User login
MD-IQ only
Pulmonology Data Trends 2024
Pulmonology Data Trends 2024 is a supplement to CHEST Physician highlighting the latest breakthroughs in pulmonology research and treatments through a series of infographics.
Read more:
Artificial Intelligence in Sleep Apnea
Ritwick Agrawal, MD, MS, FCCP
RSV Updates: Prophylaxis Approval and Hospitalization for Severe RSV
Riddhi Upadhyay, MD
Biologics in Asthma: Changing the Severe Asthma Paradigm
Shyam Subramanian, MD, FCCP
Updates in COPD Guidelines and Treatment
Dharani K. Narendra, MD, FCCP
Targeted Therapies and Surgical Resection for Lung Cancer: Evolving Treatment Options
Saadia A. Faiz, MD, FCCP
Closing the GAP in Idiopathic Pulmonary Fibrosis
Humayun Anjum, MD, FCCP
Severe Community-Acquired Pneumonia: Diagnostic Criteria, Treatment, and COVID-19
Sujith V. Cherian, MD, FCCP
Pulmonary Hypertension: Comorbidities and Novel Therapies
Mary Jo S. Farmer, MD, PhD, FCCP
The Genetic Side of Interstitial Lung Disease
Priya Balakrishnan, MD, MS, FCCP
Noninvasive Ventilation in Neuromuscular Disease
Sreelatha Naik, MD, FCCP, and Kelly Lobrutto, CRNP
Pulmonology Data Trends 2024 is a supplement to CHEST Physician highlighting the latest breakthroughs in pulmonology research and treatments through a series of infographics.
Read more:
Artificial Intelligence in Sleep Apnea
Ritwick Agrawal, MD, MS, FCCP
RSV Updates: Prophylaxis Approval and Hospitalization for Severe RSV
Riddhi Upadhyay, MD
Biologics in Asthma: Changing the Severe Asthma Paradigm
Shyam Subramanian, MD, FCCP
Updates in COPD Guidelines and Treatment
Dharani K. Narendra, MD, FCCP
Targeted Therapies and Surgical Resection for Lung Cancer: Evolving Treatment Options
Saadia A. Faiz, MD, FCCP
Closing the GAP in Idiopathic Pulmonary Fibrosis
Humayun Anjum, MD, FCCP
Severe Community-Acquired Pneumonia: Diagnostic Criteria, Treatment, and COVID-19
Sujith V. Cherian, MD, FCCP
Pulmonary Hypertension: Comorbidities and Novel Therapies
Mary Jo S. Farmer, MD, PhD, FCCP
The Genetic Side of Interstitial Lung Disease
Priya Balakrishnan, MD, MS, FCCP
Noninvasive Ventilation in Neuromuscular Disease
Sreelatha Naik, MD, FCCP, and Kelly Lobrutto, CRNP
