User login
Updating the core competencies in hospital medicine—2017 revision: Introduction and methodology
In 2006, the Society of Hospital Medicine (SHM) first published The Core Competencies in Hospital Medicine: A Framework for Curricular Development (henceforth described as the Core Competencies) to help define the role and expectations of hospitalists.1,2 The Core Competencies provided a framework for evaluating clinical skills and professional expertise within a rapidly developing field and highlighted opportunities for growth. Since the initial development and publication of the Core Competencies, changes in the healthcare landscape and hospitalist practice environment have prompted this revision.
Over the past decade, the field of hospital medicine has experienced exponential growth. In 2005, just over 16,000 hospitalists were practicing in the United States. By 2015, that number had increased to an estimated 44,000 hospitalists, accounting for approximately 6% of the physician workforce.3 Hospitalists have expanded the scope of hospital medicine in many ways. In their roles, hospitalists lead and participate in hospital-based care models that emphasize interprofessional collaboration and a focus on the delivery of high-quality and cost-effective care across a variety of clinical domains (eg, the Choosing Wisely initiative).4 They are also engaged in patient safety and quality initiatives that are increasingly being used as benchmarks to rate hospitals and as factors for hospital payment (eg, Hospital Inpatient Value-Based Purchasing Program).5 In fact, the American Board of Internal Medicine (ABIM) created a Focused Practice in Hospital Medicine Maintenance of Certification program in response to the growing number of internists choosing to concentrate their practice in the hospital setting. This decision by the ABIM underscores the value that hospitalists bring to improving patient care in the hospital setting. The ABIM also recognizes the Core Competencies as a curricular framework for a focused practice in hospital medicine.6
Changes within the educational environment have demanded attentive and active participation by many hospitalists. For example, in 2012, the Accreditation Council for Graduate Medical Education (ACGME) introduced the Milestones Project, a new outcomes-based framework designed to more effectively assess learner performance across the 6 core competencies.7 These milestones assessments create intentional opportunities to guide the development of physicians during their training, including in the inpatient environments in which hospitalists practice. Where applicable, existing Core Competencies learning objectives were compared with external sources such as the individual ACGME performance milestones for this revision.
THE CORE COMPETENCIES
The Core Competencies focus on adult hospital medicine. The Pediatric Hospital Medicine Core Competencies are published separately.8 Importantly, the Core Competencies document is not intended to define an absolute set of clinical, procedural, or system-based topics described in textbooks or used by graduate medical education training programs. It does not define or limit the scope of the practice of hospital medicine. Rather, the Core Competencies serve as measurable learning objectives that encourage teaching faculty, practicing hospitalists, and administrators to develop individual skill sets and programs to improve patient care contextualized to the needs of an individual, care setting, or institution. To permit this flexibility, individual chapter-specific objectives are intentionally general in nature. Finally, the Core Competencies document is not a set of practice guidelines, nor does it offer any representation of a “standard of care.” Readers are encouraged to explore the article by McKean et al.9 to review examples of application of the Core Competencies and suggestions for curricular development.
The purpose of this article is to describe the criteria for inclusion of new chapters in the Core Competencies and the methodology of the review and revision process. It outlines the process of initial review and editing of the existing chapters; needs assessment for new topics; new chapter production; and the process of review and revision of individual chapters to create the complete document. The revised Core Competencies document is available online at http://www.journalofhospitalmedicine.com/jhospmed/issue/134981/journal-hospital-medicine-124-suppl-1.
REVIEW AND REVISION PROCESS
In 2012, the Society of Hospital Medicine (SHM) Education Committee created a Core Competencies Task Force (CCTF) in response to the SHM Board of Directors’ charge that it review and update the initial Core Competencies document. The CCTF comprised of 5 physician SHM Education Committee members and one SHM staff representative. CCTF membership included hospitalists with an interest and familiarity with the Core Competencies document. The SHM Education Committee nominated the CCTF chair, who determined the optimal size, qualifications, and composition of the task force with approval from the Committee. The CCTF communicated through frequent conference calls and via e-mail correspondence to conduct an initial review of the existing chapters and to perform a needs assessment for new topics.
Individual Chapter Review
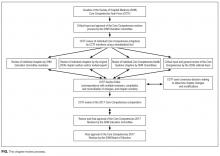
The SHM Education Committee provided critical input and approved the chapter review process designed by the CCTF (Figure). The CCTF reviewed each chapter of the Core Competencies document to assess its continuing relevance to the field of hospital medicine with a standardized tool (Appendix 1). The process required that at least 2 CCTF members reviewed each chapter. Preliminary reviewers assessed the current relevance of each chapter, determined whether individual learning objectives required additional investigation or modification, and developed new learning objectives to fill any educational gaps. All CCTF members then discussed assimilated feedback from the initial CCTF review, using consensus decision making to determine chapter changes and modifications. The CCTF found each of the existing chapters to be relevant to the field and identified none for removal.
The CCTF rewrote all chapters. It then disseminated proposed chapter changes to a panel of diverse independent reviewers to solicit suggestions and comments to ensure a multidisciplinary and balanced review process. Independent reviewers included authors of the original Core Competencies chapters, invited content experts, and members of the SHM Education Committee. When appropriate, corresponding SHM Committees reviewed individual chapters for updates and revisions. For example, the SHM Hospital Quality and Patient Safety Committee reviewed the chapters on patient safety and quality improvement, and the SHM Practice Management Committee reviewed the chapter on management practices. Four CCTF section editors managed an independent portfolio of chapters. Each CCTF section editor assimilated the various draft versions, corresponded with individual reviewers when necessary, and compiled the changes into a subsequent draft. This process ensured that the final version of every chapter reflected the thoughtful input from all parties involved in the review. Throughout the process, the CCTF used consensus decision making to adjudicate chapter changes and modifications. The 2006 Core Competencies Editorial team also reviewed the revision and provided critical input. The SHM Education Committee and the SHM Board of Directors reviewed and approved the final version of the Core Competencies document.
Needs Assessment and Selection of New Core Competency Chapters
The CCTF issued a call for new topics to the members of the SHM Education Committee for inclusion in the Core Competencies. Topics were also identified from the following sources: the top 100 adult medical diagnoses at hospital discharge in the Healthcare Cost and Utilization Project database in 2010; topics in hospital medicine textbooks; curricula presented at the 3 most recent SHM annual meetings; and responses from SHM annual meeting surveys. Table 1 lists the topics considered for addition.
Members of the SHM Education Committee rated each of the potential topics considered for inclusion based on the following characteristics: relevance to the field of hospital medicine; intersection of the topic with medical subspecialties; and its appropriateness as a separate, stand-alone chapter. In addition, topics more frequently encountered by hospitalists, those deemed clinically important with a known risk of complications or management inconsistencies, and those with significant opportunities for quality improvement initiatives carried more weight. Syncope and hyponatremia were the only 2 clinical conditions identified that met all of the inclusion criteria. No additional topics met the criteria for new chapter development in the Procedures or Healthcare Systems sections. The SHM Education Committee identified the use of point-of-care ultrasonography as an important advancement in the field. Where appropriate, the individual procedure chapters now include a new competency-based objective highlighting its role. In addition, a separate SHM task force is working to develop a practice guideline for the use of point-of-care ultrasonography by hospitalists.
Contributors
The SHM Education Committee determined authorship for the new chapters (syncope and hyponatremia). It assigned 2 CCTF members with content expertise and familiarity with the Core Competencies to each author one chapter. Given the limited number of new chapters, it made a decision to develop the content internally rather than through an open-call for authorship nominations to practicing SHM members. The authors made an effort to maintain consistency with the educational theory used to develop the initial Core Competencies. Each of the new topics underwent rigorous review as previously described, including additional independent reviews by hospitalists with content expertise in these areas.
CHAPTER FORMAT AND CONTENT CHANGES
Following the same format as the earlier version, the 2017 Core Competencies revision contains 53 chapters, divided into 3 sections—Clinical Conditions, Procedures, and Healthcare Systems (Table 2) —all integral components of the practice of hospital medicine. The design allows individual chapters to stand alone. However, each chapter should be considered in the context of the entire document because a particular concept may be only briefly discussed in one chapter, but described in greater depth in another given the potential overlap across topics.
The chapters maintain the same content structure as the original version. Each chapter begins with an introductory paragraph followed by a list of competency-based objectives grouped in subsections according to the educational theory of learning domains: cognitive (knowledge), psychomotor (skills), and affective (attitudes).10 In addition, a subsection for System Organization and Improvement is included in the Clinical Conditions and Procedure chapters to emphasize the importance of interprofessional collaboration for optimal patient care. These subsections were not included in the Healthcare Systems chapters, as system organization and improvement is intrinsic to these subjects.
The introductory paragraph provides background information and describes how the chapter remains relevant to the current practice of hospital medicine. Individual competency-based objectives outline a relevant concept and expected level of proficiency as defined by Bloom’s taxonomy.10 New objectives reflect changes in the healthcare landscape over the past decade or further enhance each chapter’s concepts. Chapter authors made an effort to develop chapter and learning objective concepts that are consistent with external resources such as the ACGME Milestones Project and practice guideline objectives developed by a variety of professional organizations.
SUMMARY AND FUTURE DIRECTIONS
The Core Competencies document serves as a resource for hospitalists and hospital medicine programs to evaluate, develop, and improve individual and collective skills and the practice environment. The Core Competencies also provide a framework for medical school clerkship directors and residency and fellowship program directors, as well as course directors of Continuing Medical Education programs, to develop curricula to enhance educational experiences for trainees and hospital medicine providers. The updates in every chapter in this revision to the Core Competencies reflects the changes in the healthcare landscape and hospitalist practice environment over the past decade, and we encourage readers to revisit the entire compendium. Table 3 highlights some of the salient changes in this revision.
Hospital medicine continues to evolve as a specialty. The Core Competencies define hospitalists as agents of change and foster the development of a culture of safe and effective patient care within the hospital environment. Although the CCTF hopes that the Core Competencies will preserve their relevance over time, it recognizes the importance of their periodic reevaluation and adaptation. Additionally, SHM developed the Core Competencies primarily for physicians practicing as hospitalists. As the number of physician assistants and nurse practitioners engaged in the practice of hospital medicine increases, and hospital medicine expands into nontraditional specialties such as surgical comanagement, it may be necessary to consider the development of additional or separate Hospital Medicine Core Competencies tailored to the needs of these subsets of clinicians.
Acknowledgments
The authors and the CCTF are immensely grateful to Nick Marzano for project coordination and Abbie Young for her assistance with medical editing and chapter formatting. We extend our sincerest appreciation and gratitude to the index team of authors and editors whose efforts laid the foundation for this body of work. The initial development and this revision of the Core Competencies would not have been possible without the support and assistance of the SHM staff, the SHM Education Committee, and the scores of contributors and reviewers who participated in its creation (complete list of individuals is available in Appendix 2). We thank everyone for his or her invaluable input and effort.
Disclosures
The Society of Hospital Medicine (SHM) provided administrative support for project coordination. SHM, or any of its representatives, had no role in the development of topic areas, refinement, or vetting of the topic list. No member of the Core Competencies Task Force or the SHM Education Committee received compensation for their participation in revising the Core Competencies. The authors report no conflicts of inte
1. The core competencies in hospital medicine: a framework for curriculum development by the society of hospital medicine. J Hosp Med. 2006;1 Suppl 1:2-95.
2. Dressler DD, Pistoria MJ, Budnitz TL, McKean SCW, Amin AN. Core competencies in hospital medicine: development and methodology. J Hosp Med. 2006;1(1):48-56.
3. Hospital Medicine News, Society of Hospital Medicine. http://www.hospitalmedicine.org/press. Accessed June 16, 2016.
4. Bulger J, Nickel W, Messler J, et al. Choosing wisely in adult hospital medicine: five opportunities for improved healthcare value. J Hosp Med. 2013;8(9):486-492.
5. Conway PH. Value-driven health care: implications for hospitals and hospitalists. J Hosp Med. 2009;4(8):507-511.
6. American Board of Internal Medicine. Questions and Answers Regarding ABIM’s Maintenance of Certification in Internal Medicine with a Focused Practice in Hospital Medicine Program. 2009. http://www.abim.org/news/focused-practice-hospital-medicine-questions-answers.aspx. Accessed November 11, 2016.
7. The Internal Medicine Milestone Project. http://www.acgme.org/acgmeweb/portals/0/pdfs/milestones/internalmedicinemilestones.pdf. Accessed February 29, 2016.
8. Stucky ER, Ottolini MC, Maniscalco J. Pediatric hospital medicine core competencies: development and methodology. J Hosp Med. 2010;5(6):339-343.
9. McKean SC, Budnitz TL, Dressler DD, Amin AN, Pistoria MJ. How to use the core competencies in hospital medicine: a framework for curriculum development. J Hosp Med. 2006;1 Suppl 1:57-67.
10. Anderson LW, Krathwohl DR (eds). A Taxonomy for Learning, Teaching and Assessing: A Revision of Bloom’s Taxonomy of Educational Outcomes. Complete edition. New York, NY: Longman; 2001.
In 2006, the Society of Hospital Medicine (SHM) first published The Core Competencies in Hospital Medicine: A Framework for Curricular Development (henceforth described as the Core Competencies) to help define the role and expectations of hospitalists.1,2 The Core Competencies provided a framework for evaluating clinical skills and professional expertise within a rapidly developing field and highlighted opportunities for growth. Since the initial development and publication of the Core Competencies, changes in the healthcare landscape and hospitalist practice environment have prompted this revision.
Over the past decade, the field of hospital medicine has experienced exponential growth. In 2005, just over 16,000 hospitalists were practicing in the United States. By 2015, that number had increased to an estimated 44,000 hospitalists, accounting for approximately 6% of the physician workforce.3 Hospitalists have expanded the scope of hospital medicine in many ways. In their roles, hospitalists lead and participate in hospital-based care models that emphasize interprofessional collaboration and a focus on the delivery of high-quality and cost-effective care across a variety of clinical domains (eg, the Choosing Wisely initiative).4 They are also engaged in patient safety and quality initiatives that are increasingly being used as benchmarks to rate hospitals and as factors for hospital payment (eg, Hospital Inpatient Value-Based Purchasing Program).5 In fact, the American Board of Internal Medicine (ABIM) created a Focused Practice in Hospital Medicine Maintenance of Certification program in response to the growing number of internists choosing to concentrate their practice in the hospital setting. This decision by the ABIM underscores the value that hospitalists bring to improving patient care in the hospital setting. The ABIM also recognizes the Core Competencies as a curricular framework for a focused practice in hospital medicine.6
Changes within the educational environment have demanded attentive and active participation by many hospitalists. For example, in 2012, the Accreditation Council for Graduate Medical Education (ACGME) introduced the Milestones Project, a new outcomes-based framework designed to more effectively assess learner performance across the 6 core competencies.7 These milestones assessments create intentional opportunities to guide the development of physicians during their training, including in the inpatient environments in which hospitalists practice. Where applicable, existing Core Competencies learning objectives were compared with external sources such as the individual ACGME performance milestones for this revision.
THE CORE COMPETENCIES
The Core Competencies focus on adult hospital medicine. The Pediatric Hospital Medicine Core Competencies are published separately.8 Importantly, the Core Competencies document is not intended to define an absolute set of clinical, procedural, or system-based topics described in textbooks or used by graduate medical education training programs. It does not define or limit the scope of the practice of hospital medicine. Rather, the Core Competencies serve as measurable learning objectives that encourage teaching faculty, practicing hospitalists, and administrators to develop individual skill sets and programs to improve patient care contextualized to the needs of an individual, care setting, or institution. To permit this flexibility, individual chapter-specific objectives are intentionally general in nature. Finally, the Core Competencies document is not a set of practice guidelines, nor does it offer any representation of a “standard of care.” Readers are encouraged to explore the article by McKean et al.9 to review examples of application of the Core Competencies and suggestions for curricular development.
The purpose of this article is to describe the criteria for inclusion of new chapters in the Core Competencies and the methodology of the review and revision process. It outlines the process of initial review and editing of the existing chapters; needs assessment for new topics; new chapter production; and the process of review and revision of individual chapters to create the complete document. The revised Core Competencies document is available online at http://www.journalofhospitalmedicine.com/jhospmed/issue/134981/journal-hospital-medicine-124-suppl-1.
REVIEW AND REVISION PROCESS
In 2012, the Society of Hospital Medicine (SHM) Education Committee created a Core Competencies Task Force (CCTF) in response to the SHM Board of Directors’ charge that it review and update the initial Core Competencies document. The CCTF comprised of 5 physician SHM Education Committee members and one SHM staff representative. CCTF membership included hospitalists with an interest and familiarity with the Core Competencies document. The SHM Education Committee nominated the CCTF chair, who determined the optimal size, qualifications, and composition of the task force with approval from the Committee. The CCTF communicated through frequent conference calls and via e-mail correspondence to conduct an initial review of the existing chapters and to perform a needs assessment for new topics.
Individual Chapter Review
The SHM Education Committee provided critical input and approved the chapter review process designed by the CCTF (Figure). The CCTF reviewed each chapter of the Core Competencies document to assess its continuing relevance to the field of hospital medicine with a standardized tool (Appendix 1). The process required that at least 2 CCTF members reviewed each chapter. Preliminary reviewers assessed the current relevance of each chapter, determined whether individual learning objectives required additional investigation or modification, and developed new learning objectives to fill any educational gaps. All CCTF members then discussed assimilated feedback from the initial CCTF review, using consensus decision making to determine chapter changes and modifications. The CCTF found each of the existing chapters to be relevant to the field and identified none for removal.
The CCTF rewrote all chapters. It then disseminated proposed chapter changes to a panel of diverse independent reviewers to solicit suggestions and comments to ensure a multidisciplinary and balanced review process. Independent reviewers included authors of the original Core Competencies chapters, invited content experts, and members of the SHM Education Committee. When appropriate, corresponding SHM Committees reviewed individual chapters for updates and revisions. For example, the SHM Hospital Quality and Patient Safety Committee reviewed the chapters on patient safety and quality improvement, and the SHM Practice Management Committee reviewed the chapter on management practices. Four CCTF section editors managed an independent portfolio of chapters. Each CCTF section editor assimilated the various draft versions, corresponded with individual reviewers when necessary, and compiled the changes into a subsequent draft. This process ensured that the final version of every chapter reflected the thoughtful input from all parties involved in the review. Throughout the process, the CCTF used consensus decision making to adjudicate chapter changes and modifications. The 2006 Core Competencies Editorial team also reviewed the revision and provided critical input. The SHM Education Committee and the SHM Board of Directors reviewed and approved the final version of the Core Competencies document.
Needs Assessment and Selection of New Core Competency Chapters
The CCTF issued a call for new topics to the members of the SHM Education Committee for inclusion in the Core Competencies. Topics were also identified from the following sources: the top 100 adult medical diagnoses at hospital discharge in the Healthcare Cost and Utilization Project database in 2010; topics in hospital medicine textbooks; curricula presented at the 3 most recent SHM annual meetings; and responses from SHM annual meeting surveys. Table 1 lists the topics considered for addition.
Members of the SHM Education Committee rated each of the potential topics considered for inclusion based on the following characteristics: relevance to the field of hospital medicine; intersection of the topic with medical subspecialties; and its appropriateness as a separate, stand-alone chapter. In addition, topics more frequently encountered by hospitalists, those deemed clinically important with a known risk of complications or management inconsistencies, and those with significant opportunities for quality improvement initiatives carried more weight. Syncope and hyponatremia were the only 2 clinical conditions identified that met all of the inclusion criteria. No additional topics met the criteria for new chapter development in the Procedures or Healthcare Systems sections. The SHM Education Committee identified the use of point-of-care ultrasonography as an important advancement in the field. Where appropriate, the individual procedure chapters now include a new competency-based objective highlighting its role. In addition, a separate SHM task force is working to develop a practice guideline for the use of point-of-care ultrasonography by hospitalists.
Contributors
The SHM Education Committee determined authorship for the new chapters (syncope and hyponatremia). It assigned 2 CCTF members with content expertise and familiarity with the Core Competencies to each author one chapter. Given the limited number of new chapters, it made a decision to develop the content internally rather than through an open-call for authorship nominations to practicing SHM members. The authors made an effort to maintain consistency with the educational theory used to develop the initial Core Competencies. Each of the new topics underwent rigorous review as previously described, including additional independent reviews by hospitalists with content expertise in these areas.
CHAPTER FORMAT AND CONTENT CHANGES
Following the same format as the earlier version, the 2017 Core Competencies revision contains 53 chapters, divided into 3 sections—Clinical Conditions, Procedures, and Healthcare Systems (Table 2) —all integral components of the practice of hospital medicine. The design allows individual chapters to stand alone. However, each chapter should be considered in the context of the entire document because a particular concept may be only briefly discussed in one chapter, but described in greater depth in another given the potential overlap across topics.
The chapters maintain the same content structure as the original version. Each chapter begins with an introductory paragraph followed by a list of competency-based objectives grouped in subsections according to the educational theory of learning domains: cognitive (knowledge), psychomotor (skills), and affective (attitudes).10 In addition, a subsection for System Organization and Improvement is included in the Clinical Conditions and Procedure chapters to emphasize the importance of interprofessional collaboration for optimal patient care. These subsections were not included in the Healthcare Systems chapters, as system organization and improvement is intrinsic to these subjects.
The introductory paragraph provides background information and describes how the chapter remains relevant to the current practice of hospital medicine. Individual competency-based objectives outline a relevant concept and expected level of proficiency as defined by Bloom’s taxonomy.10 New objectives reflect changes in the healthcare landscape over the past decade or further enhance each chapter’s concepts. Chapter authors made an effort to develop chapter and learning objective concepts that are consistent with external resources such as the ACGME Milestones Project and practice guideline objectives developed by a variety of professional organizations.
SUMMARY AND FUTURE DIRECTIONS
The Core Competencies document serves as a resource for hospitalists and hospital medicine programs to evaluate, develop, and improve individual and collective skills and the practice environment. The Core Competencies also provide a framework for medical school clerkship directors and residency and fellowship program directors, as well as course directors of Continuing Medical Education programs, to develop curricula to enhance educational experiences for trainees and hospital medicine providers. The updates in every chapter in this revision to the Core Competencies reflects the changes in the healthcare landscape and hospitalist practice environment over the past decade, and we encourage readers to revisit the entire compendium. Table 3 highlights some of the salient changes in this revision.
Hospital medicine continues to evolve as a specialty. The Core Competencies define hospitalists as agents of change and foster the development of a culture of safe and effective patient care within the hospital environment. Although the CCTF hopes that the Core Competencies will preserve their relevance over time, it recognizes the importance of their periodic reevaluation and adaptation. Additionally, SHM developed the Core Competencies primarily for physicians practicing as hospitalists. As the number of physician assistants and nurse practitioners engaged in the practice of hospital medicine increases, and hospital medicine expands into nontraditional specialties such as surgical comanagement, it may be necessary to consider the development of additional or separate Hospital Medicine Core Competencies tailored to the needs of these subsets of clinicians.
Acknowledgments
The authors and the CCTF are immensely grateful to Nick Marzano for project coordination and Abbie Young for her assistance with medical editing and chapter formatting. We extend our sincerest appreciation and gratitude to the index team of authors and editors whose efforts laid the foundation for this body of work. The initial development and this revision of the Core Competencies would not have been possible without the support and assistance of the SHM staff, the SHM Education Committee, and the scores of contributors and reviewers who participated in its creation (complete list of individuals is available in Appendix 2). We thank everyone for his or her invaluable input and effort.
Disclosures
The Society of Hospital Medicine (SHM) provided administrative support for project coordination. SHM, or any of its representatives, had no role in the development of topic areas, refinement, or vetting of the topic list. No member of the Core Competencies Task Force or the SHM Education Committee received compensation for their participation in revising the Core Competencies. The authors report no conflicts of inte
In 2006, the Society of Hospital Medicine (SHM) first published The Core Competencies in Hospital Medicine: A Framework for Curricular Development (henceforth described as the Core Competencies) to help define the role and expectations of hospitalists.1,2 The Core Competencies provided a framework for evaluating clinical skills and professional expertise within a rapidly developing field and highlighted opportunities for growth. Since the initial development and publication of the Core Competencies, changes in the healthcare landscape and hospitalist practice environment have prompted this revision.
