User login
Society for Pediatric Dermatology (SPD): Annual Meeting
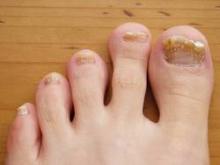
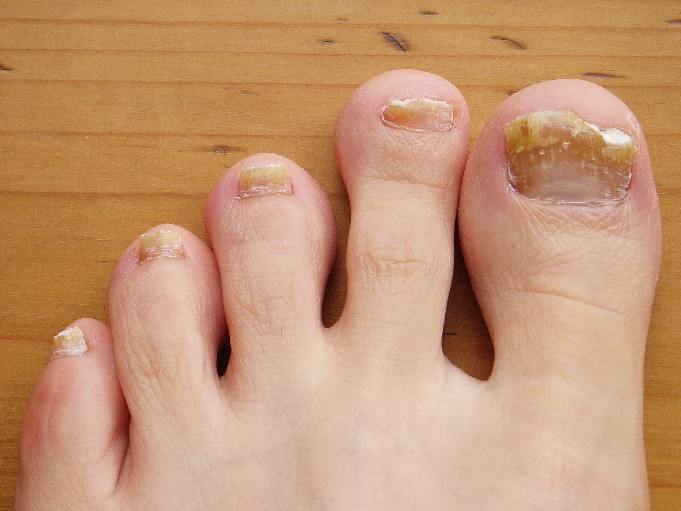
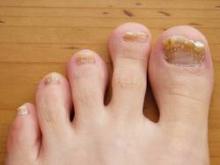
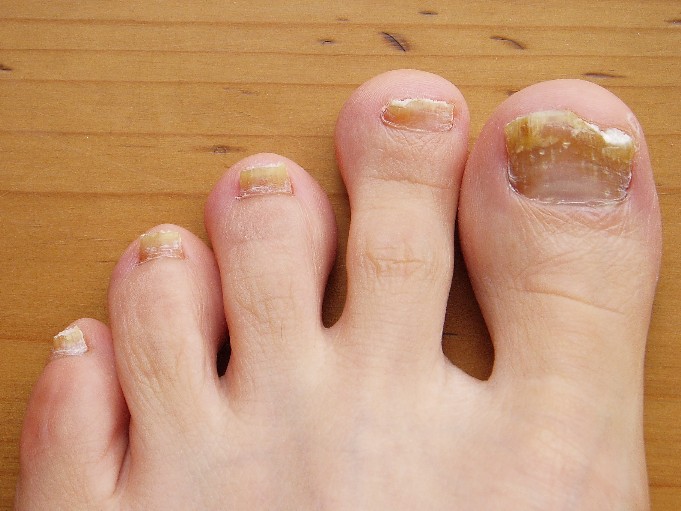
Onychomycosis: Not Just For Adults
COEUR D’ALENE, IDAHO – Conventional wisdom holds that onychomycosis is rare in children. Not so.
Fully one-third of children and adolescents who presented with a nail complaint to a prominent dermatologic nail disorders center were diagnosed with mycologically confirmed onychomycosis, Dr. Julie Jefferson reported at the annual meeting of the Society for Pediatric Dermatology.
That’s a substantially higher prevalence than the 15.5% figure reported by Spanish investigators in a 20-year retrospective study (Mycoses 2011;54:450-3). It’s also well below the 47% prevalence recently reported in Denver in a 5-year retrospective study, where Trychophyton rubrum was the most common pathogen, and the highest prevalence of onychomycosis in the pediatric population was seen in 6- to 10-year-olds (Pediatr. Dermatol. 2014;31:106-8), noted Dr. Jefferson of Johns Hopkins University, Baltimore.
The investigators in both Spain and Denver observed that the prevalence of pediatric onychomycosis appears to be increasing in recent years.
Dr. Jefferson presented a retrospective study that included 917 patients up to age 18 years who presented to the Oregon Dermatology and Research Center, Portland, in a recent 6-year period. One or more nail disorders were diagnosed in 11%. The mean age at presentation was 9.4 years, with a mean 2.4-year duration of the condition prior to presentation. Toenails were affected in 47 patients, fingernails in 37, and both in 18. Fourteen patients had two nail disorders, 9 had three, and 2 had four distinct nail disorders.
The etiologies ranged widely, from infections to congenital and hereditary malformations, tumors, inflammatory processes, and systemic diseases.
The most common nail condition was onychomycosis, diagnosed in 34 patients. Thus, 3.7% of all pediatric patients presenting to the dermatology center for any reason were diagnosed with onychomycosis, a higher rate than previously reported by others.
Other conditions included 13 cases of longitudinal melanonychia, 11 of disappearing nail bed, 9 of retronychia, 8 cases of congenital malalignment, 8 cases of trachyonychia, 7 of paronychia, 5 cases of psoriasis, and 2 of lichen planus.
Three of four patients with an ingrown toenail also had congenital malalignment of the affected great toenail, supporting the notion put forth by other investigators that congenital malalignment of the great toenail predisposes to ingrown toenails, according to Dr. Jefferson.
She reported having no financial conflicts related to this study.
COEUR D’ALENE, IDAHO – Conventional wisdom holds that onychomycosis is rare in children. Not so.
Fully one-third of children and adolescents who presented with a nail complaint to a prominent dermatologic nail disorders center were diagnosed with mycologically confirmed onychomycosis, Dr. Julie Jefferson reported at the annual meeting of the Society for Pediatric Dermatology.
That’s a substantially higher prevalence than the 15.5% figure reported by Spanish investigators in a 20-year retrospective study (Mycoses 2011;54:450-3). It’s also well below the 47% prevalence recently reported in Denver in a 5-year retrospective study, where Trychophyton rubrum was the most common pathogen, and the highest prevalence of onychomycosis in the pediatric population was seen in 6- to 10-year-olds (Pediatr. Dermatol. 2014;31:106-8), noted Dr. Jefferson of Johns Hopkins University, Baltimore.
The investigators in both Spain and Denver observed that the prevalence of pediatric onychomycosis appears to be increasing in recent years.
Dr. Jefferson presented a retrospective study that included 917 patients up to age 18 years who presented to the Oregon Dermatology and Research Center, Portland, in a recent 6-year period. One or more nail disorders were diagnosed in 11%. The mean age at presentation was 9.4 years, with a mean 2.4-year duration of the condition prior to presentation. Toenails were affected in 47 patients, fingernails in 37, and both in 18. Fourteen patients had two nail disorders, 9 had three, and 2 had four distinct nail disorders.
The etiologies ranged widely, from infections to congenital and hereditary malformations, tumors, inflammatory processes, and systemic diseases.
The most common nail condition was onychomycosis, diagnosed in 34 patients. Thus, 3.7% of all pediatric patients presenting to the dermatology center for any reason were diagnosed with onychomycosis, a higher rate than previously reported by others.
Other conditions included 13 cases of longitudinal melanonychia, 11 of disappearing nail bed, 9 of retronychia, 8 cases of congenital malalignment, 8 cases of trachyonychia, 7 of paronychia, 5 cases of psoriasis, and 2 of lichen planus.
Three of four patients with an ingrown toenail also had congenital malalignment of the affected great toenail, supporting the notion put forth by other investigators that congenital malalignment of the great toenail predisposes to ingrown toenails, according to Dr. Jefferson.
She reported having no financial conflicts related to this study.
COEUR D’ALENE, IDAHO – Conventional wisdom holds that onychomycosis is rare in children. Not so.
Fully one-third of children and adolescents who presented with a nail complaint to a prominent dermatologic nail disorders center were diagnosed with mycologically confirmed onychomycosis, Dr. Julie Jefferson reported at the annual meeting of the Society for Pediatric Dermatology.
That’s a substantially higher prevalence than the 15.5% figure reported by Spanish investigators in a 20-year retrospective study (Mycoses 2011;54:450-3). It’s also well below the 47% prevalence recently reported in Denver in a 5-year retrospective study, where Trychophyton rubrum was the most common pathogen, and the highest prevalence of onychomycosis in the pediatric population was seen in 6- to 10-year-olds (Pediatr. Dermatol. 2014;31:106-8), noted Dr. Jefferson of Johns Hopkins University, Baltimore.
The investigators in both Spain and Denver observed that the prevalence of pediatric onychomycosis appears to be increasing in recent years.
Dr. Jefferson presented a retrospective study that included 917 patients up to age 18 years who presented to the Oregon Dermatology and Research Center, Portland, in a recent 6-year period. One or more nail disorders were diagnosed in 11%. The mean age at presentation was 9.4 years, with a mean 2.4-year duration of the condition prior to presentation. Toenails were affected in 47 patients, fingernails in 37, and both in 18. Fourteen patients had two nail disorders, 9 had three, and 2 had four distinct nail disorders.
The etiologies ranged widely, from infections to congenital and hereditary malformations, tumors, inflammatory processes, and systemic diseases.
The most common nail condition was onychomycosis, diagnosed in 34 patients. Thus, 3.7% of all pediatric patients presenting to the dermatology center for any reason were diagnosed with onychomycosis, a higher rate than previously reported by others.
Other conditions included 13 cases of longitudinal melanonychia, 11 of disappearing nail bed, 9 of retronychia, 8 cases of congenital malalignment, 8 cases of trachyonychia, 7 of paronychia, 5 cases of psoriasis, and 2 of lichen planus.
Three of four patients with an ingrown toenail also had congenital malalignment of the affected great toenail, supporting the notion put forth by other investigators that congenital malalignment of the great toenail predisposes to ingrown toenails, according to Dr. Jefferson.
She reported having no financial conflicts related to this study.
AT THE SPD ANNUAL MEETING
Juvenile facial linear scleroderma is a neurocutaneous disease
COEUR D’ALENE, IDAHO – Juvenile linear scleroderma of the face – traditionally considered a disease limited to the skin – now is more properly viewed as a neurocutaneous disorder, Dr. Francesco Zulian said in his Sidney Hurwitz Memorial Lecture at the annual meeting of the Society for Pediatric Dermatology.
"We need acute neurologic screening at onset of juvenile linear scleroderma of the face and careful monitoring during follow-up," according to Dr. Zulian, chief of pediatric rheumatology at the University of Padua (Italy).
In his series of 77 patients with juvenile linear scleroderma of the face, 46 (60%) proved to have CNS involvement based upon positive brain MRI or EEG findings. The MRI was positive in 73% of patients with neurologic symptoms, which included seizures, chronic headache, cranial nerve palsy, hemiparesis, and behavioral abnormalities.
Moreover, one-quarter of the 24 neurologically asymptomatic patients had organic brain lesions, as evidenced by multiple white and gray matter involvement on MRI. Similarly, 27% of neurologically asymptomatic patients had abnormal EEG findings.
"I am thinking – and other people agree with me – that probably we are dealing with a neurocutaneous condition," he said.
The onset of localized scleroderma of the face occurred at an average age of 5.3 years, which is 2 years earlier than disease onset in patients with juvenile localized scleroderma at other sites. Patients with linear scleroderma of the face who developed neurologic symptoms did so within 5 years of skin disease onset.
How best to monitor patients with juvenile linear scleroderma of the face for occult brain involvement remains an unresolved issue. MRI is expensive and requires sedation in children. Dr. Zulian has turned instead to cone beam CT, a relatively new imaging technique used in maxillofacial surgery and dentistry. It differs from standard CT in that it uses less radiation, takes less time, doesn’t involve sedation, and costs significantly less than MRI or conventional CT. These advantages permit more frequent patient monitoring.
Cone beam CT allows for the creation of three-dimensional images so that physicians can calculate the size of soft tissue and bone abnormalities and monitor their changes over time. Currently, Dr. Zulian is evaluating the utility of this imaging method in a prospective study involving 15 patients. He said he plans to complete the study and present the findings next year.