Pulmonology Data Trends 2024 is a supplement to CHEST Physician highlighting the latest breakthroughs in pulmonology research and treatments through a series of infographics.
Read more:
Artificial Intelligence in Sleep Apnea
Ritwick Agrawal, MD, MS, FCCP
RSV Updates: Prophylaxis Approval and Hospitalization for Severe RSV
Riddhi Upadhyay, MD
Biologics in Asthma: Changing the Severe Asthma Paradigm
Shyam Subramanian, MD, FCCP
Updates in COPD Guidelines and Treatment
Dharani K. Narendra, MD, FCCP
Targeted Therapies and Surgical Resection for Lung Cancer: Evolving Treatment Options
Saadia A. Faiz, MD, FCCP
Closing the GAP in Idiopathic Pulmonary Fibrosis
Humayun Anjum, MD, FCCP
Severe Community-Acquired Pneumonia: Diagnostic Criteria, Treatment, and COVID-19
Sujith V. Cherian, MD, FCCP
Pulmonary Hypertension: Comorbidities and Novel Therapies
Mary Jo S. Farmer, MD, PhD, FCCP
The Genetic Side of Interstitial Lung Disease
Priya Balakrishnan, MD, MS, FCCP
Noninvasive Ventilation in Neuromuscular Disease
Sreelatha Naik, MD, FCCP, and Kelly Lobrutto, CRNP
Pulmonary Hypertension: Comorbidities and Novel Therapeutics
- Cullivan S, Gaine S, Sitbon O. New trends in pulmonary hypertension. Eur Respir Rev. 2023;32(167):220211. doi:10.1183/16000617.0211-2022
- Mocumbi A, Humbert M, Saxena A, et al. Pulmonary hypertension [published correction appears in Nat Rev Dis Primers. 2024;10(1):5]. Nat Rev Dis Primers. 2024;10(1):1. doi:10.1038/s41572-023-00486-7
- Lang IM, Palazzini M. The burden of comorbidities in pulmonary arterial hypertension. Eur Heart J Suppl. 2019;21(suppl K):K21-K28. doi:10.1093/ eurheartj/suz205
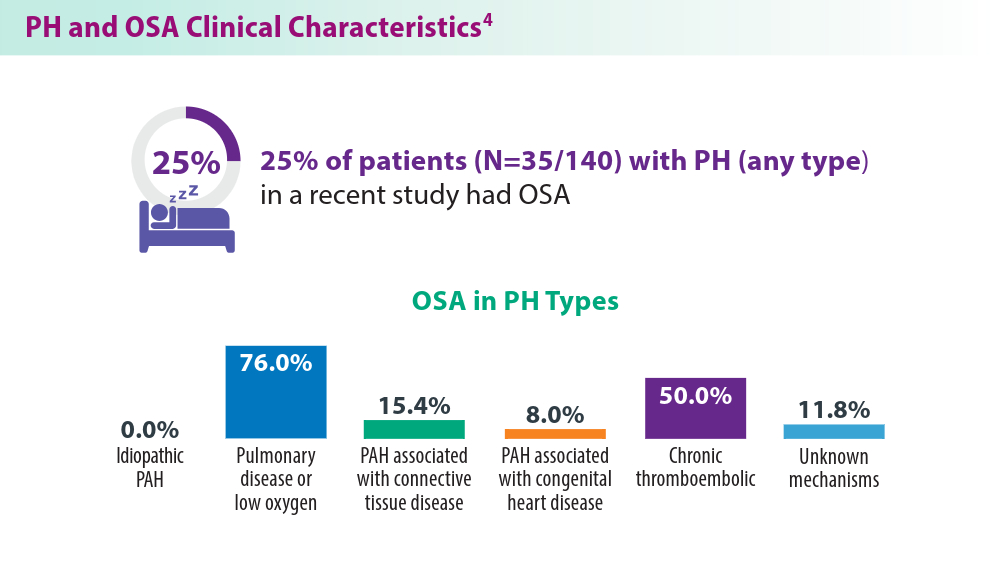
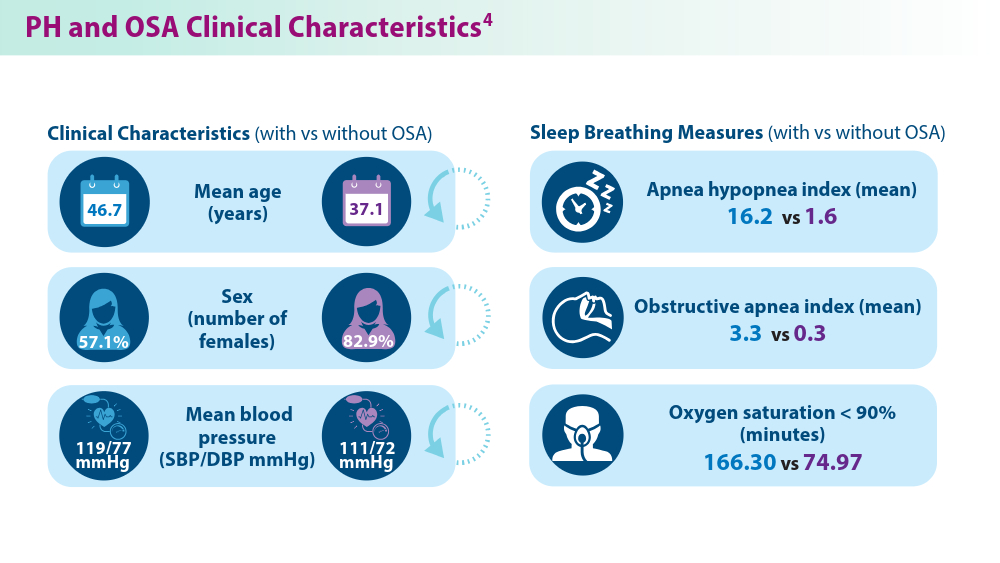
- Yan L, Zhao Z, Zhao Q, et al. The clinical characteristics of patients with pulmonary hypertension combined with obstructive sleep apnoea. BMC Pulm Med. 2021;21(1):378. doi:10.1186/s12890-021-01755-5
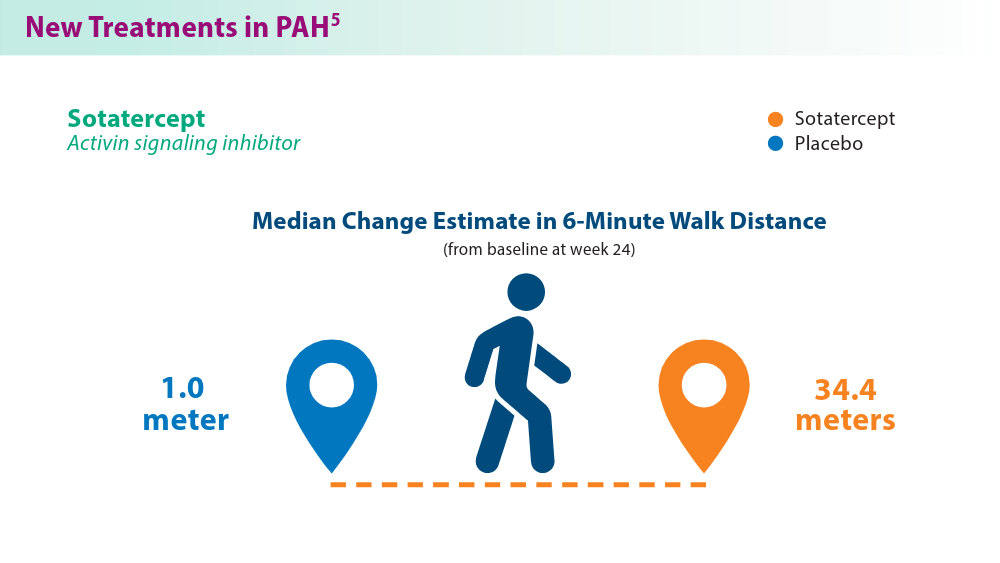
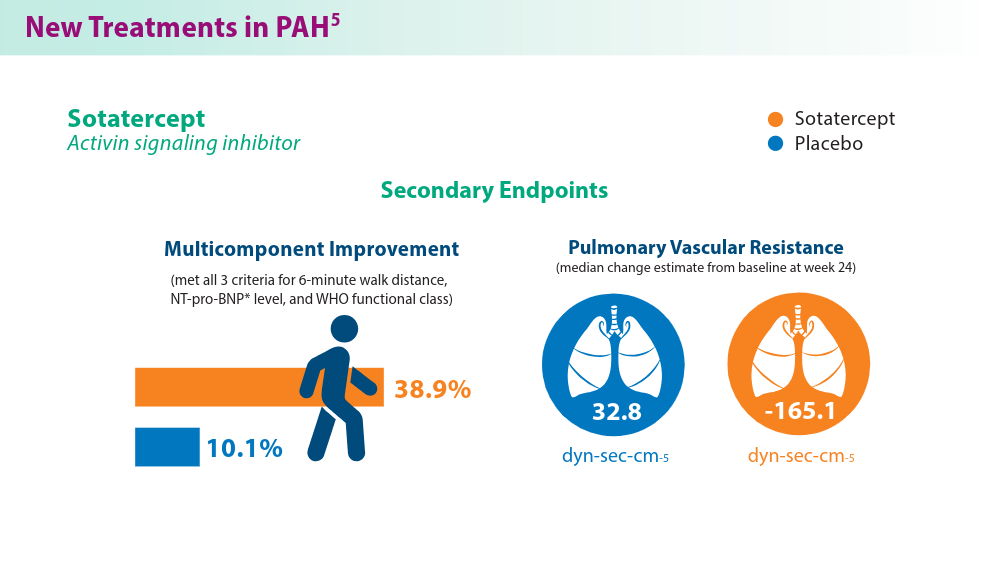
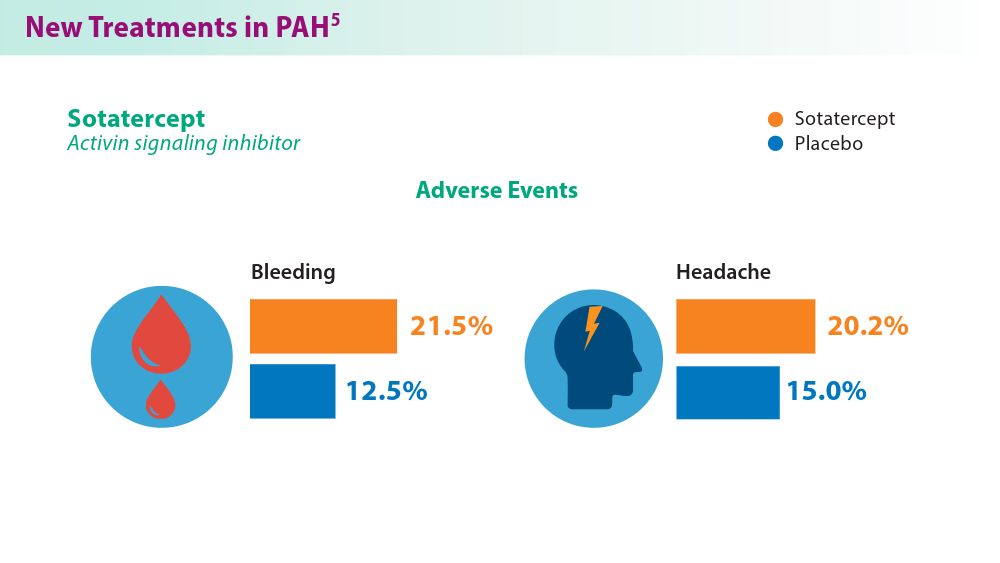
- Hoeper MM, Badesch DB, Ghofrani HA, et al; for the STELLAR Trial Investigators. Phase 3 trial of sotatercept for treatment of pulmonary arterial hypertension. N Engl J Med. 2023;388(16):1478-1490. doi:10.1056/NEJMoa2213558
