User login
Expert highlights rare causes of stroke to keep in mind
ATLANTA – According to Peter Berlit, MD, clinicians should .
Other factors include combination of ischemic and hemorrhagic stroke, exclusive involvement of intracranial vessels, systemic signs, and lab tests indicating inflammation.
At the annual meeting of the American Neurological Association, Dr. Berlit, secretary general of the German Society of Neurology in Berlin, discussed the diagnosis and management of rare causes of stroke.
Giant cell arteritis (GCA)
One of the rare causes of stroke, GCA can be diagnosed when three of five criteria are met: being 50 years of age or older, having a newly developed headache, tenderness of the superficial temporal artery, elevated sedimentation rate of at least 50 mm per hour, and GCA in a biopsy specimen from the temporal artery.
“What we fear most is sudden blindness due to involvement of arteries serving the eyes, which appears in up to 30% of GCA patients,” said Dr. Berlit, who formerly chaired the department of neurology at Alfried Krupp Hospital, Essen, Germany. “Stroke occurs in approximately 2% of GCA patients, so it’s a lot rarer.” GCA can also be diagnosed by ultrasound. One meta-analysis of 23 studies using halo, stenosis, and occlusion as ultrasound criteria found a sensitivity of 87% and a specificity of 96% (Ann Intern Med. 2005;142[5]:359-69). “You can also use 3-Tesla MRI with the use of contrast agent, which shows inflammation of the temporal artery, but also other large vessels including the aortic arch,” he said. “The treatment of GCA has changed since the end of 2017 and involves starting with prednisolone 1 mg/kg body weight.” After a dose of 30 mg for 4 weeks, reduce the dose by 2.5 mg every 2 weeks. After reaching the dose of 15 mg daily, reduce by 1 mg per month. “The recommended steroid-sparing treatment is subcutaneous tocilizumab at a dose of 162 mg weekly or every other week, combined with a prednisone taper for a minimum of 26 weeks,” he said. Supportive therapies include pantoprazole 20 mg, aspirin 100 mg, calcium, vitamin D, and bisphosphonates.
Primary angiitis of the central nervous system (PACNS)
Next, Dr. Berlit discussed diagnostic criteria for PACNS, an acquired neurological deficit unexplained after complete evaluation. “You should have a diagnostic cerebral angiogram or biopsy demonstrating vasculitis,” he said. “There should be no evidence of systemic vasculitis or any other conditions that could mimic the angiogram findings. Usually you have abnormal CSF findings, including pleocytosis and protein elevation, and a biopsy demonstrating vasculitis.”
MRI studies in suspected vasculitis include fluid-attenuated inversion recovery (FLAIR), diffusion imaging with apparent diffusion coefficient (ADC) maps, gradient ECHO, MR angiography, and contrast-enhanced imaging. “These usually show multifocal lesions of different ages, and hemorrhages occur in about 10% of lesions,” Dr. Berlit said. “Leptomeningeal enhancement is an indicator of good treatment response.”
A brain and leptomeningeal biopsy demonstrating the angiitis remains the preferred method for diagnosis of PACNS. “Open biopsies out of recent MRI lesions are especially diagnostic,” he said. “If there are no lesions accessible for surgery in noneloquent brain areas, a biopsy from the right frontal lobe is recommended.” The histologic findings of PACNS consist of granulomatous inflammation, fibrinoid necrosis of vessel walls, or exclusively lymphocytic cellular infiltrates. “The treatment of choice in PACNS is the combination of steroids and cyclophosphamide pulse therapy,” he said. “There are also data showing that rituximab or methotrexate might be treatment options. With a relapse rate of 25% and a reduced survival rate, a close follow-up of suspected PACNS is mandatory.”
Reversible cerebral vasoconstriction syndrome (RCVS)
Another rare cause of stroke is RCVS, which typically presents as thunderclap headaches with or without neurologic symptoms. MRI may be normal, but symmetric border zone infarctions and small subarachnoid hemorrhages are possible. Catheter, CT, or MR angiography show segmental arterial vasoconstriction. “You always have to exclude cerebral aneurysm,” Dr. Berlit said. “There is reversibility of RCVS within 3 months.” RCVS is often associated with a long list of drugs, including phenylpropanolamine, Methergine (methylergonovine), bromocriptine, lisuride, SSRIs, triptans, isometheptene, tacrolimus, cyclophosphamide, erythropoietin, intravenous immunoglobulins, erythrocyte concentrates, nasal sprays, cocaine, ecstasy, amphetamines, cannabis, and LSD. “After stopping responsible medications, treatment involves a course of nimodipine,” he said.
Moyamoya disease (MMD)
Dr. Berlit closed his presentation by discussing MMD, a rare occlusive cerebrovascular disorder characterized by progressive stenosis or occlusion of the intracranial portion of the internal carotid artery and proximal cerebral arteries with an extensive network of fine collaterals. “This is an idiopathic vasculopathy with remarkable regional and racial differences worldwide; it’s most frequently found in Asians, especially in Japan and Korea,” he said. “In Europe, there is about one-tenth the incidence, compared with that of Japan. In Asian MMD, about 15% of cases follow an autosomal dominant inheritance. The collaterals in MMD present histologically as a thin media, a fragmented elastic laminae, and the formation of microaneurysms. There is no inflammation.”
MMD diagnostic criteria include stenosis or occlusion of the terminal portion of the internal carotid artery and at the proximal portion of the anterior and middle cerebral arteries. Abnormal vascular networks are present in the basal ganglia and angiographic findings present bilaterally. Cases with unilateral angiographic findings are considered probable. Clinicians should exclude the following conditions: arteriosclerosis, autoimmune disease, brain neoplasm, history of cranial irradiation, Down syndrome, head trauma, neurofibromatosis, and meningitis. “If the angiographic pattern is resembled by one of these conditions, this is called moyamoya syndrome,” Dr. Berlit noted. “MMD is a progressive disorder. Within a few months you can see occlusion of the middle cerebral artery and the anterior cerebral artery, so you have to treat these patients.”
In patients who are white, MMD presents with lower rates of hemorrhage, but in Asians, microbleeds occur in up to 44% of patients and hemorrhages in up to 65% patients. “Both subarachnoidal and intracerebral hemorrhages occur, especially in connection with pregnancy and delivery,” he said. “The risk of both cerebral ischemia and hemorrhagic complications increases with stages of MMD.”
Direct or indirect intracranial bypass surgery is recommended in stages 3 or more, and has been shown to significantly reduce the 5-year stroke risk. To date, Dr. Berlit and his associates have treated 86 hemispheres in 56 patients. The average age of the patients was 42 years, 70% were female, and the average follow-up was 39 months. All intracranial bypasses were open on follow-up, and a decrease of the typical moyamoya vessels was observed in 81% of patients.
Dr. Berlit reported having no financial disclosures.
ATLANTA – According to Peter Berlit, MD, clinicians should .
Other factors include combination of ischemic and hemorrhagic stroke, exclusive involvement of intracranial vessels, systemic signs, and lab tests indicating inflammation.
At the annual meeting of the American Neurological Association, Dr. Berlit, secretary general of the German Society of Neurology in Berlin, discussed the diagnosis and management of rare causes of stroke.
Giant cell arteritis (GCA)
One of the rare causes of stroke, GCA can be diagnosed when three of five criteria are met: being 50 years of age or older, having a newly developed headache, tenderness of the superficial temporal artery, elevated sedimentation rate of at least 50 mm per hour, and GCA in a biopsy specimen from the temporal artery.
