59
- 2.8% at age 60 to 69
- 9.9% at age 70 to 79
- 23.5% at age 80 to 89.2
Numerous large randomized trials have shown that anticoagulation with warfarin reduces the risk of stroke by about two-thirds in patients with atrial fibrillation, and that this benefit extends to the elderly.
In the Birmingham Atrial Fibrillation Treatment of the Aged trial,3 973 patients at least 75 years old (mean age 81.5, 55% male) were randomized to receive either warfarin with a target international normalized ratio of 2.0 to 3.0 or aspirin 75 mg/day. Over an average follow-up of 2.7 years, the composite outcome of fatal or disabling stroke, arterial embolism, or intracranial hemorrhage occurred in 24 (4.9%) of the 488 patients in the warfarin group and 48 (9.9%) of the 485 patients in the aspirin group (absolute yearly risk reduction 2%, 95% confidence interval 0.7–3.2, number needed to treat 50 for 1 year). Importantly, the benefit of warfarin was similar in men and women, and in patients ages 75 to 79, 80 to 84, and 85 and older.
More recently, the oral anticoagulants dabigatran, rivaroxaban, apixaban, and edoxaban have been shown to be at least as effective as warfarin with respect to both stroke prevention and major bleeding complications, and subgroup analyses have confirmed similar outcomes in older and younger patients.4,5
But despite the proven value of anticoagulation for stroke prevention in older adults, only 40% to 60% of older patients who are suitable candidates for anticoagulation actually receive it.6 Moreover, the proportion of patients who are treated declines progressively with age. The most frequently cited reason for nontreatment is perception of a high risk of falls and associated concerns about bleeding, especially intracranial hemorrhage.7–10
BALANCING STROKE RISK VS BLEEDING RISK
Balancing the risk of stroke against the risk of bleeding related to falls is a commonly encountered conundrum in older patients with atrial fibrillation.
Stroke risk
The CHADS2 score was, until recently, the most widely used method for assessing stroke risk in patients with nonvalvular atrial fibrillation. CHADS2 assigns 1 point each for congestive heart failure, hypertension, age ≥ 75, and diabetes, and 2 points for prior stroke or transient ischemic attack (range 0–6 points). Annual stroke risk based on the CHADS2 score ranges from about 2% to about 18%
(Table 1).11
The CHA2DS2-VASc score,12 a modification of CHADS2, appears to assess the risk of stroke more accurately, especially at the lower end of the scale, and recent guidelines for managing atrial fibrillation recommend using the CHA2DS2-VASc algorithm.13 CHA2DS2-VASc is similar to CHADS2, except that it assigns 1 point for ages 65 to 74, 2 points for ages 75 and older, 1 point for vascular disease (coronary artery disease, peripheral arterial disease, aortic aneurysm), and 1 point for female sex (Table 1).11,12
For both CHADS2 and CHA2DS2-VASc, systemic anticoagulation is recommended for patients who have a score of 2 or higher. Our patient’s CHADS2 score is 2, and her CHA2DS2-VASc score is 4, corresponding to an annual estimated stroke risk of 4% with both scores (Table 1). Note, however, that the CHA2DS2-VASc score provides more information at the lower end of the spectrum.
Bleeding risk
Several scoring systems for assessing bleeding risk have also been developed (Table 2).14–16 Of these, the HAS-BLED score has come to be used more widely in recent years.
Perhaps not surprisingly, some of the same factors associated with risk of stroke also predict increased risk of bleeding (eg, older age, hypertension, prior stroke).14 Note, however, that history of falling or high risk of falling is only included in one of the bleeding risk models (HEMORR2HAGES).15
These tools are somewhat limited by their lack of consideration of concomitant antiplatelet therapy (only included in HAS-BLED) or history of bleeding (only ATRIA16 considers major and minor bleeding, HEMORR2HAGES does not specify bleeding severity, and HAS-BLED only considers major bleeding). The models also fail to include medications such as antibiotics or antiarrhythmic agents, which are commonly used by older patients with atrial fibrillation and may increase bleeding risk. In addition, all bleeding risk scores were developed for warfarin, and their applicability to patients treated with the newer oral anticoagulants has not been established.
At the time of presentation, our patient has a HAS-BLED score of 2 (1 point each for age and hypertension), placing her at intermediate risk of bleeding.14
Fear the clot, not the bleed
So how does one balance the risk of stroke vs the risk of bleeding? An adage from the early days of thrombolytic therapy for acute myocardial infarction was “fear the clot, not the bleed.” In other words, in the present context the consequences of a thrombus embolizing from the heart to the brain are likely to be more devastating and more permanent than the consequences of anticoagulation-associated hemorrhage.
Support for this view is underscored by a 2015 study by Lip et al,17 who examined stroke and bleeding risks and outcomes in a large real-world population of patients age 75 and older. The analysis included 819 patients ages 85 to 89 and 386 patients age 90 and older. The key finding was that the oldest patients derived the greatest net benefit from anticoagulation.
Moreover, the Canadian stroke registry of 3,197 patients, mean age 79, showed that advanced age was a more potent risk factor for ischemic stroke than it was for hemorrhagic stroke.18
Thus, the benefit from anticoagulation in patients with atrial fibrillation does not appear to have an upper age limit.
FALLS AND ANTICOAGULATION
Falls are an important source of morbidity, disability, and activity curtailment in older adults and, like atrial fibrillation, the incidence and prevalence of falls increase with age. In community-dwelling adults age 65 and older, the overall proportion with at least 1 fall in the preceding year ranges from about 30% to 40%.19 However, the rate increases with age and exceeds 50% in nursing home residents.20
Although anticoagulation is associated with a higher risk of bleeding in patients who fall, the absolute risk is small.
In a study of older adults with nonvalvular atrial fibrillation, a history of falls or documented high risk of falling was associated with a risk of intracranial hemorrhage during follow-up that was 1.9 times higher.21 Importantly, however, this risk did not differ among patients treated with warfarin, aspirin, or no antithrombotic therapy. In this analysis, patients with a CHADS2 score of 2 or higher benefited from anticoagulation, whether or not they were considered to be at risk for falls.
In another study,22 it was estimated that an individual would have to fall 295 times in 1 year for the risk of fall-related major bleeding to outweigh the benefit of warfarin in reducing the risk of stroke.
Thus, based on available evidence, perception of a high risk of falling should not be construed as justification for withholding anticoagulation in older patients who are otherwise suitable candidates for such therapy.
AT WHAT POINT DOES BLEEDING RISK OUTWEIGH ANTICOAGULATION BENEFIT?
Absolute contraindications to anticoagulation include an intracranial hemorrhage or neurosurgical procedure with high risk for bleeding within the past 30 days, an intracranial neoplasm or vascular abnormality with high risk of bleeding, recurrent life-threatening gastrointestinal or other bleeding events, and severe bleeding disorders, including severe thrombocytopenia.
In patients with atrial fibrillation at high risk of bleeding as assessed by one of the bleeding risk scores and relatively low risk of ischemic stroke, the risk of anticoagulation may outweigh the benefit, although no studies have specifically addressed this issue.
In patients with frequent falls, including injurious falls, the benefits of anticoagulation usually outweigh the risks of bleeding, but management should incorporate interventions designed to mitigate fall risk.
Finally, in patients with a poor prognosis approaching the end of life, the risks and burdens of anticoagulation may exceed the perceived benefits, in which case discontinuation of anticoagulation may be appropriate.
SHOULD OUR PATIENT RECEIVE ANTICOAGULATION?
As noted above, our patient has a high risk of stroke and a moderate risk of bleeding, and multiple lines of evidence indicate that the benefits of anticoagulation (ie, prevention of stroke and systemic embolization) substantially outweigh the risks of bleeding. Although she has a history of falls, which may seem to muddy the waters, this factor should not play a major role in decision-making. Moreover, her advanced age should, if anything, be considered a point in favor of anticoagulation. So from the scientific standpoint, anticoagulation is the clear winner.
A shared decision
But that is not the end of the story. Since there is tension between benefits and risks with either approach (ie, anticoagulation or no anticoagulation), it is important to discuss the issues and options with the patient and relevant caregivers. Most older adults have witnessed the ravages of stroke in a friend or relative, and a recent study showed that most would be willing to accept a modest risk of bleeding to prevent a stroke.23
However, this is ultimately a personal decision for each patient, and in accordance with the principle of patient autonomy, the patient’s expressed wishes should be honored by using a process of shared decision-making.
Which anticoagulant?
Finally, what about the choice of anticoagulation? The complexities of using warfarin, including its narrow therapeutic range and myriad interactions with other medications and foods, can make it a less appealing option for both patient and provider.
We recommend a novel oral anticoagulant as first-line therapy in the absence of contraindications such as severe renal insufficiency, and prefer apixaban because it is the only agent shown to be superior to warfarin with respect to both stroke prevention and bleeding risk.24
Important disadvantages of the novel oral anticoagulants include their higher cost and lack of an effective antidote in the event of clinically significant bleeding (with the exception of idarucizumab, which was recently approved for reversal of serious bleeding associated with dabigatran), issues that may be of particular concern to older adults. While there is no therapeutic range to monitor for the newer agents, more frequent monitoring for occult anemia may be needed.
Thus, selection of an anticoagulant should also be individualized through shared decision-making.
Is aspirin alone an alternative?
And what if the patient chooses to forgo anticoagulation? In that case, aspirin 75 to 325 mg/day may seem reasonable, but there is scant evidence that aspirin is beneficial for stroke prevention in patients with atrial fibrillation in this age group, and aspirin, too, is associated with an increased risk of bleeding.25
As a result, current US and European guidelines recommend a very limited role for aspirin as a single agent in the management of atrial fibrillation.26 The joint 2014 guidelines of the American Heart Association, American College of Cardiology, and Heart Rhythm Society give aspirin a class IIB recommendation (ie, it “may” be considered), level of evidence C (ie, very limited) for use as an alternative to no antithrombotic therapy or systemic anticoagulation only in patients with a CHA2DS2-VASc score of 1, thereby excluding all patients age 75 and older.13
In most cases, aspirin as sole prophylaxis against stroke in atrial fibrillation should be avoided in the absence of another indication for its use, such as coexisting coronary artery disease or peripheral arterial disease.
A COMPLEX DECISION
In summary, the decisions surrounding anticoagulation of elderly patients with nonvalvular atrial fibrillation are complex. Accurate assessment of stroke risk is key, and although bleeding risk is also an essential consideration, it is important not to overemphasize bleeding and fall risks in the decision-making process.
- Go AS, Hylek EM, Phillips KA, et al. Prevalence of diagnosed atrial fibrillation in adults: national implications for rhythm management and stroke prevention: the AnTicoagulation and Risk Factors in Atrial Fibrillation (ATRIA) Study. JAMA 2001; 285:2370–2375.
