User login
-
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'main-prefix')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
div[contains(@class, 'view-medstat-quiz-listing-panes')]
div[contains(@class, 'pane-article-sidebar-latest-news')]


Stroke Risk from Atrial Fibrillation Rises in Presence of Rheumatoid Arthritis
TOPLINE:
Patients with both rheumatoid arthritis (RA) and atrial fibrillation (AF) have a higher risk for ischemic stroke than those with only AF. They are also less likely to receive oral anticoagulant treatment, which may contribute to this increased stroke risk.
METHODOLOGY:
- Researchers conducted a registry-based retrospective cohort study using the Norwegian Cardio-Rheuma Register to evaluate the risk for ischemic stroke following the diagnosis of AF in patients with or without RA.
- They included 163,595 patients with newly diagnosed AF between 2010 and 2017, of whom 2750 had RA. Patients had to be diagnosed with RA before the diagnosis of AF.
- They also assessed whether patients with RA were less likely to receive oral anticoagulants for stroke prevention within 3 months of AF diagnosis than those without RA.
- The median follow-up time was 2.5 years for patients with RA and 3.0 years for those without RA.
- The primary endpoint was ischemic stroke, which was identified through hospital admissions and visits.
TAKEAWAY:
- At 5 years, patients with both RA and AF showed a higher cumulative incidence of ischemic stroke than those with only AF (7.3% vs 5.0%).
- Among patients with AF, the risk of having a stroke was 25% higher in those with RA than in those without RA (adjusted hazard ratio, 1.25; 95% CI, 1.05-1.50).
- Patients with RA were also less likely to receive treatment with oral anticoagulants than those without RA, driven by concerns over potential interactions with RA medications, bleeding risk, or other factors (adjusted odds ratio, 0.88; 95% CI, 0.80-0.97).
IN PRACTICE:
“Our study prompts preventive measures such as meticulous cardiovascular risk factor control among patients with RA and AF and raises the question whether the presence of RA should be taken into account when considering OAC [oral anticoagulant] treatment for AF patients,” the authors wrote.
SOURCE:
This study was led by Anne M. Kerola, MD, PhD, Helsinki University Hospital and University of Helsinki in Finland. It was published online in Rheumatology.
LIMITATIONS:
This study lacked data on smoking, blood pressure measurements, alcohol use, and obesity, which may have affected the comprehensiveness of the findings. The study population was limited to Norway and may not be generalizable to other populations.
DISCLOSURES:
This study was supported by the Olav Thon Foundation, the Research Council of Norway, and the Foundation for Research in Rheumatology. Some authors received speaker fees, participated in advisory boards, served as consultants, or had other ties with some pharmaceutical companies and institutions.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
Patients with both rheumatoid arthritis (RA) and atrial fibrillation (AF) have a higher risk for ischemic stroke than those with only AF. They are also less likely to receive oral anticoagulant treatment, which may contribute to this increased stroke risk.
METHODOLOGY:
- Researchers conducted a registry-based retrospective cohort study using the Norwegian Cardio-Rheuma Register to evaluate the risk for ischemic stroke following the diagnosis of AF in patients with or without RA.
- They included 163,595 patients with newly diagnosed AF between 2010 and 2017, of whom 2750 had RA. Patients had to be diagnosed with RA before the diagnosis of AF.
- They also assessed whether patients with RA were less likely to receive oral anticoagulants for stroke prevention within 3 months of AF diagnosis than those without RA.
- The median follow-up time was 2.5 years for patients with RA and 3.0 years for those without RA.
- The primary endpoint was ischemic stroke, which was identified through hospital admissions and visits.
TAKEAWAY:
- At 5 years, patients with both RA and AF showed a higher cumulative incidence of ischemic stroke than those with only AF (7.3% vs 5.0%).
- Among patients with AF, the risk of having a stroke was 25% higher in those with RA than in those without RA (adjusted hazard ratio, 1.25; 95% CI, 1.05-1.50).
- Patients with RA were also less likely to receive treatment with oral anticoagulants than those without RA, driven by concerns over potential interactions with RA medications, bleeding risk, or other factors (adjusted odds ratio, 0.88; 95% CI, 0.80-0.97).
IN PRACTICE:
“Our study prompts preventive measures such as meticulous cardiovascular risk factor control among patients with RA and AF and raises the question whether the presence of RA should be taken into account when considering OAC [oral anticoagulant] treatment for AF patients,” the authors wrote.
SOURCE:
This study was led by Anne M. Kerola, MD, PhD, Helsinki University Hospital and University of Helsinki in Finland. It was published online in Rheumatology.
LIMITATIONS:
This study lacked data on smoking, blood pressure measurements, alcohol use, and obesity, which may have affected the comprehensiveness of the findings. The study population was limited to Norway and may not be generalizable to other populations.
DISCLOSURES:
This study was supported by the Olav Thon Foundation, the Research Council of Norway, and the Foundation for Research in Rheumatology. Some authors received speaker fees, participated in advisory boards, served as consultants, or had other ties with some pharmaceutical companies and institutions.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
Patients with both rheumatoid arthritis (RA) and atrial fibrillation (AF) have a higher risk for ischemic stroke than those with only AF. They are also less likely to receive oral anticoagulant treatment, which may contribute to this increased stroke risk.
METHODOLOGY:
- Researchers conducted a registry-based retrospective cohort study using the Norwegian Cardio-Rheuma Register to evaluate the risk for ischemic stroke following the diagnosis of AF in patients with or without RA.
- They included 163,595 patients with newly diagnosed AF between 2010 and 2017, of whom 2750 had RA. Patients had to be diagnosed with RA before the diagnosis of AF.
- They also assessed whether patients with RA were less likely to receive oral anticoagulants for stroke prevention within 3 months of AF diagnosis than those without RA.
- The median follow-up time was 2.5 years for patients with RA and 3.0 years for those without RA.
- The primary endpoint was ischemic stroke, which was identified through hospital admissions and visits.
TAKEAWAY:
- At 5 years, patients with both RA and AF showed a higher cumulative incidence of ischemic stroke than those with only AF (7.3% vs 5.0%).
- Among patients with AF, the risk of having a stroke was 25% higher in those with RA than in those without RA (adjusted hazard ratio, 1.25; 95% CI, 1.05-1.50).
- Patients with RA were also less likely to receive treatment with oral anticoagulants than those without RA, driven by concerns over potential interactions with RA medications, bleeding risk, or other factors (adjusted odds ratio, 0.88; 95% CI, 0.80-0.97).
IN PRACTICE:
“Our study prompts preventive measures such as meticulous cardiovascular risk factor control among patients with RA and AF and raises the question whether the presence of RA should be taken into account when considering OAC [oral anticoagulant] treatment for AF patients,” the authors wrote.
SOURCE:
This study was led by Anne M. Kerola, MD, PhD, Helsinki University Hospital and University of Helsinki in Finland. It was published online in Rheumatology.
LIMITATIONS:
This study lacked data on smoking, blood pressure measurements, alcohol use, and obesity, which may have affected the comprehensiveness of the findings. The study population was limited to Norway and may not be generalizable to other populations.
DISCLOSURES:
This study was supported by the Olav Thon Foundation, the Research Council of Norway, and the Foundation for Research in Rheumatology. Some authors received speaker fees, participated in advisory boards, served as consultants, or had other ties with some pharmaceutical companies and institutions.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
As Interest From Families Wanes, Pediatricians Scale Back on COVID Shots
When pediatrician Eric Ball, MD, opened a refrigerator full of childhood vaccines, all the expected shots were there — DTaP, polio, pneumococcal vaccine — except one.
“This is where we usually store our COVID vaccines, but we don’t have any right now because they all expired at the end of last year and we had to dispose of them,” said Dr. Ball, who is part of a pediatric practice in Orange County, California.
“We thought demand would be way higher than it was.”
Providers like Dr. Ball don’t want to waste money ordering doses that won’t be used, but they need enough on hand to vaccinate vulnerable children.
The Centers for Disease Control and Prevention recommends that anyone 6 months or older get the updated COVID vaccination, but in the 2023-24 vaccination season only about 15% of eligible children in the United States got a shot.
Dr. Ball said it was difficult to let vaccines go to waste in 2023. It was the first time the federal government was no longer picking up the tab for the shots, and providers had to pay upfront for the vaccines. Parents would often skip the COVID shot, which can have a very short shelf life, compared with other vaccines.
“Watching it sitting on our shelves expiring every 30 days, that’s like throwing away $150 repeatedly every day, multiple times a month,” Dr. Ball said.
in 2024, Dr. Ball slashed his fall vaccine order to the bare minimum to avoid another costly mistake.
“We took the number of flu vaccines that we order, and then we ordered 5% of that in COVID vaccines,” Dr. Ball said. “It’s a guess.”
That small vaccine order cost more than $63,000, he said.
Pharmacists, pharmacy interns, and techs are allowed to give COVID vaccines only to children age 3 and up, meaning babies and toddlers would need to visit a doctor’s office for inoculation.
It’s difficult to predict how parents will feel about the shots this fall, said Chicago pediatrician Scott Goldstein, MD. Unlike other vaccinations, COVID shots aren’t required for kids to attend school, and parental interest seems to wane with each new formulation. For a physician-owned practice such as Dr. Goldstein’s, the upfront cost of the vaccine can be a gamble.
“The cost of vaccines, that’s far and away our biggest expense. But it’s also the most important thing we do, you could argue, is vaccinating kids,” Dr. Goldstein said.
Insurance doesn’t necessarily cover vaccine storage accidents, which can put the practice at risk of financial ruin.
“We’ve had things happen like a refrigerator gets unplugged. And then we’re all of a sudden out $80,000 overnight,” Dr. Goldstein said.
South Carolina pediatrician Deborah Greenhouse, MD, said she would order more COVID vaccines for older children if the pharmaceutical companies that she buys from had a more forgiving return policy.
“Pfizer is creating that situation. If you’re only going to let us return 30%, we’re not going to buy much,” she said. “We can’t.”
Greenhouse owns her practice, so the remaining 70% of leftover shots would come out of her pocket.
Vaccine maker Pfizer will take back all unused COVID shots for young children, but only 30% of doses for people 12 and older.
Pfizer said in an Aug. 20 emailed statement, “The return policy was instituted as we recognize both the importance and the complexity of pediatric vaccination and wanted to ensure that pediatric offices did not have hurdles to providing vaccine to their young patients.”
Pfizer’s return policy is similar to policies from other drugmakers for pediatric flu vaccines, also recommended during the fall season. Physicians who are worried about unwanted COVID vaccines expiring on the shelves said flu shots cost them about $20 per dose, while COVID shots cost around $150 per dose.
“We run on a very thin margin. If we get stuck holding a ton of vaccine that we cannot return, we can’t absorb that kind of cost,” Dr. Greenhouse said.
Vaccine maker Moderna will accept COVID vaccine returns, but the amount depends on the individual contract with a provider. Novavax will accept the return of only unopened vaccines and doesn’t specify the amount they’ll accept.
Dr. Greenhouse wants to vaccinate as many children as possible but said she can’t afford to stock shots with a short shelf life. Once she runs out of the doses she’s ordered, Dr. Greenhouse plans to tell families to go to a pharmacy to get older children vaccinated. If pediatricians around the country are making the same calculations, doses for very small children could be harder to find at doctors’ offices.
“Frankly, it’s not an ideal situation, but it’s what we have to do to stay in business,” she said.
Dr. Ball worries that parents’ limited interest has caused pediatricians to minimize their vaccine orders, in turn making the newest COVID shots difficult to find once they become available.
“I think there’s just a misperception that it’s less of a big deal to get COVID, but I’m still sending babies to the hospital with COVID,” Dr. Ball said. “We’re still seeing kids with long COVID. This is with us forever.”
KFF Health News is a national newsroom that produces in-depth journalism about health issues and is one of the core operating programs at KFF — the independent source for health policy research, polling, and journalism.
When pediatrician Eric Ball, MD, opened a refrigerator full of childhood vaccines, all the expected shots were there — DTaP, polio, pneumococcal vaccine — except one.
“This is where we usually store our COVID vaccines, but we don’t have any right now because they all expired at the end of last year and we had to dispose of them,” said Dr. Ball, who is part of a pediatric practice in Orange County, California.
“We thought demand would be way higher than it was.”
Providers like Dr. Ball don’t want to waste money ordering doses that won’t be used, but they need enough on hand to vaccinate vulnerable children.
The Centers for Disease Control and Prevention recommends that anyone 6 months or older get the updated COVID vaccination, but in the 2023-24 vaccination season only about 15% of eligible children in the United States got a shot.
Dr. Ball said it was difficult to let vaccines go to waste in 2023. It was the first time the federal government was no longer picking up the tab for the shots, and providers had to pay upfront for the vaccines. Parents would often skip the COVID shot, which can have a very short shelf life, compared with other vaccines.
“Watching it sitting on our shelves expiring every 30 days, that’s like throwing away $150 repeatedly every day, multiple times a month,” Dr. Ball said.
in 2024, Dr. Ball slashed his fall vaccine order to the bare minimum to avoid another costly mistake.
“We took the number of flu vaccines that we order, and then we ordered 5% of that in COVID vaccines,” Dr. Ball said. “It’s a guess.”
That small vaccine order cost more than $63,000, he said.
Pharmacists, pharmacy interns, and techs are allowed to give COVID vaccines only to children age 3 and up, meaning babies and toddlers would need to visit a doctor’s office for inoculation.
It’s difficult to predict how parents will feel about the shots this fall, said Chicago pediatrician Scott Goldstein, MD. Unlike other vaccinations, COVID shots aren’t required for kids to attend school, and parental interest seems to wane with each new formulation. For a physician-owned practice such as Dr. Goldstein’s, the upfront cost of the vaccine can be a gamble.
“The cost of vaccines, that’s far and away our biggest expense. But it’s also the most important thing we do, you could argue, is vaccinating kids,” Dr. Goldstein said.
Insurance doesn’t necessarily cover vaccine storage accidents, which can put the practice at risk of financial ruin.
“We’ve had things happen like a refrigerator gets unplugged. And then we’re all of a sudden out $80,000 overnight,” Dr. Goldstein said.
South Carolina pediatrician Deborah Greenhouse, MD, said she would order more COVID vaccines for older children if the pharmaceutical companies that she buys from had a more forgiving return policy.
“Pfizer is creating that situation. If you’re only going to let us return 30%, we’re not going to buy much,” she said. “We can’t.”
Greenhouse owns her practice, so the remaining 70% of leftover shots would come out of her pocket.
Vaccine maker Pfizer will take back all unused COVID shots for young children, but only 30% of doses for people 12 and older.
