User login
What Are the Strategies for Secondary Stroke Prevention after Transient Ischemic Attack?
Case
Mr. G is an 80-year-old man with a pacemaker, peripheral artery disease, atrial fibrillation (AF) on warfarin, and tachy-brady syndrome. He presented after experiencing episodes in which he was unable to speak and had weakness on his right side. He had a normal neurological exam upon arrival to the ED, and his blood pressure was 160/80 mm Hg.
Overview
Transient ischemic attacks (TIAs) are brief interruptions in brain perfusion that do not result in permanent neurologic damage. Up to half a million TIAs occur each year in the U.S., and they account for one third of acute cerebrovascular disease.1 While the term suggests that TIAs are benign, they are in fact an important warning sign of impending stroke and are essentially analogous to unstable angina. Some 10% of TIAs convert to full strokes within 90 days, but growing evidence suggests appropriate interventions can decrease this risk to 3%.2
Unfortunately, the symptoms of TIA have usually resolved by the time patients arrive at the hospital, which makes them challenging to diagnose. This article provides a summary of how to diagnose TIA accurately, using a focused history informed by cerebrovascular localization; how to triage, evaluate, and risk stratify patients; and how to implement preventative strategies.
Review of the Data
Classically, TIAs are defined as lasting less than 24 hours; however, 24 hours is an arbitrary number, and most TIAs last less than one hour.1 Furthermore, this definition has evolved with advances in neuroimaging that reveal that up to 50% of classically defined TIAs have evidence of infarct on MRI.1 There is no absolute temporal cut-off after which infarct is always seen on MRI, but longer duration of symptoms correlates with a higher likelihood of infarct. To reconcile these observations, a recently proposed definition stipulates that a true TIA lasts no more than one hour and does not show evidence of infarct on MRI.3
The causes of TIA are identical to those for ischemic stroke. Cerebral ischemia can result from an embolus, arterial thrombosis, or hypoperfusion due to arterial stenosis. Emboli can be cardiac, most commonly due to AF, or non-cardiac, stemming from a ruptured atherosclerotic plaque in the aortic arch, the carotid or vertebral artery, or an intracranial vessel. Atherosclerotic disease in the carotid arteries or intracranial vessels can also lead to thrombosis and occlusion or flow-related TIAs as a result of severe stenosis.
Risk factors for TIA mirror those for heart disease. Non-modifiable risk factors include older age, black race, male sex, and family history of stroke. Modifiable factors include hypertension, hyperlipidemia, tobacco smoking, diabetes, and AF.4
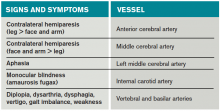
Most of the time, patients’ symptoms will have resolved by the time they are evaluated by a physician. Therefore, the diagnosis of TIA relies almost exclusively on the patient history. Eliciting a good history helps physicians determine whether the episode of transient neurologic dysfunction was caused by cerebral ischemia, as opposed to another mechanism, such as migraine or seizure. This calls for a basic understanding of cerebrovascular anatomy (see Table 1).
Types of Ischemia
Anterior cerebral artery ischemia causes contralateral leg weakness because it supplies the medial frontal and parietal lobes, where the legs in the sensorimotor homunculus are represented. Middle cerebral artery (MCA) ischemia causes contralateral face and arm weakness out of proportion to leg weakness. Ischemia in Broca’s area of the brain, which is supplied by the left MCA, may also cause expressive aphasia. Transient monocular blindness is a TIA of the retina due to atheroemboli originating from the internal carotid artery. Vertebrobasilar TIA is less common than anterior circulation TIA and manifests with brainstem symptoms that include diplopia, dysarthria, dysphagia, vertigo, gait imbalance, and weakness. In general, language and motor symptoms are more specific for cerebral ischemia and therefore more worrisome for TIA than sensory symptoms.5
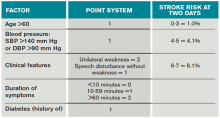
Once a clinical diagnosis of TIA is made, an ABCD2 score (age, blood pressure, clinical features, duration of TIA, presence of diabetes) can be used to predict the short-term risk of subsequent stroke (see Table 2).6,7 A general rule of thumb is to admit patients who present within 72 hours of the event and have an ABCD2 score of three or higher for observation, work-up, and initiation of secondary prevention.1
Although only a small percentage of patients with TIA will have a stroke during the period of observation in the hospital, this approach may be cost effective based on the assumption that hospitalized patients are more likely to receive intravenous tissue plasminogen activator.8 The decision should also be guided by clinical judgment. It is reasonable to admit a patient whose diagnostic workup cannot be rapidly completed.1
The workup for TIA includes routine labs, EKG with cardiac monitoring, and brain imaging. Labs are useful to evaluate for other mimics of TIA such as hyponatremia and glucose abnormalities. In addition, risk factors such as hyperlipidemia and diabetes should be evaluated with fasting lipid panel and blood glucose. The purpose of EKG and telemetry is to identify MI and capture paroxysmal AF. The goal of imaging is to ascertain the presence of vascular disease and to exclude a non-ischemic etiology. While less likely to cause transient neurologic symptoms, a hemorrhagic event must be ruled out, as it would trigger a different management pathway.