- Wolf PA, Abbott RD, Kannel WB. Atrial fibrillation as an independent risk factor for stroke: the Framingham Study. Stroke 1991; 22:983–988.
- Mant J, Hobbs FD, Fletcher K, et al; BAFTA investigators; Midland Research Practices Network (MidReC). Warfarin versus aspirin for stroke prevention in an elderly community population with atrial fibrillation (the Birmingham Atrial Fibrillation Treatment of the Aged Study, BAFTA): a randomised controlled trial. Lancet 2007; 370:493–503.
- Chatterjee S, Sardar P, Biondi-Zoccai G, Kumbhani DJ. New oral anticoagulants and the risk of intracranial hemorrhage: traditional and Bayesian meta-analysis and mixed treatment comparison of randomized trials of new oral anticoagulants in atrial fibrillation. JAMA Neurology 2013; 70:1486–1490.
- Sardar P, Chatterjee S, Chaudhari S, Lip GY. New oral anticoagulants in elderly adults: evidence from a meta-analysis of randomized trials. J Am Geriatr Soc 2014; 62:857–864.
- Rich MW. Atrial fibrillation in long term care. J Am Med Dir Assoc 2012; 13:688–691.
- McCrory DC, Matchar DB, Samsa G, Sanders LL, Pritchett EL. Physician attitudes about anticoagulation for nonvalvular atrial fibrillation in the elderly. Arch Intern Med 1995; 155:277–281.
- Pugh D, Pugh J, Mead GE. Attitudes of physicians regarding anticoagulation for atrial fibrillation: a systematic review. Age Ageing 2011; 40:675–683.
- Sellers MB, Newby LK. Atrial fibrillation, anticoagulation, fall risk, and outcomes in elderly patients. Am Heart J 2011; 161:241–246.
- Bahri O, Roca F, Lechani T, et al. Underuse of oral anticoagulation for individuals with atrial fibrillation in a nursing home setting in France: comparisons of resident characteristics and physician attitude. J Am Geriatr Soc 2015; 63:71–76.
- Gage BF, Waterman AD, Shannon W, Boechler M, Rich MW, Radford MJ. Validation of clinical classification schemes for predicting stroke: results from the National Registry of Atrial Fibrillation. JAMA 2001; 285:2864–2870.
- Lip GY, Nieuwlaat R, Pisters R, Lane DA, Crijns HJ. Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor-based approach: the Euro Heart Survey on Atrial Fibrillation. Chest 2010; 137:263–272.
- January CT, Wann LS, Alpert JS, et al; American College of Cardiology/American Heart Association Task Force on Practice Guidelines. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol 2014; 64:e1–e76.
- Pisters R, Lane DA, Nieuwlaat R, de Vos CB, Crijns HJ, Lip GY. A novel user-friendly score (HAS-BLED) to assess 1-year risk of major bleeding in patients with atrial fibrillation: the Euro Heart Survey. Chest 2010; 138:1093–1100.
- Gage BF, Yan Y, Milligan PE, et al. Clinical classification schemes for predicting hemorrhage: results from the National Registry of Atrial Fibrillation (NRAF). Am Heart J 2006; 151:713–719.
- Fang MC, Go AS, Chang Y, et al. A new risk scheme to predict warfarin-associated hemorrhage: The ATRIA (Anticoagulation and Risk Factors in Atrial Fibrillation) Study. J Am Coll Cardiol 2011; 58:395–401.
- Lip GY, Clementy N, Pericart L, Banerjee A, Fauchier L. Stroke and major bleeding risk in elderly patients aged ≥ 75 years with atrial fibrillation: the Loire Valley atrial fibrillation project. Stroke 2015; 46:143–150.
- McGrath ER, Kapral MK, Fang J, et al; Investigators of the Registry of the Canadian Stroke Network. Which risk factors are more associated with ischemic stroke than intracerebral hemorrhage in patients with atrial fibrillation? Stroke 2012; 43:2048–2054.
- Phelan EA, Mahoney JE, Voit JC, Stevens JA. Assessment and management of fall risk in primary care settings. Med Clin North Am 2015; 99:281–293.
- Deandrea S, Bravi F, Turati F, Lucenteforte E, La Vecchia C, Negri E. Risk factors for falls in older people in nursing homes and hospitals. A systematic review and meta-analysis. Arch Gerontol Geriatr 2013; 56:407–415.
- Gage BF, Birman-Deych E, Kerzner R, Radford MJ, Nilasena DS, Rich MW. Incidence of intracranial hemorrhage in patients with atrial fibrillation who are prone to fall. Am J Med 2005; 118:612–617.
- Man-Son-Hing M, Nichol G, Lau A, Laupacis A. Choosing antithrombotic therapy for elderly patients with atrial fibrillation who are at risk for falls. Arch Intern Med 1999; 159:677–685.
- Riva N, Smith DE, Lip GY, Lane DA. Advancing age and bleeding risk are the strongest barriers to anticoagulant prescription in atrial fibrillation. Age Ageing 2011; 40:653–655.
- De Caterina R, Andersson U, Alexander JH, et al; ARISTOTLE Investigators. History of bleeding and outcomes with apixaban versus warfarin in patients with atrial fibrillation in the Apixaban for Reduction in Stroke and Other Thromboembolic Events in Atrial Fibrillation trial. Am Heart J 2016; 175:175–183.
- Ben Freedman S, Gersh BJ, Lip GY. Misperceptions of aspirin efficacy and safety may perpetuate anticoagulant underutilization in atrial fibrillation. Eur Heart J 2015; 36:653–656.
- Camm AJ, Lip GY, De Caterina R, et al; ESC Committee for Practice Guidelines (CPG). 2012 focused update of the ESC Guidelines for the management of atrial fibrillation: an update of the 2010 ESC Guidelines for the management of atrial fibrillation. Developed with the special contribution of the European Heart Rhythm Association. Eur Heart J 2012; 33:2719–2747.
- Go AS, Hylek EM, Phillips KA, et al. Prevalence of diagnosed atrial fibrillation in adults: national implications for rhythm management and stroke prevention: the AnTicoagulation and Risk Factors in Atrial Fibrillation (ATRIA) Study. JAMA 2001; 285:2370–2375.
- Wolf PA, Abbott RD, Kannel WB. Atrial fibrillation as an independent risk factor for stroke: the Framingham Study. Stroke 1991; 22:983–988.
- Mant J, Hobbs FD, Fletcher K, et al; BAFTA investigators; Midland Research Practices Network (MidReC). Warfarin versus aspirin for stroke prevention in an elderly community population with atrial fibrillation (the Birmingham Atrial Fibrillation Treatment of the Aged Study, BAFTA): a randomised controlled trial. Lancet 2007; 370:493–503.
- Chatterjee S, Sardar P, Biondi-Zoccai G, Kumbhani DJ. New oral anticoagulants and the risk of intracranial hemorrhage: traditional and Bayesian meta-analysis and mixed treatment comparison of randomized trials of new oral anticoagulants in atrial fibrillation. JAMA Neurology 2013; 70:1486–1490.
- Sardar P, Chatterjee S, Chaudhari S, Lip GY. New oral anticoagulants in elderly adults: evidence from a meta-analysis of randomized trials. J Am Geriatr Soc 2014; 62:857–864.
- Rich MW. Atrial fibrillation in long term care. J Am Med Dir Assoc 2012; 13:688–691.
- McCrory DC, Matchar DB, Samsa G, Sanders LL, Pritchett EL. Physician attitudes about anticoagulation for nonvalvular atrial fibrillation in the elderly. Arch Intern Med 1995; 155:277–281.
- Pugh D, Pugh J, Mead GE. Attitudes of physicians regarding anticoagulation for atrial fibrillation: a systematic review. Age Ageing 2011; 40:675–683.
- Sellers MB, Newby LK. Atrial fibrillation, anticoagulation, fall risk, and outcomes in elderly patients. Am Heart J 2011; 161:241–246.
- Bahri O, Roca F, Lechani T, et al. Underuse of oral anticoagulation for individuals with atrial fibrillation in a nursing home setting in France: comparisons of resident characteristics and physician attitude. J Am Geriatr Soc 2015; 63:71–76.
- Gage BF, Waterman AD, Shannon W, Boechler M, Rich MW, Radford MJ. Validation of clinical classification schemes for predicting stroke: results from the National Registry of Atrial Fibrillation. JAMA 2001; 285:2864–2870.
- Lip GY, Nieuwlaat R, Pisters R, Lane DA, Crijns HJ. Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor-based approach: the Euro Heart Survey on Atrial Fibrillation. Chest 2010; 137:263–272.
- January CT, Wann LS, Alpert JS, et al; American College of Cardiology/American Heart Association Task Force on Practice Guidelines. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol 2014; 64:e1–e76.
- Pisters R, Lane DA, Nieuwlaat R, de Vos CB, Crijns HJ, Lip GY. A novel user-friendly score (HAS-BLED) to assess 1-year risk of major bleeding in patients with atrial fibrillation: the Euro Heart Survey. Chest 2010; 138:1093–1100.
- Gage BF, Yan Y, Milligan PE, et al. Clinical classification schemes for predicting hemorrhage: results from the National Registry of Atrial Fibrillation (NRAF). Am Heart J 2006; 151:713–719.
- Fang MC, Go AS, Chang Y, et al. A new risk scheme to predict warfarin-associated hemorrhage: The ATRIA (Anticoagulation and Risk Factors in Atrial Fibrillation) Study. J Am Coll Cardiol 2011; 58:395–401.
- Lip GY, Clementy N, Pericart L, Banerjee A, Fauchier L. Stroke and major bleeding risk in elderly patients aged ≥ 75 years with atrial fibrillation: the Loire Valley atrial fibrillation project. Stroke 2015; 46:143–150.
- McGrath ER, Kapral MK, Fang J, et al; Investigators of the Registry of the Canadian Stroke Network. Which risk factors are more associated with ischemic stroke than intracerebral hemorrhage in patients with atrial fibrillation? Stroke 2012; 43:2048–2054.
- Phelan EA, Mahoney JE, Voit JC, Stevens JA. Assessment and management of fall risk in primary care settings. Med Clin North Am 2015; 99:281–293.
- Deandrea S, Bravi F, Turati F, Lucenteforte E, La Vecchia C, Negri E. Risk factors for falls in older people in nursing homes and hospitals. A systematic review and meta-analysis. Arch Gerontol Geriatr 2013; 56:407–415.
- Gage BF, Birman-Deych E, Kerzner R, Radford MJ, Nilasena DS, Rich MW. Incidence of intracranial hemorrhage in patients with atrial fibrillation who are prone to fall. Am J Med 2005; 118:612–617.
- Man-Son-Hing M, Nichol G, Lau A, Laupacis A. Choosing antithrombotic therapy for elderly patients with atrial fibrillation who are at risk for falls. Arch Intern Med 1999; 159:677–685.
