User login
Neurostimulation May Work When Migraine Drugs Fail
LOS ANGELES – Occipital nerve stimulation provided relief from chronic migraine in a randomized, double-blind trial of patients who had not responded to currently available pharmacologic therapies.
The 12-week trial of 125 patients found that patients who received active neurostimulation were about four times more likely to rate their pain relief as excellent or fair than were those who received sham neurostimulation. The actively-stimulated patients also had more than double the reduction in number of days with headache each month.
"This is the first large-scale study of peripheral nerve stimulation for intractable chronic migraine that showed a significant reduction in pain, number of headache-days, and migraine-related disability," said lead investigator Dr. Stephen D. Silberstein of the Jefferson Headache Center in Philadelphia.
"These results suggest that patients who have exhausted all treatment options may benefit from peripheral nerve stimulation of the occipital nerves," he added. "These results provide the strongest evidence to date to support the safety and efficacy of peripheral nerve stimulation for intractable chronic migraine."
Session attendee Dr. Peter Goadsby of the University of California, San Francisco, questioned whether the trial was truly blinded, given the challenge of concealing group assignment when using neurostimulation. "It is very difficult. ... Much of the blinding in these studies is compromised," he said at the annual meeting of the American Headache Society. "I think it’s not fair to say this is the strongest evidence for peripheral nerve, occipital nerve stimulation. I think it’s another piece of evidence, but I don’t think that we have hit a home run here. ... I don’t think this is prime time yet."
"I’m not disagreeing with you," Dr. Silberstein replied. "I am saying of all the trials, in the subset of patients we defined as intractable chronic migraine, this is the best evidence so far. ... How do you blind brain stimulation trials? That’s a fundamental problem," he acknowledged, noting that patients in the trial were told that they were receiving neurostimulation with different parameters. "It’s very difficult and very complicated to control with any active stimulation device. We tried the best we could."
In the trial, the investigators enrolled patients with chronic, intractable migraines who had headache at least 15 days a month, had experienced a failure of at least three preventive medications, and had moderate headache-related disability. Their pain had to be at least 6 cm out of 10 cm on a visual analog scale and localized to the posterior head or originating from the cervical region.
Patients were not required to have responded previously to an occipital nerve block, Dr. Silberstein noted, explaining that "there is no evidence at all that occipital nerve block is predictive of the response to stimulation." Patients who overused medication were included, but those taking opioids were not.
All patients underwent implantation of St. Jude Medical’s neurostimulation system and were randomized to the neurostimulation group (n = 88) or the control group (n = 37).
Results showed that about 70% of patients in the neurostimulation group reported excellent or fair pain relief at 12 weeks, compared with only 20% of their counterparts in the control group (P = .001).
Patients who received active neurostimulation were also more likely to achieve a reduction in mean daily pain intensity on the visual analogue scale of 10%, 20%, or 30% (P less than .05 for each). "Looking at 40%, 50%, and 60% differences in this particular trial, they were not statistically significant differences, but again, remember, this was a disabling migraine population that had failed three therapies," Dr. Silberstein commented.
The neurostimulation group also had a greater mean reduction in monthly number of headache-days (7.0 vs. 2.7 days; P = .03) and were more likely to have a 10%, 20%, 30%, and 40% reduction in the number (P less than .05 for each).
In terms of headache-related disability, patients in the neurostimulation group also had a greater reduction in MIDAS (Migraine Disability Assessment) scores (a drop of 73 points vs. a drop of 27 points; P = .001) and in Zung Pain and Distress Scale scores (by 15 vs. 6 points, P = .001).
The overall rate of adverse events was about 61% in the neurostimulation group and 49% in the control group.
The leading hardware-related adverse event was lead migration, accounting for 15% of all events, with a higher rate in the neurostimulation group. "If you have a device in and it’s actively stimulating and you are getting pain relief, and then the pain relief ceases, it’s easier to identify the fact that you have had lead migration," Dr. Silberstein explained, adding that lead migration has become much less problematic as surgical techniques have improved.
The leading biological adverse event was persistent pain and/or numbness at the implantable pulse generator or lead site, which accounted for 22% of all events.
Dr. Silberstein disclosed that he receives consulting fees/honoraria and research support from St. Jude Medical Neuromodulation Division, which funded the trial.
neurostimulation, pain relief, chronic migraine, Dr. Stephen D. Silberstein, Jefferson Headache Center in Philadelphia,
LOS ANGELES – Occipital nerve stimulation provided relief from chronic migraine in a randomized, double-blind trial of patients who had not responded to currently available pharmacologic therapies.
The 12-week trial of 125 patients found that patients who received active neurostimulation were about four times more likely to rate their pain relief as excellent or fair than were those who received sham neurostimulation. The actively-stimulated patients also had more than double the reduction in number of days with headache each month.
"This is the first large-scale study of peripheral nerve stimulation for intractable chronic migraine that showed a significant reduction in pain, number of headache-days, and migraine-related disability," said lead investigator Dr. Stephen D. Silberstein of the Jefferson Headache Center in Philadelphia.
"These results suggest that patients who have exhausted all treatment options may benefit from peripheral nerve stimulation of the occipital nerves," he added. "These results provide the strongest evidence to date to support the safety and efficacy of peripheral nerve stimulation for intractable chronic migraine."
Session attendee Dr. Peter Goadsby of the University of California, San Francisco, questioned whether the trial was truly blinded, given the challenge of concealing group assignment when using neurostimulation. "It is very difficult. ... Much of the blinding in these studies is compromised," he said at the annual meeting of the American Headache Society. "I think it’s not fair to say this is the strongest evidence for peripheral nerve, occipital nerve stimulation. I think it’s another piece of evidence, but I don’t think that we have hit a home run here. ... I don’t think this is prime time yet."
"I’m not disagreeing with you," Dr. Silberstein replied. "I am saying of all the trials, in the subset of patients we defined as intractable chronic migraine, this is the best evidence so far. ... How do you blind brain stimulation trials? That’s a fundamental problem," he acknowledged, noting that patients in the trial were told that they were receiving neurostimulation with different parameters. "It’s very difficult and very complicated to control with any active stimulation device. We tried the best we could."
In the trial, the investigators enrolled patients with chronic, intractable migraines who had headache at least 15 days a month, had experienced a failure of at least three preventive medications, and had moderate headache-related disability. Their pain had to be at least 6 cm out of 10 cm on a visual analog scale and localized to the posterior head or originating from the cervical region.
Patients were not required to have responded previously to an occipital nerve block, Dr. Silberstein noted, explaining that "there is no evidence at all that occipital nerve block is predictive of the response to stimulation." Patients who overused medication were included, but those taking opioids were not.
All patients underwent implantation of St. Jude Medical’s neurostimulation system and were randomized to the neurostimulation group (n = 88) or the control group (n = 37).
Results showed that about 70% of patients in the neurostimulation group reported excellent or fair pain relief at 12 weeks, compared with only 20% of their counterparts in the control group (P = .001).
Patients who received active neurostimulation were also more likely to achieve a reduction in mean daily pain intensity on the visual analogue scale of 10%, 20%, or 30% (P less than .05 for each). "Looking at 40%, 50%, and 60% differences in this particular trial, they were not statistically significant differences, but again, remember, this was a disabling migraine population that had failed three therapies," Dr. Silberstein commented.
The neurostimulation group also had a greater mean reduction in monthly number of headache-days (7.0 vs. 2.7 days; P = .03) and were more likely to have a 10%, 20%, 30%, and 40% reduction in the number (P less than .05 for each).
In terms of headache-related disability, patients in the neurostimulation group also had a greater reduction in MIDAS (Migraine Disability Assessment) scores (a drop of 73 points vs. a drop of 27 points; P = .001) and in Zung Pain and Distress Scale scores (by 15 vs. 6 points, P = .001).
The overall rate of adverse events was about 61% in the neurostimulation group and 49% in the control group.
The leading hardware-related adverse event was lead migration, accounting for 15% of all events, with a higher rate in the neurostimulation group. "If you have a device in and it’s actively stimulating and you are getting pain relief, and then the pain relief ceases, it’s easier to identify the fact that you have had lead migration," Dr. Silberstein explained, adding that lead migration has become much less problematic as surgical techniques have improved.
The leading biological adverse event was persistent pain and/or numbness at the implantable pulse generator or lead site, which accounted for 22% of all events.
Dr. Silberstein disclosed that he receives consulting fees/honoraria and research support from St. Jude Medical Neuromodulation Division, which funded the trial.
LOS ANGELES – Occipital nerve stimulation provided relief from chronic migraine in a randomized, double-blind trial of patients who had not responded to currently available pharmacologic therapies.
The 12-week trial of 125 patients found that patients who received active neurostimulation were about four times more likely to rate their pain relief as excellent or fair than were those who received sham neurostimulation. The actively-stimulated patients also had more than double the reduction in number of days with headache each month.
"This is the first large-scale study of peripheral nerve stimulation for intractable chronic migraine that showed a significant reduction in pain, number of headache-days, and migraine-related disability," said lead investigator Dr. Stephen D. Silberstein of the Jefferson Headache Center in Philadelphia.
"These results suggest that patients who have exhausted all treatment options may benefit from peripheral nerve stimulation of the occipital nerves," he added. "These results provide the strongest evidence to date to support the safety and efficacy of peripheral nerve stimulation for intractable chronic migraine."
Session attendee Dr. Peter Goadsby of the University of California, San Francisco, questioned whether the trial was truly blinded, given the challenge of concealing group assignment when using neurostimulation. "It is very difficult. ... Much of the blinding in these studies is compromised," he said at the annual meeting of the American Headache Society. "I think it’s not fair to say this is the strongest evidence for peripheral nerve, occipital nerve stimulation. I think it’s another piece of evidence, but I don’t think that we have hit a home run here. ... I don’t think this is prime time yet."
"I’m not disagreeing with you," Dr. Silberstein replied. "I am saying of all the trials, in the subset of patients we defined as intractable chronic migraine, this is the best evidence so far. ... How do you blind brain stimulation trials? That’s a fundamental problem," he acknowledged, noting that patients in the trial were told that they were receiving neurostimulation with different parameters. "It’s very difficult and very complicated to control with any active stimulation device. We tried the best we could."
In the trial, the investigators enrolled patients with chronic, intractable migraines who had headache at least 15 days a month, had experienced a failure of at least three preventive medications, and had moderate headache-related disability. Their pain had to be at least 6 cm out of 10 cm on a visual analog scale and localized to the posterior head or originating from the cervical region.
Patients were not required to have responded previously to an occipital nerve block, Dr. Silberstein noted, explaining that "there is no evidence at all that occipital nerve block is predictive of the response to stimulation." Patients who overused medication were included, but those taking opioids were not.
All patients underwent implantation of St. Jude Medical’s neurostimulation system and were randomized to the neurostimulation group (n = 88) or the control group (n = 37).
Results showed that about 70% of patients in the neurostimulation group reported excellent or fair pain relief at 12 weeks, compared with only 20% of their counterparts in the control group (P = .001).
Patients who received active neurostimulation were also more likely to achieve a reduction in mean daily pain intensity on the visual analogue scale of 10%, 20%, or 30% (P less than .05 for each). "Looking at 40%, 50%, and 60% differences in this particular trial, they were not statistically significant differences, but again, remember, this was a disabling migraine population that had failed three therapies," Dr. Silberstein commented.
The neurostimulation group also had a greater mean reduction in monthly number of headache-days (7.0 vs. 2.7 days; P = .03) and were more likely to have a 10%, 20%, 30%, and 40% reduction in the number (P less than .05 for each).
In terms of headache-related disability, patients in the neurostimulation group also had a greater reduction in MIDAS (Migraine Disability Assessment) scores (a drop of 73 points vs. a drop of 27 points; P = .001) and in Zung Pain and Distress Scale scores (by 15 vs. 6 points, P = .001).
The overall rate of adverse events was about 61% in the neurostimulation group and 49% in the control group.
The leading hardware-related adverse event was lead migration, accounting for 15% of all events, with a higher rate in the neurostimulation group. "If you have a device in and it’s actively stimulating and you are getting pain relief, and then the pain relief ceases, it’s easier to identify the fact that you have had lead migration," Dr. Silberstein explained, adding that lead migration has become much less problematic as surgical techniques have improved.
The leading biological adverse event was persistent pain and/or numbness at the implantable pulse generator or lead site, which accounted for 22% of all events.
Dr. Silberstein disclosed that he receives consulting fees/honoraria and research support from St. Jude Medical Neuromodulation Division, which funded the trial.
neurostimulation, pain relief, chronic migraine, Dr. Stephen D. Silberstein, Jefferson Headache Center in Philadelphia,
neurostimulation, pain relief, chronic migraine, Dr. Stephen D. Silberstein, Jefferson Headache Center in Philadelphia,
AT THE ANNUAL MEETING OF THE AMERICAN HEADACHE SOCIETY
Major Finding: About 70% of patients in the neurostimulation group reported excellent or fair pain relief at 12 weeks, compared with only 20% of their counterparts in the control group (P = .001).
Data Source: This was a multicenter, randomized, double-blind trial of 125 patients with intractable chronic migraine.
Disclosures: Dr. Silberstein disclosed that he receives consulting fees/honoraria and research support from St. Jude Medical Neuromodulation Division, which funded the trial.
Olaparib Defers Progression of Serous Ovarian Cancer - Again
CHICAGO – Olaparib, the novel PARP inhibitor that came up short of a survival advantage in a much-watched trial in ovarian cancer, is again showing positive results in that disease.
Olaparib was effective and well tolerated as an adjunctive targeted therapy in women with platinum-sensitive advanced serous ovarian cancer, regardless of BRCA mutational status, according to new results from a different randomized, open-label phase II trial.
Among the 162 women studied, those treated with olaparib – an oral investigational inhibitor of PARP, or poly(ADP-ribose) polymerase – along with chemotherapy and also as maintenance therapy had a nearly one-half reduction in the risk of progression or death relative to their counterparts treated with chemotherapy alone and no maintenance therapy.
The absolute difference in the risk of events was about 3 months, investigators reported at the annual meeting of the American Society of Clinical Oncology.
"There is an active biomarker analysis program to analyze tumor tissue, and the analysis of samples from this study will be combined with the samples from [a monotherapy maintenance study] to look at potential predictive biomarkers for olaparib efficacy," noted first author Dr. Amit M. Oza of Princess Margaret Hospital in Toronto.
"Olaparib clinical development continues, and the immediate aims are to determine the optimal patient population and to find an acceptable tablet dose and schedule for long-term treatment, to enable the next generation of clinical studies," he added.
Dr. Michael V. Seiden of the Fox Chase Cancer Center in Philadelphia cited the earlier study of olaparib maintenance, which showed improved progression-free survival but, at least as of an interim analysis, not overall survival in his invited discussion of the current study (N. Engl. J. Med. 2012;366:1382-92).
"If we as a community are going to bend the survival curves of ovarian cancer upward, we are going to need to find a therapeutic strategy that works in advanced serous carcinoma," he said. Although the study was "positive ... perhaps a bit provocatively, I am going to argue that I suspect this study will ultimately be negative – negative not in that it didn’t meet its therapeutic end point, but in that it will fail to bend the ovarian cancer survival curve upward."
Serous ovarian cancers have extremely diverse genomic aberrations, so it is unlikely that there are large groups of patients with similar tumors, according to Dr. Seiden.
"The genomics of serous carcinoma argue against phase III studies at least of what I would argue are molecularly targeted agents. Bringing new drugs to our patients will require new strategies in clinical trial design, new strategies in genomic stratification, and new strategies in FDA approval," he maintained.
The women studied in the trial were 60 years old on average, Dr. Oza reported at the meeting. In 80% of cases, the cancer’s status regarding mutations of the BRCA genes (which have been associated with greater PARP inhibitor efficacy in some cancers) was unknown. The majority of women had received only a single platinum-containing regimen.
The investigators assigned the women evenly to chemotherapy (paclitaxel plus carboplatin) with concurrent olaparib, followed by olaparib maintenance, or to chemotherapy alone followed by no maintenance therapy. The carboplatin dose was one-third lower in the group given olaparib. "In this study, we used the capsule formulation of olaparib," he noted.
Study results showed that median progression-free survival was 12.2 months with olaparib and 9.6 months without it (hazard ratio, 0.51; P = .001).
"The curves begin to separate at 6 months and after that. So there was no separation of the two curves during the concurrent portion of the treatment," Dr. Oza pointed out.
"There were no specific subgroups that did not seem to benefit from the addition of olaparib," he continued, although benefit was somewhat greater in women who had a progression-free interval exceeding 12 months before the trial as compared with a shorter duration.
Immature data for overall survival showed a rate of about 15% with olaparib and 14% without it. There were no significant differences between the groups in terms of response rate whether assessed with RECIST criteria (64% vs. 58%) or cancer antigen 125 (CA-125) levels (86% vs. 74%).
Women known to have BRCA mutations appeared to be scattered evenly throughout a waterfall plot showing the degree of response (or lack thereof), Dr. Oza noted.
The combination regimen "was well tolerated and deliverable," with similar rates of dose modifications and completion of chemotherapy," he said. During the concurrent phase of treatment, "the rates of grade 3 or worse neutropenia and thrombocytopenia are not dramatically different between the two arms, which is reassuring."
During the maintenance phase of treatment, the overall rate of grade 3 or worse adverse events was 29% in the olaparib group and 16% in the control group, and "there were no additional safety concerns or safety signals that emerged related to toxicity of olaparib."
Dr. Oza disclosed that he receives research funding from AstraZeneca. Dr. Seiden disclosed no relevant conflicts of interest.
CHICAGO – Olaparib, the novel PARP inhibitor that came up short of a survival advantage in a much-watched trial in ovarian cancer, is again showing positive results in that disease.
Olaparib was effective and well tolerated as an adjunctive targeted therapy in women with platinum-sensitive advanced serous ovarian cancer, regardless of BRCA mutational status, according to new results from a different randomized, open-label phase II trial.
Among the 162 women studied, those treated with olaparib – an oral investigational inhibitor of PARP, or poly(ADP-ribose) polymerase – along with chemotherapy and also as maintenance therapy had a nearly one-half reduction in the risk of progression or death relative to their counterparts treated with chemotherapy alone and no maintenance therapy.
The absolute difference in the risk of events was about 3 months, investigators reported at the annual meeting of the American Society of Clinical Oncology.
"There is an active biomarker analysis program to analyze tumor tissue, and the analysis of samples from this study will be combined with the samples from [a monotherapy maintenance study] to look at potential predictive biomarkers for olaparib efficacy," noted first author Dr. Amit M. Oza of Princess Margaret Hospital in Toronto.
"Olaparib clinical development continues, and the immediate aims are to determine the optimal patient population and to find an acceptable tablet dose and schedule for long-term treatment, to enable the next generation of clinical studies," he added.