Imaging for TIA
There are two primary modes of brain imaging: computed tomography (CT) and MRI. Most patients who are suspected to have had a TIA undergo CT scan, and an infarct is seen about 20% of the time.1 The presence of an infarct usually correlates with the duration of symptoms and has prognostic value. In one study, a new infarct was associated with four times higher risk of stroke in the subsequent 90 days.9 Diffusion-weighted imaging, an MR-based technique, is the preferred modality when it is available because of its higher sensitivity and specificity for identifying acute lesions.1 In an international and multicenter study, incorporating imaging data increased the discriminatory power of stroke prediction.10
Extracranial imaging is mandatory to rule out carotid stenosis as a potential etiology of TIA. The least invasive modality is ultrasound, which can detect carotid stenosis with a sensitivity and specificity approaching 80%.1 While both the intra- and extracranial vasculature can be concurrently assessed using MR- or CT-angiography (CTA), this is not usually necessary in the acute setting, because only detecting carotid stenosis will result in a management change.1
Carotid endarterectomy is standard for symptomatic patients with greater than 70% stenosis and is a consideration for symptomatic patients with greater than 50% stenosis if it is the most probable explanation for the ischemic event.11 Despite a comprehensive workup, about 50% of TIA cases remain cryptogenic.12 In some of these patients, AF can be detected using extended ambulatory cardiac monitoring.12
The goal of admitting high-risk patients is to expedite workup and initiate therapy. Two studies have shown that immediate initiation of preventative treatment significantly reduces the risk of stroke by as much as 80%.13,14 Unless there is a specific indication for anticoagulation, all TIA patients should be started on an antiplatelet agent such as aspirin or clopidogrel. A large randomized trial conducted in China and published in 2013 demonstrated that dual antiplatelet therapy with aspirin and clopidogrel for 21 days, followed by clopidogrel monotherapy, reduced the risk of stroke compared to aspirin monotherapy. An international multicenter trial designed to test the efficacy of short-term dual antiplatelet therapy is ongoing, and if the benefit of this approach is confirmed, this will likely become the standard of care. Evidence-based indications for anticoagulation after TIA are restricted to AF and mural thrombus in the setting of recent MI. Patients with implanted mechanical devices, including left ventricular assist devices and metal heart valves, should also receive anticoagulation.15
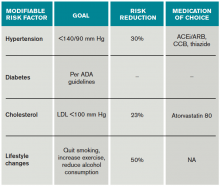
Risk factors should also be targeted in every case. Hypertension should be treated with a goal of lower than 140/90 mm Hg (or 130/80 mm Hg in diabetics and those with renal disease). Studies have shown that patients who are discharged with a blood pressure lower than 140/90 mm Hg are more likely to maintain this blood pressure at one-year follow-up.16 The choice of medication is less well studied, but drugs that act on the renin-angiotensin-aldosterone system and thiazides are generally preferred.15 Treatment with a statin is recommended after cerebrovascular ischemic events, with a goal LDL under 100. This reduces risk of secondary stroke by about 20%.17
At discharge, it is also important to counsel patients on their role in preventing strokes. As with many diseases, making lifestyle changes is key to stroke prevention. Encourage smoking cessation and an increase in physical activity, and discourage heavy alcohol use. The association between smoking and the risk for first stroke is well established. Moderate to high-intensity exercise can reduce secondary stroke risk by as much as 50%18 (see Table 3). While light alcohol consumption can be protective against strokes, heavy use is strongly discouraged. Emerging data suggest obstructive sleep apnea (OSA) may be another modifiable risk factor for stroke and TIA, so screening for potential OSA and referral may be needed.15
Back to the Case
When Mr. G arrived at the ED, his symptoms had resolved. Based on the history of expressive aphasia and right-sided weakness, he most likely had a TIA in the left MCA territory. Hemorrhage was ruled out with a non-contrast head CT. His pacemaker precluded obtaining an MRI. CTA revealed diffuse atherosclerotic disease without evidence of carotid stenosis. His ABCD2 score was six given his age, blood pressure, weakness, and symptom duration, and he was admitted for an expedited workup. His sodium and glucose were within normal limits. His hemoglobin A1c was 6.5%, his LDL was 120, and his international normalized ratio (INR) was therapeutic at 2.1. His TIA may have been due to AF, despite a therapeutic INR, because warfarin does not fully eliminate the stroke risk. It might also have been caused by intracranial atherosclerosis.
Two days later, the patient was discharged on atorvastatin at 80 mg, and his lisinopril was increased for blood pressure control. For his age group, A1c of 6.5% was acceptable, and he was not initiated on glycemic control.
Bottom Line
TIAs are diagnosed based on patient history. Urgent initiation of secondary prevention is important to reduce the short-term risk of stroke and should be implemented by the time of discharge from the hospital.
Dr. Zeng is a hospitalist in the department of internal medicine at Vanderbilt University Medical Center in Nashville, and Dr. Douglas is associate professor in the department of neurology at the University of California at San Francisco.
