User login
American Society of Hematology (ASH): ASH 2016
Ibrutinib, palbociclib yield durable complete responses in pretreated mantle cell lymphoma
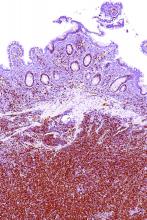
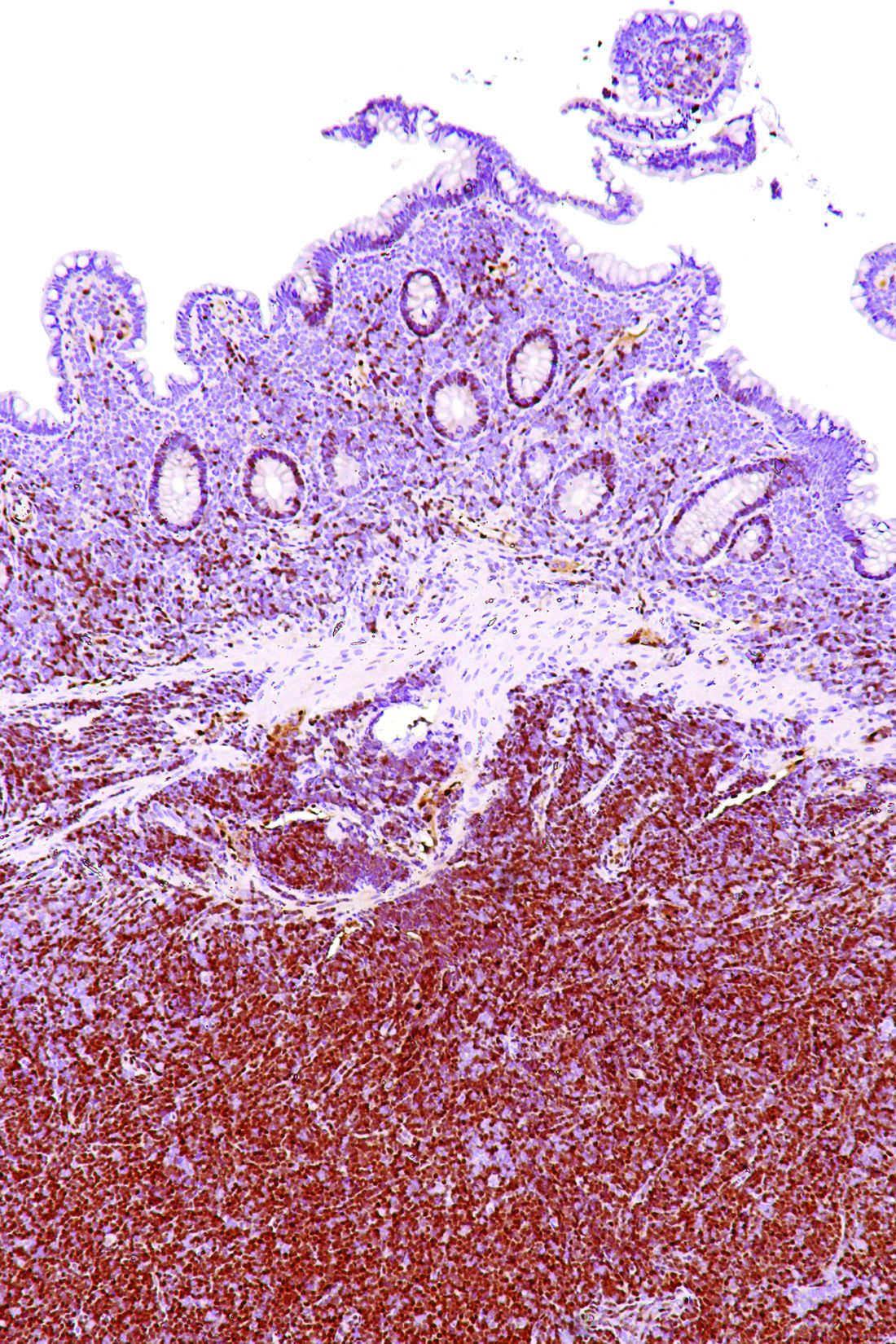
SAN DIEGO – A “mechanism-based” combination of ibrutinib and palbociclib was reasonably well tolerated and induced complete responses in 44% of patients with previously treated mantle cell lymphoma, Peter Martin, MD, reported at the annual meeting of the American Society of Hematology.
Fully 67% of patients remained alive and progression-free after a median of 11 months of follow-up, and no responders progressed during this phase I trial, added Dr. Martin of Weill Cornell Medical College in New York. These rates “appear better than those reported in studies of single-agent ibrutinib, although the number of patients was very small,” he acknowledged. Most patients tolerated therapy, although 25% developed dose-limiting toxicities or stopped treatment because of adverse effects. Based on these results, the investigators are studying biomarkers for resistance and are planning a phase II, multicenter trial to evaluate time to progression.
Single-agent ibrutinib (Imbruvica) has shown promise in mantle cell lymphoma, but treatment failure affects about half of patients within 1 year, Dr. Martin noted. The CDK4/6 inhibitor palbociclib (Ibrance) induces prolonged arrest early in the G1 phase of the cell cycle, which overcame ibrutinib resistance in mantle cell lymphoma cell lines in a prior study (Cancer Discov. 2014;4[9]:1022-35).
To test the maximum tolerated dose of combination therapy, Dr. Martin and his associates enrolled 20 adults with previously treated mantle cell lymphoma who were naive to ibrutinib and CD4/6 inhibitors. The patients had received a median of one and up to five prior lines of therapy, and six (30%) were refractory to their most recent therapy. They received ibrutinib daily and palbociclib on the first 21 days of each 28-day treatment cycle. Dosing began at one of five levels, ranging from 280 mg ibrutinib/75 mg palbociclib to 560 mg ibrutinib/125 mg palbociclib. Doses were escalated based on a standard phase I 3+3 design.
Among 18 patients evaluated, 12 (67%) responded to treatment, and 8 (44%) had a complete response. Median time to complete response was three cycles. The most common grade 1-2 adverse events were diarrhea, fatigue, rash, and bruising. Three patients (15%) developed dose-limiting toxicities. These included one case of grade 4 thrombocytopenia at 420 mg ibrutinib/100 mg palbociclib and two cases of grade 3 rash at 560 mg ibrutinib/125 mg palbociclib. The grade 3 rashes led to dose reductions, and six patients needed dose interruptions. Also, four patients stopped treatment because of disease progression, two did so because of elevated liver enzymes or prolonged cytopenia, and one did so to undergo allogeneic stem cell transplantation.
The National Cancer Institute sponsored the study. Dr. Martin disclosed ties to Janssen, which makes ibrutinib, and to Celgene, Gilead, Novartis, Acerta, and Teva. Senior author John P. Leonard, MD, and one of 10 coinvestigators disclosed ties to several pharmaceutical companies.
SAN DIEGO – A “mechanism-based” combination of ibrutinib and palbociclib was reasonably well tolerated and induced complete responses in 44% of patients with previously treated mantle cell lymphoma, Peter Martin, MD, reported at the annual meeting of the American Society of Hematology.
Fully 67% of patients remained alive and progression-free after a median of 11 months of follow-up, and no responders progressed during this phase I trial, added Dr. Martin of Weill Cornell Medical College in New York. These rates “appear better than those reported in studies of single-agent ibrutinib, although the number of patients was very small,” he acknowledged. Most patients tolerated therapy, although 25% developed dose-limiting toxicities or stopped treatment because of adverse effects. Based on these results, the investigators are studying biomarkers for resistance and are planning a phase II, multicenter trial to evaluate time to progression.
Single-agent ibrutinib (Imbruvica) has shown promise in mantle cell lymphoma, but treatment failure affects about half of patients within 1 year, Dr. Martin noted. The CDK4/6 inhibitor palbociclib (Ibrance) induces prolonged arrest early in the G1 phase of the cell cycle, which overcame ibrutinib resistance in mantle cell lymphoma cell lines in a prior study (Cancer Discov. 2014;4[9]:1022-35).
To test the maximum tolerated dose of combination therapy, Dr. Martin and his associates enrolled 20 adults with previously treated mantle cell lymphoma who were naive to ibrutinib and CD4/6 inhibitors. The patients had received a median of one and up to five prior lines of therapy, and six (30%) were refractory to their most recent therapy. They received ibrutinib daily and palbociclib on the first 21 days of each 28-day treatment cycle. Dosing began at one of five levels, ranging from 280 mg ibrutinib/75 mg palbociclib to 560 mg ibrutinib/125 mg palbociclib. Doses were escalated based on a standard phase I 3+3 design.
Among 18 patients evaluated, 12 (67%) responded to treatment, and 8 (44%) had a complete response. Median time to complete response was three cycles. The most common grade 1-2 adverse events were diarrhea, fatigue, rash, and bruising. Three patients (15%) developed dose-limiting toxicities. These included one case of grade 4 thrombocytopenia at 420 mg ibrutinib/100 mg palbociclib and two cases of grade 3 rash at 560 mg ibrutinib/125 mg palbociclib. The grade 3 rashes led to dose reductions, and six patients needed dose interruptions. Also, four patients stopped treatment because of disease progression, two did so because of elevated liver enzymes or prolonged cytopenia, and one did so to undergo allogeneic stem cell transplantation.
The National Cancer Institute sponsored the study. Dr. Martin disclosed ties to Janssen, which makes ibrutinib, and to Celgene, Gilead, Novartis, Acerta, and Teva. Senior author John P. Leonard, MD, and one of 10 coinvestigators disclosed ties to several pharmaceutical companies.
SAN DIEGO – A “mechanism-based” combination of ibrutinib and palbociclib was reasonably well tolerated and induced complete responses in 44% of patients with previously treated mantle cell lymphoma, Peter Martin, MD, reported at the annual meeting of the American Society of Hematology.
Fully 67% of patients remained alive and progression-free after a median of 11 months of follow-up, and no responders progressed during this phase I trial, added Dr. Martin of Weill Cornell Medical College in New York. These rates “appear better than those reported in studies of single-agent ibrutinib, although the number of patients was very small,” he acknowledged. Most patients tolerated therapy, although 25% developed dose-limiting toxicities or stopped treatment because of adverse effects. Based on these results, the investigators are studying biomarkers for resistance and are planning a phase II, multicenter trial to evaluate time to progression.
Single-agent ibrutinib (Imbruvica) has shown promise in mantle cell lymphoma, but treatment failure affects about half of patients within 1 year, Dr. Martin noted. The CDK4/6 inhibitor palbociclib (Ibrance) induces prolonged arrest early in the G1 phase of the cell cycle, which overcame ibrutinib resistance in mantle cell lymphoma cell lines in a prior study (Cancer Discov. 2014;4[9]:1022-35).
To test the maximum tolerated dose of combination therapy, Dr. Martin and his associates enrolled 20 adults with previously treated mantle cell lymphoma who were naive to ibrutinib and CD4/6 inhibitors. The patients had received a median of one and up to five prior lines of therapy, and six (30%) were refractory to their most recent therapy. They received ibrutinib daily and palbociclib on the first 21 days of each 28-day treatment cycle. Dosing began at one of five levels, ranging from 280 mg ibrutinib/75 mg palbociclib to 560 mg ibrutinib/125 mg palbociclib. Doses were escalated based on a standard phase I 3+3 design.
Among 18 patients evaluated, 12 (67%) responded to treatment, and 8 (44%) had a complete response. Median time to complete response was three cycles. The most common grade 1-2 adverse events were diarrhea, fatigue, rash, and bruising. Three patients (15%) developed dose-limiting toxicities. These included one case of grade 4 thrombocytopenia at 420 mg ibrutinib/100 mg palbociclib and two cases of grade 3 rash at 560 mg ibrutinib/125 mg palbociclib. The grade 3 rashes led to dose reductions, and six patients needed dose interruptions. Also, four patients stopped treatment because of disease progression, two did so because of elevated liver enzymes or prolonged cytopenia, and one did so to undergo allogeneic stem cell transplantation.
The National Cancer Institute sponsored the study. Dr. Martin disclosed ties to Janssen, which makes ibrutinib, and to Celgene, Gilead, Novartis, Acerta, and Teva. Senior author John P. Leonard, MD, and one of 10 coinvestigators disclosed ties to several pharmaceutical companies.
AT ASH 2016
Key clinical point: Combination therapy with ibrutinib and palbociclib was generally well tolerated and induced complete responses in patients with pretreated mantle cell lymphoma.
