User login
Apremilast and Phototherapy for Treatment of Psoriasis in a Patient With Human Immunodeficiency Virus
To the Editor:
A 50-year old man with Fitzpatrick skin type IV, human immunodeficiency virus (HIV), fatty liver disease, and moderate psoriasis (10% body surface area [BSA] affected) currently treated with clobetasol spray and calcitriol ointment presented with persistent psoriatic lesions on the trunk, arms, legs, and buttocks. His CD4 count was 460 and his HIV RNA count was 48 copies/mL on polymerase chain reaction 2 months prior to the current presentation. He had been undergoing phototherapy 3 times weekly for the last 5 months for treatment of psoriasis.
At the current presentation, he was started on an apremilast starter pack with the dosage titrated from 10 mg to 30 mg over the course of 1 week. He was maintained on a dose of 30 mg twice daily after 1 week and continued clobetasol spray, calcitriol ointment, and phototherapy 3 times weekly with the intent to reduce the frequency after adequate control of psoriasis was achieved. After 3 months of treatment, the affected BSA was 0%. He continued apremilast, and phototherapy was reduced to once weekly. Phototherapy was discontinued after 7 months of concomitant treatment with apremilast after clearance was maintained. It was reinitiated twice weekly after a mild flare (3% BSA affected). After 20 total months of treatment, the patient was no longer able to afford apremilast treatment and presented with a severe psoriasis flare (40% BSA affected). He was switched to acitretin with a plan to apply for apremilast financial assistance programs.
Psoriasis treatment in the HIV population poses a challenge given the immunosuppressed state of these patients, the risk of reactivation of latent infections, and the refractory nature of psoriasis in the setting of HIV. Two of the authors (S.P.R. and J.J.W.) previously reported a case of moderate to severe psoriasis in a patient with HIV and hepatitis C who demonstrated treatment success with apremilast until it was discontinued due to financial implications.1 Currently, apremilast is not widely used to treat psoriasis in the HIV population. The National Psoriasis Foundation 2010 guidelines recommended UV light therapy for treatment of moderate to severe psoriasis in HIV-positive patients, with oral retinoids as the second-line treatment.2 There remains a need for updated guidelines on the use of systemic agents for psoriasis treatment in the HIV population.
Apremilast, a phosphodiesterase 4 inhibitor, is an oral therapy that restores the balance of proinflammatory and anti-inflammatory cytokines by inhibiting inflammatory cytokine (eg, tumor necrosis factor α, IFN-γ, IL-2, IL-12, IL-23) secretion and stimulating anti-inflammatory cytokine (eg, IL-6, IL-10) production. In 2015, the phase 3 ESTEEM 13 and ESTEEM 24 trials demonstrated the efficacy of apremilast 30 mg twice daily for treatment of psoriasis. In both trials, the psoriasis area and severity index 75 response rate at week 16 was significantly
Use of other systemic agents such as tumor necrosis factor α inhibitors and ustekinumab has been reported in HIV-positive patients.5-7 There is no current data on IL-17 and IL-23 inhibitors. Acitretin generally is recommended as a second-line agent in HIV patients given its lack of immunosuppression2; however, methotrexate and cyclosporine should be avoided given the risk of opportunistic infections.8
Apremilast is a promising therapy with a favorable safety profile that should be considered as an adjuvant treatment to first-line agents such as phototherapy in HIV-positive patients. Apremilast has been successfully used in an HIV patient with a concomitant chronic hepatitis C infection.1 Systemic medications such as apremilast should be managed in coordination with infectious disease specialists with close monitoring of CD4 levels and viral loads as well as prophylactic agents.
- Reddy SP, Shah VV, Wu JJ. Apremilast for a psoriasis patient with HIV and hepatitis C. J Eur Acad Dermatol Venereol. 2017;31:e481-e482.
- Menon K, Van Voorhees AS, Bebo BF Jr, et al. Psoriasis in patients with HIV infection: from the medical board of the National Psoriasis Foundation [published online July 31, 2009]. J Am Acad Dermatol. 2010;62:291-299.
- Papp K, Reich K, Leonardi CL, et al. Apremilast, an oral phosphodiesterase 4 (PDE4) inhibitor, in patients with moderate to severe plaque psoriasis: results of a phase III, randomized, controlled trial (Efficacy and Safety Trial Evaluating the Effects of Apremilast in Psoriasis [ESTEEM] 1). J Am Acad Dermatol. 2015;73:37-49.
