User login
An overlooked cause of catatonia
CASE Agitation and bizarre behavior
Ms. L, age 40, presents to the emergency department (ED) for altered mental status and bizarre behavior. Before arriving at the ED, she had experienced a severe headache and an episode of vomiting. At home she had been irritable and agitated, repetitively dressing and undressing, urinating outside the toilet, and opening and closing water faucets in the house. She also had stopped eating and drinking. Ms. L’s home medications consist of levothyroxine 100 mcg/d for hypothyroidism.
In the ED, Ms. L has severe psychomotor agitation. She is restless and displays purposeless repetitive movements with her hands. She is mostly mute, but does groan at times.
HISTORY Multiple trips to the ED
In addition to hypothyroidism, Ms. L has a history of migraines and asthma. Four days before presenting to the ED, she complained of a severe headache and generalized fatigue, with vomiting and nausea. Two days later, she presented to the ED at a different hospital and underwent a brain CT scan; the results were unremarkable. At that facility, a laboratory work-up—including complete blood count, urea, creatinine, C-reactive protein, electrolytes, magnesium, phosphorus, calcium, full liver function tests, amylase, lipase, bilirubin, thyroid function test, and beta-human chorionic gonadotropin—was normal except for low thyroid-stimulating hormone levels (0.016 mIU/L). Ms. L was diagnosed with a severe migraine attack and discharged home with instructions to follow up with her endocrinologist.
Ms. L has no previous psychiatric history. Her family’s psychiatric history includes depression with psychotic features (mother), depression (maternal aunt), and generalized anxiety disorder (mother’s maternal aunt).
[polldaddy:11252938]
The authors’ observations
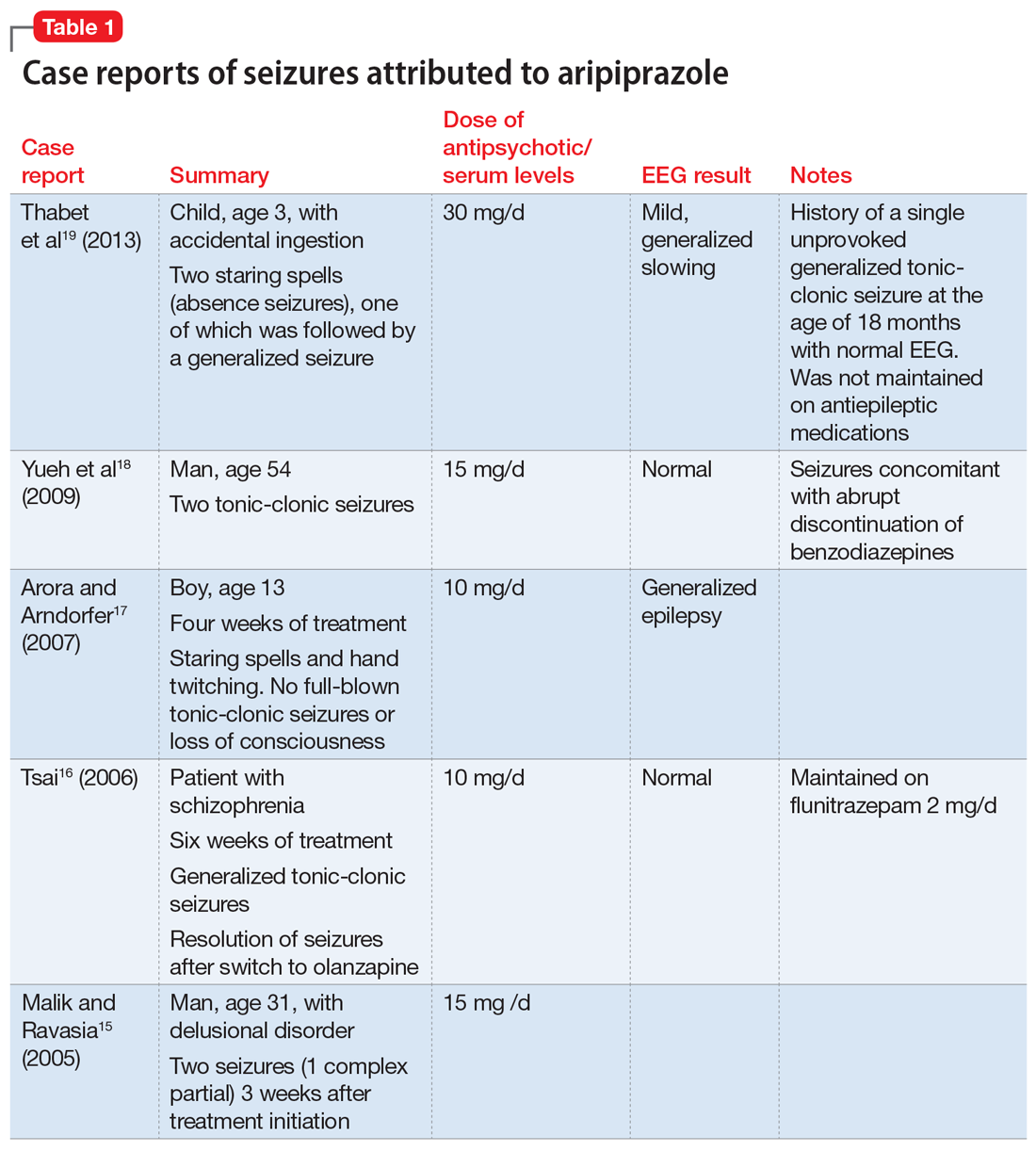
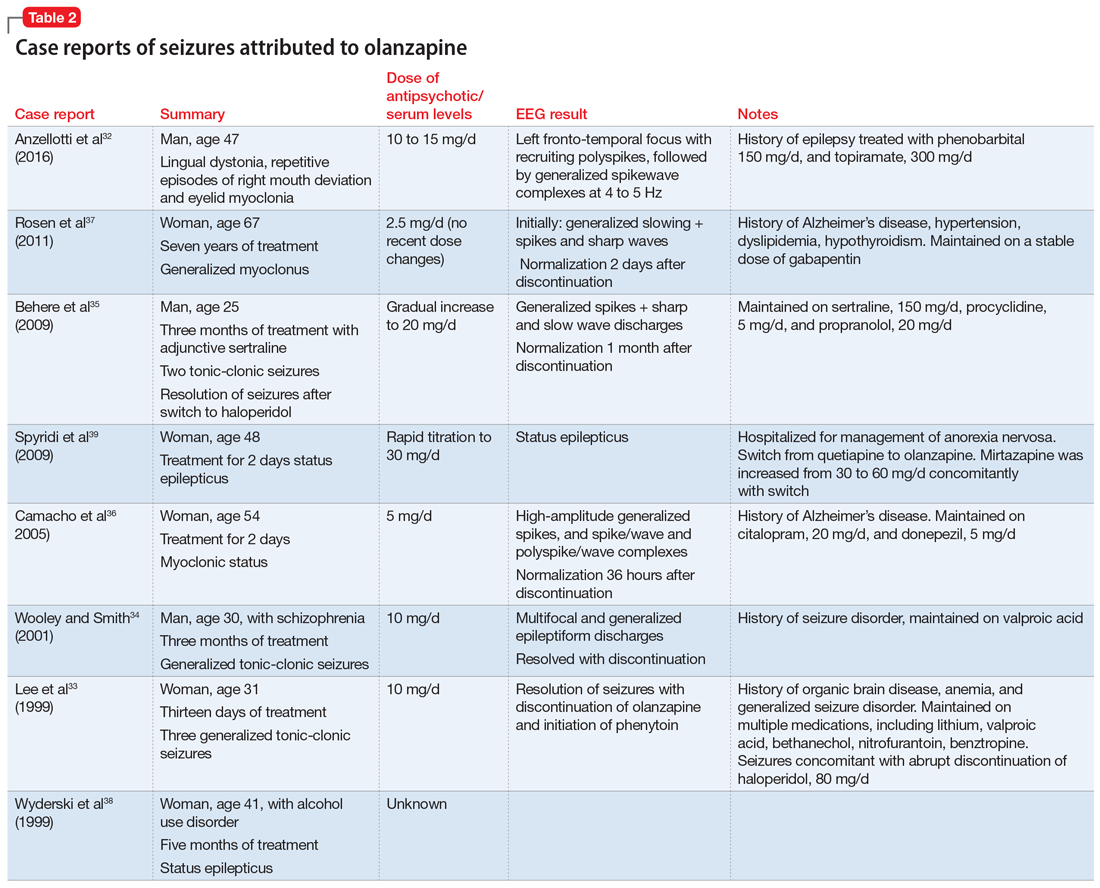
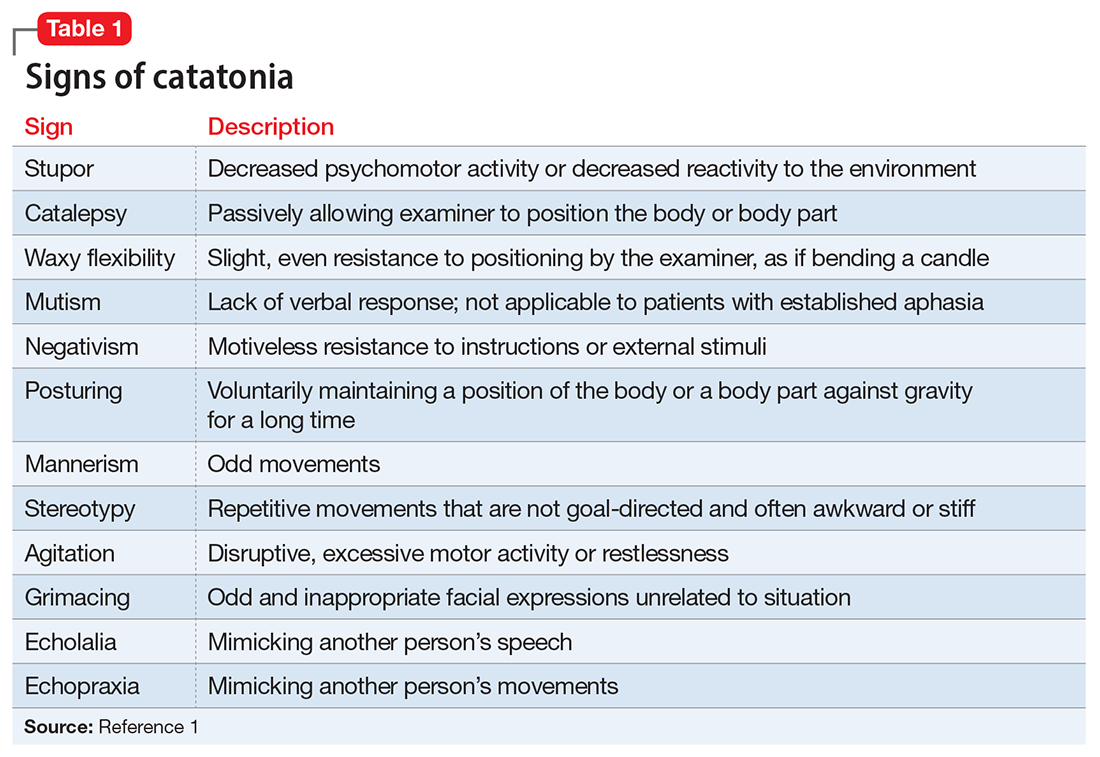
Catatonia is a behavioral syndrome with heterogeneous signs and symptoms. According to DSM-5, the diagnosis is considered when a patient presents with ≥3 of the 12 signs outlined in Table 1.1 It usually occurs in the context of an underlying psychiatric disorder such as schizophrenia or depression, or a medical disorder such as CNS infection or encephalopathy due to metabolic causes.1 Ms. L exhibited mutism, negativism, mannerism, stereotypy, and agitation and thus met the criteria for a catatonia diagnosis.
EVALUATION Unexpected finding on physical exam
In the ED, Ms. L is hemodynamically stable. Her blood pressure is 140/80 mm Hg; heart rate is 103 beats per minute; oxygen saturation is 98%; respiratory rate is 14 breaths per minute; and temperature is 37.5° C. Results from a brain MRI and total body scan performed prior to admission are unremarkable.
Ms. L is admitted to the psychiatric ward under the care of neurology for a psychiatry consultation. For approximately 24 hours, she receives IV diazepam 5 mg every 8 hours (due to the unavailability of lorazepam) for management of her catatonic symptoms, and olanzapine 10 mg every 8 hours orally as needed for agitation. Collateral history rules out a current mood episode or onset of psychosis in the weeks before she came to the ED. Diazepam improves Ms. L’s psychomotor agitation, which allows the primary team an opportunity to examine her.
Continue to: A physical exam reveals...
A physical exam reveals small vesicular lesions (1 to 2 cm in diameter) on an erythematous base on the left breast associated with an erythematous plaque with no evident vesicles on the left inner arm. The vesicular lesions display in a segmented pattern of dermatomal distribution.
[polldaddy:11252941]
The authors’ observations
Catatonic symptoms, coupled with psychomotor agitation in an immunocompetent middle-aged adult with a history of migraine headaches, strong family history of severe mental illness, and noncontributory findings on brain imaging, prompted a Psychiatry consultation and administration of psychotropic medications. A thorough physical exam revealing the small area of shingles and acute altered mental status prompted more aggressive investigations to explore the possibility of encephalitis.
Physicians should have a low index of suspicion for encephalitis (viral, bacterial, autoimmune, etc) and perform a lumbar puncture (LP) when necessary, despite the invasiveness of this test. A direct physical examination is often underutilized, notably in psychiatric patients, which can lead to the omission of important clinical information.2 Normal vital signs, blood workup, and MRI before admission are not sufficient to correctly guide diagnosis.
EVALUATION Additional lab results establish the diagnosis
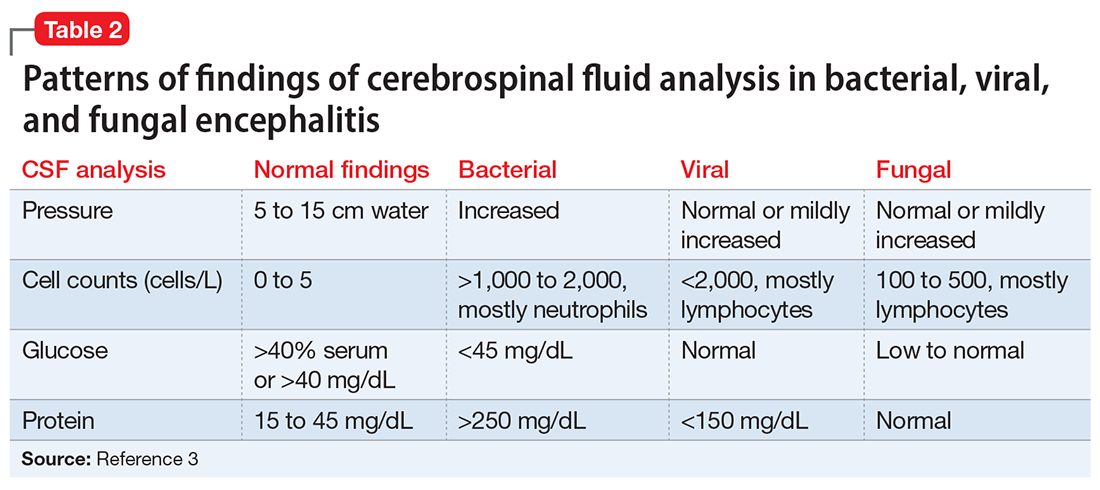
An LP reveals Ms. L’s protein levels are 44 mg/dL, her glucose levels are 85 mg/dL, red blood cell count is 4/µL, and white blood cell count is 200/µL with 92% lymphocytes and 1% neutrophils. Ms. L’s CSF analysis profile indicates a viral CNS infection (Table 23).
[polldaddy:11252943]
The authors’ observations
Varicella-zoster virus (VZV) and herpes simplex virus (HSV) are human neurotropic alphaherpesviruses that cause lifelong infections in ganglia, and their reactivation can come in the form of encephalitis.4
Continue to: Ms. L's clinical presentation...
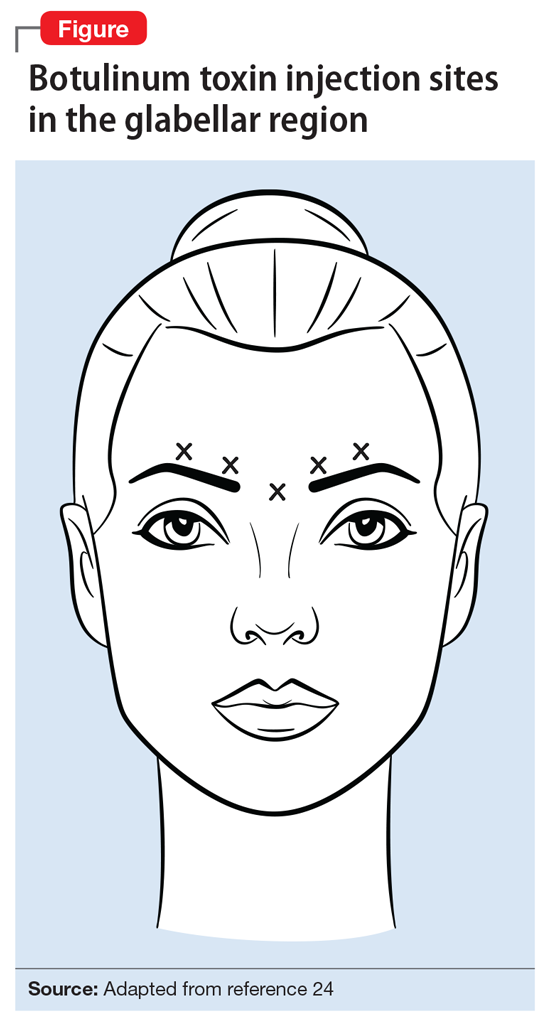
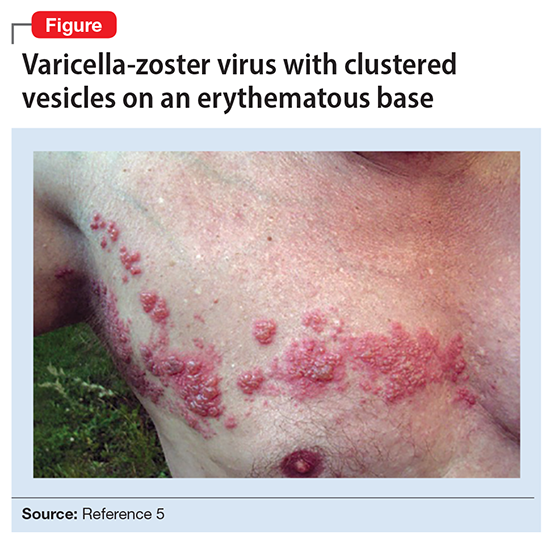
Ms. L’s clinical presentation most likely implicated VZV. Skin lesions of VZV may look exactly like HSV, with clustered vesicles on an erythematous base (Figure5). However, VZV rash tends to follow a dermatomal distribution (as in Ms. L’s case), which can help distinguish it from herpetic lesions.
Cases of VZV infection have been increasing worldwide. It is usually seen in older adults or those with compromised immunity.6 Significantly higher rates of VZV complications have been reported in such patients. A serious complication is VZV encephalitis, which is rare but possible, even in healthy individuals.6 VZV encephalitis can present with atypical psychiatric features. Ms. L exhibited several symptoms of VZV encephalitis, which include headache, fever, vomiting, altered level of consciousness, and seizures. An EEG also showed intermittent generalized slow waves in the range of theta commonly seen in encephalitis.
Ms. L’s case shows the importance of early recognition of VZV infection. The diagnosis is confirmed through CSF analysis. There is an urgency to promptly conduct the LP to confirm the diagnosis and quickly initiate antiviral treatment to stop the progression of the infection and its life-threatening sequelae.
In the absence of underlying medical cause, typical treatment of catatonia involves the sublingual or IM administration of 1 to 2 mg lorazepam that can be repeated twice at 3-hour intervals if the patient’s symptoms do not resolve. ECT is indicated if the patient experiences minimal or no response to lorazepam.
The use of antipsychotics for catatonia is controversial. High-potency antipsychotics such as haloperidol and risperidone are not recommended due to increased risk of the progression of catatonia into neuroleptic malignant syndrome.7
Continue to: OUTCOME Prompt recovery with an antiviral
OUTCOME Prompt recovery with an antiviral
Ms. L receives IV acyclovir 1,200 mg every 8 hours for 14 days. Just 48 hours after starting this antiviral medication, her bizarre behavior and catatonic features cease, and she returns to her baseline mental functioning. Olanzapine is discontinued, and lorazepam is progressively decreased. The CSF polymerase chain reaction assay indicates Ms. L is positive for VZV, which confirms the diagnosis of VZV encephalitis. A spine MRI is also performed and rules out myelitis as a sequela of the infection.
The authors’ observations
Chickenpox is caused by a primary encounter with VZV. Inside the ganglions of neurons, a dormant form of VZV resides. Its reactivation leads to the spread of the infection to the skin innervated by these neurons, causing shingles. Reactivation occurs in approximately 1 million people in the United States each year. The annual incidence is 5 to 6.5 cases per 1,000 people at age 60, and 8 to 11 cases per 1,000 people at age 70.8
In 2006, the FDA approved the first zoster vaccine (Zostavax) for use in nonimmunocompromised, VZV-seropositive adults age >60 (later lowered to age 50). This vaccine reduces the incidence of shingles by 51%, the incidence of postherpetic neuralgia by 66%, and the burden of illness by 61%. In 2017, the FDA approved a second VZV vaccine (Shingrix, recombinant nonlive vaccine). In 2021, Shingrix was approved for use in immunosuppressed patients.9
Reactivation of VZV starts with a prodromal phase, characterized by pain, itching, numbness, and dysesthesias in 1 to 3 dermatomes. A maculopapular rash appears on the affected area a few days later, evolving into vesicles that scab over in 10 days.10
Dissemination of the virus leading specifically to VZV encephalitis typically occurs in immunosuppressed individuals and older patients. According to the World Health Organization, encephalitis is a life-threatening complication of VZV and occurs in 1 of 33,000 to 50,000 cases.11
Continue to: Delay in the diagnosis...
Delay in the diagnosis and treatment of VZV encephalitis can be detrimental or even fatal. Kodadhala et al12 found that the mortality rate for VZV encephalitis is 5% to 10% and ≤80% in immunosuppressed individuals.
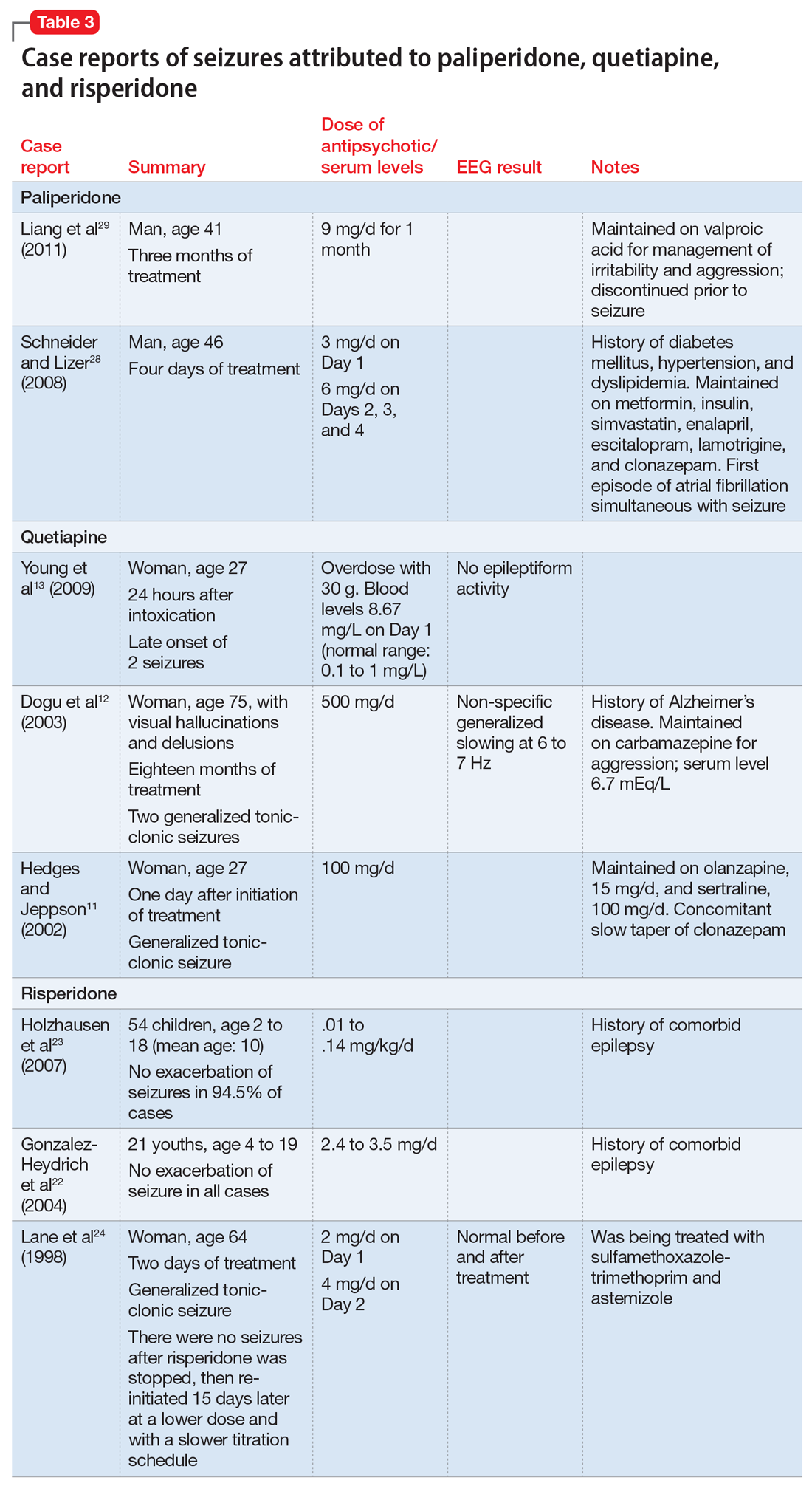
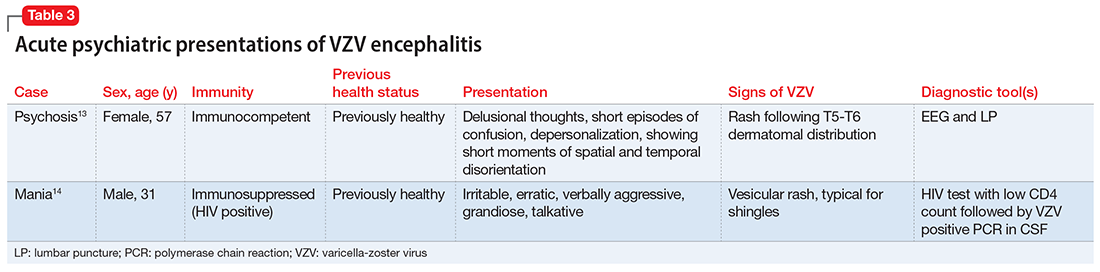
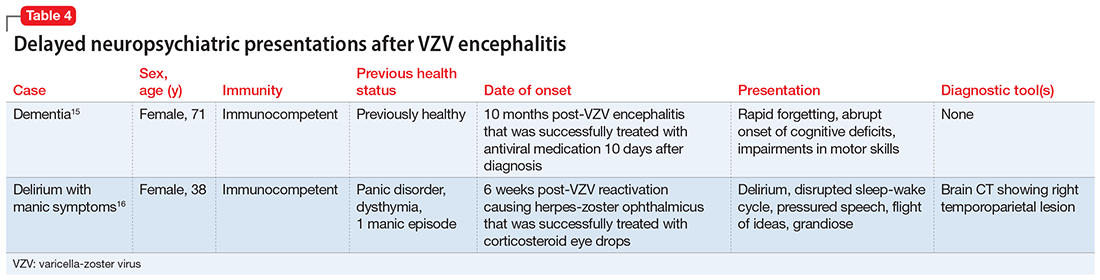
Sometimes, VZV encephalitis can masquerade as a psychiatric presentation. Few cases presenting with acute or delayed neuropsychiatric symptoms related to VZV encephalitis have been previously reported in the literature. Some are summarized in Table 313,14 and Table 4.15,16
To our knowledge, this is the first case report of catatonia as a presentation of VZV encephalitis. The catatonic presentation has been previously described in autoimmune encephalitis such as N-methyl-
Bottom Line
In the setting of a patient with an abrupt change in mental status/behavior, physicians must be aware of the importance of a thorough physical examination to better ascertain a diagnosis and to rule out an underlying medical disorder. Reactivation of varicella-zoster virus (VZV) can result in encephalitis that might masquerade as a psychiatric presentation, including symptoms of catatonia.
Related Resources
- Baum ML, Johnson MC, Lizano P. Is it psychosis, or an autoimmune encephalitis? Current Psychiatry. 2022;21(8): 31-38,44. doi:10.12788/cp.0273
- Reinfold S. Are we failing to diagnose and treat the many faces of catatonia? Current Psychiatry. 2022;21(1):e3-e5. doi:10.12788/cp.0208
Drug Brand Names
Acyclovir • Sitavig
Diazepam • Valium
Haloperidol • Haldol
Lorazepam • Ativan
Levothyroxine • Levoxyl
Olanzapine • Zyprexa
Risperidone • Risperdal
1. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. American Psychiatric Association; 2013.
2. Sanders RD, Keshavan MS. Physical and neurologic examinations in neuropsychiatry. Semin Clin Neuropsychiatry. 2002;7(1):18-29.
3. Howes DS, Lazoff M. Encephalitis workup. Medscape. Updated August 7, 2018. Accessed August 9, 2022. https://emedicine.medscape.com/article/791896-workup#c11
4. Kennedy PG, Rovnak J, Badani H, et al. A comparison of herpes simplex virus type 1 and varicella-zoster virus latency and reactivation. J Gen Virol. 2015;96(Pt 7):1581-1602.
5. Fisle, CC BY-SA 3.0 (https://creativecommons.org/licenses/by-sa/3.0). Wikimedia Commons. https://upload.wikimedia.org/wikipedia/commons/1/19/Herpes_zoster_chest.png
6. John AR, Canaday DH. Herpes zoster in the older adult. Infect Dis Clin North Am. 2017;31(4):811-826.
7. Rosebush PI, Mazurek MF. Catatonia and its treatment. Schizophr Bull. 2010;36(2):239-242.
8. Gershon AA, Breuer J, Cohen JI, et al. Varicella zoster virus infection. Nat Rev Dis Primers. 2015;1:15016.
9. Raedler LA. Shingrix (zoster vaccine recombinant) a new vaccine approved for herpes zoster prevention in older adults. American Health & Drug Benefits, Ninth Annual Payers’ Guide. March 2018. Updated August 30, 2021. Accessed August 9, 2022. https://www.ahdbonline.com/issues/2018/april-2018-vol-11-ninth-annual-payers-guide/2567-shingrix-zoster-vaccine-recombinant-a-new-vaccine-approved-for-herpes-zoster-prevention-in-older-adults
10. Nair PA, Patel BC. Herpes zoster. StatPearls [Internet]. StatPearls Publishing; 2022. https://www.ncbi.nlm.nih.gov/books/NBK441824/
11. Lizzi J, Hill T, Jakubowski J. Varicella zoster virus encephalitis. Clin Pract Cases Emerg Med. 2019;3(4):380-382.
12. Kodadhala V, Dessalegn M, Barned S, et al. 578: Varicella encephalitis: a rare complication of herpes zoster in an elderly patient. Crit Care Med. 2019;47(1):269.
13. Tremolizzo L, Tremolizzo S, Beghi M, et al. Mood disorder with psychotic symptoms and overlooked skin lesions: the strange case of Mrs. O. Riv Psichiatr. 2012;47(5):447-450.
14. George O, Daniel J, Forsyth S, et al. Mania presenting as a VZV encephalitis in the context of HIV. BMJ Case Rep. 2020;13(9):e230512.
15. Bangen KJ, Delano-Wood L, Wierenga CE, et al. Dementia following herpes zoster encephalitis. Clin Neuropsychol. 2010;24(7):1193-1203.
16. McKenna KF, Warneke LB. Encephalitis associated with herpes zoster: a case report and review. Can J Psychiatry. 1992;37(4):271-273.
17. Rogers JP, Pollak TA, Blackman G, et al. Catatonia and the immune system: a review. Lancet Psychiatry. 2019;6(7):620-630.
CASE Agitation and bizarre behavior
Ms. L, age 40, presents to the emergency department (ED) for altered mental status and bizarre behavior. Before arriving at the ED, she had experienced a severe headache and an episode of vomiting. At home she had been irritable and agitated, repetitively dressing and undressing, urinating outside the toilet, and opening and closing water faucets in the house. She also had stopped eating and drinking. Ms. L’s home medications consist of levothyroxine 100 mcg/d for hypothyroidism.
In the ED, Ms. L has severe psychomotor agitation. She is restless and displays purposeless repetitive movements with her hands. She is mostly mute, but does groan at times.
HISTORY Multiple trips to the ED
In addition to hypothyroidism, Ms. L has a history of migraines and asthma. Four days before presenting to the ED, she complained of a severe headache and generalized fatigue, with vomiting and nausea. Two days later, she presented to the ED at a different hospital and underwent a brain CT scan; the results were unremarkable. At that facility, a laboratory work-up—including complete blood count, urea, creatinine, C-reactive protein, electrolytes, magnesium, phosphorus, calcium, full liver function tests, amylase, lipase, bilirubin, thyroid function test, and beta-human chorionic gonadotropin—was normal except for low thyroid-stimulating hormone levels (0.016 mIU/L). Ms. L was diagnosed with a severe migraine attack and discharged home with instructions to follow up with her endocrinologist.
Ms. L has no previous psychiatric history. Her family’s psychiatric history includes depression with psychotic features (mother), depression (maternal aunt), and generalized anxiety disorder (mother’s maternal aunt).
[polldaddy:11252938]
The authors’ observations
Catatonia is a behavioral syndrome with heterogeneous signs and symptoms. According to DSM-5, the diagnosis is considered when a patient presents with ≥3 of the 12 signs outlined in Table 1.1 It usually occurs in the context of an underlying psychiatric disorder such as schizophrenia or depression, or a medical disorder such as CNS infection or encephalopathy due to metabolic causes.1 Ms. L exhibited mutism, negativism, mannerism, stereotypy, and agitation and thus met the criteria for a catatonia diagnosis.

EVALUATION Unexpected finding on physical exam
In the ED, Ms. L is hemodynamically stable. Her blood pressure is 140/80 mm Hg; heart rate is 103 beats per minute; oxygen saturation is 98%; respiratory rate is 14 breaths per minute; and temperature is 37.5° C. Results from a brain MRI and total body scan performed prior to admission are unremarkable.
Ms. L is admitted to the psychiatric ward under the care of neurology for a psychiatry consultation. For approximately 24 hours, she receives IV diazepam 5 mg every 8 hours (due to the unavailability of lorazepam) for management of her catatonic symptoms, and olanzapine 10 mg every 8 hours orally as needed for agitation. Collateral history rules out a current mood episode or onset of psychosis in the weeks before she came to the ED. Diazepam improves Ms. L’s psychomotor agitation, which allows the primary team an opportunity to examine her.
Continue to: A physical exam reveals...
A physical exam reveals small vesicular lesions (1 to 2 cm in diameter) on an erythematous base on the left breast associated with an erythematous plaque with no evident vesicles on the left inner arm. The vesicular lesions display in a segmented pattern of dermatomal distribution.
[polldaddy:11252941]
The authors’ observations
Catatonic symptoms, coupled with psychomotor agitation in an immunocompetent middle-aged adult with a history of migraine headaches, strong family history of severe mental illness, and noncontributory findings on brain imaging, prompted a Psychiatry consultation and administration of psychotropic medications. A thorough physical exam revealing the small area of shingles and acute altered mental status prompted more aggressive investigations to explore the possibility of encephalitis.
Physicians should have a low index of suspicion for encephalitis (viral, bacterial, autoimmune, etc) and perform a lumbar puncture (LP) when necessary, despite the invasiveness of this test. A direct physical examination is often underutilized, notably in psychiatric patients, which can lead to the omission of important clinical information.2 Normal vital signs, blood workup, and MRI before admission are not sufficient to correctly guide diagnosis.
EVALUATION Additional lab results establish the diagnosis
An LP reveals Ms. L’s protein levels are 44 mg/dL, her glucose levels are 85 mg/dL, red blood cell count is 4/µL, and white blood cell count is 200/µL with 92% lymphocytes and 1% neutrophils. Ms. L’s CSF analysis profile indicates a viral CNS infection (Table 23).

[polldaddy:11252943]
The authors’ observations
Varicella-zoster virus (VZV) and herpes simplex virus (HSV) are human neurotropic alphaherpesviruses that cause lifelong infections in ganglia, and their reactivation can come in the form of encephalitis.4
Continue to: Ms. L's clinical presentation...
Ms. L’s clinical presentation most likely implicated VZV. Skin lesions of VZV may look exactly like HSV, with clustered vesicles on an erythematous base (Figure5). However, VZV rash tends to follow a dermatomal distribution (as in Ms. L’s case), which can help distinguish it from herpetic lesions.

Cases of VZV infection have been increasing worldwide. It is usually seen in older adults or those with compromised immunity.6 Significantly higher rates of VZV complications have been reported in such patients. A serious complication is VZV encephalitis, which is rare but possible, even in healthy individuals.6 VZV encephalitis can present with atypical psychiatric features. Ms. L exhibited several symptoms of VZV encephalitis, which include headache, fever, vomiting, altered level of consciousness, and seizures. An EEG also showed intermittent generalized slow waves in the range of theta commonly seen in encephalitis.
Ms. L’s case shows the importance of early recognition of VZV infection. The diagnosis is confirmed through CSF analysis. There is an urgency to promptly conduct the LP to confirm the diagnosis and quickly initiate antiviral treatment to stop the progression of the infection and its life-threatening sequelae.
In the absence of underlying medical cause, typical treatment of catatonia involves the sublingual or IM administration of 1 to 2 mg lorazepam that can be repeated twice at 3-hour intervals if the patient’s symptoms do not resolve. ECT is indicated if the patient experiences minimal or no response to lorazepam.
The use of antipsychotics for catatonia is controversial. High-potency antipsychotics such as haloperidol and risperidone are not recommended due to increased risk of the progression of catatonia into neuroleptic malignant syndrome.7
Continue to: OUTCOME Prompt recovery with an antiviral
OUTCOME Prompt recovery with an antiviral
Ms. L receives IV acyclovir 1,200 mg every 8 hours for 14 days. Just 48 hours after starting this antiviral medication, her bizarre behavior and catatonic features cease, and she returns to her baseline mental functioning. Olanzapine is discontinued, and lorazepam is progressively decreased. The CSF polymerase chain reaction assay indicates Ms. L is positive for VZV, which confirms the diagnosis of VZV encephalitis. A spine MRI is also performed and rules out myelitis as a sequela of the infection.
The authors’ observations
Chickenpox is caused by a primary encounter with VZV. Inside the ganglions of neurons, a dormant form of VZV resides. Its reactivation leads to the spread of the infection to the skin innervated by these neurons, causing shingles. Reactivation occurs in approximately 1 million people in the United States each year. The annual incidence is 5 to 6.5 cases per 1,000 people at age 60, and 8 to 11 cases per 1,000 people at age 70.8
In 2006, the FDA approved the first zoster vaccine (Zostavax) for use in nonimmunocompromised, VZV-seropositive adults age >60 (later lowered to age 50). This vaccine reduces the incidence of shingles by 51%, the incidence of postherpetic neuralgia by 66%, and the burden of illness by 61%. In 2017, the FDA approved a second VZV vaccine (Shingrix, recombinant nonlive vaccine). In 2021, Shingrix was approved for use in immunosuppressed patients.9
Reactivation of VZV starts with a prodromal phase, characterized by pain, itching, numbness, and dysesthesias in 1 to 3 dermatomes. A maculopapular rash appears on the affected area a few days later, evolving into vesicles that scab over in 10 days.10
Dissemination of the virus leading specifically to VZV encephalitis typically occurs in immunosuppressed individuals and older patients. According to the World Health Organization, encephalitis is a life-threatening complication of VZV and occurs in 1 of 33,000 to 50,000 cases.11
Continue to: Delay in the diagnosis...
Delay in the diagnosis and treatment of VZV encephalitis can be detrimental or even fatal. Kodadhala et al12 found that the mortality rate for VZV encephalitis is 5% to 10% and ≤80% in immunosuppressed individuals.
Sometimes, VZV encephalitis can masquerade as a psychiatric presentation. Few cases presenting with acute or delayed neuropsychiatric symptoms related to VZV encephalitis have been previously reported in the literature. Some are summarized in Table 313,14 and Table 4.15,16

To our knowledge, this is the first case report of catatonia as a presentation of VZV encephalitis. The catatonic presentation has been previously described in autoimmune encephalitis such as N-methyl-

Bottom Line
In the setting of a patient with an abrupt change in mental status/behavior, physicians must be aware of the importance of a thorough physical examination to better ascertain a diagnosis and to rule out an underlying medical disorder. Reactivation of varicella-zoster virus (VZV) can result in encephalitis that might masquerade as a psychiatric presentation, including symptoms of catatonia.
Related Resources
- Baum ML, Johnson MC, Lizano P. Is it psychosis, or an autoimmune encephalitis? Current Psychiatry. 2022;21(8): 31-38,44. doi:10.12788/cp.0273
- Reinfold S. Are we failing to diagnose and treat the many faces of catatonia? Current Psychiatry. 2022;21(1):e3-e5. doi:10.12788/cp.0208
Drug Brand Names
Acyclovir • Sitavig
Diazepam • Valium
Haloperidol • Haldol
Lorazepam • Ativan
Levothyroxine • Levoxyl
Olanzapine • Zyprexa
Risperidone • Risperdal
CASE Agitation and bizarre behavior
Ms. L, age 40, presents to the emergency department (ED) for altered mental status and bizarre behavior. Before arriving at the ED, she had experienced a severe headache and an episode of vomiting. At home she had been irritable and agitated, repetitively dressing and undressing, urinating outside the toilet, and opening and closing water faucets in the house. She also had stopped eating and drinking. Ms. L’s home medications consist of levothyroxine 100 mcg/d for hypothyroidism.
In the ED, Ms. L has severe psychomotor agitation. She is restless and displays purposeless repetitive movements with her hands. She is mostly mute, but does groan at times.
HISTORY Multiple trips to the ED
In addition to hypothyroidism, Ms. L has a history of migraines and asthma. Four days before presenting to the ED, she complained of a severe headache and generalized fatigue, with vomiting and nausea. Two days later, she presented to the ED at a different hospital and underwent a brain CT scan; the results were unremarkable. At that facility, a laboratory work-up—including complete blood count, urea, creatinine, C-reactive protein, electrolytes, magnesium, phosphorus, calcium, full liver function tests, amylase, lipase, bilirubin, thyroid function test, and beta-human chorionic gonadotropin—was normal except for low thyroid-stimulating hormone levels (0.016 mIU/L). Ms. L was diagnosed with a severe migraine attack and discharged home with instructions to follow up with her endocrinologist.
Ms. L has no previous psychiatric history. Her family’s psychiatric history includes depression with psychotic features (mother), depression (maternal aunt), and generalized anxiety disorder (mother’s maternal aunt).
[polldaddy:11252938]
The authors’ observations
Catatonia is a behavioral syndrome with heterogeneous signs and symptoms. According to DSM-5, the diagnosis is considered when a patient presents with ≥3 of the 12 signs outlined in Table 1.1 It usually occurs in the context of an underlying psychiatric disorder such as schizophrenia or depression, or a medical disorder such as CNS infection or encephalopathy due to metabolic causes.1 Ms. L exhibited mutism, negativism, mannerism, stereotypy, and agitation and thus met the criteria for a catatonia diagnosis.

EVALUATION Unexpected finding on physical exam
In the ED, Ms. L is hemodynamically stable. Her blood pressure is 140/80 mm Hg; heart rate is 103 beats per minute; oxygen saturation is 98%; respiratory rate is 14 breaths per minute; and temperature is 37.5° C. Results from a brain MRI and total body scan performed prior to admission are unremarkable.
Ms. L is admitted to the psychiatric ward under the care of neurology for a psychiatry consultation. For approximately 24 hours, she receives IV diazepam 5 mg every 8 hours (due to the unavailability of lorazepam) for management of her catatonic symptoms, and olanzapine 10 mg every 8 hours orally as needed for agitation. Collateral history rules out a current mood episode or onset of psychosis in the weeks before she came to the ED. Diazepam improves Ms. L’s psychomotor agitation, which allows the primary team an opportunity to examine her.
Continue to: A physical exam reveals...
A physical exam reveals small vesicular lesions (1 to 2 cm in diameter) on an erythematous base on the left breast associated with an erythematous plaque with no evident vesicles on the left inner arm. The vesicular lesions display in a segmented pattern of dermatomal distribution.
[polldaddy:11252941]
The authors’ observations
Catatonic symptoms, coupled with psychomotor agitation in an immunocompetent middle-aged adult with a history of migraine headaches, strong family history of severe mental illness, and noncontributory findings on brain imaging, prompted a Psychiatry consultation and administration of psychotropic medications. A thorough physical exam revealing the small area of shingles and acute altered mental status prompted more aggressive investigations to explore the possibility of encephalitis.
Physicians should have a low index of suspicion for encephalitis (viral, bacterial, autoimmune, etc) and perform a lumbar puncture (LP) when necessary, despite the invasiveness of this test. A direct physical examination is often underutilized, notably in psychiatric patients, which can lead to the omission of important clinical information.2 Normal vital signs, blood workup, and MRI before admission are not sufficient to correctly guide diagnosis.
EVALUATION Additional lab results establish the diagnosis
An LP reveals Ms. L’s protein levels are 44 mg/dL, her glucose levels are 85 mg/dL, red blood cell count is 4/µL, and white blood cell count is 200/µL with 92% lymphocytes and 1% neutrophils. Ms. L’s CSF analysis profile indicates a viral CNS infection (Table 23).

[polldaddy:11252943]
The authors’ observations
Varicella-zoster virus (VZV) and herpes simplex virus (HSV) are human neurotropic alphaherpesviruses that cause lifelong infections in ganglia, and their reactivation can come in the form of encephalitis.4
Continue to: Ms. L's clinical presentation...
Ms. L’s clinical presentation most likely implicated VZV. Skin lesions of VZV may look exactly like HSV, with clustered vesicles on an erythematous base (Figure5). However, VZV rash tends to follow a dermatomal distribution (as in Ms. L’s case), which can help distinguish it from herpetic lesions.

Cases of VZV infection have been increasing worldwide. It is usually seen in older adults or those with compromised immunity.6 Significantly higher rates of VZV complications have been reported in such patients. A serious complication is VZV encephalitis, which is rare but possible, even in healthy individuals.6 VZV encephalitis can present with atypical psychiatric features. Ms. L exhibited several symptoms of VZV encephalitis, which include headache, fever, vomiting, altered level of consciousness, and seizures. An EEG also showed intermittent generalized slow waves in the range of theta commonly seen in encephalitis.
Ms. L’s case shows the importance of early recognition of VZV infection. The diagnosis is confirmed through CSF analysis. There is an urgency to promptly conduct the LP to confirm the diagnosis and quickly initiate antiviral treatment to stop the progression of the infection and its life-threatening sequelae.
In the absence of underlying medical cause, typical treatment of catatonia involves the sublingual or IM administration of 1 to 2 mg lorazepam that can be repeated twice at 3-hour intervals if the patient’s symptoms do not resolve. ECT is indicated if the patient experiences minimal or no response to lorazepam.
The use of antipsychotics for catatonia is controversial. High-potency antipsychotics such as haloperidol and risperidone are not recommended due to increased risk of the progression of catatonia into neuroleptic malignant syndrome.7
Continue to: OUTCOME Prompt recovery with an antiviral
OUTCOME Prompt recovery with an antiviral
Ms. L receives IV acyclovir 1,200 mg every 8 hours for 14 days. Just 48 hours after starting this antiviral medication, her bizarre behavior and catatonic features cease, and she returns to her baseline mental functioning. Olanzapine is discontinued, and lorazepam is progressively decreased. The CSF polymerase chain reaction assay indicates Ms. L is positive for VZV, which confirms the diagnosis of VZV encephalitis. A spine MRI is also performed and rules out myelitis as a sequela of the infection.
The authors’ observations
Chickenpox is caused by a primary encounter with VZV. Inside the ganglions of neurons, a dormant form of VZV resides. Its reactivation leads to the spread of the infection to the skin innervated by these neurons, causing shingles. Reactivation occurs in approximately 1 million people in the United States each year. The annual incidence is 5 to 6.5 cases per 1,000 people at age 60, and 8 to 11 cases per 1,000 people at age 70.8
In 2006, the FDA approved the first zoster vaccine (Zostavax) for use in nonimmunocompromised, VZV-seropositive adults age >60 (later lowered to age 50). This vaccine reduces the incidence of shingles by 51%, the incidence of postherpetic neuralgia by 66%, and the burden of illness by 61%. In 2017, the FDA approved a second VZV vaccine (Shingrix, recombinant nonlive vaccine). In 2021, Shingrix was approved for use in immunosuppressed patients.9
Reactivation of VZV starts with a prodromal phase, characterized by pain, itching, numbness, and dysesthesias in 1 to 3 dermatomes. A maculopapular rash appears on the affected area a few days later, evolving into vesicles that scab over in 10 days.10
Dissemination of the virus leading specifically to VZV encephalitis typically occurs in immunosuppressed individuals and older patients. According to the World Health Organization, encephalitis is a life-threatening complication of VZV and occurs in 1 of 33,000 to 50,000 cases.11
Continue to: Delay in the diagnosis...
Delay in the diagnosis and treatment of VZV encephalitis can be detrimental or even fatal. Kodadhala et al12 found that the mortality rate for VZV encephalitis is 5% to 10% and ≤80% in immunosuppressed individuals.
Sometimes, VZV encephalitis can masquerade as a psychiatric presentation. Few cases presenting with acute or delayed neuropsychiatric symptoms related to VZV encephalitis have been previously reported in the literature. Some are summarized in Table 313,14 and Table 4.15,16

To our knowledge, this is the first case report of catatonia as a presentation of VZV encephalitis. The catatonic presentation has been previously described in autoimmune encephalitis such as N-methyl-

Bottom Line
In the setting of a patient with an abrupt change in mental status/behavior, physicians must be aware of the importance of a thorough physical examination to better ascertain a diagnosis and to rule out an underlying medical disorder. Reactivation of varicella-zoster virus (VZV) can result in encephalitis that might masquerade as a psychiatric presentation, including symptoms of catatonia.
Related Resources
- Baum ML, Johnson MC, Lizano P. Is it psychosis, or an autoimmune encephalitis? Current Psychiatry. 2022;21(8): 31-38,44. doi:10.12788/cp.0273
- Reinfold S. Are we failing to diagnose and treat the many faces of catatonia? Current Psychiatry. 2022;21(1):e3-e5. doi:10.12788/cp.0208
Drug Brand Names
Acyclovir • Sitavig
Diazepam • Valium
Haloperidol • Haldol
Lorazepam • Ativan
Levothyroxine • Levoxyl
Olanzapine • Zyprexa
Risperidone • Risperdal
1. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. American Psychiatric Association; 2013.
2. Sanders RD, Keshavan MS. Physical and neurologic examinations in neuropsychiatry. Semin Clin Neuropsychiatry. 2002;7(1):18-29.
3. Howes DS, Lazoff M. Encephalitis workup. Medscape. Updated August 7, 2018. Accessed August 9, 2022. https://emedicine.medscape.com/article/791896-workup#c11
4. Kennedy PG, Rovnak J, Badani H, et al. A comparison of herpes simplex virus type 1 and varicella-zoster virus latency and reactivation. J Gen Virol. 2015;96(Pt 7):1581-1602.
5. Fisle, CC BY-SA 3.0 (https://creativecommons.org/licenses/by-sa/3.0). Wikimedia Commons. https://upload.wikimedia.org/wikipedia/commons/1/19/Herpes_zoster_chest.png
6. John AR, Canaday DH. Herpes zoster in the older adult. Infect Dis Clin North Am. 2017;31(4):811-826.
7. Rosebush PI, Mazurek MF. Catatonia and its treatment. Schizophr Bull. 2010;36(2):239-242.
8. Gershon AA, Breuer J, Cohen JI, et al. Varicella zoster virus infection. Nat Rev Dis Primers. 2015;1:15016.
9. Raedler LA. Shingrix (zoster vaccine recombinant) a new vaccine approved for herpes zoster prevention in older adults. American Health & Drug Benefits, Ninth Annual Payers’ Guide. March 2018. Updated August 30, 2021. Accessed August 9, 2022. https://www.ahdbonline.com/issues/2018/april-2018-vol-11-ninth-annual-payers-guide/2567-shingrix-zoster-vaccine-recombinant-a-new-vaccine-approved-for-herpes-zoster-prevention-in-older-adults
10. Nair PA, Patel BC. Herpes zoster. StatPearls [Internet]. StatPearls Publishing; 2022. https://www.ncbi.nlm.nih.gov/books/NBK441824/
11. Lizzi J, Hill T, Jakubowski J. Varicella zoster virus encephalitis. Clin Pract Cases Emerg Med. 2019;3(4):380-382.
12. Kodadhala V, Dessalegn M, Barned S, et al. 578: Varicella encephalitis: a rare complication of herpes zoster in an elderly patient. Crit Care Med. 2019;47(1):269.
13. Tremolizzo L, Tremolizzo S, Beghi M, et al. Mood disorder with psychotic symptoms and overlooked skin lesions: the strange case of Mrs. O. Riv Psichiatr. 2012;47(5):447-450.
14. George O, Daniel J, Forsyth S, et al. Mania presenting as a VZV encephalitis in the context of HIV. BMJ Case Rep. 2020;13(9):e230512.
15. Bangen KJ, Delano-Wood L, Wierenga CE, et al. Dementia following herpes zoster encephalitis. Clin Neuropsychol. 2010;24(7):1193-1203.
16. McKenna KF, Warneke LB. Encephalitis associated with herpes zoster: a case report and review. Can J Psychiatry. 1992;37(4):271-273.
17. Rogers JP, Pollak TA, Blackman G, et al. Catatonia and the immune system: a review. Lancet Psychiatry. 2019;6(7):620-630.
1. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. American Psychiatric Association; 2013.
2. Sanders RD, Keshavan MS. Physical and neurologic examinations in neuropsychiatry. Semin Clin Neuropsychiatry. 2002;7(1):18-29.
3. Howes DS, Lazoff M. Encephalitis workup. Medscape. Updated August 7, 2018. Accessed August 9, 2022. https://emedicine.medscape.com/article/791896-workup#c11
4. Kennedy PG, Rovnak J, Badani H, et al. A comparison of herpes simplex virus type 1 and varicella-zoster virus latency and reactivation. J Gen Virol. 2015;96(Pt 7):1581-1602.
5. Fisle, CC BY-SA 3.0 (https://creativecommons.org/licenses/by-sa/3.0). Wikimedia Commons. https://upload.wikimedia.org/wikipedia/commons/1/19/Herpes_zoster_chest.png
6. John AR, Canaday DH. Herpes zoster in the older adult. Infect Dis Clin North Am. 2017;31(4):811-826.
7. Rosebush PI, Mazurek MF. Catatonia and its treatment. Schizophr Bull. 2010;36(2):239-242.
8. Gershon AA, Breuer J, Cohen JI, et al. Varicella zoster virus infection. Nat Rev Dis Primers. 2015;1:15016.
9. Raedler LA. Shingrix (zoster vaccine recombinant) a new vaccine approved for herpes zoster prevention in older adults. American Health & Drug Benefits, Ninth Annual Payers’ Guide. March 2018. Updated August 30, 2021. Accessed August 9, 2022. https://www.ahdbonline.com/issues/2018/april-2018-vol-11-ninth-annual-payers-guide/2567-shingrix-zoster-vaccine-recombinant-a-new-vaccine-approved-for-herpes-zoster-prevention-in-older-adults
10. Nair PA, Patel BC. Herpes zoster. StatPearls [Internet]. StatPearls Publishing; 2022. https://www.ncbi.nlm.nih.gov/books/NBK441824/
11. Lizzi J, Hill T, Jakubowski J. Varicella zoster virus encephalitis. Clin Pract Cases Emerg Med. 2019;3(4):380-382.
12. Kodadhala V, Dessalegn M, Barned S, et al. 578: Varicella encephalitis: a rare complication of herpes zoster in an elderly patient. Crit Care Med. 2019;47(1):269.
13. Tremolizzo L, Tremolizzo S, Beghi M, et al. Mood disorder with psychotic symptoms and overlooked skin lesions: the strange case of Mrs. O. Riv Psichiatr. 2012;47(5):447-450.
14. George O, Daniel J, Forsyth S, et al. Mania presenting as a VZV encephalitis in the context of HIV. BMJ Case Rep. 2020;13(9):e230512.
15. Bangen KJ, Delano-Wood L, Wierenga CE, et al. Dementia following herpes zoster encephalitis. Clin Neuropsychol. 2010;24(7):1193-1203.
16. McKenna KF, Warneke LB. Encephalitis associated with herpes zoster: a case report and review. Can J Psychiatry. 1992;37(4):271-273.
17. Rogers JP, Pollak TA, Blackman G, et al. Catatonia and the immune system: a review. Lancet Psychiatry. 2019;6(7):620-630.
Sleep disorders in older adults
As humans live longer, a renewed focus on quality of life has made the prompt diagnosis and treatment of sleep-related disorders in older adults increasingly necessary.1 Normative aging results in multiple changes in sleep architecture, including decreased total sleep time, decreased sleep efficiency, decreased slow-wave sleep (SWS), and increased awakenings after sleep onset.2 Sleep disturbances in older adults are increasingly recognized as multifactorial health conditions requiring comprehensive modification of risk factors, diagnosis, and treatment.3
In this article, we discuss the effects of aging on sleep architecture and provide an overview of primary sleep disorders in older adults. We also summarize strategies for diagnosing and treating sleep disorders in these patients.
