User login
-
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'main-prefix')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
div[contains(@class, 'view-medstat-quiz-listing-panes')]
div[contains(@class, 'pane-article-sidebar-latest-news')]


HALT early recognition is key
DIFFUSE LUNG DISEASE AND LUNG TRANSPLANT NETWORK
Lung Transplant Section
Hyperammonemia after lung transplantation (HALT) is a rare but serious complication occurring in 1% to 4% of patients with high morbidity and mortality. Early recognition is crucial, as mortality rates can reach 75%.1
HALT arises from excess ammonia production or decreased clearance and is often linked to infections by urea-splitting organisms, including mycoplasma and ureaplasma. Prompt, aggressive treatment is essential and typically includes dietary protein restriction, renal replacement therapy (ideally intermittent hemodialysis), bowel decontamination (lactulose, rifaximin, metronidazole, or neomycin), amino acids (arginine and levocarnitine), nitrogen scavengers (sodium phenylbutyrate or glycerol phenylbutyrate), and empiric antimicrobial coverage for urea-splitting organisms.2 Given concerns for calcineurin inhibitor-induced hyperammonemia, transition to an alternative agent may be considered.
Given the severe risks associated with HALT, vigilance is vital, particularly in intubated and sedated patients where monitoring of neurologic status is more challenging. Protocols may involve routine serum ammonia monitoring, polymerase chain reaction testing for mycoplasma and ureaplasma at the time of transplant or with postoperative bronchoscopy, and empiric antimicrobial treatment. No definitive ammonia threshold exists, but altered sensorium with elevated levels warrants immediate and more aggressive treatment with levels >75 μmol/L. Early testing and symptom recognition can significantly improve survival rates in this potentially devastating condition.
References
1. Leger RF, Silverman MS, Hauck ES, Guvakova KD. Hyperammonemia post lung transplantation: a review. Clin Med Insights Circ Respir Pulm Med. 2020;14:1179548420966234. doi:10.1177/1179548420966234
2. Chen C, Bain KB, Luppa JA. Hyperammonemia syndrome after lung transplantation: a single center experience. Transplantation. 2016;100(3):678-684. doi:10.1097/TP.0000000000000868
DIFFUSE LUNG DISEASE AND LUNG TRANSPLANT NETWORK
Lung Transplant Section
Hyperammonemia after lung transplantation (HALT) is a rare but serious complication occurring in 1% to 4% of patients with high morbidity and mortality. Early recognition is crucial, as mortality rates can reach 75%.1
HALT arises from excess ammonia production or decreased clearance and is often linked to infections by urea-splitting organisms, including mycoplasma and ureaplasma. Prompt, aggressive treatment is essential and typically includes dietary protein restriction, renal replacement therapy (ideally intermittent hemodialysis), bowel decontamination (lactulose, rifaximin, metronidazole, or neomycin), amino acids (arginine and levocarnitine), nitrogen scavengers (sodium phenylbutyrate or glycerol phenylbutyrate), and empiric antimicrobial coverage for urea-splitting organisms.2 Given concerns for calcineurin inhibitor-induced hyperammonemia, transition to an alternative agent may be considered.
Given the severe risks associated with HALT, vigilance is vital, particularly in intubated and sedated patients where monitoring of neurologic status is more challenging. Protocols may involve routine serum ammonia monitoring, polymerase chain reaction testing for mycoplasma and ureaplasma at the time of transplant or with postoperative bronchoscopy, and empiric antimicrobial treatment. No definitive ammonia threshold exists, but altered sensorium with elevated levels warrants immediate and more aggressive treatment with levels >75 μmol/L. Early testing and symptom recognition can significantly improve survival rates in this potentially devastating condition.
References
1. Leger RF, Silverman MS, Hauck ES, Guvakova KD. Hyperammonemia post lung transplantation: a review. Clin Med Insights Circ Respir Pulm Med. 2020;14:1179548420966234. doi:10.1177/1179548420966234
2. Chen C, Bain KB, Luppa JA. Hyperammonemia syndrome after lung transplantation: a single center experience. Transplantation. 2016;100(3):678-684. doi:10.1097/TP.0000000000000868
DIFFUSE LUNG DISEASE AND LUNG TRANSPLANT NETWORK
Lung Transplant Section
Hyperammonemia after lung transplantation (HALT) is a rare but serious complication occurring in 1% to 4% of patients with high morbidity and mortality. Early recognition is crucial, as mortality rates can reach 75%.1
HALT arises from excess ammonia production or decreased clearance and is often linked to infections by urea-splitting organisms, including mycoplasma and ureaplasma. Prompt, aggressive treatment is essential and typically includes dietary protein restriction, renal replacement therapy (ideally intermittent hemodialysis), bowel decontamination (lactulose, rifaximin, metronidazole, or neomycin), amino acids (arginine and levocarnitine), nitrogen scavengers (sodium phenylbutyrate or glycerol phenylbutyrate), and empiric antimicrobial coverage for urea-splitting organisms.2 Given concerns for calcineurin inhibitor-induced hyperammonemia, transition to an alternative agent may be considered.
Given the severe risks associated with HALT, vigilance is vital, particularly in intubated and sedated patients where monitoring of neurologic status is more challenging. Protocols may involve routine serum ammonia monitoring, polymerase chain reaction testing for mycoplasma and ureaplasma at the time of transplant or with postoperative bronchoscopy, and empiric antimicrobial treatment. No definitive ammonia threshold exists, but altered sensorium with elevated levels warrants immediate and more aggressive treatment with levels >75 μmol/L. Early testing and symptom recognition can significantly improve survival rates in this potentially devastating condition.
References
1. Leger RF, Silverman MS, Hauck ES, Guvakova KD. Hyperammonemia post lung transplantation: a review. Clin Med Insights Circ Respir Pulm Med. 2020;14:1179548420966234. doi:10.1177/1179548420966234
2. Chen C, Bain KB, Luppa JA. Hyperammonemia syndrome after lung transplantation: a single center experience. Transplantation. 2016;100(3):678-684. doi:10.1097/TP.0000000000000868
SURMOUNT-OSA Results: ‘Impressive’ in Improving Sleep Apnea
This transcript has been edited for clarity.
Akshay B. Jain, MD: Welcome. I’m Dr. Akshay Jain, an endocrinologist in Vancouver, Canada, and with me is a very special guest. Today we have Dr. James Kim, a primary care physician working in Calgary, Canada. Both Dr. Kim and I were fortunate to attend the recently concluded American Diabetes Association annual conference in Orlando in June.
We thought we could share with you some of the key learnings that we found very insightful and clinically quite relevant. We were hoping to bring our own conclusion regarding what these findings were, both from a primary care perspective and an endocrinology perspective.
There were so many different studies that, frankly, it was difficult to pick them, but we handpicked a few studies we felt we could do a bit of a deeper dive on, and we’ll talk about each of these studies.
Welcome, Dr. Kim, and thanks for joining us.
James W. Kim, MBBCh, PgDip, MScCH: Thank you so much, Dr Jain. It’s a pleasure to be here.
Dr. Jain: Probably the best place to start would be with the SURMOUNT-OSA study. This was highlighted at the American Diabetes Association conference. Essentially, it looked at people who are living with obesity who also had obstructive sleep apnea.
This was a randomized controlled trial where individuals tested either got tirzepatide (trade name, Mounjaro) or placebo treatment. They looked at the change in their apnea-hypopnea index at the end of the study.
This included both people who were using CPAP machines and those who were not using CPAP machines at baseline. We do know that many individuals with sleep apnea may not use these machines.
That was a big reduction.
Dr. Kim, what’s the relevance of this study in primary care?
Dr. Kim: Oh, it’s massive. Obstructive sleep apnea is probably one of the most underdiagnosed yet huge cardiac risk factors that we tend to overlook in primary care. We sometimes say, oh, it’s just sleep apnea; what’s the big deal? We know it’s a big problem. We know that more than 50% of people with type 2 diabetes have obstructive sleep apnea, and some studies have even quoted that 90% of their population cohorts had sleep apnea. This is a big deal.
What do we know so far? We know that obstructive sleep apnea, which I’m just going to call OSA, increases the risk for hypertension, bad cholesterol, and worsening blood glucose in terms of A1c and fasting glucose, which eventually leads to myocardial infarction, arrhythmia, stroke, and eventually cardiovascular death.
We also know that people with type 2 diabetes have an increased risk for OSA. There seems to be a bidirectional relationship between diabetes and OSA. It seems like weight plays the biggest role in terms of developing OSA, and numerous studies have shown this.
Also, thankfully, some of the studies showed that weight loss improves not just OSA but also blood pressure, cholesterol, blood glucose, and insulin sensitivities. These have been fascinating. We see these patients every single day. If you think about it in your population, for 50%-90% of the patients to have OSA is a large number. If you haven’t seen a person with OSA this week, you probably missed them, very likely.
Therefore, the SURMOUNT-OSA trial was quite fascinating with, as you mentioned, 50%-60% reduction in the severity of OSA, which is very impressive. Even more impressive, I think, is that for about 50% of the patients on tirzepatide, the OSA improves so much that they may not even need to be on CPAP machines.
Those who were on CPAP may not need to be on CPAP any longer. These are huge data, especially for primary care, because as you mentioned, we see these people every single day.
Dr. Jain: Thanks for pointing that out. Clearly, it’s very clinically relevant. I think the most important takeaway for me from this study was the correlation between weight loss and AHI improvement.
Clearly, it showed that placebo had about a 6% drop in AHI, whereas there was a 60% drop in the tirzepatide group, so you can see that it’s significantly different. The placebo group did not have any significant degree of weight loss, whereas the tirzepatide group had nearly 20% weight loss. This again goes to show that there is a very close correlation between weight loss and improvement in OSA.
What’s very important to note is that we’ve seen this in the past as well. We had seen some of these data with other GLP-1 agents, but the extent of improvement that we have seen in the SURMOUNT-OSA trial is significantly more than what we’ve seen in previous studies. There is a ray of hope now where we have medical management to offer people who are living with obesity and obstructive sleep apnea.
Dr. Kim: I want to add that, from a primary care perspective, this study also showed the improvement of the sleep apnea–related symptoms as well. The biggest problem with sleep apnea — or at least what patients’ spouses complain of, is the person snoring too much; it’s a symptom.
It’s the next-day symptoms that really do disturb people, like chronic fatigue. I have numerous patients who say that, once they’ve been treated for sleep apnea, they feel like a brand-new person. They have sudden bursts of energy that they never felt before, and over 50% of these people have huge improvements in the symptoms as well.
This is a huge trial. The only thing that I wish this study included were people with mild obstructive sleep apnea who were symptomatic. I do understand that, with other studies in this population, the data have been conflicting, but it would have been really awesome if they had those patients included. However, it is still a significant study for primary care.
Dr. Jain: That’s a really good point. Fatigue improves and overall quality of life improves. That’s very important from a primary care perspective.
From an endocrinology perspective, we know that management of sleep apnea can often lead to improvement in male hypogonadism, polycystic ovary syndrome, and insulin resistance. The amount of insulin required, or the number of medications needed for managing diabetes, can improve. Hypertension can improve as well. There are multiple benefits that you can get from appropriate management of sleep apnea.
Thanks, Dr. Kim. We really appreciate your insights on SURMOUNT-OSA.
Dr. Jain is a clinical instructor, Department of Endocrinology, University of British Columbia, Vancouver. Dr. Kim is a clinical assistant professor, Department of Family Medicine, University of Calgary in Alberta. Both disclosed conflicts of interest with numerous pharmaceutical companies.
A version of this article appeared on Medscape.com.
This transcript has been edited for clarity.
Akshay B. Jain, MD: Welcome. I’m Dr. Akshay Jain, an endocrinologist in Vancouver, Canada, and with me is a very special guest. Today we have Dr. James Kim, a primary care physician working in Calgary, Canada. Both Dr. Kim and I were fortunate to attend the recently concluded American Diabetes Association annual conference in Orlando in June.
We thought we could share with you some of the key learnings that we found very insightful and clinically quite relevant. We were hoping to bring our own conclusion regarding what these findings were, both from a primary care perspective and an endocrinology perspective.
There were so many different studies that, frankly, it was difficult to pick them, but we handpicked a few studies we felt we could do a bit of a deeper dive on, and we’ll talk about each of these studies.
Welcome, Dr. Kim, and thanks for joining us.
James W. Kim, MBBCh, PgDip, MScCH: Thank you so much, Dr Jain. It’s a pleasure to be here.
Dr. Jain: Probably the best place to start would be with the SURMOUNT-OSA study. This was highlighted at the American Diabetes Association conference. Essentially, it looked at people who are living with obesity who also had obstructive sleep apnea.
This was a randomized controlled trial where individuals tested either got tirzepatide (trade name, Mounjaro) or placebo treatment. They looked at the change in their apnea-hypopnea index at the end of the study.
This included both people who were using CPAP machines and those who were not using CPAP machines at baseline. We do know that many individuals with sleep apnea may not use these machines.
That was a big reduction.
Dr. Kim, what’s the relevance of this study in primary care?
Dr. Kim: Oh, it’s massive. Obstructive sleep apnea is probably one of the most underdiagnosed yet huge cardiac risk factors that we tend to overlook in primary care. We sometimes say, oh, it’s just sleep apnea; what’s the big deal? We know it’s a big problem. We know that more than 50% of people with type 2 diabetes have obstructive sleep apnea, and some studies have even quoted that 90% of their population cohorts had sleep apnea. This is a big deal.
What do we know so far? We know that obstructive sleep apnea, which I’m just going to call OSA, increases the risk for hypertension, bad cholesterol, and worsening blood glucose in terms of A1c and fasting glucose, which eventually leads to myocardial infarction, arrhythmia, stroke, and eventually cardiovascular death.
We also know that people with type 2 diabetes have an increased risk for OSA. There seems to be a bidirectional relationship between diabetes and OSA. It seems like weight plays the biggest role in terms of developing OSA, and numerous studies have shown this.
Also, thankfully, some of the studies showed that weight loss improves not just OSA but also blood pressure, cholesterol, blood glucose, and insulin sensitivities. These have been fascinating. We see these patients every single day. If you think about it in your population, for 50%-90% of the patients to have OSA is a large number. If you haven’t seen a person with OSA this week, you probably missed them, very likely.
Therefore, the SURMOUNT-OSA trial was quite fascinating with, as you mentioned, 50%-60% reduction in the severity of OSA, which is very impressive. Even more impressive, I think, is that for about 50% of the patients on tirzepatide, the OSA improves so much that they may not even need to be on CPAP machines.
Those who were on CPAP may not need to be on CPAP any longer. These are huge data, especially for primary care, because as you mentioned, we see these people every single day.