- Paul C, Cather J, Gooderham M, et al. Efficacy and safety of apremilast, an oral phosphodiesterase 4 inhibitor, in patients with moderate-to-severe plaque psoriasis over 52 weeks: a phase III, randomized controlled trial (ESTEEM 2). Br J Dermatol. 2015;173:1387-1399.
- Lindsey SF, Weiss J, Lee ES, et al. Treatment of severe psoriasis and psoriatic arthritis with adalimumab in an HIV-positive patient. J Drugs Dermatol. 2014;13:869-871.
- Saeki H, Ito T, Hayashi M, et al. Successful treatment of ustekinumab in a severe psoriasis patient with human immunodeficiency virus infection. J Eur Acad Dermatol Venereol. 2015;29:1653-1655.
- Paparizos V, Rallis E, Kirsten L, et al. Ustekinumab for the treatment of HIV psoriasis. J Dermatolog Treat. 2012;23:398-399.
- Kaushik SB, Lebwohl MG. Psoriasis: which therapy for which patient: focus on special populations and chronic infections [published online July 11, 2018]. J Am Acad Dermatol. 2019;80:43-53.
To the Editor:
A 50-year old man with Fitzpatrick skin type IV, human immunodeficiency virus (HIV), fatty liver disease, and moderate psoriasis (10% body surface area [BSA] affected) currently treated with clobetasol spray and calcitriol ointment presented with persistent psoriatic lesions on the trunk, arms, legs, and buttocks. His CD4 count was 460 and his HIV RNA count was 48 copies/mL on polymerase chain reaction 2 months prior to the current presentation. He had been undergoing phototherapy 3 times weekly for the last 5 months for treatment of psoriasis.
At the current presentation, he was started on an apremilast starter pack with the dosage titrated from 10 mg to 30 mg over the course of 1 week. He was maintained on a dose of 30 mg twice daily after 1 week and continued clobetasol spray, calcitriol ointment, and phototherapy 3 times weekly with the intent to reduce the frequency after adequate control of psoriasis was achieved. After 3 months of treatment, the affected BSA was 0%. He continued apremilast, and phototherapy was reduced to once weekly. Phototherapy was discontinued after 7 months of concomitant treatment with apremilast after clearance was maintained. It was reinitiated twice weekly after a mild flare (3% BSA affected). After 20 total months of treatment, the patient was no longer able to afford apremilast treatment and presented with a severe psoriasis flare (40% BSA affected). He was switched to acitretin with a plan to apply for apremilast financial assistance programs.
Psoriasis treatment in the HIV population poses a challenge given the immunosuppressed state of these patients, the risk of reactivation of latent infections, and the refractory nature of psoriasis in the setting of HIV. Two of the authors (S.P.R. and J.J.W.) previously reported a case of moderate to severe psoriasis in a patient with HIV and hepatitis C who demonstrated treatment success with apremilast until it was discontinued due to financial implications.1 Currently, apremilast is not widely used to treat psoriasis in the HIV population. The National Psoriasis Foundation 2010 guidelines recommended UV light therapy for treatment of moderate to severe psoriasis in HIV-positive patients, with oral retinoids as the second-line treatment.2 There remains a need for updated guidelines on the use of systemic agents for psoriasis treatment in the HIV population.
Apremilast, a phosphodiesterase 4 inhibitor, is an oral therapy that restores the balance of proinflammatory and anti-inflammatory cytokines by inhibiting inflammatory cytokine (eg, tumor necrosis factor α, IFN-γ, IL-2, IL-12, IL-23) secretion and stimulating anti-inflammatory cytokine (eg, IL-6, IL-10) production. In 2015, the phase 3 ESTEEM 13 and ESTEEM 24 trials demonstrated the efficacy of apremilast 30 mg twice daily for treatment of psoriasis. In both trials, the psoriasis area and severity index 75 response rate at week 16 was significantly
Use of other systemic agents such as tumor necrosis factor α inhibitors and ustekinumab has been reported in HIV-positive patients.5-7 There is no current data on IL-17 and IL-23 inhibitors. Acitretin generally is recommended as a second-line agent in HIV patients given its lack of immunosuppression2; however, methotrexate and cyclosporine should be avoided given the risk of opportunistic infections.8
Apremilast is a promising therapy with a favorable safety profile that should be considered as an adjuvant treatment to first-line agents such as phototherapy in HIV-positive patients. Apremilast has been successfully used in an HIV patient with a concomitant chronic hepatitis C infection.1 Systemic medications such as apremilast should be managed in coordination with infectious disease specialists with close monitoring of CD4 levels and viral loads as well as prophylactic agents.
