User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
R-CHOP and intensive R-HDS comparable in DLBCL
Two front-line treatment regimens for patients with high-risk diffuse large B-cell lymphomas (DLBCL) produced comparable outcomes, according to new data.
Patients who received rituximab combined with high-dose sequential chemotherapy (R-HDS) plus autologous stem-cell transplantation (ASCT) had similar results in terms of overall response rate and long-term outcomes, compared to patients treated with rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone (R-CHOP).
It was presumed that event-free survival would be improved with chemotherapy and ASCT, but that result was not observed at 3-year follow up, wrote Sergio Cortelazzo, MD, of Humanitas Gavazzeni, Bergamo, Italy, and coauthors.
“Our results indicate that CHOP chemotherapy, optimally supplemented by eight doses of rituximab, remains the standard of care also for this group of patients at higher risk for disease resistance or recurrence,” they wrote in a study published online ahead of print in the Journal of Clinical Oncology (J Clin Oncol. 2016 Oct 3. doi: 10.1200/JCO.2016.67.2980).
The benefit of R-HDS chemotherapy with ASCT as front-line therapy for this patient population is still a matter of debate. To address that issue, Dr. Cortelazzo and his colleagues conducted a phase III randomized trial in which 246 high-risk patients with a high-intermediate (56%) or high (44%) International Prognostic Index (IPI) score were assigned to receive either R-CHOP or R-HDS.
The primary efficacy endpoint was 3-year, event-free survival, and the results were analyzed on an intent-to-treat basis.
At a median follow-up of 5 years (range, 0.05-9.49), with an intent-to-treat analysis, the 3-year, event-free survival was 62% (95% CI, 54%-71%) for the R-CHOP arm, compared with 65% (95% CI, 56% to 74%) for those treated with R-HDS (P = .83; hazard ratio, 0.99; 95% CI, 0.66-1.48).
There was no difference in event-free survival even when analyzed within the IPI subgroups.
The 3-year progression free survival also did not significantly differ between groups; 65% in the R-CHOP arm (95% CI, 57% to 74%) versus 75% (95% CI, 67%-83%; P = .119) for the R-HDS arm in the whole population, as well as within IPI subgroups.
Of note, the 3-year disease-free survival was better in the R-HDS group (79% vs. 91%, respectively; P = .034), but this difference subsequently disappeared with longer follow-up.
Grade 3-4 hematologic toxicity was lower in the R-CHOP arm compared with the R-HDS arm, with at least one episode of neutropenia in 34% versus 84% of patients (P less than .001), anemia in 15% versus 71% of patients (P less than .001), and thrombocytopenia in 5% versus 86% (P less than .001).
The study was supported in part by the Associazione Italiana Lotta alla Leucemia sezione di Bergamo, the Associazione Italiana per la Ricerca sul Cancro, Ministero Istruzione, Universita e Ricerca and unrestricted grants from Roche SpA and Amgen, Italy. Dr Cortelazzo had no relevant disclosures. Several coauthors indicated relationships with industry.
Two front-line treatment regimens for patients with high-risk diffuse large B-cell lymphomas (DLBCL) produced comparable outcomes, according to new data.
Patients who received rituximab combined with high-dose sequential chemotherapy (R-HDS) plus autologous stem-cell transplantation (ASCT) had similar results in terms of overall response rate and long-term outcomes, compared to patients treated with rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone (R-CHOP).
It was presumed that event-free survival would be improved with chemotherapy and ASCT, but that result was not observed at 3-year follow up, wrote Sergio Cortelazzo, MD, of Humanitas Gavazzeni, Bergamo, Italy, and coauthors.
“Our results indicate that CHOP chemotherapy, optimally supplemented by eight doses of rituximab, remains the standard of care also for this group of patients at higher risk for disease resistance or recurrence,” they wrote in a study published online ahead of print in the Journal of Clinical Oncology (J Clin Oncol. 2016 Oct 3. doi: 10.1200/JCO.2016.67.2980).
The benefit of R-HDS chemotherapy with ASCT as front-line therapy for this patient population is still a matter of debate. To address that issue, Dr. Cortelazzo and his colleagues conducted a phase III randomized trial in which 246 high-risk patients with a high-intermediate (56%) or high (44%) International Prognostic Index (IPI) score were assigned to receive either R-CHOP or R-HDS.
The primary efficacy endpoint was 3-year, event-free survival, and the results were analyzed on an intent-to-treat basis.
At a median follow-up of 5 years (range, 0.05-9.49), with an intent-to-treat analysis, the 3-year, event-free survival was 62% (95% CI, 54%-71%) for the R-CHOP arm, compared with 65% (95% CI, 56% to 74%) for those treated with R-HDS (P = .83; hazard ratio, 0.99; 95% CI, 0.66-1.48).
There was no difference in event-free survival even when analyzed within the IPI subgroups.
The 3-year progression free survival also did not significantly differ between groups; 65% in the R-CHOP arm (95% CI, 57% to 74%) versus 75% (95% CI, 67%-83%; P = .119) for the R-HDS arm in the whole population, as well as within IPI subgroups.
Of note, the 3-year disease-free survival was better in the R-HDS group (79% vs. 91%, respectively; P = .034), but this difference subsequently disappeared with longer follow-up.
Grade 3-4 hematologic toxicity was lower in the R-CHOP arm compared with the R-HDS arm, with at least one episode of neutropenia in 34% versus 84% of patients (P less than .001), anemia in 15% versus 71% of patients (P less than .001), and thrombocytopenia in 5% versus 86% (P less than .001).
The study was supported in part by the Associazione Italiana Lotta alla Leucemia sezione di Bergamo, the Associazione Italiana per la Ricerca sul Cancro, Ministero Istruzione, Universita e Ricerca and unrestricted grants from Roche SpA and Amgen, Italy. Dr Cortelazzo had no relevant disclosures. Several coauthors indicated relationships with industry.
Two front-line treatment regimens for patients with high-risk diffuse large B-cell lymphomas (DLBCL) produced comparable outcomes, according to new data.
Patients who received rituximab combined with high-dose sequential chemotherapy (R-HDS) plus autologous stem-cell transplantation (ASCT) had similar results in terms of overall response rate and long-term outcomes, compared to patients treated with rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone (R-CHOP).
It was presumed that event-free survival would be improved with chemotherapy and ASCT, but that result was not observed at 3-year follow up, wrote Sergio Cortelazzo, MD, of Humanitas Gavazzeni, Bergamo, Italy, and coauthors.
“Our results indicate that CHOP chemotherapy, optimally supplemented by eight doses of rituximab, remains the standard of care also for this group of patients at higher risk for disease resistance or recurrence,” they wrote in a study published online ahead of print in the Journal of Clinical Oncology (J Clin Oncol. 2016 Oct 3. doi: 10.1200/JCO.2016.67.2980).
The benefit of R-HDS chemotherapy with ASCT as front-line therapy for this patient population is still a matter of debate. To address that issue, Dr. Cortelazzo and his colleagues conducted a phase III randomized trial in which 246 high-risk patients with a high-intermediate (56%) or high (44%) International Prognostic Index (IPI) score were assigned to receive either R-CHOP or R-HDS.
The primary efficacy endpoint was 3-year, event-free survival, and the results were analyzed on an intent-to-treat basis.
At a median follow-up of 5 years (range, 0.05-9.49), with an intent-to-treat analysis, the 3-year, event-free survival was 62% (95% CI, 54%-71%) for the R-CHOP arm, compared with 65% (95% CI, 56% to 74%) for those treated with R-HDS (P = .83; hazard ratio, 0.99; 95% CI, 0.66-1.48).
There was no difference in event-free survival even when analyzed within the IPI subgroups.
The 3-year progression free survival also did not significantly differ between groups; 65% in the R-CHOP arm (95% CI, 57% to 74%) versus 75% (95% CI, 67%-83%; P = .119) for the R-HDS arm in the whole population, as well as within IPI subgroups.
Of note, the 3-year disease-free survival was better in the R-HDS group (79% vs. 91%, respectively; P = .034), but this difference subsequently disappeared with longer follow-up.
Grade 3-4 hematologic toxicity was lower in the R-CHOP arm compared with the R-HDS arm, with at least one episode of neutropenia in 34% versus 84% of patients (P less than .001), anemia in 15% versus 71% of patients (P less than .001), and thrombocytopenia in 5% versus 86% (P less than .001).
The study was supported in part by the Associazione Italiana Lotta alla Leucemia sezione di Bergamo, the Associazione Italiana per la Ricerca sul Cancro, Ministero Istruzione, Universita e Ricerca and unrestricted grants from Roche SpA and Amgen, Italy. Dr Cortelazzo had no relevant disclosures. Several coauthors indicated relationships with industry.
Key clinical point:
Major finding: At a median follow-up of 5 years, the 3-year event-free survival was similar for both groups: 62% versus 65% (P = .83).
Data source: A randomized phase III trial that included 246 patients with diffuse large B-cell lymphomas.
Disclosures: The study was supported in part by the Associazione Italiana Lotta alla Leucemia sezione di Bergamo, the Associazione Italiana per la Ricerca sul Cancro, Ministero Istruzione, Universita e Ricerca and unrestricted grants from Roche SpA and Amgen, Italy. Dr Cortelazzo has no disclosures. Several coauthors indicate relationships with industry.
Expert panel offers treatment recommendations in Waldenström macroglobulinemia
Treatment recommendations for Waldenström macroglobulinemia have been updated based on the advice of a task force convened at the Eighth International Workshop on Waldenström Macroglobulinemia; the guidelines have been published in Blood.
The task force was impaneled to review recently published and ongoing clinical trial data as well as the impact of the newly recognized mutations MYD88 and CXCR4 on treatment decisions, indications for B-cell receptor and proteasome inhibitors, and future clinical trial initiatives.
The panel reiterated that the criteria for initiating therapy include immunoglobulin M (IgM)-related complications and/or symptoms that are related to direct involvement of the bone marrow by tumor cells, constitutional symptoms, and bulky extramedullary disease. Patients presenting with symptoms that include symptomatic hyperviscosity, moderate to severe hemolytic anemia, and symptomatic cryoglobulinemia need immediate treatment.
Close observation is recommended for the subgroup of patients who do not really fulfill the criteria for a diagnosis of Waldenström macroglobulinemia (WM), and whose laboratory findings may be the only indicator of the presence of a progressive disease.
Treatment recommendations
For symptomatic patients in the first-line setting, “anti-CD20 monoclonal antibody therapy alone or in combination with chemotherapy is an important standard of care for most patients with WM,” the authors, led by Veronique Leblond, MD, of Pitié-Salpêtrière Hôpital, Paris, wrote.
Rituximab is frequently used in WM, either as monotherapy or in combination with chemotherapeutic agents. The panel cautions that rituximab as monotherapy should be avoided in patients with high IgM levels, because of a lower chance of response and the risk of an IgM flare.
In patients with high IgM levels (typically around 4,000 mg/dL), plasmapheresis can be initiated before rituximab therapy, and plasmapheresis should always and immediately be used when symptomatic hyperviscosity is present. However, plasmapheresis alone is not an effective treatment for WM and must be followed by a rapidly acting cytoreductive regimen.
Several rituximab combinations are recommended by the panel. These include:
• Dexamethasone-rituximab-cyclophosphamide, which is an active and safe option, has a manageable toxicity, and can be considered for frail patients who need combination therapy.
• Bendamustine-rituximab is effective for front-line treatment and is well tolerated even in elderly patients who experience limited episodes of myelosuppression and infections.
Other therapeutic regimens include bortezomib-based therapy, which is recommended for patients with high IgM levels, symptomatic hyperviscosity, cryoglobulinemia or cold agglutinemia, amyloidosis, and renal impairment or in young patients who prefer to avoid alkylator or nucleoside analogue therapy.
Another option is carfilzomib-based therapy, which is an emerging “neuropathy-sparing” regimen for proteasome-inhibitor–based therapy, although it may not be the best choice for elderly patients with preexisting cardiac conditions due to potential cardiac toxicity.
Ibrutinib has been approved as a primary therapy for patients who are not candidates for chemoimmunotherapy, but the authors point out that the optimal use of this agent is still being investigated.
“The aim of the first-line treatments is to reach a high response rate with a prolonged progression-free survival,” write the authors. “The panel agrees that there is need to perform clinical trials with chemotherapy-free combinations with new compounds alone or in combination with anti-CD20 antibodies.”
