User login
Childhood Bacterial Meningitis Brings Hefty Late Sequelae
VAIL, COLO. – Half of all childhood bacterial meningitis survivors have sequelae 5 years or more afterward, according to a systematic literature review.
Intellectual and/or behavioral deficits accounted for 78% of the long-term sequelae. These are lingering aftereffects that impose academic and behavioral limitations, not the very common minor neurologic deficits that often resolve soon after discharge, Dr. Ann-Christine Nyquist noted at a conference on pediatric infectious diseases sponsored by Children’s Hospital Colorado.
She said she presented highlights of a literature analysis conducted by Dr. Aruna Chandran and coworkers at Johns Hopkins University, Baltimore, because she considers this to be the first good-quality, comprehensive data on the long-term sequelae of childhood bacterial meningitis.
Most prior studies have focused on sequelae present in the first months following a childhood episode of bacterial meningitis; the Johns Hopkins analysis was restricted to studies featuring a minimum follow-up of 5 years. It provides a far more complete picture of the disease’s underappreciated impact, noted Dr. Nyquist, a pediatric infectious diseases specialist at the University of Colorado at Denver.
The analysis included 1,433 children who survived an episode of bacterial meningitis, 49% of whom had one or more long-term sequelae. Seventy-eight percent of the 1,012 recorded long-term sequelae were behavioral and/or intellectual deficits. Specifically, 45% of all long-term sequelae were categorized as low intelligence quotient/cognitive impairment, 7.6% as behavioral deficits, and 2.4% as attention-deficit/hyperactivity disorder.
Gross neurologic deficits accounted for 14.3% of long-term sequelae. Another 6.7% consisted of hearing deficits (Pediatr. Infect. Dis. J. 2011;30:3-6).
These data underscore the importance of prompt diagnosis and effective treatment of pediatric bacterial meningitis, Dr. Nyquist emphasized.
She pointed out that the most recent Cochrane review of corticosteroids for acute bacterial meningitis came down squarely on the pro side because of the significant benefit in terms of reduced sequelae. The analysis included 24 randomized, placebo-controlled clinical trials in 4,041 subjects, both children and adults.
The Cochrane group found that in high-income countries, the use of adjunctive dexamethasone reduced the risk of severe hearing loss by 49%, of any hearing loss by 42%, and of short-term neurologic sequelae by 36%. Adjunctive steroids weren’t associated with significant decreases in overall mortality or long-term neurologic sequelae; however, in the subgroup with Streptococcus pneumoniae meningitis, the trend for reduced mortality did achieve statistical significance (Cochrane Database Syst. Rev. 2010 Sept. 8;CD004405).
"I think within our infectious disease practice group, most of us would probably go on the side of giving dexamethasone in all cases of suspected bacterial meningitis in infants and children if we have the opportunity to make that recommendation," Dr. Nyquist said.
The dose is 0.4 mg/kg of dexamethasone given every 12 hours for a total of four doses over a period of 2 days, administered starting before or at initiation of parenteral antibiotic therapy.
A couple of caveats: Only limited data exist on the use of adjunctive corticosteroids in neonates with acute bacterial meningitis. And steroid therapy in any age group poses a challenge because its anti-inflammatory action reduces the permeability of the damaged CNS capillary membrane – the blood-brain barrier – at just the time when the physician is trying to get antibiotics into the CNS.
The inhibitory effect of steroids is greatest on large-molecular-weight or hydrophobic antibiotics. Vancomycin is the antibiotic affected most; levels are reduced by 42%-77%. Ampicillin levels are decreased by 57%, gentamicin levels are reduced by 30%, and ceftriaxone levels have been shown in various studies to be either unaffected or decreased by 45%, according to Dr. Nyquist.
She said that that she serves as a consultant to and has received speaking honoraria from Merck, Sanofi Pasteur, and Novartis.
VAIL, COLO. – Half of all childhood bacterial meningitis survivors have sequelae 5 years or more afterward, according to a systematic literature review.
Intellectual and/or behavioral deficits accounted for 78% of the long-term sequelae. These are lingering aftereffects that impose academic and behavioral limitations, not the very common minor neurologic deficits that often resolve soon after discharge, Dr. Ann-Christine Nyquist noted at a conference on pediatric infectious diseases sponsored by Children’s Hospital Colorado.
She said she presented highlights of a literature analysis conducted by Dr. Aruna Chandran and coworkers at Johns Hopkins University, Baltimore, because she considers this to be the first good-quality, comprehensive data on the long-term sequelae of childhood bacterial meningitis.
Most prior studies have focused on sequelae present in the first months following a childhood episode of bacterial meningitis; the Johns Hopkins analysis was restricted to studies featuring a minimum follow-up of 5 years. It provides a far more complete picture of the disease’s underappreciated impact, noted Dr. Nyquist, a pediatric infectious diseases specialist at the University of Colorado at Denver.
The analysis included 1,433 children who survived an episode of bacterial meningitis, 49% of whom had one or more long-term sequelae. Seventy-eight percent of the 1,012 recorded long-term sequelae were behavioral and/or intellectual deficits. Specifically, 45% of all long-term sequelae were categorized as low intelligence quotient/cognitive impairment, 7.6% as behavioral deficits, and 2.4% as attention-deficit/hyperactivity disorder.
Gross neurologic deficits accounted for 14.3% of long-term sequelae. Another 6.7% consisted of hearing deficits (Pediatr. Infect. Dis. J. 2011;30:3-6).
These data underscore the importance of prompt diagnosis and effective treatment of pediatric bacterial meningitis, Dr. Nyquist emphasized.
She pointed out that the most recent Cochrane review of corticosteroids for acute bacterial meningitis came down squarely on the pro side because of the significant benefit in terms of reduced sequelae. The analysis included 24 randomized, placebo-controlled clinical trials in 4,041 subjects, both children and adults.
The Cochrane group found that in high-income countries, the use of adjunctive dexamethasone reduced the risk of severe hearing loss by 49%, of any hearing loss by 42%, and of short-term neurologic sequelae by 36%. Adjunctive steroids weren’t associated with significant decreases in overall mortality or long-term neurologic sequelae; however, in the subgroup with Streptococcus pneumoniae meningitis, the trend for reduced mortality did achieve statistical significance (Cochrane Database Syst. Rev. 2010 Sept. 8;CD004405).
"I think within our infectious disease practice group, most of us would probably go on the side of giving dexamethasone in all cases of suspected bacterial meningitis in infants and children if we have the opportunity to make that recommendation," Dr. Nyquist said.
The dose is 0.4 mg/kg of dexamethasone given every 12 hours for a total of four doses over a period of 2 days, administered starting before or at initiation of parenteral antibiotic therapy.
A couple of caveats: Only limited data exist on the use of adjunctive corticosteroids in neonates with acute bacterial meningitis. And steroid therapy in any age group poses a challenge because its anti-inflammatory action reduces the permeability of the damaged CNS capillary membrane – the blood-brain barrier – at just the time when the physician is trying to get antibiotics into the CNS.
The inhibitory effect of steroids is greatest on large-molecular-weight or hydrophobic antibiotics. Vancomycin is the antibiotic affected most; levels are reduced by 42%-77%. Ampicillin levels are decreased by 57%, gentamicin levels are reduced by 30%, and ceftriaxone levels have been shown in various studies to be either unaffected or decreased by 45%, according to Dr. Nyquist.
She said that that she serves as a consultant to and has received speaking honoraria from Merck, Sanofi Pasteur, and Novartis.
VAIL, COLO. – Half of all childhood bacterial meningitis survivors have sequelae 5 years or more afterward, according to a systematic literature review.
Intellectual and/or behavioral deficits accounted for 78% of the long-term sequelae. These are lingering aftereffects that impose academic and behavioral limitations, not the very common minor neurologic deficits that often resolve soon after discharge, Dr. Ann-Christine Nyquist noted at a conference on pediatric infectious diseases sponsored by Children’s Hospital Colorado.
She said she presented highlights of a literature analysis conducted by Dr. Aruna Chandran and coworkers at Johns Hopkins University, Baltimore, because she considers this to be the first good-quality, comprehensive data on the long-term sequelae of childhood bacterial meningitis.
Most prior studies have focused on sequelae present in the first months following a childhood episode of bacterial meningitis; the Johns Hopkins analysis was restricted to studies featuring a minimum follow-up of 5 years. It provides a far more complete picture of the disease’s underappreciated impact, noted Dr. Nyquist, a pediatric infectious diseases specialist at the University of Colorado at Denver.
The analysis included 1,433 children who survived an episode of bacterial meningitis, 49% of whom had one or more long-term sequelae. Seventy-eight percent of the 1,012 recorded long-term sequelae were behavioral and/or intellectual deficits. Specifically, 45% of all long-term sequelae were categorized as low intelligence quotient/cognitive impairment, 7.6% as behavioral deficits, and 2.4% as attention-deficit/hyperactivity disorder.
Gross neurologic deficits accounted for 14.3% of long-term sequelae. Another 6.7% consisted of hearing deficits (Pediatr. Infect. Dis. J. 2011;30:3-6).
These data underscore the importance of prompt diagnosis and effective treatment of pediatric bacterial meningitis, Dr. Nyquist emphasized.
She pointed out that the most recent Cochrane review of corticosteroids for acute bacterial meningitis came down squarely on the pro side because of the significant benefit in terms of reduced sequelae. The analysis included 24 randomized, placebo-controlled clinical trials in 4,041 subjects, both children and adults.
The Cochrane group found that in high-income countries, the use of adjunctive dexamethasone reduced the risk of severe hearing loss by 49%, of any hearing loss by 42%, and of short-term neurologic sequelae by 36%. Adjunctive steroids weren’t associated with significant decreases in overall mortality or long-term neurologic sequelae; however, in the subgroup with Streptococcus pneumoniae meningitis, the trend for reduced mortality did achieve statistical significance (Cochrane Database Syst. Rev. 2010 Sept. 8;CD004405).
"I think within our infectious disease practice group, most of us would probably go on the side of giving dexamethasone in all cases of suspected bacterial meningitis in infants and children if we have the opportunity to make that recommendation," Dr. Nyquist said.
The dose is 0.4 mg/kg of dexamethasone given every 12 hours for a total of four doses over a period of 2 days, administered starting before or at initiation of parenteral antibiotic therapy.
A couple of caveats: Only limited data exist on the use of adjunctive corticosteroids in neonates with acute bacterial meningitis. And steroid therapy in any age group poses a challenge because its anti-inflammatory action reduces the permeability of the damaged CNS capillary membrane – the blood-brain barrier – at just the time when the physician is trying to get antibiotics into the CNS.
The inhibitory effect of steroids is greatest on large-molecular-weight or hydrophobic antibiotics. Vancomycin is the antibiotic affected most; levels are reduced by 42%-77%. Ampicillin levels are decreased by 57%, gentamicin levels are reduced by 30%, and ceftriaxone levels have been shown in various studies to be either unaffected or decreased by 45%, according to Dr. Nyquist.
She said that that she serves as a consultant to and has received speaking honoraria from Merck, Sanofi Pasteur, and Novartis.
EXPERT ANALYSIS FROM A CONFERENCE ON PEDIATRIC INFECTIOUS DISEASES
Prolonged Fever is Typhoid’s Hallmark
VAIL, COLO. – Two-thirds of all cases of typhoid fever seen in the United States result from travel to India or neighboring Pakistan or Bangladesh.
Add in Mexico, the Philippines, and Guatemala, and travel to just those six countries accounts for 80% of all U.S. cases of typhoid fever, according to a Centers for Disease Control and Prevention study.
Some 41% of cases in the United States during 1999-2006 occurred in patients aged 17 years or younger.
"This is a very important disease in children," Dr. Jay S. Keystone noted in highlighting the CDC report at a conference on pediatric infectious diseases sponsored by Children’s Hospital, Colorado, Aurora.
The infection has substantial morbidity, as 73% of patients were hospitalized, half for longer than a week. The mortality rate, however, was only 0.2%.
Dr. Keystone stressed that prolonged fever is the key distinguishing feature of typhoid. The fever lasts weeks longer than those of dengue or chikungunya, which persist for about 1 week. So once malaria has been ruled out, weeks of fever have accrued, and the clinical picture isn’t consistent with mononucleosis, cytomegalovirus, or other viral illnesses, it’s time to think about typhoid fever.
Other than prolonged fever, the classic symptoms include headache, cough, diarrhea, and abdominal pain.
Only 5% of patients in the CDC study had been vaccinated against typhoid fever prior to their foreign travel. However, it’s important to recognize that the vaccine is only about 70% effective, so immunization against typhoid – unlike the other standard travel vaccines, which are 95% or more effective – does not rule out the disease, noted Dr. Keystone, professor of medicine at the University of Toronto and a past president of the International Society of Travel Medicine.
A relatively long period of effective antibiotic therapy (average, 3-5 days) is required before patients with typhoid become afebrile. Azithromycin or ceftriaxone are good choices for empiric therapy during the 3-day wait for results of blood, stool, and urine cultures, the physician said.
Azithromycin gets the edge because only 5-7 days of therapy are required, in contrast to 14 days for ceftriaxone, he added.
In the CDC series, 13% of Salmonella ser Typhi isolates were multidrug-resistant to ampicillin, chloramphenicol, and trimethoprim-sulfamethoxazole. Some 38% were resistant to nalidixic acid, which is a marker for reduced susceptibility to fluoroquinolones. The proportion of nalidixic acid-resistant isolates tripled from 1999 to 2006 (JAMA 2009;302:859-65).
Dr. Keystone declared having no relevant financial interests.
VAIL, COLO. – Two-thirds of all cases of typhoid fever seen in the United States result from travel to India or neighboring Pakistan or Bangladesh.
Add in Mexico, the Philippines, and Guatemala, and travel to just those six countries accounts for 80% of all U.S. cases of typhoid fever, according to a Centers for Disease Control and Prevention study.
Some 41% of cases in the United States during 1999-2006 occurred in patients aged 17 years or younger.
"This is a very important disease in children," Dr. Jay S. Keystone noted in highlighting the CDC report at a conference on pediatric infectious diseases sponsored by Children’s Hospital, Colorado, Aurora.
The infection has substantial morbidity, as 73% of patients were hospitalized, half for longer than a week. The mortality rate, however, was only 0.2%.
Dr. Keystone stressed that prolonged fever is the key distinguishing feature of typhoid. The fever lasts weeks longer than those of dengue or chikungunya, which persist for about 1 week. So once malaria has been ruled out, weeks of fever have accrued, and the clinical picture isn’t consistent with mononucleosis, cytomegalovirus, or other viral illnesses, it’s time to think about typhoid fever.
Other than prolonged fever, the classic symptoms include headache, cough, diarrhea, and abdominal pain.
Only 5% of patients in the CDC study had been vaccinated against typhoid fever prior to their foreign travel. However, it’s important to recognize that the vaccine is only about 70% effective, so immunization against typhoid – unlike the other standard travel vaccines, which are 95% or more effective – does not rule out the disease, noted Dr. Keystone, professor of medicine at the University of Toronto and a past president of the International Society of Travel Medicine.
A relatively long period of effective antibiotic therapy (average, 3-5 days) is required before patients with typhoid become afebrile. Azithromycin or ceftriaxone are good choices for empiric therapy during the 3-day wait for results of blood, stool, and urine cultures, the physician said.
