User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
nav[contains(@class, 'nav-ce-stack nav-ce-stack__large-screen')]
header[@id='header']
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'main-prefix')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
div[contains(@class, 'view-medstat-quiz-listing-panes')]
div[contains(@class, 'pane-article-sidebar-latest-news')]
LDL cholesterol lowering tied to less risk of first CVD events in patients older than 70
TOPLINE:
, which is similar to the benefit seen among younger patients in primary prevention, new research shows.
METHODOLOGY:
- Using various cross-linked Danish registries, researchers analyzed 65,190 participants aged 50 years and older (49,155 aged 50-69 and 16,035 aged 70+) without a history of atherosclerotic cardiovascular disease (ASCVD) who initiated new lipid-lowering treatment and had a baseline LDL cholesterol measurement and a subsequent measurement within a year.
- The primary outcome was hospitalization for a major vascular event, defined as a composite of acute coronary syndrome, nonhemorrhagic stroke, and coronary revascularization. Secondary outcomes included individual cardiovascular components of the primary outcome and all-cause mortality.
TAKEAWAY:
- During a median follow-up of 2.5 years, 626 older (70 years and over) and 1,123 younger (aged 50-69) participants had a major vascular event, with crude incidence rates of 13.4 and 7.1 per 1000 person-years, respectively.
- After adjustment for potential confounders, each 1-mmol/L reduction in LDL cholesterol in people aged 70 and older was associated with a significant 23% lower risk for major vascular events (hazard ratio [HR] 0.77; 95% confidence interval [CI], 0.71-0.83), similar to results for those younger than 70 (HR, 0.76; 95% CI, 0.71-0.80; P value for the difference between the age groups, 0.79).
- Results across all cardiovascular secondary analyses supported the main findings, and there was no significant difference between older and younger participants across all subgroup analyses, including using 75 years as the age cutoff.
- There was no association with all-cause mortality for either the older (HR, 1.03; 95% CI, 0.98-1.09) or younger (HR, 1.00; 95% CI, 0.95-1.06) groups.
IN PRACTICE:
“Our results, based on a substantial sample size representative of a contemporary general population, may contribute to informing future guideline recommendations,” and to discussions with older patients about the benefits of LDL lowering therapy, the authors wrote. They stressed that any potential benefits should be balanced against potential harms in this population, as these individuals may have comorbidities and may be taking multiple medications.
In an accompanying editorial, Safi U. Khan, MD, from the department of cardiology at Houston Methodist DeBakey Heart and Vascular Center, said the study “contributes valuable insights regarding the effects of LDL-C-lowering therapy, especially as the burgeoning aging population faces escalating burden of ASCVD,” and added future research “should focus on corroborating these findings and addressing the safety of lipid-lowering treatments in older individuals.”
SOURCE:
The study was conducted by Niklas Worm Andersson, MD, department of epidemiology research, Statens Serum Institut, Copenhagen, and colleagues. It was published online Journal of the American College of Cardiology.
LIMITATIONS:
The results may not apply to individuals without LDL monitoring when receiving lipid-lowering treatment. Outcomes relied on the validity of recorded diagnostic codes in the registries, and medical record review of cases was not done. Residual confounding can’t be ruled out, in part because data on potentially important risk factors such as smoking, blood pressure, and body mass index weren’t available. The results may not generalize to clinical scenarios or subpopulations not directly studied.
DISCLOSURES:
Dr. Andersson has no relevant conflicts of interest. Author Tine Lovsø Dohlmann, PhD, was employed by Statens Serum Institut during the study, but has been employed by Novo Nordisk since January 2023. All other study authors and the editorialist Dr. Khan have no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
TOPLINE:
, which is similar to the benefit seen among younger patients in primary prevention, new research shows.
METHODOLOGY:
- Using various cross-linked Danish registries, researchers analyzed 65,190 participants aged 50 years and older (49,155 aged 50-69 and 16,035 aged 70+) without a history of atherosclerotic cardiovascular disease (ASCVD) who initiated new lipid-lowering treatment and had a baseline LDL cholesterol measurement and a subsequent measurement within a year.
- The primary outcome was hospitalization for a major vascular event, defined as a composite of acute coronary syndrome, nonhemorrhagic stroke, and coronary revascularization. Secondary outcomes included individual cardiovascular components of the primary outcome and all-cause mortality.
TAKEAWAY:
- During a median follow-up of 2.5 years, 626 older (70 years and over) and 1,123 younger (aged 50-69) participants had a major vascular event, with crude incidence rates of 13.4 and 7.1 per 1000 person-years, respectively.
- After adjustment for potential confounders, each 1-mmol/L reduction in LDL cholesterol in people aged 70 and older was associated with a significant 23% lower risk for major vascular events (hazard ratio [HR] 0.77; 95% confidence interval [CI], 0.71-0.83), similar to results for those younger than 70 (HR, 0.76; 95% CI, 0.71-0.80; P value for the difference between the age groups, 0.79).
- Results across all cardiovascular secondary analyses supported the main findings, and there was no significant difference between older and younger participants across all subgroup analyses, including using 75 years as the age cutoff.
- There was no association with all-cause mortality for either the older (HR, 1.03; 95% CI, 0.98-1.09) or younger (HR, 1.00; 95% CI, 0.95-1.06) groups.
IN PRACTICE:
“Our results, based on a substantial sample size representative of a contemporary general population, may contribute to informing future guideline recommendations,” and to discussions with older patients about the benefits of LDL lowering therapy, the authors wrote. They stressed that any potential benefits should be balanced against potential harms in this population, as these individuals may have comorbidities and may be taking multiple medications.
In an accompanying editorial, Safi U. Khan, MD, from the department of cardiology at Houston Methodist DeBakey Heart and Vascular Center, said the study “contributes valuable insights regarding the effects of LDL-C-lowering therapy, especially as the burgeoning aging population faces escalating burden of ASCVD,” and added future research “should focus on corroborating these findings and addressing the safety of lipid-lowering treatments in older individuals.”
SOURCE:
The study was conducted by Niklas Worm Andersson, MD, department of epidemiology research, Statens Serum Institut, Copenhagen, and colleagues. It was published online Journal of the American College of Cardiology.
LIMITATIONS:
The results may not apply to individuals without LDL monitoring when receiving lipid-lowering treatment. Outcomes relied on the validity of recorded diagnostic codes in the registries, and medical record review of cases was not done. Residual confounding can’t be ruled out, in part because data on potentially important risk factors such as smoking, blood pressure, and body mass index weren’t available. The results may not generalize to clinical scenarios or subpopulations not directly studied.
DISCLOSURES:
Dr. Andersson has no relevant conflicts of interest. Author Tine Lovsø Dohlmann, PhD, was employed by Statens Serum Institut during the study, but has been employed by Novo Nordisk since January 2023. All other study authors and the editorialist Dr. Khan have no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
TOPLINE:
, which is similar to the benefit seen among younger patients in primary prevention, new research shows.
METHODOLOGY:
- Using various cross-linked Danish registries, researchers analyzed 65,190 participants aged 50 years and older (49,155 aged 50-69 and 16,035 aged 70+) without a history of atherosclerotic cardiovascular disease (ASCVD) who initiated new lipid-lowering treatment and had a baseline LDL cholesterol measurement and a subsequent measurement within a year.
- The primary outcome was hospitalization for a major vascular event, defined as a composite of acute coronary syndrome, nonhemorrhagic stroke, and coronary revascularization. Secondary outcomes included individual cardiovascular components of the primary outcome and all-cause mortality.
TAKEAWAY:
- During a median follow-up of 2.5 years, 626 older (70 years and over) and 1,123 younger (aged 50-69) participants had a major vascular event, with crude incidence rates of 13.4 and 7.1 per 1000 person-years, respectively.
- After adjustment for potential confounders, each 1-mmol/L reduction in LDL cholesterol in people aged 70 and older was associated with a significant 23% lower risk for major vascular events (hazard ratio [HR] 0.77; 95% confidence interval [CI], 0.71-0.83), similar to results for those younger than 70 (HR, 0.76; 95% CI, 0.71-0.80; P value for the difference between the age groups, 0.79).
- Results across all cardiovascular secondary analyses supported the main findings, and there was no significant difference between older and younger participants across all subgroup analyses, including using 75 years as the age cutoff.
- There was no association with all-cause mortality for either the older (HR, 1.03; 95% CI, 0.98-1.09) or younger (HR, 1.00; 95% CI, 0.95-1.06) groups.
IN PRACTICE:
“Our results, based on a substantial sample size representative of a contemporary general population, may contribute to informing future guideline recommendations,” and to discussions with older patients about the benefits of LDL lowering therapy, the authors wrote. They stressed that any potential benefits should be balanced against potential harms in this population, as these individuals may have comorbidities and may be taking multiple medications.
In an accompanying editorial, Safi U. Khan, MD, from the department of cardiology at Houston Methodist DeBakey Heart and Vascular Center, said the study “contributes valuable insights regarding the effects of LDL-C-lowering therapy, especially as the burgeoning aging population faces escalating burden of ASCVD,” and added future research “should focus on corroborating these findings and addressing the safety of lipid-lowering treatments in older individuals.”
SOURCE:
The study was conducted by Niklas Worm Andersson, MD, department of epidemiology research, Statens Serum Institut, Copenhagen, and colleagues. It was published online Journal of the American College of Cardiology.
LIMITATIONS:
The results may not apply to individuals without LDL monitoring when receiving lipid-lowering treatment. Outcomes relied on the validity of recorded diagnostic codes in the registries, and medical record review of cases was not done. Residual confounding can’t be ruled out, in part because data on potentially important risk factors such as smoking, blood pressure, and body mass index weren’t available. The results may not generalize to clinical scenarios or subpopulations not directly studied.
DISCLOSURES:
Dr. Andersson has no relevant conflicts of interest. Author Tine Lovsø Dohlmann, PhD, was employed by Statens Serum Institut during the study, but has been employed by Novo Nordisk since January 2023. All other study authors and the editorialist Dr. Khan have no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
FROM THE JOURNAL OF THE AMERICAN COLLEGE OF CARDIOLOGY
People with long COVID have specific blood biomarkers, study says
The findings may be a step toward creating blood tests to positively identify people with long COVID so specialized treatments can be employed, researchers said.
“This is a decisive step forward in the development of valid and reliable blood testing protocols for long COVID,” said David Putrino, PhD., lead author and professor of rehabilitation and human performance and director of the Abilities Research Center at Icahn Mount Sinai Health System, New York.
Researchers from the Icahn School of Medicine at Mount Sinai and Yale School of Medicine looked at blood samples from about 270 people between January 2021 and June 2022. The people had never been infected with COVID, had fully recovered from an infection, or still showed symptoms at least four months after infection.
Using machine learning, the research teams were able to differentiate between people with and without long COVID with 96% accuracy based on distinctive features in the blood samples, according to a news release from Mount Sinai.
