User login
MRD better measure of ALL remission than morphology
MONTREAL – In children with acute lymphoblastic leukemia, minimal residual disease findings appear to be better at defining remission than morphology, Children’s Oncology Group investigators reported.
A study of outcomes of more than 9,000 children and young adults with B-lineage or T-lineage acute lymphoblastic leukemia (ALL) showed that patients who would be defined as being in remission by morphology but have minimal residual disease (MRD) of 5% or greater have survival outcomes similar to those of patients who do get a morphologic remission. Additionally, patients with discordant morphologic and MRD findings have significantly worse outcomes than do patients who were in morphologic remission and had concordant MRD findings, said Sumit Gupta, MD, PhD, from the Hospital for Sick Children in Toronto.
“Given that, however, although MRD is used to measure the depth of remission either using flow cytometry or PCR [polymerase chain reaction]-based methods, remission itself continues to be defined by basic morphological assessment, whether that’s in clinical practice or clinical trials,” he added.
To see whether the practice of declaring remissions by morphology still makes sense, Dr. Gupta and his colleagues in the Children’s Oncology Group looked at outcomes for children and young adults with discordant ALL remissions as assessed by morphology, compared with MRD.
They looked at data on 9,350 patients from the ages of 1 to 31 years who were enrolled in one of three Children’s Oncology Group trials for patients with newly diagnosed ALL. Two of the trials (AALL0331 and AALL0232) were for patients with B-lineage ALL, and one (AALL0434) was for patients with T-lineage ALL.
They looked at morphologic responses as assessed by local centers, with M1 responses defined as less than 5% leukemic blasts (remission), M2 defined as 5% to less than 25% blasts, and M3 as 25% or more blasts. MRD was measured by flow cytometry at one of two central labs.
They found that discordant results (M1 morphology but MRD of 5% or greater) occurred in only 0.9% of patients with B-ALL, but in 6.9% of patients with T-ALL (P less than .0001).
In multivariate analysis, significant predictors of discordance in patients with B-ALL were patients age 10 years or older (P = .03), white blood cell counts of 50,000/mcL or greater (P = .005), and neutral or unfavorable cytogenetics vs. favorable (P less than .0001 for each).
Among patients with T-ALL, the only significant predictor of discordant results was the early T-precursor phenotype, with an odds ratio of 4.7 (P less than .0001).
Comparing event-free survival (EFS) between patients with concordant remission findings (M1/MRD less than 5%), they investigators saw that for patients with B-ALL, the 5-year EFS was 87%, compared with 59% for patients with discordant findings (M1/MRD 5% or greater, P less than .0001 vs. concordant remissions), and 39% for patients with concordant results showing a lack of remission (P = .009 vs. discordant findings).
Similarly, respective EFS rates for patients with T-ALL were 88%, 80% (P = .011) and 63% (not significant).
In a subanalysis of EFS by risk category, they found no differences according to concordance/discordance among patients with standard-risk B-ALL but a significant difference among patients with high-risk disease.
Attempting to determine what was driving the intermediate outcomes of patients with discordant findings, “we hypothesized that maybe it’s a difference in their actual MRD levels.” Specifically, they found that while both discordant and concordant not-in-remission patients had MRD levels of 5% or higher, the MRD levels were higher among those patients who were conclusively not in remission, Dr. Gupta said.
Finally, they found that for those patients with known overall survival data, concordant in remission patients with B-ALL had a 94% rate out to 12 years, compared with 73% for those with discordant results (P less than .0001). There was no significant difference in OS among patients with T-ALL, however.
“Should MRD assessment actually replace morphology in defining remission in subjects with ALL? I think these data strongly support that,” Dr. Gupta said.
The study was supported by the National Institutes of Health. Dr. Gupta reported having no conflicts of interest.
MONTREAL – In children with acute lymphoblastic leukemia, minimal residual disease findings appear to be better at defining remission than morphology, Children’s Oncology Group investigators reported.
A study of outcomes of more than 9,000 children and young adults with B-lineage or T-lineage acute lymphoblastic leukemia (ALL) showed that patients who would be defined as being in remission by morphology but have minimal residual disease (MRD) of 5% or greater have survival outcomes similar to those of patients who do get a morphologic remission. Additionally, patients with discordant morphologic and MRD findings have significantly worse outcomes than do patients who were in morphologic remission and had concordant MRD findings, said Sumit Gupta, MD, PhD, from the Hospital for Sick Children in Toronto.
“Given that, however, although MRD is used to measure the depth of remission either using flow cytometry or PCR [polymerase chain reaction]-based methods, remission itself continues to be defined by basic morphological assessment, whether that’s in clinical practice or clinical trials,” he added.
To see whether the practice of declaring remissions by morphology still makes sense, Dr. Gupta and his colleagues in the Children’s Oncology Group looked at outcomes for children and young adults with discordant ALL remissions as assessed by morphology, compared with MRD.
They looked at data on 9,350 patients from the ages of 1 to 31 years who were enrolled in one of three Children’s Oncology Group trials for patients with newly diagnosed ALL. Two of the trials (AALL0331 and AALL0232) were for patients with B-lineage ALL, and one (AALL0434) was for patients with T-lineage ALL.
They looked at morphologic responses as assessed by local centers, with M1 responses defined as less than 5% leukemic blasts (remission), M2 defined as 5% to less than 25% blasts, and M3 as 25% or more blasts. MRD was measured by flow cytometry at one of two central labs.
They found that discordant results (M1 morphology but MRD of 5% or greater) occurred in only 0.9% of patients with B-ALL, but in 6.9% of patients with T-ALL (P less than .0001).
In multivariate analysis, significant predictors of discordance in patients with B-ALL were patients age 10 years or older (P = .03), white blood cell counts of 50,000/mcL or greater (P = .005), and neutral or unfavorable cytogenetics vs. favorable (P less than .0001 for each).
Among patients with T-ALL, the only significant predictor of discordant results was the early T-precursor phenotype, with an odds ratio of 4.7 (P less than .0001).
Comparing event-free survival (EFS) between patients with concordant remission findings (M1/MRD less than 5%), they investigators saw that for patients with B-ALL, the 5-year EFS was 87%, compared with 59% for patients with discordant findings (M1/MRD 5% or greater, P less than .0001 vs. concordant remissions), and 39% for patients with concordant results showing a lack of remission (P = .009 vs. discordant findings).
Similarly, respective EFS rates for patients with T-ALL were 88%, 80% (P = .011) and 63% (not significant).
In a subanalysis of EFS by risk category, they found no differences according to concordance/discordance among patients with standard-risk B-ALL but a significant difference among patients with high-risk disease.
Attempting to determine what was driving the intermediate outcomes of patients with discordant findings, “we hypothesized that maybe it’s a difference in their actual MRD levels.” Specifically, they found that while both discordant and concordant not-in-remission patients had MRD levels of 5% or higher, the MRD levels were higher among those patients who were conclusively not in remission, Dr. Gupta said.
Finally, they found that for those patients with known overall survival data, concordant in remission patients with B-ALL had a 94% rate out to 12 years, compared with 73% for those with discordant results (P less than .0001). There was no significant difference in OS among patients with T-ALL, however.
“Should MRD assessment actually replace morphology in defining remission in subjects with ALL? I think these data strongly support that,” Dr. Gupta said.
The study was supported by the National Institutes of Health. Dr. Gupta reported having no conflicts of interest.
MONTREAL – In children with acute lymphoblastic leukemia, minimal residual disease findings appear to be better at defining remission than morphology, Children’s Oncology Group investigators reported.
A study of outcomes of more than 9,000 children and young adults with B-lineage or T-lineage acute lymphoblastic leukemia (ALL) showed that patients who would be defined as being in remission by morphology but have minimal residual disease (MRD) of 5% or greater have survival outcomes similar to those of patients who do get a morphologic remission. Additionally, patients with discordant morphologic and MRD findings have significantly worse outcomes than do patients who were in morphologic remission and had concordant MRD findings, said Sumit Gupta, MD, PhD, from the Hospital for Sick Children in Toronto.
“Given that, however, although MRD is used to measure the depth of remission either using flow cytometry or PCR [polymerase chain reaction]-based methods, remission itself continues to be defined by basic morphological assessment, whether that’s in clinical practice or clinical trials,” he added.
To see whether the practice of declaring remissions by morphology still makes sense, Dr. Gupta and his colleagues in the Children’s Oncology Group looked at outcomes for children and young adults with discordant ALL remissions as assessed by morphology, compared with MRD.
They looked at data on 9,350 patients from the ages of 1 to 31 years who were enrolled in one of three Children’s Oncology Group trials for patients with newly diagnosed ALL. Two of the trials (AALL0331 and AALL0232) were for patients with B-lineage ALL, and one (AALL0434) was for patients with T-lineage ALL.
They looked at morphologic responses as assessed by local centers, with M1 responses defined as less than 5% leukemic blasts (remission), M2 defined as 5% to less than 25% blasts, and M3 as 25% or more blasts. MRD was measured by flow cytometry at one of two central labs.
They found that discordant results (M1 morphology but MRD of 5% or greater) occurred in only 0.9% of patients with B-ALL, but in 6.9% of patients with T-ALL (P less than .0001).
In multivariate analysis, significant predictors of discordance in patients with B-ALL were patients age 10 years or older (P = .03), white blood cell counts of 50,000/mcL or greater (P = .005), and neutral or unfavorable cytogenetics vs. favorable (P less than .0001 for each).
Among patients with T-ALL, the only significant predictor of discordant results was the early T-precursor phenotype, with an odds ratio of 4.7 (P less than .0001).
Comparing event-free survival (EFS) between patients with concordant remission findings (M1/MRD less than 5%), they investigators saw that for patients with B-ALL, the 5-year EFS was 87%, compared with 59% for patients with discordant findings (M1/MRD 5% or greater, P less than .0001 vs. concordant remissions), and 39% for patients with concordant results showing a lack of remission (P = .009 vs. discordant findings).
Similarly, respective EFS rates for patients with T-ALL were 88%, 80% (P = .011) and 63% (not significant).
In a subanalysis of EFS by risk category, they found no differences according to concordance/discordance among patients with standard-risk B-ALL but a significant difference among patients with high-risk disease.
Attempting to determine what was driving the intermediate outcomes of patients with discordant findings, “we hypothesized that maybe it’s a difference in their actual MRD levels.” Specifically, they found that while both discordant and concordant not-in-remission patients had MRD levels of 5% or higher, the MRD levels were higher among those patients who were conclusively not in remission, Dr. Gupta said.
Finally, they found that for those patients with known overall survival data, concordant in remission patients with B-ALL had a 94% rate out to 12 years, compared with 73% for those with discordant results (P less than .0001). There was no significant difference in OS among patients with T-ALL, however.
“Should MRD assessment actually replace morphology in defining remission in subjects with ALL? I think these data strongly support that,” Dr. Gupta said.
The study was supported by the National Institutes of Health. Dr. Gupta reported having no conflicts of interest.
FROM ASPHO 2017
Key clinical point: Patients with ALL determined to be in remission by both morphology and minimal residual disease had better outcomes than did those with discordant results.
Major finding: Event-free survival of B-ALL was 87% for patients with concordant remission findings vs. 59% for patients with discordant findings and 39% for concordant not-in-remission findings.
Data source: Retrospective review of data on 9,350 children and young adults with ALL.
Disclosures: The study was supported by the National Institutes of Health. Dr. Gupta reported having no conflicts of interest.
Game over: VTE is a risk in obese, sedentary teens
MONTREAL – It’s well known that airplane passengers, condemned to sit for endless hours in the claustrophobic cabins of the unfriendly skies, are at increased risk for venous thromboembolic events (VTEs). Less well documented, however, is the VTE risk encountered by overweight or obese teens who while their hours away playing video games.
“This is becoming a sedentary-type risk factor,” said Mira A. Kohorst, MD, from the division of pediatric hematology-oncology at the Mayo Clinic in Rochester, Minn.
Dr. Kohorst and her colleagues reported on a small but troubling trend of VTE episodes that they observed in teen boys over the last few years. They refer to obesity, sedentary lifestyle, and gaming as “the new thrombophilia cocktail in adolescent males.”
The reported incidence of pediatric VTE ranges from 0.7 to 4.9 per 100,000 person years, considerably lower than the 1 in 1000 estimated incidences reported in adults. But, thanks to the growing incidence of obesity in children, which more than doubled from 1980 to 2012 and quadrupled in teens age 12-19 years from 5% to 21%, youngsters appear to be catching up in the VTE department, the investigators reported.
“Given the direct mortality rate of 2% [that is] associated with VTE and risk for postthrombotic syndrome of 26%, it is important to understand underlying modifiable risk factors,” they wrote.
To do this, they retrospectively reviewed records of children who presented with VTE in their center.
All play, no exercise
The authors described three cases, including that of an 18-year old boy with a body mass index (BMI) of 37 kg/m2, putting him squarely in the obese category. This lad, who spent 12 or more hours a day playing video games and was sedentary at other times as well, presented with bilateral pulmonary emboli and an associated right lower lobe infarction. Testing for thrombophilia showed that he was heterozygous for factor V Leiden but did not have other coagulation abnormalities. He was started on enoxaparin (Lovenox) and then transitioned to apixaban (Eliquis) for a total of 6 months of thromboprophylaxis. He was counseled about modifying his lifestyle and did not have a recurrence after 14 months of follow-up.
