User login
Making a List, Check It Twice
In October, a 36‐year‐old woman with no significant past medical history presented to the Emergency Department (ED) with a 3‐day history of headache and fever. The headache was severe, throbbing, and frontal in location. She also complained of daily fevers measured up to 103F, generalized malaise, and fatigue. She did not report neck stiffness or photophobia. She felt better after receiving intravenous fluids and was discharged home with a diagnosis of a nonspecific viral illness. Two days later, she returned to the ED with worsening headache, fever, mild photophobia, and poor oral intake. She also complained of a dry cough that made her headache worse, as did bending over. She did not report confusion, neck stiffness, shortness of breath, sore throat, runny nose, abdominal symptoms, or rash.
This patient presents a second time to the ED with worsening headache and fever raising concerns about meningitis. At the time of her first ED visit, it can be assumed that she had a nontoxic appearance because she was discharged shortly thereafter. Thus, acute bacterial meningitis seems less likely, but occasionally patients with meningococcal meningitis may not appear significantly ill until later in the process. Nonetheless, acute meningitis, possibly viral, is the initial concern. The time of the year is an important variable because many viral infections are seasonal. Enteroviruses are the most common cause of viral meningitis in the United States, particularly in the summer and fall. In contrast, mumps, measles, and varicella zoster viruses occur more commonly in winter and spring. Herpetic meningoencephalitis is a life‐threatening condition with a guarded prognosis. Therefore, early recognition and treatment is necessary to decrease morbidity and mortality. Drugs such as nonsteroidal anti‐inflammatory agents, trimethoprim‐sulfamethoxazole, amoxicillin, and rarely vaccines can also cause aseptic meningitis. Infections from fungi, spirochetes, mycobacteria, and rarely parasites also cause meningitis, but would be of greater concern in a patient with risk factors such as recent travel or an immunocompromised state.
Increased headache with bending and cough might indicate elevated intracranial pressure. However, this is a nonspecific complaint, and headache is often worse with the Valsalva maneuver. Because she reports a cough, a chest x‐ray would be useful. In addition to routine initial tests, cerebrospinal fluid (CSF) analysis and human immunodeficiency virus (HIV) testing is recommended.
Her past medical history was notable for depression. Her medications included bupropion, multivitamins, and fish oil. She was also taking milk thistle pills daily to protect her liver because she had been drinking alcohol heavily for the past 2 weeks since her husband left her. She smoked 1 pack of cigarettes daily. She had not traveled recently. She reported no recent animal or wildlife exposure but did recall falling into a midwestern river while canoeing 2 weeks prior to presentation. She worked as a hairstylist and described no sick contacts or risk factors for HIV disease.
An important new historical element is that the patient fell into a river. If she swallowed a significant amount of water during her fall overboard, meningitis from waterborne infections such as Aeromonas, Acanthamoeba, and Naegleria need to be considered. Fortunately, these are rare in the Midwest. Her canoeing history may suggest exposure to wooded areas. Certainly, tickborne infections such as ehrlichiosis, babesiosis, Lyme disease, and Rocky Mountain spotted fever can also cause meningitis. Histoplasmosis and blastomycosis are also endemic to the midwestern United States and can disseminate and cause central nervous system disease.
At this time, viral and bacterial infections are highest on the differential diagnosis. However, the microbiology laboratory needs to be alerted to the possibility of fungal or parasitic organisms depending on the initial CSF analysis results.
The patient was a Caucasian woman who appeared comfortable. Her blood pressure was 130/62 mm Hg, heart rate was 83 beats per minute, respiratory rate was 18 per minute, temperature was 100.8F, and oxygen saturation was 98% on room air. She was fully alert and oriented. Her pupils were bilaterally equal, reactive to light and accommodation with intact extraocular movement and no nystagmus. There was conjunctival injection bilaterally without noticeable pallor or icterus. Fundoscopic examination, which the patient tolerated without difficulty, was normal. Inspection of the oral cavity showed mild tonsillar enlargement. The neck was supple with no stiffness. No cervical, axillary, or inguinal lymph nodes were palpable. Faint bilateral basilar crackles were audible over the posterior chest. There was very mild right upper quadrant abdominal tenderness without guarding. The liver and spleen were normal size and bowel sounds were present. No rash, peripheral edema, or spinal tenderness was noted. A complete neurological examination was normal.
Her general appearance and vital signs seem reassuring. Conjunctival injection and mild tonsillar enlargement are nonspecific findings and may occur in systemic inflammatory states especially viral infections. Atelectasis may account for faint bilateral basilar crackles especially if associated with post‐tussive change. Her alcohol use puts her at risk of aspiration. A right lower lobe process (pneumonia) can sometimes present with right upper quadrant tenderness. However, this tenderness may also represent muscle soreness from repeated coughing, liver, or gallbladder disease. The same infectious process affecting the central nervous system and possibly her lungs, may also be affecting the liver.
A complete blood count revealed a white blood cell count of 3000/mm3 (79% neutrophils, 15% lymphocytes, 5% monocytes), hemoglobin of 11.7 g/dL, and platelets of 110,000/mm3. The serum sodium was 133 mmol/L, potassium was 3.7 mmol/L, bicarbonate was 22 mmol/L, and blood urea nitrogen was 20 mg/dL. The serum creatinine was 1.5 compared to 1.0 mg/dL on testing 2 days prior. A liver function panel showed protein of 5.1 g/dL, albumin of 3 g/dL, aspartate aminotransferase (AST) of 576 IU/L, alanine aminotransferase (ALT) of 584 IU/L, alkaline phosphatase of 282 IU/L, and total bilirubin of 1 mg/dL. The coagulation profile, creatinine phosphokinase, acetaminophen level, urine pregnancy test, urine drug screen, and urinalysis (including urine microscopy) were normal.
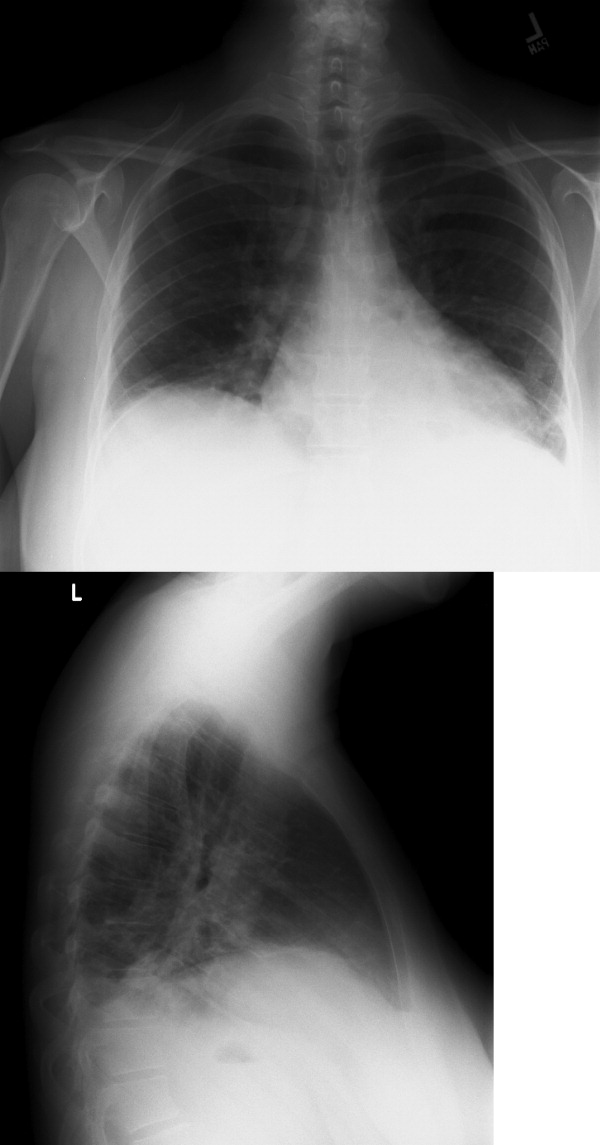
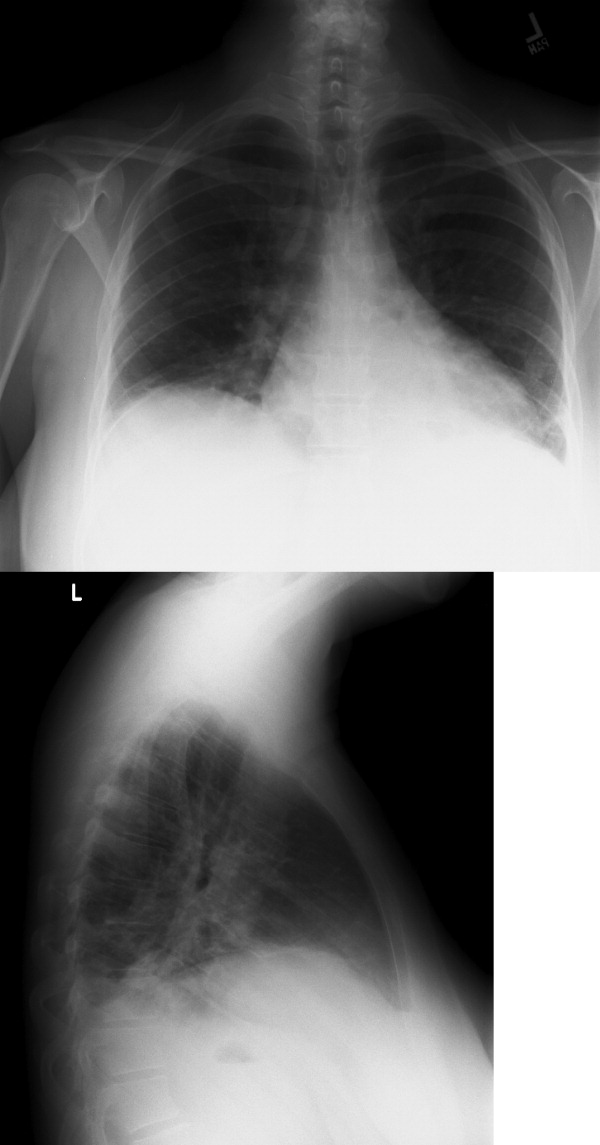
The CSF opening pressure was 13 cm H2O. CSF analysis showed 4 mononuclear leukocytes per high‐power field, CSF protein was 27 mg/dL, and glucose was 76 mg/dL. No organisms were noted on gram stain. A chest x‐ray showed focal airspace opacity in the left lower lobe (Figure 1) and the patient was hospitalized for further management.
The normal CSF analysis makes acute meningitis much less likely. It is interesting to note that the aminotransferase levels are nearly equal. Usually, in viral and many other causes of hepatitis, the ALT is higher than the AST, whereas the contrary is true in alcoholic hepatitis. Because the patient has been consuming significant amounts of alcohol recently, these levels may become equal in the setting of another primary liver process. The elevation in liver enzymes also raises the possibility of autoimmune hepatitis secondary to a systemic vasculitis such as systemic lupus erythematosus. Nonetheless, the focus should be on infectious causes of hepatitis such as hepatitis C, adenovirus, parvovirus, Epstein‐Barr virus (EBV), cytomegalovirus, and herpes simplex virus that can cause pneumonia either as a primary or secondary infection. Acute HIV infection can also present in this fashion, and anti‐HIV antibody testing may be negative early in the disease. In the setting of a normal urinalysis and bland urine sediment, prerenal azotemia is the most likely cause of her acute renal injury and can be confirmed by testing the urinary sodium and creatinine. A peripheral smear should be reviewed to evaluate the pancytopenia.
Severe headache, fever, conjunctival injection, pancytopenia, acute kidney injury, hepatitis, and pneumonia may occur in leptospirosis, particularly in a patient with recent freshwater exposure. Alternatively, ehrlichiosis can also account for fever, headache, pancytopenia, renal failure, hepatitis, and pneumonia, but conjunctival suffusion is not often present. At this time, treatment for community‐acquired pneumonia that includes coverage for leptospirosis should be started.
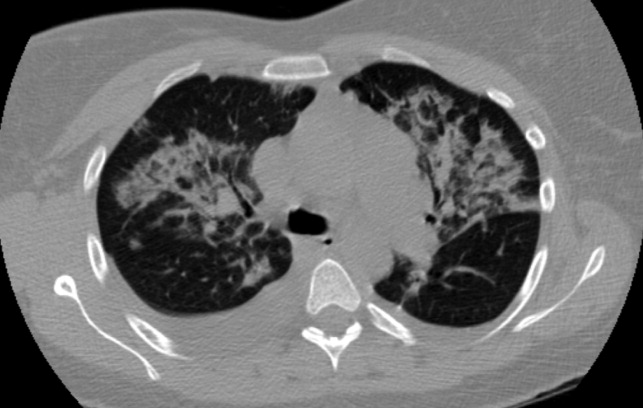
The patient was hydrated with intravenous fluids and treated with intravenous ceftriaxone and azithromycin for community‐acquired pneumonia. An abdominal ultrasound was normal. The serologic assays for acute hepatitis A, B, and C infection were negative. The following morning, she reported worsening headache, increased cough now productive of whitish‐yellow sputum, and diffuse body aches. She appeared more lethargic and toxic. Her blood pressure was 100/83 mm Hg, heart rate was 84 beats per minute, respiratory rate was 24 per minute, and temperature was 101.3F. She had increased crackles on chest auscultation bilaterally and required supplemental oxygen at 4 L/minute by nasal cannula. Examination of both legs now revealed multiple scattered, faintly erythematous, 2‐cm‐sized patches overlying tender subtle subcutaneous nodules. Additionally, a mildly pruritic, V‐shaped area of blanchable erythema was also seen on her chest. The white blood cell count was 2500/mm3 (77% neutrophils, 15% lymphocytes), serum creatinine was 1.8 mg/dL, AST was 351 IU/L, and ALT was 485 IU/L. Blood cultures showed no growth and a peripheral smear examination was unrevealing. A noncontrast chest computed tomographic scan showed findings consistent with multifocal pneumonia (Figure 2).