Major finding: A total of 44% of patients had complete responses, and 67% remained alive and progression-free after a median of 11 months of follow-up. Severe rashes occurred at the highest dose studied (420 mg ibrutinib/100 mg palbociclib).
Data source: A phase I trial of 20 patients with previously treated mantle cell lymphoma.
Disclosures: The National Cancer Institute sponsored the study. Dr. Martin disclosed ties to Janssen, which makes ibrutinib, and to Celgene, Gilead, Novartis, Acerta, and Teva. Senior author John P. Leonard, MD, and one of 10 coinvestigators disclosed ties to several pharmaceutical companies.
Bortezomib-based regimen led to durable remissions in mantle cell lymphoma
SAN DIEGO – For adults with mantle cell lymphoma, adding bortezomib to a modified hyper-CVAD (VcR-CVAD) regimen followed by rituximab maintenance induced durable remissions at rates resembling those seen with more intensive chemotherapy followed by autologous hematopoietic stem cell transplantation, according to long-term results from a multicenter phase II trial.
Two-thirds of patients were alive and 50% remained in remission after a median follow-up period of 7.8 years, said Julie E. Chang, MD, who reported the results of the study at the annual meeting of the American Society of Hematology. “VcR-CVAD is a moderate-intensity chemotherapy regimen that is tolerable for many older and less fit adult patients as first-line therapy of mantle cell lymphoma,” she emphasized.
Mantle cell lymphoma lacks a clear standard first-line therapy, noted Dr. Chang of the University of Wisconsin in Madison. “We hypothesized that the addition of bortezomib would improve the complete response rate, and maintenance rituximab would improve the remission duration,” she said.
To test that idea, she and her associates enrolled 30 adults with histologically confirmed mantle cell lymphoma who had received either no treatment or just one cycle of CHOP or CHOP-like chemotherapy.
Patients received six 21-day cycles of VcR-CVAD induction chemotherapy. This regimen consisted of rituximab (375 mg/m2 IV) on day 1; bortezomib (1.3 mg/m2 IV) on days 1 and 4; cyclophosphamide (300 mg/m2 IV every 12 hours) on days 1 through 3; doxorubicin (50 mg/m2 IV given as a continuous infusion) on days 1 and 2; vincristine (1 mg IV) on day 3; and dexamethasone (40 mg orally) on days 1 through 4.
Patients were permitted all supportive care measures, including prophylaxis for tumor lysis syndrome, transfusions, and antibiotics. Those with at least a partial response received rituximab consolidation (375 mg/m2 IV per week for 4 weeks) followed by rituximab maintenance (375 mg/m2 IV every 12 weeks for 5 years).
Median age was 61 years (range, 48-74 years), 80% of patients were male, all had advanced-stage disease, 60% were mantle cell lymphoma international prognostic index (MIPI) medium or high risk, and six had blastic morphology, the researchers noted.
Estimated 6-year rates of progression-free and overall survival were 53% (95% confidence interval, 38%-75%) and 70% (95% CI, 55%-84%), respectively. Neither age nor MIPI score significantly affected the chances of progression-free or overall survival, but there was a trend toward worse survival among MIPI high-risk patients.
The 10 deaths included 5 from progressive disease, 3 from complications after allogeneic transplant, and 2 from unrelated causes. No patients who remained progression free for 5 years subsequently relapsed, nor were there late toxicities related to treatment.
A recent phase III trial (N Engl J Med. 2015 Mar 5;372[10]:944-53) confirmed the benefits of adding bortezomib to standard immunochemotherapy in mantle cell lymphoma, Dr. Chang noted. “VcR-CVAD remains an effective therapy choice for initial treatment of MCL, both in younger and older MCL populations,” she concluded.
Dr. Chang had no relevant disclosures. Senior author Brad S. Kahl, MD, disclosed ties to Celgene, Gilead, Infinity, Juno, Pharmacyclics, and Seattle Genetics.
SAN DIEGO – For adults with mantle cell lymphoma, adding bortezomib to a modified hyper-CVAD (VcR-CVAD) regimen followed by rituximab maintenance induced durable remissions at rates resembling those seen with more intensive chemotherapy followed by autologous hematopoietic stem cell transplantation, according to long-term results from a multicenter phase II trial.
Two-thirds of patients were alive and 50% remained in remission after a median follow-up period of 7.8 years, said Julie E. Chang, MD, who reported the results of the study at the annual meeting of the American Society of Hematology. “VcR-CVAD is a moderate-intensity chemotherapy regimen that is tolerable for many older and less fit adult patients as first-line therapy of mantle cell lymphoma,” she emphasized.
Mantle cell lymphoma lacks a clear standard first-line therapy, noted Dr. Chang of the University of Wisconsin in Madison. “We hypothesized that the addition of bortezomib would improve the complete response rate, and maintenance rituximab would improve the remission duration,” she said.
To test that idea, she and her associates enrolled 30 adults with histologically confirmed mantle cell lymphoma who had received either no treatment or just one cycle of CHOP or CHOP-like chemotherapy.
Patients received six 21-day cycles of VcR-CVAD induction chemotherapy. This regimen consisted of rituximab (375 mg/m2 IV) on day 1; bortezomib (1.3 mg/m2 IV) on days 1 and 4; cyclophosphamide (300 mg/m2 IV every 12 hours) on days 1 through 3; doxorubicin (50 mg/m2 IV given as a continuous infusion) on days 1 and 2; vincristine (1 mg IV) on day 3; and dexamethasone (40 mg orally) on days 1 through 4.
Patients were permitted all supportive care measures, including prophylaxis for tumor lysis syndrome, transfusions, and antibiotics. Those with at least a partial response received rituximab consolidation (375 mg/m2 IV per week for 4 weeks) followed by rituximab maintenance (375 mg/m2 IV every 12 weeks for 5 years).
Median age was 61 years (range, 48-74 years), 80% of patients were male, all had advanced-stage disease, 60% were mantle cell lymphoma international prognostic index (MIPI) medium or high risk, and six had blastic morphology, the researchers noted.
Estimated 6-year rates of progression-free and overall survival were 53% (95% confidence interval, 38%-75%) and 70% (95% CI, 55%-84%), respectively. Neither age nor MIPI score significantly affected the chances of progression-free or overall survival, but there was a trend toward worse survival among MIPI high-risk patients.
The 10 deaths included 5 from progressive disease, 3 from complications after allogeneic transplant, and 2 from unrelated causes. No patients who remained progression free for 5 years subsequently relapsed, nor were there late toxicities related to treatment.
A recent phase III trial (N Engl J Med. 2015 Mar 5;372[10]:944-53) confirmed the benefits of adding bortezomib to standard immunochemotherapy in mantle cell lymphoma, Dr. Chang noted. “VcR-CVAD remains an effective therapy choice for initial treatment of MCL, both in younger and older MCL populations,” she concluded.
Dr. Chang had no relevant disclosures. Senior author Brad S. Kahl, MD, disclosed ties to Celgene, Gilead, Infinity, Juno, Pharmacyclics, and Seattle Genetics.
SAN DIEGO – For adults with mantle cell lymphoma, adding bortezomib to a modified hyper-CVAD (VcR-CVAD) regimen followed by rituximab maintenance induced durable remissions at rates resembling those seen with more intensive chemotherapy followed by autologous hematopoietic stem cell transplantation, according to long-term results from a multicenter phase II trial.
Two-thirds of patients were alive and 50% remained in remission after a median follow-up period of 7.8 years, said Julie E. Chang, MD, who reported the results of the study at the annual meeting of the American Society of Hematology. “VcR-CVAD is a moderate-intensity chemotherapy regimen that is tolerable for many older and less fit adult patients as first-line therapy of mantle cell lymphoma,” she emphasized.
Mantle cell lymphoma lacks a clear standard first-line therapy, noted Dr. Chang of the University of Wisconsin in Madison. “We hypothesized that the addition of bortezomib would improve the complete response rate, and maintenance rituximab would improve the remission duration,” she said.
To test that idea, she and her associates enrolled 30 adults with histologically confirmed mantle cell lymphoma who had received either no treatment or just one cycle of CHOP or CHOP-like chemotherapy.
Patients received six 21-day cycles of VcR-CVAD induction chemotherapy. This regimen consisted of rituximab (375 mg/m2 IV) on day 1; bortezomib (1.3 mg/m2 IV) on days 1 and 4; cyclophosphamide (300 mg/m2 IV every 12 hours) on days 1 through 3; doxorubicin (50 mg/m2 IV given as a continuous infusion) on days 1 and 2; vincristine (1 mg IV) on day 3; and dexamethasone (40 mg orally) on days 1 through 4.
Patients were permitted all supportive care measures, including prophylaxis for tumor lysis syndrome, transfusions, and antibiotics. Those with at least a partial response received rituximab consolidation (375 mg/m2 IV per week for 4 weeks) followed by rituximab maintenance (375 mg/m2 IV every 12 weeks for 5 years).
Median age was 61 years (range, 48-74 years), 80% of patients were male, all had advanced-stage disease, 60% were mantle cell lymphoma international prognostic index (MIPI) medium or high risk, and six had blastic morphology, the researchers noted.
Estimated 6-year rates of progression-free and overall survival were 53% (95% confidence interval, 38%-75%) and 70% (95% CI, 55%-84%), respectively. Neither age nor MIPI score significantly affected the chances of progression-free or overall survival, but there was a trend toward worse survival among MIPI high-risk patients.
The 10 deaths included 5 from progressive disease, 3 from complications after allogeneic transplant, and 2 from unrelated causes. No patients who remained progression free for 5 years subsequently relapsed, nor were there late toxicities related to treatment.
A recent phase III trial (N Engl J Med. 2015 Mar 5;372[10]:944-53) confirmed the benefits of adding bortezomib to standard immunochemotherapy in mantle cell lymphoma, Dr. Chang noted. “VcR-CVAD remains an effective therapy choice for initial treatment of MCL, both in younger and older MCL populations,” she concluded.
Dr. Chang had no relevant disclosures. Senior author Brad S. Kahl, MD, disclosed ties to Celgene, Gilead, Infinity, Juno, Pharmacyclics, and Seattle Genetics.
Key clinical point: First-line therapy with bortezomib plus modified hyper-CVAD followed by rituximab maintenance induced durable remissions in mantle cell lymphoma.
Major finding: Two-thirds of patients were alive and 50% remained in remission after a median follow-up period of 7.8 years.
Data source: A multicenter phase II trial of 30 adults with mantle cell lymphoma who were treatment-naïve or had received only one cycle of CHOP or CHOP-like chemotherapy.
Disclosures: Dr. Chang had no relevant disclosures. Senior author Brad S. Kahl, MD, disclosed ties to Celgene, Gilead, Infinity, Juno, Pharmacyclics, and Seattle Genetics.
Rituximab after ASCT boosted survival in mantle cell lymphoma
SAN DIEGO – Maintenance therapy every other month with rituximab significantly prolonged event-free and overall survival after autologous stem cell transplantation among younger patients with mantle cell lymphoma, based on results from a multicenter, randomized, phase 3 trial.
After a median follow-up period of 50 months, 79% of the rituximab maintenance arm remained alive and free of progression, relapse, and severe infection, compared with 61% of the no-maintenance arm (P = .001), said Steven Le Gouill, MD, PhD, at the 2016 meeting of the American Society of Hematology.
This is the first study linking rituximab maintenance after ASCT to improved survival in younger patients with mantle cell lymphoma, said Dr. Le Gouill of Nantes University Hospital in Nantes, France.
The trial “demonstrates for the first time that rituximab maintenance after ASCT prolongs event-free survival, progression-free survival, and overall survival” in younger patients with treatment-naïve mantle cell lymphoma, Dr. Le Gouill said. The findings confirm rituximab maintenance as “a new standard of care” in younger patients with mantle cell lymphoma, he concluded.
Prior research http://www.nejm.org/doi/full/10.1056/NEJMoa1200920#t=abstract supports maintenance therapy with rituximab rather than interferon alfa for older patients whose mantle cell lymphoma responded to induction with rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone (R-CHOP), noted Dr. Le Gouill. To examine outcomes in younger patients with treatment-naïve mantle cell lymphoma, he and his associates treated 299 individuals aged 65 years and younger (median age, 57 years) with standard induction consisting of 4 courses of rituximab, dexamethasone, high-dose cytarabine, and salt platinum (R-DHAP) every 21 days, followed by conditioning with rituximab plus BiCNU, etoposide, cytarabine, and melphalan (R-BEAM) and ASCT. Patients without at least a partial response to R-DHAP received 4 additional courses of R-CHOP-14 before ASCT. Patients then were randomized either to no maintenance or to infusions of 375 mg R per m2 every 2 months for 3 years.