- Riva N, Smith DE, Lip GY, Lane DA. Advancing age and bleeding risk are the strongest barriers to anticoagulant prescription in atrial fibrillation. Age Ageing 2011; 40:653–655.
- De Caterina R, Andersson U, Alexander JH, et al; ARISTOTLE Investigators. History of bleeding and outcomes with apixaban versus warfarin in patients with atrial fibrillation in the Apixaban for Reduction in Stroke and Other Thromboembolic Events in Atrial Fibrillation trial. Am Heart J 2016; 175:175–183.
- Ben Freedman S, Gersh BJ, Lip GY. Misperceptions of aspirin efficacy and safety may perpetuate anticoagulant underutilization in atrial fibrillation. Eur Heart J 2015; 36:653–656.
- Camm AJ, Lip GY, De Caterina R, et al; ESC Committee for Practice Guidelines (CPG). 2012 focused update of the ESC Guidelines for the management of atrial fibrillation: an update of the 2010 ESC Guidelines for the management of atrial fibrillation. Developed with the special contribution of the European Heart Rhythm Association. Eur Heart J 2012; 33:2719–2747.
KEY POINTS
- For most patients in this category, the benefits of anticoagulation outweigh the risks.
- Although they are not perfect, scoring systems have been developed to predict the risk of stroke without anticoagulation and the risk of bleeding with anticoagulation.
- The decision-making process is complex and should be shared with the patient and the patient’s family and caregivers.
Geriatric Syndromes in Older Cardiology Patients
Utilizing hospitalist physicians as the primary providers of inpatient care is a rapidly growing trend. In the United States the number of hospitalists now approaches 12,000 and may reach 30,000 by 2010.1 Simultaneously, by 2030 the proportion of adults aged 65 and older will have more than doubled to make up 20% of the U.S. population. Currently, patients aged 65 and older account for approximately 49% of hospital days.2 Congestive heart failure is the most common discharge diagnosis and cardiovascular disease is the leading cause of death of these older adults.3 Given current trends in aging demographics, hospitalists can expect an increasing proportion of their practices to consist of frail older adults with cardiovascular disease.
Hospitalization for any acute illness predisposes elderly patients to increased disability.4 Studies have demonstrated that underrecognition of geriatric syndromes is common and contributes to hospitalized older adults having poor outcomes.5, 6, 7 Between 35% and 50% of elderly patients will experience functional decline while hospitalized,4, 8 and up to 50% will develop hospital‐acquired delirium.6 The risk of experiencing an iatrogenic event while hospitalized is 2‐fold higher for older adults than for those younger than age 65.7, 9 These adverse outcomes lead to longer length of stay (LOS), higher hospital costs, and, for patients able to live at home prior to admission, increased risk of temporary or permanent institutionalization.10, 11
The objective of this study was to characterize a population of acutely ill older adults with known cardiovascular disease admitted to a specialty cardiac ward, to determine the prevalence of geriatric syndromes (ie, functional impairment, cognitive impairment, depression, polypharmacy), and to record the incidence of hospital‐acquired adverse events (urinary tract infection, falls, use of restraints). We hypothesized that these syndromes would be prevalent and underrecognized by the patients' physicians.
METHODS
At Barnes‐Jewish Hospital, an academic medical center in St. Louis, Missouri, patients hospitalized for an acute cardiovascular disorder are preferentially admitted to a cardiac ward with a cardiologist as the attending physician. We conducted a prospective cohort study of 100 patients aged 70 and older admitted to the cardiac ward between January and December of 2003. Participation in the study was not offered to patients who were nonverbal, non‐English‐speaking, or unavailable for screening because of being hospitalized on weekends, holidays, or other days when the research nurse was not available. Participants provided written informed consent. If a patient did not demonstrate an understanding of his or her role in the study, a surrogate decision maker was identified who provided consent in addition to the patient's assent. If a surrogate decision maker was not present, the patient was not enrolled in the study. In addition, patients could decline to continue participating in the study at any time. The institutional review board of the Human Studies Committee at Washington University School of Medicine approved this study.
Data Collection
A trained research nurse administered the following geriatric screening questionnaires: (1) the Katz Index of basic activities of daily living (ADLs)12; (2) the Vulnerable Elders Survey (VES)13; (3) the Short Blessed Test of Orientation, Memory, and Concentration (SBT)14; (4) the Clock Completion Test (CCT)15; and (5) the 15‐item Yesavage Geriatric Depression Scale (GDS).16 The Katz Index (score range 6‐18) assesses the performance of 6 basic ADLs (bathing, continence, dressing, feeding, toileting, and transferring) based on a report by the patient or a collateral source about the patient's level of dependence. Performance of each activity is rated on a scale from 1 (completely dependent) to 3 (completely independent). For this study, patients were considered dependent in any activity if the performance score was less than 3. The Vulnerable Elders Survey (score range 0‐10) utilizes patient age and self‐reported health and functional status to identify frail older adults. A VES score of 3 or greater correlates with a 4‐fold increased risk of death or functional decline over a 2‐year period. Cognition was assessed with the Short Blessed Test of Orientation, Memory, and Concentration and the Clock Completion Test. The Short Blessed Test score ranges from 0 to 28, with a score of 9 or greater indicating increasing severity of cognitive impairment. The Clock Completion Test is scored by evaluating whether the digits in the 4 quadrants of a predrawn circle are accurately placed. The CCT score can range from 0 to 7, with a score of 4 or more indicating cognitive impairment. The 15‐item Geriatric Depression Scale was administered to screen for depressive symptoms. The GDS score can range from 0 to 15, with a score of 6 or more indicating increasing severity of depressive symptoms.
Demographic, psychosocial, and medical data were abstracted by review of patients' hospital records (A.R., C.L.). Medical data obtained from the medical charts included medical diagnoses, number and classes of medications prescribed, and physician documentation of prior or newly diagnosed geriatric syndromes. These geriatric syndromes included dementia, delirium, depression, falls, malnutrition/weight loss, pressure sores, osteoporosis and/or hip fracture, urinary incontinence, and polypharmacy (4 routine medications). A patient was recorded as having documented dementia and/or delirium if the terms dementia, memory loss, cognitive impairment, delirium/delirious, confusion, mental status change, or similar were recorded in physician notes. Admission and discharge orders were reviewed for classes of medications cited in Beers criteria as potentially inappropriate medications for older adults.17 For this study, these high‐risk medications included benzodiazepines, diphenhydramine, propoxyphene, hypnotics, anticholingeric/antidopaminergic medications, and tricyclic antidepressants. Patients' medical charts were reviewed for adverse events such as falls and development of pressure sores or use of restraints. A patient was recorded as having a urinary tract infection (UTI) if a physician documented a UTI in the medical record at any time during hospitalization.
Statistical Analysis
Descriptive statistics were generated using SPSS version 12.0. For continuous measures, values were dichotomized for analytic purposes using standard cutoff scores. Fisher's exact test was used to compare the UTI rate of patients who received a Foley catheter with that of those who did not.
A P value < .05 was considered statistically significant.
RESULTS
Sample Characteristics
Descriptive characteristics for the population are summarized in Table 1. The mean age of the patients was 79.2 5.5 years. The sample was predominantly female and white and had an average stay of 7 days on the cardiac ward. Most patients were admitted for management of heart failure, an arrhythmia, acute myocardial infarction, or angina. Twelve patients had a history of cardiovascular disease (CVD) but were admitted for a noncardiovascular complaint. Only 4 patients did not have a history of CVD.
| Patient characteristic | |
|---|---|
| |
| Age, years (mean SD) | 79.2 5.5 |
| Sex (% female) | 61% |
| Race (% white) | 68% |
| Percent admitted to cardiac ward from: | |
| Home | 69% |
| Outside hospital | 21% |
| Nursing home/skilled nursing facility | 8% |
| ICU | 2% |
| Discharged home from cardiac ward (%) | 84% |
| Length of hospital stay (days), mean SD | 7.4 5.9 |
| Length of cardiac ward stay (days), mean SD | 7.0 5.5 |
| Died during hospitalization (%) | 3% |
| Admitting diagnoses as determined by ICD9 codes (%) | |
| Heart failure | 23% |
| Arrhythmia | 19% |
| Acute myocardial infarction | 10% |
| Chest pain/stable or unstable angina | 10% |
| Coronary artery disease | 9% |
| Syncope | 6% |
| Other cardiovascular diagnoses* | 7% |
| Noncardiovascular diagnoses in patients with history of CVD | 12% |
| Noncardiovascular diagnoses in patients without history of CVD | 4% |
| Comorbidities (%) | |
| Hypertension | 83% |
| Coronary artery disease | 67% |
| History of CABG and/or percutaneous intervention | 54% |
| Hyperlipidemia | 53% |
| Atrial fibrillation | 50% |
| Heart failure | 46% |
| Myocardial infarction | 38% |
| Diabetes mellitus | 37% |
| Chronic renal insufficiency | 29% |
| Stroke or transient ischemic attack | 25% |
| Chronic obstructive pulmonary disease | 23% |
Functional Status and Geriatric Syndromes
Forty‐one percent of patients had a history of 2 or more geriatric syndromes, as documented in their medical record (Table 2). Thirty‐five percent of patients were dependent in at least 1 basic ADL, and 85% had a VES score that indicated an increased risk of functional decline and mortality over the next 2 years. Only 6% of all patients had dementia and only 9% had delirium documented by their physicians in the medical record. Abnormal cognition as detected by screening tests was prevalent. Screening showed that 19% of the patients who completed the SBT and 59% of those who completed the CCT had cognitive impairment. Only 14% of patients with an abnormal CCT and 42% with an abnormal SBT had dementia and/or delirium documented in their hospital chart.
| |
| Katz Index of Basic Activities of Daily Living* (n = 100) | |
| Mean score SD (range 0‐18) | 17.0 1.9 |
| Dependent in 1 ADL (%) | 35% |
| Dependent in 2 ADLs (%) | 20% |
| Vulnerable Elders Survey (n = 100) | |
| Mean score SD (range 0‐10) | 4.6 3.0 |
| Patients with score 3 (%) | 85% |
| Abnormal geriatric screens (%) | |
| Short Blessed Test score 9 (n = 98) | 19% |
| Clock Construction Test score 4 (n = 95) | 59% |
| Geriatric Depression Scale score‖ 6 (n = 99) | 7% |
| Geriatric syndromes documented in cardiology physician notes (%) | |
| Polypharmacy | 95% |
| Depression | 18% |
| History of a prior fall | 17% |
| Delirium | 9% |
| Dementia | 6% |
| Other | 21% |
| Patients with 2 geriatric syndromes | 41% |
| Polypharmacy | |
| Routine medications (range 0‐17) on admission, (n = 100), mean SD | 8.2 3.2 |
| Routine medications (range 3‐17) at discharge, (n = 97), mean SD | 9.0 3.0 |
| Patients taking 12 routine medications on admission (%) | 15% |
| Patients taking 12 routine medications at discharge (%) | 19% |
| Patients with 1 potentially inappropriate medication# ordered on admission or discharge, routine or PRN (%) | 37% |
Polypharmacy was also prevalent. Patients had an average of 9 routine discharge medications, with 19% of patients prescribed at least 12 routine medications at discharge. Thirty‐seven percent of patients were prescribed at least 1 high‐risk medication. Of the 6 patients prescribed a tricyclic antidepressant, 3 had a history of atrial fibrillation/flutter, and 4 had a history of coronary artery disease.
