User login
Safety Assessment of a Noninvasive Respiratory Protocol for Adults With COVID-19
Hypoxemic respiratory failure is a hallmark of severe coronavirus disease 2019 (COVID-19). Initial guidelines favored early mechanical ventilation (MV) over traditional noninvasive strategies, such as high-flow nasal cannula (HFNC) and noninvasive positive pressure ventilation (NIV), based on perceived ineffectiveness and dangers extrapolated from severe acute respiratory syndrome coronavirus (SARS-CoV) and Middle East respiratory syndrome coronavirus (MERS-CoV) patients.1,2 As COVID-19 progressed, early MV became associated with prolonged ventilator courses and high mortality.3-6 Simultaneously, data emerged that HFNC/NIV and self-proning, could successfully stabilize some COVID-19 patients.7-10 Based on evolving evidence, we implemented a noninvasive COVID-19 respiratory protocol (NCRP) that promoted the early use of HFNC, NIV, and self-proning for hypoxemia in patients with COVID-19, with the intention of avoiding MV in some patients. The protocol was implemented throughout our hospital system, from the Emergency Departments (EDs) to the medical floors and critical care units.
Although preliminary evidence supported the use of HFNC, NIV, and self-proning, the impact of a system-wide noninvasive COVID-19 respiratory protocol on safety has not been well described. The objective of this study was to evaluate patient safety outcomes after implementation of the NCRP, including intubation rate and mortality.
METHODS
Study Design and Setting
We performed a retrospective chart review, adhering to SQUIRE (Standards for Quality Improvement Reporting Excellence) Guidelines, to assess safety outcomes after implementation of the NCRP.11 Baystate Health is a not-for-profit, integrated healthcare system in western Massachusetts composed of four hospitals and one free-standing ED with 980 beds serving over 800,000 people. The Baystate Health IRB determined that this project did not meet criteria for Human Subjects Research.
Selection of Participants
A consecutive sample of adults (≥18 years old) admitted to the hospital with a positive nucleic acid test for SARS-CoV-2 (reverse transcriptase–polymerase chain reaction [RT-PCR]) test via nasopharyngeal swab (Cepheid or Roche Cobas 6800) between March 15, 2020, and April 15, 2020, were included. Participants were identified by either an order for the COVID-19 test with a positive result or a discharge diagnosis of COVID-19. Daily rapid response team (RRT), intensive care unit (ICU), and COVID-19 unit logs were reviewed to ensure all COVID-19 patients were included. Patients with positive tests admitted for reasons unrelated to COVID-19 infections, such as patients in labor, were excluded.
Interventions
At the start of the COVID-19 pandemic, the Baystate Health system adopted a conservative approach to the respiratory management of patients with COVID-19. This approach started with nasal cannula up to 6 L/min or nonrebreather up to 15 L/min. If the patient remained in respiratory distress, intubation was recommended.
Based on emerging evidence, the NCRP was created. The details of the NCRP implementation have been previously described.12 Briefly, over a 4-day period (April 3, 2020, to April 7, 2020), a multidisciplinary team developed, refined, and rapidly implemented a COVID-19 respiratory protocol that encouraged the early use of HFNC, NIV, and self-proning in clinically appropriate patients with hypoxemia and respiratory distress due to COVID-19 prior to intubation across all departments of the Baystate Health system (Appendix 1).
Measurements
A chart review was performed using a structured data collection form (Appendix 2). The data collection form was piloted by three physician-researchers. Data abstraction was performed by 16 clinicians. Abstractors were practicing emergency providers and hospitalists and were blinded to the study outcomes. Abstractors received a 1-hour training and abstracted data from at least five charts in parallel with investigators. An additional 10% of charts were double abstracted to calculate interrater reliability for five variables determined a priori.
To validate the capture of outcomes of interest, we triangulated data sources by cross-referencing the monthly RRT log, the ICU list, all orders for HFNC, and RRT activations. Data abstraction occurred from April 21, 2020, to April 30, 2020. Patients who were still hospitalized after April 30,2020, were followed until hospital discharge, ending July 1, 2020.
Outcomes and Analysis
The primary outcome was mortality, defined as the proportion of deaths by admissions during the post–NCRP implementation period (April 3, 2020, to April 15, 2020), compared with the preimplementation period (March 15, 2020, to April 2, 2020). Deaths were stratified by patient code status (do not resuscitate/do not intubate [DNR/DNI] established prior to admission vs Full Code or presumed Full Code). Mortality outcomes were evaluated using one-sided Fisher exact tests.
To assess whether the protocol led to an increase in the use of the interventions and a decrease in intubations, we compared the use of proning, HFNC, NIV, and intubation before the protocol was implemented and with use after. Intubation rates were analyzed using interrupted time series (piecemeal regression), without adjustments, using a cut point of April 2, 2020.
Secondary outcomes included unexpected cardiac arrests, ICU transfers and consultations, and RRT activations during the postimplementation period, compared with the preimplementation period. Secondary outcomes were evaluated using standard chi-square tests (χ2). Additional descriptive outcomes included use of the NCRP, overall and by components, and in-hospital rates of MV.
RESULTS
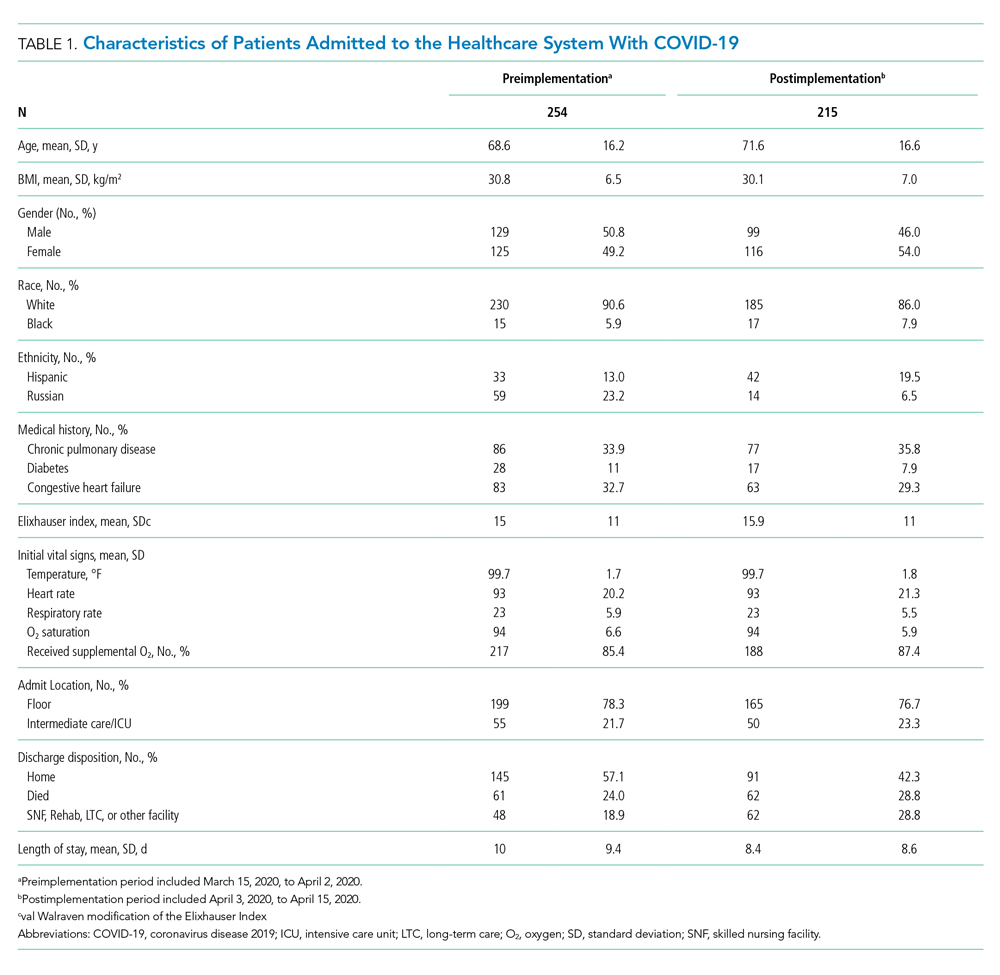
From March 15, 2020, through April 15, 2020, there were 469 patients with COVID-19 admitted to the four hospitals of the Baystate Health system. Patients had an average age of 70 years (SD, 16.4), 241 (52%) were female, and 336 (72%) spoke English as their primary language. Most patients, 405 (86.4%), required supplemental oxygen upon being admitted to the hospital (Table 1).
Postimplementation Mortality
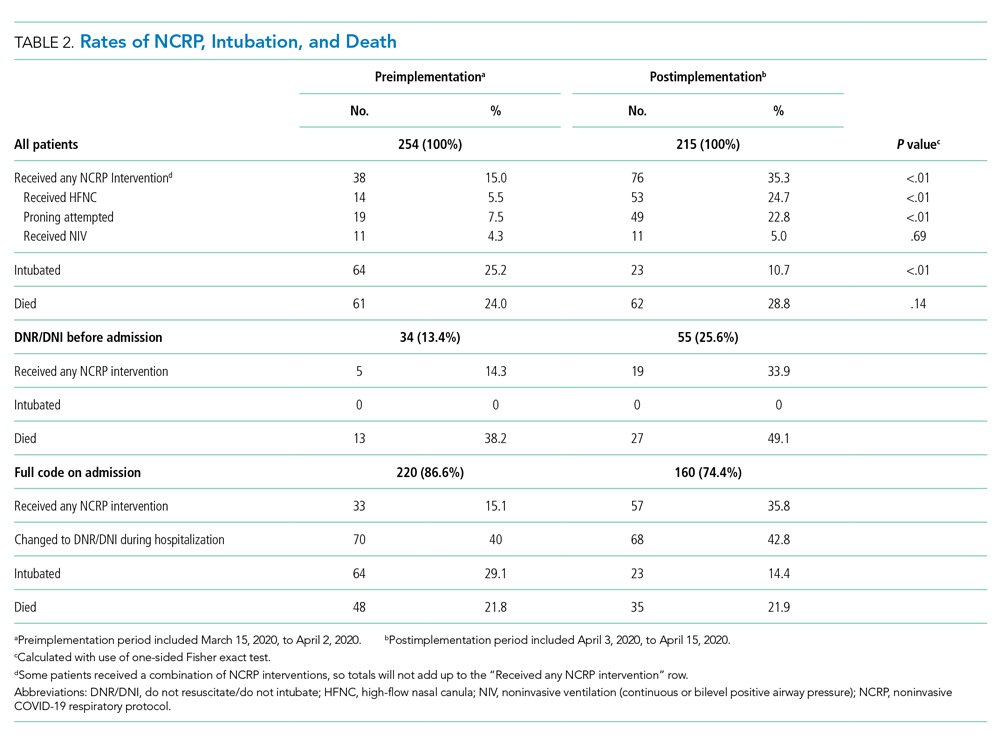
Overall, 123 (26.2%) patients died during the study period. In the preimplementation cohort, 24% (61 of 254) of patients died, compared with 28.8% (62 of 215) in the postimplementation cohort (one-sided Fisher exact, P = .14). Excluding patients with an established DNR/DNI prior to admission, 21.8% (48 of 220) patients died in the preimplementation period vs 21.9% (35 of 160) patients after implementation of the NCRP (Table 2).
Secondary Safety Outcomes
There was no increase in RRT activations (preimplementation, 16.5% [42 of 254], vs postimplementation, 11.6% [25 of 215]; χ2P = 0.17) or ICU consultations (preimplementation, 18.1% [47 of 254], vs postimplementation, 16.3% [35 of 215]; χ2P = 0.52). ICU transfers decreased in the postimplementation period (preimplementation, 26.8% [68 of 254], vs postimplementation, 13.5% [29 of 215], χ2P < .001). There was one unexpected cardiac arrest documented in the postimplementation period, compared to none before implementation.
NCRP Protocol Implementation
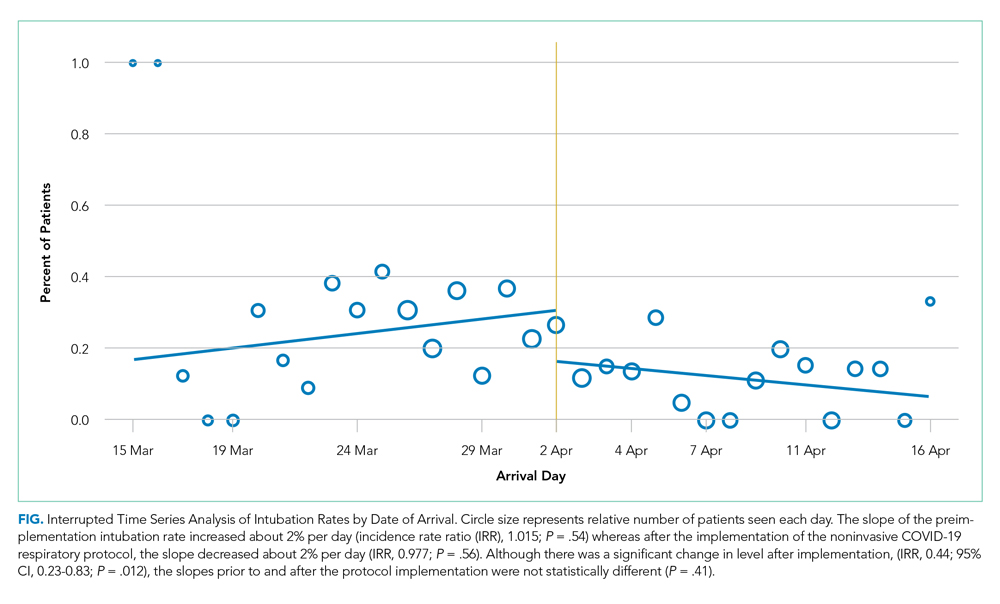
After implementation, the proportion of patients using HFNC increased from 5.5% (14 of 254) to 24.7% (53 of 215), and self-proning increased from 7.5% (19 of 254) to 22.8% (49 of 215). The proportion of patients who were intubated (MV) decreased from 25.2% (64 of 254) to 10.7% (23 of 215) (χ2P < .01). Interrupted time series analysis demonstrated an immediate reduction in the proportion of patients intubated after the intervention (incident rate ratio, 0.44; 95% CI, 0.23-0.83; P = .012) (Figure). The median time from admission to MV was longer in the postimplementation period patients (postimplementation, 1.4 days; interquartile range, 0.21-2.9; vs preimplementation, 0.66 days; IQR 0.23-1.69).
Interrater Reliability
Interrater reliability for variables chosen a priori was k = 1.0 for self-proning, k = 1.0 for intubation, k = 0.95 for discharge disposition, k = 0.94 for nasal cannula, and k = 0.74 for HFNC.
DISCUSSION
The rapid spread of SARS-CoV-2 led to early recommendations based on minimal data. As evidence emerged, hospitals were forced to adapt to protect patients and medical providers. As a healthcare system, we incorporated emerging evidence to rapidly implement a noninvasive respiratory treatment protocol. Aware of the methodological problems in evaluating the NCRP itself, we integrated best practices of quality improvement to examine multiple patient safety outcomes after NCRP implementation. We found the rate of intubation decreased with no significant increase in mortality, ICU transfers, RRT activations, or unexpected deaths after the implementation of the NCRP.
Although we were unable to measure all confounders and changes that co-occurred during the study period, initial vital signs, age, BMI, past medical history, and use of oxygen were similar between the pre- and postimplementation cohorts. Further, there were many constants worth noting. First, COVID-19 respiratory protocols were highly regulated to ensure patient safety and minimize COVID-19 transmission. Second, there were no new nonrespiratory treatments or medications during the study. Third, although the COVID-19 hospital census rose during the study, it never overwhelmed resources; there was no rationing of clinical care.
The nonsignificant increase in mortality in the postimplementation period was limited to patients with an established DNR/DNI prior to admission. Established DNR/DNI patients were largely from skilled nursing facilities that were disproportionally impacted in the postimplementation period through clustered outbreaks of COVID-19 in our region, which likely contributed to the increased mortality.13
Additionally, despite decreased MV rates in the postimplementation period, we did not find a concurrent decrease in mortality. We do not believe this is a failure of noninvasive treatments. Rather, the increased proportion of DNR/DNI patients, combined with increased nursing home outbreaks in the postimplementation period likely influenced mortality. The postimplementation decreases in ICU transfers and RRT activations supports this hypothesis.
Finally, it is worth nothing that, although the goal of decreasing intubations was to improve patient care and decrease mortality, a decrease in intubations alone, without a change in mortality, may be important because mechanical ventilation has been associated with increased morbidity, such as posttraumatic stress disorder.14
Taken together, the post–NCRP implementation period appears to have been safe for patients, compared to the preimplementation period’s protocol. Future research may help understand the impact of specific noninvasive interventions on COVID-19–related MV and mortality.
Limitations
Given the urgency of COVID-19 treatment, the NCRP was designed as a quality improvement initiative rather than a prospective trial. Issues of selection bias and confounding limit our ability to evaluate the effect of the NCRP itself. Additionally, unmeasured patient and provider factors may have influenced outcomes. For example, increased provider knowledge and experience treating COVID-19 may have improved outcomes over time, and unmeasured patient characteristics may have been different in the pre- and postimplementation groups. Finally, our study was limited to a single healthcare system, which may limit generalizability
That said, the objective of our study was to evaluate patient safety outcomes of the NCRP, an important first step while other hospital systems continue to confront increasing rates of COVID-19 and must decide on appropriate respiratory management. To that end, our enrollment captured 469 COVID-19 admissions across four diverse hospitals without obvious differences in initial measured covariates. Further, the strict protocolization of respiratory treatments, the evaluation of multiple safety outcomes, and the complete patient follow-up all support the conclusion that NCRP in the postimplementation period did not increase adverse patient outcomes. Further studies are needed to determine the efficacy of the NCRP protocol itself.
CONCLUSION
In our health system, patients with COVID-19 did not experience a significant increase in mortality, RRT activations, or ICU admissions despite decreased rates of MV after implementation of a respiratory protocol that encouraged early noninvasive management of COVID-19 respiratory distress.
ACKNOWLEDGEMENTS
The authors would like to acknowledge Elizabeth Coray, Joseph Lahey, Richard Gabor, Cheryl Greenstein, Sarah Badach, Marie Boutin, Adrienne Wurl, Anthony Kitchen, Michelle Holton, Matthew Shapiro, Eleanor Ragone, Nageshwar Jonnalagadda, Ryan Flynn, Raghuveer Rakasi, and Jasmine Paadam.