A total of 53% of patients were mantle cell lymphoma international prognostic index (MIPI) low risk, 27% were intermediate and 19% were high-risk. Rituximab maintenance was associated with a 60% lower risk of progression (HR, 0.4; 95% CI, 0.23 to 0.68; P = .0007) and a 50% lower risk of death (HR, 0.5; 95% CI, 0.25 to 0.98; P = .04).
The French Innovative Leukemia Organisation sponsored the trial. Dr. Le Gouill disclosed ties to Roche, Janssen-Cilag, and Celgene.
SAN DIEGO – Maintenance therapy every other month with rituximab significantly prolonged event-free and overall survival after autologous stem cell transplantation among younger patients with mantle cell lymphoma, based on results from a multicenter, randomized, phase 3 trial.
After a median follow-up period of 50 months, 79% of the rituximab maintenance arm remained alive and free of progression, relapse, and severe infection, compared with 61% of the no-maintenance arm (P = .001), said Steven Le Gouill, MD, PhD, at the 2016 meeting of the American Society of Hematology.
This is the first study linking rituximab maintenance after ASCT to improved survival in younger patients with mantle cell lymphoma, said Dr. Le Gouill of Nantes University Hospital in Nantes, France.
The trial “demonstrates for the first time that rituximab maintenance after ASCT prolongs event-free survival, progression-free survival, and overall survival” in younger patients with treatment-naïve mantle cell lymphoma, Dr. Le Gouill said. The findings confirm rituximab maintenance as “a new standard of care” in younger patients with mantle cell lymphoma, he concluded.
Prior research http://www.nejm.org/doi/full/10.1056/NEJMoa1200920#t=abstract supports maintenance therapy with rituximab rather than interferon alfa for older patients whose mantle cell lymphoma responded to induction with rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone (R-CHOP), noted Dr. Le Gouill. To examine outcomes in younger patients with treatment-naïve mantle cell lymphoma, he and his associates treated 299 individuals aged 65 years and younger (median age, 57 years) with standard induction consisting of 4 courses of rituximab, dexamethasone, high-dose cytarabine, and salt platinum (R-DHAP) every 21 days, followed by conditioning with rituximab plus BiCNU, etoposide, cytarabine, and melphalan (R-BEAM) and ASCT. Patients without at least a partial response to R-DHAP received 4 additional courses of R-CHOP-14 before ASCT. Patients then were randomized either to no maintenance or to infusions of 375 mg R per m2 every 2 months for 3 years.
A total of 53% of patients were mantle cell lymphoma international prognostic index (MIPI) low risk, 27% were intermediate and 19% were high-risk. Rituximab maintenance was associated with a 60% lower risk of progression (HR, 0.4; 95% CI, 0.23 to 0.68; P = .0007) and a 50% lower risk of death (HR, 0.5; 95% CI, 0.25 to 0.98; P = .04).
The French Innovative Leukemia Organisation sponsored the trial. Dr. Le Gouill disclosed ties to Roche, Janssen-Cilag, and Celgene.
SAN DIEGO – Maintenance therapy every other month with rituximab significantly prolonged event-free and overall survival after autologous stem cell transplantation among younger patients with mantle cell lymphoma, based on results from a multicenter, randomized, phase 3 trial.
After a median follow-up period of 50 months, 79% of the rituximab maintenance arm remained alive and free of progression, relapse, and severe infection, compared with 61% of the no-maintenance arm (P = .001), said Steven Le Gouill, MD, PhD, at the 2016 meeting of the American Society of Hematology.
This is the first study linking rituximab maintenance after ASCT to improved survival in younger patients with mantle cell lymphoma, said Dr. Le Gouill of Nantes University Hospital in Nantes, France.
The trial “demonstrates for the first time that rituximab maintenance after ASCT prolongs event-free survival, progression-free survival, and overall survival” in younger patients with treatment-naïve mantle cell lymphoma, Dr. Le Gouill said. The findings confirm rituximab maintenance as “a new standard of care” in younger patients with mantle cell lymphoma, he concluded.
Prior research http://www.nejm.org/doi/full/10.1056/NEJMoa1200920#t=abstract supports maintenance therapy with rituximab rather than interferon alfa for older patients whose mantle cell lymphoma responded to induction with rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone (R-CHOP), noted Dr. Le Gouill. To examine outcomes in younger patients with treatment-naïve mantle cell lymphoma, he and his associates treated 299 individuals aged 65 years and younger (median age, 57 years) with standard induction consisting of 4 courses of rituximab, dexamethasone, high-dose cytarabine, and salt platinum (R-DHAP) every 21 days, followed by conditioning with rituximab plus BiCNU, etoposide, cytarabine, and melphalan (R-BEAM) and ASCT. Patients without at least a partial response to R-DHAP received 4 additional courses of R-CHOP-14 before ASCT. Patients then were randomized either to no maintenance or to infusions of 375 mg R per m2 every 2 months for 3 years.
A total of 53% of patients were mantle cell lymphoma international prognostic index (MIPI) low risk, 27% were intermediate and 19% were high-risk. Rituximab maintenance was associated with a 60% lower risk of progression (HR, 0.4; 95% CI, 0.23 to 0.68; P = .0007) and a 50% lower risk of death (HR, 0.5; 95% CI, 0.25 to 0.98; P = .04).
The French Innovative Leukemia Organisation sponsored the trial. Dr. Le Gouill disclosed ties to Roche, Janssen-Cilag, and Celgene.
AT ASH 2016
Key clinical point: Maintenance therapy with rituximab after autologous stem cell transplantation was associated with significantly increased survival among younger patients with mantle cell lymphoma.
Major finding: After a median follow-up time of 50 months, 79% of patients who received rituximab maintenance remained alive and free of progression, relapse, and severe infection, compared with 61% of those who received no maintenance therapy (P = .001).
Data source: A multicenter randomized phase 3 trial of 299 adults up to 65 years old with mantle cell lymphoma.
Disclosures: The French Innovative Leukemia Organisation sponsored the trial. Dr. Le Gouill disclosed ties to Roche, Janssen-Cilag, and Celgene.
VIDEO: Despite toxicities, ibrutinib is beneficial for treatment-resistant graft-vs.-host disease
SAN DIEGO – An oral regimen of 420 mg ibrutinib achieved complete response in one-third of allogeneic stem cell recipients with chronic graft-vs.-host disease, David Miklos, MD, reported during a late-breaker session at the annual meeting of the American Society of Hematology.
Fully 79% of patients in this open-label phase II study were considered responders when first assessed, 71% of responses lasted at least 5 months, and patients whose disease involved multiple organs generally showed responses in at least two organs, said Dr. Miklos of Stanford (Calif.) University.
Ibrutinib is a first-in-class Bruton’s tyrosine kinase (BTK) inhibitor. Cardiotoxicities have been a concern with ibrutinib, but were not observed in this cohort of 42 patients whose graft-vs.-host disease had not benefited from frontline therapy, Dr. Miklos said during a video interview. However, 52% of patients in this study developed other serious adverse events that are typical with ibrutinib, including pneumonia, septic shock, and fever, he said.
Chronic graft-vs.-host disease is the most common morbidity after allogeneic transplant. This is an “orphan disease” – there are no approved therapies for patients for whom corticosteroids are ineffective, Dr. Miklos noted. Based on these results, investigators are planning a randomized, placebo-controlled, phase III study, he added.
Ibrutinib is jointly commercialized and developed by Janssen Biotech and by Pharmacyclics LLC, an Abbvie company. Dr. Miklos disclosed a consulting relationship, travel and expenses reimbursements, and research funding from Pharmacyclics.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
SAN DIEGO – An oral regimen of 420 mg ibrutinib achieved complete response in one-third of allogeneic stem cell recipients with chronic graft-vs.-host disease, David Miklos, MD, reported during a late-breaker session at the annual meeting of the American Society of Hematology.
Fully 79% of patients in this open-label phase II study were considered responders when first assessed, 71% of responses lasted at least 5 months, and patients whose disease involved multiple organs generally showed responses in at least two organs, said Dr. Miklos of Stanford (Calif.) University.
Ibrutinib is a first-in-class Bruton’s tyrosine kinase (BTK) inhibitor. Cardiotoxicities have been a concern with ibrutinib, but were not observed in this cohort of 42 patients whose graft-vs.-host disease had not benefited from frontline therapy, Dr. Miklos said during a video interview. However, 52% of patients in this study developed other serious adverse events that are typical with ibrutinib, including pneumonia, septic shock, and fever, he said.
Chronic graft-vs.-host disease is the most common morbidity after allogeneic transplant. This is an “orphan disease” – there are no approved therapies for patients for whom corticosteroids are ineffective, Dr. Miklos noted. Based on these results, investigators are planning a randomized, placebo-controlled, phase III study, he added.
Ibrutinib is jointly commercialized and developed by Janssen Biotech and by Pharmacyclics LLC, an Abbvie company. Dr. Miklos disclosed a consulting relationship, travel and expenses reimbursements, and research funding from Pharmacyclics.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
SAN DIEGO – An oral regimen of 420 mg ibrutinib achieved complete response in one-third of allogeneic stem cell recipients with chronic graft-vs.-host disease, David Miklos, MD, reported during a late-breaker session at the annual meeting of the American Society of Hematology.
Fully 79% of patients in this open-label phase II study were considered responders when first assessed, 71% of responses lasted at least 5 months, and patients whose disease involved multiple organs generally showed responses in at least two organs, said Dr. Miklos of Stanford (Calif.) University.
Ibrutinib is a first-in-class Bruton’s tyrosine kinase (BTK) inhibitor. Cardiotoxicities have been a concern with ibrutinib, but were not observed in this cohort of 42 patients whose graft-vs.-host disease had not benefited from frontline therapy, Dr. Miklos said during a video interview. However, 52% of patients in this study developed other serious adverse events that are typical with ibrutinib, including pneumonia, septic shock, and fever, he said.
Chronic graft-vs.-host disease is the most common morbidity after allogeneic transplant. This is an “orphan disease” – there are no approved therapies for patients for whom corticosteroids are ineffective, Dr. Miklos noted. Based on these results, investigators are planning a randomized, placebo-controlled, phase III study, he added.
Ibrutinib is jointly commercialized and developed by Janssen Biotech and by Pharmacyclics LLC, an Abbvie company. Dr. Miklos disclosed a consulting relationship, travel and expenses reimbursements, and research funding from Pharmacyclics.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
AT ASH 2016
Key clinical point: Ibrutinib (420 mg) led to complete responses in one-third of patients with chronic, treatment-resistant graft-vs-host disease.
Major finding: No cardiotoxicities were observed, but 52% of patients had other serious adverse effects, such as sepsis, pyrexia, and pneumonia.
Data source: An open-label phase II study of 42 patients who developed chronic, treatment-resistant graft-vs.-host disease after undergoing allogeneic stem cell transplantation.
Disclosures: Ibrutinib is jointly commercialized and developed by Janssen Biotech and by Pharmacyclics LLC, an Abbvie company. Dr. Miklos disclosed a consulting relationship, reimbursement for travel and expenses, and research funding from Pharmacyclics.
Bortezomib bolsters hematologic response in AL amyloidosis
SAN DIEGO – Adding bortezomib (B) to melphalan and dexamethasone (MDex) increased the frequency and depth of hematologic responses in a phase III trial of patients with previously untreated immunoglobulin light-chain (AL) amyloidosis.
Rates of hematologic response after three treatment cycles were 79% for BMDex and 52% for MDex (P = .002), Efstathios Kastritis, MD, said at the annual meeting of the American Society of Hematology. Very good partial responses accounted for most of this difference, with rates of 45% and 25%, respectively (P = .02). This first-in-kind trial establishes BMDex “as a novel standard of care in AL amyloidosis,” Dr. Kastritis concluded.
MDex is a standard regimen in intermediate-risk AL amyloidosis, while single-agent therapy with bortezomib yielded a median overall survival time of more than 5 years in one study, noted Dr. Kastritis of the University of Athens. In another matched case-control study, BMDex outperformed MDex based on overall response (69% vs. 51%; P = .01) and complete response (42% vs. 19%; P = .002).