A similarly sedentary 17-year old male with an even higher BMI (39 kg/m2) presented with bilateral basilar pulmonary emboli and infarctions in association with a left femoral deep vein thrombosis. This patients also had factor V Leiden heterozygosity and the May-Thurner (iliac vein compression) syndrome. He was treated for a total of 6 months with warfarin followed by rivaroxaban (Xarelto) and was counseled about lifestyle changes but was unable to lose weight. Eight months after completing therapy, he had a second extensive deep vein thrombosis, this time in his right leg, and was restarted on rivaroxaban.
The third patient, a morbidly obese (BMI 56 kg/m2) 13-year-old boy, presented with left lower lobe pulmonary embolism following 3 weeks of immobility caused by the Guillain-Barré syndrome. As in the other cases, he confessed to a sedentary lifestyle and a predilection for gaming. His father had previously developed a line-associated thrombus. The family declined thrombophilia testing. The patient received 3 months of enoxaparin. He has not been followed since discontinuing therapy.
Move it, kid!
The risk of VTE in adolescent boys, especially obese and extreme gamers who spend most of their waking hours in a chair staring at a screen, is similar to that for adolescent girls who use oral contraceptives, Dr. Kohorst and her colleagues said.
“Many case reports link prolonged ‘gaming’ to thrombosis and fatal pulmonary emboli. Additionally, prolonged television viewing has become a documented risk factor for mortality from pulmonary emboli,” the investigators wrote.
They recommend that clinicians ask adolescents about their gaming and TV-watching habits and encourage them to become more active to lower their risk for VTE.
The study was internally supported. Dr. Kohorst and colleagues reported no relevant disclosures.
MONTREAL – It’s well known that airplane passengers, condemned to sit for endless hours in the claustrophobic cabins of the unfriendly skies, are at increased risk for venous thromboembolic events (VTEs). Less well documented, however, is the VTE risk encountered by overweight or obese teens who while their hours away playing video games.
“This is becoming a sedentary-type risk factor,” said Mira A. Kohorst, MD, from the division of pediatric hematology-oncology at the Mayo Clinic in Rochester, Minn.
Dr. Kohorst and her colleagues reported on a small but troubling trend of VTE episodes that they observed in teen boys over the last few years. They refer to obesity, sedentary lifestyle, and gaming as “the new thrombophilia cocktail in adolescent males.”
The reported incidence of pediatric VTE ranges from 0.7 to 4.9 per 100,000 person years, considerably lower than the 1 in 1000 estimated incidences reported in adults. But, thanks to the growing incidence of obesity in children, which more than doubled from 1980 to 2012 and quadrupled in teens age 12-19 years from 5% to 21%, youngsters appear to be catching up in the VTE department, the investigators reported.
“Given the direct mortality rate of 2% [that is] associated with VTE and risk for postthrombotic syndrome of 26%, it is important to understand underlying modifiable risk factors,” they wrote.
To do this, they retrospectively reviewed records of children who presented with VTE in their center.
All play, no exercise
The authors described three cases, including that of an 18-year old boy with a body mass index (BMI) of 37 kg/m2, putting him squarely in the obese category. This lad, who spent 12 or more hours a day playing video games and was sedentary at other times as well, presented with bilateral pulmonary emboli and an associated right lower lobe infarction. Testing for thrombophilia showed that he was heterozygous for factor V Leiden but did not have other coagulation abnormalities. He was started on enoxaparin (Lovenox) and then transitioned to apixaban (Eliquis) for a total of 6 months of thromboprophylaxis. He was counseled about modifying his lifestyle and did not have a recurrence after 14 months of follow-up.
A similarly sedentary 17-year old male with an even higher BMI (39 kg/m2) presented with bilateral basilar pulmonary emboli and infarctions in association with a left femoral deep vein thrombosis. This patients also had factor V Leiden heterozygosity and the May-Thurner (iliac vein compression) syndrome. He was treated for a total of 6 months with warfarin followed by rivaroxaban (Xarelto) and was counseled about lifestyle changes but was unable to lose weight. Eight months after completing therapy, he had a second extensive deep vein thrombosis, this time in his right leg, and was restarted on rivaroxaban.
The third patient, a morbidly obese (BMI 56 kg/m2) 13-year-old boy, presented with left lower lobe pulmonary embolism following 3 weeks of immobility caused by the Guillain-Barré syndrome. As in the other cases, he confessed to a sedentary lifestyle and a predilection for gaming. His father had previously developed a line-associated thrombus. The family declined thrombophilia testing. The patient received 3 months of enoxaparin. He has not been followed since discontinuing therapy.
Move it, kid!
The risk of VTE in adolescent boys, especially obese and extreme gamers who spend most of their waking hours in a chair staring at a screen, is similar to that for adolescent girls who use oral contraceptives, Dr. Kohorst and her colleagues said.
“Many case reports link prolonged ‘gaming’ to thrombosis and fatal pulmonary emboli. Additionally, prolonged television viewing has become a documented risk factor for mortality from pulmonary emboli,” the investigators wrote.
They recommend that clinicians ask adolescents about their gaming and TV-watching habits and encourage them to become more active to lower their risk for VTE.
The study was internally supported. Dr. Kohorst and colleagues reported no relevant disclosures.
MONTREAL – It’s well known that airplane passengers, condemned to sit for endless hours in the claustrophobic cabins of the unfriendly skies, are at increased risk for venous thromboembolic events (VTEs). Less well documented, however, is the VTE risk encountered by overweight or obese teens who while their hours away playing video games.
“This is becoming a sedentary-type risk factor,” said Mira A. Kohorst, MD, from the division of pediatric hematology-oncology at the Mayo Clinic in Rochester, Minn.
Dr. Kohorst and her colleagues reported on a small but troubling trend of VTE episodes that they observed in teen boys over the last few years. They refer to obesity, sedentary lifestyle, and gaming as “the new thrombophilia cocktail in adolescent males.”
The reported incidence of pediatric VTE ranges from 0.7 to 4.9 per 100,000 person years, considerably lower than the 1 in 1000 estimated incidences reported in adults. But, thanks to the growing incidence of obesity in children, which more than doubled from 1980 to 2012 and quadrupled in teens age 12-19 years from 5% to 21%, youngsters appear to be catching up in the VTE department, the investigators reported.
“Given the direct mortality rate of 2% [that is] associated with VTE and risk for postthrombotic syndrome of 26%, it is important to understand underlying modifiable risk factors,” they wrote.
To do this, they retrospectively reviewed records of children who presented with VTE in their center.
All play, no exercise
The authors described three cases, including that of an 18-year old boy with a body mass index (BMI) of 37 kg/m2, putting him squarely in the obese category. This lad, who spent 12 or more hours a day playing video games and was sedentary at other times as well, presented with bilateral pulmonary emboli and an associated right lower lobe infarction. Testing for thrombophilia showed that he was heterozygous for factor V Leiden but did not have other coagulation abnormalities. He was started on enoxaparin (Lovenox) and then transitioned to apixaban (Eliquis) for a total of 6 months of thromboprophylaxis. He was counseled about modifying his lifestyle and did not have a recurrence after 14 months of follow-up.
A similarly sedentary 17-year old male with an even higher BMI (39 kg/m2) presented with bilateral basilar pulmonary emboli and infarctions in association with a left femoral deep vein thrombosis. This patients also had factor V Leiden heterozygosity and the May-Thurner (iliac vein compression) syndrome. He was treated for a total of 6 months with warfarin followed by rivaroxaban (Xarelto) and was counseled about lifestyle changes but was unable to lose weight. Eight months after completing therapy, he had a second extensive deep vein thrombosis, this time in his right leg, and was restarted on rivaroxaban.
The third patient, a morbidly obese (BMI 56 kg/m2) 13-year-old boy, presented with left lower lobe pulmonary embolism following 3 weeks of immobility caused by the Guillain-Barré syndrome. As in the other cases, he confessed to a sedentary lifestyle and a predilection for gaming. His father had previously developed a line-associated thrombus. The family declined thrombophilia testing. The patient received 3 months of enoxaparin. He has not been followed since discontinuing therapy.
Move it, kid!
The risk of VTE in adolescent boys, especially obese and extreme gamers who spend most of their waking hours in a chair staring at a screen, is similar to that for adolescent girls who use oral contraceptives, Dr. Kohorst and her colleagues said.
“Many case reports link prolonged ‘gaming’ to thrombosis and fatal pulmonary emboli. Additionally, prolonged television viewing has become a documented risk factor for mortality from pulmonary emboli,” the investigators wrote.
They recommend that clinicians ask adolescents about their gaming and TV-watching habits and encourage them to become more active to lower their risk for VTE.
The study was internally supported. Dr. Kohorst and colleagues reported no relevant disclosures.
FROM ASPHO 2017
Key clinical point: Obesity and a sedentary lifestyle are risk factors for venous thromboembolic events in teens, as well as adults.
Major finding: Teen boys who were obese and spent much of their day playing video games presented with VTE.
Data source: Retrospective review and case series.
Disclosures: The study was internally supported. Dr. Kohorst and colleagues reported no relevant disclosures.
Could refractory T-ALL be daratumumab’s next frontier?
MONTREAL – Daratumumab may do for patients with T-cell acute lymphoblastic leukemia (T-ALL) what it has done for those with multiple myeloma. That, at least, is the hope of a team of investigators who are conducting preclinical studies and planning human trials of the CD38 inhibitor in leukemia.
“We believe daratumumab significantly inhibits disease progression as shown in our different [patient-derived xenograft] models,” said Karen L. Bride, MD, of Children’s Hospital of Philadelphia.
The Food and Drug Administration approved daratumumab (Darzalex) in November 2015 for the treatment of patients with multiple myeloma who had received at least three prior lines of therapy. They then amended the approval last fall to “at least one prior medicine.”
When added to a standard regimen of bortezomib and dexamethasone in patients with relapsed or refractory multiple myeloma in the phase III CASTOR trial, daratumumab reduced the risk of disease progression or death by 61% with little increase in toxicity.
The drug is believed to work against multiple myeloma through both an on-target (anti-CD38) mechanism, and through off-target promotion of increases in T-helper cells, cytotoxic T-lymphocytes, T-cell function response, and T-cell receptor clonality (Blood. 2016 Jan. doi: 10.1182/blood-2015-12-687749).
CD38 in T-ALL
Dr. Bride and her colleagues hope to bring daratumumab’s anti-CD38 action to bear on relapsed or refractory T-ALL.
“One of the reasons this is particularly challenging is that we find T-ALL is clinically and genetically heterogeneous,” she said. “With a number of different genetic mutations that have been identified, there are certainly some potentially targetable pathways. However, finding an appropriate target that can be broadly applicable is still needed.”
CD38 may be one such target. It is expressed at relatively high levels on both T-ALL and B-precursor ALL blasts but at only low levels on normal immune cells.
The investigators first used flow cytometry to measure CD38 levels in samples from 10 patients with early T-cell precursor (ETP) T-ALL and 11 with non-ETP disease, both at diagnosis and after 1 month of induction chemotherapy. CD38 expression was detectable in all of the samples and did not change significantly after chemotherapy, suggesting that CD38 was indeed a valid target in T-ALL.
They then grafted primary ALL blasts from patients with ETP-ALL and non-ETP-ALL into mice and randomly assigned them to be treated for 3 to 5 weeks with daratumumab or to serve as controls. The mice were initially treated after they developed more than 1% of peripheral blood blasts.
Daratumumab-treated models had significant reductions in disease burden as measured by blasts in both peripheral blood (P = .0112) and spleen (P = .0003).
There were six responses to daratumumab in the seven treated mice grafted with ETP-ALL and no cases of toxicity. Among the eight mice with non-ETP ALL, however, there was only one response, and five animals became moribund roughly 1 hour after injection.
The investigators could not find an explanation for these reactions either on necropsy or pathology studies.
“We hypothesized that there was potentially massive tumor lysis syndrome being experienced by the mice, and, as a consequence, they were becoming moribund,” Dr. Bride said.
In subsequent experiments, they have begun introducing the drug within 5 days of adoptive transfer, prior to full engraftment. This is akin to treating during a minimal residual disease phase, she said.
Despite the observed but unexplained toxicities in some animals, “our data are promising enough that we’re hopeful that we will open a phase I/II trial of daratumumab starting next year,” Dr. Bride said.
Not so fast
However, a pediatric hematologist/oncologist who was not involved in the study said in an interview that Dr. Bride and her colleagues would be wise not to proceed too quickly into human trials, at least until the potential toxicities of daratumumab in T-ALL have been more fully elucidated.
“I found it very striking that the mice responded the way they did, and that was just from receiving the drug. So, there is something else that’s going on, and I think it behooves them to investigate further. It’s not that I’m skeptical about the activity of the drug; I just don’t want studies to be shut down because the investigators didn’t have the best trial design,” said Valerie I. Brown, MD.