Adverse Events
Thirty‐eight of the 100 patients in the study received a Foley catheter during hospitalization (Table 3). These patients were significantly more likely to have a UTI during their hospitalization than those who did not have a catheter placed (risk ratio 6.0, 95% CI 1.8‐20, P = .002). Other adverse events were rare. Three patients experienced a fall while hospitalized, and 1 patient was restrained (soft limb restraint applied to left upper extremity).
| Developed a UTI (n) | Did not develop a UTI (n) | Risk ratio* (95% confidence interval) | |
|---|---|---|---|
| |||
| Received a Foley | |||
| Yes | 11 | 27 | 6.0 (1.8‐20) |
| No | 3 | 59 | P = .002 (Fisher's exact test) |
DISCUSSION
The goal of this pilot study was to determine the prevalence of geriatric syndromes and the incidence of selected adverse events in hospitalized older patients with cardiovascular disease. We are unaware of another study documenting these syndromes specifically in hospitalized elderly patients with cardiovascular disease. We found that geriatric syndromes were prevalent in this patient population and often unrecognized by physicians. In 1 study of hospitalized frail elderly cardiovascular patients with long hospital stays, physician failure to recognize poor functional status on admission was an independent predictor of patients experiencing a preventable iatrogenic event.7 Brown et al. documented the prevalence and impact of poor mobility in hospitalized adults aged 70 and older. In this study, low mobility was associated with increased risk of further decline in ADL performance, institutionalization, and death; however, it was common for these patients to have bed rest orders (33%), usually without medical indication (60%), indicating underrecognition of functional impairment by attending physicians.18 The proportion of our patients with dependence in at least 1 ADL (35%) and/or at increased risk of functional decline and death based on VES scores (85%) indicates that our patients were already experiencing significant disability at the time of admission, yet these disabilities were rarely documented in the medical record.
In addition to physical frailty, elderly patients with cardiovascular disease may be at increased risk of cognitive impairment. The ongoing Cognitive and Emotional Health Project survey of 36 large cohort studies noted shared risk factors for cardiovascular disease and cognitive impairment in older adults.19 In our study abnormal scores were found for 19% and 59% of the patients who completed the SBT and the CCT, respectively. Several factors may explain the difference in the proportion of patients scoring abnormally on these 2 cognitive screens. We did not measure the visual acuity of our participants, so the number of patients with an abnormal CCT (which relies more on visual cues than the SBT does) may overrepresent the true prevalence of cognitive impairment in our sample. Also, the CCT is a more sensitive indicator of impairments in the visuospatial and executive function domains of cognition than is the SBT and is more likely to be abnormal in vascular dementia.20 Thus, differences in the SBT and CCT scores in our sample may also reflect a higher proportion of patients with a vascular component to their dementia. However, even the number of patients with an abnormal SBT score likely underrepresents the prevalence of underlying cognitive impairment in this sample because of selection bias introduced in obtaining informed consent (ie, the most cognitively impaired patients and/or those deemed to not have decision‐making capacity were excluded or were more likely to decline participation in this study). Consistent with the results of studies of other inpatient populations, cognitive impairment (dementia and/or delirium) was documented in our patients' medical charts far less frequently than detected by either cognitive screen.5, 21 Patients with unrecognized dementia are at increased risk for incident delirium during hospitalization.6
Another common geriatric syndrome in patients with cardiovascular disease is polypharmacy. According to current guidelines, heart failure and coronary artery disease each require multiple medications for optimal therapy. Our patient population were prescribed an average of 9 routine medications at discharge, with nearly 20% prescribed 12 or more routine medications (in addition to as‐needed medications). In comparison, a cohort of hospitalized elderly oncology patients were prescribed an average of 6 routine medications at discharge.22 Thirty‐seven percent of the patients in our study had at least 1 potentially inappropriate medication ordered on admission or at discharge. Although this study was not able to monitor prospectively for adverse drug events, the potential for harm from drug prescribing is substantial in this sample of frail older adult patients. This remains a fruitful area for research.
Thirty‐eight percent of patients in our study received a Foley catheter and were therefore at increased risk of developing a UTI. We did not document the indications for catheterization in this patient population. Studies indicate that up to 20% of urinary catheters are placed without a specific medical indication23 and that hospitalized older adults receiving unwarranted urinary catheterization are at increased risk of prolonged length of stay and death.24
Interventions that increase recognition of geriatric syndromes have been shown to improve the outcomes of hospitalized older adults. The Hospital Elder Life Program demonstrated a 40% reduction in hospital‐acquired delirium in patients aged 70 and older by enhancing recognition and management of geriatric syndromes such as cognitive impairment, immobility, visual/hearing impairment, and polypharmacy.6, 8 Other studies have demonstrated that use of inappropriate medications in hospitalized older adults can be reduced with nonpharmacologic and physician‐education interventions.25, 26 In a broader effort to address multiple geriatric syndromes simultaneously, Acute Care for Elders (ACE) Units have been developed in medical centers worldwide. The ACE Unit model of care emphasizes patient‐centered care, nurse‐driven prevention protocols, frequent interdisciplinary team rounds addressing geriatric syndromes, and discharge planning beginning the day of admission. Studies evaluating outcomes in patients admitted to an ACE Unit have found preservation of physical functioning and independence in ADLs,27, 28 reduced LOS,21 improved patient and provider satisfaction,29 and reduced rates of restraint use,29, 30 institutionalization,27, 29 and mortality.31 This model should be considered for older adults admitted to a cardiac ward. However, other care models could include utilization of inpatient geriatric consultation, hiring a gerontological nurse specialist, or educational programs focused on recognizing and managing geriatric syndromes and designed for the physicians and nurses who care for these patients.
Our study had several limitations. The sample size and number of serious adverse outcomes were small. We did not have adequate power to detect clinically significant differences in length of stay between patients with and without selected geriatric syndromes (0.5 days). The process of informed consent likely selected for a greater number of cognitively intact and fewer depressed patients. The results of the ADL screens may be limited because they were mostly based on patient self‐report of functional status without informant corroboration. Specifically, self‐report may overestimate functional status.
Despite these limitations, we found that functional dependence and geriatric syndromes were prevalent in older cardiovascular patients and that these conditions were rarely documented by the attending physicians or house staff. Over the next decades, an increasing proportion of older adults will be admitted and cared for by hospitalist physicians. Interventions utilizing comprehensive geriatric assessments and interdisciplinary models of care could assist hospitalists in recognizing and managing geriatric syndromes in their frail elderly patients. Future studies are needed to confirm the prevalence of geriatric syndromes and to evaluate the impact of an interdisciplinary model of care on clinical outcomes in hospitalized elderly cardiovascular patients.
Acknowledgements
The authors thank Valerie Emory for her invaluable assistance in collecting data for this study.
- ,.The expanding role of hospitalists in the United States.Swiss Med Wkly.2006;136:591–596.
- American Association of Retired Persons and the Administration on Aging.A Profile of Older Americans: 1999.Washington, DC:American Association of Retired Persons;1999.
- Federal Interagency Forum on Aging Related Statistics. Older Americans 2004: key indicators of well‐being. Available at: http://www.agingstats.gov
- ,,,.Hospitalization, restricted activity, and the development of disability among older persons.JAMA.2004;292:2115–2124.
- ,,,.Cognitively impaired older adults: From hospital to home.Am J Nurs.2005;105:52–61.
- .Prevention of delirium in hospitalized older patients: risk factors and targeted intervention strategies.Ann Med.2000;32:257–263.
- ,,, et al.Iatrogenic complications in high‐risk, elderly patients.Arch Intern Med.1992;152:2074–2080.
- ,,,,.The hospital elder life program: A model of care to prevent cognitive and functional decline in older hospitalized patients.J Am Geriatr Soc.2000;48:1697–1706.
- ,.Incidence and types of preventable adverse events in elderly patients: population based review of medical records.BMJ.2000;320:741–744.
- ,Landefeld S: Improving care for hospitalized elders.Ann Long Term Care: Clin Care Aging.2001;9:35–40.
- :Acute hospital care of the elderly: minimizing the risk of functional decline.Cleve Clin J Med.1995;62:117–128.
- ,,,,.Studies of illness in the aged: the index of ADL: a standardized measure of biological and psychosocial function.JAMA.1963;185:914–919.
- ,,, et al.The Vulnerable Elders Survey: a tool for identifying vulnerable older people in the community.J Am Geriatr Soc.2001;49:1691–1699.
- ,,,,,.Validation of a short orientation memory‐concentration test of cognitive impairment.Am J Psychiatry.1983;140:734–739.
- ,,.Clock completion: an objective screening test for dementia.J Am Geriatr Soc.1993;41:1235–1240.
- .Geriatric depression scale.Psychopharmacol Bull.1988;24:709–711.
- ,,,,,.Updating the Beers Criteria for potentially inappropriate medication use in older adults.Arch Intern Med.2003;163:2716–2724.
- ,,.Prevalence and outcomes of low mobility in hospitalized older patients.J Am Geriatr Soc.2004;52:1263–1270.
- ,,, et al.The NIH cognitive and emotional health project: Report of the critical evaluation study committee.Alzheimers Dement.2006;2:12–32.
- ,,,.Can clock drawing test help to differentiate between dementia of the Alzheimer's type and vascular dementia? A preliminary study.Int J Geriatr Psychiatry.2002;17:699–703.
- ,,, et al.Geriatric‐based versus general wards for older acute medical patients: a randomized comparison of outcomes and use of resources.J Am Geriatr Soc.2000;48:1381–1388.
- ,,,,,.Geriatric syndromes in elderly patients admitted to an oncology‐acute care for elders unit.J Clin Oncol.2006;24:2298–2303.
- ,,,.Overuse of the indwelling urinary tract catheter in hospitalized medical patients.Arch Intern Med.1995;155:1425–1429.
- ,,, et al.The relationship of indwelling urinary catheters to death, length of hospital stay, functional decline, and nursing home admission in hospitalized older medical patients.J Am Geriatr Soc.2007;55:227–233.
- ,,,.A strategy to decrease the use of risky drugs in the elderly.Cleve Clin J Med.2004;71:561–568.