Therefore, Dr. Kastritis and his associates in Europe and Australia randomly assigned 110 patients with newly diagnosed AL amyloidosis to receive either MDex (0.22 mg/kg melphalan plus 40 mg dexamethasone daily for 4 consecutive days every 28 days) or BMDex (MDex plus 1.3 mg/m2 bortezomib on days 1, 4, 8, and 11 during cycles one and two, and on days 1, 8, 15, and 22 during subsequent cycles). Treatment continued through nine cycles of MDex or eight cycles of BMDex, or through cycle six if patients had either a complete response or a partial response plus an organ response. Patients stopped treatment after three cycles if they did not have at least a partial response.
After a median of five treatment cycles, BMDex and MDex led to similar rates of complete response (23% vs. 20%), partial response (19% and 17%), cardiac response (38% vs. 29%), and renal response (44% vs. 44%). Twenty-eight patients died, with no significant difference in overall survival between treatment arms. However, overall survival did favor BMDex (P = .03) among the 77 patients who were in cardiac stage II. Also, median time to second-line therapy was not reached for BMDex but was only 12 months with MDex (P less than .001).
The BMDex regimen was associated with higher rates of peripheral neuropathy (19% vs. 4% for MDex; P less than .001). Furthermore, three patients (1%) developed severe peripheral neuropathy on BMDex, while none did so on MDex. Grade 3 or higher adverse events were more common with BMDex than with MDex, but the difference did not reach statistical significance (52% vs. 40%; P = .13). There were four cardiac deaths in the first 100 days of the trial, three in the BMDex arm and one in the MDex arm (P = .31).
The European Myeloma Network sponsored the trial. Dr. Kastritis disclosed ties to Genesis, Takeda, Janssen, and Amgen.
SAN DIEGO – Adding bortezomib (B) to melphalan and dexamethasone (MDex) increased the frequency and depth of hematologic responses in a phase III trial of patients with previously untreated immunoglobulin light-chain (AL) amyloidosis.
Rates of hematologic response after three treatment cycles were 79% for BMDex and 52% for MDex (P = .002), Efstathios Kastritis, MD, said at the annual meeting of the American Society of Hematology. Very good partial responses accounted for most of this difference, with rates of 45% and 25%, respectively (P = .02). This first-in-kind trial establishes BMDex “as a novel standard of care in AL amyloidosis,” Dr. Kastritis concluded.
MDex is a standard regimen in intermediate-risk AL amyloidosis, while single-agent therapy with bortezomib yielded a median overall survival time of more than 5 years in one study, noted Dr. Kastritis of the University of Athens. In another matched case-control study, BMDex outperformed MDex based on overall response (69% vs. 51%; P = .01) and complete response (42% vs. 19%; P = .002).
Therefore, Dr. Kastritis and his associates in Europe and Australia randomly assigned 110 patients with newly diagnosed AL amyloidosis to receive either MDex (0.22 mg/kg melphalan plus 40 mg dexamethasone daily for 4 consecutive days every 28 days) or BMDex (MDex plus 1.3 mg/m2 bortezomib on days 1, 4, 8, and 11 during cycles one and two, and on days 1, 8, 15, and 22 during subsequent cycles). Treatment continued through nine cycles of MDex or eight cycles of BMDex, or through cycle six if patients had either a complete response or a partial response plus an organ response. Patients stopped treatment after three cycles if they did not have at least a partial response.
After a median of five treatment cycles, BMDex and MDex led to similar rates of complete response (23% vs. 20%), partial response (19% and 17%), cardiac response (38% vs. 29%), and renal response (44% vs. 44%). Twenty-eight patients died, with no significant difference in overall survival between treatment arms. However, overall survival did favor BMDex (P = .03) among the 77 patients who were in cardiac stage II. Also, median time to second-line therapy was not reached for BMDex but was only 12 months with MDex (P less than .001).
The BMDex regimen was associated with higher rates of peripheral neuropathy (19% vs. 4% for MDex; P less than .001). Furthermore, three patients (1%) developed severe peripheral neuropathy on BMDex, while none did so on MDex. Grade 3 or higher adverse events were more common with BMDex than with MDex, but the difference did not reach statistical significance (52% vs. 40%; P = .13). There were four cardiac deaths in the first 100 days of the trial, three in the BMDex arm and one in the MDex arm (P = .31).
The European Myeloma Network sponsored the trial. Dr. Kastritis disclosed ties to Genesis, Takeda, Janssen, and Amgen.
SAN DIEGO – Adding bortezomib (B) to melphalan and dexamethasone (MDex) increased the frequency and depth of hematologic responses in a phase III trial of patients with previously untreated immunoglobulin light-chain (AL) amyloidosis.
Rates of hematologic response after three treatment cycles were 79% for BMDex and 52% for MDex (P = .002), Efstathios Kastritis, MD, said at the annual meeting of the American Society of Hematology. Very good partial responses accounted for most of this difference, with rates of 45% and 25%, respectively (P = .02). This first-in-kind trial establishes BMDex “as a novel standard of care in AL amyloidosis,” Dr. Kastritis concluded.
MDex is a standard regimen in intermediate-risk AL amyloidosis, while single-agent therapy with bortezomib yielded a median overall survival time of more than 5 years in one study, noted Dr. Kastritis of the University of Athens. In another matched case-control study, BMDex outperformed MDex based on overall response (69% vs. 51%; P = .01) and complete response (42% vs. 19%; P = .002).
Therefore, Dr. Kastritis and his associates in Europe and Australia randomly assigned 110 patients with newly diagnosed AL amyloidosis to receive either MDex (0.22 mg/kg melphalan plus 40 mg dexamethasone daily for 4 consecutive days every 28 days) or BMDex (MDex plus 1.3 mg/m2 bortezomib on days 1, 4, 8, and 11 during cycles one and two, and on days 1, 8, 15, and 22 during subsequent cycles). Treatment continued through nine cycles of MDex or eight cycles of BMDex, or through cycle six if patients had either a complete response or a partial response plus an organ response. Patients stopped treatment after three cycles if they did not have at least a partial response.
After a median of five treatment cycles, BMDex and MDex led to similar rates of complete response (23% vs. 20%), partial response (19% and 17%), cardiac response (38% vs. 29%), and renal response (44% vs. 44%). Twenty-eight patients died, with no significant difference in overall survival between treatment arms. However, overall survival did favor BMDex (P = .03) among the 77 patients who were in cardiac stage II. Also, median time to second-line therapy was not reached for BMDex but was only 12 months with MDex (P less than .001).
The BMDex regimen was associated with higher rates of peripheral neuropathy (19% vs. 4% for MDex; P less than .001). Furthermore, three patients (1%) developed severe peripheral neuropathy on BMDex, while none did so on MDex. Grade 3 or higher adverse events were more common with BMDex than with MDex, but the difference did not reach statistical significance (52% vs. 40%; P = .13). There were four cardiac deaths in the first 100 days of the trial, three in the BMDex arm and one in the MDex arm (P = .31).
The European Myeloma Network sponsored the trial. Dr. Kastritis disclosed ties to Genesis, Takeda, Janssen, and Amgen.
AT ASH 2016
Key clinical point: Adding bortezomib (B) to melphalan and dexamethasone (MDex) bolstered hematologic response in immunoglobulin light-chain (AL) amyloidosis.
Major finding: After three treatment cycles, rates of hematologic response were 79% in the BMDex arm and 52% for MDex (P = .002).
Data source: A multicenter randomized trial of 110 patients with newly diagnosed AL amyloidosis.
Disclosures: The European Myeloma Network sponsored the trial. Dr. Kastritis disclosed ties to Genesis, Takeda, Janssen, and Amgen.
FDG-PET/CT at maintenance predicts myeloma survival
SAN DIEGO – Having less than three focal lesions on FDG-PET/CT (fluorodeoxyglucose positron emission tomography integrated with computed tomography) when beginning lenalidomide maintenance therapy predicted significantly higher rates of progression-free and overall survival among patients with newly diagnosed multiple myeloma.
Survival in this prospective study of 102 patients also correlated with FDG uptake that did not exceed the level of the liver (Deauville score less than 3), reported Elena Zamagni, MD, PhD, of Bologna (Italy) University. The findings highlight FDG-PET/CT as “a powerful prognostic marker for survival, both in terms of number and score of focal lesions,” she said during an oral presentation at the annual meeting of the American Society of Hematology.
Although FDG-PET/CT is “well recognized” for staging and evaluating prognosis in multiple myeloma, a “major inconsistency in methodology between studies” inspired the current analysis, Dr. Zamagni said. “Different groups have used different interpretation criteria and arbitrary cutoffs with very variable results, especially in terms of posttreatment and borderline cases,” she noted.
Therefore, researchers from eight participating centers evaluated FDG-PET/CT scans from 103 patients with newly diagnosed multiple myeloma who were part of the randomized phase III EMN02 trial. Scans were performed at diagnosis, at the start of induction therapy, and just before patients started maintenance therapy with lenalidomide. Five nuclear medicine experts reviewed the scans in a blinded manner.
About 34% of patients had positive focal lesions on FDG-PET/CT at the start of maintenance, Dr. Zamagni said. After a median follow-up of 2 years, rates of progression-free survival were 84% in those with fewer than three focal lesions and 47% in those with three or more lesions (hazard ratio, 3.5; P = .01). Rates of overall survival followed the same trend at 98% and 68%, respectively (HR, 13.6; P = .0002).
Likewise, among patients whose FDG uptake did not exceed that of the liver, 2-year rates of progression-free and overall survival were 87% and 100%, compared with 69% and 45% in patients with new focal lesions or slightly, moderately, or markedly greater FDG uptake than the liver (Deauville scores of 4 and 5; P less than .001 for each comparison).
Normalization of FDG-PET/CT after induction also predicted improved survival, Dr. Zamagni said. Two-year progression-free survival rates were 85% among patients who had become PET-negative by the time they began maintenance, but were only 66% among patients who remained PET-positive (HR, 1.5; P less than .01). Rates of overall survival at 2 years were 98% among PET-negative patients and 87% among PET-positive patients (HR, 1.6; P = .03).
The study also confirmed the value of performing FDG-PET/CT at de novo myeloma diagnosis. Strikingly, only 20% of patients with baseline FDG-PET/CT evidence of extramedullary disease at this time point were alive and progression free 2 years later, compared with 81% of those without extramedullary disease (HR, 5.0; P = .001). Once again, the same trend emerged for Deauville scores – 99% of patients with scores of 3 or lower at diagnosis were alive 2 years later, compared with 83% of those who scored 4 or 5 (HR, 5.6; P = .03).
The EMN02 trial included 714 patients with newly diagnosed multiple myeloma who underwent induction with bortezomib-cyclophosphamide-dexamethasone (VCD), followed by either standard-dose intensification therapy with bortezomib-melphalan-prednisone (VMP) or high-dose intensification therapy with melphalan followed by single or double autologous stem cell transplantation. After that, patients either underwent consolidation therapy followed by lenalidomide maintenance therapy or proceeded directly to maintenance. Among the study subgroup of 103 patients, median age was 58 years, 25% of patients had high-risk cytogenetics, and 15% were ISS stage III.
“FDG-PET/CT is by now the preferred imaging technique for evaluating and monitoring response to therapy,” Dr. Zamagni concluded. She and her associates will use data from four other trials to further validate prognostic criteria and more precisely define cutoff points, she said.
Fondazione del Monte di Bologna e Ravenna partially supported the study. Dr. Zamagni had no relevant financial conflicts of interest.
SAN DIEGO – Having less than three focal lesions on FDG-PET/CT (fluorodeoxyglucose positron emission tomography integrated with computed tomography) when beginning lenalidomide maintenance therapy predicted significantly higher rates of progression-free and overall survival among patients with newly diagnosed multiple myeloma.
Survival in this prospective study of 102 patients also correlated with FDG uptake that did not exceed the level of the liver (Deauville score less than 3), reported Elena Zamagni, MD, PhD, of Bologna (Italy) University. The findings highlight FDG-PET/CT as “a powerful prognostic marker for survival, both in terms of number and score of focal lesions,” she said during an oral presentation at the annual meeting of the American Society of Hematology.
Although FDG-PET/CT is “well recognized” for staging and evaluating prognosis in multiple myeloma, a “major inconsistency in methodology between studies” inspired the current analysis, Dr. Zamagni said. “Different groups have used different interpretation criteria and arbitrary cutoffs with very variable results, especially in terms of posttreatment and borderline cases,” she noted.