To the Editor:
A 50-year old man with Fitzpatrick skin type IV, human immunodeficiency virus (HIV), fatty liver disease, and moderate psoriasis (10% body surface area [BSA] affected) currently treated with clobetasol spray and calcitriol ointment presented with persistent psoriatic lesions on the trunk, arms, legs, and buttocks. His CD4 count was 460 and his HIV RNA count was 48 copies/mL on polymerase chain reaction 2 months prior to the current presentation. He had been undergoing phototherapy 3 times weekly for the last 5 months for treatment of psoriasis.
At the current presentation, he was started on an apremilast starter pack with the dosage titrated from 10 mg to 30 mg over the course of 1 week. He was maintained on a dose of 30 mg twice daily after 1 week and continued clobetasol spray, calcitriol ointment, and phototherapy 3 times weekly with the intent to reduce the frequency after adequate control of psoriasis was achieved. After 3 months of treatment, the affected BSA was 0%. He continued apremilast, and phototherapy was reduced to once weekly. Phototherapy was discontinued after 7 months of concomitant treatment with apremilast after clearance was maintained. It was reinitiated twice weekly after a mild flare (3% BSA affected). After 20 total months of treatment, the patient was no longer able to afford apremilast treatment and presented with a severe psoriasis flare (40% BSA affected). He was switched to acitretin with a plan to apply for apremilast financial assistance programs.
Psoriasis treatment in the HIV population poses a challenge given the immunosuppressed state of these patients, the risk of reactivation of latent infections, and the refractory nature of psoriasis in the setting of HIV. Two of the authors (S.P.R. and J.J.W.) previously reported a case of moderate to severe psoriasis in a patient with HIV and hepatitis C who demonstrated treatment success with apremilast until it was discontinued due to financial implications.1 Currently, apremilast is not widely used to treat psoriasis in the HIV population. The National Psoriasis Foundation 2010 guidelines recommended UV light therapy for treatment of moderate to severe psoriasis in HIV-positive patients, with oral retinoids as the second-line treatment.2 There remains a need for updated guidelines on the use of systemic agents for psoriasis treatment in the HIV population.
Apremilast, a phosphodiesterase 4 inhibitor, is an oral therapy that restores the balance of proinflammatory and anti-inflammatory cytokines by inhibiting inflammatory cytokine (eg, tumor necrosis factor α, IFN-γ, IL-2, IL-12, IL-23) secretion and stimulating anti-inflammatory cytokine (eg, IL-6, IL-10) production. In 2015, the phase 3 ESTEEM 13 and ESTEEM 24 trials demonstrated the efficacy of apremilast 30 mg twice daily for treatment of psoriasis. In both trials, the psoriasis area and severity index 75 response rate at week 16 was significantly
Use of other systemic agents such as tumor necrosis factor α inhibitors and ustekinumab has been reported in HIV-positive patients.5-7 There is no current data on IL-17 and IL-23 inhibitors. Acitretin generally is recommended as a second-line agent in HIV patients given its lack of immunosuppression2; however, methotrexate and cyclosporine should be avoided given the risk of opportunistic infections.8
Apremilast is a promising therapy with a favorable safety profile that should be considered as an adjuvant treatment to first-line agents such as phototherapy in HIV-positive patients. Apremilast has been successfully used in an HIV patient with a concomitant chronic hepatitis C infection.1 Systemic medications such as apremilast should be managed in coordination with infectious disease specialists with close monitoring of CD4 levels and viral loads as well as prophylactic agents.
- Reddy SP, Shah VV, Wu JJ. Apremilast for a psoriasis patient with HIV and hepatitis C. J Eur Acad Dermatol Venereol. 2017;31:e481-e482.
- Menon K, Van Voorhees AS, Bebo BF Jr, et al. Psoriasis in patients with HIV infection: from the medical board of the National Psoriasis Foundation [published online July 31, 2009]. J Am Acad Dermatol. 2010;62:291-299.