- ,,,,,.A multifactorial intervention to reduce prevalence of delirium and shorten hospital length of stay.J Am Geriatr Soc.2005;53:18–23.
- ,,,,.A randomized trial of care in a hospital medical unit especially designed to improve the functional outcomes of acutely ill older patients.N Engl J Med.1995;332:1338–1344.
- ,,, et al.A controlled trial of inpatient and outpatient geriatric evaluation and management.N Engl J Med.2002;346:905–912.
- ,,, et al.Effects of a multicomponent intervention on functional outcomes and process of care in hospitalized older patients: A randomized controlled trial of Acute Care for Elders (ACE) in a community hospital.J Am Geriatr Soc.2000;48:1572–1581.
- ,,,,,.A model for managing delirious older inpatients.J Am Geriatr Soc.2003;51:1031–1035.
- ,,,,.Reduced mortality in treating acutely sick, frail older patients in a geriatric evaluation and management unit. A prospective randomized trial.J Am Geriatr Soc.2002;50:792–798.
Utilizing hospitalist physicians as the primary providers of inpatient care is a rapidly growing trend. In the United States the number of hospitalists now approaches 12,000 and may reach 30,000 by 2010.1 Simultaneously, by 2030 the proportion of adults aged 65 and older will have more than doubled to make up 20% of the U.S. population. Currently, patients aged 65 and older account for approximately 49% of hospital days.2 Congestive heart failure is the most common discharge diagnosis and cardiovascular disease is the leading cause of death of these older adults.3 Given current trends in aging demographics, hospitalists can expect an increasing proportion of their practices to consist of frail older adults with cardiovascular disease.
Hospitalization for any acute illness predisposes elderly patients to increased disability.4 Studies have demonstrated that underrecognition of geriatric syndromes is common and contributes to hospitalized older adults having poor outcomes.5, 6, 7 Between 35% and 50% of elderly patients will experience functional decline while hospitalized,4, 8 and up to 50% will develop hospital‐acquired delirium.6 The risk of experiencing an iatrogenic event while hospitalized is 2‐fold higher for older adults than for those younger than age 65.7, 9 These adverse outcomes lead to longer length of stay (LOS), higher hospital costs, and, for patients able to live at home prior to admission, increased risk of temporary or permanent institutionalization.10, 11
The objective of this study was to characterize a population of acutely ill older adults with known cardiovascular disease admitted to a specialty cardiac ward, to determine the prevalence of geriatric syndromes (ie, functional impairment, cognitive impairment, depression, polypharmacy), and to record the incidence of hospital‐acquired adverse events (urinary tract infection, falls, use of restraints). We hypothesized that these syndromes would be prevalent and underrecognized by the patients' physicians.
METHODS
At Barnes‐Jewish Hospital, an academic medical center in St. Louis, Missouri, patients hospitalized for an acute cardiovascular disorder are preferentially admitted to a cardiac ward with a cardiologist as the attending physician. We conducted a prospective cohort study of 100 patients aged 70 and older admitted to the cardiac ward between January and December of 2003. Participation in the study was not offered to patients who were nonverbal, non‐English‐speaking, or unavailable for screening because of being hospitalized on weekends, holidays, or other days when the research nurse was not available. Participants provided written informed consent. If a patient did not demonstrate an understanding of his or her role in the study, a surrogate decision maker was identified who provided consent in addition to the patient's assent. If a surrogate decision maker was not present, the patient was not enrolled in the study. In addition, patients could decline to continue participating in the study at any time. The institutional review board of the Human Studies Committee at Washington University School of Medicine approved this study.
Data Collection
A trained research nurse administered the following geriatric screening questionnaires: (1) the Katz Index of basic activities of daily living (ADLs)12; (2) the Vulnerable Elders Survey (VES)13; (3) the Short Blessed Test of Orientation, Memory, and Concentration (SBT)14; (4) the Clock Completion Test (CCT)15; and (5) the 15‐item Yesavage Geriatric Depression Scale (GDS).16 The Katz Index (score range 6‐18) assesses the performance of 6 basic ADLs (bathing, continence, dressing, feeding, toileting, and transferring) based on a report by the patient or a collateral source about the patient's level of dependence. Performance of each activity is rated on a scale from 1 (completely dependent) to 3 (completely independent). For this study, patients were considered dependent in any activity if the performance score was less than 3. The Vulnerable Elders Survey (score range 0‐10) utilizes patient age and self‐reported health and functional status to identify frail older adults. A VES score of 3 or greater correlates with a 4‐fold increased risk of death or functional decline over a 2‐year period. Cognition was assessed with the Short Blessed Test of Orientation, Memory, and Concentration and the Clock Completion Test. The Short Blessed Test score ranges from 0 to 28, with a score of 9 or greater indicating increasing severity of cognitive impairment. The Clock Completion Test is scored by evaluating whether the digits in the 4 quadrants of a predrawn circle are accurately placed. The CCT score can range from 0 to 7, with a score of 4 or more indicating cognitive impairment. The 15‐item Geriatric Depression Scale was administered to screen for depressive symptoms. The GDS score can range from 0 to 15, with a score of 6 or more indicating increasing severity of depressive symptoms.
Demographic, psychosocial, and medical data were abstracted by review of patients' hospital records (A.R., C.L.). Medical data obtained from the medical charts included medical diagnoses, number and classes of medications prescribed, and physician documentation of prior or newly diagnosed geriatric syndromes. These geriatric syndromes included dementia, delirium, depression, falls, malnutrition/weight loss, pressure sores, osteoporosis and/or hip fracture, urinary incontinence, and polypharmacy (4 routine medications). A patient was recorded as having documented dementia and/or delirium if the terms dementia, memory loss, cognitive impairment, delirium/delirious, confusion, mental status change, or similar were recorded in physician notes. Admission and discharge orders were reviewed for classes of medications cited in Beers criteria as potentially inappropriate medications for older adults.17 For this study, these high‐risk medications included benzodiazepines, diphenhydramine, propoxyphene, hypnotics, anticholingeric/antidopaminergic medications, and tricyclic antidepressants. Patients' medical charts were reviewed for adverse events such as falls and development of pressure sores or use of restraints. A patient was recorded as having a urinary tract infection (UTI) if a physician documented a UTI in the medical record at any time during hospitalization.
Statistical Analysis
Descriptive statistics were generated using SPSS version 12.0. For continuous measures, values were dichotomized for analytic purposes using standard cutoff scores. Fisher's exact test was used to compare the UTI rate of patients who received a Foley catheter with that of those who did not.
A P value < .05 was considered statistically significant.
RESULTS
Sample Characteristics
Descriptive characteristics for the population are summarized in Table 1. The mean age of the patients was 79.2 5.5 years. The sample was predominantly female and white and had an average stay of 7 days on the cardiac ward. Most patients were admitted for management of heart failure, an arrhythmia, acute myocardial infarction, or angina. Twelve patients had a history of cardiovascular disease (CVD) but were admitted for a noncardiovascular complaint. Only 4 patients did not have a history of CVD.
| Patient characteristic | |
|---|---|
| |
| Age, years (mean SD) | 79.2 5.5 |
| Sex (% female) | 61% |
| Race (% white) | 68% |
| Percent admitted to cardiac ward from: | |
| Home | 69% |
| Outside hospital | 21% |
| Nursing home/skilled nursing facility | 8% |
| ICU | 2% |
| Discharged home from cardiac ward (%) | 84% |
| Length of hospital stay (days), mean SD | 7.4 5.9 |
| Length of cardiac ward stay (days), mean SD | 7.0 5.5 |
| Died during hospitalization (%) | 3% |
| Admitting diagnoses as determined by ICD9 codes (%) | |
| Heart failure | 23% |
| Arrhythmia | 19% |
| Acute myocardial infarction | 10% |
| Chest pain/stable or unstable angina | 10% |
| Coronary artery disease | 9% |
| Syncope | 6% |
| Other cardiovascular diagnoses* | 7% |
| Noncardiovascular diagnoses in patients with history of CVD | 12% |
| Noncardiovascular diagnoses in patients without history of CVD | 4% |
| Comorbidities (%) | |
| Hypertension | 83% |
| Coronary artery disease | 67% |
| History of CABG and/or percutaneous intervention | 54% |
| Hyperlipidemia | 53% |
| Atrial fibrillation | 50% |
| Heart failure | 46% |
| Myocardial infarction | 38% |
| Diabetes mellitus | 37% |
| Chronic renal insufficiency | 29% |
| Stroke or transient ischemic attack | 25% |
| Chronic obstructive pulmonary disease | 23% |
Functional Status and Geriatric Syndromes
Forty‐one percent of patients had a history of 2 or more geriatric syndromes, as documented in their medical record (Table 2). Thirty‐five percent of patients were dependent in at least 1 basic ADL, and 85% had a VES score that indicated an increased risk of functional decline and mortality over the next 2 years. Only 6% of all patients had dementia and only 9% had delirium documented by their physicians in the medical record. Abnormal cognition as detected by screening tests was prevalent. Screening showed that 19% of the patients who completed the SBT and 59% of those who completed the CCT had cognitive impairment. Only 14% of patients with an abnormal CCT and 42% with an abnormal SBT had dementia and/or delirium documented in their hospital chart.
| |
| Katz Index of Basic Activities of Daily Living* (n = 100) | |
| Mean score SD (range 0‐18) | 17.0 1.9 |
| Dependent in 1 ADL (%) | 35% |
| Dependent in 2 ADLs (%) | 20% |
| Vulnerable Elders Survey (n = 100) | |
| Mean score SD (range 0‐10) | 4.6 3.0 |
| Patients with score 3 (%) | 85% |
| Abnormal geriatric screens (%) | |
| Short Blessed Test score 9 (n = 98) | 19% |
| Clock Construction Test score 4 (n = 95) | 59% |
| Geriatric Depression Scale score‖ 6 (n = 99) | 7% |
| Geriatric syndromes documented in cardiology physician notes (%) | |
| Polypharmacy | 95% |
| Depression | 18% |
| History of a prior fall | 17% |
| Delirium | 9% |
| Dementia | 6% |
| Other | 21% |
| Patients with 2 geriatric syndromes | 41% |
| Polypharmacy | |
| Routine medications (range 0‐17) on admission, (n = 100), mean SD | 8.2 3.2 |
| Routine medications (range 3‐17) at discharge, (n = 97), mean SD | 9.0 3.0 |
| Patients taking 12 routine medications on admission (%) | 15% |
| Patients taking 12 routine medications at discharge (%) | 19% |
| Patients with 1 potentially inappropriate medication# ordered on admission or discharge, routine or PRN (%) | 37% |
Polypharmacy was also prevalent. Patients had an average of 9 routine discharge medications, with 19% of patients prescribed at least 12 routine medications at discharge. Thirty‐seven percent of patients were prescribed at least 1 high‐risk medication. Of the 6 patients prescribed a tricyclic antidepressant, 3 had a history of atrial fibrillation/flutter, and 4 had a history of coronary artery disease.
