User login
A Transdisciplinary COVID-19 Early Respiratory Intervention Protocol: An Implementation Story
My colleague asked, “Do you remember that patient?” I froze because, like most emergency physicians, this phrase haunts me. It was the early days of the COVID-19 epidemic, and the story that followed was upsetting. A patient who looked comfortable when I admitted him was intubated hours later by the rapid response team who was called to the floor. All I could think was, “But he looked so comfortable when I admitted him; he was just on a couple of liters of oxygen. Why was he intubated?”
In the days after COVID-19 arrived in our region, there were many such stories of patients sent to the floor from the Emergency Department who were intubated shortly after admission. Many of those patients subsequently endured prolonged and complicated courses on the ventilator. While we would typically use noninvasive modalities such as high-flow nasal cannula (HFNC) or noninvasive ventilation (NIV) for acute respiratory failure, our quickness to intubate was driven by two factors: (1) early reports that noninvasive modalities posed a high risk of failure and subsequent intubation and (2) fear that HFNC and NIV would aerosolize SARS-CoV-2 and unnecessarily expose the heath care team.1 We would soon find out that our thinking was flawed on both accounts.
RETHINKING INITIAL ASSUMPTIONS
When we dug into the evidence for early intubation, we realized that these recommendations were based on a 12-patient series in which 5 patients were trialed on NIV but ultimately intubated and placed on invasive mechanical ventilation (IMV). As the pandemic progressed, more case series and small studies were published, revealing a different picture.2 Sun and colleagues reported a multifaceted intervention of 610 inpatients, of whom 10% were critically ill, that identified at-risk patients and used NIV or HFNC and awake proning. Reportedly, fewer than 1% required IMV.3 Similarly, a small study found intubation was avoided in 85% of patients with severe acute respiratory failure caused by COVID-19 with use of HFNC and NIV.4 Early findings from New York University in New York, New York, where only 8.5% of patients undergoing IMV were extubated by the time of outcome reporting, suggest early IMV could lead to poor outcomes.5
Still, we had concerns about use of HFNC and NIV because of worries about the health and safety of other patients and particularly that of healthcare workers (HCWs) because they have been disproportionately affected by the disease.6 Fortunately, we identified emerging data that revealed that HFNC is no more aerosolizing than low-flow nasal cannula or a nonrebreather mask and droplet spread is reduced with a surgical mask.7,8 In light of these new studies and our own developing experience with the disease, we felt that there was insufficient evidence to continue following the “early intubation” protocol in patients with COVID-19. It was time for a new paradigm.
GATHERING EVIDENCE AND STAKEHOLDERS
In order to effectively and quickly change our respiratory pathway for these patients, we initially sought out protocols from other institutions through social media. These protocols, supported by early data from those sites, informed our process. We considered data from various sources, including emergency medicine, hospital medicine, and critical care. We then assembled stakeholders within our organization from emergency medicine, hospital medicine, critical care, and respiratory therapy because our protocol would need endorsement from all key players within our organization who cared for these patients across the potential spectrum of care. We made sure that all stakeholders understood that the quality of the evidence for treatment of this novel disease was much lower than our typical threshold to change practice, but that we aimed to reflect the best evidence to date. We also were careful to identify pathways that would be amenable to near-immediate implementation.
UNVEILING A NOVEL PROTOCOL
Our group reached consensus within 48 hours and quickly disseminated our first draft of the protocol (Appendix Figure). Dubbed the “Early Intervention Respiratory Protocol,” it differed from usual management in several ways. First, we had consistently observed (and confirmed from the literature) a phenotype of patients with “silent hypoxemia”9 (that is, a subset of patients who presented with profound hypoxemia but minimally increased work of breathing). The protocol encouraged tolerance of lower oxygen saturations than is usually seen on inpatient units. This required ensuring all stakeholders were comfortable with a target oxygen saturation of 88%. Second, the protocol leveraged early “awake” proning by patients. Historically, proning is used in mechanically ventilated patients with acute respiratory distress syndrome (ARDS) to improve ventilation-perfusion matching, promote more uniform ventilation, and increase end-expiratory lung volume.10 Prior literature was limited to the use of awake proning in small case series of ARDS, but given our limitations in terms of ICU capacity, we agreed to trial awake proning in a sizable proportion of our COVID-19 patients outside the ICU.11,12 Finally, we clarified safe practices regarding the risk of aerosolization with noninvasive modalities. Local infection control determined that HFNC wa not aerosol generating, and use of surgical masks was added for further protection from respiratory droplets. In addition, airborne personal protective equipment was to be worn on the inpatient ward, and we used NIV sparingly and preferentially placed these patients in negative pressure rooms, if available.13
Implementation of the protocol involved aggressive dissemination and education (Table). A single-page protocol was designed for ease of use at the bedside that included anticipatory guidance regarding aerosolization and addressed potential resistance to awake proning because of concerns regarding safety and hassle. Departmental leaders disseminated the protocol throughout the institution with tailored education on the rationale and acknowledgment of a reversal in approach. In addition to email, we used text messaging (WhatsApp) and a comprehensive living document (Google Drive) to reach clinicians.
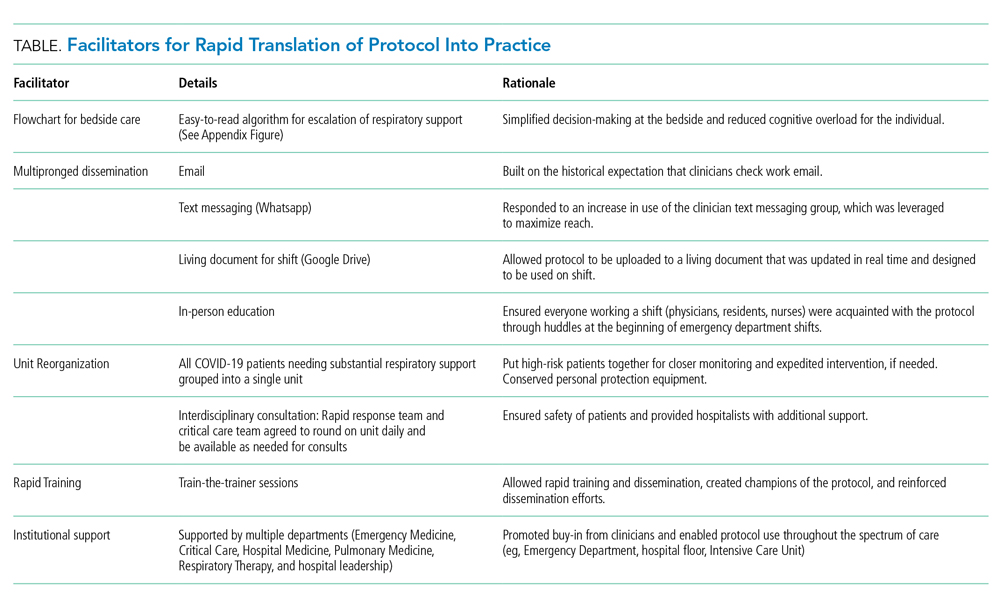
For ease of monitoring and safety, we designated a COVID-19 intermediate care unit. We partnered with the unit medical director, nurse educator, and a focused group of hospitalists, conducting individual train-the-trainer sessions. This training was carried forward, and all nurses, respiratory therapists, and clinicians were trained on the early aggressive respiratory protocol within 12 hours of protocol approval. In addition, the rapid response and critical care teams agreed to round on the COVID-19 intermediate care unit daily.
As a result of these efforts, adoption of the protocol was essentially immediate across the institution. We had shifted the mindset of a diverse group of clinicians regarding how to support the respiratory status of these patients, but also detected reductions in the proportion of patients undergoing IMV and ICU admission (we are planning to report these results separately).
TRANSLATING KNOWLEDGE INTO PRACTICE
The COVID-19 pandemic has highlighted the importance of having cognitive flexibility when the evidence base is rapidly changing and there is a need for rapid dissemination of knowledge. Even in clinical scenarios with an abundance of high-quality evidence, a gap in knowledge translation on the order of a decade often exists. In contrast, a pandemic involving a novel virus highlights an urgent need for adaptive knowledge translation in the present moment rather than a decade later. In the absence of robust evidence regarding SARS-CoV-2, early management of COVID-19 was based on expert recommendations and experience with other disease processes. Even so, we should anticipate that management paradigms may shift, and we should constantly seek out emerging evidence to adjust our mindset (and protocols like this) accordingly. Our original protocol was based on nearly nonexistent evidence, but we anticipated that, in a pandemic, data would accumulate quickly, so we prioritized rapid translation of new information into practice. In fact, further evidence has emerged regarding the improvement in oxygenation in COVID-19 patients with self-proning.14
The final step is evaluating the success of both clinical and implementation outcomes. We are attempting to identify changes in intubation, length of stay, days on ventilator, and days in ICU. In addition, we will measure feasibility and adaptability. We are also attempting, in real time, to identify barriers to its use, including conducting qualitative interviews to understand whether there were unintended consequences to use of the protocol. This endeavor highlights how the COVID-19 pandemic, for all its tragedy, may represent an important era for implementation science: a time when emerging literature from a variety of sources can be implemented in days rather than years.
1. World Health Organization. Clinical management of severe acute respiratory infection (SARI) when COVID-19 disease is suspected. 2020. https://www.who.int/publications-detail/clinical-management-of-severe-acute-respiratory-infection-when-novel-coronavirus-(ncov)-infection-is-suspected. Accessed March 25, 2020.
2. Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054-1062. https://doi.org/10.1016/s0140-6736(20)30566-3.
3. Sun Q, Qiu H, Huang M, Yang Y. Lower mortality of COVID-19 by early recognition and intervention: experience from Jiangsu Province. Ann Intensive Care. 2020;10(1):33. https://doi.org/10.1186/s13613-020-00650-2.
4. Wang K, Zhao W, Li J, Shu W, Duan J. The experience of high-flow nasal cannula in hospitalized patients with 2019 novel coronavirus-infected pneumonia in two hospitals of Chongqing, China. Ann Intensive Care. 2020;10(1):37. https://doi.org/10.1186/s13613-020-00653-z.
5. Petrilli C, Jones SA, Yang J, Rajagopalan H, et al. Factors associated with hospitalization and critical illness among 4,103 patients with Covid-19 disease in New York City [preprint]. medRxiv. 2020. https://doi.org/10.1101/2020.04.08.20057794. Accessed April 12, 2020.
6. Wang D, Hu B, Hu C, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus–infected pneumonia in Wuhan, China. JAMA. 2020;323(11):1061-1069. https://doi.org/10.1001/jama.2020.1585.
7. Leonard S, Volakis L, DeBellis R, Kahlon A, Mayar S. Transmission Assessment Report: High Velocity Nasal Insufflation (HVNI) Therapy Application in Management of COVID-19. March 25, 2020. Vapotherm Blog. 2020. https://vapotherm.com/blog/transmission-assessment-report/. Accessed March 25, 2020.
8. Iwashyna TJ, Boehman A, Capecelatro J, Cohn A, JM. C. Variation in aerosol production across oxygen delivery devices in 2 spontaneously breathing human subjects [preprint]. medRxiv. 2020. https://doi.org/10.1101/2020.04.15.20066688. Accessed April 20, 2020.
9. Meng L, Qiu H, Wan L, et al. Intubation and ventilation amid the COVID-19 outbreak [online ahead of print]. Anesthesiology. 2020. https://doi.org/10.1097/aln.0000000000003296.
10. Munshi L, Del Sorbo L, Adhikari NKJ, et al. Prone position for acute respiratory distress syndrome: a systematic review and meta-analysis. Ann Am Thorac Soc. 2017;14(suppl 4):S280-S288. https://doi.org/10.1513/annalsats.201704-343ot.
11. Scaravilli V, Grasselli G, Castagna L, et al. Prone positioning improves oxygenation in spontaneously breathing nonintubated patients with hypoxemic acute respiratory failure: a retrospective study. J Crit Care. 2015;30(6):1390-1394. https://doi.org/10.1016/j.jcrc.2015.07.008
12. Ding L, Wang L, Ma W, He H. Efficacy and safety of early prone positioning combined with HFNC or NIV in moderate to severe ARDS: a multi-center prospective cohort study. Crit Care. 2020;24(1):28. https://doi.org/10.1186/s13054-020-2738-5.
13. Alhazzani W, Møller MH, Arabi YM, et al. Surviving Sepsis Campaign: guidelines on the management of critically ill adults with coronavirus disease 2019 (COVID-19). Intensive Care Med. 2020;1‐34. https://doi.org/10.1007/s00134-020-06022-5.
14. Caputo ND, Strayer RJ, Levitan R. Early self-proning in awake, non-intubated patients in the emergency department: a single ED’s experience during the COVID-19 pandemic [online ahead of print]. Acad Emerg Med. 2020. https://doi.org/10.1111/acem.13994.
My colleague asked, “Do you remember that patient?” I froze because, like most emergency physicians, this phrase haunts me. It was the early days of the COVID-19 epidemic, and the story that followed was upsetting. A patient who looked comfortable when I admitted him was intubated hours later by the rapid response team who was called to the floor. All I could think was, “But he looked so comfortable when I admitted him; he was just on a couple of liters of oxygen. Why was he intubated?”
In the days after COVID-19 arrived in our region, there were many such stories of patients sent to the floor from the Emergency Department who were intubated shortly after admission. Many of those patients subsequently endured prolonged and complicated courses on the ventilator. While we would typically use noninvasive modalities such as high-flow nasal cannula (HFNC) or noninvasive ventilation (NIV) for acute respiratory failure, our quickness to intubate was driven by two factors: (1) early reports that noninvasive modalities posed a high risk of failure and subsequent intubation and (2) fear that HFNC and NIV would aerosolize SARS-CoV-2 and unnecessarily expose the heath care team.1 We would soon find out that our thinking was flawed on both accounts.
RETHINKING INITIAL ASSUMPTIONS
When we dug into the evidence for early intubation, we realized that these recommendations were based on a 12-patient series in which 5 patients were trialed on NIV but ultimately intubated and placed on invasive mechanical ventilation (IMV). As the pandemic progressed, more case series and small studies were published, revealing a different picture.2 Sun and colleagues reported a multifaceted intervention of 610 inpatients, of whom 10% were critically ill, that identified at-risk patients and used NIV or HFNC and awake proning. Reportedly, fewer than 1% required IMV.3 Similarly, a small study found intubation was avoided in 85% of patients with severe acute respiratory failure caused by COVID-19 with use of HFNC and NIV.4 Early findings from New York University in New York, New York, where only 8.5% of patients undergoing IMV were extubated by the time of outcome reporting, suggest early IMV could lead to poor outcomes.5
Still, we had concerns about use of HFNC and NIV because of worries about the health and safety of other patients and particularly that of healthcare workers (HCWs) because they have been disproportionately affected by the disease.6 Fortunately, we identified emerging data that revealed that HFNC is no more aerosolizing than low-flow nasal cannula or a nonrebreather mask and droplet spread is reduced with a surgical mask.7,8 In light of these new studies and our own developing experience with the disease, we felt that there was insufficient evidence to continue following the “early intubation” protocol in patients with COVID-19. It was time for a new paradigm.
GATHERING EVIDENCE AND STAKEHOLDERS
In order to effectively and quickly change our respiratory pathway for these patients, we initially sought out protocols from other institutions through social media. These protocols, supported by early data from those sites, informed our process. We considered data from various sources, including emergency medicine, hospital medicine, and critical care. We then assembled stakeholders within our organization from emergency medicine, hospital medicine, critical care, and respiratory therapy because our protocol would need endorsement from all key players within our organization who cared for these patients across the potential spectrum of care. We made sure that all stakeholders understood that the quality of the evidence for treatment of this novel disease was much lower than our typical threshold to change practice, but that we aimed to reflect the best evidence to date. We also were careful to identify pathways that would be amenable to near-immediate implementation.