Pfizer said in an Aug. 20 emailed statement, “The return policy was instituted as we recognize both the importance and the complexity of pediatric vaccination and wanted to ensure that pediatric offices did not have hurdles to providing vaccine to their young patients.”
Pfizer’s return policy is similar to policies from other drugmakers for pediatric flu vaccines, also recommended during the fall season. Physicians who are worried about unwanted COVID vaccines expiring on the shelves said flu shots cost them about $20 per dose, while COVID shots cost around $150 per dose.
“We run on a very thin margin. If we get stuck holding a ton of vaccine that we cannot return, we can’t absorb that kind of cost,” Dr. Greenhouse said.
Vaccine maker Moderna will accept COVID vaccine returns, but the amount depends on the individual contract with a provider. Novavax will accept the return of only unopened vaccines and doesn’t specify the amount they’ll accept.
Dr. Greenhouse wants to vaccinate as many children as possible but said she can’t afford to stock shots with a short shelf life. Once she runs out of the doses she’s ordered, Dr. Greenhouse plans to tell families to go to a pharmacy to get older children vaccinated. If pediatricians around the country are making the same calculations, doses for very small children could be harder to find at doctors’ offices.
“Frankly, it’s not an ideal situation, but it’s what we have to do to stay in business,” she said.
Dr. Ball worries that parents’ limited interest has caused pediatricians to minimize their vaccine orders, in turn making the newest COVID shots difficult to find once they become available.
“I think there’s just a misperception that it’s less of a big deal to get COVID, but I’m still sending babies to the hospital with COVID,” Dr. Ball said. “We’re still seeing kids with long COVID. This is with us forever.”
KFF Health News is a national newsroom that produces in-depth journalism about health issues and is one of the core operating programs at KFF — the independent source for health policy research, polling, and journalism.
When pediatrician Eric Ball, MD, opened a refrigerator full of childhood vaccines, all the expected shots were there — DTaP, polio, pneumococcal vaccine — except one.
“This is where we usually store our COVID vaccines, but we don’t have any right now because they all expired at the end of last year and we had to dispose of them,” said Dr. Ball, who is part of a pediatric practice in Orange County, California.
“We thought demand would be way higher than it was.”
Providers like Dr. Ball don’t want to waste money ordering doses that won’t be used, but they need enough on hand to vaccinate vulnerable children.
The Centers for Disease Control and Prevention recommends that anyone 6 months or older get the updated COVID vaccination, but in the 2023-24 vaccination season only about 15% of eligible children in the United States got a shot.
Dr. Ball said it was difficult to let vaccines go to waste in 2023. It was the first time the federal government was no longer picking up the tab for the shots, and providers had to pay upfront for the vaccines. Parents would often skip the COVID shot, which can have a very short shelf life, compared with other vaccines.
“Watching it sitting on our shelves expiring every 30 days, that’s like throwing away $150 repeatedly every day, multiple times a month,” Dr. Ball said.
in 2024, Dr. Ball slashed his fall vaccine order to the bare minimum to avoid another costly mistake.
“We took the number of flu vaccines that we order, and then we ordered 5% of that in COVID vaccines,” Dr. Ball said. “It’s a guess.”
That small vaccine order cost more than $63,000, he said.
Pharmacists, pharmacy interns, and techs are allowed to give COVID vaccines only to children age 3 and up, meaning babies and toddlers would need to visit a doctor’s office for inoculation.
It’s difficult to predict how parents will feel about the shots this fall, said Chicago pediatrician Scott Goldstein, MD. Unlike other vaccinations, COVID shots aren’t required for kids to attend school, and parental interest seems to wane with each new formulation. For a physician-owned practice such as Dr. Goldstein’s, the upfront cost of the vaccine can be a gamble.
“The cost of vaccines, that’s far and away our biggest expense. But it’s also the most important thing we do, you could argue, is vaccinating kids,” Dr. Goldstein said.
Insurance doesn’t necessarily cover vaccine storage accidents, which can put the practice at risk of financial ruin.
“We’ve had things happen like a refrigerator gets unplugged. And then we’re all of a sudden out $80,000 overnight,” Dr. Goldstein said.
South Carolina pediatrician Deborah Greenhouse, MD, said she would order more COVID vaccines for older children if the pharmaceutical companies that she buys from had a more forgiving return policy.
“Pfizer is creating that situation. If you’re only going to let us return 30%, we’re not going to buy much,” she said. “We can’t.”
Greenhouse owns her practice, so the remaining 70% of leftover shots would come out of her pocket.
Vaccine maker Pfizer will take back all unused COVID shots for young children, but only 30% of doses for people 12 and older.
Pfizer said in an Aug. 20 emailed statement, “The return policy was instituted as we recognize both the importance and the complexity of pediatric vaccination and wanted to ensure that pediatric offices did not have hurdles to providing vaccine to their young patients.”
Pfizer’s return policy is similar to policies from other drugmakers for pediatric flu vaccines, also recommended during the fall season. Physicians who are worried about unwanted COVID vaccines expiring on the shelves said flu shots cost them about $20 per dose, while COVID shots cost around $150 per dose.
“We run on a very thin margin. If we get stuck holding a ton of vaccine that we cannot return, we can’t absorb that kind of cost,” Dr. Greenhouse said.
Vaccine maker Moderna will accept COVID vaccine returns, but the amount depends on the individual contract with a provider. Novavax will accept the return of only unopened vaccines and doesn’t specify the amount they’ll accept.
Dr. Greenhouse wants to vaccinate as many children as possible but said she can’t afford to stock shots with a short shelf life. Once she runs out of the doses she’s ordered, Dr. Greenhouse plans to tell families to go to a pharmacy to get older children vaccinated. If pediatricians around the country are making the same calculations, doses for very small children could be harder to find at doctors’ offices.
“Frankly, it’s not an ideal situation, but it’s what we have to do to stay in business,” she said.
Dr. Ball worries that parents’ limited interest has caused pediatricians to minimize their vaccine orders, in turn making the newest COVID shots difficult to find once they become available.
“I think there’s just a misperception that it’s less of a big deal to get COVID, but I’m still sending babies to the hospital with COVID,” Dr. Ball said. “We’re still seeing kids with long COVID. This is with us forever.”
KFF Health News is a national newsroom that produces in-depth journalism about health issues and is one of the core operating programs at KFF — the independent source for health policy research, polling, and journalism.
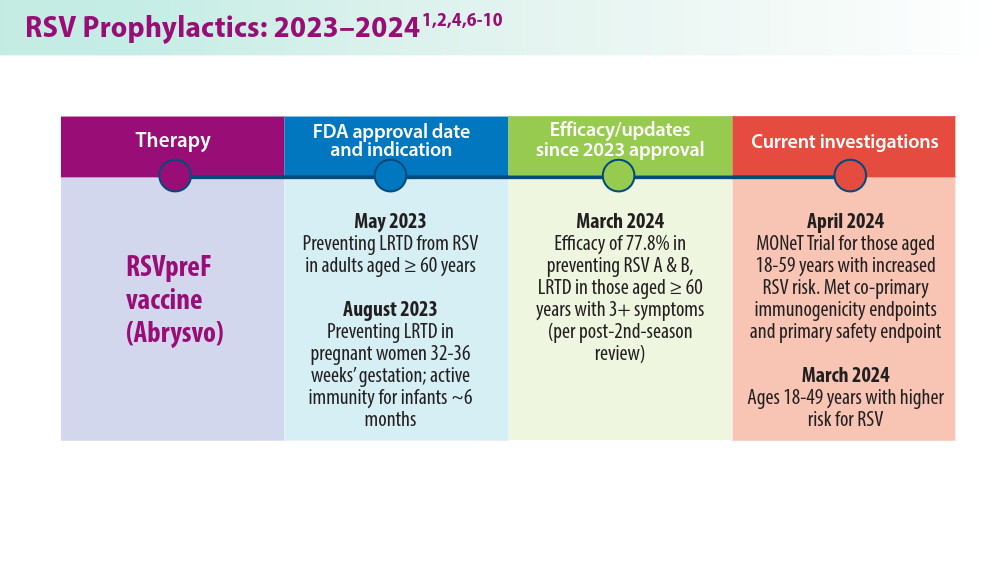
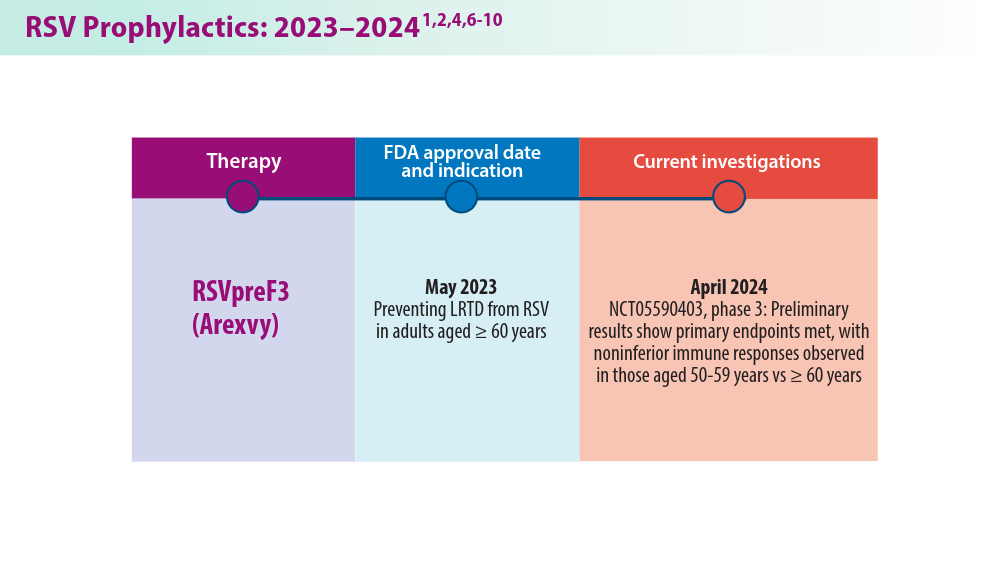
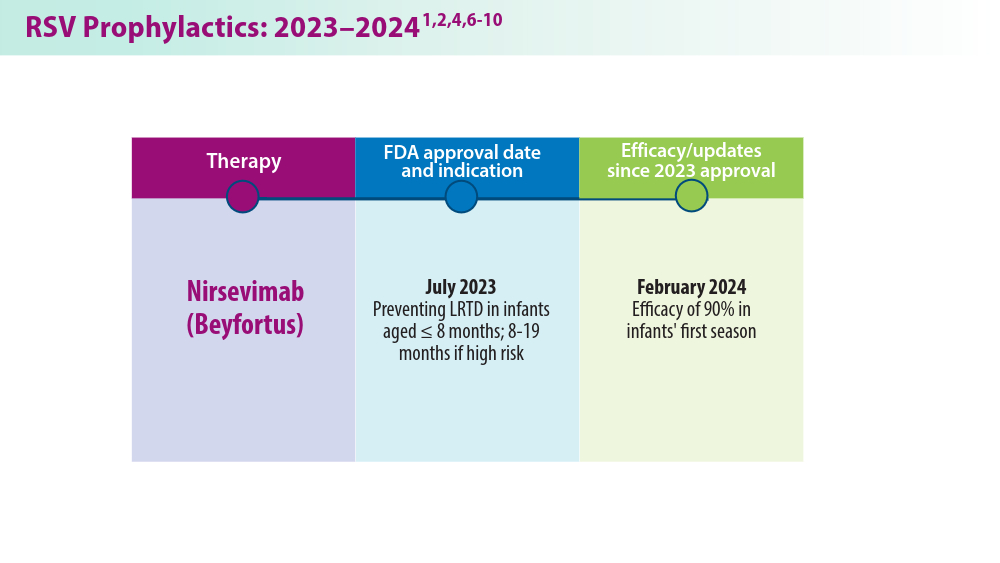
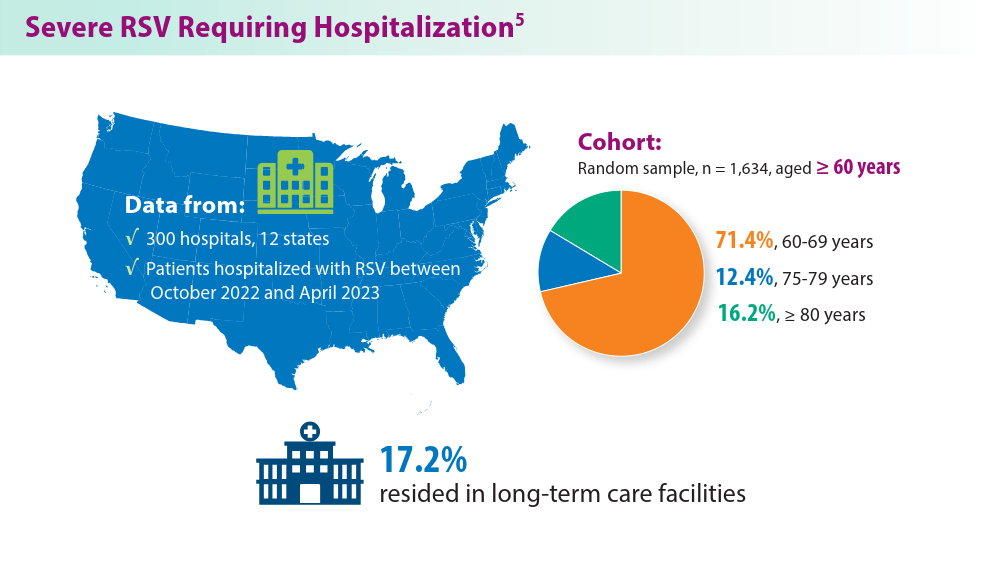
RSV Updates: Prophylaxis Approval and Hospitalization for Severe RSV
1. Pfizer announces positive top-line results from phase 3 study of ABRYSVO® in adults aged 18 to 59 at increased risk for RSV disease. Press release. Pfizer; April 9, 2024. Accessed May 22, 2024. https://www.pfizer.com/news/press-release/press-release-detail/pfizer-announces-positive-top-line-results-phase-3-study-1
2. Pfizer announces positive top-line data for full season two efficacy of ABRYSVO® for RSV in older adults. Press release. Pfizer; February 29, 2024. Accessed May 22, 2024. https://www.pfizer.com/news/press-release/press-release-detail/pfizer-announces-positive-top-line-data-full-season-two
3. CDC study shows effectiveness of RSV immunization for infants. Press release. US Centers for Disease Control and Prevention; March 7, 2024. Accessed May 22, 2024. https://www.cdc.gov/media/releases/2024/s0307-rsv-immunization.html
4. Moline HL, Tannis A, Toepfer AP, et al. Early estimate of nirsevimab effectiveness for prevention of respiratory syncytial virus–associated hospitalization among infants entering their first respiratory syncytial virus season — new vaccine surveillance network, October 2023–February 2024. MMWR Morb Mortal Wkly Rep. 2024;73(9):209-214. doi:10.15585/mmwr.mm7309a4
5. Havers FP, Whitaker M, Melgar M, et al; for the RSV-NET Surveillance Team. Characteristics and outcomes among adults aged ≥60 years hospitalized with laboratory-confirmed respiratory syncytial virus ─ RSV-NET, 12 states, July 2022–June 2023. MMWR Morb Mortal Wkly Rep. 2023;72(40):1075-1082. doi:10.15585/mmwr.mm7240a1
6. Walsh EE, Pérez Marc G, Zareba AM, et al; for the RENOIR Clinical Trial Group. Efficacy and safety of a bivalent RSV prefusion F vaccine in older adults. N Engl J Med. 2023;388(16):1465-1477. doi:10.1056/NEJMoa2213836