It would be prudent at this time to expand her antimicrobial coverage (such as with vancomycin and piperacillin‐tazobactam) for activity against methicillin‐resistant Staphylococcus aureus and Pseudomonas because of her clinical worsening. Although ceftriaxone or piperacillin would cover leptospirosis, given the possibility of ehrlichiosis, the addition of doxycycline should be strongly considered.
The description of the rash on her legs seems consistent with erythema nodosum, which is associated with a number of infections (streptococcal, fungal, syphilis, EBV, cat‐scratch disease, tuberculosis), inflammatory conditions (inflammatory bowel disease, autoimmune disease, malignancy), and pregnancy. The blanchable rash on the chest is also a cause of concern for a possible drug reaction (ceftriaxone). A Jarisch‐Herxheimer reaction is possible given her acute worsening of symptoms with initiation of antibiotic therapy.
An antineutrophil cytoplasmic antibodyassociated vasculitis or another autoimmune condition such as systemic lupus erythematosus can account for erythema nodosum, rash, pancytopenia, and hepatitis. This diagnosis might also fit if she had a vasculitic pulmonary hemorrhage that caused her lung infiltrates and worsening hypoxia. A complete antinuclear antibody panel, antineutrophil cytoplasmic antibody, and antismooth muscle antibody testing is recommended. A skin and bronchoscopic biopsy should be considered.
Her dose of ceftriaxone was increased for possible severe pneumococcal pneumonia. The dermatology consultant felt that her leg lesions were consistent with erythema nodosum and the chest rash consistent with cutaneous photodamage. Bronchoscopic examination was normal and a bronchoalveolar lavage sample showed 2905 red blood cells/mm3 and 605 white blood cells/mm3 (70% neutrophils, 7% lymphocytes, 16% histiocytes), normal cytology, and negative cultures. There was no significant clinical improvement by the fourth hospital day and oral doxycycline was started. The next day, her skin lesions had resolved and she felt better. The serologic tests for Legionella, Mycoplasma, cytomegalovirus, EBV, Toxoplasma, Chlamydophila, Ehrlichia, Leptospira, Q‐fever, parvovirus, and adenovirus were negative. A fungal serology panel, HIV polymerase chain reaction, cryoglobulin level, and several rheumatologic tests (antinuclear antibody, extractable nuclear antigen panel, rheumatoid factor, antineutrophil cytoplasmic antibody, antiproteinase 3, and antiglomerular basement membrane antibodies) were normal. Blood cultures continued to show no growth.
The apparent response to doxycycline suggests she might have ehrlichiosis. A buffy coat review for morulae should be done. It is also possible that she may have improved on her initial therapy alone before starting doxycycline and her clinical worsening (including the chest rash) was due to a Jarisch‐Herxheimer reaction. Serologic tests for leptospirosis and ehrlichiosis should be repeated in 12 weeks because such infections may not cause detectable antibody levels early in the illness.
Ceftriaxone and doxycycline were continued and she showed rapid and significant clinical improvement. She was discharged 4 days later with instructions to complete a 10‐day course of antibiotics. At her 3‐month follow‐up, she was doing well and a repeat Leptospira antibody test by the Indirect Hemagglutination Assay (MRL Diagnostics, Cypress, California; normal titer <1:50) was positive at a titer of 1:100, which is highly suggestive of leptospirosis.
Commentary
Leptospirosis is a zoonotic infection caused by spirochetes of the genus Leptospira. The infection is usually transmitted indirectly to humans through contact with water, food, or soil contaminated with the urine of infected mammals.1 Risk factors for infection include participation in recreational activities (such as freshwater swimming, canoeing, and camping), occupational exposure, and exposure to infected pets or domesticated livestock. Approximately 100200 cases are identified annually in the United States, and approximately half occur in the state of Hawaii.2 Outbreaks of leptospirosis have been reported previously in the Midwest.3 These organisms inoculate humans through contact with mucous membranes or broken skin, or enter by swallowing infected food or water. A large number of these infections remain subclinical or result in a very mild illness with spontaneous clearance by the host's immune mechanism. Following an incubation period of 230 days, infected individuals may develop clinically significant disease (Table 1). Clinical presentations may overlap as the disease progresses. Although much remains to be learned about the exact pathogenic mechanism, disruption of the cell membranes of small vessel endothelia (a toxin‐like effect), and cytokine‐mediated tissue injury are believed to cause organ hemorrhage and ischemia.4
|
| 1. Mild influenza‐like self‐remitting disease (90% of cases) |
| Undifferentiated fever (usually 100F105F), severe headache, and myalgia (especially lower limbs). |
| 2. Moderately severe disease usually requiring hospitalization (5%9% of cases) |
| Marked prostration, anorexia, nausea, and vomiting, conjunctival suffusion, transient rash, frequently abdominal pain, constipation or diarrhea, and occasionally epistaxis. |
| 3. Severe disease involving multiple organ systems (1%5% of cases) |
| Hepatorenal Syndrome (Weil's syndrome) |
| Constellation of jaundice, hemorrhagic diathesis, and acute renal failure. Hepatic failure is rarely fatal. Renal involvement is usually more severe and the common cause of death. Cardiac (myocarditis with arrhythmias) and pulmonary complications are frequent. Confusion and restlessness may occur. |
| Hemorrhagic pneumonitis |
| Usually presents as a dry cough initially but becomes blood‐streaked after 23 days. Often characterized by a rapid progression to involve extensive areas of lungs, massive intra‐alveolar hemorrhage, acute respiratory failure, and death. |
| Central nervous system involvement |
| Meningismus, meningitis, or meningoencephalitis. |
The clinical diagnosis of leptospirosis is difficult because of its protean manifestations. Although nonspecific, 2 clinical features may provide a clue to the clinical diagnosis. First, the presence of conjunctival suffusion occurs in the early stage of the disease and is often associated with subconjunctival hemorrhage. Second, severe myalgia, commonly involving the lower limbs, is also characteristically present.1, 5 In 1 series of 58 patients with acute leptospirosis, conjunctival suffusion was observed in 50% of cases, and subconjunctival hemorrhage in 29%. Body ache and muscle tenderness was described in almost all cases.6
As seen in this case, the presence of a rash may pose a clinical challenge. A transient macular, maculopapular, purpuric, or urticarial rash may be seen in acute leptospirosis, but rashes may also be representative of a complication of treatment.1 First described in 1895 in patients with syphilis treated with mercury, the Jarisch‐Herxheimer reaction typically occurs within a few hours of antimicrobial treatment of spirochete infections and often presents with a rash, headache, fever, rigors, hypotension, sweating, and worsening symptoms of the underlying illness.7 Other skin findings such as the occurrence of erythema nodosum have been previously reported in cases of leptospirosis.8
Human ehrlichiosis (HE) is caused by tickborne, obligatory intracellular bacteria that infect leukocytes. There are 3 distinct clinical conditions: human monocytic ehrlichiosis (HME, caused by Ehrlichia chaffeensis), human granulocytic anaplasmosis (HGA, caused by Anaplasma phagocytophilum), and human ewingii ehrlichiosis (HEE, caused by E. ewingii). Although most cases of HME and HEE are seen in the southeastern and south‐central United States and California, the highest incidence of HGA is reported in the northeastern and upper Midwest regions.9 As with leptospirosis, the clinical range of HE spans from asymptomatic infection to life‐threatening illness. Following an incubation period of 12 weeks, symptomatic cases usually present with nonspecific complaints such as high fevers, chills, headache, nausea, arthralgia, myalgia, and malaise.10 The majority of cases will report a tick bite or an exposure to ticks. Laboratory tests often reveal leukopenia (white blood cell count < 4000/mm3), thrombocytopenia, hyponatremia, and elevated AST and ALT. Patients with severe disease may develop renal, respiratory, and hepatic failure. Thus, differentiating ehrlichiosis from leptospirosis is often challenging for the clinician.
However, there are a few clinical clues that help distinguish between these illnesses in this case. HGA as a cause of HE would be more likely in the Midwest. Although a rash is present in one‐third of patients with HME, it is seldom present in HGA unless coinfected with Borrelia burgdorferi, the causative agent for Lyme disease. Additionally, her history of freshwater exposure and the absence of a history of a tick bite also favor leptospirosis. As noted previously, conjunctival suffusion, a characteristic clinical feature of leptospirosis, has only been described in case reports of HE.11, 12
Serologic tests are often used to establish the diagnosis of leptospirosis and ehrlichiosis. Leptospires are fastidious organisms that are difficult to isolate on inoculated growth media. The microscopic agglutination test for leptospirosis is considered the diagnostic gold standard due to its high specificity, but its use is limited by its technical complexity, lack of availability (other than in reference laboratories), and low sensitivity early in the disease (antibody levels detected by this method usually do not appear until 7 days after symptom onset).13 A variety of rapid serologic assays are also available. Although these tests have good overall sensitivity (ranging between 79% and 93%), they perform relatively poorly for acute‐phase sera (sensitivity of 38.5%52.7%).13 The high early false negative rate is believed to be a result of inadequate Leptospira antibody titers in the acute phase of the illness. Seroconversion or a 4‐fold rise between acute and convalescent‐phase antibody titers is the most definitive criterion for the diagnosis of leptospirosis. However, without paired sera samples, a single high microscopic agglutination test titer can be taken as diagnostic for leptospirosis depending on the degree of regional endemicity.14
Similarly, currently available serologic assays for ehrlichiosis produce negative results in most patients in the first week of illness, and it is important to obtain a convalescent phase serum specimen for confirmatory diagnosis of HME and HGA. Seroconversion or a 4‐fold increase in titer between acute and convalescent phase sera is considered diagnostic. The sensitivity of finding morulae (intracytoplasmic vacuolar microcolonies of Ehrlichia) on a peripheral smear is unknown, and data suggest that this finding is more common in cases of HGA compared to HME.15
Although doxycycline is the drug of choice for the treatment of ehrlichiosis, Leptospira is susceptible to a wide variety of antibiotics because it exhibits a double membrane surface architecture with components common to both gram‐negative and gram‐positive bacteria.1 Recommended treatment regimens for severe leptospirosis include the use of high‐dose intravenous penicillin or a third‐generation cephalosporin. Less severe cases can be treated with oral amoxicillin or doxycycline.16 The fact that this patient's clinical improvement appeared to lag after initiation of ceftriaxone does not necessarily indicate a lack of efficacy but perhaps a Jarisch‐Herxheimer reaction in response to appropriate antibiotic therapy.
Teaching Points
-
Establishing a diagnosis of leptospirosis is challenging and requires a high index of suspicion. Clinicians should be aware of the limitations of the diagnostic accuracy of the serologic assays for leptospirosis because they are frequently negative in the first week after symptom onset.
-
The classic finding of conjunctival suffusion is helpful in differentiating leptospirosis from human ehrlichiosis.
-
This case also highlights the importance of the clinical practice of making a list of suspected diagnoses, remaining open to these possibilities, and checking serologic tests again in convalescence to confirm the diagnosis.
The approach to clinical conundrums by an expert clinician is revealed through the presentation of an actual patient's case in an approach typical of a morning report. Similarly to patient care, sequential pieces of information are provided to the clinician, who is unfamiliar with the case. The focus is on the thought processes of both the clinical team caring for the patient and the discussant.
Acknowledgements
The authors thank Dr. Brian Harte for his valuable guidance in the preparation of this manuscript.
- ,,.Leptospirosis: an emerging global public health problem.J Biosci.2008;33:557–569.
- Centers for Disease Control and Prevention. Leptospirosis.2005. http://www.cdc.gov/ncidod/dbmd/diseaseinfo/leptospirosis_t.htm. Accessed November 15,year="2010"2010.
- Morbidity and Mortality Weekly Report.From the Centers for Disease Control and Prevention. Update: leptospirosis and unexplained acute febrile illness among athletes participating in triathlons—Illinois and Wisconsin, 1998.JAMA.1998;280:1474–1475.
- ,.Optimal treatment of leptospirosis: queries and projections.Int J Antimicrob Agents.2006;28:491–496.
- ,.Leptospirosis in the tropics and in travelers.Curr Infect Dis Rep.2006;8:51–58.
- ,,,,,.Clinico‐epidemiological study of hospitalized cases of severe leptospirosis.Indian J Med Res.1999;109:94–99.
- ,.Proposed mechanisms and preventative options of Jarisch‐Herxheimer reactions.J Clin Pharm Ther.2005;30:291–295.
- .Leptospirosis presenting with erythema nodosum.Arch Dis Child.1977;52:418–419.
- ,,.Emerging and re‐emerging tick‐transmitted rickettsial and ehrlichial infections.Med Clin N Am.2008;92:1345–1361.
- ,.Tick‐borne ehrlichiosis infection in human beings.J Vector Borne Dis.2008;45:273–280.
- ,.Ehrlichia in Tennessee.South Med J.1989;82:669.
- ,,,,,.Ehrlichial meningitis with cerebrospinal fluid morulae.Pediatr Infect Dis J.1999;18:552–555.
- ,,, et al.Evaluation of four commercially available rapid serologic tests for diagnosis of leptospirosis.J Clin Microbiol.2003;41:803–809.
- ,.Diagnosis of leptospirosis utilizing modified Faine's criteria.J Assoc Physicians India.2004;52:678–679.
- ,.Ehrlichiosis in children.J Pediatr.1997;131:184–192.
- ,World Health Organization, International Leptospirosis Society. Human leptospirosis: guidance for diagnosis, surveillance and control.Geneva, Switzerland:World Health Organization;2003.