UNVEILING A NOVEL PROTOCOL
Our group reached consensus within 48 hours and quickly disseminated our first draft of the protocol (Appendix Figure). Dubbed the “Early Intervention Respiratory Protocol,” it differed from usual management in several ways. First, we had consistently observed (and confirmed from the literature) a phenotype of patients with “silent hypoxemia”9 (that is, a subset of patients who presented with profound hypoxemia but minimally increased work of breathing). The protocol encouraged tolerance of lower oxygen saturations than is usually seen on inpatient units. This required ensuring all stakeholders were comfortable with a target oxygen saturation of 88%. Second, the protocol leveraged early “awake” proning by patients. Historically, proning is used in mechanically ventilated patients with acute respiratory distress syndrome (ARDS) to improve ventilation-perfusion matching, promote more uniform ventilation, and increase end-expiratory lung volume.10 Prior literature was limited to the use of awake proning in small case series of ARDS, but given our limitations in terms of ICU capacity, we agreed to trial awake proning in a sizable proportion of our COVID-19 patients outside the ICU.11,12 Finally, we clarified safe practices regarding the risk of aerosolization with noninvasive modalities. Local infection control determined that HFNC wa not aerosol generating, and use of surgical masks was added for further protection from respiratory droplets. In addition, airborne personal protective equipment was to be worn on the inpatient ward, and we used NIV sparingly and preferentially placed these patients in negative pressure rooms, if available.13
Implementation of the protocol involved aggressive dissemination and education (Table). A single-page protocol was designed for ease of use at the bedside that included anticipatory guidance regarding aerosolization and addressed potential resistance to awake proning because of concerns regarding safety and hassle. Departmental leaders disseminated the protocol throughout the institution with tailored education on the rationale and acknowledgment of a reversal in approach. In addition to email, we used text messaging (WhatsApp) and a comprehensive living document (Google Drive) to reach clinicians.

For ease of monitoring and safety, we designated a COVID-19 intermediate care unit. We partnered with the unit medical director, nurse educator, and a focused group of hospitalists, conducting individual train-the-trainer sessions. This training was carried forward, and all nurses, respiratory therapists, and clinicians were trained on the early aggressive respiratory protocol within 12 hours of protocol approval. In addition, the rapid response and critical care teams agreed to round on the COVID-19 intermediate care unit daily.
As a result of these efforts, adoption of the protocol was essentially immediate across the institution. We had shifted the mindset of a diverse group of clinicians regarding how to support the respiratory status of these patients, but also detected reductions in the proportion of patients undergoing IMV and ICU admission (we are planning to report these results separately).
TRANSLATING KNOWLEDGE INTO PRACTICE
The COVID-19 pandemic has highlighted the importance of having cognitive flexibility when the evidence base is rapidly changing and there is a need for rapid dissemination of knowledge. Even in clinical scenarios with an abundance of high-quality evidence, a gap in knowledge translation on the order of a decade often exists. In contrast, a pandemic involving a novel virus highlights an urgent need for adaptive knowledge translation in the present moment rather than a decade later. In the absence of robust evidence regarding SARS-CoV-2, early management of COVID-19 was based on expert recommendations and experience with other disease processes. Even so, we should anticipate that management paradigms may shift, and we should constantly seek out emerging evidence to adjust our mindset (and protocols like this) accordingly. Our original protocol was based on nearly nonexistent evidence, but we anticipated that, in a pandemic, data would accumulate quickly, so we prioritized rapid translation of new information into practice. In fact, further evidence has emerged regarding the improvement in oxygenation in COVID-19 patients with self-proning.14
The final step is evaluating the success of both clinical and implementation outcomes. We are attempting to identify changes in intubation, length of stay, days on ventilator, and days in ICU. In addition, we will measure feasibility and adaptability. We are also attempting, in real time, to identify barriers to its use, including conducting qualitative interviews to understand whether there were unintended consequences to use of the protocol. This endeavor highlights how the COVID-19 pandemic, for all its tragedy, may represent an important era for implementation science: a time when emerging literature from a variety of sources can be implemented in days rather than years.
My colleague asked, “Do you remember that patient?” I froze because, like most emergency physicians, this phrase haunts me. It was the early days of the COVID-19 epidemic, and the story that followed was upsetting. A patient who looked comfortable when I admitted him was intubated hours later by the rapid response team who was called to the floor. All I could think was, “But he looked so comfortable when I admitted him; he was just on a couple of liters of oxygen. Why was he intubated?”
In the days after COVID-19 arrived in our region, there were many such stories of patients sent to the floor from the Emergency Department who were intubated shortly after admission. Many of those patients subsequently endured prolonged and complicated courses on the ventilator. While we would typically use noninvasive modalities such as high-flow nasal cannula (HFNC) or noninvasive ventilation (NIV) for acute respiratory failure, our quickness to intubate was driven by two factors: (1) early reports that noninvasive modalities posed a high risk of failure and subsequent intubation and (2) fear that HFNC and NIV would aerosolize SARS-CoV-2 and unnecessarily expose the heath care team.1 We would soon find out that our thinking was flawed on both accounts.
RETHINKING INITIAL ASSUMPTIONS
When we dug into the evidence for early intubation, we realized that these recommendations were based on a 12-patient series in which 5 patients were trialed on NIV but ultimately intubated and placed on invasive mechanical ventilation (IMV). As the pandemic progressed, more case series and small studies were published, revealing a different picture.2 Sun and colleagues reported a multifaceted intervention of 610 inpatients, of whom 10% were critically ill, that identified at-risk patients and used NIV or HFNC and awake proning. Reportedly, fewer than 1% required IMV.3 Similarly, a small study found intubation was avoided in 85% of patients with severe acute respiratory failure caused by COVID-19 with use of HFNC and NIV.4 Early findings from New York University in New York, New York, where only 8.5% of patients undergoing IMV were extubated by the time of outcome reporting, suggest early IMV could lead to poor outcomes.5
Still, we had concerns about use of HFNC and NIV because of worries about the health and safety of other patients and particularly that of healthcare workers (HCWs) because they have been disproportionately affected by the disease.6 Fortunately, we identified emerging data that revealed that HFNC is no more aerosolizing than low-flow nasal cannula or a nonrebreather mask and droplet spread is reduced with a surgical mask.7,8 In light of these new studies and our own developing experience with the disease, we felt that there was insufficient evidence to continue following the “early intubation” protocol in patients with COVID-19. It was time for a new paradigm.
GATHERING EVIDENCE AND STAKEHOLDERS
In order to effectively and quickly change our respiratory pathway for these patients, we initially sought out protocols from other institutions through social media. These protocols, supported by early data from those sites, informed our process. We considered data from various sources, including emergency medicine, hospital medicine, and critical care. We then assembled stakeholders within our organization from emergency medicine, hospital medicine, critical care, and respiratory therapy because our protocol would need endorsement from all key players within our organization who cared for these patients across the potential spectrum of care. We made sure that all stakeholders understood that the quality of the evidence for treatment of this novel disease was much lower than our typical threshold to change practice, but that we aimed to reflect the best evidence to date. We also were careful to identify pathways that would be amenable to near-immediate implementation.
UNVEILING A NOVEL PROTOCOL
Our group reached consensus within 48 hours and quickly disseminated our first draft of the protocol (Appendix Figure). Dubbed the “Early Intervention Respiratory Protocol,” it differed from usual management in several ways. First, we had consistently observed (and confirmed from the literature) a phenotype of patients with “silent hypoxemia”9 (that is, a subset of patients who presented with profound hypoxemia but minimally increased work of breathing). The protocol encouraged tolerance of lower oxygen saturations than is usually seen on inpatient units. This required ensuring all stakeholders were comfortable with a target oxygen saturation of 88%. Second, the protocol leveraged early “awake” proning by patients. Historically, proning is used in mechanically ventilated patients with acute respiratory distress syndrome (ARDS) to improve ventilation-perfusion matching, promote more uniform ventilation, and increase end-expiratory lung volume.10 Prior literature was limited to the use of awake proning in small case series of ARDS, but given our limitations in terms of ICU capacity, we agreed to trial awake proning in a sizable proportion of our COVID-19 patients outside the ICU.11,12 Finally, we clarified safe practices regarding the risk of aerosolization with noninvasive modalities. Local infection control determined that HFNC wa not aerosol generating, and use of surgical masks was added for further protection from respiratory droplets. In addition, airborne personal protective equipment was to be worn on the inpatient ward, and we used NIV sparingly and preferentially placed these patients in negative pressure rooms, if available.13
Implementation of the protocol involved aggressive dissemination and education (Table). A single-page protocol was designed for ease of use at the bedside that included anticipatory guidance regarding aerosolization and addressed potential resistance to awake proning because of concerns regarding safety and hassle. Departmental leaders disseminated the protocol throughout the institution with tailored education on the rationale and acknowledgment of a reversal in approach. In addition to email, we used text messaging (WhatsApp) and a comprehensive living document (Google Drive) to reach clinicians.

For ease of monitoring and safety, we designated a COVID-19 intermediate care unit. We partnered with the unit medical director, nurse educator, and a focused group of hospitalists, conducting individual train-the-trainer sessions. This training was carried forward, and all nurses, respiratory therapists, and clinicians were trained on the early aggressive respiratory protocol within 12 hours of protocol approval. In addition, the rapid response and critical care teams agreed to round on the COVID-19 intermediate care unit daily.
As a result of these efforts, adoption of the protocol was essentially immediate across the institution. We had shifted the mindset of a diverse group of clinicians regarding how to support the respiratory status of these patients, but also detected reductions in the proportion of patients undergoing IMV and ICU admission (we are planning to report these results separately).
TRANSLATING KNOWLEDGE INTO PRACTICE
The COVID-19 pandemic has highlighted the importance of having cognitive flexibility when the evidence base is rapidly changing and there is a need for rapid dissemination of knowledge. Even in clinical scenarios with an abundance of high-quality evidence, a gap in knowledge translation on the order of a decade often exists. In contrast, a pandemic involving a novel virus highlights an urgent need for adaptive knowledge translation in the present moment rather than a decade later. In the absence of robust evidence regarding SARS-CoV-2, early management of COVID-19 was based on expert recommendations and experience with other disease processes. Even so, we should anticipate that management paradigms may shift, and we should constantly seek out emerging evidence to adjust our mindset (and protocols like this) accordingly. Our original protocol was based on nearly nonexistent evidence, but we anticipated that, in a pandemic, data would accumulate quickly, so we prioritized rapid translation of new information into practice. In fact, further evidence has emerged regarding the improvement in oxygenation in COVID-19 patients with self-proning.14
The final step is evaluating the success of both clinical and implementation outcomes. We are attempting to identify changes in intubation, length of stay, days on ventilator, and days in ICU. In addition, we will measure feasibility and adaptability. We are also attempting, in real time, to identify barriers to its use, including conducting qualitative interviews to understand whether there were unintended consequences to use of the protocol. This endeavor highlights how the COVID-19 pandemic, for all its tragedy, may represent an important era for implementation science: a time when emerging literature from a variety of sources can be implemented in days rather than years.
1. World Health Organization. Clinical management of severe acute respiratory infection (SARI) when COVID-19 disease is suspected. 2020. https://www.who.int/publications-detail/clinical-management-of-severe-acute-respiratory-infection-when-novel-coronavirus-(ncov)-infection-is-suspected. Accessed March 25, 2020.
2. Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054-1062. https://doi.org/10.1016/s0140-6736(20)30566-3.
3. Sun Q, Qiu H, Huang M, Yang Y. Lower mortality of COVID-19 by early recognition and intervention: experience from Jiangsu Province. Ann Intensive Care. 2020;10(1):33. https://doi.org/10.1186/s13613-020-00650-2.
4. Wang K, Zhao W, Li J, Shu W, Duan J. The experience of high-flow nasal cannula in hospitalized patients with 2019 novel coronavirus-infected pneumonia in two hospitals of Chongqing, China. Ann Intensive Care. 2020;10(1):37. https://doi.org/10.1186/s13613-020-00653-z.
5. Petrilli C, Jones SA, Yang J, Rajagopalan H, et al. Factors associated with hospitalization and critical illness among 4,103 patients with Covid-19 disease in New York City [preprint]. medRxiv. 2020. https://doi.org/10.1101/2020.04.08.20057794. Accessed April 12, 2020.
6. Wang D, Hu B, Hu C, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus–infected pneumonia in Wuhan, China. JAMA. 2020;323(11):1061-1069. https://doi.org/10.1001/jama.2020.1585.
7. Leonard S, Volakis L, DeBellis R, Kahlon A, Mayar S. Transmission Assessment Report: High Velocity Nasal Insufflation (HVNI) Therapy Application in Management of COVID-19. March 25, 2020. Vapotherm Blog. 2020. https://vapotherm.com/blog/transmission-assessment-report/. Accessed March 25, 2020.
8. Iwashyna TJ, Boehman A, Capecelatro J, Cohn A, JM. C. Variation in aerosol production across oxygen delivery devices in 2 spontaneously breathing human subjects [preprint]. medRxiv. 2020. https://doi.org/10.1101/2020.04.15.20066688. Accessed April 20, 2020.
9. Meng L, Qiu H, Wan L, et al. Intubation and ventilation amid the COVID-19 outbreak [online ahead of print]. Anesthesiology. 2020. https://doi.org/10.1097/aln.0000000000003296.
10. Munshi L, Del Sorbo L, Adhikari NKJ, et al. Prone position for acute respiratory distress syndrome: a systematic review and meta-analysis. Ann Am Thorac Soc. 2017;14(suppl 4):S280-S288. https://doi.org/10.1513/annalsats.201704-343ot.
11. Scaravilli V, Grasselli G, Castagna L, et al. Prone positioning improves oxygenation in spontaneously breathing nonintubated patients with hypoxemic acute respiratory failure: a retrospective study. J Crit Care. 2015;30(6):1390-1394. https://doi.org/10.1016/j.jcrc.2015.07.008
12. Ding L, Wang L, Ma W, He H. Efficacy and safety of early prone positioning combined with HFNC or NIV in moderate to severe ARDS: a multi-center prospective cohort study. Crit Care. 2020;24(1):28. https://doi.org/10.1186/s13054-020-2738-5.
13. Alhazzani W, Møller MH, Arabi YM, et al. Surviving Sepsis Campaign: guidelines on the management of critically ill adults with coronavirus disease 2019 (COVID-19). Intensive Care Med. 2020;1‐34. https://doi.org/10.1007/s00134-020-06022-5.
14. Caputo ND, Strayer RJ, Levitan R. Early self-proning in awake, non-intubated patients in the emergency department: a single ED’s experience during the COVID-19 pandemic [online ahead of print]. Acad Emerg Med. 2020. https://doi.org/10.1111/acem.13994.
1. World Health Organization. Clinical management of severe acute respiratory infection (SARI) when COVID-19 disease is suspected. 2020. https://www.who.int/publications-detail/clinical-management-of-severe-acute-respiratory-infection-when-novel-coronavirus-(ncov)-infection-is-suspected. Accessed March 25, 2020.
2. Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054-1062. https://doi.org/10.1016/s0140-6736(20)30566-3.
3. Sun Q, Qiu H, Huang M, Yang Y. Lower mortality of COVID-19 by early recognition and intervention: experience from Jiangsu Province. Ann Intensive Care. 2020;10(1):33. https://doi.org/10.1186/s13613-020-00650-2.
4. Wang K, Zhao W, Li J, Shu W, Duan J. The experience of high-flow nasal cannula in hospitalized patients with 2019 novel coronavirus-infected pneumonia in two hospitals of Chongqing, China. Ann Intensive Care. 2020;10(1):37. https://doi.org/10.1186/s13613-020-00653-z.
5. Petrilli C, Jones SA, Yang J, Rajagopalan H, et al. Factors associated with hospitalization and critical illness among 4,103 patients with Covid-19 disease in New York City [preprint]. medRxiv. 2020. https://doi.org/10.1101/2020.04.08.20057794. Accessed April 12, 2020.