Dr. Brown, director of experimental therapeutics at Penn State Health Milton S. Hershey (Penn.) Medical Center, was a comoderator of the session where Dr. Bride presented the study findings.
Asked about her response to Dr. Brown’s comments in an interview, Dr. Bride said that “because of the success of daratumumab in humans already, I think I’m a bit less worried about this agent. You can’t necessarily translate exactly across diseases, but I do think it’s very promising, and I don’t think [the toxicity] is a reason to pull back.”
The study was supported by grants from the Leukemia and Lymphoma Society and the National Institutes of Health. Janssen donated the daratumumab. Dr. Bride and Dr. Brown reported no conflicts of interest to disclose.
MONTREAL – Daratumumab may do for patients with T-cell acute lymphoblastic leukemia (T-ALL) what it has done for those with multiple myeloma. That, at least, is the hope of a team of investigators who are conducting preclinical studies and planning human trials of the CD38 inhibitor in leukemia.
“We believe daratumumab significantly inhibits disease progression as shown in our different [patient-derived xenograft] models,” said Karen L. Bride, MD, of Children’s Hospital of Philadelphia.
The Food and Drug Administration approved daratumumab (Darzalex) in November 2015 for the treatment of patients with multiple myeloma who had received at least three prior lines of therapy. They then amended the approval last fall to “at least one prior medicine.”
When added to a standard regimen of bortezomib and dexamethasone in patients with relapsed or refractory multiple myeloma in the phase III CASTOR trial, daratumumab reduced the risk of disease progression or death by 61% with little increase in toxicity.
The drug is believed to work against multiple myeloma through both an on-target (anti-CD38) mechanism, and through off-target promotion of increases in T-helper cells, cytotoxic T-lymphocytes, T-cell function response, and T-cell receptor clonality (Blood. 2016 Jan. doi: 10.1182/blood-2015-12-687749).
CD38 in T-ALL
Dr. Bride and her colleagues hope to bring daratumumab’s anti-CD38 action to bear on relapsed or refractory T-ALL.
“One of the reasons this is particularly challenging is that we find T-ALL is clinically and genetically heterogeneous,” she said. “With a number of different genetic mutations that have been identified, there are certainly some potentially targetable pathways. However, finding an appropriate target that can be broadly applicable is still needed.”
CD38 may be one such target. It is expressed at relatively high levels on both T-ALL and B-precursor ALL blasts but at only low levels on normal immune cells.
The investigators first used flow cytometry to measure CD38 levels in samples from 10 patients with early T-cell precursor (ETP) T-ALL and 11 with non-ETP disease, both at diagnosis and after 1 month of induction chemotherapy. CD38 expression was detectable in all of the samples and did not change significantly after chemotherapy, suggesting that CD38 was indeed a valid target in T-ALL.
They then grafted primary ALL blasts from patients with ETP-ALL and non-ETP-ALL into mice and randomly assigned them to be treated for 3 to 5 weeks with daratumumab or to serve as controls. The mice were initially treated after they developed more than 1% of peripheral blood blasts.
Daratumumab-treated models had significant reductions in disease burden as measured by blasts in both peripheral blood (P = .0112) and spleen (P = .0003).
There were six responses to daratumumab in the seven treated mice grafted with ETP-ALL and no cases of toxicity. Among the eight mice with non-ETP ALL, however, there was only one response, and five animals became moribund roughly 1 hour after injection.
The investigators could not find an explanation for these reactions either on necropsy or pathology studies.
“We hypothesized that there was potentially massive tumor lysis syndrome being experienced by the mice, and, as a consequence, they were becoming moribund,” Dr. Bride said.
In subsequent experiments, they have begun introducing the drug within 5 days of adoptive transfer, prior to full engraftment. This is akin to treating during a minimal residual disease phase, she said.
Despite the observed but unexplained toxicities in some animals, “our data are promising enough that we’re hopeful that we will open a phase I/II trial of daratumumab starting next year,” Dr. Bride said.
Not so fast
However, a pediatric hematologist/oncologist who was not involved in the study said in an interview that Dr. Bride and her colleagues would be wise not to proceed too quickly into human trials, at least until the potential toxicities of daratumumab in T-ALL have been more fully elucidated.
“I found it very striking that the mice responded the way they did, and that was just from receiving the drug. So, there is something else that’s going on, and I think it behooves them to investigate further. It’s not that I’m skeptical about the activity of the drug; I just don’t want studies to be shut down because the investigators didn’t have the best trial design,” said Valerie I. Brown, MD.
Dr. Brown, director of experimental therapeutics at Penn State Health Milton S. Hershey (Penn.) Medical Center, was a comoderator of the session where Dr. Bride presented the study findings.
Asked about her response to Dr. Brown’s comments in an interview, Dr. Bride said that “because of the success of daratumumab in humans already, I think I’m a bit less worried about this agent. You can’t necessarily translate exactly across diseases, but I do think it’s very promising, and I don’t think [the toxicity] is a reason to pull back.”
The study was supported by grants from the Leukemia and Lymphoma Society and the National Institutes of Health. Janssen donated the daratumumab. Dr. Bride and Dr. Brown reported no conflicts of interest to disclose.
MONTREAL – Daratumumab may do for patients with T-cell acute lymphoblastic leukemia (T-ALL) what it has done for those with multiple myeloma. That, at least, is the hope of a team of investigators who are conducting preclinical studies and planning human trials of the CD38 inhibitor in leukemia.
“We believe daratumumab significantly inhibits disease progression as shown in our different [patient-derived xenograft] models,” said Karen L. Bride, MD, of Children’s Hospital of Philadelphia.
The Food and Drug Administration approved daratumumab (Darzalex) in November 2015 for the treatment of patients with multiple myeloma who had received at least three prior lines of therapy. They then amended the approval last fall to “at least one prior medicine.”
When added to a standard regimen of bortezomib and dexamethasone in patients with relapsed or refractory multiple myeloma in the phase III CASTOR trial, daratumumab reduced the risk of disease progression or death by 61% with little increase in toxicity.
The drug is believed to work against multiple myeloma through both an on-target (anti-CD38) mechanism, and through off-target promotion of increases in T-helper cells, cytotoxic T-lymphocytes, T-cell function response, and T-cell receptor clonality (Blood. 2016 Jan. doi: 10.1182/blood-2015-12-687749).
CD38 in T-ALL
Dr. Bride and her colleagues hope to bring daratumumab’s anti-CD38 action to bear on relapsed or refractory T-ALL.
“One of the reasons this is particularly challenging is that we find T-ALL is clinically and genetically heterogeneous,” she said. “With a number of different genetic mutations that have been identified, there are certainly some potentially targetable pathways. However, finding an appropriate target that can be broadly applicable is still needed.”
CD38 may be one such target. It is expressed at relatively high levels on both T-ALL and B-precursor ALL blasts but at only low levels on normal immune cells.
The investigators first used flow cytometry to measure CD38 levels in samples from 10 patients with early T-cell precursor (ETP) T-ALL and 11 with non-ETP disease, both at diagnosis and after 1 month of induction chemotherapy. CD38 expression was detectable in all of the samples and did not change significantly after chemotherapy, suggesting that CD38 was indeed a valid target in T-ALL.
They then grafted primary ALL blasts from patients with ETP-ALL and non-ETP-ALL into mice and randomly assigned them to be treated for 3 to 5 weeks with daratumumab or to serve as controls. The mice were initially treated after they developed more than 1% of peripheral blood blasts.
Daratumumab-treated models had significant reductions in disease burden as measured by blasts in both peripheral blood (P = .0112) and spleen (P = .0003).
There were six responses to daratumumab in the seven treated mice grafted with ETP-ALL and no cases of toxicity. Among the eight mice with non-ETP ALL, however, there was only one response, and five animals became moribund roughly 1 hour after injection.
The investigators could not find an explanation for these reactions either on necropsy or pathology studies.
“We hypothesized that there was potentially massive tumor lysis syndrome being experienced by the mice, and, as a consequence, they were becoming moribund,” Dr. Bride said.
In subsequent experiments, they have begun introducing the drug within 5 days of adoptive transfer, prior to full engraftment. This is akin to treating during a minimal residual disease phase, she said.
Despite the observed but unexplained toxicities in some animals, “our data are promising enough that we’re hopeful that we will open a phase I/II trial of daratumumab starting next year,” Dr. Bride said.
Not so fast
However, a pediatric hematologist/oncologist who was not involved in the study said in an interview that Dr. Bride and her colleagues would be wise not to proceed too quickly into human trials, at least until the potential toxicities of daratumumab in T-ALL have been more fully elucidated.
“I found it very striking that the mice responded the way they did, and that was just from receiving the drug. So, there is something else that’s going on, and I think it behooves them to investigate further. It’s not that I’m skeptical about the activity of the drug; I just don’t want studies to be shut down because the investigators didn’t have the best trial design,” said Valerie I. Brown, MD.
Dr. Brown, director of experimental therapeutics at Penn State Health Milton S. Hershey (Penn.) Medical Center, was a comoderator of the session where Dr. Bride presented the study findings.
Asked about her response to Dr. Brown’s comments in an interview, Dr. Bride said that “because of the success of daratumumab in humans already, I think I’m a bit less worried about this agent. You can’t necessarily translate exactly across diseases, but I do think it’s very promising, and I don’t think [the toxicity] is a reason to pull back.”
The study was supported by grants from the Leukemia and Lymphoma Society and the National Institutes of Health. Janssen donated the daratumumab. Dr. Bride and Dr. Brown reported no conflicts of interest to disclose.
Key clinical point: CD38 may be a valid target for therapy against relapsed/refractory T-cell acute lymphoblastic leukemia.
Major finding: Six of seven models of early T-precursor T-ALL responded to daratumumab injections.
Data source: In vitro and in vivo studies evaluating the potential of daratumumab for treatment of T-ALL.
Disclosures: The study was supported by grants from the Leukemia and Lymphoma Society and the National Institutes of Health. Janssen donated the daratumumab. Dr. Bride and Dr. Brown reported having no conflicts of interest.
Watchful waiting a suitable option for pediatric acute ITP
MONTREAL – Clinicians who manage the care of children with newly diagnosed idiopathic thrombocytopenic purpura (ITP) have to decide whether to treat patients early and possibly increase risk for chronic ITP down the road or to leave well enough alone and treat only as needed.
Evidence from a retrospective study suggests that, for most patients, early treatment of acute ITP does not appear to increase the risk for chronic ITP in the future, reported Chelsea L. Grama, a fourth-year medical student, and her colleagues from Penn State Health Children’s Hospital in Hershey, Pennsylvania.
“It’s controversial whether to treat or not. Some people think steroids are the best way to go, some think IVIG [intravenous immunoglobulin] is the best way to go, and some think that no treatment could be the best option. There is really no consensus in the literature to say what the ideal treatment for ITP is,” she commented in an interview at the annual meeting of the American Society of Pediatric Hematology/Oncology.
That lack of consensus is the result of the fact that serious consequences of ITP, such as intracranial hemorrhage, are rare, making it difficult for investigators to compare clinical outcomes with various forms of treatment. It’s also unclear whether early interventions could lead to later chronic disease.
In hopes of answering this question, Ms. Grama and her coinvestigators, led by Andrew S. Freiberg, MD, took a retrospective look at data on 249 patients with ITP diagnosed from birth through age 21 from 1994 to 2011.
They looked at demographic variables, treatments received, and subsequent platelet counts. Outcomes included intracranial hemorrhage and long-term improvements in platelet counts. They defined chronic ITP as a platelet count below 140,000/mcL for at least 6 months following diagnosis.
Of the 249 patients, 126 (66 boys and 60 girls) progressed to chronic ITP. The incidence of chronic ITP among treated patients was 51.1% and, in untreated patients, 49.3%.
However, in two subgroups, there was a significant association between treatment and chronic ITP. Among girls from birth through age 3 years, 58% of those treated went on to chronic ITP, compared with 0% for untreated patients (P = .008). When the age cohort was expanded to girls younger than 6 years, a similar pattern emerged, with chronic ITP rates of 45% for treated patients, compared with 0% for untreated patients. There were only 44 total patients in this age and sex subgroup, however, making it difficult to draw strong inferences about a potential relationship between treatment and chronic ITP, Ms. Grama noted.
Only three patients had cerebral hemorrhage. Two had hemorrhage as their initial ITP presentation, and the third had bleeding despite being on treatment. The sample size was too small to correlate the outcome with treatment, the authors noted.
In multivariate analysis controlling for demographic, clinical, and treatment factors, only age and platelet count at presentation independently predicted chronic thrombocytopenia (P less than .0001 for each). Steroid therapy was the only independent predictor for acute ITP (P = .0001).
Although their data suggest that there could be a benefit to treating patients who first present with acute ITP at age 12 years or older, “I do think it’s reasonable to wait and watch these patients,” Ms. Grama said.
“The incidence of brain bleeds, which is the really scary complication, was so low in these patients that I think watchful waiting would be a better way to go, just monitoring patients very closely. Hopefully, they will resolve without having treatment,” she added.
The study was internally supported. The authors reported no relevant disclosures.
