User login
The Johns Hopkins VTE Collaborative
Venous thromboembolism (VTE), which encompasses deep venous thrombosis and pulmonary embolism, is an important cause of preventable morbidity and mortality.[1] Each year it is estimated as many as 600,000 American's suffer VTE and as many as 100,000 die.[2] Consequently, patient safety and healthcare quality, accrediting organizations such as The Joint Commission, and federal agencies such as the Centers for Disease Control and Prevention and Agency for Healthcare Research and Quality (AHRQ) have made VTE prevention a priority.[3, 4, 5]
Despite widespread recognition that VTE prophylaxis is an important patient safety measure, poor performance is common. The ENDORSE (Epidemiologic International Day for the Evaluation of Patients at Risk for Venous Thromboembolism in the Acute Hospital Care Setting) study of over 68,000 hospitalized patients in 32 countries noted only 58.5% of surgical patients and 39.5% medical patients received American College of Chest Physicians (ACCP) guideline‐appropriate VTE prophylaxis.[6] In 2005, an audit of the surgical services at The Johns Hopkins Hospital found that only 33% of 322 randomly selected patients were prescribed prophylaxis consistent with the ACCP guidelines.
Achieving defect‐free VTE prevention requires attention to each step in the process: (1) assessment of both VTE and bleeding risk, (2) prescription of risk‐appropriate VTE prophylaxis, and (3) administration of risk‐appropriate VTE prophylaxis. In 2005, to improve our VTE prevention performance at Johns Hopkins Hospital, the Center for Innovations organized a VTE Collaborative of 2 physicians, 1 nurse, and 1 pharmacist dedicated to VTE quality improvement. Since then, the group has grown dramatically, adding a clinical informatics expert and numerous other members and coming under the auspices of The Armstrong Institute for Patient Safety. Recognizing that many, though not all, VTEs are potentially preventable,[7, 8] the mission of the Johns Hopkins VTE Collaborative is to ensure that all hospitalized patients receive risk‐appropriate, best‐practice VTE prophylaxis. This article chronicles the innovative strategies that the Johns Hopkins VTE Collaborative has employed over the past decade to improve our hospital's performance in VTE prevention (Table 1).
|
| Strategies to improve VTE prophylaxis ordering |
| Paper‐based patient risk assessment forms (before computer order entry) |
| Mandatory evidence‐based specialty‐specific computer clinical decision support smart order sets |
| Group data and competitions |
| 1‐on‐1 provider feedback |
| Pay for performance |
| Individualized feedback with resident scorecards |
| Strategies to improve VTE prophylaxis administration |
| Identification of missed doses as a major contributor to preventable VTE |
| Identification of physician, nurse and patient contributors to missed doses |
| Collaboration with patients to create patient‐centered educational materials |
| Novel web‐based module for nursing education |
| Real‐time missed doses alert |
| Targeted 1‐on‐1 patient education |
ENSURING EVERY PATIENT IS PRESCRIBED RISK‐APPROPRIATE PROPHYLAXIS
With the support of hospital leadership, the VTE Collaborative held a series of events in 2005 with medical and surgical providers to review the current evidence supporting VTE prophylaxis and achieve consensus on appropriate practice based upon the 2004 ACCP VTE Prophylaxis Guideline. The result was the development of 5 evidence‐based, paper VTE prophylaxis order sets that guided the ordering provider on the assessment of VTE and bleeding risk and facilitated the selection of risk‐appropriate VTE prophylaxis. Because there were no validated VTE or bleeding risk assessment tools at the time we developed our order sets, we used specialty‐specific VTE risk factors derived from the 2004 ACCP Guideline. To identify patients inappropriate for pharmacologic prophylaxis, we used exclusion criteria derived from contemporary randomized clinical trials of pharmacologic prophylaxis in the target populations (ie, active bleeding, abnormal activated partial thromboplastin time not due to a lupus inhibitor) or mutually agreed upon thresholds after discussion with individual provider groups (platelet count <50,000/L). On the Johns Hopkins Hospital inpatient acute rehabilitation unit, introduction of the paper order sets increased adherence with ACCP guidelines from 27% to 98% (P < 0.0001) and reduced symptomatic VTE from 49 per 1000 admissions to 8 per 1000 admissions (P = 0.0001).[9] This study demonstrated that paper order sets used consistently by a dedicated group of providers can result in sustained improvements in practice. Paper order sets remain a low‐tech, easy‐to‐implement strategy that can be applied in any healthcare setting. Other services also saw improvements in risk‐appropriate prophylaxis prescription. In a follow‐up cross‐sectional analysis of the surgical services at Johns Hopkins, we found that appropriate VTE prophylaxis prescription improved from 33% to 62% in a sample of 226 patients. Unfortunately, paper order sets had several disadvantages including (1) the inherent difficulty of making them a mandatory part of the admission or transfer process, (2) their existence outside the usual clinical workflow, and (3) the labor‐ and time‐intensive data collection that made it difficult to provide credible, timely performance reports to providers and leadership.
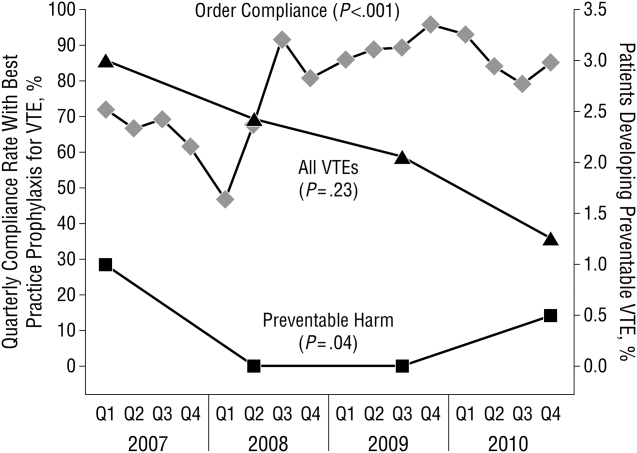
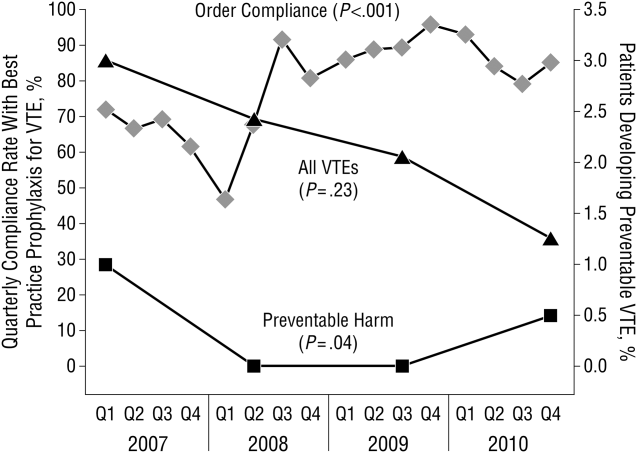
These disadvantages and our adoption of a computerized provider order entry system prompted us to pursue the development and implementation of mandatory, evidence‐based, specialty‐specific computerized clinical decision support (CCDS) VTE prophylaxis order sets. Using the Translating Research Into Practice approach to quality improvement,[10] we collaborated with providers to design 16 different evidence‐based specialty‐specific CCDS VTE order sets. These CCDS VTE order sets, which are imbedded in the specialty‐specific admission and transfer order sets, assist providers in assessing patients' VTE and bleeding risk factors and provide evidence‐based risk‐appropriate VTE prophylaxis (see Supporting Figure 1 in the online version of this article). Individual patient data are saved in an administrative database and can be easily aggregated for research analyses and quality improvement/performance reporting. A detailed discussion of our strategy for change is discussed in Streiff et al.[11] Because pharmacologic prophylaxis is not appropriate for every patient, and not all VTE are preventable, even with perfect prophylaxis, the goals of our collaborative are to ensure that every patient is ordered VTE prophylaxis consistent with their risk profile (risk‐appropriate prophylaxis) and to eliminate preventable episodes of VTE (VTE that occurs in the setting of suboptimal prophylaxis). In a prepost quasi‐experimental study of 1599 trauma patients, the CCDS VTE order set increased risk‐appropriate prophylaxis prescription from 66.2% to 84.4% (P < .001) and reduced the incidence of potentially preventable harm from VTE from 1% to 0.17% (P = 0.04) (Figure 1).[12] On the medical service, the CCDS VTE order set improved risk‐appropriate VTE prophylaxis prescription from 65.6% to 90.1% (P < 0.0001) and reduced the incidence of potentially preventable harm attributable to VTE from 1.1% to 0% (P = 0.001). There was no increase in major bleeding (International Society of Thrombosis and Hemostasis definition: hemoglobin decline of 2 grams/dL or transfusion of 2 or more units of blood or bleeding into a critical organ such as brain, gastrointestinal tract, or eye) postorder set implementation (0.3% vs 0.1%, P = 0.625) or all‐cause mortality (1.3% vs 2.0%, P = 0.285).[13]
These order sets demonstrated that CCDS tools can lead to significant improvements in prescribing practices and reductions in preventable harm from VTE without increasing the risk of major bleeding complications. In addition to improving the quality of care, the order sets also improved the consistency of care. In a retrospective analysis, we found that implementation of CCDS VTE order sets eliminated racial disparities in prescribing practices. In the preimplementation group, risk‐appropriate VTE prophylaxis was prescribed for 70.1% of black patients and 56.6% of white patients on the trauma service (P = 0.025) and 69.5% of black patients and 61.7% of white patients on the medical service (P = 0.015). After implementation of the CCDS VTE order sets, care improved for all patients such that the previously observed disparities were eliminated (trauma service 84.5% vs 85.5%, P = 0.99 and medical service 91.8% vs 88.0%, P = 0.082).[14] These data indicate that standardizing care can potentially eliminate disparities in clinical practice.
Although implementation of mandatory evidence‐based, specialty‐specific CDSS VTE order sets led to substantial improvements in VTE prophylaxis ordering, high performance was not uniform across our institution. On the medical service, substantial disparities in adherence to order set recommendations existed. On the housestaff services, over 90% of patients consistently received risk‐appropriate VTE prophylaxis compared with only 85% on the hospitalist service. Examination of individual provider performance found that some providers only ordered risk‐appropriate prophylaxis 50% of the time, whereas others were doing so 98% of the time. To address this disparity, we conducted a retrospective analysis of a prospective performance improvement project conducted on the Johns Hopkins Hospitalist service studying the impact of individualized hospitalist attending feedback on VTE prevention practices. During the preintervention period (January 2009December 2010), guideline‐adherent VTE prophylaxis was ordered for 86% (95% confidence interval [CI]: 85%‐88%) of patients. Six months after initiation of direct face‐to‐face provider feedback (January 2011June 2011), guideline‐adherent VTE prophylaxis rates rose to 90% (95% CI: 88‐93). Subsequently (July 2011December 2012), a pay‐for‐performance (P4P) initiative was added to direct face‐to‐face provider feedback. During the P4P initiative, provider incentive per relative value unit (RVU) was progressively increased with increasing performance on provision of risk‐appropriate VTE prophylaxis (adherence <80% = no bonus to $0.50 per RVU for adherence 95%). During this period, prescription of guideline‐adherent prophylaxis rose to 94% (95% CI: 93%‐96%).[15] These initiatives transformed the hospitalist unit from a consistently low‐performance unit to a high‐performance unit.
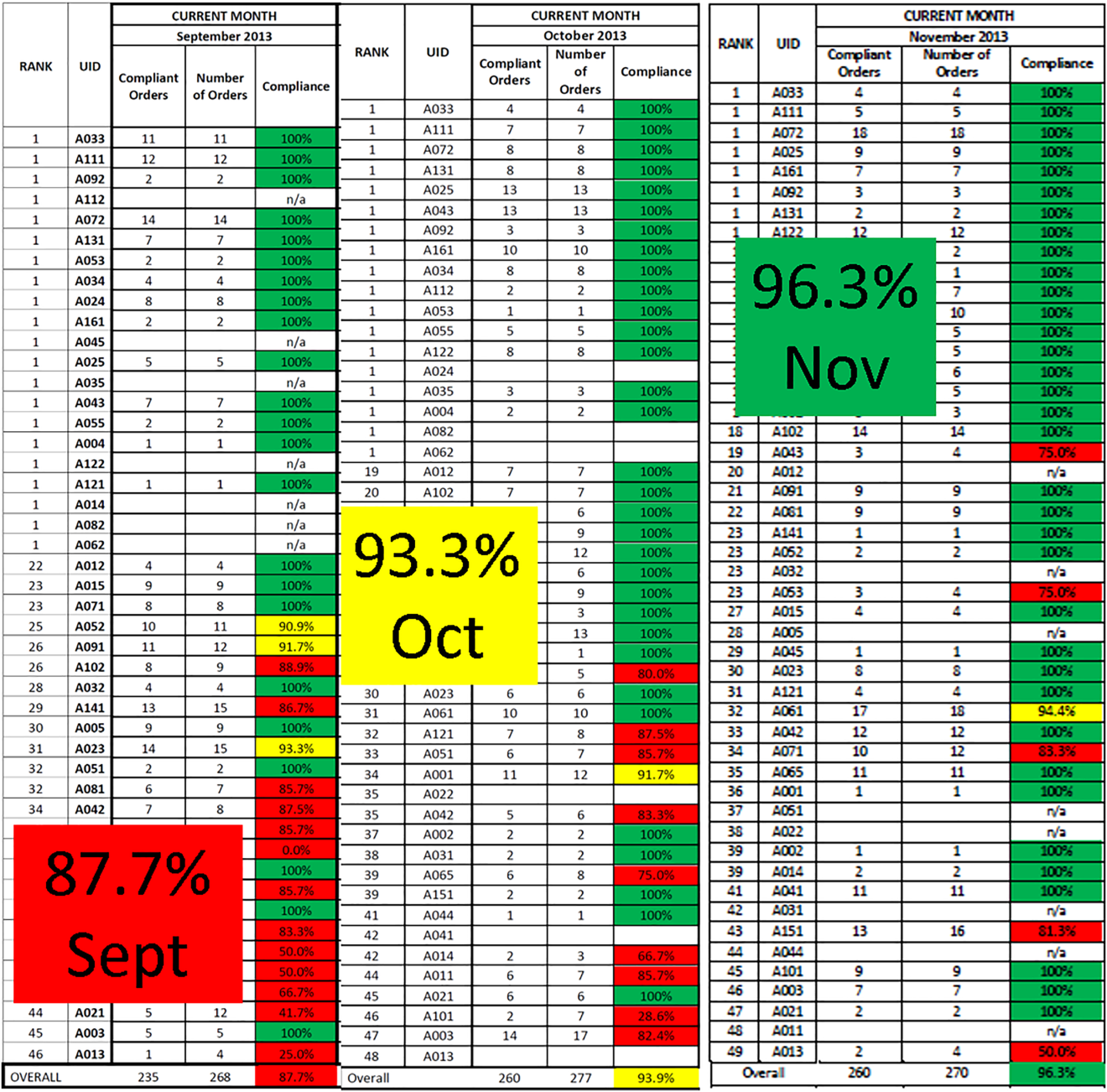
Similar findings were noted on the trauma service. Although the original plan was to provide feedback to attending trauma surgeons, that plan changed when we found that performance was driven entirely by resident practice; residents write the VTE prophylaxis orders, which is then attributed to attending performance. Resident performance varied widely; 42 of 75 (56%) residents on the trauma service ordered risk‐appropriate prophylaxis for 100% of their patients. In contrast, 7 (9.3%) residents never ordered optimal prophylaxis for any of their patients.[16] To motivate all residents to prescribe optimal prophylaxis, we developed an individualized resident VTE prophylaxis scorecard (Figure 2). This prospective cohort study of 2420 patients and 49 general surgery residents compared resident VTE prophylaxis performance on the general surgery service during 3 periods: period 1 (baseline, July 2013September 2013), period 2 (surgery resident scorecard, October 2013December 2013), period 3 (resident scorecard plus individualized 1‐on‐1 coaching, January 2014March 2014). At baseline, 89.4% of patients were prescribed appropriate VTE prophylaxis, and only 45% of residents prescribed risk‐appropriate prophylaxis for all their patients. During the scorecard period, 95.4% of patients were prescribed risk‐appropriate VTE prophylaxis (P < 0.001). During the scorecard plus coaching period, risk‐appropriate prophylaxis rose to 96.4%. These prescribing practice changes were durable. During the 15 months prior to issuing scorecards, 88.0% of patients (3718/4226) were prescribed risk‐appropriate prophylaxis. After implementation, 95.8% of patients (3799/3966) were prescribed risk‐appropriate prophylaxis (P < 0.001) (see Supporting Figure 2 in the online version of this article). During the baseline period, 7 of 865 patients (0.81%) had a VTE during their hospital stay, of which 3 (0.35%) were potentially preventable. In contrast, none of the 3 of 784 patients who suffered VTE during the postimplementation period had a potentially preventable event (0.35% vs 0%, P = 0.046).[17] These studies demonstrate that providing physicians with their own specific data can be a powerful tool for performance improvement that may be applicable to many other quality and safety measures. Our group recently received funding from the AHRQ to scale this work to other residents, nurse practitioners, physician assistants, and attending physicians (1R01HS024547, Individualized Performance Feedback on Venous Thromboembolism Prevention Practice).