7. Fleming-Dutra KE, Jones JM, Roper LE, et al. Use of the Pfizer respiratory syncytial virus vaccine during pregnancy for the prevention of respiratory syncytial virus–associated lower respiratory tract disease in infants: recommendations of the Advisory Committee on Immunization Practices — United States, 2023. MMWR Morb Mortal Wkly Rep. 2023;72(41):1115-1122. doi:10.15585/mmwr.mm7241e1
8. Baker J, Aliabadi N, Munjal I, et al. Equivalent immunogenicity across three RSVpreF vaccine lots in healthy adults 18-49 years of age: results of a randomized phase 3 study. Vaccine. 2024;42(13):3172-3179. doi:10.1016/j.vaccine.2024.03.070
9. New data for AREXVY, GSK’s RSV vaccine, show potential to help protect adults aged 50 to 59 at increased risk for RSV disease. Press release. GSK; October 25, 2023. Accessed May 22, 2024. https://us.gsk.com/en-us/media/press-releases/new-data-for-arexvy/
1. Pfizer announces positive top-line results from phase 3 study of ABRYSVO® in adults aged 18 to 59 at increased risk for RSV disease. Press release. Pfizer; April 9, 2024. Accessed May 22, 2024. https://www.pfizer.com/news/press-release/press-release-detail/pfizer-announces-positive-top-line-results-phase-3-study-1
2. Pfizer announces positive top-line data for full season two efficacy of ABRYSVO® for RSV in older adults. Press release. Pfizer; February 29, 2024. Accessed May 22, 2024. https://www.pfizer.com/news/press-release/press-release-detail/pfizer-announces-positive-top-line-data-full-season-two
3. CDC study shows effectiveness of RSV immunization for infants. Press release. US Centers for Disease Control and Prevention; March 7, 2024. Accessed May 22, 2024. https://www.cdc.gov/media/releases/2024/s0307-rsv-immunization.html
4. Moline HL, Tannis A, Toepfer AP, et al. Early estimate of nirsevimab effectiveness for prevention of respiratory syncytial virus–associated hospitalization among infants entering their first respiratory syncytial virus season — new vaccine surveillance network, October 2023–February 2024. MMWR Morb Mortal Wkly Rep. 2024;73(9):209-214. doi:10.15585/mmwr.mm7309a4
5. Havers FP, Whitaker M, Melgar M, et al; for the RSV-NET Surveillance Team. Characteristics and outcomes among adults aged ≥60 years hospitalized with laboratory-confirmed respiratory syncytial virus ─ RSV-NET, 12 states, July 2022–June 2023. MMWR Morb Mortal Wkly Rep. 2023;72(40):1075-1082. doi:10.15585/mmwr.mm7240a1
6. Walsh EE, Pérez Marc G, Zareba AM, et al; for the RENOIR Clinical Trial Group. Efficacy and safety of a bivalent RSV prefusion F vaccine in older adults. N Engl J Med. 2023;388(16):1465-1477. doi:10.1056/NEJMoa2213836
7. Fleming-Dutra KE, Jones JM, Roper LE, et al. Use of the Pfizer respiratory syncytial virus vaccine during pregnancy for the prevention of respiratory syncytial virus–associated lower respiratory tract disease in infants: recommendations of the Advisory Committee on Immunization Practices — United States, 2023. MMWR Morb Mortal Wkly Rep. 2023;72(41):1115-1122. doi:10.15585/mmwr.mm7241e1
8. Baker J, Aliabadi N, Munjal I, et al. Equivalent immunogenicity across three RSVpreF vaccine lots in healthy adults 18-49 years of age: results of a randomized phase 3 study. Vaccine. 2024;42(13):3172-3179. doi:10.1016/j.vaccine.2024.03.070
9. New data for AREXVY, GSK’s RSV vaccine, show potential to help protect adults aged 50 to 59 at increased risk for RSV disease. Press release. GSK; October 25, 2023. Accessed May 22, 2024. https://us.gsk.com/en-us/media/press-releases/new-data-for-arexvy/
1. Pfizer announces positive top-line results from phase 3 study of ABRYSVO® in adults aged 18 to 59 at increased risk for RSV disease. Press release. Pfizer; April 9, 2024. Accessed May 22, 2024. https://www.pfizer.com/news/press-release/press-release-detail/pfizer-announces-positive-top-line-results-phase-3-study-1
2. Pfizer announces positive top-line data for full season two efficacy of ABRYSVO® for RSV in older adults. Press release. Pfizer; February 29, 2024. Accessed May 22, 2024. https://www.pfizer.com/news/press-release/press-release-detail/pfizer-announces-positive-top-line-data-full-season-two
3. CDC study shows effectiveness of RSV immunization for infants. Press release. US Centers for Disease Control and Prevention; March 7, 2024. Accessed May 22, 2024. https://www.cdc.gov/media/releases/2024/s0307-rsv-immunization.html
4. Moline HL, Tannis A, Toepfer AP, et al. Early estimate of nirsevimab effectiveness for prevention of respiratory syncytial virus–associated hospitalization among infants entering their first respiratory syncytial virus season — new vaccine surveillance network, October 2023–February 2024. MMWR Morb Mortal Wkly Rep. 2024;73(9):209-214. doi:10.15585/mmwr.mm7309a4
5. Havers FP, Whitaker M, Melgar M, et al; for the RSV-NET Surveillance Team. Characteristics and outcomes among adults aged ≥60 years hospitalized with laboratory-confirmed respiratory syncytial virus ─ RSV-NET, 12 states, July 2022–June 2023. MMWR Morb Mortal Wkly Rep. 2023;72(40):1075-1082. doi:10.15585/mmwr.mm7240a1
6. Walsh EE, Pérez Marc G, Zareba AM, et al; for the RENOIR Clinical Trial Group. Efficacy and safety of a bivalent RSV prefusion F vaccine in older adults. N Engl J Med. 2023;388(16):1465-1477. doi:10.1056/NEJMoa2213836
7. Fleming-Dutra KE, Jones JM, Roper LE, et al. Use of the Pfizer respiratory syncytial virus vaccine during pregnancy for the prevention of respiratory syncytial virus–associated lower respiratory tract disease in infants: recommendations of the Advisory Committee on Immunization Practices — United States, 2023. MMWR Morb Mortal Wkly Rep. 2023;72(41):1115-1122. doi:10.15585/mmwr.mm7241e1
8. Baker J, Aliabadi N, Munjal I, et al. Equivalent immunogenicity across three RSVpreF vaccine lots in healthy adults 18-49 years of age: results of a randomized phase 3 study. Vaccine. 2024;42(13):3172-3179. doi:10.1016/j.vaccine.2024.03.070
9. New data for AREXVY, GSK’s RSV vaccine, show potential to help protect adults aged 50 to 59 at increased risk for RSV disease. Press release. GSK; October 25, 2023. Accessed May 22, 2024. https://us.gsk.com/en-us/media/press-releases/new-data-for-arexvy/
Targeted Therapies and Surgical Resection for Lung Cancer: Evolving Treatment Options
- American Cancer Society. Key statistics for lung cancer. Revised January 29, 2024. Accessed June 10, 2024. https://www.cancer.org/cancer/types/lung-cancer/about/key-statistics.html
- Drilon A, Camidge DR, Lin JJ, et al; for the TRIDENT-1 Investigators. Repotrectinib in ROS1 fusion-positive non-small-cell lung cancer. N Engl J Med. 2024;390(2):118-131. doi:10.1056/NEJMoa2302299
- Wu YL, Dziadziuszko R, Ahn JS, et al; for the ALINA Investigators. Alectinib in resected ALK-positive non-small-cell lung cancer. N Engl J Med. 2024;390(14):1265-1276.
- Mulligan L. Selective RET kinase inhibitors and lung cancer. N Engl J Med. 2023;389(20):1913-1916. doi:10.1056/NEJMe2311295
- Zhou C, Soloman B, Loong HH, et al; for the LIBRETTO-432 Trial Investigators. First-line selpercatinib or chemotherapy and pembrolizumab in RET fusion-positive NSCLC. N Engl J Med. 2023:389(20):1839-1850. doi:10.1056/NEJMoa239457
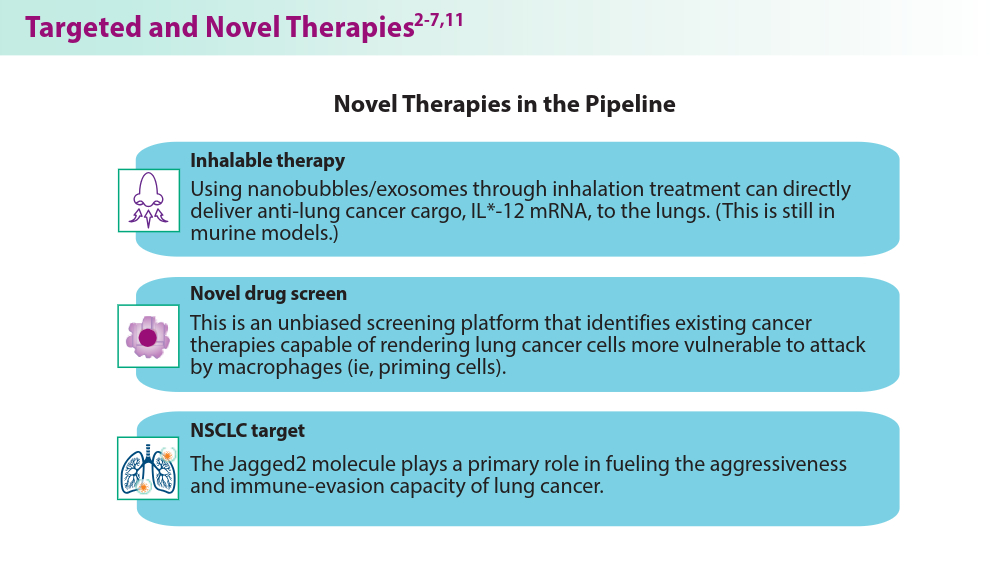
- Vaccaro K, Allen J, Whitfield TW, et al. Targeted therapies prime oncogene-driven lung cancers for macrophage-mediated destruction. bioRxiv. Preprint posted online March 6, 2023. doi:10.1101/2023.03.03.531059
- Liu M, Hu S, Yan N, Popowski KD, Cheng K. Inhalable extracellular vesicle delivery of IL-12 mRNA to treat lung cancer and promote systemic immunity. Nat Nanotechnol. 2024;19(4):565-575. doi:10.1038/s41565-023-01580-3
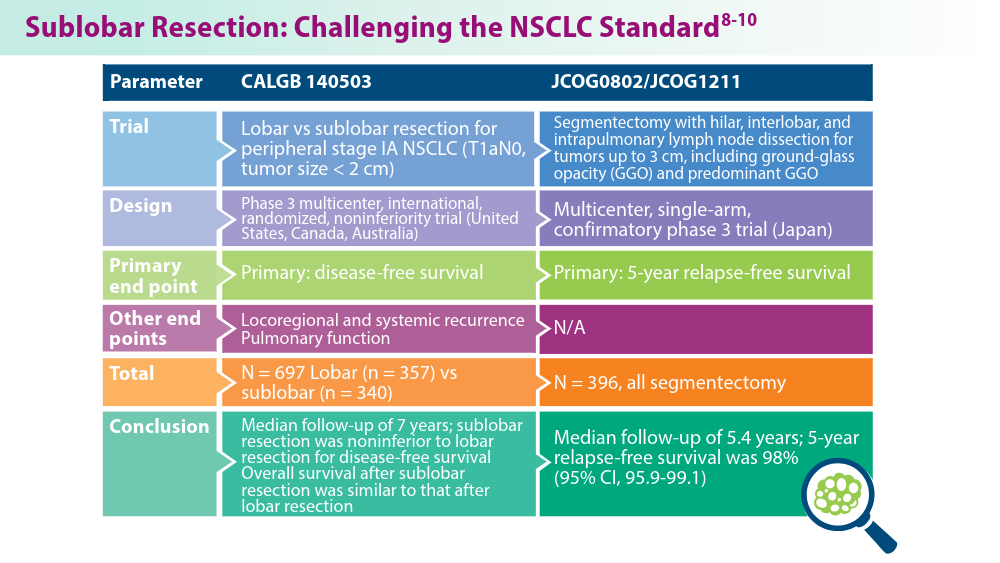
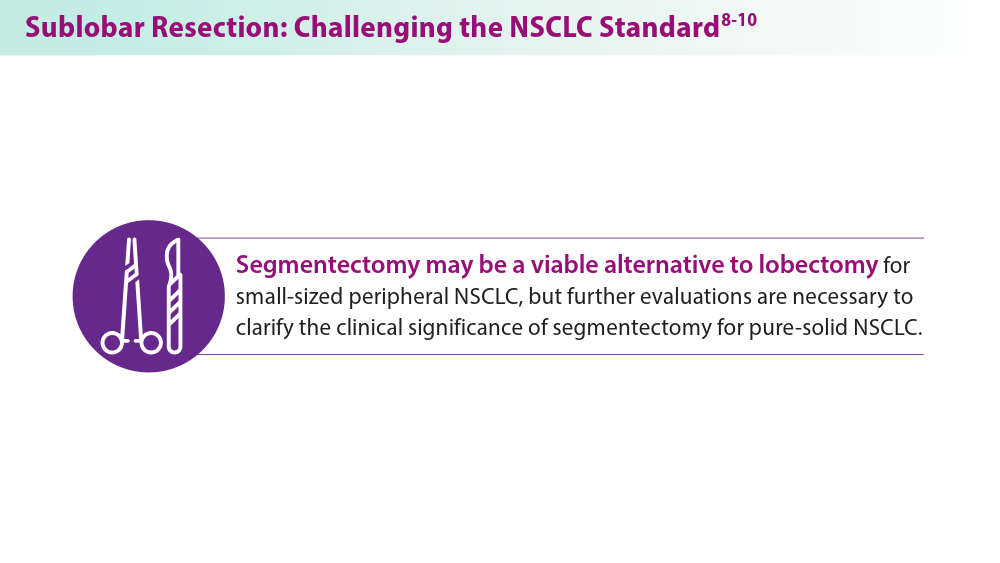
- Altorki N, Wang X, Kozono D, et al. Lobar or sublobar resection for peripheral stage IA non-small-cell lung cancer. N Engl J Med. 2023;388(6):489-498. doi:10.1056/NEJMoa2212083
- Koike T, Hasebe T, Nakamura M, Shimizu Y, Goto T, Tsuchida M. Towards better outcomes: segmentectomy for ground-glass opacity-dominant non-small cell lung cancer 3 cm or less─insights form JCOG1211 [editorial commentary]. AME Clin Trials Rev. 2023;1:5. doi:10.21037/actr-23-10
- Aokage K, Suzuki K, Saji H, et al; for the Japan Clinical Oncology Group. Segmentectomy for ground-glass-dominant lung cancer with a tumour diameter of 3 cm or less including groundglass opacity (JCOG1211): a multicentre, single-arm, confirmatory phase 3 trial. Lancet Respir Med. 2023;11(6):540-549. doi:10.1016/S2213-2600(23)00041-3
- Mandula JK, Sierra-Mondragon RA, Jimenez RV, et al. Jagged2 targeting in lung cancer activates anti-tumor immunity via Notch-induced functional reprogramming of tumor-associated macrophages. Immunity. 2024;57(5):1124-1140.e9. doi:10.1016/j.immuni.2024.03.020
- American Cancer Society. Key statistics for lung cancer. Revised January 29, 2024. Accessed June 10, 2024. https://www.cancer.org/cancer/types/lung-cancer/about/key-statistics.html
- Drilon A, Camidge DR, Lin JJ, et al; for the TRIDENT-1 Investigators. Repotrectinib in ROS1 fusion-positive non-small-cell lung cancer. N Engl J Med. 2024;390(2):118-131. doi:10.1056/NEJMoa2302299
- Wu YL, Dziadziuszko R, Ahn JS, et al; for the ALINA Investigators. Alectinib in resected ALK-positive non-small-cell lung cancer. N Engl J Med. 2024;390(14):1265-1276.