References
- Easton JD, Saver JL, Albers GW, et al. Definition and evaluation of transient ischemic attack: a scientific statement for healthcare professionals from the American Heart Association/American Stroke Association Stroke Council; Council on Cardiovascular Surgery and Anesthesia; Council on Cardiovascular Radiology and Intervention; Council on Cardiovascular Nursing; and the Interdisciplinary Council on Peripheral Vascular Disease. The American Academy of Neurology affirms the value of this statement as an educational tool for neurologists. Stroke. 2009;40(6):2276-2293.
- Sundararajan V, Thrift AG, Phan TG, Choi PM, Clissold B, Srikanth VK. Trends over time in the risk of stroke after an incident transient ischemic attack. Stroke. 2014;45(11):3214-3218.
- Albers GW, Caplan LR, Easton JD, et al. Transient ischemic attack–proposal for a new definition. N Engl J Med. 2002;347(21):1713-1716.
- Grysiewicz RA, Thomas K, Pandey DK. Epidemiology of ischemic and hemorrhagic stroke: incidence, prevalence, mortality, and risk factors. Neurol Clin. 2008;26(4):871-895, vii.
- Johnston SC, Sidney S, Bernstein AL, Gress DR. A comparison of risk factors for recurrent TIA and stroke in patients diagnosed with TIA. Neurology. 2003;60(2):280-285.
- Tsivgoulis G, Stamboulis E, Sharma VK, et al. Multicenter external validation of the ABCD2 score in triaging TIA patients. Neurology. 2010;74(17):1351-1357.
- Johnston SC, Rothwell PM, Nguyen-Huynh MN, et al. Validation and refinement of scores to predict very early stroke risk after transient ischaemic attack. Lancet. 2007;369(9558):283-292.
- Nguyen-Huynh MN, Johnston SC. Is hospitalization after TIA cost-effective on the basis of treatment with tPA? Neurology. 2005;65(11):1799-1801.
- Douglas VC, Johnston CM, Elkins J, Sidney S, Gress DR, Johnston SC. Head computed tomography findings predict short-term stroke risk after transient ischemic attack. Stroke. 2003;34(12):2894-2898.
- Giles MF, Albers GW, Amarenco P, et al. Addition of brain infarction to the ABCD2 Score (ABCD2I): a collaborative analysis of unpublished data on 4574 patients. Stroke. 2010;41(9):1907-1913.
- Lanzino G, Rabinstein AA, Brown RD Jr. Treatment of carotid artery stenosis: medical therapy, surgery, or stenting? Mayo Clin Proc. 2009;84(4):362-387; quiz 367-368.
- Gladstone DJ, Spring M, Dorian P, et al. Atrial fibrillation in patients with cryptogenic stroke. N Engl J Med. 2014;370(26):2467-2477.
- Lavallée PC, Meseguer E, Abboud H, et al. A transient ischaemic attack clinic with round-the-clock access (SOS-TIA): feasibility and effects. Lancet Neurol. 2007;6(11):953-960.
- Rothwell PM, Giles MF, Chandratheva A, et al. Effect of urgent treatment of transient ischaemic attack and minor stroke on early recurrent stroke (EXPRESS study): a prospective population-based sequential comparison. Lancet. 2007;370(9596):1432-1442.
- Kernan WN, Ovbiagele B, Black HR, et al. Guidelines for the prevention of stroke in patients with stroke and transient ischemic attack: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2014;45(7):2160-2236.
- Roumie CL, Zillich AJ, Bravata DM, et al. Hypertension treatment intensification among stroke survivors with uncontrolled blood pressure. Stroke. 2015;46(2):465-470.
- Amarenco P, Bogousslavsky J, Callahan A, et al. High-dose atorvastatin after stroke or transient ischemic attack. N Engl J Med. 2006;355(6):549-559.
- Lennon O, Galvin R, Smith K, Doody C, Blake C. Lifestyle interventions for secondary disease prevention in stroke and transient ischaemic attack: a systematic review. Eur J Prev Cardiol. 2014;21(8):1026-1039.
Case
Mr. G is an 80-year-old man with a pacemaker, peripheral artery disease, atrial fibrillation (AF) on warfarin, and tachy-brady syndrome. He presented after experiencing episodes in which he was unable to speak and had weakness on his right side. He had a normal neurological exam upon arrival to the ED, and his blood pressure was 160/80 mm Hg.
Overview
Transient ischemic attacks (TIAs) are brief interruptions in brain perfusion that do not result in permanent neurologic damage. Up to half a million TIAs occur each year in the U.S., and they account for one third of acute cerebrovascular disease.1 While the term suggests that TIAs are benign, they are in fact an important warning sign of impending stroke and are essentially analogous to unstable angina. Some 10% of TIAs convert to full strokes within 90 days, but growing evidence suggests appropriate interventions can decrease this risk to 3%.2
Unfortunately, the symptoms of TIA have usually resolved by the time patients arrive at the hospital, which makes them challenging to diagnose. This article provides a summary of how to diagnose TIA accurately, using a focused history informed by cerebrovascular localization; how to triage, evaluate, and risk stratify patients; and how to implement preventative strategies.