People with long COVID had abnormal T-cell activity and low levels of the hormone cortisol. Cortisol helps people feel alert and awake, which would explain why people with long COVID often report fatigue, NBC News said in a report on the study.
“It was one of the findings that most definitively separated the folks with long Covid from the people without long Covid,” Dr. Putrino told NBC News.
The study also found that long COVID appears to reactivate latent viruses including Epstein-Barr and mononucleosis, the study said.
The blood tests could allow doctors to come up with specialized treatments in people who report a wide variety of long COVID symptoms, Dr. Putrino said.
“There is no ‘silver bullet’ for treating long COVID, because it is an illness that infiltrates complex systems such as the immune and hormonal regulation,” he said.
The Centers for Disease Control and Prevention says about one in five Americans who had COVID still have long COVID. Symptoms include fatigue, brain fog, dizziness, digestive problems, and loss of smell and taste.
A version of this article appeared on WebMD.com.
The findings may be a step toward creating blood tests to positively identify people with long COVID so specialized treatments can be employed, researchers said.
“This is a decisive step forward in the development of valid and reliable blood testing protocols for long COVID,” said David Putrino, PhD., lead author and professor of rehabilitation and human performance and director of the Abilities Research Center at Icahn Mount Sinai Health System, New York.
Researchers from the Icahn School of Medicine at Mount Sinai and Yale School of Medicine looked at blood samples from about 270 people between January 2021 and June 2022. The people had never been infected with COVID, had fully recovered from an infection, or still showed symptoms at least four months after infection.
Using machine learning, the research teams were able to differentiate between people with and without long COVID with 96% accuracy based on distinctive features in the blood samples, according to a news release from Mount Sinai.
People with long COVID had abnormal T-cell activity and low levels of the hormone cortisol. Cortisol helps people feel alert and awake, which would explain why people with long COVID often report fatigue, NBC News said in a report on the study.
“It was one of the findings that most definitively separated the folks with long Covid from the people without long Covid,” Dr. Putrino told NBC News.
The study also found that long COVID appears to reactivate latent viruses including Epstein-Barr and mononucleosis, the study said.
The blood tests could allow doctors to come up with specialized treatments in people who report a wide variety of long COVID symptoms, Dr. Putrino said.
“There is no ‘silver bullet’ for treating long COVID, because it is an illness that infiltrates complex systems such as the immune and hormonal regulation,” he said.
The Centers for Disease Control and Prevention says about one in five Americans who had COVID still have long COVID. Symptoms include fatigue, brain fog, dizziness, digestive problems, and loss of smell and taste.
A version of this article appeared on WebMD.com.
The findings may be a step toward creating blood tests to positively identify people with long COVID so specialized treatments can be employed, researchers said.
“This is a decisive step forward in the development of valid and reliable blood testing protocols for long COVID,” said David Putrino, PhD., lead author and professor of rehabilitation and human performance and director of the Abilities Research Center at Icahn Mount Sinai Health System, New York.
Researchers from the Icahn School of Medicine at Mount Sinai and Yale School of Medicine looked at blood samples from about 270 people between January 2021 and June 2022. The people had never been infected with COVID, had fully recovered from an infection, or still showed symptoms at least four months after infection.
Using machine learning, the research teams were able to differentiate between people with and without long COVID with 96% accuracy based on distinctive features in the blood samples, according to a news release from Mount Sinai.
People with long COVID had abnormal T-cell activity and low levels of the hormone cortisol. Cortisol helps people feel alert and awake, which would explain why people with long COVID often report fatigue, NBC News said in a report on the study.
“It was one of the findings that most definitively separated the folks with long Covid from the people without long Covid,” Dr. Putrino told NBC News.
The study also found that long COVID appears to reactivate latent viruses including Epstein-Barr and mononucleosis, the study said.
The blood tests could allow doctors to come up with specialized treatments in people who report a wide variety of long COVID symptoms, Dr. Putrino said.
“There is no ‘silver bullet’ for treating long COVID, because it is an illness that infiltrates complex systems such as the immune and hormonal regulation,” he said.
The Centers for Disease Control and Prevention says about one in five Americans who had COVID still have long COVID. Symptoms include fatigue, brain fog, dizziness, digestive problems, and loss of smell and taste.
A version of this article appeared on WebMD.com.
Endoscopic monitoring may not be needed for nonerosive GERD
Patients with confirmed nonerosive gastroesophageal reflux disease (GERD) are not at greater risk for esophageal cancer compared with the general population and are unlikely to need additional endoscopic monitoring for cancer, new research suggests.
By contrast, patients with erosive disease had more than double the incidence of esophageal cancer.
“We expected a less-strong association with cancer among patients with nonerosive GERD compared to those with erosive GERD, [and] the results do make sense in view of the fact that the nonerosive GERD patients had normal esophageal mucosa at endoscopy,” Jesper Lagergren, MD, PhD, of Karolinska Institutet, Stockholm, told this news organization.
The findings “suggest that in patients with GERD, a normal endoscopy indicates that the risk of cancer development in the esophagus is low,” he said. “If future research confirms our results, no monitoring would be needed for patients with known nonerosive GERD.”
However, a related editorial suggests there may be other reasons to endoscopically monitor patients with nonerosive GERD.
The study was published online in the BMJ, as was the editorial.
Erosive GERD raises risk
To assess the incidence rate of esophageal cancer among patients with nonerosive GERD compared with the general population, the investigators analyzed records from 486,556 patients in hospital and specialized outpatient centers in Denmark, Finland, and Sweden who underwent endoscopy from 1987 to 2019.
A total of 285,811 patients were included in the nonerosive GERD cohort, and 200,745 were included in a validation cohort of patients with erosive GERD.
Nonerosive GERD was defined by the absence of esophagitis and any other esophageal disorder at endoscopy. Erosive GERD was defined by esophagitis at endoscopy.
The incidence rate of esophageal cancer was assessed for up to 31 years of follow-up, with the median being 6.3 years.
In the nonerosive GERD cohort, 228 patients developed esophageal cancer during nearly 2.1 million person-years of follow-up. The incidence rate was 11 per 100,000 person-years, similar to that of the general population (standardized incidence ratio, 1.04) and did not increase with longer follow-up.
In the erosive GERD cohort, 542 patients developed esophageal cancer over almost 1.8 million person-years. This corresponded to an incidence rate of 31 per 100,000 person-years, or an increased overall standardized incidence ratio of 2.36, which became more pronounced with longer follow-up.
“This finding suggests that endoscopically confirmed non-erosive [GERD] does not require additional endoscopic monitoring for esophageal adenocarcinoma,” the authors concluded.
‘Dynamic’ progression
In a related editorial, Jerry Zhou, PhD, and Vincent Ho, MD, both of Western Sydney University, Penrith, New South Wales, Australia, wrote that the finding that patients with nonerosive disease do not have to undergo additional endoscopic evaluations for cancer is in line with previous research.
However, they added, “the more pressing rationale for reevaluating these patients would be the potential for progression to conditions such as erosive reflux disease or Barrett’s esophagus.” Longitudinal studies have shown that GERD progression is dynamic, and so the development of erosive disease after nonerosive disease is feasible.
“Widespread use of proton-pump inhibitors complicates our understanding” of GERD progression, they noted. Although study participants were advised not to take antireflux medications in the weeks prior to their endoscopy, “uncertainties about previous treatments remain due to the study’s design.” Some participants without erosive disease at baseline may have had it in the past.
Dr. Zhou and Dr. Ho also postulated that rather than being a progressive disease, nonerosive and erosive GERD might be two distinct conditions with different features and underpinnings.
Although valuable, the study “prompts reflection on the limitations of relying on the absence of esophageal erosions as the sole diagnostic criterion for non-erosive disease. The changing progression of gastroesophageal reflux disease, the complex influence of proton pump inhibitors, and the potential for a range of underlying pathophysiological causes requires a more comprehensive diagnostic perspective,” they concluded.
Dr. Lagergren said that his group plans to assess whether treatment of nonerosive GERD should be different from erosive GERD.
The study was funded by the Swedish Research Council, Swedish Cancer Society, and Nordic Cancer Union. No competing interests were declared.
A version of this article appeared on Medscape.com.
Patients with confirmed nonerosive gastroesophageal reflux disease (GERD) are not at greater risk for esophageal cancer compared with the general population and are unlikely to need additional endoscopic monitoring for cancer, new research suggests.
By contrast, patients with erosive disease had more than double the incidence of esophageal cancer.
“We expected a less-strong association with cancer among patients with nonerosive GERD compared to those with erosive GERD, [and] the results do make sense in view of the fact that the nonerosive GERD patients had normal esophageal mucosa at endoscopy,” Jesper Lagergren, MD, PhD, of Karolinska Institutet, Stockholm, told this news organization.
The findings “suggest that in patients with GERD, a normal endoscopy indicates that the risk of cancer development in the esophagus is low,” he said. “If future research confirms our results, no monitoring would be needed for patients with known nonerosive GERD.”
However, a related editorial suggests there may be other reasons to endoscopically monitor patients with nonerosive GERD.
The study was published online in the BMJ, as was the editorial.
Erosive GERD raises risk
To assess the incidence rate of esophageal cancer among patients with nonerosive GERD compared with the general population, the investigators analyzed records from 486,556 patients in hospital and specialized outpatient centers in Denmark, Finland, and Sweden who underwent endoscopy from 1987 to 2019.
A total of 285,811 patients were included in the nonerosive GERD cohort, and 200,745 were included in a validation cohort of patients with erosive GERD.
Nonerosive GERD was defined by the absence of esophagitis and any other esophageal disorder at endoscopy. Erosive GERD was defined by esophagitis at endoscopy.
The incidence rate of esophageal cancer was assessed for up to 31 years of follow-up, with the median being 6.3 years.
In the nonerosive GERD cohort, 228 patients developed esophageal cancer during nearly 2.1 million person-years of follow-up. The incidence rate was 11 per 100,000 person-years, similar to that of the general population (standardized incidence ratio, 1.04) and did not increase with longer follow-up.
In the erosive GERD cohort, 542 patients developed esophageal cancer over almost 1.8 million person-years. This corresponded to an incidence rate of 31 per 100,000 person-years, or an increased overall standardized incidence ratio of 2.36, which became more pronounced with longer follow-up.
“This finding suggests that endoscopically confirmed non-erosive [GERD] does not require additional endoscopic monitoring for esophageal adenocarcinoma,” the authors concluded.
‘Dynamic’ progression
In a related editorial, Jerry Zhou, PhD, and Vincent Ho, MD, both of Western Sydney University, Penrith, New South Wales, Australia, wrote that the finding that patients with nonerosive disease do not have to undergo additional endoscopic evaluations for cancer is in line with previous research.
However, they added, “the more pressing rationale for reevaluating these patients would be the potential for progression to conditions such as erosive reflux disease or Barrett’s esophagus.” Longitudinal studies have shown that GERD progression is dynamic, and so the development of erosive disease after nonerosive disease is feasible.