Dr. Michael V. Seiden of the Fox Chase Cancer Center in Philadelphia cited the earlier study of olaparib maintenance, which showed improved progression-free survival but, at least as of an interim analysis, not overall survival in his invited discussion of the current study (N. Engl. J. Med. 2012;366:1382-92).
"If we as a community are going to bend the survival curves of ovarian cancer upward, we are going to need to find a therapeutic strategy that works in advanced serous carcinoma," he said. Although the study was "positive ... perhaps a bit provocatively, I am going to argue that I suspect this study will ultimately be negative – negative not in that it didn’t meet its therapeutic end point, but in that it will fail to bend the ovarian cancer survival curve upward."
Serous ovarian cancers have extremely diverse genomic aberrations, so it is unlikely that there are large groups of patients with similar tumors, according to Dr. Seiden.
"The genomics of serous carcinoma argue against phase III studies at least of what I would argue are molecularly targeted agents. Bringing new drugs to our patients will require new strategies in clinical trial design, new strategies in genomic stratification, and new strategies in FDA approval," he maintained.
The women studied in the trial were 60 years old on average, Dr. Oza reported at the meeting. In 80% of cases, the cancer’s status regarding mutations of the BRCA genes (which have been associated with greater PARP inhibitor efficacy in some cancers) was unknown. The majority of women had received only a single platinum-containing regimen.
The investigators assigned the women evenly to chemotherapy (paclitaxel plus carboplatin) with concurrent olaparib, followed by olaparib maintenance, or to chemotherapy alone followed by no maintenance therapy. The carboplatin dose was one-third lower in the group given olaparib. "In this study, we used the capsule formulation of olaparib," he noted.
Study results showed that median progression-free survival was 12.2 months with olaparib and 9.6 months without it (hazard ratio, 0.51; P = .001).
"The curves begin to separate at 6 months and after that. So there was no separation of the two curves during the concurrent portion of the treatment," Dr. Oza pointed out.
"There were no specific subgroups that did not seem to benefit from the addition of olaparib," he continued, although benefit was somewhat greater in women who had a progression-free interval exceeding 12 months before the trial as compared with a shorter duration.
Immature data for overall survival showed a rate of about 15% with olaparib and 14% without it. There were no significant differences between the groups in terms of response rate whether assessed with RECIST criteria (64% vs. 58%) or cancer antigen 125 (CA-125) levels (86% vs. 74%).
Women known to have BRCA mutations appeared to be scattered evenly throughout a waterfall plot showing the degree of response (or lack thereof), Dr. Oza noted.
The combination regimen "was well tolerated and deliverable," with similar rates of dose modifications and completion of chemotherapy," he said. During the concurrent phase of treatment, "the rates of grade 3 or worse neutropenia and thrombocytopenia are not dramatically different between the two arms, which is reassuring."
During the maintenance phase of treatment, the overall rate of grade 3 or worse adverse events was 29% in the olaparib group and 16% in the control group, and "there were no additional safety concerns or safety signals that emerged related to toxicity of olaparib."
Dr. Oza disclosed that he receives research funding from AstraZeneca. Dr. Seiden disclosed no relevant conflicts of interest.
CHICAGO – Olaparib, the novel PARP inhibitor that came up short of a survival advantage in a much-watched trial in ovarian cancer, is again showing positive results in that disease.
Olaparib was effective and well tolerated as an adjunctive targeted therapy in women with platinum-sensitive advanced serous ovarian cancer, regardless of BRCA mutational status, according to new results from a different randomized, open-label phase II trial.
Among the 162 women studied, those treated with olaparib – an oral investigational inhibitor of PARP, or poly(ADP-ribose) polymerase – along with chemotherapy and also as maintenance therapy had a nearly one-half reduction in the risk of progression or death relative to their counterparts treated with chemotherapy alone and no maintenance therapy.
The absolute difference in the risk of events was about 3 months, investigators reported at the annual meeting of the American Society of Clinical Oncology.
"There is an active biomarker analysis program to analyze tumor tissue, and the analysis of samples from this study will be combined with the samples from [a monotherapy maintenance study] to look at potential predictive biomarkers for olaparib efficacy," noted first author Dr. Amit M. Oza of Princess Margaret Hospital in Toronto.
"Olaparib clinical development continues, and the immediate aims are to determine the optimal patient population and to find an acceptable tablet dose and schedule for long-term treatment, to enable the next generation of clinical studies," he added.
Dr. Michael V. Seiden of the Fox Chase Cancer Center in Philadelphia cited the earlier study of olaparib maintenance, which showed improved progression-free survival but, at least as of an interim analysis, not overall survival in his invited discussion of the current study (N. Engl. J. Med. 2012;366:1382-92).
"If we as a community are going to bend the survival curves of ovarian cancer upward, we are going to need to find a therapeutic strategy that works in advanced serous carcinoma," he said. Although the study was "positive ... perhaps a bit provocatively, I am going to argue that I suspect this study will ultimately be negative – negative not in that it didn’t meet its therapeutic end point, but in that it will fail to bend the ovarian cancer survival curve upward."
Serous ovarian cancers have extremely diverse genomic aberrations, so it is unlikely that there are large groups of patients with similar tumors, according to Dr. Seiden.
"The genomics of serous carcinoma argue against phase III studies at least of what I would argue are molecularly targeted agents. Bringing new drugs to our patients will require new strategies in clinical trial design, new strategies in genomic stratification, and new strategies in FDA approval," he maintained.
The women studied in the trial were 60 years old on average, Dr. Oza reported at the meeting. In 80% of cases, the cancer’s status regarding mutations of the BRCA genes (which have been associated with greater PARP inhibitor efficacy in some cancers) was unknown. The majority of women had received only a single platinum-containing regimen.
The investigators assigned the women evenly to chemotherapy (paclitaxel plus carboplatin) with concurrent olaparib, followed by olaparib maintenance, or to chemotherapy alone followed by no maintenance therapy. The carboplatin dose was one-third lower in the group given olaparib. "In this study, we used the capsule formulation of olaparib," he noted.
Study results showed that median progression-free survival was 12.2 months with olaparib and 9.6 months without it (hazard ratio, 0.51; P = .001).
"The curves begin to separate at 6 months and after that. So there was no separation of the two curves during the concurrent portion of the treatment," Dr. Oza pointed out.
"There were no specific subgroups that did not seem to benefit from the addition of olaparib," he continued, although benefit was somewhat greater in women who had a progression-free interval exceeding 12 months before the trial as compared with a shorter duration.
Immature data for overall survival showed a rate of about 15% with olaparib and 14% without it. There were no significant differences between the groups in terms of response rate whether assessed with RECIST criteria (64% vs. 58%) or cancer antigen 125 (CA-125) levels (86% vs. 74%).
Women known to have BRCA mutations appeared to be scattered evenly throughout a waterfall plot showing the degree of response (or lack thereof), Dr. Oza noted.
The combination regimen "was well tolerated and deliverable," with similar rates of dose modifications and completion of chemotherapy," he said. During the concurrent phase of treatment, "the rates of grade 3 or worse neutropenia and thrombocytopenia are not dramatically different between the two arms, which is reassuring."
During the maintenance phase of treatment, the overall rate of grade 3 or worse adverse events was 29% in the olaparib group and 16% in the control group, and "there were no additional safety concerns or safety signals that emerged related to toxicity of olaparib."
Dr. Oza disclosed that he receives research funding from AstraZeneca. Dr. Seiden disclosed no relevant conflicts of interest.
AT THE ANNUAL MEETING OF THE AMERICAN SOCIETY OF CLINICAL ONCOLOGY
Major Finding: Addition of olaparib concurrently to paclitaxel-carboplatin chemotherapy and as maintenance therapy reduced the risk of progression or death by a relative 49%.
Data Source: Investigators conducted a randomized, open-label phase II trial among 162 women with platinum-sensitive advanced serous ovarian cancer.
Disclosures: Dr. Oza disclosed that he receives research funding from AstraZeneca, which sponsored the trial. Dr. Seiden disclosed no relevant conflicts of interest.
Vemurafenib's Efficacy in Melanoma Wanes Over Time
CHICAGO – Vemurafenib has a persistent survival benefit when given as first-line therapy for advanced BRAF-mutant melanoma, but the magnitude of benefit diminishes over time, according to an update of the BRIM-3 randomized trial.
With a median follow-up of about a year, vemurafenib was associated with a 30% relative reduction in the risk of death, relative to dacarbazine, presenting author Dr. Paul Chapman reported at the annual meeting of the American Society of Clinical Oncology. This is roughly half of the 63% relative risk reduction seen at a median follow-up of about 3 months, as reported at last year’s meeting.
The safety profile of vemurafenib was largely consistent with the earlier experience; the main high-grade adverse effects were skin toxicity (seen in about a third of patients) and liver toxicity (seen in about a tenth). But a few patients have developed second primary melanomas.
The observed temporal trend for overall survival benefit suggests "perhaps that most of the benefit in overall survival may be due to decreasing early deaths," said Dr. Chapman, an oncologist at Memorial Sloan-Kettering Cancer Center in New York.
Discussant Dr. Michael B. Atkins of the Lombardi Comprehensive Cancer Center at Georgetown University in Washington maintained that "the mature overall survival data [confirm] that vemurafenib is better than dacarbazine," and noted that it was "reassuring" that findings were similar, whether patients who crossed over to the vemurafenib arm were censored or not, "probably the most stringent test of survival."
However, he called attention to the fact that the median survival of 13-plus months with vemurafenib seen in BRIM-3 falls short of the 16-plus months seen in BRIM-2, a phase II trial in patients with previously treated advanced melanoma. "Did first-line patients have more aggressive disease and therefore fall off early?" he wondered. "Alternatively, was there less continuation of vemurafenib after progressive disease in the BRIM-3 trial relative to the BRIM-2 trial? If that was the case, some sort of confirmatory analysis should be done to see whether that can impact overall survival."
The decrease in vemurafenib efficacy seen over time might have several explanations, Dr. Atkins proposed. "Is it that the early, more robust hazard ratio was a manifestation of the so-called Lazarus effect, that these targeted therapies can cause patients who are really in bad shape and would have a short survival to have their survival prolonged, even though it was less than the survival of patients with less-aggressive tumors?" he wondered, echoing Dr. Chapman’s suggestion. Alternatively, maturation of the survival data might have contributed to the change in efficacy in patients with stage IIIC, M1a, and M1b disease who did not appear to benefit from vemurafenib.
In the larger context, the findings raise an important question pertaining to the clinical trials process generally, according to Dr. Atkins: "What does this say about the early analysis of trials like this, and reporting forever more that the hazard ratio is 0.35 or so, rather than what the more accurate 0.70 is?"
A Genentech spokesperson said that "due to differences in the study populations and trial designs, the results from these trials cannot be directly compared. BRIM3 was a global, randomized, Phase III trial comparing Zelboraf (vemurafenib) to DTIC chemotherapy in people with newly diagnosed BRAF V600 mutation-positive metastatic melanoma, while BRIM2 was a Phase II study that evaluated patients with previously treated disease."
The 675 patients in BRIM-3 had stage IIIC or IV melanoma harboring the BRAF V600E mutation. They were assigned evenly to intravenous dacarbazine or oral vemurafenib, the only targeted agent approved specifically for treating BRAF-mutant melanoma.
Large proportions of patients in both the vemurafenib and dacarbazine arms (36% and 44%, respectively) received other anticancer therapies after coming off the trial, Dr. Chapman reported. About a fifth of all patients in each arm went on to receive ipilimumab, and a fourth in the dacarbazine arm crossed over to vemurafenib.
Updated results – after a median follow-up of 9.5 months in the dacarbazine arm and 12.5 months in the vemurafenib arm – showed persistence of a progression-free survival benefit in favor of vemurafenib with censoring of patients at crossover (6.9 vs. 1.6 months; hazard ratio, 0.38; P less than .001).
There was also still an improvement in overall survival, regardless of whether patients were censored at crossover (13.6 vs. 9.7 months; HR, 0.70; P less than .001) or not (13.6 vs. 10.3 months; HR, 0.76; P less than .01).
The leading grade 3 or worse adverse events with vemurafenib remained cutaneous squamous cell carcinomas (seen in 19% of patients), keratoacanthomas (10%), and elevations of liver function tests (10%). In addition, eight patients developed new primary melanomas.
Dr. Chapman disclosed that he receives honoraria and research funding from Roche/Genentech. The trial was sponsored by Hoffman-LaRoche. Dr. Atkins disclosed that he is a consultant to Bristol-Myers Squibb, Celgene, Curetech, Genentech, Merck, Novartis, and Prometheus.
This article was updated on July 3, 2012.
CHICAGO – Vemurafenib has a persistent survival benefit when given as first-line therapy for advanced BRAF-mutant melanoma, but the magnitude of benefit diminishes over time, according to an update of the BRIM-3 randomized trial.
With a median follow-up of about a year, vemurafenib was associated with a 30% relative reduction in the risk of death, relative to dacarbazine, presenting author Dr. Paul Chapman reported at the annual meeting of the American Society of Clinical Oncology. This is roughly half of the 63% relative risk reduction seen at a median follow-up of about 3 months, as reported at last year’s meeting.
The safety profile of vemurafenib was largely consistent with the earlier experience; the main high-grade adverse effects were skin toxicity (seen in about a third of patients) and liver toxicity (seen in about a tenth). But a few patients have developed second primary melanomas.
The observed temporal trend for overall survival benefit suggests "perhaps that most of the benefit in overall survival may be due to decreasing early deaths," said Dr. Chapman, an oncologist at Memorial Sloan-Kettering Cancer Center in New York.
Discussant Dr. Michael B. Atkins of the Lombardi Comprehensive Cancer Center at Georgetown University in Washington maintained that "the mature overall survival data [confirm] that vemurafenib is better than dacarbazine," and noted that it was "reassuring" that findings were similar, whether patients who crossed over to the vemurafenib arm were censored or not, "probably the most stringent test of survival."
However, he called attention to the fact that the median survival of 13-plus months with vemurafenib seen in BRIM-3 falls short of the 16-plus months seen in BRIM-2, a phase II trial in patients with previously treated advanced melanoma. "Did first-line patients have more aggressive disease and therefore fall off early?" he wondered. "Alternatively, was there less continuation of vemurafenib after progressive disease in the BRIM-3 trial relative to the BRIM-2 trial? If that was the case, some sort of confirmatory analysis should be done to see whether that can impact overall survival."
The decrease in vemurafenib efficacy seen over time might have several explanations, Dr. Atkins proposed. "Is it that the early, more robust hazard ratio was a manifestation of the so-called Lazarus effect, that these targeted therapies can cause patients who are really in bad shape and would have a short survival to have their survival prolonged, even though it was less than the survival of patients with less-aggressive tumors?" he wondered, echoing Dr. Chapman’s suggestion. Alternatively, maturation of the survival data might have contributed to the change in efficacy in patients with stage IIIC, M1a, and M1b disease who did not appear to benefit from vemurafenib.
In the larger context, the findings raise an important question pertaining to the clinical trials process generally, according to Dr. Atkins: "What does this say about the early analysis of trials like this, and reporting forever more that the hazard ratio is 0.35 or so, rather than what the more accurate 0.70 is?"
A Genentech spokesperson said that "due to differences in the study populations and trial designs, the results from these trials cannot be directly compared. BRIM3 was a global, randomized, Phase III trial comparing Zelboraf (vemurafenib) to DTIC chemotherapy in people with newly diagnosed BRAF V600 mutation-positive metastatic melanoma, while BRIM2 was a Phase II study that evaluated patients with previously treated disease."
The 675 patients in BRIM-3 had stage IIIC or IV melanoma harboring the BRAF V600E mutation. They were assigned evenly to intravenous dacarbazine or oral vemurafenib, the only targeted agent approved specifically for treating BRAF-mutant melanoma.
Large proportions of patients in both the vemurafenib and dacarbazine arms (36% and 44%, respectively) received other anticancer therapies after coming off the trial, Dr. Chapman reported. About a fifth of all patients in each arm went on to receive ipilimumab, and a fourth in the dacarbazine arm crossed over to vemurafenib.
Updated results – after a median follow-up of 9.5 months in the dacarbazine arm and 12.5 months in the vemurafenib arm – showed persistence of a progression-free survival benefit in favor of vemurafenib with censoring of patients at crossover (6.9 vs. 1.6 months; hazard ratio, 0.38; P less than .001).
There was also still an improvement in overall survival, regardless of whether patients were censored at crossover (13.6 vs. 9.7 months; HR, 0.70; P less than .001) or not (13.6 vs. 10.3 months; HR, 0.76; P less than .01).
The leading grade 3 or worse adverse events with vemurafenib remained cutaneous squamous cell carcinomas (seen in 19% of patients), keratoacanthomas (10%), and elevations of liver function tests (10%). In addition, eight patients developed new primary melanomas.
Dr. Chapman disclosed that he receives honoraria and research funding from Roche/Genentech. The trial was sponsored by Hoffman-LaRoche. Dr. Atkins disclosed that he is a consultant to Bristol-Myers Squibb, Celgene, Curetech, Genentech, Merck, Novartis, and Prometheus.
This article was updated on July 3, 2012.
CHICAGO – Vemurafenib has a persistent survival benefit when given as first-line therapy for advanced BRAF-mutant melanoma, but the magnitude of benefit diminishes over time, according to an update of the BRIM-3 randomized trial.
With a median follow-up of about a year, vemurafenib was associated with a 30% relative reduction in the risk of death, relative to dacarbazine, presenting author Dr. Paul Chapman reported at the annual meeting of the American Society of Clinical Oncology. This is roughly half of the 63% relative risk reduction seen at a median follow-up of about 3 months, as reported at last year’s meeting.
The safety profile of vemurafenib was largely consistent with the earlier experience; the main high-grade adverse effects were skin toxicity (seen in about a third of patients) and liver toxicity (seen in about a tenth). But a few patients have developed second primary melanomas.
The observed temporal trend for overall survival benefit suggests "perhaps that most of the benefit in overall survival may be due to decreasing early deaths," said Dr. Chapman, an oncologist at Memorial Sloan-Kettering Cancer Center in New York.
Discussant Dr. Michael B. Atkins of the Lombardi Comprehensive Cancer Center at Georgetown University in Washington maintained that "the mature overall survival data [confirm] that vemurafenib is better than dacarbazine," and noted that it was "reassuring" that findings were similar, whether patients who crossed over to the vemurafenib arm were censored or not, "probably the most stringent test of survival."
However, he called attention to the fact that the median survival of 13-plus months with vemurafenib seen in BRIM-3 falls short of the 16-plus months seen in BRIM-2, a phase II trial in patients with previously treated advanced melanoma. "Did first-line patients have more aggressive disease and therefore fall off early?" he wondered. "Alternatively, was there less continuation of vemurafenib after progressive disease in the BRIM-3 trial relative to the BRIM-2 trial? If that was the case, some sort of confirmatory analysis should be done to see whether that can impact overall survival."