1. Brown CA 3rd, Mosier JM, Carlson JN, Gibbs MA. Pragmatic recommendations for intubating critically ill patients with suspected COVID-19. J Am Coll Emerg Physicians Open. 2020;1(2):80-84. https://doi.org/10.1002/emp2.12063
2. Arabi YM, Arifi AA, Balkhy HH, et al. Clinical course and outcomes of critically ill patients with middle east respiratory syndrome coronavirus infection. Ann Intern Med. 2014;160(6):389-397. https://doi.org/10.7326/m13-2486
3. Ziehr DR, Alladina J, Petri CR, et al. Respiratory pathophysiology of mechanically ventilated patients with COVID-19: a cohort study. Am J Respir Crit Care Med. 2020;201(12):1560-1564. https://doi.org/10.1164/rccm.202004-1163le
4. Richardson S, Hirsch JS, Narasimhan M, et al. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City area. JAMA. 2020;323(20):2052-2059. https://doi.org/10.1001/jama.2020.6775
5. Cummings MJ, Baldwin MR, Abrams D, et al. Epidemiology, clinical course, and outcomes of critically ill adults with COVID-19 in New York City: a prospective cohort study. Lancet. 2020;395(10239):1763-1770. https://doi.org/10.1016/s0140-6736(20)31189-2
6. Farfel JM, Franca SA, Sitta Mdo C, Filho WJ, Carvalho CR. Age, invasive ventilatory support and outcomes in elderly patients admitted to intensive care units. Age Ageing. 2009;38(5):515-520. https://doi.org/10.1093/ageing/afp119
7. Caputo ND, Strayer RJ, Levitan R. Early self-proning in awake, non-intubated patients in the emergency department: a single ED’s experience during the COVID-19 pandemic. Acad Emerg Med. 2020;27(5):375-378. https://doi.org/10.1111/acem.13994
8. Sun Q, Qiu H, Huang M, Yang Y. Lower mortality of COVID-19 by early recognition and intervention: experience from Jiangsu Province. Ann Intensive Care. 2020;10(1):33. https://doi.org/10.1186/s13613-020-00650-2
9. Wang K, Zhao W, Li J, Shu W, Duan J. The experience of high-flow nasal cannula in hospitalized patients with 2019 novel coronavirus-infected pneumonia in two hospitals of Chongqing, China. Ann Intensive Care. 2020;10(1):37. https://doi.org/10.1186/s13613-020-00653-z
10. Alhazzani W, Møller MH, Arabi YM, et al. Surviving Sepsis Campaign: guidelines on the management of critically ill adults with coronavirus disease 2019 (COVID-19). Intensive Care Med. 2020;46(5):854-887 https://doi.org/10.1007/s00134-020-06022-5
11. Ogrinc G, Davies L, Goodman D, Batalden P, Davidoff F, Stevens D. SQUIRE 2.0 (standards for quality improvement reporting excellence): revised publication guidelines from a detailed consensus process. BMJ Qual Saf. 2016;25(12):986-992. https://doi.org/10.1136/bmjqs-2015-004411
12. Westafer LM, Elia T, Medarametla V, Lagu T. A transdiciplinary COVID-19 early respiratory intervention protocol: an implementation story. J Hosp Med. 2020;15(6):372-374. https://doi.org/10.12788/jhm.3456
13. COVID-19 Response Reporting. Mass.gov. Accessed July 20, 2020. https://www.mass.gov/info-details/covid-19-response-reporting#covid-19-daily-dashboard-
14. Shaw RJ, Harvey JE, Bernard R, Gunary R, Tiley M, Steiner H. Comparison of short-term psychological outcomes of respiratory failure treated by either invasive or non-invasive ventilation. Psychosomatics. 2009;50(6):586-591. https://doi.org/10.1176/appi.psy.50.6.586
Hypoxemic respiratory failure is a hallmark of severe coronavirus disease 2019 (COVID-19). Initial guidelines favored early mechanical ventilation (MV) over traditional noninvasive strategies, such as high-flow nasal cannula (HFNC) and noninvasive positive pressure ventilation (NIV), based on perceived ineffectiveness and dangers extrapolated from severe acute respiratory syndrome coronavirus (SARS-CoV) and Middle East respiratory syndrome coronavirus (MERS-CoV) patients.1,2 As COVID-19 progressed, early MV became associated with prolonged ventilator courses and high mortality.3-6 Simultaneously, data emerged that HFNC/NIV and self-proning, could successfully stabilize some COVID-19 patients.7-10 Based on evolving evidence, we implemented a noninvasive COVID-19 respiratory protocol (NCRP) that promoted the early use of HFNC, NIV, and self-proning for hypoxemia in patients with COVID-19, with the intention of avoiding MV in some patients. The protocol was implemented throughout our hospital system, from the Emergency Departments (EDs) to the medical floors and critical care units.
Although preliminary evidence supported the use of HFNC, NIV, and self-proning, the impact of a system-wide noninvasive COVID-19 respiratory protocol on safety has not been well described. The objective of this study was to evaluate patient safety outcomes after implementation of the NCRP, including intubation rate and mortality.
METHODS
Study Design and Setting
We performed a retrospective chart review, adhering to SQUIRE (Standards for Quality Improvement Reporting Excellence) Guidelines, to assess safety outcomes after implementation of the NCRP.11 Baystate Health is a not-for-profit, integrated healthcare system in western Massachusetts composed of four hospitals and one free-standing ED with 980 beds serving over 800,000 people. The Baystate Health IRB determined that this project did not meet criteria for Human Subjects Research.
Selection of Participants
A consecutive sample of adults (≥18 years old) admitted to the hospital with a positive nucleic acid test for SARS-CoV-2 (reverse transcriptase–polymerase chain reaction [RT-PCR]) test via nasopharyngeal swab (Cepheid or Roche Cobas 6800) between March 15, 2020, and April 15, 2020, were included. Participants were identified by either an order for the COVID-19 test with a positive result or a discharge diagnosis of COVID-19. Daily rapid response team (RRT), intensive care unit (ICU), and COVID-19 unit logs were reviewed to ensure all COVID-19 patients were included. Patients with positive tests admitted for reasons unrelated to COVID-19 infections, such as patients in labor, were excluded.
Interventions
At the start of the COVID-19 pandemic, the Baystate Health system adopted a conservative approach to the respiratory management of patients with COVID-19. This approach started with nasal cannula up to 6 L/min or nonrebreather up to 15 L/min. If the patient remained in respiratory distress, intubation was recommended.
Based on emerging evidence, the NCRP was created. The details of the NCRP implementation have been previously described.12 Briefly, over a 4-day period (April 3, 2020, to April 7, 2020), a multidisciplinary team developed, refined, and rapidly implemented a COVID-19 respiratory protocol that encouraged the early use of HFNC, NIV, and self-proning in clinically appropriate patients with hypoxemia and respiratory distress due to COVID-19 prior to intubation across all departments of the Baystate Health system (Appendix 1).
Measurements
A chart review was performed using a structured data collection form (Appendix 2). The data collection form was piloted by three physician-researchers. Data abstraction was performed by 16 clinicians. Abstractors were practicing emergency providers and hospitalists and were blinded to the study outcomes. Abstractors received a 1-hour training and abstracted data from at least five charts in parallel with investigators. An additional 10% of charts were double abstracted to calculate interrater reliability for five variables determined a priori.
To validate the capture of outcomes of interest, we triangulated data sources by cross-referencing the monthly RRT log, the ICU list, all orders for HFNC, and RRT activations. Data abstraction occurred from April 21, 2020, to April 30, 2020. Patients who were still hospitalized after April 30,2020, were followed until hospital discharge, ending July 1, 2020.
Outcomes and Analysis
The primary outcome was mortality, defined as the proportion of deaths by admissions during the post–NCRP implementation period (April 3, 2020, to April 15, 2020), compared with the preimplementation period (March 15, 2020, to April 2, 2020). Deaths were stratified by patient code status (do not resuscitate/do not intubate [DNR/DNI] established prior to admission vs Full Code or presumed Full Code). Mortality outcomes were evaluated using one-sided Fisher exact tests.
To assess whether the protocol led to an increase in the use of the interventions and a decrease in intubations, we compared the use of proning, HFNC, NIV, and intubation before the protocol was implemented and with use after. Intubation rates were analyzed using interrupted time series (piecemeal regression), without adjustments, using a cut point of April 2, 2020.
Secondary outcomes included unexpected cardiac arrests, ICU transfers and consultations, and RRT activations during the postimplementation period, compared with the preimplementation period. Secondary outcomes were evaluated using standard chi-square tests (χ2). Additional descriptive outcomes included use of the NCRP, overall and by components, and in-hospital rates of MV.
RESULTS
From March 15, 2020, through April 15, 2020, there were 469 patients with COVID-19 admitted to the four hospitals of the Baystate Health system. Patients had an average age of 70 years (SD, 16.4), 241 (52%) were female, and 336 (72%) spoke English as their primary language. Most patients, 405 (86.4%), required supplemental oxygen upon being admitted to the hospital (Table 1).

Postimplementation Mortality
Overall, 123 (26.2%) patients died during the study period. In the preimplementation cohort, 24% (61 of 254) of patients died, compared with 28.8% (62 of 215) in the postimplementation cohort (one-sided Fisher exact, P = .14). Excluding patients with an established DNR/DNI prior to admission, 21.8% (48 of 220) patients died in the preimplementation period vs 21.9% (35 of 160) patients after implementation of the NCRP (Table 2).

Secondary Safety Outcomes
There was no increase in RRT activations (preimplementation, 16.5% [42 of 254], vs postimplementation, 11.6% [25 of 215]; χ2P = 0.17) or ICU consultations (preimplementation, 18.1% [47 of 254], vs postimplementation, 16.3% [35 of 215]; χ2P = 0.52). ICU transfers decreased in the postimplementation period (preimplementation, 26.8% [68 of 254], vs postimplementation, 13.5% [29 of 215], χ2P < .001). There was one unexpected cardiac arrest documented in the postimplementation period, compared to none before implementation.
NCRP Protocol Implementation
After implementation, the proportion of patients using HFNC increased from 5.5% (14 of 254) to 24.7% (53 of 215), and self-proning increased from 7.5% (19 of 254) to 22.8% (49 of 215). The proportion of patients who were intubated (MV) decreased from 25.2% (64 of 254) to 10.7% (23 of 215) (χ2P < .01). Interrupted time series analysis demonstrated an immediate reduction in the proportion of patients intubated after the intervention (incident rate ratio, 0.44; 95% CI, 0.23-0.83; P = .012) (Figure). The median time from admission to MV was longer in the postimplementation period patients (postimplementation, 1.4 days; interquartile range, 0.21-2.9; vs preimplementation, 0.66 days; IQR 0.23-1.69).

Interrater Reliability
Interrater reliability for variables chosen a priori was k = 1.0 for self-proning, k = 1.0 for intubation, k = 0.95 for discharge disposition, k = 0.94 for nasal cannula, and k = 0.74 for HFNC.
DISCUSSION
The rapid spread of SARS-CoV-2 led to early recommendations based on minimal data. As evidence emerged, hospitals were forced to adapt to protect patients and medical providers. As a healthcare system, we incorporated emerging evidence to rapidly implement a noninvasive respiratory treatment protocol. Aware of the methodological problems in evaluating the NCRP itself, we integrated best practices of quality improvement to examine multiple patient safety outcomes after NCRP implementation. We found the rate of intubation decreased with no significant increase in mortality, ICU transfers, RRT activations, or unexpected deaths after the implementation of the NCRP.
Although we were unable to measure all confounders and changes that co-occurred during the study period, initial vital signs, age, BMI, past medical history, and use of oxygen were similar between the pre- and postimplementation cohorts. Further, there were many constants worth noting. First, COVID-19 respiratory protocols were highly regulated to ensure patient safety and minimize COVID-19 transmission. Second, there were no new nonrespiratory treatments or medications during the study. Third, although the COVID-19 hospital census rose during the study, it never overwhelmed resources; there was no rationing of clinical care.
The nonsignificant increase in mortality in the postimplementation period was limited to patients with an established DNR/DNI prior to admission. Established DNR/DNI patients were largely from skilled nursing facilities that were disproportionally impacted in the postimplementation period through clustered outbreaks of COVID-19 in our region, which likely contributed to the increased mortality.13
Additionally, despite decreased MV rates in the postimplementation period, we did not find a concurrent decrease in mortality. We do not believe this is a failure of noninvasive treatments. Rather, the increased proportion of DNR/DNI patients, combined with increased nursing home outbreaks in the postimplementation period likely influenced mortality. The postimplementation decreases in ICU transfers and RRT activations supports this hypothesis.
Finally, it is worth nothing that, although the goal of decreasing intubations was to improve patient care and decrease mortality, a decrease in intubations alone, without a change in mortality, may be important because mechanical ventilation has been associated with increased morbidity, such as posttraumatic stress disorder.14
Taken together, the post–NCRP implementation period appears to have been safe for patients, compared to the preimplementation period’s protocol. Future research may help understand the impact of specific noninvasive interventions on COVID-19–related MV and mortality.
Limitations
Given the urgency of COVID-19 treatment, the NCRP was designed as a quality improvement initiative rather than a prospective trial. Issues of selection bias and confounding limit our ability to evaluate the effect of the NCRP itself. Additionally, unmeasured patient and provider factors may have influenced outcomes. For example, increased provider knowledge and experience treating COVID-19 may have improved outcomes over time, and unmeasured patient characteristics may have been different in the pre- and postimplementation groups. Finally, our study was limited to a single healthcare system, which may limit generalizability
That said, the objective of our study was to evaluate patient safety outcomes of the NCRP, an important first step while other hospital systems continue to confront increasing rates of COVID-19 and must decide on appropriate respiratory management. To that end, our enrollment captured 469 COVID-19 admissions across four diverse hospitals without obvious differences in initial measured covariates. Further, the strict protocolization of respiratory treatments, the evaluation of multiple safety outcomes, and the complete patient follow-up all support the conclusion that NCRP in the postimplementation period did not increase adverse patient outcomes. Further studies are needed to determine the efficacy of the NCRP protocol itself.
CONCLUSION
In our health system, patients with COVID-19 did not experience a significant increase in mortality, RRT activations, or ICU admissions despite decreased rates of MV after implementation of a respiratory protocol that encouraged early noninvasive management of COVID-19 respiratory distress.
ACKNOWLEDGEMENTS
The authors would like to acknowledge Elizabeth Coray, Joseph Lahey, Richard Gabor, Cheryl Greenstein, Sarah Badach, Marie Boutin, Adrienne Wurl, Anthony Kitchen, Michelle Holton, Matthew Shapiro, Eleanor Ragone, Nageshwar Jonnalagadda, Ryan Flynn, Raghuveer Rakasi, and Jasmine Paadam.
Hypoxemic respiratory failure is a hallmark of severe coronavirus disease 2019 (COVID-19). Initial guidelines favored early mechanical ventilation (MV) over traditional noninvasive strategies, such as high-flow nasal cannula (HFNC) and noninvasive positive pressure ventilation (NIV), based on perceived ineffectiveness and dangers extrapolated from severe acute respiratory syndrome coronavirus (SARS-CoV) and Middle East respiratory syndrome coronavirus (MERS-CoV) patients.1,2 As COVID-19 progressed, early MV became associated with prolonged ventilator courses and high mortality.3-6 Simultaneously, data emerged that HFNC/NIV and self-proning, could successfully stabilize some COVID-19 patients.7-10 Based on evolving evidence, we implemented a noninvasive COVID-19 respiratory protocol (NCRP) that promoted the early use of HFNC, NIV, and self-proning for hypoxemia in patients with COVID-19, with the intention of avoiding MV in some patients. The protocol was implemented throughout our hospital system, from the Emergency Departments (EDs) to the medical floors and critical care units.
Although preliminary evidence supported the use of HFNC, NIV, and self-proning, the impact of a system-wide noninvasive COVID-19 respiratory protocol on safety has not been well described. The objective of this study was to evaluate patient safety outcomes after implementation of the NCRP, including intubation rate and mortality.
METHODS
Study Design and Setting
We performed a retrospective chart review, adhering to SQUIRE (Standards for Quality Improvement Reporting Excellence) Guidelines, to assess safety outcomes after implementation of the NCRP.11 Baystate Health is a not-for-profit, integrated healthcare system in western Massachusetts composed of four hospitals and one free-standing ED with 980 beds serving over 800,000 people. The Baystate Health IRB determined that this project did not meet criteria for Human Subjects Research.
Selection of Participants
A consecutive sample of adults (≥18 years old) admitted to the hospital with a positive nucleic acid test for SARS-CoV-2 (reverse transcriptase–polymerase chain reaction [RT-PCR]) test via nasopharyngeal swab (Cepheid or Roche Cobas 6800) between March 15, 2020, and April 15, 2020, were included. Participants were identified by either an order for the COVID-19 test with a positive result or a discharge diagnosis of COVID-19. Daily rapid response team (RRT), intensive care unit (ICU), and COVID-19 unit logs were reviewed to ensure all COVID-19 patients were included. Patients with positive tests admitted for reasons unrelated to COVID-19 infections, such as patients in labor, were excluded.
Interventions
At the start of the COVID-19 pandemic, the Baystate Health system adopted a conservative approach to the respiratory management of patients with COVID-19. This approach started with nasal cannula up to 6 L/min or nonrebreather up to 15 L/min. If the patient remained in respiratory distress, intubation was recommended.
Based on emerging evidence, the NCRP was created. The details of the NCRP implementation have been previously described.12 Briefly, over a 4-day period (April 3, 2020, to April 7, 2020), a multidisciplinary team developed, refined, and rapidly implemented a COVID-19 respiratory protocol that encouraged the early use of HFNC, NIV, and self-proning in clinically appropriate patients with hypoxemia and respiratory distress due to COVID-19 prior to intubation across all departments of the Baystate Health system (Appendix 1).
Measurements
A chart review was performed using a structured data collection form (Appendix 2). The data collection form was piloted by three physician-researchers. Data abstraction was performed by 16 clinicians. Abstractors were practicing emergency providers and hospitalists and were blinded to the study outcomes. Abstractors received a 1-hour training and abstracted data from at least five charts in parallel with investigators. An additional 10% of charts were double abstracted to calculate interrater reliability for five variables determined a priori.
To validate the capture of outcomes of interest, we triangulated data sources by cross-referencing the monthly RRT log, the ICU list, all orders for HFNC, and RRT activations. Data abstraction occurred from April 21, 2020, to April 30, 2020. Patients who were still hospitalized after April 30,2020, were followed until hospital discharge, ending July 1, 2020.
Outcomes and Analysis
The primary outcome was mortality, defined as the proportion of deaths by admissions during the post–NCRP implementation period (April 3, 2020, to April 15, 2020), compared with the preimplementation period (March 15, 2020, to April 2, 2020). Deaths were stratified by patient code status (do not resuscitate/do not intubate [DNR/DNI] established prior to admission vs Full Code or presumed Full Code). Mortality outcomes were evaluated using one-sided Fisher exact tests.