“What we fear most is sudden blindness due to involvement of arteries serving the eyes, which appears in up to 30% of GCA patients,” said Dr. Berlit, who formerly chaired the department of neurology at Alfried Krupp Hospital, Essen, Germany. “Stroke occurs in approximately 2% of GCA patients, so it’s a lot rarer.” GCA can also be diagnosed by ultrasound. One meta-analysis of 23 studies using halo, stenosis, and occlusion as ultrasound criteria found a sensitivity of 87% and a specificity of 96% (Ann Intern Med. 2005;142[5]:359-69). “You can also use 3-Tesla MRI with the use of contrast agent, which shows inflammation of the temporal artery, but also other large vessels including the aortic arch,” he said. “The treatment of GCA has changed since the end of 2017 and involves starting with prednisolone 1 mg/kg body weight.” After a dose of 30 mg for 4 weeks, reduce the dose by 2.5 mg every 2 weeks. After reaching the dose of 15 mg daily, reduce by 1 mg per month. “The recommended steroid-sparing treatment is subcutaneous tocilizumab at a dose of 162 mg weekly or every other week, combined with a prednisone taper for a minimum of 26 weeks,” he said. Supportive therapies include pantoprazole 20 mg, aspirin 100 mg, calcium, vitamin D, and bisphosphonates.
Primary angiitis of the central nervous system (PACNS)
Next, Dr. Berlit discussed diagnostic criteria for PACNS, an acquired neurological deficit unexplained after complete evaluation. “You should have a diagnostic cerebral angiogram or biopsy demonstrating vasculitis,” he said. “There should be no evidence of systemic vasculitis or any other conditions that could mimic the angiogram findings. Usually you have abnormal CSF findings, including pleocytosis and protein elevation, and a biopsy demonstrating vasculitis.”
MRI studies in suspected vasculitis include fluid-attenuated inversion recovery (FLAIR), diffusion imaging with apparent diffusion coefficient (ADC) maps, gradient ECHO, MR angiography, and contrast-enhanced imaging. “These usually show multifocal lesions of different ages, and hemorrhages occur in about 10% of lesions,” Dr. Berlit said. “Leptomeningeal enhancement is an indicator of good treatment response.”
A brain and leptomeningeal biopsy demonstrating the angiitis remains the preferred method for diagnosis of PACNS. “Open biopsies out of recent MRI lesions are especially diagnostic,” he said. “If there are no lesions accessible for surgery in noneloquent brain areas, a biopsy from the right frontal lobe is recommended.” The histologic findings of PACNS consist of granulomatous inflammation, fibrinoid necrosis of vessel walls, or exclusively lymphocytic cellular infiltrates. “The treatment of choice in PACNS is the combination of steroids and cyclophosphamide pulse therapy,” he said. “There are also data showing that rituximab or methotrexate might be treatment options. With a relapse rate of 25% and a reduced survival rate, a close follow-up of suspected PACNS is mandatory.”
Reversible cerebral vasoconstriction syndrome (RCVS)
Another rare cause of stroke is RCVS, which typically presents as thunderclap headaches with or without neurologic symptoms. MRI may be normal, but symmetric border zone infarctions and small subarachnoid hemorrhages are possible. Catheter, CT, or MR angiography show segmental arterial vasoconstriction. “You always have to exclude cerebral aneurysm,” Dr. Berlit said. “There is reversibility of RCVS within 3 months.” RCVS is often associated with a long list of drugs, including phenylpropanolamine, Methergine (methylergonovine), bromocriptine, lisuride, SSRIs, triptans, isometheptene, tacrolimus, cyclophosphamide, erythropoietin, intravenous immunoglobulins, erythrocyte concentrates, nasal sprays, cocaine, ecstasy, amphetamines, cannabis, and LSD. “After stopping responsible medications, treatment involves a course of nimodipine,” he said.
Moyamoya disease (MMD)
Dr. Berlit closed his presentation by discussing MMD, a rare occlusive cerebrovascular disorder characterized by progressive stenosis or occlusion of the intracranial portion of the internal carotid artery and proximal cerebral arteries with an extensive network of fine collaterals. “This is an idiopathic vasculopathy with remarkable regional and racial differences worldwide; it’s most frequently found in Asians, especially in Japan and Korea,” he said. “In Europe, there is about one-tenth the incidence, compared with that of Japan. In Asian MMD, about 15% of cases follow an autosomal dominant inheritance. The collaterals in MMD present histologically as a thin media, a fragmented elastic laminae, and the formation of microaneurysms. There is no inflammation.”
MMD diagnostic criteria include stenosis or occlusion of the terminal portion of the internal carotid artery and at the proximal portion of the anterior and middle cerebral arteries. Abnormal vascular networks are present in the basal ganglia and angiographic findings present bilaterally. Cases with unilateral angiographic findings are considered probable. Clinicians should exclude the following conditions: arteriosclerosis, autoimmune disease, brain neoplasm, history of cranial irradiation, Down syndrome, head trauma, neurofibromatosis, and meningitis. “If the angiographic pattern is resembled by one of these conditions, this is called moyamoya syndrome,” Dr. Berlit noted. “MMD is a progressive disorder. Within a few months you can see occlusion of the middle cerebral artery and the anterior cerebral artery, so you have to treat these patients.”
In patients who are white, MMD presents with lower rates of hemorrhage, but in Asians, microbleeds occur in up to 44% of patients and hemorrhages in up to 65% patients. “Both subarachnoidal and intracerebral hemorrhages occur, especially in connection with pregnancy and delivery,” he said. “The risk of both cerebral ischemia and hemorrhagic complications increases with stages of MMD.”
Direct or indirect intracranial bypass surgery is recommended in stages 3 or more, and has been shown to significantly reduce the 5-year stroke risk. To date, Dr. Berlit and his associates have treated 86 hemispheres in 56 patients. The average age of the patients was 42 years, 70% were female, and the average follow-up was 39 months. All intracranial bypasses were open on follow-up, and a decrease of the typical moyamoya vessels was observed in 81% of patients.
Dr. Berlit reported having no financial disclosures.
ATLANTA – According to Peter Berlit, MD, clinicians should .
Other factors include combination of ischemic and hemorrhagic stroke, exclusive involvement of intracranial vessels, systemic signs, and lab tests indicating inflammation.
At the annual meeting of the American Neurological Association, Dr. Berlit, secretary general of the German Society of Neurology in Berlin, discussed the diagnosis and management of rare causes of stroke.
Giant cell arteritis (GCA)
One of the rare causes of stroke, GCA can be diagnosed when three of five criteria are met: being 50 years of age or older, having a newly developed headache, tenderness of the superficial temporal artery, elevated sedimentation rate of at least 50 mm per hour, and GCA in a biopsy specimen from the temporal artery.
“What we fear most is sudden blindness due to involvement of arteries serving the eyes, which appears in up to 30% of GCA patients,” said Dr. Berlit, who formerly chaired the department of neurology at Alfried Krupp Hospital, Essen, Germany. “Stroke occurs in approximately 2% of GCA patients, so it’s a lot rarer.” GCA can also be diagnosed by ultrasound. One meta-analysis of 23 studies using halo, stenosis, and occlusion as ultrasound criteria found a sensitivity of 87% and a specificity of 96% (Ann Intern Med. 2005;142[5]:359-69). “You can also use 3-Tesla MRI with the use of contrast agent, which shows inflammation of the temporal artery, but also other large vessels including the aortic arch,” he said. “The treatment of GCA has changed since the end of 2017 and involves starting with prednisolone 1 mg/kg body weight.” After a dose of 30 mg for 4 weeks, reduce the dose by 2.5 mg every 2 weeks. After reaching the dose of 15 mg daily, reduce by 1 mg per month. “The recommended steroid-sparing treatment is subcutaneous tocilizumab at a dose of 162 mg weekly or every other week, combined with a prednisone taper for a minimum of 26 weeks,” he said. Supportive therapies include pantoprazole 20 mg, aspirin 100 mg, calcium, vitamin D, and bisphosphonates.
Primary angiitis of the central nervous system (PACNS)
Next, Dr. Berlit discussed diagnostic criteria for PACNS, an acquired neurological deficit unexplained after complete evaluation. “You should have a diagnostic cerebral angiogram or biopsy demonstrating vasculitis,” he said. “There should be no evidence of systemic vasculitis or any other conditions that could mimic the angiogram findings. Usually you have abnormal CSF findings, including pleocytosis and protein elevation, and a biopsy demonstrating vasculitis.”
