User login
Red patches on the tongue with white borders • history of geographic tongue • incompletely treated celiac disease • Dx?
THE CASE
A 49-year-old woman presented to our clinic with concerns about the changing appearance of her tongue over the past 2 to 3 weeks. She had been given a diagnosis of celiac disease by her gastroenterologist approximately 5 years earlier. At the time of that diagnosis, she had smooth patches on the surface of her tongue with missing papillae and slightly raised borders. (This gave her tongue a map-like appearance, consistent with geographic tongue [GT].) The patient’s symptoms improved after she started a gluten-free diet, but she reported occasional noncompliance over the past year.
At the current presentation, the patient noted that new lesions on the tongue had started as diffuse shiny red patches surrounded by clearly delineated white borders, ultimately progressing to structural changes. She denied any burning of the tongue or other oral symptoms but reported feelings of anxiety, a “foggy mind,” and diffuse arthralgia for the past several weeks. The patient’s list of medications included vitamin D and magnesium supplements, a multivitamin, and probiotics.
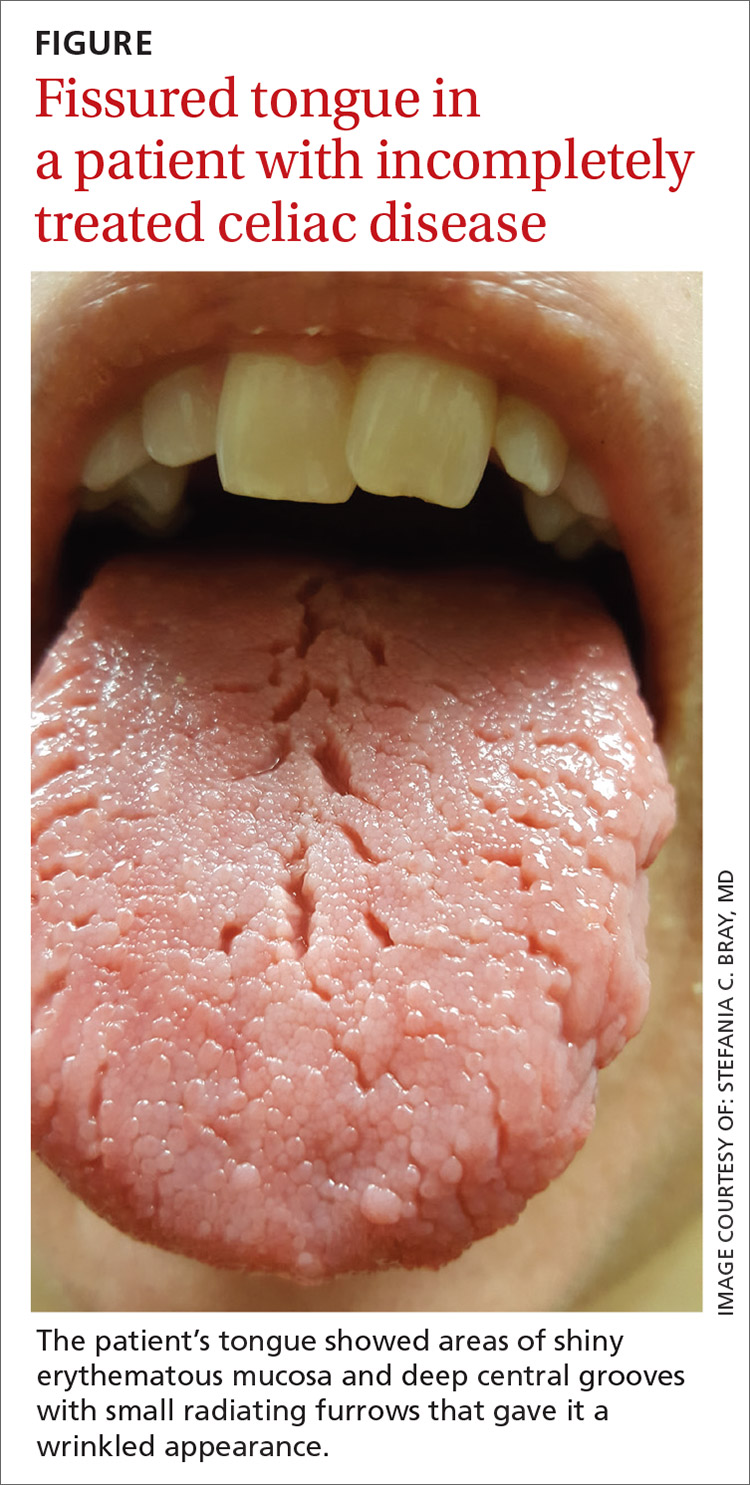
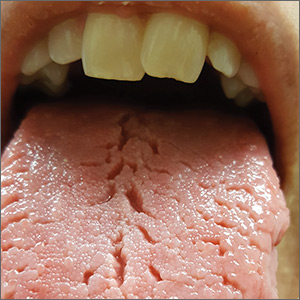
On physical examination, her tongue showed areas of shiny erythematous mucosa and deep central grooves with small radiating furrows giving a wrinkled appearance (FIGURE). A review of systems revealed nonspecific abdominal pain including bloating, cramping, and gas for the previous few months. An examination of her throat and oral cavity was unremarkable, and the remainder of the physical examination was normal.
THE DIAGNOSIS
A diagnosis of fissured tongue (FT) was suspected based on the clinical appearance of the patient’s tongue. Laboratory studies including a complete blood count; antinuclear antibody test; rheumatoid factor test; anticyclic citrullinated peptide test; a comprehensive metabolic panel; and thyroid-stimulating hormone, 25-hydroxyvitamin D, and vitamin B₁₂ level tests were performed based on her symptoms and current medications to rule out any other potential diagnoses. All laboratory results were normal, and a tissue transglutaminase IgA test was not repeated because it was positive when previously tested by the gastroenterologist at the time of her celiac disease diagnosis. A diagnosis of FT due to incompletely treated celiac disease was confirmed.
DISCUSSION
Clinical presentation. FT commonly presents in association with GT,1,2 with some cases of GT naturally progressing to FT.3,4 In most cases, FT is asymptomatic unless debris becomes entrapped in the fissures. Rarely, patients may complain of a burning sensation on the tongue. The clinical appearance of the tongue includes deep grooves with possible malodor or halitosis along with discoloration if trapping of debris and subsequent inflammation occurs.1
Etiology. FT has been linked to celiac disease; systemic conditions such as arthritis, iron deficiency, depression, anxiety, and neuropathy; and poor oral hygiene. Genetics also may play a role, as some cases of FT may be inherited. Getting to the source requires a careful history to uncover signs and symptoms (that may not have been reported until now) and to determine if other family members also have FT. A careful examination of the oral cavity, with an eye toward the patient’s oral hygiene, is also instructive (TABLE).5-8 In general, FT is believed to be a normal tongue variant in less than 10% of the general population.5,6 Additionally, local factors such as ill-fitting prosthesis, infection, parafunctional habits, allergic reaction, xerostomia, and galvanism have been implicated in the etiology of FT.5
In our patient, progression of GT to FT was caused by incompletely treated celiac disease. Both FT and GT may represent different reaction patterns caused by the same hematologic and immunologic diseases.3 In fact, the appearance of the tongue may aid in the diagnosis of celiac disease, which has been observed in 15% of patients with GT.7 Fissured tongue also may indicate an inability of the gastrointestinal mucosa to absorb nutrients; therefore, close nutrition monitoring is recommended.9
Continue to: Other oral and dental manifestations...
Other oral and dental manifestations of celiac disease include enamel defects, delayed tooth eruption, recurrent aphthous ulcers, cheilosis, oral lichen planus, and atrophic glossitis.10 Our patient also reported anxiety, “foggy mind,” diffuse arthralgia, and abdominal pain, which are symptoms of uncontrolled celiac disease. There is no known etiology of tongue manifestations in patients with incompletely treated celiac disease.
Treatment. FT generally does not require specific therapy other than the treatment of the underlying inflammatory condition. It is important to maintain proper oral and dental care, such as brushing the top surface of the tongue to clean and remove food debris. Bacteria and plaque can collect in the fissures, leading to bad breath and an increased potential for tooth decay.
Our patient was referred to a dietitian to assist with adherence to the gluten-free diet. At follow-up 3 months later, the appearance of her tongue had improved and fewer fissures were visible. The majority of her other symptoms also had resolved.
THE TAKEAWAY
FT may be a normal variant of the tongue in some patients or may be associated with poor oral hygiene. Additionally, FT often is associated with an underlying medical or inherited condition and may serve as a marker for an untreated or partially treated condition such as celiac disease, as was the case with our patient. When other signs or symptoms of systemic disease are present, further laboratory and endoscopic workup is necessary to rule out other causes and to diagnose celiac disease, if present.
As FT has been reported to be a natural progression from GT, the appearance of FT may indicate partial treatment of the underlying disease process and therefore more intensive therapy and follow-up would be needed. In this case, more intensive dietary guidance was provided with subsequent improvement of symptoms.
CORRESPONDENCE
Peter J. Carek, MD, MS, Department of Community Health and Family Medicine, College of Medicine, University of Florida, P.O. Box 100237, Gainesville, FL 32610-0237; carek@ufl.edu
1. Reamy BV, Cerby R, Bunt CW. Common tongue conditions in primary care. Am Fam Physician. 2010;81:627-634.
2. Yarom N, Cantony U, Gorsky M. Prevalence of fissured tongue, geographic tongue and median rhomboid glossitis among Israeli adults of different ethnic origins. Dermatology. 2004;209:88-94.
3. Dafar A, Cevik-Aras H, Robledo-Sierra J, et al. Factors associated with geographic tongue and fissured tongue. Acta Odontol Scad. 2016;74:210-216.
4. Hume WJ. Geographic stomatitis: a critical review. J Dent. 1975;3:25-43.
5. Sudarshan R, Sree Vijayabala G, Samata Y, et al. Newer classification system for fissured tongue: an epidemiological approach. J Tropical Med. doi:10.1155/2015/262079.
6. Mangold AR, Torgerson RR, Rogers RS. Diseases of the tongue. Clin Dermatol. 2016;34:458-469.
7. Cigic L, Galic T, Kero D, et al. The prevalence of celiac disease in patients with geographic tongue. J Oral Pathol Med. 2016;45:791-796.
8. Zargari O. The prevalence and significance of fissured tongue and geographical tongue in psoriatic patients. Clin Exp Dermatology. 2006;31:192-195.
9. Kullaa-Mikkonen A, Penttila I, Kotilainen R, et al. Haematological and immunological features of patients with fissured tongue syndrome. Br J Oral Maxillofac Surg. 1987;25:481-487.
10. Rashid M, Zarkadas M, Anca A, et al. Oral manifestations of celiac disease: a clinical guide for dentists. J Can Dent Assoc. 2011;77:b39.
THE CASE
A 49-year-old woman presented to our clinic with concerns about the changing appearance of her tongue over the past 2 to 3 weeks. She had been given a diagnosis of celiac disease by her gastroenterologist approximately 5 years earlier. At the time of that diagnosis, she had smooth patches on the surface of her tongue with missing papillae and slightly raised borders. (This gave her tongue a map-like appearance, consistent with geographic tongue [GT].) The patient’s symptoms improved after she started a gluten-free diet, but she reported occasional noncompliance over the past year.
At the current presentation, the patient noted that new lesions on the tongue had started as diffuse shiny red patches surrounded by clearly delineated white borders, ultimately progressing to structural changes. She denied any burning of the tongue or other oral symptoms but reported feelings of anxiety, a “foggy mind,” and diffuse arthralgia for the past several weeks. The patient’s list of medications included vitamin D and magnesium supplements, a multivitamin, and probiotics.
On physical examination, her tongue showed areas of shiny erythematous mucosa and deep central grooves with small radiating furrows giving a wrinkled appearance (FIGURE). A review of systems revealed nonspecific abdominal pain including bloating, cramping, and gas for the previous few months. An examination of her throat and oral cavity was unremarkable, and the remainder of the physical examination was normal.
THE DIAGNOSIS
A diagnosis of fissured tongue (FT) was suspected based on the clinical appearance of the patient’s tongue. Laboratory studies including a complete blood count; antinuclear antibody test; rheumatoid factor test; anticyclic citrullinated peptide test; a comprehensive metabolic panel; and thyroid-stimulating hormone, 25-hydroxyvitamin D, and vitamin B₁₂ level tests were performed based on her symptoms and current medications to rule out any other potential diagnoses. All laboratory results were normal, and a tissue transglutaminase IgA test was not repeated because it was positive when previously tested by the gastroenterologist at the time of her celiac disease diagnosis. A diagnosis of FT due to incompletely treated celiac disease was confirmed.
DISCUSSION
Clinical presentation. FT commonly presents in association with GT,1,2 with some cases of GT naturally progressing to FT.3,4 In most cases, FT is asymptomatic unless debris becomes entrapped in the fissures. Rarely, patients may complain of a burning sensation on the tongue. The clinical appearance of the tongue includes deep grooves with possible malodor or halitosis along with discoloration if trapping of debris and subsequent inflammation occurs.1
Etiology. FT has been linked to celiac disease; systemic conditions such as arthritis, iron deficiency, depression, anxiety, and neuropathy; and poor oral hygiene. Genetics also may play a role, as some cases of FT may be inherited. Getting to the source requires a careful history to uncover signs and symptoms (that may not have been reported until now) and to determine if other family members also have FT. A careful examination of the oral cavity, with an eye toward the patient’s oral hygiene, is also instructive (TABLE).5-8 In general, FT is believed to be a normal tongue variant in less than 10% of the general population.5,6 Additionally, local factors such as ill-fitting prosthesis, infection, parafunctional habits, allergic reaction, xerostomia, and galvanism have been implicated in the etiology of FT.5
In our patient, progression of GT to FT was caused by incompletely treated celiac disease. Both FT and GT may represent different reaction patterns caused by the same hematologic and immunologic diseases.3 In fact, the appearance of the tongue may aid in the diagnosis of celiac disease, which has been observed in 15% of patients with GT.7 Fissured tongue also may indicate an inability of the gastrointestinal mucosa to absorb nutrients; therefore, close nutrition monitoring is recommended.9
Continue to: Other oral and dental manifestations...
Other oral and dental manifestations of celiac disease include enamel defects, delayed tooth eruption, recurrent aphthous ulcers, cheilosis, oral lichen planus, and atrophic glossitis.10 Our patient also reported anxiety, “foggy mind,” diffuse arthralgia, and abdominal pain, which are symptoms of uncontrolled celiac disease. There is no known etiology of tongue manifestations in patients with incompletely treated celiac disease.
Treatment. FT generally does not require specific therapy other than the treatment of the underlying inflammatory condition. It is important to maintain proper oral and dental care, such as brushing the top surface of the tongue to clean and remove food debris. Bacteria and plaque can collect in the fissures, leading to bad breath and an increased potential for tooth decay.
Our patient was referred to a dietitian to assist with adherence to the gluten-free diet. At follow-up 3 months later, the appearance of her tongue had improved and fewer fissures were visible. The majority of her other symptoms also had resolved.
THE TAKEAWAY
FT may be a normal variant of the tongue in some patients or may be associated with poor oral hygiene. Additionally, FT often is associated with an underlying medical or inherited condition and may serve as a marker for an untreated or partially treated condition such as celiac disease, as was the case with our patient. When other signs or symptoms of systemic disease are present, further laboratory and endoscopic workup is necessary to rule out other causes and to diagnose celiac disease, if present.
As FT has been reported to be a natural progression from GT, the appearance of FT may indicate partial treatment of the underlying disease process and therefore more intensive therapy and follow-up would be needed. In this case, more intensive dietary guidance was provided with subsequent improvement of symptoms.
CORRESPONDENCE
Peter J. Carek, MD, MS, Department of Community Health and Family Medicine, College of Medicine, University of Florida, P.O. Box 100237, Gainesville, FL 32610-0237; carek@ufl.edu
THE CASE
A 49-year-old woman presented to our clinic with concerns about the changing appearance of her tongue over the past 2 to 3 weeks. She had been given a diagnosis of celiac disease by her gastroenterologist approximately 5 years earlier. At the time of that diagnosis, she had smooth patches on the surface of her tongue with missing papillae and slightly raised borders. (This gave her tongue a map-like appearance, consistent with geographic tongue [GT].) The patient’s symptoms improved after she started a gluten-free diet, but she reported occasional noncompliance over the past year.
At the current presentation, the patient noted that new lesions on the tongue had started as diffuse shiny red patches surrounded by clearly delineated white borders, ultimately progressing to structural changes. She denied any burning of the tongue or other oral symptoms but reported feelings of anxiety, a “foggy mind,” and diffuse arthralgia for the past several weeks. The patient’s list of medications included vitamin D and magnesium supplements, a multivitamin, and probiotics.
On physical examination, her tongue showed areas of shiny erythematous mucosa and deep central grooves with small radiating furrows giving a wrinkled appearance (FIGURE). A review of systems revealed nonspecific abdominal pain including bloating, cramping, and gas for the previous few months. An examination of her throat and oral cavity was unremarkable, and the remainder of the physical examination was normal.
THE DIAGNOSIS
A diagnosis of fissured tongue (FT) was suspected based on the clinical appearance of the patient’s tongue. Laboratory studies including a complete blood count; antinuclear antibody test; rheumatoid factor test; anticyclic citrullinated peptide test; a comprehensive metabolic panel; and thyroid-stimulating hormone, 25-hydroxyvitamin D, and vitamin B₁₂ level tests were performed based on her symptoms and current medications to rule out any other potential diagnoses. All laboratory results were normal, and a tissue transglutaminase IgA test was not repeated because it was positive when previously tested by the gastroenterologist at the time of her celiac disease diagnosis. A diagnosis of FT due to incompletely treated celiac disease was confirmed.
DISCUSSION
Clinical presentation. FT commonly presents in association with GT,1,2 with some cases of GT naturally progressing to FT.3,4 In most cases, FT is asymptomatic unless debris becomes entrapped in the fissures. Rarely, patients may complain of a burning sensation on the tongue. The clinical appearance of the tongue includes deep grooves with possible malodor or halitosis along with discoloration if trapping of debris and subsequent inflammation occurs.1
Etiology. FT has been linked to celiac disease; systemic conditions such as arthritis, iron deficiency, depression, anxiety, and neuropathy; and poor oral hygiene. Genetics also may play a role, as some cases of FT may be inherited. Getting to the source requires a careful history to uncover signs and symptoms (that may not have been reported until now) and to determine if other family members also have FT. A careful examination of the oral cavity, with an eye toward the patient’s oral hygiene, is also instructive (TABLE).5-8 In general, FT is believed to be a normal tongue variant in less than 10% of the general population.5,6 Additionally, local factors such as ill-fitting prosthesis, infection, parafunctional habits, allergic reaction, xerostomia, and galvanism have been implicated in the etiology of FT.5
In our patient, progression of GT to FT was caused by incompletely treated celiac disease. Both FT and GT may represent different reaction patterns caused by the same hematologic and immunologic diseases.3 In fact, the appearance of the tongue may aid in the diagnosis of celiac disease, which has been observed in 15% of patients with GT.7 Fissured tongue also may indicate an inability of the gastrointestinal mucosa to absorb nutrients; therefore, close nutrition monitoring is recommended.9
Continue to: Other oral and dental manifestations...
Other oral and dental manifestations of celiac disease include enamel defects, delayed tooth eruption, recurrent aphthous ulcers, cheilosis, oral lichen planus, and atrophic glossitis.10 Our patient also reported anxiety, “foggy mind,” diffuse arthralgia, and abdominal pain, which are symptoms of uncontrolled celiac disease. There is no known etiology of tongue manifestations in patients with incompletely treated celiac disease.
Treatment. FT generally does not require specific therapy other than the treatment of the underlying inflammatory condition. It is important to maintain proper oral and dental care, such as brushing the top surface of the tongue to clean and remove food debris. Bacteria and plaque can collect in the fissures, leading to bad breath and an increased potential for tooth decay.
Our patient was referred to a dietitian to assist with adherence to the gluten-free diet. At follow-up 3 months later, the appearance of her tongue had improved and fewer fissures were visible. The majority of her other symptoms also had resolved.
THE TAKEAWAY
FT may be a normal variant of the tongue in some patients or may be associated with poor oral hygiene. Additionally, FT often is associated with an underlying medical or inherited condition and may serve as a marker for an untreated or partially treated condition such as celiac disease, as was the case with our patient. When other signs or symptoms of systemic disease are present, further laboratory and endoscopic workup is necessary to rule out other causes and to diagnose celiac disease, if present.
As FT has been reported to be a natural progression from GT, the appearance of FT may indicate partial treatment of the underlying disease process and therefore more intensive therapy and follow-up would be needed. In this case, more intensive dietary guidance was provided with subsequent improvement of symptoms.
CORRESPONDENCE
Peter J. Carek, MD, MS, Department of Community Health and Family Medicine, College of Medicine, University of Florida, P.O. Box 100237, Gainesville, FL 32610-0237; carek@ufl.edu
1. Reamy BV, Cerby R, Bunt CW. Common tongue conditions in primary care. Am Fam Physician. 2010;81:627-634.
2. Yarom N, Cantony U, Gorsky M. Prevalence of fissured tongue, geographic tongue and median rhomboid glossitis among Israeli adults of different ethnic origins. Dermatology. 2004;209:88-94.
3. Dafar A, Cevik-Aras H, Robledo-Sierra J, et al. Factors associated with geographic tongue and fissured tongue. Acta Odontol Scad. 2016;74:210-216.
4. Hume WJ. Geographic stomatitis: a critical review. J Dent. 1975;3:25-43.
5. Sudarshan R, Sree Vijayabala G, Samata Y, et al. Newer classification system for fissured tongue: an epidemiological approach. J Tropical Med. doi:10.1155/2015/262079.
6. Mangold AR, Torgerson RR, Rogers RS. Diseases of the tongue. Clin Dermatol. 2016;34:458-469.
7. Cigic L, Galic T, Kero D, et al. The prevalence of celiac disease in patients with geographic tongue. J Oral Pathol Med. 2016;45:791-796.
8. Zargari O. The prevalence and significance of fissured tongue and geographical tongue in psoriatic patients. Clin Exp Dermatology. 2006;31:192-195.
9. Kullaa-Mikkonen A, Penttila I, Kotilainen R, et al. Haematological and immunological features of patients with fissured tongue syndrome. Br J Oral Maxillofac Surg. 1987;25:481-487.
10. Rashid M, Zarkadas M, Anca A, et al. Oral manifestations of celiac disease: a clinical guide for dentists. J Can Dent Assoc. 2011;77:b39.
1. Reamy BV, Cerby R, Bunt CW. Common tongue conditions in primary care. Am Fam Physician. 2010;81:627-634.
2. Yarom N, Cantony U, Gorsky M. Prevalence of fissured tongue, geographic tongue and median rhomboid glossitis among Israeli adults of different ethnic origins. Dermatology. 2004;209:88-94.
3. Dafar A, Cevik-Aras H, Robledo-Sierra J, et al. Factors associated with geographic tongue and fissured tongue. Acta Odontol Scad. 2016;74:210-216.
4. Hume WJ. Geographic stomatitis: a critical review. J Dent. 1975;3:25-43.
5. Sudarshan R, Sree Vijayabala G, Samata Y, et al. Newer classification system for fissured tongue: an epidemiological approach. J Tropical Med. doi:10.1155/2015/262079.
6. Mangold AR, Torgerson RR, Rogers RS. Diseases of the tongue. Clin Dermatol. 2016;34:458-469.
7. Cigic L, Galic T, Kero D, et al. The prevalence of celiac disease in patients with geographic tongue. J Oral Pathol Med. 2016;45:791-796.
8. Zargari O. The prevalence and significance of fissured tongue and geographical tongue in psoriatic patients. Clin Exp Dermatology. 2006;31:192-195.
9. Kullaa-Mikkonen A, Penttila I, Kotilainen R, et al. Haematological and immunological features of patients with fissured tongue syndrome. Br J Oral Maxillofac Surg. 1987;25:481-487.
10. Rashid M, Zarkadas M, Anca A, et al. Oral manifestations of celiac disease: a clinical guide for dentists. J Can Dent Assoc. 2011;77:b39.
Consider these exercises for chronic musculoskeletal conditions
Regular exercise confers several well-established benefits. In such conditions as coronary heart disease, stroke, heart failure, and diabetes, exercise has led to a reduction in mortality similar to that seen with pharmacotherapy.1 For patients with chronic musculoskeletal conditions, the benefits of exercise-based interventions are measurably reduced pain and improved daily function.2 However, prescribing of exercise is often neglected, with preference given to pharmacologic or surgical interventions.3 In part, the disregard of exercise as therapy results from unfamiliarity with appropriate exercise prescriptions,3 which include various forms of aerobic exercise, strength training, and stretching to increase flexibility (TABLE).