Azithromycin gets the edge because only 5-7 days of therapy are required, in contrast to 14 days for ceftriaxone, he added.
In the CDC series, 13% of Salmonella ser Typhi isolates were multidrug-resistant to ampicillin, chloramphenicol, and trimethoprim-sulfamethoxazole. Some 38% were resistant to nalidixic acid, which is a marker for reduced susceptibility to fluoroquinolones. The proportion of nalidixic acid-resistant isolates tripled from 1999 to 2006 (JAMA 2009;302:859-65).
Dr. Keystone declared having no relevant financial interests.
VAIL, COLO. – Two-thirds of all cases of typhoid fever seen in the United States result from travel to India or neighboring Pakistan or Bangladesh.
Add in Mexico, the Philippines, and Guatemala, and travel to just those six countries accounts for 80% of all U.S. cases of typhoid fever, according to a Centers for Disease Control and Prevention study.
Some 41% of cases in the United States during 1999-2006 occurred in patients aged 17 years or younger.
"This is a very important disease in children," Dr. Jay S. Keystone noted in highlighting the CDC report at a conference on pediatric infectious diseases sponsored by Children’s Hospital, Colorado, Aurora.
The infection has substantial morbidity, as 73% of patients were hospitalized, half for longer than a week. The mortality rate, however, was only 0.2%.
Dr. Keystone stressed that prolonged fever is the key distinguishing feature of typhoid. The fever lasts weeks longer than those of dengue or chikungunya, which persist for about 1 week. So once malaria has been ruled out, weeks of fever have accrued, and the clinical picture isn’t consistent with mononucleosis, cytomegalovirus, or other viral illnesses, it’s time to think about typhoid fever.
Other than prolonged fever, the classic symptoms include headache, cough, diarrhea, and abdominal pain.
Only 5% of patients in the CDC study had been vaccinated against typhoid fever prior to their foreign travel. However, it’s important to recognize that the vaccine is only about 70% effective, so immunization against typhoid – unlike the other standard travel vaccines, which are 95% or more effective – does not rule out the disease, noted Dr. Keystone, professor of medicine at the University of Toronto and a past president of the International Society of Travel Medicine.
A relatively long period of effective antibiotic therapy (average, 3-5 days) is required before patients with typhoid become afebrile. Azithromycin or ceftriaxone are good choices for empiric therapy during the 3-day wait for results of blood, stool, and urine cultures, the physician said.
Azithromycin gets the edge because only 5-7 days of therapy are required, in contrast to 14 days for ceftriaxone, he added.
In the CDC series, 13% of Salmonella ser Typhi isolates were multidrug-resistant to ampicillin, chloramphenicol, and trimethoprim-sulfamethoxazole. Some 38% were resistant to nalidixic acid, which is a marker for reduced susceptibility to fluoroquinolones. The proportion of nalidixic acid-resistant isolates tripled from 1999 to 2006 (JAMA 2009;302:859-65).
Dr. Keystone declared having no relevant financial interests.
EXPERT ANALYSIS FROM A CONFERENCE ON PEDIATRIC INFECTIOUS DISEASES
Kocher Criteria Still Best Way to ID Septic Arthritis in Kids
VAIL, COLO. – Four simple criteria are useful in distinguishing septic arthritis from transient synovitis in a child with an inflamed hip.
The criteria are known as the Kocher criteria, for Dr. Mininder S. Kocher, associate director of sports medicine at Children’s Hospital Boston, who was first author of the study that introduced the criteria and an associated evidence-based, predictive algorithm.
The criteria are inability to tolerate weight bearing, fever greater than 38.5° C (101.3° F), an ESR (erythrocyte sedimentation rate) in excess of 40 mm/hour, and a peripheral WBC count greater than 12,000 cells/mm3.
Dr. Kocher and his coworkers showed in a retrospective study that a child who meets none of these four criteria has just a 0.2% chance of having septic arthritis. With one criterion present, there’s a 3% chance. With two criteria, it’s 40%. With any three, the probability of septic arthritis jumps to 93%. And when all four criteria are present, the probability of septic arthritis is 99.6% (J. Bone Joint Surg. Am. 1999;81:1662-70).
"Looking back on our own cases, this has been really helpful," Heather R. Heizer said at a conference on pediatric infectious diseases sponsored by Children’s Hospital Colorado.
The Kocher criteria deserve to be more widely known. Septic arthritis is an orthopedic emergency. Delayed treatment can lead to irreversible joint damage. And septic arthritis occurs more often in childhood than at any other period of life, observed Ms. Heizer, a physician assistant in the department of pediatrics at the University of Colorado at Denver.
The most common site for septic arthritis is the hip, followed by the knee, then the ankle. Together these three sites account for 80% of all cases.
In addition to the Kocher criteria, other signs and symptoms of septic arthritis include limb pain, joint effusion, and a strong tendency for the patient to hold the affected joint in the position of maximal intracapsular volume in order to minimize discomfort. Neonates and infants may present with pseudoparalysis in response to the joint pain.
The log-roll technique is an effective way to detect hip effusion on clinical examination. With the patient lying supine on the examination table, the examiner places one hand at the ankle and another on the thigh and rolls the leg back and forth. Hip effusion can also be detected by ultrasound or MRI. However, it’s important to recognize that the presence of hip effusion doesn’t distinguish septic arthritis from transient synovitis.
Diagnosis of septic arthritis is based upon a combination of clinical findings and analysis of synovial fluid obtained via joint aspiration. Septic arthritis is suggested by an opaque, yellow-to-green synovial fluid with an elevated WBC, at least 75% polymorphonuclear leukocytes, and a glucose concentration of only about 30% of that in blood.
The initial antibiotic therapy should be selected before synovial fluid culture results are available. The child’s age is an important consideration, as the causative organisms vary. Haemophilus influenzae was the most common pathogen in children younger than 5 years of age until the vaccine entered wide use. Now Staphylococcus aureus is No. 1 in all age groups, and community-acquired MRSA (methicillin-resistant S. aureus) is an important consideration.
In neonates, other organisms include group B streptococcus, Streptococcus viridans, Streptococcus pneumoniae, Neisseria gonorrhoeae, gram-negative enteric bacteria including Escherichia coli and group A streptococcus.
An important and underappreciated cause of septic arthritis in non-neonates younger than age 5 years is Kingella kingae. It is typically culture-negative but can be detected by polymerase chain reaction. In addition, Neisseria meningitidis is a consideration in this age group and all the way through adolescence, as well.
Ms. Heizer declared having no financial conflicts.
VAIL, COLO. – Four simple criteria are useful in distinguishing septic arthritis from transient synovitis in a child with an inflamed hip.
The criteria are known as the Kocher criteria, for Dr. Mininder S. Kocher, associate director of sports medicine at Children’s Hospital Boston, who was first author of the study that introduced the criteria and an associated evidence-based, predictive algorithm.
The criteria are inability to tolerate weight bearing, fever greater than 38.5° C (101.3° F), an ESR (erythrocyte sedimentation rate) in excess of 40 mm/hour, and a peripheral WBC count greater than 12,000 cells/mm3.
Dr. Kocher and his coworkers showed in a retrospective study that a child who meets none of these four criteria has just a 0.2% chance of having septic arthritis. With one criterion present, there’s a 3% chance. With two criteria, it’s 40%. With any three, the probability of septic arthritis jumps to 93%. And when all four criteria are present, the probability of septic arthritis is 99.6% (J. Bone Joint Surg. Am. 1999;81:1662-70).
"Looking back on our own cases, this has been really helpful," Heather R. Heizer said at a conference on pediatric infectious diseases sponsored by Children’s Hospital Colorado.
The Kocher criteria deserve to be more widely known. Septic arthritis is an orthopedic emergency. Delayed treatment can lead to irreversible joint damage. And septic arthritis occurs more often in childhood than at any other period of life, observed Ms. Heizer, a physician assistant in the department of pediatrics at the University of Colorado at Denver.
The most common site for septic arthritis is the hip, followed by the knee, then the ankle. Together these three sites account for 80% of all cases.
In addition to the Kocher criteria, other signs and symptoms of septic arthritis include limb pain, joint effusion, and a strong tendency for the patient to hold the affected joint in the position of maximal intracapsular volume in order to minimize discomfort. Neonates and infants may present with pseudoparalysis in response to the joint pain.
The log-roll technique is an effective way to detect hip effusion on clinical examination. With the patient lying supine on the examination table, the examiner places one hand at the ankle and another on the thigh and rolls the leg back and forth. Hip effusion can also be detected by ultrasound or MRI. However, it’s important to recognize that the presence of hip effusion doesn’t distinguish septic arthritis from transient synovitis.
Diagnosis of septic arthritis is based upon a combination of clinical findings and analysis of synovial fluid obtained via joint aspiration. Septic arthritis is suggested by an opaque, yellow-to-green synovial fluid with an elevated WBC, at least 75% polymorphonuclear leukocytes, and a glucose concentration of only about 30% of that in blood.
The initial antibiotic therapy should be selected before synovial fluid culture results are available. The child’s age is an important consideration, as the causative organisms vary. Haemophilus influenzae was the most common pathogen in children younger than 5 years of age until the vaccine entered wide use. Now Staphylococcus aureus is No. 1 in all age groups, and community-acquired MRSA (methicillin-resistant S. aureus) is an important consideration.
In neonates, other organisms include group B streptococcus, Streptococcus viridans, Streptococcus pneumoniae, Neisseria gonorrhoeae, gram-negative enteric bacteria including Escherichia coli and group A streptococcus.
An important and underappreciated cause of septic arthritis in non-neonates younger than age 5 years is Kingella kingae. It is typically culture-negative but can be detected by polymerase chain reaction. In addition, Neisseria meningitidis is a consideration in this age group and all the way through adolescence, as well.
Ms. Heizer declared having no financial conflicts.
VAIL, COLO. – Four simple criteria are useful in distinguishing septic arthritis from transient synovitis in a child with an inflamed hip.
The criteria are known as the Kocher criteria, for Dr. Mininder S. Kocher, associate director of sports medicine at Children’s Hospital Boston, who was first author of the study that introduced the criteria and an associated evidence-based, predictive algorithm.
The criteria are inability to tolerate weight bearing, fever greater than 38.5° C (101.3° F), an ESR (erythrocyte sedimentation rate) in excess of 40 mm/hour, and a peripheral WBC count greater than 12,000 cells/mm3.
Dr. Kocher and his coworkers showed in a retrospective study that a child who meets none of these four criteria has just a 0.2% chance of having septic arthritis. With one criterion present, there’s a 3% chance. With two criteria, it’s 40%. With any three, the probability of septic arthritis jumps to 93%. And when all four criteria are present, the probability of septic arthritis is 99.6% (J. Bone Joint Surg. Am. 1999;81:1662-70).
"Looking back on our own cases, this has been really helpful," Heather R. Heizer said at a conference on pediatric infectious diseases sponsored by Children’s Hospital Colorado.
The Kocher criteria deserve to be more widely known. Septic arthritis is an orthopedic emergency. Delayed treatment can lead to irreversible joint damage. And septic arthritis occurs more often in childhood than at any other period of life, observed Ms. Heizer, a physician assistant in the department of pediatrics at the University of Colorado at Denver.
The most common site for septic arthritis is the hip, followed by the knee, then the ankle. Together these three sites account for 80% of all cases.
In addition to the Kocher criteria, other signs and symptoms of septic arthritis include limb pain, joint effusion, and a strong tendency for the patient to hold the affected joint in the position of maximal intracapsular volume in order to minimize discomfort. Neonates and infants may present with pseudoparalysis in response to the joint pain.
The log-roll technique is an effective way to detect hip effusion on clinical examination. With the patient lying supine on the examination table, the examiner places one hand at the ankle and another on the thigh and rolls the leg back and forth. Hip effusion can also be detected by ultrasound or MRI. However, it’s important to recognize that the presence of hip effusion doesn’t distinguish septic arthritis from transient synovitis.
Diagnosis of septic arthritis is based upon a combination of clinical findings and analysis of synovial fluid obtained via joint aspiration. Septic arthritis is suggested by an opaque, yellow-to-green synovial fluid with an elevated WBC, at least 75% polymorphonuclear leukocytes, and a glucose concentration of only about 30% of that in blood.
The initial antibiotic therapy should be selected before synovial fluid culture results are available. The child’s age is an important consideration, as the causative organisms vary. Haemophilus influenzae was the most common pathogen in children younger than 5 years of age until the vaccine entered wide use. Now Staphylococcus aureus is No. 1 in all age groups, and community-acquired MRSA (methicillin-resistant S. aureus) is an important consideration.
In neonates, other organisms include group B streptococcus, Streptococcus viridans, Streptococcus pneumoniae, Neisseria gonorrhoeae, gram-negative enteric bacteria including Escherichia coli and group A streptococcus.
An important and underappreciated cause of septic arthritis in non-neonates younger than age 5 years is Kingella kingae. It is typically culture-negative but can be detected by polymerase chain reaction. In addition, Neisseria meningitidis is a consideration in this age group and all the way through adolescence, as well.
Ms. Heizer declared having no financial conflicts.
EXPERT OPINION FROM A CONFERENCE ON PEDIATRIC INFECTIOUS DISEASES SPONSORED BY CHILDREN'S HOSPITAL COLORADO
Differentiating Dengue from Chikungunya in Returned Travelers
VAIL, COLO. – Dengue fever and chikungunya have much in common in terms of symptoms, incubation period, clinical course, vector, and geographical distribution.
There is, however, a good way to distinguish the two tropical fevers based upon presenting symptoms: Dengue plus arthritis equals chikungunya, Dr. Jay S. Keystone said at a conference on pediatric infectious diseases sponsored by Children’s Hospital Colorado.
For fever in a traveler returned from Southeast Asia, the Caribbean, or Latin America, think "dengue" after first ruling out malaria. Studies show that dengue is in fact the most common cause of fever in returned travelers from those areas. There is not so much malaria in those parts of the world, in contrast to sub-Saharan Africa, where malaria accounts for nearly two-thirds of febrile illnesses in returned travelers, noted Dr. Keystone, professor of medicine at the University of Toronto and a past president of the International Society of Travel Medicine.
Both dengue and chikungunya are viral diseases whose vector is the Aedes aegypti mosquito. Chikungunya has as an additional vector, the Asian tiger mosquito (Aedes albopictus). Chikungunya is a problem in Southeast Asia, as is dengue, but also in India and sub-Saharan Africa.
A key feature shared by both diseases is that the fever comes on sharply about a week after exposure and lasts about a week before resolving. Dengue fever classically is accompanied by a headache, retro-orbital pain, prominent muscle aches, and adenopathy. Similarly, chikungunya features prominent headache and adenopathy, but instead of the myalgia that is so characteristic of dengue, chikungunya entails marked joint pain and arthritis.
"Joint pain is the difference between the two. In adults, the arthritis can go on for many months," according to Dr. Keystone.
A maculopapular rash is common beginning on about day 3 in patients with chikungunya, especially so in children.
Diagnosis of dengue fever is made serologically on the basis of the detection of antibodies to dengue virus. Chikungunya, too, is diagnosed serologically.
Treatment of both tropical fevers is symptomatic. However, NSAIDs are to be avoided because of capillary fragility and increased bleeding risk.