For symptomatic previously treated patients
The panel also offered recommendations for previously treated symptomatic patients who have relapsed or are refractory to treatment.
Any of the interventions recommended for symptomatic, untreated patients can be considered for those who have already gone through first line therapy. Retreatment can be considered with a specific intervention if a response was achieved for 2 or more years with that therapy, although they caution that patients who have progressed on first-line ibrutinib should not use it again.
Ofatumumab is a potential option for patients who are unable to tolerate rituximab, and nucleoside analogues can be considered in fit patients who have not responded to less-toxic treatments.
Another option in this setting is everolimus, although since it is associated with considerable toxicities, the best candidates for this drug are those who have not responded to or have progressed after multiple lines of other better-tolerated regimens.
Immunomodulatory agents can also be considered, but in the context of a clinical trial only, because of their potential adverse events.
Finally, the panel also agreed that stem cell transplantation should be discussed with select patients, and while it is a feasible and effective treatment option for high-risk WM patients, it should be ideally offered at early relapse.
Investigating B-cell receptor (BCR) pathway inhibitors along with existing and novel compounds in patients in the relapsed/refractory setting should be a priority, according to the panel.
“BCR inhibitors, combined with proteasome inhibitors, would be of interest for overcoming resistance by interfering with the two key pathways that are affected by MYD88,” wrote Dr. Leblond and coauthors.
Treatment recommendations for Waldenström macroglobulinemia have been updated based on the advice of a task force convened at the Eighth International Workshop on Waldenström Macroglobulinemia; the guidelines have been published in Blood.
The task force was impaneled to review recently published and ongoing clinical trial data as well as the impact of the newly recognized mutations MYD88 and CXCR4 on treatment decisions, indications for B-cell receptor and proteasome inhibitors, and future clinical trial initiatives.
The panel reiterated that the criteria for initiating therapy include immunoglobulin M (IgM)-related complications and/or symptoms that are related to direct involvement of the bone marrow by tumor cells, constitutional symptoms, and bulky extramedullary disease. Patients presenting with symptoms that include symptomatic hyperviscosity, moderate to severe hemolytic anemia, and symptomatic cryoglobulinemia need immediate treatment.
Close observation is recommended for the subgroup of patients who do not really fulfill the criteria for a diagnosis of Waldenström macroglobulinemia (WM), and whose laboratory findings may be the only indicator of the presence of a progressive disease.
Treatment recommendations
For symptomatic patients in the first-line setting, “anti-CD20 monoclonal antibody therapy alone or in combination with chemotherapy is an important standard of care for most patients with WM,” the authors, led by Veronique Leblond, MD, of Pitié-Salpêtrière Hôpital, Paris, wrote.
Rituximab is frequently used in WM, either as monotherapy or in combination with chemotherapeutic agents. The panel cautions that rituximab as monotherapy should be avoided in patients with high IgM levels, because of a lower chance of response and the risk of an IgM flare.
In patients with high IgM levels (typically around 4,000 mg/dL), plasmapheresis can be initiated before rituximab therapy, and plasmapheresis should always and immediately be used when symptomatic hyperviscosity is present. However, plasmapheresis alone is not an effective treatment for WM and must be followed by a rapidly acting cytoreductive regimen.
Several rituximab combinations are recommended by the panel. These include:
• Dexamethasone-rituximab-cyclophosphamide, which is an active and safe option, has a manageable toxicity, and can be considered for frail patients who need combination therapy.
• Bendamustine-rituximab is effective for front-line treatment and is well tolerated even in elderly patients who experience limited episodes of myelosuppression and infections.
Other therapeutic regimens include bortezomib-based therapy, which is recommended for patients with high IgM levels, symptomatic hyperviscosity, cryoglobulinemia or cold agglutinemia, amyloidosis, and renal impairment or in young patients who prefer to avoid alkylator or nucleoside analogue therapy.
Another option is carfilzomib-based therapy, which is an emerging “neuropathy-sparing” regimen for proteasome-inhibitor–based therapy, although it may not be the best choice for elderly patients with preexisting cardiac conditions due to potential cardiac toxicity.
Ibrutinib has been approved as a primary therapy for patients who are not candidates for chemoimmunotherapy, but the authors point out that the optimal use of this agent is still being investigated.
“The aim of the first-line treatments is to reach a high response rate with a prolonged progression-free survival,” write the authors. “The panel agrees that there is need to perform clinical trials with chemotherapy-free combinations with new compounds alone or in combination with anti-CD20 antibodies.”
For symptomatic previously treated patients
The panel also offered recommendations for previously treated symptomatic patients who have relapsed or are refractory to treatment.
Any of the interventions recommended for symptomatic, untreated patients can be considered for those who have already gone through first line therapy. Retreatment can be considered with a specific intervention if a response was achieved for 2 or more years with that therapy, although they caution that patients who have progressed on first-line ibrutinib should not use it again.
Ofatumumab is a potential option for patients who are unable to tolerate rituximab, and nucleoside analogues can be considered in fit patients who have not responded to less-toxic treatments.
Another option in this setting is everolimus, although since it is associated with considerable toxicities, the best candidates for this drug are those who have not responded to or have progressed after multiple lines of other better-tolerated regimens.
Immunomodulatory agents can also be considered, but in the context of a clinical trial only, because of their potential adverse events.
Finally, the panel also agreed that stem cell transplantation should be discussed with select patients, and while it is a feasible and effective treatment option for high-risk WM patients, it should be ideally offered at early relapse.
Investigating B-cell receptor (BCR) pathway inhibitors along with existing and novel compounds in patients in the relapsed/refractory setting should be a priority, according to the panel.
“BCR inhibitors, combined with proteasome inhibitors, would be of interest for overcoming resistance by interfering with the two key pathways that are affected by MYD88,” wrote Dr. Leblond and coauthors.
Treatment recommendations for Waldenström macroglobulinemia have been updated based on the advice of a task force convened at the Eighth International Workshop on Waldenström Macroglobulinemia; the guidelines have been published in Blood.
The task force was impaneled to review recently published and ongoing clinical trial data as well as the impact of the newly recognized mutations MYD88 and CXCR4 on treatment decisions, indications for B-cell receptor and proteasome inhibitors, and future clinical trial initiatives.
The panel reiterated that the criteria for initiating therapy include immunoglobulin M (IgM)-related complications and/or symptoms that are related to direct involvement of the bone marrow by tumor cells, constitutional symptoms, and bulky extramedullary disease. Patients presenting with symptoms that include symptomatic hyperviscosity, moderate to severe hemolytic anemia, and symptomatic cryoglobulinemia need immediate treatment.
Close observation is recommended for the subgroup of patients who do not really fulfill the criteria for a diagnosis of Waldenström macroglobulinemia (WM), and whose laboratory findings may be the only indicator of the presence of a progressive disease.
Treatment recommendations
For symptomatic patients in the first-line setting, “anti-CD20 monoclonal antibody therapy alone or in combination with chemotherapy is an important standard of care for most patients with WM,” the authors, led by Veronique Leblond, MD, of Pitié-Salpêtrière Hôpital, Paris, wrote.
Rituximab is frequently used in WM, either as monotherapy or in combination with chemotherapeutic agents. The panel cautions that rituximab as monotherapy should be avoided in patients with high IgM levels, because of a lower chance of response and the risk of an IgM flare.
In patients with high IgM levels (typically around 4,000 mg/dL), plasmapheresis can be initiated before rituximab therapy, and plasmapheresis should always and immediately be used when symptomatic hyperviscosity is present. However, plasmapheresis alone is not an effective treatment for WM and must be followed by a rapidly acting cytoreductive regimen.
Several rituximab combinations are recommended by the panel. These include:
• Dexamethasone-rituximab-cyclophosphamide, which is an active and safe option, has a manageable toxicity, and can be considered for frail patients who need combination therapy.
• Bendamustine-rituximab is effective for front-line treatment and is well tolerated even in elderly patients who experience limited episodes of myelosuppression and infections.
Other therapeutic regimens include bortezomib-based therapy, which is recommended for patients with high IgM levels, symptomatic hyperviscosity, cryoglobulinemia or cold agglutinemia, amyloidosis, and renal impairment or in young patients who prefer to avoid alkylator or nucleoside analogue therapy.
Another option is carfilzomib-based therapy, which is an emerging “neuropathy-sparing” regimen for proteasome-inhibitor–based therapy, although it may not be the best choice for elderly patients with preexisting cardiac conditions due to potential cardiac toxicity.
Ibrutinib has been approved as a primary therapy for patients who are not candidates for chemoimmunotherapy, but the authors point out that the optimal use of this agent is still being investigated.
“The aim of the first-line treatments is to reach a high response rate with a prolonged progression-free survival,” write the authors. “The panel agrees that there is need to perform clinical trials with chemotherapy-free combinations with new compounds alone or in combination with anti-CD20 antibodies.”
For symptomatic previously treated patients
The panel also offered recommendations for previously treated symptomatic patients who have relapsed or are refractory to treatment.
Any of the interventions recommended for symptomatic, untreated patients can be considered for those who have already gone through first line therapy. Retreatment can be considered with a specific intervention if a response was achieved for 2 or more years with that therapy, although they caution that patients who have progressed on first-line ibrutinib should not use it again.
Ofatumumab is a potential option for patients who are unable to tolerate rituximab, and nucleoside analogues can be considered in fit patients who have not responded to less-toxic treatments.
Another option in this setting is everolimus, although since it is associated with considerable toxicities, the best candidates for this drug are those who have not responded to or have progressed after multiple lines of other better-tolerated regimens.
Immunomodulatory agents can also be considered, but in the context of a clinical trial only, because of their potential adverse events.
Finally, the panel also agreed that stem cell transplantation should be discussed with select patients, and while it is a feasible and effective treatment option for high-risk WM patients, it should be ideally offered at early relapse.
Investigating B-cell receptor (BCR) pathway inhibitors along with existing and novel compounds in patients in the relapsed/refractory setting should be a priority, according to the panel.
“BCR inhibitors, combined with proteasome inhibitors, would be of interest for overcoming resistance by interfering with the two key pathways that are affected by MYD88,” wrote Dr. Leblond and coauthors.
FROM BLOOD
Is stem-cell transplant curative for HIV infection?
DURBAN, SOUTH AFRICA – The 15 HIV-infected patients who have undergone allogeneic stem-cell transplant for life-threatening hematologic cancers under the auspices of the European EpiStem Consortium have uniformly demonstrated a profound and durable reduction in viral reservoir to a degree that hasn’t been approached by any other investigational cure strategy, Annemarie Wensing, MD, said at the 21st International AIDS Conference.
“We see an enormous reduction in the viral reservoir, and in two patients we cannot find any viable HIV in the blood using ultrasensitive tests. But we don’t know whether these patients are cured because they are still on antiretroviral therapy,” said Dr. Wensing of Utrecht (The Netherlands) University.
Non-Hodgkin’s lymphoma and Hodgkin’s lymphoma are 7-9 times more frequent in HIV-positive patients than in the general population. But allogeneic stem cell transplantation is an even higher-risk treatment in HIV-positive patients with life-threatening leukemia or lymphoma than in the HIV-negative population. Only 6 of the 15 EuroStem patients remain alive. Eight died within 4 months of the procedure and another died 2.5 years post-transplant, all from progression of their cancer or as a result of opportunistic infections arising during the immunosuppressive chemoablation that’s central to stem-cell transplantation. However, 3 of the 15 patients have survived longer than 3 years. In two of them, no HIV can be detected in blood or intestinal tissue using ultrasensitive tests, while in the third there is “only a slight trace,” according to Dr. Wensing, a clinical virologist.
EpiStem (the European Project to Guide and Investigate the Potential for HIV Cure by Stem-Cell Transplantation) is a multinational collaboration of European oncologists, infectious disease physicians, and other specialists. It was formed in response to the successful outcome of allogeneic stem cell transplantation for acute myeloid leukemia in HIV-positive Timothy Brown, more famously known as “the Berlin patient” (N Engl J Med. 2009 Feb 12;360(7):692-8). He has thus far survived 7 years off antiretroviral therapy.
