User login
Anti-Smith and Anti–Double-Stranded DNA Antibodies in a Patient With Henoch-Schönlein Purpura Following COVID-19 Vaccination
To the Editor:
Henoch-Schönlein purpura (HSP)(also known as IgA vasculitis) is a small vessel vasculitis characterized by deposition of IgA in small vessels, resulting in the development of purpura on the legs. Based on the European Alliance of Associations for Rheumatology criteria,1 the patient also must have at least 1 of the following: arthritis, arthralgia, abdominal pain, leukocytoclastic vasculitis with IgA deposition, or kidney involvement. The disease can be triggered by infection—with more than 75% of patients reporting an antecedent upper respiratory tract infection2—as well as medications, circulating immune complexes, certain foods, vaccines, and rarely cancer.3,4 The disease more commonly occurs in children but also can affect adults.
Several cases of HSP have been reported following COVID-19 vaccination.5 We report a case of HSP developing days after the messenger RNA Pfizer-BioNTech COVID-19 vaccine booster that was associated with anti-Smith and anti–double-stranded DNA (dsDNA) antibodies as well as antineutrophil cytoplasmic antibodies (ANCAs).
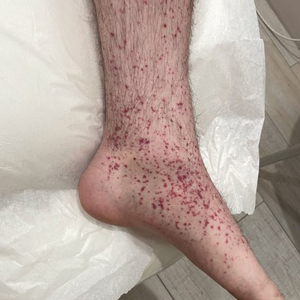
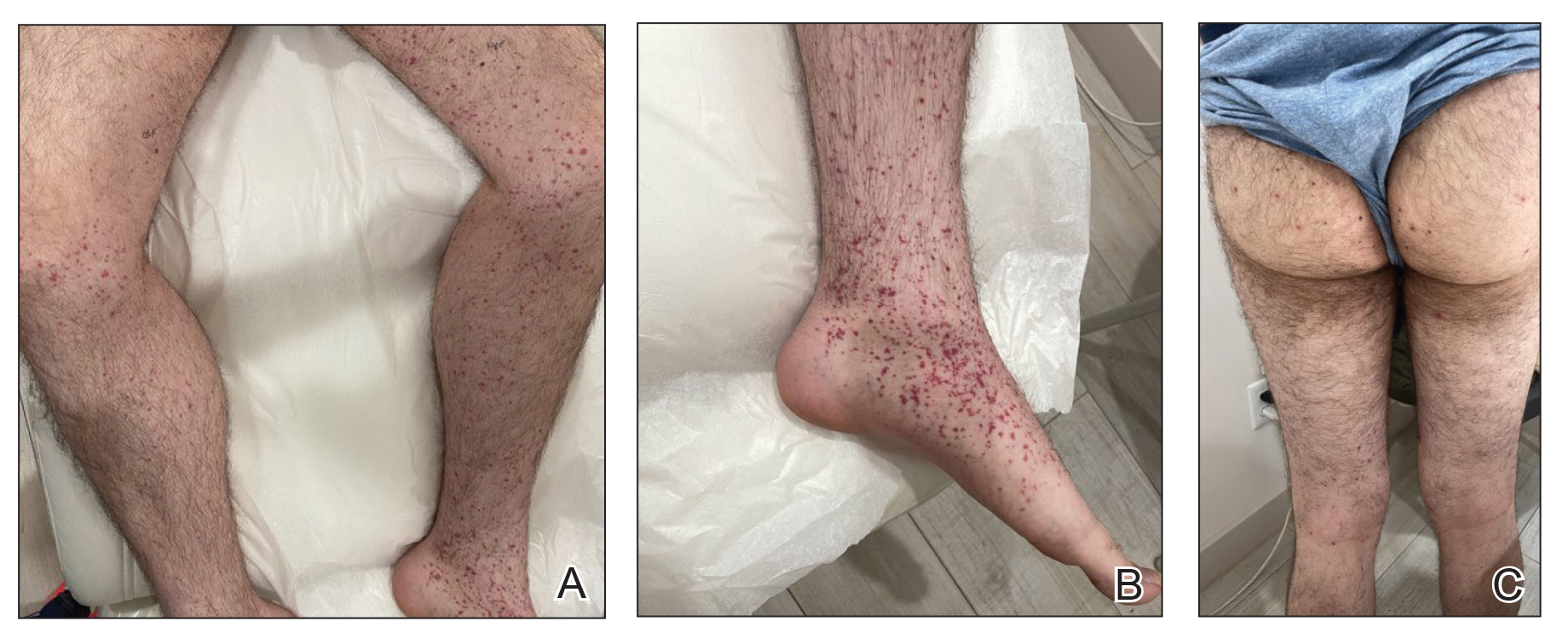
A 24-year-old man presented to dermatology with a rash of 3 weeks’ duration that first appeared 1 week after receiving his second booster of the messenger RNA Pfizer-BioNTech COVID-19 vaccine. Physical examination revealed petechiae with nonblanching erythematous macules and papules covering the legs below the knees (Figure 1) as well as the back of the right arm. A few days later, he developed arthralgia in the knees, hands, and feet. The patient denied any recent infections as well as respiratory and urinary tract symptoms. Approximately 10 days after the rash appeared, he developed epigastric abdominal pain that gradually worsened and sought care from his primary care physician, who ordered computed tomography and referred him for endoscopy. Computed tomography with and without contrast was suspicious for colitis. Colonoscopy and endoscopy were unremarkable. Laboratory tests were notable for elevated white blood cell count (17.08×103/µL [reference range, 3.66–10.60×103/µL]), serum IgA (437 mg/dL [reference range, 70–400 mg/dL]), C-reactive protein (1.5 mg/dL [reference range, <0.5 mg/dL]), anti-Smith antibody (28.1 CU [reference range, <20 CU), positive antinuclear antibody with titer (1:160 [reference range, <1:80]), anti-dsDNA (40.4 IU/mL [reference range, <27 IU/mL]), and cytoplasmic ANCA (c-ANCA) titer (1:320 [reference range, <1:20]). Blood urea nitrogen, creatinine, and estimated glomerular filtration rate were all within reference range. Urinalysis with microscopic examination was notable for 2 to 5 red blood cells per high-power field (reference range, 0) and proteinuria of 1+ (reference range, negative for protein).
The patient’s rash progressively worsened over the next few weeks, spreading proximally on the legs to the buttocks and the back of both elbows. A repeat complete blood cell count showed resolution of the leukocytosis. Two biopsies were taken from a lesion on the left proximal thigh: 1 for hematoxylin and eosin stain for histopathologic examination and 1 for direct immunofluorescence examination.
The patient was preliminarily diagnosed with HSP, and dermatology prescribed oral tofacitinib 5 mg twice daily for 5 days, which was supposed to be increased to 10 mg twice daily on the sixth day of treatment; however, the patient discontinued the medication after 4 days based on his primary care physician’s recommendation due to clotting concerns. The rash and arthralgia temporarily improved for 1 week, then relapsed.
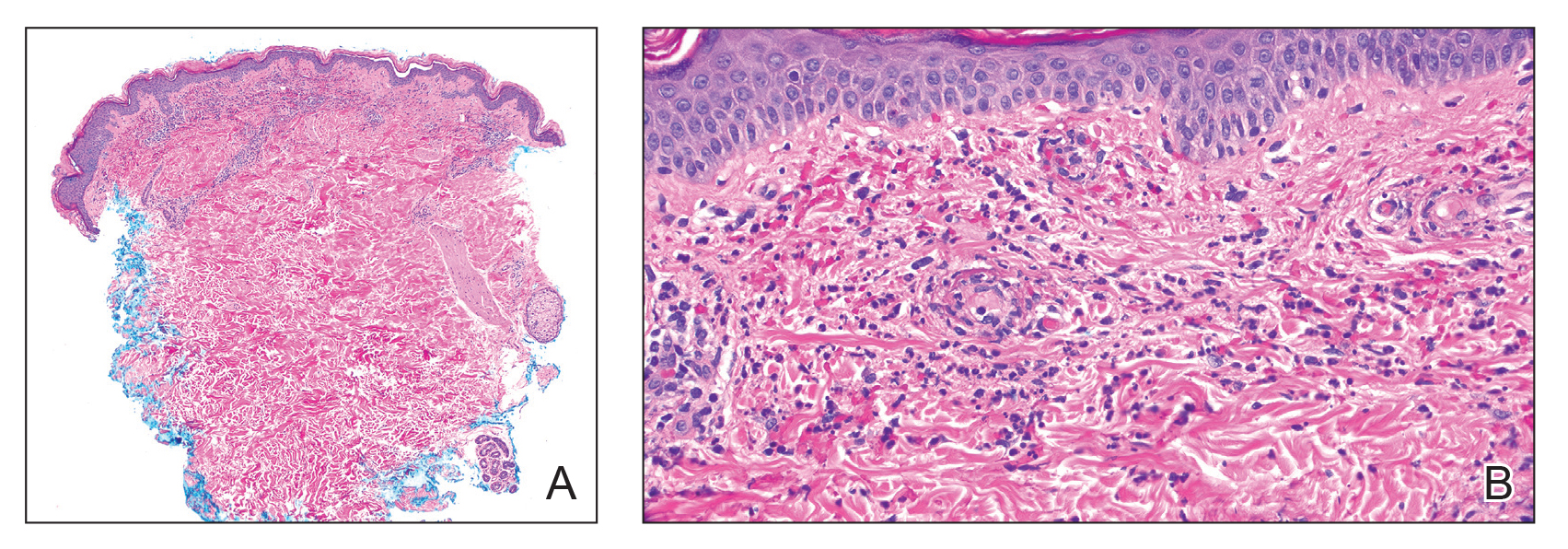
Histopathology revealed neutrophils surrounding and infiltrating small dermal blood vessel walls as well as associated neutrophilic debris and erythrocytes, consistent with leukocytoclastic vasculitis (Figure 2). Direct immunofluorescence was negative for IgA antibodies. His primary care physician, in consultation with his dermatologist, then started the patient on oral prednisone 70 mg once daily for 7 days with a plan to taper. Three days after prednisone was started, the arthralgia and abdominal pain resolved, and the rash became lighter in color. After 1 week, the rash resolved completely.
Due to the unusual antibodies, the patient was referred to a rheumatologist, who repeated the blood tests approximately 1 week after the patient started prednisone. The tests were negative for anti-Smith, anti-dsDNA, and c-ANCA but showed an elevated atypical perinuclear ANCA (p-ANCA) titer of 1:80 (reference range [negative], <1:20). A repeat urinalysis was unremarkable. The patient slowly tapered the prednisone over the course of 3 months and was subsequently lost to follow-up. The rash and other symptoms had not recurred as of the patient’s last physician contact. The most recent laboratory results showed a white blood cell count of 14.0×103/µL (reference range, 3.4–10.8×103/µL), likely due to the prednisone; blood urea nitrogen, creatinine, and estimated glomerular filtration rate were within reference range. The urinalysis was notable for occult blood and was negative for protein. C-reactive protein was 1 mg/dL (reference range, 0–10 mg/dL); p-ANCA, c-ANCA, and atypical p-ANCA, as well as antinuclear antibody, were negative. As of his last follow-up, the patient felt well.