Over the past decade, the field of hospital medicine has experienced exponential growth. In 2005, just over 16,000 hospitalists were practicing in the United States. By 2015, that number had increased to an estimated 44,000 hospitalists, accounting for approximately 6% of the physician workforce.3 Hospitalists have expanded the scope of hospital medicine in many ways. In their roles, hospitalists lead and participate in hospital-based care models that emphasize interprofessional collaboration and a focus on the delivery of high-quality and cost-effective care across a variety of clinical domains (eg, the Choosing Wisely initiative).4 They are also engaged in patient safety and quality initiatives that are increasingly being used as benchmarks to rate hospitals and as factors for hospital payment (eg, Hospital Inpatient Value-Based Purchasing Program).5 In fact, the American Board of Internal Medicine (ABIM) created a Focused Practice in Hospital Medicine Maintenance of Certification program in response to the growing number of internists choosing to concentrate their practice in the hospital setting. This decision by the ABIM underscores the value that hospitalists bring to improving patient care in the hospital setting. The ABIM also recognizes the Core Competencies as a curricular framework for a focused practice in hospital medicine.6
Changes within the educational environment have demanded attentive and active participation by many hospitalists. For example, in 2012, the Accreditation Council for Graduate Medical Education (ACGME) introduced the Milestones Project, a new outcomes-based framework designed to more effectively assess learner performance across the 6 core competencies.7 These milestones assessments create intentional opportunities to guide the development of physicians during their training, including in the inpatient environments in which hospitalists practice. Where applicable, existing Core Competencies learning objectives were compared with external sources such as the individual ACGME performance milestones for this revision.
THE CORE COMPETENCIES
The Core Competencies focus on adult hospital medicine. The Pediatric Hospital Medicine Core Competencies are published separately.8 Importantly, the Core Competencies document is not intended to define an absolute set of clinical, procedural, or system-based topics described in textbooks or used by graduate medical education training programs. It does not define or limit the scope of the practice of hospital medicine. Rather, the Core Competencies serve as measurable learning objectives that encourage teaching faculty, practicing hospitalists, and administrators to develop individual skill sets and programs to improve patient care contextualized to the needs of an individual, care setting, or institution. To permit this flexibility, individual chapter-specific objectives are intentionally general in nature. Finally, the Core Competencies document is not a set of practice guidelines, nor does it offer any representation of a “standard of care.” Readers are encouraged to explore the article by McKean et al.9 to review examples of application of the Core Competencies and suggestions for curricular development.
The purpose of this article is to describe the criteria for inclusion of new chapters in the Core Competencies and the methodology of the review and revision process. It outlines the process of initial review and editing of the existing chapters; needs assessment for new topics; new chapter production; and the process of review and revision of individual chapters to create the complete document. The revised Core Competencies document is available online at http://www.journalofhospitalmedicine.com/jhospmed/issue/134981/journal-hospital-medicine-124-suppl-1.
REVIEW AND REVISION PROCESS
In 2012, the Society of Hospital Medicine (SHM) Education Committee created a Core Competencies Task Force (CCTF) in response to the SHM Board of Directors’ charge that it review and update the initial Core Competencies document. The CCTF comprised of 5 physician SHM Education Committee members and one SHM staff representative. CCTF membership included hospitalists with an interest and familiarity with the Core Competencies document. The SHM Education Committee nominated the CCTF chair, who determined the optimal size, qualifications, and composition of the task force with approval from the Committee. The CCTF communicated through frequent conference calls and via e-mail correspondence to conduct an initial review of the existing chapters and to perform a needs assessment for new topics.
Individual Chapter Review
The SHM Education Committee provided critical input and approved the chapter review process designed by the CCTF (Figure). The CCTF reviewed each chapter of the Core Competencies document to assess its continuing relevance to the field of hospital medicine with a standardized tool (Appendix 1). The process required that at least 2 CCTF members reviewed each chapter. Preliminary reviewers assessed the current relevance of each chapter, determined whether individual learning objectives required additional investigation or modification, and developed new learning objectives to fill any educational gaps. All CCTF members then discussed assimilated feedback from the initial CCTF review, using consensus decision making to determine chapter changes and modifications. The CCTF found each of the existing chapters to be relevant to the field and identified none for removal.
The CCTF rewrote all chapters. It then disseminated proposed chapter changes to a panel of diverse independent reviewers to solicit suggestions and comments to ensure a multidisciplinary and balanced review process. Independent reviewers included authors of the original Core Competencies chapters, invited content experts, and members of the SHM Education Committee. When appropriate, corresponding SHM Committees reviewed individual chapters for updates and revisions. For example, the SHM Hospital Quality and Patient Safety Committee reviewed the chapters on patient safety and quality improvement, and the SHM Practice Management Committee reviewed the chapter on management practices. Four CCTF section editors managed an independent portfolio of chapters. Each CCTF section editor assimilated the various draft versions, corresponded with individual reviewers when necessary, and compiled the changes into a subsequent draft. This process ensured that the final version of every chapter reflected the thoughtful input from all parties involved in the review. Throughout the process, the CCTF used consensus decision making to adjudicate chapter changes and modifications. The 2006 Core Competencies Editorial team also reviewed the revision and provided critical input. The SHM Education Committee and the SHM Board of Directors reviewed and approved the final version of the Core Competencies document.
Needs Assessment and Selection of New Core Competency Chapters
The CCTF issued a call for new topics to the members of the SHM Education Committee for inclusion in the Core Competencies. Topics were also identified from the following sources: the top 100 adult medical diagnoses at hospital discharge in the Healthcare Cost and Utilization Project database in 2010; topics in hospital medicine textbooks; curricula presented at the 3 most recent SHM annual meetings; and responses from SHM annual meeting surveys. Table 1 lists the topics considered for addition.
Members of the SHM Education Committee rated each of the potential topics considered for inclusion based on the following characteristics: relevance to the field of hospital medicine; intersection of the topic with medical subspecialties; and its appropriateness as a separate, stand-alone chapter. In addition, topics more frequently encountered by hospitalists, those deemed clinically important with a known risk of complications or management inconsistencies, and those with significant opportunities for quality improvement initiatives carried more weight. Syncope and hyponatremia were the only 2 clinical conditions identified that met all of the inclusion criteria. No additional topics met the criteria for new chapter development in the Procedures or Healthcare Systems sections. The SHM Education Committee identified the use of point-of-care ultrasonography as an important advancement in the field. Where appropriate, the individual procedure chapters now include a new competency-based objective highlighting its role. In addition, a separate SHM task force is working to develop a practice guideline for the use of point-of-care ultrasonography by hospitalists.
Contributors
The SHM Education Committee determined authorship for the new chapters (syncope and hyponatremia). It assigned 2 CCTF members with content expertise and familiarity with the Core Competencies to each author one chapter. Given the limited number of new chapters, it made a decision to develop the content internally rather than through an open-call for authorship nominations to practicing SHM members. The authors made an effort to maintain consistency with the educational theory used to develop the initial Core Competencies. Each of the new topics underwent rigorous review as previously described, including additional independent reviews by hospitalists with content expertise in these areas.
CHAPTER FORMAT AND CONTENT CHANGES
Following the same format as the earlier version, the 2017 Core Competencies revision contains 53 chapters, divided into 3 sections—Clinical Conditions, Procedures, and Healthcare Systems (Table 2) —all integral components of the practice of hospital medicine. The design allows individual chapters to stand alone. However, each chapter should be considered in the context of the entire document because a particular concept may be only briefly discussed in one chapter, but described in greater depth in another given the potential overlap across topics.
The chapters maintain the same content structure as the original version. Each chapter begins with an introductory paragraph followed by a list of competency-based objectives grouped in subsections according to the educational theory of learning domains: cognitive (knowledge), psychomotor (skills), and affective (attitudes).10 In addition, a subsection for System Organization and Improvement is included in the Clinical Conditions and Procedure chapters to emphasize the importance of interprofessional collaboration for optimal patient care. These subsections were not included in the Healthcare Systems chapters, as system organization and improvement is intrinsic to these subjects.
The introductory paragraph provides background information and describes how the chapter remains relevant to the current practice of hospital medicine. Individual competency-based objectives outline a relevant concept and expected level of proficiency as defined by Bloom’s taxonomy.10 New objectives reflect changes in the healthcare landscape over the past decade or further enhance each chapter’s concepts. Chapter authors made an effort to develop chapter and learning objective concepts that are consistent with external resources such as the ACGME Milestones Project and practice guideline objectives developed by a variety of professional organizations.
SUMMARY AND FUTURE DIRECTIONS
The Core Competencies document serves as a resource for hospitalists and hospital medicine programs to evaluate, develop, and improve individual and collective skills and the practice environment. The Core Competencies also provide a framework for medical school clerkship directors and residency and fellowship program directors, as well as course directors of Continuing Medical Education programs, to develop curricula to enhance educational experiences for trainees and hospital medicine providers. The updates in every chapter in this revision to the Core Competencies reflects the changes in the healthcare landscape and hospitalist practice environment over the past decade, and we encourage readers to revisit the entire compendium. Table 3 highlights some of the salient changes in this revision.
Hospital medicine continues to evolve as a specialty. The Core Competencies define hospitalists as agents of change and foster the development of a culture of safe and effective patient care within the hospital environment. Although the CCTF hopes that the Core Competencies will preserve their relevance over time, it recognizes the importance of their periodic reevaluation and adaptation. Additionally, SHM developed the Core Competencies primarily for physicians practicing as hospitalists. As the number of physician assistants and nurse practitioners engaged in the practice of hospital medicine increases, and hospital medicine expands into nontraditional specialties such as surgical comanagement, it may be necessary to consider the development of additional or separate Hospital Medicine Core Competencies tailored to the needs of these subsets of clinicians.
Acknowledgments
The authors and the CCTF are immensely grateful to Nick Marzano for project coordination and Abbie Young for her assistance with medical editing and chapter formatting. We extend our sincerest appreciation and gratitude to the index team of authors and editors whose efforts laid the foundation for this body of work. The initial development and this revision of the Core Competencies would not have been possible without the support and assistance of the SHM staff, the SHM Education Committee, and the scores of contributors and reviewers who participated in its creation (complete list of individuals is available in Appendix 2). We thank everyone for his or her invaluable input and effort.
Disclosures
The Society of Hospital Medicine (SHM) provided administrative support for project coordination. SHM, or any of its representatives, had no role in the development of topic areas, refinement, or vetting of the topic list. No member of the Core Competencies Task Force or the SHM Education Committee received compensation for their participation in revising the Core Competencies. The authors report no conflicts of inte
1. The core competencies in hospital medicine: a framework for curriculum development by the society of hospital medicine. J Hosp Med. 2006;1 Suppl 1:2-95.
2. Dressler DD, Pistoria MJ, Budnitz TL, McKean SCW, Amin AN. Core competencies in hospital medicine: development and methodology. J Hosp Med. 2006;1(1):48-56.
3. Hospital Medicine News, Society of Hospital Medicine. http://www.hospitalmedicine.org/press. Accessed June 16, 2016.
4. Bulger J, Nickel W, Messler J, et al. Choosing wisely in adult hospital medicine: five opportunities for improved healthcare value. J Hosp Med. 2013;8(9):486-492.
5. Conway PH. Value-driven health care: implications for hospitals and hospitalists. J Hosp Med. 2009;4(8):507-511.
6. American Board of Internal Medicine. Questions and Answers Regarding ABIM’s Maintenance of Certification in Internal Medicine with a Focused Practice in Hospital Medicine Program. 2009. http://www.abim.org/news/focused-practice-hospital-medicine-questions-answers.aspx. Accessed November 11, 2016.
7. The Internal Medicine Milestone Project. http://www.acgme.org/acgmeweb/portals/0/pdfs/milestones/internalmedicinemilestones.pdf. Accessed February 29, 2016.
8. Stucky ER, Ottolini MC, Maniscalco J. Pediatric hospital medicine core competencies: development and methodology. J Hosp Med. 2010;5(6):339-343.
9. McKean SC, Budnitz TL, Dressler DD, Amin AN, Pistoria MJ. How to use the core competencies in hospital medicine: a framework for curriculum development. J Hosp Med. 2006;1 Suppl 1:57-67.
10. Anderson LW, Krathwohl DR (eds). A Taxonomy for Learning, Teaching and Assessing: A Revision of Bloom’s Taxonomy of Educational Outcomes. Complete edition. New York, NY: Longman; 2001.
1. The core competencies in hospital medicine: a framework for curriculum development by the society of hospital medicine. J Hosp Med. 2006;1 Suppl 1:2-95.
2. Dressler DD, Pistoria MJ, Budnitz TL, McKean SCW, Amin AN. Core competencies in hospital medicine: development and methodology. J Hosp Med. 2006;1(1):48-56.
3. Hospital Medicine News, Society of Hospital Medicine. http://www.hospitalmedicine.org/press. Accessed June 16, 2016.
4. Bulger J, Nickel W, Messler J, et al. Choosing wisely in adult hospital medicine: five opportunities for improved healthcare value. J Hosp Med. 2013;8(9):486-492.
5. Conway PH. Value-driven health care: implications for hospitals and hospitalists. J Hosp Med. 2009;4(8):507-511.
6. American Board of Internal Medicine. Questions and Answers Regarding ABIM’s Maintenance of Certification in Internal Medicine with a Focused Practice in Hospital Medicine Program. 2009. http://www.abim.org/news/focused-practice-hospital-medicine-questions-answers.aspx. Accessed November 11, 2016.
7. The Internal Medicine Milestone Project. http://www.acgme.org/acgmeweb/portals/0/pdfs/milestones/internalmedicinemilestones.pdf. Accessed February 29, 2016.
8. Stucky ER, Ottolini MC, Maniscalco J. Pediatric hospital medicine core competencies: development and methodology. J Hosp Med. 2010;5(6):339-343.
9. McKean SC, Budnitz TL, Dressler DD, Amin AN, Pistoria MJ. How to use the core competencies in hospital medicine: a framework for curriculum development. J Hosp Med. 2006;1 Suppl 1:57-67.
10. Anderson LW, Krathwohl DR (eds). A Taxonomy for Learning, Teaching and Assessing: A Revision of Bloom’s Taxonomy of Educational Outcomes. Complete edition. New York, NY: Longman; 2001.
© 2017 Society of Hospital Medicine
How Is SIADH Diagnosed and Managed?
Case
A 70-year-old woman with hypertension presents after a fall. Her medications include hydrochlorothiazide. Her blood pressure is 130/70 mm/Hg, with heart rate of 86. She has normal orthostatic vital signs. Her mucus membranes are moist and she has no jugular venous distension, edema, or ascites. Her plasma sodium (PNa) is 125 mmol/L, potassium 3.6 mmol/L, blood urea nitrogen (BUN) 30 mg/dL, and creatinine 0.8 mg/dL. Additional labs include serum thyroid stimulating hormone 1.12 mIU/L, cortisol 15 mcg/dL, serum osmolality 270 mOsm/kg, uric acid 4 mg/dL, urine osmolality 300 mOsm/kg, urine sodium (UNa) 40 mmol/L, fractional excretion of sodium 1.0%, and fractional excretion of urate (FEUrate) 13%. She receives 2 L isotonic saline intravenously over 24 hours, with resulting PNa of 127.
What is the cause of her hyponatremia, and how should her hyponatremia be managed?
Overview
Hyponatremia is one of the most common electrolyte abnormalities; it has a prevalence as high as 30% upon admission to the hospital.1 Hyponatremia is important clinically because of its high risk of mortality in the acute and symptomatic setting, and the risk of central pontine myelinolysis (CPM), or death with too rapid correction.2 Even so-called “asymptomatic” mild hyponatremia is associated with increased falls and impairments in gait and attention in the elderly.3
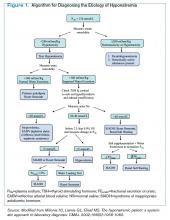
Hyponatremia is a state of excess water compared with the amount of solute in the extracellular fluid. To aid in diagnosing the etiology of hypotonic hyponatremia, the differential is traditionally divided into categories based on extracellular fluid volume (ECV) status, as shown in Table 1 (below), with syndrome of inappropriate antidiuretic hormone secretion (SIADH) being the most common cause of euvolemic hyponatremia.2 However, data show that clinical determination of volume status is often flawed,4 and an algorithmic approach to diagnosis and treatment yields improved results.5
Review of the Data
Diagnosis of SIADH. The original diagnostic criteria for SIADH, with minor modifications, are presented in Table 2, page 18).6,7,8 However, applying these criteria in clinical settings presents several difficulties, most notably a determination of ECV. The gold standard for assessing ECV status is by radioisotope, which is not practically feasible.9 Therefore, clinicians must rely on surrogate clinical markers of ECV (orthostatic hypotension, skin turgor, mucus membrane dryness, central venous pressure, BUN, BUN-creatinine ratio, and serum uric acid levels), which lack both sensitivity and specificity.4 Astoundingly, clinical assessment of ECV has been demonstrated to be accurate only 50% of the time when differentiating euvolemic patients from those with hypovolemia.4
Another challenge lies in the interpretation of UNa, which frequently is used as a surrogate for extra-arterial blood volume (EABV) status.10 Unfortunately, in the setting of diuretic use, UNa becomes inaccurate. The FEUrate, however, is unaffected by diuretic use and can be helpful in distinguishing between etiologies of hyponatremia with UNa greater than 30 mmol/L.11 The FEUrate is about 10% in normal euvolemic subjects and is reduced (usually <8%) in patients with low effective arterial blood volume.11,12 A trial of 86 patients demonstrated that a FEUrate of 12% had a specificity and positive predictive value of 100% in accurately identifying SIADH from diuretic-induced hyponatremia in patients on diuretics.11,12 Therefore, the UNa is a valid marker of EABV status when patients are not on diuretics; however, the FEUrate should be used in the setting of diuretic use.
Yet another pitfall is differentiating patients with salt depletion from those with SIADH. In these situations, measurement of the change in PNa concentration after a test infusion of isotonic saline is helpful. In salt depletion, PNa usually increases ≥5 mmol/L after 2 L saline infusion, which is not the case with SIADH.13 Incorrectly diagnosing renal salt wasting (RSW) as SIADH results in fluid restriction and, consequently, ECV depletion and increased morbidity.14 The persistence of hypouricemia and elevated FEUrate after correction of the hyponatremia in RSW differentiates it from SIADH.13, 14
Given these challenges, recommendations to use an algorithmic approach for the evaluation and diagnosis of hyponatremia have surfaced. In a study of 121 patients admitted with hyponatremia, an algorithm-based approach to the diagnosis of hyponatremia yielded an overall diagnostic accuracy of 71%, compared with an accuracy of 32% by experienced clinicians.5 This study also highlighted SIADH as the most frequent false-positive diagnosis that was expected whenever the combination of euvolemia and a UNa >30 mmol/L was present.5 Cases of diuretic-induced hyponatremia often were misclassified due to errors in the accurate assessment of ECV status, as most of these patients appeared clinically euvolemic or hypervolemic.5 Therefore, it is important to use an algorithm in identifying SIADH and to use one that does not rely solely on clinical estimation of ECV status (see Figure 1, below).
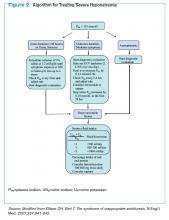
Management of acute and symptomatic hyponatremia. When hyponatremia develops acutely, urgent treatment is required (see Figure 2, below).15 Hyponatremia is considered acute when the onset is within 48 hours.15 Acute hyponatremia is most easily identified in the hospital and is commonly iatrogenic. Small case reviews in the 1980s began to associate postoperative deaths with the administration of hypotonic fluids.16 Asymptomatic patients with hyponatremia presenting from home should be considered chronic hyponatremias as the duration often is unclear.
Acute hyponatremia or neurologically symptomatic hyponatremia regardless of duration requires the use of hypertonic saline.15 Traditional sodium correction algorithms are based on early case series, which were focused on limiting neurologic complications from sodium overcorrection.17 This resulted in protocols recommending a conservative rate of correction spread over a 24- to 48-hour period.17 Infusing 3% saline at a rate of 1 ml/kg/hr to 2 ml/kg/hr results in a 1 mmol/L/hr to 2 mmol/L/hr increase in PNa.15 This simplified formula results in similar correction rates as more complex calculations.15 Correction should not exceed 8 mmol/L to 10 mmol/L within the first 24 hours, and 18 mmol/L to 25 mmol/L by 48 hours to avoid CPM.15 PNa should be checked every two hours to ensure that the correction rate is not exceeding the predicted rate, as the formulas do not take into account oral intake and ongoing losses.15
Recent observations focused on the initial four hours from onset of hyponatremia suggest a higher rate of correction can be tolerated without complications.18 Rapid sodium correction of 4 mmol/L to 6 mmol/L often is enough to stop neurologic complications.18 This can be accomplished with a bolus infusion of 100 mL of 3% saline.19 This may be repeated twice at 10-minute intervals until there is neurologic improvement.19 This might sound aggressive, but this would correspond to a rise in PNa of 5 mmol/L to 6 mmol/L in a 50 kg woman. Subsequent treatment with hypertonic fluid might not be needed if symptoms resolve.
Management of chronic hyponatremia. Hyponatremia secondary to SIADH improves with the treatment of the underlying cause, thus an active search for a causative medication or condition should be sought (see Table 1, p. 17).20
Water restriction. Restriction of fluid intake is the first-line treatment for SIADH in patients without hypovolemia. The severity of fluid restriction is guided by the concentration of the urinary solutes.15 Restriction of water intake to 500 ml/day to 1,000 ml/day is generally advised for many patients, as losses from the skin, lungs, and urine exceed this amount, leading to a gradual reduction in total body water.21 The main drawback of fluid restriction is poor compliance due to an intact thirst mechanism.
Saline infusion. The infusion of normal saline theoretically worsens hyponatremia due to SIADH because the water is retained while the salt is excreted. However, a trial of normal saline sometimes is attempted in patients in whom the differentiation between hypovolemia and euvolemia is difficult. From a study of a series of 17 patients with chronic SIADH, Musch and Decaux concluded that the infusion of intravenous normal (0.9%) saline raises PNa when the urine osmolality is less than 530 mosm/L.22
Oral solutes (urea and salt). The oral intake of salt augments water excretion23, and salt tablets are used as a second-line agent in patients with persistent hyponatremia despite fluid restriction.23 The oral administration of urea also results in increased free-water excretion via osmotic diuresis,24 but its poor palatability, lack of availability in the U.S., and limited user experience has restricted its usage.24
Demeclocycline. Demeclo-cycline is a tetracycline derivative that causes a partial nephrogenic diabetes insipidus.25 Its limitations include a slow onset of action (two to five days) and an unpredictable treatment effect with the possibility of causing profound polyuria and hypernatremia. It is also associated with reversible azotemia and sometimes nephrotoxicity, especially in patients with cirrhosis.
Lithium. Lithium also causes nephrogenic diabetes insipidus by downregulating vasopressin-stimulated aquaporin-2 expression and thus improves hyponatremia in SIADH.26 However, its use is significantly limited by its unpredictable response and the risks of interstitial nephritis and end-stage renal disease with chronic use. Therefore, it is no longer recommended for the treatment of SIADH.
Vasopressin receptor antagonists. Due to the role of excessive levels of vasopressin in the pathophysiology of most types of SIADH, antagonists of the vasopressin receptor were developed with the goal of preventing the excess water absorption that causes hyponatremia. Two vasopressin receptor antagonists, or vaptans, have been approved by the FDA for the treatment of nonemergent euvolemic and hypervolemic hyponatremia. Conivaptan is a nonselective vasopressin receptor antagonist that is for IV use only. Tolvaptan is a selective V2 receptor antagonist that is taken orally. Both conivaptan and tolvaptan successfully increase PNa levels while the drugs are being taken.27,28,29,30 Tolvaptan increases PNa levels in hyponatremia due to SIADH and CHF, and modestly so in cirrhosis.30
The most common side effects of the vaptans include dry mouth, increased thirst, and increased urination, although serious side effects (hypernatremia or too-rapid rate of increase in PNa) are possible.29 It is unclear if treating stable, asymptomatic hyponatremia with vaptans has any reduction in morbidity or mortality. One study found that tolvaptan increased the patients’ self-evaluations of mental functioning, but a study of tolvaptan used in combination with diuretics in the setting of CHF did not result in decreased mortality.29,31 Due to their expense, necessity of being started in the hospital, and unclear long-term benefit, the vaptans are only recommended when traditional measures such as fluid restriction and salt tablets have been unsuccessful.
Back to the Case
Our patient has hypotonic hyponatremia based on her low serum osmolality. The duration of her hyponatremia is unclear, but the patient is not experiencing seizures or coma. Therefore, her hyponatremia should be corrected slowly, and hypertonic saline is not indicated.
As is common in clinical practice, her true volume status is difficult to clinically ascertain. By physical exam, she appears euvolemic, but because she is on hydrochlorothiazide, she might be subtly hypovolemic. The UNa of 40 mmol/L is not consistent with hypovolemia, but its accuracy is limited in the setting of diuretics. The failure to improve her sodium by at least 5 mmol/L after a 2 L normal saline infusion argues against low effective arterial blood volume and indicates that the hydrochlorothiazide is unlikely to be the cause of her hyponatremia.