- Mulligan L. Selective RET kinase inhibitors and lung cancer. N Engl J Med. 2023;389(20):1913-1916. doi:10.1056/NEJMe2311295
- Zhou C, Soloman B, Loong HH, et al; for the LIBRETTO-432 Trial Investigators. First-line selpercatinib or chemotherapy and pembrolizumab in RET fusion-positive NSCLC. N Engl J Med. 2023:389(20):1839-1850. doi:10.1056/NEJMoa239457
- Vaccaro K, Allen J, Whitfield TW, et al. Targeted therapies prime oncogene-driven lung cancers for macrophage-mediated destruction. bioRxiv. Preprint posted online March 6, 2023. doi:10.1101/2023.03.03.531059
- Liu M, Hu S, Yan N, Popowski KD, Cheng K. Inhalable extracellular vesicle delivery of IL-12 mRNA to treat lung cancer and promote systemic immunity. Nat Nanotechnol. 2024;19(4):565-575. doi:10.1038/s41565-023-01580-3
- Altorki N, Wang X, Kozono D, et al. Lobar or sublobar resection for peripheral stage IA non-small-cell lung cancer. N Engl J Med. 2023;388(6):489-498. doi:10.1056/NEJMoa2212083
- Koike T, Hasebe T, Nakamura M, Shimizu Y, Goto T, Tsuchida M. Towards better outcomes: segmentectomy for ground-glass opacity-dominant non-small cell lung cancer 3 cm or less─insights form JCOG1211 [editorial commentary]. AME Clin Trials Rev. 2023;1:5. doi:10.21037/actr-23-10
- Aokage K, Suzuki K, Saji H, et al; for the Japan Clinical Oncology Group. Segmentectomy for ground-glass-dominant lung cancer with a tumour diameter of 3 cm or less including groundglass opacity (JCOG1211): a multicentre, single-arm, confirmatory phase 3 trial. Lancet Respir Med. 2023;11(6):540-549. doi:10.1016/S2213-2600(23)00041-3
- Mandula JK, Sierra-Mondragon RA, Jimenez RV, et al. Jagged2 targeting in lung cancer activates anti-tumor immunity via Notch-induced functional reprogramming of tumor-associated macrophages. Immunity. 2024;57(5):1124-1140.e9. doi:10.1016/j.immuni.2024.03.020
- American Cancer Society. Key statistics for lung cancer. Revised January 29, 2024. Accessed June 10, 2024. https://www.cancer.org/cancer/types/lung-cancer/about/key-statistics.html
- Drilon A, Camidge DR, Lin JJ, et al; for the TRIDENT-1 Investigators. Repotrectinib in ROS1 fusion-positive non-small-cell lung cancer. N Engl J Med. 2024;390(2):118-131. doi:10.1056/NEJMoa2302299
- Wu YL, Dziadziuszko R, Ahn JS, et al; for the ALINA Investigators. Alectinib in resected ALK-positive non-small-cell lung cancer. N Engl J Med. 2024;390(14):1265-1276.
- Mulligan L. Selective RET kinase inhibitors and lung cancer. N Engl J Med. 2023;389(20):1913-1916. doi:10.1056/NEJMe2311295
- Zhou C, Soloman B, Loong HH, et al; for the LIBRETTO-432 Trial Investigators. First-line selpercatinib or chemotherapy and pembrolizumab in RET fusion-positive NSCLC. N Engl J Med. 2023:389(20):1839-1850. doi:10.1056/NEJMoa239457
- Vaccaro K, Allen J, Whitfield TW, et al. Targeted therapies prime oncogene-driven lung cancers for macrophage-mediated destruction. bioRxiv. Preprint posted online March 6, 2023. doi:10.1101/2023.03.03.531059
- Liu M, Hu S, Yan N, Popowski KD, Cheng K. Inhalable extracellular vesicle delivery of IL-12 mRNA to treat lung cancer and promote systemic immunity. Nat Nanotechnol. 2024;19(4):565-575. doi:10.1038/s41565-023-01580-3
- Altorki N, Wang X, Kozono D, et al. Lobar or sublobar resection for peripheral stage IA non-small-cell lung cancer. N Engl J Med. 2023;388(6):489-498. doi:10.1056/NEJMoa2212083
- Koike T, Hasebe T, Nakamura M, Shimizu Y, Goto T, Tsuchida M. Towards better outcomes: segmentectomy for ground-glass opacity-dominant non-small cell lung cancer 3 cm or less─insights form JCOG1211 [editorial commentary]. AME Clin Trials Rev. 2023;1:5. doi:10.21037/actr-23-10
- Aokage K, Suzuki K, Saji H, et al; for the Japan Clinical Oncology Group. Segmentectomy for ground-glass-dominant lung cancer with a tumour diameter of 3 cm or less including groundglass opacity (JCOG1211): a multicentre, single-arm, confirmatory phase 3 trial. Lancet Respir Med. 2023;11(6):540-549. doi:10.1016/S2213-2600(23)00041-3
- Mandula JK, Sierra-Mondragon RA, Jimenez RV, et al. Jagged2 targeting in lung cancer activates anti-tumor immunity via Notch-induced functional reprogramming of tumor-associated macrophages. Immunity. 2024;57(5):1124-1140.e9. doi:10.1016/j.immuni.2024.03.020
Closing the GAP in Idiopathic Pulmonary Fibrosis
5 things you should know about IPF. American Lung Association. April 12, 2023. Accessed June 21, 2024. https://www.lung.org/blog/idiopathic-pulmonary-fibrosis-things-to-know
Raghu G, Chen SY, Yeh WS, et al. Idiopathic pulmonary fibrosis in US Medicare beneficiaries aged 65 years and older: incidence, prevalence, and survival, 2001-11. Lancet Respir Med. 2014;2(7):566-572. doi:10.1016/S2213-2600(14)70101-8
Morrow T. Improving outcomes and managing costs in idiopathic pulmonary fibrosis. Am J Manag Care. 2019;25(11 suppl):S204-S209. PMID: 31419090
Man RK, Gogikar A, Nanda A, et al. A comparison of the effectiveness of nintedanib and pirfenidone in treating idiopathic pulmonary fibrosis: a systematic review. Cureus. 2024;16(2):e54268. doi:10.7759/cureus.54268
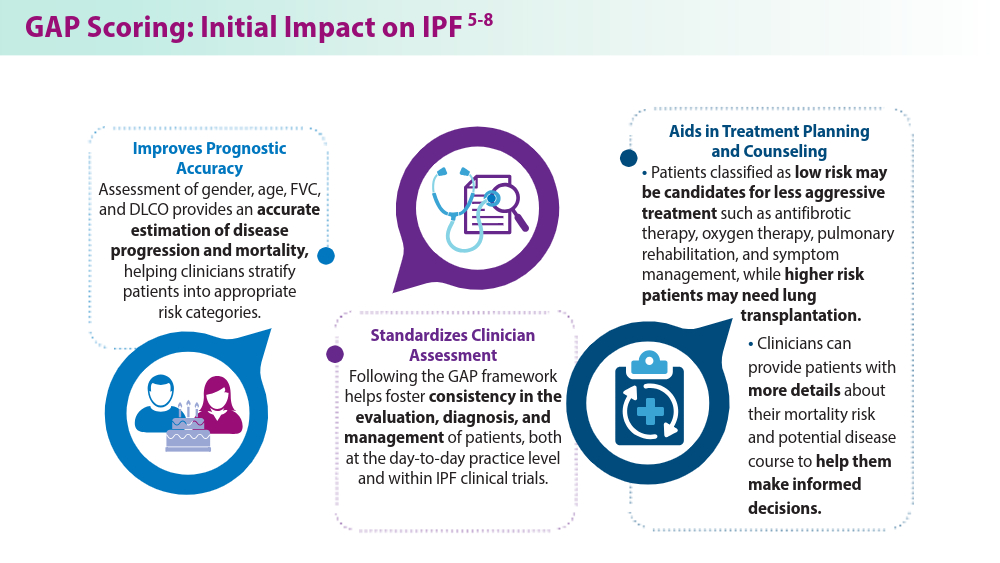
Ley B, Ryerson CJ, Vittinghoff E, et al. A multidimensional index and staging system for idiopathic pulmonary fibrosis. Ann Intern Med. 2012;156(10):684-691. doi:10.7326/0003-4819-156-10-201205150-00004
Raghu G, Remy-Jardin M, Myers JL, et al. Diagnosis of idiopathic pulmonary fibrosis. An official ATS/ERS/JRS/ALAT clinical practice guideline. Am J Respir Crit Care Med. 2018;198(5):e44-e68. doi:10.1164/rccm.201807-1255ST
Collard HR, Ryerson CJ, Corte TJ, et al. Acute exacerbation of idiopathic pulmonary fibrosis. An International Working Group report. Am J Respir Crit Care Med. 2016;194(3):265-275. doi:10.1164/rccm.201604-0801CI
Abuserewa ST, Duff R, Becker G. Treatment of idiopathic pulmonary fibrosis. Cureus. 2021;13(5):e15360. doi:10.7759/cureus.15360
Lee JH, Jang JH, Jang HJ, et al. New prognostic scoring system for mortality in idiopathic pulmonary fibrosis by modifying the gender, age, and physiology model with desaturation during the six-minute walk test. Front Med (Lausanne). 2023;10:1052129. doi:10.3389/fmed.2023.1052129
Chandel A, Pastre J, Valery S, King CS, Nathan SD. Derivation and validation of a simple multidimensional index incorporating exercise capacity parameters for survival prediction in idiopathic pulmonary fibrosis. Thorax. 2023;78(4):368-375. doi:10.1136/thoraxjnl-2021-218440
Chandel A, King CS, Ignacio RV, et al. External validation and longitudinal application of the DO-GAP index to individualise survival prediction in idiopathic pulmonary fibrosis. ERJ Open Res. 2023;9(3):00124-2023. doi:10.1183/23120541.00124-2023
Suzuki Y, Mori K, Aono Y, et al. Combined assessment of the GAP index and body mass index at antifibrotic therapy initiation for prognosis of idiopathic pulmonary fibrosis. Sci Rep. 2021;11(1):18579. doi:10.1038/s41598-021-98161-y
Lacedonia D, De Pace CC, Rea G, et al. Machine learning and BMI improve the prognostic value of GAP index in treated IPF patients. Bioengineering (Basel). 2023;10(2):251. doi:10.3390/bioengineering10020251
Fujii H, Hara Y, Saigusa Y, et al. ILD-GAP combined with the Charlson Comorbidity Index score (ILD-GAPC) as a prognostic prediction model in patients with interstitial lung disease. Can Respir J. 2023;2023:5088207. doi:10.1155/2023/5088207
Ley B, Bradford WZ, Weycker D, Vittinghoff E, du Bois RM, Collard HR. Unified baseline and longitudinal mortality prediction in idiopathic pulmonary fibrosis. Eur Respir J. 2015;45(5):1374-1381. doi:10.1183/09031936.00146314
Kreuter M, Lee JS, Tzouvelekis A, et al. Monocyte count as a prognostic biomarker in patients with idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2021;204(1):74-81. doi:10.1164/rccm.202003-0669OC
Kreuter M, Lee JS, Tzouvelekis A, et al. A modified blood cell GAP (cGAP) to prognosticate outcomes in IPF. Poster presented at: European Respiratory Society International Congress; September 4-6, 2022. https://medically.gene.com/global/en/unrestricted/respiratory/ERS-2022/ers-2022-poster-kreuter-a-modified-blood-cell-gap.html
Nishikiori H, Chiba H, Lee SH, et al. A modified GAP model for East-Asian populations with idiopathic pulmonary fibrosis. Respir Investig. 2020;58(5):395-402. doi:10.1016/j.resinv.2020.04.001
5 things you should know about IPF. American Lung Association. April 12, 2023. Accessed June 21, 2024. https://www.lung.org/blog/idiopathic-pulmonary-fibrosis-things-to-know
Raghu G, Chen SY, Yeh WS, et al. Idiopathic pulmonary fibrosis in US Medicare beneficiaries aged 65 years and older: incidence, prevalence, and survival, 2001-11. Lancet Respir Med. 2014;2(7):566-572. doi:10.1016/S2213-2600(14)70101-8
Morrow T. Improving outcomes and managing costs in idiopathic pulmonary fibrosis. Am J Manag Care. 2019;25(11 suppl):S204-S209. PMID: 31419090
Man RK, Gogikar A, Nanda A, et al. A comparison of the effectiveness of nintedanib and pirfenidone in treating idiopathic pulmonary fibrosis: a systematic review. Cureus. 2024;16(2):e54268. doi:10.7759/cureus.54268
Ley B, Ryerson CJ, Vittinghoff E, et al. A multidimensional index and staging system for idiopathic pulmonary fibrosis. Ann Intern Med. 2012;156(10):684-691. doi:10.7326/0003-4819-156-10-201205150-00004