The major differential diagnoses for our patient included HSP, ANCA vasculitis, and systemic lupus erythematosus. Although ANCA vasculitis has been reported after SARS-CoV-2 infection,6 the lack of pulmonary symptoms made this diagnosis unlikely.7 Although our patient initially had elevated anti-Smith and anti-dsDNA antibodies as well as mild renal involvement, he fulfilled at most only 2 of the 11 criteria necessary for diagnosing lupus: malar rash, discoid rash (includes alopecia), photosensitivity, ocular ulcers, nonerosive arthritis, serositis, renal disorder (protein >500 mg/24 h, red blood cells, casts), neurologic disorder (seizures, psychosis), hematologic disorders (hemolytic anemia, leukopenia), ANA, and immunologic disorder (anti-Smith). Four of the 11 criteria are necessary for the diagnosis of lupus.8
Torraca et al7 reported a case of HSP with positive c-ANCA (1:640) in a patient lacking pulmonary symptoms who was diagnosed with HSP. Cytoplasmic ANCA is not a typical finding in HSP. However, the additional findings of anti-Smith, anti-dsDNA, and mildly elevated atypical p-ANCA antibodies in our patient were unexpected and could be explained by the proposed pathogenesis of HSP—an overzealous immune response resulting in aberrant antibody complex deposition with ensuing complement activation.5,9 Production of these additional antibodies could be part of the overzealous response to COVID-19 vaccination.
Of all the COVID-19 vaccines, messenger RNA–based vaccines have been associated with the majority of cutaneous reactions, including local injection-site reactions (most common), delayed local reactions, urticaria, angioedema, morbilliform eruption, herpes zoster eruption, bullous eruptions, dermal filler reactions, chilblains, and pityriasis rosea. Less common reactions have included acute generalized exanthematous pustulosis, Stevens-Johnson syndrome, erythema multiforme, Sweet Syndrome, lichen planus, papulovesicular eruptions, pityriasis rosea–like eruptions, generalized annular lesions, facial pustular neutrophilic eruptions, and flares of underlying autoimmune skin conditions.10 Multiple cases of HSP have been reported following COVID-19 vaccination from all the major vaccine companies.5
In our patient, laboratory tests were repeated by a rheumatologist and were negative for anti-Smith and anti-dsDNA antibodies as well as c-ANCA, most likely because he started taking prednisone approximately 1 week prior, which may have resulted in decreased antibodies. Also, the patient’s symptoms resolved after 1 week of steroid therapy. Therefore, the diagnosis is most consistent with HSP associated with COVID-19 vaccination. The clinical presentation, microscopic hematuria and proteinuria, and histopathology were consistent with the European Alliance of Associations for Rheumatology criteria for HSP.1
Although direct immunofluorescence typically is positive for IgA deposition on biopsies, it can be negative for IgA, especially in lesions that are biopsied more than 7 days after their appearance, as shown in our case; a negative IgA on immunofluorescence does not rule out HSP.4 Elevated serum IgA is seen in more than 50% of cases of HSP.11 Although the disease typically is self-limited, glucocorticoids are used if the disease course is prolonged or if there is evidence of kidney involvement.9 The unique combination of anti-Smith and anti-dsDNA antibodies as well as ANCAs associated with HSP with negative IgA on direct immunofluorescence has been reported with lupus.12 Clinicians should be aware of COVID-19 vaccine–associated HSP that is negative for IgA deposition and positive for anti-Smith and anti-dsDNA antibodies as well as ANCAs.
Acknowledgment—We thank our patient for granting permission to publish this information.
- Ozen S, Ruperto N, Dillon MJ, et al. EULAR/PReS endorsed consensus criteria for the classification of childhood vasculitides. Ann Rheum Dis. 2006;65:936-941. doi:10.1136/ard.2005.046300
- Rai A, Nast C, Adler S. Henoch–Schönlein purpura nephritis. J Am Soc Nephrol. 1999;10:2637-2644.
- Casini F, Magenes VC, De Sanctis M, et al. Henoch-Schönlein purpura following COVID-19 vaccine in a child: a case report. Ital J Pediatr. 2022;48:158. doi:10.1186/s13052-022-01351-1
- Poudel P, Adams SH, Mirchia K, et al. IgA negative immunofluorescence in diagnoses of adult-onset Henoch-Schönlein purpura. Proc (Bayl Univ Med Cent). 2020;33:436-437. doi:10.1080/08998280.2020.1770526
- Maronese CA, Zelin E, Avallone G, et al. Cutaneous vasculitis and vasculopathy in the era of COVID-19 pandemic. Front Med (Lausanne). 2022;9:996288. doi:10.3389/fmed.2022.996288
- Bryant MC, Spencer LT, Yalcindag A. A case of ANCA-associated vasculitis in a 16-year-old female following SARS-COV-2 infection and a systematic review of the literature. Pediatr Rheumatol Online J. 2022;20:65. doi:10.1186/s12969-022-00727-1
- Torraca PFS, Castro BC, Hans Filho G. Henoch-Schönlein purpura with c-ANCA antibody in adult. An Bras Dermatol. 2016;91:667-669. doi:10.1590/abd1806-4841.20164181
- Agabegi SS, Agabegi ED. Step-Up to Medicine. 4th ed. Wolters Kluwer; 2015.
- Ball-Burack MR, Kosowsky JM. A Case of leukocytoclastic vasculitis following SARS-CoV-2 vaccination. J Emerg Med. 2022;63:E62-E65. doi:10.1016/j.jemermed.2021.10.005
- Tan SW, Tam YC, Pang SM. Cutaneous reactions to COVID-19 vaccines: a review. JAAD Int. 2022;7:178-186. doi:10.1016/j.jdin.2022.01.011
- Calviño MC, Llorca J, García-Porrúa C, et al. Henoch-Schönlein purpura in children from northwestern Spain: a 20-year epidemiologic and clinical study. Medicine (Baltimore). 2001;80:279-290.
- Hu P, Huang BY, Zhang DD, et al. Henoch-Schönlein purpura in a pediatric patient with lupus. Arch Med Sci. 2017;13:689-690. doi:10.5114/aoms.2017.67288
To the Editor:
Henoch-Schönlein purpura (HSP)(also known as IgA vasculitis) is a small vessel vasculitis characterized by deposition of IgA in small vessels, resulting in the development of purpura on the legs. Based on the European Alliance of Associations for Rheumatology criteria,1 the patient also must have at least 1 of the following: arthritis, arthralgia, abdominal pain, leukocytoclastic vasculitis with IgA deposition, or kidney involvement. The disease can be triggered by infection—with more than 75% of patients reporting an antecedent upper respiratory tract infection2—as well as medications, circulating immune complexes, certain foods, vaccines, and rarely cancer.3,4 The disease more commonly occurs in children but also can affect adults.
Several cases of HSP have been reported following COVID-19 vaccination.5 We report a case of HSP developing days after the messenger RNA Pfizer-BioNTech COVID-19 vaccine booster that was associated with anti-Smith and anti–double-stranded DNA (dsDNA) antibodies as well as antineutrophil cytoplasmic antibodies (ANCAs).
A 24-year-old man presented to dermatology with a rash of 3 weeks’ duration that first appeared 1 week after receiving his second booster of the messenger RNA Pfizer-BioNTech COVID-19 vaccine. Physical examination revealed petechiae with nonblanching erythematous macules and papules covering the legs below the knees (Figure 1) as well as the back of the right arm. A few days later, he developed arthralgia in the knees, hands, and feet. The patient denied any recent infections as well as respiratory and urinary tract symptoms. Approximately 10 days after the rash appeared, he developed epigastric abdominal pain that gradually worsened and sought care from his primary care physician, who ordered computed tomography and referred him for endoscopy. Computed tomography with and without contrast was suspicious for colitis. Colonoscopy and endoscopy were unremarkable. Laboratory tests were notable for elevated white blood cell count (17.08×103/µL [reference range, 3.66–10.60×103/µL]), serum IgA (437 mg/dL [reference range, 70–400 mg/dL]), C-reactive protein (1.5 mg/dL [reference range, <0.5 mg/dL]), anti-Smith antibody (28.1 CU [reference range, <20 CU), positive antinuclear antibody with titer (1:160 [reference range, <1:80]), anti-dsDNA (40.4 IU/mL [reference range, <27 IU/mL]), and cytoplasmic ANCA (c-ANCA) titer (1:320 [reference range, <1:20]). Blood urea nitrogen, creatinine, and estimated glomerular filtration rate were all within reference range. Urinalysis with microscopic examination was notable for 2 to 5 red blood cells per high-power field (reference range, 0) and proteinuria of 1+ (reference range, negative for protein).
The patient’s rash progressively worsened over the next few weeks, spreading proximally on the legs to the buttocks and the back of both elbows. A repeat complete blood cell count showed resolution of the leukocytosis. Two biopsies were taken from a lesion on the left proximal thigh: 1 for hematoxylin and eosin stain for histopathologic examination and 1 for direct immunofluorescence examination.
The patient was preliminarily diagnosed with HSP, and dermatology prescribed oral tofacitinib 5 mg twice daily for 5 days, which was supposed to be increased to 10 mg twice daily on the sixth day of treatment; however, the patient discontinued the medication after 4 days based on his primary care physician’s recommendation due to clotting concerns. The rash and arthralgia temporarily improved for 1 week, then relapsed.
Histopathology revealed neutrophils surrounding and infiltrating small dermal blood vessel walls as well as associated neutrophilic debris and erythrocytes, consistent with leukocytoclastic vasculitis (Figure 2). Direct immunofluorescence was negative for IgA antibodies. His primary care physician, in consultation with his dermatologist, then started the patient on oral prednisone 70 mg once daily for 7 days with a plan to taper. Three days after prednisone was started, the arthralgia and abdominal pain resolved, and the rash became lighter in color. After 1 week, the rash resolved completely.
Due to the unusual antibodies, the patient was referred to a rheumatologist, who repeated the blood tests approximately 1 week after the patient started prednisone. The tests were negative for anti-Smith, anti-dsDNA, and c-ANCA but showed an elevated atypical perinuclear ANCA (p-ANCA) titer of 1:80 (reference range [negative], <1:20). A repeat urinalysis was unremarkable. The patient slowly tapered the prednisone over the course of 3 months and was subsequently lost to follow-up. The rash and other symptoms had not recurred as of the patient’s last physician contact. The most recent laboratory results showed a white blood cell count of 14.0×103/µL (reference range, 3.4–10.8×103/µL), likely due to the prednisone; blood urea nitrogen, creatinine, and estimated glomerular filtration rate were within reference range. The urinalysis was notable for occult blood and was negative for protein. C-reactive protein was 1 mg/dL (reference range, 0–10 mg/dL); p-ANCA, c-ANCA, and atypical p-ANCA, as well as antinuclear antibody, were negative. As of his last follow-up, the patient felt well.
The major differential diagnoses for our patient included HSP, ANCA vasculitis, and systemic lupus erythematosus. Although ANCA vasculitis has been reported after SARS-CoV-2 infection,6 the lack of pulmonary symptoms made this diagnosis unlikely.7 Although our patient initially had elevated anti-Smith and anti-dsDNA antibodies as well as mild renal involvement, he fulfilled at most only 2 of the 11 criteria necessary for diagnosing lupus: malar rash, discoid rash (includes alopecia), photosensitivity, ocular ulcers, nonerosive arthritis, serositis, renal disorder (protein >500 mg/24 h, red blood cells, casts), neurologic disorder (seizures, psychosis), hematologic disorders (hemolytic anemia, leukopenia), ANA, and immunologic disorder (anti-Smith). Four of the 11 criteria are necessary for the diagnosis of lupus.8
Torraca et al7 reported a case of HSP with positive c-ANCA (1:640) in a patient lacking pulmonary symptoms who was diagnosed with HSP. Cytoplasmic ANCA is not a typical finding in HSP. However, the additional findings of anti-Smith, anti-dsDNA, and mildly elevated atypical p-ANCA antibodies in our patient were unexpected and could be explained by the proposed pathogenesis of HSP—an overzealous immune response resulting in aberrant antibody complex deposition with ensuing complement activation.5,9 Production of these additional antibodies could be part of the overzealous response to COVID-19 vaccination.