Dr. Jain: Thanks for pointing that out. Clearly, it’s very clinically relevant. I think the most important takeaway for me from this study was the correlation between weight loss and AHI improvement.
Clearly, it showed that placebo had about a 6% drop in AHI, whereas there was a 60% drop in the tirzepatide group, so you can see that it’s significantly different. The placebo group did not have any significant degree of weight loss, whereas the tirzepatide group had nearly 20% weight loss. This again goes to show that there is a very close correlation between weight loss and improvement in OSA.
What’s very important to note is that we’ve seen this in the past as well. We had seen some of these data with other GLP-1 agents, but the extent of improvement that we have seen in the SURMOUNT-OSA trial is significantly more than what we’ve seen in previous studies. There is a ray of hope now where we have medical management to offer people who are living with obesity and obstructive sleep apnea.
Dr. Kim: I want to add that, from a primary care perspective, this study also showed the improvement of the sleep apnea–related symptoms as well. The biggest problem with sleep apnea — or at least what patients’ spouses complain of, is the person snoring too much; it’s a symptom.
It’s the next-day symptoms that really do disturb people, like chronic fatigue. I have numerous patients who say that, once they’ve been treated for sleep apnea, they feel like a brand-new person. They have sudden bursts of energy that they never felt before, and over 50% of these people have huge improvements in the symptoms as well.
This is a huge trial. The only thing that I wish this study included were people with mild obstructive sleep apnea who were symptomatic. I do understand that, with other studies in this population, the data have been conflicting, but it would have been really awesome if they had those patients included. However, it is still a significant study for primary care.
Dr. Jain: That’s a really good point. Fatigue improves and overall quality of life improves. That’s very important from a primary care perspective.
From an endocrinology perspective, we know that management of sleep apnea can often lead to improvement in male hypogonadism, polycystic ovary syndrome, and insulin resistance. The amount of insulin required, or the number of medications needed for managing diabetes, can improve. Hypertension can improve as well. There are multiple benefits that you can get from appropriate management of sleep apnea.
Thanks, Dr. Kim. We really appreciate your insights on SURMOUNT-OSA.
Dr. Jain is a clinical instructor, Department of Endocrinology, University of British Columbia, Vancouver. Dr. Kim is a clinical assistant professor, Department of Family Medicine, University of Calgary in Alberta. Both disclosed conflicts of interest with numerous pharmaceutical companies.
A version of this article appeared on Medscape.com.
This transcript has been edited for clarity.
Akshay B. Jain, MD: Welcome. I’m Dr. Akshay Jain, an endocrinologist in Vancouver, Canada, and with me is a very special guest. Today we have Dr. James Kim, a primary care physician working in Calgary, Canada. Both Dr. Kim and I were fortunate to attend the recently concluded American Diabetes Association annual conference in Orlando in June.
We thought we could share with you some of the key learnings that we found very insightful and clinically quite relevant. We were hoping to bring our own conclusion regarding what these findings were, both from a primary care perspective and an endocrinology perspective.
There were so many different studies that, frankly, it was difficult to pick them, but we handpicked a few studies we felt we could do a bit of a deeper dive on, and we’ll talk about each of these studies.
Welcome, Dr. Kim, and thanks for joining us.
James W. Kim, MBBCh, PgDip, MScCH: Thank you so much, Dr Jain. It’s a pleasure to be here.
Dr. Jain: Probably the best place to start would be with the SURMOUNT-OSA study. This was highlighted at the American Diabetes Association conference. Essentially, it looked at people who are living with obesity who also had obstructive sleep apnea.
This was a randomized controlled trial where individuals tested either got tirzepatide (trade name, Mounjaro) or placebo treatment. They looked at the change in their apnea-hypopnea index at the end of the study.
This included both people who were using CPAP machines and those who were not using CPAP machines at baseline. We do know that many individuals with sleep apnea may not use these machines.
That was a big reduction.
Dr. Kim, what’s the relevance of this study in primary care?
Dr. Kim: Oh, it’s massive. Obstructive sleep apnea is probably one of the most underdiagnosed yet huge cardiac risk factors that we tend to overlook in primary care. We sometimes say, oh, it’s just sleep apnea; what’s the big deal? We know it’s a big problem. We know that more than 50% of people with type 2 diabetes have obstructive sleep apnea, and some studies have even quoted that 90% of their population cohorts had sleep apnea. This is a big deal.
What do we know so far? We know that obstructive sleep apnea, which I’m just going to call OSA, increases the risk for hypertension, bad cholesterol, and worsening blood glucose in terms of A1c and fasting glucose, which eventually leads to myocardial infarction, arrhythmia, stroke, and eventually cardiovascular death.
We also know that people with type 2 diabetes have an increased risk for OSA. There seems to be a bidirectional relationship between diabetes and OSA. It seems like weight plays the biggest role in terms of developing OSA, and numerous studies have shown this.
Also, thankfully, some of the studies showed that weight loss improves not just OSA but also blood pressure, cholesterol, blood glucose, and insulin sensitivities. These have been fascinating. We see these patients every single day. If you think about it in your population, for 50%-90% of the patients to have OSA is a large number. If you haven’t seen a person with OSA this week, you probably missed them, very likely.
Therefore, the SURMOUNT-OSA trial was quite fascinating with, as you mentioned, 50%-60% reduction in the severity of OSA, which is very impressive. Even more impressive, I think, is that for about 50% of the patients on tirzepatide, the OSA improves so much that they may not even need to be on CPAP machines.
Those who were on CPAP may not need to be on CPAP any longer. These are huge data, especially for primary care, because as you mentioned, we see these people every single day.
Dr. Jain: Thanks for pointing that out. Clearly, it’s very clinically relevant. I think the most important takeaway for me from this study was the correlation between weight loss and AHI improvement.
Clearly, it showed that placebo had about a 6% drop in AHI, whereas there was a 60% drop in the tirzepatide group, so you can see that it’s significantly different. The placebo group did not have any significant degree of weight loss, whereas the tirzepatide group had nearly 20% weight loss. This again goes to show that there is a very close correlation between weight loss and improvement in OSA.
What’s very important to note is that we’ve seen this in the past as well. We had seen some of these data with other GLP-1 agents, but the extent of improvement that we have seen in the SURMOUNT-OSA trial is significantly more than what we’ve seen in previous studies. There is a ray of hope now where we have medical management to offer people who are living with obesity and obstructive sleep apnea.
Dr. Kim: I want to add that, from a primary care perspective, this study also showed the improvement of the sleep apnea–related symptoms as well. The biggest problem with sleep apnea — or at least what patients’ spouses complain of, is the person snoring too much; it’s a symptom.
It’s the next-day symptoms that really do disturb people, like chronic fatigue. I have numerous patients who say that, once they’ve been treated for sleep apnea, they feel like a brand-new person. They have sudden bursts of energy that they never felt before, and over 50% of these people have huge improvements in the symptoms as well.
This is a huge trial. The only thing that I wish this study included were people with mild obstructive sleep apnea who were symptomatic. I do understand that, with other studies in this population, the data have been conflicting, but it would have been really awesome if they had those patients included. However, it is still a significant study for primary care.
Dr. Jain: That’s a really good point. Fatigue improves and overall quality of life improves. That’s very important from a primary care perspective.
From an endocrinology perspective, we know that management of sleep apnea can often lead to improvement in male hypogonadism, polycystic ovary syndrome, and insulin resistance. The amount of insulin required, or the number of medications needed for managing diabetes, can improve. Hypertension can improve as well. There are multiple benefits that you can get from appropriate management of sleep apnea.
Thanks, Dr. Kim. We really appreciate your insights on SURMOUNT-OSA.
Dr. Jain is a clinical instructor, Department of Endocrinology, University of British Columbia, Vancouver. Dr. Kim is a clinical assistant professor, Department of Family Medicine, University of Calgary in Alberta. Both disclosed conflicts of interest with numerous pharmaceutical companies.
A version of this article appeared on Medscape.com.
Pulmonary Hypertension: Comorbidities and Novel Therapeutics