Raghu G, Remy-Jardin M, Myers JL, et al. Diagnosis of idiopathic pulmonary fibrosis. An official ATS/ERS/JRS/ALAT clinical practice guideline. Am J Respir Crit Care Med. 2018;198(5):e44-e68. doi:10.1164/rccm.201807-1255ST
Collard HR, Ryerson CJ, Corte TJ, et al. Acute exacerbation of idiopathic pulmonary fibrosis. An International Working Group report. Am J Respir Crit Care Med. 2016;194(3):265-275. doi:10.1164/rccm.201604-0801CI
Abuserewa ST, Duff R, Becker G. Treatment of idiopathic pulmonary fibrosis. Cureus. 2021;13(5):e15360. doi:10.7759/cureus.15360
Lee JH, Jang JH, Jang HJ, et al. New prognostic scoring system for mortality in idiopathic pulmonary fibrosis by modifying the gender, age, and physiology model with desaturation during the six-minute walk test. Front Med (Lausanne). 2023;10:1052129. doi:10.3389/fmed.2023.1052129
Chandel A, Pastre J, Valery S, King CS, Nathan SD. Derivation and validation of a simple multidimensional index incorporating exercise capacity parameters for survival prediction in idiopathic pulmonary fibrosis. Thorax. 2023;78(4):368-375. doi:10.1136/thoraxjnl-2021-218440
Chandel A, King CS, Ignacio RV, et al. External validation and longitudinal application of the DO-GAP index to individualise survival prediction in idiopathic pulmonary fibrosis. ERJ Open Res. 2023;9(3):00124-2023. doi:10.1183/23120541.00124-2023
Suzuki Y, Mori K, Aono Y, et al. Combined assessment of the GAP index and body mass index at antifibrotic therapy initiation for prognosis of idiopathic pulmonary fibrosis. Sci Rep. 2021;11(1):18579. doi:10.1038/s41598-021-98161-y
Lacedonia D, De Pace CC, Rea G, et al. Machine learning and BMI improve the prognostic value of GAP index in treated IPF patients. Bioengineering (Basel). 2023;10(2):251. doi:10.3390/bioengineering10020251
Fujii H, Hara Y, Saigusa Y, et al. ILD-GAP combined with the Charlson Comorbidity Index score (ILD-GAPC) as a prognostic prediction model in patients with interstitial lung disease. Can Respir J. 2023;2023:5088207. doi:10.1155/2023/5088207
Ley B, Bradford WZ, Weycker D, Vittinghoff E, du Bois RM, Collard HR. Unified baseline and longitudinal mortality prediction in idiopathic pulmonary fibrosis. Eur Respir J. 2015;45(5):1374-1381. doi:10.1183/09031936.00146314
Kreuter M, Lee JS, Tzouvelekis A, et al. Monocyte count as a prognostic biomarker in patients with idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2021;204(1):74-81. doi:10.1164/rccm.202003-0669OC
Kreuter M, Lee JS, Tzouvelekis A, et al. A modified blood cell GAP (cGAP) to prognosticate outcomes in IPF. Poster presented at: European Respiratory Society International Congress; September 4-6, 2022. https://medically.gene.com/global/en/unrestricted/respiratory/ERS-2022/ers-2022-poster-kreuter-a-modified-blood-cell-gap.html
Nishikiori H, Chiba H, Lee SH, et al. A modified GAP model for East-Asian populations with idiopathic pulmonary fibrosis. Respir Investig. 2020;58(5):395-402. doi:10.1016/j.resinv.2020.04.001
5 things you should know about IPF. American Lung Association. April 12, 2023. Accessed June 21, 2024. https://www.lung.org/blog/idiopathic-pulmonary-fibrosis-things-to-know
Raghu G, Chen SY, Yeh WS, et al. Idiopathic pulmonary fibrosis in US Medicare beneficiaries aged 65 years and older: incidence, prevalence, and survival, 2001-11. Lancet Respir Med. 2014;2(7):566-572. doi:10.1016/S2213-2600(14)70101-8
Morrow T. Improving outcomes and managing costs in idiopathic pulmonary fibrosis. Am J Manag Care. 2019;25(11 suppl):S204-S209. PMID: 31419090
Man RK, Gogikar A, Nanda A, et al. A comparison of the effectiveness of nintedanib and pirfenidone in treating idiopathic pulmonary fibrosis: a systematic review. Cureus. 2024;16(2):e54268. doi:10.7759/cureus.54268
Ley B, Ryerson CJ, Vittinghoff E, et al. A multidimensional index and staging system for idiopathic pulmonary fibrosis. Ann Intern Med. 2012;156(10):684-691. doi:10.7326/0003-4819-156-10-201205150-00004
Raghu G, Remy-Jardin M, Myers JL, et al. Diagnosis of idiopathic pulmonary fibrosis. An official ATS/ERS/JRS/ALAT clinical practice guideline. Am J Respir Crit Care Med. 2018;198(5):e44-e68. doi:10.1164/rccm.201807-1255ST
Collard HR, Ryerson CJ, Corte TJ, et al. Acute exacerbation of idiopathic pulmonary fibrosis. An International Working Group report. Am J Respir Crit Care Med. 2016;194(3):265-275. doi:10.1164/rccm.201604-0801CI
Abuserewa ST, Duff R, Becker G. Treatment of idiopathic pulmonary fibrosis. Cureus. 2021;13(5):e15360. doi:10.7759/cureus.15360
Lee JH, Jang JH, Jang HJ, et al. New prognostic scoring system for mortality in idiopathic pulmonary fibrosis by modifying the gender, age, and physiology model with desaturation during the six-minute walk test. Front Med (Lausanne). 2023;10:1052129. doi:10.3389/fmed.2023.1052129
Chandel A, Pastre J, Valery S, King CS, Nathan SD. Derivation and validation of a simple multidimensional index incorporating exercise capacity parameters for survival prediction in idiopathic pulmonary fibrosis. Thorax. 2023;78(4):368-375. doi:10.1136/thoraxjnl-2021-218440
Chandel A, King CS, Ignacio RV, et al. External validation and longitudinal application of the DO-GAP index to individualise survival prediction in idiopathic pulmonary fibrosis. ERJ Open Res. 2023;9(3):00124-2023. doi:10.1183/23120541.00124-2023
Suzuki Y, Mori K, Aono Y, et al. Combined assessment of the GAP index and body mass index at antifibrotic therapy initiation for prognosis of idiopathic pulmonary fibrosis. Sci Rep. 2021;11(1):18579. doi:10.1038/s41598-021-98161-y
Lacedonia D, De Pace CC, Rea G, et al. Machine learning and BMI improve the prognostic value of GAP index in treated IPF patients. Bioengineering (Basel). 2023;10(2):251. doi:10.3390/bioengineering10020251
Fujii H, Hara Y, Saigusa Y, et al. ILD-GAP combined with the Charlson Comorbidity Index score (ILD-GAPC) as a prognostic prediction model in patients with interstitial lung disease. Can Respir J. 2023;2023:5088207. doi:10.1155/2023/5088207
Ley B, Bradford WZ, Weycker D, Vittinghoff E, du Bois RM, Collard HR. Unified baseline and longitudinal mortality prediction in idiopathic pulmonary fibrosis. Eur Respir J. 2015;45(5):1374-1381. doi:10.1183/09031936.00146314
Kreuter M, Lee JS, Tzouvelekis A, et al. Monocyte count as a prognostic biomarker in patients with idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2021;204(1):74-81. doi:10.1164/rccm.202003-0669OC
Kreuter M, Lee JS, Tzouvelekis A, et al. A modified blood cell GAP (cGAP) to prognosticate outcomes in IPF. Poster presented at: European Respiratory Society International Congress; September 4-6, 2022. https://medically.gene.com/global/en/unrestricted/respiratory/ERS-2022/ers-2022-poster-kreuter-a-modified-blood-cell-gap.html
Nishikiori H, Chiba H, Lee SH, et al. A modified GAP model for East-Asian populations with idiopathic pulmonary fibrosis. Respir Investig. 2020;58(5):395-402. doi:10.1016/j.resinv.2020.04.001
Pulmonology Data Trends 2024
Pulmonology Data Trends 2024 is a supplement to CHEST Physician highlighting the latest breakthroughs in pulmonology research and treatments through a series of infographics.
Read more:
Artificial Intelligence in Sleep Apnea
Ritwick Agrawal, MD, MS, FCCP
RSV Updates: Prophylaxis Approval and Hospitalization for Severe RSV
Riddhi Upadhyay, MD
Biologics in Asthma: Changing the Severe Asthma Paradigm
Shyam Subramanian, MD, FCCP
Updates in COPD Guidelines and Treatment
Dharani K. Narendra, MD, FCCP
Targeted Therapies and Surgical Resection for Lung Cancer: Evolving Treatment Options
Saadia A. Faiz, MD, FCCP
Closing the GAP in Idiopathic Pulmonary Fibrosis
Humayun Anjum, MD, FCCP
Severe Community-Acquired Pneumonia: Diagnostic Criteria, Treatment, and COVID-19
Sujith V. Cherian, MD, FCCP
Pulmonary Hypertension: Comorbidities and Novel Therapies
Mary Jo S. Farmer, MD, PhD, FCCP
The Genetic Side of Interstitial Lung Disease
Priya Balakrishnan, MD, MS, FCCP
Noninvasive Ventilation in Neuromuscular Disease
Sreelatha Naik, MD, FCCP, and Kelly Lobrutto, CRNP
Pulmonology Data Trends 2024 is a supplement to CHEST Physician highlighting the latest breakthroughs in pulmonology research and treatments through a series of infographics.
Read more:
Artificial Intelligence in Sleep Apnea
Ritwick Agrawal, MD, MS, FCCP
RSV Updates: Prophylaxis Approval and Hospitalization for Severe RSV
Riddhi Upadhyay, MD
Biologics in Asthma: Changing the Severe Asthma Paradigm
Shyam Subramanian, MD, FCCP
Updates in COPD Guidelines and Treatment
Dharani K. Narendra, MD, FCCP
Targeted Therapies and Surgical Resection for Lung Cancer: Evolving Treatment Options
Saadia A. Faiz, MD, FCCP
Closing the GAP in Idiopathic Pulmonary Fibrosis
Humayun Anjum, MD, FCCP
Severe Community-Acquired Pneumonia: Diagnostic Criteria, Treatment, and COVID-19
Sujith V. Cherian, MD, FCCP
Pulmonary Hypertension: Comorbidities and Novel Therapies
Mary Jo S. Farmer, MD, PhD, FCCP
The Genetic Side of Interstitial Lung Disease
Priya Balakrishnan, MD, MS, FCCP
Noninvasive Ventilation in Neuromuscular Disease
Sreelatha Naik, MD, FCCP, and Kelly Lobrutto, CRNP
Pulmonology Data Trends 2024 is a supplement to CHEST Physician highlighting the latest breakthroughs in pulmonology research and treatments through a series of infographics.