MONTREAL – Clinicians who manage the care of children with newly diagnosed idiopathic thrombocytopenic purpura (ITP) have to decide whether to treat patients early and possibly increase risk for chronic ITP down the road or to leave well enough alone and treat only as needed.
Evidence from a retrospective study suggests that, for most patients, early treatment of acute ITP does not appear to increase the risk for chronic ITP in the future, reported Chelsea L. Grama, a fourth-year medical student, and her colleagues from Penn State Health Children’s Hospital in Hershey, Pennsylvania.
“It’s controversial whether to treat or not. Some people think steroids are the best way to go, some think IVIG [intravenous immunoglobulin] is the best way to go, and some think that no treatment could be the best option. There is really no consensus in the literature to say what the ideal treatment for ITP is,” she commented in an interview at the annual meeting of the American Society of Pediatric Hematology/Oncology.
That lack of consensus is the result of the fact that serious consequences of ITP, such as intracranial hemorrhage, are rare, making it difficult for investigators to compare clinical outcomes with various forms of treatment. It’s also unclear whether early interventions could lead to later chronic disease.
In hopes of answering this question, Ms. Grama and her coinvestigators, led by Andrew S. Freiberg, MD, took a retrospective look at data on 249 patients with ITP diagnosed from birth through age 21 from 1994 to 2011.
They looked at demographic variables, treatments received, and subsequent platelet counts. Outcomes included intracranial hemorrhage and long-term improvements in platelet counts. They defined chronic ITP as a platelet count below 140,000/mcL for at least 6 months following diagnosis.
Of the 249 patients, 126 (66 boys and 60 girls) progressed to chronic ITP. The incidence of chronic ITP among treated patients was 51.1% and, in untreated patients, 49.3%.
However, in two subgroups, there was a significant association between treatment and chronic ITP. Among girls from birth through age 3 years, 58% of those treated went on to chronic ITP, compared with 0% for untreated patients (P = .008). When the age cohort was expanded to girls younger than 6 years, a similar pattern emerged, with chronic ITP rates of 45% for treated patients, compared with 0% for untreated patients. There were only 44 total patients in this age and sex subgroup, however, making it difficult to draw strong inferences about a potential relationship between treatment and chronic ITP, Ms. Grama noted.
Only three patients had cerebral hemorrhage. Two had hemorrhage as their initial ITP presentation, and the third had bleeding despite being on treatment. The sample size was too small to correlate the outcome with treatment, the authors noted.
In multivariate analysis controlling for demographic, clinical, and treatment factors, only age and platelet count at presentation independently predicted chronic thrombocytopenia (P less than .0001 for each). Steroid therapy was the only independent predictor for acute ITP (P = .0001).
Although their data suggest that there could be a benefit to treating patients who first present with acute ITP at age 12 years or older, “I do think it’s reasonable to wait and watch these patients,” Ms. Grama said.
“The incidence of brain bleeds, which is the really scary complication, was so low in these patients that I think watchful waiting would be a better way to go, just monitoring patients very closely. Hopefully, they will resolve without having treatment,” she added.
The study was internally supported. The authors reported no relevant disclosures.
MONTREAL – Clinicians who manage the care of children with newly diagnosed idiopathic thrombocytopenic purpura (ITP) have to decide whether to treat patients early and possibly increase risk for chronic ITP down the road or to leave well enough alone and treat only as needed.
Evidence from a retrospective study suggests that, for most patients, early treatment of acute ITP does not appear to increase the risk for chronic ITP in the future, reported Chelsea L. Grama, a fourth-year medical student, and her colleagues from Penn State Health Children’s Hospital in Hershey, Pennsylvania.
“It’s controversial whether to treat or not. Some people think steroids are the best way to go, some think IVIG [intravenous immunoglobulin] is the best way to go, and some think that no treatment could be the best option. There is really no consensus in the literature to say what the ideal treatment for ITP is,” she commented in an interview at the annual meeting of the American Society of Pediatric Hematology/Oncology.
That lack of consensus is the result of the fact that serious consequences of ITP, such as intracranial hemorrhage, are rare, making it difficult for investigators to compare clinical outcomes with various forms of treatment. It’s also unclear whether early interventions could lead to later chronic disease.
In hopes of answering this question, Ms. Grama and her coinvestigators, led by Andrew S. Freiberg, MD, took a retrospective look at data on 249 patients with ITP diagnosed from birth through age 21 from 1994 to 2011.
They looked at demographic variables, treatments received, and subsequent platelet counts. Outcomes included intracranial hemorrhage and long-term improvements in platelet counts. They defined chronic ITP as a platelet count below 140,000/mcL for at least 6 months following diagnosis.
Of the 249 patients, 126 (66 boys and 60 girls) progressed to chronic ITP. The incidence of chronic ITP among treated patients was 51.1% and, in untreated patients, 49.3%.
However, in two subgroups, there was a significant association between treatment and chronic ITP. Among girls from birth through age 3 years, 58% of those treated went on to chronic ITP, compared with 0% for untreated patients (P = .008). When the age cohort was expanded to girls younger than 6 years, a similar pattern emerged, with chronic ITP rates of 45% for treated patients, compared with 0% for untreated patients. There were only 44 total patients in this age and sex subgroup, however, making it difficult to draw strong inferences about a potential relationship between treatment and chronic ITP, Ms. Grama noted.
Only three patients had cerebral hemorrhage. Two had hemorrhage as their initial ITP presentation, and the third had bleeding despite being on treatment. The sample size was too small to correlate the outcome with treatment, the authors noted.
In multivariate analysis controlling for demographic, clinical, and treatment factors, only age and platelet count at presentation independently predicted chronic thrombocytopenia (P less than .0001 for each). Steroid therapy was the only independent predictor for acute ITP (P = .0001).
Although their data suggest that there could be a benefit to treating patients who first present with acute ITP at age 12 years or older, “I do think it’s reasonable to wait and watch these patients,” Ms. Grama said.
“The incidence of brain bleeds, which is the really scary complication, was so low in these patients that I think watchful waiting would be a better way to go, just monitoring patients very closely. Hopefully, they will resolve without having treatment,” she added.
The study was internally supported. The authors reported no relevant disclosures.
FROM ASPHO 2017
Key clinical point: Treatment of acute idiopathic thrombocytopenic purpura does not appear to increase risk of later chronic ITP in children older than 6 years.
Major finding: Girls younger than 6 years were the only group in which early treatment of acute ITP may have increased risk for chronic ITP.
Data source: Retrospective data review on 249 children and young adults with ITP.
Disclosures: The study was internally supported. The authors reported no relevant disclosures.
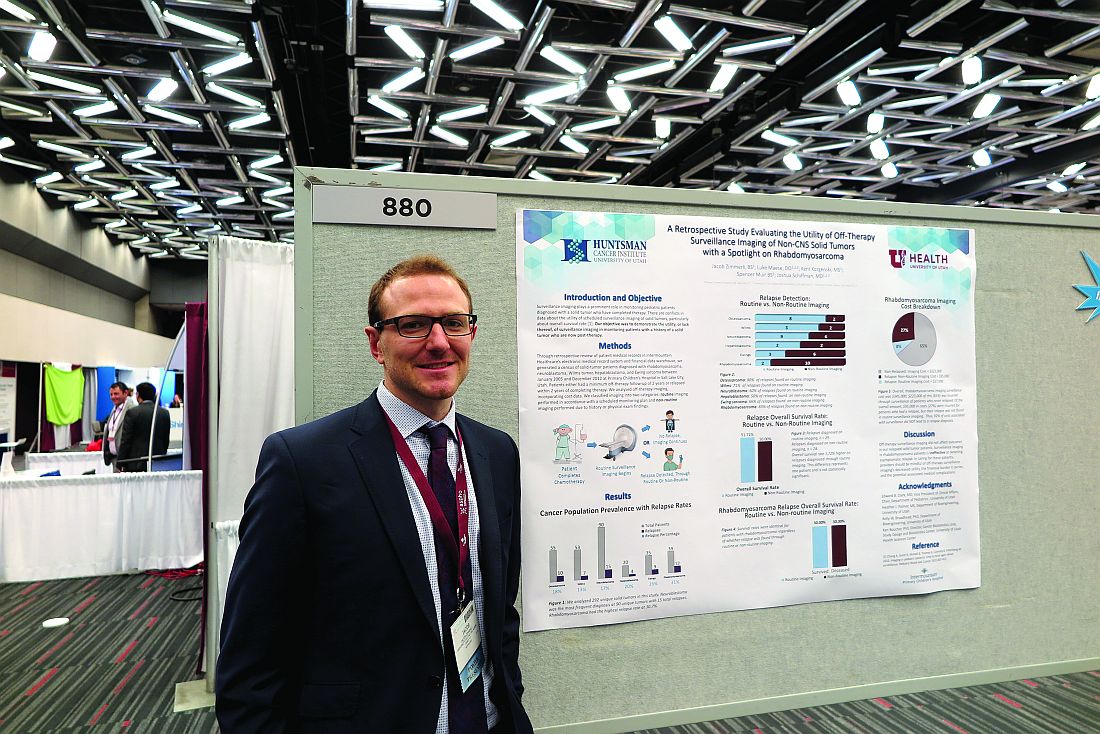
In sickle cell disease, osteomyelitis is a tough call
MONTREAL – Osteomyelitis is an especially challenging diagnosis in children with sickle cell disease (SCD) because the bone and joint signs, elevated white cell counts, and C-reactive protein levels that are commonly used to diagnose bone infection are frequently features of SCD as well.
As a result, most patients with SCD and suspected osteomyelitis are treated without a confirmation of the diagnosis.
Among 30 patients with SCD who were followed at a single center over a decade, 29 patients had elevated ESR, but only 13 patients had leukocytosis, and only 13 had elevated CRP.
“Prior studies on sickle cell disease have shown that it is very difficult to differentiate between osteoarticular infection and bone infarction. Therefore, oftentimes, this diagnosis is very difficult to make,” Dr. Weisman said at the annual meeting of the American Society of Pediatric Hematology/Oncology.
Laboratory findings for osteomyelitis in SCD are often nonspecific, including leukocytosis, elevated CRP and ESR, and blood cultures positive for Staphylococcus aureus (the predominant pathogen in children with osteomyelitis), or, in children with hemoglobinopathies, salmonella.
In children with SCD, CRP levels can vary from normal to elevated. ESR is similarly variable, as low hematocrit values can result in higher ESR values. Additionally, sickle erythrocytes can fail to aggregate, which can lead to lower ESR values.
A decade of data
The researchers set out to get a better handle on the characteristics and outcomes of osteomyelitis in patients with SCD and to see which laboratory and imaging findings might prove most useful for diagnosing osteomyelitis in this population. They reviewed data on 59 patients who were identified with indeterminate or likely osteomyelitis over a 10-year span. Of those, 30 were diagnosed and treated for osteomyelitis, and 29 were tentatively diagnosed but not treated. The latter group likely had symptoms caused by a bone infarction or vaso-occlusive crisis, Dr. Weisman said.
Among the 30 treated patients, osteomyelitis was confirmed by bone biopsy in 3, and an organism was isolated from blood or an abscess in 6. In the other 21, osteomyelitis was presumed based on clinical, laboratory, and MRI findings.
The median patient age was 12 years (range, 8 months to 18 years), 18 were male, and all but three patients have the HbSS genotype. Of the remaining patients, two had the HbSC and one the HbSF genotypes.
Infections occurred in the lower extremities in 11 patients, in the upper extremities in 10, in the pelvis or vertebrae in 2 each, and in the scapula, clavicle, hand, rib, or mandible in 1 patient each.
Just 13 of the 30 patients (43%) had lab findings of leukocytosis (more than 15,000 cells/mm2), and an equal number had elevated CRP (greater than 10 mg/L).
In contrast, 29 patients had an ESR above 20 mm/hour, and, in three of these patients, the rate was higher than 100 mm/hour.
When the researchers compared white blood cell counts and CRP levels between the treated patients and the 29 untreated controls, they found no significant differences for either measure of inflammation. In contrast, ESR was significantly higher among treated patients (P = .03).
Looking at the receiver operating characteristic curve for ESR, they found that an ESR of more than 100 mm/hour had 100% specificity for osteomyelitis in this group of patients.
Only 6 of the 30 (20%) had bacteremia. In 9 patients, nontyphoidal salmonella was isolated from cultures of either bone biopsy (3), abscess (3), or blood (6), but no possible causative organism could be isolated in the remaining 21 patients.
All patients were treated with prolonged antibiotic therapy. Surgical drainage and/or debridement were required in 6 patients. Two patients developed chronic osteomyelitis, but infection eventually resolved in all patients.
Recommendations
Dr. Weisman recommended early consultation with infectious disease experts and orthopedists; labs studies with complete blood counts, CRP, and ESR; and imaging studies with MRI when there is clinical suspicion of osteomyelitis in patients with SCD.
When an SCD patient has indeterminate findings, a blood culture can be performed. If it is positive for salmonella and the ESR is above 100 mm/hour, the patient can then go on to treatment. If the blood culture is negative and the ESR is below 100 mm hour but the suspicion of osteomyelitis remains high, a bone biopsy can be considered, they concluded.