Dr. Zulian reported having no relevant financial conflicts.
COEUR D’ALENE, IDAHO – Juvenile linear scleroderma of the face – traditionally considered a disease limited to the skin – now is more properly viewed as a neurocutaneous disorder, Dr. Francesco Zulian said in his Sidney Hurwitz Memorial Lecture at the annual meeting of the Society for Pediatric Dermatology.
"We need acute neurologic screening at onset of juvenile linear scleroderma of the face and careful monitoring during follow-up," according to Dr. Zulian, chief of pediatric rheumatology at the University of Padua (Italy).
In his series of 77 patients with juvenile linear scleroderma of the face, 46 (60%) proved to have CNS involvement based upon positive brain MRI or EEG findings. The MRI was positive in 73% of patients with neurologic symptoms, which included seizures, chronic headache, cranial nerve palsy, hemiparesis, and behavioral abnormalities.
Moreover, one-quarter of the 24 neurologically asymptomatic patients had organic brain lesions, as evidenced by multiple white and gray matter involvement on MRI. Similarly, 27% of neurologically asymptomatic patients had abnormal EEG findings.
"I am thinking – and other people agree with me – that probably we are dealing with a neurocutaneous condition," he said.
The onset of localized scleroderma of the face occurred at an average age of 5.3 years, which is 2 years earlier than disease onset in patients with juvenile localized scleroderma at other sites. Patients with linear scleroderma of the face who developed neurologic symptoms did so within 5 years of skin disease onset.
How best to monitor patients with juvenile linear scleroderma of the face for occult brain involvement remains an unresolved issue. MRI is expensive and requires sedation in children. Dr. Zulian has turned instead to cone beam CT, a relatively new imaging technique used in maxillofacial surgery and dentistry. It differs from standard CT in that it uses less radiation, takes less time, doesn’t involve sedation, and costs significantly less than MRI or conventional CT. These advantages permit more frequent patient monitoring.
Cone beam CT allows for the creation of three-dimensional images so that physicians can calculate the size of soft tissue and bone abnormalities and monitor their changes over time. Currently, Dr. Zulian is evaluating the utility of this imaging method in a prospective study involving 15 patients. He said he plans to complete the study and present the findings next year.
Dr. Zulian reported having no relevant financial conflicts.
COEUR D’ALENE, IDAHO – Juvenile linear scleroderma of the face – traditionally considered a disease limited to the skin – now is more properly viewed as a neurocutaneous disorder, Dr. Francesco Zulian said in his Sidney Hurwitz Memorial Lecture at the annual meeting of the Society for Pediatric Dermatology.
"We need acute neurologic screening at onset of juvenile linear scleroderma of the face and careful monitoring during follow-up," according to Dr. Zulian, chief of pediatric rheumatology at the University of Padua (Italy).
In his series of 77 patients with juvenile linear scleroderma of the face, 46 (60%) proved to have CNS involvement based upon positive brain MRI or EEG findings. The MRI was positive in 73% of patients with neurologic symptoms, which included seizures, chronic headache, cranial nerve palsy, hemiparesis, and behavioral abnormalities.
Moreover, one-quarter of the 24 neurologically asymptomatic patients had organic brain lesions, as evidenced by multiple white and gray matter involvement on MRI. Similarly, 27% of neurologically asymptomatic patients had abnormal EEG findings.
"I am thinking – and other people agree with me – that probably we are dealing with a neurocutaneous condition," he said.
The onset of localized scleroderma of the face occurred at an average age of 5.3 years, which is 2 years earlier than disease onset in patients with juvenile localized scleroderma at other sites. Patients with linear scleroderma of the face who developed neurologic symptoms did so within 5 years of skin disease onset.
How best to monitor patients with juvenile linear scleroderma of the face for occult brain involvement remains an unresolved issue. MRI is expensive and requires sedation in children. Dr. Zulian has turned instead to cone beam CT, a relatively new imaging technique used in maxillofacial surgery and dentistry. It differs from standard CT in that it uses less radiation, takes less time, doesn’t involve sedation, and costs significantly less than MRI or conventional CT. These advantages permit more frequent patient monitoring.
Cone beam CT allows for the creation of three-dimensional images so that physicians can calculate the size of soft tissue and bone abnormalities and monitor their changes over time. Currently, Dr. Zulian is evaluating the utility of this imaging method in a prospective study involving 15 patients. He said he plans to complete the study and present the findings next year.
Dr. Zulian reported having no relevant financial conflicts.
EXPERT ANALYSIS FROM THE SPD ANNUAL MEETING
Autologous punch grafts unequalled for segmental vitiligo
COEUR D’ALENE, IDAHO – Autologous grafting is a treatment of unmatched effectiveness for pediatric patients with segmental vitiligo unresponsive to medical modalities, according to Dr. Pearl E. Grimes, director of the Vitiligo and Pigmentation Institute of Southern California and a dermatologist at the University of California, Los Angeles.
"Segmental vitiligo is really the only type of vitiligo I think I can cure, and that’s because we get our absolute best responses with autologous grafting in combination with phototherapy," she said at the annual meeting of the Society for Pediatric Dermatology.
She favors the use of 1-mm punch grafts over more elaborate techniques such as split-thickness grafts, blister roof grafts, and cultured cell transplantation. Autologous grafting using 1-mm punch grafts is simple, and long-term follow-up studies have documented the persistence of repigmentation for more than 5 years in patients with segmental vitiligo.
"It’s so, so easy," Dr. Grimes said. "And the great thing about punch grafts is this technique doesn’t require a lot of special equipment, unlike sheet grafts and the other methods. All you need are iris scissors and jeweler’s forceps."
"I’ve harvested anywhere from 10 to 250 grafts at one setting. We anesthetize a donor area on the hip, harvest the 1-mm punch grafts, place them in saline in sterile petri dishes, remove 1-mm punch grafts from the anesthetized recipient site, and then with jeweler’s forceps you place those harvested 1-mm grafts in those 1-mm recipient holes. You cover the areas with Steri-Strips, which stay in place for 7 days. Then you remove them and start phototherapy, either narrow-band UVB, PUVA [psoralen and UVA], or with an excimer laser," she explained.
In her experience, aggressive topical therapy using high-potency corticosteroids and tacrolimus (Protopic) often provides good results in treating segmental vitiligo on the face.
"If you have segmental vitiligo on the trunk and extremity areas, it’s much more difficult to get good results with topical therapies. Those are the patients who ultimately will need to be grafted," according to the dermatologist.
She stressed that when using autologous grafting to treat patients with generalized vitiligo, it’s imperative to stabilize them beforehand. This is accomplished using systemic steroids: for example, 5-10 mg/day of prednisone for 2 weeks, or mini-pulse dosing of betamethasone at 5 mg twice weekly.
"When I see kids with segmental vitiligo in my initial consultation, I always tell them and their parents that if I were going to get vitiligo, I’d choose segmental versus the generalized variety. That’s because of all the types of vitiligo that we see, the segmental variety is the most predictable. It burns itself out in about 95% of patients in that first year, so we know that in the overwhelming majority of patients it’s not going to spread beyond that dermatome," Dr. Grimes said.
She made a plea for physicians to be more aggressive in treating childhood vitiligo. Often they are reluctant to treat. Yet it has been shown across the board, for all treatment modalities, that efficacy is significantly greater in children than adults.
"Children are the ones who are more likely to give you that greater than 75% repigmentation. It’s a great reason to treat children early," Dr. Grimes said.
She reported having performed clinical research and/or serving as a consultant to a dozen pharmaceutical and cosmetics companies.
COEUR D’ALENE, IDAHO – Autologous grafting is a treatment of unmatched effectiveness for pediatric patients with segmental vitiligo unresponsive to medical modalities, according to Dr. Pearl E. Grimes, director of the Vitiligo and Pigmentation Institute of Southern California and a dermatologist at the University of California, Los Angeles.
"Segmental vitiligo is really the only type of vitiligo I think I can cure, and that’s because we get our absolute best responses with autologous grafting in combination with phototherapy," she said at the annual meeting of the Society for Pediatric Dermatology.
She favors the use of 1-mm punch grafts over more elaborate techniques such as split-thickness grafts, blister roof grafts, and cultured cell transplantation. Autologous grafting using 1-mm punch grafts is simple, and long-term follow-up studies have documented the persistence of repigmentation for more than 5 years in patients with segmental vitiligo.
"It’s so, so easy," Dr. Grimes said. "And the great thing about punch grafts is this technique doesn’t require a lot of special equipment, unlike sheet grafts and the other methods. All you need are iris scissors and jeweler’s forceps."
"I’ve harvested anywhere from 10 to 250 grafts at one setting. We anesthetize a donor area on the hip, harvest the 1-mm punch grafts, place them in saline in sterile petri dishes, remove 1-mm punch grafts from the anesthetized recipient site, and then with jeweler’s forceps you place those harvested 1-mm grafts in those 1-mm recipient holes. You cover the areas with Steri-Strips, which stay in place for 7 days. Then you remove them and start phototherapy, either narrow-band UVB, PUVA [psoralen and UVA], or with an excimer laser," she explained.
In her experience, aggressive topical therapy using high-potency corticosteroids and tacrolimus (Protopic) often provides good results in treating segmental vitiligo on the face.
"If you have segmental vitiligo on the trunk and extremity areas, it’s much more difficult to get good results with topical therapies. Those are the patients who ultimately will need to be grafted," according to the dermatologist.
She stressed that when using autologous grafting to treat patients with generalized vitiligo, it’s imperative to stabilize them beforehand. This is accomplished using systemic steroids: for example, 5-10 mg/day of prednisone for 2 weeks, or mini-pulse dosing of betamethasone at 5 mg twice weekly.
"When I see kids with segmental vitiligo in my initial consultation, I always tell them and their parents that if I were going to get vitiligo, I’d choose segmental versus the generalized variety. That’s because of all the types of vitiligo that we see, the segmental variety is the most predictable. It burns itself out in about 95% of patients in that first year, so we know that in the overwhelming majority of patients it’s not going to spread beyond that dermatome," Dr. Grimes said.
She made a plea for physicians to be more aggressive in treating childhood vitiligo. Often they are reluctant to treat. Yet it has been shown across the board, for all treatment modalities, that efficacy is significantly greater in children than adults.
"Children are the ones who are more likely to give you that greater than 75% repigmentation. It’s a great reason to treat children early," Dr. Grimes said.
She reported having performed clinical research and/or serving as a consultant to a dozen pharmaceutical and cosmetics companies.
COEUR D’ALENE, IDAHO – Autologous grafting is a treatment of unmatched effectiveness for pediatric patients with segmental vitiligo unresponsive to medical modalities, according to Dr. Pearl E. Grimes, director of the Vitiligo and Pigmentation Institute of Southern California and a dermatologist at the University of California, Los Angeles.
"Segmental vitiligo is really the only type of vitiligo I think I can cure, and that’s because we get our absolute best responses with autologous grafting in combination with phototherapy," she said at the annual meeting of the Society for Pediatric Dermatology.
She favors the use of 1-mm punch grafts over more elaborate techniques such as split-thickness grafts, blister roof grafts, and cultured cell transplantation. Autologous grafting using 1-mm punch grafts is simple, and long-term follow-up studies have documented the persistence of repigmentation for more than 5 years in patients with segmental vitiligo.
"It’s so, so easy," Dr. Grimes said. "And the great thing about punch grafts is this technique doesn’t require a lot of special equipment, unlike sheet grafts and the other methods. All you need are iris scissors and jeweler’s forceps."