- Cullivan S, Gaine S, Sitbon O. New trends in pulmonary hypertension. Eur Respir Rev. 2023;32(167):220211. doi:10.1183/16000617.0211-2022
- Mocumbi A, Humbert M, Saxena A, et al. Pulmonary hypertension [published correction appears in Nat Rev Dis Primers. 2024;10(1):5]. Nat Rev Dis Primers. 2024;10(1):1. doi:10.1038/s41572-023-00486-7
- Lang IM, Palazzini M. The burden of comorbidities in pulmonary arterial hypertension. Eur Heart J Suppl. 2019;21(suppl K):K21-K28. doi:10.1093/ eurheartj/suz205
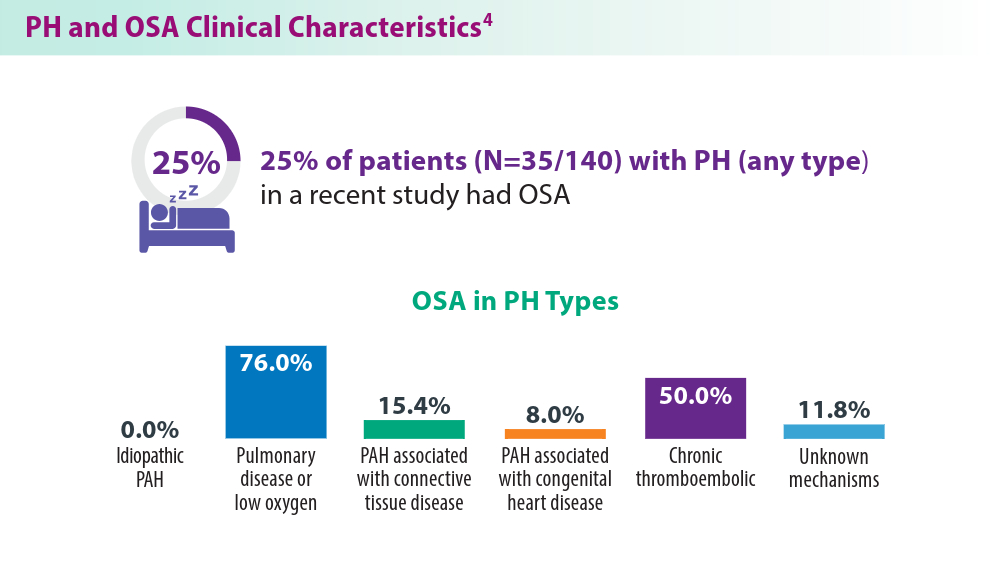
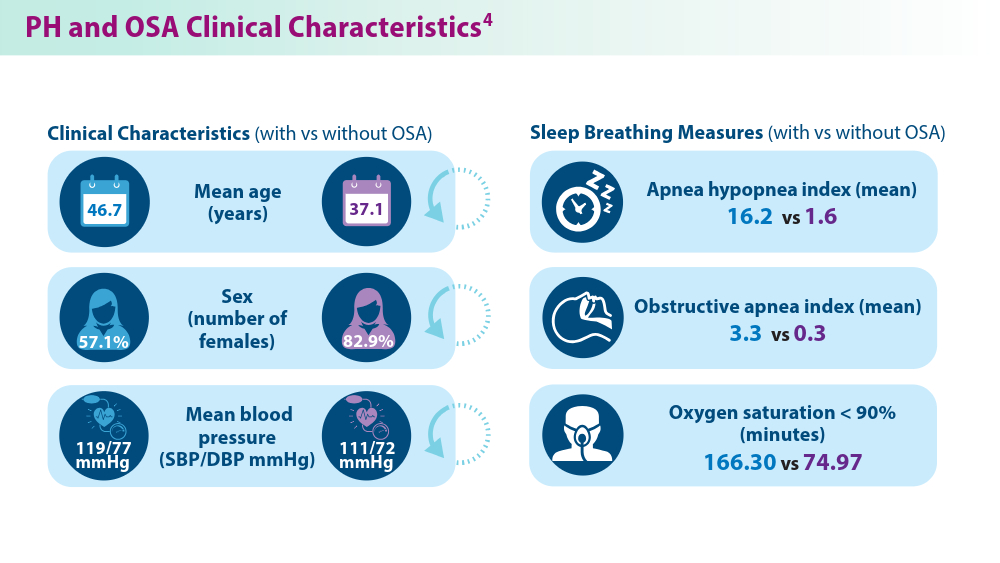
- Yan L, Zhao Z, Zhao Q, et al. The clinical characteristics of patients with pulmonary hypertension combined with obstructive sleep apnoea. BMC Pulm Med. 2021;21(1):378. doi:10.1186/s12890-021-01755-5
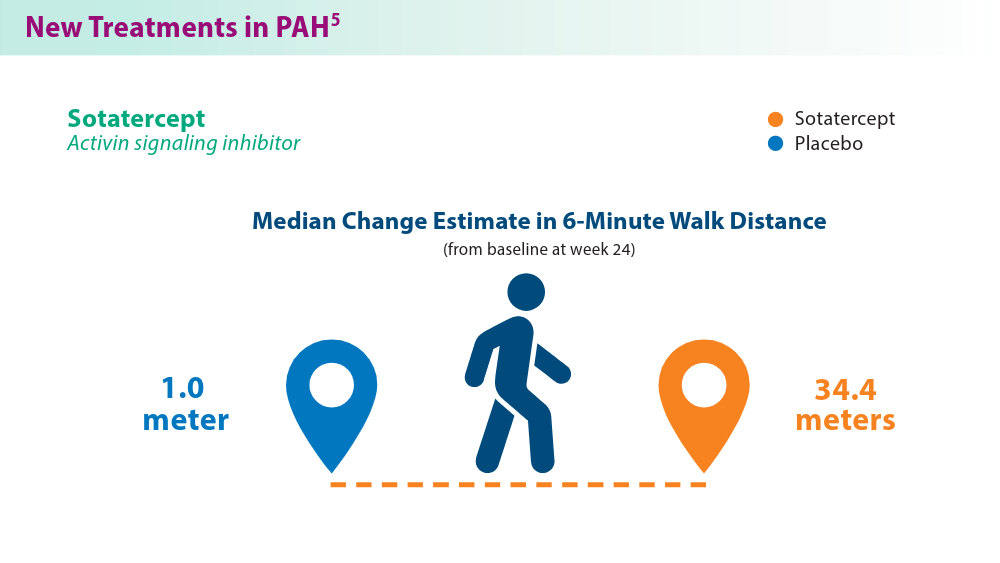
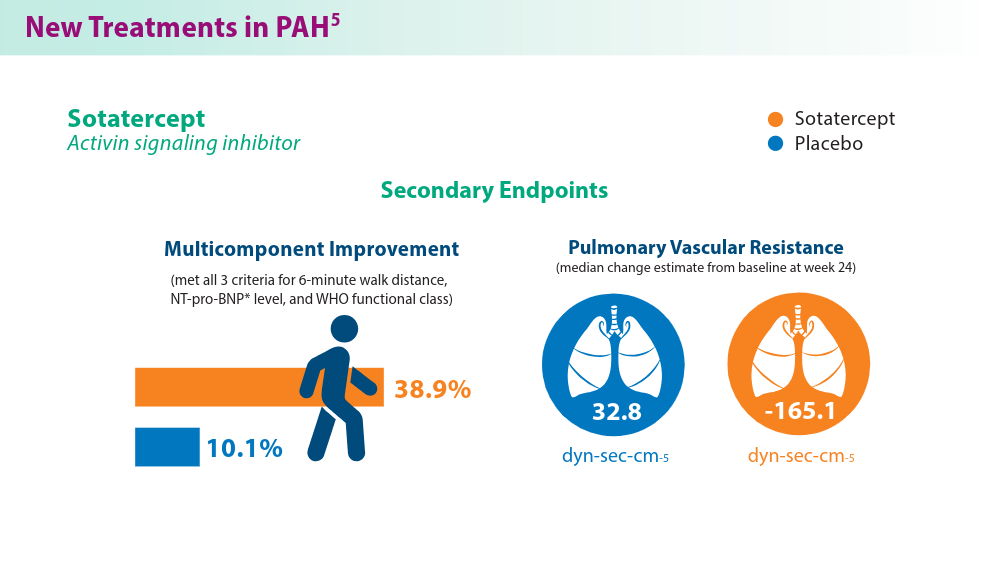
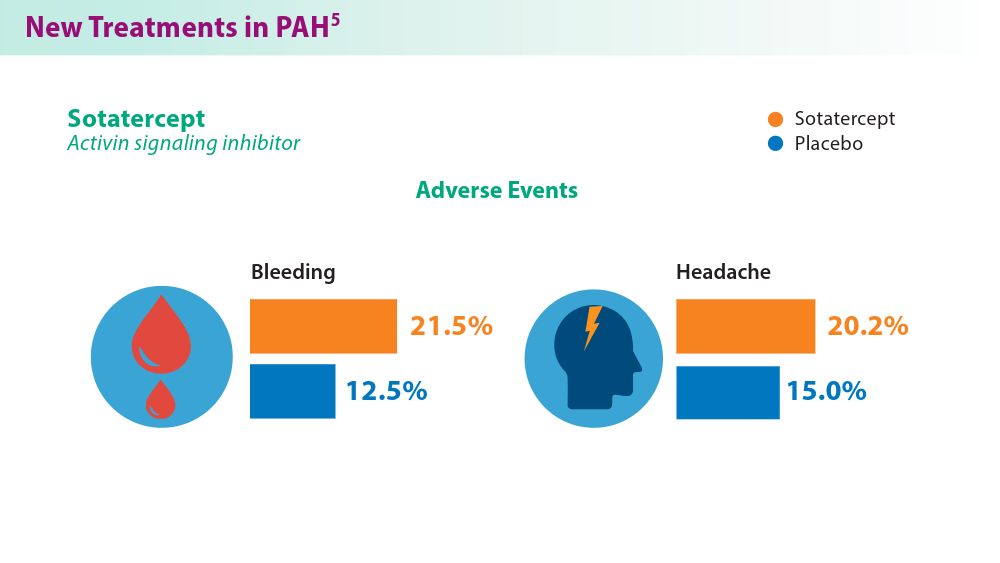
- Hoeper MM, Badesch DB, Ghofrani HA, et al; for the STELLAR Trial Investigators. Phase 3 trial of sotatercept for treatment of pulmonary arterial hypertension. N Engl J Med. 2023;388(16):1478-1490. doi:10.1056/NEJMoa2213558
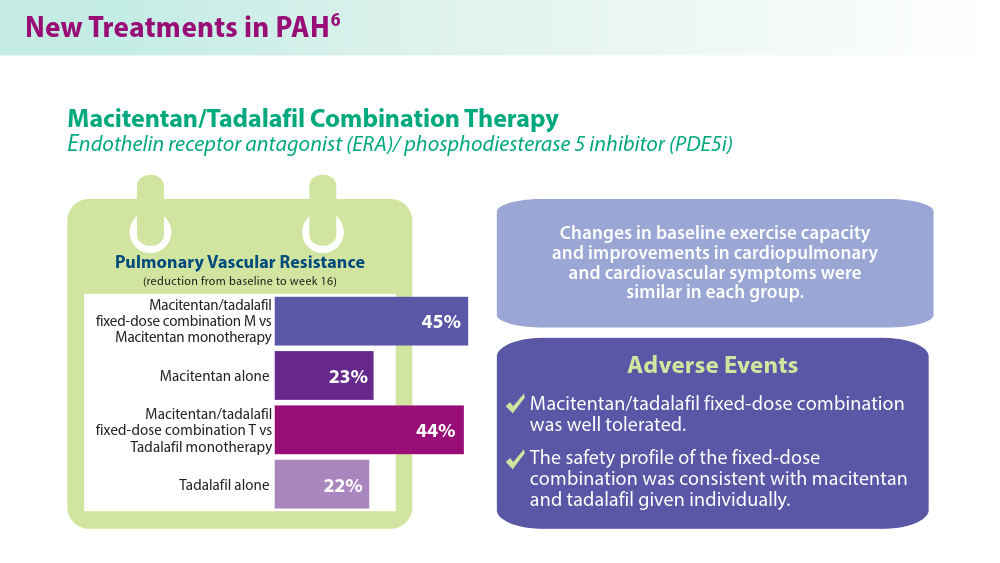
- Grünig E, Jansa P, Fan F, et al. Randomized trial of macitentan/tadalafil single-tablet combination therapy for pulmonary arterial hypertension. J Am Coll Cardiol. 2024;83(4):473-484. doi:10.1016/j.jacc.2023.10.045
- Higuchi S, Horinouchi H, Aoki T, et al. Balloon pulmonary angioplasty in the management of chronic thromboembolic pulmonary hypertension. Radiographics. 2022;42(6):1881-1896. doi:10.1148/rg.210102
- Cullivan S, Gaine S, Sitbon O. New trends in pulmonary hypertension. Eur Respir Rev. 2023;32(167):220211. doi:10.1183/16000617.0211-2022
- Mocumbi A, Humbert M, Saxena A, et al. Pulmonary hypertension [published correction appears in Nat Rev Dis Primers. 2024;10(1):5]. Nat Rev Dis Primers. 2024;10(1):1. doi:10.1038/s41572-023-00486-7
- Lang IM, Palazzini M. The burden of comorbidities in pulmonary arterial hypertension. Eur Heart J Suppl. 2019;21(suppl K):K21-K28. doi:10.1093/ eurheartj/suz205
- Yan L, Zhao Z, Zhao Q, et al. The clinical characteristics of patients with pulmonary hypertension combined with obstructive sleep apnoea. BMC Pulm Med. 2021;21(1):378. doi:10.1186/s12890-021-01755-5
- Hoeper MM, Badesch DB, Ghofrani HA, et al; for the STELLAR Trial Investigators. Phase 3 trial of sotatercept for treatment of pulmonary arterial hypertension. N Engl J Med. 2023;388(16):1478-1490. doi:10.1056/NEJMoa2213558
- Grünig E, Jansa P, Fan F, et al. Randomized trial of macitentan/tadalafil single-tablet combination therapy for pulmonary arterial hypertension. J Am Coll Cardiol. 2024;83(4):473-484. doi:10.1016/j.jacc.2023.10.045
- Higuchi S, Horinouchi H, Aoki T, et al. Balloon pulmonary angioplasty in the management of chronic thromboembolic pulmonary hypertension. Radiographics. 2022;42(6):1881-1896. doi:10.1148/rg.210102
- Cullivan S, Gaine S, Sitbon O. New trends in pulmonary hypertension. Eur Respir Rev. 2023;32(167):220211. doi:10.1183/16000617.0211-2022
- Mocumbi A, Humbert M, Saxena A, et al. Pulmonary hypertension [published correction appears in Nat Rev Dis Primers. 2024;10(1):5]. Nat Rev Dis Primers. 2024;10(1):1. doi:10.1038/s41572-023-00486-7
- Lang IM, Palazzini M. The burden of comorbidities in pulmonary arterial hypertension. Eur Heart J Suppl. 2019;21(suppl K):K21-K28. doi:10.1093/ eurheartj/suz205
- Yan L, Zhao Z, Zhao Q, et al. The clinical characteristics of patients with pulmonary hypertension combined with obstructive sleep apnoea. BMC Pulm Med. 2021;21(1):378. doi:10.1186/s12890-021-01755-5
- Hoeper MM, Badesch DB, Ghofrani HA, et al; for the STELLAR Trial Investigators. Phase 3 trial of sotatercept for treatment of pulmonary arterial hypertension. N Engl J Med. 2023;388(16):1478-1490. doi:10.1056/NEJMoa2213558
- Grünig E, Jansa P, Fan F, et al. Randomized trial of macitentan/tadalafil single-tablet combination therapy for pulmonary arterial hypertension. J Am Coll Cardiol. 2024;83(4):473-484. doi:10.1016/j.jacc.2023.10.045
- Higuchi S, Horinouchi H, Aoki T, et al. Balloon pulmonary angioplasty in the management of chronic thromboembolic pulmonary hypertension. Radiographics. 2022;42(6):1881-1896. doi:10.1148/rg.210102
Advancements in nutritional management for critically ill patients
CRITICAL CARE NETWORK
Nonrespiratory Critical Care Section
Nutrition plays an important role in the management and recovery of critically ill patients admitted to the ICU. Major guidelines recommend that critically ill patients should receive 1.2 to 2.0 g/kg/day of protein, with an emphasis on early (within 48 hours of ICU admission) enteral nutrition.1-3
In a randomized controlled trial involving 173 critically ill patients who stayed in the ICU in Zhejiang, China, Wang and colleagues studied the impact of early high protein intake (1.5 g/kg/day vs 0.8 g/kg/day).4 The primary outcome of 28-day mortality was lower among the high protein intake group (8.14% vs 19.54%). Still, this intention-to-treat analysis did not reach a statistical significance (P = .051). However, a time-to-event analysis using the Cox proportional hazard model showed that the high protein intake group had a significantly lower 28-day mortality rate, shorter ICU stays, and improved nutritional status, particularly in patients with sepsis (P = .045).
In a systematic review and meta-analysis involving 19 randomized controlled trials and 1,731 patients, there was no definitive evidence that higher protein intake significantly reduces mortality. However, it may improve specific clinical outcomes like muscle mass retention and shorter duration of mechanical ventilation.5 Similarly, a post hoc analysis on the EFFORT Protein Trial focusing on critically ill patients with acute kidney injury (AKI) showed that higher protein intake did not significantly impact the duration of kidney replacement therapy but was associated with higher serum urea levels and slower time-to-discharge-alive among patients with AKI.6
For critically ill patients, increasing early protein intake to 1.5 g/kg/day is safe and may be beneficial. We still need more data to guide the best approach to determining the protein intake.
References
1. Taylor BE, McClave SA, Martindale RG, et al. Guidelines for the provision and assessment of nutrition support therapy in the adult critically ill patient: Society of Critical Care Medicine (SCCM) and American Society for Parenteral and Enteral Nutrition (ASPEN). Crit Care Med. 2016;44(2):390-438. doi:10.1097/CCM.0000000000001525
2. Singer P, Blaser AR, Berger MM, et al. ESPEN guideline on clinical nutrition in the intensive care unit. Clin Nutr. 2019;38(1):48-79. doi:10.1016/j.clnu.2018.08.037
3. McClave SA, Taylor BE, Martindale RG, et al. Guidelines for the provision and assessment of nutrition support therapy in the adult critically ill patient: Society of Critical Care Medicine (SCCM) and American Society for Parenteral and Enteral Nutrition (ASPEN). JJPEN J Parenter Enteral Nutr. 2016;40(2):159-211. doi:10.1177/0148607115621863
4. Wang Y, Ye Y, Xuan L, et al. Impact of early high protein intake in critically ill patients: a randomized controlled trial. Nutr Metab. 2024;21(1):39. doi.org/10.1186/s12986-024-00818-8
5. Lee ZY, Yap CSL, Hasan MS, et al. The effect of higher versus lower protein delivery in critically ill patients: a systematic review and meta-analysis of randomized controlled trials. Crit Care. 2021;25(1):260. doi.org/10.1186/s13054-021-03693-4
6. Stoppe C, Patel JJ, Zarbock A, et al. The impact of higher protein dosing on outcomes in critically ill patients with acute kidney injury: a post hoc analysis of the EFFORT protein trial. Crit Care. 2023;27(1):399. doi.org/10.1186/s13054-023-04663-8
CRITICAL CARE NETWORK
Nonrespiratory Critical Care Section
Nutrition plays an important role in the management and recovery of critically ill patients admitted to the ICU. Major guidelines recommend that critically ill patients should receive 1.2 to 2.0 g/kg/day of protein, with an emphasis on early (within 48 hours of ICU admission) enteral nutrition.1-3
In a randomized controlled trial involving 173 critically ill patients who stayed in the ICU in Zhejiang, China, Wang and colleagues studied the impact of early high protein intake (1.5 g/kg/day vs 0.8 g/kg/day).4 The primary outcome of 28-day mortality was lower among the high protein intake group (8.14% vs 19.54%). Still, this intention-to-treat analysis did not reach a statistical significance (P = .051). However, a time-to-event analysis using the Cox proportional hazard model showed that the high protein intake group had a significantly lower 28-day mortality rate, shorter ICU stays, and improved nutritional status, particularly in patients with sepsis (P = .045).
In a systematic review and meta-analysis involving 19 randomized controlled trials and 1,731 patients, there was no definitive evidence that higher protein intake significantly reduces mortality. However, it may improve specific clinical outcomes like muscle mass retention and shorter duration of mechanical ventilation.5 Similarly, a post hoc analysis on the EFFORT Protein Trial focusing on critically ill patients with acute kidney injury (AKI) showed that higher protein intake did not significantly impact the duration of kidney replacement therapy but was associated with higher serum urea levels and slower time-to-discharge-alive among patients with AKI.6
For critically ill patients, increasing early protein intake to 1.5 g/kg/day is safe and may be beneficial. We still need more data to guide the best approach to determining the protein intake.