As is true of many therapeutic modalities, exercise must be tailored to the condition and to a patient’s preferences to optimize its benefits. In this review, we describe exercise regimens well suited for common musculoskeletal conditions, examine the effectiveness of exercise in each condition, and provide examples for use in treating patients.
Osteoarthritis of the hip and knee
Osteoarthritis (OA), one of the most common chronic joint diseases, erodes the articular cartilage and subchondral bone of a synovial joint, eventually leading to joint failure. Pain and diminished muscle strength restrict physical activity and can lead to decreased fitness and impaired muscle function. Exercise helps reduce pain and improve muscle function and quality of life in patients with hip or knee OA regardless of age, disease severity, or level of pain and dysfunction.2
Knee exercises. Activities suitable for patients with OA include muscle strengthening, aerobic conditioning, and range-of-motion (ROM) exercises.4-6 A 2015 Cochrane review of OA of the knee showed that exercise reduced pain and improved physical function and quality of life in patients who completed a treatment program, and that pain relief persisted up to 6 months after intervention.5
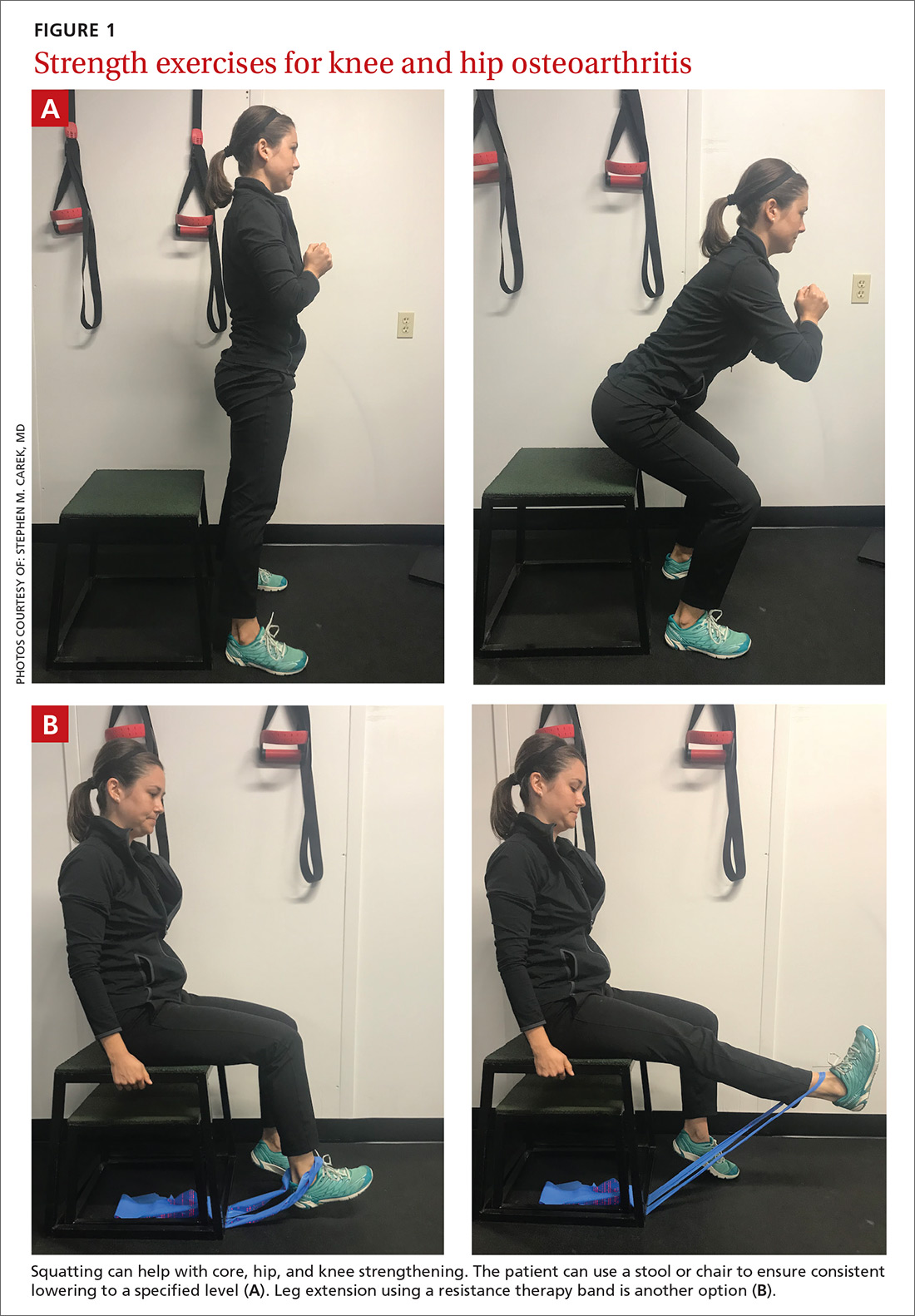
When designing an exercise prescription for patients with knee OA, consider quadriceps strengthening with an initial period of supervision, which may provide greater pain relief than nonspecific, unsupervised lower limb exercises.4 Enhanced strength of the lower limb may lessen force through the knee, thereby decreasing pain and improving overall physical function.7 Simple, teachable exercises include squats, step-ups, knee extension/flexion while sitting in a chair, and hip abduction/adduction while standing or lying down. Elastic bands, dumbbells, or cuff weights may be used to increase resistance.
Hip exercises. Exercise can significantly reduce pain and improve function for up to 6 months for patients with mild-to-moderate symptomatic hip OA.6 Types of exercise for hip OA include strength training of hip and core muscles, functional exercises that imitate movements in daily activities, and flexibility training. These exercises help reduce pain and increase ROM. Exercise should include resistance training and should not exceed the limit for acceptable pain.8
Aquatic therapy is also appropriate for exercise and strength training and can decrease pain and disability and improve quality of life.9 Supervised physical therapy, including strength training, manual therapy, and balance training, are important for reducing pain and improving function. Physical therapy can also enhance adherence to a prescribed exercise program.10
Continue to: Appropriate exercise prescriptions...
Appropriate exercise prescriptions for patients with knee or hip OA should focus on low-impact activities that can improve strength, flexibility, and function (FIGURE 1). A typical regimen would be 30 or more cumulative minutes daily of stationary cycling, water-based exercises, or strength training, 3 to 5 days per week. Individualize workout intensity for each patient, emphasizing that high-intensity, low-impact effort may yield greater strength gains and take less time to perform.11 A high-intensity exercise prescription focusing on quadriceps, hip, and core strengthening may consist of 3 sets of 8 repetitions with resistance set at 40% of the maximum resistance against which the patient can perform 1 repetition.7
Barriers to exercise in knee and hip OA include negative patient and provider perspectives on exercise and patients’ fear that increased activity may actually worsen OA.12 Depending on a patient’s personal preferences, ways to overcome these barriers and encourage adherence might be supervised exercises in an individual or group setting or audiotapes or videos of recommended exercises.10
Chronic low back pain
Chronic low back pain (LBP) is a large socioeconomic burden in the United States, with upward of $100 billion per year accounted for in health care costs and decreased worker productivity.13 The etiology of chronic LBP can be multifactorial and due to any of several conditions such as degenerative disc disease, spinal stenosis, spondylolisthesis, and facet arthropathy. Treatment is difficult, given that many common interventions—medications, massage, manipulation—have limited efficacy.14 However, for patients with nonspecific chronic LBP, exercise is an effective intervention for reducing pain and improving physical function.15
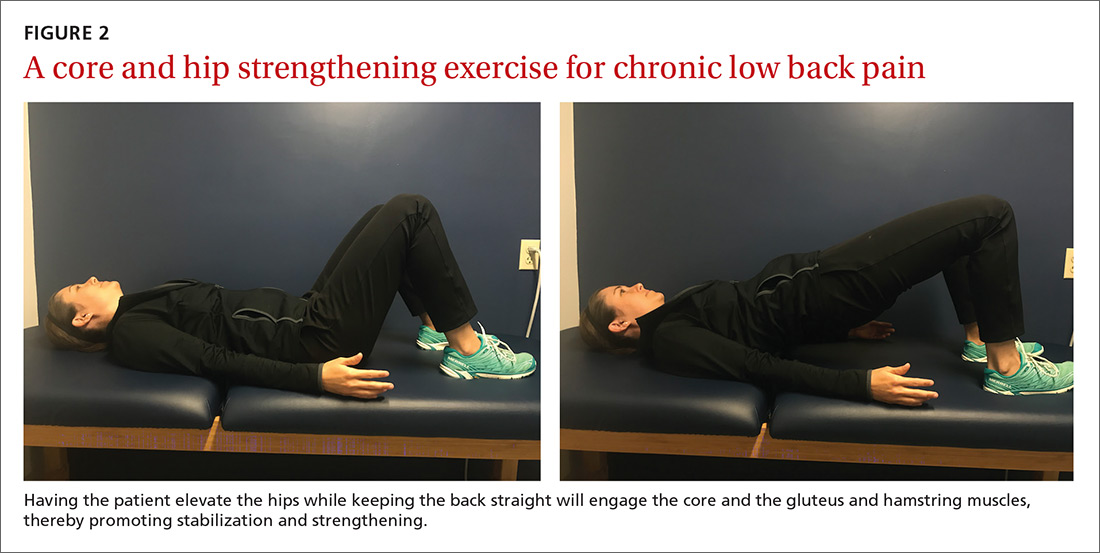
An effective approach is to design an exercise regimen for the individual by type, duration, and frequency of activity, administered under supervision to encourage adherence.16 Appropriate exercises emphasize resistance, strength training, and core stabilization, often focusing on whole body and trunk motion (FIGURE 2).17
Although yoga or Pilates classes may have a small effect on function, no high-quality evidence exists for their superiority to other forms of exercise.18,19 Back School, a therapeutic program that includes education on anatomy and biomechanics, optimal posture, ergonomics, and back exercises, has limited, low-quality evidence for treatment comparisons.20 Aerobic exercise, including treadmill, elliptical, or cycling exercises or walking outdoors can reduce pain and improve physical and psychologic functioning.21
Continue to: The most common reported adverse effect...
The most common reported adverse effect of exercise is a temporary exacerbation of back pain. However, having patients continue daily activities within the permitted limits of pain leads to more rapid recovery than rest or back-mobilizing exercises.15,22,23
Cautions. Exercise is contraindicated in patients with LBP arising from a serious medical condition, such as fracture, infection, cancer, or cauda equina syndrome.24 Importantly, exercise interventions recommended for acute LBP have not shown benefit for chronic LBP.
Chronic shoulder pain
With a prevalence ranging from 7% to 26% in the general population,25 chronic shoulder pain often interferes with essential activities of daily living. The etiology of chronic shoulder pain is broad and most commonly involves disorders of the rotator cuff, which functions in both motion and dynamic stabilization of the shoulder. The common term “rotator cuff pain syndrome” can cover such disorders as subacromial impingement syndrome, rotator cuff tendinopathy or tendinitis, partial or full thickness rotator cuff tears, calcific tendinitis, and subacromial bursitis. These pathologies may have overlapping presentations. Manual therapy and exercise, usually delivered as a component of structured physical therapy, focus on stretches and other exercises to increase ROM, stability, and strength of the rotator cuff musculature.26
A 2016 Cochrane review that evaluated manual therapy and exercise for chronic shoulder pain yielded limited high-quality evidence for effectiveness compared with placebo.27 Five trials found no important differences between manual therapy and exercise compared with glucocorticoid injection relative to overall pain, function, active shoulder abduction, and quality of life from 4 weeks up to 12 months.27 But compared with placebo, exercise has been more effective in reducing reported pain, especially in the context of strengthening regimens focused on flexion, extension, and internal and external rotation.28
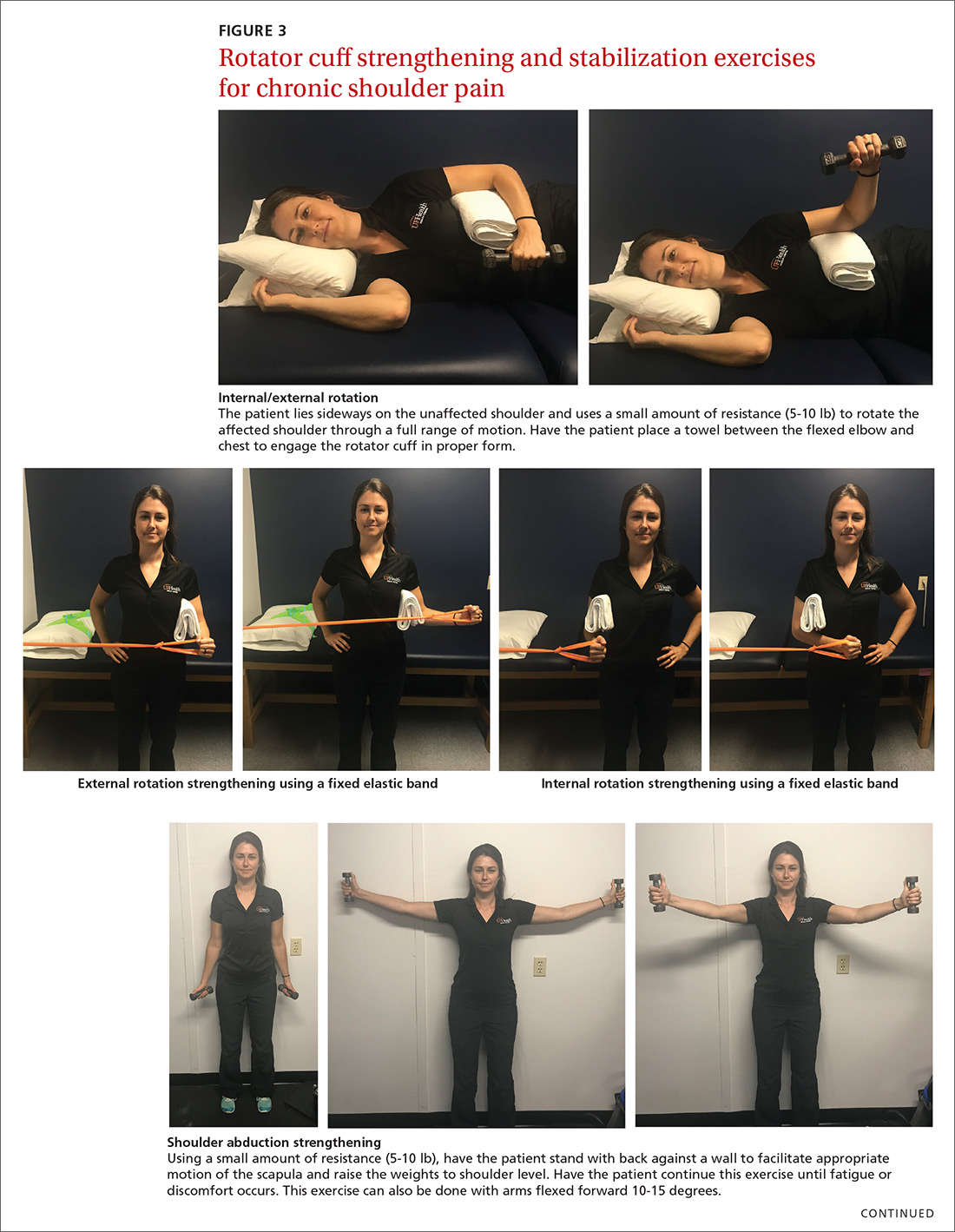
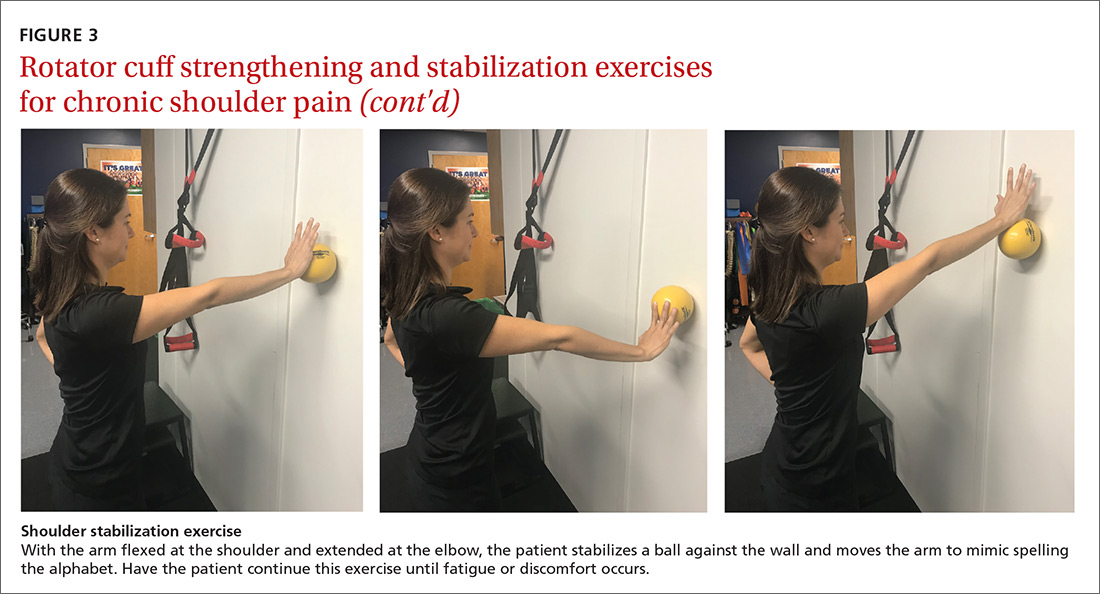
For subacromial impingement syndrome, a 2017 meta-analysis found that a generalized exercise program relieves pain and improves function, ROM, and strength.29 A generalized shoulder-strengthening program includes exercises that focus on internal and external rotation, horizontal abduction, and shoulder stabilization (FIGURE 3). These exercises can be completed with 3 sets of 15 to 20 repetitions, which create a fatigue response that improves strength and targets local muscular endurance.30
Continue to: Achilles tendinopathy
Achilles tendinopathy
Achilles tendinopathy (also referred to as chronic Achilles tendinitis) is a degenerative condition of the Achilles tendon related to overuse that leads to pain, swelling, and impaired performance. It accounts for approximately 18% of injuries in runners and 4% of all patients presenting to sports medicine clinics.31 Eccentric muscle loading has become the dominant conservative intervention strategy for chronic Achilles tendinopathy.
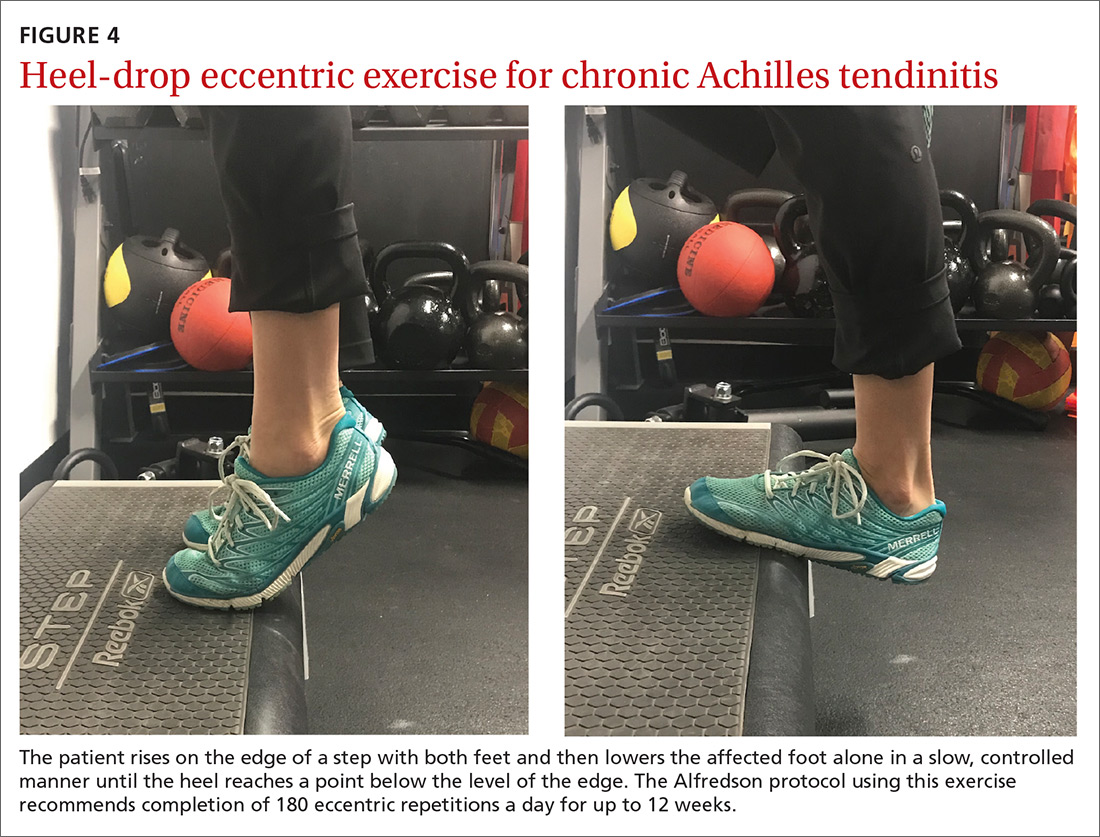
For chronic tendinopathies, eccentric exercises subject greater force than concentric exercises through a controlled lengthening of a muscle-tendon unit, resulting in a greater remodeling stimulus of the tendon.32 Classically, the Alfredson protocol has been used to treat chronic Achilles tendinopathy. This program of eccentric heel-drop exercises recommends completion of 180 eccentric repetitions a day for up to 12 weeks (FIGURE 4).33 Exercises are performed slowly, and load can be increased when exercises are performed without pain or perhaps with mild nondisabling pain.
A variation of this protocol has allowed a gradual escalation of repetitions over a week up to the recommended 180 repetitions, and has shown improvements in pain reduction and function similar to that achieved with the primary protocol.34 Additionally, a 6-week “do as tolerated” program of eccentric exercises did not lead to lesser improvement for individuals with midportion Achilles tendinopathy.35
Several systematic reviews have supported the use of eccentric exercises for chronic Achilles tendinopathy,31,36,37 but no specific protocol or exercise regimen has demonstrated superiority. However, with the Alfredson protocol, improvement in pain and function in patients with chronic Achilles tendinopathy has persisted for up to 5 years.38
Lateral epicondylitis
Lateral epicondylitis (also called lateral epicondylosis or “tennis elbow”) is a disabling musculoskeletal condition that leads to pain and tenderness around the extensor mass of the lateral elbow. It is caused by microtrauma to the tendon, usually sustained through repetitive movement in a sporting activity, industrial work, or hobby. Affecting up to 3% of the US population, lateral epicondylitis is associated with pain and functional disability, as well as emotional and psychosocial consequences.39
Continue to: Proposed treatment and rehabilitation options...