"I’ve harvested anywhere from 10 to 250 grafts at one setting. We anesthetize a donor area on the hip, harvest the 1-mm punch grafts, place them in saline in sterile petri dishes, remove 1-mm punch grafts from the anesthetized recipient site, and then with jeweler’s forceps you place those harvested 1-mm grafts in those 1-mm recipient holes. You cover the areas with Steri-Strips, which stay in place for 7 days. Then you remove them and start phototherapy, either narrow-band UVB, PUVA [psoralen and UVA], or with an excimer laser," she explained.
In her experience, aggressive topical therapy using high-potency corticosteroids and tacrolimus (Protopic) often provides good results in treating segmental vitiligo on the face.
"If you have segmental vitiligo on the trunk and extremity areas, it’s much more difficult to get good results with topical therapies. Those are the patients who ultimately will need to be grafted," according to the dermatologist.
She stressed that when using autologous grafting to treat patients with generalized vitiligo, it’s imperative to stabilize them beforehand. This is accomplished using systemic steroids: for example, 5-10 mg/day of prednisone for 2 weeks, or mini-pulse dosing of betamethasone at 5 mg twice weekly.
"When I see kids with segmental vitiligo in my initial consultation, I always tell them and their parents that if I were going to get vitiligo, I’d choose segmental versus the generalized variety. That’s because of all the types of vitiligo that we see, the segmental variety is the most predictable. It burns itself out in about 95% of patients in that first year, so we know that in the overwhelming majority of patients it’s not going to spread beyond that dermatome," Dr. Grimes said.
She made a plea for physicians to be more aggressive in treating childhood vitiligo. Often they are reluctant to treat. Yet it has been shown across the board, for all treatment modalities, that efficacy is significantly greater in children than adults.
"Children are the ones who are more likely to give you that greater than 75% repigmentation. It’s a great reason to treat children early," Dr. Grimes said.
She reported having performed clinical research and/or serving as a consultant to a dozen pharmaceutical and cosmetics companies.
EXPERT ANALYSIS FROM THE SPD ANNUAL MEETING
Effective treatments abound for children with heavy sweating in the hands and feet
COEUR D’ALENE, IDAHO – By the time patients seek a physician’s help for palmar/plantar focal hyperhidrosis, they may be well beyond the point at which even potent topical antiperspirants will help.
"Most people think there is nothing that can be done at all [for these patients]. But there are effective options. Even if you’re not going to offer them, get your patients to someone who will. We can make a profound difference for children and adolescents," Dr. Jane S. Bellet said at the annual meeting of the Society for Pediatric Dermatology.
In fact, more potent therapies are available, ranging from iontophoresis to oral medications, botulinum toxin A injections, and surgical thoracic sympathetectomy.
Oral medications: "I’ve really changed my practice in the last 5 years. I didn’t use systemic medications very much. I use them much more frequently now. I think you can get a very nice response," said Dr. Bellet, a pediatric dermatologist at Duke University in Durham, N.C.
She typically turns to the anticholinergic agents glycopyrrolate and oxybutynin. Glycopyrrolate, marketed as Cuvposa in a cherry-flavored solution at 1 mg/5 mL, does not have an indication from the Food and Drug Administration for treatment of pediatric hyperhidrosis, she noted. However, it is FDA-approved in 3- to 16-year-olds for severe chronic drooling caused by neurologic disorders.
"Although we don’t have an indication for hyperhidrosis, we do have a pediatric indication, and I think many times that puts our parents at ease," she observed.
Glycopyrrolate is cost effective and painless, although approximately 30% of children treated for hyperhidrosis will develop dry mouth and/or dry eyes.
"I would definitely add glycopyrrolate to your armamentarium. I think the biggest concern most of us have is the side effects. Speak about them with the family ahead of time, guide them as to what to expect, and stop if it becomes intolerable," Dr. Bellet said.
A recent randomized, prospective, controlled clinical trial in 45 children aged 7-14 years with palmar hyperhidrosis showed excellent outcomes with oxybutynin (Ditropan), with more than 85% experiencing at least moderate improvement in sweating and 80% gaining improved quality of life (Pediatr. Dermatol. 2014;31:48-53).
Iontophoresis: "This is a wonderful treatment for palms and soles," said Dr. Bellet.
The treatment entails placing the hands or feet in a water bath tray filled with tap water through which direct electric current is running. The proposed mechanism of benefit is that hydrolysis of the bath water results in accumulation of hydrogen ions, which then induce sweat gland destruction.
The chief disadvantage of iontophoresis is that it is labor intensive. Unless the family rents or buys a home unit, the patient typically must visit the physician’s office on a daily basis initially, placing the hands or feet in the bath for 10 minutes, followed by a second 10-minute round after the polarity is switched. Eventually, many patients can step down to two or three 20- to 30-minute sessions per week, then perhaps once-weekly maintenance therapy, she said.
Adding glycopyrrolate to the water bath has been shown to result in longer improvement in a study in both children and adults (Australas. J. Dermatol. 2004;45:208-12); however, this poses a greater risk of systemic absorption in children.
"Interestingly enough, some insurance companies will cover iontophoresis if you add glycopyrrolate to the water, but they won’t cover regular iontophoresis," Dr. Bellet said.
In response to audience inquiries, she indicated she uses the Fischer MD-1a galvanic unit. It’s simple to operate, costs about $700 including accessories, and can be rented with the payments applied to a later purchase.
Botulinum toxin A: First using this product for hyperhidrosis is off-label therapy, Dr. Bellet emphasized. Second, the doses required to treat palmar/plantar hyperhidrosis – 75-100 units per palm or sole – are much higher than in treating the axillae. Also, injecting botulinum toxin A into the palms and soles is extraordinarily painful. Children typically require general anesthesia, because the alternative methods employed with mixed results in adults, including EMLA cream, ice packs, and ethyl chloride spray, don’t cut it in younger patients, Dr. Bellet noted.
That being said, botulinum toxin A therapy works quite well in children and adolescents. However, it’s important to explain to patients and parents up front that the injections can cause transient weakness of the hand muscles because of the diffusion of the toxin from dermis to muscles; for a budding pianist or baseball pitcher, that can be a deal-breaker, she said. Also, Dr. Bellet added, repeated injections can result in atrophy of the thenar and hypothenar eminences, with resultant irreversible weakness.
Thoracic sympathectomy: Not many American thoracic surgeons do sympathectomy to treat hyperhidrosis on a frequent basis, but those with extensive experience obtain outstanding results, Dr. Bellet said. To treat palmar hyperhidrosis, the nerve is clipped at the top of the third or fourth rib; for the soles, it’s at the fourth and fifth rib. The youngest reported treated patients have been 8 years old.
Dr. Bellet recommended the International Hyperhidrosis Society (www.sweathelp.org) as an excellent source of further information.
Dr. Bellet reported having no financial conflicts of interest regarding her presentation.
How do you treat palmar/plantar hyperhidrosis in children? Take our Quick Poll on the Skin & Allergy News homepage.
COEUR D’ALENE, IDAHO – By the time patients seek a physician’s help for palmar/plantar focal hyperhidrosis, they may be well beyond the point at which even potent topical antiperspirants will help.
"Most people think there is nothing that can be done at all [for these patients]. But there are effective options. Even if you’re not going to offer them, get your patients to someone who will. We can make a profound difference for children and adolescents," Dr. Jane S. Bellet said at the annual meeting of the Society for Pediatric Dermatology.
In fact, more potent therapies are available, ranging from iontophoresis to oral medications, botulinum toxin A injections, and surgical thoracic sympathetectomy.
Oral medications: "I’ve really changed my practice in the last 5 years. I didn’t use systemic medications very much. I use them much more frequently now. I think you can get a very nice response," said Dr. Bellet, a pediatric dermatologist at Duke University in Durham, N.C.
She typically turns to the anticholinergic agents glycopyrrolate and oxybutynin. Glycopyrrolate, marketed as Cuvposa in a cherry-flavored solution at 1 mg/5 mL, does not have an indication from the Food and Drug Administration for treatment of pediatric hyperhidrosis, she noted. However, it is FDA-approved in 3- to 16-year-olds for severe chronic drooling caused by neurologic disorders.
"Although we don’t have an indication for hyperhidrosis, we do have a pediatric indication, and I think many times that puts our parents at ease," she observed.
Glycopyrrolate is cost effective and painless, although approximately 30% of children treated for hyperhidrosis will develop dry mouth and/or dry eyes.
"I would definitely add glycopyrrolate to your armamentarium. I think the biggest concern most of us have is the side effects. Speak about them with the family ahead of time, guide them as to what to expect, and stop if it becomes intolerable," Dr. Bellet said.
A recent randomized, prospective, controlled clinical trial in 45 children aged 7-14 years with palmar hyperhidrosis showed excellent outcomes with oxybutynin (Ditropan), with more than 85% experiencing at least moderate improvement in sweating and 80% gaining improved quality of life (Pediatr. Dermatol. 2014;31:48-53).
Iontophoresis: "This is a wonderful treatment for palms and soles," said Dr. Bellet.
The treatment entails placing the hands or feet in a water bath tray filled with tap water through which direct electric current is running. The proposed mechanism of benefit is that hydrolysis of the bath water results in accumulation of hydrogen ions, which then induce sweat gland destruction.
The chief disadvantage of iontophoresis is that it is labor intensive. Unless the family rents or buys a home unit, the patient typically must visit the physician’s office on a daily basis initially, placing the hands or feet in the bath for 10 minutes, followed by a second 10-minute round after the polarity is switched. Eventually, many patients can step down to two or three 20- to 30-minute sessions per week, then perhaps once-weekly maintenance therapy, she said.
Adding glycopyrrolate to the water bath has been shown to result in longer improvement in a study in both children and adults (Australas. J. Dermatol. 2004;45:208-12); however, this poses a greater risk of systemic absorption in children.
"Interestingly enough, some insurance companies will cover iontophoresis if you add glycopyrrolate to the water, but they won’t cover regular iontophoresis," Dr. Bellet said.
In response to audience inquiries, she indicated she uses the Fischer MD-1a galvanic unit. It’s simple to operate, costs about $700 including accessories, and can be rented with the payments applied to a later purchase.
Botulinum toxin A: First using this product for hyperhidrosis is off-label therapy, Dr. Bellet emphasized. Second, the doses required to treat palmar/plantar hyperhidrosis – 75-100 units per palm or sole – are much higher than in treating the axillae. Also, injecting botulinum toxin A into the palms and soles is extraordinarily painful. Children typically require general anesthesia, because the alternative methods employed with mixed results in adults, including EMLA cream, ice packs, and ethyl chloride spray, don’t cut it in younger patients, Dr. Bellet noted.
That being said, botulinum toxin A therapy works quite well in children and adolescents. However, it’s important to explain to patients and parents up front that the injections can cause transient weakness of the hand muscles because of the diffusion of the toxin from dermis to muscles; for a budding pianist or baseball pitcher, that can be a deal-breaker, she said. Also, Dr. Bellet added, repeated injections can result in atrophy of the thenar and hypothenar eminences, with resultant irreversible weakness.
Thoracic sympathectomy: Not many American thoracic surgeons do sympathectomy to treat hyperhidrosis on a frequent basis, but those with extensive experience obtain outstanding results, Dr. Bellet said. To treat palmar hyperhidrosis, the nerve is clipped at the top of the third or fourth rib; for the soles, it’s at the fourth and fifth rib. The youngest reported treated patients have been 8 years old.
Dr. Bellet recommended the International Hyperhidrosis Society (www.sweathelp.org) as an excellent source of further information.
Dr. Bellet reported having no financial conflicts of interest regarding her presentation.
How do you treat palmar/plantar hyperhidrosis in children? Take our Quick Poll on the Skin & Allergy News homepage.
COEUR D’ALENE, IDAHO – By the time patients seek a physician’s help for palmar/plantar focal hyperhidrosis, they may be well beyond the point at which even potent topical antiperspirants will help.