“Widespread use of proton-pump inhibitors complicates our understanding” of GERD progression, they noted. Although study participants were advised not to take antireflux medications in the weeks prior to their endoscopy, “uncertainties about previous treatments remain due to the study’s design.” Some participants without erosive disease at baseline may have had it in the past.
Dr. Zhou and Dr. Ho also postulated that rather than being a progressive disease, nonerosive and erosive GERD might be two distinct conditions with different features and underpinnings.
Although valuable, the study “prompts reflection on the limitations of relying on the absence of esophageal erosions as the sole diagnostic criterion for non-erosive disease. The changing progression of gastroesophageal reflux disease, the complex influence of proton pump inhibitors, and the potential for a range of underlying pathophysiological causes requires a more comprehensive diagnostic perspective,” they concluded.
Dr. Lagergren said that his group plans to assess whether treatment of nonerosive GERD should be different from erosive GERD.
The study was funded by the Swedish Research Council, Swedish Cancer Society, and Nordic Cancer Union. No competing interests were declared.
A version of this article appeared on Medscape.com.
Patients with confirmed nonerosive gastroesophageal reflux disease (GERD) are not at greater risk for esophageal cancer compared with the general population and are unlikely to need additional endoscopic monitoring for cancer, new research suggests.
By contrast, patients with erosive disease had more than double the incidence of esophageal cancer.
“We expected a less-strong association with cancer among patients with nonerosive GERD compared to those with erosive GERD, [and] the results do make sense in view of the fact that the nonerosive GERD patients had normal esophageal mucosa at endoscopy,” Jesper Lagergren, MD, PhD, of Karolinska Institutet, Stockholm, told this news organization.
The findings “suggest that in patients with GERD, a normal endoscopy indicates that the risk of cancer development in the esophagus is low,” he said. “If future research confirms our results, no monitoring would be needed for patients with known nonerosive GERD.”
However, a related editorial suggests there may be other reasons to endoscopically monitor patients with nonerosive GERD.
The study was published online in the BMJ, as was the editorial.
Erosive GERD raises risk
To assess the incidence rate of esophageal cancer among patients with nonerosive GERD compared with the general population, the investigators analyzed records from 486,556 patients in hospital and specialized outpatient centers in Denmark, Finland, and Sweden who underwent endoscopy from 1987 to 2019.
A total of 285,811 patients were included in the nonerosive GERD cohort, and 200,745 were included in a validation cohort of patients with erosive GERD.
Nonerosive GERD was defined by the absence of esophagitis and any other esophageal disorder at endoscopy. Erosive GERD was defined by esophagitis at endoscopy.
The incidence rate of esophageal cancer was assessed for up to 31 years of follow-up, with the median being 6.3 years.
In the nonerosive GERD cohort, 228 patients developed esophageal cancer during nearly 2.1 million person-years of follow-up. The incidence rate was 11 per 100,000 person-years, similar to that of the general population (standardized incidence ratio, 1.04) and did not increase with longer follow-up.
In the erosive GERD cohort, 542 patients developed esophageal cancer over almost 1.8 million person-years. This corresponded to an incidence rate of 31 per 100,000 person-years, or an increased overall standardized incidence ratio of 2.36, which became more pronounced with longer follow-up.
“This finding suggests that endoscopically confirmed non-erosive [GERD] does not require additional endoscopic monitoring for esophageal adenocarcinoma,” the authors concluded.
‘Dynamic’ progression
In a related editorial, Jerry Zhou, PhD, and Vincent Ho, MD, both of Western Sydney University, Penrith, New South Wales, Australia, wrote that the finding that patients with nonerosive disease do not have to undergo additional endoscopic evaluations for cancer is in line with previous research.
However, they added, “the more pressing rationale for reevaluating these patients would be the potential for progression to conditions such as erosive reflux disease or Barrett’s esophagus.” Longitudinal studies have shown that GERD progression is dynamic, and so the development of erosive disease after nonerosive disease is feasible.
“Widespread use of proton-pump inhibitors complicates our understanding” of GERD progression, they noted. Although study participants were advised not to take antireflux medications in the weeks prior to their endoscopy, “uncertainties about previous treatments remain due to the study’s design.” Some participants without erosive disease at baseline may have had it in the past.
Dr. Zhou and Dr. Ho also postulated that rather than being a progressive disease, nonerosive and erosive GERD might be two distinct conditions with different features and underpinnings.
Although valuable, the study “prompts reflection on the limitations of relying on the absence of esophageal erosions as the sole diagnostic criterion for non-erosive disease. The changing progression of gastroesophageal reflux disease, the complex influence of proton pump inhibitors, and the potential for a range of underlying pathophysiological causes requires a more comprehensive diagnostic perspective,” they concluded.
Dr. Lagergren said that his group plans to assess whether treatment of nonerosive GERD should be different from erosive GERD.
The study was funded by the Swedish Research Council, Swedish Cancer Society, and Nordic Cancer Union. No competing interests were declared.
A version of this article appeared on Medscape.com.
Study: Antiviral med linked to COVID mutations that can spread
published in the online journal Nature.
There’s no evidence that molnupiravir, sold under the brand name Lagevrio, has caused the creation of more transmissible or severe variants of COVID, the study says, but researchers called for more scrutiny of the drug.
Researchers looked at 15 million COVID genomes and discovered that hallmark mutations linked to molnupiravir increased in 2022, especially in places where the drug was widely used, such as the United States and the United Kingdom. Levels of the mutations were also found in populations where the drug was heavily prescribed, such as seniors.
Molnupiravir is an antiviral given to people after they show signs of having COVID-19. It interferes with the COVID-19 virus’s ability to make copies of itself, thus stopping the spread of the virus throughout the body and keeping the virus level low.
The study found the virus can sometimes survive molnupiravir, resulting in mutations that have spread to other people.
Theo Sanderson, PhD, the lead author on the study and a postdoctoral researcher at the Francis Crick Institute in London, told The Guardian that the implications of the mutations were unclear.
“The signature is very clear, but there aren’t any widely circulating variants that have the signature. At the moment there’s nothing that’s transmitted very widely that’s due to molnupiravir,” he said.
The study doesn’t say people should not use molnupiravir but calls for public health officials to scrutinize it.
“The observation that molnupiravir treatment has left a visible trace in global sequencing databases, including onwards transmission of molnupiravir-derived sequences, will be an important consideration for assessing the effects and evolutionary safety of this drug,” the researchers concluded.
When reached for comment, Merck questioned the evidence.
“The authors assume these mutations were associated with viral spread from molnupiravir-treated patients without documented evidence of that transmission. Instead, the authors rely on circumstantial associations between the region from which the sequence was identified and time frame of sequence collection in countries where molnupiravir is available to draw their conclusions,” the company said.
The Food and Drug Administration authorized the use of molnupiravir for the treatment of mild to moderate COVID-19 in adults in December 2021. The FDA has also authorized the use of nirmatrelvir/ritonavir (Paxlovid), an antiviral made by Pfizer.
A version of this article appeared on WebMD.com.
published in the online journal Nature.
There’s no evidence that molnupiravir, sold under the brand name Lagevrio, has caused the creation of more transmissible or severe variants of COVID, the study says, but researchers called for more scrutiny of the drug.
Researchers looked at 15 million COVID genomes and discovered that hallmark mutations linked to molnupiravir increased in 2022, especially in places where the drug was widely used, such as the United States and the United Kingdom. Levels of the mutations were also found in populations where the drug was heavily prescribed, such as seniors.
Molnupiravir is an antiviral given to people after they show signs of having COVID-19. It interferes with the COVID-19 virus’s ability to make copies of itself, thus stopping the spread of the virus throughout the body and keeping the virus level low.
The study found the virus can sometimes survive molnupiravir, resulting in mutations that have spread to other people.
Theo Sanderson, PhD, the lead author on the study and a postdoctoral researcher at the Francis Crick Institute in London, told The Guardian that the implications of the mutations were unclear.
“The signature is very clear, but there aren’t any widely circulating variants that have the signature. At the moment there’s nothing that’s transmitted very widely that’s due to molnupiravir,” he said.
The study doesn’t say people should not use molnupiravir but calls for public health officials to scrutinize it.
“The observation that molnupiravir treatment has left a visible trace in global sequencing databases, including onwards transmission of molnupiravir-derived sequences, will be an important consideration for assessing the effects and evolutionary safety of this drug,” the researchers concluded.
When reached for comment, Merck questioned the evidence.
“The authors assume these mutations were associated with viral spread from molnupiravir-treated patients without documented evidence of that transmission. Instead, the authors rely on circumstantial associations between the region from which the sequence was identified and time frame of sequence collection in countries where molnupiravir is available to draw their conclusions,” the company said.
The Food and Drug Administration authorized the use of molnupiravir for the treatment of mild to moderate COVID-19 in adults in December 2021. The FDA has also authorized the use of nirmatrelvir/ritonavir (Paxlovid), an antiviral made by Pfizer.
A version of this article appeared on WebMD.com.
published in the online journal Nature.
There’s no evidence that molnupiravir, sold under the brand name Lagevrio, has caused the creation of more transmissible or severe variants of COVID, the study says, but researchers called for more scrutiny of the drug.
Researchers looked at 15 million COVID genomes and discovered that hallmark mutations linked to molnupiravir increased in 2022, especially in places where the drug was widely used, such as the United States and the United Kingdom. Levels of the mutations were also found in populations where the drug was heavily prescribed, such as seniors.
Molnupiravir is an antiviral given to people after they show signs of having COVID-19. It interferes with the COVID-19 virus’s ability to make copies of itself, thus stopping the spread of the virus throughout the body and keeping the virus level low.
The study found the virus can sometimes survive molnupiravir, resulting in mutations that have spread to other people.
Theo Sanderson, PhD, the lead author on the study and a postdoctoral researcher at the Francis Crick Institute in London, told The Guardian that the implications of the mutations were unclear.
“The signature is very clear, but there aren’t any widely circulating variants that have the signature. At the moment there’s nothing that’s transmitted very widely that’s due to molnupiravir,” he said.
The study doesn’t say people should not use molnupiravir but calls for public health officials to scrutinize it.
“The observation that molnupiravir treatment has left a visible trace in global sequencing databases, including onwards transmission of molnupiravir-derived sequences, will be an important consideration for assessing the effects and evolutionary safety of this drug,” the researchers concluded.
When reached for comment, Merck questioned the evidence.
“The authors assume these mutations were associated with viral spread from molnupiravir-treated patients without documented evidence of that transmission. Instead, the authors rely on circumstantial associations between the region from which the sequence was identified and time frame of sequence collection in countries where molnupiravir is available to draw their conclusions,” the company said.
The Food and Drug Administration authorized the use of molnupiravir for the treatment of mild to moderate COVID-19 in adults in December 2021. The FDA has also authorized the use of nirmatrelvir/ritonavir (Paxlovid), an antiviral made by Pfizer.