The study was internally funded. Dr. Weisman reported no conflicts of interest to disclose.
MONTREAL – Osteomyelitis is an especially challenging diagnosis in children with sickle cell disease (SCD) because the bone and joint signs, elevated white cell counts, and C-reactive protein levels that are commonly used to diagnose bone infection are frequently features of SCD as well.
As a result, most patients with SCD and suspected osteomyelitis are treated without a confirmation of the diagnosis.
Among 30 patients with SCD who were followed at a single center over a decade, 29 patients had elevated ESR, but only 13 patients had leukocytosis, and only 13 had elevated CRP.
“Prior studies on sickle cell disease have shown that it is very difficult to differentiate between osteoarticular infection and bone infarction. Therefore, oftentimes, this diagnosis is very difficult to make,” Dr. Weisman said at the annual meeting of the American Society of Pediatric Hematology/Oncology.
Laboratory findings for osteomyelitis in SCD are often nonspecific, including leukocytosis, elevated CRP and ESR, and blood cultures positive for Staphylococcus aureus (the predominant pathogen in children with osteomyelitis), or, in children with hemoglobinopathies, salmonella.
In children with SCD, CRP levels can vary from normal to elevated. ESR is similarly variable, as low hematocrit values can result in higher ESR values. Additionally, sickle erythrocytes can fail to aggregate, which can lead to lower ESR values.
A decade of data
The researchers set out to get a better handle on the characteristics and outcomes of osteomyelitis in patients with SCD and to see which laboratory and imaging findings might prove most useful for diagnosing osteomyelitis in this population. They reviewed data on 59 patients who were identified with indeterminate or likely osteomyelitis over a 10-year span. Of those, 30 were diagnosed and treated for osteomyelitis, and 29 were tentatively diagnosed but not treated. The latter group likely had symptoms caused by a bone infarction or vaso-occlusive crisis, Dr. Weisman said.
Among the 30 treated patients, osteomyelitis was confirmed by bone biopsy in 3, and an organism was isolated from blood or an abscess in 6. In the other 21, osteomyelitis was presumed based on clinical, laboratory, and MRI findings.
The median patient age was 12 years (range, 8 months to 18 years), 18 were male, and all but three patients have the HbSS genotype. Of the remaining patients, two had the HbSC and one the HbSF genotypes.
Infections occurred in the lower extremities in 11 patients, in the upper extremities in 10, in the pelvis or vertebrae in 2 each, and in the scapula, clavicle, hand, rib, or mandible in 1 patient each.
Just 13 of the 30 patients (43%) had lab findings of leukocytosis (more than 15,000 cells/mm2), and an equal number had elevated CRP (greater than 10 mg/L).
In contrast, 29 patients had an ESR above 20 mm/hour, and, in three of these patients, the rate was higher than 100 mm/hour.
When the researchers compared white blood cell counts and CRP levels between the treated patients and the 29 untreated controls, they found no significant differences for either measure of inflammation. In contrast, ESR was significantly higher among treated patients (P = .03).
Looking at the receiver operating characteristic curve for ESR, they found that an ESR of more than 100 mm/hour had 100% specificity for osteomyelitis in this group of patients.
Only 6 of the 30 (20%) had bacteremia. In 9 patients, nontyphoidal salmonella was isolated from cultures of either bone biopsy (3), abscess (3), or blood (6), but no possible causative organism could be isolated in the remaining 21 patients.
All patients were treated with prolonged antibiotic therapy. Surgical drainage and/or debridement were required in 6 patients. Two patients developed chronic osteomyelitis, but infection eventually resolved in all patients.
Recommendations
Dr. Weisman recommended early consultation with infectious disease experts and orthopedists; labs studies with complete blood counts, CRP, and ESR; and imaging studies with MRI when there is clinical suspicion of osteomyelitis in patients with SCD.
When an SCD patient has indeterminate findings, a blood culture can be performed. If it is positive for salmonella and the ESR is above 100 mm/hour, the patient can then go on to treatment. If the blood culture is negative and the ESR is below 100 mm hour but the suspicion of osteomyelitis remains high, a bone biopsy can be considered, they concluded.
The study was internally funded. Dr. Weisman reported no conflicts of interest to disclose.
MONTREAL – Osteomyelitis is an especially challenging diagnosis in children with sickle cell disease (SCD) because the bone and joint signs, elevated white cell counts, and C-reactive protein levels that are commonly used to diagnose bone infection are frequently features of SCD as well.
As a result, most patients with SCD and suspected osteomyelitis are treated without a confirmation of the diagnosis.
Among 30 patients with SCD who were followed at a single center over a decade, 29 patients had elevated ESR, but only 13 patients had leukocytosis, and only 13 had elevated CRP.
“Prior studies on sickle cell disease have shown that it is very difficult to differentiate between osteoarticular infection and bone infarction. Therefore, oftentimes, this diagnosis is very difficult to make,” Dr. Weisman said at the annual meeting of the American Society of Pediatric Hematology/Oncology.
Laboratory findings for osteomyelitis in SCD are often nonspecific, including leukocytosis, elevated CRP and ESR, and blood cultures positive for Staphylococcus aureus (the predominant pathogen in children with osteomyelitis), or, in children with hemoglobinopathies, salmonella.
In children with SCD, CRP levels can vary from normal to elevated. ESR is similarly variable, as low hematocrit values can result in higher ESR values. Additionally, sickle erythrocytes can fail to aggregate, which can lead to lower ESR values.
A decade of data
The researchers set out to get a better handle on the characteristics and outcomes of osteomyelitis in patients with SCD and to see which laboratory and imaging findings might prove most useful for diagnosing osteomyelitis in this population. They reviewed data on 59 patients who were identified with indeterminate or likely osteomyelitis over a 10-year span. Of those, 30 were diagnosed and treated for osteomyelitis, and 29 were tentatively diagnosed but not treated. The latter group likely had symptoms caused by a bone infarction or vaso-occlusive crisis, Dr. Weisman said.
Among the 30 treated patients, osteomyelitis was confirmed by bone biopsy in 3, and an organism was isolated from blood or an abscess in 6. In the other 21, osteomyelitis was presumed based on clinical, laboratory, and MRI findings.
The median patient age was 12 years (range, 8 months to 18 years), 18 were male, and all but three patients have the HbSS genotype. Of the remaining patients, two had the HbSC and one the HbSF genotypes.
Infections occurred in the lower extremities in 11 patients, in the upper extremities in 10, in the pelvis or vertebrae in 2 each, and in the scapula, clavicle, hand, rib, or mandible in 1 patient each.
Just 13 of the 30 patients (43%) had lab findings of leukocytosis (more than 15,000 cells/mm2), and an equal number had elevated CRP (greater than 10 mg/L).
In contrast, 29 patients had an ESR above 20 mm/hour, and, in three of these patients, the rate was higher than 100 mm/hour.
When the researchers compared white blood cell counts and CRP levels between the treated patients and the 29 untreated controls, they found no significant differences for either measure of inflammation. In contrast, ESR was significantly higher among treated patients (P = .03).
Looking at the receiver operating characteristic curve for ESR, they found that an ESR of more than 100 mm/hour had 100% specificity for osteomyelitis in this group of patients.
Only 6 of the 30 (20%) had bacteremia. In 9 patients, nontyphoidal salmonella was isolated from cultures of either bone biopsy (3), abscess (3), or blood (6), but no possible causative organism could be isolated in the remaining 21 patients.
All patients were treated with prolonged antibiotic therapy. Surgical drainage and/or debridement were required in 6 patients. Two patients developed chronic osteomyelitis, but infection eventually resolved in all patients.
Recommendations
Dr. Weisman recommended early consultation with infectious disease experts and orthopedists; labs studies with complete blood counts, CRP, and ESR; and imaging studies with MRI when there is clinical suspicion of osteomyelitis in patients with SCD.
When an SCD patient has indeterminate findings, a blood culture can be performed. If it is positive for salmonella and the ESR is above 100 mm/hour, the patient can then go on to treatment. If the blood culture is negative and the ESR is below 100 mm hour but the suspicion of osteomyelitis remains high, a bone biopsy can be considered, they concluded.
The study was internally funded. Dr. Weisman reported no conflicts of interest to disclose.
Key clinical point: ESR may be a better lab marker for osteomyelitis in sickle cell disease than either WBC or CRP.
Major finding: An ESR greater than 100 mm/hr was 100% specific for osteomyelitis in this study.
Data source: A retrospective review of data on 59 patients with sickle cell disease and suspected or probable osteomyelitis.
Disclosures: The study was internally funded. Dr. Weisman reported having no conflicts of interest to disclose.
Cord blood/placental cell combo induces rapid immune recovery
MONTREAL – A combination of placenta-derived stem cells and umbilical cord blood was associated with early engraftment and high degrees of cord blood donor chimerism in the treatment of children with both malignant and nonmalignant hematologic conditions requiring stem cell transplantation, updated results of a pilot study show.
Among 16 children treated with the combination, the probability of neutrophil engraftment was 87.5%, and all patients who had neutrophil engraftment went on to have platelet engraftment. The probability of 12-month overall survival was 81.2%, reported Allyson Flower, MD, from Boston Children’s Health Physicians in Hawthorne, N.Y. “The probability of grade II-IV acute graft vs. host disease was 12.5%, compared with 32.5% seen with unrelated cord blood in our group’s previous studies. Cellular immune reconstitution was robust,” she said at the annual meeting of the American Society of Pediatric Hematology/Oncology.
Augmenting cord blood
Although unrelated donor cord blood transplantation expands the donor pool, is rapidly available, and is associated with decreases in both severe acute graft vs. host disease (GVHD) and chronic GVHD, compared with other stem cell sources, the technique is hampered by limited cell doses, prolonged immune reconstitution time, delays in hematopoietic recovery, and a higher incidence of graft failure.
Early studies of myeloablative conditioning followed by unrelated umbilical or placental blood transplantation showed a median of 22-24 days to neutrophil engraftment (Blood 1996 88:795-802; N Engl J Med. 1996;335:157-66), Dr. Flower noted.
More recently, a multivariate analysis of patients who underwent reduced-intensity conditioning followed by hematopoietic stem cell transplant with unrelated cord blood showed that graft failure was an independent risk factor for worse overall survival (Biol Blood Marrow Transplant. 2013 Apr;19:4;552-61).
Multiple groups have shown that adding human placenta–derived stem cells (HPDSC) to cord blood transplantation can facilitate more rapid hematopoietic engraftment by increasing the number of stem cells, increasing the proportion of hematopoietic progenitor cells, and providing additional, immature CD34+/CD45– progenitor cells.
In a single-arm, nonrandomized study, the investigators enrolled 16 patients ranging in age from 0.3 to 15.7 years with inborn errors of metabolism, marrow failure syndromes, severe immunodeficiency states, or hematologic malignancies.
Malignant conditions included B-cell precursor acute lymphoblastic leukemia (B-ALL; four patients), acute myeloid leukemia (AML; two), and T-cell ALL (one) in first complete remission, and T-cell lymphoblastic lymphoma following induction failure (one). Nonmalignant conditions included adrenoleukodystrophy (two patients), amegakaryotic thrombocytopenia (one), severe combined immunodeficiency (SCID; two), dyskeratosis congenita (one), chronic granulomatous disease (one), and severe congenital neutropenia (one).
The patients first underwent either myeloablative or reduced-intensity conditioning, followed 10 days later by infusion of unrelated cord blood and HPDSCs. Prior to HPDSC infusion, patients were medicated with diphenhydramine and hydrocortisone to prevent or reduce potential sensitivity reactions. HPDSCs were infused no sooner than 4 hours after the end of the cord blood infusion.
Patients received GVHD prophylaxis with either tacrolimus or cyclosporine, plus mycophenolate mofetil.
The combination appeared to be safe, with no cases of grade 3 or 4 toxicity secondary to HPDSC infusion.
The probability of neutrophil engraftment was 87.5%, with engraftment occurring at a median of 23 days (range 13-53). As noted before, all patients who had neutrophil engraftment had platelet engraftment, which was achieved at a median of 47 days (range, 20-98). In the group’s previous studies, median time to platelet engraftment was 53 days for patients who had undergone reduced-intensity conditioning, and 118 days for patients who had undergone myeloablation.
The probability of grade 2-4 acute GVHD within 100 days was 12.5%, and there were no cases of chronic GVHD.
Respective percentages of cord blood donor chimerism at days 30, 60, 100, and 180 were 88%, 98%, 99%, and 99%.
Immune reconstitution was strong, with normalization of mean CD3+, CD19+, and CD56+ cells occurring by day 100, CD8+ cells by day 180, and CD4+ cells by day 270.
There were three patient deaths: one from adenoviremia in a patient with B-ALL and CNS relapse, who had neutrophil engraftment at day 21; one in a patient with SCID, from adenoviremia and multiple system organ failure, who did not have engraftment before death; and one in a patient with severe congenital neutrophilia, who also did not have neutrophil engraftment.
None of the eight patients with malignant disease have experienced relapse to date, Dr. Flower noted.