Therefore, the most likely cause of the hyponatremia is SIADH, a diagnosis further corroborated by the elevated FEUrate of 13%. Her chronic hyponatremia should be managed initially with fluid restriction while an investigation for an underlying cause of SIADH is initiated.
Bottom Line
The diagnosis of SIADH relies on the careful evaluation of laboratory values, use of an algorithm, and recognizing the limitations of clinically assessing volume status. The underlying cause of SIADH must also be sought and treated. TH
Dr. Grant is a clinical lecturer in internal medicine, Dr. Cho is a clinical instructor in internal medicine, and Dr. Nichani is an assistant professor of internal medicine at the University of Michigan Hospital and Health Systems in Ann Arbor.
References
- Upadhyay A, Jaber BL, Madias NE. Incidence and prevalence of hyponatremia. Am J Med. 2006;119(7 Suppl 1):S30-35.
- Verbalis JG, Goldsmith SR, Greenberg A, Schrier RW, Sterns RH. Hyponatremia treatment guidelines 2007: expert panel recommendations. Am J Med. 2007;120(11 Suppl 1):S1-21.
- Renneboog B, Musch W, Vandemergel X, Manto MU, Decaux G. Mild chronic hyponatremia is associated with falls, unsteadiness, and attention deficits. Am J Med. 2006;119(1):71.e71-78.
- Chung HM, Kluge R, Schrier RW, Anderson RJ. Clinical assessment of extracellular fluid volume in hyponatremia. Am J Med. 1987;83(5):905-908.
- Fenske W, Maier SK, Blechschmidt A, Allolio B, Störk S. Utility and limitations of the traditional diagnostic approach to hyponatremia: a diagnostic study. Am J Med. 2010;123(7):652-657.
- Bartter FC, Schwartz WB. The syndrome of inappropriate secretion of antidiuretic hormone. Am J Med. 1967;42(5):790-806.
- Smith DM, McKenna K, Thompson CJ. Hyponatraemia. Clin Endocrinol (Oxf). 2000;52(6):667-678.
- Verbalis JG. Hyponatraemia. Baillieres Clin Endocrinol Metab. Aug 1989;3(2):499-530.
- Maesaka JK, Imbriano LJ, Ali NM, Ilamathi E. Is it cerebral or renal salt wasting? Kidney Int. 2009;76(9):934-938.
- Verbalis JG. Disorders of body water homeostasis. Best Pract Res Clin Endocrinol Metab. 2003;17(4):471-503.
- Fenske W, Störk S, Koschker AC, et al. Value of fractional uric acid excretion in differential diagnosis of hyponatremic patients on diuretics. J Clin Endocrinol Metab. 2008;93(8):2991-2997.
- Maesaka JK, Fishbane S. Regulation of renal urate excretion: a critical review. Am J Kidney Dis. 1998;32(6):917-933.
- Milionis HJ, Liamis GL, Elisaf MS. The hyponatremic patient: a systematic approach to laboratory diagnosis. CMAJ. 2002;166(8):1056-1062.
- Bitew S, Imbriano L, Miyawaki N, Fishbane S, Maesaka JK. More on renal salt wasting without cerebral disease: response to saline infusion. Clin J Am Soc Nephrol. 2009;4(2):309-315.
- Ellison DH, Berl T. Clinical practice. The syndrome of inappropriate antidiuresis. N Engl J Med. 2007;356(20):2064-2072.
- Arieff AI. Hyponatremia, convulsions, respiratory arrest, and permanent brain damage after elective surgery in healthy women. N Engl J Med. 1986;314(24):1529-1535.
- Ayus JC, Krothapalli RK, Arieff AI. Treatment of symptomatic hyponatremia and its relation to brain damage. A prospective study. N Engl J Med. 1987;317(19):1190-1195.
- Sterns RH, Nigwekar SU, Hix JK. The treatment of hyponatremia. Semin Nephrol. 2009;29(3):282-299.
- Hew-Butler T, Ayus JC, Kipps C, et al. Statement of the Second International Exercise-Associated Hyponatremia Consensus Development Conference, New Zealand, 2007. Clin J Sport Med. 2008;18(2):111-121.
- List AF, Hainsworth JD, Davis BW, Hande KR, Greco FA, Johnson DH. The syndrome of inappropriate secretion of antidiuretic hormone (SIADH) in small-cell lung cancer. J Clin Oncol. 1986;4(8):1191-1198.
- Verbalis JG. Managing hyponatremia in patients with syndrome of inappropriate antidiuretic hormone secretion. J Hosp Med. 2010;5 Suppl 3:S18-S26.
- Musch W, Decaux G. Treating the syndrome of inappropriate ADH secretion with isotonic saline. QJM. 1998;91(11):749-753.
- Berl T. Impact of solute intake on urine flow and water excretion. J Am Soc Nephrol. 2008;19(6):1076-1078.
- Decaux G, Brimioulle S, Genette F, Mockel J. Treatment of the syndrome of inappropriate secretion of antidiuretic hormone by urea. Am J Med. 1980;69(1):99-106.
- Forrest JN Jr., Cox M, Hong C, Morrison G, Bia M, Singer I. Superiority of demeclocycline over lithium in the treatment of chronic syndrome of inappropriate secretion of antidiuretic hormone. N Engl J Med. 1978;298(4):173-177.
- Nielsen J, Hoffert JD, Knepper MA, Agre P, Nielsen S, Fenton RA. Proteomic analysis of lithium-induced nephrogenic diabetes insipidus: mechanisms for aquaporin 2 down-regulation and cellular proliferation. Proc Natl Acad Sci U S A. 2008;105(9):3634-3639.
- Zeltser D, Rosansky S, van Rensburg H, Verbalis JG, Smith N. Assessment of the efficacy and safety of intravenous conivaptan in euvolemic and hypervolemic hyponatremia. Am J Nephrol. 2007;27(5):447-457.
- Verbalis JG, Zeltser D, Smith N, Barve A, Andoh M. Assessment of the efficacy and safety of intravenous conivaptan in patients with euvolaemic hyponatraemia: subgroup analysis of a randomized, controlled study. Clin Endocrinol (Oxf). 2008;69(1):159-168.
- Schrier RW, Gross P, Gheorghiade M, et al. Tolvaptan, a selective oral vasopressin V2-receptor antagonist, for hyponatremia. N Engl J Med. 2006;355(20):2099-2112.
- Berl T, Quittnat-Pelletier F, Verbalis JG, et al. Oral tolvaptan is safe and effective in chronic hyponatremia. J Am Soc Nephrol. 2010;21(4):705-712.
- Konstam MA, Gheorghiade M, Burnett JC Jr., et al. Effects of oral tolvaptan in patients hospitalized for worsening heart failure: the EVEREST Outcome Trial. JAMA. 2007;297(12):1319-1331.
Case
A 70-year-old woman with hypertension presents after a fall. Her medications include hydrochlorothiazide. Her blood pressure is 130/70 mm/Hg, with heart rate of 86. She has normal orthostatic vital signs. Her mucus membranes are moist and she has no jugular venous distension, edema, or ascites. Her plasma sodium (PNa) is 125 mmol/L, potassium 3.6 mmol/L, blood urea nitrogen (BUN) 30 mg/dL, and creatinine 0.8 mg/dL. Additional labs include serum thyroid stimulating hormone 1.12 mIU/L, cortisol 15 mcg/dL, serum osmolality 270 mOsm/kg, uric acid 4 mg/dL, urine osmolality 300 mOsm/kg, urine sodium (UNa) 40 mmol/L, fractional excretion of sodium 1.0%, and fractional excretion of urate (FEUrate) 13%. She receives 2 L isotonic saline intravenously over 24 hours, with resulting PNa of 127.
What is the cause of her hyponatremia, and how should her hyponatremia be managed?
Overview
Hyponatremia is one of the most common electrolyte abnormalities; it has a prevalence as high as 30% upon admission to the hospital.1 Hyponatremia is important clinically because of its high risk of mortality in the acute and symptomatic setting, and the risk of central pontine myelinolysis (CPM), or death with too rapid correction.2 Even so-called “asymptomatic” mild hyponatremia is associated with increased falls and impairments in gait and attention in the elderly.3
Hyponatremia is a state of excess water compared with the amount of solute in the extracellular fluid. To aid in diagnosing the etiology of hypotonic hyponatremia, the differential is traditionally divided into categories based on extracellular fluid volume (ECV) status, as shown in Table 1 (below), with syndrome of inappropriate antidiuretic hormone secretion (SIADH) being the most common cause of euvolemic hyponatremia.2 However, data show that clinical determination of volume status is often flawed,4 and an algorithmic approach to diagnosis and treatment yields improved results.5
Review of the Data
Diagnosis of SIADH. The original diagnostic criteria for SIADH, with minor modifications, are presented in Table 2, page 18).6,7,8 However, applying these criteria in clinical settings presents several difficulties, most notably a determination of ECV. The gold standard for assessing ECV status is by radioisotope, which is not practically feasible.9 Therefore, clinicians must rely on surrogate clinical markers of ECV (orthostatic hypotension, skin turgor, mucus membrane dryness, central venous pressure, BUN, BUN-creatinine ratio, and serum uric acid levels), which lack both sensitivity and specificity.4 Astoundingly, clinical assessment of ECV has been demonstrated to be accurate only 50% of the time when differentiating euvolemic patients from those with hypovolemia.4
Another challenge lies in the interpretation of UNa, which frequently is used as a surrogate for extra-arterial blood volume (EABV) status.10 Unfortunately, in the setting of diuretic use, UNa becomes inaccurate. The FEUrate, however, is unaffected by diuretic use and can be helpful in distinguishing between etiologies of hyponatremia with UNa greater than 30 mmol/L.11 The FEUrate is about 10% in normal euvolemic subjects and is reduced (usually <8%) in patients with low effective arterial blood volume.11,12 A trial of 86 patients demonstrated that a FEUrate of 12% had a specificity and positive predictive value of 100% in accurately identifying SIADH from diuretic-induced hyponatremia in patients on diuretics.11,12 Therefore, the UNa is a valid marker of EABV status when patients are not on diuretics; however, the FEUrate should be used in the setting of diuretic use.
Yet another pitfall is differentiating patients with salt depletion from those with SIADH. In these situations, measurement of the change in PNa concentration after a test infusion of isotonic saline is helpful. In salt depletion, PNa usually increases ≥5 mmol/L after 2 L saline infusion, which is not the case with SIADH.13 Incorrectly diagnosing renal salt wasting (RSW) as SIADH results in fluid restriction and, consequently, ECV depletion and increased morbidity.14 The persistence of hypouricemia and elevated FEUrate after correction of the hyponatremia in RSW differentiates it from SIADH.13, 14
Given these challenges, recommendations to use an algorithmic approach for the evaluation and diagnosis of hyponatremia have surfaced. In a study of 121 patients admitted with hyponatremia, an algorithm-based approach to the diagnosis of hyponatremia yielded an overall diagnostic accuracy of 71%, compared with an accuracy of 32% by experienced clinicians.5 This study also highlighted SIADH as the most frequent false-positive diagnosis that was expected whenever the combination of euvolemia and a UNa >30 mmol/L was present.5 Cases of diuretic-induced hyponatremia often were misclassified due to errors in the accurate assessment of ECV status, as most of these patients appeared clinically euvolemic or hypervolemic.5 Therefore, it is important to use an algorithm in identifying SIADH and to use one that does not rely solely on clinical estimation of ECV status (see Figure 1, below).
Management of acute and symptomatic hyponatremia. When hyponatremia develops acutely, urgent treatment is required (see Figure 2, below).15 Hyponatremia is considered acute when the onset is within 48 hours.15 Acute hyponatremia is most easily identified in the hospital and is commonly iatrogenic. Small case reviews in the 1980s began to associate postoperative deaths with the administration of hypotonic fluids.16 Asymptomatic patients with hyponatremia presenting from home should be considered chronic hyponatremias as the duration often is unclear.
Acute hyponatremia or neurologically symptomatic hyponatremia regardless of duration requires the use of hypertonic saline.15 Traditional sodium correction algorithms are based on early case series, which were focused on limiting neurologic complications from sodium overcorrection.17 This resulted in protocols recommending a conservative rate of correction spread over a 24- to 48-hour period.17 Infusing 3% saline at a rate of 1 ml/kg/hr to 2 ml/kg/hr results in a 1 mmol/L/hr to 2 mmol/L/hr increase in PNa.15 This simplified formula results in similar correction rates as more complex calculations.15 Correction should not exceed 8 mmol/L to 10 mmol/L within the first 24 hours, and 18 mmol/L to 25 mmol/L by 48 hours to avoid CPM.15 PNa should be checked every two hours to ensure that the correction rate is not exceeding the predicted rate, as the formulas do not take into account oral intake and ongoing losses.15
Recent observations focused on the initial four hours from onset of hyponatremia suggest a higher rate of correction can be tolerated without complications.18 Rapid sodium correction of 4 mmol/L to 6 mmol/L often is enough to stop neurologic complications.18 This can be accomplished with a bolus infusion of 100 mL of 3% saline.19 This may be repeated twice at 10-minute intervals until there is neurologic improvement.19 This might sound aggressive, but this would correspond to a rise in PNa of 5 mmol/L to 6 mmol/L in a 50 kg woman. Subsequent treatment with hypertonic fluid might not be needed if symptoms resolve.
Management of chronic hyponatremia. Hyponatremia secondary to SIADH improves with the treatment of the underlying cause, thus an active search for a causative medication or condition should be sought (see Table 1, p. 17).20
Water restriction. Restriction of fluid intake is the first-line treatment for SIADH in patients without hypovolemia. The severity of fluid restriction is guided by the concentration of the urinary solutes.15 Restriction of water intake to 500 ml/day to 1,000 ml/day is generally advised for many patients, as losses from the skin, lungs, and urine exceed this amount, leading to a gradual reduction in total body water.21 The main drawback of fluid restriction is poor compliance due to an intact thirst mechanism.
Saline infusion. The infusion of normal saline theoretically worsens hyponatremia due to SIADH because the water is retained while the salt is excreted. However, a trial of normal saline sometimes is attempted in patients in whom the differentiation between hypovolemia and euvolemia is difficult. From a study of a series of 17 patients with chronic SIADH, Musch and Decaux concluded that the infusion of intravenous normal (0.9%) saline raises PNa when the urine osmolality is less than 530 mosm/L.22
Oral solutes (urea and salt). The oral intake of salt augments water excretion23, and salt tablets are used as a second-line agent in patients with persistent hyponatremia despite fluid restriction.23 The oral administration of urea also results in increased free-water excretion via osmotic diuresis,24 but its poor palatability, lack of availability in the U.S., and limited user experience has restricted its usage.24
Demeclocycline. Demeclo-cycline is a tetracycline derivative that causes a partial nephrogenic diabetes insipidus.25 Its limitations include a slow onset of action (two to five days) and an unpredictable treatment effect with the possibility of causing profound polyuria and hypernatremia. It is also associated with reversible azotemia and sometimes nephrotoxicity, especially in patients with cirrhosis.
Lithium. Lithium also causes nephrogenic diabetes insipidus by downregulating vasopressin-stimulated aquaporin-2 expression and thus improves hyponatremia in SIADH.26 However, its use is significantly limited by its unpredictable response and the risks of interstitial nephritis and end-stage renal disease with chronic use. Therefore, it is no longer recommended for the treatment of SIADH.
Vasopressin receptor antagonists. Due to the role of excessive levels of vasopressin in the pathophysiology of most types of SIADH, antagonists of the vasopressin receptor were developed with the goal of preventing the excess water absorption that causes hyponatremia. Two vasopressin receptor antagonists, or vaptans, have been approved by the FDA for the treatment of nonemergent euvolemic and hypervolemic hyponatremia. Conivaptan is a nonselective vasopressin receptor antagonist that is for IV use only. Tolvaptan is a selective V2 receptor antagonist that is taken orally. Both conivaptan and tolvaptan successfully increase PNa levels while the drugs are being taken.27,28,29,30 Tolvaptan increases PNa levels in hyponatremia due to SIADH and CHF, and modestly so in cirrhosis.30
The most common side effects of the vaptans include dry mouth, increased thirst, and increased urination, although serious side effects (hypernatremia or too-rapid rate of increase in PNa) are possible.29 It is unclear if treating stable, asymptomatic hyponatremia with vaptans has any reduction in morbidity or mortality. One study found that tolvaptan increased the patients’ self-evaluations of mental functioning, but a study of tolvaptan used in combination with diuretics in the setting of CHF did not result in decreased mortality.29,31 Due to their expense, necessity of being started in the hospital, and unclear long-term benefit, the vaptans are only recommended when traditional measures such as fluid restriction and salt tablets have been unsuccessful.
Back to the Case
Our patient has hypotonic hyponatremia based on her low serum osmolality. The duration of her hyponatremia is unclear, but the patient is not experiencing seizures or coma. Therefore, her hyponatremia should be corrected slowly, and hypertonic saline is not indicated.
As is common in clinical practice, her true volume status is difficult to clinically ascertain. By physical exam, she appears euvolemic, but because she is on hydrochlorothiazide, she might be subtly hypovolemic. The UNa of 40 mmol/L is not consistent with hypovolemia, but its accuracy is limited in the setting of diuretics. The failure to improve her sodium by at least 5 mmol/L after a 2 L normal saline infusion argues against low effective arterial blood volume and indicates that the hydrochlorothiazide is unlikely to be the cause of her hyponatremia.
Therefore, the most likely cause of the hyponatremia is SIADH, a diagnosis further corroborated by the elevated FEUrate of 13%. Her chronic hyponatremia should be managed initially with fluid restriction while an investigation for an underlying cause of SIADH is initiated.
Bottom Line
The diagnosis of SIADH relies on the careful evaluation of laboratory values, use of an algorithm, and recognizing the limitations of clinically assessing volume status. The underlying cause of SIADH must also be sought and treated. TH
Dr. Grant is a clinical lecturer in internal medicine, Dr. Cho is a clinical instructor in internal medicine, and Dr. Nichani is an assistant professor of internal medicine at the University of Michigan Hospital and Health Systems in Ann Arbor.
References
- Upadhyay A, Jaber BL, Madias NE. Incidence and prevalence of hyponatremia. Am J Med. 2006;119(7 Suppl 1):S30-35.
- Verbalis JG, Goldsmith SR, Greenberg A, Schrier RW, Sterns RH. Hyponatremia treatment guidelines 2007: expert panel recommendations. Am J Med. 2007;120(11 Suppl 1):S1-21.
- Renneboog B, Musch W, Vandemergel X, Manto MU, Decaux G. Mild chronic hyponatremia is associated with falls, unsteadiness, and attention deficits. Am J Med. 2006;119(1):71.e71-78.
- Chung HM, Kluge R, Schrier RW, Anderson RJ. Clinical assessment of extracellular fluid volume in hyponatremia. Am J Med. 1987;83(5):905-908.
- Fenske W, Maier SK, Blechschmidt A, Allolio B, Störk S. Utility and limitations of the traditional diagnostic approach to hyponatremia: a diagnostic study. Am J Med. 2010;123(7):652-657.
- Bartter FC, Schwartz WB. The syndrome of inappropriate secretion of antidiuretic hormone. Am J Med. 1967;42(5):790-806.
- Smith DM, McKenna K, Thompson CJ. Hyponatraemia. Clin Endocrinol (Oxf). 2000;52(6):667-678.
- Verbalis JG. Hyponatraemia. Baillieres Clin Endocrinol Metab. Aug 1989;3(2):499-530.
- Maesaka JK, Imbriano LJ, Ali NM, Ilamathi E. Is it cerebral or renal salt wasting? Kidney Int. 2009;76(9):934-938.
- Verbalis JG. Disorders of body water homeostasis. Best Pract Res Clin Endocrinol Metab. 2003;17(4):471-503.
- Fenske W, Störk S, Koschker AC, et al. Value of fractional uric acid excretion in differential diagnosis of hyponatremic patients on diuretics. J Clin Endocrinol Metab. 2008;93(8):2991-2997.
- Maesaka JK, Fishbane S. Regulation of renal urate excretion: a critical review. Am J Kidney Dis. 1998;32(6):917-933.
- Milionis HJ, Liamis GL, Elisaf MS. The hyponatremic patient: a systematic approach to laboratory diagnosis. CMAJ. 2002;166(8):1056-1062.
- Bitew S, Imbriano L, Miyawaki N, Fishbane S, Maesaka JK. More on renal salt wasting without cerebral disease: response to saline infusion. Clin J Am Soc Nephrol. 2009;4(2):309-315.
- Ellison DH, Berl T. Clinical practice. The syndrome of inappropriate antidiuresis. N Engl J Med. 2007;356(20):2064-2072.
- Arieff AI. Hyponatremia, convulsions, respiratory arrest, and permanent brain damage after elective surgery in healthy women. N Engl J Med. 1986;314(24):1529-1535.
- Ayus JC, Krothapalli RK, Arieff AI. Treatment of symptomatic hyponatremia and its relation to brain damage. A prospective study. N Engl J Med. 1987;317(19):1190-1195.
- Sterns RH, Nigwekar SU, Hix JK. The treatment of hyponatremia. Semin Nephrol. 2009;29(3):282-299.
- Hew-Butler T, Ayus JC, Kipps C, et al. Statement of the Second International Exercise-Associated Hyponatremia Consensus Development Conference, New Zealand, 2007. Clin J Sport Med. 2008;18(2):111-121.
- List AF, Hainsworth JD, Davis BW, Hande KR, Greco FA, Johnson DH. The syndrome of inappropriate secretion of antidiuretic hormone (SIADH) in small-cell lung cancer. J Clin Oncol. 1986;4(8):1191-1198.
- Verbalis JG. Managing hyponatremia in patients with syndrome of inappropriate antidiuretic hormone secretion. J Hosp Med. 2010;5 Suppl 3:S18-S26.
- Musch W, Decaux G. Treating the syndrome of inappropriate ADH secretion with isotonic saline. QJM. 1998;91(11):749-753.
- Berl T. Impact of solute intake on urine flow and water excretion. J Am Soc Nephrol. 2008;19(6):1076-1078.
- Decaux G, Brimioulle S, Genette F, Mockel J. Treatment of the syndrome of inappropriate secretion of antidiuretic hormone by urea. Am J Med. 1980;69(1):99-106.
- Forrest JN Jr., Cox M, Hong C, Morrison G, Bia M, Singer I. Superiority of demeclocycline over lithium in the treatment of chronic syndrome of inappropriate secretion of antidiuretic hormone. N Engl J Med. 1978;298(4):173-177.
- Nielsen J, Hoffert JD, Knepper MA, Agre P, Nielsen S, Fenton RA. Proteomic analysis of lithium-induced nephrogenic diabetes insipidus: mechanisms for aquaporin 2 down-regulation and cellular proliferation. Proc Natl Acad Sci U S A. 2008;105(9):3634-3639.
- Zeltser D, Rosansky S, van Rensburg H, Verbalis JG, Smith N. Assessment of the efficacy and safety of intravenous conivaptan in euvolemic and hypervolemic hyponatremia. Am J Nephrol. 2007;27(5):447-457.
- Verbalis JG, Zeltser D, Smith N, Barve A, Andoh M. Assessment of the efficacy and safety of intravenous conivaptan in patients with euvolaemic hyponatraemia: subgroup analysis of a randomized, controlled study. Clin Endocrinol (Oxf). 2008;69(1):159-168.
- Schrier RW, Gross P, Gheorghiade M, et al. Tolvaptan, a selective oral vasopressin V2-receptor antagonist, for hyponatremia. N Engl J Med. 2006;355(20):2099-2112.
- Berl T, Quittnat-Pelletier F, Verbalis JG, et al. Oral tolvaptan is safe and effective in chronic hyponatremia. J Am Soc Nephrol. 2010;21(4):705-712.
- Konstam MA, Gheorghiade M, Burnett JC Jr., et al. Effects of oral tolvaptan in patients hospitalized for worsening heart failure: the EVEREST Outcome Trial. JAMA. 2007;297(12):1319-1331.
Case
A 70-year-old woman with hypertension presents after a fall. Her medications include hydrochlorothiazide. Her blood pressure is 130/70 mm/Hg, with heart rate of 86. She has normal orthostatic vital signs. Her mucus membranes are moist and she has no jugular venous distension, edema, or ascites. Her plasma sodium (PNa) is 125 mmol/L, potassium 3.6 mmol/L, blood urea nitrogen (BUN) 30 mg/dL, and creatinine 0.8 mg/dL. Additional labs include serum thyroid stimulating hormone 1.12 mIU/L, cortisol 15 mcg/dL, serum osmolality 270 mOsm/kg, uric acid 4 mg/dL, urine osmolality 300 mOsm/kg, urine sodium (UNa) 40 mmol/L, fractional excretion of sodium 1.0%, and fractional excretion of urate (FEUrate) 13%. She receives 2 L isotonic saline intravenously over 24 hours, with resulting PNa of 127.
