User login
Pulmonology Data Trends 2024
Pulmonology Data Trends 2024 is a supplement to CHEST Physician highlighting the latest breakthroughs in pulmonology research and treatments through a series of infographics.
Read more:
Artificial Intelligence in Sleep Apnea
Ritwick Agrawal, MD, MS, FCCP
RSV Updates: Prophylaxis Approval and Hospitalization for Severe RSV
Riddhi Upadhyay, MD
Biologics in Asthma: Changing the Severe Asthma Paradigm
Shyam Subramanian, MD, FCCP
Updates in COPD Guidelines and Treatment
Dharani K. Narendra, MD, FCCP
Targeted Therapies and Surgical Resection for Lung Cancer: Evolving Treatment Options
Saadia A. Faiz, MD, FCCP
Closing the GAP in Idiopathic Pulmonary Fibrosis
Humayun Anjum, MD, FCCP
Severe Community-Acquired Pneumonia: Diagnostic Criteria, Treatment, and COVID-19
Sujith V. Cherian, MD, FCCP
Pulmonary Hypertension: Comorbidities and Novel Therapies
Mary Jo S. Farmer, MD, PhD, FCCP
The Genetic Side of Interstitial Lung Disease
Priya Balakrishnan, MD, MS, FCCP
Noninvasive Ventilation in Neuromuscular Disease
Sreelatha Naik, MD, FCCP, and Kelly Lobrutto, CRNP
Pulmonology Data Trends 2024 is a supplement to CHEST Physician highlighting the latest breakthroughs in pulmonology research and treatments through a series of infographics.
Read more:
Artificial Intelligence in Sleep Apnea
Ritwick Agrawal, MD, MS, FCCP
RSV Updates: Prophylaxis Approval and Hospitalization for Severe RSV
Riddhi Upadhyay, MD
Biologics in Asthma: Changing the Severe Asthma Paradigm
Shyam Subramanian, MD, FCCP
Updates in COPD Guidelines and Treatment
Dharani K. Narendra, MD, FCCP
Targeted Therapies and Surgical Resection for Lung Cancer: Evolving Treatment Options
Saadia A. Faiz, MD, FCCP
Closing the GAP in Idiopathic Pulmonary Fibrosis
Humayun Anjum, MD, FCCP
Severe Community-Acquired Pneumonia: Diagnostic Criteria, Treatment, and COVID-19
Sujith V. Cherian, MD, FCCP
Pulmonary Hypertension: Comorbidities and Novel Therapies
Mary Jo S. Farmer, MD, PhD, FCCP
The Genetic Side of Interstitial Lung Disease
Priya Balakrishnan, MD, MS, FCCP
Noninvasive Ventilation in Neuromuscular Disease
Sreelatha Naik, MD, FCCP, and Kelly Lobrutto, CRNP
Pulmonology Data Trends 2024 is a supplement to CHEST Physician highlighting the latest breakthroughs in pulmonology research and treatments through a series of infographics.
Read more:
Artificial Intelligence in Sleep Apnea
Ritwick Agrawal, MD, MS, FCCP
RSV Updates: Prophylaxis Approval and Hospitalization for Severe RSV
Riddhi Upadhyay, MD
Biologics in Asthma: Changing the Severe Asthma Paradigm
Shyam Subramanian, MD, FCCP
Updates in COPD Guidelines and Treatment
Dharani K. Narendra, MD, FCCP
Targeted Therapies and Surgical Resection for Lung Cancer: Evolving Treatment Options
Saadia A. Faiz, MD, FCCP
Closing the GAP in Idiopathic Pulmonary Fibrosis
Humayun Anjum, MD, FCCP
Severe Community-Acquired Pneumonia: Diagnostic Criteria, Treatment, and COVID-19
Sujith V. Cherian, MD, FCCP
Pulmonary Hypertension: Comorbidities and Novel Therapies
Mary Jo S. Farmer, MD, PhD, FCCP
The Genetic Side of Interstitial Lung Disease
Priya Balakrishnan, MD, MS, FCCP
Noninvasive Ventilation in Neuromuscular Disease
Sreelatha Naik, MD, FCCP, and Kelly Lobrutto, CRNP
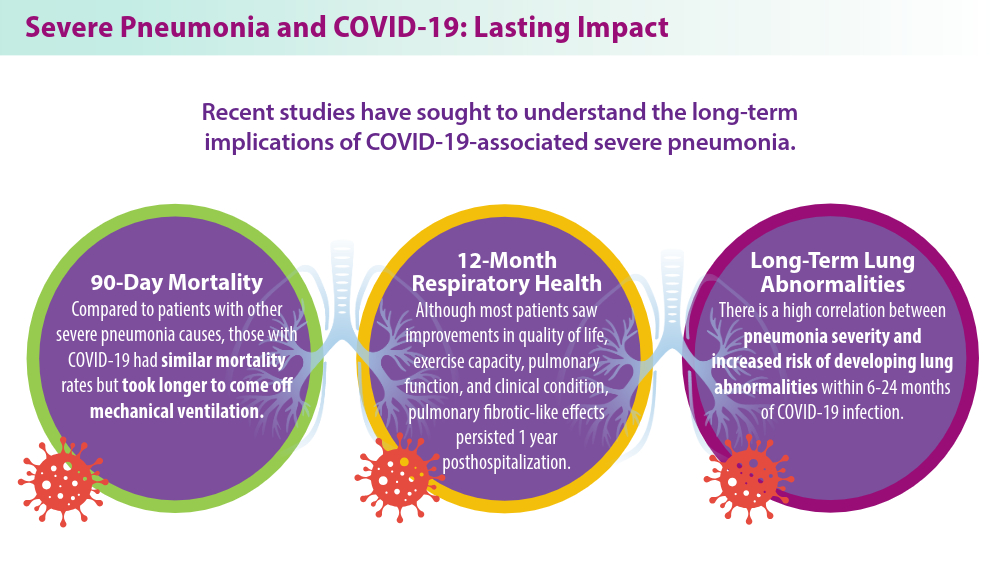
Severe Community-Acquired Pneumonia: Diagnostic Criteria, Treatment, and COVID-19
- Torres A, Cilloniz C, Niederman MS, et al. Pneumonia. Nat Rev Dis Primers. 2021;7(1):25. doi:10.1038/s41572-021-00259-0
- Niederman MS, Torres A. Severe community-acquired pneumonia. Eur Respir Rev. 2022;31(166):220123. doi:10.1183/16000617.0123-2022
- Metlay JP, Waterer GW, Long AC, et al. Diagnosis and treatment of adults with community-acquired pneumonia. An official clinical practice guideline of the American Thoracic Society and Infectious Diseases Society of America. Am J Respir Crit Care Med. 2019;200(7):e45-e67. doi:10.1164/rccm.201908-1581ST
- Memon RA, Rashid MA, Avva S, et al. The use of the SMART-COP score in predicting severity outcomes among patients with community-acquired pneumonia: a meta-analysis. Cureus. 2022;14(7):e27248. doi:10.7759/cureus.27248
- Regunath H, Oba Y. Community-acquired pneumonia. StatPearls [Internet]. Treasure Island, FL: StatPearls Publishing; 2024. Updated January 26, 2024. Accessed May 14, 2024. https://www.ncbi.nlm.nih.gov/books/NBK430749/
- Dequin PF, Meziani F, Quenot JP, et al; for the CRICS-TriGGERSep Network. Hydrocortisone in severe community-acquired pneumonia. N Engl J Med. 2023;388(21):1931-1941. doi:10.1056/NEJMoa2215145
- Eizaguirre S, Sabater G, Belda S, et al. Long-term respiratory consequences of COVID-19 related pneumonia: a cohort study. BMC Pulm Med. 2023;23(1):439. doi:10.1186/s12890-023-02627-w
- Ramirez JA, Wiemken TL, Peyrani P, et al; for the University of Louisville Pneumonia Study Group. Adults hospitalized with pneumonia in the United States: incidence, epidemiology, and mortality. Clin Infect Dis. 2017;65(11):1806-1812. doi:10.1093/cid/cix647
- Morgan AJ, Glossop AJ. Severe community-acquired pneumonia. BJA Educ. 2016;16(5):167-172. doi:10.1093/bjaed/mkv052
- Haessler S, Guo N, Deshpande A, et al. Etiology, treatments, and outcomes of patients with severe community-acquired pneumonia in a large U.S. sample. Crit Care Med. 2022;50(7):1063-1071. doi:10.1097/CCM.0000000000005498
- Nolley EP, Sahetya SK, Hochberg CH, et al. Outcomes among mechanically ventilated patients with severe pneumonia and acute hypoxemic respiratory failure from SARS-CoV-2 and other etiologies. JAMA Netw Open. 2023;6(1):e2250401. doi:10.1001/jamanetworkopen.2022.50401
- Hino T, Nishino M, Valtchinov VI, et al. Severe COVID-19 pneumonia leads to post-COVID-19 lung abnormalities on follow-up CT scans. Eur J Radiol Open. 2023;10:100483. doi:10.1016/j.ejro.2023.100483
- Torres A, Cilloniz C, Niederman MS, et al. Pneumonia. Nat Rev Dis Primers. 2021;7(1):25. doi:10.1038/s41572-021-00259-0
- Niederman MS, Torres A. Severe community-acquired pneumonia. Eur Respir Rev. 2022;31(166):220123. doi:10.1183/16000617.0123-2022
- Metlay JP, Waterer GW, Long AC, et al. Diagnosis and treatment of adults with community-acquired pneumonia. An official clinical practice guideline of the American Thoracic Society and Infectious Diseases Society of America. Am J Respir Crit Care Med. 2019;200(7):e45-e67. doi:10.1164/rccm.201908-1581ST
- Memon RA, Rashid MA, Avva S, et al. The use of the SMART-COP score in predicting severity outcomes among patients with community-acquired pneumonia: a meta-analysis. Cureus. 2022;14(7):e27248. doi:10.7759/cureus.27248
- Regunath H, Oba Y. Community-acquired pneumonia. StatPearls [Internet]. Treasure Island, FL: StatPearls Publishing; 2024. Updated January 26, 2024. Accessed May 14, 2024. https://www.ncbi.nlm.nih.gov/books/NBK430749/
- Dequin PF, Meziani F, Quenot JP, et al; for the CRICS-TriGGERSep Network. Hydrocortisone in severe community-acquired pneumonia. N Engl J Med. 2023;388(21):1931-1941. doi:10.1056/NEJMoa2215145
- Eizaguirre S, Sabater G, Belda S, et al. Long-term respiratory consequences of COVID-19 related pneumonia: a cohort study. BMC Pulm Med. 2023;23(1):439. doi:10.1186/s12890-023-02627-w
- Ramirez JA, Wiemken TL, Peyrani P, et al; for the University of Louisville Pneumonia Study Group. Adults hospitalized with pneumonia in the United States: incidence, epidemiology, and mortality. Clin Infect Dis. 2017;65(11):1806-1812. doi:10.1093/cid/cix647
- Morgan AJ, Glossop AJ. Severe community-acquired pneumonia. BJA Educ. 2016;16(5):167-172. doi:10.1093/bjaed/mkv052
- Haessler S, Guo N, Deshpande A, et al. Etiology, treatments, and outcomes of patients with severe community-acquired pneumonia in a large U.S. sample. Crit Care Med. 2022;50(7):1063-1071. doi:10.1097/CCM.0000000000005498
- Nolley EP, Sahetya SK, Hochberg CH, et al. Outcomes among mechanically ventilated patients with severe pneumonia and acute hypoxemic respiratory failure from SARS-CoV-2 and other etiologies. JAMA Netw Open. 2023;6(1):e2250401. doi:10.1001/jamanetworkopen.2022.50401
- Hino T, Nishino M, Valtchinov VI, et al. Severe COVID-19 pneumonia leads to post-COVID-19 lung abnormalities on follow-up CT scans. Eur J Radiol Open. 2023;10:100483. doi:10.1016/j.ejro.2023.100483
- Torres A, Cilloniz C, Niederman MS, et al. Pneumonia. Nat Rev Dis Primers. 2021;7(1):25. doi:10.1038/s41572-021-00259-0
- Niederman MS, Torres A. Severe community-acquired pneumonia. Eur Respir Rev. 2022;31(166):220123. doi:10.1183/16000617.0123-2022
- Metlay JP, Waterer GW, Long AC, et al. Diagnosis and treatment of adults with community-acquired pneumonia. An official clinical practice guideline of the American Thoracic Society and Infectious Diseases Society of America. Am J Respir Crit Care Med. 2019;200(7):e45-e67. doi:10.1164/rccm.201908-1581ST
- Memon RA, Rashid MA, Avva S, et al. The use of the SMART-COP score in predicting severity outcomes among patients with community-acquired pneumonia: a meta-analysis. Cureus. 2022;14(7):e27248. doi:10.7759/cureus.27248
- Regunath H, Oba Y. Community-acquired pneumonia. StatPearls [Internet]. Treasure Island, FL: StatPearls Publishing; 2024. Updated January 26, 2024. Accessed May 14, 2024. https://www.ncbi.nlm.nih.gov/books/NBK430749/
- Dequin PF, Meziani F, Quenot JP, et al; for the CRICS-TriGGERSep Network. Hydrocortisone in severe community-acquired pneumonia. N Engl J Med. 2023;388(21):1931-1941. doi:10.1056/NEJMoa2215145
- Eizaguirre S, Sabater G, Belda S, et al. Long-term respiratory consequences of COVID-19 related pneumonia: a cohort study. BMC Pulm Med. 2023;23(1):439. doi:10.1186/s12890-023-02627-w
- Ramirez JA, Wiemken TL, Peyrani P, et al; for the University of Louisville Pneumonia Study Group. Adults hospitalized with pneumonia in the United States: incidence, epidemiology, and mortality. Clin Infect Dis. 2017;65(11):1806-1812. doi:10.1093/cid/cix647
- Morgan AJ, Glossop AJ. Severe community-acquired pneumonia. BJA Educ. 2016;16(5):167-172. doi:10.1093/bjaed/mkv052
- Haessler S, Guo N, Deshpande A, et al. Etiology, treatments, and outcomes of patients with severe community-acquired pneumonia in a large U.S. sample. Crit Care Med. 2022;50(7):1063-1071. doi:10.1097/CCM.0000000000005498
- Nolley EP, Sahetya SK, Hochberg CH, et al. Outcomes among mechanically ventilated patients with severe pneumonia and acute hypoxemic respiratory failure from SARS-CoV-2 and other etiologies. JAMA Netw Open. 2023;6(1):e2250401. doi:10.1001/jamanetworkopen.2022.50401
- Hino T, Nishino M, Valtchinov VI, et al. Severe COVID-19 pneumonia leads to post-COVID-19 lung abnormalities on follow-up CT scans. Eur J Radiol Open. 2023;10:100483. doi:10.1016/j.ejro.2023.100483
Specific Antipsychotics Linked to Increased Pneumonia Risk
TOPLINE:
High-dose antipsychotics, particularly quetiapine, clozapine, and olanzapine, are linked to increased pneumonia risk in patients with schizophrenia, new data show. Monotherapy with high anticholinergic burden also raises pneumonia risk.