MRI studies in suspected vasculitis include fluid-attenuated inversion recovery (FLAIR), diffusion imaging with apparent diffusion coefficient (ADC) maps, gradient ECHO, MR angiography, and contrast-enhanced imaging. “These usually show multifocal lesions of different ages, and hemorrhages occur in about 10% of lesions,” Dr. Berlit said. “Leptomeningeal enhancement is an indicator of good treatment response.”
A brain and leptomeningeal biopsy demonstrating the angiitis remains the preferred method for diagnosis of PACNS. “Open biopsies out of recent MRI lesions are especially diagnostic,” he said. “If there are no lesions accessible for surgery in noneloquent brain areas, a biopsy from the right frontal lobe is recommended.” The histologic findings of PACNS consist of granulomatous inflammation, fibrinoid necrosis of vessel walls, or exclusively lymphocytic cellular infiltrates. “The treatment of choice in PACNS is the combination of steroids and cyclophosphamide pulse therapy,” he said. “There are also data showing that rituximab or methotrexate might be treatment options. With a relapse rate of 25% and a reduced survival rate, a close follow-up of suspected PACNS is mandatory.”
Reversible cerebral vasoconstriction syndrome (RCVS)
Another rare cause of stroke is RCVS, which typically presents as thunderclap headaches with or without neurologic symptoms. MRI may be normal, but symmetric border zone infarctions and small subarachnoid hemorrhages are possible. Catheter, CT, or MR angiography show segmental arterial vasoconstriction. “You always have to exclude cerebral aneurysm,” Dr. Berlit said. “There is reversibility of RCVS within 3 months.” RCVS is often associated with a long list of drugs, including phenylpropanolamine, Methergine (methylergonovine), bromocriptine, lisuride, SSRIs, triptans, isometheptene, tacrolimus, cyclophosphamide, erythropoietin, intravenous immunoglobulins, erythrocyte concentrates, nasal sprays, cocaine, ecstasy, amphetamines, cannabis, and LSD. “After stopping responsible medications, treatment involves a course of nimodipine,” he said.
Moyamoya disease (MMD)
Dr. Berlit closed his presentation by discussing MMD, a rare occlusive cerebrovascular disorder characterized by progressive stenosis or occlusion of the intracranial portion of the internal carotid artery and proximal cerebral arteries with an extensive network of fine collaterals. “This is an idiopathic vasculopathy with remarkable regional and racial differences worldwide; it’s most frequently found in Asians, especially in Japan and Korea,” he said. “In Europe, there is about one-tenth the incidence, compared with that of Japan. In Asian MMD, about 15% of cases follow an autosomal dominant inheritance. The collaterals in MMD present histologically as a thin media, a fragmented elastic laminae, and the formation of microaneurysms. There is no inflammation.”
MMD diagnostic criteria include stenosis or occlusion of the terminal portion of the internal carotid artery and at the proximal portion of the anterior and middle cerebral arteries. Abnormal vascular networks are present in the basal ganglia and angiographic findings present bilaterally. Cases with unilateral angiographic findings are considered probable. Clinicians should exclude the following conditions: arteriosclerosis, autoimmune disease, brain neoplasm, history of cranial irradiation, Down syndrome, head trauma, neurofibromatosis, and meningitis. “If the angiographic pattern is resembled by one of these conditions, this is called moyamoya syndrome,” Dr. Berlit noted. “MMD is a progressive disorder. Within a few months you can see occlusion of the middle cerebral artery and the anterior cerebral artery, so you have to treat these patients.”
In patients who are white, MMD presents with lower rates of hemorrhage, but in Asians, microbleeds occur in up to 44% of patients and hemorrhages in up to 65% patients. “Both subarachnoidal and intracerebral hemorrhages occur, especially in connection with pregnancy and delivery,” he said. “The risk of both cerebral ischemia and hemorrhagic complications increases with stages of MMD.”
Direct or indirect intracranial bypass surgery is recommended in stages 3 or more, and has been shown to significantly reduce the 5-year stroke risk. To date, Dr. Berlit and his associates have treated 86 hemispheres in 56 patients. The average age of the patients was 42 years, 70% were female, and the average follow-up was 39 months. All intracranial bypasses were open on follow-up, and a decrease of the typical moyamoya vessels was observed in 81% of patients.
Dr. Berlit reported having no financial disclosures.
EXPERT ANALYSIS FROM ANA 2018
Brain stimulation device improved fluency in persons who stutter
ATLANTA – After undergoing 10 days of noninvasive magnetic brain stimulation with a novel device, eight of nine persons who stutter experienced improvements in speech fluency.
“This is the first step in what we believe is a major breakthrough in treatment,” lead study author David B. Rosenfield, MD, said in an interview at the annual meeting of the American Neurological Association.
In previously published work, Dr. Rosenfield, Director of the Speech and Language Center at Houston Methodist Neurological Institute, and his colleagues showed significant reduction in functional connectivity between Broca’s and Wernicke’s areas in persons who stutter, compared with normal speakers, by performing resting-state functional MRI of the brain. In the current open-label pilot trial, they tested the hypothesis that using direct noninvasive synchronous bifocal stimulation to potentiate the strength of connectivity between Broca’s and Wernicke’s areas in persons who stutter should improve their fluency.
Researchers enrolled nine persons who stutter who ranged in age from 18 to 80 years. For 40 minutes each consecutive weekday over the course of 2 weeks, the participants wore a compact, portable device known as a Transcranial Rotating Permanent Magnetic Stimulator (TRPMS) to deliver highly focal stimuli to Broca’s and Wernicke’s area locations specified by 10-20 international electroencephalographic electrode sites on the left side. Magnetic stimuli were 100 milliseconds in duration and delivered at 0.2 Hz. Next, a certified speech-language pathologist viewed video recordings of the study subjects both speaking spontaneously and reading a passage aloud on day 1 (before stimulation), day 5 (after stimulation), and day 10 (after stimulation), to assess fluency using the Stuttering Speech Severity Instrument version 4 (SSI-4).
The researchers found that all study participants significantly improved in fluency on either day 5 (P = 0.01) or day 10 (P = 0.02). Only one subject failed to show improvement on day 10 compared with day 1 after showing it on day 5. “This wasn’t meant to be a 10-day treatment that would last forever; this was meant to be a 10-day treatment to see whether the magnetic stimulation would work,” Dr. Rosenfield said. “It might well be that patients need treatment every day, once a week, or once a month. All we can say is that we have an input and an output. We gave them the treatment and they improved. One patient was so happy with it that he begged us to come back for additional treatment. It seems as though it’s a robust therapy.”
Going forward, the researchers plan to study fMRI brain imaging before and following external magnetic treatments to speech and language areas to confirm the efficacy of this therapy in a randomized, double-blind, sham treatment-controlled trial.
The TRPMS device was coinvented by Santosh A. Helekar, MD, PhD, and Henning Voss, PhD, both at Weill Cornell Medical College. The study received funding support from The Houston Methodist Hospital System Physicians Organization. Dr. Rosenfield reported having no financial disclosures. He noted that commercialization of the patented technology underlying the TRPMS device used in this study and in other diseases is currently being advanced by Seraya Medical Systems LLC.
dbrunk@mdedge.com
Source: Ann Neurol. 2018;84[S22]:S45-6. Abstract S115.
ATLANTA – After undergoing 10 days of noninvasive magnetic brain stimulation with a novel device, eight of nine persons who stutter experienced improvements in speech fluency.
“This is the first step in what we believe is a major breakthrough in treatment,” lead study author David B. Rosenfield, MD, said in an interview at the annual meeting of the American Neurological Association.
In previously published work, Dr. Rosenfield, Director of the Speech and Language Center at Houston Methodist Neurological Institute, and his colleagues showed significant reduction in functional connectivity between Broca’s and Wernicke’s areas in persons who stutter, compared with normal speakers, by performing resting-state functional MRI of the brain. In the current open-label pilot trial, they tested the hypothesis that using direct noninvasive synchronous bifocal stimulation to potentiate the strength of connectivity between Broca’s and Wernicke’s areas in persons who stutter should improve their fluency.