"Most people think there is nothing that can be done at all [for these patients]. But there are effective options. Even if you’re not going to offer them, get your patients to someone who will. We can make a profound difference for children and adolescents," Dr. Jane S. Bellet said at the annual meeting of the Society for Pediatric Dermatology.
In fact, more potent therapies are available, ranging from iontophoresis to oral medications, botulinum toxin A injections, and surgical thoracic sympathetectomy.
Oral medications: "I’ve really changed my practice in the last 5 years. I didn’t use systemic medications very much. I use them much more frequently now. I think you can get a very nice response," said Dr. Bellet, a pediatric dermatologist at Duke University in Durham, N.C.
She typically turns to the anticholinergic agents glycopyrrolate and oxybutynin. Glycopyrrolate, marketed as Cuvposa in a cherry-flavored solution at 1 mg/5 mL, does not have an indication from the Food and Drug Administration for treatment of pediatric hyperhidrosis, she noted. However, it is FDA-approved in 3- to 16-year-olds for severe chronic drooling caused by neurologic disorders.
"Although we don’t have an indication for hyperhidrosis, we do have a pediatric indication, and I think many times that puts our parents at ease," she observed.
Glycopyrrolate is cost effective and painless, although approximately 30% of children treated for hyperhidrosis will develop dry mouth and/or dry eyes.
"I would definitely add glycopyrrolate to your armamentarium. I think the biggest concern most of us have is the side effects. Speak about them with the family ahead of time, guide them as to what to expect, and stop if it becomes intolerable," Dr. Bellet said.
A recent randomized, prospective, controlled clinical trial in 45 children aged 7-14 years with palmar hyperhidrosis showed excellent outcomes with oxybutynin (Ditropan), with more than 85% experiencing at least moderate improvement in sweating and 80% gaining improved quality of life (Pediatr. Dermatol. 2014;31:48-53).
Iontophoresis: "This is a wonderful treatment for palms and soles," said Dr. Bellet.
The treatment entails placing the hands or feet in a water bath tray filled with tap water through which direct electric current is running. The proposed mechanism of benefit is that hydrolysis of the bath water results in accumulation of hydrogen ions, which then induce sweat gland destruction.
The chief disadvantage of iontophoresis is that it is labor intensive. Unless the family rents or buys a home unit, the patient typically must visit the physician’s office on a daily basis initially, placing the hands or feet in the bath for 10 minutes, followed by a second 10-minute round after the polarity is switched. Eventually, many patients can step down to two or three 20- to 30-minute sessions per week, then perhaps once-weekly maintenance therapy, she said.
Adding glycopyrrolate to the water bath has been shown to result in longer improvement in a study in both children and adults (Australas. J. Dermatol. 2004;45:208-12); however, this poses a greater risk of systemic absorption in children.
"Interestingly enough, some insurance companies will cover iontophoresis if you add glycopyrrolate to the water, but they won’t cover regular iontophoresis," Dr. Bellet said.
In response to audience inquiries, she indicated she uses the Fischer MD-1a galvanic unit. It’s simple to operate, costs about $700 including accessories, and can be rented with the payments applied to a later purchase.
Botulinum toxin A: First using this product for hyperhidrosis is off-label therapy, Dr. Bellet emphasized. Second, the doses required to treat palmar/plantar hyperhidrosis – 75-100 units per palm or sole – are much higher than in treating the axillae. Also, injecting botulinum toxin A into the palms and soles is extraordinarily painful. Children typically require general anesthesia, because the alternative methods employed with mixed results in adults, including EMLA cream, ice packs, and ethyl chloride spray, don’t cut it in younger patients, Dr. Bellet noted.
That being said, botulinum toxin A therapy works quite well in children and adolescents. However, it’s important to explain to patients and parents up front that the injections can cause transient weakness of the hand muscles because of the diffusion of the toxin from dermis to muscles; for a budding pianist or baseball pitcher, that can be a deal-breaker, she said. Also, Dr. Bellet added, repeated injections can result in atrophy of the thenar and hypothenar eminences, with resultant irreversible weakness.
Thoracic sympathectomy: Not many American thoracic surgeons do sympathectomy to treat hyperhidrosis on a frequent basis, but those with extensive experience obtain outstanding results, Dr. Bellet said. To treat palmar hyperhidrosis, the nerve is clipped at the top of the third or fourth rib; for the soles, it’s at the fourth and fifth rib. The youngest reported treated patients have been 8 years old.
Dr. Bellet recommended the International Hyperhidrosis Society (www.sweathelp.org) as an excellent source of further information.
Dr. Bellet reported having no financial conflicts of interest regarding her presentation.
How do you treat palmar/plantar hyperhidrosis in children? Take our Quick Poll on the Skin & Allergy News homepage.
EXPERT ANALYSIS FROM THE SPD ANNUAL MEETING
Onychomycosis: Not just for adults
COEUR D’ALENE, IDAHO – Conventional wisdom holds that onychomycosis is rare in children. Not so.
Fully one-third of children and adolescents who presented with a nail complaint to a prominent dermatologic nail disorders center were diagnosed with mycologically confirmed onychomycosis, Dr. Julie Jefferson reported at the annual meeting of the Society for Pediatric Dermatology.
That’s a substantially higher prevalence than the 15.5% figure reported by Spanish investigators in a 20-year retrospective study (Mycoses 2011;54:450-3). It’s also well below the 47% prevalence recently reported in Denver in a 5-year retrospective study, where Trychophyton rubrum was the most common pathogen, and the highest prevalence of onychomycosis in the pediatric population was seen in 6- to 10-year-olds (Pediatr. Dermatol. 2014;31:106-8), noted Dr. Jefferson of Johns Hopkins University, Baltimore.
The investigators in both Span and Denver observed that the prevalence of pediatric onychomycosis appears to be increasing in recent years.
Dr. Jefferson presented a retrospective study that included 917 patients up to age 18 years who presented to the Oregon Dermatology and Research Center, Portland, in a recent 6-year period. One or more nail disorders were diagnosed in 11%. The mean age at presentation was 9.4 years, with a mean 2.4-year duration of the condition prior to presentation. Toenails were affected in 47 patients, fingernails in 37, and both in 18. Fourteen patients had two nail disorders, 9 had three, and 2 had four distinct nail disorders.
The etiologies ranged widely, from infections to congenital and hereditary malformations, tumors, inflammatory processes, and systemic diseases.
The most common nail condition was onychomycosis, diagnosed in 34 patients. Thus, 3.7% of all pediatric patients presenting to the dermatology center for any reason were diagnosed with onychomycosis, a higher rate than previously reported by others.
Other conditions included 13 cases of longitudinal melanonychia, 11 of disappearing nail bed, 9 of retronychia, 8 cases of congenital malalignment, 8 cases of trachyonychia, 7 of paronychia, 5 cases of psoriasis, and 2 of lichen planus.
Three of four patients with an ingrown toenail also had congenital malalignment of the affected great toenail, supporting the notion put forth by other investigators that congenital malalignment of the great toenail predisposes to ingrown toenails, according to Dr. Jefferson.
She reported having no financial conflicts related to this study.
COEUR D’ALENE, IDAHO – Conventional wisdom holds that onychomycosis is rare in children. Not so.
Fully one-third of children and adolescents who presented with a nail complaint to a prominent dermatologic nail disorders center were diagnosed with mycologically confirmed onychomycosis, Dr. Julie Jefferson reported at the annual meeting of the Society for Pediatric Dermatology.
That’s a substantially higher prevalence than the 15.5% figure reported by Spanish investigators in a 20-year retrospective study (Mycoses 2011;54:450-3). It’s also well below the 47% prevalence recently reported in Denver in a 5-year retrospective study, where Trychophyton rubrum was the most common pathogen, and the highest prevalence of onychomycosis in the pediatric population was seen in 6- to 10-year-olds (Pediatr. Dermatol. 2014;31:106-8), noted Dr. Jefferson of Johns Hopkins University, Baltimore.
The investigators in both Span and Denver observed that the prevalence of pediatric onychomycosis appears to be increasing in recent years.
Dr. Jefferson presented a retrospective study that included 917 patients up to age 18 years who presented to the Oregon Dermatology and Research Center, Portland, in a recent 6-year period. One or more nail disorders were diagnosed in 11%. The mean age at presentation was 9.4 years, with a mean 2.4-year duration of the condition prior to presentation. Toenails were affected in 47 patients, fingernails in 37, and both in 18. Fourteen patients had two nail disorders, 9 had three, and 2 had four distinct nail disorders.
The etiologies ranged widely, from infections to congenital and hereditary malformations, tumors, inflammatory processes, and systemic diseases.
The most common nail condition was onychomycosis, diagnosed in 34 patients. Thus, 3.7% of all pediatric patients presenting to the dermatology center for any reason were diagnosed with onychomycosis, a higher rate than previously reported by others.
Other conditions included 13 cases of longitudinal melanonychia, 11 of disappearing nail bed, 9 of retronychia, 8 cases of congenital malalignment, 8 cases of trachyonychia, 7 of paronychia, 5 cases of psoriasis, and 2 of lichen planus.
Three of four patients with an ingrown toenail also had congenital malalignment of the affected great toenail, supporting the notion put forth by other investigators that congenital malalignment of the great toenail predisposes to ingrown toenails, according to Dr. Jefferson.
She reported having no financial conflicts related to this study.
COEUR D’ALENE, IDAHO – Conventional wisdom holds that onychomycosis is rare in children. Not so.
Fully one-third of children and adolescents who presented with a nail complaint to a prominent dermatologic nail disorders center were diagnosed with mycologically confirmed onychomycosis, Dr. Julie Jefferson reported at the annual meeting of the Society for Pediatric Dermatology.
That’s a substantially higher prevalence than the 15.5% figure reported by Spanish investigators in a 20-year retrospective study (Mycoses 2011;54:450-3). It’s also well below the 47% prevalence recently reported in Denver in a 5-year retrospective study, where Trychophyton rubrum was the most common pathogen, and the highest prevalence of onychomycosis in the pediatric population was seen in 6- to 10-year-olds (Pediatr. Dermatol. 2014;31:106-8), noted Dr. Jefferson of Johns Hopkins University, Baltimore.
The investigators in both Span and Denver observed that the prevalence of pediatric onychomycosis appears to be increasing in recent years.
Dr. Jefferson presented a retrospective study that included 917 patients up to age 18 years who presented to the Oregon Dermatology and Research Center, Portland, in a recent 6-year period. One or more nail disorders were diagnosed in 11%. The mean age at presentation was 9.4 years, with a mean 2.4-year duration of the condition prior to presentation. Toenails were affected in 47 patients, fingernails in 37, and both in 18. Fourteen patients had two nail disorders, 9 had three, and 2 had four distinct nail disorders.
The etiologies ranged widely, from infections to congenital and hereditary malformations, tumors, inflammatory processes, and systemic diseases.
The most common nail condition was onychomycosis, diagnosed in 34 patients. Thus, 3.7% of all pediatric patients presenting to the dermatology center for any reason were diagnosed with onychomycosis, a higher rate than previously reported by others.
Other conditions included 13 cases of longitudinal melanonychia, 11 of disappearing nail bed, 9 of retronychia, 8 cases of congenital malalignment, 8 cases of trachyonychia, 7 of paronychia, 5 cases of psoriasis, and 2 of lichen planus.
Three of four patients with an ingrown toenail also had congenital malalignment of the affected great toenail, supporting the notion put forth by other investigators that congenital malalignment of the great toenail predisposes to ingrown toenails, according to Dr. Jefferson.
She reported having no financial conflicts related to this study.