Proposed treatment and rehabilitation options for patients with lateral epicondylitis have included massage, manipulation, taping, acupuncture, orthotic devices, ultrasound, activity modification, and rest. Exercise programs incorporating eccentric muscle activity are becoming increasingly popular for such conditions as Achilles and patellar tendinopathies, and they may translate well to other chronic tendinopathies, such as lateral epicondylitis.32
An eccentric exercise program for lateral epicondylitis, either in isolation or as an adjunct to other therapies, has decreased pain and improved function and grip strength from baseline measures.40 Compared with a standard exercise regimen without eccentric strength training, use of eccentric training improves such clinical measures as pain intensity and disability status, as it decreases tendon thickness and aids in recovering homogenous tendon structure.41
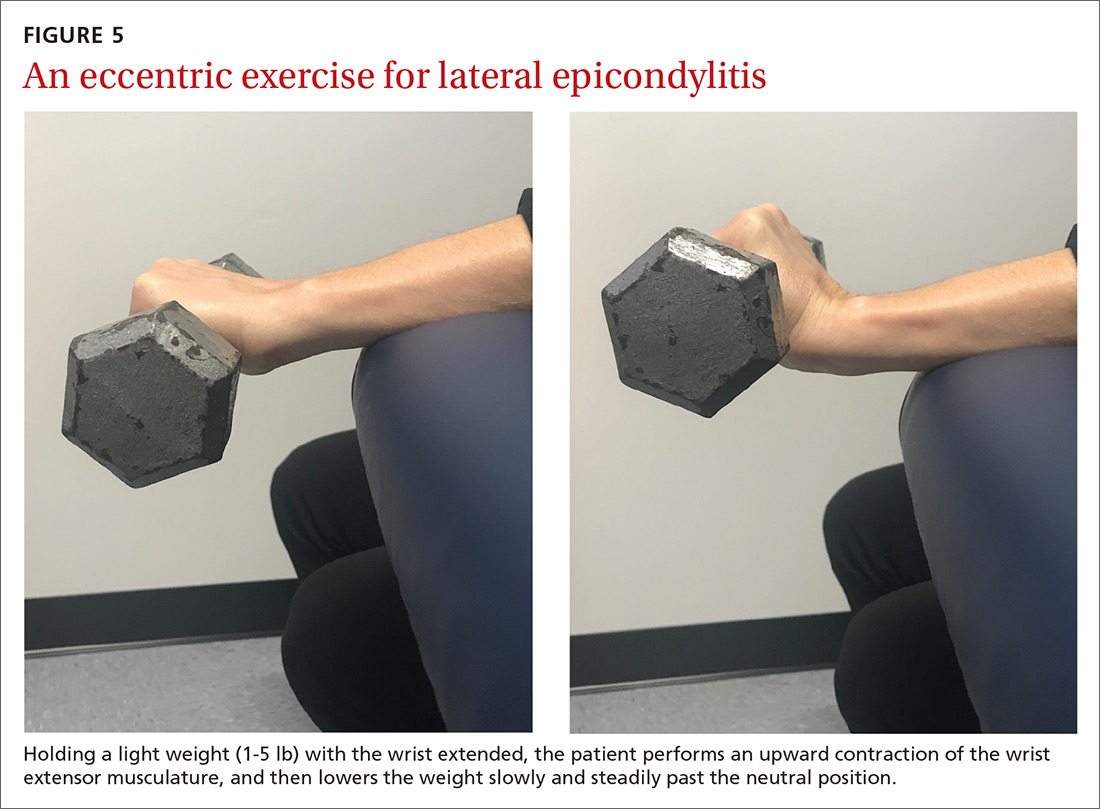
A sample exercise. The patient may sit in a chair and, with the forearm flexed and pronated over the edge of a table, grasp some form of resistance (bucket of water, training weight, resistance band) (FIGURE 5). The nonaffected hand can be used to help lift the affected wrist into full extension and then removed to allow lowering of the hand over several seconds into flexion. This activity can be performed in sets of 8 to 12 repetitions, 2 to 3 times a day, until the patient’s pain and function have improved.42
Overcoming barriers to exercise
A major concern across all studies assessing the therapeutic value of exercise is patient compliance and adherence to prescribed programs. Compliance and adherence are affected in part by psychosocial factors such as low literacy and poor social support. From a physician’s perspective, direct and indirect costs of treatment and rehabilitation of chronic musculoskeletal conditions may discourage the prescribing of supervised physical therapy.3
Steps to consider in overcoming these barriers would be advising an exercise regimen that requires only an initial period of supervision; educating patients about the benefits of an exercise program; exploring a patient’s expectations, beliefs, and fears; and developing strategies for long-term adherence.16 Supervision through physical therapy is often suggested. However, significant barriers may exist that impede a patient’s ability to attend or participate, in which case physician observation in the course of regularly scheduled clinical examinations could be considered.
Continue to: When prescribing exercises...
When prescribing exercises, be sure to address patient expectations regarding pain, duration, and limitations of exercise. It would be helpful for patients to know, for instance, that working through mild-to-moderate pain during exercise has been shown to shorten post-exercise recovery time and, in the short-term, improve relief from pain.43
Tailoring specific exercise prescriptions for a patient will make the regimen more satisfying for the individual and optimize adherence, which in turn will increase the potential for pain reduction and improved function. Secondary benefits would likely be weight loss and prevention (or regression) of cardiovascular disease. Continued evaluation by the physician or physical therapist should be part of ongoing management, as well as “refresher courses” to ensure understanding of, and adherence to, the exercise program. The potential benefits and limited risks of exercise, if done properly, make it a primary intervention for specific musculoskeletal conditions.
CORRESPONDENCE
Peter J. Carek, MD, MS, Department of Community Health and Family Medicine, College of Medicine, University of Florida, PO Box 100237, Gainesville, FL 32610-0237; carek@ufl.edu.
1. Naci H, Ioannidis JP. Comparative effectiveness of exercise and drug interventions on mortality outcomes: metaepidemiological study. BMJ. 2013;347:f5577.
2. Babatunde OO, Jordan JL, van der Windt DA, et al. Effective treatment options for musculoskeletal pain in primary care: a systematic overview of current evidence. PLoS One. 2017;12:e0178621.
3. Persson G, Brorsson A, Ekvall Hansson E, et al. Physical activity on prescription (PAP) from the general practitioner’s perspective - a qualitative study. BMC Fam Pract. 2013;14:128.
4. Juhl C, Christensen R, Roos EM, et al. Impact of exercise type and dose on pain and disability in knee osteoarthritis: a systematic review and meta-regression analysis of randomized controlled trials. Arthritis Rheumatol. 2014;66:622-636.
5. Fransen M, McConnell S, Harmer AR, et al. Exercise for osteoarthritis of the knee. Cochrane Database Syst Rev. 2015;1:CD004376.
6. Fransen M, McConnell S, Hernandez-Molina G, et al. Exercise for osteoarthritis of the hip. Cochrane Database Syst Rev. 2014;(4):CD007912.
7. Vincent KR, Vincent HK. Resistance exercise for knee osteoarthritis. PM R. 2012;4(suppl 5):S45-S52.
8. Fernandes L, Storheim K, Nordsletten L, et al. Development of a therapeutic exercise program for patients with osteoarthritis of the hip. Phys Ther. 2010;90:592-601.
9. Bartels EM, Juhl CB, Christensen R, et al. Aquatic exercise for the treatment of knee and hip osteoarthritis. Cochrane Database Syst Rev. 2016;(3):CD005523.
10. Jordan JL, Holden MA, Mason EE, et al. Interventions to improve adherence to exercise for chronic musculoskeletal pain in adults. Cochrane Database Syst Rev. 2010;(1):CD005956.
11. Baker KR, Nelson ME, Felson DT, et al. The efficacy of home based progressive strength training in older adults with knee osteoarthritis: a randomized controlled trial. J Rheumatol. 2001;28:1655-1665.
12. Brakke R, Singh J, Sullivan W. Physical therapy in persons with osteoarthritis. PM R. 2012;4:S53-S58.
13. Katz JN. Lumbar disc disorders and low-back pain: socioeconomic factors and consequences. J Bone Joint Surg Am. 2006;88(suppl 2):21-24.
14. Qaseem A, Wilt TJ, McLean RM, et al. Noninvasive treatments for acute, subacute, and chronic low back pain: a clinical practice guideline from the American College of Physicians. Ann Intern Med. 2017;166:514-530.
15. Hayden JA, van Tulder MW, Malmivaara A, et al. Exercise therapy for treatment of non-specific low back pain. Cochrane Database Syst Rev. 2005;(3):CD000335.
16. Hayden JA, van Tulder MW, Tomlinson G. Systematic review: strategies for using exercise therapy to improve outcomes in chronic low back pain. Ann Intern Med. 2005;142:776-785.
17. Searle A, Spink M, Ho A, et al. Exercise interventions for the treatment of chronic low back pain: a systematic review and meta-analysis of randomised controlled trials. Clin Rehabil. 2015;29:1155-1167.
18. Yamato TP, Maher CG, Saragiotto BT, et al. Pilates for low back pain. Cochrane Database Syst Rev. 2015;(7):CD010265.
19. Wieland LS, Skoetz N, Pilkington K, et al. Yoga treatment for chronic non-specific low back pain. Cochrane Database Syst Rev. 2017;(1):CD010671.
20. Parreira P, Heymans MW, van Tulder MW, et al. Back Schools for chronic non-specific low back pain. Cochrane Database Syst Rev. 2017;(8):CD011674.
21. Meng XG, Yue SW. Efficacy of aerobic exercise for treatment of chronic low back pain: a meta-analysis. Am J Phys Med Rehabil. 2015;94:358-365.
22. Malmivaara A, Häkkinen U, Aro T, et al. The treatment of acute low back pain--bed rest, exercises, or ordinary activity? N Engl J Med. 1995;332:351-355.
23. van Tulder M, Malmivaara A, Esmail R, Koes B. Exercise therapy for low back pain: a systematic review within the framework of the cochrane collaboration back review group. Spine (Phila Pa 1976). 2000;25:2784-2796.
24. Hoffmann TC, Maher CG, Briffa T, et al. Prescribing exercise interventions for patients with chronic conditions. CMAJ. 2016;188:510-518.
25. Luime JJ, Koes BW, Hendriksen IJ, et al. Prevalence and incidence of shoulder pain in the general population; a systematic review. Scand J Rheumatol. 2004;33:73-81.
26. Kuhn JE. Exercise in the treatment of rotator cuff impingement: a systematic review and a synthesized evidence-based rehabilitation protocol. J Shoulder Elbow Surg. 2009;18:138-160.
27. Page MJ, Green S, McBain B, et al. Manual therapy and exercise for rotator cuff disease. Cochrane Database Syst Rev. 2016;(6):CD012224.
28. van den Dolder PA, Ferreira PH, Refshauge KM. Effectiveness of soft tissue massage and exercise for the treatment of non-specific shoulder pain: a systematic review with meta-analysis. Br J Sports Med. 2014;48:1216-1226.
29. Shire AR, Stæhr TAB, Overby JB, et al. Specific or general exercise strategy for subacromial impingement syndrome-does it matter? A systematic literature review and meta analysis. BMC Musculoskelet Disord. 2017;18:158.
30. Ellenbecker TS, Cools A. Rehabilitation of shoulder impingement syndrome and rotator cuff injuries: an evidence-based review. Br J Sports Med. 2010;44:319-327.
31. Magnussen RA, Dunn WR, Thomson AB. Nonoperative treatment of midportion Achilles tendinopathy: a systematic review. Clin J Sport Med. 2009;19:54-64.
32. Rees JD, Wolman RL, Wilson A. Eccentric exercises; why do they work, what are the problems and how can we improve them? Br J Sports Med. 2009;43:242-246.
33. Alfredson H, Pietilä T, Jonsson P, et al. Heavy-load eccentric calf muscle training for the treatment of chronic Achilles tendinosis. Am J Sports Med. 1998;26:360-366.
34. Rompe JD, Nafe B, Furia JP, et al. Eccentric loading, shock-wave treatment, or a wait-and-see policy for tendinopathy of the main body of tendo Achillis: a randomized controlled trial. Am J Sports Med. 2007;35:374-383.
35. Stevens M, Tan CW. Effectiveness of the Alfredson protocol compared with a lower repetition-volume protocol for midportion Achilles tendinopathy: a randomized controlled trial. J Orthop Sports Phys Ther. 2014;44:59-67.
36. Habets B, van Cingel RE. Eccentric exercise training in chronic mid-portion Achilles tendinopathy: a systematic review on different protocols. Scand J Med Sci Sports. 2015;25:3-15.
37. Malliaras P, Barton CJ, Reeves ND, et al. Achilles and patellar tendinopathy loading programmes : a systematic review comparing clinical outcomes and identifying potential mechanisms for effectiveness. Sports Med. 2013;43:267-286.
38. van der Plas A, de Jonge S, de Vos RJ, et al. A 5-year follow-up study of Alfredson’s heel-drop exercise programme in chronic midportion Achilles tendinopathy. Br J Sports Med. 2012;46:214-218.
39. Alizadehkhaiyat O, Fisher AC, Kemp GJ, et al. Pain, functional disability, and psychologic status in tennis elbow. Clin J Pain. 2007;23:482-489.
40. Cullinane FL, Boocock MG, Trevelyan FC. Is eccentric exercise an effective treatment for lateral epicondylitis? A systematic review. Clin Rehabil. 2014;28:3-19.
41. Croisier JL, Foidart-Dessalle M, Tinant F, et al. An isokinetic eccentric programme for the management of chronic lateral epicondylar tendinopathy. Br J Sports Med. 2007;41:269-275.
42. Söderberg J, Grooten WJ, Ang BO. Effects of eccentric training on hand strength in subjects with lateral epicondylalgia: a randomized-controlled trial. Scand J Med Sci Sports. 2012;22:797-803.
43. Smith BE, Hendrick P, Smith TO, et al. Should exercises be painful in the management of chronic musculoskeletal pain? A systematic review and meta-analysis. Br J Sports Med. 2017;51:1679-1687.
Regular exercise confers several well-established benefits. In such conditions as coronary heart disease, stroke, heart failure, and diabetes, exercise has led to a reduction in mortality similar to that seen with pharmacotherapy.1 For patients with chronic musculoskeletal conditions, the benefits of exercise-based interventions are measurably reduced pain and improved daily function.2 However, prescribing of exercise is often neglected, with preference given to pharmacologic or surgical interventions.3 In part, the disregard of exercise as therapy results from unfamiliarity with appropriate exercise prescriptions,3 which include various forms of aerobic exercise, strength training, and stretching to increase flexibility (TABLE).
As is true of many therapeutic modalities, exercise must be tailored to the condition and to a patient’s preferences to optimize its benefits. In this review, we describe exercise regimens well suited for common musculoskeletal conditions, examine the effectiveness of exercise in each condition, and provide examples for use in treating patients.
Osteoarthritis of the hip and knee
Osteoarthritis (OA), one of the most common chronic joint diseases, erodes the articular cartilage and subchondral bone of a synovial joint, eventually leading to joint failure. Pain and diminished muscle strength restrict physical activity and can lead to decreased fitness and impaired muscle function. Exercise helps reduce pain and improve muscle function and quality of life in patients with hip or knee OA regardless of age, disease severity, or level of pain and dysfunction.2
Knee exercises. Activities suitable for patients with OA include muscle strengthening, aerobic conditioning, and range-of-motion (ROM) exercises.4-6 A 2015 Cochrane review of OA of the knee showed that exercise reduced pain and improved physical function and quality of life in patients who completed a treatment program, and that pain relief persisted up to 6 months after intervention.5
When designing an exercise prescription for patients with knee OA, consider quadriceps strengthening with an initial period of supervision, which may provide greater pain relief than nonspecific, unsupervised lower limb exercises.4 Enhanced strength of the lower limb may lessen force through the knee, thereby decreasing pain and improving overall physical function.7 Simple, teachable exercises include squats, step-ups, knee extension/flexion while sitting in a chair, and hip abduction/adduction while standing or lying down. Elastic bands, dumbbells, or cuff weights may be used to increase resistance.
Hip exercises. Exercise can significantly reduce pain and improve function for up to 6 months for patients with mild-to-moderate symptomatic hip OA.6 Types of exercise for hip OA include strength training of hip and core muscles, functional exercises that imitate movements in daily activities, and flexibility training. These exercises help reduce pain and increase ROM. Exercise should include resistance training and should not exceed the limit for acceptable pain.8
Aquatic therapy is also appropriate for exercise and strength training and can decrease pain and disability and improve quality of life.9 Supervised physical therapy, including strength training, manual therapy, and balance training, are important for reducing pain and improving function. Physical therapy can also enhance adherence to a prescribed exercise program.10
Continue to: Appropriate exercise prescriptions...
Appropriate exercise prescriptions for patients with knee or hip OA should focus on low-impact activities that can improve strength, flexibility, and function (FIGURE 1). A typical regimen would be 30 or more cumulative minutes daily of stationary cycling, water-based exercises, or strength training, 3 to 5 days per week. Individualize workout intensity for each patient, emphasizing that high-intensity, low-impact effort may yield greater strength gains and take less time to perform.11 A high-intensity exercise prescription focusing on quadriceps, hip, and core strengthening may consist of 3 sets of 8 repetitions with resistance set at 40% of the maximum resistance against which the patient can perform 1 repetition.7
Barriers to exercise in knee and hip OA include negative patient and provider perspectives on exercise and patients’ fear that increased activity may actually worsen OA.12 Depending on a patient’s personal preferences, ways to overcome these barriers and encourage adherence might be supervised exercises in an individual or group setting or audiotapes or videos of recommended exercises.10
Chronic low back pain
Chronic low back pain (LBP) is a large socioeconomic burden in the United States, with upward of $100 billion per year accounted for in health care costs and decreased worker productivity.13 The etiology of chronic LBP can be multifactorial and due to any of several conditions such as degenerative disc disease, spinal stenosis, spondylolisthesis, and facet arthropathy. Treatment is difficult, given that many common interventions—medications, massage, manipulation—have limited efficacy.14 However, for patients with nonspecific chronic LBP, exercise is an effective intervention for reducing pain and improving physical function.15
An effective approach is to design an exercise regimen for the individual by type, duration, and frequency of activity, administered under supervision to encourage adherence.16 Appropriate exercises emphasize resistance, strength training, and core stabilization, often focusing on whole body and trunk motion (FIGURE 2).17
Although yoga or Pilates classes may have a small effect on function, no high-quality evidence exists for their superiority to other forms of exercise.18,19 Back School, a therapeutic program that includes education on anatomy and biomechanics, optimal posture, ergonomics, and back exercises, has limited, low-quality evidence for treatment comparisons.20 Aerobic exercise, including treadmill, elliptical, or cycling exercises or walking outdoors can reduce pain and improve physical and psychologic functioning.21
Continue to: The most common reported adverse effect...
The most common reported adverse effect of exercise is a temporary exacerbation of back pain. However, having patients continue daily activities within the permitted limits of pain leads to more rapid recovery than rest or back-mobilizing exercises.15,22,23
Cautions. Exercise is contraindicated in patients with LBP arising from a serious medical condition, such as fracture, infection, cancer, or cauda equina syndrome.24 Importantly, exercise interventions recommended for acute LBP have not shown benefit for chronic LBP.
Chronic shoulder pain
With a prevalence ranging from 7% to 26% in the general population,25 chronic shoulder pain often interferes with essential activities of daily living. The etiology of chronic shoulder pain is broad and most commonly involves disorders of the rotator cuff, which functions in both motion and dynamic stabilization of the shoulder. The common term “rotator cuff pain syndrome” can cover such disorders as subacromial impingement syndrome, rotator cuff tendinopathy or tendinitis, partial or full thickness rotator cuff tears, calcific tendinitis, and subacromial bursitis. These pathologies may have overlapping presentations. Manual therapy and exercise, usually delivered as a component of structured physical therapy, focus on stretches and other exercises to increase ROM, stability, and strength of the rotator cuff musculature.26
A 2016 Cochrane review that evaluated manual therapy and exercise for chronic shoulder pain yielded limited high-quality evidence for effectiveness compared with placebo.27 Five trials found no important differences between manual therapy and exercise compared with glucocorticoid injection relative to overall pain, function, active shoulder abduction, and quality of life from 4 weeks up to 12 months.27 But compared with placebo, exercise has been more effective in reducing reported pain, especially in the context of strengthening regimens focused on flexion, extension, and internal and external rotation.28
For subacromial impingement syndrome, a 2017 meta-analysis found that a generalized exercise program relieves pain and improves function, ROM, and strength.29 A generalized shoulder-strengthening program includes exercises that focus on internal and external rotation, horizontal abduction, and shoulder stabilization (FIGURE 3). These exercises can be completed with 3 sets of 15 to 20 repetitions, which create a fatigue response that improves strength and targets local muscular endurance.30
Continue to: Achilles tendinopathy
Achilles tendinopathy
Achilles tendinopathy (also referred to as chronic Achilles tendinitis) is a degenerative condition of the Achilles tendon related to overuse that leads to pain, swelling, and impaired performance. It accounts for approximately 18% of injuries in runners and 4% of all patients presenting to sports medicine clinics.31 Eccentric muscle loading has become the dominant conservative intervention strategy for chronic Achilles tendinopathy.
For chronic tendinopathies, eccentric exercises subject greater force than concentric exercises through a controlled lengthening of a muscle-tendon unit, resulting in a greater remodeling stimulus of the tendon.32 Classically, the Alfredson protocol has been used to treat chronic Achilles tendinopathy. This program of eccentric heel-drop exercises recommends completion of 180 eccentric repetitions a day for up to 12 weeks (FIGURE 4).33 Exercises are performed slowly, and load can be increased when exercises are performed without pain or perhaps with mild nondisabling pain.
A variation of this protocol has allowed a gradual escalation of repetitions over a week up to the recommended 180 repetitions, and has shown improvements in pain reduction and function similar to that achieved with the primary protocol.34 Additionally, a 6-week “do as tolerated” program of eccentric exercises did not lead to lesser improvement for individuals with midportion Achilles tendinopathy.35
Several systematic reviews have supported the use of eccentric exercises for chronic Achilles tendinopathy,31,36,37 but no specific protocol or exercise regimen has demonstrated superiority. However, with the Alfredson protocol, improvement in pain and function in patients with chronic Achilles tendinopathy has persisted for up to 5 years.38
Lateral epicondylitis
Lateral epicondylitis (also called lateral epicondylosis or “tennis elbow”) is a disabling musculoskeletal condition that leads to pain and tenderness around the extensor mass of the lateral elbow. It is caused by microtrauma to the tendon, usually sustained through repetitive movement in a sporting activity, industrial work, or hobby. Affecting up to 3% of the US population, lateral epicondylitis is associated with pain and functional disability, as well as emotional and psychosocial consequences.39
Continue to: Proposed treatment and rehabilitation options...
Proposed treatment and rehabilitation options for patients with lateral epicondylitis have included massage, manipulation, taping, acupuncture, orthotic devices, ultrasound, activity modification, and rest. Exercise programs incorporating eccentric muscle activity are becoming increasingly popular for such conditions as Achilles and patellar tendinopathies, and they may translate well to other chronic tendinopathies, such as lateral epicondylitis.32
An eccentric exercise program for lateral epicondylitis, either in isolation or as an adjunct to other therapies, has decreased pain and improved function and grip strength from baseline measures.40 Compared with a standard exercise regimen without eccentric strength training, use of eccentric training improves such clinical measures as pain intensity and disability status, as it decreases tendon thickness and aids in recovering homogenous tendon structure.41
A sample exercise. The patient may sit in a chair and, with the forearm flexed and pronated over the edge of a table, grasp some form of resistance (bucket of water, training weight, resistance band) (FIGURE 5). The nonaffected hand can be used to help lift the affected wrist into full extension and then removed to allow lowering of the hand over several seconds into flexion. This activity can be performed in sets of 8 to 12 repetitions, 2 to 3 times a day, until the patient’s pain and function have improved.42
Overcoming barriers to exercise
A major concern across all studies assessing the therapeutic value of exercise is patient compliance and adherence to prescribed programs. Compliance and adherence are affected in part by psychosocial factors such as low literacy and poor social support. From a physician’s perspective, direct and indirect costs of treatment and rehabilitation of chronic musculoskeletal conditions may discourage the prescribing of supervised physical therapy.3
Steps to consider in overcoming these barriers would be advising an exercise regimen that requires only an initial period of supervision; educating patients about the benefits of an exercise program; exploring a patient’s expectations, beliefs, and fears; and developing strategies for long-term adherence.16 Supervision through physical therapy is often suggested. However, significant barriers may exist that impede a patient’s ability to attend or participate, in which case physician observation in the course of regularly scheduled clinical examinations could be considered.
Continue to: When prescribing exercises...
When prescribing exercises, be sure to address patient expectations regarding pain, duration, and limitations of exercise. It would be helpful for patients to know, for instance, that working through mild-to-moderate pain during exercise has been shown to shorten post-exercise recovery time and, in the short-term, improve relief from pain.43
Tailoring specific exercise prescriptions for a patient will make the regimen more satisfying for the individual and optimize adherence, which in turn will increase the potential for pain reduction and improved function. Secondary benefits would likely be weight loss and prevention (or regression) of cardiovascular disease. Continued evaluation by the physician or physical therapist should be part of ongoing management, as well as “refresher courses” to ensure understanding of, and adherence to, the exercise program. The potential benefits and limited risks of exercise, if done properly, make it a primary intervention for specific musculoskeletal conditions.
CORRESPONDENCE
Peter J. Carek, MD, MS, Department of Community Health and Family Medicine, College of Medicine, University of Florida, PO Box 100237, Gainesville, FL 32610-0237; carek@ufl.edu.
Regular exercise confers several well-established benefits. In such conditions as coronary heart disease, stroke, heart failure, and diabetes, exercise has led to a reduction in mortality similar to that seen with pharmacotherapy.1 For patients with chronic musculoskeletal conditions, the benefits of exercise-based interventions are measurably reduced pain and improved daily function.2 However, prescribing of exercise is often neglected, with preference given to pharmacologic or surgical interventions.3 In part, the disregard of exercise as therapy results from unfamiliarity with appropriate exercise prescriptions,3 which include various forms of aerobic exercise, strength training, and stretching to increase flexibility (TABLE).