Of all the COVID-19 vaccines, messenger RNA–based vaccines have been associated with the majority of cutaneous reactions, including local injection-site reactions (most common), delayed local reactions, urticaria, angioedema, morbilliform eruption, herpes zoster eruption, bullous eruptions, dermal filler reactions, chilblains, and pityriasis rosea. Less common reactions have included acute generalized exanthematous pustulosis, Stevens-Johnson syndrome, erythema multiforme, Sweet Syndrome, lichen planus, papulovesicular eruptions, pityriasis rosea–like eruptions, generalized annular lesions, facial pustular neutrophilic eruptions, and flares of underlying autoimmune skin conditions.10 Multiple cases of HSP have been reported following COVID-19 vaccination from all the major vaccine companies.5
In our patient, laboratory tests were repeated by a rheumatologist and were negative for anti-Smith and anti-dsDNA antibodies as well as c-ANCA, most likely because he started taking prednisone approximately 1 week prior, which may have resulted in decreased antibodies. Also, the patient’s symptoms resolved after 1 week of steroid therapy. Therefore, the diagnosis is most consistent with HSP associated with COVID-19 vaccination. The clinical presentation, microscopic hematuria and proteinuria, and histopathology were consistent with the European Alliance of Associations for Rheumatology criteria for HSP.1
Although direct immunofluorescence typically is positive for IgA deposition on biopsies, it can be negative for IgA, especially in lesions that are biopsied more than 7 days after their appearance, as shown in our case; a negative IgA on immunofluorescence does not rule out HSP.4 Elevated serum IgA is seen in more than 50% of cases of HSP.11 Although the disease typically is self-limited, glucocorticoids are used if the disease course is prolonged or if there is evidence of kidney involvement.9 The unique combination of anti-Smith and anti-dsDNA antibodies as well as ANCAs associated with HSP with negative IgA on direct immunofluorescence has been reported with lupus.12 Clinicians should be aware of COVID-19 vaccine–associated HSP that is negative for IgA deposition and positive for anti-Smith and anti-dsDNA antibodies as well as ANCAs.
Acknowledgment—We thank our patient for granting permission to publish this information.
To the Editor:
Henoch-Schönlein purpura (HSP)(also known as IgA vasculitis) is a small vessel vasculitis characterized by deposition of IgA in small vessels, resulting in the development of purpura on the legs. Based on the European Alliance of Associations for Rheumatology criteria,1 the patient also must have at least 1 of the following: arthritis, arthralgia, abdominal pain, leukocytoclastic vasculitis with IgA deposition, or kidney involvement. The disease can be triggered by infection—with more than 75% of patients reporting an antecedent upper respiratory tract infection2—as well as medications, circulating immune complexes, certain foods, vaccines, and rarely cancer.3,4 The disease more commonly occurs in children but also can affect adults.
Several cases of HSP have been reported following COVID-19 vaccination.5 We report a case of HSP developing days after the messenger RNA Pfizer-BioNTech COVID-19 vaccine booster that was associated with anti-Smith and anti–double-stranded DNA (dsDNA) antibodies as well as antineutrophil cytoplasmic antibodies (ANCAs).
A 24-year-old man presented to dermatology with a rash of 3 weeks’ duration that first appeared 1 week after receiving his second booster of the messenger RNA Pfizer-BioNTech COVID-19 vaccine. Physical examination revealed petechiae with nonblanching erythematous macules and papules covering the legs below the knees (Figure 1) as well as the back of the right arm. A few days later, he developed arthralgia in the knees, hands, and feet. The patient denied any recent infections as well as respiratory and urinary tract symptoms. Approximately 10 days after the rash appeared, he developed epigastric abdominal pain that gradually worsened and sought care from his primary care physician, who ordered computed tomography and referred him for endoscopy. Computed tomography with and without contrast was suspicious for colitis. Colonoscopy and endoscopy were unremarkable. Laboratory tests were notable for elevated white blood cell count (17.08×103/µL [reference range, 3.66–10.60×103/µL]), serum IgA (437 mg/dL [reference range, 70–400 mg/dL]), C-reactive protein (1.5 mg/dL [reference range, <0.5 mg/dL]), anti-Smith antibody (28.1 CU [reference range, <20 CU), positive antinuclear antibody with titer (1:160 [reference range, <1:80]), anti-dsDNA (40.4 IU/mL [reference range, <27 IU/mL]), and cytoplasmic ANCA (c-ANCA) titer (1:320 [reference range, <1:20]). Blood urea nitrogen, creatinine, and estimated glomerular filtration rate were all within reference range. Urinalysis with microscopic examination was notable for 2 to 5 red blood cells per high-power field (reference range, 0) and proteinuria of 1+ (reference range, negative for protein).
The patient’s rash progressively worsened over the next few weeks, spreading proximally on the legs to the buttocks and the back of both elbows. A repeat complete blood cell count showed resolution of the leukocytosis. Two biopsies were taken from a lesion on the left proximal thigh: 1 for hematoxylin and eosin stain for histopathologic examination and 1 for direct immunofluorescence examination.
The patient was preliminarily diagnosed with HSP, and dermatology prescribed oral tofacitinib 5 mg twice daily for 5 days, which was supposed to be increased to 10 mg twice daily on the sixth day of treatment; however, the patient discontinued the medication after 4 days based on his primary care physician’s recommendation due to clotting concerns. The rash and arthralgia temporarily improved for 1 week, then relapsed.
Histopathology revealed neutrophils surrounding and infiltrating small dermal blood vessel walls as well as associated neutrophilic debris and erythrocytes, consistent with leukocytoclastic vasculitis (Figure 2). Direct immunofluorescence was negative for IgA antibodies. His primary care physician, in consultation with his dermatologist, then started the patient on oral prednisone 70 mg once daily for 7 days with a plan to taper. Three days after prednisone was started, the arthralgia and abdominal pain resolved, and the rash became lighter in color. After 1 week, the rash resolved completely.
Due to the unusual antibodies, the patient was referred to a rheumatologist, who repeated the blood tests approximately 1 week after the patient started prednisone. The tests were negative for anti-Smith, anti-dsDNA, and c-ANCA but showed an elevated atypical perinuclear ANCA (p-ANCA) titer of 1:80 (reference range [negative], <1:20). A repeat urinalysis was unremarkable. The patient slowly tapered the prednisone over the course of 3 months and was subsequently lost to follow-up. The rash and other symptoms had not recurred as of the patient’s last physician contact. The most recent laboratory results showed a white blood cell count of 14.0×103/µL (reference range, 3.4–10.8×103/µL), likely due to the prednisone; blood urea nitrogen, creatinine, and estimated glomerular filtration rate were within reference range. The urinalysis was notable for occult blood and was negative for protein. C-reactive protein was 1 mg/dL (reference range, 0–10 mg/dL); p-ANCA, c-ANCA, and atypical p-ANCA, as well as antinuclear antibody, were negative. As of his last follow-up, the patient felt well.
The major differential diagnoses for our patient included HSP, ANCA vasculitis, and systemic lupus erythematosus. Although ANCA vasculitis has been reported after SARS-CoV-2 infection,6 the lack of pulmonary symptoms made this diagnosis unlikely.7 Although our patient initially had elevated anti-Smith and anti-dsDNA antibodies as well as mild renal involvement, he fulfilled at most only 2 of the 11 criteria necessary for diagnosing lupus: malar rash, discoid rash (includes alopecia), photosensitivity, ocular ulcers, nonerosive arthritis, serositis, renal disorder (protein >500 mg/24 h, red blood cells, casts), neurologic disorder (seizures, psychosis), hematologic disorders (hemolytic anemia, leukopenia), ANA, and immunologic disorder (anti-Smith). Four of the 11 criteria are necessary for the diagnosis of lupus.8
Torraca et al7 reported a case of HSP with positive c-ANCA (1:640) in a patient lacking pulmonary symptoms who was diagnosed with HSP. Cytoplasmic ANCA is not a typical finding in HSP. However, the additional findings of anti-Smith, anti-dsDNA, and mildly elevated atypical p-ANCA antibodies in our patient were unexpected and could be explained by the proposed pathogenesis of HSP—an overzealous immune response resulting in aberrant antibody complex deposition with ensuing complement activation.5,9 Production of these additional antibodies could be part of the overzealous response to COVID-19 vaccination.


Of all the COVID-19 vaccines, messenger RNA–based vaccines have been associated with the majority of cutaneous reactions, including local injection-site reactions (most common), delayed local reactions, urticaria, angioedema, morbilliform eruption, herpes zoster eruption, bullous eruptions, dermal filler reactions, chilblains, and pityriasis rosea. Less common reactions have included acute generalized exanthematous pustulosis, Stevens-Johnson syndrome, erythema multiforme, Sweet Syndrome, lichen planus, papulovesicular eruptions, pityriasis rosea–like eruptions, generalized annular lesions, facial pustular neutrophilic eruptions, and flares of underlying autoimmune skin conditions.10 Multiple cases of HSP have been reported following COVID-19 vaccination from all the major vaccine companies.5
In our patient, laboratory tests were repeated by a rheumatologist and were negative for anti-Smith and anti-dsDNA antibodies as well as c-ANCA, most likely because he started taking prednisone approximately 1 week prior, which may have resulted in decreased antibodies. Also, the patient’s symptoms resolved after 1 week of steroid therapy. Therefore, the diagnosis is most consistent with HSP associated with COVID-19 vaccination. The clinical presentation, microscopic hematuria and proteinuria, and histopathology were consistent with the European Alliance of Associations for Rheumatology criteria for HSP.1
Although direct immunofluorescence typically is positive for IgA deposition on biopsies, it can be negative for IgA, especially in lesions that are biopsied more than 7 days after their appearance, as shown in our case; a negative IgA on immunofluorescence does not rule out HSP.4 Elevated serum IgA is seen in more than 50% of cases of HSP.11 Although the disease typically is self-limited, glucocorticoids are used if the disease course is prolonged or if there is evidence of kidney involvement.9 The unique combination of anti-Smith and anti-dsDNA antibodies as well as ANCAs associated with HSP with negative IgA on direct immunofluorescence has been reported with lupus.12 Clinicians should be aware of COVID-19 vaccine–associated HSP that is negative for IgA deposition and positive for anti-Smith and anti-dsDNA antibodies as well as ANCAs.
Acknowledgment—We thank our patient for granting permission to publish this information.
- Ozen S, Ruperto N, Dillon MJ, et al. EULAR/PReS endorsed consensus criteria for the classification of childhood vasculitides. Ann Rheum Dis. 2006;65:936-941. doi:10.1136/ard.2005.046300
- Rai A, Nast C, Adler S. Henoch–Schönlein purpura nephritis. J Am Soc Nephrol. 1999;10:2637-2644.