6. Wang D, Hu B, Hu C, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus–infected pneumonia in Wuhan, China. JAMA. 2020;323(11):1061-1069. https://doi.org/10.1001/jama.2020.1585.
7. Leonard S, Volakis L, DeBellis R, Kahlon A, Mayar S. Transmission Assessment Report: High Velocity Nasal Insufflation (HVNI) Therapy Application in Management of COVID-19. March 25, 2020. Vapotherm Blog. 2020. https://vapotherm.com/blog/transmission-assessment-report/. Accessed March 25, 2020.
8. Iwashyna TJ, Boehman A, Capecelatro J, Cohn A, JM. C. Variation in aerosol production across oxygen delivery devices in 2 spontaneously breathing human subjects [preprint]. medRxiv. 2020. https://doi.org/10.1101/2020.04.15.20066688. Accessed April 20, 2020.
9. Meng L, Qiu H, Wan L, et al. Intubation and ventilation amid the COVID-19 outbreak [online ahead of print]. Anesthesiology. 2020. https://doi.org/10.1097/aln.0000000000003296.
10. Munshi L, Del Sorbo L, Adhikari NKJ, et al. Prone position for acute respiratory distress syndrome: a systematic review and meta-analysis. Ann Am Thorac Soc. 2017;14(suppl 4):S280-S288. https://doi.org/10.1513/annalsats.201704-343ot.
11. Scaravilli V, Grasselli G, Castagna L, et al. Prone positioning improves oxygenation in spontaneously breathing nonintubated patients with hypoxemic acute respiratory failure: a retrospective study. J Crit Care. 2015;30(6):1390-1394. https://doi.org/10.1016/j.jcrc.2015.07.008
12. Ding L, Wang L, Ma W, He H. Efficacy and safety of early prone positioning combined with HFNC or NIV in moderate to severe ARDS: a multi-center prospective cohort study. Crit Care. 2020;24(1):28. https://doi.org/10.1186/s13054-020-2738-5.
13. Alhazzani W, Møller MH, Arabi YM, et al. Surviving Sepsis Campaign: guidelines on the management of critically ill adults with coronavirus disease 2019 (COVID-19). Intensive Care Med. 2020;1‐34. https://doi.org/10.1007/s00134-020-06022-5.
14. Caputo ND, Strayer RJ, Levitan R. Early self-proning in awake, non-intubated patients in the emergency department: a single ED’s experience during the COVID-19 pandemic [online ahead of print]. Acad Emerg Med. 2020. https://doi.org/10.1111/acem.13994.
© 2020 Society of Hospital Medicine
Give serious thought before starting antipsychotics in elders
The page filled me with dread: “Your elderly patient is confused, getting out of bed, and threw her entire dinner tray at a nurse just now.” Because this morning my patient was polite and appropriate, the now-angry, dinner-splattered nurse means only one thing: delirium.
Delirium is one of the most difficult problems for hospitalists to manage, in part because our management of delirium is often learned on the fly during residency and early years of practice. This post-hoc approach toward delirium misses the most important aspect of treatment: Prevention.
Interventions like early mobilization, environmental interventions, careful oversight of drugs, hydration protocols, and reinforcing the day/night cycle are crucial. Unfortunately, few hospitals can provide these resources or the trained multidisciplinary team with geriatrics expertise to administer them. The result is that delirium occurs more frequently than it should, and hospitalists often face a patient who is a risk to themselves or others.
In this situation, antipsychotics (APs) are often prescribed. However, long term use of APs by elders is highly discouraged by many organizations, including the Society of Hospital Medicine, because of risks like cardiac events (e.g., QT prolongation), stroke, extrapyramidal symptoms, falls, somnolence, and increased mortality in older patients with dementia.
One unanswered question regarding the use of APs is whether starting the medications in the hospital results in long-term use. To answer this, we performed a retrospective study of 300 elderly hospitalized patients who were treated for the first time with APs during their hospitalization. Of these 300 patients, 10% died during that first hospitalization, and almost half (48%) remained on APs at discharge. We found that most of the prescriptions were to treat delirium (J Hosp Med. 2014 Dec;9[12]:802-4. doi:10.1002/jhm.2277).
In a more recent study, we looked at outcomes for patients discharged from Baystate Medical Center (Springfield, Mass.) on APs. Within a year of discharge, 40% of these patients were readmitted at least once and approximately two-thirds were still taking the same APs on which they had been discharged (J Hosp Med. 2016 Apr 6. doi: 10.1002/jhm.2585). Thus, if I start my patient described above (who threw the dinner tray) on an AP today, she is very likely to be readmitted the next year still taking that same medication. Starting an AP in the short term can lead to the very thing we have been warned against: long-term use of an AP in an elderly person.
Even more striking than the continuation rate was the incredibly high 1-year mortality rate. Of the 260 patients discharged from the original admission on an AP, one-third had died at the 1-year mark. This group of patients had a wide range of diagnoses, but nearly as many died as if they all had stage IV heart failure. Because most had an agitated delirium at the time of AP prescription, these findings suggest that onset of in-hospital delirium should trigger a closer examination of the patient’s current burden of illness, prognosis, functional and cognitive status, treatment options, and goals of care.
Prevention is key
Our study also supports the prevention of delirium as the most important strategy to improve patient outcomes. Since conducting this study, Baystate Medical Center has implemented an “ACE” (Acute Care for Elders) pilot project and will soon open a full ACE unit. This unit, which employs many of the behavioral interventions described above (early discharge planning, drug oversight, team-based care, early mobilization, optimizing vision and hearing, sleep-wake cycle preservation, and hydration) has resulted in declines in both delirium rates and use of APs. Use of restraints has been virtually eliminated.
Our ACE program was a combined effort between geriatrics, hospital medicine, nursing, pharmacy, and others, but hospitalists often lead acute care quality improvement (QI) initiatives, and are superbly positioned to champion programs like ACE, NICHE (Nurses Improving Care for Health System Elders), and HELP (Hospital Elder Life Program) to benefit this vulnerable population.
Some important questions about AP use remain unanswered. First, there is very limited clinical trial evidence that APs actually improve outcomes in patients with delirium. Second, our study fails to answer one all-important question: does long-term AP use increase mortality in elders? Prior studies are largely retrospective, and results have been mixed.
Our study highlights the difficulty of teasing apart the baseline risks of the patients, the risk of the medications themselves, and confounding variables. There may be an association between APs and death, but it is quite possible that patients who require APs are simply at higher risk of death independent of the drugs’ effect; this confounding by indication cannot be adjusted away.
This leaves hospitalists in a difficult position. At Baystate Medical Center, hospitalists have opted to focus on prevention, but when delirium occurs, some patients are still treated with APs. Clinicians reserve the medications for patients who are suffering and fail to respond to nonpharmacologic interventions or are a risk to themselves or others. Still, Baystate plans to reduce use even in this population by instituting behavioral response teams to devise nondrug care plans, and hospitalists are encouraged to avoid discharging patients on APs.
Finally, even though patients who require APs may lack a clear terminal diagnosis, we encourage clinicians to recognize that delirium should prompt a discussion of prognosis and clarification of values, goals, and realistic treatment options.
Dr. Loh is a fellow at the James Wilmot Cancer Center, University of Rochester (N.Y.) Medical Center. Dr. Brennan is chief of geriatrics and post-acute medicine at Baystate Medical Center, Springfield, Mass. Dr. Lagu is an academic hospitalist in the Center for Quality of Care Research at Baystate Medical Center.
The page filled me with dread: “Your elderly patient is confused, getting out of bed, and threw her entire dinner tray at a nurse just now.” Because this morning my patient was polite and appropriate, the now-angry, dinner-splattered nurse means only one thing: delirium.
Delirium is one of the most difficult problems for hospitalists to manage, in part because our management of delirium is often learned on the fly during residency and early years of practice. This post-hoc approach toward delirium misses the most important aspect of treatment: Prevention.
Interventions like early mobilization, environmental interventions, careful oversight of drugs, hydration protocols, and reinforcing the day/night cycle are crucial. Unfortunately, few hospitals can provide these resources or the trained multidisciplinary team with geriatrics expertise to administer them. The result is that delirium occurs more frequently than it should, and hospitalists often face a patient who is a risk to themselves or others.
In this situation, antipsychotics (APs) are often prescribed. However, long term use of APs by elders is highly discouraged by many organizations, including the Society of Hospital Medicine, because of risks like cardiac events (e.g., QT prolongation), stroke, extrapyramidal symptoms, falls, somnolence, and increased mortality in older patients with dementia.
One unanswered question regarding the use of APs is whether starting the medications in the hospital results in long-term use. To answer this, we performed a retrospective study of 300 elderly hospitalized patients who were treated for the first time with APs during their hospitalization. Of these 300 patients, 10% died during that first hospitalization, and almost half (48%) remained on APs at discharge. We found that most of the prescriptions were to treat delirium (J Hosp Med. 2014 Dec;9[12]:802-4. doi:10.1002/jhm.2277).
In a more recent study, we looked at outcomes for patients discharged from Baystate Medical Center (Springfield, Mass.) on APs. Within a year of discharge, 40% of these patients were readmitted at least once and approximately two-thirds were still taking the same APs on which they had been discharged (J Hosp Med. 2016 Apr 6. doi: 10.1002/jhm.2585). Thus, if I start my patient described above (who threw the dinner tray) on an AP today, she is very likely to be readmitted the next year still taking that same medication. Starting an AP in the short term can lead to the very thing we have been warned against: long-term use of an AP in an elderly person.
Even more striking than the continuation rate was the incredibly high 1-year mortality rate. Of the 260 patients discharged from the original admission on an AP, one-third had died at the 1-year mark. This group of patients had a wide range of diagnoses, but nearly as many died as if they all had stage IV heart failure. Because most had an agitated delirium at the time of AP prescription, these findings suggest that onset of in-hospital delirium should trigger a closer examination of the patient’s current burden of illness, prognosis, functional and cognitive status, treatment options, and goals of care.
Prevention is key
Our study also supports the prevention of delirium as the most important strategy to improve patient outcomes. Since conducting this study, Baystate Medical Center has implemented an “ACE” (Acute Care for Elders) pilot project and will soon open a full ACE unit. This unit, which employs many of the behavioral interventions described above (early discharge planning, drug oversight, team-based care, early mobilization, optimizing vision and hearing, sleep-wake cycle preservation, and hydration) has resulted in declines in both delirium rates and use of APs. Use of restraints has been virtually eliminated.
Our ACE program was a combined effort between geriatrics, hospital medicine, nursing, pharmacy, and others, but hospitalists often lead acute care quality improvement (QI) initiatives, and are superbly positioned to champion programs like ACE, NICHE (Nurses Improving Care for Health System Elders), and HELP (Hospital Elder Life Program) to benefit this vulnerable population.
Some important questions about AP use remain unanswered. First, there is very limited clinical trial evidence that APs actually improve outcomes in patients with delirium. Second, our study fails to answer one all-important question: does long-term AP use increase mortality in elders? Prior studies are largely retrospective, and results have been mixed.
Our study highlights the difficulty of teasing apart the baseline risks of the patients, the risk of the medications themselves, and confounding variables. There may be an association between APs and death, but it is quite possible that patients who require APs are simply at higher risk of death independent of the drugs’ effect; this confounding by indication cannot be adjusted away.
This leaves hospitalists in a difficult position. At Baystate Medical Center, hospitalists have opted to focus on prevention, but when delirium occurs, some patients are still treated with APs. Clinicians reserve the medications for patients who are suffering and fail to respond to nonpharmacologic interventions or are a risk to themselves or others. Still, Baystate plans to reduce use even in this population by instituting behavioral response teams to devise nondrug care plans, and hospitalists are encouraged to avoid discharging patients on APs.
Finally, even though patients who require APs may lack a clear terminal diagnosis, we encourage clinicians to recognize that delirium should prompt a discussion of prognosis and clarification of values, goals, and realistic treatment options.
Dr. Loh is a fellow at the James Wilmot Cancer Center, University of Rochester (N.Y.) Medical Center. Dr. Brennan is chief of geriatrics and post-acute medicine at Baystate Medical Center, Springfield, Mass. Dr. Lagu is an academic hospitalist in the Center for Quality of Care Research at Baystate Medical Center.
The page filled me with dread: “Your elderly patient is confused, getting out of bed, and threw her entire dinner tray at a nurse just now.” Because this morning my patient was polite and appropriate, the now-angry, dinner-splattered nurse means only one thing: delirium.
Delirium is one of the most difficult problems for hospitalists to manage, in part because our management of delirium is often learned on the fly during residency and early years of practice. This post-hoc approach toward delirium misses the most important aspect of treatment: Prevention.
Interventions like early mobilization, environmental interventions, careful oversight of drugs, hydration protocols, and reinforcing the day/night cycle are crucial. Unfortunately, few hospitals can provide these resources or the trained multidisciplinary team with geriatrics expertise to administer them. The result is that delirium occurs more frequently than it should, and hospitalists often face a patient who is a risk to themselves or others.
In this situation, antipsychotics (APs) are often prescribed. However, long term use of APs by elders is highly discouraged by many organizations, including the Society of Hospital Medicine, because of risks like cardiac events (e.g., QT prolongation), stroke, extrapyramidal symptoms, falls, somnolence, and increased mortality in older patients with dementia.
One unanswered question regarding the use of APs is whether starting the medications in the hospital results in long-term use. To answer this, we performed a retrospective study of 300 elderly hospitalized patients who were treated for the first time with APs during their hospitalization. Of these 300 patients, 10% died during that first hospitalization, and almost half (48%) remained on APs at discharge. We found that most of the prescriptions were to treat delirium (J Hosp Med. 2014 Dec;9[12]:802-4. doi:10.1002/jhm.2277).
In a more recent study, we looked at outcomes for patients discharged from Baystate Medical Center (Springfield, Mass.) on APs. Within a year of discharge, 40% of these patients were readmitted at least once and approximately two-thirds were still taking the same APs on which they had been discharged (J Hosp Med. 2016 Apr 6. doi: 10.1002/jhm.2585). Thus, if I start my patient described above (who threw the dinner tray) on an AP today, she is very likely to be readmitted the next year still taking that same medication. Starting an AP in the short term can lead to the very thing we have been warned against: long-term use of an AP in an elderly person.
Even more striking than the continuation rate was the incredibly high 1-year mortality rate. Of the 260 patients discharged from the original admission on an AP, one-third had died at the 1-year mark. This group of patients had a wide range of diagnoses, but nearly as many died as if they all had stage IV heart failure. Because most had an agitated delirium at the time of AP prescription, these findings suggest that onset of in-hospital delirium should trigger a closer examination of the patient’s current burden of illness, prognosis, functional and cognitive status, treatment options, and goals of care.
Prevention is key
Our study also supports the prevention of delirium as the most important strategy to improve patient outcomes. Since conducting this study, Baystate Medical Center has implemented an “ACE” (Acute Care for Elders) pilot project and will soon open a full ACE unit. This unit, which employs many of the behavioral interventions described above (early discharge planning, drug oversight, team-based care, early mobilization, optimizing vision and hearing, sleep-wake cycle preservation, and hydration) has resulted in declines in both delirium rates and use of APs. Use of restraints has been virtually eliminated.
Our ACE program was a combined effort between geriatrics, hospital medicine, nursing, pharmacy, and others, but hospitalists often lead acute care quality improvement (QI) initiatives, and are superbly positioned to champion programs like ACE, NICHE (Nurses Improving Care for Health System Elders), and HELP (Hospital Elder Life Program) to benefit this vulnerable population.
Some important questions about AP use remain unanswered. First, there is very limited clinical trial evidence that APs actually improve outcomes in patients with delirium. Second, our study fails to answer one all-important question: does long-term AP use increase mortality in elders? Prior studies are largely retrospective, and results have been mixed.