A version of this article appeared on WebMD.com.
FROM NATURE
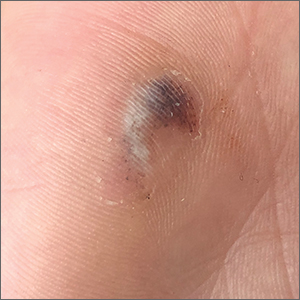
Hyperpigmented lesion on palm
This patient had a posttraumatic tache noir (also known as talon noir on the volar aspect of the feet); it is a subcorneal hematoma. The diagnosis is made clinically. Dermoscopic evaluation of tache/talon noir will reveal “pebbles on a ridge” or “satellite globules.” Confirmation of tache/talon noir can be made by paring the corneum with a #15 blade, which will reveal blood in the shavings and punctate lesions.1
This patient noted that the knob of his baseball bat rubbed the hypothenar eminence of his nondominant hand when he took a swing. The sheer force of the knob led to the subcorneal hematoma. Tache noir was high on the differential due to his physician’s clinical experience with similar cases. Tache noir occurs predominantly in people ages 12 to 24 years, without regard to gender.2 The condition is commonly found in athletes who participate in baseball, cricket, racquet sports, weightlifting, and rock climbing.2-4
Talon noir occurs most commonly in athletes who are frequently jumping, turning, and pivoting, as in football, basketball, tennis, and lacrosse.
Tache noir can be differentiated from other conditions by the presence of preserved architecture of the skin surface and punctate capillaries beneath the stratum corneum. The differential diagnosis includes verruca vulgaris, acral melanoma, and a traumatic tattoo.
Talon/tache noir are benign conditions that do not require treatment and do not affect sports performance. The lesion will usually self-resolve within a matter of weeks from onset or can even be gently scraped with a sterile needle or blade.
This patient was advised that the lesion would resolve on its own. His knee pain was determined to be a simple case of patellofemoral syndrome or “runner’s knee” and he opted to complete a home exercise program to obtain relief.
This case was adapted from: Warden D. Hyperpigmented lesion on left palm. J Fam Pract. 2021;70:459-460. Photos courtesy of Daniel Warden, MD
1. Googe AB, Schulmeier JS, Jackson AR, et al. Talon noir: paring can eliminate the need for biopsy. Postgrad Med J. 2014;90:730-731. doi: 10.1136/postgradmedj-2014-132996
2. Burkhart C, Nguyen N. Talon noire. Dermatology Advisor. Accessed October 19, 2021. www.dermatologyadvisor.com/home/decision-support-in-medicine/dermatology/talon-noire-black-heel-calcaneal-petechiae-runners-heel-basketball-heel-tennis-heel-hyperkeratosis-hemorrhagica-pseudochromhidrosis-plantaris-chromidrose-plantaire-eccrine-intracorne/
3. Talon noir. Primary Care Dermatology Society. Updated August 1, 2021. Accessed October 19, 2021. www.pcds.org.uk/clinical-guidance/talon-noir
4. Birrer RB, Griesemer BA, Cataletto MB, eds. Pediatric Sports Medicine for Primary Care. Lippincott Williams & Wilkins; 2002.

This patient had a posttraumatic tache noir (also known as talon noir on the volar aspect of the feet); it is a subcorneal hematoma. The diagnosis is made clinically. Dermoscopic evaluation of tache/talon noir will reveal “pebbles on a ridge” or “satellite globules.” Confirmation of tache/talon noir can be made by paring the corneum with a #15 blade, which will reveal blood in the shavings and punctate lesions.1
This patient noted that the knob of his baseball bat rubbed the hypothenar eminence of his nondominant hand when he took a swing. The sheer force of the knob led to the subcorneal hematoma. Tache noir was high on the differential due to his physician’s clinical experience with similar cases. Tache noir occurs predominantly in people ages 12 to 24 years, without regard to gender.2 The condition is commonly found in athletes who participate in baseball, cricket, racquet sports, weightlifting, and rock climbing.2-4
Talon noir occurs most commonly in athletes who are frequently jumping, turning, and pivoting, as in football, basketball, tennis, and lacrosse.
Tache noir can be differentiated from other conditions by the presence of preserved architecture of the skin surface and punctate capillaries beneath the stratum corneum. The differential diagnosis includes verruca vulgaris, acral melanoma, and a traumatic tattoo.
Talon/tache noir are benign conditions that do not require treatment and do not affect sports performance. The lesion will usually self-resolve within a matter of weeks from onset or can even be gently scraped with a sterile needle or blade.
This patient was advised that the lesion would resolve on its own. His knee pain was determined to be a simple case of patellofemoral syndrome or “runner’s knee” and he opted to complete a home exercise program to obtain relief.
This case was adapted from: Warden D. Hyperpigmented lesion on left palm. J Fam Pract. 2021;70:459-460. Photos courtesy of Daniel Warden, MD

This patient had a posttraumatic tache noir (also known as talon noir on the volar aspect of the feet); it is a subcorneal hematoma. The diagnosis is made clinically. Dermoscopic evaluation of tache/talon noir will reveal “pebbles on a ridge” or “satellite globules.” Confirmation of tache/talon noir can be made by paring the corneum with a #15 blade, which will reveal blood in the shavings and punctate lesions.1
This patient noted that the knob of his baseball bat rubbed the hypothenar eminence of his nondominant hand when he took a swing. The sheer force of the knob led to the subcorneal hematoma. Tache noir was high on the differential due to his physician’s clinical experience with similar cases. Tache noir occurs predominantly in people ages 12 to 24 years, without regard to gender.2 The condition is commonly found in athletes who participate in baseball, cricket, racquet sports, weightlifting, and rock climbing.2-4
Talon noir occurs most commonly in athletes who are frequently jumping, turning, and pivoting, as in football, basketball, tennis, and lacrosse.
Tache noir can be differentiated from other conditions by the presence of preserved architecture of the skin surface and punctate capillaries beneath the stratum corneum. The differential diagnosis includes verruca vulgaris, acral melanoma, and a traumatic tattoo.
Talon/tache noir are benign conditions that do not require treatment and do not affect sports performance. The lesion will usually self-resolve within a matter of weeks from onset or can even be gently scraped with a sterile needle or blade.
This patient was advised that the lesion would resolve on its own. His knee pain was determined to be a simple case of patellofemoral syndrome or “runner’s knee” and he opted to complete a home exercise program to obtain relief.
This case was adapted from: Warden D. Hyperpigmented lesion on left palm. J Fam Pract. 2021;70:459-460. Photos courtesy of Daniel Warden, MD
1. Googe AB, Schulmeier JS, Jackson AR, et al. Talon noir: paring can eliminate the need for biopsy. Postgrad Med J. 2014;90:730-731. doi: 10.1136/postgradmedj-2014-132996
2. Burkhart C, Nguyen N. Talon noire. Dermatology Advisor. Accessed October 19, 2021. www.dermatologyadvisor.com/home/decision-support-in-medicine/dermatology/talon-noire-black-heel-calcaneal-petechiae-runners-heel-basketball-heel-tennis-heel-hyperkeratosis-hemorrhagica-pseudochromhidrosis-plantaris-chromidrose-plantaire-eccrine-intracorne/
3. Talon noir. Primary Care Dermatology Society. Updated August 1, 2021. Accessed October 19, 2021. www.pcds.org.uk/clinical-guidance/talon-noir
4. Birrer RB, Griesemer BA, Cataletto MB, eds. Pediatric Sports Medicine for Primary Care. Lippincott Williams & Wilkins; 2002.
1. Googe AB, Schulmeier JS, Jackson AR, et al. Talon noir: paring can eliminate the need for biopsy. Postgrad Med J. 2014;90:730-731. doi: 10.1136/postgradmedj-2014-132996
2. Burkhart C, Nguyen N. Talon noire. Dermatology Advisor. Accessed October 19, 2021. www.dermatologyadvisor.com/home/decision-support-in-medicine/dermatology/talon-noire-black-heel-calcaneal-petechiae-runners-heel-basketball-heel-tennis-heel-hyperkeratosis-hemorrhagica-pseudochromhidrosis-plantaris-chromidrose-plantaire-eccrine-intracorne/
3. Talon noir. Primary Care Dermatology Society. Updated August 1, 2021. Accessed October 19, 2021. www.pcds.org.uk/clinical-guidance/talon-noir
4. Birrer RB, Griesemer BA, Cataletto MB, eds. Pediatric Sports Medicine for Primary Care. Lippincott Williams & Wilkins; 2002.
Weekly insulin with dosing app beneficial in type 2 diabetes
TOPLINE:
with improved treatment satisfaction and compliance scores and similarly low hypoglycemia rates.
METHODOLOGY:
- A 52-week, randomized, open-label, parallel-group, phase 3a trial with real-world elements was conducted at 176 sites in seven countries.
- A total of 1,085 insulin-naive patients with type 2 diabetes were randomly assigned to receive icodec with a dosing guide app or daily analogs (U100 glargine, U300 glargine, or icodec).
TAKEAWAY:
- A1c levels dropped from 8.96% at baseline to 7.24% at week 52 with icodec and from 8.88% to 7.61% with the daily analog, a treatment difference of 0.37 percentage point (P < .001 for noninferiority and P = .009 for superiority in favor of icodec plus the app).
- Patient-reported outcomes were more favorable with icodec plus the app vs. daily analogs, with estimated treatment differences that were significant for the Treatment Related Impact Measure for Diabetes (3.04) but not the Diabetes Treatment Satisfaction Questionnaire (0.78).
- Observed rates of combined clinically significant or severe hypoglycemia were low (0.19 event per patient-year of exposure for icodec plus the app vs. 0.14 for daily analogs; estimated rate ratio, 1.17).
IN PRACTICE:
“Once-weekly icodec with a dosing guide app could conceivably address several challenges seen in everyday practice, including inadequate dose titration and nonadherence to prescribed treatment regimens.”
SOURCE:
The study was conducted by Harpreet S. Bajaj, MD, MPH, of LMC Diabetes and Endocrinology, Brampton, Ontario, and colleagues. It was published online in Annals of Internal Medicine.
LIMITATIONS:
The research could not differentiate between the effects of icodec and those of the dosing guide app. The study had an open-label design. A 1-year duration is insufficient to assess long-term diabetes- and cardiovascular-related outcomes.
DISCLOSURES:
The study was funded by Novo Nordisk A/S.
A version of this article appeared on Medscape.com.
TOPLINE:
with improved treatment satisfaction and compliance scores and similarly low hypoglycemia rates.
METHODOLOGY:
- A 52-week, randomized, open-label, parallel-group, phase 3a trial with real-world elements was conducted at 176 sites in seven countries.
- A total of 1,085 insulin-naive patients with type 2 diabetes were randomly assigned to receive icodec with a dosing guide app or daily analogs (U100 glargine, U300 glargine, or icodec).