Researchers enrolled nine persons who stutter who ranged in age from 18 to 80 years. For 40 minutes each consecutive weekday over the course of 2 weeks, the participants wore a compact, portable device known as a Transcranial Rotating Permanent Magnetic Stimulator (TRPMS) to deliver highly focal stimuli to Broca’s and Wernicke’s area locations specified by 10-20 international electroencephalographic electrode sites on the left side. Magnetic stimuli were 100 milliseconds in duration and delivered at 0.2 Hz. Next, a certified speech-language pathologist viewed video recordings of the study subjects both speaking spontaneously and reading a passage aloud on day 1 (before stimulation), day 5 (after stimulation), and day 10 (after stimulation), to assess fluency using the Stuttering Speech Severity Instrument version 4 (SSI-4).
The researchers found that all study participants significantly improved in fluency on either day 5 (P = 0.01) or day 10 (P = 0.02). Only one subject failed to show improvement on day 10 compared with day 1 after showing it on day 5. “This wasn’t meant to be a 10-day treatment that would last forever; this was meant to be a 10-day treatment to see whether the magnetic stimulation would work,” Dr. Rosenfield said. “It might well be that patients need treatment every day, once a week, or once a month. All we can say is that we have an input and an output. We gave them the treatment and they improved. One patient was so happy with it that he begged us to come back for additional treatment. It seems as though it’s a robust therapy.”
Going forward, the researchers plan to study fMRI brain imaging before and following external magnetic treatments to speech and language areas to confirm the efficacy of this therapy in a randomized, double-blind, sham treatment-controlled trial.
The TRPMS device was coinvented by Santosh A. Helekar, MD, PhD, and Henning Voss, PhD, both at Weill Cornell Medical College. The study received funding support from The Houston Methodist Hospital System Physicians Organization. Dr. Rosenfield reported having no financial disclosures. He noted that commercialization of the patented technology underlying the TRPMS device used in this study and in other diseases is currently being advanced by Seraya Medical Systems LLC.
dbrunk@mdedge.com
Source: Ann Neurol. 2018;84[S22]:S45-6. Abstract S115.
ATLANTA – After undergoing 10 days of noninvasive magnetic brain stimulation with a novel device, eight of nine persons who stutter experienced improvements in speech fluency.
“This is the first step in what we believe is a major breakthrough in treatment,” lead study author David B. Rosenfield, MD, said in an interview at the annual meeting of the American Neurological Association.
In previously published work, Dr. Rosenfield, Director of the Speech and Language Center at Houston Methodist Neurological Institute, and his colleagues showed significant reduction in functional connectivity between Broca’s and Wernicke’s areas in persons who stutter, compared with normal speakers, by performing resting-state functional MRI of the brain. In the current open-label pilot trial, they tested the hypothesis that using direct noninvasive synchronous bifocal stimulation to potentiate the strength of connectivity between Broca’s and Wernicke’s areas in persons who stutter should improve their fluency.
Researchers enrolled nine persons who stutter who ranged in age from 18 to 80 years. For 40 minutes each consecutive weekday over the course of 2 weeks, the participants wore a compact, portable device known as a Transcranial Rotating Permanent Magnetic Stimulator (TRPMS) to deliver highly focal stimuli to Broca’s and Wernicke’s area locations specified by 10-20 international electroencephalographic electrode sites on the left side. Magnetic stimuli were 100 milliseconds in duration and delivered at 0.2 Hz. Next, a certified speech-language pathologist viewed video recordings of the study subjects both speaking spontaneously and reading a passage aloud on day 1 (before stimulation), day 5 (after stimulation), and day 10 (after stimulation), to assess fluency using the Stuttering Speech Severity Instrument version 4 (SSI-4).
The researchers found that all study participants significantly improved in fluency on either day 5 (P = 0.01) or day 10 (P = 0.02). Only one subject failed to show improvement on day 10 compared with day 1 after showing it on day 5. “This wasn’t meant to be a 10-day treatment that would last forever; this was meant to be a 10-day treatment to see whether the magnetic stimulation would work,” Dr. Rosenfield said. “It might well be that patients need treatment every day, once a week, or once a month. All we can say is that we have an input and an output. We gave them the treatment and they improved. One patient was so happy with it that he begged us to come back for additional treatment. It seems as though it’s a robust therapy.”
Going forward, the researchers plan to study fMRI brain imaging before and following external magnetic treatments to speech and language areas to confirm the efficacy of this therapy in a randomized, double-blind, sham treatment-controlled trial.
The TRPMS device was coinvented by Santosh A. Helekar, MD, PhD, and Henning Voss, PhD, both at Weill Cornell Medical College. The study received funding support from The Houston Methodist Hospital System Physicians Organization. Dr. Rosenfield reported having no financial disclosures. He noted that commercialization of the patented technology underlying the TRPMS device used in this study and in other diseases is currently being advanced by Seraya Medical Systems LLC.
dbrunk@mdedge.com
Source: Ann Neurol. 2018;84[S22]:S45-6. Abstract S115.
AT ANA 2018
Key clinical point: .
Major finding: All study participants significantly improved in speech fluency on either day 5 of treatment or on day 10.
Study details: An open-label pilot trial of a novel devices used in nine persons who stutter.
Disclosures: The study received funding support from The Houston Methodist Hospital System Physicians Organization. Dr. Rosenfield reported having no financial disclosures. He noted that commercialization of the patented technology underlying the TRPMS device used in this study and in other diseases is currently being advanced by Seraya Medical Systems LLC.
Source: Ann Neurol. 2018;84[S22]:S45-6. Abstract S115.
Think research is just for MD-PhDs? Think again
ATLANTA – You don’t have to hold an advanced research degree or secure National Institutes of Health funding in order to contribute to neurology research in a meaningful way.
That’s a key finding from an analysis of 244 neurology residency program graduates.
“Science as a whole is trying to get better,” lead study author Wyatt P. Bensken said in an interview at the annual meeting of the American Neurological Association. “If your goal is to be a clinician, that doesn’t mean you can’t contribute to research. If your goal is to see patients for 80% of your time, that doesn’t mean that other 20% – which is research – disqualifies you from being a physician-scientist.”
In an effort to better understand the current status of the physician-scientist workforce in the neurology field, Mr. Bensken and his colleagues identified neurology residency graduates from the top National Institute of Neurological Disorders and Stroke–funded institutions for 2003, 2004, and 2005 via program websites. Data points collected for each individual included complete NIH and other government funding history, number of post-residency publications by year, and the Hirsch-index, or h-index, which measures an individual’s research publication impact. The researchers conducted data analysis via visualization and ANOVA testing.
Mr. Bensken, a research collaborator with the NINDS who is also a PhD student at Case Western Reserve University in Cleveland, reported that 186 of the 244 neurology residency program graduates had demonstrated interest in research based on their publication activity findings. Specifically, 26 had obtained an R01 grant, 31 were non–R01-funded, and 129 were nonfunded. Of the 26 individuals who had obtained an R01, 15 (58%) were MD-PhDs, from a total of 50 MD‐PhDs in the cohort. In addition, 43 individuals had a K‐series award, with 18 going on to receive R01 funding.
Of those with non‐R01 funding or no funding, a number of individuals performed as well as R01‐funded individuals with respect to post‐residency publication rate and impact factor. However, the publication rate and impact factor were highest in the R01-funded group (6.4 and 28.6, respectively), followed by those in the non‐R01 group (3.0 and 15.9), and those in the nonfunded group (1.2 and 8.0). Further, the publications‐per‐research hour for the three groups revealed varied productivity levels. Specifically, those in the R01-funded group with 80% protected research time produced 3.2 publications per 1,000 research hours, while those in the non–R01-funded group with 40% protected research time produced 3.0 publications per 1,000 research hours. Meanwhile, those without R01 funding overall (those with non-RO1 funding and those without funding) performed at a higher per-hour rate, when estimating 10% or 15% protected time (4.9 and 3.3 publications per 1,000 research hours, respectively).