What is the cause of her hyponatremia, and how should her hyponatremia be managed?
Overview
Hyponatremia is one of the most common electrolyte abnormalities; it has a prevalence as high as 30% upon admission to the hospital.1 Hyponatremia is important clinically because of its high risk of mortality in the acute and symptomatic setting, and the risk of central pontine myelinolysis (CPM), or death with too rapid correction.2 Even so-called “asymptomatic” mild hyponatremia is associated with increased falls and impairments in gait and attention in the elderly.3
Hyponatremia is a state of excess water compared with the amount of solute in the extracellular fluid. To aid in diagnosing the etiology of hypotonic hyponatremia, the differential is traditionally divided into categories based on extracellular fluid volume (ECV) status, as shown in Table 1 (below), with syndrome of inappropriate antidiuretic hormone secretion (SIADH) being the most common cause of euvolemic hyponatremia.2 However, data show that clinical determination of volume status is often flawed,4 and an algorithmic approach to diagnosis and treatment yields improved results.5
Review of the Data
Diagnosis of SIADH. The original diagnostic criteria for SIADH, with minor modifications, are presented in Table 2, page 18).6,7,8 However, applying these criteria in clinical settings presents several difficulties, most notably a determination of ECV. The gold standard for assessing ECV status is by radioisotope, which is not practically feasible.9 Therefore, clinicians must rely on surrogate clinical markers of ECV (orthostatic hypotension, skin turgor, mucus membrane dryness, central venous pressure, BUN, BUN-creatinine ratio, and serum uric acid levels), which lack both sensitivity and specificity.4 Astoundingly, clinical assessment of ECV has been demonstrated to be accurate only 50% of the time when differentiating euvolemic patients from those with hypovolemia.4
Another challenge lies in the interpretation of UNa, which frequently is used as a surrogate for extra-arterial blood volume (EABV) status.10 Unfortunately, in the setting of diuretic use, UNa becomes inaccurate. The FEUrate, however, is unaffected by diuretic use and can be helpful in distinguishing between etiologies of hyponatremia with UNa greater than 30 mmol/L.11 The FEUrate is about 10% in normal euvolemic subjects and is reduced (usually <8%) in patients with low effective arterial blood volume.11,12 A trial of 86 patients demonstrated that a FEUrate of 12% had a specificity and positive predictive value of 100% in accurately identifying SIADH from diuretic-induced hyponatremia in patients on diuretics.11,12 Therefore, the UNa is a valid marker of EABV status when patients are not on diuretics; however, the FEUrate should be used in the setting of diuretic use.
Yet another pitfall is differentiating patients with salt depletion from those with SIADH. In these situations, measurement of the change in PNa concentration after a test infusion of isotonic saline is helpful. In salt depletion, PNa usually increases ≥5 mmol/L after 2 L saline infusion, which is not the case with SIADH.13 Incorrectly diagnosing renal salt wasting (RSW) as SIADH results in fluid restriction and, consequently, ECV depletion and increased morbidity.14 The persistence of hypouricemia and elevated FEUrate after correction of the hyponatremia in RSW differentiates it from SIADH.13, 14
Given these challenges, recommendations to use an algorithmic approach for the evaluation and diagnosis of hyponatremia have surfaced. In a study of 121 patients admitted with hyponatremia, an algorithm-based approach to the diagnosis of hyponatremia yielded an overall diagnostic accuracy of 71%, compared with an accuracy of 32% by experienced clinicians.5 This study also highlighted SIADH as the most frequent false-positive diagnosis that was expected whenever the combination of euvolemia and a UNa >30 mmol/L was present.5 Cases of diuretic-induced hyponatremia often were misclassified due to errors in the accurate assessment of ECV status, as most of these patients appeared clinically euvolemic or hypervolemic.5 Therefore, it is important to use an algorithm in identifying SIADH and to use one that does not rely solely on clinical estimation of ECV status (see Figure 1, below).
Management of acute and symptomatic hyponatremia. When hyponatremia develops acutely, urgent treatment is required (see Figure 2, below).15 Hyponatremia is considered acute when the onset is within 48 hours.15 Acute hyponatremia is most easily identified in the hospital and is commonly iatrogenic. Small case reviews in the 1980s began to associate postoperative deaths with the administration of hypotonic fluids.16 Asymptomatic patients with hyponatremia presenting from home should be considered chronic hyponatremias as the duration often is unclear.
Acute hyponatremia or neurologically symptomatic hyponatremia regardless of duration requires the use of hypertonic saline.15 Traditional sodium correction algorithms are based on early case series, which were focused on limiting neurologic complications from sodium overcorrection.17 This resulted in protocols recommending a conservative rate of correction spread over a 24- to 48-hour period.17 Infusing 3% saline at a rate of 1 ml/kg/hr to 2 ml/kg/hr results in a 1 mmol/L/hr to 2 mmol/L/hr increase in PNa.15 This simplified formula results in similar correction rates as more complex calculations.15 Correction should not exceed 8 mmol/L to 10 mmol/L within the first 24 hours, and 18 mmol/L to 25 mmol/L by 48 hours to avoid CPM.15 PNa should be checked every two hours to ensure that the correction rate is not exceeding the predicted rate, as the formulas do not take into account oral intake and ongoing losses.15
Recent observations focused on the initial four hours from onset of hyponatremia suggest a higher rate of correction can be tolerated without complications.18 Rapid sodium correction of 4 mmol/L to 6 mmol/L often is enough to stop neurologic complications.18 This can be accomplished with a bolus infusion of 100 mL of 3% saline.19 This may be repeated twice at 10-minute intervals until there is neurologic improvement.19 This might sound aggressive, but this would correspond to a rise in PNa of 5 mmol/L to 6 mmol/L in a 50 kg woman. Subsequent treatment with hypertonic fluid might not be needed if symptoms resolve.
Management of chronic hyponatremia. Hyponatremia secondary to SIADH improves with the treatment of the underlying cause, thus an active search for a causative medication or condition should be sought (see Table 1, p. 17).20
Water restriction. Restriction of fluid intake is the first-line treatment for SIADH in patients without hypovolemia. The severity of fluid restriction is guided by the concentration of the urinary solutes.15 Restriction of water intake to 500 ml/day to 1,000 ml/day is generally advised for many patients, as losses from the skin, lungs, and urine exceed this amount, leading to a gradual reduction in total body water.21 The main drawback of fluid restriction is poor compliance due to an intact thirst mechanism.
Saline infusion. The infusion of normal saline theoretically worsens hyponatremia due to SIADH because the water is retained while the salt is excreted. However, a trial of normal saline sometimes is attempted in patients in whom the differentiation between hypovolemia and euvolemia is difficult. From a study of a series of 17 patients with chronic SIADH, Musch and Decaux concluded that the infusion of intravenous normal (0.9%) saline raises PNa when the urine osmolality is less than 530 mosm/L.22
Oral solutes (urea and salt). The oral intake of salt augments water excretion23, and salt tablets are used as a second-line agent in patients with persistent hyponatremia despite fluid restriction.23 The oral administration of urea also results in increased free-water excretion via osmotic diuresis,24 but its poor palatability, lack of availability in the U.S., and limited user experience has restricted its usage.24
Demeclocycline. Demeclo-cycline is a tetracycline derivative that causes a partial nephrogenic diabetes insipidus.25 Its limitations include a slow onset of action (two to five days) and an unpredictable treatment effect with the possibility of causing profound polyuria and hypernatremia. It is also associated with reversible azotemia and sometimes nephrotoxicity, especially in patients with cirrhosis.
Lithium. Lithium also causes nephrogenic diabetes insipidus by downregulating vasopressin-stimulated aquaporin-2 expression and thus improves hyponatremia in SIADH.26 However, its use is significantly limited by its unpredictable response and the risks of interstitial nephritis and end-stage renal disease with chronic use. Therefore, it is no longer recommended for the treatment of SIADH.
Vasopressin receptor antagonists. Due to the role of excessive levels of vasopressin in the pathophysiology of most types of SIADH, antagonists of the vasopressin receptor were developed with the goal of preventing the excess water absorption that causes hyponatremia. Two vasopressin receptor antagonists, or vaptans, have been approved by the FDA for the treatment of nonemergent euvolemic and hypervolemic hyponatremia. Conivaptan is a nonselective vasopressin receptor antagonist that is for IV use only. Tolvaptan is a selective V2 receptor antagonist that is taken orally. Both conivaptan and tolvaptan successfully increase PNa levels while the drugs are being taken.27,28,29,30 Tolvaptan increases PNa levels in hyponatremia due to SIADH and CHF, and modestly so in cirrhosis.30
The most common side effects of the vaptans include dry mouth, increased thirst, and increased urination, although serious side effects (hypernatremia or too-rapid rate of increase in PNa) are possible.29 It is unclear if treating stable, asymptomatic hyponatremia with vaptans has any reduction in morbidity or mortality. One study found that tolvaptan increased the patients’ self-evaluations of mental functioning, but a study of tolvaptan used in combination with diuretics in the setting of CHF did not result in decreased mortality.29,31 Due to their expense, necessity of being started in the hospital, and unclear long-term benefit, the vaptans are only recommended when traditional measures such as fluid restriction and salt tablets have been unsuccessful.
Back to the Case
Our patient has hypotonic hyponatremia based on her low serum osmolality. The duration of her hyponatremia is unclear, but the patient is not experiencing seizures or coma. Therefore, her hyponatremia should be corrected slowly, and hypertonic saline is not indicated.
As is common in clinical practice, her true volume status is difficult to clinically ascertain. By physical exam, she appears euvolemic, but because she is on hydrochlorothiazide, she might be subtly hypovolemic. The UNa of 40 mmol/L is not consistent with hypovolemia, but its accuracy is limited in the setting of diuretics. The failure to improve her sodium by at least 5 mmol/L after a 2 L normal saline infusion argues against low effective arterial blood volume and indicates that the hydrochlorothiazide is unlikely to be the cause of her hyponatremia.
Therefore, the most likely cause of the hyponatremia is SIADH, a diagnosis further corroborated by the elevated FEUrate of 13%. Her chronic hyponatremia should be managed initially with fluid restriction while an investigation for an underlying cause of SIADH is initiated.
Bottom Line
The diagnosis of SIADH relies on the careful evaluation of laboratory values, use of an algorithm, and recognizing the limitations of clinically assessing volume status. The underlying cause of SIADH must also be sought and treated. TH
Dr. Grant is a clinical lecturer in internal medicine, Dr. Cho is a clinical instructor in internal medicine, and Dr. Nichani is an assistant professor of internal medicine at the University of Michigan Hospital and Health Systems in Ann Arbor.
References
- Upadhyay A, Jaber BL, Madias NE. Incidence and prevalence of hyponatremia. Am J Med. 2006;119(7 Suppl 1):S30-35.
- Verbalis JG, Goldsmith SR, Greenberg A, Schrier RW, Sterns RH. Hyponatremia treatment guidelines 2007: expert panel recommendations. Am J Med. 2007;120(11 Suppl 1):S1-21.
- Renneboog B, Musch W, Vandemergel X, Manto MU, Decaux G. Mild chronic hyponatremia is associated with falls, unsteadiness, and attention deficits. Am J Med. 2006;119(1):71.e71-78.
- Chung HM, Kluge R, Schrier RW, Anderson RJ. Clinical assessment of extracellular fluid volume in hyponatremia. Am J Med. 1987;83(5):905-908.
- Fenske W, Maier SK, Blechschmidt A, Allolio B, Störk S. Utility and limitations of the traditional diagnostic approach to hyponatremia: a diagnostic study. Am J Med. 2010;123(7):652-657.
- Bartter FC, Schwartz WB. The syndrome of inappropriate secretion of antidiuretic hormone. Am J Med. 1967;42(5):790-806.
- Smith DM, McKenna K, Thompson CJ. Hyponatraemia. Clin Endocrinol (Oxf). 2000;52(6):667-678.
- Verbalis JG. Hyponatraemia. Baillieres Clin Endocrinol Metab. Aug 1989;3(2):499-530.
- Maesaka JK, Imbriano LJ, Ali NM, Ilamathi E. Is it cerebral or renal salt wasting? Kidney Int. 2009;76(9):934-938.
- Verbalis JG. Disorders of body water homeostasis. Best Pract Res Clin Endocrinol Metab. 2003;17(4):471-503.
- Fenske W, Störk S, Koschker AC, et al. Value of fractional uric acid excretion in differential diagnosis of hyponatremic patients on diuretics. J Clin Endocrinol Metab. 2008;93(8):2991-2997.
- Maesaka JK, Fishbane S. Regulation of renal urate excretion: a critical review. Am J Kidney Dis. 1998;32(6):917-933.
- Milionis HJ, Liamis GL, Elisaf MS. The hyponatremic patient: a systematic approach to laboratory diagnosis. CMAJ. 2002;166(8):1056-1062.
- Bitew S, Imbriano L, Miyawaki N, Fishbane S, Maesaka JK. More on renal salt wasting without cerebral disease: response to saline infusion. Clin J Am Soc Nephrol. 2009;4(2):309-315.
- Ellison DH, Berl T. Clinical practice. The syndrome of inappropriate antidiuresis. N Engl J Med. 2007;356(20):2064-2072.
- Arieff AI. Hyponatremia, convulsions, respiratory arrest, and permanent brain damage after elective surgery in healthy women. N Engl J Med. 1986;314(24):1529-1535.
- Ayus JC, Krothapalli RK, Arieff AI. Treatment of symptomatic hyponatremia and its relation to brain damage. A prospective study. N Engl J Med. 1987;317(19):1190-1195.
- Sterns RH, Nigwekar SU, Hix JK. The treatment of hyponatremia. Semin Nephrol. 2009;29(3):282-299.
- Hew-Butler T, Ayus JC, Kipps C, et al. Statement of the Second International Exercise-Associated Hyponatremia Consensus Development Conference, New Zealand, 2007. Clin J Sport Med. 2008;18(2):111-121.
- List AF, Hainsworth JD, Davis BW, Hande KR, Greco FA, Johnson DH. The syndrome of inappropriate secretion of antidiuretic hormone (SIADH) in small-cell lung cancer. J Clin Oncol. 1986;4(8):1191-1198.
- Verbalis JG. Managing hyponatremia in patients with syndrome of inappropriate antidiuretic hormone secretion. J Hosp Med. 2010;5 Suppl 3:S18-S26.
- Musch W, Decaux G. Treating the syndrome of inappropriate ADH secretion with isotonic saline. QJM. 1998;91(11):749-753.
- Berl T. Impact of solute intake on urine flow and water excretion. J Am Soc Nephrol. 2008;19(6):1076-1078.
- Decaux G, Brimioulle S, Genette F, Mockel J. Treatment of the syndrome of inappropriate secretion of antidiuretic hormone by urea. Am J Med. 1980;69(1):99-106.
- Forrest JN Jr., Cox M, Hong C, Morrison G, Bia M, Singer I. Superiority of demeclocycline over lithium in the treatment of chronic syndrome of inappropriate secretion of antidiuretic hormone. N Engl J Med. 1978;298(4):173-177.
- Nielsen J, Hoffert JD, Knepper MA, Agre P, Nielsen S, Fenton RA. Proteomic analysis of lithium-induced nephrogenic diabetes insipidus: mechanisms for aquaporin 2 down-regulation and cellular proliferation. Proc Natl Acad Sci U S A. 2008;105(9):3634-3639.
- Zeltser D, Rosansky S, van Rensburg H, Verbalis JG, Smith N. Assessment of the efficacy and safety of intravenous conivaptan in euvolemic and hypervolemic hyponatremia. Am J Nephrol. 2007;27(5):447-457.
- Verbalis JG, Zeltser D, Smith N, Barve A, Andoh M. Assessment of the efficacy and safety of intravenous conivaptan in patients with euvolaemic hyponatraemia: subgroup analysis of a randomized, controlled study. Clin Endocrinol (Oxf). 2008;69(1):159-168.
- Schrier RW, Gross P, Gheorghiade M, et al. Tolvaptan, a selective oral vasopressin V2-receptor antagonist, for hyponatremia. N Engl J Med. 2006;355(20):2099-2112.
- Berl T, Quittnat-Pelletier F, Verbalis JG, et al. Oral tolvaptan is safe and effective in chronic hyponatremia. J Am Soc Nephrol. 2010;21(4):705-712.
- Konstam MA, Gheorghiade M, Burnett JC Jr., et al. Effects of oral tolvaptan in patients hospitalized for worsening heart failure: the EVEREST Outcome Trial. JAMA. 2007;297(12):1319-1331.
Making a List, Check It Twice
In October, a 36‐year‐old woman with no significant past medical history presented to the Emergency Department (ED) with a 3‐day history of headache and fever. The headache was severe, throbbing, and frontal in location. She also complained of daily fevers measured up to 103F, generalized malaise, and fatigue. She did not report neck stiffness or photophobia. She felt better after receiving intravenous fluids and was discharged home with a diagnosis of a nonspecific viral illness. Two days later, she returned to the ED with worsening headache, fever, mild photophobia, and poor oral intake. She also complained of a dry cough that made her headache worse, as did bending over. She did not report confusion, neck stiffness, shortness of breath, sore throat, runny nose, abdominal symptoms, or rash.
This patient presents a second time to the ED with worsening headache and fever raising concerns about meningitis. At the time of her first ED visit, it can be assumed that she had a nontoxic appearance because she was discharged shortly thereafter. Thus, acute bacterial meningitis seems less likely, but occasionally patients with meningococcal meningitis may not appear significantly ill until later in the process. Nonetheless, acute meningitis, possibly viral, is the initial concern. The time of the year is an important variable because many viral infections are seasonal. Enteroviruses are the most common cause of viral meningitis in the United States, particularly in the summer and fall. In contrast, mumps, measles, and varicella zoster viruses occur more commonly in winter and spring. Herpetic meningoencephalitis is a life‐threatening condition with a guarded prognosis. Therefore, early recognition and treatment is necessary to decrease morbidity and mortality. Drugs such as nonsteroidal anti‐inflammatory agents, trimethoprim‐sulfamethoxazole, amoxicillin, and rarely vaccines can also cause aseptic meningitis. Infections from fungi, spirochetes, mycobacteria, and rarely parasites also cause meningitis, but would be of greater concern in a patient with risk factors such as recent travel or an immunocompromised state.
Increased headache with bending and cough might indicate elevated intracranial pressure. However, this is a nonspecific complaint, and headache is often worse with the Valsalva maneuver. Because she reports a cough, a chest x‐ray would be useful. In addition to routine initial tests, cerebrospinal fluid (CSF) analysis and human immunodeficiency virus (HIV) testing is recommended.
Her past medical history was notable for depression. Her medications included bupropion, multivitamins, and fish oil. She was also taking milk thistle pills daily to protect her liver because she had been drinking alcohol heavily for the past 2 weeks since her husband left her. She smoked 1 pack of cigarettes daily. She had not traveled recently. She reported no recent animal or wildlife exposure but did recall falling into a midwestern river while canoeing 2 weeks prior to presentation. She worked as a hairstylist and described no sick contacts or risk factors for HIV disease.
An important new historical element is that the patient fell into a river. If she swallowed a significant amount of water during her fall overboard, meningitis from waterborne infections such as Aeromonas, Acanthamoeba, and Naegleria need to be considered. Fortunately, these are rare in the Midwest. Her canoeing history may suggest exposure to wooded areas. Certainly, tickborne infections such as ehrlichiosis, babesiosis, Lyme disease, and Rocky Mountain spotted fever can also cause meningitis. Histoplasmosis and blastomycosis are also endemic to the midwestern United States and can disseminate and cause central nervous system disease.
At this time, viral and bacterial infections are highest on the differential diagnosis. However, the microbiology laboratory needs to be alerted to the possibility of fungal or parasitic organisms depending on the initial CSF analysis results.
The patient was a Caucasian woman who appeared comfortable. Her blood pressure was 130/62 mm Hg, heart rate was 83 beats per minute, respiratory rate was 18 per minute, temperature was 100.8F, and oxygen saturation was 98% on room air. She was fully alert and oriented. Her pupils were bilaterally equal, reactive to light and accommodation with intact extraocular movement and no nystagmus. There was conjunctival injection bilaterally without noticeable pallor or icterus. Fundoscopic examination, which the patient tolerated without difficulty, was normal. Inspection of the oral cavity showed mild tonsillar enlargement. The neck was supple with no stiffness. No cervical, axillary, or inguinal lymph nodes were palpable. Faint bilateral basilar crackles were audible over the posterior chest. There was very mild right upper quadrant abdominal tenderness without guarding. The liver and spleen were normal size and bowel sounds were present. No rash, peripheral edema, or spinal tenderness was noted. A complete neurological examination was normal.
Her general appearance and vital signs seem reassuring. Conjunctival injection and mild tonsillar enlargement are nonspecific findings and may occur in systemic inflammatory states especially viral infections. Atelectasis may account for faint bilateral basilar crackles especially if associated with post‐tussive change. Her alcohol use puts her at risk of aspiration. A right lower lobe process (pneumonia) can sometimes present with right upper quadrant tenderness. However, this tenderness may also represent muscle soreness from repeated coughing, liver, or gallbladder disease. The same infectious process affecting the central nervous system and possibly her lungs, may also be affecting the liver.
A complete blood count revealed a white blood cell count of 3000/mm3 (79% neutrophils, 15% lymphocytes, 5% monocytes), hemoglobin of 11.7 g/dL, and platelets of 110,000/mm3. The serum sodium was 133 mmol/L, potassium was 3.7 mmol/L, bicarbonate was 22 mmol/L, and blood urea nitrogen was 20 mg/dL. The serum creatinine was 1.5 compared to 1.0 mg/dL on testing 2 days prior. A liver function panel showed protein of 5.1 g/dL, albumin of 3 g/dL, aspartate aminotransferase (AST) of 576 IU/L, alanine aminotransferase (ALT) of 584 IU/L, alkaline phosphatase of 282 IU/L, and total bilirubin of 1 mg/dL. The coagulation profile, creatinine phosphokinase, acetaminophen level, urine pregnancy test, urine drug screen, and urinalysis (including urine microscopy) were normal.
The CSF opening pressure was 13 cm H2O. CSF analysis showed 4 mononuclear leukocytes per high‐power field, CSF protein was 27 mg/dL, and glucose was 76 mg/dL. No organisms were noted on gram stain. A chest x‐ray showed focal airspace opacity in the left lower lobe (Figure 1) and the patient was hospitalized for further management.
The normal CSF analysis makes acute meningitis much less likely. It is interesting to note that the aminotransferase levels are nearly equal. Usually, in viral and many other causes of hepatitis, the ALT is higher than the AST, whereas the contrary is true in alcoholic hepatitis. Because the patient has been consuming significant amounts of alcohol recently, these levels may become equal in the setting of another primary liver process. The elevation in liver enzymes also raises the possibility of autoimmune hepatitis secondary to a systemic vasculitis such as systemic lupus erythematosus. Nonetheless, the focus should be on infectious causes of hepatitis such as hepatitis C, adenovirus, parvovirus, Epstein‐Barr virus (EBV), cytomegalovirus, and herpes simplex virus that can cause pneumonia either as a primary or secondary infection. Acute HIV infection can also present in this fashion, and anti‐HIV antibody testing may be negative early in the disease. In the setting of a normal urinalysis and bland urine sediment, prerenal azotemia is the most likely cause of her acute renal injury and can be confirmed by testing the urinary sodium and creatinine. A peripheral smear should be reviewed to evaluate the pancytopenia.
Severe headache, fever, conjunctival injection, pancytopenia, acute kidney injury, hepatitis, and pneumonia may occur in leptospirosis, particularly in a patient with recent freshwater exposure. Alternatively, ehrlichiosis can also account for fever, headache, pancytopenia, renal failure, hepatitis, and pneumonia, but conjunctival suffusion is not often present. At this time, treatment for community‐acquired pneumonia that includes coverage for leptospirosis should be started.