Dengue and chikungunya share one more thing: Medical epidemiologists and entomologists are worried that both diseases may be on the march. The North American habitat of A. aegypti extends north as far as Chicago, and last year a first-ever outbreak of 28 cases of dengue fever was reported in the Florida Keys. Similarly, a substantial outbreak of chikungunya occurred several years ago in Italy, probably as a result of the chikungunya virus spreading through the local Asian tiger mosquito population via exposure to an infected returned traveler.
Dr. Keystone declared having no relevant financial disclosures.
VAIL, COLO. – Dengue fever and chikungunya have much in common in terms of symptoms, incubation period, clinical course, vector, and geographical distribution.
There is, however, a good way to distinguish the two tropical fevers based upon presenting symptoms: Dengue plus arthritis equals chikungunya, Dr. Jay S. Keystone said at a conference on pediatric infectious diseases sponsored by Children’s Hospital Colorado.
For fever in a traveler returned from Southeast Asia, the Caribbean, or Latin America, think "dengue" after first ruling out malaria. Studies show that dengue is in fact the most common cause of fever in returned travelers from those areas. There is not so much malaria in those parts of the world, in contrast to sub-Saharan Africa, where malaria accounts for nearly two-thirds of febrile illnesses in returned travelers, noted Dr. Keystone, professor of medicine at the University of Toronto and a past president of the International Society of Travel Medicine.
Both dengue and chikungunya are viral diseases whose vector is the Aedes aegypti mosquito. Chikungunya has as an additional vector, the Asian tiger mosquito (Aedes albopictus). Chikungunya is a problem in Southeast Asia, as is dengue, but also in India and sub-Saharan Africa.
A key feature shared by both diseases is that the fever comes on sharply about a week after exposure and lasts about a week before resolving. Dengue fever classically is accompanied by a headache, retro-orbital pain, prominent muscle aches, and adenopathy. Similarly, chikungunya features prominent headache and adenopathy, but instead of the myalgia that is so characteristic of dengue, chikungunya entails marked joint pain and arthritis.
"Joint pain is the difference between the two. In adults, the arthritis can go on for many months," according to Dr. Keystone.
A maculopapular rash is common beginning on about day 3 in patients with chikungunya, especially so in children.
Diagnosis of dengue fever is made serologically on the basis of the detection of antibodies to dengue virus. Chikungunya, too, is diagnosed serologically.
Treatment of both tropical fevers is symptomatic. However, NSAIDs are to be avoided because of capillary fragility and increased bleeding risk.
Dengue and chikungunya share one more thing: Medical epidemiologists and entomologists are worried that both diseases may be on the march. The North American habitat of A. aegypti extends north as far as Chicago, and last year a first-ever outbreak of 28 cases of dengue fever was reported in the Florida Keys. Similarly, a substantial outbreak of chikungunya occurred several years ago in Italy, probably as a result of the chikungunya virus spreading through the local Asian tiger mosquito population via exposure to an infected returned traveler.
Dr. Keystone declared having no relevant financial disclosures.
VAIL, COLO. – Dengue fever and chikungunya have much in common in terms of symptoms, incubation period, clinical course, vector, and geographical distribution.
There is, however, a good way to distinguish the two tropical fevers based upon presenting symptoms: Dengue plus arthritis equals chikungunya, Dr. Jay S. Keystone said at a conference on pediatric infectious diseases sponsored by Children’s Hospital Colorado.
For fever in a traveler returned from Southeast Asia, the Caribbean, or Latin America, think "dengue" after first ruling out malaria. Studies show that dengue is in fact the most common cause of fever in returned travelers from those areas. There is not so much malaria in those parts of the world, in contrast to sub-Saharan Africa, where malaria accounts for nearly two-thirds of febrile illnesses in returned travelers, noted Dr. Keystone, professor of medicine at the University of Toronto and a past president of the International Society of Travel Medicine.
Both dengue and chikungunya are viral diseases whose vector is the Aedes aegypti mosquito. Chikungunya has as an additional vector, the Asian tiger mosquito (Aedes albopictus). Chikungunya is a problem in Southeast Asia, as is dengue, but also in India and sub-Saharan Africa.
A key feature shared by both diseases is that the fever comes on sharply about a week after exposure and lasts about a week before resolving. Dengue fever classically is accompanied by a headache, retro-orbital pain, prominent muscle aches, and adenopathy. Similarly, chikungunya features prominent headache and adenopathy, but instead of the myalgia that is so characteristic of dengue, chikungunya entails marked joint pain and arthritis.
"Joint pain is the difference between the two. In adults, the arthritis can go on for many months," according to Dr. Keystone.
A maculopapular rash is common beginning on about day 3 in patients with chikungunya, especially so in children.
Diagnosis of dengue fever is made serologically on the basis of the detection of antibodies to dengue virus. Chikungunya, too, is diagnosed serologically.
Treatment of both tropical fevers is symptomatic. However, NSAIDs are to be avoided because of capillary fragility and increased bleeding risk.
Dengue and chikungunya share one more thing: Medical epidemiologists and entomologists are worried that both diseases may be on the march. The North American habitat of A. aegypti extends north as far as Chicago, and last year a first-ever outbreak of 28 cases of dengue fever was reported in the Florida Keys. Similarly, a substantial outbreak of chikungunya occurred several years ago in Italy, probably as a result of the chikungunya virus spreading through the local Asian tiger mosquito population via exposure to an infected returned traveler.
Dr. Keystone declared having no relevant financial disclosures.
EXPERT ANALYSIS FROM A CONFERENCE ON PEDIATRIC INFECTIOUS DISEASES SPONSORED BY CHILDREN'S HOSPITAL COLORADO
Fever in Returned Traveler: Think Malaria First
VAIL, COLO. – Fever in a traveler back from the tropics is malaria until proven otherwise – and it’s a medical emergency, Dr. Jay S. Keystone said at a conference on pediatric infectious diseases sponsored by Children’s Hospital Colorado.
"Death from malaria can occur in 3-4 days. Not always, but it can. That’s all it takes," said Dr. Keystone, professor of medicine at the University of Toronto and a past president of the International Society of Travel Medicine.
If it’s malaria due to Plasmodium falciparum in a nonimmune patient – and children should be assumed to be nonimmune – hospital admission for up to 48 hours is warranted, even if only minimal parasitemia is present. There’s no need to keep the patient in the hospital until the parasitemia is zero; once the parasitemia is falling in response to therapy, monitoring can safely be accomplished on an outpatient basis with daily blood films until the patient has fully recovered.
Why admit a patient with mere low-level P. falciparum parasitemia? Dr. Keystone has seen returned travelers with a several-day history of fever go from 1% to 30% parasitemia in the course of just 9 hours.
"You really don’t know the parasitemia mass when you’re looking at the blood film because most of the falciparum develops in the microcirculation," he explained.
The one exception to his maximum 48-hour hospitalization rule is in patients with a heavy P. falciparum infection as defined by 5% or greater parasitemia. Onset of adult respiratory distress syndrome in such patients often occurs on day 3-4 of treatment, just as they’re starting to look markedly better and their parasitemia is coming down.
The clinical hallmark of malaria is fever. "That’s the only thing you have to know about what malaria looks like. And if there’s no periodicity to the fever, ignore that; malaria is still in the differential diagnosis," Dr. Keystone said. "For falciparum, the fever you’re most worried about, there really is only rarely periodicity, especially in children. If, however, there is an exact periodicity – fever every other day, every third day, et cetera – it can only be malaria."
In a study Dr. Keystone coauthored that provided the first systematic evaluation of illness in returned pediatric travelers, febrile illness was the presenting complaint in 23% of 1,591 ill children seen at 51 tropical medicine clinics in the GeoSentinel Global Surveillance Network maintained by the International Society of Travel Medicine and U.S. Centers for Disease Control and Prevention. In fact, fever was the third most common presenting complaint, after diarrhea in 28% of patients and dermatologic conditions in 25%.
Of 358 ill returned pediatric travelers with fever, malaria was the No. 1 cause, accounting for 35% of cases, followed by upper respiratory tract infections and other viral illnesses in 28% (Pediatrics 2010;125: 1072-80).
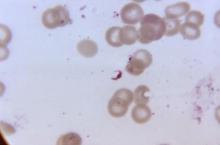
The diagnosis of malaria is made based upon thick blood films; speciation is based upon thin films. If the first blood film is negative, testing should be repeated daily for 2 additional days; otherwise, it’s quite possible to miss parasitemia.
As an alternative to the time-honored thick blood films, Dr. Keystone strongly recommended the use of rapid diagnostic tests, in which malaria is diagnosed based upon detection of malaria antigen in the blood. These tests have been shown to have 99% sensitivity and 94% specificity for P. falciparum infection, and 94% sensitivity and 100% specificity for Plasmodium vivax.
"In Ontario, that’s all we do – we do the rapid diagnostic test, then thin blood films looking for the species and the parasitemia. Rapid diagnostic tests are especially good if your lab doesn’t have a lot of experience with malaria, as you’d expect in a place such as Colorado," he noted.
Parasitemia of 1% or greater is due to P. falciparum more than 90% of the time. In parasitemia, 5% is a critical number; at that level, parenteral therapy is warranted and there is a risk of death.
"At 10% or more, it’s time to change your underwear," Dr. Keystone quipped. "You’ve got a serious problem. At that point, you’re always thinking about exchange transfusion."
Recognizing and Treating Malaria in the United States
Who brings malaria back to the United States from abroad? A recent CDC analysis of cases imported during 2009 concluded that U.S. immigrants who had been visiting friends and relatives abroad accounted for 63% of cases. Missionaries made up another 10%, with the remainder being divided between tourists, business travelers, and students (MMWR Surveill. Summ. 2011 Apr;60:1-15).
A review of 13 North American and European clinical studies highlighted differences between children and adults in the presenting signs and symptoms of malaria. The majority of children had fever in excess of 40°C, compared with just a quarter of adults. The same was true of hepatosplenomegaly and anemia. Nausea, vomiting, and diarrhea were very common in children, whereas the most common nonspecific symptoms in adults were headache and myalgia. Thrombocytopenia was extremely common in both groups (Lancet Infect. Dis. 2007;7:349-57).
Every medical student has to learn the four human malaria parasites: P. falciparum, vivax, malariae, and ovale. Recently a fifth has been identified: Plasmodium knowlesi. And it’s a pip.
"It’s a monkey malaria that’s now in the human population. Knowlesi is found mainly in Southeast Asia, especially in the border areas of Thailand. It is one of the fatal malarias. It looks benign and like [Plasmodium] malariae under the microscope, but it kills like P. falciparum. So if you see someone from Southeast Asia who’s very sick and it looks like malariae, think about knowlesi," Dr. Keystone urged.
Two very good medications are available in the United States for treatment of malaria. For patients with heavy parasitemia or severe malaria as defined by cerebral, respiratory, or renal manifestations or severe anemia, intravenous artemether/lumefantrine (Coartem) is the treatment of choice because it’s faster acting, with an average parasite clearance time of 2 days, compared to 4 days with atovaquone/proguanil (Malarone). However, atovaquone/proguanil is better tolerated and thus a good choice in patients with less than 5% parasitemia who don’t have severe disease, he said.
For more information on travel medicine, Dr. Keystone recommends the following resources:
• Text: "Control of Communicable Diseases Manual," 19th Edition, edited by Dr. David L. Heymann. An official report of the American Public Health Association. Available in paperback at Amazon.com for about $23.
"It’s a brilliant book," Dr. Keystone said. "The beauty of this book is it doesn’t just give you the epidemiology and incubation, it gives you the period of communicability, and it’s all laid out in easy form. I would highly advise you that if you don’t have one of these in the office, it’s definitely worth having."
• Web: Every infectious diseases program should have a subscription to Gideon, Dr. Keystone said. Plug in the pertinent facts regarding a case and Gideon spits out the complete differential diagnosis, with the possibilities rank-ordered and accompanying treatment recommendations. Physicians can obtain a free 15-day trial through the website.
"Gideon is probably the best geographic, computer-based, online program for infectious diseases that I know of. You don’t have to be smart to know everything about tropical medicine. If you’re looking for something to help you diagnose tropical diseases, this would be the one," he said.
• Phone: 770-488-7788 connects you to the CDC’s malaria branch.
Dr. Keystone disclosed that he is a consultant to Gideon.
VAIL, COLO. – Fever in a traveler back from the tropics is malaria until proven otherwise – and it’s a medical emergency, Dr. Jay S. Keystone said at a conference on pediatric infectious diseases sponsored by Children’s Hospital Colorado.
"Death from malaria can occur in 3-4 days. Not always, but it can. That’s all it takes," said Dr. Keystone, professor of medicine at the University of Toronto and a past president of the International Society of Travel Medicine.
If it’s malaria due to Plasmodium falciparum in a nonimmune patient – and children should be assumed to be nonimmune – hospital admission for up to 48 hours is warranted, even if only minimal parasitemia is present. There’s no need to keep the patient in the hospital until the parasitemia is zero; once the parasitemia is falling in response to therapy, monitoring can safely be accomplished on an outpatient basis with daily blood films until the patient has fully recovered.
Why admit a patient with mere low-level P. falciparum parasitemia? Dr. Keystone has seen returned travelers with a several-day history of fever go from 1% to 30% parasitemia in the course of just 9 hours.
"You really don’t know the parasitemia mass when you’re looking at the blood film because most of the falciparum develops in the microcirculation," he explained.
The one exception to his maximum 48-hour hospitalization rule is in patients with a heavy P. falciparum infection as defined by 5% or greater parasitemia. Onset of adult respiratory distress syndrome in such patients often occurs on day 3-4 of treatment, just as they’re starting to look markedly better and their parasitemia is coming down.
The clinical hallmark of malaria is fever. "That’s the only thing you have to know about what malaria looks like. And if there’s no periodicity to the fever, ignore that; malaria is still in the differential diagnosis," Dr. Keystone said. "For falciparum, the fever you’re most worried about, there really is only rarely periodicity, especially in children. If, however, there is an exact periodicity – fever every other day, every third day, et cetera – it can only be malaria."
In a study Dr. Keystone coauthored that provided the first systematic evaluation of illness in returned pediatric travelers, febrile illness was the presenting complaint in 23% of 1,591 ill children seen at 51 tropical medicine clinics in the GeoSentinel Global Surveillance Network maintained by the International Society of Travel Medicine and U.S. Centers for Disease Control and Prevention. In fact, fever was the third most common presenting complaint, after diarrhea in 28% of patients and dermatologic conditions in 25%.
Of 358 ill returned pediatric travelers with fever, malaria was the No. 1 cause, accounting for 35% of cases, followed by upper respiratory tract infections and other viral illnesses in 28% (Pediatrics 2010;125: 1072-80).
The diagnosis of malaria is made based upon thick blood films; speciation is based upon thin films. If the first blood film is negative, testing should be repeated daily for 2 additional days; otherwise, it’s quite possible to miss parasitemia.
As an alternative to the time-honored thick blood films, Dr. Keystone strongly recommended the use of rapid diagnostic tests, in which malaria is diagnosed based upon detection of malaria antigen in the blood. These tests have been shown to have 99% sensitivity and 94% specificity for P. falciparum infection, and 94% sensitivity and 100% specificity for Plasmodium vivax.
"In Ontario, that’s all we do – we do the rapid diagnostic test, then thin blood films looking for the species and the parasitemia. Rapid diagnostic tests are especially good if your lab doesn’t have a lot of experience with malaria, as you’d expect in a place such as Colorado," he noted.