“I think this reinforces the notion that there are far more neurologists out there who aren’t trained as MD-PhDs, who aren’t receiving R01s, but who are making meaningful contributions,” Mr. Bensken said. “Our ultimate goal is to maximize the potential of everybody in this environment to contribute. If everyone was able to contribute what they could, I think research would be far more successful and far more impactful than it is now.”
The study was funded by the NINDS. Mr. Bensken reported having no financial disclosures.
SOURCE: Bensken WP et al. Ann Neurol. 2018;84[S22]:S72-3, Abstract S176.
ATLANTA – You don’t have to hold an advanced research degree or secure National Institutes of Health funding in order to contribute to neurology research in a meaningful way.
That’s a key finding from an analysis of 244 neurology residency program graduates.
“Science as a whole is trying to get better,” lead study author Wyatt P. Bensken said in an interview at the annual meeting of the American Neurological Association. “If your goal is to be a clinician, that doesn’t mean you can’t contribute to research. If your goal is to see patients for 80% of your time, that doesn’t mean that other 20% – which is research – disqualifies you from being a physician-scientist.”
In an effort to better understand the current status of the physician-scientist workforce in the neurology field, Mr. Bensken and his colleagues identified neurology residency graduates from the top National Institute of Neurological Disorders and Stroke–funded institutions for 2003, 2004, and 2005 via program websites. Data points collected for each individual included complete NIH and other government funding history, number of post-residency publications by year, and the Hirsch-index, or h-index, which measures an individual’s research publication impact. The researchers conducted data analysis via visualization and ANOVA testing.
Mr. Bensken, a research collaborator with the NINDS who is also a PhD student at Case Western Reserve University in Cleveland, reported that 186 of the 244 neurology residency program graduates had demonstrated interest in research based on their publication activity findings. Specifically, 26 had obtained an R01 grant, 31 were non–R01-funded, and 129 were nonfunded. Of the 26 individuals who had obtained an R01, 15 (58%) were MD-PhDs, from a total of 50 MD‐PhDs in the cohort. In addition, 43 individuals had a K‐series award, with 18 going on to receive R01 funding.
Of those with non‐R01 funding or no funding, a number of individuals performed as well as R01‐funded individuals with respect to post‐residency publication rate and impact factor. However, the publication rate and impact factor were highest in the R01-funded group (6.4 and 28.6, respectively), followed by those in the non‐R01 group (3.0 and 15.9), and those in the nonfunded group (1.2 and 8.0). Further, the publications‐per‐research hour for the three groups revealed varied productivity levels. Specifically, those in the R01-funded group with 80% protected research time produced 3.2 publications per 1,000 research hours, while those in the non–R01-funded group with 40% protected research time produced 3.0 publications per 1,000 research hours. Meanwhile, those without R01 funding overall (those with non-RO1 funding and those without funding) performed at a higher per-hour rate, when estimating 10% or 15% protected time (4.9 and 3.3 publications per 1,000 research hours, respectively).
“I think this reinforces the notion that there are far more neurologists out there who aren’t trained as MD-PhDs, who aren’t receiving R01s, but who are making meaningful contributions,” Mr. Bensken said. “Our ultimate goal is to maximize the potential of everybody in this environment to contribute. If everyone was able to contribute what they could, I think research would be far more successful and far more impactful than it is now.”
The study was funded by the NINDS. Mr. Bensken reported having no financial disclosures.
SOURCE: Bensken WP et al. Ann Neurol. 2018;84[S22]:S72-3, Abstract S176.
ATLANTA – You don’t have to hold an advanced research degree or secure National Institutes of Health funding in order to contribute to neurology research in a meaningful way.
That’s a key finding from an analysis of 244 neurology residency program graduates.
“Science as a whole is trying to get better,” lead study author Wyatt P. Bensken said in an interview at the annual meeting of the American Neurological Association. “If your goal is to be a clinician, that doesn’t mean you can’t contribute to research. If your goal is to see patients for 80% of your time, that doesn’t mean that other 20% – which is research – disqualifies you from being a physician-scientist.”
In an effort to better understand the current status of the physician-scientist workforce in the neurology field, Mr. Bensken and his colleagues identified neurology residency graduates from the top National Institute of Neurological Disorders and Stroke–funded institutions for 2003, 2004, and 2005 via program websites. Data points collected for each individual included complete NIH and other government funding history, number of post-residency publications by year, and the Hirsch-index, or h-index, which measures an individual’s research publication impact. The researchers conducted data analysis via visualization and ANOVA testing.
Mr. Bensken, a research collaborator with the NINDS who is also a PhD student at Case Western Reserve University in Cleveland, reported that 186 of the 244 neurology residency program graduates had demonstrated interest in research based on their publication activity findings. Specifically, 26 had obtained an R01 grant, 31 were non–R01-funded, and 129 were nonfunded. Of the 26 individuals who had obtained an R01, 15 (58%) were MD-PhDs, from a total of 50 MD‐PhDs in the cohort. In addition, 43 individuals had a K‐series award, with 18 going on to receive R01 funding.
Of those with non‐R01 funding or no funding, a number of individuals performed as well as R01‐funded individuals with respect to post‐residency publication rate and impact factor. However, the publication rate and impact factor were highest in the R01-funded group (6.4 and 28.6, respectively), followed by those in the non‐R01 group (3.0 and 15.9), and those in the nonfunded group (1.2 and 8.0). Further, the publications‐per‐research hour for the three groups revealed varied productivity levels. Specifically, those in the R01-funded group with 80% protected research time produced 3.2 publications per 1,000 research hours, while those in the non–R01-funded group with 40% protected research time produced 3.0 publications per 1,000 research hours. Meanwhile, those without R01 funding overall (those with non-RO1 funding and those without funding) performed at a higher per-hour rate, when estimating 10% or 15% protected time (4.9 and 3.3 publications per 1,000 research hours, respectively).
“I think this reinforces the notion that there are far more neurologists out there who aren’t trained as MD-PhDs, who aren’t receiving R01s, but who are making meaningful contributions,” Mr. Bensken said. “Our ultimate goal is to maximize the potential of everybody in this environment to contribute. If everyone was able to contribute what they could, I think research would be far more successful and far more impactful than it is now.”
The study was funded by the NINDS. Mr. Bensken reported having no financial disclosures.
SOURCE: Bensken WP et al. Ann Neurol. 2018;84[S22]:S72-3, Abstract S176.
REPORTING FROM ANA 2018
Key clinical point:
Major finding: Those in the R01-funded group with 80% protected research time produced 3.2 publications per 1,000 research hours, while those in the non–R01-funded group with 40% protected research time produced 3.0 publications per 1,000 research hours.
Study details: An analysis of 244 neurology residency program graduates.
Disclosures: The study was funded by the NINDS. Mr. Bensken reported having no financial disclosures.
Source: Bensken WP et al. Ann Neurol. 2018;84[S 22]:S72-3, Abstract S176.
Robin Williams’ widow recounts ‘terror’ of late husband’s Lewy body dementia
ATLANTA –
“With our medical team’s care, for the next 10 months we chased symptoms, but they were so elusive,” Mr. Williams’ widow, Susan Schneider Williams, said during a keynote address at the annual meeting of the American Neurological Association. “One hallmark of LBD is that symptoms appear and disappear randomly. The game whack-a-mole comes to mind. As soon as you think you are about to figure out a symptom, it disappears, and another one pops up.”