METHODOLOGY:
- Using several nationwide data registers, investigators pulled data on individuals who received inpatient care for schizophrenia or schizoaffective disorder (n = 61,889) between 1972 and 2014.
- Data on drug use were gathered from a prescription register and included dispensing dates, cost, dose, package size, and drug formulation. Data on dates and causes of death were obtained from the Causes of Death register.
- After entering the cohort, follow-up started in January 1996 or after the first diagnosis of schizophrenia for those diagnosed between 1996 and 2014.
- The primary outcome was hospitalization caused by pneumonia as the main diagnosis for hospital admission.
TAKEAWAY:
- During 22 years of follow-up, 8917 patients (14.4%) had one or more hospitalizations for pneumonia, and 1137 (12.8%) died within 30 days of admission.
- Pneumonia risk was the highest with the use of high-dose (> 440 mg/d) quetiapine (P = .003), followed by high- (≥ 330 mg/d) and medium-dose (180 to < 330 mg/d) clozapine (both P < .001) and high-dose (≥ 11 mg/d) olanzapine (P = .02).
- Compared with no antipsychotic use, antipsychotic monotherapy was associated with an increased pneumonia risk (P = .03), whereas antipsychotic polytherapy was not.
- Only the use of antipsychotics with high anticholinergic potency was associated with pneumonia risk (P < .001).
IN PRACTICE:
“Identification of antipsychotic drugs that are associated with pneumonia risk may better inform prevention programs (eg, vaccinations),” the researchers noted. “Second, the availability of pneumonia risk estimates for individual antipsychotics and for groups of antipsychotics may foster personalized prescribing guidelines.”
SOURCE:
The study was led by Jurjen Luykx, MD, Amsterdam University Medical Center, Amsterdam, the Netherlands. It was published online in JAMA Psychiatry.
LIMITATIONS:
The investigators could not correct for all possible risk factors that may increase pneumonia risk in individuals with schizophrenia, such as smoking and lifestyle habits. Also, cases of pneumonia that didn’t require hospital admission couldn’t be included in the analysis, so the findings may generalize only to cases of severe pneumonia.
DISCLOSURES:
The study was funded by the Finnish Ministry of Social Affairs and Health.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication.
A version of this article first appeared on Medscape.com.
TOPLINE:
High-dose antipsychotics, particularly quetiapine, clozapine, and olanzapine, are linked to increased pneumonia risk in patients with schizophrenia, new data show. Monotherapy with high anticholinergic burden also raises pneumonia risk.
METHODOLOGY:
- Using several nationwide data registers, investigators pulled data on individuals who received inpatient care for schizophrenia or schizoaffective disorder (n = 61,889) between 1972 and 2014.
- Data on drug use were gathered from a prescription register and included dispensing dates, cost, dose, package size, and drug formulation. Data on dates and causes of death were obtained from the Causes of Death register.
- After entering the cohort, follow-up started in January 1996 or after the first diagnosis of schizophrenia for those diagnosed between 1996 and 2014.
- The primary outcome was hospitalization caused by pneumonia as the main diagnosis for hospital admission.
TAKEAWAY:
- During 22 years of follow-up, 8917 patients (14.4%) had one or more hospitalizations for pneumonia, and 1137 (12.8%) died within 30 days of admission.
- Pneumonia risk was the highest with the use of high-dose (> 440 mg/d) quetiapine (P = .003), followed by high- (≥ 330 mg/d) and medium-dose (180 to < 330 mg/d) clozapine (both P < .001) and high-dose (≥ 11 mg/d) olanzapine (P = .02).
- Compared with no antipsychotic use, antipsychotic monotherapy was associated with an increased pneumonia risk (P = .03), whereas antipsychotic polytherapy was not.
- Only the use of antipsychotics with high anticholinergic potency was associated with pneumonia risk (P < .001).
IN PRACTICE:
“Identification of antipsychotic drugs that are associated with pneumonia risk may better inform prevention programs (eg, vaccinations),” the researchers noted. “Second, the availability of pneumonia risk estimates for individual antipsychotics and for groups of antipsychotics may foster personalized prescribing guidelines.”
SOURCE:
The study was led by Jurjen Luykx, MD, Amsterdam University Medical Center, Amsterdam, the Netherlands. It was published online in JAMA Psychiatry.
LIMITATIONS:
The investigators could not correct for all possible risk factors that may increase pneumonia risk in individuals with schizophrenia, such as smoking and lifestyle habits. Also, cases of pneumonia that didn’t require hospital admission couldn’t be included in the analysis, so the findings may generalize only to cases of severe pneumonia.
DISCLOSURES:
The study was funded by the Finnish Ministry of Social Affairs and Health.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication.
A version of this article first appeared on Medscape.com.
TOPLINE:
High-dose antipsychotics, particularly quetiapine, clozapine, and olanzapine, are linked to increased pneumonia risk in patients with schizophrenia, new data show. Monotherapy with high anticholinergic burden also raises pneumonia risk.
METHODOLOGY:
- Using several nationwide data registers, investigators pulled data on individuals who received inpatient care for schizophrenia or schizoaffective disorder (n = 61,889) between 1972 and 2014.
- Data on drug use were gathered from a prescription register and included dispensing dates, cost, dose, package size, and drug formulation. Data on dates and causes of death were obtained from the Causes of Death register.
- After entering the cohort, follow-up started in January 1996 or after the first diagnosis of schizophrenia for those diagnosed between 1996 and 2014.
- The primary outcome was hospitalization caused by pneumonia as the main diagnosis for hospital admission.
TAKEAWAY:
- During 22 years of follow-up, 8917 patients (14.4%) had one or more hospitalizations for pneumonia, and 1137 (12.8%) died within 30 days of admission.
- Pneumonia risk was the highest with the use of high-dose (> 440 mg/d) quetiapine (P = .003), followed by high- (≥ 330 mg/d) and medium-dose (180 to < 330 mg/d) clozapine (both P < .001) and high-dose (≥ 11 mg/d) olanzapine (P = .02).
- Compared with no antipsychotic use, antipsychotic monotherapy was associated with an increased pneumonia risk (P = .03), whereas antipsychotic polytherapy was not.
- Only the use of antipsychotics with high anticholinergic potency was associated with pneumonia risk (P < .001).
IN PRACTICE:
“Identification of antipsychotic drugs that are associated with pneumonia risk may better inform prevention programs (eg, vaccinations),” the researchers noted. “Second, the availability of pneumonia risk estimates for individual antipsychotics and for groups of antipsychotics may foster personalized prescribing guidelines.”
SOURCE:
The study was led by Jurjen Luykx, MD, Amsterdam University Medical Center, Amsterdam, the Netherlands. It was published online in JAMA Psychiatry.
LIMITATIONS:
The investigators could not correct for all possible risk factors that may increase pneumonia risk in individuals with schizophrenia, such as smoking and lifestyle habits. Also, cases of pneumonia that didn’t require hospital admission couldn’t be included in the analysis, so the findings may generalize only to cases of severe pneumonia.
DISCLOSURES:
The study was funded by the Finnish Ministry of Social Affairs and Health.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication.
A version of this article first appeared on Medscape.com.
Systemic Bias in AI Models May Undermine Diagnostic Accuracy
Systematically biased artificial intelligence (AI) models did not improve clinicians’ accuracy in diagnosing hospitalized patients, based on data from more than 450 clinicians.
“Artificial Intelligence (AI) could support clinicians in their diagnostic decisions of hospitalized patients but could also be biased and cause potential harm,” said Sarah Jabbour, MSE, a PhD candidate in computer science and engineering at the University of Michigan, Ann Arbor, in an interview.
“Regulatory guidance has suggested that the use of AI explanations could mitigate these harms, but the effectiveness of using AI explanations has not been established,” she said.
To examine whether AI explanations can be effective in mitigating the potential harms of systemic bias in AI models, Ms. Jabbour and colleagues conducted a randomized clinical vignette survey study. The survey was administered between April 2022 and January 2023 across 13 states, and the study population included hospitalist physicians, nurse practitioners, and physician assistants. The results were published in JAMA.
Participants were randomized to AI predictions with AI explanations (226 clinicians) or without AI explanations (231 clinicians).
The primary outcome was diagnostic accuracy for pneumonia, heart failure, and chronic obstructive pulmonary disease, defined as the number of correct diagnoses over the total number of assessments, the researchers wrote.
The clinicians viewed nine clinical vignettes of patients hospitalized with acute respiratory failure, including their presenting symptoms, physical examination, laboratory results, and chest radiographs. Clinicians viewed two vignettes with no AI model input to establish baseline diagnostic accuracy. They made three assessments in each vignette, one for each diagnosis. The order of the vignettes was two without AI predictions (to establish baseline diagnostic accuracy), six with AI predictions, and one with a clinical consultation by a hypothetical colleague. The vignettes included standard and systematically biased AI models.
The baseline diagnostic accuracy was 73% for the diagnoses of pneumonia, heart failure, and chronic obstructive pulmonary disease. Clinicians’ accuracy increased by 2.9% when they viewed a standard diagnostic AI model without explanations and by 4.4% when they viewed models with AI explanations.
However, clinicians’ accuracy decreased by 11.3% after viewing systematically biased AI model predictions without explanations compared with baseline, and biased AI model predictions with explanations decreased accuracy by 9.1%.
The decrease in accuracy with systematically biased AI predictions without explanations was mainly attributable to a decrease in the participants’ diagnostic specificity, the researchers noted, but the addition of explanations did little to improve it, the researchers said.
Potentially Useful but Still Imperfect
The findings were limited by several factors including the use of a web-based survey, which differs from surveys in a clinical setting, the researchers wrote. Other limitations included the younger than average study population, and the focus on the clinicians making treatment decisions, vs other clinicians who might have a better understanding of the AI explanations.
“In our study, explanations were presented in a way that were considered to be obvious, where the AI model was completely focused on areas of the chest X-rays unrelated to the clinical condition,” Ms. Jabbour told this news organization. “We hypothesized that if presented with such explanations, the participants in our study would notice that the model was behaving incorrectly and not rely on its predictions. This was surprisingly not the case, and the explanations when presented alongside biased AI predictions had seemingly no effect in mitigating clinicians’ overreliance on biased AI,” she said.
“AI is being developed at an extraordinary rate, and our study shows that it has the potential to improve clinical decision-making. At the same time, it could harm clinical decision-making when biased,” Ms. Jabbour said. “We must be thoughtful about how to carefully integrate AI into clinical workflows, with the goal of improving clinical care while not introducing systematic errors or harming patients,” she added.
Looking ahead, “There are several potential research areas that could be explored,” said Ms. Jabbour. “Researchers should focus on careful validation of AI models to identify biased model behavior prior to deployment. AI researchers should also continue including and communicating with clinicians during the development of AI tools to better understand clinicians’ needs and how they interact with AI,” she said. “This is not an exhaustive list of research directions, and it will take much discussion between experts across disciplines such as AI, human computer interaction, and medicine to ultimately deploy AI safely into clinical care.”
Don’t Overestimate AI
“With the increasing use of artificial intelligence and machine learning in other spheres, there has been an increase in interest in exploring how they can be utilized to improve clinical outcomes,” said Suman Pal, MD, assistant professor in the division of hospital medicine at the University of New Mexico, Albuquerque, in an interview. “However, concerns remain regarding the possible harms and ways to mitigate them,” said Dr. Pal, who was not involved in the current study.
In the current study, “It was interesting to note that explanations did not significantly mitigate the decrease in clinician accuracy from systematically biased AI model predictions,” Dr. Pal said.
“For the clinician, the findings of this study caution against overreliance on AI in clinical decision-making, especially because of the risk of exacerbating existing health disparities due to systemic inequities in existing literature,” Dr. Pal told this news organization.
“Additional research is needed to explore how clinicians can be better trained in identifying both the utility and the limitations of AI and into methods of validation and continuous quality checks with integration of AI into clinical workflows,” he noted.
The study was funded by the National Heart, Lung, and Blood Institute. The researchers had no financial conflicts to disclose. Dr. Pal had no financial conflicts to disclose.
A version of this article first appeared on Medscape.com.
Systematically biased artificial intelligence (AI) models did not improve clinicians’ accuracy in diagnosing hospitalized patients, based on data from more than 450 clinicians.
“Artificial Intelligence (AI) could support clinicians in their diagnostic decisions of hospitalized patients but could also be biased and cause potential harm,” said Sarah Jabbour, MSE, a PhD candidate in computer science and engineering at the University of Michigan, Ann Arbor, in an interview.
“Regulatory guidance has suggested that the use of AI explanations could mitigate these harms, but the effectiveness of using AI explanations has not been established,” she said.