To assess whether the protocol led to an increase in the use of the interventions and a decrease in intubations, we compared the use of proning, HFNC, NIV, and intubation before the protocol was implemented and with use after. Intubation rates were analyzed using interrupted time series (piecemeal regression), without adjustments, using a cut point of April 2, 2020.
Secondary outcomes included unexpected cardiac arrests, ICU transfers and consultations, and RRT activations during the postimplementation period, compared with the preimplementation period. Secondary outcomes were evaluated using standard chi-square tests (χ2). Additional descriptive outcomes included use of the NCRP, overall and by components, and in-hospital rates of MV.
RESULTS
From March 15, 2020, through April 15, 2020, there were 469 patients with COVID-19 admitted to the four hospitals of the Baystate Health system. Patients had an average age of 70 years (SD, 16.4), 241 (52%) were female, and 336 (72%) spoke English as their primary language. Most patients, 405 (86.4%), required supplemental oxygen upon being admitted to the hospital (Table 1).

Postimplementation Mortality
Overall, 123 (26.2%) patients died during the study period. In the preimplementation cohort, 24% (61 of 254) of patients died, compared with 28.8% (62 of 215) in the postimplementation cohort (one-sided Fisher exact, P = .14). Excluding patients with an established DNR/DNI prior to admission, 21.8% (48 of 220) patients died in the preimplementation period vs 21.9% (35 of 160) patients after implementation of the NCRP (Table 2).

Secondary Safety Outcomes
There was no increase in RRT activations (preimplementation, 16.5% [42 of 254], vs postimplementation, 11.6% [25 of 215]; χ2P = 0.17) or ICU consultations (preimplementation, 18.1% [47 of 254], vs postimplementation, 16.3% [35 of 215]; χ2P = 0.52). ICU transfers decreased in the postimplementation period (preimplementation, 26.8% [68 of 254], vs postimplementation, 13.5% [29 of 215], χ2P < .001). There was one unexpected cardiac arrest documented in the postimplementation period, compared to none before implementation.
NCRP Protocol Implementation
After implementation, the proportion of patients using HFNC increased from 5.5% (14 of 254) to 24.7% (53 of 215), and self-proning increased from 7.5% (19 of 254) to 22.8% (49 of 215). The proportion of patients who were intubated (MV) decreased from 25.2% (64 of 254) to 10.7% (23 of 215) (χ2P < .01). Interrupted time series analysis demonstrated an immediate reduction in the proportion of patients intubated after the intervention (incident rate ratio, 0.44; 95% CI, 0.23-0.83; P = .012) (Figure). The median time from admission to MV was longer in the postimplementation period patients (postimplementation, 1.4 days; interquartile range, 0.21-2.9; vs preimplementation, 0.66 days; IQR 0.23-1.69).

Interrater Reliability
Interrater reliability for variables chosen a priori was k = 1.0 for self-proning, k = 1.0 for intubation, k = 0.95 for discharge disposition, k = 0.94 for nasal cannula, and k = 0.74 for HFNC.
DISCUSSION
The rapid spread of SARS-CoV-2 led to early recommendations based on minimal data. As evidence emerged, hospitals were forced to adapt to protect patients and medical providers. As a healthcare system, we incorporated emerging evidence to rapidly implement a noninvasive respiratory treatment protocol. Aware of the methodological problems in evaluating the NCRP itself, we integrated best practices of quality improvement to examine multiple patient safety outcomes after NCRP implementation. We found the rate of intubation decreased with no significant increase in mortality, ICU transfers, RRT activations, or unexpected deaths after the implementation of the NCRP.
Although we were unable to measure all confounders and changes that co-occurred during the study period, initial vital signs, age, BMI, past medical history, and use of oxygen were similar between the pre- and postimplementation cohorts. Further, there were many constants worth noting. First, COVID-19 respiratory protocols were highly regulated to ensure patient safety and minimize COVID-19 transmission. Second, there were no new nonrespiratory treatments or medications during the study. Third, although the COVID-19 hospital census rose during the study, it never overwhelmed resources; there was no rationing of clinical care.
The nonsignificant increase in mortality in the postimplementation period was limited to patients with an established DNR/DNI prior to admission. Established DNR/DNI patients were largely from skilled nursing facilities that were disproportionally impacted in the postimplementation period through clustered outbreaks of COVID-19 in our region, which likely contributed to the increased mortality.13
Additionally, despite decreased MV rates in the postimplementation period, we did not find a concurrent decrease in mortality. We do not believe this is a failure of noninvasive treatments. Rather, the increased proportion of DNR/DNI patients, combined with increased nursing home outbreaks in the postimplementation period likely influenced mortality. The postimplementation decreases in ICU transfers and RRT activations supports this hypothesis.
Finally, it is worth nothing that, although the goal of decreasing intubations was to improve patient care and decrease mortality, a decrease in intubations alone, without a change in mortality, may be important because mechanical ventilation has been associated with increased morbidity, such as posttraumatic stress disorder.14
Taken together, the post–NCRP implementation period appears to have been safe for patients, compared to the preimplementation period’s protocol. Future research may help understand the impact of specific noninvasive interventions on COVID-19–related MV and mortality.
Limitations
Given the urgency of COVID-19 treatment, the NCRP was designed as a quality improvement initiative rather than a prospective trial. Issues of selection bias and confounding limit our ability to evaluate the effect of the NCRP itself. Additionally, unmeasured patient and provider factors may have influenced outcomes. For example, increased provider knowledge and experience treating COVID-19 may have improved outcomes over time, and unmeasured patient characteristics may have been different in the pre- and postimplementation groups. Finally, our study was limited to a single healthcare system, which may limit generalizability
That said, the objective of our study was to evaluate patient safety outcomes of the NCRP, an important first step while other hospital systems continue to confront increasing rates of COVID-19 and must decide on appropriate respiratory management. To that end, our enrollment captured 469 COVID-19 admissions across four diverse hospitals without obvious differences in initial measured covariates. Further, the strict protocolization of respiratory treatments, the evaluation of multiple safety outcomes, and the complete patient follow-up all support the conclusion that NCRP in the postimplementation period did not increase adverse patient outcomes. Further studies are needed to determine the efficacy of the NCRP protocol itself.
CONCLUSION
In our health system, patients with COVID-19 did not experience a significant increase in mortality, RRT activations, or ICU admissions despite decreased rates of MV after implementation of a respiratory protocol that encouraged early noninvasive management of COVID-19 respiratory distress.
ACKNOWLEDGEMENTS
The authors would like to acknowledge Elizabeth Coray, Joseph Lahey, Richard Gabor, Cheryl Greenstein, Sarah Badach, Marie Boutin, Adrienne Wurl, Anthony Kitchen, Michelle Holton, Matthew Shapiro, Eleanor Ragone, Nageshwar Jonnalagadda, Ryan Flynn, Raghuveer Rakasi, and Jasmine Paadam.
1. Brown CA 3rd, Mosier JM, Carlson JN, Gibbs MA. Pragmatic recommendations for intubating critically ill patients with suspected COVID-19. J Am Coll Emerg Physicians Open. 2020;1(2):80-84. https://doi.org/10.1002/emp2.12063
2. Arabi YM, Arifi AA, Balkhy HH, et al. Clinical course and outcomes of critically ill patients with middle east respiratory syndrome coronavirus infection. Ann Intern Med. 2014;160(6):389-397. https://doi.org/10.7326/m13-2486
3. Ziehr DR, Alladina J, Petri CR, et al. Respiratory pathophysiology of mechanically ventilated patients with COVID-19: a cohort study. Am J Respir Crit Care Med. 2020;201(12):1560-1564. https://doi.org/10.1164/rccm.202004-1163le
4. Richardson S, Hirsch JS, Narasimhan M, et al. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City area. JAMA. 2020;323(20):2052-2059. https://doi.org/10.1001/jama.2020.6775
5. Cummings MJ, Baldwin MR, Abrams D, et al. Epidemiology, clinical course, and outcomes of critically ill adults with COVID-19 in New York City: a prospective cohort study. Lancet. 2020;395(10239):1763-1770. https://doi.org/10.1016/s0140-6736(20)31189-2
6. Farfel JM, Franca SA, Sitta Mdo C, Filho WJ, Carvalho CR. Age, invasive ventilatory support and outcomes in elderly patients admitted to intensive care units. Age Ageing. 2009;38(5):515-520. https://doi.org/10.1093/ageing/afp119
7. Caputo ND, Strayer RJ, Levitan R. Early self-proning in awake, non-intubated patients in the emergency department: a single ED’s experience during the COVID-19 pandemic. Acad Emerg Med. 2020;27(5):375-378. https://doi.org/10.1111/acem.13994
8. Sun Q, Qiu H, Huang M, Yang Y. Lower mortality of COVID-19 by early recognition and intervention: experience from Jiangsu Province. Ann Intensive Care. 2020;10(1):33. https://doi.org/10.1186/s13613-020-00650-2
9. Wang K, Zhao W, Li J, Shu W, Duan J. The experience of high-flow nasal cannula in hospitalized patients with 2019 novel coronavirus-infected pneumonia in two hospitals of Chongqing, China. Ann Intensive Care. 2020;10(1):37. https://doi.org/10.1186/s13613-020-00653-z
10. Alhazzani W, Møller MH, Arabi YM, et al. Surviving Sepsis Campaign: guidelines on the management of critically ill adults with coronavirus disease 2019 (COVID-19). Intensive Care Med. 2020;46(5):854-887 https://doi.org/10.1007/s00134-020-06022-5
11. Ogrinc G, Davies L, Goodman D, Batalden P, Davidoff F, Stevens D. SQUIRE 2.0 (standards for quality improvement reporting excellence): revised publication guidelines from a detailed consensus process. BMJ Qual Saf. 2016;25(12):986-992. https://doi.org/10.1136/bmjqs-2015-004411
12. Westafer LM, Elia T, Medarametla V, Lagu T. A transdiciplinary COVID-19 early respiratory intervention protocol: an implementation story. J Hosp Med. 2020;15(6):372-374. https://doi.org/10.12788/jhm.3456
13. COVID-19 Response Reporting. Mass.gov. Accessed July 20, 2020. https://www.mass.gov/info-details/covid-19-response-reporting#covid-19-daily-dashboard-
14. Shaw RJ, Harvey JE, Bernard R, Gunary R, Tiley M, Steiner H. Comparison of short-term psychological outcomes of respiratory failure treated by either invasive or non-invasive ventilation. Psychosomatics. 2009;50(6):586-591. https://doi.org/10.1176/appi.psy.50.6.586
1. Brown CA 3rd, Mosier JM, Carlson JN, Gibbs MA. Pragmatic recommendations for intubating critically ill patients with suspected COVID-19. J Am Coll Emerg Physicians Open. 2020;1(2):80-84. https://doi.org/10.1002/emp2.12063
2. Arabi YM, Arifi AA, Balkhy HH, et al. Clinical course and outcomes of critically ill patients with middle east respiratory syndrome coronavirus infection. Ann Intern Med. 2014;160(6):389-397. https://doi.org/10.7326/m13-2486
3. Ziehr DR, Alladina J, Petri CR, et al. Respiratory pathophysiology of mechanically ventilated patients with COVID-19: a cohort study. Am J Respir Crit Care Med. 2020;201(12):1560-1564. https://doi.org/10.1164/rccm.202004-1163le
4. Richardson S, Hirsch JS, Narasimhan M, et al. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City area. JAMA. 2020;323(20):2052-2059. https://doi.org/10.1001/jama.2020.6775
5. Cummings MJ, Baldwin MR, Abrams D, et al. Epidemiology, clinical course, and outcomes of critically ill adults with COVID-19 in New York City: a prospective cohort study. Lancet. 2020;395(10239):1763-1770. https://doi.org/10.1016/s0140-6736(20)31189-2
6. Farfel JM, Franca SA, Sitta Mdo C, Filho WJ, Carvalho CR. Age, invasive ventilatory support and outcomes in elderly patients admitted to intensive care units. Age Ageing. 2009;38(5):515-520. https://doi.org/10.1093/ageing/afp119
7. Caputo ND, Strayer RJ, Levitan R. Early self-proning in awake, non-intubated patients in the emergency department: a single ED’s experience during the COVID-19 pandemic. Acad Emerg Med. 2020;27(5):375-378. https://doi.org/10.1111/acem.13994
8. Sun Q, Qiu H, Huang M, Yang Y. Lower mortality of COVID-19 by early recognition and intervention: experience from Jiangsu Province. Ann Intensive Care. 2020;10(1):33. https://doi.org/10.1186/s13613-020-00650-2
9. Wang K, Zhao W, Li J, Shu W, Duan J. The experience of high-flow nasal cannula in hospitalized patients with 2019 novel coronavirus-infected pneumonia in two hospitals of Chongqing, China. Ann Intensive Care. 2020;10(1):37. https://doi.org/10.1186/s13613-020-00653-z
10. Alhazzani W, Møller MH, Arabi YM, et al. Surviving Sepsis Campaign: guidelines on the management of critically ill adults with coronavirus disease 2019 (COVID-19). Intensive Care Med. 2020;46(5):854-887 https://doi.org/10.1007/s00134-020-06022-5
11. Ogrinc G, Davies L, Goodman D, Batalden P, Davidoff F, Stevens D. SQUIRE 2.0 (standards for quality improvement reporting excellence): revised publication guidelines from a detailed consensus process. BMJ Qual Saf. 2016;25(12):986-992. https://doi.org/10.1136/bmjqs-2015-004411
12. Westafer LM, Elia T, Medarametla V, Lagu T. A transdiciplinary COVID-19 early respiratory intervention protocol: an implementation story. J Hosp Med. 2020;15(6):372-374. https://doi.org/10.12788/jhm.3456
13. COVID-19 Response Reporting. Mass.gov. Accessed July 20, 2020. https://www.mass.gov/info-details/covid-19-response-reporting#covid-19-daily-dashboard-
14. Shaw RJ, Harvey JE, Bernard R, Gunary R, Tiley M, Steiner H. Comparison of short-term psychological outcomes of respiratory failure treated by either invasive or non-invasive ventilation. Psychosomatics. 2009;50(6):586-591. https://doi.org/10.1176/appi.psy.50.6.586
© 2020 Society of Hospital Medicine
The evolution of “COVIDists”
Adapting to the demands placed on hospital resources by COVID-19
The challenges posed by COVID-19 have crippled health care systems around the globe. By February 2020, the first outbreak in the United States had been set off in Washington State. We quickly became the world’s epicenter of the epidemic, with over 1.8 million patients and over 110,000 deaths.1 The rapidity of spread and the severity of the disease created a tremendous strain on resources. It blindsided policymakers and hospital administrators, which left little time to react to the challenges placed on hospital operations all over the country.
The necessity of a new care model
Although health systems in the United States are adept in managing complications of common seasonal viral respiratory illnesses, COVID-19 presented an entirely different challenge with its significantly higher mortality rate. A respiratory disease turning into a multiorgan disease that causes debilitating cardiac, renal, neurological, hematological, and psychosocial complications2 was not something we had experience managing effectively. Additional challenges included a massive surge of COVID-19 patients, a limited supply of personal protective equipment (PPE), an inadequate number of intensivists for managing the anticipated ventilated patients, and most importantly, the potential of losing some of our workforce if they became infected.
Based on the experiences in China and Italy, and various predictive models, the division of hospital medicine at Baystate Health quickly realized the necessity of a new model of care for COVID-19 patients. We came up with an elaborate plan to manage the disease burden and the strain on resources effectively. The measures we put in place could be broadly divided into three categories following the timeline of the disease: the preparatory phase, the execution phase, and the maintenance phase.
The preparatory phase: From “Hospitalists” to “COVIDists”
As in most hospitals around the country, hospitalists are the backbone of inpatient clinical operations at our health system. A focused group of 10 hospitalists who volunteered to take care of COVID-19 patients with a particular interest in the pandemic and experience in critical care were selected, and the term “COVIDists” was coined to refer to them.
COVIDists were trained in various treatment protocols and ongoing clinical trials. They were given refresher training in Advanced Cardiac Life Support (ACLS) and Fundamental Critical Care Support (FCCS) courses and were taught in critical care/ventilator management by the intensivists through rapid indoctrination in the ICU. All of them had their N-95 mask fitting updated and were trained in the safe donning and doffing of all kinds of PPE by PPE coaches. The palliative care team trained them in conducting end-of-life/code status discussions with a focus on being unable to speak with family members at the bedside. COVIDists were also assigned as Code Blue leaders for any “COVID code blue” in the hospital.
In addition to the rapid training course, COVID-related updates were disseminated daily using three different modalities: brief huddles at the start of the day with the COVIDists; a COVID-19 newsletter summarizing daily updates, new treatments, strategies, and policies; and a WhatsApp group for instantly broadcasting information to the COVIDists (Table 1).
The execution phase
All the hospitalized COVID-19 patients were grouped together to COVID units, and the COVIDists were deployed to those units geographically. COVIDists were given lighter than usual patient loads to deal with the extra time needed for donning and doffing of PPE and for coordination with specialists. COVIDists were almost the only clinicians physically visiting the patients in most cases, and they became the “eyes and ears” of specialists since the specialists were advised to minimize exposure and pursue telemedicine consults. The COVIDists were also undertaking the most challenging part of the care – talking to families about end-of-life issues and the futility of aggressive care in certain patients with preexisting conditions.