The decrease in vemurafenib efficacy seen over time might have several explanations, Dr. Atkins proposed. "Is it that the early, more robust hazard ratio was a manifestation of the so-called Lazarus effect, that these targeted therapies can cause patients who are really in bad shape and would have a short survival to have their survival prolonged, even though it was less than the survival of patients with less-aggressive tumors?" he wondered, echoing Dr. Chapman’s suggestion. Alternatively, maturation of the survival data might have contributed to the change in efficacy in patients with stage IIIC, M1a, and M1b disease who did not appear to benefit from vemurafenib.
In the larger context, the findings raise an important question pertaining to the clinical trials process generally, according to Dr. Atkins: "What does this say about the early analysis of trials like this, and reporting forever more that the hazard ratio is 0.35 or so, rather than what the more accurate 0.70 is?"
A Genentech spokesperson said that "due to differences in the study populations and trial designs, the results from these trials cannot be directly compared. BRIM3 was a global, randomized, Phase III trial comparing Zelboraf (vemurafenib) to DTIC chemotherapy in people with newly diagnosed BRAF V600 mutation-positive metastatic melanoma, while BRIM2 was a Phase II study that evaluated patients with previously treated disease."
The 675 patients in BRIM-3 had stage IIIC or IV melanoma harboring the BRAF V600E mutation. They were assigned evenly to intravenous dacarbazine or oral vemurafenib, the only targeted agent approved specifically for treating BRAF-mutant melanoma.
Large proportions of patients in both the vemurafenib and dacarbazine arms (36% and 44%, respectively) received other anticancer therapies after coming off the trial, Dr. Chapman reported. About a fifth of all patients in each arm went on to receive ipilimumab, and a fourth in the dacarbazine arm crossed over to vemurafenib.
Updated results – after a median follow-up of 9.5 months in the dacarbazine arm and 12.5 months in the vemurafenib arm – showed persistence of a progression-free survival benefit in favor of vemurafenib with censoring of patients at crossover (6.9 vs. 1.6 months; hazard ratio, 0.38; P less than .001).
There was also still an improvement in overall survival, regardless of whether patients were censored at crossover (13.6 vs. 9.7 months; HR, 0.70; P less than .001) or not (13.6 vs. 10.3 months; HR, 0.76; P less than .01).
The leading grade 3 or worse adverse events with vemurafenib remained cutaneous squamous cell carcinomas (seen in 19% of patients), keratoacanthomas (10%), and elevations of liver function tests (10%). In addition, eight patients developed new primary melanomas.
Dr. Chapman disclosed that he receives honoraria and research funding from Roche/Genentech. The trial was sponsored by Hoffman-LaRoche. Dr. Atkins disclosed that he is a consultant to Bristol-Myers Squibb, Celgene, Curetech, Genentech, Merck, Novartis, and Prometheus.
This article was updated on July 3, 2012.
AT THE ANNUAL MEETING OF THE AMERICAN SOCIETY OF CLINICAL ONCOLOGY
Major Finding: At a median follow-up of 9.5-12.5 months, vemurafenib conferred a 30% reduction in the risk of death vs. dacarbazine, about half the 63% reduction reported at a median follow-up of 2.3-3.8 months.
Data Source: The phase III, randomized BRIM-3 trial randomized 675 patients with V600E BRAF-mutant melanoma.
Disclosures: Dr. Chapman disclosed that he receives honoraria and research funding from Roche/Genentech. The trial was sponsored by Hoffman-LaRoche. Dr. Atkins disclosed that he is a consultant to Bristol-Myers Squibb, Celgene, Curetech, Genentech, Merck, Novartis, and Prometheus.
Studies Examine Scenarios of Changing Triptan Regimens
LOS ANGELES – In patients using triptans for the acute treatment of migraine, the impact on headache-related disability of switching or adding medications depends on the frequency of attacks and the type of medication, new data show.
Dr. Richard B. Lipton and Dawn C. Buse, Ph.D., both of the Albert Einstein College of Medicine in New York, and their colleagues assessed associations between treatment changes and disability among more than 1,500 triptan users from the longitudinal, population-based AMPP (American Migraine Prevalence and Prevention) study, reporting their findings at the annual meeting of the American Headache Society.
In one analysis, patients switching to NSAIDs or to combination analgesics containing an opioid or a barbiturate had respective 31% and 48% worsening in scores for headache-related disability, compared with nonswitchers, but patients staying on the triptan or switching to another one did not have any significant change in score. In stratified analyses, switching to an NSAID was associated with a worsening among the subset of patients having the most frequent attacks.
The second study found that adding an NSAID to the triptan improved scores for patients with medium-frequency episodic migraines, while adding an NSAID or another triptan worsened scores for those with high-frequency episodic migraines or chronic migraines. Patients with low-frequency episodic migraines did not have any change regardless of the medication added.
"On average, the changes clinicians make in prescription drug therapy in the real world, switching from one triptan to another or adding a second triptan, are not helpful," Dr. Lipton commented. "That is not to say that when we switch individual patients, there isn’t a benefit to that or that optimized algorithms for choosing treatments have no effect on patient care. But it is to say that maybe when we think about what to do with patients who don’t respond to medications, it’s worth considering that switching triptans on average doesn’t have the benefits for disability that I certainly previously thought."
The studies may have had the bias of confounding by indication, whereby the reasons for medication changes (which were unknown) influenced outcomes, he acknowledged. "But they do have the strength of generalizability and reflecting what actually goes on in the real world."
Session attendee Dr. James Couch of the University of Oklahoma, Oklahoma City, expressed concern about the possible confounding.
"Most of us have found that all the triptans are not equal, and if you go through the whole list of seven, it’s not unlikely that you will find one that works better than others and so on," he elaborated. Thus, some patients on one triptan "may have gotten a moderate effect, and they were still looking for a triptan that was going to have that whiz-bang effect." Also, insurance company requirements may dictate medication switches in some cases.
Dr. Peter Goadsby of the University of California, San Francisco, asked whether analyses were masking the heterogeneity of response in the study sample. "It strikes me that in practice, it’s quite heterogeneous: You make a change in some patients, they do spectacularly well, or maybe it’s just luck, and other patients don’t," he commented. "So is there more than one population, and are you losing some granularity in what we do by having them all lumped together?"
"Our reason for stratifying by attack frequency was to try to reduce that heterogeneity," Dr. Lipton replied. "And when we stratify by attack frequency, we see some pretty robust effects."
Finally, session co-moderator Dr. Andrew Hershey of the Cincinnati Children’s Hospital wondered if the directionality of association was perhaps reversed, and headache-related disability had instead prompted the medication changes.
Dr. Lipton noted that the investigators assessed changes in score from before to after a medication switch, in addition to using stratification. "So that’s our attempt to take baseline differences into account, though it’s certainly imperfect," he acknowledged.
Switching Medications
Dr. Buse and her colleagues studied the impact of medication switches from one year to the next in 799 patients with migraine taking triptans for acute treatment.
"Providers commonly switch patients in their acute pharmacologic regimens, we know that. We switch medications for a variety of reasons: patient preference, nonresponse, what is allowed by third-party payers," she commented. However, most studies of switching medications have looked at short-term outcomes, and few have looked at switches to or from triptans.
Fully 83% of the patients studied continued on the same triptan, 10% switched to another triptan, 4% switched to a combination analgesic containing an opioid or barbiturate, and 3% switched to an NSAID.
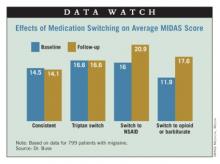
Results showed that headache-related disability, assessed from Migraine Disability Assessment (MIDAS) scores, was essentially unchanged from one year to the next in patients who stayed on the same triptan (14.5 vs. 14.1) or who switched from one triptan to another (16.6 vs. 16.6). In contrast, scores increased (worsened) in patients who switched to NSAIDs (16.0 vs. 20.9) and who switched to combination therapy containing opioids or barbiturates (11.9 vs. 17.6).
Additional analyses showed that the impact of switching to NSAIDs varied according to migraine frequency. Specifically, patients with high-frequency episodic migraines (10-14 headache-days monthly) or chronic migraines (15 or more headache-days monthly) had a significant increase in MIDAS scores when switching to NSAIDs relative to their counterparts with low-frequency episodic migraines (0-4 headache-days monthly) or moderate-frequency episodic migraines (5-9 headache-days monthly).
"Not only did the treatment matter, but the average number of days of headache at baseline matters quite importantly here," Dr. Buse commented.
"In this observational study, switching triptan regimens or switching from a triptan to an alternative pharmacologic therapy for acute migraine does not appear to be associated with improvements in headache-related disability and, in some cases, is associated with increased headache-related disability over the course of 1 year to the second year," she concluded.
Adding medications
Dr. Lipton’s team studied the impact of medication additions from one year to the next in 960 patients with migraine taking triptans for acute treatment.
"Most people with migraine in the population use more than one acute treatment, and triptan users often use more than one triptan or use a triptan in combination with other medications, or even use combination products, such as Treximet [sumatriptan and naproxen], which contains a triptan and a nonsteroidal [anti-inflammatory drug]," he noted.
"By and large, we have not done much in terms of studying combination acute treatment in migraine, and certainly combining triptans has not been studied very often," he noted.
Most patients, 68%, did not change their acute treatment, while 13% added a combination analgesic containing opioids or barbiturates, 12% added another triptan, and 7% added an NSAID.
Results showed that among patients having low-frequency episodic migraines, adding another medication to their triptan did not significantly affect MIDAS score from one year to the next, regardless of the type of medication added.
Among patients with medium-frequency episodic migraines, adding an NSAID was associated with a significant decrease in MIDAS scores, whereas adding other medications did not affect scores.
But among patients with high-frequency episodic migraines or chronic migraines, MIDAS scores actually increased significantly with addition of an NSAID or another triptan. They were unaffected by addition of a combination analgesic containing opioids or barbiturates.
Further analyses confirmed that the impact of adding NSAIDs varied according to migraine frequency: Patients with high-frequency episodic migraines or chronic migraines had a significant increase in MIDAS scores when switching to NSAIDs relative to their counterparts with low-frequency episodic migraines.
"This is analogous to the finding in the Bigal paper [Headache 2008;48:1157-68] showing that NSAIDs are associated with an increased risk of transition to chronic migraine in people who have high-frequency episodic migraine," Dr. Lipton commented.
Dr. Lipton disclosed that he receives research grant support from the National Institutes of Health, the National Headache Foundation, and the Migraine Research Fund, and serves as a consultant or advisor to or has received honoraria from the American Headache Society and various pharmaceutical companies manufacturing drugs for migraine. Dr. Buse disclosed that she has received grant support and honoraria from Endo Pharmaceuticals and other pharmaceutical companies manufacturing drugs for migraine. The AMPP study is funded through a research grant to the National Headache Foundation from Ortho-McNeil Neurologics; additional analyses and manuscript preparation were supported through a grant from MAP Pharmaceuticals and Allergan to the National Headache Foundation.
LOS ANGELES – In patients using triptans for the acute treatment of migraine, the impact on headache-related disability of switching or adding medications depends on the frequency of attacks and the type of medication, new data show.
Dr. Richard B. Lipton and Dawn C. Buse, Ph.D., both of the Albert Einstein College of Medicine in New York, and their colleagues assessed associations between treatment changes and disability among more than 1,500 triptan users from the longitudinal, population-based AMPP (American Migraine Prevalence and Prevention) study, reporting their findings at the annual meeting of the American Headache Society.
In one analysis, patients switching to NSAIDs or to combination analgesics containing an opioid or a barbiturate had respective 31% and 48% worsening in scores for headache-related disability, compared with nonswitchers, but patients staying on the triptan or switching to another one did not have any significant change in score. In stratified analyses, switching to an NSAID was associated with a worsening among the subset of patients having the most frequent attacks.
The second study found that adding an NSAID to the triptan improved scores for patients with medium-frequency episodic migraines, while adding an NSAID or another triptan worsened scores for those with high-frequency episodic migraines or chronic migraines. Patients with low-frequency episodic migraines did not have any change regardless of the medication added.
"On average, the changes clinicians make in prescription drug therapy in the real world, switching from one triptan to another or adding a second triptan, are not helpful," Dr. Lipton commented. "That is not to say that when we switch individual patients, there isn’t a benefit to that or that optimized algorithms for choosing treatments have no effect on patient care. But it is to say that maybe when we think about what to do with patients who don’t respond to medications, it’s worth considering that switching triptans on average doesn’t have the benefits for disability that I certainly previously thought."
The studies may have had the bias of confounding by indication, whereby the reasons for medication changes (which were unknown) influenced outcomes, he acknowledged. "But they do have the strength of generalizability and reflecting what actually goes on in the real world."
Session attendee Dr. James Couch of the University of Oklahoma, Oklahoma City, expressed concern about the possible confounding.
"Most of us have found that all the triptans are not equal, and if you go through the whole list of seven, it’s not unlikely that you will find one that works better than others and so on," he elaborated. Thus, some patients on one triptan "may have gotten a moderate effect, and they were still looking for a triptan that was going to have that whiz-bang effect." Also, insurance company requirements may dictate medication switches in some cases.
Dr. Peter Goadsby of the University of California, San Francisco, asked whether analyses were masking the heterogeneity of response in the study sample. "It strikes me that in practice, it’s quite heterogeneous: You make a change in some patients, they do spectacularly well, or maybe it’s just luck, and other patients don’t," he commented. "So is there more than one population, and are you losing some granularity in what we do by having them all lumped together?"
"Our reason for stratifying by attack frequency was to try to reduce that heterogeneity," Dr. Lipton replied. "And when we stratify by attack frequency, we see some pretty robust effects."
Finally, session co-moderator Dr. Andrew Hershey of the Cincinnati Children’s Hospital wondered if the directionality of association was perhaps reversed, and headache-related disability had instead prompted the medication changes.
Dr. Lipton noted that the investigators assessed changes in score from before to after a medication switch, in addition to using stratification. "So that’s our attempt to take baseline differences into account, though it’s certainly imperfect," he acknowledged.
Switching Medications
Dr. Buse and her colleagues studied the impact of medication switches from one year to the next in 799 patients with migraine taking triptans for acute treatment.
"Providers commonly switch patients in their acute pharmacologic regimens, we know that. We switch medications for a variety of reasons: patient preference, nonresponse, what is allowed by third-party payers," she commented. However, most studies of switching medications have looked at short-term outcomes, and few have looked at switches to or from triptans.
Fully 83% of the patients studied continued on the same triptan, 10% switched to another triptan, 4% switched to a combination analgesic containing an opioid or barbiturate, and 3% switched to an NSAID.
Results showed that headache-related disability, assessed from Migraine Disability Assessment (MIDAS) scores, was essentially unchanged from one year to the next in patients who stayed on the same triptan (14.5 vs. 14.1) or who switched from one triptan to another (16.6 vs. 16.6). In contrast, scores increased (worsened) in patients who switched to NSAIDs (16.0 vs. 20.9) and who switched to combination therapy containing opioids or barbiturates (11.9 vs. 17.6).
Additional analyses showed that the impact of switching to NSAIDs varied according to migraine frequency. Specifically, patients with high-frequency episodic migraines (10-14 headache-days monthly) or chronic migraines (15 or more headache-days monthly) had a significant increase in MIDAS scores when switching to NSAIDs relative to their counterparts with low-frequency episodic migraines (0-4 headache-days monthly) or moderate-frequency episodic migraines (5-9 headache-days monthly).
"Not only did the treatment matter, but the average number of days of headache at baseline matters quite importantly here," Dr. Buse commented.
"In this observational study, switching triptan regimens or switching from a triptan to an alternative pharmacologic therapy for acute migraine does not appear to be associated with improvements in headache-related disability and, in some cases, is associated with increased headache-related disability over the course of 1 year to the second year," she concluded.
Adding medications
Dr. Lipton’s team studied the impact of medication additions from one year to the next in 960 patients with migraine taking triptans for acute treatment.
"Most people with migraine in the population use more than one acute treatment, and triptan users often use more than one triptan or use a triptan in combination with other medications, or even use combination products, such as Treximet [sumatriptan and naproxen], which contains a triptan and a nonsteroidal [anti-inflammatory drug]," he noted.
"By and large, we have not done much in terms of studying combination acute treatment in migraine, and certainly combining triptans has not been studied very often," he noted.
Most patients, 68%, did not change their acute treatment, while 13% added a combination analgesic containing opioids or barbiturates, 12% added another triptan, and 7% added an NSAID.
Results showed that among patients having low-frequency episodic migraines, adding another medication to their triptan did not significantly affect MIDAS score from one year to the next, regardless of the type of medication added.
Among patients with medium-frequency episodic migraines, adding an NSAID was associated with a significant decrease in MIDAS scores, whereas adding other medications did not affect scores.
But among patients with high-frequency episodic migraines or chronic migraines, MIDAS scores actually increased significantly with addition of an NSAID or another triptan. They were unaffected by addition of a combination analgesic containing opioids or barbiturates.
Further analyses confirmed that the impact of adding NSAIDs varied according to migraine frequency: Patients with high-frequency episodic migraines or chronic migraines had a significant increase in MIDAS scores when switching to NSAIDs relative to their counterparts with low-frequency episodic migraines.
"This is analogous to the finding in the Bigal paper [Headache 2008;48:1157-68] showing that NSAIDs are associated with an increased risk of transition to chronic migraine in people who have high-frequency episodic migraine," Dr. Lipton commented.
Dr. Lipton disclosed that he receives research grant support from the National Institutes of Health, the National Headache Foundation, and the Migraine Research Fund, and serves as a consultant or advisor to or has received honoraria from the American Headache Society and various pharmaceutical companies manufacturing drugs for migraine. Dr. Buse disclosed that she has received grant support and honoraria from Endo Pharmaceuticals and other pharmaceutical companies manufacturing drugs for migraine. The AMPP study is funded through a research grant to the National Headache Foundation from Ortho-McNeil Neurologics; additional analyses and manuscript preparation were supported through a grant from MAP Pharmaceuticals and Allergan to the National Headache Foundation.
LOS ANGELES – In patients using triptans for the acute treatment of migraine, the impact on headache-related disability of switching or adding medications depends on the frequency of attacks and the type of medication, new data show.
Dr. Richard B. Lipton and Dawn C. Buse, Ph.D., both of the Albert Einstein College of Medicine in New York, and their colleagues assessed associations between treatment changes and disability among more than 1,500 triptan users from the longitudinal, population-based AMPP (American Migraine Prevalence and Prevention) study, reporting their findings at the annual meeting of the American Headache Society.
In one analysis, patients switching to NSAIDs or to combination analgesics containing an opioid or a barbiturate had respective 31% and 48% worsening in scores for headache-related disability, compared with nonswitchers, but patients staying on the triptan or switching to another one did not have any significant change in score. In stratified analyses, switching to an NSAID was associated with a worsening among the subset of patients having the most frequent attacks.