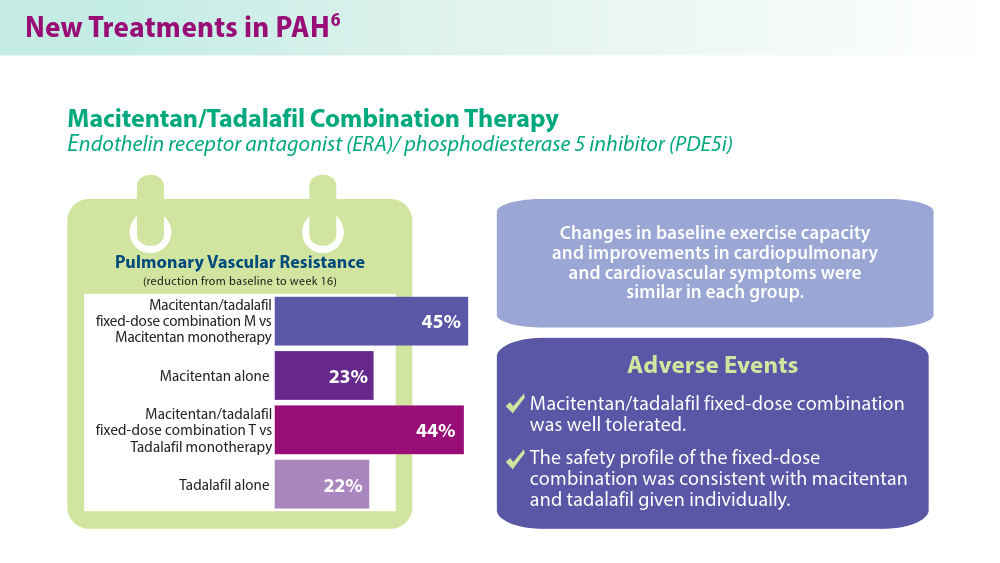
- Grünig E, Jansa P, Fan F, et al. Randomized trial of macitentan/tadalafil single-tablet combination therapy for pulmonary arterial hypertension. J Am Coll Cardiol. 2024;83(4):473-484. doi:10.1016/j.jacc.2023.10.045
- Higuchi S, Horinouchi H, Aoki T, et al. Balloon pulmonary angioplasty in the management of chronic thromboembolic pulmonary hypertension. Radiographics. 2022;42(6):1881-1896. doi:10.1148/rg.210102
- Cullivan S, Gaine S, Sitbon O. New trends in pulmonary hypertension. Eur Respir Rev. 2023;32(167):220211. doi:10.1183/16000617.0211-2022
- Mocumbi A, Humbert M, Saxena A, et al. Pulmonary hypertension [published correction appears in Nat Rev Dis Primers. 2024;10(1):5]. Nat Rev Dis Primers. 2024;10(1):1. doi:10.1038/s41572-023-00486-7
- Lang IM, Palazzini M. The burden of comorbidities in pulmonary arterial hypertension. Eur Heart J Suppl. 2019;21(suppl K):K21-K28. doi:10.1093/ eurheartj/suz205
- Yan L, Zhao Z, Zhao Q, et al. The clinical characteristics of patients with pulmonary hypertension combined with obstructive sleep apnoea. BMC Pulm Med. 2021;21(1):378. doi:10.1186/s12890-021-01755-5
- Hoeper MM, Badesch DB, Ghofrani HA, et al; for the STELLAR Trial Investigators. Phase 3 trial of sotatercept for treatment of pulmonary arterial hypertension. N Engl J Med. 2023;388(16):1478-1490. doi:10.1056/NEJMoa2213558
- Grünig E, Jansa P, Fan F, et al. Randomized trial of macitentan/tadalafil single-tablet combination therapy for pulmonary arterial hypertension. J Am Coll Cardiol. 2024;83(4):473-484. doi:10.1016/j.jacc.2023.10.045
- Higuchi S, Horinouchi H, Aoki T, et al. Balloon pulmonary angioplasty in the management of chronic thromboembolic pulmonary hypertension. Radiographics. 2022;42(6):1881-1896. doi:10.1148/rg.210102
- Cullivan S, Gaine S, Sitbon O. New trends in pulmonary hypertension. Eur Respir Rev. 2023;32(167):220211. doi:10.1183/16000617.0211-2022
- Mocumbi A, Humbert M, Saxena A, et al. Pulmonary hypertension [published correction appears in Nat Rev Dis Primers. 2024;10(1):5]. Nat Rev Dis Primers. 2024;10(1):1. doi:10.1038/s41572-023-00486-7