To examine whether AI explanations can be effective in mitigating the potential harms of systemic bias in AI models, Ms. Jabbour and colleagues conducted a randomized clinical vignette survey study. The survey was administered between April 2022 and January 2023 across 13 states, and the study population included hospitalist physicians, nurse practitioners, and physician assistants. The results were published in JAMA.
Participants were randomized to AI predictions with AI explanations (226 clinicians) or without AI explanations (231 clinicians).
The primary outcome was diagnostic accuracy for pneumonia, heart failure, and chronic obstructive pulmonary disease, defined as the number of correct diagnoses over the total number of assessments, the researchers wrote.
The clinicians viewed nine clinical vignettes of patients hospitalized with acute respiratory failure, including their presenting symptoms, physical examination, laboratory results, and chest radiographs. Clinicians viewed two vignettes with no AI model input to establish baseline diagnostic accuracy. They made three assessments in each vignette, one for each diagnosis. The order of the vignettes was two without AI predictions (to establish baseline diagnostic accuracy), six with AI predictions, and one with a clinical consultation by a hypothetical colleague. The vignettes included standard and systematically biased AI models.
The baseline diagnostic accuracy was 73% for the diagnoses of pneumonia, heart failure, and chronic obstructive pulmonary disease. Clinicians’ accuracy increased by 2.9% when they viewed a standard diagnostic AI model without explanations and by 4.4% when they viewed models with AI explanations.
However, clinicians’ accuracy decreased by 11.3% after viewing systematically biased AI model predictions without explanations compared with baseline, and biased AI model predictions with explanations decreased accuracy by 9.1%.
The decrease in accuracy with systematically biased AI predictions without explanations was mainly attributable to a decrease in the participants’ diagnostic specificity, the researchers noted, but the addition of explanations did little to improve it, the researchers said.
Potentially Useful but Still Imperfect
The findings were limited by several factors including the use of a web-based survey, which differs from surveys in a clinical setting, the researchers wrote. Other limitations included the younger than average study population, and the focus on the clinicians making treatment decisions, vs other clinicians who might have a better understanding of the AI explanations.
“In our study, explanations were presented in a way that were considered to be obvious, where the AI model was completely focused on areas of the chest X-rays unrelated to the clinical condition,” Ms. Jabbour told this news organization. “We hypothesized that if presented with such explanations, the participants in our study would notice that the model was behaving incorrectly and not rely on its predictions. This was surprisingly not the case, and the explanations when presented alongside biased AI predictions had seemingly no effect in mitigating clinicians’ overreliance on biased AI,” she said.
“AI is being developed at an extraordinary rate, and our study shows that it has the potential to improve clinical decision-making. At the same time, it could harm clinical decision-making when biased,” Ms. Jabbour said. “We must be thoughtful about how to carefully integrate AI into clinical workflows, with the goal of improving clinical care while not introducing systematic errors or harming patients,” she added.
Looking ahead, “There are several potential research areas that could be explored,” said Ms. Jabbour. “Researchers should focus on careful validation of AI models to identify biased model behavior prior to deployment. AI researchers should also continue including and communicating with clinicians during the development of AI tools to better understand clinicians’ needs and how they interact with AI,” she said. “This is not an exhaustive list of research directions, and it will take much discussion between experts across disciplines such as AI, human computer interaction, and medicine to ultimately deploy AI safely into clinical care.”
Don’t Overestimate AI
“With the increasing use of artificial intelligence and machine learning in other spheres, there has been an increase in interest in exploring how they can be utilized to improve clinical outcomes,” said Suman Pal, MD, assistant professor in the division of hospital medicine at the University of New Mexico, Albuquerque, in an interview. “However, concerns remain regarding the possible harms and ways to mitigate them,” said Dr. Pal, who was not involved in the current study.
In the current study, “It was interesting to note that explanations did not significantly mitigate the decrease in clinician accuracy from systematically biased AI model predictions,” Dr. Pal said.
“For the clinician, the findings of this study caution against overreliance on AI in clinical decision-making, especially because of the risk of exacerbating existing health disparities due to systemic inequities in existing literature,” Dr. Pal told this news organization.
“Additional research is needed to explore how clinicians can be better trained in identifying both the utility and the limitations of AI and into methods of validation and continuous quality checks with integration of AI into clinical workflows,” he noted.
The study was funded by the National Heart, Lung, and Blood Institute. The researchers had no financial conflicts to disclose. Dr. Pal had no financial conflicts to disclose.
A version of this article first appeared on Medscape.com.
Systematically biased artificial intelligence (AI) models did not improve clinicians’ accuracy in diagnosing hospitalized patients, based on data from more than 450 clinicians.
“Artificial Intelligence (AI) could support clinicians in their diagnostic decisions of hospitalized patients but could also be biased and cause potential harm,” said Sarah Jabbour, MSE, a PhD candidate in computer science and engineering at the University of Michigan, Ann Arbor, in an interview.
“Regulatory guidance has suggested that the use of AI explanations could mitigate these harms, but the effectiveness of using AI explanations has not been established,” she said.
To examine whether AI explanations can be effective in mitigating the potential harms of systemic bias in AI models, Ms. Jabbour and colleagues conducted a randomized clinical vignette survey study. The survey was administered between April 2022 and January 2023 across 13 states, and the study population included hospitalist physicians, nurse practitioners, and physician assistants. The results were published in JAMA.
Participants were randomized to AI predictions with AI explanations (226 clinicians) or without AI explanations (231 clinicians).
The primary outcome was diagnostic accuracy for pneumonia, heart failure, and chronic obstructive pulmonary disease, defined as the number of correct diagnoses over the total number of assessments, the researchers wrote.
The clinicians viewed nine clinical vignettes of patients hospitalized with acute respiratory failure, including their presenting symptoms, physical examination, laboratory results, and chest radiographs. Clinicians viewed two vignettes with no AI model input to establish baseline diagnostic accuracy. They made three assessments in each vignette, one for each diagnosis. The order of the vignettes was two without AI predictions (to establish baseline diagnostic accuracy), six with AI predictions, and one with a clinical consultation by a hypothetical colleague. The vignettes included standard and systematically biased AI models.
The baseline diagnostic accuracy was 73% for the diagnoses of pneumonia, heart failure, and chronic obstructive pulmonary disease. Clinicians’ accuracy increased by 2.9% when they viewed a standard diagnostic AI model without explanations and by 4.4% when they viewed models with AI explanations.
However, clinicians’ accuracy decreased by 11.3% after viewing systematically biased AI model predictions without explanations compared with baseline, and biased AI model predictions with explanations decreased accuracy by 9.1%.
The decrease in accuracy with systematically biased AI predictions without explanations was mainly attributable to a decrease in the participants’ diagnostic specificity, the researchers noted, but the addition of explanations did little to improve it, the researchers said.
Potentially Useful but Still Imperfect
The findings were limited by several factors including the use of a web-based survey, which differs from surveys in a clinical setting, the researchers wrote. Other limitations included the younger than average study population, and the focus on the clinicians making treatment decisions, vs other clinicians who might have a better understanding of the AI explanations.
“In our study, explanations were presented in a way that were considered to be obvious, where the AI model was completely focused on areas of the chest X-rays unrelated to the clinical condition,” Ms. Jabbour told this news organization. “We hypothesized that if presented with such explanations, the participants in our study would notice that the model was behaving incorrectly and not rely on its predictions. This was surprisingly not the case, and the explanations when presented alongside biased AI predictions had seemingly no effect in mitigating clinicians’ overreliance on biased AI,” she said.
“AI is being developed at an extraordinary rate, and our study shows that it has the potential to improve clinical decision-making. At the same time, it could harm clinical decision-making when biased,” Ms. Jabbour said. “We must be thoughtful about how to carefully integrate AI into clinical workflows, with the goal of improving clinical care while not introducing systematic errors or harming patients,” she added.
Looking ahead, “There are several potential research areas that could be explored,” said Ms. Jabbour. “Researchers should focus on careful validation of AI models to identify biased model behavior prior to deployment. AI researchers should also continue including and communicating with clinicians during the development of AI tools to better understand clinicians’ needs and how they interact with AI,” she said. “This is not an exhaustive list of research directions, and it will take much discussion between experts across disciplines such as AI, human computer interaction, and medicine to ultimately deploy AI safely into clinical care.”
Don’t Overestimate AI
“With the increasing use of artificial intelligence and machine learning in other spheres, there has been an increase in interest in exploring how they can be utilized to improve clinical outcomes,” said Suman Pal, MD, assistant professor in the division of hospital medicine at the University of New Mexico, Albuquerque, in an interview. “However, concerns remain regarding the possible harms and ways to mitigate them,” said Dr. Pal, who was not involved in the current study.
In the current study, “It was interesting to note that explanations did not significantly mitigate the decrease in clinician accuracy from systematically biased AI model predictions,” Dr. Pal said.
“For the clinician, the findings of this study caution against overreliance on AI in clinical decision-making, especially because of the risk of exacerbating existing health disparities due to systemic inequities in existing literature,” Dr. Pal told this news organization.
“Additional research is needed to explore how clinicians can be better trained in identifying both the utility and the limitations of AI and into methods of validation and continuous quality checks with integration of AI into clinical workflows,” he noted.
The study was funded by the National Heart, Lung, and Blood Institute. The researchers had no financial conflicts to disclose. Dr. Pal had no financial conflicts to disclose.
A version of this article first appeared on Medscape.com.
FROM JAMA
Breast implants used in double lung transplant post infection
An innovative surgical procedure combining breast implants and an artificial lung may help more patients with severe lung disease survive to receive transplants. The case was described in a press conference sponsored by Northwestern University, Evanston, Ill.
In May 2023, a surgical team at Northwestern removed both infected lungs from David “Davey” Bauer, aged 34 years, and temporarily used breast implants to hold his heart in place until new lungs were available.
In April 2023, Mr. Bauer, a longtime smoker and vaper, experienced shortness of breath. His girlfriend, Susan Gore, took him to an urgent care center, and he returned home, but “the next morning he couldn’t walk,” Ms. Gore said in the press conference. A trip to the ED yielded a diagnosis of influenza A, followed rapidly by a bacterial lung infection that proved resistant to antibiotics. Mr. Bauer had no prior medical history of serious illness, but he was soon in an intensive care unit. His condition continued to decline, and a double lung transplant was his only option.

The Northwestern Medicine Canning Thoracic Institute specializes in challenging cases, and Mr. Bauer was transferred there.
Back from the brink
Mr. Bauer made the transfer to Chicago despite being critically ill. He was in dire need of a lung transplant, and the only way to resolve his infection was to remove the lungs, said Ankit Bharat, MD, chief of thoracic surgery and director of Northwestern Medicine Canning Thoracic Institute, in the press conference.
“Something needed to be done right away,” Dr. Bharat said. Mr. Bauer’s lungs were removed and the chest cavity was extensively debrided to remove the infection.
Then it was time for outside-the-box thinking. “With the lungs taken out, we needed something to support the heart,” he said. Breast implants came to mind, and double Ds were the largest available.
In addition, the surgeons created an artificial lung system of conduits to keep Mr. Bauer’s blood pumping. “We wanted to maintain the natural blood flow in the body that would be present if the lungs were there,” Dr. Bharat explained.
Plastic surgeons at Northwestern gave Mr. Bauer’s surgical team “a crash course” in managing the breast implants, Dr. Bharat said. The team anticipated that their novel surgical solution would need to last for weeks, but Mr. Bauer’s condition improved immediately once the infected lungs were removed. He was placed on a double-lung transplant list, and the team received an offer of new lungs within 24 hours.
The breast implants were removed, the new lungs were implanted, and Bauer spent several months in the ICU before his discharge to rehabilitation therapy at the end of September, according to a Northwestern press release.
This type of procedure could help patients with infections who need transplants but are too sick to undergo them, Dr. Bharat said in the press conference. In Mr. Bauer’s case, “a lot of stars aligned,” including Bauer’s rapid improvement and the quick availability of a perfect lung match, Dr. Bharat said. Many patients don’t survive to the point of transplant.
“We were surprised how quickly he recovered once we removed the infected lungs,” Dr. Bharat noted. The quick recovery may be in part because of Bauer’s youth and relative good health, but “this was uncharted territory.”
Mr. Bauer’s case is the first use of this particular surgical technique, although the team drew on lessons learned in other surgical settings, such as removal of both lungs to prevent cross-contamination in patients with cancer, he added.
Causes and effects
As for the factors that contributed to Mr. Bauer’s initial infection, “there is a lot we don’t know, but we can try to put things together,” said Dr. Bharat. Just as many factors lined up to promote Mr. Bauer’s recovery, many factors lined up to cause the problem, including long-standing smoking and vaping. Although some still view vaping as a safer alternative to smoking, patient data and experiences do not support this claim. “We know for a fact that both of them cause harm,” he added.
Mr. Bauer started smoking cigarettes at age 21 and typically smoked a pack of cigarettes each day before switching to vaping in 2014. In addition, Mr. Bauer had not been vaccinated against the flu, and his flu infection was followed by a bacterial infection.
Bacterial infections followed by hospitalizations are not new as an effect of vaping; a series of articles described the ongoing epidemic of e-cigarette or vaping product use–associated lung injury (EVALI). Patients with EVALI often present at urgent care centers, as Bauer did, with symptoms of flu or pneumonia, and they are often given medication and sent home.
Looking ahead: “We expect that Davey will fully recover and live a normal life,” although he will remain in Chicago for another year for monitoring, said Rade Tomic, MD, pulmonologist and medical director of the Northwestern Medicine Canning Thoracic Institute lung transplant program, in the press conference.
Mr. Bauer expressed his thanks to the surgical team, who also presented him with another gift: a T-shirt with his newly chosen nickname, “DD Davey.” “I feel so blessed, I got a second chance at life,” Mr. Bauer said in the press conference. “You should not inhale anything into your lungs except oxygen.”
A version of this article first appeared on Medscape.com.
An innovative surgical procedure combining breast implants and an artificial lung may help more patients with severe lung disease survive to receive transplants. The case was described in a press conference sponsored by Northwestern University, Evanston, Ill.