Adverse Events
Thirty‐eight of the 100 patients in the study received a Foley catheter during hospitalization (Table 3). These patients were significantly more likely to have a UTI during their hospitalization than those who did not have a catheter placed (risk ratio 6.0, 95% CI 1.8‐20, P = .002). Other adverse events were rare. Three patients experienced a fall while hospitalized, and 1 patient was restrained (soft limb restraint applied to left upper extremity).
| Developed a UTI (n) | Did not develop a UTI (n) | Risk ratio* (95% confidence interval) | |
|---|---|---|---|
| |||
| Received a Foley | |||
| Yes | 11 | 27 | 6.0 (1.8‐20) |
| No | 3 | 59 | P = .002 (Fisher's exact test) |
DISCUSSION
The goal of this pilot study was to determine the prevalence of geriatric syndromes and the incidence of selected adverse events in hospitalized older patients with cardiovascular disease. We are unaware of another study documenting these syndromes specifically in hospitalized elderly patients with cardiovascular disease. We found that geriatric syndromes were prevalent in this patient population and often unrecognized by physicians. In 1 study of hospitalized frail elderly cardiovascular patients with long hospital stays, physician failure to recognize poor functional status on admission was an independent predictor of patients experiencing a preventable iatrogenic event.7 Brown et al. documented the prevalence and impact of poor mobility in hospitalized adults aged 70 and older. In this study, low mobility was associated with increased risk of further decline in ADL performance, institutionalization, and death; however, it was common for these patients to have bed rest orders (33%), usually without medical indication (60%), indicating underrecognition of functional impairment by attending physicians.18 The proportion of our patients with dependence in at least 1 ADL (35%) and/or at increased risk of functional decline and death based on VES scores (85%) indicates that our patients were already experiencing significant disability at the time of admission, yet these disabilities were rarely documented in the medical record.
In addition to physical frailty, elderly patients with cardiovascular disease may be at increased risk of cognitive impairment. The ongoing Cognitive and Emotional Health Project survey of 36 large cohort studies noted shared risk factors for cardiovascular disease and cognitive impairment in older adults.19 In our study abnormal scores were found for 19% and 59% of the patients who completed the SBT and the CCT, respectively. Several factors may explain the difference in the proportion of patients scoring abnormally on these 2 cognitive screens. We did not measure the visual acuity of our participants, so the number of patients with an abnormal CCT (which relies more on visual cues than the SBT does) may overrepresent the true prevalence of cognitive impairment in our sample. Also, the CCT is a more sensitive indicator of impairments in the visuospatial and executive function domains of cognition than is the SBT and is more likely to be abnormal in vascular dementia.20 Thus, differences in the SBT and CCT scores in our sample may also reflect a higher proportion of patients with a vascular component to their dementia. However, even the number of patients with an abnormal SBT score likely underrepresents the prevalence of underlying cognitive impairment in this sample because of selection bias introduced in obtaining informed consent (ie, the most cognitively impaired patients and/or those deemed to not have decision‐making capacity were excluded or were more likely to decline participation in this study). Consistent with the results of studies of other inpatient populations, cognitive impairment (dementia and/or delirium) was documented in our patients' medical charts far less frequently than detected by either cognitive screen.5, 21 Patients with unrecognized dementia are at increased risk for incident delirium during hospitalization.6
Another common geriatric syndrome in patients with cardiovascular disease is polypharmacy. According to current guidelines, heart failure and coronary artery disease each require multiple medications for optimal therapy. Our patient population were prescribed an average of 9 routine medications at discharge, with nearly 20% prescribed 12 or more routine medications (in addition to as‐needed medications). In comparison, a cohort of hospitalized elderly oncology patients were prescribed an average of 6 routine medications at discharge.22 Thirty‐seven percent of the patients in our study had at least 1 potentially inappropriate medication ordered on admission or at discharge. Although this study was not able to monitor prospectively for adverse drug events, the potential for harm from drug prescribing is substantial in this sample of frail older adult patients. This remains a fruitful area for research.
Thirty‐eight percent of patients in our study received a Foley catheter and were therefore at increased risk of developing a UTI. We did not document the indications for catheterization in this patient population. Studies indicate that up to 20% of urinary catheters are placed without a specific medical indication23 and that hospitalized older adults receiving unwarranted urinary catheterization are at increased risk of prolonged length of stay and death.24
Interventions that increase recognition of geriatric syndromes have been shown to improve the outcomes of hospitalized older adults. The Hospital Elder Life Program demonstrated a 40% reduction in hospital‐acquired delirium in patients aged 70 and older by enhancing recognition and management of geriatric syndromes such as cognitive impairment, immobility, visual/hearing impairment, and polypharmacy.6, 8 Other studies have demonstrated that use of inappropriate medications in hospitalized older adults can be reduced with nonpharmacologic and physician‐education interventions.25, 26 In a broader effort to address multiple geriatric syndromes simultaneously, Acute Care for Elders (ACE) Units have been developed in medical centers worldwide. The ACE Unit model of care emphasizes patient‐centered care, nurse‐driven prevention protocols, frequent interdisciplinary team rounds addressing geriatric syndromes, and discharge planning beginning the day of admission. Studies evaluating outcomes in patients admitted to an ACE Unit have found preservation of physical functioning and independence in ADLs,27, 28 reduced LOS,21 improved patient and provider satisfaction,29 and reduced rates of restraint use,29, 30 institutionalization,27, 29 and mortality.31 This model should be considered for older adults admitted to a cardiac ward. However, other care models could include utilization of inpatient geriatric consultation, hiring a gerontological nurse specialist, or educational programs focused on recognizing and managing geriatric syndromes and designed for the physicians and nurses who care for these patients.
Our study had several limitations. The sample size and number of serious adverse outcomes were small. We did not have adequate power to detect clinically significant differences in length of stay between patients with and without selected geriatric syndromes (0.5 days). The process of informed consent likely selected for a greater number of cognitively intact and fewer depressed patients. The results of the ADL screens may be limited because they were mostly based on patient self‐report of functional status without informant corroboration. Specifically, self‐report may overestimate functional status.
Despite these limitations, we found that functional dependence and geriatric syndromes were prevalent in older cardiovascular patients and that these conditions were rarely documented by the attending physicians or house staff. Over the next decades, an increasing proportion of older adults will be admitted and cared for by hospitalist physicians. Interventions utilizing comprehensive geriatric assessments and interdisciplinary models of care could assist hospitalists in recognizing and managing geriatric syndromes in their frail elderly patients. Future studies are needed to confirm the prevalence of geriatric syndromes and to evaluate the impact of an interdisciplinary model of care on clinical outcomes in hospitalized elderly cardiovascular patients.
Acknowledgements
The authors thank Valerie Emory for her invaluable assistance in collecting data for this study.
Utilizing hospitalist physicians as the primary providers of inpatient care is a rapidly growing trend. In the United States the number of hospitalists now approaches 12,000 and may reach 30,000 by 2010.1 Simultaneously, by 2030 the proportion of adults aged 65 and older will have more than doubled to make up 20% of the U.S. population. Currently, patients aged 65 and older account for approximately 49% of hospital days.2 Congestive heart failure is the most common discharge diagnosis and cardiovascular disease is the leading cause of death of these older adults.3 Given current trends in aging demographics, hospitalists can expect an increasing proportion of their practices to consist of frail older adults with cardiovascular disease.
Hospitalization for any acute illness predisposes elderly patients to increased disability.4 Studies have demonstrated that underrecognition of geriatric syndromes is common and contributes to hospitalized older adults having poor outcomes.5, 6, 7 Between 35% and 50% of elderly patients will experience functional decline while hospitalized,4, 8 and up to 50% will develop hospital‐acquired delirium.6 The risk of experiencing an iatrogenic event while hospitalized is 2‐fold higher for older adults than for those younger than age 65.7, 9 These adverse outcomes lead to longer length of stay (LOS), higher hospital costs, and, for patients able to live at home prior to admission, increased risk of temporary or permanent institutionalization.10, 11
The objective of this study was to characterize a population of acutely ill older adults with known cardiovascular disease admitted to a specialty cardiac ward, to determine the prevalence of geriatric syndromes (ie, functional impairment, cognitive impairment, depression, polypharmacy), and to record the incidence of hospital‐acquired adverse events (urinary tract infection, falls, use of restraints). We hypothesized that these syndromes would be prevalent and underrecognized by the patients' physicians.
METHODS
At Barnes‐Jewish Hospital, an academic medical center in St. Louis, Missouri, patients hospitalized for an acute cardiovascular disorder are preferentially admitted to a cardiac ward with a cardiologist as the attending physician. We conducted a prospective cohort study of 100 patients aged 70 and older admitted to the cardiac ward between January and December of 2003. Participation in the study was not offered to patients who were nonverbal, non‐English‐speaking, or unavailable for screening because of being hospitalized on weekends, holidays, or other days when the research nurse was not available. Participants provided written informed consent. If a patient did not demonstrate an understanding of his or her role in the study, a surrogate decision maker was identified who provided consent in addition to the patient's assent. If a surrogate decision maker was not present, the patient was not enrolled in the study. In addition, patients could decline to continue participating in the study at any time. The institutional review board of the Human Studies Committee at Washington University School of Medicine approved this study.
Data Collection
A trained research nurse administered the following geriatric screening questionnaires: (1) the Katz Index of basic activities of daily living (ADLs)12; (2) the Vulnerable Elders Survey (VES)13; (3) the Short Blessed Test of Orientation, Memory, and Concentration (SBT)14; (4) the Clock Completion Test (CCT)15; and (5) the 15‐item Yesavage Geriatric Depression Scale (GDS).16 The Katz Index (score range 6‐18) assesses the performance of 6 basic ADLs (bathing, continence, dressing, feeding, toileting, and transferring) based on a report by the patient or a collateral source about the patient's level of dependence. Performance of each activity is rated on a scale from 1 (completely dependent) to 3 (completely independent). For this study, patients were considered dependent in any activity if the performance score was less than 3. The Vulnerable Elders Survey (score range 0‐10) utilizes patient age and self‐reported health and functional status to identify frail older adults. A VES score of 3 or greater correlates with a 4‐fold increased risk of death or functional decline over a 2‐year period. Cognition was assessed with the Short Blessed Test of Orientation, Memory, and Concentration and the Clock Completion Test. The Short Blessed Test score ranges from 0 to 28, with a score of 9 or greater indicating increasing severity of cognitive impairment. The Clock Completion Test is scored by evaluating whether the digits in the 4 quadrants of a predrawn circle are accurately placed. The CCT score can range from 0 to 7, with a score of 4 or more indicating cognitive impairment. The 15‐item Geriatric Depression Scale was administered to screen for depressive symptoms. The GDS score can range from 0 to 15, with a score of 6 or more indicating increasing severity of depressive symptoms.