- Lang IM, Palazzini M. The burden of comorbidities in pulmonary arterial hypertension. Eur Heart J Suppl. 2019;21(suppl K):K21-K28. doi:10.1093/ eurheartj/suz205
- Yan L, Zhao Z, Zhao Q, et al. The clinical characteristics of patients with pulmonary hypertension combined with obstructive sleep apnoea. BMC Pulm Med. 2021;21(1):378. doi:10.1186/s12890-021-01755-5
- Hoeper MM, Badesch DB, Ghofrani HA, et al; for the STELLAR Trial Investigators. Phase 3 trial of sotatercept for treatment of pulmonary arterial hypertension. N Engl J Med. 2023;388(16):1478-1490. doi:10.1056/NEJMoa2213558
- Grünig E, Jansa P, Fan F, et al. Randomized trial of macitentan/tadalafil single-tablet combination therapy for pulmonary arterial hypertension. J Am Coll Cardiol. 2024;83(4):473-484. doi:10.1016/j.jacc.2023.10.045
- Higuchi S, Horinouchi H, Aoki T, et al. Balloon pulmonary angioplasty in the management of chronic thromboembolic pulmonary hypertension. Radiographics. 2022;42(6):1881-1896. doi:10.1148/rg.210102
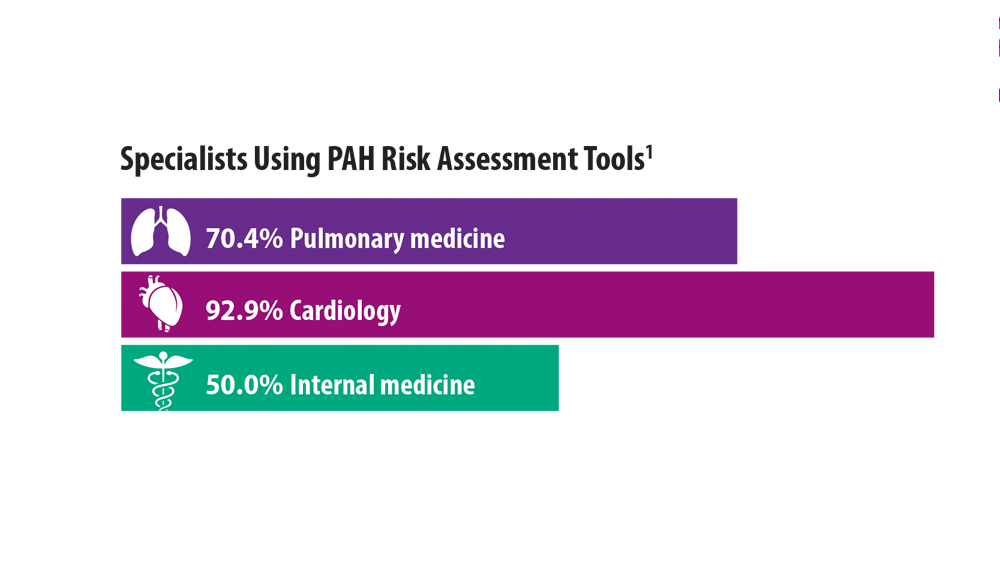
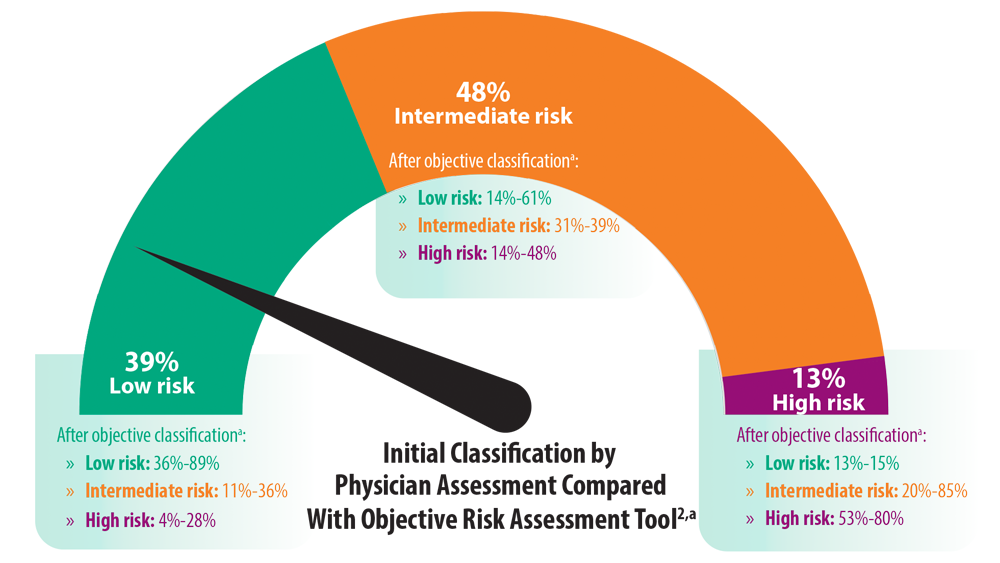
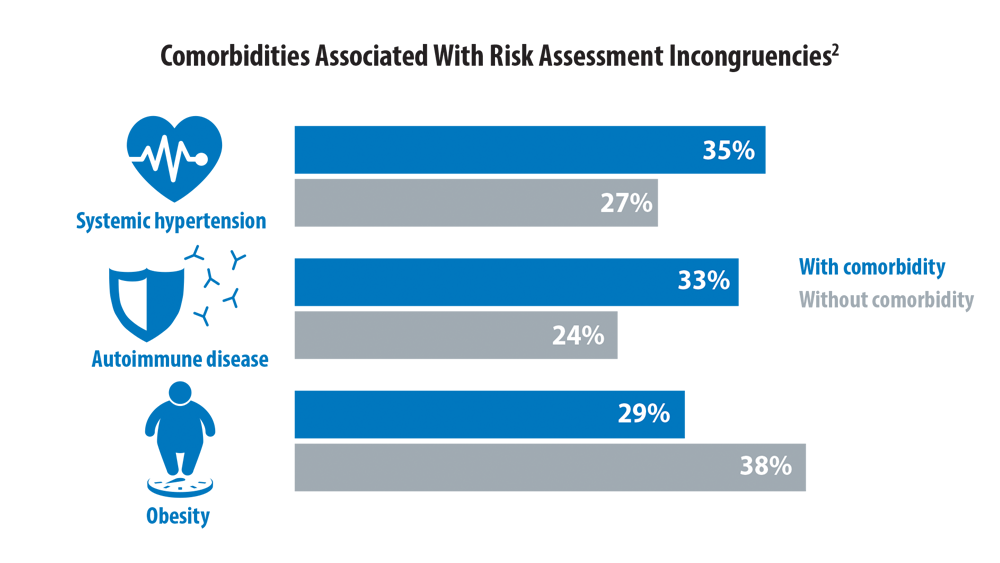
Risk Assessment in Pulmonary Arterial Hypertension
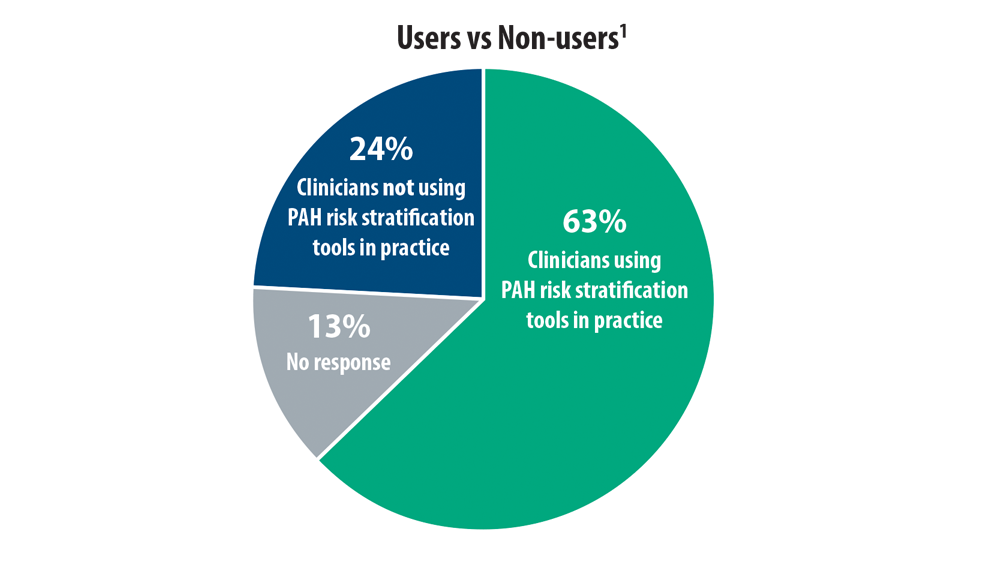
- Sahay S, Balasubramanian V, Memon H, et al. Utilization of risk assessment tools in management of PAH: a PAH provider survey. Pulm Circ. 2022;12(2):e12057. doi:10.1002/pul2.12057
- Sahay S, Tonelli AR, Selej M, Watson Z, Benza RL. Risk assessment in patients with functional class II pulmonary arterial hypertension: comparison of physician gestalt with ESC/ERS and the REVEAL 2.0 risk score. PLoS One. 2020;15(11):e0241504. doi:10.1371/journal.pone.0241504
- Galiè N, Channick RN, Frantz RP, et al. Risk stratification and medical therapy of pulmonary arterial hypertension. Eur Respir J. 2019;53(1):1801889. doi:10.1183/13993003.01889-2018
- Boucly A, Weatherald J, Savale L, et al. Risk assessment, prognosis and guideline implementation in pulmonary arterial hypertension. Eur Respir J. 2017;50(2):1700889. doi:10.1183/13993003.00889-2017
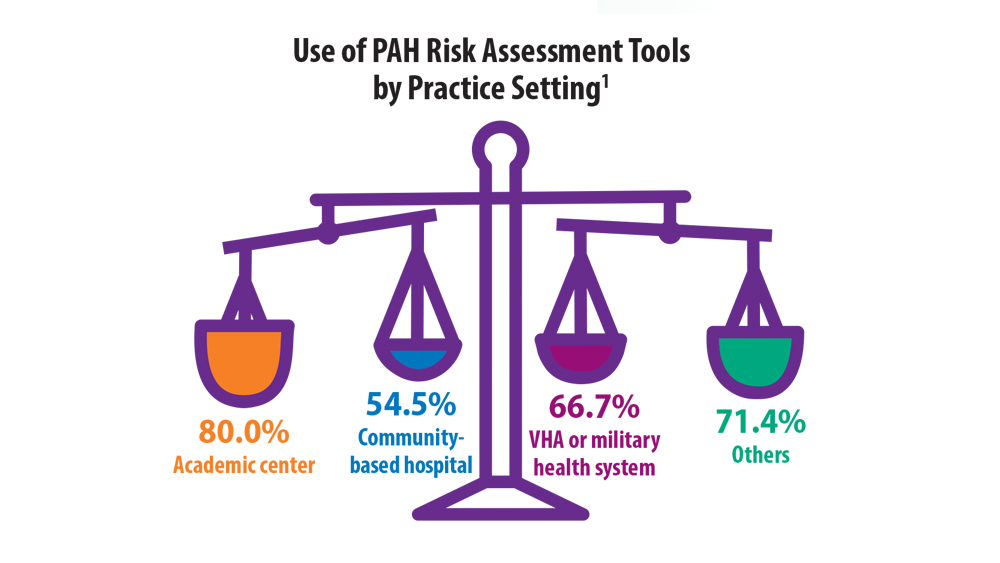