Mr. Williams’ medical team included one general physician, one neurologist, one motor specialist, two psychiatrists, one hypnotherapist, one physical trainer, and assorted alternative specialists. “We had been celebrating our second wedding anniversary when Robin started having gut discomfort,” Ms. Williams recalled. “He was tested for diverticulitis [but] the results came back negative. The pain eventually subsided but what was alarming was Robin’s reaction to it. He had a sudden and sustained spike in fear and anxiety unlike anything I’d seen before. By that point, we’d been by each other’s side long enough that I knew his normal baseline moods, fears, and anxieties. This was totally out of character, and I wondered privately: ‘Is my husband a hypochondriac?’ What I know now is that he was exhibiting a notable hallmark of LBD: new onset anxiety, sustained.” Lewy body disease is characterized by more than 40 symptoms, she continued, “and Robin experienced nearly all of them. He was particularly debilitated by fear, anxiety, delusions, paranoia, and as I came to find out later, hallucinations.”
The medical team continued running all sorts of tests, but everything kept came back negative, except for a very high cortisol count. By the late spring of 2014, however, Mr. Williams was diagnosed with Parkinson’s disease. “I was relieved to find out we finally had an answer, but I could tell, Robin was not buying it,” said Ms. Williams, who is a California-based fine artist, author, and brain health advocate. “The motor specialist said it was early and mild and that he’d be feeling better once he adjusted to the medications, [that] he had another 10 good years.”
In an attempt to treat the Parkinson’s and what was assumed to be depression, his care plan involved adjusting Parkinson’s medications, combined with an antidepressant. His physician also recommended a visit to the Dan Anderson Renewal Center in Minnesota, “for enhanced 12-step work to augment his sobriety,” Ms. Williams said. “The hope was this might help with fear and anxiety. Robin was clean and sober for 8 continuous years when he passed. I watched how he gained spiritually in so many ways from all the work he’d been doing, but his brain biology was going in the exact opposite direction. He tried desperately to join the parts of his heart, mind, and spirit, but his brain was pulling him apart. I felt like I was watching my husband disintegrate before my eyes, and there was nothing anyone could do about it. There came a day when we were getting ready to go to one of our dear friend’s birthday party. I came and saw Robin as he lay on our bed, imprisoned by fear and anxiety. Through tears, he pleaded, ‘I just want to reboot my brain!’ I promised him, ‘I know, honey. I swear we’re going to get to the bottom of this.’ ”
The couple was about a week away from choosing which neurocognitive testing facility to go to for further evaluation when Mr. Williams took his own life in his Paradise Cay, Calif., home on Aug. 11, 2014. “Robin was exhausted from the terror coming from his brain,” Ms. Williams said. “He took [his own life] before it could take any more of him.”
About 3 months later, the underlying cause of death was revealed: diffuse Lewy body dementia, “one of the worst cases they’d ever seen,” she said. “Because Robin’s disease pathway was extreme and unfolded the way it did, it highlights quite strikingly this disease spectrum. He had a perfusion of Lewy bodies, the essential underlying shared biology between Parkinson’s and Lewy body disease, scattered throughout his entire brain and brain stem.” She added that her husband’s prior history of depression from earlier in life “added to the challenge of getting a proper diagnosis. That single symptom of depression was being treated as its own illness, rather than part of the larger neurocognitive disease. It seems that one of the biggest challenges to getting an accurate diagnosis is that LBD symptoms have tremendous crossover with normal human psychology and behavior, mood, cognition and sleep issues. All of us experience fear, stress, anxiety, paranoia, trouble sleeping, mild depression, and other issues from time to time. We would hardly be human if we didn’t. The challenge of LBD is seeing the giant constellation that it is, rather than just a few of its stars.”
In early 2016, Ms. Williams received the “Commitment to Cures Award” from American Brain Foundation, honoring work she’s done raising awareness for Lewy body disease since her husband’s death. “The day I accepted that award and told our story to a room full of neurologists, my path was forever changed,” she said. “The ABF’s mission of connecting donors to researchers and curing brain disease was an alignment with my mission and hope.” She currently serves as vice chair of the ABF’s board of directors.
“From my own research and from the myriad of letters and information that has come to me, I have distilled what I think are the top three overlooked ideas in this disease space,” Ms. Williams said. “1. Diagnosis: The norm seems to be misdiagnosis, switched diagnosis, or no diagnosis at all. 2. Symptoms: They are being treated independently, apart from the neurological disorder. 3. Suicides: If more autopsies were done, more suicides would be attributed to this disease.”
She concluded her address by reflecting on the impact of her husband’s death has had in bringing an international spotlight to LBD. “When I meet individuals who have lost someone they loved to LBD, I see the pain in their eyes, but I hear the determination in their voice as they chart their own course toward making a difference,” Ms. Williams said. “I have been blessed to learn over and over again that I am not alone. I believe that Robin’s death in this battle against these diseases holds a profound purpose. There was tremendous power in what he suffered, and I saw that power up close. I’m here doing all that I can to see that power transformed into something good.”
ATLANTA –
“With our medical team’s care, for the next 10 months we chased symptoms, but they were so elusive,” Mr. Williams’ widow, Susan Schneider Williams, said during a keynote address at the annual meeting of the American Neurological Association. “One hallmark of LBD is that symptoms appear and disappear randomly. The game whack-a-mole comes to mind. As soon as you think you are about to figure out a symptom, it disappears, and another one pops up.”
Mr. Williams’ medical team included one general physician, one neurologist, one motor specialist, two psychiatrists, one hypnotherapist, one physical trainer, and assorted alternative specialists. “We had been celebrating our second wedding anniversary when Robin started having gut discomfort,” Ms. Williams recalled. “He was tested for diverticulitis [but] the results came back negative. The pain eventually subsided but what was alarming was Robin’s reaction to it. He had a sudden and sustained spike in fear and anxiety unlike anything I’d seen before. By that point, we’d been by each other’s side long enough that I knew his normal baseline moods, fears, and anxieties. This was totally out of character, and I wondered privately: ‘Is my husband a hypochondriac?’ What I know now is that he was exhibiting a notable hallmark of LBD: new onset anxiety, sustained.” Lewy body disease is characterized by more than 40 symptoms, she continued, “and Robin experienced nearly all of them. He was particularly debilitated by fear, anxiety, delusions, paranoia, and as I came to find out later, hallucinations.”
The medical team continued running all sorts of tests, but everything kept came back negative, except for a very high cortisol count. By the late spring of 2014, however, Mr. Williams was diagnosed with Parkinson’s disease. “I was relieved to find out we finally had an answer, but I could tell, Robin was not buying it,” said Ms. Williams, who is a California-based fine artist, author, and brain health advocate. “The motor specialist said it was early and mild and that he’d be feeling better once he adjusted to the medications, [that] he had another 10 good years.”
In an attempt to treat the Parkinson’s and what was assumed to be depression, his care plan involved adjusting Parkinson’s medications, combined with an antidepressant. His physician also recommended a visit to the Dan Anderson Renewal Center in Minnesota, “for enhanced 12-step work to augment his sobriety,” Ms. Williams said. “The hope was this might help with fear and anxiety. Robin was clean and sober for 8 continuous years when he passed. I watched how he gained spiritually in so many ways from all the work he’d been doing, but his brain biology was going in the exact opposite direction. He tried desperately to join the parts of his heart, mind, and spirit, but his brain was pulling him apart. I felt like I was watching my husband disintegrate before my eyes, and there was nothing anyone could do about it. There came a day when we were getting ready to go to one of our dear friend’s birthday party. I came and saw Robin as he lay on our bed, imprisoned by fear and anxiety. Through tears, he pleaded, ‘I just want to reboot my brain!’ I promised him, ‘I know, honey. I swear we’re going to get to the bottom of this.’ ”
The couple was about a week away from choosing which neurocognitive testing facility to go to for further evaluation when Mr. Williams took his own life in his Paradise Cay, Calif., home on Aug. 11, 2014. “Robin was exhausted from the terror coming from his brain,” Ms. Williams said. “He took [his own life] before it could take any more of him.”