- Papp K, Reich K, Leonardi CL, et al. Apremilast, an oral phosphodiesterase 4 (PDE4) inhibitor, in patients with moderate to severe plaque psoriasis: results of a phase III, randomized, controlled trial (Efficacy and Safety Trial Evaluating the Effects of Apremilast in Psoriasis [ESTEEM] 1). J Am Acad Dermatol. 2015;73:37-49.
- Paul C, Cather J, Gooderham M, et al. Efficacy and safety of apremilast, an oral phosphodiesterase 4 inhibitor, in patients with moderate-to-severe plaque psoriasis over 52 weeks: a phase III, randomized controlled trial (ESTEEM 2). Br J Dermatol. 2015;173:1387-1399.
- Lindsey SF, Weiss J, Lee ES, et al. Treatment of severe psoriasis and psoriatic arthritis with adalimumab in an HIV-positive patient. J Drugs Dermatol. 2014;13:869-871.
- Saeki H, Ito T, Hayashi M, et al. Successful treatment of ustekinumab in a severe psoriasis patient with human immunodeficiency virus infection. J Eur Acad Dermatol Venereol. 2015;29:1653-1655.
- Paparizos V, Rallis E, Kirsten L, et al. Ustekinumab for the treatment of HIV psoriasis. J Dermatolog Treat. 2012;23:398-399.
- Kaushik SB, Lebwohl MG. Psoriasis: which therapy for which patient: focus on special populations and chronic infections [published online July 11, 2018]. J Am Acad Dermatol. 2019;80:43-53.
- Reddy SP, Shah VV, Wu JJ. Apremilast for a psoriasis patient with HIV and hepatitis C. J Eur Acad Dermatol Venereol. 2017;31:e481-e482.
- Menon K, Van Voorhees AS, Bebo BF Jr, et al. Psoriasis in patients with HIV infection: from the medical board of the National Psoriasis Foundation [published online July 31, 2009]. J Am Acad Dermatol. 2010;62:291-299.
- Papp K, Reich K, Leonardi CL, et al. Apremilast, an oral phosphodiesterase 4 (PDE4) inhibitor, in patients with moderate to severe plaque psoriasis: results of a phase III, randomized, controlled trial (Efficacy and Safety Trial Evaluating the Effects of Apremilast in Psoriasis [ESTEEM] 1). J Am Acad Dermatol. 2015;73:37-49.
- Paul C, Cather J, Gooderham M, et al. Efficacy and safety of apremilast, an oral phosphodiesterase 4 inhibitor, in patients with moderate-to-severe plaque psoriasis over 52 weeks: a phase III, randomized controlled trial (ESTEEM 2). Br J Dermatol. 2015;173:1387-1399.
- Lindsey SF, Weiss J, Lee ES, et al. Treatment of severe psoriasis and psoriatic arthritis with adalimumab in an HIV-positive patient. J Drugs Dermatol. 2014;13:869-871.
- Saeki H, Ito T, Hayashi M, et al. Successful treatment of ustekinumab in a severe psoriasis patient with human immunodeficiency virus infection. J Eur Acad Dermatol Venereol. 2015;29:1653-1655.
- Paparizos V, Rallis E, Kirsten L, et al. Ustekinumab for the treatment of HIV psoriasis. J Dermatolog Treat. 2012;23:398-399.
- Kaushik SB, Lebwohl MG. Psoriasis: which therapy for which patient: focus on special populations and chronic infections [published online July 11, 2018]. J Am Acad Dermatol. 2019;80:43-53.
Practice Point
- Apremilast may be considered as a first-line therapy in the human immunodeficiency virus population due to decreased immunosuppression.
Scalp Psoriasis With Increased Hair Density
Case Report
A 19-year-old man first presented to our outpatient dermatology clinic for evaluation of a rash on the elbows and knees of 2 to 3 months’ duration. The lesions were asymptomatic. A review of symptoms including joint pain was largely negative. His medical history was remarkable for terminal ileitis, Crohn disease, anal fissure, rhabdomyolysis, and viral gastroenteritis. Physical examination revealed a well-nourished man with red, scaly, indurated papules and plaques involving approximately 0.5% of the body surface area. A diagnosis of plaque psoriasis was made, and he was treated with topical corticosteroids for 2 weeks and as needed thereafter.