In October, a 36‐year‐old woman with no significant past medical history presented to the Emergency Department (ED) with a 3‐day history of headache and fever. The headache was severe, throbbing, and frontal in location. She also complained of daily fevers measured up to 103F, generalized malaise, and fatigue. She did not report neck stiffness or photophobia. She felt better after receiving intravenous fluids and was discharged home with a diagnosis of a nonspecific viral illness. Two days later, she returned to the ED with worsening headache, fever, mild photophobia, and poor oral intake. She also complained of a dry cough that made her headache worse, as did bending over. She did not report confusion, neck stiffness, shortness of breath, sore throat, runny nose, abdominal symptoms, or rash.
This patient presents a second time to the ED with worsening headache and fever raising concerns about meningitis. At the time of her first ED visit, it can be assumed that she had a nontoxic appearance because she was discharged shortly thereafter. Thus, acute bacterial meningitis seems less likely, but occasionally patients with meningococcal meningitis may not appear significantly ill until later in the process. Nonetheless, acute meningitis, possibly viral, is the initial concern. The time of the year is an important variable because many viral infections are seasonal. Enteroviruses are the most common cause of viral meningitis in the United States, particularly in the summer and fall. In contrast, mumps, measles, and varicella zoster viruses occur more commonly in winter and spring. Herpetic meningoencephalitis is a life‐threatening condition with a guarded prognosis. Therefore, early recognition and treatment is necessary to decrease morbidity and mortality. Drugs such as nonsteroidal anti‐inflammatory agents, trimethoprim‐sulfamethoxazole, amoxicillin, and rarely vaccines can also cause aseptic meningitis. Infections from fungi, spirochetes, mycobacteria, and rarely parasites also cause meningitis, but would be of greater concern in a patient with risk factors such as recent travel or an immunocompromised state.
Increased headache with bending and cough might indicate elevated intracranial pressure. However, this is a nonspecific complaint, and headache is often worse with the Valsalva maneuver. Because she reports a cough, a chest x‐ray would be useful. In addition to routine initial tests, cerebrospinal fluid (CSF) analysis and human immunodeficiency virus (HIV) testing is recommended.
Her past medical history was notable for depression. Her medications included bupropion, multivitamins, and fish oil. She was also taking milk thistle pills daily to protect her liver because she had been drinking alcohol heavily for the past 2 weeks since her husband left her. She smoked 1 pack of cigarettes daily. She had not traveled recently. She reported no recent animal or wildlife exposure but did recall falling into a midwestern river while canoeing 2 weeks prior to presentation. She worked as a hairstylist and described no sick contacts or risk factors for HIV disease.
An important new historical element is that the patient fell into a river. If she swallowed a significant amount of water during her fall overboard, meningitis from waterborne infections such as Aeromonas, Acanthamoeba, and Naegleria need to be considered. Fortunately, these are rare in the Midwest. Her canoeing history may suggest exposure to wooded areas. Certainly, tickborne infections such as ehrlichiosis, babesiosis, Lyme disease, and Rocky Mountain spotted fever can also cause meningitis. Histoplasmosis and blastomycosis are also endemic to the midwestern United States and can disseminate and cause central nervous system disease.
At this time, viral and bacterial infections are highest on the differential diagnosis. However, the microbiology laboratory needs to be alerted to the possibility of fungal or parasitic organisms depending on the initial CSF analysis results.
The patient was a Caucasian woman who appeared comfortable. Her blood pressure was 130/62 mm Hg, heart rate was 83 beats per minute, respiratory rate was 18 per minute, temperature was 100.8F, and oxygen saturation was 98% on room air. She was fully alert and oriented. Her pupils were bilaterally equal, reactive to light and accommodation with intact extraocular movement and no nystagmus. There was conjunctival injection bilaterally without noticeable pallor or icterus. Fundoscopic examination, which the patient tolerated without difficulty, was normal. Inspection of the oral cavity showed mild tonsillar enlargement. The neck was supple with no stiffness. No cervical, axillary, or inguinal lymph nodes were palpable. Faint bilateral basilar crackles were audible over the posterior chest. There was very mild right upper quadrant abdominal tenderness without guarding. The liver and spleen were normal size and bowel sounds were present. No rash, peripheral edema, or spinal tenderness was noted. A complete neurological examination was normal.
Her general appearance and vital signs seem reassuring. Conjunctival injection and mild tonsillar enlargement are nonspecific findings and may occur in systemic inflammatory states especially viral infections. Atelectasis may account for faint bilateral basilar crackles especially if associated with post‐tussive change. Her alcohol use puts her at risk of aspiration. A right lower lobe process (pneumonia) can sometimes present with right upper quadrant tenderness. However, this tenderness may also represent muscle soreness from repeated coughing, liver, or gallbladder disease. The same infectious process affecting the central nervous system and possibly her lungs, may also be affecting the liver.
A complete blood count revealed a white blood cell count of 3000/mm3 (79% neutrophils, 15% lymphocytes, 5% monocytes), hemoglobin of 11.7 g/dL, and platelets of 110,000/mm3. The serum sodium was 133 mmol/L, potassium was 3.7 mmol/L, bicarbonate was 22 mmol/L, and blood urea nitrogen was 20 mg/dL. The serum creatinine was 1.5 compared to 1.0 mg/dL on testing 2 days prior. A liver function panel showed protein of 5.1 g/dL, albumin of 3 g/dL, aspartate aminotransferase (AST) of 576 IU/L, alanine aminotransferase (ALT) of 584 IU/L, alkaline phosphatase of 282 IU/L, and total bilirubin of 1 mg/dL. The coagulation profile, creatinine phosphokinase, acetaminophen level, urine pregnancy test, urine drug screen, and urinalysis (including urine microscopy) were normal.
The CSF opening pressure was 13 cm H2O. CSF analysis showed 4 mononuclear leukocytes per high‐power field, CSF protein was 27 mg/dL, and glucose was 76 mg/dL. No organisms were noted on gram stain. A chest x‐ray showed focal airspace opacity in the left lower lobe (Figure 1) and the patient was hospitalized for further management.

The normal CSF analysis makes acute meningitis much less likely. It is interesting to note that the aminotransferase levels are nearly equal. Usually, in viral and many other causes of hepatitis, the ALT is higher than the AST, whereas the contrary is true in alcoholic hepatitis. Because the patient has been consuming significant amounts of alcohol recently, these levels may become equal in the setting of another primary liver process. The elevation in liver enzymes also raises the possibility of autoimmune hepatitis secondary to a systemic vasculitis such as systemic lupus erythematosus. Nonetheless, the focus should be on infectious causes of hepatitis such as hepatitis C, adenovirus, parvovirus, Epstein‐Barr virus (EBV), cytomegalovirus, and herpes simplex virus that can cause pneumonia either as a primary or secondary infection. Acute HIV infection can also present in this fashion, and anti‐HIV antibody testing may be negative early in the disease. In the setting of a normal urinalysis and bland urine sediment, prerenal azotemia is the most likely cause of her acute renal injury and can be confirmed by testing the urinary sodium and creatinine. A peripheral smear should be reviewed to evaluate the pancytopenia.
Severe headache, fever, conjunctival injection, pancytopenia, acute kidney injury, hepatitis, and pneumonia may occur in leptospirosis, particularly in a patient with recent freshwater exposure. Alternatively, ehrlichiosis can also account for fever, headache, pancytopenia, renal failure, hepatitis, and pneumonia, but conjunctival suffusion is not often present. At this time, treatment for community‐acquired pneumonia that includes coverage for leptospirosis should be started.
The patient was hydrated with intravenous fluids and treated with intravenous ceftriaxone and azithromycin for community‐acquired pneumonia. An abdominal ultrasound was normal. The serologic assays for acute hepatitis A, B, and C infection were negative. The following morning, she reported worsening headache, increased cough now productive of whitish‐yellow sputum, and diffuse body aches. She appeared more lethargic and toxic. Her blood pressure was 100/83 mm Hg, heart rate was 84 beats per minute, respiratory rate was 24 per minute, and temperature was 101.3F. She had increased crackles on chest auscultation bilaterally and required supplemental oxygen at 4 L/minute by nasal cannula. Examination of both legs now revealed multiple scattered, faintly erythematous, 2‐cm‐sized patches overlying tender subtle subcutaneous nodules. Additionally, a mildly pruritic, V‐shaped area of blanchable erythema was also seen on her chest. The white blood cell count was 2500/mm3 (77% neutrophils, 15% lymphocytes), serum creatinine was 1.8 mg/dL, AST was 351 IU/L, and ALT was 485 IU/L. Blood cultures showed no growth and a peripheral smear examination was unrevealing. A noncontrast chest computed tomographic scan showed findings consistent with multifocal pneumonia (Figure 2).

It would be prudent at this time to expand her antimicrobial coverage (such as with vancomycin and piperacillin‐tazobactam) for activity against methicillin‐resistant Staphylococcus aureus and Pseudomonas because of her clinical worsening. Although ceftriaxone or piperacillin would cover leptospirosis, given the possibility of ehrlichiosis, the addition of doxycycline should be strongly considered.
The description of the rash on her legs seems consistent with erythema nodosum, which is associated with a number of infections (streptococcal, fungal, syphilis, EBV, cat‐scratch disease, tuberculosis), inflammatory conditions (inflammatory bowel disease, autoimmune disease, malignancy), and pregnancy. The blanchable rash on the chest is also a cause of concern for a possible drug reaction (ceftriaxone). A Jarisch‐Herxheimer reaction is possible given her acute worsening of symptoms with initiation of antibiotic therapy.
An antineutrophil cytoplasmic antibodyassociated vasculitis or another autoimmune condition such as systemic lupus erythematosus can account for erythema nodosum, rash, pancytopenia, and hepatitis. This diagnosis might also fit if she had a vasculitic pulmonary hemorrhage that caused her lung infiltrates and worsening hypoxia. A complete antinuclear antibody panel, antineutrophil cytoplasmic antibody, and antismooth muscle antibody testing is recommended. A skin and bronchoscopic biopsy should be considered.
Her dose of ceftriaxone was increased for possible severe pneumococcal pneumonia. The dermatology consultant felt that her leg lesions were consistent with erythema nodosum and the chest rash consistent with cutaneous photodamage. Bronchoscopic examination was normal and a bronchoalveolar lavage sample showed 2905 red blood cells/mm3 and 605 white blood cells/mm3 (70% neutrophils, 7% lymphocytes, 16% histiocytes), normal cytology, and negative cultures. There was no significant clinical improvement by the fourth hospital day and oral doxycycline was started. The next day, her skin lesions had resolved and she felt better. The serologic tests for Legionella, Mycoplasma, cytomegalovirus, EBV, Toxoplasma, Chlamydophila, Ehrlichia, Leptospira, Q‐fever, parvovirus, and adenovirus were negative. A fungal serology panel, HIV polymerase chain reaction, cryoglobulin level, and several rheumatologic tests (antinuclear antibody, extractable nuclear antigen panel, rheumatoid factor, antineutrophil cytoplasmic antibody, antiproteinase 3, and antiglomerular basement membrane antibodies) were normal. Blood cultures continued to show no growth.
The apparent response to doxycycline suggests she might have ehrlichiosis. A buffy coat review for morulae should be done. It is also possible that she may have improved on her initial therapy alone before starting doxycycline and her clinical worsening (including the chest rash) was due to a Jarisch‐Herxheimer reaction. Serologic tests for leptospirosis and ehrlichiosis should be repeated in 12 weeks because such infections may not cause detectable antibody levels early in the illness.
Ceftriaxone and doxycycline were continued and she showed rapid and significant clinical improvement. She was discharged 4 days later with instructions to complete a 10‐day course of antibiotics. At her 3‐month follow‐up, she was doing well and a repeat Leptospira antibody test by the Indirect Hemagglutination Assay (MRL Diagnostics, Cypress, California; normal titer <1:50) was positive at a titer of 1:100, which is highly suggestive of leptospirosis.
Commentary
Leptospirosis is a zoonotic infection caused by spirochetes of the genus Leptospira. The infection is usually transmitted indirectly to humans through contact with water, food, or soil contaminated with the urine of infected mammals.1 Risk factors for infection include participation in recreational activities (such as freshwater swimming, canoeing, and camping), occupational exposure, and exposure to infected pets or domesticated livestock. Approximately 100200 cases are identified annually in the United States, and approximately half occur in the state of Hawaii.2 Outbreaks of leptospirosis have been reported previously in the Midwest.3 These organisms inoculate humans through contact with mucous membranes or broken skin, or enter by swallowing infected food or water. A large number of these infections remain subclinical or result in a very mild illness with spontaneous clearance by the host's immune mechanism. Following an incubation period of 230 days, infected individuals may develop clinically significant disease (Table 1). Clinical presentations may overlap as the disease progresses. Although much remains to be learned about the exact pathogenic mechanism, disruption of the cell membranes of small vessel endothelia (a toxin‐like effect), and cytokine‐mediated tissue injury are believed to cause organ hemorrhage and ischemia.4
|
| 1. Mild influenza‐like self‐remitting disease (90% of cases) |
| Undifferentiated fever (usually 100F105F), severe headache, and myalgia (especially lower limbs). |
| 2. Moderately severe disease usually requiring hospitalization (5%9% of cases) |
| Marked prostration, anorexia, nausea, and vomiting, conjunctival suffusion, transient rash, frequently abdominal pain, constipation or diarrhea, and occasionally epistaxis. |
| 3. Severe disease involving multiple organ systems (1%5% of cases) |
| Hepatorenal Syndrome (Weil's syndrome) |
| Constellation of jaundice, hemorrhagic diathesis, and acute renal failure. Hepatic failure is rarely fatal. Renal involvement is usually more severe and the common cause of death. Cardiac (myocarditis with arrhythmias) and pulmonary complications are frequent. Confusion and restlessness may occur. |
| Hemorrhagic pneumonitis |
| Usually presents as a dry cough initially but becomes blood‐streaked after 23 days. Often characterized by a rapid progression to involve extensive areas of lungs, massive intra‐alveolar hemorrhage, acute respiratory failure, and death. |
| Central nervous system involvement |
| Meningismus, meningitis, or meningoencephalitis. |
The clinical diagnosis of leptospirosis is difficult because of its protean manifestations. Although nonspecific, 2 clinical features may provide a clue to the clinical diagnosis. First, the presence of conjunctival suffusion occurs in the early stage of the disease and is often associated with subconjunctival hemorrhage. Second, severe myalgia, commonly involving the lower limbs, is also characteristically present.1, 5 In 1 series of 58 patients with acute leptospirosis, conjunctival suffusion was observed in 50% of cases, and subconjunctival hemorrhage in 29%. Body ache and muscle tenderness was described in almost all cases.6
As seen in this case, the presence of a rash may pose a clinical challenge. A transient macular, maculopapular, purpuric, or urticarial rash may be seen in acute leptospirosis, but rashes may also be representative of a complication of treatment.1 First described in 1895 in patients with syphilis treated with mercury, the Jarisch‐Herxheimer reaction typically occurs within a few hours of antimicrobial treatment of spirochete infections and often presents with a rash, headache, fever, rigors, hypotension, sweating, and worsening symptoms of the underlying illness.7 Other skin findings such as the occurrence of erythema nodosum have been previously reported in cases of leptospirosis.8
Human ehrlichiosis (HE) is caused by tickborne, obligatory intracellular bacteria that infect leukocytes. There are 3 distinct clinical conditions: human monocytic ehrlichiosis (HME, caused by Ehrlichia chaffeensis), human granulocytic anaplasmosis (HGA, caused by Anaplasma phagocytophilum), and human ewingii ehrlichiosis (HEE, caused by E. ewingii). Although most cases of HME and HEE are seen in the southeastern and south‐central United States and California, the highest incidence of HGA is reported in the northeastern and upper Midwest regions.9 As with leptospirosis, the clinical range of HE spans from asymptomatic infection to life‐threatening illness. Following an incubation period of 12 weeks, symptomatic cases usually present with nonspecific complaints such as high fevers, chills, headache, nausea, arthralgia, myalgia, and malaise.10 The majority of cases will report a tick bite or an exposure to ticks. Laboratory tests often reveal leukopenia (white blood cell count < 4000/mm3), thrombocytopenia, hyponatremia, and elevated AST and ALT. Patients with severe disease may develop renal, respiratory, and hepatic failure. Thus, differentiating ehrlichiosis from leptospirosis is often challenging for the clinician.