About 3 months later, the underlying cause of death was revealed: diffuse Lewy body dementia, “one of the worst cases they’d ever seen,” she said. “Because Robin’s disease pathway was extreme and unfolded the way it did, it highlights quite strikingly this disease spectrum. He had a perfusion of Lewy bodies, the essential underlying shared biology between Parkinson’s and Lewy body disease, scattered throughout his entire brain and brain stem.” She added that her husband’s prior history of depression from earlier in life “added to the challenge of getting a proper diagnosis. That single symptom of depression was being treated as its own illness, rather than part of the larger neurocognitive disease. It seems that one of the biggest challenges to getting an accurate diagnosis is that LBD symptoms have tremendous crossover with normal human psychology and behavior, mood, cognition and sleep issues. All of us experience fear, stress, anxiety, paranoia, trouble sleeping, mild depression, and other issues from time to time. We would hardly be human if we didn’t. The challenge of LBD is seeing the giant constellation that it is, rather than just a few of its stars.”
In early 2016, Ms. Williams received the “Commitment to Cures Award” from American Brain Foundation, honoring work she’s done raising awareness for Lewy body disease since her husband’s death. “The day I accepted that award and told our story to a room full of neurologists, my path was forever changed,” she said. “The ABF’s mission of connecting donors to researchers and curing brain disease was an alignment with my mission and hope.” She currently serves as vice chair of the ABF’s board of directors.
“From my own research and from the myriad of letters and information that has come to me, I have distilled what I think are the top three overlooked ideas in this disease space,” Ms. Williams said. “1. Diagnosis: The norm seems to be misdiagnosis, switched diagnosis, or no diagnosis at all. 2. Symptoms: They are being treated independently, apart from the neurological disorder. 3. Suicides: If more autopsies were done, more suicides would be attributed to this disease.”
She concluded her address by reflecting on the impact of her husband’s death has had in bringing an international spotlight to LBD. “When I meet individuals who have lost someone they loved to LBD, I see the pain in their eyes, but I hear the determination in their voice as they chart their own course toward making a difference,” Ms. Williams said. “I have been blessed to learn over and over again that I am not alone. I believe that Robin’s death in this battle against these diseases holds a profound purpose. There was tremendous power in what he suffered, and I saw that power up close. I’m here doing all that I can to see that power transformed into something good.”
ATLANTA –
“With our medical team’s care, for the next 10 months we chased symptoms, but they were so elusive,” Mr. Williams’ widow, Susan Schneider Williams, said during a keynote address at the annual meeting of the American Neurological Association. “One hallmark of LBD is that symptoms appear and disappear randomly. The game whack-a-mole comes to mind. As soon as you think you are about to figure out a symptom, it disappears, and another one pops up.”
Mr. Williams’ medical team included one general physician, one neurologist, one motor specialist, two psychiatrists, one hypnotherapist, one physical trainer, and assorted alternative specialists. “We had been celebrating our second wedding anniversary when Robin started having gut discomfort,” Ms. Williams recalled. “He was tested for diverticulitis [but] the results came back negative. The pain eventually subsided but what was alarming was Robin’s reaction to it. He had a sudden and sustained spike in fear and anxiety unlike anything I’d seen before. By that point, we’d been by each other’s side long enough that I knew his normal baseline moods, fears, and anxieties. This was totally out of character, and I wondered privately: ‘Is my husband a hypochondriac?’ What I know now is that he was exhibiting a notable hallmark of LBD: new onset anxiety, sustained.” Lewy body disease is characterized by more than 40 symptoms, she continued, “and Robin experienced nearly all of them. He was particularly debilitated by fear, anxiety, delusions, paranoia, and as I came to find out later, hallucinations.”
The medical team continued running all sorts of tests, but everything kept came back negative, except for a very high cortisol count. By the late spring of 2014, however, Mr. Williams was diagnosed with Parkinson’s disease. “I was relieved to find out we finally had an answer, but I could tell, Robin was not buying it,” said Ms. Williams, who is a California-based fine artist, author, and brain health advocate. “The motor specialist said it was early and mild and that he’d be feeling better once he adjusted to the medications, [that] he had another 10 good years.”
In an attempt to treat the Parkinson’s and what was assumed to be depression, his care plan involved adjusting Parkinson’s medications, combined with an antidepressant. His physician also recommended a visit to the Dan Anderson Renewal Center in Minnesota, “for enhanced 12-step work to augment his sobriety,” Ms. Williams said. “The hope was this might help with fear and anxiety. Robin was clean and sober for 8 continuous years when he passed. I watched how he gained spiritually in so many ways from all the work he’d been doing, but his brain biology was going in the exact opposite direction. He tried desperately to join the parts of his heart, mind, and spirit, but his brain was pulling him apart. I felt like I was watching my husband disintegrate before my eyes, and there was nothing anyone could do about it. There came a day when we were getting ready to go to one of our dear friend’s birthday party. I came and saw Robin as he lay on our bed, imprisoned by fear and anxiety. Through tears, he pleaded, ‘I just want to reboot my brain!’ I promised him, ‘I know, honey. I swear we’re going to get to the bottom of this.’ ”
The couple was about a week away from choosing which neurocognitive testing facility to go to for further evaluation when Mr. Williams took his own life in his Paradise Cay, Calif., home on Aug. 11, 2014. “Robin was exhausted from the terror coming from his brain,” Ms. Williams said. “He took [his own life] before it could take any more of him.”
About 3 months later, the underlying cause of death was revealed: diffuse Lewy body dementia, “one of the worst cases they’d ever seen,” she said. “Because Robin’s disease pathway was extreme and unfolded the way it did, it highlights quite strikingly this disease spectrum. He had a perfusion of Lewy bodies, the essential underlying shared biology between Parkinson’s and Lewy body disease, scattered throughout his entire brain and brain stem.” She added that her husband’s prior history of depression from earlier in life “added to the challenge of getting a proper diagnosis. That single symptom of depression was being treated as its own illness, rather than part of the larger neurocognitive disease. It seems that one of the biggest challenges to getting an accurate diagnosis is that LBD symptoms have tremendous crossover with normal human psychology and behavior, mood, cognition and sleep issues. All of us experience fear, stress, anxiety, paranoia, trouble sleeping, mild depression, and other issues from time to time. We would hardly be human if we didn’t. The challenge of LBD is seeing the giant constellation that it is, rather than just a few of its stars.”
In early 2016, Ms. Williams received the “Commitment to Cures Award” from American Brain Foundation, honoring work she’s done raising awareness for Lewy body disease since her husband’s death. “The day I accepted that award and told our story to a room full of neurologists, my path was forever changed,” she said. “The ABF’s mission of connecting donors to researchers and curing brain disease was an alignment with my mission and hope.” She currently serves as vice chair of the ABF’s board of directors.
“From my own research and from the myriad of letters and information that has come to me, I have distilled what I think are the top three overlooked ideas in this disease space,” Ms. Williams said. “1. Diagnosis: The norm seems to be misdiagnosis, switched diagnosis, or no diagnosis at all. 2. Symptoms: They are being treated independently, apart from the neurological disorder. 3. Suicides: If more autopsies were done, more suicides would be attributed to this disease.”
She concluded her address by reflecting on the impact of her husband’s death has had in bringing an international spotlight to LBD. “When I meet individuals who have lost someone they loved to LBD, I see the pain in their eyes, but I hear the determination in their voice as they chart their own course toward making a difference,” Ms. Williams said. “I have been blessed to learn over and over again that I am not alone. I believe that Robin’s death in this battle against these diseases holds a profound purpose. There was tremendous power in what he suffered, and I saw that power up close. I’m here doing all that I can to see that power transformed into something good.”
REPORTING FROM ANA 2018
Management of Lewy body dementia remains complex
ATLANTA – In the not-so-distant past, neurologists viewed dementia with Lewy bodies as a disorder primarily of the brain, but it turned out to be far more complex than that.
At the annual meeting of the American Neurological Association, Bradley F. Boeve, MD, described dementia with Lewy bodies (DLB) as a systemic neurologic disorder affecting the brain, including brain stem, spinal cord, and peripheral nervous system, especially the autonomic nervous system. “This leads to the complex array of clinical manifestations, which are quite different from patient to patient cross-sectionally and longitudinally,” said Dr. Boeve, the Little Family Foundation Professor of Lewy Body Dementia in the department of neurology at the Mayo Clinic, Rochester, Minn.