The second study found that adding an NSAID to the triptan improved scores for patients with medium-frequency episodic migraines, while adding an NSAID or another triptan worsened scores for those with high-frequency episodic migraines or chronic migraines. Patients with low-frequency episodic migraines did not have any change regardless of the medication added.
"On average, the changes clinicians make in prescription drug therapy in the real world, switching from one triptan to another or adding a second triptan, are not helpful," Dr. Lipton commented. "That is not to say that when we switch individual patients, there isn’t a benefit to that or that optimized algorithms for choosing treatments have no effect on patient care. But it is to say that maybe when we think about what to do with patients who don’t respond to medications, it’s worth considering that switching triptans on average doesn’t have the benefits for disability that I certainly previously thought."
The studies may have had the bias of confounding by indication, whereby the reasons for medication changes (which were unknown) influenced outcomes, he acknowledged. "But they do have the strength of generalizability and reflecting what actually goes on in the real world."
Session attendee Dr. James Couch of the University of Oklahoma, Oklahoma City, expressed concern about the possible confounding.
"Most of us have found that all the triptans are not equal, and if you go through the whole list of seven, it’s not unlikely that you will find one that works better than others and so on," he elaborated. Thus, some patients on one triptan "may have gotten a moderate effect, and they were still looking for a triptan that was going to have that whiz-bang effect." Also, insurance company requirements may dictate medication switches in some cases.
Dr. Peter Goadsby of the University of California, San Francisco, asked whether analyses were masking the heterogeneity of response in the study sample. "It strikes me that in practice, it’s quite heterogeneous: You make a change in some patients, they do spectacularly well, or maybe it’s just luck, and other patients don’t," he commented. "So is there more than one population, and are you losing some granularity in what we do by having them all lumped together?"
"Our reason for stratifying by attack frequency was to try to reduce that heterogeneity," Dr. Lipton replied. "And when we stratify by attack frequency, we see some pretty robust effects."
Finally, session co-moderator Dr. Andrew Hershey of the Cincinnati Children’s Hospital wondered if the directionality of association was perhaps reversed, and headache-related disability had instead prompted the medication changes.
Dr. Lipton noted that the investigators assessed changes in score from before to after a medication switch, in addition to using stratification. "So that’s our attempt to take baseline differences into account, though it’s certainly imperfect," he acknowledged.
Switching Medications
Dr. Buse and her colleagues studied the impact of medication switches from one year to the next in 799 patients with migraine taking triptans for acute treatment.
"Providers commonly switch patients in their acute pharmacologic regimens, we know that. We switch medications for a variety of reasons: patient preference, nonresponse, what is allowed by third-party payers," she commented. However, most studies of switching medications have looked at short-term outcomes, and few have looked at switches to or from triptans.
Fully 83% of the patients studied continued on the same triptan, 10% switched to another triptan, 4% switched to a combination analgesic containing an opioid or barbiturate, and 3% switched to an NSAID.
Results showed that headache-related disability, assessed from Migraine Disability Assessment (MIDAS) scores, was essentially unchanged from one year to the next in patients who stayed on the same triptan (14.5 vs. 14.1) or who switched from one triptan to another (16.6 vs. 16.6). In contrast, scores increased (worsened) in patients who switched to NSAIDs (16.0 vs. 20.9) and who switched to combination therapy containing opioids or barbiturates (11.9 vs. 17.6).
Additional analyses showed that the impact of switching to NSAIDs varied according to migraine frequency. Specifically, patients with high-frequency episodic migraines (10-14 headache-days monthly) or chronic migraines (15 or more headache-days monthly) had a significant increase in MIDAS scores when switching to NSAIDs relative to their counterparts with low-frequency episodic migraines (0-4 headache-days monthly) or moderate-frequency episodic migraines (5-9 headache-days monthly).
"Not only did the treatment matter, but the average number of days of headache at baseline matters quite importantly here," Dr. Buse commented.
"In this observational study, switching triptan regimens or switching from a triptan to an alternative pharmacologic therapy for acute migraine does not appear to be associated with improvements in headache-related disability and, in some cases, is associated with increased headache-related disability over the course of 1 year to the second year," she concluded.
Adding medications
Dr. Lipton’s team studied the impact of medication additions from one year to the next in 960 patients with migraine taking triptans for acute treatment.
"Most people with migraine in the population use more than one acute treatment, and triptan users often use more than one triptan or use a triptan in combination with other medications, or even use combination products, such as Treximet [sumatriptan and naproxen], which contains a triptan and a nonsteroidal [anti-inflammatory drug]," he noted.
"By and large, we have not done much in terms of studying combination acute treatment in migraine, and certainly combining triptans has not been studied very often," he noted.
Most patients, 68%, did not change their acute treatment, while 13% added a combination analgesic containing opioids or barbiturates, 12% added another triptan, and 7% added an NSAID.
Results showed that among patients having low-frequency episodic migraines, adding another medication to their triptan did not significantly affect MIDAS score from one year to the next, regardless of the type of medication added.
Among patients with medium-frequency episodic migraines, adding an NSAID was associated with a significant decrease in MIDAS scores, whereas adding other medications did not affect scores.
But among patients with high-frequency episodic migraines or chronic migraines, MIDAS scores actually increased significantly with addition of an NSAID or another triptan. They were unaffected by addition of a combination analgesic containing opioids or barbiturates.
Further analyses confirmed that the impact of adding NSAIDs varied according to migraine frequency: Patients with high-frequency episodic migraines or chronic migraines had a significant increase in MIDAS scores when switching to NSAIDs relative to their counterparts with low-frequency episodic migraines.
"This is analogous to the finding in the Bigal paper [Headache 2008;48:1157-68] showing that NSAIDs are associated with an increased risk of transition to chronic migraine in people who have high-frequency episodic migraine," Dr. Lipton commented.
Dr. Lipton disclosed that he receives research grant support from the National Institutes of Health, the National Headache Foundation, and the Migraine Research Fund, and serves as a consultant or advisor to or has received honoraria from the American Headache Society and various pharmaceutical companies manufacturing drugs for migraine. Dr. Buse disclosed that she has received grant support and honoraria from Endo Pharmaceuticals and other pharmaceutical companies manufacturing drugs for migraine. The AMPP study is funded through a research grant to the National Headache Foundation from Ortho-McNeil Neurologics; additional analyses and manuscript preparation were supported through a grant from MAP Pharmaceuticals and Allergan to the National Headache Foundation.
AT THE ANNUAL MEETING OF THE AMERICAN HEADACHE SOCIETY
Major Finding: Headache-related disability among patients taking a triptan worsened by 31% in patients who switched to an NSAID and was worst among those with high-frequency episodic migraines or chronic migraines. Adding an NSAID benefited only medium-frequency patients and worsened high-frequency patients.
Data Source: A pair of studies in 799 and 960 triptan users with migraine from the longitudinal, population-based American Migraine Prevalence and Prevention (AMPP) study
Disclosures: Dr. Lipton disclosed that he receives research grant support from the National Institutes of Health, the National Headache Foundation, and the Migraine Research Fund, and serves as a consultant or advisor to or has received honoraria from the American Headache Society and various pharmaceutical companies manufacturing drugs for migraine. Dr. Buse disclosed that she has received grant support and honoraria from Endo Pharmaceuticals and other pharmaceutical companies manufacturing drugs for migraine. The AMPP study is funded through a research grant to the National Headache Foundation from Ortho-McNeil Neurologics; additional analyses and manuscript preparation were supported through a grant from MAP Pharmaceuticals and Allergan to the National Headache Foundation.
Neoadjuvant Lapatinib Fails to Surpass Trastuzumab in HER2+ Breast Cancer
CHICAGO – Lapatinib was not superior to trastuzumab when used as a component of neoadjuvant therapy for operable HER2-positive breast cancer in a randomized cooperative group trial. And neither was a combination of both drugs in the population overall.
About 50%-60% of the 529 patients studied had a pathological complete response (pCR) whether they received trastuzumab (Herceptin) only, lapatinib (Tykerb) only, or lapatinib plus trastuzumab, each in addition to standard chemotherapy in the NSABP (National Surgical Adjuvant Breast and Bowel Project) B-41 trial, investigators reported.
However, patients whose tumors had a high level of HER2 overexpression on immunohistochemistry, scored as 3+ staining, were more likely to have a pCR if they received lapatinib plus trastuzumab than if they received trastuzumab alone, Dr. Andre Robidoux told attendees at the annual meeting of the American Society of Clinical Oncology.
Of note, the rate of grade 3 or worse diarrhea was higher with lapatinib alone or in combination, the investigators found. And patients in those groups were less likely to complete their treatment.
"Combined HER2-targeted therapy produced a numerically higher pCR percentage than single-agent HER2-directed therapy, but the difference was not statistically significant," said Dr. Robidoux of the Centre Hospitalier de l’Université de Montréal
"Unplanned analysis based on immunohistochemical staining intensity suggests that combined HER2-targeted therapy may be of greatest value in patients with tumors with a high degree of protein overexpression," he added.
Dual Blockade Supported
Discussant Dr. Gunter von Minckwitz of the German Breast Group and the University of Frankfurt noted that neoadjuvant trastuzumab therapy increases the likelihood of residual ductal carcinoma in situ (DCIS) at surgery, which is a negative prognostic factor for disease-free survival (J. Clin. Oncol. 2012;30:901-3). "So maybe the NSABP investigators should also analyze their data for an even more pure pCR definition," he proposed.
"We have seen now a series of studies either combining lapatinib and trastuzumab or combining trastuzumab and pertuzumab [Perjeta] showing higher pCR rates compared to patients who got only one of the anti-HER2 treatments," he maintained. "So this is the way we have to go. ... Dual blockade appears to be the most promising approach for the future."
"We have to wait, of course, to see how far the neoadjuvant results are predicting the outcome of adjuvant treatment; therefore, ALTTO and APHINITY are crucial," Dr. von Minckwitz added, referring to two trials.
"However, I believe the results from the neoadjuvant setting are quite consistent with the results that are now obtained in other settings – the adjuvant and metastatic settings. Therefore, this might open a regulatory path for conditional approval of agents in early development," as recently proposed by the Food and Drug Administration (N. Engl. J. Med. 2012 May 30).
Giving background on NSABP B-41, Dr. Robidoux noted that doxorubicin (Adriamycin) plus cyclophosphamide, followed by weekly paclitaxel plus trastuzumab, has been a standard neoadjuvant therapy for HER2-positive breast cancer since 2005. But lapatinib, an oral dual tyrosine kinase inhibitor of HER2 and the epidermal growth factor receptor (EGFR), has a different mechanism of action and shows synergy with trastuzumab.
"The next logical step was to evaluate the addition of a second agent that interrupts HER2 signaling through a different mechanism than trastuzumab," he said.
PCR Rates Indistinguishable
Women enrolled in the trial had a palpable tumor measuring at least 2 cm, a left ventricular ejection fraction of at least 50%, and HER2-positive disease, defined as gene amplification by fluorescence in situ hybridization (FISH) or a score of 3+ by immunohistochemistry.
All women received four cycles of doxorubicin plus cyclophosphamide. Thereafter, they received weekly paclitaxel plus trastuzumab only (as a control), plus lapatinib only, or plus both. They then underwent surgery and received trastuzumab postoperatively.
The main results showed that the rate of clinical complete response with lapatinib alone (69.9%) was lower than that with trastuzumab alone (82%) (P = .014). But it did not differ significantly between trastuzumab and lapatinib plus trastuzumab (76.8%).
The rates of pCR in the breast with lapatinib alone (53.2%) or lapatinib combined with trastuzumab (62%) were statistically indistinguishable from that seen with trastuzumab alone (52.5%).
This pattern was the same in subgroups having hormone receptor–positive and negative disease. However, in a finding that Dr. Robidoux called "intriguing," the pCR rate among those with 3+ immunohistochemical staining for HER2 was higher with lapatinib plus trastuzumab (71%) than with trastuzumab alone (54.7%) (P = .006).
The overall rate of grade 3 or worse toxicity was 50% with trastuzumab alone, 62% with lapatinib alone, and 61% with lapatinib plus trastuzumab. Grade 3 or worse diarrhea was by far more common in both lapatinib groups (P less than .001), but grade 3 or worse febrile neutropenia and hepatotoxicity were similarly common.
The rate of New York Heart Association class III or IV congestive heart failure was 4% with trastuzumab alone, 4% with lapatinib alone, and 1% with the combination, differences that were not statistically significant.
Patients in the trastuzumab group were more likely to complete their therapy (78%) than their counterparts in the lapatinib group (68%) and in the combination group (63%) (P = .01).
About half of patients were able to have breast-conserving surgery, whether they received trastuzumab alone (54%), lapatinib alone (46%), or both drugs (50%).
Dr. Robidoux disclosed that he is a consultant to GlaxoSmithKline and receives honoraria and research funding from GlaxoSmithKline and Roche. Dr. von Minckwitz disclosed no relevant conflicts of interest.
CHICAGO – Lapatinib was not superior to trastuzumab when used as a component of neoadjuvant therapy for operable HER2-positive breast cancer in a randomized cooperative group trial. And neither was a combination of both drugs in the population overall.
About 50%-60% of the 529 patients studied had a pathological complete response (pCR) whether they received trastuzumab (Herceptin) only, lapatinib (Tykerb) only, or lapatinib plus trastuzumab, each in addition to standard chemotherapy in the NSABP (National Surgical Adjuvant Breast and Bowel Project) B-41 trial, investigators reported.
However, patients whose tumors had a high level of HER2 overexpression on immunohistochemistry, scored as 3+ staining, were more likely to have a pCR if they received lapatinib plus trastuzumab than if they received trastuzumab alone, Dr. Andre Robidoux told attendees at the annual meeting of the American Society of Clinical Oncology.
Of note, the rate of grade 3 or worse diarrhea was higher with lapatinib alone or in combination, the investigators found. And patients in those groups were less likely to complete their treatment.
"Combined HER2-targeted therapy produced a numerically higher pCR percentage than single-agent HER2-directed therapy, but the difference was not statistically significant," said Dr. Robidoux of the Centre Hospitalier de l’Université de Montréal
"Unplanned analysis based on immunohistochemical staining intensity suggests that combined HER2-targeted therapy may be of greatest value in patients with tumors with a high degree of protein overexpression," he added.
Dual Blockade Supported
Discussant Dr. Gunter von Minckwitz of the German Breast Group and the University of Frankfurt noted that neoadjuvant trastuzumab therapy increases the likelihood of residual ductal carcinoma in situ (DCIS) at surgery, which is a negative prognostic factor for disease-free survival (J. Clin. Oncol. 2012;30:901-3). "So maybe the NSABP investigators should also analyze their data for an even more pure pCR definition," he proposed.
"We have seen now a series of studies either combining lapatinib and trastuzumab or combining trastuzumab and pertuzumab [Perjeta] showing higher pCR rates compared to patients who got only one of the anti-HER2 treatments," he maintained. "So this is the way we have to go. ... Dual blockade appears to be the most promising approach for the future."
"We have to wait, of course, to see how far the neoadjuvant results are predicting the outcome of adjuvant treatment; therefore, ALTTO and APHINITY are crucial," Dr. von Minckwitz added, referring to two trials.
"However, I believe the results from the neoadjuvant setting are quite consistent with the results that are now obtained in other settings – the adjuvant and metastatic settings. Therefore, this might open a regulatory path for conditional approval of agents in early development," as recently proposed by the Food and Drug Administration (N. Engl. J. Med. 2012 May 30).
Giving background on NSABP B-41, Dr. Robidoux noted that doxorubicin (Adriamycin) plus cyclophosphamide, followed by weekly paclitaxel plus trastuzumab, has been a standard neoadjuvant therapy for HER2-positive breast cancer since 2005. But lapatinib, an oral dual tyrosine kinase inhibitor of HER2 and the epidermal growth factor receptor (EGFR), has a different mechanism of action and shows synergy with trastuzumab.
"The next logical step was to evaluate the addition of a second agent that interrupts HER2 signaling through a different mechanism than trastuzumab," he said.
PCR Rates Indistinguishable
Women enrolled in the trial had a palpable tumor measuring at least 2 cm, a left ventricular ejection fraction of at least 50%, and HER2-positive disease, defined as gene amplification by fluorescence in situ hybridization (FISH) or a score of 3+ by immunohistochemistry.
All women received four cycles of doxorubicin plus cyclophosphamide. Thereafter, they received weekly paclitaxel plus trastuzumab only (as a control), plus lapatinib only, or plus both. They then underwent surgery and received trastuzumab postoperatively.
The main results showed that the rate of clinical complete response with lapatinib alone (69.9%) was lower than that with trastuzumab alone (82%) (P = .014). But it did not differ significantly between trastuzumab and lapatinib plus trastuzumab (76.8%).
The rates of pCR in the breast with lapatinib alone (53.2%) or lapatinib combined with trastuzumab (62%) were statistically indistinguishable from that seen with trastuzumab alone (52.5%).
This pattern was the same in subgroups having hormone receptor–positive and negative disease. However, in a finding that Dr. Robidoux called "intriguing," the pCR rate among those with 3+ immunohistochemical staining for HER2 was higher with lapatinib plus trastuzumab (71%) than with trastuzumab alone (54.7%) (P = .006).
The overall rate of grade 3 or worse toxicity was 50% with trastuzumab alone, 62% with lapatinib alone, and 61% with lapatinib plus trastuzumab. Grade 3 or worse diarrhea was by far more common in both lapatinib groups (P less than .001), but grade 3 or worse febrile neutropenia and hepatotoxicity were similarly common.
The rate of New York Heart Association class III or IV congestive heart failure was 4% with trastuzumab alone, 4% with lapatinib alone, and 1% with the combination, differences that were not statistically significant.
Patients in the trastuzumab group were more likely to complete their therapy (78%) than their counterparts in the lapatinib group (68%) and in the combination group (63%) (P = .01).
About half of patients were able to have breast-conserving surgery, whether they received trastuzumab alone (54%), lapatinib alone (46%), or both drugs (50%).
Dr. Robidoux disclosed that he is a consultant to GlaxoSmithKline and receives honoraria and research funding from GlaxoSmithKline and Roche. Dr. von Minckwitz disclosed no relevant conflicts of interest.
CHICAGO – Lapatinib was not superior to trastuzumab when used as a component of neoadjuvant therapy for operable HER2-positive breast cancer in a randomized cooperative group trial. And neither was a combination of both drugs in the population overall.
About 50%-60% of the 529 patients studied had a pathological complete response (pCR) whether they received trastuzumab (Herceptin) only, lapatinib (Tykerb) only, or lapatinib plus trastuzumab, each in addition to standard chemotherapy in the NSABP (National Surgical Adjuvant Breast and Bowel Project) B-41 trial, investigators reported.
However, patients whose tumors had a high level of HER2 overexpression on immunohistochemistry, scored as 3+ staining, were more likely to have a pCR if they received lapatinib plus trastuzumab than if they received trastuzumab alone, Dr. Andre Robidoux told attendees at the annual meeting of the American Society of Clinical Oncology.