References
1. Taylor BE, McClave SA, Martindale RG, et al. Guidelines for the provision and assessment of nutrition support therapy in the adult critically ill patient: Society of Critical Care Medicine (SCCM) and American Society for Parenteral and Enteral Nutrition (ASPEN). Crit Care Med. 2016;44(2):390-438. doi:10.1097/CCM.0000000000001525
2. Singer P, Blaser AR, Berger MM, et al. ESPEN guideline on clinical nutrition in the intensive care unit. Clin Nutr. 2019;38(1):48-79. doi:10.1016/j.clnu.2018.08.037
3. McClave SA, Taylor BE, Martindale RG, et al. Guidelines for the provision and assessment of nutrition support therapy in the adult critically ill patient: Society of Critical Care Medicine (SCCM) and American Society for Parenteral and Enteral Nutrition (ASPEN). JJPEN J Parenter Enteral Nutr. 2016;40(2):159-211. doi:10.1177/0148607115621863
4. Wang Y, Ye Y, Xuan L, et al. Impact of early high protein intake in critically ill patients: a randomized controlled trial. Nutr Metab. 2024;21(1):39. doi.org/10.1186/s12986-024-00818-8
5. Lee ZY, Yap CSL, Hasan MS, et al. The effect of higher versus lower protein delivery in critically ill patients: a systematic review and meta-analysis of randomized controlled trials. Crit Care. 2021;25(1):260. doi.org/10.1186/s13054-021-03693-4
6. Stoppe C, Patel JJ, Zarbock A, et al. The impact of higher protein dosing on outcomes in critically ill patients with acute kidney injury: a post hoc analysis of the EFFORT protein trial. Crit Care. 2023;27(1):399. doi.org/10.1186/s13054-023-04663-8
CRITICAL CARE NETWORK
Nonrespiratory Critical Care Section
Nutrition plays an important role in the management and recovery of critically ill patients admitted to the ICU. Major guidelines recommend that critically ill patients should receive 1.2 to 2.0 g/kg/day of protein, with an emphasis on early (within 48 hours of ICU admission) enteral nutrition.1-3
In a randomized controlled trial involving 173 critically ill patients who stayed in the ICU in Zhejiang, China, Wang and colleagues studied the impact of early high protein intake (1.5 g/kg/day vs 0.8 g/kg/day).4 The primary outcome of 28-day mortality was lower among the high protein intake group (8.14% vs 19.54%). Still, this intention-to-treat analysis did not reach a statistical significance (P = .051). However, a time-to-event analysis using the Cox proportional hazard model showed that the high protein intake group had a significantly lower 28-day mortality rate, shorter ICU stays, and improved nutritional status, particularly in patients with sepsis (P = .045).
In a systematic review and meta-analysis involving 19 randomized controlled trials and 1,731 patients, there was no definitive evidence that higher protein intake significantly reduces mortality. However, it may improve specific clinical outcomes like muscle mass retention and shorter duration of mechanical ventilation.5 Similarly, a post hoc analysis on the EFFORT Protein Trial focusing on critically ill patients with acute kidney injury (AKI) showed that higher protein intake did not significantly impact the duration of kidney replacement therapy but was associated with higher serum urea levels and slower time-to-discharge-alive among patients with AKI.6
For critically ill patients, increasing early protein intake to 1.5 g/kg/day is safe and may be beneficial. We still need more data to guide the best approach to determining the protein intake.
References
1. Taylor BE, McClave SA, Martindale RG, et al. Guidelines for the provision and assessment of nutrition support therapy in the adult critically ill patient: Society of Critical Care Medicine (SCCM) and American Society for Parenteral and Enteral Nutrition (ASPEN). Crit Care Med. 2016;44(2):390-438. doi:10.1097/CCM.0000000000001525
2. Singer P, Blaser AR, Berger MM, et al. ESPEN guideline on clinical nutrition in the intensive care unit. Clin Nutr. 2019;38(1):48-79. doi:10.1016/j.clnu.2018.08.037
3. McClave SA, Taylor BE, Martindale RG, et al. Guidelines for the provision and assessment of nutrition support therapy in the adult critically ill patient: Society of Critical Care Medicine (SCCM) and American Society for Parenteral and Enteral Nutrition (ASPEN). JJPEN J Parenter Enteral Nutr. 2016;40(2):159-211. doi:10.1177/0148607115621863
4. Wang Y, Ye Y, Xuan L, et al. Impact of early high protein intake in critically ill patients: a randomized controlled trial. Nutr Metab. 2024;21(1):39. doi.org/10.1186/s12986-024-00818-8
5. Lee ZY, Yap CSL, Hasan MS, et al. The effect of higher versus lower protein delivery in critically ill patients: a systematic review and meta-analysis of randomized controlled trials. Crit Care. 2021;25(1):260. doi.org/10.1186/s13054-021-03693-4
6. Stoppe C, Patel JJ, Zarbock A, et al. The impact of higher protein dosing on outcomes in critically ill patients with acute kidney injury: a post hoc analysis of the EFFORT protein trial. Crit Care. 2023;27(1):399. doi.org/10.1186/s13054-023-04663-8
New developments on the forefront of intermediate-risk pulmonary embolism
PULMONARY VASCULAR AND CARDIOVASCULAR NETWORK
Cardiovascular Medicine and Surgery Section
Patients with intermediate-risk pulmonary embolism (IRPE), or those with right ventricular dysfunction without overt hemodynamic instability, represent a heterogenous population with short-term mortality ranging from 2% to 17%.1 While systemic anticoagulation is the mainstay therapy, select individuals may benefit from more immediate reperfusion. Unfortunately, only small, randomized trials exploring surrogate outcomes are available to guide modality and patient selection.2
To better define which patients with IRPE are best managed with which therapy, several large-scale randomized controlled trials are underway. PE-TRACT, a study funded by the National Institutes of Health, aims to randomize 500 patients with IRPE to anticoagulation alone vs one of several modalities of CBT with a focus on long-term functional outcomes, including peak oxygen consumption at 3 months and functional class at 1 year. Aspiration thrombectomy with the FlowTriever® device is being compared with anticoagulation alone in a study of 1,200 patients examining short-term composite end points.
While full-dose thrombolysis may decrease the composite outcome of death or hemodynamic deterioration in this population, the benefit is counterbalanced by the risk of significant bleeding. Whether reduced-dose thrombolysis is associated with improved outcomes has been questioned in several small studies. The PEITHO-3 trial plans to randomize 650 patients with IRPE to reduced-dose thrombolytics vs placebo, exploring several outcomes at 30 days. With multiple large trials ongoing, we anticipate important changes to the landscape of IRPE care over the coming years.
References
1. Fernández C, Bova C, Sanchez O, et al. Validation of a model for identification of patients at intermediate to high risk for complications associated with acute symptomatic pulmonary embolism. Chest. 2015;148(1):211-218. doi:10.1378/chest.14-2551
2. Yuriditsky E, Horowitz JM. The role of the PERT in the management and therapeutic decision-making in pulmonary embolism. Eur Heart J Acute Cardiovasc Care. 2022;11(9):693-694. doi:10.1093/ehjacc/zuac102
3. National Library of Medicine (US). A Randomized Trial of Ultrasound-facilitated, Catheter-directed, Thrombolysis Versus Anticoagulation for Acute Intermediate-high Risk Pulmonary Embolism: The Higher-risk Pulmonary Embolism Thrombolysis Study. Updated July 16, 2024. https://clinicaltrials.gov/study/NCT04790370
4. National Library of Medicine (US). PEERLESS II: RCT of FlowTriever vs. Anticoagulation Alone in Pulmonary Embolism. Updated July 17, 2024. https://clinicaltrials.gov/study/NCT06055920
5. National Library of Medicine (US). A Reduced Dose of Thrombolytic Treatment for Patients With Intermediate High-risk Acute Pulmonary Embolism: a Randomized Controled Trial. Updated July 17, 2024. https://clinicaltrials.gov/study/NCT04430569
PULMONARY VASCULAR AND CARDIOVASCULAR NETWORK
Cardiovascular Medicine and Surgery Section
Patients with intermediate-risk pulmonary embolism (IRPE), or those with right ventricular dysfunction without overt hemodynamic instability, represent a heterogenous population with short-term mortality ranging from 2% to 17%.1 While systemic anticoagulation is the mainstay therapy, select individuals may benefit from more immediate reperfusion. Unfortunately, only small, randomized trials exploring surrogate outcomes are available to guide modality and patient selection.2
To better define which patients with IRPE are best managed with which therapy, several large-scale randomized controlled trials are underway. PE-TRACT, a study funded by the National Institutes of Health, aims to randomize 500 patients with IRPE to anticoagulation alone vs one of several modalities of CBT with a focus on long-term functional outcomes, including peak oxygen consumption at 3 months and functional class at 1 year. Aspiration thrombectomy with the FlowTriever® device is being compared with anticoagulation alone in a study of 1,200 patients examining short-term composite end points.
While full-dose thrombolysis may decrease the composite outcome of death or hemodynamic deterioration in this population, the benefit is counterbalanced by the risk of significant bleeding. Whether reduced-dose thrombolysis is associated with improved outcomes has been questioned in several small studies. The PEITHO-3 trial plans to randomize 650 patients with IRPE to reduced-dose thrombolytics vs placebo, exploring several outcomes at 30 days. With multiple large trials ongoing, we anticipate important changes to the landscape of IRPE care over the coming years.
References
1. Fernández C, Bova C, Sanchez O, et al. Validation of a model for identification of patients at intermediate to high risk for complications associated with acute symptomatic pulmonary embolism. Chest. 2015;148(1):211-218. doi:10.1378/chest.14-2551
2. Yuriditsky E, Horowitz JM. The role of the PERT in the management and therapeutic decision-making in pulmonary embolism. Eur Heart J Acute Cardiovasc Care. 2022;11(9):693-694. doi:10.1093/ehjacc/zuac102
3. National Library of Medicine (US). A Randomized Trial of Ultrasound-facilitated, Catheter-directed, Thrombolysis Versus Anticoagulation for Acute Intermediate-high Risk Pulmonary Embolism: The Higher-risk Pulmonary Embolism Thrombolysis Study. Updated July 16, 2024. https://clinicaltrials.gov/study/NCT04790370
4. National Library of Medicine (US). PEERLESS II: RCT of FlowTriever vs. Anticoagulation Alone in Pulmonary Embolism. Updated July 17, 2024. https://clinicaltrials.gov/study/NCT06055920
5. National Library of Medicine (US). A Reduced Dose of Thrombolytic Treatment for Patients With Intermediate High-risk Acute Pulmonary Embolism: a Randomized Controled Trial. Updated July 17, 2024. https://clinicaltrials.gov/study/NCT04430569
PULMONARY VASCULAR AND CARDIOVASCULAR NETWORK
Cardiovascular Medicine and Surgery Section
Patients with intermediate-risk pulmonary embolism (IRPE), or those with right ventricular dysfunction without overt hemodynamic instability, represent a heterogenous population with short-term mortality ranging from 2% to 17%.1 While systemic anticoagulation is the mainstay therapy, select individuals may benefit from more immediate reperfusion. Unfortunately, only small, randomized trials exploring surrogate outcomes are available to guide modality and patient selection.2
To better define which patients with IRPE are best managed with which therapy, several large-scale randomized controlled trials are underway. PE-TRACT, a study funded by the National Institutes of Health, aims to randomize 500 patients with IRPE to anticoagulation alone vs one of several modalities of CBT with a focus on long-term functional outcomes, including peak oxygen consumption at 3 months and functional class at 1 year. Aspiration thrombectomy with the FlowTriever® device is being compared with anticoagulation alone in a study of 1,200 patients examining short-term composite end points.
While full-dose thrombolysis may decrease the composite outcome of death or hemodynamic deterioration in this population, the benefit is counterbalanced by the risk of significant bleeding. Whether reduced-dose thrombolysis is associated with improved outcomes has been questioned in several small studies. The PEITHO-3 trial plans to randomize 650 patients with IRPE to reduced-dose thrombolytics vs placebo, exploring several outcomes at 30 days. With multiple large trials ongoing, we anticipate important changes to the landscape of IRPE care over the coming years.