In May 2023, a surgical team at Northwestern removed both infected lungs from David “Davey” Bauer, aged 34 years, and temporarily used breast implants to hold his heart in place until new lungs were available.
In April 2023, Mr. Bauer, a longtime smoker and vaper, experienced shortness of breath. His girlfriend, Susan Gore, took him to an urgent care center, and he returned home, but “the next morning he couldn’t walk,” Ms. Gore said in the press conference. A trip to the ED yielded a diagnosis of influenza A, followed rapidly by a bacterial lung infection that proved resistant to antibiotics. Mr. Bauer had no prior medical history of serious illness, but he was soon in an intensive care unit. His condition continued to decline, and a double lung transplant was his only option.

The Northwestern Medicine Canning Thoracic Institute specializes in challenging cases, and Mr. Bauer was transferred there.
Back from the brink
Mr. Bauer made the transfer to Chicago despite being critically ill. He was in dire need of a lung transplant, and the only way to resolve his infection was to remove the lungs, said Ankit Bharat, MD, chief of thoracic surgery and director of Northwestern Medicine Canning Thoracic Institute, in the press conference.
“Something needed to be done right away,” Dr. Bharat said. Mr. Bauer’s lungs were removed and the chest cavity was extensively debrided to remove the infection.
Then it was time for outside-the-box thinking. “With the lungs taken out, we needed something to support the heart,” he said. Breast implants came to mind, and double Ds were the largest available.
In addition, the surgeons created an artificial lung system of conduits to keep Mr. Bauer’s blood pumping. “We wanted to maintain the natural blood flow in the body that would be present if the lungs were there,” Dr. Bharat explained.
Plastic surgeons at Northwestern gave Mr. Bauer’s surgical team “a crash course” in managing the breast implants, Dr. Bharat said. The team anticipated that their novel surgical solution would need to last for weeks, but Mr. Bauer’s condition improved immediately once the infected lungs were removed. He was placed on a double-lung transplant list, and the team received an offer of new lungs within 24 hours.
The breast implants were removed, the new lungs were implanted, and Bauer spent several months in the ICU before his discharge to rehabilitation therapy at the end of September, according to a Northwestern press release.
This type of procedure could help patients with infections who need transplants but are too sick to undergo them, Dr. Bharat said in the press conference. In Mr. Bauer’s case, “a lot of stars aligned,” including Bauer’s rapid improvement and the quick availability of a perfect lung match, Dr. Bharat said. Many patients don’t survive to the point of transplant.
“We were surprised how quickly he recovered once we removed the infected lungs,” Dr. Bharat noted. The quick recovery may be in part because of Bauer’s youth and relative good health, but “this was uncharted territory.”
Mr. Bauer’s case is the first use of this particular surgical technique, although the team drew on lessons learned in other surgical settings, such as removal of both lungs to prevent cross-contamination in patients with cancer, he added.
Causes and effects
As for the factors that contributed to Mr. Bauer’s initial infection, “there is a lot we don’t know, but we can try to put things together,” said Dr. Bharat. Just as many factors lined up to promote Mr. Bauer’s recovery, many factors lined up to cause the problem, including long-standing smoking and vaping. Although some still view vaping as a safer alternative to smoking, patient data and experiences do not support this claim. “We know for a fact that both of them cause harm,” he added.
Mr. Bauer started smoking cigarettes at age 21 and typically smoked a pack of cigarettes each day before switching to vaping in 2014. In addition, Mr. Bauer had not been vaccinated against the flu, and his flu infection was followed by a bacterial infection.
Bacterial infections followed by hospitalizations are not new as an effect of vaping; a series of articles described the ongoing epidemic of e-cigarette or vaping product use–associated lung injury (EVALI). Patients with EVALI often present at urgent care centers, as Bauer did, with symptoms of flu or pneumonia, and they are often given medication and sent home.
Looking ahead: “We expect that Davey will fully recover and live a normal life,” although he will remain in Chicago for another year for monitoring, said Rade Tomic, MD, pulmonologist and medical director of the Northwestern Medicine Canning Thoracic Institute lung transplant program, in the press conference.
Mr. Bauer expressed his thanks to the surgical team, who also presented him with another gift: a T-shirt with his newly chosen nickname, “DD Davey.” “I feel so blessed, I got a second chance at life,” Mr. Bauer said in the press conference. “You should not inhale anything into your lungs except oxygen.”
A version of this article first appeared on Medscape.com.
An innovative surgical procedure combining breast implants and an artificial lung may help more patients with severe lung disease survive to receive transplants. The case was described in a press conference sponsored by Northwestern University, Evanston, Ill.
In May 2023, a surgical team at Northwestern removed both infected lungs from David “Davey” Bauer, aged 34 years, and temporarily used breast implants to hold his heart in place until new lungs were available.
In April 2023, Mr. Bauer, a longtime smoker and vaper, experienced shortness of breath. His girlfriend, Susan Gore, took him to an urgent care center, and he returned home, but “the next morning he couldn’t walk,” Ms. Gore said in the press conference. A trip to the ED yielded a diagnosis of influenza A, followed rapidly by a bacterial lung infection that proved resistant to antibiotics. Mr. Bauer had no prior medical history of serious illness, but he was soon in an intensive care unit. His condition continued to decline, and a double lung transplant was his only option.

The Northwestern Medicine Canning Thoracic Institute specializes in challenging cases, and Mr. Bauer was transferred there.
Back from the brink
Mr. Bauer made the transfer to Chicago despite being critically ill. He was in dire need of a lung transplant, and the only way to resolve his infection was to remove the lungs, said Ankit Bharat, MD, chief of thoracic surgery and director of Northwestern Medicine Canning Thoracic Institute, in the press conference.
“Something needed to be done right away,” Dr. Bharat said. Mr. Bauer’s lungs were removed and the chest cavity was extensively debrided to remove the infection.
Then it was time for outside-the-box thinking. “With the lungs taken out, we needed something to support the heart,” he said. Breast implants came to mind, and double Ds were the largest available.
In addition, the surgeons created an artificial lung system of conduits to keep Mr. Bauer’s blood pumping. “We wanted to maintain the natural blood flow in the body that would be present if the lungs were there,” Dr. Bharat explained.
Plastic surgeons at Northwestern gave Mr. Bauer’s surgical team “a crash course” in managing the breast implants, Dr. Bharat said. The team anticipated that their novel surgical solution would need to last for weeks, but Mr. Bauer’s condition improved immediately once the infected lungs were removed. He was placed on a double-lung transplant list, and the team received an offer of new lungs within 24 hours.
The breast implants were removed, the new lungs were implanted, and Bauer spent several months in the ICU before his discharge to rehabilitation therapy at the end of September, according to a Northwestern press release.
This type of procedure could help patients with infections who need transplants but are too sick to undergo them, Dr. Bharat said in the press conference. In Mr. Bauer’s case, “a lot of stars aligned,” including Bauer’s rapid improvement and the quick availability of a perfect lung match, Dr. Bharat said. Many patients don’t survive to the point of transplant.
“We were surprised how quickly he recovered once we removed the infected lungs,” Dr. Bharat noted. The quick recovery may be in part because of Bauer’s youth and relative good health, but “this was uncharted territory.”
Mr. Bauer’s case is the first use of this particular surgical technique, although the team drew on lessons learned in other surgical settings, such as removal of both lungs to prevent cross-contamination in patients with cancer, he added.
Causes and effects
As for the factors that contributed to Mr. Bauer’s initial infection, “there is a lot we don’t know, but we can try to put things together,” said Dr. Bharat. Just as many factors lined up to promote Mr. Bauer’s recovery, many factors lined up to cause the problem, including long-standing smoking and vaping. Although some still view vaping as a safer alternative to smoking, patient data and experiences do not support this claim. “We know for a fact that both of them cause harm,” he added.
Mr. Bauer started smoking cigarettes at age 21 and typically smoked a pack of cigarettes each day before switching to vaping in 2014. In addition, Mr. Bauer had not been vaccinated against the flu, and his flu infection was followed by a bacterial infection.
Bacterial infections followed by hospitalizations are not new as an effect of vaping; a series of articles described the ongoing epidemic of e-cigarette or vaping product use–associated lung injury (EVALI). Patients with EVALI often present at urgent care centers, as Bauer did, with symptoms of flu or pneumonia, and they are often given medication and sent home.
Looking ahead: “We expect that Davey will fully recover and live a normal life,” although he will remain in Chicago for another year for monitoring, said Rade Tomic, MD, pulmonologist and medical director of the Northwestern Medicine Canning Thoracic Institute lung transplant program, in the press conference.
Mr. Bauer expressed his thanks to the surgical team, who also presented him with another gift: a T-shirt with his newly chosen nickname, “DD Davey.” “I feel so blessed, I got a second chance at life,” Mr. Bauer said in the press conference. “You should not inhale anything into your lungs except oxygen.”
A version of this article first appeared on Medscape.com.
Three antibiotic regimens show similar effectiveness for CAP
Adults with nonsevere community-acquired pneumonia (CAP) responded nearly equally to three first-line and alternative antibiotic regimens, based on data from more than 23,000 individuals.
Current recommendations for the treatment of CAP vary across guidelines, wrote Anthony D. Bai, MD, of Queen’s University, Kingston, Ont., and colleagues. However, most guidelines were based on studies that were not powered to examine the effect of treatments on mortality, they said.
“Large observational studies could fill this gap by comparing multiple treatment arms, including patients not well represented in trials, and having a large sample size powered to detect a difference in mortality,” they noted.
In a study published in Chest, the researchers reviewed data from 23,512 consecutive patients admitted to 19 hospitals in Canada for CAP between 2015 and 2021. Patients were treated with one of four initial antibiotic regimens: beta-lactam plus macrolide (BL+M), beta-lactam alone (BL), respiratory fluoroquinolone (FQ), or beta-lactam plus doxycycline (BL+D). Of these, BL+M is generally considered the first-line regimen, the researchers noted.
Patients were divided into four groups according to their initial antibiotic treatment within 48 hours of admission; 9,340 patients received BL+M, 9,146 received BL, 4,510 received FQ, and 516 received BL+D. The duration of any antibiotic that was active against CAP was at least 4 days, or until hospital discharge or death.
The primary outcome was all-cause in-hospital mortality, which was 7.5%, 9.7%, 6.7%, and 6.0% for patients in each of the four treatment groups, respectively. Relative to the first-line therapy of BL+M, the adjusted risk differences for BL, FQ, and BL+D were 1.5%, –0.9%, and –1.9%, respectively.
The adjusted in-hospital mortality was not significantly different between BL+M and either FQ or BL+D, but the difference of 1.5% seen with BL alone suggested a “small but clinically important difference,” the researchers noted.
Key secondary outcomes were the length of hospital stay and being discharged alive. The median length of stay was 4.6 days for BL+M, 5.2 days for BL, 4.6 days for FQ, and 6.0 days for BL+D. Patients treated with BL also had a longer time to hospital discharge, which suggests that BL may not be as effective as the other regimens, the researchers said. In addition, patients in the BL group had a subdistribution hazard ratio of 0.90 for being discharged alive, compared with the BL+M group after adjustment with propensity scores and overlap weighting.
Overall, the results support dropping BL as a first-line regimen in the current ATS/IDSA guidelines, and support the recommendation of BL+M, FQ, and BL+D as similarly effective options as listed in other guidelines, applied according to other patient characteristics. For example, “Doxycycline may be preferred over a macrolide in many cases such as macrolide allergy, prolonged QT, or high [Clostridioides] difficile risk,” the researchers said.
The findings were limited by several factors including the lack of follow-up data after hospital discharge.
However, the results were strengthened by the large sample size and use of a comprehensive database that allowed adjustment for many variables, as well as the availability of complete follow-up data for the time spent in the hospital. Based on this study, clinicians may choose a respiratory fluoroquinolone, a beta-lactam plus macrolide, or a beta-lactam plus doxycycline for equally effective antibiotic treatment of CAP, based on the best fit for each individual patient, the researchers concluded.
The study received no outside funding. The researchers had no financial conflicts to disclose.
Adults with nonsevere community-acquired pneumonia (CAP) responded nearly equally to three first-line and alternative antibiotic regimens, based on data from more than 23,000 individuals.
Current recommendations for the treatment of CAP vary across guidelines, wrote Anthony D. Bai, MD, of Queen’s University, Kingston, Ont., and colleagues. However, most guidelines were based on studies that were not powered to examine the effect of treatments on mortality, they said.
“Large observational studies could fill this gap by comparing multiple treatment arms, including patients not well represented in trials, and having a large sample size powered to detect a difference in mortality,” they noted.
In a study published in Chest, the researchers reviewed data from 23,512 consecutive patients admitted to 19 hospitals in Canada for CAP between 2015 and 2021. Patients were treated with one of four initial antibiotic regimens: beta-lactam plus macrolide (BL+M), beta-lactam alone (BL), respiratory fluoroquinolone (FQ), or beta-lactam plus doxycycline (BL+D). Of these, BL+M is generally considered the first-line regimen, the researchers noted.
Patients were divided into four groups according to their initial antibiotic treatment within 48 hours of admission; 9,340 patients received BL+M, 9,146 received BL, 4,510 received FQ, and 516 received BL+D. The duration of any antibiotic that was active against CAP was at least 4 days, or until hospital discharge or death.
The primary outcome was all-cause in-hospital mortality, which was 7.5%, 9.7%, 6.7%, and 6.0% for patients in each of the four treatment groups, respectively. Relative to the first-line therapy of BL+M, the adjusted risk differences for BL, FQ, and BL+D were 1.5%, –0.9%, and –1.9%, respectively.
The adjusted in-hospital mortality was not significantly different between BL+M and either FQ or BL+D, but the difference of 1.5% seen with BL alone suggested a “small but clinically important difference,” the researchers noted.