Much has been made of the fact that Mr. Brown’s donor cells were homozygous for the CCR5 delta32 mutation, which confers natural resistance to HIV infection because it prevents the virus from infecting T cells. Only 1% or less of the population is homozygous for this mutation. But Dr. Wensing isn’t convinced that using donor cells with the mutation is a prerequisite for success. Indeed, while 4 of the 15 EpiStem patients received stem cells from donors homozygous for the mutation and another got donor cells heterozygous for the CCR5 delta32 mutation, the other 10 received stem cells capable of being infected by HIV – yet all 15 experienced an enormous reduction in their viral reservoir. And two of the three patients who have survived longer than 3 years got stem cells without the CCR5 delta32 mutation.
Dr. Wensing observed that a common denominator shared by Timothy Brown and the two EpiStem patients who have trace or undetectable HIV in blood or tissue samples more than 3 years post-transplant is that all three developed severe graft-versus-host disease in conjunction with their stem cell transplantation. She suspects this may have helped them to clear the infection, a hypothesis she intends to pursue further as EpiStem gathers more patients.
Eventually, if patients continue to test negative for HIV using ultrasensitive tests, it will be time to have a discussion with patients and their treating physicians as to whether they should continue on antiretroviral therapy.
“In the end it’s the patients’ decision, but they should be very well counseled because it can have medical and also psychological consequences if HIV returns,” she said.
EpiStem is funded by the American Foundation for AIDS Research Conssortium on HIV Eradication. Dr. Wensing reported having no financial conflicts regarding her presentation.
DURBAN, SOUTH AFRICA – The 15 HIV-infected patients who have undergone allogeneic stem-cell transplant for life-threatening hematologic cancers under the auspices of the European EpiStem Consortium have uniformly demonstrated a profound and durable reduction in viral reservoir to a degree that hasn’t been approached by any other investigational cure strategy, Annemarie Wensing, MD, said at the 21st International AIDS Conference.
“We see an enormous reduction in the viral reservoir, and in two patients we cannot find any viable HIV in the blood using ultrasensitive tests. But we don’t know whether these patients are cured because they are still on antiretroviral therapy,” said Dr. Wensing of Utrecht (The Netherlands) University.
Non-Hodgkin’s lymphoma and Hodgkin’s lymphoma are 7-9 times more frequent in HIV-positive patients than in the general population. But allogeneic stem cell transplantation is an even higher-risk treatment in HIV-positive patients with life-threatening leukemia or lymphoma than in the HIV-negative population. Only 6 of the 15 EuroStem patients remain alive. Eight died within 4 months of the procedure and another died 2.5 years post-transplant, all from progression of their cancer or as a result of opportunistic infections arising during the immunosuppressive chemoablation that’s central to stem-cell transplantation. However, 3 of the 15 patients have survived longer than 3 years. In two of them, no HIV can be detected in blood or intestinal tissue using ultrasensitive tests, while in the third there is “only a slight trace,” according to Dr. Wensing, a clinical virologist.
EpiStem (the European Project to Guide and Investigate the Potential for HIV Cure by Stem-Cell Transplantation) is a multinational collaboration of European oncologists, infectious disease physicians, and other specialists. It was formed in response to the successful outcome of allogeneic stem cell transplantation for acute myeloid leukemia in HIV-positive Timothy Brown, more famously known as “the Berlin patient” (N Engl J Med. 2009 Feb 12;360(7):692-8). He has thus far survived 7 years off antiretroviral therapy.
Much has been made of the fact that Mr. Brown’s donor cells were homozygous for the CCR5 delta32 mutation, which confers natural resistance to HIV infection because it prevents the virus from infecting T cells. Only 1% or less of the population is homozygous for this mutation. But Dr. Wensing isn’t convinced that using donor cells with the mutation is a prerequisite for success. Indeed, while 4 of the 15 EpiStem patients received stem cells from donors homozygous for the mutation and another got donor cells heterozygous for the CCR5 delta32 mutation, the other 10 received stem cells capable of being infected by HIV – yet all 15 experienced an enormous reduction in their viral reservoir. And two of the three patients who have survived longer than 3 years got stem cells without the CCR5 delta32 mutation.
Dr. Wensing observed that a common denominator shared by Timothy Brown and the two EpiStem patients who have trace or undetectable HIV in blood or tissue samples more than 3 years post-transplant is that all three developed severe graft-versus-host disease in conjunction with their stem cell transplantation. She suspects this may have helped them to clear the infection, a hypothesis she intends to pursue further as EpiStem gathers more patients.
Eventually, if patients continue to test negative for HIV using ultrasensitive tests, it will be time to have a discussion with patients and their treating physicians as to whether they should continue on antiretroviral therapy.
“In the end it’s the patients’ decision, but they should be very well counseled because it can have medical and also psychological consequences if HIV returns,” she said.
EpiStem is funded by the American Foundation for AIDS Research Conssortium on HIV Eradication. Dr. Wensing reported having no financial conflicts regarding her presentation.
DURBAN, SOUTH AFRICA – The 15 HIV-infected patients who have undergone allogeneic stem-cell transplant for life-threatening hematologic cancers under the auspices of the European EpiStem Consortium have uniformly demonstrated a profound and durable reduction in viral reservoir to a degree that hasn’t been approached by any other investigational cure strategy, Annemarie Wensing, MD, said at the 21st International AIDS Conference.
“We see an enormous reduction in the viral reservoir, and in two patients we cannot find any viable HIV in the blood using ultrasensitive tests. But we don’t know whether these patients are cured because they are still on antiretroviral therapy,” said Dr. Wensing of Utrecht (The Netherlands) University.
Non-Hodgkin’s lymphoma and Hodgkin’s lymphoma are 7-9 times more frequent in HIV-positive patients than in the general population. But allogeneic stem cell transplantation is an even higher-risk treatment in HIV-positive patients with life-threatening leukemia or lymphoma than in the HIV-negative population. Only 6 of the 15 EuroStem patients remain alive. Eight died within 4 months of the procedure and another died 2.5 years post-transplant, all from progression of their cancer or as a result of opportunistic infections arising during the immunosuppressive chemoablation that’s central to stem-cell transplantation. However, 3 of the 15 patients have survived longer than 3 years. In two of them, no HIV can be detected in blood or intestinal tissue using ultrasensitive tests, while in the third there is “only a slight trace,” according to Dr. Wensing, a clinical virologist.
EpiStem (the European Project to Guide and Investigate the Potential for HIV Cure by Stem-Cell Transplantation) is a multinational collaboration of European oncologists, infectious disease physicians, and other specialists. It was formed in response to the successful outcome of allogeneic stem cell transplantation for acute myeloid leukemia in HIV-positive Timothy Brown, more famously known as “the Berlin patient” (N Engl J Med. 2009 Feb 12;360(7):692-8). He has thus far survived 7 years off antiretroviral therapy.
Much has been made of the fact that Mr. Brown’s donor cells were homozygous for the CCR5 delta32 mutation, which confers natural resistance to HIV infection because it prevents the virus from infecting T cells. Only 1% or less of the population is homozygous for this mutation. But Dr. Wensing isn’t convinced that using donor cells with the mutation is a prerequisite for success. Indeed, while 4 of the 15 EpiStem patients received stem cells from donors homozygous for the mutation and another got donor cells heterozygous for the CCR5 delta32 mutation, the other 10 received stem cells capable of being infected by HIV – yet all 15 experienced an enormous reduction in their viral reservoir. And two of the three patients who have survived longer than 3 years got stem cells without the CCR5 delta32 mutation.
Dr. Wensing observed that a common denominator shared by Timothy Brown and the two EpiStem patients who have trace or undetectable HIV in blood or tissue samples more than 3 years post-transplant is that all three developed severe graft-versus-host disease in conjunction with their stem cell transplantation. She suspects this may have helped them to clear the infection, a hypothesis she intends to pursue further as EpiStem gathers more patients.
Eventually, if patients continue to test negative for HIV using ultrasensitive tests, it will be time to have a discussion with patients and their treating physicians as to whether they should continue on antiretroviral therapy.
“In the end it’s the patients’ decision, but they should be very well counseled because it can have medical and also psychological consequences if HIV returns,” she said.
EpiStem is funded by the American Foundation for AIDS Research Conssortium on HIV Eradication. Dr. Wensing reported having no financial conflicts regarding her presentation.
AT AIDS 2016
Key clinical point: It doesn’t appear to be necessary to use donor stem cells that are homozygous for the CCR5 delta32 mutation to achieve enormous sustained reductions in the viral reservoir in HIV-infected patients undergoing allogeneic stem cell transplantation for hematologic cancers.
Major finding: Two of three patients in a European series who have survived for longer than 3 years after stem-cell transplantation with undetectable or only trace HIV in their blood received donor cells lacking the rare CCR5 delta32 mutation.
Data source: EpiStem is an ongoing observational study of HIV-infected patients who undergo allogeneic stem cell transplantation for life-threatening hematologic cancers.
Disclosures: The EpiStem project is funded by the American Foundation for AIDS Research Conssortium on HIV Eradication. The presenter reported having no financial conflicts regarding her presentation.
Treatment may allow HSCT without radiation, chemotherapy
A new therapy combining an anti-c-Kit monoclonal antibody with a CD47 blocker allowed hematopoietic stem cell engraftment in immunocompetent mice without the need for toxic preconditioning using radiation or chemotherapy, according to a report published in Science Translational Medicine.
Until now, hematopoietic stem cell transplantation has required rigorous conditioning regimens to clear out the host’s bone marrow, which can cause lifelong complications. So the procedure has been reserved for patients whose life-threatening disorders justified such toxicity. “Safer and more targeted conditioning protocols could both improve the safety of transplantation and extend the existing clinical utility of this powerful form of cell therapy,” said Akanksha Chhabra, PhD, of the department of blood and marrow transplantation, Stanford (Calif.) University, and her associates.
They assessed the new combined treatment in a series of laboratory and mouse studies. The opsonizing anti-c-Kit monoclonal antibodies induced robust depletion of functional hematopoietic stem cells in immunocompetent mice, which allowed donor stem cells to engraft in these hosts. Adding the T-cell–depleting CD47-antagonists further facilitated immune ablation of host stem cells and progenitor cells. Combined, the two agents eliminated more than 99% of host hematopoietic stem cells in the bone marrow and enabled strong engraftment of the donor stem cells, while avoiding radiation- and chemotherapy-related adverse effects.
The main toxicities that occurred in treated mice were, as expected, reductions in hematologic parameters, especially red blood cell indices. This may be related to a factor in mouse physiology that is not present in humans. But if such toxicities do develop in human subjects, they can be mitigated by careful monitoring and occasional supportive transfusions, Dr. Chhabra and her associates said (Sci Transl Med. 2016;8:351ra105).
These two types of antibodies are already being investigated separately in early-phase clinical trials. If the combined treatment proves effective and safe in humans – a question that awaits further clinical studies – hematopoietic stem cell transplantation might be extended to nonmalignant conditions such as inherited immunodeficiency, inborn errors of metabolism, and hemoglobinopathies. It might also be adapted for use in solid-organ transplants, the researchers added.
This work was supported by the Virginia and D.K. Ludwig Fund for Cancer Research and several other nonprofit organizations, the California Institute for Regenerative Medicine, and the National Institutes of Health. Dr. Chhabra is a coinventor on a patent described in this article, and her associates are cofounders of Forty Seven, the company that licensed the technology for radiation- and chemotherapy-free stem-cell transplantation. Two associates also serve as advisors for Alexo Therapeutics, which develops CD47-based treatments.
A new therapy combining an anti-c-Kit monoclonal antibody with a CD47 blocker allowed hematopoietic stem cell engraftment in immunocompetent mice without the need for toxic preconditioning using radiation or chemotherapy, according to a report published in Science Translational Medicine.
Until now, hematopoietic stem cell transplantation has required rigorous conditioning regimens to clear out the host’s bone marrow, which can cause lifelong complications. So the procedure has been reserved for patients whose life-threatening disorders justified such toxicity. “Safer and more targeted conditioning protocols could both improve the safety of transplantation and extend the existing clinical utility of this powerful form of cell therapy,” said Akanksha Chhabra, PhD, of the department of blood and marrow transplantation, Stanford (Calif.) University, and her associates.
They assessed the new combined treatment in a series of laboratory and mouse studies. The opsonizing anti-c-Kit monoclonal antibodies induced robust depletion of functional hematopoietic stem cells in immunocompetent mice, which allowed donor stem cells to engraft in these hosts. Adding the T-cell–depleting CD47-antagonists further facilitated immune ablation of host stem cells and progenitor cells. Combined, the two agents eliminated more than 99% of host hematopoietic stem cells in the bone marrow and enabled strong engraftment of the donor stem cells, while avoiding radiation- and chemotherapy-related adverse effects.