IMPROVING VTE PROPHYLAXIS ADMINISTRATION
Ordering VTE prophylaxis does not ensure its administration. We conducted a retrospective review of electronic administration records of 10,526 consecutive patients admitted over a 7‐month period at The Johns Hopkins Hospital. Twelve percent of the over 100,000 ordered doses of VTE prophylaxis were not administered, and the proportion of nonadministered doses on individual floors varied 5‐fold from 5.4% to 26.9%. The proportion of nonadministered doses was significantly higher on medical floors compared with all other services (17.5% vs 8.1%, odds ratio [OR]: 2.1 [95% CI: 2.0‐2.2]). Patient or family member refusal was the most common cause for nonadministered doses of VTE prophylaxis accounting for 59% of all missed doses. Eight percent of patients missed more than half their prescribed doses, and 5% of patients missed over 75% of ordered doses of VTE prophylaxis. Consistent with the Pareto principle, over 80% of the missed doses of prophylaxis were accounted for by just 20% of the patients.[18] A retrospective analysis of hospital‐acquired VTE at Johns Hopkins found that 39% of events occurred in patients who missed 1 or more doses of appropriate VTE prophylaxis.[19] Louis et al. noted that nonadministration of 1 dose of VTE prophylaxis was associated with a significant increase in risk for hospital acquired VTE.[20] These data indicate the need for more aggressive interventions to reduce missed doses to improve VTE prevention.
To fully understand the root causes of VTE prophylaxis non‐administration, we conducted a series of studies examining each of the participants in the VTE prevention care pathway, physicians, nurses, and patients. In a survey of 122 resident physicians, we found significant differences in clinical practice between medicine and surgery residents. Medicine residents were more likely to believe that VTE prophylaxis was overprescribed, and that it was appropriate for nurses to make judgement calls about whether patients needed the prophylaxis that was prescribed.[21] In a mixed methods study that included a written survey and qualitative observations of nursing practice, we found that some nurses presented pharmacologic VTE prophylaxis injections as optional to patients. Furthermore, nurses on units where nonadministration was higher were more likely to believe that VTE prophylaxis was prescribed for patients unnecessarily, and that they could use their clinical judgement to determine when it was appropriate to omit doses of pharmacologic prophylaxis.[22] Our team also examined patient preferences in regard to VTE prophylaxis. In a survey of 227 consecutive medical and surgical inpatients, we found that 60% of patients would prefer an oral route of administration if available. Patients with a preference for a parenteral route of administration (27.5%) were less likely to refuse prophylaxis (37.5% vs 51.3%, P < 0.0001).[23] These findings underscore the fact that unit culture, nursing attitudes and beliefs, and patient preferences have an important influence on medication administration, and that nursepatient communication is an important target for modifying adherence.
PATIENT‐CENTERED APPROACHES TO IMPROVE VTE PROPHYLAXIS ADMINISTRATION
To address nurse‐ and patient‐related factors that influence VTE prophylaxis administration, we applied for and received a Patient Centered Outcomes Research Institute contract to develop patient‐centered interventions to engage and empower patients to take an active role in their preventive care. To achieve these aims, we partnered with 3 national patient advocacy organizations, the National Blood Clot Alliance, the North American Thrombosis Forum, and ClotCare, as well as our local Johns Hopkins Patient and Family Advisory Council. Using a modified Delphi method, we engaged patient stakeholders from the 4 collaborating organizations to build consensus on patient‐centered VTE education methods. Input from this Delphi assessment was used to build educational materials including paper brochures published in 8 different languages and a 10‐minute educational video filmed by an Oscar‐winning documentary director featuring both clinicians and patients relating their VTE experience and the importance of VTE prevention.[24] These educational materials are available for public use (
ENGAGING TRAINEES IN MULTIDISCIPLINARY PATIENT SAFETY/QUALITY IMPROVEMENT INITIATIVES
Trainees from many healthcare‐related disciplines have played a critical role in our quest to improve VTE prevention. Over the past 10 years, we have mentored countless medical students, public health graduate students, nursing students, residents, and postdoctoral fellows in research projects that have resulted in numerous high‐quality publications. Trainees have helped to dispel staff concerns about patient falls in connection of intermittent pneumatic compression devices,[25] identify the weaknesses of current publicly reported VTE measures,[26, 27, 28, 29] identify opportunities to improve VTE prevention practices within clinical specialties,[30, 31, 32] define the role of surveillance bias in VTE outcomes reporting,[33, 34, 35] discover and fully explore the important problem of missed doses of VTE prophylaxis,[18, 21, 22, 23, 36] and summarize knowledge about VTE prevention via systematic reviews and meta‐analyses.[37, 38, 39] These collaborations have been a classic win‐win. The mentees learn critical skills while growing their curriculum vitae with contributions to the literature, allowing them to progress in their careers (ie, obtain a residency match, faculty positions). The faculty have leveraged this work to obtain over $3 million in extramural funding to develop interventions to study and improve the quality of VTE preventive care for hospitalized patients.
In healthcare, we have not yet achieved defect‐free VTE prevention; however, we have a better understanding of the path to accomplishing this goal. In this article we describe our goal of zero harm from VTE and our learning journey to realize that goal. Although the journey never ends, a critical ingredient to the success of our program has been the multidisciplinary nature of our VTE collaborative team. The combination of expertise from medicine, surgery, nursing, pharmacy, clinical informatics, and public health has facilitated the development of innovative strategies to improve VTE prevention that integrate seamlessly into clinical workflow. The approach used for VTE can be applied to eliminate other types of harms.
Disclosures
Mr. Lau, Dr. Streiff, and Dr. Haut are supported by a grant from the Agency for Healthcare Research and Quality (1R01HS024547) titled Individualized Performance Feedback on Venous Thromboembolism Prevention Practice and a contract from the Patient‐Centered Outcomes Research Institute titled Preventing Venous Thromboembolism: Empowering Patients and Enabling Patient‐Centered Care via Health Information Technology (CE‐12‐11‐4489). Mr. Lau is supported by the Institute for Excellence in Education Berkheimer Faculty Education Scholar Grant and a contract (AD‐1306‐03980) from the Patient‐Centered Outcomes Research Institute titled Patient Centered Approaches to Collect Sexual Orientation/Gender Identity Information in the Emergency Department. Ms. Hobson has given expert witness testimony in various medical malpractice cases. Dr. Streiff has received research funding from Portola and Janssen; consulted for Bio2Medical, CSL Behring, Merck, and Janssen HealthCare; and has given expert witness testimony in various medical malpractice cases. Dr. Haut receives royalties from Lippincott, Williams, and Wilkins for a book titled Avoiding Common ICU Errors. Dr. Haut is a paid consultant and speaker for the Preventing Avoidable Venous ThromboembolismEvery Patient, Every Time VHA/Vizient IMPERATIV Advantage Performance Improvement Collaborative. Dr. Haut is a paid consultant and speaker for the Illinois Surgical Quality Improvement Collaborative. All other authors report no disclosures.
- , . Thromboprophylaxis in nonsurgical patients. Hematology Am Soc Hematol Educ Program. 2012;2012:631–637.
- Office of the Surgeon General (US); National Heart, Lung, and Blood Institute (US). The Surgeon General's Call to Action to Prevent Deep Vein Thrombosis and Pulmonary Embolism. Rockville, MD: Office of the Surgeon General; 2008.
- , . Prevention of venous thromboembolism: brief update review. In: Making Health Care Safer II: An Updated Critical Analysis of the Evidence for Patient Safety Practices. Rockville, MD: Agency for Healthcare Research and Quality; 2013.
- , , , et al. The top patient safety strategies that can be encouraged for adoption now. Ann Intern Med. 2013;158:365–368.
- , . Practices to prevent venous thromboembolism: a brief review. BMJ Qual Saf. 2014;23:187–195.
- , , , et al. Venous thromboembolism risk and prophylaxis in the acute hospital care setting (ENDORSE study): a multinational cross‐sectional study. Lancet. 2008;371:387–394.
- , . The CMS ruling on venous thromboembolism after total knee or hip arthroplasty: weighing risks and benefits. JAMA. 2009;301:1063–1065.
- , , , et al. ICD‐9 code‐based venous thromboembolism performance targets fail to measure up. Am J Med Qual. 2016;31(5):448–453.
- , , , , . Evidence‐based venous thromboembolism prophylaxis is associated with a six‐fold decrease in numbers of symptomatic venous thromboembolisms in rehabilitation inpatients. PM R. 2011;3:1111–1115.e1.
- , , . Translating evidence into practice: a model for large scale knowledge translation. BMJ. 2008;337:a1714.
- , , , et al. Lessons from the Johns Hopkins Multi‐Disciplinary Venous Thromboembolism (VTE) Prevention Collaborative. BMJ. 2012;344:e3935.
- , , , et al. Improved prophylaxis and decreased rates of preventable harm with the use of a mandatory computerized clinical decision support tool for prophylaxis for venous thromboembolism in trauma. Arch Surg. 2012;147:901–907.
- , , , et al. Impact of a venous thromboembolism prophylaxis “smart order set”: improved compliance, fewer events. Am J Hematol. 2013;88(7):545–549.
- , , , et al. Eliminating health care disparities with mandatory clinical decision support: the venous thromboembolism (VTE) example. Med Care. 2015;53:18–24.
- , , , et al. Use of provider‐level dashboards and pay‐for‐performance in venous thromboembolism prophylaxis. J Hosp Med. 2015;10:172–178.
- , , , , , . Attending physician performance measure scores and resident physicians' ordering practices. JAMA Surg. 2015;150:813–814.
- , , , et al. Individualized performance feedback to surgical residents improves appropriate venous thromboembolism prophylaxis prescription and reduces potentially preventable VTE: a prospective cohort study [published online November 25, 2015]. Ann Surg. doi: 10.1097/SLA.0000000000001512.
- , , , et al. Patterns of non‐administration of ordered doses of venous thromboembolism prophylaxis: implications for novel intervention strategies. PLoS One. 2013;8:e66311.
- , , , et al. Preventability of hospital‐acquired venous thromboembolism. JAMA Surg. 2015;150(9):912–915.
- , , , et al. Correlation of missed doses of enoxaparin with increased incidence of deep vein thrombosis in trauma and general surgery patients. JAMA Surg. 2014;149:365–370.
- , , , et al. Prescriber knowledge and attitudes regarding non‐administration of prescribed pharmacologic venous thromboembolism prophylaxis [published online May 21, 2016]. J Thromb Thrombolysis. doi:10.1007/s11239-016-1378-8.
- , , , et al. Hidden barriers to delivery of pharmacological venous thromboembolism prophylaxis: the role of nursing beliefs and practices. J Patient Saf. 2016;12:63–68.
- , , , et al. Patient preferences regarding pharmacologic venous thromboembolism prophylaxis. J Hosp Med. 2015;10:108–111.
- , , , et al. Patient preferences for receiving education on venous thromboembolism prevention—a survey of stakeholder organizations. PLoS One. 2016;11:e0152084.
- , , , , , . Are sequential compression devices commonly associated with in‐hospital falls? A myth‐busters review using the patient safety net database. J Patient Saf. 2011;7:77–79.
- , , , , . No association between hospital‐reported perioperative venous thromboembolism prophylaxis and outcome rates in publicly reported data. JAMA Surg. 2014;149:400–401.
- , , , , . Linking processes and outcomes: a key strategy to prevent and report harm from venous thromboembolism in surgical patients. JAMA Surg. 2013;148:299–300.
- , , , et al. Hazards of benchmarking complications with the National Trauma Data Bank: numerators in search of denominators. J Trauma. 2008;64:273–277; discussion 277–279.
- , , , et al. Is the meaningful use venous thromboembolism VTE‐6 measure meaningful? A retrospective analysis of one hospital's VTE‐6 cases. Jt Comm J Qual Patient Saf. 2016;42(9):410–416.
- , , , et al. Is venous thromboembolism in colorectal surgery patients preventable or inevitable? One institution's experience. J Am Coll Surg. 2013;216:395–401.e1.
- , , , et al. Venous thromboembolic prophylaxis after a hepatic resection: patterns of care among liver surgeons. HPB (Oxford). 2014;16:892–898.
- , , , et al. Defining incidence and risk factors of venous thromboembolism after hepatectomy. J Gastrointest Surg. 2014;18:1116–1124.
- , , , et al. Can increased incidence of deep vein thrombosis (DVT) be used as a marker of quality of care in the absence of standardized screening? The potential effect of surveillance bias on reported DVT rates after trauma. J Trauma. 2007;63:1132–1135; discussion 1135–1137.
- , . Surveillance bias in outcomes reporting. JAMA. 2011;305:2462–2463.
- , , , et al. Surveillance bias and deep vein thrombosis in the national trauma data bank: the more we look, the more we find. J Trauma. 2008;64:932–936; discussion 936–937.
- , , , et al. Nonadministration of thromboprophylaxis in hospitalized patients with HIV: a missed opportunity for prevention? J Hosp Med. 2014;9:215–220.
- , , , et al. Pharmacologic and mechanical prophylaxis of venous thromboembolism among special populations. Comparative effectiveness review No. 116. Prepared by the Johns Hopkins University Evidence‐based Practice Center under Contract No. 290‐2007‐10061‐I.) AHRQ Publication No. 13‐EHC082–1. Rockville, MD: Agency for Healthcare Research and Quality; 2013.
- , , , et al. Pharmacologic and mechanical strategies for preventing venous thromboembolism after bariatric surgery: a systematic review and meta‐analysis. JAMA Surg. 2013;148:675–686.
- , , , et al. The effectiveness of prophylactic inferior vena cava filters in trauma patients: a systematic review and meta‐analysis. JAMA Surg. 2014;149:194–202.
Venous thromboembolism (VTE), which encompasses deep venous thrombosis and pulmonary embolism, is an important cause of preventable morbidity and mortality.[1] Each year it is estimated as many as 600,000 American's suffer VTE and as many as 100,000 die.[2] Consequently, patient safety and healthcare quality, accrediting organizations such as The Joint Commission, and federal agencies such as the Centers for Disease Control and Prevention and Agency for Healthcare Research and Quality (AHRQ) have made VTE prevention a priority.[3, 4, 5]
Despite widespread recognition that VTE prophylaxis is an important patient safety measure, poor performance is common. The ENDORSE (Epidemiologic International Day for the Evaluation of Patients at Risk for Venous Thromboembolism in the Acute Hospital Care Setting) study of over 68,000 hospitalized patients in 32 countries noted only 58.5% of surgical patients and 39.5% medical patients received American College of Chest Physicians (ACCP) guideline‐appropriate VTE prophylaxis.[6] In 2005, an audit of the surgical services at The Johns Hopkins Hospital found that only 33% of 322 randomly selected patients were prescribed prophylaxis consistent with the ACCP guidelines.