The patient was hydrated with intravenous fluids and treated with intravenous ceftriaxone and azithromycin for community‐acquired pneumonia. An abdominal ultrasound was normal. The serologic assays for acute hepatitis A, B, and C infection were negative. The following morning, she reported worsening headache, increased cough now productive of whitish‐yellow sputum, and diffuse body aches. She appeared more lethargic and toxic. Her blood pressure was 100/83 mm Hg, heart rate was 84 beats per minute, respiratory rate was 24 per minute, and temperature was 101.3F. She had increased crackles on chest auscultation bilaterally and required supplemental oxygen at 4 L/minute by nasal cannula. Examination of both legs now revealed multiple scattered, faintly erythematous, 2‐cm‐sized patches overlying tender subtle subcutaneous nodules. Additionally, a mildly pruritic, V‐shaped area of blanchable erythema was also seen on her chest. The white blood cell count was 2500/mm3 (77% neutrophils, 15% lymphocytes), serum creatinine was 1.8 mg/dL, AST was 351 IU/L, and ALT was 485 IU/L. Blood cultures showed no growth and a peripheral smear examination was unrevealing. A noncontrast chest computed tomographic scan showed findings consistent with multifocal pneumonia (Figure 2).
It would be prudent at this time to expand her antimicrobial coverage (such as with vancomycin and piperacillin‐tazobactam) for activity against methicillin‐resistant Staphylococcus aureus and Pseudomonas because of her clinical worsening. Although ceftriaxone or piperacillin would cover leptospirosis, given the possibility of ehrlichiosis, the addition of doxycycline should be strongly considered.
The description of the rash on her legs seems consistent with erythema nodosum, which is associated with a number of infections (streptococcal, fungal, syphilis, EBV, cat‐scratch disease, tuberculosis), inflammatory conditions (inflammatory bowel disease, autoimmune disease, malignancy), and pregnancy. The blanchable rash on the chest is also a cause of concern for a possible drug reaction (ceftriaxone). A Jarisch‐Herxheimer reaction is possible given her acute worsening of symptoms with initiation of antibiotic therapy.
An antineutrophil cytoplasmic antibodyassociated vasculitis or another autoimmune condition such as systemic lupus erythematosus can account for erythema nodosum, rash, pancytopenia, and hepatitis. This diagnosis might also fit if she had a vasculitic pulmonary hemorrhage that caused her lung infiltrates and worsening hypoxia. A complete antinuclear antibody panel, antineutrophil cytoplasmic antibody, and antismooth muscle antibody testing is recommended. A skin and bronchoscopic biopsy should be considered.
Her dose of ceftriaxone was increased for possible severe pneumococcal pneumonia. The dermatology consultant felt that her leg lesions were consistent with erythema nodosum and the chest rash consistent with cutaneous photodamage. Bronchoscopic examination was normal and a bronchoalveolar lavage sample showed 2905 red blood cells/mm3 and 605 white blood cells/mm3 (70% neutrophils, 7% lymphocytes, 16% histiocytes), normal cytology, and negative cultures. There was no significant clinical improvement by the fourth hospital day and oral doxycycline was started. The next day, her skin lesions had resolved and she felt better. The serologic tests for Legionella, Mycoplasma, cytomegalovirus, EBV, Toxoplasma, Chlamydophila, Ehrlichia, Leptospira, Q‐fever, parvovirus, and adenovirus were negative. A fungal serology panel, HIV polymerase chain reaction, cryoglobulin level, and several rheumatologic tests (antinuclear antibody, extractable nuclear antigen panel, rheumatoid factor, antineutrophil cytoplasmic antibody, antiproteinase 3, and antiglomerular basement membrane antibodies) were normal. Blood cultures continued to show no growth.
The apparent response to doxycycline suggests she might have ehrlichiosis. A buffy coat review for morulae should be done. It is also possible that she may have improved on her initial therapy alone before starting doxycycline and her clinical worsening (including the chest rash) was due to a Jarisch‐Herxheimer reaction. Serologic tests for leptospirosis and ehrlichiosis should be repeated in 12 weeks because such infections may not cause detectable antibody levels early in the illness.
Ceftriaxone and doxycycline were continued and she showed rapid and significant clinical improvement. She was discharged 4 days later with instructions to complete a 10‐day course of antibiotics. At her 3‐month follow‐up, she was doing well and a repeat Leptospira antibody test by the Indirect Hemagglutination Assay (MRL Diagnostics, Cypress, California; normal titer <1:50) was positive at a titer of 1:100, which is highly suggestive of leptospirosis.
Commentary
Leptospirosis is a zoonotic infection caused by spirochetes of the genus Leptospira. The infection is usually transmitted indirectly to humans through contact with water, food, or soil contaminated with the urine of infected mammals.1 Risk factors for infection include participation in recreational activities (such as freshwater swimming, canoeing, and camping), occupational exposure, and exposure to infected pets or domesticated livestock. Approximately 100200 cases are identified annually in the United States, and approximately half occur in the state of Hawaii.2 Outbreaks of leptospirosis have been reported previously in the Midwest.3 These organisms inoculate humans through contact with mucous membranes or broken skin, or enter by swallowing infected food or water. A large number of these infections remain subclinical or result in a very mild illness with spontaneous clearance by the host's immune mechanism. Following an incubation period of 230 days, infected individuals may develop clinically significant disease (Table 1). Clinical presentations may overlap as the disease progresses. Although much remains to be learned about the exact pathogenic mechanism, disruption of the cell membranes of small vessel endothelia (a toxin‐like effect), and cytokine‐mediated tissue injury are believed to cause organ hemorrhage and ischemia.4
|
| 1. Mild influenza‐like self‐remitting disease (90% of cases) |
| Undifferentiated fever (usually 100F105F), severe headache, and myalgia (especially lower limbs). |
| 2. Moderately severe disease usually requiring hospitalization (5%9% of cases) |
| Marked prostration, anorexia, nausea, and vomiting, conjunctival suffusion, transient rash, frequently abdominal pain, constipation or diarrhea, and occasionally epistaxis. |
| 3. Severe disease involving multiple organ systems (1%5% of cases) |
| Hepatorenal Syndrome (Weil's syndrome) |
| Constellation of jaundice, hemorrhagic diathesis, and acute renal failure. Hepatic failure is rarely fatal. Renal involvement is usually more severe and the common cause of death. Cardiac (myocarditis with arrhythmias) and pulmonary complications are frequent. Confusion and restlessness may occur. |
| Hemorrhagic pneumonitis |
| Usually presents as a dry cough initially but becomes blood‐streaked after 23 days. Often characterized by a rapid progression to involve extensive areas of lungs, massive intra‐alveolar hemorrhage, acute respiratory failure, and death. |
| Central nervous system involvement |
| Meningismus, meningitis, or meningoencephalitis. |
The clinical diagnosis of leptospirosis is difficult because of its protean manifestations. Although nonspecific, 2 clinical features may provide a clue to the clinical diagnosis. First, the presence of conjunctival suffusion occurs in the early stage of the disease and is often associated with subconjunctival hemorrhage. Second, severe myalgia, commonly involving the lower limbs, is also characteristically present.1, 5 In 1 series of 58 patients with acute leptospirosis, conjunctival suffusion was observed in 50% of cases, and subconjunctival hemorrhage in 29%. Body ache and muscle tenderness was described in almost all cases.6
As seen in this case, the presence of a rash may pose a clinical challenge. A transient macular, maculopapular, purpuric, or urticarial rash may be seen in acute leptospirosis, but rashes may also be representative of a complication of treatment.1 First described in 1895 in patients with syphilis treated with mercury, the Jarisch‐Herxheimer reaction typically occurs within a few hours of antimicrobial treatment of spirochete infections and often presents with a rash, headache, fever, rigors, hypotension, sweating, and worsening symptoms of the underlying illness.7 Other skin findings such as the occurrence of erythema nodosum have been previously reported in cases of leptospirosis.8
Human ehrlichiosis (HE) is caused by tickborne, obligatory intracellular bacteria that infect leukocytes. There are 3 distinct clinical conditions: human monocytic ehrlichiosis (HME, caused by Ehrlichia chaffeensis), human granulocytic anaplasmosis (HGA, caused by Anaplasma phagocytophilum), and human ewingii ehrlichiosis (HEE, caused by E. ewingii). Although most cases of HME and HEE are seen in the southeastern and south‐central United States and California, the highest incidence of HGA is reported in the northeastern and upper Midwest regions.9 As with leptospirosis, the clinical range of HE spans from asymptomatic infection to life‐threatening illness. Following an incubation period of 12 weeks, symptomatic cases usually present with nonspecific complaints such as high fevers, chills, headache, nausea, arthralgia, myalgia, and malaise.10 The majority of cases will report a tick bite or an exposure to ticks. Laboratory tests often reveal leukopenia (white blood cell count < 4000/mm3), thrombocytopenia, hyponatremia, and elevated AST and ALT. Patients with severe disease may develop renal, respiratory, and hepatic failure. Thus, differentiating ehrlichiosis from leptospirosis is often challenging for the clinician.
However, there are a few clinical clues that help distinguish between these illnesses in this case. HGA as a cause of HE would be more likely in the Midwest. Although a rash is present in one‐third of patients with HME, it is seldom present in HGA unless coinfected with Borrelia burgdorferi, the causative agent for Lyme disease. Additionally, her history of freshwater exposure and the absence of a history of a tick bite also favor leptospirosis. As noted previously, conjunctival suffusion, a characteristic clinical feature of leptospirosis, has only been described in case reports of HE.11, 12
Serologic tests are often used to establish the diagnosis of leptospirosis and ehrlichiosis. Leptospires are fastidious organisms that are difficult to isolate on inoculated growth media. The microscopic agglutination test for leptospirosis is considered the diagnostic gold standard due to its high specificity, but its use is limited by its technical complexity, lack of availability (other than in reference laboratories), and low sensitivity early in the disease (antibody levels detected by this method usually do not appear until 7 days after symptom onset).13 A variety of rapid serologic assays are also available. Although these tests have good overall sensitivity (ranging between 79% and 93%), they perform relatively poorly for acute‐phase sera (sensitivity of 38.5%52.7%).13 The high early false negative rate is believed to be a result of inadequate Leptospira antibody titers in the acute phase of the illness. Seroconversion or a 4‐fold rise between acute and convalescent‐phase antibody titers is the most definitive criterion for the diagnosis of leptospirosis. However, without paired sera samples, a single high microscopic agglutination test titer can be taken as diagnostic for leptospirosis depending on the degree of regional endemicity.14
Similarly, currently available serologic assays for ehrlichiosis produce negative results in most patients in the first week of illness, and it is important to obtain a convalescent phase serum specimen for confirmatory diagnosis of HME and HGA. Seroconversion or a 4‐fold increase in titer between acute and convalescent phase sera is considered diagnostic. The sensitivity of finding morulae (intracytoplasmic vacuolar microcolonies of Ehrlichia) on a peripheral smear is unknown, and data suggest that this finding is more common in cases of HGA compared to HME.15
Although doxycycline is the drug of choice for the treatment of ehrlichiosis, Leptospira is susceptible to a wide variety of antibiotics because it exhibits a double membrane surface architecture with components common to both gram‐negative and gram‐positive bacteria.1 Recommended treatment regimens for severe leptospirosis include the use of high‐dose intravenous penicillin or a third‐generation cephalosporin. Less severe cases can be treated with oral amoxicillin or doxycycline.16 The fact that this patient's clinical improvement appeared to lag after initiation of ceftriaxone does not necessarily indicate a lack of efficacy but perhaps a Jarisch‐Herxheimer reaction in response to appropriate antibiotic therapy.
Teaching Points
-
Establishing a diagnosis of leptospirosis is challenging and requires a high index of suspicion. Clinicians should be aware of the limitations of the diagnostic accuracy of the serologic assays for leptospirosis because they are frequently negative in the first week after symptom onset.
-
The classic finding of conjunctival suffusion is helpful in differentiating leptospirosis from human ehrlichiosis.
-
This case also highlights the importance of the clinical practice of making a list of suspected diagnoses, remaining open to these possibilities, and checking serologic tests again in convalescence to confirm the diagnosis.
The approach to clinical conundrums by an expert clinician is revealed through the presentation of an actual patient's case in an approach typical of a morning report. Similarly to patient care, sequential pieces of information are provided to the clinician, who is unfamiliar with the case. The focus is on the thought processes of both the clinical team caring for the patient and the discussant.
Acknowledgements
The authors thank Dr. Brian Harte for his valuable guidance in the preparation of this manuscript.
- ,,.Leptospirosis: an emerging global public health problem.J Biosci.2008;33:557–569.
- Centers for Disease Control and Prevention. Leptospirosis.2005. http://www.cdc.gov/ncidod/dbmd/diseaseinfo/leptospirosis_t.htm. Accessed November 15,year="2010"2010.
- Morbidity and Mortality Weekly Report.From the Centers for Disease Control and Prevention. Update: leptospirosis and unexplained acute febrile illness among athletes participating in triathlons—Illinois and Wisconsin, 1998.JAMA.1998;280:1474–1475.
- ,.Optimal treatment of leptospirosis: queries and projections.Int J Antimicrob Agents.2006;28:491–496.
- ,.Leptospirosis in the tropics and in travelers.Curr Infect Dis Rep.2006;8:51–58.
- ,,,,,.Clinico‐epidemiological study of hospitalized cases of severe leptospirosis.Indian J Med Res.1999;109:94–99.
- ,.Proposed mechanisms and preventative options of Jarisch‐Herxheimer reactions.J Clin Pharm Ther.2005;30:291–295.
- .Leptospirosis presenting with erythema nodosum.Arch Dis Child.1977;52:418–419.
- ,,.Emerging and re‐emerging tick‐transmitted rickettsial and ehrlichial infections.Med Clin N Am.2008;92:1345–1361.
- ,.Tick‐borne ehrlichiosis infection in human beings.J Vector Borne Dis.2008;45:273–280.
- ,.Ehrlichia in Tennessee.South Med J.1989;82:669.
- ,,,,,.Ehrlichial meningitis with cerebrospinal fluid morulae.Pediatr Infect Dis J.1999;18:552–555.
- ,,, et al.Evaluation of four commercially available rapid serologic tests for diagnosis of leptospirosis.J Clin Microbiol.2003;41:803–809.
- ,.Diagnosis of leptospirosis utilizing modified Faine's criteria.J Assoc Physicians India.2004;52:678–679.
- ,.Ehrlichiosis in children.J Pediatr.1997;131:184–192.
- ,World Health Organization, International Leptospirosis Society. Human leptospirosis: guidance for diagnosis, surveillance and control.Geneva, Switzerland:World Health Organization;2003.
In October, a 36‐year‐old woman with no significant past medical history presented to the Emergency Department (ED) with a 3‐day history of headache and fever. The headache was severe, throbbing, and frontal in location. She also complained of daily fevers measured up to 103F, generalized malaise, and fatigue. She did not report neck stiffness or photophobia. She felt better after receiving intravenous fluids and was discharged home with a diagnosis of a nonspecific viral illness. Two days later, she returned to the ED with worsening headache, fever, mild photophobia, and poor oral intake. She also complained of a dry cough that made her headache worse, as did bending over. She did not report confusion, neck stiffness, shortness of breath, sore throat, runny nose, abdominal symptoms, or rash.
This patient presents a second time to the ED with worsening headache and fever raising concerns about meningitis. At the time of her first ED visit, it can be assumed that she had a nontoxic appearance because she was discharged shortly thereafter. Thus, acute bacterial meningitis seems less likely, but occasionally patients with meningococcal meningitis may not appear significantly ill until later in the process. Nonetheless, acute meningitis, possibly viral, is the initial concern. The time of the year is an important variable because many viral infections are seasonal. Enteroviruses are the most common cause of viral meningitis in the United States, particularly in the summer and fall. In contrast, mumps, measles, and varicella zoster viruses occur more commonly in winter and spring. Herpetic meningoencephalitis is a life‐threatening condition with a guarded prognosis. Therefore, early recognition and treatment is necessary to decrease morbidity and mortality. Drugs such as nonsteroidal anti‐inflammatory agents, trimethoprim‐sulfamethoxazole, amoxicillin, and rarely vaccines can also cause aseptic meningitis. Infections from fungi, spirochetes, mycobacteria, and rarely parasites also cause meningitis, but would be of greater concern in a patient with risk factors such as recent travel or an immunocompromised state.
Increased headache with bending and cough might indicate elevated intracranial pressure. However, this is a nonspecific complaint, and headache is often worse with the Valsalva maneuver. Because she reports a cough, a chest x‐ray would be useful. In addition to routine initial tests, cerebrospinal fluid (CSF) analysis and human immunodeficiency virus (HIV) testing is recommended.
Her past medical history was notable for depression. Her medications included bupropion, multivitamins, and fish oil. She was also taking milk thistle pills daily to protect her liver because she had been drinking alcohol heavily for the past 2 weeks since her husband left her. She smoked 1 pack of cigarettes daily. She had not traveled recently. She reported no recent animal or wildlife exposure but did recall falling into a midwestern river while canoeing 2 weeks prior to presentation. She worked as a hairstylist and described no sick contacts or risk factors for HIV disease.
An important new historical element is that the patient fell into a river. If she swallowed a significant amount of water during her fall overboard, meningitis from waterborne infections such as Aeromonas, Acanthamoeba, and Naegleria need to be considered. Fortunately, these are rare in the Midwest. Her canoeing history may suggest exposure to wooded areas. Certainly, tickborne infections such as ehrlichiosis, babesiosis, Lyme disease, and Rocky Mountain spotted fever can also cause meningitis. Histoplasmosis and blastomycosis are also endemic to the midwestern United States and can disseminate and cause central nervous system disease.
At this time, viral and bacterial infections are highest on the differential diagnosis. However, the microbiology laboratory needs to be alerted to the possibility of fungal or parasitic organisms depending on the initial CSF analysis results.
The patient was a Caucasian woman who appeared comfortable. Her blood pressure was 130/62 mm Hg, heart rate was 83 beats per minute, respiratory rate was 18 per minute, temperature was 100.8F, and oxygen saturation was 98% on room air. She was fully alert and oriented. Her pupils were bilaterally equal, reactive to light and accommodation with intact extraocular movement and no nystagmus. There was conjunctival injection bilaterally without noticeable pallor or icterus. Fundoscopic examination, which the patient tolerated without difficulty, was normal. Inspection of the oral cavity showed mild tonsillar enlargement. The neck was supple with no stiffness. No cervical, axillary, or inguinal lymph nodes were palpable. Faint bilateral basilar crackles were audible over the posterior chest. There was very mild right upper quadrant abdominal tenderness without guarding. The liver and spleen were normal size and bowel sounds were present. No rash, peripheral edema, or spinal tenderness was noted. A complete neurological examination was normal.
Her general appearance and vital signs seem reassuring. Conjunctival injection and mild tonsillar enlargement are nonspecific findings and may occur in systemic inflammatory states especially viral infections. Atelectasis may account for faint bilateral basilar crackles especially if associated with post‐tussive change. Her alcohol use puts her at risk of aspiration. A right lower lobe process (pneumonia) can sometimes present with right upper quadrant tenderness. However, this tenderness may also represent muscle soreness from repeated coughing, liver, or gallbladder disease. The same infectious process affecting the central nervous system and possibly her lungs, may also be affecting the liver.
A complete blood count revealed a white blood cell count of 3000/mm3 (79% neutrophils, 15% lymphocytes, 5% monocytes), hemoglobin of 11.7 g/dL, and platelets of 110,000/mm3. The serum sodium was 133 mmol/L, potassium was 3.7 mmol/L, bicarbonate was 22 mmol/L, and blood urea nitrogen was 20 mg/dL. The serum creatinine was 1.5 compared to 1.0 mg/dL on testing 2 days prior. A liver function panel showed protein of 5.1 g/dL, albumin of 3 g/dL, aspartate aminotransferase (AST) of 576 IU/L, alanine aminotransferase (ALT) of 584 IU/L, alkaline phosphatase of 282 IU/L, and total bilirubin of 1 mg/dL. The coagulation profile, creatinine phosphokinase, acetaminophen level, urine pregnancy test, urine drug screen, and urinalysis (including urine microscopy) were normal.
The CSF opening pressure was 13 cm H2O. CSF analysis showed 4 mononuclear leukocytes per high‐power field, CSF protein was 27 mg/dL, and glucose was 76 mg/dL. No organisms were noted on gram stain. A chest x‐ray showed focal airspace opacity in the left lower lobe (Figure 1) and the patient was hospitalized for further management.

The normal CSF analysis makes acute meningitis much less likely. It is interesting to note that the aminotransferase levels are nearly equal. Usually, in viral and many other causes of hepatitis, the ALT is higher than the AST, whereas the contrary is true in alcoholic hepatitis. Because the patient has been consuming significant amounts of alcohol recently, these levels may become equal in the setting of another primary liver process. The elevation in liver enzymes also raises the possibility of autoimmune hepatitis secondary to a systemic vasculitis such as systemic lupus erythematosus. Nonetheless, the focus should be on infectious causes of hepatitis such as hepatitis C, adenovirus, parvovirus, Epstein‐Barr virus (EBV), cytomegalovirus, and herpes simplex virus that can cause pneumonia either as a primary or secondary infection. Acute HIV infection can also present in this fashion, and anti‐HIV antibody testing may be negative early in the disease. In the setting of a normal urinalysis and bland urine sediment, prerenal azotemia is the most likely cause of her acute renal injury and can be confirmed by testing the urinary sodium and creatinine. A peripheral smear should be reviewed to evaluate the pancytopenia.
Severe headache, fever, conjunctival injection, pancytopenia, acute kidney injury, hepatitis, and pneumonia may occur in leptospirosis, particularly in a patient with recent freshwater exposure. Alternatively, ehrlichiosis can also account for fever, headache, pancytopenia, renal failure, hepatitis, and pneumonia, but conjunctival suffusion is not often present. At this time, treatment for community‐acquired pneumonia that includes coverage for leptospirosis should be started.
The patient was hydrated with intravenous fluids and treated with intravenous ceftriaxone and azithromycin for community‐acquired pneumonia. An abdominal ultrasound was normal. The serologic assays for acute hepatitis A, B, and C infection were negative. The following morning, she reported worsening headache, increased cough now productive of whitish‐yellow sputum, and diffuse body aches. She appeared more lethargic and toxic. Her blood pressure was 100/83 mm Hg, heart rate was 84 beats per minute, respiratory rate was 24 per minute, and temperature was 101.3F. She had increased crackles on chest auscultation bilaterally and required supplemental oxygen at 4 L/minute by nasal cannula. Examination of both legs now revealed multiple scattered, faintly erythematous, 2‐cm‐sized patches overlying tender subtle subcutaneous nodules. Additionally, a mildly pruritic, V‐shaped area of blanchable erythema was also seen on her chest. The white blood cell count was 2500/mm3 (77% neutrophils, 15% lymphocytes), serum creatinine was 1.8 mg/dL, AST was 351 IU/L, and ALT was 485 IU/L. Blood cultures showed no growth and a peripheral smear examination was unrevealing. A noncontrast chest computed tomographic scan showed findings consistent with multifocal pneumonia (Figure 2).

It would be prudent at this time to expand her antimicrobial coverage (such as with vancomycin and piperacillin‐tazobactam) for activity against methicillin‐resistant Staphylococcus aureus and Pseudomonas because of her clinical worsening. Although ceftriaxone or piperacillin would cover leptospirosis, given the possibility of ehrlichiosis, the addition of doxycycline should be strongly considered.
The description of the rash on her legs seems consistent with erythema nodosum, which is associated with a number of infections (streptococcal, fungal, syphilis, EBV, cat‐scratch disease, tuberculosis), inflammatory conditions (inflammatory bowel disease, autoimmune disease, malignancy), and pregnancy. The blanchable rash on the chest is also a cause of concern for a possible drug reaction (ceftriaxone). A Jarisch‐Herxheimer reaction is possible given her acute worsening of symptoms with initiation of antibiotic therapy.
An antineutrophil cytoplasmic antibodyassociated vasculitis or another autoimmune condition such as systemic lupus erythematosus can account for erythema nodosum, rash, pancytopenia, and hepatitis. This diagnosis might also fit if she had a vasculitic pulmonary hemorrhage that caused her lung infiltrates and worsening hypoxia. A complete antinuclear antibody panel, antineutrophil cytoplasmic antibody, and antismooth muscle antibody testing is recommended. A skin and bronchoscopic biopsy should be considered.
Her dose of ceftriaxone was increased for possible severe pneumococcal pneumonia. The dermatology consultant felt that her leg lesions were consistent with erythema nodosum and the chest rash consistent with cutaneous photodamage. Bronchoscopic examination was normal and a bronchoalveolar lavage sample showed 2905 red blood cells/mm3 and 605 white blood cells/mm3 (70% neutrophils, 7% lymphocytes, 16% histiocytes), normal cytology, and negative cultures. There was no significant clinical improvement by the fourth hospital day and oral doxycycline was started. The next day, her skin lesions had resolved and she felt better. The serologic tests for Legionella, Mycoplasma, cytomegalovirus, EBV, Toxoplasma, Chlamydophila, Ehrlichia, Leptospira, Q‐fever, parvovirus, and adenovirus were negative. A fungal serology panel, HIV polymerase chain reaction, cryoglobulin level, and several rheumatologic tests (antinuclear antibody, extractable nuclear antigen panel, rheumatoid factor, antineutrophil cytoplasmic antibody, antiproteinase 3, and antiglomerular basement membrane antibodies) were normal. Blood cultures continued to show no growth.
