Psoriasis is an inflammatory autoimmune skin condition that affects approximately 125 million individuals worldwide, with approximately 8 million patients in the United States.1 Psoriasis not only involves a cosmetic component but also comprises other comorbidities, such as psoriatic arthritis, cardiovascular disease, and psychiatric disorders, that can influence patient quality of life.2-4 In addition, the costs associated with psoriasis are substantial, with an estimated economic burden of $35.2 billion in the United States in 2015.5 Given the prevalence of psoriasis and its many effects on patients, it is important that providers have high-quality evidence regarding efficacious treatment options.
Systematic reviews, which compile all available evidence on a subject to answer a specific question, represent the gold standard of research.6 However, studies have demonstrated that when referencing research literature, physicians tend to read only the abstract of a study rather than the entire article.7,8 A study by Marcelo et al8 showed that residents at a tertiary care center answered clinical questions using only the abstract of a paper 69% of the time. Based on these findings, it is imperative that the results of systematic reviews be accurately reported in their abstracts because they can influence patient care.
Referencing only the abstracts of systematic reviews can be problematic if the abstract contains spin. Spin is a form of reporting that inappropriately highlights the benefits of a treatment with greater emphasis than what is shown by the results.9 Research has identified the presence of spin in the abstracts of randomized controlled trials.10-12 For example, Cooper et al10 found that 70% (33/47) of abstracts in otolaryngology randomized controlled trials contained spin. Additionally, Arthur et al11 and Austin et al12 had similar findings within abstracts of orthopedic and obesity trials, where 44.8% (112/250) and 46.7% (21/45) contained spin, respectively. Ottwell et al13 found that the presence of spin in abstracts is not limited to randomized controlled trials; they demonstrated that the abstracts of nearly one-third (31% [11/36]) of systematic reviews focused on the treatment of acne vulgaris contained spin.
In our study, we aimed to evaluate the presence of spin in the abstracts of systematic reviews focused on the treatment of psoriasis.
Methods
Reproducibility and Reporting—Our study did not meet the regulatory definition for human subjects research per the US Code of Federal Regulations because the study did not involve human research subjects. The study also was not subject to review by the institutional review board. Our protocol, data set, analysis scripts, extraction forms, and other material related to the study have been placed on Open Science Framework to provide transparency and ensure reproducibility. To further allow for analytic reproducibility, our data set was given to an independent laboratory and reanalyzed with a masked approach. Our study was carried out alongside other studies assessing spin in systematic reviews regarding different specialties and disease states. Because these studies were similar in design, this methodology also has been reported elsewhere. The Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA)14 and the guidelines for meta-epidemiological studies developed by Murad and Wang15 were used in drafting this article.
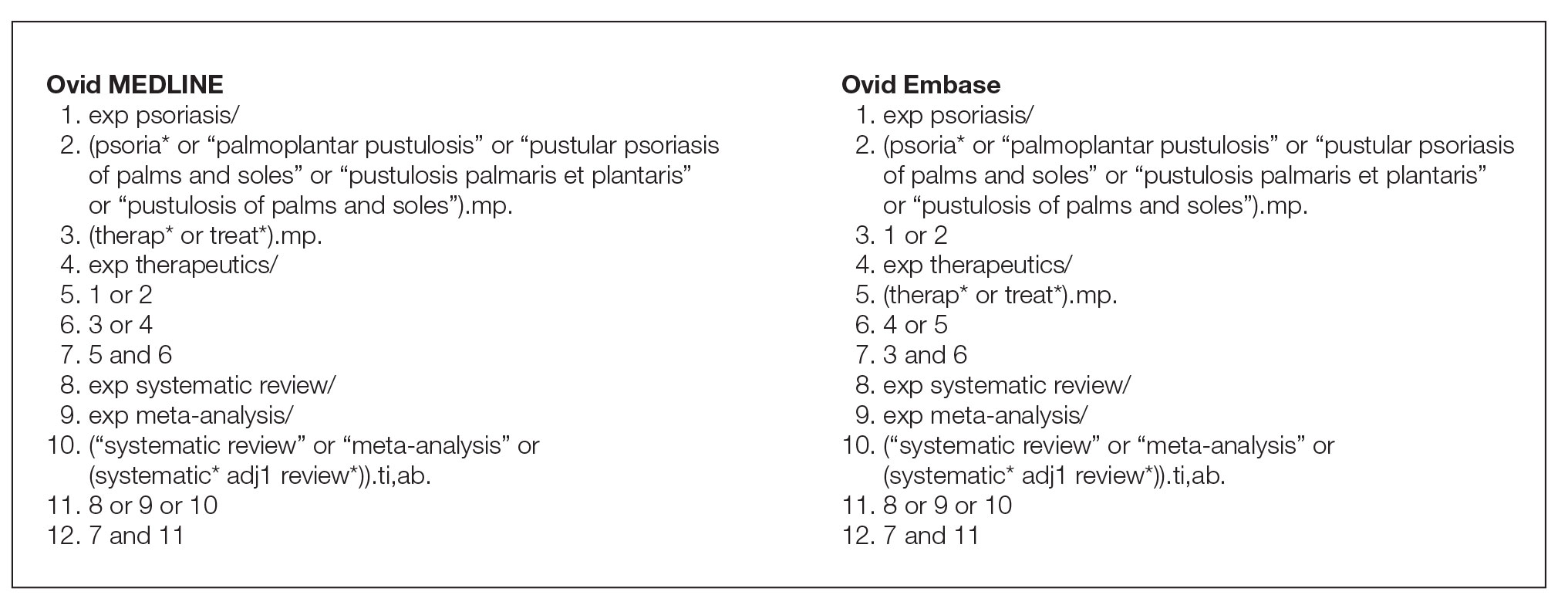
Search Strategy—The search strategies for the MEDLINE (Ovid) and Embase (Ovid) databases were created by a systematic review librarian (D.N.W.) to identify systematic reviews and meta-analyses regarding treatments for psoriasis (Figure 1). The searches were performed on June 2, 2020, and uploaded to Rayyan, a systematic review screening platform.16 After duplicates were removed, the records were screened for eligibility by 2 authors (C.H. and A.L.) using the titles and abstracts. Screening was conducted independently while each of these authors was masked to the other’s results; disagreements were resolved through discussion.
Eligibility Criteria—An article had to meet the following criteria for inclusion in our study: (1) be a systematic review with or without a meta-analysis; (2) relate to the treatment of psoriasis; and (3) be written in English and include human patients only. The PRISMA definition of systematic reviews and meta-analyses was applied.17