Elements of the sleep cycle
The human sleep cycle begins with light sleep (sleep stages 1 and 2), progresses into SWS (sleep stage 3), and culminates in rapid eye movement (REM) sleep. The first 3 stages are referred to as non-rapid eye movement sleep (NREM). Throughout the night, this coupling of NREM and REM cycles occurs 4 to 6 times, with each successive cycle decreasing in length until awakening.4
Two complex neurologic pathways intersect to regulate the timing of sleep and wakefulness on arousal. The first pathway, the circadian system, is located within the suprachiasmatic nucleus of the hypothalamus and is highly dependent on external stimuli (light, food, etc.) to synchronize sleep/wake cycles. The suprachiasmatic nucleus regulates melatonin secretion by the pineal gland, which signals day-night transitions. The other pathway, the homeostatic system, modifies the amount of sleep needed daily. When multiple days of poor sleep occur, homeostatic sleep pressure (colloquially described as sleep debt) compensates by increasing the amount of sleep required in the following days. Together, the circadian and homeostatic systems work in conjunction to regulate sleep quantity to approximately one-third of the total sleep-wake cycle.2,5
Age-related dysfunction of the regulatory sleep pathways leads to blunting of the ability to initiate and sustain high-quality sleep.6 Dysregulation of homeostatic sleep pressure decreases time spent in SWS, and failure of the circadian signaling apparatus results in delays in sleep/wake timing.2 While research into the underlying neurobiology of sleep reveals that some of these changes are inherent to aging (Box7-14), significant underdiagnosed pathologies may adversely affect sleep architecture, including polypharmacy, comorbid neuropathology (eg, synucleinopathies, tauopathies, etc.), and primary sleep disorders (insomnias, hypersomnias, and parasomnias).15
Box
It has long been known that sleep architecture changes significantly with age. One of the largest meta-analyses of sleep changes in healthy individuals throughout childhood into old age found that total sleep time, sleep efficiency, percentage of slow-wave sleep, percentage of rapid eye movement sleep (REM), and REM latency all decreased with normative aging.7 Other studies have also found a decreased ability to maintain sleep (increased frequency of awakenings and prolonged nocturnal awakenings).8
Based on several meta-analyses, the average total sleep time at night in the adult population decreases by approximately 10 minutes per decade in both men and women.7,9-11 However, this pattern is not observed after age 60, when the total sleep time plateaus.7 Similarly, the duration of wake after sleep onset increases by approximately 10 minutes every decade for adults age 30 to 60, and plateaus after that.7,8
Epidemiologic studies have suggested that the prevalence of daytime napping increases with age.8 This trend continues into older age without a noticeable plateau.
A study of a nationally representative sample of >7,000 Japanese participants found that a significantly higher proportion of older adults take daytime naps (27.4%) compared with middle-age adults (14.4%).12 Older adults nap more frequently because of both lifestyle and biologic changes that accompany normative aging. Polls in the United States have shown a correlation between frequent napping and an increase in excessive daytime sleepiness, depression, pain, and nocturia.13
While sleep latency steadily increases after age 50, recent studies have shown that in healthy individuals, these changes are modest at best,7,9,14 which suggests that other pathologic factors may be contributing to this problem. Although healthy older people were found to have more frequent arousals throughout the night, they retained the ability to reinitiate sleep as rapidly as younger adults.7,9
Primary sleep disorders
Obstructive sleep apnea (OSA) is one of the most common, yet frequently underdiagnosed reversible causes of sleep disturbances. It is characterized by partial or complete airway obstruction culminating in periods of involuntary cessation of respirations during sleep. The resultant fragmentation in sleep leads to significant downstream effects over time, including excessive daytime sleepiness and fatigue, poor occupational and social performance, and substantial cognitive impairment.3 While it is well known that OSA increases in prevalence throughout middle age, this relationship plateaus after age 60.16 An estimated 40% to 60% of Americans age >60 are affected by OSA.17 The hypoxemia and fragmented sleep caused by unrecognized OSA are associated with a significant decline in activities of daily living (ADL).18 Untreated OSA is strongly linked to the development and progression of several major health conditions, including cardiovascular disease, diabetes mellitus, hypertension, stroke, and depression.19 In studies of long-term care facility residents—many of whom may have comorbid cognitive decline—researchers found that unrecognized OSA often mimics the progressive cognitive decline seen in major neurocognitive disorders.20 However, classic symptoms of OSA may not always be present in these patients, and their daytime sleepiness is often attributed to old age rather than to a pathological etiology.16 Screening for OSA and prompt initiation of the appropriate treatment may reverse OSA-induced cognitive changes in these patients.21
The primary presenting symptom of OSA is snoring, which is correlated with pauses in breathing. Risk factors include increased body mass index (BMI), thick neck circumference, male sex, and advanced age. In older adults, BMI has a lower impact on the Apnea-Hypopnea Index, an indicator of the number of pauses in breathing per hour, when compared with young and middle-age adults.16 Validated screening questionnaires for OSA include the STOP-Bang Questionnaire (Table 122), OSA50, Berlin Questionnaire, and Epworth Sleepiness Scale, each of which is used in different subpopulations. The current diagnostic standard for OSA is nocturnal polysomnography in a sleep laboratory, but recent advances in home sleep apnea testing have made it a viable, low-cost alternative for patients who do not have significant medical comorbidities.23 Standard utilized cutoffs for diagnosis are ≥5 events/hour (hypopneas associated with at least 4% oxygen desaturations) in conjunction with clinical symptoms of OSA.24
Continue to: Treatment
Treatment. First-line treatment for OSA is continuous positive airway pressure therapy, but adherence rates vary widely with patient education and regular follow-up.25 Adjunctive therapy includes weight loss, oral appliances, and uvulopalatopharyngoplasty, a procedure in which tissue in the throat is remodeled or removed.
Central sleep apnea (CSA) is a pause in breathing without evidence of associated respiratory effort. In adults, the development of CSA is indicative of underlying lower brainstem dysfunction, due to intermittent failures in the pontomedullary centers responsible for regulation of rhythmic breathing.26 This can occur as a consequence of multiple diseases, including congestive heart failure, stroke, renal failure, chronic medication use (opioids), and brain tumors.
The Sleep Heart Health Study—the largest community-based cohort study to date examining CSA—estimated that the prevalence of CSA among adults age >65 was 1.1% (compared with 0.4% in those age <65).27 Subgroup analysis revealed that men had significantly higher rates of CSA compared with women (2.7% vs 0.2%, respectively).
CSA may present similarly to OSA (excessive daytime somnolence, insomnia, poor sleep quality, difficulties with attention and concentration). Symptoms may also mimic those of coexisting medical conditions in older adults, such as nocturnal angina or paroxysmal nocturnal dyspnea.27 Any older patient with daytime sleepiness and risk factors for CSA should be referred for in-laboratory nocturnal polysomnography, the gold standard diagnostic test. Unlike in OSA, ambulatory diagnostic measures (home sleep apnea testing) have not been validated for this disorder.27
Treatment. The primary treatment for CSA is to address the underlying medical problem. Positive pressure ventilation has been attempted with mixed results. Supplemental oxygen and medical management (acetazolamide or theophylline) can help stimulate breathing. Newer studies have shown favorable outcomes with transvenous neurostimulation or adaptive servoventilation.28-30
Continue to: Insomnia
Insomnia. For a primary diagnosis of insomnia, DSM-5 requires at least 3 nights per week of sleep disturbances that induce distress or functional impairment for at least 3 months.31 The International Classification of Disease, 10th Edition requires at least 1 month of symptoms (lying awake for a long time before falling asleep, sleeping for short periods, being awake for most of the night, feeling lack of sleep, waking up early) after ruling out other sleep disorders, substance use, or other medical conditions.4 Clinically, insomnia tends to present in older adults as a subjective complaint of dissatisfaction with the quality and/or quantity of their sleep. Insomnia has been consistently shown to be a significant risk factor for both the development or exacerbation of depression in older adults.32-34
While the diagnosis of insomnia is mainly clinical via a thorough sleep and medication history, assistive ancillary testing can include wrist actigraphy and screening questionnaires (the Insomnia Severity Index and the Pittsburgh Sleep Quality Index).4 Because population studies of older adults have found discrepancies between objective and subjective methods of assessing sleep quality, relying on the accuracy of self-reported symptoms alone is questionable.35
Treatment. Given that drug elimination half-life increases with age, and the risks of adverse effects are increased in older adults, the preferred treatment modalities for insomnia are nonpharmacologic.4 Sleep hygiene education (Table 2) and cognitive-behavioral therapy (CBT) for insomnia are often the first-line therapies.4,36,37 It is crucial to manage comorbidities such as heart disease and obesity, as well as sources of discomfort from conditions such as arthritic pain.38,39 If nonpharmacologic therapies are not effective, pharmacologic options can be considered.4 Before prescribing sleep medications, it may be more fruitful to treat underlying psychiatric disorders such as depression and anxiety with antidepressants.4 Although benzodiazepines are helpful for their sedative effects, they are not recommended for older adults because of an increased risk of falls, rebound insomnia, potential tolerance, and associated cognitive impairment.40 Benzodiazepine receptor agonists (eg, zolpidem, eszopiclone, zaleplon) were initially developed as a first-line treatment for insomnia to replace the reliance on benzodiazepines, but these medications have a “black-box” warning of a serious risk of complex sleep behaviors, including life-threatening parasomnias.41 As a result, guidelines suggest a shorter duration of treatment with a benzodiazepine receptor agonist may still provide benefit while limiting the risk of adverse effects.42
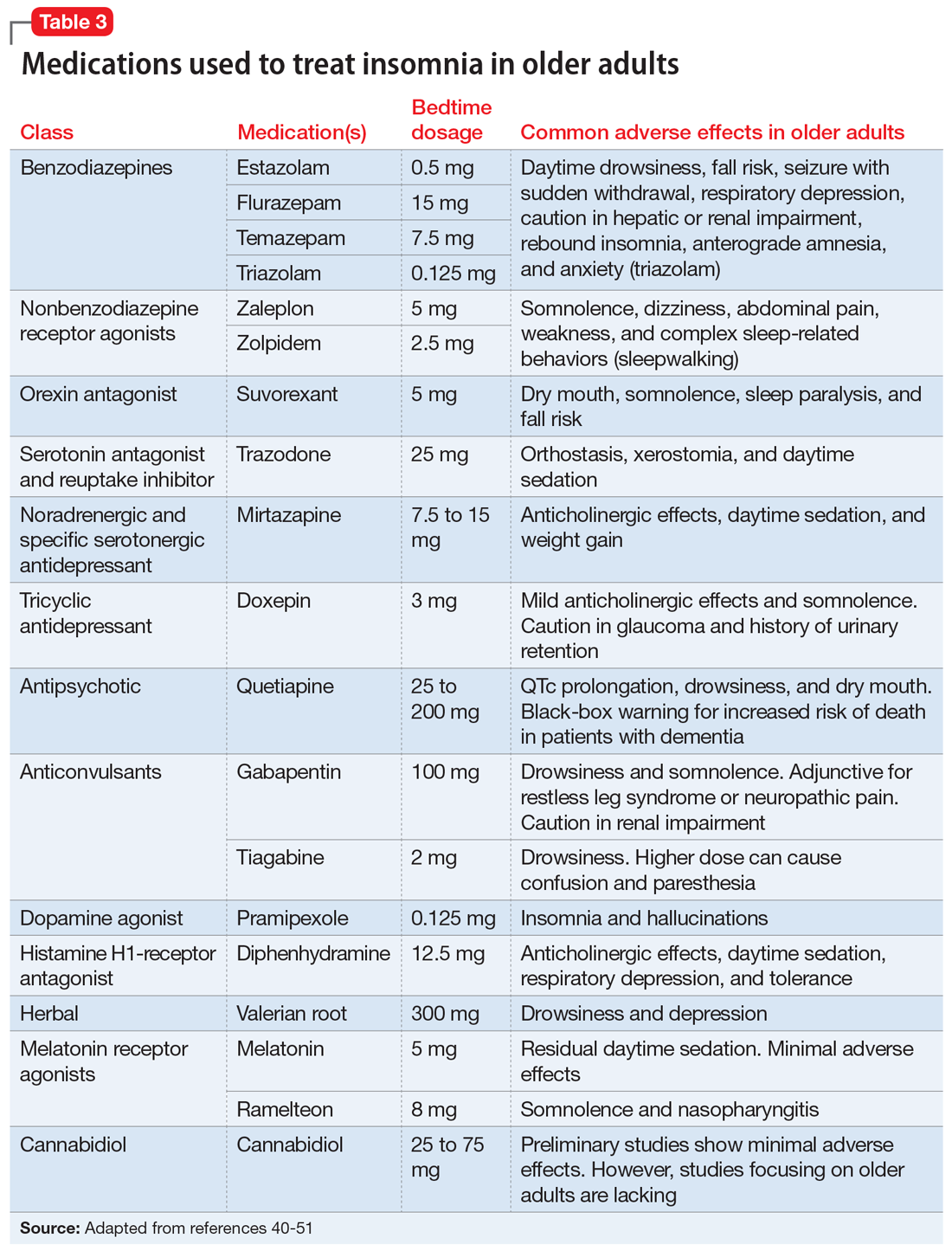
Doxepin is the only antidepressant FDA-approved for insomnia; it improves sleep latency (time taken to initiate sleep after lying down), duration, and quality in adults age >65.43 Melatonin receptor agonists such as ramelteon and melatonin have shown positive results in older patients with insomnia. In clinical trials of patients age ≥65, ramelteon, which is FDA-approved for insomnia, produced no rebound insomnia, withdrawal effects, memory impairment, or gait instability.44-46 Suvorexant, an orexin receptor antagonist, decreases sleep latency and increases total sleep time equally in both young and older adults.47-49Table 340-51 provides a list of medications used to treat insomnia (including off-label agents) and their common adverse effects in older adults.
Parasomnias are undesirable behaviors that occur during sleep, commonly associated with the sleep-wake transition period. These behaviors can occur during REM sleep (nightmare disorder, sleep paralysis, REM sleep behavior disorder) or NREM sleep (somnambulism [sleepwalking], confusional arousals, sleep terrors). According to a cross-sectional Norwegian study of parasomnias, the estimated lifetime prevalence of sleep walking is 22.4%; sleep talking, 66.8%; confusional arousal, 18.5%; and sleep terror, 10.4%.52
Continue to: When evaluating a patient...
When evaluating a patient with parasomnias, it is important to review their drug and substance use as well as coexisting medical conditions. Drugs and substances that can affect sleep include prescription medications (second-generation antidepressants, stimulants, dopamine agonists), excessive caffeine, alcohol, certain foods (coffee, chocolate milk, black tea, caffeinated soft drinks), environmental exposures (smoking, pesticides), and recreational drugs (amphetamines).53-56 Certain medical conditions are correlated with specific parasomnias (eg, sleep paralysis and narcolepsy, REM sleep behavior disorder and Parkinson’s disease [PD], etc.).54 Diagnosis of parasomnias is mainly clinical but supporting evidence can be obtained through in-lab polysomnography.
Treatment. For parasomnias, treatment is primarily supportive and includes creating a safe sleeping environment to reduce the risk of self-harm. Recommendations include sleeping in a room on the ground floor, minimizing furniture in the bedroom, padding any bedside furniture, child-proofing doorknobs, and locking up weapons and other dangerous household items.54
REM sleep behavior disorder (RBD). This disorder is characterized by a loss of the typical REM sleep-associated atonia and the presence of motor activity during dreaming (dream-enacted behaviors). While the estimated incidence of RBD in the general adult population is approximately 0.5%, it increases to 7.7% among those age >60.57 RBD occurs most commonly in the setting of the alpha-synucleinopathies (PD, Lewy body dementia, multisystem atrophy), but can also be found in patients with cerebral ischemia, demyelinating disorders, or alcohol misuse, or can be medication-induced (primarily antidepressants and antipsychotics).58 In patients with PD, the presence of RBD is associated with a more impaired cognitive profile, suggestive of widespread neurodegeneration.59 Recent studies revealed that RBD may also be a prodromal state of neurodegenerative diseases such as PD, which should prompt close monitoring and long-term follow up.60 Similar to other parasomnias, the diagnosis of RBD is primarily clinical, but polysomnography plays an important role in demonstrating loss of REM-related atonia.54
Treatment. Clonazepam and melatonin have been shown to be effective in treating the symptoms of RBD.54
Depression, anxiety, and sleep disturbances
Major depressive disorder (MDD) and generalized anxiety disorder (GAD) affect sleep in patients of all ages, but are underreported in older adults. According to national epidemiologic surveys, the estimated prevalence of MDD and GAD among older adults is 13% and 11.4%, respectively.61,62 Rates as high as 42% and 39% have been reported in meta-regression analyses among patients with Alzheimer’s dementia.63
Continue to: Depression and anxiety
Depression and anxiety may have additive effects and manifest as poor sleep satisfaction, increased sleep latency, insomnia, and daytime sleepiness.64 However, they may also have independent effects. Studies showed that patients with depression alone reported overall poor sleep satisfaction, whereas patients with anxiety alone reported problems with sleep latency, daytime drowsiness, and waking up at night in addition to their overall poor sleep satisfaction.65-67 Both depression and anxiety are risk factors for developing cognitive decline, and may be an early sign/prodrome of neurodegenerative diseases (dementias).68 The bidirectional relationship between depression/anxiety and sleep is complex and needs further investigation.
Treatment. Pharmacologic treatments for patients with depression/anxiety and sleep disturbances include selective serotonin reuptake inhibitors, serotonin-norepinephrine reuptake inhibitors, tricyclic antidepressants, and other serotonin receptor agonists.69-72 Nonpharmacologic treatments include CBT for both depression and anxiety, and problem-solving therapy for patients with mild cognitive impairment and depression.73,74 For severe depression and/or anxiety, electroconvulsive therapy is effective.75
Bottom Line
Sleep disorders in older adults are common but often underdiagnosed. Timely recognition of obstructive sleep apnea, central sleep apnea, insomnia, parasomnias, and other sleep disturbances can facilitate effective treatment and greatly improve older adults’ quality of life.
Related Resources
- American Academy of Sleep Medicine. International Classification of Sleep Disorders—Third Edition. https://aasm.org
- SleepFoundation.org. Sleep hygiene. https://www.sleepfoundation.org/articles/sleep-hygiene
Drug Brand Names
Acetazolamide • Diamox
Clonazepam • Klonopin
Doxepin • Silenor
Eszopiclone • Lunesta
Gabapentin • Neurontin
Mirtazapine • Remeron
Pramipexole • Mirapex
Quetiapine • Seroquel
Ramelteon • Rozerem
Suvorexant • Belsomra
Temazepam • Restoril
Theophylline • Elixophyllin
Tiagabine • Gabitril
Trazadone • Desyrel
Triazolam • Halcion
Zaleplon • Sonata
Zolpidem • Ambien
1. Centers for Disease Control and Prevention. The state of aging and health in America. 2013. Accessed January 27, 2021. https://www.cdc.gov/aging/pdf/state-aging-health-in-america-2013.pdf
2. Suzuki K, Miyamoto M, Hirata K. Sleep disorders in the elderly: diagnosis and management. J Gen Fam Med. 2017;18(2):61-71.
3. Inouye SK, Studenski S, Tinetti ME, et al. Geriatric syndromes: clinical, research, and policy implications of a core geriatric concept. J Am Geriatr Soc. 2007;55(5):780-791.
4. Patel D, Steinberg J, Patel P. Insomnia in the elderly: a review. J Clin Sleep Med. 2018;14(6):1017-1024.
5. Neubauer DN. A review of ramelteon in the treatment of sleep disorders. Neuropsychiatr Dis Treat. 2008;4(1):69-79.
6. Mander BA, Winer JR, Walker MP. Sleep and human aging. Neuron. 2017;94(1):19-36.
7. Ohayon MM, Carskadon MA, Guilleminault C, et al. Meta-analysis of quantitative sleep parameters from childhood to old age in healthy individuals: developing normative sleep values across the human lifespan. Sleep. 2004;27:1255-1273.
8. Li J, Vitiello MV, Gooneratne NS. Sleep in normal aging. Sleep Med Clin. 2018;13(1):1-11.
9. Floyd JA, Medler SM, Ager JW, et al. Age-related changes in initiation and maintenance of sleep: a meta-analysis. Res Nurs Health. 2000;23(2):106-117.
10. Floyd JA, Janisse JJ, Jenuwine ES, et al. Changes in REM-sleep percentage over the adult lifespan. Sleep. 2007;30(7):829-836.
11. Dorffner G, Vitr M, Anderer P. The effects of aging on sleep architecture in healthy subjects. Adv Exp Med Biol. 2015;821:93-100.
12. Furihata R, Kaneita Y, Jike M, et al. Napping and associated factors: a Japanese nationwide general population survey. Sleep Med. 2016;20:72-79.
13. Foley DJ, Vitiello MV, Bliwise DL, et al. Frequent napping is associated with excessive daytime sleepiness, depression, pain, and nocturia in older adults: findings from the National Sleep Foundation ‘2003 Sleep in America’ Poll. Am J Geriatr Psychiatry. 2007;15(4):344-350.
14. Floyd JA, Janisse JJ, Marshall Medler S, et al. Nonlinear components of age-related change in sleep initiation. Nurs Res. 2000;49(5):290-294.
15. Miner B, Kryger MH. Sleep in the aging population. Sleep Med Clin. 2017;12(1):31-38.
16. Young T, Peppard PE, Gottlieb DJ. Epidemiology of obstructive sleep apnea: a population health perspective. Am J Respir Crit Care Med. 2002;165(9):1217-1239.
17. Ancoli-Israel S, Klauber MR, Butters N, et al. Dementia in institutionalized elderly: relation to sleep apnea. J Am Geriatr Soc. 1991;39(3):258-263.
18. Spira AP, Stone KL, Rebok GW, et al. Sleep-disordered breathing and functional decline in older women. J Am Geriatr Soc. 2014;62(11):2040-2046.
19. Vijayan VK. Morbidities associated with obstructive sleep apnea. Expert Rev Respir Med. 2012;6(5):557-566.
20. Kerner NA, Roose SP. Obstructive sleep apnea is linked to depression and cognitive impairment: evidence and potential mechanisms. Am J Geriatr Psychiatry. 2016;24(6):496-508.
21. Dalmases M, Solé-Padullés C, Torres M, et al. Effect of CPAP on cognition, brain function, and structure among elderly patients with OSA: a randomized pilot study. Chest. 2015;148(5):1214-1223.
22. Toronto Western Hospital, University Health Network. University of Toronto. STOP-Bang Questionnaire. 2012. Accessed January 26, 2021. www.stopbang.ca
23. Freedman N. Doing it better for less: incorporating OSA management into alternative payment models. Chest. 2019;155(1):227-233.
24. Kapur VK, Auckley DH, Chowdhuri S, et al. Clinical practice guideline for diagnostic testing for adult obstructive sleep apnea: an American Academy of Sleep Medicine clinical practice guideline. J Clin Sleep Med. 2017;13(3):479-504.
25. Semelka M, Wilson J, Floyd R. Diagnosis and treatment of obstructive sleep apnea in adults. Am Fam Physician. 2016;94(5):355-360.
26. Javaheri S, Dempsey JA. Central sleep apnea. Compr Physiol. 2013;3(1):141-163.
27. Donovan LM, Kapur VK. Prevalence and characteristics of central compared to obstructive sleep apnea: analyses from the Sleep Heart Health Study cohort. Sleep. 2016;39(7):1353-1359.
28. Cao M, Cardell CY, Willes L, et al. A novel adaptive servoventilation (ASVAuto) for the treatment of central sleep apnea associated with chronic use of opioids. J Clin Sleep Med. 2014;10(8):855-861.
29. Oldenburg O, Spießhöfer J, Fox H, et al. Performance of conventional and enhanced adaptive servoventilation (ASV) in heart failure patients with central sleep apnea who have adapted to conventional ASV. Sleep Breath. 2015;19(3):795-800.
30. Costanzo MR, Ponikowski P, Javaheri S, et al. Transvenous neurostimulation for central sleep apnoea: a randomised controlled trial. Lancet. 2016;388(10048):974-982.
31. Diagnostic and statistical manual of mental disorders, 5th ed. American Psychiatric Association; 2013:362.
32. Perlis ML, Smith LJ, Lyness JM, et al. Insomnia as a risk factor for onset of depression in the elderly. Behav Sleep Med. 2006;4(2):104-113.
33. Cole MG, Dendukuri N. Risk factors for depression among elderly community subjects: a systematic review and meta-analysis. Am J Psychiatry. 2003;160(6):1147-1156.
34. Pigeon WR, Hegel M, Unützer J, et al. Is insomnia a perpetuating factor for late-life depression in the IMPACT cohort? Sleep. 2008;31(4):481-488.
35. Hughes JM, Song Y, Fung CH, et al. Measuring sleep in vulnerable older adults: a comparison of subjective and objective sleep measures. Clin Gerontol. 2018;41(2):145-157.
36. Irish LA, Kline CE, Gunn HE, et al. The role of sleep hygiene in promoting public health: a review of empirical evidence. Sleep Med Rev. 2015;22:23-36.
37. Sleep Foundation. Sleep hygiene. Accessed January 27, 2021. https://www.sleepfoundation.org/articles/sleep-hygiene
38. Foley D, Ancoli-Israel S, Britz P, et al. Sleep disturbances and chronic disease in older adults: results of the 2003 National Sleep Foundation Sleep in America Survey. J Psychosom Res. 2004;56(5):497-502.
39. Eslami V, Zimmerman ME, Grewal T, et al. Pain grade and sleep disturbance in older adults: evaluation the role of pain, and stress for depressed and non-depressed individuals. Int J Geriatr Psychiatry. 2016;31(5):450-457.
40. American Geriatrics Society Beers Criteria Update Expert Panel. American Geriatrics Society 2015 updated Beers Criteria for potentially inappropriate medication use in older adults. J Am Geriatr Soc. 2015;63(11):2227-2246.
41. United States Food & Drug Administration. FDA adds Boxed Warning for risk of serious injuries caused by sleepwalking with certain prescription insomnia medicines. 2019. Accessed January 27, 2021. https://www.fda.gov/drugs/drug-safety-and-availability/fda-adds-boxed-warning-risk-serious-injuries-caused-sleepwalking-certain-prescription-insomnia
42. Schroeck JL, Ford J, Conway EL, et al. Review of safety and efficacy of sleep medicines in older adults. Clin Ther. 2016;38(11):2340-2372.
43. Krystal AD, Lankford A, Durrence HH, et al. Efficacy and safety of doxepin 3 and 6 mg in a 35-day sleep laboratory trial in adults with chronic primary insomnia. Sleep. 2011;34(10):1433-1442.
44. Roth T, Seiden D, Sainati S, et al. Effects of ramelteon on patient-reported sleep latency in older adults with chronic insomnia. Sleep Med. 2006;7(4):312-318.
45. Zammit G, Wang-Weigand S, Rosenthal M, et al. Effect of ramelteon on middle-of-the-night balance in older adults with chronic insomnia. J Clin Sleep Med. 2009;5(1):34-40.
46. Mets MAJ, de Vries JM, de Senerpont Domis LM, et al. Next-day effects of ramelteon (8 mg), zopiclone (7.5 mg), and placebo on highway driving performance, memory functioning, psychomotor performance, and mood in healthy adult subjects. Sleep. 2011;34(10):1327-1334.
47. Rhyne DN, Anderson SL. Suvorexant in insomnia: efficacy, safety and place in therapy. Ther Adv Drug Saf. 2015;6(5):189-195.
48. Norman JL, Anderson SL. Novel class of medications, orexin receptor antagonists, in the treatment of insomnia - critical appraisal of suvorexant. Nat Sci Sleep. 2016;8:239-247.
49. Owen RT. Suvorexant: efficacy and safety profile of a dual orexin receptor antagonist in treating insomnia. Drugs Today (Barc). 2016;52(1):29-40.
50. Shannon S, Lewis N, Lee H, et al. Cannabidiol in anxiety and sleep: a large case series. Perm J. 2019;23:18-041. doi: 10.7812/TPP/18-041
51. Yunusa I, Alsumali A, Garba AE, et al. Assessment of reported comparative effectiveness and safety of atypical antipsychotics in the treatment of behavioral and psychological symptoms of dementia: a network meta-analysis. JAMA Netw Open. 2019;2(3):e190828.
52. Bjorvatn B, Gronli J, Pallesen S. Prevalence of different parasomnias in the general population. Sleep Med. 2010;11(10):1031-1034.
53. Postuma RB, Montplaisir JY, Pelletier A, et al. Environmental risk factors for REM sleep behavior disorder: a multicenter case-control study. Neurology. 2012;79(5):428-434.
54. Fleetham JA, Fleming JA. Parasomnias. CMAJ. 2014;186(8):E273-E280.
55. Dinis-Oliveira RJ, Caldas I, Carvalho F, et al. Bruxism after 3,4-methylenedioxymethamphetamine (ecstasy) abuse. Clin Toxicol (Phila.) 2010;48(8):863-864.
56. Irfan MH, Howell MJ. Rapid eye movement sleep behavior disorder: overview and current perspective. Curr Sleep Medicine Rep. 2016;2:64-73.
57. Mahlknecht P, Seppi K, Frauscher B, et al. Probable RBD and association with neurodegenerative disease markers: a population-based study. Mov Disord. 2015;30(10):1417-1421.
58. Oertel WH, Depboylu C, Krenzer M, et al. [REM sleep behavior disorder as a prodromal stage of α-synucleinopathies: symptoms, epidemiology, pathophysiology, diagnosis and therapy]. Nervenarzt. 2014;85:19-25. German.
59. Jozwiak N, Postuma RB, Montplaisir J, et al. REM sleep behavior disorder and cognitive impairment in Parkinson’s disease. Sleep. 2017;40(8):zsx101. doi: 10.1093/sleep/zsx101
60. Claassen DO, Josephs KA, Ahlskog JE, et al. REM sleep behavior disorder preceding other aspects of synucleinopathies by up to half a century. Neurology 2010;75(6):494-499.
61. Reynolds K, Pietrzak RH, El-Gabalawy R, et al. Prevalence of psychiatric disorders in U.S. older adults: findings from a nationally representative survey. World Psychiatry. 2015;14(1):74-81.
62. Lohman MC, Mezuk B, Dumenci L. Depression and frailty: concurrent risks for adverse health outcomes. Aging Ment Health. 2017;21(4):399-408.
63. Zhao QF, Tan L, Wang HF, et al. The prevalence of neuropsychiatric symptoms in Alzheimer’s disease: systematic review and meta-analysis. J Affect Disord. 2016;190:264-271.
64. Furihata R, Hall MH, Stone KL, et al. An aggregate measure of sleep health is associated with prevalent and incident clinically significant depression symptoms among community-dwelling older women. Sleep. 2017;40(3):zsw075. doi: 10.1093/sleep/zsw075
65. Spira AP, Stone K, Beaudreau SA, et al. Anxiety symptoms and objectively measured sleep quality in older women. Am J Geriatr Psychiatry. 2009;17(2):136-143.
66. Press Y, Punchik B, Freud T. The association between subjectively impaired sleep and symptoms of depression and anxiety in a frail elderly population. Aging Clin Exp Res. 2018;30(7):755-765.
67. Gould CE, Spira AP, Liou-Johnson V, et al. Association of anxiety symptom clusters with sleep quality and daytime sleepiness. J Gerontol B Psychol Sci Soc Sci. 2018;73(3):413-420.
68. Kassem AM, Ganguli M, Yaffe K, et al. Anxiety symptoms and risk of cognitive decline in older community-dwelling men. Int Psychogeriatr. 2017;29(7):1137-1145.
69. Frank C. Pharmacologic treatment of depression in the elderly. Can Fam Physician. 2014;60(2):121-126.
70. Subramanyam AA, Kedare J, Singh OP, et al. Clinical practice guidelines for geriatric anxiety disorders. Indian J Psychiatry. 2018;60(suppl 3):S371-S382.
71. Emsley R, Ahokas A, Suarez A, et al. Efficacy of tianeptine 25-50 mg in elderly patients with recurrent major depressive disorder: an 8-week placebo- and escitalopram-controlled study. J Clin Psychiatry. 2018;79(4):17m11741. doi: 10.4088/JCP.17m11741
72. Semel D, Murphy TK, Zlateva G, et al. Evaluation of the safety and efficacy of pregabalin in older patients with neuropathic pain: results from a pooled analysis of 11 clinical studies. BMC Fam Pract. 2010;11:85.
73. Orgeta V, Qazi A, Spector A, et al. Psychological treatments for depression and anxiety in dementia and mild cognitive impairment: systematic review and meta-analysis. Br J Psychiatry. 2015;207(4):293-298.
74. Morimoto SS, Kanellopoulos D, Manning KJ, et al. Diagnosis and treatment of depression and cognitive impairment in late life. Ann N Y Acad Sci. 2015;1345(1):36-46.
75. Casey DA. Depression in older adults: a treatable medical condition. Prim Care. 2017;44(3):499-510.
As humans live longer, a renewed focus on quality of life has made the prompt diagnosis and treatment of sleep-related disorders in older adults increasingly necessary.1 Normative aging results in multiple changes in sleep architecture, including decreased total sleep time, decreased sleep efficiency, decreased slow-wave sleep (SWS), and increased awakenings after sleep onset.2 Sleep disturbances in older adults are increasingly recognized as multifactorial health conditions requiring comprehensive modification of risk factors, diagnosis, and treatment.3
In this article, we discuss the effects of aging on sleep architecture and provide an overview of primary sleep disorders in older adults. We also summarize strategies for diagnosing and treating sleep disorders in these patients.
Elements of the sleep cycle
The human sleep cycle begins with light sleep (sleep stages 1 and 2), progresses into SWS (sleep stage 3), and culminates in rapid eye movement (REM) sleep. The first 3 stages are referred to as non-rapid eye movement sleep (NREM). Throughout the night, this coupling of NREM and REM cycles occurs 4 to 6 times, with each successive cycle decreasing in length until awakening.4
Two complex neurologic pathways intersect to regulate the timing of sleep and wakefulness on arousal. The first pathway, the circadian system, is located within the suprachiasmatic nucleus of the hypothalamus and is highly dependent on external stimuli (light, food, etc.) to synchronize sleep/wake cycles. The suprachiasmatic nucleus regulates melatonin secretion by the pineal gland, which signals day-night transitions. The other pathway, the homeostatic system, modifies the amount of sleep needed daily. When multiple days of poor sleep occur, homeostatic sleep pressure (colloquially described as sleep debt) compensates by increasing the amount of sleep required in the following days. Together, the circadian and homeostatic systems work in conjunction to regulate sleep quantity to approximately one-third of the total sleep-wake cycle.2,5
Age-related dysfunction of the regulatory sleep pathways leads to blunting of the ability to initiate and sustain high-quality sleep.6 Dysregulation of homeostatic sleep pressure decreases time spent in SWS, and failure of the circadian signaling apparatus results in delays in sleep/wake timing.2 While research into the underlying neurobiology of sleep reveals that some of these changes are inherent to aging (Box7-14), significant underdiagnosed pathologies may adversely affect sleep architecture, including polypharmacy, comorbid neuropathology (eg, synucleinopathies, tauopathies, etc.), and primary sleep disorders (insomnias, hypersomnias, and parasomnias).15
Box
It has long been known that sleep architecture changes significantly with age. One of the largest meta-analyses of sleep changes in healthy individuals throughout childhood into old age found that total sleep time, sleep efficiency, percentage of slow-wave sleep, percentage of rapid eye movement sleep (REM), and REM latency all decreased with normative aging.7 Other studies have also found a decreased ability to maintain sleep (increased frequency of awakenings and prolonged nocturnal awakenings).8
Based on several meta-analyses, the average total sleep time at night in the adult population decreases by approximately 10 minutes per decade in both men and women.7,9-11 However, this pattern is not observed after age 60, when the total sleep time plateaus.7 Similarly, the duration of wake after sleep onset increases by approximately 10 minutes every decade for adults age 30 to 60, and plateaus after that.7,8
Epidemiologic studies have suggested that the prevalence of daytime napping increases with age.8 This trend continues into older age without a noticeable plateau.
A study of a nationally representative sample of >7,000 Japanese participants found that a significantly higher proportion of older adults take daytime naps (27.4%) compared with middle-age adults (14.4%).12 Older adults nap more frequently because of both lifestyle and biologic changes that accompany normative aging. Polls in the United States have shown a correlation between frequent napping and an increase in excessive daytime sleepiness, depression, pain, and nocturia.13
While sleep latency steadily increases after age 50, recent studies have shown that in healthy individuals, these changes are modest at best,7,9,14 which suggests that other pathologic factors may be contributing to this problem. Although healthy older people were found to have more frequent arousals throughout the night, they retained the ability to reinitiate sleep as rapidly as younger adults.7,9
Primary sleep disorders
Obstructive sleep apnea (OSA) is one of the most common, yet frequently underdiagnosed reversible causes of sleep disturbances. It is characterized by partial or complete airway obstruction culminating in periods of involuntary cessation of respirations during sleep. The resultant fragmentation in sleep leads to significant downstream effects over time, including excessive daytime sleepiness and fatigue, poor occupational and social performance, and substantial cognitive impairment.3 While it is well known that OSA increases in prevalence throughout middle age, this relationship plateaus after age 60.16 An estimated 40% to 60% of Americans age >60 are affected by OSA.17 The hypoxemia and fragmented sleep caused by unrecognized OSA are associated with a significant decline in activities of daily living (ADL).18 Untreated OSA is strongly linked to the development and progression of several major health conditions, including cardiovascular disease, diabetes mellitus, hypertension, stroke, and depression.19 In studies of long-term care facility residents—many of whom may have comorbid cognitive decline—researchers found that unrecognized OSA often mimics the progressive cognitive decline seen in major neurocognitive disorders.20 However, classic symptoms of OSA may not always be present in these patients, and their daytime sleepiness is often attributed to old age rather than to a pathological etiology.16 Screening for OSA and prompt initiation of the appropriate treatment may reverse OSA-induced cognitive changes in these patients.21
The primary presenting symptom of OSA is snoring, which is correlated with pauses in breathing. Risk factors include increased body mass index (BMI), thick neck circumference, male sex, and advanced age. In older adults, BMI has a lower impact on the Apnea-Hypopnea Index, an indicator of the number of pauses in breathing per hour, when compared with young and middle-age adults.16 Validated screening questionnaires for OSA include the STOP-Bang Questionnaire (Table 122), OSA50, Berlin Questionnaire, and Epworth Sleepiness Scale, each of which is used in different subpopulations. The current diagnostic standard for OSA is nocturnal polysomnography in a sleep laboratory, but recent advances in home sleep apnea testing have made it a viable, low-cost alternative for patients who do not have significant medical comorbidities.23 Standard utilized cutoffs for diagnosis are ≥5 events/hour (hypopneas associated with at least 4% oxygen desaturations) in conjunction with clinical symptoms of OSA.24
Continue to: Treatment
Treatment. First-line treatment for OSA is continuous positive airway pressure therapy, but adherence rates vary widely with patient education and regular follow-up.25 Adjunctive therapy includes weight loss, oral appliances, and uvulopalatopharyngoplasty, a procedure in which tissue in the throat is remodeled or removed.
Central sleep apnea (CSA) is a pause in breathing without evidence of associated respiratory effort. In adults, the development of CSA is indicative of underlying lower brainstem dysfunction, due to intermittent failures in the pontomedullary centers responsible for regulation of rhythmic breathing.26 This can occur as a consequence of multiple diseases, including congestive heart failure, stroke, renal failure, chronic medication use (opioids), and brain tumors.
The Sleep Heart Health Study—the largest community-based cohort study to date examining CSA—estimated that the prevalence of CSA among adults age >65 was 1.1% (compared with 0.4% in those age <65).27 Subgroup analysis revealed that men had significantly higher rates of CSA compared with women (2.7% vs 0.2%, respectively).
CSA may present similarly to OSA (excessive daytime somnolence, insomnia, poor sleep quality, difficulties with attention and concentration). Symptoms may also mimic those of coexisting medical conditions in older adults, such as nocturnal angina or paroxysmal nocturnal dyspnea.27 Any older patient with daytime sleepiness and risk factors for CSA should be referred for in-laboratory nocturnal polysomnography, the gold standard diagnostic test. Unlike in OSA, ambulatory diagnostic measures (home sleep apnea testing) have not been validated for this disorder.27
Treatment. The primary treatment for CSA is to address the underlying medical problem. Positive pressure ventilation has been attempted with mixed results. Supplemental oxygen and medical management (acetazolamide or theophylline) can help stimulate breathing. Newer studies have shown favorable outcomes with transvenous neurostimulation or adaptive servoventilation.28-30
Continue to: Insomnia
Insomnia. For a primary diagnosis of insomnia, DSM-5 requires at least 3 nights per week of sleep disturbances that induce distress or functional impairment for at least 3 months.31 The International Classification of Disease, 10th Edition requires at least 1 month of symptoms (lying awake for a long time before falling asleep, sleeping for short periods, being awake for most of the night, feeling lack of sleep, waking up early) after ruling out other sleep disorders, substance use, or other medical conditions.4 Clinically, insomnia tends to present in older adults as a subjective complaint of dissatisfaction with the quality and/or quantity of their sleep. Insomnia has been consistently shown to be a significant risk factor for both the development or exacerbation of depression in older adults.32-34
While the diagnosis of insomnia is mainly clinical via a thorough sleep and medication history, assistive ancillary testing can include wrist actigraphy and screening questionnaires (the Insomnia Severity Index and the Pittsburgh Sleep Quality Index).4 Because population studies of older adults have found discrepancies between objective and subjective methods of assessing sleep quality, relying on the accuracy of self-reported symptoms alone is questionable.35
Treatment. Given that drug elimination half-life increases with age, and the risks of adverse effects are increased in older adults, the preferred treatment modalities for insomnia are nonpharmacologic.4 Sleep hygiene education (Table 2) and cognitive-behavioral therapy (CBT) for insomnia are often the first-line therapies.4,36,37 It is crucial to manage comorbidities such as heart disease and obesity, as well as sources of discomfort from conditions such as arthritic pain.38,39 If nonpharmacologic therapies are not effective, pharmacologic options can be considered.4 Before prescribing sleep medications, it may be more fruitful to treat underlying psychiatric disorders such as depression and anxiety with antidepressants.4 Although benzodiazepines are helpful for their sedative effects, they are not recommended for older adults because of an increased risk of falls, rebound insomnia, potential tolerance, and associated cognitive impairment.40 Benzodiazepine receptor agonists (eg, zolpidem, eszopiclone, zaleplon) were initially developed as a first-line treatment for insomnia to replace the reliance on benzodiazepines, but these medications have a “black-box” warning of a serious risk of complex sleep behaviors, including life-threatening parasomnias.41 As a result, guidelines suggest a shorter duration of treatment with a benzodiazepine receptor agonist may still provide benefit while limiting the risk of adverse effects.42
Doxepin is the only antidepressant FDA-approved for insomnia; it improves sleep latency (time taken to initiate sleep after lying down), duration, and quality in adults age >65.43 Melatonin receptor agonists such as ramelteon and melatonin have shown positive results in older patients with insomnia. In clinical trials of patients age ≥65, ramelteon, which is FDA-approved for insomnia, produced no rebound insomnia, withdrawal effects, memory impairment, or gait instability.44-46 Suvorexant, an orexin receptor antagonist, decreases sleep latency and increases total sleep time equally in both young and older adults.47-49Table 340-51 provides a list of medications used to treat insomnia (including off-label agents) and their common adverse effects in older adults.
Parasomnias are undesirable behaviors that occur during sleep, commonly associated with the sleep-wake transition period. These behaviors can occur during REM sleep (nightmare disorder, sleep paralysis, REM sleep behavior disorder) or NREM sleep (somnambulism [sleepwalking], confusional arousals, sleep terrors). According to a cross-sectional Norwegian study of parasomnias, the estimated lifetime prevalence of sleep walking is 22.4%; sleep talking, 66.8%; confusional arousal, 18.5%; and sleep terror, 10.4%.52
Continue to: When evaluating a patient...
When evaluating a patient with parasomnias, it is important to review their drug and substance use as well as coexisting medical conditions. Drugs and substances that can affect sleep include prescription medications (second-generation antidepressants, stimulants, dopamine agonists), excessive caffeine, alcohol, certain foods (coffee, chocolate milk, black tea, caffeinated soft drinks), environmental exposures (smoking, pesticides), and recreational drugs (amphetamines).53-56 Certain medical conditions are correlated with specific parasomnias (eg, sleep paralysis and narcolepsy, REM sleep behavior disorder and Parkinson’s disease [PD], etc.).54 Diagnosis of parasomnias is mainly clinical but supporting evidence can be obtained through in-lab polysomnography.
Treatment. For parasomnias, treatment is primarily supportive and includes creating a safe sleeping environment to reduce the risk of self-harm. Recommendations include sleeping in a room on the ground floor, minimizing furniture in the bedroom, padding any bedside furniture, child-proofing doorknobs, and locking up weapons and other dangerous household items.54
REM sleep behavior disorder (RBD). This disorder is characterized by a loss of the typical REM sleep-associated atonia and the presence of motor activity during dreaming (dream-enacted behaviors). While the estimated incidence of RBD in the general adult population is approximately 0.5%, it increases to 7.7% among those age >60.57 RBD occurs most commonly in the setting of the alpha-synucleinopathies (PD, Lewy body dementia, multisystem atrophy), but can also be found in patients with cerebral ischemia, demyelinating disorders, or alcohol misuse, or can be medication-induced (primarily antidepressants and antipsychotics).58 In patients with PD, the presence of RBD is associated with a more impaired cognitive profile, suggestive of widespread neurodegeneration.59 Recent studies revealed that RBD may also be a prodromal state of neurodegenerative diseases such as PD, which should prompt close monitoring and long-term follow up.60 Similar to other parasomnias, the diagnosis of RBD is primarily clinical, but polysomnography plays an important role in demonstrating loss of REM-related atonia.54
Treatment. Clonazepam and melatonin have been shown to be effective in treating the symptoms of RBD.54
Depression, anxiety, and sleep disturbances
Major depressive disorder (MDD) and generalized anxiety disorder (GAD) affect sleep in patients of all ages, but are underreported in older adults. According to national epidemiologic surveys, the estimated prevalence of MDD and GAD among older adults is 13% and 11.4%, respectively.61,62 Rates as high as 42% and 39% have been reported in meta-regression analyses among patients with Alzheimer’s dementia.63
Continue to: Depression and anxiety
Depression and anxiety may have additive effects and manifest as poor sleep satisfaction, increased sleep latency, insomnia, and daytime sleepiness.64 However, they may also have independent effects. Studies showed that patients with depression alone reported overall poor sleep satisfaction, whereas patients with anxiety alone reported problems with sleep latency, daytime drowsiness, and waking up at night in addition to their overall poor sleep satisfaction.65-67 Both depression and anxiety are risk factors for developing cognitive decline, and may be an early sign/prodrome of neurodegenerative diseases (dementias).68 The bidirectional relationship between depression/anxiety and sleep is complex and needs further investigation.
Treatment. Pharmacologic treatments for patients with depression/anxiety and sleep disturbances include selective serotonin reuptake inhibitors, serotonin-norepinephrine reuptake inhibitors, tricyclic antidepressants, and other serotonin receptor agonists.69-72 Nonpharmacologic treatments include CBT for both depression and anxiety, and problem-solving therapy for patients with mild cognitive impairment and depression.73,74 For severe depression and/or anxiety, electroconvulsive therapy is effective.75
Bottom Line
Sleep disorders in older adults are common but often underdiagnosed. Timely recognition of obstructive sleep apnea, central sleep apnea, insomnia, parasomnias, and other sleep disturbances can facilitate effective treatment and greatly improve older adults’ quality of life.
Related Resources
- American Academy of Sleep Medicine. International Classification of Sleep Disorders—Third Edition. https://aasm.org
- SleepFoundation.org. Sleep hygiene. https://www.sleepfoundation.org/articles/sleep-hygiene
Drug Brand Names
Acetazolamide • Diamox
Clonazepam • Klonopin
Doxepin • Silenor
Eszopiclone • Lunesta
Gabapentin • Neurontin
Mirtazapine • Remeron
Pramipexole • Mirapex
Quetiapine • Seroquel
Ramelteon • Rozerem
Suvorexant • Belsomra
Temazepam • Restoril
Theophylline • Elixophyllin
Tiagabine • Gabitril
Trazadone • Desyrel
Triazolam • Halcion
Zaleplon • Sonata
Zolpidem • Ambien
As humans live longer, a renewed focus on quality of life has made the prompt diagnosis and treatment of sleep-related disorders in older adults increasingly necessary.1 Normative aging results in multiple changes in sleep architecture, including decreased total sleep time, decreased sleep efficiency, decreased slow-wave sleep (SWS), and increased awakenings after sleep onset.2 Sleep disturbances in older adults are increasingly recognized as multifactorial health conditions requiring comprehensive modification of risk factors, diagnosis, and treatment.3
In this article, we discuss the effects of aging on sleep architecture and provide an overview of primary sleep disorders in older adults. We also summarize strategies for diagnosing and treating sleep disorders in these patients.
Elements of the sleep cycle
The human sleep cycle begins with light sleep (sleep stages 1 and 2), progresses into SWS (sleep stage 3), and culminates in rapid eye movement (REM) sleep. The first 3 stages are referred to as non-rapid eye movement sleep (NREM). Throughout the night, this coupling of NREM and REM cycles occurs 4 to 6 times, with each successive cycle decreasing in length until awakening.4
Two complex neurologic pathways intersect to regulate the timing of sleep and wakefulness on arousal. The first pathway, the circadian system, is located within the suprachiasmatic nucleus of the hypothalamus and is highly dependent on external stimuli (light, food, etc.) to synchronize sleep/wake cycles. The suprachiasmatic nucleus regulates melatonin secretion by the pineal gland, which signals day-night transitions. The other pathway, the homeostatic system, modifies the amount of sleep needed daily. When multiple days of poor sleep occur, homeostatic sleep pressure (colloquially described as sleep debt) compensates by increasing the amount of sleep required in the following days. Together, the circadian and homeostatic systems work in conjunction to regulate sleep quantity to approximately one-third of the total sleep-wake cycle.2,5
Age-related dysfunction of the regulatory sleep pathways leads to blunting of the ability to initiate and sustain high-quality sleep.6 Dysregulation of homeostatic sleep pressure decreases time spent in SWS, and failure of the circadian signaling apparatus results in delays in sleep/wake timing.2 While research into the underlying neurobiology of sleep reveals that some of these changes are inherent to aging (Box7-14), significant underdiagnosed pathologies may adversely affect sleep architecture, including polypharmacy, comorbid neuropathology (eg, synucleinopathies, tauopathies, etc.), and primary sleep disorders (insomnias, hypersomnias, and parasomnias).15
Box
It has long been known that sleep architecture changes significantly with age. One of the largest meta-analyses of sleep changes in healthy individuals throughout childhood into old age found that total sleep time, sleep efficiency, percentage of slow-wave sleep, percentage of rapid eye movement sleep (REM), and REM latency all decreased with normative aging.7 Other studies have also found a decreased ability to maintain sleep (increased frequency of awakenings and prolonged nocturnal awakenings).8
Based on several meta-analyses, the average total sleep time at night in the adult population decreases by approximately 10 minutes per decade in both men and women.7,9-11 However, this pattern is not observed after age 60, when the total sleep time plateaus.7 Similarly, the duration of wake after sleep onset increases by approximately 10 minutes every decade for adults age 30 to 60, and plateaus after that.7,8
Epidemiologic studies have suggested that the prevalence of daytime napping increases with age.8 This trend continues into older age without a noticeable plateau.
A study of a nationally representative sample of >7,000 Japanese participants found that a significantly higher proportion of older adults take daytime naps (27.4%) compared with middle-age adults (14.4%).12 Older adults nap more frequently because of both lifestyle and biologic changes that accompany normative aging. Polls in the United States have shown a correlation between frequent napping and an increase in excessive daytime sleepiness, depression, pain, and nocturia.13
While sleep latency steadily increases after age 50, recent studies have shown that in healthy individuals, these changes are modest at best,7,9,14 which suggests that other pathologic factors may be contributing to this problem. Although healthy older people were found to have more frequent arousals throughout the night, they retained the ability to reinitiate sleep as rapidly as younger adults.7,9
Primary sleep disorders
Obstructive sleep apnea (OSA) is one of the most common, yet frequently underdiagnosed reversible causes of sleep disturbances. It is characterized by partial or complete airway obstruction culminating in periods of involuntary cessation of respirations during sleep. The resultant fragmentation in sleep leads to significant downstream effects over time, including excessive daytime sleepiness and fatigue, poor occupational and social performance, and substantial cognitive impairment.3 While it is well known that OSA increases in prevalence throughout middle age, this relationship plateaus after age 60.16 An estimated 40% to 60% of Americans age >60 are affected by OSA.17 The hypoxemia and fragmented sleep caused by unrecognized OSA are associated with a significant decline in activities of daily living (ADL).18 Untreated OSA is strongly linked to the development and progression of several major health conditions, including cardiovascular disease, diabetes mellitus, hypertension, stroke, and depression.19 In studies of long-term care facility residents—many of whom may have comorbid cognitive decline—researchers found that unrecognized OSA often mimics the progressive cognitive decline seen in major neurocognitive disorders.20 However, classic symptoms of OSA may not always be present in these patients, and their daytime sleepiness is often attributed to old age rather than to a pathological etiology.16 Screening for OSA and prompt initiation of the appropriate treatment may reverse OSA-induced cognitive changes in these patients.21
The primary presenting symptom of OSA is snoring, which is correlated with pauses in breathing. Risk factors include increased body mass index (BMI), thick neck circumference, male sex, and advanced age. In older adults, BMI has a lower impact on the Apnea-Hypopnea Index, an indicator of the number of pauses in breathing per hour, when compared with young and middle-age adults.16 Validated screening questionnaires for OSA include the STOP-Bang Questionnaire (Table 122), OSA50, Berlin Questionnaire, and Epworth Sleepiness Scale, each of which is used in different subpopulations. The current diagnostic standard for OSA is nocturnal polysomnography in a sleep laboratory, but recent advances in home sleep apnea testing have made it a viable, low-cost alternative for patients who do not have significant medical comorbidities.23 Standard utilized cutoffs for diagnosis are ≥5 events/hour (hypopneas associated with at least 4% oxygen desaturations) in conjunction with clinical symptoms of OSA.24
Continue to: Treatment
Treatment. First-line treatment for OSA is continuous positive airway pressure therapy, but adherence rates vary widely with patient education and regular follow-up.25 Adjunctive therapy includes weight loss, oral appliances, and uvulopalatopharyngoplasty, a procedure in which tissue in the throat is remodeled or removed.
Central sleep apnea (CSA) is a pause in breathing without evidence of associated respiratory effort. In adults, the development of CSA is indicative of underlying lower brainstem dysfunction, due to intermittent failures in the pontomedullary centers responsible for regulation of rhythmic breathing.26 This can occur as a consequence of multiple diseases, including congestive heart failure, stroke, renal failure, chronic medication use (opioids), and brain tumors.
The Sleep Heart Health Study—the largest community-based cohort study to date examining CSA—estimated that the prevalence of CSA among adults age >65 was 1.1% (compared with 0.4% in those age <65).27 Subgroup analysis revealed that men had significantly higher rates of CSA compared with women (2.7% vs 0.2%, respectively).
CSA may present similarly to OSA (excessive daytime somnolence, insomnia, poor sleep quality, difficulties with attention and concentration). Symptoms may also mimic those of coexisting medical conditions in older adults, such as nocturnal angina or paroxysmal nocturnal dyspnea.27 Any older patient with daytime sleepiness and risk factors for CSA should be referred for in-laboratory nocturnal polysomnography, the gold standard diagnostic test. Unlike in OSA, ambulatory diagnostic measures (home sleep apnea testing) have not been validated for this disorder.27
Treatment. The primary treatment for CSA is to address the underlying medical problem. Positive pressure ventilation has been attempted with mixed results. Supplemental oxygen and medical management (acetazolamide or theophylline) can help stimulate breathing. Newer studies have shown favorable outcomes with transvenous neurostimulation or adaptive servoventilation.28-30
Continue to: Insomnia
Insomnia. For a primary diagnosis of insomnia, DSM-5 requires at least 3 nights per week of sleep disturbances that induce distress or functional impairment for at least 3 months.31 The International Classification of Disease, 10th Edition requires at least 1 month of symptoms (lying awake for a long time before falling asleep, sleeping for short periods, being awake for most of the night, feeling lack of sleep, waking up early) after ruling out other sleep disorders, substance use, or other medical conditions.4 Clinically, insomnia tends to present in older adults as a subjective complaint of dissatisfaction with the quality and/or quantity of their sleep. Insomnia has been consistently shown to be a significant risk factor for both the development or exacerbation of depression in older adults.32-34
While the diagnosis of insomnia is mainly clinical via a thorough sleep and medication history, assistive ancillary testing can include wrist actigraphy and screening questionnaires (the Insomnia Severity Index and the Pittsburgh Sleep Quality Index).4 Because population studies of older adults have found discrepancies between objective and subjective methods of assessing sleep quality, relying on the accuracy of self-reported symptoms alone is questionable.35
Treatment. Given that drug elimination half-life increases with age, and the risks of adverse effects are increased in older adults, the preferred treatment modalities for insomnia are nonpharmacologic.4 Sleep hygiene education (Table 2) and cognitive-behavioral therapy (CBT) for insomnia are often the first-line therapies.4,36,37 It is crucial to manage comorbidities such as heart disease and obesity, as well as sources of discomfort from conditions such as arthritic pain.38,39 If nonpharmacologic therapies are not effective, pharmacologic options can be considered.4 Before prescribing sleep medications, it may be more fruitful to treat underlying psychiatric disorders such as depression and anxiety with antidepressants.4 Although benzodiazepines are helpful for their sedative effects, they are not recommended for older adults because of an increased risk of falls, rebound insomnia, potential tolerance, and associated cognitive impairment.40 Benzodiazepine receptor agonists (eg, zolpidem, eszopiclone, zaleplon) were initially developed as a first-line treatment for insomnia to replace the reliance on benzodiazepines, but these medications have a “black-box” warning of a serious risk of complex sleep behaviors, including life-threatening parasomnias.41 As a result, guidelines suggest a shorter duration of treatment with a benzodiazepine receptor agonist may still provide benefit while limiting the risk of adverse effects.42
Doxepin is the only antidepressant FDA-approved for insomnia; it improves sleep latency (time taken to initiate sleep after lying down), duration, and quality in adults age >65.43 Melatonin receptor agonists such as ramelteon and melatonin have shown positive results in older patients with insomnia. In clinical trials of patients age ≥65, ramelteon, which is FDA-approved for insomnia, produced no rebound insomnia, withdrawal effects, memory impairment, or gait instability.44-46 Suvorexant, an orexin receptor antagonist, decreases sleep latency and increases total sleep time equally in both young and older adults.47-49Table 340-51 provides a list of medications used to treat insomnia (including off-label agents) and their common adverse effects in older adults.