Some COVIDists were deployed to the ICU to work alongside the intensivists and became an invaluable resource in ICU management when the ICU census skyrocketed during the initial phase of the outbreak. This helped in tiding the health system over during the initial crisis. Within a short time, we shifted away from an early intubation strategy, and most of the ICU patients were managed in the intermediate care units on high flow oxygen along with the awake-proning protocol. The COVIDists exclusively managed these units. They led multidisciplinary rounds two times a day with the ICU, rapid response team (RRT), the palliative care team, and the nursing team. This step drastically decreased the number of intubations, RRT activations, reduced ICU census,3 and helped with hospital capacity and patient flow (Tables 2 and 3).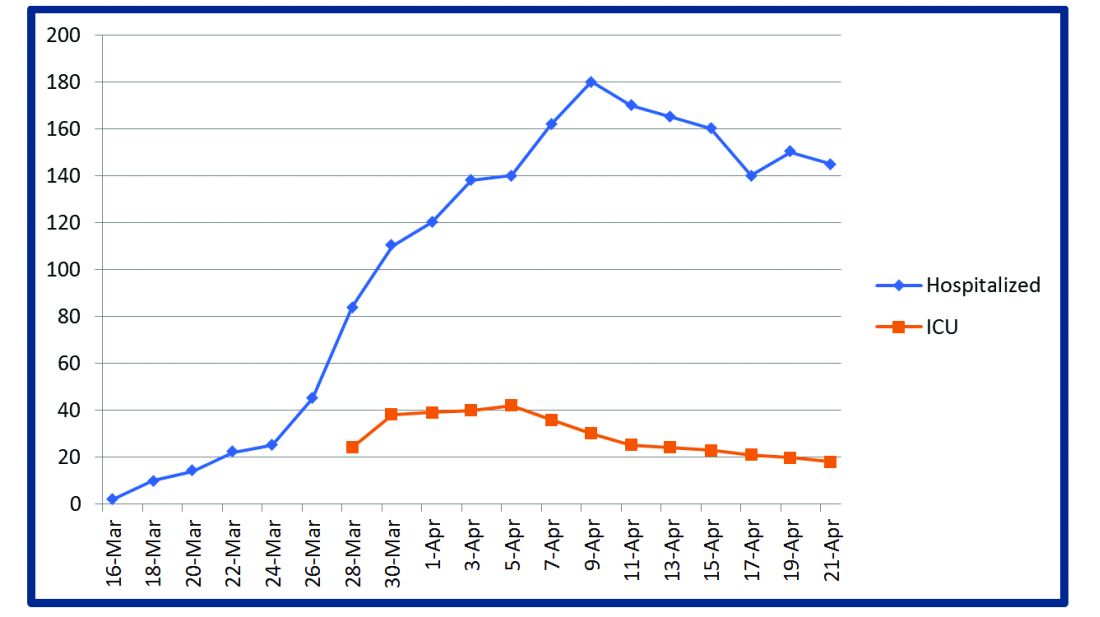
This strategy also helped build solidarity and camaraderie between all these groups, making the COVIDists feel that they were never alone and that the whole hospital supported them. We are currently evaluating clinical outcomes and attempting to identify effects on mortality, length of stay, days on the ventilator, and days in ICU. 
The maintenance phase
It is already 2 months since the first devising COVIDists. There is no difference in sick callouts between COVIDists and non-COVIDists. One COVIDist and one non-COVIDist contracted the disease, but none of them required hospitalization. Although we initially thought that COVIDists would be needed for only a short period of time, the evolution of the disease is showing signs that it might be prolonged over the next several months. Hence, we are planning to continue COVIDist service for at least the next 6 months and reevaluate the need.
Hospital medicine leadership checked on COVIDists daily in regard to their physical health and, more importantly, their mental well-being. They were offered the chance to be taken off the schedule if they felt burned out, but no one wanted to come off their scheduled service before finishing their shifts. BlueCross MA recognized one of the COVIDists, Raghuveer Rakasi, MD, as a “hero on the front line.”4 In Dr. Rakasi’s words, “We took a nosedive into something without knowing its depth, and aware that we could have fatalities among ourselves. We took up new roles, faced new challenges, learned new things every day, evolving every step of the way. We had to change the way we practice medicine, finding new ways to treat patients, and protecting the workforce by limiting patient exposure, prioritizing investigations.” He added that “we have to adapt to a new normal; we should be prepared for this to come in waves. Putting aside our political views, we should stand united 6 feet apart, with a mask covering our brave faces, frequently washing our helping hands to overcome these uncertain times.”
Conclusion
The creation of a focused group of hospitalists called COVIDists and providing them with structured and rapid training (in various aspects of clinical care of COVID-19 patients, critical care/ventilator management, efficient and safe use of PPE) and daily information dissemination allowed our health system to prepare for the large volume of COVID-19 patients. It also helped in preserving the larger hospital workforce for a possible future surge.
The rapid development and implementation of the COVIDist strategy succeeded because of the intrinsic motivation of the providers to improve the outcomes of this high-risk patient population and the close collaboration of the stakeholders. Our institution remains successful in managing the pandemic in Western Massachusetts, with reserve capacity remaining even during the peak of the epidemic. A large part of this was because of creating and training a pool of COVIDists.
Dr. Medarametla is medical director, clinical operations, in the division of hospital medicine at Baystate Health, and assistant professor at University of Massachusetts, Worcester. Readers can contact him at Venkatrao.MedarametlaMD@Baystatehealth.org. Dr. Prabhakaran is unit medical director, geriatrics unit, in the division of hospital medicine at Baystate Health and assistant professor at University of Massachusetts. Dr. Bryson is associate program director of the Internal Medicine Residency at Baystate Health and assistant professor at University of Massachusetts. Dr. Umar is medical director, clinical operations, in the division of hospital medicine at Baystate Health. Dr. Natanasabapathy is division chief of hospital medicine at Baystate Health and assistant professor at University of Massachusetts.
References
1. Centers for Disease Control and Prevention. Coronavirus Disease 2019 (COVID-19). Updated Jun 10, 2020. https://www.cdc.gov/coronavirus/2019-ncov/cases-updates/cases-in-us.html.
2. Zhou F et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: A retrospective cohort study. Lancet. 2020 Mar 28;395(10229):1054-62.
3. Westafer LM et al. A transdisciplinary COVID-19 early respiratory intervention protocol: An implementation story. J Hosp Med. 2020 May 21;15(6):372-374.
4. Miller J. “Heroes on the front line: Dr. Raghuveer Rakasi.” Coverage. May 18, 2020. https://coverage.bluecrossma.com/article/heroes-front-line-dr-raghuveer-rakasi
Adapting to the demands placed on hospital resources by COVID-19
Adapting to the demands placed on hospital resources by COVID-19
The challenges posed by COVID-19 have crippled health care systems around the globe. By February 2020, the first outbreak in the United States had been set off in Washington State. We quickly became the world’s epicenter of the epidemic, with over 1.8 million patients and over 110,000 deaths.1 The rapidity of spread and the severity of the disease created a tremendous strain on resources. It blindsided policymakers and hospital administrators, which left little time to react to the challenges placed on hospital operations all over the country.
The necessity of a new care model
Although health systems in the United States are adept in managing complications of common seasonal viral respiratory illnesses, COVID-19 presented an entirely different challenge with its significantly higher mortality rate. A respiratory disease turning into a multiorgan disease that causes debilitating cardiac, renal, neurological, hematological, and psychosocial complications2 was not something we had experience managing effectively. Additional challenges included a massive surge of COVID-19 patients, a limited supply of personal protective equipment (PPE), an inadequate number of intensivists for managing the anticipated ventilated patients, and most importantly, the potential of losing some of our workforce if they became infected.
Based on the experiences in China and Italy, and various predictive models, the division of hospital medicine at Baystate Health quickly realized the necessity of a new model of care for COVID-19 patients. We came up with an elaborate plan to manage the disease burden and the strain on resources effectively. The measures we put in place could be broadly divided into three categories following the timeline of the disease: the preparatory phase, the execution phase, and the maintenance phase.
The preparatory phase: From “Hospitalists” to “COVIDists”
As in most hospitals around the country, hospitalists are the backbone of inpatient clinical operations at our health system. A focused group of 10 hospitalists who volunteered to take care of COVID-19 patients with a particular interest in the pandemic and experience in critical care were selected, and the term “COVIDists” was coined to refer to them.
COVIDists were trained in various treatment protocols and ongoing clinical trials. They were given refresher training in Advanced Cardiac Life Support (ACLS) and Fundamental Critical Care Support (FCCS) courses and were taught in critical care/ventilator management by the intensivists through rapid indoctrination in the ICU. All of them had their N-95 mask fitting updated and were trained in the safe donning and doffing of all kinds of PPE by PPE coaches. The palliative care team trained them in conducting end-of-life/code status discussions with a focus on being unable to speak with family members at the bedside. COVIDists were also assigned as Code Blue leaders for any “COVID code blue” in the hospital.
In addition to the rapid training course, COVID-related updates were disseminated daily using three different modalities: brief huddles at the start of the day with the COVIDists; a COVID-19 newsletter summarizing daily updates, new treatments, strategies, and policies; and a WhatsApp group for instantly broadcasting information to the COVIDists (Table 1).
The execution phase
All the hospitalized COVID-19 patients were grouped together to COVID units, and the COVIDists were deployed to those units geographically. COVIDists were given lighter than usual patient loads to deal with the extra time needed for donning and doffing of PPE and for coordination with specialists. COVIDists were almost the only clinicians physically visiting the patients in most cases, and they became the “eyes and ears” of specialists since the specialists were advised to minimize exposure and pursue telemedicine consults. The COVIDists were also undertaking the most challenging part of the care – talking to families about end-of-life issues and the futility of aggressive care in certain patients with preexisting conditions.
Some COVIDists were deployed to the ICU to work alongside the intensivists and became an invaluable resource in ICU management when the ICU census skyrocketed during the initial phase of the outbreak. This helped in tiding the health system over during the initial crisis. Within a short time, we shifted away from an early intubation strategy, and most of the ICU patients were managed in the intermediate care units on high flow oxygen along with the awake-proning protocol. The COVIDists exclusively managed these units. They led multidisciplinary rounds two times a day with the ICU, rapid response team (RRT), the palliative care team, and the nursing team. This step drastically decreased the number of intubations, RRT activations, reduced ICU census,3 and helped with hospital capacity and patient flow (Tables 2 and 3).
This strategy also helped build solidarity and camaraderie between all these groups, making the COVIDists feel that they were never alone and that the whole hospital supported them. We are currently evaluating clinical outcomes and attempting to identify effects on mortality, length of stay, days on the ventilator, and days in ICU. 
The maintenance phase
It is already 2 months since the first devising COVIDists. There is no difference in sick callouts between COVIDists and non-COVIDists. One COVIDist and one non-COVIDist contracted the disease, but none of them required hospitalization. Although we initially thought that COVIDists would be needed for only a short period of time, the evolution of the disease is showing signs that it might be prolonged over the next several months. Hence, we are planning to continue COVIDist service for at least the next 6 months and reevaluate the need.
Hospital medicine leadership checked on COVIDists daily in regard to their physical health and, more importantly, their mental well-being. They were offered the chance to be taken off the schedule if they felt burned out, but no one wanted to come off their scheduled service before finishing their shifts. BlueCross MA recognized one of the COVIDists, Raghuveer Rakasi, MD, as a “hero on the front line.”4 In Dr. Rakasi’s words, “We took a nosedive into something without knowing its depth, and aware that we could have fatalities among ourselves. We took up new roles, faced new challenges, learned new things every day, evolving every step of the way. We had to change the way we practice medicine, finding new ways to treat patients, and protecting the workforce by limiting patient exposure, prioritizing investigations.” He added that “we have to adapt to a new normal; we should be prepared for this to come in waves. Putting aside our political views, we should stand united 6 feet apart, with a mask covering our brave faces, frequently washing our helping hands to overcome these uncertain times.”
Conclusion
The creation of a focused group of hospitalists called COVIDists and providing them with structured and rapid training (in various aspects of clinical care of COVID-19 patients, critical care/ventilator management, efficient and safe use of PPE) and daily information dissemination allowed our health system to prepare for the large volume of COVID-19 patients. It also helped in preserving the larger hospital workforce for a possible future surge.
The rapid development and implementation of the COVIDist strategy succeeded because of the intrinsic motivation of the providers to improve the outcomes of this high-risk patient population and the close collaboration of the stakeholders. Our institution remains successful in managing the pandemic in Western Massachusetts, with reserve capacity remaining even during the peak of the epidemic. A large part of this was because of creating and training a pool of COVIDists.
Dr. Medarametla is medical director, clinical operations, in the division of hospital medicine at Baystate Health, and assistant professor at University of Massachusetts, Worcester. Readers can contact him at Venkatrao.MedarametlaMD@Baystatehealth.org. Dr. Prabhakaran is unit medical director, geriatrics unit, in the division of hospital medicine at Baystate Health and assistant professor at University of Massachusetts. Dr. Bryson is associate program director of the Internal Medicine Residency at Baystate Health and assistant professor at University of Massachusetts. Dr. Umar is medical director, clinical operations, in the division of hospital medicine at Baystate Health. Dr. Natanasabapathy is division chief of hospital medicine at Baystate Health and assistant professor at University of Massachusetts.
References
1. Centers for Disease Control and Prevention. Coronavirus Disease 2019 (COVID-19). Updated Jun 10, 2020. https://www.cdc.gov/coronavirus/2019-ncov/cases-updates/cases-in-us.html.
2. Zhou F et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: A retrospective cohort study. Lancet. 2020 Mar 28;395(10229):1054-62.
3. Westafer LM et al. A transdisciplinary COVID-19 early respiratory intervention protocol: An implementation story. J Hosp Med. 2020 May 21;15(6):372-374.
4. Miller J. “Heroes on the front line: Dr. Raghuveer Rakasi.” Coverage. May 18, 2020. https://coverage.bluecrossma.com/article/heroes-front-line-dr-raghuveer-rakasi
The challenges posed by COVID-19 have crippled health care systems around the globe. By February 2020, the first outbreak in the United States had been set off in Washington State. We quickly became the world’s epicenter of the epidemic, with over 1.8 million patients and over 110,000 deaths.1 The rapidity of spread and the severity of the disease created a tremendous strain on resources. It blindsided policymakers and hospital administrators, which left little time to react to the challenges placed on hospital operations all over the country.
The necessity of a new care model
Although health systems in the United States are adept in managing complications of common seasonal viral respiratory illnesses, COVID-19 presented an entirely different challenge with its significantly higher mortality rate. A respiratory disease turning into a multiorgan disease that causes debilitating cardiac, renal, neurological, hematological, and psychosocial complications2 was not something we had experience managing effectively. Additional challenges included a massive surge of COVID-19 patients, a limited supply of personal protective equipment (PPE), an inadequate number of intensivists for managing the anticipated ventilated patients, and most importantly, the potential of losing some of our workforce if they became infected.
Based on the experiences in China and Italy, and various predictive models, the division of hospital medicine at Baystate Health quickly realized the necessity of a new model of care for COVID-19 patients. We came up with an elaborate plan to manage the disease burden and the strain on resources effectively. The measures we put in place could be broadly divided into three categories following the timeline of the disease: the preparatory phase, the execution phase, and the maintenance phase.
The preparatory phase: From “Hospitalists” to “COVIDists”
As in most hospitals around the country, hospitalists are the backbone of inpatient clinical operations at our health system. A focused group of 10 hospitalists who volunteered to take care of COVID-19 patients with a particular interest in the pandemic and experience in critical care were selected, and the term “COVIDists” was coined to refer to them.
COVIDists were trained in various treatment protocols and ongoing clinical trials. They were given refresher training in Advanced Cardiac Life Support (ACLS) and Fundamental Critical Care Support (FCCS) courses and were taught in critical care/ventilator management by the intensivists through rapid indoctrination in the ICU. All of them had their N-95 mask fitting updated and were trained in the safe donning and doffing of all kinds of PPE by PPE coaches. The palliative care team trained them in conducting end-of-life/code status discussions with a focus on being unable to speak with family members at the bedside. COVIDists were also assigned as Code Blue leaders for any “COVID code blue” in the hospital.
In addition to the rapid training course, COVID-related updates were disseminated daily using three different modalities: brief huddles at the start of the day with the COVIDists; a COVID-19 newsletter summarizing daily updates, new treatments, strategies, and policies; and a WhatsApp group for instantly broadcasting information to the COVIDists (Table 1).
The execution phase
All the hospitalized COVID-19 patients were grouped together to COVID units, and the COVIDists were deployed to those units geographically. COVIDists were given lighter than usual patient loads to deal with the extra time needed for donning and doffing of PPE and for coordination with specialists. COVIDists were almost the only clinicians physically visiting the patients in most cases, and they became the “eyes and ears” of specialists since the specialists were advised to minimize exposure and pursue telemedicine consults. The COVIDists were also undertaking the most challenging part of the care – talking to families about end-of-life issues and the futility of aggressive care in certain patients with preexisting conditions.
Some COVIDists were deployed to the ICU to work alongside the intensivists and became an invaluable resource in ICU management when the ICU census skyrocketed during the initial phase of the outbreak. This helped in tiding the health system over during the initial crisis. Within a short time, we shifted away from an early intubation strategy, and most of the ICU patients were managed in the intermediate care units on high flow oxygen along with the awake-proning protocol. The COVIDists exclusively managed these units. They led multidisciplinary rounds two times a day with the ICU, rapid response team (RRT), the palliative care team, and the nursing team. This step drastically decreased the number of intubations, RRT activations, reduced ICU census,3 and helped with hospital capacity and patient flow (Tables 2 and 3).
This strategy also helped build solidarity and camaraderie between all these groups, making the COVIDists feel that they were never alone and that the whole hospital supported them. We are currently evaluating clinical outcomes and attempting to identify effects on mortality, length of stay, days on the ventilator, and days in ICU. 
The maintenance phase
It is already 2 months since the first devising COVIDists. There is no difference in sick callouts between COVIDists and non-COVIDists. One COVIDist and one non-COVIDist contracted the disease, but none of them required hospitalization. Although we initially thought that COVIDists would be needed for only a short period of time, the evolution of the disease is showing signs that it might be prolonged over the next several months. Hence, we are planning to continue COVIDist service for at least the next 6 months and reevaluate the need.
Hospital medicine leadership checked on COVIDists daily in regard to their physical health and, more importantly, their mental well-being. They were offered the chance to be taken off the schedule if they felt burned out, but no one wanted to come off their scheduled service before finishing their shifts. BlueCross MA recognized one of the COVIDists, Raghuveer Rakasi, MD, as a “hero on the front line.”4 In Dr. Rakasi’s words, “We took a nosedive into something without knowing its depth, and aware that we could have fatalities among ourselves. We took up new roles, faced new challenges, learned new things every day, evolving every step of the way. We had to change the way we practice medicine, finding new ways to treat patients, and protecting the workforce by limiting patient exposure, prioritizing investigations.” He added that “we have to adapt to a new normal; we should be prepared for this to come in waves. Putting aside our political views, we should stand united 6 feet apart, with a mask covering our brave faces, frequently washing our helping hands to overcome these uncertain times.”
Conclusion
The creation of a focused group of hospitalists called COVIDists and providing them with structured and rapid training (in various aspects of clinical care of COVID-19 patients, critical care/ventilator management, efficient and safe use of PPE) and daily information dissemination allowed our health system to prepare for the large volume of COVID-19 patients. It also helped in preserving the larger hospital workforce for a possible future surge.
The rapid development and implementation of the COVIDist strategy succeeded because of the intrinsic motivation of the providers to improve the outcomes of this high-risk patient population and the close collaboration of the stakeholders. Our institution remains successful in managing the pandemic in Western Massachusetts, with reserve capacity remaining even during the peak of the epidemic. A large part of this was because of creating and training a pool of COVIDists.
Dr. Medarametla is medical director, clinical operations, in the division of hospital medicine at Baystate Health, and assistant professor at University of Massachusetts, Worcester. Readers can contact him at Venkatrao.MedarametlaMD@Baystatehealth.org. Dr. Prabhakaran is unit medical director, geriatrics unit, in the division of hospital medicine at Baystate Health and assistant professor at University of Massachusetts. Dr. Bryson is associate program director of the Internal Medicine Residency at Baystate Health and assistant professor at University of Massachusetts. Dr. Umar is medical director, clinical operations, in the division of hospital medicine at Baystate Health. Dr. Natanasabapathy is division chief of hospital medicine at Baystate Health and assistant professor at University of Massachusetts.