Key secondary outcomes were the length of hospital stay and being discharged alive. The median length of stay was 4.6 days for BL+M, 5.2 days for BL, 4.6 days for FQ, and 6.0 days for BL+D. Patients treated with BL also had a longer time to hospital discharge, which suggests that BL may not be as effective as the other regimens, the researchers said. In addition, patients in the BL group had a subdistribution hazard ratio of 0.90 for being discharged alive, compared with the BL+M group after adjustment with propensity scores and overlap weighting.
Overall, the results support dropping BL as a first-line regimen in the current ATS/IDSA guidelines, and support the recommendation of BL+M, FQ, and BL+D as similarly effective options as listed in other guidelines, applied according to other patient characteristics. For example, “Doxycycline may be preferred over a macrolide in many cases such as macrolide allergy, prolonged QT, or high [Clostridioides] difficile risk,” the researchers said.
The findings were limited by several factors including the lack of follow-up data after hospital discharge.
However, the results were strengthened by the large sample size and use of a comprehensive database that allowed adjustment for many variables, as well as the availability of complete follow-up data for the time spent in the hospital. Based on this study, clinicians may choose a respiratory fluoroquinolone, a beta-lactam plus macrolide, or a beta-lactam plus doxycycline for equally effective antibiotic treatment of CAP, based on the best fit for each individual patient, the researchers concluded.
The study received no outside funding. The researchers had no financial conflicts to disclose.
Adults with nonsevere community-acquired pneumonia (CAP) responded nearly equally to three first-line and alternative antibiotic regimens, based on data from more than 23,000 individuals.
Current recommendations for the treatment of CAP vary across guidelines, wrote Anthony D. Bai, MD, of Queen’s University, Kingston, Ont., and colleagues. However, most guidelines were based on studies that were not powered to examine the effect of treatments on mortality, they said.
“Large observational studies could fill this gap by comparing multiple treatment arms, including patients not well represented in trials, and having a large sample size powered to detect a difference in mortality,” they noted.
In a study published in Chest, the researchers reviewed data from 23,512 consecutive patients admitted to 19 hospitals in Canada for CAP between 2015 and 2021. Patients were treated with one of four initial antibiotic regimens: beta-lactam plus macrolide (BL+M), beta-lactam alone (BL), respiratory fluoroquinolone (FQ), or beta-lactam plus doxycycline (BL+D). Of these, BL+M is generally considered the first-line regimen, the researchers noted.
Patients were divided into four groups according to their initial antibiotic treatment within 48 hours of admission; 9,340 patients received BL+M, 9,146 received BL, 4,510 received FQ, and 516 received BL+D. The duration of any antibiotic that was active against CAP was at least 4 days, or until hospital discharge or death.
The primary outcome was all-cause in-hospital mortality, which was 7.5%, 9.7%, 6.7%, and 6.0% for patients in each of the four treatment groups, respectively. Relative to the first-line therapy of BL+M, the adjusted risk differences for BL, FQ, and BL+D were 1.5%, –0.9%, and –1.9%, respectively.
The adjusted in-hospital mortality was not significantly different between BL+M and either FQ or BL+D, but the difference of 1.5% seen with BL alone suggested a “small but clinically important difference,” the researchers noted.
Key secondary outcomes were the length of hospital stay and being discharged alive. The median length of stay was 4.6 days for BL+M, 5.2 days for BL, 4.6 days for FQ, and 6.0 days for BL+D. Patients treated with BL also had a longer time to hospital discharge, which suggests that BL may not be as effective as the other regimens, the researchers said. In addition, patients in the BL group had a subdistribution hazard ratio of 0.90 for being discharged alive, compared with the BL+M group after adjustment with propensity scores and overlap weighting.
Overall, the results support dropping BL as a first-line regimen in the current ATS/IDSA guidelines, and support the recommendation of BL+M, FQ, and BL+D as similarly effective options as listed in other guidelines, applied according to other patient characteristics. For example, “Doxycycline may be preferred over a macrolide in many cases such as macrolide allergy, prolonged QT, or high [Clostridioides] difficile risk,” the researchers said.
The findings were limited by several factors including the lack of follow-up data after hospital discharge.
However, the results were strengthened by the large sample size and use of a comprehensive database that allowed adjustment for many variables, as well as the availability of complete follow-up data for the time spent in the hospital. Based on this study, clinicians may choose a respiratory fluoroquinolone, a beta-lactam plus macrolide, or a beta-lactam plus doxycycline for equally effective antibiotic treatment of CAP, based on the best fit for each individual patient, the researchers concluded.
The study received no outside funding. The researchers had no financial conflicts to disclose.
FROM CHEST
Pneumonia decision tool reduces death in ED patients
a 3-year, pragmatic, cluster-controlled study shows.
“We designed the ePNa specifically to require minimal input from the clinician so everything it does is already in the electronic medical record,” Nathan Dean, MD, University of Utah, Salt Lake City, told this news organization.
“So it’s actually putting the guideline recommendations into effect for physicians so that they can make better decisions by having all this information – it’s a comprehensive best practice kind of tool where best practices are likely to make the biggest difference for patients with a high severity of illness,” he added.
The study was published online in the American Journal of Respiratory and Critical Care Medicine.
Guideline-based tool
The ePNa makes use of pneumonia guidelines of 2007 and 2019 from the American Thoracic Society/Infectious Disease Society of America. The system was deployed into six geographic clusters of 16 Intermountain hospital EDs at 2-month intervals between December 2017 and November 2018. Simultaneous deployment was impractical, as implementation of the tool takes education, monitoring, and feedback that can be facilitated by focusing on only a few hospitals at a time.
The decision support tool gathers key patient indicators including age, fever, oxygen saturation, vital signs, and laboratory and chest imaging results to offer recommendations on care, including appropriate antibiotic therapy, microbiology studies, and whether a given patient should be sent to the intensive care unit, admitted to hospital, or may safely be discharged home.
Investigators analyzed a total of 6,848 patients, of whom 4,536 were managed for pneumonia before the ePNa was deployed and 2,312 after deployment.
The median age of patients was 67 years (interquartile range, 50-79 years). Roughly half were female and almost all were White. “Observed 30-day all-cause mortality including both outpatients and inpatients was 8.6% before deployment versus 4.8% after deployment of ePNa,” Dr. Dean and colleagues reported.
Adjusted for severity of illness, the odds ratio for lower mortality post-ePNa launch was 0.62 (95% confidence interval, 0.49-0.79; P < .0010) “and lower morality was consistent across hospital clusters.”
Compared with patients who were discharged home, reductions in mortality were greatest in patients who were directly admitted to ICUs from the ED (OR, 0.32; 95% CI, 0.14-0.77; P = .01). The OR for patients admitted to the medical floor was 0.53 (95% CI, 0.25-1.1; P = .09), which did not reach statistical significance.
Dr. Dean explained that the reductions in mortality were seen among those with the most severe illness, in whom best practices would benefit the most. In contrast, patients who are sent home on an antibiotic are at low risk for mortality while patients admitted to the medical floor may well have another, more lethal illness from which they end up dying, rather than simple pneumonia.
“For me, this was a clear demonstration that these best practices made the biggest difference in patients who were sick and who did not have any underlying disease that was going to kill them anyway,” he emphasized. On the other hand, both 30-day mortality and 7-day secondary hospital admission were higher among patients the tool recommended for hospital ward admission but who were discharged home from the ED.
“This was an unexpected finding,” Dr. Dean observed. However, as he explained, the authors reviewed 25% of randomly selected patients who fell into this subgroup and discovered that the ePNa tool was used in only about 20% of patients – “so doctors did not use the tool in the majority of this group.”
In addition, some of these patients declined hospital admission, so the doctors may have recommended that they be admitted but the patients said no. “The hypothesis here is that if they had been admitted to the hospital, they may have had a lower mortality risk,” Dr. Dean said.
Noticeable changes
Another noticeable change following the introduction of the ePNa tool was that guideline-concordant antibiotic prescribing increased in the 8 hours after patients presented to the ED, from 79.5% prior to the tool’s launch to 87.9%, again after adjusting for pneumonia severity (P < .001). Use of broad-spectrum antibiotics was not significantly different between the two treatment intervals, but administration of antibiotics active against methicillin-resistant Staphylococcus aureus dropped significantly between the two treatment intervals (P < .001). And the mean time from admission to the ED to the first antibiotic taken was slightly faster, improving from 159.4 minutes (95% CI, 156.9-161.9 minutes) prior to the ePNa launch to 150.9 minutes (95% CI, 144.1-157.8) post deployment (P < .001).
“Overall outpatient disposition for treatment of pneumonia from the emergency department increased from 29.2% before ePNa to 46.9% [post ePNA],” the authors noted, while a similar increase was observed in patients for whom ePNA recommended outpatient care – from 49.2% pre-ePNA to 66.6% after ePNA.
Both hospital ward admission and admission to the ICU decreased after ePNa had been introduced. Despite a significant increase in the percentage of patients being discharged home, neither 7-day secondary hospital admission nor severity-adjusted, 30-day mortality were significantly different before versus after the introduction of ePNa, the authors stressed.
A limitation of the study was that the trial was confined to a single health care system in one region of the United States with a patient population that may differ from that in other regions.
Reason for its success
Asked to comment on the findings, Adam Balls, MD, emergency department chair, Intermountain Medical Center, Murray, Utah, suggested that the reason the ePNa tool has been so successful at improving care for pneumonia patients is that it puts the guidelines directly into the hands of individual providers and tells them what’s going on. (Dr. Balls was not involved in the study.) “The tool allows us to take into consideration various clinical features – a patient’s oxygen requirements and whether or not they had prior complicated pneumonias that required additional antibiotics, for example – and then it makes the best determination for not only the disposition for that patient but antibiotic treatment as well,” he said in an interview.
This then allows physicians to either appropriately discharge less severely ill patients and admit those who are more ill – “and in general, just do a better job of treating pneumonia with this tool,” Dr. Balls said. He himself uses the decision support tool when attending to his own patients with pneumonia, as he feels that the tool really does make his care of these patients better. “There is a disparity around how we treat pneumonia in the U.S.
“Clinicians sometimes have a bias or a preference for certain antibiotics and we may not be appropriately treating these patients with broad-spectrum antibiotics or are perhaps using antibiotics that are not as effective based on an individual patient scenario so this is definitely a user-friendly tool that hopefully can be deployed throughout other health care systems to improve the treatment of pneumonia overall,” Dr. Balls emphasized.
A version of this article first appeared on Medscape.com.
a 3-year, pragmatic, cluster-controlled study shows.
“We designed the ePNa specifically to require minimal input from the clinician so everything it does is already in the electronic medical record,” Nathan Dean, MD, University of Utah, Salt Lake City, told this news organization.
“So it’s actually putting the guideline recommendations into effect for physicians so that they can make better decisions by having all this information – it’s a comprehensive best practice kind of tool where best practices are likely to make the biggest difference for patients with a high severity of illness,” he added.
The study was published online in the American Journal of Respiratory and Critical Care Medicine.
Guideline-based tool
The ePNa makes use of pneumonia guidelines of 2007 and 2019 from the American Thoracic Society/Infectious Disease Society of America. The system was deployed into six geographic clusters of 16 Intermountain hospital EDs at 2-month intervals between December 2017 and November 2018. Simultaneous deployment was impractical, as implementation of the tool takes education, monitoring, and feedback that can be facilitated by focusing on only a few hospitals at a time.
The decision support tool gathers key patient indicators including age, fever, oxygen saturation, vital signs, and laboratory and chest imaging results to offer recommendations on care, including appropriate antibiotic therapy, microbiology studies, and whether a given patient should be sent to the intensive care unit, admitted to hospital, or may safely be discharged home.
Investigators analyzed a total of 6,848 patients, of whom 4,536 were managed for pneumonia before the ePNa was deployed and 2,312 after deployment.
The median age of patients was 67 years (interquartile range, 50-79 years). Roughly half were female and almost all were White. “Observed 30-day all-cause mortality including both outpatients and inpatients was 8.6% before deployment versus 4.8% after deployment of ePNa,” Dr. Dean and colleagues reported.
Adjusted for severity of illness, the odds ratio for lower mortality post-ePNa launch was 0.62 (95% confidence interval, 0.49-0.79; P < .0010) “and lower morality was consistent across hospital clusters.”
Compared with patients who were discharged home, reductions in mortality were greatest in patients who were directly admitted to ICUs from the ED (OR, 0.32; 95% CI, 0.14-0.77; P = .01). The OR for patients admitted to the medical floor was 0.53 (95% CI, 0.25-1.1; P = .09), which did not reach statistical significance.
Dr. Dean explained that the reductions in mortality were seen among those with the most severe illness, in whom best practices would benefit the most. In contrast, patients who are sent home on an antibiotic are at low risk for mortality while patients admitted to the medical floor may well have another, more lethal illness from which they end up dying, rather than simple pneumonia.
“For me, this was a clear demonstration that these best practices made the biggest difference in patients who were sick and who did not have any underlying disease that was going to kill them anyway,” he emphasized. On the other hand, both 30-day mortality and 7-day secondary hospital admission were higher among patients the tool recommended for hospital ward admission but who were discharged home from the ED.
“This was an unexpected finding,” Dr. Dean observed. However, as he explained, the authors reviewed 25% of randomly selected patients who fell into this subgroup and discovered that the ePNa tool was used in only about 20% of patients – “so doctors did not use the tool in the majority of this group.”
In addition, some of these patients declined hospital admission, so the doctors may have recommended that they be admitted but the patients said no. “The hypothesis here is that if they had been admitted to the hospital, they may have had a lower mortality risk,” Dr. Dean said.
Noticeable changes
Another noticeable change following the introduction of the ePNa tool was that guideline-concordant antibiotic prescribing increased in the 8 hours after patients presented to the ED, from 79.5% prior to the tool’s launch to 87.9%, again after adjusting for pneumonia severity (P < .001). Use of broad-spectrum antibiotics was not significantly different between the two treatment intervals, but administration of antibiotics active against methicillin-resistant Staphylococcus aureus dropped significantly between the two treatment intervals (P < .001). And the mean time from admission to the ED to the first antibiotic taken was slightly faster, improving from 159.4 minutes (95% CI, 156.9-161.9 minutes) prior to the ePNa launch to 150.9 minutes (95% CI, 144.1-157.8) post deployment (P < .001).