- Wilson M, Keeley J, Kingman M, Wang J, Rogers F. Current clinical utilization of risk assessment tools in pulmonary arterial hypertension: a descriptive survey of facilitation strategies, patterns, and barriers to use in the United States. Pulm Circ. 2020;10(3):2045894020950186. doi:10.1177/2045894020950186
- Sahay S, Balasubramanian V, Memon H, et al. Utilization of risk assessment tools in management of PAH: a PAH provider survey. Pulm Circ. 2022;12(2):e12057. doi:10.1002/pul2.12057
- Sahay S, Tonelli AR, Selej M, Watson Z, Benza RL. Risk assessment in patients with functional class II pulmonary arterial hypertension: comparison of physician gestalt with ESC/ERS and the REVEAL 2.0 risk score. PLoS One. 2020;15(11):e0241504. doi:10.1371/journal.pone.0241504
- Galiè N, Channick RN, Frantz RP, et al. Risk stratification and medical therapy of pulmonary arterial hypertension. Eur Respir J. 2019;53(1):1801889. doi:10.1183/13993003.01889-2018
- Boucly A, Weatherald J, Savale L, et al. Risk assessment, prognosis and guideline implementation in pulmonary arterial hypertension. Eur Respir J. 2017;50(2):1700889. doi:10.1183/13993003.00889-2017
- Wilson M, Keeley J, Kingman M, Wang J, Rogers F. Current clinical utilization of risk assessment tools in pulmonary arterial hypertension: a descriptive survey of facilitation strategies, patterns, and barriers to use in the United States. Pulm Circ. 2020;10(3):2045894020950186. doi:10.1177/2045894020950186
- Sahay S, Balasubramanian V, Memon H, et al. Utilization of risk assessment tools in management of PAH: a PAH provider survey. Pulm Circ. 2022;12(2):e12057. doi:10.1002/pul2.12057
- Sahay S, Tonelli AR, Selej M, Watson Z, Benza RL. Risk assessment in patients with functional class II pulmonary arterial hypertension: comparison of physician gestalt with ESC/ERS and the REVEAL 2.0 risk score. PLoS One. 2020;15(11):e0241504. doi:10.1371/journal.pone.0241504
- Galiè N, Channick RN, Frantz RP, et al. Risk stratification and medical therapy of pulmonary arterial hypertension. Eur Respir J. 2019;53(1):1801889. doi:10.1183/13993003.01889-2018
- Boucly A, Weatherald J, Savale L, et al. Risk assessment, prognosis and guideline implementation in pulmonary arterial hypertension. Eur Respir J. 2017;50(2):1700889. doi:10.1183/13993003.00889-2017