Parasomnias are undesirable behaviors that occur during sleep, commonly associated with the sleep-wake transition period. These behaviors can occur during REM sleep (nightmare disorder, sleep paralysis, REM sleep behavior disorder) or NREM sleep (somnambulism [sleepwalking], confusional arousals, sleep terrors). According to a cross-sectional Norwegian study of parasomnias, the estimated lifetime prevalence of sleep walking is 22.4%; sleep talking, 66.8%; confusional arousal, 18.5%; and sleep terror, 10.4%.52
Continue to: When evaluating a patient...
When evaluating a patient with parasomnias, it is important to review their drug and substance use as well as coexisting medical conditions. Drugs and substances that can affect sleep include prescription medications (second-generation antidepressants, stimulants, dopamine agonists), excessive caffeine, alcohol, certain foods (coffee, chocolate milk, black tea, caffeinated soft drinks), environmental exposures (smoking, pesticides), and recreational drugs (amphetamines).53-56 Certain medical conditions are correlated with specific parasomnias (eg, sleep paralysis and narcolepsy, REM sleep behavior disorder and Parkinson’s disease [PD], etc.).54 Diagnosis of parasomnias is mainly clinical but supporting evidence can be obtained through in-lab polysomnography.
Treatment. For parasomnias, treatment is primarily supportive and includes creating a safe sleeping environment to reduce the risk of self-harm. Recommendations include sleeping in a room on the ground floor, minimizing furniture in the bedroom, padding any bedside furniture, child-proofing doorknobs, and locking up weapons and other dangerous household items.54
REM sleep behavior disorder (RBD). This disorder is characterized by a loss of the typical REM sleep-associated atonia and the presence of motor activity during dreaming (dream-enacted behaviors). While the estimated incidence of RBD in the general adult population is approximately 0.5%, it increases to 7.7% among those age >60.57 RBD occurs most commonly in the setting of the alpha-synucleinopathies (PD, Lewy body dementia, multisystem atrophy), but can also be found in patients with cerebral ischemia, demyelinating disorders, or alcohol misuse, or can be medication-induced (primarily antidepressants and antipsychotics).58 In patients with PD, the presence of RBD is associated with a more impaired cognitive profile, suggestive of widespread neurodegeneration.59 Recent studies revealed that RBD may also be a prodromal state of neurodegenerative diseases such as PD, which should prompt close monitoring and long-term follow up.60 Similar to other parasomnias, the diagnosis of RBD is primarily clinical, but polysomnography plays an important role in demonstrating loss of REM-related atonia.54
Treatment. Clonazepam and melatonin have been shown to be effective in treating the symptoms of RBD.54
Depression, anxiety, and sleep disturbances
Major depressive disorder (MDD) and generalized anxiety disorder (GAD) affect sleep in patients of all ages, but are underreported in older adults. According to national epidemiologic surveys, the estimated prevalence of MDD and GAD among older adults is 13% and 11.4%, respectively.61,62 Rates as high as 42% and 39% have been reported in meta-regression analyses among patients with Alzheimer’s dementia.63
Continue to: Depression and anxiety
Depression and anxiety may have additive effects and manifest as poor sleep satisfaction, increased sleep latency, insomnia, and daytime sleepiness.64 However, they may also have independent effects. Studies showed that patients with depression alone reported overall poor sleep satisfaction, whereas patients with anxiety alone reported problems with sleep latency, daytime drowsiness, and waking up at night in addition to their overall poor sleep satisfaction.65-67 Both depression and anxiety are risk factors for developing cognitive decline, and may be an early sign/prodrome of neurodegenerative diseases (dementias).68 The bidirectional relationship between depression/anxiety and sleep is complex and needs further investigation.
Treatment. Pharmacologic treatments for patients with depression/anxiety and sleep disturbances include selective serotonin reuptake inhibitors, serotonin-norepinephrine reuptake inhibitors, tricyclic antidepressants, and other serotonin receptor agonists.69-72 Nonpharmacologic treatments include CBT for both depression and anxiety, and problem-solving therapy for patients with mild cognitive impairment and depression.73,74 For severe depression and/or anxiety, electroconvulsive therapy is effective.75
Bottom Line
Sleep disorders in older adults are common but often underdiagnosed. Timely recognition of obstructive sleep apnea, central sleep apnea, insomnia, parasomnias, and other sleep disturbances can facilitate effective treatment and greatly improve older adults’ quality of life.
Related Resources
- American Academy of Sleep Medicine. International Classification of Sleep Disorders—Third Edition. https://aasm.org
- SleepFoundation.org. Sleep hygiene. https://www.sleepfoundation.org/articles/sleep-hygiene
Drug Brand Names
Acetazolamide • Diamox
Clonazepam • Klonopin
Doxepin • Silenor
Eszopiclone • Lunesta
Gabapentin • Neurontin
Mirtazapine • Remeron
Pramipexole • Mirapex
Quetiapine • Seroquel
Ramelteon • Rozerem
Suvorexant • Belsomra
Temazepam • Restoril
Theophylline • Elixophyllin
Tiagabine • Gabitril
Trazadone • Desyrel
Triazolam • Halcion
Zaleplon • Sonata
Zolpidem • Ambien
1. Centers for Disease Control and Prevention. The state of aging and health in America. 2013. Accessed January 27, 2021. https://www.cdc.gov/aging/pdf/state-aging-health-in-america-2013.pdf
2. Suzuki K, Miyamoto M, Hirata K. Sleep disorders in the elderly: diagnosis and management. J Gen Fam Med. 2017;18(2):61-71.
3. Inouye SK, Studenski S, Tinetti ME, et al. Geriatric syndromes: clinical, research, and policy implications of a core geriatric concept. J Am Geriatr Soc. 2007;55(5):780-791.
4. Patel D, Steinberg J, Patel P. Insomnia in the elderly: a review. J Clin Sleep Med. 2018;14(6):1017-1024.
5. Neubauer DN. A review of ramelteon in the treatment of sleep disorders. Neuropsychiatr Dis Treat. 2008;4(1):69-79.
6. Mander BA, Winer JR, Walker MP. Sleep and human aging. Neuron. 2017;94(1):19-36.
7. Ohayon MM, Carskadon MA, Guilleminault C, et al. Meta-analysis of quantitative sleep parameters from childhood to old age in healthy individuals: developing normative sleep values across the human lifespan. Sleep. 2004;27:1255-1273.
8. Li J, Vitiello MV, Gooneratne NS. Sleep in normal aging. Sleep Med Clin. 2018;13(1):1-11.
9. Floyd JA, Medler SM, Ager JW, et al. Age-related changes in initiation and maintenance of sleep: a meta-analysis. Res Nurs Health. 2000;23(2):106-117.
10. Floyd JA, Janisse JJ, Jenuwine ES, et al. Changes in REM-sleep percentage over the adult lifespan. Sleep. 2007;30(7):829-836.
11. Dorffner G, Vitr M, Anderer P. The effects of aging on sleep architecture in healthy subjects. Adv Exp Med Biol. 2015;821:93-100.
12. Furihata R, Kaneita Y, Jike M, et al. Napping and associated factors: a Japanese nationwide general population survey. Sleep Med. 2016;20:72-79.
13. Foley DJ, Vitiello MV, Bliwise DL, et al. Frequent napping is associated with excessive daytime sleepiness, depression, pain, and nocturia in older adults: findings from the National Sleep Foundation ‘2003 Sleep in America’ Poll. Am J Geriatr Psychiatry. 2007;15(4):344-350.
14. Floyd JA, Janisse JJ, Marshall Medler S, et al. Nonlinear components of age-related change in sleep initiation. Nurs Res. 2000;49(5):290-294.
15. Miner B, Kryger MH. Sleep in the aging population. Sleep Med Clin. 2017;12(1):31-38.
16. Young T, Peppard PE, Gottlieb DJ. Epidemiology of obstructive sleep apnea: a population health perspective. Am J Respir Crit Care Med. 2002;165(9):1217-1239.
17. Ancoli-Israel S, Klauber MR, Butters N, et al. Dementia in institutionalized elderly: relation to sleep apnea. J Am Geriatr Soc. 1991;39(3):258-263.
18. Spira AP, Stone KL, Rebok GW, et al. Sleep-disordered breathing and functional decline in older women. J Am Geriatr Soc. 2014;62(11):2040-2046.
19. Vijayan VK. Morbidities associated with obstructive sleep apnea. Expert Rev Respir Med. 2012;6(5):557-566.
20. Kerner NA, Roose SP. Obstructive sleep apnea is linked to depression and cognitive impairment: evidence and potential mechanisms. Am J Geriatr Psychiatry. 2016;24(6):496-508.
21. Dalmases M, Solé-Padullés C, Torres M, et al. Effect of CPAP on cognition, brain function, and structure among elderly patients with OSA: a randomized pilot study. Chest. 2015;148(5):1214-1223.
22. Toronto Western Hospital, University Health Network. University of Toronto. STOP-Bang Questionnaire. 2012. Accessed January 26, 2021. www.stopbang.ca
23. Freedman N. Doing it better for less: incorporating OSA management into alternative payment models. Chest. 2019;155(1):227-233.
24. Kapur VK, Auckley DH, Chowdhuri S, et al. Clinical practice guideline for diagnostic testing for adult obstructive sleep apnea: an American Academy of Sleep Medicine clinical practice guideline. J Clin Sleep Med. 2017;13(3):479-504.
25. Semelka M, Wilson J, Floyd R. Diagnosis and treatment of obstructive sleep apnea in adults. Am Fam Physician. 2016;94(5):355-360.
26. Javaheri S, Dempsey JA. Central sleep apnea. Compr Physiol. 2013;3(1):141-163.
27. Donovan LM, Kapur VK. Prevalence and characteristics of central compared to obstructive sleep apnea: analyses from the Sleep Heart Health Study cohort. Sleep. 2016;39(7):1353-1359.
28. Cao M, Cardell CY, Willes L, et al. A novel adaptive servoventilation (ASVAuto) for the treatment of central sleep apnea associated with chronic use of opioids. J Clin Sleep Med. 2014;10(8):855-861.
29. Oldenburg O, Spießhöfer J, Fox H, et al. Performance of conventional and enhanced adaptive servoventilation (ASV) in heart failure patients with central sleep apnea who have adapted to conventional ASV. Sleep Breath. 2015;19(3):795-800.
30. Costanzo MR, Ponikowski P, Javaheri S, et al. Transvenous neurostimulation for central sleep apnoea: a randomised controlled trial. Lancet. 2016;388(10048):974-982.
31. Diagnostic and statistical manual of mental disorders, 5th ed. American Psychiatric Association; 2013:362.
32. Perlis ML, Smith LJ, Lyness JM, et al. Insomnia as a risk factor for onset of depression in the elderly. Behav Sleep Med. 2006;4(2):104-113.
33. Cole MG, Dendukuri N. Risk factors for depression among elderly community subjects: a systematic review and meta-analysis. Am J Psychiatry. 2003;160(6):1147-1156.
34. Pigeon WR, Hegel M, Unützer J, et al. Is insomnia a perpetuating factor for late-life depression in the IMPACT cohort? Sleep. 2008;31(4):481-488.
35. Hughes JM, Song Y, Fung CH, et al. Measuring sleep in vulnerable older adults: a comparison of subjective and objective sleep measures. Clin Gerontol. 2018;41(2):145-157.
36. Irish LA, Kline CE, Gunn HE, et al. The role of sleep hygiene in promoting public health: a review of empirical evidence. Sleep Med Rev. 2015;22:23-36.
37. Sleep Foundation. Sleep hygiene. Accessed January 27, 2021. https://www.sleepfoundation.org/articles/sleep-hygiene
38. Foley D, Ancoli-Israel S, Britz P, et al. Sleep disturbances and chronic disease in older adults: results of the 2003 National Sleep Foundation Sleep in America Survey. J Psychosom Res. 2004;56(5):497-502.
39. Eslami V, Zimmerman ME, Grewal T, et al. Pain grade and sleep disturbance in older adults: evaluation the role of pain, and stress for depressed and non-depressed individuals. Int J Geriatr Psychiatry. 2016;31(5):450-457.
40. American Geriatrics Society Beers Criteria Update Expert Panel. American Geriatrics Society 2015 updated Beers Criteria for potentially inappropriate medication use in older adults. J Am Geriatr Soc. 2015;63(11):2227-2246.
41. United States Food & Drug Administration. FDA adds Boxed Warning for risk of serious injuries caused by sleepwalking with certain prescription insomnia medicines. 2019. Accessed January 27, 2021. https://www.fda.gov/drugs/drug-safety-and-availability/fda-adds-boxed-warning-risk-serious-injuries-caused-sleepwalking-certain-prescription-insomnia
42. Schroeck JL, Ford J, Conway EL, et al. Review of safety and efficacy of sleep medicines in older adults. Clin Ther. 2016;38(11):2340-2372.
43. Krystal AD, Lankford A, Durrence HH, et al. Efficacy and safety of doxepin 3 and 6 mg in a 35-day sleep laboratory trial in adults with chronic primary insomnia. Sleep. 2011;34(10):1433-1442.
44. Roth T, Seiden D, Sainati S, et al. Effects of ramelteon on patient-reported sleep latency in older adults with chronic insomnia. Sleep Med. 2006;7(4):312-318.
45. Zammit G, Wang-Weigand S, Rosenthal M, et al. Effect of ramelteon on middle-of-the-night balance in older adults with chronic insomnia. J Clin Sleep Med. 2009;5(1):34-40.
46. Mets MAJ, de Vries JM, de Senerpont Domis LM, et al. Next-day effects of ramelteon (8 mg), zopiclone (7.5 mg), and placebo on highway driving performance, memory functioning, psychomotor performance, and mood in healthy adult subjects. Sleep. 2011;34(10):1327-1334.
47. Rhyne DN, Anderson SL. Suvorexant in insomnia: efficacy, safety and place in therapy. Ther Adv Drug Saf. 2015;6(5):189-195.
48. Norman JL, Anderson SL. Novel class of medications, orexin receptor antagonists, in the treatment of insomnia - critical appraisal of suvorexant. Nat Sci Sleep. 2016;8:239-247.
49. Owen RT. Suvorexant: efficacy and safety profile of a dual orexin receptor antagonist in treating insomnia. Drugs Today (Barc). 2016;52(1):29-40.
50. Shannon S, Lewis N, Lee H, et al. Cannabidiol in anxiety and sleep: a large case series. Perm J. 2019;23:18-041. doi: 10.7812/TPP/18-041
51. Yunusa I, Alsumali A, Garba AE, et al. Assessment of reported comparative effectiveness and safety of atypical antipsychotics in the treatment of behavioral and psychological symptoms of dementia: a network meta-analysis. JAMA Netw Open. 2019;2(3):e190828.
52. Bjorvatn B, Gronli J, Pallesen S. Prevalence of different parasomnias in the general population. Sleep Med. 2010;11(10):1031-1034.
53. Postuma RB, Montplaisir JY, Pelletier A, et al. Environmental risk factors for REM sleep behavior disorder: a multicenter case-control study. Neurology. 2012;79(5):428-434.
54. Fleetham JA, Fleming JA. Parasomnias. CMAJ. 2014;186(8):E273-E280.
55. Dinis-Oliveira RJ, Caldas I, Carvalho F, et al. Bruxism after 3,4-methylenedioxymethamphetamine (ecstasy) abuse. Clin Toxicol (Phila.) 2010;48(8):863-864.
56. Irfan MH, Howell MJ. Rapid eye movement sleep behavior disorder: overview and current perspective. Curr Sleep Medicine Rep. 2016;2:64-73.
57. Mahlknecht P, Seppi K, Frauscher B, et al. Probable RBD and association with neurodegenerative disease markers: a population-based study. Mov Disord. 2015;30(10):1417-1421.
58. Oertel WH, Depboylu C, Krenzer M, et al. [REM sleep behavior disorder as a prodromal stage of α-synucleinopathies: symptoms, epidemiology, pathophysiology, diagnosis and therapy]. Nervenarzt. 2014;85:19-25. German.
59. Jozwiak N, Postuma RB, Montplaisir J, et al. REM sleep behavior disorder and cognitive impairment in Parkinson’s disease. Sleep. 2017;40(8):zsx101. doi: 10.1093/sleep/zsx101
60. Claassen DO, Josephs KA, Ahlskog JE, et al. REM sleep behavior disorder preceding other aspects of synucleinopathies by up to half a century. Neurology 2010;75(6):494-499.
61. Reynolds K, Pietrzak RH, El-Gabalawy R, et al. Prevalence of psychiatric disorders in U.S. older adults: findings from a nationally representative survey. World Psychiatry. 2015;14(1):74-81.
62. Lohman MC, Mezuk B, Dumenci L. Depression and frailty: concurrent risks for adverse health outcomes. Aging Ment Health. 2017;21(4):399-408.
63. Zhao QF, Tan L, Wang HF, et al. The prevalence of neuropsychiatric symptoms in Alzheimer’s disease: systematic review and meta-analysis. J Affect Disord. 2016;190:264-271.
64. Furihata R, Hall MH, Stone KL, et al. An aggregate measure of sleep health is associated with prevalent and incident clinically significant depression symptoms among community-dwelling older women. Sleep. 2017;40(3):zsw075. doi: 10.1093/sleep/zsw075
65. Spira AP, Stone K, Beaudreau SA, et al. Anxiety symptoms and objectively measured sleep quality in older women. Am J Geriatr Psychiatry. 2009;17(2):136-143.
66. Press Y, Punchik B, Freud T. The association between subjectively impaired sleep and symptoms of depression and anxiety in a frail elderly population. Aging Clin Exp Res. 2018;30(7):755-765.
67. Gould CE, Spira AP, Liou-Johnson V, et al. Association of anxiety symptom clusters with sleep quality and daytime sleepiness. J Gerontol B Psychol Sci Soc Sci. 2018;73(3):413-420.
68. Kassem AM, Ganguli M, Yaffe K, et al. Anxiety symptoms and risk of cognitive decline in older community-dwelling men. Int Psychogeriatr. 2017;29(7):1137-1145.
69. Frank C. Pharmacologic treatment of depression in the elderly. Can Fam Physician. 2014;60(2):121-126.
70. Subramanyam AA, Kedare J, Singh OP, et al. Clinical practice guidelines for geriatric anxiety disorders. Indian J Psychiatry. 2018;60(suppl 3):S371-S382.
71. Emsley R, Ahokas A, Suarez A, et al. Efficacy of tianeptine 25-50 mg in elderly patients with recurrent major depressive disorder: an 8-week placebo- and escitalopram-controlled study. J Clin Psychiatry. 2018;79(4):17m11741. doi: 10.4088/JCP.17m11741
72. Semel D, Murphy TK, Zlateva G, et al. Evaluation of the safety and efficacy of pregabalin in older patients with neuropathic pain: results from a pooled analysis of 11 clinical studies. BMC Fam Pract. 2010;11:85.
73. Orgeta V, Qazi A, Spector A, et al. Psychological treatments for depression and anxiety in dementia and mild cognitive impairment: systematic review and meta-analysis. Br J Psychiatry. 2015;207(4):293-298.
74. Morimoto SS, Kanellopoulos D, Manning KJ, et al. Diagnosis and treatment of depression and cognitive impairment in late life. Ann N Y Acad Sci. 2015;1345(1):36-46.
75. Casey DA. Depression in older adults: a treatable medical condition. Prim Care. 2017;44(3):499-510.
1. Centers for Disease Control and Prevention. The state of aging and health in America. 2013. Accessed January 27, 2021. https://www.cdc.gov/aging/pdf/state-aging-health-in-america-2013.pdf
2. Suzuki K, Miyamoto M, Hirata K. Sleep disorders in the elderly: diagnosis and management. J Gen Fam Med. 2017;18(2):61-71.
3. Inouye SK, Studenski S, Tinetti ME, et al. Geriatric syndromes: clinical, research, and policy implications of a core geriatric concept. J Am Geriatr Soc. 2007;55(5):780-791.
4. Patel D, Steinberg J, Patel P. Insomnia in the elderly: a review. J Clin Sleep Med. 2018;14(6):1017-1024.
5. Neubauer DN. A review of ramelteon in the treatment of sleep disorders. Neuropsychiatr Dis Treat. 2008;4(1):69-79.
6. Mander BA, Winer JR, Walker MP. Sleep and human aging. Neuron. 2017;94(1):19-36.
7. Ohayon MM, Carskadon MA, Guilleminault C, et al. Meta-analysis of quantitative sleep parameters from childhood to old age in healthy individuals: developing normative sleep values across the human lifespan. Sleep. 2004;27:1255-1273.
8. Li J, Vitiello MV, Gooneratne NS. Sleep in normal aging. Sleep Med Clin. 2018;13(1):1-11.
9. Floyd JA, Medler SM, Ager JW, et al. Age-related changes in initiation and maintenance of sleep: a meta-analysis. Res Nurs Health. 2000;23(2):106-117.
10. Floyd JA, Janisse JJ, Jenuwine ES, et al. Changes in REM-sleep percentage over the adult lifespan. Sleep. 2007;30(7):829-836.
11. Dorffner G, Vitr M, Anderer P. The effects of aging on sleep architecture in healthy subjects. Adv Exp Med Biol. 2015;821:93-100.
12. Furihata R, Kaneita Y, Jike M, et al. Napping and associated factors: a Japanese nationwide general population survey. Sleep Med. 2016;20:72-79.
13. Foley DJ, Vitiello MV, Bliwise DL, et al. Frequent napping is associated with excessive daytime sleepiness, depression, pain, and nocturia in older adults: findings from the National Sleep Foundation ‘2003 Sleep in America’ Poll. Am J Geriatr Psychiatry. 2007;15(4):344-350.
14. Floyd JA, Janisse JJ, Marshall Medler S, et al. Nonlinear components of age-related change in sleep initiation. Nurs Res. 2000;49(5):290-294.
15. Miner B, Kryger MH. Sleep in the aging population. Sleep Med Clin. 2017;12(1):31-38.
16. Young T, Peppard PE, Gottlieb DJ. Epidemiology of obstructive sleep apnea: a population health perspective. Am J Respir Crit Care Med. 2002;165(9):1217-1239.
17. Ancoli-Israel S, Klauber MR, Butters N, et al. Dementia in institutionalized elderly: relation to sleep apnea. J Am Geriatr Soc. 1991;39(3):258-263.
18. Spira AP, Stone KL, Rebok GW, et al. Sleep-disordered breathing and functional decline in older women. J Am Geriatr Soc. 2014;62(11):2040-2046.
19. Vijayan VK. Morbidities associated with obstructive sleep apnea. Expert Rev Respir Med. 2012;6(5):557-566.
20. Kerner NA, Roose SP. Obstructive sleep apnea is linked to depression and cognitive impairment: evidence and potential mechanisms. Am J Geriatr Psychiatry. 2016;24(6):496-508.
21. Dalmases M, Solé-Padullés C, Torres M, et al. Effect of CPAP on cognition, brain function, and structure among elderly patients with OSA: a randomized pilot study. Chest. 2015;148(5):1214-1223.
22. Toronto Western Hospital, University Health Network. University of Toronto. STOP-Bang Questionnaire. 2012. Accessed January 26, 2021. www.stopbang.ca
23. Freedman N. Doing it better for less: incorporating OSA management into alternative payment models. Chest. 2019;155(1):227-233.
24. Kapur VK, Auckley DH, Chowdhuri S, et al. Clinical practice guideline for diagnostic testing for adult obstructive sleep apnea: an American Academy of Sleep Medicine clinical practice guideline. J Clin Sleep Med. 2017;13(3):479-504.
25. Semelka M, Wilson J, Floyd R. Diagnosis and treatment of obstructive sleep apnea in adults. Am Fam Physician. 2016;94(5):355-360.
26. Javaheri S, Dempsey JA. Central sleep apnea. Compr Physiol. 2013;3(1):141-163.
27. Donovan LM, Kapur VK. Prevalence and characteristics of central compared to obstructive sleep apnea: analyses from the Sleep Heart Health Study cohort. Sleep. 2016;39(7):1353-1359.
28. Cao M, Cardell CY, Willes L, et al. A novel adaptive servoventilation (ASVAuto) for the treatment of central sleep apnea associated with chronic use of opioids. J Clin Sleep Med. 2014;10(8):855-861.
29. Oldenburg O, Spießhöfer J, Fox H, et al. Performance of conventional and enhanced adaptive servoventilation (ASV) in heart failure patients with central sleep apnea who have adapted to conventional ASV. Sleep Breath. 2015;19(3):795-800.
30. Costanzo MR, Ponikowski P, Javaheri S, et al. Transvenous neurostimulation for central sleep apnoea: a randomised controlled trial. Lancet. 2016;388(10048):974-982.
31. Diagnostic and statistical manual of mental disorders, 5th ed. American Psychiatric Association; 2013:362.
32. Perlis ML, Smith LJ, Lyness JM, et al. Insomnia as a risk factor for onset of depression in the elderly. Behav Sleep Med. 2006;4(2):104-113.
33. Cole MG, Dendukuri N. Risk factors for depression among elderly community subjects: a systematic review and meta-analysis. Am J Psychiatry. 2003;160(6):1147-1156.
34. Pigeon WR, Hegel M, Unützer J, et al. Is insomnia a perpetuating factor for late-life depression in the IMPACT cohort? Sleep. 2008;31(4):481-488.
35. Hughes JM, Song Y, Fung CH, et al. Measuring sleep in vulnerable older adults: a comparison of subjective and objective sleep measures. Clin Gerontol. 2018;41(2):145-157.
36. Irish LA, Kline CE, Gunn HE, et al. The role of sleep hygiene in promoting public health: a review of empirical evidence. Sleep Med Rev. 2015;22:23-36.
37. Sleep Foundation. Sleep hygiene. Accessed January 27, 2021. https://www.sleepfoundation.org/articles/sleep-hygiene
38. Foley D, Ancoli-Israel S, Britz P, et al. Sleep disturbances and chronic disease in older adults: results of the 2003 National Sleep Foundation Sleep in America Survey. J Psychosom Res. 2004;56(5):497-502.
39. Eslami V, Zimmerman ME, Grewal T, et al. Pain grade and sleep disturbance in older adults: evaluation the role of pain, and stress for depressed and non-depressed individuals. Int J Geriatr Psychiatry. 2016;31(5):450-457.
40. American Geriatrics Society Beers Criteria Update Expert Panel. American Geriatrics Society 2015 updated Beers Criteria for potentially inappropriate medication use in older adults. J Am Geriatr Soc. 2015;63(11):2227-2246.
41. United States Food & Drug Administration. FDA adds Boxed Warning for risk of serious injuries caused by sleepwalking with certain prescription insomnia medicines. 2019. Accessed January 27, 2021. https://www.fda.gov/drugs/drug-safety-and-availability/fda-adds-boxed-warning-risk-serious-injuries-caused-sleepwalking-certain-prescription-insomnia
42. Schroeck JL, Ford J, Conway EL, et al. Review of safety and efficacy of sleep medicines in older adults. Clin Ther. 2016;38(11):2340-2372.
43. Krystal AD, Lankford A, Durrence HH, et al. Efficacy and safety of doxepin 3 and 6 mg in a 35-day sleep laboratory trial in adults with chronic primary insomnia. Sleep. 2011;34(10):1433-1442.
44. Roth T, Seiden D, Sainati S, et al. Effects of ramelteon on patient-reported sleep latency in older adults with chronic insomnia. Sleep Med. 2006;7(4):312-318.
45. Zammit G, Wang-Weigand S, Rosenthal M, et al. Effect of ramelteon on middle-of-the-night balance in older adults with chronic insomnia. J Clin Sleep Med. 2009;5(1):34-40.
46. Mets MAJ, de Vries JM, de Senerpont Domis LM, et al. Next-day effects of ramelteon (8 mg), zopiclone (7.5 mg), and placebo on highway driving performance, memory functioning, psychomotor performance, and mood in healthy adult subjects. Sleep. 2011;34(10):1327-1334.
47. Rhyne DN, Anderson SL. Suvorexant in insomnia: efficacy, safety and place in therapy. Ther Adv Drug Saf. 2015;6(5):189-195.
48. Norman JL, Anderson SL. Novel class of medications, orexin receptor antagonists, in the treatment of insomnia - critical appraisal of suvorexant. Nat Sci Sleep. 2016;8:239-247.
49. Owen RT. Suvorexant: efficacy and safety profile of a dual orexin receptor antagonist in treating insomnia. Drugs Today (Barc). 2016;52(1):29-40.
50. Shannon S, Lewis N, Lee H, et al. Cannabidiol in anxiety and sleep: a large case series. Perm J. 2019;23:18-041. doi: 10.7812/TPP/18-041
51. Yunusa I, Alsumali A, Garba AE, et al. Assessment of reported comparative effectiveness and safety of atypical antipsychotics in the treatment of behavioral and psychological symptoms of dementia: a network meta-analysis. JAMA Netw Open. 2019;2(3):e190828.
52. Bjorvatn B, Gronli J, Pallesen S. Prevalence of different parasomnias in the general population. Sleep Med. 2010;11(10):1031-1034.
53. Postuma RB, Montplaisir JY, Pelletier A, et al. Environmental risk factors for REM sleep behavior disorder: a multicenter case-control study. Neurology. 2012;79(5):428-434.
54. Fleetham JA, Fleming JA. Parasomnias. CMAJ. 2014;186(8):E273-E280.
55. Dinis-Oliveira RJ, Caldas I, Carvalho F, et al. Bruxism after 3,4-methylenedioxymethamphetamine (ecstasy) abuse. Clin Toxicol (Phila.) 2010;48(8):863-864.
56. Irfan MH, Howell MJ. Rapid eye movement sleep behavior disorder: overview and current perspective. Curr Sleep Medicine Rep. 2016;2:64-73.
57. Mahlknecht P, Seppi K, Frauscher B, et al. Probable RBD and association with neurodegenerative disease markers: a population-based study. Mov Disord. 2015;30(10):1417-1421.
58. Oertel WH, Depboylu C, Krenzer M, et al. [REM sleep behavior disorder as a prodromal stage of α-synucleinopathies: symptoms, epidemiology, pathophysiology, diagnosis and therapy]. Nervenarzt. 2014;85:19-25. German.
59. Jozwiak N, Postuma RB, Montplaisir J, et al. REM sleep behavior disorder and cognitive impairment in Parkinson’s disease. Sleep. 2017;40(8):zsx101. doi: 10.1093/sleep/zsx101
60. Claassen DO, Josephs KA, Ahlskog JE, et al. REM sleep behavior disorder preceding other aspects of synucleinopathies by up to half a century. Neurology 2010;75(6):494-499.
61. Reynolds K, Pietrzak RH, El-Gabalawy R, et al. Prevalence of psychiatric disorders in U.S. older adults: findings from a nationally representative survey. World Psychiatry. 2015;14(1):74-81.
62. Lohman MC, Mezuk B, Dumenci L. Depression and frailty: concurrent risks for adverse health outcomes. Aging Ment Health. 2017;21(4):399-408.
63. Zhao QF, Tan L, Wang HF, et al. The prevalence of neuropsychiatric symptoms in Alzheimer’s disease: systematic review and meta-analysis. J Affect Disord. 2016;190:264-271.
64. Furihata R, Hall MH, Stone KL, et al. An aggregate measure of sleep health is associated with prevalent and incident clinically significant depression symptoms among community-dwelling older women. Sleep. 2017;40(3):zsw075. doi: 10.1093/sleep/zsw075
65. Spira AP, Stone K, Beaudreau SA, et al. Anxiety symptoms and objectively measured sleep quality in older women. Am J Geriatr Psychiatry. 2009;17(2):136-143.
66. Press Y, Punchik B, Freud T. The association between subjectively impaired sleep and symptoms of depression and anxiety in a frail elderly population. Aging Clin Exp Res. 2018;30(7):755-765.
67. Gould CE, Spira AP, Liou-Johnson V, et al. Association of anxiety symptom clusters with sleep quality and daytime sleepiness. J Gerontol B Psychol Sci Soc Sci. 2018;73(3):413-420.
68. Kassem AM, Ganguli M, Yaffe K, et al. Anxiety symptoms and risk of cognitive decline in older community-dwelling men. Int Psychogeriatr. 2017;29(7):1137-1145.
69. Frank C. Pharmacologic treatment of depression in the elderly. Can Fam Physician. 2014;60(2):121-126.
70. Subramanyam AA, Kedare J, Singh OP, et al. Clinical practice guidelines for geriatric anxiety disorders. Indian J Psychiatry. 2018;60(suppl 3):S371-S382.
71. Emsley R, Ahokas A, Suarez A, et al. Efficacy of tianeptine 25-50 mg in elderly patients with recurrent major depressive disorder: an 8-week placebo- and escitalopram-controlled study. J Clin Psychiatry. 2018;79(4):17m11741. doi: 10.4088/JCP.17m11741
72. Semel D, Murphy TK, Zlateva G, et al. Evaluation of the safety and efficacy of pregabalin in older patients with neuropathic pain: results from a pooled analysis of 11 clinical studies. BMC Fam Pract. 2010;11:85.
73. Orgeta V, Qazi A, Spector A, et al. Psychological treatments for depression and anxiety in dementia and mild cognitive impairment: systematic review and meta-analysis. Br J Psychiatry. 2015;207(4):293-298.
74. Morimoto SS, Kanellopoulos D, Manning KJ, et al. Diagnosis and treatment of depression and cognitive impairment in late life. Ann N Y Acad Sci. 2015;1345(1):36-46.
75. Casey DA. Depression in older adults: a treatable medical condition. Prim Care. 2017;44(3):499-510.
COVID-19 and the risk of homicide-suicide among older adults
On March 25, 2020, in Cambridge, United Kingdom, a 71-year-old man stabbed his 71-year-old wife before suffocating himself to death. The couple was reportedly anxious about the coronavirus disease 2019 (COVID-19) pandemic lockdown measures and were on the verge of running out of food and medicine.1
One week later, in Chicago, Illinois, a 54-year-old man shot and killed his female partner, age 54, before killing himself. The couple was tested for COVID-19 2 days earlier and the man believed they had contracted the virus; however, the test results for both of them had come back negative.2
Intimate partner homicide-suicide is the most dramatic domestic abuse outcome.3 Homicide-suicide is defined as “homicide committed by a person who subsequently commits suicide within one week of the homicide. In most cases the subsequent suicide occurs within a 24-hour period.”4 Approximately one-quarter of all homicide-suicides are committed by persons age ≥55 years.5,6 We believe that during the COVID-19 pandemic, the risk of homicide-suicide among older adults may be increased due to several factors, including:
- physical distancing and quarantine measures. Protocols established to slow the spread of the virus may be associated with increased rates of depression and anxiety7 and an increased risk of suicide among older adults8
- increased intimate partner violence9
- increased firearm ownership rates in the United States.10
In this article, we review studies that identified risk factors for homicide-suicide among older adults, discuss the impact the COVID-19 pandemic has had on these risks, and describe steps clinicians can take to intervene.
A review of the literature
To better characterize the perpetrators of older adult homicide-suicide, we conducted a literature search of relevant terms. We identified 9 original research publications that examined homicide-suicide in older adults.
Bourget et al11 (2010) reviewed coroners’ charts of individuals killed by an older (age ≥65) spouse or family member from 1992 through 2007 in Quebec, Canada. They identified 19 cases of homicide-suicide, 17 (90%) of which were perpetrated by men. Perpetrators and victims were married (63%), in common-law relationships (16%), or separated/divorced (16%). A history of domestic violence was documented in 4 (21%) cases. The authors found that 13 of 15 perpetrators (87%) had “major depression” and 2 perpetrators had a psychotic disorder. Substance use at the time of the event was confirmed in 6 (32%) cases. Firearms and strangulation were the top methods used to carry out the homicide-suicide.11
Cheung et al12 (2016) conducted a review of coroners’ records of homicide-suicide cases among individuals age ≥65 in New Zealand from 2007 through 2012. In all 4 cases, the perpetrators were men, and their victims were predominantly female, live-in family members. Two cases involved men with a history of domestic violence who were undergoing significant changes in their home and social lives. Both men had a history suggestive of depression and used a firearm to carry out the homicide-suicide.12
Continue to: Cohen et al
Cohen et al13 (1998) conducted a review of coroners’ records from 1988 through 1994 in 2 regions in Florida. They found 48 intimate partner homicide-suicide cases among “old couples” (age ≥55). All were perpetrated by men. The authors identified sociocultural differences in risk factors between the 2 regions. In west-central Florida, perpetrators and victims were predominantly white and in a spousal relationship. Domestic violence was documented in <4% of cases. Approximately 55% of the couples were reported to be ill, and a substantial proportion were documented to be declining in health. One-quarter of the perpetrators and one-third of the victims had “pain and suffering.” More than one-third of perpetrators were reported to have “depression,” 15% were reported to have talked about suicide, and 4% had a history of a suicide attempt. Only 11% of perpetrators were described as abusing substances.
The authors noted several differences in cases in southeastern Florida. Approximately two-thirds of the couples were Hispanic, and 14% had a history of domestic violence. A minority of the couples were in a live-in relationship. Less than 15% of the perpetrators and victims were described as having a decline in health. Additionally, only 19% of perpetrators were reported to have “depression,” and none of the perpetrators had a documented history of attempted suicide or substance abuse. No information was provided regarding the methods used to carry out the homicide-suicide in the southeastern region.13 Financial stress was not a factor in either region.
Malphurs et al14 (2001) used the same database described in the Cohen et al13 study to compare 27 perpetrators of homicide-suicide to 36 age-matched suicide decedents in west central Florida. They found that homicide-suicide perpetrators were significantly less likely to have health problems and were 3 times more likely to be caregivers to their spouses. Approximately 52% of perpetrators had at least 1 documented psychiatric symptom (“depression” and/or substance abuse or other), but only 5% were seeking mental health services at the time of death.14
De Koning and Piette15 (2014) conducted a retrospective medicolegal chart review from 1935 to 2010 to identify homicide-suicide cases in West and East Flanders, Belgium. They found 19 cases of intimate partner homicide-suicide committed by offenders age ≥55 years. Ninety-five percent of the perpetrators were men who killed their female partners. In one-quarter of the cases, either the perpetrator or the victim had a health issue; 21% of the perpetrators were documented as having depression and 27% had alcohol intoxication at the time of death. A motive was documented in 14 out of 19 cases; “mercy killing” was determined as the motive in 6 (43%) cases and “amorous jealousy” in 5 cases (36%).15 Starting in the 1970s, firearms were the most prevalent method used to kill a partner.
Logan et al16 (2019) used data from the National Violent Death Reporting System between 2003 and 2015 to identify characteristics that differentiated male suicide decedents from male perpetrators of intimate partner homicide-suicide. They found that men age 50 to 64 years were 3 times more likely than men age 18 to 34 years to commit intimate partner homicide-suicide, and that men age ≥65 years were approximately 5 times more likely than men age 18 to 34 years to commit intimate partner homicide-suicide. The authors found that approximately 22% of all perpetrators had a documented history of physical domestic violence, and close to 17% had a prior interaction with the criminal justice system. Furthermore, one-third of perpetrators had relationship difficulties and were in the process of a breakup. Health issues were prevalent in 34% of the victims and 26% of the perpetrators. Perpetrator-caregiver burden was reported as a contributing factor for homicide-suicide in 16% of cases. In 27% of cases, multiple health-related contributing factors were mentioned.16
Continue to: Malphurs and Cohen
Malphurs and Cohen5 (2002) reviewed American newspapers from 1997 through 1999 and identified 673 homicide-suicide events, of which 152 (27%) were committed by individuals age ≥55 years. The victims and perpetrators (95% of which were men) were intimate partners in three-quarters of cases. In nearly one-third of cases, caregiving was a contributing factor for the homicide-suicide. A history of or a pending divorce was reported in nearly 14% of cases. Substance use history was rarely recorded. Firearms were used in 88% of the homicide-suicide cases.5
Malphurs and Cohen17 (2005) reviewed coroner records between 1998 and 1999 in Florida and compared 20 cases of intimate partner homicide-suicide involving perpetrators age ≥55 years with matched suicide decedents. They found that 60% of homicide-suicide perpetrators had documented health issues. The authors reported that a “recent change in health status” was more prevalent among perpetrators compared with decedents. Perpetrators were also more likely to be caregivers to their spouses. The authors found that 65% of perpetrators were reported to have a “depressed mood” and 15% of perpetrators had reportedly threatened suicide prior to the incident. However, none of the perpetrators tested positive for antidepressants as documented on post-mortem toxicology reports. Firearms were used in 100% of homicide-suicide cases.17
Salari3 (2007) reviewed multiple American media sources and published police reports between 1999 and 2005 to retrieve data about intimate partner homicide-suicide events in the United States. There were 225 events identified where the perpetrator and/or the victim were age ≥60 years. Ninety-six percent of the perpetrators were men and most homicide-suicide events were committed at the home. A history of domestic violence was reported in 14% of homicide-suicide cases. Thirteen percent of couples were separated or divorced. The perpetrator and/or victim had health issues in 124 (55%) events. Dementia was reported in 7.5% of cases, but overwhelmingly among the victims. Substance abuse was rarely mentioned as a contributing factor. In three-quarters of cases where a motive was described, the perpetrator was “suicidal”; however, a “suicide pact” was mentioned in only 4% of cases. Firearms were used in 87% of cases.3
Focus on common risk factors
The scarcity and heterogeneity of research regarding older adult homicide-suicide were major limitations to our review. Because most of the studies we identified had a small sample size and limited information regarding the mental health of victims and perpetrators, it would be an overreach to claim to have identified a typical profile of an older perpetrator of homicide-suicide. However, the literature has repeatedly identified several common characteristics of such perpetrators. They are significantly more likely to be men who use firearms to murder their intimate partners and then die by suicide in their home (Table3,5,11-17). Health issues afflicting 1 or both individuals in the couple appear to be a contributing factor, particularly when the perpetrator is in a caregiving role. Relational discord, with or without a history of domestic violence, increases the risk of homicide-suicide. Finally, older perpetrators are highly likely to be depressed and have suicidal ideations.
How COVID-19 affects these risks
Although it is too early to determine if there is a causal relationship between the COVID-19 pandemic and an increase in homicide-suicide, the pandemic is likely to promote risk factors for these events, especially among older adults. Confinement measures put into place during the pandemic context have already been shown to increase rates of domestic violence18 and depression and anxiety among older individuals.7 Furthermore,
Continue to: Late-life psychiatric disorders
Late-life psychiatric disorders
Early recognition and effective treatment of late-life psychiatric disorders is crucial. Unfortunately, depression in geriatric patients is often underdiagnosed and undertreated.20 Older adults have more frequent contact with their primary care physicians, and rarely consult mental health professionals.21,22 Several models of integrated depression care within primary care settings have shown the positive impact of this collaborative approach in treating late-life depression and preventing suicide in older individuals.23 Additionally, because alcohol abuse is also a risk factor for domestic violence and breaking the law in this population,24,25 older adults should be screened for alcohol use disorders, and referred to treatment when necessary.
Take steps to keep patients safe
In the context of the COVID-19 pandemic, there are several steps clinicians need to keep in mind when interacting with older patients:
- Screen for depressive symptoms, suicidality, and alcohol and substance use disorders. Individuals who have tested positive for COVID-19 or who have been in contact with a carrier are a particularly vulnerable population.
- Screen for domestic violence and access to weapons at home.4 Any older adult who has a psychiatric disorder and/or suicide ideation should receive immediate intervention through a social worker that includes providing gun-risk education to other family members or contacting law-enforcement officials.26
- Refer patients with a suspected psychiatric disorder to specialized mental health clinicians. Telemental health services can provide rapid access to subspecialists, allowing patients to be treated from their homes.27 These services need to be promoted among older adults during this critical period and reimbursed by public and private insurance systems to ensure accessibility and affordability.28
- Create psychiatric inpatient units specifically designed for suicidal and/or homicidal patients with COVID-19.
Additionally, informing the public about these major health issues is crucial. The media can raise awareness about domestic violence and depression among older adults; however, this should be done responsibly and with accuracy to prevent the spread of misinformation, confusion, fear, and panic.29
Bottom Line
The mental health burden of the coronavirus disease 2019 pandemic has significantly impacted individuals who are older and most vulnerable. Reducing the incidence of homicide-suicide among older adults requires timely screening and interventions by primary care providers, mental health specialists, social workers, media, and governmental agencies.
Related Resources
- Saeed SA, Hebishi K. The psychiatric consequences of COVID-19: 8 studies. Current Psychiatry. 2020;19(11):22-24,28-30,32-35.
- Schwab-Reese LM, Murfree L, Coppola EC, et al. Homicidesuicide across the lifespan: a mixed methods examination of factors contributing to older adult perpetration. Aging Ment Health. 2020;20:1-9.
1. Christodoulou H. LOCKDOWN ‘MURDER-SUICIDE’ OAP, 71, ‘stabbed wife to death then killed himself as he worried about coping with coronavirus lockdown.’ The Sun. Updated April 4, 2020. Accessed December 22, 2020. https://www.thesun.co.uk/news/11327095/coronavirus-lockdown-murder-suicide-cambridge/
2. Farberov S. Illinois man, 54, shoots dead his wife then kills himself in murder-suicide because he feared they had coronavirus - but tests later show the couple were NOT ill. Updated April 6, 2020. Accessed December 22, 2020. https://www.dailymail.co.uk/news/article-8191933/Man-kills-wife-feared-coronavirus.html
3. Salari S. Patterns of intimate partner homicide suicide in later life: strategies for prevention. Clin Interv Aging. 2007;2(3):441-452.
4. Kotzé C, Roos JL. Homicide–suicide: practical implications for risk reduction and support services at primary care level. South African Family Practice. 2019;61(4):165-169.
5. Malphurs JE, Cohen D. A newspaper surveillance study of homicide-suicide in the United States. Am J Forensic Med Pathol. 2002;23(2):142-148.
6. Eliason S. Murder-suicide: a review of the recent literature. J Am Acad Psychiatry Law. 2009;37(3):371-376.
7. Armitage R, Nellums LB. COVID-19 and the consequences of isolating the elderly. Lancet Public Health. 2020;5(5):e256. doi: 10.1016/S2468-2667(20)30061-X
8. Gunnell D, Appleby L, Arensman E, et al. Suicide risk and prevention during the COVID-19 pandemic. Lancet Psychiatry 2020;7(6):468-471.
9. Gosangi B, Park H, Thomas R, et al. Exacerbation of physical intimate partner violence during COVID-19 pandemic. Radiology. 2021;298(1):E38-E45.
10. Mannix R, Lee LK, Fleegler EW. Coronavirus disease 2019 (COVID-19) and firearms in the United States: will an epidemic of suicide follow? Ann Intern Med. 2020;173(3):228-229.
11. Bourget D, Gagne P, Whitehurst L. Domestic homicide and homicide-suicide: the older offender. J Am Acad Psychiatry Law. 2010;38(3):305-311.
12. Cheung G, Hatters Friedman S, Sundram F. Late-life homicide-suicide: a national case series in New Zealand. Psychogeriatrics. 2016;16(1):76-81.
13. Cohen D, Llorente M, Eisdorfer C. Homicide-suicide in older persons. Am J Psychiatry. 1998;155(3):390-396.
14. Malphurs JE, Eisdorfer C, Cohen D. A comparison of antecedents of homicide-suicide and suicide in older married men. Am J Geriatr Psychiatry. 2001;9(1):49-57.
15. De Koning E, Piette MHA. A retrospective study of murder–suicide at the Forensic Institute of Ghent University, Belgium: 1935–2010. Med Sci Law. 2014;54(2):88-98.
16. Logan JE, Ertl A, Bossarte R. Correlates of intimate partner homicide among male suicide decedents with known intimate partner problems. Suicide Life Threat Behav. 2019;49(6):1693-1706.
17. Malphurs JE, Cohen D. A statewide case-control study of spousal homicide-suicide in older persons. Am J Geriatr Psychiatry. 2005;13(3):211-217.
18. Sanford A. ‘Horrifying surge in domestic violence’ against women amid coronavirus-lockdowns, UN chief warns. Euronews. Published June 4, 2020. Accessed December 22, 2020. https://www.euronews.com/2020/04/06/horrifying-surge-in-domestic-violence-against-women-amid-coronavirus-lockdowns-un-chief-w
19. Appel JM. Intimate partner homicide in elderly populations. In: Friedman SH, ed. Family murder: pathologies of love and hate. American Psychiatric Association Publishing; 2019:131-142.
20. Hall CA, Reynolds-III CF. Late-life depression in the primary care setting: challenges, collaborative care, and prevention. Maturitas. 2014;79(2):147-152.
21. Unützer J. Diagnosis and treatment of older adults with depression in primary care. Biological Psychiatry. 2002;52(3):285-292.
22. Byers AL, Arean PA, Yaffe K. Low use of mental health services among older Americans with mood and anxiety disorders. Psychiatr Serv. 2012;63(1):66-72.
23. Bruce ML, Sirey JA. Integrated care for depression in older primary care patients. Can J Psychiatry. 2018;63(7):439-446.
24. Rao R, Roche A. Substance misuse in older people. BMJ. 2017;358:j3885. doi: 10.1136/bmj.j3885
25. Ghossoub E, Khoury R. Prevalence and correlates of criminal behavior among the non-institutionalized elderly: results from the National Survey on Drug Use and Health. J Geriatr Psychiatry Neurol. 2018;31(4):211-222.
26. Slater MAG. Older adults at risk for suicide. In: Berkman B. Handbook of social work in health and aging. Oxford University Press; 2006:149-161.
27. Hollander JE, Carr BG. Virtually perfect? Telemedicine for Covid-19. N Engl J Med. 2020;382(18):1679-1681.
28. Centers for Medicare & Medicaid Services. President Trump expands telehealth benefits for Medicare beneficiaries during COVID-19 outbreak. Published March 17, 2020. Accessed December 23, 2020. https://www.cms.gov/newsroom/press-releases/president-trump-expands-telehealth-benefits-medicare-beneficiaries-during-covid-19-outbreak
29. Mian A, Khan S. Coronavirus: the spread of misinformation. BMC Med. 2020;18(1):89.
On March 25, 2020, in Cambridge, United Kingdom, a 71-year-old man stabbed his 71-year-old wife before suffocating himself to death. The couple was reportedly anxious about the coronavirus disease 2019 (COVID-19) pandemic lockdown measures and were on the verge of running out of food and medicine.1
One week later, in Chicago, Illinois, a 54-year-old man shot and killed his female partner, age 54, before killing himself. The couple was tested for COVID-19 2 days earlier and the man believed they had contracted the virus; however, the test results for both of them had come back negative.2
Intimate partner homicide-suicide is the most dramatic domestic abuse outcome.3 Homicide-suicide is defined as “homicide committed by a person who subsequently commits suicide within one week of the homicide. In most cases the subsequent suicide occurs within a 24-hour period.”4 Approximately one-quarter of all homicide-suicides are committed by persons age ≥55 years.5,6 We believe that during the COVID-19 pandemic, the risk of homicide-suicide among older adults may be increased due to several factors, including:
- physical distancing and quarantine measures. Protocols established to slow the spread of the virus may be associated with increased rates of depression and anxiety7 and an increased risk of suicide among older adults8
- increased intimate partner violence9
- increased firearm ownership rates in the United States.10
In this article, we review studies that identified risk factors for homicide-suicide among older adults, discuss the impact the COVID-19 pandemic has had on these risks, and describe steps clinicians can take to intervene.
A review of the literature
To better characterize the perpetrators of older adult homicide-suicide, we conducted a literature search of relevant terms. We identified 9 original research publications that examined homicide-suicide in older adults.
Bourget et al11 (2010) reviewed coroners’ charts of individuals killed by an older (age ≥65) spouse or family member from 1992 through 2007 in Quebec, Canada. They identified 19 cases of homicide-suicide, 17 (90%) of which were perpetrated by men. Perpetrators and victims were married (63%), in common-law relationships (16%), or separated/divorced (16%). A history of domestic violence was documented in 4 (21%) cases. The authors found that 13 of 15 perpetrators (87%) had “major depression” and 2 perpetrators had a psychotic disorder. Substance use at the time of the event was confirmed in 6 (32%) cases. Firearms and strangulation were the top methods used to carry out the homicide-suicide.11
Cheung et al12 (2016) conducted a review of coroners’ records of homicide-suicide cases among individuals age ≥65 in New Zealand from 2007 through 2012. In all 4 cases, the perpetrators were men, and their victims were predominantly female, live-in family members. Two cases involved men with a history of domestic violence who were undergoing significant changes in their home and social lives. Both men had a history suggestive of depression and used a firearm to carry out the homicide-suicide.12
Continue to: Cohen et al
Cohen et al13 (1998) conducted a review of coroners’ records from 1988 through 1994 in 2 regions in Florida. They found 48 intimate partner homicide-suicide cases among “old couples” (age ≥55). All were perpetrated by men. The authors identified sociocultural differences in risk factors between the 2 regions. In west-central Florida, perpetrators and victims were predominantly white and in a spousal relationship. Domestic violence was documented in <4% of cases. Approximately 55% of the couples were reported to be ill, and a substantial proportion were documented to be declining in health. One-quarter of the perpetrators and one-third of the victims had “pain and suffering.” More than one-third of perpetrators were reported to have “depression,” 15% were reported to have talked about suicide, and 4% had a history of a suicide attempt. Only 11% of perpetrators were described as abusing substances.
The authors noted several differences in cases in southeastern Florida. Approximately two-thirds of the couples were Hispanic, and 14% had a history of domestic violence. A minority of the couples were in a live-in relationship. Less than 15% of the perpetrators and victims were described as having a decline in health. Additionally, only 19% of perpetrators were reported to have “depression,” and none of the perpetrators had a documented history of attempted suicide or substance abuse. No information was provided regarding the methods used to carry out the homicide-suicide in the southeastern region.13 Financial stress was not a factor in either region.
Malphurs et al14 (2001) used the same database described in the Cohen et al13 study to compare 27 perpetrators of homicide-suicide to 36 age-matched suicide decedents in west central Florida. They found that homicide-suicide perpetrators were significantly less likely to have health problems and were 3 times more likely to be caregivers to their spouses. Approximately 52% of perpetrators had at least 1 documented psychiatric symptom (“depression” and/or substance abuse or other), but only 5% were seeking mental health services at the time of death.14
De Koning and Piette15 (2014) conducted a retrospective medicolegal chart review from 1935 to 2010 to identify homicide-suicide cases in West and East Flanders, Belgium. They found 19 cases of intimate partner homicide-suicide committed by offenders age ≥55 years. Ninety-five percent of the perpetrators were men who killed their female partners. In one-quarter of the cases, either the perpetrator or the victim had a health issue; 21% of the perpetrators were documented as having depression and 27% had alcohol intoxication at the time of death. A motive was documented in 14 out of 19 cases; “mercy killing” was determined as the motive in 6 (43%) cases and “amorous jealousy” in 5 cases (36%).15 Starting in the 1970s, firearms were the most prevalent method used to kill a partner.
Logan et al16 (2019) used data from the National Violent Death Reporting System between 2003 and 2015 to identify characteristics that differentiated male suicide decedents from male perpetrators of intimate partner homicide-suicide. They found that men age 50 to 64 years were 3 times more likely than men age 18 to 34 years to commit intimate partner homicide-suicide, and that men age ≥65 years were approximately 5 times more likely than men age 18 to 34 years to commit intimate partner homicide-suicide. The authors found that approximately 22% of all perpetrators had a documented history of physical domestic violence, and close to 17% had a prior interaction with the criminal justice system. Furthermore, one-third of perpetrators had relationship difficulties and were in the process of a breakup. Health issues were prevalent in 34% of the victims and 26% of the perpetrators. Perpetrator-caregiver burden was reported as a contributing factor for homicide-suicide in 16% of cases. In 27% of cases, multiple health-related contributing factors were mentioned.16
Continue to: Malphurs and Cohen
Malphurs and Cohen5 (2002) reviewed American newspapers from 1997 through 1999 and identified 673 homicide-suicide events, of which 152 (27%) were committed by individuals age ≥55 years. The victims and perpetrators (95% of which were men) were intimate partners in three-quarters of cases. In nearly one-third of cases, caregiving was a contributing factor for the homicide-suicide. A history of or a pending divorce was reported in nearly 14% of cases. Substance use history was rarely recorded. Firearms were used in 88% of the homicide-suicide cases.5
Malphurs and Cohen17 (2005) reviewed coroner records between 1998 and 1999 in Florida and compared 20 cases of intimate partner homicide-suicide involving perpetrators age ≥55 years with matched suicide decedents. They found that 60% of homicide-suicide perpetrators had documented health issues. The authors reported that a “recent change in health status” was more prevalent among perpetrators compared with decedents. Perpetrators were also more likely to be caregivers to their spouses. The authors found that 65% of perpetrators were reported to have a “depressed mood” and 15% of perpetrators had reportedly threatened suicide prior to the incident. However, none of the perpetrators tested positive for antidepressants as documented on post-mortem toxicology reports. Firearms were used in 100% of homicide-suicide cases.17
Salari3 (2007) reviewed multiple American media sources and published police reports between 1999 and 2005 to retrieve data about intimate partner homicide-suicide events in the United States. There were 225 events identified where the perpetrator and/or the victim were age ≥60 years. Ninety-six percent of the perpetrators were men and most homicide-suicide events were committed at the home. A history of domestic violence was reported in 14% of homicide-suicide cases. Thirteen percent of couples were separated or divorced. The perpetrator and/or victim had health issues in 124 (55%) events. Dementia was reported in 7.5% of cases, but overwhelmingly among the victims. Substance abuse was rarely mentioned as a contributing factor. In three-quarters of cases where a motive was described, the perpetrator was “suicidal”; however, a “suicide pact” was mentioned in only 4% of cases. Firearms were used in 87% of cases.3
Focus on common risk factors
The scarcity and heterogeneity of research regarding older adult homicide-suicide were major limitations to our review. Because most of the studies we identified had a small sample size and limited information regarding the mental health of victims and perpetrators, it would be an overreach to claim to have identified a typical profile of an older perpetrator of homicide-suicide. However, the literature has repeatedly identified several common characteristics of such perpetrators. They are significantly more likely to be men who use firearms to murder their intimate partners and then die by suicide in their home (Table3,5,11-17). Health issues afflicting 1 or both individuals in the couple appear to be a contributing factor, particularly when the perpetrator is in a caregiving role. Relational discord, with or without a history of domestic violence, increases the risk of homicide-suicide. Finally, older perpetrators are highly likely to be depressed and have suicidal ideations.