As is true of many therapeutic modalities, exercise must be tailored to the condition and to a patient’s preferences to optimize its benefits. In this review, we describe exercise regimens well suited for common musculoskeletal conditions, examine the effectiveness of exercise in each condition, and provide examples for use in treating patients.
Osteoarthritis of the hip and knee
Osteoarthritis (OA), one of the most common chronic joint diseases, erodes the articular cartilage and subchondral bone of a synovial joint, eventually leading to joint failure. Pain and diminished muscle strength restrict physical activity and can lead to decreased fitness and impaired muscle function. Exercise helps reduce pain and improve muscle function and quality of life in patients with hip or knee OA regardless of age, disease severity, or level of pain and dysfunction.2
Knee exercises. Activities suitable for patients with OA include muscle strengthening, aerobic conditioning, and range-of-motion (ROM) exercises.4-6 A 2015 Cochrane review of OA of the knee showed that exercise reduced pain and improved physical function and quality of life in patients who completed a treatment program, and that pain relief persisted up to 6 months after intervention.5
When designing an exercise prescription for patients with knee OA, consider quadriceps strengthening with an initial period of supervision, which may provide greater pain relief than nonspecific, unsupervised lower limb exercises.4 Enhanced strength of the lower limb may lessen force through the knee, thereby decreasing pain and improving overall physical function.7 Simple, teachable exercises include squats, step-ups, knee extension/flexion while sitting in a chair, and hip abduction/adduction while standing or lying down. Elastic bands, dumbbells, or cuff weights may be used to increase resistance.
Hip exercises. Exercise can significantly reduce pain and improve function for up to 6 months for patients with mild-to-moderate symptomatic hip OA.6 Types of exercise for hip OA include strength training of hip and core muscles, functional exercises that imitate movements in daily activities, and flexibility training. These exercises help reduce pain and increase ROM. Exercise should include resistance training and should not exceed the limit for acceptable pain.8
Aquatic therapy is also appropriate for exercise and strength training and can decrease pain and disability and improve quality of life.9 Supervised physical therapy, including strength training, manual therapy, and balance training, are important for reducing pain and improving function. Physical therapy can also enhance adherence to a prescribed exercise program.10
Continue to: Appropriate exercise prescriptions...
Appropriate exercise prescriptions for patients with knee or hip OA should focus on low-impact activities that can improve strength, flexibility, and function (FIGURE 1). A typical regimen would be 30 or more cumulative minutes daily of stationary cycling, water-based exercises, or strength training, 3 to 5 days per week. Individualize workout intensity for each patient, emphasizing that high-intensity, low-impact effort may yield greater strength gains and take less time to perform.11 A high-intensity exercise prescription focusing on quadriceps, hip, and core strengthening may consist of 3 sets of 8 repetitions with resistance set at 40% of the maximum resistance against which the patient can perform 1 repetition.7
Barriers to exercise in knee and hip OA include negative patient and provider perspectives on exercise and patients’ fear that increased activity may actually worsen OA.12 Depending on a patient’s personal preferences, ways to overcome these barriers and encourage adherence might be supervised exercises in an individual or group setting or audiotapes or videos of recommended exercises.10
Chronic low back pain
Chronic low back pain (LBP) is a large socioeconomic burden in the United States, with upward of $100 billion per year accounted for in health care costs and decreased worker productivity.13 The etiology of chronic LBP can be multifactorial and due to any of several conditions such as degenerative disc disease, spinal stenosis, spondylolisthesis, and facet arthropathy. Treatment is difficult, given that many common interventions—medications, massage, manipulation—have limited efficacy.14 However, for patients with nonspecific chronic LBP, exercise is an effective intervention for reducing pain and improving physical function.15
An effective approach is to design an exercise regimen for the individual by type, duration, and frequency of activity, administered under supervision to encourage adherence.16 Appropriate exercises emphasize resistance, strength training, and core stabilization, often focusing on whole body and trunk motion (FIGURE 2).17
Although yoga or Pilates classes may have a small effect on function, no high-quality evidence exists for their superiority to other forms of exercise.18,19 Back School, a therapeutic program that includes education on anatomy and biomechanics, optimal posture, ergonomics, and back exercises, has limited, low-quality evidence for treatment comparisons.20 Aerobic exercise, including treadmill, elliptical, or cycling exercises or walking outdoors can reduce pain and improve physical and psychologic functioning.21
Continue to: The most common reported adverse effect...
The most common reported adverse effect of exercise is a temporary exacerbation of back pain. However, having patients continue daily activities within the permitted limits of pain leads to more rapid recovery than rest or back-mobilizing exercises.15,22,23
Cautions. Exercise is contraindicated in patients with LBP arising from a serious medical condition, such as fracture, infection, cancer, or cauda equina syndrome.24 Importantly, exercise interventions recommended for acute LBP have not shown benefit for chronic LBP.
Chronic shoulder pain
With a prevalence ranging from 7% to 26% in the general population,25 chronic shoulder pain often interferes with essential activities of daily living. The etiology of chronic shoulder pain is broad and most commonly involves disorders of the rotator cuff, which functions in both motion and dynamic stabilization of the shoulder. The common term “rotator cuff pain syndrome” can cover such disorders as subacromial impingement syndrome, rotator cuff tendinopathy or tendinitis, partial or full thickness rotator cuff tears, calcific tendinitis, and subacromial bursitis. These pathologies may have overlapping presentations. Manual therapy and exercise, usually delivered as a component of structured physical therapy, focus on stretches and other exercises to increase ROM, stability, and strength of the rotator cuff musculature.26
A 2016 Cochrane review that evaluated manual therapy and exercise for chronic shoulder pain yielded limited high-quality evidence for effectiveness compared with placebo.27 Five trials found no important differences between manual therapy and exercise compared with glucocorticoid injection relative to overall pain, function, active shoulder abduction, and quality of life from 4 weeks up to 12 months.27 But compared with placebo, exercise has been more effective in reducing reported pain, especially in the context of strengthening regimens focused on flexion, extension, and internal and external rotation.28
For subacromial impingement syndrome, a 2017 meta-analysis found that a generalized exercise program relieves pain and improves function, ROM, and strength.29 A generalized shoulder-strengthening program includes exercises that focus on internal and external rotation, horizontal abduction, and shoulder stabilization (FIGURE 3). These exercises can be completed with 3 sets of 15 to 20 repetitions, which create a fatigue response that improves strength and targets local muscular endurance.30
Continue to: Achilles tendinopathy
Achilles tendinopathy
Achilles tendinopathy (also referred to as chronic Achilles tendinitis) is a degenerative condition of the Achilles tendon related to overuse that leads to pain, swelling, and impaired performance. It accounts for approximately 18% of injuries in runners and 4% of all patients presenting to sports medicine clinics.31 Eccentric muscle loading has become the dominant conservative intervention strategy for chronic Achilles tendinopathy.
For chronic tendinopathies, eccentric exercises subject greater force than concentric exercises through a controlled lengthening of a muscle-tendon unit, resulting in a greater remodeling stimulus of the tendon.32 Classically, the Alfredson protocol has been used to treat chronic Achilles tendinopathy. This program of eccentric heel-drop exercises recommends completion of 180 eccentric repetitions a day for up to 12 weeks (FIGURE 4).33 Exercises are performed slowly, and load can be increased when exercises are performed without pain or perhaps with mild nondisabling pain.
A variation of this protocol has allowed a gradual escalation of repetitions over a week up to the recommended 180 repetitions, and has shown improvements in pain reduction and function similar to that achieved with the primary protocol.34 Additionally, a 6-week “do as tolerated” program of eccentric exercises did not lead to lesser improvement for individuals with midportion Achilles tendinopathy.35
Several systematic reviews have supported the use of eccentric exercises for chronic Achilles tendinopathy,31,36,37 but no specific protocol or exercise regimen has demonstrated superiority. However, with the Alfredson protocol, improvement in pain and function in patients with chronic Achilles tendinopathy has persisted for up to 5 years.38
Lateral epicondylitis
Lateral epicondylitis (also called lateral epicondylosis or “tennis elbow”) is a disabling musculoskeletal condition that leads to pain and tenderness around the extensor mass of the lateral elbow. It is caused by microtrauma to the tendon, usually sustained through repetitive movement in a sporting activity, industrial work, or hobby. Affecting up to 3% of the US population, lateral epicondylitis is associated with pain and functional disability, as well as emotional and psychosocial consequences.39
Continue to: Proposed treatment and rehabilitation options...
Proposed treatment and rehabilitation options for patients with lateral epicondylitis have included massage, manipulation, taping, acupuncture, orthotic devices, ultrasound, activity modification, and rest. Exercise programs incorporating eccentric muscle activity are becoming increasingly popular for such conditions as Achilles and patellar tendinopathies, and they may translate well to other chronic tendinopathies, such as lateral epicondylitis.32
An eccentric exercise program for lateral epicondylitis, either in isolation or as an adjunct to other therapies, has decreased pain and improved function and grip strength from baseline measures.40 Compared with a standard exercise regimen without eccentric strength training, use of eccentric training improves such clinical measures as pain intensity and disability status, as it decreases tendon thickness and aids in recovering homogenous tendon structure.41
A sample exercise. The patient may sit in a chair and, with the forearm flexed and pronated over the edge of a table, grasp some form of resistance (bucket of water, training weight, resistance band) (FIGURE 5). The nonaffected hand can be used to help lift the affected wrist into full extension and then removed to allow lowering of the hand over several seconds into flexion. This activity can be performed in sets of 8 to 12 repetitions, 2 to 3 times a day, until the patient’s pain and function have improved.42
Overcoming barriers to exercise
A major concern across all studies assessing the therapeutic value of exercise is patient compliance and adherence to prescribed programs. Compliance and adherence are affected in part by psychosocial factors such as low literacy and poor social support. From a physician’s perspective, direct and indirect costs of treatment and rehabilitation of chronic musculoskeletal conditions may discourage the prescribing of supervised physical therapy.3
Steps to consider in overcoming these barriers would be advising an exercise regimen that requires only an initial period of supervision; educating patients about the benefits of an exercise program; exploring a patient’s expectations, beliefs, and fears; and developing strategies for long-term adherence.16 Supervision through physical therapy is often suggested. However, significant barriers may exist that impede a patient’s ability to attend or participate, in which case physician observation in the course of regularly scheduled clinical examinations could be considered.
Continue to: When prescribing exercises...
When prescribing exercises, be sure to address patient expectations regarding pain, duration, and limitations of exercise. It would be helpful for patients to know, for instance, that working through mild-to-moderate pain during exercise has been shown to shorten post-exercise recovery time and, in the short-term, improve relief from pain.43
Tailoring specific exercise prescriptions for a patient will make the regimen more satisfying for the individual and optimize adherence, which in turn will increase the potential for pain reduction and improved function. Secondary benefits would likely be weight loss and prevention (or regression) of cardiovascular disease. Continued evaluation by the physician or physical therapist should be part of ongoing management, as well as “refresher courses” to ensure understanding of, and adherence to, the exercise program. The potential benefits and limited risks of exercise, if done properly, make it a primary intervention for specific musculoskeletal conditions.
CORRESPONDENCE
Peter J. Carek, MD, MS, Department of Community Health and Family Medicine, College of Medicine, University of Florida, PO Box 100237, Gainesville, FL 32610-0237; carek@ufl.edu.
1. Naci H, Ioannidis JP. Comparative effectiveness of exercise and drug interventions on mortality outcomes: metaepidemiological study. BMJ. 2013;347:f5577.
2. Babatunde OO, Jordan JL, van der Windt DA, et al. Effective treatment options for musculoskeletal pain in primary care: a systematic overview of current evidence. PLoS One. 2017;12:e0178621.
3. Persson G, Brorsson A, Ekvall Hansson E, et al. Physical activity on prescription (PAP) from the general practitioner’s perspective - a qualitative study. BMC Fam Pract. 2013;14:128.
4. Juhl C, Christensen R, Roos EM, et al. Impact of exercise type and dose on pain and disability in knee osteoarthritis: a systematic review and meta-regression analysis of randomized controlled trials. Arthritis Rheumatol. 2014;66:622-636.
5. Fransen M, McConnell S, Harmer AR, et al. Exercise for osteoarthritis of the knee. Cochrane Database Syst Rev. 2015;1:CD004376.
6. Fransen M, McConnell S, Hernandez-Molina G, et al. Exercise for osteoarthritis of the hip. Cochrane Database Syst Rev. 2014;(4):CD007912.
7. Vincent KR, Vincent HK. Resistance exercise for knee osteoarthritis. PM R. 2012;4(suppl 5):S45-S52.
8. Fernandes L, Storheim K, Nordsletten L, et al. Development of a therapeutic exercise program for patients with osteoarthritis of the hip. Phys Ther. 2010;90:592-601.
9. Bartels EM, Juhl CB, Christensen R, et al. Aquatic exercise for the treatment of knee and hip osteoarthritis. Cochrane Database Syst Rev. 2016;(3):CD005523.
10. Jordan JL, Holden MA, Mason EE, et al. Interventions to improve adherence to exercise for chronic musculoskeletal pain in adults. Cochrane Database Syst Rev. 2010;(1):CD005956.
11. Baker KR, Nelson ME, Felson DT, et al. The efficacy of home based progressive strength training in older adults with knee osteoarthritis: a randomized controlled trial. J Rheumatol. 2001;28:1655-1665.
12. Brakke R, Singh J, Sullivan W. Physical therapy in persons with osteoarthritis. PM R. 2012;4:S53-S58.
13. Katz JN. Lumbar disc disorders and low-back pain: socioeconomic factors and consequences. J Bone Joint Surg Am. 2006;88(suppl 2):21-24.
14. Qaseem A, Wilt TJ, McLean RM, et al. Noninvasive treatments for acute, subacute, and chronic low back pain: a clinical practice guideline from the American College of Physicians. Ann Intern Med. 2017;166:514-530.
15. Hayden JA, van Tulder MW, Malmivaara A, et al. Exercise therapy for treatment of non-specific low back pain. Cochrane Database Syst Rev. 2005;(3):CD000335.
16. Hayden JA, van Tulder MW, Tomlinson G. Systematic review: strategies for using exercise therapy to improve outcomes in chronic low back pain. Ann Intern Med. 2005;142:776-785.
17. Searle A, Spink M, Ho A, et al. Exercise interventions for the treatment of chronic low back pain: a systematic review and meta-analysis of randomised controlled trials. Clin Rehabil. 2015;29:1155-1167.
18. Yamato TP, Maher CG, Saragiotto BT, et al. Pilates for low back pain. Cochrane Database Syst Rev. 2015;(7):CD010265.
19. Wieland LS, Skoetz N, Pilkington K, et al. Yoga treatment for chronic non-specific low back pain. Cochrane Database Syst Rev. 2017;(1):CD010671.
20. Parreira P, Heymans MW, van Tulder MW, et al. Back Schools for chronic non-specific low back pain. Cochrane Database Syst Rev. 2017;(8):CD011674.
21. Meng XG, Yue SW. Efficacy of aerobic exercise for treatment of chronic low back pain: a meta-analysis. Am J Phys Med Rehabil. 2015;94:358-365.
22. Malmivaara A, Häkkinen U, Aro T, et al. The treatment of acute low back pain--bed rest, exercises, or ordinary activity? N Engl J Med. 1995;332:351-355.
23. van Tulder M, Malmivaara A, Esmail R, Koes B. Exercise therapy for low back pain: a systematic review within the framework of the cochrane collaboration back review group. Spine (Phila Pa 1976). 2000;25:2784-2796.
24. Hoffmann TC, Maher CG, Briffa T, et al. Prescribing exercise interventions for patients with chronic conditions. CMAJ. 2016;188:510-518.
25. Luime JJ, Koes BW, Hendriksen IJ, et al. Prevalence and incidence of shoulder pain in the general population; a systematic review. Scand J Rheumatol. 2004;33:73-81.
26. Kuhn JE. Exercise in the treatment of rotator cuff impingement: a systematic review and a synthesized evidence-based rehabilitation protocol. J Shoulder Elbow Surg. 2009;18:138-160.
27. Page MJ, Green S, McBain B, et al. Manual therapy and exercise for rotator cuff disease. Cochrane Database Syst Rev. 2016;(6):CD012224.
28. van den Dolder PA, Ferreira PH, Refshauge KM. Effectiveness of soft tissue massage and exercise for the treatment of non-specific shoulder pain: a systematic review with meta-analysis. Br J Sports Med. 2014;48:1216-1226.
29. Shire AR, Stæhr TAB, Overby JB, et al. Specific or general exercise strategy for subacromial impingement syndrome-does it matter? A systematic literature review and meta analysis. BMC Musculoskelet Disord. 2017;18:158.
30. Ellenbecker TS, Cools A. Rehabilitation of shoulder impingement syndrome and rotator cuff injuries: an evidence-based review. Br J Sports Med. 2010;44:319-327.
31. Magnussen RA, Dunn WR, Thomson AB. Nonoperative treatment of midportion Achilles tendinopathy: a systematic review. Clin J Sport Med. 2009;19:54-64.
32. Rees JD, Wolman RL, Wilson A. Eccentric exercises; why do they work, what are the problems and how can we improve them? Br J Sports Med. 2009;43:242-246.
33. Alfredson H, Pietilä T, Jonsson P, et al. Heavy-load eccentric calf muscle training for the treatment of chronic Achilles tendinosis. Am J Sports Med. 1998;26:360-366.
34. Rompe JD, Nafe B, Furia JP, et al. Eccentric loading, shock-wave treatment, or a wait-and-see policy for tendinopathy of the main body of tendo Achillis: a randomized controlled trial. Am J Sports Med. 2007;35:374-383.
35. Stevens M, Tan CW. Effectiveness of the Alfredson protocol compared with a lower repetition-volume protocol for midportion Achilles tendinopathy: a randomized controlled trial. J Orthop Sports Phys Ther. 2014;44:59-67.
36. Habets B, van Cingel RE. Eccentric exercise training in chronic mid-portion Achilles tendinopathy: a systematic review on different protocols. Scand J Med Sci Sports. 2015;25:3-15.
37. Malliaras P, Barton CJ, Reeves ND, et al. Achilles and patellar tendinopathy loading programmes : a systematic review comparing clinical outcomes and identifying potential mechanisms for effectiveness. Sports Med. 2013;43:267-286.
38. van der Plas A, de Jonge S, de Vos RJ, et al. A 5-year follow-up study of Alfredson’s heel-drop exercise programme in chronic midportion Achilles tendinopathy. Br J Sports Med. 2012;46:214-218.
39. Alizadehkhaiyat O, Fisher AC, Kemp GJ, et al. Pain, functional disability, and psychologic status in tennis elbow. Clin J Pain. 2007;23:482-489.
40. Cullinane FL, Boocock MG, Trevelyan FC. Is eccentric exercise an effective treatment for lateral epicondylitis? A systematic review. Clin Rehabil. 2014;28:3-19.
41. Croisier JL, Foidart-Dessalle M, Tinant F, et al. An isokinetic eccentric programme for the management of chronic lateral epicondylar tendinopathy. Br J Sports Med. 2007;41:269-275.
42. Söderberg J, Grooten WJ, Ang BO. Effects of eccentric training on hand strength in subjects with lateral epicondylalgia: a randomized-controlled trial. Scand J Med Sci Sports. 2012;22:797-803.
43. Smith BE, Hendrick P, Smith TO, et al. Should exercises be painful in the management of chronic musculoskeletal pain? A systematic review and meta-analysis. Br J Sports Med. 2017;51:1679-1687.
1. Naci H, Ioannidis JP. Comparative effectiveness of exercise and drug interventions on mortality outcomes: metaepidemiological study. BMJ. 2013;347:f5577.
2. Babatunde OO, Jordan JL, van der Windt DA, et al. Effective treatment options for musculoskeletal pain in primary care: a systematic overview of current evidence. PLoS One. 2017;12:e0178621.
3. Persson G, Brorsson A, Ekvall Hansson E, et al. Physical activity on prescription (PAP) from the general practitioner’s perspective - a qualitative study. BMC Fam Pract. 2013;14:128.
4. Juhl C, Christensen R, Roos EM, et al. Impact of exercise type and dose on pain and disability in knee osteoarthritis: a systematic review and meta-regression analysis of randomized controlled trials. Arthritis Rheumatol. 2014;66:622-636.
5. Fransen M, McConnell S, Harmer AR, et al. Exercise for osteoarthritis of the knee. Cochrane Database Syst Rev. 2015;1:CD004376.
6. Fransen M, McConnell S, Hernandez-Molina G, et al. Exercise for osteoarthritis of the hip. Cochrane Database Syst Rev. 2014;(4):CD007912.
7. Vincent KR, Vincent HK. Resistance exercise for knee osteoarthritis. PM R. 2012;4(suppl 5):S45-S52.
8. Fernandes L, Storheim K, Nordsletten L, et al. Development of a therapeutic exercise program for patients with osteoarthritis of the hip. Phys Ther. 2010;90:592-601.
9. Bartels EM, Juhl CB, Christensen R, et al. Aquatic exercise for the treatment of knee and hip osteoarthritis. Cochrane Database Syst Rev. 2016;(3):CD005523.
10. Jordan JL, Holden MA, Mason EE, et al. Interventions to improve adherence to exercise for chronic musculoskeletal pain in adults. Cochrane Database Syst Rev. 2010;(1):CD005956.
11. Baker KR, Nelson ME, Felson DT, et al. The efficacy of home based progressive strength training in older adults with knee osteoarthritis: a randomized controlled trial. J Rheumatol. 2001;28:1655-1665.
12. Brakke R, Singh J, Sullivan W. Physical therapy in persons with osteoarthritis. PM R. 2012;4:S53-S58.
13. Katz JN. Lumbar disc disorders and low-back pain: socioeconomic factors and consequences. J Bone Joint Surg Am. 2006;88(suppl 2):21-24.
14. Qaseem A, Wilt TJ, McLean RM, et al. Noninvasive treatments for acute, subacute, and chronic low back pain: a clinical practice guideline from the American College of Physicians. Ann Intern Med. 2017;166:514-530.
15. Hayden JA, van Tulder MW, Malmivaara A, et al. Exercise therapy for treatment of non-specific low back pain. Cochrane Database Syst Rev. 2005;(3):CD000335.
16. Hayden JA, van Tulder MW, Tomlinson G. Systematic review: strategies for using exercise therapy to improve outcomes in chronic low back pain. Ann Intern Med. 2005;142:776-785.
17. Searle A, Spink M, Ho A, et al. Exercise interventions for the treatment of chronic low back pain: a systematic review and meta-analysis of randomised controlled trials. Clin Rehabil. 2015;29:1155-1167.
18. Yamato TP, Maher CG, Saragiotto BT, et al. Pilates for low back pain. Cochrane Database Syst Rev. 2015;(7):CD010265.
19. Wieland LS, Skoetz N, Pilkington K, et al. Yoga treatment for chronic non-specific low back pain. Cochrane Database Syst Rev. 2017;(1):CD010671.
20. Parreira P, Heymans MW, van Tulder MW, et al. Back Schools for chronic non-specific low back pain. Cochrane Database Syst Rev. 2017;(8):CD011674.
21. Meng XG, Yue SW. Efficacy of aerobic exercise for treatment of chronic low back pain: a meta-analysis. Am J Phys Med Rehabil. 2015;94:358-365.
22. Malmivaara A, Häkkinen U, Aro T, et al. The treatment of acute low back pain--bed rest, exercises, or ordinary activity? N Engl J Med. 1995;332:351-355.
23. van Tulder M, Malmivaara A, Esmail R, Koes B. Exercise therapy for low back pain: a systematic review within the framework of the cochrane collaboration back review group. Spine (Phila Pa 1976). 2000;25:2784-2796.
24. Hoffmann TC, Maher CG, Briffa T, et al. Prescribing exercise interventions for patients with chronic conditions. CMAJ. 2016;188:510-518.
25. Luime JJ, Koes BW, Hendriksen IJ, et al. Prevalence and incidence of shoulder pain in the general population; a systematic review. Scand J Rheumatol. 2004;33:73-81.
26. Kuhn JE. Exercise in the treatment of rotator cuff impingement: a systematic review and a synthesized evidence-based rehabilitation protocol. J Shoulder Elbow Surg. 2009;18:138-160.
27. Page MJ, Green S, McBain B, et al. Manual therapy and exercise for rotator cuff disease. Cochrane Database Syst Rev. 2016;(6):CD012224.
28. van den Dolder PA, Ferreira PH, Refshauge KM. Effectiveness of soft tissue massage and exercise for the treatment of non-specific shoulder pain: a systematic review with meta-analysis. Br J Sports Med. 2014;48:1216-1226.