Review of the Data
Classically, TIAs are defined as lasting less than 24 hours; however, 24 hours is an arbitrary number, and most TIAs last less than one hour.1 Furthermore, this definition has evolved with advances in neuroimaging that reveal that up to 50% of classically defined TIAs have evidence of infarct on MRI.1 There is no absolute temporal cut-off after which infarct is always seen on MRI, but longer duration of symptoms correlates with a higher likelihood of infarct. To reconcile these observations, a recently proposed definition stipulates that a true TIA lasts no more than one hour and does not show evidence of infarct on MRI.3
The causes of TIA are identical to those for ischemic stroke. Cerebral ischemia can result from an embolus, arterial thrombosis, or hypoperfusion due to arterial stenosis. Emboli can be cardiac, most commonly due to AF, or non-cardiac, stemming from a ruptured atherosclerotic plaque in the aortic arch, the carotid or vertebral artery, or an intracranial vessel. Atherosclerotic disease in the carotid arteries or intracranial vessels can also lead to thrombosis and occlusion or flow-related TIAs as a result of severe stenosis.
Risk factors for TIA mirror those for heart disease. Non-modifiable risk factors include older age, black race, male sex, and family history of stroke. Modifiable factors include hypertension, hyperlipidemia, tobacco smoking, diabetes, and AF.4
Most of the time, patients’ symptoms will have resolved by the time they are evaluated by a physician. Therefore, the diagnosis of TIA relies almost exclusively on the patient history. Eliciting a good history helps physicians determine whether the episode of transient neurologic dysfunction was caused by cerebral ischemia, as opposed to another mechanism, such as migraine or seizure. This calls for a basic understanding of cerebrovascular anatomy (see Table 1).
Types of Ischemia
Anterior cerebral artery ischemia causes contralateral leg weakness because it supplies the medial frontal and parietal lobes, where the legs in the sensorimotor homunculus are represented. Middle cerebral artery (MCA) ischemia causes contralateral face and arm weakness out of proportion to leg weakness. Ischemia in Broca’s area of the brain, which is supplied by the left MCA, may also cause expressive aphasia. Transient monocular blindness is a TIA of the retina due to atheroemboli originating from the internal carotid artery. Vertebrobasilar TIA is less common than anterior circulation TIA and manifests with brainstem symptoms that include diplopia, dysarthria, dysphagia, vertigo, gait imbalance, and weakness. In general, language and motor symptoms are more specific for cerebral ischemia and therefore more worrisome for TIA than sensory symptoms.5
Once a clinical diagnosis of TIA is made, an ABCD2 score (age, blood pressure, clinical features, duration of TIA, presence of diabetes) can be used to predict the short-term risk of subsequent stroke (see Table 2).6,7 A general rule of thumb is to admit patients who present within 72 hours of the event and have an ABCD2 score of three or higher for observation, work-up, and initiation of secondary prevention.1
Although only a small percentage of patients with TIA will have a stroke during the period of observation in the hospital, this approach may be cost effective based on the assumption that hospitalized patients are more likely to receive intravenous tissue plasminogen activator.8 The decision should also be guided by clinical judgment. It is reasonable to admit a patient whose diagnostic workup cannot be rapidly completed.1
The workup for TIA includes routine labs, EKG with cardiac monitoring, and brain imaging. Labs are useful to evaluate for other mimics of TIA such as hyponatremia and glucose abnormalities. In addition, risk factors such as hyperlipidemia and diabetes should be evaluated with fasting lipid panel and blood glucose. The purpose of EKG and telemetry is to identify MI and capture paroxysmal AF. The goal of imaging is to ascertain the presence of vascular disease and to exclude a non-ischemic etiology. While less likely to cause transient neurologic symptoms, a hemorrhagic event must be ruled out, as it would trigger a different management pathway.
Imaging for TIA
There are two primary modes of brain imaging: computed tomography (CT) and MRI. Most patients who are suspected to have had a TIA undergo CT scan, and an infarct is seen about 20% of the time.1 The presence of an infarct usually correlates with the duration of symptoms and has prognostic value. In one study, a new infarct was associated with four times higher risk of stroke in the subsequent 90 days.9 Diffusion-weighted imaging, an MR-based technique, is the preferred modality when it is available because of its higher sensitivity and specificity for identifying acute lesions.1 In an international and multicenter study, incorporating imaging data increased the discriminatory power of stroke prediction.10
Extracranial imaging is mandatory to rule out carotid stenosis as a potential etiology of TIA. The least invasive modality is ultrasound, which can detect carotid stenosis with a sensitivity and specificity approaching 80%.1 While both the intra- and extracranial vasculature can be concurrently assessed using MR- or CT-angiography (CTA), this is not usually necessary in the acute setting, because only detecting carotid stenosis will result in a management change.1
Carotid endarterectomy is standard for symptomatic patients with greater than 70% stenosis and is a consideration for symptomatic patients with greater than 50% stenosis if it is the most probable explanation for the ischemic event.11 Despite a comprehensive workup, about 50% of TIA cases remain cryptogenic.12 In some of these patients, AF can be detected using extended ambulatory cardiac monitoring.12