Of note, the rate of grade 3 or worse diarrhea was higher with lapatinib alone or in combination, the investigators found. And patients in those groups were less likely to complete their treatment.
"Combined HER2-targeted therapy produced a numerically higher pCR percentage than single-agent HER2-directed therapy, but the difference was not statistically significant," said Dr. Robidoux of the Centre Hospitalier de l’Université de Montréal
"Unplanned analysis based on immunohistochemical staining intensity suggests that combined HER2-targeted therapy may be of greatest value in patients with tumors with a high degree of protein overexpression," he added.
Dual Blockade Supported
Discussant Dr. Gunter von Minckwitz of the German Breast Group and the University of Frankfurt noted that neoadjuvant trastuzumab therapy increases the likelihood of residual ductal carcinoma in situ (DCIS) at surgery, which is a negative prognostic factor for disease-free survival (J. Clin. Oncol. 2012;30:901-3). "So maybe the NSABP investigators should also analyze their data for an even more pure pCR definition," he proposed.
"We have seen now a series of studies either combining lapatinib and trastuzumab or combining trastuzumab and pertuzumab [Perjeta] showing higher pCR rates compared to patients who got only one of the anti-HER2 treatments," he maintained. "So this is the way we have to go. ... Dual blockade appears to be the most promising approach for the future."
"We have to wait, of course, to see how far the neoadjuvant results are predicting the outcome of adjuvant treatment; therefore, ALTTO and APHINITY are crucial," Dr. von Minckwitz added, referring to two trials.
"However, I believe the results from the neoadjuvant setting are quite consistent with the results that are now obtained in other settings – the adjuvant and metastatic settings. Therefore, this might open a regulatory path for conditional approval of agents in early development," as recently proposed by the Food and Drug Administration (N. Engl. J. Med. 2012 May 30).
Giving background on NSABP B-41, Dr. Robidoux noted that doxorubicin (Adriamycin) plus cyclophosphamide, followed by weekly paclitaxel plus trastuzumab, has been a standard neoadjuvant therapy for HER2-positive breast cancer since 2005. But lapatinib, an oral dual tyrosine kinase inhibitor of HER2 and the epidermal growth factor receptor (EGFR), has a different mechanism of action and shows synergy with trastuzumab.
"The next logical step was to evaluate the addition of a second agent that interrupts HER2 signaling through a different mechanism than trastuzumab," he said.
PCR Rates Indistinguishable
Women enrolled in the trial had a palpable tumor measuring at least 2 cm, a left ventricular ejection fraction of at least 50%, and HER2-positive disease, defined as gene amplification by fluorescence in situ hybridization (FISH) or a score of 3+ by immunohistochemistry.
All women received four cycles of doxorubicin plus cyclophosphamide. Thereafter, they received weekly paclitaxel plus trastuzumab only (as a control), plus lapatinib only, or plus both. They then underwent surgery and received trastuzumab postoperatively.
The main results showed that the rate of clinical complete response with lapatinib alone (69.9%) was lower than that with trastuzumab alone (82%) (P = .014). But it did not differ significantly between trastuzumab and lapatinib plus trastuzumab (76.8%).
The rates of pCR in the breast with lapatinib alone (53.2%) or lapatinib combined with trastuzumab (62%) were statistically indistinguishable from that seen with trastuzumab alone (52.5%).
This pattern was the same in subgroups having hormone receptor–positive and negative disease. However, in a finding that Dr. Robidoux called "intriguing," the pCR rate among those with 3+ immunohistochemical staining for HER2 was higher with lapatinib plus trastuzumab (71%) than with trastuzumab alone (54.7%) (P = .006).
The overall rate of grade 3 or worse toxicity was 50% with trastuzumab alone, 62% with lapatinib alone, and 61% with lapatinib plus trastuzumab. Grade 3 or worse diarrhea was by far more common in both lapatinib groups (P less than .001), but grade 3 or worse febrile neutropenia and hepatotoxicity were similarly common.
The rate of New York Heart Association class III or IV congestive heart failure was 4% with trastuzumab alone, 4% with lapatinib alone, and 1% with the combination, differences that were not statistically significant.
Patients in the trastuzumab group were more likely to complete their therapy (78%) than their counterparts in the lapatinib group (68%) and in the combination group (63%) (P = .01).
About half of patients were able to have breast-conserving surgery, whether they received trastuzumab alone (54%), lapatinib alone (46%), or both drugs (50%).
Dr. Robidoux disclosed that he is a consultant to GlaxoSmithKline and receives honoraria and research funding from GlaxoSmithKline and Roche. Dr. von Minckwitz disclosed no relevant conflicts of interest.
AT THE ANNUAL MEETING OF THE AMERICAN SOCIETY OF CLINICAL ONCOLOGY
Radiation Therapy Beneficial Even for 'Good-Risk' Ductal Carcinoma
CHICAGO – Radiation therapy dramatically improves local control in ductal carcinoma in situ, even in cases having favorable features, investigators reported.
The randomized Radiation Therapy Oncology Group (RTOG) 9804 trial was conducted among 585 women who had "good-risk" ductal carcinoma in situ (DCIS), meaning small, asymptomatic tumors of low grade and with adequate resection margins after lumpectomy.
The main results showed that women had a significant 86% relative reduction in the risk of local failure if they received radiation therapy instead of observation. But the absolute 5-year rate of local failure was only about 3%, even without this intervention.
"We were able, using standard pathology methods and our Web-based RTOG pathology tool, to define good-risk or low-risk DCIS patients who had an extremely low event rate even with observation," said lead investigator Dr. Beryl McCormick at the annual meeting of the American Society of Clinical Oncology.
Nonetheless, "for this good-risk disease, the addition of radiation significantly reduced the risk of local failure. Clearly, we are expecting to follow this group out longer," added Dr. McCormick, a radiation oncologist and chief of the external beam radiotherapy service at Memorial Sloan-Kettering Cancer Center in New York.
"The study was a positive study," commented discussant Dr. Eun-Sil Shelley Hwang of Duke University Medical Center in Durham, N.C. "The reasons for that are debatable. On this analysis, there was a lower recurrence rate in the excision-only group than had been predicted, but what drove the positive findings was the much lower rate in the radiation therapy group."
Dr. Hwang speculated that the use of tamoxifen by about two-thirds of women may have contributed to the low rate of local recurrence seen even in the absence of radiation therapy.
"Further follow-up is required, because many of these patients [had] a short follow-up that may explain why we saw such low recurrence risks in this study," she proposed.
Women with DCIS who were above age 26 were eligible for RTOG 9804 if they had no symptoms (their tumors had been found mammographically or incidentally), had only low or intermediate tumor grade, had a tumor size of 2.5 cm or less, and had a resection margin width of at least 3 mm.
The 585 study participants were randomly assigned in balanced fashion to observation or radiation therapy, each with or without tamoxifen. (Overall, 62% received the drug.) Radiation therapy began within 12 weeks of final surgery, and consisted of 42.5-50.4 Gy, with no boost.
The main trial results showed that the actuarial 5-year rate of local failure (invasive or noninvasive) in the treated breast was 3.2% in the observation group and 0.4% in the radiation therapy group, corresponding to an 86% reduction in risk (hazard ratio, 0.14; P = .002), Dr. McCormick reported.
In the radiation therapy group, there were no local failures in the same quadrant as the original tumor; in contrast, in the observation group, two-thirds of the failures were in the same quadrant.
The two groups were statistically indistinguishable with respect to the rate of contralateral breast events, disease-free survival, and overall survival.
The rate of acute grade 3 or worse nonhematologic toxicities was similar in the observation group and radiation therapy group (4.0% vs. 4.2%, respectively), although lower-grade toxicities were more common in the latter. The rate of late grade 3 or worse radiation therapy toxicity was 0.7% in the group given this therapy.
Dr. McCormick disclosed no relevant conflicts of interest. Dr. Hwang disclosed that she is a consultant to Genomic Health and receives research funding from Merck and Novartis.
CHICAGO – Radiation therapy dramatically improves local control in ductal carcinoma in situ, even in cases having favorable features, investigators reported.
The randomized Radiation Therapy Oncology Group (RTOG) 9804 trial was conducted among 585 women who had "good-risk" ductal carcinoma in situ (DCIS), meaning small, asymptomatic tumors of low grade and with adequate resection margins after lumpectomy.
The main results showed that women had a significant 86% relative reduction in the risk of local failure if they received radiation therapy instead of observation. But the absolute 5-year rate of local failure was only about 3%, even without this intervention.
"We were able, using standard pathology methods and our Web-based RTOG pathology tool, to define good-risk or low-risk DCIS patients who had an extremely low event rate even with observation," said lead investigator Dr. Beryl McCormick at the annual meeting of the American Society of Clinical Oncology.
Nonetheless, "for this good-risk disease, the addition of radiation significantly reduced the risk of local failure. Clearly, we are expecting to follow this group out longer," added Dr. McCormick, a radiation oncologist and chief of the external beam radiotherapy service at Memorial Sloan-Kettering Cancer Center in New York.
"The study was a positive study," commented discussant Dr. Eun-Sil Shelley Hwang of Duke University Medical Center in Durham, N.C. "The reasons for that are debatable. On this analysis, there was a lower recurrence rate in the excision-only group than had been predicted, but what drove the positive findings was the much lower rate in the radiation therapy group."
Dr. Hwang speculated that the use of tamoxifen by about two-thirds of women may have contributed to the low rate of local recurrence seen even in the absence of radiation therapy.
"Further follow-up is required, because many of these patients [had] a short follow-up that may explain why we saw such low recurrence risks in this study," she proposed.
Women with DCIS who were above age 26 were eligible for RTOG 9804 if they had no symptoms (their tumors had been found mammographically or incidentally), had only low or intermediate tumor grade, had a tumor size of 2.5 cm or less, and had a resection margin width of at least 3 mm.
The 585 study participants were randomly assigned in balanced fashion to observation or radiation therapy, each with or without tamoxifen. (Overall, 62% received the drug.) Radiation therapy began within 12 weeks of final surgery, and consisted of 42.5-50.4 Gy, with no boost.
The main trial results showed that the actuarial 5-year rate of local failure (invasive or noninvasive) in the treated breast was 3.2% in the observation group and 0.4% in the radiation therapy group, corresponding to an 86% reduction in risk (hazard ratio, 0.14; P = .002), Dr. McCormick reported.
In the radiation therapy group, there were no local failures in the same quadrant as the original tumor; in contrast, in the observation group, two-thirds of the failures were in the same quadrant.
The two groups were statistically indistinguishable with respect to the rate of contralateral breast events, disease-free survival, and overall survival.
The rate of acute grade 3 or worse nonhematologic toxicities was similar in the observation group and radiation therapy group (4.0% vs. 4.2%, respectively), although lower-grade toxicities were more common in the latter. The rate of late grade 3 or worse radiation therapy toxicity was 0.7% in the group given this therapy.
Dr. McCormick disclosed no relevant conflicts of interest. Dr. Hwang disclosed that she is a consultant to Genomic Health and receives research funding from Merck and Novartis.
CHICAGO – Radiation therapy dramatically improves local control in ductal carcinoma in situ, even in cases having favorable features, investigators reported.
The randomized Radiation Therapy Oncology Group (RTOG) 9804 trial was conducted among 585 women who had "good-risk" ductal carcinoma in situ (DCIS), meaning small, asymptomatic tumors of low grade and with adequate resection margins after lumpectomy.
The main results showed that women had a significant 86% relative reduction in the risk of local failure if they received radiation therapy instead of observation. But the absolute 5-year rate of local failure was only about 3%, even without this intervention.
"We were able, using standard pathology methods and our Web-based RTOG pathology tool, to define good-risk or low-risk DCIS patients who had an extremely low event rate even with observation," said lead investigator Dr. Beryl McCormick at the annual meeting of the American Society of Clinical Oncology.
Nonetheless, "for this good-risk disease, the addition of radiation significantly reduced the risk of local failure. Clearly, we are expecting to follow this group out longer," added Dr. McCormick, a radiation oncologist and chief of the external beam radiotherapy service at Memorial Sloan-Kettering Cancer Center in New York.
"The study was a positive study," commented discussant Dr. Eun-Sil Shelley Hwang of Duke University Medical Center in Durham, N.C. "The reasons for that are debatable. On this analysis, there was a lower recurrence rate in the excision-only group than had been predicted, but what drove the positive findings was the much lower rate in the radiation therapy group."
Dr. Hwang speculated that the use of tamoxifen by about two-thirds of women may have contributed to the low rate of local recurrence seen even in the absence of radiation therapy.
"Further follow-up is required, because many of these patients [had] a short follow-up that may explain why we saw such low recurrence risks in this study," she proposed.
Women with DCIS who were above age 26 were eligible for RTOG 9804 if they had no symptoms (their tumors had been found mammographically or incidentally), had only low or intermediate tumor grade, had a tumor size of 2.5 cm or less, and had a resection margin width of at least 3 mm.
The 585 study participants were randomly assigned in balanced fashion to observation or radiation therapy, each with or without tamoxifen. (Overall, 62% received the drug.) Radiation therapy began within 12 weeks of final surgery, and consisted of 42.5-50.4 Gy, with no boost.
The main trial results showed that the actuarial 5-year rate of local failure (invasive or noninvasive) in the treated breast was 3.2% in the observation group and 0.4% in the radiation therapy group, corresponding to an 86% reduction in risk (hazard ratio, 0.14; P = .002), Dr. McCormick reported.
In the radiation therapy group, there were no local failures in the same quadrant as the original tumor; in contrast, in the observation group, two-thirds of the failures were in the same quadrant.
The two groups were statistically indistinguishable with respect to the rate of contralateral breast events, disease-free survival, and overall survival.
The rate of acute grade 3 or worse nonhematologic toxicities was similar in the observation group and radiation therapy group (4.0% vs. 4.2%, respectively), although lower-grade toxicities were more common in the latter. The rate of late grade 3 or worse radiation therapy toxicity was 0.7% in the group given this therapy.
Dr. McCormick disclosed no relevant conflicts of interest. Dr. Hwang disclosed that she is a consultant to Genomic Health and receives research funding from Merck and Novartis.
AT THE ANNUAL MEETING OF THE AMERICAN SOCIETY OF CLINICAL ONCOLOGY
DCIS Score Independently Predicts Ipsilateral Breast Events
CHICAGO – The new DCIS score, an offshoot of the Oncotype DX recurrence score, independently predicts the risk of ipsilateral recurrence or invasive disease in patients with ductal carcinoma in situ, according to a subgroup analysis from the prospective validation study.
The score correlated only moderately, poorly, or not at all with measures such as menopausal status and histologic grade among 327 patients with ductal carcinoma in situ (DCIS) treated with wide local excision and without radiation therapy, investigators reported.
And even after such measures were taken into account, each 50-point increase in the 100-point score was significantly associated with more than a doubling of the 10-year risk of recurrence or invasive disease in the same breast.
"The DCIS score provides independent information on ipsilateral breast risk beyond clinical and pathologic variables," lead author Dr. Sunil S. Badve said at the annual meeting of the American Society of Clinical Oncology.
"One can use the DCIS score to select patients with ... a clinically good prognosis who may have a higher DCIS score and may have a slightly [greater] risk than one would have estimated by traditional criteria," added Dr. Badve, a professor in the department of pathology and laboratory medicine and the department of internal medicine, and director of the Translational Genomics Core at Indiana University, Indianapolis.
"So particularly for the subset with a clinically good prognosis, we may be able to dissect which patients really might have a better prognosis and which patients may need to be more cautious."
Discussant Dr. Eun-Sil Shelley Hwang of Duke University Medical Center in Durham, N.C., called particular attention to the weak association between nuclear grade and DCIS Score.
"This is a very important point to make and a response to the criticism that’s often heard that the DCIS score or the Oncotype score may just be a surrogate – and a very expensive one – for proliferative index or Ki-67," she commented.
Next-generation sequencing and advances in epigenomics, proteomics, and molecular characterization of stroma are likely to make it possible to further risk-stratify DCIS in the future, according to Dr. Hwang.
"But none of this is important unless we can implement it into treatment of patients. So how this is implemented in our everyday practice will be very important to analyze – whether it impacts decision making, and whether it gives patients satisfaction with the decisions they make will also be very important to look at," she added.
"Finally, in this increasingly resource-constrained environment, cost-effectiveness issues will also be very important to analyze as we go forward," she noted.
Study analyses were based on a subset of women from the E5194 trial, a prospective study to validate the DCIS score, which contains 12 of the 21 genes included in the Oncotype DX recurrence score used in estrogen receptor–positive early-stage breast cancer.
All of the women had DCIS treated with wide local excision. They had a median age of 61 years and a median tumor size of 7 mm. Twenty-nine percent received tamoxifen. The median duration of follow-up was 8.8 years.
Results showed that the DCIS score was only moderately correlated with histologic grade (r = 0.41-0.46) and with the percentage of cells showing comedo necrosis (r = 0.49), and was poorly correlated with tumor size (r = 0.18), Dr. Badve reported. And there was no correlation with age, menopausal status, DCIS histologic pattern, or margin status.
In a multivariate analysis, each 50-point increase in DCIS score was associated with more than a doubling of the risk of a recurrence of DCIS or the occurrence of invasive disease in the ipsilateral breast (hazard ratio, 2.41; P = .02).
In addition, tumor size was an independent positive predictor of risk (HR for each 5-mm increase, 1.52; P = .01), and postmenopausal status was an independent negative predictor (HR, 0.49; P = .02).
Dr. Badve disclosed that he is a consultant to Genomic Health, which makes the assay. Dr. Hwang disclosed that she is a consultant to Genomic Health and receives research funding from Merck and Novartis.
CHICAGO – The new DCIS score, an offshoot of the Oncotype DX recurrence score, independently predicts the risk of ipsilateral recurrence or invasive disease in patients with ductal carcinoma in situ, according to a subgroup analysis from the prospective validation study.
The score correlated only moderately, poorly, or not at all with measures such as menopausal status and histologic grade among 327 patients with ductal carcinoma in situ (DCIS) treated with wide local excision and without radiation therapy, investigators reported.
And even after such measures were taken into account, each 50-point increase in the 100-point score was significantly associated with more than a doubling of the 10-year risk of recurrence or invasive disease in the same breast.
"The DCIS score provides independent information on ipsilateral breast risk beyond clinical and pathologic variables," lead author Dr. Sunil S. Badve said at the annual meeting of the American Society of Clinical Oncology.
"One can use the DCIS score to select patients with ... a clinically good prognosis who may have a higher DCIS score and may have a slightly [greater] risk than one would have estimated by traditional criteria," added Dr. Badve, a professor in the department of pathology and laboratory medicine and the department of internal medicine, and director of the Translational Genomics Core at Indiana University, Indianapolis.
"So particularly for the subset with a clinically good prognosis, we may be able to dissect which patients really might have a better prognosis and which patients may need to be more cautious."