Therefore, researchers from eight participating centers evaluated FDG-PET/CT scans from 103 patients with newly diagnosed multiple myeloma who were part of the randomized phase III EMN02 trial. Scans were performed at diagnosis, at the start of induction therapy, and just before patients started maintenance therapy with lenalidomide. Five nuclear medicine experts reviewed the scans in a blinded manner.
About 34% of patients had positive focal lesions on FDG-PET/CT at the start of maintenance, Dr. Zamagni said. After a median follow-up of 2 years, rates of progression-free survival were 84% in those with fewer than three focal lesions and 47% in those with three or more lesions (hazard ratio, 3.5; P = .01). Rates of overall survival followed the same trend at 98% and 68%, respectively (HR, 13.6; P = .0002).
Likewise, among patients whose FDG uptake did not exceed that of the liver, 2-year rates of progression-free and overall survival were 87% and 100%, compared with 69% and 45% in patients with new focal lesions or slightly, moderately, or markedly greater FDG uptake than the liver (Deauville scores of 4 and 5; P less than .001 for each comparison).
Normalization of FDG-PET/CT after induction also predicted improved survival, Dr. Zamagni said. Two-year progression-free survival rates were 85% among patients who had become PET-negative by the time they began maintenance, but were only 66% among patients who remained PET-positive (HR, 1.5; P less than .01). Rates of overall survival at 2 years were 98% among PET-negative patients and 87% among PET-positive patients (HR, 1.6; P = .03).
The study also confirmed the value of performing FDG-PET/CT at de novo myeloma diagnosis. Strikingly, only 20% of patients with baseline FDG-PET/CT evidence of extramedullary disease at this time point were alive and progression free 2 years later, compared with 81% of those without extramedullary disease (HR, 5.0; P = .001). Once again, the same trend emerged for Deauville scores – 99% of patients with scores of 3 or lower at diagnosis were alive 2 years later, compared with 83% of those who scored 4 or 5 (HR, 5.6; P = .03).
The EMN02 trial included 714 patients with newly diagnosed multiple myeloma who underwent induction with bortezomib-cyclophosphamide-dexamethasone (VCD), followed by either standard-dose intensification therapy with bortezomib-melphalan-prednisone (VMP) or high-dose intensification therapy with melphalan followed by single or double autologous stem cell transplantation. After that, patients either underwent consolidation therapy followed by lenalidomide maintenance therapy or proceeded directly to maintenance. Among the study subgroup of 103 patients, median age was 58 years, 25% of patients had high-risk cytogenetics, and 15% were ISS stage III.
“FDG-PET/CT is by now the preferred imaging technique for evaluating and monitoring response to therapy,” Dr. Zamagni concluded. She and her associates will use data from four other trials to further validate prognostic criteria and more precisely define cutoff points, she said.
Fondazione del Monte di Bologna e Ravenna partially supported the study. Dr. Zamagni had no relevant financial conflicts of interest.
SAN DIEGO – Having less than three focal lesions on FDG-PET/CT (fluorodeoxyglucose positron emission tomography integrated with computed tomography) when beginning lenalidomide maintenance therapy predicted significantly higher rates of progression-free and overall survival among patients with newly diagnosed multiple myeloma.
Survival in this prospective study of 102 patients also correlated with FDG uptake that did not exceed the level of the liver (Deauville score less than 3), reported Elena Zamagni, MD, PhD, of Bologna (Italy) University. The findings highlight FDG-PET/CT as “a powerful prognostic marker for survival, both in terms of number and score of focal lesions,” she said during an oral presentation at the annual meeting of the American Society of Hematology.
Although FDG-PET/CT is “well recognized” for staging and evaluating prognosis in multiple myeloma, a “major inconsistency in methodology between studies” inspired the current analysis, Dr. Zamagni said. “Different groups have used different interpretation criteria and arbitrary cutoffs with very variable results, especially in terms of posttreatment and borderline cases,” she noted.
Therefore, researchers from eight participating centers evaluated FDG-PET/CT scans from 103 patients with newly diagnosed multiple myeloma who were part of the randomized phase III EMN02 trial. Scans were performed at diagnosis, at the start of induction therapy, and just before patients started maintenance therapy with lenalidomide. Five nuclear medicine experts reviewed the scans in a blinded manner.
About 34% of patients had positive focal lesions on FDG-PET/CT at the start of maintenance, Dr. Zamagni said. After a median follow-up of 2 years, rates of progression-free survival were 84% in those with fewer than three focal lesions and 47% in those with three or more lesions (hazard ratio, 3.5; P = .01). Rates of overall survival followed the same trend at 98% and 68%, respectively (HR, 13.6; P = .0002).
Likewise, among patients whose FDG uptake did not exceed that of the liver, 2-year rates of progression-free and overall survival were 87% and 100%, compared with 69% and 45% in patients with new focal lesions or slightly, moderately, or markedly greater FDG uptake than the liver (Deauville scores of 4 and 5; P less than .001 for each comparison).
Normalization of FDG-PET/CT after induction also predicted improved survival, Dr. Zamagni said. Two-year progression-free survival rates were 85% among patients who had become PET-negative by the time they began maintenance, but were only 66% among patients who remained PET-positive (HR, 1.5; P less than .01). Rates of overall survival at 2 years were 98% among PET-negative patients and 87% among PET-positive patients (HR, 1.6; P = .03).
The study also confirmed the value of performing FDG-PET/CT at de novo myeloma diagnosis. Strikingly, only 20% of patients with baseline FDG-PET/CT evidence of extramedullary disease at this time point were alive and progression free 2 years later, compared with 81% of those without extramedullary disease (HR, 5.0; P = .001). Once again, the same trend emerged for Deauville scores – 99% of patients with scores of 3 or lower at diagnosis were alive 2 years later, compared with 83% of those who scored 4 or 5 (HR, 5.6; P = .03).
The EMN02 trial included 714 patients with newly diagnosed multiple myeloma who underwent induction with bortezomib-cyclophosphamide-dexamethasone (VCD), followed by either standard-dose intensification therapy with bortezomib-melphalan-prednisone (VMP) or high-dose intensification therapy with melphalan followed by single or double autologous stem cell transplantation. After that, patients either underwent consolidation therapy followed by lenalidomide maintenance therapy or proceeded directly to maintenance. Among the study subgroup of 103 patients, median age was 58 years, 25% of patients had high-risk cytogenetics, and 15% were ISS stage III.
“FDG-PET/CT is by now the preferred imaging technique for evaluating and monitoring response to therapy,” Dr. Zamagni concluded. She and her associates will use data from four other trials to further validate prognostic criteria and more precisely define cutoff points, she said.
Fondazione del Monte di Bologna e Ravenna partially supported the study. Dr. Zamagni had no relevant financial conflicts of interest.
AT ASH 2016
Key clinical point: FDG-PET/CT helped predict survival in newly diagnosed multiple myeloma, regardless of induction regimen.
Major finding: Progression-free survival at 2 years was 84% when patients had less than three focal lesions at the start of maintenance, vs. 47% when they had three or more lesions (hazard ratio, 3.5; P = .01).
Data source: A prospective study of 103 patients with newly diagnosed, transplant-eligible multiple myeloma from a randomized phase III trial.
Disclosures: Fondazione del Monte di Bologna e Ravenna partially supported the study. Dr. Zamagni had no conflicts of interest.
Chemo-free induction regimen shines in MCL
SAN DIEGO – A chemotherapy-free induction regimen of ibrutinib and rituximab was well tolerated and achieved an overall response rate of 100% among patients with newly diagnosed mantle cell lymphoma (MCL), according to results of a small single-center phase II trial.
A total of 72% of patients had complete responses to induction, while 28% had partial responses, and 100% had complete responses to consolidation, reported Michael Wang, MD, at the annual meeting of the American Society of Hematology. The findings highlight a “window of opportunity” to effectively treat de novo MCL in young, fit patients while potentially sparing them from repeated cycles of intensive chemoimmunotherapy, the investigators noted.
Established treatments for MCL include eight cycles of rituximab–hyper-CVAD (hyper-fractionated cyclophosphamide, vincristine, doxorubicin, and dexamethasone) alternating with rituximab–methotrexate–Ara-C (cytarabine). Median overall survival with this regimen exceeds 10 years, but during that decade, more than 6% of patients will develop myeloid neoplasms related to treatment, noted Dr. Wang of the University of Texas MD Anderson Cancer Center in Houston.
For the study, patients up to 65 years old with newly diagnosed, untreated MCL underwent induction with continuous daily ibrutinib (560 mg), plus rituximab (375 mg/m2) administered weekly for 4 weeks during cycle 1 and on day 1 of cycles 3-12. Consolidation consisted of rituximab plus hyper-CVAD, alternating every 28 days with rituximab plus high-dose methotrexate–Ara-C. Complete responders to induction received four cycles of chemoimmunotherapy, while progressors and partial responders received chemoimmunotherapy for two cycles beyond the point of complete remission.
Among 36 evaluable patients, 28% had a partial response and the rest had complete responses to induction. Moreover, all 19 patients who completed consolidation had a complete response. During induction, the most common toxicities were fatigue, diarrhea, rash, and myalgia, nearly all of which were mild or moderate in severity. During consolidation, two patients developed severe neutropenia, one patient developed severe febrile neutropenia, and one developed a severe increase in liver enzymes. There were no treatment-related deaths.
“The toxicity after intensive immune-chemotherapy in shortened cycles [is] much improved compared to historical controls, but longer follow-up is needed,” the researchers wrote. “This unprecedented efficacy and safety may provide a window of opportunity for less chemoimmunotherapy needed for consolidation.”
Dr. Wang disclosed ties to Janssen, Onyx, BeiGene, Kite Pharma, Asana Biosciences, Juno Therapeutics, Acerta Pharma, and Celgene.
SAN DIEGO – A chemotherapy-free induction regimen of ibrutinib and rituximab was well tolerated and achieved an overall response rate of 100% among patients with newly diagnosed mantle cell lymphoma (MCL), according to results of a small single-center phase II trial.
A total of 72% of patients had complete responses to induction, while 28% had partial responses, and 100% had complete responses to consolidation, reported Michael Wang, MD, at the annual meeting of the American Society of Hematology. The findings highlight a “window of opportunity” to effectively treat de novo MCL in young, fit patients while potentially sparing them from repeated cycles of intensive chemoimmunotherapy, the investigators noted.
Established treatments for MCL include eight cycles of rituximab–hyper-CVAD (hyper-fractionated cyclophosphamide, vincristine, doxorubicin, and dexamethasone) alternating with rituximab–methotrexate–Ara-C (cytarabine). Median overall survival with this regimen exceeds 10 years, but during that decade, more than 6% of patients will develop myeloid neoplasms related to treatment, noted Dr. Wang of the University of Texas MD Anderson Cancer Center in Houston.
For the study, patients up to 65 years old with newly diagnosed, untreated MCL underwent induction with continuous daily ibrutinib (560 mg), plus rituximab (375 mg/m2) administered weekly for 4 weeks during cycle 1 and on day 1 of cycles 3-12. Consolidation consisted of rituximab plus hyper-CVAD, alternating every 28 days with rituximab plus high-dose methotrexate–Ara-C. Complete responders to induction received four cycles of chemoimmunotherapy, while progressors and partial responders received chemoimmunotherapy for two cycles beyond the point of complete remission.
Among 36 evaluable patients, 28% had a partial response and the rest had complete responses to induction. Moreover, all 19 patients who completed consolidation had a complete response. During induction, the most common toxicities were fatigue, diarrhea, rash, and myalgia, nearly all of which were mild or moderate in severity. During consolidation, two patients developed severe neutropenia, one patient developed severe febrile neutropenia, and one developed a severe increase in liver enzymes. There were no treatment-related deaths.
“The toxicity after intensive immune-chemotherapy in shortened cycles [is] much improved compared to historical controls, but longer follow-up is needed,” the researchers wrote. “This unprecedented efficacy and safety may provide a window of opportunity for less chemoimmunotherapy needed for consolidation.”
Dr. Wang disclosed ties to Janssen, Onyx, BeiGene, Kite Pharma, Asana Biosciences, Juno Therapeutics, Acerta Pharma, and Celgene.
SAN DIEGO – A chemotherapy-free induction regimen of ibrutinib and rituximab was well tolerated and achieved an overall response rate of 100% among patients with newly diagnosed mantle cell lymphoma (MCL), according to results of a small single-center phase II trial.