AT THE SPD ANNUAL MEETING
Key clinical point: Onychomycosis appears to be rising in children and adolescents, and needs to be considered in the differential diagnosis of pediatric nail abnormalities.
Major finding: Onychomycosis was diagnosed in 3.7% of all children and adolescents who presented for any reason to a tertiary dermatology center, and in 33% of the 102 who presented for evaluation of a nail disorder.
Data source: This was a 6-year, single-center, retrospective study of 917 patients up to 18 years of age.
Disclosures: The presenter reported having no financial conflicts with regard to this study.
Check thyroid function in children with vitiligo
COEUR D’ALENE, IDAHO – Every child and adolescent with vitiligo should undergo a laboratory evaluation that includes thyroid function tests and antibody levels, Dr. Pearl E. Grimes advised at the annual meeting of the Society for Pediatric Dermatology.
"At some point, we used to debate whether thyroid disease really is more common in the pediatric population with vitiligo. But now we have multiple studies in the literature to suggest that it is. And that certainly has also been my own clinical experience in looking at thyroid function tests as well as thyroid antibodies for the past 25 years in the pediatric population," said Dr. Grimes, director of the Vitiligo and Pigmentation Institute of Southern California and a dermatologist at the University of California, Los Angeles.
Vitiligo is an autoimmune disease. As such, it is well known to be associated with other autoimmune diseases in adults, with Hashimoto’s thyroiditis and other forms of thyroid disease being the most common comorbid autoimmune conditions.
Dr. Grimes cited two recent studies showing a sharply increased prevalence of thyroid abnormalities as well in the pediatric population with vitiligo.
Investigators at the Netherlands Institute for Pigment Disorders in Amsterdam reported on 260 children and adolescents with vitiligo who underwent measurement of thyroid-stimulating hormone, free thyroxine, and antithyroid peroxidase antibody levels. The results indicated 6.2% had autoimmune thyroiditis with thyroid hormone disturbances, a prevalence far greater than that seen in the general pediatric population. Moreover, 10.5% of patients had elevated levels of antithyroid peroxidase antibodies without disturbance of thyroid hormone, a condition known to be associated with an increased risk of developing overt thyroid disease down the road (Horm. Res. Paediatr. 2013;79:137-44).
In another study, investigators at Children’s Hospital in Izmir, Turkey, retrospectively reviewed laboratory findings in 79 vitiligo patients, aged 2-15 years. Fully one-quarter had abnormal results on thyroid function tests and/or elevated thyroid autoantibodies (Indian J. Endocrinol. Metab. 2013;1096-9).
Dr. Grimes recommended that a routine laboratory screening panel for children and adolescents with vitiligo should "at a bare minimum" consist of a CBC with differential, a thyroid panel, thyroid antibodies, and an anti-nuclear antibody level.
"I’ve been looking at ANAs [antinuclear antibody test results] in children and adults with vitiligo for a very long time. I find that about 30% of patients, including kids, will have a positive ANA. What’s the significance? A small cohort of vitiligo patients will go on to develop lupus. Although lupus is not one of our more common autoimmune diseases, it happens. If the ANA titer is less than 1:160, I typically don’t get a lupus panel; but if it is greater than 1:160, I will follow up with a lupus panel – and that determines whether I will send that patient for a rheumatologic consultation. The ANA is also very important in determining whether a vitiligo patient is a candidate for phototherapy. If there’s a high-titer ANA, I will not place a patient on narrow band UVB (therapy)," according to Dr. Grimes.
She reported having performed clinical research for and serving as a consultant to several pharmaceutical and cosmetics companies.
COEUR D’ALENE, IDAHO – Every child and adolescent with vitiligo should undergo a laboratory evaluation that includes thyroid function tests and antibody levels, Dr. Pearl E. Grimes advised at the annual meeting of the Society for Pediatric Dermatology.
"At some point, we used to debate whether thyroid disease really is more common in the pediatric population with vitiligo. But now we have multiple studies in the literature to suggest that it is. And that certainly has also been my own clinical experience in looking at thyroid function tests as well as thyroid antibodies for the past 25 years in the pediatric population," said Dr. Grimes, director of the Vitiligo and Pigmentation Institute of Southern California and a dermatologist at the University of California, Los Angeles.
Vitiligo is an autoimmune disease. As such, it is well known to be associated with other autoimmune diseases in adults, with Hashimoto’s thyroiditis and other forms of thyroid disease being the most common comorbid autoimmune conditions.
Dr. Grimes cited two recent studies showing a sharply increased prevalence of thyroid abnormalities as well in the pediatric population with vitiligo.
Investigators at the Netherlands Institute for Pigment Disorders in Amsterdam reported on 260 children and adolescents with vitiligo who underwent measurement of thyroid-stimulating hormone, free thyroxine, and antithyroid peroxidase antibody levels. The results indicated 6.2% had autoimmune thyroiditis with thyroid hormone disturbances, a prevalence far greater than that seen in the general pediatric population. Moreover, 10.5% of patients had elevated levels of antithyroid peroxidase antibodies without disturbance of thyroid hormone, a condition known to be associated with an increased risk of developing overt thyroid disease down the road (Horm. Res. Paediatr. 2013;79:137-44).
In another study, investigators at Children’s Hospital in Izmir, Turkey, retrospectively reviewed laboratory findings in 79 vitiligo patients, aged 2-15 years. Fully one-quarter had abnormal results on thyroid function tests and/or elevated thyroid autoantibodies (Indian J. Endocrinol. Metab. 2013;1096-9).
Dr. Grimes recommended that a routine laboratory screening panel for children and adolescents with vitiligo should "at a bare minimum" consist of a CBC with differential, a thyroid panel, thyroid antibodies, and an anti-nuclear antibody level.
"I’ve been looking at ANAs [antinuclear antibody test results] in children and adults with vitiligo for a very long time. I find that about 30% of patients, including kids, will have a positive ANA. What’s the significance? A small cohort of vitiligo patients will go on to develop lupus. Although lupus is not one of our more common autoimmune diseases, it happens. If the ANA titer is less than 1:160, I typically don’t get a lupus panel; but if it is greater than 1:160, I will follow up with a lupus panel – and that determines whether I will send that patient for a rheumatologic consultation. The ANA is also very important in determining whether a vitiligo patient is a candidate for phototherapy. If there’s a high-titer ANA, I will not place a patient on narrow band UVB (therapy)," according to Dr. Grimes.
She reported having performed clinical research for and serving as a consultant to several pharmaceutical and cosmetics companies.
COEUR D’ALENE, IDAHO – Every child and adolescent with vitiligo should undergo a laboratory evaluation that includes thyroid function tests and antibody levels, Dr. Pearl E. Grimes advised at the annual meeting of the Society for Pediatric Dermatology.
"At some point, we used to debate whether thyroid disease really is more common in the pediatric population with vitiligo. But now we have multiple studies in the literature to suggest that it is. And that certainly has also been my own clinical experience in looking at thyroid function tests as well as thyroid antibodies for the past 25 years in the pediatric population," said Dr. Grimes, director of the Vitiligo and Pigmentation Institute of Southern California and a dermatologist at the University of California, Los Angeles.
Vitiligo is an autoimmune disease. As such, it is well known to be associated with other autoimmune diseases in adults, with Hashimoto’s thyroiditis and other forms of thyroid disease being the most common comorbid autoimmune conditions.
Dr. Grimes cited two recent studies showing a sharply increased prevalence of thyroid abnormalities as well in the pediatric population with vitiligo.
Investigators at the Netherlands Institute for Pigment Disorders in Amsterdam reported on 260 children and adolescents with vitiligo who underwent measurement of thyroid-stimulating hormone, free thyroxine, and antithyroid peroxidase antibody levels. The results indicated 6.2% had autoimmune thyroiditis with thyroid hormone disturbances, a prevalence far greater than that seen in the general pediatric population. Moreover, 10.5% of patients had elevated levels of antithyroid peroxidase antibodies without disturbance of thyroid hormone, a condition known to be associated with an increased risk of developing overt thyroid disease down the road (Horm. Res. Paediatr. 2013;79:137-44).
In another study, investigators at Children’s Hospital in Izmir, Turkey, retrospectively reviewed laboratory findings in 79 vitiligo patients, aged 2-15 years. Fully one-quarter had abnormal results on thyroid function tests and/or elevated thyroid autoantibodies (Indian J. Endocrinol. Metab. 2013;1096-9).
Dr. Grimes recommended that a routine laboratory screening panel for children and adolescents with vitiligo should "at a bare minimum" consist of a CBC with differential, a thyroid panel, thyroid antibodies, and an anti-nuclear antibody level.
"I’ve been looking at ANAs [antinuclear antibody test results] in children and adults with vitiligo for a very long time. I find that about 30% of patients, including kids, will have a positive ANA. What’s the significance? A small cohort of vitiligo patients will go on to develop lupus. Although lupus is not one of our more common autoimmune diseases, it happens. If the ANA titer is less than 1:160, I typically don’t get a lupus panel; but if it is greater than 1:160, I will follow up with a lupus panel – and that determines whether I will send that patient for a rheumatologic consultation. The ANA is also very important in determining whether a vitiligo patient is a candidate for phototherapy. If there’s a high-titer ANA, I will not place a patient on narrow band UVB (therapy)," according to Dr. Grimes.
She reported having performed clinical research for and serving as a consultant to several pharmaceutical and cosmetics companies.
EXPERT ANALYSIS FROM THE SPD ANNUAL MEETING
Maintenance therapy options for childhood alopecia areata
COEUR D’ALENE, IDAHO – Systemic corticosteroids usually bring an impressive initial response for children with alopecia areata. The big question is, ‘What do you try next?’
Children can’t safely remain on long-term systemic steroids. And the great majority of responders will relapse once steroids are discontinued. The challenge is to find a safe, well-tolerated drug with long-term effectiveness as a maintenance therapy.
"For me, when I look at the list of drugs for maintenance therapy in alopecia areata, which includes cyclosporine, azathioprine, and sulfasalazine, the drug I’m most comfortable giving to children for a prolonged time is methotrexate. That’s why this drug has really become my go-to maintenance therapy for patients with alopecia areata," Dr. Ruth Ann Vleugels said at the annual meeting of the Society for Pediatric Dermatology.
"I will give them 2-3 months of systemic steroids as a boost while their methotrexate kicks in; but after that, it’s maintenance therapy with methotrexate at 1 mg/kg/week," added Dr. Vleugels, director of the Autoimmune Skin Disease Program at Brigham and Women’s Hospital, Boston.
Of course, methotrexate doesn’t work as maintenance therapy for all children with alopecia areata. Patients who don’t respond to the initial several months of combination therapy with systemic steroids won’t respond to maintenance therapy with methotrexate alone.
For those few patients who need second-line maintenance therapy, Dr. Vleugels’ drug of choice is the transplant anti-rejection medication mycophenolate mofetil (CellCept), which is used off-label for alopecia areata. Mycophenolate mofetil also is her rescue agent as maintenance therapy for children with systemic lupus erythematosus, cutaneous lupus erythematosus, juvenile dermatomyositis, and linear morphea with progressive hemifacial atrophy.
In patients with progressive juvenile systemic sclerosis, Dr. Vleugels and her Boston colleagues now use mycophenolate mofetil, rather than methotrexate, as their first-line therapy based upon favorable results in their practice and prospective studies in adults (J. Rheumatol. 2012;39:1241-7).
The mycophenolate mofetil dosing in children with alopecia areata is 600 mg/m2 given twice daily, or 1 g twice daily in patients bigger than 1.5 m2. She uses half the dose for the first 2-3 weeks, then titrates up to full-dose therapy in order to minimize GI side effects, which she said are far and away the most common adverse effects. Smaller children do best with the liquid formulation.