References
1. Fernández C, Bova C, Sanchez O, et al. Validation of a model for identification of patients at intermediate to high risk for complications associated with acute symptomatic pulmonary embolism. Chest. 2015;148(1):211-218. doi:10.1378/chest.14-2551
2. Yuriditsky E, Horowitz JM. The role of the PERT in the management and therapeutic decision-making in pulmonary embolism. Eur Heart J Acute Cardiovasc Care. 2022;11(9):693-694. doi:10.1093/ehjacc/zuac102
3. National Library of Medicine (US). A Randomized Trial of Ultrasound-facilitated, Catheter-directed, Thrombolysis Versus Anticoagulation for Acute Intermediate-high Risk Pulmonary Embolism: The Higher-risk Pulmonary Embolism Thrombolysis Study. Updated July 16, 2024. https://clinicaltrials.gov/study/NCT04790370
4. National Library of Medicine (US). PEERLESS II: RCT of FlowTriever vs. Anticoagulation Alone in Pulmonary Embolism. Updated July 17, 2024. https://clinicaltrials.gov/study/NCT06055920
5. National Library of Medicine (US). A Reduced Dose of Thrombolytic Treatment for Patients With Intermediate High-risk Acute Pulmonary Embolism: a Randomized Controled Trial. Updated July 17, 2024. https://clinicaltrials.gov/study/NCT04430569
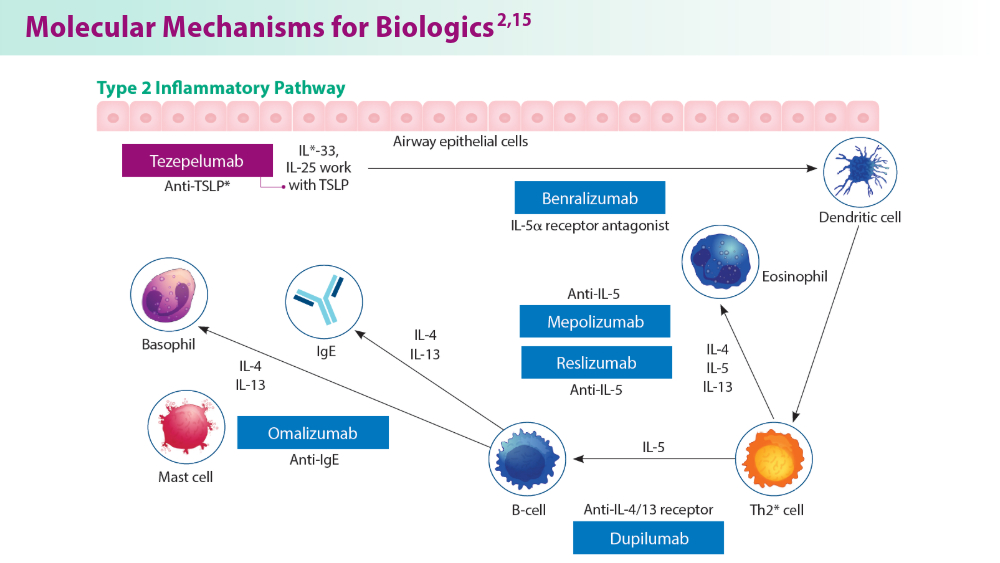
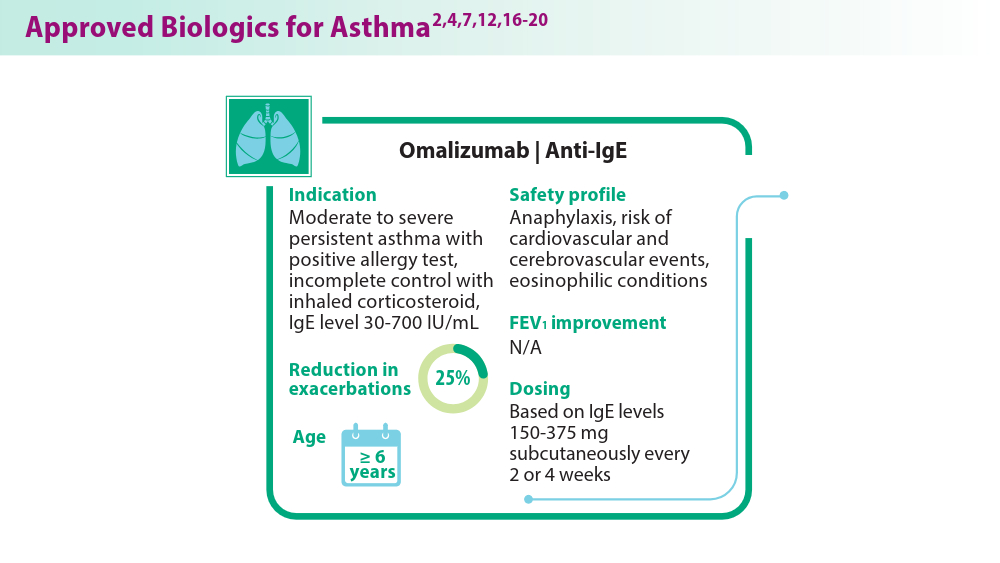
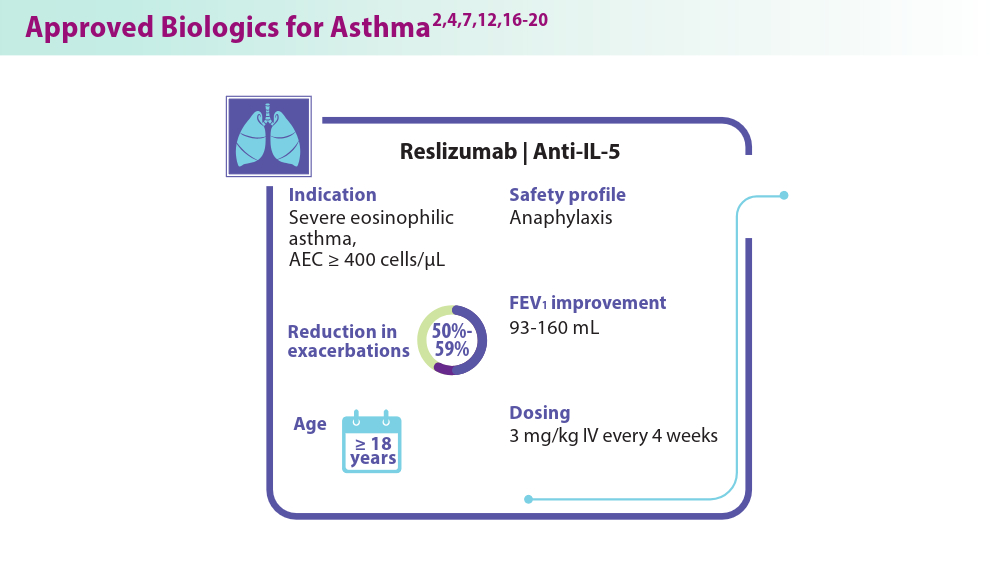
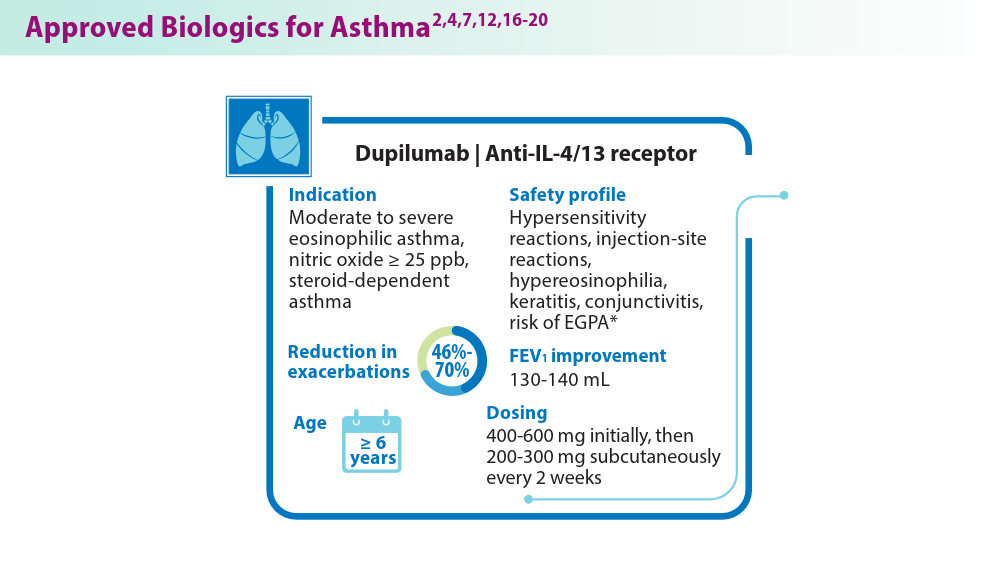
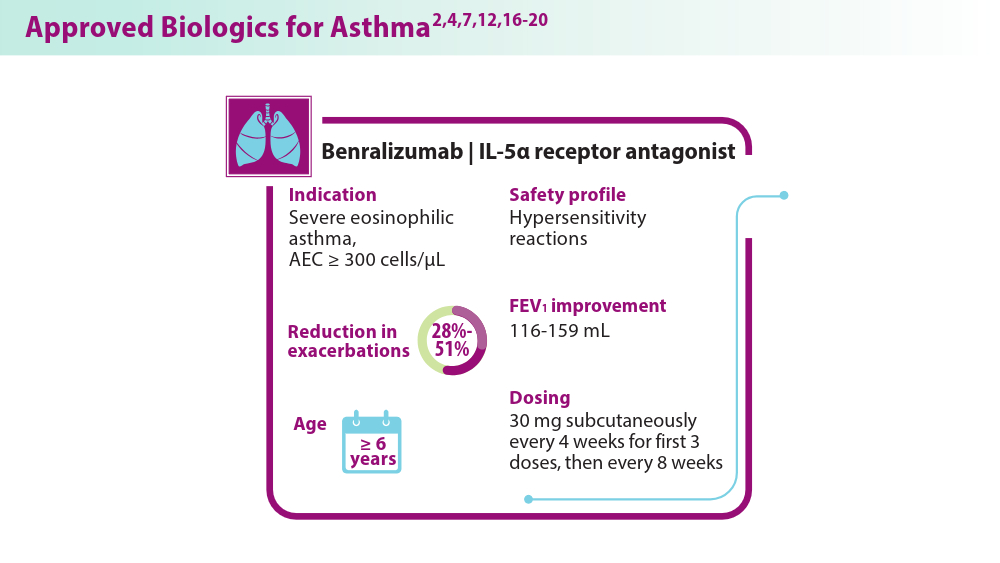
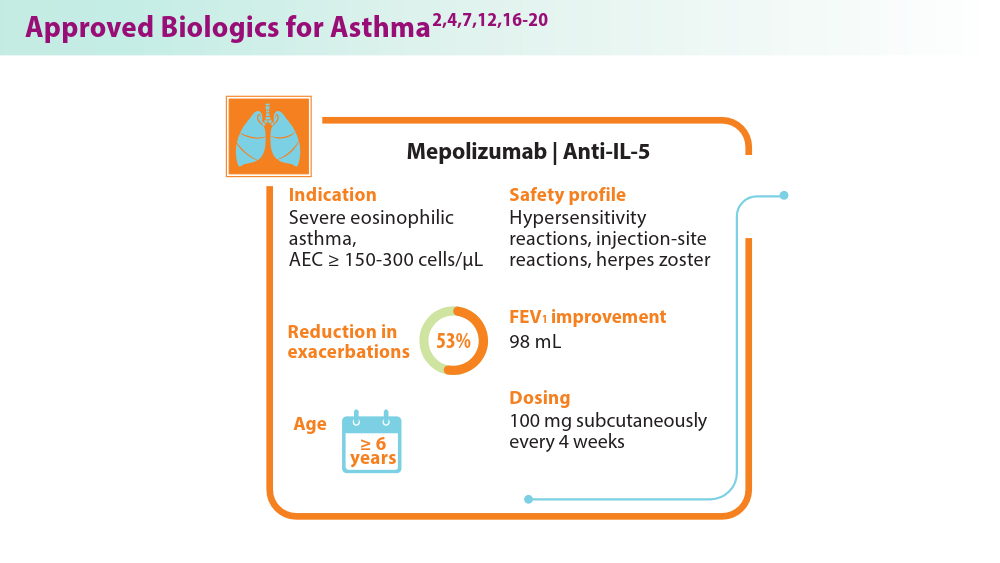
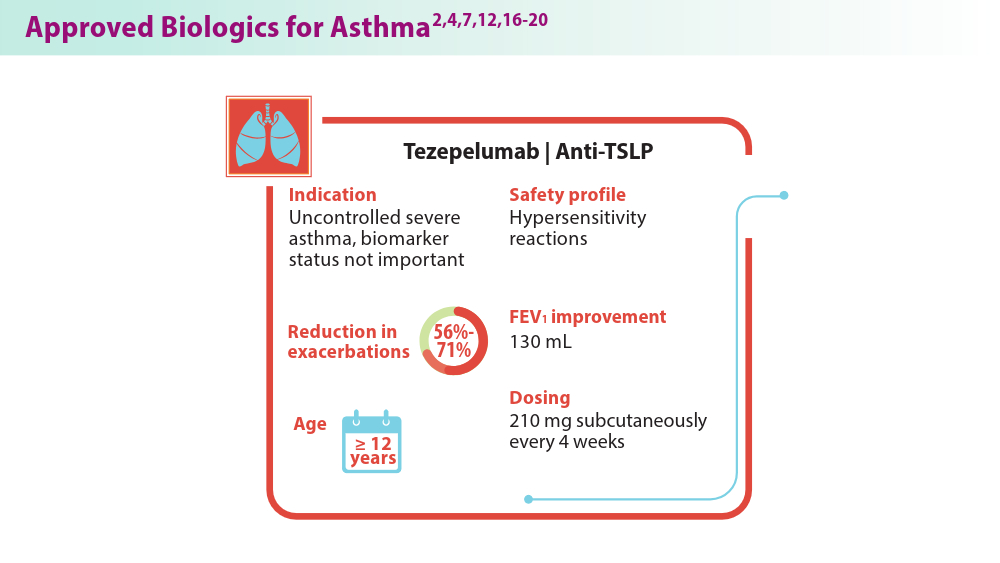
Biologics in Asthma: Changing the Severe Asthma Paradigm
- Shah PA, Brightling C. Biologics for severe asthma—which, when and why? Respirology. 2023;28(8):709-721. doi:10.1111/resp.14520
- Rogers L, Jesenak M, Bjermer L, Hanania NA, Seys SF, Diamant Z. Biologics in severe asthma: a pragmatic approach for choosing the right treatment for the right patient. Respir Med. 2023;218:107414. doi:10.1016/j.rmed.2023.107414
- Frøssing L, Silberbrandt A, Von Bülow A, Backer V, Porsbjerg C. The Prevalence of Subtypes of Type 2 Inflammation in an Unselected Population of Patients with Severe Asthma. J Allergy Clin Immunol Pract. 2021;9(3):1267-1275. doi:10.1016/j.jaip.2020.09.051
- McGregor MC, Krings JG, Nair P, Castro M. Role of biologics in asthma. Am J Respir Crit Care Med. 2019;199(4):433-445. doi:10.1164/rccm.201810-1944CI
- d'Ancona G, Kavanagh J, Roxas C, et al. Adherence to corticosteroids and clinical outcomes in mepolizumab therapy for severe asthma. Eur Respir J. 2020;55(5):1902259. Published 2020 May 7. doi:10.1183/13993003.02259-2019
- Exacerbation reduction & other clinical information | TEZSPIRE® (tezepelumab-Ekko) for hcps. Accessed July 25, 2024. https://www.tezspirehcp.com/efficacy-and-clinical-data/exacerbation-reductions-and-clinical-in-formation.html
- Exacerbation reduction in patients 12+ years. DUPIXENT® (dupilumab) for healthcare providers. Accessed June 18, 2024. https://www.dupixenthcp.com/asthma/efficacy/exacerbations
- Korn S, Bourdin A, Chupp G, et al. Integrated Safety and Efficacy Among Patients Receiving Benralizumab for Up to 5 Years. J Allergy Clin Immunol Pract. 2021;9(12):4381-4392.e4. doi:10.1016/j.jaip.2021.07.058
- Jackson DJ, Heaney LG, Humbert M, et al; for the SHAMAL Investigators. Reduction of daily maintenance inhaled corticosteroids in patients with severe eosinophilic asthma treated with benralizumab (SHAMAL): a randomised, multicentre, open-label, phase 4 study [published correction appears in Lancet. 2024;403(10432):1140]. Lancet. 2024;403(10423):271-281. doi:10.1016/S0140-6736(23)02284-5
- Thomas D, McDonald VM, Stevens S, et al. Biologics (mepolizumab and omalizumab) induced remission in severe asthma patients. Allergy. 2024;79(2):384-392. doi:10.1111/all.15867
- Hansen S, Baastrup Søndergaard M, von Bülow A, et al. Clinical response and remission in patients with severe asthma treated with biologic therapies. Chest. 2024;165(2):253-266. doi:10.1016/j.chest.2023.10.046
- Bagnasco D, Savarino EV, Yacoub MR, et al. Personalized and precision medicine in asthma and eosinophilic esophagitis: the role of T2 target therapy. Pharmaceutics. 2023;15(9):2359. doi:10.3390/pharmaceutics15092359
- Wang E, Wechsler ME, Tran TN, et al. Characterization of severe asthma worldwide: data from the International Severe Asthma Registry [published correction appears in Chest. 2021;160(5):1989.]. Chest. 2020;157(4):790-804. doi:10.1016/j.chest.2019.10.053
- Inselman JW, Jeffery MM, Maddux JT, Shah NS, Rank MA. Trends and Disparities in Asthma Biologic Use in the United States. J Allergy Clin Immunol Pract. 2020;8(2):549-554.e1. doi:10.1016/j.jaip.2019.08.024
- Pelaia C, Crimi C, Vatrella A, Tinello C, Terracciano R, Pelaia G. Molecular targets for biological therapies of severe asthma. Front Immunol. 2020;11:603312. doi:10.3389/fimmu.2020.603312
- Biologics for the treatment of asthma. Asthma and Allergy Foundation of America. Reviewed November 2023. Accessed June 18, 2024. https://aafa.org/asthma/asthma-treatment/biologics-asthma-treatment/
- Safety profile. TEZSPIRE® (tezepelumab-ekko) for healthcare providers. Accessed June 18, 2024. https://www.tezspirehcp.com/safety-profile.html
- Nucala (mepolizumab) for hcps. Severe Eosinophilic Asthma | NUCALA (mepolizumab) for HCPs. Accessed August 1, 2024. https://nucalahcp.com/severe-eosinophilic-asthma/.