“Overall outpatient disposition for treatment of pneumonia from the emergency department increased from 29.2% before ePNa to 46.9% [post ePNA],” the authors noted, while a similar increase was observed in patients for whom ePNA recommended outpatient care – from 49.2% pre-ePNA to 66.6% after ePNA.
Both hospital ward admission and admission to the ICU decreased after ePNa had been introduced. Despite a significant increase in the percentage of patients being discharged home, neither 7-day secondary hospital admission nor severity-adjusted, 30-day mortality were significantly different before versus after the introduction of ePNa, the authors stressed.
A limitation of the study was that the trial was confined to a single health care system in one region of the United States with a patient population that may differ from that in other regions.
Reason for its success
Asked to comment on the findings, Adam Balls, MD, emergency department chair, Intermountain Medical Center, Murray, Utah, suggested that the reason the ePNa tool has been so successful at improving care for pneumonia patients is that it puts the guidelines directly into the hands of individual providers and tells them what’s going on. (Dr. Balls was not involved in the study.) “The tool allows us to take into consideration various clinical features – a patient’s oxygen requirements and whether or not they had prior complicated pneumonias that required additional antibiotics, for example – and then it makes the best determination for not only the disposition for that patient but antibiotic treatment as well,” he said in an interview.
This then allows physicians to either appropriately discharge less severely ill patients and admit those who are more ill – “and in general, just do a better job of treating pneumonia with this tool,” Dr. Balls said. He himself uses the decision support tool when attending to his own patients with pneumonia, as he feels that the tool really does make his care of these patients better. “There is a disparity around how we treat pneumonia in the U.S.
“Clinicians sometimes have a bias or a preference for certain antibiotics and we may not be appropriately treating these patients with broad-spectrum antibiotics or are perhaps using antibiotics that are not as effective based on an individual patient scenario so this is definitely a user-friendly tool that hopefully can be deployed throughout other health care systems to improve the treatment of pneumonia overall,” Dr. Balls emphasized.
A version of this article first appeared on Medscape.com.
a 3-year, pragmatic, cluster-controlled study shows.
“We designed the ePNa specifically to require minimal input from the clinician so everything it does is already in the electronic medical record,” Nathan Dean, MD, University of Utah, Salt Lake City, told this news organization.
“So it’s actually putting the guideline recommendations into effect for physicians so that they can make better decisions by having all this information – it’s a comprehensive best practice kind of tool where best practices are likely to make the biggest difference for patients with a high severity of illness,” he added.
The study was published online in the American Journal of Respiratory and Critical Care Medicine.
Guideline-based tool
The ePNa makes use of pneumonia guidelines of 2007 and 2019 from the American Thoracic Society/Infectious Disease Society of America. The system was deployed into six geographic clusters of 16 Intermountain hospital EDs at 2-month intervals between December 2017 and November 2018. Simultaneous deployment was impractical, as implementation of the tool takes education, monitoring, and feedback that can be facilitated by focusing on only a few hospitals at a time.
The decision support tool gathers key patient indicators including age, fever, oxygen saturation, vital signs, and laboratory and chest imaging results to offer recommendations on care, including appropriate antibiotic therapy, microbiology studies, and whether a given patient should be sent to the intensive care unit, admitted to hospital, or may safely be discharged home.
Investigators analyzed a total of 6,848 patients, of whom 4,536 were managed for pneumonia before the ePNa was deployed and 2,312 after deployment.
The median age of patients was 67 years (interquartile range, 50-79 years). Roughly half were female and almost all were White. “Observed 30-day all-cause mortality including both outpatients and inpatients was 8.6% before deployment versus 4.8% after deployment of ePNa,” Dr. Dean and colleagues reported.
Adjusted for severity of illness, the odds ratio for lower mortality post-ePNa launch was 0.62 (95% confidence interval, 0.49-0.79; P < .0010) “and lower morality was consistent across hospital clusters.”
Compared with patients who were discharged home, reductions in mortality were greatest in patients who were directly admitted to ICUs from the ED (OR, 0.32; 95% CI, 0.14-0.77; P = .01). The OR for patients admitted to the medical floor was 0.53 (95% CI, 0.25-1.1; P = .09), which did not reach statistical significance.
Dr. Dean explained that the reductions in mortality were seen among those with the most severe illness, in whom best practices would benefit the most. In contrast, patients who are sent home on an antibiotic are at low risk for mortality while patients admitted to the medical floor may well have another, more lethal illness from which they end up dying, rather than simple pneumonia.
“For me, this was a clear demonstration that these best practices made the biggest difference in patients who were sick and who did not have any underlying disease that was going to kill them anyway,” he emphasized. On the other hand, both 30-day mortality and 7-day secondary hospital admission were higher among patients the tool recommended for hospital ward admission but who were discharged home from the ED.
“This was an unexpected finding,” Dr. Dean observed. However, as he explained, the authors reviewed 25% of randomly selected patients who fell into this subgroup and discovered that the ePNa tool was used in only about 20% of patients – “so doctors did not use the tool in the majority of this group.”
In addition, some of these patients declined hospital admission, so the doctors may have recommended that they be admitted but the patients said no. “The hypothesis here is that if they had been admitted to the hospital, they may have had a lower mortality risk,” Dr. Dean said.
Noticeable changes
Another noticeable change following the introduction of the ePNa tool was that guideline-concordant antibiotic prescribing increased in the 8 hours after patients presented to the ED, from 79.5% prior to the tool’s launch to 87.9%, again after adjusting for pneumonia severity (P < .001). Use of broad-spectrum antibiotics was not significantly different between the two treatment intervals, but administration of antibiotics active against methicillin-resistant Staphylococcus aureus dropped significantly between the two treatment intervals (P < .001). And the mean time from admission to the ED to the first antibiotic taken was slightly faster, improving from 159.4 minutes (95% CI, 156.9-161.9 minutes) prior to the ePNa launch to 150.9 minutes (95% CI, 144.1-157.8) post deployment (P < .001).
“Overall outpatient disposition for treatment of pneumonia from the emergency department increased from 29.2% before ePNa to 46.9% [post ePNA],” the authors noted, while a similar increase was observed in patients for whom ePNA recommended outpatient care – from 49.2% pre-ePNA to 66.6% after ePNA.
Both hospital ward admission and admission to the ICU decreased after ePNa had been introduced. Despite a significant increase in the percentage of patients being discharged home, neither 7-day secondary hospital admission nor severity-adjusted, 30-day mortality were significantly different before versus after the introduction of ePNa, the authors stressed.
A limitation of the study was that the trial was confined to a single health care system in one region of the United States with a patient population that may differ from that in other regions.
Reason for its success
Asked to comment on the findings, Adam Balls, MD, emergency department chair, Intermountain Medical Center, Murray, Utah, suggested that the reason the ePNa tool has been so successful at improving care for pneumonia patients is that it puts the guidelines directly into the hands of individual providers and tells them what’s going on. (Dr. Balls was not involved in the study.) “The tool allows us to take into consideration various clinical features – a patient’s oxygen requirements and whether or not they had prior complicated pneumonias that required additional antibiotics, for example – and then it makes the best determination for not only the disposition for that patient but antibiotic treatment as well,” he said in an interview.
This then allows physicians to either appropriately discharge less severely ill patients and admit those who are more ill – “and in general, just do a better job of treating pneumonia with this tool,” Dr. Balls said. He himself uses the decision support tool when attending to his own patients with pneumonia, as he feels that the tool really does make his care of these patients better. “There is a disparity around how we treat pneumonia in the U.S.
“Clinicians sometimes have a bias or a preference for certain antibiotics and we may not be appropriately treating these patients with broad-spectrum antibiotics or are perhaps using antibiotics that are not as effective based on an individual patient scenario so this is definitely a user-friendly tool that hopefully can be deployed throughout other health care systems to improve the treatment of pneumonia overall,” Dr. Balls emphasized.
A version of this article first appeared on Medscape.com.
FROM THE AMERICAN JOURNAL OF RESPIRATORY AND CRITICAL CARE MEDICINE
Legionnaires’ disease shows steady increase in U.S. over 15+ years
Legionnaires’ disease (LD) in the United States appears to be on an upswing that started in 2003, according to a study from the Centers for Disease Control and Prevention.
The reasons for this increased incidence are unclear, the researchers write in Emerging Infectious Diseases.
“The findings revealed a rising national trend in cases, widening racial disparities between Black or African American persons and White persons, and an increasing geographic focus in the Middle Atlantic, the East North Central, and New England,” lead author Albert E. Barskey, MPH, an epidemiologist in CDC’s Division of Bacterial Diseases, Atlanta, said in an email.
“Legionnaires’ disease cannot be diagnosed based on clinical features alone, and studies estimate that it is underdiagnosed, perhaps by 50%,” he added. “Our findings may serve to heighten clinicians’ awareness of this severe pneumonia’s etiology, so with an earlier correct diagnosis, appropriate treatment can be rendered sooner.”
Mr. Barskey and his coauthors at CDC – mathematical statistician Gordana Derado, PhD, and epidemiologist Chris Edens, PhD – used surveillance data to investigate the incidence of LD in the U.S. over time. They compared LD incidence in 2018 with average incidence between 1992 and 2002. The incidence data, from over 80,000 LD cases, were age-standardized using the 2005 U.S. standard population as the reference.
The researchers analyzed LD data reported to CDC by the 50 states, New York City, and Washington, D.C., through the National Notifiable Diseases Surveillance System. They performed regression analysis to identify the optimal year when population parameters changed, and for most analyses, they compared 1992-2002 data with 2003-2018 data.
Legionnaires’ disease up in various groups
- The overall age-standardized average incidence grew from 0.48 per 100,000 people during 1992-2002 to 2.71 per 100,000 in 2018 (incidence risk ratio, 5.67; 95% confidence interval, 5.52-5.83).
- LD incidence more than quintupled for people over 34 years of age, with the largest relative increase in those over 85 (RR, 6.50; 95% CI, 5.82-7.27).
- Incidence in men increased slightly more (RR, 5.86; 95% CI, 5.67-6.05) than in women (RR, 5.29; 95% CI, 5.06-5.53).
- Over the years, the racial disparity in incidence grew markedly. Incidence in Black persons increased from 0.47 to 5.21 per 100,000 (RR, 11.04; 95% CI, 10.39-11.73), compared with an increase from 0.37 to 1.99 per 100,000 in White persons (RR, 5.30; 95% CI, 5.12-5.49).
- The relative increase in incidence was highest in the Northeast (RR, 7.04; 95% CI, 6.70-7.40), followed by the Midwest (RR, 6.13; 95% CI, 5.85-6.42), the South (RR, 5.97; 95% CI, 5.67-6.29), and the West (RR, 3.39; 95% CI, 3.11-3.68).
Most LD cases occurred in summer or fall, and the seasonal pattern became more pronounced over time. The average of 57.8% of cases between June and November during 1992-2002 grew to 68.9% in 2003-2018.
Although the study “was hindered by incomplete race and ethnicity data,” Mr. Barskey said, “its breadth was a strength.”
Consider legionella in your diagnosis
In an interview, Paul G. Auwaerter, MD, a professor of medicine and the clinical director of the Division of Infectious Diseases at Johns Hopkins University School of Medicine, Baltimore, said he was not surprised by the results. “CDC has been reporting increased incidence of Legionnaires’ disease from water source outbreaks over the years. As a clinician, I very much depend on epidemiologic trends to help me understand the patient in front of me.
“The key point is that there’s more of it around, so consider it in your diagnosis,” he advised.
“Physicians are increasingly beginning to consider Legionella. Because LD is difficult to diagnose by traditional methods such as culture, they may use a PCR test,” said Dr. Auwaerter, who was not involved in the study. “Legionella needs antibiotics that differ a bit from traditional antibiotics used to treat bacterial pneumonia, so a correct diagnosis can inform a more directed therapy.”
“Why the incidence is increasing is the big question, and the authors nicely outline a litany of things,” he said.
The authors and Dr. Auwaerter proposed a number of possible contributing factors to the increased incidence:
- an aging population
- aging municipal and residential water sources that may harbor more organisms
- racial disparities and poverty
- underlying conditions, including diabetes, end-stage renal disease, and some cancers
- occupations in transportation, repair, cleaning services, and construction
- weather patterns
- improved surveillance and reporting
“Why Legionella appears in some locations more than others has not been explained,” Dr. Auwaerter added. “For example, Pittsburgh always seemed to have much more Legionella than Baltimore.”
Mr. Barskey and his team are planning further research into racial disparities and links between weather and climate and Legionnaires’ disease.
The authors are employees of CDC. Dr. Auwaerter has disclosed no relevant financial realtionships.
A version of this article first appeared on Medscape.com.
Legionnaires’ disease (LD) in the United States appears to be on an upswing that started in 2003, according to a study from the Centers for Disease Control and Prevention.
The reasons for this increased incidence are unclear, the researchers write in Emerging Infectious Diseases.
“The findings revealed a rising national trend in cases, widening racial disparities between Black or African American persons and White persons, and an increasing geographic focus in the Middle Atlantic, the East North Central, and New England,” lead author Albert E. Barskey, MPH, an epidemiologist in CDC’s Division of Bacterial Diseases, Atlanta, said in an email.
“Legionnaires’ disease cannot be diagnosed based on clinical features alone, and studies estimate that it is underdiagnosed, perhaps by 50%,” he added. “Our findings may serve to heighten clinicians’ awareness of this severe pneumonia’s etiology, so with an earlier correct diagnosis, appropriate treatment can be rendered sooner.”
Mr. Barskey and his coauthors at CDC – mathematical statistician Gordana Derado, PhD, and epidemiologist Chris Edens, PhD – used surveillance data to investigate the incidence of LD in the U.S. over time. They compared LD incidence in 2018 with average incidence between 1992 and 2002. The incidence data, from over 80,000 LD cases, were age-standardized using the 2005 U.S. standard population as the reference.