A total of 72% of patients had complete responses to induction, while 28% had partial responses, and 100% had complete responses to consolidation, reported Michael Wang, MD, at the annual meeting of the American Society of Hematology. The findings highlight a “window of opportunity” to effectively treat de novo MCL in young, fit patients while potentially sparing them from repeated cycles of intensive chemoimmunotherapy, the investigators noted.
Established treatments for MCL include eight cycles of rituximab–hyper-CVAD (hyper-fractionated cyclophosphamide, vincristine, doxorubicin, and dexamethasone) alternating with rituximab–methotrexate–Ara-C (cytarabine). Median overall survival with this regimen exceeds 10 years, but during that decade, more than 6% of patients will develop myeloid neoplasms related to treatment, noted Dr. Wang of the University of Texas MD Anderson Cancer Center in Houston.
For the study, patients up to 65 years old with newly diagnosed, untreated MCL underwent induction with continuous daily ibrutinib (560 mg), plus rituximab (375 mg/m2) administered weekly for 4 weeks during cycle 1 and on day 1 of cycles 3-12. Consolidation consisted of rituximab plus hyper-CVAD, alternating every 28 days with rituximab plus high-dose methotrexate–Ara-C. Complete responders to induction received four cycles of chemoimmunotherapy, while progressors and partial responders received chemoimmunotherapy for two cycles beyond the point of complete remission.
Among 36 evaluable patients, 28% had a partial response and the rest had complete responses to induction. Moreover, all 19 patients who completed consolidation had a complete response. During induction, the most common toxicities were fatigue, diarrhea, rash, and myalgia, nearly all of which were mild or moderate in severity. During consolidation, two patients developed severe neutropenia, one patient developed severe febrile neutropenia, and one developed a severe increase in liver enzymes. There were no treatment-related deaths.
“The toxicity after intensive immune-chemotherapy in shortened cycles [is] much improved compared to historical controls, but longer follow-up is needed,” the researchers wrote. “This unprecedented efficacy and safety may provide a window of opportunity for less chemoimmunotherapy needed for consolidation.”
Dr. Wang disclosed ties to Janssen, Onyx, BeiGene, Kite Pharma, Asana Biosciences, Juno Therapeutics, Acerta Pharma, and Celgene.
AT ASH 2016
Key clinical point: Induction with ibrutinib and rituximab achieved a 100% response rate in newly diagnosed mantle cell lymphoma, enabling patients to receive less intensive consolidation.
Major finding: Rates of complete response were 72% for induction and 100% for induction plus consolidation.
Data source: A single-center phase II trial of 36 patients with newly diagnosed, untreated mantle cell lymphoma.
Disclosures: Dr. Wang disclosed ties to Janssen, Onyx, BeiGene, Kite Pharma, Asana Biosciences, Juno Therapeutics, Acerta Pharma, and Celgene.
Lenalidomide maintenance extended progression-free survival in high-risk CLL
SAN DIEGO – Patients with chronic lymphocytic leukemia at risk for early relapse were about 85% less likely to progress on lenalidomide maintenance therapy compared with placebo, based on an interim analysis of the randomized phase III CLLM1 study.
After a typical follow-up time of 17.5 months, median durations of progression-free survival were not reached with lenalidomide and were 13 months with placebo, for a hazard ratio of 0.15 (95% confidence interval, 0.06-0.35). These results were “statistically significant, robust, and reliable in favor of lenalidomide,” Anna Fink, MD, said at the 2016 meeting of the American Society of Hematology. Several patients in the lenalidomide arm also converted to minimal residual disease (MRD) negativity, added Dr. Fink of University Hospital Cologne (Germany).
The study included patients whose CLL responded at least partially to front-line chemoimmunotherapy, but who were at high risk of progression – minimal residual disease levels were at least 10–2, or were between 10–4 and 10–2 in patients who also had an unmutated IGHV gene status or del(17p) or TP53 mutations at baseline. Among 89 patients who met these criteria, 60 received lenalidomide maintenance and 20 received placebo.
The initial lenalidomide cycle consisted of 5 mg daily for 28 days. To achieve MRD negativity, clinicians increased this dose during subsequent cycles to a maximum of 15 mg daily for patients who were MRD negative after cycle 12, 20 mg for patients who were MRD negative after cycle 18, and 25 mg for patients who remained MRD positive. Median age was 64 years, more than 80% of patients were male, and the median cumulative illness rating was relatively low at 2, ranging between 0 and 8.
A total of 37% of patients had a high MRD level and 63% had an intermediate level. For both cohorts, lenalidomide maintenance significantly prolonged progression-free survival, compared with placebo, with hazard ratios of 0.17 and 0.13, respectively. Patients received a median of 11 and up to 40 cycles of lenalidomide, but a median of only 8 cycles of placebo.
In all, 43% of lenalidomide patients and 72% of placebo patients stopped maintenance. Nearly a third of those who discontinued lenalidomide did so because of adverse events, but 45% of patients who stopped placebo did so because of progressive disease, Dr. Fink said. Lenalidomide was associated with more neutropenia, gastrointestinal disorders, nervous system disorders, and respiratory and skin disorders than was placebo, but the events were usually mild to moderate in severity, she added.
The three deaths in this study yielded no treatment-based difference in rates of overall survival. Causes of death included acute lymphoblastic leukemia (lenalidomide arm), progressive multifocal leukoencephalopathy (placebo), and Richter’s syndrome (placebo). Venous thromboembolic events were uncommon because patients were given low-dose aspirin or anticoagulant therapy, Dr. Fink noted.
“Lenalidomide is a feasible and efficacious maintenance option for high-risk CLL after chemoimmunotherapy,” she concluded. The low duration of progression-free survival in the placebo group confirms the prognostic utility of assessing risk based on MRD, which might be useful in future studies, she added.
The German CLL Study Group sponsored the trial. Dr. Fink disclosed ties to Mundipharma, Roche, Celgene, and AbbVie.
SAN DIEGO – Patients with chronic lymphocytic leukemia at risk for early relapse were about 85% less likely to progress on lenalidomide maintenance therapy compared with placebo, based on an interim analysis of the randomized phase III CLLM1 study.
After a typical follow-up time of 17.5 months, median durations of progression-free survival were not reached with lenalidomide and were 13 months with placebo, for a hazard ratio of 0.15 (95% confidence interval, 0.06-0.35). These results were “statistically significant, robust, and reliable in favor of lenalidomide,” Anna Fink, MD, said at the 2016 meeting of the American Society of Hematology. Several patients in the lenalidomide arm also converted to minimal residual disease (MRD) negativity, added Dr. Fink of University Hospital Cologne (Germany).
The study included patients whose CLL responded at least partially to front-line chemoimmunotherapy, but who were at high risk of progression – minimal residual disease levels were at least 10–2, or were between 10–4 and 10–2 in patients who also had an unmutated IGHV gene status or del(17p) or TP53 mutations at baseline. Among 89 patients who met these criteria, 60 received lenalidomide maintenance and 20 received placebo.
The initial lenalidomide cycle consisted of 5 mg daily for 28 days. To achieve MRD negativity, clinicians increased this dose during subsequent cycles to a maximum of 15 mg daily for patients who were MRD negative after cycle 12, 20 mg for patients who were MRD negative after cycle 18, and 25 mg for patients who remained MRD positive. Median age was 64 years, more than 80% of patients were male, and the median cumulative illness rating was relatively low at 2, ranging between 0 and 8.
A total of 37% of patients had a high MRD level and 63% had an intermediate level. For both cohorts, lenalidomide maintenance significantly prolonged progression-free survival, compared with placebo, with hazard ratios of 0.17 and 0.13, respectively. Patients received a median of 11 and up to 40 cycles of lenalidomide, but a median of only 8 cycles of placebo.
In all, 43% of lenalidomide patients and 72% of placebo patients stopped maintenance. Nearly a third of those who discontinued lenalidomide did so because of adverse events, but 45% of patients who stopped placebo did so because of progressive disease, Dr. Fink said. Lenalidomide was associated with more neutropenia, gastrointestinal disorders, nervous system disorders, and respiratory and skin disorders than was placebo, but the events were usually mild to moderate in severity, she added.
The three deaths in this study yielded no treatment-based difference in rates of overall survival. Causes of death included acute lymphoblastic leukemia (lenalidomide arm), progressive multifocal leukoencephalopathy (placebo), and Richter’s syndrome (placebo). Venous thromboembolic events were uncommon because patients were given low-dose aspirin or anticoagulant therapy, Dr. Fink noted.
“Lenalidomide is a feasible and efficacious maintenance option for high-risk CLL after chemoimmunotherapy,” she concluded. The low duration of progression-free survival in the placebo group confirms the prognostic utility of assessing risk based on MRD, which might be useful in future studies, she added.
The German CLL Study Group sponsored the trial. Dr. Fink disclosed ties to Mundipharma, Roche, Celgene, and AbbVie.
SAN DIEGO – Patients with chronic lymphocytic leukemia at risk for early relapse were about 85% less likely to progress on lenalidomide maintenance therapy compared with placebo, based on an interim analysis of the randomized phase III CLLM1 study.
After a typical follow-up time of 17.5 months, median durations of progression-free survival were not reached with lenalidomide and were 13 months with placebo, for a hazard ratio of 0.15 (95% confidence interval, 0.06-0.35). These results were “statistically significant, robust, and reliable in favor of lenalidomide,” Anna Fink, MD, said at the 2016 meeting of the American Society of Hematology. Several patients in the lenalidomide arm also converted to minimal residual disease (MRD) negativity, added Dr. Fink of University Hospital Cologne (Germany).
The study included patients whose CLL responded at least partially to front-line chemoimmunotherapy, but who were at high risk of progression – minimal residual disease levels were at least 10–2, or were between 10–4 and 10–2 in patients who also had an unmutated IGHV gene status or del(17p) or TP53 mutations at baseline. Among 89 patients who met these criteria, 60 received lenalidomide maintenance and 20 received placebo.
The initial lenalidomide cycle consisted of 5 mg daily for 28 days. To achieve MRD negativity, clinicians increased this dose during subsequent cycles to a maximum of 15 mg daily for patients who were MRD negative after cycle 12, 20 mg for patients who were MRD negative after cycle 18, and 25 mg for patients who remained MRD positive. Median age was 64 years, more than 80% of patients were male, and the median cumulative illness rating was relatively low at 2, ranging between 0 and 8.
A total of 37% of patients had a high MRD level and 63% had an intermediate level. For both cohorts, lenalidomide maintenance significantly prolonged progression-free survival, compared with placebo, with hazard ratios of 0.17 and 0.13, respectively. Patients received a median of 11 and up to 40 cycles of lenalidomide, but a median of only 8 cycles of placebo.
In all, 43% of lenalidomide patients and 72% of placebo patients stopped maintenance. Nearly a third of those who discontinued lenalidomide did so because of adverse events, but 45% of patients who stopped placebo did so because of progressive disease, Dr. Fink said. Lenalidomide was associated with more neutropenia, gastrointestinal disorders, nervous system disorders, and respiratory and skin disorders than was placebo, but the events were usually mild to moderate in severity, she added.
The three deaths in this study yielded no treatment-based difference in rates of overall survival. Causes of death included acute lymphoblastic leukemia (lenalidomide arm), progressive multifocal leukoencephalopathy (placebo), and Richter’s syndrome (placebo). Venous thromboembolic events were uncommon because patients were given low-dose aspirin or anticoagulant therapy, Dr. Fink noted.
“Lenalidomide is a feasible and efficacious maintenance option for high-risk CLL after chemoimmunotherapy,” she concluded. The low duration of progression-free survival in the placebo group confirms the prognostic utility of assessing risk based on MRD, which might be useful in future studies, she added.
The German CLL Study Group sponsored the trial. Dr. Fink disclosed ties to Mundipharma, Roche, Celgene, and AbbVie.
AT ASH 2016
Key clinical point: Lenalidomide maintenance may be an option for patients with chronic lymphocytic leukemia at risk of early progression.
Major finding: Median progression-free survival times were 13 months with placebo but were not reached with lenalidomide, for a hazard ratio of 0.15 (95% confidence interval, 0.06-0.35).
Data source: An interim analysis of 89 partial responders to frontline chemotherapy in the randomized, phase III CLLM1 study.
Disclosures: The German CLL Study Group sponsored the trial. Dr. Fink disclosed ties to Mundipharma, Roche, Celgene, and AbbVie.