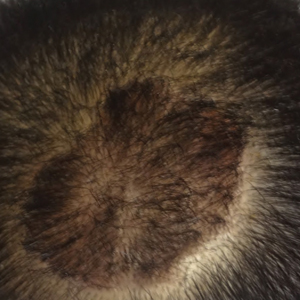
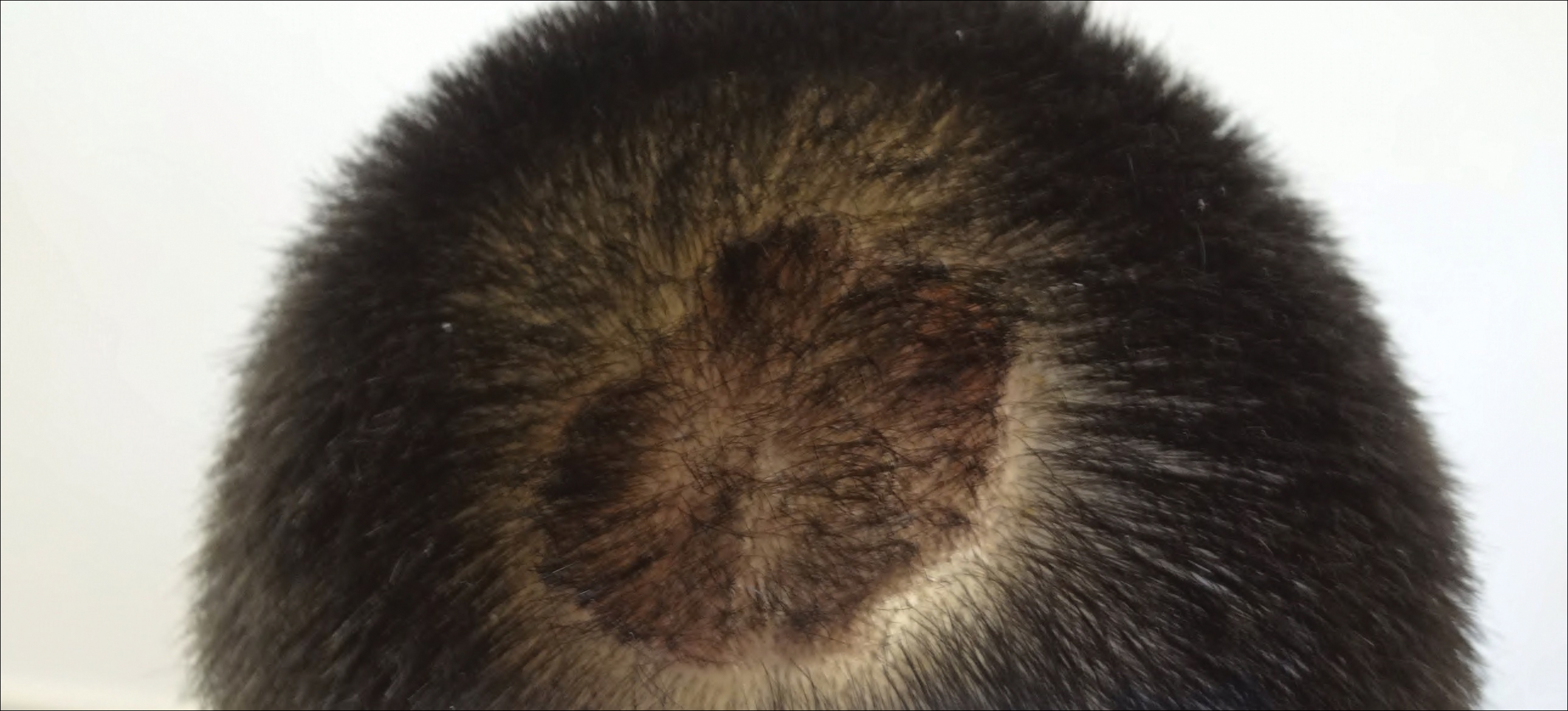
The patient remained stable for 5 years before presenting again to the dermatology clinic for psoriasis that had now spread to the scalp. Clinical examination revealed a very thin, faintly erythematous, scaly patch associated with increased hair density of the right frontal and parietal scalp (Figure). The patient denied any trauma or injury to the area or application of hair dye. We prescribed clobetasol solution 0.05% twice daily to the affected area of the scalp for 2 weeks, which resulted in minimal resolution of the psoriatic scalp lesion.