29. Shire AR, Stæhr TAB, Overby JB, et al. Specific or general exercise strategy for subacromial impingement syndrome-does it matter? A systematic literature review and meta analysis. BMC Musculoskelet Disord. 2017;18:158.
30. Ellenbecker TS, Cools A. Rehabilitation of shoulder impingement syndrome and rotator cuff injuries: an evidence-based review. Br J Sports Med. 2010;44:319-327.
31. Magnussen RA, Dunn WR, Thomson AB. Nonoperative treatment of midportion Achilles tendinopathy: a systematic review. Clin J Sport Med. 2009;19:54-64.
32. Rees JD, Wolman RL, Wilson A. Eccentric exercises; why do they work, what are the problems and how can we improve them? Br J Sports Med. 2009;43:242-246.
33. Alfredson H, Pietilä T, Jonsson P, et al. Heavy-load eccentric calf muscle training for the treatment of chronic Achilles tendinosis. Am J Sports Med. 1998;26:360-366.
34. Rompe JD, Nafe B, Furia JP, et al. Eccentric loading, shock-wave treatment, or a wait-and-see policy for tendinopathy of the main body of tendo Achillis: a randomized controlled trial. Am J Sports Med. 2007;35:374-383.
35. Stevens M, Tan CW. Effectiveness of the Alfredson protocol compared with a lower repetition-volume protocol for midportion Achilles tendinopathy: a randomized controlled trial. J Orthop Sports Phys Ther. 2014;44:59-67.
36. Habets B, van Cingel RE. Eccentric exercise training in chronic mid-portion Achilles tendinopathy: a systematic review on different protocols. Scand J Med Sci Sports. 2015;25:3-15.
37. Malliaras P, Barton CJ, Reeves ND, et al. Achilles and patellar tendinopathy loading programmes : a systematic review comparing clinical outcomes and identifying potential mechanisms for effectiveness. Sports Med. 2013;43:267-286.
38. van der Plas A, de Jonge S, de Vos RJ, et al. A 5-year follow-up study of Alfredson’s heel-drop exercise programme in chronic midportion Achilles tendinopathy. Br J Sports Med. 2012;46:214-218.
39. Alizadehkhaiyat O, Fisher AC, Kemp GJ, et al. Pain, functional disability, and psychologic status in tennis elbow. Clin J Pain. 2007;23:482-489.
40. Cullinane FL, Boocock MG, Trevelyan FC. Is eccentric exercise an effective treatment for lateral epicondylitis? A systematic review. Clin Rehabil. 2014;28:3-19.
41. Croisier JL, Foidart-Dessalle M, Tinant F, et al. An isokinetic eccentric programme for the management of chronic lateral epicondylar tendinopathy. Br J Sports Med. 2007;41:269-275.
42. Söderberg J, Grooten WJ, Ang BO. Effects of eccentric training on hand strength in subjects with lateral epicondylalgia: a randomized-controlled trial. Scand J Med Sci Sports. 2012;22:797-803.
43. Smith BE, Hendrick P, Smith TO, et al. Should exercises be painful in the management of chronic musculoskeletal pain? A systematic review and meta-analysis. Br J Sports Med. 2017;51:1679-1687.
From The Journal of Family Practice | 2018;67(9):534-538,540-543.
PRACTICE RECOMMENDATIONS
› Consider quadriceps strengthening for knee osteoarthritis with an initial period of supervision, which can provide greater pain relief than nonspecific, unsupervised lower limb exercises. B
› Consider a generalized exercise program for subacromial impingement syndrome, to relieve shoulder pain and improve function, range of motion, and strength. A
› Bear in mind that the Alfredson protocol for Achilles tendinopathy has yielded improvement in pain and function for up to 5 years, although other exercise regimens have also proven initially effective. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
A thorough yet efficient exam identifies most problems in school athletes
- A complete medical history, preferably from the student and a parent, will reveal approximately 75% of problems affecting initial athletic participation (D).
- For asymptomatic athletes with no previous injuries, a 90-second screening musculoskeletal test will detect 90% of significant musculoskeletal injuries (A).
- A routine screening need not include noninvasive cardiac testing or laboratory tests such as ur inalysis, blood count, chemistry profile, lipid profile, ferritin level, or spirometry (B).
Is the preparticipation physical examination the best way to determine whether a student athlete can participate fully in his or her chosen sport? This examination has become the standard of care for the over 6 million high school and college students. While most athletes pass the exam without significant medical or orthopedic abnormalities being noted, it often detects conditions that may predispose an athlete to injury or limit full participation in certain activities. We describe an efficient approach to the preparticipation examination.
Although many organizations have adopted the preparticipation exam there has been considerable debate on its content and usefulness.1-4 Nevertheless, sponsoring institutions continue to require the medical evaluation prior to competition in organized athletics, so family physicians should be knowledgeable about the objectives and limitations of the exam.
The American Academy of Family Physicians, the American Academy of Pediatrics, the American Medical Society for Sports Medicine, the American Orthopedic Society for Sports Medicine, and the American Osteopathic Academy of Sports Medicine established the Preparticipation Physical Examination Task Force. The recommendations of this task force serve as a guide for the physician conducting these examinations for high school and collegiate athletes.5,6
Assessing risks of mortality and morbidity
The mortality associated with athletic participation is most often the result of sudden cardiac death, which occurs in about 0.5 per 100,000 high school athletes per academic year and is most commonly due to hypertrophic cardiomyopathy.7,8 Screening for predisposing conditions is limited by the low prevalence of relevant cardiovascular lesions in the general youth population, the low risk of sudden death even among persons with an unsuspected abnormality, and the large number of school athletes.7-9
An estimated 200,000 children and adolescents would have to be screened to detect the 500 athletes who are at risk for sudden cardiac death and the 1 person who would actually experience it.10 Even when cardiac abnormalities are detected, the findings leading to disqualification are most often rhythm and conduction abnormalities, valvular abnormalities, and systemic hypertension, which are not the cardiac abnormalities usually associated with sudden cardiac death in athletes.11,12
The majority of sudden deaths are associated with 4 sports: football, basketball, track, and soccer. Approximately 90% of athletic-field deaths have occurred in males, mostly high school athletes.7,13
More frequently than mortality, athletic participation places the individual at risk for acute injury or worsening of an underlying medical condition. These conditions are most commonly musculoskeletal, cardiovascular, or ophthalmologic (Table 1).5,9,21
Nine studies of the preparticipation exam done between 1980 and 1999 show general agreement on the rates at which it qualifies (84.8% to 96.6%), qualifies with conditions (3.1% to 13.9%), and disqualifies students for sports participation (0.2% to 2.6%).14-22
TABLE 1
Medical and orthopedic conditions resulting in additional evaluations
| Rifat, 1995* | Lively, 1999† | ||
|---|---|---|---|
| n=2,574 | n=596 | ||
| Pass with follow-up and/or restriction (12.6%) | Fail with follow-up (2.6%) | Follow-up or restriction (14.1%) | |
| Medical (% of overall total) | 76.6 | 74.1 | 55.4 |
| Cardiovascular | 18.3 | 35.0 | 63.0 |
| Dermatologic | 6.8 | ||
| Endocrinologic | 0.4 | ||
| Ear, nose, and throat | 9.6 | 2.5 | |
| Gastrointestinal | 0.9 | 2.2 | |
| Genitourinary | 9.6 | 12.5 | 8.7 |
| Gynecologic | 4.4 | ||
| Infectious | 0.4 | 6.5 | |
| Neurologic | 6.5 | ||
| Ophthalmologic | 26.0 | 25.0 | 6.5 |
| Psychological | 2.2 | ||
| Pulmonary | 14.2 | 2.5 | |
| Other‡ | 13.7 | 22.5 | |
| Total medical (%) | 100.0 | 100.0 | 100.0 |
| Orthopedic (% of overall total) | 23.4 | 25.9 | 44.6 |
| Ankle/Foot | 14.9 | 7.7 | 2.7 |
| Back/Neck | 22.4 | 14.3 | 5.4 |
| Elbow | 5.4 | ||
| Hand/Wrist | 1.5 | 10.9 | |
| Knee | 41.8 | 7.1 | 43.2 |
| Leg | 5.4 | ||
| Shoulder | 27.0 | ||
| Nonspecific pain/injury | 19.4 | 71.4 | |
| Total orthopedic (%) | 100.0 | 100.0 | 100.0 |
| ∗Studied junior high and high school students. Two individual s failed (nonspecific pain/injury). | |||
| †Studied college-aged students. One individual failed (complicated pregnancy). | |||
| ‡“Other ” includes abdominal pain, allergy, bruising, chest pain, chronic/recurrent illness, dizziness/syncope with exercise, surgery (recent). | |||
What should the medical history include?
The examining physician should obtain a medical history from each participant (strength of recommendation [SOR]: D). A complete medical history will identify approximately 75% of problems that will affect initial athletic participation and serves as the cornerstone of the exam.14,19 Most conditions requiring further evaluation or restriction will be identified from the medical history. Rifat and colleagues21 noted that a complete medical history accounted for 88% of the abnormal findings and 57% of the reasons cited for activity restriction. The Preparticipation Physical Evaluation Task Force has developed a history form that emphasizes the areas of greatest concern.5
In particular, examining physicians should ask regarding risk factors and symptoms of cardiovascular disease ( Table 2 ). You should confirm a positive response to any of these questions, and conduct further evaluation if necessary. Unfortunately, most athletes with hypertrophic cardiomyopathy do not report a history of syncope with exercise or a family history of premature sudden cardiac death due to the disease.
Musculoskeletal injury is a common cause for disqualification of an athlete.14,19,21 The most common injury to restrict participation is a knee injury, with an ankle injury the next most common.23 The strongest independent predictor of sports injuries is a previous injury (odds ratio [OR]=9.4) and exposure time (OR=6.9).24 DuRant and colleagues23 found that a previous knee injury or knee surgery was significantly associated with further knee injuries during the subsequent sports season when compared with individuals who did not report previous knee injury or surgery (30.6% vs. 7.2%, P=.0001).
Additional historical information has been recommended for inclusion (SOR: D). For example, the examining physician should question the athlete about wheezing during exercise. Due to the high rate of recurrence and potential for long-term adverse effects, he or she should also obtain a history of previous concussions. Other issues to be addressed include presence of a single bilateral organ and use of performance-enhancing medication. Finally, physicians should question female athletes regarding their menstrual history and other symptoms or signs of the female athletic triad (eating disorder, amenorrhea, and osteoporosis).
Always carefully review the information provided by the athlete and his or her parents. In 2 separate studies, minimal agreement was found between histories obtained from athletes and parents independently.19,25 We do not know which source provides the most accurate history; therefore, both the parents and student athlete should be questioned.
TABLE 2
Questions to help discern cardiovascular risk
| Have you ever passed out during or after exercise? |
| Have you ever been dizzy during or after exercise? |
| Have you ever had chest pain during or after exercise? |
| Do you get tired more quickly than your friends during exercise? |
| Have you ever had racing of your heart or skipped heartbeats? |
| Have you ever had high blood pressure or high cholesterol? |
| Have you been told you have a heart murmur? |
| Has any family member or relative died of heart problems or of sudden death before age 50? |
| Have you had a severe viral infection (for example, myocarditis or mononucleosis) within the last month? |
| Has a physician ever denied or restricted your participation in sports for any heart problem? |
What should the physical examination include ?
A complete physical examination is not necessary (SOR: D).5 The screening physical examination should include vital signs (ie, height, weight, and blood pressure) and visual acuity testing as well as a cardiovascular, pulmonary, abdominal, skin, genital (for males), and musculoskeletal examination. Further examination should be based on issues elicited during the history.
Cardiovascular examination
The cardiovascular examination requires an additional level of detail. Perform auscultation of the heart initially with the patient in both standing and supine position, and during various maneuvers (squat-to-stand, deep inspiration, or Valsalva’s maneuver), as these maneuvers can clarify the type of murmur.
Any systolic murmur grade III/VI or louder, any murmur that disrupts normal heart sounds, any diastolic murmur, or any murmur that intensifies with the previously described maneuvers should be evaluated further through diagnostic studies (echocardiography) or consultation prior to participation. Sinus bradycardia and systolic murmurs are commonly found, occurring in over 50% and between 30% and 50% of athletes, respectively; they do not warrant further evaluation in the asymptomatic athlete.26 Third and fourth heart sounds are also commonly found in asymptomatic athletes without underlying heart disease.26,27
Noninvasive cardiac testing (eg, electrocardiography, echocardiography, or exercise stress testing) should not be a routine part of the screening preparticipation exam (SOR: B ).7 These tests are not cost-effective in a population at relatively low risk for cardiac abnormalities and cannot consistently identify athletes at actual risk.28-32 For example, a substantial minority of subjects (11%) were found to have a clinically significant increased ventricular wall thickness, which made clinical interpretation of the echocardiographic findings difficult in individual athletes.28 Furthermore, some patients with hypertrophic cardiomyopathy are able to tolerate particularly intense athletic training and competition for many years, and even maintain high levels of achievement without incurring symptoms, disease progression, or sudden death.29
Echocardiography and stress testing are the most commonly recommended diagnostic tests for patients with an abnormal cardiovascular history or examination. With the assistance of clinical information, echocardiography is able to distinguish the nonobstructive hypertrophic cardiomyopathy from the athletic heart syndrome.33
Musculos keletal examination
A screening musculoskeletal history and examination in combination can be used for asymptomatic athletes with no previous injuries (Table 3) (SOR: A).34 An accurate history is able to detect over 90% of significant musculoskeletal injuries. The screening physical examination is 51% sensitive and 97% specific.34 If the athlete has either a previous injury or other signs or symptoms (ie, pain; tenderness; asymmetries in muscle bulk, strength, or range of motion; any obvious deformity) detected by the general screening examination or history, the general screening should be supplemented with relevant elements of a site-specific examination.
Additional forms of musculoskeletal evaluation are often performed for athletes to determine their general state of flexibility and muscular strength. While various degrees of hyperlaxity, muscular tightness, weakness, asymmetry of strength or flexibility, poor endurance, and abnormal foot configuration may predispose an athlete to increased risk of injury during sports competition, studies have failed to demonstrate conclusively that injuries are prevented by interventions aimed at correcting such abnormalities.35-37
TABLE 3
The “90-second” musculoskeletal screening examination
| Instruction | Observations |
|---|---|
| Stand facing examiner | Acromiclavicular joints: general habitus |
| Look at ceiling, floor, over both shoulders, touch ears to shoulder | Cervical spine motion |
| Shrug shoulders (resistance) | Trapezius strength |
| Abduct shoulders to 90° (resistance at 90°) | Deltoid strength |
| Full external rotation of arms | Shoulder motion |
| Flex and extend elbows | Elbow motion |
| Arms at sides, elbows at 90° flexed; pronate and supinate wrists | Elbow and wrist motion |
| Spread fingers; make fist | Hand and finger motion, strength, and deformities |
| Tighten (contract) quadriceps; quadriceps | Symmetry and knee effusions, ankle effusion relax |
| “Duck walk” away and towards examiner | Hip, knee, and ankle motions |
| Back to examiner | Shoulder symmetry; scoliosis |
| Knees straight, touch toes | Scoliosis, hip motion, hamstring tightness |
| Raise upon toes, heels | Calf symmetry, leg strength |
Role for lab tests?
Studies do not support the use of routine laboratory or other screening tests such as urinalysis, complete blood count, chemistry profile, lipid profile, ferritin level, or spirometry as part of the exam (SOR: B).38-41
Determining clearance
Occasionally, an abnormality or condition is found that may limit an athlete’s participation or predispose him or her to further injury. In these cases, the examining physician should review the following questions:5
- Does the problem place the athlete at increased risk for injury?
- Is another participant at risk for injury because of the problem?
- Can the athlete safely participate with treatment (ie, medication, rehabilitation, bracing, or padding)?
- Can limited participation be allowed while treatment is being completed?
- If clearance is denied only for certain sports or sport categories, in what activities can the athlete safely participate?
Physicians should base clearance to participate in a particular sport on previously published guidelines, such as the recommendations by the American Academy of Pediatrics, the 26th Bethesda Conference, and the American Heart Association.7,43,44 Participation recommendations are based on the specific diagnosis, though multiple factors such as the classification of the sport and the specific health status of the athlete affect the decision.44
Approach to the patient
While current research demonstrates that the preparticipation physical examination has no effect on the overall morbidity and mortality rates in athletes, these exams may fulfill other objectives. Furthermore, no harmful effects of these examinations have been reported, and the exam has become institutionalized in the athletic and sports medicine community. As such, physicians should base their evaluation on the best available evidence using the standard form shown in “Preparticipation physical evaluation for athletics.”6 (A copy of the Preparticipation Physical Evaluation form can be found at www.jfponline.com.) This may require that the physician work with local school systems to assure that they understand what constitutes an appropriate examination.
To assist future patient care decisions and research efforts, a standardized preparticipation physical examination with an associated form similar to the evaluation recommended by the Preparticipation Physical Evaluation Task Force should be uniformly implemented throughout the country. The use of consistent clearance criteria as recommended by the Preparticipation Physical Evaluation Task Force or the American Academy of Pediatrics (“Medical conditions and sports participation,” also available at www.jfponline.com) should be used, studied, and revised as needed.5,44
In addition to the exam, physicians should consider exploring other aspects of sports participation to assist athletes in reducing the risk of injury. Rules, equipment, or other factors may have a greater effect on decreasing the mortality and morbidity associated with athletic participation. A marked decrease in cervical spine injuries occurred following the rule change in football banning deliberate “spearing”—the use of the top of the helmet as the initial point of contact in making a tackle.41
1. MacAuley D. Does the preseason screening for cardiac disease really work?: the British perspective. Med Sci Sports Exerc 1998;30(Suppl):S345-S350.
2. Glover DW, Maron BJ. Profile of preparticipation cardiovascular screening for high school athletes. JAMA 1998;279:1817-9.
3. Pfister GC, Puffer JC, Maron BJ. Preparticipation cardiovascular screening for US collegiate student-athletes. JAMA 2000;283:1597-9.
4. Reich JD. It won’t be me next time: an opinion on preparticipation sports physicals. Am Fam Physician 2000;61:2618, 2620, 2625, 2629.-
5. Smith DM, Kovan JR, Rich BSE, Tanner SM. Preparticipation Physical Evaluation. 2nd ed. Minneapolis, Minn: McGraw-Hill Co; 1997;1-46.
6. Lombardo JA, Robinson JB, Smith DM, et al. Preparticipation physical examination. 1st ed. Kansas City, Mo: American Academy of Family Physicians, American Academy of Pediatrics, American Medical Society for Sports Medicine, American Orthopedic Society for Sports Medicine, American Osteopathic Academy of Sports Medicine; 1992.
7. Maron BJ, Thompson PD, Puffer JC, et al. Cardiovascular preparticipation screening of competitive athletes. A statement for health professionals from the Sudden Death Committee (clinical cardiology) and Congenital Cardiac Defects Committee (cardiovascular disease in the young), American Heart Association. Circulation 1996;94:850-6.
8. Maron BJ, Gohman TE, Aeppli D. Prevalence of sudden cardiac death during competitive sports activities in Minnesota high school athletes. J Am Coll Cardiol 1998;32:1881-4.
9. American Medical Association Board of Trustees, Group on Science and Technology. Athletic participation examinations for adolescents. Arch Pediatr Adolesc Med 1994;148:93-8.
10. Epstein SE, Maron BJ. Sudden death and the competitive athlete: perspectives on preparticipation screening studies. J Am Coll Cardiol 1986;7:220-30.
11. Pelliccia A, Maron BJ. Preparticipation cardiovascular evaluation of the competitive athlete: Perspectives from the 30-year Italian experience. Am J Cardiol 1995;75:827-9.
12. Corrado D, Basso C, Schiavon M, Thiene G. Screening for hypertrophic cardiomyopathy in young athletes. N Engl J Med 1998;339:364-9.
13. Cantu RC, Mueller FO. Fatalities and catastrophic injuries in high school and college sports, 1982-1997. Phys Sportsmed 1999;27:35-48.
14. Goldberg B, Saraniti A, Witman P, et al. Preparticipation sports assessment: an objective evaluation. Pediatrics 1980;66:736-45.
15. Linder CW, DuRant RH, Seklecki RM, Strong WB. Preparticipation health screening of young athletes: results of 1268 examinations. Am J Sports Med 1981;9:187-93.
16. Tennant FS, Jr, Sorenson K, Day CM. Benefits of preparticipation sports examinations. J Fam Pract 1981;13:287-8.
17. Thompson TR, Andrish JT, Bergfeld JA. A prospective study of preparticipation sports examinations of 2670 young athletes: method and results. Cleve Clin Q 1982;49:225-33.
18. DuRant R, Seymore C, Linder CW, Jay S. The preparticipation examination of athletes. Comparison of single and multiple examiners. Am J Dis Child. 1985;139:657-61.
19. Risser WL, Hoffman HM, Bellah GG, Jr. Frequency of preparticipation sports examinations in secondary school athletes: are the University Interscholastic League guidelines appropriate? Tex Med 1985;81:35-9.
20. Magnes SA, Henderson JM, Hunter SC. What limits sports participation: experience with 10,540 athletes. Phys Sportsmed 1992;20:143-60.
21. Rifat SF, Ruffin MT, Gorenflo DW. Disqualifying criteria in preparticipation sports evaluation. J Fam Pract 1995;41:42-50.
22. Lively MW. Preparticipation physical examinations: a collegiate experience. Clin J Sports Med 1999;9:38.-
23. DuRant RH, Pendergrast RA, Seymore C, Gaillard G, Donner J. Findings from the preparticipation athletic examination and athletic injuries. Am J Dis Child 1992;146:85-91.
24. Van Mechelen W, Twisk J, Molendijk A, Blom B, Snel J, Kemper HC. Subject-related risk factors for sports injuries: a 1-yr prospective study in young adults. Med Sci Sports Exerc 1996;28:1171-9.
25. Carek PJ, Futrell MA. Athlete’s view of the preparticipation physical examination: Attitudes toward certain health screening questions. Arch Fam Med 1999;8:307-12.
26. Huston TP, Puffer JC, Rodney WM. The athletic heart syndrome. N Engl J Med 1985;313:24-32.
27. Crawford MH, O’Rourke RA. The athlete’s heart. Adv Intern Med 1979;24:311-29.
28. Lewis JF, Maron BJ, Diggs JA, Spencer JE, Mehrotra PP, Curry CL. Preparticipation echocardiographic screening for cardiovascular disease in a large, predominately black population of collegiate athletes. Am J Cardiol 1989;64:1029-33.
29. Maron BJ, Klues HG. Surviving competitive athletes with hypertrophic cardiomyopathy. Am J Cardiol 1994;73:1098-104.
30. Fuller CM, McNulty CM, Spring DA, et al. Prospective screening of 5,615 high school athletes for risk of sudden death. Med Sci Sports Exer 1997;29:1131-8.
31. Fuller CM. Cost effectiveness of analysis of high school athletes for risks of sudden cardiac death. Med Sci Sports Exer 2000;32:887-90.
32. Pelliccia A, Maron BJ, Culasso F, et al. Clinical significance of abnormal electrocardiographic patterns in trained athletes. Circulation 2000;102:278-84.
33. Maron BJ, Pelliccia A, Spirito P. Cardiac disease in young trained athletes: insights into methods for distinguishing athlete’s heart from structural heart disease with particular emphasis on hypertrophic cardiomyopathy. Circulation 1995;91:1596-1601.
34. Gomez JE, Landry GL, Bernhardt DT. Critical evaluation of the 2-minute orthopedic screening examination. Am J Dis Child 1993;147:1109-13.
35. Abbott HG, Kress JB. Preconditioning in the prevention of knee injuries. Arch Phys Med Rehabil 1969;50:326-33.
36. Jackson DW, Jarrett H, Bailey D, Kausek J, Swanson J, Powell JW. Injury prediction in the young athlete: a preliminary report. Am J Sports Med 1978;6:6-14.
37. Nicholas JA. Injuries in knee ligaments: Relationship to looseness and tightness in football players. JAMA 1970;212:2236-9.
38. Dodge WF, West EF, Smith EH, Harvey B 3rd. Proteinuria and hematuria in schoolchildren: epidemiology and early natural history. J Pediatr 1976;88:327-47.
39. Peggs JF, Reinhardt RW, O’Brien JM. Proteinuria in adolescent sports physical examinations. J Fam Pract 1986;22:80-1.
40. Rupp NT, Brudno DS, Guill MF. The value of screening for risk of exercise-induced asthma in high school athletes. Ann Allergy 1993;70:339-42.
41. Feinstein RA, LaRussa J, Wang-Dohlman A, Bartolucci AA. Screening adolescent athletes for exercise-induced asthma. Clin J Sports Med 1996;6:119-23.