Our study highlights the difficulty of teasing apart the baseline risks of the patients, the risk of the medications themselves, and confounding variables. There may be an association between APs and death, but it is quite possible that patients who require APs are simply at higher risk of death independent of the drugs’ effect; this confounding by indication cannot be adjusted away.
This leaves hospitalists in a difficult position. At Baystate Medical Center, hospitalists have opted to focus on prevention, but when delirium occurs, some patients are still treated with APs. Clinicians reserve the medications for patients who are suffering and fail to respond to nonpharmacologic interventions or are a risk to themselves or others. Still, Baystate plans to reduce use even in this population by instituting behavioral response teams to devise nondrug care plans, and hospitalists are encouraged to avoid discharging patients on APs.
Finally, even though patients who require APs may lack a clear terminal diagnosis, we encourage clinicians to recognize that delirium should prompt a discussion of prognosis and clarification of values, goals, and realistic treatment options.
Dr. Loh is a fellow at the James Wilmot Cancer Center, University of Rochester (N.Y.) Medical Center. Dr. Brennan is chief of geriatrics and post-acute medicine at Baystate Medical Center, Springfield, Mass. Dr. Lagu is an academic hospitalist in the Center for Quality of Care Research at Baystate Medical Center.
Impact of MC Intervention on QIs
Decompensated cirrhosis (DC) is defined as cirrhosis with at least 1 of the following complications: ascites, hepatocellular carcinoma, bleeding from portal hypertension, or hepatic encephalopathy. Patients with DC have a median survival estimated at 2 years compared to the 12‐year median survival of compensated cirrhotics.[1] In an era where quality of hospital care is being measured, and where progress is being made in the management of several conditions including congestive heart failure and nosocomial infections, little attention has been paid to DC. The burden of chronic liver failure is clear in the United States, where DC leads to more than 150,000 annual admissions to the hospital and accounts for 40,000 deaths annually.[2]
This burden of disease spurred quality improvement efforts in 2010, when a team of experts identified a set of literature‐based parameters or quality indicators (QI) for patients with cirrhosis.[3] We have demonstrated that adherence to these indicators fell far short of desired targets.[4] A year before their publication, an overall compliance of <50% with these metrics was measured at a single medical center.
We sought to improve the quality of care for patients with DC through implementation of mandatory consultation (MC) with a gastroenterologist for all patients admitted with DC. We assessed whether MC was associated with better care and improved outcomes (hospitalization length of stay [LOS], 30‐day readmission, and inpatient mortality) when compared to usual care (UC).[4]
MATERIALS AND METHODS
Design, Setting, and Patients
We conducted a cohort study comparing adherence to QI and outcomes of patients admitted with DC after the institution of an MC to a historical cohort of patients managed with UC (ie, before MC, adherence to QI for this group has been reported elsewhere).[4] Both cohorts included all patients aged >18 years with DC admitted to Baystate Medical Center, a tertiary care medical center in western Massachusetts. The UC cohort was collected between January 1, 2009 and December 31, 2009, and the MC cohort was assembled between June 1, 2011 and June 30, 2012.
As previously reported,[4] patients were considered for inclusion in the historical cohort if their International Classification of DiseasesNinth Revision discharge code pertained to chronic liver disease (see Supporting Information, Appendix 1, in the online version of this article). This list was broad by design to identify all patients with decompensated cirrhosis. A gastroenterologist (R.G.) then manually extracted charts from electronic medical records (EMRs) using a set of predefined clinical criteria, the same in both cohorts, to identify the patients with DC: cirrhosis with concomitant ascites, hepatic encephalopathy, or gastrointestinal (GI) bleeding secondary to portal hypertension. Other types of decompensated states, such as hepatocellular carcinoma, were not included as their management was not detailed in the QI.[3]
We included patients with suspected or established cirrhosis who had ascites confirmed radiographically or by exam, noting shifting dullness or fluid wave. However, patients were excluded if they lacked sufficient peritoneal fluid for bedside or image‐guided paracentesis. Cirrhotic patients were defined as having hepatic encephalopathy if the patient had altered mental status not secondary to seizures, cerebrovascular accident, or alcohol withdrawal. Finally, gastrointestinal bleeding in cirrhotic patients was defined as any upper or lower bleeding prompting hospital admission, or identified in the medical record as clinically significant by the attending physician.
The same QIs were measured in both cohorts. From the QI set,[3] we selected the 16 QIs that would apply to the management of inpatients (see Supporting Information, Appendix 2, in the online version of this article). Indicators developed for outpatient settings were not included. A quality score was calculated for each admission, defined as the proportion of QIs met divided by the number of QIs for which the patient was eligible. For example, a patient with hepatic encephalopathy but without GI bleeding or ascites would have a score calculated as the number of QIs met for hepatic encephalopathy and documentation of transplant evaluation divided by 3 (2 QIs for hepatic encephalopathy and 1 QI for transplant evaluation). If the patient met both QIs for hepatic encephalopathy, but the consultant failed to address liver transplant eligibility, the score would be 2/3=0.666.
After the institution of the MC, all inpatients with DC were identified within 24 hours of admission by a gastroenterologist (R.G., D.D.), who manually reviewed on a daily basis all admissions from EMRs. An author (R.G.) would then contact the admitting team (hospitalist or resident) to make sure that a gastroenterology consult was called and would then obtain the QI by manual extraction from the EMRs.
Of the 16 gastroenterologists who work at the hospital, 12 of them belong to several private practice groups, whereas 4 are employed by the hospital. As part of the intervention, all gastroenterologists were made aware of the intervention 1 month before the starting date, were provided with a checklist of the QIs of interest, and were encouraged to work with the hospitalist attendings to achieve compliance with the QIs. We reminded the gastroenterologists of the ongoing study during routine division meetings and regularly sought feedback from the hospitalists
The MC consisted of a systematic consultation by a gastroenterologist: any identified patient with DC would generate a mandatory GI consultation and would be assigned to a specialist depending on the roster coverage for that day. A close monitoring of the process allowed us to confirm that all patients admitted with DC were seen by a gastroenterologist. Patients were followed until their discharge, death, or readmission to our institution during the study period.
Outcomes
The primary outcome was defined as the rate of adherence to the QIs and overall QI score expressed as a proportion as noted above. Secondary outcomes included in‐hospital mortality, LOS, and 30‐day readmission rate. These parameters were abstracted from the medical record.
Covariates
The hospital EMR (Cerner Corporation, North Kansas City, MO) was used to extract patient demographic parameters such as gender, race, language, and age at time of admission. Other admission‐level details were extracted from the EMR including Model for End‐Stage Liver Disease (MELD) scores, documented comorbidities (including substance abuse, psychiatric diagnosis, diabetes mellitus, renal failure, congestive heart failure, coronary artery disease, and cancer), underlying etiology for cirrhosis, and reason for admission.
The study was approved by Baystate Medical Center's institutional review board.
Statistical Analysis
Summary statistics for outcomes and covariates were calculated as means/standard deviations (SDs), medians/emnterquartile range, and proportions. Univariable statistics (unpaired t tests, 1‐way analysis of variance, Fisher exact test, Spearman correlation) were used to identify possible demographic (eg, age, race) and clinical (eg, admission complaint) predictors of quality score and with 30‐day outcomes. For each admission, a composite quality score, also known as an opportunity model score,[5, 6] was calculated as a fraction (ie, the number of QIs met divided by the total number of possible QIs indicated by the patient's presentation). This fraction was then multiplied by 100 so as to express the QI score as a percent. Possible scores, therefore, ranged from 0 to 100%.
Calculation of the 30‐day incidence proportion of readmission after the first admission was restricted to patients whose readmission occurred in this hospital, and occurring up to 30‐days before study closure (June 1, 2012). In‐hospital death was examined as a function of QI score during that admission. To derive an unbiased, risk‐adjusted estimate of the association between quality score and outcomes, multiple linear regression (opportunity model score [OMS], LOS) or multiple Poisson regression models (30‐day readmission, in‐hospital death) were built. These included a dummy variable for the study period, as well as any potential confounder that was associated at P0.10, with both study period and the outcome in univariable analyses. Robust standard errors were specified to account for multiple admissions within patients. Marginal means or proportions were then estimated with 95% confidence intervals derived using the delta method. All analyses were performed using Stata 12.1 for Windows (StataCorp, College Station, TX).
RESULTS
A total of 303 patients were observed in 695 hospitalizations;149 patients in 379 admissions were observed in the UC cohort, and 154 patients in 316 admissions were observed in the MC cohort. Baseline demographics of all study admissions appear in Table 1. Patients seen in the MC cohort were younger, more likely to speak English, and less likely to be male or have comorbid diabetes mellitus. Most admissions (n=217, 57.2%; 95% confidence interval: 52.3%‐62.3%) were not evaluated by a gastroenterologist in the UC cohort but all were in the MC cohort.
| UC, N=379, N (%) or Mean/SD | MC, N=316, N (%) or Mean/SD | P Value* | |
|---|---|---|---|
| |||
| Age, y | 55.3/12.1 | 53.3/13.6 | 0.05 |
| English speaking | 261 (68.9%) | 261 (82.6%) | <0.001 |
| Male | 251 (66.2%) | 163 (53.5%) | 0.001 |
| Race | <0.001 | ||
| White | 301 (79.4%) | 262 (82.9%) | |
| Black | 31 (8.2%) | 40 (12.7%) | |
| Asian | 16 (4.2%) | 0 (0.0%) | |
| Other | 31 (8.2%) | 14 (4.4%) | |
| Comorbidities | |||
| Substance | 75 (19.8%) | 58 (18.4%) | 0.70 |
| abuse | |||
| Psychiatric | 123 (32.5%) | 103 (32.9%) | 0.94 |
| Diabetes mellitus | 175 (45.4%) | 115 (36.5%) | 0.02 |
| Renal failure | 74 (19.3%) | 55 (17.4%) | 0.50 |
| CHF | 38 (10.0%) | 24 (7.6%) | 0.35 |
| CAD | 26 (6.9%) | 17 (5.4%) | 0.43 |
| Cancer | 48 (12.7%) | 40 (12.7%) | 1.00 |
| Admission MELD | 15.6/6.9 | 17.0/7.0 | 0.006 |
| Serum creatinine | 1.43/1.94 | 1.42/1.30 | 0.91 |
| Reason for admission | |||
| Hepatology/GI | 318 (83.9%) | 257 (81.3%) | 0.42 |
| Renal failure | 85 (22.4%) | 90 (28.5%) | 0.08 |
| Encephalopathy | 151 (39.3%) | 113 (34.9%) | 0.24 |
| GI bleed | 78 (20.5%) | 57 (18.0%) | 1.00 |
| Abdominal pain | 116 (30.7%) | 114 (36.2%) | 0.15 |
| Ascites | 246 (64.9%) | 185 (58.5%) | 0.10 |
Admission Characteristics
The baseline clinical measures of all study admissions appear in Table 1. The UC and MC cohorts had similar characteristics, with the majority of patients with DC admitted for a gastrointestinal/hepatology‐related reason specifically for the management of ascites and hepatic encephalopathy. The patients in the MC cohort had a statistically higher MELD score on admission, which was not clinically relevant.
Quality Measures
Adherence to individual quality indices is shown in Table 2.
| Condition (Denominator) | Quality Indicator (Numerator) | UC (n=379), Met/Indicated | MC (n=316), Met/Indicated | P Value | |
|---|---|---|---|---|---|
| |||||
| Admissions with ascites | |||||
| 1 | Admissions to the hospital because of ascites or encephalopathy. | Diagnostic paracentesis during admission. | 77/193, 39.9%, (32.9%, 46.9%) | 111/135, 82.2% (75.7%, 88.8%) | <0.001 |
| 2 | No fibrinolysis or disseminated intravascular coagulation before paracentesis INR <2.5, >100,000 platelets. | No fresh frozen plasma or platelet replacement given. | 36/37, 97.3% (91.8%, 103.0%) | 41/42, 97.6% (92.8%, 102.4%) | 1.00 |
| 3 | All admissions with diagnostic paracentesis (not limited to admissions for ascites or hepatic encephalopathy). | Cell count differential, total protein, albumin, and culture/sensitivity all performed. | 31/49, 63.3% (49.3%‐77.3%) | 46/72 63.9% (52.7%, 75.0%) | 1.00 |
| 4 | Admissions with known portal hypertension‐related ascites receiving a paracentesis. | Ascitic fluid cell count and differential performed. | 15/104, 14.4% (7.6%‐ 21.3%) | 47/62, 75.8% (63.2%, 88.4%) | <0.001 |
| 5 | Serum sodium 110 mEq/L. | Fluid restriction and discontinuation of diuretics. | NA | NA | NA |
| 6 | Polymorphonuclear count of 250/mm3 in ascites. | Empiric antibiotics, 6 hours of results. | 10/13, 76.9% (50.4%‐ 103.4%) | 16/20, 80.0% (60.8%, 99.2%) | 1.00 |
| 7 | Ascitic fluid, total protein 1.1 gm/dL, serum bilirubin 2.5 mg/dL. | Prophylactic antibiotics. | 4/12, 33.3% (2.0%‐ 64.6%) | 18/30, 60.0%, (41.4%, 78.6%) | 0.18 |
| 8 | Normal renal function. | Salt restriction and diuretics (spironolactone and loop diuretics). | 57/186, 30.6%, (24.0%‐ 37.3%) | 81/122, 66.4%, (57.9%, 74.9%) | <0.001 |
| Total ascites subscore, mean/SD | 30%/36% | 67%/34% | <0.001 | ||
| GI bleeding | |||||
| 9 | Admissions with GI bleeding: variceal and nonvariceal, hematemesis and melena. | Upper endoscopy 24 hours of presentation. | 60/78, 76.9% (67.4%, 86.4%) | 52/57, 91.2% (83.7%, 98.8%) | 0.04 |
| 10 | Esophageal varices (active, stigmata of recent bleeding, or no other causes to explain bleeding). | Endoscopic variceal ligation/sublerotherapy. | 40/46, 87.0% (76.8%‐97.1%) | 30/32, 93.8% (84.9%, 100.0%) | 0.46 |
| 11 | Admissions with established/suspected upper GI bleeding. | Antibiotics within 24 hours of admission. | 27/69, 39.1% (27.3%‐ 50.9%) | 26/58, 44.8% (31.6%, 58.0%) | 0.59 |
| 12 | Admissions with established/suspected variceal bleeding. | Somatostatin/octreotide given within 12 hours of presentation. | 53/69, 76.8%, (66.6%‐ 87.0%) | 49/58, 84.5% (73.8%, 95.2%) | 0.37 |
| 13 | Recurrent bleeding within 72 hours of initial endoscopic hemostasis. | Repeat endoscopy or transjugular intrahepatic portosystemic shunt. | 5/5 100% | 2/3, 66.7% (76.8%, 210.0%) | 0.38 |
| Total GI subscore, mean/SD | 61%/38% | 74%/28% | 0.04 | ||
| Liver transplantation | |||||
| 14 | Admissions with MELD 15 or MELD 15 and decompensated status (ie, all admissions in our study). | Documented evaluation for liver transplantation. | 112/379, 29.6% (24.9%‐ 34.2%) | 231/316, 73.6% (68.7%, 78.5%) | <0.001 |
| Hepatic encephalopathy | |||||
| 15 | Admissions with hepatic encephalopathy. | Search for reversible factors documented. | 81/151, 53.6% (45.6%‐ 61.7 %) | 97/113, 85.8% (79.4%, 92.3%) | <0.001 |
| 16 | Admissions with hepatic encephalopathy. | Oral disaccharides/ rifaximin. | 144/151, 95.3% (91.9 %‐ 98.7 %) | 107/113, 94.7% (90.7%. 98.69%) | 1.00 |
| Total encephalopathy subscore, mean/SD | 75%/28% | 90%/24% | <0.001 | ||
Ascites
The management of ascites yielded 3 main differences between the 2 cohorts. Following the implementation of the MC, 82.2 % (111/135) of ascites‐related admissions led to a diagnostic paracentesis as compared to 39.9% (77/193) in the UC group (P<0.001).