Comment
The scalp is a site of predilection in psoriasis, as approximately 80% of psoriasis patients report involvement of the scalp.1 Scalp involvement can dramatically affect a patient’s quality of life and often poses considerable therapeutic challenges for dermatologists.1 Alopecia in the setting of scalp psoriasis is common but is not well understood.2 First described by Shuster3 in 1972, psoriatic alopecia is associated with diminished hair density, follicular miniaturization, sebaceous gland atrophy, and an increased number of dystrophic bulbs in psoriatic plaques.4 It clinically presents as pink scaly plaques consistent with psoriasis with overlying alopecia. There are few instances of psoriatic alopecia reported as cicatricial hair loss and generalized telogen effluvium.2 It is known that a higher proportion of telogen and catagen hairs exist in patients with psoriatic alopecia.5 Additionally, psoriasis patients have more dystrophic hairs in affected and unaffected skin despite no differences in skin when compared to unaffected patients. Many patients achieve hair regrowth following treatment of psoriasis.2
We described a patient with scalp psoriasis who had increased and preserved hair density. Our case suggests that while most patients with scalp psoriasis experience psoriatic alopecia of the lesional skin, some may unconventionally experience increased hair density, which is contradictory to propositions that the friction associated with the application of topical treatments results in breakage of telogen hairs.2 Additionally, the presence of increased hair density in scalp psoriasis can further complicate antipsoriatic treatment by making the scalp inaccessible and topical therapies even more difficult to apply.
- Krueger G, Koo J, Lebwohl M, et al. The impact of psoriasis on quality of life: results of a 1998 National Psoriasis Foundation patient-membership survey. Arch Dermatol. 2001;137:280-284.
- George SM, Taylor MR, Farrant PB. Psoriatic alopecia. Clin Exp Dermatol. 2015;40:717-721.
- Shuster S. Psoriatic alopecia. Br J Dermatol. 1972;87:73-77.
- Wyatt E, Bottoms E, Comaish S. Abnormal hair shafts in psoriasis on scanning electron microscopy. Br J Dermatol. 1972;87:368-373.
- Schoorl WJ, van Baar HJ, van de Kerkhof PC. The hair root pattern in psoriasis of the scalp. Acta Derm Venereol. 1992;72:141-142.
Case Report
A 19-year-old man first presented to our outpatient dermatology clinic for evaluation of a rash on the elbows and knees of 2 to 3 months’ duration. The lesions were asymptomatic. A review of symptoms including joint pain was largely negative. His medical history was remarkable for terminal ileitis, Crohn disease, anal fissure, rhabdomyolysis, and viral gastroenteritis. Physical examination revealed a well-nourished man with red, scaly, indurated papules and plaques involving approximately 0.5% of the body surface area. A diagnosis of plaque psoriasis was made, and he was treated with topical corticosteroids for 2 weeks and as needed thereafter.
The patient remained stable for 5 years before presenting again to the dermatology clinic for psoriasis that had now spread to the scalp. Clinical examination revealed a very thin, faintly erythematous, scaly patch associated with increased hair density of the right frontal and parietal scalp (Figure). The patient denied any trauma or injury to the area or application of hair dye. We prescribed clobetasol solution 0.05% twice daily to the affected area of the scalp for 2 weeks, which resulted in minimal resolution of the psoriatic scalp lesion.

Comment
The scalp is a site of predilection in psoriasis, as approximately 80% of psoriasis patients report involvement of the scalp.1 Scalp involvement can dramatically affect a patient’s quality of life and often poses considerable therapeutic challenges for dermatologists.1 Alopecia in the setting of scalp psoriasis is common but is not well understood.2 First described by Shuster3 in 1972, psoriatic alopecia is associated with diminished hair density, follicular miniaturization, sebaceous gland atrophy, and an increased number of dystrophic bulbs in psoriatic plaques.4 It clinically presents as pink scaly plaques consistent with psoriasis with overlying alopecia. There are few instances of psoriatic alopecia reported as cicatricial hair loss and generalized telogen effluvium.2 It is known that a higher proportion of telogen and catagen hairs exist in patients with psoriatic alopecia.5 Additionally, psoriasis patients have more dystrophic hairs in affected and unaffected skin despite no differences in skin when compared to unaffected patients. Many patients achieve hair regrowth following treatment of psoriasis.2
We described a patient with scalp psoriasis who had increased and preserved hair density. Our case suggests that while most patients with scalp psoriasis experience psoriatic alopecia of the lesional skin, some may unconventionally experience increased hair density, which is contradictory to propositions that the friction associated with the application of topical treatments results in breakage of telogen hairs.2 Additionally, the presence of increased hair density in scalp psoriasis can further complicate antipsoriatic treatment by making the scalp inaccessible and topical therapies even more difficult to apply.