The study was funded by a grant from Celgene Cellular Therapeutics. Dr. Flower reported having no conflicts of interest.
MONTREAL – A combination of placenta-derived stem cells and umbilical cord blood was associated with early engraftment and high degrees of cord blood donor chimerism in the treatment of children with both malignant and nonmalignant hematologic conditions requiring stem cell transplantation, updated results of a pilot study show.
Among 16 children treated with the combination, the probability of neutrophil engraftment was 87.5%, and all patients who had neutrophil engraftment went on to have platelet engraftment. The probability of 12-month overall survival was 81.2%, reported Allyson Flower, MD, from Boston Children’s Health Physicians in Hawthorne, N.Y. “The probability of grade II-IV acute graft vs. host disease was 12.5%, compared with 32.5% seen with unrelated cord blood in our group’s previous studies. Cellular immune reconstitution was robust,” she said at the annual meeting of the American Society of Pediatric Hematology/Oncology.
Augmenting cord blood
Although unrelated donor cord blood transplantation expands the donor pool, is rapidly available, and is associated with decreases in both severe acute graft vs. host disease (GVHD) and chronic GVHD, compared with other stem cell sources, the technique is hampered by limited cell doses, prolonged immune reconstitution time, delays in hematopoietic recovery, and a higher incidence of graft failure.
Early studies of myeloablative conditioning followed by unrelated umbilical or placental blood transplantation showed a median of 22-24 days to neutrophil engraftment (Blood 1996 88:795-802; N Engl J Med. 1996;335:157-66), Dr. Flower noted.
More recently, a multivariate analysis of patients who underwent reduced-intensity conditioning followed by hematopoietic stem cell transplant with unrelated cord blood showed that graft failure was an independent risk factor for worse overall survival (Biol Blood Marrow Transplant. 2013 Apr;19:4;552-61).
Multiple groups have shown that adding human placenta–derived stem cells (HPDSC) to cord blood transplantation can facilitate more rapid hematopoietic engraftment by increasing the number of stem cells, increasing the proportion of hematopoietic progenitor cells, and providing additional, immature CD34+/CD45– progenitor cells.
In a single-arm, nonrandomized study, the investigators enrolled 16 patients ranging in age from 0.3 to 15.7 years with inborn errors of metabolism, marrow failure syndromes, severe immunodeficiency states, or hematologic malignancies.
Malignant conditions included B-cell precursor acute lymphoblastic leukemia (B-ALL; four patients), acute myeloid leukemia (AML; two), and T-cell ALL (one) in first complete remission, and T-cell lymphoblastic lymphoma following induction failure (one). Nonmalignant conditions included adrenoleukodystrophy (two patients), amegakaryotic thrombocytopenia (one), severe combined immunodeficiency (SCID; two), dyskeratosis congenita (one), chronic granulomatous disease (one), and severe congenital neutropenia (one).
The patients first underwent either myeloablative or reduced-intensity conditioning, followed 10 days later by infusion of unrelated cord blood and HPDSCs. Prior to HPDSC infusion, patients were medicated with diphenhydramine and hydrocortisone to prevent or reduce potential sensitivity reactions. HPDSCs were infused no sooner than 4 hours after the end of the cord blood infusion.
Patients received GVHD prophylaxis with either tacrolimus or cyclosporine, plus mycophenolate mofetil.
The combination appeared to be safe, with no cases of grade 3 or 4 toxicity secondary to HPDSC infusion.
The probability of neutrophil engraftment was 87.5%, with engraftment occurring at a median of 23 days (range 13-53). As noted before, all patients who had neutrophil engraftment had platelet engraftment, which was achieved at a median of 47 days (range, 20-98). In the group’s previous studies, median time to platelet engraftment was 53 days for patients who had undergone reduced-intensity conditioning, and 118 days for patients who had undergone myeloablation.
The probability of grade 2-4 acute GVHD within 100 days was 12.5%, and there were no cases of chronic GVHD.
Respective percentages of cord blood donor chimerism at days 30, 60, 100, and 180 were 88%, 98%, 99%, and 99%.
Immune reconstitution was strong, with normalization of mean CD3+, CD19+, and CD56+ cells occurring by day 100, CD8+ cells by day 180, and CD4+ cells by day 270.
There were three patient deaths: one from adenoviremia in a patient with B-ALL and CNS relapse, who had neutrophil engraftment at day 21; one in a patient with SCID, from adenoviremia and multiple system organ failure, who did not have engraftment before death; and one in a patient with severe congenital neutrophilia, who also did not have neutrophil engraftment.
None of the eight patients with malignant disease have experienced relapse to date, Dr. Flower noted.
The study was funded by a grant from Celgene Cellular Therapeutics. Dr. Flower reported having no conflicts of interest.
MONTREAL – A combination of placenta-derived stem cells and umbilical cord blood was associated with early engraftment and high degrees of cord blood donor chimerism in the treatment of children with both malignant and nonmalignant hematologic conditions requiring stem cell transplantation, updated results of a pilot study show.
Among 16 children treated with the combination, the probability of neutrophil engraftment was 87.5%, and all patients who had neutrophil engraftment went on to have platelet engraftment. The probability of 12-month overall survival was 81.2%, reported Allyson Flower, MD, from Boston Children’s Health Physicians in Hawthorne, N.Y. “The probability of grade II-IV acute graft vs. host disease was 12.5%, compared with 32.5% seen with unrelated cord blood in our group’s previous studies. Cellular immune reconstitution was robust,” she said at the annual meeting of the American Society of Pediatric Hematology/Oncology.
Augmenting cord blood
Although unrelated donor cord blood transplantation expands the donor pool, is rapidly available, and is associated with decreases in both severe acute graft vs. host disease (GVHD) and chronic GVHD, compared with other stem cell sources, the technique is hampered by limited cell doses, prolonged immune reconstitution time, delays in hematopoietic recovery, and a higher incidence of graft failure.
Early studies of myeloablative conditioning followed by unrelated umbilical or placental blood transplantation showed a median of 22-24 days to neutrophil engraftment (Blood 1996 88:795-802; N Engl J Med. 1996;335:157-66), Dr. Flower noted.
More recently, a multivariate analysis of patients who underwent reduced-intensity conditioning followed by hematopoietic stem cell transplant with unrelated cord blood showed that graft failure was an independent risk factor for worse overall survival (Biol Blood Marrow Transplant. 2013 Apr;19:4;552-61).
Multiple groups have shown that adding human placenta–derived stem cells (HPDSC) to cord blood transplantation can facilitate more rapid hematopoietic engraftment by increasing the number of stem cells, increasing the proportion of hematopoietic progenitor cells, and providing additional, immature CD34+/CD45– progenitor cells.
In a single-arm, nonrandomized study, the investigators enrolled 16 patients ranging in age from 0.3 to 15.7 years with inborn errors of metabolism, marrow failure syndromes, severe immunodeficiency states, or hematologic malignancies.
Malignant conditions included B-cell precursor acute lymphoblastic leukemia (B-ALL; four patients), acute myeloid leukemia (AML; two), and T-cell ALL (one) in first complete remission, and T-cell lymphoblastic lymphoma following induction failure (one). Nonmalignant conditions included adrenoleukodystrophy (two patients), amegakaryotic thrombocytopenia (one), severe combined immunodeficiency (SCID; two), dyskeratosis congenita (one), chronic granulomatous disease (one), and severe congenital neutropenia (one).
The patients first underwent either myeloablative or reduced-intensity conditioning, followed 10 days later by infusion of unrelated cord blood and HPDSCs. Prior to HPDSC infusion, patients were medicated with diphenhydramine and hydrocortisone to prevent or reduce potential sensitivity reactions. HPDSCs were infused no sooner than 4 hours after the end of the cord blood infusion.
Patients received GVHD prophylaxis with either tacrolimus or cyclosporine, plus mycophenolate mofetil.
The combination appeared to be safe, with no cases of grade 3 or 4 toxicity secondary to HPDSC infusion.
The probability of neutrophil engraftment was 87.5%, with engraftment occurring at a median of 23 days (range 13-53). As noted before, all patients who had neutrophil engraftment had platelet engraftment, which was achieved at a median of 47 days (range, 20-98). In the group’s previous studies, median time to platelet engraftment was 53 days for patients who had undergone reduced-intensity conditioning, and 118 days for patients who had undergone myeloablation.
The probability of grade 2-4 acute GVHD within 100 days was 12.5%, and there were no cases of chronic GVHD.
Respective percentages of cord blood donor chimerism at days 30, 60, 100, and 180 were 88%, 98%, 99%, and 99%.
Immune reconstitution was strong, with normalization of mean CD3+, CD19+, and CD56+ cells occurring by day 100, CD8+ cells by day 180, and CD4+ cells by day 270.
There were three patient deaths: one from adenoviremia in a patient with B-ALL and CNS relapse, who had neutrophil engraftment at day 21; one in a patient with SCID, from adenoviremia and multiple system organ failure, who did not have engraftment before death; and one in a patient with severe congenital neutrophilia, who also did not have neutrophil engraftment.
None of the eight patients with malignant disease have experienced relapse to date, Dr. Flower noted.
The study was funded by a grant from Celgene Cellular Therapeutics. Dr. Flower reported having no conflicts of interest.
Key clinical point: A combination of donor cord blood and human placenta–derived stem cells induced more rapid engraftment than cord blood alone.
Major finding: The probability of 12-month overall survival was 81%.
Data source: Open-label single-arm study in 16 children with severe malignant and nonmalignant diseases requiring hematopoietic stem cell transplants.
Disclosures: The study was funded by a grant from Celgene Cellular Therapeutics. Dr. Flower reported having no conflicts of interest.
Routine surveillance adds cost but little benefit following a pediatric tumor
MONTREAL – It can be a tough sell to worried patients or their caregivers, but surveillance imaging in children with a history of some treated solid tumors may not add anything to care except costs, a team of investigators suggested.
A study of the relationship between surveillance imaging and outcomes in patients who were followed after therapy for solid tumors found that “off therapy surveillance imaging did not affect outcomes in our relapsed solid tumor patients,” wrote Jacob Zimmerli and his coinvestigators at the University of Utah Huntsman Cancer Institute, Salt Lake City.
“In the case of rhabdomyosarcoma, we had a 31% relapse rate of our 39 unique cancer diagnoses that we looked at over a 10-year period. We had 12 relapses, and, of those relapses, 10 were found through nonroutine imaging,” Mr. Zimmerli said in an interview.
Survival rates for patients with rhabdomyosarcoma were identical whether their relapsed disease was detected via surveillance imaging or imaging performed when relapse was directly suspected because of clinical or physical findings, he said.
Surveillance imaging also came at a significant financial cost. Of the $345,000 total expenditure for imaging of rhabdomyosarcoma, $223,000 was spent on imaging for patients who never experienced a relapse. Additionally, $95,000 was spent on imaging for patients who had a relapse but whose relapses were not detected by routine surveillance imaging. Therefore, the investigators calculated, 92% of costs associated with surveillance did not lead to a relapse diagnosis.
“This finding in itself will lead us to look at a few more specific cancer subgroups and maybe do more cost analysis to see if there is a better way to treat these patients,” Mr. Zimmerli said.
To see whether surveillance imaging was associated with outcomes, the investigators performed a retrospective review of data on 292 patients treated at Primary Children’s Hospital in Salt Lake City for osteosarcoma, Wilms’ tumor, neuroblastoma, hepatoblastoma, Ewing sarcoma , or rhabdomyosarcoma.
All patients had either completed a minimum of 2 years of posttherapy follow-up or had a disease relapse within 2 years of completing therapy.
The investigators found that 8 of 10 osteosarcoma relapses, five of seven Wilms’ tumor relapses, and 9 of 15 neuroblastoma relapses were detected on routine imaging.
In contrast, only two of four hepatoblastoma relapses, three of nine Ewings sarcoma relapses, and 2 of 12 rhabdomyosarcoma relapses were found on routine imaging.
The overall survival rate for patients with relapses diagnosed on routine imaging was 51.72%, compared with 50% for patients whose relapses were diagnosed on imaging following the appearance of symptoms. The difference represented only one patient and was not statistically significant.
Mr. Zimmerli acknowledged that many routine posttherapy imaging studies are performed to assuage patient concerns and that it may be difficult to convince parents of children with a history of cancer that routine imaging of some tumors may not offer the assurances of relapse-free survival that they seek.
The study was supported by Intermountain Healthcare. The investigators reported no conflicts of interest.
MONTREAL – It can be a tough sell to worried patients or their caregivers, but surveillance imaging in children with a history of some treated solid tumors may not add anything to care except costs, a team of investigators suggested.
A study of the relationship between surveillance imaging and outcomes in patients who were followed after therapy for solid tumors found that “off therapy surveillance imaging did not affect outcomes in our relapsed solid tumor patients,” wrote Jacob Zimmerli and his coinvestigators at the University of Utah Huntsman Cancer Institute, Salt Lake City.
“In the case of rhabdomyosarcoma, we had a 31% relapse rate of our 39 unique cancer diagnoses that we looked at over a 10-year period. We had 12 relapses, and, of those relapses, 10 were found through nonroutine imaging,” Mr. Zimmerli said in an interview.