The apparent response to doxycycline suggests she might have ehrlichiosis. A buffy coat review for morulae should be done. It is also possible that she may have improved on her initial therapy alone before starting doxycycline and her clinical worsening (including the chest rash) was due to a Jarisch‐Herxheimer reaction. Serologic tests for leptospirosis and ehrlichiosis should be repeated in 12 weeks because such infections may not cause detectable antibody levels early in the illness.
Ceftriaxone and doxycycline were continued and she showed rapid and significant clinical improvement. She was discharged 4 days later with instructions to complete a 10‐day course of antibiotics. At her 3‐month follow‐up, she was doing well and a repeat Leptospira antibody test by the Indirect Hemagglutination Assay (MRL Diagnostics, Cypress, California; normal titer <1:50) was positive at a titer of 1:100, which is highly suggestive of leptospirosis.
Commentary
Leptospirosis is a zoonotic infection caused by spirochetes of the genus Leptospira. The infection is usually transmitted indirectly to humans through contact with water, food, or soil contaminated with the urine of infected mammals.1 Risk factors for infection include participation in recreational activities (such as freshwater swimming, canoeing, and camping), occupational exposure, and exposure to infected pets or domesticated livestock. Approximately 100200 cases are identified annually in the United States, and approximately half occur in the state of Hawaii.2 Outbreaks of leptospirosis have been reported previously in the Midwest.3 These organisms inoculate humans through contact with mucous membranes or broken skin, or enter by swallowing infected food or water. A large number of these infections remain subclinical or result in a very mild illness with spontaneous clearance by the host's immune mechanism. Following an incubation period of 230 days, infected individuals may develop clinically significant disease (Table 1). Clinical presentations may overlap as the disease progresses. Although much remains to be learned about the exact pathogenic mechanism, disruption of the cell membranes of small vessel endothelia (a toxin‐like effect), and cytokine‐mediated tissue injury are believed to cause organ hemorrhage and ischemia.4
|
| 1. Mild influenza‐like self‐remitting disease (90% of cases) |
| Undifferentiated fever (usually 100F105F), severe headache, and myalgia (especially lower limbs). |
| 2. Moderately severe disease usually requiring hospitalization (5%9% of cases) |
| Marked prostration, anorexia, nausea, and vomiting, conjunctival suffusion, transient rash, frequently abdominal pain, constipation or diarrhea, and occasionally epistaxis. |
| 3. Severe disease involving multiple organ systems (1%5% of cases) |
| Hepatorenal Syndrome (Weil's syndrome) |
| Constellation of jaundice, hemorrhagic diathesis, and acute renal failure. Hepatic failure is rarely fatal. Renal involvement is usually more severe and the common cause of death. Cardiac (myocarditis with arrhythmias) and pulmonary complications are frequent. Confusion and restlessness may occur. |
| Hemorrhagic pneumonitis |
| Usually presents as a dry cough initially but becomes blood‐streaked after 23 days. Often characterized by a rapid progression to involve extensive areas of lungs, massive intra‐alveolar hemorrhage, acute respiratory failure, and death. |
| Central nervous system involvement |
| Meningismus, meningitis, or meningoencephalitis. |
The clinical diagnosis of leptospirosis is difficult because of its protean manifestations. Although nonspecific, 2 clinical features may provide a clue to the clinical diagnosis. First, the presence of conjunctival suffusion occurs in the early stage of the disease and is often associated with subconjunctival hemorrhage. Second, severe myalgia, commonly involving the lower limbs, is also characteristically present.1, 5 In 1 series of 58 patients with acute leptospirosis, conjunctival suffusion was observed in 50% of cases, and subconjunctival hemorrhage in 29%. Body ache and muscle tenderness was described in almost all cases.6
As seen in this case, the presence of a rash may pose a clinical challenge. A transient macular, maculopapular, purpuric, or urticarial rash may be seen in acute leptospirosis, but rashes may also be representative of a complication of treatment.1 First described in 1895 in patients with syphilis treated with mercury, the Jarisch‐Herxheimer reaction typically occurs within a few hours of antimicrobial treatment of spirochete infections and often presents with a rash, headache, fever, rigors, hypotension, sweating, and worsening symptoms of the underlying illness.7 Other skin findings such as the occurrence of erythema nodosum have been previously reported in cases of leptospirosis.8
Human ehrlichiosis (HE) is caused by tickborne, obligatory intracellular bacteria that infect leukocytes. There are 3 distinct clinical conditions: human monocytic ehrlichiosis (HME, caused by Ehrlichia chaffeensis), human granulocytic anaplasmosis (HGA, caused by Anaplasma phagocytophilum), and human ewingii ehrlichiosis (HEE, caused by E. ewingii). Although most cases of HME and HEE are seen in the southeastern and south‐central United States and California, the highest incidence of HGA is reported in the northeastern and upper Midwest regions.9 As with leptospirosis, the clinical range of HE spans from asymptomatic infection to life‐threatening illness. Following an incubation period of 12 weeks, symptomatic cases usually present with nonspecific complaints such as high fevers, chills, headache, nausea, arthralgia, myalgia, and malaise.10 The majority of cases will report a tick bite or an exposure to ticks. Laboratory tests often reveal leukopenia (white blood cell count < 4000/mm3), thrombocytopenia, hyponatremia, and elevated AST and ALT. Patients with severe disease may develop renal, respiratory, and hepatic failure. Thus, differentiating ehrlichiosis from leptospirosis is often challenging for the clinician.
However, there are a few clinical clues that help distinguish between these illnesses in this case. HGA as a cause of HE would be more likely in the Midwest. Although a rash is present in one‐third of patients with HME, it is seldom present in HGA unless coinfected with Borrelia burgdorferi, the causative agent for Lyme disease. Additionally, her history of freshwater exposure and the absence of a history of a tick bite also favor leptospirosis. As noted previously, conjunctival suffusion, a characteristic clinical feature of leptospirosis, has only been described in case reports of HE.11, 12
Serologic tests are often used to establish the diagnosis of leptospirosis and ehrlichiosis. Leptospires are fastidious organisms that are difficult to isolate on inoculated growth media. The microscopic agglutination test for leptospirosis is considered the diagnostic gold standard due to its high specificity, but its use is limited by its technical complexity, lack of availability (other than in reference laboratories), and low sensitivity early in the disease (antibody levels detected by this method usually do not appear until 7 days after symptom onset).13 A variety of rapid serologic assays are also available. Although these tests have good overall sensitivity (ranging between 79% and 93%), they perform relatively poorly for acute‐phase sera (sensitivity of 38.5%52.7%).13 The high early false negative rate is believed to be a result of inadequate Leptospira antibody titers in the acute phase of the illness. Seroconversion or a 4‐fold rise between acute and convalescent‐phase antibody titers is the most definitive criterion for the diagnosis of leptospirosis. However, without paired sera samples, a single high microscopic agglutination test titer can be taken as diagnostic for leptospirosis depending on the degree of regional endemicity.14
Similarly, currently available serologic assays for ehrlichiosis produce negative results in most patients in the first week of illness, and it is important to obtain a convalescent phase serum specimen for confirmatory diagnosis of HME and HGA. Seroconversion or a 4‐fold increase in titer between acute and convalescent phase sera is considered diagnostic. The sensitivity of finding morulae (intracytoplasmic vacuolar microcolonies of Ehrlichia) on a peripheral smear is unknown, and data suggest that this finding is more common in cases of HGA compared to HME.15
Although doxycycline is the drug of choice for the treatment of ehrlichiosis, Leptospira is susceptible to a wide variety of antibiotics because it exhibits a double membrane surface architecture with components common to both gram‐negative and gram‐positive bacteria.1 Recommended treatment regimens for severe leptospirosis include the use of high‐dose intravenous penicillin or a third‐generation cephalosporin. Less severe cases can be treated with oral amoxicillin or doxycycline.16 The fact that this patient's clinical improvement appeared to lag after initiation of ceftriaxone does not necessarily indicate a lack of efficacy but perhaps a Jarisch‐Herxheimer reaction in response to appropriate antibiotic therapy.
Teaching Points
-
Establishing a diagnosis of leptospirosis is challenging and requires a high index of suspicion. Clinicians should be aware of the limitations of the diagnostic accuracy of the serologic assays for leptospirosis because they are frequently negative in the first week after symptom onset.
-
The classic finding of conjunctival suffusion is helpful in differentiating leptospirosis from human ehrlichiosis.
-
This case also highlights the importance of the clinical practice of making a list of suspected diagnoses, remaining open to these possibilities, and checking serologic tests again in convalescence to confirm the diagnosis.
The approach to clinical conundrums by an expert clinician is revealed through the presentation of an actual patient's case in an approach typical of a morning report. Similarly to patient care, sequential pieces of information are provided to the clinician, who is unfamiliar with the case. The focus is on the thought processes of both the clinical team caring for the patient and the discussant.
Acknowledgements
The authors thank Dr. Brian Harte for his valuable guidance in the preparation of this manuscript.
In October, a 36‐year‐old woman with no significant past medical history presented to the Emergency Department (ED) with a 3‐day history of headache and fever. The headache was severe, throbbing, and frontal in location. She also complained of daily fevers measured up to 103F, generalized malaise, and fatigue. She did not report neck stiffness or photophobia. She felt better after receiving intravenous fluids and was discharged home with a diagnosis of a nonspecific viral illness. Two days later, she returned to the ED with worsening headache, fever, mild photophobia, and poor oral intake. She also complained of a dry cough that made her headache worse, as did bending over. She did not report confusion, neck stiffness, shortness of breath, sore throat, runny nose, abdominal symptoms, or rash.
This patient presents a second time to the ED with worsening headache and fever raising concerns about meningitis. At the time of her first ED visit, it can be assumed that she had a nontoxic appearance because she was discharged shortly thereafter. Thus, acute bacterial meningitis seems less likely, but occasionally patients with meningococcal meningitis may not appear significantly ill until later in the process. Nonetheless, acute meningitis, possibly viral, is the initial concern. The time of the year is an important variable because many viral infections are seasonal. Enteroviruses are the most common cause of viral meningitis in the United States, particularly in the summer and fall. In contrast, mumps, measles, and varicella zoster viruses occur more commonly in winter and spring. Herpetic meningoencephalitis is a life‐threatening condition with a guarded prognosis. Therefore, early recognition and treatment is necessary to decrease morbidity and mortality. Drugs such as nonsteroidal anti‐inflammatory agents, trimethoprim‐sulfamethoxazole, amoxicillin, and rarely vaccines can also cause aseptic meningitis. Infections from fungi, spirochetes, mycobacteria, and rarely parasites also cause meningitis, but would be of greater concern in a patient with risk factors such as recent travel or an immunocompromised state.
Increased headache with bending and cough might indicate elevated intracranial pressure. However, this is a nonspecific complaint, and headache is often worse with the Valsalva maneuver. Because she reports a cough, a chest x‐ray would be useful. In addition to routine initial tests, cerebrospinal fluid (CSF) analysis and human immunodeficiency virus (HIV) testing is recommended.
Her past medical history was notable for depression. Her medications included bupropion, multivitamins, and fish oil. She was also taking milk thistle pills daily to protect her liver because she had been drinking alcohol heavily for the past 2 weeks since her husband left her. She smoked 1 pack of cigarettes daily. She had not traveled recently. She reported no recent animal or wildlife exposure but did recall falling into a midwestern river while canoeing 2 weeks prior to presentation. She worked as a hairstylist and described no sick contacts or risk factors for HIV disease.
An important new historical element is that the patient fell into a river. If she swallowed a significant amount of water during her fall overboard, meningitis from waterborne infections such as Aeromonas, Acanthamoeba, and Naegleria need to be considered. Fortunately, these are rare in the Midwest. Her canoeing history may suggest exposure to wooded areas. Certainly, tickborne infections such as ehrlichiosis, babesiosis, Lyme disease, and Rocky Mountain spotted fever can also cause meningitis. Histoplasmosis and blastomycosis are also endemic to the midwestern United States and can disseminate and cause central nervous system disease.
At this time, viral and bacterial infections are highest on the differential diagnosis. However, the microbiology laboratory needs to be alerted to the possibility of fungal or parasitic organisms depending on the initial CSF analysis results.
The patient was a Caucasian woman who appeared comfortable. Her blood pressure was 130/62 mm Hg, heart rate was 83 beats per minute, respiratory rate was 18 per minute, temperature was 100.8F, and oxygen saturation was 98% on room air. She was fully alert and oriented. Her pupils were bilaterally equal, reactive to light and accommodation with intact extraocular movement and no nystagmus. There was conjunctival injection bilaterally without noticeable pallor or icterus. Fundoscopic examination, which the patient tolerated without difficulty, was normal. Inspection of the oral cavity showed mild tonsillar enlargement. The neck was supple with no stiffness. No cervical, axillary, or inguinal lymph nodes were palpable. Faint bilateral basilar crackles were audible over the posterior chest. There was very mild right upper quadrant abdominal tenderness without guarding. The liver and spleen were normal size and bowel sounds were present. No rash, peripheral edema, or spinal tenderness was noted. A complete neurological examination was normal.
Her general appearance and vital signs seem reassuring. Conjunctival injection and mild tonsillar enlargement are nonspecific findings and may occur in systemic inflammatory states especially viral infections. Atelectasis may account for faint bilateral basilar crackles especially if associated with post‐tussive change. Her alcohol use puts her at risk of aspiration. A right lower lobe process (pneumonia) can sometimes present with right upper quadrant tenderness. However, this tenderness may also represent muscle soreness from repeated coughing, liver, or gallbladder disease. The same infectious process affecting the central nervous system and possibly her lungs, may also be affecting the liver.
A complete blood count revealed a white blood cell count of 3000/mm3 (79% neutrophils, 15% lymphocytes, 5% monocytes), hemoglobin of 11.7 g/dL, and platelets of 110,000/mm3. The serum sodium was 133 mmol/L, potassium was 3.7 mmol/L, bicarbonate was 22 mmol/L, and blood urea nitrogen was 20 mg/dL. The serum creatinine was 1.5 compared to 1.0 mg/dL on testing 2 days prior. A liver function panel showed protein of 5.1 g/dL, albumin of 3 g/dL, aspartate aminotransferase (AST) of 576 IU/L, alanine aminotransferase (ALT) of 584 IU/L, alkaline phosphatase of 282 IU/L, and total bilirubin of 1 mg/dL. The coagulation profile, creatinine phosphokinase, acetaminophen level, urine pregnancy test, urine drug screen, and urinalysis (including urine microscopy) were normal.
The CSF opening pressure was 13 cm H2O. CSF analysis showed 4 mononuclear leukocytes per high‐power field, CSF protein was 27 mg/dL, and glucose was 76 mg/dL. No organisms were noted on gram stain. A chest x‐ray showed focal airspace opacity in the left lower lobe (Figure 1) and the patient was hospitalized for further management.

The normal CSF analysis makes acute meningitis much less likely. It is interesting to note that the aminotransferase levels are nearly equal. Usually, in viral and many other causes of hepatitis, the ALT is higher than the AST, whereas the contrary is true in alcoholic hepatitis. Because the patient has been consuming significant amounts of alcohol recently, these levels may become equal in the setting of another primary liver process. The elevation in liver enzymes also raises the possibility of autoimmune hepatitis secondary to a systemic vasculitis such as systemic lupus erythematosus. Nonetheless, the focus should be on infectious causes of hepatitis such as hepatitis C, adenovirus, parvovirus, Epstein‐Barr virus (EBV), cytomegalovirus, and herpes simplex virus that can cause pneumonia either as a primary or secondary infection. Acute HIV infection can also present in this fashion, and anti‐HIV antibody testing may be negative early in the disease. In the setting of a normal urinalysis and bland urine sediment, prerenal azotemia is the most likely cause of her acute renal injury and can be confirmed by testing the urinary sodium and creatinine. A peripheral smear should be reviewed to evaluate the pancytopenia.
Severe headache, fever, conjunctival injection, pancytopenia, acute kidney injury, hepatitis, and pneumonia may occur in leptospirosis, particularly in a patient with recent freshwater exposure. Alternatively, ehrlichiosis can also account for fever, headache, pancytopenia, renal failure, hepatitis, and pneumonia, but conjunctival suffusion is not often present. At this time, treatment for community‐acquired pneumonia that includes coverage for leptospirosis should be started.
The patient was hydrated with intravenous fluids and treated with intravenous ceftriaxone and azithromycin for community‐acquired pneumonia. An abdominal ultrasound was normal. The serologic assays for acute hepatitis A, B, and C infection were negative. The following morning, she reported worsening headache, increased cough now productive of whitish‐yellow sputum, and diffuse body aches. She appeared more lethargic and toxic. Her blood pressure was 100/83 mm Hg, heart rate was 84 beats per minute, respiratory rate was 24 per minute, and temperature was 101.3F. She had increased crackles on chest auscultation bilaterally and required supplemental oxygen at 4 L/minute by nasal cannula. Examination of both legs now revealed multiple scattered, faintly erythematous, 2‐cm‐sized patches overlying tender subtle subcutaneous nodules. Additionally, a mildly pruritic, V‐shaped area of blanchable erythema was also seen on her chest. The white blood cell count was 2500/mm3 (77% neutrophils, 15% lymphocytes), serum creatinine was 1.8 mg/dL, AST was 351 IU/L, and ALT was 485 IU/L. Blood cultures showed no growth and a peripheral smear examination was unrevealing. A noncontrast chest computed tomographic scan showed findings consistent with multifocal pneumonia (Figure 2).

It would be prudent at this time to expand her antimicrobial coverage (such as with vancomycin and piperacillin‐tazobactam) for activity against methicillin‐resistant Staphylococcus aureus and Pseudomonas because of her clinical worsening. Although ceftriaxone or piperacillin would cover leptospirosis, given the possibility of ehrlichiosis, the addition of doxycycline should be strongly considered.
The description of the rash on her legs seems consistent with erythema nodosum, which is associated with a number of infections (streptococcal, fungal, syphilis, EBV, cat‐scratch disease, tuberculosis), inflammatory conditions (inflammatory bowel disease, autoimmune disease, malignancy), and pregnancy. The blanchable rash on the chest is also a cause of concern for a possible drug reaction (ceftriaxone). A Jarisch‐Herxheimer reaction is possible given her acute worsening of symptoms with initiation of antibiotic therapy.
An antineutrophil cytoplasmic antibodyassociated vasculitis or another autoimmune condition such as systemic lupus erythematosus can account for erythema nodosum, rash, pancytopenia, and hepatitis. This diagnosis might also fit if she had a vasculitic pulmonary hemorrhage that caused her lung infiltrates and worsening hypoxia. A complete antinuclear antibody panel, antineutrophil cytoplasmic antibody, and antismooth muscle antibody testing is recommended. A skin and bronchoscopic biopsy should be considered.
Her dose of ceftriaxone was increased for possible severe pneumococcal pneumonia. The dermatology consultant felt that her leg lesions were consistent with erythema nodosum and the chest rash consistent with cutaneous photodamage. Bronchoscopic examination was normal and a bronchoalveolar lavage sample showed 2905 red blood cells/mm3 and 605 white blood cells/mm3 (70% neutrophils, 7% lymphocytes, 16% histiocytes), normal cytology, and negative cultures. There was no significant clinical improvement by the fourth hospital day and oral doxycycline was started. The next day, her skin lesions had resolved and she felt better. The serologic tests for Legionella, Mycoplasma, cytomegalovirus, EBV, Toxoplasma, Chlamydophila, Ehrlichia, Leptospira, Q‐fever, parvovirus, and adenovirus were negative. A fungal serology panel, HIV polymerase chain reaction, cryoglobulin level, and several rheumatologic tests (antinuclear antibody, extractable nuclear antigen panel, rheumatoid factor, antineutrophil cytoplasmic antibody, antiproteinase 3, and antiglomerular basement membrane antibodies) were normal. Blood cultures continued to show no growth.
The apparent response to doxycycline suggests she might have ehrlichiosis. A buffy coat review for morulae should be done. It is also possible that she may have improved on her initial therapy alone before starting doxycycline and her clinical worsening (including the chest rash) was due to a Jarisch‐Herxheimer reaction. Serologic tests for leptospirosis and ehrlichiosis should be repeated in 12 weeks because such infections may not cause detectable antibody levels early in the illness.
Ceftriaxone and doxycycline were continued and she showed rapid and significant clinical improvement. She was discharged 4 days later with instructions to complete a 10‐day course of antibiotics. At her 3‐month follow‐up, she was doing well and a repeat Leptospira antibody test by the Indirect Hemagglutination Assay (MRL Diagnostics, Cypress, California; normal titer <1:50) was positive at a titer of 1:100, which is highly suggestive of leptospirosis.
Commentary
Leptospirosis is a zoonotic infection caused by spirochetes of the genus Leptospira. The infection is usually transmitted indirectly to humans through contact with water, food, or soil contaminated with the urine of infected mammals.1 Risk factors for infection include participation in recreational activities (such as freshwater swimming, canoeing, and camping), occupational exposure, and exposure to infected pets or domesticated livestock. Approximately 100200 cases are identified annually in the United States, and approximately half occur in the state of Hawaii.2 Outbreaks of leptospirosis have been reported previously in the Midwest.3 These organisms inoculate humans through contact with mucous membranes or broken skin, or enter by swallowing infected food or water. A large number of these infections remain subclinical or result in a very mild illness with spontaneous clearance by the host's immune mechanism. Following an incubation period of 230 days, infected individuals may develop clinically significant disease (Table 1). Clinical presentations may overlap as the disease progresses. Although much remains to be learned about the exact pathogenic mechanism, disruption of the cell membranes of small vessel endothelia (a toxin‐like effect), and cytokine‐mediated tissue injury are believed to cause organ hemorrhage and ischemia.4
|
| 1. Mild influenza‐like self‐remitting disease (90% of cases) |
| Undifferentiated fever (usually 100F105F), severe headache, and myalgia (especially lower limbs). |
| 2. Moderately severe disease usually requiring hospitalization (5%9% of cases) |
| Marked prostration, anorexia, nausea, and vomiting, conjunctival suffusion, transient rash, frequently abdominal pain, constipation or diarrhea, and occasionally epistaxis. |
| 3. Severe disease involving multiple organ systems (1%5% of cases) |
| Hepatorenal Syndrome (Weil's syndrome) |
| Constellation of jaundice, hemorrhagic diathesis, and acute renal failure. Hepatic failure is rarely fatal. Renal involvement is usually more severe and the common cause of death. Cardiac (myocarditis with arrhythmias) and pulmonary complications are frequent. Confusion and restlessness may occur. |
| Hemorrhagic pneumonitis |
| Usually presents as a dry cough initially but becomes blood‐streaked after 23 days. Often characterized by a rapid progression to involve extensive areas of lungs, massive intra‐alveolar hemorrhage, acute respiratory failure, and death. |
| Central nervous system involvement |
| Meningismus, meningitis, or meningoencephalitis. |
The clinical diagnosis of leptospirosis is difficult because of its protean manifestations. Although nonspecific, 2 clinical features may provide a clue to the clinical diagnosis. First, the presence of conjunctival suffusion occurs in the early stage of the disease and is often associated with subconjunctival hemorrhage. Second, severe myalgia, commonly involving the lower limbs, is also characteristically present.1, 5 In 1 series of 58 patients with acute leptospirosis, conjunctival suffusion was observed in 50% of cases, and subconjunctival hemorrhage in 29%. Body ache and muscle tenderness was described in almost all cases.6
As seen in this case, the presence of a rash may pose a clinical challenge. A transient macular, maculopapular, purpuric, or urticarial rash may be seen in acute leptospirosis, but rashes may also be representative of a complication of treatment.1 First described in 1895 in patients with syphilis treated with mercury, the Jarisch‐Herxheimer reaction typically occurs within a few hours of antimicrobial treatment of spirochete infections and often presents with a rash, headache, fever, rigors, hypotension, sweating, and worsening symptoms of the underlying illness.7 Other skin findings such as the occurrence of erythema nodosum have been previously reported in cases of leptospirosis.8
Human ehrlichiosis (HE) is caused by tickborne, obligatory intracellular bacteria that infect leukocytes. There are 3 distinct clinical conditions: human monocytic ehrlichiosis (HME, caused by Ehrlichia chaffeensis), human granulocytic anaplasmosis (HGA, caused by Anaplasma phagocytophilum), and human ewingii ehrlichiosis (HEE, caused by E. ewingii). Although most cases of HME and HEE are seen in the southeastern and south‐central United States and California, the highest incidence of HGA is reported in the northeastern and upper Midwest regions.9 As with leptospirosis, the clinical range of HE spans from asymptomatic infection to life‐threatening illness. Following an incubation period of 12 weeks, symptomatic cases usually present with nonspecific complaints such as high fevers, chills, headache, nausea, arthralgia, myalgia, and malaise.10 The majority of cases will report a tick bite or an exposure to ticks. Laboratory tests often reveal leukopenia (white blood cell count < 4000/mm3), thrombocytopenia, hyponatremia, and elevated AST and ALT. Patients with severe disease may develop renal, respiratory, and hepatic failure. Thus, differentiating ehrlichiosis from leptospirosis is often challenging for the clinician.