- Casini F, Magenes VC, De Sanctis M, et al. Henoch-Schönlein purpura following COVID-19 vaccine in a child: a case report. Ital J Pediatr. 2022;48:158. doi:10.1186/s13052-022-01351-1
- Poudel P, Adams SH, Mirchia K, et al. IgA negative immunofluorescence in diagnoses of adult-onset Henoch-Schönlein purpura. Proc (Bayl Univ Med Cent). 2020;33:436-437. doi:10.1080/08998280.2020.1770526
- Maronese CA, Zelin E, Avallone G, et al. Cutaneous vasculitis and vasculopathy in the era of COVID-19 pandemic. Front Med (Lausanne). 2022;9:996288. doi:10.3389/fmed.2022.996288
- Bryant MC, Spencer LT, Yalcindag A. A case of ANCA-associated vasculitis in a 16-year-old female following SARS-COV-2 infection and a systematic review of the literature. Pediatr Rheumatol Online J. 2022;20:65. doi:10.1186/s12969-022-00727-1
- Torraca PFS, Castro BC, Hans Filho G. Henoch-Schönlein purpura with c-ANCA antibody in adult. An Bras Dermatol. 2016;91:667-669. doi:10.1590/abd1806-4841.20164181
- Agabegi SS, Agabegi ED. Step-Up to Medicine. 4th ed. Wolters Kluwer; 2015.
- Ball-Burack MR, Kosowsky JM. A Case of leukocytoclastic vasculitis following SARS-CoV-2 vaccination. J Emerg Med. 2022;63:E62-E65. doi:10.1016/j.jemermed.2021.10.005
- Tan SW, Tam YC, Pang SM. Cutaneous reactions to COVID-19 vaccines: a review. JAAD Int. 2022;7:178-186. doi:10.1016/j.jdin.2022.01.011
- Calviño MC, Llorca J, García-Porrúa C, et al. Henoch-Schönlein purpura in children from northwestern Spain: a 20-year epidemiologic and clinical study. Medicine (Baltimore). 2001;80:279-290.
- Hu P, Huang BY, Zhang DD, et al. Henoch-Schönlein purpura in a pediatric patient with lupus. Arch Med Sci. 2017;13:689-690. doi:10.5114/aoms.2017.67288
- Ozen S, Ruperto N, Dillon MJ, et al. EULAR/PReS endorsed consensus criteria for the classification of childhood vasculitides. Ann Rheum Dis. 2006;65:936-941. doi:10.1136/ard.2005.046300
- Rai A, Nast C, Adler S. Henoch–Schönlein purpura nephritis. J Am Soc Nephrol. 1999;10:2637-2644.
- Casini F, Magenes VC, De Sanctis M, et al. Henoch-Schönlein purpura following COVID-19 vaccine in a child: a case report. Ital J Pediatr. 2022;48:158. doi:10.1186/s13052-022-01351-1
- Poudel P, Adams SH, Mirchia K, et al. IgA negative immunofluorescence in diagnoses of adult-onset Henoch-Schönlein purpura. Proc (Bayl Univ Med Cent). 2020;33:436-437. doi:10.1080/08998280.2020.1770526
- Maronese CA, Zelin E, Avallone G, et al. Cutaneous vasculitis and vasculopathy in the era of COVID-19 pandemic. Front Med (Lausanne). 2022;9:996288. doi:10.3389/fmed.2022.996288
- Bryant MC, Spencer LT, Yalcindag A. A case of ANCA-associated vasculitis in a 16-year-old female following SARS-COV-2 infection and a systematic review of the literature. Pediatr Rheumatol Online J. 2022;20:65. doi:10.1186/s12969-022-00727-1
- Torraca PFS, Castro BC, Hans Filho G. Henoch-Schönlein purpura with c-ANCA antibody in adult. An Bras Dermatol. 2016;91:667-669. doi:10.1590/abd1806-4841.20164181
- Agabegi SS, Agabegi ED. Step-Up to Medicine. 4th ed. Wolters Kluwer; 2015.
- Ball-Burack MR, Kosowsky JM. A Case of leukocytoclastic vasculitis following SARS-CoV-2 vaccination. J Emerg Med. 2022;63:E62-E65. doi:10.1016/j.jemermed.2021.10.005
- Tan SW, Tam YC, Pang SM. Cutaneous reactions to COVID-19 vaccines: a review. JAAD Int. 2022;7:178-186. doi:10.1016/j.jdin.2022.01.011
- Calviño MC, Llorca J, García-Porrúa C, et al. Henoch-Schönlein purpura in children from northwestern Spain: a 20-year epidemiologic and clinical study. Medicine (Baltimore). 2001;80:279-290.
- Hu P, Huang BY, Zhang DD, et al. Henoch-Schönlein purpura in a pediatric patient with lupus. Arch Med Sci. 2017;13:689-690. doi:10.5114/aoms.2017.67288
Practice Points
- Dermatologists should be vigilant for Henoch-Schönlein purpura (HSP) despite negative direct immunofluorescence of IgA deposition and unusual antibodies.
- Messenger RNA–based COVID-19 vaccines are associated with various cutaneous reactions, including HSP.
- Anti-Smith and anti–double-stranded DNA antibodies typically are not associated with HSP but may be seen in patients with coexisting systemic lupus erythematosus.
Multiple Keratoacanthomas Arising Within Red Tattoo Pigment
To the Editor:
Keratoacanthoma (KA)–type squamous cell carcinomas (SCCs) are rapidly evolving neoplasms of the epithelium that often spontaneously regress but rarely metastasize.1,2 Keratoacanthomas are thought to ascend from the hair follicle,1 and they clinically present as an enlarging solitary crateriform nodule with a keratin-filled center. Multiple KAs are rare2; histologically, KAs can be difficult to distinguish from conventional SCCs and are frequently treated by standard surgical excision.1 Reactive KAs are a subtype of KA that are induced by trauma including UV exposure, electromagnetic radiation, surgical procedures, chemical peels, laser treatments, and rarely tattoos.3-5
A 56-year-old man presented to the clinic with 3 asymptomatic enlarging papulonodules within a multicolored tattoo along the right forearm and elbow of 5 months’ duration (Figure 1). The lesions developed 1 month after the tattoo was placed and were localized to the areas of red pigment. The patient had several other tattoos. Histologic examination of the lesions revealed a well-differentiated squamous neoplasm with a crateriform invagination consistent with the superficial portion of a KA (Figures 2A–C). The specimen also revealed exogenous red pigment that was consistent with the background tattoo (Figure 2D). The patient underwent excisions of all 3 KAs, and free surgical margins were obtained.
Tattooing is a popular practice dating back to 3000
Cipollaro10 reported the first case of a KA in a tattoo in 1973. Although there have been reports of melanoma and basal cell carcinoma occurring within tattoos, KAs and conventional SCCs are the most common cutaneous neoplasms arising in tattoos.
The pathogenesis underlying the development of malignancies in tattoos is unclear. It has been hypothesized that trauma from tattooing may play a role given the temporal relationship between tattoo placement and malignancy development.11 Another theory is that tattoo pigment causes a chronic inflammatory foreign body reaction that triggers carcinogenesis.12 Lastly, it has been postulated that tattoo pigment may alter UV light absorption in the skin that could potentially impact mutagenesis.11
The most common treatment of KAs is standard surgical excision.4 Mohs micrographic surgery is an option if the KA is located in a cosmetically sensitive area. Although there are no reports of recurrence after excision of tattoo-related KAs, new KAs forming adjacent to a previously excised KA have been reported.13
Currently, tattoos are not regulated by the US Food and Drug Administration before going to market. Although many states regulate the practice of tattooing, few regulate the contents of tattoo ink, and ink is only investigated when safety issues arise.14 This case provides further evidence of an association between KAs, tattooing, and potentially carcinogenic pigments, especially in red dye, supporting the need for further research on the safety of pigment components and more regulation of tattoo ink.
- Schwartz RA. Keratoacanthoma: a clinico-pathologic enigma. Dermatol Surg. 2004;30:326-333.
- Kwiek B, Schwartz RA. Keratoacanthoma (KA): an update and review. J Am Acad Dermatol. 2016;74:1220-1233.
- McGrouther DA, Downie PA, Thompson WD. Reactions to red tattoos. Br J Plas Surg. 1977;30:84-85.
- Sowden JM, Byrne JP, Smith AG, et al. Red tattoo reactions: x-ray microanalysis and patch-test studies. Br J Dermatol. 1991;124:576-580.
- Wiener DA, Scher RK. Basal cell carcinoma arising in a tattoo. Cutis. 1987;39:125-126.
- Pesapane F, Nazzaro G, Gianotti R, et al. A short history of tattoo. JAMA Dermatol. 2014;150:145.
- Junqueira AL, Wanat, KA, Farah RS. Squamous neoplasms arising within tattoos: clinical presentation, histopathology and management. Clin Exp Dermatol. 2017;42:601-606.
- Tammaro A, Toniolo C, Giulianelli V, et al. Chemical research on red pigments after adverse reactions to tattoo. Eur Ann Allergy Clin Immunol. 2016;48:46-48.
- Forbat E, Al-Niaimi F. Patterns of reactions to red pigment tattoo and treatment methods. Dermatol Therapy (Heidelb). 2016;6:13-23.
- Cipollaro VA. Keratoacanthoma developing in a tattoo. Cutis. 1973;11:809.
- Kluger N, Koljonen V. Tattoos, inks, and cancer. Lancet Oncol. 2012;13:E161-E168.
- Müller KM, Schmitz I, Hupe-Nörenberg L. Reaction patterns to cutaneous particulate and ornamental tattoos. Pathologe. 2002;23:46-53.
- Maxim E, Higgins H, D’Souza L. A case of multiple squamous cell carcinomas arising from red tattoo pigment. Int J Womens Dermatol. 2017;3:228-230.
- MacDonald J. Why doesn’t the FDA regulate tattoo ink? JSTOR Daily. September 21, 2017. https://daily.jstor.org/why-doesnt-the-fda-regulate-tattoo-ink/. Accessed October 15, 2019.
To the Editor:
Keratoacanthoma (KA)–type squamous cell carcinomas (SCCs) are rapidly evolving neoplasms of the epithelium that often spontaneously regress but rarely metastasize.1,2 Keratoacanthomas are thought to ascend from the hair follicle,1 and they clinically present as an enlarging solitary crateriform nodule with a keratin-filled center. Multiple KAs are rare2; histologically, KAs can be difficult to distinguish from conventional SCCs and are frequently treated by standard surgical excision.1 Reactive KAs are a subtype of KA that are induced by trauma including UV exposure, electromagnetic radiation, surgical procedures, chemical peels, laser treatments, and rarely tattoos.3-5
A 56-year-old man presented to the clinic with 3 asymptomatic enlarging papulonodules within a multicolored tattoo along the right forearm and elbow of 5 months’ duration (Figure 1). The lesions developed 1 month after the tattoo was placed and were localized to the areas of red pigment. The patient had several other tattoos. Histologic examination of the lesions revealed a well-differentiated squamous neoplasm with a crateriform invagination consistent with the superficial portion of a KA (Figures 2A–C). The specimen also revealed exogenous red pigment that was consistent with the background tattoo (Figure 2D). The patient underwent excisions of all 3 KAs, and free surgical margins were obtained.
Tattooing is a popular practice dating back to 3000
Cipollaro10 reported the first case of a KA in a tattoo in 1973. Although there have been reports of melanoma and basal cell carcinoma occurring within tattoos, KAs and conventional SCCs are the most common cutaneous neoplasms arising in tattoos.