In the MC cohort, 75.8% (47/62) of admissions with known portal hypertensionrelated ascites who received a paracentesis had an ascites cell count checked. In contrast, only 14.4% (15/104) in the UC group receiving paracentesis had a fluid cell count (P<0.001). The management of ascites in patients with normal renal function was optimal, with sodium restriction and diuretics combination in 66.4% (81/122) of the MC cohort, whereas this parameter in the UC cohort was only 30.6% (57/186) (P<0.001). There were no significant differences between the groups for the other QIs.
Variceal Bleeding
The MC group had a higher frequency of endoscopy within 24 hours of admissions than the UC group (91.2% [52/57] vs 76.9% [60/78], respectively; P<0.04). The rest had endoscopy later in the admission. Among admissions with bleeding from varices, banding was done 93.8% of the time for patients in the MC group (30/32), which was not statistically different than 87.0% (40/46) for patients seen in the UC group. In the remaining admissions, endoscopy only revealed nonbleeding large esophageal varices, and the endoscopist opted not to proceed with therapy. There were no statistically significant differences in the rest of the management.
Hepatic Encephalopathy
For hepatic encephalopathy, an empirical treatment was given to 95.3 % (144/151) patients in the UC group and 94.7% (107/113) of the patients in the MC group. We found better documentation of a search for underlying etiologies leading to hepatic encephalopathy in the MC cohort 85.8% (97/113) versus the UC cohort, which was only 53.6% (81/151) (P<0.001).
Evaluation for Liver Transplantation
Better documentation of evaluation for liver transplantation was seen in the MC group 73.6% (231/316) in comparison to the UC group 29.4% (111/379) (P<0.001).
Opportunity Score and Clinical Outcomes
As detailed above, care provided during the MC achieved a higher compliance with the QI shown with the QI score or OMS (Table 3). These improvements were not associated with statistically significant differences in in‐hospital death, LOS, or 30‐day readmission. To explore this further we also examined the direct association between the OMS and outcomes in the MC group by dividing patients into 2 groups: patients whose OMS was 80% and those whose OMS was <80% (see Supporting Information, Appendix 4, in the online version of this article). Although there were trends toward decreased in‐hospital death (6.4% vs 8.6%, P=0.26), increased 30‐day readmission (33.8% vs 23.0%, P=0.27), and decreased LOS (6.2 days vs 6.6 days, P=0.77), none of these differences achieved statistical significance.
| Unadjusted | Adjusted* | |||||
|---|---|---|---|---|---|---|
| UC | MC | Difference | UC | MC | Difference | |
| ||||||
| Opportunity model score | 0.46 | 0.77 | +0.31 (0.24, 0.39) | 0.46 | 0.77 | +0.30(0.23, 0.37) |
| In‐hospital death | 7.1% | 8.5% | +1.4 (0.3, +5.6) | 7.5% | 7.9% | +0.4% (4.0%, +4.5%) |
| Readmission within 30 days | 39.6% | 32.6% | 7.0% (16.4%, +2.5%) | 40.0% | 31.8% | 8.2%(18.0%, +1.5%) |
| Length of stay | 6.1d | 6.2d | +0.1d (1.0 d, +1.2 d) | 6.1d | 6.2d | +0.1d (1.0 d, +1.2d) |
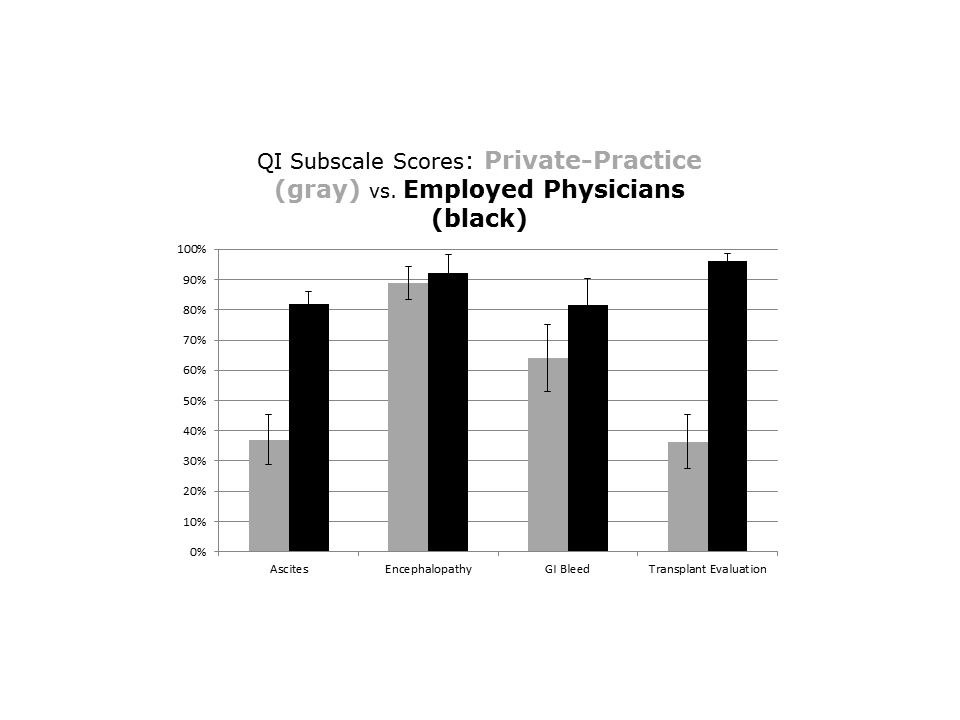
Mandatory Consultation Subgroups: Employed Versus Private Physicians
The type of employment of the gastroenterologist on consultation (employed by the hospital vs private practice) affected the management of the patients admitted with DC (see Supporting Information, Appendix 3, in the online version of this article). Patients seen by a hospital‐employed gastroenterologist were more likely to have a better documentation in regard to evaluation for liver transplantation and better management of ascites. Except for the prescription of antibiotics in patients presenting with GI bleeding, which were more often given by the employed physician (63% vs 23%, P=0.004), the management of hepatic encephalopathy and GI bleeding was similar between employed and private‐practice physicians.
DISCUSSION
In this evaluation of an MC intervention for patients with DC cared for at a large tertiary academic medical center, we found that the implementation of a routine consultation by a gastroenterologist led to greater adherence to recommended care processes when compared to UC. Overall, the management of ascites and the documentation of evaluation for liver transplantation were statistically superior in the intervention (MC) group. UC and MC were similar with respect to treatment of variceal bleeding and hepatic encephalopathy. Although we did not demonstrate changes in mortality, readmission, or LOS as a result of the MC intervention, our study was underpowered to detect clinically meaningful effects.
The gaps in care of patients with cirrhosis were reported before and after the publication of the formal QIs.[7, 8, 9, 10] These gaps remain relevant in the face of an increasing prevalence of DC along with a recent publication suggesting an underestimation of the burden of liver disease in the United States.[11] Ours is the first study to evaluate the impact on inpatients with DC of a liver service with a systematic, mandatory, specialist consultation. A previous study[12] had shown that a GI consultation would improve the care of patients with DC, but excluded patients with variceal bleeding, did not specifically measure the compliance with QIs, and more important, the GI consult was not mandatory.
Our study has several limitations that must be considered while weighing its findings. The patients were not randomly assigned but followed a pre‐established distribution depending on the call schedule. Some of the improvement we noted might be the result of secular trends; however, this remains unlikely given the lack of national initiatives or pay for performance programs. In the UC cohort, patients who were nonEnglish‐speaking were associated with a lower QI score, which could account for part of the improvement seen in the MC group that has a more prominent English‐speaking cohort. Readmissions could have occurred at other hospitals, and patients were not monitored in an outpatient setting. We did not observe a change in the secondary outcomes (30‐day readmission, LOS, in‐hospital death); however, our study was underpowered for that purpose. Given the complexity of the billing process we did not collect the costs of the MC, which is another limitation of our work. Future studies are needed to determine the cost‐effectiveness of the intervention.
This study shows that a dedicated team of physicians focused on compliance with QIs can achieve a rapid improvement, over a year, in providing higher‐quality care. This may be relevant at other institutions. The strength of our study is that our large tertiary academic medical center serves a large catchment area, with a mix of patients from both rural and urban communities. It is located in Massachusetts, where most of the population has had access to healthcare since 2006. Therefore, although this is a single‐center study, we expect our findings to be more generalizable and less subject to selection bias than other single‐center studies.
Importantly, the compliance with QIs was often far from being perfect in the MC group and was different across type of employment of providers, reflecting the challenges in changing practice among physicians.[13] In fact the QI scores of the private practice group did not change, and mirror the compliance observed at our institution in the previous study, before the implementation of the MC.[4] The difference in performance according to the type of employment of providers stems from 2 factors. First, a better documentation of the need of formal evaluation for liver transplantation by the employed gastroenterologists resulted in better compliance with this QI. Second, and more important, among the employed physicians, there was a readiness to assist the hospitalist with diagnostic/therapeutic paracentesis without relying on, for example, an interventional radiologist. This is reflected by the higher score in the management of ascites. Although our study was not designed to answer this directly, employed physicians may have been more engaged in the project and showed a greater willingness to change practice. In the future, linking reimbursement to quality of care will lead to improved accountability of consultants.
In this study we show that a direct involvement of a gastroenterologist improves the care of inpatients as measured by QIs. We theorize that a better coordination of the transition to outpatient care involving the specialist should lead to better outcomes, specifically a reduction in the 22% observed readmission rate within 30 days of patients with DC.[14, 15] As we move forward, a broader definition of outcomes should be addressed, taking into account patient‐related outcomes and preferences.[16] Future studies should define the relationship between the gastroenterologist and the hospitalist service, the role of physician assistants and nurse practitioners in implementing and monitoring compliance with QIs, and define how physicians and patients can be made accountable in the transition to the outpatient setting.
Disclosures
R.G.: Conception, data collection and interpretation, manuscript. J.F.: Data management, data analysis, manuscript. P.V.: Conception, data analysis, manuscript. P.L.: Conception, data interpretation, manuscript. T.L.: Dr. Lagu is supported by the National Heart, Lung, and Blood Institute of the National Institutes of Health under award number K01HL114745. Conception, data interpretation, manuscript. D.D.: Conception, data collection and interpretation, manuscript. A.B.: Data collection. J.S.: Data collection. Source of funding: internal. The authors report no conflicts of interest.
- , , . Natural history and prognostic indicators of survival in cirrhosis: a systematic review of 118 studies. J Hepatol. 2006;44:217–231.
- , , , . Efficacy of a chronic disease management model for patients with chronic liver failure. Clin Gastroenterol Hepatol. 2013;11:850–858.
- , , , et al. An Explicit Quality Indicator Set for Measurement of Quality of Care in Patients with Cirrhosis. Clin Gastroenterol Hepatol. 2010;8:709–717.
- , , , , , Desilets D. Measurement of the quality of care of patients admitted with decompensated cirrhosis. Liver Int. 2014:34:204–210.
- , . All‐or‐none measurement raises the bar on performance. JAMA. 2006;295:1168–1170.
- Joint Commission on Accreditation of Healthcare Organizations. Quality report user guide. Available at: http://www.jointcommission.org. Accessed May 30, 2011.
- , , , et al. Management of patients with cirrhosis in Southern California: results of a practitioner survey. J Clin Gastroenterol. 2006;40:156–161.
- , , , et al. Spanish Collaborative Study Group on Therapeutic Management In Liver Disease. Multicenter hospital study on prescribing patterns for prophylaxis and treatment of complications of cirrhosis. Eur J Clin Pharmacol. 2002;58:435–440.
- , , , et al. The quality of care provided to patients with cirrhosis and ascites in the Department of Veterans Affairs. Gastroenterology. 2012 143(1):70–77.
- , , , et al. Improved patient survival after acute variceal bleeding: a multicenter, cohort study. Am J Gastroenterol. 2003;98:653–659.
- , , , , . Underestimation of liver‐related mortality in the United States. Gastroenterology. 2013;145:375–382.
- , , , et al. Impact of gastroenterology consultation on the outcomes of patients admitted to the hospital with decompensated cirrhosis. Hepatology. 2001;34:1089–1095.
- , , , et al. Why don't physicians follow clinical practice guidelines? A framework for improvement. JAMA. 1999;282:1458–1465.
- , , , et al. Incidence and predictors of 30‐day readmission among patients hospitalized with advanced liver disease. Clin Gastroenterol Hepatol. 2011;9:254–259.
- , , , et al. Hospital Readmissions among patients with decompensated cirrhosis. Am J Gastroenterol. 2012;107:247–252.
- . Patient‐reported outcomes of cirrhosis. Clin Gastroenterol Hepatol. 2013;11:1043–1045.
Decompensated cirrhosis (DC) is defined as cirrhosis with at least 1 of the following complications: ascites, hepatocellular carcinoma, bleeding from portal hypertension, or hepatic encephalopathy. Patients with DC have a median survival estimated at 2 years compared to the 12‐year median survival of compensated cirrhotics.[1] In an era where quality of hospital care is being measured, and where progress is being made in the management of several conditions including congestive heart failure and nosocomial infections, little attention has been paid to DC. The burden of chronic liver failure is clear in the United States, where DC leads to more than 150,000 annual admissions to the hospital and accounts for 40,000 deaths annually.[2]
This burden of disease spurred quality improvement efforts in 2010, when a team of experts identified a set of literature‐based parameters or quality indicators (QI) for patients with cirrhosis.[3] We have demonstrated that adherence to these indicators fell far short of desired targets.[4] A year before their publication, an overall compliance of <50% with these metrics was measured at a single medical center.
We sought to improve the quality of care for patients with DC through implementation of mandatory consultation (MC) with a gastroenterologist for all patients admitted with DC. We assessed whether MC was associated with better care and improved outcomes (hospitalization length of stay [LOS], 30‐day readmission, and inpatient mortality) when compared to usual care (UC).[4]
MATERIALS AND METHODS
Design, Setting, and Patients
We conducted a cohort study comparing adherence to QI and outcomes of patients admitted with DC after the institution of an MC to a historical cohort of patients managed with UC (ie, before MC, adherence to QI for this group has been reported elsewhere).[4] Both cohorts included all patients aged >18 years with DC admitted to Baystate Medical Center, a tertiary care medical center in western Massachusetts. The UC cohort was collected between January 1, 2009 and December 31, 2009, and the MC cohort was assembled between June 1, 2011 and June 30, 2012.
As previously reported,[4] patients were considered for inclusion in the historical cohort if their International Classification of DiseasesNinth Revision discharge code pertained to chronic liver disease (see Supporting Information, Appendix 1, in the online version of this article). This list was broad by design to identify all patients with decompensated cirrhosis. A gastroenterologist (R.G.) then manually extracted charts from electronic medical records (EMRs) using a set of predefined clinical criteria, the same in both cohorts, to identify the patients with DC: cirrhosis with concomitant ascites, hepatic encephalopathy, or gastrointestinal (GI) bleeding secondary to portal hypertension. Other types of decompensated states, such as hepatocellular carcinoma, were not included as their management was not detailed in the QI.[3]
We included patients with suspected or established cirrhosis who had ascites confirmed radiographically or by exam, noting shifting dullness or fluid wave. However, patients were excluded if they lacked sufficient peritoneal fluid for bedside or image‐guided paracentesis. Cirrhotic patients were defined as having hepatic encephalopathy if the patient had altered mental status not secondary to seizures, cerebrovascular accident, or alcohol withdrawal. Finally, gastrointestinal bleeding in cirrhotic patients was defined as any upper or lower bleeding prompting hospital admission, or identified in the medical record as clinically significant by the attending physician.