However, there are a few clinical clues that help distinguish between these illnesses in this case. HGA as a cause of HE would be more likely in the Midwest. Although a rash is present in one‐third of patients with HME, it is seldom present in HGA unless coinfected with Borrelia burgdorferi, the causative agent for Lyme disease. Additionally, her history of freshwater exposure and the absence of a history of a tick bite also favor leptospirosis. As noted previously, conjunctival suffusion, a characteristic clinical feature of leptospirosis, has only been described in case reports of HE.11, 12
Serologic tests are often used to establish the diagnosis of leptospirosis and ehrlichiosis. Leptospires are fastidious organisms that are difficult to isolate on inoculated growth media. The microscopic agglutination test for leptospirosis is considered the diagnostic gold standard due to its high specificity, but its use is limited by its technical complexity, lack of availability (other than in reference laboratories), and low sensitivity early in the disease (antibody levels detected by this method usually do not appear until 7 days after symptom onset).13 A variety of rapid serologic assays are also available. Although these tests have good overall sensitivity (ranging between 79% and 93%), they perform relatively poorly for acute‐phase sera (sensitivity of 38.5%52.7%).13 The high early false negative rate is believed to be a result of inadequate Leptospira antibody titers in the acute phase of the illness. Seroconversion or a 4‐fold rise between acute and convalescent‐phase antibody titers is the most definitive criterion for the diagnosis of leptospirosis. However, without paired sera samples, a single high microscopic agglutination test titer can be taken as diagnostic for leptospirosis depending on the degree of regional endemicity.14
Similarly, currently available serologic assays for ehrlichiosis produce negative results in most patients in the first week of illness, and it is important to obtain a convalescent phase serum specimen for confirmatory diagnosis of HME and HGA. Seroconversion or a 4‐fold increase in titer between acute and convalescent phase sera is considered diagnostic. The sensitivity of finding morulae (intracytoplasmic vacuolar microcolonies of Ehrlichia) on a peripheral smear is unknown, and data suggest that this finding is more common in cases of HGA compared to HME.15
Although doxycycline is the drug of choice for the treatment of ehrlichiosis, Leptospira is susceptible to a wide variety of antibiotics because it exhibits a double membrane surface architecture with components common to both gram‐negative and gram‐positive bacteria.1 Recommended treatment regimens for severe leptospirosis include the use of high‐dose intravenous penicillin or a third‐generation cephalosporin. Less severe cases can be treated with oral amoxicillin or doxycycline.16 The fact that this patient's clinical improvement appeared to lag after initiation of ceftriaxone does not necessarily indicate a lack of efficacy but perhaps a Jarisch‐Herxheimer reaction in response to appropriate antibiotic therapy.
Teaching Points
-
Establishing a diagnosis of leptospirosis is challenging and requires a high index of suspicion. Clinicians should be aware of the limitations of the diagnostic accuracy of the serologic assays for leptospirosis because they are frequently negative in the first week after symptom onset.
-
The classic finding of conjunctival suffusion is helpful in differentiating leptospirosis from human ehrlichiosis.
-
This case also highlights the importance of the clinical practice of making a list of suspected diagnoses, remaining open to these possibilities, and checking serologic tests again in convalescence to confirm the diagnosis.
The approach to clinical conundrums by an expert clinician is revealed through the presentation of an actual patient's case in an approach typical of a morning report. Similarly to patient care, sequential pieces of information are provided to the clinician, who is unfamiliar with the case. The focus is on the thought processes of both the clinical team caring for the patient and the discussant.
Acknowledgements
The authors thank Dr. Brian Harte for his valuable guidance in the preparation of this manuscript.
- ,,.Leptospirosis: an emerging global public health problem.J Biosci.2008;33:557–569.
- Centers for Disease Control and Prevention. Leptospirosis.2005. http://www.cdc.gov/ncidod/dbmd/diseaseinfo/leptospirosis_t.htm. Accessed November 15,year="2010"2010.
- Morbidity and Mortality Weekly Report.From the Centers for Disease Control and Prevention. Update: leptospirosis and unexplained acute febrile illness among athletes participating in triathlons—Illinois and Wisconsin, 1998.JAMA.1998;280:1474–1475.
- ,.Optimal treatment of leptospirosis: queries and projections.Int J Antimicrob Agents.2006;28:491–496.
- ,.Leptospirosis in the tropics and in travelers.Curr Infect Dis Rep.2006;8:51–58.
- ,,,,,.Clinico‐epidemiological study of hospitalized cases of severe leptospirosis.Indian J Med Res.1999;109:94–99.
- ,.Proposed mechanisms and preventative options of Jarisch‐Herxheimer reactions.J Clin Pharm Ther.2005;30:291–295.
- .Leptospirosis presenting with erythema nodosum.Arch Dis Child.1977;52:418–419.
- ,,.Emerging and re‐emerging tick‐transmitted rickettsial and ehrlichial infections.Med Clin N Am.2008;92:1345–1361.
- ,.Tick‐borne ehrlichiosis infection in human beings.J Vector Borne Dis.2008;45:273–280.
- ,.Ehrlichia in Tennessee.South Med J.1989;82:669.
- ,,,,,.Ehrlichial meningitis with cerebrospinal fluid morulae.Pediatr Infect Dis J.1999;18:552–555.
- ,,, et al.Evaluation of four commercially available rapid serologic tests for diagnosis of leptospirosis.J Clin Microbiol.2003;41:803–809.
- ,.Diagnosis of leptospirosis utilizing modified Faine's criteria.J Assoc Physicians India.2004;52:678–679.
- ,.Ehrlichiosis in children.J Pediatr.1997;131:184–192.
- ,World Health Organization, International Leptospirosis Society. Human leptospirosis: guidance for diagnosis, surveillance and control.Geneva, Switzerland:World Health Organization;2003.
- ,,.Leptospirosis: an emerging global public health problem.J Biosci.2008;33:557–569.
- Centers for Disease Control and Prevention. Leptospirosis.2005. http://www.cdc.gov/ncidod/dbmd/diseaseinfo/leptospirosis_t.htm. Accessed November 15,year="2010"2010.
- Morbidity and Mortality Weekly Report.From the Centers for Disease Control and Prevention. Update: leptospirosis and unexplained acute febrile illness among athletes participating in triathlons—Illinois and Wisconsin, 1998.JAMA.1998;280:1474–1475.
- ,.Optimal treatment of leptospirosis: queries and projections.Int J Antimicrob Agents.2006;28:491–496.
- ,.Leptospirosis in the tropics and in travelers.Curr Infect Dis Rep.2006;8:51–58.
- ,,,,,.Clinico‐epidemiological study of hospitalized cases of severe leptospirosis.Indian J Med Res.1999;109:94–99.
- ,.Proposed mechanisms and preventative options of Jarisch‐Herxheimer reactions.J Clin Pharm Ther.2005;30:291–295.
- .Leptospirosis presenting with erythema nodosum.Arch Dis Child.1977;52:418–419.
- ,,.Emerging and re‐emerging tick‐transmitted rickettsial and ehrlichial infections.Med Clin N Am.2008;92:1345–1361.
- ,.Tick‐borne ehrlichiosis infection in human beings.J Vector Borne Dis.2008;45:273–280.
- ,.Ehrlichia in Tennessee.South Med J.1989;82:669.
- ,,,,,.Ehrlichial meningitis with cerebrospinal fluid morulae.Pediatr Infect Dis J.1999;18:552–555.
- ,,, et al.Evaluation of four commercially available rapid serologic tests for diagnosis of leptospirosis.J Clin Microbiol.2003;41:803–809.
- ,.Diagnosis of leptospirosis utilizing modified Faine's criteria.J Assoc Physicians India.2004;52:678–679.
- ,.Ehrlichiosis in children.J Pediatr.1997;131:184–192.
- ,World Health Organization, International Leptospirosis Society. Human leptospirosis: guidance for diagnosis, surveillance and control.Geneva, Switzerland:World Health Organization;2003.
In the Literature
Literature at a Glance
A guide to this month’s abstracts
- Steroids reduce mortality only in patients with confirmed bacterial meningitis.
- Probiotics can be useful in the treatment of acute diarrhea in children.
- CT pulmonary angiography is not inferior to V/Q scanning for exclusion of PE.
- Hospitalist care results in shorter LOS compared with care by traditional general internists and family practice physicians.
- The early risk of stroke after TIA is approximately 15% to 20% at 90 days after the sentinel event.
- Different anti-thrombotic strategies produce no difference in outcomes of early acute coronary syndromes.
- The risk of fatal PE is highest in the first year after medication is stopped.
- Beers criteria medications are associated with fewer ED visits by elderly patients compared with warfarin, digoxin, and insulin.
Do Steroids Affect the Outcome in Patients with Meningitis?
Background: Pyogenic (bacterial) meningitis has high morbidity and mortality. Studies suggest some benefit of steroids in children but provide limited evidence for adult use.
Study design: Intention-to-treat, randomized control trial.
Setting: Single hospital in Vietnam.
Synopsis: Of 435 patients older than 14 with suspected meningitis all received lumbar puncture with randomization to IV dexamethasone or placebo for four days. Results showed 69% of patients had definite meningitis, 28.3% were probable, and 2.8% had an alternative diagnosis based on culture results.
The primary outcome was death after one month, which did not differ among groups (risk ratio [RR] 0.79, confidence interval [CI] 0.45-1.39).
Predefined subgroup analysis of patients with definitive meningitis showed a significant reduction in mortality at one month (RR 0.43, CI 0.2-0.94) and death/disability at six months (odds ratio [OR] 0.56, CI 0.32-0.98).
In patients with probable meningitis, those who received steroids demonstrated a trend toward harm (OR 2.65, CI 0.73-9.63).
Probable versus definite meningitis was determined retrospectively based on cultures. The most common isolate was Streptococcus suis.
Bottom line: This study provides some evidence for using steroids in adults with confirmed bacterial meningitis. Clinical application is limited by bacterial epidemiology and the difficulty of prospectively separating patients who would benefit from those who might be harmed.
Citation: Nguyen TH, Tran TH, Thwaites G, et. al. Dexamethasone in Vietnamese adolescents and adults with bacterial meningitis. N Engl J Med. 2007;357:2431-2439.
Which Probiotic Preparations Best Reduce the Duration of Acute Diarrhea in Children?
Background: Probiotics have been suggested as an adjunctive therapy to reduce the severity and duration of acute diarrhea in children. However, there are no clear data to suggest if specific probiotic agents are superior to others.
Study design: Prospective single-blind, randomized, controlled trial.
Setting: Outpatient primary care in Naples, Italy.
Synopsis: This study compared five commercially available probiotic preparations (mix of Lactobacillus delbrueckii var bulgaricus/Streptococcus thermophilus/L. acidophilus/ Bifido-bacterium bifidum; L. rhamnosus strain GG; Saccharomyces boulardii; Bacillus clausii; or Enterococcus faecium SF68) and a control group in the treatment of outpatient acute diarrhea in 571 children age 3 months to 36 months.
The primary outcomes were the duration of diarrhea and the number and consistency of stools. The groups receiving Lactobacillus GG and the mixture had a shorter total duration of diarrhea (78.5 and 70 hours, respectively), decreased total number of stools, and improved stool consistency when compared with the control (115.5 hours). The other therapies showed no improvement over the control group. These data report on products commercially available in Italy, which may differ greatly from products available locally.
Bottom line: Probiotic preparations for the treatment of acute diarrhea in children should be chosen based on effectiveness data.
Citation: Canani RB, Cirillo P, Terrin G, et al. Probiotics for treatment of acute diarrhoea in children: randomised clinical trial of five different preparations. BMJ 2007;335:340-345.
Is CTPA a Reliable Alternative to V/Q Scan for Diagnosing PE?
Background: Computed tomography pulmonary angiogram (CTPA) has replaced ventilation/perfusion (V/Q) scanning at many hospitals as the test of choice for ruling out pulmonary embolism (PE). But limited clinical data compare CTPA with V/Q scanning in those suspected of having venous thromboembolism (VTE).
Study design: Randomized, investigator blinded, controlled trial.
Setting: The emergency departments (ED), inpatient wards, and outpatient clinics of five academic centers.
Synopsis: In the study, 1,411 patients were enrolled from five medical centers. Of 694 patients randomized to CTPA, 133 (19.2%) were diagnosed with VTE in the initial evaluation period, while 101 of 712 patients (14.2%) receiving a V/Q scan were diagnosed with VTE.
Patients not initially diagnosed with VTE were monitored. At three-month follow-up, 0.4% of the CTPA group and 1.0% of the V/Q group had a diagnosed VTE.
The overall rate of VTE found in the initial diagnostic period was significantly greater in patients randomized to CTPA (19.2% vs. 14.2%; difference, 5.0%; 95% CI; 1.1% to 8.9% p=.01). This suggests CTPA has a higher false positive rate or detects clinically insignificant thrombi.
Bottom line: CTPA was not inferior to V/Q scanning for excluding clinically meaningful PE, but CTPA diagnosed about 30% more patients with VTE than did V/Q scanning.
Citation: Anderson DR, Kahn SR, Rodger MA, et al. Computed tomographic pulmonary angiography vs. ventilation-perfusion lung scanning in patients with suspected pulmonary embolism: a randomized controlled trial. JAMA. 2007;298(23):2743-2753.
Does the Hospitalist Model Improve Length of Stay, Quality, and Cost of Care?
Background: The hospitalist model, with increased physician availability and expertise but greater discontinuity of care, is becoming more prevalent in U.S. medicine. What little is known about how this model will affect patient care is derived from a number of small studies.
Study design: Retrospective cohort study.
Setting: 45 small to midsize, predominantly nonteaching hospitals throughout the U.S.
Synopsis: Using the Premier Healthcare Informatics database, this study examined information on 76,926 patients admitted for seven common diagnoses to one of three services: hospitalist, general internist, or family physician. Analysis showed that patients on a hospitalist service had a 0.4-day shorter length of stay (p<0.001) compared with those on a general internist or family physician service.
The cost to patients cared for by a hospitalist was lower than the cost of family physicians ($125 less, p=0.33) and internists ($268 less, p=0.02). There was no difference found in death rate or 14-day readmission rate among the three services.
Given the retrospective design of this study, no causal relationship can be deduced. This study is further limited by its lack of specific data on the physicians categorized into one of the three groups solely by administrative data. The authors had concerns that the biases inherent to the retrospective nature of their work accounted for the significant difference found between hospitalists and internists.
Bottom line: The hospitalist model is associated with modest improvements in length of stay as compared with traditional inpatient approaches.
Citation: Lindenauer PK, Rothberg MB, Pekow PS, et. al. Outcomes of care by hospitalists, general internists, and family physicians. N Engl J Med. 2007;357:2589-2600.
What Is the Stroke Risk Soon after TIA, and What Factors Drive the Variability of Previous Findings?
Background: Many studies have attempted to estimate the risk of stroke in the early period after a transient ischemic attack (TIA). These studies vary widely in their calculation of the estimated risk. Further, the clinical and methodological factors underlying this variability are unclear.
Study design: Systematic review and meta-analysis.
Setting: Community and hospital.
Synopsis: Searching the Cochrane review database, MEDLINE, EMBASE, CINAHL, and BIOSIS, 11 studies from 1973 to 2006 were included for meta-analysis, selected from 694 potential candidate studies identified on initial screening. The studies ranged in size from 62 to 2,285 patients.
The pooled estimate of risk for stroke following TIA was found to be 3.5%, 8%, and 9.2% at two, 30, and 90 days following TIA, respectively. However, there was significant heterogeneity for all periods considered (p<0.001).
Outcome ascertainment was identified as a major source of methodological heterogeneity. When risk of stroke at follow-up was determined by passive ascertainment (e.g., administrative documentation) the early risk of stroke was 3.1% two days after TIA, 6.4% at 30 days, and 8.7% at 90 days. But active ascertainment (e.g., direct, personal contact with study participants) determined stroke risk to be 9.9%, 13.4%, and 17.4% at two, 30, and 90 days after TIA, respectively.
Bottom line: Based on analysis of completed studies that included directly observed follow-up of study participants, the early risk of stroke after TIA is approximately 15% to 20% at 90 days following the sentinel event.
Citation: Wu CM, McLaughlin K, Lorenzetti DL, Hill MD, Manns BJ, Ghali WA. Early risk of stroke after transient ischemic attack. Arch Intern Med. 2007;167:2417-2422.
What Is the 1-year Ischemia and Mortality Rate for Three Anti-thrombotic Therapies for Early Invasive Management of ACS?
Background: Early interventional or surgical revascularization has improved morbidity and mortality in patients with acute coronary syndrome (ACS). The optimal anti-thrombotic regimen to reduce late ischemic and death rates has not been determined.
Study design: Prospective, open-label randomized control trial.
Setting: 450 academic and community-based institutions in 17 countries.
Synopsis: A total of 13,819 patients were enrolled between August 2003 and December 2005. They were assigned to heparin plus glycoprotein (GP) IIb/IIIa inhibitors (n=4,603), bivalirudin (Angiomax) plus IIb/IIIa inhibitors (n=4,604), or bivalirudin monotherapy (n=4,612).
For patients receiving GP IIb/IIIa inhibitors, a 2x2 factorial design assigned half the heparin and bivalirudin groups to routine upstream GP inhibitor administration (4,605 patients). The other half received selective GP IIb/IIIa inhibitors administration if PCI was indicated (4,602 patients).
At one year, there was no statistically significant difference in ischemia or mortality rate among the three therapy groups. No difference in ischemia rate was detected between the two GP IIb/IIIa inhibitor utilization strategies.
Since the hypotheses and the power for the one-year analysis in this trial were not prospectively determined, the results are considered to be exploratory and hypothesis generating.
Bottom line: At one year, there is no statistically significant difference in ischemia or mortality rate for the three antithrombotic regiments and the two glycoprotein utilization strategies.
Citation: Stone GW, Ware JH, Bertrand ME, et. al. Antithrombotic strategies in patients with acute coronary syndromes undergoing early invasive management. One-year results from the ACUITY trial. JAMA 2007;298:2497-2505.
What Is the PE Risk after Discontinuing Anticoagulation in Patients with Symptomatic VTE?
Background: The natural history of patients with symptomatic VTE who have completed anticoagulation is not well understood.
Study design: Inception cohort using pooled data from a prospective cohort study and one arm of an open-label randomized trial.
Setting: Academic medical centers in Canada, Sweden, and Italy.
Synopsis: Using pooled data from two previous studies, 2,052 patients with a first diagnosis of symptomatic VTE (lower-extremity deep-vein thrombosis [DVT], PE, or both) were evaluated for fatal PE after a standard course of therapy (mean of six months) with a vitamin K antagonist.
Patients were followed for up to 120 months. The investigators found an annual event risk of 0.19-0.49 per 100 person-years for fatal PE. Patients with prolonged immobility, active cancer, and thrombophilia were excluded, as were those with recurrent acute DVT.
Secondary analysis revealed an incidence of any fatal, definite or probable PE within the first year of discontinuing therapy of 0.35%-0.81%.
After the first year, the annual event risk ranged from 0.15-0.40 events per 100 person-years. Patients with advanced age, idiopathic VTE as well as those presenting with PE had higher rates of fatal PE.
Bottom line: There is a real though small (less than 1%) risk of fatal PE in the first year following discontinuation of anticoagulation for the first VTE episode. The optimal course of treatment for patients with idiopathic VTE is yet to be determined.
Citation: Douketis JD, Gu CS, Schulman S, et al. The risk for fatal pulmonary embolism after discontinuing anticoagulant therapy for venous thromboembolism. Ann Intern Med. 2007;147(11):766-774.
Do the Beers Criteria Predict ED Visits Associated with Adverse Drug Events?
Background: Adverse drug events are common in the elderly. The Beers criteria are a consensus-based list of 41 medications that are considered inappropriate for use in older adults and often lead to poor outcomes.
Study design: Retrospective medical record review and data analysis.
Setting: Three nationally representative, U.S. public health surveillance systems: the National Electronic Injury Surveillance System-Cooperative Adverse Drug Event Surveillance System (NEISS-CADES), 2004-2005; the National Ambulatory Medical Care Survey (NAMCS), 2004; and National Hospital Ambulatory Medical Care Survey (NHAMCS), 2004.
Synopsis: Using data collected from ED visits at 58 hospitals in the NEISS-CADES system, this study estimated that 177,504 visits for adverse drug events occur annually in the United States. Only 8.8% of such visits were attributable to the 41 medications included in the Beers criteria. Three drug classes (anticoagulant and antiplatelet agents, antidiabetic agents, and narrow therapeutic index agents) accounted for nearly half of all such ED visits. Warfarin (17.3%), insulin (13%), and digoxin (3.2%) were the most commonly implicated medications, collectively accounting for 33% of visits (CI, 27.8% to 38.7%).
This study suggests that because of the common use and high risk of adverse events associated with these three drugs, interventions targeting their use may prevent ED visits for adverse drug events in the elderly, compared with interventions aimed at reducing the use of medications identified in the Beers criteria.
This study only included adverse drug events identified in the ED and relied on the diagnosis and documentation of such events by the ED physician.
Bottom line: Beers criteria medications, although considered inappropriate for use in the elderly, were associated with significantly fewer ED visits for adverse events compared with warfarin, digoxin, and insulin.
Citation: Budnitz DS, Shehab N, Kegler SR, et. al. Medication use leading to emergency department visits for adverse drug events in older adults. Ann Intern Med. 2007;147:755-765. TH
Literature at a Glance
A guide to this month’s abstracts
- Steroids reduce mortality only in patients with confirmed bacterial meningitis.
- Probiotics can be useful in the treatment of acute diarrhea in children.
- CT pulmonary angiography is not inferior to V/Q scanning for exclusion of PE.
- Hospitalist care results in shorter LOS compared with care by traditional general internists and family practice physicians.
- The early risk of stroke after TIA is approximately 15% to 20% at 90 days after the sentinel event.
- Different anti-thrombotic strategies produce no difference in outcomes of early acute coronary syndromes.
- The risk of fatal PE is highest in the first year after medication is stopped.
- Beers criteria medications are associated with fewer ED visits by elderly patients compared with warfarin, digoxin, and insulin.
Do Steroids Affect the Outcome in Patients with Meningitis?
Background: Pyogenic (bacterial) meningitis has high morbidity and mortality. Studies suggest some benefit of steroids in children but provide limited evidence for adult use.
Study design: Intention-to-treat, randomized control trial.
Setting: Single hospital in Vietnam.
Synopsis: Of 435 patients older than 14 with suspected meningitis all received lumbar puncture with randomization to IV dexamethasone or placebo for four days. Results showed 69% of patients had definite meningitis, 28.3% were probable, and 2.8% had an alternative diagnosis based on culture results.
The primary outcome was death after one month, which did not differ among groups (risk ratio [RR] 0.79, confidence interval [CI] 0.45-1.39).
Predefined subgroup analysis of patients with definitive meningitis showed a significant reduction in mortality at one month (RR 0.43, CI 0.2-0.94) and death/disability at six months (odds ratio [OR] 0.56, CI 0.32-0.98).
In patients with probable meningitis, those who received steroids demonstrated a trend toward harm (OR 2.65, CI 0.73-9.63).
Probable versus definite meningitis was determined retrospectively based on cultures. The most common isolate was Streptococcus suis.
Bottom line: This study provides some evidence for using steroids in adults with confirmed bacterial meningitis. Clinical application is limited by bacterial epidemiology and the difficulty of prospectively separating patients who would benefit from those who might be harmed.
Citation: Nguyen TH, Tran TH, Thwaites G, et. al. Dexamethasone in Vietnamese adolescents and adults with bacterial meningitis. N Engl J Med. 2007;357:2431-2439.
Which Probiotic Preparations Best Reduce the Duration of Acute Diarrhea in Children?
Background: Probiotics have been suggested as an adjunctive therapy to reduce the severity and duration of acute diarrhea in children. However, there are no clear data to suggest if specific probiotic agents are superior to others.