TAKEAWAY:
- A1c levels dropped from 8.96% at baseline to 7.24% at week 52 with icodec and from 8.88% to 7.61% with the daily analog, a treatment difference of 0.37 percentage point (P < .001 for noninferiority and P = .009 for superiority in favor of icodec plus the app).
- Patient-reported outcomes were more favorable with icodec plus the app vs. daily analogs, with estimated treatment differences that were significant for the Treatment Related Impact Measure for Diabetes (3.04) but not the Diabetes Treatment Satisfaction Questionnaire (0.78).
- Observed rates of combined clinically significant or severe hypoglycemia were low (0.19 event per patient-year of exposure for icodec plus the app vs. 0.14 for daily analogs; estimated rate ratio, 1.17).
IN PRACTICE:
“Once-weekly icodec with a dosing guide app could conceivably address several challenges seen in everyday practice, including inadequate dose titration and nonadherence to prescribed treatment regimens.”
SOURCE:
The study was conducted by Harpreet S. Bajaj, MD, MPH, of LMC Diabetes and Endocrinology, Brampton, Ontario, and colleagues. It was published online in Annals of Internal Medicine.
LIMITATIONS:
The research could not differentiate between the effects of icodec and those of the dosing guide app. The study had an open-label design. A 1-year duration is insufficient to assess long-term diabetes- and cardiovascular-related outcomes.
DISCLOSURES:
The study was funded by Novo Nordisk A/S.
A version of this article appeared on Medscape.com.
TOPLINE:
with improved treatment satisfaction and compliance scores and similarly low hypoglycemia rates.
METHODOLOGY:
- A 52-week, randomized, open-label, parallel-group, phase 3a trial with real-world elements was conducted at 176 sites in seven countries.
- A total of 1,085 insulin-naive patients with type 2 diabetes were randomly assigned to receive icodec with a dosing guide app or daily analogs (U100 glargine, U300 glargine, or icodec).
TAKEAWAY:
- A1c levels dropped from 8.96% at baseline to 7.24% at week 52 with icodec and from 8.88% to 7.61% with the daily analog, a treatment difference of 0.37 percentage point (P < .001 for noninferiority and P = .009 for superiority in favor of icodec plus the app).
- Patient-reported outcomes were more favorable with icodec plus the app vs. daily analogs, with estimated treatment differences that were significant for the Treatment Related Impact Measure for Diabetes (3.04) but not the Diabetes Treatment Satisfaction Questionnaire (0.78).
- Observed rates of combined clinically significant or severe hypoglycemia were low (0.19 event per patient-year of exposure for icodec plus the app vs. 0.14 for daily analogs; estimated rate ratio, 1.17).
IN PRACTICE:
“Once-weekly icodec with a dosing guide app could conceivably address several challenges seen in everyday practice, including inadequate dose titration and nonadherence to prescribed treatment regimens.”
SOURCE:
The study was conducted by Harpreet S. Bajaj, MD, MPH, of LMC Diabetes and Endocrinology, Brampton, Ontario, and colleagues. It was published online in Annals of Internal Medicine.
LIMITATIONS:
The research could not differentiate between the effects of icodec and those of the dosing guide app. The study had an open-label design. A 1-year duration is insufficient to assess long-term diabetes- and cardiovascular-related outcomes.
DISCLOSURES:
The study was funded by Novo Nordisk A/S.
A version of this article appeared on Medscape.com.
FROM ANNALS OF INTERNAL MEDICINE
Don’t miss type 1 diabetes in adults
Approximately 4 in 10 cases of type 1 diabetes in adults are diagnosed at age 30 years and older, based on data from nearly 1,000 individuals.
New-onset type 1 diabetes in adults is often misdiagnosed as type 2 diabetes, which may lead to inappropriate care, wrote Michael Fang, PhD, of Johns Hopkins University, Baltimore, and colleagues.
Previous research suggests that more than half of type 1 diabetes cases develop in adults, but data on variations in clinical characteristics and age at diagnosis are limited, the researchers said. “Clarifying the burden of adult-onset type 1 diabetes in the general population may help reduce misdiagnosis.”
In a study published in Annals of Internal Medicine, the researchers identified 947 adults aged 18 years and older with newly diagnosed type 1 diabetes, by using data from the National Health Interview Survey between 2016 and 2022. The subjects’ mean age at the time of the survey was 49 years and 48% were women. The racial/ethnic distribution was 73% non-Hispanic White, 10% non-Hispanic Black, 12% Hispanic, 3%, non-Hispanic Asian, and 3% other race/ethnicity.
Overall, 37% of participants were diagnosed with type 1 diabetes after age 30 years, with an overall median age at diagnosis of 24 years.
Type 1 diabetes was diagnosed later in men than in women, at a median age of 27 years vs. 22 years, respectively, and later in racial/ethnic minorities than in non-Hispanic Whites, with a median age of 26-30 years versus 21 years, respectively.
Autoantibody and C-peptide tests are recommended to confirm type 1 diabetes in adults with a suspected diagnosis, but the best method to identify high-risk adults remains unclear, the researchers wrote in their discussion.
“Traditional markers used to differentiate type 1 and type 2 diabetes, such as body mass index, may have limited utility, especially because obesity is now common in the type 1 diabetes population,” they said. New tools combining clinical features and biomarkers may improve accuracy of diagnosis of type 1 diabetes in the adult population, but more research is needed.
The findings were limited by several factors including misclassification based on self-reports of diagnosis and age, the researchers noted. Other limitations included lack of data on diagnostic measures such as levels of autoantibodies, C-peptides, and other indicators of diabetes, as well as inexact subgroup estimates because of small sample sizes.
“We extended existing research by characterizing the age at diagnosis in a nationally representative sample and by documenting variation across race/ethnicity and clinical characteristics,” they said.
The study was supported by grants from the National Heart, Lung, and Blood Institute. The lead authors had no financial conflicts to disclose. Corresponding author Elizabeth Selvin, PhD, disclosed grants from NIH and FNIH, personal fees from Novo Nordisk, other financial relationships with Wolters Kluwer, and nonfinancial support from many pharmaceutical companies outside the current study; she also serves as deputy editor of Diabetes Care and a member of the editorial board of Diabetologia.
Approximately 4 in 10 cases of type 1 diabetes in adults are diagnosed at age 30 years and older, based on data from nearly 1,000 individuals.
New-onset type 1 diabetes in adults is often misdiagnosed as type 2 diabetes, which may lead to inappropriate care, wrote Michael Fang, PhD, of Johns Hopkins University, Baltimore, and colleagues.
Previous research suggests that more than half of type 1 diabetes cases develop in adults, but data on variations in clinical characteristics and age at diagnosis are limited, the researchers said. “Clarifying the burden of adult-onset type 1 diabetes in the general population may help reduce misdiagnosis.”
In a study published in Annals of Internal Medicine, the researchers identified 947 adults aged 18 years and older with newly diagnosed type 1 diabetes, by using data from the National Health Interview Survey between 2016 and 2022. The subjects’ mean age at the time of the survey was 49 years and 48% were women. The racial/ethnic distribution was 73% non-Hispanic White, 10% non-Hispanic Black, 12% Hispanic, 3%, non-Hispanic Asian, and 3% other race/ethnicity.
Overall, 37% of participants were diagnosed with type 1 diabetes after age 30 years, with an overall median age at diagnosis of 24 years.
Type 1 diabetes was diagnosed later in men than in women, at a median age of 27 years vs. 22 years, respectively, and later in racial/ethnic minorities than in non-Hispanic Whites, with a median age of 26-30 years versus 21 years, respectively.
Autoantibody and C-peptide tests are recommended to confirm type 1 diabetes in adults with a suspected diagnosis, but the best method to identify high-risk adults remains unclear, the researchers wrote in their discussion.
“Traditional markers used to differentiate type 1 and type 2 diabetes, such as body mass index, may have limited utility, especially because obesity is now common in the type 1 diabetes population,” they said. New tools combining clinical features and biomarkers may improve accuracy of diagnosis of type 1 diabetes in the adult population, but more research is needed.
The findings were limited by several factors including misclassification based on self-reports of diagnosis and age, the researchers noted. Other limitations included lack of data on diagnostic measures such as levels of autoantibodies, C-peptides, and other indicators of diabetes, as well as inexact subgroup estimates because of small sample sizes.
“We extended existing research by characterizing the age at diagnosis in a nationally representative sample and by documenting variation across race/ethnicity and clinical characteristics,” they said.
The study was supported by grants from the National Heart, Lung, and Blood Institute. The lead authors had no financial conflicts to disclose. Corresponding author Elizabeth Selvin, PhD, disclosed grants from NIH and FNIH, personal fees from Novo Nordisk, other financial relationships with Wolters Kluwer, and nonfinancial support from many pharmaceutical companies outside the current study; she also serves as deputy editor of Diabetes Care and a member of the editorial board of Diabetologia.
Approximately 4 in 10 cases of type 1 diabetes in adults are diagnosed at age 30 years and older, based on data from nearly 1,000 individuals.
New-onset type 1 diabetes in adults is often misdiagnosed as type 2 diabetes, which may lead to inappropriate care, wrote Michael Fang, PhD, of Johns Hopkins University, Baltimore, and colleagues.
Previous research suggests that more than half of type 1 diabetes cases develop in adults, but data on variations in clinical characteristics and age at diagnosis are limited, the researchers said. “Clarifying the burden of adult-onset type 1 diabetes in the general population may help reduce misdiagnosis.”
In a study published in Annals of Internal Medicine, the researchers identified 947 adults aged 18 years and older with newly diagnosed type 1 diabetes, by using data from the National Health Interview Survey between 2016 and 2022. The subjects’ mean age at the time of the survey was 49 years and 48% were women. The racial/ethnic distribution was 73% non-Hispanic White, 10% non-Hispanic Black, 12% Hispanic, 3%, non-Hispanic Asian, and 3% other race/ethnicity.
Overall, 37% of participants were diagnosed with type 1 diabetes after age 30 years, with an overall median age at diagnosis of 24 years.
Type 1 diabetes was diagnosed later in men than in women, at a median age of 27 years vs. 22 years, respectively, and later in racial/ethnic minorities than in non-Hispanic Whites, with a median age of 26-30 years versus 21 years, respectively.
Autoantibody and C-peptide tests are recommended to confirm type 1 diabetes in adults with a suspected diagnosis, but the best method to identify high-risk adults remains unclear, the researchers wrote in their discussion.
“Traditional markers used to differentiate type 1 and type 2 diabetes, such as body mass index, may have limited utility, especially because obesity is now common in the type 1 diabetes population,” they said. New tools combining clinical features and biomarkers may improve accuracy of diagnosis of type 1 diabetes in the adult population, but more research is needed.
The findings were limited by several factors including misclassification based on self-reports of diagnosis and age, the researchers noted. Other limitations included lack of data on diagnostic measures such as levels of autoantibodies, C-peptides, and other indicators of diabetes, as well as inexact subgroup estimates because of small sample sizes.