Read more:
Artificial Intelligence in Sleep Apnea
Ritwick Agrawal, MD, MS, FCCP
RSV Updates: Prophylaxis Approval and Hospitalization for Severe RSV
Riddhi Upadhyay, MD
Biologics in Asthma: Changing the Severe Asthma Paradigm
Shyam Subramanian, MD, FCCP
Updates in COPD Guidelines and Treatment
Dharani K. Narendra, MD, FCCP
Targeted Therapies and Surgical Resection for Lung Cancer: Evolving Treatment Options
Saadia A. Faiz, MD, FCCP
Closing the GAP in Idiopathic Pulmonary Fibrosis
Humayun Anjum, MD, FCCP
Severe Community-Acquired Pneumonia: Diagnostic Criteria, Treatment, and COVID-19
Sujith V. Cherian, MD, FCCP
Pulmonary Hypertension: Comorbidities and Novel Therapies
Mary Jo S. Farmer, MD, PhD, FCCP
The Genetic Side of Interstitial Lung Disease
Priya Balakrishnan, MD, MS, FCCP
Noninvasive Ventilation in Neuromuscular Disease
Sreelatha Naik, MD, FCCP, and Kelly Lobrutto, CRNP
Updates in COPD Guidelines and Treatment
Al Wachami N, Guennouni M, Iderdar Y, et al. Estimating the global prevalence of chronic obstructive pulmonary disease (COPD): a systematic review and meta-analysis. BMC Public Health. 2024;24(1):297. doi:10.1186/s12889-024-17686-9
COPD trends brief. American Lung Association. Accessed July 11, 2024. https://www.lung.org/research/trends-in-lung-disease/copd-trends-brief
Chronic obstructive pulmonary disease (COPD). World Health Organization. March 16, 2023. Accessed July 11, 2024. https://www.who.int/news-room/fact-sheets/detail/chronic-obstructive-pulmonary-disease-(copd)
Shalabi MS, Aqdi SW, Alfort OA, et al. Effectiveness and safety of bronchodilators and inhaled corticosteroids in the management of chronic obstructive pulmonary disease. Int J Commun Med Public Health. 2023;10(8):2955-2959. doi:10.18203/2394-6040.ijcmph20232392
McCormick B. FDA approves ensifentrine for maintenance treatment of adult patients with COPD. AJMC. June 26, 2024. Accessed July 11, 2024. https://www.ajmc.com/view/fda-approves-ensifentrine-for-maintenance-treatment-of-adult-patients-with-copd
Kersul AL, Cosio BG. Biologics in COPD. Open Resp Arch. 2024;6(2):100306. doi:10.1016/j.opresp.2024.100306
2023 GOLD Report. Global Initiative for Chronic Obstructive Lung Disease. Accessed July 11, 2024. https://goldcopd.org/2023-gold-report-2
2024 GOLD Report. Global Initiative for Chronic Obstructive Lung Disease. Accessed July 11, 2024. https://goldcopd.org/2024-gold-report/
Regeneron Pharmaceuticals Inc. Dupixent® (dupilumab) late-breaking data from NOTUS confirmatory phase 3 COPD trial presented at ATS and published in the New England Journal of Medicine [press release]. May 20, 2024. Accessed July 11, 2024. https://investor.regeneron.com/news-releases/news-release-details/dupixentr-dupilumab-late-breaking-data-notus-confirmatory-phase
Pavord ID, Chapman KR, Bafadhel M, et al. Mepolizumab for eosinophil-associated COPD: analysis of METREX and METREO. Int J Chron Obstruct Pulmon Dis. 2021;16:1755-1770. doi:10.2147/COPD.S294333
Mepolizumab as add-on treatment in participants with COPD characterized by frequent exacerbations and eosinophil level (MATINEE). Clinicaltrials.gov. Updated August 28, 2023. Accessed July 11, 2024. https://clinicaltrials.gov/study/NCT04133909
Singh D, Criner GJ, Agustí A, et al. Benralizumab prevents recurrent exacerbations in patients with chronic obstructive pulmonary disease: a post hoc analysis. Int J Chron Obstruct Pulmon Dis. 2023;18:1595-1599. doi:10.2147/COPD.S418944
Efficacy and safety of benralizumab in moderate to very severe chronic obstructive pulmonary disease (COPD) with a history of frequent exacerbations (RESOLUTE). Clinicaltrials.gov. Updated May 8, 2024. Accessed July 11, 2024. https://clinicaltrials.gov/study/NCT04053634
Efficacy and safety of tozorakimab in symptomatic chronic obstructive pulmonary disease with a history of exacerbations (TITANIA). Clinicaltrials.gov. Updated June 27, 2024. Accessed July 11, 2024. https://clinicaltrials.gov/study/NCT05158387
Efficacy and safety of tozorakimab in symptomatic chronic obstructive pulmonary disease with a history of exacerbations (OBERON). Clinicaltrials.gov. Updated June 21, 2024. Accessed July 11, 2024. https://clinicaltrials.gov/study/NCT05166889
Long-term efficacy and safety of tozorakimab in participants with chronic obstructive pulmonary disease with a history of exacerbations (PROSPERO). Clinicaltrials.gov. Updated June 20, 2024. Accessed July 11, 2024. https://clinicaltrials.gov/study/NCT05742802
Efficacy and safety of tozorakimab in symptomatic chronic obstructive pulmonary disease with a history of exacerbations (MIRANDA). Clinicaltrials.gov. Updated June 4, 2024. Accessed July 11, 2024. https://clinicaltrials.gov/study/NCT06040086
Study to assess the efficacy, safety, and tolerability of SAR440340/REGN3500/itepekimab in chronic obstructive pulmonary disease (COPD) (AERIFY-1). ClinicalTrials.gov. Updated June 21, 2024. Accessed July 11, 2024. https://classic.clinicaltrials.gov/ct2/show/NCT04701983
Study to assess the efficacy, safety, and tolerability of SAR440340/REGN3500/itepekimab in chronic obstructive pulmonary disease (COPD) (AERIFY-2). ClinicalTrials.gov. Updated May 9, 2024. Accessed July 11, 2024. https://classic.clinicaltrials.gov/ct2/show/NCT04751487
ALIENTO and ARNASA: study designs of two randomised, double-blind, placebo-controlled trials of astegolimab in patients with COPD. Medically. 2023. Accessed July 11, 2024. https://medically.gene.com/global/en/unrestricted/respiratory/ERS-2023/ers-2023-poster-brightling-aliento-and-arnasa-study-des.html
Anzueto A, Barjaktarevic IZ, Siler TM, et al. Ensifentrine, a novel phosphodiesterase 3 and 4 inhibitor for the treatment of chronic obstructive pulmonary disease: randomized, double-blind, placebo-controlled, multicenter phase III trials (the ENHANCE trials). Am J Respir Crit Care Med. 2023;208(4):406-416. doi:10.1164/rccm.202306-0944OC
US Preventive Services Taskforce. Lung cancer: screening. March 9, 2021. Accessed July 11, 2024. https://www.uspreventiveservicestaskforce.org/uspstf/recommendation/lung-cancer-screening
Al Wachami N, Guennouni M, Iderdar Y, et al. Estimating the global prevalence of chronic obstructive pulmonary disease (COPD): a systematic review and meta-analysis. BMC Public Health. 2024;24(1):297. doi:10.1186/s12889-024-17686-9
COPD trends brief. American Lung Association. Accessed July 11, 2024. https://www.lung.org/research/trends-in-lung-disease/copd-trends-brief
Chronic obstructive pulmonary disease (COPD). World Health Organization. March 16, 2023. Accessed July 11, 2024. https://www.who.int/news-room/fact-sheets/detail/chronic-obstructive-pulmonary-disease-(copd)
Shalabi MS, Aqdi SW, Alfort OA, et al. Effectiveness and safety of bronchodilators and inhaled corticosteroids in the management of chronic obstructive pulmonary disease. Int J Commun Med Public Health. 2023;10(8):2955-2959. doi:10.18203/2394-6040.ijcmph20232392
McCormick B. FDA approves ensifentrine for maintenance treatment of adult patients with COPD. AJMC. June 26, 2024. Accessed July 11, 2024. https://www.ajmc.com/view/fda-approves-ensifentrine-for-maintenance-treatment-of-adult-patients-with-copd
Kersul AL, Cosio BG. Biologics in COPD. Open Resp Arch. 2024;6(2):100306. doi:10.1016/j.opresp.2024.100306
2023 GOLD Report. Global Initiative for Chronic Obstructive Lung Disease. Accessed July 11, 2024. https://goldcopd.org/2023-gold-report-2
2024 GOLD Report. Global Initiative for Chronic Obstructive Lung Disease. Accessed July 11, 2024. https://goldcopd.org/2024-gold-report/
Regeneron Pharmaceuticals Inc. Dupixent® (dupilumab) late-breaking data from NOTUS confirmatory phase 3 COPD trial presented at ATS and published in the New England Journal of Medicine [press release]. May 20, 2024. Accessed July 11, 2024. https://investor.regeneron.com/news-releases/news-release-details/dupixentr-dupilumab-late-breaking-data-notus-confirmatory-phase
Pavord ID, Chapman KR, Bafadhel M, et al. Mepolizumab for eosinophil-associated COPD: analysis of METREX and METREO. Int J Chron Obstruct Pulmon Dis. 2021;16:1755-1770. doi:10.2147/COPD.S294333
Mepolizumab as add-on treatment in participants with COPD characterized by frequent exacerbations and eosinophil level (MATINEE). Clinicaltrials.gov. Updated August 28, 2023. Accessed July 11, 2024. https://clinicaltrials.gov/study/NCT04133909
Singh D, Criner GJ, Agustí A, et al. Benralizumab prevents recurrent exacerbations in patients with chronic obstructive pulmonary disease: a post hoc analysis. Int J Chron Obstruct Pulmon Dis. 2023;18:1595-1599. doi:10.2147/COPD.S418944
Efficacy and safety of benralizumab in moderate to very severe chronic obstructive pulmonary disease (COPD) with a history of frequent exacerbations (RESOLUTE). Clinicaltrials.gov. Updated May 8, 2024. Accessed July 11, 2024. https://clinicaltrials.gov/study/NCT04053634
Efficacy and safety of tozorakimab in symptomatic chronic obstructive pulmonary disease with a history of exacerbations (TITANIA). Clinicaltrials.gov. Updated June 27, 2024. Accessed July 11, 2024. https://clinicaltrials.gov/study/NCT05158387
Efficacy and safety of tozorakimab in symptomatic chronic obstructive pulmonary disease with a history of exacerbations (OBERON). Clinicaltrials.gov. Updated June 21, 2024. Accessed July 11, 2024. https://clinicaltrials.gov/study/NCT05166889
Long-term efficacy and safety of tozorakimab in participants with chronic obstructive pulmonary disease with a history of exacerbations (PROSPERO). Clinicaltrials.gov. Updated June 20, 2024. Accessed July 11, 2024. https://clinicaltrials.gov/study/NCT05742802
Efficacy and safety of tozorakimab in symptomatic chronic obstructive pulmonary disease with a history of exacerbations (MIRANDA). Clinicaltrials.gov. Updated June 4, 2024. Accessed July 11, 2024. https://clinicaltrials.gov/study/NCT06040086
Study to assess the efficacy, safety, and tolerability of SAR440340/REGN3500/itepekimab in chronic obstructive pulmonary disease (COPD) (AERIFY-1). ClinicalTrials.gov. Updated June 21, 2024. Accessed July 11, 2024. https://classic.clinicaltrials.gov/ct2/show/NCT04701983
Study to assess the efficacy, safety, and tolerability of SAR440340/REGN3500/itepekimab in chronic obstructive pulmonary disease (COPD) (AERIFY-2). ClinicalTrials.gov. Updated May 9, 2024. Accessed July 11, 2024. https://classic.clinicaltrials.gov/ct2/show/NCT04751487
ALIENTO and ARNASA: study designs of two randomised, double-blind, placebo-controlled trials of astegolimab in patients with COPD. Medically. 2023. Accessed July 11, 2024. https://medically.gene.com/global/en/unrestricted/respiratory/ERS-2023/ers-2023-poster-brightling-aliento-and-arnasa-study-des.html
Anzueto A, Barjaktarevic IZ, Siler TM, et al. Ensifentrine, a novel phosphodiesterase 3 and 4 inhibitor for the treatment of chronic obstructive pulmonary disease: randomized, double-blind, placebo-controlled, multicenter phase III trials (the ENHANCE trials). Am J Respir Crit Care Med. 2023;208(4):406-416. doi:10.1164/rccm.202306-0944OC
US Preventive Services Taskforce. Lung cancer: screening. March 9, 2021. Accessed July 11, 2024. https://www.uspreventiveservicestaskforce.org/uspstf/recommendation/lung-cancer-screening
Al Wachami N, Guennouni M, Iderdar Y, et al. Estimating the global prevalence of chronic obstructive pulmonary disease (COPD): a systematic review and meta-analysis. BMC Public Health. 2024;24(1):297. doi:10.1186/s12889-024-17686-9
COPD trends brief. American Lung Association. Accessed July 11, 2024. https://www.lung.org/research/trends-in-lung-disease/copd-trends-brief
Chronic obstructive pulmonary disease (COPD). World Health Organization. March 16, 2023. Accessed July 11, 2024. https://www.who.int/news-room/fact-sheets/detail/chronic-obstructive-pulmonary-disease-(copd)
Shalabi MS, Aqdi SW, Alfort OA, et al. Effectiveness and safety of bronchodilators and inhaled corticosteroids in the management of chronic obstructive pulmonary disease. Int J Commun Med Public Health. 2023;10(8):2955-2959. doi:10.18203/2394-6040.ijcmph20232392
McCormick B. FDA approves ensifentrine for maintenance treatment of adult patients with COPD. AJMC. June 26, 2024. Accessed July 11, 2024. https://www.ajmc.com/view/fda-approves-ensifentrine-for-maintenance-treatment-of-adult-patients-with-copd
Kersul AL, Cosio BG. Biologics in COPD. Open Resp Arch. 2024;6(2):100306. doi:10.1016/j.opresp.2024.100306
2023 GOLD Report. Global Initiative for Chronic Obstructive Lung Disease. Accessed July 11, 2024. https://goldcopd.org/2023-gold-report-2
2024 GOLD Report. Global Initiative for Chronic Obstructive Lung Disease. Accessed July 11, 2024. https://goldcopd.org/2024-gold-report/
Regeneron Pharmaceuticals Inc. Dupixent® (dupilumab) late-breaking data from NOTUS confirmatory phase 3 COPD trial presented at ATS and published in the New England Journal of Medicine [press release]. May 20, 2024. Accessed July 11, 2024. https://investor.regeneron.com/news-releases/news-release-details/dupixentr-dupilumab-late-breaking-data-notus-confirmatory-phase
Pavord ID, Chapman KR, Bafadhel M, et al. Mepolizumab for eosinophil-associated COPD: analysis of METREX and METREO. Int J Chron Obstruct Pulmon Dis. 2021;16:1755-1770. doi:10.2147/COPD.S294333
Mepolizumab as add-on treatment in participants with COPD characterized by frequent exacerbations and eosinophil level (MATINEE). Clinicaltrials.gov. Updated August 28, 2023. Accessed July 11, 2024. https://clinicaltrials.gov/study/NCT04133909
Singh D, Criner GJ, Agustí A, et al. Benralizumab prevents recurrent exacerbations in patients with chronic obstructive pulmonary disease: a post hoc analysis. Int J Chron Obstruct Pulmon Dis. 2023;18:1595-1599. doi:10.2147/COPD.S418944
Efficacy and safety of benralizumab in moderate to very severe chronic obstructive pulmonary disease (COPD) with a history of frequent exacerbations (RESOLUTE). Clinicaltrials.gov. Updated May 8, 2024. Accessed July 11, 2024. https://clinicaltrials.gov/study/NCT04053634
Efficacy and safety of tozorakimab in symptomatic chronic obstructive pulmonary disease with a history of exacerbations (TITANIA). Clinicaltrials.gov. Updated June 27, 2024. Accessed July 11, 2024. https://clinicaltrials.gov/study/NCT05158387
Efficacy and safety of tozorakimab in symptomatic chronic obstructive pulmonary disease with a history of exacerbations (OBERON). Clinicaltrials.gov. Updated June 21, 2024. Accessed July 11, 2024. https://clinicaltrials.gov/study/NCT05166889
Long-term efficacy and safety of tozorakimab in participants with chronic obstructive pulmonary disease with a history of exacerbations (PROSPERO). Clinicaltrials.gov. Updated June 20, 2024. Accessed July 11, 2024. https://clinicaltrials.gov/study/NCT05742802
Efficacy and safety of tozorakimab in symptomatic chronic obstructive pulmonary disease with a history of exacerbations (MIRANDA). Clinicaltrials.gov. Updated June 4, 2024. Accessed July 11, 2024. https://clinicaltrials.gov/study/NCT06040086
Study to assess the efficacy, safety, and tolerability of SAR440340/REGN3500/itepekimab in chronic obstructive pulmonary disease (COPD) (AERIFY-1). ClinicalTrials.gov. Updated June 21, 2024. Accessed July 11, 2024. https://classic.clinicaltrials.gov/ct2/show/NCT04701983
Study to assess the efficacy, safety, and tolerability of SAR440340/REGN3500/itepekimab in chronic obstructive pulmonary disease (COPD) (AERIFY-2). ClinicalTrials.gov. Updated May 9, 2024. Accessed July 11, 2024. https://classic.clinicaltrials.gov/ct2/show/NCT04751487
ALIENTO and ARNASA: study designs of two randomised, double-blind, placebo-controlled trials of astegolimab in patients with COPD. Medically. 2023. Accessed July 11, 2024. https://medically.gene.com/global/en/unrestricted/respiratory/ERS-2023/ers-2023-poster-brightling-aliento-and-arnasa-study-des.html
Anzueto A, Barjaktarevic IZ, Siler TM, et al. Ensifentrine, a novel phosphodiesterase 3 and 4 inhibitor for the treatment of chronic obstructive pulmonary disease: randomized, double-blind, placebo-controlled, multicenter phase III trials (the ENHANCE trials). Am J Respir Crit Care Med. 2023;208(4):406-416. doi:10.1164/rccm.202306-0944OC
US Preventive Services Taskforce. Lung cancer: screening. March 9, 2021. Accessed July 11, 2024. https://www.uspreventiveservicestaskforce.org/uspstf/recommendation/lung-cancer-screening
Noninvasive Ventilation in Neuromuscular Disease
- Gong Y, Sankari A. Noninvasive ventilation. StatPearls [Internet]. Treasure Island, FL: StatPearls Publishing; 2024.Updated December 11, 2022. Accessed June 19, 2024. https://www.ncbi.nlm.nih.gov/books/NBK578188/
- Khan A, Frazer-Green L, Amin R, et al. Respiratory management of patients with neuromuscular weakness: an American College of Chest Physicians clinical practice guideline and expert panel report. Chest. 2023;164(2):394-413. doi:10.1016/j.chest.2023.03.011
- Taran S, McCredie VA, Goligher EC. Noninvasive and invasive mechanical ventilation for neurologic disorders. Handb Clin Neurol. 2022;189:361-386. doi:10.1016/B978-0-323-91532-8.00015-X
- Rao F, Garuti G, Vitacca M, et al; for the UILDM Respiratory Group. Management of respiratory complications and rehabilitation in individuals with muscular dystrophies: 1st Consensus Conference report from UILDM - Italian Muscular Dystrophy Association (Milan, January 25-26, 2019). Acta Myol. 2021;40(1):8-42. doi:10.36185/2532-1900-045
- Respiratory assist devices. Centers for Medicare & Medicaid Services. Revised January 1, 2024. Accessed June 19, 2024. https://www.cms.gov/ medicare-coverage-database/view/lcd.aspx?lcdid=33800
- What you need to know about the Philips PAP device recalls. American College of Chest Physicians. February 1, 2024. Accessed June 19, 2024. https://www.chestnet.org/Newsroom/CHEST-News/2021/07/What-YouNeed-to-Know-About-the-Philips-PAP-Device-Recall
- Orr JE, Chen K, Vaida F, et al. Effectiveness of long-term noninvasive ventilation measured by remote monitoring in neuromuscular disease. ERJ Open Res. 2023;9(5):00163-2023. doi:10.1183/23120541.00163-2023
- Kapur VK, Auckley DH, Chowdhuri S, et al. Clinical practice guideline for diagnostic testing for adult obstructive sleep apnea: an American Academy of Sleep Medicine clinical practice guideline. J Clin Sleep Med. 2017;13(3):479-504. doi:10.5664/jcsm.6506
- Phillips Respironics. Trilogy Evo Clinical Manual. 2019
- ResMed. Astral Series Clinical Guide. 2018
- Breas. Vivo 45 LS User Manual. 2023
- Lowenstein Medical. Luisa Life Support Ventilation.