Achieving defect‐free VTE prevention requires attention to each step in the process: (1) assessment of both VTE and bleeding risk, (2) prescription of risk‐appropriate VTE prophylaxis, and (3) administration of risk‐appropriate VTE prophylaxis. In 2005, to improve our VTE prevention performance at Johns Hopkins Hospital, the Center for Innovations organized a VTE Collaborative of 2 physicians, 1 nurse, and 1 pharmacist dedicated to VTE quality improvement. Since then, the group has grown dramatically, adding a clinical informatics expert and numerous other members and coming under the auspices of The Armstrong Institute for Patient Safety. Recognizing that many, though not all, VTEs are potentially preventable,[7, 8] the mission of the Johns Hopkins VTE Collaborative is to ensure that all hospitalized patients receive risk‐appropriate, best‐practice VTE prophylaxis. This article chronicles the innovative strategies that the Johns Hopkins VTE Collaborative has employed over the past decade to improve our hospital's performance in VTE prevention (Table 1).
|
| Strategies to improve VTE prophylaxis ordering |
| Paper‐based patient risk assessment forms (before computer order entry) |
| Mandatory evidence‐based specialty‐specific computer clinical decision support smart order sets |
| Group data and competitions |
| 1‐on‐1 provider feedback |
| Pay for performance |
| Individualized feedback with resident scorecards |
| Strategies to improve VTE prophylaxis administration |
| Identification of missed doses as a major contributor to preventable VTE |
| Identification of physician, nurse and patient contributors to missed doses |
| Collaboration with patients to create patient‐centered educational materials |
| Novel web‐based module for nursing education |
| Real‐time missed doses alert |
| Targeted 1‐on‐1 patient education |
ENSURING EVERY PATIENT IS PRESCRIBED RISK‐APPROPRIATE PROPHYLAXIS
With the support of hospital leadership, the VTE Collaborative held a series of events in 2005 with medical and surgical providers to review the current evidence supporting VTE prophylaxis and achieve consensus on appropriate practice based upon the 2004 ACCP VTE Prophylaxis Guideline. The result was the development of 5 evidence‐based, paper VTE prophylaxis order sets that guided the ordering provider on the assessment of VTE and bleeding risk and facilitated the selection of risk‐appropriate VTE prophylaxis. Because there were no validated VTE or bleeding risk assessment tools at the time we developed our order sets, we used specialty‐specific VTE risk factors derived from the 2004 ACCP Guideline. To identify patients inappropriate for pharmacologic prophylaxis, we used exclusion criteria derived from contemporary randomized clinical trials of pharmacologic prophylaxis in the target populations (ie, active bleeding, abnormal activated partial thromboplastin time not due to a lupus inhibitor) or mutually agreed upon thresholds after discussion with individual provider groups (platelet count <50,000/L). On the Johns Hopkins Hospital inpatient acute rehabilitation unit, introduction of the paper order sets increased adherence with ACCP guidelines from 27% to 98% (P < 0.0001) and reduced symptomatic VTE from 49 per 1000 admissions to 8 per 1000 admissions (P = 0.0001).[9] This study demonstrated that paper order sets used consistently by a dedicated group of providers can result in sustained improvements in practice. Paper order sets remain a low‐tech, easy‐to‐implement strategy that can be applied in any healthcare setting. Other services also saw improvements in risk‐appropriate prophylaxis prescription. In a follow‐up cross‐sectional analysis of the surgical services at Johns Hopkins, we found that appropriate VTE prophylaxis prescription improved from 33% to 62% in a sample of 226 patients. Unfortunately, paper order sets had several disadvantages including (1) the inherent difficulty of making them a mandatory part of the admission or transfer process, (2) their existence outside the usual clinical workflow, and (3) the labor‐ and time‐intensive data collection that made it difficult to provide credible, timely performance reports to providers and leadership.
These disadvantages and our adoption of a computerized provider order entry system prompted us to pursue the development and implementation of mandatory, evidence‐based, specialty‐specific computerized clinical decision support (CCDS) VTE prophylaxis order sets. Using the Translating Research Into Practice approach to quality improvement,[10] we collaborated with providers to design 16 different evidence‐based specialty‐specific CCDS VTE order sets. These CCDS VTE order sets, which are imbedded in the specialty‐specific admission and transfer order sets, assist providers in assessing patients' VTE and bleeding risk factors and provide evidence‐based risk‐appropriate VTE prophylaxis (see Supporting Figure 1 in the online version of this article). Individual patient data are saved in an administrative database and can be easily aggregated for research analyses and quality improvement/performance reporting. A detailed discussion of our strategy for change is discussed in Streiff et al.[11] Because pharmacologic prophylaxis is not appropriate for every patient, and not all VTE are preventable, even with perfect prophylaxis, the goals of our collaborative are to ensure that every patient is ordered VTE prophylaxis consistent with their risk profile (risk‐appropriate prophylaxis) and to eliminate preventable episodes of VTE (VTE that occurs in the setting of suboptimal prophylaxis). In a prepost quasi‐experimental study of 1599 trauma patients, the CCDS VTE order set increased risk‐appropriate prophylaxis prescription from 66.2% to 84.4% (P < .001) and reduced the incidence of potentially preventable harm from VTE from 1% to 0.17% (P = 0.04) (Figure 1).[12] On the medical service, the CCDS VTE order set improved risk‐appropriate VTE prophylaxis prescription from 65.6% to 90.1% (P < 0.0001) and reduced the incidence of potentially preventable harm attributable to VTE from 1.1% to 0% (P = 0.001). There was no increase in major bleeding (International Society of Thrombosis and Hemostasis definition: hemoglobin decline of 2 grams/dL or transfusion of 2 or more units of blood or bleeding into a critical organ such as brain, gastrointestinal tract, or eye) postorder set implementation (0.3% vs 0.1%, P = 0.625) or all‐cause mortality (1.3% vs 2.0%, P = 0.285).[13]

These order sets demonstrated that CCDS tools can lead to significant improvements in prescribing practices and reductions in preventable harm from VTE without increasing the risk of major bleeding complications. In addition to improving the quality of care, the order sets also improved the consistency of care. In a retrospective analysis, we found that implementation of CCDS VTE order sets eliminated racial disparities in prescribing practices. In the preimplementation group, risk‐appropriate VTE prophylaxis was prescribed for 70.1% of black patients and 56.6% of white patients on the trauma service (P = 0.025) and 69.5% of black patients and 61.7% of white patients on the medical service (P = 0.015). After implementation of the CCDS VTE order sets, care improved for all patients such that the previously observed disparities were eliminated (trauma service 84.5% vs 85.5%, P = 0.99 and medical service 91.8% vs 88.0%, P = 0.082).[14] These data indicate that standardizing care can potentially eliminate disparities in clinical practice.
Although implementation of mandatory evidence‐based, specialty‐specific CDSS VTE order sets led to substantial improvements in VTE prophylaxis ordering, high performance was not uniform across our institution. On the medical service, substantial disparities in adherence to order set recommendations existed. On the housestaff services, over 90% of patients consistently received risk‐appropriate VTE prophylaxis compared with only 85% on the hospitalist service. Examination of individual provider performance found that some providers only ordered risk‐appropriate prophylaxis 50% of the time, whereas others were doing so 98% of the time. To address this disparity, we conducted a retrospective analysis of a prospective performance improvement project conducted on the Johns Hopkins Hospitalist service studying the impact of individualized hospitalist attending feedback on VTE prevention practices. During the preintervention period (January 2009December 2010), guideline‐adherent VTE prophylaxis was ordered for 86% (95% confidence interval [CI]: 85%‐88%) of patients. Six months after initiation of direct face‐to‐face provider feedback (January 2011June 2011), guideline‐adherent VTE prophylaxis rates rose to 90% (95% CI: 88‐93). Subsequently (July 2011December 2012), a pay‐for‐performance (P4P) initiative was added to direct face‐to‐face provider feedback. During the P4P initiative, provider incentive per relative value unit (RVU) was progressively increased with increasing performance on provision of risk‐appropriate VTE prophylaxis (adherence <80% = no bonus to $0.50 per RVU for adherence 95%). During this period, prescription of guideline‐adherent prophylaxis rose to 94% (95% CI: 93%‐96%).[15] These initiatives transformed the hospitalist unit from a consistently low‐performance unit to a high‐performance unit.
Similar findings were noted on the trauma service. Although the original plan was to provide feedback to attending trauma surgeons, that plan changed when we found that performance was driven entirely by resident practice; residents write the VTE prophylaxis orders, which is then attributed to attending performance. Resident performance varied widely; 42 of 75 (56%) residents on the trauma service ordered risk‐appropriate prophylaxis for 100% of their patients. In contrast, 7 (9.3%) residents never ordered optimal prophylaxis for any of their patients.[16] To motivate all residents to prescribe optimal prophylaxis, we developed an individualized resident VTE prophylaxis scorecard (Figure 2). This prospective cohort study of 2420 patients and 49 general surgery residents compared resident VTE prophylaxis performance on the general surgery service during 3 periods: period 1 (baseline, July 2013September 2013), period 2 (surgery resident scorecard, October 2013December 2013), period 3 (resident scorecard plus individualized 1‐on‐1 coaching, January 2014March 2014). At baseline, 89.4% of patients were prescribed appropriate VTE prophylaxis, and only 45% of residents prescribed risk‐appropriate prophylaxis for all their patients. During the scorecard period, 95.4% of patients were prescribed risk‐appropriate VTE prophylaxis (P < 0.001). During the scorecard plus coaching period, risk‐appropriate prophylaxis rose to 96.4%. These prescribing practice changes were durable. During the 15 months prior to issuing scorecards, 88.0% of patients (3718/4226) were prescribed risk‐appropriate prophylaxis. After implementation, 95.8% of patients (3799/3966) were prescribed risk‐appropriate prophylaxis (P < 0.001) (see Supporting Figure 2 in the online version of this article). During the baseline period, 7 of 865 patients (0.81%) had a VTE during their hospital stay, of which 3 (0.35%) were potentially preventable. In contrast, none of the 3 of 784 patients who suffered VTE during the postimplementation period had a potentially preventable event (0.35% vs 0%, P = 0.046).[17] These studies demonstrate that providing physicians with their own specific data can be a powerful tool for performance improvement that may be applicable to many other quality and safety measures. Our group recently received funding from the AHRQ to scale this work to other residents, nurse practitioners, physician assistants, and attending physicians (1R01HS024547, Individualized Performance Feedback on Venous Thromboembolism Prevention Practice).

IMPROVING VTE PROPHYLAXIS ADMINISTRATION
Ordering VTE prophylaxis does not ensure its administration. We conducted a retrospective review of electronic administration records of 10,526 consecutive patients admitted over a 7‐month period at The Johns Hopkins Hospital. Twelve percent of the over 100,000 ordered doses of VTE prophylaxis were not administered, and the proportion of nonadministered doses on individual floors varied 5‐fold from 5.4% to 26.9%. The proportion of nonadministered doses was significantly higher on medical floors compared with all other services (17.5% vs 8.1%, odds ratio [OR]: 2.1 [95% CI: 2.0‐2.2]). Patient or family member refusal was the most common cause for nonadministered doses of VTE prophylaxis accounting for 59% of all missed doses. Eight percent of patients missed more than half their prescribed doses, and 5% of patients missed over 75% of ordered doses of VTE prophylaxis. Consistent with the Pareto principle, over 80% of the missed doses of prophylaxis were accounted for by just 20% of the patients.[18] A retrospective analysis of hospital‐acquired VTE at Johns Hopkins found that 39% of events occurred in patients who missed 1 or more doses of appropriate VTE prophylaxis.[19] Louis et al. noted that nonadministration of 1 dose of VTE prophylaxis was associated with a significant increase in risk for hospital acquired VTE.[20] These data indicate the need for more aggressive interventions to reduce missed doses to improve VTE prevention.
To fully understand the root causes of VTE prophylaxis non‐administration, we conducted a series of studies examining each of the participants in the VTE prevention care pathway, physicians, nurses, and patients. In a survey of 122 resident physicians, we found significant differences in clinical practice between medicine and surgery residents. Medicine residents were more likely to believe that VTE prophylaxis was overprescribed, and that it was appropriate for nurses to make judgement calls about whether patients needed the prophylaxis that was prescribed.[21] In a mixed methods study that included a written survey and qualitative observations of nursing practice, we found that some nurses presented pharmacologic VTE prophylaxis injections as optional to patients. Furthermore, nurses on units where nonadministration was higher were more likely to believe that VTE prophylaxis was prescribed for patients unnecessarily, and that they could use their clinical judgement to determine when it was appropriate to omit doses of pharmacologic prophylaxis.[22] Our team also examined patient preferences in regard to VTE prophylaxis. In a survey of 227 consecutive medical and surgical inpatients, we found that 60% of patients would prefer an oral route of administration if available. Patients with a preference for a parenteral route of administration (27.5%) were less likely to refuse prophylaxis (37.5% vs 51.3%, P < 0.0001).[23] These findings underscore the fact that unit culture, nursing attitudes and beliefs, and patient preferences have an important influence on medication administration, and that nursepatient communication is an important target for modifying adherence.
PATIENT‐CENTERED APPROACHES TO IMPROVE VTE PROPHYLAXIS ADMINISTRATION
To address nurse‐ and patient‐related factors that influence VTE prophylaxis administration, we applied for and received a Patient Centered Outcomes Research Institute contract to develop patient‐centered interventions to engage and empower patients to take an active role in their preventive care. To achieve these aims, we partnered with 3 national patient advocacy organizations, the National Blood Clot Alliance, the North American Thrombosis Forum, and ClotCare, as well as our local Johns Hopkins Patient and Family Advisory Council. Using a modified Delphi method, we engaged patient stakeholders from the 4 collaborating organizations to build consensus on patient‐centered VTE education methods. Input from this Delphi assessment was used to build educational materials including paper brochures published in 8 different languages and a 10‐minute educational video filmed by an Oscar‐winning documentary director featuring both clinicians and patients relating their VTE experience and the importance of VTE prevention.[24] These educational materials are available for public use (
ENGAGING TRAINEES IN MULTIDISCIPLINARY PATIENT SAFETY/QUALITY IMPROVEMENT INITIATIVES
Trainees from many healthcare‐related disciplines have played a critical role in our quest to improve VTE prevention. Over the past 10 years, we have mentored countless medical students, public health graduate students, nursing students, residents, and postdoctoral fellows in research projects that have resulted in numerous high‐quality publications. Trainees have helped to dispel staff concerns about patient falls in connection of intermittent pneumatic compression devices,[25] identify the weaknesses of current publicly reported VTE measures,[26, 27, 28, 29] identify opportunities to improve VTE prevention practices within clinical specialties,[30, 31, 32] define the role of surveillance bias in VTE outcomes reporting,[33, 34, 35] discover and fully explore the important problem of missed doses of VTE prophylaxis,[18, 21, 22, 23, 36] and summarize knowledge about VTE prevention via systematic reviews and meta‐analyses.[37, 38, 39] These collaborations have been a classic win‐win. The mentees learn critical skills while growing their curriculum vitae with contributions to the literature, allowing them to progress in their careers (ie, obtain a residency match, faculty positions). The faculty have leveraged this work to obtain over $3 million in extramural funding to develop interventions to study and improve the quality of VTE preventive care for hospitalized patients.
In healthcare, we have not yet achieved defect‐free VTE prevention; however, we have a better understanding of the path to accomplishing this goal. In this article we describe our goal of zero harm from VTE and our learning journey to realize that goal. Although the journey never ends, a critical ingredient to the success of our program has been the multidisciplinary nature of our VTE collaborative team. The combination of expertise from medicine, surgery, nursing, pharmacy, clinical informatics, and public health has facilitated the development of innovative strategies to improve VTE prevention that integrate seamlessly into clinical workflow. The approach used for VTE can be applied to eliminate other types of harms.