References
1. Centers for Disease Control and Prevention. Coronavirus Disease 2019 (COVID-19). Updated Jun 10, 2020. https://www.cdc.gov/coronavirus/2019-ncov/cases-updates/cases-in-us.html.
2. Zhou F et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: A retrospective cohort study. Lancet. 2020 Mar 28;395(10229):1054-62.
3. Westafer LM et al. A transdisciplinary COVID-19 early respiratory intervention protocol: An implementation story. J Hosp Med. 2020 May 21;15(6):372-374.
4. Miller J. “Heroes on the front line: Dr. Raghuveer Rakasi.” Coverage. May 18, 2020. https://coverage.bluecrossma.com/article/heroes-front-line-dr-raghuveer-rakasi
A Transdisciplinary COVID-19 Early Respiratory Intervention Protocol: An Implementation Story
My colleague asked, “Do you remember that patient?” I froze because, like most emergency physicians, this phrase haunts me. It was the early days of the COVID-19 epidemic, and the story that followed was upsetting. A patient who looked comfortable when I admitted him was intubated hours later by the rapid response team who was called to the floor. All I could think was, “But he looked so comfortable when I admitted him; he was just on a couple of liters of oxygen. Why was he intubated?”
In the days after COVID-19 arrived in our region, there were many such stories of patients sent to the floor from the Emergency Department who were intubated shortly after admission. Many of those patients subsequently endured prolonged and complicated courses on the ventilator. While we would typically use noninvasive modalities such as high-flow nasal cannula (HFNC) or noninvasive ventilation (NIV) for acute respiratory failure, our quickness to intubate was driven by two factors: (1) early reports that noninvasive modalities posed a high risk of failure and subsequent intubation and (2) fear that HFNC and NIV would aerosolize SARS-CoV-2 and unnecessarily expose the heath care team.1 We would soon find out that our thinking was flawed on both accounts.
RETHINKING INITIAL ASSUMPTIONS
When we dug into the evidence for early intubation, we realized that these recommendations were based on a 12-patient series in which 5 patients were trialed on NIV but ultimately intubated and placed on invasive mechanical ventilation (IMV). As the pandemic progressed, more case series and small studies were published, revealing a different picture.2 Sun and colleagues reported a multifaceted intervention of 610 inpatients, of whom 10% were critically ill, that identified at-risk patients and used NIV or HFNC and awake proning. Reportedly, fewer than 1% required IMV.3 Similarly, a small study found intubation was avoided in 85% of patients with severe acute respiratory failure caused by COVID-19 with use of HFNC and NIV.4 Early findings from New York University in New York, New York, where only 8.5% of patients undergoing IMV were extubated by the time of outcome reporting, suggest early IMV could lead to poor outcomes.5
Still, we had concerns about use of HFNC and NIV because of worries about the health and safety of other patients and particularly that of healthcare workers (HCWs) because they have been disproportionately affected by the disease.6 Fortunately, we identified emerging data that revealed that HFNC is no more aerosolizing than low-flow nasal cannula or a nonrebreather mask and droplet spread is reduced with a surgical mask.7,8 In light of these new studies and our own developing experience with the disease, we felt that there was insufficient evidence to continue following the “early intubation” protocol in patients with COVID-19. It was time for a new paradigm.
GATHERING EVIDENCE AND STAKEHOLDERS
In order to effectively and quickly change our respiratory pathway for these patients, we initially sought out protocols from other institutions through social media. These protocols, supported by early data from those sites, informed our process. We considered data from various sources, including emergency medicine, hospital medicine, and critical care. We then assembled stakeholders within our organization from emergency medicine, hospital medicine, critical care, and respiratory therapy because our protocol would need endorsement from all key players within our organization who cared for these patients across the potential spectrum of care. We made sure that all stakeholders understood that the quality of the evidence for treatment of this novel disease was much lower than our typical threshold to change practice, but that we aimed to reflect the best evidence to date. We also were careful to identify pathways that would be amenable to near-immediate implementation.
UNVEILING A NOVEL PROTOCOL
Our group reached consensus within 48 hours and quickly disseminated our first draft of the protocol (Appendix Figure). Dubbed the “Early Intervention Respiratory Protocol,” it differed from usual management in several ways. First, we had consistently observed (and confirmed from the literature) a phenotype of patients with “silent hypoxemia”9 (that is, a subset of patients who presented with profound hypoxemia but minimally increased work of breathing). The protocol encouraged tolerance of lower oxygen saturations than is usually seen on inpatient units. This required ensuring all stakeholders were comfortable with a target oxygen saturation of 88%. Second, the protocol leveraged early “awake” proning by patients. Historically, proning is used in mechanically ventilated patients with acute respiratory distress syndrome (ARDS) to improve ventilation-perfusion matching, promote more uniform ventilation, and increase end-expiratory lung volume.10 Prior literature was limited to the use of awake proning in small case series of ARDS, but given our limitations in terms of ICU capacity, we agreed to trial awake proning in a sizable proportion of our COVID-19 patients outside the ICU.11,12 Finally, we clarified safe practices regarding the risk of aerosolization with noninvasive modalities. Local infection control determined that HFNC wa not aerosol generating, and use of surgical masks was added for further protection from respiratory droplets. In addition, airborne personal protective equipment was to be worn on the inpatient ward, and we used NIV sparingly and preferentially placed these patients in negative pressure rooms, if available.13
Implementation of the protocol involved aggressive dissemination and education (Table). A single-page protocol was designed for ease of use at the bedside that included anticipatory guidance regarding aerosolization and addressed potential resistance to awake proning because of concerns regarding safety and hassle. Departmental leaders disseminated the protocol throughout the institution with tailored education on the rationale and acknowledgment of a reversal in approach. In addition to email, we used text messaging (WhatsApp) and a comprehensive living document (Google Drive) to reach clinicians.
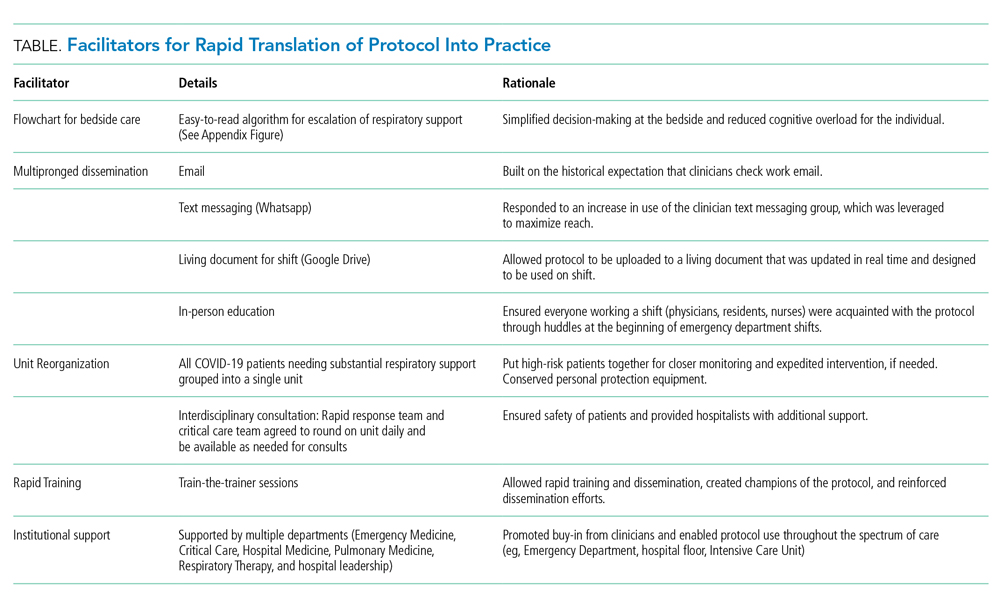
For ease of monitoring and safety, we designated a COVID-19 intermediate care unit. We partnered with the unit medical director, nurse educator, and a focused group of hospitalists, conducting individual train-the-trainer sessions. This training was carried forward, and all nurses, respiratory therapists, and clinicians were trained on the early aggressive respiratory protocol within 12 hours of protocol approval. In addition, the rapid response and critical care teams agreed to round on the COVID-19 intermediate care unit daily.
As a result of these efforts, adoption of the protocol was essentially immediate across the institution. We had shifted the mindset of a diverse group of clinicians regarding how to support the respiratory status of these patients, but also detected reductions in the proportion of patients undergoing IMV and ICU admission (we are planning to report these results separately).
TRANSLATING KNOWLEDGE INTO PRACTICE
The COVID-19 pandemic has highlighted the importance of having cognitive flexibility when the evidence base is rapidly changing and there is a need for rapid dissemination of knowledge. Even in clinical scenarios with an abundance of high-quality evidence, a gap in knowledge translation on the order of a decade often exists. In contrast, a pandemic involving a novel virus highlights an urgent need for adaptive knowledge translation in the present moment rather than a decade later. In the absence of robust evidence regarding SARS-CoV-2, early management of COVID-19 was based on expert recommendations and experience with other disease processes. Even so, we should anticipate that management paradigms may shift, and we should constantly seek out emerging evidence to adjust our mindset (and protocols like this) accordingly. Our original protocol was based on nearly nonexistent evidence, but we anticipated that, in a pandemic, data would accumulate quickly, so we prioritized rapid translation of new information into practice. In fact, further evidence has emerged regarding the improvement in oxygenation in COVID-19 patients with self-proning.14
The final step is evaluating the success of both clinical and implementation outcomes. We are attempting to identify changes in intubation, length of stay, days on ventilator, and days in ICU. In addition, we will measure feasibility and adaptability. We are also attempting, in real time, to identify barriers to its use, including conducting qualitative interviews to understand whether there were unintended consequences to use of the protocol. This endeavor highlights how the COVID-19 pandemic, for all its tragedy, may represent an important era for implementation science: a time when emerging literature from a variety of sources can be implemented in days rather than years.
1. World Health Organization. Clinical management of severe acute respiratory infection (SARI) when COVID-19 disease is suspected. 2020. https://www.who.int/publications-detail/clinical-management-of-severe-acute-respiratory-infection-when-novel-coronavirus-(ncov)-infection-is-suspected. Accessed March 25, 2020.
2. Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054-1062. https://doi.org/10.1016/s0140-6736(20)30566-3.
3. Sun Q, Qiu H, Huang M, Yang Y. Lower mortality of COVID-19 by early recognition and intervention: experience from Jiangsu Province. Ann Intensive Care. 2020;10(1):33. https://doi.org/10.1186/s13613-020-00650-2.
4. Wang K, Zhao W, Li J, Shu W, Duan J. The experience of high-flow nasal cannula in hospitalized patients with 2019 novel coronavirus-infected pneumonia in two hospitals of Chongqing, China. Ann Intensive Care. 2020;10(1):37. https://doi.org/10.1186/s13613-020-00653-z.
5. Petrilli C, Jones SA, Yang J, Rajagopalan H, et al. Factors associated with hospitalization and critical illness among 4,103 patients with Covid-19 disease in New York City [preprint]. medRxiv. 2020. https://doi.org/10.1101/2020.04.08.20057794. Accessed April 12, 2020.
6. Wang D, Hu B, Hu C, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus–infected pneumonia in Wuhan, China. JAMA. 2020;323(11):1061-1069. https://doi.org/10.1001/jama.2020.1585.
7. Leonard S, Volakis L, DeBellis R, Kahlon A, Mayar S. Transmission Assessment Report: High Velocity Nasal Insufflation (HVNI) Therapy Application in Management of COVID-19. March 25, 2020. Vapotherm Blog. 2020. https://vapotherm.com/blog/transmission-assessment-report/. Accessed March 25, 2020.
8. Iwashyna TJ, Boehman A, Capecelatro J, Cohn A, JM. C. Variation in aerosol production across oxygen delivery devices in 2 spontaneously breathing human subjects [preprint]. medRxiv. 2020. https://doi.org/10.1101/2020.04.15.20066688. Accessed April 20, 2020.
9. Meng L, Qiu H, Wan L, et al. Intubation and ventilation amid the COVID-19 outbreak [online ahead of print]. Anesthesiology. 2020. https://doi.org/10.1097/aln.0000000000003296.
10. Munshi L, Del Sorbo L, Adhikari NKJ, et al. Prone position for acute respiratory distress syndrome: a systematic review and meta-analysis. Ann Am Thorac Soc. 2017;14(suppl 4):S280-S288. https://doi.org/10.1513/annalsats.201704-343ot.
11. Scaravilli V, Grasselli G, Castagna L, et al. Prone positioning improves oxygenation in spontaneously breathing nonintubated patients with hypoxemic acute respiratory failure: a retrospective study. J Crit Care. 2015;30(6):1390-1394. https://doi.org/10.1016/j.jcrc.2015.07.008
12. Ding L, Wang L, Ma W, He H. Efficacy and safety of early prone positioning combined with HFNC or NIV in moderate to severe ARDS: a multi-center prospective cohort study. Crit Care. 2020;24(1):28. https://doi.org/10.1186/s13054-020-2738-5.
13. Alhazzani W, Møller MH, Arabi YM, et al. Surviving Sepsis Campaign: guidelines on the management of critically ill adults with coronavirus disease 2019 (COVID-19). Intensive Care Med. 2020;1‐34. https://doi.org/10.1007/s00134-020-06022-5.
14. Caputo ND, Strayer RJ, Levitan R. Early self-proning in awake, non-intubated patients in the emergency department: a single ED’s experience during the COVID-19 pandemic [online ahead of print]. Acad Emerg Med. 2020. https://doi.org/10.1111/acem.13994.
My colleague asked, “Do you remember that patient?” I froze because, like most emergency physicians, this phrase haunts me. It was the early days of the COVID-19 epidemic, and the story that followed was upsetting. A patient who looked comfortable when I admitted him was intubated hours later by the rapid response team who was called to the floor. All I could think was, “But he looked so comfortable when I admitted him; he was just on a couple of liters of oxygen. Why was he intubated?”
In the days after COVID-19 arrived in our region, there were many such stories of patients sent to the floor from the Emergency Department who were intubated shortly after admission. Many of those patients subsequently endured prolonged and complicated courses on the ventilator. While we would typically use noninvasive modalities such as high-flow nasal cannula (HFNC) or noninvasive ventilation (NIV) for acute respiratory failure, our quickness to intubate was driven by two factors: (1) early reports that noninvasive modalities posed a high risk of failure and subsequent intubation and (2) fear that HFNC and NIV would aerosolize SARS-CoV-2 and unnecessarily expose the heath care team.1 We would soon find out that our thinking was flawed on both accounts.
RETHINKING INITIAL ASSUMPTIONS
When we dug into the evidence for early intubation, we realized that these recommendations were based on a 12-patient series in which 5 patients were trialed on NIV but ultimately intubated and placed on invasive mechanical ventilation (IMV). As the pandemic progressed, more case series and small studies were published, revealing a different picture.2 Sun and colleagues reported a multifaceted intervention of 610 inpatients, of whom 10% were critically ill, that identified at-risk patients and used NIV or HFNC and awake proning. Reportedly, fewer than 1% required IMV.3 Similarly, a small study found intubation was avoided in 85% of patients with severe acute respiratory failure caused by COVID-19 with use of HFNC and NIV.4 Early findings from New York University in New York, New York, where only 8.5% of patients undergoing IMV were extubated by the time of outcome reporting, suggest early IMV could lead to poor outcomes.5
Still, we had concerns about use of HFNC and NIV because of worries about the health and safety of other patients and particularly that of healthcare workers (HCWs) because they have been disproportionately affected by the disease.6 Fortunately, we identified emerging data that revealed that HFNC is no more aerosolizing than low-flow nasal cannula or a nonrebreather mask and droplet spread is reduced with a surgical mask.7,8 In light of these new studies and our own developing experience with the disease, we felt that there was insufficient evidence to continue following the “early intubation” protocol in patients with COVID-19. It was time for a new paradigm.
GATHERING EVIDENCE AND STAKEHOLDERS
In order to effectively and quickly change our respiratory pathway for these patients, we initially sought out protocols from other institutions through social media. These protocols, supported by early data from those sites, informed our process. We considered data from various sources, including emergency medicine, hospital medicine, and critical care. We then assembled stakeholders within our organization from emergency medicine, hospital medicine, critical care, and respiratory therapy because our protocol would need endorsement from all key players within our organization who cared for these patients across the potential spectrum of care. We made sure that all stakeholders understood that the quality of the evidence for treatment of this novel disease was much lower than our typical threshold to change practice, but that we aimed to reflect the best evidence to date. We also were careful to identify pathways that would be amenable to near-immediate implementation.
UNVEILING A NOVEL PROTOCOL
Our group reached consensus within 48 hours and quickly disseminated our first draft of the protocol (Appendix Figure). Dubbed the “Early Intervention Respiratory Protocol,” it differed from usual management in several ways. First, we had consistently observed (and confirmed from the literature) a phenotype of patients with “silent hypoxemia”9 (that is, a subset of patients who presented with profound hypoxemia but minimally increased work of breathing). The protocol encouraged tolerance of lower oxygen saturations than is usually seen on inpatient units. This required ensuring all stakeholders were comfortable with a target oxygen saturation of 88%. Second, the protocol leveraged early “awake” proning by patients. Historically, proning is used in mechanically ventilated patients with acute respiratory distress syndrome (ARDS) to improve ventilation-perfusion matching, promote more uniform ventilation, and increase end-expiratory lung volume.10 Prior literature was limited to the use of awake proning in small case series of ARDS, but given our limitations in terms of ICU capacity, we agreed to trial awake proning in a sizable proportion of our COVID-19 patients outside the ICU.11,12 Finally, we clarified safe practices regarding the risk of aerosolization with noninvasive modalities. Local infection control determined that HFNC wa not aerosol generating, and use of surgical masks was added for further protection from respiratory droplets. In addition, airborne personal protective equipment was to be worn on the inpatient ward, and we used NIV sparingly and preferentially placed these patients in negative pressure rooms, if available.13
Implementation of the protocol involved aggressive dissemination and education (Table). A single-page protocol was designed for ease of use at the bedside that included anticipatory guidance regarding aerosolization and addressed potential resistance to awake proning because of concerns regarding safety and hassle. Departmental leaders disseminated the protocol throughout the institution with tailored education on the rationale and acknowledgment of a reversal in approach. In addition to email, we used text messaging (WhatsApp) and a comprehensive living document (Google Drive) to reach clinicians.

For ease of monitoring and safety, we designated a COVID-19 intermediate care unit. We partnered with the unit medical director, nurse educator, and a focused group of hospitalists, conducting individual train-the-trainer sessions. This training was carried forward, and all nurses, respiratory therapists, and clinicians were trained on the early aggressive respiratory protocol within 12 hours of protocol approval. In addition, the rapid response and critical care teams agreed to round on the COVID-19 intermediate care unit daily.