The main toxicities that occurred in treated mice were, as expected, reductions in hematologic parameters, especially red blood cell indices. This may be related to a factor in mouse physiology that is not present in humans. But if such toxicities do develop in human subjects, they can be mitigated by careful monitoring and occasional supportive transfusions, Dr. Chhabra and her associates said (Sci Transl Med. 2016;8:351ra105).
These two types of antibodies are already being investigated separately in early-phase clinical trials. If the combined treatment proves effective and safe in humans – a question that awaits further clinical studies – hematopoietic stem cell transplantation might be extended to nonmalignant conditions such as inherited immunodeficiency, inborn errors of metabolism, and hemoglobinopathies. It might also be adapted for use in solid-organ transplants, the researchers added.
This work was supported by the Virginia and D.K. Ludwig Fund for Cancer Research and several other nonprofit organizations, the California Institute for Regenerative Medicine, and the National Institutes of Health. Dr. Chhabra is a coinventor on a patent described in this article, and her associates are cofounders of Forty Seven, the company that licensed the technology for radiation- and chemotherapy-free stem-cell transplantation. Two associates also serve as advisors for Alexo Therapeutics, which develops CD47-based treatments.
A new therapy combining an anti-c-Kit monoclonal antibody with a CD47 blocker allowed hematopoietic stem cell engraftment in immunocompetent mice without the need for toxic preconditioning using radiation or chemotherapy, according to a report published in Science Translational Medicine.
Until now, hematopoietic stem cell transplantation has required rigorous conditioning regimens to clear out the host’s bone marrow, which can cause lifelong complications. So the procedure has been reserved for patients whose life-threatening disorders justified such toxicity. “Safer and more targeted conditioning protocols could both improve the safety of transplantation and extend the existing clinical utility of this powerful form of cell therapy,” said Akanksha Chhabra, PhD, of the department of blood and marrow transplantation, Stanford (Calif.) University, and her associates.
They assessed the new combined treatment in a series of laboratory and mouse studies. The opsonizing anti-c-Kit monoclonal antibodies induced robust depletion of functional hematopoietic stem cells in immunocompetent mice, which allowed donor stem cells to engraft in these hosts. Adding the T-cell–depleting CD47-antagonists further facilitated immune ablation of host stem cells and progenitor cells. Combined, the two agents eliminated more than 99% of host hematopoietic stem cells in the bone marrow and enabled strong engraftment of the donor stem cells, while avoiding radiation- and chemotherapy-related adverse effects.
The main toxicities that occurred in treated mice were, as expected, reductions in hematologic parameters, especially red blood cell indices. This may be related to a factor in mouse physiology that is not present in humans. But if such toxicities do develop in human subjects, they can be mitigated by careful monitoring and occasional supportive transfusions, Dr. Chhabra and her associates said (Sci Transl Med. 2016;8:351ra105).
These two types of antibodies are already being investigated separately in early-phase clinical trials. If the combined treatment proves effective and safe in humans – a question that awaits further clinical studies – hematopoietic stem cell transplantation might be extended to nonmalignant conditions such as inherited immunodeficiency, inborn errors of metabolism, and hemoglobinopathies. It might also be adapted for use in solid-organ transplants, the researchers added.
This work was supported by the Virginia and D.K. Ludwig Fund for Cancer Research and several other nonprofit organizations, the California Institute for Regenerative Medicine, and the National Institutes of Health. Dr. Chhabra is a coinventor on a patent described in this article, and her associates are cofounders of Forty Seven, the company that licensed the technology for radiation- and chemotherapy-free stem-cell transplantation. Two associates also serve as advisors for Alexo Therapeutics, which develops CD47-based treatments.
FROM SCIENCE TRANSLATIONAL MEDICINE
Key clinical point: A new treatment allowed hematopoietic stem cell engraftment in immunocompetent mice without the need for toxic preconditioning using radiation or chemotherapy.
Major finding: The combined therapy eliminated more than 99% of host hematopoietic stem cells.
Data source: A series of laboratory and mouse studies of combined treatment with anti-c-Kit monoclonal antibodies plus CD47 blockers.
Disclosures: This work was supported by the Virginia and D.K. Ludwig Fund for Cancer Research and several other nonprofit organizations, the California Institute for Regenerative Medicine, and the National Institutes of Health. Dr. Chhabra is a coinventor on a patent described in this article, and her associates are cofounders of Forty Seven, the company that licensed the technology for radiation- and chemotherapy-free stem-cell transplantation. Two associates also serve as advisors for Alexo Therapeutics, which develops CD47-based treatments.
Pretransplantation mogamulizumab for ATLL raises risk of GVHD
The use of mogamulizumab before allogeneic hematopoietic stem-cell transplantation in aggressive adult T-cell leukemia/lymphoma is associated with an increased risk of acute graft-versus-host disease (GVHD), which leads to an inferior overall survival, investigators report in the Journal of Clinical Oncology.
Mogamulizumab is an anti-CCR4 monoclonal antibody that showed promise in small clinical studies when added to conventional chemotherapy as first-line treatment. It was recently approved for the treatment of adult T-cell leukemia/lymphoma in Japan, and eventually may be approved in the U.S. and other countries, said Shigeo Fuji, MD, of the department of hematopoietic stem-cell transplantation, National Cancer Center Hospital, Tokyo, and his associates.
The agent significantly depleted regulatory T cells for several months in animal models. This prompted concern regarding the possibility of exacerbating GVHD in human patients who don’t respond completely to first-line chemotherapy and then undergo stem-cell transplantation. “However, no direct evidence has demonstrated [regulatory T-cell] depletion in humans,” the investigators noted.
To examine this issue, they assessed clinical outcomes in a cohort of 996 patients across Japan who had aggressive adult T-cell leukemia/lymphoma, were aged 20-69 years, were diagnosed in 2000-2013, and received intensive, multiagent chemotherapy before undergoing allogeneic hematopoietic stem-cell transplantation.
Grade 2-4 acute GVHD developed in 381 of 873 patients who didn’t receive mogamulizumab (43.6%), compared with 47 of 81 patients who did receive the agent (58.0%), for a relative risk of 1.33 (P = .01). Grade 3-4 acute GVHD developed in 150 patients who didn’t receive mogamulizumab (17.2%), compared with 25 who did (30.9%), for an RR of 1.80 (P less than .01) .
The agent not only raised the rate of GVHD, it also increased the severity of the disorder. GVHD was refractory to systemic corticosteroids in 23.5% of patients who didn’t receive mogamulizumab, compared with 48.9% of those who did, for an RR of 2.09 (P less than .01), the investigators reported (J Clin Oncol. 2016. doi: 10.1200/JCO.2016.67.8250).
In addition, 1-year disease-free mortality was 25.1% without mogamulizumab, compared with 43.7% with it. The estimated 1-year overall survival was 49.4% without mogamulizumab, compared with 32.3% with it. And in multivariable analyses, receiving mogamulizumab before undergoing stem-cell transplantation was a significant risk factor for both disease-free mortality (hazard ratio, 1.93) and overall mortality (HR, 1.67).
“All hematologists should take the risks and benefits of mogamulizumab into consideration before they use [it] in transplantation-eligible patients,” Dr. Fuji and his associates said.
The use of mogamulizumab before allogeneic hematopoietic stem-cell transplantation in aggressive adult T-cell leukemia/lymphoma is associated with an increased risk of acute graft-versus-host disease (GVHD), which leads to an inferior overall survival, investigators report in the Journal of Clinical Oncology.
Mogamulizumab is an anti-CCR4 monoclonal antibody that showed promise in small clinical studies when added to conventional chemotherapy as first-line treatment. It was recently approved for the treatment of adult T-cell leukemia/lymphoma in Japan, and eventually may be approved in the U.S. and other countries, said Shigeo Fuji, MD, of the department of hematopoietic stem-cell transplantation, National Cancer Center Hospital, Tokyo, and his associates.
The agent significantly depleted regulatory T cells for several months in animal models. This prompted concern regarding the possibility of exacerbating GVHD in human patients who don’t respond completely to first-line chemotherapy and then undergo stem-cell transplantation. “However, no direct evidence has demonstrated [regulatory T-cell] depletion in humans,” the investigators noted.
To examine this issue, they assessed clinical outcomes in a cohort of 996 patients across Japan who had aggressive adult T-cell leukemia/lymphoma, were aged 20-69 years, were diagnosed in 2000-2013, and received intensive, multiagent chemotherapy before undergoing allogeneic hematopoietic stem-cell transplantation.
Grade 2-4 acute GVHD developed in 381 of 873 patients who didn’t receive mogamulizumab (43.6%), compared with 47 of 81 patients who did receive the agent (58.0%), for a relative risk of 1.33 (P = .01). Grade 3-4 acute GVHD developed in 150 patients who didn’t receive mogamulizumab (17.2%), compared with 25 who did (30.9%), for an RR of 1.80 (P less than .01) .
The agent not only raised the rate of GVHD, it also increased the severity of the disorder. GVHD was refractory to systemic corticosteroids in 23.5% of patients who didn’t receive mogamulizumab, compared with 48.9% of those who did, for an RR of 2.09 (P less than .01), the investigators reported (J Clin Oncol. 2016. doi: 10.1200/JCO.2016.67.8250).
In addition, 1-year disease-free mortality was 25.1% without mogamulizumab, compared with 43.7% with it. The estimated 1-year overall survival was 49.4% without mogamulizumab, compared with 32.3% with it. And in multivariable analyses, receiving mogamulizumab before undergoing stem-cell transplantation was a significant risk factor for both disease-free mortality (hazard ratio, 1.93) and overall mortality (HR, 1.67).
“All hematologists should take the risks and benefits of mogamulizumab into consideration before they use [it] in transplantation-eligible patients,” Dr. Fuji and his associates said.
The use of mogamulizumab before allogeneic hematopoietic stem-cell transplantation in aggressive adult T-cell leukemia/lymphoma is associated with an increased risk of acute graft-versus-host disease (GVHD), which leads to an inferior overall survival, investigators report in the Journal of Clinical Oncology.
Mogamulizumab is an anti-CCR4 monoclonal antibody that showed promise in small clinical studies when added to conventional chemotherapy as first-line treatment. It was recently approved for the treatment of adult T-cell leukemia/lymphoma in Japan, and eventually may be approved in the U.S. and other countries, said Shigeo Fuji, MD, of the department of hematopoietic stem-cell transplantation, National Cancer Center Hospital, Tokyo, and his associates.
The agent significantly depleted regulatory T cells for several months in animal models. This prompted concern regarding the possibility of exacerbating GVHD in human patients who don’t respond completely to first-line chemotherapy and then undergo stem-cell transplantation. “However, no direct evidence has demonstrated [regulatory T-cell] depletion in humans,” the investigators noted.
To examine this issue, they assessed clinical outcomes in a cohort of 996 patients across Japan who had aggressive adult T-cell leukemia/lymphoma, were aged 20-69 years, were diagnosed in 2000-2013, and received intensive, multiagent chemotherapy before undergoing allogeneic hematopoietic stem-cell transplantation.
Grade 2-4 acute GVHD developed in 381 of 873 patients who didn’t receive mogamulizumab (43.6%), compared with 47 of 81 patients who did receive the agent (58.0%), for a relative risk of 1.33 (P = .01). Grade 3-4 acute GVHD developed in 150 patients who didn’t receive mogamulizumab (17.2%), compared with 25 who did (30.9%), for an RR of 1.80 (P less than .01) .
The agent not only raised the rate of GVHD, it also increased the severity of the disorder. GVHD was refractory to systemic corticosteroids in 23.5% of patients who didn’t receive mogamulizumab, compared with 48.9% of those who did, for an RR of 2.09 (P less than .01), the investigators reported (J Clin Oncol. 2016. doi: 10.1200/JCO.2016.67.8250).
In addition, 1-year disease-free mortality was 25.1% without mogamulizumab, compared with 43.7% with it. The estimated 1-year overall survival was 49.4% without mogamulizumab, compared with 32.3% with it. And in multivariable analyses, receiving mogamulizumab before undergoing stem-cell transplantation was a significant risk factor for both disease-free mortality (hazard ratio, 1.93) and overall mortality (HR, 1.67).