42. Torg JS, Vegso JJ, Sennett B, Das M. The National Football Head and Neck Injury Registry. 14-year report on cervical quadriplegia, 1971 through 1984. JAMA 1985;254:3439-43.
43. 26th Bethesda Conference: Recommendations for determining eligibility for competition in athletes with cardiovascular abnormalities Med Sci Sports Exerc 1994;26(Suppl):S223-S283.
44. American Academy of Pediatrics. Medical conditions affecting sports participation. Pediatrics 2001;107:1206-7.
- A complete medical history, preferably from the student and a parent, will reveal approximately 75% of problems affecting initial athletic participation (D).
- For asymptomatic athletes with no previous injuries, a 90-second screening musculoskeletal test will detect 90% of significant musculoskeletal injuries (A).
- A routine screening need not include noninvasive cardiac testing or laboratory tests such as ur inalysis, blood count, chemistry profile, lipid profile, ferritin level, or spirometry (B).
Is the preparticipation physical examination the best way to determine whether a student athlete can participate fully in his or her chosen sport? This examination has become the standard of care for the over 6 million high school and college students. While most athletes pass the exam without significant medical or orthopedic abnormalities being noted, it often detects conditions that may predispose an athlete to injury or limit full participation in certain activities. We describe an efficient approach to the preparticipation examination.
Although many organizations have adopted the preparticipation exam there has been considerable debate on its content and usefulness.1-4 Nevertheless, sponsoring institutions continue to require the medical evaluation prior to competition in organized athletics, so family physicians should be knowledgeable about the objectives and limitations of the exam.
The American Academy of Family Physicians, the American Academy of Pediatrics, the American Medical Society for Sports Medicine, the American Orthopedic Society for Sports Medicine, and the American Osteopathic Academy of Sports Medicine established the Preparticipation Physical Examination Task Force. The recommendations of this task force serve as a guide for the physician conducting these examinations for high school and collegiate athletes.5,6
Assessing risks of mortality and morbidity
The mortality associated with athletic participation is most often the result of sudden cardiac death, which occurs in about 0.5 per 100,000 high school athletes per academic year and is most commonly due to hypertrophic cardiomyopathy.7,8 Screening for predisposing conditions is limited by the low prevalence of relevant cardiovascular lesions in the general youth population, the low risk of sudden death even among persons with an unsuspected abnormality, and the large number of school athletes.7-9
An estimated 200,000 children and adolescents would have to be screened to detect the 500 athletes who are at risk for sudden cardiac death and the 1 person who would actually experience it.10 Even when cardiac abnormalities are detected, the findings leading to disqualification are most often rhythm and conduction abnormalities, valvular abnormalities, and systemic hypertension, which are not the cardiac abnormalities usually associated with sudden cardiac death in athletes.11,12
The majority of sudden deaths are associated with 4 sports: football, basketball, track, and soccer. Approximately 90% of athletic-field deaths have occurred in males, mostly high school athletes.7,13
More frequently than mortality, athletic participation places the individual at risk for acute injury or worsening of an underlying medical condition. These conditions are most commonly musculoskeletal, cardiovascular, or ophthalmologic (Table 1).5,9,21
Nine studies of the preparticipation exam done between 1980 and 1999 show general agreement on the rates at which it qualifies (84.8% to 96.6%), qualifies with conditions (3.1% to 13.9%), and disqualifies students for sports participation (0.2% to 2.6%).14-22
TABLE 1
Medical and orthopedic conditions resulting in additional evaluations
| Rifat, 1995* | Lively, 1999† | ||
|---|---|---|---|
| n=2,574 | n=596 | ||
| Pass with follow-up and/or restriction (12.6%) | Fail with follow-up (2.6%) | Follow-up or restriction (14.1%) | |
| Medical (% of overall total) | 76.6 | 74.1 | 55.4 |
| Cardiovascular | 18.3 | 35.0 | 63.0 |
| Dermatologic | 6.8 | ||
| Endocrinologic | 0.4 | ||
| Ear, nose, and throat | 9.6 | 2.5 | |
| Gastrointestinal | 0.9 | 2.2 | |
| Genitourinary | 9.6 | 12.5 | 8.7 |
| Gynecologic | 4.4 | ||
| Infectious | 0.4 | 6.5 | |
| Neurologic | 6.5 | ||
| Ophthalmologic | 26.0 | 25.0 | 6.5 |
| Psychological | 2.2 | ||
| Pulmonary | 14.2 | 2.5 | |
| Other‡ | 13.7 | 22.5 | |
| Total medical (%) | 100.0 | 100.0 | 100.0 |
| Orthopedic (% of overall total) | 23.4 | 25.9 | 44.6 |
| Ankle/Foot | 14.9 | 7.7 | 2.7 |
| Back/Neck | 22.4 | 14.3 | 5.4 |
| Elbow | 5.4 | ||
| Hand/Wrist | 1.5 | 10.9 | |
| Knee | 41.8 | 7.1 | 43.2 |
| Leg | 5.4 | ||
| Shoulder | 27.0 | ||
| Nonspecific pain/injury | 19.4 | 71.4 | |
| Total orthopedic (%) | 100.0 | 100.0 | 100.0 |
| ∗Studied junior high and high school students. Two individual s failed (nonspecific pain/injury). | |||
| †Studied college-aged students. One individual failed (complicated pregnancy). | |||
| ‡“Other ” includes abdominal pain, allergy, bruising, chest pain, chronic/recurrent illness, dizziness/syncope with exercise, surgery (recent). | |||
What should the medical history include?
The examining physician should obtain a medical history from each participant (strength of recommendation [SOR]: D). A complete medical history will identify approximately 75% of problems that will affect initial athletic participation and serves as the cornerstone of the exam.14,19 Most conditions requiring further evaluation or restriction will be identified from the medical history. Rifat and colleagues21 noted that a complete medical history accounted for 88% of the abnormal findings and 57% of the reasons cited for activity restriction. The Preparticipation Physical Evaluation Task Force has developed a history form that emphasizes the areas of greatest concern.5
In particular, examining physicians should ask regarding risk factors and symptoms of cardiovascular disease ( Table 2 ). You should confirm a positive response to any of these questions, and conduct further evaluation if necessary. Unfortunately, most athletes with hypertrophic cardiomyopathy do not report a history of syncope with exercise or a family history of premature sudden cardiac death due to the disease.
Musculoskeletal injury is a common cause for disqualification of an athlete.14,19,21 The most common injury to restrict participation is a knee injury, with an ankle injury the next most common.23 The strongest independent predictor of sports injuries is a previous injury (odds ratio [OR]=9.4) and exposure time (OR=6.9).24 DuRant and colleagues23 found that a previous knee injury or knee surgery was significantly associated with further knee injuries during the subsequent sports season when compared with individuals who did not report previous knee injury or surgery (30.6% vs. 7.2%, P=.0001).
Additional historical information has been recommended for inclusion (SOR: D). For example, the examining physician should question the athlete about wheezing during exercise. Due to the high rate of recurrence and potential for long-term adverse effects, he or she should also obtain a history of previous concussions. Other issues to be addressed include presence of a single bilateral organ and use of performance-enhancing medication. Finally, physicians should question female athletes regarding their menstrual history and other symptoms or signs of the female athletic triad (eating disorder, amenorrhea, and osteoporosis).
Always carefully review the information provided by the athlete and his or her parents. In 2 separate studies, minimal agreement was found between histories obtained from athletes and parents independently.19,25 We do not know which source provides the most accurate history; therefore, both the parents and student athlete should be questioned.
TABLE 2
Questions to help discern cardiovascular risk
| Have you ever passed out during or after exercise? |
| Have you ever been dizzy during or after exercise? |
| Have you ever had chest pain during or after exercise? |
| Do you get tired more quickly than your friends during exercise? |
| Have you ever had racing of your heart or skipped heartbeats? |
| Have you ever had high blood pressure or high cholesterol? |
| Have you been told you have a heart murmur? |
| Has any family member or relative died of heart problems or of sudden death before age 50? |
| Have you had a severe viral infection (for example, myocarditis or mononucleosis) within the last month? |
| Has a physician ever denied or restricted your participation in sports for any heart problem? |
What should the physical examination include ?
A complete physical examination is not necessary (SOR: D).5 The screening physical examination should include vital signs (ie, height, weight, and blood pressure) and visual acuity testing as well as a cardiovascular, pulmonary, abdominal, skin, genital (for males), and musculoskeletal examination. Further examination should be based on issues elicited during the history.
Cardiovascular examination
The cardiovascular examination requires an additional level of detail. Perform auscultation of the heart initially with the patient in both standing and supine position, and during various maneuvers (squat-to-stand, deep inspiration, or Valsalva’s maneuver), as these maneuvers can clarify the type of murmur.
Any systolic murmur grade III/VI or louder, any murmur that disrupts normal heart sounds, any diastolic murmur, or any murmur that intensifies with the previously described maneuvers should be evaluated further through diagnostic studies (echocardiography) or consultation prior to participation. Sinus bradycardia and systolic murmurs are commonly found, occurring in over 50% and between 30% and 50% of athletes, respectively; they do not warrant further evaluation in the asymptomatic athlete.26 Third and fourth heart sounds are also commonly found in asymptomatic athletes without underlying heart disease.26,27
Noninvasive cardiac testing (eg, electrocardiography, echocardiography, or exercise stress testing) should not be a routine part of the screening preparticipation exam (SOR: B ).7 These tests are not cost-effective in a population at relatively low risk for cardiac abnormalities and cannot consistently identify athletes at actual risk.28-32 For example, a substantial minority of subjects (11%) were found to have a clinically significant increased ventricular wall thickness, which made clinical interpretation of the echocardiographic findings difficult in individual athletes.28 Furthermore, some patients with hypertrophic cardiomyopathy are able to tolerate particularly intense athletic training and competition for many years, and even maintain high levels of achievement without incurring symptoms, disease progression, or sudden death.29
Echocardiography and stress testing are the most commonly recommended diagnostic tests for patients with an abnormal cardiovascular history or examination. With the assistance of clinical information, echocardiography is able to distinguish the nonobstructive hypertrophic cardiomyopathy from the athletic heart syndrome.33
Musculos keletal examination
A screening musculoskeletal history and examination in combination can be used for asymptomatic athletes with no previous injuries (Table 3) (SOR: A).34 An accurate history is able to detect over 90% of significant musculoskeletal injuries. The screening physical examination is 51% sensitive and 97% specific.34 If the athlete has either a previous injury or other signs or symptoms (ie, pain; tenderness; asymmetries in muscle bulk, strength, or range of motion; any obvious deformity) detected by the general screening examination or history, the general screening should be supplemented with relevant elements of a site-specific examination.
Additional forms of musculoskeletal evaluation are often performed for athletes to determine their general state of flexibility and muscular strength. While various degrees of hyperlaxity, muscular tightness, weakness, asymmetry of strength or flexibility, poor endurance, and abnormal foot configuration may predispose an athlete to increased risk of injury during sports competition, studies have failed to demonstrate conclusively that injuries are prevented by interventions aimed at correcting such abnormalities.35-37
TABLE 3
The “90-second” musculoskeletal screening examination
| Instruction | Observations |
|---|---|
| Stand facing examiner | Acromiclavicular joints: general habitus |
| Look at ceiling, floor, over both shoulders, touch ears to shoulder | Cervical spine motion |
| Shrug shoulders (resistance) | Trapezius strength |
| Abduct shoulders to 90° (resistance at 90°) | Deltoid strength |
| Full external rotation of arms | Shoulder motion |
| Flex and extend elbows | Elbow motion |
| Arms at sides, elbows at 90° flexed; pronate and supinate wrists | Elbow and wrist motion |
| Spread fingers; make fist | Hand and finger motion, strength, and deformities |
| Tighten (contract) quadriceps; quadriceps | Symmetry and knee effusions, ankle effusion relax |
| “Duck walk” away and towards examiner | Hip, knee, and ankle motions |
| Back to examiner | Shoulder symmetry; scoliosis |
| Knees straight, touch toes | Scoliosis, hip motion, hamstring tightness |
| Raise upon toes, heels | Calf symmetry, leg strength |
Role for lab tests?
Studies do not support the use of routine laboratory or other screening tests such as urinalysis, complete blood count, chemistry profile, lipid profile, ferritin level, or spirometry as part of the exam (SOR: B).38-41
Determining clearance
Occasionally, an abnormality or condition is found that may limit an athlete’s participation or predispose him or her to further injury. In these cases, the examining physician should review the following questions:5
- Does the problem place the athlete at increased risk for injury?
- Is another participant at risk for injury because of the problem?
- Can the athlete safely participate with treatment (ie, medication, rehabilitation, bracing, or padding)?
- Can limited participation be allowed while treatment is being completed?
- If clearance is denied only for certain sports or sport categories, in what activities can the athlete safely participate?
Physicians should base clearance to participate in a particular sport on previously published guidelines, such as the recommendations by the American Academy of Pediatrics, the 26th Bethesda Conference, and the American Heart Association.7,43,44 Participation recommendations are based on the specific diagnosis, though multiple factors such as the classification of the sport and the specific health status of the athlete affect the decision.44
Approach to the patient
While current research demonstrates that the preparticipation physical examination has no effect on the overall morbidity and mortality rates in athletes, these exams may fulfill other objectives. Furthermore, no harmful effects of these examinations have been reported, and the exam has become institutionalized in the athletic and sports medicine community. As such, physicians should base their evaluation on the best available evidence using the standard form shown in “Preparticipation physical evaluation for athletics.”6 (A copy of the Preparticipation Physical Evaluation form can be found at www.jfponline.com.) This may require that the physician work with local school systems to assure that they understand what constitutes an appropriate examination.
To assist future patient care decisions and research efforts, a standardized preparticipation physical examination with an associated form similar to the evaluation recommended by the Preparticipation Physical Evaluation Task Force should be uniformly implemented throughout the country. The use of consistent clearance criteria as recommended by the Preparticipation Physical Evaluation Task Force or the American Academy of Pediatrics (“Medical conditions and sports participation,” also available at www.jfponline.com) should be used, studied, and revised as needed.5,44
In addition to the exam, physicians should consider exploring other aspects of sports participation to assist athletes in reducing the risk of injury. Rules, equipment, or other factors may have a greater effect on decreasing the mortality and morbidity associated with athletic participation. A marked decrease in cervical spine injuries occurred following the rule change in football banning deliberate “spearing”—the use of the top of the helmet as the initial point of contact in making a tackle.41
- A complete medical history, preferably from the student and a parent, will reveal approximately 75% of problems affecting initial athletic participation (D).
- For asymptomatic athletes with no previous injuries, a 90-second screening musculoskeletal test will detect 90% of significant musculoskeletal injuries (A).
- A routine screening need not include noninvasive cardiac testing or laboratory tests such as ur inalysis, blood count, chemistry profile, lipid profile, ferritin level, or spirometry (B).
Is the preparticipation physical examination the best way to determine whether a student athlete can participate fully in his or her chosen sport? This examination has become the standard of care for the over 6 million high school and college students. While most athletes pass the exam without significant medical or orthopedic abnormalities being noted, it often detects conditions that may predispose an athlete to injury or limit full participation in certain activities. We describe an efficient approach to the preparticipation examination.
Although many organizations have adopted the preparticipation exam there has been considerable debate on its content and usefulness.1-4 Nevertheless, sponsoring institutions continue to require the medical evaluation prior to competition in organized athletics, so family physicians should be knowledgeable about the objectives and limitations of the exam.
The American Academy of Family Physicians, the American Academy of Pediatrics, the American Medical Society for Sports Medicine, the American Orthopedic Society for Sports Medicine, and the American Osteopathic Academy of Sports Medicine established the Preparticipation Physical Examination Task Force. The recommendations of this task force serve as a guide for the physician conducting these examinations for high school and collegiate athletes.5,6
Assessing risks of mortality and morbidity
The mortality associated with athletic participation is most often the result of sudden cardiac death, which occurs in about 0.5 per 100,000 high school athletes per academic year and is most commonly due to hypertrophic cardiomyopathy.7,8 Screening for predisposing conditions is limited by the low prevalence of relevant cardiovascular lesions in the general youth population, the low risk of sudden death even among persons with an unsuspected abnormality, and the large number of school athletes.7-9
An estimated 200,000 children and adolescents would have to be screened to detect the 500 athletes who are at risk for sudden cardiac death and the 1 person who would actually experience it.10 Even when cardiac abnormalities are detected, the findings leading to disqualification are most often rhythm and conduction abnormalities, valvular abnormalities, and systemic hypertension, which are not the cardiac abnormalities usually associated with sudden cardiac death in athletes.11,12
The majority of sudden deaths are associated with 4 sports: football, basketball, track, and soccer. Approximately 90% of athletic-field deaths have occurred in males, mostly high school athletes.7,13
More frequently than mortality, athletic participation places the individual at risk for acute injury or worsening of an underlying medical condition. These conditions are most commonly musculoskeletal, cardiovascular, or ophthalmologic (Table 1).5,9,21
Nine studies of the preparticipation exam done between 1980 and 1999 show general agreement on the rates at which it qualifies (84.8% to 96.6%), qualifies with conditions (3.1% to 13.9%), and disqualifies students for sports participation (0.2% to 2.6%).14-22
TABLE 1
Medical and orthopedic conditions resulting in additional evaluations
| Rifat, 1995* | Lively, 1999† | ||
|---|---|---|---|
| n=2,574 | n=596 | ||
| Pass with follow-up and/or restriction (12.6%) | Fail with follow-up (2.6%) | Follow-up or restriction (14.1%) | |
| Medical (% of overall total) | 76.6 | 74.1 | 55.4 |
| Cardiovascular | 18.3 | 35.0 | 63.0 |
| Dermatologic | 6.8 | ||
| Endocrinologic | 0.4 | ||
| Ear, nose, and throat | 9.6 | 2.5 | |
| Gastrointestinal | 0.9 | 2.2 | |
| Genitourinary | 9.6 | 12.5 | 8.7 |
| Gynecologic | 4.4 | ||
| Infectious | 0.4 | 6.5 | |
| Neurologic | 6.5 | ||
| Ophthalmologic | 26.0 | 25.0 | 6.5 |
| Psychological | 2.2 | ||
| Pulmonary | 14.2 | 2.5 | |
| Other‡ | 13.7 | 22.5 | |
| Total medical (%) | 100.0 | 100.0 | 100.0 |
| Orthopedic (% of overall total) | 23.4 | 25.9 | 44.6 |
| Ankle/Foot | 14.9 | 7.7 | 2.7 |
| Back/Neck | 22.4 | 14.3 | 5.4 |
| Elbow | 5.4 | ||
| Hand/Wrist | 1.5 | 10.9 | |
| Knee | 41.8 | 7.1 | 43.2 |
| Leg | 5.4 | ||
| Shoulder | 27.0 | ||
| Nonspecific pain/injury | 19.4 | 71.4 | |
| Total orthopedic (%) | 100.0 | 100.0 | 100.0 |
| ∗Studied junior high and high school students. Two individual s failed (nonspecific pain/injury). | |||
| †Studied college-aged students. One individual failed (complicated pregnancy). | |||
| ‡“Other ” includes abdominal pain, allergy, bruising, chest pain, chronic/recurrent illness, dizziness/syncope with exercise, surgery (recent). | |||
What should the medical history include?
The examining physician should obtain a medical history from each participant (strength of recommendation [SOR]: D). A complete medical history will identify approximately 75% of problems that will affect initial athletic participation and serves as the cornerstone of the exam.14,19 Most conditions requiring further evaluation or restriction will be identified from the medical history. Rifat and colleagues21 noted that a complete medical history accounted for 88% of the abnormal findings and 57% of the reasons cited for activity restriction. The Preparticipation Physical Evaluation Task Force has developed a history form that emphasizes the areas of greatest concern.5
In particular, examining physicians should ask regarding risk factors and symptoms of cardiovascular disease ( Table 2 ). You should confirm a positive response to any of these questions, and conduct further evaluation if necessary. Unfortunately, most athletes with hypertrophic cardiomyopathy do not report a history of syncope with exercise or a family history of premature sudden cardiac death due to the disease.
Musculoskeletal injury is a common cause for disqualification of an athlete.14,19,21 The most common injury to restrict participation is a knee injury, with an ankle injury the next most common.23 The strongest independent predictor of sports injuries is a previous injury (odds ratio [OR]=9.4) and exposure time (OR=6.9).24 DuRant and colleagues23 found that a previous knee injury or knee surgery was significantly associated with further knee injuries during the subsequent sports season when compared with individuals who did not report previous knee injury or surgery (30.6% vs. 7.2%, P=.0001).
Additional historical information has been recommended for inclusion (SOR: D). For example, the examining physician should question the athlete about wheezing during exercise. Due to the high rate of recurrence and potential for long-term adverse effects, he or she should also obtain a history of previous concussions. Other issues to be addressed include presence of a single bilateral organ and use of performance-enhancing medication. Finally, physicians should question female athletes regarding their menstrual history and other symptoms or signs of the female athletic triad (eating disorder, amenorrhea, and osteoporosis).
Always carefully review the information provided by the athlete and his or her parents. In 2 separate studies, minimal agreement was found between histories obtained from athletes and parents independently.19,25 We do not know which source provides the most accurate history; therefore, both the parents and student athlete should be questioned.
TABLE 2
Questions to help discern cardiovascular risk
| Have you ever passed out during or after exercise? |
| Have you ever been dizzy during or after exercise? |
| Have you ever had chest pain during or after exercise? |
| Do you get tired more quickly than your friends during exercise? |
| Have you ever had racing of your heart or skipped heartbeats? |
| Have you ever had high blood pressure or high cholesterol? |
| Have you been told you have a heart murmur? |
| Has any family member or relative died of heart problems or of sudden death before age 50? |
| Have you had a severe viral infection (for example, myocarditis or mononucleosis) within the last month? |
| Has a physician ever denied or restricted your participation in sports for any heart problem? |
What should the physical examination include ?
A complete physical examination is not necessary (SOR: D).5 The screening physical examination should include vital signs (ie, height, weight, and blood pressure) and visual acuity testing as well as a cardiovascular, pulmonary, abdominal, skin, genital (for males), and musculoskeletal examination. Further examination should be based on issues elicited during the history.
Cardiovascular examination
The cardiovascular examination requires an additional level of detail. Perform auscultation of the heart initially with the patient in both standing and supine position, and during various maneuvers (squat-to-stand, deep inspiration, or Valsalva’s maneuver), as these maneuvers can clarify the type of murmur.
Any systolic murmur grade III/VI or louder, any murmur that disrupts normal heart sounds, any diastolic murmur, or any murmur that intensifies with the previously described maneuvers should be evaluated further through diagnostic studies (echocardiography) or consultation prior to participation. Sinus bradycardia and systolic murmurs are commonly found, occurring in over 50% and between 30% and 50% of athletes, respectively; they do not warrant further evaluation in the asymptomatic athlete.26 Third and fourth heart sounds are also commonly found in asymptomatic athletes without underlying heart disease.26,27
Noninvasive cardiac testing (eg, electrocardiography, echocardiography, or exercise stress testing) should not be a routine part of the screening preparticipation exam (SOR: B ).7 These tests are not cost-effective in a population at relatively low risk for cardiac abnormalities and cannot consistently identify athletes at actual risk.28-32 For example, a substantial minority of subjects (11%) were found to have a clinically significant increased ventricular wall thickness, which made clinical interpretation of the echocardiographic findings difficult in individual athletes.28 Furthermore, some patients with hypertrophic cardiomyopathy are able to tolerate particularly intense athletic training and competition for many years, and even maintain high levels of achievement without incurring symptoms, disease progression, or sudden death.29
Echocardiography and stress testing are the most commonly recommended diagnostic tests for patients with an abnormal cardiovascular history or examination. With the assistance of clinical information, echocardiography is able to distinguish the nonobstructive hypertrophic cardiomyopathy from the athletic heart syndrome.33
Musculos keletal examination
A screening musculoskeletal history and examination in combination can be used for asymptomatic athletes with no previous injuries (Table 3) (SOR: A).34 An accurate history is able to detect over 90% of significant musculoskeletal injuries. The screening physical examination is 51% sensitive and 97% specific.34 If the athlete has either a previous injury or other signs or symptoms (ie, pain; tenderness; asymmetries in muscle bulk, strength, or range of motion; any obvious deformity) detected by the general screening examination or history, the general screening should be supplemented with relevant elements of a site-specific examination.