However, there are a few clinical clues that help distinguish between these illnesses in this case. HGA as a cause of HE would be more likely in the Midwest. Although a rash is present in one‐third of patients with HME, it is seldom present in HGA unless coinfected with Borrelia burgdorferi, the causative agent for Lyme disease. Additionally, her history of freshwater exposure and the absence of a history of a tick bite also favor leptospirosis. As noted previously, conjunctival suffusion, a characteristic clinical feature of leptospirosis, has only been described in case reports of HE.11, 12
Serologic tests are often used to establish the diagnosis of leptospirosis and ehrlichiosis. Leptospires are fastidious organisms that are difficult to isolate on inoculated growth media. The microscopic agglutination test for leptospirosis is considered the diagnostic gold standard due to its high specificity, but its use is limited by its technical complexity, lack of availability (other than in reference laboratories), and low sensitivity early in the disease (antibody levels detected by this method usually do not appear until 7 days after symptom onset).13 A variety of rapid serologic assays are also available. Although these tests have good overall sensitivity (ranging between 79% and 93%), they perform relatively poorly for acute‐phase sera (sensitivity of 38.5%52.7%).13 The high early false negative rate is believed to be a result of inadequate Leptospira antibody titers in the acute phase of the illness. Seroconversion or a 4‐fold rise between acute and convalescent‐phase antibody titers is the most definitive criterion for the diagnosis of leptospirosis. However, without paired sera samples, a single high microscopic agglutination test titer can be taken as diagnostic for leptospirosis depending on the degree of regional endemicity.14
Similarly, currently available serologic assays for ehrlichiosis produce negative results in most patients in the first week of illness, and it is important to obtain a convalescent phase serum specimen for confirmatory diagnosis of HME and HGA. Seroconversion or a 4‐fold increase in titer between acute and convalescent phase sera is considered diagnostic. The sensitivity of finding morulae (intracytoplasmic vacuolar microcolonies of Ehrlichia) on a peripheral smear is unknown, and data suggest that this finding is more common in cases of HGA compared to HME.15
Although doxycycline is the drug of choice for the treatment of ehrlichiosis, Leptospira is susceptible to a wide variety of antibiotics because it exhibits a double membrane surface architecture with components common to both gram‐negative and gram‐positive bacteria.1 Recommended treatment regimens for severe leptospirosis include the use of high‐dose intravenous penicillin or a third‐generation cephalosporin. Less severe cases can be treated with oral amoxicillin or doxycycline.16 The fact that this patient's clinical improvement appeared to lag after initiation of ceftriaxone does not necessarily indicate a lack of efficacy but perhaps a Jarisch‐Herxheimer reaction in response to appropriate antibiotic therapy.
Teaching Points
-
Establishing a diagnosis of leptospirosis is challenging and requires a high index of suspicion. Clinicians should be aware of the limitations of the diagnostic accuracy of the serologic assays for leptospirosis because they are frequently negative in the first week after symptom onset.
-
The classic finding of conjunctival suffusion is helpful in differentiating leptospirosis from human ehrlichiosis.
-
This case also highlights the importance of the clinical practice of making a list of suspected diagnoses, remaining open to these possibilities, and checking serologic tests again in convalescence to confirm the diagnosis.
The approach to clinical conundrums by an expert clinician is revealed through the presentation of an actual patient's case in an approach typical of a morning report. Similarly to patient care, sequential pieces of information are provided to the clinician, who is unfamiliar with the case. The focus is on the thought processes of both the clinical team caring for the patient and the discussant.
Acknowledgements
The authors thank Dr. Brian Harte for his valuable guidance in the preparation of this manuscript.
In October, a 36‐year‐old woman with no significant past medical history presented to the Emergency Department (ED) with a 3‐day history of headache and fever. The headache was severe, throbbing, and frontal in location. She also complained of daily fevers measured up to 103F, generalized malaise, and fatigue. She did not report neck stiffness or photophobia. She felt better after receiving intravenous fluids and was discharged home with a diagnosis of a nonspecific viral illness. Two days later, she returned to the ED with worsening headache, fever, mild photophobia, and poor oral intake. She also complained of a dry cough that made her headache worse, as did bending over. She did not report confusion, neck stiffness, shortness of breath, sore throat, runny nose, abdominal symptoms, or rash.
This patient presents a second time to the ED with worsening headache and fever raising concerns about meningitis. At the time of her first ED visit, it can be assumed that she had a nontoxic appearance because she was discharged shortly thereafter. Thus, acute bacterial meningitis seems less likely, but occasionally patients with meningococcal meningitis may not appear significantly ill until later in the process. Nonetheless, acute meningitis, possibly viral, is the initial concern. The time of the year is an important variable because many viral infections are seasonal. Enteroviruses are the most common cause of viral meningitis in the United States, particularly in the summer and fall. In contrast, mumps, measles, and varicella zoster viruses occur more commonly in winter and spring. Herpetic meningoencephalitis is a life‐threatening condition with a guarded prognosis. Therefore, early recognition and treatment is necessary to decrease morbidity and mortality. Drugs such as nonsteroidal anti‐inflammatory agents, trimethoprim‐sulfamethoxazole, amoxicillin, and rarely vaccines can also cause aseptic meningitis. Infections from fungi, spirochetes, mycobacteria, and rarely parasites also cause meningitis, but would be of greater concern in a patient with risk factors such as recent travel or an immunocompromised state.
Increased headache with bending and cough might indicate elevated intracranial pressure. However, this is a nonspecific complaint, and headache is often worse with the Valsalva maneuver. Because she reports a cough, a chest x‐ray would be useful. In addition to routine initial tests, cerebrospinal fluid (CSF) analysis and human immunodeficiency virus (HIV) testing is recommended.
Her past medical history was notable for depression. Her medications included bupropion, multivitamins, and fish oil. She was also taking milk thistle pills daily to protect her liver because she had been drinking alcohol heavily for the past 2 weeks since her husband left her. She smoked 1 pack of cigarettes daily. She had not traveled recently. She reported no recent animal or wildlife exposure but did recall falling into a midwestern river while canoeing 2 weeks prior to presentation. She worked as a hairstylist and described no sick contacts or risk factors for HIV disease.
An important new historical element is that the patient fell into a river. If she swallowed a significant amount of water during her fall overboard, meningitis from waterborne infections such as Aeromonas, Acanthamoeba, and Naegleria need to be considered. Fortunately, these are rare in the Midwest. Her canoeing history may suggest exposure to wooded areas. Certainly, tickborne infections such as ehrlichiosis, babesiosis, Lyme disease, and Rocky Mountain spotted fever can also cause meningitis. Histoplasmosis and blastomycosis are also endemic to the midwestern United States and can disseminate and cause central nervous system disease.
At this time, viral and bacterial infections are highest on the differential diagnosis. However, the microbiology laboratory needs to be alerted to the possibility of fungal or parasitic organisms depending on the initial CSF analysis results.
The patient was a Caucasian woman who appeared comfortable. Her blood pressure was 130/62 mm Hg, heart rate was 83 beats per minute, respiratory rate was 18 per minute, temperature was 100.8F, and oxygen saturation was 98% on room air. She was fully alert and oriented. Her pupils were bilaterally equal, reactive to light and accommodation with intact extraocular movement and no nystagmus. There was conjunctival injection bilaterally without noticeable pallor or icterus. Fundoscopic examination, which the patient tolerated without difficulty, was normal. Inspection of the oral cavity showed mild tonsillar enlargement. The neck was supple with no stiffness. No cervical, axillary, or inguinal lymph nodes were palpable. Faint bilateral basilar crackles were audible over the posterior chest. There was very mild right upper quadrant abdominal tenderness without guarding. The liver and spleen were normal size and bowel sounds were present. No rash, peripheral edema, or spinal tenderness was noted. A complete neurological examination was normal.
Her general appearance and vital signs seem reassuring. Conjunctival injection and mild tonsillar enlargement are nonspecific findings and may occur in systemic inflammatory states especially viral infections. Atelectasis may account for faint bilateral basilar crackles especially if associated with post‐tussive change. Her alcohol use puts her at risk of aspiration. A right lower lobe process (pneumonia) can sometimes present with right upper quadrant tenderness. However, this tenderness may also represent muscle soreness from repeated coughing, liver, or gallbladder disease. The same infectious process affecting the central nervous system and possibly her lungs, may also be affecting the liver.
A complete blood count revealed a white blood cell count of 3000/mm3 (79% neutrophils, 15% lymphocytes, 5% monocytes), hemoglobin of 11.7 g/dL, and platelets of 110,000/mm3. The serum sodium was 133 mmol/L, potassium was 3.7 mmol/L, bicarbonate was 22 mmol/L, and blood urea nitrogen was 20 mg/dL. The serum creatinine was 1.5 compared to 1.0 mg/dL on testing 2 days prior. A liver function panel showed protein of 5.1 g/dL, albumin of 3 g/dL, aspartate aminotransferase (AST) of 576 IU/L, alanine aminotransferase (ALT) of 584 IU/L, alkaline phosphatase of 282 IU/L, and total bilirubin of 1 mg/dL. The coagulation profile, creatinine phosphokinase, acetaminophen level, urine pregnancy test, urine drug screen, and urinalysis (including urine microscopy) were normal.
The CSF opening pressure was 13 cm H2O. CSF analysis showed 4 mononuclear leukocytes per high‐power field, CSF protein was 27 mg/dL, and glucose was 76 mg/dL. No organisms were noted on gram stain. A chest x‐ray showed focal airspace opacity in the left lower lobe (Figure 1) and the patient was hospitalized for further management.

The normal CSF analysis makes acute meningitis much less likely. It is interesting to note that the aminotransferase levels are nearly equal. Usually, in viral and many other causes of hepatitis, the ALT is higher than the AST, whereas the contrary is true in alcoholic hepatitis. Because the patient has been consuming significant amounts of alcohol recently, these levels may become equal in the setting of another primary liver process. The elevation in liver enzymes also raises the possibility of autoimmune hepatitis secondary to a systemic vasculitis such as systemic lupus erythematosus. Nonetheless, the focus should be on infectious causes of hepatitis such as hepatitis C, adenovirus, parvovirus, Epstein‐Barr virus (EBV), cytomegalovirus, and herpes simplex virus that can cause pneumonia either as a primary or secondary infection. Acute HIV infection can also present in this fashion, and anti‐HIV antibody testing may be negative early in the disease. In the setting of a normal urinalysis and bland urine sediment, prerenal azotemia is the most likely cause of her acute renal injury and can be confirmed by testing the urinary sodium and creatinine. A peripheral smear should be reviewed to evaluate the pancytopenia.
Severe headache, fever, conjunctival injection, pancytopenia, acute kidney injury, hepatitis, and pneumonia may occur in leptospirosis, particularly in a patient with recent freshwater exposure. Alternatively, ehrlichiosis can also account for fever, headache, pancytopenia, renal failure, hepatitis, and pneumonia, but conjunctival suffusion is not often present. At this time, treatment for community‐acquired pneumonia that includes coverage for leptospirosis should be started.
The patient was hydrated with intravenous fluids and treated with intravenous ceftriaxone and azithromycin for community‐acquired pneumonia. An abdominal ultrasound was normal. The serologic assays for acute hepatitis A, B, and C infection were negative. The following morning, she reported worsening headache, increased cough now productive of whitish‐yellow sputum, and diffuse body aches. She appeared more lethargic and toxic. Her blood pressure was 100/83 mm Hg, heart rate was 84 beats per minute, respiratory rate was 24 per minute, and temperature was 101.3F. She had increased crackles on chest auscultation bilaterally and required supplemental oxygen at 4 L/minute by nasal cannula. Examination of both legs now revealed multiple scattered, faintly erythematous, 2‐cm‐sized patches overlying tender subtle subcutaneous nodules. Additionally, a mildly pruritic, V‐shaped area of blanchable erythema was also seen on her chest. The white blood cell count was 2500/mm3 (77% neutrophils, 15% lymphocytes), serum creatinine was 1.8 mg/dL, AST was 351 IU/L, and ALT was 485 IU/L. Blood cultures showed no growth and a peripheral smear examination was unrevealing. A noncontrast chest computed tomographic scan showed findings consistent with multifocal pneumonia (Figure 2).