Discussant Dr. Eun-Sil Shelley Hwang of Duke University Medical Center in Durham, N.C., called particular attention to the weak association between nuclear grade and DCIS Score.
"This is a very important point to make and a response to the criticism that’s often heard that the DCIS score or the Oncotype score may just be a surrogate – and a very expensive one – for proliferative index or Ki-67," she commented.
Next-generation sequencing and advances in epigenomics, proteomics, and molecular characterization of stroma are likely to make it possible to further risk-stratify DCIS in the future, according to Dr. Hwang.
"But none of this is important unless we can implement it into treatment of patients. So how this is implemented in our everyday practice will be very important to analyze – whether it impacts decision making, and whether it gives patients satisfaction with the decisions they make will also be very important to look at," she added.
"Finally, in this increasingly resource-constrained environment, cost-effectiveness issues will also be very important to analyze as we go forward," she noted.
Study analyses were based on a subset of women from the E5194 trial, a prospective study to validate the DCIS score, which contains 12 of the 21 genes included in the Oncotype DX recurrence score used in estrogen receptor–positive early-stage breast cancer.
All of the women had DCIS treated with wide local excision. They had a median age of 61 years and a median tumor size of 7 mm. Twenty-nine percent received tamoxifen. The median duration of follow-up was 8.8 years.
Results showed that the DCIS score was only moderately correlated with histologic grade (r = 0.41-0.46) and with the percentage of cells showing comedo necrosis (r = 0.49), and was poorly correlated with tumor size (r = 0.18), Dr. Badve reported. And there was no correlation with age, menopausal status, DCIS histologic pattern, or margin status.
In a multivariate analysis, each 50-point increase in DCIS score was associated with more than a doubling of the risk of a recurrence of DCIS or the occurrence of invasive disease in the ipsilateral breast (hazard ratio, 2.41; P = .02).
In addition, tumor size was an independent positive predictor of risk (HR for each 5-mm increase, 1.52; P = .01), and postmenopausal status was an independent negative predictor (HR, 0.49; P = .02).
Dr. Badve disclosed that he is a consultant to Genomic Health, which makes the assay. Dr. Hwang disclosed that she is a consultant to Genomic Health and receives research funding from Merck and Novartis.
CHICAGO – The new DCIS score, an offshoot of the Oncotype DX recurrence score, independently predicts the risk of ipsilateral recurrence or invasive disease in patients with ductal carcinoma in situ, according to a subgroup analysis from the prospective validation study.
The score correlated only moderately, poorly, or not at all with measures such as menopausal status and histologic grade among 327 patients with ductal carcinoma in situ (DCIS) treated with wide local excision and without radiation therapy, investigators reported.
And even after such measures were taken into account, each 50-point increase in the 100-point score was significantly associated with more than a doubling of the 10-year risk of recurrence or invasive disease in the same breast.
"The DCIS score provides independent information on ipsilateral breast risk beyond clinical and pathologic variables," lead author Dr. Sunil S. Badve said at the annual meeting of the American Society of Clinical Oncology.
"One can use the DCIS score to select patients with ... a clinically good prognosis who may have a higher DCIS score and may have a slightly [greater] risk than one would have estimated by traditional criteria," added Dr. Badve, a professor in the department of pathology and laboratory medicine and the department of internal medicine, and director of the Translational Genomics Core at Indiana University, Indianapolis.
"So particularly for the subset with a clinically good prognosis, we may be able to dissect which patients really might have a better prognosis and which patients may need to be more cautious."
Discussant Dr. Eun-Sil Shelley Hwang of Duke University Medical Center in Durham, N.C., called particular attention to the weak association between nuclear grade and DCIS Score.
"This is a very important point to make and a response to the criticism that’s often heard that the DCIS score or the Oncotype score may just be a surrogate – and a very expensive one – for proliferative index or Ki-67," she commented.
Next-generation sequencing and advances in epigenomics, proteomics, and molecular characterization of stroma are likely to make it possible to further risk-stratify DCIS in the future, according to Dr. Hwang.
"But none of this is important unless we can implement it into treatment of patients. So how this is implemented in our everyday practice will be very important to analyze – whether it impacts decision making, and whether it gives patients satisfaction with the decisions they make will also be very important to look at," she added.
"Finally, in this increasingly resource-constrained environment, cost-effectiveness issues will also be very important to analyze as we go forward," she noted.
Study analyses were based on a subset of women from the E5194 trial, a prospective study to validate the DCIS score, which contains 12 of the 21 genes included in the Oncotype DX recurrence score used in estrogen receptor–positive early-stage breast cancer.
All of the women had DCIS treated with wide local excision. They had a median age of 61 years and a median tumor size of 7 mm. Twenty-nine percent received tamoxifen. The median duration of follow-up was 8.8 years.
Results showed that the DCIS score was only moderately correlated with histologic grade (r = 0.41-0.46) and with the percentage of cells showing comedo necrosis (r = 0.49), and was poorly correlated with tumor size (r = 0.18), Dr. Badve reported. And there was no correlation with age, menopausal status, DCIS histologic pattern, or margin status.
In a multivariate analysis, each 50-point increase in DCIS score was associated with more than a doubling of the risk of a recurrence of DCIS or the occurrence of invasive disease in the ipsilateral breast (hazard ratio, 2.41; P = .02).
In addition, tumor size was an independent positive predictor of risk (HR for each 5-mm increase, 1.52; P = .01), and postmenopausal status was an independent negative predictor (HR, 0.49; P = .02).
Dr. Badve disclosed that he is a consultant to Genomic Health, which makes the assay. Dr. Hwang disclosed that she is a consultant to Genomic Health and receives research funding from Merck and Novartis.
AT THE ANNUAL MEETING OF THE AMERICAN SOCIETY OF CLINICAL ONCOLOGY
At 6 Months Postcesarean, 3% Have Chronic Pain
MONTEREY, CALIF. – Chronic pain after cesarean delivery is uncommon and generally mild, new data show. Additionally, women undergoing repeat cesarean do not have worse pain than their counterparts undergoing primary cesarean.
These were among the key findings of a pair of prospective longitudinal studies that investigators reported at the annual meeting of the Society for Obstetric Anesthesia and Perinatology.
The studies took place among healthy women from Brazil and the United States who were having scheduled elective cesarean deliveries with no prior labor, using standardized spinal anesthesia, surgical techniques, and multimodal postoperative analgesia.
Prevalence and description of chronic pain
In the first study, investigators led by Dr. Clemens M. Ortner of the department of anesthesiology and pain medicine at the University of Washington Medical Center in Seattle, followed 360 women from São Paulo, Brazil.
They used two tools recommended by the Initiative on Methods, Measurement, and Pain Assessment in Clinical Trials (IMMPACT), which is endorsed by the Food and Drug Administration: the Brief Pain Inventory (BPI), which captures the effect of pain on physical functioning, and the Short-Form McGill Pain Questionnaire 2 (SF-MPQ-2), which evaluates the nature of pain using 22 descriptors that are grouped into four overarching types of pain.
The women were a mean 31 years. For the large majority (70%), the cesarean was their first. Mean surgical time was 40 minutes.
Results with the BPI showed that 3% of women had pain at 6 months, Dr. Ortner reported. However, in the majority of cases, the pain was rated as mild.
Data from the SF-MPQ-2 suggested that the pain was most often of the neuropathic type and seldom of the continuous, intermittent, or affective types.
The leading descriptor the women selected for the pain, cited by 38% of those affected, was numbness. "Some would actually even argue [that numbness] does not qualify as a pain symptom," commented Dr. Ortner. Descriptors such as throbbing, tender, cramping, stabbing, and piercing were rarely used.
"This is the first prospective longitudinal study to characterize chronic pain after cesarean delivery using the SF-MPQ-2, and to our knowledge, is actually the first study that uses the SF-MPQ-2 that tries to characterize chronic postoperative pain," Dr. Ortner noted.
"Our incidence of chronic pain was very low at 6 months: It was 3%. However, this population was a very healthy population that had no other risk factors, like prior sensitization or other chronic pain syndromes," he cautioned.
"We can take this information from our study as a control for future analysis where we can use the SF-MPQ-2 to investigate chronic pain in high-risk populations undergoing cesarean delivery," he added.
Dr. Kenneth E. Nelson, session moderator and an obstetrical anesthesiologist at Wake Forest Baptist Medical Center in Winston Salem, N.C., wondered if the pain descriptors of the SF-MPQ-2 had the same meaning in the Brazilian context. "Some of those words are pretty esoteric. How culturally sensitive are they? Do you see a difference in different cultures using those words to describe pain, and is that potentially an issue with comparing one of these studies to another?" he asked.
"It is true that the SF-MPQ-2 was validated in an English-speaking population. However, these descriptors have been retrieved from a very, very large population of patients, how they describe their pain," Dr. Ortner replied. Additionally, the questionnaire underwent a rigorous validation process, whereby it was translated for a Portuguese-speaking population and then translated back to English. "However, of course, cultural differences can have an impact on these results, yes."
Pain with primary vs. repeat cesarean
In the second study – the only prospective evaluation of pain among healthy women after primary vs. repeat cesareans – a team led by Dr. Ruth Landau studied 451 women from São Paulo, Brazil, and Seattle.
Subsequent cesarean deliveries are noteworthy among surgical procedures in that surgeons typically go through the same scar again, a process that some speculate may contribute to greater pain, she noted.
"The hypothesis is that central sensitization as can be elicited by scar hyperalgesia after a previous cesarean section could result in increased pain if the surgery is repeated," she explained. "And the clinical implications for that would be that analgesic regimens may need to be adapted in women undergoing a repeat cesarean delivery, and that we should tailor our postoperative analgesia based on whether it’s a primary or repeat cesarean section."
Overall, 67% of the women studied were undergoing a primary cesarean delivery, whereas 33% were undergoing a repeat cesarean delivery. They had a mean age of about 30 years.
Results on the BPI showed relatively low scores (2-5 points on a 10-point scale) for pain – at rest, while sitting, and specifically for uterine cramping – at 12 hours and at 24 hours postoperatively, with no significant differences between the primary and repeat cesarean groups, reported Dr. Landau, who is professor and director of obstetric anesthesiology and clinical genetics, anesthesiology, and pain medicine at the University of Washington Medical Center.
At 48 hours, there was a trend whereby women undergoing repeat cesarean had a higher level of pain while at rest relative to women undergoing primary cesarean, but pain while sitting, uterine cramping, wound hyperalgesia, and opioid use were essentially the same.
There also were no significant differences between the primary and repeat cesarean groups with respect to average pain in the past week, worst pain in the past week, and pain now, at either 8 weeks or at 6 months postoperatively.
"In this cohort of healthy women undergoing an elective cesarean delivery under what is considered the best modality to have good intraoperative anesthesia and postoperative analgesia, we did not find a relevant difference in pain scores or analgesic use in the first 48 hours. Therefore, I cannot recommend we should change our practice in giving everybody the same thing," said Dr. Landau.
"I would however caution that we shouldn’t yet extrapolate these results to other clinical contexts, in particular, women who have high risk for pain, prior pain, a nonelective primary cesarean delivery, or women [operated on] with different surgical techniques," she added. "And I would propose ... that we do look into scar hyperalgesia in those who have had a previous cesarean delivery, in trying to tease out women who are potentially at risk for more severe pain postoperatively."
Session moderator Dr. Dennis C. Shay in a group anesthesia practice in San Diego asked, "Did you look at activity levels? I would imagine with a repeat C-section, they have a young child, so if the child is visiting, maybe they are getting up and walking around a lot more than if it was just their first child. And maybe that would make a difference."
It would be challenging to interpret how activity data relate to pain data, Dr. Landau replied. For example, women could be less active because they have more pain or because they are more comfortable.
Dr. Ortner and Dr. Landau said they had no relevant financial conflicts of interest.
MONTEREY, CALIF. – Chronic pain after cesarean delivery is uncommon and generally mild, new data show. Additionally, women undergoing repeat cesarean do not have worse pain than their counterparts undergoing primary cesarean.
These were among the key findings of a pair of prospective longitudinal studies that investigators reported at the annual meeting of the Society for Obstetric Anesthesia and Perinatology.
The studies took place among healthy women from Brazil and the United States who were having scheduled elective cesarean deliveries with no prior labor, using standardized spinal anesthesia, surgical techniques, and multimodal postoperative analgesia.
Prevalence and description of chronic pain
In the first study, investigators led by Dr. Clemens M. Ortner of the department of anesthesiology and pain medicine at the University of Washington Medical Center in Seattle, followed 360 women from São Paulo, Brazil.
They used two tools recommended by the Initiative on Methods, Measurement, and Pain Assessment in Clinical Trials (IMMPACT), which is endorsed by the Food and Drug Administration: the Brief Pain Inventory (BPI), which captures the effect of pain on physical functioning, and the Short-Form McGill Pain Questionnaire 2 (SF-MPQ-2), which evaluates the nature of pain using 22 descriptors that are grouped into four overarching types of pain.
The women were a mean 31 years. For the large majority (70%), the cesarean was their first. Mean surgical time was 40 minutes.
Results with the BPI showed that 3% of women had pain at 6 months, Dr. Ortner reported. However, in the majority of cases, the pain was rated as mild.
Data from the SF-MPQ-2 suggested that the pain was most often of the neuropathic type and seldom of the continuous, intermittent, or affective types.
The leading descriptor the women selected for the pain, cited by 38% of those affected, was numbness. "Some would actually even argue [that numbness] does not qualify as a pain symptom," commented Dr. Ortner. Descriptors such as throbbing, tender, cramping, stabbing, and piercing were rarely used.
"This is the first prospective longitudinal study to characterize chronic pain after cesarean delivery using the SF-MPQ-2, and to our knowledge, is actually the first study that uses the SF-MPQ-2 that tries to characterize chronic postoperative pain," Dr. Ortner noted.
"Our incidence of chronic pain was very low at 6 months: It was 3%. However, this population was a very healthy population that had no other risk factors, like prior sensitization or other chronic pain syndromes," he cautioned.
"We can take this information from our study as a control for future analysis where we can use the SF-MPQ-2 to investigate chronic pain in high-risk populations undergoing cesarean delivery," he added.
Dr. Kenneth E. Nelson, session moderator and an obstetrical anesthesiologist at Wake Forest Baptist Medical Center in Winston Salem, N.C., wondered if the pain descriptors of the SF-MPQ-2 had the same meaning in the Brazilian context. "Some of those words are pretty esoteric. How culturally sensitive are they? Do you see a difference in different cultures using those words to describe pain, and is that potentially an issue with comparing one of these studies to another?" he asked.
"It is true that the SF-MPQ-2 was validated in an English-speaking population. However, these descriptors have been retrieved from a very, very large population of patients, how they describe their pain," Dr. Ortner replied. Additionally, the questionnaire underwent a rigorous validation process, whereby it was translated for a Portuguese-speaking population and then translated back to English. "However, of course, cultural differences can have an impact on these results, yes."
Pain with primary vs. repeat cesarean
In the second study – the only prospective evaluation of pain among healthy women after primary vs. repeat cesareans – a team led by Dr. Ruth Landau studied 451 women from São Paulo, Brazil, and Seattle.
Subsequent cesarean deliveries are noteworthy among surgical procedures in that surgeons typically go through the same scar again, a process that some speculate may contribute to greater pain, she noted.
"The hypothesis is that central sensitization as can be elicited by scar hyperalgesia after a previous cesarean section could result in increased pain if the surgery is repeated," she explained. "And the clinical implications for that would be that analgesic regimens may need to be adapted in women undergoing a repeat cesarean delivery, and that we should tailor our postoperative analgesia based on whether it’s a primary or repeat cesarean section."
Overall, 67% of the women studied were undergoing a primary cesarean delivery, whereas 33% were undergoing a repeat cesarean delivery. They had a mean age of about 30 years.
Results on the BPI showed relatively low scores (2-5 points on a 10-point scale) for pain – at rest, while sitting, and specifically for uterine cramping – at 12 hours and at 24 hours postoperatively, with no significant differences between the primary and repeat cesarean groups, reported Dr. Landau, who is professor and director of obstetric anesthesiology and clinical genetics, anesthesiology, and pain medicine at the University of Washington Medical Center.
At 48 hours, there was a trend whereby women undergoing repeat cesarean had a higher level of pain while at rest relative to women undergoing primary cesarean, but pain while sitting, uterine cramping, wound hyperalgesia, and opioid use were essentially the same.
There also were no significant differences between the primary and repeat cesarean groups with respect to average pain in the past week, worst pain in the past week, and pain now, at either 8 weeks or at 6 months postoperatively.
"In this cohort of healthy women undergoing an elective cesarean delivery under what is considered the best modality to have good intraoperative anesthesia and postoperative analgesia, we did not find a relevant difference in pain scores or analgesic use in the first 48 hours. Therefore, I cannot recommend we should change our practice in giving everybody the same thing," said Dr. Landau.
"I would however caution that we shouldn’t yet extrapolate these results to other clinical contexts, in particular, women who have high risk for pain, prior pain, a nonelective primary cesarean delivery, or women [operated on] with different surgical techniques," she added. "And I would propose ... that we do look into scar hyperalgesia in those who have had a previous cesarean delivery, in trying to tease out women who are potentially at risk for more severe pain postoperatively."
Session moderator Dr. Dennis C. Shay in a group anesthesia practice in San Diego asked, "Did you look at activity levels? I would imagine with a repeat C-section, they have a young child, so if the child is visiting, maybe they are getting up and walking around a lot more than if it was just their first child. And maybe that would make a difference."
It would be challenging to interpret how activity data relate to pain data, Dr. Landau replied. For example, women could be less active because they have more pain or because they are more comfortable.
Dr. Ortner and Dr. Landau said they had no relevant financial conflicts of interest.
MONTEREY, CALIF. – Chronic pain after cesarean delivery is uncommon and generally mild, new data show. Additionally, women undergoing repeat cesarean do not have worse pain than their counterparts undergoing primary cesarean.
These were among the key findings of a pair of prospective longitudinal studies that investigators reported at the annual meeting of the Society for Obstetric Anesthesia and Perinatology.
The studies took place among healthy women from Brazil and the United States who were having scheduled elective cesarean deliveries with no prior labor, using standardized spinal anesthesia, surgical techniques, and multimodal postoperative analgesia.
Prevalence and description of chronic pain
In the first study, investigators led by Dr. Clemens M. Ortner of the department of anesthesiology and pain medicine at the University of Washington Medical Center in Seattle, followed 360 women from São Paulo, Brazil.
They used two tools recommended by the Initiative on Methods, Measurement, and Pain Assessment in Clinical Trials (IMMPACT), which is endorsed by the Food and Drug Administration: the Brief Pain Inventory (BPI), which captures the effect of pain on physical functioning, and the Short-Form McGill Pain Questionnaire 2 (SF-MPQ-2), which evaluates the nature of pain using 22 descriptors that are grouped into four overarching types of pain.
The women were a mean 31 years. For the large majority (70%), the cesarean was their first. Mean surgical time was 40 minutes.