“We extended existing research by characterizing the age at diagnosis in a nationally representative sample and by documenting variation across race/ethnicity and clinical characteristics,” they said.
The study was supported by grants from the National Heart, Lung, and Blood Institute. The lead authors had no financial conflicts to disclose. Corresponding author Elizabeth Selvin, PhD, disclosed grants from NIH and FNIH, personal fees from Novo Nordisk, other financial relationships with Wolters Kluwer, and nonfinancial support from many pharmaceutical companies outside the current study; she also serves as deputy editor of Diabetes Care and a member of the editorial board of Diabetologia.
FROM ANNALS OF INTERNAL MEDICINE
The ‘triple-G’ agonist for obesity management: Five things to know
The complex pathophysiology of obesity requires a multidisciplinary approach that includes lifestyle and medical interventions for successful management. Antiobesity medications (AOMs) have emerged as a powerful and life-changing tool for many individuals with obesity who are unable to sustain long-term weight loss through lifestyle changes alone. As with other chronic diseases such as hypertension and hyperlipidemia, the goal of decades of research has been to develop antiobesity medications with long-term efficacy and safety. Recent groundbreaking findings from a phase 2 trial show immense potential for a new AOM.
1. Gut hormone physiology informs the development of AOMs.
The three hormones associated with obesity or diabetes are glucagonlike peptide 1 (GLP-1), glucose-dependent insulinotropic peptide (GIP), and glucagon. GLP-1, a peptide released from the intestines in response to food ingestion, increases insulin production, reduces gut motility, and suppresses appetite. GIP is also an intestinal hormone that increases meal-stimulated insulin production and additionally facilitates lipolysis. Glucagon is known to increase hepatic glucose output but will also increase insulin secretion in the setting of hyperglycemia. Glucagon also promotes lipolysis.
Though these hormones are more commonly thought of as incretins, gut hormones that stimulate postprandial insulin secretion, their role in energy physiology is more diverse. Because of multiple mechanisms of action, incretins are increasingly referred to as nutrient-stimulated hormones (NuSH), a term which encompasses other peptides with therapeutic potential (e.g., amylin, oxyntomodulin, peptide tyrosine–tyrosine).
2. Studies have shown that NuSH therapies are highly effective AOMs.
In 2021 the Food and Drug Administration approved subcutaneous semaglutide 2.4 mg, a GLP-1 receptor agonist, for the treatment of obesity. Clinical trials demonstrating an average weight loss of 15% in patients taking semaglutide ushered in a new era of AOMs associated with significant weight loss that not only improve disease activity but also have the potential to achieve diabetes remission. Recent findings from the OASIS I trial demonstrated an average weight loss of 15.1% from baseline in patients treated with oral semaglutide for 68 weeks. Medical societies, including the American Diabetes Association and the American Association for the Study of Liver Diseases, recommend 10%-15% weight loss to fully treat weight-related comorbidities like type 2 diabetes and nonalcoholic fatty liver disease. In 2022, tirzepatide, a dual GLP-1 and GIP receptor agonist, demonstrated an average weight loss of 22.5% in phase 3 of the SURMOUNT-1 trial for obesity – a weight loss approaching that of some bariatric surgeries.
3. Clinical trial data show that the novel triple agonist retatrutide induces significant weight loss.
Preclinical studies on the newest NuSH therapy, triple GLP-1–GIP–glucagon receptor agonist retatrutide, showed predominant activity at the GIP receptor, with less GLP-1– and glucagon-receptor agonism than that of endogenous GLP-1 and GIP. Results from a phase 2 trial published in June 2023 showed a weight loss of 24% at 48 weeks in adults with obesity treated with retatrutide, which is the greatest weight loss reported in an obesity trial so far. Moreover, for the first time in obesity pharmacotherapy research, 100% of participants achieved clinically significant weight loss (defined as ≥ 5% of baseline weight).
4. Retatrutide may improve lipid metabolism.
In the phase 2 trial, retatrutide reduced low-density lipoprotein cholesterol levels by approximately 20%. This degree of reduced plasma LDL-C is dramatic in weight loss studies. Typically, weight loss significantly reduces triglyceride levels, increases high-density lipoprotein cholesterol levels, and has a modest effect on LDL-C reduction of about 5%.
A 20% reduction in LDL-C with retatrutide is hypothesis generating. Preclinical studies have shown glucagon to be an important regulator of proprotein convertase subtilisin/kexin type 9 degradation, with the lack of glucagon resulting in increased PCSK9 levels, decreased LDL receptors, and increased plasma LDL; conversely, treatment with glucagon decreased plasma LDL.
5. The long-term safety of retatrutide still needs to be determined.
In the 48-week phase 2 trial, retatrutide was observed to have a side-effect profile largely similar to other NuSH therapies (e.g., semaglutide 2.4 mg, tirzepatide), with a predominance of gastrointestinal symptoms including nausea, diarrhea, vomiting, and constipation. However, side effects potentially unique to retatrutide also emerged. Cutaneous hyperesthesia and skin sensitivity were reported in 7% of participants in the retatrutide group vs. 1% in the placebo group; none of these effects were associated with physical skin findings. Of note, 17 out of 198 (9%) participants in the retatrutide group developed cardiac arrhythmia vs. two out of 70 (3%) in the placebo group. There was no consistent pattern of arrhythmia type (e.g., supraventricular, ventricular) observed, and some of these events were reported as “palpitations” or “increased heart rate” without further detail. Phase 3 clinical trial data will provide further insight into the long-term safety of retatrutide.
Dr. Tchang is assistant professor of clinical medicine, division of endocrinology, Weill Cornell Medicine and physician, department of medicine, New York-Presbyterian/Weill Cornell Medical Center, both in New York. She has disclosed ties with Gelesis and Novo Nordisk.
A version of this article appeared on Medscape.com.
The complex pathophysiology of obesity requires a multidisciplinary approach that includes lifestyle and medical interventions for successful management. Antiobesity medications (AOMs) have emerged as a powerful and life-changing tool for many individuals with obesity who are unable to sustain long-term weight loss through lifestyle changes alone. As with other chronic diseases such as hypertension and hyperlipidemia, the goal of decades of research has been to develop antiobesity medications with long-term efficacy and safety. Recent groundbreaking findings from a phase 2 trial show immense potential for a new AOM.
1. Gut hormone physiology informs the development of AOMs.
The three hormones associated with obesity or diabetes are glucagonlike peptide 1 (GLP-1), glucose-dependent insulinotropic peptide (GIP), and glucagon. GLP-1, a peptide released from the intestines in response to food ingestion, increases insulin production, reduces gut motility, and suppresses appetite. GIP is also an intestinal hormone that increases meal-stimulated insulin production and additionally facilitates lipolysis. Glucagon is known to increase hepatic glucose output but will also increase insulin secretion in the setting of hyperglycemia. Glucagon also promotes lipolysis.
Though these hormones are more commonly thought of as incretins, gut hormones that stimulate postprandial insulin secretion, their role in energy physiology is more diverse. Because of multiple mechanisms of action, incretins are increasingly referred to as nutrient-stimulated hormones (NuSH), a term which encompasses other peptides with therapeutic potential (e.g., amylin, oxyntomodulin, peptide tyrosine–tyrosine).
2. Studies have shown that NuSH therapies are highly effective AOMs.
In 2021 the Food and Drug Administration approved subcutaneous semaglutide 2.4 mg, a GLP-1 receptor agonist, for the treatment of obesity. Clinical trials demonstrating an average weight loss of 15% in patients taking semaglutide ushered in a new era of AOMs associated with significant weight loss that not only improve disease activity but also have the potential to achieve diabetes remission. Recent findings from the OASIS I trial demonstrated an average weight loss of 15.1% from baseline in patients treated with oral semaglutide for 68 weeks. Medical societies, including the American Diabetes Association and the American Association for the Study of Liver Diseases, recommend 10%-15% weight loss to fully treat weight-related comorbidities like type 2 diabetes and nonalcoholic fatty liver disease. In 2022, tirzepatide, a dual GLP-1 and GIP receptor agonist, demonstrated an average weight loss of 22.5% in phase 3 of the SURMOUNT-1 trial for obesity – a weight loss approaching that of some bariatric surgeries.
3. Clinical trial data show that the novel triple agonist retatrutide induces significant weight loss.
Preclinical studies on the newest NuSH therapy, triple GLP-1–GIP–glucagon receptor agonist retatrutide, showed predominant activity at the GIP receptor, with less GLP-1– and glucagon-receptor agonism than that of endogenous GLP-1 and GIP. Results from a phase 2 trial published in June 2023 showed a weight loss of 24% at 48 weeks in adults with obesity treated with retatrutide, which is the greatest weight loss reported in an obesity trial so far. Moreover, for the first time in obesity pharmacotherapy research, 100% of participants achieved clinically significant weight loss (defined as ≥ 5% of baseline weight).
4. Retatrutide may improve lipid metabolism.
In the phase 2 trial, retatrutide reduced low-density lipoprotein cholesterol levels by approximately 20%. This degree of reduced plasma LDL-C is dramatic in weight loss studies. Typically, weight loss significantly reduces triglyceride levels, increases high-density lipoprotein cholesterol levels, and has a modest effect on LDL-C reduction of about 5%.
A 20% reduction in LDL-C with retatrutide is hypothesis generating. Preclinical studies have shown glucagon to be an important regulator of proprotein convertase subtilisin/kexin type 9 degradation, with the lack of glucagon resulting in increased PCSK9 levels, decreased LDL receptors, and increased plasma LDL; conversely, treatment with glucagon decreased plasma LDL.
5. The long-term safety of retatrutide still needs to be determined.
In the 48-week phase 2 trial, retatrutide was observed to have a side-effect profile largely similar to other NuSH therapies (e.g., semaglutide 2.4 mg, tirzepatide), with a predominance of gastrointestinal symptoms including nausea, diarrhea, vomiting, and constipation. However, side effects potentially unique to retatrutide also emerged. Cutaneous hyperesthesia and skin sensitivity were reported in 7% of participants in the retatrutide group vs. 1% in the placebo group; none of these effects were associated with physical skin findings. Of note, 17 out of 198 (9%) participants in the retatrutide group developed cardiac arrhythmia vs. two out of 70 (3%) in the placebo group. There was no consistent pattern of arrhythmia type (e.g., supraventricular, ventricular) observed, and some of these events were reported as “palpitations” or “increased heart rate” without further detail. Phase 3 clinical trial data will provide further insight into the long-term safety of retatrutide.
Dr. Tchang is assistant professor of clinical medicine, division of endocrinology, Weill Cornell Medicine and physician, department of medicine, New York-Presbyterian/Weill Cornell Medical Center, both in New York. She has disclosed ties with Gelesis and Novo Nordisk.
A version of this article appeared on Medscape.com.