The pathogenesis underlying the development of malignancies in tattoos is unclear. It has been hypothesized that trauma from tattooing may play a role given the temporal relationship between tattoo placement and malignancy development.11 Another theory is that tattoo pigment causes a chronic inflammatory foreign body reaction that triggers carcinogenesis.12 Lastly, it has been postulated that tattoo pigment may alter UV light absorption in the skin that could potentially impact mutagenesis.11
The most common treatment of KAs is standard surgical excision.4 Mohs micrographic surgery is an option if the KA is located in a cosmetically sensitive area. Although there are no reports of recurrence after excision of tattoo-related KAs, new KAs forming adjacent to a previously excised KA have been reported.13
Currently, tattoos are not regulated by the US Food and Drug Administration before going to market. Although many states regulate the practice of tattooing, few regulate the contents of tattoo ink, and ink is only investigated when safety issues arise.14 This case provides further evidence of an association between KAs, tattooing, and potentially carcinogenic pigments, especially in red dye, supporting the need for further research on the safety of pigment components and more regulation of tattoo ink.
To the Editor:
Keratoacanthoma (KA)–type squamous cell carcinomas (SCCs) are rapidly evolving neoplasms of the epithelium that often spontaneously regress but rarely metastasize.1,2 Keratoacanthomas are thought to ascend from the hair follicle,1 and they clinically present as an enlarging solitary crateriform nodule with a keratin-filled center. Multiple KAs are rare2; histologically, KAs can be difficult to distinguish from conventional SCCs and are frequently treated by standard surgical excision.1 Reactive KAs are a subtype of KA that are induced by trauma including UV exposure, electromagnetic radiation, surgical procedures, chemical peels, laser treatments, and rarely tattoos.3-5
A 56-year-old man presented to the clinic with 3 asymptomatic enlarging papulonodules within a multicolored tattoo along the right forearm and elbow of 5 months’ duration (Figure 1). The lesions developed 1 month after the tattoo was placed and were localized to the areas of red pigment. The patient had several other tattoos. Histologic examination of the lesions revealed a well-differentiated squamous neoplasm with a crateriform invagination consistent with the superficial portion of a KA (Figures 2A–C). The specimen also revealed exogenous red pigment that was consistent with the background tattoo (Figure 2D). The patient underwent excisions of all 3 KAs, and free surgical margins were obtained.
Tattooing is a popular practice dating back to 3000
Cipollaro10 reported the first case of a KA in a tattoo in 1973. Although there have been reports of melanoma and basal cell carcinoma occurring within tattoos, KAs and conventional SCCs are the most common cutaneous neoplasms arising in tattoos.
The pathogenesis underlying the development of malignancies in tattoos is unclear. It has been hypothesized that trauma from tattooing may play a role given the temporal relationship between tattoo placement and malignancy development.11 Another theory is that tattoo pigment causes a chronic inflammatory foreign body reaction that triggers carcinogenesis.12 Lastly, it has been postulated that tattoo pigment may alter UV light absorption in the skin that could potentially impact mutagenesis.11
The most common treatment of KAs is standard surgical excision.4 Mohs micrographic surgery is an option if the KA is located in a cosmetically sensitive area. Although there are no reports of recurrence after excision of tattoo-related KAs, new KAs forming adjacent to a previously excised KA have been reported.13
Currently, tattoos are not regulated by the US Food and Drug Administration before going to market. Although many states regulate the practice of tattooing, few regulate the contents of tattoo ink, and ink is only investigated when safety issues arise.14 This case provides further evidence of an association between KAs, tattooing, and potentially carcinogenic pigments, especially in red dye, supporting the need for further research on the safety of pigment components and more regulation of tattoo ink.
- Schwartz RA. Keratoacanthoma: a clinico-pathologic enigma. Dermatol Surg. 2004;30:326-333.
- Kwiek B, Schwartz RA. Keratoacanthoma (KA): an update and review. J Am Acad Dermatol. 2016;74:1220-1233.
- McGrouther DA, Downie PA, Thompson WD. Reactions to red tattoos. Br J Plas Surg. 1977;30:84-85.
- Sowden JM, Byrne JP, Smith AG, et al. Red tattoo reactions: x-ray microanalysis and patch-test studies. Br J Dermatol. 1991;124:576-580.
- Wiener DA, Scher RK. Basal cell carcinoma arising in a tattoo. Cutis. 1987;39:125-126.
- Pesapane F, Nazzaro G, Gianotti R, et al. A short history of tattoo. JAMA Dermatol. 2014;150:145.
- Junqueira AL, Wanat, KA, Farah RS. Squamous neoplasms arising within tattoos: clinical presentation, histopathology and management. Clin Exp Dermatol. 2017;42:601-606.
- Tammaro A, Toniolo C, Giulianelli V, et al. Chemical research on red pigments after adverse reactions to tattoo. Eur Ann Allergy Clin Immunol. 2016;48:46-48.
- Forbat E, Al-Niaimi F. Patterns of reactions to red pigment tattoo and treatment methods. Dermatol Therapy (Heidelb). 2016;6:13-23.
- Cipollaro VA. Keratoacanthoma developing in a tattoo. Cutis. 1973;11:809.
- Kluger N, Koljonen V. Tattoos, inks, and cancer. Lancet Oncol. 2012;13:E161-E168.
- Müller KM, Schmitz I, Hupe-Nörenberg L. Reaction patterns to cutaneous particulate and ornamental tattoos. Pathologe. 2002;23:46-53.
- Maxim E, Higgins H, D’Souza L. A case of multiple squamous cell carcinomas arising from red tattoo pigment. Int J Womens Dermatol. 2017;3:228-230.
- MacDonald J. Why doesn’t the FDA regulate tattoo ink? JSTOR Daily. September 21, 2017. https://daily.jstor.org/why-doesnt-the-fda-regulate-tattoo-ink/. Accessed October 15, 2019.
- Schwartz RA. Keratoacanthoma: a clinico-pathologic enigma. Dermatol Surg. 2004;30:326-333.
- Kwiek B, Schwartz RA. Keratoacanthoma (KA): an update and review. J Am Acad Dermatol. 2016;74:1220-1233.
- McGrouther DA, Downie PA, Thompson WD. Reactions to red tattoos. Br J Plas Surg. 1977;30:84-85.
- Sowden JM, Byrne JP, Smith AG, et al. Red tattoo reactions: x-ray microanalysis and patch-test studies. Br J Dermatol. 1991;124:576-580.
- Wiener DA, Scher RK. Basal cell carcinoma arising in a tattoo. Cutis. 1987;39:125-126.
- Pesapane F, Nazzaro G, Gianotti R, et al. A short history of tattoo. JAMA Dermatol. 2014;150:145.
- Junqueira AL, Wanat, KA, Farah RS. Squamous neoplasms arising within tattoos: clinical presentation, histopathology and management. Clin Exp Dermatol. 2017;42:601-606.
- Tammaro A, Toniolo C, Giulianelli V, et al. Chemical research on red pigments after adverse reactions to tattoo. Eur Ann Allergy Clin Immunol. 2016;48:46-48.
- Forbat E, Al-Niaimi F. Patterns of reactions to red pigment tattoo and treatment methods. Dermatol Therapy (Heidelb). 2016;6:13-23.
- Cipollaro VA. Keratoacanthoma developing in a tattoo. Cutis. 1973;11:809.
- Kluger N, Koljonen V. Tattoos, inks, and cancer. Lancet Oncol. 2012;13:E161-E168.
- Müller KM, Schmitz I, Hupe-Nörenberg L. Reaction patterns to cutaneous particulate and ornamental tattoos. Pathologe. 2002;23:46-53.
- Maxim E, Higgins H, D’Souza L. A case of multiple squamous cell carcinomas arising from red tattoo pigment. Int J Womens Dermatol. 2017;3:228-230.
- MacDonald J. Why doesn’t the FDA regulate tattoo ink? JSTOR Daily. September 21, 2017. https://daily.jstor.org/why-doesnt-the-fda-regulate-tattoo-ink/. Accessed October 15, 2019.
Practice Points
- Tattoo reactions range from infectious and inflammatory dermatoses to the development of malignant neoplasms.
- Red pigment is the most common cause of adverse tattoo reactions.
- The management of tattoo-associated keratoacanthoma (KA)–type squamous cell carcinomas (SCCs) has not been widely published, but they can be approached similarly to nontattoo-associated KA-SCCs.
Cystic Nodule on the Palm
The Diagnosis: Nodular Hidradenoma
Nodular hidradenomas (NHs) are rare benign cutaneous adnexal neoplasms first described in 1949 as clear cell papillary carcinomas.1 Since then, various terms have been used to describe this entity, such as eccrine acrospiroma, solid-cystic hidradenoma, and clear cell hidradenoma.2 Review of the literature revealed a female predominance (2:1 ratio) and a mean age at presentation of 37.2 years.3,4 Nodular hidradenoma presents as an asymptomatic, solitary, mobile, firm nodule with intact overlying skin. Rarely, multiple nodules may occur.3 Some tumors display ulceration and serous fluid leakage.5 They occur most commonly on the scalp, face, and upper extremities with an average size of 2 cm.3 Rapid growth of the tumor may signal a malignant change.6
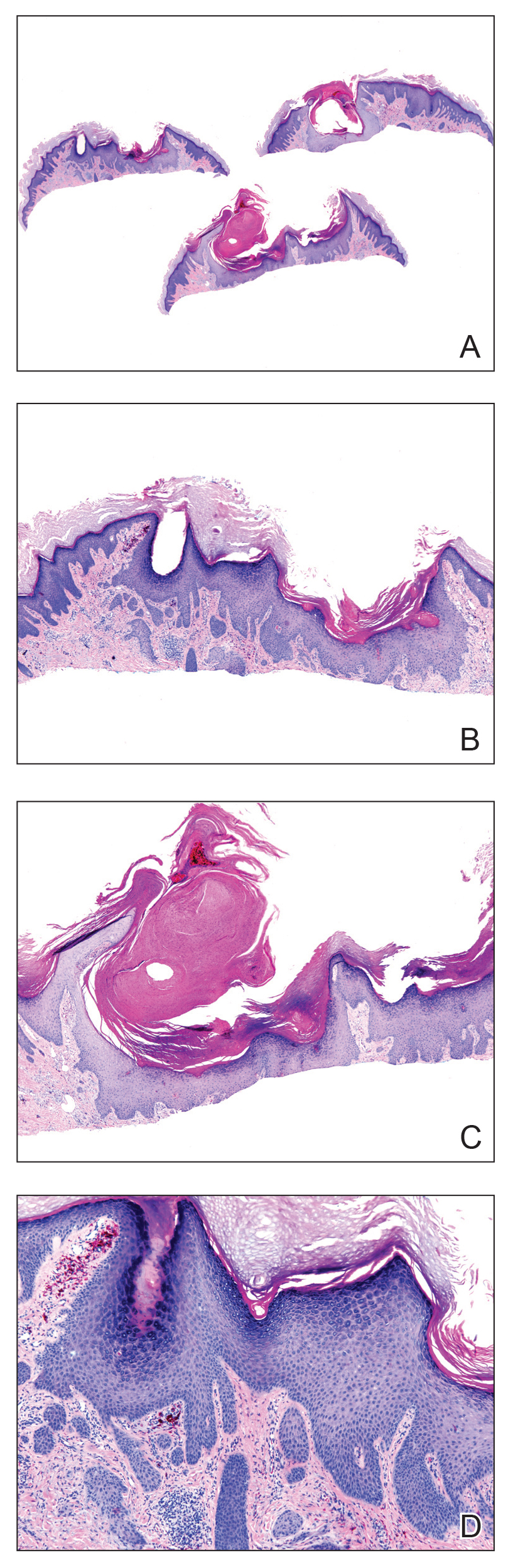
Histopathology reveals a lobulated, circumscribed, symmetrical tumor with dermal nests of epithelial cells that are polygonal with eosinophilic cytoplasm forming ductlike spaces (Figure). However, clear cell changes and squamous differentiation may be prominent features. Cystic spaces may result from tumor cell degeneration. Most tumors are encased by collagenous fibrous tissue and rarely have epidermal attachments.3
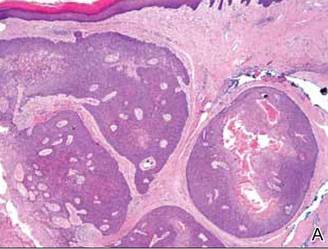 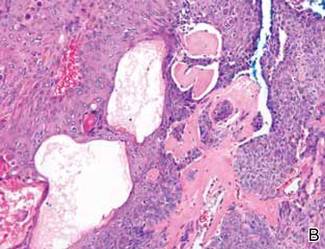 Anastomosing aggregates of squamous cells forming ductlike spaces were viewed on low-power magnification (A)(H&E, original magnification ×10). On higher power there were ductlike spaces and eosinophilic hyalinized stroma entrapped by the bland-appearing squamous proliferation (B)(H&E, original magnification ×20). |
Nodular hidradenoma traditionally has been considered to be of eccrine origin, but more recent literature indicates that the majority of NHs are of apocrine origin. Histologically, apocrine tumors display eosinophilic secretion, mucinous epithelium, squamous or sebaceous differentiation, and decapitation secretion, whereas eccrine tumors are identified by their lack of specific features.3
Nodular hidradenoma may recur after excision. Malignant transformation is rare. In one review, 6.7% (6/89) of NHs were malignant, characterized by abnormal mitoses, nuclear atypia, and necrosis.4 Malignant NH or nodular hidradenocarcinoma behaves aggressively with up to an 86% local recurrence and 60% rate of metastasis within 2 years.6 Survival time is inversely proportional to the size of the tumor and is generally poor, with a 5-year disease-free survival of less than 30%.6,7
Treatment of NH is achieved through primary excision or Mohs micrographic surgery; however, treatment of nodular hidradenocarcinoma is controversial and typically begins with wide local excision but may involve lymph node dissection if necessary. Use of adjuvant chemotherapy and radiation therapy for metastases warrants more clinical studies, as it is a rare occurrence.6 Our patient planned to undergo a total excision of the benign nodule once she healed from the biopsy; however, she was lost to follow-up, as she moved out of state.