Disclosures
Mr. Lau, Dr. Streiff, and Dr. Haut are supported by a grant from the Agency for Healthcare Research and Quality (1R01HS024547) titled Individualized Performance Feedback on Venous Thromboembolism Prevention Practice and a contract from the Patient‐Centered Outcomes Research Institute titled Preventing Venous Thromboembolism: Empowering Patients and Enabling Patient‐Centered Care via Health Information Technology (CE‐12‐11‐4489). Mr. Lau is supported by the Institute for Excellence in Education Berkheimer Faculty Education Scholar Grant and a contract (AD‐1306‐03980) from the Patient‐Centered Outcomes Research Institute titled Patient Centered Approaches to Collect Sexual Orientation/Gender Identity Information in the Emergency Department. Ms. Hobson has given expert witness testimony in various medical malpractice cases. Dr. Streiff has received research funding from Portola and Janssen; consulted for Bio2Medical, CSL Behring, Merck, and Janssen HealthCare; and has given expert witness testimony in various medical malpractice cases. Dr. Haut receives royalties from Lippincott, Williams, and Wilkins for a book titled Avoiding Common ICU Errors. Dr. Haut is a paid consultant and speaker for the Preventing Avoidable Venous ThromboembolismEvery Patient, Every Time VHA/Vizient IMPERATIV Advantage Performance Improvement Collaborative. Dr. Haut is a paid consultant and speaker for the Illinois Surgical Quality Improvement Collaborative. All other authors report no disclosures.
Venous thromboembolism (VTE), which encompasses deep venous thrombosis and pulmonary embolism, is an important cause of preventable morbidity and mortality.[1] Each year it is estimated as many as 600,000 American's suffer VTE and as many as 100,000 die.[2] Consequently, patient safety and healthcare quality, accrediting organizations such as The Joint Commission, and federal agencies such as the Centers for Disease Control and Prevention and Agency for Healthcare Research and Quality (AHRQ) have made VTE prevention a priority.[3, 4, 5]
Despite widespread recognition that VTE prophylaxis is an important patient safety measure, poor performance is common. The ENDORSE (Epidemiologic International Day for the Evaluation of Patients at Risk for Venous Thromboembolism in the Acute Hospital Care Setting) study of over 68,000 hospitalized patients in 32 countries noted only 58.5% of surgical patients and 39.5% medical patients received American College of Chest Physicians (ACCP) guideline‐appropriate VTE prophylaxis.[6] In 2005, an audit of the surgical services at The Johns Hopkins Hospital found that only 33% of 322 randomly selected patients were prescribed prophylaxis consistent with the ACCP guidelines.
Achieving defect‐free VTE prevention requires attention to each step in the process: (1) assessment of both VTE and bleeding risk, (2) prescription of risk‐appropriate VTE prophylaxis, and (3) administration of risk‐appropriate VTE prophylaxis. In 2005, to improve our VTE prevention performance at Johns Hopkins Hospital, the Center for Innovations organized a VTE Collaborative of 2 physicians, 1 nurse, and 1 pharmacist dedicated to VTE quality improvement. Since then, the group has grown dramatically, adding a clinical informatics expert and numerous other members and coming under the auspices of The Armstrong Institute for Patient Safety. Recognizing that many, though not all, VTEs are potentially preventable,[7, 8] the mission of the Johns Hopkins VTE Collaborative is to ensure that all hospitalized patients receive risk‐appropriate, best‐practice VTE prophylaxis. This article chronicles the innovative strategies that the Johns Hopkins VTE Collaborative has employed over the past decade to improve our hospital's performance in VTE prevention (Table 1).
|
| Strategies to improve VTE prophylaxis ordering |
| Paper‐based patient risk assessment forms (before computer order entry) |
| Mandatory evidence‐based specialty‐specific computer clinical decision support smart order sets |
| Group data and competitions |
| 1‐on‐1 provider feedback |
| Pay for performance |
| Individualized feedback with resident scorecards |
| Strategies to improve VTE prophylaxis administration |
| Identification of missed doses as a major contributor to preventable VTE |
| Identification of physician, nurse and patient contributors to missed doses |
| Collaboration with patients to create patient‐centered educational materials |
| Novel web‐based module for nursing education |
| Real‐time missed doses alert |
| Targeted 1‐on‐1 patient education |
ENSURING EVERY PATIENT IS PRESCRIBED RISK‐APPROPRIATE PROPHYLAXIS
With the support of hospital leadership, the VTE Collaborative held a series of events in 2005 with medical and surgical providers to review the current evidence supporting VTE prophylaxis and achieve consensus on appropriate practice based upon the 2004 ACCP VTE Prophylaxis Guideline. The result was the development of 5 evidence‐based, paper VTE prophylaxis order sets that guided the ordering provider on the assessment of VTE and bleeding risk and facilitated the selection of risk‐appropriate VTE prophylaxis. Because there were no validated VTE or bleeding risk assessment tools at the time we developed our order sets, we used specialty‐specific VTE risk factors derived from the 2004 ACCP Guideline. To identify patients inappropriate for pharmacologic prophylaxis, we used exclusion criteria derived from contemporary randomized clinical trials of pharmacologic prophylaxis in the target populations (ie, active bleeding, abnormal activated partial thromboplastin time not due to a lupus inhibitor) or mutually agreed upon thresholds after discussion with individual provider groups (platelet count <50,000/L). On the Johns Hopkins Hospital inpatient acute rehabilitation unit, introduction of the paper order sets increased adherence with ACCP guidelines from 27% to 98% (P < 0.0001) and reduced symptomatic VTE from 49 per 1000 admissions to 8 per 1000 admissions (P = 0.0001).[9] This study demonstrated that paper order sets used consistently by a dedicated group of providers can result in sustained improvements in practice. Paper order sets remain a low‐tech, easy‐to‐implement strategy that can be applied in any healthcare setting. Other services also saw improvements in risk‐appropriate prophylaxis prescription. In a follow‐up cross‐sectional analysis of the surgical services at Johns Hopkins, we found that appropriate VTE prophylaxis prescription improved from 33% to 62% in a sample of 226 patients. Unfortunately, paper order sets had several disadvantages including (1) the inherent difficulty of making them a mandatory part of the admission or transfer process, (2) their existence outside the usual clinical workflow, and (3) the labor‐ and time‐intensive data collection that made it difficult to provide credible, timely performance reports to providers and leadership.
These disadvantages and our adoption of a computerized provider order entry system prompted us to pursue the development and implementation of mandatory, evidence‐based, specialty‐specific computerized clinical decision support (CCDS) VTE prophylaxis order sets. Using the Translating Research Into Practice approach to quality improvement,[10] we collaborated with providers to design 16 different evidence‐based specialty‐specific CCDS VTE order sets. These CCDS VTE order sets, which are imbedded in the specialty‐specific admission and transfer order sets, assist providers in assessing patients' VTE and bleeding risk factors and provide evidence‐based risk‐appropriate VTE prophylaxis (see Supporting Figure 1 in the online version of this article). Individual patient data are saved in an administrative database and can be easily aggregated for research analyses and quality improvement/performance reporting. A detailed discussion of our strategy for change is discussed in Streiff et al.[11] Because pharmacologic prophylaxis is not appropriate for every patient, and not all VTE are preventable, even with perfect prophylaxis, the goals of our collaborative are to ensure that every patient is ordered VTE prophylaxis consistent with their risk profile (risk‐appropriate prophylaxis) and to eliminate preventable episodes of VTE (VTE that occurs in the setting of suboptimal prophylaxis). In a prepost quasi‐experimental study of 1599 trauma patients, the CCDS VTE order set increased risk‐appropriate prophylaxis prescription from 66.2% to 84.4% (P < .001) and reduced the incidence of potentially preventable harm from VTE from 1% to 0.17% (P = 0.04) (Figure 1).[12] On the medical service, the CCDS VTE order set improved risk‐appropriate VTE prophylaxis prescription from 65.6% to 90.1% (P < 0.0001) and reduced the incidence of potentially preventable harm attributable to VTE from 1.1% to 0% (P = 0.001). There was no increase in major bleeding (International Society of Thrombosis and Hemostasis definition: hemoglobin decline of 2 grams/dL or transfusion of 2 or more units of blood or bleeding into a critical organ such as brain, gastrointestinal tract, or eye) postorder set implementation (0.3% vs 0.1%, P = 0.625) or all‐cause mortality (1.3% vs 2.0%, P = 0.285).[13]

These order sets demonstrated that CCDS tools can lead to significant improvements in prescribing practices and reductions in preventable harm from VTE without increasing the risk of major bleeding complications. In addition to improving the quality of care, the order sets also improved the consistency of care. In a retrospective analysis, we found that implementation of CCDS VTE order sets eliminated racial disparities in prescribing practices. In the preimplementation group, risk‐appropriate VTE prophylaxis was prescribed for 70.1% of black patients and 56.6% of white patients on the trauma service (P = 0.025) and 69.5% of black patients and 61.7% of white patients on the medical service (P = 0.015). After implementation of the CCDS VTE order sets, care improved for all patients such that the previously observed disparities were eliminated (trauma service 84.5% vs 85.5%, P = 0.99 and medical service 91.8% vs 88.0%, P = 0.082).[14] These data indicate that standardizing care can potentially eliminate disparities in clinical practice.
Although implementation of mandatory evidence‐based, specialty‐specific CDSS VTE order sets led to substantial improvements in VTE prophylaxis ordering, high performance was not uniform across our institution. On the medical service, substantial disparities in adherence to order set recommendations existed. On the housestaff services, over 90% of patients consistently received risk‐appropriate VTE prophylaxis compared with only 85% on the hospitalist service. Examination of individual provider performance found that some providers only ordered risk‐appropriate prophylaxis 50% of the time, whereas others were doing so 98% of the time. To address this disparity, we conducted a retrospective analysis of a prospective performance improvement project conducted on the Johns Hopkins Hospitalist service studying the impact of individualized hospitalist attending feedback on VTE prevention practices. During the preintervention period (January 2009December 2010), guideline‐adherent VTE prophylaxis was ordered for 86% (95% confidence interval [CI]: 85%‐88%) of patients. Six months after initiation of direct face‐to‐face provider feedback (January 2011June 2011), guideline‐adherent VTE prophylaxis rates rose to 90% (95% CI: 88‐93). Subsequently (July 2011December 2012), a pay‐for‐performance (P4P) initiative was added to direct face‐to‐face provider feedback. During the P4P initiative, provider incentive per relative value unit (RVU) was progressively increased with increasing performance on provision of risk‐appropriate VTE prophylaxis (adherence <80% = no bonus to $0.50 per RVU for adherence 95%). During this period, prescription of guideline‐adherent prophylaxis rose to 94% (95% CI: 93%‐96%).[15] These initiatives transformed the hospitalist unit from a consistently low‐performance unit to a high‐performance unit.
Similar findings were noted on the trauma service. Although the original plan was to provide feedback to attending trauma surgeons, that plan changed when we found that performance was driven entirely by resident practice; residents write the VTE prophylaxis orders, which is then attributed to attending performance. Resident performance varied widely; 42 of 75 (56%) residents on the trauma service ordered risk‐appropriate prophylaxis for 100% of their patients. In contrast, 7 (9.3%) residents never ordered optimal prophylaxis for any of their patients.[16] To motivate all residents to prescribe optimal prophylaxis, we developed an individualized resident VTE prophylaxis scorecard (Figure 2). This prospective cohort study of 2420 patients and 49 general surgery residents compared resident VTE prophylaxis performance on the general surgery service during 3 periods: period 1 (baseline, July 2013September 2013), period 2 (surgery resident scorecard, October 2013December 2013), period 3 (resident scorecard plus individualized 1‐on‐1 coaching, January 2014March 2014). At baseline, 89.4% of patients were prescribed appropriate VTE prophylaxis, and only 45% of residents prescribed risk‐appropriate prophylaxis for all their patients. During the scorecard period, 95.4% of patients were prescribed risk‐appropriate VTE prophylaxis (P < 0.001). During the scorecard plus coaching period, risk‐appropriate prophylaxis rose to 96.4%. These prescribing practice changes were durable. During the 15 months prior to issuing scorecards, 88.0% of patients (3718/4226) were prescribed risk‐appropriate prophylaxis. After implementation, 95.8% of patients (3799/3966) were prescribed risk‐appropriate prophylaxis (P < 0.001) (see Supporting Figure 2 in the online version of this article). During the baseline period, 7 of 865 patients (0.81%) had a VTE during their hospital stay, of which 3 (0.35%) were potentially preventable. In contrast, none of the 3 of 784 patients who suffered VTE during the postimplementation period had a potentially preventable event (0.35% vs 0%, P = 0.046).[17] These studies demonstrate that providing physicians with their own specific data can be a powerful tool for performance improvement that may be applicable to many other quality and safety measures. Our group recently received funding from the AHRQ to scale this work to other residents, nurse practitioners, physician assistants, and attending physicians (1R01HS024547, Individualized Performance Feedback on Venous Thromboembolism Prevention Practice).

IMPROVING VTE PROPHYLAXIS ADMINISTRATION
Ordering VTE prophylaxis does not ensure its administration. We conducted a retrospective review of electronic administration records of 10,526 consecutive patients admitted over a 7‐month period at The Johns Hopkins Hospital. Twelve percent of the over 100,000 ordered doses of VTE prophylaxis were not administered, and the proportion of nonadministered doses on individual floors varied 5‐fold from 5.4% to 26.9%. The proportion of nonadministered doses was significantly higher on medical floors compared with all other services (17.5% vs 8.1%, odds ratio [OR]: 2.1 [95% CI: 2.0‐2.2]). Patient or family member refusal was the most common cause for nonadministered doses of VTE prophylaxis accounting for 59% of all missed doses. Eight percent of patients missed more than half their prescribed doses, and 5% of patients missed over 75% of ordered doses of VTE prophylaxis. Consistent with the Pareto principle, over 80% of the missed doses of prophylaxis were accounted for by just 20% of the patients.[18] A retrospective analysis of hospital‐acquired VTE at Johns Hopkins found that 39% of events occurred in patients who missed 1 or more doses of appropriate VTE prophylaxis.[19] Louis et al. noted that nonadministration of 1 dose of VTE prophylaxis was associated with a significant increase in risk for hospital acquired VTE.[20] These data indicate the need for more aggressive interventions to reduce missed doses to improve VTE prevention.
To fully understand the root causes of VTE prophylaxis non‐administration, we conducted a series of studies examining each of the participants in the VTE prevention care pathway, physicians, nurses, and patients. In a survey of 122 resident physicians, we found significant differences in clinical practice between medicine and surgery residents. Medicine residents were more likely to believe that VTE prophylaxis was overprescribed, and that it was appropriate for nurses to make judgement calls about whether patients needed the prophylaxis that was prescribed.[21] In a mixed methods study that included a written survey and qualitative observations of nursing practice, we found that some nurses presented pharmacologic VTE prophylaxis injections as optional to patients. Furthermore, nurses on units where nonadministration was higher were more likely to believe that VTE prophylaxis was prescribed for patients unnecessarily, and that they could use their clinical judgement to determine when it was appropriate to omit doses of pharmacologic prophylaxis.[22] Our team also examined patient preferences in regard to VTE prophylaxis. In a survey of 227 consecutive medical and surgical inpatients, we found that 60% of patients would prefer an oral route of administration if available. Patients with a preference for a parenteral route of administration (27.5%) were less likely to refuse prophylaxis (37.5% vs 51.3%, P < 0.0001).[23] These findings underscore the fact that unit culture, nursing attitudes and beliefs, and patient preferences have an important influence on medication administration, and that nursepatient communication is an important target for modifying adherence.