How COVID-19 affects these risks
Although it is too early to determine if there is a causal relationship between the COVID-19 pandemic and an increase in homicide-suicide, the pandemic is likely to promote risk factors for these events, especially among older adults. Confinement measures put into place during the pandemic context have already been shown to increase rates of domestic violence18 and depression and anxiety among older individuals.7 Furthermore,
Continue to: Late-life psychiatric disorders
Late-life psychiatric disorders
Early recognition and effective treatment of late-life psychiatric disorders is crucial. Unfortunately, depression in geriatric patients is often underdiagnosed and undertreated.20 Older adults have more frequent contact with their primary care physicians, and rarely consult mental health professionals.21,22 Several models of integrated depression care within primary care settings have shown the positive impact of this collaborative approach in treating late-life depression and preventing suicide in older individuals.23 Additionally, because alcohol abuse is also a risk factor for domestic violence and breaking the law in this population,24,25 older adults should be screened for alcohol use disorders, and referred to treatment when necessary.
Take steps to keep patients safe
In the context of the COVID-19 pandemic, there are several steps clinicians need to keep in mind when interacting with older patients:
- Screen for depressive symptoms, suicidality, and alcohol and substance use disorders. Individuals who have tested positive for COVID-19 or who have been in contact with a carrier are a particularly vulnerable population.
- Screen for domestic violence and access to weapons at home.4 Any older adult who has a psychiatric disorder and/or suicide ideation should receive immediate intervention through a social worker that includes providing gun-risk education to other family members or contacting law-enforcement officials.26
- Refer patients with a suspected psychiatric disorder to specialized mental health clinicians. Telemental health services can provide rapid access to subspecialists, allowing patients to be treated from their homes.27 These services need to be promoted among older adults during this critical period and reimbursed by public and private insurance systems to ensure accessibility and affordability.28
- Create psychiatric inpatient units specifically designed for suicidal and/or homicidal patients with COVID-19.
Additionally, informing the public about these major health issues is crucial. The media can raise awareness about domestic violence and depression among older adults; however, this should be done responsibly and with accuracy to prevent the spread of misinformation, confusion, fear, and panic.29
Bottom Line
The mental health burden of the coronavirus disease 2019 pandemic has significantly impacted individuals who are older and most vulnerable. Reducing the incidence of homicide-suicide among older adults requires timely screening and interventions by primary care providers, mental health specialists, social workers, media, and governmental agencies.
Related Resources
- Saeed SA, Hebishi K. The psychiatric consequences of COVID-19: 8 studies. Current Psychiatry. 2020;19(11):22-24,28-30,32-35.
- Schwab-Reese LM, Murfree L, Coppola EC, et al. Homicidesuicide across the lifespan: a mixed methods examination of factors contributing to older adult perpetration. Aging Ment Health. 2020;20:1-9.
On March 25, 2020, in Cambridge, United Kingdom, a 71-year-old man stabbed his 71-year-old wife before suffocating himself to death. The couple was reportedly anxious about the coronavirus disease 2019 (COVID-19) pandemic lockdown measures and were on the verge of running out of food and medicine.1
One week later, in Chicago, Illinois, a 54-year-old man shot and killed his female partner, age 54, before killing himself. The couple was tested for COVID-19 2 days earlier and the man believed they had contracted the virus; however, the test results for both of them had come back negative.2
Intimate partner homicide-suicide is the most dramatic domestic abuse outcome.3 Homicide-suicide is defined as “homicide committed by a person who subsequently commits suicide within one week of the homicide. In most cases the subsequent suicide occurs within a 24-hour period.”4 Approximately one-quarter of all homicide-suicides are committed by persons age ≥55 years.5,6 We believe that during the COVID-19 pandemic, the risk of homicide-suicide among older adults may be increased due to several factors, including:
- physical distancing and quarantine measures. Protocols established to slow the spread of the virus may be associated with increased rates of depression and anxiety7 and an increased risk of suicide among older adults8
- increased intimate partner violence9
- increased firearm ownership rates in the United States.10
In this article, we review studies that identified risk factors for homicide-suicide among older adults, discuss the impact the COVID-19 pandemic has had on these risks, and describe steps clinicians can take to intervene.
A review of the literature
To better characterize the perpetrators of older adult homicide-suicide, we conducted a literature search of relevant terms. We identified 9 original research publications that examined homicide-suicide in older adults.
Bourget et al11 (2010) reviewed coroners’ charts of individuals killed by an older (age ≥65) spouse or family member from 1992 through 2007 in Quebec, Canada. They identified 19 cases of homicide-suicide, 17 (90%) of which were perpetrated by men. Perpetrators and victims were married (63%), in common-law relationships (16%), or separated/divorced (16%). A history of domestic violence was documented in 4 (21%) cases. The authors found that 13 of 15 perpetrators (87%) had “major depression” and 2 perpetrators had a psychotic disorder. Substance use at the time of the event was confirmed in 6 (32%) cases. Firearms and strangulation were the top methods used to carry out the homicide-suicide.11
Cheung et al12 (2016) conducted a review of coroners’ records of homicide-suicide cases among individuals age ≥65 in New Zealand from 2007 through 2012. In all 4 cases, the perpetrators were men, and their victims were predominantly female, live-in family members. Two cases involved men with a history of domestic violence who were undergoing significant changes in their home and social lives. Both men had a history suggestive of depression and used a firearm to carry out the homicide-suicide.12
Continue to: Cohen et al
Cohen et al13 (1998) conducted a review of coroners’ records from 1988 through 1994 in 2 regions in Florida. They found 48 intimate partner homicide-suicide cases among “old couples” (age ≥55). All were perpetrated by men. The authors identified sociocultural differences in risk factors between the 2 regions. In west-central Florida, perpetrators and victims were predominantly white and in a spousal relationship. Domestic violence was documented in <4% of cases. Approximately 55% of the couples were reported to be ill, and a substantial proportion were documented to be declining in health. One-quarter of the perpetrators and one-third of the victims had “pain and suffering.” More than one-third of perpetrators were reported to have “depression,” 15% were reported to have talked about suicide, and 4% had a history of a suicide attempt. Only 11% of perpetrators were described as abusing substances.
The authors noted several differences in cases in southeastern Florida. Approximately two-thirds of the couples were Hispanic, and 14% had a history of domestic violence. A minority of the couples were in a live-in relationship. Less than 15% of the perpetrators and victims were described as having a decline in health. Additionally, only 19% of perpetrators were reported to have “depression,” and none of the perpetrators had a documented history of attempted suicide or substance abuse. No information was provided regarding the methods used to carry out the homicide-suicide in the southeastern region.13 Financial stress was not a factor in either region.
Malphurs et al14 (2001) used the same database described in the Cohen et al13 study to compare 27 perpetrators of homicide-suicide to 36 age-matched suicide decedents in west central Florida. They found that homicide-suicide perpetrators were significantly less likely to have health problems and were 3 times more likely to be caregivers to their spouses. Approximately 52% of perpetrators had at least 1 documented psychiatric symptom (“depression” and/or substance abuse or other), but only 5% were seeking mental health services at the time of death.14
De Koning and Piette15 (2014) conducted a retrospective medicolegal chart review from 1935 to 2010 to identify homicide-suicide cases in West and East Flanders, Belgium. They found 19 cases of intimate partner homicide-suicide committed by offenders age ≥55 years. Ninety-five percent of the perpetrators were men who killed their female partners. In one-quarter of the cases, either the perpetrator or the victim had a health issue; 21% of the perpetrators were documented as having depression and 27% had alcohol intoxication at the time of death. A motive was documented in 14 out of 19 cases; “mercy killing” was determined as the motive in 6 (43%) cases and “amorous jealousy” in 5 cases (36%).15 Starting in the 1970s, firearms were the most prevalent method used to kill a partner.
Logan et al16 (2019) used data from the National Violent Death Reporting System between 2003 and 2015 to identify characteristics that differentiated male suicide decedents from male perpetrators of intimate partner homicide-suicide. They found that men age 50 to 64 years were 3 times more likely than men age 18 to 34 years to commit intimate partner homicide-suicide, and that men age ≥65 years were approximately 5 times more likely than men age 18 to 34 years to commit intimate partner homicide-suicide. The authors found that approximately 22% of all perpetrators had a documented history of physical domestic violence, and close to 17% had a prior interaction with the criminal justice system. Furthermore, one-third of perpetrators had relationship difficulties and were in the process of a breakup. Health issues were prevalent in 34% of the victims and 26% of the perpetrators. Perpetrator-caregiver burden was reported as a contributing factor for homicide-suicide in 16% of cases. In 27% of cases, multiple health-related contributing factors were mentioned.16
Continue to: Malphurs and Cohen
Malphurs and Cohen5 (2002) reviewed American newspapers from 1997 through 1999 and identified 673 homicide-suicide events, of which 152 (27%) were committed by individuals age ≥55 years. The victims and perpetrators (95% of which were men) were intimate partners in three-quarters of cases. In nearly one-third of cases, caregiving was a contributing factor for the homicide-suicide. A history of or a pending divorce was reported in nearly 14% of cases. Substance use history was rarely recorded. Firearms were used in 88% of the homicide-suicide cases.5
Malphurs and Cohen17 (2005) reviewed coroner records between 1998 and 1999 in Florida and compared 20 cases of intimate partner homicide-suicide involving perpetrators age ≥55 years with matched suicide decedents. They found that 60% of homicide-suicide perpetrators had documented health issues. The authors reported that a “recent change in health status” was more prevalent among perpetrators compared with decedents. Perpetrators were also more likely to be caregivers to their spouses. The authors found that 65% of perpetrators were reported to have a “depressed mood” and 15% of perpetrators had reportedly threatened suicide prior to the incident. However, none of the perpetrators tested positive for antidepressants as documented on post-mortem toxicology reports. Firearms were used in 100% of homicide-suicide cases.17
Salari3 (2007) reviewed multiple American media sources and published police reports between 1999 and 2005 to retrieve data about intimate partner homicide-suicide events in the United States. There were 225 events identified where the perpetrator and/or the victim were age ≥60 years. Ninety-six percent of the perpetrators were men and most homicide-suicide events were committed at the home. A history of domestic violence was reported in 14% of homicide-suicide cases. Thirteen percent of couples were separated or divorced. The perpetrator and/or victim had health issues in 124 (55%) events. Dementia was reported in 7.5% of cases, but overwhelmingly among the victims. Substance abuse was rarely mentioned as a contributing factor. In three-quarters of cases where a motive was described, the perpetrator was “suicidal”; however, a “suicide pact” was mentioned in only 4% of cases. Firearms were used in 87% of cases.3
Focus on common risk factors
The scarcity and heterogeneity of research regarding older adult homicide-suicide were major limitations to our review. Because most of the studies we identified had a small sample size and limited information regarding the mental health of victims and perpetrators, it would be an overreach to claim to have identified a typical profile of an older perpetrator of homicide-suicide. However, the literature has repeatedly identified several common characteristics of such perpetrators. They are significantly more likely to be men who use firearms to murder their intimate partners and then die by suicide in their home (Table3,5,11-17). Health issues afflicting 1 or both individuals in the couple appear to be a contributing factor, particularly when the perpetrator is in a caregiving role. Relational discord, with or without a history of domestic violence, increases the risk of homicide-suicide. Finally, older perpetrators are highly likely to be depressed and have suicidal ideations.
How COVID-19 affects these risks
Although it is too early to determine if there is a causal relationship between the COVID-19 pandemic and an increase in homicide-suicide, the pandemic is likely to promote risk factors for these events, especially among older adults. Confinement measures put into place during the pandemic context have already been shown to increase rates of domestic violence18 and depression and anxiety among older individuals.7 Furthermore,
Continue to: Late-life psychiatric disorders
Late-life psychiatric disorders
Early recognition and effective treatment of late-life psychiatric disorders is crucial. Unfortunately, depression in geriatric patients is often underdiagnosed and undertreated.20 Older adults have more frequent contact with their primary care physicians, and rarely consult mental health professionals.21,22 Several models of integrated depression care within primary care settings have shown the positive impact of this collaborative approach in treating late-life depression and preventing suicide in older individuals.23 Additionally, because alcohol abuse is also a risk factor for domestic violence and breaking the law in this population,24,25 older adults should be screened for alcohol use disorders, and referred to treatment when necessary.
Take steps to keep patients safe
In the context of the COVID-19 pandemic, there are several steps clinicians need to keep in mind when interacting with older patients:
- Screen for depressive symptoms, suicidality, and alcohol and substance use disorders. Individuals who have tested positive for COVID-19 or who have been in contact with a carrier are a particularly vulnerable population.
- Screen for domestic violence and access to weapons at home.4 Any older adult who has a psychiatric disorder and/or suicide ideation should receive immediate intervention through a social worker that includes providing gun-risk education to other family members or contacting law-enforcement officials.26
- Refer patients with a suspected psychiatric disorder to specialized mental health clinicians. Telemental health services can provide rapid access to subspecialists, allowing patients to be treated from their homes.27 These services need to be promoted among older adults during this critical period and reimbursed by public and private insurance systems to ensure accessibility and affordability.28
- Create psychiatric inpatient units specifically designed for suicidal and/or homicidal patients with COVID-19.
Additionally, informing the public about these major health issues is crucial. The media can raise awareness about domestic violence and depression among older adults; however, this should be done responsibly and with accuracy to prevent the spread of misinformation, confusion, fear, and panic.29
Bottom Line
The mental health burden of the coronavirus disease 2019 pandemic has significantly impacted individuals who are older and most vulnerable. Reducing the incidence of homicide-suicide among older adults requires timely screening and interventions by primary care providers, mental health specialists, social workers, media, and governmental agencies.
Related Resources
- Saeed SA, Hebishi K. The psychiatric consequences of COVID-19: 8 studies. Current Psychiatry. 2020;19(11):22-24,28-30,32-35.
- Schwab-Reese LM, Murfree L, Coppola EC, et al. Homicidesuicide across the lifespan: a mixed methods examination of factors contributing to older adult perpetration. Aging Ment Health. 2020;20:1-9.
1. Christodoulou H. LOCKDOWN ‘MURDER-SUICIDE’ OAP, 71, ‘stabbed wife to death then killed himself as he worried about coping with coronavirus lockdown.’ The Sun. Updated April 4, 2020. Accessed December 22, 2020. https://www.thesun.co.uk/news/11327095/coronavirus-lockdown-murder-suicide-cambridge/
2. Farberov S. Illinois man, 54, shoots dead his wife then kills himself in murder-suicide because he feared they had coronavirus - but tests later show the couple were NOT ill. Updated April 6, 2020. Accessed December 22, 2020. https://www.dailymail.co.uk/news/article-8191933/Man-kills-wife-feared-coronavirus.html
3. Salari S. Patterns of intimate partner homicide suicide in later life: strategies for prevention. Clin Interv Aging. 2007;2(3):441-452.
4. Kotzé C, Roos JL. Homicide–suicide: practical implications for risk reduction and support services at primary care level. South African Family Practice. 2019;61(4):165-169.
5. Malphurs JE, Cohen D. A newspaper surveillance study of homicide-suicide in the United States. Am J Forensic Med Pathol. 2002;23(2):142-148.
6. Eliason S. Murder-suicide: a review of the recent literature. J Am Acad Psychiatry Law. 2009;37(3):371-376.
7. Armitage R, Nellums LB. COVID-19 and the consequences of isolating the elderly. Lancet Public Health. 2020;5(5):e256. doi: 10.1016/S2468-2667(20)30061-X
8. Gunnell D, Appleby L, Arensman E, et al. Suicide risk and prevention during the COVID-19 pandemic. Lancet Psychiatry 2020;7(6):468-471.
9. Gosangi B, Park H, Thomas R, et al. Exacerbation of physical intimate partner violence during COVID-19 pandemic. Radiology. 2021;298(1):E38-E45.
10. Mannix R, Lee LK, Fleegler EW. Coronavirus disease 2019 (COVID-19) and firearms in the United States: will an epidemic of suicide follow? Ann Intern Med. 2020;173(3):228-229.
11. Bourget D, Gagne P, Whitehurst L. Domestic homicide and homicide-suicide: the older offender. J Am Acad Psychiatry Law. 2010;38(3):305-311.
12. Cheung G, Hatters Friedman S, Sundram F. Late-life homicide-suicide: a national case series in New Zealand. Psychogeriatrics. 2016;16(1):76-81.
13. Cohen D, Llorente M, Eisdorfer C. Homicide-suicide in older persons. Am J Psychiatry. 1998;155(3):390-396.
14. Malphurs JE, Eisdorfer C, Cohen D. A comparison of antecedents of homicide-suicide and suicide in older married men. Am J Geriatr Psychiatry. 2001;9(1):49-57.
15. De Koning E, Piette MHA. A retrospective study of murder–suicide at the Forensic Institute of Ghent University, Belgium: 1935–2010. Med Sci Law. 2014;54(2):88-98.
16. Logan JE, Ertl A, Bossarte R. Correlates of intimate partner homicide among male suicide decedents with known intimate partner problems. Suicide Life Threat Behav. 2019;49(6):1693-1706.
17. Malphurs JE, Cohen D. A statewide case-control study of spousal homicide-suicide in older persons. Am J Geriatr Psychiatry. 2005;13(3):211-217.
18. Sanford A. ‘Horrifying surge in domestic violence’ against women amid coronavirus-lockdowns, UN chief warns. Euronews. Published June 4, 2020. Accessed December 22, 2020. https://www.euronews.com/2020/04/06/horrifying-surge-in-domestic-violence-against-women-amid-coronavirus-lockdowns-un-chief-w
19. Appel JM. Intimate partner homicide in elderly populations. In: Friedman SH, ed. Family murder: pathologies of love and hate. American Psychiatric Association Publishing; 2019:131-142.
20. Hall CA, Reynolds-III CF. Late-life depression in the primary care setting: challenges, collaborative care, and prevention. Maturitas. 2014;79(2):147-152.
21. Unützer J. Diagnosis and treatment of older adults with depression in primary care. Biological Psychiatry. 2002;52(3):285-292.
22. Byers AL, Arean PA, Yaffe K. Low use of mental health services among older Americans with mood and anxiety disorders. Psychiatr Serv. 2012;63(1):66-72.
23. Bruce ML, Sirey JA. Integrated care for depression in older primary care patients. Can J Psychiatry. 2018;63(7):439-446.
24. Rao R, Roche A. Substance misuse in older people. BMJ. 2017;358:j3885. doi: 10.1136/bmj.j3885
25. Ghossoub E, Khoury R. Prevalence and correlates of criminal behavior among the non-institutionalized elderly: results from the National Survey on Drug Use and Health. J Geriatr Psychiatry Neurol. 2018;31(4):211-222.
26. Slater MAG. Older adults at risk for suicide. In: Berkman B. Handbook of social work in health and aging. Oxford University Press; 2006:149-161.
27. Hollander JE, Carr BG. Virtually perfect? Telemedicine for Covid-19. N Engl J Med. 2020;382(18):1679-1681.
28. Centers for Medicare & Medicaid Services. President Trump expands telehealth benefits for Medicare beneficiaries during COVID-19 outbreak. Published March 17, 2020. Accessed December 23, 2020. https://www.cms.gov/newsroom/press-releases/president-trump-expands-telehealth-benefits-medicare-beneficiaries-during-covid-19-outbreak
29. Mian A, Khan S. Coronavirus: the spread of misinformation. BMC Med. 2020;18(1):89.
1. Christodoulou H. LOCKDOWN ‘MURDER-SUICIDE’ OAP, 71, ‘stabbed wife to death then killed himself as he worried about coping with coronavirus lockdown.’ The Sun. Updated April 4, 2020. Accessed December 22, 2020. https://www.thesun.co.uk/news/11327095/coronavirus-lockdown-murder-suicide-cambridge/
2. Farberov S. Illinois man, 54, shoots dead his wife then kills himself in murder-suicide because he feared they had coronavirus - but tests later show the couple were NOT ill. Updated April 6, 2020. Accessed December 22, 2020. https://www.dailymail.co.uk/news/article-8191933/Man-kills-wife-feared-coronavirus.html
3. Salari S. Patterns of intimate partner homicide suicide in later life: strategies for prevention. Clin Interv Aging. 2007;2(3):441-452.
4. Kotzé C, Roos JL. Homicide–suicide: practical implications for risk reduction and support services at primary care level. South African Family Practice. 2019;61(4):165-169.
5. Malphurs JE, Cohen D. A newspaper surveillance study of homicide-suicide in the United States. Am J Forensic Med Pathol. 2002;23(2):142-148.
6. Eliason S. Murder-suicide: a review of the recent literature. J Am Acad Psychiatry Law. 2009;37(3):371-376.
7. Armitage R, Nellums LB. COVID-19 and the consequences of isolating the elderly. Lancet Public Health. 2020;5(5):e256. doi: 10.1016/S2468-2667(20)30061-X
8. Gunnell D, Appleby L, Arensman E, et al. Suicide risk and prevention during the COVID-19 pandemic. Lancet Psychiatry 2020;7(6):468-471.
9. Gosangi B, Park H, Thomas R, et al. Exacerbation of physical intimate partner violence during COVID-19 pandemic. Radiology. 2021;298(1):E38-E45.
10. Mannix R, Lee LK, Fleegler EW. Coronavirus disease 2019 (COVID-19) and firearms in the United States: will an epidemic of suicide follow? Ann Intern Med. 2020;173(3):228-229.
11. Bourget D, Gagne P, Whitehurst L. Domestic homicide and homicide-suicide: the older offender. J Am Acad Psychiatry Law. 2010;38(3):305-311.
12. Cheung G, Hatters Friedman S, Sundram F. Late-life homicide-suicide: a national case series in New Zealand. Psychogeriatrics. 2016;16(1):76-81.
13. Cohen D, Llorente M, Eisdorfer C. Homicide-suicide in older persons. Am J Psychiatry. 1998;155(3):390-396.
14. Malphurs JE, Eisdorfer C, Cohen D. A comparison of antecedents of homicide-suicide and suicide in older married men. Am J Geriatr Psychiatry. 2001;9(1):49-57.
15. De Koning E, Piette MHA. A retrospective study of murder–suicide at the Forensic Institute of Ghent University, Belgium: 1935–2010. Med Sci Law. 2014;54(2):88-98.
16. Logan JE, Ertl A, Bossarte R. Correlates of intimate partner homicide among male suicide decedents with known intimate partner problems. Suicide Life Threat Behav. 2019;49(6):1693-1706.
17. Malphurs JE, Cohen D. A statewide case-control study of spousal homicide-suicide in older persons. Am J Geriatr Psychiatry. 2005;13(3):211-217.
18. Sanford A. ‘Horrifying surge in domestic violence’ against women amid coronavirus-lockdowns, UN chief warns. Euronews. Published June 4, 2020. Accessed December 22, 2020. https://www.euronews.com/2020/04/06/horrifying-surge-in-domestic-violence-against-women-amid-coronavirus-lockdowns-un-chief-w
19. Appel JM. Intimate partner homicide in elderly populations. In: Friedman SH, ed. Family murder: pathologies of love and hate. American Psychiatric Association Publishing; 2019:131-142.
20. Hall CA, Reynolds-III CF. Late-life depression in the primary care setting: challenges, collaborative care, and prevention. Maturitas. 2014;79(2):147-152.
21. Unützer J. Diagnosis and treatment of older adults with depression in primary care. Biological Psychiatry. 2002;52(3):285-292.
22. Byers AL, Arean PA, Yaffe K. Low use of mental health services among older Americans with mood and anxiety disorders. Psychiatr Serv. 2012;63(1):66-72.
23. Bruce ML, Sirey JA. Integrated care for depression in older primary care patients. Can J Psychiatry. 2018;63(7):439-446.
24. Rao R, Roche A. Substance misuse in older people. BMJ. 2017;358:j3885. doi: 10.1136/bmj.j3885
25. Ghossoub E, Khoury R. Prevalence and correlates of criminal behavior among the non-institutionalized elderly: results from the National Survey on Drug Use and Health. J Geriatr Psychiatry Neurol. 2018;31(4):211-222.
26. Slater MAG. Older adults at risk for suicide. In: Berkman B. Handbook of social work in health and aging. Oxford University Press; 2006:149-161.
27. Hollander JE, Carr BG. Virtually perfect? Telemedicine for Covid-19. N Engl J Med. 2020;382(18):1679-1681.
28. Centers for Medicare & Medicaid Services. President Trump expands telehealth benefits for Medicare beneficiaries during COVID-19 outbreak. Published March 17, 2020. Accessed December 23, 2020. https://www.cms.gov/newsroom/press-releases/president-trump-expands-telehealth-benefits-medicare-beneficiaries-during-covid-19-outbreak
29. Mian A, Khan S. Coronavirus: the spread of misinformation. BMC Med. 2020;18(1):89.
Antipsychotics and seizures: What are the risks?
Antipsychotics, especially second-generation antipsychotics (SGAs), have been proven effective for treating psychosis as well as mood disorders.1,2 Because antipsychotics can lower the epileptogenic threshold, seizures are a serious potential adverse effect. Antipsychotics can cause isolated EEG abnormalities in 7% of patients with no history of epilepsy, and clinical seizures in .5% to 1.2% of such patients.3 Additionally, the neuropathophysiology underlying epilepsy can predispose patients to psychiatric disorders4; the estimated prevalence of psychosis in patients with epilepsy is approximately 7%.5 This review will shed light on the risk of clinical seizures related to antipsychotics.
Comparing seizure risk among antipsychotics
In a review of the World Health Organization’s adverse drug reactions database, Kumlien and Lundberg6 calculated the ratio of the number of reports of seizures to the total number of reports for each drug. They found that approximately 9% of all adverse drug reaction reports involving clozapine were due to seizures. Equivalent ratios were 5.90% for quetiapine, 4.91% for olanzapine, 3.68% for risperidone, 3.27% for haloperidol, and 2.59% for aripiprazole. Using the database of the Pharmacovigilance Unit of the Basque Country, Lertxundi et al7 reported a 3.2-fold increased risk of seizure with SGAs in comparison with first-generation antipsychotics (FGAs) (95% confidence interval [CI], 2.21 to 4.63), which went down to 2.08 (CI, 1.39 to 3.12) once clozapine was excluded. However, as the authors of both studies noted, the quality and relevance of this data are limited because it relies on spontaneous reporting.
Overall, the evidence regarding the seizure risk associated with antipsychotics is scarce. To the best of our knowledge, only 2 large observational studies have compared the seizure risks associated with different antipsychotics.
Using data from the UK-based Clinical Practice Research Datalink between 1998 and 2013, Bloechlinger et al8 examined the incidence rates of seizures among patients newly diagnosed with schizophrenia, affective disorders, or dementia who were prescribed antipsychotics. They excluded patients with a history of seizures or antiepileptic use. In the cohort of 60,121 patients, the incidence rates of seizures per 10,000 person-years were 11.7 (CI, 10.0 to 13.4) for those who did not use antipsychotics, 12.4 (CI, 10.9 to 13.8) for past users, 115.4 (CI, 50.1 to 180.7) for current users of haloperidol, 48.8 (CI, 30.7 to 66.9) for current users of quetiapine, 25.9 (CI, 11.8 to 40.0) for current users of risperidone, and 19.0 (CI, 8.7 to 29.3) for current users of olanzapine. No data were available about clozapine use.
In subsequent analyses, the authors found that among patients with affective disorders, only current use of medium- to high-potency FGAs (haloperidol, prochlorperazine, and trifluoperazine) was associated with a significantly increased risk of seizures (adjusted odds ratio: 2.51, CI, 1.51 to 4.18) compared with non-users.8 Among patients with dementia, current use of olanzapine or quetiapine and current use of any FGAs were associated with significantly increased odds of seizures. This study suggests that the underlying mental illness might modulate the seizure risk associated with antipsychotics.8
Wu et al9 conducted a study based on the National Health Insurance Research Database in Taiwan. They examined the 1-year incidence of new-onset seizures among patients diagnosed with schizophrenia or mood disorders who were new to antipsychotic treatment, and calculated the risk of seizure associated with each antipsychotic in reference to risperidone. They found that those receiving clozapine, thioridazine, and haloperidol were 2 to 3 times more likely to develop seizures than those treated with risperidone; risks associated with the rest of the FGAs were similar to that of risperidone.
The results of these 2 large cohort studies are somewhat concurrent in indicating that, other than clozapine, SGAs incur similar risks of seizures; furthermore, they specify that, contrary to earlier studies,10 haloperidol is associated with significantly higher odds of seizures. While both of these cohort studies controlled for several sociodemographic and clinical confounders, they have several limitations. First, diagnoses of seizures were based on information available in databases, which might be subject to inaccuracies. Second, neither study evaluated the effect of drug dosage and duration of exposure on new-onset seizures.
Continue to: Most evidence is from case reports
Most evidence is from case reports
Other than these 2 large studies, most of the evidence addressing the relationship between the use of antipsychotics and incidence of seizures is low quality and relies on case reports or expert opinions. Older studies found that, among FGAs, seizure risk is highest with chlorpromazine and promazine, and lowest with thioridazine and haloperidol.10 As for SGAs, case reports have described seizuresassociated with the use of quetiapine, aripiprazole, risperidone, paliperidone, and olanzapine.
Quetiapine. Three case reports published between 2002 and 2010 describe generalized
Aripiprazole. Five case reports described staring spells and tonic-clonic seizures in patients receiving 10 to 15 mg of aripiprazole.15-19 In the New Drug Application (NDA) for aripiprazole, the incidence of seizures was estimated to be .11% (1 of 926 patients) in placebo-controlled trials and .46% (3 of 859 patients) in haloperidol-controlled trials.20
Risperidone’s product labeling suggests the drug should be used with caution in patients with a history of seizures or conditions that could result in a lower seizure threshold. In Phase III placebo-controlled trials, seizures occurred in .3% of patients treated with risperidone, although in some cases, the seizures were induced by electrolyte disturbances such as hyponatremia.21 Gonzalez-Heydrich et al22 and Holzhausen et al23 found no increase in seizure activity among patients with epilepsy who were receiving risperidone. Lane et al24 published a case report of a geriatric woman who presented with a generalized tonic-clonic seizure related to rapid titration of risperidone; however, with slower titration and lower doses, she stopped having seizures without adding any antiepileptic drugs. Komossa et al25 found that risperidone is less epileptogenic than clozapine, with a relative risk of .22.
Paliperidone is the active metabolite of risperidone and does not have pharmacokinetic interactions with drugs metabolized by the cytochrome P450 (CYP) enzymes. Its labeling indicates that the drug should be used with caution in patients with a history of seizures.26 In Phase III placebo-controlled trials of paliperidone, the rate of seizures was .22%.27 Two case reports suggest close monitoring of seizure risk in patients receiving paliperidone.28,29 Liang et al29 reported that co-administration of valproic acid could mask an underlying decrease of the seizure threshold caused by antipsychotics such as paliperidone.
Continue to: Olanzapine
Olanzapine is a thienobenzodiazepine derivative and is chemically related to clozapine.30 The olanzapine NDA31 shows that 23 of 3,139 patients developed seizures, mainly tonic-clonic, with evidence suggesting that the seizures may have been due to confounding factors such as a history of seizures or metabolic abnormalities. There were no statistically significant differences in the rate of seizures associated with olanzapine compared with placebo or haloperidol (P = .252 and .168, respectively).
A literature review for olanzapine yielded 1 case report of repetitive focal seizures and lingual dystonia,32 5 case reports of generalized tonic-clonic seizures and myoclonus,33-37 and 2 case reports of status epilepticus.38,39 Olanzapine’s clearance is 25% to 30% lower in women, and most of these case reports occurred women.40
Details of the above case reports are summarized in Table 1 (aripiprazole15-19), Table 2 (olanzapine32-39), and Table 3 (paliperidone,28,29 quetiapine,11-13 and risperidone22-24).
Ziprasidone. According to the NDA safety database, the seizure rate attributed to ziprasidone was 1.8 per 100 subject-years or 0.54% of participants (12 of 2,588).41 No additional studies have been published regarding its seizure risk.
Clozapine has a black-box warning
To the best of our knowledge, clozapine is the only antipsychotic that carries an FDA “black-box” warning regarding its risk of inducing seizures.42 Devinsky and Pacia43 reported a cumulative risk of 10% after 3.8 years of treatment. The literature has described clozapine-induced generalized tonic-clonic, myoclonic, simple and complex partial, and absence seizures.44 Table 445 lists the estimated frequency of each seizure type based on 101 cases of clozapine-induced seizures. Myoclonic seizures and drop attacks could be precursors/warning signs of grand mal tonic-clonic seizures.46,47 Seizures have been observed at all stages of treatment, but were more common during initiation of clozapine, which emphasizes the importance of a progressive and slow titration.43,48 The incidence of seizures was estimated to be 6% in a sample of 216 patients with schizophrenia with no history of epilepsy who were prescribed clozapine.49
Continue to: Regarding a possible association between...
Regarding a possible association between clozapine dose or clozapine plasma levels and seizure risk, there is a positive linear relationship between the dose of clozapine and its serum concentration over a dosing range of 25 to 800 mg/d.50 However, the plasma concentration is also significantly affected by factors such as smoking, gender, age, drug interactions, and CYP genotypes. Therefore, the same clozapine dose will yield a lower serum concentration in an older male who smokes compared with a younger, non-smoking female.51 Perry et al52 suggested a dosing nomogram to calculate the influence of gender and smoking. Seizure risk, especially for tonic-clonic seizures, has been reported to increase with clozapine doses >600 mg/d,53 and with plasma concentrations exceeding 1,000 to 1,300 mg/L.54 However, in a 2011 regression analysis, Varma et al55 found no statistically significant relationship between seizure risk and clozapine oral dose; there was not enough data to test a correlation between clozapine plasma levels and the incidence of seizures.
How antipsychotics might lower the seizure threshold
Researchers have suggested several possible mechanisms to explain how antipsychotics might lower the seizure threshold. Antagonism of dopamine D4, histamine H1, and acetylcholine-muscarinic receptors seems to induce EEG alterations and increase the risk of seizures.56 Additionally, modulation of the N-methyl-
Watch for pharmacokinetic interactions
The CYP enzymes involved in drug metabolism include CYP1A2, CYP2C9, CYP2C19, CYP2D6, and CYP3A4. Most commonly used antiepileptics and antipsychotics are metabolized by CYP enzymes, and may also act as inhibitors or inducers of these enzymes.61 Drug interactions may impair seizure control, which is why monotherapy is preferable to combination treatment in patients with epilepsy.62 Carbamazepine and phenytoin are inducers of both CYP1A2 (which metabolizes olanzapine and clozapine), and CYP3A4 (which metabolizes haloperidol, risperidone, quetiapine, ziprasidone and clozapine). Paliperidone is not metabolized by CYP enzymes.62 Discontinuing an enzyme-inducing agent may result in increased antipsychotic plasma concentrations, which might lead to an increased risk of seizures.
Valproic acid, which is often used to prevent or treat clozapine-induced seizures, has an unclear effect on clozapine plasma concentrations.63 Although valproic acid is known to inhibit clozapine metabolism, 2 reports have suggested that the plasma concentrations of clozapine and its metabolites may decrease after adding valproic acid.64,65 Other studies have found that valproic acid increases plasma concentrations of clozapine while it decreases plasma concentrations of norclozapine; norclozapine is the main clozapine metabolite responsible for inducing seizures.66,67
Steps for minimizing seizure risk
Determining the seizure risk for a patient taking an antipsychotic is challenging because doing so depends not only on the seizurogenic potential of each drug but also on individualized predisposing factors.11,57,68 Choosing the “best” antipsychotic therefore largely depends on each patient’s profile. The predisposing factors consist mainly of the individually inherited seizure threshold (personal history of febrile convulsions or a family history of seizures) and other comorbid seizurogenic conditions, such as a history of head trauma, brain injury, intellectual disability, cerebral arteriosclerosis, neurodegenerative diseases, encephalopathy, chronic renal insufficiency, and hyponatremia. Furthermore, seizure risk depends on the antipsychotic dose administered and the rate of titration.11
Continue to: There is not enough evidence...
There is not enough evidence to recommend performing an EEG in all patients taking antipsychotics. Such testing is recommended only for patients who have predisposing factors for seizures. If an EEG shows any abnormality in a patient taking clozapine, consider decreasing the clozapine dose69,70 or adding an antiepileptic drug such as valproic acid or lamotrigine.44,70
Although clozapine carries a black-box warning of increased risk of causing seizures, there is no consensus regarding the efficacy of co-prescribing an antiepileptic. Some studies have suggested prescribing valproic acid prophylactically,71 after the occurrence of 1 seizure,59 or after 2 seizures.54,72 Others have recommended prescribing prophylactic valproic acid for patients taking ≥600 mg/d of clozapine or whose clozapine plasma levels are >500 mg/L.73 Varma et al55 recommended starting an antiepileptic medication if there are clear epileptiform discharges on EEG, if the patient develops stuttering or speech difficulties, or if seizures occur. Liukkonen et al72 advised initiating an antiepileptic at the start of clozapine treatment in patients who are taking other epileptogenic medications, patients with pre-existing seizure disorder, and patients with neurologic abnormalities. On the other hand, Caetano51 argued against primary prevention of seizures for patients receiving >600 mg/d of clozapine, suggesting that “the risk of seizures would be better managed by close clinical monitoring and measures of clozapine serum concentration rather than adding an anticonvulsant drug.”
Current recommendations for primary and secondary prevention of clozapine-induced seizures are detailed in Table 5.42,44,45,51,55,57,69,74,75
Studies addressing the seizurogenic potential of SGAs other than clozapine have a low level of evidence and include patients who had comorbid conditions and were taking other medications that could cause seizures. Additionally, clinical trials of SGAs rarely include patients with seizure disorders; this might underestimate the risk of seizures.4
The effect of the mental illness itself on the seizure threshold needs to be considered.43 Bloechlinger et al8 found that dementia might be inherently associated with a higher risk of antipsychotic-related seizures. Moreover, numerous qualitative EEG studies have found abnormalities in 20% to 60% of patients with schizophrenia.56 Other quantitative studies have reported mild and nonspecific EEG abnormalities, such as increased delta and/or theta activity, in many non-medicated patients with schizophrenia.10,76 Additionally, brain tissue analysis of deceased patients who had schizophrenia has shown a significant increase in dopamine concentrations in the left amygdala compared with controls, and this might be responsible for enhanced electrical activity in this region.10 Some studies have described EEG slowing in the frontal brain regions of patients with schizophrenia,77 and was selectively normalized in these areas with antipsychotics.78
As always, start low, go slow
Mounting evidence suggests that antipsychotic medications decrease the seizure threshold. Practitioners should thus be cautious in prescribing antipsychotics and should target reaching the minimal effective dose with slow titration, especially in patients with predisposing factors for epilepsy.
Continue to: Although evidence suggests...
Although evidence suggests antipsychotics can induce different types of epileptic seizures, the quality of this evidence is low. Randomized controlled trials are needed to determine which antipsychotics increase seizure risk and whether there is a dose-effect relationship.
Bottom Line
Among second-generation antipsychotics, clozapine appears to increase the risk of clinical seizure the most. Correlations with dosage and/or plasma levels have not been proven. Psychiatrists should be vigilant for pharmacokinetic interactions between antipsychotics and antiepileptics, notably via CYP1A2 and CYP3A4.
Related Resources
- Druschky K, Bleich S, Grohmann R, et al. Seizure rates under treatment with antipsychotic drugs: Data from the AMSP project. World J Biol Psychiatry. 2018;15:1-10.
- Epilepsy Foundation. For professionals: Antipsychotics. https://www.epilepsy.com/learn/professionals/diagnosistreatment/psychotropic-drugs-developmental-disabilities/comorbid-5.
Drug Brand Names
Aripiprazole • Abilify
Benztropine • Cogentin
Bethanechol • Duvoid
Carbamazepine • Carbatrol, Tegretol
Chlorpromazine • Thorazine
Cimetidine • Tagamet
Ciprofloxacin • Cipro
Citalopram • Celexa
Clonazepam • Klonopin
Clozapine • Clozaril
Donepezil • Aricept
Enalapril • Vasotec
Erythromycin • Erythrocin
Escitalopram • Lexapro
Flunitrazepam • Rohypnol
Fluvoxamine • Luvox
Gabapentin • Neurontin
Haloperidol • Haldol
Lamotrigine • Lamictal
Lithium • Eskalith, Lithobid
Metformin • Fortamet, Glucophage
Mirtazapine • Remeron
Nitrofurantoin • Furadantin
Olanzapine • Zyprexa
Paliperidone • Invega
Phenobarbital • Luminal
Phenytoin • Dilantin
Prochlorperazine • Compazine
Procyclidine • Kemadrin
Propranolol • Inderal
Quetiapine • Seroquel
Risperidone • Risperdal
Sertraline • Zoloft
Simvastatin • Zocor
Sulfamethoxazole/trimethoprim • Bactrim, Sulfatrim
Topiramate • Topamax
Trifluoperazine • Stelazine
Valproic acid • Depakene, Depakote
Ziprasidone • Geodon
1. Bruijnzeel D, Suryadevara U, Tandon R. Antipsychotic treatment of schizophrenia: an update. Asian J Psychiatr. 2014;11:3-7.
2. Hrdlicka M, Dudova I. Atypical antipsychotics in the treatment of early-onset schizophrenia. Neuropsychiatr Dis Treat. 2015;11:907-913.
3. Koch-Stoecker S. Antipsychotic drugs and epilepsy: indications and treatment guidelines. Epilepsia. 2002;43(suppl 2):19-24.
4. Alper K, Schwartz KA, Kolts RL, et al. Seizure incidence in psychopharmacological clinical trials: an analysis of Food and Drug Administration (FDA) summary basis of approval reports. Biol Psychiatry. 2007;62(4):345-354.
5. Torta R, Keller R. Behavioral, psychotic, and anxiety disorders in epilepsy: etiology, clinical features, and therapeutic implications. Epilepsia. 1999;40(suppl 10):S2-S20.
6. Kumlien E, Lundberg PO. Seizure risk associated with neuroactive drugs: data from the WHO adverse drug reactions database. Seizure. 2010;19(2):69-73.
7. Lertxundi U, Hernandez R, Medrano J, et al. Antipsychotics and seizures: higher risk with atypicals? Seizure. 2013;22(2):141-143.
8. Bloechliger M, Rüegg S, Jick SS, et al. Antipsychotic drug use and the risk of seizures: follow-up study with a nested case-control analysis. CNS Drugs. 2015;29(7):591-603.
9. Wu CS, Wang SC, Yeh IJ, et al. Comparative risk of seizure with use of first- and second-generation antipsychotics in patients with schizophrenia and mood disorders. J Clin Psychiatry. 2016;77(5):e573-e579.
10. Cold JA, Wells BG, Froemming JH. Seizure activity associated with antipsychotic therapy. [Erratum in DICP. 1990;24(10):1012.] DICP. 1990;24(6):601-606.
11. Hedges DW, Jeppson KG. New-onset seizure associated with quetiapine and olanzapine. Ann Pharmacother. 2002;36(3):437-439.
12. Dogu O, Sevim S, Kaleagasi HS. Seizures associated with quetiapine treatment. Ann Pharmacother. 2003;37(9):1224-1227.
13. Young AC, Kleinschmidt KC, Wax PM. Late-onset seizures associated with quetiapine poisoning. J Med Toxicol. 2009;5(1):24-26.
14. US Food and Drug Administration. Recommendation of approvable action for quetiapine fumarate extended release (Seroquel® XR) for the treatment of schizophrenia. http://www.accessdata.fda.gov/drugsatfda_docs/nda/2007/022047Orig1s000MedR.pdf. April 24, 2007. Accessed January 28, 2019.
15. Malik AR, Ravasia S. Aripiprazole-induced seizure. Can J Psychiatry. 2005;50(3):186.
16. Tsai JF. Aripiprazole-associated seizure. J Clin Psychiatry. 2006;67(6):995-996.
17. Arora M, Arndorfer L. EEG abnormalities in a patient taking aripiprazole. Psychiatry (Edgmont). 2007;4(7):18-19.
18. Yueh CL, Yu SL, Chen HM, et al. Aripiprazole-induced seizure: a second case report. BMJ case reports. 2009;2009:bcr03.2009.1693. doi: 10.1136/bcr.03.2009.1693.
19. Thabet FI, Sweis RT, Joseph SA. Aripiprazole-induced seizure in a 3-year-old child: a case report and literature review. Clin Neuropharmacol. 2013;36(1):29-30.
20. US Food and Drug Administration. Abilify (Aripiprazole) tablets. Medical Review Part 2. http://www.accessdata.fda.gov/drugsatfda_docs/nda/2002/21-436_Abilify_medr_P2.pdf. Published March 07, 2003. Accessed January 28, 2019.
21. US Food and Drug Administration. Drugs@FDA: FDA approved drug products. Risperdal tablets, Risperdal oral solution & Risperdal M-tab orally disintegrating tablets. http://www.accessdata.fda.gov/drugsatfda_docs/nda/2003/021444_S004_RISPERDAL_TABLETS.pdf. Published September 10, 2003. Accessed January 28, 2019.
22. Gonzalez-Heydrich J, Pandina GJ, Fleisher CA, et al. No seizure exacerbation from risperidone in youth with comorbid epilepsy and psychiatric disorders: a case series. J Child Adolesc Psychopharmacol. 2004;14(2):295-310.
23. Holzhausen SPF, Guerreiro MM, Baccin CE, et al. Use of risperidone in children with epilepsy. Epilepsy Behav. 2007;10(3):412-416.
24. Lane HY, Chang WH, Chou JC. Seizure during risperidone treatment in an elderly woman treated with concomitant medications. J Clinl Psychiatry. 1998;59(2):81-82.
25. Komossa K, Rummel-Kluge C, Schwarz S, et al. Risperidone versus other atypical antipsychotics for schizophrenia. Cochrane Database Syst Rev. 2011;(1):19:CD006626.
26. Paliperidone [package insert]. Mountainville, CA: Janssen Pharmaceuticals, Inc.; 2007.
27. Brugge, MD; US Food and Drug Administration. Paliperidone OROS oral formulation. http://www.accessdata.fda.gov/drugsatfda_docs/nda/2006/021999s000_MedR_Part4.pdf. Accessed January 28, 2019.
28. Schneider RA, Lizer MH. Apparent seizure and atrial fibrillation associated with paliperidone. Am J Health System Pharm. 2008;65(22):2122-2125.
29. Liang CS, Yang FW, Chiang KT. Paliperidone-associated seizure after discontinuation of sodium valproate: a case report. J Clin Psychopharmacol. 2011;31(2):246-247.
30. Fulton B, Goa KL. Olanzapine. A review of its pharmacological properties and therapeutic efficacy in the management of schizophrenia and related psychoses. Drugs. 1997;53(2):281-298.
31. US Food and Drug Administration. Drugs@FDA: FDA approved drug products: Zyprexa (olanzapine). ORIG-1. http://www.accessdata.fda.gov/drugsatfda_docs/nda/96/020592_Original_Approval_Pkg%20.pdf. Published September 30, 1996. Accessed January 28, 2019.
32. Anzellotti F, Capasso M, Frazzini V, et al. Olanzapine-related repetitive focal seizures with lingual dystonia. Epileptic Disord. 2016;18(1):83-86.
33. Lee JW, Crismon ML, Dorson PG. Seizure associated with olanzapine. Ann Pharmac. 1999;33(5):554-556.
34. Woolley J, Smith S. Lowered seizure threshold on olanzapine. Br J Psychiatry. 2001;178(1):85-86.
35. Behere RV, Anjith D, Rao NP, et al. Olanzapine-induced clinical seizure: a case report. Clin Neuropharmacol. 2009;32(5):297-298.
36. Camacho A, García-Navarro M, Martínez B, et al. Olanzapine-induced myoclonic status. Clin Neuropharmacol. 2005;28(3):145-147.
37. Rosen JB, Milstein MJ, Haut SR. Olanzapine-associated myoclonus. Epilepsy Res. 2012;98(2-3):247-250.
38. Wyderski RJ, Starrett WG, Abou-Saif A. Fatal status epilepticus associated with olanzapine therapy. Ann Pharmacother. 1999;33(7-8):787-789.
39. Spyridi S, Sokolaki S, Nimatoudis J, et al. Status epilepticus in a patient treated with olanzapine and mirtazapine. Int J Clin Pharmacol Ther. 2009;47(2):120-123.
40. Schatzberg AF, Nemeroff CB. Essentials of clinical psychopharmacology. 2nd ed. Arlington, Virginia: American Psychiatric Publishing; 2006.
41. US Food and Drug Administration. Drug approval package: Geodon (Ziprasidone HCI) Capsules. Medical Review Part 2. http://www.accessdata.fda.gov/drugsatfda_docs/nda/2001/20-825_Geodan_medr_P2.pdf. Published February 5, 2001. Accessed January 29, 2019.
42. Clozaril [package insert]. East Hanover, NJ: Novartis; 2008.
43. Devinsky O, Pacia SV. Seizures during clozapine therapy. J Clin Psychiatry. 1994;55(suppl B):153-156.
44. Williams AM, Park SH. Seizure associated with clozapine: incidence, etiology, and management. CNS Drugs. 2015;29(2):101-111.
45. Wong J, Delva N. Clozapine-induced seizures: recognition and treatment. Can J Psychiatry. 2007;52(7):457-463.
46. Berman I, Zalma A, DuRand CJ, et al. Clozapine-induced myoclonic jerks and drop attacks. J Clin Psychiatry. 1992;53(9):329-330.
47. Gouzoulis E, Ozdaglar A, Kasper J. Myoclonic seizures followed by grand mal seizures during clozapine treatment. Am J Psychiatry. 1993;150(7):1128.
48. Sajatovic M, Meltzer HY. Clozapine-induced myoclonus and generalized seizures. Biol Psychiatry. 1996;39(5):367-370.
49. Grover S, Hazari N, Chakrabarti S, et al. Association of clozapine with seizures: a brief report involving 222 patients prescribed clozapine. East Asian Arch Psychiatry. 2015;25(2):73-78.
50. Byerly MJ, DeVane CL. Pharmacokinetics of clozapine and risperidone: a review of recent literature. J Clin Psychopharmacol. 1996;16(2):177-187.
51. Caetano D. Use of anticonvulsants as prophylaxis for seizures in patients on clozapine. Australas Psychiatry. 2014;22(1):78-83.
52. Perry PJ, Bever KA, Arndt S, et al. Relationship between patient variables and plasma clozapine concentrations: a dosing nomogram. Biol Psychiatry.1998;44(8):733-738.
53. Dumortier G, Mahé V, Pons D, et al. Clonic seizure associated with high clozapine plasma level. J Neuropsychiatry Clin Neurosci. 2001;13(2):302-303.
54. Funderburg LG, Vertrees JE, True JE, et al. Seizure following addition of erythromycin to clozapine treatment. Am J Psychiatry. 1994;151(12):1840-1841.
55. Varma S, Bishara D, Besag FMC, et al. Clozapine-related EEG changes and seizures: dose and plasma-level relationships. Ther Adv Psychopharmacol. 2011;1(2):47-66.
56. Amann BL, Pogarell O, Mergl R, et al. EEG abnormalities associated with antipsychotics: a comparison of quetiapine, olanzapine, haloperidol and healthy subjects. Hum Psychopharmacol. 2003;18(8):641-646.
57. Pisani F, Oteri G, Costa C, et al. Effects of psychotropic drugs on seizure threshold. Drug Saf. 2002;25(2):91-110.
58. Maurice T, Phan VL, Urani A, et al. Neuroactive neurosteroids as endogenous effectors for the sigma1 (sigma1) receptor: pharmacological evidence and therapeutic opportunities. Jpn J Pharmacol. 1999;81(2):125-155.
59. Haller E, Binder RL. Clozapine and seizures. Am J Psychiatry. 1990;147(8):1069-1071.
60. Torta R, Monaco F. Atypical antipsychotics and serotoninergic antidepressants in patients with epilepsy: pharmacodynamic considerations. Epilepsia. 2002;43(suppl 2):8-13.
61. Spina E. Drug interactions. In: Shorvon S, Perucca E, Engel J Jr, eds. The treatment of epilepsy. 3rd ed. Oxford, UK: Blackwell Publishing; 2009:361-377.
62. Spina E, Perucca E. Clinical significance of pharmacokinetic interactions between antiepileptic and psychotropic drugs. Epilepsia. 2002;43(suppl 2):37-44.
63. de Leon J, Santoro V, D’Arrigo C, et al. Interactions between antiepileptics and second-generation antipsychotics. Expert Opin Drug Metab Toxicol. 2012;8(3):311-334.
64. Finley P, Warner D. Potential impact of valproic acid therapy on clozapine disposition. Biol Psychiatry. 1994;36(7):487-488.
65. Longo LP, Salzman C. Valproic acid effects on serum concentrations of clozapine and norclozapine. Am J Psychiatry. 1995;152(4):650.
66. Centorrino F, Baldessarini RJ, Kando J, et al. Serum concentrations of clozapine and its major metabolites: effects of cotreatment with fluoxetine or valproate. Am J Psychiatry. 1994;151(1):123-125.
67. Facciolà G, Avenoso A, Scordo MG, et al. Small effects of valproic acid on the plasma concentrations of clozapine and its major metabolites in patients with schizophrenic or affective disorders. Ther Drug Monit. 1999;21(3):341-345.
68. Hyde TM, Weinberger DR. Seizures and schizophrenia. Schizophr Bull. 1997;23(4):611-622.
69. Muzyk A, Gala G, Kahn DA. Use of lamotrigine in a patient with a clozapine-related seizure. J Psychiatr Pract. 2010;16(2):125-128.
70. Kikuchi YS, Sato W, Ataka K, et al. Clozapine-induced seizures, electroencephalography abnormalities, and clinical responses in Japanese patients with schizophrenia. Neuropsychiatr Dis Treat. 2014;10:1973-1978.
71. Taner E, Coşar B, Işik E. Clozapine-induced myoclonic seizures and valproic acid. Int J Psychiatry Clin Pract. 1998;2(1):53-55.
72. Liukkonen J, Koponen HJ, Nousiainen U. Clinical picture and long-term course of epileptic seizures that occur during clozapine treatment. Psychiatry Res. 1992;44(2):107-112.
73. Devinsky O, Honigfeld G, Patin J. Clozapine-related seizures. Neurology. 1991;41(3):369-371.
74. Foster R, Olajide D. A case of clozapine-induced tonic-clonic seizures managed with valproate: implications for clinical care. J Psychopharmacol. 2005;19(1):93-96.
75. Gandelman-Marton R, Theitler J, Klein C, et al. Phenytoin intoxication in a clozapine-related prolonged seizure. J Emerg Med. 2008;35(4):407-409.
76. Primavera A, Giberti L, Scotto P, et al. Nonconvulsive status epilepticus as a cause of confusion in later life: a report of 5 cases. Neuropsychobiology. 1994;30(2-3):148-152.
77. Boutros NN, Arfken C, Galderisi S, et al. The status of spectral EEG abnormality as a diagnostic test for schizophrenia. Schizophrenia Res. 2008;99(1-3):225-237.
78. Takahashi T, Cho RY, Mizuno T, et al. Antipsychotics reverse abnormal EEG complexity in drug-naïve schizophrenia: a multiscale entropy analysis. Neuroimage. 2010;51(1):173-182.
Antipsychotics, especially second-generation antipsychotics (SGAs), have been proven effective for treating psychosis as well as mood disorders.1,2 Because antipsychotics can lower the epileptogenic threshold, seizures are a serious potential adverse effect. Antipsychotics can cause isolated EEG abnormalities in 7% of patients with no history of epilepsy, and clinical seizures in .5% to 1.2% of such patients.3 Additionally, the neuropathophysiology underlying epilepsy can predispose patients to psychiatric disorders4; the estimated prevalence of psychosis in patients with epilepsy is approximately 7%.5 This review will shed light on the risk of clinical seizures related to antipsychotics.
Comparing seizure risk among antipsychotics
In a review of the World Health Organization’s adverse drug reactions database, Kumlien and Lundberg6 calculated the ratio of the number of reports of seizures to the total number of reports for each drug. They found that approximately 9% of all adverse drug reaction reports involving clozapine were due to seizures. Equivalent ratios were 5.90% for quetiapine, 4.91% for olanzapine, 3.68% for risperidone, 3.27% for haloperidol, and 2.59% for aripiprazole. Using the database of the Pharmacovigilance Unit of the Basque Country, Lertxundi et al7 reported a 3.2-fold increased risk of seizure with SGAs in comparison with first-generation antipsychotics (FGAs) (95% confidence interval [CI], 2.21 to 4.63), which went down to 2.08 (CI, 1.39 to 3.12) once clozapine was excluded. However, as the authors of both studies noted, the quality and relevance of this data are limited because it relies on spontaneous reporting.
Overall, the evidence regarding the seizure risk associated with antipsychotics is scarce. To the best of our knowledge, only 2 large observational studies have compared the seizure risks associated with different antipsychotics.
Using data from the UK-based Clinical Practice Research Datalink between 1998 and 2013, Bloechlinger et al8 examined the incidence rates of seizures among patients newly diagnosed with schizophrenia, affective disorders, or dementia who were prescribed antipsychotics. They excluded patients with a history of seizures or antiepileptic use. In the cohort of 60,121 patients, the incidence rates of seizures per 10,000 person-years were 11.7 (CI, 10.0 to 13.4) for those who did not use antipsychotics, 12.4 (CI, 10.9 to 13.8) for past users, 115.4 (CI, 50.1 to 180.7) for current users of haloperidol, 48.8 (CI, 30.7 to 66.9) for current users of quetiapine, 25.9 (CI, 11.8 to 40.0) for current users of risperidone, and 19.0 (CI, 8.7 to 29.3) for current users of olanzapine. No data were available about clozapine use.