The goal of admitting high-risk patients is to expedite workup and initiate therapy. Two studies have shown that immediate initiation of preventative treatment significantly reduces the risk of stroke by as much as 80%.13,14 Unless there is a specific indication for anticoagulation, all TIA patients should be started on an antiplatelet agent such as aspirin or clopidogrel. A large randomized trial conducted in China and published in 2013 demonstrated that dual antiplatelet therapy with aspirin and clopidogrel for 21 days, followed by clopidogrel monotherapy, reduced the risk of stroke compared to aspirin monotherapy. An international multicenter trial designed to test the efficacy of short-term dual antiplatelet therapy is ongoing, and if the benefit of this approach is confirmed, this will likely become the standard of care. Evidence-based indications for anticoagulation after TIA are restricted to AF and mural thrombus in the setting of recent MI. Patients with implanted mechanical devices, including left ventricular assist devices and metal heart valves, should also receive anticoagulation.15
Risk factors should also be targeted in every case. Hypertension should be treated with a goal of lower than 140/90 mm Hg (or 130/80 mm Hg in diabetics and those with renal disease). Studies have shown that patients who are discharged with a blood pressure lower than 140/90 mm Hg are more likely to maintain this blood pressure at one-year follow-up.16 The choice of medication is less well studied, but drugs that act on the renin-angiotensin-aldosterone system and thiazides are generally preferred.15 Treatment with a statin is recommended after cerebrovascular ischemic events, with a goal LDL under 100. This reduces risk of secondary stroke by about 20%.17
At discharge, it is also important to counsel patients on their role in preventing strokes. As with many diseases, making lifestyle changes is key to stroke prevention. Encourage smoking cessation and an increase in physical activity, and discourage heavy alcohol use. The association between smoking and the risk for first stroke is well established. Moderate to high-intensity exercise can reduce secondary stroke risk by as much as 50%18 (see Table 3). While light alcohol consumption can be protective against strokes, heavy use is strongly discouraged. Emerging data suggest obstructive sleep apnea (OSA) may be another modifiable risk factor for stroke and TIA, so screening for potential OSA and referral may be needed.15
Back to the Case
When Mr. G arrived at the ED, his symptoms had resolved. Based on the history of expressive aphasia and right-sided weakness, he most likely had a TIA in the left MCA territory. Hemorrhage was ruled out with a non-contrast head CT. His pacemaker precluded obtaining an MRI. CTA revealed diffuse atherosclerotic disease without evidence of carotid stenosis. His ABCD2 score was six given his age, blood pressure, weakness, and symptom duration, and he was admitted for an expedited workup. His sodium and glucose were within normal limits. His hemoglobin A1c was 6.5%, his LDL was 120, and his international normalized ratio (INR) was therapeutic at 2.1. His TIA may have been due to AF, despite a therapeutic INR, because warfarin does not fully eliminate the stroke risk. It might also have been caused by intracranial atherosclerosis.
Two days later, the patient was discharged on atorvastatin at 80 mg, and his lisinopril was increased for blood pressure control. For his age group, A1c of 6.5% was acceptable, and he was not initiated on glycemic control.
Bottom Line
TIAs are diagnosed based on patient history. Urgent initiation of secondary prevention is important to reduce the short-term risk of stroke and should be implemented by the time of discharge from the hospital.
Dr. Zeng is a hospitalist in the department of internal medicine at Vanderbilt University Medical Center in Nashville, and Dr. Douglas is associate professor in the department of neurology at the University of California at San Francisco.
References
- Easton JD, Saver JL, Albers GW, et al. Definition and evaluation of transient ischemic attack: a scientific statement for healthcare professionals from the American Heart Association/American Stroke Association Stroke Council; Council on Cardiovascular Surgery and Anesthesia; Council on Cardiovascular Radiology and Intervention; Council on Cardiovascular Nursing; and the Interdisciplinary Council on Peripheral Vascular Disease. The American Academy of Neurology affirms the value of this statement as an educational tool for neurologists. Stroke. 2009;40(6):2276-2293.
- Sundararajan V, Thrift AG, Phan TG, Choi PM, Clissold B, Srikanth VK. Trends over time in the risk of stroke after an incident transient ischemic attack. Stroke. 2014;45(11):3214-3218.
- Albers GW, Caplan LR, Easton JD, et al. Transient ischemic attack–proposal for a new definition. N Engl J Med. 2002;347(21):1713-1716.
- Grysiewicz RA, Thomas K, Pandey DK. Epidemiology of ischemic and hemorrhagic stroke: incidence, prevalence, mortality, and risk factors. Neurol Clin. 2008;26(4):871-895, vii.
- Johnston SC, Sidney S, Bernstein AL, Gress DR. A comparison of risk factors for recurrent TIA and stroke in patients diagnosed with TIA. Neurology. 2003;60(2):280-285.
- Tsivgoulis G, Stamboulis E, Sharma VK, et al. Multicenter external validation of the ABCD2 score in triaging TIA patients. Neurology. 2010;74(17):1351-1357.
- Johnston SC, Rothwell PM, Nguyen-Huynh MN, et al. Validation and refinement of scores to predict very early stroke risk after transient ischaemic attack. Lancet. 2007;369(9558):283-292.
- Nguyen-Huynh MN, Johnston SC. Is hospitalization after TIA cost-effective on the basis of treatment with tPA? Neurology. 2005;65(11):1799-1801.
- Douglas VC, Johnston CM, Elkins J, Sidney S, Gress DR, Johnston SC. Head computed tomography findings predict short-term stroke risk after transient ischemic attack. Stroke. 2003;34(12):2894-2898.