As a result of these efforts, adoption of the protocol was essentially immediate across the institution. We had shifted the mindset of a diverse group of clinicians regarding how to support the respiratory status of these patients, but also detected reductions in the proportion of patients undergoing IMV and ICU admission (we are planning to report these results separately).
TRANSLATING KNOWLEDGE INTO PRACTICE
The COVID-19 pandemic has highlighted the importance of having cognitive flexibility when the evidence base is rapidly changing and there is a need for rapid dissemination of knowledge. Even in clinical scenarios with an abundance of high-quality evidence, a gap in knowledge translation on the order of a decade often exists. In contrast, a pandemic involving a novel virus highlights an urgent need for adaptive knowledge translation in the present moment rather than a decade later. In the absence of robust evidence regarding SARS-CoV-2, early management of COVID-19 was based on expert recommendations and experience with other disease processes. Even so, we should anticipate that management paradigms may shift, and we should constantly seek out emerging evidence to adjust our mindset (and protocols like this) accordingly. Our original protocol was based on nearly nonexistent evidence, but we anticipated that, in a pandemic, data would accumulate quickly, so we prioritized rapid translation of new information into practice. In fact, further evidence has emerged regarding the improvement in oxygenation in COVID-19 patients with self-proning.14
The final step is evaluating the success of both clinical and implementation outcomes. We are attempting to identify changes in intubation, length of stay, days on ventilator, and days in ICU. In addition, we will measure feasibility and adaptability. We are also attempting, in real time, to identify barriers to its use, including conducting qualitative interviews to understand whether there were unintended consequences to use of the protocol. This endeavor highlights how the COVID-19 pandemic, for all its tragedy, may represent an important era for implementation science: a time when emerging literature from a variety of sources can be implemented in days rather than years.
My colleague asked, “Do you remember that patient?” I froze because, like most emergency physicians, this phrase haunts me. It was the early days of the COVID-19 epidemic, and the story that followed was upsetting. A patient who looked comfortable when I admitted him was intubated hours later by the rapid response team who was called to the floor. All I could think was, “But he looked so comfortable when I admitted him; he was just on a couple of liters of oxygen. Why was he intubated?”
In the days after COVID-19 arrived in our region, there were many such stories of patients sent to the floor from the Emergency Department who were intubated shortly after admission. Many of those patients subsequently endured prolonged and complicated courses on the ventilator. While we would typically use noninvasive modalities such as high-flow nasal cannula (HFNC) or noninvasive ventilation (NIV) for acute respiratory failure, our quickness to intubate was driven by two factors: (1) early reports that noninvasive modalities posed a high risk of failure and subsequent intubation and (2) fear that HFNC and NIV would aerosolize SARS-CoV-2 and unnecessarily expose the heath care team.1 We would soon find out that our thinking was flawed on both accounts.
RETHINKING INITIAL ASSUMPTIONS
When we dug into the evidence for early intubation, we realized that these recommendations were based on a 12-patient series in which 5 patients were trialed on NIV but ultimately intubated and placed on invasive mechanical ventilation (IMV). As the pandemic progressed, more case series and small studies were published, revealing a different picture.2 Sun and colleagues reported a multifaceted intervention of 610 inpatients, of whom 10% were critically ill, that identified at-risk patients and used NIV or HFNC and awake proning. Reportedly, fewer than 1% required IMV.3 Similarly, a small study found intubation was avoided in 85% of patients with severe acute respiratory failure caused by COVID-19 with use of HFNC and NIV.4 Early findings from New York University in New York, New York, where only 8.5% of patients undergoing IMV were extubated by the time of outcome reporting, suggest early IMV could lead to poor outcomes.5
Still, we had concerns about use of HFNC and NIV because of worries about the health and safety of other patients and particularly that of healthcare workers (HCWs) because they have been disproportionately affected by the disease.6 Fortunately, we identified emerging data that revealed that HFNC is no more aerosolizing than low-flow nasal cannula or a nonrebreather mask and droplet spread is reduced with a surgical mask.7,8 In light of these new studies and our own developing experience with the disease, we felt that there was insufficient evidence to continue following the “early intubation” protocol in patients with COVID-19. It was time for a new paradigm.
GATHERING EVIDENCE AND STAKEHOLDERS
In order to effectively and quickly change our respiratory pathway for these patients, we initially sought out protocols from other institutions through social media. These protocols, supported by early data from those sites, informed our process. We considered data from various sources, including emergency medicine, hospital medicine, and critical care. We then assembled stakeholders within our organization from emergency medicine, hospital medicine, critical care, and respiratory therapy because our protocol would need endorsement from all key players within our organization who cared for these patients across the potential spectrum of care. We made sure that all stakeholders understood that the quality of the evidence for treatment of this novel disease was much lower than our typical threshold to change practice, but that we aimed to reflect the best evidence to date. We also were careful to identify pathways that would be amenable to near-immediate implementation.
UNVEILING A NOVEL PROTOCOL
Our group reached consensus within 48 hours and quickly disseminated our first draft of the protocol (Appendix Figure). Dubbed the “Early Intervention Respiratory Protocol,” it differed from usual management in several ways. First, we had consistently observed (and confirmed from the literature) a phenotype of patients with “silent hypoxemia”9 (that is, a subset of patients who presented with profound hypoxemia but minimally increased work of breathing). The protocol encouraged tolerance of lower oxygen saturations than is usually seen on inpatient units. This required ensuring all stakeholders were comfortable with a target oxygen saturation of 88%. Second, the protocol leveraged early “awake” proning by patients. Historically, proning is used in mechanically ventilated patients with acute respiratory distress syndrome (ARDS) to improve ventilation-perfusion matching, promote more uniform ventilation, and increase end-expiratory lung volume.10 Prior literature was limited to the use of awake proning in small case series of ARDS, but given our limitations in terms of ICU capacity, we agreed to trial awake proning in a sizable proportion of our COVID-19 patients outside the ICU.11,12 Finally, we clarified safe practices regarding the risk of aerosolization with noninvasive modalities. Local infection control determined that HFNC wa not aerosol generating, and use of surgical masks was added for further protection from respiratory droplets. In addition, airborne personal protective equipment was to be worn on the inpatient ward, and we used NIV sparingly and preferentially placed these patients in negative pressure rooms, if available.13
Implementation of the protocol involved aggressive dissemination and education (Table). A single-page protocol was designed for ease of use at the bedside that included anticipatory guidance regarding aerosolization and addressed potential resistance to awake proning because of concerns regarding safety and hassle. Departmental leaders disseminated the protocol throughout the institution with tailored education on the rationale and acknowledgment of a reversal in approach. In addition to email, we used text messaging (WhatsApp) and a comprehensive living document (Google Drive) to reach clinicians.

For ease of monitoring and safety, we designated a COVID-19 intermediate care unit. We partnered with the unit medical director, nurse educator, and a focused group of hospitalists, conducting individual train-the-trainer sessions. This training was carried forward, and all nurses, respiratory therapists, and clinicians were trained on the early aggressive respiratory protocol within 12 hours of protocol approval. In addition, the rapid response and critical care teams agreed to round on the COVID-19 intermediate care unit daily.
As a result of these efforts, adoption of the protocol was essentially immediate across the institution. We had shifted the mindset of a diverse group of clinicians regarding how to support the respiratory status of these patients, but also detected reductions in the proportion of patients undergoing IMV and ICU admission (we are planning to report these results separately).
TRANSLATING KNOWLEDGE INTO PRACTICE
The COVID-19 pandemic has highlighted the importance of having cognitive flexibility when the evidence base is rapidly changing and there is a need for rapid dissemination of knowledge. Even in clinical scenarios with an abundance of high-quality evidence, a gap in knowledge translation on the order of a decade often exists. In contrast, a pandemic involving a novel virus highlights an urgent need for adaptive knowledge translation in the present moment rather than a decade later. In the absence of robust evidence regarding SARS-CoV-2, early management of COVID-19 was based on expert recommendations and experience with other disease processes. Even so, we should anticipate that management paradigms may shift, and we should constantly seek out emerging evidence to adjust our mindset (and protocols like this) accordingly. Our original protocol was based on nearly nonexistent evidence, but we anticipated that, in a pandemic, data would accumulate quickly, so we prioritized rapid translation of new information into practice. In fact, further evidence has emerged regarding the improvement in oxygenation in COVID-19 patients with self-proning.14
The final step is evaluating the success of both clinical and implementation outcomes. We are attempting to identify changes in intubation, length of stay, days on ventilator, and days in ICU. In addition, we will measure feasibility and adaptability. We are also attempting, in real time, to identify barriers to its use, including conducting qualitative interviews to understand whether there were unintended consequences to use of the protocol. This endeavor highlights how the COVID-19 pandemic, for all its tragedy, may represent an important era for implementation science: a time when emerging literature from a variety of sources can be implemented in days rather than years.
1. World Health Organization. Clinical management of severe acute respiratory infection (SARI) when COVID-19 disease is suspected. 2020. https://www.who.int/publications-detail/clinical-management-of-severe-acute-respiratory-infection-when-novel-coronavirus-(ncov)-infection-is-suspected. Accessed March 25, 2020.
2. Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054-1062. https://doi.org/10.1016/s0140-6736(20)30566-3.
3. Sun Q, Qiu H, Huang M, Yang Y. Lower mortality of COVID-19 by early recognition and intervention: experience from Jiangsu Province. Ann Intensive Care. 2020;10(1):33. https://doi.org/10.1186/s13613-020-00650-2.
4. Wang K, Zhao W, Li J, Shu W, Duan J. The experience of high-flow nasal cannula in hospitalized patients with 2019 novel coronavirus-infected pneumonia in two hospitals of Chongqing, China. Ann Intensive Care. 2020;10(1):37. https://doi.org/10.1186/s13613-020-00653-z.
5. Petrilli C, Jones SA, Yang J, Rajagopalan H, et al. Factors associated with hospitalization and critical illness among 4,103 patients with Covid-19 disease in New York City [preprint]. medRxiv. 2020. https://doi.org/10.1101/2020.04.08.20057794. Accessed April 12, 2020.
6. Wang D, Hu B, Hu C, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus–infected pneumonia in Wuhan, China. JAMA. 2020;323(11):1061-1069. https://doi.org/10.1001/jama.2020.1585.
7. Leonard S, Volakis L, DeBellis R, Kahlon A, Mayar S. Transmission Assessment Report: High Velocity Nasal Insufflation (HVNI) Therapy Application in Management of COVID-19. March 25, 2020. Vapotherm Blog. 2020. https://vapotherm.com/blog/transmission-assessment-report/. Accessed March 25, 2020.
8. Iwashyna TJ, Boehman A, Capecelatro J, Cohn A, JM. C. Variation in aerosol production across oxygen delivery devices in 2 spontaneously breathing human subjects [preprint]. medRxiv. 2020. https://doi.org/10.1101/2020.04.15.20066688. Accessed April 20, 2020.
9. Meng L, Qiu H, Wan L, et al. Intubation and ventilation amid the COVID-19 outbreak [online ahead of print]. Anesthesiology. 2020. https://doi.org/10.1097/aln.0000000000003296.
10. Munshi L, Del Sorbo L, Adhikari NKJ, et al. Prone position for acute respiratory distress syndrome: a systematic review and meta-analysis. Ann Am Thorac Soc. 2017;14(suppl 4):S280-S288. https://doi.org/10.1513/annalsats.201704-343ot.
11. Scaravilli V, Grasselli G, Castagna L, et al. Prone positioning improves oxygenation in spontaneously breathing nonintubated patients with hypoxemic acute respiratory failure: a retrospective study. J Crit Care. 2015;30(6):1390-1394. https://doi.org/10.1016/j.jcrc.2015.07.008
12. Ding L, Wang L, Ma W, He H. Efficacy and safety of early prone positioning combined with HFNC or NIV in moderate to severe ARDS: a multi-center prospective cohort study. Crit Care. 2020;24(1):28. https://doi.org/10.1186/s13054-020-2738-5.
13. Alhazzani W, Møller MH, Arabi YM, et al. Surviving Sepsis Campaign: guidelines on the management of critically ill adults with coronavirus disease 2019 (COVID-19). Intensive Care Med. 2020;1‐34. https://doi.org/10.1007/s00134-020-06022-5.
14. Caputo ND, Strayer RJ, Levitan R. Early self-proning in awake, non-intubated patients in the emergency department: a single ED’s experience during the COVID-19 pandemic [online ahead of print]. Acad Emerg Med. 2020. https://doi.org/10.1111/acem.13994.
1. World Health Organization. Clinical management of severe acute respiratory infection (SARI) when COVID-19 disease is suspected. 2020. https://www.who.int/publications-detail/clinical-management-of-severe-acute-respiratory-infection-when-novel-coronavirus-(ncov)-infection-is-suspected. Accessed March 25, 2020.
2. Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054-1062. https://doi.org/10.1016/s0140-6736(20)30566-3.
3. Sun Q, Qiu H, Huang M, Yang Y. Lower mortality of COVID-19 by early recognition and intervention: experience from Jiangsu Province. Ann Intensive Care. 2020;10(1):33. https://doi.org/10.1186/s13613-020-00650-2.
4. Wang K, Zhao W, Li J, Shu W, Duan J. The experience of high-flow nasal cannula in hospitalized patients with 2019 novel coronavirus-infected pneumonia in two hospitals of Chongqing, China. Ann Intensive Care. 2020;10(1):37. https://doi.org/10.1186/s13613-020-00653-z.
5. Petrilli C, Jones SA, Yang J, Rajagopalan H, et al. Factors associated with hospitalization and critical illness among 4,103 patients with Covid-19 disease in New York City [preprint]. medRxiv. 2020. https://doi.org/10.1101/2020.04.08.20057794. Accessed April 12, 2020.
6. Wang D, Hu B, Hu C, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus–infected pneumonia in Wuhan, China. JAMA. 2020;323(11):1061-1069. https://doi.org/10.1001/jama.2020.1585.
7. Leonard S, Volakis L, DeBellis R, Kahlon A, Mayar S. Transmission Assessment Report: High Velocity Nasal Insufflation (HVNI) Therapy Application in Management of COVID-19. March 25, 2020. Vapotherm Blog. 2020. https://vapotherm.com/blog/transmission-assessment-report/. Accessed March 25, 2020.
8. Iwashyna TJ, Boehman A, Capecelatro J, Cohn A, JM. C. Variation in aerosol production across oxygen delivery devices in 2 spontaneously breathing human subjects [preprint]. medRxiv. 2020. https://doi.org/10.1101/2020.04.15.20066688. Accessed April 20, 2020.
9. Meng L, Qiu H, Wan L, et al. Intubation and ventilation amid the COVID-19 outbreak [online ahead of print]. Anesthesiology. 2020. https://doi.org/10.1097/aln.0000000000003296.
10. Munshi L, Del Sorbo L, Adhikari NKJ, et al. Prone position for acute respiratory distress syndrome: a systematic review and meta-analysis. Ann Am Thorac Soc. 2017;14(suppl 4):S280-S288. https://doi.org/10.1513/annalsats.201704-343ot.
11. Scaravilli V, Grasselli G, Castagna L, et al. Prone positioning improves oxygenation in spontaneously breathing nonintubated patients with hypoxemic acute respiratory failure: a retrospective study. J Crit Care. 2015;30(6):1390-1394. https://doi.org/10.1016/j.jcrc.2015.07.008
12. Ding L, Wang L, Ma W, He H. Efficacy and safety of early prone positioning combined with HFNC or NIV in moderate to severe ARDS: a multi-center prospective cohort study. Crit Care. 2020;24(1):28. https://doi.org/10.1186/s13054-020-2738-5.
13. Alhazzani W, Møller MH, Arabi YM, et al. Surviving Sepsis Campaign: guidelines on the management of critically ill adults with coronavirus disease 2019 (COVID-19). Intensive Care Med. 2020;1‐34. https://doi.org/10.1007/s00134-020-06022-5.
14. Caputo ND, Strayer RJ, Levitan R. Early self-proning in awake, non-intubated patients in the emergency department: a single ED’s experience during the COVID-19 pandemic [online ahead of print]. Acad Emerg Med. 2020. https://doi.org/10.1111/acem.13994.
© 2020 Society of Hospital Medicine
Culture: An unseen force in the hospital workplace
Parallels from the airline industry
“Workplace culture” has a profound influence on the success or failure of a team in the modern-day work environment, where teamwork and interpersonal interactions have paramount importance. Crew resource management (CRM), a technique developed originally by the airline industry, has been used as a tool to improve safety and quality in ICUs, trauma rooms, and operating rooms.1,2 This article discusses the use of CRM in hospital medicine as a tool for training and maintaining a favorable workplace culture.
Origin and evolution of CRM
United Airlines instituted the airline industry’s first crew resource management for pilots in 1981, following the 1978 crash of United Flight 173 in Portland, Ore. CRM was created based on recommendations from the National Transportation Safety Board and from a NASA workshop held subsequently.3 CRM has since evolved through five generations, and is a required annual training for most major commercial airline companies around the world. It also has been adapted for personnel training by several modern international industries.4
From the airline industry to the hospital
The health care industry is similar to the airline industry in that there is absolutely no margin of error, and that workplace culture plays a very important role. The culture being referred to here is the sum total of values, beliefs, work ethics, work strategies, strengths, and weaknesses of a group of people, and how they interact as a group. In other words, it is the dynamics of a group.
According to Donelson R. Forsyth, a social and personality psychologist at the University of Richmond (Virginia), the two key determinants of successful teamwork are a “shared mental representation of the task,” which refers to an in-depth understanding of the team and the tasks they are attempting; and “group unity/cohesion,” which means that, generally, members of cohesive groups like each other and the group, and they also are united in their pursuit of collective, group-level goals.5
Understanding the culture of a hospitalist team
Analyzing group dynamics and actively managing them toward both the institutional and global goals of health care is critical for the success of an organization. This is the core of successfully managing any team in any industry.
Additionally, the rapidly changing health care climate and insurance payment systems requires hospital medicine groups to rapidly adapt to the constantly changing health care business environment. As a result, there are a couple of ways to evaluate the effectiveness of the team:
- Measure tangible outcomes. The outcomes have to be well defined, important and measurable. These could be cost of care, quality of care, engagement of the team etc. These tangible measures’ outcome over a period of time can be used as a measure of how effective the team is.
- Simply ask your team! It is very important to know what core values the team holds dear. The best way to get that information from the team is to find out the de facto leaders of the team. They should be involved in the decision-making process, thus making them valuable to the management as well as the team.