In subsequent analyses, the authors found that among patients with affective disorders, only current use of medium- to high-potency FGAs (haloperidol, prochlorperazine, and trifluoperazine) was associated with a significantly increased risk of seizures (adjusted odds ratio: 2.51, CI, 1.51 to 4.18) compared with non-users.8 Among patients with dementia, current use of olanzapine or quetiapine and current use of any FGAs were associated with significantly increased odds of seizures. This study suggests that the underlying mental illness might modulate the seizure risk associated with antipsychotics.8
Wu et al9 conducted a study based on the National Health Insurance Research Database in Taiwan. They examined the 1-year incidence of new-onset seizures among patients diagnosed with schizophrenia or mood disorders who were new to antipsychotic treatment, and calculated the risk of seizure associated with each antipsychotic in reference to risperidone. They found that those receiving clozapine, thioridazine, and haloperidol were 2 to 3 times more likely to develop seizures than those treated with risperidone; risks associated with the rest of the FGAs were similar to that of risperidone.
The results of these 2 large cohort studies are somewhat concurrent in indicating that, other than clozapine, SGAs incur similar risks of seizures; furthermore, they specify that, contrary to earlier studies,10 haloperidol is associated with significantly higher odds of seizures. While both of these cohort studies controlled for several sociodemographic and clinical confounders, they have several limitations. First, diagnoses of seizures were based on information available in databases, which might be subject to inaccuracies. Second, neither study evaluated the effect of drug dosage and duration of exposure on new-onset seizures.
Continue to: Most evidence is from case reports
Most evidence is from case reports
Other than these 2 large studies, most of the evidence addressing the relationship between the use of antipsychotics and incidence of seizures is low quality and relies on case reports or expert opinions. Older studies found that, among FGAs, seizure risk is highest with chlorpromazine and promazine, and lowest with thioridazine and haloperidol.10 As for SGAs, case reports have described seizuresassociated with the use of quetiapine, aripiprazole, risperidone, paliperidone, and olanzapine.
Quetiapine. Three case reports published between 2002 and 2010 describe generalized
Aripiprazole. Five case reports described staring spells and tonic-clonic seizures in patients receiving 10 to 15 mg of aripiprazole.15-19 In the New Drug Application (NDA) for aripiprazole, the incidence of seizures was estimated to be .11% (1 of 926 patients) in placebo-controlled trials and .46% (3 of 859 patients) in haloperidol-controlled trials.20
Risperidone’s product labeling suggests the drug should be used with caution in patients with a history of seizures or conditions that could result in a lower seizure threshold. In Phase III placebo-controlled trials, seizures occurred in .3% of patients treated with risperidone, although in some cases, the seizures were induced by electrolyte disturbances such as hyponatremia.21 Gonzalez-Heydrich et al22 and Holzhausen et al23 found no increase in seizure activity among patients with epilepsy who were receiving risperidone. Lane et al24 published a case report of a geriatric woman who presented with a generalized tonic-clonic seizure related to rapid titration of risperidone; however, with slower titration and lower doses, she stopped having seizures without adding any antiepileptic drugs. Komossa et al25 found that risperidone is less epileptogenic than clozapine, with a relative risk of .22.
Paliperidone is the active metabolite of risperidone and does not have pharmacokinetic interactions with drugs metabolized by the cytochrome P450 (CYP) enzymes. Its labeling indicates that the drug should be used with caution in patients with a history of seizures.26 In Phase III placebo-controlled trials of paliperidone, the rate of seizures was .22%.27 Two case reports suggest close monitoring of seizure risk in patients receiving paliperidone.28,29 Liang et al29 reported that co-administration of valproic acid could mask an underlying decrease of the seizure threshold caused by antipsychotics such as paliperidone.
Continue to: Olanzapine
Olanzapine is a thienobenzodiazepine derivative and is chemically related to clozapine.30 The olanzapine NDA31 shows that 23 of 3,139 patients developed seizures, mainly tonic-clonic, with evidence suggesting that the seizures may have been due to confounding factors such as a history of seizures or metabolic abnormalities. There were no statistically significant differences in the rate of seizures associated with olanzapine compared with placebo or haloperidol (P = .252 and .168, respectively).
A literature review for olanzapine yielded 1 case report of repetitive focal seizures and lingual dystonia,32 5 case reports of generalized tonic-clonic seizures and myoclonus,33-37 and 2 case reports of status epilepticus.38,39 Olanzapine’s clearance is 25% to 30% lower in women, and most of these case reports occurred women.40
Details of the above case reports are summarized in Table 1 (aripiprazole15-19), Table 2 (olanzapine32-39), and Table 3 (paliperidone,28,29 quetiapine,11-13 and risperidone22-24).
Ziprasidone. According to the NDA safety database, the seizure rate attributed to ziprasidone was 1.8 per 100 subject-years or 0.54% of participants (12 of 2,588).41 No additional studies have been published regarding its seizure risk.
Clozapine has a black-box warning
To the best of our knowledge, clozapine is the only antipsychotic that carries an FDA “black-box” warning regarding its risk of inducing seizures.42 Devinsky and Pacia43 reported a cumulative risk of 10% after 3.8 years of treatment. The literature has described clozapine-induced generalized tonic-clonic, myoclonic, simple and complex partial, and absence seizures.44 Table 445 lists the estimated frequency of each seizure type based on 101 cases of clozapine-induced seizures. Myoclonic seizures and drop attacks could be precursors/warning signs of grand mal tonic-clonic seizures.46,47 Seizures have been observed at all stages of treatment, but were more common during initiation of clozapine, which emphasizes the importance of a progressive and slow titration.43,48 The incidence of seizures was estimated to be 6% in a sample of 216 patients with schizophrenia with no history of epilepsy who were prescribed clozapine.49
Continue to: Regarding a possible association between...
Regarding a possible association between clozapine dose or clozapine plasma levels and seizure risk, there is a positive linear relationship between the dose of clozapine and its serum concentration over a dosing range of 25 to 800 mg/d.50 However, the plasma concentration is also significantly affected by factors such as smoking, gender, age, drug interactions, and CYP genotypes. Therefore, the same clozapine dose will yield a lower serum concentration in an older male who smokes compared with a younger, non-smoking female.51 Perry et al52 suggested a dosing nomogram to calculate the influence of gender and smoking. Seizure risk, especially for tonic-clonic seizures, has been reported to increase with clozapine doses >600 mg/d,53 and with plasma concentrations exceeding 1,000 to 1,300 mg/L.54 However, in a 2011 regression analysis, Varma et al55 found no statistically significant relationship between seizure risk and clozapine oral dose; there was not enough data to test a correlation between clozapine plasma levels and the incidence of seizures.
How antipsychotics might lower the seizure threshold
Researchers have suggested several possible mechanisms to explain how antipsychotics might lower the seizure threshold. Antagonism of dopamine D4, histamine H1, and acetylcholine-muscarinic receptors seems to induce EEG alterations and increase the risk of seizures.56 Additionally, modulation of the N-methyl-
Watch for pharmacokinetic interactions
The CYP enzymes involved in drug metabolism include CYP1A2, CYP2C9, CYP2C19, CYP2D6, and CYP3A4. Most commonly used antiepileptics and antipsychotics are metabolized by CYP enzymes, and may also act as inhibitors or inducers of these enzymes.61 Drug interactions may impair seizure control, which is why monotherapy is preferable to combination treatment in patients with epilepsy.62 Carbamazepine and phenytoin are inducers of both CYP1A2 (which metabolizes olanzapine and clozapine), and CYP3A4 (which metabolizes haloperidol, risperidone, quetiapine, ziprasidone and clozapine). Paliperidone is not metabolized by CYP enzymes.62 Discontinuing an enzyme-inducing agent may result in increased antipsychotic plasma concentrations, which might lead to an increased risk of seizures.
Valproic acid, which is often used to prevent or treat clozapine-induced seizures, has an unclear effect on clozapine plasma concentrations.63 Although valproic acid is known to inhibit clozapine metabolism, 2 reports have suggested that the plasma concentrations of clozapine and its metabolites may decrease after adding valproic acid.64,65 Other studies have found that valproic acid increases plasma concentrations of clozapine while it decreases plasma concentrations of norclozapine; norclozapine is the main clozapine metabolite responsible for inducing seizures.66,67
Steps for minimizing seizure risk
Determining the seizure risk for a patient taking an antipsychotic is challenging because doing so depends not only on the seizurogenic potential of each drug but also on individualized predisposing factors.11,57,68 Choosing the “best” antipsychotic therefore largely depends on each patient’s profile. The predisposing factors consist mainly of the individually inherited seizure threshold (personal history of febrile convulsions or a family history of seizures) and other comorbid seizurogenic conditions, such as a history of head trauma, brain injury, intellectual disability, cerebral arteriosclerosis, neurodegenerative diseases, encephalopathy, chronic renal insufficiency, and hyponatremia. Furthermore, seizure risk depends on the antipsychotic dose administered and the rate of titration.11
Continue to: There is not enough evidence...
There is not enough evidence to recommend performing an EEG in all patients taking antipsychotics. Such testing is recommended only for patients who have predisposing factors for seizures. If an EEG shows any abnormality in a patient taking clozapine, consider decreasing the clozapine dose69,70 or adding an antiepileptic drug such as valproic acid or lamotrigine.44,70
Although clozapine carries a black-box warning of increased risk of causing seizures, there is no consensus regarding the efficacy of co-prescribing an antiepileptic. Some studies have suggested prescribing valproic acid prophylactically,71 after the occurrence of 1 seizure,59 or after 2 seizures.54,72 Others have recommended prescribing prophylactic valproic acid for patients taking ≥600 mg/d of clozapine or whose clozapine plasma levels are >500 mg/L.73 Varma et al55 recommended starting an antiepileptic medication if there are clear epileptiform discharges on EEG, if the patient develops stuttering or speech difficulties, or if seizures occur. Liukkonen et al72 advised initiating an antiepileptic at the start of clozapine treatment in patients who are taking other epileptogenic medications, patients with pre-existing seizure disorder, and patients with neurologic abnormalities. On the other hand, Caetano51 argued against primary prevention of seizures for patients receiving >600 mg/d of clozapine, suggesting that “the risk of seizures would be better managed by close clinical monitoring and measures of clozapine serum concentration rather than adding an anticonvulsant drug.”
Current recommendations for primary and secondary prevention of clozapine-induced seizures are detailed in Table 5.42,44,45,51,55,57,69,74,75
Studies addressing the seizurogenic potential of SGAs other than clozapine have a low level of evidence and include patients who had comorbid conditions and were taking other medications that could cause seizures. Additionally, clinical trials of SGAs rarely include patients with seizure disorders; this might underestimate the risk of seizures.4
The effect of the mental illness itself on the seizure threshold needs to be considered.43 Bloechlinger et al8 found that dementia might be inherently associated with a higher risk of antipsychotic-related seizures. Moreover, numerous qualitative EEG studies have found abnormalities in 20% to 60% of patients with schizophrenia.56 Other quantitative studies have reported mild and nonspecific EEG abnormalities, such as increased delta and/or theta activity, in many non-medicated patients with schizophrenia.10,76 Additionally, brain tissue analysis of deceased patients who had schizophrenia has shown a significant increase in dopamine concentrations in the left amygdala compared with controls, and this might be responsible for enhanced electrical activity in this region.10 Some studies have described EEG slowing in the frontal brain regions of patients with schizophrenia,77 and was selectively normalized in these areas with antipsychotics.78
As always, start low, go slow
Mounting evidence suggests that antipsychotic medications decrease the seizure threshold. Practitioners should thus be cautious in prescribing antipsychotics and should target reaching the minimal effective dose with slow titration, especially in patients with predisposing factors for epilepsy.
Continue to: Although evidence suggests...
Although evidence suggests antipsychotics can induce different types of epileptic seizures, the quality of this evidence is low. Randomized controlled trials are needed to determine which antipsychotics increase seizure risk and whether there is a dose-effect relationship.
Bottom Line
Among second-generation antipsychotics, clozapine appears to increase the risk of clinical seizure the most. Correlations with dosage and/or plasma levels have not been proven. Psychiatrists should be vigilant for pharmacokinetic interactions between antipsychotics and antiepileptics, notably via CYP1A2 and CYP3A4.
Related Resources
- Druschky K, Bleich S, Grohmann R, et al. Seizure rates under treatment with antipsychotic drugs: Data from the AMSP project. World J Biol Psychiatry. 2018;15:1-10.
- Epilepsy Foundation. For professionals: Antipsychotics. https://www.epilepsy.com/learn/professionals/diagnosistreatment/psychotropic-drugs-developmental-disabilities/comorbid-5.
Drug Brand Names
Aripiprazole • Abilify
Benztropine • Cogentin
Bethanechol • Duvoid
Carbamazepine • Carbatrol, Tegretol
Chlorpromazine • Thorazine
Cimetidine • Tagamet
Ciprofloxacin • Cipro
Citalopram • Celexa
Clonazepam • Klonopin
Clozapine • Clozaril
Donepezil • Aricept
Enalapril • Vasotec
Erythromycin • Erythrocin
Escitalopram • Lexapro
Flunitrazepam • Rohypnol
Fluvoxamine • Luvox
Gabapentin • Neurontin
Haloperidol • Haldol
Lamotrigine • Lamictal
Lithium • Eskalith, Lithobid
Metformin • Fortamet, Glucophage
Mirtazapine • Remeron
Nitrofurantoin • Furadantin
Olanzapine • Zyprexa
Paliperidone • Invega
Phenobarbital • Luminal
Phenytoin • Dilantin
Prochlorperazine • Compazine
Procyclidine • Kemadrin
Propranolol • Inderal
Quetiapine • Seroquel
Risperidone • Risperdal
Sertraline • Zoloft
Simvastatin • Zocor
Sulfamethoxazole/trimethoprim • Bactrim, Sulfatrim
Topiramate • Topamax
Trifluoperazine • Stelazine
Valproic acid • Depakene, Depakote
Ziprasidone • Geodon
Antipsychotics, especially second-generation antipsychotics (SGAs), have been proven effective for treating psychosis as well as mood disorders.1,2 Because antipsychotics can lower the epileptogenic threshold, seizures are a serious potential adverse effect. Antipsychotics can cause isolated EEG abnormalities in 7% of patients with no history of epilepsy, and clinical seizures in .5% to 1.2% of such patients.3 Additionally, the neuropathophysiology underlying epilepsy can predispose patients to psychiatric disorders4; the estimated prevalence of psychosis in patients with epilepsy is approximately 7%.5 This review will shed light on the risk of clinical seizures related to antipsychotics.
Comparing seizure risk among antipsychotics
In a review of the World Health Organization’s adverse drug reactions database, Kumlien and Lundberg6 calculated the ratio of the number of reports of seizures to the total number of reports for each drug. They found that approximately 9% of all adverse drug reaction reports involving clozapine were due to seizures. Equivalent ratios were 5.90% for quetiapine, 4.91% for olanzapine, 3.68% for risperidone, 3.27% for haloperidol, and 2.59% for aripiprazole. Using the database of the Pharmacovigilance Unit of the Basque Country, Lertxundi et al7 reported a 3.2-fold increased risk of seizure with SGAs in comparison with first-generation antipsychotics (FGAs) (95% confidence interval [CI], 2.21 to 4.63), which went down to 2.08 (CI, 1.39 to 3.12) once clozapine was excluded. However, as the authors of both studies noted, the quality and relevance of this data are limited because it relies on spontaneous reporting.
Overall, the evidence regarding the seizure risk associated with antipsychotics is scarce. To the best of our knowledge, only 2 large observational studies have compared the seizure risks associated with different antipsychotics.
Using data from the UK-based Clinical Practice Research Datalink between 1998 and 2013, Bloechlinger et al8 examined the incidence rates of seizures among patients newly diagnosed with schizophrenia, affective disorders, or dementia who were prescribed antipsychotics. They excluded patients with a history of seizures or antiepileptic use. In the cohort of 60,121 patients, the incidence rates of seizures per 10,000 person-years were 11.7 (CI, 10.0 to 13.4) for those who did not use antipsychotics, 12.4 (CI, 10.9 to 13.8) for past users, 115.4 (CI, 50.1 to 180.7) for current users of haloperidol, 48.8 (CI, 30.7 to 66.9) for current users of quetiapine, 25.9 (CI, 11.8 to 40.0) for current users of risperidone, and 19.0 (CI, 8.7 to 29.3) for current users of olanzapine. No data were available about clozapine use.
In subsequent analyses, the authors found that among patients with affective disorders, only current use of medium- to high-potency FGAs (haloperidol, prochlorperazine, and trifluoperazine) was associated with a significantly increased risk of seizures (adjusted odds ratio: 2.51, CI, 1.51 to 4.18) compared with non-users.8 Among patients with dementia, current use of olanzapine or quetiapine and current use of any FGAs were associated with significantly increased odds of seizures. This study suggests that the underlying mental illness might modulate the seizure risk associated with antipsychotics.8
Wu et al9 conducted a study based on the National Health Insurance Research Database in Taiwan. They examined the 1-year incidence of new-onset seizures among patients diagnosed with schizophrenia or mood disorders who were new to antipsychotic treatment, and calculated the risk of seizure associated with each antipsychotic in reference to risperidone. They found that those receiving clozapine, thioridazine, and haloperidol were 2 to 3 times more likely to develop seizures than those treated with risperidone; risks associated with the rest of the FGAs were similar to that of risperidone.
The results of these 2 large cohort studies are somewhat concurrent in indicating that, other than clozapine, SGAs incur similar risks of seizures; furthermore, they specify that, contrary to earlier studies,10 haloperidol is associated with significantly higher odds of seizures. While both of these cohort studies controlled for several sociodemographic and clinical confounders, they have several limitations. First, diagnoses of seizures were based on information available in databases, which might be subject to inaccuracies. Second, neither study evaluated the effect of drug dosage and duration of exposure on new-onset seizures.
Continue to: Most evidence is from case reports
Most evidence is from case reports
Other than these 2 large studies, most of the evidence addressing the relationship between the use of antipsychotics and incidence of seizures is low quality and relies on case reports or expert opinions. Older studies found that, among FGAs, seizure risk is highest with chlorpromazine and promazine, and lowest with thioridazine and haloperidol.10 As for SGAs, case reports have described seizuresassociated with the use of quetiapine, aripiprazole, risperidone, paliperidone, and olanzapine.
Quetiapine. Three case reports published between 2002 and 2010 describe generalized
Aripiprazole. Five case reports described staring spells and tonic-clonic seizures in patients receiving 10 to 15 mg of aripiprazole.15-19 In the New Drug Application (NDA) for aripiprazole, the incidence of seizures was estimated to be .11% (1 of 926 patients) in placebo-controlled trials and .46% (3 of 859 patients) in haloperidol-controlled trials.20
Risperidone’s product labeling suggests the drug should be used with caution in patients with a history of seizures or conditions that could result in a lower seizure threshold. In Phase III placebo-controlled trials, seizures occurred in .3% of patients treated with risperidone, although in some cases, the seizures were induced by electrolyte disturbances such as hyponatremia.21 Gonzalez-Heydrich et al22 and Holzhausen et al23 found no increase in seizure activity among patients with epilepsy who were receiving risperidone. Lane et al24 published a case report of a geriatric woman who presented with a generalized tonic-clonic seizure related to rapid titration of risperidone; however, with slower titration and lower doses, she stopped having seizures without adding any antiepileptic drugs. Komossa et al25 found that risperidone is less epileptogenic than clozapine, with a relative risk of .22.
Paliperidone is the active metabolite of risperidone and does not have pharmacokinetic interactions with drugs metabolized by the cytochrome P450 (CYP) enzymes. Its labeling indicates that the drug should be used with caution in patients with a history of seizures.26 In Phase III placebo-controlled trials of paliperidone, the rate of seizures was .22%.27 Two case reports suggest close monitoring of seizure risk in patients receiving paliperidone.28,29 Liang et al29 reported that co-administration of valproic acid could mask an underlying decrease of the seizure threshold caused by antipsychotics such as paliperidone.
Continue to: Olanzapine
Olanzapine is a thienobenzodiazepine derivative and is chemically related to clozapine.30 The olanzapine NDA31 shows that 23 of 3,139 patients developed seizures, mainly tonic-clonic, with evidence suggesting that the seizures may have been due to confounding factors such as a history of seizures or metabolic abnormalities. There were no statistically significant differences in the rate of seizures associated with olanzapine compared with placebo or haloperidol (P = .252 and .168, respectively).
A literature review for olanzapine yielded 1 case report of repetitive focal seizures and lingual dystonia,32 5 case reports of generalized tonic-clonic seizures and myoclonus,33-37 and 2 case reports of status epilepticus.38,39 Olanzapine’s clearance is 25% to 30% lower in women, and most of these case reports occurred women.40
Details of the above case reports are summarized in Table 1 (aripiprazole15-19), Table 2 (olanzapine32-39), and Table 3 (paliperidone,28,29 quetiapine,11-13 and risperidone22-24).
Ziprasidone. According to the NDA safety database, the seizure rate attributed to ziprasidone was 1.8 per 100 subject-years or 0.54% of participants (12 of 2,588).41 No additional studies have been published regarding its seizure risk.
Clozapine has a black-box warning
To the best of our knowledge, clozapine is the only antipsychotic that carries an FDA “black-box” warning regarding its risk of inducing seizures.42 Devinsky and Pacia43 reported a cumulative risk of 10% after 3.8 years of treatment. The literature has described clozapine-induced generalized tonic-clonic, myoclonic, simple and complex partial, and absence seizures.44 Table 445 lists the estimated frequency of each seizure type based on 101 cases of clozapine-induced seizures. Myoclonic seizures and drop attacks could be precursors/warning signs of grand mal tonic-clonic seizures.46,47 Seizures have been observed at all stages of treatment, but were more common during initiation of clozapine, which emphasizes the importance of a progressive and slow titration.43,48 The incidence of seizures was estimated to be 6% in a sample of 216 patients with schizophrenia with no history of epilepsy who were prescribed clozapine.49
Continue to: Regarding a possible association between...
Regarding a possible association between clozapine dose or clozapine plasma levels and seizure risk, there is a positive linear relationship between the dose of clozapine and its serum concentration over a dosing range of 25 to 800 mg/d.50 However, the plasma concentration is also significantly affected by factors such as smoking, gender, age, drug interactions, and CYP genotypes. Therefore, the same clozapine dose will yield a lower serum concentration in an older male who smokes compared with a younger, non-smoking female.51 Perry et al52 suggested a dosing nomogram to calculate the influence of gender and smoking. Seizure risk, especially for tonic-clonic seizures, has been reported to increase with clozapine doses >600 mg/d,53 and with plasma concentrations exceeding 1,000 to 1,300 mg/L.54 However, in a 2011 regression analysis, Varma et al55 found no statistically significant relationship between seizure risk and clozapine oral dose; there was not enough data to test a correlation between clozapine plasma levels and the incidence of seizures.
How antipsychotics might lower the seizure threshold
Researchers have suggested several possible mechanisms to explain how antipsychotics might lower the seizure threshold. Antagonism of dopamine D4, histamine H1, and acetylcholine-muscarinic receptors seems to induce EEG alterations and increase the risk of seizures.56 Additionally, modulation of the N-methyl-
Watch for pharmacokinetic interactions
The CYP enzymes involved in drug metabolism include CYP1A2, CYP2C9, CYP2C19, CYP2D6, and CYP3A4. Most commonly used antiepileptics and antipsychotics are metabolized by CYP enzymes, and may also act as inhibitors or inducers of these enzymes.61 Drug interactions may impair seizure control, which is why monotherapy is preferable to combination treatment in patients with epilepsy.62 Carbamazepine and phenytoin are inducers of both CYP1A2 (which metabolizes olanzapine and clozapine), and CYP3A4 (which metabolizes haloperidol, risperidone, quetiapine, ziprasidone and clozapine). Paliperidone is not metabolized by CYP enzymes.62 Discontinuing an enzyme-inducing agent may result in increased antipsychotic plasma concentrations, which might lead to an increased risk of seizures.
Valproic acid, which is often used to prevent or treat clozapine-induced seizures, has an unclear effect on clozapine plasma concentrations.63 Although valproic acid is known to inhibit clozapine metabolism, 2 reports have suggested that the plasma concentrations of clozapine and its metabolites may decrease after adding valproic acid.64,65 Other studies have found that valproic acid increases plasma concentrations of clozapine while it decreases plasma concentrations of norclozapine; norclozapine is the main clozapine metabolite responsible for inducing seizures.66,67
Steps for minimizing seizure risk
Determining the seizure risk for a patient taking an antipsychotic is challenging because doing so depends not only on the seizurogenic potential of each drug but also on individualized predisposing factors.11,57,68 Choosing the “best” antipsychotic therefore largely depends on each patient’s profile. The predisposing factors consist mainly of the individually inherited seizure threshold (personal history of febrile convulsions or a family history of seizures) and other comorbid seizurogenic conditions, such as a history of head trauma, brain injury, intellectual disability, cerebral arteriosclerosis, neurodegenerative diseases, encephalopathy, chronic renal insufficiency, and hyponatremia. Furthermore, seizure risk depends on the antipsychotic dose administered and the rate of titration.11
Continue to: There is not enough evidence...
There is not enough evidence to recommend performing an EEG in all patients taking antipsychotics. Such testing is recommended only for patients who have predisposing factors for seizures. If an EEG shows any abnormality in a patient taking clozapine, consider decreasing the clozapine dose69,70 or adding an antiepileptic drug such as valproic acid or lamotrigine.44,70
Although clozapine carries a black-box warning of increased risk of causing seizures, there is no consensus regarding the efficacy of co-prescribing an antiepileptic. Some studies have suggested prescribing valproic acid prophylactically,71 after the occurrence of 1 seizure,59 or after 2 seizures.54,72 Others have recommended prescribing prophylactic valproic acid for patients taking ≥600 mg/d of clozapine or whose clozapine plasma levels are >500 mg/L.73 Varma et al55 recommended starting an antiepileptic medication if there are clear epileptiform discharges on EEG, if the patient develops stuttering or speech difficulties, or if seizures occur. Liukkonen et al72 advised initiating an antiepileptic at the start of clozapine treatment in patients who are taking other epileptogenic medications, patients with pre-existing seizure disorder, and patients with neurologic abnormalities. On the other hand, Caetano51 argued against primary prevention of seizures for patients receiving >600 mg/d of clozapine, suggesting that “the risk of seizures would be better managed by close clinical monitoring and measures of clozapine serum concentration rather than adding an anticonvulsant drug.”
Current recommendations for primary and secondary prevention of clozapine-induced seizures are detailed in Table 5.42,44,45,51,55,57,69,74,75
Studies addressing the seizurogenic potential of SGAs other than clozapine have a low level of evidence and include patients who had comorbid conditions and were taking other medications that could cause seizures. Additionally, clinical trials of SGAs rarely include patients with seizure disorders; this might underestimate the risk of seizures.4
The effect of the mental illness itself on the seizure threshold needs to be considered.43 Bloechlinger et al8 found that dementia might be inherently associated with a higher risk of antipsychotic-related seizures. Moreover, numerous qualitative EEG studies have found abnormalities in 20% to 60% of patients with schizophrenia.56 Other quantitative studies have reported mild and nonspecific EEG abnormalities, such as increased delta and/or theta activity, in many non-medicated patients with schizophrenia.10,76 Additionally, brain tissue analysis of deceased patients who had schizophrenia has shown a significant increase in dopamine concentrations in the left amygdala compared with controls, and this might be responsible for enhanced electrical activity in this region.10 Some studies have described EEG slowing in the frontal brain regions of patients with schizophrenia,77 and was selectively normalized in these areas with antipsychotics.78
As always, start low, go slow
Mounting evidence suggests that antipsychotic medications decrease the seizure threshold. Practitioners should thus be cautious in prescribing antipsychotics and should target reaching the minimal effective dose with slow titration, especially in patients with predisposing factors for epilepsy.
Continue to: Although evidence suggests...
Although evidence suggests antipsychotics can induce different types of epileptic seizures, the quality of this evidence is low. Randomized controlled trials are needed to determine which antipsychotics increase seizure risk and whether there is a dose-effect relationship.
Bottom Line
Among second-generation antipsychotics, clozapine appears to increase the risk of clinical seizure the most. Correlations with dosage and/or plasma levels have not been proven. Psychiatrists should be vigilant for pharmacokinetic interactions between antipsychotics and antiepileptics, notably via CYP1A2 and CYP3A4.
Related Resources
- Druschky K, Bleich S, Grohmann R, et al. Seizure rates under treatment with antipsychotic drugs: Data from the AMSP project. World J Biol Psychiatry. 2018;15:1-10.
- Epilepsy Foundation. For professionals: Antipsychotics. https://www.epilepsy.com/learn/professionals/diagnosistreatment/psychotropic-drugs-developmental-disabilities/comorbid-5.
Drug Brand Names
Aripiprazole • Abilify
Benztropine • Cogentin
Bethanechol • Duvoid
Carbamazepine • Carbatrol, Tegretol
Chlorpromazine • Thorazine
Cimetidine • Tagamet
Ciprofloxacin • Cipro
Citalopram • Celexa
Clonazepam • Klonopin
Clozapine • Clozaril
Donepezil • Aricept
Enalapril • Vasotec
Erythromycin • Erythrocin
Escitalopram • Lexapro
Flunitrazepam • Rohypnol
Fluvoxamine • Luvox
Gabapentin • Neurontin
Haloperidol • Haldol
Lamotrigine • Lamictal
Lithium • Eskalith, Lithobid
Metformin • Fortamet, Glucophage
Mirtazapine • Remeron
Nitrofurantoin • Furadantin
Olanzapine • Zyprexa
Paliperidone • Invega
Phenobarbital • Luminal
Phenytoin • Dilantin
Prochlorperazine • Compazine
Procyclidine • Kemadrin
Propranolol • Inderal
Quetiapine • Seroquel
Risperidone • Risperdal
Sertraline • Zoloft
Simvastatin • Zocor
Sulfamethoxazole/trimethoprim • Bactrim, Sulfatrim
Topiramate • Topamax
Trifluoperazine • Stelazine
Valproic acid • Depakene, Depakote
Ziprasidone • Geodon
1. Bruijnzeel D, Suryadevara U, Tandon R. Antipsychotic treatment of schizophrenia: an update. Asian J Psychiatr. 2014;11:3-7.
2. Hrdlicka M, Dudova I. Atypical antipsychotics in the treatment of early-onset schizophrenia. Neuropsychiatr Dis Treat. 2015;11:907-913.
3. Koch-Stoecker S. Antipsychotic drugs and epilepsy: indications and treatment guidelines. Epilepsia. 2002;43(suppl 2):19-24.
4. Alper K, Schwartz KA, Kolts RL, et al. Seizure incidence in psychopharmacological clinical trials: an analysis of Food and Drug Administration (FDA) summary basis of approval reports. Biol Psychiatry. 2007;62(4):345-354.
5. Torta R, Keller R. Behavioral, psychotic, and anxiety disorders in epilepsy: etiology, clinical features, and therapeutic implications. Epilepsia. 1999;40(suppl 10):S2-S20.
6. Kumlien E, Lundberg PO. Seizure risk associated with neuroactive drugs: data from the WHO adverse drug reactions database. Seizure. 2010;19(2):69-73.
7. Lertxundi U, Hernandez R, Medrano J, et al. Antipsychotics and seizures: higher risk with atypicals? Seizure. 2013;22(2):141-143.
8. Bloechliger M, Rüegg S, Jick SS, et al. Antipsychotic drug use and the risk of seizures: follow-up study with a nested case-control analysis. CNS Drugs. 2015;29(7):591-603.
9. Wu CS, Wang SC, Yeh IJ, et al. Comparative risk of seizure with use of first- and second-generation antipsychotics in patients with schizophrenia and mood disorders. J Clin Psychiatry. 2016;77(5):e573-e579.
10. Cold JA, Wells BG, Froemming JH. Seizure activity associated with antipsychotic therapy. [Erratum in DICP. 1990;24(10):1012.] DICP. 1990;24(6):601-606.
11. Hedges DW, Jeppson KG. New-onset seizure associated with quetiapine and olanzapine. Ann Pharmacother. 2002;36(3):437-439.
12. Dogu O, Sevim S, Kaleagasi HS. Seizures associated with quetiapine treatment. Ann Pharmacother. 2003;37(9):1224-1227.
13. Young AC, Kleinschmidt KC, Wax PM. Late-onset seizures associated with quetiapine poisoning. J Med Toxicol. 2009;5(1):24-26.
14. US Food and Drug Administration. Recommendation of approvable action for quetiapine fumarate extended release (Seroquel® XR) for the treatment of schizophrenia. http://www.accessdata.fda.gov/drugsatfda_docs/nda/2007/022047Orig1s000MedR.pdf. April 24, 2007. Accessed January 28, 2019.
15. Malik AR, Ravasia S. Aripiprazole-induced seizure. Can J Psychiatry. 2005;50(3):186.
16. Tsai JF. Aripiprazole-associated seizure. J Clin Psychiatry. 2006;67(6):995-996.
17. Arora M, Arndorfer L. EEG abnormalities in a patient taking aripiprazole. Psychiatry (Edgmont). 2007;4(7):18-19.
18. Yueh CL, Yu SL, Chen HM, et al. Aripiprazole-induced seizure: a second case report. BMJ case reports. 2009;2009:bcr03.2009.1693. doi: 10.1136/bcr.03.2009.1693.
19. Thabet FI, Sweis RT, Joseph SA. Aripiprazole-induced seizure in a 3-year-old child: a case report and literature review. Clin Neuropharmacol. 2013;36(1):29-30.
20. US Food and Drug Administration. Abilify (Aripiprazole) tablets. Medical Review Part 2. http://www.accessdata.fda.gov/drugsatfda_docs/nda/2002/21-436_Abilify_medr_P2.pdf. Published March 07, 2003. Accessed January 28, 2019.
21. US Food and Drug Administration. Drugs@FDA: FDA approved drug products. Risperdal tablets, Risperdal oral solution & Risperdal M-tab orally disintegrating tablets. http://www.accessdata.fda.gov/drugsatfda_docs/nda/2003/021444_S004_RISPERDAL_TABLETS.pdf. Published September 10, 2003. Accessed January 28, 2019.
22. Gonzalez-Heydrich J, Pandina GJ, Fleisher CA, et al. No seizure exacerbation from risperidone in youth with comorbid epilepsy and psychiatric disorders: a case series. J Child Adolesc Psychopharmacol. 2004;14(2):295-310.
23. Holzhausen SPF, Guerreiro MM, Baccin CE, et al. Use of risperidone in children with epilepsy. Epilepsy Behav. 2007;10(3):412-416.
24. Lane HY, Chang WH, Chou JC. Seizure during risperidone treatment in an elderly woman treated with concomitant medications. J Clinl Psychiatry. 1998;59(2):81-82.
25. Komossa K, Rummel-Kluge C, Schwarz S, et al. Risperidone versus other atypical antipsychotics for schizophrenia. Cochrane Database Syst Rev. 2011;(1):19:CD006626.
26. Paliperidone [package insert]. Mountainville, CA: Janssen Pharmaceuticals, Inc.; 2007.
27. Brugge, MD; US Food and Drug Administration. Paliperidone OROS oral formulation. http://www.accessdata.fda.gov/drugsatfda_docs/nda/2006/021999s000_MedR_Part4.pdf. Accessed January 28, 2019.
28. Schneider RA, Lizer MH. Apparent seizure and atrial fibrillation associated with paliperidone. Am J Health System Pharm. 2008;65(22):2122-2125.
29. Liang CS, Yang FW, Chiang KT. Paliperidone-associated seizure after discontinuation of sodium valproate: a case report. J Clin Psychopharmacol. 2011;31(2):246-247.
30. Fulton B, Goa KL. Olanzapine. A review of its pharmacological properties and therapeutic efficacy in the management of schizophrenia and related psychoses. Drugs. 1997;53(2):281-298.
31. US Food and Drug Administration. Drugs@FDA: FDA approved drug products: Zyprexa (olanzapine). ORIG-1. http://www.accessdata.fda.gov/drugsatfda_docs/nda/96/020592_Original_Approval_Pkg%20.pdf. Published September 30, 1996. Accessed January 28, 2019.
32. Anzellotti F, Capasso M, Frazzini V, et al. Olanzapine-related repetitive focal seizures with lingual dystonia. Epileptic Disord. 2016;18(1):83-86.
33. Lee JW, Crismon ML, Dorson PG. Seizure associated with olanzapine. Ann Pharmac. 1999;33(5):554-556.
34. Woolley J, Smith S. Lowered seizure threshold on olanzapine. Br J Psychiatry. 2001;178(1):85-86.
35. Behere RV, Anjith D, Rao NP, et al. Olanzapine-induced clinical seizure: a case report. Clin Neuropharmacol. 2009;32(5):297-298.
36. Camacho A, García-Navarro M, Martínez B, et al. Olanzapine-induced myoclonic status. Clin Neuropharmacol. 2005;28(3):145-147.
37. Rosen JB, Milstein MJ, Haut SR. Olanzapine-associated myoclonus. Epilepsy Res. 2012;98(2-3):247-250.
38. Wyderski RJ, Starrett WG, Abou-Saif A. Fatal status epilepticus associated with olanzapine therapy. Ann Pharmacother. 1999;33(7-8):787-789.
39. Spyridi S, Sokolaki S, Nimatoudis J, et al. Status epilepticus in a patient treated with olanzapine and mirtazapine. Int J Clin Pharmacol Ther. 2009;47(2):120-123.
40. Schatzberg AF, Nemeroff CB. Essentials of clinical psychopharmacology. 2nd ed. Arlington, Virginia: American Psychiatric Publishing; 2006.
41. US Food and Drug Administration. Drug approval package: Geodon (Ziprasidone HCI) Capsules. Medical Review Part 2. http://www.accessdata.fda.gov/drugsatfda_docs/nda/2001/20-825_Geodan_medr_P2.pdf. Published February 5, 2001. Accessed January 29, 2019.
42. Clozaril [package insert]. East Hanover, NJ: Novartis; 2008.
43. Devinsky O, Pacia SV. Seizures during clozapine therapy. J Clin Psychiatry. 1994;55(suppl B):153-156.
44. Williams AM, Park SH. Seizure associated with clozapine: incidence, etiology, and management. CNS Drugs. 2015;29(2):101-111.
45. Wong J, Delva N. Clozapine-induced seizures: recognition and treatment. Can J Psychiatry. 2007;52(7):457-463.
46. Berman I, Zalma A, DuRand CJ, et al. Clozapine-induced myoclonic jerks and drop attacks. J Clin Psychiatry. 1992;53(9):329-330.
47. Gouzoulis E, Ozdaglar A, Kasper J. Myoclonic seizures followed by grand mal seizures during clozapine treatment. Am J Psychiatry. 1993;150(7):1128.
48. Sajatovic M, Meltzer HY. Clozapine-induced myoclonus and generalized seizures. Biol Psychiatry. 1996;39(5):367-370.
49. Grover S, Hazari N, Chakrabarti S, et al. Association of clozapine with seizures: a brief report involving 222 patients prescribed clozapine. East Asian Arch Psychiatry. 2015;25(2):73-78.
50. Byerly MJ, DeVane CL. Pharmacokinetics of clozapine and risperidone: a review of recent literature. J Clin Psychopharmacol. 1996;16(2):177-187.
51. Caetano D. Use of anticonvulsants as prophylaxis for seizures in patients on clozapine. Australas Psychiatry. 2014;22(1):78-83.
52. Perry PJ, Bever KA, Arndt S, et al. Relationship between patient variables and plasma clozapine concentrations: a dosing nomogram. Biol Psychiatry.1998;44(8):733-738.
53. Dumortier G, Mahé V, Pons D, et al. Clonic seizure associated with high clozapine plasma level. J Neuropsychiatry Clin Neurosci. 2001;13(2):302-303.
54. Funderburg LG, Vertrees JE, True JE, et al. Seizure following addition of erythromycin to clozapine treatment. Am J Psychiatry. 1994;151(12):1840-1841.
55. Varma S, Bishara D, Besag FMC, et al. Clozapine-related EEG changes and seizures: dose and plasma-level relationships. Ther Adv Psychopharmacol. 2011;1(2):47-66.
56. Amann BL, Pogarell O, Mergl R, et al. EEG abnormalities associated with antipsychotics: a comparison of quetiapine, olanzapine, haloperidol and healthy subjects. Hum Psychopharmacol. 2003;18(8):641-646.
57. Pisani F, Oteri G, Costa C, et al. Effects of psychotropic drugs on seizure threshold. Drug Saf. 2002;25(2):91-110.
58. Maurice T, Phan VL, Urani A, et al. Neuroactive neurosteroids as endogenous effectors for the sigma1 (sigma1) receptor: pharmacological evidence and therapeutic opportunities. Jpn J Pharmacol. 1999;81(2):125-155.
59. Haller E, Binder RL. Clozapine and seizures. Am J Psychiatry. 1990;147(8):1069-1071.
60. Torta R, Monaco F. Atypical antipsychotics and serotoninergic antidepressants in patients with epilepsy: pharmacodynamic considerations. Epilepsia. 2002;43(suppl 2):8-13.
61. Spina E. Drug interactions. In: Shorvon S, Perucca E, Engel J Jr, eds. The treatment of epilepsy. 3rd ed. Oxford, UK: Blackwell Publishing; 2009:361-377.
62. Spina E, Perucca E. Clinical significance of pharmacokinetic interactions between antiepileptic and psychotropic drugs. Epilepsia. 2002;43(suppl 2):37-44.
63. de Leon J, Santoro V, D’Arrigo C, et al. Interactions between antiepileptics and second-generation antipsychotics. Expert Opin Drug Metab Toxicol. 2012;8(3):311-334.
64. Finley P, Warner D. Potential impact of valproic acid therapy on clozapine disposition. Biol Psychiatry. 1994;36(7):487-488.
65. Longo LP, Salzman C. Valproic acid effects on serum concentrations of clozapine and norclozapine. Am J Psychiatry. 1995;152(4):650.
66. Centorrino F, Baldessarini RJ, Kando J, et al. Serum concentrations of clozapine and its major metabolites: effects of cotreatment with fluoxetine or valproate. Am J Psychiatry. 1994;151(1):123-125.
67. Facciolà G, Avenoso A, Scordo MG, et al. Small effects of valproic acid on the plasma concentrations of clozapine and its major metabolites in patients with schizophrenic or affective disorders. Ther Drug Monit. 1999;21(3):341-345.
68. Hyde TM, Weinberger DR. Seizures and schizophrenia. Schizophr Bull. 1997;23(4):611-622.
69. Muzyk A, Gala G, Kahn DA. Use of lamotrigine in a patient with a clozapine-related seizure. J Psychiatr Pract. 2010;16(2):125-128.
70. Kikuchi YS, Sato W, Ataka K, et al. Clozapine-induced seizures, electroencephalography abnormalities, and clinical responses in Japanese patients with schizophrenia. Neuropsychiatr Dis Treat. 2014;10:1973-1978.
71. Taner E, Coşar B, Işik E. Clozapine-induced myoclonic seizures and valproic acid. Int J Psychiatry Clin Pract. 1998;2(1):53-55.
72. Liukkonen J, Koponen HJ, Nousiainen U. Clinical picture and long-term course of epileptic seizures that occur during clozapine treatment. Psychiatry Res. 1992;44(2):107-112.
73. Devinsky O, Honigfeld G, Patin J. Clozapine-related seizures. Neurology. 1991;41(3):369-371.
74. Foster R, Olajide D. A case of clozapine-induced tonic-clonic seizures managed with valproate: implications for clinical care. J Psychopharmacol. 2005;19(1):93-96.
75. Gandelman-Marton R, Theitler J, Klein C, et al. Phenytoin intoxication in a clozapine-related prolonged seizure. J Emerg Med. 2008;35(4):407-409.
76. Primavera A, Giberti L, Scotto P, et al. Nonconvulsive status epilepticus as a cause of confusion in later life: a report of 5 cases. Neuropsychobiology. 1994;30(2-3):148-152.
77. Boutros NN, Arfken C, Galderisi S, et al. The status of spectral EEG abnormality as a diagnostic test for schizophrenia. Schizophrenia Res. 2008;99(1-3):225-237.
78. Takahashi T, Cho RY, Mizuno T, et al. Antipsychotics reverse abnormal EEG complexity in drug-naïve schizophrenia: a multiscale entropy analysis. Neuroimage. 2010;51(1):173-182.
1. Bruijnzeel D, Suryadevara U, Tandon R. Antipsychotic treatment of schizophrenia: an update. Asian J Psychiatr. 2014;11:3-7.
2. Hrdlicka M, Dudova I. Atypical antipsychotics in the treatment of early-onset schizophrenia. Neuropsychiatr Dis Treat. 2015;11:907-913.
3. Koch-Stoecker S. Antipsychotic drugs and epilepsy: indications and treatment guidelines. Epilepsia. 2002;43(suppl 2):19-24.
4. Alper K, Schwartz KA, Kolts RL, et al. Seizure incidence in psychopharmacological clinical trials: an analysis of Food and Drug Administration (FDA) summary basis of approval reports. Biol Psychiatry. 2007;62(4):345-354.
5. Torta R, Keller R. Behavioral, psychotic, and anxiety disorders in epilepsy: etiology, clinical features, and therapeutic implications. Epilepsia. 1999;40(suppl 10):S2-S20.
6. Kumlien E, Lundberg PO. Seizure risk associated with neuroactive drugs: data from the WHO adverse drug reactions database. Seizure. 2010;19(2):69-73.
7. Lertxundi U, Hernandez R, Medrano J, et al. Antipsychotics and seizures: higher risk with atypicals? Seizure. 2013;22(2):141-143.
8. Bloechliger M, Rüegg S, Jick SS, et al. Antipsychotic drug use and the risk of seizures: follow-up study with a nested case-control analysis. CNS Drugs. 2015;29(7):591-603.
9. Wu CS, Wang SC, Yeh IJ, et al. Comparative risk of seizure with use of first- and second-generation antipsychotics in patients with schizophrenia and mood disorders. J Clin Psychiatry. 2016;77(5):e573-e579.
10. Cold JA, Wells BG, Froemming JH. Seizure activity associated with antipsychotic therapy. [Erratum in DICP. 1990;24(10):1012.] DICP. 1990;24(6):601-606.
11. Hedges DW, Jeppson KG. New-onset seizure associated with quetiapine and olanzapine. Ann Pharmacother. 2002;36(3):437-439.
12. Dogu O, Sevim S, Kaleagasi HS. Seizures associated with quetiapine treatment. Ann Pharmacother. 2003;37(9):1224-1227.
13. Young AC, Kleinschmidt KC, Wax PM. Late-onset seizures associated with quetiapine poisoning. J Med Toxicol. 2009;5(1):24-26.
14. US Food and Drug Administration. Recommendation of approvable action for quetiapine fumarate extended release (Seroquel® XR) for the treatment of schizophrenia. http://www.accessdata.fda.gov/drugsatfda_docs/nda/2007/022047Orig1s000MedR.pdf. April 24, 2007. Accessed January 28, 2019.
15. Malik AR, Ravasia S. Aripiprazole-induced seizure. Can J Psychiatry. 2005;50(3):186.
16. Tsai JF. Aripiprazole-associated seizure. J Clin Psychiatry. 2006;67(6):995-996.
17. Arora M, Arndorfer L. EEG abnormalities in a patient taking aripiprazole. Psychiatry (Edgmont). 2007;4(7):18-19.
18. Yueh CL, Yu SL, Chen HM, et al. Aripiprazole-induced seizure: a second case report. BMJ case reports. 2009;2009:bcr03.2009.1693. doi: 10.1136/bcr.03.2009.1693.
19. Thabet FI, Sweis RT, Joseph SA. Aripiprazole-induced seizure in a 3-year-old child: a case report and literature review. Clin Neuropharmacol. 2013;36(1):29-30.
20. US Food and Drug Administration. Abilify (Aripiprazole) tablets. Medical Review Part 2. http://www.accessdata.fda.gov/drugsatfda_docs/nda/2002/21-436_Abilify_medr_P2.pdf. Published March 07, 2003. Accessed January 28, 2019.
21. US Food and Drug Administration. Drugs@FDA: FDA approved drug products. Risperdal tablets, Risperdal oral solution & Risperdal M-tab orally disintegrating tablets. http://www.accessdata.fda.gov/drugsatfda_docs/nda/2003/021444_S004_RISPERDAL_TABLETS.pdf. Published September 10, 2003. Accessed January 28, 2019.
22. Gonzalez-Heydrich J, Pandina GJ, Fleisher CA, et al. No seizure exacerbation from risperidone in youth with comorbid epilepsy and psychiatric disorders: a case series. J Child Adolesc Psychopharmacol. 2004;14(2):295-310.
23. Holzhausen SPF, Guerreiro MM, Baccin CE, et al. Use of risperidone in children with epilepsy. Epilepsy Behav. 2007;10(3):412-416.
24. Lane HY, Chang WH, Chou JC. Seizure during risperidone treatment in an elderly woman treated with concomitant medications. J Clinl Psychiatry. 1998;59(2):81-82.
25. Komossa K, Rummel-Kluge C, Schwarz S, et al. Risperidone versus other atypical antipsychotics for schizophrenia. Cochrane Database Syst Rev. 2011;(1):19:CD006626.
26. Paliperidone [package insert]. Mountainville, CA: Janssen Pharmaceuticals, Inc.; 2007.
27. Brugge, MD; US Food and Drug Administration. Paliperidone OROS oral formulation. http://www.accessdata.fda.gov/drugsatfda_docs/nda/2006/021999s000_MedR_Part4.pdf. Accessed January 28, 2019.
28. Schneider RA, Lizer MH. Apparent seizure and atrial fibrillation associated with paliperidone. Am J Health System Pharm. 2008;65(22):2122-2125.
29. Liang CS, Yang FW, Chiang KT. Paliperidone-associated seizure after discontinuation of sodium valproate: a case report. J Clin Psychopharmacol. 2011;31(2):246-247.
30. Fulton B, Goa KL. Olanzapine. A review of its pharmacological properties and therapeutic efficacy in the management of schizophrenia and related psychoses. Drugs. 1997;53(2):281-298.
31. US Food and Drug Administration. Drugs@FDA: FDA approved drug products: Zyprexa (olanzapine). ORIG-1. http://www.accessdata.fda.gov/drugsatfda_docs/nda/96/020592_Original_Approval_Pkg%20.pdf. Published September 30, 1996. Accessed January 28, 2019.
32. Anzellotti F, Capasso M, Frazzini V, et al. Olanzapine-related repetitive focal seizures with lingual dystonia. Epileptic Disord. 2016;18(1):83-86.
33. Lee JW, Crismon ML, Dorson PG. Seizure associated with olanzapine. Ann Pharmac. 1999;33(5):554-556.
34. Woolley J, Smith S. Lowered seizure threshold on olanzapine. Br J Psychiatry. 2001;178(1):85-86.
35. Behere RV, Anjith D, Rao NP, et al. Olanzapine-induced clinical seizure: a case report. Clin Neuropharmacol. 2009;32(5):297-298.
36. Camacho A, García-Navarro M, Martínez B, et al. Olanzapine-induced myoclonic status. Clin Neuropharmacol. 2005;28(3):145-147.
37. Rosen JB, Milstein MJ, Haut SR. Olanzapine-associated myoclonus. Epilepsy Res. 2012;98(2-3):247-250.
38. Wyderski RJ, Starrett WG, Abou-Saif A. Fatal status epilepticus associated with olanzapine therapy. Ann Pharmacother. 1999;33(7-8):787-789.
39. Spyridi S, Sokolaki S, Nimatoudis J, et al. Status epilepticus in a patient treated with olanzapine and mirtazapine. Int J Clin Pharmacol Ther. 2009;47(2):120-123.
40. Schatzberg AF, Nemeroff CB. Essentials of clinical psychopharmacology. 2nd ed. Arlington, Virginia: American Psychiatric Publishing; 2006.
41. US Food and Drug Administration. Drug approval package: Geodon (Ziprasidone HCI) Capsules. Medical Review Part 2. http://www.accessdata.fda.gov/drugsatfda_docs/nda/2001/20-825_Geodan_medr_P2.pdf. Published February 5, 2001. Accessed January 29, 2019.
42. Clozaril [package insert]. East Hanover, NJ: Novartis; 2008.
43. Devinsky O, Pacia SV. Seizures during clozapine therapy. J Clin Psychiatry. 1994;55(suppl B):153-156.
44. Williams AM, Park SH. Seizure associated with clozapine: incidence, etiology, and management. CNS Drugs. 2015;29(2):101-111.
45. Wong J, Delva N. Clozapine-induced seizures: recognition and treatment. Can J Psychiatry. 2007;52(7):457-463.
46. Berman I, Zalma A, DuRand CJ, et al. Clozapine-induced myoclonic jerks and drop attacks. J Clin Psychiatry. 1992;53(9):329-330.
47. Gouzoulis E, Ozdaglar A, Kasper J. Myoclonic seizures followed by grand mal seizures during clozapine treatment. Am J Psychiatry. 1993;150(7):1128.
48. Sajatovic M, Meltzer HY. Clozapine-induced myoclonus and generalized seizures. Biol Psychiatry. 1996;39(5):367-370.
49. Grover S, Hazari N, Chakrabarti S, et al. Association of clozapine with seizures: a brief report involving 222 patients prescribed clozapine. East Asian Arch Psychiatry. 2015;25(2):73-78.
50. Byerly MJ, DeVane CL. Pharmacokinetics of clozapine and risperidone: a review of recent literature. J Clin Psychopharmacol. 1996;16(2):177-187.
51. Caetano D. Use of anticonvulsants as prophylaxis for seizures in patients on clozapine. Australas Psychiatry. 2014;22(1):78-83.
52. Perry PJ, Bever KA, Arndt S, et al. Relationship between patient variables and plasma clozapine concentrations: a dosing nomogram. Biol Psychiatry.1998;44(8):733-738.
53. Dumortier G, Mahé V, Pons D, et al. Clonic seizure associated with high clozapine plasma level. J Neuropsychiatry Clin Neurosci. 2001;13(2):302-303.
54. Funderburg LG, Vertrees JE, True JE, et al. Seizure following addition of erythromycin to clozapine treatment. Am J Psychiatry. 1994;151(12):1840-1841.
55. Varma S, Bishara D, Besag FMC, et al. Clozapine-related EEG changes and seizures: dose and plasma-level relationships. Ther Adv Psychopharmacol. 2011;1(2):47-66.
56. Amann BL, Pogarell O, Mergl R, et al. EEG abnormalities associated with antipsychotics: a comparison of quetiapine, olanzapine, haloperidol and healthy subjects. Hum Psychopharmacol. 2003;18(8):641-646.
57. Pisani F, Oteri G, Costa C, et al. Effects of psychotropic drugs on seizure threshold. Drug Saf. 2002;25(2):91-110.
58. Maurice T, Phan VL, Urani A, et al. Neuroactive neurosteroids as endogenous effectors for the sigma1 (sigma1) receptor: pharmacological evidence and therapeutic opportunities. Jpn J Pharmacol. 1999;81(2):125-155.
59. Haller E, Binder RL. Clozapine and seizures. Am J Psychiatry. 1990;147(8):1069-1071.
60. Torta R, Monaco F. Atypical antipsychotics and serotoninergic antidepressants in patients with epilepsy: pharmacodynamic considerations. Epilepsia. 2002;43(suppl 2):8-13.
61. Spina E. Drug interactions. In: Shorvon S, Perucca E, Engel J Jr, eds. The treatment of epilepsy. 3rd ed. Oxford, UK: Blackwell Publishing; 2009:361-377.
62. Spina E, Perucca E. Clinical significance of pharmacokinetic interactions between antiepileptic and psychotropic drugs. Epilepsia. 2002;43(suppl 2):37-44.
63. de Leon J, Santoro V, D’Arrigo C, et al. Interactions between antiepileptics and second-generation antipsychotics. Expert Opin Drug Metab Toxicol. 2012;8(3):311-334.
64. Finley P, Warner D. Potential impact of valproic acid therapy on clozapine disposition. Biol Psychiatry. 1994;36(7):487-488.
65. Longo LP, Salzman C. Valproic acid effects on serum concentrations of clozapine and norclozapine. Am J Psychiatry. 1995;152(4):650.
66. Centorrino F, Baldessarini RJ, Kando J, et al. Serum concentrations of clozapine and its major metabolites: effects of cotreatment with fluoxetine or valproate. Am J Psychiatry. 1994;151(1):123-125.
67. Facciolà G, Avenoso A, Scordo MG, et al. Small effects of valproic acid on the plasma concentrations of clozapine and its major metabolites in patients with schizophrenic or affective disorders. Ther Drug Monit. 1999;21(3):341-345.
68. Hyde TM, Weinberger DR. Seizures and schizophrenia. Schizophr Bull. 1997;23(4):611-622.
69. Muzyk A, Gala G, Kahn DA. Use of lamotrigine in a patient with a clozapine-related seizure. J Psychiatr Pract. 2010;16(2):125-128.
70. Kikuchi YS, Sato W, Ataka K, et al. Clozapine-induced seizures, electroencephalography abnormalities, and clinical responses in Japanese patients with schizophrenia. Neuropsychiatr Dis Treat. 2014;10:1973-1978.
71. Taner E, Coşar B, Işik E. Clozapine-induced myoclonic seizures and valproic acid. Int J Psychiatry Clin Pract. 1998;2(1):53-55.
72. Liukkonen J, Koponen HJ, Nousiainen U. Clinical picture and long-term course of epileptic seizures that occur during clozapine treatment. Psychiatry Res. 1992;44(2):107-112.
73. Devinsky O, Honigfeld G, Patin J. Clozapine-related seizures. Neurology. 1991;41(3):369-371.
74. Foster R, Olajide D. A case of clozapine-induced tonic-clonic seizures managed with valproate: implications for clinical care. J Psychopharmacol. 2005;19(1):93-96.