It would be prudent at this time to expand her antimicrobial coverage (such as with vancomycin and piperacillin‐tazobactam) for activity against methicillin‐resistant Staphylococcus aureus and Pseudomonas because of her clinical worsening. Although ceftriaxone or piperacillin would cover leptospirosis, given the possibility of ehrlichiosis, the addition of doxycycline should be strongly considered.
The description of the rash on her legs seems consistent with erythema nodosum, which is associated with a number of infections (streptococcal, fungal, syphilis, EBV, cat‐scratch disease, tuberculosis), inflammatory conditions (inflammatory bowel disease, autoimmune disease, malignancy), and pregnancy. The blanchable rash on the chest is also a cause of concern for a possible drug reaction (ceftriaxone). A Jarisch‐Herxheimer reaction is possible given her acute worsening of symptoms with initiation of antibiotic therapy.
An antineutrophil cytoplasmic antibodyassociated vasculitis or another autoimmune condition such as systemic lupus erythematosus can account for erythema nodosum, rash, pancytopenia, and hepatitis. This diagnosis might also fit if she had a vasculitic pulmonary hemorrhage that caused her lung infiltrates and worsening hypoxia. A complete antinuclear antibody panel, antineutrophil cytoplasmic antibody, and antismooth muscle antibody testing is recommended. A skin and bronchoscopic biopsy should be considered.
Her dose of ceftriaxone was increased for possible severe pneumococcal pneumonia. The dermatology consultant felt that her leg lesions were consistent with erythema nodosum and the chest rash consistent with cutaneous photodamage. Bronchoscopic examination was normal and a bronchoalveolar lavage sample showed 2905 red blood cells/mm3 and 605 white blood cells/mm3 (70% neutrophils, 7% lymphocytes, 16% histiocytes), normal cytology, and negative cultures. There was no significant clinical improvement by the fourth hospital day and oral doxycycline was started. The next day, her skin lesions had resolved and she felt better. The serologic tests for Legionella, Mycoplasma, cytomegalovirus, EBV, Toxoplasma, Chlamydophila, Ehrlichia, Leptospira, Q‐fever, parvovirus, and adenovirus were negative. A fungal serology panel, HIV polymerase chain reaction, cryoglobulin level, and several rheumatologic tests (antinuclear antibody, extractable nuclear antigen panel, rheumatoid factor, antineutrophil cytoplasmic antibody, antiproteinase 3, and antiglomerular basement membrane antibodies) were normal. Blood cultures continued to show no growth.
The apparent response to doxycycline suggests she might have ehrlichiosis. A buffy coat review for morulae should be done. It is also possible that she may have improved on her initial therapy alone before starting doxycycline and her clinical worsening (including the chest rash) was due to a Jarisch‐Herxheimer reaction. Serologic tests for leptospirosis and ehrlichiosis should be repeated in 12 weeks because such infections may not cause detectable antibody levels early in the illness.
Ceftriaxone and doxycycline were continued and she showed rapid and significant clinical improvement. She was discharged 4 days later with instructions to complete a 10‐day course of antibiotics. At her 3‐month follow‐up, she was doing well and a repeat Leptospira antibody test by the Indirect Hemagglutination Assay (MRL Diagnostics, Cypress, California; normal titer <1:50) was positive at a titer of 1:100, which is highly suggestive of leptospirosis.
Commentary
Leptospirosis is a zoonotic infection caused by spirochetes of the genus Leptospira. The infection is usually transmitted indirectly to humans through contact with water, food, or soil contaminated with the urine of infected mammals.1 Risk factors for infection include participation in recreational activities (such as freshwater swimming, canoeing, and camping), occupational exposure, and exposure to infected pets or domesticated livestock. Approximately 100200 cases are identified annually in the United States, and approximately half occur in the state of Hawaii.2 Outbreaks of leptospirosis have been reported previously in the Midwest.3 These organisms inoculate humans through contact with mucous membranes or broken skin, or enter by swallowing infected food or water. A large number of these infections remain subclinical or result in a very mild illness with spontaneous clearance by the host's immune mechanism. Following an incubation period of 230 days, infected individuals may develop clinically significant disease (Table 1). Clinical presentations may overlap as the disease progresses. Although much remains to be learned about the exact pathogenic mechanism, disruption of the cell membranes of small vessel endothelia (a toxin‐like effect), and cytokine‐mediated tissue injury are believed to cause organ hemorrhage and ischemia.4
|
| 1. Mild influenza‐like self‐remitting disease (90% of cases) |
| Undifferentiated fever (usually 100F105F), severe headache, and myalgia (especially lower limbs). |
| 2. Moderately severe disease usually requiring hospitalization (5%9% of cases) |
| Marked prostration, anorexia, nausea, and vomiting, conjunctival suffusion, transient rash, frequently abdominal pain, constipation or diarrhea, and occasionally epistaxis. |
| 3. Severe disease involving multiple organ systems (1%5% of cases) |
| Hepatorenal Syndrome (Weil's syndrome) |
| Constellation of jaundice, hemorrhagic diathesis, and acute renal failure. Hepatic failure is rarely fatal. Renal involvement is usually more severe and the common cause of death. Cardiac (myocarditis with arrhythmias) and pulmonary complications are frequent. Confusion and restlessness may occur. |
| Hemorrhagic pneumonitis |
| Usually presents as a dry cough initially but becomes blood‐streaked after 23 days. Often characterized by a rapid progression to involve extensive areas of lungs, massive intra‐alveolar hemorrhage, acute respiratory failure, and death. |
| Central nervous system involvement |
| Meningismus, meningitis, or meningoencephalitis. |
The clinical diagnosis of leptospirosis is difficult because of its protean manifestations. Although nonspecific, 2 clinical features may provide a clue to the clinical diagnosis. First, the presence of conjunctival suffusion occurs in the early stage of the disease and is often associated with subconjunctival hemorrhage. Second, severe myalgia, commonly involving the lower limbs, is also characteristically present.1, 5 In 1 series of 58 patients with acute leptospirosis, conjunctival suffusion was observed in 50% of cases, and subconjunctival hemorrhage in 29%. Body ache and muscle tenderness was described in almost all cases.6
As seen in this case, the presence of a rash may pose a clinical challenge. A transient macular, maculopapular, purpuric, or urticarial rash may be seen in acute leptospirosis, but rashes may also be representative of a complication of treatment.1 First described in 1895 in patients with syphilis treated with mercury, the Jarisch‐Herxheimer reaction typically occurs within a few hours of antimicrobial treatment of spirochete infections and often presents with a rash, headache, fever, rigors, hypotension, sweating, and worsening symptoms of the underlying illness.7 Other skin findings such as the occurrence of erythema nodosum have been previously reported in cases of leptospirosis.8
Human ehrlichiosis (HE) is caused by tickborne, obligatory intracellular bacteria that infect leukocytes. There are 3 distinct clinical conditions: human monocytic ehrlichiosis (HME, caused by Ehrlichia chaffeensis), human granulocytic anaplasmosis (HGA, caused by Anaplasma phagocytophilum), and human ewingii ehrlichiosis (HEE, caused by E. ewingii). Although most cases of HME and HEE are seen in the southeastern and south‐central United States and California, the highest incidence of HGA is reported in the northeastern and upper Midwest regions.9 As with leptospirosis, the clinical range of HE spans from asymptomatic infection to life‐threatening illness. Following an incubation period of 12 weeks, symptomatic cases usually present with nonspecific complaints such as high fevers, chills, headache, nausea, arthralgia, myalgia, and malaise.10 The majority of cases will report a tick bite or an exposure to ticks. Laboratory tests often reveal leukopenia (white blood cell count < 4000/mm3), thrombocytopenia, hyponatremia, and elevated AST and ALT. Patients with severe disease may develop renal, respiratory, and hepatic failure. Thus, differentiating ehrlichiosis from leptospirosis is often challenging for the clinician.
However, there are a few clinical clues that help distinguish between these illnesses in this case. HGA as a cause of HE would be more likely in the Midwest. Although a rash is present in one‐third of patients with HME, it is seldom present in HGA unless coinfected with Borrelia burgdorferi, the causative agent for Lyme disease. Additionally, her history of freshwater exposure and the absence of a history of a tick bite also favor leptospirosis. As noted previously, conjunctival suffusion, a characteristic clinical feature of leptospirosis, has only been described in case reports of HE.11, 12
Serologic tests are often used to establish the diagnosis of leptospirosis and ehrlichiosis. Leptospires are fastidious organisms that are difficult to isolate on inoculated growth media. The microscopic agglutination test for leptospirosis is considered the diagnostic gold standard due to its high specificity, but its use is limited by its technical complexity, lack of availability (other than in reference laboratories), and low sensitivity early in the disease (antibody levels detected by this method usually do not appear until 7 days after symptom onset).13 A variety of rapid serologic assays are also available. Although these tests have good overall sensitivity (ranging between 79% and 93%), they perform relatively poorly for acute‐phase sera (sensitivity of 38.5%52.7%).13 The high early false negative rate is believed to be a result of inadequate Leptospira antibody titers in the acute phase of the illness. Seroconversion or a 4‐fold rise between acute and convalescent‐phase antibody titers is the most definitive criterion for the diagnosis of leptospirosis. However, without paired sera samples, a single high microscopic agglutination test titer can be taken as diagnostic for leptospirosis depending on the degree of regional endemicity.14
Similarly, currently available serologic assays for ehrlichiosis produce negative results in most patients in the first week of illness, and it is important to obtain a convalescent phase serum specimen for confirmatory diagnosis of HME and HGA. Seroconversion or a 4‐fold increase in titer between acute and convalescent phase sera is considered diagnostic. The sensitivity of finding morulae (intracytoplasmic vacuolar microcolonies of Ehrlichia) on a peripheral smear is unknown, and data suggest that this finding is more common in cases of HGA compared to HME.15
Although doxycycline is the drug of choice for the treatment of ehrlichiosis, Leptospira is susceptible to a wide variety of antibiotics because it exhibits a double membrane surface architecture with components common to both gram‐negative and gram‐positive bacteria.1 Recommended treatment regimens for severe leptospirosis include the use of high‐dose intravenous penicillin or a third‐generation cephalosporin. Less severe cases can be treated with oral amoxicillin or doxycycline.16 The fact that this patient's clinical improvement appeared to lag after initiation of ceftriaxone does not necessarily indicate a lack of efficacy but perhaps a Jarisch‐Herxheimer reaction in response to appropriate antibiotic therapy.
Teaching Points
-
Establishing a diagnosis of leptospirosis is challenging and requires a high index of suspicion. Clinicians should be aware of the limitations of the diagnostic accuracy of the serologic assays for leptospirosis because they are frequently negative in the first week after symptom onset.
-
The classic finding of conjunctival suffusion is helpful in differentiating leptospirosis from human ehrlichiosis.
-
This case also highlights the importance of the clinical practice of making a list of suspected diagnoses, remaining open to these possibilities, and checking serologic tests again in convalescence to confirm the diagnosis.
The approach to clinical conundrums by an expert clinician is revealed through the presentation of an actual patient's case in an approach typical of a morning report. Similarly to patient care, sequential pieces of information are provided to the clinician, who is unfamiliar with the case. The focus is on the thought processes of both the clinical team caring for the patient and the discussant.
Acknowledgements
The authors thank Dr. Brian Harte for his valuable guidance in the preparation of this manuscript.
- ,,.Leptospirosis: an emerging global public health problem.J Biosci.2008;33:557–569.
- Centers for Disease Control and Prevention. Leptospirosis.2005. http://www.cdc.gov/ncidod/dbmd/diseaseinfo/leptospirosis_t.htm. Accessed November 15,year="2010"2010.
- Morbidity and Mortality Weekly Report.From the Centers for Disease Control and Prevention. Update: leptospirosis and unexplained acute febrile illness among athletes participating in triathlons—Illinois and Wisconsin, 1998.JAMA.1998;280:1474–1475.
- ,.Optimal treatment of leptospirosis: queries and projections.Int J Antimicrob Agents.2006;28:491–496.
- ,.Leptospirosis in the tropics and in travelers.Curr Infect Dis Rep.2006;8:51–58.
- ,,,,,.Clinico‐epidemiological study of hospitalized cases of severe leptospirosis.Indian J Med Res.1999;109:94–99.
- ,.Proposed mechanisms and preventative options of Jarisch‐Herxheimer reactions.J Clin Pharm Ther.2005;30:291–295.
- .Leptospirosis presenting with erythema nodosum.Arch Dis Child.1977;52:418–419.
- ,,.Emerging and re‐emerging tick‐transmitted rickettsial and ehrlichial infections.Med Clin N Am.2008;92:1345–1361.
- ,.Tick‐borne ehrlichiosis infection in human beings.J Vector Borne Dis.2008;45:273–280.
- ,.Ehrlichia in Tennessee.South Med J.1989;82:669.
- ,,,,,.Ehrlichial meningitis with cerebrospinal fluid morulae.Pediatr Infect Dis J.1999;18:552–555.
- ,,, et al.Evaluation of four commercially available rapid serologic tests for diagnosis of leptospirosis.J Clin Microbiol.2003;41:803–809.
- ,.Diagnosis of leptospirosis utilizing modified Faine's criteria.J Assoc Physicians India.2004;52:678–679.
- ,.Ehrlichiosis in children.J Pediatr.1997;131:184–192.