The researchers analyzed LD data reported to CDC by the 50 states, New York City, and Washington, D.C., through the National Notifiable Diseases Surveillance System. They performed regression analysis to identify the optimal year when population parameters changed, and for most analyses, they compared 1992-2002 data with 2003-2018 data.
Legionnaires’ disease up in various groups
- The overall age-standardized average incidence grew from 0.48 per 100,000 people during 1992-2002 to 2.71 per 100,000 in 2018 (incidence risk ratio, 5.67; 95% confidence interval, 5.52-5.83).
- LD incidence more than quintupled for people over 34 years of age, with the largest relative increase in those over 85 (RR, 6.50; 95% CI, 5.82-7.27).
- Incidence in men increased slightly more (RR, 5.86; 95% CI, 5.67-6.05) than in women (RR, 5.29; 95% CI, 5.06-5.53).
- Over the years, the racial disparity in incidence grew markedly. Incidence in Black persons increased from 0.47 to 5.21 per 100,000 (RR, 11.04; 95% CI, 10.39-11.73), compared with an increase from 0.37 to 1.99 per 100,000 in White persons (RR, 5.30; 95% CI, 5.12-5.49).
- The relative increase in incidence was highest in the Northeast (RR, 7.04; 95% CI, 6.70-7.40), followed by the Midwest (RR, 6.13; 95% CI, 5.85-6.42), the South (RR, 5.97; 95% CI, 5.67-6.29), and the West (RR, 3.39; 95% CI, 3.11-3.68).
Most LD cases occurred in summer or fall, and the seasonal pattern became more pronounced over time. The average of 57.8% of cases between June and November during 1992-2002 grew to 68.9% in 2003-2018.
Although the study “was hindered by incomplete race and ethnicity data,” Mr. Barskey said, “its breadth was a strength.”
Consider legionella in your diagnosis
In an interview, Paul G. Auwaerter, MD, a professor of medicine and the clinical director of the Division of Infectious Diseases at Johns Hopkins University School of Medicine, Baltimore, said he was not surprised by the results. “CDC has been reporting increased incidence of Legionnaires’ disease from water source outbreaks over the years. As a clinician, I very much depend on epidemiologic trends to help me understand the patient in front of me.
“The key point is that there’s more of it around, so consider it in your diagnosis,” he advised.
“Physicians are increasingly beginning to consider Legionella. Because LD is difficult to diagnose by traditional methods such as culture, they may use a PCR test,” said Dr. Auwaerter, who was not involved in the study. “Legionella needs antibiotics that differ a bit from traditional antibiotics used to treat bacterial pneumonia, so a correct diagnosis can inform a more directed therapy.”
“Why the incidence is increasing is the big question, and the authors nicely outline a litany of things,” he said.
The authors and Dr. Auwaerter proposed a number of possible contributing factors to the increased incidence:
- an aging population
- aging municipal and residential water sources that may harbor more organisms
- racial disparities and poverty
- underlying conditions, including diabetes, end-stage renal disease, and some cancers
- occupations in transportation, repair, cleaning services, and construction
- weather patterns
- improved surveillance and reporting
“Why Legionella appears in some locations more than others has not been explained,” Dr. Auwaerter added. “For example, Pittsburgh always seemed to have much more Legionella than Baltimore.”
Mr. Barskey and his team are planning further research into racial disparities and links between weather and climate and Legionnaires’ disease.
The authors are employees of CDC. Dr. Auwaerter has disclosed no relevant financial realtionships.
A version of this article first appeared on Medscape.com.
Legionnaires’ disease (LD) in the United States appears to be on an upswing that started in 2003, according to a study from the Centers for Disease Control and Prevention.
The reasons for this increased incidence are unclear, the researchers write in Emerging Infectious Diseases.
“The findings revealed a rising national trend in cases, widening racial disparities between Black or African American persons and White persons, and an increasing geographic focus in the Middle Atlantic, the East North Central, and New England,” lead author Albert E. Barskey, MPH, an epidemiologist in CDC’s Division of Bacterial Diseases, Atlanta, said in an email.
“Legionnaires’ disease cannot be diagnosed based on clinical features alone, and studies estimate that it is underdiagnosed, perhaps by 50%,” he added. “Our findings may serve to heighten clinicians’ awareness of this severe pneumonia’s etiology, so with an earlier correct diagnosis, appropriate treatment can be rendered sooner.”
Mr. Barskey and his coauthors at CDC – mathematical statistician Gordana Derado, PhD, and epidemiologist Chris Edens, PhD – used surveillance data to investigate the incidence of LD in the U.S. over time. They compared LD incidence in 2018 with average incidence between 1992 and 2002. The incidence data, from over 80,000 LD cases, were age-standardized using the 2005 U.S. standard population as the reference.
The researchers analyzed LD data reported to CDC by the 50 states, New York City, and Washington, D.C., through the National Notifiable Diseases Surveillance System. They performed regression analysis to identify the optimal year when population parameters changed, and for most analyses, they compared 1992-2002 data with 2003-2018 data.
Legionnaires’ disease up in various groups
- The overall age-standardized average incidence grew from 0.48 per 100,000 people during 1992-2002 to 2.71 per 100,000 in 2018 (incidence risk ratio, 5.67; 95% confidence interval, 5.52-5.83).
- LD incidence more than quintupled for people over 34 years of age, with the largest relative increase in those over 85 (RR, 6.50; 95% CI, 5.82-7.27).
- Incidence in men increased slightly more (RR, 5.86; 95% CI, 5.67-6.05) than in women (RR, 5.29; 95% CI, 5.06-5.53).
- Over the years, the racial disparity in incidence grew markedly. Incidence in Black persons increased from 0.47 to 5.21 per 100,000 (RR, 11.04; 95% CI, 10.39-11.73), compared with an increase from 0.37 to 1.99 per 100,000 in White persons (RR, 5.30; 95% CI, 5.12-5.49).
- The relative increase in incidence was highest in the Northeast (RR, 7.04; 95% CI, 6.70-7.40), followed by the Midwest (RR, 6.13; 95% CI, 5.85-6.42), the South (RR, 5.97; 95% CI, 5.67-6.29), and the West (RR, 3.39; 95% CI, 3.11-3.68).
Most LD cases occurred in summer or fall, and the seasonal pattern became more pronounced over time. The average of 57.8% of cases between June and November during 1992-2002 grew to 68.9% in 2003-2018.
Although the study “was hindered by incomplete race and ethnicity data,” Mr. Barskey said, “its breadth was a strength.”
Consider legionella in your diagnosis
In an interview, Paul G. Auwaerter, MD, a professor of medicine and the clinical director of the Division of Infectious Diseases at Johns Hopkins University School of Medicine, Baltimore, said he was not surprised by the results. “CDC has been reporting increased incidence of Legionnaires’ disease from water source outbreaks over the years. As a clinician, I very much depend on epidemiologic trends to help me understand the patient in front of me.
“The key point is that there’s more of it around, so consider it in your diagnosis,” he advised.
“Physicians are increasingly beginning to consider Legionella. Because LD is difficult to diagnose by traditional methods such as culture, they may use a PCR test,” said Dr. Auwaerter, who was not involved in the study. “Legionella needs antibiotics that differ a bit from traditional antibiotics used to treat bacterial pneumonia, so a correct diagnosis can inform a more directed therapy.”
“Why the incidence is increasing is the big question, and the authors nicely outline a litany of things,” he said.
The authors and Dr. Auwaerter proposed a number of possible contributing factors to the increased incidence:
- an aging population
- aging municipal and residential water sources that may harbor more organisms
- racial disparities and poverty
- underlying conditions, including diabetes, end-stage renal disease, and some cancers
- occupations in transportation, repair, cleaning services, and construction
- weather patterns
- improved surveillance and reporting
“Why Legionella appears in some locations more than others has not been explained,” Dr. Auwaerter added. “For example, Pittsburgh always seemed to have much more Legionella than Baltimore.”
Mr. Barskey and his team are planning further research into racial disparities and links between weather and climate and Legionnaires’ disease.
The authors are employees of CDC. Dr. Auwaerter has disclosed no relevant financial realtionships.
A version of this article first appeared on Medscape.com.
Clinical Edge Journal Scan Commentary: CAP January 2022
The practitioner’s primary responsibility in managing respiratory infection is to determine which patients with community acquired pneumonia (CAP) warrant hospitalization and more aggressive management. Standard practice for mild infection dictates empiric administration of 5 days of antimicrobial agents1 particularly for the youngest, oldest and patients with underlying comorbid conditions, usually on an outpatient basis with careful follow-up.
Variables for hospitalization and for the selection of antibiotic therapy include the likelihood of a bacterial etiology, epidemiologic considerations, clinical symptoms, predisposing host factors, age, and radiographic findings. Patients who have viral processes generally have low-grade fever and are usually uncomfortable, although they may not appear toxic. However, it is not possible to distinguish between viral and bacterial pneumonia on clinical grounds alone, particularly with the emergence of COVID-19 (SARS-CoV-2), as rapid progression of an initially mild viral upper respiratory infection may become life threatening, particularly in patients with comorbid conditions, such as pulmonary disease, diabetes, and immunodeficiency. All patients with severe CAP should be tested for this viral agent. A recent study of 96 patients with chronic obstructive pulmonary disease (COPD) and COVID-19 infection showed they had higher intensive care unit (ICU) admissions, ventilation requirements, cardiovascular events, and mortality as compared to 1,129 hospitalized patients with COPD and non-COVID-19 infection.2 In addition to COVID-19, type 2 diabetes mellitus must also be considered, as such patients developing severe CAP from all causes have a higher mortality and poorer clinical outcomes than those without diabetes.3
Predicting which patients are likely to progress to severe disease from CAP is a challenging task. In the past clinicians relied on clinical assessment, along with some inflammatory lab studies such as the complete blood count (CBC), erythrocyte sedimentation rate (ESR), C-reactive protein (CRP), and procalcitonin. Newer tests have recently been studied for their ability to inform prognosis and likely mortality. A very simple test is the admission blood glucose level, which, if increased at hospital admission, is associated with a higher mortality.4 More recently the quick sequential organ failure assessment (qSOFA) score was shown to be better than the Infectious Diseases Society of America/American Thoracic Society (IDSA/ATS) minor criteria score in predicting mortality.5 A potential new laboratory test, serum IL-17 concentrations, demonstrated a positive correlation with CAP severity, ICU admission, longer hospital stay, mechanical ventilation, and mortality.6
Current studies have identified some potential advantages of newer antibiotics. Cefoperazone-sulbactam was shown to be equivalent to piperacillin-tazobactam (PIP-TAZO) for treating severe CAP in elderly patients, thereby avoiding the potential acute kidney injury (AKI) of the PIP-TAZO – vancomycin combination.7 This benefit of reduced AKI was supported by a retrospective cohort study that included 449,535 hospitalized adult patients with CAP.8
In a phase 3 study, lefamulin was as effective as moxifloxacin in treating bacterial CAP, including drug-resistant strains and typical, atypical, and polymicrobial infections.9 Of 1,289 study patients, a pathogen was identified in 709. Side effects were minor. This antibiotic has a unique mechanism of action through inhibition of protein synthesis, preventing the binding of transfer RNA.
Adjunctive oral dexamethasone therapy for CAP has been advocated by some experts. In a study of 354 non-ICU patients, a shorter hospital stay was seen in patients who received dexamethasone vs. placebo (4 vs. 6 days) but only in patients who had elevated neutrophil or WBC counts, or high neutrophil-lymphocyte ratios; otherwise there was no difference.10
References
- Vaughn VM et al. a statewide collaborative quality initiative to improve antibiotic duration and outcomes in patients hospitalized with uncomplicated community-acquired pneumonia. Clin Infect Dis. 2021(Nov 13):ciab950 (Nov 13).
- Sheikh D et al. Clinical outcomes in patients with COPD hospitalized with SARS-CoV-2 versus non-SARS-CoV-2 community-acquired pneumonia. Respir Med. 2021(Dec 8);191:106714.
- Huang D et al. Clinical characteristics and risk factors associated with mortality in patients with severe community-acquired pneumonia and type 2 diabetes mellitus. Crit Care. 2021(Dec 7);25:419.
- Shen Y et al. Association of admission blood glucose level with all-cause mortality according to age in patients with community acquired pneumonia. Int J Gen Med. 2021(Nov 6);14:7775-7781.
- Guo Q et al. qSOFA predicted pneumonia mortality better than minor criteria and worse than CURB-65 with robust elements and higher convergence. Am J Emerg Med. 2022(Feb);52:1-7.
- Feng CM et al. Serum interleukin-17 predicts severity and prognosis in patients with community acquired pneumonia: a prospective cohort study. BMC Pulm Med. 2021(Dec 2);21:393.
- Huang CT et al. Clinical effectiveness of cefoperazone-sulbactam versus piperacillin-tazobactam for the treatment of pneumonia in the elderly population. Int J Antimicrob Agents. 2021(Dec 4);106491.
- Le P et al. Association of antibiotic use and acute kidney injury in patients hospitalized with community-acquired pneumonia. Curr Med Res Opin. 2021 (Nov 15).
- Paukner S et al. Pooled microbiological findings and efficacy outcomes by pathogen in adults with community-acquired bacterial pneumonia from the Lefamulin Evaluation Against Pneumonia (LEAP) 1 and LEAP 2 phase 3 trials of lefamulin versus moxifloxacin. J Glob Antimicrob Resist. 2021 (Nov 14).
- Wittermans E et al. Neutrophil count, lymphocyte count and neutrophil-to-lymphocyte ratio in relation to response to adjunctive dexamethasone treatment in community-acquired pneumonia. Eur J Intern Med. 2021 (Nov 12).
The practitioner’s primary responsibility in managing respiratory infection is to determine which patients with community acquired pneumonia (CAP) warrant hospitalization and more aggressive management. Standard practice for mild infection dictates empiric administration of 5 days of antimicrobial agents1 particularly for the youngest, oldest and patients with underlying comorbid conditions, usually on an outpatient basis with careful follow-up.