Survival rates for patients with rhabdomyosarcoma were identical whether their relapsed disease was detected via surveillance imaging or imaging performed when relapse was directly suspected because of clinical or physical findings, he said.
Surveillance imaging also came at a significant financial cost. Of the $345,000 total expenditure for imaging of rhabdomyosarcoma, $223,000 was spent on imaging for patients who never experienced a relapse. Additionally, $95,000 was spent on imaging for patients who had a relapse but whose relapses were not detected by routine surveillance imaging. Therefore, the investigators calculated, 92% of costs associated with surveillance did not lead to a relapse diagnosis.
“This finding in itself will lead us to look at a few more specific cancer subgroups and maybe do more cost analysis to see if there is a better way to treat these patients,” Mr. Zimmerli said.
To see whether surveillance imaging was associated with outcomes, the investigators performed a retrospective review of data on 292 patients treated at Primary Children’s Hospital in Salt Lake City for osteosarcoma, Wilms’ tumor, neuroblastoma, hepatoblastoma, Ewing sarcoma , or rhabdomyosarcoma.
All patients had either completed a minimum of 2 years of posttherapy follow-up or had a disease relapse within 2 years of completing therapy.
The investigators found that 8 of 10 osteosarcoma relapses, five of seven Wilms’ tumor relapses, and 9 of 15 neuroblastoma relapses were detected on routine imaging.
In contrast, only two of four hepatoblastoma relapses, three of nine Ewings sarcoma relapses, and 2 of 12 rhabdomyosarcoma relapses were found on routine imaging.
The overall survival rate for patients with relapses diagnosed on routine imaging was 51.72%, compared with 50% for patients whose relapses were diagnosed on imaging following the appearance of symptoms. The difference represented only one patient and was not statistically significant.
Mr. Zimmerli acknowledged that many routine posttherapy imaging studies are performed to assuage patient concerns and that it may be difficult to convince parents of children with a history of cancer that routine imaging of some tumors may not offer the assurances of relapse-free survival that they seek.
The study was supported by Intermountain Healthcare. The investigators reported no conflicts of interest.
MONTREAL – It can be a tough sell to worried patients or their caregivers, but surveillance imaging in children with a history of some treated solid tumors may not add anything to care except costs, a team of investigators suggested.
A study of the relationship between surveillance imaging and outcomes in patients who were followed after therapy for solid tumors found that “off therapy surveillance imaging did not affect outcomes in our relapsed solid tumor patients,” wrote Jacob Zimmerli and his coinvestigators at the University of Utah Huntsman Cancer Institute, Salt Lake City.
“In the case of rhabdomyosarcoma, we had a 31% relapse rate of our 39 unique cancer diagnoses that we looked at over a 10-year period. We had 12 relapses, and, of those relapses, 10 were found through nonroutine imaging,” Mr. Zimmerli said in an interview.
Survival rates for patients with rhabdomyosarcoma were identical whether their relapsed disease was detected via surveillance imaging or imaging performed when relapse was directly suspected because of clinical or physical findings, he said.
Surveillance imaging also came at a significant financial cost. Of the $345,000 total expenditure for imaging of rhabdomyosarcoma, $223,000 was spent on imaging for patients who never experienced a relapse. Additionally, $95,000 was spent on imaging for patients who had a relapse but whose relapses were not detected by routine surveillance imaging. Therefore, the investigators calculated, 92% of costs associated with surveillance did not lead to a relapse diagnosis.
“This finding in itself will lead us to look at a few more specific cancer subgroups and maybe do more cost analysis to see if there is a better way to treat these patients,” Mr. Zimmerli said.
To see whether surveillance imaging was associated with outcomes, the investigators performed a retrospective review of data on 292 patients treated at Primary Children’s Hospital in Salt Lake City for osteosarcoma, Wilms’ tumor, neuroblastoma, hepatoblastoma, Ewing sarcoma , or rhabdomyosarcoma.
All patients had either completed a minimum of 2 years of posttherapy follow-up or had a disease relapse within 2 years of completing therapy.
The investigators found that 8 of 10 osteosarcoma relapses, five of seven Wilms’ tumor relapses, and 9 of 15 neuroblastoma relapses were detected on routine imaging.
In contrast, only two of four hepatoblastoma relapses, three of nine Ewings sarcoma relapses, and 2 of 12 rhabdomyosarcoma relapses were found on routine imaging.
The overall survival rate for patients with relapses diagnosed on routine imaging was 51.72%, compared with 50% for patients whose relapses were diagnosed on imaging following the appearance of symptoms. The difference represented only one patient and was not statistically significant.
Mr. Zimmerli acknowledged that many routine posttherapy imaging studies are performed to assuage patient concerns and that it may be difficult to convince parents of children with a history of cancer that routine imaging of some tumors may not offer the assurances of relapse-free survival that they seek.
The study was supported by Intermountain Healthcare. The investigators reported no conflicts of interest.
FROM ASPHO
Key clinical point: Routine surveillance was less effective at detecting relapse of some pediatric solid tumors than imaging performed to confirm a clinical finding.
Major finding: Using imaging performed after symptoms appeared, 10 of 12 rhabdomyosarcoma relapses were detected.
Data source: A retrospective review of outcomes following routine and nonroutine imaging among children followed after treatment for one of six solid tumor types.
Disclosures: The study was supported by Intermountain Healthcare. The investigators reported no conflicts of interest.
Treatment-related hypertension, kidney injury are undertreated in kids with leukemia
MONTREAL – Hypertension is a frequent, but underrecognized and undertreated, complication of chemotherapy for acute lymphoblastic leukemia (ALL), the most common childhood malignancy, investigators in a single-center U.S. study reported.
Additionally, there is a “concerningly high” incidence of acute kidney injury among children and young adults who undergo multiagent chemotherapy for acute myeloid leukemia (AML), said the authors of a study conducted in Canada.
Both studies were reported in a scientific poster session at the annual meeting of the American Society for Pediatric Hematology/Oncology.
Hypertension in ALL
Although standard induction regimens for childhood ALL contain high-dose steroids, which are known to be associated with increased risk for hypertension, the incidence of hypertension throughout induction, consolidation, and maintenance for ALL in children has not been adequately evaluated, according to Andrew S. Freiberg, MD, and his colleagues at Penn State Children’s Hospital in Hershey, Penn.
“The incidence of hypertension was much higher than the under 5% expected for a healthy pediatric population,” the investigators found.
Of 562 total readings taken during induction, 56% were in the normo- or prehypertensive range, but 30% were classified as stage 1 and 14% as stage II hypertension.
The combined percentage of stage 1 and 2 readings declined slightly over the year from 44% during induction to 35% during Q4 “but remained well above expected for the pediatric population,” the investigators reported.
Despite the high incidence, “I was surprised at how few of the patients with hypertension we actually treated,” Dr. Freiberg said in an interview.
Just 3 of the 36 patients studied received treatment for hypertension, he said, possibly because clinicians assumed that the effect was steroid related and transient.
“Now that we’re paying attention, however, we’re treating more of these patients,” Dr. Freiberg said.
The electronic record system used at his institution now alerts clinicians to hypertensive episodes during treatment, he added.
Kidney injury in AML
Like Dr. Freiberg and his colleagues, Liezl du Plessis, MBChB, from the British Columbia Children’s Hospital in Vancouver, Canada, and her colleagues were similarly taken aback when they looked into the incidence of acute kidney injury (AKI) in children and adolescents undergoing multidrug chemotherapy for AML.
“Chemotherapy agents that are used in acute myeloid leukemia are not considered to be nephrotoxic, so it was quite alarming to us to see that there is such a high rate of kidney injury in these patients,” Dr. du Plessis said in an interview.
They found that 34 of the 53 patients (64%) had AKI, with 11 patients having stage 1 (rise in serum creatinine of 1.5 or more times the baseline level), 11 having stage 2 (SCr 2 or more times baseline), and 12 having stage 3 AKI (SCr 3 or more times baseline or the need for dialysis).
Creatinine changes were counted only if they occurred within 7 days from nadir to peak.
AKI occurred in all chemotherapy cycles, with severe injury having the highest frequency in cycle 1.
In a logistical regression model, factors significantly associated with risk for AKI were male sex (odds ratio, 0.2; P = .03) and age 10 years or older (OR, 17.3; P less than .01). Neither sepsis nor aminoglycoside or vancomycin use for more than 3 days was significantly associated with risk for AKI, however.
“I think people need to realize that many of these injuries are happening on the oncology ward, and some of these kids may not even look acutely unwell, so people need to take note.” Dr. du Plessis said.
She recommended curtailing use of nephrotoxic agents whenever possible, and emphasized that clinicians need to document AKI in the medical record.
“A big portion of our AML population goes on to bone marrow transplant, and that is known to be a high risk for further kidney injury. So, I think it would be important to know that before your patient goes for his transplant that he already has certain toxicities and organ injury, so that you can limit things like fluids and get the nephrology team on board to help with the management of these patients,” she said.
The study by Dr. Freiberg was supported by the Four Diamonds Fund. The study by Dr. du Plessis was internally funded. The investigators for each study reported having no relevant financial disclosures.
MONTREAL – Hypertension is a frequent, but underrecognized and undertreated, complication of chemotherapy for acute lymphoblastic leukemia (ALL), the most common childhood malignancy, investigators in a single-center U.S. study reported.
Additionally, there is a “concerningly high” incidence of acute kidney injury among children and young adults who undergo multiagent chemotherapy for acute myeloid leukemia (AML), said the authors of a study conducted in Canada.
Both studies were reported in a scientific poster session at the annual meeting of the American Society for Pediatric Hematology/Oncology.
Hypertension in ALL
Although standard induction regimens for childhood ALL contain high-dose steroids, which are known to be associated with increased risk for hypertension, the incidence of hypertension throughout induction, consolidation, and maintenance for ALL in children has not been adequately evaluated, according to Andrew S. Freiberg, MD, and his colleagues at Penn State Children’s Hospital in Hershey, Penn.
“The incidence of hypertension was much higher than the under 5% expected for a healthy pediatric population,” the investigators found.
Of 562 total readings taken during induction, 56% were in the normo- or prehypertensive range, but 30% were classified as stage 1 and 14% as stage II hypertension.
The combined percentage of stage 1 and 2 readings declined slightly over the year from 44% during induction to 35% during Q4 “but remained well above expected for the pediatric population,” the investigators reported.
Despite the high incidence, “I was surprised at how few of the patients with hypertension we actually treated,” Dr. Freiberg said in an interview.
Just 3 of the 36 patients studied received treatment for hypertension, he said, possibly because clinicians assumed that the effect was steroid related and transient.
“Now that we’re paying attention, however, we’re treating more of these patients,” Dr. Freiberg said.
The electronic record system used at his institution now alerts clinicians to hypertensive episodes during treatment, he added.
Kidney injury in AML
Like Dr. Freiberg and his colleagues, Liezl du Plessis, MBChB, from the British Columbia Children’s Hospital in Vancouver, Canada, and her colleagues were similarly taken aback when they looked into the incidence of acute kidney injury (AKI) in children and adolescents undergoing multidrug chemotherapy for AML.
“Chemotherapy agents that are used in acute myeloid leukemia are not considered to be nephrotoxic, so it was quite alarming to us to see that there is such a high rate of kidney injury in these patients,” Dr. du Plessis said in an interview.
They found that 34 of the 53 patients (64%) had AKI, with 11 patients having stage 1 (rise in serum creatinine of 1.5 or more times the baseline level), 11 having stage 2 (SCr 2 or more times baseline), and 12 having stage 3 AKI (SCr 3 or more times baseline or the need for dialysis).
Creatinine changes were counted only if they occurred within 7 days from nadir to peak.
AKI occurred in all chemotherapy cycles, with severe injury having the highest frequency in cycle 1.
In a logistical regression model, factors significantly associated with risk for AKI were male sex (odds ratio, 0.2; P = .03) and age 10 years or older (OR, 17.3; P less than .01). Neither sepsis nor aminoglycoside or vancomycin use for more than 3 days was significantly associated with risk for AKI, however.
“I think people need to realize that many of these injuries are happening on the oncology ward, and some of these kids may not even look acutely unwell, so people need to take note.” Dr. du Plessis said.
She recommended curtailing use of nephrotoxic agents whenever possible, and emphasized that clinicians need to document AKI in the medical record.
“A big portion of our AML population goes on to bone marrow transplant, and that is known to be a high risk for further kidney injury. So, I think it would be important to know that before your patient goes for his transplant that he already has certain toxicities and organ injury, so that you can limit things like fluids and get the nephrology team on board to help with the management of these patients,” she said.
The study by Dr. Freiberg was supported by the Four Diamonds Fund. The study by Dr. du Plessis was internally funded. The investigators for each study reported having no relevant financial disclosures.
MONTREAL – Hypertension is a frequent, but underrecognized and undertreated, complication of chemotherapy for acute lymphoblastic leukemia (ALL), the most common childhood malignancy, investigators in a single-center U.S. study reported.