“All hematologists should take the risks and benefits of mogamulizumab into consideration before they use [it] in transplantation-eligible patients,” Dr. Fuji and his associates said.
FROM THE JOURNAL OF CLINICAL ONCOLOGY
Key clinical point: The use of mogamulizumab before allogeneic hematopoietic stem-cell transplantation in aggressive adult T-cell leukemia/lymphoma was associated with GVHD and increased mortality.
Major finding: Grade 3-4 acute GVHD developed in 17.2% of patients who didn’t receive mogamulizumab, compared with 30.9% who did, for a relative risk of 1.80.
Data source: A retrospective cohort study involving 996 patients with adult T-cell leukemia/lymphoma in Japan.
Disclosures: This study was supported in part by Practical Research for Innovative Cancer Control and the Japan Agency for Medical Research and Development. Dr. Fuji and one associate reported receiving honoraria from Kyowa Hakko Kirin; another associate reported ties to numerous industry sources.
Metabolic tumor volume predicts outcome in follicular lymphoma
The total metabolic tumor volume, as quantified on PET scanning at the time that follicular lymphoma is diagnosed, is a strong independent predictor of treatment response and patient outcome, according to a report published online in Journal of Clinical Oncology.
Until now, no study has specifically examined the prognostic possibilities of PET-derived total metabolic tumor volume (TMTV) for this malignancy, either on its own or in combination with any of several existing prognostic indices. Those tools use a variety of surrogates to estimate tumor burden. Now that PET is recommended at diagnosis for all cases of follicular lymphoma and anatomic CT data are also available, it is much easier to estimate total tumor burden than it was when those indices were developed, said Michel Meignan, MD, PhD, of Hôpital Henri Mondor, Crétiel (France) and his associates.
It is crucial to identify patients likely to have a poor response to standard treatment, both to spare them the considerable adverse effects of that treatment and to select them for alternative first-line approaches. Even though patient survival has improved markedly during the past decade with the introduction of combined treatment using rituximab plus chemotherapy, approximately 20% of patients still show disease progression within 2 years, and the 5-year overall survival is only 50%, the investigators noted.
To assess the prognostic value of TMTV as assessed by PET, they pooled data from three multicenter prospective studies involving 185 patients with either a high tumor burden or advanced-stage follicular lymphoma. These participants were followed for a median of 63.5 months at 56 medical centers in France, Belgium, Australia, and Italy.
A TMTV threshold of 510 cm3 was found to have the optimal sensitivity (0.46), specificity (0.83), positive predictive value (0.67), and negative predictive value (0.67) for predicting both progression-free and overall survival. The 30% of patients who had a TMTV greater than that cutoff point had markedly inferior 5-year progression-free survival (less than 3 years), while the 70% who had a smaller TMTV had median progression-free survival of more than 6 years, Dr. Meignan and his associates said (J Clin Oncol. 2016 Aug 22. doi:10.1200/JCO.2016.66.9440).
Combining TMTV with other prognostic measures improved predictions even further. Patients who had both a high TMTV and an intermediate to high score on the Follicular Lymphoma International Prognostic Index 2 showed extremely poor outcomes, with a median progression-free survival of only 19 months. “This population can no longer be characterized as having an indolent lymphoma,” the investigators said.
No sponsor or funding source was cited for this study. Dr. Meignan reported receiving fees for travel and expenses from Roche; his associates reported ties to numerous industry sources.
The total metabolic tumor volume, as quantified on PET scanning at the time that follicular lymphoma is diagnosed, is a strong independent predictor of treatment response and patient outcome, according to a report published online in Journal of Clinical Oncology.
Until now, no study has specifically examined the prognostic possibilities of PET-derived total metabolic tumor volume (TMTV) for this malignancy, either on its own or in combination with any of several existing prognostic indices. Those tools use a variety of surrogates to estimate tumor burden. Now that PET is recommended at diagnosis for all cases of follicular lymphoma and anatomic CT data are also available, it is much easier to estimate total tumor burden than it was when those indices were developed, said Michel Meignan, MD, PhD, of Hôpital Henri Mondor, Crétiel (France) and his associates.
It is crucial to identify patients likely to have a poor response to standard treatment, both to spare them the considerable adverse effects of that treatment and to select them for alternative first-line approaches. Even though patient survival has improved markedly during the past decade with the introduction of combined treatment using rituximab plus chemotherapy, approximately 20% of patients still show disease progression within 2 years, and the 5-year overall survival is only 50%, the investigators noted.
To assess the prognostic value of TMTV as assessed by PET, they pooled data from three multicenter prospective studies involving 185 patients with either a high tumor burden or advanced-stage follicular lymphoma. These participants were followed for a median of 63.5 months at 56 medical centers in France, Belgium, Australia, and Italy.
A TMTV threshold of 510 cm3 was found to have the optimal sensitivity (0.46), specificity (0.83), positive predictive value (0.67), and negative predictive value (0.67) for predicting both progression-free and overall survival. The 30% of patients who had a TMTV greater than that cutoff point had markedly inferior 5-year progression-free survival (less than 3 years), while the 70% who had a smaller TMTV had median progression-free survival of more than 6 years, Dr. Meignan and his associates said (J Clin Oncol. 2016 Aug 22. doi:10.1200/JCO.2016.66.9440).
Combining TMTV with other prognostic measures improved predictions even further. Patients who had both a high TMTV and an intermediate to high score on the Follicular Lymphoma International Prognostic Index 2 showed extremely poor outcomes, with a median progression-free survival of only 19 months. “This population can no longer be characterized as having an indolent lymphoma,” the investigators said.
No sponsor or funding source was cited for this study. Dr. Meignan reported receiving fees for travel and expenses from Roche; his associates reported ties to numerous industry sources.
The total metabolic tumor volume, as quantified on PET scanning at the time that follicular lymphoma is diagnosed, is a strong independent predictor of treatment response and patient outcome, according to a report published online in Journal of Clinical Oncology.
Until now, no study has specifically examined the prognostic possibilities of PET-derived total metabolic tumor volume (TMTV) for this malignancy, either on its own or in combination with any of several existing prognostic indices. Those tools use a variety of surrogates to estimate tumor burden. Now that PET is recommended at diagnosis for all cases of follicular lymphoma and anatomic CT data are also available, it is much easier to estimate total tumor burden than it was when those indices were developed, said Michel Meignan, MD, PhD, of Hôpital Henri Mondor, Crétiel (France) and his associates.
It is crucial to identify patients likely to have a poor response to standard treatment, both to spare them the considerable adverse effects of that treatment and to select them for alternative first-line approaches. Even though patient survival has improved markedly during the past decade with the introduction of combined treatment using rituximab plus chemotherapy, approximately 20% of patients still show disease progression within 2 years, and the 5-year overall survival is only 50%, the investigators noted.
To assess the prognostic value of TMTV as assessed by PET, they pooled data from three multicenter prospective studies involving 185 patients with either a high tumor burden or advanced-stage follicular lymphoma. These participants were followed for a median of 63.5 months at 56 medical centers in France, Belgium, Australia, and Italy.
A TMTV threshold of 510 cm3 was found to have the optimal sensitivity (0.46), specificity (0.83), positive predictive value (0.67), and negative predictive value (0.67) for predicting both progression-free and overall survival. The 30% of patients who had a TMTV greater than that cutoff point had markedly inferior 5-year progression-free survival (less than 3 years), while the 70% who had a smaller TMTV had median progression-free survival of more than 6 years, Dr. Meignan and his associates said (J Clin Oncol. 2016 Aug 22. doi:10.1200/JCO.2016.66.9440).
Combining TMTV with other prognostic measures improved predictions even further. Patients who had both a high TMTV and an intermediate to high score on the Follicular Lymphoma International Prognostic Index 2 showed extremely poor outcomes, with a median progression-free survival of only 19 months. “This population can no longer be characterized as having an indolent lymphoma,” the investigators said.
No sponsor or funding source was cited for this study. Dr. Meignan reported receiving fees for travel and expenses from Roche; his associates reported ties to numerous industry sources.
FROM JOURNAL OF CLINICAL ONCOLOGY
Key clinical point: At diagnosis, the total metabolic tumor volume of follicular lymphoma predicts treatment response and patient outcome.
Major finding: A TMTV threshold of 510 cm3 was found to have the optimal sensitivity (0.46), specificity (0.83), positive predictive value (0.67), and negative predictive value (0.67) for predicting both progression-free and overall survival.
Data source: A pooled analysis of three multicenter prospective studies involving 185 patients with a high burden of disease.
Disclosures: No sponsor or funding source was cited for this study. Dr. Meignan reported receiving fees for travel and expenses from Roche; his associates reported ties to numerous industry sources.
Cancer trends shifting in HIV-positive patients
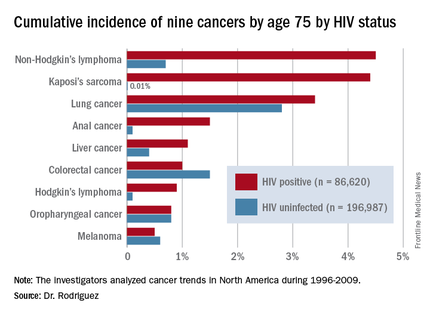
DURBAN, SOUTH AFRICA – The rates of the AIDS-defining cancers Kaposi’s sarcoma and non-Hodgkin’s lymphoma have plummeted in the antiretroviral era, yet they are still the two top cancers in terms of cumulative incidence in HIV-infected patients by age 75, Benigno Rodriguez, MD, said at the 21st International AIDS Conference.
Lung cancer also remains a major concern in the HIV-infected population. Each of these three cancers carries about a 1 in 25 lifetime risk to age 75, the estimated lifespan of HIV-positive patients on combination antiretroviral therapy (ART), according to Dr. Rodriguez of Case Western Reserve University in Cleveland.
ART has resulted in a marked change in cancer trends among HIV-infected patients. The incidence of AIDS-defining cancers has decreased “massively,” Dr. Rodriguez observed; but because ART has extended the lifespan, patients are now living long enough to get other cancers. And they do so at a higher rate than that of the general population because of their impaired immune function, higher rate of risk factors such as smoking, and greater prevalence of oncogenic viral coinfections such as hepatitis B and C and Epstein-Barr virus.
The increase in the incidence and risk of colorectal, liver, and anal cancers among HIV-positive individuals since the introduction of combination ART can largely be explained by the population’s longer exposure to risk due to increasing survival, he said.
Dr. Rodriguez presented highlights of an analysis of cancer trends in North America during 1996-2009. The study was based on 86,620 HIV-infected persons with 475,660 person-years of follow-up and 196,987 subjects without HIV infection and with more than 1.8 million person-years of follow-up. Trends over time were assessed for nine cancers: Kaposi’s sarcoma, non-Hodgkin’s lymphoma, Hodgkin’s lymphoma, and melanoma, as well as anal, lung, colorectal, liver, and oropharyngeal cancers (Ann Intern Med. 2015;163:507-18).
Examining trends in three periods – 1996-1999, 2000-2004, and 2005-2009 – the investigators looked at the impact of ART over time on rates of AIDS-related and non–AIDS-related cancers in HIV-infected patients and compared them to results in the general HIV-uninfected population.
By conducting a competing risk analysis, Dr. Rodriguez and his coworkers were able to estimate the cumulative lifetime risk of the various cancers by age 75, a metric that provides readily understandable information for counseling HIV-infected patients about their long-term cancer risk.
The measure “is more intuitive than using incidence rates,” Dr. Rodriguez said. In a study of 1,578 HIV-infected patients who received the hepatitis B vaccine, for example, those patients whose immune function did not to respond to the vaccine were more likely to be among the 6% of patients who subsequently developed cancer during up to 20 years of follow-up.
The findings on cancer trends in the ART era have clinical implications for cancer screening and prevention in HIV-infected patients. The high rates of smoking and lung cancer in this population make HIV-positive smokers a logical target for lung cancer screening. The rising risk of colorectal cancer – the cumulative lifetime risk to age 75 was 0.4% in 1996-1999, 0.7% in 2000-2004, and 1.3% in 2005-2009 – suggests a need for increased colorectal cancer screening in the older HIV-positive population.