- Ventec Life Systems. VOCSN Clinical and Technical Manual. 2019
- Gong Y, Sankari A. Noninvasive ventilation. StatPearls [Internet]. Treasure Island, FL: StatPearls Publishing; 2024.Updated December 11, 2022. Accessed June 19, 2024. https://www.ncbi.nlm.nih.gov/books/NBK578188/
- Khan A, Frazer-Green L, Amin R, et al. Respiratory management of patients with neuromuscular weakness: an American College of Chest Physicians clinical practice guideline and expert panel report. Chest. 2023;164(2):394-413. doi:10.1016/j.chest.2023.03.011
- Taran S, McCredie VA, Goligher EC. Noninvasive and invasive mechanical ventilation for neurologic disorders. Handb Clin Neurol. 2022;189:361-386. doi:10.1016/B978-0-323-91532-8.00015-X
- Rao F, Garuti G, Vitacca M, et al; for the UILDM Respiratory Group. Management of respiratory complications and rehabilitation in individuals with muscular dystrophies: 1st Consensus Conference report from UILDM - Italian Muscular Dystrophy Association (Milan, January 25-26, 2019). Acta Myol. 2021;40(1):8-42. doi:10.36185/2532-1900-045
- Respiratory assist devices. Centers for Medicare & Medicaid Services. Revised January 1, 2024. Accessed June 19, 2024. https://www.cms.gov/ medicare-coverage-database/view/lcd.aspx?lcdid=33800
- What you need to know about the Philips PAP device recalls. American College of Chest Physicians. February 1, 2024. Accessed June 19, 2024. https://www.chestnet.org/Newsroom/CHEST-News/2021/07/What-YouNeed-to-Know-About-the-Philips-PAP-Device-Recall
- Orr JE, Chen K, Vaida F, et al. Effectiveness of long-term noninvasive ventilation measured by remote monitoring in neuromuscular disease. ERJ Open Res. 2023;9(5):00163-2023. doi:10.1183/23120541.00163-2023
- Kapur VK, Auckley DH, Chowdhuri S, et al. Clinical practice guideline for diagnostic testing for adult obstructive sleep apnea: an American Academy of Sleep Medicine clinical practice guideline. J Clin Sleep Med. 2017;13(3):479-504. doi:10.5664/jcsm.6506
- Phillips Respironics. Trilogy Evo Clinical Manual. 2019
- ResMed. Astral Series Clinical Guide. 2018
- Breas. Vivo 45 LS User Manual. 2023
- Lowenstein Medical. Luisa Life Support Ventilation.
- Ventec Life Systems. VOCSN Clinical and Technical Manual. 2019
- Gong Y, Sankari A. Noninvasive ventilation. StatPearls [Internet]. Treasure Island, FL: StatPearls Publishing; 2024.Updated December 11, 2022. Accessed June 19, 2024. https://www.ncbi.nlm.nih.gov/books/NBK578188/
- Khan A, Frazer-Green L, Amin R, et al. Respiratory management of patients with neuromuscular weakness: an American College of Chest Physicians clinical practice guideline and expert panel report. Chest. 2023;164(2):394-413. doi:10.1016/j.chest.2023.03.011
- Taran S, McCredie VA, Goligher EC. Noninvasive and invasive mechanical ventilation for neurologic disorders. Handb Clin Neurol. 2022;189:361-386. doi:10.1016/B978-0-323-91532-8.00015-X
- Rao F, Garuti G, Vitacca M, et al; for the UILDM Respiratory Group. Management of respiratory complications and rehabilitation in individuals with muscular dystrophies: 1st Consensus Conference report from UILDM - Italian Muscular Dystrophy Association (Milan, January 25-26, 2019). Acta Myol. 2021;40(1):8-42. doi:10.36185/2532-1900-045
- Respiratory assist devices. Centers for Medicare & Medicaid Services. Revised January 1, 2024. Accessed June 19, 2024. https://www.cms.gov/ medicare-coverage-database/view/lcd.aspx?lcdid=33800
- What you need to know about the Philips PAP device recalls. American College of Chest Physicians. February 1, 2024. Accessed June 19, 2024. https://www.chestnet.org/Newsroom/CHEST-News/2021/07/What-YouNeed-to-Know-About-the-Philips-PAP-Device-Recall
- Orr JE, Chen K, Vaida F, et al. Effectiveness of long-term noninvasive ventilation measured by remote monitoring in neuromuscular disease. ERJ Open Res. 2023;9(5):00163-2023. doi:10.1183/23120541.00163-2023
- Kapur VK, Auckley DH, Chowdhuri S, et al. Clinical practice guideline for diagnostic testing for adult obstructive sleep apnea: an American Academy of Sleep Medicine clinical practice guideline. J Clin Sleep Med. 2017;13(3):479-504. doi:10.5664/jcsm.6506
- Phillips Respironics. Trilogy Evo Clinical Manual. 2019
- ResMed. Astral Series Clinical Guide. 2018
- Breas. Vivo 45 LS User Manual. 2023
- Lowenstein Medical. Luisa Life Support Ventilation.
- Ventec Life Systems. VOCSN Clinical and Technical Manual. 2019
The Wellness Industry: Financially Toxic, Says Ethicist
This transcript has been edited for clarity.
Hi. I’m Art Caplan. I’m at the Division of Medical Ethics at the NYU Grossman School of Medicine in New York City.
We have many debates and arguments that are swirling around about the out-of-control costs of Medicare. Many people are arguing we’ve got to trim it and cut back, and many people note that we can’t just go on and on with that kind of expenditure.
People look around for savings. Rightly, we can’t go on with the prices that we’re paying. No system could. We’ll bankrupt ourselves if we don’t drive prices down.
There’s another area that is driving up cost where, despite the fact that Medicare doesn’t pay for it, we could capture resources and hopefully shift them back to things like Medicare coverage or the insurance of other efficacious procedures. That area is the wellness industry.
That’s money coming out of people’s pockets that we could hopefully aim at the payment of things that we know work, not seeing the money drain out to cover bunk, nonsense, and charlatanism.
Does any or most of this stuff work? Do anything? Help anybody? No. We are spending money on charlatans and quacks. The US Food and Drug Administration (FDA), which you might think is the agency that could step in and start to get rid of some of this nonsense, is just too overwhelmed trying to track drugs, devices, and vaccines to give much attention to the wellness industry.
What am I talking about specifically? I’m talking about everything from gut probiotics that are sold in sodas to probiotic facial creams and the Goop industry of Gwyneth Paltrow, where you have people buying things like wellness mats or vaginal eggs that are supposed to maintain gynecologic health.
We’re talking about things like PEMF, or pulse electronic magnetic fields, where you buy a machine and expose yourself to mild magnetic pulses. I went online to look them up, and the machines cost $5000-$50,000. There’s no evidence that it works. By the way, the machines are not only out there as being sold for pain relief and many other things to humans, but also they’re being sold for your pets.
That industry is completely out of control. Wellness interventions, whether it’s transcranial magnetism or all manner of supplements that are sold in health food stores, over and over again, we see a world in which wellness is promoted but no data are introduced to show that any of it helps, works, or does anybody any good.
It may not be all that harmful, but it’s certainly financially toxic to many people who end up spending good amounts of money using these things. I think doctors need to ask patients if they are using any of these things, particularly if they have chronic conditions. They’re likely, many of them, to be seduced by online advertisement to get involved with this stuff because it’s preventive or it’ll help treat some condition that they have.
The industry is out of control. We’re trying to figure out how to spend money on things we know work in medicine, and yet we continue to tolerate bunk, nonsense, quackery, and charlatanism, just letting it grow and grow and grow in terms of cost.
That’s money that could go elsewhere. That is money that is being taken out of the pockets of patients. They’re doing things that may even delay medical treatment, which won’t really help them, and they are doing things that perhaps might even interfere with medical care that really is known to be beneficial.
I think it’s time to push for more money for the FDA to regulate the wellness side. I think it’s time for the Federal Trade Commission to go after ads that promise health benefits. I think it’s time to have some honest conversations with patients: What are you using? What are you doing? Tell me about it, and here’s why I think you could probably spend your money in a better way.
Dr. Caplan, director, Division of Medical Ethics, New York University Langone Medical Center, New York, disclosed ties with Johnson & Johnson’s Panel for Compassionate Drug Use (unpaid position). He serves as a contributing author and adviser for Medscape.
A version of this article appeared on Medscape.com.
This transcript has been edited for clarity.
Hi. I’m Art Caplan. I’m at the Division of Medical Ethics at the NYU Grossman School of Medicine in New York City.
We have many debates and arguments that are swirling around about the out-of-control costs of Medicare. Many people are arguing we’ve got to trim it and cut back, and many people note that we can’t just go on and on with that kind of expenditure.
People look around for savings. Rightly, we can’t go on with the prices that we’re paying. No system could. We’ll bankrupt ourselves if we don’t drive prices down.
There’s another area that is driving up cost where, despite the fact that Medicare doesn’t pay for it, we could capture resources and hopefully shift them back to things like Medicare coverage or the insurance of other efficacious procedures. That area is the wellness industry.
That’s money coming out of people’s pockets that we could hopefully aim at the payment of things that we know work, not seeing the money drain out to cover bunk, nonsense, and charlatanism.
Does any or most of this stuff work? Do anything? Help anybody? No. We are spending money on charlatans and quacks. The US Food and Drug Administration (FDA), which you might think is the agency that could step in and start to get rid of some of this nonsense, is just too overwhelmed trying to track drugs, devices, and vaccines to give much attention to the wellness industry.
What am I talking about specifically? I’m talking about everything from gut probiotics that are sold in sodas to probiotic facial creams and the Goop industry of Gwyneth Paltrow, where you have people buying things like wellness mats or vaginal eggs that are supposed to maintain gynecologic health.
We’re talking about things like PEMF, or pulse electronic magnetic fields, where you buy a machine and expose yourself to mild magnetic pulses. I went online to look them up, and the machines cost $5000-$50,000. There’s no evidence that it works. By the way, the machines are not only out there as being sold for pain relief and many other things to humans, but also they’re being sold for your pets.
That industry is completely out of control. Wellness interventions, whether it’s transcranial magnetism or all manner of supplements that are sold in health food stores, over and over again, we see a world in which wellness is promoted but no data are introduced to show that any of it helps, works, or does anybody any good.
It may not be all that harmful, but it’s certainly financially toxic to many people who end up spending good amounts of money using these things. I think doctors need to ask patients if they are using any of these things, particularly if they have chronic conditions. They’re likely, many of them, to be seduced by online advertisement to get involved with this stuff because it’s preventive or it’ll help treat some condition that they have.
The industry is out of control. We’re trying to figure out how to spend money on things we know work in medicine, and yet we continue to tolerate bunk, nonsense, quackery, and charlatanism, just letting it grow and grow and grow in terms of cost.
That’s money that could go elsewhere. That is money that is being taken out of the pockets of patients. They’re doing things that may even delay medical treatment, which won’t really help them, and they are doing things that perhaps might even interfere with medical care that really is known to be beneficial.