The complex pathophysiology of obesity requires a multidisciplinary approach that includes lifestyle and medical interventions for successful management. Antiobesity medications (AOMs) have emerged as a powerful and life-changing tool for many individuals with obesity who are unable to sustain long-term weight loss through lifestyle changes alone. As with other chronic diseases such as hypertension and hyperlipidemia, the goal of decades of research has been to develop antiobesity medications with long-term efficacy and safety. Recent groundbreaking findings from a phase 2 trial show immense potential for a new AOM.
1. Gut hormone physiology informs the development of AOMs.
The three hormones associated with obesity or diabetes are glucagonlike peptide 1 (GLP-1), glucose-dependent insulinotropic peptide (GIP), and glucagon. GLP-1, a peptide released from the intestines in response to food ingestion, increases insulin production, reduces gut motility, and suppresses appetite. GIP is also an intestinal hormone that increases meal-stimulated insulin production and additionally facilitates lipolysis. Glucagon is known to increase hepatic glucose output but will also increase insulin secretion in the setting of hyperglycemia. Glucagon also promotes lipolysis.
Though these hormones are more commonly thought of as incretins, gut hormones that stimulate postprandial insulin secretion, their role in energy physiology is more diverse. Because of multiple mechanisms of action, incretins are increasingly referred to as nutrient-stimulated hormones (NuSH), a term which encompasses other peptides with therapeutic potential (e.g., amylin, oxyntomodulin, peptide tyrosine–tyrosine).
2. Studies have shown that NuSH therapies are highly effective AOMs.
In 2021 the Food and Drug Administration approved subcutaneous semaglutide 2.4 mg, a GLP-1 receptor agonist, for the treatment of obesity. Clinical trials demonstrating an average weight loss of 15% in patients taking semaglutide ushered in a new era of AOMs associated with significant weight loss that not only improve disease activity but also have the potential to achieve diabetes remission. Recent findings from the OASIS I trial demonstrated an average weight loss of 15.1% from baseline in patients treated with oral semaglutide for 68 weeks. Medical societies, including the American Diabetes Association and the American Association for the Study of Liver Diseases, recommend 10%-15% weight loss to fully treat weight-related comorbidities like type 2 diabetes and nonalcoholic fatty liver disease. In 2022, tirzepatide, a dual GLP-1 and GIP receptor agonist, demonstrated an average weight loss of 22.5% in phase 3 of the SURMOUNT-1 trial for obesity – a weight loss approaching that of some bariatric surgeries.
3. Clinical trial data show that the novel triple agonist retatrutide induces significant weight loss.
Preclinical studies on the newest NuSH therapy, triple GLP-1–GIP–glucagon receptor agonist retatrutide, showed predominant activity at the GIP receptor, with less GLP-1– and glucagon-receptor agonism than that of endogenous GLP-1 and GIP. Results from a phase 2 trial published in June 2023 showed a weight loss of 24% at 48 weeks in adults with obesity treated with retatrutide, which is the greatest weight loss reported in an obesity trial so far. Moreover, for the first time in obesity pharmacotherapy research, 100% of participants achieved clinically significant weight loss (defined as ≥ 5% of baseline weight).
4. Retatrutide may improve lipid metabolism.
In the phase 2 trial, retatrutide reduced low-density lipoprotein cholesterol levels by approximately 20%. This degree of reduced plasma LDL-C is dramatic in weight loss studies. Typically, weight loss significantly reduces triglyceride levels, increases high-density lipoprotein cholesterol levels, and has a modest effect on LDL-C reduction of about 5%.
A 20% reduction in LDL-C with retatrutide is hypothesis generating. Preclinical studies have shown glucagon to be an important regulator of proprotein convertase subtilisin/kexin type 9 degradation, with the lack of glucagon resulting in increased PCSK9 levels, decreased LDL receptors, and increased plasma LDL; conversely, treatment with glucagon decreased plasma LDL.
5. The long-term safety of retatrutide still needs to be determined.
In the 48-week phase 2 trial, retatrutide was observed to have a side-effect profile largely similar to other NuSH therapies (e.g., semaglutide 2.4 mg, tirzepatide), with a predominance of gastrointestinal symptoms including nausea, diarrhea, vomiting, and constipation. However, side effects potentially unique to retatrutide also emerged. Cutaneous hyperesthesia and skin sensitivity were reported in 7% of participants in the retatrutide group vs. 1% in the placebo group; none of these effects were associated with physical skin findings. Of note, 17 out of 198 (9%) participants in the retatrutide group developed cardiac arrhythmia vs. two out of 70 (3%) in the placebo group. There was no consistent pattern of arrhythmia type (e.g., supraventricular, ventricular) observed, and some of these events were reported as “palpitations” or “increased heart rate” without further detail. Phase 3 clinical trial data will provide further insight into the long-term safety of retatrutide.
Dr. Tchang is assistant professor of clinical medicine, division of endocrinology, Weill Cornell Medicine and physician, department of medicine, New York-Presbyterian/Weill Cornell Medical Center, both in New York. She has disclosed ties with Gelesis and Novo Nordisk.
A version of this article appeared on Medscape.com.
Paxlovid weaker against current COVID-19 variants
But when looking at death alone, the antiviral was still highly effective.
Paxlovid was about 37% effective at preventing death or hospitalization in high-risk patients, compared with no treatment. The study also looked at the antiviral Lagevrio, made by Merck, and found it was about 41% effective. In preventing death alone, Paxlovid was about 84% effective, compared with no treatment, and Lagevrio was about 77% effective.
The investigators, of the University of North Carolina at Chapel Hill and the Cleveland Clinic, examined electronic health records of 68,867 patients at hospitals in Cleveland and Florida who were diagnosed with COVID from April 1, 2022, to Feb. 20, 2023.
For Paxlovid, the effectiveness against death and hospitalization was lower than the effectiveness rate of about 86% found in clinical trials in 2021, according to Bloomberg.
The difference in effectiveness in the real-world and clinical studies may have occurred because the early studies were conducted with unvaccinated people. Also, the virus has evolved since those first studies, Bloomberg reported.
The researchers said Paxlovid and Lagevrio are recommended for use because they reduce hospitalization and death among high-risk patients who get COVID, even taking recent Omicron subvariants into account.
“These findings suggest that the use of either nirmatrelvir (Paxlovid) or molnupiravir (Lagevrio) is associated with reductions in mortality and hospitalization in patients infected with Omicron, regardless of age, race and ethnicity, virus strain, vaccination status, previous infection status, or coexisting conditions,” the researchers wrote. “Both drugs can, therefore, be used to treat nonhospitalized patients who are at high risk of progressing to severe COVID-19.”
Both drugs should be taken within 5 days of the onset of COVID symptoms.
The study was supported by the National Institutes of Health. Three coauthors reported conflicts of interest with various companies and organizations.
A version of this article first appeared on WebMD.com.
But when looking at death alone, the antiviral was still highly effective.
Paxlovid was about 37% effective at preventing death or hospitalization in high-risk patients, compared with no treatment. The study also looked at the antiviral Lagevrio, made by Merck, and found it was about 41% effective. In preventing death alone, Paxlovid was about 84% effective, compared with no treatment, and Lagevrio was about 77% effective.
The investigators, of the University of North Carolina at Chapel Hill and the Cleveland Clinic, examined electronic health records of 68,867 patients at hospitals in Cleveland and Florida who were diagnosed with COVID from April 1, 2022, to Feb. 20, 2023.
For Paxlovid, the effectiveness against death and hospitalization was lower than the effectiveness rate of about 86% found in clinical trials in 2021, according to Bloomberg.
The difference in effectiveness in the real-world and clinical studies may have occurred because the early studies were conducted with unvaccinated people. Also, the virus has evolved since those first studies, Bloomberg reported.
The researchers said Paxlovid and Lagevrio are recommended for use because they reduce hospitalization and death among high-risk patients who get COVID, even taking recent Omicron subvariants into account.
“These findings suggest that the use of either nirmatrelvir (Paxlovid) or molnupiravir (Lagevrio) is associated with reductions in mortality and hospitalization in patients infected with Omicron, regardless of age, race and ethnicity, virus strain, vaccination status, previous infection status, or coexisting conditions,” the researchers wrote. “Both drugs can, therefore, be used to treat nonhospitalized patients who are at high risk of progressing to severe COVID-19.”
Both drugs should be taken within 5 days of the onset of COVID symptoms.
The study was supported by the National Institutes of Health. Three coauthors reported conflicts of interest with various companies and organizations.
A version of this article first appeared on WebMD.com.
But when looking at death alone, the antiviral was still highly effective.
Paxlovid was about 37% effective at preventing death or hospitalization in high-risk patients, compared with no treatment. The study also looked at the antiviral Lagevrio, made by Merck, and found it was about 41% effective. In preventing death alone, Paxlovid was about 84% effective, compared with no treatment, and Lagevrio was about 77% effective.
The investigators, of the University of North Carolina at Chapel Hill and the Cleveland Clinic, examined electronic health records of 68,867 patients at hospitals in Cleveland and Florida who were diagnosed with COVID from April 1, 2022, to Feb. 20, 2023.
For Paxlovid, the effectiveness against death and hospitalization was lower than the effectiveness rate of about 86% found in clinical trials in 2021, according to Bloomberg.
The difference in effectiveness in the real-world and clinical studies may have occurred because the early studies were conducted with unvaccinated people. Also, the virus has evolved since those first studies, Bloomberg reported.
The researchers said Paxlovid and Lagevrio are recommended for use because they reduce hospitalization and death among high-risk patients who get COVID, even taking recent Omicron subvariants into account.
“These findings suggest that the use of either nirmatrelvir (Paxlovid) or molnupiravir (Lagevrio) is associated with reductions in mortality and hospitalization in patients infected with Omicron, regardless of age, race and ethnicity, virus strain, vaccination status, previous infection status, or coexisting conditions,” the researchers wrote. “Both drugs can, therefore, be used to treat nonhospitalized patients who are at high risk of progressing to severe COVID-19.”
Both drugs should be taken within 5 days of the onset of COVID symptoms.
The study was supported by the National Institutes of Health. Three coauthors reported conflicts of interest with various companies and organizations.
A version of this article first appeared on WebMD.com.
FROM JAMA NETWORK OPEN
Are vitamin D levels key to canagliflozin’s fracture risk?
Sodium-glucose cotransporter 2 (SGLT2) inhibitors are beneficial for treating type 2 diabetes and reducing cardiovascular and kidney disease risk. However, some, but not all, trial data have linked the SLGT2 inhibitor canagliflozin to increased fracture risk. That particular agent has been reported to accelerate loss of bone mineral density, which could contribute to fracture risk. Other drugs in the class have also been implicated in worsening markers of bone health.
The new findings, from a small study of Amish adults with vitamin D deficiency (≤ 20 ng/mL) but without diabetes or osteoporosis, suggest that physicians consider screening for vitamin D deficiency prior to prescribing SGLT2 inhibitor. Alternatively, these patients can simply be prescribed safe, inexpensive, OTC vitamin D supplements without being screening, Zhinous Shahidzadeh Yazdi, MD, of the division of endocrinology, diabetes, and nutrition at the University of Maryland, Baltimore, and colleagues wrote.