Additionally, there is a “concerningly high” incidence of acute kidney injury among children and young adults who undergo multiagent chemotherapy for acute myeloid leukemia (AML), said the authors of a study conducted in Canada.
Both studies were reported in a scientific poster session at the annual meeting of the American Society for Pediatric Hematology/Oncology.
Hypertension in ALL
Although standard induction regimens for childhood ALL contain high-dose steroids, which are known to be associated with increased risk for hypertension, the incidence of hypertension throughout induction, consolidation, and maintenance for ALL in children has not been adequately evaluated, according to Andrew S. Freiberg, MD, and his colleagues at Penn State Children’s Hospital in Hershey, Penn.
“The incidence of hypertension was much higher than the under 5% expected for a healthy pediatric population,” the investigators found.
Of 562 total readings taken during induction, 56% were in the normo- or prehypertensive range, but 30% were classified as stage 1 and 14% as stage II hypertension.
The combined percentage of stage 1 and 2 readings declined slightly over the year from 44% during induction to 35% during Q4 “but remained well above expected for the pediatric population,” the investigators reported.
Despite the high incidence, “I was surprised at how few of the patients with hypertension we actually treated,” Dr. Freiberg said in an interview.
Just 3 of the 36 patients studied received treatment for hypertension, he said, possibly because clinicians assumed that the effect was steroid related and transient.
“Now that we’re paying attention, however, we’re treating more of these patients,” Dr. Freiberg said.
The electronic record system used at his institution now alerts clinicians to hypertensive episodes during treatment, he added.
Kidney injury in AML
Like Dr. Freiberg and his colleagues, Liezl du Plessis, MBChB, from the British Columbia Children’s Hospital in Vancouver, Canada, and her colleagues were similarly taken aback when they looked into the incidence of acute kidney injury (AKI) in children and adolescents undergoing multidrug chemotherapy for AML.
“Chemotherapy agents that are used in acute myeloid leukemia are not considered to be nephrotoxic, so it was quite alarming to us to see that there is such a high rate of kidney injury in these patients,” Dr. du Plessis said in an interview.
They found that 34 of the 53 patients (64%) had AKI, with 11 patients having stage 1 (rise in serum creatinine of 1.5 or more times the baseline level), 11 having stage 2 (SCr 2 or more times baseline), and 12 having stage 3 AKI (SCr 3 or more times baseline or the need for dialysis).
Creatinine changes were counted only if they occurred within 7 days from nadir to peak.
AKI occurred in all chemotherapy cycles, with severe injury having the highest frequency in cycle 1.
In a logistical regression model, factors significantly associated with risk for AKI were male sex (odds ratio, 0.2; P = .03) and age 10 years or older (OR, 17.3; P less than .01). Neither sepsis nor aminoglycoside or vancomycin use for more than 3 days was significantly associated with risk for AKI, however.
“I think people need to realize that many of these injuries are happening on the oncology ward, and some of these kids may not even look acutely unwell, so people need to take note.” Dr. du Plessis said.
She recommended curtailing use of nephrotoxic agents whenever possible, and emphasized that clinicians need to document AKI in the medical record.
“A big portion of our AML population goes on to bone marrow transplant, and that is known to be a high risk for further kidney injury. So, I think it would be important to know that before your patient goes for his transplant that he already has certain toxicities and organ injury, so that you can limit things like fluids and get the nephrology team on board to help with the management of these patients,” she said.
The study by Dr. Freiberg was supported by the Four Diamonds Fund. The study by Dr. du Plessis was internally funded. The investigators for each study reported having no relevant financial disclosures.
At ASPHO 2017
Key clinical point:
Major finding: The combined incidence of stage 1 or 2 hypertension in children during induction therapy for acute lymphoblastic leukemia was 44%.
Data source: Two retrospective institutional studies.
Disclosures: The study by Dr. Freiberg was supported by the Four Diamonds Fund. The study by Dr. du Plessis was internally funded. The investigators for each study reported having no relevant financial disclosures.
Modifying CAR-T with IL-15 improved activity in glioma models
MONTREAL – Adding an immunostimulatory cytokine to chimeric antigen receptor–T cells (CAR-T) improved the adaptive immunotherapy’s activity against aggressive pediatric brain malignancies both in vitro and in animal models, an investigator reported.
CAR-T cells engineered to express interleukin-15 (IL-15), an inducer of T-cell proliferation and survival, were associated with significantly longer progression-free survival (PFS) and overall survival in mouse models of glioma, compared with regular CAR-T cells, said Giedre Krenciute, PhD, of Baylor College of Medicine in Houston.
The improved T-cell persistence, however, still resulted in the eventual loss of both targeted and nontargeted tumor-associated antigens and tumor recurrence, Dr. Krenciute noted.
The results suggest that T-cell persistence is critical for antitumor activity, and that it will be necessary to perform antigen profiling of recurrent tumors in order to develop follow-on therapies targeting multiple tumor-associated antigens, she said.
Her team had previously reported on the development of a CAR-T cell directed specifically against the IL-13 receptor alpha-2, which is expressed at high frequency in diffuse intrinsic pontine glioma and glioblastoma tumors but not in normal brain tissues.
In preclinical models, the construct had potent antiglioblastoma activity. However, the T-cells had only limited persistence, and tumors positive for IL-13 receptor alpha-2 recurred.
To see whether they could improve on T-cell persistence, they took their CARs back to the shop and modified them with a retroviral vector to express IL-15 transgenes. They then tested the altered cells in vitro using standard assays and found that the addition of IL-15 did not change the T-cell phenotype or affect the cells’ cytotoxicity.
The addition of IL-15 significantly improved the persistence of the T cells when they were injected into the tumors of mice with human glioblastoma xenografts (P less than .05), and this persistence translated into a near-doubling of PFS, compared with regular CAR-T cells (media, 84 days vs. 49 days; P = .008), as well as improved overall survival (P = .02).
Of 10 mice that received the IL-15–expressing CAR-Ts, 4 were free of glioma through at least 80 days of follow-up.
Of five mice with recurring gliomas, three had down-regulated expression of the IL-13 receptor alpha-2 target, indicating immune escape, and all recurring tumors had reduced expression of the human epidermal growth factor receptor-2 antigen, which is associated with gliomas.
The investigators are currently test-driving a new CD20-targeted CAR, transduced to express IL-15, and have seen good expansion and persistence of the T cells for up to 15 days in culture, with in vivo tests in the planning stage, Dr. Krenciute said.
The work is supported by the National Institutes of Health, Alex’s Lemonade Stand Foundation, the James S. McDonnell Foundation, and the American Brain Tumor Association. The laboratory, the Center for Cell and Gene Therapy at Baylor, has or had research collaborations with Celgene, Bluebird Bio, and Tessa Therapeutics, and investigators at the center hold or have applied for patents in T-cell and gene-modified T-cell therapies for cancer.
MONTREAL – Adding an immunostimulatory cytokine to chimeric antigen receptor–T cells (CAR-T) improved the adaptive immunotherapy’s activity against aggressive pediatric brain malignancies both in vitro and in animal models, an investigator reported.
CAR-T cells engineered to express interleukin-15 (IL-15), an inducer of T-cell proliferation and survival, were associated with significantly longer progression-free survival (PFS) and overall survival in mouse models of glioma, compared with regular CAR-T cells, said Giedre Krenciute, PhD, of Baylor College of Medicine in Houston.
The improved T-cell persistence, however, still resulted in the eventual loss of both targeted and nontargeted tumor-associated antigens and tumor recurrence, Dr. Krenciute noted.
The results suggest that T-cell persistence is critical for antitumor activity, and that it will be necessary to perform antigen profiling of recurrent tumors in order to develop follow-on therapies targeting multiple tumor-associated antigens, she said.
Her team had previously reported on the development of a CAR-T cell directed specifically against the IL-13 receptor alpha-2, which is expressed at high frequency in diffuse intrinsic pontine glioma and glioblastoma tumors but not in normal brain tissues.
In preclinical models, the construct had potent antiglioblastoma activity. However, the T-cells had only limited persistence, and tumors positive for IL-13 receptor alpha-2 recurred.
To see whether they could improve on T-cell persistence, they took their CARs back to the shop and modified them with a retroviral vector to express IL-15 transgenes. They then tested the altered cells in vitro using standard assays and found that the addition of IL-15 did not change the T-cell phenotype or affect the cells’ cytotoxicity.
The addition of IL-15 significantly improved the persistence of the T cells when they were injected into the tumors of mice with human glioblastoma xenografts (P less than .05), and this persistence translated into a near-doubling of PFS, compared with regular CAR-T cells (media, 84 days vs. 49 days; P = .008), as well as improved overall survival (P = .02).
Of 10 mice that received the IL-15–expressing CAR-Ts, 4 were free of glioma through at least 80 days of follow-up.
Of five mice with recurring gliomas, three had down-regulated expression of the IL-13 receptor alpha-2 target, indicating immune escape, and all recurring tumors had reduced expression of the human epidermal growth factor receptor-2 antigen, which is associated with gliomas.
The investigators are currently test-driving a new CD20-targeted CAR, transduced to express IL-15, and have seen good expansion and persistence of the T cells for up to 15 days in culture, with in vivo tests in the planning stage, Dr. Krenciute said.
The work is supported by the National Institutes of Health, Alex’s Lemonade Stand Foundation, the James S. McDonnell Foundation, and the American Brain Tumor Association. The laboratory, the Center for Cell and Gene Therapy at Baylor, has or had research collaborations with Celgene, Bluebird Bio, and Tessa Therapeutics, and investigators at the center hold or have applied for patents in T-cell and gene-modified T-cell therapies for cancer.
MONTREAL – Adding an immunostimulatory cytokine to chimeric antigen receptor–T cells (CAR-T) improved the adaptive immunotherapy’s activity against aggressive pediatric brain malignancies both in vitro and in animal models, an investigator reported.
CAR-T cells engineered to express interleukin-15 (IL-15), an inducer of T-cell proliferation and survival, were associated with significantly longer progression-free survival (PFS) and overall survival in mouse models of glioma, compared with regular CAR-T cells, said Giedre Krenciute, PhD, of Baylor College of Medicine in Houston.
The improved T-cell persistence, however, still resulted in the eventual loss of both targeted and nontargeted tumor-associated antigens and tumor recurrence, Dr. Krenciute noted.
The results suggest that T-cell persistence is critical for antitumor activity, and that it will be necessary to perform antigen profiling of recurrent tumors in order to develop follow-on therapies targeting multiple tumor-associated antigens, she said.
Her team had previously reported on the development of a CAR-T cell directed specifically against the IL-13 receptor alpha-2, which is expressed at high frequency in diffuse intrinsic pontine glioma and glioblastoma tumors but not in normal brain tissues.
In preclinical models, the construct had potent antiglioblastoma activity. However, the T-cells had only limited persistence, and tumors positive for IL-13 receptor alpha-2 recurred.
To see whether they could improve on T-cell persistence, they took their CARs back to the shop and modified them with a retroviral vector to express IL-15 transgenes. They then tested the altered cells in vitro using standard assays and found that the addition of IL-15 did not change the T-cell phenotype or affect the cells’ cytotoxicity.
The addition of IL-15 significantly improved the persistence of the T cells when they were injected into the tumors of mice with human glioblastoma xenografts (P less than .05), and this persistence translated into a near-doubling of PFS, compared with regular CAR-T cells (media, 84 days vs. 49 days; P = .008), as well as improved overall survival (P = .02).
Of 10 mice that received the IL-15–expressing CAR-Ts, 4 were free of glioma through at least 80 days of follow-up.
Of five mice with recurring gliomas, three had down-regulated expression of the IL-13 receptor alpha-2 target, indicating immune escape, and all recurring tumors had reduced expression of the human epidermal growth factor receptor-2 antigen, which is associated with gliomas.
The investigators are currently test-driving a new CD20-targeted CAR, transduced to express IL-15, and have seen good expansion and persistence of the T cells for up to 15 days in culture, with in vivo tests in the planning stage, Dr. Krenciute said.
The work is supported by the National Institutes of Health, Alex’s Lemonade Stand Foundation, the James S. McDonnell Foundation, and the American Brain Tumor Association. The laboratory, the Center for Cell and Gene Therapy at Baylor, has or had research collaborations with Celgene, Bluebird Bio, and Tessa Therapeutics, and investigators at the center hold or have applied for patents in T-cell and gene-modified T-cell therapies for cancer.
FROM ASPHO
Key clinical point: CAR-T cells, modified to express IL-15, have improved activity against aggressive pediatric gliomas.
Major finding: IL-15 expressed T cells were associated with improved progression-free and overall survival in animal models of glioblastoma.
Data source: In vitro and in vivo experiments of CAR-T cells modified to improve T-cell persistence and clinical efficacy.
Disclosures: The work is supported by the National Institutes of Health, Alex’s Lemonade Stand Foundation, the James S. McDonnell Foundation, and the American Brain Tumor Association. The laboratory, the Center for Cell and Gene Therapy at Baylor, has or had research collaborations with Celgene, Bluebird Bio, and Tessa Therapeutics, and investigators at the center hold or have applied for patents in T-cell and gene-modified T-cell therapies for cancer.