75. Gandelman-Marton R, Theitler J, Klein C, et al. Phenytoin intoxication in a clozapine-related prolonged seizure. J Emerg Med. 2008;35(4):407-409.
76. Primavera A, Giberti L, Scotto P, et al. Nonconvulsive status epilepticus as a cause of confusion in later life: a report of 5 cases. Neuropsychobiology. 1994;30(2-3):148-152.
77. Boutros NN, Arfken C, Galderisi S, et al. The status of spectral EEG abnormality as a diagnostic test for schizophrenia. Schizophrenia Res. 2008;99(1-3):225-237.
78. Takahashi T, Cho RY, Mizuno T, et al. Antipsychotics reverse abnormal EEG complexity in drug-naïve schizophrenia: a multiscale entropy analysis. Neuroimage. 2010;51(1):173-182.
Can lifestyle modifications delay or prevent Alzheimer’s disease?
Clinicians have devoted strenuous efforts to secondary prevention of Alzheimer’s disease (AD) by diagnosing and treating patients as early as possible. Unfortunately, there is no cure for AD, and the field has witnessed recurrent failures of several pharmacotherapy candidates with either symptomatic or disease-modifying properties.1 An estimated one-third of AD cases can be attributed to modifiable risk factors.2 Thus, implementing primary prevention measures by addressing modifiable risk factors thought to contribute to the disease, with the goal of reducing the risk of developing AD, or at least delaying its onset, is a crucial public health strategy.
Cardiovascular risk factors, such as hypertension, hyperlipidemia, diabetes, hyperhomocysteinemia, obesity, and smoking, have emerged as substantive risk factors for AD.3 Optimal management of these major risk factors, especially in mid-life, may be a preventive approach against AD. Although detailing the evidence on the impact of managing cardiovascular risk factors to delay or prevent AD is beyond the scope of this article, it is becoming clear that “what is good for the heart is good for the brain.”
Additional modifiable risk factors are related to lifestyle habits, such as physical exercise, mental and social activity, meditation/spiritual activity, and diet. This article reviews the importance of pursuing a healthy lifestyle in delaying AD, with the corresponding levels of evidence that support each specific lifestyle modification. The levels of evidence are defined in Table 1.4
Physical exercise
Twenty-one percent of AD cases in the United States are attributable to physical inactivity.5 In addition to its beneficial effect on metabolic syndrome, in animal and human research, regular exercise has been shown to have direct neuroprotective effects. High levels of physical activity increase hippocampal neurogenesis and neuroplasticity, increase vascular circulation in the brain regions implicated in AD, and modulate inflammatory mediators as well as brain growth factors such as brain-derived neurotrophic factor (BDNF) and insulin-like growth factor-1 (IGF-1).6
The definition of regular physical exercise varies across the literature, but usually implies aerobic exercise—an ongoing activity sufficient to increase the heart rate and the need for oxygen, sustained for 20 to 30 minutes per session.7 Modalities include household activities and leisure-time activities. In a large prospective cohort study, Scarmeas et al8 categorized leisure-time activities into 3 types:
- light (walking, dancing, calisthenics, golfing, bowling, gardening, horseback riding)
- moderate (bicycling, swimming, hiking, playing tennis)
- vigorous (aerobic dancing, jogging, playing handball).
These types of physical exercise were weighed by the frequency of participation per week. Compared with being physically inactive, low levels of weekly physical activity (0.1 hours of vigorous, 0.8 hours of moderate, or 1.3 hours of light exercise) were associated with a 29% to 41% lower risk of developing AD, while higher weekly physical activity (1.3 hours of vigorous, 2.3 hours of moderate, or 3.8 hours of light exercise) were associated with a 37% to 50% lower risk (level III).8
In another 20-year cohort study, engaging in leisure-time physical activity at least twice a week in mid-life was significantly associated with a reduced risk of AD, after adjusting for age, sex, education, follow-up time, locomotor disorders, apolipoprotein E (ApoE) genotype, vascular disorders, smoking, and alcohol intake (level III).9 Moreover, a systematic review of 29 randomized controlled trials (RCTs) showed that aerobic exercise training, such as brisk walking, jogging, and biking, was associated with improvements in attention, processing speed, executive function, and memory among healthy older adults and those with mild cognitive impairment (MCI; level IA).10
Continue to: From a pathophysiological standpoint...
From a pathophysiological standpoint, higher levels of physical exercise in cognitively intact older adults have been associated with reduced brain amyloid beta deposits, especially in ApoE4 carriers.11 This inverse relationship also has been demonstrated in patients who are presymptomatic who carry 1 of the 3 known autosomal dominant mutations for the familial forms of AD.12
Overall, physicians should recommend that patients—especially those with cardiovascular risk factors that increase their risk for AD—exercise regularly by following the guidelines of the American Heart Association or the American College of Sports Medicine.13 These include muscle-strengthening activities (legs, hips, back, abdomen, shoulders, and arms) at least 2 days/week, in addition to either 30 minutes/day of moderate-intensity aerobic activity such as brisk walking, 5 days/week; or 25 minutes of vigorous aerobic activity such as jogging and running, 3 days/week14 (level IA evidence for overall improvement in cognitive function; level III evidence for AD delay/risk reduction). Neuromotor exercise, such as yoga and tai chi, and flexibility exercise such as muscle stretching, especially after a hot bath, 2 to 3 days/week are also recommended (level III).15
Mental activity
Nineteen percent of AD cases worldwide and 7% in the United States. can be attributed to low educational attainment, which is associated with low brain cognitive reserve.5 Cognitive resilience in later life may be enhanced by building brain reserves through intellectual stimulation, which affects neuronal branching and plasticity.16 Higher levels of complex mental activities measured across the lifespan, such as education, occupation, reading, and writing, are correlated with significantly less hippocampal volume shrinkage over time.17 Frequent participation in mentally stimulating activities—such as listening to the radio; reading newspapers, magazines, or books; playing games (cards, checkers, crosswords or other puzzles); and visiting museums—was associated with an up to 64% reduction in the odds of developing AD in a cohort of cognitively intact older adults followed for 4 years.18 The correlation between mental activity and AD was found to be independent of physical activity, social activity, or baseline cognitive function.19
In a large cohort of cognitively intact older adults (mean age 70), engaging in a mentally stimulating activity (craft activities, computer use, or going to the theater/movies) once to twice a week was significantly associated with a reduced incidence of amnestic MCI.20 Another prospective 21-year study demonstrated a significant reduction in AD risk in community-dwelling cognitively intact older adults (age 75 to 85) who participated in cognitively stimulating activities, such as reading books or newspapers, writing for pleasure, doing crossword puzzles, playing board games or cards, or playing musical instruments, several times/week.21
Growing scientific evidence also suggests that lifelong multilingualism can delay AD onset by 4 to 5 years.22 Multilingualism is associated with greater cognitive reserve, gray matter volume, functional connectivity and white matter density.23
Continue to: Physicians should encourage their patients...
Physicians should encourage their patients to engage in intellectually stimulating activities and creative leisure-time activities several times/week to enhance their cognitive reserves and delay AD onset (level III evidence with respect to AD risk reduction/delay).
Social activity
Social engagement may be an additional protective factor against AD. In a large 4-year prospective study, increased loneliness in cognitively intact older adults doubled the risk of AD.24 Data from the large French cohort PAQUID (Personnes Agées QUID) emphasized the importance of a patient’s social network as a protective factor against AD. In this cohort, the perception of reciprocity in relationships with others (the perception that a person had received more than he or she had given) was associated with a 53% reduction in AD risk (level III).25 In another longitudinal cohort study, social activity was found to decrease the incidence of subjective cognitive decline, which is a prodromal syndrome for MCI and AD (level III).26
A major confounder in studies assessing for social activity is the uncertainty if social withdrawal is a modifiable risk factor or an early manifestation of AD, since apathetic patients with AD tend to be socially withdrawn.27 Another limitation of measuring the impact of social activity relative to AD risk is the difficulty in isolating social activities from activities that have physical and mental activity components, such as leisure-time activities.28
Meditation/spiritual activity
Chronic psychological stress is believed to compromise limbic structures that regulate stress-related behaviors and the memory network, which might explain how being prone to psychological distress may be associated with MCI or AD.29 Cognitive stress may increase the oxidative stress and telomere shortening implicated in the neurodegenerative processes of AD.30 In one study, participants who were highly prone to psychological distress were found to be at 3 times increased risk for developing AD, after adjusting for depression symptoms and physical and mental activities (level III).31 By reducing chronic psychological stress, meditation techniques offer a promising preventive option against AD.
Mindfulness-based interventions (MBI) have gained increased attention in the past decade. They entail directing one’s attention towards the present moment, thereby decreasing ruminative thoughts and stress arousal.32 Recent RCTs have shown that MBI may promote brain health in older adults not only by improving psychological well-being but also by improving attentional control33 and functional connectivity in brain regions implicated in executive functioning,34 as well as by modulating inflammatory processes implicated in AD.35 Furthermore, an RCT of patients diagnosed with MCI found that compared with memory enhancement training, a weekly 60-minute yoga session improved memory and executive functioning.36
Continue to: Kirtan Kriya is a medication technique...
Kirtan Kriya is a meditation technique that is easy to learn and practice by older adults and can improve memory in patients at risk for developing AD.37 However, more rigorous RCTs conducted in larger samples of older adults are needed to better evaluate the effect of all meditation techniques for delaying or preventing AD (level IB with respect to improvement in cognitive functioning/level III for AD delay/risk reduction).38
Spiritual activities, such as going to places of worship or religious meditation, have been associated with a lower prevalence of AD. Attending religious services, gatherings, or retreats involves a social component because these activities often are practiced in groups. They also confer a method of dealing with psychological distress and depression. Additionally, frequent readings of religious texts represents a mentally stimulating activity that may also contribute to delaying/preventing AD (level III).39
Diet
In the past decade, a growing body of evidence has linked diet to cognition. Individuals with a higher intake of calories and fat are at higher risk for developing AD.40 The incidence of AD rose in Japan after the country transitioned to a more Westernized diet.41 A modern Western diet rich in saturated fatty acids and simple carbohydrates may negatively impact hippocampus-mediated functions such as memory and learning, and is associated with an increased risk of AD.42 In contrast with high-glycemic and fatty diets, a “healthy diet” is associated with a decrease in beta-amyloid burden, inflammation, and oxidative stress.43,44
Studies focusing on dietary patterns rather than a single nutrient for delaying or preventing AD have yielded more robust and consistent results.45 In a recent meta-analysis, adhering to a Mediterranean diet—which is rich in fruits and vegetables, whole grains, olive oil, and fish; moderate in some dairy products and wine; and low in red meat—was associated with a decreased risk of AD; this evidence was derived mostly from epidemiologic studies.46 Scarmeas et al8 found that high adherence to the Mediterranean diet was associated with 32% to 40% reduced risk of AD. Combining this diet with physical exercise was associated with an up to 67% reduced risk (level III). The Dietary Approaches to Stop Hypertension (DASH) diet, which is rich in total grains, fruits, vegetables, and dairy products, but low in sodium and sweets, correlated with neurocognitive improvement in patients with hypertension.47 Both the Mediterranean and DASH diets have been associated with better cognitive function48 and slower cognitive decline.49 Thus, an attempt to combine the neuroprotective components from both diets led to the creation of the MIND (Mediterranean-DASH Intervention for Neurodegenerative Delay) diet, which also has been associated with a lower incidence of AD.50
Besides specific diets, some food groups have also been found to promote brain health and may help delay or prevent AD. Berries have the highest amount of antioxidants of all fruit. Among vegetables, tomatoes and green leafy vegetables have the highest amount of nutrients for the brain. Nuts, such as walnuts, which are rich in omega-3 fatty acids, are also considered “power foods” for the brain; however, they should be consumed in moderation because they are also rich in fat. Monounsaturated fatty acids, which are found in olives and olive oil, are also beneficial for the brain. Among the 3 types of omega-3 fatty acids, the most important for cognition is docosahexaenoic acid (DHA) because it constitutes 40% of all fatty acids in the brain. Mainly found in oily fish, DHA has antioxidant and anti-inflammatory properties that may delay or prevent AD. Low levels of DHA have been found in patients with AD.51
Continue to: Curcumin, which is derived from...
Curcumin, which is derived from the curry spice turmeric, is a polyphenol with anti-inflammatory, antioxidant, and anti-amyloid properties that may have a promising role in preventing AD in cognitively intact individuals. Initial trials with curcumin have yielded mixed results on cognition, which was partly related to the low solubility and bioavailability of its formulation.52 However, a recent 18-month double-blind randomized placebo-controlled trial found positive effects on memory and attention, as well as reduction of amyloid plaques and tau tangles deposition in the brain, in non-demented older adults age 51 to 84 who took Theracumin, a highly absorptive oral form of curcumin dispersed with colloidal nanoparticles.53 A longer follow-up is required to determine if curcumin can delay or prevent AD.
Alcohol
The role of alcohol in AD prevention is controversial. Overall, data from prospective studies has shown that low to moderate alcohol consumption may be associated with a reduced risk of AD (level III).54 Alcohol drinking in mid-life showed a U-shaped relationship with cognitive impairment; both abstainers and heavy drinkers had an increased risk of cognitive decline compared with light to moderate drinkers (level III).55 Binge drinking significantly increased the odds of cognitive decline, even after controlling for total alcohol consumption per week.55
The definition of low-to-moderate drinking varies substantially among countries. In addition, the size and amount of alcohol contained in a standard drink may differ.56 According to the National Institute on Alcohol Abuse and Alcoholism (NIAAA),57 moderate drinking is defined as up to 1 drink daily for women and 2 drinks daily for men. Binge drinking involves drinking >4 drinks for women and >5 drinks for men, in approximately 2 hours, at least monthly. In the United States, one standard drink contains 14 grams of pure alcohol, which is usually found in 12 ounces of regular beer, 5 ounces of wine, and 1.5 ounces of distilled spirits (vodka or whiskey).58
In a 5-year prospective Canadian study, having 1 drink weekly (especially wine) was associated with an up to 50% reduced risk of AD (level III).59 In the French cohort PAQUID, mild drinkers (<1 to 2 drinks/day) and moderate drinkers (3 to 4 drinks daily) had a reduced incidence of AD compared with non-drinkers. Wine was the most frequently consumed beverage in this study.60 Other studies have found cognitive benefits from mild to moderate drinking regardless of beverage type.54 However, a recent study that included a 30-year follow-up failed to find a significant protective effect of light drinking over abstinence in terms of hippocampal atrophy.61 Atrophy of the hippocampus was correlated with increasing alcohol amounts in a dose-dependent manner, starting at 7 to 14 drinks/week (level III).61
Research has shown that moderate and heavy alcohol use or misuse can directly induce microglial activation and inflammatory mediators’ release, which induce amyloid beta pathology and leads to brain atrophy.62 Hence, non-drinkers should not be advised to begin drinking, because of the lack of RCTs and the concern that beginning to drink may lead to heavy drinking. All drinkers should be advised to adhere to the NIAAA recommendations.13
Continue to: Coffee/tea
Coffee/tea
Although studies of caffeinated coffee have been heterogeneous and yielded mixed results (beneficial effect vs no effect on delaying cognitive decline), systematic reviews and meta-analyses of cross-sectional, case-control, and longitudinal cohort studies have found a general trend towards a favorable preventive role (level III).63-65 Caffeine exhibits its neuroprotective effect by increasing brain serotonin and acetylcholine, and by stabilizing blood-brain-barrier integrity.66 Moreover, in an animal study, mice given caffeine in their drinking water from young adulthood into older age had lower amyloid beta plasma levels compared with those given decaffeinated water.67 These findings suggest that in humans, 5 cups of regular caffeinated coffee daily, equivalent to 500 mg of caffeine,
An Italian study showed that older adults who don’t or rarely drink coffee (<1 cup daily) and those who recently increased their consumption pattern to >1 cup daily had a higher incidence of MCI than those who habitually consumed 1 to 2 cups daily.69 Therefore, it is not recommended to advise a change in coffee drinking pattern in old age. Older adults who are coffee drinkers should, however, be educated about the association between heavier caffeine intake and anxiety, insomnia, and cardiac arrhythmias.70
Despite its more modest caffeine levels, green tea is rich in polyphenols, which belong to the family of catechins and are characterized by antioxidant and anti-inflammatory properties.71 In a Japanese cohort, higher green tea consumption (up to 1 cup daily) was associated with a decreased incidence of MCI in older adults.72 More studies are needed to confirm its potential preventative role in AD.
Which lifestyle change is the most important?
Focusing on a single lifestyle change may be insufficient, especially because the bulk of evidence for individual interventions comes from population-based cohort studies (level III), rather than strong RCTs with a long follow-up. There is increasing evidence that combining multiple lifestyle modifications may yield better outcomes in maintaining or improving cognition.73
The Finnish Geriatric Intervention Study to Prevent Cognitive Impairment and Disability (FINGER), a large, 2-year RCT that included community-dwelling older adults (age 60 to 77) with no diagnosis of major neurocognitive disorder, found that compared with regular health advice, multi-domain interventions reduced cognitive decline and improved overall cognition, executive functioning, and processing speed. The interventions evaluated in this study combined the following 4 modalities74:
- a healthy diet according to the Finnish nutrition recommendations (eating vegetables, fruits, and berries [minimum: 500 g/d], whole grain cereals [several times a day], and fish [2 to 3 times/week]; using low-salt products; consuming fat-free or low-fat milk products; and limiting red meat consumption to <500 g/week
- regular physical exercise tailored for improving muscle strength (1 to 3 times/week) coupled with aerobic exercise (2 to 5 times/week)
- cognitive training, including group sessions that have a social activity component and computer-based individual sessions 3 times/week that target episodic and working memory and executive functioning
- optimal management of cardiovascular risk factors.
Continue to: This multi-domain approach...
This multi-domain approach for lifestyle modification should be strongly recommended to cognitively intact older patients (level IB).
Modeled after the FINGER study, the Alzheimer’s Association U.S. Study to Protect Brain Health Through Lifestyle Intervention to Reduce Risk (U.S. POINTER) is a 2-year, multicenter, controlled clinical trial aimed at testing the ability of a multidimensional lifestyle intervention to prevent AD in at-risk older adults (age 60 to 79, with established metabolic and cardiovascular risk factors). Interventions include a combination of physical exercise, nutritional counseling and management, cognitive and social stimulation, and improved management of cardiovascular risk factors. Recruitment for this large-scale trial was estimated to begin in January 2019 (NCT03688126).75
On a practical basis, Desai et al13 have proposed a checklist (Table 213) that physicians can use in their routine consultations to improve primary prevention of AD among their older patients.
Bottom Line
Advise patients that pursuing a healthy lifestyle is a key to delaying or preventing Alzheimer’s disease. This involves managing cardiovascular risk factors and a combination of staying physically, mentally, socially, and spiritually active, in addition to adhering to a healthy diet such as the Mediterranean diet.
Related Resources
- Anderson K, Grossberg GT. Brain games to slow cognitive decline in Alzheimer’s disease. J Am Med Dir Assoc. 2014;15(8):536-537.
- Small G, Vorgan G. The memory prescription: Dr. Garry Small’s 14-day plan to keep your brain and body young. New York, NY: Hyperion; 2004.
- Small G, Vorgan G. The Alzheimer’s prevention program; keep your brain healthy for the rest of your life. New York, NY: Workman Publishing Company, Inc.; 2012.
Drug Brand Name
Curcumin • Theracurmin
1. Mehta D, Jackson R, Paul G, et al. Why do trials for Alzheimer’s disease drugs keep failing? A discontinued drug perspective for 2010-2015. Expert Opin Investig Drugs. 2017;26(6):735-739.
2. Norton S, Matthews FE, Barnes DE, et al. Potential for primary prevention of Alzheimer’s disease: an analysis of population-based data. Lancet Neurol. 2014;13(8):788-794.
3. Meng XF, Yu JT, Wang HF, et al. Midlife vascular risk factors and the risk of Alzheimer’s disease: a systematic review and meta-analysis. J Alzheimers Dis. 2014;42(4):1295-1310.
4. Shekelle PG, Woolf SH, Eccles M, et al. Developing clinical guidelines. West J Med. 1999;170(6):348-351.
5. Barnes DE, Yaffe Y. The projected impact of risk factor reduction on Alzheimer’s disease prevalence. Lancet Neurol. 2011;10(9):819-828.
6. Cotman CW, Berchtold NC, Christie LA. Exercise builds brain health: key roles of growth factor cascades and inflammation. Trends Neurosci. 2007;30(9):464-472.
7. Ahlskog JE, Geda YE, Graff-Radford NR, et al. Physical exercise as a preventive or disease-modifying treatment of dementia and brain aging. Mayo Clin Proc. 2011;86(9):876-884.
8. Scarmeas N, Luchsinger JA, Schupf N, et al. Physical activity, diet, and risk of Alzheimer Disease. JAMA. 2009;302(6):627-637.
9. Rovio S, Kåreholt I, Helkala EL, et al. Leisure-time physical activity at midlife and the risk of dementia and Alzheimer’s disease. Lancet Neurol. 2005;4(11):705-711.
10. Smith PJ et al. Aerobic exercise and neurocognitive performance: a meta-analytic review of randomized controlled trials. Psychosom Med. 2010;72(3):239-252.
11. Brown BM, Peiffer JJ, Taddei K, et al. Physical activity and amyloid-beta plasma and brain levels: results from the Australian imaging, biomarkers and lifestyle study of ageing. Mol Psychiatry. 2013;18(8):875-881.
12. Brown BM, Sohrabi HR, Taddei K, et al. Habitual exercise levels are associated with cerebral amyloid load in presymptomatic autosomal dominant Alzheimer’s disease. Alzheimers Dement. 2017;13(11):1197-1206.
13. Desai AK, Grossberg GT, Chibnall JT. Healthy brain aging: a road map. Clin Geriatr Med. 2010;26(1):1-16.
14. Centers for Disease Control and Prevention. Physical activity: how much physical activity do older adults need?
15. Garber CE, Blissmer B, Deschenes MR, et al; American College of Sports Medicine. American College of Sports Medicine position stand. Quantity and quality of exercise for developing and maintaining cardiorespiratory, musculoskeletal, and neuromotor fitness in apparently healthy adults: guidance for prescribing exercise. Med Sci Sports Exerc. 2011;43(7):1334-1359.
16. Livingston G, Sommerlad A, Orgeta V, et al. Dementia prevention, intervention, and care. Lancet. 2017;390(10113);2673-2734.
17. Valenzuela MJ, Sachdev P, Wen W, et al. Lifespan mental activity predicts diminished rate of hippocampal atrophy. PLoS One. 2008;3(7):e2598. doi.org/10.1371/journal.pone.0002598.
18. Wilson RS, Bennett DA, Bienias JL, et al. Cognitive activity and incident AD in a population-based sample of older persons. Neurology. 2002;59(12):1910-1914.
19. Wilson RS, Scherr PA, Schneider JA, et al. Relation of cognitive activity to risk of developing Alzheimer disease. Neurology. 2007;69(20):1911-1920.
20. Krell-Roesch J, Vemuri P, Pink A, et al. Association between mentally stimulating activities in late life and the outcome of incident mild cognitive impairment, with an analysis of the apoe ε4 genotype. JAMA Neurol. 2017;74(3):332-338.
21. Verghese J, Lipton RB, Katz MJ, et al. Leisure activities and the risk of dementia in the elderly. N Engl J Med. 2003;348(25):2508-2516.
22. Klein RM, Christie J, Parkvall M. Does multilingualism affect the incidence of Alzheimer’s disease?: a worldwide analysis by country. SSM Popul Health. 2016;2:463-467.
23. Grundy JG, Anderson JAE, Bialystok E. Neural correlates of cognitive processing in monolinguals and bilinguals. Ann N Y Acad Sci. 2017;1396(1):183-201.
24. Wilson RS, Krueger KR, Arnold SE, et al. Loneliness and risk of Alzheimer disease. Arch Gen Psychiatry. 2007;64(2):234-240.
25. Amieva H, Stoykova R, Matharan F, et al. What aspects of social network are protective for dementia? Not the quantity but the quality of social interactions is protective up to 15 years later. Psychosom Med. 2010;72(9):905-911.
26. Kuiper JS, Oude Voshaar RC, Zuidema SU, et al. The relationship between social functioning and subjective memory complaints in older persons: a population-based longitudinal cohort study. Int J Geriatr Psychiatry. 2017;32(10):1059-1071.
27. Robert P, Onyike CU, Leentjens AF, et al. Proposed diagnostic criteria for apathy in Alzheimer’s disease and other neuropsychiatric disorders. Eur Psychiatry. 2009;24(2):98-104.
28. Marioni RE, Proust-Lima C, Amieva H, et al. Social activity, cognitive decline and dementia risk: a 20-year prospective cohort study. BMC Public Health. 2015;15:1089.
29. Wilson RS, Schneider JA, Boyle PA, et al. Chronic distress and incidence of mild cognitive impairment. Neurology. 2007;68(24):2085-2092.
30. Cai Z, Yan LJ, Ratka A. Telomere shortening and Alzheimer’s disease. Neuromolecular Med. 2013;15(1):25-48.
31. Wilson RS, Arnold SE, Schneider JA, et al. Chronic psychological distress and risk of Alzheimer’s disease in old age. Neuroepidemiology. 2006;27(3):143-153.
32. Epel E, Daubenmier J, Moskowitz JT, et al. Can meditation slow rate of cellular aging? Cognitive stress, mindfulness, and telomeres. Ann N Y Acad Sci. 2009;1172:34-53.
33. Malinowski P, Moore AW, Mead Br, et al. Mindful aging: the effects of regular brief mindfulness practice on electrophysiological markers of cognitive and affective processing in older adults. Mindfulness (N Y). 2017;8(1):78-94.
34. Taren AA, Gianaros PJ, Greco CM, et al. Mindfulness meditation training and executive control network resting state functional connectivity: a randomized controlled trial. Psychosom Med. 2017;79(6):674-683.
35. Fountain-Zaragoza S, Prakash RS. Mindfulness training for healthy aging: impact on attention, well-being, and inflammation. Front in Aging Neurosci. 2017;9:11.
36. Eyre HA, Siddarth P, Acevedo B, et al. A randomized controlled trial of Kundalini yoga in mild cognitive impairment. Int Psychogeriatr. 2017;29(4):557-567.
37. Khalsa DS. Stress, meditation, and Alzheimer’s disease prevention: where the evidence stands. J Alzheimers Dis. 2015;48(1):1-12.
38. Berk L, van Boxtel M, van Os J. Can mindfulness-based interventions influence cognitive functioning in older adults? A review and considerations for future research. Aging Ment Health. 2017;21(11):1113-1120.
39. Hosseini S, Chaurasia A, Oremus M. The effect of religion and spirituality on cognitive function: a systematic review. Gerontologist. 2017. doi: 10.1093/geront/gnx024.
40. Luchsinger JA, Tang MX, Shea S, et al. Caloric intake and the risk of Alzheimer disease. Arch Neurol. 2002;59(8):1258-1263.
41. Grant WB. Trends in diet and Alzheimer’s disease during the nutrition transition in Japan and developing countries. J Alzheimers Dis. 2014;38(3):611-620.
42. Kanoski SE, Davidson TL. Western diet consumption and cognitive impairment: links to hippocampal dysfunction and obesity. Physiol Behav. 2011;103(1):59-68.
43. Hu N, Yu JT, Tan L, et al. Nutrition and the risk of Alzheimer’s disease. Biomed Res Int. 2013;2013:524820. doi: 10.1155/2013/524820.
44. Taylor MK, Sullivan DK, Swerdlow RH, et al. A high-glycemic diet is associated with cerebral amyloid burden in cognitively normal older adults. Am J Clin Nutr. 2017;106(6):1463-1470.
45. van de Rest O, Berendsen AM, Haveman-Nies A, et al. Dietary patterns, cognitive decline, and dementia: a systematic review. Adv Nutr. 2015;6(2):154-168.
46. Petersson SD, Philippou E. Mediterranean diet, cognitive function, and dementia: a systematic review of the evidence. Adv Nutr. 2016;7(5):889-904.
47. Smith PJ, Blumenthal JA, Babyak MA, et al. Effects of the dietary approaches to stop hypertension diet, exercise, and caloric restriction on neurocognition in overweight adults with high blood pressure. Hypertension. 2010;55(6):1331-1338.
48. Wengreen H, Munger RG, Cutler A, et al. Prospective study of dietary approaches to stop hypertension- and Mediterranean-style dietary patterns and age-related cognitive change: the Cache County study on memory, health and aging. Am J Clin Nutr. 2013;98(5):1263-1271.
49. Tangney CC, Li H, Wang Y, et al. Relation of DASH- and Mediterranean-like dietary patterns to cognitive decline in older persons. Neurology. 2014;83(16):1410-1416.
50. Morris MC, Tangney CC, Wang Y, et al. MIND diet associated with reduced incidence of Alzheimer’s disease. Alzheimers Dement. 2015;11(9):1007-1014.
51. Desai AK, Rush J, Naveen L, et al. Nutrition and nutritional supplements to promote brain health. In: Hartman-Stein PE, Rue AL, eds. Enhancing cognitive fitness in adults: a guide to the use and development of community-based programs. New York, NY: Springer; 2011:249-269.
52. Goozee KG, Shah TM, Sohrabi HR, et al. Examining the potential clinical value of curcumin in the prevention and diagnosis of Alzheimer’s disease. Br J Nutr. 2016;115(3):449-465.
53. Small GW, Siddarth P, Li Z, et al. Memory and brain amyloid and tau effects of a bioavailable form of curcumin in non-demented adults: a double-blind, placebo-controlled 18-month trial. Am J Geriatr Psychiatry. 2018;26(3):266-277.
54. Kim JW, Lee DY, Lee BC, et al. Alcohol and cognition in the elderly: a review. Psychiatry Investig. 2012;9(1):8-16.
55. Virtaa JJ, Järvenpää T, Heikkilä K, et al. Midlife alcohol consumption and later risk of cognitive impairment: a twin follow-up study. J Alzheimers Dis. 2010;22(3):939-948.
56. Kerr WC, Stockwell T. Understanding standard drinks and drinking guidelines. Drug and Alcohol Rev. 2012;31(2):200-205.
57. National Institute on Alcohol Abuse and Alcoholism. Drinking levels defined. https://www.niaaa.nih.gov/alcohol-health/overview-alcohol-consumption/moderate-binge-drinking. Accessed December 9, 2017.
58. National Institute on Alcohol Abuse and Alcoholism. What is a standard drink? https://www.niaaa.nih.gov/alcohol-health/overview-alcohol-consumption/what-standard-drink. Accessed November 9, 2017.
59. Lindsay J, Laurin D, Verreault R, et al. Risk factors for Alzheimer’s disease: a prospective analysis from the Canadian study of health and aging. Am J Epidemiol. 2002;156(5):445-453.
60. Orgogozo JM, Dartigues JF, Lafont S, et al. Wine consumption and dementia in the elderly: a prospective community study in the Bordeaux area. Rev Neurol (Paris). 1997;153(3):185-192.
61. Topiwala A, Allan CL, Valkanova V, et al. Moderate alcohol consumption as risk factor for adverse brain outcomes and cognitive decline: longitudinal cohort study. BMJ. 2017;357.
62. Venkataraman A, Kalk N, Sewell G, et al. Alcohol and Alzheimer’s disease-does alcohol dependence contribute to beta-amyloid deposition, neuroinflammation and neurodegeneration in Alzheimer’s Disease? Alcohol Alcohol. 2017;52(2):151-158.
63. Ma QP, Huang C, Cui QY, et al. Meta-analysis of the association between tea intake and the risk of cognitive disorders. PLoS One. 2016;11(11):e0165861. doi: 10.1371/journal.pone.0165861.
64. Santos C, Costa J, Santos J, et al. Caffeine intake and dementia: systematic review and meta-analysis. J Alzheimers Dis. 2010;20(Suppl 1):S187-204.
65. Panza F, Solfrizzi V, Barulli MR, et al. Coffee, tea, and caffeine consumption and prevention of late-life cognitive decline and dementia: a systematic review. J Nutr Health Aging. 2015;19(3):313-328.
66. Wierzejska R. Can coffee consumption lower the risk of Alzheimer’s disease and Parkinson’s disease? A literature review. Arch Med Sci. 2017;13(3):507-514.
67. Arendash GW, Cao C. Caffeine and coffee as therapeutics against Alzheimer’s disease. J Alzheimers Dis. 2010;20 (Suppl 1):S117-S126.
68. Eskelinen MH, Ngandu T, Tuomilehto J, et al. Midlife coffee and tea drinking and the risk of late-life dementia: a population-based CAIDE study. J Alzheimers Dis. 2009;16(1):85-91.
69. Solfrizzi V, Panza F, Imbimbo BP, et al. Coffee consumption habits and the risk of mild cognitive impairment: the Italian longitudinal study on aging. J Alzheimers Dis. 2015;47(4):889-899.
70. Vittoria Mattioli. Beverages of daily life: impact of caffeine on atrial fibrillation. J Atr Fibrillation. 2014;7(2):1133.
71. Chacko SM, Thambi PT, Kuttan R, et al. Beneficial effects of green tea: a literature review. Chin Med. 2010;5:13.
72. Noguchi-Shinohara M, Yuki S, Dohmoto C, et al. Consumption of green tea, but not black tea or coffee, is associated with reduced risk of cognitive decline. PLoS One. 2014;9(5):e96013. doi: 10.1371/journal.pone.0096013.
73. Schneider N, Yvon C. A review of multidomain interventions to support healthy cognitive ageing. J Nutr Health Aging. 2013;17(3):252-257.
74. Ngandu T, Lehitsalo J, Solomon A, et al. A 2 year multidomain intervention of diet, exercise, cognitive training, and vascular risk monitoring versus control to prevent cognitive decline in at-risk elderly people (FINGER): a randomised controlled trial. Lancet. 2015;385(9984):2255-2263.
75. U.S. National Library of Medicing. ClinicalTrials.gov. U.S. study to protect brain health through lifestyle intervention to reduce risk (POINTER). https://clinicaltrials.gov/ct2/show/NCT03688126?term=pointer&cond=Alzheimer+Disease&rank=1. Published September 28, 2018. Accessed November 3, 2018.
Clinicians have devoted strenuous efforts to secondary prevention of Alzheimer’s disease (AD) by diagnosing and treating patients as early as possible. Unfortunately, there is no cure for AD, and the field has witnessed recurrent failures of several pharmacotherapy candidates with either symptomatic or disease-modifying properties.1 An estimated one-third of AD cases can be attributed to modifiable risk factors.2 Thus, implementing primary prevention measures by addressing modifiable risk factors thought to contribute to the disease, with the goal of reducing the risk of developing AD, or at least delaying its onset, is a crucial public health strategy.
Cardiovascular risk factors, such as hypertension, hyperlipidemia, diabetes, hyperhomocysteinemia, obesity, and smoking, have emerged as substantive risk factors for AD.3 Optimal management of these major risk factors, especially in mid-life, may be a preventive approach against AD. Although detailing the evidence on the impact of managing cardiovascular risk factors to delay or prevent AD is beyond the scope of this article, it is becoming clear that “what is good for the heart is good for the brain.”
Additional modifiable risk factors are related to lifestyle habits, such as physical exercise, mental and social activity, meditation/spiritual activity, and diet. This article reviews the importance of pursuing a healthy lifestyle in delaying AD, with the corresponding levels of evidence that support each specific lifestyle modification. The levels of evidence are defined in Table 1.4
Physical exercise
Twenty-one percent of AD cases in the United States are attributable to physical inactivity.5 In addition to its beneficial effect on metabolic syndrome, in animal and human research, regular exercise has been shown to have direct neuroprotective effects. High levels of physical activity increase hippocampal neurogenesis and neuroplasticity, increase vascular circulation in the brain regions implicated in AD, and modulate inflammatory mediators as well as brain growth factors such as brain-derived neurotrophic factor (BDNF) and insulin-like growth factor-1 (IGF-1).6
The definition of regular physical exercise varies across the literature, but usually implies aerobic exercise—an ongoing activity sufficient to increase the heart rate and the need for oxygen, sustained for 20 to 30 minutes per session.7 Modalities include household activities and leisure-time activities. In a large prospective cohort study, Scarmeas et al8 categorized leisure-time activities into 3 types:
- light (walking, dancing, calisthenics, golfing, bowling, gardening, horseback riding)
- moderate (bicycling, swimming, hiking, playing tennis)
- vigorous (aerobic dancing, jogging, playing handball).
These types of physical exercise were weighed by the frequency of participation per week. Compared with being physically inactive, low levels of weekly physical activity (0.1 hours of vigorous, 0.8 hours of moderate, or 1.3 hours of light exercise) were associated with a 29% to 41% lower risk of developing AD, while higher weekly physical activity (1.3 hours of vigorous, 2.3 hours of moderate, or 3.8 hours of light exercise) were associated with a 37% to 50% lower risk (level III).8
In another 20-year cohort study, engaging in leisure-time physical activity at least twice a week in mid-life was significantly associated with a reduced risk of AD, after adjusting for age, sex, education, follow-up time, locomotor disorders, apolipoprotein E (ApoE) genotype, vascular disorders, smoking, and alcohol intake (level III).9 Moreover, a systematic review of 29 randomized controlled trials (RCTs) showed that aerobic exercise training, such as brisk walking, jogging, and biking, was associated with improvements in attention, processing speed, executive function, and memory among healthy older adults and those with mild cognitive impairment (MCI; level IA).10
Continue to: From a pathophysiological standpoint...
From a pathophysiological standpoint, higher levels of physical exercise in cognitively intact older adults have been associated with reduced brain amyloid beta deposits, especially in ApoE4 carriers.11 This inverse relationship also has been demonstrated in patients who are presymptomatic who carry 1 of the 3 known autosomal dominant mutations for the familial forms of AD.12
Overall, physicians should recommend that patients—especially those with cardiovascular risk factors that increase their risk for AD—exercise regularly by following the guidelines of the American Heart Association or the American College of Sports Medicine.13 These include muscle-strengthening activities (legs, hips, back, abdomen, shoulders, and arms) at least 2 days/week, in addition to either 30 minutes/day of moderate-intensity aerobic activity such as brisk walking, 5 days/week; or 25 minutes of vigorous aerobic activity such as jogging and running, 3 days/week14 (level IA evidence for overall improvement in cognitive function; level III evidence for AD delay/risk reduction). Neuromotor exercise, such as yoga and tai chi, and flexibility exercise such as muscle stretching, especially after a hot bath, 2 to 3 days/week are also recommended (level III).15
Mental activity
Nineteen percent of AD cases worldwide and 7% in the United States. can be attributed to low educational attainment, which is associated with low brain cognitive reserve.5 Cognitive resilience in later life may be enhanced by building brain reserves through intellectual stimulation, which affects neuronal branching and plasticity.16 Higher levels of complex mental activities measured across the lifespan, such as education, occupation, reading, and writing, are correlated with significantly less hippocampal volume shrinkage over time.17 Frequent participation in mentally stimulating activities—such as listening to the radio; reading newspapers, magazines, or books; playing games (cards, checkers, crosswords or other puzzles); and visiting museums—was associated with an up to 64% reduction in the odds of developing AD in a cohort of cognitively intact older adults followed for 4 years.18 The correlation between mental activity and AD was found to be independent of physical activity, social activity, or baseline cognitive function.19
In a large cohort of cognitively intact older adults (mean age 70), engaging in a mentally stimulating activity (craft activities, computer use, or going to the theater/movies) once to twice a week was significantly associated with a reduced incidence of amnestic MCI.20 Another prospective 21-year study demonstrated a significant reduction in AD risk in community-dwelling cognitively intact older adults (age 75 to 85) who participated in cognitively stimulating activities, such as reading books or newspapers, writing for pleasure, doing crossword puzzles, playing board games or cards, or playing musical instruments, several times/week.21
Growing scientific evidence also suggests that lifelong multilingualism can delay AD onset by 4 to 5 years.22 Multilingualism is associated with greater cognitive reserve, gray matter volume, functional connectivity and white matter density.23
Continue to: Physicians should encourage their patients...
Physicians should encourage their patients to engage in intellectually stimulating activities and creative leisure-time activities several times/week to enhance their cognitive reserves and delay AD onset (level III evidence with respect to AD risk reduction/delay).
Social activity
Social engagement may be an additional protective factor against AD. In a large 4-year prospective study, increased loneliness in cognitively intact older adults doubled the risk of AD.24 Data from the large French cohort PAQUID (Personnes Agées QUID) emphasized the importance of a patient’s social network as a protective factor against AD. In this cohort, the perception of reciprocity in relationships with others (the perception that a person had received more than he or she had given) was associated with a 53% reduction in AD risk (level III).25 In another longitudinal cohort study, social activity was found to decrease the incidence of subjective cognitive decline, which is a prodromal syndrome for MCI and AD (level III).26
A major confounder in studies assessing for social activity is the uncertainty if social withdrawal is a modifiable risk factor or an early manifestation of AD, since apathetic patients with AD tend to be socially withdrawn.27 Another limitation of measuring the impact of social activity relative to AD risk is the difficulty in isolating social activities from activities that have physical and mental activity components, such as leisure-time activities.28
Meditation/spiritual activity
Chronic psychological stress is believed to compromise limbic structures that regulate stress-related behaviors and the memory network, which might explain how being prone to psychological distress may be associated with MCI or AD.29 Cognitive stress may increase the oxidative stress and telomere shortening implicated in the neurodegenerative processes of AD.30 In one study, participants who were highly prone to psychological distress were found to be at 3 times increased risk for developing AD, after adjusting for depression symptoms and physical and mental activities (level III).31 By reducing chronic psychological stress, meditation techniques offer a promising preventive option against AD.
Mindfulness-based interventions (MBI) have gained increased attention in the past decade. They entail directing one’s attention towards the present moment, thereby decreasing ruminative thoughts and stress arousal.32 Recent RCTs have shown that MBI may promote brain health in older adults not only by improving psychological well-being but also by improving attentional control33 and functional connectivity in brain regions implicated in executive functioning,34 as well as by modulating inflammatory processes implicated in AD.35 Furthermore, an RCT of patients diagnosed with MCI found that compared with memory enhancement training, a weekly 60-minute yoga session improved memory and executive functioning.36
Continue to: Kirtan Kriya is a medication technique...
Kirtan Kriya is a meditation technique that is easy to learn and practice by older adults and can improve memory in patients at risk for developing AD.37 However, more rigorous RCTs conducted in larger samples of older adults are needed to better evaluate the effect of all meditation techniques for delaying or preventing AD (level IB with respect to improvement in cognitive functioning/level III for AD delay/risk reduction).38
Spiritual activities, such as going to places of worship or religious meditation, have been associated with a lower prevalence of AD. Attending religious services, gatherings, or retreats involves a social component because these activities often are practiced in groups. They also confer a method of dealing with psychological distress and depression. Additionally, frequent readings of religious texts represents a mentally stimulating activity that may also contribute to delaying/preventing AD (level III).39
Diet
In the past decade, a growing body of evidence has linked diet to cognition. Individuals with a higher intake of calories and fat are at higher risk for developing AD.40 The incidence of AD rose in Japan after the country transitioned to a more Westernized diet.41 A modern Western diet rich in saturated fatty acids and simple carbohydrates may negatively impact hippocampus-mediated functions such as memory and learning, and is associated with an increased risk of AD.42 In contrast with high-glycemic and fatty diets, a “healthy diet” is associated with a decrease in beta-amyloid burden, inflammation, and oxidative stress.43,44
Studies focusing on dietary patterns rather than a single nutrient for delaying or preventing AD have yielded more robust and consistent results.45 In a recent meta-analysis, adhering to a Mediterranean diet—which is rich in fruits and vegetables, whole grains, olive oil, and fish; moderate in some dairy products and wine; and low in red meat—was associated with a decreased risk of AD; this evidence was derived mostly from epidemiologic studies.46 Scarmeas et al8 found that high adherence to the Mediterranean diet was associated with 32% to 40% reduced risk of AD. Combining this diet with physical exercise was associated with an up to 67% reduced risk (level III). The Dietary Approaches to Stop Hypertension (DASH) diet, which is rich in total grains, fruits, vegetables, and dairy products, but low in sodium and sweets, correlated with neurocognitive improvement in patients with hypertension.47 Both the Mediterranean and DASH diets have been associated with better cognitive function48 and slower cognitive decline.49 Thus, an attempt to combine the neuroprotective components from both diets led to the creation of the MIND (Mediterranean-DASH Intervention for Neurodegenerative Delay) diet, which also has been associated with a lower incidence of AD.50
Besides specific diets, some food groups have also been found to promote brain health and may help delay or prevent AD. Berries have the highest amount of antioxidants of all fruit. Among vegetables, tomatoes and green leafy vegetables have the highest amount of nutrients for the brain. Nuts, such as walnuts, which are rich in omega-3 fatty acids, are also considered “power foods” for the brain; however, they should be consumed in moderation because they are also rich in fat. Monounsaturated fatty acids, which are found in olives and olive oil, are also beneficial for the brain. Among the 3 types of omega-3 fatty acids, the most important for cognition is docosahexaenoic acid (DHA) because it constitutes 40% of all fatty acids in the brain. Mainly found in oily fish, DHA has antioxidant and anti-inflammatory properties that may delay or prevent AD. Low levels of DHA have been found in patients with AD.51
Continue to: Curcumin, which is derived from...
Curcumin, which is derived from the curry spice turmeric, is a polyphenol with anti-inflammatory, antioxidant, and anti-amyloid properties that may have a promising role in preventing AD in cognitively intact individuals. Initial trials with curcumin have yielded mixed results on cognition, which was partly related to the low solubility and bioavailability of its formulation.52 However, a recent 18-month double-blind randomized placebo-controlled trial found positive effects on memory and attention, as well as reduction of amyloid plaques and tau tangles deposition in the brain, in non-demented older adults age 51 to 84 who took Theracumin, a highly absorptive oral form of curcumin dispersed with colloidal nanoparticles.53 A longer follow-up is required to determine if curcumin can delay or prevent AD.
Alcohol
The role of alcohol in AD prevention is controversial. Overall, data from prospective studies has shown that low to moderate alcohol consumption may be associated with a reduced risk of AD (level III).54 Alcohol drinking in mid-life showed a U-shaped relationship with cognitive impairment; both abstainers and heavy drinkers had an increased risk of cognitive decline compared with light to moderate drinkers (level III).55 Binge drinking significantly increased the odds of cognitive decline, even after controlling for total alcohol consumption per week.55
The definition of low-to-moderate drinking varies substantially among countries. In addition, the size and amount of alcohol contained in a standard drink may differ.56 According to the National Institute on Alcohol Abuse and Alcoholism (NIAAA),57 moderate drinking is defined as up to 1 drink daily for women and 2 drinks daily for men. Binge drinking involves drinking >4 drinks for women and >5 drinks for men, in approximately 2 hours, at least monthly. In the United States, one standard drink contains 14 grams of pure alcohol, which is usually found in 12 ounces of regular beer, 5 ounces of wine, and 1.5 ounces of distilled spirits (vodka or whiskey).58
In a 5-year prospective Canadian study, having 1 drink weekly (especially wine) was associated with an up to 50% reduced risk of AD (level III).59 In the French cohort PAQUID, mild drinkers (<1 to 2 drinks/day) and moderate drinkers (3 to 4 drinks daily) had a reduced incidence of AD compared with non-drinkers. Wine was the most frequently consumed beverage in this study.60 Other studies have found cognitive benefits from mild to moderate drinking regardless of beverage type.54 However, a recent study that included a 30-year follow-up failed to find a significant protective effect of light drinking over abstinence in terms of hippocampal atrophy.61 Atrophy of the hippocampus was correlated with increasing alcohol amounts in a dose-dependent manner, starting at 7 to 14 drinks/week (level III).61
Research has shown that moderate and heavy alcohol use or misuse can directly induce microglial activation and inflammatory mediators’ release, which induce amyloid beta pathology and leads to brain atrophy.62 Hence, non-drinkers should not be advised to begin drinking, because of the lack of RCTs and the concern that beginning to drink may lead to heavy drinking. All drinkers should be advised to adhere to the NIAAA recommendations.13
Continue to: Coffee/tea
Coffee/tea
Although studies of caffeinated coffee have been heterogeneous and yielded mixed results (beneficial effect vs no effect on delaying cognitive decline), systematic reviews and meta-analyses of cross-sectional, case-control, and longitudinal cohort studies have found a general trend towards a favorable preventive role (level III).63-65 Caffeine exhibits its neuroprotective effect by increasing brain serotonin and acetylcholine, and by stabilizing blood-brain-barrier integrity.66 Moreover, in an animal study, mice given caffeine in their drinking water from young adulthood into older age had lower amyloid beta plasma levels compared with those given decaffeinated water.67 These findings suggest that in humans, 5 cups of regular caffeinated coffee daily, equivalent to 500 mg of caffeine,
An Italian study showed that older adults who don’t or rarely drink coffee (<1 cup daily) and those who recently increased their consumption pattern to >1 cup daily had a higher incidence of MCI than those who habitually consumed 1 to 2 cups daily.69 Therefore, it is not recommended to advise a change in coffee drinking pattern in old age. Older adults who are coffee drinkers should, however, be educated about the association between heavier caffeine intake and anxiety, insomnia, and cardiac arrhythmias.70
Despite its more modest caffeine levels, green tea is rich in polyphenols, which belong to the family of catechins and are characterized by antioxidant and anti-inflammatory properties.71 In a Japanese cohort, higher green tea consumption (up to 1 cup daily) was associated with a decreased incidence of MCI in older adults.72 More studies are needed to confirm its potential preventative role in AD.
Which lifestyle change is the most important?
Focusing on a single lifestyle change may be insufficient, especially because the bulk of evidence for individual interventions comes from population-based cohort studies (level III), rather than strong RCTs with a long follow-up. There is increasing evidence that combining multiple lifestyle modifications may yield better outcomes in maintaining or improving cognition.73
The Finnish Geriatric Intervention Study to Prevent Cognitive Impairment and Disability (FINGER), a large, 2-year RCT that included community-dwelling older adults (age 60 to 77) with no diagnosis of major neurocognitive disorder, found that compared with regular health advice, multi-domain interventions reduced cognitive decline and improved overall cognition, executive functioning, and processing speed. The interventions evaluated in this study combined the following 4 modalities74:
- a healthy diet according to the Finnish nutrition recommendations (eating vegetables, fruits, and berries [minimum: 500 g/d], whole grain cereals [several times a day], and fish [2 to 3 times/week]; using low-salt products; consuming fat-free or low-fat milk products; and limiting red meat consumption to <500 g/week
- regular physical exercise tailored for improving muscle strength (1 to 3 times/week) coupled with aerobic exercise (2 to 5 times/week)
- cognitive training, including group sessions that have a social activity component and computer-based individual sessions 3 times/week that target episodic and working memory and executive functioning
- optimal management of cardiovascular risk factors.
Continue to: This multi-domain approach...
This multi-domain approach for lifestyle modification should be strongly recommended to cognitively intact older patients (level IB).
Modeled after the FINGER study, the Alzheimer’s Association U.S. Study to Protect Brain Health Through Lifestyle Intervention to Reduce Risk (U.S. POINTER) is a 2-year, multicenter, controlled clinical trial aimed at testing the ability of a multidimensional lifestyle intervention to prevent AD in at-risk older adults (age 60 to 79, with established metabolic and cardiovascular risk factors). Interventions include a combination of physical exercise, nutritional counseling and management, cognitive and social stimulation, and improved management of cardiovascular risk factors. Recruitment for this large-scale trial was estimated to begin in January 2019 (NCT03688126).75
On a practical basis, Desai et al13 have proposed a checklist (Table 213) that physicians can use in their routine consultations to improve primary prevention of AD among their older patients.
Bottom Line
Advise patients that pursuing a healthy lifestyle is a key to delaying or preventing Alzheimer’s disease. This involves managing cardiovascular risk factors and a combination of staying physically, mentally, socially, and spiritually active, in addition to adhering to a healthy diet such as the Mediterranean diet.
Related Resources
- Anderson K, Grossberg GT. Brain games to slow cognitive decline in Alzheimer’s disease. J Am Med Dir Assoc. 2014;15(8):536-537.
- Small G, Vorgan G. The memory prescription: Dr. Garry Small’s 14-day plan to keep your brain and body young. New York, NY: Hyperion; 2004.
- Small G, Vorgan G. The Alzheimer’s prevention program; keep your brain healthy for the rest of your life. New York, NY: Workman Publishing Company, Inc.; 2012.
Drug Brand Name
Curcumin • Theracurmin
Clinicians have devoted strenuous efforts to secondary prevention of Alzheimer’s disease (AD) by diagnosing and treating patients as early as possible. Unfortunately, there is no cure for AD, and the field has witnessed recurrent failures of several pharmacotherapy candidates with either symptomatic or disease-modifying properties.1 An estimated one-third of AD cases can be attributed to modifiable risk factors.2 Thus, implementing primary prevention measures by addressing modifiable risk factors thought to contribute to the disease, with the goal of reducing the risk of developing AD, or at least delaying its onset, is a crucial public health strategy.
Cardiovascular risk factors, such as hypertension, hyperlipidemia, diabetes, hyperhomocysteinemia, obesity, and smoking, have emerged as substantive risk factors for AD.3 Optimal management of these major risk factors, especially in mid-life, may be a preventive approach against AD. Although detailing the evidence on the impact of managing cardiovascular risk factors to delay or prevent AD is beyond the scope of this article, it is becoming clear that “what is good for the heart is good for the brain.”
Additional modifiable risk factors are related to lifestyle habits, such as physical exercise, mental and social activity, meditation/spiritual activity, and diet. This article reviews the importance of pursuing a healthy lifestyle in delaying AD, with the corresponding levels of evidence that support each specific lifestyle modification. The levels of evidence are defined in Table 1.4
Physical exercise
Twenty-one percent of AD cases in the United States are attributable to physical inactivity.5 In addition to its beneficial effect on metabolic syndrome, in animal and human research, regular exercise has been shown to have direct neuroprotective effects. High levels of physical activity increase hippocampal neurogenesis and neuroplasticity, increase vascular circulation in the brain regions implicated in AD, and modulate inflammatory mediators as well as brain growth factors such as brain-derived neurotrophic factor (BDNF) and insulin-like growth factor-1 (IGF-1).6
The definition of regular physical exercise varies across the literature, but usually implies aerobic exercise—an ongoing activity sufficient to increase the heart rate and the need for oxygen, sustained for 20 to 30 minutes per session.7 Modalities include household activities and leisure-time activities. In a large prospective cohort study, Scarmeas et al8 categorized leisure-time activities into 3 types:
- light (walking, dancing, calisthenics, golfing, bowling, gardening, horseback riding)
- moderate (bicycling, swimming, hiking, playing tennis)
- vigorous (aerobic dancing, jogging, playing handball).
These types of physical exercise were weighed by the frequency of participation per week. Compared with being physically inactive, low levels of weekly physical activity (0.1 hours of vigorous, 0.8 hours of moderate, or 1.3 hours of light exercise) were associated with a 29% to 41% lower risk of developing AD, while higher weekly physical activity (1.3 hours of vigorous, 2.3 hours of moderate, or 3.8 hours of light exercise) were associated with a 37% to 50% lower risk (level III).8
In another 20-year cohort study, engaging in leisure-time physical activity at least twice a week in mid-life was significantly associated with a reduced risk of AD, after adjusting for age, sex, education, follow-up time, locomotor disorders, apolipoprotein E (ApoE) genotype, vascular disorders, smoking, and alcohol intake (level III).9 Moreover, a systematic review of 29 randomized controlled trials (RCTs) showed that aerobic exercise training, such as brisk walking, jogging, and biking, was associated with improvements in attention, processing speed, executive function, and memory among healthy older adults and those with mild cognitive impairment (MCI; level IA).10
Continue to: From a pathophysiological standpoint...
From a pathophysiological standpoint, higher levels of physical exercise in cognitively intact older adults have been associated with reduced brain amyloid beta deposits, especially in ApoE4 carriers.11 This inverse relationship also has been demonstrated in patients who are presymptomatic who carry 1 of the 3 known autosomal dominant mutations for the familial forms of AD.12
Overall, physicians should recommend that patients—especially those with cardiovascular risk factors that increase their risk for AD—exercise regularly by following the guidelines of the American Heart Association or the American College of Sports Medicine.13 These include muscle-strengthening activities (legs, hips, back, abdomen, shoulders, and arms) at least 2 days/week, in addition to either 30 minutes/day of moderate-intensity aerobic activity such as brisk walking, 5 days/week; or 25 minutes of vigorous aerobic activity such as jogging and running, 3 days/week14 (level IA evidence for overall improvement in cognitive function; level III evidence for AD delay/risk reduction). Neuromotor exercise, such as yoga and tai chi, and flexibility exercise such as muscle stretching, especially after a hot bath, 2 to 3 days/week are also recommended (level III).15
Mental activity
Nineteen percent of AD cases worldwide and 7% in the United States. can be attributed to low educational attainment, which is associated with low brain cognitive reserve.5 Cognitive resilience in later life may be enhanced by building brain reserves through intellectual stimulation, which affects neuronal branching and plasticity.16 Higher levels of complex mental activities measured across the lifespan, such as education, occupation, reading, and writing, are correlated with significantly less hippocampal volume shrinkage over time.17 Frequent participation in mentally stimulating activities—such as listening to the radio; reading newspapers, magazines, or books; playing games (cards, checkers, crosswords or other puzzles); and visiting museums—was associated with an up to 64% reduction in the odds of developing AD in a cohort of cognitively intact older adults followed for 4 years.18 The correlation between mental activity and AD was found to be independent of physical activity, social activity, or baseline cognitive function.19
In a large cohort of cognitively intact older adults (mean age 70), engaging in a mentally stimulating activity (craft activities, computer use, or going to the theater/movies) once to twice a week was significantly associated with a reduced incidence of amnestic MCI.20 Another prospective 21-year study demonstrated a significant reduction in AD risk in community-dwelling cognitively intact older adults (age 75 to 85) who participated in cognitively stimulating activities, such as reading books or newspapers, writing for pleasure, doing crossword puzzles, playing board games or cards, or playing musical instruments, several times/week.21
Growing scientific evidence also suggests that lifelong multilingualism can delay AD onset by 4 to 5 years.22 Multilingualism is associated with greater cognitive reserve, gray matter volume, functional connectivity and white matter density.23
Continue to: Physicians should encourage their patients...