A common clinical dilemma involves the child who experiences intolerable GI upset on mycophenolate mofetil. Dr. Vleugels offered two suggestions: One is to tell the parents to ignore the package insert and have their child take the drug with meals.
"No one can handle this drug on an empty stomach," she said.
A caveat: mycophenolate mofetil cannot be taken with antacids.
If mealtime dosing doesn’t solve the GI upset problem, the alternative is to switch to mycophenolic acid (Myfortic), which is enteric-coated, has better bioavailability, and is better tolerated. It’s also more expensive than mycophenolate mofetil and will require prior authorization from payers. The dose conversion is as follows: 500 mg mycophenolate mofetil equals 360 mg of mycophenolic acid.
Dr. Vleugels reported having no financial conflicts with regard to her presentation.
COEUR D’ALENE, IDAHO – Systemic corticosteroids usually bring an impressive initial response for children with alopecia areata. The big question is, ‘What do you try next?’
Children can’t safely remain on long-term systemic steroids. And the great majority of responders will relapse once steroids are discontinued. The challenge is to find a safe, well-tolerated drug with long-term effectiveness as a maintenance therapy.
"For me, when I look at the list of drugs for maintenance therapy in alopecia areata, which includes cyclosporine, azathioprine, and sulfasalazine, the drug I’m most comfortable giving to children for a prolonged time is methotrexate. That’s why this drug has really become my go-to maintenance therapy for patients with alopecia areata," Dr. Ruth Ann Vleugels said at the annual meeting of the Society for Pediatric Dermatology.
"I will give them 2-3 months of systemic steroids as a boost while their methotrexate kicks in; but after that, it’s maintenance therapy with methotrexate at 1 mg/kg/week," added Dr. Vleugels, director of the Autoimmune Skin Disease Program at Brigham and Women’s Hospital, Boston.
Of course, methotrexate doesn’t work as maintenance therapy for all children with alopecia areata. Patients who don’t respond to the initial several months of combination therapy with systemic steroids won’t respond to maintenance therapy with methotrexate alone.
For those few patients who need second-line maintenance therapy, Dr. Vleugels’ drug of choice is the transplant anti-rejection medication mycophenolate mofetil (CellCept), which is used off-label for alopecia areata. Mycophenolate mofetil also is her rescue agent as maintenance therapy for children with systemic lupus erythematosus, cutaneous lupus erythematosus, juvenile dermatomyositis, and linear morphea with progressive hemifacial atrophy.
In patients with progressive juvenile systemic sclerosis, Dr. Vleugels and her Boston colleagues now use mycophenolate mofetil, rather than methotrexate, as their first-line therapy based upon favorable results in their practice and prospective studies in adults (J. Rheumatol. 2012;39:1241-7).
The mycophenolate mofetil dosing in children with alopecia areata is 600 mg/m2 given twice daily, or 1 g twice daily in patients bigger than 1.5 m2. She uses half the dose for the first 2-3 weeks, then titrates up to full-dose therapy in order to minimize GI side effects, which she said are far and away the most common adverse effects. Smaller children do best with the liquid formulation.
A common clinical dilemma involves the child who experiences intolerable GI upset on mycophenolate mofetil. Dr. Vleugels offered two suggestions: One is to tell the parents to ignore the package insert and have their child take the drug with meals.
"No one can handle this drug on an empty stomach," she said.
A caveat: mycophenolate mofetil cannot be taken with antacids.
If mealtime dosing doesn’t solve the GI upset problem, the alternative is to switch to mycophenolic acid (Myfortic), which is enteric-coated, has better bioavailability, and is better tolerated. It’s also more expensive than mycophenolate mofetil and will require prior authorization from payers. The dose conversion is as follows: 500 mg mycophenolate mofetil equals 360 mg of mycophenolic acid.
Dr. Vleugels reported having no financial conflicts with regard to her presentation.
COEUR D’ALENE, IDAHO – Systemic corticosteroids usually bring an impressive initial response for children with alopecia areata. The big question is, ‘What do you try next?’
Children can’t safely remain on long-term systemic steroids. And the great majority of responders will relapse once steroids are discontinued. The challenge is to find a safe, well-tolerated drug with long-term effectiveness as a maintenance therapy.
"For me, when I look at the list of drugs for maintenance therapy in alopecia areata, which includes cyclosporine, azathioprine, and sulfasalazine, the drug I’m most comfortable giving to children for a prolonged time is methotrexate. That’s why this drug has really become my go-to maintenance therapy for patients with alopecia areata," Dr. Ruth Ann Vleugels said at the annual meeting of the Society for Pediatric Dermatology.
"I will give them 2-3 months of systemic steroids as a boost while their methotrexate kicks in; but after that, it’s maintenance therapy with methotrexate at 1 mg/kg/week," added Dr. Vleugels, director of the Autoimmune Skin Disease Program at Brigham and Women’s Hospital, Boston.
Of course, methotrexate doesn’t work as maintenance therapy for all children with alopecia areata. Patients who don’t respond to the initial several months of combination therapy with systemic steroids won’t respond to maintenance therapy with methotrexate alone.
For those few patients who need second-line maintenance therapy, Dr. Vleugels’ drug of choice is the transplant anti-rejection medication mycophenolate mofetil (CellCept), which is used off-label for alopecia areata. Mycophenolate mofetil also is her rescue agent as maintenance therapy for children with systemic lupus erythematosus, cutaneous lupus erythematosus, juvenile dermatomyositis, and linear morphea with progressive hemifacial atrophy.
In patients with progressive juvenile systemic sclerosis, Dr. Vleugels and her Boston colleagues now use mycophenolate mofetil, rather than methotrexate, as their first-line therapy based upon favorable results in their practice and prospective studies in adults (J. Rheumatol. 2012;39:1241-7).
The mycophenolate mofetil dosing in children with alopecia areata is 600 mg/m2 given twice daily, or 1 g twice daily in patients bigger than 1.5 m2. She uses half the dose for the first 2-3 weeks, then titrates up to full-dose therapy in order to minimize GI side effects, which she said are far and away the most common adverse effects. Smaller children do best with the liquid formulation.
A common clinical dilemma involves the child who experiences intolerable GI upset on mycophenolate mofetil. Dr. Vleugels offered two suggestions: One is to tell the parents to ignore the package insert and have their child take the drug with meals.
"No one can handle this drug on an empty stomach," she said.
A caveat: mycophenolate mofetil cannot be taken with antacids.
If mealtime dosing doesn’t solve the GI upset problem, the alternative is to switch to mycophenolic acid (Myfortic), which is enteric-coated, has better bioavailability, and is better tolerated. It’s also more expensive than mycophenolate mofetil and will require prior authorization from payers. The dose conversion is as follows: 500 mg mycophenolate mofetil equals 360 mg of mycophenolic acid.
Dr. Vleugels reported having no financial conflicts with regard to her presentation.
EXPERT ANALYSIS FROM THE SPD ANNUAL MEETING
Think methotrexate for juvenile localized scleroderma
COEUR D’ALENE, IDAHO – Long-term use of methotrexate has a lot going for it as first-line therapy for active juvenile localized scleroderma, according to Dr. Francesco Zulian, chief of pediatric rheumatology at the University of Padua (Italy).
"It’s a drug that’s very old, it’s not expensive, it’s used in many dermatologic and rheumatologic conditions – and it is very useful in patients with scleroderma," he observed in his Sidney Hurwitz Memorial Lecture at the annual meeting of the Society for Pediatric Dermatology.
The initial studies of methotrexate in scleroderma were conducted in adults. Dr. Zulian and colleagues are credited with performing the first randomized, double-blind, prospective clinical trial in pediatric patients, building upon other investigators’ favorable earlier nonrandomized results.
Based upon the randomized trial findings and the subsequent long-term follow-up study, his recommendation for patients with active juvenile localized scleroderma – whether of the linear, pansclerotic, or generalized morphea subtype – is 3 months of initial bridging therapy with a combination of methotrexate plus systemic corticosteroids, followed by at least 24 months of methotrexate without systemic steroids.
In the long-term follow-up study involving 65 patients, treatment was associated with a 74% clinical remission rate. This broke down as approximately a 54% complete remission rate maintained for at least 6 months without treatment, and a 20% clinical remission rate on treatment. Treatment for less than 24 months yielded lesser long-term benefit. Adverse effects were seen in nearly half of patients; however, they were typically mild, and no patients discontinued treatment as a result (J. Am. Acad. Dermatol. 2012;67:1151-6).
"This study shows there is a large group of patients who get better with a relatively mild treatment," Dr. Zulian noted.
The bridging therapy regimen employed in the landmark double-blind, placebo-controlled trial involved oral methotrexate at 15 mg/m2 or a maximum of 20 mg per week, along with prednisone at 1 mg/kg/day or a maximum of 50 mg daily for 3 months (Arthritis Rheum. 2011;63:1998-2006).
In his own clinical practice, Dr. Zulian said, he turns to mycophenolate mofetil (CellCept) in the minority of cases in which bridging therapy with methotrexate and prednisone proves inadequate.
In patients with circumscribed morphea as defined in the international Padua consensus conference guidelines, subsequently formalized by the American College of Rheumatology, the European League Against Rheumatism, and the Pediatric Rheumatism European Society (Arthritis Rheum. 2007;203-12), his recommended treatment is topical corticosteroids, calcipotriol, or phototherapy.
While scleroderma is a rare condition in children, it is nonetheless the third most frequent condition within pediatric rheumatology. For physicians with affected patients who are interested in collaborative research to advance the treatment and understanding of this disease, Dr. Zulian recommended contacting the Juvenile Scleroderma International Network (www.jusinet.org).
Dr. Zulian reported having no financial conflicts with regard to his presentation.
COEUR D’ALENE, IDAHO – Long-term use of methotrexate has a lot going for it as first-line therapy for active juvenile localized scleroderma, according to Dr. Francesco Zulian, chief of pediatric rheumatology at the University of Padua (Italy).
"It’s a drug that’s very old, it’s not expensive, it’s used in many dermatologic and rheumatologic conditions – and it is very useful in patients with scleroderma," he observed in his Sidney Hurwitz Memorial Lecture at the annual meeting of the Society for Pediatric Dermatology.
The initial studies of methotrexate in scleroderma were conducted in adults. Dr. Zulian and colleagues are credited with performing the first randomized, double-blind, prospective clinical trial in pediatric patients, building upon other investigators’ favorable earlier nonrandomized results.
Based upon the randomized trial findings and the subsequent long-term follow-up study, his recommendation for patients with active juvenile localized scleroderma – whether of the linear, pansclerotic, or generalized morphea subtype – is 3 months of initial bridging therapy with a combination of methotrexate plus systemic corticosteroids, followed by at least 24 months of methotrexate without systemic steroids.
In the long-term follow-up study involving 65 patients, treatment was associated with a 74% clinical remission rate. This broke down as approximately a 54% complete remission rate maintained for at least 6 months without treatment, and a 20% clinical remission rate on treatment. Treatment for less than 24 months yielded lesser long-term benefit. Adverse effects were seen in nearly half of patients; however, they were typically mild, and no patients discontinued treatment as a result (J. Am. Acad. Dermatol. 2012;67:1151-6).
"This study shows there is a large group of patients who get better with a relatively mild treatment," Dr. Zulian noted.
The bridging therapy regimen employed in the landmark double-blind, placebo-controlled trial involved oral methotrexate at 15 mg/m2 or a maximum of 20 mg per week, along with prednisone at 1 mg/kg/day or a maximum of 50 mg daily for 3 months (Arthritis Rheum. 2011;63:1998-2006).
In his own clinical practice, Dr. Zulian said, he turns to mycophenolate mofetil (CellCept) in the minority of cases in which bridging therapy with methotrexate and prednisone proves inadequate.