Demographic, psychosocial, and medical data were abstracted by review of patients' hospital records (A.R., C.L.). Medical data obtained from the medical charts included medical diagnoses, number and classes of medications prescribed, and physician documentation of prior or newly diagnosed geriatric syndromes. These geriatric syndromes included dementia, delirium, depression, falls, malnutrition/weight loss, pressure sores, osteoporosis and/or hip fracture, urinary incontinence, and polypharmacy (4 routine medications). A patient was recorded as having documented dementia and/or delirium if the terms dementia, memory loss, cognitive impairment, delirium/delirious, confusion, mental status change, or similar were recorded in physician notes. Admission and discharge orders were reviewed for classes of medications cited in Beers criteria as potentially inappropriate medications for older adults.17 For this study, these high‐risk medications included benzodiazepines, diphenhydramine, propoxyphene, hypnotics, anticholingeric/antidopaminergic medications, and tricyclic antidepressants. Patients' medical charts were reviewed for adverse events such as falls and development of pressure sores or use of restraints. A patient was recorded as having a urinary tract infection (UTI) if a physician documented a UTI in the medical record at any time during hospitalization.
Statistical Analysis
Descriptive statistics were generated using SPSS version 12.0. For continuous measures, values were dichotomized for analytic purposes using standard cutoff scores. Fisher's exact test was used to compare the UTI rate of patients who received a Foley catheter with that of those who did not.
A P value < .05 was considered statistically significant.
RESULTS
Sample Characteristics
Descriptive characteristics for the population are summarized in Table 1. The mean age of the patients was 79.2 5.5 years. The sample was predominantly female and white and had an average stay of 7 days on the cardiac ward. Most patients were admitted for management of heart failure, an arrhythmia, acute myocardial infarction, or angina. Twelve patients had a history of cardiovascular disease (CVD) but were admitted for a noncardiovascular complaint. Only 4 patients did not have a history of CVD.
| Patient characteristic | |
|---|---|
| |
| Age, years (mean SD) | 79.2 5.5 |
| Sex (% female) | 61% |
| Race (% white) | 68% |
| Percent admitted to cardiac ward from: | |
| Home | 69% |
| Outside hospital | 21% |
| Nursing home/skilled nursing facility | 8% |
| ICU | 2% |
| Discharged home from cardiac ward (%) | 84% |
| Length of hospital stay (days), mean SD | 7.4 5.9 |
| Length of cardiac ward stay (days), mean SD | 7.0 5.5 |
| Died during hospitalization (%) | 3% |
| Admitting diagnoses as determined by ICD9 codes (%) | |
| Heart failure | 23% |
| Arrhythmia | 19% |
| Acute myocardial infarction | 10% |
| Chest pain/stable or unstable angina | 10% |
| Coronary artery disease | 9% |
| Syncope | 6% |
| Other cardiovascular diagnoses* | 7% |
| Noncardiovascular diagnoses in patients with history of CVD | 12% |
| Noncardiovascular diagnoses in patients without history of CVD | 4% |
| Comorbidities (%) | |
| Hypertension | 83% |
| Coronary artery disease | 67% |
| History of CABG and/or percutaneous intervention | 54% |
| Hyperlipidemia | 53% |
| Atrial fibrillation | 50% |
| Heart failure | 46% |
| Myocardial infarction | 38% |
| Diabetes mellitus | 37% |
| Chronic renal insufficiency | 29% |
| Stroke or transient ischemic attack | 25% |
| Chronic obstructive pulmonary disease | 23% |
Functional Status and Geriatric Syndromes
Forty‐one percent of patients had a history of 2 or more geriatric syndromes, as documented in their medical record (Table 2). Thirty‐five percent of patients were dependent in at least 1 basic ADL, and 85% had a VES score that indicated an increased risk of functional decline and mortality over the next 2 years. Only 6% of all patients had dementia and only 9% had delirium documented by their physicians in the medical record. Abnormal cognition as detected by screening tests was prevalent. Screening showed that 19% of the patients who completed the SBT and 59% of those who completed the CCT had cognitive impairment. Only 14% of patients with an abnormal CCT and 42% with an abnormal SBT had dementia and/or delirium documented in their hospital chart.
| |
| Katz Index of Basic Activities of Daily Living* (n = 100) | |
| Mean score SD (range 0‐18) | 17.0 1.9 |
| Dependent in 1 ADL (%) | 35% |
| Dependent in 2 ADLs (%) | 20% |
| Vulnerable Elders Survey (n = 100) | |
| Mean score SD (range 0‐10) | 4.6 3.0 |
| Patients with score 3 (%) | 85% |
| Abnormal geriatric screens (%) | |
| Short Blessed Test score 9 (n = 98) | 19% |
| Clock Construction Test score 4 (n = 95) | 59% |
| Geriatric Depression Scale score‖ 6 (n = 99) | 7% |
| Geriatric syndromes documented in cardiology physician notes (%) | |
| Polypharmacy | 95% |
| Depression | 18% |
| History of a prior fall | 17% |
| Delirium | 9% |
| Dementia | 6% |
| Other | 21% |
| Patients with 2 geriatric syndromes | 41% |
| Polypharmacy | |
| Routine medications (range 0‐17) on admission, (n = 100), mean SD | 8.2 3.2 |
| Routine medications (range 3‐17) at discharge, (n = 97), mean SD | 9.0 3.0 |
| Patients taking 12 routine medications on admission (%) | 15% |
| Patients taking 12 routine medications at discharge (%) | 19% |
| Patients with 1 potentially inappropriate medication# ordered on admission or discharge, routine or PRN (%) | 37% |
Polypharmacy was also prevalent. Patients had an average of 9 routine discharge medications, with 19% of patients prescribed at least 12 routine medications at discharge. Thirty‐seven percent of patients were prescribed at least 1 high‐risk medication. Of the 6 patients prescribed a tricyclic antidepressant, 3 had a history of atrial fibrillation/flutter, and 4 had a history of coronary artery disease.
Adverse Events
Thirty‐eight of the 100 patients in the study received a Foley catheter during hospitalization (Table 3). These patients were significantly more likely to have a UTI during their hospitalization than those who did not have a catheter placed (risk ratio 6.0, 95% CI 1.8‐20, P = .002). Other adverse events were rare. Three patients experienced a fall while hospitalized, and 1 patient was restrained (soft limb restraint applied to left upper extremity).
| Developed a UTI (n) | Did not develop a UTI (n) | Risk ratio* (95% confidence interval) | |
|---|---|---|---|
| |||
| Received a Foley | |||
| Yes | 11 | 27 | 6.0 (1.8‐20) |
| No | 3 | 59 | P = .002 (Fisher's exact test) |
DISCUSSION
The goal of this pilot study was to determine the prevalence of geriatric syndromes and the incidence of selected adverse events in hospitalized older patients with cardiovascular disease. We are unaware of another study documenting these syndromes specifically in hospitalized elderly patients with cardiovascular disease. We found that geriatric syndromes were prevalent in this patient population and often unrecognized by physicians. In 1 study of hospitalized frail elderly cardiovascular patients with long hospital stays, physician failure to recognize poor functional status on admission was an independent predictor of patients experiencing a preventable iatrogenic event.7 Brown et al. documented the prevalence and impact of poor mobility in hospitalized adults aged 70 and older. In this study, low mobility was associated with increased risk of further decline in ADL performance, institutionalization, and death; however, it was common for these patients to have bed rest orders (33%), usually without medical indication (60%), indicating underrecognition of functional impairment by attending physicians.18 The proportion of our patients with dependence in at least 1 ADL (35%) and/or at increased risk of functional decline and death based on VES scores (85%) indicates that our patients were already experiencing significant disability at the time of admission, yet these disabilities were rarely documented in the medical record.
In addition to physical frailty, elderly patients with cardiovascular disease may be at increased risk of cognitive impairment. The ongoing Cognitive and Emotional Health Project survey of 36 large cohort studies noted shared risk factors for cardiovascular disease and cognitive impairment in older adults.19 In our study abnormal scores were found for 19% and 59% of the patients who completed the SBT and the CCT, respectively. Several factors may explain the difference in the proportion of patients scoring abnormally on these 2 cognitive screens. We did not measure the visual acuity of our participants, so the number of patients with an abnormal CCT (which relies more on visual cues than the SBT does) may overrepresent the true prevalence of cognitive impairment in our sample. Also, the CCT is a more sensitive indicator of impairments in the visuospatial and executive function domains of cognition than is the SBT and is more likely to be abnormal in vascular dementia.20 Thus, differences in the SBT and CCT scores in our sample may also reflect a higher proportion of patients with a vascular component to their dementia. However, even the number of patients with an abnormal SBT score likely underrepresents the prevalence of underlying cognitive impairment in this sample because of selection bias introduced in obtaining informed consent (ie, the most cognitively impaired patients and/or those deemed to not have decision‐making capacity were excluded or were more likely to decline participation in this study). Consistent with the results of studies of other inpatient populations, cognitive impairment (dementia and/or delirium) was documented in our patients' medical charts far less frequently than detected by either cognitive screen.5, 21 Patients with unrecognized dementia are at increased risk for incident delirium during hospitalization.6
Another common geriatric syndrome in patients with cardiovascular disease is polypharmacy. According to current guidelines, heart failure and coronary artery disease each require multiple medications for optimal therapy. Our patient population were prescribed an average of 9 routine medications at discharge, with nearly 20% prescribed 12 or more routine medications (in addition to as‐needed medications). In comparison, a cohort of hospitalized elderly oncology patients were prescribed an average of 6 routine medications at discharge.22 Thirty‐seven percent of the patients in our study had at least 1 potentially inappropriate medication ordered on admission or at discharge. Although this study was not able to monitor prospectively for adverse drug events, the potential for harm from drug prescribing is substantial in this sample of frail older adult patients. This remains a fruitful area for research.