- ,World Health Organization, International Leptospirosis Society. Human leptospirosis: guidance for diagnosis, surveillance and control.Geneva, Switzerland:World Health Organization;2003.
- ,,.Leptospirosis: an emerging global public health problem.J Biosci.2008;33:557–569.
- Centers for Disease Control and Prevention. Leptospirosis.2005. http://www.cdc.gov/ncidod/dbmd/diseaseinfo/leptospirosis_t.htm. Accessed November 15,year="2010"2010.
- Morbidity and Mortality Weekly Report.From the Centers for Disease Control and Prevention. Update: leptospirosis and unexplained acute febrile illness among athletes participating in triathlons—Illinois and Wisconsin, 1998.JAMA.1998;280:1474–1475.
- ,.Optimal treatment of leptospirosis: queries and projections.Int J Antimicrob Agents.2006;28:491–496.
- ,.Leptospirosis in the tropics and in travelers.Curr Infect Dis Rep.2006;8:51–58.
- ,,,,,.Clinico‐epidemiological study of hospitalized cases of severe leptospirosis.Indian J Med Res.1999;109:94–99.
- ,.Proposed mechanisms and preventative options of Jarisch‐Herxheimer reactions.J Clin Pharm Ther.2005;30:291–295.
- .Leptospirosis presenting with erythema nodosum.Arch Dis Child.1977;52:418–419.
- ,,.Emerging and re‐emerging tick‐transmitted rickettsial and ehrlichial infections.Med Clin N Am.2008;92:1345–1361.
- ,.Tick‐borne ehrlichiosis infection in human beings.J Vector Borne Dis.2008;45:273–280.
- ,.Ehrlichia in Tennessee.South Med J.1989;82:669.
- ,,,,,.Ehrlichial meningitis with cerebrospinal fluid morulae.Pediatr Infect Dis J.1999;18:552–555.
- ,,, et al.Evaluation of four commercially available rapid serologic tests for diagnosis of leptospirosis.J Clin Microbiol.2003;41:803–809.
- ,.Diagnosis of leptospirosis utilizing modified Faine's criteria.J Assoc Physicians India.2004;52:678–679.
- ,.Ehrlichiosis in children.J Pediatr.1997;131:184–192.
- ,World Health Organization, International Leptospirosis Society. Human leptospirosis: guidance for diagnosis, surveillance and control.Geneva, Switzerland:World Health Organization;2003.
Hospitalists and ACC in Pandemic Flu
Major natural disasters, such as Hurricane Rita and Hurricane Katrina in 2005, have reinforced the reality that health care workers may be asked to treat patients outside the traditional hospital setting.1 The emergence of H5N1 avian influenza in Southeast Asia has also raised concerns about a potential worldwide pandemic influenza.2 Since 2003, the number of avian influenza cases in humans has totaled 387, with 245 deaths.3 While H5N1 influenza has thus far been largely confined to avian populations, the virulence of this strain has raised concern regarding the possible emergence of enhanced human transmission.4 While impossible to accurately forecast the devastation of the next pandemic on the health system, anything similar to the pandemics of the past century will require a large coordinated response by the health system. The most severe pandemic in the past century occurred in 1918 to 1919. The estimated deaths attributed to this worldwide ranges from 20 to 100 million persons,57 with >500,000 of these deaths in the United States.6, 7 In comparison, the annual rate of deaths related to influenza in the United States ranges from 30,000 to 50,000.2, 5 It has been estimated that the next pandemic influenza could cause 75 to 100 million people to become ill, and lead to as many as 1.9 million deaths in the United States.8 In response, the Department of Health and Human Services (HHS) has stressed the importance of advanced planning,9 and the most recent Homeland Security Presidential Directive (HSPD‐21) directs health care organizations and the federal government to develop preparedness plans to provide surge capacity care in times of a catastrophic health event.10 A previous report by one of the authors emphasized the need for hospitalists to play a major role in institutional planning for a pandemic influenza.11
The Alternate Care Center
The concept of offsite care in an influenza pandemic has previously been described, and we will refer to these as Alternate Care Centers (ACCs). Although the literature describes different models of care at an ACC (Table 1),12 we believe an ACC should be activated as an extension of the supporting hospital, once the hospital becomes over capacity despite measures to grow its inpatient service volume.
| Overflow hospital providing full range of care |
| Patient isolation and alternative to home care for infectious patients |
| Expanded ambulatory care |
| Care for recovering, noninfectious patients |
| Limited supportive care for noncritical patients |
| Primary triage and rapid patient screening |
| Quarantine |
Our health system is a large academic medical center, and we have been working with our state to develop a plan to establish and operate an ACC for the next pandemic influenza. Our plans call for an ACC to be activated as an overflow hospital once our hospitals are beyond 120% capacity. We have gone through several functional and tabletop exercises to help identify critical issues that are likely to arise during a real pandemic. Subsequent to these exercises, we have convened an ACC Planning Work Group, reviewed the available literature on surge hospitals, and have focused our recent efforts on several key areas.13 First, it will be important to clearly outline the general services that will be available at this offsite location (Table 2), and this information should be disseminated to the local medical community and the general public. An informed public, with a clear understanding that the ACC is an extension of the hospital with hospitalists in charge of medical care, is more likely to accept getting healthcare in this setting.
|
| IVF administration |
| Parenteral medication administration (eg, antibiotics, steroids, narcotic analgesics, antiemetics) |
| Oxygen support |
| Palliative care services |
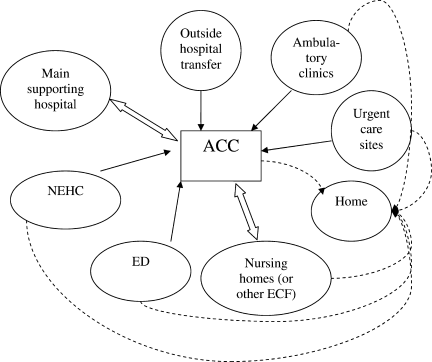
Second, hospitals and the ACCas an extension to the main hospitalwill be asked to provide care to patients referred from several external facilities. Thus, the relationship between the ACC and the main hospital is critical. In a situation where local and even national health care assets will be overwhelmed, having a traditional hospital take full ownership of the ACC and facilitate the transport of patients in and out of the center will be vital to the maintenance of operations. Figure 1 illustrates an example of how patients may be transitioned from 1 site of care to another.
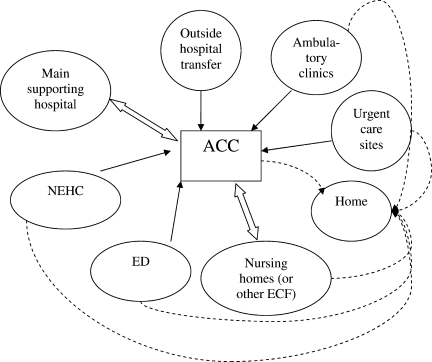
Third, the logistics of establishing an ACC should include details regarding: (1) securing a location that is able to accommodate the needs of the ACC; (2) predetermining the scope of care that can be provided; (3) procuring the necessary equipment and supplies; (4) planning for an adequate number of workforce and staff members; and (5) ensuring a reliable communication plan within the local health system and with state and federal public health officials.14 Staffing shortages and communication barriers are worthy of further emphasis. Given conservative estimates that up to 35% of staff may become ill, refuse to work, or remain home to care for ill family members,15 it is essential that hospitals and regional emergency planners develop a staffing model for the ACC, well in advance of a pandemic. These may include scenarios in which the recommended provider‐to‐patient ratio can not be met. Among the essential lessons learned from the severe acute respiratory syndrome (SARS) outbreak in Toronto (Ontario, Canada) was the importance of developing redundant and reliable communication plans among the healthcare providers.16, 17
Last, healthcare workers' concerns about occupational health and safety must be addressed, and strict measures to protect providers in the ACC need to be implemented.16 This includes providing all exposed staff with adequate personal protective equipment (eg, N‐95 masks), ensuring that all staff are vaccinated against the influenza virus, and implementing strict infection control (eg, hand washing) practices.
For more information, we refer the reader to references that contain further details on our ACC exercises13 and documents that outline concepts of operations in an ACC, developed by the Joint Commission and a multiagency working group.1, 14
The Hospitalist Physician and the ACC
During an influenza pandemic, physicians from all specialties will be vital to the success of the health systems' response. General internists,18 family practitioners, and pediatricians will be overextended in the ambulatory setting to provide intravenous (IV) fluids, antibiotics, and vaccines. Emergency physicians will be called upon to provide care for a burgeoning number of patient arrivals to the Emergency Department (ED), whose acuity is higher than in nonpandemic times. These physicians' clinical expertise at their sites of practice may be severely tested. Hospitalists, given their inpatient focus will be ideally suited to provide medical care to patients admitted to the ACC.
Previous physician leadership at surge hospitals has come from multiple specialties. Case studies describing the heroic physician leadership after Hurricane Katrina and Hurricane Rita represented pediatricians, family physicians, emergency department physicians, and internists.1 In an influenza pandemic, patients in the ACC will require medical care that would, under nonsurge situations, warrant inpatient care. Hospitalists are well poised to lead the response in the ACC for pandemic flu. Hospitalists have expanded their presence into many clinical and administrative responsibilities in their local health systems,19 and the specialty of hospital medicine has evolved to incorporate many of the skills and expertise that would be required of physician leaders who manage an ACC during an influenza pandemic.
While the actual morbidity and mortality associated with the next pandemic are uncertain, it is likely that the number of patients who seek out medical care will exceed current capacity. With constrained space and resources, patients will require appropriate and safe transition to and from the hospital and the ACC. Hospitalists have become leaders in developing and promoting quality transition of care out of acute care settings.20, 21 Their expertise in optimizing this vulnerable time period in patients' healthcare experience should help hospitalists make efficient and appropriate transition care decisions even during busy times and in an alternate care location. Many hospitalists have also developed local and national expertise in quality improvement (QI) and patient safety (PS) initiatives in acute care settings.22 Hospitalists can lead the efforts to apply QI and PS practices in the ACC. These interventions should focus on the potential to be effective in improving patient care, but also consider issues such as ease of implementation, cost, and potential for harm.23
An influenza pandemic will require all levels of the healthcare system to work together to develop a coordinated approach to patient care. Previously, Kisuule et al.24 described how hospitalists can expand their role to include public health. The hospitalists' leadership in the ACC fits well with their descriptions, and hospitalists should work with local, state, and national public health officials in pandemic flu planning. Their scope of practice and clinical expertise will call on them to play key roles in recognition of the development of a pandemic; help lead the response efforts; provide education to staff, patients, and family members; develop clinical care guidelines and pathways for patients; utilize best practices in the use of antimicrobial therapy; and provide appropriate palliative care. Depending on the severity of the influenza pandemic, mortality could be considerable. Many hospitalists have expertise in palliative care at their hospitals,2527 and this skill set will be invaluable in providing compassionate end‐of‐life care to patients in the ACC.
In a pandemic, the most vulnerable patient populations will likely be disproportionately affected, including the elderly, children, and the immune‐compromised. Hospitalists who care regularly for these diverse groups of patients through the spectrum of illness and recovery will be able to address the variety of clinical and nonclinical issues that arise. If the ACC will provide care for children, hospitalists with training in pediatrics, medicine‐pediatrics, or family medicine should be available.
Additional Considerations
While many unanswered questions remain about how to best utilize the ACC, hospitalists are ideally suited to help lead planning efforts for an ACC for pandemic flu. Other issues that may require additional considerations include: (1) whether to strictly care for patients with influenza symptoms and influenza‐related illnesses or to provide care for all patients at the ACC; (2) what to do when patients refuse transfer to and from the ACC; (3) determining the optimal staffing model for patient care providers and to provide care for a wide range of age groups; (4) how the ACC will be funded; (5) how and where to store stockpiles; (6) developing redundant and coordinated communication plans; and (7) planning for reliable access to information and technology from the ACC.
Conclusions
We have introduced the concept of the ACC for the hospitalist community, and emphasized the benefits of engaging hospitalists to lead the ACC initiative at their own health organizations during pandemic flu. As hospitalists currently serve in many of these roles and possess the skills to provide care and lead these initiatives, we encourage hospitalists to contact their hospital administrators to volunteer to assist with preparation efforts.
- Joint Commission on Accreditation of Healthcare Organizations. Surge Hospitals: Providing Safe Care in Emergencies;2006. Available at: http://www.jointcommission.org/NR/rdonlyres/802E9DA4‐AE80‐4584‐A205‐48989C5BD684/0/surge_hospital.pdf. Accessed May 2009.
- .Pandemic influenza: are we ready?Disaster Manag Response.2005;3(3):61–67.
- Cumulative Number of Confirmed Human Cases of Avian Influenza A/(H5N1) Reported to WHO.2008. Available at: http://www.who.int/csr/disease/avian_influenza/country/cases_table_2008_09_10/en/index.html. Accessed May 2009.
- ,,, et al.Human infection with highly pathogenic H5N1 influenza virus.Lancet.2008;371(9622):1464–1475.
- .Preparing for the next pandemic.N Engl J Med.2005;352(18):1839–1842.
- ,,.Influenza pandemic preparedness action plan for the United States: 2002 update.Clin Infect Dis.2002;35(5):590–596.
- ,,, et al.Nonpharmaceutical interventions implemented by US cities during the 1918‐1919 influenza pandemic.JAMA.2007;298(6):644–654.
- The Health Care Response to Pandemic Influenza: Position Paper.Philadelphia, PA:American College of Physicians;2006.
- U.S. Department of Health and Human Services (HHS). HHS Pandemic Influenza Plan. November2005. Available at: http://www.hhs.gov/pandemicflu/plan. Accessed May 2009.
- Homeland Security Presidential Directive/HSPD‐21.2007. Available at: http://www.whitehouse.gov/news/releases/2007/10/20071018‐10.html. Accessed May 2009.