In patients with circumscribed morphea as defined in the international Padua consensus conference guidelines, subsequently formalized by the American College of Rheumatology, the European League Against Rheumatism, and the Pediatric Rheumatism European Society (Arthritis Rheum. 2007;203-12), his recommended treatment is topical corticosteroids, calcipotriol, or phototherapy.
While scleroderma is a rare condition in children, it is nonetheless the third most frequent condition within pediatric rheumatology. For physicians with affected patients who are interested in collaborative research to advance the treatment and understanding of this disease, Dr. Zulian recommended contacting the Juvenile Scleroderma International Network (www.jusinet.org).
Dr. Zulian reported having no financial conflicts with regard to his presentation.
COEUR D’ALENE, IDAHO – Long-term use of methotrexate has a lot going for it as first-line therapy for active juvenile localized scleroderma, according to Dr. Francesco Zulian, chief of pediatric rheumatology at the University of Padua (Italy).
"It’s a drug that’s very old, it’s not expensive, it’s used in many dermatologic and rheumatologic conditions – and it is very useful in patients with scleroderma," he observed in his Sidney Hurwitz Memorial Lecture at the annual meeting of the Society for Pediatric Dermatology.
The initial studies of methotrexate in scleroderma were conducted in adults. Dr. Zulian and colleagues are credited with performing the first randomized, double-blind, prospective clinical trial in pediatric patients, building upon other investigators’ favorable earlier nonrandomized results.
Based upon the randomized trial findings and the subsequent long-term follow-up study, his recommendation for patients with active juvenile localized scleroderma – whether of the linear, pansclerotic, or generalized morphea subtype – is 3 months of initial bridging therapy with a combination of methotrexate plus systemic corticosteroids, followed by at least 24 months of methotrexate without systemic steroids.
In the long-term follow-up study involving 65 patients, treatment was associated with a 74% clinical remission rate. This broke down as approximately a 54% complete remission rate maintained for at least 6 months without treatment, and a 20% clinical remission rate on treatment. Treatment for less than 24 months yielded lesser long-term benefit. Adverse effects were seen in nearly half of patients; however, they were typically mild, and no patients discontinued treatment as a result (J. Am. Acad. Dermatol. 2012;67:1151-6).
"This study shows there is a large group of patients who get better with a relatively mild treatment," Dr. Zulian noted.
The bridging therapy regimen employed in the landmark double-blind, placebo-controlled trial involved oral methotrexate at 15 mg/m2 or a maximum of 20 mg per week, along with prednisone at 1 mg/kg/day or a maximum of 50 mg daily for 3 months (Arthritis Rheum. 2011;63:1998-2006).
In his own clinical practice, Dr. Zulian said, he turns to mycophenolate mofetil (CellCept) in the minority of cases in which bridging therapy with methotrexate and prednisone proves inadequate.
In patients with circumscribed morphea as defined in the international Padua consensus conference guidelines, subsequently formalized by the American College of Rheumatology, the European League Against Rheumatism, and the Pediatric Rheumatism European Society (Arthritis Rheum. 2007;203-12), his recommended treatment is topical corticosteroids, calcipotriol, or phototherapy.
While scleroderma is a rare condition in children, it is nonetheless the third most frequent condition within pediatric rheumatology. For physicians with affected patients who are interested in collaborative research to advance the treatment and understanding of this disease, Dr. Zulian recommended contacting the Juvenile Scleroderma International Network (www.jusinet.org).
Dr. Zulian reported having no financial conflicts with regard to his presentation.
EXPERT ANALYSIS FROM THE SPD ANNUAL MEETING
Ask about sleep sweat before diagnosing primary focal hyperhidrosis
COEUR D’ALENE, IDAHO – An individual who complains of excessive focal sweating that continues during sleep does not – repeat, not – have primary focal hyperhidrosis, according to pediatric dermatologist Dr. Jane S. Bellet.
"If you remember nothing else I tell you today, the sweating must cease during sleep. This is absolutely critical. Otherwise you’re dealing with something completely different. So I really press every single patient on that question," she emphasized at the annual meeting of the Society for Pediatric Dermatology.
Primary focal hyperhidrosis occurs in 1.6% of children and adolescents. Two-thirds of affected patients have a positive family history.
"This is really a life-altering condition, and if untreated it will continue unabated into adulthood. There are effective treatment options. We can make a profound difference for these children and adolescents," she said.
Primary focal hyperhidrosis is a clinical diagnosis. It’s based upon the patient’s history, a review of symptoms, and physical examination. No tests are needed, although Minor’s starch iodine test or the older quinizarin test can be useful as documentation for insurance purposes or to guide botulinum toxin A therapy, according to Dr. Bellet of Duke University, Durham, N.C.
According to the widely accepted, decade-old diagnostic criteria developed by a multispecialty expert working group, the diagnosis requires visible, excessive, focal sweating of the axillae, palms, soles, or face/head for at least 6 months with no apparent cause, plus at least two of six additional criteria. These are onset before age 25 years; bilateral, relatively symmetric sweating; impairment of daily activities; cessation during sleep; a positive family history for the disorder; and at least one episode per week (J. Am. Acad. Dermatol. 2004;51:274-86).
Sweating that continues during sleep is due to one of many possible secondary causes, which are elaborated upon in the multispecialty working group’s report.
"It’s a completely separate algorithm, but if you’re dealing with secondary hyperhidrosis there will always be clues that will send you down that pathway," she said.
The patient history is critical in establishing the diagnosis of primary focal hyperhidrosis and its adverse effect on quality of life and daily activities, which is important to document for insurance purposes.
The physical exam is "pretty basic," according to Dr. Bellet. She uses it as an opportunity to touch the patient and establish a bond.
"Shake hands. It shows the patient you understand their condition, and you know their embarrassment. Resist the temptation to wipe your hands on your pants afterward," she advised.
The review of symptoms is essentially a screen for secondary causes of hyperhidrosis to make sure that the correct diagnosis is indeed primary focal hyperhidrosis. Fever, headache, weight loss, abdominal pain, vomiting, palpitations, anorexia – these point toward a secondary cause. Medications that can cause a generalized sweating problem include antidepressants, antimigraine medications, beta-agonists, pilocarpine, insulin, and GnRH agonists.
Dr. Bellet reported having no financial conflicts of interest regarding her presentation.
COEUR D’ALENE, IDAHO – An individual who complains of excessive focal sweating that continues during sleep does not – repeat, not – have primary focal hyperhidrosis, according to pediatric dermatologist Dr. Jane S. Bellet.
"If you remember nothing else I tell you today, the sweating must cease during sleep. This is absolutely critical. Otherwise you’re dealing with something completely different. So I really press every single patient on that question," she emphasized at the annual meeting of the Society for Pediatric Dermatology.
Primary focal hyperhidrosis occurs in 1.6% of children and adolescents. Two-thirds of affected patients have a positive family history.
"This is really a life-altering condition, and if untreated it will continue unabated into adulthood. There are effective treatment options. We can make a profound difference for these children and adolescents," she said.
Primary focal hyperhidrosis is a clinical diagnosis. It’s based upon the patient’s history, a review of symptoms, and physical examination. No tests are needed, although Minor’s starch iodine test or the older quinizarin test can be useful as documentation for insurance purposes or to guide botulinum toxin A therapy, according to Dr. Bellet of Duke University, Durham, N.C.
According to the widely accepted, decade-old diagnostic criteria developed by a multispecialty expert working group, the diagnosis requires visible, excessive, focal sweating of the axillae, palms, soles, or face/head for at least 6 months with no apparent cause, plus at least two of six additional criteria. These are onset before age 25 years; bilateral, relatively symmetric sweating; impairment of daily activities; cessation during sleep; a positive family history for the disorder; and at least one episode per week (J. Am. Acad. Dermatol. 2004;51:274-86).
Sweating that continues during sleep is due to one of many possible secondary causes, which are elaborated upon in the multispecialty working group’s report.
"It’s a completely separate algorithm, but if you’re dealing with secondary hyperhidrosis there will always be clues that will send you down that pathway," she said.
The patient history is critical in establishing the diagnosis of primary focal hyperhidrosis and its adverse effect on quality of life and daily activities, which is important to document for insurance purposes.
The physical exam is "pretty basic," according to Dr. Bellet. She uses it as an opportunity to touch the patient and establish a bond.
"Shake hands. It shows the patient you understand their condition, and you know their embarrassment. Resist the temptation to wipe your hands on your pants afterward," she advised.
The review of symptoms is essentially a screen for secondary causes of hyperhidrosis to make sure that the correct diagnosis is indeed primary focal hyperhidrosis. Fever, headache, weight loss, abdominal pain, vomiting, palpitations, anorexia – these point toward a secondary cause. Medications that can cause a generalized sweating problem include antidepressants, antimigraine medications, beta-agonists, pilocarpine, insulin, and GnRH agonists.
Dr. Bellet reported having no financial conflicts of interest regarding her presentation.
COEUR D’ALENE, IDAHO – An individual who complains of excessive focal sweating that continues during sleep does not – repeat, not – have primary focal hyperhidrosis, according to pediatric dermatologist Dr. Jane S. Bellet.
"If you remember nothing else I tell you today, the sweating must cease during sleep. This is absolutely critical. Otherwise you’re dealing with something completely different. So I really press every single patient on that question," she emphasized at the annual meeting of the Society for Pediatric Dermatology.
Primary focal hyperhidrosis occurs in 1.6% of children and adolescents. Two-thirds of affected patients have a positive family history.
"This is really a life-altering condition, and if untreated it will continue unabated into adulthood. There are effective treatment options. We can make a profound difference for these children and adolescents," she said.
Primary focal hyperhidrosis is a clinical diagnosis. It’s based upon the patient’s history, a review of symptoms, and physical examination. No tests are needed, although Minor’s starch iodine test or the older quinizarin test can be useful as documentation for insurance purposes or to guide botulinum toxin A therapy, according to Dr. Bellet of Duke University, Durham, N.C.
According to the widely accepted, decade-old diagnostic criteria developed by a multispecialty expert working group, the diagnosis requires visible, excessive, focal sweating of the axillae, palms, soles, or face/head for at least 6 months with no apparent cause, plus at least two of six additional criteria. These are onset before age 25 years; bilateral, relatively symmetric sweating; impairment of daily activities; cessation during sleep; a positive family history for the disorder; and at least one episode per week (J. Am. Acad. Dermatol. 2004;51:274-86).
Sweating that continues during sleep is due to one of many possible secondary causes, which are elaborated upon in the multispecialty working group’s report.
"It’s a completely separate algorithm, but if you’re dealing with secondary hyperhidrosis there will always be clues that will send you down that pathway," she said.
The patient history is critical in establishing the diagnosis of primary focal hyperhidrosis and its adverse effect on quality of life and daily activities, which is important to document for insurance purposes.
The physical exam is "pretty basic," according to Dr. Bellet. She uses it as an opportunity to touch the patient and establish a bond.
"Shake hands. It shows the patient you understand their condition, and you know their embarrassment. Resist the temptation to wipe your hands on your pants afterward," she advised.
The review of symptoms is essentially a screen for secondary causes of hyperhidrosis to make sure that the correct diagnosis is indeed primary focal hyperhidrosis. Fever, headache, weight loss, abdominal pain, vomiting, palpitations, anorexia – these point toward a secondary cause. Medications that can cause a generalized sweating problem include antidepressants, antimigraine medications, beta-agonists, pilocarpine, insulin, and GnRH agonists.
Dr. Bellet reported having no financial conflicts of interest regarding her presentation.