PATIENT‐CENTERED APPROACHES TO IMPROVE VTE PROPHYLAXIS ADMINISTRATION
To address nurse‐ and patient‐related factors that influence VTE prophylaxis administration, we applied for and received a Patient Centered Outcomes Research Institute contract to develop patient‐centered interventions to engage and empower patients to take an active role in their preventive care. To achieve these aims, we partnered with 3 national patient advocacy organizations, the National Blood Clot Alliance, the North American Thrombosis Forum, and ClotCare, as well as our local Johns Hopkins Patient and Family Advisory Council. Using a modified Delphi method, we engaged patient stakeholders from the 4 collaborating organizations to build consensus on patient‐centered VTE education methods. Input from this Delphi assessment was used to build educational materials including paper brochures published in 8 different languages and a 10‐minute educational video filmed by an Oscar‐winning documentary director featuring both clinicians and patients relating their VTE experience and the importance of VTE prevention.[24] These educational materials are available for public use (
ENGAGING TRAINEES IN MULTIDISCIPLINARY PATIENT SAFETY/QUALITY IMPROVEMENT INITIATIVES
Trainees from many healthcare‐related disciplines have played a critical role in our quest to improve VTE prevention. Over the past 10 years, we have mentored countless medical students, public health graduate students, nursing students, residents, and postdoctoral fellows in research projects that have resulted in numerous high‐quality publications. Trainees have helped to dispel staff concerns about patient falls in connection of intermittent pneumatic compression devices,[25] identify the weaknesses of current publicly reported VTE measures,[26, 27, 28, 29] identify opportunities to improve VTE prevention practices within clinical specialties,[30, 31, 32] define the role of surveillance bias in VTE outcomes reporting,[33, 34, 35] discover and fully explore the important problem of missed doses of VTE prophylaxis,[18, 21, 22, 23, 36] and summarize knowledge about VTE prevention via systematic reviews and meta‐analyses.[37, 38, 39] These collaborations have been a classic win‐win. The mentees learn critical skills while growing their curriculum vitae with contributions to the literature, allowing them to progress in their careers (ie, obtain a residency match, faculty positions). The faculty have leveraged this work to obtain over $3 million in extramural funding to develop interventions to study and improve the quality of VTE preventive care for hospitalized patients.
In healthcare, we have not yet achieved defect‐free VTE prevention; however, we have a better understanding of the path to accomplishing this goal. In this article we describe our goal of zero harm from VTE and our learning journey to realize that goal. Although the journey never ends, a critical ingredient to the success of our program has been the multidisciplinary nature of our VTE collaborative team. The combination of expertise from medicine, surgery, nursing, pharmacy, clinical informatics, and public health has facilitated the development of innovative strategies to improve VTE prevention that integrate seamlessly into clinical workflow. The approach used for VTE can be applied to eliminate other types of harms.
Disclosures
Mr. Lau, Dr. Streiff, and Dr. Haut are supported by a grant from the Agency for Healthcare Research and Quality (1R01HS024547) titled Individualized Performance Feedback on Venous Thromboembolism Prevention Practice and a contract from the Patient‐Centered Outcomes Research Institute titled Preventing Venous Thromboembolism: Empowering Patients and Enabling Patient‐Centered Care via Health Information Technology (CE‐12‐11‐4489). Mr. Lau is supported by the Institute for Excellence in Education Berkheimer Faculty Education Scholar Grant and a contract (AD‐1306‐03980) from the Patient‐Centered Outcomes Research Institute titled Patient Centered Approaches to Collect Sexual Orientation/Gender Identity Information in the Emergency Department. Ms. Hobson has given expert witness testimony in various medical malpractice cases. Dr. Streiff has received research funding from Portola and Janssen; consulted for Bio2Medical, CSL Behring, Merck, and Janssen HealthCare; and has given expert witness testimony in various medical malpractice cases. Dr. Haut receives royalties from Lippincott, Williams, and Wilkins for a book titled Avoiding Common ICU Errors. Dr. Haut is a paid consultant and speaker for the Preventing Avoidable Venous ThromboembolismEvery Patient, Every Time VHA/Vizient IMPERATIV Advantage Performance Improvement Collaborative. Dr. Haut is a paid consultant and speaker for the Illinois Surgical Quality Improvement Collaborative. All other authors report no disclosures.
- , . Thromboprophylaxis in nonsurgical patients. Hematology Am Soc Hematol Educ Program. 2012;2012:631–637.
- Office of the Surgeon General (US); National Heart, Lung, and Blood Institute (US). The Surgeon General's Call to Action to Prevent Deep Vein Thrombosis and Pulmonary Embolism. Rockville, MD: Office of the Surgeon General; 2008.
- , . Prevention of venous thromboembolism: brief update review. In: Making Health Care Safer II: An Updated Critical Analysis of the Evidence for Patient Safety Practices. Rockville, MD: Agency for Healthcare Research and Quality; 2013.
- , , , et al. The top patient safety strategies that can be encouraged for adoption now. Ann Intern Med. 2013;158:365–368.
- , . Practices to prevent venous thromboembolism: a brief review. BMJ Qual Saf. 2014;23:187–195.
- , , , et al. Venous thromboembolism risk and prophylaxis in the acute hospital care setting (ENDORSE study): a multinational cross‐sectional study. Lancet. 2008;371:387–394.
- , . The CMS ruling on venous thromboembolism after total knee or hip arthroplasty: weighing risks and benefits. JAMA. 2009;301:1063–1065.
- , , , et al. ICD‐9 code‐based venous thromboembolism performance targets fail to measure up. Am J Med Qual. 2016;31(5):448–453.
- , , , , . Evidence‐based venous thromboembolism prophylaxis is associated with a six‐fold decrease in numbers of symptomatic venous thromboembolisms in rehabilitation inpatients. PM R. 2011;3:1111–1115.e1.
- , , . Translating evidence into practice: a model for large scale knowledge translation. BMJ. 2008;337:a1714.
- , , , et al. Lessons from the Johns Hopkins Multi‐Disciplinary Venous Thromboembolism (VTE) Prevention Collaborative. BMJ. 2012;344:e3935.
- , , , et al. Improved prophylaxis and decreased rates of preventable harm with the use of a mandatory computerized clinical decision support tool for prophylaxis for venous thromboembolism in trauma. Arch Surg. 2012;147:901–907.
- , , , et al. Impact of a venous thromboembolism prophylaxis “smart order set”: improved compliance, fewer events. Am J Hematol. 2013;88(7):545–549.
- , , , et al. Eliminating health care disparities with mandatory clinical decision support: the venous thromboembolism (VTE) example. Med Care. 2015;53:18–24.
- , , , et al. Use of provider‐level dashboards and pay‐for‐performance in venous thromboembolism prophylaxis. J Hosp Med. 2015;10:172–178.
- , , , , , . Attending physician performance measure scores and resident physicians' ordering practices. JAMA Surg. 2015;150:813–814.
- , , , et al. Individualized performance feedback to surgical residents improves appropriate venous thromboembolism prophylaxis prescription and reduces potentially preventable VTE: a prospective cohort study [published online November 25, 2015]. Ann Surg. doi: 10.1097/SLA.0000000000001512.
- , , , et al. Patterns of non‐administration of ordered doses of venous thromboembolism prophylaxis: implications for novel intervention strategies. PLoS One. 2013;8:e66311.
- , , , et al. Preventability of hospital‐acquired venous thromboembolism. JAMA Surg. 2015;150(9):912–915.
- , , , et al. Correlation of missed doses of enoxaparin with increased incidence of deep vein thrombosis in trauma and general surgery patients. JAMA Surg. 2014;149:365–370.
- , , , et al. Prescriber knowledge and attitudes regarding non‐administration of prescribed pharmacologic venous thromboembolism prophylaxis [published online May 21, 2016]. J Thromb Thrombolysis. doi:10.1007/s11239-016-1378-8.
- , , , et al. Hidden barriers to delivery of pharmacological venous thromboembolism prophylaxis: the role of nursing beliefs and practices. J Patient Saf. 2016;12:63–68.
- , , , et al. Patient preferences regarding pharmacologic venous thromboembolism prophylaxis. J Hosp Med. 2015;10:108–111.
- , , , et al. Patient preferences for receiving education on venous thromboembolism prevention—a survey of stakeholder organizations. PLoS One. 2016;11:e0152084.
- , , , , , . Are sequential compression devices commonly associated with in‐hospital falls? A myth‐busters review using the patient safety net database. J Patient Saf. 2011;7:77–79.
- , , , , . No association between hospital‐reported perioperative venous thromboembolism prophylaxis and outcome rates in publicly reported data. JAMA Surg. 2014;149:400–401.
- , , , , . Linking processes and outcomes: a key strategy to prevent and report harm from venous thromboembolism in surgical patients. JAMA Surg. 2013;148:299–300.
- , , , et al. Hazards of benchmarking complications with the National Trauma Data Bank: numerators in search of denominators. J Trauma. 2008;64:273–277; discussion 277–279.
- , , , et al. Is the meaningful use venous thromboembolism VTE‐6 measure meaningful? A retrospective analysis of one hospital's VTE‐6 cases. Jt Comm J Qual Patient Saf. 2016;42(9):410–416.
- , , , et al. Is venous thromboembolism in colorectal surgery patients preventable or inevitable? One institution's experience. J Am Coll Surg. 2013;216:395–401.e1.
- , , , et al. Venous thromboembolic prophylaxis after a hepatic resection: patterns of care among liver surgeons. HPB (Oxford). 2014;16:892–898.
- , , , et al. Defining incidence and risk factors of venous thromboembolism after hepatectomy. J Gastrointest Surg. 2014;18:1116–1124.
- , , , et al. Can increased incidence of deep vein thrombosis (DVT) be used as a marker of quality of care in the absence of standardized screening? The potential effect of surveillance bias on reported DVT rates after trauma. J Trauma. 2007;63:1132–1135; discussion 1135–1137.
- , . Surveillance bias in outcomes reporting. JAMA. 2011;305:2462–2463.
- , , , et al. Surveillance bias and deep vein thrombosis in the national trauma data bank: the more we look, the more we find. J Trauma. 2008;64:932–936; discussion 936–937.
- , , , et al. Nonadministration of thromboprophylaxis in hospitalized patients with HIV: a missed opportunity for prevention? J Hosp Med. 2014;9:215–220.
- , , , et al. Pharmacologic and mechanical prophylaxis of venous thromboembolism among special populations. Comparative effectiveness review No. 116. Prepared by the Johns Hopkins University Evidence‐based Practice Center under Contract No. 290‐2007‐10061‐I.) AHRQ Publication No. 13‐EHC082–1. Rockville, MD: Agency for Healthcare Research and Quality; 2013.
- , , , et al. Pharmacologic and mechanical strategies for preventing venous thromboembolism after bariatric surgery: a systematic review and meta‐analysis. JAMA Surg. 2013;148:675–686.
- , , , et al. The effectiveness of prophylactic inferior vena cava filters in trauma patients: a systematic review and meta‐analysis. JAMA Surg. 2014;149:194–202.
- , . Thromboprophylaxis in nonsurgical patients. Hematology Am Soc Hematol Educ Program. 2012;2012:631–637.
- Office of the Surgeon General (US); National Heart, Lung, and Blood Institute (US). The Surgeon General's Call to Action to Prevent Deep Vein Thrombosis and Pulmonary Embolism. Rockville, MD: Office of the Surgeon General; 2008.
- , . Prevention of venous thromboembolism: brief update review. In: Making Health Care Safer II: An Updated Critical Analysis of the Evidence for Patient Safety Practices. Rockville, MD: Agency for Healthcare Research and Quality; 2013.
- , , , et al. The top patient safety strategies that can be encouraged for adoption now. Ann Intern Med. 2013;158:365–368.
- , . Practices to prevent venous thromboembolism: a brief review. BMJ Qual Saf. 2014;23:187–195.
- , , , et al. Venous thromboembolism risk and prophylaxis in the acute hospital care setting (ENDORSE study): a multinational cross‐sectional study. Lancet. 2008;371:387–394.
- , . The CMS ruling on venous thromboembolism after total knee or hip arthroplasty: weighing risks and benefits. JAMA. 2009;301:1063–1065.
- , , , et al. ICD‐9 code‐based venous thromboembolism performance targets fail to measure up. Am J Med Qual. 2016;31(5):448–453.
- , , , , . Evidence‐based venous thromboembolism prophylaxis is associated with a six‐fold decrease in numbers of symptomatic venous thromboembolisms in rehabilitation inpatients. PM R. 2011;3:1111–1115.e1.
- , , . Translating evidence into practice: a model for large scale knowledge translation. BMJ. 2008;337:a1714.
- , , , et al. Lessons from the Johns Hopkins Multi‐Disciplinary Venous Thromboembolism (VTE) Prevention Collaborative. BMJ. 2012;344:e3935.
- , , , et al. Improved prophylaxis and decreased rates of preventable harm with the use of a mandatory computerized clinical decision support tool for prophylaxis for venous thromboembolism in trauma. Arch Surg. 2012;147:901–907.
- , , , et al. Impact of a venous thromboembolism prophylaxis “smart order set”: improved compliance, fewer events. Am J Hematol. 2013;88(7):545–549.
- , , , et al. Eliminating health care disparities with mandatory clinical decision support: the venous thromboembolism (VTE) example. Med Care. 2015;53:18–24.
- , , , et al. Use of provider‐level dashboards and pay‐for‐performance in venous thromboembolism prophylaxis. J Hosp Med. 2015;10:172–178.
- , , , , , . Attending physician performance measure scores and resident physicians' ordering practices. JAMA Surg. 2015;150:813–814.
- , , , et al. Individualized performance feedback to surgical residents improves appropriate venous thromboembolism prophylaxis prescription and reduces potentially preventable VTE: a prospective cohort study [published online November 25, 2015]. Ann Surg. doi: 10.1097/SLA.0000000000001512.
- , , , et al. Patterns of non‐administration of ordered doses of venous thromboembolism prophylaxis: implications for novel intervention strategies. PLoS One. 2013;8:e66311.
- , , , et al. Preventability of hospital‐acquired venous thromboembolism. JAMA Surg. 2015;150(9):912–915.
- , , , et al. Correlation of missed doses of enoxaparin with increased incidence of deep vein thrombosis in trauma and general surgery patients. JAMA Surg. 2014;149:365–370.
- , , , et al. Prescriber knowledge and attitudes regarding non‐administration of prescribed pharmacologic venous thromboembolism prophylaxis [published online May 21, 2016]. J Thromb Thrombolysis. doi:10.1007/s11239-016-1378-8.
- , , , et al. Hidden barriers to delivery of pharmacological venous thromboembolism prophylaxis: the role of nursing beliefs and practices. J Patient Saf. 2016;12:63–68.
- , , , et al. Patient preferences regarding pharmacologic venous thromboembolism prophylaxis. J Hosp Med. 2015;10:108–111.
- , , , et al. Patient preferences for receiving education on venous thromboembolism prevention—a survey of stakeholder organizations. PLoS One. 2016;11:e0152084.
- , , , , , . Are sequential compression devices commonly associated with in‐hospital falls? A myth‐busters review using the patient safety net database. J Patient Saf. 2011;7:77–79.
- , , , , . No association between hospital‐reported perioperative venous thromboembolism prophylaxis and outcome rates in publicly reported data. JAMA Surg. 2014;149:400–401.
- , , , , . Linking processes and outcomes: a key strategy to prevent and report harm from venous thromboembolism in surgical patients. JAMA Surg. 2013;148:299–300.
- , , , et al. Hazards of benchmarking complications with the National Trauma Data Bank: numerators in search of denominators. J Trauma. 2008;64:273–277; discussion 277–279.
- , , , et al. Is the meaningful use venous thromboembolism VTE‐6 measure meaningful? A retrospective analysis of one hospital's VTE‐6 cases. Jt Comm J Qual Patient Saf. 2016;42(9):410–416.
- , , , et al. Is venous thromboembolism in colorectal surgery patients preventable or inevitable? One institution's experience. J Am Coll Surg. 2013;216:395–401.e1.