- Giles MF, Albers GW, Amarenco P, et al. Addition of brain infarction to the ABCD2 Score (ABCD2I): a collaborative analysis of unpublished data on 4574 patients. Stroke. 2010;41(9):1907-1913.
- Lanzino G, Rabinstein AA, Brown RD Jr. Treatment of carotid artery stenosis: medical therapy, surgery, or stenting? Mayo Clin Proc. 2009;84(4):362-387; quiz 367-368.
- Gladstone DJ, Spring M, Dorian P, et al. Atrial fibrillation in patients with cryptogenic stroke. N Engl J Med. 2014;370(26):2467-2477.
- Lavallée PC, Meseguer E, Abboud H, et al. A transient ischaemic attack clinic with round-the-clock access (SOS-TIA): feasibility and effects. Lancet Neurol. 2007;6(11):953-960.
- Rothwell PM, Giles MF, Chandratheva A, et al. Effect of urgent treatment of transient ischaemic attack and minor stroke on early recurrent stroke (EXPRESS study): a prospective population-based sequential comparison. Lancet. 2007;370(9596):1432-1442.
- Kernan WN, Ovbiagele B, Black HR, et al. Guidelines for the prevention of stroke in patients with stroke and transient ischemic attack: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2014;45(7):2160-2236.
- Roumie CL, Zillich AJ, Bravata DM, et al. Hypertension treatment intensification among stroke survivors with uncontrolled blood pressure. Stroke. 2015;46(2):465-470.
- Amarenco P, Bogousslavsky J, Callahan A, et al. High-dose atorvastatin after stroke or transient ischemic attack. N Engl J Med. 2006;355(6):549-559.
- Lennon O, Galvin R, Smith K, Doody C, Blake C. Lifestyle interventions for secondary disease prevention in stroke and transient ischaemic attack: a systematic review. Eur J Prev Cardiol. 2014;21(8):1026-1039.
Case
Mr. G is an 80-year-old man with a pacemaker, peripheral artery disease, atrial fibrillation (AF) on warfarin, and tachy-brady syndrome. He presented after experiencing episodes in which he was unable to speak and had weakness on his right side. He had a normal neurological exam upon arrival to the ED, and his blood pressure was 160/80 mm Hg.
Overview
Transient ischemic attacks (TIAs) are brief interruptions in brain perfusion that do not result in permanent neurologic damage. Up to half a million TIAs occur each year in the U.S., and they account for one third of acute cerebrovascular disease.1 While the term suggests that TIAs are benign, they are in fact an important warning sign of impending stroke and are essentially analogous to unstable angina. Some 10% of TIAs convert to full strokes within 90 days, but growing evidence suggests appropriate interventions can decrease this risk to 3%.2
Unfortunately, the symptoms of TIA have usually resolved by the time patients arrive at the hospital, which makes them challenging to diagnose. This article provides a summary of how to diagnose TIA accurately, using a focused history informed by cerebrovascular localization; how to triage, evaluate, and risk stratify patients; and how to implement preventative strategies.
Review of the Data
Classically, TIAs are defined as lasting less than 24 hours; however, 24 hours is an arbitrary number, and most TIAs last less than one hour.1 Furthermore, this definition has evolved with advances in neuroimaging that reveal that up to 50% of classically defined TIAs have evidence of infarct on MRI.1 There is no absolute temporal cut-off after which infarct is always seen on MRI, but longer duration of symptoms correlates with a higher likelihood of infarct. To reconcile these observations, a recently proposed definition stipulates that a true TIA lasts no more than one hour and does not show evidence of infarct on MRI.3
The causes of TIA are identical to those for ischemic stroke. Cerebral ischemia can result from an embolus, arterial thrombosis, or hypoperfusion due to arterial stenosis. Emboli can be cardiac, most commonly due to AF, or non-cardiac, stemming from a ruptured atherosclerotic plaque in the aortic arch, the carotid or vertebral artery, or an intracranial vessel. Atherosclerotic disease in the carotid arteries or intracranial vessels can also lead to thrombosis and occlusion or flow-related TIAs as a result of severe stenosis.
Risk factors for TIA mirror those for heart disease. Non-modifiable risk factors include older age, black race, male sex, and family history of stroke. Modifiable factors include hypertension, hyperlipidemia, tobacco smoking, diabetes, and AF.4
Most of the time, patients’ symptoms will have resolved by the time they are evaluated by a physician. Therefore, the diagnosis of TIA relies almost exclusively on the patient history. Eliciting a good history helps physicians determine whether the episode of transient neurologic dysfunction was caused by cerebral ischemia, as opposed to another mechanism, such as migraine or seizure. This calls for a basic understanding of cerebrovascular anatomy (see Table 1).