Rituximab vanquished MRD in mantle cell lymphoma
SAN DIEGO – Rituximab can at least temporarily vanquish minimal residual disease (MRD) in mantle cell lymphoma (MCL) patients who relapse after induction therapy and autologous stem cell transplantation (ASCT), researchers reported at the annual meeting of the American Society of Hematology.
Of 58 patients whose MCL relapsed after induction therapy and ASCT, 82% converted back to an MRD-negative state after receiving 4 weekly doses of rituximab (375 mg/m2), Arne Kolstad, MD, PhD, and his associates. The data “strongly suggest that preemptive rituximab treatment delayed clinical relapse in MCL,” they wrote in their abstract. They recommended molecular and clinical monitoring after ASCT, not only “as an alternative to maintenance therapy for all MCL patients” but to identify MRD-positive candidates for clinical trials.
The study was an analysis of the Nordic Lymphoma Group phase II MCL2 and MCL3 trials (NTC 00514475), in which patients received six alternating cycles of R-CHOP (cyclophosphamide, doxorubicin, vincristine, and prednisone plus rituximab) and R-Ara-C (rituximab-cytarabine). followed by high-dose ASCT. In MCL3, responders who fell short of complete remission also received intensification with yttium-90 ibritumomab tiuxetan (0.4 mCi/kg) 1 week before treatment with BEAM/C (carmustine, etoposide, cytarabine, and melphalan or cyclophosphamide). Patients were evaluated 2-3 months after completing ASCT, and then every 6 months for 5 years or until relapse. Survivors were followed for a median of 8.5 years, noted Dr. Kolstad, who is with Oslo University Hospital in Norway.
Among 183 patients who underwent polymerase chain reaction–based testing for markers of MRD, median time to molecular relapse was 55 months. However, the relapse-free survival curve did not plateau – patients in all risk groups continued to relapse 5-10 years after undergoing ASCT, the researchers said. “Hence, it is fair to consider MCL as a chronic incurable lymphoma entity, and novel approaches will be necessary to change the natural course of this disease,” they wrote.
After controlling for potential confounders, significant predictors of molecular relapse included high MCL international prognostic index at diagnosis (hazard ratio, 1.9; 95% confidence interval 1.4-2.7; P = .0001) and detection of MRD before patients underwent ASCT (HR, 2.5; 95% CI, 1.5-4.1; P = .0005). Minimal residual disease predicted clinical relapse and shorter survival (P less than .001 for both associations). In contrast, the 86 patients who remained in continuous molecular remission had a 76% chance of having at least a 10-year clinical remission, the investigators said.
Minimal residual disease was assessed by testing bone marrow and blood samples with combined standard nested and quantitative real-time polymerase chain reaction (PCR) for Bcl-1 or IgH rearrangement. They defined molecular relapse as conversion from a negative to a positive result on standard nested PCR, or, for patients who were MRD positive after ASCT, as a more than fivefold rise in real-time quantitative PCR levels in two consecutive bone marrow samples.
Oslo University sponsored the trials. Dr. Kolstad reported ties to Nordic Nanovector, Bayer Schering Pharma, Merck, and Roche.
SAN DIEGO – Rituximab can at least temporarily vanquish minimal residual disease (MRD) in mantle cell lymphoma (MCL) patients who relapse after induction therapy and autologous stem cell transplantation (ASCT), researchers reported at the annual meeting of the American Society of Hematology.
Of 58 patients whose MCL relapsed after induction therapy and ASCT, 82% converted back to an MRD-negative state after receiving 4 weekly doses of rituximab (375 mg/m2), Arne Kolstad, MD, PhD, and his associates. The data “strongly suggest that preemptive rituximab treatment delayed clinical relapse in MCL,” they wrote in their abstract. They recommended molecular and clinical monitoring after ASCT, not only “as an alternative to maintenance therapy for all MCL patients” but to identify MRD-positive candidates for clinical trials.
The study was an analysis of the Nordic Lymphoma Group phase II MCL2 and MCL3 trials (NTC 00514475), in which patients received six alternating cycles of R-CHOP (cyclophosphamide, doxorubicin, vincristine, and prednisone plus rituximab) and R-Ara-C (rituximab-cytarabine). followed by high-dose ASCT. In MCL3, responders who fell short of complete remission also received intensification with yttium-90 ibritumomab tiuxetan (0.4 mCi/kg) 1 week before treatment with BEAM/C (carmustine, etoposide, cytarabine, and melphalan or cyclophosphamide). Patients were evaluated 2-3 months after completing ASCT, and then every 6 months for 5 years or until relapse. Survivors were followed for a median of 8.5 years, noted Dr. Kolstad, who is with Oslo University Hospital in Norway.
Among 183 patients who underwent polymerase chain reaction–based testing for markers of MRD, median time to molecular relapse was 55 months. However, the relapse-free survival curve did not plateau – patients in all risk groups continued to relapse 5-10 years after undergoing ASCT, the researchers said. “Hence, it is fair to consider MCL as a chronic incurable lymphoma entity, and novel approaches will be necessary to change the natural course of this disease,” they wrote.
After controlling for potential confounders, significant predictors of molecular relapse included high MCL international prognostic index at diagnosis (hazard ratio, 1.9; 95% confidence interval 1.4-2.7; P = .0001) and detection of MRD before patients underwent ASCT (HR, 2.5; 95% CI, 1.5-4.1; P = .0005). Minimal residual disease predicted clinical relapse and shorter survival (P less than .001 for both associations). In contrast, the 86 patients who remained in continuous molecular remission had a 76% chance of having at least a 10-year clinical remission, the investigators said.
Minimal residual disease was assessed by testing bone marrow and blood samples with combined standard nested and quantitative real-time polymerase chain reaction (PCR) for Bcl-1 or IgH rearrangement. They defined molecular relapse as conversion from a negative to a positive result on standard nested PCR, or, for patients who were MRD positive after ASCT, as a more than fivefold rise in real-time quantitative PCR levels in two consecutive bone marrow samples.
Oslo University sponsored the trials. Dr. Kolstad reported ties to Nordic Nanovector, Bayer Schering Pharma, Merck, and Roche.
SAN DIEGO – Rituximab can at least temporarily vanquish minimal residual disease (MRD) in mantle cell lymphoma (MCL) patients who relapse after induction therapy and autologous stem cell transplantation (ASCT), researchers reported at the annual meeting of the American Society of Hematology.
Of 58 patients whose MCL relapsed after induction therapy and ASCT, 82% converted back to an MRD-negative state after receiving 4 weekly doses of rituximab (375 mg/m2), Arne Kolstad, MD, PhD, and his associates. The data “strongly suggest that preemptive rituximab treatment delayed clinical relapse in MCL,” they wrote in their abstract. They recommended molecular and clinical monitoring after ASCT, not only “as an alternative to maintenance therapy for all MCL patients” but to identify MRD-positive candidates for clinical trials.
The study was an analysis of the Nordic Lymphoma Group phase II MCL2 and MCL3 trials (NTC 00514475), in which patients received six alternating cycles of R-CHOP (cyclophosphamide, doxorubicin, vincristine, and prednisone plus rituximab) and R-Ara-C (rituximab-cytarabine). followed by high-dose ASCT. In MCL3, responders who fell short of complete remission also received intensification with yttium-90 ibritumomab tiuxetan (0.4 mCi/kg) 1 week before treatment with BEAM/C (carmustine, etoposide, cytarabine, and melphalan or cyclophosphamide). Patients were evaluated 2-3 months after completing ASCT, and then every 6 months for 5 years or until relapse. Survivors were followed for a median of 8.5 years, noted Dr. Kolstad, who is with Oslo University Hospital in Norway.
Among 183 patients who underwent polymerase chain reaction–based testing for markers of MRD, median time to molecular relapse was 55 months. However, the relapse-free survival curve did not plateau – patients in all risk groups continued to relapse 5-10 years after undergoing ASCT, the researchers said. “Hence, it is fair to consider MCL as a chronic incurable lymphoma entity, and novel approaches will be necessary to change the natural course of this disease,” they wrote.
After controlling for potential confounders, significant predictors of molecular relapse included high MCL international prognostic index at diagnosis (hazard ratio, 1.9; 95% confidence interval 1.4-2.7; P = .0001) and detection of MRD before patients underwent ASCT (HR, 2.5; 95% CI, 1.5-4.1; P = .0005). Minimal residual disease predicted clinical relapse and shorter survival (P less than .001 for both associations). In contrast, the 86 patients who remained in continuous molecular remission had a 76% chance of having at least a 10-year clinical remission, the investigators said.
Minimal residual disease was assessed by testing bone marrow and blood samples with combined standard nested and quantitative real-time polymerase chain reaction (PCR) for Bcl-1 or IgH rearrangement. They defined molecular relapse as conversion from a negative to a positive result on standard nested PCR, or, for patients who were MRD positive after ASCT, as a more than fivefold rise in real-time quantitative PCR levels in two consecutive bone marrow samples.
Oslo University sponsored the trials. Dr. Kolstad reported ties to Nordic Nanovector, Bayer Schering Pharma, Merck, and Roche.
AT ASH 2016
Key clinical point:
Major finding: Among 58 patients who relapsed after induction therapy and autologous stem cell transplantation, 82% converted back to an MRD-negative state with 4 weekly doses of rituximab (375 mg/m2).
Data source: A study of 183 patients with mantle cell lymphoma from the Nordic MCL2 and MCL3 trials.
Disclosures: Oslo University sponsored the trials. Dr. Kolstad reported ties to Nordic Nanovector, Bayer Schering Pharma, Merck, and Roche.
Ibrutinib continues to wow in CLL/SLL
SAN DIEGO – More than 90% of the first patients with previously untreated chronic lymphocytic leukemia who received ibrutinib in an early study are alive and without disease progression 5 years later, investigators reported.
Among 31 treatment-naive patients with chronic lymphocytic leukemia/small lymphocytic leukemia (CLL/SLL) who were started on the Bruton’s tyrosine kinase inhibitor ibrutinib (Imbruvica) in the phase Ib/II PCYC-1102/1103 study, the 5-year progression-free survival (PFS) rate was 92%, with the median PFS not reached. Estimated 5-year overall survival (OS) among these patients was also 92%, with the median not reached, Susan O’Brien, MD, of the University of California, Irvine, reported at the annual meeting of the American Society of Hematology.
The 5-year PFS rate for patients with relapsed/refractory disease who had received a median of four prior lines of therapy before starting on single-agent ibrutinib was 43%, with a median PFS of 52 months. In this group, the overall survival (OS) rate was 57%, with the median not reached.
“At 5 years of follow-up, there are very durable responses in treatment-naive and relapsed refractory patients. You saw that, in the treatment-naive group, there is, in fact, only one patient who has progressed so far,” Dr. O’Brien said.
In a separate study, investigators from the phase III RESONATE-2 trial reported updated safety and efficacy data showing that in first-line therapy for patients aged 65 years and older with CLL/SLL with active disease, ibrutinib was associated with significantly better PFS at 24 months, compared with chlorambucil.
In this study by Dr. O’Brien and colleagues, 31 patients with previously untreated CLL/SLL and 101 patients with relapsed/refractory disease (progression or no objective response within 24 months of starting on chemoimmunotherapy) were treated with oral ibrutinib once daily at doses of 420 mg or 840 mg until disease progression or unacceptable toxicity.
After 5 years of follow-up, 20 of the 31 treatment-naive patients (65%) and 30 of the 101 relapsed/refractory patients (30%) remained on ibrutinib therapy. The primary reasons for discontinuation among relapsed/refractory patients included progressive disease in 33%, adverse events in 21%, and investigator decision in 11%.
Best response rates in treatment-naive patients were 87% (29% complete response, 55% partial response, 3% partial response-L) and 89% in relapsed/refractory patients (10%, 76%, and 3%, respectively).
An analysis of survival by IGHV (immunoglobulin heavy chain variable) mutational status in patients with relapsed/refractory disease showed a 53% 5-year PFS rate among patients with mutated IGHV and a median PFS of 63 months, compared with 38% and 43 months for patients with unmutated IGHV. Respective 5-year overall survival rates were 66% (median OS, 63 months) and 55% (median not reached).
In an analysis of survival outcomes by chromosomal abnormalities detected by FISH (fluorescent in situ hybridization) among patients with relapsed/refractory disease, median PFS and OS rates were highest among patients with the 13q deletion, at 91% for both PFS and OS, compared with 80% for each in patients with trisomy 12, 33% and 61% for patients with deletion 11q, and 19% and 32% for patients with deletion 17p. In patients with no chromosomal abnormalities, the 5-year PFS rate was 66% (median not reached), and the 5-year OS rate was 83% (median not reached).