Results with the BPI showed that 3% of women had pain at 6 months, Dr. Ortner reported. However, in the majority of cases, the pain was rated as mild.
Data from the SF-MPQ-2 suggested that the pain was most often of the neuropathic type and seldom of the continuous, intermittent, or affective types.
The leading descriptor the women selected for the pain, cited by 38% of those affected, was numbness. "Some would actually even argue [that numbness] does not qualify as a pain symptom," commented Dr. Ortner. Descriptors such as throbbing, tender, cramping, stabbing, and piercing were rarely used.
"This is the first prospective longitudinal study to characterize chronic pain after cesarean delivery using the SF-MPQ-2, and to our knowledge, is actually the first study that uses the SF-MPQ-2 that tries to characterize chronic postoperative pain," Dr. Ortner noted.
"Our incidence of chronic pain was very low at 6 months: It was 3%. However, this population was a very healthy population that had no other risk factors, like prior sensitization or other chronic pain syndromes," he cautioned.
"We can take this information from our study as a control for future analysis where we can use the SF-MPQ-2 to investigate chronic pain in high-risk populations undergoing cesarean delivery," he added.
Dr. Kenneth E. Nelson, session moderator and an obstetrical anesthesiologist at Wake Forest Baptist Medical Center in Winston Salem, N.C., wondered if the pain descriptors of the SF-MPQ-2 had the same meaning in the Brazilian context. "Some of those words are pretty esoteric. How culturally sensitive are they? Do you see a difference in different cultures using those words to describe pain, and is that potentially an issue with comparing one of these studies to another?" he asked.
"It is true that the SF-MPQ-2 was validated in an English-speaking population. However, these descriptors have been retrieved from a very, very large population of patients, how they describe their pain," Dr. Ortner replied. Additionally, the questionnaire underwent a rigorous validation process, whereby it was translated for a Portuguese-speaking population and then translated back to English. "However, of course, cultural differences can have an impact on these results, yes."
Pain with primary vs. repeat cesarean
In the second study – the only prospective evaluation of pain among healthy women after primary vs. repeat cesareans – a team led by Dr. Ruth Landau studied 451 women from São Paulo, Brazil, and Seattle.
Subsequent cesarean deliveries are noteworthy among surgical procedures in that surgeons typically go through the same scar again, a process that some speculate may contribute to greater pain, she noted.
"The hypothesis is that central sensitization as can be elicited by scar hyperalgesia after a previous cesarean section could result in increased pain if the surgery is repeated," she explained. "And the clinical implications for that would be that analgesic regimens may need to be adapted in women undergoing a repeat cesarean delivery, and that we should tailor our postoperative analgesia based on whether it’s a primary or repeat cesarean section."
Overall, 67% of the women studied were undergoing a primary cesarean delivery, whereas 33% were undergoing a repeat cesarean delivery. They had a mean age of about 30 years.
Results on the BPI showed relatively low scores (2-5 points on a 10-point scale) for pain – at rest, while sitting, and specifically for uterine cramping – at 12 hours and at 24 hours postoperatively, with no significant differences between the primary and repeat cesarean groups, reported Dr. Landau, who is professor and director of obstetric anesthesiology and clinical genetics, anesthesiology, and pain medicine at the University of Washington Medical Center.
At 48 hours, there was a trend whereby women undergoing repeat cesarean had a higher level of pain while at rest relative to women undergoing primary cesarean, but pain while sitting, uterine cramping, wound hyperalgesia, and opioid use were essentially the same.
There also were no significant differences between the primary and repeat cesarean groups with respect to average pain in the past week, worst pain in the past week, and pain now, at either 8 weeks or at 6 months postoperatively.
"In this cohort of healthy women undergoing an elective cesarean delivery under what is considered the best modality to have good intraoperative anesthesia and postoperative analgesia, we did not find a relevant difference in pain scores or analgesic use in the first 48 hours. Therefore, I cannot recommend we should change our practice in giving everybody the same thing," said Dr. Landau.
"I would however caution that we shouldn’t yet extrapolate these results to other clinical contexts, in particular, women who have high risk for pain, prior pain, a nonelective primary cesarean delivery, or women [operated on] with different surgical techniques," she added. "And I would propose ... that we do look into scar hyperalgesia in those who have had a previous cesarean delivery, in trying to tease out women who are potentially at risk for more severe pain postoperatively."
Session moderator Dr. Dennis C. Shay in a group anesthesia practice in San Diego asked, "Did you look at activity levels? I would imagine with a repeat C-section, they have a young child, so if the child is visiting, maybe they are getting up and walking around a lot more than if it was just their first child. And maybe that would make a difference."
It would be challenging to interpret how activity data relate to pain data, Dr. Landau replied. For example, women could be less active because they have more pain or because they are more comfortable.
Dr. Ortner and Dr. Landau said they had no relevant financial conflicts of interest.
FROM THE ANNUAL MEETING OF THE SOCIETY FOR OBSTETRIC ANESTHESIA AND PERINATOLOGY
Major Finding: Only 3% of women in the first study had pain at 6 months, and it was usually mild.
Data Source: Results were taken from one of a pair of prospective longitudinal studies. The first study was based on a cohort of 360 healthy women undergoing elective cesarean delivery with standardized spinal anesthesia, surgical techniques, and multimodal postoperative analgesia.
Disclosures: Dr. Ortner and Dr. Landau disclosed no relevant conflicts of interest.
Drug Combination Improved Local Analgesia After Cesarean
MONTEREY, CALIF. – Two drugs work better than one when it comes to reducing pain and inflammation with local therapy after cesarean delivery, researchers reported.
In a randomized trial of 60 women who used subcutaneous drug delivery, the area under the curve for postoperative pain scores while sitting was roughly 30% less and supplemental opioid requirements were roughly 40% less when the nonsteroidal anti-inflammatory drug ketorolac was added to bupivacaine. Wound exudate levels of the inflammatory markers interleukin-6 and -10 also were lower.
"Giving a low dose peripherally of nonsteroidal ketorolac has both an anti-inflammatory and an analgesic effect," commented lead author Dr. Brendan Carvalho, an anesthesiologist at Lucile Packard Children’s Hospital in Stanford, Calif.
"This suggests that there is a local mediation effect – this is not a systemic effect – and it may give birth to the whole concept of being able to give very small doses directly in the wound," he said at the annual meeting of the Society for Obstetric Anesthesia and Perinatology.
"You could even create a multimodal analgesic protocol with small doses of drugs that will not have the high side effects based on big systemic doses, and they can work exactly in the area that we know is most important for inflammation and pain propagation."
In contrast, there were no significant improvements in study outcomes when subcutaneous hydromorphone was added to bupivacaine. "This probably could have been anticipated if you look at the balance of the literature, looking at the efficacy of opioids and nonsteroidals peripherally administered," Dr. Carvalho said.
Session attendee Dr. David R. Gambling of the Sharp Mary Birch Hospital for Women in San Diego questioned the impact of the catheter used to deliver the drugs. "Do you think that having that foreign body in place affects the release of the mediators you were measuring, and if that’s the case, do you think patient movement and irritation of the foreign body could have had an effect on the result?" he asked.
The point is a good one, Dr. Carvalho acknowledged. However, "to be able to follow someone continuously [without a catheter in place] would require invasive [needle procedures] as well as punch biopsies. ... There’s sort of a long way to go, and maybe the whole idea behind this is to encourage people to think of better ways of doing this."
Dr. Andrea Fuller of the University of Colorado Hospital in Aurora, who also attended the session, said, "I’m wondering clinically how to use this. If you don’t have an ON-Q pump [to deliver the drug], are you doing things like asking your obstetricians to put some ketorolac in infiltrate right at the site, or even putting it in a TAP [transversus abdominus plane] block – it might be close enough."
"It’s way too early to say," Dr. Carvalho replied. "I am not recommending the use [of a nonsteroidal agent peripherally] yet, until we truly understand what these drugs are doing. ... It’s a growing area, and I think a few years from now, a lot of the drug administration will be where it should be as opposed to systemically."
The trial participants were 60 healthy women with term singleton pregnancies who underwent elective cesarean delivery with spinal anesthesia. The investigators placed elastomeric ON-Q pumps subcutaneously in the incisional wound to permit collection of wound exudate and instillation of agents.
The women were randomized equally into three groups given continuous subcutaneous instillation for 48 hours after surgery of bupivacaine at 10 mg/hr only (as an active control); bupivacaine plus ketorolac at 0.6 mg/hr; or bupivacaine plus hydromorphone at 0.04 mg/hr.
The drugs were intentionally "given in very small doses, which we believe would not be systemically effective, so it’s not a systemic absorption effect," Dr. Carvalho noted.
Pain intensity was measured at 4, 24, and 48 hours post surgery, both at rest and during activity, using the verbal pain scale, on which values range from 0 to 10. Wound exudate was collected at the same time points and assayed for a wide variety of cytokines.
On average, the women were about 32 years old and had a parity of 1. Eighty percent had had a previous cesarean delivery.
Trial results showed that the area under the curve for postoperative pain scores while sitting was approximately 250 for women given bupivacaine only, versus 175 for women given bupivacaine plus ketorolac (P = .018), Dr. Carvalho reported. There were no significant differences in pain scores while at rest.
Compared with bupivacaine alone, bupivacaine plus ketorolac was associated with lower levels of interleukin-6 (P = .012) and interleukin-10 (P = .004) in wound exudate.
Postoperative supplemental opioid analgesic use was approximately 25 mg of morphine equivalents for women in the bupivacaine-ketorolac group, compared with 40 mg for their counterparts in the bupivacaine-only group (P = .020).
None of the women had significant adverse events or study-related complications, and there were no hematomas, infections, or delays in wound healing. One woman developed a subincisional fluid collection.
"The majority of patients we enrolled had had previous cesarean deliveries, which may [have introduced] bias, as patients having second C-sections may respond differently from someone who is undergoing a cesarean for the first time," Dr. Carvalho acknowledged. Additionally, there was some "empiric guessing" involved in the selection of drug doses, and the study focused only on the acute inflammatory period.
"The next plan is to compare systemic versus peripheral administration of various other drugs, and to start understanding the mechanisms behind what is happening in the periphery," he concluded. "We have a very delicate balance where if we inhibit inflammation too much, then we have delayed wound healing. If we accelerate it too much, we might get hypertrophic scarring. So it’s a delicate balance that we can intervene on and maybe improve analgesia, but we mustn’t affect the balance of wound healing."
Dr. Carvalho disclosed that he has a relationship with I-Flow, manufacturer of the pump used in the study.
MONTEREY, CALIF. – Two drugs work better than one when it comes to reducing pain and inflammation with local therapy after cesarean delivery, researchers reported.
In a randomized trial of 60 women who used subcutaneous drug delivery, the area under the curve for postoperative pain scores while sitting was roughly 30% less and supplemental opioid requirements were roughly 40% less when the nonsteroidal anti-inflammatory drug ketorolac was added to bupivacaine. Wound exudate levels of the inflammatory markers interleukin-6 and -10 also were lower.
"Giving a low dose peripherally of nonsteroidal ketorolac has both an anti-inflammatory and an analgesic effect," commented lead author Dr. Brendan Carvalho, an anesthesiologist at Lucile Packard Children’s Hospital in Stanford, Calif.
"This suggests that there is a local mediation effect – this is not a systemic effect – and it may give birth to the whole concept of being able to give very small doses directly in the wound," he said at the annual meeting of the Society for Obstetric Anesthesia and Perinatology.
"You could even create a multimodal analgesic protocol with small doses of drugs that will not have the high side effects based on big systemic doses, and they can work exactly in the area that we know is most important for inflammation and pain propagation."
In contrast, there were no significant improvements in study outcomes when subcutaneous hydromorphone was added to bupivacaine. "This probably could have been anticipated if you look at the balance of the literature, looking at the efficacy of opioids and nonsteroidals peripherally administered," Dr. Carvalho said.
Session attendee Dr. David R. Gambling of the Sharp Mary Birch Hospital for Women in San Diego questioned the impact of the catheter used to deliver the drugs. "Do you think that having that foreign body in place affects the release of the mediators you were measuring, and if that’s the case, do you think patient movement and irritation of the foreign body could have had an effect on the result?" he asked.
The point is a good one, Dr. Carvalho acknowledged. However, "to be able to follow someone continuously [without a catheter in place] would require invasive [needle procedures] as well as punch biopsies. ... There’s sort of a long way to go, and maybe the whole idea behind this is to encourage people to think of better ways of doing this."
Dr. Andrea Fuller of the University of Colorado Hospital in Aurora, who also attended the session, said, "I’m wondering clinically how to use this. If you don’t have an ON-Q pump [to deliver the drug], are you doing things like asking your obstetricians to put some ketorolac in infiltrate right at the site, or even putting it in a TAP [transversus abdominus plane] block – it might be close enough."
"It’s way too early to say," Dr. Carvalho replied. "I am not recommending the use [of a nonsteroidal agent peripherally] yet, until we truly understand what these drugs are doing. ... It’s a growing area, and I think a few years from now, a lot of the drug administration will be where it should be as opposed to systemically."
The trial participants were 60 healthy women with term singleton pregnancies who underwent elective cesarean delivery with spinal anesthesia. The investigators placed elastomeric ON-Q pumps subcutaneously in the incisional wound to permit collection of wound exudate and instillation of agents.
The women were randomized equally into three groups given continuous subcutaneous instillation for 48 hours after surgery of bupivacaine at 10 mg/hr only (as an active control); bupivacaine plus ketorolac at 0.6 mg/hr; or bupivacaine plus hydromorphone at 0.04 mg/hr.
The drugs were intentionally "given in very small doses, which we believe would not be systemically effective, so it’s not a systemic absorption effect," Dr. Carvalho noted.
Pain intensity was measured at 4, 24, and 48 hours post surgery, both at rest and during activity, using the verbal pain scale, on which values range from 0 to 10. Wound exudate was collected at the same time points and assayed for a wide variety of cytokines.
On average, the women were about 32 years old and had a parity of 1. Eighty percent had had a previous cesarean delivery.
Trial results showed that the area under the curve for postoperative pain scores while sitting was approximately 250 for women given bupivacaine only, versus 175 for women given bupivacaine plus ketorolac (P = .018), Dr. Carvalho reported. There were no significant differences in pain scores while at rest.
Compared with bupivacaine alone, bupivacaine plus ketorolac was associated with lower levels of interleukin-6 (P = .012) and interleukin-10 (P = .004) in wound exudate.
Postoperative supplemental opioid analgesic use was approximately 25 mg of morphine equivalents for women in the bupivacaine-ketorolac group, compared with 40 mg for their counterparts in the bupivacaine-only group (P = .020).
None of the women had significant adverse events or study-related complications, and there were no hematomas, infections, or delays in wound healing. One woman developed a subincisional fluid collection.
"The majority of patients we enrolled had had previous cesarean deliveries, which may [have introduced] bias, as patients having second C-sections may respond differently from someone who is undergoing a cesarean for the first time," Dr. Carvalho acknowledged. Additionally, there was some "empiric guessing" involved in the selection of drug doses, and the study focused only on the acute inflammatory period.
"The next plan is to compare systemic versus peripheral administration of various other drugs, and to start understanding the mechanisms behind what is happening in the periphery," he concluded. "We have a very delicate balance where if we inhibit inflammation too much, then we have delayed wound healing. If we accelerate it too much, we might get hypertrophic scarring. So it’s a delicate balance that we can intervene on and maybe improve analgesia, but we mustn’t affect the balance of wound healing."
Dr. Carvalho disclosed that he has a relationship with I-Flow, manufacturer of the pump used in the study.
MONTEREY, CALIF. – Two drugs work better than one when it comes to reducing pain and inflammation with local therapy after cesarean delivery, researchers reported.
In a randomized trial of 60 women who used subcutaneous drug delivery, the area under the curve for postoperative pain scores while sitting was roughly 30% less and supplemental opioid requirements were roughly 40% less when the nonsteroidal anti-inflammatory drug ketorolac was added to bupivacaine. Wound exudate levels of the inflammatory markers interleukin-6 and -10 also were lower.
"Giving a low dose peripherally of nonsteroidal ketorolac has both an anti-inflammatory and an analgesic effect," commented lead author Dr. Brendan Carvalho, an anesthesiologist at Lucile Packard Children’s Hospital in Stanford, Calif.
"This suggests that there is a local mediation effect – this is not a systemic effect – and it may give birth to the whole concept of being able to give very small doses directly in the wound," he said at the annual meeting of the Society for Obstetric Anesthesia and Perinatology.
"You could even create a multimodal analgesic protocol with small doses of drugs that will not have the high side effects based on big systemic doses, and they can work exactly in the area that we know is most important for inflammation and pain propagation."
In contrast, there were no significant improvements in study outcomes when subcutaneous hydromorphone was added to bupivacaine. "This probably could have been anticipated if you look at the balance of the literature, looking at the efficacy of opioids and nonsteroidals peripherally administered," Dr. Carvalho said.
Session attendee Dr. David R. Gambling of the Sharp Mary Birch Hospital for Women in San Diego questioned the impact of the catheter used to deliver the drugs. "Do you think that having that foreign body in place affects the release of the mediators you were measuring, and if that’s the case, do you think patient movement and irritation of the foreign body could have had an effect on the result?" he asked.
The point is a good one, Dr. Carvalho acknowledged. However, "to be able to follow someone continuously [without a catheter in place] would require invasive [needle procedures] as well as punch biopsies. ... There’s sort of a long way to go, and maybe the whole idea behind this is to encourage people to think of better ways of doing this."
Dr. Andrea Fuller of the University of Colorado Hospital in Aurora, who also attended the session, said, "I’m wondering clinically how to use this. If you don’t have an ON-Q pump [to deliver the drug], are you doing things like asking your obstetricians to put some ketorolac in infiltrate right at the site, or even putting it in a TAP [transversus abdominus plane] block – it might be close enough."
"It’s way too early to say," Dr. Carvalho replied. "I am not recommending the use [of a nonsteroidal agent peripherally] yet, until we truly understand what these drugs are doing. ... It’s a growing area, and I think a few years from now, a lot of the drug administration will be where it should be as opposed to systemically."
The trial participants were 60 healthy women with term singleton pregnancies who underwent elective cesarean delivery with spinal anesthesia. The investigators placed elastomeric ON-Q pumps subcutaneously in the incisional wound to permit collection of wound exudate and instillation of agents.
The women were randomized equally into three groups given continuous subcutaneous instillation for 48 hours after surgery of bupivacaine at 10 mg/hr only (as an active control); bupivacaine plus ketorolac at 0.6 mg/hr; or bupivacaine plus hydromorphone at 0.04 mg/hr.
The drugs were intentionally "given in very small doses, which we believe would not be systemically effective, so it’s not a systemic absorption effect," Dr. Carvalho noted.