Culture shapes outcomes
We have used the analogy of a convex and concave lens to help understand this better. A well-developed and well-coordinated team is like convex lens. A lens’ ability to converge or diverge light rays depends on certain characteristics like the curvature of surfaces and refractory index. Likewise, the culture of a group determines its ability to transform all the demands of the collective workload toward a unified goal/outcome. If it is favorable, the group will work as one and success will happen automatically.
Unfortunately, the opposite of this, (the concave lens effect), is more commonplace, where the dynamics of a team prevent the goals being achieved, as there is discordance, poor coordination of ideas and values, and team members not liking each other.
Most teams would fall somewhere within this spectrum, spanning the most favorable convex lens–like group to the least favorable concave lens–like group.
Change team dynamics using CRM principles
The concept of using CRM principles in health care is not entirely new. Such agencies as the Joint Commission and the Agency for Healthcare Research and Quality recommend using principles of CRM to improve communications, and as an error-prevention tool in health care.6
This approach can be broken down into four important steps:
1. Recruit right. It is important to make sure that the new recruit is the right fit for the team and that the de facto leaders and a few other team members are involved in interviewing the candidates. Their assessment should be given due consideration in making the decision to give the new recruit the job.
Every program looks for aspects like clinical competence, interpersonal communication, teamwork, etc., in a candidate, but it is even more important to make sure the candidate has the tenets that would make him/her a part of that particular team.
2. Train well. The newly recruited providers should be given focused training and the seasoned providers should be given refresher training at regular intervals. Care should be taken in designing the training programs in such a way that the providers are trained in skills that they don’t always think about, things that aren’t readily obvious, and in skills that they never get trained in during medical school and residency.
Specifically, they should be trained in:
- Values. These should include the values of both the organization and the team.
- Safety. This should include all the safety protocols that are in place in the organization - where to get help, how to report unsafe events etc.
- Communication.
Within the group: Have a mentor for the new provider, and also develop a culture where he/she feels comfortable to reach out to anyone in the team for help.
With patients and families: This training should ideally be done in a simulated environment if possible.
With other groups in the hospital: Consultants, nurses, other ancillary staff. Give them an idea about the prevailing culture in the organization with regard to these groups, so that they know what to expect when dealing with them.
- Managing perceptions. How the providers are viewed in the hospital, and how to improve it or maintain it.
- Nurturing the good. Use positive reinforcements to solidify the positive aspects of group dynamics these individuals might possess.
- Weeding out the bad. Use training and feedback to alter the negative group dynamic aspects.
3. Intervene. This is necessary either to maintain the positive aspects of a team that is already high-functioning, or to transform a poorly functioning team into a well-coordinated team. This is where the principles of CRM are going to be most useful.
There are five generations of CRM, each with a different focus.6 Only the aspects relevant to hospital medicine training are mentioned here.
- Communication. Address the gaps in communication. It is important to include people who are trusted by the team in designing and executing these sessions.
- Leadership. The goal should be to encourage the team to take ownership of the program. This will make a tremendous change in the ability of a team to deliver and rise up to challenges. The organizational leadership has to be willing to elevate the leaders of the group to positions where they can meaningfully take part in managing the team and making decisions that are critical to the team.
- Burnout management. Providers getting disillusioned: having no work-life balance; not getting enough respect from management, as well as other groups of doctors/nurses/etc. in the hospital; they are subject to bad scheduling and poor pay – all of which can all lead to career-ending burnout. It is important to recognize this and mitigate the factors that cause burnout.
- Organizational culture. If the team feels valued and supported, they will, in turn, work hard toward success. Creative leadership and a willingness to accommodate what matters the most to the team is essential for achieving this.
- Simulated training. These can be done in simulation labs, or in-group sessions with the team, re-creating difficult scenarios or problems in which the whole team can come together and solve them.
- Error containment and management. The team needs to identify possible sources of error and contain them before errors happen. The group should get together if a serious event happens and brainstorm why it happened and take measures to prevent it.
4. Reevaluate. Team dynamics tend to change over time. It is important to constantly re-evaluate the team and make sure that the team’s culture remains favorable. There should be recurrent cycles of retraining and interventions to maintain the positive growth that has been attained, as depicted in the schematic below:
Conclusion
CRM is widely accepted as an effective tool in training individuals in many high performing industries. This article describes a framework in which the principles of CRM can be applied to hospital medicine to maintain positive work culture.
Dr. Prabhakaran is director of hospital medicine transitions of care, Baystate Medical Center, Springfield, Mass., and assistant professor of medicine, University of Massachusetts, Worcester. Dr. Medarametla is medical director, hospital medicine, Baystate Medical Center, and assistant professor of medicine, University of Massachusetts.
References
1. Haerkens MH et al. Crew Resource Management in the ICU: The need for culture change. Ann Intensive Care. 2012 Aug 22;2:39.
2. Haerkens MH et al. Crew Resource Management in the trauma room: A prospective 3-year cohort study. Eur J Emerg Med. 2018 Aug;25(4):281-7.
3. Malcolm Gladwell. The ethnic theory of plane crashes. Outliers: The Story of Success. (Boston: Little, Brown and Company; 2008:177-223).
4. Helmreich RL et al. The evolution of Crew Resource Management training in commercial aviation. Int J Aviat Psychol. 1999;9(1):19-32.
5. Forsyth DR. The psychology of groups. In R. Biswas-Diener & E. Diener (eds), Noba textbook series: Psychology. Champaign, Ill: DEF publishers; 2017.
6. Crew Resource Management. Available at Aviation Knowledge. Accessed Dec. 20, 2017.
Parallels from the airline industry
Parallels from the airline industry
“Workplace culture” has a profound influence on the success or failure of a team in the modern-day work environment, where teamwork and interpersonal interactions have paramount importance. Crew resource management (CRM), a technique developed originally by the airline industry, has been used as a tool to improve safety and quality in ICUs, trauma rooms, and operating rooms.1,2 This article discusses the use of CRM in hospital medicine as a tool for training and maintaining a favorable workplace culture.
Origin and evolution of CRM
United Airlines instituted the airline industry’s first crew resource management for pilots in 1981, following the 1978 crash of United Flight 173 in Portland, Ore. CRM was created based on recommendations from the National Transportation Safety Board and from a NASA workshop held subsequently.3 CRM has since evolved through five generations, and is a required annual training for most major commercial airline companies around the world. It also has been adapted for personnel training by several modern international industries.4
From the airline industry to the hospital
The health care industry is similar to the airline industry in that there is absolutely no margin of error, and that workplace culture plays a very important role. The culture being referred to here is the sum total of values, beliefs, work ethics, work strategies, strengths, and weaknesses of a group of people, and how they interact as a group. In other words, it is the dynamics of a group.
According to Donelson R. Forsyth, a social and personality psychologist at the University of Richmond (Virginia), the two key determinants of successful teamwork are a “shared mental representation of the task,” which refers to an in-depth understanding of the team and the tasks they are attempting; and “group unity/cohesion,” which means that, generally, members of cohesive groups like each other and the group, and they also are united in their pursuit of collective, group-level goals.5
Understanding the culture of a hospitalist team
Analyzing group dynamics and actively managing them toward both the institutional and global goals of health care is critical for the success of an organization. This is the core of successfully managing any team in any industry.
Additionally, the rapidly changing health care climate and insurance payment systems requires hospital medicine groups to rapidly adapt to the constantly changing health care business environment. As a result, there are a couple of ways to evaluate the effectiveness of the team:
- Measure tangible outcomes. The outcomes have to be well defined, important and measurable. These could be cost of care, quality of care, engagement of the team etc. These tangible measures’ outcome over a period of time can be used as a measure of how effective the team is.
- Simply ask your team! It is very important to know what core values the team holds dear. The best way to get that information from the team is to find out the de facto leaders of the team. They should be involved in the decision-making process, thus making them valuable to the management as well as the team.
Culture shapes outcomes
We have used the analogy of a convex and concave lens to help understand this better. A well-developed and well-coordinated team is like convex lens. A lens’ ability to converge or diverge light rays depends on certain characteristics like the curvature of surfaces and refractory index. Likewise, the culture of a group determines its ability to transform all the demands of the collective workload toward a unified goal/outcome. If it is favorable, the group will work as one and success will happen automatically.
Unfortunately, the opposite of this, (the concave lens effect), is more commonplace, where the dynamics of a team prevent the goals being achieved, as there is discordance, poor coordination of ideas and values, and team members not liking each other.
Most teams would fall somewhere within this spectrum, spanning the most favorable convex lens–like group to the least favorable concave lens–like group.
Change team dynamics using CRM principles
The concept of using CRM principles in health care is not entirely new. Such agencies as the Joint Commission and the Agency for Healthcare Research and Quality recommend using principles of CRM to improve communications, and as an error-prevention tool in health care.6
This approach can be broken down into four important steps:
1. Recruit right. It is important to make sure that the new recruit is the right fit for the team and that the de facto leaders and a few other team members are involved in interviewing the candidates. Their assessment should be given due consideration in making the decision to give the new recruit the job.
Every program looks for aspects like clinical competence, interpersonal communication, teamwork, etc., in a candidate, but it is even more important to make sure the candidate has the tenets that would make him/her a part of that particular team.
2. Train well. The newly recruited providers should be given focused training and the seasoned providers should be given refresher training at regular intervals. Care should be taken in designing the training programs in such a way that the providers are trained in skills that they don’t always think about, things that aren’t readily obvious, and in skills that they never get trained in during medical school and residency.
Specifically, they should be trained in:
- Values. These should include the values of both the organization and the team.
- Safety. This should include all the safety protocols that are in place in the organization - where to get help, how to report unsafe events etc.
- Communication.
Within the group: Have a mentor for the new provider, and also develop a culture where he/she feels comfortable to reach out to anyone in the team for help.
With patients and families: This training should ideally be done in a simulated environment if possible.
With other groups in the hospital: Consultants, nurses, other ancillary staff. Give them an idea about the prevailing culture in the organization with regard to these groups, so that they know what to expect when dealing with them.
- Managing perceptions. How the providers are viewed in the hospital, and how to improve it or maintain it.
- Nurturing the good. Use positive reinforcements to solidify the positive aspects of group dynamics these individuals might possess.
- Weeding out the bad. Use training and feedback to alter the negative group dynamic aspects.
3. Intervene. This is necessary either to maintain the positive aspects of a team that is already high-functioning, or to transform a poorly functioning team into a well-coordinated team. This is where the principles of CRM are going to be most useful.
There are five generations of CRM, each with a different focus.6 Only the aspects relevant to hospital medicine training are mentioned here.
- Communication. Address the gaps in communication. It is important to include people who are trusted by the team in designing and executing these sessions.
- Leadership. The goal should be to encourage the team to take ownership of the program. This will make a tremendous change in the ability of a team to deliver and rise up to challenges. The organizational leadership has to be willing to elevate the leaders of the group to positions where they can meaningfully take part in managing the team and making decisions that are critical to the team.
- Burnout management. Providers getting disillusioned: having no work-life balance; not getting enough respect from management, as well as other groups of doctors/nurses/etc. in the hospital; they are subject to bad scheduling and poor pay – all of which can all lead to career-ending burnout. It is important to recognize this and mitigate the factors that cause burnout.
- Organizational culture. If the team feels valued and supported, they will, in turn, work hard toward success. Creative leadership and a willingness to accommodate what matters the most to the team is essential for achieving this.
- Simulated training. These can be done in simulation labs, or in-group sessions with the team, re-creating difficult scenarios or problems in which the whole team can come together and solve them.
- Error containment and management. The team needs to identify possible sources of error and contain them before errors happen. The group should get together if a serious event happens and brainstorm why it happened and take measures to prevent it.
4. Reevaluate. Team dynamics tend to change over time. It is important to constantly re-evaluate the team and make sure that the team’s culture remains favorable. There should be recurrent cycles of retraining and interventions to maintain the positive growth that has been attained, as depicted in the schematic below:
Conclusion
CRM is widely accepted as an effective tool in training individuals in many high performing industries. This article describes a framework in which the principles of CRM can be applied to hospital medicine to maintain positive work culture.
Dr. Prabhakaran is director of hospital medicine transitions of care, Baystate Medical Center, Springfield, Mass., and assistant professor of medicine, University of Massachusetts, Worcester. Dr. Medarametla is medical director, hospital medicine, Baystate Medical Center, and assistant professor of medicine, University of Massachusetts.
References
1. Haerkens MH et al. Crew Resource Management in the ICU: The need for culture change. Ann Intensive Care. 2012 Aug 22;2:39.
2. Haerkens MH et al. Crew Resource Management in the trauma room: A prospective 3-year cohort study. Eur J Emerg Med. 2018 Aug;25(4):281-7.
3. Malcolm Gladwell. The ethnic theory of plane crashes. Outliers: The Story of Success. (Boston: Little, Brown and Company; 2008:177-223).
4. Helmreich RL et al. The evolution of Crew Resource Management training in commercial aviation. Int J Aviat Psychol. 1999;9(1):19-32.
5. Forsyth DR. The psychology of groups. In R. Biswas-Diener & E. Diener (eds), Noba textbook series: Psychology. Champaign, Ill: DEF publishers; 2017.
6. Crew Resource Management. Available at Aviation Knowledge. Accessed Dec. 20, 2017.
“Workplace culture” has a profound influence on the success or failure of a team in the modern-day work environment, where teamwork and interpersonal interactions have paramount importance. Crew resource management (CRM), a technique developed originally by the airline industry, has been used as a tool to improve safety and quality in ICUs, trauma rooms, and operating rooms.1,2 This article discusses the use of CRM in hospital medicine as a tool for training and maintaining a favorable workplace culture.
Origin and evolution of CRM
United Airlines instituted the airline industry’s first crew resource management for pilots in 1981, following the 1978 crash of United Flight 173 in Portland, Ore. CRM was created based on recommendations from the National Transportation Safety Board and from a NASA workshop held subsequently.3 CRM has since evolved through five generations, and is a required annual training for most major commercial airline companies around the world. It also has been adapted for personnel training by several modern international industries.4
From the airline industry to the hospital
The health care industry is similar to the airline industry in that there is absolutely no margin of error, and that workplace culture plays a very important role. The culture being referred to here is the sum total of values, beliefs, work ethics, work strategies, strengths, and weaknesses of a group of people, and how they interact as a group. In other words, it is the dynamics of a group.
According to Donelson R. Forsyth, a social and personality psychologist at the University of Richmond (Virginia), the two key determinants of successful teamwork are a “shared mental representation of the task,” which refers to an in-depth understanding of the team and the tasks they are attempting; and “group unity/cohesion,” which means that, generally, members of cohesive groups like each other and the group, and they also are united in their pursuit of collective, group-level goals.5
Understanding the culture of a hospitalist team
Analyzing group dynamics and actively managing them toward both the institutional and global goals of health care is critical for the success of an organization. This is the core of successfully managing any team in any industry.
Additionally, the rapidly changing health care climate and insurance payment systems requires hospital medicine groups to rapidly adapt to the constantly changing health care business environment. As a result, there are a couple of ways to evaluate the effectiveness of the team:
- Measure tangible outcomes. The outcomes have to be well defined, important and measurable. These could be cost of care, quality of care, engagement of the team etc. These tangible measures’ outcome over a period of time can be used as a measure of how effective the team is.
- Simply ask your team! It is very important to know what core values the team holds dear. The best way to get that information from the team is to find out the de facto leaders of the team. They should be involved in the decision-making process, thus making them valuable to the management as well as the team.
Culture shapes outcomes
We have used the analogy of a convex and concave lens to help understand this better. A well-developed and well-coordinated team is like convex lens. A lens’ ability to converge or diverge light rays depends on certain characteristics like the curvature of surfaces and refractory index. Likewise, the culture of a group determines its ability to transform all the demands of the collective workload toward a unified goal/outcome. If it is favorable, the group will work as one and success will happen automatically.
Unfortunately, the opposite of this, (the concave lens effect), is more commonplace, where the dynamics of a team prevent the goals being achieved, as there is discordance, poor coordination of ideas and values, and team members not liking each other.
Most teams would fall somewhere within this spectrum, spanning the most favorable convex lens–like group to the least favorable concave lens–like group.
Change team dynamics using CRM principles
The concept of using CRM principles in health care is not entirely new. Such agencies as the Joint Commission and the Agency for Healthcare Research and Quality recommend using principles of CRM to improve communications, and as an error-prevention tool in health care.6
This approach can be broken down into four important steps:
1. Recruit right. It is important to make sure that the new recruit is the right fit for the team and that the de facto leaders and a few other team members are involved in interviewing the candidates. Their assessment should be given due consideration in making the decision to give the new recruit the job.
Every program looks for aspects like clinical competence, interpersonal communication, teamwork, etc., in a candidate, but it is even more important to make sure the candidate has the tenets that would make him/her a part of that particular team.
2. Train well. The newly recruited providers should be given focused training and the seasoned providers should be given refresher training at regular intervals. Care should be taken in designing the training programs in such a way that the providers are trained in skills that they don’t always think about, things that aren’t readily obvious, and in skills that they never get trained in during medical school and residency.
Specifically, they should be trained in:
- Values. These should include the values of both the organization and the team.
- Safety. This should include all the safety protocols that are in place in the organization - where to get help, how to report unsafe events etc.
- Communication.
Within the group: Have a mentor for the new provider, and also develop a culture where he/she feels comfortable to reach out to anyone in the team for help.
With patients and families: This training should ideally be done in a simulated environment if possible.
With other groups in the hospital: Consultants, nurses, other ancillary staff. Give them an idea about the prevailing culture in the organization with regard to these groups, so that they know what to expect when dealing with them.
- Managing perceptions. How the providers are viewed in the hospital, and how to improve it or maintain it.
- Nurturing the good. Use positive reinforcements to solidify the positive aspects of group dynamics these individuals might possess.
- Weeding out the bad. Use training and feedback to alter the negative group dynamic aspects.
3. Intervene. This is necessary either to maintain the positive aspects of a team that is already high-functioning, or to transform a poorly functioning team into a well-coordinated team. This is where the principles of CRM are going to be most useful.
There are five generations of CRM, each with a different focus.6 Only the aspects relevant to hospital medicine training are mentioned here.
- Communication. Address the gaps in communication. It is important to include people who are trusted by the team in designing and executing these sessions.
- Leadership. The goal should be to encourage the team to take ownership of the program. This will make a tremendous change in the ability of a team to deliver and rise up to challenges. The organizational leadership has to be willing to elevate the leaders of the group to positions where they can meaningfully take part in managing the team and making decisions that are critical to the team.
- Burnout management. Providers getting disillusioned: having no work-life balance; not getting enough respect from management, as well as other groups of doctors/nurses/etc. in the hospital; they are subject to bad scheduling and poor pay – all of which can all lead to career-ending burnout. It is important to recognize this and mitigate the factors that cause burnout.
- Organizational culture. If the team feels valued and supported, they will, in turn, work hard toward success. Creative leadership and a willingness to accommodate what matters the most to the team is essential for achieving this.