Case Report
A 19-year-old man first presented to our outpatient dermatology clinic for evaluation of a rash on the elbows and knees of 2 to 3 months’ duration. The lesions were asymptomatic. A review of symptoms including joint pain was largely negative. His medical history was remarkable for terminal ileitis, Crohn disease, anal fissure, rhabdomyolysis, and viral gastroenteritis. Physical examination revealed a well-nourished man with red, scaly, indurated papules and plaques involving approximately 0.5% of the body surface area. A diagnosis of plaque psoriasis was made, and he was treated with topical corticosteroids for 2 weeks and as needed thereafter.
The patient remained stable for 5 years before presenting again to the dermatology clinic for psoriasis that had now spread to the scalp. Clinical examination revealed a very thin, faintly erythematous, scaly patch associated with increased hair density of the right frontal and parietal scalp (Figure). The patient denied any trauma or injury to the area or application of hair dye. We prescribed clobetasol solution 0.05% twice daily to the affected area of the scalp for 2 weeks, which resulted in minimal resolution of the psoriatic scalp lesion.

Comment
The scalp is a site of predilection in psoriasis, as approximately 80% of psoriasis patients report involvement of the scalp.1 Scalp involvement can dramatically affect a patient’s quality of life and often poses considerable therapeutic challenges for dermatologists.1 Alopecia in the setting of scalp psoriasis is common but is not well understood.2 First described by Shuster3 in 1972, psoriatic alopecia is associated with diminished hair density, follicular miniaturization, sebaceous gland atrophy, and an increased number of dystrophic bulbs in psoriatic plaques.4 It clinically presents as pink scaly plaques consistent with psoriasis with overlying alopecia. There are few instances of psoriatic alopecia reported as cicatricial hair loss and generalized telogen effluvium.2 It is known that a higher proportion of telogen and catagen hairs exist in patients with psoriatic alopecia.5 Additionally, psoriasis patients have more dystrophic hairs in affected and unaffected skin despite no differences in skin when compared to unaffected patients. Many patients achieve hair regrowth following treatment of psoriasis.2
We described a patient with scalp psoriasis who had increased and preserved hair density. Our case suggests that while most patients with scalp psoriasis experience psoriatic alopecia of the lesional skin, some may unconventionally experience increased hair density, which is contradictory to propositions that the friction associated with the application of topical treatments results in breakage of telogen hairs.2 Additionally, the presence of increased hair density in scalp psoriasis can further complicate antipsoriatic treatment by making the scalp inaccessible and topical therapies even more difficult to apply.
- Krueger G, Koo J, Lebwohl M, et al. The impact of psoriasis on quality of life: results of a 1998 National Psoriasis Foundation patient-membership survey. Arch Dermatol. 2001;137:280-284.
- George SM, Taylor MR, Farrant PB. Psoriatic alopecia. Clin Exp Dermatol. 2015;40:717-721.
- Shuster S. Psoriatic alopecia. Br J Dermatol. 1972;87:73-77.
- Wyatt E, Bottoms E, Comaish S. Abnormal hair shafts in psoriasis on scanning electron microscopy. Br J Dermatol. 1972;87:368-373.
- Schoorl WJ, van Baar HJ, van de Kerkhof PC. The hair root pattern in psoriasis of the scalp. Acta Derm Venereol. 1992;72:141-142.
- Krueger G, Koo J, Lebwohl M, et al. The impact of psoriasis on quality of life: results of a 1998 National Psoriasis Foundation patient-membership survey. Arch Dermatol. 2001;137:280-284.
- George SM, Taylor MR, Farrant PB. Psoriatic alopecia. Clin Exp Dermatol. 2015;40:717-721.
- Shuster S. Psoriatic alopecia. Br J Dermatol. 1972;87:73-77.
- Wyatt E, Bottoms E, Comaish S. Abnormal hair shafts in psoriasis on scanning electron microscopy. Br J Dermatol. 1972;87:368-373.
- Schoorl WJ, van Baar HJ, van de Kerkhof PC. The hair root pattern in psoriasis of the scalp. Acta Derm Venereol. 1992;72:141-142.
Practice Points
- Scalp psoriasis may present with hair loss or increased hair density.
- Psoriasis with increased hair density may make topical medications more difficult to apply.