- Wilson M, Keeley J, Kingman M, Wang J, Rogers F. Current clinical utilization of risk assessment tools in pulmonary arterial hypertension: a descriptive survey of facilitation strategies, patterns, and barriers to use in the United States. Pulm Circ. 2020;10(3):2045894020950186. doi:10.1177/2045894020950186
In PAH trials, clinical worsening risk rose with time
Current clinical trials evaluating combination therapy for pulmonary artery hypertension (PAH) may be longer than what is needed to demonstrate treatment benefit, results of a recent meta-analysis suggest.
, according to results of the study published in the May issue of the journal Chest®.
The meta-analysis by Dr. Lajoie and her colleagues included 3,801 patients enrolled in one of 15 previously published randomized clinical trials. Of those trials, four were long-term, event driven studies, with a mean duration of 87 weeks, while the remainder were shorter studies with a mean duration of 15 weeks.
For the long-term, event-driven trials, the mean number needed to treat (NNT) was 17.4 at week 16, gradually decreasing to 8.8 at 52 weeks of follow-up, remaining stable after that, according to investigators.
Consistent with that finding, the mean relative risk of clinical worsening was 0.38 at 16 weeks, and similarly, 0.41 at 26 weeks, investigators reported. After that, the relative risk progressively increased to 0.54 at 52 weeks and 0.68 at 104 weeks.
Looking at all trials combined, Dr. Lajoie and her colleagues observed that longer trial duration had a positive correlation with relative risk of clinical worsening (P = .0002).
Pragmatically, these results raise the possibility that PAH combination therapy trials could be shorter in duration. Some recent event-driven studies have lasted up to 6 years, with patients on treatment for about 2 of those years, investigators noted.
“In the context of an orphan disease with limited and competing recruitment for trials and the rapidly changing treatment paradigm in PAH, the optimal duration of future trials should be revisited,” Dr. Lajoie and her colleagues wrote in a discussion of their findings.