“Something as simple as OTC vitamin D might protect against bone fractures caused by chronic multiyear treatment with a drug,” study lead author Simeon I. Taylor, MD, PhD, professor of medicine at the University of Maryland, said in an interview.
In the study, published in the Journal of Clinical Endocrinology and Metabolism, 11 adults with vitamin D deficiency underwent two canagliflozin challenge protocols of 300 mg/d for 5 days, once before and once after vitamin D3 supplementation (either 50,000 IU per week or twice weekly for body mass index < 30 kg/m2 or ≥ 30 kg/m2, respectively), to achieve 25(OH)D of at least 30 ng/mL.
When the participants were vitamin D deficient, canagliflozin significantly decreased 1,25(OH)2D levels by 31.3%, from 43.8 pg/mL on day 1 to 29.1 pg/mL on day 3 (P = .0003). In contrast, after receiving the vitamin D3 supplements, canagliflozin reduced mean 1,25(OH)2D levels by a nonsignificant 9.3%, from 45 pg/mL on day 1 to 41 pg/mL on day 3 (P = .3).
“Thus, [vitamin D3] supplementation provided statistically significant protection from the adverse effect of canagliflozin to decrease mean plasma levels of 1,25(OH)2D (P = .04),” Yazdi and colleagues wrote.
Similarly, when the participants were vitamin D deficient, canagliflozin was associated with a significant 36.2% increase in mean parathyroid hormone (PTH) levels, from 47.5 pg/mL on day 1 to 58.5 pg/mL on day 6 (P = .0009). In contrast, after vitamin D3 supplementation, the increase in PTH was far less, from 48.4 pg/mL on day 1 to 53.3 pg/mL on day 6 (P = .02).
Therefore, the supplementation “significantly decreased the magnitude of the canagliflozin-induced increase in mean levels of PTH (P = .005),” they wrote.
Also, in the vitamin D deficient state, canagliflozin significantly increased mean serum phosphorous on day 3 in comparison with day 1 (P = .007), while after supplementation, that change was also insignificant (P = .8).
“We are saying that SGLT2 inhibitors, when superimposed on vitamin D deficiency, is bad for bone health. This group of people have two important risk factors – vitamin D deficiency and SGLT2 inhibitors – and are distinct from the general population of people who are not vitamin D deficient,” Dr. Taylor noted.
The findings “raise interesting questions about how to proceed,” he said in an interview, since “the gold standard study – in this case, a fracture prevention study – will never be done because it would cost more than $100 million. Vitamin D costs only $10-$20 per year, and at appropriate doses, is extremely safe. At worst, vitamin D supplements are unnecessary. At best, vitamin D supplements can protect some patients against a serious drug toxicity, bone fracture.”
The study was funded by the National Institutes of Health. Dr. Taylor serves as a consultant for Ionis Pharmaceuticals and receives an inventor’s share of royalties from the National Institute of Diabetes, Digestive, and Kidney Diseases for metreleptin as a treatment for generalized lipodystrophy. Dr. Yazdi disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Sodium-glucose cotransporter 2 (SGLT2) inhibitors are beneficial for treating type 2 diabetes and reducing cardiovascular and kidney disease risk. However, some, but not all, trial data have linked the SLGT2 inhibitor canagliflozin to increased fracture risk. That particular agent has been reported to accelerate loss of bone mineral density, which could contribute to fracture risk. Other drugs in the class have also been implicated in worsening markers of bone health.
The new findings, from a small study of Amish adults with vitamin D deficiency (≤ 20 ng/mL) but without diabetes or osteoporosis, suggest that physicians consider screening for vitamin D deficiency prior to prescribing SGLT2 inhibitor. Alternatively, these patients can simply be prescribed safe, inexpensive, OTC vitamin D supplements without being screening, Zhinous Shahidzadeh Yazdi, MD, of the division of endocrinology, diabetes, and nutrition at the University of Maryland, Baltimore, and colleagues wrote.
“Something as simple as OTC vitamin D might protect against bone fractures caused by chronic multiyear treatment with a drug,” study lead author Simeon I. Taylor, MD, PhD, professor of medicine at the University of Maryland, said in an interview.
In the study, published in the Journal of Clinical Endocrinology and Metabolism, 11 adults with vitamin D deficiency underwent two canagliflozin challenge protocols of 300 mg/d for 5 days, once before and once after vitamin D3 supplementation (either 50,000 IU per week or twice weekly for body mass index < 30 kg/m2 or ≥ 30 kg/m2, respectively), to achieve 25(OH)D of at least 30 ng/mL.
When the participants were vitamin D deficient, canagliflozin significantly decreased 1,25(OH)2D levels by 31.3%, from 43.8 pg/mL on day 1 to 29.1 pg/mL on day 3 (P = .0003). In contrast, after receiving the vitamin D3 supplements, canagliflozin reduced mean 1,25(OH)2D levels by a nonsignificant 9.3%, from 45 pg/mL on day 1 to 41 pg/mL on day 3 (P = .3).
“Thus, [vitamin D3] supplementation provided statistically significant protection from the adverse effect of canagliflozin to decrease mean plasma levels of 1,25(OH)2D (P = .04),” Yazdi and colleagues wrote.
Similarly, when the participants were vitamin D deficient, canagliflozin was associated with a significant 36.2% increase in mean parathyroid hormone (PTH) levels, from 47.5 pg/mL on day 1 to 58.5 pg/mL on day 6 (P = .0009). In contrast, after vitamin D3 supplementation, the increase in PTH was far less, from 48.4 pg/mL on day 1 to 53.3 pg/mL on day 6 (P = .02).
Therefore, the supplementation “significantly decreased the magnitude of the canagliflozin-induced increase in mean levels of PTH (P = .005),” they wrote.
Also, in the vitamin D deficient state, canagliflozin significantly increased mean serum phosphorous on day 3 in comparison with day 1 (P = .007), while after supplementation, that change was also insignificant (P = .8).
“We are saying that SGLT2 inhibitors, when superimposed on vitamin D deficiency, is bad for bone health. This group of people have two important risk factors – vitamin D deficiency and SGLT2 inhibitors – and are distinct from the general population of people who are not vitamin D deficient,” Dr. Taylor noted.
The findings “raise interesting questions about how to proceed,” he said in an interview, since “the gold standard study – in this case, a fracture prevention study – will never be done because it would cost more than $100 million. Vitamin D costs only $10-$20 per year, and at appropriate doses, is extremely safe. At worst, vitamin D supplements are unnecessary. At best, vitamin D supplements can protect some patients against a serious drug toxicity, bone fracture.”
The study was funded by the National Institutes of Health. Dr. Taylor serves as a consultant for Ionis Pharmaceuticals and receives an inventor’s share of royalties from the National Institute of Diabetes, Digestive, and Kidney Diseases for metreleptin as a treatment for generalized lipodystrophy. Dr. Yazdi disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Sodium-glucose cotransporter 2 (SGLT2) inhibitors are beneficial for treating type 2 diabetes and reducing cardiovascular and kidney disease risk. However, some, but not all, trial data have linked the SLGT2 inhibitor canagliflozin to increased fracture risk. That particular agent has been reported to accelerate loss of bone mineral density, which could contribute to fracture risk. Other drugs in the class have also been implicated in worsening markers of bone health.
The new findings, from a small study of Amish adults with vitamin D deficiency (≤ 20 ng/mL) but without diabetes or osteoporosis, suggest that physicians consider screening for vitamin D deficiency prior to prescribing SGLT2 inhibitor. Alternatively, these patients can simply be prescribed safe, inexpensive, OTC vitamin D supplements without being screening, Zhinous Shahidzadeh Yazdi, MD, of the division of endocrinology, diabetes, and nutrition at the University of Maryland, Baltimore, and colleagues wrote.
“Something as simple as OTC vitamin D might protect against bone fractures caused by chronic multiyear treatment with a drug,” study lead author Simeon I. Taylor, MD, PhD, professor of medicine at the University of Maryland, said in an interview.
In the study, published in the Journal of Clinical Endocrinology and Metabolism, 11 adults with vitamin D deficiency underwent two canagliflozin challenge protocols of 300 mg/d for 5 days, once before and once after vitamin D3 supplementation (either 50,000 IU per week or twice weekly for body mass index < 30 kg/m2 or ≥ 30 kg/m2, respectively), to achieve 25(OH)D of at least 30 ng/mL.
When the participants were vitamin D deficient, canagliflozin significantly decreased 1,25(OH)2D levels by 31.3%, from 43.8 pg/mL on day 1 to 29.1 pg/mL on day 3 (P = .0003). In contrast, after receiving the vitamin D3 supplements, canagliflozin reduced mean 1,25(OH)2D levels by a nonsignificant 9.3%, from 45 pg/mL on day 1 to 41 pg/mL on day 3 (P = .3).
“Thus, [vitamin D3] supplementation provided statistically significant protection from the adverse effect of canagliflozin to decrease mean plasma levels of 1,25(OH)2D (P = .04),” Yazdi and colleagues wrote.
Similarly, when the participants were vitamin D deficient, canagliflozin was associated with a significant 36.2% increase in mean parathyroid hormone (PTH) levels, from 47.5 pg/mL on day 1 to 58.5 pg/mL on day 6 (P = .0009). In contrast, after vitamin D3 supplementation, the increase in PTH was far less, from 48.4 pg/mL on day 1 to 53.3 pg/mL on day 6 (P = .02).
Therefore, the supplementation “significantly decreased the magnitude of the canagliflozin-induced increase in mean levels of PTH (P = .005),” they wrote.
Also, in the vitamin D deficient state, canagliflozin significantly increased mean serum phosphorous on day 3 in comparison with day 1 (P = .007), while after supplementation, that change was also insignificant (P = .8).
“We are saying that SGLT2 inhibitors, when superimposed on vitamin D deficiency, is bad for bone health. This group of people have two important risk factors – vitamin D deficiency and SGLT2 inhibitors – and are distinct from the general population of people who are not vitamin D deficient,” Dr. Taylor noted.
The findings “raise interesting questions about how to proceed,” he said in an interview, since “the gold standard study – in this case, a fracture prevention study – will never be done because it would cost more than $100 million. Vitamin D costs only $10-$20 per year, and at appropriate doses, is extremely safe. At worst, vitamin D supplements are unnecessary. At best, vitamin D supplements can protect some patients against a serious drug toxicity, bone fracture.”
The study was funded by the National Institutes of Health. Dr. Taylor serves as a consultant for Ionis Pharmaceuticals and receives an inventor’s share of royalties from the National Institute of Diabetes, Digestive, and Kidney Diseases for metreleptin as a treatment for generalized lipodystrophy. Dr. Yazdi disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM THE JOURNAL OF CLINICAL ENDOCRINOLOGY AND METABOLISM