- Xolair® (omalizumab). xolair. Accessed August 1, 2024. https://www.xolairhcp.com/allergic-asthma/side-effects/summary.html.
- Cinqair. Cinqairhcp.com. Accessed August 1, 2024. https://www.cinqairhcp.com/efficacy-and-safety-profiles/.
- Shah PA, Brightling C. Biologics for severe asthma—which, when and why? Respirology. 2023;28(8):709-721. doi:10.1111/resp.14520
- Rogers L, Jesenak M, Bjermer L, Hanania NA, Seys SF, Diamant Z. Biologics in severe asthma: a pragmatic approach for choosing the right treatment for the right patient. Respir Med. 2023;218:107414. doi:10.1016/j.rmed.2023.107414
- Frøssing L, Silberbrandt A, Von Bülow A, Backer V, Porsbjerg C. The Prevalence of Subtypes of Type 2 Inflammation in an Unselected Population of Patients with Severe Asthma. J Allergy Clin Immunol Pract. 2021;9(3):1267-1275. doi:10.1016/j.jaip.2020.09.051
- McGregor MC, Krings JG, Nair P, Castro M. Role of biologics in asthma. Am J Respir Crit Care Med. 2019;199(4):433-445. doi:10.1164/rccm.201810-1944CI
- d'Ancona G, Kavanagh J, Roxas C, et al. Adherence to corticosteroids and clinical outcomes in mepolizumab therapy for severe asthma. Eur Respir J. 2020;55(5):1902259. Published 2020 May 7. doi:10.1183/13993003.02259-2019
- Exacerbation reduction & other clinical information | TEZSPIRE® (tezepelumab-Ekko) for hcps. Accessed July 25, 2024. https://www.tezspirehcp.com/efficacy-and-clinical-data/exacerbation-reductions-and-clinical-in-formation.html
- Exacerbation reduction in patients 12+ years. DUPIXENT® (dupilumab) for healthcare providers. Accessed June 18, 2024. https://www.dupixenthcp.com/asthma/efficacy/exacerbations
- Korn S, Bourdin A, Chupp G, et al. Integrated Safety and Efficacy Among Patients Receiving Benralizumab for Up to 5 Years. J Allergy Clin Immunol Pract. 2021;9(12):4381-4392.e4. doi:10.1016/j.jaip.2021.07.058
- Jackson DJ, Heaney LG, Humbert M, et al; for the SHAMAL Investigators. Reduction of daily maintenance inhaled corticosteroids in patients with severe eosinophilic asthma treated with benralizumab (SHAMAL): a randomised, multicentre, open-label, phase 4 study [published correction appears in Lancet. 2024;403(10432):1140]. Lancet. 2024;403(10423):271-281. doi:10.1016/S0140-6736(23)02284-5
- Thomas D, McDonald VM, Stevens S, et al. Biologics (mepolizumab and omalizumab) induced remission in severe asthma patients. Allergy. 2024;79(2):384-392. doi:10.1111/all.15867
- Hansen S, Baastrup Søndergaard M, von Bülow A, et al. Clinical response and remission in patients with severe asthma treated with biologic therapies. Chest. 2024;165(2):253-266. doi:10.1016/j.chest.2023.10.046
- Bagnasco D, Savarino EV, Yacoub MR, et al. Personalized and precision medicine in asthma and eosinophilic esophagitis: the role of T2 target therapy. Pharmaceutics. 2023;15(9):2359. doi:10.3390/pharmaceutics15092359
- Wang E, Wechsler ME, Tran TN, et al. Characterization of severe asthma worldwide: data from the International Severe Asthma Registry [published correction appears in Chest. 2021;160(5):1989.]. Chest. 2020;157(4):790-804. doi:10.1016/j.chest.2019.10.053
- Inselman JW, Jeffery MM, Maddux JT, Shah NS, Rank MA. Trends and Disparities in Asthma Biologic Use in the United States. J Allergy Clin Immunol Pract. 2020;8(2):549-554.e1. doi:10.1016/j.jaip.2019.08.024
- Pelaia C, Crimi C, Vatrella A, Tinello C, Terracciano R, Pelaia G. Molecular targets for biological therapies of severe asthma. Front Immunol. 2020;11:603312. doi:10.3389/fimmu.2020.603312
- Biologics for the treatment of asthma. Asthma and Allergy Foundation of America. Reviewed November 2023. Accessed June 18, 2024. https://aafa.org/asthma/asthma-treatment/biologics-asthma-treatment/
- Safety profile. TEZSPIRE® (tezepelumab-ekko) for healthcare providers. Accessed June 18, 2024. https://www.tezspirehcp.com/safety-profile.html
- Nucala (mepolizumab) for hcps. Severe Eosinophilic Asthma | NUCALA (mepolizumab) for HCPs. Accessed August 1, 2024. https://nucalahcp.com/severe-eosinophilic-asthma/.
- Xolair® (omalizumab). xolair. Accessed August 1, 2024. https://www.xolairhcp.com/allergic-asthma/side-effects/summary.html.
- Cinqair. Cinqairhcp.com. Accessed August 1, 2024. https://www.cinqairhcp.com/efficacy-and-safety-profiles/.
- Shah PA, Brightling C. Biologics for severe asthma—which, when and why? Respirology. 2023;28(8):709-721. doi:10.1111/resp.14520
- Rogers L, Jesenak M, Bjermer L, Hanania NA, Seys SF, Diamant Z. Biologics in severe asthma: a pragmatic approach for choosing the right treatment for the right patient. Respir Med. 2023;218:107414. doi:10.1016/j.rmed.2023.107414
- Frøssing L, Silberbrandt A, Von Bülow A, Backer V, Porsbjerg C. The Prevalence of Subtypes of Type 2 Inflammation in an Unselected Population of Patients with Severe Asthma. J Allergy Clin Immunol Pract. 2021;9(3):1267-1275. doi:10.1016/j.jaip.2020.09.051
- McGregor MC, Krings JG, Nair P, Castro M. Role of biologics in asthma. Am J Respir Crit Care Med. 2019;199(4):433-445. doi:10.1164/rccm.201810-1944CI
- d'Ancona G, Kavanagh J, Roxas C, et al. Adherence to corticosteroids and clinical outcomes in mepolizumab therapy for severe asthma. Eur Respir J. 2020;55(5):1902259. Published 2020 May 7. doi:10.1183/13993003.02259-2019
- Exacerbation reduction & other clinical information | TEZSPIRE® (tezepelumab-Ekko) for hcps. Accessed July 25, 2024. https://www.tezspirehcp.com/efficacy-and-clinical-data/exacerbation-reductions-and-clinical-in-formation.html
- Exacerbation reduction in patients 12+ years. DUPIXENT® (dupilumab) for healthcare providers. Accessed June 18, 2024. https://www.dupixenthcp.com/asthma/efficacy/exacerbations
- Korn S, Bourdin A, Chupp G, et al. Integrated Safety and Efficacy Among Patients Receiving Benralizumab for Up to 5 Years. J Allergy Clin Immunol Pract. 2021;9(12):4381-4392.e4. doi:10.1016/j.jaip.2021.07.058
- Jackson DJ, Heaney LG, Humbert M, et al; for the SHAMAL Investigators. Reduction of daily maintenance inhaled corticosteroids in patients with severe eosinophilic asthma treated with benralizumab (SHAMAL): a randomised, multicentre, open-label, phase 4 study [published correction appears in Lancet. 2024;403(10432):1140]. Lancet. 2024;403(10423):271-281. doi:10.1016/S0140-6736(23)02284-5
- Thomas D, McDonald VM, Stevens S, et al. Biologics (mepolizumab and omalizumab) induced remission in severe asthma patients. Allergy. 2024;79(2):384-392. doi:10.1111/all.15867
- Hansen S, Baastrup Søndergaard M, von Bülow A, et al. Clinical response and remission in patients with severe asthma treated with biologic therapies. Chest. 2024;165(2):253-266. doi:10.1016/j.chest.2023.10.046
- Bagnasco D, Savarino EV, Yacoub MR, et al. Personalized and precision medicine in asthma and eosinophilic esophagitis: the role of T2 target therapy. Pharmaceutics. 2023;15(9):2359. doi:10.3390/pharmaceutics15092359
- Wang E, Wechsler ME, Tran TN, et al. Characterization of severe asthma worldwide: data from the International Severe Asthma Registry [published correction appears in Chest. 2021;160(5):1989.]. Chest. 2020;157(4):790-804. doi:10.1016/j.chest.2019.10.053
- Inselman JW, Jeffery MM, Maddux JT, Shah NS, Rank MA. Trends and Disparities in Asthma Biologic Use in the United States. J Allergy Clin Immunol Pract. 2020;8(2):549-554.e1. doi:10.1016/j.jaip.2019.08.024
- Pelaia C, Crimi C, Vatrella A, Tinello C, Terracciano R, Pelaia G. Molecular targets for biological therapies of severe asthma. Front Immunol. 2020;11:603312. doi:10.3389/fimmu.2020.603312
- Biologics for the treatment of asthma. Asthma and Allergy Foundation of America. Reviewed November 2023. Accessed June 18, 2024. https://aafa.org/asthma/asthma-treatment/biologics-asthma-treatment/
- Safety profile. TEZSPIRE® (tezepelumab-ekko) for healthcare providers. Accessed June 18, 2024. https://www.tezspirehcp.com/safety-profile.html
- Nucala (mepolizumab) for hcps. Severe Eosinophilic Asthma | NUCALA (mepolizumab) for HCPs. Accessed August 1, 2024. https://nucalahcp.com/severe-eosinophilic-asthma/.
- Xolair® (omalizumab). xolair. Accessed August 1, 2024. https://www.xolairhcp.com/allergic-asthma/side-effects/summary.html.
- Cinqair. Cinqairhcp.com. Accessed August 1, 2024. https://www.cinqairhcp.com/efficacy-and-safety-profiles/.
Bronchiectasis: A call to action
AIRWAYS DISORDERS NETWORK
Bronchiectasis Section
For years, the noncystic fibrosis (CF) bronchiectasis community has been trying to organize to provide better care for more than half a million adults with bronchiectasis in the United States. Internationally, the Europeans created the European Bronchiectasis Registry, which has been a powerful tool including nearly 20,000 patients, to answer important epidemiologic and management questions. We must do more for the bronchiectasis community.
Clinicaltrials.gov indicates that there are 8 international phase 3 or 4 clinical trials that are currently enrolling; 3 of those have enrollment sites in the United States. One such study from University of North Carolina at Chapel Hill is looking at the use of nebulized hypertonic saline in patients with non-CF bronchiectasis to understand the effect it has on mucociliary clearance. Emory University is looking at the use of elexacaftor/tezacaftor/ivacaftor (Trikafta) in patients with non-CF bronchiectasis; these patients have only 1 targetable mutation and a phenotype that resembles CF. This 8-week, open-label, single-center study aims to measure both clinical and biomarker outcomes after treatment with Trikafta. Finally, a phase 3 trial out of Florida, the ICoN-1 study, is examining the efficacy and safety of inhaled clofazimine in the treatment of nontuberculous mycobacteria (NTM). This double-blind, randomized trial will look at culture conversion and quality of life measures. Additionally, the COPD Foundation has created the Bronchiectasis and NTM Research Registry, an American cohort containing more than 5,000 patients and data from 22 different sites, to answer some of the most important questions for clinicians and patients.
We have made significant progress in bronchiectasis research; however, there is still much to learn. Together, we must make a concerted effort to enroll patients in clinical trials. Doing so will allow us to define our epidemiologic profile more precisely and explore new treatments and airway clearance techniques.
AIRWAYS DISORDERS NETWORK
Bronchiectasis Section
For years, the noncystic fibrosis (CF) bronchiectasis community has been trying to organize to provide better care for more than half a million adults with bronchiectasis in the United States. Internationally, the Europeans created the European Bronchiectasis Registry, which has been a powerful tool including nearly 20,000 patients, to answer important epidemiologic and management questions. We must do more for the bronchiectasis community.
Clinicaltrials.gov indicates that there are 8 international phase 3 or 4 clinical trials that are currently enrolling; 3 of those have enrollment sites in the United States. One such study from University of North Carolina at Chapel Hill is looking at the use of nebulized hypertonic saline in patients with non-CF bronchiectasis to understand the effect it has on mucociliary clearance. Emory University is looking at the use of elexacaftor/tezacaftor/ivacaftor (Trikafta) in patients with non-CF bronchiectasis; these patients have only 1 targetable mutation and a phenotype that resembles CF. This 8-week, open-label, single-center study aims to measure both clinical and biomarker outcomes after treatment with Trikafta. Finally, a phase 3 trial out of Florida, the ICoN-1 study, is examining the efficacy and safety of inhaled clofazimine in the treatment of nontuberculous mycobacteria (NTM). This double-blind, randomized trial will look at culture conversion and quality of life measures. Additionally, the COPD Foundation has created the Bronchiectasis and NTM Research Registry, an American cohort containing more than 5,000 patients and data from 22 different sites, to answer some of the most important questions for clinicians and patients.
We have made significant progress in bronchiectasis research; however, there is still much to learn. Together, we must make a concerted effort to enroll patients in clinical trials. Doing so will allow us to define our epidemiologic profile more precisely and explore new treatments and airway clearance techniques.