Parasitemia of 1% or greater is due to P. falciparum more than 90% of the time. In parasitemia, 5% is a critical number; at that level, parenteral therapy is warranted and there is a risk of death.
"At 10% or more, it’s time to change your underwear," Dr. Keystone quipped. "You’ve got a serious problem. At that point, you’re always thinking about exchange transfusion."
Recognizing and Treating Malaria in the United States
Who brings malaria back to the United States from abroad? A recent CDC analysis of cases imported during 2009 concluded that U.S. immigrants who had been visiting friends and relatives abroad accounted for 63% of cases. Missionaries made up another 10%, with the remainder being divided between tourists, business travelers, and students (MMWR Surveill. Summ. 2011 Apr;60:1-15).
A review of 13 North American and European clinical studies highlighted differences between children and adults in the presenting signs and symptoms of malaria. The majority of children had fever in excess of 40°C, compared with just a quarter of adults. The same was true of hepatosplenomegaly and anemia. Nausea, vomiting, and diarrhea were very common in children, whereas the most common nonspecific symptoms in adults were headache and myalgia. Thrombocytopenia was extremely common in both groups (Lancet Infect. Dis. 2007;7:349-57).
Every medical student has to learn the four human malaria parasites: P. falciparum, vivax, malariae, and ovale. Recently a fifth has been identified: Plasmodium knowlesi. And it’s a pip.
"It’s a monkey malaria that’s now in the human population. Knowlesi is found mainly in Southeast Asia, especially in the border areas of Thailand. It is one of the fatal malarias. It looks benign and like [Plasmodium] malariae under the microscope, but it kills like P. falciparum. So if you see someone from Southeast Asia who’s very sick and it looks like malariae, think about knowlesi," Dr. Keystone urged.
Two very good medications are available in the United States for treatment of malaria. For patients with heavy parasitemia or severe malaria as defined by cerebral, respiratory, or renal manifestations or severe anemia, intravenous artemether/lumefantrine (Coartem) is the treatment of choice because it’s faster acting, with an average parasite clearance time of 2 days, compared to 4 days with atovaquone/proguanil (Malarone). However, atovaquone/proguanil is better tolerated and thus a good choice in patients with less than 5% parasitemia who don’t have severe disease, he said.
For more information on travel medicine, Dr. Keystone recommends the following resources:
• Text: "Control of Communicable Diseases Manual," 19th Edition, edited by Dr. David L. Heymann. An official report of the American Public Health Association. Available in paperback at Amazon.com for about $23.
"It’s a brilliant book," Dr. Keystone said. "The beauty of this book is it doesn’t just give you the epidemiology and incubation, it gives you the period of communicability, and it’s all laid out in easy form. I would highly advise you that if you don’t have one of these in the office, it’s definitely worth having."
• Web: Every infectious diseases program should have a subscription to Gideon, Dr. Keystone said. Plug in the pertinent facts regarding a case and Gideon spits out the complete differential diagnosis, with the possibilities rank-ordered and accompanying treatment recommendations. Physicians can obtain a free 15-day trial through the website.
"Gideon is probably the best geographic, computer-based, online program for infectious diseases that I know of. You don’t have to be smart to know everything about tropical medicine. If you’re looking for something to help you diagnose tropical diseases, this would be the one," he said.
• Phone: 770-488-7788 connects you to the CDC’s malaria branch.
Dr. Keystone disclosed that he is a consultant to Gideon.
VAIL, COLO. – Fever in a traveler back from the tropics is malaria until proven otherwise – and it’s a medical emergency, Dr. Jay S. Keystone said at a conference on pediatric infectious diseases sponsored by Children’s Hospital Colorado.
"Death from malaria can occur in 3-4 days. Not always, but it can. That’s all it takes," said Dr. Keystone, professor of medicine at the University of Toronto and a past president of the International Society of Travel Medicine.
If it’s malaria due to Plasmodium falciparum in a nonimmune patient – and children should be assumed to be nonimmune – hospital admission for up to 48 hours is warranted, even if only minimal parasitemia is present. There’s no need to keep the patient in the hospital until the parasitemia is zero; once the parasitemia is falling in response to therapy, monitoring can safely be accomplished on an outpatient basis with daily blood films until the patient has fully recovered.
Why admit a patient with mere low-level P. falciparum parasitemia? Dr. Keystone has seen returned travelers with a several-day history of fever go from 1% to 30% parasitemia in the course of just 9 hours.
"You really don’t know the parasitemia mass when you’re looking at the blood film because most of the falciparum develops in the microcirculation," he explained.
The one exception to his maximum 48-hour hospitalization rule is in patients with a heavy P. falciparum infection as defined by 5% or greater parasitemia. Onset of adult respiratory distress syndrome in such patients often occurs on day 3-4 of treatment, just as they’re starting to look markedly better and their parasitemia is coming down.
The clinical hallmark of malaria is fever. "That’s the only thing you have to know about what malaria looks like. And if there’s no periodicity to the fever, ignore that; malaria is still in the differential diagnosis," Dr. Keystone said. "For falciparum, the fever you’re most worried about, there really is only rarely periodicity, especially in children. If, however, there is an exact periodicity – fever every other day, every third day, et cetera – it can only be malaria."
In a study Dr. Keystone coauthored that provided the first systematic evaluation of illness in returned pediatric travelers, febrile illness was the presenting complaint in 23% of 1,591 ill children seen at 51 tropical medicine clinics in the GeoSentinel Global Surveillance Network maintained by the International Society of Travel Medicine and U.S. Centers for Disease Control and Prevention. In fact, fever was the third most common presenting complaint, after diarrhea in 28% of patients and dermatologic conditions in 25%.
Of 358 ill returned pediatric travelers with fever, malaria was the No. 1 cause, accounting for 35% of cases, followed by upper respiratory tract infections and other viral illnesses in 28% (Pediatrics 2010;125: 1072-80).
The diagnosis of malaria is made based upon thick blood films; speciation is based upon thin films. If the first blood film is negative, testing should be repeated daily for 2 additional days; otherwise, it’s quite possible to miss parasitemia.
As an alternative to the time-honored thick blood films, Dr. Keystone strongly recommended the use of rapid diagnostic tests, in which malaria is diagnosed based upon detection of malaria antigen in the blood. These tests have been shown to have 99% sensitivity and 94% specificity for P. falciparum infection, and 94% sensitivity and 100% specificity for Plasmodium vivax.
"In Ontario, that’s all we do – we do the rapid diagnostic test, then thin blood films looking for the species and the parasitemia. Rapid diagnostic tests are especially good if your lab doesn’t have a lot of experience with malaria, as you’d expect in a place such as Colorado," he noted.
Parasitemia of 1% or greater is due to P. falciparum more than 90% of the time. In parasitemia, 5% is a critical number; at that level, parenteral therapy is warranted and there is a risk of death.
"At 10% or more, it’s time to change your underwear," Dr. Keystone quipped. "You’ve got a serious problem. At that point, you’re always thinking about exchange transfusion."
Recognizing and Treating Malaria in the United States
Who brings malaria back to the United States from abroad? A recent CDC analysis of cases imported during 2009 concluded that U.S. immigrants who had been visiting friends and relatives abroad accounted for 63% of cases. Missionaries made up another 10%, with the remainder being divided between tourists, business travelers, and students (MMWR Surveill. Summ. 2011 Apr;60:1-15).
A review of 13 North American and European clinical studies highlighted differences between children and adults in the presenting signs and symptoms of malaria. The majority of children had fever in excess of 40°C, compared with just a quarter of adults. The same was true of hepatosplenomegaly and anemia. Nausea, vomiting, and diarrhea were very common in children, whereas the most common nonspecific symptoms in adults were headache and myalgia. Thrombocytopenia was extremely common in both groups (Lancet Infect. Dis. 2007;7:349-57).
Every medical student has to learn the four human malaria parasites: P. falciparum, vivax, malariae, and ovale. Recently a fifth has been identified: Plasmodium knowlesi. And it’s a pip.
"It’s a monkey malaria that’s now in the human population. Knowlesi is found mainly in Southeast Asia, especially in the border areas of Thailand. It is one of the fatal malarias. It looks benign and like [Plasmodium] malariae under the microscope, but it kills like P. falciparum. So if you see someone from Southeast Asia who’s very sick and it looks like malariae, think about knowlesi," Dr. Keystone urged.
Two very good medications are available in the United States for treatment of malaria. For patients with heavy parasitemia or severe malaria as defined by cerebral, respiratory, or renal manifestations or severe anemia, intravenous artemether/lumefantrine (Coartem) is the treatment of choice because it’s faster acting, with an average parasite clearance time of 2 days, compared to 4 days with atovaquone/proguanil (Malarone). However, atovaquone/proguanil is better tolerated and thus a good choice in patients with less than 5% parasitemia who don’t have severe disease, he said.
For more information on travel medicine, Dr. Keystone recommends the following resources:
• Text: "Control of Communicable Diseases Manual," 19th Edition, edited by Dr. David L. Heymann. An official report of the American Public Health Association. Available in paperback at Amazon.com for about $23.
"It’s a brilliant book," Dr. Keystone said. "The beauty of this book is it doesn’t just give you the epidemiology and incubation, it gives you the period of communicability, and it’s all laid out in easy form. I would highly advise you that if you don’t have one of these in the office, it’s definitely worth having."
• Web: Every infectious diseases program should have a subscription to Gideon, Dr. Keystone said. Plug in the pertinent facts regarding a case and Gideon spits out the complete differential diagnosis, with the possibilities rank-ordered and accompanying treatment recommendations. Physicians can obtain a free 15-day trial through the website.
"Gideon is probably the best geographic, computer-based, online program for infectious diseases that I know of. You don’t have to be smart to know everything about tropical medicine. If you’re looking for something to help you diagnose tropical diseases, this would be the one," he said.
• Phone: 770-488-7788 connects you to the CDC’s malaria branch.
Dr. Keystone disclosed that he is a consultant to Gideon.
EXPERT OPINION FROM A CONFERENCE ON PEDIATRIC INFECTIOUS DISEASES
IGRAs Are TB Test Alternative After Age 5 Years
VAIL, COLO. – Interferon-gamma release assays offer significant advantages over conventional tuberculin skin testing under certain circumstances, but not in children younger than 5 years old.
The IFN-gamma release assays (IGRAs) have not been well studied in children younger than age 5 years, plus the available data suggest that the test results are less reliable in this age group. Given the high rate of progression from latent to active disease as well as the high rates of severe disease in this younger population, the Centers for Disease Control and Prevention recommends using the tuberculin skin test (TST) for these young children, Dr. Donna Curtis said at a conference on pediatric infectious diseases sponsored by Children’s Hospital Colorado.
Beyond age 5 years, however, the sensitivity and specificity of IGRAs and the TST are similar, and the tests can be used interchangeably, added Dr. Curtis of the University of Colorado at Denver.
IGRAs are blood tests that measure IFN-gamma production by WBCs in response to stimulation by TB antigens. The two Food and Drug Administration–approved IGRAs on the market are Cellistis’s QuantiFERON–TB Gold In-Tube test and Oxford Immunotec’s T-SPOT.TB test. The test results are reported from the laboratory as positive, negative, or indeterminate.
Among the IGRAs’ advantages are that only a single patient visit is required and results are available 24 hours after laboratory processing. Also, there are no false-positives due to previous BCG (bacille Calmette-Guérin) vaccination, a big problem with TSTs in foreign-born patients. And unlike the TST, there is no boosting phenomenon with repeated IGRA tests.
On the other hand, an IGRA requires fresh blood and plenty of it – 4 tubes’ worth – and it must be transported promptly to the laboratory for processing. The cost, albeit variable, is often more than that of a TST.
An IGRA is clearly the preferred test in patients older than age 5 years who have received a BCG vaccine, and in those with a reduced likelihood of returning for a second visit to have their TST read (such as substance abusers, people with transportation difficulties, and patients in homeless shelters).
As with TSTs, IGRAs should be used only to test patients with a TB exposure or risk factors. Otherwise, the odds of a false-positive test are greatly increased. After all, fewer than 1% of the noninstitutionalized U.S. population with no risk factors has a latent TB infection.
The CDC recommends both the IGRA and TST in patients whose first test is negative but who have a high risk of infection or progression to active disease. The health agency also recommends both tests as useful when the first test is positive and additional evidence is needed to convince the patient or family to undergo treatment.
Dr. Curtis noted that a recent Croatian study involving 142 BCG-vaccinated children younger than age 5 with a known TB exposure – all of whom had both a TST and IGRA – found a high rate of discordant results. The investigators concluded that both tests should routinely be used in this age group, and that a child should be considered infected if either or both results are positive (Pediatr. Infect. Dis. J. 2011 May 12 [Epub ahead of print]).
However, Dr. John W. Ogle commented that the dual-test strategy for younger children is fraught with problems.
"There are no normative data for IGRAs in kids under age 5 years. The IGRAs are standardized on adult patients. The amount of interferon that you make in response to an antigen is age dependent; kids less than age 5 make much less compared to adults. So if you do an IGRA in a young kid, you’re much more likely to have a false-negative result. Families will beg you to do an IGRA because they’ve learned this on the Internet and they don’t want their child to have to be exposed to the medical treatment," explained Dr. Ogle, professor and vice chair of pediatrics at the University of Colorado at Denver and director of pediatric services at Denver Health Medical Center.
A year ago, the CDC issued updated guidelines for the use of IGRAs (MMWR 2010; 59[RR-5];1-25).Dr. Curtis said physicians with any questions about IGRAs will find this publication quite helpful, even though it includes studies published only through 2008.
She declared having no financial conflicts.
VAIL, COLO. – Interferon-gamma release assays offer significant advantages over conventional tuberculin skin testing under certain circumstances, but not in children younger than 5 years old.
The IFN-gamma release assays (IGRAs) have not been well studied in children younger than age 5 years, plus the available data suggest that the test results are less reliable in this age group. Given the high rate of progression from latent to active disease as well as the high rates of severe disease in this younger population, the Centers for Disease Control and Prevention recommends using the tuberculin skin test (TST) for these young children, Dr. Donna Curtis said at a conference on pediatric infectious diseases sponsored by Children’s Hospital Colorado.
Beyond age 5 years, however, the sensitivity and specificity of IGRAs and the TST are similar, and the tests can be used interchangeably, added Dr. Curtis of the University of Colorado at Denver.
IGRAs are blood tests that measure IFN-gamma production by WBCs in response to stimulation by TB antigens. The two Food and Drug Administration–approved IGRAs on the market are Cellistis’s QuantiFERON–TB Gold In-Tube test and Oxford Immunotec’s T-SPOT.TB test. The test results are reported from the laboratory as positive, negative, or indeterminate.
Among the IGRAs’ advantages are that only a single patient visit is required and results are available 24 hours after laboratory processing. Also, there are no false-positives due to previous BCG (bacille Calmette-Guérin) vaccination, a big problem with TSTs in foreign-born patients. And unlike the TST, there is no boosting phenomenon with repeated IGRA tests.
On the other hand, an IGRA requires fresh blood and plenty of it – 4 tubes’ worth – and it must be transported promptly to the laboratory for processing. The cost, albeit variable, is often more than that of a TST.