They also cautioned that NNT, a measure of how many patient treatments are needed to prevent one additional adverse event, could be “misleading” despite its value as a simple measure of treatment impact.
Likewise, relative risk can be misleading; for example, a treatment reducing event risk from 30% to 20% represents a relative risk reduction of approximately 35%, but so does a treatment reducing event risk from 3% to 2%, the researchers noted.
“Multiple factors, in addition to the efficacy of the therapy and the comparator, may directly influence the NNT and relative risk and should be taken into account in their interpretation,” they said in their report.
Dr. Lajoie had no disclosures related to the study. Her coauthors had disclosures related to Actelion Pharmaceuticals, Bayer, and GlaxoSmithKline, among others.
SOURCE: Lajoie AC et al. Chest. 2017 May;153(5):1142-52.
Key clinical point: A meta-analysis calls into question the need to perform pulmonary arterial hypertension (PAH) trials beyond 6 to 12 months of treatment in the future.
Major finding: The mean number needed to treat was stable at 52 weeks of follow-up and thereafter, while the mean relative risk of clinical worsening progressively increased from approximately 0.38 at week 16 to 0.68 at week 104.
Study details: A systematic review of 3,801 patients enrolled in 15 randomized clinical trials.
Disclosures: The authors reported disclosures related to Actelion Pharmaceuticals, Bayer, and GlaxoSmithKline, among other entities.
Source: Lajoie AC et al. Chest. 2017 May;153(5):1142-52.
Key clinical point: A meta-analysis calls into question the need to perform pulmonary arterial hypertension (PAH) trials beyond 6 to 12 months of treatment in the future.
Major finding: The mean number needed to treat was stable at 52 weeks of follow-up and thereafter, while the mean relative risk of clinical worsening progressively increased from approximately 0.38 at week 16 to 0.68 at week 104.
Study details: A systematic review of 3,801 patients enrolled in 15 randomized clinical trials.
Disclosures: The authors reported disclosures related to Actelion Pharmaceuticals, Bayer, and GlaxoSmithKline, among other entities.