- ,.Pandemic influenza and the hospitalist: apocalypse when?J Hosp Med.2006;1(2):118–123.
- ,,,,.The prospect of using alternative medical care facilities in an influenza pandemic.Biosecur Bioterror.2006;4(4):384–390.
- ,,, et al.Pandemic influenza and acute care centers (ACCs): taking care of sick patients in a non‐hospital setting.Biosecur Bioterror.2008;6(4):335–348.
- ,,.Acute Care Center. Modular Emergency Medical System: Concept of Operations for the Acute Care Center (ACC).Mass Casualty Care Strategy for A Biological Terrorism Incident. May2003. Available at: http://dms.dartmouth.edu/nnemmrs/resources/surge_capacity_guidance/documents/acute_care_center__concept_ of_operations. pdf. Accessed May 2009.
- Illinois Department of Public Health. Influenza.2007. Available at: http://www.idph.state.il.us/flu/pandemicfs.htm. Accessed May 2009.
- ,,.Learning from SARS in Hong Kong and Toronto.JAMA.2004;291(20):2483–2487.
- .Planning for epidemics—the lessons of SARS.N Engl J Med.2004;350(23):2332–2334.
- .The role of internists during epidemics, outbreaks, and bioterrorist attacks.J Gen Intern Med.2007;22(1):131–136.
- ,.The expanding role of hospitalists in the United States.Swiss Med Wkly.2006;136(37‐38):591–596.
- ,,,.Promoting effective transitions of care at hospital discharge: a review of key issues for hospitalists.J Hosp Med.2007;2(5):314–323.
- ,.Executing high‐quality care transitions: a call to do it right.J Hosp Med.2007;2(5):287–290.
- .Reflections: the hospitalist movement a decade later.J Hosp Med.2006;1(4):248–252.
- ,.Implementing patient safety interventions in your hospital: what to try and what to avoid.Med Clin North Am.2008;92(2):275–293, vii‐viii.
- ,,,.Expanding the roles of hospitalist physicians to include public health.J Hosp Med.2007;2(,2):93–101.
- ,,,,,.Evaluating the California hospital initiative in palliative services.Arch Intern Med.2006;166(2):227–230.
- .Palliative care and hospitalists: a partnership for hope.J Hosp Med.2006;1(1):5–6.
- .Palliative care in hospitals.J Hosp Med.2006;1(1):21–28.
Major natural disasters, such as Hurricane Rita and Hurricane Katrina in 2005, have reinforced the reality that health care workers may be asked to treat patients outside the traditional hospital setting.1 The emergence of H5N1 avian influenza in Southeast Asia has also raised concerns about a potential worldwide pandemic influenza.2 Since 2003, the number of avian influenza cases in humans has totaled 387, with 245 deaths.3 While H5N1 influenza has thus far been largely confined to avian populations, the virulence of this strain has raised concern regarding the possible emergence of enhanced human transmission.4 While impossible to accurately forecast the devastation of the next pandemic on the health system, anything similar to the pandemics of the past century will require a large coordinated response by the health system. The most severe pandemic in the past century occurred in 1918 to 1919. The estimated deaths attributed to this worldwide ranges from 20 to 100 million persons,57 with >500,000 of these deaths in the United States.6, 7 In comparison, the annual rate of deaths related to influenza in the United States ranges from 30,000 to 50,000.2, 5 It has been estimated that the next pandemic influenza could cause 75 to 100 million people to become ill, and lead to as many as 1.9 million deaths in the United States.8 In response, the Department of Health and Human Services (HHS) has stressed the importance of advanced planning,9 and the most recent Homeland Security Presidential Directive (HSPD‐21) directs health care organizations and the federal government to develop preparedness plans to provide surge capacity care in times of a catastrophic health event.10 A previous report by one of the authors emphasized the need for hospitalists to play a major role in institutional planning for a pandemic influenza.11
The Alternate Care Center
The concept of offsite care in an influenza pandemic has previously been described, and we will refer to these as Alternate Care Centers (ACCs). Although the literature describes different models of care at an ACC (Table 1),12 we believe an ACC should be activated as an extension of the supporting hospital, once the hospital becomes over capacity despite measures to grow its inpatient service volume.
| Overflow hospital providing full range of care |
| Patient isolation and alternative to home care for infectious patients |
| Expanded ambulatory care |
| Care for recovering, noninfectious patients |
| Limited supportive care for noncritical patients |
| Primary triage and rapid patient screening |
| Quarantine |
Our health system is a large academic medical center, and we have been working with our state to develop a plan to establish and operate an ACC for the next pandemic influenza. Our plans call for an ACC to be activated as an overflow hospital once our hospitals are beyond 120% capacity. We have gone through several functional and tabletop exercises to help identify critical issues that are likely to arise during a real pandemic. Subsequent to these exercises, we have convened an ACC Planning Work Group, reviewed the available literature on surge hospitals, and have focused our recent efforts on several key areas.13 First, it will be important to clearly outline the general services that will be available at this offsite location (Table 2), and this information should be disseminated to the local medical community and the general public. An informed public, with a clear understanding that the ACC is an extension of the hospital with hospitalists in charge of medical care, is more likely to accept getting healthcare in this setting.
|
| IVF administration |
| Parenteral medication administration (eg, antibiotics, steroids, narcotic analgesics, antiemetics) |
| Oxygen support |
| Palliative care services |
Second, hospitals and the ACCas an extension to the main hospitalwill be asked to provide care to patients referred from several external facilities. Thus, the relationship between the ACC and the main hospital is critical. In a situation where local and even national health care assets will be overwhelmed, having a traditional hospital take full ownership of the ACC and facilitate the transport of patients in and out of the center will be vital to the maintenance of operations. Figure 1 illustrates an example of how patients may be transitioned from 1 site of care to another.

Third, the logistics of establishing an ACC should include details regarding: (1) securing a location that is able to accommodate the needs of the ACC; (2) predetermining the scope of care that can be provided; (3) procuring the necessary equipment and supplies; (4) planning for an adequate number of workforce and staff members; and (5) ensuring a reliable communication plan within the local health system and with state and federal public health officials.14 Staffing shortages and communication barriers are worthy of further emphasis. Given conservative estimates that up to 35% of staff may become ill, refuse to work, or remain home to care for ill family members,15 it is essential that hospitals and regional emergency planners develop a staffing model for the ACC, well in advance of a pandemic. These may include scenarios in which the recommended provider‐to‐patient ratio can not be met. Among the essential lessons learned from the severe acute respiratory syndrome (SARS) outbreak in Toronto (Ontario, Canada) was the importance of developing redundant and reliable communication plans among the healthcare providers.16, 17
Last, healthcare workers' concerns about occupational health and safety must be addressed, and strict measures to protect providers in the ACC need to be implemented.16 This includes providing all exposed staff with adequate personal protective equipment (eg, N‐95 masks), ensuring that all staff are vaccinated against the influenza virus, and implementing strict infection control (eg, hand washing) practices.
For more information, we refer the reader to references that contain further details on our ACC exercises13 and documents that outline concepts of operations in an ACC, developed by the Joint Commission and a multiagency working group.1, 14
The Hospitalist Physician and the ACC
During an influenza pandemic, physicians from all specialties will be vital to the success of the health systems' response. General internists,18 family practitioners, and pediatricians will be overextended in the ambulatory setting to provide intravenous (IV) fluids, antibiotics, and vaccines. Emergency physicians will be called upon to provide care for a burgeoning number of patient arrivals to the Emergency Department (ED), whose acuity is higher than in nonpandemic times. These physicians' clinical expertise at their sites of practice may be severely tested. Hospitalists, given their inpatient focus will be ideally suited to provide medical care to patients admitted to the ACC.
Previous physician leadership at surge hospitals has come from multiple specialties. Case studies describing the heroic physician leadership after Hurricane Katrina and Hurricane Rita represented pediatricians, family physicians, emergency department physicians, and internists.1 In an influenza pandemic, patients in the ACC will require medical care that would, under nonsurge situations, warrant inpatient care. Hospitalists are well poised to lead the response in the ACC for pandemic flu. Hospitalists have expanded their presence into many clinical and administrative responsibilities in their local health systems,19 and the specialty of hospital medicine has evolved to incorporate many of the skills and expertise that would be required of physician leaders who manage an ACC during an influenza pandemic.
While the actual morbidity and mortality associated with the next pandemic are uncertain, it is likely that the number of patients who seek out medical care will exceed current capacity. With constrained space and resources, patients will require appropriate and safe transition to and from the hospital and the ACC. Hospitalists have become leaders in developing and promoting quality transition of care out of acute care settings.20, 21 Their expertise in optimizing this vulnerable time period in patients' healthcare experience should help hospitalists make efficient and appropriate transition care decisions even during busy times and in an alternate care location. Many hospitalists have also developed local and national expertise in quality improvement (QI) and patient safety (PS) initiatives in acute care settings.22 Hospitalists can lead the efforts to apply QI and PS practices in the ACC. These interventions should focus on the potential to be effective in improving patient care, but also consider issues such as ease of implementation, cost, and potential for harm.23
An influenza pandemic will require all levels of the healthcare system to work together to develop a coordinated approach to patient care. Previously, Kisuule et al.24 described how hospitalists can expand their role to include public health. The hospitalists' leadership in the ACC fits well with their descriptions, and hospitalists should work with local, state, and national public health officials in pandemic flu planning. Their scope of practice and clinical expertise will call on them to play key roles in recognition of the development of a pandemic; help lead the response efforts; provide education to staff, patients, and family members; develop clinical care guidelines and pathways for patients; utilize best practices in the use of antimicrobial therapy; and provide appropriate palliative care. Depending on the severity of the influenza pandemic, mortality could be considerable. Many hospitalists have expertise in palliative care at their hospitals,2527 and this skill set will be invaluable in providing compassionate end‐of‐life care to patients in the ACC.
In a pandemic, the most vulnerable patient populations will likely be disproportionately affected, including the elderly, children, and the immune‐compromised. Hospitalists who care regularly for these diverse groups of patients through the spectrum of illness and recovery will be able to address the variety of clinical and nonclinical issues that arise. If the ACC will provide care for children, hospitalists with training in pediatrics, medicine‐pediatrics, or family medicine should be available.
Additional Considerations
While many unanswered questions remain about how to best utilize the ACC, hospitalists are ideally suited to help lead planning efforts for an ACC for pandemic flu. Other issues that may require additional considerations include: (1) whether to strictly care for patients with influenza symptoms and influenza‐related illnesses or to provide care for all patients at the ACC; (2) what to do when patients refuse transfer to and from the ACC; (3) determining the optimal staffing model for patient care providers and to provide care for a wide range of age groups; (4) how the ACC will be funded; (5) how and where to store stockpiles; (6) developing redundant and coordinated communication plans; and (7) planning for reliable access to information and technology from the ACC.
Conclusions
We have introduced the concept of the ACC for the hospitalist community, and emphasized the benefits of engaging hospitalists to lead the ACC initiative at their own health organizations during pandemic flu. As hospitalists currently serve in many of these roles and possess the skills to provide care and lead these initiatives, we encourage hospitalists to contact their hospital administrators to volunteer to assist with preparation efforts.
Major natural disasters, such as Hurricane Rita and Hurricane Katrina in 2005, have reinforced the reality that health care workers may be asked to treat patients outside the traditional hospital setting.1 The emergence of H5N1 avian influenza in Southeast Asia has also raised concerns about a potential worldwide pandemic influenza.2 Since 2003, the number of avian influenza cases in humans has totaled 387, with 245 deaths.3 While H5N1 influenza has thus far been largely confined to avian populations, the virulence of this strain has raised concern regarding the possible emergence of enhanced human transmission.4 While impossible to accurately forecast the devastation of the next pandemic on the health system, anything similar to the pandemics of the past century will require a large coordinated response by the health system. The most severe pandemic in the past century occurred in 1918 to 1919. The estimated deaths attributed to this worldwide ranges from 20 to 100 million persons,57 with >500,000 of these deaths in the United States.6, 7 In comparison, the annual rate of deaths related to influenza in the United States ranges from 30,000 to 50,000.2, 5 It has been estimated that the next pandemic influenza could cause 75 to 100 million people to become ill, and lead to as many as 1.9 million deaths in the United States.8 In response, the Department of Health and Human Services (HHS) has stressed the importance of advanced planning,9 and the most recent Homeland Security Presidential Directive (HSPD‐21) directs health care organizations and the federal government to develop preparedness plans to provide surge capacity care in times of a catastrophic health event.10 A previous report by one of the authors emphasized the need for hospitalists to play a major role in institutional planning for a pandemic influenza.11
The Alternate Care Center
The concept of offsite care in an influenza pandemic has previously been described, and we will refer to these as Alternate Care Centers (ACCs). Although the literature describes different models of care at an ACC (Table 1),12 we believe an ACC should be activated as an extension of the supporting hospital, once the hospital becomes over capacity despite measures to grow its inpatient service volume.
| Overflow hospital providing full range of care |
| Patient isolation and alternative to home care for infectious patients |
| Expanded ambulatory care |
| Care for recovering, noninfectious patients |
| Limited supportive care for noncritical patients |
| Primary triage and rapid patient screening |
| Quarantine |
Our health system is a large academic medical center, and we have been working with our state to develop a plan to establish and operate an ACC for the next pandemic influenza. Our plans call for an ACC to be activated as an overflow hospital once our hospitals are beyond 120% capacity. We have gone through several functional and tabletop exercises to help identify critical issues that are likely to arise during a real pandemic. Subsequent to these exercises, we have convened an ACC Planning Work Group, reviewed the available literature on surge hospitals, and have focused our recent efforts on several key areas.13 First, it will be important to clearly outline the general services that will be available at this offsite location (Table 2), and this information should be disseminated to the local medical community and the general public. An informed public, with a clear understanding that the ACC is an extension of the hospital with hospitalists in charge of medical care, is more likely to accept getting healthcare in this setting.
|
| IVF administration |
| Parenteral medication administration (eg, antibiotics, steroids, narcotic analgesics, antiemetics) |
| Oxygen support |
| Palliative care services |
Second, hospitals and the ACCas an extension to the main hospitalwill be asked to provide care to patients referred from several external facilities. Thus, the relationship between the ACC and the main hospital is critical. In a situation where local and even national health care assets will be overwhelmed, having a traditional hospital take full ownership of the ACC and facilitate the transport of patients in and out of the center will be vital to the maintenance of operations. Figure 1 illustrates an example of how patients may be transitioned from 1 site of care to another.