Early and sustained HIV suppression with combination ART remains the only known method of preventing AIDS-defining cancers. Dr. Rodriguez and his coinvestigators in the Centers for AIDS Research Network of Integrated Clinical Systems demonstrated the crucial role of suppressing HIV in a study of 6,036 HIV-infected patients who started on ART and were followed for more than 21,000 person-years. Compared with HIV-infected patients with a 3-month lagged HIV viremia of no more than 50 copies/mL, patients’ risk of developing non-Hodgkin’s lymphoma was 2.1-fold greater if their 3-month lagged HIV viremia was 51-500 copies/mL and 3.56-fold greater if it exceeded 500 copies/mL (Clin Infect Dis. 2014 Jun;58[11]:1599-606).
The study on cancer trends over time was funded by the National Institutes of Health. Dr. Rodriguez reported receiving honoraria from Gilead Sciences.
DURBAN, SOUTH AFRICA – The rates of the AIDS-defining cancers Kaposi’s sarcoma and non-Hodgkin’s lymphoma have plummeted in the antiretroviral era, yet they are still the two top cancers in terms of cumulative incidence in HIV-infected patients by age 75, Benigno Rodriguez, MD, said at the 21st International AIDS Conference.
Lung cancer also remains a major concern in the HIV-infected population. Each of these three cancers carries about a 1 in 25 lifetime risk to age 75, the estimated lifespan of HIV-positive patients on combination antiretroviral therapy (ART), according to Dr. Rodriguez of Case Western Reserve University in Cleveland.
ART has resulted in a marked change in cancer trends among HIV-infected patients. The incidence of AIDS-defining cancers has decreased “massively,” Dr. Rodriguez observed; but because ART has extended the lifespan, patients are now living long enough to get other cancers. And they do so at a higher rate than that of the general population because of their impaired immune function, higher rate of risk factors such as smoking, and greater prevalence of oncogenic viral coinfections such as hepatitis B and C and Epstein-Barr virus.
The increase in the incidence and risk of colorectal, liver, and anal cancers among HIV-positive individuals since the introduction of combination ART can largely be explained by the population’s longer exposure to risk due to increasing survival, he said.
Dr. Rodriguez presented highlights of an analysis of cancer trends in North America during 1996-2009. The study was based on 86,620 HIV-infected persons with 475,660 person-years of follow-up and 196,987 subjects without HIV infection and with more than 1.8 million person-years of follow-up. Trends over time were assessed for nine cancers: Kaposi’s sarcoma, non-Hodgkin’s lymphoma, Hodgkin’s lymphoma, and melanoma, as well as anal, lung, colorectal, liver, and oropharyngeal cancers (Ann Intern Med. 2015;163:507-18).
Examining trends in three periods – 1996-1999, 2000-2004, and 2005-2009 – the investigators looked at the impact of ART over time on rates of AIDS-related and non–AIDS-related cancers in HIV-infected patients and compared them to results in the general HIV-uninfected population.

By conducting a competing risk analysis, Dr. Rodriguez and his coworkers were able to estimate the cumulative lifetime risk of the various cancers by age 75, a metric that provides readily understandable information for counseling HIV-infected patients about their long-term cancer risk.
The measure “is more intuitive than using incidence rates,” Dr. Rodriguez said. In a study of 1,578 HIV-infected patients who received the hepatitis B vaccine, for example, those patients whose immune function did not to respond to the vaccine were more likely to be among the 6% of patients who subsequently developed cancer during up to 20 years of follow-up.
The findings on cancer trends in the ART era have clinical implications for cancer screening and prevention in HIV-infected patients. The high rates of smoking and lung cancer in this population make HIV-positive smokers a logical target for lung cancer screening. The rising risk of colorectal cancer – the cumulative lifetime risk to age 75 was 0.4% in 1996-1999, 0.7% in 2000-2004, and 1.3% in 2005-2009 – suggests a need for increased colorectal cancer screening in the older HIV-positive population.
Early and sustained HIV suppression with combination ART remains the only known method of preventing AIDS-defining cancers. Dr. Rodriguez and his coinvestigators in the Centers for AIDS Research Network of Integrated Clinical Systems demonstrated the crucial role of suppressing HIV in a study of 6,036 HIV-infected patients who started on ART and were followed for more than 21,000 person-years. Compared with HIV-infected patients with a 3-month lagged HIV viremia of no more than 50 copies/mL, patients’ risk of developing non-Hodgkin’s lymphoma was 2.1-fold greater if their 3-month lagged HIV viremia was 51-500 copies/mL and 3.56-fold greater if it exceeded 500 copies/mL (Clin Infect Dis. 2014 Jun;58[11]:1599-606).
The study on cancer trends over time was funded by the National Institutes of Health. Dr. Rodriguez reported receiving honoraria from Gilead Sciences.
DURBAN, SOUTH AFRICA – The rates of the AIDS-defining cancers Kaposi’s sarcoma and non-Hodgkin’s lymphoma have plummeted in the antiretroviral era, yet they are still the two top cancers in terms of cumulative incidence in HIV-infected patients by age 75, Benigno Rodriguez, MD, said at the 21st International AIDS Conference.
Lung cancer also remains a major concern in the HIV-infected population. Each of these three cancers carries about a 1 in 25 lifetime risk to age 75, the estimated lifespan of HIV-positive patients on combination antiretroviral therapy (ART), according to Dr. Rodriguez of Case Western Reserve University in Cleveland.
ART has resulted in a marked change in cancer trends among HIV-infected patients. The incidence of AIDS-defining cancers has decreased “massively,” Dr. Rodriguez observed; but because ART has extended the lifespan, patients are now living long enough to get other cancers. And they do so at a higher rate than that of the general population because of their impaired immune function, higher rate of risk factors such as smoking, and greater prevalence of oncogenic viral coinfections such as hepatitis B and C and Epstein-Barr virus.
The increase in the incidence and risk of colorectal, liver, and anal cancers among HIV-positive individuals since the introduction of combination ART can largely be explained by the population’s longer exposure to risk due to increasing survival, he said.
Dr. Rodriguez presented highlights of an analysis of cancer trends in North America during 1996-2009. The study was based on 86,620 HIV-infected persons with 475,660 person-years of follow-up and 196,987 subjects without HIV infection and with more than 1.8 million person-years of follow-up. Trends over time were assessed for nine cancers: Kaposi’s sarcoma, non-Hodgkin’s lymphoma, Hodgkin’s lymphoma, and melanoma, as well as anal, lung, colorectal, liver, and oropharyngeal cancers (Ann Intern Med. 2015;163:507-18).
Examining trends in three periods – 1996-1999, 2000-2004, and 2005-2009 – the investigators looked at the impact of ART over time on rates of AIDS-related and non–AIDS-related cancers in HIV-infected patients and compared them to results in the general HIV-uninfected population.

By conducting a competing risk analysis, Dr. Rodriguez and his coworkers were able to estimate the cumulative lifetime risk of the various cancers by age 75, a metric that provides readily understandable information for counseling HIV-infected patients about their long-term cancer risk.
The measure “is more intuitive than using incidence rates,” Dr. Rodriguez said. In a study of 1,578 HIV-infected patients who received the hepatitis B vaccine, for example, those patients whose immune function did not to respond to the vaccine were more likely to be among the 6% of patients who subsequently developed cancer during up to 20 years of follow-up.
The findings on cancer trends in the ART era have clinical implications for cancer screening and prevention in HIV-infected patients. The high rates of smoking and lung cancer in this population make HIV-positive smokers a logical target for lung cancer screening. The rising risk of colorectal cancer – the cumulative lifetime risk to age 75 was 0.4% in 1996-1999, 0.7% in 2000-2004, and 1.3% in 2005-2009 – suggests a need for increased colorectal cancer screening in the older HIV-positive population.
Early and sustained HIV suppression with combination ART remains the only known method of preventing AIDS-defining cancers. Dr. Rodriguez and his coinvestigators in the Centers for AIDS Research Network of Integrated Clinical Systems demonstrated the crucial role of suppressing HIV in a study of 6,036 HIV-infected patients who started on ART and were followed for more than 21,000 person-years. Compared with HIV-infected patients with a 3-month lagged HIV viremia of no more than 50 copies/mL, patients’ risk of developing non-Hodgkin’s lymphoma was 2.1-fold greater if their 3-month lagged HIV viremia was 51-500 copies/mL and 3.56-fold greater if it exceeded 500 copies/mL (Clin Infect Dis. 2014 Jun;58[11]:1599-606).
The study on cancer trends over time was funded by the National Institutes of Health. Dr. Rodriguez reported receiving honoraria from Gilead Sciences.
AT AIDS 2016
Key clinical point: HIV-infected persons in North America have roughly a 1 in 25 cumulative lifetime risk of developing lung cancer, Kaposi’s sarcoma, or non-Hodgkin’s lymphoma.
Major finding: The cumulative lifetime risk of developing non-Hodgkin’s lymphoma in HIV-infected patients on combination antiretroviral therapy is sevenfold greater than the risk in the general population.
Data source: A competing risk analysis for nine cancers based upon 86,620 HIV-infected persons followed for 475,660 person-years and 196,987 subjects not infected with HIV and with more than 1.8 million person-years of follow-up.
Disclosures: The National Institutes of Health funded the study. The presenter reported receiving honoraria from Gilead Sciences.
HIV-related lymphoma rate remains sky-high despite ART
DURBAN, SOUTH AFRICA – The good news about non-Hodgkin lymphoma in the setting of HIV infection is that the risk drops dramatically after several years of antiretroviral therapy. The bad news? The risk still remains extraordinarily high, compared with the risk seen in the general population, Mathias Egger, MD, reported at the 21st International AIDS Conference.
That was a key finding in a new analysis of lymphoma trends in more than 210,000 HIV-positive adults on combination antiretroviral therapy (ART) during more than 1.1 million person-years of follow-up in North America, Europe, Latin America, and South Africa.
The non-Hodgkin lymphoma (NHL) incidence rate standardized to 40 years of age was 287 cases per 100,000 person-years. From a pre-ART baseline of about 500 cases per 100,000 person-years, it dropped “massively” within a year after going on ART. Even after 5 years of ART, however, the rate remained in the range of 60-200 cases per 100,000 person-years, depending upon geographic location and HIV transmission route. In contrast, the incidence rate among the general population of the U.S. and Canada, which is among the world’s highest, is less than 10 per 100,000 person-years, according to Dr. Egger, professor of epidemiology and public health at the University of Bern, Switzerland.
The risk of developing NHL in the setting of HIV infection varied by continent. It was slightly higher in HIV-infected patients in North America than in Europe or South Africa, although the South African data are considered unreliable due to underascertainment of cancers.
In Latin American HIV-infected adults the NHL rate was lowest of all, fully 54% lower than in Europe after adjustment for current CD4 cell count, ART regimen and duration, and transmission risk group. The low NHL rate in Latin America was driven by a very low risk in HIV-infected women.
Across the world, NHL rates in patients on ART were consistently lowest in women, intermediate in heterosexual men, and highest in men who have sex with men.
The explanation for the regional variation in NHL trends might plausibly involve differing prevalences of Epstein-Barr virus–2 and other oncogenic viruses as well as differences in the completeness of cancer ascertainment, Dr. Egger said.
While NHL is categorized as an AIDS-defining condition, Hodgkin lymphoma is not. Nonetheless, the risk of Hodgkin lymphoma is markedly increased in the setting of HIV infection. In one classic meta-analysis, it was increased by 11-fold, compared with that seen in the general population (Lancet. 2007 Jul 7;370(9581):59-67).
In a study of more than 41,000 HIV-infected European adults by Dr. Egger and his coworkers, the incidence of Hodgkin lymphoma was 49 cases per 100,000 person-years. Importantly, unlike in NHL, the cumulative incidence and mortality of Hodgkin lymphoma were unaffected by ART (Blood. 2011 Jun 9;117(23):6100-8).
The clinical implication of these trends in HIV-related lymphomas is clear: with more than 2 million new cases of HIV infection occurring annually worldwide, and with infected patients living far longer as ART transforms HIV infection into a chronic manageable condition, physicians can anticipate encountering a steadily growing number of patients with NHL and Hodgkin lymphoma, he said.
The NHL study was funded by the European Union and the U.S. National Institutes of Health. The analysis utilized data collected by the Collaboration of Observational HIV Epidemiology and Research Europe (COHERE) and the International Epidemiologic Databases to Evaluate AIDS (IeDEA). Dr. Egger reported having no financial conflicts of interest.