Thirty‐eight percent of patients in our study received a Foley catheter and were therefore at increased risk of developing a UTI. We did not document the indications for catheterization in this patient population. Studies indicate that up to 20% of urinary catheters are placed without a specific medical indication23 and that hospitalized older adults receiving unwarranted urinary catheterization are at increased risk of prolonged length of stay and death.24
Interventions that increase recognition of geriatric syndromes have been shown to improve the outcomes of hospitalized older adults. The Hospital Elder Life Program demonstrated a 40% reduction in hospital‐acquired delirium in patients aged 70 and older by enhancing recognition and management of geriatric syndromes such as cognitive impairment, immobility, visual/hearing impairment, and polypharmacy.6, 8 Other studies have demonstrated that use of inappropriate medications in hospitalized older adults can be reduced with nonpharmacologic and physician‐education interventions.25, 26 In a broader effort to address multiple geriatric syndromes simultaneously, Acute Care for Elders (ACE) Units have been developed in medical centers worldwide. The ACE Unit model of care emphasizes patient‐centered care, nurse‐driven prevention protocols, frequent interdisciplinary team rounds addressing geriatric syndromes, and discharge planning beginning the day of admission. Studies evaluating outcomes in patients admitted to an ACE Unit have found preservation of physical functioning and independence in ADLs,27, 28 reduced LOS,21 improved patient and provider satisfaction,29 and reduced rates of restraint use,29, 30 institutionalization,27, 29 and mortality.31 This model should be considered for older adults admitted to a cardiac ward. However, other care models could include utilization of inpatient geriatric consultation, hiring a gerontological nurse specialist, or educational programs focused on recognizing and managing geriatric syndromes and designed for the physicians and nurses who care for these patients.
Our study had several limitations. The sample size and number of serious adverse outcomes were small. We did not have adequate power to detect clinically significant differences in length of stay between patients with and without selected geriatric syndromes (0.5 days). The process of informed consent likely selected for a greater number of cognitively intact and fewer depressed patients. The results of the ADL screens may be limited because they were mostly based on patient self‐report of functional status without informant corroboration. Specifically, self‐report may overestimate functional status.
Despite these limitations, we found that functional dependence and geriatric syndromes were prevalent in older cardiovascular patients and that these conditions were rarely documented by the attending physicians or house staff. Over the next decades, an increasing proportion of older adults will be admitted and cared for by hospitalist physicians. Interventions utilizing comprehensive geriatric assessments and interdisciplinary models of care could assist hospitalists in recognizing and managing geriatric syndromes in their frail elderly patients. Future studies are needed to confirm the prevalence of geriatric syndromes and to evaluate the impact of an interdisciplinary model of care on clinical outcomes in hospitalized elderly cardiovascular patients.
Acknowledgements
The authors thank Valerie Emory for her invaluable assistance in collecting data for this study.
- ,.The expanding role of hospitalists in the United States.Swiss Med Wkly.2006;136:591–596.
- American Association of Retired Persons and the Administration on Aging.A Profile of Older Americans: 1999.Washington, DC:American Association of Retired Persons;1999.
- Federal Interagency Forum on Aging Related Statistics. Older Americans 2004: key indicators of well‐being. Available at: http://www.agingstats.gov
- ,,,.Hospitalization, restricted activity, and the development of disability among older persons.JAMA.2004;292:2115–2124.
- ,,,.Cognitively impaired older adults: From hospital to home.Am J Nurs.2005;105:52–61.
- .Prevention of delirium in hospitalized older patients: risk factors and targeted intervention strategies.Ann Med.2000;32:257–263.
- ,,, et al.Iatrogenic complications in high‐risk, elderly patients.Arch Intern Med.1992;152:2074–2080.
- ,,,,.The hospital elder life program: A model of care to prevent cognitive and functional decline in older hospitalized patients.J Am Geriatr Soc.2000;48:1697–1706.
- ,.Incidence and types of preventable adverse events in elderly patients: population based review of medical records.BMJ.2000;320:741–744.
- ,Landefeld S: Improving care for hospitalized elders.Ann Long Term Care: Clin Care Aging.2001;9:35–40.
- :Acute hospital care of the elderly: minimizing the risk of functional decline.Cleve Clin J Med.1995;62:117–128.
- ,,,,.Studies of illness in the aged: the index of ADL: a standardized measure of biological and psychosocial function.JAMA.1963;185:914–919.
- ,,, et al.The Vulnerable Elders Survey: a tool for identifying vulnerable older people in the community.J Am Geriatr Soc.2001;49:1691–1699.
- ,,,,,.Validation of a short orientation memory‐concentration test of cognitive impairment.Am J Psychiatry.1983;140:734–739.
- ,,.Clock completion: an objective screening test for dementia.J Am Geriatr Soc.1993;41:1235–1240.
- .Geriatric depression scale.Psychopharmacol Bull.1988;24:709–711.
- ,,,,,.Updating the Beers Criteria for potentially inappropriate medication use in older adults.Arch Intern Med.2003;163:2716–2724.
- ,,.Prevalence and outcomes of low mobility in hospitalized older patients.J Am Geriatr Soc.2004;52:1263–1270.
- ,,, et al.The NIH cognitive and emotional health project: Report of the critical evaluation study committee.Alzheimers Dement.2006;2:12–32.
- ,,,.Can clock drawing test help to differentiate between dementia of the Alzheimer's type and vascular dementia? A preliminary study.Int J Geriatr Psychiatry.2002;17:699–703.
- ,,, et al.Geriatric‐based versus general wards for older acute medical patients: a randomized comparison of outcomes and use of resources.J Am Geriatr Soc.2000;48:1381–1388.
- ,,,,,.Geriatric syndromes in elderly patients admitted to an oncology‐acute care for elders unit.J Clin Oncol.2006;24:2298–2303.
- ,,,.Overuse of the indwelling urinary tract catheter in hospitalized medical patients.Arch Intern Med.1995;155:1425–1429.
- ,,, et al.The relationship of indwelling urinary catheters to death, length of hospital stay, functional decline, and nursing home admission in hospitalized older medical patients.J Am Geriatr Soc.2007;55:227–233.
- ,,,.A strategy to decrease the use of risky drugs in the elderly.Cleve Clin J Med.2004;71:561–568.
- ,,,,,.A multifactorial intervention to reduce prevalence of delirium and shorten hospital length of stay.J Am Geriatr Soc.2005;53:18–23.
- ,,,,.A randomized trial of care in a hospital medical unit especially designed to improve the functional outcomes of acutely ill older patients.N Engl J Med.1995;332:1338–1344.
- ,,, et al.A controlled trial of inpatient and outpatient geriatric evaluation and management.N Engl J Med.2002;346:905–912.
- ,,, et al.Effects of a multicomponent intervention on functional outcomes and process of care in hospitalized older patients: A randomized controlled trial of Acute Care for Elders (ACE) in a community hospital.J Am Geriatr Soc.2000;48:1572–1581.
- ,,,,,.A model for managing delirious older inpatients.J Am Geriatr Soc.2003;51:1031–1035.
- ,,,,.Reduced mortality in treating acutely sick, frail older patients in a geriatric evaluation and management unit. A prospective randomized trial.J Am Geriatr Soc.2002;50:792–798.
- ,.The expanding role of hospitalists in the United States.Swiss Med Wkly.2006;136:591–596.
- American Association of Retired Persons and the Administration on Aging.A Profile of Older Americans: 1999.Washington, DC:American Association of Retired Persons;1999.
- Federal Interagency Forum on Aging Related Statistics. Older Americans 2004: key indicators of well‐being. Available at: http://www.agingstats.gov
- ,,,.Hospitalization, restricted activity, and the development of disability among older persons.JAMA.2004;292:2115–2124.
- ,,,.Cognitively impaired older adults: From hospital to home.Am J Nurs.2005;105:52–61.
- .Prevention of delirium in hospitalized older patients: risk factors and targeted intervention strategies.Ann Med.2000;32:257–263.
- ,,, et al.Iatrogenic complications in high‐risk, elderly patients.Arch Intern Med.1992;152:2074–2080.
- ,,,,.The hospital elder life program: A model of care to prevent cognitive and functional decline in older hospitalized patients.J Am Geriatr Soc.2000;48:1697–1706.
- ,.Incidence and types of preventable adverse events in elderly patients: population based review of medical records.BMJ.2000;320:741–744.
- ,Landefeld S: Improving care for hospitalized elders.Ann Long Term Care: Clin Care Aging.2001;9:35–40.
- :Acute hospital care of the elderly: minimizing the risk of functional decline.Cleve Clin J Med.1995;62:117–128.
- ,,,,.Studies of illness in the aged: the index of ADL: a standardized measure of biological and psychosocial function.JAMA.1963;185:914–919.
- ,,, et al.The Vulnerable Elders Survey: a tool for identifying vulnerable older people in the community.J Am Geriatr Soc.2001;49:1691–1699.
- ,,,,,.Validation of a short orientation memory‐concentration test of cognitive impairment.Am J Psychiatry.1983;140:734–739.
- ,,.Clock completion: an objective screening test for dementia.J Am Geriatr Soc.1993;41:1235–1240.
- .Geriatric depression scale.Psychopharmacol Bull.1988;24:709–711.
- ,,,,,.Updating the Beers Criteria for potentially inappropriate medication use in older adults.Arch Intern Med.2003;163:2716–2724.
- ,,.Prevalence and outcomes of low mobility in hospitalized older patients.J Am Geriatr Soc.2004;52:1263–1270.
- ,,, et al.The NIH cognitive and emotional health project: Report of the critical evaluation study committee.Alzheimers Dement.2006;2:12–32.
- ,,,.Can clock drawing test help to differentiate between dementia of the Alzheimer's type and vascular dementia? A preliminary study.Int J Geriatr Psychiatry.2002;17:699–703.
- ,,, et al.Geriatric‐based versus general wards for older acute medical patients: a randomized comparison of outcomes and use of resources.J Am Geriatr Soc.2000;48:1381–1388.
- ,,,,,.Geriatric syndromes in elderly patients admitted to an oncology‐acute care for elders unit.J Clin Oncol.2006;24:2298–2303.
- ,,,.Overuse of the indwelling urinary tract catheter in hospitalized medical patients.Arch Intern Med.1995;155:1425–1429.
- ,,, et al.The relationship of indwelling urinary catheters to death, length of hospital stay, functional decline, and nursing home admission in hospitalized older medical patients.J Am Geriatr Soc.2007;55:227–233.
- ,,,.A strategy to decrease the use of risky drugs in the elderly.Cleve Clin J Med.2004;71:561–568.
- ,,,,,.A multifactorial intervention to reduce prevalence of delirium and shorten hospital length of stay.J Am Geriatr Soc.2005;53:18–23.
- ,,,,.A randomized trial of care in a hospital medical unit especially designed to improve the functional outcomes of acutely ill older patients.N Engl J Med.1995;332:1338–1344.
- ,,, et al.A controlled trial of inpatient and outpatient geriatric evaluation and management.N Engl J Med.2002;346:905–912.
- ,,, et al.Effects of a multicomponent intervention on functional outcomes and process of care in hospitalized older patients: A randomized controlled trial of Acute Care for Elders (ACE) in a community hospital.J Am Geriatr Soc.2000;48:1572–1581.
- ,,,,,.A model for managing delirious older inpatients.J Am Geriatr Soc.2003;51:1031–1035.
- ,,,,.Reduced mortality in treating acutely sick, frail older patients in a geriatric evaluation and management unit. A prospective randomized trial.J Am Geriatr Soc.2002;50:792–798.
Copyright © 2007 Society of Hospital Medicine