1. Lui Y. The histogenesis of clear cell papillary carcinoma of the skin. Am J Pathol. 1949;25:93-103.
2. Obaidat NA, Khaled OA, Ghazarian D. Skin adnexal neoplasms–part 2: an approach to tumours of cutaneous sweat glands. J Clin Pathol. 2007;60:145-159.
3. Nandeesh BN, Rajalakshmi T. A study of histopathologic spectrum of nodular hidradenoma. Am J Dermatopathol. 2012;34:461-470.
4. Hernández-Pérez E, Cestoni-Parducci R. Nodular hidradenoma and hidradenocarcinoma: a 10-year review. J Am Acad Dermatol. 1985;12:15-20.
5. Sirinoglu H, Celebiler O. Benign nodular hidradenoma of the face. J Craniofac Surg. 2011;22:750-751.
6. Souvatzidis P, Sbano P, Mandato F, et al. Malignant nodular hidradenoma of the skin: report of seven cases. J Eur Acad Dermatol Venereol. 2008;22:549-554.
7. Ko CJ, Cochran AJ, Eng W, et al. Hidradenocarcinoma: a histological and immunohistochemical study. J Cutan Pathol. 2006;33:726-730.
The Diagnosis: Nodular Hidradenoma
Nodular hidradenomas (NHs) are rare benign cutaneous adnexal neoplasms first described in 1949 as clear cell papillary carcinomas.1 Since then, various terms have been used to describe this entity, such as eccrine acrospiroma, solid-cystic hidradenoma, and clear cell hidradenoma.2 Review of the literature revealed a female predominance (2:1 ratio) and a mean age at presentation of 37.2 years.3,4 Nodular hidradenoma presents as an asymptomatic, solitary, mobile, firm nodule with intact overlying skin. Rarely, multiple nodules may occur.3 Some tumors display ulceration and serous fluid leakage.5 They occur most commonly on the scalp, face, and upper extremities with an average size of 2 cm.3 Rapid growth of the tumor may signal a malignant change.6
Histopathology reveals a lobulated, circumscribed, symmetrical tumor with dermal nests of epithelial cells that are polygonal with eosinophilic cytoplasm forming ductlike spaces (Figure). However, clear cell changes and squamous differentiation may be prominent features. Cystic spaces may result from tumor cell degeneration. Most tumors are encased by collagenous fibrous tissue and rarely have epidermal attachments.3
  Anastomosing aggregates of squamous cells forming ductlike spaces were viewed on low-power magnification (A)(H&E, original magnification ×10). On higher power there were ductlike spaces and eosinophilic hyalinized stroma entrapped by the bland-appearing squamous proliferation (B)(H&E, original magnification ×20). |
Nodular hidradenoma traditionally has been considered to be of eccrine origin, but more recent literature indicates that the majority of NHs are of apocrine origin. Histologically, apocrine tumors display eosinophilic secretion, mucinous epithelium, squamous or sebaceous differentiation, and decapitation secretion, whereas eccrine tumors are identified by their lack of specific features.3
Nodular hidradenoma may recur after excision. Malignant transformation is rare. In one review, 6.7% (6/89) of NHs were malignant, characterized by abnormal mitoses, nuclear atypia, and necrosis.4 Malignant NH or nodular hidradenocarcinoma behaves aggressively with up to an 86% local recurrence and 60% rate of metastasis within 2 years.6 Survival time is inversely proportional to the size of the tumor and is generally poor, with a 5-year disease-free survival of less than 30%.6,7
Treatment of NH is achieved through primary excision or Mohs micrographic surgery; however, treatment of nodular hidradenocarcinoma is controversial and typically begins with wide local excision but may involve lymph node dissection if necessary. Use of adjuvant chemotherapy and radiation therapy for metastases warrants more clinical studies, as it is a rare occurrence.6 Our patient planned to undergo a total excision of the benign nodule once she healed from the biopsy; however, she was lost to follow-up, as she moved out of state.
The Diagnosis: Nodular Hidradenoma
Nodular hidradenomas (NHs) are rare benign cutaneous adnexal neoplasms first described in 1949 as clear cell papillary carcinomas.1 Since then, various terms have been used to describe this entity, such as eccrine acrospiroma, solid-cystic hidradenoma, and clear cell hidradenoma.2 Review of the literature revealed a female predominance (2:1 ratio) and a mean age at presentation of 37.2 years.3,4 Nodular hidradenoma presents as an asymptomatic, solitary, mobile, firm nodule with intact overlying skin. Rarely, multiple nodules may occur.3 Some tumors display ulceration and serous fluid leakage.5 They occur most commonly on the scalp, face, and upper extremities with an average size of 2 cm.3 Rapid growth of the tumor may signal a malignant change.6
Histopathology reveals a lobulated, circumscribed, symmetrical tumor with dermal nests of epithelial cells that are polygonal with eosinophilic cytoplasm forming ductlike spaces (Figure). However, clear cell changes and squamous differentiation may be prominent features. Cystic spaces may result from tumor cell degeneration. Most tumors are encased by collagenous fibrous tissue and rarely have epidermal attachments.3
  Anastomosing aggregates of squamous cells forming ductlike spaces were viewed on low-power magnification (A)(H&E, original magnification ×10). On higher power there were ductlike spaces and eosinophilic hyalinized stroma entrapped by the bland-appearing squamous proliferation (B)(H&E, original magnification ×20). |
Nodular hidradenoma traditionally has been considered to be of eccrine origin, but more recent literature indicates that the majority of NHs are of apocrine origin. Histologically, apocrine tumors display eosinophilic secretion, mucinous epithelium, squamous or sebaceous differentiation, and decapitation secretion, whereas eccrine tumors are identified by their lack of specific features.3
Nodular hidradenoma may recur after excision. Malignant transformation is rare. In one review, 6.7% (6/89) of NHs were malignant, characterized by abnormal mitoses, nuclear atypia, and necrosis.4 Malignant NH or nodular hidradenocarcinoma behaves aggressively with up to an 86% local recurrence and 60% rate of metastasis within 2 years.6 Survival time is inversely proportional to the size of the tumor and is generally poor, with a 5-year disease-free survival of less than 30%.6,7
Treatment of NH is achieved through primary excision or Mohs micrographic surgery; however, treatment of nodular hidradenocarcinoma is controversial and typically begins with wide local excision but may involve lymph node dissection if necessary. Use of adjuvant chemotherapy and radiation therapy for metastases warrants more clinical studies, as it is a rare occurrence.6 Our patient planned to undergo a total excision of the benign nodule once she healed from the biopsy; however, she was lost to follow-up, as she moved out of state.
1. Lui Y. The histogenesis of clear cell papillary carcinoma of the skin. Am J Pathol. 1949;25:93-103.
2. Obaidat NA, Khaled OA, Ghazarian D. Skin adnexal neoplasms–part 2: an approach to tumours of cutaneous sweat glands. J Clin Pathol. 2007;60:145-159.
3. Nandeesh BN, Rajalakshmi T. A study of histopathologic spectrum of nodular hidradenoma. Am J Dermatopathol. 2012;34:461-470.
4. Hernández-Pérez E, Cestoni-Parducci R. Nodular hidradenoma and hidradenocarcinoma: a 10-year review. J Am Acad Dermatol. 1985;12:15-20.
5. Sirinoglu H, Celebiler O. Benign nodular hidradenoma of the face. J Craniofac Surg. 2011;22:750-751.
6. Souvatzidis P, Sbano P, Mandato F, et al. Malignant nodular hidradenoma of the skin: report of seven cases. J Eur Acad Dermatol Venereol. 2008;22:549-554.
7. Ko CJ, Cochran AJ, Eng W, et al. Hidradenocarcinoma: a histological and immunohistochemical study. J Cutan Pathol. 2006;33:726-730.
1. Lui Y. The histogenesis of clear cell papillary carcinoma of the skin. Am J Pathol. 1949;25:93-103.
2. Obaidat NA, Khaled OA, Ghazarian D. Skin adnexal neoplasms–part 2: an approach to tumours of cutaneous sweat glands. J Clin Pathol. 2007;60:145-159.
3. Nandeesh BN, Rajalakshmi T. A study of histopathologic spectrum of nodular hidradenoma. Am J Dermatopathol. 2012;34:461-470.
4. Hernández-Pérez E, Cestoni-Parducci R. Nodular hidradenoma and hidradenocarcinoma: a 10-year review. J Am Acad Dermatol. 1985;12:15-20.
5. Sirinoglu H, Celebiler O. Benign nodular hidradenoma of the face. J Craniofac Surg. 2011;22:750-751.
6. Souvatzidis P, Sbano P, Mandato F, et al. Malignant nodular hidradenoma of the skin: report of seven cases. J Eur Acad Dermatol Venereol. 2008;22:549-554.