I think it’s time to push for more money for the FDA to regulate the wellness side. I think it’s time for the Federal Trade Commission to go after ads that promise health benefits. I think it’s time to have some honest conversations with patients: What are you using? What are you doing? Tell me about it, and here’s why I think you could probably spend your money in a better way.
Dr. Caplan, director, Division of Medical Ethics, New York University Langone Medical Center, New York, disclosed ties with Johnson & Johnson’s Panel for Compassionate Drug Use (unpaid position). He serves as a contributing author and adviser for Medscape.
A version of this article appeared on Medscape.com.
This transcript has been edited for clarity.
Hi. I’m Art Caplan. I’m at the Division of Medical Ethics at the NYU Grossman School of Medicine in New York City.
We have many debates and arguments that are swirling around about the out-of-control costs of Medicare. Many people are arguing we’ve got to trim it and cut back, and many people note that we can’t just go on and on with that kind of expenditure.
People look around for savings. Rightly, we can’t go on with the prices that we’re paying. No system could. We’ll bankrupt ourselves if we don’t drive prices down.
There’s another area that is driving up cost where, despite the fact that Medicare doesn’t pay for it, we could capture resources and hopefully shift them back to things like Medicare coverage or the insurance of other efficacious procedures. That area is the wellness industry.
That’s money coming out of people’s pockets that we could hopefully aim at the payment of things that we know work, not seeing the money drain out to cover bunk, nonsense, and charlatanism.
Does any or most of this stuff work? Do anything? Help anybody? No. We are spending money on charlatans and quacks. The US Food and Drug Administration (FDA), which you might think is the agency that could step in and start to get rid of some of this nonsense, is just too overwhelmed trying to track drugs, devices, and vaccines to give much attention to the wellness industry.
What am I talking about specifically? I’m talking about everything from gut probiotics that are sold in sodas to probiotic facial creams and the Goop industry of Gwyneth Paltrow, where you have people buying things like wellness mats or vaginal eggs that are supposed to maintain gynecologic health.
We’re talking about things like PEMF, or pulse electronic magnetic fields, where you buy a machine and expose yourself to mild magnetic pulses. I went online to look them up, and the machines cost $5000-$50,000. There’s no evidence that it works. By the way, the machines are not only out there as being sold for pain relief and many other things to humans, but also they’re being sold for your pets.
That industry is completely out of control. Wellness interventions, whether it’s transcranial magnetism or all manner of supplements that are sold in health food stores, over and over again, we see a world in which wellness is promoted but no data are introduced to show that any of it helps, works, or does anybody any good.
It may not be all that harmful, but it’s certainly financially toxic to many people who end up spending good amounts of money using these things. I think doctors need to ask patients if they are using any of these things, particularly if they have chronic conditions. They’re likely, many of them, to be seduced by online advertisement to get involved with this stuff because it’s preventive or it’ll help treat some condition that they have.
The industry is out of control. We’re trying to figure out how to spend money on things we know work in medicine, and yet we continue to tolerate bunk, nonsense, quackery, and charlatanism, just letting it grow and grow and grow in terms of cost.
That’s money that could go elsewhere. That is money that is being taken out of the pockets of patients. They’re doing things that may even delay medical treatment, which won’t really help them, and they are doing things that perhaps might even interfere with medical care that really is known to be beneficial.
I think it’s time to push for more money for the FDA to regulate the wellness side. I think it’s time for the Federal Trade Commission to go after ads that promise health benefits. I think it’s time to have some honest conversations with patients: What are you using? What are you doing? Tell me about it, and here’s why I think you could probably spend your money in a better way.
Dr. Caplan, director, Division of Medical Ethics, New York University Langone Medical Center, New York, disclosed ties with Johnson & Johnson’s Panel for Compassionate Drug Use (unpaid position). He serves as a contributing author and adviser for Medscape.
A version of this article appeared on Medscape.com.
Hospital to home tracheostomy care
SLEEP MEDICINE NETWORK
Home-Based Mechanical Ventilation and Neuromuscular Section
Technological improvement has enhanced our ability to support these patients with complex conditions in their home settings. However, clinical practice guidelines are lacking, and current practice relies on a consensus of expert opinions.1-3
Once a patient who has had a tracheostomy begins transitioning care to home, identifying caregivers is vital.
Caregivers need to be educated on daily tracheostomy care, airway clearance, and ventilator management.
Protocols to standardize this transition, such as the “Trach Trail” protocol, help reduce ICU readmissions with new tracheostomies (P = .05), eliminate predischarge mortality (P = .05), and may decrease ICU length of stay (P = 0.72).4 Standardized protocols for aspects of tracheostomy care, such as the “Go-Bag” from Boston Children’s Hospital, ensure that a consistent approach keeps providers, families, and patients familiar with their equipment and safety procedures, improving outcomes and decreasing tracheostomy-related adverse events.4-6
Understanding the landscape surrounding which equipment companies have trained field respiratory therapists is crucial. Airway clearance is key to improving ventilation and oxygenation and maintaining tracheostomy patency. Knowing the types of airway clearance modalities used for each patient remains critical.
Trach care may look substantially different for some populations, like patients in the neonatal ICU. Trach changes may happen more frequently. Speaking valve times may be gradually increased while planning for possible decannulation. Skin care involving granulation tissue and stoma complications is particularly important for this population. Active infants need well-fitting trach ties to balance enough support to maintain their trach without causing skin breakdown or discomfort. Securing the trach to prevent pulling or dislodgement as infants become more active is crucial as developmental milestones are achieved.
We hope national societies prioritize standardizing care for this vulnerable population while promoting additional high-quality, patient-centered outcomes in research studies. Implementation strategies to promote interprofessional teams to enhance education, communication, and outcomes will reduce health care disparities.
References
1. Am J Respir Crit Care Med Vol 161. pp Sherman JM, Davis S, Albamonte-Petrick S, et al. Care of the child with a chronic tracheostomy. This official statement of the American Thoracic Society was adopted by the ATS Board of Directors, July 1999. Am J Respir Crit Care Med. 2000;161(1):297-308. doi: 10.1164/ajrccm.161.1.ats1-00 297-308, 2000
2. Mitchell RB, Hussey HM, Setzen G, et al. Clinical consensus statement: tracheostomy care. Otolaryngol Head Neck Surg. 2013;148(1):6-20. Preprint. Posted online September 18, 2012. PMID: 22990518. doi: 10.1177/0194599812460376
3. Sterni LM, Collaco JM, Baker CD, et al; ATS Pediatric Chronic Home Ventilation Workgroup. An official American Thoracic Society clinical practice guideline: pediatric chronic home invasive ventilation. Am J Respir Crit Care Med. 2016;193(8):e16-35. PMID: 27082538; PMCID: PMC5439679. doi: 10.1164/rccm.201602-0276ST
4. Cherney RL, Pandian V, Ninan A, et al. The Trach Trail: a systems-based pathway to improve quality of tracheostomy care and interdisciplinary collaboration. Otolaryngol Head Neck Surg. 2020;163(2):232-243. doi: 10.1177/0194599820917427
5. Brown J. Tracheostomy to noninvasive ventilation: from acute care to home. Sleep Med Clin. 2020;15(4):593-598. doi: 10.1016/j.jsmc.2020.08.003
6. Kohn J, McKeon M, Munhall D, Blanchette S, Wells S, Watters K. Standardization of pediatric tracheostomy care with “Go-bags.” Int J Pediatr Otorhinolaryngol. 2019;121:154-156. doi: 10.1016/j.ijporl.2019.03.022
SLEEP MEDICINE NETWORK
Home-Based Mechanical Ventilation and Neuromuscular Section
Technological improvement has enhanced our ability to support these patients with complex conditions in their home settings. However, clinical practice guidelines are lacking, and current practice relies on a consensus of expert opinions.1-3
Once a patient who has had a tracheostomy begins transitioning care to home, identifying caregivers is vital.
Caregivers need to be educated on daily tracheostomy care, airway clearance, and ventilator management.
Protocols to standardize this transition, such as the “Trach Trail” protocol, help reduce ICU readmissions with new tracheostomies (P = .05), eliminate predischarge mortality (P = .05), and may decrease ICU length of stay (P = 0.72).4 Standardized protocols for aspects of tracheostomy care, such as the “Go-Bag” from Boston Children’s Hospital, ensure that a consistent approach keeps providers, families, and patients familiar with their equipment and safety procedures, improving outcomes and decreasing tracheostomy-related adverse events.4-6
Understanding the landscape surrounding which equipment companies have trained field respiratory therapists is crucial. Airway clearance is key to improving ventilation and oxygenation and maintaining tracheostomy patency. Knowing the types of airway clearance modalities used for each patient remains critical.
Trach care may look substantially different for some populations, like patients in the neonatal ICU. Trach changes may happen more frequently. Speaking valve times may be gradually increased while planning for possible decannulation. Skin care involving granulation tissue and stoma complications is particularly important for this population. Active infants need well-fitting trach ties to balance enough support to maintain their trach without causing skin breakdown or discomfort. Securing the trach to prevent pulling or dislodgement as infants become more active is crucial as developmental milestones are achieved.
We hope national societies prioritize standardizing care for this vulnerable population while promoting additional high-quality, patient-centered outcomes in research studies. Implementation strategies to promote interprofessional teams to enhance education, communication, and outcomes will reduce health care disparities.
References
1. Am J Respir Crit Care Med Vol 161. pp Sherman JM, Davis S, Albamonte-Petrick S, et al. Care of the child with a chronic tracheostomy. This official statement of the American Thoracic Society was adopted by the ATS Board of Directors, July 1999. Am J Respir Crit Care Med. 2000;161(1):297-308. doi: 10.1164/ajrccm.161.1.ats1-00 297-308, 2000
2. Mitchell RB, Hussey HM, Setzen G, et al. Clinical consensus statement: tracheostomy care. Otolaryngol Head Neck Surg. 2013;148(1):6-20. Preprint. Posted online September 18, 2012. PMID: 22990518. doi: 10.1177/0194599812460376
3. Sterni LM, Collaco JM, Baker CD, et al; ATS Pediatric Chronic Home Ventilation Workgroup. An official American Thoracic Society clinical practice guideline: pediatric chronic home invasive ventilation. Am J Respir Crit Care Med. 2016;193(8):e16-35. PMID: 27082538; PMCID: PMC5439679. doi: 10.1164/rccm.201602-0276ST
4. Cherney RL, Pandian V, Ninan A, et al. The Trach Trail: a systems-based pathway to improve quality of tracheostomy care and interdisciplinary collaboration. Otolaryngol Head Neck Surg. 2020;163(2):232-243. doi: 10.1177/0194599820917427
5. Brown J. Tracheostomy to noninvasive ventilation: from acute care to home. Sleep Med Clin. 2020;15(4):593-598. doi: 10.1016/j.jsmc.2020.08.003
6. Kohn J, McKeon M, Munhall D, Blanchette S, Wells S, Watters K. Standardization of pediatric tracheostomy care with “Go-bags.” Int J Pediatr Otorhinolaryngol. 2019;121:154-156. doi: 10.1016/j.ijporl.2019.03.022
SLEEP MEDICINE NETWORK
Home-Based Mechanical Ventilation and Neuromuscular Section
Technological improvement has enhanced our ability to support these patients with complex conditions in their home settings. However, clinical practice guidelines are lacking, and current practice relies on a consensus of expert opinions.1-3
Once a patient who has had a tracheostomy begins transitioning care to home, identifying caregivers is vital.
Caregivers need to be educated on daily tracheostomy care, airway clearance, and ventilator management.
Protocols to standardize this transition, such as the “Trach Trail” protocol, help reduce ICU readmissions with new tracheostomies (P = .05), eliminate predischarge mortality (P = .05), and may decrease ICU length of stay (P = 0.72).4 Standardized protocols for aspects of tracheostomy care, such as the “Go-Bag” from Boston Children’s Hospital, ensure that a consistent approach keeps providers, families, and patients familiar with their equipment and safety procedures, improving outcomes and decreasing tracheostomy-related adverse events.4-6
Understanding the landscape surrounding which equipment companies have trained field respiratory therapists is crucial. Airway clearance is key to improving ventilation and oxygenation and maintaining tracheostomy patency. Knowing the types of airway clearance modalities used for each patient remains critical.
Trach care may look substantially different for some populations, like patients in the neonatal ICU. Trach changes may happen more frequently. Speaking valve times may be gradually increased while planning for possible decannulation. Skin care involving granulation tissue and stoma complications is particularly important for this population. Active infants need well-fitting trach ties to balance enough support to maintain their trach without causing skin breakdown or discomfort. Securing the trach to prevent pulling or dislodgement as infants become more active is crucial as developmental milestones are achieved.
We hope national societies prioritize standardizing care for this vulnerable population while promoting additional high-quality, patient-centered outcomes in research studies. Implementation strategies to promote interprofessional teams to enhance education, communication, and outcomes will reduce health care disparities.
References
1. Am J Respir Crit Care Med Vol 161. pp Sherman JM, Davis S, Albamonte-Petrick S, et al. Care of the child with a chronic tracheostomy. This official statement of the American Thoracic Society was adopted by the ATS Board of Directors, July 1999. Am J Respir Crit Care Med. 2000;161(1):297-308. doi: 10.1164/ajrccm.161.1.ats1-00 297-308, 2000
2. Mitchell RB, Hussey HM, Setzen G, et al. Clinical consensus statement: tracheostomy care. Otolaryngol Head Neck Surg. 2013;148(1):6-20. Preprint. Posted online September 18, 2012. PMID: 22990518. doi: 10.1177/0194599812460376
3. Sterni LM, Collaco JM, Baker CD, et al; ATS Pediatric Chronic Home Ventilation Workgroup. An official American Thoracic Society clinical practice guideline: pediatric chronic home invasive ventilation. Am J Respir Crit Care Med. 2016;193(8):e16-35. PMID: 27082538; PMCID: PMC5439679. doi: 10.1164/rccm.201602-0276ST
4. Cherney RL, Pandian V, Ninan A, et al. The Trach Trail: a systems-based pathway to improve quality of tracheostomy care and interdisciplinary collaboration. Otolaryngol Head Neck Surg. 2020;163(2):232-243. doi: 10.1177/0194599820917427
5. Brown J. Tracheostomy to noninvasive ventilation: from acute care to home. Sleep Med Clin. 2020;15(4):593-598. doi: 10.1016/j.jsmc.2020.08.003
6. Kohn J, McKeon M, Munhall D, Blanchette S, Wells S, Watters K. Standardization of pediatric tracheostomy care with “Go-bags.” Int J Pediatr Otorhinolaryngol. 2019;121:154-156. doi: 10.1016/j.ijporl.2019.03.022