Additional forms of musculoskeletal evaluation are often performed for athletes to determine their general state of flexibility and muscular strength. While various degrees of hyperlaxity, muscular tightness, weakness, asymmetry of strength or flexibility, poor endurance, and abnormal foot configuration may predispose an athlete to increased risk of injury during sports competition, studies have failed to demonstrate conclusively that injuries are prevented by interventions aimed at correcting such abnormalities.35-37
TABLE 3
The “90-second” musculoskeletal screening examination
| Instruction | Observations |
|---|---|
| Stand facing examiner | Acromiclavicular joints: general habitus |
| Look at ceiling, floor, over both shoulders, touch ears to shoulder | Cervical spine motion |
| Shrug shoulders (resistance) | Trapezius strength |
| Abduct shoulders to 90° (resistance at 90°) | Deltoid strength |
| Full external rotation of arms | Shoulder motion |
| Flex and extend elbows | Elbow motion |
| Arms at sides, elbows at 90° flexed; pronate and supinate wrists | Elbow and wrist motion |
| Spread fingers; make fist | Hand and finger motion, strength, and deformities |
| Tighten (contract) quadriceps; quadriceps | Symmetry and knee effusions, ankle effusion relax |
| “Duck walk” away and towards examiner | Hip, knee, and ankle motions |
| Back to examiner | Shoulder symmetry; scoliosis |
| Knees straight, touch toes | Scoliosis, hip motion, hamstring tightness |
| Raise upon toes, heels | Calf symmetry, leg strength |
Role for lab tests?
Studies do not support the use of routine laboratory or other screening tests such as urinalysis, complete blood count, chemistry profile, lipid profile, ferritin level, or spirometry as part of the exam (SOR: B).38-41
Determining clearance
Occasionally, an abnormality or condition is found that may limit an athlete’s participation or predispose him or her to further injury. In these cases, the examining physician should review the following questions:5
- Does the problem place the athlete at increased risk for injury?
- Is another participant at risk for injury because of the problem?
- Can the athlete safely participate with treatment (ie, medication, rehabilitation, bracing, or padding)?
- Can limited participation be allowed while treatment is being completed?
- If clearance is denied only for certain sports or sport categories, in what activities can the athlete safely participate?
Physicians should base clearance to participate in a particular sport on previously published guidelines, such as the recommendations by the American Academy of Pediatrics, the 26th Bethesda Conference, and the American Heart Association.7,43,44 Participation recommendations are based on the specific diagnosis, though multiple factors such as the classification of the sport and the specific health status of the athlete affect the decision.44
Approach to the patient
While current research demonstrates that the preparticipation physical examination has no effect on the overall morbidity and mortality rates in athletes, these exams may fulfill other objectives. Furthermore, no harmful effects of these examinations have been reported, and the exam has become institutionalized in the athletic and sports medicine community. As such, physicians should base their evaluation on the best available evidence using the standard form shown in “Preparticipation physical evaluation for athletics.”6 (A copy of the Preparticipation Physical Evaluation form can be found at www.jfponline.com.) This may require that the physician work with local school systems to assure that they understand what constitutes an appropriate examination.
To assist future patient care decisions and research efforts, a standardized preparticipation physical examination with an associated form similar to the evaluation recommended by the Preparticipation Physical Evaluation Task Force should be uniformly implemented throughout the country. The use of consistent clearance criteria as recommended by the Preparticipation Physical Evaluation Task Force or the American Academy of Pediatrics (“Medical conditions and sports participation,” also available at www.jfponline.com) should be used, studied, and revised as needed.5,44
In addition to the exam, physicians should consider exploring other aspects of sports participation to assist athletes in reducing the risk of injury. Rules, equipment, or other factors may have a greater effect on decreasing the mortality and morbidity associated with athletic participation. A marked decrease in cervical spine injuries occurred following the rule change in football banning deliberate “spearing”—the use of the top of the helmet as the initial point of contact in making a tackle.41
1. MacAuley D. Does the preseason screening for cardiac disease really work?: the British perspective. Med Sci Sports Exerc 1998;30(Suppl):S345-S350.
2. Glover DW, Maron BJ. Profile of preparticipation cardiovascular screening for high school athletes. JAMA 1998;279:1817-9.
3. Pfister GC, Puffer JC, Maron BJ. Preparticipation cardiovascular screening for US collegiate student-athletes. JAMA 2000;283:1597-9.
4. Reich JD. It won’t be me next time: an opinion on preparticipation sports physicals. Am Fam Physician 2000;61:2618, 2620, 2625, 2629.-
5. Smith DM, Kovan JR, Rich BSE, Tanner SM. Preparticipation Physical Evaluation. 2nd ed. Minneapolis, Minn: McGraw-Hill Co; 1997;1-46.
6. Lombardo JA, Robinson JB, Smith DM, et al. Preparticipation physical examination. 1st ed. Kansas City, Mo: American Academy of Family Physicians, American Academy of Pediatrics, American Medical Society for Sports Medicine, American Orthopedic Society for Sports Medicine, American Osteopathic Academy of Sports Medicine; 1992.
7. Maron BJ, Thompson PD, Puffer JC, et al. Cardiovascular preparticipation screening of competitive athletes. A statement for health professionals from the Sudden Death Committee (clinical cardiology) and Congenital Cardiac Defects Committee (cardiovascular disease in the young), American Heart Association. Circulation 1996;94:850-6.
8. Maron BJ, Gohman TE, Aeppli D. Prevalence of sudden cardiac death during competitive sports activities in Minnesota high school athletes. J Am Coll Cardiol 1998;32:1881-4.
9. American Medical Association Board of Trustees, Group on Science and Technology. Athletic participation examinations for adolescents. Arch Pediatr Adolesc Med 1994;148:93-8.
10. Epstein SE, Maron BJ. Sudden death and the competitive athlete: perspectives on preparticipation screening studies. J Am Coll Cardiol 1986;7:220-30.
11. Pelliccia A, Maron BJ. Preparticipation cardiovascular evaluation of the competitive athlete: Perspectives from the 30-year Italian experience. Am J Cardiol 1995;75:827-9.
12. Corrado D, Basso C, Schiavon M, Thiene G. Screening for hypertrophic cardiomyopathy in young athletes. N Engl J Med 1998;339:364-9.
13. Cantu RC, Mueller FO. Fatalities and catastrophic injuries in high school and college sports, 1982-1997. Phys Sportsmed 1999;27:35-48.
14. Goldberg B, Saraniti A, Witman P, et al. Preparticipation sports assessment: an objective evaluation. Pediatrics 1980;66:736-45.
15. Linder CW, DuRant RH, Seklecki RM, Strong WB. Preparticipation health screening of young athletes: results of 1268 examinations. Am J Sports Med 1981;9:187-93.
16. Tennant FS, Jr, Sorenson K, Day CM. Benefits of preparticipation sports examinations. J Fam Pract 1981;13:287-8.
17. Thompson TR, Andrish JT, Bergfeld JA. A prospective study of preparticipation sports examinations of 2670 young athletes: method and results. Cleve Clin Q 1982;49:225-33.
18. DuRant R, Seymore C, Linder CW, Jay S. The preparticipation examination of athletes. Comparison of single and multiple examiners. Am J Dis Child. 1985;139:657-61.
19. Risser WL, Hoffman HM, Bellah GG, Jr. Frequency of preparticipation sports examinations in secondary school athletes: are the University Interscholastic League guidelines appropriate? Tex Med 1985;81:35-9.
20. Magnes SA, Henderson JM, Hunter SC. What limits sports participation: experience with 10,540 athletes. Phys Sportsmed 1992;20:143-60.
21. Rifat SF, Ruffin MT, Gorenflo DW. Disqualifying criteria in preparticipation sports evaluation. J Fam Pract 1995;41:42-50.
22. Lively MW. Preparticipation physical examinations: a collegiate experience. Clin J Sports Med 1999;9:38.-
23. DuRant RH, Pendergrast RA, Seymore C, Gaillard G, Donner J. Findings from the preparticipation athletic examination and athletic injuries. Am J Dis Child 1992;146:85-91.
24. Van Mechelen W, Twisk J, Molendijk A, Blom B, Snel J, Kemper HC. Subject-related risk factors for sports injuries: a 1-yr prospective study in young adults. Med Sci Sports Exerc 1996;28:1171-9.
25. Carek PJ, Futrell MA. Athlete’s view of the preparticipation physical examination: Attitudes toward certain health screening questions. Arch Fam Med 1999;8:307-12.
26. Huston TP, Puffer JC, Rodney WM. The athletic heart syndrome. N Engl J Med 1985;313:24-32.
27. Crawford MH, O’Rourke RA. The athlete’s heart. Adv Intern Med 1979;24:311-29.
28. Lewis JF, Maron BJ, Diggs JA, Spencer JE, Mehrotra PP, Curry CL. Preparticipation echocardiographic screening for cardiovascular disease in a large, predominately black population of collegiate athletes. Am J Cardiol 1989;64:1029-33.
29. Maron BJ, Klues HG. Surviving competitive athletes with hypertrophic cardiomyopathy. Am J Cardiol 1994;73:1098-104.
30. Fuller CM, McNulty CM, Spring DA, et al. Prospective screening of 5,615 high school athletes for risk of sudden death. Med Sci Sports Exer 1997;29:1131-8.
31. Fuller CM. Cost effectiveness of analysis of high school athletes for risks of sudden cardiac death. Med Sci Sports Exer 2000;32:887-90.
32. Pelliccia A, Maron BJ, Culasso F, et al. Clinical significance of abnormal electrocardiographic patterns in trained athletes. Circulation 2000;102:278-84.
33. Maron BJ, Pelliccia A, Spirito P. Cardiac disease in young trained athletes: insights into methods for distinguishing athlete’s heart from structural heart disease with particular emphasis on hypertrophic cardiomyopathy. Circulation 1995;91:1596-1601.
34. Gomez JE, Landry GL, Bernhardt DT. Critical evaluation of the 2-minute orthopedic screening examination. Am J Dis Child 1993;147:1109-13.
35. Abbott HG, Kress JB. Preconditioning in the prevention of knee injuries. Arch Phys Med Rehabil 1969;50:326-33.
36. Jackson DW, Jarrett H, Bailey D, Kausek J, Swanson J, Powell JW. Injury prediction in the young athlete: a preliminary report. Am J Sports Med 1978;6:6-14.
37. Nicholas JA. Injuries in knee ligaments: Relationship to looseness and tightness in football players. JAMA 1970;212:2236-9.
38. Dodge WF, West EF, Smith EH, Harvey B 3rd. Proteinuria and hematuria in schoolchildren: epidemiology and early natural history. J Pediatr 1976;88:327-47.
39. Peggs JF, Reinhardt RW, O’Brien JM. Proteinuria in adolescent sports physical examinations. J Fam Pract 1986;22:80-1.
40. Rupp NT, Brudno DS, Guill MF. The value of screening for risk of exercise-induced asthma in high school athletes. Ann Allergy 1993;70:339-42.
41. Feinstein RA, LaRussa J, Wang-Dohlman A, Bartolucci AA. Screening adolescent athletes for exercise-induced asthma. Clin J Sports Med 1996;6:119-23.
42. Torg JS, Vegso JJ, Sennett B, Das M. The National Football Head and Neck Injury Registry. 14-year report on cervical quadriplegia, 1971 through 1984. JAMA 1985;254:3439-43.
43. 26th Bethesda Conference: Recommendations for determining eligibility for competition in athletes with cardiovascular abnormalities Med Sci Sports Exerc 1994;26(Suppl):S223-S283.
44. American Academy of Pediatrics. Medical conditions affecting sports participation. Pediatrics 2001;107:1206-7.
1. MacAuley D. Does the preseason screening for cardiac disease really work?: the British perspective. Med Sci Sports Exerc 1998;30(Suppl):S345-S350.
2. Glover DW, Maron BJ. Profile of preparticipation cardiovascular screening for high school athletes. JAMA 1998;279:1817-9.
3. Pfister GC, Puffer JC, Maron BJ. Preparticipation cardiovascular screening for US collegiate student-athletes. JAMA 2000;283:1597-9.
4. Reich JD. It won’t be me next time: an opinion on preparticipation sports physicals. Am Fam Physician 2000;61:2618, 2620, 2625, 2629.-
5. Smith DM, Kovan JR, Rich BSE, Tanner SM. Preparticipation Physical Evaluation. 2nd ed. Minneapolis, Minn: McGraw-Hill Co; 1997;1-46.
6. Lombardo JA, Robinson JB, Smith DM, et al. Preparticipation physical examination. 1st ed. Kansas City, Mo: American Academy of Family Physicians, American Academy of Pediatrics, American Medical Society for Sports Medicine, American Orthopedic Society for Sports Medicine, American Osteopathic Academy of Sports Medicine; 1992.
7. Maron BJ, Thompson PD, Puffer JC, et al. Cardiovascular preparticipation screening of competitive athletes. A statement for health professionals from the Sudden Death Committee (clinical cardiology) and Congenital Cardiac Defects Committee (cardiovascular disease in the young), American Heart Association. Circulation 1996;94:850-6.
8. Maron BJ, Gohman TE, Aeppli D. Prevalence of sudden cardiac death during competitive sports activities in Minnesota high school athletes. J Am Coll Cardiol 1998;32:1881-4.
9. American Medical Association Board of Trustees, Group on Science and Technology. Athletic participation examinations for adolescents. Arch Pediatr Adolesc Med 1994;148:93-8.
10. Epstein SE, Maron BJ. Sudden death and the competitive athlete: perspectives on preparticipation screening studies. J Am Coll Cardiol 1986;7:220-30.
11. Pelliccia A, Maron BJ. Preparticipation cardiovascular evaluation of the competitive athlete: Perspectives from the 30-year Italian experience. Am J Cardiol 1995;75:827-9.
12. Corrado D, Basso C, Schiavon M, Thiene G. Screening for hypertrophic cardiomyopathy in young athletes. N Engl J Med 1998;339:364-9.
13. Cantu RC, Mueller FO. Fatalities and catastrophic injuries in high school and college sports, 1982-1997. Phys Sportsmed 1999;27:35-48.
14. Goldberg B, Saraniti A, Witman P, et al. Preparticipation sports assessment: an objective evaluation. Pediatrics 1980;66:736-45.
15. Linder CW, DuRant RH, Seklecki RM, Strong WB. Preparticipation health screening of young athletes: results of 1268 examinations. Am J Sports Med 1981;9:187-93.
16. Tennant FS, Jr, Sorenson K, Day CM. Benefits of preparticipation sports examinations. J Fam Pract 1981;13:287-8.
17. Thompson TR, Andrish JT, Bergfeld JA. A prospective study of preparticipation sports examinations of 2670 young athletes: method and results. Cleve Clin Q 1982;49:225-33.
18. DuRant R, Seymore C, Linder CW, Jay S. The preparticipation examination of athletes. Comparison of single and multiple examiners. Am J Dis Child. 1985;139:657-61.
19. Risser WL, Hoffman HM, Bellah GG, Jr. Frequency of preparticipation sports examinations in secondary school athletes: are the University Interscholastic League guidelines appropriate? Tex Med 1985;81:35-9.
20. Magnes SA, Henderson JM, Hunter SC. What limits sports participation: experience with 10,540 athletes. Phys Sportsmed 1992;20:143-60.
21. Rifat SF, Ruffin MT, Gorenflo DW. Disqualifying criteria in preparticipation sports evaluation. J Fam Pract 1995;41:42-50.
22. Lively MW. Preparticipation physical examinations: a collegiate experience. Clin J Sports Med 1999;9:38.-
23. DuRant RH, Pendergrast RA, Seymore C, Gaillard G, Donner J. Findings from the preparticipation athletic examination and athletic injuries. Am J Dis Child 1992;146:85-91.
24. Van Mechelen W, Twisk J, Molendijk A, Blom B, Snel J, Kemper HC. Subject-related risk factors for sports injuries: a 1-yr prospective study in young adults. Med Sci Sports Exerc 1996;28:1171-9.
25. Carek PJ, Futrell MA. Athlete’s view of the preparticipation physical examination: Attitudes toward certain health screening questions. Arch Fam Med 1999;8:307-12.
26. Huston TP, Puffer JC, Rodney WM. The athletic heart syndrome. N Engl J Med 1985;313:24-32.
27. Crawford MH, O’Rourke RA. The athlete’s heart. Adv Intern Med 1979;24:311-29.
28. Lewis JF, Maron BJ, Diggs JA, Spencer JE, Mehrotra PP, Curry CL. Preparticipation echocardiographic screening for cardiovascular disease in a large, predominately black population of collegiate athletes. Am J Cardiol 1989;64:1029-33.
29. Maron BJ, Klues HG. Surviving competitive athletes with hypertrophic cardiomyopathy. Am J Cardiol 1994;73:1098-104.
30. Fuller CM, McNulty CM, Spring DA, et al. Prospective screening of 5,615 high school athletes for risk of sudden death. Med Sci Sports Exer 1997;29:1131-8.
31. Fuller CM. Cost effectiveness of analysis of high school athletes for risks of sudden cardiac death. Med Sci Sports Exer 2000;32:887-90.
32. Pelliccia A, Maron BJ, Culasso F, et al. Clinical significance of abnormal electrocardiographic patterns in trained athletes. Circulation 2000;102:278-84.
33. Maron BJ, Pelliccia A, Spirito P. Cardiac disease in young trained athletes: insights into methods for distinguishing athlete’s heart from structural heart disease with particular emphasis on hypertrophic cardiomyopathy. Circulation 1995;91:1596-1601.
34. Gomez JE, Landry GL, Bernhardt DT. Critical evaluation of the 2-minute orthopedic screening examination. Am J Dis Child 1993;147:1109-13.
35. Abbott HG, Kress JB. Preconditioning in the prevention of knee injuries. Arch Phys Med Rehabil 1969;50:326-33.
36. Jackson DW, Jarrett H, Bailey D, Kausek J, Swanson J, Powell JW. Injury prediction in the young athlete: a preliminary report. Am J Sports Med 1978;6:6-14.
37. Nicholas JA. Injuries in knee ligaments: Relationship to looseness and tightness in football players. JAMA 1970;212:2236-9.
38. Dodge WF, West EF, Smith EH, Harvey B 3rd. Proteinuria and hematuria in schoolchildren: epidemiology and early natural history. J Pediatr 1976;88:327-47.
39. Peggs JF, Reinhardt RW, O’Brien JM. Proteinuria in adolescent sports physical examinations. J Fam Pract 1986;22:80-1.
40. Rupp NT, Brudno DS, Guill MF. The value of screening for risk of exercise-induced asthma in high school athletes. Ann Allergy 1993;70:339-42.
41. Feinstein RA, LaRussa J, Wang-Dohlman A, Bartolucci AA. Screening adolescent athletes for exercise-induced asthma. Clin J Sports Med 1996;6:119-23.
42. Torg JS, Vegso JJ, Sennett B, Das M. The National Football Head and Neck Injury Registry. 14-year report on cervical quadriplegia, 1971 through 1984. JAMA 1985;254:3439-43.
43. 26th Bethesda Conference: Recommendations for determining eligibility for competition in athletes with cardiovascular abnormalities Med Sci Sports Exerc 1994;26(Suppl):S223-S283.
44. American Academy of Pediatrics. Medical conditions affecting sports participation. Pediatrics 2001;107:1206-7.
Are new antihypertensive agents better than old antihypertensive agents in preventing cardiovascular complications?
ABSTRACT
BACKGROUND: It has not been established whether antihypertensive agents provide a benefit beyond their blood pressure lowering effects. The investigators conducted a meta-analysis to determine whether old or new antihypertensive agents are more effective in preventing cardiovascular complications.
POPULATION STUDIED: The investigators included 9 studies enrolling 62,605 middle-aged patients (53-76 years) with a mean blood pressure at entry ranging from 145 to 194 mm Hg systolic and 83 to 106 mm Hg diastolic. The proportion of women ranged from 22% to 67%, and the proportion of patients with cardiovascular complications and diabetes varied (4% to 45% and 4% to 100%, respectively).
STUDY DESIGN AND VALIDITY: The meta-analysis compared older antihypertensive agents (β-blockers and diuretics) with new antihypertensive agents (angiotensin-converting enzyme [ACE] inhibitors, calcium channel blockers, and (β-blockers) for the prevention of cardiovascular complications. All studies were randomized controlled trials, published in peer-reviewed journals, included an assessment of blood pressure and cardiovascular events, were at least 2 years in duration, and enrolled at least 100 patients.
OUTCOMES MEASURED: The researchers determined cardiovascular mortality, cardiovascular events, fatal and nonfatal stroke, fatal and nonfatal myocardial infarction (MI), fatal and nonfatal congestive heart failure (CHF) rates with old versus new antihypertensive agents.
RESULTS: The new antihypertensive agents were as effective as the old antihypertensive agents in the prevention of cardiovascular mortality, fatal and nonfatal stroke, and fatal and nonfatal MI. Calcium channel blockers provided more reduction in the risk of stroke than the older antihypertensive agents (absolute risk reduction [ARR]=13.5%, P <.03; number needed to treat [NNT]=7) but were associated with an increase in risk of fatal and nonfatal MI (absolute risk increase [ARI]=19.2%, P <.01; number needed to harm [NNH]=5). Older antihypertensive agents were more effective in preventing cardiovascular events (ARR=11.2%, P <.001; NNT=9). Newer antihypertensive agents were less effective in preventing fatal and nonfatal CHF (ARI=52.4%, P <.001; NNH=2), but this result was attributed to the higher risk of events with the (β-blocker doxazosin compared with the diuretic, chlorthalidone, in the Antihypertensive and Lipid Lowering Treatment to Prevent Heart Attack Trial.
This study confirms that blood pressure control reduces the risk of cardiovascular complications in patients with hypertension. As a group, newer antihypertensive agents are as effective as the older antihypertensive agents in the prevention of cardiovascular mortality, fatal and nonfatal stroke, and fatal and nonfatal MI. However, the (β-blockers and diuretics are more effective in preventing cardiovascular events than ACE inhibitors and calcium channel blockers. Considering that (β-blockers and diuretics are much less expensive than the newer antihypertensive agents, they should remain first line in the treatment of hypertension.
ABSTRACT
BACKGROUND: It has not been established whether antihypertensive agents provide a benefit beyond their blood pressure lowering effects. The investigators conducted a meta-analysis to determine whether old or new antihypertensive agents are more effective in preventing cardiovascular complications.
POPULATION STUDIED: The investigators included 9 studies enrolling 62,605 middle-aged patients (53-76 years) with a mean blood pressure at entry ranging from 145 to 194 mm Hg systolic and 83 to 106 mm Hg diastolic. The proportion of women ranged from 22% to 67%, and the proportion of patients with cardiovascular complications and diabetes varied (4% to 45% and 4% to 100%, respectively).
STUDY DESIGN AND VALIDITY: The meta-analysis compared older antihypertensive agents (β-blockers and diuretics) with new antihypertensive agents (angiotensin-converting enzyme [ACE] inhibitors, calcium channel blockers, and (β-blockers) for the prevention of cardiovascular complications. All studies were randomized controlled trials, published in peer-reviewed journals, included an assessment of blood pressure and cardiovascular events, were at least 2 years in duration, and enrolled at least 100 patients.
OUTCOMES MEASURED: The researchers determined cardiovascular mortality, cardiovascular events, fatal and nonfatal stroke, fatal and nonfatal myocardial infarction (MI), fatal and nonfatal congestive heart failure (CHF) rates with old versus new antihypertensive agents.
RESULTS: The new antihypertensive agents were as effective as the old antihypertensive agents in the prevention of cardiovascular mortality, fatal and nonfatal stroke, and fatal and nonfatal MI. Calcium channel blockers provided more reduction in the risk of stroke than the older antihypertensive agents (absolute risk reduction [ARR]=13.5%, P <.03; number needed to treat [NNT]=7) but were associated with an increase in risk of fatal and nonfatal MI (absolute risk increase [ARI]=19.2%, P <.01; number needed to harm [NNH]=5). Older antihypertensive agents were more effective in preventing cardiovascular events (ARR=11.2%, P <.001; NNT=9). Newer antihypertensive agents were less effective in preventing fatal and nonfatal CHF (ARI=52.4%, P <.001; NNH=2), but this result was attributed to the higher risk of events with the (β-blocker doxazosin compared with the diuretic, chlorthalidone, in the Antihypertensive and Lipid Lowering Treatment to Prevent Heart Attack Trial.
This study confirms that blood pressure control reduces the risk of cardiovascular complications in patients with hypertension. As a group, newer antihypertensive agents are as effective as the older antihypertensive agents in the prevention of cardiovascular mortality, fatal and nonfatal stroke, and fatal and nonfatal MI. However, the (β-blockers and diuretics are more effective in preventing cardiovascular events than ACE inhibitors and calcium channel blockers. Considering that (β-blockers and diuretics are much less expensive than the newer antihypertensive agents, they should remain first line in the treatment of hypertension.