Third, the logistics of establishing an ACC should include details regarding: (1) securing a location that is able to accommodate the needs of the ACC; (2) predetermining the scope of care that can be provided; (3) procuring the necessary equipment and supplies; (4) planning for an adequate number of workforce and staff members; and (5) ensuring a reliable communication plan within the local health system and with state and federal public health officials.14 Staffing shortages and communication barriers are worthy of further emphasis. Given conservative estimates that up to 35% of staff may become ill, refuse to work, or remain home to care for ill family members,15 it is essential that hospitals and regional emergency planners develop a staffing model for the ACC, well in advance of a pandemic. These may include scenarios in which the recommended provider‐to‐patient ratio can not be met. Among the essential lessons learned from the severe acute respiratory syndrome (SARS) outbreak in Toronto (Ontario, Canada) was the importance of developing redundant and reliable communication plans among the healthcare providers.16, 17
Last, healthcare workers' concerns about occupational health and safety must be addressed, and strict measures to protect providers in the ACC need to be implemented.16 This includes providing all exposed staff with adequate personal protective equipment (eg, N‐95 masks), ensuring that all staff are vaccinated against the influenza virus, and implementing strict infection control (eg, hand washing) practices.
For more information, we refer the reader to references that contain further details on our ACC exercises13 and documents that outline concepts of operations in an ACC, developed by the Joint Commission and a multiagency working group.1, 14
The Hospitalist Physician and the ACC
During an influenza pandemic, physicians from all specialties will be vital to the success of the health systems' response. General internists,18 family practitioners, and pediatricians will be overextended in the ambulatory setting to provide intravenous (IV) fluids, antibiotics, and vaccines. Emergency physicians will be called upon to provide care for a burgeoning number of patient arrivals to the Emergency Department (ED), whose acuity is higher than in nonpandemic times. These physicians' clinical expertise at their sites of practice may be severely tested. Hospitalists, given their inpatient focus will be ideally suited to provide medical care to patients admitted to the ACC.
Previous physician leadership at surge hospitals has come from multiple specialties. Case studies describing the heroic physician leadership after Hurricane Katrina and Hurricane Rita represented pediatricians, family physicians, emergency department physicians, and internists.1 In an influenza pandemic, patients in the ACC will require medical care that would, under nonsurge situations, warrant inpatient care. Hospitalists are well poised to lead the response in the ACC for pandemic flu. Hospitalists have expanded their presence into many clinical and administrative responsibilities in their local health systems,19 and the specialty of hospital medicine has evolved to incorporate many of the skills and expertise that would be required of physician leaders who manage an ACC during an influenza pandemic.
While the actual morbidity and mortality associated with the next pandemic are uncertain, it is likely that the number of patients who seek out medical care will exceed current capacity. With constrained space and resources, patients will require appropriate and safe transition to and from the hospital and the ACC. Hospitalists have become leaders in developing and promoting quality transition of care out of acute care settings.20, 21 Their expertise in optimizing this vulnerable time period in patients' healthcare experience should help hospitalists make efficient and appropriate transition care decisions even during busy times and in an alternate care location. Many hospitalists have also developed local and national expertise in quality improvement (QI) and patient safety (PS) initiatives in acute care settings.22 Hospitalists can lead the efforts to apply QI and PS practices in the ACC. These interventions should focus on the potential to be effective in improving patient care, but also consider issues such as ease of implementation, cost, and potential for harm.23
An influenza pandemic will require all levels of the healthcare system to work together to develop a coordinated approach to patient care. Previously, Kisuule et al.24 described how hospitalists can expand their role to include public health. The hospitalists' leadership in the ACC fits well with their descriptions, and hospitalists should work with local, state, and national public health officials in pandemic flu planning. Their scope of practice and clinical expertise will call on them to play key roles in recognition of the development of a pandemic; help lead the response efforts; provide education to staff, patients, and family members; develop clinical care guidelines and pathways for patients; utilize best practices in the use of antimicrobial therapy; and provide appropriate palliative care. Depending on the severity of the influenza pandemic, mortality could be considerable. Many hospitalists have expertise in palliative care at their hospitals,2527 and this skill set will be invaluable in providing compassionate end‐of‐life care to patients in the ACC.
In a pandemic, the most vulnerable patient populations will likely be disproportionately affected, including the elderly, children, and the immune‐compromised. Hospitalists who care regularly for these diverse groups of patients through the spectrum of illness and recovery will be able to address the variety of clinical and nonclinical issues that arise. If the ACC will provide care for children, hospitalists with training in pediatrics, medicine‐pediatrics, or family medicine should be available.
Additional Considerations
While many unanswered questions remain about how to best utilize the ACC, hospitalists are ideally suited to help lead planning efforts for an ACC for pandemic flu. Other issues that may require additional considerations include: (1) whether to strictly care for patients with influenza symptoms and influenza‐related illnesses or to provide care for all patients at the ACC; (2) what to do when patients refuse transfer to and from the ACC; (3) determining the optimal staffing model for patient care providers and to provide care for a wide range of age groups; (4) how the ACC will be funded; (5) how and where to store stockpiles; (6) developing redundant and coordinated communication plans; and (7) planning for reliable access to information and technology from the ACC.
Conclusions
We have introduced the concept of the ACC for the hospitalist community, and emphasized the benefits of engaging hospitalists to lead the ACC initiative at their own health organizations during pandemic flu. As hospitalists currently serve in many of these roles and possess the skills to provide care and lead these initiatives, we encourage hospitalists to contact their hospital administrators to volunteer to assist with preparation efforts.
- Joint Commission on Accreditation of Healthcare Organizations. Surge Hospitals: Providing Safe Care in Emergencies;2006. Available at: http://www.jointcommission.org/NR/rdonlyres/802E9DA4‐AE80‐4584‐A205‐48989C5BD684/0/surge_hospital.pdf. Accessed May 2009.
- .Pandemic influenza: are we ready?Disaster Manag Response.2005;3(3):61–67.
- Cumulative Number of Confirmed Human Cases of Avian Influenza A/(H5N1) Reported to WHO.2008. Available at: http://www.who.int/csr/disease/avian_influenza/country/cases_table_2008_09_10/en/index.html. Accessed May 2009.
- ,,, et al.Human infection with highly pathogenic H5N1 influenza virus.Lancet.2008;371(9622):1464–1475.
- .Preparing for the next pandemic.N Engl J Med.2005;352(18):1839–1842.
- ,,.Influenza pandemic preparedness action plan for the United States: 2002 update.Clin Infect Dis.2002;35(5):590–596.
- ,,, et al.Nonpharmaceutical interventions implemented by US cities during the 1918‐1919 influenza pandemic.JAMA.2007;298(6):644–654.
- The Health Care Response to Pandemic Influenza: Position Paper.Philadelphia, PA:American College of Physicians;2006.
- U.S. Department of Health and Human Services (HHS). HHS Pandemic Influenza Plan. November2005. Available at: http://www.hhs.gov/pandemicflu/plan. Accessed May 2009.
- Homeland Security Presidential Directive/HSPD‐21.2007. Available at: http://www.whitehouse.gov/news/releases/2007/10/20071018‐10.html. Accessed May 2009.
- ,.Pandemic influenza and the hospitalist: apocalypse when?J Hosp Med.2006;1(2):118–123.
- ,,,,.The prospect of using alternative medical care facilities in an influenza pandemic.Biosecur Bioterror.2006;4(4):384–390.
- ,,, et al.Pandemic influenza and acute care centers (ACCs): taking care of sick patients in a non‐hospital setting.Biosecur Bioterror.2008;6(4):335–348.
- ,,.Acute Care Center. Modular Emergency Medical System: Concept of Operations for the Acute Care Center (ACC).Mass Casualty Care Strategy for A Biological Terrorism Incident. May2003. Available at: http://dms.dartmouth.edu/nnemmrs/resources/surge_capacity_guidance/documents/acute_care_center__concept_ of_operations. pdf. Accessed May 2009.
- Illinois Department of Public Health. Influenza.2007. Available at: http://www.idph.state.il.us/flu/pandemicfs.htm. Accessed May 2009.
- ,,.Learning from SARS in Hong Kong and Toronto.JAMA.2004;291(20):2483–2487.
- .Planning for epidemics—the lessons of SARS.N Engl J Med.2004;350(23):2332–2334.
- .The role of internists during epidemics, outbreaks, and bioterrorist attacks.J Gen Intern Med.2007;22(1):131–136.
- ,.The expanding role of hospitalists in the United States.Swiss Med Wkly.2006;136(37‐38):591–596.
- ,,,.Promoting effective transitions of care at hospital discharge: a review of key issues for hospitalists.J Hosp Med.2007;2(5):314–323.
- ,.Executing high‐quality care transitions: a call to do it right.J Hosp Med.2007;2(5):287–290.
- .Reflections: the hospitalist movement a decade later.J Hosp Med.2006;1(4):248–252.
- ,.Implementing patient safety interventions in your hospital: what to try and what to avoid.Med Clin North Am.2008;92(2):275–293, vii‐viii.
- ,,,.Expanding the roles of hospitalist physicians to include public health.J Hosp Med.2007;2(,2):93–101.
- ,,,,,.Evaluating the California hospital initiative in palliative services.Arch Intern Med.2006;166(2):227–230.
- .Palliative care and hospitalists: a partnership for hope.J Hosp Med.2006;1(1):5–6.
- .Palliative care in hospitals.J Hosp Med.2006;1(1):21–28.
- Joint Commission on Accreditation of Healthcare Organizations. Surge Hospitals: Providing Safe Care in Emergencies;2006. Available at: http://www.jointcommission.org/NR/rdonlyres/802E9DA4‐AE80‐4584‐A205‐48989C5BD684/0/surge_hospital.pdf. Accessed May 2009.
- .Pandemic influenza: are we ready?Disaster Manag Response.2005;3(3):61–67.
- Cumulative Number of Confirmed Human Cases of Avian Influenza A/(H5N1) Reported to WHO.2008. Available at: http://www.who.int/csr/disease/avian_influenza/country/cases_table_2008_09_10/en/index.html. Accessed May 2009.
- ,,, et al.Human infection with highly pathogenic H5N1 influenza virus.Lancet.2008;371(9622):1464–1475.
- .Preparing for the next pandemic.N Engl J Med.2005;352(18):1839–1842.
- ,,.Influenza pandemic preparedness action plan for the United States: 2002 update.Clin Infect Dis.2002;35(5):590–596.
- ,,, et al.Nonpharmaceutical interventions implemented by US cities during the 1918‐1919 influenza pandemic.JAMA.2007;298(6):644–654.
- The Health Care Response to Pandemic Influenza: Position Paper.Philadelphia, PA:American College of Physicians;2006.
- U.S. Department of Health and Human Services (HHS). HHS Pandemic Influenza Plan. November2005. Available at: http://www.hhs.gov/pandemicflu/plan. Accessed May 2009.
- Homeland Security Presidential Directive/HSPD‐21.2007. Available at: http://www.whitehouse.gov/news/releases/2007/10/20071018‐10.html. Accessed May 2009.
- ,.Pandemic influenza and the hospitalist: apocalypse when?J Hosp Med.2006;1(2):118–123.
- ,,,,.The prospect of using alternative medical care facilities in an influenza pandemic.Biosecur Bioterror.2006;4(4):384–390.
- ,,, et al.Pandemic influenza and acute care centers (ACCs): taking care of sick patients in a non‐hospital setting.Biosecur Bioterror.2008;6(4):335–348.
- ,,.Acute Care Center. Modular Emergency Medical System: Concept of Operations for the Acute Care Center (ACC).Mass Casualty Care Strategy for A Biological Terrorism Incident. May2003. Available at: http://dms.dartmouth.edu/nnemmrs/resources/surge_capacity_guidance/documents/acute_care_center__concept_ of_operations. pdf. Accessed May 2009.
- Illinois Department of Public Health. Influenza.2007. Available at: http://www.idph.state.il.us/flu/pandemicfs.htm. Accessed May 2009.
- ,,.Learning from SARS in Hong Kong and Toronto.JAMA.2004;291(20):2483–2487.
- .Planning for epidemics—the lessons of SARS.N Engl J Med.2004;350(23):2332–2334.
- .The role of internists during epidemics, outbreaks, and bioterrorist attacks.J Gen Intern Med.2007;22(1):131–136.
- ,.The expanding role of hospitalists in the United States.Swiss Med Wkly.2006;136(37‐38):591–596.
- ,,,.Promoting effective transitions of care at hospital discharge: a review of key issues for hospitalists.J Hosp Med.2007;2(5):314–323.
- ,.Executing high‐quality care transitions: a call to do it right.J Hosp Med.2007;2(5):287–290.
- .Reflections: the hospitalist movement a decade later.J Hosp Med.2006;1(4):248–252.
- ,.Implementing patient safety interventions in your hospital: what to try and what to avoid.Med Clin North Am.2008;92(2):275–293, vii‐viii.
- ,,,.Expanding the roles of hospitalist physicians to include public health.J Hosp Med.2007;2(,2):93–101.
- ,,,,,.Evaluating the California hospital initiative in palliative services.Arch Intern Med.2006;166(2):227–230.
- .Palliative care and hospitalists: a partnership for hope.J Hosp Med.2006;1(1):5–6.
- .Palliative care in hospitals.J Hosp Med.2006;1(1):21–28.