7. Ko CJ, Cochran AJ, Eng W, et al. Hidradenocarcinoma: a histological and immunohistochemical study. J Cutan Pathol. 2006;33:726-730.
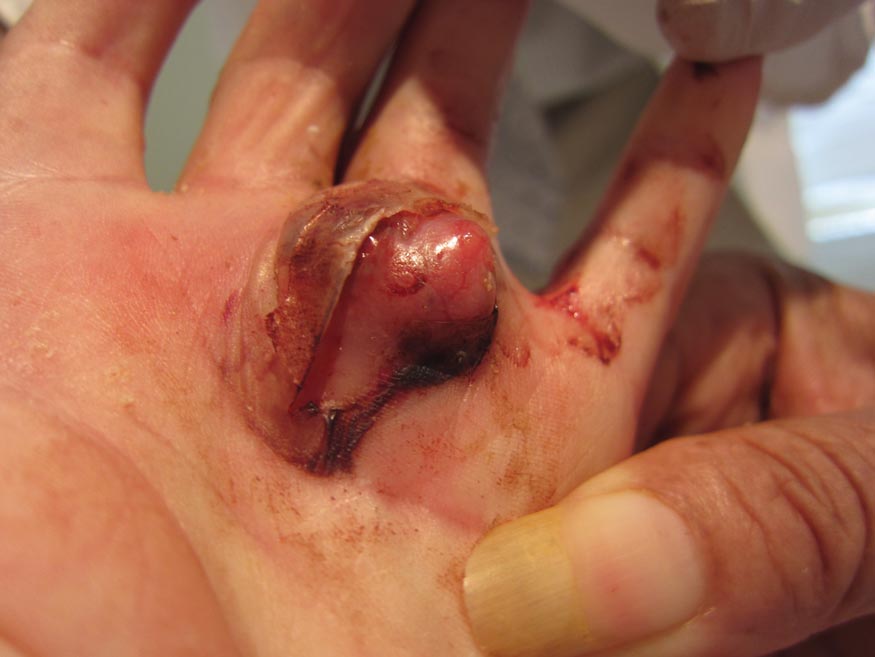
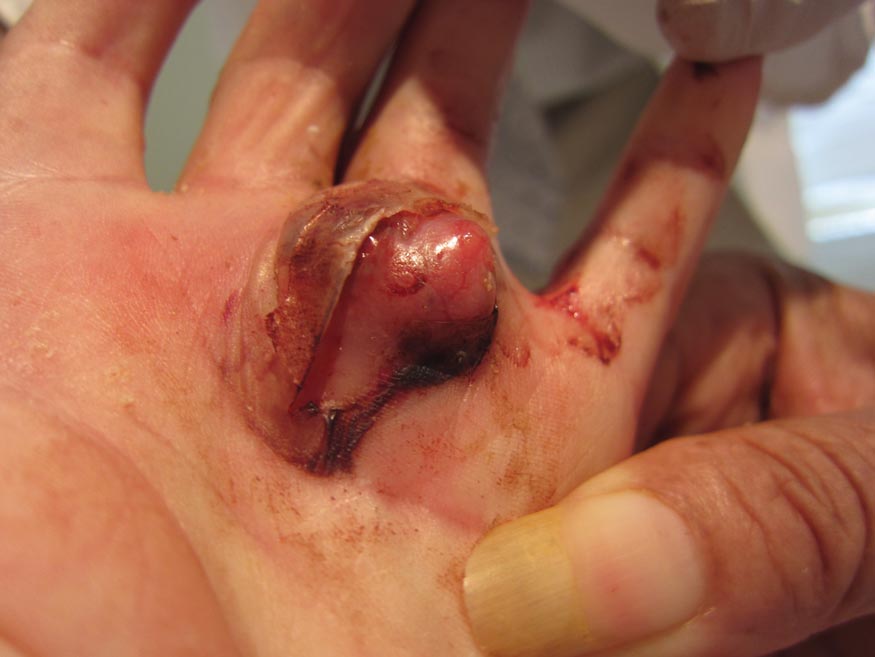
A 73-year-old woman with a history of multiple strokes with residual left-sided motor deficits and resultant left-hand contracture, type 2 diabetes mellitus, hypertension, and a remote history of treated colon cancer and breast cancer presented with hypertensive urgency and neck pain. Upon admission, the nursing staff found an “unusual growth” on the patient’s left hand. Dermatology was consulted and a 2×1.5×1.5-cm multilobulated, malodorous, slightly tender, nonfluctuant, gelatinous, mobile, cystic nodule overlying the fourth metacarpal palmar head was examined. The patient reported the lesion was present for more than a year. Imaging was pursued, but radiography, ultrasonography, and magnetic resonance imaging could not be performed adequately due to the patient’s severe contracture. Given the extensive differential diagnoses, an orthopedic hand surgeon performed a large incisional biopsy to obtain tissue diagnosis.
Indurated Erythematous Papules and Plaques on the Forearm
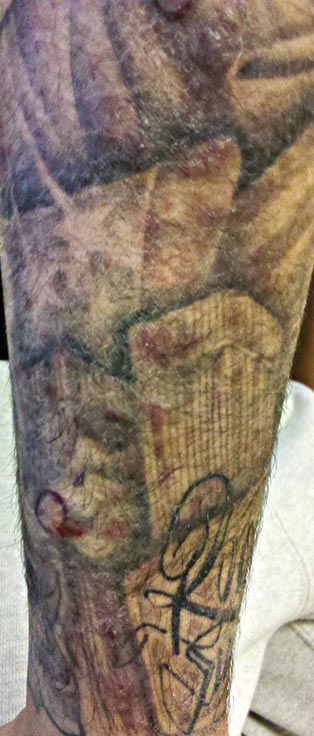
The Diagnosis: Mycobacterium chelonae Arising Within a Tattoo
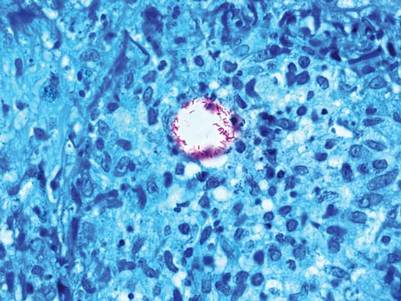
A 3-mm punch biopsy specimen was obtained from one of the plaques. Histopathology revealed an unremarkable epidermis with granulomatous collections of epithelioid histiocytes in association with neutrophils and lymphocytes in the dermis (Figure 1). Periodic acid–Schiff stain was negative for fungal organisms. Gram stain was negative for bacteria. Fite stain was positive for acid-fast bacilli (Figure 2). Clinical and histopathologic findings led to the initial diagnosis of a mycobacterial infection within the tattoo. The patient was empirically started on oral doxycycline 100 mg twice daily and oral clarithromycin 500 mg twice daily with mild improvement of the lesions over the next month. After 6 weeks the mycobacterial cultures were persistently negative and high-performance liquid chromatography was performed verifying the presence of Mycobacterium chelonae. Clarithromycin was continued and the doxycycline was replaced with oral trimethoprim-sulfamethoxazole (double strength) twice daily due to resistance. The patient was referred to an infectious disease specialist who concurred with the treatment regimen for a duration of 6 months to a year. A chest radiograph also was performed to rule out disseminated disease. Several months later the patient’s close friend presented with a similar infection that was acquired on the same day as our patient at the same tattoo parlor. The New York City Department of Health and Mental Hygiene was notified about these cases and informed us of other cases in New York State linked to contaminated tattoo ink.
|
Mycobacterium chelonae is a rapidly growing nontuberculous mycobacteria (Runyon group IV) that is found in nature and contaminated sources such as soil, lakes, sewage, and tap water.1 Inoculation ofM chelonae through contaminated instruments leads to the formation of painful lesions, abscesses, fistulas, and granulomas that are extremely difficult to treat.2 In our patient, M chelonae was most likely transmitted via contaminated tap water that was used to dilute the black tattoo ink to yield a gray color. Alternative sources are the ink itself or the container used to mix the ink.3Mycobacterium chelonae can cause infections in the skin, lungs, joints, bones, and eyes.4 With the exception of lung disease, trauma is the usual inciting factor. Disseminated infections are almost exclusively found in immunosuppressed individuals. Mycobacterium chelonae is typically found to grow on culture within 7 days. However, in our case there was no growth after 6 weeks of incubation. High-performance liquid chromatography analysis of mycolic acid is an alternative method of identifying mycobacteria. This technique verified the presence of M chelonae in our case when cultures were persistently negative.5
Mycobacterium chelonae is difficult to treat. The most common antibiotics used for treatment are clarithromycin, azithromycin, doxycycline, and linezolid.6 Studies of various antibiotics have shown that clarithromycin is the most effective macrolide against M chelonae.7 Tetracyclines also were studied in their effectiveness at treating nontuberculous mycobacteria infections but were found to have increased resistance to M chelonae.8 Although treatment regimens vary, the highest success rates are achieved with a minimum of 6 months of therapy using at least 2 drugs. Longer treatment is recommended for immunocompromised individuals.9
The case we present is important from a public health perspective. The tattoo industry and ink manufacturers should bemade aware of the risks of various infections from nonsterile techniques. Tattoo parlor employees should be advised of the risks of using nonsterile water for ink dilution and cleaning tattoo equipment. They also should be continuously educated on aseptic techniques.
1. Lee RP, Cheung KW, Chiu KH, et al. Mycobacterium chelonae infection after total knee arthroplasty: a case report. J Orthop Surg (Hong Kong). 2012;20:134-136.
2. Camargo D, Saad C, Ruiz F, et al. latrogenic outbreak of M. chelonae skin abscesses. Epidemiol Infect. 1996;117:113-119.
3. Rodríguez-Blanco I, Fernández LC, Suárez-Peñaranda JM, et al. Mycobacterium chelonae infection associated with tattoos. Acta Derm Venereol. 2011;91:61-62.
4. Karak K, Bhattacharyya S, Majumdar S, et al. Pulmonary infections caused by mycobacteria other than M. tuberculosis in and around Calcutta. Indian J Pathol Microbiol. 1996;39:131-134.
5. Butler WR, Floyd MM, Silcox V, et al. Standardized Method for HPLC Identification of Mycobacteria. Atlanta, GA: Centers for Disease Control and Prevention, US Department of Health and Human Services; 1996.
6. Brown-Elliot BA, Wallace RJ Jr, Blinkhorn R, et al. Successful treatment of disseminated Mycobacterium chelonae infection with linezolid. Clin Infect Dis. 2001;33:1433-1434.
7. Brown BA, Wallace RJ Jr, Onyi GO, et al. Activities of four macrolides, including clarithromycin, against Mycobacterium fortuitum, Mycobacterium chelonae, and Mycobacterium chelonae-like organisms. Antimicrob Agents Chemother. 1992;36:180-184.
8. Swenson JM, Wallace RJ Jr, Silcox VA, et al. Antimicrobial susceptibility of five subgroups of Mycobacterium fortuitum and Mycobacterium chelonae. Antimicrob Agents Chemother. 1985;28:807-811.
9. Leung YY, Choi KW, Ho KM, et al. Disseminated cutaneous infection with Mycobacterium chelonae mimicking panniculitis in a patient with dermatomyositis. Hong Kong Med J. 2005;11:515-519.