An IGRA is clearly the preferred test in patients older than age 5 years who have received a BCG vaccine, and in those with a reduced likelihood of returning for a second visit to have their TST read (such as substance abusers, people with transportation difficulties, and patients in homeless shelters).
As with TSTs, IGRAs should be used only to test patients with a TB exposure or risk factors. Otherwise, the odds of a false-positive test are greatly increased. After all, fewer than 1% of the noninstitutionalized U.S. population with no risk factors has a latent TB infection.
The CDC recommends both the IGRA and TST in patients whose first test is negative but who have a high risk of infection or progression to active disease. The health agency also recommends both tests as useful when the first test is positive and additional evidence is needed to convince the patient or family to undergo treatment.
Dr. Curtis noted that a recent Croatian study involving 142 BCG-vaccinated children younger than age 5 with a known TB exposure – all of whom had both a TST and IGRA – found a high rate of discordant results. The investigators concluded that both tests should routinely be used in this age group, and that a child should be considered infected if either or both results are positive (Pediatr. Infect. Dis. J. 2011 May 12 [Epub ahead of print]).
However, Dr. John W. Ogle commented that the dual-test strategy for younger children is fraught with problems.
"There are no normative data for IGRAs in kids under age 5 years. The IGRAs are standardized on adult patients. The amount of interferon that you make in response to an antigen is age dependent; kids less than age 5 make much less compared to adults. So if you do an IGRA in a young kid, you’re much more likely to have a false-negative result. Families will beg you to do an IGRA because they’ve learned this on the Internet and they don’t want their child to have to be exposed to the medical treatment," explained Dr. Ogle, professor and vice chair of pediatrics at the University of Colorado at Denver and director of pediatric services at Denver Health Medical Center.
A year ago, the CDC issued updated guidelines for the use of IGRAs (MMWR 2010; 59[RR-5];1-25).Dr. Curtis said physicians with any questions about IGRAs will find this publication quite helpful, even though it includes studies published only through 2008.
She declared having no financial conflicts.
VAIL, COLO. – Interferon-gamma release assays offer significant advantages over conventional tuberculin skin testing under certain circumstances, but not in children younger than 5 years old.
The IFN-gamma release assays (IGRAs) have not been well studied in children younger than age 5 years, plus the available data suggest that the test results are less reliable in this age group. Given the high rate of progression from latent to active disease as well as the high rates of severe disease in this younger population, the Centers for Disease Control and Prevention recommends using the tuberculin skin test (TST) for these young children, Dr. Donna Curtis said at a conference on pediatric infectious diseases sponsored by Children’s Hospital Colorado.
Beyond age 5 years, however, the sensitivity and specificity of IGRAs and the TST are similar, and the tests can be used interchangeably, added Dr. Curtis of the University of Colorado at Denver.
IGRAs are blood tests that measure IFN-gamma production by WBCs in response to stimulation by TB antigens. The two Food and Drug Administration–approved IGRAs on the market are Cellistis’s QuantiFERON–TB Gold In-Tube test and Oxford Immunotec’s T-SPOT.TB test. The test results are reported from the laboratory as positive, negative, or indeterminate.
Among the IGRAs’ advantages are that only a single patient visit is required and results are available 24 hours after laboratory processing. Also, there are no false-positives due to previous BCG (bacille Calmette-Guérin) vaccination, a big problem with TSTs in foreign-born patients. And unlike the TST, there is no boosting phenomenon with repeated IGRA tests.
On the other hand, an IGRA requires fresh blood and plenty of it – 4 tubes’ worth – and it must be transported promptly to the laboratory for processing. The cost, albeit variable, is often more than that of a TST.
An IGRA is clearly the preferred test in patients older than age 5 years who have received a BCG vaccine, and in those with a reduced likelihood of returning for a second visit to have their TST read (such as substance abusers, people with transportation difficulties, and patients in homeless shelters).
As with TSTs, IGRAs should be used only to test patients with a TB exposure or risk factors. Otherwise, the odds of a false-positive test are greatly increased. After all, fewer than 1% of the noninstitutionalized U.S. population with no risk factors has a latent TB infection.
The CDC recommends both the IGRA and TST in patients whose first test is negative but who have a high risk of infection or progression to active disease. The health agency also recommends both tests as useful when the first test is positive and additional evidence is needed to convince the patient or family to undergo treatment.
Dr. Curtis noted that a recent Croatian study involving 142 BCG-vaccinated children younger than age 5 with a known TB exposure – all of whom had both a TST and IGRA – found a high rate of discordant results. The investigators concluded that both tests should routinely be used in this age group, and that a child should be considered infected if either or both results are positive (Pediatr. Infect. Dis. J. 2011 May 12 [Epub ahead of print]).
However, Dr. John W. Ogle commented that the dual-test strategy for younger children is fraught with problems.
"There are no normative data for IGRAs in kids under age 5 years. The IGRAs are standardized on adult patients. The amount of interferon that you make in response to an antigen is age dependent; kids less than age 5 make much less compared to adults. So if you do an IGRA in a young kid, you’re much more likely to have a false-negative result. Families will beg you to do an IGRA because they’ve learned this on the Internet and they don’t want their child to have to be exposed to the medical treatment," explained Dr. Ogle, professor and vice chair of pediatrics at the University of Colorado at Denver and director of pediatric services at Denver Health Medical Center.
A year ago, the CDC issued updated guidelines for the use of IGRAs (MMWR 2010; 59[RR-5];1-25).Dr. Curtis said physicians with any questions about IGRAs will find this publication quite helpful, even though it includes studies published only through 2008.
She declared having no financial conflicts.
EXPERT ANALYSIS FROM A CONFERENCE ON PEDIATRIC INFECTIOUS DISEASES SPONSORED BY CHILDREN'S HOSPITAL COLORADO
Reasons Behind ACIP's Off-Label Vaccine Recommendations
VAIL, COLO. – Earlier this year, the Advisory Committee on Immunization Practices took the unusual step of updating recommendations for the use of the meningococcal conjugate vaccines and Tdap vaccine that are at odds with the Food and Drug Administration–approved licensed indications, Dr. Marc Fisher said.
"It’s sort of unusual for ACIP to make off-label indications," Dr. Fischer observed in presenting an update from the Centers for Disease Control and Prevention’s ACIP at a conference on pediatric infectious diseases sponsored by Children’s Hospital Colorado.
The extraordinary step wasn’t taken lightly. The ACIP issued its off-label recommendations after careful deliberation in an attempt to address several public health problems related to shortcomings in vaccination coverage. These concerns have come to the fore only since licensure of the meningococcal conjugate and Tdap (tetanus toxoid with reduced diphtheria toxoid and acellular pertussis) vaccines.
The committee weighed new data supporting the safety and efficacy of the vaccines in populations that were not included in the licensed indications, explained Dr. Fischer, surveillance and epidemiology chief at the CDC’s Division of Vector-Borne Infectious Diseases in Ft. Collins, Colo. He serves as an ACIP adviser.
For example, the package insert for the MCV4-D meningococcal conjugate vaccine (Menactra) lists a history of Guillain-Barré syndrome as a contraindication to giving the vaccine. However, given the reassuring findings of two population-based studies totaling more than 10 million teens – data which became available only after the product labeling was created – the ACIP has concluded there is no increased risk of Guillain-Barré syndrome after vaccination with MCV4-D. Also, the latest results from the Vaccine Adverse Event Reporting System (VAERS) show no signal of a relationship between the meningococcal conjugate vaccine and Guillain-Barré syndrome.
"So if you’re following the package insert you wouldn’t give the vaccine to an 18-year-old headed off to college who has a history of [Guillain-Barré syndrome], and if you’re following the ACIP recommendation you ignore the history of [Guillain-Barré syndrome] and give the vaccine," Dr. Fischer explained.
Off-label ACIP recommendations for the Tdap vaccine published this year (MMWR 2011;60:13-5) after deliberations at the fall 2010 ACIP meeting include the following:
• Children aged 7-10 years who aren’t fully vaccinated against pertussis should receive a single dose of Tdap.
• Adults aged 65 or older who anticipate having close contact with an infant should get a single dose of Tdap.
• Tdap can now be given regardless of how long it has been since the last dose of a tetanus- or diphtheria-containing vaccine. Tdap is actually licensed for use only 5 years or longer after the last dose.
ACIP took these actions in order to improve pertussis vaccine coverage rates and create a coordinated strategy for preventing disease in young infants. Such a strategy is sorely needed. The incidence of pertussis is highest in children 1 month of age or younger. So is the number of deaths. Moreover, both the absolute number of deaths and the proportion of pertussis-related deaths occurring before age 1 month have increased in each of the past 3 decades. During 2000-2009, fully 78% of all pertussis-related deaths in the United States occurred in infants aged 1 month or younger. "We’re not really solving this problem of deaths in young infants," Dr. Fischer commented.
The new off-label Tdap recommendations address two major gaps in pertussis vaccine coverage stemming from the fact that no pertussis vaccine is licensed for use in children aged 7-10 years or in adults aged 65 and older, even though grandparents and other older adults are now believed to be one of the main sources of infection in young infants.
Filling these two gaps in coverage is a strategy known in immunization circles as cocooning. Cocooning is half of a dual ACIP approach aimed at preventing pertussis in young infants. The other half is embodied in an ACIP recommendation that ob.gyns. should routinely administer Tdap to pregnant women who haven’t previously received the vaccine. This should be done after 20 weeks’ gestation, or – as a second-best alternative –immediately post partum.
"This is a pretty big step, recommending a vaccine during pregnancy," the medical epidemiologist commented.
The committee was swayed in part by a cost-effectiveness analysis which estimated that maternal Tdap vaccination would cost $400,000 per quality-adjusted life-year saved. That’s threefold less than for postpartum maternal vaccination, and 13-fold less costly per quality-adjusted life-year saved than for a cocooning strategy involving vaccination of the child’s parents and grandparents.
ACIP recommended maternal vaccination during pregnancy despite some cautionary evidence that transplacental antibodies may blunt levels of active antibody produced when infants later undergo primary pertussis vaccination. The committee nonetheless decided to act because it’s unclear whether this blunted infant immune response to Tdap is actually clinically significant – and even if it is, the net effect could be to shift cases from the youngest infants to older infants, a group with less-severe disease and a lower case fatality rate. Still, the committee is looking forward to the guidance anticipated to come from an ongoing study of infants born to mothers who received Tdap or tetanus/diphtheria vaccine during their third trimester.
One of the barriers to more effective prevention of invasive meningococcal disease is lack of herd immunity because of insufficient coverage. Only 54% of U.S. 13- to 17-year-olds had received meningococcal vaccine in 2009.
Also, the vaccine’s protective effect appears to be considerably less durable than initially thought. There is mounting evidence of waning immunity by 2-5 years post vaccination. In sum, the evidence suggests that the ACIP’s recommendation at the time of Menactra’s licensure that vaccination routinely occur at the preventive care visit at age 11-12 years "maybe wasn’t the best decision" because it may not protect through the period of highest risk, Dr. Fischer said.
For this reason, this year ACIP has published revised recommendations for the use of meningococcal conjugate vaccines (Menveo and Menactra) in teens, based on a decision made at the fall 2010 ACIP meeting (MMWR 2011;60:72-6). Patients who receive the vaccine at age 11-12 years should get a booster dose at age 16, whereas those who get their first dose at age 13-15 years should have a booster dose at age 16-18. Those who get their first dose at age 16 or later don’t need a booster dose.
The currently available conjugate vaccines cover Neisseria meningitidis serogroups A, C, Y, and W135, but not serogroup B, which predominates among infants. Developing a conjugate vaccine that protects against serogroup B is a high priority. Initial efforts failed because candidate polysaccharide vaccines had molecular mimicry issues predisposing to adverse events. However, several non-polysaccharide vaccines that sidestep such concerns are now in late-stage clinical trials and – if the data prove positive – could be licensed in the next couple of years, according to Dr. Fischer.
Issues of particular interest to primary care physicians that the ACIP is likely to take under consideration within the next year include routine meningococcal conjugate vaccine for infants, hepatitis B vaccine for diabetic adults, and pneumococcal conjugate vaccine for adults, according to Dr. Fischer.
Two additional meningococcal conjugate vaccines are now under Food and Drug Administration review and are likely to be licensed by the end of the year. "I think ACIP is likely to reconsider the issue of routine meningococcal conjugate vaccine for infants in October, with a possible vote on the matter in February," he predicted.
A pneumococcal conjugate vaccine for adults is also likely to be licensed in the coming months, he said. That’s another vaccine the ACIP will consider recommending.
Also on tap for ACIP consideration is the use of human papillomavirus vaccine in males. There’s already a permissive ACIP recommendation for use of the HPV vaccine in males, but since this relatively weak endorsement was issued there have been new data demonstrating the vaccine’s effectiveness in preventing penile cancer, as well as a cost-effectiveness analysis.
Use of the herpes zoster vaccine in 50- to 59-year-olds isn’t recommended by the ACIP at this time. It’s an issue that’s likely to be raised at an upcoming meeting, according to Dr. Fischer.
Routine hepatitis B vaccination in adult diabetic patients as a means of preventing hepatitis outbreaks could come up for a vote as early as ACIP’s October meeting, he said.
Dr. Fischer said he had no relevant financial disclosures.
VAIL, COLO. – Earlier this year, the Advisory Committee on Immunization Practices took the unusual step of updating recommendations for the use of the meningococcal conjugate vaccines and Tdap vaccine that are at odds with the Food and Drug Administration–approved licensed indications, Dr. Marc Fisher said.
"It’s sort of unusual for ACIP to make off-label indications," Dr. Fischer observed in presenting an update from the Centers for Disease Control and Prevention’s ACIP at a conference on pediatric infectious diseases sponsored by Children’s Hospital Colorado.
The extraordinary step wasn’t taken lightly. The ACIP issued its off-label recommendations after careful deliberation in an attempt to address several public health problems related to shortcomings in vaccination coverage. These concerns have come to the fore only since licensure of the meningococcal conjugate and Tdap (tetanus toxoid with reduced diphtheria toxoid and acellular pertussis) vaccines.
The committee weighed new data supporting the safety and efficacy of the vaccines in populations that were not included in the licensed indications, explained Dr. Fischer, surveillance and epidemiology chief at the CDC’s Division of Vector-Borne Infectious Diseases in Ft. Collins, Colo. He serves as an ACIP adviser.
For example, the package insert for the MCV4-D meningococcal conjugate vaccine (Menactra) lists a history of Guillain-Barré syndrome as a contraindication to giving the vaccine. However, given the reassuring findings of two population-based studies totaling more than 10 million teens – data which became available only after the product labeling was created – the ACIP has concluded there is no increased risk of Guillain-Barré syndrome after vaccination with MCV4-D. Also, the latest results from the Vaccine Adverse Event Reporting System (VAERS) show no signal of a relationship between the meningococcal conjugate vaccine and Guillain-Barré syndrome.
"So if you’re following the package insert you wouldn’t give the vaccine to an 18-year-old headed off to college who has a history of [Guillain-Barré syndrome], and if you’re following the ACIP recommendation you ignore the history of [Guillain-Barré syndrome] and give the vaccine," Dr. Fischer explained.
Off-label ACIP recommendations for the Tdap vaccine published this year (MMWR 2011;60:13-5) after deliberations at the fall 2010 ACIP meeting include the following:
• Children aged 7-10 years who aren’t fully vaccinated against pertussis should receive a single dose of Tdap.