- , , , et al. Venous thromboembolic prophylaxis after a hepatic resection: patterns of care among liver surgeons. HPB (Oxford). 2014;16:892–898.
- , , , et al. Defining incidence and risk factors of venous thromboembolism after hepatectomy. J Gastrointest Surg. 2014;18:1116–1124.
- , , , et al. Can increased incidence of deep vein thrombosis (DVT) be used as a marker of quality of care in the absence of standardized screening? The potential effect of surveillance bias on reported DVT rates after trauma. J Trauma. 2007;63:1132–1135; discussion 1135–1137.
- , . Surveillance bias in outcomes reporting. JAMA. 2011;305:2462–2463.
- , , , et al. Surveillance bias and deep vein thrombosis in the national trauma data bank: the more we look, the more we find. J Trauma. 2008;64:932–936; discussion 936–937.
- , , , et al. Nonadministration of thromboprophylaxis in hospitalized patients with HIV: a missed opportunity for prevention? J Hosp Med. 2014;9:215–220.
- , , , et al. Pharmacologic and mechanical prophylaxis of venous thromboembolism among special populations. Comparative effectiveness review No. 116. Prepared by the Johns Hopkins University Evidence‐based Practice Center under Contract No. 290‐2007‐10061‐I.) AHRQ Publication No. 13‐EHC082–1. Rockville, MD: Agency for Healthcare Research and Quality; 2013.
- , , , et al. Pharmacologic and mechanical strategies for preventing venous thromboembolism after bariatric surgery: a systematic review and meta‐analysis. JAMA Surg. 2013;148:675–686.
- , , , et al. The effectiveness of prophylactic inferior vena cava filters in trauma patients: a systematic review and meta‐analysis. JAMA Surg. 2014;149:194–202.
© 2016 Society of Hospital Medicine
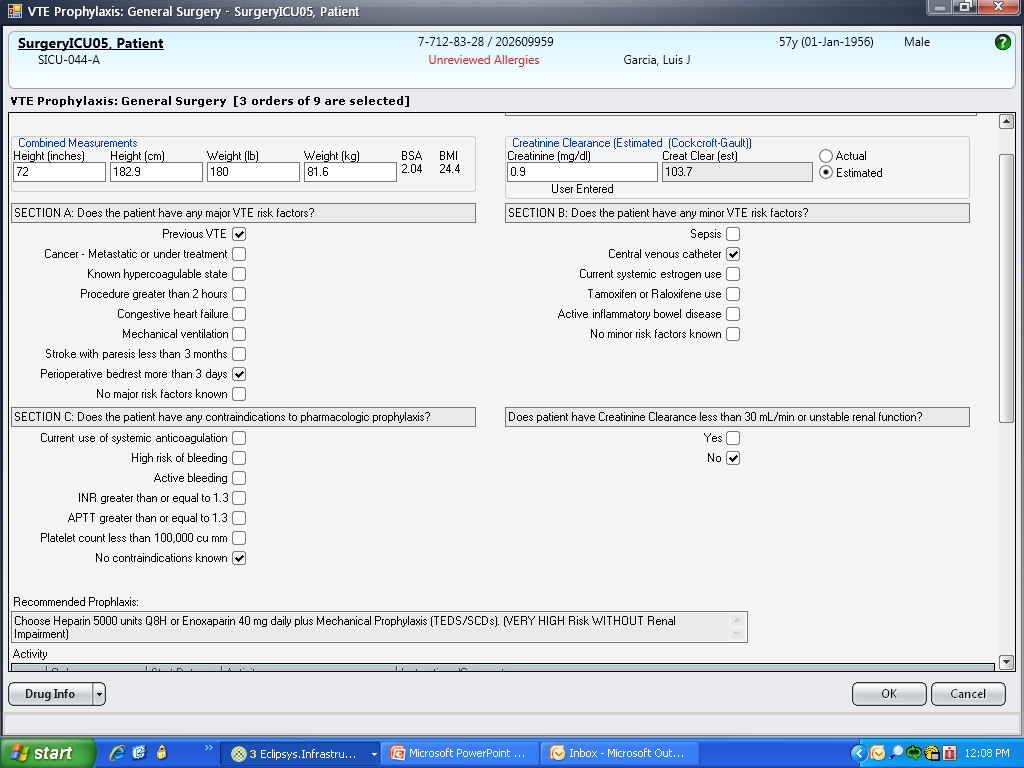
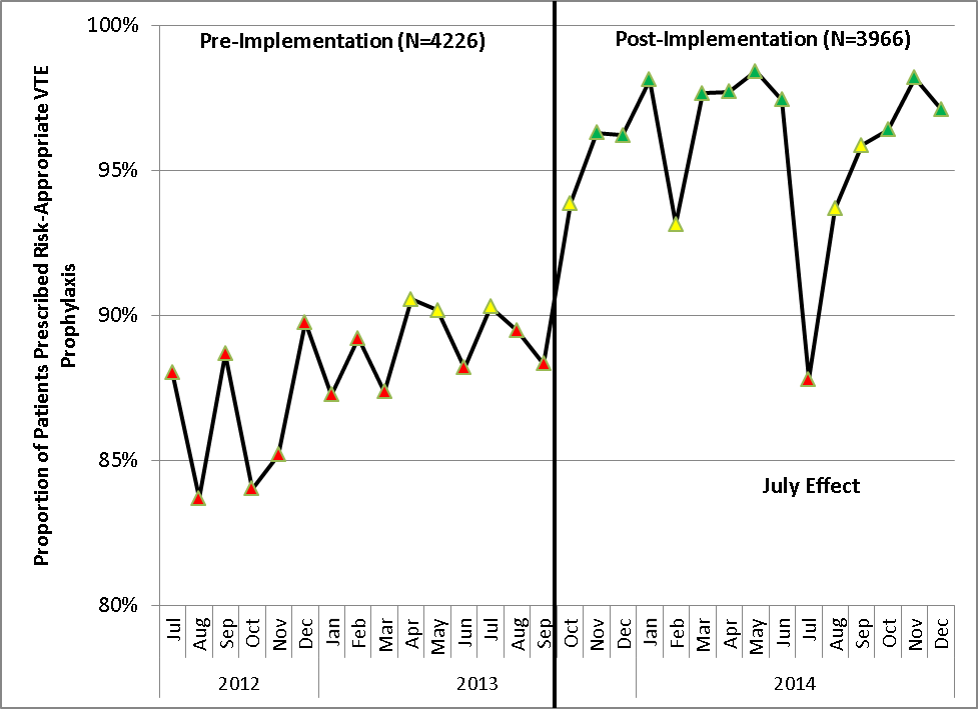
Thromboembolism Prophylaxis Preferences
The 2012 American College of Chest Physicians (ACCP) guidelines on antithrombotic and thrombolytic therapy conducted a systematic review focusing on patient values and preferences regarding antithrombotic therapy, including thromboprophylaxis.[1] They found that patient values and preferences are highly variable and should be considered when developing future clinical practice guidelines. Notably, there were no studies evaluating patient preferences for venous thromboembolism (VTE) prophylaxis, which is prescribed for the vast majority of hospitalized patients.
Historically, interventions to prevent VTE have focused on increasing prescriptions of prophylaxis. At the Johns Hopkins Hospital, we implemented a mandatory clinical decision support tool in our computerized provider order entry system.[2] Following implementation of this tool, prescription of risk‐appropriate VTE prophylaxis dramatically increased for both medical and surgical patients.[3, 4, 5] These efforts were made with the implicit and incorrect assumption that prescribed medication doses will always be administered to patients, when in fact patient refusal is a leading cause of nonadministration. Studies of VTE prophylaxis administration have reported that 10% to 12% of doses are not administered to patients.[6] Alarmingly, it has been reported that among medically ill patients, between 10% and 30% of doses are not administered, with patient refusal as the most frequently documented reason.
The purpose of this study was to assess patient preferences regarding pharmacological VTE prophylaxis.
METHODS
Study Design
A sample of consecutive hospitalized patients on select medicine and surgical floors previously identified as low‐ and high‐performing units at our institution in regard to administration rates of pharmacologic VTE prophylaxis was assembled from a daily electronic report of patients prescribed pharmacological VTE prophylaxis (Allscripts Sunrise, Chicago, IL) from December 2012 to March 2013. These units were identified in a study conducted at our institution as the lowest‐ and highest‐performing units in regard to incidence of administration of ordered pharmacologic VTE prophylaxis. From this data analysis, we chose the 2 lowest‐performing and 2 highest‐performing units on the medical and surgical service. To be eligible for this study, patients had to have an active order for 1 of the following VTE prophylaxis regimens: unfractionated heparin 5000 units or 7500 units administered subcutaneously every 8 or 12 hours, enoxaparin 30 mg administered subcutaneously every 12 hours or 40 mg administered subcutaneously every 24 hours. Participants had to be at least 18 years of age and hospitalized for at least 2 days on their respective units. Patients who were nonEnglish speaking, those previously enrolled in this study, or those unable to provide consent were excluded from the study.
Data Collection
Demographic information was collected, including patient‐reported education level. To determine their preference for VTE prophylaxis, patients were provided a survey, which included being asked, Would you prefer a pill or a shot to prevent blood clots, if they both worked equally well. The survey was created by the study team to collect information from patients regarding their baseline knowledge of VTE and preference regarding pharmacologic prophylaxis. Additional data included the patient's education level to determine potential association with preference. The survey was verbally administered by 1 investigator (A.W.) to all patients. Patients were asked to explain their rationale for their stated preference in regard to VTE prophylaxis. Patient rationale was subsequently coded to allow for uniformity among patient responses based on patterns in responses. Our electronic medication record allows us to identify patients who refused their medication through nursing documentation. Patients with documented refusal of ordered pharmacologic VTE prophylaxis were asked about the rationale for their refusal. This study was approved by the Johns Hopkins Medicine Institutional Review Board.
Statistical Analysis
Quantitative data from the surveys were analyzed using Minitab (Minitab Inc., State College, PA). A [2] test analysis was performed for categorical data, as appropriate. A P value <0.05 was considered to be statistically significant.
RESULTS
Quantitative Results
We interviewed patients regarding their preferred route of administration of VTE prophylaxis. Overall, 339 patients were screened for this study. Sixty patients were not eligible to participate. Forty‐seven were unable to provide consent, and 13 were nonEnglish speaking. Of the 269 remaining eligible patients, 227 (84.4%) consented to participate.
Baseline demographics of the participants are presented in Table 1, categorized on the basis of their preferred route of administration for VTE prophylaxis. A majority of patients indicated a preference for an oral formulation of pharmacologic VTE prophylaxis. There was no association between education level or service type on preference. Preference for an oral formulation was largely influenced by patient‐reported pain and bruising associated with subcutaneous administration (Table 2). A substantial majority of patients reporting a preference for a subcutaneous formulation and emphasized a belief that this route was associated with a faster onset of action. Among patients who preferred an oral formulation (n=137), 71 patients (51.8%) were documented as having refused at least 1 dose of ordered VTE prophylaxis. Patients who preferred a subcutaneous route of VTE prophylaxis were less likely to refuse prophylaxis, with only 22 patients (35.5%) having a documented refusal of at least 1 dose (P<0.0001).
| Enteral, n=137 | Parenteral, n=62 | No Preference, n=28 | |
|---|---|---|---|
| |||
| Age, y, mean ( SD) | 49.5 (14.7) | 51.7 (16.1) | 48.9 (14.6) |
| Male, n (%) | 74 (54.0) | 38 (61.3) | 15 (53.6) |
| Race n (%) | |||
| Caucasian | 81 (59.1) | 31 (50.0) | 14 (50.0) |
| African American | 50 (36.5) | 28 (45.2) | 14 (50.0) |
| Education level, n (%) | |||
| High school or less | 46 (33.6) | 27 (43.5) | 14 (50.0) |
| College | 68 (49.6) | 21 (33.9) | 9 (32.1) |
| Advanced degree | 10 (7.3) | 8 (12.9) | 2 (7.1) |
| Unable to obtain | 13 (9.5) | 6 (9.7) | 3 (10.8) |
| Past history of VTE, n (%) | 12 (8.8) | 9 (14.5) | 2 (7.1) |
| Type of unit, n (%) | |||
| Medical | 59 (43.1) | 24 (38.7) | 17 (60.7) |
| Surgical | 78 (56.9) | 38 (61.3) | 11 (39.3) |
| Documented refusal of ordered prophylaxis, n (%) | 71 (51.8) | 20 (32.3) | 9 (32.1) |
| Length of hospital stay prior to inclusion in study, d, median (IQR) | 4.0 (3.07.0) | 3.0 (3.05.0) | 4.0 (2.05.0) |
| Patients preferring enteral route, n (%) | 137 (60.4) |
| Dislike of needles | 41 (30.0) |
| Pain from injection | 38 (27.7) |
| Ease of use | 18 (13.1) |
| Bruising from injection | 9 (6.6) |
| Other/no rationale | 31 (22.6) |
| Patients preferring injection route, n (%) | 62 (27.5) |
| Faster onset of action | 25 (40.3) |
| Pill burden | 11 (17.7) |
| Ease of use | 9 (14.5) |
| Other/no rationale | 17 (27.5) |
| Patients with no preference, n (%) | 28 (12.4) |
DISCUSSION
Using a mixed‐methods approach, we report the first survey evaluating patient preferences regarding pharmacologic VTE prophylaxis. We found that a majority of patients preferred an oral route of administration. Nevertheless, a substantial number of patients favored a subcutaneous route of administration believing it to be associated with a faster onset of action. Of interest, patients favoring subcutaneous injections were significantly less likely to refuse doses of ordered VTE prophylaxis. Given that all patients were prescribed a subcutaneous form of VTE prophylaxis, matching patient preference to VTE prophylaxis prescription could potentially increase adherence and reduce patient refusal of ordered prophylaxis. Considering the large number of patients who preferred an oral route of administration, the availability of an oral formulation may potentially result in improved adherence to inpatient VTE prophylaxis.
Our findings have significant implications for healthcare providers, and for patient safety and quality‐improvement researchers. VTE prophylaxis is an important patient‐safety practice, particularly for medically ill patients, which is believed to be underprescribed.[7] Recent studies have demonstrated that a significant number of doses of VTE prophylaxis are not administered, primarily due to patient refusal.[6] Our data indicate that tailoring the route of prophylaxis administration to patient preference may represent a feasible strategy to improve VTE prophylaxis administration rates. Recently, several target‐specific oral anticoagulants (TSOACs) have been approved for a variety of clinical indications, and all have been investigated for VTE prophylaxis.[7, 8, 9, 10, 11, 12, 13, 14, 15] However, no agent is currently US Food & Drug Administration (FDA) approved for primary prevention of VTE, although apixaban and rivaroxaban are FDA approved for VTE prevention in joint replacement.[13, 14] Although in some instances these TSOACs were noted to demonstrate only equivalent efficacy to standard subcutaneous forms of VTE prophylaxis, our data suggest that perhaps in some patients, use of these agents may result in better outcomes due to improved adherence to therapy due to a preferred oral route of administration. We think this hypothesis warrants further investigation.
Our study also underscores the importance of considering patient preferences when caring for patients as emphasized by the 2012 ACCP guidelines.[1] Our results indicate that consideration of patient preferences may lead to better patient care and better outcomes. Interestingly, there were no differences in preference based on education level or the type of service to which the patient was admitted. Clarification of uninformed opinions regarding the rationale for preference may also lead to more informed decisions by patients.
This study has a number of limitations. We only included patients on the internal medicine and general surgical services. It is possible that patients on other specialty services may have different opinions regarding prophylaxis that were not captured in our sample. Similarly, our sample size was limited, and approximately 15% of potential subjects did not participate. We do believe that our population is reflective of our institution based upon our previously published evaluation of multiple hospital units and the inclusion of low‐ and high‐performing units on both the medical and surgical services. Nevertheless, we believe that much more investigation of patient perspectives on VTE prophylaxis needs to be done to inform decision making, including the impact of patient preferences on VTE‐related outcomes. Additionally, we did not evaluate potential predictors of preference including admission diagnosis and duration of hospital length of stay.