Types of Ischemia
Anterior cerebral artery ischemia causes contralateral leg weakness because it supplies the medial frontal and parietal lobes, where the legs in the sensorimotor homunculus are represented. Middle cerebral artery (MCA) ischemia causes contralateral face and arm weakness out of proportion to leg weakness. Ischemia in Broca’s area of the brain, which is supplied by the left MCA, may also cause expressive aphasia. Transient monocular blindness is a TIA of the retina due to atheroemboli originating from the internal carotid artery. Vertebrobasilar TIA is less common than anterior circulation TIA and manifests with brainstem symptoms that include diplopia, dysarthria, dysphagia, vertigo, gait imbalance, and weakness. In general, language and motor symptoms are more specific for cerebral ischemia and therefore more worrisome for TIA than sensory symptoms.5
Once a clinical diagnosis of TIA is made, an ABCD2 score (age, blood pressure, clinical features, duration of TIA, presence of diabetes) can be used to predict the short-term risk of subsequent stroke (see Table 2).6,7 A general rule of thumb is to admit patients who present within 72 hours of the event and have an ABCD2 score of three or higher for observation, work-up, and initiation of secondary prevention.1
Although only a small percentage of patients with TIA will have a stroke during the period of observation in the hospital, this approach may be cost effective based on the assumption that hospitalized patients are more likely to receive intravenous tissue plasminogen activator.8 The decision should also be guided by clinical judgment. It is reasonable to admit a patient whose diagnostic workup cannot be rapidly completed.1
The workup for TIA includes routine labs, EKG with cardiac monitoring, and brain imaging. Labs are useful to evaluate for other mimics of TIA such as hyponatremia and glucose abnormalities. In addition, risk factors such as hyperlipidemia and diabetes should be evaluated with fasting lipid panel and blood glucose. The purpose of EKG and telemetry is to identify MI and capture paroxysmal AF. The goal of imaging is to ascertain the presence of vascular disease and to exclude a non-ischemic etiology. While less likely to cause transient neurologic symptoms, a hemorrhagic event must be ruled out, as it would trigger a different management pathway.
Imaging for TIA
There are two primary modes of brain imaging: computed tomography (CT) and MRI. Most patients who are suspected to have had a TIA undergo CT scan, and an infarct is seen about 20% of the time.1 The presence of an infarct usually correlates with the duration of symptoms and has prognostic value. In one study, a new infarct was associated with four times higher risk of stroke in the subsequent 90 days.9 Diffusion-weighted imaging, an MR-based technique, is the preferred modality when it is available because of its higher sensitivity and specificity for identifying acute lesions.1 In an international and multicenter study, incorporating imaging data increased the discriminatory power of stroke prediction.10
Extracranial imaging is mandatory to rule out carotid stenosis as a potential etiology of TIA. The least invasive modality is ultrasound, which can detect carotid stenosis with a sensitivity and specificity approaching 80%.1 While both the intra- and extracranial vasculature can be concurrently assessed using MR- or CT-angiography (CTA), this is not usually necessary in the acute setting, because only detecting carotid stenosis will result in a management change.1
Carotid endarterectomy is standard for symptomatic patients with greater than 70% stenosis and is a consideration for symptomatic patients with greater than 50% stenosis if it is the most probable explanation for the ischemic event.11 Despite a comprehensive workup, about 50% of TIA cases remain cryptogenic.12 In some of these patients, AF can be detected using extended ambulatory cardiac monitoring.12
The goal of admitting high-risk patients is to expedite workup and initiate therapy. Two studies have shown that immediate initiation of preventative treatment significantly reduces the risk of stroke by as much as 80%.13,14 Unless there is a specific indication for anticoagulation, all TIA patients should be started on an antiplatelet agent such as aspirin or clopidogrel. A large randomized trial conducted in China and published in 2013 demonstrated that dual antiplatelet therapy with aspirin and clopidogrel for 21 days, followed by clopidogrel monotherapy, reduced the risk of stroke compared to aspirin monotherapy. An international multicenter trial designed to test the efficacy of short-term dual antiplatelet therapy is ongoing, and if the benefit of this approach is confirmed, this will likely become the standard of care. Evidence-based indications for anticoagulation after TIA are restricted to AF and mural thrombus in the setting of recent MI. Patients with implanted mechanical devices, including left ventricular assist devices and metal heart valves, should also receive anticoagulation.15
Risk factors should also be targeted in every case. Hypertension should be treated with a goal of lower than 140/90 mm Hg (or 130/80 mm Hg in diabetics and those with renal disease). Studies have shown that patients who are discharged with a blood pressure lower than 140/90 mm Hg are more likely to maintain this blood pressure at one-year follow-up.16 The choice of medication is less well studied, but drugs that act on the renin-angiotensin-aldosterone system and thiazides are generally preferred.15 Treatment with a statin is recommended after cerebrovascular ischemic events, with a goal LDL under 100. This reduces risk of secondary stroke by about 20%.17
At discharge, it is also important to counsel patients on their role in preventing strokes. As with many diseases, making lifestyle changes is key to stroke prevention. Encourage smoking cessation and an increase in physical activity, and discourage heavy alcohol use. The association between smoking and the risk for first stroke is well established. Moderate to high-intensity exercise can reduce secondary stroke risk by as much as 50%18 (see Table 3). While light alcohol consumption can be protective against strokes, heavy use is strongly discouraged. Emerging data suggest obstructive sleep apnea (OSA) may be another modifiable risk factor for stroke and TIA, so screening for potential OSA and referral may be needed.15
Back to the Case
When Mr. G arrived at the ED, his symptoms had resolved. Based on the history of expressive aphasia and right-sided weakness, he most likely had a TIA in the left MCA territory. Hemorrhage was ruled out with a non-contrast head CT. His pacemaker precluded obtaining an MRI. CTA revealed diffuse atherosclerotic disease without evidence of carotid stenosis. His ABCD2 score was six given his age, blood pressure, weakness, and symptom duration, and he was admitted for an expedited workup. His sodium and glucose were within normal limits. His hemoglobin A1c was 6.5%, his LDL was 120, and his international normalized ratio (INR) was therapeutic at 2.1. His TIA may have been due to AF, despite a therapeutic INR, because warfarin does not fully eliminate the stroke risk. It might also have been caused by intracranial atherosclerosis.