The Diagnosis: Mycobacterium chelonae Arising Within a Tattoo
A 3-mm punch biopsy specimen was obtained from one of the plaques. Histopathology revealed an unremarkable epidermis with granulomatous collections of epithelioid histiocytes in association with neutrophils and lymphocytes in the dermis (Figure 1). Periodic acid–Schiff stain was negative for fungal organisms. Gram stain was negative for bacteria. Fite stain was positive for acid-fast bacilli (Figure 2). Clinical and histopathologic findings led to the initial diagnosis of a mycobacterial infection within the tattoo. The patient was empirically started on oral doxycycline 100 mg twice daily and oral clarithromycin 500 mg twice daily with mild improvement of the lesions over the next month. After 6 weeks the mycobacterial cultures were persistently negative and high-performance liquid chromatography was performed verifying the presence of Mycobacterium chelonae. Clarithromycin was continued and the doxycycline was replaced with oral trimethoprim-sulfamethoxazole (double strength) twice daily due to resistance. The patient was referred to an infectious disease specialist who concurred with the treatment regimen for a duration of 6 months to a year. A chest radiograph also was performed to rule out disseminated disease. Several months later the patient’s close friend presented with a similar infection that was acquired on the same day as our patient at the same tattoo parlor. The New York City Department of Health and Mental Hygiene was notified about these cases and informed us of other cases in New York State linked to contaminated tattoo ink.
|
Mycobacterium chelonae is a rapidly growing nontuberculous mycobacteria (Runyon group IV) that is found in nature and contaminated sources such as soil, lakes, sewage, and tap water.1 Inoculation ofM chelonae through contaminated instruments leads to the formation of painful lesions, abscesses, fistulas, and granulomas that are extremely difficult to treat.2 In our patient, M chelonae was most likely transmitted via contaminated tap water that was used to dilute the black tattoo ink to yield a gray color. Alternative sources are the ink itself or the container used to mix the ink.3Mycobacterium chelonae can cause infections in the skin, lungs, joints, bones, and eyes.4 With the exception of lung disease, trauma is the usual inciting factor. Disseminated infections are almost exclusively found in immunosuppressed individuals. Mycobacterium chelonae is typically found to grow on culture within 7 days. However, in our case there was no growth after 6 weeks of incubation. High-performance liquid chromatography analysis of mycolic acid is an alternative method of identifying mycobacteria. This technique verified the presence of M chelonae in our case when cultures were persistently negative.5
Mycobacterium chelonae is difficult to treat. The most common antibiotics used for treatment are clarithromycin, azithromycin, doxycycline, and linezolid.6 Studies of various antibiotics have shown that clarithromycin is the most effective macrolide against M chelonae.7 Tetracyclines also were studied in their effectiveness at treating nontuberculous mycobacteria infections but were found to have increased resistance to M chelonae.8 Although treatment regimens vary, the highest success rates are achieved with a minimum of 6 months of therapy using at least 2 drugs. Longer treatment is recommended for immunocompromised individuals.9
The case we present is important from a public health perspective. The tattoo industry and ink manufacturers should bemade aware of the risks of various infections from nonsterile techniques. Tattoo parlor employees should be advised of the risks of using nonsterile water for ink dilution and cleaning tattoo equipment. They also should be continuously educated on aseptic techniques.
The Diagnosis: Mycobacterium chelonae Arising Within a Tattoo
A 3-mm punch biopsy specimen was obtained from one of the plaques. Histopathology revealed an unremarkable epidermis with granulomatous collections of epithelioid histiocytes in association with neutrophils and lymphocytes in the dermis (Figure 1). Periodic acid–Schiff stain was negative for fungal organisms. Gram stain was negative for bacteria. Fite stain was positive for acid-fast bacilli (Figure 2). Clinical and histopathologic findings led to the initial diagnosis of a mycobacterial infection within the tattoo. The patient was empirically started on oral doxycycline 100 mg twice daily and oral clarithromycin 500 mg twice daily with mild improvement of the lesions over the next month. After 6 weeks the mycobacterial cultures were persistently negative and high-performance liquid chromatography was performed verifying the presence of Mycobacterium chelonae. Clarithromycin was continued and the doxycycline was replaced with oral trimethoprim-sulfamethoxazole (double strength) twice daily due to resistance. The patient was referred to an infectious disease specialist who concurred with the treatment regimen for a duration of 6 months to a year. A chest radiograph also was performed to rule out disseminated disease. Several months later the patient’s close friend presented with a similar infection that was acquired on the same day as our patient at the same tattoo parlor. The New York City Department of Health and Mental Hygiene was notified about these cases and informed us of other cases in New York State linked to contaminated tattoo ink.
|
Mycobacterium chelonae is a rapidly growing nontuberculous mycobacteria (Runyon group IV) that is found in nature and contaminated sources such as soil, lakes, sewage, and tap water.1 Inoculation ofM chelonae through contaminated instruments leads to the formation of painful lesions, abscesses, fistulas, and granulomas that are extremely difficult to treat.2 In our patient, M chelonae was most likely transmitted via contaminated tap water that was used to dilute the black tattoo ink to yield a gray color. Alternative sources are the ink itself or the container used to mix the ink.3Mycobacterium chelonae can cause infections in the skin, lungs, joints, bones, and eyes.4 With the exception of lung disease, trauma is the usual inciting factor. Disseminated infections are almost exclusively found in immunosuppressed individuals. Mycobacterium chelonae is typically found to grow on culture within 7 days. However, in our case there was no growth after 6 weeks of incubation. High-performance liquid chromatography analysis of mycolic acid is an alternative method of identifying mycobacteria. This technique verified the presence of M chelonae in our case when cultures were persistently negative.5
Mycobacterium chelonae is difficult to treat. The most common antibiotics used for treatment are clarithromycin, azithromycin, doxycycline, and linezolid.6 Studies of various antibiotics have shown that clarithromycin is the most effective macrolide against M chelonae.7 Tetracyclines also were studied in their effectiveness at treating nontuberculous mycobacteria infections but were found to have increased resistance to M chelonae.8 Although treatment regimens vary, the highest success rates are achieved with a minimum of 6 months of therapy using at least 2 drugs. Longer treatment is recommended for immunocompromised individuals.9
The case we present is important from a public health perspective. The tattoo industry and ink manufacturers should bemade aware of the risks of various infections from nonsterile techniques. Tattoo parlor employees should be advised of the risks of using nonsterile water for ink dilution and cleaning tattoo equipment. They also should be continuously educated on aseptic techniques.
1. Lee RP, Cheung KW, Chiu KH, et al. Mycobacterium chelonae infection after total knee arthroplasty: a case report. J Orthop Surg (Hong Kong). 2012;20:134-136.
2. Camargo D, Saad C, Ruiz F, et al. latrogenic outbreak of M. chelonae skin abscesses. Epidemiol Infect. 1996;117:113-119.
3. Rodríguez-Blanco I, Fernández LC, Suárez-Peñaranda JM, et al. Mycobacterium chelonae infection associated with tattoos. Acta Derm Venereol. 2011;91:61-62.
4. Karak K, Bhattacharyya S, Majumdar S, et al. Pulmonary infections caused by mycobacteria other than M. tuberculosis in and around Calcutta. Indian J Pathol Microbiol. 1996;39:131-134.
5. Butler WR, Floyd MM, Silcox V, et al. Standardized Method for HPLC Identification of Mycobacteria. Atlanta, GA: Centers for Disease Control and Prevention, US Department of Health and Human Services; 1996.
6. Brown-Elliot BA, Wallace RJ Jr, Blinkhorn R, et al. Successful treatment of disseminated Mycobacterium chelonae infection with linezolid. Clin Infect Dis. 2001;33:1433-1434.
7. Brown BA, Wallace RJ Jr, Onyi GO, et al. Activities of four macrolides, including clarithromycin, against Mycobacterium fortuitum, Mycobacterium chelonae, and Mycobacterium chelonae-like organisms. Antimicrob Agents Chemother. 1992;36:180-184.
8. Swenson JM, Wallace RJ Jr, Silcox VA, et al. Antimicrobial susceptibility of five subgroups of Mycobacterium fortuitum and Mycobacterium chelonae. Antimicrob Agents Chemother. 1985;28:807-811.
9. Leung YY, Choi KW, Ho KM, et al. Disseminated cutaneous infection with Mycobacterium chelonae mimicking panniculitis in a patient with dermatomyositis. Hong Kong Med J. 2005;11:515-519.
1. Lee RP, Cheung KW, Chiu KH, et al. Mycobacterium chelonae infection after total knee arthroplasty: a case report. J Orthop Surg (Hong Kong). 2012;20:134-136.
2. Camargo D, Saad C, Ruiz F, et al. latrogenic outbreak of M. chelonae skin abscesses. Epidemiol Infect. 1996;117:113-119.
3. Rodríguez-Blanco I, Fernández LC, Suárez-Peñaranda JM, et al. Mycobacterium chelonae infection associated with tattoos. Acta Derm Venereol. 2011;91:61-62.
4. Karak K, Bhattacharyya S, Majumdar S, et al. Pulmonary infections caused by mycobacteria other than M. tuberculosis in and around Calcutta. Indian J Pathol Microbiol. 1996;39:131-134.
5. Butler WR, Floyd MM, Silcox V, et al. Standardized Method for HPLC Identification of Mycobacteria. Atlanta, GA: Centers for Disease Control and Prevention, US Department of Health and Human Services; 1996.
6. Brown-Elliot BA, Wallace RJ Jr, Blinkhorn R, et al. Successful treatment of disseminated Mycobacterium chelonae infection with linezolid. Clin Infect Dis. 2001;33:1433-1434.
7. Brown BA, Wallace RJ Jr, Onyi GO, et al. Activities of four macrolides, including clarithromycin, against Mycobacterium fortuitum, Mycobacterium chelonae, and Mycobacterium chelonae-like organisms. Antimicrob Agents Chemother. 1992;36:180-184.
8. Swenson JM, Wallace RJ Jr, Silcox VA, et al. Antimicrobial susceptibility of five subgroups of Mycobacterium fortuitum and Mycobacterium chelonae. Antimicrob Agents Chemother. 1985;28:807-811.
9. Leung YY, Choi KW, Ho KM, et al. Disseminated cutaneous infection with Mycobacterium chelonae mimicking panniculitis in a patient with dermatomyositis. Hong Kong Med J. 2005;11:515-519.
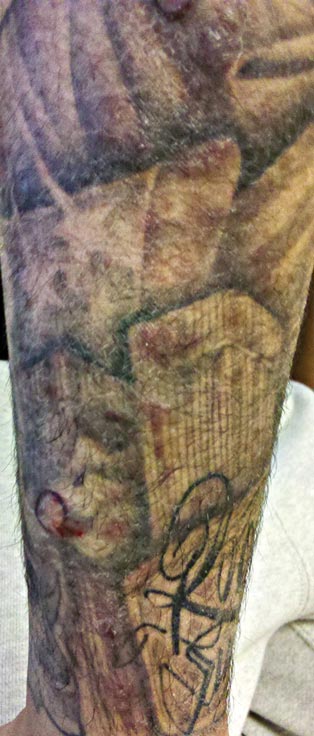
A 21-year-old man presented with growing, mildly pruritic, cutaneous papules and plaques on the right extensor forearm of 3 weeks’ duration. The lesions appeared 1 week after receiving a tattoo on the arm. One year prior the patient had a similar tattoo placed on another section of the right arm without any complications. The patient was afebrile and denied a history of sarcoidosis. Physical examination revealed indurated erythematous papules and plaques on the right extensor forearm that were most prominent in the gray-colored areas of the tattoo.