Variables for hospitalization and for the selection of antibiotic therapy include the likelihood of a bacterial etiology, epidemiologic considerations, clinical symptoms, predisposing host factors, age, and radiographic findings. Patients who have viral processes generally have low-grade fever and are usually uncomfortable, although they may not appear toxic. However, it is not possible to distinguish between viral and bacterial pneumonia on clinical grounds alone, particularly with the emergence of COVID-19 (SARS-CoV-2), as rapid progression of an initially mild viral upper respiratory infection may become life threatening, particularly in patients with comorbid conditions, such as pulmonary disease, diabetes, and immunodeficiency. All patients with severe CAP should be tested for this viral agent. A recent study of 96 patients with chronic obstructive pulmonary disease (COPD) and COVID-19 infection showed they had higher intensive care unit (ICU) admissions, ventilation requirements, cardiovascular events, and mortality as compared to 1,129 hospitalized patients with COPD and non-COVID-19 infection.2 In addition to COVID-19, type 2 diabetes mellitus must also be considered, as such patients developing severe CAP from all causes have a higher mortality and poorer clinical outcomes than those without diabetes.3
Predicting which patients are likely to progress to severe disease from CAP is a challenging task. In the past clinicians relied on clinical assessment, along with some inflammatory lab studies such as the complete blood count (CBC), erythrocyte sedimentation rate (ESR), C-reactive protein (CRP), and procalcitonin. Newer tests have recently been studied for their ability to inform prognosis and likely mortality. A very simple test is the admission blood glucose level, which, if increased at hospital admission, is associated with a higher mortality.4 More recently the quick sequential organ failure assessment (qSOFA) score was shown to be better than the Infectious Diseases Society of America/American Thoracic Society (IDSA/ATS) minor criteria score in predicting mortality.5 A potential new laboratory test, serum IL-17 concentrations, demonstrated a positive correlation with CAP severity, ICU admission, longer hospital stay, mechanical ventilation, and mortality.6
Current studies have identified some potential advantages of newer antibiotics. Cefoperazone-sulbactam was shown to be equivalent to piperacillin-tazobactam (PIP-TAZO) for treating severe CAP in elderly patients, thereby avoiding the potential acute kidney injury (AKI) of the PIP-TAZO – vancomycin combination.7 This benefit of reduced AKI was supported by a retrospective cohort study that included 449,535 hospitalized adult patients with CAP.8
In a phase 3 study, lefamulin was as effective as moxifloxacin in treating bacterial CAP, including drug-resistant strains and typical, atypical, and polymicrobial infections.9 Of 1,289 study patients, a pathogen was identified in 709. Side effects were minor. This antibiotic has a unique mechanism of action through inhibition of protein synthesis, preventing the binding of transfer RNA.
Adjunctive oral dexamethasone therapy for CAP has been advocated by some experts. In a study of 354 non-ICU patients, a shorter hospital stay was seen in patients who received dexamethasone vs. placebo (4 vs. 6 days) but only in patients who had elevated neutrophil or WBC counts, or high neutrophil-lymphocyte ratios; otherwise there was no difference.10
References
- Vaughn VM et al. a statewide collaborative quality initiative to improve antibiotic duration and outcomes in patients hospitalized with uncomplicated community-acquired pneumonia. Clin Infect Dis. 2021(Nov 13):ciab950 (Nov 13).
- Sheikh D et al. Clinical outcomes in patients with COPD hospitalized with SARS-CoV-2 versus non-SARS-CoV-2 community-acquired pneumonia. Respir Med. 2021(Dec 8);191:106714.
- Huang D et al. Clinical characteristics and risk factors associated with mortality in patients with severe community-acquired pneumonia and type 2 diabetes mellitus. Crit Care. 2021(Dec 7);25:419.
- Shen Y et al. Association of admission blood glucose level with all-cause mortality according to age in patients with community acquired pneumonia. Int J Gen Med. 2021(Nov 6);14:7775-7781.
- Guo Q et al. qSOFA predicted pneumonia mortality better than minor criteria and worse than CURB-65 with robust elements and higher convergence. Am J Emerg Med. 2022(Feb);52:1-7.
- Feng CM et al. Serum interleukin-17 predicts severity and prognosis in patients with community acquired pneumonia: a prospective cohort study. BMC Pulm Med. 2021(Dec 2);21:393.
- Huang CT et al. Clinical effectiveness of cefoperazone-sulbactam versus piperacillin-tazobactam for the treatment of pneumonia in the elderly population. Int J Antimicrob Agents. 2021(Dec 4);106491.
- Le P et al. Association of antibiotic use and acute kidney injury in patients hospitalized with community-acquired pneumonia. Curr Med Res Opin. 2021 (Nov 15).
- Paukner S et al. Pooled microbiological findings and efficacy outcomes by pathogen in adults with community-acquired bacterial pneumonia from the Lefamulin Evaluation Against Pneumonia (LEAP) 1 and LEAP 2 phase 3 trials of lefamulin versus moxifloxacin. J Glob Antimicrob Resist. 2021 (Nov 14).
- Wittermans E et al. Neutrophil count, lymphocyte count and neutrophil-to-lymphocyte ratio in relation to response to adjunctive dexamethasone treatment in community-acquired pneumonia. Eur J Intern Med. 2021 (Nov 12).
The practitioner’s primary responsibility in managing respiratory infection is to determine which patients with community acquired pneumonia (CAP) warrant hospitalization and more aggressive management. Standard practice for mild infection dictates empiric administration of 5 days of antimicrobial agents1 particularly for the youngest, oldest and patients with underlying comorbid conditions, usually on an outpatient basis with careful follow-up.
Variables for hospitalization and for the selection of antibiotic therapy include the likelihood of a bacterial etiology, epidemiologic considerations, clinical symptoms, predisposing host factors, age, and radiographic findings. Patients who have viral processes generally have low-grade fever and are usually uncomfortable, although they may not appear toxic. However, it is not possible to distinguish between viral and bacterial pneumonia on clinical grounds alone, particularly with the emergence of COVID-19 (SARS-CoV-2), as rapid progression of an initially mild viral upper respiratory infection may become life threatening, particularly in patients with comorbid conditions, such as pulmonary disease, diabetes, and immunodeficiency. All patients with severe CAP should be tested for this viral agent. A recent study of 96 patients with chronic obstructive pulmonary disease (COPD) and COVID-19 infection showed they had higher intensive care unit (ICU) admissions, ventilation requirements, cardiovascular events, and mortality as compared to 1,129 hospitalized patients with COPD and non-COVID-19 infection.2 In addition to COVID-19, type 2 diabetes mellitus must also be considered, as such patients developing severe CAP from all causes have a higher mortality and poorer clinical outcomes than those without diabetes.3
Predicting which patients are likely to progress to severe disease from CAP is a challenging task. In the past clinicians relied on clinical assessment, along with some inflammatory lab studies such as the complete blood count (CBC), erythrocyte sedimentation rate (ESR), C-reactive protein (CRP), and procalcitonin. Newer tests have recently been studied for their ability to inform prognosis and likely mortality. A very simple test is the admission blood glucose level, which, if increased at hospital admission, is associated with a higher mortality.4 More recently the quick sequential organ failure assessment (qSOFA) score was shown to be better than the Infectious Diseases Society of America/American Thoracic Society (IDSA/ATS) minor criteria score in predicting mortality.5 A potential new laboratory test, serum IL-17 concentrations, demonstrated a positive correlation with CAP severity, ICU admission, longer hospital stay, mechanical ventilation, and mortality.6
Current studies have identified some potential advantages of newer antibiotics. Cefoperazone-sulbactam was shown to be equivalent to piperacillin-tazobactam (PIP-TAZO) for treating severe CAP in elderly patients, thereby avoiding the potential acute kidney injury (AKI) of the PIP-TAZO – vancomycin combination.7 This benefit of reduced AKI was supported by a retrospective cohort study that included 449,535 hospitalized adult patients with CAP.8
In a phase 3 study, lefamulin was as effective as moxifloxacin in treating bacterial CAP, including drug-resistant strains and typical, atypical, and polymicrobial infections.9 Of 1,289 study patients, a pathogen was identified in 709. Side effects were minor. This antibiotic has a unique mechanism of action through inhibition of protein synthesis, preventing the binding of transfer RNA.
Adjunctive oral dexamethasone therapy for CAP has been advocated by some experts. In a study of 354 non-ICU patients, a shorter hospital stay was seen in patients who received dexamethasone vs. placebo (4 vs. 6 days) but only in patients who had elevated neutrophil or WBC counts, or high neutrophil-lymphocyte ratios; otherwise there was no difference.10
References
- Vaughn VM et al. a statewide collaborative quality initiative to improve antibiotic duration and outcomes in patients hospitalized with uncomplicated community-acquired pneumonia. Clin Infect Dis. 2021(Nov 13):ciab950 (Nov 13).
- Sheikh D et al. Clinical outcomes in patients with COPD hospitalized with SARS-CoV-2 versus non-SARS-CoV-2 community-acquired pneumonia. Respir Med. 2021(Dec 8);191:106714.
- Huang D et al. Clinical characteristics and risk factors associated with mortality in patients with severe community-acquired pneumonia and type 2 diabetes mellitus. Crit Care. 2021(Dec 7);25:419.
- Shen Y et al. Association of admission blood glucose level with all-cause mortality according to age in patients with community acquired pneumonia. Int J Gen Med. 2021(Nov 6);14:7775-7781.
- Guo Q et al. qSOFA predicted pneumonia mortality better than minor criteria and worse than CURB-65 with robust elements and higher convergence. Am J Emerg Med. 2022(Feb);52:1-7.
- Feng CM et al. Serum interleukin-17 predicts severity and prognosis in patients with community acquired pneumonia: a prospective cohort study. BMC Pulm Med. 2021(Dec 2);21:393.
- Huang CT et al. Clinical effectiveness of cefoperazone-sulbactam versus piperacillin-tazobactam for the treatment of pneumonia in the elderly population. Int J Antimicrob Agents. 2021(Dec 4);106491.
- Le P et al. Association of antibiotic use and acute kidney injury in patients hospitalized with community-acquired pneumonia. Curr Med Res Opin. 2021 (Nov 15).
- Paukner S et al. Pooled microbiological findings and efficacy outcomes by pathogen in adults with community-acquired bacterial pneumonia from the Lefamulin Evaluation Against Pneumonia (LEAP) 1 and LEAP 2 phase 3 trials of lefamulin versus moxifloxacin. J Glob Antimicrob Resist. 2021 (Nov 14).
- Wittermans E et al. Neutrophil count, lymphocyte count and neutrophil-to-lymphocyte ratio in relation to response to adjunctive dexamethasone treatment in community-acquired pneumonia. Eur J Intern Med. 2021 (Nov 12).
Not all patients with community-acquired pneumonia respond to adjunctive dexamethasone
Key clinical point: Neutrophil count, white blood cell (WBC) count, or neutrophil-lymphocyte ratio (NLR) may help in ascertaining which patients hospitalized for community-acquired pneumonia (CAP) would benefit from adjunctive oral dexamethasone therapy.
Main finding: Among patients with a high neutrophil count, WBC count, or NLR who did not die in hospital, those who received dexamethasone had a shorter median length of stay (LOS) than those who received placebo (4 days vs. 6 days), whereas patients with low values for these parameters showed no difference in LOS between those receiving placebo and dexamethasone (P < .05).
Study details: Findings are from a post hoc analysis on 354 out of the 401 non-ICU patients with CAP included in the randomized placebo-controlled Santeon-CAP trial who were randomly assigned to receive either placebo or oral dexamethasone.
Disclosures: The study was sponsored by St. Antonius research fund. The authors declared no conflicts of interests.
Source: Wittermans E et al. Eur J Intern Med. 2021 (Nov 12). Doi: 10.1016/j.ejim.2021.10.030.
Key clinical point: Neutrophil count, white blood cell (WBC) count, or neutrophil-lymphocyte ratio (NLR) may help in ascertaining which patients hospitalized for community-acquired pneumonia (CAP) would benefit from adjunctive oral dexamethasone therapy.
Main finding: Among patients with a high neutrophil count, WBC count, or NLR who did not die in hospital, those who received dexamethasone had a shorter median length of stay (LOS) than those who received placebo (4 days vs. 6 days), whereas patients with low values for these parameters showed no difference in LOS between those receiving placebo and dexamethasone (P < .05).
Study details: Findings are from a post hoc analysis on 354 out of the 401 non-ICU patients with CAP included in the randomized placebo-controlled Santeon-CAP trial who were randomly assigned to receive either placebo or oral dexamethasone.
Disclosures: The study was sponsored by St. Antonius research fund. The authors declared no conflicts of interests.
Source: Wittermans E et al. Eur J Intern Med. 2021 (Nov 12). Doi: 10.1016/j.ejim.2021.10.030.
Key clinical point: Neutrophil count, white blood cell (WBC) count, or neutrophil-lymphocyte ratio (NLR) may help in ascertaining which patients hospitalized for community-acquired pneumonia (CAP) would benefit from adjunctive oral dexamethasone therapy.
Main finding: Among patients with a high neutrophil count, WBC count, or NLR who did not die in hospital, those who received dexamethasone had a shorter median length of stay (LOS) than those who received placebo (4 days vs. 6 days), whereas patients with low values for these parameters showed no difference in LOS between those receiving placebo and dexamethasone (P < .05).
Study details: Findings are from a post hoc analysis on 354 out of the 401 non-ICU patients with CAP included in the randomized placebo-controlled Santeon-CAP trial who were randomly assigned to receive either placebo or oral dexamethasone.
Disclosures: The study was sponsored by St. Antonius research fund. The authors declared no conflicts of interests.
Source: Wittermans E et al. Eur J Intern Med. 2021 (Nov 12). Doi: 10.1016/j.ejim.2021.10.030.