- Simulated training. These can be done in simulation labs, or in-group sessions with the team, re-creating difficult scenarios or problems in which the whole team can come together and solve them.
- Error containment and management. The team needs to identify possible sources of error and contain them before errors happen. The group should get together if a serious event happens and brainstorm why it happened and take measures to prevent it.
4. Reevaluate. Team dynamics tend to change over time. It is important to constantly re-evaluate the team and make sure that the team’s culture remains favorable. There should be recurrent cycles of retraining and interventions to maintain the positive growth that has been attained, as depicted in the schematic below:
Conclusion
CRM is widely accepted as an effective tool in training individuals in many high performing industries. This article describes a framework in which the principles of CRM can be applied to hospital medicine to maintain positive work culture.
Dr. Prabhakaran is director of hospital medicine transitions of care, Baystate Medical Center, Springfield, Mass., and assistant professor of medicine, University of Massachusetts, Worcester. Dr. Medarametla is medical director, hospital medicine, Baystate Medical Center, and assistant professor of medicine, University of Massachusetts.
References
1. Haerkens MH et al. Crew Resource Management in the ICU: The need for culture change. Ann Intensive Care. 2012 Aug 22;2:39.
2. Haerkens MH et al. Crew Resource Management in the trauma room: A prospective 3-year cohort study. Eur J Emerg Med. 2018 Aug;25(4):281-7.
3. Malcolm Gladwell. The ethnic theory of plane crashes. Outliers: The Story of Success. (Boston: Little, Brown and Company; 2008:177-223).
4. Helmreich RL et al. The evolution of Crew Resource Management training in commercial aviation. Int J Aviat Psychol. 1999;9(1):19-32.
5. Forsyth DR. The psychology of groups. In R. Biswas-Diener & E. Diener (eds), Noba textbook series: Psychology. Champaign, Ill: DEF publishers; 2017.
6. Crew Resource Management. Available at Aviation Knowledge. Accessed Dec. 20, 2017.
Immigration reforms: Repercussions for hospitalists and the health care industry
International medical graduates (IMGs) have been playing a crucial role in clinician staffing needs for U.S. hospitals, especially in hospital medicine and internal medicine. According to a study, IMGs comprise 25% of the total U.S. physician workforce and 36% of internists.1,2 According to data from the 2008 Today’s Hospitalist Compensation & Career Survey, 32% of practicing hospitalists are IMGs.3
Many IMGs come to work in the U.S. via one of three paths. Just like all roads lead to Rome, all visas lead to a permanent residency pathway, eventually based on the country of origin and number of years waiting. The first path is a green card – cases where IMGs were on a visa and within a certain amount of time they received a green card. The second path is J-1 visa waivers for physicians who trained in the U.S. under a J-1 Visa. Typically, physicians on J-1 Visa waivers need to provide their services for a minimum of 3 years working in underserved areas – where there’s a shortage of health professionals – before they can apply for permanent residency.
The third and most popular path is the H-1B visa, which hospitalists traditionally use as a springboard to apply for permanent residency. Studies have shown that IMGs are more likely to practice medicine in rural and underserved areas. In many instances, physicians end up working in these areas for long periods of time.4
The Department of Homeland Security has considered creating another visa pathway for the technology industry, whereby an alien graduating from a U.S. university with an advanced degree in a STEM (Science, Technology, Engineering, and Math) course of study would receive a new visa and pathway to permanent residency. We believe hospitalists and other physicians should also have an expedited pathway to permanent residency. This step benefits both the U.S. health care system and hospitalists in many ways. It increases hospitalists’ portability and flexibility with schedules. With a traditional H-1B visa, hospitalists are bound to work with the one hospital/system that sponsors the H-1B, and would not be able to work at any other hospital without another extension/addendum to current visa status, even in cases where a physician had time off and would like to offer services at another facility. It is a well-known fact that hospitalist teams are understaffed and try to bring on per-diem staff to fill holes in schedules. The majority of hospitalists are working week-on/week-off schedules, and with an expedited pathway to a green card they would be able to work in different hospitals. They would also be able to move to remote places, or “doctor deserts,” and offer their services, helping to ensure the quality and safety of patient care to which all Americans are entitled.
In 2016 alone, around 1,500 H-1B visas were filed for hospitalist physicians.7 Each hospitalist has an average of 15 patient encounters per day, and for 1,500 physicians that amounts to about 4 million patient encounters annually.8 These data account for only new 2016 visa-holding physicians, and do not account for already approved or renewed visas. It would be very challenging to count the number of patient encounters by hospitalists who are on a visa, but 1 billion patient encounters is not overestimating. Recent studies show that quality of care provided by IMGs is not inferior to that of U.S. medical graduates. The study showed that patients cared for by IMGs have lesser mortality, compared with those cared by U.S. medical graduates.9
In this era of hospital medicine, hospitalists are focusing not only on clinical aspects of patient care but also on efficacy, quality of care, and patient safety and satisfaction, and they are working with the Centers for Medicare & Medicaid Services to develop cost-cutting programs to save billions of dollars in health care expenses. This is the primary reason a majority of hospitals are focused on developing a hospitalist track, and encouraging hospitalists to pursue leadership roles in managing hospitals effectively.
The U.S. health care system is starved for hospitalists and primary care physicians, and IMGs will continue to play a pivotal role. Yet IMGs must deal with shifting trends in immigration policy, and in some recent instances immigrant physicians have been asked to leave the U.S. because of immigration reforms.10,11 We would like the Society of Hospital Medicine to take a stand on behalf of IMG hospitalists and ask the U.S. Department of Labor and Homeland Security for an expedited permanent residency pathway for IMG hospitalists. We are certain that our request will get a fair hearing, as the former U.S. surgeon general was a hospitalist and, indeed, an immigrant.
Dr. Medarametla is medical director, Intermediate Care Unit, Baystate Medical Center, Springfield, Mass., and assistant professor of medicine, University of Massachusetts Medical School. Dr. Pamerla is a hospitalist at Wilson Medical Center, Wilson, N.C.
References
1. Educational Commission for Foreign Medical Graduates; ECFMG 2015 Annual Report. April 2016 http://www.ecfmg.org/resources/ECFMG-2015-annual-report.pdf.
2. Pinsky WW. The Importance of International Medical Graduates in the United States. Ann Intern Med. 2017. doi: 10.7326/M17-0505.
3. Hart LG, Skillman SM, Fordyce M, et al. International medical graduate physicians in the United States: changes since 1981. Health Aff. 2007 July/August;26(4):1159-69.
4. Goodfellow A1, Ulloa JG, Dowling PT, et al. Predictors of Primary Care Physician Practice Location in Underserved Urban or Rural Areas in the United States: A Systematic Literature Review. Acad Med. 2016 Sep;91(9):1313-21.
5. https://www.uscis.gov/working-united-states/temporary-workers/h-1b-specialty-occupations-and-fashion-models/h-1b-fiscal-year-fy-2018-cap-season#count
6. https://www.graphiq.com/vlp/bCIqXCpVqF7
7. http://www.myvisajobs.com/Reports/2017-H1B-Visa-Category.aspx?T=JT&P=2
8. Steven M Harris: http://www.the-hospitalist.org/hospitalist/article/125455/appropriate-patient-census-hospital-medicines-holy-grail
9. Tsugawa Y, Jena AB, Orav EJ, Jha AK. Quality of care delivered by general internists in US hospitals who graduated from foreign versus US medical schools: observational study. BMJ. 2017;356:j273.
10. https://www.propublica.org/article/cleveland-clinic-doctor-forced-to-leave-country-after-trump-order
11. http://www.houstonchronicle.com/news/houston-texas/houston/article/Houston-immigrant-doctors-given-24-hours-to-leave-11040259.php
International medical graduates (IMGs) have been playing a crucial role in clinician staffing needs for U.S. hospitals, especially in hospital medicine and internal medicine. According to a study, IMGs comprise 25% of the total U.S. physician workforce and 36% of internists.1,2 According to data from the 2008 Today’s Hospitalist Compensation & Career Survey, 32% of practicing hospitalists are IMGs.3
Many IMGs come to work in the U.S. via one of three paths. Just like all roads lead to Rome, all visas lead to a permanent residency pathway, eventually based on the country of origin and number of years waiting. The first path is a green card – cases where IMGs were on a visa and within a certain amount of time they received a green card. The second path is J-1 visa waivers for physicians who trained in the U.S. under a J-1 Visa. Typically, physicians on J-1 Visa waivers need to provide their services for a minimum of 3 years working in underserved areas – where there’s a shortage of health professionals – before they can apply for permanent residency.
The third and most popular path is the H-1B visa, which hospitalists traditionally use as a springboard to apply for permanent residency. Studies have shown that IMGs are more likely to practice medicine in rural and underserved areas. In many instances, physicians end up working in these areas for long periods of time.4
The Department of Homeland Security has considered creating another visa pathway for the technology industry, whereby an alien graduating from a U.S. university with an advanced degree in a STEM (Science, Technology, Engineering, and Math) course of study would receive a new visa and pathway to permanent residency. We believe hospitalists and other physicians should also have an expedited pathway to permanent residency. This step benefits both the U.S. health care system and hospitalists in many ways. It increases hospitalists’ portability and flexibility with schedules. With a traditional H-1B visa, hospitalists are bound to work with the one hospital/system that sponsors the H-1B, and would not be able to work at any other hospital without another extension/addendum to current visa status, even in cases where a physician had time off and would like to offer services at another facility. It is a well-known fact that hospitalist teams are understaffed and try to bring on per-diem staff to fill holes in schedules. The majority of hospitalists are working week-on/week-off schedules, and with an expedited pathway to a green card they would be able to work in different hospitals. They would also be able to move to remote places, or “doctor deserts,” and offer their services, helping to ensure the quality and safety of patient care to which all Americans are entitled.
In 2016 alone, around 1,500 H-1B visas were filed for hospitalist physicians.7 Each hospitalist has an average of 15 patient encounters per day, and for 1,500 physicians that amounts to about 4 million patient encounters annually.8 These data account for only new 2016 visa-holding physicians, and do not account for already approved or renewed visas. It would be very challenging to count the number of patient encounters by hospitalists who are on a visa, but 1 billion patient encounters is not overestimating. Recent studies show that quality of care provided by IMGs is not inferior to that of U.S. medical graduates. The study showed that patients cared for by IMGs have lesser mortality, compared with those cared by U.S. medical graduates.9
In this era of hospital medicine, hospitalists are focusing not only on clinical aspects of patient care but also on efficacy, quality of care, and patient safety and satisfaction, and they are working with the Centers for Medicare & Medicaid Services to develop cost-cutting programs to save billions of dollars in health care expenses. This is the primary reason a majority of hospitals are focused on developing a hospitalist track, and encouraging hospitalists to pursue leadership roles in managing hospitals effectively.
The U.S. health care system is starved for hospitalists and primary care physicians, and IMGs will continue to play a pivotal role. Yet IMGs must deal with shifting trends in immigration policy, and in some recent instances immigrant physicians have been asked to leave the U.S. because of immigration reforms.10,11 We would like the Society of Hospital Medicine to take a stand on behalf of IMG hospitalists and ask the U.S. Department of Labor and Homeland Security for an expedited permanent residency pathway for IMG hospitalists. We are certain that our request will get a fair hearing, as the former U.S. surgeon general was a hospitalist and, indeed, an immigrant.
Dr. Medarametla is medical director, Intermediate Care Unit, Baystate Medical Center, Springfield, Mass., and assistant professor of medicine, University of Massachusetts Medical School. Dr. Pamerla is a hospitalist at Wilson Medical Center, Wilson, N.C.
References
1. Educational Commission for Foreign Medical Graduates; ECFMG 2015 Annual Report. April 2016 http://www.ecfmg.org/resources/ECFMG-2015-annual-report.pdf.
2. Pinsky WW. The Importance of International Medical Graduates in the United States. Ann Intern Med. 2017. doi: 10.7326/M17-0505.
3. Hart LG, Skillman SM, Fordyce M, et al. International medical graduate physicians in the United States: changes since 1981. Health Aff. 2007 July/August;26(4):1159-69.
4. Goodfellow A1, Ulloa JG, Dowling PT, et al. Predictors of Primary Care Physician Practice Location in Underserved Urban or Rural Areas in the United States: A Systematic Literature Review. Acad Med. 2016 Sep;91(9):1313-21.
5. https://www.uscis.gov/working-united-states/temporary-workers/h-1b-specialty-occupations-and-fashion-models/h-1b-fiscal-year-fy-2018-cap-season#count
6. https://www.graphiq.com/vlp/bCIqXCpVqF7
7. http://www.myvisajobs.com/Reports/2017-H1B-Visa-Category.aspx?T=JT&P=2
8. Steven M Harris: http://www.the-hospitalist.org/hospitalist/article/125455/appropriate-patient-census-hospital-medicines-holy-grail
9. Tsugawa Y, Jena AB, Orav EJ, Jha AK. Quality of care delivered by general internists in US hospitals who graduated from foreign versus US medical schools: observational study. BMJ. 2017;356:j273.
10. https://www.propublica.org/article/cleveland-clinic-doctor-forced-to-leave-country-after-trump-order
11. http://www.houstonchronicle.com/news/houston-texas/houston/article/Houston-immigrant-doctors-given-24-hours-to-leave-11040259.php
International medical graduates (IMGs) have been playing a crucial role in clinician staffing needs for U.S. hospitals, especially in hospital medicine and internal medicine. According to a study, IMGs comprise 25% of the total U.S. physician workforce and 36% of internists.1,2 According to data from the 2008 Today’s Hospitalist Compensation & Career Survey, 32% of practicing hospitalists are IMGs.3
Many IMGs come to work in the U.S. via one of three paths. Just like all roads lead to Rome, all visas lead to a permanent residency pathway, eventually based on the country of origin and number of years waiting. The first path is a green card – cases where IMGs were on a visa and within a certain amount of time they received a green card. The second path is J-1 visa waivers for physicians who trained in the U.S. under a J-1 Visa. Typically, physicians on J-1 Visa waivers need to provide their services for a minimum of 3 years working in underserved areas – where there’s a shortage of health professionals – before they can apply for permanent residency.
The third and most popular path is the H-1B visa, which hospitalists traditionally use as a springboard to apply for permanent residency. Studies have shown that IMGs are more likely to practice medicine in rural and underserved areas. In many instances, physicians end up working in these areas for long periods of time.4
The Department of Homeland Security has considered creating another visa pathway for the technology industry, whereby an alien graduating from a U.S. university with an advanced degree in a STEM (Science, Technology, Engineering, and Math) course of study would receive a new visa and pathway to permanent residency. We believe hospitalists and other physicians should also have an expedited pathway to permanent residency. This step benefits both the U.S. health care system and hospitalists in many ways. It increases hospitalists’ portability and flexibility with schedules. With a traditional H-1B visa, hospitalists are bound to work with the one hospital/system that sponsors the H-1B, and would not be able to work at any other hospital without another extension/addendum to current visa status, even in cases where a physician had time off and would like to offer services at another facility. It is a well-known fact that hospitalist teams are understaffed and try to bring on per-diem staff to fill holes in schedules. The majority of hospitalists are working week-on/week-off schedules, and with an expedited pathway to a green card they would be able to work in different hospitals. They would also be able to move to remote places, or “doctor deserts,” and offer their services, helping to ensure the quality and safety of patient care to which all Americans are entitled.
In 2016 alone, around 1,500 H-1B visas were filed for hospitalist physicians.7 Each hospitalist has an average of 15 patient encounters per day, and for 1,500 physicians that amounts to about 4 million patient encounters annually.8 These data account for only new 2016 visa-holding physicians, and do not account for already approved or renewed visas. It would be very challenging to count the number of patient encounters by hospitalists who are on a visa, but 1 billion patient encounters is not overestimating. Recent studies show that quality of care provided by IMGs is not inferior to that of U.S. medical graduates. The study showed that patients cared for by IMGs have lesser mortality, compared with those cared by U.S. medical graduates.9
In this era of hospital medicine, hospitalists are focusing not only on clinical aspects of patient care but also on efficacy, quality of care, and patient safety and satisfaction, and they are working with the Centers for Medicare & Medicaid Services to develop cost-cutting programs to save billions of dollars in health care expenses. This is the primary reason a majority of hospitals are focused on developing a hospitalist track, and encouraging hospitalists to pursue leadership roles in managing hospitals effectively.
The U.S. health care system is starved for hospitalists and primary care physicians, and IMGs will continue to play a pivotal role. Yet IMGs must deal with shifting trends in immigration policy, and in some recent instances immigrant physicians have been asked to leave the U.S. because of immigration reforms.10,11 We would like the Society of Hospital Medicine to take a stand on behalf of IMG hospitalists and ask the U.S. Department of Labor and Homeland Security for an expedited permanent residency pathway for IMG hospitalists. We are certain that our request will get a fair hearing, as the former U.S. surgeon general was a hospitalist and, indeed, an immigrant.
Dr. Medarametla is medical director, Intermediate Care Unit, Baystate Medical Center, Springfield, Mass., and assistant professor of medicine, University of Massachusetts Medical School. Dr. Pamerla is a hospitalist at Wilson Medical Center, Wilson, N.C.
References
1. Educational Commission for Foreign Medical Graduates; ECFMG 2015 Annual Report. April 2016 http://www.ecfmg.org/resources/ECFMG-2015-annual-report.pdf.
2. Pinsky WW. The Importance of International Medical Graduates in the United States. Ann Intern Med. 2017. doi: 10.7326/M17-0505.
3. Hart LG, Skillman SM, Fordyce M, et al. International medical graduate physicians in the United States: changes since 1981. Health Aff. 2007 July/August;26(4):1159-69.
4. Goodfellow A1, Ulloa JG, Dowling PT, et al. Predictors of Primary Care Physician Practice Location in Underserved Urban or Rural Areas in the United States: A Systematic Literature Review. Acad Med. 2016 Sep;91(9):1313-21.
5. https://www.uscis.gov/working-united-states/temporary-workers/h-1b-specialty-occupations-and-fashion-models/h-1b-fiscal-year-fy-2018-cap-season#count
6. https://www.graphiq.com/vlp/bCIqXCpVqF7
7. http://www.myvisajobs.com/Reports/2017-H1B-Visa-Category.aspx?T=JT&P=2
8. Steven M Harris: http://www.the-hospitalist.org/hospitalist/article/125455/appropriate-patient-census-hospital-medicines-holy-grail
9. Tsugawa Y, Jena AB, Orav EJ, Jha AK. Quality of care delivered by general internists in US hospitals who graduated from foreign versus US medical schools: observational study. BMJ. 2017;356:j273.
10. https://www.propublica.org/article/cleveland-clinic-doctor-forced-to-leave-country-after-trump-order
11. http://www.houstonchronicle.com/news/houston-texas/houston/article/Houston-immigrant-doctors-given-24-hours-to-leave-11040259.php