AIRWAYS DISORDERS NETWORK
Bronchiectasis Section
For years, the noncystic fibrosis (CF) bronchiectasis community has been trying to organize to provide better care for more than half a million adults with bronchiectasis in the United States. Internationally, the Europeans created the European Bronchiectasis Registry, which has been a powerful tool including nearly 20,000 patients, to answer important epidemiologic and management questions. We must do more for the bronchiectasis community.
Clinicaltrials.gov indicates that there are 8 international phase 3 or 4 clinical trials that are currently enrolling; 3 of those have enrollment sites in the United States. One such study from University of North Carolina at Chapel Hill is looking at the use of nebulized hypertonic saline in patients with non-CF bronchiectasis to understand the effect it has on mucociliary clearance. Emory University is looking at the use of elexacaftor/tezacaftor/ivacaftor (Trikafta) in patients with non-CF bronchiectasis; these patients have only 1 targetable mutation and a phenotype that resembles CF. This 8-week, open-label, single-center study aims to measure both clinical and biomarker outcomes after treatment with Trikafta. Finally, a phase 3 trial out of Florida, the ICoN-1 study, is examining the efficacy and safety of inhaled clofazimine in the treatment of nontuberculous mycobacteria (NTM). This double-blind, randomized trial will look at culture conversion and quality of life measures. Additionally, the COPD Foundation has created the Bronchiectasis and NTM Research Registry, an American cohort containing more than 5,000 patients and data from 22 different sites, to answer some of the most important questions for clinicians and patients.
We have made significant progress in bronchiectasis research; however, there is still much to learn. Together, we must make a concerted effort to enroll patients in clinical trials. Doing so will allow us to define our epidemiologic profile more precisely and explore new treatments and airway clearance techniques.
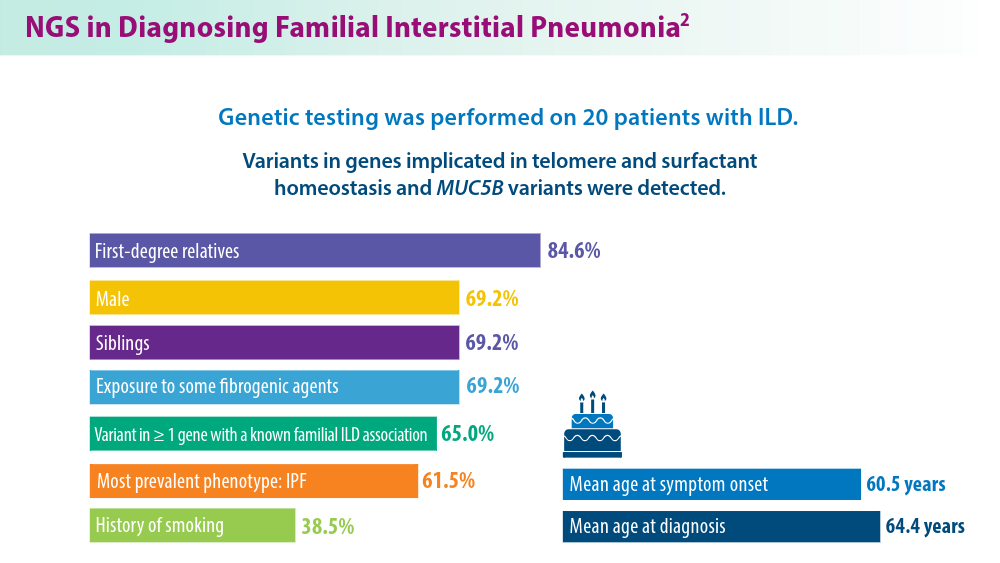
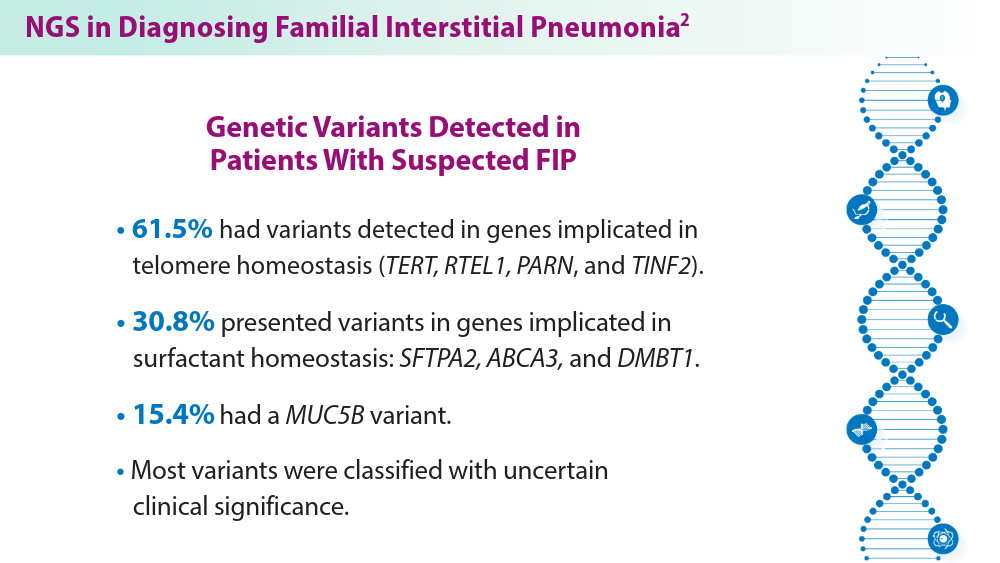
The Genetic Side of Interstitial Lung Disease
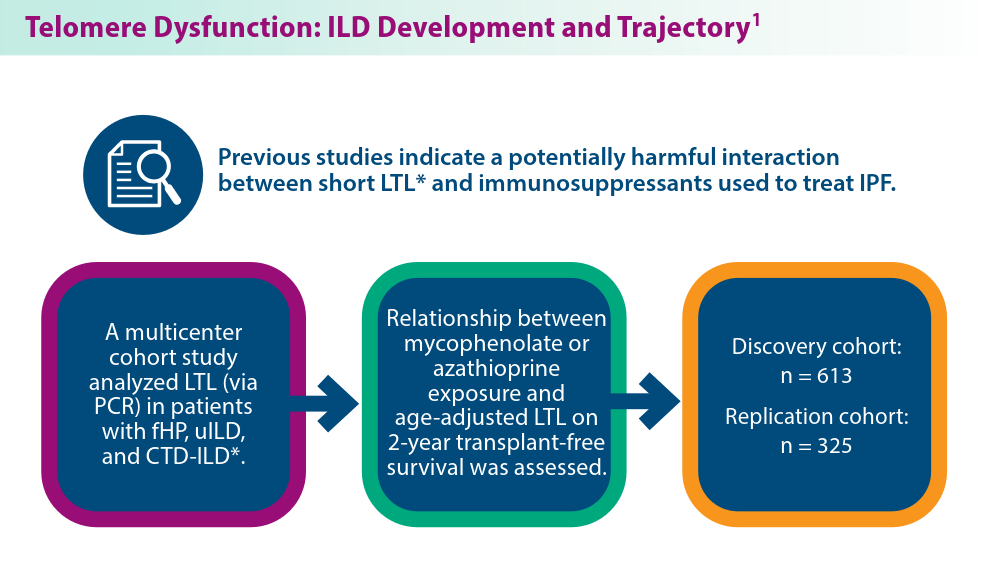
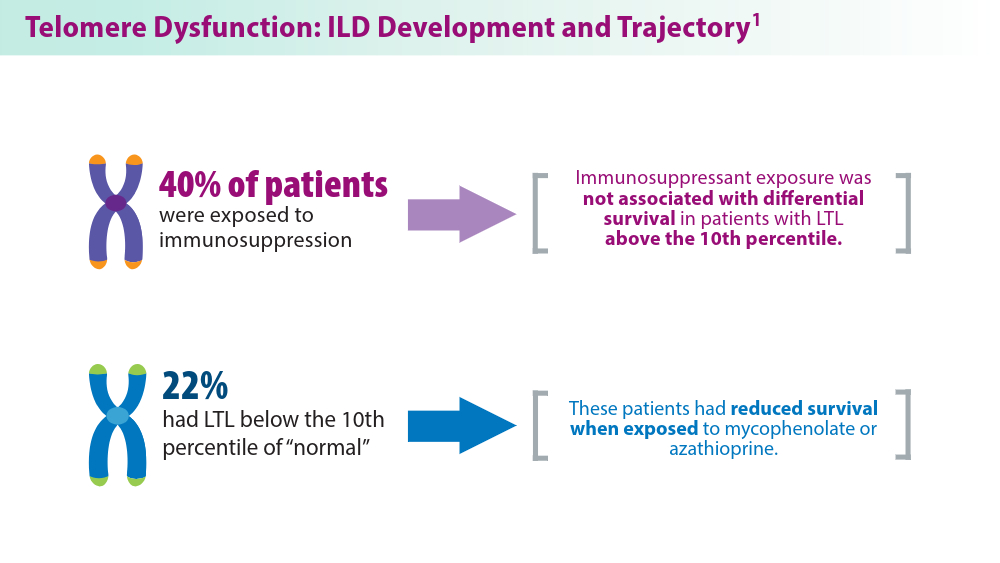
- Zhang D, Adegunsoye A, Oldham JM, et al. Telomere length and immunosuppression in non-idiopathic pulmonary fibrosis interstitial lung disease. Eur Respir J. 2023;62(5):2300441. doi:10.1183/13993003.00441-2023
- Gigante AR, Tinoco EM, Fonseca A, et al. Use of next-generation sequencing to support the diagnosis of familial interstitial pneumonia. Genes (Basel). 2023;14(2):326. doi:10.3390/genes14020326
- Adegunsoye A, Kropski JA, Behr J, et al. Genetics and genomics of pulmonary fibrosis: charting the molecular landscape and shaping precision medicine. Am J Respir Crit Care Med. Published online April 4, 2024. doi:10.1164/rccm.202401-0238SO
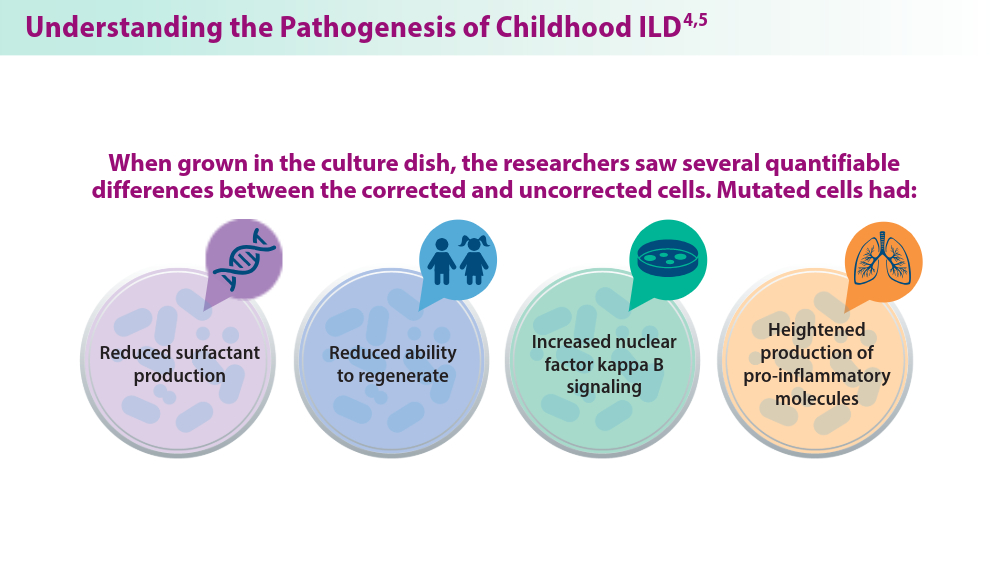
- Sun YL, Hennessey EE, Heins H, et al. Human pluripotent stem cell modeling of alveolar type 2 cell dysfunction caused by ABCA3 mutations. J Clin Invest. 2024;134(2):e164274. doi:10.1172/JCI164274
- Raghu G, Torres JM, Bennett RL. Genetic factors for ILD—the path of precision medicine. Lancet Respir Med. Published online March 20, 2024. doi:10.1016/S2213-2600(24)00071-7
- Zhang D, Adegunsoye A, Oldham JM, et al. Telomere length and immunosuppression in non-idiopathic pulmonary fibrosis interstitial lung disease. Eur Respir J. 2023;62(5):2300441. doi:10.1183/13993003.00441-2023
- Gigante AR, Tinoco EM, Fonseca A, et al. Use of next-generation sequencing to support the diagnosis of familial interstitial pneumonia. Genes (Basel). 2023;14(2):326. doi:10.3390/genes14020326
- Adegunsoye A, Kropski JA, Behr J, et al. Genetics and genomics of pulmonary fibrosis: charting the molecular landscape and shaping precision medicine. Am J Respir Crit Care Med. Published online April 4, 2024. doi:10.1164/rccm.202401-0238SO
- Sun YL, Hennessey EE, Heins H, et al. Human pluripotent stem cell modeling of alveolar type 2 cell dysfunction caused by ABCA3 mutations. J Clin Invest. 2024;134(2):e164274. doi:10.1172/JCI164274
- Raghu G, Torres JM, Bennett RL. Genetic factors for ILD—the path of precision medicine. Lancet Respir Med. Published online March 20, 2024. doi:10.1016/S2213-2600(24)00071-7
- Zhang D, Adegunsoye A, Oldham JM, et al. Telomere length and immunosuppression in non-idiopathic pulmonary fibrosis interstitial lung disease. Eur Respir J. 2023;62(5):2300441. doi:10.1183/13993003.00441-2023
- Gigante AR, Tinoco EM, Fonseca A, et al. Use of next-generation sequencing to support the diagnosis of familial interstitial pneumonia. Genes (Basel). 2023;14(2):326. doi:10.3390/genes14020326
- Adegunsoye A, Kropski JA, Behr J, et al. Genetics and genomics of pulmonary fibrosis: charting the molecular landscape and shaping precision medicine. Am J Respir Crit Care Med. Published online April 4, 2024. doi:10.1164/rccm.202401-0238SO
- Sun YL, Hennessey EE, Heins H, et al. Human pluripotent stem cell modeling of alveolar type 2 cell dysfunction caused by ABCA3 mutations. J Clin Invest. 2024;134(2):e164274. doi:10.1172/JCI164274
- Raghu G, Torres JM, Bennett RL. Genetic factors for ILD—the path of precision medicine. Lancet Respir Med. Published online March 20, 2024. doi:10.1016/S2213-2600(24)00071-7
The countdown to CHEST 2024 begins
As we find ourselves in September, I cannot help but dedicate my column to the upcoming CHEST Annual Meeting quickly approaching, October 6 to 9, in Boston.