Two days later, the patient was discharged on atorvastatin at 80 mg, and his lisinopril was increased for blood pressure control. For his age group, A1c of 6.5% was acceptable, and he was not initiated on glycemic control.
Bottom Line
TIAs are diagnosed based on patient history. Urgent initiation of secondary prevention is important to reduce the short-term risk of stroke and should be implemented by the time of discharge from the hospital.
Dr. Zeng is a hospitalist in the department of internal medicine at Vanderbilt University Medical Center in Nashville, and Dr. Douglas is associate professor in the department of neurology at the University of California at San Francisco.
References
- Easton JD, Saver JL, Albers GW, et al. Definition and evaluation of transient ischemic attack: a scientific statement for healthcare professionals from the American Heart Association/American Stroke Association Stroke Council; Council on Cardiovascular Surgery and Anesthesia; Council on Cardiovascular Radiology and Intervention; Council on Cardiovascular Nursing; and the Interdisciplinary Council on Peripheral Vascular Disease. The American Academy of Neurology affirms the value of this statement as an educational tool for neurologists. Stroke. 2009;40(6):2276-2293.
- Sundararajan V, Thrift AG, Phan TG, Choi PM, Clissold B, Srikanth VK. Trends over time in the risk of stroke after an incident transient ischemic attack. Stroke. 2014;45(11):3214-3218.
- Albers GW, Caplan LR, Easton JD, et al. Transient ischemic attack–proposal for a new definition. N Engl J Med. 2002;347(21):1713-1716.
- Grysiewicz RA, Thomas K, Pandey DK. Epidemiology of ischemic and hemorrhagic stroke: incidence, prevalence, mortality, and risk factors. Neurol Clin. 2008;26(4):871-895, vii.
- Johnston SC, Sidney S, Bernstein AL, Gress DR. A comparison of risk factors for recurrent TIA and stroke in patients diagnosed with TIA. Neurology. 2003;60(2):280-285.
- Tsivgoulis G, Stamboulis E, Sharma VK, et al. Multicenter external validation of the ABCD2 score in triaging TIA patients. Neurology. 2010;74(17):1351-1357.
- Johnston SC, Rothwell PM, Nguyen-Huynh MN, et al. Validation and refinement of scores to predict very early stroke risk after transient ischaemic attack. Lancet. 2007;369(9558):283-292.
- Nguyen-Huynh MN, Johnston SC. Is hospitalization after TIA cost-effective on the basis of treatment with tPA? Neurology. 2005;65(11):1799-1801.
- Douglas VC, Johnston CM, Elkins J, Sidney S, Gress DR, Johnston SC. Head computed tomography findings predict short-term stroke risk after transient ischemic attack. Stroke. 2003;34(12):2894-2898.
- Giles MF, Albers GW, Amarenco P, et al. Addition of brain infarction to the ABCD2 Score (ABCD2I): a collaborative analysis of unpublished data on 4574 patients. Stroke. 2010;41(9):1907-1913.
- Lanzino G, Rabinstein AA, Brown RD Jr. Treatment of carotid artery stenosis: medical therapy, surgery, or stenting? Mayo Clin Proc. 2009;84(4):362-387; quiz 367-368.
- Gladstone DJ, Spring M, Dorian P, et al. Atrial fibrillation in patients with cryptogenic stroke. N Engl J Med. 2014;370(26):2467-2477.
- Lavallée PC, Meseguer E, Abboud H, et al. A transient ischaemic attack clinic with round-the-clock access (SOS-TIA): feasibility and effects. Lancet Neurol. 2007;6(11):953-960.
- Rothwell PM, Giles MF, Chandratheva A, et al. Effect of urgent treatment of transient ischaemic attack and minor stroke on early recurrent stroke (EXPRESS study): a prospective population-based sequential comparison. Lancet. 2007;370(9596):1432-1442.
- Kernan WN, Ovbiagele B, Black HR, et al. Guidelines for the prevention of stroke in patients with stroke and transient ischemic attack: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2014;45(7):2160-2236.
- Roumie CL, Zillich AJ, Bravata DM, et al. Hypertension treatment intensification among stroke survivors with uncontrolled blood pressure. Stroke. 2015;46(2):465-470.
- Amarenco P, Bogousslavsky J, Callahan A, et al. High-dose atorvastatin after stroke or transient ischemic attack. N Engl J Med. 2006;355(6):549-559.
- Lennon O, Galvin R, Smith K, Doody C, Blake C. Lifestyle interventions for secondary disease prevention in stroke and transient ischaemic attack: a systematic review. Eur J Prev Cardiol. 2014;21(8):1026-1039.