• Adults aged 65 or older who anticipate having close contact with an infant should get a single dose of Tdap.
• Tdap can now be given regardless of how long it has been since the last dose of a tetanus- or diphtheria-containing vaccine. Tdap is actually licensed for use only 5 years or longer after the last dose.
ACIP took these actions in order to improve pertussis vaccine coverage rates and create a coordinated strategy for preventing disease in young infants. Such a strategy is sorely needed. The incidence of pertussis is highest in children 1 month of age or younger. So is the number of deaths. Moreover, both the absolute number of deaths and the proportion of pertussis-related deaths occurring before age 1 month have increased in each of the past 3 decades. During 2000-2009, fully 78% of all pertussis-related deaths in the United States occurred in infants aged 1 month or younger. "We’re not really solving this problem of deaths in young infants," Dr. Fischer commented.
The new off-label Tdap recommendations address two major gaps in pertussis vaccine coverage stemming from the fact that no pertussis vaccine is licensed for use in children aged 7-10 years or in adults aged 65 and older, even though grandparents and other older adults are now believed to be one of the main sources of infection in young infants.
Filling these two gaps in coverage is a strategy known in immunization circles as cocooning. Cocooning is half of a dual ACIP approach aimed at preventing pertussis in young infants. The other half is embodied in an ACIP recommendation that ob.gyns. should routinely administer Tdap to pregnant women who haven’t previously received the vaccine. This should be done after 20 weeks’ gestation, or – as a second-best alternative –immediately post partum.
"This is a pretty big step, recommending a vaccine during pregnancy," the medical epidemiologist commented.
The committee was swayed in part by a cost-effectiveness analysis which estimated that maternal Tdap vaccination would cost $400,000 per quality-adjusted life-year saved. That’s threefold less than for postpartum maternal vaccination, and 13-fold less costly per quality-adjusted life-year saved than for a cocooning strategy involving vaccination of the child’s parents and grandparents.
ACIP recommended maternal vaccination during pregnancy despite some cautionary evidence that transplacental antibodies may blunt levels of active antibody produced when infants later undergo primary pertussis vaccination. The committee nonetheless decided to act because it’s unclear whether this blunted infant immune response to Tdap is actually clinically significant – and even if it is, the net effect could be to shift cases from the youngest infants to older infants, a group with less-severe disease and a lower case fatality rate. Still, the committee is looking forward to the guidance anticipated to come from an ongoing study of infants born to mothers who received Tdap or tetanus/diphtheria vaccine during their third trimester.
One of the barriers to more effective prevention of invasive meningococcal disease is lack of herd immunity because of insufficient coverage. Only 54% of U.S. 13- to 17-year-olds had received meningococcal vaccine in 2009.
Also, the vaccine’s protective effect appears to be considerably less durable than initially thought. There is mounting evidence of waning immunity by 2-5 years post vaccination. In sum, the evidence suggests that the ACIP’s recommendation at the time of Menactra’s licensure that vaccination routinely occur at the preventive care visit at age 11-12 years "maybe wasn’t the best decision" because it may not protect through the period of highest risk, Dr. Fischer said.
For this reason, this year ACIP has published revised recommendations for the use of meningococcal conjugate vaccines (Menveo and Menactra) in teens, based on a decision made at the fall 2010 ACIP meeting (MMWR 2011;60:72-6). Patients who receive the vaccine at age 11-12 years should get a booster dose at age 16, whereas those who get their first dose at age 13-15 years should have a booster dose at age 16-18. Those who get their first dose at age 16 or later don’t need a booster dose.
The currently available conjugate vaccines cover Neisseria meningitidis serogroups A, C, Y, and W135, but not serogroup B, which predominates among infants. Developing a conjugate vaccine that protects against serogroup B is a high priority. Initial efforts failed because candidate polysaccharide vaccines had molecular mimicry issues predisposing to adverse events. However, several non-polysaccharide vaccines that sidestep such concerns are now in late-stage clinical trials and – if the data prove positive – could be licensed in the next couple of years, according to Dr. Fischer.
Issues of particular interest to primary care physicians that the ACIP is likely to take under consideration within the next year include routine meningococcal conjugate vaccine for infants, hepatitis B vaccine for diabetic adults, and pneumococcal conjugate vaccine for adults, according to Dr. Fischer.
Two additional meningococcal conjugate vaccines are now under Food and Drug Administration review and are likely to be licensed by the end of the year. "I think ACIP is likely to reconsider the issue of routine meningococcal conjugate vaccine for infants in October, with a possible vote on the matter in February," he predicted.
A pneumococcal conjugate vaccine for adults is also likely to be licensed in the coming months, he said. That’s another vaccine the ACIP will consider recommending.
Also on tap for ACIP consideration is the use of human papillomavirus vaccine in males. There’s already a permissive ACIP recommendation for use of the HPV vaccine in males, but since this relatively weak endorsement was issued there have been new data demonstrating the vaccine’s effectiveness in preventing penile cancer, as well as a cost-effectiveness analysis.
Use of the herpes zoster vaccine in 50- to 59-year-olds isn’t recommended by the ACIP at this time. It’s an issue that’s likely to be raised at an upcoming meeting, according to Dr. Fischer.
Routine hepatitis B vaccination in adult diabetic patients as a means of preventing hepatitis outbreaks could come up for a vote as early as ACIP’s October meeting, he said.
Dr. Fischer said he had no relevant financial disclosures.
VAIL, COLO. – Earlier this year, the Advisory Committee on Immunization Practices took the unusual step of updating recommendations for the use of the meningococcal conjugate vaccines and Tdap vaccine that are at odds with the Food and Drug Administration–approved licensed indications, Dr. Marc Fisher said.
"It’s sort of unusual for ACIP to make off-label indications," Dr. Fischer observed in presenting an update from the Centers for Disease Control and Prevention’s ACIP at a conference on pediatric infectious diseases sponsored by Children’s Hospital Colorado.
The extraordinary step wasn’t taken lightly. The ACIP issued its off-label recommendations after careful deliberation in an attempt to address several public health problems related to shortcomings in vaccination coverage. These concerns have come to the fore only since licensure of the meningococcal conjugate and Tdap (tetanus toxoid with reduced diphtheria toxoid and acellular pertussis) vaccines.
The committee weighed new data supporting the safety and efficacy of the vaccines in populations that were not included in the licensed indications, explained Dr. Fischer, surveillance and epidemiology chief at the CDC’s Division of Vector-Borne Infectious Diseases in Ft. Collins, Colo. He serves as an ACIP adviser.
For example, the package insert for the MCV4-D meningococcal conjugate vaccine (Menactra) lists a history of Guillain-Barré syndrome as a contraindication to giving the vaccine. However, given the reassuring findings of two population-based studies totaling more than 10 million teens – data which became available only after the product labeling was created – the ACIP has concluded there is no increased risk of Guillain-Barré syndrome after vaccination with MCV4-D. Also, the latest results from the Vaccine Adverse Event Reporting System (VAERS) show no signal of a relationship between the meningococcal conjugate vaccine and Guillain-Barré syndrome.
"So if you’re following the package insert you wouldn’t give the vaccine to an 18-year-old headed off to college who has a history of [Guillain-Barré syndrome], and if you’re following the ACIP recommendation you ignore the history of [Guillain-Barré syndrome] and give the vaccine," Dr. Fischer explained.
Off-label ACIP recommendations for the Tdap vaccine published this year (MMWR 2011;60:13-5) after deliberations at the fall 2010 ACIP meeting include the following:
• Children aged 7-10 years who aren’t fully vaccinated against pertussis should receive a single dose of Tdap.
• Adults aged 65 or older who anticipate having close contact with an infant should get a single dose of Tdap.
• Tdap can now be given regardless of how long it has been since the last dose of a tetanus- or diphtheria-containing vaccine. Tdap is actually licensed for use only 5 years or longer after the last dose.
ACIP took these actions in order to improve pertussis vaccine coverage rates and create a coordinated strategy for preventing disease in young infants. Such a strategy is sorely needed. The incidence of pertussis is highest in children 1 month of age or younger. So is the number of deaths. Moreover, both the absolute number of deaths and the proportion of pertussis-related deaths occurring before age 1 month have increased in each of the past 3 decades. During 2000-2009, fully 78% of all pertussis-related deaths in the United States occurred in infants aged 1 month or younger. "We’re not really solving this problem of deaths in young infants," Dr. Fischer commented.
The new off-label Tdap recommendations address two major gaps in pertussis vaccine coverage stemming from the fact that no pertussis vaccine is licensed for use in children aged 7-10 years or in adults aged 65 and older, even though grandparents and other older adults are now believed to be one of the main sources of infection in young infants.
Filling these two gaps in coverage is a strategy known in immunization circles as cocooning. Cocooning is half of a dual ACIP approach aimed at preventing pertussis in young infants. The other half is embodied in an ACIP recommendation that ob.gyns. should routinely administer Tdap to pregnant women who haven’t previously received the vaccine. This should be done after 20 weeks’ gestation, or – as a second-best alternative –immediately post partum.
"This is a pretty big step, recommending a vaccine during pregnancy," the medical epidemiologist commented.
The committee was swayed in part by a cost-effectiveness analysis which estimated that maternal Tdap vaccination would cost $400,000 per quality-adjusted life-year saved. That’s threefold less than for postpartum maternal vaccination, and 13-fold less costly per quality-adjusted life-year saved than for a cocooning strategy involving vaccination of the child’s parents and grandparents.
ACIP recommended maternal vaccination during pregnancy despite some cautionary evidence that transplacental antibodies may blunt levels of active antibody produced when infants later undergo primary pertussis vaccination. The committee nonetheless decided to act because it’s unclear whether this blunted infant immune response to Tdap is actually clinically significant – and even if it is, the net effect could be to shift cases from the youngest infants to older infants, a group with less-severe disease and a lower case fatality rate. Still, the committee is looking forward to the guidance anticipated to come from an ongoing study of infants born to mothers who received Tdap or tetanus/diphtheria vaccine during their third trimester.
One of the barriers to more effective prevention of invasive meningococcal disease is lack of herd immunity because of insufficient coverage. Only 54% of U.S. 13- to 17-year-olds had received meningococcal vaccine in 2009.
Also, the vaccine’s protective effect appears to be considerably less durable than initially thought. There is mounting evidence of waning immunity by 2-5 years post vaccination. In sum, the evidence suggests that the ACIP’s recommendation at the time of Menactra’s licensure that vaccination routinely occur at the preventive care visit at age 11-12 years "maybe wasn’t the best decision" because it may not protect through the period of highest risk, Dr. Fischer said.
For this reason, this year ACIP has published revised recommendations for the use of meningococcal conjugate vaccines (Menveo and Menactra) in teens, based on a decision made at the fall 2010 ACIP meeting (MMWR 2011;60:72-6). Patients who receive the vaccine at age 11-12 years should get a booster dose at age 16, whereas those who get their first dose at age 13-15 years should have a booster dose at age 16-18. Those who get their first dose at age 16 or later don’t need a booster dose.
The currently available conjugate vaccines cover Neisseria meningitidis serogroups A, C, Y, and W135, but not serogroup B, which predominates among infants. Developing a conjugate vaccine that protects against serogroup B is a high priority. Initial efforts failed because candidate polysaccharide vaccines had molecular mimicry issues predisposing to adverse events. However, several non-polysaccharide vaccines that sidestep such concerns are now in late-stage clinical trials and – if the data prove positive – could be licensed in the next couple of years, according to Dr. Fischer.
Issues of particular interest to primary care physicians that the ACIP is likely to take under consideration within the next year include routine meningococcal conjugate vaccine for infants, hepatitis B vaccine for diabetic adults, and pneumococcal conjugate vaccine for adults, according to Dr. Fischer.
Two additional meningococcal conjugate vaccines are now under Food and Drug Administration review and are likely to be licensed by the end of the year. "I think ACIP is likely to reconsider the issue of routine meningococcal conjugate vaccine for infants in October, with a possible vote on the matter in February," he predicted.
A pneumococcal conjugate vaccine for adults is also likely to be licensed in the coming months, he said. That’s another vaccine the ACIP will consider recommending.
Also on tap for ACIP consideration is the use of human papillomavirus vaccine in males. There’s already a permissive ACIP recommendation for use of the HPV vaccine in males, but since this relatively weak endorsement was issued there have been new data demonstrating the vaccine’s effectiveness in preventing penile cancer, as well as a cost-effectiveness analysis.
Use of the herpes zoster vaccine in 50- to 59-year-olds isn’t recommended by the ACIP at this time. It’s an issue that’s likely to be raised at an upcoming meeting, according to Dr. Fischer.
Routine hepatitis B vaccination in adult diabetic patients as a means of preventing hepatitis outbreaks could come up for a vote as early as ACIP’s October meeting, he said.
Dr. Fischer said he had no relevant financial disclosures.
EXPERT ANALYSIS FROM A CONFERENCE ON PEDIATRIC INFECTIOUS DISEASES SPONSORED BY CHILDREN'S HOSPITAL COLORADO
New Latent TB Therapy Is on the Horizon
VAIL, COLO. – Results of a landmark study of a far simpler and more convenient treatment regimen for latent tuberculosis infection are expected to transform clinical pediatric practice in relatively short order, Dr. John W. Ogle said.
The Centers for Disease Control and Prevention–sponsored PREVENT TB trial included 8,053 participants with latent TB, of whom nearly 1,000 were children older than age 2 years. Participants were randomized to either the standard treatment regimen of daily self-administered isoniazid for 9 months, or a novel regimen consisting of directly observed therapy with isoniazid (INH) and rifapentine (INN) once-weekly for 3 months. With 33 months of follow-up, the new regimen was as effective in preventing cases of active TB as was the standard regimen. However, the once-weekly therapy had significantly better adherence and fewer side effects, which was not surprising given that it entailed only 12 total doses of medication, compared with 270 doses in the standard regimen.
The soon-to-be-published study was presented earlier this year in Denver at a meeting of the American Thoracic Society. The CDC has called the PREVENT TB findings "one of the most significant advances in TB research in decades." An expert panel has been convened to analyze the data and begin working on new U.S. treatment guidelines.
The shorter-course regimen appeared to be as effective as standard therapy in children as well as in adults. The increased rate of hepatitis and other isoniazid-related side effects that is seen with standard therapy was more striking in adults than in children, but that’s to be expected because adults typically have more toxicities than do children, Dr. Ogle observed at a conference on pediatric infectious diseases sponsored by Children’s Hospital Colorado.
"It’s very likely that we’re going to be urged to adopt this INH/INN regimen as routine therapy because it’s so much easier," predicted Dr. Ogle, professor of pediatrics at the University of Colorado at Denver and director of pediatric services at Denver Health Medical Center.
That being said, he urged his pediatric colleagues to remain vigilant as they adopt the anticipated new guidelines.
"When this regimen is broadened to a bigger population, we’ll undoubtedly learn new things. That’s what always happens when you take a moderately or even a big-powered study and adopt it in another population. No one knows the incidence of side effects in the general population. We’ve seen a couple of kids who’ve had rifapentine reactions," Dr. Ogle said.
As an aside, Dr. Ogle noted that he hasn’t prescribed liquid isoniazid in more than 10 years. "I’ve found it to be a reliable way to give a child diarrhea. We’ll crush the tablets instead, even down to a 1-month-old infant," he said.