The same QIs were measured in both cohorts. From the QI set,[3] we selected the 16 QIs that would apply to the management of inpatients (see Supporting Information, Appendix 2, in the online version of this article). Indicators developed for outpatient settings were not included. A quality score was calculated for each admission, defined as the proportion of QIs met divided by the number of QIs for which the patient was eligible. For example, a patient with hepatic encephalopathy but without GI bleeding or ascites would have a score calculated as the number of QIs met for hepatic encephalopathy and documentation of transplant evaluation divided by 3 (2 QIs for hepatic encephalopathy and 1 QI for transplant evaluation). If the patient met both QIs for hepatic encephalopathy, but the consultant failed to address liver transplant eligibility, the score would be 2/3=0.666.
After the institution of the MC, all inpatients with DC were identified within 24 hours of admission by a gastroenterologist (R.G., D.D.), who manually reviewed on a daily basis all admissions from EMRs. An author (R.G.) would then contact the admitting team (hospitalist or resident) to make sure that a gastroenterology consult was called and would then obtain the QI by manual extraction from the EMRs.
Of the 16 gastroenterologists who work at the hospital, 12 of them belong to several private practice groups, whereas 4 are employed by the hospital. As part of the intervention, all gastroenterologists were made aware of the intervention 1 month before the starting date, were provided with a checklist of the QIs of interest, and were encouraged to work with the hospitalist attendings to achieve compliance with the QIs. We reminded the gastroenterologists of the ongoing study during routine division meetings and regularly sought feedback from the hospitalists
The MC consisted of a systematic consultation by a gastroenterologist: any identified patient with DC would generate a mandatory GI consultation and would be assigned to a specialist depending on the roster coverage for that day. A close monitoring of the process allowed us to confirm that all patients admitted with DC were seen by a gastroenterologist. Patients were followed until their discharge, death, or readmission to our institution during the study period.
Outcomes
The primary outcome was defined as the rate of adherence to the QIs and overall QI score expressed as a proportion as noted above. Secondary outcomes included in‐hospital mortality, LOS, and 30‐day readmission rate. These parameters were abstracted from the medical record.
Covariates
The hospital EMR (Cerner Corporation, North Kansas City, MO) was used to extract patient demographic parameters such as gender, race, language, and age at time of admission. Other admission‐level details were extracted from the EMR including Model for End‐Stage Liver Disease (MELD) scores, documented comorbidities (including substance abuse, psychiatric diagnosis, diabetes mellitus, renal failure, congestive heart failure, coronary artery disease, and cancer), underlying etiology for cirrhosis, and reason for admission.
The study was approved by Baystate Medical Center's institutional review board.
Statistical Analysis
Summary statistics for outcomes and covariates were calculated as means/standard deviations (SDs), medians/emnterquartile range, and proportions. Univariable statistics (unpaired t tests, 1‐way analysis of variance, Fisher exact test, Spearman correlation) were used to identify possible demographic (eg, age, race) and clinical (eg, admission complaint) predictors of quality score and with 30‐day outcomes. For each admission, a composite quality score, also known as an opportunity model score,[5, 6] was calculated as a fraction (ie, the number of QIs met divided by the total number of possible QIs indicated by the patient's presentation). This fraction was then multiplied by 100 so as to express the QI score as a percent. Possible scores, therefore, ranged from 0 to 100%.
Calculation of the 30‐day incidence proportion of readmission after the first admission was restricted to patients whose readmission occurred in this hospital, and occurring up to 30‐days before study closure (June 1, 2012). In‐hospital death was examined as a function of QI score during that admission. To derive an unbiased, risk‐adjusted estimate of the association between quality score and outcomes, multiple linear regression (opportunity model score [OMS], LOS) or multiple Poisson regression models (30‐day readmission, in‐hospital death) were built. These included a dummy variable for the study period, as well as any potential confounder that was associated at P0.10, with both study period and the outcome in univariable analyses. Robust standard errors were specified to account for multiple admissions within patients. Marginal means or proportions were then estimated with 95% confidence intervals derived using the delta method. All analyses were performed using Stata 12.1 for Windows (StataCorp, College Station, TX).
RESULTS
A total of 303 patients were observed in 695 hospitalizations;149 patients in 379 admissions were observed in the UC cohort, and 154 patients in 316 admissions were observed in the MC cohort. Baseline demographics of all study admissions appear in Table 1. Patients seen in the MC cohort were younger, more likely to speak English, and less likely to be male or have comorbid diabetes mellitus. Most admissions (n=217, 57.2%; 95% confidence interval: 52.3%‐62.3%) were not evaluated by a gastroenterologist in the UC cohort but all were in the MC cohort.
| UC, N=379, N (%) or Mean/SD | MC, N=316, N (%) or Mean/SD | P Value* | |
|---|---|---|---|
| |||
| Age, y | 55.3/12.1 | 53.3/13.6 | 0.05 |
| English speaking | 261 (68.9%) | 261 (82.6%) | <0.001 |
| Male | 251 (66.2%) | 163 (53.5%) | 0.001 |
| Race | <0.001 | ||
| White | 301 (79.4%) | 262 (82.9%) | |
| Black | 31 (8.2%) | 40 (12.7%) | |
| Asian | 16 (4.2%) | 0 (0.0%) | |
| Other | 31 (8.2%) | 14 (4.4%) | |
| Comorbidities | |||
| Substance | 75 (19.8%) | 58 (18.4%) | 0.70 |
| abuse | |||
| Psychiatric | 123 (32.5%) | 103 (32.9%) | 0.94 |
| Diabetes mellitus | 175 (45.4%) | 115 (36.5%) | 0.02 |
| Renal failure | 74 (19.3%) | 55 (17.4%) | 0.50 |
| CHF | 38 (10.0%) | 24 (7.6%) | 0.35 |
| CAD | 26 (6.9%) | 17 (5.4%) | 0.43 |
| Cancer | 48 (12.7%) | 40 (12.7%) | 1.00 |
| Admission MELD | 15.6/6.9 | 17.0/7.0 | 0.006 |
| Serum creatinine | 1.43/1.94 | 1.42/1.30 | 0.91 |
| Reason for admission | |||
| Hepatology/GI | 318 (83.9%) | 257 (81.3%) | 0.42 |
| Renal failure | 85 (22.4%) | 90 (28.5%) | 0.08 |
| Encephalopathy | 151 (39.3%) | 113 (34.9%) | 0.24 |
| GI bleed | 78 (20.5%) | 57 (18.0%) | 1.00 |
| Abdominal pain | 116 (30.7%) | 114 (36.2%) | 0.15 |
| Ascites | 246 (64.9%) | 185 (58.5%) | 0.10 |
Admission Characteristics
The baseline clinical measures of all study admissions appear in Table 1. The UC and MC cohorts had similar characteristics, with the majority of patients with DC admitted for a gastrointestinal/hepatology‐related reason specifically for the management of ascites and hepatic encephalopathy. The patients in the MC cohort had a statistically higher MELD score on admission, which was not clinically relevant.
Quality Measures
Adherence to individual quality indices is shown in Table 2.
| Condition (Denominator) | Quality Indicator (Numerator) | UC (n=379), Met/Indicated | MC (n=316), Met/Indicated | P Value | |
|---|---|---|---|---|---|
| |||||
| Admissions with ascites | |||||
| 1 | Admissions to the hospital because of ascites or encephalopathy. | Diagnostic paracentesis during admission. | 77/193, 39.9%, (32.9%, 46.9%) | 111/135, 82.2% (75.7%, 88.8%) | <0.001 |
| 2 | No fibrinolysis or disseminated intravascular coagulation before paracentesis INR <2.5, >100,000 platelets. | No fresh frozen plasma or platelet replacement given. | 36/37, 97.3% (91.8%, 103.0%) | 41/42, 97.6% (92.8%, 102.4%) | 1.00 |
| 3 | All admissions with diagnostic paracentesis (not limited to admissions for ascites or hepatic encephalopathy). | Cell count differential, total protein, albumin, and culture/sensitivity all performed. | 31/49, 63.3% (49.3%‐77.3%) | 46/72 63.9% (52.7%, 75.0%) | 1.00 |
| 4 | Admissions with known portal hypertension‐related ascites receiving a paracentesis. | Ascitic fluid cell count and differential performed. | 15/104, 14.4% (7.6%‐ 21.3%) | 47/62, 75.8% (63.2%, 88.4%) | <0.001 |
| 5 | Serum sodium 110 mEq/L. | Fluid restriction and discontinuation of diuretics. | NA | NA | NA |
| 6 | Polymorphonuclear count of 250/mm3 in ascites. | Empiric antibiotics, 6 hours of results. | 10/13, 76.9% (50.4%‐ 103.4%) | 16/20, 80.0% (60.8%, 99.2%) | 1.00 |
| 7 | Ascitic fluid, total protein 1.1 gm/dL, serum bilirubin 2.5 mg/dL. | Prophylactic antibiotics. | 4/12, 33.3% (2.0%‐ 64.6%) | 18/30, 60.0%, (41.4%, 78.6%) | 0.18 |
| 8 | Normal renal function. | Salt restriction and diuretics (spironolactone and loop diuretics). | 57/186, 30.6%, (24.0%‐ 37.3%) | 81/122, 66.4%, (57.9%, 74.9%) | <0.001 |
| Total ascites subscore, mean/SD | 30%/36% | 67%/34% | <0.001 | ||
| GI bleeding | |||||
| 9 | Admissions with GI bleeding: variceal and nonvariceal, hematemesis and melena. | Upper endoscopy 24 hours of presentation. | 60/78, 76.9% (67.4%, 86.4%) | 52/57, 91.2% (83.7%, 98.8%) | 0.04 |
| 10 | Esophageal varices (active, stigmata of recent bleeding, or no other causes to explain bleeding). | Endoscopic variceal ligation/sublerotherapy. | 40/46, 87.0% (76.8%‐97.1%) | 30/32, 93.8% (84.9%, 100.0%) | 0.46 |
| 11 | Admissions with established/suspected upper GI bleeding. | Antibiotics within 24 hours of admission. | 27/69, 39.1% (27.3%‐ 50.9%) | 26/58, 44.8% (31.6%, 58.0%) | 0.59 |
| 12 | Admissions with established/suspected variceal bleeding. | Somatostatin/octreotide given within 12 hours of presentation. | 53/69, 76.8%, (66.6%‐ 87.0%) | 49/58, 84.5% (73.8%, 95.2%) | 0.37 |
| 13 | Recurrent bleeding within 72 hours of initial endoscopic hemostasis. | Repeat endoscopy or transjugular intrahepatic portosystemic shunt. | 5/5 100% | 2/3, 66.7% (76.8%, 210.0%) | 0.38 |
| Total GI subscore, mean/SD | 61%/38% | 74%/28% | 0.04 | ||
| Liver transplantation | |||||
| 14 | Admissions with MELD 15 or MELD 15 and decompensated status (ie, all admissions in our study). | Documented evaluation for liver transplantation. | 112/379, 29.6% (24.9%‐ 34.2%) | 231/316, 73.6% (68.7%, 78.5%) | <0.001 |
| Hepatic encephalopathy | |||||
| 15 | Admissions with hepatic encephalopathy. | Search for reversible factors documented. | 81/151, 53.6% (45.6%‐ 61.7 %) | 97/113, 85.8% (79.4%, 92.3%) | <0.001 |
| 16 | Admissions with hepatic encephalopathy. | Oral disaccharides/ rifaximin. | 144/151, 95.3% (91.9 %‐ 98.7 %) | 107/113, 94.7% (90.7%. 98.69%) | 1.00 |
| Total encephalopathy subscore, mean/SD | 75%/28% | 90%/24% | <0.001 | ||
Ascites
The management of ascites yielded 3 main differences between the 2 cohorts. Following the implementation of the MC, 82.2 % (111/135) of ascites‐related admissions led to a diagnostic paracentesis as compared to 39.9% (77/193) in the UC group (P<0.001).
In the MC cohort, 75.8% (47/62) of admissions with known portal hypertensionrelated ascites who received a paracentesis had an ascites cell count checked. In contrast, only 14.4% (15/104) in the UC group receiving paracentesis had a fluid cell count (P<0.001). The management of ascites in patients with normal renal function was optimal, with sodium restriction and diuretics combination in 66.4% (81/122) of the MC cohort, whereas this parameter in the UC cohort was only 30.6% (57/186) (P<0.001). There were no significant differences between the groups for the other QIs.
Variceal Bleeding
The MC group had a higher frequency of endoscopy within 24 hours of admissions than the UC group (91.2% [52/57] vs 76.9% [60/78], respectively; P<0.04). The rest had endoscopy later in the admission. Among admissions with bleeding from varices, banding was done 93.8% of the time for patients in the MC group (30/32), which was not statistically different than 87.0% (40/46) for patients seen in the UC group. In the remaining admissions, endoscopy only revealed nonbleeding large esophageal varices, and the endoscopist opted not to proceed with therapy. There were no statistically significant differences in the rest of the management.
Hepatic Encephalopathy
For hepatic encephalopathy, an empirical treatment was given to 95.3 % (144/151) patients in the UC group and 94.7% (107/113) of the patients in the MC group. We found better documentation of a search for underlying etiologies leading to hepatic encephalopathy in the MC cohort 85.8% (97/113) versus the UC cohort, which was only 53.6% (81/151) (P<0.001).
Evaluation for Liver Transplantation
Better documentation of evaluation for liver transplantation was seen in the MC group 73.6% (231/316) in comparison to the UC group 29.4% (111/379) (P<0.001).
Opportunity Score and Clinical Outcomes
As detailed above, care provided during the MC achieved a higher compliance with the QI shown with the QI score or OMS (Table 3). These improvements were not associated with statistically significant differences in in‐hospital death, LOS, or 30‐day readmission. To explore this further we also examined the direct association between the OMS and outcomes in the MC group by dividing patients into 2 groups: patients whose OMS was 80% and those whose OMS was <80% (see Supporting Information, Appendix 4, in the online version of this article). Although there were trends toward decreased in‐hospital death (6.4% vs 8.6%, P=0.26), increased 30‐day readmission (33.8% vs 23.0%, P=0.27), and decreased LOS (6.2 days vs 6.6 days, P=0.77), none of these differences achieved statistical significance.
| Unadjusted | Adjusted* | |||||
|---|---|---|---|---|---|---|
| UC | MC | Difference | UC | MC | Difference | |
| ||||||
| Opportunity model score | 0.46 | 0.77 | +0.31 (0.24, 0.39) | 0.46 | 0.77 | +0.30(0.23, 0.37) |
| In‐hospital death | 7.1% | 8.5% | +1.4 (0.3, +5.6) | 7.5% | 7.9% | +0.4% (4.0%, +4.5%) |
| Readmission within 30 days | 39.6% | 32.6% | 7.0% (16.4%, +2.5%) | 40.0% | 31.8% | 8.2%(18.0%, +1.5%) |
| Length of stay | 6.1d | 6.2d | +0.1d (1.0 d, +1.2 d) | 6.1d | 6.2d | +0.1d (1.0 d, +1.2d) |
Mandatory Consultation Subgroups: Employed Versus Private Physicians
The type of employment of the gastroenterologist on consultation (employed by the hospital vs private practice) affected the management of the patients admitted with DC (see Supporting Information, Appendix 3, in the online version of this article). Patients seen by a hospital‐employed gastroenterologist were more likely to have a better documentation in regard to evaluation for liver transplantation and better management of ascites. Except for the prescription of antibiotics in patients presenting with GI bleeding, which were more often given by the employed physician (63% vs 23%, P=0.004), the management of hepatic encephalopathy and GI bleeding was similar between employed and private‐practice physicians.