If you haven’t yet been to a CHEST Annual Meeting, it’s an unmatched experience.
For those who have attended, there’s always something new to see. Every year is different, with the culture of the location guiding the way and new opportunities to network while engaging in activity. No matter how many times you have been, attending the CHEST Annual Meeting never gets old.
Leveraging CHEST 2024’s location, we’ll be hosting a Grand Rounds event days before the meeting starts with pulmonary and critical care medicine fellows from the regional Boston programs to learn from visiting CHEST leadership on a variety of influential topics. These fellowship programs held events like this prepandemic, so I’m truly excited we could help restart the tradition and give the local fellows an opportunity to interact with each other from both an academic and social perspective. Personally, I am very much looking forward to meeting and getting to know the fellows from the Boston area.
The meeting has a lot of notable opportunities lined up (see my official “President’s checklist”), including the third year of CHEST After Hours (Monday, October 7)—a unique storytelling event focusing on the humanities of medicine in partnership with The Nocturnists podcast. And for the first time in recent years, CHEST 2024 will feature a 5K run/walk (Tuesday, October 8) in support of CHEST philanthropy and its work to fuel breakthroughs, empower innovation, and drive toward a future where every patient’s well-being is safeguarded. I encourage you to register in advance of the meeting to secure your space and snag a souvenir T-shirt.
First thing Sunday morning (October 6), the meeting kicks off with the Opening Session where we will be celebrating the new fellows of the college (FCCP), honoring trailblazers in chest medicine, and welcoming this year’s keynote speaker.
This year’s keynote address will come from Vanessa Kerry, MD, who will speak on environmental issues and her work to raise awareness of the impact of climate change on health.
With so many things to look forward to, this meeting will be one to remember for all in attendance.
I look forward to seeing you in Boston,
Jack
As we find ourselves in September, I cannot help but dedicate my column to the upcoming CHEST Annual Meeting quickly approaching, October 6 to 9, in Boston.
If you haven’t yet been to a CHEST Annual Meeting, it’s an unmatched experience.
For those who have attended, there’s always something new to see. Every year is different, with the culture of the location guiding the way and new opportunities to network while engaging in activity. No matter how many times you have been, attending the CHEST Annual Meeting never gets old.
Leveraging CHEST 2024’s location, we’ll be hosting a Grand Rounds event days before the meeting starts with pulmonary and critical care medicine fellows from the regional Boston programs to learn from visiting CHEST leadership on a variety of influential topics. These fellowship programs held events like this prepandemic, so I’m truly excited we could help restart the tradition and give the local fellows an opportunity to interact with each other from both an academic and social perspective. Personally, I am very much looking forward to meeting and getting to know the fellows from the Boston area.
The meeting has a lot of notable opportunities lined up (see my official “President’s checklist”), including the third year of CHEST After Hours (Monday, October 7)—a unique storytelling event focusing on the humanities of medicine in partnership with The Nocturnists podcast. And for the first time in recent years, CHEST 2024 will feature a 5K run/walk (Tuesday, October 8) in support of CHEST philanthropy and its work to fuel breakthroughs, empower innovation, and drive toward a future where every patient’s well-being is safeguarded. I encourage you to register in advance of the meeting to secure your space and snag a souvenir T-shirt.
First thing Sunday morning (October 6), the meeting kicks off with the Opening Session where we will be celebrating the new fellows of the college (FCCP), honoring trailblazers in chest medicine, and welcoming this year’s keynote speaker.
This year’s keynote address will come from Vanessa Kerry, MD, who will speak on environmental issues and her work to raise awareness of the impact of climate change on health.
With so many things to look forward to, this meeting will be one to remember for all in attendance.
I look forward to seeing you in Boston,
Jack
As we find ourselves in September, I cannot help but dedicate my column to the upcoming CHEST Annual Meeting quickly approaching, October 6 to 9, in Boston.
If you haven’t yet been to a CHEST Annual Meeting, it’s an unmatched experience.
For those who have attended, there’s always something new to see. Every year is different, with the culture of the location guiding the way and new opportunities to network while engaging in activity. No matter how many times you have been, attending the CHEST Annual Meeting never gets old.
Leveraging CHEST 2024’s location, we’ll be hosting a Grand Rounds event days before the meeting starts with pulmonary and critical care medicine fellows from the regional Boston programs to learn from visiting CHEST leadership on a variety of influential topics. These fellowship programs held events like this prepandemic, so I’m truly excited we could help restart the tradition and give the local fellows an opportunity to interact with each other from both an academic and social perspective. Personally, I am very much looking forward to meeting and getting to know the fellows from the Boston area.
The meeting has a lot of notable opportunities lined up (see my official “President’s checklist”), including the third year of CHEST After Hours (Monday, October 7)—a unique storytelling event focusing on the humanities of medicine in partnership with The Nocturnists podcast. And for the first time in recent years, CHEST 2024 will feature a 5K run/walk (Tuesday, October 8) in support of CHEST philanthropy and its work to fuel breakthroughs, empower innovation, and drive toward a future where every patient’s well-being is safeguarded. I encourage you to register in advance of the meeting to secure your space and snag a souvenir T-shirt.
First thing Sunday morning (October 6), the meeting kicks off with the Opening Session where we will be celebrating the new fellows of the college (FCCP), honoring trailblazers in chest medicine, and welcoming this year’s keynote speaker.
This year’s keynote address will come from Vanessa Kerry, MD, who will speak on environmental issues and her work to raise awareness of the impact of climate change on health.
With so many things to look forward to, this meeting will be one to remember for all in attendance.
I look forward to seeing you in Boston,
Jack
Severe Community-Acquired Pneumonia: Diagnostic Criteria, Treatment, and COVID-19
- Torres A, Cilloniz C, Niederman MS, et al. Pneumonia. Nat Rev Dis Primers. 2021;7(1):25. doi:10.1038/s41572-021-00259-0
- Niederman MS, Torres A. Severe community-acquired pneumonia. Eur Respir Rev. 2022;31(166):220123. doi:10.1183/16000617.0123-2022
- Metlay JP, Waterer GW, Long AC, et al. Diagnosis and treatment of adults with community-acquired pneumonia. An official clinical practice guideline of the American Thoracic Society and Infectious Diseases Society of America. Am J Respir Crit Care Med. 2019;200(7):e45-e67. doi:10.1164/rccm.201908-1581ST
- Memon RA, Rashid MA, Avva S, et al. The use of the SMART-COP score in predicting severity outcomes among patients with community-acquired pneumonia: a meta-analysis. Cureus. 2022;14(7):e27248. doi:10.7759/cureus.27248
- Regunath H, Oba Y. Community-acquired pneumonia. StatPearls [Internet]. Treasure Island, FL: StatPearls Publishing; 2024. Updated January 26, 2024. Accessed May 14, 2024. https://www.ncbi.nlm.nih.gov/books/NBK430749/
- Dequin PF, Meziani F, Quenot JP, et al; for the CRICS-TriGGERSep Network. Hydrocortisone in severe community-acquired pneumonia. N Engl J Med. 2023;388(21):1931-1941. doi:10.1056/NEJMoa2215145
- Eizaguirre S, Sabater G, Belda S, et al. Long-term respiratory consequences of COVID-19 related pneumonia: a cohort study. BMC Pulm Med. 2023;23(1):439. doi:10.1186/s12890-023-02627-w
- Ramirez JA, Wiemken TL, Peyrani P, et al; for the University of Louisville Pneumonia Study Group. Adults hospitalized with pneumonia in the United States: incidence, epidemiology, and mortality. Clin Infect Dis. 2017;65(11):1806-1812. doi:10.1093/cid/cix647
- Morgan AJ, Glossop AJ. Severe community-acquired pneumonia. BJA Educ. 2016;16(5):167-172. doi:10.1093/bjaed/mkv052
- Haessler S, Guo N, Deshpande A, et al. Etiology, treatments, and outcomes of patients with severe community-acquired pneumonia in a large U.S. sample. Crit Care Med. 2022;50(7):1063-1071. doi:10.1097/CCM.0000000000005498
- Nolley EP, Sahetya SK, Hochberg CH, et al. Outcomes among mechanically ventilated patients with severe pneumonia and acute hypoxemic respiratory failure from SARS-CoV-2 and other etiologies. JAMA Netw Open. 2023;6(1):e2250401. doi:10.1001/jamanetworkopen.2022.50401
- Hino T, Nishino M, Valtchinov VI, et al. Severe COVID-19 pneumonia leads to post-COVID-19 lung abnormalities on follow-up CT scans. Eur J Radiol Open. 2023;10:100483. doi:10.1016/j.ejro.2023.100483
- Torres A, Cilloniz C, Niederman MS, et al. Pneumonia. Nat Rev Dis Primers. 2021;7(1):25. doi:10.1038/s41572-021-00259-0
- Niederman MS, Torres A. Severe community-acquired pneumonia. Eur Respir Rev. 2022;31(166):220123. doi:10.1183/16000617.0123-2022
- Metlay JP, Waterer GW, Long AC, et al. Diagnosis and treatment of adults with community-acquired pneumonia. An official clinical practice guideline of the American Thoracic Society and Infectious Diseases Society of America. Am J Respir Crit Care Med. 2019;200(7):e45-e67. doi:10.1164/rccm.201908-1581ST
- Memon RA, Rashid MA, Avva S, et al. The use of the SMART-COP score in predicting severity outcomes among patients with community-acquired pneumonia: a meta-analysis. Cureus. 2022;14(7):e27248. doi:10.7759/cureus.27248
- Regunath H, Oba Y. Community-acquired pneumonia. StatPearls [Internet]. Treasure Island, FL: StatPearls Publishing; 2024. Updated January 26, 2024. Accessed May 14, 2024. https://www.ncbi.nlm.nih.gov/books/NBK430749/
- Dequin PF, Meziani F, Quenot JP, et al; for the CRICS-TriGGERSep Network. Hydrocortisone in severe community-acquired pneumonia. N Engl J Med. 2023;388(21):1931-1941. doi:10.1056/NEJMoa2215145
- Eizaguirre S, Sabater G, Belda S, et al. Long-term respiratory consequences of COVID-19 related pneumonia: a cohort study. BMC Pulm Med. 2023;23(1):439. doi:10.1186/s12890-023-02627-w
- Ramirez JA, Wiemken TL, Peyrani P, et al; for the University of Louisville Pneumonia Study Group. Adults hospitalized with pneumonia in the United States: incidence, epidemiology, and mortality. Clin Infect Dis. 2017;65(11):1806-1812. doi:10.1093/cid/cix647
- Morgan AJ, Glossop AJ. Severe community-acquired pneumonia. BJA Educ. 2016;16(5):167-172. doi:10.1093/bjaed/mkv052
- Haessler S, Guo N, Deshpande A, et al. Etiology, treatments, and outcomes of patients with severe community-acquired pneumonia in a large U.S. sample. Crit Care Med. 2022;50(7):1063-1071. doi:10.1097/CCM.0000000000005498
- Nolley EP, Sahetya SK, Hochberg CH, et al. Outcomes among mechanically ventilated patients with severe pneumonia and acute hypoxemic respiratory failure from SARS-CoV-2 and other etiologies. JAMA Netw Open. 2023;6(1):e2250401. doi:10.1001/jamanetworkopen.2022.50401
- Hino T, Nishino M, Valtchinov VI, et al. Severe COVID-19 pneumonia leads to post-COVID-19 lung abnormalities on follow-up CT scans. Eur J Radiol Open. 2023;10:100483. doi:10.1016/j.ejro.2023.100483
- Torres A, Cilloniz C, Niederman MS, et al. Pneumonia. Nat Rev Dis Primers. 2021;7(1):25. doi:10.1038/s41572-021-00259-0
- Niederman MS, Torres A. Severe community-acquired pneumonia. Eur Respir Rev. 2022;31(166):220123. doi:10.1183/16000617.0123-2022
- Metlay JP, Waterer GW, Long AC, et al. Diagnosis and treatment of adults with community-acquired pneumonia. An official clinical practice guideline of the American Thoracic Society and Infectious Diseases Society of America. Am J Respir Crit Care Med. 2019;200(7):e45-e67. doi:10.1164/rccm.201908-1581ST
- Memon RA, Rashid MA, Avva S, et al. The use of the SMART-COP score in predicting severity outcomes among patients with community-acquired pneumonia: a meta-analysis. Cureus. 2022;14(7):e27248. doi:10.7759/cureus.27248
- Regunath H, Oba Y. Community-acquired pneumonia. StatPearls [Internet]. Treasure Island, FL: StatPearls Publishing; 2024. Updated January 26, 2024. Accessed May 14, 2024. https://www.ncbi.nlm.nih.gov/books/NBK430749/
- Dequin PF, Meziani F, Quenot JP, et al; for the CRICS-TriGGERSep Network. Hydrocortisone in severe community-acquired pneumonia. N Engl J Med. 2023;388(21):1931-1941. doi:10.1056/NEJMoa2215145
- Eizaguirre S, Sabater G, Belda S, et al. Long-term respiratory consequences of COVID-19 related pneumonia: a cohort study. BMC Pulm Med. 2023;23(1):439. doi:10.1186/s12890-023-02627-w
- Ramirez JA, Wiemken TL, Peyrani P, et al; for the University of Louisville Pneumonia Study Group. Adults hospitalized with pneumonia in the United States: incidence, epidemiology, and mortality. Clin Infect Dis. 2017;65(11):1806-1812. doi:10.1093/cid/cix647
- Morgan AJ, Glossop AJ. Severe community-acquired pneumonia. BJA Educ. 2016;16(5):167-172. doi:10.1093/bjaed/mkv052
- Haessler S, Guo N, Deshpande A, et al. Etiology, treatments, and outcomes of patients with severe community-acquired pneumonia in a large U.S. sample. Crit Care Med. 2022;50(7):1063-1071. doi:10.1097/CCM.0000000000005498
- Nolley EP, Sahetya SK, Hochberg CH, et al. Outcomes among mechanically ventilated patients with severe pneumonia and acute hypoxemic respiratory failure from SARS-CoV-2 and other etiologies. JAMA Netw Open. 2023;6(1):e2250401. doi:10.1001/jamanetworkopen.2022.50401
- Hino T, Nishino M, Valtchinov VI, et al. Severe COVID-19 pneumonia leads to post-COVID-19 lung abnormalities on follow-up CT scans. Eur J Radiol Open. 2023;10:100483. doi:10.1016/j.ejro.2023.100483