In conclusion, we conducted a mixed‐methods analysis of patient preferences regarding pharmacologic VTE prophylaxis. Matching patient preference to ordered VTE prophylaxis may increase adherence to ordered prophylaxis. In this era of increasingly patient‐centered healthcare and expanding options for VTE prophylaxis, we believe information on patient preferences will be helpful to tailoring options for prevention and treatment.
ACKNOWLEDGMENTS
Disclosures: Dr. Haut is the primary investigator of the Mentored Clinician Scientist Development Award K08 1K08HS017952‐01 from the Agency for Healthcare Research and Quality entitled Does Screening Variability Make DVT an Unreliable Quality Measure of Trauma Care? Dr. Haut receives royalties from Lippincott, Williams, & Wilkins for a book he coauthored (Avoiding Common ICU Errors). He has received honoraria for various speaking engagements regarding clinical, quality, and safety topics and has given expert witness testimony in various medical malpractice cases. Dr. Streiff has received research funding from Sanofi‐Aventis and Bristol‐Myers Squibb; honoraria for Continuing Medial Education lectures from Sanofi‐Aventis and Ortho‐McNeil; consulted for Sanofi‐Aventis, Eisai, Daiichi‐Sankyo, and Janssen HealthCare; and has given expert witness testimony in various medical malpractice cases. Mr. Lau, Drs. Haut, Streiff, and Shermock are supported by a contract from the Patient‐Centered Outcomes Research Institute titled Preventing Venous Thromboembolism: Empowering Patients and Enabling Patient‐Centered Care via Health Information Technology (CE‐12‐11‐4489). Ms. Hobson has given expert witness testimony in various medical malpractice cases. All others have no relevant funding or conflicts of interest to report.
- , , , et al. Patient values and preferences in decision making for antithrombotic therapy: a systematic review. Chest. 2012;141(2):e1S–e23S.
- , , , et al. Lessons from the Johns Hopkins Multi‐Disciplinary Venous Thromboembolism (VTE) Prevention Collaborative. BMJ. 2012;344:e3935.
- , , , et al. Impact of a venous thromboembolism (VTE) prophylaxis “smart order set”: improved compliance, fewer events. Am J Hematol. 2013;88(7):545–549.
- , , , et al. Improved prophylaxis and decreased preventable harm with a mandatory computerized clinical decision support tool for venous thromboembolism (VTE) prophylaxis in trauma patients. Arch Surg. 2012;147(10):901–907.
- , , , , . Linking processes and outcomes: a key strategy to prevent and report harm from venous thromboembolism in surgical patients. JAMA Surg. 2013;148(3):299–300.
- , , , et al. Patterns of non‐administration of ordered doses of venous thromboembolism prophylaxis: implications for intervention strategies. PLoS One. 2013;8(6):e66311.
- , , , et al. Venous thromboembolism risk and prophylaxis in the acute hospital care setting (ENDORSE study): a multinational cross‐sectional study. Lancet. 2008;371:387–394.
- , , , et al. Rivaroxaban versus enoxaparin for thromboprophylaxis after hip arthroplasty. N Engl J Med. 2008;358:2765–2775.
- , , , et al. Rivaroxaban versus enoxaparin for thromboprophylaxis after total knee arthoplasty. N Engl J Med. 2008;358:2776–2786.
- , , , , , . Apixaban or enoxaparin for thromboprophylaxis after knee replacement. N Engl J Med. 2009;361:594–604.
- , , , , , . Apixaban versus enoxaparin for thromboprophylaxis after knee replacement (ADVANCE‐2): a randomized double‐blind trial. Lancet. 2010;275:807–815.
- , , , et al. Rivaroxaban for the prevention of venous thromboembolism after hip or knee arthroplasty. Pooled analysis of four studies. Thromb Haemost. 2011;105:444–453.
- , , , et al. Apixaban versus enoxaparin for thromboprophylaxis in medically ill patients. N Engl J Med. 2011;365:2167–2177.
- , , , et al. Efficacy and safety of thromboprophylaxis with low‐molecular‐weight heparin or rivaroxaban in hip and knee replacement surgery: findings from the ORTHO‐TEP registry. Thromb Haemost. 2013;109:154–163.
- , , , et al. Rivaroxaban for thromboprophylaxis in acutely ill medical patients. N Engl J Med. 2013;368:513–523.
The 2012 American College of Chest Physicians (ACCP) guidelines on antithrombotic and thrombolytic therapy conducted a systematic review focusing on patient values and preferences regarding antithrombotic therapy, including thromboprophylaxis.[1] They found that patient values and preferences are highly variable and should be considered when developing future clinical practice guidelines. Notably, there were no studies evaluating patient preferences for venous thromboembolism (VTE) prophylaxis, which is prescribed for the vast majority of hospitalized patients.
Historically, interventions to prevent VTE have focused on increasing prescriptions of prophylaxis. At the Johns Hopkins Hospital, we implemented a mandatory clinical decision support tool in our computerized provider order entry system.[2] Following implementation of this tool, prescription of risk‐appropriate VTE prophylaxis dramatically increased for both medical and surgical patients.[3, 4, 5] These efforts were made with the implicit and incorrect assumption that prescribed medication doses will always be administered to patients, when in fact patient refusal is a leading cause of nonadministration. Studies of VTE prophylaxis administration have reported that 10% to 12% of doses are not administered to patients.[6] Alarmingly, it has been reported that among medically ill patients, between 10% and 30% of doses are not administered, with patient refusal as the most frequently documented reason.
The purpose of this study was to assess patient preferences regarding pharmacological VTE prophylaxis.
METHODS
Study Design
A sample of consecutive hospitalized patients on select medicine and surgical floors previously identified as low‐ and high‐performing units at our institution in regard to administration rates of pharmacologic VTE prophylaxis was assembled from a daily electronic report of patients prescribed pharmacological VTE prophylaxis (Allscripts Sunrise, Chicago, IL) from December 2012 to March 2013. These units were identified in a study conducted at our institution as the lowest‐ and highest‐performing units in regard to incidence of administration of ordered pharmacologic VTE prophylaxis. From this data analysis, we chose the 2 lowest‐performing and 2 highest‐performing units on the medical and surgical service. To be eligible for this study, patients had to have an active order for 1 of the following VTE prophylaxis regimens: unfractionated heparin 5000 units or 7500 units administered subcutaneously every 8 or 12 hours, enoxaparin 30 mg administered subcutaneously every 12 hours or 40 mg administered subcutaneously every 24 hours. Participants had to be at least 18 years of age and hospitalized for at least 2 days on their respective units. Patients who were nonEnglish speaking, those previously enrolled in this study, or those unable to provide consent were excluded from the study.
Data Collection
Demographic information was collected, including patient‐reported education level. To determine their preference for VTE prophylaxis, patients were provided a survey, which included being asked, Would you prefer a pill or a shot to prevent blood clots, if they both worked equally well. The survey was created by the study team to collect information from patients regarding their baseline knowledge of VTE and preference regarding pharmacologic prophylaxis. Additional data included the patient's education level to determine potential association with preference. The survey was verbally administered by 1 investigator (A.W.) to all patients. Patients were asked to explain their rationale for their stated preference in regard to VTE prophylaxis. Patient rationale was subsequently coded to allow for uniformity among patient responses based on patterns in responses. Our electronic medication record allows us to identify patients who refused their medication through nursing documentation. Patients with documented refusal of ordered pharmacologic VTE prophylaxis were asked about the rationale for their refusal. This study was approved by the Johns Hopkins Medicine Institutional Review Board.
Statistical Analysis
Quantitative data from the surveys were analyzed using Minitab (Minitab Inc., State College, PA). A [2] test analysis was performed for categorical data, as appropriate. A P value <0.05 was considered to be statistically significant.
RESULTS
Quantitative Results
We interviewed patients regarding their preferred route of administration of VTE prophylaxis. Overall, 339 patients were screened for this study. Sixty patients were not eligible to participate. Forty‐seven were unable to provide consent, and 13 were nonEnglish speaking. Of the 269 remaining eligible patients, 227 (84.4%) consented to participate.
Baseline demographics of the participants are presented in Table 1, categorized on the basis of their preferred route of administration for VTE prophylaxis. A majority of patients indicated a preference for an oral formulation of pharmacologic VTE prophylaxis. There was no association between education level or service type on preference. Preference for an oral formulation was largely influenced by patient‐reported pain and bruising associated with subcutaneous administration (Table 2). A substantial majority of patients reporting a preference for a subcutaneous formulation and emphasized a belief that this route was associated with a faster onset of action. Among patients who preferred an oral formulation (n=137), 71 patients (51.8%) were documented as having refused at least 1 dose of ordered VTE prophylaxis. Patients who preferred a subcutaneous route of VTE prophylaxis were less likely to refuse prophylaxis, with only 22 patients (35.5%) having a documented refusal of at least 1 dose (P<0.0001).
| Enteral, n=137 | Parenteral, n=62 | No Preference, n=28 | |
|---|---|---|---|
| |||
| Age, y, mean ( SD) | 49.5 (14.7) | 51.7 (16.1) | 48.9 (14.6) |
| Male, n (%) | 74 (54.0) | 38 (61.3) | 15 (53.6) |
| Race n (%) | |||
| Caucasian | 81 (59.1) | 31 (50.0) | 14 (50.0) |
| African American | 50 (36.5) | 28 (45.2) | 14 (50.0) |
| Education level, n (%) | |||
| High school or less | 46 (33.6) | 27 (43.5) | 14 (50.0) |
| College | 68 (49.6) | 21 (33.9) | 9 (32.1) |
| Advanced degree | 10 (7.3) | 8 (12.9) | 2 (7.1) |
| Unable to obtain | 13 (9.5) | 6 (9.7) | 3 (10.8) |
| Past history of VTE, n (%) | 12 (8.8) | 9 (14.5) | 2 (7.1) |
| Type of unit, n (%) | |||
| Medical | 59 (43.1) | 24 (38.7) | 17 (60.7) |
| Surgical | 78 (56.9) | 38 (61.3) | 11 (39.3) |
| Documented refusal of ordered prophylaxis, n (%) | 71 (51.8) | 20 (32.3) | 9 (32.1) |
| Length of hospital stay prior to inclusion in study, d, median (IQR) | 4.0 (3.07.0) | 3.0 (3.05.0) | 4.0 (2.05.0) |
| Patients preferring enteral route, n (%) | 137 (60.4) |
| Dislike of needles | 41 (30.0) |
| Pain from injection | 38 (27.7) |
| Ease of use | 18 (13.1) |
| Bruising from injection | 9 (6.6) |
| Other/no rationale | 31 (22.6) |
| Patients preferring injection route, n (%) | 62 (27.5) |
| Faster onset of action | 25 (40.3) |
| Pill burden | 11 (17.7) |
| Ease of use | 9 (14.5) |
| Other/no rationale | 17 (27.5) |
| Patients with no preference, n (%) | 28 (12.4) |
DISCUSSION
Using a mixed‐methods approach, we report the first survey evaluating patient preferences regarding pharmacologic VTE prophylaxis. We found that a majority of patients preferred an oral route of administration. Nevertheless, a substantial number of patients favored a subcutaneous route of administration believing it to be associated with a faster onset of action. Of interest, patients favoring subcutaneous injections were significantly less likely to refuse doses of ordered VTE prophylaxis. Given that all patients were prescribed a subcutaneous form of VTE prophylaxis, matching patient preference to VTE prophylaxis prescription could potentially increase adherence and reduce patient refusal of ordered prophylaxis. Considering the large number of patients who preferred an oral route of administration, the availability of an oral formulation may potentially result in improved adherence to inpatient VTE prophylaxis.
Our findings have significant implications for healthcare providers, and for patient safety and quality‐improvement researchers. VTE prophylaxis is an important patient‐safety practice, particularly for medically ill patients, which is believed to be underprescribed.[7] Recent studies have demonstrated that a significant number of doses of VTE prophylaxis are not administered, primarily due to patient refusal.[6] Our data indicate that tailoring the route of prophylaxis administration to patient preference may represent a feasible strategy to improve VTE prophylaxis administration rates. Recently, several target‐specific oral anticoagulants (TSOACs) have been approved for a variety of clinical indications, and all have been investigated for VTE prophylaxis.[7, 8, 9, 10, 11, 12, 13, 14, 15] However, no agent is currently US Food & Drug Administration (FDA) approved for primary prevention of VTE, although apixaban and rivaroxaban are FDA approved for VTE prevention in joint replacement.[13, 14] Although in some instances these TSOACs were noted to demonstrate only equivalent efficacy to standard subcutaneous forms of VTE prophylaxis, our data suggest that perhaps in some patients, use of these agents may result in better outcomes due to improved adherence to therapy due to a preferred oral route of administration. We think this hypothesis warrants further investigation.
Our study also underscores the importance of considering patient preferences when caring for patients as emphasized by the 2012 ACCP guidelines.[1] Our results indicate that consideration of patient preferences may lead to better patient care and better outcomes. Interestingly, there were no differences in preference based on education level or the type of service to which the patient was admitted. Clarification of uninformed opinions regarding the rationale for preference may also lead to more informed decisions by patients.
This study has a number of limitations. We only included patients on the internal medicine and general surgical services. It is possible that patients on other specialty services may have different opinions regarding prophylaxis that were not captured in our sample. Similarly, our sample size was limited, and approximately 15% of potential subjects did not participate. We do believe that our population is reflective of our institution based upon our previously published evaluation of multiple hospital units and the inclusion of low‐ and high‐performing units on both the medical and surgical services. Nevertheless, we believe that much more investigation of patient perspectives on VTE prophylaxis needs to be done to inform decision making, including the impact of patient preferences on VTE‐related outcomes. Additionally, we did not evaluate potential predictors of preference including admission diagnosis and duration of hospital length of stay.
In conclusion, we conducted a mixed‐methods analysis of patient preferences regarding pharmacologic VTE prophylaxis. Matching patient preference to ordered VTE prophylaxis may increase adherence to ordered prophylaxis. In this era of increasingly patient‐centered healthcare and expanding options for VTE prophylaxis, we believe information on patient preferences will be helpful to tailoring options for prevention and treatment.
ACKNOWLEDGMENTS
Disclosures: Dr. Haut is the primary investigator of the Mentored Clinician Scientist Development Award K08 1K08HS017952‐01 from the Agency for Healthcare Research and Quality entitled Does Screening Variability Make DVT an Unreliable Quality Measure of Trauma Care? Dr. Haut receives royalties from Lippincott, Williams, & Wilkins for a book he coauthored (Avoiding Common ICU Errors). He has received honoraria for various speaking engagements regarding clinical, quality, and safety topics and has given expert witness testimony in various medical malpractice cases. Dr. Streiff has received research funding from Sanofi‐Aventis and Bristol‐Myers Squibb; honoraria for Continuing Medial Education lectures from Sanofi‐Aventis and Ortho‐McNeil; consulted for Sanofi‐Aventis, Eisai, Daiichi‐Sankyo, and Janssen HealthCare; and has given expert witness testimony in various medical malpractice cases. Mr. Lau, Drs. Haut, Streiff, and Shermock are supported by a contract from the Patient‐Centered Outcomes Research Institute titled Preventing Venous Thromboembolism: Empowering Patients and Enabling Patient‐Centered Care via Health Information Technology (CE‐12‐11‐4489). Ms. Hobson has given expert witness testimony in various medical malpractice cases. All others have no relevant funding or conflicts of interest to report.
The 2012 American College of Chest Physicians (ACCP) guidelines on antithrombotic and thrombolytic therapy conducted a systematic review focusing on patient values and preferences regarding antithrombotic therapy, including thromboprophylaxis.[1] They found that patient values and preferences are highly variable and should be considered when developing future clinical practice guidelines. Notably, there were no studies evaluating patient preferences for venous thromboembolism (VTE) prophylaxis, which is prescribed for the vast majority of hospitalized patients.