DISCUSSION
In this evaluation of an MC intervention for patients with DC cared for at a large tertiary academic medical center, we found that the implementation of a routine consultation by a gastroenterologist led to greater adherence to recommended care processes when compared to UC. Overall, the management of ascites and the documentation of evaluation for liver transplantation were statistically superior in the intervention (MC) group. UC and MC were similar with respect to treatment of variceal bleeding and hepatic encephalopathy. Although we did not demonstrate changes in mortality, readmission, or LOS as a result of the MC intervention, our study was underpowered to detect clinically meaningful effects.
The gaps in care of patients with cirrhosis were reported before and after the publication of the formal QIs.[7, 8, 9, 10] These gaps remain relevant in the face of an increasing prevalence of DC along with a recent publication suggesting an underestimation of the burden of liver disease in the United States.[11] Ours is the first study to evaluate the impact on inpatients with DC of a liver service with a systematic, mandatory, specialist consultation. A previous study[12] had shown that a GI consultation would improve the care of patients with DC, but excluded patients with variceal bleeding, did not specifically measure the compliance with QIs, and more important, the GI consult was not mandatory.
Our study has several limitations that must be considered while weighing its findings. The patients were not randomly assigned but followed a pre‐established distribution depending on the call schedule. Some of the improvement we noted might be the result of secular trends; however, this remains unlikely given the lack of national initiatives or pay for performance programs. In the UC cohort, patients who were nonEnglish‐speaking were associated with a lower QI score, which could account for part of the improvement seen in the MC group that has a more prominent English‐speaking cohort. Readmissions could have occurred at other hospitals, and patients were not monitored in an outpatient setting. We did not observe a change in the secondary outcomes (30‐day readmission, LOS, in‐hospital death); however, our study was underpowered for that purpose. Given the complexity of the billing process we did not collect the costs of the MC, which is another limitation of our work. Future studies are needed to determine the cost‐effectiveness of the intervention.
This study shows that a dedicated team of physicians focused on compliance with QIs can achieve a rapid improvement, over a year, in providing higher‐quality care. This may be relevant at other institutions. The strength of our study is that our large tertiary academic medical center serves a large catchment area, with a mix of patients from both rural and urban communities. It is located in Massachusetts, where most of the population has had access to healthcare since 2006. Therefore, although this is a single‐center study, we expect our findings to be more generalizable and less subject to selection bias than other single‐center studies.
Importantly, the compliance with QIs was often far from being perfect in the MC group and was different across type of employment of providers, reflecting the challenges in changing practice among physicians.[13] In fact the QI scores of the private practice group did not change, and mirror the compliance observed at our institution in the previous study, before the implementation of the MC.[4] The difference in performance according to the type of employment of providers stems from 2 factors. First, a better documentation of the need of formal evaluation for liver transplantation by the employed gastroenterologists resulted in better compliance with this QI. Second, and more important, among the employed physicians, there was a readiness to assist the hospitalist with diagnostic/therapeutic paracentesis without relying on, for example, an interventional radiologist. This is reflected by the higher score in the management of ascites. Although our study was not designed to answer this directly, employed physicians may have been more engaged in the project and showed a greater willingness to change practice. In the future, linking reimbursement to quality of care will lead to improved accountability of consultants.
In this study we show that a direct involvement of a gastroenterologist improves the care of inpatients as measured by QIs. We theorize that a better coordination of the transition to outpatient care involving the specialist should lead to better outcomes, specifically a reduction in the 22% observed readmission rate within 30 days of patients with DC.[14, 15] As we move forward, a broader definition of outcomes should be addressed, taking into account patient‐related outcomes and preferences.[16] Future studies should define the relationship between the gastroenterologist and the hospitalist service, the role of physician assistants and nurse practitioners in implementing and monitoring compliance with QIs, and define how physicians and patients can be made accountable in the transition to the outpatient setting.
Disclosures
R.G.: Conception, data collection and interpretation, manuscript. J.F.: Data management, data analysis, manuscript. P.V.: Conception, data analysis, manuscript. P.L.: Conception, data interpretation, manuscript. T.L.: Dr. Lagu is supported by the National Heart, Lung, and Blood Institute of the National Institutes of Health under award number K01HL114745. Conception, data interpretation, manuscript. D.D.: Conception, data collection and interpretation, manuscript. A.B.: Data collection. J.S.: Data collection. Source of funding: internal. The authors report no conflicts of interest.
Decompensated cirrhosis (DC) is defined as cirrhosis with at least 1 of the following complications: ascites, hepatocellular carcinoma, bleeding from portal hypertension, or hepatic encephalopathy. Patients with DC have a median survival estimated at 2 years compared to the 12‐year median survival of compensated cirrhotics.[1] In an era where quality of hospital care is being measured, and where progress is being made in the management of several conditions including congestive heart failure and nosocomial infections, little attention has been paid to DC. The burden of chronic liver failure is clear in the United States, where DC leads to more than 150,000 annual admissions to the hospital and accounts for 40,000 deaths annually.[2]
This burden of disease spurred quality improvement efforts in 2010, when a team of experts identified a set of literature‐based parameters or quality indicators (QI) for patients with cirrhosis.[3] We have demonstrated that adherence to these indicators fell far short of desired targets.[4] A year before their publication, an overall compliance of <50% with these metrics was measured at a single medical center.
We sought to improve the quality of care for patients with DC through implementation of mandatory consultation (MC) with a gastroenterologist for all patients admitted with DC. We assessed whether MC was associated with better care and improved outcomes (hospitalization length of stay [LOS], 30‐day readmission, and inpatient mortality) when compared to usual care (UC).[4]
MATERIALS AND METHODS
Design, Setting, and Patients
We conducted a cohort study comparing adherence to QI and outcomes of patients admitted with DC after the institution of an MC to a historical cohort of patients managed with UC (ie, before MC, adherence to QI for this group has been reported elsewhere).[4] Both cohorts included all patients aged >18 years with DC admitted to Baystate Medical Center, a tertiary care medical center in western Massachusetts. The UC cohort was collected between January 1, 2009 and December 31, 2009, and the MC cohort was assembled between June 1, 2011 and June 30, 2012.
As previously reported,[4] patients were considered for inclusion in the historical cohort if their International Classification of DiseasesNinth Revision discharge code pertained to chronic liver disease (see Supporting Information, Appendix 1, in the online version of this article). This list was broad by design to identify all patients with decompensated cirrhosis. A gastroenterologist (R.G.) then manually extracted charts from electronic medical records (EMRs) using a set of predefined clinical criteria, the same in both cohorts, to identify the patients with DC: cirrhosis with concomitant ascites, hepatic encephalopathy, or gastrointestinal (GI) bleeding secondary to portal hypertension. Other types of decompensated states, such as hepatocellular carcinoma, were not included as their management was not detailed in the QI.[3]
We included patients with suspected or established cirrhosis who had ascites confirmed radiographically or by exam, noting shifting dullness or fluid wave. However, patients were excluded if they lacked sufficient peritoneal fluid for bedside or image‐guided paracentesis. Cirrhotic patients were defined as having hepatic encephalopathy if the patient had altered mental status not secondary to seizures, cerebrovascular accident, or alcohol withdrawal. Finally, gastrointestinal bleeding in cirrhotic patients was defined as any upper or lower bleeding prompting hospital admission, or identified in the medical record as clinically significant by the attending physician.
The same QIs were measured in both cohorts. From the QI set,[3] we selected the 16 QIs that would apply to the management of inpatients (see Supporting Information, Appendix 2, in the online version of this article). Indicators developed for outpatient settings were not included. A quality score was calculated for each admission, defined as the proportion of QIs met divided by the number of QIs for which the patient was eligible. For example, a patient with hepatic encephalopathy but without GI bleeding or ascites would have a score calculated as the number of QIs met for hepatic encephalopathy and documentation of transplant evaluation divided by 3 (2 QIs for hepatic encephalopathy and 1 QI for transplant evaluation). If the patient met both QIs for hepatic encephalopathy, but the consultant failed to address liver transplant eligibility, the score would be 2/3=0.666.
After the institution of the MC, all inpatients with DC were identified within 24 hours of admission by a gastroenterologist (R.G., D.D.), who manually reviewed on a daily basis all admissions from EMRs. An author (R.G.) would then contact the admitting team (hospitalist or resident) to make sure that a gastroenterology consult was called and would then obtain the QI by manual extraction from the EMRs.
Of the 16 gastroenterologists who work at the hospital, 12 of them belong to several private practice groups, whereas 4 are employed by the hospital. As part of the intervention, all gastroenterologists were made aware of the intervention 1 month before the starting date, were provided with a checklist of the QIs of interest, and were encouraged to work with the hospitalist attendings to achieve compliance with the QIs. We reminded the gastroenterologists of the ongoing study during routine division meetings and regularly sought feedback from the hospitalists
The MC consisted of a systematic consultation by a gastroenterologist: any identified patient with DC would generate a mandatory GI consultation and would be assigned to a specialist depending on the roster coverage for that day. A close monitoring of the process allowed us to confirm that all patients admitted with DC were seen by a gastroenterologist. Patients were followed until their discharge, death, or readmission to our institution during the study period.
Outcomes
The primary outcome was defined as the rate of adherence to the QIs and overall QI score expressed as a proportion as noted above. Secondary outcomes included in‐hospital mortality, LOS, and 30‐day readmission rate. These parameters were abstracted from the medical record.
Covariates
The hospital EMR (Cerner Corporation, North Kansas City, MO) was used to extract patient demographic parameters such as gender, race, language, and age at time of admission. Other admission‐level details were extracted from the EMR including Model for End‐Stage Liver Disease (MELD) scores, documented comorbidities (including substance abuse, psychiatric diagnosis, diabetes mellitus, renal failure, congestive heart failure, coronary artery disease, and cancer), underlying etiology for cirrhosis, and reason for admission.
The study was approved by Baystate Medical Center's institutional review board.
Statistical Analysis
Summary statistics for outcomes and covariates were calculated as means/standard deviations (SDs), medians/emnterquartile range, and proportions. Univariable statistics (unpaired t tests, 1‐way analysis of variance, Fisher exact test, Spearman correlation) were used to identify possible demographic (eg, age, race) and clinical (eg, admission complaint) predictors of quality score and with 30‐day outcomes. For each admission, a composite quality score, also known as an opportunity model score,[5, 6] was calculated as a fraction (ie, the number of QIs met divided by the total number of possible QIs indicated by the patient's presentation). This fraction was then multiplied by 100 so as to express the QI score as a percent. Possible scores, therefore, ranged from 0 to 100%.
Calculation of the 30‐day incidence proportion of readmission after the first admission was restricted to patients whose readmission occurred in this hospital, and occurring up to 30‐days before study closure (June 1, 2012). In‐hospital death was examined as a function of QI score during that admission. To derive an unbiased, risk‐adjusted estimate of the association between quality score and outcomes, multiple linear regression (opportunity model score [OMS], LOS) or multiple Poisson regression models (30‐day readmission, in‐hospital death) were built. These included a dummy variable for the study period, as well as any potential confounder that was associated at P0.10, with both study period and the outcome in univariable analyses. Robust standard errors were specified to account for multiple admissions within patients. Marginal means or proportions were then estimated with 95% confidence intervals derived using the delta method. All analyses were performed using Stata 12.1 for Windows (StataCorp, College Station, TX).
RESULTS
A total of 303 patients were observed in 695 hospitalizations;149 patients in 379 admissions were observed in the UC cohort, and 154 patients in 316 admissions were observed in the MC cohort. Baseline demographics of all study admissions appear in Table 1. Patients seen in the MC cohort were younger, more likely to speak English, and less likely to be male or have comorbid diabetes mellitus. Most admissions (n=217, 57.2%; 95% confidence interval: 52.3%‐62.3%) were not evaluated by a gastroenterologist in the UC cohort but all were in the MC cohort.
| UC, N=379, N (%) or Mean/SD | MC, N=316, N (%) or Mean/SD | P Value* | |
|---|---|---|---|
| |||
| Age, y | 55.3/12.1 | 53.3/13.6 | 0.05 |
| English speaking | 261 (68.9%) | 261 (82.6%) | <0.001 |
| Male | 251 (66.2%) | 163 (53.5%) | 0.001 |
| Race | <0.001 | ||
| White | 301 (79.4%) | 262 (82.9%) | |
| Black | 31 (8.2%) | 40 (12.7%) | |
| Asian | 16 (4.2%) | 0 (0.0%) | |
| Other | 31 (8.2%) | 14 (4.4%) | |
| Comorbidities | |||
| Substance | 75 (19.8%) | 58 (18.4%) | 0.70 |
| abuse | |||
| Psychiatric | 123 (32.5%) | 103 (32.9%) | 0.94 |
| Diabetes mellitus | 175 (45.4%) | 115 (36.5%) | 0.02 |
| Renal failure | 74 (19.3%) | 55 (17.4%) | 0.50 |
| CHF | 38 (10.0%) | 24 (7.6%) | 0.35 |
| CAD | 26 (6.9%) | 17 (5.4%) | 0.43 |
| Cancer | 48 (12.7%) | 40 (12.7%) | 1.00 |
| Admission MELD | 15.6/6.9 | 17.0/7.0 | 0.006 |
| Serum creatinine | 1.43/1.94 | 1.42/1.30 | 0.91 |
| Reason for admission | |||
| Hepatology/GI | 318 (83.9%) | 257 (81.3%) | 0.42 |
| Renal failure | 85 (22.4%) | 90 (28.5%) | 0.08 |
| Encephalopathy | 151 (39.3%) | 113 (34.9%) | 0.24 |
| GI bleed | 78 (20.5%) | 57 (18.0%) | 1.00 |
| Abdominal pain | 116 (30.7%) | 114 (36.2%) | 0.15 |
| Ascites | 246 (64.9%) | 185 (58.5%) | 0.10 |
Admission Characteristics
The baseline clinical measures of all study admissions appear in Table 1. The UC and MC cohorts had similar characteristics, with the majority of patients with DC admitted for a gastrointestinal/hepatology‐related reason specifically for the management of ascites and hepatic encephalopathy. The patients in the MC cohort had a statistically higher MELD score on admission, which was not clinically relevant.