Dr. Ogle declared having no relevant financial disclosures.
VAIL, COLO. – Results of a landmark study of a far simpler and more convenient treatment regimen for latent tuberculosis infection are expected to transform clinical pediatric practice in relatively short order, Dr. John W. Ogle said.
The Centers for Disease Control and Prevention–sponsored PREVENT TB trial included 8,053 participants with latent TB, of whom nearly 1,000 were children older than age 2 years. Participants were randomized to either the standard treatment regimen of daily self-administered isoniazid for 9 months, or a novel regimen consisting of directly observed therapy with isoniazid (INH) and rifapentine (INN) once-weekly for 3 months. With 33 months of follow-up, the new regimen was as effective in preventing cases of active TB as was the standard regimen. However, the once-weekly therapy had significantly better adherence and fewer side effects, which was not surprising given that it entailed only 12 total doses of medication, compared with 270 doses in the standard regimen.
The soon-to-be-published study was presented earlier this year in Denver at a meeting of the American Thoracic Society. The CDC has called the PREVENT TB findings "one of the most significant advances in TB research in decades." An expert panel has been convened to analyze the data and begin working on new U.S. treatment guidelines.
The shorter-course regimen appeared to be as effective as standard therapy in children as well as in adults. The increased rate of hepatitis and other isoniazid-related side effects that is seen with standard therapy was more striking in adults than in children, but that’s to be expected because adults typically have more toxicities than do children, Dr. Ogle observed at a conference on pediatric infectious diseases sponsored by Children’s Hospital Colorado.
"It’s very likely that we’re going to be urged to adopt this INH/INN regimen as routine therapy because it’s so much easier," predicted Dr. Ogle, professor of pediatrics at the University of Colorado at Denver and director of pediatric services at Denver Health Medical Center.
That being said, he urged his pediatric colleagues to remain vigilant as they adopt the anticipated new guidelines.
"When this regimen is broadened to a bigger population, we’ll undoubtedly learn new things. That’s what always happens when you take a moderately or even a big-powered study and adopt it in another population. No one knows the incidence of side effects in the general population. We’ve seen a couple of kids who’ve had rifapentine reactions," Dr. Ogle said.
As an aside, Dr. Ogle noted that he hasn’t prescribed liquid isoniazid in more than 10 years. "I’ve found it to be a reliable way to give a child diarrhea. We’ll crush the tablets instead, even down to a 1-month-old infant," he said.
Dr. Ogle declared having no relevant financial disclosures.
VAIL, COLO. – Results of a landmark study of a far simpler and more convenient treatment regimen for latent tuberculosis infection are expected to transform clinical pediatric practice in relatively short order, Dr. John W. Ogle said.
The Centers for Disease Control and Prevention–sponsored PREVENT TB trial included 8,053 participants with latent TB, of whom nearly 1,000 were children older than age 2 years. Participants were randomized to either the standard treatment regimen of daily self-administered isoniazid for 9 months, or a novel regimen consisting of directly observed therapy with isoniazid (INH) and rifapentine (INN) once-weekly for 3 months. With 33 months of follow-up, the new regimen was as effective in preventing cases of active TB as was the standard regimen. However, the once-weekly therapy had significantly better adherence and fewer side effects, which was not surprising given that it entailed only 12 total doses of medication, compared with 270 doses in the standard regimen.
The soon-to-be-published study was presented earlier this year in Denver at a meeting of the American Thoracic Society. The CDC has called the PREVENT TB findings "one of the most significant advances in TB research in decades." An expert panel has been convened to analyze the data and begin working on new U.S. treatment guidelines.
The shorter-course regimen appeared to be as effective as standard therapy in children as well as in adults. The increased rate of hepatitis and other isoniazid-related side effects that is seen with standard therapy was more striking in adults than in children, but that’s to be expected because adults typically have more toxicities than do children, Dr. Ogle observed at a conference on pediatric infectious diseases sponsored by Children’s Hospital Colorado.
"It’s very likely that we’re going to be urged to adopt this INH/INN regimen as routine therapy because it’s so much easier," predicted Dr. Ogle, professor of pediatrics at the University of Colorado at Denver and director of pediatric services at Denver Health Medical Center.
That being said, he urged his pediatric colleagues to remain vigilant as they adopt the anticipated new guidelines.
"When this regimen is broadened to a bigger population, we’ll undoubtedly learn new things. That’s what always happens when you take a moderately or even a big-powered study and adopt it in another population. No one knows the incidence of side effects in the general population. We’ve seen a couple of kids who’ve had rifapentine reactions," Dr. Ogle said.
As an aside, Dr. Ogle noted that he hasn’t prescribed liquid isoniazid in more than 10 years. "I’ve found it to be a reliable way to give a child diarrhea. We’ll crush the tablets instead, even down to a 1-month-old infant," he said.
Dr. Ogle declared having no relevant financial disclosures.
EXPERT ANALYSIS FROM A CONFERENCE ON PEDIATRIC INFECTIOUS DISEASES SPONSORED BY CHILDREN'S HOSPITAL COLORADO
Incise, Drain for Most MRSA Skin Infections
VAIL, COLO. – Current broadly endorsed guidelines for outpatient management of skin and soft tissue infections in an era of rampant community-acquired methicillin-resistant Staphylococcus aureus emphasize incision and drainage without routine antibiotic coverage.
"In general, for a boil or abscess or pimple that a parent is complaining about, if you drain it, that’s good enough. Patients don’t need antibiotics unless they’re presenting with more severe systemic signs of infection," Dr. James K. Todd explained at a conference on pediatric infectious diseases, which was sponsored by Children’s Hospital Colorado in Aurora.
The guidelines were jointly developed by the American Medical Association, the Centers for Disease Control and Prevention, and the Infectious Disease Society of America in 2007. The underlying strategy is to avoid unnecessary prescribing of antibiotics, with all of its attendant problems.
The effectiveness of this guideline-recommended, drainage-only approach was underscored in a recent randomized, controlled trial that was published after the guidelines release. The study involved 200 pediatric patients with uncomplicated skin infections, 69% of whom had methicillin-resistant S. aureus (MRSA) cultured from their wounds. All participants underwent drainage of their focal infection and were randomized to 7 days of clindamycin or cephalexin. Clindamycin (Cleocin) is effective against MRSA; cephalexin (Keflex) is not.
Complete resolution of the skin infection was observed by day 7 in 94% of those who received clindamycin and similarly in 97% of patients in the cephalexin arm (Pediatrics 2011;127:e573-80).
"This study shows that there’s no need for antibiotics because Keflex is not effective for MRSA," commented Dr. Todd, professor of pediatrics, microbiology, and epidemiology at the University of Colorado at Denver.
The abscess is the sine qua non or "essential element" of S. aureus infections. The organism has an affinity for injured tissue, and it likes to localize. These focal infections must be drained in order to expose S. aureus harbors.
"As Johnnie Cochran might have said, ‘Antibiotics contain, but you still have to drain.’ Keep that in mind," Dr. Todd added.
The guidelines recommend incision and drainage of purulent skin infections, with the aspirated pus sent off for culture and susceptibility testing. Supplemental antimicrobial treatment with coverage for MRSA is to be considered only in patients who have systemic or quite severe local symptoms, who are immunosuppressed, or whose infection isn’t responsive to drainage.
On the other hand, the guidelines note, in cases of possible cellulitis without abscess, then group A streptococcus infection becomes a consideration and antimicrobial coverage for that pathogen is appropriate. Clindamycin is a good choice because it is effective against both group A streptococcus and MRSA, Dr. Todd continued.
In confabbing with other pediatric infectious disease specialists, he said the shared anecdotal experience has been that the kids who develop severe MRSA infections almost never previously came in for the occasional skin infection with MRSA.
"What this implies is that when you get exposed to MRSA, even though we know that there are some more virulent strains, there are also different host responses, so if you get buttock lesions initially that’s probably all you’re going to get. You’re not going to go on to develop a necrotizing pneumonia or septic thrombophlebitis," according to Dr. Todd. "I think there’s some relief in the idea you can be patient with kids with recurrent boils, even though the mother is going crazy about it. You can recommend simple measures, and in time the problem is going to go away, and it’s not likely to be going to cause serious complications."
He’s not a fan of MRSA carriage eradication regimens. Like cockroaches, MRSA is very difficult to get rid of. Most attempts at eradication fail, with recolonization occurring by about 6 months. A Cochrane review found no evidence that any antimicrobial regimen for eradication is better than placebo (Cochrane Database Syst. Rev. 2003 [doi:10.1002/14651858.CD003340]).
Nevertheless, he’ll consider an attempt at eradication in patients who’ve had three or more episodes of skin or soft-tissue infection, those who have multiple family members with recurrent MRSA disease, and patients who have not responded to hygienic measures and bleach baths.
"Most methods really don’t work in the long term, but we might recommend considering an attempt at eradication in those situations, while recognizing that we’re really just buying time until the MRSA goes away on its own," Dr. Todd said.
Dr. Todd said that he had no relevant financial disclosures.
VAIL, COLO. – Current broadly endorsed guidelines for outpatient management of skin and soft tissue infections in an era of rampant community-acquired methicillin-resistant Staphylococcus aureus emphasize incision and drainage without routine antibiotic coverage.
"In general, for a boil or abscess or pimple that a parent is complaining about, if you drain it, that’s good enough. Patients don’t need antibiotics unless they’re presenting with more severe systemic signs of infection," Dr. James K. Todd explained at a conference on pediatric infectious diseases, which was sponsored by Children’s Hospital Colorado in Aurora.
The guidelines were jointly developed by the American Medical Association, the Centers for Disease Control and Prevention, and the Infectious Disease Society of America in 2007. The underlying strategy is to avoid unnecessary prescribing of antibiotics, with all of its attendant problems.
The effectiveness of this guideline-recommended, drainage-only approach was underscored in a recent randomized, controlled trial that was published after the guidelines release. The study involved 200 pediatric patients with uncomplicated skin infections, 69% of whom had methicillin-resistant S. aureus (MRSA) cultured from their wounds. All participants underwent drainage of their focal infection and were randomized to 7 days of clindamycin or cephalexin. Clindamycin (Cleocin) is effective against MRSA; cephalexin (Keflex) is not.
Complete resolution of the skin infection was observed by day 7 in 94% of those who received clindamycin and similarly in 97% of patients in the cephalexin arm (Pediatrics 2011;127:e573-80).
"This study shows that there’s no need for antibiotics because Keflex is not effective for MRSA," commented Dr. Todd, professor of pediatrics, microbiology, and epidemiology at the University of Colorado at Denver.
The abscess is the sine qua non or "essential element" of S. aureus infections. The organism has an affinity for injured tissue, and it likes to localize. These focal infections must be drained in order to expose S. aureus harbors.
"As Johnnie Cochran might have said, ‘Antibiotics contain, but you still have to drain.’ Keep that in mind," Dr. Todd added.
The guidelines recommend incision and drainage of purulent skin infections, with the aspirated pus sent off for culture and susceptibility testing. Supplemental antimicrobial treatment with coverage for MRSA is to be considered only in patients who have systemic or quite severe local symptoms, who are immunosuppressed, or whose infection isn’t responsive to drainage.
On the other hand, the guidelines note, in cases of possible cellulitis without abscess, then group A streptococcus infection becomes a consideration and antimicrobial coverage for that pathogen is appropriate. Clindamycin is a good choice because it is effective against both group A streptococcus and MRSA, Dr. Todd continued.
In confabbing with other pediatric infectious disease specialists, he said the shared anecdotal experience has been that the kids who develop severe MRSA infections almost never previously came in for the occasional skin infection with MRSA.
"What this implies is that when you get exposed to MRSA, even though we know that there are some more virulent strains, there are also different host responses, so if you get buttock lesions initially that’s probably all you’re going to get. You’re not going to go on to develop a necrotizing pneumonia or septic thrombophlebitis," according to Dr. Todd. "I think there’s some relief in the idea you can be patient with kids with recurrent boils, even though the mother is going crazy about it. You can recommend simple measures, and in time the problem is going to go away, and it’s not likely to be going to cause serious complications."
He’s not a fan of MRSA carriage eradication regimens. Like cockroaches, MRSA is very difficult to get rid of. Most attempts at eradication fail, with recolonization occurring by about 6 months. A Cochrane review found no evidence that any antimicrobial regimen for eradication is better than placebo (Cochrane Database Syst. Rev. 2003 [doi:10.1002/14651858.CD003340]).
Nevertheless, he’ll consider an attempt at eradication in patients who’ve had three or more episodes of skin or soft-tissue infection, those who have multiple family members with recurrent MRSA disease, and patients who have not responded to hygienic measures and bleach baths.
"Most methods really don’t work in the long term, but we might recommend considering an attempt at eradication in those situations, while recognizing that we’re really just buying time until the MRSA goes away on its own," Dr. Todd said.
Dr. Todd said that he had no relevant financial disclosures.
VAIL, COLO. – Current broadly endorsed guidelines for outpatient management of skin and soft tissue infections in an era of rampant community-acquired methicillin-resistant Staphylococcus aureus emphasize incision and drainage without routine antibiotic coverage.
"In general, for a boil or abscess or pimple that a parent is complaining about, if you drain it, that’s good enough. Patients don’t need antibiotics unless they’re presenting with more severe systemic signs of infection," Dr. James K. Todd explained at a conference on pediatric infectious diseases, which was sponsored by Children’s Hospital Colorado in Aurora.
The guidelines were jointly developed by the American Medical Association, the Centers for Disease Control and Prevention, and the Infectious Disease Society of America in 2007. The underlying strategy is to avoid unnecessary prescribing of antibiotics, with all of its attendant problems.
The effectiveness of this guideline-recommended, drainage-only approach was underscored in a recent randomized, controlled trial that was published after the guidelines release. The study involved 200 pediatric patients with uncomplicated skin infections, 69% of whom had methicillin-resistant S. aureus (MRSA) cultured from their wounds. All participants underwent drainage of their focal infection and were randomized to 7 days of clindamycin or cephalexin. Clindamycin (Cleocin) is effective against MRSA; cephalexin (Keflex) is not.
Complete resolution of the skin infection was observed by day 7 in 94% of those who received clindamycin and similarly in 97% of patients in the cephalexin arm (Pediatrics 2011;127:e573-80).
"This study shows that there’s no need for antibiotics because Keflex is not effective for MRSA," commented Dr. Todd, professor of pediatrics, microbiology, and epidemiology at the University of Colorado at Denver.
The abscess is the sine qua non or "essential element" of S. aureus infections. The organism has an affinity for injured tissue, and it likes to localize. These focal infections must be drained in order to expose S. aureus harbors.
"As Johnnie Cochran might have said, ‘Antibiotics contain, but you still have to drain.’ Keep that in mind," Dr. Todd added.
The guidelines recommend incision and drainage of purulent skin infections, with the aspirated pus sent off for culture and susceptibility testing. Supplemental antimicrobial treatment with coverage for MRSA is to be considered only in patients who have systemic or quite severe local symptoms, who are immunosuppressed, or whose infection isn’t responsive to drainage.
On the other hand, the guidelines note, in cases of possible cellulitis without abscess, then group A streptococcus infection becomes a consideration and antimicrobial coverage for that pathogen is appropriate. Clindamycin is a good choice because it is effective against both group A streptococcus and MRSA, Dr. Todd continued.