Source: Lajoie AC et al. Chest. 2017 May;153(5):1142-52.
Key clinical point: A meta-analysis calls into question the need to perform pulmonary arterial hypertension (PAH) trials beyond 6 to 12 months of treatment in the future.
Major finding: The mean number needed to treat was stable at 52 weeks of follow-up and thereafter, while the mean relative risk of clinical worsening progressively increased from approximately 0.38 at week 16 to 0.68 at week 104.
Study details: A systematic review of 3,801 patients enrolled in 15 randomized clinical trials.
Disclosures: The authors reported disclosures related to Actelion Pharmaceuticals, Bayer, and GlaxoSmithKline, among other entities.
Source: Lajoie AC et al. Chest. 2017 May;153(5):1142-52.
Current clinical trials evaluating combination therapy for pulmonary artery hypertension (PAH) may be longer than what is needed to demonstrate treatment benefit, results of a recent meta-analysis suggest.
, according to results of the study published in the May issue of the journal Chest®.
The meta-analysis by Dr. Lajoie and her colleagues included 3,801 patients enrolled in one of 15 previously published randomized clinical trials. Of those trials, four were long-term, event driven studies, with a mean duration of 87 weeks, while the remainder were shorter studies with a mean duration of 15 weeks.
For the long-term, event-driven trials, the mean number needed to treat (NNT) was 17.4 at week 16, gradually decreasing to 8.8 at 52 weeks of follow-up, remaining stable after that, according to investigators.
Consistent with that finding, the mean relative risk of clinical worsening was 0.38 at 16 weeks, and similarly, 0.41 at 26 weeks, investigators reported. After that, the relative risk progressively increased to 0.54 at 52 weeks and 0.68 at 104 weeks.
Looking at all trials combined, Dr. Lajoie and her colleagues observed that longer trial duration had a positive correlation with relative risk of clinical worsening (P = .0002).
Pragmatically, these results raise the possibility that PAH combination therapy trials could be shorter in duration. Some recent event-driven studies have lasted up to 6 years, with patients on treatment for about 2 of those years, investigators noted.
“In the context of an orphan disease with limited and competing recruitment for trials and the rapidly changing treatment paradigm in PAH, the optimal duration of future trials should be revisited,” Dr. Lajoie and her colleagues wrote in a discussion of their findings.
They also cautioned that NNT, a measure of how many patient treatments are needed to prevent one additional adverse event, could be “misleading” despite its value as a simple measure of treatment impact.
Likewise, relative risk can be misleading; for example, a treatment reducing event risk from 30% to 20% represents a relative risk reduction of approximately 35%, but so does a treatment reducing event risk from 3% to 2%, the researchers noted.
“Multiple factors, in addition to the efficacy of the therapy and the comparator, may directly influence the NNT and relative risk and should be taken into account in their interpretation,” they said in their report.
Dr. Lajoie had no disclosures related to the study. Her coauthors had disclosures related to Actelion Pharmaceuticals, Bayer, and GlaxoSmithKline, among others.
SOURCE: Lajoie AC et al. Chest. 2017 May;153(5):1142-52.
Current clinical trials evaluating combination therapy for pulmonary artery hypertension (PAH) may be longer than what is needed to demonstrate treatment benefit, results of a recent meta-analysis suggest.
, according to results of the study published in the May issue of the journal Chest®.
The meta-analysis by Dr. Lajoie and her colleagues included 3,801 patients enrolled in one of 15 previously published randomized clinical trials. Of those trials, four were long-term, event driven studies, with a mean duration of 87 weeks, while the remainder were shorter studies with a mean duration of 15 weeks.
For the long-term, event-driven trials, the mean number needed to treat (NNT) was 17.4 at week 16, gradually decreasing to 8.8 at 52 weeks of follow-up, remaining stable after that, according to investigators.
Consistent with that finding, the mean relative risk of clinical worsening was 0.38 at 16 weeks, and similarly, 0.41 at 26 weeks, investigators reported. After that, the relative risk progressively increased to 0.54 at 52 weeks and 0.68 at 104 weeks.
Looking at all trials combined, Dr. Lajoie and her colleagues observed that longer trial duration had a positive correlation with relative risk of clinical worsening (P = .0002).
Pragmatically, these results raise the possibility that PAH combination therapy trials could be shorter in duration. Some recent event-driven studies have lasted up to 6 years, with patients on treatment for about 2 of those years, investigators noted.
“In the context of an orphan disease with limited and competing recruitment for trials and the rapidly changing treatment paradigm in PAH, the optimal duration of future trials should be revisited,” Dr. Lajoie and her colleagues wrote in a discussion of their findings.
They also cautioned that NNT, a measure of how many patient treatments are needed to prevent one additional adverse event, could be “misleading” despite its value as a simple measure of treatment impact.
Likewise, relative risk can be misleading; for example, a treatment reducing event risk from 30% to 20% represents a relative risk reduction of approximately 35%, but so does a treatment reducing event risk from 3% to 2%, the researchers noted.
“Multiple factors, in addition to the efficacy of the therapy and the comparator, may directly influence the NNT and relative risk and should be taken into account in their interpretation,” they said in their report.
Dr. Lajoie had no disclosures related to the study. Her coauthors had disclosures related to Actelion Pharmaceuticals, Bayer, and GlaxoSmithKline, among others.
SOURCE: Lajoie AC et al. Chest. 2017 May;153(5):1142-52.
FROM CHEST®