DURBAN, SOUTH AFRICA – The good news about non-Hodgkin lymphoma in the setting of HIV infection is that the risk drops dramatically after several years of antiretroviral therapy. The bad news? The risk still remains extraordinarily high, compared with the risk seen in the general population, Mathias Egger, MD, reported at the 21st International AIDS Conference.
That was a key finding in a new analysis of lymphoma trends in more than 210,000 HIV-positive adults on combination antiretroviral therapy (ART) during more than 1.1 million person-years of follow-up in North America, Europe, Latin America, and South Africa.
The non-Hodgkin lymphoma (NHL) incidence rate standardized to 40 years of age was 287 cases per 100,000 person-years. From a pre-ART baseline of about 500 cases per 100,000 person-years, it dropped “massively” within a year after going on ART. Even after 5 years of ART, however, the rate remained in the range of 60-200 cases per 100,000 person-years, depending upon geographic location and HIV transmission route. In contrast, the incidence rate among the general population of the U.S. and Canada, which is among the world’s highest, is less than 10 per 100,000 person-years, according to Dr. Egger, professor of epidemiology and public health at the University of Bern, Switzerland.
The risk of developing NHL in the setting of HIV infection varied by continent. It was slightly higher in HIV-infected patients in North America than in Europe or South Africa, although the South African data are considered unreliable due to underascertainment of cancers.
In Latin American HIV-infected adults the NHL rate was lowest of all, fully 54% lower than in Europe after adjustment for current CD4 cell count, ART regimen and duration, and transmission risk group. The low NHL rate in Latin America was driven by a very low risk in HIV-infected women.
Across the world, NHL rates in patients on ART were consistently lowest in women, intermediate in heterosexual men, and highest in men who have sex with men.
The explanation for the regional variation in NHL trends might plausibly involve differing prevalences of Epstein-Barr virus–2 and other oncogenic viruses as well as differences in the completeness of cancer ascertainment, Dr. Egger said.
While NHL is categorized as an AIDS-defining condition, Hodgkin lymphoma is not. Nonetheless, the risk of Hodgkin lymphoma is markedly increased in the setting of HIV infection. In one classic meta-analysis, it was increased by 11-fold, compared with that seen in the general population (Lancet. 2007 Jul 7;370(9581):59-67).
In a study of more than 41,000 HIV-infected European adults by Dr. Egger and his coworkers, the incidence of Hodgkin lymphoma was 49 cases per 100,000 person-years. Importantly, unlike in NHL, the cumulative incidence and mortality of Hodgkin lymphoma were unaffected by ART (Blood. 2011 Jun 9;117(23):6100-8).
The clinical implication of these trends in HIV-related lymphomas is clear: with more than 2 million new cases of HIV infection occurring annually worldwide, and with infected patients living far longer as ART transforms HIV infection into a chronic manageable condition, physicians can anticipate encountering a steadily growing number of patients with NHL and Hodgkin lymphoma, he said.
The NHL study was funded by the European Union and the U.S. National Institutes of Health. The analysis utilized data collected by the Collaboration of Observational HIV Epidemiology and Research Europe (COHERE) and the International Epidemiologic Databases to Evaluate AIDS (IeDEA). Dr. Egger reported having no financial conflicts of interest.
DURBAN, SOUTH AFRICA – The good news about non-Hodgkin lymphoma in the setting of HIV infection is that the risk drops dramatically after several years of antiretroviral therapy. The bad news? The risk still remains extraordinarily high, compared with the risk seen in the general population, Mathias Egger, MD, reported at the 21st International AIDS Conference.
That was a key finding in a new analysis of lymphoma trends in more than 210,000 HIV-positive adults on combination antiretroviral therapy (ART) during more than 1.1 million person-years of follow-up in North America, Europe, Latin America, and South Africa.
The non-Hodgkin lymphoma (NHL) incidence rate standardized to 40 years of age was 287 cases per 100,000 person-years. From a pre-ART baseline of about 500 cases per 100,000 person-years, it dropped “massively” within a year after going on ART. Even after 5 years of ART, however, the rate remained in the range of 60-200 cases per 100,000 person-years, depending upon geographic location and HIV transmission route. In contrast, the incidence rate among the general population of the U.S. and Canada, which is among the world’s highest, is less than 10 per 100,000 person-years, according to Dr. Egger, professor of epidemiology and public health at the University of Bern, Switzerland.
The risk of developing NHL in the setting of HIV infection varied by continent. It was slightly higher in HIV-infected patients in North America than in Europe or South Africa, although the South African data are considered unreliable due to underascertainment of cancers.
In Latin American HIV-infected adults the NHL rate was lowest of all, fully 54% lower than in Europe after adjustment for current CD4 cell count, ART regimen and duration, and transmission risk group. The low NHL rate in Latin America was driven by a very low risk in HIV-infected women.
Across the world, NHL rates in patients on ART were consistently lowest in women, intermediate in heterosexual men, and highest in men who have sex with men.
The explanation for the regional variation in NHL trends might plausibly involve differing prevalences of Epstein-Barr virus–2 and other oncogenic viruses as well as differences in the completeness of cancer ascertainment, Dr. Egger said.
While NHL is categorized as an AIDS-defining condition, Hodgkin lymphoma is not. Nonetheless, the risk of Hodgkin lymphoma is markedly increased in the setting of HIV infection. In one classic meta-analysis, it was increased by 11-fold, compared with that seen in the general population (Lancet. 2007 Jul 7;370(9581):59-67).
In a study of more than 41,000 HIV-infected European adults by Dr. Egger and his coworkers, the incidence of Hodgkin lymphoma was 49 cases per 100,000 person-years. Importantly, unlike in NHL, the cumulative incidence and mortality of Hodgkin lymphoma were unaffected by ART (Blood. 2011 Jun 9;117(23):6100-8).
The clinical implication of these trends in HIV-related lymphomas is clear: with more than 2 million new cases of HIV infection occurring annually worldwide, and with infected patients living far longer as ART transforms HIV infection into a chronic manageable condition, physicians can anticipate encountering a steadily growing number of patients with NHL and Hodgkin lymphoma, he said.
The NHL study was funded by the European Union and the U.S. National Institutes of Health. The analysis utilized data collected by the Collaboration of Observational HIV Epidemiology and Research Europe (COHERE) and the International Epidemiologic Databases to Evaluate AIDS (IeDEA). Dr. Egger reported having no financial conflicts of interest.
AT AIDS 2016
Key clinical point: Antiretroviral therapy has had a major impact upon the incidence of HIV-related non-Hodgkin lymphoma but no effect on Hodgkin lymphoma.
Major finding: The overall incidence of non-Hodgkin lymphoma in HIV-positive adults on antiretroviral therapy is 287 cases per 100,000 person-years, varying by location and route of HIV acquisition.
Data source: This was a longitudinal analysis of non-Hodgkin lymphoma incidence in more than 210,000 HIV-infected adults on combination antiretroviral therapy on four continents.
Disclosures: The study was funded by the European Union and the U.S. National Institutes of Health. The presenter reported having no financial conflicts of interest.
Triptorelin doesn’t prevent ovarian failure in young women treated for lymphoma
Protective treatment with the GnRH agonist triptorelin failed to prevent chemotherapy-induced premature ovarian failure in young women with lymphoma, according to a report published in the Journal of Clinical Oncology.
This type of protective therapy has been used for at least 20 years but is still controversial. Some clinical practice guidelines endorse the practice, but others do not. The evidence is both sparse and ambiguous, said Isabelle Demeestere, MD, of the research laboratory on human reproduction, Université Libre de Bruxelles (Belgium), and her associates.
In what they described as “the first randomized clinical trial providing accurate information on ovarian function and fertility after a median of 5 years of follow-up,” the investigators assessed 67 young women (median age, 26 years) treated for Hodgkin’s or non-Hodgkin’s lymphoma at 15 cancer centers in France, Belgium, and Italy. These patients had been randomly assigned to receive chemotherapy plus triptorelin (32 women) or chemotherapy plus placebo injections (35 women) at baseline.
Premature ovarian failure, as measured by elevated FSH levels, occurred in six of the GnRH group and eight of the control group, a nonsignificant difference. Moreover, the rate of recovery of ovarian function, as measured by at least one FSH level of 15 IU/L or less, was the same between the two study groups. And 17 women (53%) in the GnRH group eventually achieved pregnancy, which is not significantly different from the 43% pregnancy rate in the control group (15 of 35 women).
Five women – two in the GnRH group and three in the control group – achieved pregnancy during long-term follow-up, even though they had been classified as having premature ovarian failure. This “confirms the possibility of incidental ovarian cycle recovery, leading to fertility restoration several years after treatment in this young population,” Dr. Demeestere and her associates wrote (J Clin Oncol. 2016. [doi:10.1200/JCO.2015.65.884]).
The study findings “suggest caution” regarding guidelines that recommend using GnRH agonists to preserve fertility in young women undergoing chemotherapy, they added.
Protective treatment with the GnRH agonist triptorelin failed to prevent chemotherapy-induced premature ovarian failure in young women with lymphoma, according to a report published in the Journal of Clinical Oncology.
This type of protective therapy has been used for at least 20 years but is still controversial. Some clinical practice guidelines endorse the practice, but others do not. The evidence is both sparse and ambiguous, said Isabelle Demeestere, MD, of the research laboratory on human reproduction, Université Libre de Bruxelles (Belgium), and her associates.
In what they described as “the first randomized clinical trial providing accurate information on ovarian function and fertility after a median of 5 years of follow-up,” the investigators assessed 67 young women (median age, 26 years) treated for Hodgkin’s or non-Hodgkin’s lymphoma at 15 cancer centers in France, Belgium, and Italy. These patients had been randomly assigned to receive chemotherapy plus triptorelin (32 women) or chemotherapy plus placebo injections (35 women) at baseline.
Premature ovarian failure, as measured by elevated FSH levels, occurred in six of the GnRH group and eight of the control group, a nonsignificant difference. Moreover, the rate of recovery of ovarian function, as measured by at least one FSH level of 15 IU/L or less, was the same between the two study groups. And 17 women (53%) in the GnRH group eventually achieved pregnancy, which is not significantly different from the 43% pregnancy rate in the control group (15 of 35 women).
Five women – two in the GnRH group and three in the control group – achieved pregnancy during long-term follow-up, even though they had been classified as having premature ovarian failure. This “confirms the possibility of incidental ovarian cycle recovery, leading to fertility restoration several years after treatment in this young population,” Dr. Demeestere and her associates wrote (J Clin Oncol. 2016. [doi:10.1200/JCO.2015.65.884]).
The study findings “suggest caution” regarding guidelines that recommend using GnRH agonists to preserve fertility in young women undergoing chemotherapy, they added.
Protective treatment with the GnRH agonist triptorelin failed to prevent chemotherapy-induced premature ovarian failure in young women with lymphoma, according to a report published in the Journal of Clinical Oncology.
This type of protective therapy has been used for at least 20 years but is still controversial. Some clinical practice guidelines endorse the practice, but others do not. The evidence is both sparse and ambiguous, said Isabelle Demeestere, MD, of the research laboratory on human reproduction, Université Libre de Bruxelles (Belgium), and her associates.
In what they described as “the first randomized clinical trial providing accurate information on ovarian function and fertility after a median of 5 years of follow-up,” the investigators assessed 67 young women (median age, 26 years) treated for Hodgkin’s or non-Hodgkin’s lymphoma at 15 cancer centers in France, Belgium, and Italy. These patients had been randomly assigned to receive chemotherapy plus triptorelin (32 women) or chemotherapy plus placebo injections (35 women) at baseline.
Premature ovarian failure, as measured by elevated FSH levels, occurred in six of the GnRH group and eight of the control group, a nonsignificant difference. Moreover, the rate of recovery of ovarian function, as measured by at least one FSH level of 15 IU/L or less, was the same between the two study groups. And 17 women (53%) in the GnRH group eventually achieved pregnancy, which is not significantly different from the 43% pregnancy rate in the control group (15 of 35 women).
Five women – two in the GnRH group and three in the control group – achieved pregnancy during long-term follow-up, even though they had been classified as having premature ovarian failure. This “confirms the possibility of incidental ovarian cycle recovery, leading to fertility restoration several years after treatment in this young population,” Dr. Demeestere and her associates wrote (J Clin Oncol. 2016. [doi:10.1200/JCO.2015.65.884]).
The study findings “suggest caution” regarding guidelines that recommend using GnRH agonists to preserve fertility in young women undergoing chemotherapy, they added.