In confabbing with other pediatric infectious disease specialists, he said the shared anecdotal experience has been that the kids who develop severe MRSA infections almost never previously came in for the occasional skin infection with MRSA.
"What this implies is that when you get exposed to MRSA, even though we know that there are some more virulent strains, there are also different host responses, so if you get buttock lesions initially that’s probably all you’re going to get. You’re not going to go on to develop a necrotizing pneumonia or septic thrombophlebitis," according to Dr. Todd. "I think there’s some relief in the idea you can be patient with kids with recurrent boils, even though the mother is going crazy about it. You can recommend simple measures, and in time the problem is going to go away, and it’s not likely to be going to cause serious complications."
He’s not a fan of MRSA carriage eradication regimens. Like cockroaches, MRSA is very difficult to get rid of. Most attempts at eradication fail, with recolonization occurring by about 6 months. A Cochrane review found no evidence that any antimicrobial regimen for eradication is better than placebo (Cochrane Database Syst. Rev. 2003 [doi:10.1002/14651858.CD003340]).
Nevertheless, he’ll consider an attempt at eradication in patients who’ve had three or more episodes of skin or soft-tissue infection, those who have multiple family members with recurrent MRSA disease, and patients who have not responded to hygienic measures and bleach baths.
"Most methods really don’t work in the long term, but we might recommend considering an attempt at eradication in those situations, while recognizing that we’re really just buying time until the MRSA goes away on its own," Dr. Todd said.
Dr. Todd said that he had no relevant financial disclosures.
EXPERT ANALYSIS FROM A CONFERENCE ON PEDIATRIC INFECTIOUS DISEASES
S. Aureus Vaccine: Top Priority, Big Obstacles
VAIL, COLO. – Don’t hold your breath waiting for a working Staphylococcus aureus vaccine.
Development of a successful S. aureus vaccine is a high research priority. After all, it’s estimated that more people in the United States now die annually of methicillin-resistant S. aureus than of AIDS, according to data from the Centers for Disease Control and Prevention. Pharmaceutical giants including Merck, Pfizer, and Novartis, as well as smallish biotech companies, are in the hunt for an S. aureus vaccine. But S. aureus is proving to be an elusive target.
"When you think about the bugs that we’ve been successful in developing vaccines to, they generally have a single main virulence factor that you can target: either a capsule, like Haemophilus influenzae, Streptococcus pneumoniae, or Neisseria meningitis, or a toxin, as in tetanus or diphtheria. But S. aureus has many strains with multiple virulence factors," Dr. James K. Todd explained at a conference on pediatric infectious diseases, which was sponsored by Children’s Hospital Colorado, Aurora.
What this means is that conventional active immunization strategies that zero in on a single target are unlikely to be successful in the case of S. aureus. Following the failure in a phase III trial of Nabi Biopharmaceutical’s StaphVax vaccine targeting capsular polysaccharides types 5 and 8 (Curr. Opin. Investig. Drugs 2002;3:48-50), the research thrust has shifted from the single-target approach to the development of vaccines that address composite targets encompassing multiple virulence factors.
"You’re going to have to come up with a polyvalent vaccine with a large combination of antigens if you want to successfully deal with Staphylococcus aureus infections. It’s a very time-consuming and expensive process," cautioned Dr. Todd, professor of pediatrics, microbiology, and epidemiology at the University of Colorado at Denver.
At least five such vaccines addressing composite targets in an active immunization strategy are in early stages of the developmental pipeline.
Also being aggressively pursued is a strategy involving passive immunization using therapeutic antibodies that are specifically targeted against toxins with an established role in S. aureus virulence. Again, candidate passive immunization vaccines targeting a single virulence factor have not panned out to date. Two such products have flopped in phase II trials, although roughly another half-dozen, single-target, passive immunization vaccines are in preclinical development.
Here again, the latest thinking is that passive immunization against a cocktail of virulence determinants, not just one, is likely to be necessary. The tough decision for vaccine developers is which virulence factors to include.
The appeal of passive immunization is that it’s designed to directly bottle up virulence factors and render them powerless. In contrast, active immunization aims to enhance uptake of S. aureus by white blood cells through the process of opsonization. This may be a losing proposition, in light of the nimble microorganism’s numerous means of avoiding death via phagocytosis.
For example, the highly problematic PVL (Panton-Valentine leukocidin) virulence factor, which is associated with severe invasive S. aureus disease, including pneumonia and necrotizing fasciitis, enables PVL-containing strains of S. aureus to lyse and kill WBCs that have successfully surrounded the pathogen.
"We’re going to have to deal with what goes on once the bug is inside the white blood cell, and that’s much more difficult to do with a vaccine," according to Dr. Todd.
Assuming an effective S. aureus vaccine can be developed, how might it be used? Depending upon the vaccine’s properties, it might be employed in patients who are at high risk of infection, such as those on dialysis, or patients undergoing surgery, or those with chronic diseases, or patients with a lengthy hospital stay. Health care workers might be immunized to prevent nosocomial spread. In community practice, a vaccine might be utilized to decolonize chronic carriers of S. aureus or to curb outbreaks in athletic teams.
Dr. Todd is credited with being first to describe S. aureus toxic shock syndrome. He noted that other S. aureus experts share his view that a S. aureus vaccine development faces a rocky road. For example, in a recent review, Michael Otto, Ph.D., senior investigator at the National Institute of Allergy and Infectious Diseases, observed that attempts to create an effective S. aureus vaccine have been going on for nearly a century, without success.
"The many mechanisms by which S. aureus can evade elimination by phagocytes after ingestion may help to explain why S. aureus vaccines have failed and antistaphylococcal antibodies present in many people are not protective," observed Dr. Otto (Expert Opin. Biol. Ther. 2010;10:1049-59).
Dr. Mary P. Glodé declared that a successful S. aureus vaccine is near the top of her wish list for the next 5-10 years. "There’s a true need for a S. aureus vaccine to prevent serious disease. If we look at the positive blood cultures at our hospital, it’s S. aureus leading the list, certainly for bone and joint infections, but also for bacteremia and pneumonia," according to Dr. Glodé, professor of pediatrics and head of the section of infectious disease at the University of Colorado at Denver.
Dr. Todd declared that he had no relevant financial disclosures.
VAIL, COLO. – Don’t hold your breath waiting for a working Staphylococcus aureus vaccine.
Development of a successful S. aureus vaccine is a high research priority. After all, it’s estimated that more people in the United States now die annually of methicillin-resistant S. aureus than of AIDS, according to data from the Centers for Disease Control and Prevention. Pharmaceutical giants including Merck, Pfizer, and Novartis, as well as smallish biotech companies, are in the hunt for an S. aureus vaccine. But S. aureus is proving to be an elusive target.
"When you think about the bugs that we’ve been successful in developing vaccines to, they generally have a single main virulence factor that you can target: either a capsule, like Haemophilus influenzae, Streptococcus pneumoniae, or Neisseria meningitis, or a toxin, as in tetanus or diphtheria. But S. aureus has many strains with multiple virulence factors," Dr. James K. Todd explained at a conference on pediatric infectious diseases, which was sponsored by Children’s Hospital Colorado, Aurora.
What this means is that conventional active immunization strategies that zero in on a single target are unlikely to be successful in the case of S. aureus. Following the failure in a phase III trial of Nabi Biopharmaceutical’s StaphVax vaccine targeting capsular polysaccharides types 5 and 8 (Curr. Opin. Investig. Drugs 2002;3:48-50), the research thrust has shifted from the single-target approach to the development of vaccines that address composite targets encompassing multiple virulence factors.
"You’re going to have to come up with a polyvalent vaccine with a large combination of antigens if you want to successfully deal with Staphylococcus aureus infections. It’s a very time-consuming and expensive process," cautioned Dr. Todd, professor of pediatrics, microbiology, and epidemiology at the University of Colorado at Denver.
At least five such vaccines addressing composite targets in an active immunization strategy are in early stages of the developmental pipeline.
Also being aggressively pursued is a strategy involving passive immunization using therapeutic antibodies that are specifically targeted against toxins with an established role in S. aureus virulence. Again, candidate passive immunization vaccines targeting a single virulence factor have not panned out to date. Two such products have flopped in phase II trials, although roughly another half-dozen, single-target, passive immunization vaccines are in preclinical development.
Here again, the latest thinking is that passive immunization against a cocktail of virulence determinants, not just one, is likely to be necessary. The tough decision for vaccine developers is which virulence factors to include.
The appeal of passive immunization is that it’s designed to directly bottle up virulence factors and render them powerless. In contrast, active immunization aims to enhance uptake of S. aureus by white blood cells through the process of opsonization. This may be a losing proposition, in light of the nimble microorganism’s numerous means of avoiding death via phagocytosis.
For example, the highly problematic PVL (Panton-Valentine leukocidin) virulence factor, which is associated with severe invasive S. aureus disease, including pneumonia and necrotizing fasciitis, enables PVL-containing strains of S. aureus to lyse and kill WBCs that have successfully surrounded the pathogen.
"We’re going to have to deal with what goes on once the bug is inside the white blood cell, and that’s much more difficult to do with a vaccine," according to Dr. Todd.
Assuming an effective S. aureus vaccine can be developed, how might it be used? Depending upon the vaccine’s properties, it might be employed in patients who are at high risk of infection, such as those on dialysis, or patients undergoing surgery, or those with chronic diseases, or patients with a lengthy hospital stay. Health care workers might be immunized to prevent nosocomial spread. In community practice, a vaccine might be utilized to decolonize chronic carriers of S. aureus or to curb outbreaks in athletic teams.
Dr. Todd is credited with being first to describe S. aureus toxic shock syndrome. He noted that other S. aureus experts share his view that a S. aureus vaccine development faces a rocky road. For example, in a recent review, Michael Otto, Ph.D., senior investigator at the National Institute of Allergy and Infectious Diseases, observed that attempts to create an effective S. aureus vaccine have been going on for nearly a century, without success.
"The many mechanisms by which S. aureus can evade elimination by phagocytes after ingestion may help to explain why S. aureus vaccines have failed and antistaphylococcal antibodies present in many people are not protective," observed Dr. Otto (Expert Opin. Biol. Ther. 2010;10:1049-59).
Dr. Mary P. Glodé declared that a successful S. aureus vaccine is near the top of her wish list for the next 5-10 years. "There’s a true need for a S. aureus vaccine to prevent serious disease. If we look at the positive blood cultures at our hospital, it’s S. aureus leading the list, certainly for bone and joint infections, but also for bacteremia and pneumonia," according to Dr. Glodé, professor of pediatrics and head of the section of infectious disease at the University of Colorado at Denver.
Dr. Todd declared that he had no relevant financial disclosures.
VAIL, COLO. – Don’t hold your breath waiting for a working Staphylococcus aureus vaccine.
Development of a successful S. aureus vaccine is a high research priority. After all, it’s estimated that more people in the United States now die annually of methicillin-resistant S. aureus than of AIDS, according to data from the Centers for Disease Control and Prevention. Pharmaceutical giants including Merck, Pfizer, and Novartis, as well as smallish biotech companies, are in the hunt for an S. aureus vaccine. But S. aureus is proving to be an elusive target.
"When you think about the bugs that we’ve been successful in developing vaccines to, they generally have a single main virulence factor that you can target: either a capsule, like Haemophilus influenzae, Streptococcus pneumoniae, or Neisseria meningitis, or a toxin, as in tetanus or diphtheria. But S. aureus has many strains with multiple virulence factors," Dr. James K. Todd explained at a conference on pediatric infectious diseases, which was sponsored by Children’s Hospital Colorado, Aurora.
What this means is that conventional active immunization strategies that zero in on a single target are unlikely to be successful in the case of S. aureus. Following the failure in a phase III trial of Nabi Biopharmaceutical’s StaphVax vaccine targeting capsular polysaccharides types 5 and 8 (Curr. Opin. Investig. Drugs 2002;3:48-50), the research thrust has shifted from the single-target approach to the development of vaccines that address composite targets encompassing multiple virulence factors.
"You’re going to have to come up with a polyvalent vaccine with a large combination of antigens if you want to successfully deal with Staphylococcus aureus infections. It’s a very time-consuming and expensive process," cautioned Dr. Todd, professor of pediatrics, microbiology, and epidemiology at the University of Colorado at Denver.
At least five such vaccines addressing composite targets in an active immunization strategy are in early stages of the developmental pipeline.
Also being aggressively pursued is a strategy involving passive immunization using therapeutic antibodies that are specifically targeted against toxins with an established role in S. aureus virulence. Again, candidate passive immunization vaccines targeting a single virulence factor have not panned out to date. Two such products have flopped in phase II trials, although roughly another half-dozen, single-target, passive immunization vaccines are in preclinical development.
Here again, the latest thinking is that passive immunization against a cocktail of virulence determinants, not just one, is likely to be necessary. The tough decision for vaccine developers is which virulence factors to include.
The appeal of passive immunization is that it’s designed to directly bottle up virulence factors and render them powerless. In contrast, active immunization aims to enhance uptake of S. aureus by white blood cells through the process of opsonization. This may be a losing proposition, in light of the nimble microorganism’s numerous means of avoiding death via phagocytosis.
For example, the highly problematic PVL (Panton-Valentine leukocidin) virulence factor, which is associated with severe invasive S. aureus disease, including pneumonia and necrotizing fasciitis, enables PVL-containing strains of S. aureus to lyse and kill WBCs that have successfully surrounded the pathogen.
"We’re going to have to deal with what goes on once the bug is inside the white blood cell, and that’s much more difficult to do with a vaccine," according to Dr. Todd.
Assuming an effective S. aureus vaccine can be developed, how might it be used? Depending upon the vaccine’s properties, it might be employed in patients who are at high risk of infection, such as those on dialysis, or patients undergoing surgery, or those with chronic diseases, or patients with a lengthy hospital stay. Health care workers might be immunized to prevent nosocomial spread. In community practice, a vaccine might be utilized to decolonize chronic carriers of S. aureus or to curb outbreaks in athletic teams.
Dr. Todd is credited with being first to describe S. aureus toxic shock syndrome. He noted that other S. aureus experts share his view that a S. aureus vaccine development faces a rocky road. For example, in a recent review, Michael Otto, Ph.D., senior investigator at the National Institute of Allergy and Infectious Diseases, observed that attempts to create an effective S. aureus vaccine have been going on for nearly a century, without success.
"The many mechanisms by which S. aureus can evade elimination by phagocytes after ingestion may help to explain why S. aureus vaccines have failed and antistaphylococcal antibodies present in many people are not protective," observed Dr. Otto (Expert Opin. Biol. Ther. 2010;10:1049-59).
Dr. Mary P. Glodé declared that a successful S. aureus vaccine is near the top of her wish list for the next 5-10 years. "There’s a true need for a S. aureus vaccine to prevent serious disease. If we look at the positive blood cultures at our hospital, it’s S. aureus leading the list, certainly for bone and joint infections, but also for bacteremia and pneumonia," according to Dr. Glodé, professor of pediatrics and head of the section of infectious disease at the University of Colorado at Denver.
Dr. Todd declared that he had no relevant financial disclosures.
EXPERT ANALYSIS FROM A CONFERENCE ON PEDIATRIC INFECTIOUS DISEASES