Pain intensity was measured at 4, 24, and 48 hours post surgery, both at rest and during activity, using the verbal pain scale, on which values range from 0 to 10. Wound exudate was collected at the same time points and assayed for a wide variety of cytokines.
On average, the women were about 32 years old and had a parity of 1. Eighty percent had had a previous cesarean delivery.
Trial results showed that the area under the curve for postoperative pain scores while sitting was approximately 250 for women given bupivacaine only, versus 175 for women given bupivacaine plus ketorolac (P = .018), Dr. Carvalho reported. There were no significant differences in pain scores while at rest.
Compared with bupivacaine alone, bupivacaine plus ketorolac was associated with lower levels of interleukin-6 (P = .012) and interleukin-10 (P = .004) in wound exudate.
Postoperative supplemental opioid analgesic use was approximately 25 mg of morphine equivalents for women in the bupivacaine-ketorolac group, compared with 40 mg for their counterparts in the bupivacaine-only group (P = .020).
None of the women had significant adverse events or study-related complications, and there were no hematomas, infections, or delays in wound healing. One woman developed a subincisional fluid collection.
"The majority of patients we enrolled had had previous cesarean deliveries, which may [have introduced] bias, as patients having second C-sections may respond differently from someone who is undergoing a cesarean for the first time," Dr. Carvalho acknowledged. Additionally, there was some "empiric guessing" involved in the selection of drug doses, and the study focused only on the acute inflammatory period.
"The next plan is to compare systemic versus peripheral administration of various other drugs, and to start understanding the mechanisms behind what is happening in the periphery," he concluded. "We have a very delicate balance where if we inhibit inflammation too much, then we have delayed wound healing. If we accelerate it too much, we might get hypertrophic scarring. So it’s a delicate balance that we can intervene on and maybe improve analgesia, but we mustn’t affect the balance of wound healing."
Dr. Carvalho disclosed that he has a relationship with I-Flow, manufacturer of the pump used in the study.
FROM THE ANNUAL MEETING OF THE SOCIETY FOR OBSTETRIC ANESTHESIA AND PERINATOLOGY
Paclitaxel Matches New Drugs as First-Line Breast Cancer Therapy
CHICAGO – Two fairly new and expensive drugs for breast cancer were no more effective or safer than a drug that has been used for this disease for over a decade, finds a phase III cooperative group trial among nearly 800 women with chemotherapy-naive locally recurrent or metastatic disease.
The trial pitted the newer nab-paclitaxel (Abraxane) and ixabepilone (Ixempra) against the older paclitaxel (Taxol) as a control, each given weekly, along with bevacizumab (Avastin) given every 2 weeks, as first-line therapy.
Trial results reported at the annual meeting of the American Society of Clinical Oncology showed that progression-free survival was not superior with nab-paclitaxel and was in fact inferior with ixabepilone, translating to a 53% higher risk of progression or death relative to paclitaxel, first author Dr. Hope S. Rugo reported in a press briefing.
Moreover, rates of grade 3 or worse adverse events were higher with both of the newer agents than with the old stand-by.
"These data suggest that similar patients could be appropriately treated with weekly paclitaxel," said Dr. Rugo, professor of medicine and director of breast oncology and clinical trials education at the University of California, San Francisco, Helen Diller Family Comprehensive Cancer Center, San Francisco.
"Interestingly, ixabepilone is an epothilone where preclinical and some clinical data suggest an ability to reverse resistance to the microtubule inhibitors like paclitaxel, and nab-paclitaxel has similar data," she added. "However, in this chemotherapy-naive population, these results showed equivalency or inferiority."
"These are all good drugs, these are all major-league baseball players. I want them all on my team," asserted press briefing moderator Dr. Nicholas Vogelzang, chair and medical director, Developmental Therapeutics Committee, US Oncology, Comprehensive Cancer Centers of Nevada. "And when they are used to treat breast cancer, a patient should be considered for all of them, perhaps in different sequences for different patients."
The trial’s findings do not preclude use of at least nab-paclitaxel in selected patients similar to those studied or in other treatment settings, according to Dr. Rugo.
"Based on our data, it’s very unlikely that [nab-paclitaxel] is superior to paclitaxel, but it could be used as an alternative in patients who had a reason not to receive paclitaxel," such as an allergy to cremophor (paclitaxel’s solvent), or diabetes making it hard to tolerate the steroids needed as premedication, she said. Also, the drug is effective in patients with later-stage metastatic breast cancer or with cancers that have progressed on other taxanes.
Finally, nab-paclitaxel is a useful alternative in the context of drug shortages. "In the setting where paclitaxel is not readily available, nab-paclitaxel could clearly be used in its place," she said.
Of note, however, data have shown that nab-paclitaxel given at a lower dose of 100 mg/m2 – one-third lower than the dose used in the trial – is less toxic. Therefore, "in patients in whom nab-paclitaxel is chosen ... we should be using the 100 mg/m2 dose," Dr. Rugo recommended.
The trial, formally known as CALGB 40502/NCCTG N063H and sponsored by the National Cancer Institute, randomized 799 patients to open-label treatment with one of the three chemotherapies – paclitaxel (90 mg/m2 weekly, the most commonly used dosage schedule), nab-paclitaxel (150 mg/m2 weekly), or ixabepilone (16 mg/m2 weekly) – plus bevacizumab every 2 weeks.
All chemotherapy was given on a 3-weeks-on, 1-week-off schedule. Patients who had a response or stable disease after six cycles could stop chemotherapy and continue with the bevacizumab alone.
With a median follow-up of 12 months, analyses showed that progression-free survival was no better with nab-paclitaxel (9.2 months; hazard ratio, 1.19; P = .12) and was actually worse with ixabepilone (7.6 months; hazard ratio, 1.53; P less than .0001) than with paclitaxel (10.6 months), Dr. Rugo reported.
The overall rate of grade 3 or worse adverse events was 55% with paclitaxel, compared with 79% with nab-paclitaxel and 59% with ixabepilone.
The rate of grade 3 or worse nonhematologic adverse events was significantly higher with the newer agents than with the older one. "We saw increased peripheral neuropathy in the experimental arms as compared with the control arms as well," Dr. Rugo noted. The rate of grade 3 or worse hematologic events was significantly higher with nab-paclitaxel and significantly lower with ixabepilone.
The findings are likely to remain relevant despite the Food and Drug Administration’s withdrawal of approval for bevacizumab, according to Dr. Rugo. "Interestingly, in our study, we obviously don’t have a control arm without bevacizumab, but the progression-free survival in the control arm was almost identical to ECOG 2100," which compared paclitaxel with and without bevacizumab. "And I think for that reason, it’s unlikely that there would be a big difference if you redid the whole study without bevacizumab," she said.
Dr. Rugo said she receives research funding from Abraxis BioScience, Bristol-Myers Squibb, and Roche/Genentech. Dr. Vogelzang said he is a consultant to Amgen, AVEO, Bayer, Celgene(U), Dendreon, Eisai, GE Healthcare, Genentech, GlaxoSmithKline, Johnson & Johnson, Keryx, Medscape, Novartis, Oncogenex, Pfizer, and Wilex; he receives honoraria from Amgen, Bayer, Clinical Care Options, Genentech, Imedex, Lilly, Lippincott, Williams, and Wilkins, Medscape, Novartis, Onyx, Pfizer, and Veridex; and he receives research funding from Algeta, Pfizer, Progenics, and Tokai Pharmaceuticals.
CHICAGO – Two fairly new and expensive drugs for breast cancer were no more effective or safer than a drug that has been used for this disease for over a decade, finds a phase III cooperative group trial among nearly 800 women with chemotherapy-naive locally recurrent or metastatic disease.
The trial pitted the newer nab-paclitaxel (Abraxane) and ixabepilone (Ixempra) against the older paclitaxel (Taxol) as a control, each given weekly, along with bevacizumab (Avastin) given every 2 weeks, as first-line therapy.
Trial results reported at the annual meeting of the American Society of Clinical Oncology showed that progression-free survival was not superior with nab-paclitaxel and was in fact inferior with ixabepilone, translating to a 53% higher risk of progression or death relative to paclitaxel, first author Dr. Hope S. Rugo reported in a press briefing.
Moreover, rates of grade 3 or worse adverse events were higher with both of the newer agents than with the old stand-by.
"These data suggest that similar patients could be appropriately treated with weekly paclitaxel," said Dr. Rugo, professor of medicine and director of breast oncology and clinical trials education at the University of California, San Francisco, Helen Diller Family Comprehensive Cancer Center, San Francisco.
"Interestingly, ixabepilone is an epothilone where preclinical and some clinical data suggest an ability to reverse resistance to the microtubule inhibitors like paclitaxel, and nab-paclitaxel has similar data," she added. "However, in this chemotherapy-naive population, these results showed equivalency or inferiority."
"These are all good drugs, these are all major-league baseball players. I want them all on my team," asserted press briefing moderator Dr. Nicholas Vogelzang, chair and medical director, Developmental Therapeutics Committee, US Oncology, Comprehensive Cancer Centers of Nevada. "And when they are used to treat breast cancer, a patient should be considered for all of them, perhaps in different sequences for different patients."
The trial’s findings do not preclude use of at least nab-paclitaxel in selected patients similar to those studied or in other treatment settings, according to Dr. Rugo.
"Based on our data, it’s very unlikely that [nab-paclitaxel] is superior to paclitaxel, but it could be used as an alternative in patients who had a reason not to receive paclitaxel," such as an allergy to cremophor (paclitaxel’s solvent), or diabetes making it hard to tolerate the steroids needed as premedication, she said. Also, the drug is effective in patients with later-stage metastatic breast cancer or with cancers that have progressed on other taxanes.
Finally, nab-paclitaxel is a useful alternative in the context of drug shortages. "In the setting where paclitaxel is not readily available, nab-paclitaxel could clearly be used in its place," she said.
Of note, however, data have shown that nab-paclitaxel given at a lower dose of 100 mg/m2 – one-third lower than the dose used in the trial – is less toxic. Therefore, "in patients in whom nab-paclitaxel is chosen ... we should be using the 100 mg/m2 dose," Dr. Rugo recommended.
The trial, formally known as CALGB 40502/NCCTG N063H and sponsored by the National Cancer Institute, randomized 799 patients to open-label treatment with one of the three chemotherapies – paclitaxel (90 mg/m2 weekly, the most commonly used dosage schedule), nab-paclitaxel (150 mg/m2 weekly), or ixabepilone (16 mg/m2 weekly) – plus bevacizumab every 2 weeks.
All chemotherapy was given on a 3-weeks-on, 1-week-off schedule. Patients who had a response or stable disease after six cycles could stop chemotherapy and continue with the bevacizumab alone.
With a median follow-up of 12 months, analyses showed that progression-free survival was no better with nab-paclitaxel (9.2 months; hazard ratio, 1.19; P = .12) and was actually worse with ixabepilone (7.6 months; hazard ratio, 1.53; P less than .0001) than with paclitaxel (10.6 months), Dr. Rugo reported.
The overall rate of grade 3 or worse adverse events was 55% with paclitaxel, compared with 79% with nab-paclitaxel and 59% with ixabepilone.
The rate of grade 3 or worse nonhematologic adverse events was significantly higher with the newer agents than with the older one. "We saw increased peripheral neuropathy in the experimental arms as compared with the control arms as well," Dr. Rugo noted. The rate of grade 3 or worse hematologic events was significantly higher with nab-paclitaxel and significantly lower with ixabepilone.
The findings are likely to remain relevant despite the Food and Drug Administration’s withdrawal of approval for bevacizumab, according to Dr. Rugo. "Interestingly, in our study, we obviously don’t have a control arm without bevacizumab, but the progression-free survival in the control arm was almost identical to ECOG 2100," which compared paclitaxel with and without bevacizumab. "And I think for that reason, it’s unlikely that there would be a big difference if you redid the whole study without bevacizumab," she said.
Dr. Rugo said she receives research funding from Abraxis BioScience, Bristol-Myers Squibb, and Roche/Genentech. Dr. Vogelzang said he is a consultant to Amgen, AVEO, Bayer, Celgene(U), Dendreon, Eisai, GE Healthcare, Genentech, GlaxoSmithKline, Johnson & Johnson, Keryx, Medscape, Novartis, Oncogenex, Pfizer, and Wilex; he receives honoraria from Amgen, Bayer, Clinical Care Options, Genentech, Imedex, Lilly, Lippincott, Williams, and Wilkins, Medscape, Novartis, Onyx, Pfizer, and Veridex; and he receives research funding from Algeta, Pfizer, Progenics, and Tokai Pharmaceuticals.
CHICAGO – Two fairly new and expensive drugs for breast cancer were no more effective or safer than a drug that has been used for this disease for over a decade, finds a phase III cooperative group trial among nearly 800 women with chemotherapy-naive locally recurrent or metastatic disease.
The trial pitted the newer nab-paclitaxel (Abraxane) and ixabepilone (Ixempra) against the older paclitaxel (Taxol) as a control, each given weekly, along with bevacizumab (Avastin) given every 2 weeks, as first-line therapy.
Trial results reported at the annual meeting of the American Society of Clinical Oncology showed that progression-free survival was not superior with nab-paclitaxel and was in fact inferior with ixabepilone, translating to a 53% higher risk of progression or death relative to paclitaxel, first author Dr. Hope S. Rugo reported in a press briefing.
Moreover, rates of grade 3 or worse adverse events were higher with both of the newer agents than with the old stand-by.
"These data suggest that similar patients could be appropriately treated with weekly paclitaxel," said Dr. Rugo, professor of medicine and director of breast oncology and clinical trials education at the University of California, San Francisco, Helen Diller Family Comprehensive Cancer Center, San Francisco.
"Interestingly, ixabepilone is an epothilone where preclinical and some clinical data suggest an ability to reverse resistance to the microtubule inhibitors like paclitaxel, and nab-paclitaxel has similar data," she added. "However, in this chemotherapy-naive population, these results showed equivalency or inferiority."
"These are all good drugs, these are all major-league baseball players. I want them all on my team," asserted press briefing moderator Dr. Nicholas Vogelzang, chair and medical director, Developmental Therapeutics Committee, US Oncology, Comprehensive Cancer Centers of Nevada. "And when they are used to treat breast cancer, a patient should be considered for all of them, perhaps in different sequences for different patients."
The trial’s findings do not preclude use of at least nab-paclitaxel in selected patients similar to those studied or in other treatment settings, according to Dr. Rugo.
"Based on our data, it’s very unlikely that [nab-paclitaxel] is superior to paclitaxel, but it could be used as an alternative in patients who had a reason not to receive paclitaxel," such as an allergy to cremophor (paclitaxel’s solvent), or diabetes making it hard to tolerate the steroids needed as premedication, she said. Also, the drug is effective in patients with later-stage metastatic breast cancer or with cancers that have progressed on other taxanes.
Finally, nab-paclitaxel is a useful alternative in the context of drug shortages. "In the setting where paclitaxel is not readily available, nab-paclitaxel could clearly be used in its place," she said.
Of note, however, data have shown that nab-paclitaxel given at a lower dose of 100 mg/m2 – one-third lower than the dose used in the trial – is less toxic. Therefore, "in patients in whom nab-paclitaxel is chosen ... we should be using the 100 mg/m2 dose," Dr. Rugo recommended.
The trial, formally known as CALGB 40502/NCCTG N063H and sponsored by the National Cancer Institute, randomized 799 patients to open-label treatment with one of the three chemotherapies – paclitaxel (90 mg/m2 weekly, the most commonly used dosage schedule), nab-paclitaxel (150 mg/m2 weekly), or ixabepilone (16 mg/m2 weekly) – plus bevacizumab every 2 weeks.
All chemotherapy was given on a 3-weeks-on, 1-week-off schedule. Patients who had a response or stable disease after six cycles could stop chemotherapy and continue with the bevacizumab alone.
With a median follow-up of 12 months, analyses showed that progression-free survival was no better with nab-paclitaxel (9.2 months; hazard ratio, 1.19; P = .12) and was actually worse with ixabepilone (7.6 months; hazard ratio, 1.53; P less than .0001) than with paclitaxel (10.6 months), Dr. Rugo reported.
The overall rate of grade 3 or worse adverse events was 55% with paclitaxel, compared with 79% with nab-paclitaxel and 59% with ixabepilone.
The rate of grade 3 or worse nonhematologic adverse events was significantly higher with the newer agents than with the older one. "We saw increased peripheral neuropathy in the experimental arms as compared with the control arms as well," Dr. Rugo noted. The rate of grade 3 or worse hematologic events was significantly higher with nab-paclitaxel and significantly lower with ixabepilone.
The findings are likely to remain relevant despite the Food and Drug Administration’s withdrawal of approval for bevacizumab, according to Dr. Rugo. "Interestingly, in our study, we obviously don’t have a control arm without bevacizumab, but the progression-free survival in the control arm was almost identical to ECOG 2100," which compared paclitaxel with and without bevacizumab. "And I think for that reason, it’s unlikely that there would be a big difference if you redid the whole study without bevacizumab," she said.
Dr. Rugo said she receives research funding from Abraxis BioScience, Bristol-Myers Squibb, and Roche/Genentech. Dr. Vogelzang said he is a consultant to Amgen, AVEO, Bayer, Celgene(U), Dendreon, Eisai, GE Healthcare, Genentech, GlaxoSmithKline, Johnson & Johnson, Keryx, Medscape, Novartis, Oncogenex, Pfizer, and Wilex; he receives honoraria from Amgen, Bayer, Clinical Care Options, Genentech, Imedex, Lilly, Lippincott, Williams, and Wilkins, Medscape, Novartis, Onyx, Pfizer, and Veridex; and he receives research funding from Algeta, Pfizer, Progenics, and Tokai Pharmaceuticals.
FROM THE ANNUAL MEETING OF THE AMERICAN SOCIETY OF CLINICAL ONCOLOGY
Major Finding: With a median follow-up of 12 months, progression-free survival was 10.6 months with paclitaxel, which was comparable to nab-paclitaxel (9.2 months; hazard ratio, 1.19; P = .12) and better than with ixabepilone (7.6 months; hazard ratio, 1.53; P less than .0001).
Data Source: A randomized phase III trial among 799 women with locally recurrent or metastatic breast cancer receiving bevacizumab as first line therapy (CALGB 40502/NCCTG N063H).
Disclosures: Dr. Rugo said she receives research funding from Abraxis BioScience, Bristol-Myers Squibb, and Roche/Genentech. Dr. Vogelzang said he is a consultant to Amgen, AVEO, Bayer, Celgene(U), Dendreon, Eisai, GE Healthcare, Genentech, GlaxoSmithKline, Johnson & Johnson, Keryx, Medscape, Novartis, Oncogenex, Pfizer, and Wilex; he receives honoraria from Amgen, Bayer, Clinical Care Options, Genentech, Imedex, Lilly, Lippincott, Williams, and Wilkins, Medscape, Novartis, Onyx, Pfizer, and Veridex; and he receives research funding from Algeta, Pfizer, Progenics, and Tokai Pharmaceuticals.