Historically, interventions to prevent VTE have focused on increasing prescriptions of prophylaxis. At the Johns Hopkins Hospital, we implemented a mandatory clinical decision support tool in our computerized provider order entry system.[2] Following implementation of this tool, prescription of risk‐appropriate VTE prophylaxis dramatically increased for both medical and surgical patients.[3, 4, 5] These efforts were made with the implicit and incorrect assumption that prescribed medication doses will always be administered to patients, when in fact patient refusal is a leading cause of nonadministration. Studies of VTE prophylaxis administration have reported that 10% to 12% of doses are not administered to patients.[6] Alarmingly, it has been reported that among medically ill patients, between 10% and 30% of doses are not administered, with patient refusal as the most frequently documented reason.
The purpose of this study was to assess patient preferences regarding pharmacological VTE prophylaxis.
METHODS
Study Design
A sample of consecutive hospitalized patients on select medicine and surgical floors previously identified as low‐ and high‐performing units at our institution in regard to administration rates of pharmacologic VTE prophylaxis was assembled from a daily electronic report of patients prescribed pharmacological VTE prophylaxis (Allscripts Sunrise, Chicago, IL) from December 2012 to March 2013. These units were identified in a study conducted at our institution as the lowest‐ and highest‐performing units in regard to incidence of administration of ordered pharmacologic VTE prophylaxis. From this data analysis, we chose the 2 lowest‐performing and 2 highest‐performing units on the medical and surgical service. To be eligible for this study, patients had to have an active order for 1 of the following VTE prophylaxis regimens: unfractionated heparin 5000 units or 7500 units administered subcutaneously every 8 or 12 hours, enoxaparin 30 mg administered subcutaneously every 12 hours or 40 mg administered subcutaneously every 24 hours. Participants had to be at least 18 years of age and hospitalized for at least 2 days on their respective units. Patients who were nonEnglish speaking, those previously enrolled in this study, or those unable to provide consent were excluded from the study.
Data Collection
Demographic information was collected, including patient‐reported education level. To determine their preference for VTE prophylaxis, patients were provided a survey, which included being asked, Would you prefer a pill or a shot to prevent blood clots, if they both worked equally well. The survey was created by the study team to collect information from patients regarding their baseline knowledge of VTE and preference regarding pharmacologic prophylaxis. Additional data included the patient's education level to determine potential association with preference. The survey was verbally administered by 1 investigator (A.W.) to all patients. Patients were asked to explain their rationale for their stated preference in regard to VTE prophylaxis. Patient rationale was subsequently coded to allow for uniformity among patient responses based on patterns in responses. Our electronic medication record allows us to identify patients who refused their medication through nursing documentation. Patients with documented refusal of ordered pharmacologic VTE prophylaxis were asked about the rationale for their refusal. This study was approved by the Johns Hopkins Medicine Institutional Review Board.
Statistical Analysis
Quantitative data from the surveys were analyzed using Minitab (Minitab Inc., State College, PA). A [2] test analysis was performed for categorical data, as appropriate. A P value <0.05 was considered to be statistically significant.
RESULTS
Quantitative Results
We interviewed patients regarding their preferred route of administration of VTE prophylaxis. Overall, 339 patients were screened for this study. Sixty patients were not eligible to participate. Forty‐seven were unable to provide consent, and 13 were nonEnglish speaking. Of the 269 remaining eligible patients, 227 (84.4%) consented to participate.
Baseline demographics of the participants are presented in Table 1, categorized on the basis of their preferred route of administration for VTE prophylaxis. A majority of patients indicated a preference for an oral formulation of pharmacologic VTE prophylaxis. There was no association between education level or service type on preference. Preference for an oral formulation was largely influenced by patient‐reported pain and bruising associated with subcutaneous administration (Table 2). A substantial majority of patients reporting a preference for a subcutaneous formulation and emphasized a belief that this route was associated with a faster onset of action. Among patients who preferred an oral formulation (n=137), 71 patients (51.8%) were documented as having refused at least 1 dose of ordered VTE prophylaxis. Patients who preferred a subcutaneous route of VTE prophylaxis were less likely to refuse prophylaxis, with only 22 patients (35.5%) having a documented refusal of at least 1 dose (P<0.0001).
| Enteral, n=137 | Parenteral, n=62 | No Preference, n=28 | |
|---|---|---|---|
| |||
| Age, y, mean ( SD) | 49.5 (14.7) | 51.7 (16.1) | 48.9 (14.6) |
| Male, n (%) | 74 (54.0) | 38 (61.3) | 15 (53.6) |
| Race n (%) | |||
| Caucasian | 81 (59.1) | 31 (50.0) | 14 (50.0) |
| African American | 50 (36.5) | 28 (45.2) | 14 (50.0) |
| Education level, n (%) | |||
| High school or less | 46 (33.6) | 27 (43.5) | 14 (50.0) |
| College | 68 (49.6) | 21 (33.9) | 9 (32.1) |
| Advanced degree | 10 (7.3) | 8 (12.9) | 2 (7.1) |
| Unable to obtain | 13 (9.5) | 6 (9.7) | 3 (10.8) |
| Past history of VTE, n (%) | 12 (8.8) | 9 (14.5) | 2 (7.1) |
| Type of unit, n (%) | |||
| Medical | 59 (43.1) | 24 (38.7) | 17 (60.7) |
| Surgical | 78 (56.9) | 38 (61.3) | 11 (39.3) |
| Documented refusal of ordered prophylaxis, n (%) | 71 (51.8) | 20 (32.3) | 9 (32.1) |
| Length of hospital stay prior to inclusion in study, d, median (IQR) | 4.0 (3.07.0) | 3.0 (3.05.0) | 4.0 (2.05.0) |
| Patients preferring enteral route, n (%) | 137 (60.4) |
| Dislike of needles | 41 (30.0) |
| Pain from injection | 38 (27.7) |
| Ease of use | 18 (13.1) |
| Bruising from injection | 9 (6.6) |
| Other/no rationale | 31 (22.6) |
| Patients preferring injection route, n (%) | 62 (27.5) |
| Faster onset of action | 25 (40.3) |
| Pill burden | 11 (17.7) |
| Ease of use | 9 (14.5) |
| Other/no rationale | 17 (27.5) |
| Patients with no preference, n (%) | 28 (12.4) |
DISCUSSION
Using a mixed‐methods approach, we report the first survey evaluating patient preferences regarding pharmacologic VTE prophylaxis. We found that a majority of patients preferred an oral route of administration. Nevertheless, a substantial number of patients favored a subcutaneous route of administration believing it to be associated with a faster onset of action. Of interest, patients favoring subcutaneous injections were significantly less likely to refuse doses of ordered VTE prophylaxis. Given that all patients were prescribed a subcutaneous form of VTE prophylaxis, matching patient preference to VTE prophylaxis prescription could potentially increase adherence and reduce patient refusal of ordered prophylaxis. Considering the large number of patients who preferred an oral route of administration, the availability of an oral formulation may potentially result in improved adherence to inpatient VTE prophylaxis.
Our findings have significant implications for healthcare providers, and for patient safety and quality‐improvement researchers. VTE prophylaxis is an important patient‐safety practice, particularly for medically ill patients, which is believed to be underprescribed.[7] Recent studies have demonstrated that a significant number of doses of VTE prophylaxis are not administered, primarily due to patient refusal.[6] Our data indicate that tailoring the route of prophylaxis administration to patient preference may represent a feasible strategy to improve VTE prophylaxis administration rates. Recently, several target‐specific oral anticoagulants (TSOACs) have been approved for a variety of clinical indications, and all have been investigated for VTE prophylaxis.[7, 8, 9, 10, 11, 12, 13, 14, 15] However, no agent is currently US Food & Drug Administration (FDA) approved for primary prevention of VTE, although apixaban and rivaroxaban are FDA approved for VTE prevention in joint replacement.[13, 14] Although in some instances these TSOACs were noted to demonstrate only equivalent efficacy to standard subcutaneous forms of VTE prophylaxis, our data suggest that perhaps in some patients, use of these agents may result in better outcomes due to improved adherence to therapy due to a preferred oral route of administration. We think this hypothesis warrants further investigation.
Our study also underscores the importance of considering patient preferences when caring for patients as emphasized by the 2012 ACCP guidelines.[1] Our results indicate that consideration of patient preferences may lead to better patient care and better outcomes. Interestingly, there were no differences in preference based on education level or the type of service to which the patient was admitted. Clarification of uninformed opinions regarding the rationale for preference may also lead to more informed decisions by patients.
This study has a number of limitations. We only included patients on the internal medicine and general surgical services. It is possible that patients on other specialty services may have different opinions regarding prophylaxis that were not captured in our sample. Similarly, our sample size was limited, and approximately 15% of potential subjects did not participate. We do believe that our population is reflective of our institution based upon our previously published evaluation of multiple hospital units and the inclusion of low‐ and high‐performing units on both the medical and surgical services. Nevertheless, we believe that much more investigation of patient perspectives on VTE prophylaxis needs to be done to inform decision making, including the impact of patient preferences on VTE‐related outcomes. Additionally, we did not evaluate potential predictors of preference including admission diagnosis and duration of hospital length of stay.
In conclusion, we conducted a mixed‐methods analysis of patient preferences regarding pharmacologic VTE prophylaxis. Matching patient preference to ordered VTE prophylaxis may increase adherence to ordered prophylaxis. In this era of increasingly patient‐centered healthcare and expanding options for VTE prophylaxis, we believe information on patient preferences will be helpful to tailoring options for prevention and treatment.
ACKNOWLEDGMENTS
Disclosures: Dr. Haut is the primary investigator of the Mentored Clinician Scientist Development Award K08 1K08HS017952‐01 from the Agency for Healthcare Research and Quality entitled Does Screening Variability Make DVT an Unreliable Quality Measure of Trauma Care? Dr. Haut receives royalties from Lippincott, Williams, & Wilkins for a book he coauthored (Avoiding Common ICU Errors). He has received honoraria for various speaking engagements regarding clinical, quality, and safety topics and has given expert witness testimony in various medical malpractice cases. Dr. Streiff has received research funding from Sanofi‐Aventis and Bristol‐Myers Squibb; honoraria for Continuing Medial Education lectures from Sanofi‐Aventis and Ortho‐McNeil; consulted for Sanofi‐Aventis, Eisai, Daiichi‐Sankyo, and Janssen HealthCare; and has given expert witness testimony in various medical malpractice cases. Mr. Lau, Drs. Haut, Streiff, and Shermock are supported by a contract from the Patient‐Centered Outcomes Research Institute titled Preventing Venous Thromboembolism: Empowering Patients and Enabling Patient‐Centered Care via Health Information Technology (CE‐12‐11‐4489). Ms. Hobson has given expert witness testimony in various medical malpractice cases. All others have no relevant funding or conflicts of interest to report.
- , , , et al. Patient values and preferences in decision making for antithrombotic therapy: a systematic review. Chest. 2012;141(2):e1S–e23S.
- , , , et al. Lessons from the Johns Hopkins Multi‐Disciplinary Venous Thromboembolism (VTE) Prevention Collaborative. BMJ. 2012;344:e3935.
- , , , et al. Impact of a venous thromboembolism (VTE) prophylaxis “smart order set”: improved compliance, fewer events. Am J Hematol. 2013;88(7):545–549.
- , , , et al. Improved prophylaxis and decreased preventable harm with a mandatory computerized clinical decision support tool for venous thromboembolism (VTE) prophylaxis in trauma patients. Arch Surg. 2012;147(10):901–907.
- , , , , . Linking processes and outcomes: a key strategy to prevent and report harm from venous thromboembolism in surgical patients. JAMA Surg. 2013;148(3):299–300.
- , , , et al. Patterns of non‐administration of ordered doses of venous thromboembolism prophylaxis: implications for intervention strategies. PLoS One. 2013;8(6):e66311.
- , , , et al. Venous thromboembolism risk and prophylaxis in the acute hospital care setting (ENDORSE study): a multinational cross‐sectional study. Lancet. 2008;371:387–394.
- , , , et al. Rivaroxaban versus enoxaparin for thromboprophylaxis after hip arthroplasty. N Engl J Med. 2008;358:2765–2775.
- , , , et al. Rivaroxaban versus enoxaparin for thromboprophylaxis after total knee arthoplasty. N Engl J Med. 2008;358:2776–2786.
- , , , , , . Apixaban or enoxaparin for thromboprophylaxis after knee replacement. N Engl J Med. 2009;361:594–604.
- , , , , , . Apixaban versus enoxaparin for thromboprophylaxis after knee replacement (ADVANCE‐2): a randomized double‐blind trial. Lancet. 2010;275:807–815.
- , , , et al. Rivaroxaban for the prevention of venous thromboembolism after hip or knee arthroplasty. Pooled analysis of four studies. Thromb Haemost. 2011;105:444–453.
- , , , et al. Apixaban versus enoxaparin for thromboprophylaxis in medically ill patients. N Engl J Med. 2011;365:2167–2177.
- , , , et al. Efficacy and safety of thromboprophylaxis with low‐molecular‐weight heparin or rivaroxaban in hip and knee replacement surgery: findings from the ORTHO‐TEP registry. Thromb Haemost. 2013;109:154–163.
- , , , et al. Rivaroxaban for thromboprophylaxis in acutely ill medical patients. N Engl J Med. 2013;368:513–523.
- , , , et al. Patient values and preferences in decision making for antithrombotic therapy: a systematic review. Chest. 2012;141(2):e1S–e23S.
- , , , et al. Lessons from the Johns Hopkins Multi‐Disciplinary Venous Thromboembolism (VTE) Prevention Collaborative. BMJ. 2012;344:e3935.
- , , , et al. Impact of a venous thromboembolism (VTE) prophylaxis “smart order set”: improved compliance, fewer events. Am J Hematol. 2013;88(7):545–549.
- , , , et al. Improved prophylaxis and decreased preventable harm with a mandatory computerized clinical decision support tool for venous thromboembolism (VTE) prophylaxis in trauma patients. Arch Surg. 2012;147(10):901–907.
- , , , , . Linking processes and outcomes: a key strategy to prevent and report harm from venous thromboembolism in surgical patients. JAMA Surg. 2013;148(3):299–300.
- , , , et al. Patterns of non‐administration of ordered doses of venous thromboembolism prophylaxis: implications for intervention strategies. PLoS One. 2013;8(6):e66311.
- , , , et al. Venous thromboembolism risk and prophylaxis in the acute hospital care setting (ENDORSE study): a multinational cross‐sectional study. Lancet. 2008;371:387–394.
- , , , et al. Rivaroxaban versus enoxaparin for thromboprophylaxis after hip arthroplasty. N Engl J Med. 2008;358:2765–2775.
- , , , et al. Rivaroxaban versus enoxaparin for thromboprophylaxis after total knee arthoplasty. N Engl J Med. 2008;358:2776–2786.
- , , , , , . Apixaban or enoxaparin for thromboprophylaxis after knee replacement. N Engl J Med. 2009;361:594–604.
- , , , , , . Apixaban versus enoxaparin for thromboprophylaxis after knee replacement (ADVANCE‐2): a randomized double‐blind trial. Lancet. 2010;275:807–815.
- , , , et al. Rivaroxaban for the prevention of venous thromboembolism after hip or knee arthroplasty. Pooled analysis of four studies. Thromb Haemost. 2011;105:444–453.
- , , , et al. Apixaban versus enoxaparin for thromboprophylaxis in medically ill patients. N Engl J Med. 2011;365:2167–2177.
- , , , et al. Efficacy and safety of thromboprophylaxis with low‐molecular‐weight heparin or rivaroxaban in hip and knee replacement surgery: findings from the ORTHO‐TEP registry. Thromb Haemost. 2013;109:154–163.
- , , , et al. Rivaroxaban for thromboprophylaxis in acutely ill medical patients. N Engl J Med. 2013;368:513–523.