Physicians should encourage their patients to engage in intellectually stimulating activities and creative leisure-time activities several times/week to enhance their cognitive reserves and delay AD onset (level III evidence with respect to AD risk reduction/delay).
Social activity
Social engagement may be an additional protective factor against AD. In a large 4-year prospective study, increased loneliness in cognitively intact older adults doubled the risk of AD.24 Data from the large French cohort PAQUID (Personnes Agées QUID) emphasized the importance of a patient’s social network as a protective factor against AD. In this cohort, the perception of reciprocity in relationships with others (the perception that a person had received more than he or she had given) was associated with a 53% reduction in AD risk (level III).25 In another longitudinal cohort study, social activity was found to decrease the incidence of subjective cognitive decline, which is a prodromal syndrome for MCI and AD (level III).26
A major confounder in studies assessing for social activity is the uncertainty if social withdrawal is a modifiable risk factor or an early manifestation of AD, since apathetic patients with AD tend to be socially withdrawn.27 Another limitation of measuring the impact of social activity relative to AD risk is the difficulty in isolating social activities from activities that have physical and mental activity components, such as leisure-time activities.28
Meditation/spiritual activity
Chronic psychological stress is believed to compromise limbic structures that regulate stress-related behaviors and the memory network, which might explain how being prone to psychological distress may be associated with MCI or AD.29 Cognitive stress may increase the oxidative stress and telomere shortening implicated in the neurodegenerative processes of AD.30 In one study, participants who were highly prone to psychological distress were found to be at 3 times increased risk for developing AD, after adjusting for depression symptoms and physical and mental activities (level III).31 By reducing chronic psychological stress, meditation techniques offer a promising preventive option against AD.
Mindfulness-based interventions (MBI) have gained increased attention in the past decade. They entail directing one’s attention towards the present moment, thereby decreasing ruminative thoughts and stress arousal.32 Recent RCTs have shown that MBI may promote brain health in older adults not only by improving psychological well-being but also by improving attentional control33 and functional connectivity in brain regions implicated in executive functioning,34 as well as by modulating inflammatory processes implicated in AD.35 Furthermore, an RCT of patients diagnosed with MCI found that compared with memory enhancement training, a weekly 60-minute yoga session improved memory and executive functioning.36
Continue to: Kirtan Kriya is a medication technique...
Kirtan Kriya is a meditation technique that is easy to learn and practice by older adults and can improve memory in patients at risk for developing AD.37 However, more rigorous RCTs conducted in larger samples of older adults are needed to better evaluate the effect of all meditation techniques for delaying or preventing AD (level IB with respect to improvement in cognitive functioning/level III for AD delay/risk reduction).38
Spiritual activities, such as going to places of worship or religious meditation, have been associated with a lower prevalence of AD. Attending religious services, gatherings, or retreats involves a social component because these activities often are practiced in groups. They also confer a method of dealing with psychological distress and depression. Additionally, frequent readings of religious texts represents a mentally stimulating activity that may also contribute to delaying/preventing AD (level III).39
Diet
In the past decade, a growing body of evidence has linked diet to cognition. Individuals with a higher intake of calories and fat are at higher risk for developing AD.40 The incidence of AD rose in Japan after the country transitioned to a more Westernized diet.41 A modern Western diet rich in saturated fatty acids and simple carbohydrates may negatively impact hippocampus-mediated functions such as memory and learning, and is associated with an increased risk of AD.42 In contrast with high-glycemic and fatty diets, a “healthy diet” is associated with a decrease in beta-amyloid burden, inflammation, and oxidative stress.43,44
Studies focusing on dietary patterns rather than a single nutrient for delaying or preventing AD have yielded more robust and consistent results.45 In a recent meta-analysis, adhering to a Mediterranean diet—which is rich in fruits and vegetables, whole grains, olive oil, and fish; moderate in some dairy products and wine; and low in red meat—was associated with a decreased risk of AD; this evidence was derived mostly from epidemiologic studies.46 Scarmeas et al8 found that high adherence to the Mediterranean diet was associated with 32% to 40% reduced risk of AD. Combining this diet with physical exercise was associated with an up to 67% reduced risk (level III). The Dietary Approaches to Stop Hypertension (DASH) diet, which is rich in total grains, fruits, vegetables, and dairy products, but low in sodium and sweets, correlated with neurocognitive improvement in patients with hypertension.47 Both the Mediterranean and DASH diets have been associated with better cognitive function48 and slower cognitive decline.49 Thus, an attempt to combine the neuroprotective components from both diets led to the creation of the MIND (Mediterranean-DASH Intervention for Neurodegenerative Delay) diet, which also has been associated with a lower incidence of AD.50
Besides specific diets, some food groups have also been found to promote brain health and may help delay or prevent AD. Berries have the highest amount of antioxidants of all fruit. Among vegetables, tomatoes and green leafy vegetables have the highest amount of nutrients for the brain. Nuts, such as walnuts, which are rich in omega-3 fatty acids, are also considered “power foods” for the brain; however, they should be consumed in moderation because they are also rich in fat. Monounsaturated fatty acids, which are found in olives and olive oil, are also beneficial for the brain. Among the 3 types of omega-3 fatty acids, the most important for cognition is docosahexaenoic acid (DHA) because it constitutes 40% of all fatty acids in the brain. Mainly found in oily fish, DHA has antioxidant and anti-inflammatory properties that may delay or prevent AD. Low levels of DHA have been found in patients with AD.51
Continue to: Curcumin, which is derived from...
Curcumin, which is derived from the curry spice turmeric, is a polyphenol with anti-inflammatory, antioxidant, and anti-amyloid properties that may have a promising role in preventing AD in cognitively intact individuals. Initial trials with curcumin have yielded mixed results on cognition, which was partly related to the low solubility and bioavailability of its formulation.52 However, a recent 18-month double-blind randomized placebo-controlled trial found positive effects on memory and attention, as well as reduction of amyloid plaques and tau tangles deposition in the brain, in non-demented older adults age 51 to 84 who took Theracumin, a highly absorptive oral form of curcumin dispersed with colloidal nanoparticles.53 A longer follow-up is required to determine if curcumin can delay or prevent AD.
Alcohol
The role of alcohol in AD prevention is controversial. Overall, data from prospective studies has shown that low to moderate alcohol consumption may be associated with a reduced risk of AD (level III).54 Alcohol drinking in mid-life showed a U-shaped relationship with cognitive impairment; both abstainers and heavy drinkers had an increased risk of cognitive decline compared with light to moderate drinkers (level III).55 Binge drinking significantly increased the odds of cognitive decline, even after controlling for total alcohol consumption per week.55
The definition of low-to-moderate drinking varies substantially among countries. In addition, the size and amount of alcohol contained in a standard drink may differ.56 According to the National Institute on Alcohol Abuse and Alcoholism (NIAAA),57 moderate drinking is defined as up to 1 drink daily for women and 2 drinks daily for men. Binge drinking involves drinking >4 drinks for women and >5 drinks for men, in approximately 2 hours, at least monthly. In the United States, one standard drink contains 14 grams of pure alcohol, which is usually found in 12 ounces of regular beer, 5 ounces of wine, and 1.5 ounces of distilled spirits (vodka or whiskey).58
In a 5-year prospective Canadian study, having 1 drink weekly (especially wine) was associated with an up to 50% reduced risk of AD (level III).59 In the French cohort PAQUID, mild drinkers (<1 to 2 drinks/day) and moderate drinkers (3 to 4 drinks daily) had a reduced incidence of AD compared with non-drinkers. Wine was the most frequently consumed beverage in this study.60 Other studies have found cognitive benefits from mild to moderate drinking regardless of beverage type.54 However, a recent study that included a 30-year follow-up failed to find a significant protective effect of light drinking over abstinence in terms of hippocampal atrophy.61 Atrophy of the hippocampus was correlated with increasing alcohol amounts in a dose-dependent manner, starting at 7 to 14 drinks/week (level III).61
Research has shown that moderate and heavy alcohol use or misuse can directly induce microglial activation and inflammatory mediators’ release, which induce amyloid beta pathology and leads to brain atrophy.62 Hence, non-drinkers should not be advised to begin drinking, because of the lack of RCTs and the concern that beginning to drink may lead to heavy drinking. All drinkers should be advised to adhere to the NIAAA recommendations.13
Continue to: Coffee/tea
Coffee/tea
Although studies of caffeinated coffee have been heterogeneous and yielded mixed results (beneficial effect vs no effect on delaying cognitive decline), systematic reviews and meta-analyses of cross-sectional, case-control, and longitudinal cohort studies have found a general trend towards a favorable preventive role (level III).63-65 Caffeine exhibits its neuroprotective effect by increasing brain serotonin and acetylcholine, and by stabilizing blood-brain-barrier integrity.66 Moreover, in an animal study, mice given caffeine in their drinking water from young adulthood into older age had lower amyloid beta plasma levels compared with those given decaffeinated water.67 These findings suggest that in humans, 5 cups of regular caffeinated coffee daily, equivalent to 500 mg of caffeine,
An Italian study showed that older adults who don’t or rarely drink coffee (<1 cup daily) and those who recently increased their consumption pattern to >1 cup daily had a higher incidence of MCI than those who habitually consumed 1 to 2 cups daily.69 Therefore, it is not recommended to advise a change in coffee drinking pattern in old age. Older adults who are coffee drinkers should, however, be educated about the association between heavier caffeine intake and anxiety, insomnia, and cardiac arrhythmias.70
Despite its more modest caffeine levels, green tea is rich in polyphenols, which belong to the family of catechins and are characterized by antioxidant and anti-inflammatory properties.71 In a Japanese cohort, higher green tea consumption (up to 1 cup daily) was associated with a decreased incidence of MCI in older adults.72 More studies are needed to confirm its potential preventative role in AD.
Which lifestyle change is the most important?
Focusing on a single lifestyle change may be insufficient, especially because the bulk of evidence for individual interventions comes from population-based cohort studies (level III), rather than strong RCTs with a long follow-up. There is increasing evidence that combining multiple lifestyle modifications may yield better outcomes in maintaining or improving cognition.73
The Finnish Geriatric Intervention Study to Prevent Cognitive Impairment and Disability (FINGER), a large, 2-year RCT that included community-dwelling older adults (age 60 to 77) with no diagnosis of major neurocognitive disorder, found that compared with regular health advice, multi-domain interventions reduced cognitive decline and improved overall cognition, executive functioning, and processing speed. The interventions evaluated in this study combined the following 4 modalities74:
- a healthy diet according to the Finnish nutrition recommendations (eating vegetables, fruits, and berries [minimum: 500 g/d], whole grain cereals [several times a day], and fish [2 to 3 times/week]; using low-salt products; consuming fat-free or low-fat milk products; and limiting red meat consumption to <500 g/week
- regular physical exercise tailored for improving muscle strength (1 to 3 times/week) coupled with aerobic exercise (2 to 5 times/week)
- cognitive training, including group sessions that have a social activity component and computer-based individual sessions 3 times/week that target episodic and working memory and executive functioning
- optimal management of cardiovascular risk factors.
Continue to: This multi-domain approach...
This multi-domain approach for lifestyle modification should be strongly recommended to cognitively intact older patients (level IB).
Modeled after the FINGER study, the Alzheimer’s Association U.S. Study to Protect Brain Health Through Lifestyle Intervention to Reduce Risk (U.S. POINTER) is a 2-year, multicenter, controlled clinical trial aimed at testing the ability of a multidimensional lifestyle intervention to prevent AD in at-risk older adults (age 60 to 79, with established metabolic and cardiovascular risk factors). Interventions include a combination of physical exercise, nutritional counseling and management, cognitive and social stimulation, and improved management of cardiovascular risk factors. Recruitment for this large-scale trial was estimated to begin in January 2019 (NCT03688126).75
On a practical basis, Desai et al13 have proposed a checklist (Table 213) that physicians can use in their routine consultations to improve primary prevention of AD among their older patients.
Bottom Line
Advise patients that pursuing a healthy lifestyle is a key to delaying or preventing Alzheimer’s disease. This involves managing cardiovascular risk factors and a combination of staying physically, mentally, socially, and spiritually active, in addition to adhering to a healthy diet such as the Mediterranean diet.
Related Resources
- Anderson K, Grossberg GT. Brain games to slow cognitive decline in Alzheimer’s disease. J Am Med Dir Assoc. 2014;15(8):536-537.
- Small G, Vorgan G. The memory prescription: Dr. Garry Small’s 14-day plan to keep your brain and body young. New York, NY: Hyperion; 2004.
- Small G, Vorgan G. The Alzheimer’s prevention program; keep your brain healthy for the rest of your life. New York, NY: Workman Publishing Company, Inc.; 2012.
Drug Brand Name
Curcumin • Theracurmin
1. Mehta D, Jackson R, Paul G, et al. Why do trials for Alzheimer’s disease drugs keep failing? A discontinued drug perspective for 2010-2015. Expert Opin Investig Drugs. 2017;26(6):735-739.
2. Norton S, Matthews FE, Barnes DE, et al. Potential for primary prevention of Alzheimer’s disease: an analysis of population-based data. Lancet Neurol. 2014;13(8):788-794.
3. Meng XF, Yu JT, Wang HF, et al. Midlife vascular risk factors and the risk of Alzheimer’s disease: a systematic review and meta-analysis. J Alzheimers Dis. 2014;42(4):1295-1310.
4. Shekelle PG, Woolf SH, Eccles M, et al. Developing clinical guidelines. West J Med. 1999;170(6):348-351.
5. Barnes DE, Yaffe Y. The projected impact of risk factor reduction on Alzheimer’s disease prevalence. Lancet Neurol. 2011;10(9):819-828.
6. Cotman CW, Berchtold NC, Christie LA. Exercise builds brain health: key roles of growth factor cascades and inflammation. Trends Neurosci. 2007;30(9):464-472.
7. Ahlskog JE, Geda YE, Graff-Radford NR, et al. Physical exercise as a preventive or disease-modifying treatment of dementia and brain aging. Mayo Clin Proc. 2011;86(9):876-884.
8. Scarmeas N, Luchsinger JA, Schupf N, et al. Physical activity, diet, and risk of Alzheimer Disease. JAMA. 2009;302(6):627-637.
9. Rovio S, Kåreholt I, Helkala EL, et al. Leisure-time physical activity at midlife and the risk of dementia and Alzheimer’s disease. Lancet Neurol. 2005;4(11):705-711.
10. Smith PJ et al. Aerobic exercise and neurocognitive performance: a meta-analytic review of randomized controlled trials. Psychosom Med. 2010;72(3):239-252.
11. Brown BM, Peiffer JJ, Taddei K, et al. Physical activity and amyloid-beta plasma and brain levels: results from the Australian imaging, biomarkers and lifestyle study of ageing. Mol Psychiatry. 2013;18(8):875-881.
12. Brown BM, Sohrabi HR, Taddei K, et al. Habitual exercise levels are associated with cerebral amyloid load in presymptomatic autosomal dominant Alzheimer’s disease. Alzheimers Dement. 2017;13(11):1197-1206.
13. Desai AK, Grossberg GT, Chibnall JT. Healthy brain aging: a road map. Clin Geriatr Med. 2010;26(1):1-16.
14. Centers for Disease Control and Prevention. Physical activity: how much physical activity do older adults need?
15. Garber CE, Blissmer B, Deschenes MR, et al; American College of Sports Medicine. American College of Sports Medicine position stand. Quantity and quality of exercise for developing and maintaining cardiorespiratory, musculoskeletal, and neuromotor fitness in apparently healthy adults: guidance for prescribing exercise. Med Sci Sports Exerc. 2011;43(7):1334-1359.
16. Livingston G, Sommerlad A, Orgeta V, et al. Dementia prevention, intervention, and care. Lancet. 2017;390(10113);2673-2734.
17. Valenzuela MJ, Sachdev P, Wen W, et al. Lifespan mental activity predicts diminished rate of hippocampal atrophy. PLoS One. 2008;3(7):e2598. doi.org/10.1371/journal.pone.0002598.
18. Wilson RS, Bennett DA, Bienias JL, et al. Cognitive activity and incident AD in a population-based sample of older persons. Neurology. 2002;59(12):1910-1914.
19. Wilson RS, Scherr PA, Schneider JA, et al. Relation of cognitive activity to risk of developing Alzheimer disease. Neurology. 2007;69(20):1911-1920.
20. Krell-Roesch J, Vemuri P, Pink A, et al. Association between mentally stimulating activities in late life and the outcome of incident mild cognitive impairment, with an analysis of the apoe ε4 genotype. JAMA Neurol. 2017;74(3):332-338.
21. Verghese J, Lipton RB, Katz MJ, et al. Leisure activities and the risk of dementia in the elderly. N Engl J Med. 2003;348(25):2508-2516.
22. Klein RM, Christie J, Parkvall M. Does multilingualism affect the incidence of Alzheimer’s disease?: a worldwide analysis by country. SSM Popul Health. 2016;2:463-467.
23. Grundy JG, Anderson JAE, Bialystok E. Neural correlates of cognitive processing in monolinguals and bilinguals. Ann N Y Acad Sci. 2017;1396(1):183-201.
24. Wilson RS, Krueger KR, Arnold SE, et al. Loneliness and risk of Alzheimer disease. Arch Gen Psychiatry. 2007;64(2):234-240.
25. Amieva H, Stoykova R, Matharan F, et al. What aspects of social network are protective for dementia? Not the quantity but the quality of social interactions is protective up to 15 years later. Psychosom Med. 2010;72(9):905-911.
26. Kuiper JS, Oude Voshaar RC, Zuidema SU, et al. The relationship between social functioning and subjective memory complaints in older persons: a population-based longitudinal cohort study. Int J Geriatr Psychiatry. 2017;32(10):1059-1071.
27. Robert P, Onyike CU, Leentjens AF, et al. Proposed diagnostic criteria for apathy in Alzheimer’s disease and other neuropsychiatric disorders. Eur Psychiatry. 2009;24(2):98-104.
28. Marioni RE, Proust-Lima C, Amieva H, et al. Social activity, cognitive decline and dementia risk: a 20-year prospective cohort study. BMC Public Health. 2015;15:1089.
29. Wilson RS, Schneider JA, Boyle PA, et al. Chronic distress and incidence of mild cognitive impairment. Neurology. 2007;68(24):2085-2092.
30. Cai Z, Yan LJ, Ratka A. Telomere shortening and Alzheimer’s disease. Neuromolecular Med. 2013;15(1):25-48.
31. Wilson RS, Arnold SE, Schneider JA, et al. Chronic psychological distress and risk of Alzheimer’s disease in old age. Neuroepidemiology. 2006;27(3):143-153.
32. Epel E, Daubenmier J, Moskowitz JT, et al. Can meditation slow rate of cellular aging? Cognitive stress, mindfulness, and telomeres. Ann N Y Acad Sci. 2009;1172:34-53.
33. Malinowski P, Moore AW, Mead Br, et al. Mindful aging: the effects of regular brief mindfulness practice on electrophysiological markers of cognitive and affective processing in older adults. Mindfulness (N Y). 2017;8(1):78-94.
34. Taren AA, Gianaros PJ, Greco CM, et al. Mindfulness meditation training and executive control network resting state functional connectivity: a randomized controlled trial. Psychosom Med. 2017;79(6):674-683.
35. Fountain-Zaragoza S, Prakash RS. Mindfulness training for healthy aging: impact on attention, well-being, and inflammation. Front in Aging Neurosci. 2017;9:11.
36. Eyre HA, Siddarth P, Acevedo B, et al. A randomized controlled trial of Kundalini yoga in mild cognitive impairment. Int Psychogeriatr. 2017;29(4):557-567.
37. Khalsa DS. Stress, meditation, and Alzheimer’s disease prevention: where the evidence stands. J Alzheimers Dis. 2015;48(1):1-12.
38. Berk L, van Boxtel M, van Os J. Can mindfulness-based interventions influence cognitive functioning in older adults? A review and considerations for future research. Aging Ment Health. 2017;21(11):1113-1120.
39. Hosseini S, Chaurasia A, Oremus M. The effect of religion and spirituality on cognitive function: a systematic review. Gerontologist. 2017. doi: 10.1093/geront/gnx024.
40. Luchsinger JA, Tang MX, Shea S, et al. Caloric intake and the risk of Alzheimer disease. Arch Neurol. 2002;59(8):1258-1263.
41. Grant WB. Trends in diet and Alzheimer’s disease during the nutrition transition in Japan and developing countries. J Alzheimers Dis. 2014;38(3):611-620.
42. Kanoski SE, Davidson TL. Western diet consumption and cognitive impairment: links to hippocampal dysfunction and obesity. Physiol Behav. 2011;103(1):59-68.
43. Hu N, Yu JT, Tan L, et al. Nutrition and the risk of Alzheimer’s disease. Biomed Res Int. 2013;2013:524820. doi: 10.1155/2013/524820.
44. Taylor MK, Sullivan DK, Swerdlow RH, et al. A high-glycemic diet is associated with cerebral amyloid burden in cognitively normal older adults. Am J Clin Nutr. 2017;106(6):1463-1470.
45. van de Rest O, Berendsen AM, Haveman-Nies A, et al. Dietary patterns, cognitive decline, and dementia: a systematic review. Adv Nutr. 2015;6(2):154-168.
46. Petersson SD, Philippou E. Mediterranean diet, cognitive function, and dementia: a systematic review of the evidence. Adv Nutr. 2016;7(5):889-904.
47. Smith PJ, Blumenthal JA, Babyak MA, et al. Effects of the dietary approaches to stop hypertension diet, exercise, and caloric restriction on neurocognition in overweight adults with high blood pressure. Hypertension. 2010;55(6):1331-1338.
48. Wengreen H, Munger RG, Cutler A, et al. Prospective study of dietary approaches to stop hypertension- and Mediterranean-style dietary patterns and age-related cognitive change: the Cache County study on memory, health and aging. Am J Clin Nutr. 2013;98(5):1263-1271.
49. Tangney CC, Li H, Wang Y, et al. Relation of DASH- and Mediterranean-like dietary patterns to cognitive decline in older persons. Neurology. 2014;83(16):1410-1416.
50. Morris MC, Tangney CC, Wang Y, et al. MIND diet associated with reduced incidence of Alzheimer’s disease. Alzheimers Dement. 2015;11(9):1007-1014.
51. Desai AK, Rush J, Naveen L, et al. Nutrition and nutritional supplements to promote brain health. In: Hartman-Stein PE, Rue AL, eds. Enhancing cognitive fitness in adults: a guide to the use and development of community-based programs. New York, NY: Springer; 2011:249-269.
52. Goozee KG, Shah TM, Sohrabi HR, et al. Examining the potential clinical value of curcumin in the prevention and diagnosis of Alzheimer’s disease. Br J Nutr. 2016;115(3):449-465.
53. Small GW, Siddarth P, Li Z, et al. Memory and brain amyloid and tau effects of a bioavailable form of curcumin in non-demented adults: a double-blind, placebo-controlled 18-month trial. Am J Geriatr Psychiatry. 2018;26(3):266-277.
54. Kim JW, Lee DY, Lee BC, et al. Alcohol and cognition in the elderly: a review. Psychiatry Investig. 2012;9(1):8-16.
55. Virtaa JJ, Järvenpää T, Heikkilä K, et al. Midlife alcohol consumption and later risk of cognitive impairment: a twin follow-up study. J Alzheimers Dis. 2010;22(3):939-948.
56. Kerr WC, Stockwell T. Understanding standard drinks and drinking guidelines. Drug and Alcohol Rev. 2012;31(2):200-205.
57. National Institute on Alcohol Abuse and Alcoholism. Drinking levels defined. https://www.niaaa.nih.gov/alcohol-health/overview-alcohol-consumption/moderate-binge-drinking. Accessed December 9, 2017.
58. National Institute on Alcohol Abuse and Alcoholism. What is a standard drink? https://www.niaaa.nih.gov/alcohol-health/overview-alcohol-consumption/what-standard-drink. Accessed November 9, 2017.
59. Lindsay J, Laurin D, Verreault R, et al. Risk factors for Alzheimer’s disease: a prospective analysis from the Canadian study of health and aging. Am J Epidemiol. 2002;156(5):445-453.
60. Orgogozo JM, Dartigues JF, Lafont S, et al. Wine consumption and dementia in the elderly: a prospective community study in the Bordeaux area. Rev Neurol (Paris). 1997;153(3):185-192.
61. Topiwala A, Allan CL, Valkanova V, et al. Moderate alcohol consumption as risk factor for adverse brain outcomes and cognitive decline: longitudinal cohort study. BMJ. 2017;357.
62. Venkataraman A, Kalk N, Sewell G, et al. Alcohol and Alzheimer’s disease-does alcohol dependence contribute to beta-amyloid deposition, neuroinflammation and neurodegeneration in Alzheimer’s Disease? Alcohol Alcohol. 2017;52(2):151-158.
63. Ma QP, Huang C, Cui QY, et al. Meta-analysis of the association between tea intake and the risk of cognitive disorders. PLoS One. 2016;11(11):e0165861. doi: 10.1371/journal.pone.0165861.
64. Santos C, Costa J, Santos J, et al. Caffeine intake and dementia: systematic review and meta-analysis. J Alzheimers Dis. 2010;20(Suppl 1):S187-204.
65. Panza F, Solfrizzi V, Barulli MR, et al. Coffee, tea, and caffeine consumption and prevention of late-life cognitive decline and dementia: a systematic review. J Nutr Health Aging. 2015;19(3):313-328.
66. Wierzejska R. Can coffee consumption lower the risk of Alzheimer’s disease and Parkinson’s disease? A literature review. Arch Med Sci. 2017;13(3):507-514.
67. Arendash GW, Cao C. Caffeine and coffee as therapeutics against Alzheimer’s disease. J Alzheimers Dis. 2010;20 (Suppl 1):S117-S126.
68. Eskelinen MH, Ngandu T, Tuomilehto J, et al. Midlife coffee and tea drinking and the risk of late-life dementia: a population-based CAIDE study. J Alzheimers Dis. 2009;16(1):85-91.
69. Solfrizzi V, Panza F, Imbimbo BP, et al. Coffee consumption habits and the risk of mild cognitive impairment: the Italian longitudinal study on aging. J Alzheimers Dis. 2015;47(4):889-899.
70. Vittoria Mattioli. Beverages of daily life: impact of caffeine on atrial fibrillation. J Atr Fibrillation. 2014;7(2):1133.
71. Chacko SM, Thambi PT, Kuttan R, et al. Beneficial effects of green tea: a literature review. Chin Med. 2010;5:13.
72. Noguchi-Shinohara M, Yuki S, Dohmoto C, et al. Consumption of green tea, but not black tea or coffee, is associated with reduced risk of cognitive decline. PLoS One. 2014;9(5):e96013. doi: 10.1371/journal.pone.0096013.
73. Schneider N, Yvon C. A review of multidomain interventions to support healthy cognitive ageing. J Nutr Health Aging. 2013;17(3):252-257.
74. Ngandu T, Lehitsalo J, Solomon A, et al. A 2 year multidomain intervention of diet, exercise, cognitive training, and vascular risk monitoring versus control to prevent cognitive decline in at-risk elderly people (FINGER): a randomised controlled trial. Lancet. 2015;385(9984):2255-2263.
75. U.S. National Library of Medicing. ClinicalTrials.gov. U.S. study to protect brain health through lifestyle intervention to reduce risk (POINTER). https://clinicaltrials.gov/ct2/show/NCT03688126?term=pointer&cond=Alzheimer+Disease&rank=1. Published September 28, 2018. Accessed November 3, 2018.
1. Mehta D, Jackson R, Paul G, et al. Why do trials for Alzheimer’s disease drugs keep failing? A discontinued drug perspective for 2010-2015. Expert Opin Investig Drugs. 2017;26(6):735-739.
2. Norton S, Matthews FE, Barnes DE, et al. Potential for primary prevention of Alzheimer’s disease: an analysis of population-based data. Lancet Neurol. 2014;13(8):788-794.
3. Meng XF, Yu JT, Wang HF, et al. Midlife vascular risk factors and the risk of Alzheimer’s disease: a systematic review and meta-analysis. J Alzheimers Dis. 2014;42(4):1295-1310.
4. Shekelle PG, Woolf SH, Eccles M, et al. Developing clinical guidelines. West J Med. 1999;170(6):348-351.
5. Barnes DE, Yaffe Y. The projected impact of risk factor reduction on Alzheimer’s disease prevalence. Lancet Neurol. 2011;10(9):819-828.
6. Cotman CW, Berchtold NC, Christie LA. Exercise builds brain health: key roles of growth factor cascades and inflammation. Trends Neurosci. 2007;30(9):464-472.
7. Ahlskog JE, Geda YE, Graff-Radford NR, et al. Physical exercise as a preventive or disease-modifying treatment of dementia and brain aging. Mayo Clin Proc. 2011;86(9):876-884.
8. Scarmeas N, Luchsinger JA, Schupf N, et al. Physical activity, diet, and risk of Alzheimer Disease. JAMA. 2009;302(6):627-637.
9. Rovio S, Kåreholt I, Helkala EL, et al. Leisure-time physical activity at midlife and the risk of dementia and Alzheimer’s disease. Lancet Neurol. 2005;4(11):705-711.
10. Smith PJ et al. Aerobic exercise and neurocognitive performance: a meta-analytic review of randomized controlled trials. Psychosom Med. 2010;72(3):239-252.
11. Brown BM, Peiffer JJ, Taddei K, et al. Physical activity and amyloid-beta plasma and brain levels: results from the Australian imaging, biomarkers and lifestyle study of ageing. Mol Psychiatry. 2013;18(8):875-881.
12. Brown BM, Sohrabi HR, Taddei K, et al. Habitual exercise levels are associated with cerebral amyloid load in presymptomatic autosomal dominant Alzheimer’s disease. Alzheimers Dement. 2017;13(11):1197-1206.
13. Desai AK, Grossberg GT, Chibnall JT. Healthy brain aging: a road map. Clin Geriatr Med. 2010;26(1):1-16.
14. Centers for Disease Control and Prevention. Physical activity: how much physical activity do older adults need?
15. Garber CE, Blissmer B, Deschenes MR, et al; American College of Sports Medicine. American College of Sports Medicine position stand. Quantity and quality of exercise for developing and maintaining cardiorespiratory, musculoskeletal, and neuromotor fitness in apparently healthy adults: guidance for prescribing exercise. Med Sci Sports Exerc. 2011;43(7):1334-1359.
16. Livingston G, Sommerlad A, Orgeta V, et al. Dementia prevention, intervention, and care. Lancet. 2017;390(10113);2673-2734.
17. Valenzuela MJ, Sachdev P, Wen W, et al. Lifespan mental activity predicts diminished rate of hippocampal atrophy. PLoS One. 2008;3(7):e2598. doi.org/10.1371/journal.pone.0002598.
18. Wilson RS, Bennett DA, Bienias JL, et al. Cognitive activity and incident AD in a population-based sample of older persons. Neurology. 2002;59(12):1910-1914.
19. Wilson RS, Scherr PA, Schneider JA, et al. Relation of cognitive activity to risk of developing Alzheimer disease. Neurology. 2007;69(20):1911-1920.
20. Krell-Roesch J, Vemuri P, Pink A, et al. Association between mentally stimulating activities in late life and the outcome of incident mild cognitive impairment, with an analysis of the apoe ε4 genotype. JAMA Neurol. 2017;74(3):332-338.
21. Verghese J, Lipton RB, Katz MJ, et al. Leisure activities and the risk of dementia in the elderly. N Engl J Med. 2003;348(25):2508-2516.
22. Klein RM, Christie J, Parkvall M. Does multilingualism affect the incidence of Alzheimer’s disease?: a worldwide analysis by country. SSM Popul Health. 2016;2:463-467.
23. Grundy JG, Anderson JAE, Bialystok E. Neural correlates of cognitive processing in monolinguals and bilinguals. Ann N Y Acad Sci. 2017;1396(1):183-201.
24. Wilson RS, Krueger KR, Arnold SE, et al. Loneliness and risk of Alzheimer disease. Arch Gen Psychiatry. 2007;64(2):234-240.
25. Amieva H, Stoykova R, Matharan F, et al. What aspects of social network are protective for dementia? Not the quantity but the quality of social interactions is protective up to 15 years later. Psychosom Med. 2010;72(9):905-911.
26. Kuiper JS, Oude Voshaar RC, Zuidema SU, et al. The relationship between social functioning and subjective memory complaints in older persons: a population-based longitudinal cohort study. Int J Geriatr Psychiatry. 2017;32(10):1059-1071.
27. Robert P, Onyike CU, Leentjens AF, et al. Proposed diagnostic criteria for apathy in Alzheimer’s disease and other neuropsychiatric disorders. Eur Psychiatry. 2009;24(2):98-104.
28. Marioni RE, Proust-Lima C, Amieva H, et al. Social activity, cognitive decline and dementia risk: a 20-year prospective cohort study. BMC Public Health. 2015;15:1089.
29. Wilson RS, Schneider JA, Boyle PA, et al. Chronic distress and incidence of mild cognitive impairment. Neurology. 2007;68(24):2085-2092.
30. Cai Z, Yan LJ, Ratka A. Telomere shortening and Alzheimer’s disease. Neuromolecular Med. 2013;15(1):25-48.
31. Wilson RS, Arnold SE, Schneider JA, et al. Chronic psychological distress and risk of Alzheimer’s disease in old age. Neuroepidemiology. 2006;27(3):143-153.
32. Epel E, Daubenmier J, Moskowitz JT, et al. Can meditation slow rate of cellular aging? Cognitive stress, mindfulness, and telomeres. Ann N Y Acad Sci. 2009;1172:34-53.
33. Malinowski P, Moore AW, Mead Br, et al. Mindful aging: the effects of regular brief mindfulness practice on electrophysiological markers of cognitive and affective processing in older adults. Mindfulness (N Y). 2017;8(1):78-94.
34. Taren AA, Gianaros PJ, Greco CM, et al. Mindfulness meditation training and executive control network resting state functional connectivity: a randomized controlled trial. Psychosom Med. 2017;79(6):674-683.
35. Fountain-Zaragoza S, Prakash RS. Mindfulness training for healthy aging: impact on attention, well-being, and inflammation. Front in Aging Neurosci. 2017;9:11.
36. Eyre HA, Siddarth P, Acevedo B, et al. A randomized controlled trial of Kundalini yoga in mild cognitive impairment. Int Psychogeriatr. 2017;29(4):557-567.
37. Khalsa DS. Stress, meditation, and Alzheimer’s disease prevention: where the evidence stands. J Alzheimers Dis. 2015;48(1):1-12.
38. Berk L, van Boxtel M, van Os J. Can mindfulness-based interventions influence cognitive functioning in older adults? A review and considerations for future research. Aging Ment Health. 2017;21(11):1113-1120.
39. Hosseini S, Chaurasia A, Oremus M. The effect of religion and spirituality on cognitive function: a systematic review. Gerontologist. 2017. doi: 10.1093/geront/gnx024.
40. Luchsinger JA, Tang MX, Shea S, et al. Caloric intake and the risk of Alzheimer disease. Arch Neurol. 2002;59(8):1258-1263.
41. Grant WB. Trends in diet and Alzheimer’s disease during the nutrition transition in Japan and developing countries. J Alzheimers Dis. 2014;38(3):611-620.
42. Kanoski SE, Davidson TL. Western diet consumption and cognitive impairment: links to hippocampal dysfunction and obesity. Physiol Behav. 2011;103(1):59-68.
43. Hu N, Yu JT, Tan L, et al. Nutrition and the risk of Alzheimer’s disease. Biomed Res Int. 2013;2013:524820. doi: 10.1155/2013/524820.
44. Taylor MK, Sullivan DK, Swerdlow RH, et al. A high-glycemic diet is associated with cerebral amyloid burden in cognitively normal older adults. Am J Clin Nutr. 2017;106(6):1463-1470.
45. van de Rest O, Berendsen AM, Haveman-Nies A, et al. Dietary patterns, cognitive decline, and dementia: a systematic review. Adv Nutr. 2015;6(2):154-168.
46. Petersson SD, Philippou E. Mediterranean diet, cognitive function, and dementia: a systematic review of the evidence. Adv Nutr. 2016;7(5):889-904.
47. Smith PJ, Blumenthal JA, Babyak MA, et al. Effects of the dietary approaches to stop hypertension diet, exercise, and caloric restriction on neurocognition in overweight adults with high blood pressure. Hypertension. 2010;55(6):1331-1338.
48. Wengreen H, Munger RG, Cutler A, et al. Prospective study of dietary approaches to stop hypertension- and Mediterranean-style dietary patterns and age-related cognitive change: the Cache County study on memory, health and aging. Am J Clin Nutr. 2013;98(5):1263-1271.
49. Tangney CC, Li H, Wang Y, et al. Relation of DASH- and Mediterranean-like dietary patterns to cognitive decline in older persons. Neurology. 2014;83(16):1410-1416.
50. Morris MC, Tangney CC, Wang Y, et al. MIND diet associated with reduced incidence of Alzheimer’s disease. Alzheimers Dement. 2015;11(9):1007-1014.
51. Desai AK, Rush J, Naveen L, et al. Nutrition and nutritional supplements to promote brain health. In: Hartman-Stein PE, Rue AL, eds. Enhancing cognitive fitness in adults: a guide to the use and development of community-based programs. New York, NY: Springer; 2011:249-269.
52. Goozee KG, Shah TM, Sohrabi HR, et al. Examining the potential clinical value of curcumin in the prevention and diagnosis of Alzheimer’s disease. Br J Nutr. 2016;115(3):449-465.
53. Small GW, Siddarth P, Li Z, et al. Memory and brain amyloid and tau effects of a bioavailable form of curcumin in non-demented adults: a double-blind, placebo-controlled 18-month trial. Am J Geriatr Psychiatry. 2018;26(3):266-277.
54. Kim JW, Lee DY, Lee BC, et al. Alcohol and cognition in the elderly: a review. Psychiatry Investig. 2012;9(1):8-16.
55. Virtaa JJ, Järvenpää T, Heikkilä K, et al. Midlife alcohol consumption and later risk of cognitive impairment: a twin follow-up study. J Alzheimers Dis. 2010;22(3):939-948.
56. Kerr WC, Stockwell T. Understanding standard drinks and drinking guidelines. Drug and Alcohol Rev. 2012;31(2):200-205.
57. National Institute on Alcohol Abuse and Alcoholism. Drinking levels defined. https://www.niaaa.nih.gov/alcohol-health/overview-alcohol-consumption/moderate-binge-drinking. Accessed December 9, 2017.
58. National Institute on Alcohol Abuse and Alcoholism. What is a standard drink? https://www.niaaa.nih.gov/alcohol-health/overview-alcohol-consumption/what-standard-drink. Accessed November 9, 2017.
59. Lindsay J, Laurin D, Verreault R, et al. Risk factors for Alzheimer’s disease: a prospective analysis from the Canadian study of health and aging. Am J Epidemiol. 2002;156(5):445-453.
60. Orgogozo JM, Dartigues JF, Lafont S, et al. Wine consumption and dementia in the elderly: a prospective community study in the Bordeaux area. Rev Neurol (Paris). 1997;153(3):185-192.
61. Topiwala A, Allan CL, Valkanova V, et al. Moderate alcohol consumption as risk factor for adverse brain outcomes and cognitive decline: longitudinal cohort study. BMJ. 2017;357.
62. Venkataraman A, Kalk N, Sewell G, et al. Alcohol and Alzheimer’s disease-does alcohol dependence contribute to beta-amyloid deposition, neuroinflammation and neurodegeneration in Alzheimer’s Disease? Alcohol Alcohol. 2017;52(2):151-158.
63. Ma QP, Huang C, Cui QY, et al. Meta-analysis of the association between tea intake and the risk of cognitive disorders. PLoS One. 2016;11(11):e0165861. doi: 10.1371/journal.pone.0165861.
64. Santos C, Costa J, Santos J, et al. Caffeine intake and dementia: systematic review and meta-analysis. J Alzheimers Dis. 2010;20(Suppl 1):S187-204.
65. Panza F, Solfrizzi V, Barulli MR, et al. Coffee, tea, and caffeine consumption and prevention of late-life cognitive decline and dementia: a systematic review. J Nutr Health Aging. 2015;19(3):313-328.
66. Wierzejska R. Can coffee consumption lower the risk of Alzheimer’s disease and Parkinson’s disease? A literature review. Arch Med Sci. 2017;13(3):507-514.
67. Arendash GW, Cao C. Caffeine and coffee as therapeutics against Alzheimer’s disease. J Alzheimers Dis. 2010;20 (Suppl 1):S117-S126.
68. Eskelinen MH, Ngandu T, Tuomilehto J, et al. Midlife coffee and tea drinking and the risk of late-life dementia: a population-based CAIDE study. J Alzheimers Dis. 2009;16(1):85-91.
69. Solfrizzi V, Panza F, Imbimbo BP, et al. Coffee consumption habits and the risk of mild cognitive impairment: the Italian longitudinal study on aging. J Alzheimers Dis. 2015;47(4):889-899.
70. Vittoria Mattioli. Beverages of daily life: impact of caffeine on atrial fibrillation. J Atr Fibrillation. 2014;7(2):1133.
71. Chacko SM, Thambi PT, Kuttan R, et al. Beneficial effects of green tea: a literature review. Chin Med. 2010;5:13.
72. Noguchi-Shinohara M, Yuki S, Dohmoto C, et al. Consumption of green tea, but not black tea or coffee, is associated with reduced risk of cognitive decline. PLoS One. 2014;9(5):e96013. doi: 10.1371/journal.pone.0096013.
73. Schneider N, Yvon C. A review of multidomain interventions to support healthy cognitive ageing. J Nutr Health Aging. 2013;17(3):252-257.
74. Ngandu T, Lehitsalo J, Solomon A, et al. A 2 year multidomain intervention of diet, exercise, cognitive training, and vascular risk monitoring versus control to prevent cognitive decline in at-risk elderly people (FINGER): a randomised controlled trial. Lancet. 2015;385(9984):2255-2263.
75. U.S. National Library of Medicing. ClinicalTrials.gov. U.S. study to protect brain health through lifestyle intervention to reduce risk (POINTER). https://clinicaltrials.gov/ct2/show/NCT03688126?term=pointer&cond=Alzheimer+Disease&rank=1. Published September 28, 2018. Accessed November 3, 2018.
Botulinum toxin: Emerging psychiatric indications
Botulinum toxin, a potent neurotoxic protein produced by the bacterium Clostridium botulinum, has been used as treatment for a variety of medical indications for more than 25 years (Box1-12). Recently, researchers have been exploring the role of botulinum toxin in psychiatry, primarily as an adjunctive treatment for depression, but also for several other possible indications. Several studies, including randomized controlled trials (RCTs), have provided evidence that glabellar botulinum toxin injections may be a safe and effective treatment for depression. In this article, we provide an update on the latest clinical trials that evaluated botulinum toxin for depression, and also summarize the evidence regarding other potential clinical psychiatric applications of botulinum toxin.
Several RCTs suggest efficacy for depression
The use of botulinum toxin to treat depression is based on the facial feedback hypothesis, which was first proposed by Charles Darwin in 187213 and further elaborated by William James,14,15 who emphasized the importance of the sensation of bodily changes in emotion. Contrary to the popular belief that emotions trigger physiological changes in the body, James postulated that peripheral bodily changes secondary to stimuli perception would exert a sensory feedback, generating emotions. The manipulation of human facial expression with an expression that is associated with a particular emotion (eg, holding a pen with teeth, leading to risorius/zygomaticus muscles contraction and a smile simulation) was found to influence participants’ affective responses in the presence of emotional stimuli (eg, rating cartoons as funnier), reinforcing the facial-feedback hypothesis.16,17
From a neurobiologic standpoint, facial botulinum toxin A (BTA) injections in rats were associated with increased serotonin and norepinephrine concentrations in the hypothalamus and striatum, respectively.18 Moreover, amygdala activity in response to angry vs happy faces, measured via functional magnetic resonance imaging (fMRI), was found to be attenuated after BTA applications to muscles involved in angry facial expressions.19,20 Both the neurotransmitters as well as the aforementioned brain regions have been implicated in the pathophysiology of depression.21,22
Compared with those in the placebo group, participants in the BTA group had a higher response rate as measured by the HAM-D17 at 6 weeks after treatment (P = .02), especially female patients (P = .002). Response to BTA, defined as ≥50% reduction on the HAM-D17, occurred within 2 weeks, and lasted another 6 weeks before slightly wearing off. Assessment of the CSS-GFL showed a statistically significant change at 6 weeks (P < .001). This small study failed, however, to show significant remission rates (HAM-D17 ≤7) in the BTA group compared with placebo.
Box
Botulinum toxin is a potent neurotoxin from Clostridium botulinum. Its potential for therapeutic use was first noticed in 1817 by physician Justinus Kerner, who coined the term botulism.1 In 1897, bacteriologist Emile van Ermengem isolated the causative bacterium C. botulinum.2 It was later discovered that the toxin induces muscle paralysis by inhibiting acetylcholine release from presynaptic motor neurons at the neuromuscular junction3 and was then mainly investigated as a treatment for medical conditions involving excessive or abnormal muscular contraction.
In 1989, the FDA approved botulinum toxin A (BTA) for the treatment of strabismus, blepharospasm, and other facial nerve disorders. In 2000, both BTA and botulinum toxin B (BTB) were FDA-approved for the treatment of cervical dystonia, and BTA was approved for the cosmetic treatment of frown lines (glabellar, canthal, and forehead lines).4 Other approved clinical indications for BTA include urinary incontinence due to detrusor overactivity associated with a neurologic condition such as spinal cord injury or multiple sclerosis; prophylaxis of headaches in chronic migraine patients; treatment of both upper and lower limb spasticity; severe axillary hyperhidrosis inadequately managed by topical agents; and the reduction of the severity of abnormal head position and neck pain.5 Its anticholinergic effects have been also investigated for treatment of hyperhidrosis as well as sialorrhea caused by neurodegenerative disorders such as amyotrophic lateral sclerosis.6-8 Multiple studies have shown that botulinum toxin can alleviate spasms of the gastrointestinal tract, aiding patients with dysphagia and achalasia.9-11 There is also growing evidence supporting the use of botulinum toxin in the treatment of chronic pain, including non-migraine types of headaches such as tension headaches; myofascial syndrome; and neuropathic pain.12
Continue to: In a second RCT involving 74 patients with depression...
In a second RCT involving 74 patients with depression, Finzi and Rosenthal25 observed statistically significant response and remission rates in participants who received BTA injections, as measured by the Montgomery-Åsberg Depression Rating Scale (MADRS). Participants were given either BTA or saline injections and assessed at 3 visits across 6 weeks using the MADRS, CGI, and Beck Depression Inventory-II (BDI-II). Photographs of participants’ facial expressions were assessed using frown scores to see whether changes in facial expression were associated with improvement of depression.
This study was able to reproduce on a larger scale the results observed by Wollmer et al.23 It found a statistically significant increase in the rate of remission (MADRS ≤10) at 6 weeks following BTA injections (27%, P < .02), and that even patients who were not resistant to antidepressants could benefit from BTA. However, although there was an observable trend in improvement of frown scores associated with improved depression scores, the correlation between these 2 variables was not statistically significant.
In a crossover RCT, Magid et al26 observed the response to BTA vs placebo saline injections in 30 patients with moderate to severe frown lines. The study lasted 24 weeks; participants switched treatments at Week 12. Mood improvement was assessed using the 21-item Hamilton Depression Rating Scale (HDRS-21), BDI, and Patient Health Questionnaire-9 (PHQ-9). Compared with patients who received placebo injections, those treated with BTA injections showed statistically significant response rates, but not remission rates. This study demonstrated continued improvement throughout the 24 weeks in participants who initially received BTA injections, despite having received placebo for the last 12 weeks, by which time the cosmetic effects of the initial injection had worn off. This suggests that the antidepressant effects of botulinum toxin may not depend entirely on its paralytic effects, but also on its impact on the neurotransmitters involved in the pathophysiology of depression.18 By demonstrating improvement in the placebo group once they were started on botulinum toxin, this study also was able to exclude the possibility that other variables may be responsible for the difference in the clinical course between the 2 groups. However, this study was limited by a small sample size, and it only included participants who had moderate to severe frown lines at baseline.
Zamanian et al27 examined the therapeutic effects of BTA injections in 28 Iranian patients with major depressive disorder (MDD) diagnosed according to DSM-5 criteria. At 6 weeks, there were significant improvements in BDI scores in patients who received BTA vs those receiving placebo. However, these changes were demonstrated at 6 weeks (not as early as 2 weeks), and patients didn’t achieve remission.
A large-scale, multicenter U.S. phase II RCT investigated the safety, tolerability, and efficacy of a single administration of 2 different doses of BTA (30 units or 50 units) as monotherapy for the treatment of moderate to severe depression in 258 women.28 Effects on depression were measured at 3, 6, and 9 weeks using the MADRS. Participants who received the 30-unit injection showed statistically significant improvement at 3 weeks (
More recently, in a case series, Chugh et al30 examined the effect of BTA in 42 patients (55% men) with severe treatment-resistant depression. Participants were given BTA injections in the glabellar region as an adjunctive treatment to antidepressants and observed for at least 6 weeks. Depression severity was measured using HAM-D17, MADRS, and BDI at baseline and at 3 weeks. Changes in glabellar frown lines also were assessed using the CSS-GFL. The authors reported statistically significant improvements in HAM-D17 (
A summary of the RCTs of BTA for treating depression appears in Table 1.23,25-28
Continue to: Benefits for other psychiatric indications
Benefits for other psychiatric indications
Borderline personality disorder. In a case series of 6 women, BTA injections in the glabellar region were reported to be particularly effective for the treatment of borderline personality disorder symptoms that were resistant to psychotherapy and pharmacotherapy.31 Two to 6 weeks after a 29-unit injection, borderline personality disorder symptoms as measured by the Zanarini Rating Scale for Borderline Personality Disorder and/or the Borderline Symptom List were shown to significantly improve by 49% to 94% from baseline (P ≤ .05). These findings emphasize the promising therapeutic role of BTA on depressive symptoms concomitant with the emotional lability, impulsivity, and negative emotions that usually characterize this personality disorder.31,32 A small sample size and lack of a placebo comparator are limitations of this research.
Neuroleptic-induced sialorrhea. Botulinum toxin injections in the salivary glands have been investigated for treating clozapine-induced sialorrhea because they are thought to directly inhibit the release of acetylcholine from salivary glands. One small RCT that used botulinum toxin B (BTB)33 and 1 case report that used BTA34 reported successful reduction in hypersalivation, with doses ranging from 150 to 500 units injected in each of the parotid and/or submandibular glands bilaterally. Although the treatment was well tolerated and lasted up to 16 weeks, larger studies are needed to replicate these findings.33-35
Orofacial tardive dyskinesia. Several case reports of orofacial tardive dyskinesia, including lingual dyskinesia and lingual protrusion dystonia, have found improvements in hyperkinetic movements following muscular BTA injections, such as in the genioglossus muscle in the case of tongue involvement.36-39 These cases were, however, described in the literature before the recent FDA approval of the vesicular monoamine transporter 2 inhibitors valbenazine and deutetrabenazine for the treatment of tardive dyskinesia.40,41
Studies examining botulinum toxin’s application in areas of psychiatry other than depression are summarized in Table 2.31,33,36-38
Continue to: Promising initial findings but multiple limitations
Promising initial findings but multiple limitations
Although BTA injections have been explored as a potential treatment for several psychiatric conditions, the bulk of recent evidence is derived from studies in patients with depressive disorders. BTA injections in the glabellar regions have been shown in small RCTs to be well-tolerated with overall promising improvement of depressive symptoms, optimally 6 weeks after a single injection. Moreover, BTA has been shown to be safe and long-lasting, which would be convenient for patients and might improve adherence to therapy.42-44 BTA’s antidepressant effects were shown to be independent of frown line severity or patient satisfaction with cosmetic effects.45 The trials by Wollmer et al,23 Finzi and Rosenthal,25 and Magid et al26 mainly studied BTA as an adjunctive treatment to antidepressants in patients with ongoing unipolar depression. However, Finzi and Rosenthal25 included patients who were not medicated at the time of the study.
Pooled analysis of these 3 RCTs found that patients who received BTA monotherapy improved equally to those who received it as an adjunctive treatment to antidepressants. Overall, on primary endpoint measures, a response rate of 54.2% was obtained in the BTA group compared with 10.7% among patients who received placebo saline injections (odds ratio [OR] 11.1, 95% confidence interval [CI], 4.3 to 28.8, number needed to treat [NNT] = 2.3) and a remission rate of 30.5% with BTA compared with 6.7% with placebo (OR 7.3, 95 % CI, 2.4 to 22.5, NNT = 4.2).46 However, remission rates tend to be higher in the augmentation groups, and so further studies are needed to compare both treatment strategies.
Nevertheless, these positive findings have been recently challenged by the results of the largest U.S. multicenter phase II RCT,28 which failed to find a significant antidepressant effect at 6 weeks with the 30-unit BTA injection, and also failed to prove a dose-effect relationship, as the 50-unit injection wasn’t superior to the lower dose and didn’t significantly differ from placebo. One hypothesis to explain this discrepancy may be the difference in injection sites between the treatment and placebo groups.47 Future studies need to address the various limitations of earlier clinical trials that mainly yielded promising results with BTA.
A major concern is the high rate of unblinding of participants and researchers in BTA trials, as the cosmetic effects of botulinum toxin injections make them easy to distinguish from saline injections. Ninety percent of participants in the Wollmer et al study23 were able to correctly guess their group allocation, while 60% of evaluators guessed correctly. Finzi and Rozenthal25 reported 52% of participants in the BTA group, 46% in the placebo group, and 73% of evaluators correctly guessed their allocation. Magid et al26 reported 75% of participants were able to guess the order of intervention they received. The high unblinding rates in these trials remains a significant limitation. There is a concern that this may lead to an underestimation of the placebo effect relative to clinical improvement, thus causing inflation of outcome differences between groups. Although various methods have been tried to minimize evaluator unblinding, such as placing surgical caps on participants’ faces during visits to hide the glabellar region, better methods need to be implemented to prevent unblinding of both raters and participants.
Furthermore, except for the multicenter phase II trial, most studies have been conducted in small samples, which limits their statistical power. Larger controlled trials will be needed to replicate the positive findings obtained in smaller RCTs.
Another limitation is that the majority of the well-designed RCTs were conducted in populations that were predominantly female, which makes it difficult to reliably assess treatment efficacy in men. This may be because cosmetic treatment with botulinum toxin injection is more favorably received by women than by men. A recent comparison48 of the studies by Wollmer et al23 and Finzi and Rosenthal25 discussed an interesting observation. Wollmer et al did not explicitly mention botulinum toxin when recruiting for the study, while Finzi and Rosenthal did. While approximately a quarter of the participants in the Wollmer et al study were male, Finzi and Rosenthal attracted an almost entirely female population. Perhaps there is a potential bias for females to be more attracted to these studies due to the secondary gain of receiving a cosmetic procedure.
In an attempt to understand predictors of positive response to botulinum toxin in patients with depression, Wollmer et al49 conducted a follow-up study in which they reassessed the data obtained from their initial RCT using the HAM-D agitation item scores to separate the 15 participants who received BTA into low-agitation (≤1 score on agitation item of the HAM-D scale) and high-agitation (≥2 score on agitation item of the HAM-D scale) groups. They found that the 9 participants who responded to BTA treatment had significantly higher baseline agitation scores than participants who did not respond (1.56 ± 0.88 vs 0.33 ± 0.52, P = .01). All of the participants who presented with higher agitation levels experienced response, compared with 40% of those with lower agitation levels (P = .04), although there was no significant difference in magnitude of improvement (14.2 ± 1.92 vs 8.0 ± 9.37, P = .07). The study added additional support to the facial feedback hypothesis, as it links the improvement of depression to facial muscle activation targeted by the injections. It also introduced a potential predictor of response to botulinum toxin treatment, highlighting potential factors to consider when enrolling patients in future investigations.
The case series of patients with borderline personality disorder31 also shed light on the potential positive effect of BTA treatment for a particular subtype of patients with depression—those with comorbid emotional instability—to consider as a therapeutic target for the future. Hence, inclusion criteria for future trials might potentially include patient age, gender, existence/quantification of prominent frown lines at baseline, severity of MDD, duration of depression, and personality characteristics of enrolled participants.
In conclusion, BTA injections appear promising as a treatment for depression as well as for other psychiatric disorders. Future studies should focus on identifying optimal candidates for this innovative treatment modality. Furthermore, BTA dosing and administration strategies (monotherapy vs adjunctive treatment to antidepressants) need to be further explored. As retrograde axonal transport of botulinum toxin has been demonstrated in animal studies, it would be interesting to further examine its effects in the human CNS to enhance our knowledge of the pathophysiology of botulinum and its potential applications in psychiatry.50
Bottom Line
Botulinum toxin shows promising antidepressant effects and may have a role in the treatment of several other psychiatric disorders. More research is needed to address limitations of previous studies and to establish an adequate treatment regimen.
Related Resources
- Wollmer MA, Magid M, Kruger TH. Botulinum toxin treatment in depression. In: Bewley A, Taylor RE, Reichenberg JS, et al (eds). Practical psychodermatology. Oxford, UK: Wiley; 2014.
- Wollmer MA, Neumann I, Magid M. et al. Shrink that frown! Botulinum toxin therapy is lifting the face of psychiatry. G Ital Dermatol Venereol. 2018;153(4):540-548.
Drug Brand Names
Alprazolam • Xanax
Aripiprazole • Abilify
Biperiden • Akineton
Botulinum toxin A • Botox
Botulinum toxin B • Myobloc
Clozapine • Clozaril
Deutetrabenazine • Austedo
Flupentixol • Prolixin
Imipramine • Tofranil
Olanzapine • Zyprexa
Reserpine • Serpalan, Serpasil
Tetrabenazine • Xenazine
Valbenazine • Ingrezza
Ziprasidone • Geodon
1. Erbguth FJ, Naumann M. Historical aspects of botulinum toxin. Justinus Kerner (1786-1862) and the “sausage” poison. Neurology. 1999;53(8):1850-1853.