ABSTRACT
BACKGROUND: It has not been established whether antihypertensive agents provide a benefit beyond their blood pressure lowering effects. The investigators conducted a meta-analysis to determine whether old or new antihypertensive agents are more effective in preventing cardiovascular complications.
POPULATION STUDIED: The investigators included 9 studies enrolling 62,605 middle-aged patients (53-76 years) with a mean blood pressure at entry ranging from 145 to 194 mm Hg systolic and 83 to 106 mm Hg diastolic. The proportion of women ranged from 22% to 67%, and the proportion of patients with cardiovascular complications and diabetes varied (4% to 45% and 4% to 100%, respectively).
STUDY DESIGN AND VALIDITY: The meta-analysis compared older antihypertensive agents (β-blockers and diuretics) with new antihypertensive agents (angiotensin-converting enzyme [ACE] inhibitors, calcium channel blockers, and (β-blockers) for the prevention of cardiovascular complications. All studies were randomized controlled trials, published in peer-reviewed journals, included an assessment of blood pressure and cardiovascular events, were at least 2 years in duration, and enrolled at least 100 patients.
OUTCOMES MEASURED: The researchers determined cardiovascular mortality, cardiovascular events, fatal and nonfatal stroke, fatal and nonfatal myocardial infarction (MI), fatal and nonfatal congestive heart failure (CHF) rates with old versus new antihypertensive agents.
RESULTS: The new antihypertensive agents were as effective as the old antihypertensive agents in the prevention of cardiovascular mortality, fatal and nonfatal stroke, and fatal and nonfatal MI. Calcium channel blockers provided more reduction in the risk of stroke than the older antihypertensive agents (absolute risk reduction [ARR]=13.5%, P <.03; number needed to treat [NNT]=7) but were associated with an increase in risk of fatal and nonfatal MI (absolute risk increase [ARI]=19.2%, P <.01; number needed to harm [NNH]=5). Older antihypertensive agents were more effective in preventing cardiovascular events (ARR=11.2%, P <.001; NNT=9). Newer antihypertensive agents were less effective in preventing fatal and nonfatal CHF (ARI=52.4%, P <.001; NNH=2), but this result was attributed to the higher risk of events with the (β-blocker doxazosin compared with the diuretic, chlorthalidone, in the Antihypertensive and Lipid Lowering Treatment to Prevent Heart Attack Trial.
This study confirms that blood pressure control reduces the risk of cardiovascular complications in patients with hypertension. As a group, newer antihypertensive agents are as effective as the older antihypertensive agents in the prevention of cardiovascular mortality, fatal and nonfatal stroke, and fatal and nonfatal MI. However, the (β-blockers and diuretics are more effective in preventing cardiovascular events than ACE inhibitors and calcium channel blockers. Considering that (β-blockers and diuretics are much less expensive than the newer antihypertensive agents, they should remain first line in the treatment of hypertension.
Are all b-blockers equally effective in reducing mortality after acute myocardial infarction (AMI)?
BACKGROUND: The impact of b-blockers on survival after AMI has been well demonstrated in multiple randomized controlled trials. However, differences among specific b-blockers have not been evaluated. Because b-blockers differ in b-receptor selectivity and lipophilicity, researchers and clinicians have postulated variations in efficacy due to these properties. Also, clinicians are often hesitant to use b-blockers in certain patient populations with comorbid conditions, despite the known benefit after AMI. This study was designed to compare the effectiveness of 3 b-blockers (atenolol, metoprolol, and propranolol) on survival after AMI. Atenolol and metoprolol are cardioselective b-blockers; metoprolol and propranolol are lipophilic agents.
POPULATION STUDIED: Data were obtained from the Cooperative Cardiovascular Project (CCP), a database of acute care hospital claims for Medicare patients with AMI from 1994 to 1995 (n=201,752). Of this group, 69,338 patients (34%) were prescribed b-blockers, and they comprise the population for this study. Patients were elderly (mean age = approximately 73 years), 45% men, primarily white, and had comorbidities, including illness (ie, diabetes mellitus and chronic obstructive pulmonary disease) for which b-blocker use is often considered a relative contraindication. The mean systolic blood pressure was 146 mm Hg; heart rate was 81 to 83 beats per minute; and ejection fraction was 46% to 48%.
STUDY DESIGN AND VALIDITY: This study was a retrospective chart review of hospital claims data submitted to Medicare. Survival data were based on Social Security records and were reported to be 99% accurate at 60 days. Outcomes of patients prescribed different b-blockers were compared on the basis of demographic and clinical variables. The investigators emphasized comparing the magnitude of difference between groups, given that the enormous size of the database could allow for statistical but inconsequential differences among b-blockers. Obvious limitations to this study are the lack of randomization and the use of data collected from medical records, although the authors stated that the data were very complete. Also, b-blockers prescribed in the hospital may have been changed after discharge, and these data were not collected.
OUTCOMES MEASURED: Outcomes included 1- and 2-year mortality adjusted for age, race, sex, length of hospital stay, severity of illness, type of AMI, cardiovascular interventions (ie, angioplasty, bypass surgery, thrombolytics), various comorbidities (ie, diabetes, congestive heart failure, chronic obstructive pulmonary disease, asthma), and medication use (ie, angiotensin-converting enzyme inhibitors, calcium channel blockers).
RESULTS: Sixty-five percent (n=44,865) of the CCP patients were given metoprolol; 25% (n=17,411) received atenolol; and 6% (n=4236) were given propranolol in the CCP. No other b-blocker was prescribed for more than 1% of the patients. Adjusted 1-year mortality was similar among the patient groups (8.3% for metoprolol, 8.2% for atenolol, 9.6% for propranolol, and 9.2% for other b-blockers). In comparison, adjusted 2-year mortality was similar for the cardioselective b-blockers, metoprolol and atenolol (13.52% vs 13.41%, respectively). Patients discharged while taking propranolol had a slightly increased mortality rate (15.91%), which may have been related to undetected differences in severity of illness at baseline in this group. Compared with metoprolol, patients discharged while taking propranolol had a 15% increased mortality rate at 1 year and an 18% increased mortality rate at 2 years. Overall, survival with all b-blockers was superior to the 23.2% 2-year mortality rate documented in patients not receiving therapy (number needed to treat = 10-14 over 2 years).
Overall, b-blocker therapy in patients following a myocardial infarction produces a substantial decrease in mortality over the following 2 years (13%-15% vs 23%). There is little difference between atenolol and metoprolol in reducing mortality after AMI, despite their different pharmacologic characteristics. Propranolol may be slightly less effective, but all agents resulted in improved survival when compared with patients not receiving b-blockers. Atenolol and metoprolol are both once-daily b-blockers. As atenolol is now available generically, it is considerably less expensive than metoprolol (<$10/month vs $25-$45/month).
BACKGROUND: The impact of b-blockers on survival after AMI has been well demonstrated in multiple randomized controlled trials. However, differences among specific b-blockers have not been evaluated. Because b-blockers differ in b-receptor selectivity and lipophilicity, researchers and clinicians have postulated variations in efficacy due to these properties. Also, clinicians are often hesitant to use b-blockers in certain patient populations with comorbid conditions, despite the known benefit after AMI. This study was designed to compare the effectiveness of 3 b-blockers (atenolol, metoprolol, and propranolol) on survival after AMI. Atenolol and metoprolol are cardioselective b-blockers; metoprolol and propranolol are lipophilic agents.
POPULATION STUDIED: Data were obtained from the Cooperative Cardiovascular Project (CCP), a database of acute care hospital claims for Medicare patients with AMI from 1994 to 1995 (n=201,752). Of this group, 69,338 patients (34%) were prescribed b-blockers, and they comprise the population for this study. Patients were elderly (mean age = approximately 73 years), 45% men, primarily white, and had comorbidities, including illness (ie, diabetes mellitus and chronic obstructive pulmonary disease) for which b-blocker use is often considered a relative contraindication. The mean systolic blood pressure was 146 mm Hg; heart rate was 81 to 83 beats per minute; and ejection fraction was 46% to 48%.
STUDY DESIGN AND VALIDITY: This study was a retrospective chart review of hospital claims data submitted to Medicare. Survival data were based on Social Security records and were reported to be 99% accurate at 60 days. Outcomes of patients prescribed different b-blockers were compared on the basis of demographic and clinical variables. The investigators emphasized comparing the magnitude of difference between groups, given that the enormous size of the database could allow for statistical but inconsequential differences among b-blockers. Obvious limitations to this study are the lack of randomization and the use of data collected from medical records, although the authors stated that the data were very complete. Also, b-blockers prescribed in the hospital may have been changed after discharge, and these data were not collected.
OUTCOMES MEASURED: Outcomes included 1- and 2-year mortality adjusted for age, race, sex, length of hospital stay, severity of illness, type of AMI, cardiovascular interventions (ie, angioplasty, bypass surgery, thrombolytics), various comorbidities (ie, diabetes, congestive heart failure, chronic obstructive pulmonary disease, asthma), and medication use (ie, angiotensin-converting enzyme inhibitors, calcium channel blockers).
RESULTS: Sixty-five percent (n=44,865) of the CCP patients were given metoprolol; 25% (n=17,411) received atenolol; and 6% (n=4236) were given propranolol in the CCP. No other b-blocker was prescribed for more than 1% of the patients. Adjusted 1-year mortality was similar among the patient groups (8.3% for metoprolol, 8.2% for atenolol, 9.6% for propranolol, and 9.2% for other b-blockers). In comparison, adjusted 2-year mortality was similar for the cardioselective b-blockers, metoprolol and atenolol (13.52% vs 13.41%, respectively). Patients discharged while taking propranolol had a slightly increased mortality rate (15.91%), which may have been related to undetected differences in severity of illness at baseline in this group. Compared with metoprolol, patients discharged while taking propranolol had a 15% increased mortality rate at 1 year and an 18% increased mortality rate at 2 years. Overall, survival with all b-blockers was superior to the 23.2% 2-year mortality rate documented in patients not receiving therapy (number needed to treat = 10-14 over 2 years).
Overall, b-blocker therapy in patients following a myocardial infarction produces a substantial decrease in mortality over the following 2 years (13%-15% vs 23%). There is little difference between atenolol and metoprolol in reducing mortality after AMI, despite their different pharmacologic characteristics. Propranolol may be slightly less effective, but all agents resulted in improved survival when compared with patients not receiving b-blockers. Atenolol and metoprolol are both once-daily b-blockers. As atenolol is now available generically, it is considerably less expensive than metoprolol (<$10/month vs $25-$45/month).
BACKGROUND: The impact of b-blockers on survival after AMI has been well demonstrated in multiple randomized controlled trials. However, differences among specific b-blockers have not been evaluated. Because b-blockers differ in b-receptor selectivity and lipophilicity, researchers and clinicians have postulated variations in efficacy due to these properties. Also, clinicians are often hesitant to use b-blockers in certain patient populations with comorbid conditions, despite the known benefit after AMI. This study was designed to compare the effectiveness of 3 b-blockers (atenolol, metoprolol, and propranolol) on survival after AMI. Atenolol and metoprolol are cardioselective b-blockers; metoprolol and propranolol are lipophilic agents.
POPULATION STUDIED: Data were obtained from the Cooperative Cardiovascular Project (CCP), a database of acute care hospital claims for Medicare patients with AMI from 1994 to 1995 (n=201,752). Of this group, 69,338 patients (34%) were prescribed b-blockers, and they comprise the population for this study. Patients were elderly (mean age = approximately 73 years), 45% men, primarily white, and had comorbidities, including illness (ie, diabetes mellitus and chronic obstructive pulmonary disease) for which b-blocker use is often considered a relative contraindication. The mean systolic blood pressure was 146 mm Hg; heart rate was 81 to 83 beats per minute; and ejection fraction was 46% to 48%.
STUDY DESIGN AND VALIDITY: This study was a retrospective chart review of hospital claims data submitted to Medicare. Survival data were based on Social Security records and were reported to be 99% accurate at 60 days. Outcomes of patients prescribed different b-blockers were compared on the basis of demographic and clinical variables. The investigators emphasized comparing the magnitude of difference between groups, given that the enormous size of the database could allow for statistical but inconsequential differences among b-blockers. Obvious limitations to this study are the lack of randomization and the use of data collected from medical records, although the authors stated that the data were very complete. Also, b-blockers prescribed in the hospital may have been changed after discharge, and these data were not collected.
OUTCOMES MEASURED: Outcomes included 1- and 2-year mortality adjusted for age, race, sex, length of hospital stay, severity of illness, type of AMI, cardiovascular interventions (ie, angioplasty, bypass surgery, thrombolytics), various comorbidities (ie, diabetes, congestive heart failure, chronic obstructive pulmonary disease, asthma), and medication use (ie, angiotensin-converting enzyme inhibitors, calcium channel blockers).
RESULTS: Sixty-five percent (n=44,865) of the CCP patients were given metoprolol; 25% (n=17,411) received atenolol; and 6% (n=4236) were given propranolol in the CCP. No other b-blocker was prescribed for more than 1% of the patients. Adjusted 1-year mortality was similar among the patient groups (8.3% for metoprolol, 8.2% for atenolol, 9.6% for propranolol, and 9.2% for other b-blockers). In comparison, adjusted 2-year mortality was similar for the cardioselective b-blockers, metoprolol and atenolol (13.52% vs 13.41%, respectively). Patients discharged while taking propranolol had a slightly increased mortality rate (15.91%), which may have been related to undetected differences in severity of illness at baseline in this group. Compared with metoprolol, patients discharged while taking propranolol had a 15% increased mortality rate at 1 year and an 18% increased mortality rate at 2 years. Overall, survival with all b-blockers was superior to the 23.2% 2-year mortality rate documented in patients not receiving therapy (number needed to treat = 10-14 over 2 years).
Overall, b-blocker therapy in patients following a myocardial infarction produces a substantial decrease in mortality over the following 2 years (13%-15% vs 23%). There is little difference between atenolol and metoprolol in reducing mortality after AMI, despite their different pharmacologic characteristics. Propranolol may be slightly less effective, but all agents resulted in improved survival when compared with patients not receiving b-blockers. Atenolol and metoprolol are both once-daily b-blockers. As atenolol is now available generically, it is considerably less expensive than metoprolol (<$10/month vs $25-$45/month).
Bisoprolol Prevents Mortality and Myocardial Infarction After Vascular Surgery
CLINICAL QUESTION: Does the perioperative administration of bisoprolol prevent nonfatal myocardial infarction (MI) or cardiovascular mortality in high-risk patients undergoing vascular surgery?
BACKGROUND: Many patients suffer from cardiovascular complications following major vascular surgery, and although various interventions have been proposed to improve the associated cardiovascular risks, none has been found to be efficacious. Since b-blockers demonstrate a major benefit in acute MI and other cardiovascular diseases, investigators studied the benefit of bisoprolol when given perioperatively to high-risk patients undergoing vascular surgery. Bisoprolol is a b-1 selective hydrophilic b-blocker without intrinsic sympathomimetic activity.
POPULATION STUDIED: All of the included patients were undergoing elective abdominal aortic or infrainguinal arterial reconstruction at study sites in Canada, the Netherlands, and Italy. Patients with cardiac risk factors—older than 70 years, previous MI, treatment for congestive heart failure, ventricular arrhythmias, diabetes mellitus, or reduced capacity to perform activities of daily living—underwent dobutamine stress echocardiography. Those with a positive result were considered to be high risk and were included in the study. Patients were excluded if they had significant heart wall motion abnormalities, asthma, or evidence of left main or triple coronary vessel disease during stress testing. A total of 1351 patients were screened, and 846 were found to have cardiac risk factors. Positive stress tests were documented in 173 patients, and 112 underwent randomization (59 bisoprolol plus standard care, 53 standard care alone). More than 80% of the patients were men.
STUDY DESIGN AND VALIDITY: This was a multicenter randomized controlled trial in which patients received standard perioperative care or standard care plus bisoprolol. Bisoprolol 5 mg daily was initiated at least 1 week before surgery (mean = 37 days before surgery; range = 7 to 89 days), and continued for 30 days postoperatively. Approximately 1 week after initiation, patients were reassessed and the dosage could be increased to a maximum of 10 mg daily if the heart rate remained at more than 60 beats per minute (bpm). Administration by nasogastric tube or intravenous metoprolol was substituted at times when patients were unable to take the medication orally. Bisoprolol was withheld if the heart rate dropped below 50 bpm or if the systolic blood pressure was less than 100 mm Hg.
Overall, the methods of this study were appropriate to answer the clinical question. Although physicians and patients were not blinded during the study, a monitoring committee evaluated outcomes in a masked fashion. The authors did not describe the methods used to prevent researchers from knowing to which group the patient would be assigned (concealed allocation). This lack of concealment could introduce selective enrollment of patients. The patient population studied was elderly; therefore, the impact of bisoprolol in younger or lower-risk patients undergoing vascular surgery may be less dramatic.
OUTCOMES MEASURED: The primary end points were death from cardiac causes or nonfatal myocardial infarction during the perioperative period.
RESULTS: The bisoprolol-treated group experienced fewer deaths (3.4% vs 17%, P=.02; number needed to treat [NNT]=7.3) and fewer nonfatal MIs (0% vs 17%, P <.001; NNT=5.9) than those receiving standard care alone. For the combined end point of death from cardiac causes or nonfatal MI, the overall rate in the bisoprolol group was 3.4% vs 34% in the standard care group (P <.001; NNT=3.3). The study was stopped after interim analysis because of the significant difference between groups. Investigators did not report side effects associated with the administration of bisoprolol.
This well-designed clinical trial demonstrates the major impact of perioperative administration of the b-blocker bisoprolol in high-risk patients undergoing vascular surgery. For every 3 high-risk patients treated, one death or nonfatal MI is prevented. It is not known if the benefits associated with bisoprolol will be realized with the use of other b-blockers.
CLINICAL QUESTION: Does the perioperative administration of bisoprolol prevent nonfatal myocardial infarction (MI) or cardiovascular mortality in high-risk patients undergoing vascular surgery?
BACKGROUND: Many patients suffer from cardiovascular complications following major vascular surgery, and although various interventions have been proposed to improve the associated cardiovascular risks, none has been found to be efficacious. Since b-blockers demonstrate a major benefit in acute MI and other cardiovascular diseases, investigators studied the benefit of bisoprolol when given perioperatively to high-risk patients undergoing vascular surgery. Bisoprolol is a b-1 selective hydrophilic b-blocker without intrinsic sympathomimetic activity.
POPULATION STUDIED: All of the included patients were undergoing elective abdominal aortic or infrainguinal arterial reconstruction at study sites in Canada, the Netherlands, and Italy. Patients with cardiac risk factors—older than 70 years, previous MI, treatment for congestive heart failure, ventricular arrhythmias, diabetes mellitus, or reduced capacity to perform activities of daily living—underwent dobutamine stress echocardiography. Those with a positive result were considered to be high risk and were included in the study. Patients were excluded if they had significant heart wall motion abnormalities, asthma, or evidence of left main or triple coronary vessel disease during stress testing. A total of 1351 patients were screened, and 846 were found to have cardiac risk factors. Positive stress tests were documented in 173 patients, and 112 underwent randomization (59 bisoprolol plus standard care, 53 standard care alone). More than 80% of the patients were men.
STUDY DESIGN AND VALIDITY: This was a multicenter randomized controlled trial in which patients received standard perioperative care or standard care plus bisoprolol. Bisoprolol 5 mg daily was initiated at least 1 week before surgery (mean = 37 days before surgery; range = 7 to 89 days), and continued for 30 days postoperatively. Approximately 1 week after initiation, patients were reassessed and the dosage could be increased to a maximum of 10 mg daily if the heart rate remained at more than 60 beats per minute (bpm). Administration by nasogastric tube or intravenous metoprolol was substituted at times when patients were unable to take the medication orally. Bisoprolol was withheld if the heart rate dropped below 50 bpm or if the systolic blood pressure was less than 100 mm Hg.
Overall, the methods of this study were appropriate to answer the clinical question. Although physicians and patients were not blinded during the study, a monitoring committee evaluated outcomes in a masked fashion. The authors did not describe the methods used to prevent researchers from knowing to which group the patient would be assigned (concealed allocation). This lack of concealment could introduce selective enrollment of patients. The patient population studied was elderly; therefore, the impact of bisoprolol in younger or lower-risk patients undergoing vascular surgery may be less dramatic.
OUTCOMES MEASURED: The primary end points were death from cardiac causes or nonfatal myocardial infarction during the perioperative period.
RESULTS: The bisoprolol-treated group experienced fewer deaths (3.4% vs 17%, P=.02; number needed to treat [NNT]=7.3) and fewer nonfatal MIs (0% vs 17%, P <.001; NNT=5.9) than those receiving standard care alone. For the combined end point of death from cardiac causes or nonfatal MI, the overall rate in the bisoprolol group was 3.4% vs 34% in the standard care group (P <.001; NNT=3.3). The study was stopped after interim analysis because of the significant difference between groups. Investigators did not report side effects associated with the administration of bisoprolol.
This well-designed clinical trial demonstrates the major impact of perioperative administration of the b-blocker bisoprolol in high-risk patients undergoing vascular surgery. For every 3 high-risk patients treated, one death or nonfatal MI is prevented. It is not known if the benefits associated with bisoprolol will be realized with the use of other b-blockers.
CLINICAL QUESTION: Does the perioperative administration of bisoprolol prevent nonfatal myocardial infarction (MI) or cardiovascular mortality in high-risk patients undergoing vascular surgery?
BACKGROUND: Many patients suffer from cardiovascular complications following major vascular surgery, and although various interventions have been proposed to improve the associated cardiovascular risks, none has been found to be efficacious. Since b-blockers demonstrate a major benefit in acute MI and other cardiovascular diseases, investigators studied the benefit of bisoprolol when given perioperatively to high-risk patients undergoing vascular surgery. Bisoprolol is a b-1 selective hydrophilic b-blocker without intrinsic sympathomimetic activity.
POPULATION STUDIED: All of the included patients were undergoing elective abdominal aortic or infrainguinal arterial reconstruction at study sites in Canada, the Netherlands, and Italy. Patients with cardiac risk factors—older than 70 years, previous MI, treatment for congestive heart failure, ventricular arrhythmias, diabetes mellitus, or reduced capacity to perform activities of daily living—underwent dobutamine stress echocardiography. Those with a positive result were considered to be high risk and were included in the study. Patients were excluded if they had significant heart wall motion abnormalities, asthma, or evidence of left main or triple coronary vessel disease during stress testing. A total of 1351 patients were screened, and 846 were found to have cardiac risk factors. Positive stress tests were documented in 173 patients, and 112 underwent randomization (59 bisoprolol plus standard care, 53 standard care alone). More than 80% of the patients were men.
STUDY DESIGN AND VALIDITY: This was a multicenter randomized controlled trial in which patients received standard perioperative care or standard care plus bisoprolol. Bisoprolol 5 mg daily was initiated at least 1 week before surgery (mean = 37 days before surgery; range = 7 to 89 days), and continued for 30 days postoperatively. Approximately 1 week after initiation, patients were reassessed and the dosage could be increased to a maximum of 10 mg daily if the heart rate remained at more than 60 beats per minute (bpm). Administration by nasogastric tube or intravenous metoprolol was substituted at times when patients were unable to take the medication orally. Bisoprolol was withheld if the heart rate dropped below 50 bpm or if the systolic blood pressure was less than 100 mm Hg.
Overall, the methods of this study were appropriate to answer the clinical question. Although physicians and patients were not blinded during the study, a monitoring committee evaluated outcomes in a masked fashion. The authors did not describe the methods used to prevent researchers from knowing to which group the patient would be assigned (concealed allocation). This lack of concealment could introduce selective enrollment of patients. The patient population studied was elderly; therefore, the impact of bisoprolol in younger or lower-risk patients undergoing vascular surgery may be less dramatic.
OUTCOMES MEASURED: The primary end points were death from cardiac causes or nonfatal myocardial infarction during the perioperative period.
RESULTS: The bisoprolol-treated group experienced fewer deaths (3.4% vs 17%, P=.02; number needed to treat [NNT]=7.3) and fewer nonfatal MIs (0% vs 17%, P <.001; NNT=5.9) than those receiving standard care alone. For the combined end point of death from cardiac causes or nonfatal MI, the overall rate in the bisoprolol group was 3.4% vs 34% in the standard care group (P <.001; NNT=3.3). The study was stopped after interim analysis because of the significant difference between groups. Investigators did not report side effects associated with the administration of bisoprolol.
This well-designed clinical trial demonstrates the major impact of perioperative administration of the b-blocker bisoprolol in high-risk patients undergoing vascular surgery. For every 3 high-risk patients treated, one death or nonfatal MI is prevented. It is not known if the benefits associated with bisoprolol will be realized with the use of other b-blockers.