Study design: Prospective single-blind, randomized, controlled trial.
Setting: Outpatient primary care in Naples, Italy.
Synopsis: This study compared five commercially available probiotic preparations (mix of Lactobacillus delbrueckii var bulgaricus/Streptococcus thermophilus/L. acidophilus/ Bifido-bacterium bifidum; L. rhamnosus strain GG; Saccharomyces boulardii; Bacillus clausii; or Enterococcus faecium SF68) and a control group in the treatment of outpatient acute diarrhea in 571 children age 3 months to 36 months.
The primary outcomes were the duration of diarrhea and the number and consistency of stools. The groups receiving Lactobacillus GG and the mixture had a shorter total duration of diarrhea (78.5 and 70 hours, respectively), decreased total number of stools, and improved stool consistency when compared with the control (115.5 hours). The other therapies showed no improvement over the control group. These data report on products commercially available in Italy, which may differ greatly from products available locally.
Bottom line: Probiotic preparations for the treatment of acute diarrhea in children should be chosen based on effectiveness data.
Citation: Canani RB, Cirillo P, Terrin G, et al. Probiotics for treatment of acute diarrhoea in children: randomised clinical trial of five different preparations. BMJ 2007;335:340-345.
Is CTPA a Reliable Alternative to V/Q Scan for Diagnosing PE?
Background: Computed tomography pulmonary angiogram (CTPA) has replaced ventilation/perfusion (V/Q) scanning at many hospitals as the test of choice for ruling out pulmonary embolism (PE). But limited clinical data compare CTPA with V/Q scanning in those suspected of having venous thromboembolism (VTE).
Study design: Randomized, investigator blinded, controlled trial.
Setting: The emergency departments (ED), inpatient wards, and outpatient clinics of five academic centers.
Synopsis: In the study, 1,411 patients were enrolled from five medical centers. Of 694 patients randomized to CTPA, 133 (19.2%) were diagnosed with VTE in the initial evaluation period, while 101 of 712 patients (14.2%) receiving a V/Q scan were diagnosed with VTE.
Patients not initially diagnosed with VTE were monitored. At three-month follow-up, 0.4% of the CTPA group and 1.0% of the V/Q group had a diagnosed VTE.
The overall rate of VTE found in the initial diagnostic period was significantly greater in patients randomized to CTPA (19.2% vs. 14.2%; difference, 5.0%; 95% CI; 1.1% to 8.9% p=.01). This suggests CTPA has a higher false positive rate or detects clinically insignificant thrombi.
Bottom line: CTPA was not inferior to V/Q scanning for excluding clinically meaningful PE, but CTPA diagnosed about 30% more patients with VTE than did V/Q scanning.
Citation: Anderson DR, Kahn SR, Rodger MA, et al. Computed tomographic pulmonary angiography vs. ventilation-perfusion lung scanning in patients with suspected pulmonary embolism: a randomized controlled trial. JAMA. 2007;298(23):2743-2753.
Does the Hospitalist Model Improve Length of Stay, Quality, and Cost of Care?
Background: The hospitalist model, with increased physician availability and expertise but greater discontinuity of care, is becoming more prevalent in U.S. medicine. What little is known about how this model will affect patient care is derived from a number of small studies.
Study design: Retrospective cohort study.
Setting: 45 small to midsize, predominantly nonteaching hospitals throughout the U.S.
Synopsis: Using the Premier Healthcare Informatics database, this study examined information on 76,926 patients admitted for seven common diagnoses to one of three services: hospitalist, general internist, or family physician. Analysis showed that patients on a hospitalist service had a 0.4-day shorter length of stay (p<0.001) compared with those on a general internist or family physician service.
The cost to patients cared for by a hospitalist was lower than the cost of family physicians ($125 less, p=0.33) and internists ($268 less, p=0.02). There was no difference found in death rate or 14-day readmission rate among the three services.
Given the retrospective design of this study, no causal relationship can be deduced. This study is further limited by its lack of specific data on the physicians categorized into one of the three groups solely by administrative data. The authors had concerns that the biases inherent to the retrospective nature of their work accounted for the significant difference found between hospitalists and internists.
Bottom line: The hospitalist model is associated with modest improvements in length of stay as compared with traditional inpatient approaches.
Citation: Lindenauer PK, Rothberg MB, Pekow PS, et. al. Outcomes of care by hospitalists, general internists, and family physicians. N Engl J Med. 2007;357:2589-2600.
What Is the Stroke Risk Soon after TIA, and What Factors Drive the Variability of Previous Findings?
Background: Many studies have attempted to estimate the risk of stroke in the early period after a transient ischemic attack (TIA). These studies vary widely in their calculation of the estimated risk. Further, the clinical and methodological factors underlying this variability are unclear.
Study design: Systematic review and meta-analysis.
Setting: Community and hospital.
Synopsis: Searching the Cochrane review database, MEDLINE, EMBASE, CINAHL, and BIOSIS, 11 studies from 1973 to 2006 were included for meta-analysis, selected from 694 potential candidate studies identified on initial screening. The studies ranged in size from 62 to 2,285 patients.
The pooled estimate of risk for stroke following TIA was found to be 3.5%, 8%, and 9.2% at two, 30, and 90 days following TIA, respectively. However, there was significant heterogeneity for all periods considered (p<0.001).
Outcome ascertainment was identified as a major source of methodological heterogeneity. When risk of stroke at follow-up was determined by passive ascertainment (e.g., administrative documentation) the early risk of stroke was 3.1% two days after TIA, 6.4% at 30 days, and 8.7% at 90 days. But active ascertainment (e.g., direct, personal contact with study participants) determined stroke risk to be 9.9%, 13.4%, and 17.4% at two, 30, and 90 days after TIA, respectively.
Bottom line: Based on analysis of completed studies that included directly observed follow-up of study participants, the early risk of stroke after TIA is approximately 15% to 20% at 90 days following the sentinel event.
Citation: Wu CM, McLaughlin K, Lorenzetti DL, Hill MD, Manns BJ, Ghali WA. Early risk of stroke after transient ischemic attack. Arch Intern Med. 2007;167:2417-2422.
What Is the 1-year Ischemia and Mortality Rate for Three Anti-thrombotic Therapies for Early Invasive Management of ACS?
Background: Early interventional or surgical revascularization has improved morbidity and mortality in patients with acute coronary syndrome (ACS). The optimal anti-thrombotic regimen to reduce late ischemic and death rates has not been determined.
Study design: Prospective, open-label randomized control trial.
Setting: 450 academic and community-based institutions in 17 countries.
Synopsis: A total of 13,819 patients were enrolled between August 2003 and December 2005. They were assigned to heparin plus glycoprotein (GP) IIb/IIIa inhibitors (n=4,603), bivalirudin (Angiomax) plus IIb/IIIa inhibitors (n=4,604), or bivalirudin monotherapy (n=4,612).
For patients receiving GP IIb/IIIa inhibitors, a 2x2 factorial design assigned half the heparin and bivalirudin groups to routine upstream GP inhibitor administration (4,605 patients). The other half received selective GP IIb/IIIa inhibitors administration if PCI was indicated (4,602 patients).
At one year, there was no statistically significant difference in ischemia or mortality rate among the three therapy groups. No difference in ischemia rate was detected between the two GP IIb/IIIa inhibitor utilization strategies.
Since the hypotheses and the power for the one-year analysis in this trial were not prospectively determined, the results are considered to be exploratory and hypothesis generating.
Bottom line: At one year, there is no statistically significant difference in ischemia or mortality rate for the three antithrombotic regiments and the two glycoprotein utilization strategies.
Citation: Stone GW, Ware JH, Bertrand ME, et. al. Antithrombotic strategies in patients with acute coronary syndromes undergoing early invasive management. One-year results from the ACUITY trial. JAMA 2007;298:2497-2505.
What Is the PE Risk after Discontinuing Anticoagulation in Patients with Symptomatic VTE?
Background: The natural history of patients with symptomatic VTE who have completed anticoagulation is not well understood.
Study design: Inception cohort using pooled data from a prospective cohort study and one arm of an open-label randomized trial.
Setting: Academic medical centers in Canada, Sweden, and Italy.
Synopsis: Using pooled data from two previous studies, 2,052 patients with a first diagnosis of symptomatic VTE (lower-extremity deep-vein thrombosis [DVT], PE, or both) were evaluated for fatal PE after a standard course of therapy (mean of six months) with a vitamin K antagonist.
Patients were followed for up to 120 months. The investigators found an annual event risk of 0.19-0.49 per 100 person-years for fatal PE. Patients with prolonged immobility, active cancer, and thrombophilia were excluded, as were those with recurrent acute DVT.
Secondary analysis revealed an incidence of any fatal, definite or probable PE within the first year of discontinuing therapy of 0.35%-0.81%.
After the first year, the annual event risk ranged from 0.15-0.40 events per 100 person-years. Patients with advanced age, idiopathic VTE as well as those presenting with PE had higher rates of fatal PE.
Bottom line: There is a real though small (less than 1%) risk of fatal PE in the first year following discontinuation of anticoagulation for the first VTE episode. The optimal course of treatment for patients with idiopathic VTE is yet to be determined.
Citation: Douketis JD, Gu CS, Schulman S, et al. The risk for fatal pulmonary embolism after discontinuing anticoagulant therapy for venous thromboembolism. Ann Intern Med. 2007;147(11):766-774.
Do the Beers Criteria Predict ED Visits Associated with Adverse Drug Events?
Background: Adverse drug events are common in the elderly. The Beers criteria are a consensus-based list of 41 medications that are considered inappropriate for use in older adults and often lead to poor outcomes.
Study design: Retrospective medical record review and data analysis.
Setting: Three nationally representative, U.S. public health surveillance systems: the National Electronic Injury Surveillance System-Cooperative Adverse Drug Event Surveillance System (NEISS-CADES), 2004-2005; the National Ambulatory Medical Care Survey (NAMCS), 2004; and National Hospital Ambulatory Medical Care Survey (NHAMCS), 2004.
Synopsis: Using data collected from ED visits at 58 hospitals in the NEISS-CADES system, this study estimated that 177,504 visits for adverse drug events occur annually in the United States. Only 8.8% of such visits were attributable to the 41 medications included in the Beers criteria. Three drug classes (anticoagulant and antiplatelet agents, antidiabetic agents, and narrow therapeutic index agents) accounted for nearly half of all such ED visits. Warfarin (17.3%), insulin (13%), and digoxin (3.2%) were the most commonly implicated medications, collectively accounting for 33% of visits (CI, 27.8% to 38.7%).
This study suggests that because of the common use and high risk of adverse events associated with these three drugs, interventions targeting their use may prevent ED visits for adverse drug events in the elderly, compared with interventions aimed at reducing the use of medications identified in the Beers criteria.
This study only included adverse drug events identified in the ED and relied on the diagnosis and documentation of such events by the ED physician.
Bottom line: Beers criteria medications, although considered inappropriate for use in the elderly, were associated with significantly fewer ED visits for adverse events compared with warfarin, digoxin, and insulin.
Citation: Budnitz DS, Shehab N, Kegler SR, et. al. Medication use leading to emergency department visits for adverse drug events in older adults. Ann Intern Med. 2007;147:755-765. TH
Literature at a Glance
A guide to this month’s abstracts
- Steroids reduce mortality only in patients with confirmed bacterial meningitis.
- Probiotics can be useful in the treatment of acute diarrhea in children.
- CT pulmonary angiography is not inferior to V/Q scanning for exclusion of PE.
- Hospitalist care results in shorter LOS compared with care by traditional general internists and family practice physicians.
- The early risk of stroke after TIA is approximately 15% to 20% at 90 days after the sentinel event.
- Different anti-thrombotic strategies produce no difference in outcomes of early acute coronary syndromes.
- The risk of fatal PE is highest in the first year after medication is stopped.
- Beers criteria medications are associated with fewer ED visits by elderly patients compared with warfarin, digoxin, and insulin.
Do Steroids Affect the Outcome in Patients with Meningitis?
Background: Pyogenic (bacterial) meningitis has high morbidity and mortality. Studies suggest some benefit of steroids in children but provide limited evidence for adult use.
Study design: Intention-to-treat, randomized control trial.
Setting: Single hospital in Vietnam.
Synopsis: Of 435 patients older than 14 with suspected meningitis all received lumbar puncture with randomization to IV dexamethasone or placebo for four days. Results showed 69% of patients had definite meningitis, 28.3% were probable, and 2.8% had an alternative diagnosis based on culture results.
The primary outcome was death after one month, which did not differ among groups (risk ratio [RR] 0.79, confidence interval [CI] 0.45-1.39).
Predefined subgroup analysis of patients with definitive meningitis showed a significant reduction in mortality at one month (RR 0.43, CI 0.2-0.94) and death/disability at six months (odds ratio [OR] 0.56, CI 0.32-0.98).
In patients with probable meningitis, those who received steroids demonstrated a trend toward harm (OR 2.65, CI 0.73-9.63).
Probable versus definite meningitis was determined retrospectively based on cultures. The most common isolate was Streptococcus suis.
Bottom line: This study provides some evidence for using steroids in adults with confirmed bacterial meningitis. Clinical application is limited by bacterial epidemiology and the difficulty of prospectively separating patients who would benefit from those who might be harmed.
Citation: Nguyen TH, Tran TH, Thwaites G, et. al. Dexamethasone in Vietnamese adolescents and adults with bacterial meningitis. N Engl J Med. 2007;357:2431-2439.
Which Probiotic Preparations Best Reduce the Duration of Acute Diarrhea in Children?
Background: Probiotics have been suggested as an adjunctive therapy to reduce the severity and duration of acute diarrhea in children. However, there are no clear data to suggest if specific probiotic agents are superior to others.
Study design: Prospective single-blind, randomized, controlled trial.
Setting: Outpatient primary care in Naples, Italy.
Synopsis: This study compared five commercially available probiotic preparations (mix of Lactobacillus delbrueckii var bulgaricus/Streptococcus thermophilus/L. acidophilus/ Bifido-bacterium bifidum; L. rhamnosus strain GG; Saccharomyces boulardii; Bacillus clausii; or Enterococcus faecium SF68) and a control group in the treatment of outpatient acute diarrhea in 571 children age 3 months to 36 months.
The primary outcomes were the duration of diarrhea and the number and consistency of stools. The groups receiving Lactobacillus GG and the mixture had a shorter total duration of diarrhea (78.5 and 70 hours, respectively), decreased total number of stools, and improved stool consistency when compared with the control (115.5 hours). The other therapies showed no improvement over the control group. These data report on products commercially available in Italy, which may differ greatly from products available locally.
Bottom line: Probiotic preparations for the treatment of acute diarrhea in children should be chosen based on effectiveness data.
Citation: Canani RB, Cirillo P, Terrin G, et al. Probiotics for treatment of acute diarrhoea in children: randomised clinical trial of five different preparations. BMJ 2007;335:340-345.
Is CTPA a Reliable Alternative to V/Q Scan for Diagnosing PE?
Background: Computed tomography pulmonary angiogram (CTPA) has replaced ventilation/perfusion (V/Q) scanning at many hospitals as the test of choice for ruling out pulmonary embolism (PE). But limited clinical data compare CTPA with V/Q scanning in those suspected of having venous thromboembolism (VTE).
Study design: Randomized, investigator blinded, controlled trial.
Setting: The emergency departments (ED), inpatient wards, and outpatient clinics of five academic centers.
Synopsis: In the study, 1,411 patients were enrolled from five medical centers. Of 694 patients randomized to CTPA, 133 (19.2%) were diagnosed with VTE in the initial evaluation period, while 101 of 712 patients (14.2%) receiving a V/Q scan were diagnosed with VTE.
Patients not initially diagnosed with VTE were monitored. At three-month follow-up, 0.4% of the CTPA group and 1.0% of the V/Q group had a diagnosed VTE.
The overall rate of VTE found in the initial diagnostic period was significantly greater in patients randomized to CTPA (19.2% vs. 14.2%; difference, 5.0%; 95% CI; 1.1% to 8.9% p=.01). This suggests CTPA has a higher false positive rate or detects clinically insignificant thrombi.
Bottom line: CTPA was not inferior to V/Q scanning for excluding clinically meaningful PE, but CTPA diagnosed about 30% more patients with VTE than did V/Q scanning.
Citation: Anderson DR, Kahn SR, Rodger MA, et al. Computed tomographic pulmonary angiography vs. ventilation-perfusion lung scanning in patients with suspected pulmonary embolism: a randomized controlled trial. JAMA. 2007;298(23):2743-2753.
Does the Hospitalist Model Improve Length of Stay, Quality, and Cost of Care?
Background: The hospitalist model, with increased physician availability and expertise but greater discontinuity of care, is becoming more prevalent in U.S. medicine. What little is known about how this model will affect patient care is derived from a number of small studies.
Study design: Retrospective cohort study.
Setting: 45 small to midsize, predominantly nonteaching hospitals throughout the U.S.
Synopsis: Using the Premier Healthcare Informatics database, this study examined information on 76,926 patients admitted for seven common diagnoses to one of three services: hospitalist, general internist, or family physician. Analysis showed that patients on a hospitalist service had a 0.4-day shorter length of stay (p<0.001) compared with those on a general internist or family physician service.
The cost to patients cared for by a hospitalist was lower than the cost of family physicians ($125 less, p=0.33) and internists ($268 less, p=0.02). There was no difference found in death rate or 14-day readmission rate among the three services.
Given the retrospective design of this study, no causal relationship can be deduced. This study is further limited by its lack of specific data on the physicians categorized into one of the three groups solely by administrative data. The authors had concerns that the biases inherent to the retrospective nature of their work accounted for the significant difference found between hospitalists and internists.
Bottom line: The hospitalist model is associated with modest improvements in length of stay as compared with traditional inpatient approaches.
Citation: Lindenauer PK, Rothberg MB, Pekow PS, et. al. Outcomes of care by hospitalists, general internists, and family physicians. N Engl J Med. 2007;357:2589-2600.
What Is the Stroke Risk Soon after TIA, and What Factors Drive the Variability of Previous Findings?
Background: Many studies have attempted to estimate the risk of stroke in the early period after a transient ischemic attack (TIA). These studies vary widely in their calculation of the estimated risk. Further, the clinical and methodological factors underlying this variability are unclear.
Study design: Systematic review and meta-analysis.
Setting: Community and hospital.
Synopsis: Searching the Cochrane review database, MEDLINE, EMBASE, CINAHL, and BIOSIS, 11 studies from 1973 to 2006 were included for meta-analysis, selected from 694 potential candidate studies identified on initial screening. The studies ranged in size from 62 to 2,285 patients.
The pooled estimate of risk for stroke following TIA was found to be 3.5%, 8%, and 9.2% at two, 30, and 90 days following TIA, respectively. However, there was significant heterogeneity for all periods considered (p<0.001).
Outcome ascertainment was identified as a major source of methodological heterogeneity. When risk of stroke at follow-up was determined by passive ascertainment (e.g., administrative documentation) the early risk of stroke was 3.1% two days after TIA, 6.4% at 30 days, and 8.7% at 90 days. But active ascertainment (e.g., direct, personal contact with study participants) determined stroke risk to be 9.9%, 13.4%, and 17.4% at two, 30, and 90 days after TIA, respectively.
Bottom line: Based on analysis of completed studies that included directly observed follow-up of study participants, the early risk of stroke after TIA is approximately 15% to 20% at 90 days following the sentinel event.
Citation: Wu CM, McLaughlin K, Lorenzetti DL, Hill MD, Manns BJ, Ghali WA. Early risk of stroke after transient ischemic attack. Arch Intern Med. 2007;167:2417-2422.
What Is the 1-year Ischemia and Mortality Rate for Three Anti-thrombotic Therapies for Early Invasive Management of ACS?
Background: Early interventional or surgical revascularization has improved morbidity and mortality in patients with acute coronary syndrome (ACS). The optimal anti-thrombotic regimen to reduce late ischemic and death rates has not been determined.
Study design: Prospective, open-label randomized control trial.
Setting: 450 academic and community-based institutions in 17 countries.
Synopsis: A total of 13,819 patients were enrolled between August 2003 and December 2005. They were assigned to heparin plus glycoprotein (GP) IIb/IIIa inhibitors (n=4,603), bivalirudin (Angiomax) plus IIb/IIIa inhibitors (n=4,604), or bivalirudin monotherapy (n=4,612).
For patients receiving GP IIb/IIIa inhibitors, a 2x2 factorial design assigned half the heparin and bivalirudin groups to routine upstream GP inhibitor administration (4,605 patients). The other half received selective GP IIb/IIIa inhibitors administration if PCI was indicated (4,602 patients).
At one year, there was no statistically significant difference in ischemia or mortality rate among the three therapy groups. No difference in ischemia rate was detected between the two GP IIb/IIIa inhibitor utilization strategies.
Since the hypotheses and the power for the one-year analysis in this trial were not prospectively determined, the results are considered to be exploratory and hypothesis generating.
Bottom line: At one year, there is no statistically significant difference in ischemia or mortality rate for the three antithrombotic regiments and the two glycoprotein utilization strategies.
Citation: Stone GW, Ware JH, Bertrand ME, et. al. Antithrombotic strategies in patients with acute coronary syndromes undergoing early invasive management. One-year results from the ACUITY trial. JAMA 2007;298:2497-2505.
What Is the PE Risk after Discontinuing Anticoagulation in Patients with Symptomatic VTE?
Background: The natural history of patients with symptomatic VTE who have completed anticoagulation is not well understood.
Study design: Inception cohort using pooled data from a prospective cohort study and one arm of an open-label randomized trial.
Setting: Academic medical centers in Canada, Sweden, and Italy.
Synopsis: Using pooled data from two previous studies, 2,052 patients with a first diagnosis of symptomatic VTE (lower-extremity deep-vein thrombosis [DVT], PE, or both) were evaluated for fatal PE after a standard course of therapy (mean of six months) with a vitamin K antagonist.
Patients were followed for up to 120 months. The investigators found an annual event risk of 0.19-0.49 per 100 person-years for fatal PE. Patients with prolonged immobility, active cancer, and thrombophilia were excluded, as were those with recurrent acute DVT.
Secondary analysis revealed an incidence of any fatal, definite or probable PE within the first year of discontinuing therapy of 0.35%-0.81%.
After the first year, the annual event risk ranged from 0.15-0.40 events per 100 person-years. Patients with advanced age, idiopathic VTE as well as those presenting with PE had higher rates of fatal PE.
Bottom line: There is a real though small (less than 1%) risk of fatal PE in the first year following discontinuation of anticoagulation for the first VTE episode. The optimal course of treatment for patients with idiopathic VTE is yet to be determined.
Citation: Douketis JD, Gu CS, Schulman S, et al. The risk for fatal pulmonary embolism after discontinuing anticoagulant therapy for venous thromboembolism. Ann Intern Med. 2007;147(11):766-774.
Do the Beers Criteria Predict ED Visits Associated with Adverse Drug Events?
Background: Adverse drug events are common in the elderly. The Beers criteria are a consensus-based list of 41 medications that are considered inappropriate for use in older adults and often lead to poor outcomes.
Study design: Retrospective medical record review and data analysis.
Setting: Three nationally representative, U.S. public health surveillance systems: the National Electronic Injury Surveillance System-Cooperative Adverse Drug Event Surveillance System (NEISS-CADES), 2004-2005; the National Ambulatory Medical Care Survey (NAMCS), 2004; and National Hospital Ambulatory Medical Care Survey (NHAMCS), 2004.
Synopsis: Using data collected from ED visits at 58 hospitals in the NEISS-CADES system, this study estimated that 177,504 visits for adverse drug events occur annually in the United States. Only 8.8% of such visits were attributable to the 41 medications included in the Beers criteria. Three drug classes (anticoagulant and antiplatelet agents, antidiabetic agents, and narrow therapeutic index agents) accounted for nearly half of all such ED visits. Warfarin (17.3%), insulin (13%), and digoxin (3.2%) were the most commonly implicated medications, collectively accounting for 33% of visits (CI, 27.8% to 38.7%).
This study suggests that because of the common use and high risk of adverse events associated with these three drugs, interventions targeting their use may prevent ED visits for adverse drug events in the elderly, compared with interventions aimed at reducing the use of medications identified in the Beers criteria.
This study only included adverse drug events identified in the ED and relied on the diagnosis and documentation of such events by the ED physician.
Bottom line: Beers criteria medications, although considered inappropriate for use in the elderly, were associated with significantly fewer ED visits for adverse events compared with warfarin, digoxin, and insulin.
Citation: Budnitz DS, Shehab N, Kegler SR, et. al. Medication use leading to emergency department visits for adverse drug events in older adults. Ann Intern Med. 2007;147:755-765. TH