2. Devriese PP. On the discovery of Clostridium botulinum. J History Neurosci. 1999;8(1):43-50.
3. Burgen ASV, Dickens F, Zatman LJ. The action of botulinum toxin on the neuro-muscular junction. J Physiol. 1949;109(1-2):10-24.
4. Jankovic J. Botulinum toxin in clinical practice. J Neurol Neurosurg Psychiatry. 2004;75(7):951-957.
5. BOTOX (OnabotulinumtoxinA) [package insert]. Allergan, Inc., Irvine, CA; 2015.
6. Saadia D, Voustianiouk A, Wang AK, et al. Botulinum toxin type A in primary palmar hyperhidrosis. Randomized, single-blind, two-dose study. Neurology. 2001;57(11):2095-2099.
7. Naumann MK, Lowe NJ. Effect of botulinum toxin type A on quality of life measures in patients with excessive axillary sweating: a randomized controlled trial. Br J Dermatol. 2002;147(6):1218-1226.
8. Giess R, Naumann M, Werner E, et al. Injections of botulinum toxin A into the salivary glands improve sialorrhea in amyotrophic lateral sclerosis. J Neurol Neurosurg Psychiatry. 2000;69(1):121-123.
9. Restivo DA, Palmeri A, Marchese-Ragona R. Botulinum toxin for cricopharyngeal dysfunction in Parkinson’s disease. N Engl J Med. 2002;346(15):1174-1175.
10. Pasricha PJ, Ravich WJ, Hendrix T, et al. Intrasphincteric botulinum toxin for the treatment of achalasia. N Engl J Med. 1995(12);322:774-778.
11. Schiano TD, Parkman HP, Miller LS, et al. Use of botulinum toxin in the treatment of achalasia. Dig Dis. 1998;16(1):14-22.
12. Sim WS. Application of botulinum toxin in pain management. Korean J Pain. 2011;24(1):1-6.
13. Darwin C. The expression of the emotions in man and animals. London, UK: John Murray; 1872:366.
14. James W. The principles of psychology, vol. 2. New York, NY: Henry Holt and Company; 1890.
15. James W. II. —What is an emotion? Mind. 1884;os-IX(34):188-205.
16. Strack R, Martin LL, Stepper S. Inhibiting and facilitating conditions of facial expressions: a nonobtrusive test of the facial feedback hypothesis. J Pers Soc Psychol. 1988;54(5):768-777.
17. Larsen RJ, Kasimatis M, Frey K. Facilitating the furrowed brow: an unobtrusive test of the facial feedback hypothesis applied to unpleasant affect. Cogn Emot. 1992;6(5):321-338.
18. Ibragic S, Matak I, Dracic A, et al. Effects of botulinum toxin type A facial injection on monoamines and their metabolites in sensory, limbic, and motor brain regions in rats. Neurosci Lett. 2016;617:213-217.
19. Hennenlotter A, Dresel C, Castrop F, et al. The link between facial feedback and neural activity within central circuitries of emotion—new insights from botulinum toxin-induced denervation of frown muscles. Cereb Cortex. 2009;19(3):537-42
20. Kim MJ, Neta M, Davis FC, et al. Botulinum toxin-induced facial muscle paralysis affects amygdala responses to the perception emotional expressions: preliminary findings from an A-B-A design. Biol Mood Anxiety Disord. 2014;4:11.
21. Nestler EJ, Barrot M, DiLeone RJ, et al. Neurobiology of depression. Neuron. 2002;34(1):13-25.
22. Pandya M, Altinay M, Malone DA Jr, et al. Where in the brain is depression? Curr Psychiatry Rep. 2012;14(6):634-642.
23. Wollmer MA, de Boer C, Kalak N, et al. Facing depression with botulinum toxin: a randomized controlled trial. J Psychiatr Res. 2012;46:574-581.
24. BOTOX Cosmetic [prescribing information]. Allergan, Inc., Irvine, CA; 2017.
25. Finzi E, Rosenthal NE. Treatment of depression with onabotulinumtoxinA; a randomized, double-blind, placebo controlled trial. J Psychiatr Res. 2014;52:1-6.
26. Magid M, Reichenberg JS, Poth PE, et al. The treatment of major depressive disorder using botulinum toxin A: a 24 week randomized, double-blind, placebo-controlled study. J Clin Psychiatry. 2014;75(8):837-844.
27. Zamanian A, Ghanbari Jolfaei A, Mehran G, et al. Efficacy of botox versus placebo for treatment of patients with major depression. Iran J Public Health. 2017;46(7):982-984.
28. Allergan. OnabotulinumtoxinA as treatment for major depressive disorder in adult females. 2017. https://clinicaltrials.gov/ct2/show/NCT02116361. Accessed October 26, 2018.
29. Allergan. Allergan reports topline phase II data supporting advancement of BOTOX® (onabotulinumtoxinA) for the treatment of major depressive disorder (MDD). April 5, 2017. https://www.allergan.com/news/news/thomson-reuters/allergan-reports-topline-phase-ii-data-supporting. Accessed October 26, 2018.
30. Chugh S, Chhabria A, Jung S, et al. Botulinum toxin as a treatment for depression in a real-world setting. J Psychiatr Pract. 2018;24(1):15-20.
31. Kruger TH, Magid M, Wollmer MA. Can botulinum toxin help patients with borderline personality disorder? Am J Psychiatry. 2016;173(9):940-941.
32. Baumeister JC, Papa G, Foroni F. Deeper than skin deep – the effect of botulinum toxin-A on emotion processing. Toxicon. 2016;119:86-90.
33. Steinlechner S, Klein C, Moser A, et al. Botulinum toxin B as an effective and safe treatment for neuroleptic-induced sialorrhea. Psychopharmacology (Berl). 2010;207(4):593-597.
34. Kahl KG, Hagenah J, Zapf S, et al. Botulinum toxin as an effective treatment of clozapine-induced hypersalivation. Psychopharmacology (Berl). 2004;173(1-2):229-230.
35. Bird AM, Smith TL, Walton AE. Current treatment strategies for clozapine-induced sialorrhea. Ann Pharmacother. 2011;45(5):667-675.
36. Tschopp L, Salazar Z, Micheli F. Botulinum toxin in painful tardive dyskinesia. Clin Neuropharmacol. 2009;32(3):165-166.
37. Hennings JM, Krause E, Bötzel K, et al. Successful treatment of tardive lingual dystonia with botulinum toxin: case report and review of the literature. Prog Neuropsychopharmacol Biol Psychiatry. 2008;32(5):1167-1171.
38. Slotema CW, van Harten PN, Bruggeman R, et al. Botulinum toxin in the treatment of orofacial tardive dyskinesia: a single blind study. Prog Neuropsychopharmacol Biol Psychiatry. 2008;32(2):507-509.
39. Esper CD, Freeman A, Factor SA. Lingual protrusion dystonia: frequency, etiology and botulinum toxin therapy. Parkinsonism Relat Disord. 2010;16(7):438-441.
40. Seeberger LC, Hauser RA. Valbenazine for the treatment of tardive dyskinesia. Expert Opin Pharmacother. 2017;18(12):1279-1287.
41. Citrome L. Deutetrabenazine for tardive dyskinesia: a systematic review of the efficacy and safety profile for this newly approved novel medication—What is the number needed to treat, number needed to harm and likelihood to be helped or harmed? Int J Clin Pract. 2017;71(11):e13030.
42. Brin MF, Boodhoo TI, Pogoda JM, et al. Safety and tolerability of onabotulinumtoxinA in the tretment of facial lines: a meta-analysis of individual patient data from global clinical registration studies in 1678 participants. J Am Acad Dermatol. 2009;61:961-970.
43. Beer K. Cost effectiveness of botulinum toxins for the treatment of depression: preliminary observations. J Drugs Dermatol. 2010;9(1):27-30.
44. Serna MC, Cruz I, Real J, et al. Duration and adherence of antidepressant treatment (2003-2007) based on prescription database. Eur Psychiatry. 2010;25(4):206-213.
45. Rechenberg JS, Hauptman AJ, Robertson HT, et al. Botulinum toxin for depression: Does patient appearance matter? J Am Acad Dermatol. 2016;74(1):171-173.
46. Magid M, Finzi E, Kruger THC, et al. Treating depression with botulinum toxin: a pooled analysis of randomized controlled trials. Pharmacopsychiatry. 2015;48(6):205-210.
47. Court, E. Allergan is still hopeful about using Botox to treat depression. April 8, 2017. https://www.marketwatch.com/story/allergan-is-still-hopeful-about-using-botox-to-treat-depression-2017-04-07. Accessed October 26, 2018.
48. Rudorfer MV. Botulinum toxin: does it have a place in the management of depression? CNS Drugs. 2018;32(2):97-100.
49. Wollmer MA, Kalak N, Jung S, et al. Agitation predicts response of depression to botulinum toxin treatment in a randomized controlled trial. Front Psychiatry. 2014;5:36
50. Antonucci F, Rossi C, Gianfranceschi L, et al. Long-distance retrograde effects of botulinum neurotoxin A. J Neurosci. 2008;28(14):3689-3696.
Botulinum toxin, a potent neurotoxic protein produced by the bacterium Clostridium botulinum, has been used as treatment for a variety of medical indications for more than 25 years (Box1-12). Recently, researchers have been exploring the role of botulinum toxin in psychiatry, primarily as an adjunctive treatment for depression, but also for several other possible indications. Several studies, including randomized controlled trials (RCTs), have provided evidence that glabellar botulinum toxin injections may be a safe and effective treatment for depression. In this article, we provide an update on the latest clinical trials that evaluated botulinum toxin for depression, and also summarize the evidence regarding other potential clinical psychiatric applications of botulinum toxin.
Several RCTs suggest efficacy for depression
The use of botulinum toxin to treat depression is based on the facial feedback hypothesis, which was first proposed by Charles Darwin in 187213 and further elaborated by William James,14,15 who emphasized the importance of the sensation of bodily changes in emotion. Contrary to the popular belief that emotions trigger physiological changes in the body, James postulated that peripheral bodily changes secondary to stimuli perception would exert a sensory feedback, generating emotions. The manipulation of human facial expression with an expression that is associated with a particular emotion (eg, holding a pen with teeth, leading to risorius/zygomaticus muscles contraction and a smile simulation) was found to influence participants’ affective responses in the presence of emotional stimuli (eg, rating cartoons as funnier), reinforcing the facial-feedback hypothesis.16,17
From a neurobiologic standpoint, facial botulinum toxin A (BTA) injections in rats were associated with increased serotonin and norepinephrine concentrations in the hypothalamus and striatum, respectively.18 Moreover, amygdala activity in response to angry vs happy faces, measured via functional magnetic resonance imaging (fMRI), was found to be attenuated after BTA applications to muscles involved in angry facial expressions.19,20 Both the neurotransmitters as well as the aforementioned brain regions have been implicated in the pathophysiology of depression.21,22
Compared with those in the placebo group, participants in the BTA group had a higher response rate as measured by the HAM-D17 at 6 weeks after treatment (P = .02), especially female patients (P = .002). Response to BTA, defined as ≥50% reduction on the HAM-D17, occurred within 2 weeks, and lasted another 6 weeks before slightly wearing off. Assessment of the CSS-GFL showed a statistically significant change at 6 weeks (P < .001). This small study failed, however, to show significant remission rates (HAM-D17 ≤7) in the BTA group compared with placebo.
Box
Botulinum toxin is a potent neurotoxin from Clostridium botulinum. Its potential for therapeutic use was first noticed in 1817 by physician Justinus Kerner, who coined the term botulism.1 In 1897, bacteriologist Emile van Ermengem isolated the causative bacterium C. botulinum.2 It was later discovered that the toxin induces muscle paralysis by inhibiting acetylcholine release from presynaptic motor neurons at the neuromuscular junction3 and was then mainly investigated as a treatment for medical conditions involving excessive or abnormal muscular contraction.
In 1989, the FDA approved botulinum toxin A (BTA) for the treatment of strabismus, blepharospasm, and other facial nerve disorders. In 2000, both BTA and botulinum toxin B (BTB) were FDA-approved for the treatment of cervical dystonia, and BTA was approved for the cosmetic treatment of frown lines (glabellar, canthal, and forehead lines).4 Other approved clinical indications for BTA include urinary incontinence due to detrusor overactivity associated with a neurologic condition such as spinal cord injury or multiple sclerosis; prophylaxis of headaches in chronic migraine patients; treatment of both upper and lower limb spasticity; severe axillary hyperhidrosis inadequately managed by topical agents; and the reduction of the severity of abnormal head position and neck pain.5 Its anticholinergic effects have been also investigated for treatment of hyperhidrosis as well as sialorrhea caused by neurodegenerative disorders such as amyotrophic lateral sclerosis.6-8 Multiple studies have shown that botulinum toxin can alleviate spasms of the gastrointestinal tract, aiding patients with dysphagia and achalasia.9-11 There is also growing evidence supporting the use of botulinum toxin in the treatment of chronic pain, including non-migraine types of headaches such as tension headaches; myofascial syndrome; and neuropathic pain.12
Continue to: In a second RCT involving 74 patients with depression...
In a second RCT involving 74 patients with depression, Finzi and Rosenthal25 observed statistically significant response and remission rates in participants who received BTA injections, as measured by the Montgomery-Åsberg Depression Rating Scale (MADRS). Participants were given either BTA or saline injections and assessed at 3 visits across 6 weeks using the MADRS, CGI, and Beck Depression Inventory-II (BDI-II). Photographs of participants’ facial expressions were assessed using frown scores to see whether changes in facial expression were associated with improvement of depression.
This study was able to reproduce on a larger scale the results observed by Wollmer et al.23 It found a statistically significant increase in the rate of remission (MADRS ≤10) at 6 weeks following BTA injections (27%, P < .02), and that even patients who were not resistant to antidepressants could benefit from BTA. However, although there was an observable trend in improvement of frown scores associated with improved depression scores, the correlation between these 2 variables was not statistically significant.
In a crossover RCT, Magid et al26 observed the response to BTA vs placebo saline injections in 30 patients with moderate to severe frown lines. The study lasted 24 weeks; participants switched treatments at Week 12. Mood improvement was assessed using the 21-item Hamilton Depression Rating Scale (HDRS-21), BDI, and Patient Health Questionnaire-9 (PHQ-9). Compared with patients who received placebo injections, those treated with BTA injections showed statistically significant response rates, but not remission rates. This study demonstrated continued improvement throughout the 24 weeks in participants who initially received BTA injections, despite having received placebo for the last 12 weeks, by which time the cosmetic effects of the initial injection had worn off. This suggests that the antidepressant effects of botulinum toxin may not depend entirely on its paralytic effects, but also on its impact on the neurotransmitters involved in the pathophysiology of depression.18 By demonstrating improvement in the placebo group once they were started on botulinum toxin, this study also was able to exclude the possibility that other variables may be responsible for the difference in the clinical course between the 2 groups. However, this study was limited by a small sample size, and it only included participants who had moderate to severe frown lines at baseline.
Zamanian et al27 examined the therapeutic effects of BTA injections in 28 Iranian patients with major depressive disorder (MDD) diagnosed according to DSM-5 criteria. At 6 weeks, there were significant improvements in BDI scores in patients who received BTA vs those receiving placebo. However, these changes were demonstrated at 6 weeks (not as early as 2 weeks), and patients didn’t achieve remission.
A large-scale, multicenter U.S. phase II RCT investigated the safety, tolerability, and efficacy of a single administration of 2 different doses of BTA (30 units or 50 units) as monotherapy for the treatment of moderate to severe depression in 258 women.28 Effects on depression were measured at 3, 6, and 9 weeks using the MADRS. Participants who received the 30-unit injection showed statistically significant improvement at 3 weeks (
More recently, in a case series, Chugh et al30 examined the effect of BTA in 42 patients (55% men) with severe treatment-resistant depression. Participants were given BTA injections in the glabellar region as an adjunctive treatment to antidepressants and observed for at least 6 weeks. Depression severity was measured using HAM-D17, MADRS, and BDI at baseline and at 3 weeks. Changes in glabellar frown lines also were assessed using the CSS-GFL. The authors reported statistically significant improvements in HAM-D17 (
A summary of the RCTs of BTA for treating depression appears in Table 1.23,25-28
Continue to: Benefits for other psychiatric indications
Benefits for other psychiatric indications
Borderline personality disorder. In a case series of 6 women, BTA injections in the glabellar region were reported to be particularly effective for the treatment of borderline personality disorder symptoms that were resistant to psychotherapy and pharmacotherapy.31 Two to 6 weeks after a 29-unit injection, borderline personality disorder symptoms as measured by the Zanarini Rating Scale for Borderline Personality Disorder and/or the Borderline Symptom List were shown to significantly improve by 49% to 94% from baseline (P ≤ .05). These findings emphasize the promising therapeutic role of BTA on depressive symptoms concomitant with the emotional lability, impulsivity, and negative emotions that usually characterize this personality disorder.31,32 A small sample size and lack of a placebo comparator are limitations of this research.
Neuroleptic-induced sialorrhea. Botulinum toxin injections in the salivary glands have been investigated for treating clozapine-induced sialorrhea because they are thought to directly inhibit the release of acetylcholine from salivary glands. One small RCT that used botulinum toxin B (BTB)33 and 1 case report that used BTA34 reported successful reduction in hypersalivation, with doses ranging from 150 to 500 units injected in each of the parotid and/or submandibular glands bilaterally. Although the treatment was well tolerated and lasted up to 16 weeks, larger studies are needed to replicate these findings.33-35
Orofacial tardive dyskinesia. Several case reports of orofacial tardive dyskinesia, including lingual dyskinesia and lingual protrusion dystonia, have found improvements in hyperkinetic movements following muscular BTA injections, such as in the genioglossus muscle in the case of tongue involvement.36-39 These cases were, however, described in the literature before the recent FDA approval of the vesicular monoamine transporter 2 inhibitors valbenazine and deutetrabenazine for the treatment of tardive dyskinesia.40,41
Studies examining botulinum toxin’s application in areas of psychiatry other than depression are summarized in Table 2.31,33,36-38
Continue to: Promising initial findings but multiple limitations
Promising initial findings but multiple limitations
Although BTA injections have been explored as a potential treatment for several psychiatric conditions, the bulk of recent evidence is derived from studies in patients with depressive disorders. BTA injections in the glabellar regions have been shown in small RCTs to be well-tolerated with overall promising improvement of depressive symptoms, optimally 6 weeks after a single injection. Moreover, BTA has been shown to be safe and long-lasting, which would be convenient for patients and might improve adherence to therapy.42-44 BTA’s antidepressant effects were shown to be independent of frown line severity or patient satisfaction with cosmetic effects.45 The trials by Wollmer et al,23 Finzi and Rosenthal,25 and Magid et al26 mainly studied BTA as an adjunctive treatment to antidepressants in patients with ongoing unipolar depression. However, Finzi and Rosenthal25 included patients who were not medicated at the time of the study.
Pooled analysis of these 3 RCTs found that patients who received BTA monotherapy improved equally to those who received it as an adjunctive treatment to antidepressants. Overall, on primary endpoint measures, a response rate of 54.2% was obtained in the BTA group compared with 10.7% among patients who received placebo saline injections (odds ratio [OR] 11.1, 95% confidence interval [CI], 4.3 to 28.8, number needed to treat [NNT] = 2.3) and a remission rate of 30.5% with BTA compared with 6.7% with placebo (OR 7.3, 95 % CI, 2.4 to 22.5, NNT = 4.2).46 However, remission rates tend to be higher in the augmentation groups, and so further studies are needed to compare both treatment strategies.
Nevertheless, these positive findings have been recently challenged by the results of the largest U.S. multicenter phase II RCT,28 which failed to find a significant antidepressant effect at 6 weeks with the 30-unit BTA injection, and also failed to prove a dose-effect relationship, as the 50-unit injection wasn’t superior to the lower dose and didn’t significantly differ from placebo. One hypothesis to explain this discrepancy may be the difference in injection sites between the treatment and placebo groups.47 Future studies need to address the various limitations of earlier clinical trials that mainly yielded promising results with BTA.
A major concern is the high rate of unblinding of participants and researchers in BTA trials, as the cosmetic effects of botulinum toxin injections make them easy to distinguish from saline injections. Ninety percent of participants in the Wollmer et al study23 were able to correctly guess their group allocation, while 60% of evaluators guessed correctly. Finzi and Rozenthal25 reported 52% of participants in the BTA group, 46% in the placebo group, and 73% of evaluators correctly guessed their allocation. Magid et al26 reported 75% of participants were able to guess the order of intervention they received. The high unblinding rates in these trials remains a significant limitation. There is a concern that this may lead to an underestimation of the placebo effect relative to clinical improvement, thus causing inflation of outcome differences between groups. Although various methods have been tried to minimize evaluator unblinding, such as placing surgical caps on participants’ faces during visits to hide the glabellar region, better methods need to be implemented to prevent unblinding of both raters and participants.
Furthermore, except for the multicenter phase II trial, most studies have been conducted in small samples, which limits their statistical power. Larger controlled trials will be needed to replicate the positive findings obtained in smaller RCTs.
Another limitation is that the majority of the well-designed RCTs were conducted in populations that were predominantly female, which makes it difficult to reliably assess treatment efficacy in men. This may be because cosmetic treatment with botulinum toxin injection is more favorably received by women than by men. A recent comparison48 of the studies by Wollmer et al23 and Finzi and Rosenthal25 discussed an interesting observation. Wollmer et al did not explicitly mention botulinum toxin when recruiting for the study, while Finzi and Rosenthal did. While approximately a quarter of the participants in the Wollmer et al study were male, Finzi and Rosenthal attracted an almost entirely female population. Perhaps there is a potential bias for females to be more attracted to these studies due to the secondary gain of receiving a cosmetic procedure.
In an attempt to understand predictors of positive response to botulinum toxin in patients with depression, Wollmer et al49 conducted a follow-up study in which they reassessed the data obtained from their initial RCT using the HAM-D agitation item scores to separate the 15 participants who received BTA into low-agitation (≤1 score on agitation item of the HAM-D scale) and high-agitation (≥2 score on agitation item of the HAM-D scale) groups. They found that the 9 participants who responded to BTA treatment had significantly higher baseline agitation scores than participants who did not respond (1.56 ± 0.88 vs 0.33 ± 0.52, P = .01). All of the participants who presented with higher agitation levels experienced response, compared with 40% of those with lower agitation levels (P = .04), although there was no significant difference in magnitude of improvement (14.2 ± 1.92 vs 8.0 ± 9.37, P = .07). The study added additional support to the facial feedback hypothesis, as it links the improvement of depression to facial muscle activation targeted by the injections. It also introduced a potential predictor of response to botulinum toxin treatment, highlighting potential factors to consider when enrolling patients in future investigations.
The case series of patients with borderline personality disorder31 also shed light on the potential positive effect of BTA treatment for a particular subtype of patients with depression—those with comorbid emotional instability—to consider as a therapeutic target for the future. Hence, inclusion criteria for future trials might potentially include patient age, gender, existence/quantification of prominent frown lines at baseline, severity of MDD, duration of depression, and personality characteristics of enrolled participants.
In conclusion, BTA injections appear promising as a treatment for depression as well as for other psychiatric disorders. Future studies should focus on identifying optimal candidates for this innovative treatment modality. Furthermore, BTA dosing and administration strategies (monotherapy vs adjunctive treatment to antidepressants) need to be further explored. As retrograde axonal transport of botulinum toxin has been demonstrated in animal studies, it would be interesting to further examine its effects in the human CNS to enhance our knowledge of the pathophysiology of botulinum and its potential applications in psychiatry.50
Bottom Line
Botulinum toxin shows promising antidepressant effects and may have a role in the treatment of several other psychiatric disorders. More research is needed to address limitations of previous studies and to establish an adequate treatment regimen.
Related Resources
- Wollmer MA, Magid M, Kruger TH. Botulinum toxin treatment in depression. In: Bewley A, Taylor RE, Reichenberg JS, et al (eds). Practical psychodermatology. Oxford, UK: Wiley; 2014.
- Wollmer MA, Neumann I, Magid M. et al. Shrink that frown! Botulinum toxin therapy is lifting the face of psychiatry. G Ital Dermatol Venereol. 2018;153(4):540-548.
Drug Brand Names
Alprazolam • Xanax
Aripiprazole • Abilify
Biperiden • Akineton
Botulinum toxin A • Botox
Botulinum toxin B • Myobloc
Clozapine • Clozaril
Deutetrabenazine • Austedo
Flupentixol • Prolixin
Imipramine • Tofranil
Olanzapine • Zyprexa
Reserpine • Serpalan, Serpasil
Tetrabenazine • Xenazine
Valbenazine • Ingrezza
Ziprasidone • Geodon
Botulinum toxin, a potent neurotoxic protein produced by the bacterium Clostridium botulinum, has been used as treatment for a variety of medical indications for more than 25 years (Box1-12). Recently, researchers have been exploring the role of botulinum toxin in psychiatry, primarily as an adjunctive treatment for depression, but also for several other possible indications. Several studies, including randomized controlled trials (RCTs), have provided evidence that glabellar botulinum toxin injections may be a safe and effective treatment for depression. In this article, we provide an update on the latest clinical trials that evaluated botulinum toxin for depression, and also summarize the evidence regarding other potential clinical psychiatric applications of botulinum toxin.
Several RCTs suggest efficacy for depression
The use of botulinum toxin to treat depression is based on the facial feedback hypothesis, which was first proposed by Charles Darwin in 187213 and further elaborated by William James,14,15 who emphasized the importance of the sensation of bodily changes in emotion. Contrary to the popular belief that emotions trigger physiological changes in the body, James postulated that peripheral bodily changes secondary to stimuli perception would exert a sensory feedback, generating emotions. The manipulation of human facial expression with an expression that is associated with a particular emotion (eg, holding a pen with teeth, leading to risorius/zygomaticus muscles contraction and a smile simulation) was found to influence participants’ affective responses in the presence of emotional stimuli (eg, rating cartoons as funnier), reinforcing the facial-feedback hypothesis.16,17
From a neurobiologic standpoint, facial botulinum toxin A (BTA) injections in rats were associated with increased serotonin and norepinephrine concentrations in the hypothalamus and striatum, respectively.18 Moreover, amygdala activity in response to angry vs happy faces, measured via functional magnetic resonance imaging (fMRI), was found to be attenuated after BTA applications to muscles involved in angry facial expressions.19,20 Both the neurotransmitters as well as the aforementioned brain regions have been implicated in the pathophysiology of depression.21,22
Compared with those in the placebo group, participants in the BTA group had a higher response rate as measured by the HAM-D17 at 6 weeks after treatment (P = .02), especially female patients (P = .002). Response to BTA, defined as ≥50% reduction on the HAM-D17, occurred within 2 weeks, and lasted another 6 weeks before slightly wearing off. Assessment of the CSS-GFL showed a statistically significant change at 6 weeks (P < .001). This small study failed, however, to show significant remission rates (HAM-D17 ≤7) in the BTA group compared with placebo.
Box
Botulinum toxin is a potent neurotoxin from Clostridium botulinum. Its potential for therapeutic use was first noticed in 1817 by physician Justinus Kerner, who coined the term botulism.1 In 1897, bacteriologist Emile van Ermengem isolated the causative bacterium C. botulinum.2 It was later discovered that the toxin induces muscle paralysis by inhibiting acetylcholine release from presynaptic motor neurons at the neuromuscular junction3 and was then mainly investigated as a treatment for medical conditions involving excessive or abnormal muscular contraction.
In 1989, the FDA approved botulinum toxin A (BTA) for the treatment of strabismus, blepharospasm, and other facial nerve disorders. In 2000, both BTA and botulinum toxin B (BTB) were FDA-approved for the treatment of cervical dystonia, and BTA was approved for the cosmetic treatment of frown lines (glabellar, canthal, and forehead lines).4 Other approved clinical indications for BTA include urinary incontinence due to detrusor overactivity associated with a neurologic condition such as spinal cord injury or multiple sclerosis; prophylaxis of headaches in chronic migraine patients; treatment of both upper and lower limb spasticity; severe axillary hyperhidrosis inadequately managed by topical agents; and the reduction of the severity of abnormal head position and neck pain.5 Its anticholinergic effects have been also investigated for treatment of hyperhidrosis as well as sialorrhea caused by neurodegenerative disorders such as amyotrophic lateral sclerosis.6-8 Multiple studies have shown that botulinum toxin can alleviate spasms of the gastrointestinal tract, aiding patients with dysphagia and achalasia.9-11 There is also growing evidence supporting the use of botulinum toxin in the treatment of chronic pain, including non-migraine types of headaches such as tension headaches; myofascial syndrome; and neuropathic pain.12
Continue to: In a second RCT involving 74 patients with depression...
In a second RCT involving 74 patients with depression, Finzi and Rosenthal25 observed statistically significant response and remission rates in participants who received BTA injections, as measured by the Montgomery-Åsberg Depression Rating Scale (MADRS). Participants were given either BTA or saline injections and assessed at 3 visits across 6 weeks using the MADRS, CGI, and Beck Depression Inventory-II (BDI-II). Photographs of participants’ facial expressions were assessed using frown scores to see whether changes in facial expression were associated with improvement of depression.
This study was able to reproduce on a larger scale the results observed by Wollmer et al.23 It found a statistically significant increase in the rate of remission (MADRS ≤10) at 6 weeks following BTA injections (27%, P < .02), and that even patients who were not resistant to antidepressants could benefit from BTA. However, although there was an observable trend in improvement of frown scores associated with improved depression scores, the correlation between these 2 variables was not statistically significant.
In a crossover RCT, Magid et al26 observed the response to BTA vs placebo saline injections in 30 patients with moderate to severe frown lines. The study lasted 24 weeks; participants switched treatments at Week 12. Mood improvement was assessed using the 21-item Hamilton Depression Rating Scale (HDRS-21), BDI, and Patient Health Questionnaire-9 (PHQ-9). Compared with patients who received placebo injections, those treated with BTA injections showed statistically significant response rates, but not remission rates. This study demonstrated continued improvement throughout the 24 weeks in participants who initially received BTA injections, despite having received placebo for the last 12 weeks, by which time the cosmetic effects of the initial injection had worn off. This suggests that the antidepressant effects of botulinum toxin may not depend entirely on its paralytic effects, but also on its impact on the neurotransmitters involved in the pathophysiology of depression.18 By demonstrating improvement in the placebo group once they were started on botulinum toxin, this study also was able to exclude the possibility that other variables may be responsible for the difference in the clinical course between the 2 groups. However, this study was limited by a small sample size, and it only included participants who had moderate to severe frown lines at baseline.
Zamanian et al27 examined the therapeutic effects of BTA injections in 28 Iranian patients with major depressive disorder (MDD) diagnosed according to DSM-5 criteria. At 6 weeks, there were significant improvements in BDI scores in patients who received BTA vs those receiving placebo. However, these changes were demonstrated at 6 weeks (not as early as 2 weeks), and patients didn’t achieve remission.
A large-scale, multicenter U.S. phase II RCT investigated the safety, tolerability, and efficacy of a single administration of 2 different doses of BTA (30 units or 50 units) as monotherapy for the treatment of moderate to severe depression in 258 women.28 Effects on depression were measured at 3, 6, and 9 weeks using the MADRS. Participants who received the 30-unit injection showed statistically significant improvement at 3 weeks (
More recently, in a case series, Chugh et al30 examined the effect of BTA in 42 patients (55% men) with severe treatment-resistant depression. Participants were given BTA injections in the glabellar region as an adjunctive treatment to antidepressants and observed for at least 6 weeks. Depression severity was measured using HAM-D17, MADRS, and BDI at baseline and at 3 weeks. Changes in glabellar frown lines also were assessed using the CSS-GFL. The authors reported statistically significant improvements in HAM-D17 (
A summary of the RCTs of BTA for treating depression appears in Table 1.23,25-28
Continue to: Benefits for other psychiatric indications
Benefits for other psychiatric indications
Borderline personality disorder. In a case series of 6 women, BTA injections in the glabellar region were reported to be particularly effective for the treatment of borderline personality disorder symptoms that were resistant to psychotherapy and pharmacotherapy.31 Two to 6 weeks after a 29-unit injection, borderline personality disorder symptoms as measured by the Zanarini Rating Scale for Borderline Personality Disorder and/or the Borderline Symptom List were shown to significantly improve by 49% to 94% from baseline (P ≤ .05). These findings emphasize the promising therapeutic role of BTA on depressive symptoms concomitant with the emotional lability, impulsivity, and negative emotions that usually characterize this personality disorder.31,32 A small sample size and lack of a placebo comparator are limitations of this research.
Neuroleptic-induced sialorrhea. Botulinum toxin injections in the salivary glands have been investigated for treating clozapine-induced sialorrhea because they are thought to directly inhibit the release of acetylcholine from salivary glands. One small RCT that used botulinum toxin B (BTB)33 and 1 case report that used BTA34 reported successful reduction in hypersalivation, with doses ranging from 150 to 500 units injected in each of the parotid and/or submandibular glands bilaterally. Although the treatment was well tolerated and lasted up to 16 weeks, larger studies are needed to replicate these findings.33-35
Orofacial tardive dyskinesia. Several case reports of orofacial tardive dyskinesia, including lingual dyskinesia and lingual protrusion dystonia, have found improvements in hyperkinetic movements following muscular BTA injections, such as in the genioglossus muscle in the case of tongue involvement.36-39 These cases were, however, described in the literature before the recent FDA approval of the vesicular monoamine transporter 2 inhibitors valbenazine and deutetrabenazine for the treatment of tardive dyskinesia.40,41
Studies examining botulinum toxin’s application in areas of psychiatry other than depression are summarized in Table 2.31,33,36-38
Continue to: Promising initial findings but multiple limitations
Promising initial findings but multiple limitations
Although BTA injections have been explored as a potential treatment for several psychiatric conditions, the bulk of recent evidence is derived from studies in patients with depressive disorders. BTA injections in the glabellar regions have been shown in small RCTs to be well-tolerated with overall promising improvement of depressive symptoms, optimally 6 weeks after a single injection. Moreover, BTA has been shown to be safe and long-lasting, which would be convenient for patients and might improve adherence to therapy.42-44 BTA’s antidepressant effects were shown to be independent of frown line severity or patient satisfaction with cosmetic effects.45 The trials by Wollmer et al,23 Finzi and Rosenthal,25 and Magid et al26 mainly studied BTA as an adjunctive treatment to antidepressants in patients with ongoing unipolar depression. However, Finzi and Rosenthal25 included patients who were not medicated at the time of the study.
Pooled analysis of these 3 RCTs found that patients who received BTA monotherapy improved equally to those who received it as an adjunctive treatment to antidepressants. Overall, on primary endpoint measures, a response rate of 54.2% was obtained in the BTA group compared with 10.7% among patients who received placebo saline injections (odds ratio [OR] 11.1, 95% confidence interval [CI], 4.3 to 28.8, number needed to treat [NNT] = 2.3) and a remission rate of 30.5% with BTA compared with 6.7% with placebo (OR 7.3, 95 % CI, 2.4 to 22.5, NNT = 4.2).46 However, remission rates tend to be higher in the augmentation groups, and so further studies are needed to compare both treatment strategies.
Nevertheless, these positive findings have been recently challenged by the results of the largest U.S. multicenter phase II RCT,28 which failed to find a significant antidepressant effect at 6 weeks with the 30-unit BTA injection, and also failed to prove a dose-effect relationship, as the 50-unit injection wasn’t superior to the lower dose and didn’t significantly differ from placebo. One hypothesis to explain this discrepancy may be the difference in injection sites between the treatment and placebo groups.47 Future studies need to address the various limitations of earlier clinical trials that mainly yielded promising results with BTA.
A major concern is the high rate of unblinding of participants and researchers in BTA trials, as the cosmetic effects of botulinum toxin injections make them easy to distinguish from saline injections. Ninety percent of participants in the Wollmer et al study23 were able to correctly guess their group allocation, while 60% of evaluators guessed correctly. Finzi and Rozenthal25 reported 52% of participants in the BTA group, 46% in the placebo group, and 73% of evaluators correctly guessed their allocation. Magid et al26 reported 75% of participants were able to guess the order of intervention they received. The high unblinding rates in these trials remains a significant limitation. There is a concern that this may lead to an underestimation of the placebo effect relative to clinical improvement, thus causing inflation of outcome differences between groups. Although various methods have been tried to minimize evaluator unblinding, such as placing surgical caps on participants’ faces during visits to hide the glabellar region, better methods need to be implemented to prevent unblinding of both raters and participants.
Furthermore, except for the multicenter phase II trial, most studies have been conducted in small samples, which limits their statistical power. Larger controlled trials will be needed to replicate the positive findings obtained in smaller RCTs.
Another limitation is that the majority of the well-designed RCTs were conducted in populations that were predominantly female, which makes it difficult to reliably assess treatment efficacy in men. This may be because cosmetic treatment with botulinum toxin injection is more favorably received by women than by men. A recent comparison48 of the studies by Wollmer et al23 and Finzi and Rosenthal25 discussed an interesting observation. Wollmer et al did not explicitly mention botulinum toxin when recruiting for the study, while Finzi and Rosenthal did. While approximately a quarter of the participants in the Wollmer et al study were male, Finzi and Rosenthal attracted an almost entirely female population. Perhaps there is a potential bias for females to be more attracted to these studies due to the secondary gain of receiving a cosmetic procedure.
In an attempt to understand predictors of positive response to botulinum toxin in patients with depression, Wollmer et al49 conducted a follow-up study in which they reassessed the data obtained from their initial RCT using the HAM-D agitation item scores to separate the 15 participants who received BTA into low-agitation (≤1 score on agitation item of the HAM-D scale) and high-agitation (≥2 score on agitation item of the HAM-D scale) groups. They found that the 9 participants who responded to BTA treatment had significantly higher baseline agitation scores than participants who did not respond (1.56 ± 0.88 vs 0.33 ± 0.52, P = .01). All of the participants who presented with higher agitation levels experienced response, compared with 40% of those with lower agitation levels (P = .04), although there was no significant difference in magnitude of improvement (14.2 ± 1.92 vs 8.0 ± 9.37, P = .07). The study added additional support to the facial feedback hypothesis, as it links the improvement of depression to facial muscle activation targeted by the injections. It also introduced a potential predictor of response to botulinum toxin treatment, highlighting potential factors to consider when enrolling patients in future investigations.
The case series of patients with borderline personality disorder31 also shed light on the potential positive effect of BTA treatment for a particular subtype of patients with depression—those with comorbid emotional instability—to consider as a therapeutic target for the future. Hence, inclusion criteria for future trials might potentially include patient age, gender, existence/quantification of prominent frown lines at baseline, severity of MDD, duration of depression, and personality characteristics of enrolled participants.
In conclusion, BTA injections appear promising as a treatment for depression as well as for other psychiatric disorders. Future studies should focus on identifying optimal candidates for this innovative treatment modality. Furthermore, BTA dosing and administration strategies (monotherapy vs adjunctive treatment to antidepressants) need to be further explored. As retrograde axonal transport of botulinum toxin has been demonstrated in animal studies, it would be interesting to further examine its effects in the human CNS to enhance our knowledge of the pathophysiology of botulinum and its potential applications in psychiatry.50
Bottom Line
Botulinum toxin shows promising antidepressant effects and may have a role in the treatment of several other psychiatric disorders. More research is needed to address limitations of previous studies and to establish an adequate treatment regimen.
Related Resources
- Wollmer MA, Magid M, Kruger TH. Botulinum toxin treatment in depression. In: Bewley A, Taylor RE, Reichenberg JS, et al (eds). Practical psychodermatology. Oxford, UK: Wiley; 2014.
- Wollmer MA, Neumann I, Magid M. et al. Shrink that frown! Botulinum toxin therapy is lifting the face of psychiatry. G Ital Dermatol Venereol. 2018;153(4):540-548.
Drug Brand Names
Alprazolam • Xanax
Aripiprazole • Abilify
Biperiden • Akineton
Botulinum toxin A • Botox
Botulinum toxin B • Myobloc
Clozapine • Clozaril
Deutetrabenazine • Austedo
Flupentixol • Prolixin
Imipramine • Tofranil
Olanzapine • Zyprexa
Reserpine • Serpalan, Serpasil
Tetrabenazine • Xenazine
Valbenazine • Ingrezza
Ziprasidone • Geodon
1. Erbguth FJ, Naumann M. Historical aspects of botulinum toxin. Justinus Kerner (1786-1862) and the “sausage” poison. Neurology. 1999;53(8):1850-1853.
2. Devriese PP. On the discovery of Clostridium botulinum. J History Neurosci. 1999;8(1):43-50.
3. Burgen ASV, Dickens F, Zatman LJ. The action of botulinum toxin on the neuro-muscular junction. J Physiol. 1949;109(1-2):10-24.
4. Jankovic J. Botulinum toxin in clinical practice. J Neurol Neurosurg Psychiatry. 2004;75(7):951-957.
5. BOTOX (OnabotulinumtoxinA) [package insert]. Allergan, Inc., Irvine, CA; 2015.
6. Saadia D, Voustianiouk A, Wang AK, et al. Botulinum toxin type A in primary palmar hyperhidrosis. Randomized, single-blind, two-dose study. Neurology. 2001;57(11):2095-2099.
7. Naumann MK, Lowe NJ. Effect of botulinum toxin type A on quality of life measures in patients with excessive axillary sweating: a randomized controlled trial. Br J Dermatol. 2002;147(6):1218-1226.
8. Giess R, Naumann M, Werner E, et al. Injections of botulinum toxin A into the salivary glands improve sialorrhea in amyotrophic lateral sclerosis. J Neurol Neurosurg Psychiatry. 2000;69(1):121-123.
9. Restivo DA, Palmeri A, Marchese-Ragona R. Botulinum toxin for cricopharyngeal dysfunction in Parkinson’s disease. N Engl J Med. 2002;346(15):1174-1175.
10. Pasricha PJ, Ravich WJ, Hendrix T, et al. Intrasphincteric botulinum toxin for the treatment of achalasia. N Engl J Med. 1995(12);322:774-778.
11. Schiano TD, Parkman HP, Miller LS, et al. Use of botulinum toxin in the treatment of achalasia. Dig Dis. 1998;16(1):14-22.
12. Sim WS. Application of botulinum toxin in pain management. Korean J Pain. 2011;24(1):1-6.
13. Darwin C. The expression of the emotions in man and animals. London, UK: John Murray; 1872:366.
14. James W. The principles of psychology, vol. 2. New York, NY: Henry Holt and Company; 1890.
15. James W. II. —What is an emotion? Mind. 1884;os-IX(34):188-205.
16. Strack R, Martin LL, Stepper S. Inhibiting and facilitating conditions of facial expressions: a nonobtrusive test of the facial feedback hypothesis. J Pers Soc Psychol. 1988;54(5):768-777.
17. Larsen RJ, Kasimatis M, Frey K. Facilitating the furrowed brow: an unobtrusive test of the facial feedback hypothesis applied to unpleasant affect. Cogn Emot. 1992;6(5):321-338.
18. Ibragic S, Matak I, Dracic A, et al. Effects of botulinum toxin type A facial injection on monoamines and their metabolites in sensory, limbic, and motor brain regions in rats. Neurosci Lett. 2016;617:213-217.
19. Hennenlotter A, Dresel C, Castrop F, et al. The link between facial feedback and neural activity within central circuitries of emotion—new insights from botulinum toxin-induced denervation of frown muscles. Cereb Cortex. 2009;19(3):537-42
20. Kim MJ, Neta M, Davis FC, et al. Botulinum toxin-induced facial muscle paralysis affects amygdala responses to the perception emotional expressions: preliminary findings from an A-B-A design. Biol Mood Anxiety Disord. 2014;4:11.
21. Nestler EJ, Barrot M, DiLeone RJ, et al. Neurobiology of depression. Neuron. 2002;34(1):13-25.
22. Pandya M, Altinay M, Malone DA Jr, et al. Where in the brain is depression? Curr Psychiatry Rep. 2012;14(6):634-642.
23. Wollmer MA, de Boer C, Kalak N, et al. Facing depression with botulinum toxin: a randomized controlled trial. J Psychiatr Res. 2012;46:574-581.
24. BOTOX Cosmetic [prescribing information]. Allergan, Inc., Irvine, CA; 2017.
25. Finzi E, Rosenthal NE. Treatment of depression with onabotulinumtoxinA; a randomized, double-blind, placebo controlled trial. J Psychiatr Res. 2014;52:1-6.
26. Magid M, Reichenberg JS, Poth PE, et al. The treatment of major depressive disorder using botulinum toxin A: a 24 week randomized, double-blind, placebo-controlled study. J Clin Psychiatry. 2014;75(8):837-844.
27. Zamanian A, Ghanbari Jolfaei A, Mehran G, et al. Efficacy of botox versus placebo for treatment of patients with major depression. Iran J Public Health. 2017;46(7):982-984.
28. Allergan. OnabotulinumtoxinA as treatment for major depressive disorder in adult females. 2017. https://clinicaltrials.gov/ct2/show/NCT02116361. Accessed October 26, 2018.
29. Allergan. Allergan reports topline phase II data supporting advancement of BOTOX® (onabotulinumtoxinA) for the treatment of major depressive disorder (MDD). April 5, 2017. https://www.allergan.com/news/news/thomson-reuters/allergan-reports-topline-phase-ii-data-supporting. Accessed October 26, 2018.
30. Chugh S, Chhabria A, Jung S, et al. Botulinum toxin as a treatment for depression in a real-world setting. J Psychiatr Pract. 2018;24(1):15-20.
31. Kruger TH, Magid M, Wollmer MA. Can botulinum toxin help patients with borderline personality disorder? Am J Psychiatry. 2016;173(9):940-941.
32. Baumeister JC, Papa G, Foroni F. Deeper than skin deep – the effect of botulinum toxin-A on emotion processing. Toxicon. 2016;119:86-90.
33. Steinlechner S, Klein C, Moser A, et al. Botulinum toxin B as an effective and safe treatment for neuroleptic-induced sialorrhea. Psychopharmacology (Berl). 2010;207(4):593-597.
34. Kahl KG, Hagenah J, Zapf S, et al. Botulinum toxin as an effective treatment of clozapine-induced hypersalivation. Psychopharmacology (Berl). 2004;173(1-2):229-230.
35. Bird AM, Smith TL, Walton AE. Current treatment strategies for clozapine-induced sialorrhea. Ann Pharmacother. 2011;45(5):667-675.
36. Tschopp L, Salazar Z, Micheli F. Botulinum toxin in painful tardive dyskinesia. Clin Neuropharmacol. 2009;32(3):165-166.
37. Hennings JM, Krause E, Bötzel K, et al. Successful treatment of tardive lingual dystonia with botulinum toxin: case report and review of the literature. Prog Neuropsychopharmacol Biol Psychiatry. 2008;32(5):1167-1171.
38. Slotema CW, van Harten PN, Bruggeman R, et al. Botulinum toxin in the treatment of orofacial tardive dyskinesia: a single blind study. Prog Neuropsychopharmacol Biol Psychiatry. 2008;32(2):507-509.
39. Esper CD, Freeman A, Factor SA. Lingual protrusion dystonia: frequency, etiology and botulinum toxin therapy. Parkinsonism Relat Disord. 2010;16(7):438-441.
40. Seeberger LC, Hauser RA. Valbenazine for the treatment of tardive dyskinesia. Expert Opin Pharmacother. 2017;18(12):1279-1287.
41. Citrome L. Deutetrabenazine for tardive dyskinesia: a systematic review of the efficacy and safety profile for this newly approved novel medication—What is the number needed to treat, number needed to harm and likelihood to be helped or harmed? Int J Clin Pract. 2017;71(11):e13030.
42. Brin MF, Boodhoo TI, Pogoda JM, et al. Safety and tolerability of onabotulinumtoxinA in the tretment of facial lines: a meta-analysis of individual patient data from global clinical registration studies in 1678 participants. J Am Acad Dermatol. 2009;61:961-970.
43. Beer K. Cost effectiveness of botulinum toxins for the treatment of depression: preliminary observations. J Drugs Dermatol. 2010;9(1):27-30.
44. Serna MC, Cruz I, Real J, et al. Duration and adherence of antidepressant treatment (2003-2007) based on prescription database. Eur Psychiatry. 2010;25(4):206-213.
45. Rechenberg JS, Hauptman AJ, Robertson HT, et al. Botulinum toxin for depression: Does patient appearance matter? J Am Acad Dermatol. 2016;74(1):171-173.
46. Magid M, Finzi E, Kruger THC, et al. Treating depression with botulinum toxin: a pooled analysis of randomized controlled trials. Pharmacopsychiatry. 2015;48(6):205-210.
47. Court, E. Allergan is still hopeful about using Botox to treat depression. April 8, 2017. https://www.marketwatch.com/story/allergan-is-still-hopeful-about-using-botox-to-treat-depression-2017-04-07. Accessed October 26, 2018.
48. Rudorfer MV. Botulinum toxin: does it have a place in the management of depression? CNS Drugs. 2018;32(2):97-100.
49. Wollmer MA, Kalak N, Jung S, et al. Agitation predicts response of depression to botulinum toxin treatment in a randomized controlled trial. Front Psychiatry. 2014;5:36
50. Antonucci F, Rossi C, Gianfranceschi L, et al. Long-distance retrograde effects of botulinum neurotoxin A. J Neurosci. 2008;28(14):3689-3696.
1. Erbguth FJ, Naumann M. Historical aspects of botulinum toxin. Justinus Kerner (1786-1862) and the “sausage” poison. Neurology. 1999;53(8):1850-1853.
2. Devriese PP. On the discovery of Clostridium botulinum. J History Neurosci. 1999;8(1):43-50.
3. Burgen ASV, Dickens F, Zatman LJ. The action of botulinum toxin on the neuro-muscular junction. J Physiol. 1949;109(1-2):10-24.
4. Jankovic J. Botulinum toxin in clinical practice. J Neurol Neurosurg Psychiatry. 2004;75(7):951-957.
5. BOTOX (OnabotulinumtoxinA) [package insert]. Allergan, Inc., Irvine, CA; 2015.
6. Saadia D, Voustianiouk A, Wang AK, et al. Botulinum toxin type A in primary palmar hyperhidrosis. Randomized, single-blind, two-dose study. Neurology. 2001;57(11):2095-2099.
7. Naumann MK, Lowe NJ. Effect of botulinum toxin type A on quality of life measures in patients with excessive axillary sweating: a randomized controlled trial. Br J Dermatol. 2002;147(6):1218-1226.
8. Giess R, Naumann M, Werner E, et al. Injections of botulinum toxin A into the salivary glands improve sialorrhea in amyotrophic lateral sclerosis. J Neurol Neurosurg Psychiatry. 2000;69(1):121-123.
9. Restivo DA, Palmeri A, Marchese-Ragona R. Botulinum toxin for cricopharyngeal dysfunction in Parkinson’s disease. N Engl J Med. 2002;346(15):1174-1175.
10. Pasricha PJ, Ravich WJ, Hendrix T, et al. Intrasphincteric botulinum toxin for the treatment of achalasia. N Engl J Med. 1995(12);322:774-778.
11. Schiano TD, Parkman HP, Miller LS, et al. Use of botulinum toxin in the treatment of achalasia. Dig Dis. 1998;16(1):14-22.
12. Sim WS. Application of botulinum toxin in pain management. Korean J Pain. 2011;24(1):1-6.
13. Darwin C. The expression of the emotions in man and animals. London, UK: John Murray; 1872:366.
14. James W. The principles of psychology, vol. 2. New York, NY: Henry Holt and Company; 1890.
15. James W. II. —What is an emotion? Mind. 1884;os-IX(34):188-205.
16. Strack R, Martin LL, Stepper S. Inhibiting and facilitating conditions of facial expressions: a nonobtrusive test of the facial feedback hypothesis. J Pers Soc Psychol. 1988;54(5):768-777.
17. Larsen RJ, Kasimatis M, Frey K. Facilitating the furrowed brow: an unobtrusive test of the facial feedback hypothesis applied to unpleasant affect. Cogn Emot. 1992;6(5):321-338.
18. Ibragic S, Matak I, Dracic A, et al. Effects of botulinum toxin type A facial injection on monoamines and their metabolites in sensory, limbic, and motor brain regions in rats. Neurosci Lett. 2016;617:213-217.
19. Hennenlotter A, Dresel C, Castrop F, et al. The link between facial feedback and neural activity within central circuitries of emotion—new insights from botulinum toxin-induced denervation of frown muscles. Cereb Cortex. 2009;19(3):537-42
20. Kim MJ, Neta M, Davis FC, et al. Botulinum toxin-induced facial muscle paralysis affects amygdala responses to the perception emotional expressions: preliminary findings from an A-B-A design. Biol Mood Anxiety Disord. 2014;4:11.
21. Nestler EJ, Barrot M, DiLeone RJ, et al. Neurobiology of depression. Neuron. 2002;34(1):13-25.
22. Pandya M, Altinay M, Malone DA Jr, et al. Where in the brain is depression? Curr Psychiatry Rep. 2012;14(6):634-642.
23. Wollmer MA, de Boer C, Kalak N, et al. Facing depression with botulinum toxin: a randomized controlled trial. J Psychiatr Res. 2012;46:574-581.
24. BOTOX Cosmetic [prescribing information]. Allergan, Inc., Irvine, CA; 2017.
25. Finzi E, Rosenthal NE. Treatment of depression with onabotulinumtoxinA; a randomized, double-blind, placebo controlled trial. J Psychiatr Res. 2014;52:1-6.
26. Magid M, Reichenberg JS, Poth PE, et al. The treatment of major depressive disorder using botulinum toxin A: a 24 week randomized, double-blind, placebo-controlled study. J Clin Psychiatry. 2014;75(8):837-844.
27. Zamanian A, Ghanbari Jolfaei A, Mehran G, et al. Efficacy of botox versus placebo for treatment of patients with major depression. Iran J Public Health. 2017;46(7):982-984.
28. Allergan. OnabotulinumtoxinA as treatment for major depressive disorder in adult females. 2017. https://clinicaltrials.gov/ct2/show/NCT02116361. Accessed October 26, 2018.
29. Allergan. Allergan reports topline phase II data supporting advancement of BOTOX® (onabotulinumtoxinA) for the treatment of major depressive disorder (MDD). April 5, 2017. https://www.allergan.com/news/news/thomson-reuters/allergan-reports-topline-phase-ii-data-supporting. Accessed October 26, 2018.
30. Chugh S, Chhabria A, Jung S, et al. Botulinum toxin as a treatment for depression in a real-world setting. J Psychiatr Pract. 2018;24(1):15-20.
31. Kruger TH, Magid M, Wollmer MA. Can botulinum toxin help patients with borderline personality disorder? Am J Psychiatry. 2016;173(9):940-941.
32. Baumeister JC, Papa G, Foroni F. Deeper than skin deep – the effect of botulinum toxin-A on emotion processing. Toxicon. 2016;119:86-90.
33. Steinlechner S, Klein C, Moser A, et al. Botulinum toxin B as an effective and safe treatment for neuroleptic-induced sialorrhea. Psychopharmacology (Berl). 2010;207(4):593-597.
34. Kahl KG, Hagenah J, Zapf S, et al. Botulinum toxin as an effective treatment of clozapine-induced hypersalivation. Psychopharmacology (Berl). 2004;173(1-2):229-230.
35. Bird AM, Smith TL, Walton AE. Current treatment strategies for clozapine-induced sialorrhea. Ann Pharmacother. 2011;45(5):667-675.
36. Tschopp L, Salazar Z, Micheli F. Botulinum toxin in painful tardive dyskinesia. Clin Neuropharmacol. 2009;32(3):165-166.
37. Hennings JM, Krause E, Bötzel K, et al. Successful treatment of tardive lingual dystonia with botulinum toxin: case report and review of the literature. Prog Neuropsychopharmacol Biol Psychiatry. 2008;32(5):1167-1171.
38. Slotema CW, van Harten PN, Bruggeman R, et al. Botulinum toxin in the treatment of orofacial tardive dyskinesia: a single blind study. Prog Neuropsychopharmacol Biol Psychiatry. 2008;32(2):507-509.
39. Esper CD, Freeman A, Factor SA. Lingual protrusion dystonia: frequency, etiology and botulinum toxin therapy. Parkinsonism Relat Disord. 2010;16(7):438-441.
40. Seeberger LC, Hauser RA. Valbenazine for the treatment of tardive dyskinesia. Expert Opin Pharmacother. 2017;18(12):1279-1287.
41. Citrome L. Deutetrabenazine for tardive dyskinesia: a systematic review of the efficacy and safety profile for this newly approved novel medication—What is the number needed to treat, number needed to harm and likelihood to be helped or harmed? Int J Clin Pract. 2017;71(11):e13030.
42. Brin MF, Boodhoo TI, Pogoda JM, et al. Safety and tolerability of onabotulinumtoxinA in the tretment of facial lines: a meta-analysis of individual patient data from global clinical registration studies in 1678 participants. J Am Acad Dermatol. 2009;61:961-970.
43. Beer K. Cost effectiveness of botulinum toxins for the treatment of depression: preliminary observations. J Drugs Dermatol. 2010;9(1):27-30.
44. Serna MC, Cruz I, Real J, et al. Duration and adherence of antidepressant treatment (2003-2007) based on prescription database. Eur Psychiatry. 2010;25(4):206-213.
45. Rechenberg JS, Hauptman AJ, Robertson HT, et al. Botulinum toxin for depression: Does patient appearance matter? J Am Acad Dermatol. 2016;74(1):171-173.
46. Magid M, Finzi E, Kruger THC, et al. Treating depression with botulinum toxin: a pooled analysis of randomized controlled trials. Pharmacopsychiatry. 2015;48(6):205-210.
47. Court, E. Allergan is still hopeful about using Botox to treat depression. April 8, 2017. https://www.marketwatch.com/story/allergan-is-still-hopeful-about-using-botox-to-treat-depression-2017-04-07. Accessed October 26, 2018.
48. Rudorfer MV. Botulinum toxin: does it have a place in the management of depression? CNS Drugs. 2018;32(2):97-100.
49. Wollmer MA, Kalak N, Jung S, et al. Agitation predicts response of depression to botulinum toxin treatment in a randomized controlled trial. Front Psychiatry. 2014;5:36
50. Antonucci F, Rossi C, Gianfranceschi L, et al. Long-distance retrograde effects of botulinum neurotoxin A. J Neurosci. 2008;28(14):3689-3696.