Quality Measures
Adherence to individual quality indices is shown in Table 2.
| Condition (Denominator) | Quality Indicator (Numerator) | UC (n=379), Met/Indicated | MC (n=316), Met/Indicated | P Value | |
|---|---|---|---|---|---|
| |||||
| Admissions with ascites | |||||
| 1 | Admissions to the hospital because of ascites or encephalopathy. | Diagnostic paracentesis during admission. | 77/193, 39.9%, (32.9%, 46.9%) | 111/135, 82.2% (75.7%, 88.8%) | <0.001 |
| 2 | No fibrinolysis or disseminated intravascular coagulation before paracentesis INR <2.5, >100,000 platelets. | No fresh frozen plasma or platelet replacement given. | 36/37, 97.3% (91.8%, 103.0%) | 41/42, 97.6% (92.8%, 102.4%) | 1.00 |
| 3 | All admissions with diagnostic paracentesis (not limited to admissions for ascites or hepatic encephalopathy). | Cell count differential, total protein, albumin, and culture/sensitivity all performed. | 31/49, 63.3% (49.3%‐77.3%) | 46/72 63.9% (52.7%, 75.0%) | 1.00 |
| 4 | Admissions with known portal hypertension‐related ascites receiving a paracentesis. | Ascitic fluid cell count and differential performed. | 15/104, 14.4% (7.6%‐ 21.3%) | 47/62, 75.8% (63.2%, 88.4%) | <0.001 |
| 5 | Serum sodium 110 mEq/L. | Fluid restriction and discontinuation of diuretics. | NA | NA | NA |
| 6 | Polymorphonuclear count of 250/mm3 in ascites. | Empiric antibiotics, 6 hours of results. | 10/13, 76.9% (50.4%‐ 103.4%) | 16/20, 80.0% (60.8%, 99.2%) | 1.00 |
| 7 | Ascitic fluid, total protein 1.1 gm/dL, serum bilirubin 2.5 mg/dL. | Prophylactic antibiotics. | 4/12, 33.3% (2.0%‐ 64.6%) | 18/30, 60.0%, (41.4%, 78.6%) | 0.18 |
| 8 | Normal renal function. | Salt restriction and diuretics (spironolactone and loop diuretics). | 57/186, 30.6%, (24.0%‐ 37.3%) | 81/122, 66.4%, (57.9%, 74.9%) | <0.001 |
| Total ascites subscore, mean/SD | 30%/36% | 67%/34% | <0.001 | ||
| GI bleeding | |||||
| 9 | Admissions with GI bleeding: variceal and nonvariceal, hematemesis and melena. | Upper endoscopy 24 hours of presentation. | 60/78, 76.9% (67.4%, 86.4%) | 52/57, 91.2% (83.7%, 98.8%) | 0.04 |
| 10 | Esophageal varices (active, stigmata of recent bleeding, or no other causes to explain bleeding). | Endoscopic variceal ligation/sublerotherapy. | 40/46, 87.0% (76.8%‐97.1%) | 30/32, 93.8% (84.9%, 100.0%) | 0.46 |
| 11 | Admissions with established/suspected upper GI bleeding. | Antibiotics within 24 hours of admission. | 27/69, 39.1% (27.3%‐ 50.9%) | 26/58, 44.8% (31.6%, 58.0%) | 0.59 |
| 12 | Admissions with established/suspected variceal bleeding. | Somatostatin/octreotide given within 12 hours of presentation. | 53/69, 76.8%, (66.6%‐ 87.0%) | 49/58, 84.5% (73.8%, 95.2%) | 0.37 |
| 13 | Recurrent bleeding within 72 hours of initial endoscopic hemostasis. | Repeat endoscopy or transjugular intrahepatic portosystemic shunt. | 5/5 100% | 2/3, 66.7% (76.8%, 210.0%) | 0.38 |
| Total GI subscore, mean/SD | 61%/38% | 74%/28% | 0.04 | ||
| Liver transplantation | |||||
| 14 | Admissions with MELD 15 or MELD 15 and decompensated status (ie, all admissions in our study). | Documented evaluation for liver transplantation. | 112/379, 29.6% (24.9%‐ 34.2%) | 231/316, 73.6% (68.7%, 78.5%) | <0.001 |
| Hepatic encephalopathy | |||||
| 15 | Admissions with hepatic encephalopathy. | Search for reversible factors documented. | 81/151, 53.6% (45.6%‐ 61.7 %) | 97/113, 85.8% (79.4%, 92.3%) | <0.001 |
| 16 | Admissions with hepatic encephalopathy. | Oral disaccharides/ rifaximin. | 144/151, 95.3% (91.9 %‐ 98.7 %) | 107/113, 94.7% (90.7%. 98.69%) | 1.00 |
| Total encephalopathy subscore, mean/SD | 75%/28% | 90%/24% | <0.001 | ||
Ascites
The management of ascites yielded 3 main differences between the 2 cohorts. Following the implementation of the MC, 82.2 % (111/135) of ascites‐related admissions led to a diagnostic paracentesis as compared to 39.9% (77/193) in the UC group (P<0.001).
In the MC cohort, 75.8% (47/62) of admissions with known portal hypertensionrelated ascites who received a paracentesis had an ascites cell count checked. In contrast, only 14.4% (15/104) in the UC group receiving paracentesis had a fluid cell count (P<0.001). The management of ascites in patients with normal renal function was optimal, with sodium restriction and diuretics combination in 66.4% (81/122) of the MC cohort, whereas this parameter in the UC cohort was only 30.6% (57/186) (P<0.001). There were no significant differences between the groups for the other QIs.
Variceal Bleeding
The MC group had a higher frequency of endoscopy within 24 hours of admissions than the UC group (91.2% [52/57] vs 76.9% [60/78], respectively; P<0.04). The rest had endoscopy later in the admission. Among admissions with bleeding from varices, banding was done 93.8% of the time for patients in the MC group (30/32), which was not statistically different than 87.0% (40/46) for patients seen in the UC group. In the remaining admissions, endoscopy only revealed nonbleeding large esophageal varices, and the endoscopist opted not to proceed with therapy. There were no statistically significant differences in the rest of the management.
Hepatic Encephalopathy
For hepatic encephalopathy, an empirical treatment was given to 95.3 % (144/151) patients in the UC group and 94.7% (107/113) of the patients in the MC group. We found better documentation of a search for underlying etiologies leading to hepatic encephalopathy in the MC cohort 85.8% (97/113) versus the UC cohort, which was only 53.6% (81/151) (P<0.001).
Evaluation for Liver Transplantation
Better documentation of evaluation for liver transplantation was seen in the MC group 73.6% (231/316) in comparison to the UC group 29.4% (111/379) (P<0.001).
Opportunity Score and Clinical Outcomes
As detailed above, care provided during the MC achieved a higher compliance with the QI shown with the QI score or OMS (Table 3). These improvements were not associated with statistically significant differences in in‐hospital death, LOS, or 30‐day readmission. To explore this further we also examined the direct association between the OMS and outcomes in the MC group by dividing patients into 2 groups: patients whose OMS was 80% and those whose OMS was <80% (see Supporting Information, Appendix 4, in the online version of this article). Although there were trends toward decreased in‐hospital death (6.4% vs 8.6%, P=0.26), increased 30‐day readmission (33.8% vs 23.0%, P=0.27), and decreased LOS (6.2 days vs 6.6 days, P=0.77), none of these differences achieved statistical significance.
| Unadjusted | Adjusted* | |||||
|---|---|---|---|---|---|---|
| UC | MC | Difference | UC | MC | Difference | |
| ||||||
| Opportunity model score | 0.46 | 0.77 | +0.31 (0.24, 0.39) | 0.46 | 0.77 | +0.30(0.23, 0.37) |
| In‐hospital death | 7.1% | 8.5% | +1.4 (0.3, +5.6) | 7.5% | 7.9% | +0.4% (4.0%, +4.5%) |
| Readmission within 30 days | 39.6% | 32.6% | 7.0% (16.4%, +2.5%) | 40.0% | 31.8% | 8.2%(18.0%, +1.5%) |
| Length of stay | 6.1d | 6.2d | +0.1d (1.0 d, +1.2 d) | 6.1d | 6.2d | +0.1d (1.0 d, +1.2d) |
Mandatory Consultation Subgroups: Employed Versus Private Physicians
The type of employment of the gastroenterologist on consultation (employed by the hospital vs private practice) affected the management of the patients admitted with DC (see Supporting Information, Appendix 3, in the online version of this article). Patients seen by a hospital‐employed gastroenterologist were more likely to have a better documentation in regard to evaluation for liver transplantation and better management of ascites. Except for the prescription of antibiotics in patients presenting with GI bleeding, which were more often given by the employed physician (63% vs 23%, P=0.004), the management of hepatic encephalopathy and GI bleeding was similar between employed and private‐practice physicians.
DISCUSSION
In this evaluation of an MC intervention for patients with DC cared for at a large tertiary academic medical center, we found that the implementation of a routine consultation by a gastroenterologist led to greater adherence to recommended care processes when compared to UC. Overall, the management of ascites and the documentation of evaluation for liver transplantation were statistically superior in the intervention (MC) group. UC and MC were similar with respect to treatment of variceal bleeding and hepatic encephalopathy. Although we did not demonstrate changes in mortality, readmission, or LOS as a result of the MC intervention, our study was underpowered to detect clinically meaningful effects.
The gaps in care of patients with cirrhosis were reported before and after the publication of the formal QIs.[7, 8, 9, 10] These gaps remain relevant in the face of an increasing prevalence of DC along with a recent publication suggesting an underestimation of the burden of liver disease in the United States.[11] Ours is the first study to evaluate the impact on inpatients with DC of a liver service with a systematic, mandatory, specialist consultation. A previous study[12] had shown that a GI consultation would improve the care of patients with DC, but excluded patients with variceal bleeding, did not specifically measure the compliance with QIs, and more important, the GI consult was not mandatory.
Our study has several limitations that must be considered while weighing its findings. The patients were not randomly assigned but followed a pre‐established distribution depending on the call schedule. Some of the improvement we noted might be the result of secular trends; however, this remains unlikely given the lack of national initiatives or pay for performance programs. In the UC cohort, patients who were nonEnglish‐speaking were associated with a lower QI score, which could account for part of the improvement seen in the MC group that has a more prominent English‐speaking cohort. Readmissions could have occurred at other hospitals, and patients were not monitored in an outpatient setting. We did not observe a change in the secondary outcomes (30‐day readmission, LOS, in‐hospital death); however, our study was underpowered for that purpose. Given the complexity of the billing process we did not collect the costs of the MC, which is another limitation of our work. Future studies are needed to determine the cost‐effectiveness of the intervention.
This study shows that a dedicated team of physicians focused on compliance with QIs can achieve a rapid improvement, over a year, in providing higher‐quality care. This may be relevant at other institutions. The strength of our study is that our large tertiary academic medical center serves a large catchment area, with a mix of patients from both rural and urban communities. It is located in Massachusetts, where most of the population has had access to healthcare since 2006. Therefore, although this is a single‐center study, we expect our findings to be more generalizable and less subject to selection bias than other single‐center studies.
Importantly, the compliance with QIs was often far from being perfect in the MC group and was different across type of employment of providers, reflecting the challenges in changing practice among physicians.[13] In fact the QI scores of the private practice group did not change, and mirror the compliance observed at our institution in the previous study, before the implementation of the MC.[4] The difference in performance according to the type of employment of providers stems from 2 factors. First, a better documentation of the need of formal evaluation for liver transplantation by the employed gastroenterologists resulted in better compliance with this QI. Second, and more important, among the employed physicians, there was a readiness to assist the hospitalist with diagnostic/therapeutic paracentesis without relying on, for example, an interventional radiologist. This is reflected by the higher score in the management of ascites. Although our study was not designed to answer this directly, employed physicians may have been more engaged in the project and showed a greater willingness to change practice. In the future, linking reimbursement to quality of care will lead to improved accountability of consultants.
In this study we show that a direct involvement of a gastroenterologist improves the care of inpatients as measured by QIs. We theorize that a better coordination of the transition to outpatient care involving the specialist should lead to better outcomes, specifically a reduction in the 22% observed readmission rate within 30 days of patients with DC.[14, 15] As we move forward, a broader definition of outcomes should be addressed, taking into account patient‐related outcomes and preferences.[16] Future studies should define the relationship between the gastroenterologist and the hospitalist service, the role of physician assistants and nurse practitioners in implementing and monitoring compliance with QIs, and define how physicians and patients can be made accountable in the transition to the outpatient setting.
Disclosures
R.G.: Conception, data collection and interpretation, manuscript. J.F.: Data management, data analysis, manuscript. P.V.: Conception, data analysis, manuscript. P.L.: Conception, data interpretation, manuscript. T.L.: Dr. Lagu is supported by the National Heart, Lung, and Blood Institute of the National Institutes of Health under award number K01HL114745. Conception, data interpretation, manuscript. D.D.: Conception, data collection and interpretation, manuscript. A.B.: Data collection. J.S.: Data collection. Source of funding: internal. The authors report no conflicts of interest.
- , , . Natural history and prognostic indicators of survival in cirrhosis: a systematic review of 118 studies. J Hepatol. 2006;44:217–231.
- , , , . Efficacy of a chronic disease management model for patients with chronic liver failure. Clin Gastroenterol Hepatol. 2013;11:850–858.
- , , , et al. An Explicit Quality Indicator Set for Measurement of Quality of Care in Patients with Cirrhosis. Clin Gastroenterol Hepatol. 2010;8:709–717.
- , , , , , Desilets D. Measurement of the quality of care of patients admitted with decompensated cirrhosis. Liver Int. 2014:34:204–210.
- , . All‐or‐none measurement raises the bar on performance. JAMA. 2006;295:1168–1170.
- Joint Commission on Accreditation of Healthcare Organizations. Quality report user guide. Available at: http://www.jointcommission.org. Accessed May 30, 2011.
- , , , et al. Management of patients with cirrhosis in Southern California: results of a practitioner survey. J Clin Gastroenterol. 2006;40:156–161.
- , , , et al. Spanish Collaborative Study Group on Therapeutic Management In Liver Disease. Multicenter hospital study on prescribing patterns for prophylaxis and treatment of complications of cirrhosis. Eur J Clin Pharmacol. 2002;58:435–440.
- , , , et al. The quality of care provided to patients with cirrhosis and ascites in the Department of Veterans Affairs. Gastroenterology. 2012 143(1):70–77.
- , , , et al. Improved patient survival after acute variceal bleeding: a multicenter, cohort study. Am J Gastroenterol. 2003;98:653–659.
- , , , , . Underestimation of liver‐related mortality in the United States. Gastroenterology. 2013;145:375–382.
- , , , et al. Impact of gastroenterology consultation on the outcomes of patients admitted to the hospital with decompensated cirrhosis. Hepatology. 2001;34:1089–1095.
- , , , et al. Why don't physicians follow clinical practice guidelines? A framework for improvement. JAMA. 1999;282:1458–1465.
- , , , et al. Incidence and predictors of 30‐day readmission among patients hospitalized with advanced liver disease. Clin Gastroenterol Hepatol. 2011;9:254–259.
- , , , et al. Hospital Readmissions among patients with decompensated cirrhosis. Am J Gastroenterol. 2012;107:247–252.
- . Patient‐reported outcomes of cirrhosis. Clin Gastroenterol Hepatol. 2013;11:1043–1045.
- , , . Natural history and prognostic indicators of survival in cirrhosis: a systematic review of 118 studies. J Hepatol. 2006;44:217–231.
- , , , . Efficacy of a chronic disease management model for patients with chronic liver failure. Clin Gastroenterol Hepatol. 2013;11:850–858.
- , , , et al. An Explicit Quality Indicator Set for Measurement of Quality of Care in Patients with Cirrhosis. Clin Gastroenterol Hepatol. 2010;8:709–717.
- , , , , , Desilets D. Measurement of the quality of care of patients admitted with decompensated cirrhosis. Liver Int. 2014:34:204–210.
- , . All‐or‐none measurement raises the bar on performance. JAMA. 2006;295:1168–1170.
- Joint Commission on Accreditation of Healthcare Organizations. Quality report user guide. Available at: http://www.jointcommission.org. Accessed May 30, 2011.
- , , , et al. Management of patients with cirrhosis in Southern California: results of a practitioner survey. J Clin Gastroenterol. 2006;40:156–161.
- , , , et al. Spanish Collaborative Study Group on Therapeutic Management In Liver Disease. Multicenter hospital study on prescribing patterns for prophylaxis and treatment of complications of cirrhosis. Eur J Clin Pharmacol. 2002;58:435–440.
- , , , et al. The quality of care provided to patients with cirrhosis and ascites in the Department of Veterans Affairs. Gastroenterology. 2012 143(1):70–77.
- , , , et al. Improved patient survival after acute variceal bleeding: a multicenter, cohort study. Am J Gastroenterol. 2003;98:653–659.
- , , , , . Underestimation of liver‐related mortality in the United States. Gastroenterology. 2013;145:375–382.
- , , , et al. Impact of gastroenterology consultation on the outcomes of patients admitted to the hospital with decompensated cirrhosis. Hepatology. 2001;34:1089–1095.
- , , , et al. Why don't physicians follow clinical practice guidelines? A framework for improvement. JAMA. 1999;282:1458–1465.
- , , , et al. Incidence and predictors of 30‐day readmission among patients hospitalized with advanced liver disease. Clin Gastroenterol Hepatol. 2011;9:254–259.
- , , , et al. Hospital Readmissions among patients with decompensated cirrhosis. Am J Gastroenterol. 2012;107:247–252.
- . Patient‐reported outcomes of cirrhosis. Clin Gastroenterol Hepatol. 2013;11:1043–1045.
© 2014 Society of Hospital Medicine