EXPERT ANALYSIS FROM THE SPD ANNUAL MEETING
Patch testing tricks in atopic dermatitis with concomitant contact dermatitis
COEUR D’ALENE, IDAHO– Contact dermatitis goes together with moderate-to-severe atopic dermatitis like ham and eggs. Reading patch test results in such patients poses unique challenges, because of the impaired skin barrier function intrinsic to atopic dermatitis, coupled with the moist environment created under the occlusive patches, which predisposes to Staphylococcus aureus colonization and superinfection.
All of this makes the evaluation of patch test results in atopic dermatitis more complicated than in patients without contact dermatitis. But there are tricks that greatly reduce the difficulty.
"Patch testing can play a crucial role in the work-up and management of patients with refractory atopic dermatitis. Prior to patch testing, measures should be taken to improve the skin barrier and reduce bacterial overload," Dr. Sharon E. Jacob advised at the annual meeting of the Society for Pediatric Dermatology.
Her recommended preparation program starts 3 weeks prior to the scheduled patch test. The emphasis is on beefing up the disrupted skin barrier through the use of lipid-replenishing emollients and nonalkaline soaps, avoidance of fragrances and other irritant allergens, and preemptive treatment of S. aureus colonization or superinfection, explained Dr. Jacob of Loma Linda (Calif.) University.
At the start of the 3-week countdown, a patient with no clinical signs of skin infection should be checked for nasal carriage of S. aureus. If positive, or if the patient has a remote history of S. aureus colonization or superinfection but no current signs of colonization, such as oozing or crusting, it’s appropriate to begin intranasal, perianal, and umbilical topical mupirocin twice daily for 5 days.
It’s also time to eliminate irritant allergens, start a regimen of dilute bleach baths, embrace the special emollients and soaps, and make sure areas of dermatitis are getting adequately treated with topical steroids, except for the planned patch test area, which must remain steroid free for 7 days prior to the test day.
The lipid-containing emollients, which should contain ceramides or filaggrin degradation products, are to be used all over the body, including the back, until the day before the test.
Examples of the nonalkaline soaps, which are employed to maintain an acidic skin pH, include Aveeno Moisturizing Bar soap, Cetaphil Gentle Cleansing Bar, and Dove Sensitive Skin Unscented Beauty Bar, the pediatric dermatologist continued.
Patients on UV phototherapy for their atopic dermatitis can continue except at the planned patch test site, where it should be avoided for 2 weeks beforehand.
In an adolescent with head and neck atopic dermatitis, a pre–patch test course of oral antifungal therapy is worth considering, according to Dr. Jacob.
For the atopic dermatitis patient with clinical signs of bacterial infection at the pre–patch test office visit, culture the lesions and start a preemptive 10-day course of oral cephalexin at 25-50 mg/kg per day three times daily beginning 3 days prior to patch testing, with the choice of antimicrobial adjusted as warranted by the culture results. These patients also go on the emollients, soaps, dilute bleach baths, and irritant allergen avoidance regimen.
A patient with no signs of skin infection when the test patches are removed can continue with the test readings as scheduled, with no further intervention. However, if signs of infection are present, the patient should immediately go on oral cephalexin if not already on it and take a single bleach bath the same day the patches come off. The bath should be at one-tenth to one-half the customary concentration of 0.005% sodium hypochlorite and should be followed by a fresh water rinse.
The usual patch test reading schedule in atopic dermatitis patients is at 24-48 hours, again at 72-96 hours, and once again at 120 hours. That last reading is vital because patients with atopic dermatitis can have a low irritant threshold; the delayed reading lessens the possibility of reading irritant reactions as positives.
The reading at 72-96 hours is the time for induration testing.
"When evaluating patch test reactions, palpation for induration is absolutely necessary," Dr. Jacob emphasized. "First the evaluator palpates the baseline dermatitis, and then the patch application squares, looking for areas of increased induration and effectively subtracting background."
She reported serving as a consultant to Johnson & Johnson and Medimetriks and as a clinical investigator for SmartPractice, which manufactures patch test kits, the use of which remains nonapproved by the Food and Drug Administration in children.
COEUR D’ALENE, IDAHO– Contact dermatitis goes together with moderate-to-severe atopic dermatitis like ham and eggs. Reading patch test results in such patients poses unique challenges, because of the impaired skin barrier function intrinsic to atopic dermatitis, coupled with the moist environment created under the occlusive patches, which predisposes to Staphylococcus aureus colonization and superinfection.
All of this makes the evaluation of patch test results in atopic dermatitis more complicated than in patients without contact dermatitis. But there are tricks that greatly reduce the difficulty.
"Patch testing can play a crucial role in the work-up and management of patients with refractory atopic dermatitis. Prior to patch testing, measures should be taken to improve the skin barrier and reduce bacterial overload," Dr. Sharon E. Jacob advised at the annual meeting of the Society for Pediatric Dermatology.
Her recommended preparation program starts 3 weeks prior to the scheduled patch test. The emphasis is on beefing up the disrupted skin barrier through the use of lipid-replenishing emollients and nonalkaline soaps, avoidance of fragrances and other irritant allergens, and preemptive treatment of S. aureus colonization or superinfection, explained Dr. Jacob of Loma Linda (Calif.) University.
At the start of the 3-week countdown, a patient with no clinical signs of skin infection should be checked for nasal carriage of S. aureus. If positive, or if the patient has a remote history of S. aureus colonization or superinfection but no current signs of colonization, such as oozing or crusting, it’s appropriate to begin intranasal, perianal, and umbilical topical mupirocin twice daily for 5 days.
It’s also time to eliminate irritant allergens, start a regimen of dilute bleach baths, embrace the special emollients and soaps, and make sure areas of dermatitis are getting adequately treated with topical steroids, except for the planned patch test area, which must remain steroid free for 7 days prior to the test day.
The lipid-containing emollients, which should contain ceramides or filaggrin degradation products, are to be used all over the body, including the back, until the day before the test.
Examples of the nonalkaline soaps, which are employed to maintain an acidic skin pH, include Aveeno Moisturizing Bar soap, Cetaphil Gentle Cleansing Bar, and Dove Sensitive Skin Unscented Beauty Bar, the pediatric dermatologist continued.
Patients on UV phototherapy for their atopic dermatitis can continue except at the planned patch test site, where it should be avoided for 2 weeks beforehand.
In an adolescent with head and neck atopic dermatitis, a pre–patch test course of oral antifungal therapy is worth considering, according to Dr. Jacob.
For the atopic dermatitis patient with clinical signs of bacterial infection at the pre–patch test office visit, culture the lesions and start a preemptive 10-day course of oral cephalexin at 25-50 mg/kg per day three times daily beginning 3 days prior to patch testing, with the choice of antimicrobial adjusted as warranted by the culture results. These patients also go on the emollients, soaps, dilute bleach baths, and irritant allergen avoidance regimen.
A patient with no signs of skin infection when the test patches are removed can continue with the test readings as scheduled, with no further intervention. However, if signs of infection are present, the patient should immediately go on oral cephalexin if not already on it and take a single bleach bath the same day the patches come off. The bath should be at one-tenth to one-half the customary concentration of 0.005% sodium hypochlorite and should be followed by a fresh water rinse.
The usual patch test reading schedule in atopic dermatitis patients is at 24-48 hours, again at 72-96 hours, and once again at 120 hours. That last reading is vital because patients with atopic dermatitis can have a low irritant threshold; the delayed reading lessens the possibility of reading irritant reactions as positives.
The reading at 72-96 hours is the time for induration testing.
"When evaluating patch test reactions, palpation for induration is absolutely necessary," Dr. Jacob emphasized. "First the evaluator palpates the baseline dermatitis, and then the patch application squares, looking for areas of increased induration and effectively subtracting background."
She reported serving as a consultant to Johnson & Johnson and Medimetriks and as a clinical investigator for SmartPractice, which manufactures patch test kits, the use of which remains nonapproved by the Food and Drug Administration in children.
COEUR D’ALENE, IDAHO– Contact dermatitis goes together with moderate-to-severe atopic dermatitis like ham and eggs. Reading patch test results in such patients poses unique challenges, because of the impaired skin barrier function intrinsic to atopic dermatitis, coupled with the moist environment created under the occlusive patches, which predisposes to Staphylococcus aureus colonization and superinfection.
All of this makes the evaluation of patch test results in atopic dermatitis more complicated than in patients without contact dermatitis. But there are tricks that greatly reduce the difficulty.
"Patch testing can play a crucial role in the work-up and management of patients with refractory atopic dermatitis. Prior to patch testing, measures should be taken to improve the skin barrier and reduce bacterial overload," Dr. Sharon E. Jacob advised at the annual meeting of the Society for Pediatric Dermatology.
Her recommended preparation program starts 3 weeks prior to the scheduled patch test. The emphasis is on beefing up the disrupted skin barrier through the use of lipid-replenishing emollients and nonalkaline soaps, avoidance of fragrances and other irritant allergens, and preemptive treatment of S. aureus colonization or superinfection, explained Dr. Jacob of Loma Linda (Calif.) University.
At the start of the 3-week countdown, a patient with no clinical signs of skin infection should be checked for nasal carriage of S. aureus. If positive, or if the patient has a remote history of S. aureus colonization or superinfection but no current signs of colonization, such as oozing or crusting, it’s appropriate to begin intranasal, perianal, and umbilical topical mupirocin twice daily for 5 days.
It’s also time to eliminate irritant allergens, start a regimen of dilute bleach baths, embrace the special emollients and soaps, and make sure areas of dermatitis are getting adequately treated with topical steroids, except for the planned patch test area, which must remain steroid free for 7 days prior to the test day.
The lipid-containing emollients, which should contain ceramides or filaggrin degradation products, are to be used all over the body, including the back, until the day before the test.
Examples of the nonalkaline soaps, which are employed to maintain an acidic skin pH, include Aveeno Moisturizing Bar soap, Cetaphil Gentle Cleansing Bar, and Dove Sensitive Skin Unscented Beauty Bar, the pediatric dermatologist continued.
Patients on UV phototherapy for their atopic dermatitis can continue except at the planned patch test site, where it should be avoided for 2 weeks beforehand.
In an adolescent with head and neck atopic dermatitis, a pre–patch test course of oral antifungal therapy is worth considering, according to Dr. Jacob.
For the atopic dermatitis patient with clinical signs of bacterial infection at the pre–patch test office visit, culture the lesions and start a preemptive 10-day course of oral cephalexin at 25-50 mg/kg per day three times daily beginning 3 days prior to patch testing, with the choice of antimicrobial adjusted as warranted by the culture results. These patients also go on the emollients, soaps, dilute bleach baths, and irritant allergen avoidance regimen.
A patient with no signs of skin infection when the test patches are removed can continue with the test readings as scheduled, with no further intervention. However, if signs of infection are present, the patient should immediately go on oral cephalexin if not already on it and take a single bleach bath the same day the patches come off. The bath should be at one-tenth to one-half the customary concentration of 0.005% sodium hypochlorite and should be followed by a fresh water rinse.
The usual patch test reading schedule in atopic dermatitis patients is at 24-48 hours, again at 72-96 hours, and once again at 120 hours. That last reading is vital because patients with atopic dermatitis can have a low irritant threshold; the delayed reading lessens the possibility of reading irritant reactions as positives.
The reading at 72-96 hours is the time for induration testing.
"When evaluating patch test reactions, palpation for induration is absolutely necessary," Dr. Jacob emphasized. "First the evaluator palpates the baseline dermatitis, and then the patch application squares, looking for areas of increased induration and effectively subtracting background."
She reported serving as a consultant to Johnson & Johnson and Medimetriks and as a clinical investigator for SmartPractice, which manufactures patch test kits, the use of which remains nonapproved by the Food and Drug Administration in children.
EXPERT ANALYSIS FROM THE SPD 2014