Among patients with complex karyotype, 90% of whom had relapsed refractory disease, the 5-year PFS rate was 36% (median 33 months), and the OS rate was 46% (median 57 months). In contrast, respective PFS and OS rates for patients without complex karyotype were 69% and 84%, with the median not reached in either survival category.
In multivariate analysis, only deletion 17p was identified as a significant predictor of PFS and OS.
Grade 3 or greater treatment-emergent adverse events occurred mostly frequently in the first year of therapy and declined thereafter. The most common grade 3 or greater events were hypertension, (26% of all patients), pneumonia (22%), neutropenia (17%), and atrial fibrillation (9%).
Paul M. Barr, MD, of the University of Rochester presented updated data from RESONATE-2, comparing ibrutinib with chlorambucil in patients 65 and older with newly diagnosed, active CLL/SLL.
At a median follow-up of 29 months, the rates of 2-year PFS were 89% for patients treated with ibrutinib (median PFS not reached) vs. 34% for chlorambucil (median PFS 15 months). This translated into a hazard ratio for ibrutinib of 0.121 (P less than .0001). The benefit of ibrutinib occurred without regard to IGHV status, and ibrutinib continues to demonstrate an OS benefit over chlorambucil with longer follow-up and crossover. Overall survival at 24 months was 95% for patients treated with ibrutinib (included 55 patients who were crossed over from chlorambucil) vs. 84% for patients treated with chlorambucil only.
“The depth of the responses has improved over time, now with 18% of patients achieving a CR with ibrutinib, and lastly, ibrutinib remains tolerable in this elderly population, with 79% of patients that are continuing on therapy,” Dr. Barr said.
Both studies were funded by Pharmacyclics. Dr. Barr and Dr. O’Brien disclosed serving as consultants to the company, and Dr. O’Brien further disclosed honoraria and research funding from Pharmacyclics.
SAN DIEGO – More than 90% of the first patients with previously untreated chronic lymphocytic leukemia who received ibrutinib in an early study are alive and without disease progression 5 years later, investigators reported.
Among 31 treatment-naive patients with chronic lymphocytic leukemia/small lymphocytic leukemia (CLL/SLL) who were started on the Bruton’s tyrosine kinase inhibitor ibrutinib (Imbruvica) in the phase Ib/II PCYC-1102/1103 study, the 5-year progression-free survival (PFS) rate was 92%, with the median PFS not reached. Estimated 5-year overall survival (OS) among these patients was also 92%, with the median not reached, Susan O’Brien, MD, of the University of California, Irvine, reported at the annual meeting of the American Society of Hematology.
The 5-year PFS rate for patients with relapsed/refractory disease who had received a median of four prior lines of therapy before starting on single-agent ibrutinib was 43%, with a median PFS of 52 months. In this group, the overall survival (OS) rate was 57%, with the median not reached.
“At 5 years of follow-up, there are very durable responses in treatment-naive and relapsed refractory patients. You saw that, in the treatment-naive group, there is, in fact, only one patient who has progressed so far,” Dr. O’Brien said.
In a separate study, investigators from the phase III RESONATE-2 trial reported updated safety and efficacy data showing that in first-line therapy for patients aged 65 years and older with CLL/SLL with active disease, ibrutinib was associated with significantly better PFS at 24 months, compared with chlorambucil.
In this study by Dr. O’Brien and colleagues, 31 patients with previously untreated CLL/SLL and 101 patients with relapsed/refractory disease (progression or no objective response within 24 months of starting on chemoimmunotherapy) were treated with oral ibrutinib once daily at doses of 420 mg or 840 mg until disease progression or unacceptable toxicity.
After 5 years of follow-up, 20 of the 31 treatment-naive patients (65%) and 30 of the 101 relapsed/refractory patients (30%) remained on ibrutinib therapy. The primary reasons for discontinuation among relapsed/refractory patients included progressive disease in 33%, adverse events in 21%, and investigator decision in 11%.
Best response rates in treatment-naive patients were 87% (29% complete response, 55% partial response, 3% partial response-L) and 89% in relapsed/refractory patients (10%, 76%, and 3%, respectively).
An analysis of survival by IGHV (immunoglobulin heavy chain variable) mutational status in patients with relapsed/refractory disease showed a 53% 5-year PFS rate among patients with mutated IGHV and a median PFS of 63 months, compared with 38% and 43 months for patients with unmutated IGHV. Respective 5-year overall survival rates were 66% (median OS, 63 months) and 55% (median not reached).
In an analysis of survival outcomes by chromosomal abnormalities detected by FISH (fluorescent in situ hybridization) among patients with relapsed/refractory disease, median PFS and OS rates were highest among patients with the 13q deletion, at 91% for both PFS and OS, compared with 80% for each in patients with trisomy 12, 33% and 61% for patients with deletion 11q, and 19% and 32% for patients with deletion 17p. In patients with no chromosomal abnormalities, the 5-year PFS rate was 66% (median not reached), and the 5-year OS rate was 83% (median not reached).
Among patients with complex karyotype, 90% of whom had relapsed refractory disease, the 5-year PFS rate was 36% (median 33 months), and the OS rate was 46% (median 57 months). In contrast, respective PFS and OS rates for patients without complex karyotype were 69% and 84%, with the median not reached in either survival category.
In multivariate analysis, only deletion 17p was identified as a significant predictor of PFS and OS.
Grade 3 or greater treatment-emergent adverse events occurred mostly frequently in the first year of therapy and declined thereafter. The most common grade 3 or greater events were hypertension, (26% of all patients), pneumonia (22%), neutropenia (17%), and atrial fibrillation (9%).
Paul M. Barr, MD, of the University of Rochester presented updated data from RESONATE-2, comparing ibrutinib with chlorambucil in patients 65 and older with newly diagnosed, active CLL/SLL.
At a median follow-up of 29 months, the rates of 2-year PFS were 89% for patients treated with ibrutinib (median PFS not reached) vs. 34% for chlorambucil (median PFS 15 months). This translated into a hazard ratio for ibrutinib of 0.121 (P less than .0001). The benefit of ibrutinib occurred without regard to IGHV status, and ibrutinib continues to demonstrate an OS benefit over chlorambucil with longer follow-up and crossover. Overall survival at 24 months was 95% for patients treated with ibrutinib (included 55 patients who were crossed over from chlorambucil) vs. 84% for patients treated with chlorambucil only.
“The depth of the responses has improved over time, now with 18% of patients achieving a CR with ibrutinib, and lastly, ibrutinib remains tolerable in this elderly population, with 79% of patients that are continuing on therapy,” Dr. Barr said.
Both studies were funded by Pharmacyclics. Dr. Barr and Dr. O’Brien disclosed serving as consultants to the company, and Dr. O’Brien further disclosed honoraria and research funding from Pharmacyclics.
SAN DIEGO – More than 90% of the first patients with previously untreated chronic lymphocytic leukemia who received ibrutinib in an early study are alive and without disease progression 5 years later, investigators reported.
Among 31 treatment-naive patients with chronic lymphocytic leukemia/small lymphocytic leukemia (CLL/SLL) who were started on the Bruton’s tyrosine kinase inhibitor ibrutinib (Imbruvica) in the phase Ib/II PCYC-1102/1103 study, the 5-year progression-free survival (PFS) rate was 92%, with the median PFS not reached. Estimated 5-year overall survival (OS) among these patients was also 92%, with the median not reached, Susan O’Brien, MD, of the University of California, Irvine, reported at the annual meeting of the American Society of Hematology.
The 5-year PFS rate for patients with relapsed/refractory disease who had received a median of four prior lines of therapy before starting on single-agent ibrutinib was 43%, with a median PFS of 52 months. In this group, the overall survival (OS) rate was 57%, with the median not reached.
“At 5 years of follow-up, there are very durable responses in treatment-naive and relapsed refractory patients. You saw that, in the treatment-naive group, there is, in fact, only one patient who has progressed so far,” Dr. O’Brien said.
In a separate study, investigators from the phase III RESONATE-2 trial reported updated safety and efficacy data showing that in first-line therapy for patients aged 65 years and older with CLL/SLL with active disease, ibrutinib was associated with significantly better PFS at 24 months, compared with chlorambucil.
In this study by Dr. O’Brien and colleagues, 31 patients with previously untreated CLL/SLL and 101 patients with relapsed/refractory disease (progression or no objective response within 24 months of starting on chemoimmunotherapy) were treated with oral ibrutinib once daily at doses of 420 mg or 840 mg until disease progression or unacceptable toxicity.
After 5 years of follow-up, 20 of the 31 treatment-naive patients (65%) and 30 of the 101 relapsed/refractory patients (30%) remained on ibrutinib therapy. The primary reasons for discontinuation among relapsed/refractory patients included progressive disease in 33%, adverse events in 21%, and investigator decision in 11%.
Best response rates in treatment-naive patients were 87% (29% complete response, 55% partial response, 3% partial response-L) and 89% in relapsed/refractory patients (10%, 76%, and 3%, respectively).
An analysis of survival by IGHV (immunoglobulin heavy chain variable) mutational status in patients with relapsed/refractory disease showed a 53% 5-year PFS rate among patients with mutated IGHV and a median PFS of 63 months, compared with 38% and 43 months for patients with unmutated IGHV. Respective 5-year overall survival rates were 66% (median OS, 63 months) and 55% (median not reached).
In an analysis of survival outcomes by chromosomal abnormalities detected by FISH (fluorescent in situ hybridization) among patients with relapsed/refractory disease, median PFS and OS rates were highest among patients with the 13q deletion, at 91% for both PFS and OS, compared with 80% for each in patients with trisomy 12, 33% and 61% for patients with deletion 11q, and 19% and 32% for patients with deletion 17p. In patients with no chromosomal abnormalities, the 5-year PFS rate was 66% (median not reached), and the 5-year OS rate was 83% (median not reached).
Among patients with complex karyotype, 90% of whom had relapsed refractory disease, the 5-year PFS rate was 36% (median 33 months), and the OS rate was 46% (median 57 months). In contrast, respective PFS and OS rates for patients without complex karyotype were 69% and 84%, with the median not reached in either survival category.
In multivariate analysis, only deletion 17p was identified as a significant predictor of PFS and OS.
Grade 3 or greater treatment-emergent adverse events occurred mostly frequently in the first year of therapy and declined thereafter. The most common grade 3 or greater events were hypertension, (26% of all patients), pneumonia (22%), neutropenia (17%), and atrial fibrillation (9%).
Paul M. Barr, MD, of the University of Rochester presented updated data from RESONATE-2, comparing ibrutinib with chlorambucil in patients 65 and older with newly diagnosed, active CLL/SLL.
At a median follow-up of 29 months, the rates of 2-year PFS were 89% for patients treated with ibrutinib (median PFS not reached) vs. 34% for chlorambucil (median PFS 15 months). This translated into a hazard ratio for ibrutinib of 0.121 (P less than .0001). The benefit of ibrutinib occurred without regard to IGHV status, and ibrutinib continues to demonstrate an OS benefit over chlorambucil with longer follow-up and crossover. Overall survival at 24 months was 95% for patients treated with ibrutinib (included 55 patients who were crossed over from chlorambucil) vs. 84% for patients treated with chlorambucil only.
“The depth of the responses has improved over time, now with 18% of patients achieving a CR with ibrutinib, and lastly, ibrutinib remains tolerable in this elderly population, with 79% of patients that are continuing on therapy,” Dr. Barr said.
Both studies were funded by Pharmacyclics. Dr. Barr and Dr. O’Brien disclosed serving as consultants to the company, and Dr. O’Brien further disclosed honoraria and research funding from Pharmacyclics.
AT ASH 2016
Key clinical point: Long-term follow-up of two studies shows a progression-free and overall survival advantage with ibrutinib in chronic lymphocytic leukemia/small lymphocytic leukemia.
Major finding: 5-year PFS and OS rates were 92% for treatment-naive patients with CLL/SLL treated with ibrutinib.
Data source: Phase Ib/II study and randomized phase III study of ibrutinib for treatment-naive and relapsed/refractory CLL/SLL.
Disclosures: Both studies were funded by Pharmacyclics. Dr. Barr and Dr. O’Brien disclosed serving as consultants to the company; Dr. O’Brien disclosed honoraria and research funding from the company.