FROM JOURNAL OF CLINICAL ONCOLOGY
Key clinical point: The GnRH agonist triptorelin failed to prevent chemotherapy-induced premature ovarian failure in young women with lymphoma.
Major finding: Premature ovarian failure occurred in six of the GnRH group and eight of the control group, a nonsignificant difference.
Data source: A multicenter, randomized clinical trial involving 67 women followed for 5 years.
Disclosures: This study was supported by Fonds National de la Recherche Scientifique and Ipsen Pharmaceutical Group. Dr. Demeestere reported having no relevant financial disclosures; her associates reported ties to numerous industry sources.
Long-term analysis shows dwindling RT benefit for DLBCL
Long-term follow-up from two randomized trials shows that the treatment advantage initially seen by adding radiotherapy to the CHOP regimen in patients with limited-stage diffuse large B-cell lymphoma disappeared over time.
After a median follow-up of nearly 18 years, there were no significant differences in either progression-free survival (PFS) or overall survival between patients who had received standard CHOP chemotherapy (cyclophosphamide, doxorubicin, vincristine, and prednisone) for 8 cycles (CHOP8), or 3 cycles of CHOP with involved-field radiotherapy (CHOP3RT), reported Deborah M. Stephens, DO, of the University of Utah in Salt Lake City, and her colleagues.
The addition of rituximab to CHOP plus involved-field radiotherapy (IFRT) in a separate cohort did not appear to reduce the continued relapse risk, the investigators noted (J Clin Oncol. 2016 Jul. doi: 10.1200/JCO.2015.65.4582).
The findings suggest that long-term follow-up of patients enrolled in clinical trials may provide clinically important additional information, and that limited-stage diffuse large B-cell lymphoma (DLBCL) may have a biology that is distinctly different from that of advanced stage DLBCL, the investigators wrote.
Initial results from the SWOG (Southwest Oncology Group) Study 8736, published after a median follow-up of 4.4 years, showed that CHOP3RT was associated with significantly better 5-year PFS and OS rates, compared with patients treated with CHOP8.
A second trial, SWOG Study 0014, looked at the addition of rituximab (Rituxan) to CHOP plus IFRT in patients with high-risk DLBCL with at least one adverse feature of the stage-modified International Prognostic Index. In this trial, after a median follow-up of 5.3 years, 4-year PFS was 88%, and 4-year OS was 92%.
5 years not enough
Results of both trials were published around the 5-year follow-up mark. However, an analysis of 10-year follow-up data from the S8736 trial, published only as an abstract (Blood. 98:724a-725a, 2001; abstr 3024), showed that “the survival curves for CHOP8 and CHOP3RT, surprisingly, began to overlap,” Dr. Stephens and her colleagues wrote.
“Additionally, for both S8736 and S0014, no plateau had been reached in the PFS curves. These data contrasted the expected cure rates for advanced-stage DLBCL, leading to the hypothesis that limited-stage DLBCL may have a unique biology from its advanced-stage counterpart,” they added.
Although more than half of patients with advanced-stage diffuse large B-cell lymphoma (DLBCL) can be cured with anthracycline-based chemotherapy, cure rates are markedly lower among patients with limited-stage disease, defined as Ann Arbor stage I or II, confined to a single irradiation field. Limited-stage disease accounts for approximately one-third DLBCL cases.
To see whether the trends observed at 10 years continued, the investigators conducted a survival analysis from S8736 and compared the data from similar patients in the S0014 study.
They found that after a median follow-up of 17.7 years in S8736, median PFS was 12.0 years for patients treated with CHOP8, and 11.1 years for patients treated with CHOP3RT, a difference that was not statistically significant.
Median OS was 13.0 and 13.7 years, respectively, and was also not significant.
In S0014, after a median follow-up of 12 years, the 5-year OS rate was 82%, and 10-year rate was 67%. In this trial, the investigators observed “a persistent pattern of relapse despite the addition of rituximab.”
“The populations were not entirely identical; however, even the addition of rituximab as per S0014 to combined-modality therapy did not seem to mitigate the continued relapse risk, underscoring the value of prolonged observation of clinical trial patients and possible unique biology of limited-stage DLBCL,” Dr. Stephens and her associates wrote.
The study was sponsored by grants from the National Institutes of Health. Dr. Stephens reported having no financial disclosures. Several coauthors disclosed research funding, consulting, speakers’ bureau participation or travel expenses from various oncology drug makers.
Long-term follow-up from two randomized trials shows that the treatment advantage initially seen by adding radiotherapy to the CHOP regimen in patients with limited-stage diffuse large B-cell lymphoma disappeared over time.
After a median follow-up of nearly 18 years, there were no significant differences in either progression-free survival (PFS) or overall survival between patients who had received standard CHOP chemotherapy (cyclophosphamide, doxorubicin, vincristine, and prednisone) for 8 cycles (CHOP8), or 3 cycles of CHOP with involved-field radiotherapy (CHOP3RT), reported Deborah M. Stephens, DO, of the University of Utah in Salt Lake City, and her colleagues.
The addition of rituximab to CHOP plus involved-field radiotherapy (IFRT) in a separate cohort did not appear to reduce the continued relapse risk, the investigators noted (J Clin Oncol. 2016 Jul. doi: 10.1200/JCO.2015.65.4582).
The findings suggest that long-term follow-up of patients enrolled in clinical trials may provide clinically important additional information, and that limited-stage diffuse large B-cell lymphoma (DLBCL) may have a biology that is distinctly different from that of advanced stage DLBCL, the investigators wrote.
Initial results from the SWOG (Southwest Oncology Group) Study 8736, published after a median follow-up of 4.4 years, showed that CHOP3RT was associated with significantly better 5-year PFS and OS rates, compared with patients treated with CHOP8.
A second trial, SWOG Study 0014, looked at the addition of rituximab (Rituxan) to CHOP plus IFRT in patients with high-risk DLBCL with at least one adverse feature of the stage-modified International Prognostic Index. In this trial, after a median follow-up of 5.3 years, 4-year PFS was 88%, and 4-year OS was 92%.
5 years not enough
Results of both trials were published around the 5-year follow-up mark. However, an analysis of 10-year follow-up data from the S8736 trial, published only as an abstract (Blood. 98:724a-725a, 2001; abstr 3024), showed that “the survival curves for CHOP8 and CHOP3RT, surprisingly, began to overlap,” Dr. Stephens and her colleagues wrote.
“Additionally, for both S8736 and S0014, no plateau had been reached in the PFS curves. These data contrasted the expected cure rates for advanced-stage DLBCL, leading to the hypothesis that limited-stage DLBCL may have a unique biology from its advanced-stage counterpart,” they added.
Although more than half of patients with advanced-stage diffuse large B-cell lymphoma (DLBCL) can be cured with anthracycline-based chemotherapy, cure rates are markedly lower among patients with limited-stage disease, defined as Ann Arbor stage I or II, confined to a single irradiation field. Limited-stage disease accounts for approximately one-third DLBCL cases.
To see whether the trends observed at 10 years continued, the investigators conducted a survival analysis from S8736 and compared the data from similar patients in the S0014 study.
They found that after a median follow-up of 17.7 years in S8736, median PFS was 12.0 years for patients treated with CHOP8, and 11.1 years for patients treated with CHOP3RT, a difference that was not statistically significant.
Median OS was 13.0 and 13.7 years, respectively, and was also not significant.
In S0014, after a median follow-up of 12 years, the 5-year OS rate was 82%, and 10-year rate was 67%. In this trial, the investigators observed “a persistent pattern of relapse despite the addition of rituximab.”
“The populations were not entirely identical; however, even the addition of rituximab as per S0014 to combined-modality therapy did not seem to mitigate the continued relapse risk, underscoring the value of prolonged observation of clinical trial patients and possible unique biology of limited-stage DLBCL,” Dr. Stephens and her associates wrote.
The study was sponsored by grants from the National Institutes of Health. Dr. Stephens reported having no financial disclosures. Several coauthors disclosed research funding, consulting, speakers’ bureau participation or travel expenses from various oncology drug makers.
Long-term follow-up from two randomized trials shows that the treatment advantage initially seen by adding radiotherapy to the CHOP regimen in patients with limited-stage diffuse large B-cell lymphoma disappeared over time.
After a median follow-up of nearly 18 years, there were no significant differences in either progression-free survival (PFS) or overall survival between patients who had received standard CHOP chemotherapy (cyclophosphamide, doxorubicin, vincristine, and prednisone) for 8 cycles (CHOP8), or 3 cycles of CHOP with involved-field radiotherapy (CHOP3RT), reported Deborah M. Stephens, DO, of the University of Utah in Salt Lake City, and her colleagues.
The addition of rituximab to CHOP plus involved-field radiotherapy (IFRT) in a separate cohort did not appear to reduce the continued relapse risk, the investigators noted (J Clin Oncol. 2016 Jul. doi: 10.1200/JCO.2015.65.4582).
The findings suggest that long-term follow-up of patients enrolled in clinical trials may provide clinically important additional information, and that limited-stage diffuse large B-cell lymphoma (DLBCL) may have a biology that is distinctly different from that of advanced stage DLBCL, the investigators wrote.
Initial results from the SWOG (Southwest Oncology Group) Study 8736, published after a median follow-up of 4.4 years, showed that CHOP3RT was associated with significantly better 5-year PFS and OS rates, compared with patients treated with CHOP8.
A second trial, SWOG Study 0014, looked at the addition of rituximab (Rituxan) to CHOP plus IFRT in patients with high-risk DLBCL with at least one adverse feature of the stage-modified International Prognostic Index. In this trial, after a median follow-up of 5.3 years, 4-year PFS was 88%, and 4-year OS was 92%.
5 years not enough
Results of both trials were published around the 5-year follow-up mark. However, an analysis of 10-year follow-up data from the S8736 trial, published only as an abstract (Blood. 98:724a-725a, 2001; abstr 3024), showed that “the survival curves for CHOP8 and CHOP3RT, surprisingly, began to overlap,” Dr. Stephens and her colleagues wrote.
“Additionally, for both S8736 and S0014, no plateau had been reached in the PFS curves. These data contrasted the expected cure rates for advanced-stage DLBCL, leading to the hypothesis that limited-stage DLBCL may have a unique biology from its advanced-stage counterpart,” they added.
Although more than half of patients with advanced-stage diffuse large B-cell lymphoma (DLBCL) can be cured with anthracycline-based chemotherapy, cure rates are markedly lower among patients with limited-stage disease, defined as Ann Arbor stage I or II, confined to a single irradiation field. Limited-stage disease accounts for approximately one-third DLBCL cases.
To see whether the trends observed at 10 years continued, the investigators conducted a survival analysis from S8736 and compared the data from similar patients in the S0014 study.
They found that after a median follow-up of 17.7 years in S8736, median PFS was 12.0 years for patients treated with CHOP8, and 11.1 years for patients treated with CHOP3RT, a difference that was not statistically significant.
Median OS was 13.0 and 13.7 years, respectively, and was also not significant.
In S0014, after a median follow-up of 12 years, the 5-year OS rate was 82%, and 10-year rate was 67%. In this trial, the investigators observed “a persistent pattern of relapse despite the addition of rituximab.”
“The populations were not entirely identical; however, even the addition of rituximab as per S0014 to combined-modality therapy did not seem to mitigate the continued relapse risk, underscoring the value of prolonged observation of clinical trial patients and possible unique biology of limited-stage DLBCL,” Dr. Stephens and her associates wrote.
The study was sponsored by grants from the National Institutes of Health. Dr. Stephens reported having no financial disclosures. Several coauthors disclosed research funding, consulting, speakers’ bureau participation or travel expenses from various oncology drug makers.
FROM THE JOURNAL OF CLINICAL ONCOLOGY
Key clinical point: Long-term observation of clinical trial patients revealed a dwindling of initial survival benefit from radiation added to chemotherapy in limited-stage DLBCL.
Major finding: At a median 17.7 years of follow-up, there were no significant differences in progression-free or overall survival with eight cycles of CHOP or three cycles with IFRT.
Data source: Final analysis of randomized controlled trial in 401 patients with limited-stage diffuse large B-cell lymphoma.
Disclosures: The study was sponsored by grants from the National Institutes of Health. Dr. Stephens reported having no financial disclosures. Several coauthors disclosed research funding, consulting, speakers’ bureau participation or travel expenses from various oncology drug makers.