, he said. The four core clinical features are Parkinsonism unrelated to medications; recurrent, fully-formed visual hallucinations; fluctuations in cognition and/or arousal; and rapid eye movement (REM) sleep behavior disorder. “This is the most predictive of all four features,” Dr. Boeve said. He described REM sleep behavior disorder as a parasomnia manifested by the tendency to repeatedly “act out one’s dreams.” The dreams tend to contain a chasing/attacking theme, and behaviors mirror dream content. Injuries to the patient and bed partner can occur.
Typically, patients will present with REM sleep behavior disorder in their 50s and 60s, and sometimes in their 30s and 40s, “decades before cognitive changes begin,” he said. “This is usually followed by Parkinsonism and visual hallucinations. That’s the prototypical DLB [case], but there are many examples where this is not followed. Prominent neuropsychiatric features can also begin before any cognitive changes.”
Neuropsychological features of DLB often include impairment of executive functions and visuospatial functions. “Early in the course of Alzheimer’s disease, typically performance on memory measures – especially delayed recall – are down and the other measures are borderline or mildly impaired,” Dr. Boeve noted. “By contrast, in DLB, attention, executive function, and visuospatial measures are down, but memory is often pretty good. What’s remarkable is that in the office setting, when you take a history the person often says, ‘I’m very forgetful,’ yet in the testing environment people tend to rise to the occasion pretty well.”
Imaging isn’t always helpful in establishing a diagnosis of DLB. MRI scans, for example, “can look pretty normal, including the hippocampi,” he said. “This is really the norm in DLB and it seems to be a disconnect. The person can have significant symptoms yet their MRI scan can be pretty normal.”
In Alzheimer’s disease, 18F-fluorodeoxyglucose-PET (FDG-PET) shows temporal, parietal, and frontal hypometabolism, sparing of the sensory-motor strip and sparing of the primary occipital cortex, while in DLB, FDG-PET shows marked deficits in the occipital regions with relative sparing of the frontal and temporal lobes. Another key neuroimaging sign of DLB is the posterior cingulate island sign, which is characterized by sparing of the posterior cingulate cortex relative to the precuneus plus cuneus on FDG-PET.
In 2017, the Dementia with Lewy Bodies Consortium published updated recommendations on the diagnosis and management of the disease (Neurology. 2017;89[1]:88-100). In its consensus report, the consortium defines probable DLB as dementia plus two or more clinical features or one core clinical feature plus one or more indicative biomarker. These biomarkers include reduced dopamine transport uptake in basal ganglia by SPECT or PET; abnormal (low uptake) meta-iodobenzylguanidine (MIBG) myocardial scintigraphy, and/or polysomnographic confirmation of REM sleep without atonia.
“Neuropathologically, limbic with or without neocortical Lewy bodies and Lewy neurites are the defining characteristics of pathologically-proven DLB,” added Dr. Boeve, a member of the DLB consortium. “The classic DLB phenotype can occur in limbic-predominant DLB. Lewy bodies in the neocortex are not necessary to cause a dementia syndrome.”
He characterized management of DLB as “very complicated. Consider symptoms as they relate to cognitive impairment, neuropsychiatric features, motor features, sleep disorders, and autonomic dysfunction.” He often asks the patient/family to prioritize the three most troublesome issues they seek to change, and develops a plan based on their input.
There is no Food and Drug Administration–approved medication for DLB, but the standard of care is an acetylcholinesterase inhibitor such as donepezil. “There is evidence that memantine can provide a modest benefit,” Dr. Boeve said. “Hypersomnia is quite prominent in DLB and worthy of assessing and treating.” Clinicians must weigh the pros and cons of pharmacotherapy with each patient. “For example, in the atypical neuroleptic class [of drugs], there may be a benefit to the hallucinations and delusions in DLB but hypersomnia can worsen,” he said. “Selecting agents is challenging but worth the effort.”
Survival is lower and more rapid with DLB, compared with Alzheimer’s. Most people pass away from primary DLB-related features or failure to thrive. The second most common is pneumonia or aspiration. Median survival was 4 years after diagnosis in one study, and end-of life discussions occurred in less than half of all patients. “This is a frustrating reminder that we as clinicians are not very good at discussing important topics such as end-of-life care with patients and their families,” Dr. Boeve said. Resources that he recommends for education and support include the Lewy Body Dementia Association and The Lewy Body Society.
At the 2016 Alzheimer’s Disease-Related Dementias Summit, clinicians formed a list of DLB research priorities (Neurology 2017;89[23]:2381-91). Among them were recommendations to “initiate clinical trials in diverse populations using therapies that address symptoms that have the greatest effect on patient function and caregiver burden” and “identify novel common and rare genetic variants, epigenetic changes, and environmental influences that affect the risk for and clinical features of” the disease.
Meanwhile, several research protocols are under way, including the development of a DLB module by the U.S. Alzheimer’s Research Disease Centers and a number of DLB-focused projects from the National Institute of Neurological Disorders and Stroke (NINDS) Parkinson’s Disease Biomarkers Program. In addition, the Lewy Body Dementia Association Research Centers of Excellence program is focused on optimizing clinical care and setting up the infrastructure for clinical trials, while the North American Prodromal Synucleinopathy Consortium is conducting longitudinal studies in those with REM sleep behavior disorder.
Dr. Boeve disclosed that he has been an investigator for clinical trials sponsored by GE Healthcare, Axovant, and Biogen. He is a member of the scientific advisory board for the Tau Consortium and has received research support from the National Institute on Aging, the NINDS, the Mangurian Foundation, and the Little Family Foundation.
ATLANTA – In the not-so-distant past, neurologists viewed dementia with Lewy bodies as a disorder primarily of the brain, but it turned out to be far more complex than that.
At the annual meeting of the American Neurological Association, Bradley F. Boeve, MD, described dementia with Lewy bodies (DLB) as a systemic neurologic disorder affecting the brain, including brain stem, spinal cord, and peripheral nervous system, especially the autonomic nervous system. “This leads to the complex array of clinical manifestations, which are quite different from patient to patient cross-sectionally and longitudinally,” said Dr. Boeve, the Little Family Foundation Professor of Lewy Body Dementia in the department of neurology at the Mayo Clinic, Rochester, Minn.
, he said. The four core clinical features are Parkinsonism unrelated to medications; recurrent, fully-formed visual hallucinations; fluctuations in cognition and/or arousal; and rapid eye movement (REM) sleep behavior disorder. “This is the most predictive of all four features,” Dr. Boeve said. He described REM sleep behavior disorder as a parasomnia manifested by the tendency to repeatedly “act out one’s dreams.” The dreams tend to contain a chasing/attacking theme, and behaviors mirror dream content. Injuries to the patient and bed partner can occur.
Typically, patients will present with REM sleep behavior disorder in their 50s and 60s, and sometimes in their 30s and 40s, “decades before cognitive changes begin,” he said. “This is usually followed by Parkinsonism and visual hallucinations. That’s the prototypical DLB [case], but there are many examples where this is not followed. Prominent neuropsychiatric features can also begin before any cognitive changes.”
Neuropsychological features of DLB often include impairment of executive functions and visuospatial functions. “Early in the course of Alzheimer’s disease, typically performance on memory measures – especially delayed recall – are down and the other measures are borderline or mildly impaired,” Dr. Boeve noted. “By contrast, in DLB, attention, executive function, and visuospatial measures are down, but memory is often pretty good. What’s remarkable is that in the office setting, when you take a history the person often says, ‘I’m very forgetful,’ yet in the testing environment people tend to rise to the occasion pretty well.”
Imaging isn’t always helpful in establishing a diagnosis of DLB. MRI scans, for example, “can look pretty normal, including the hippocampi,” he said. “This is really the norm in DLB and it seems to be a disconnect. The person can have significant symptoms yet their MRI scan can be pretty normal.”
In Alzheimer’s disease, 18F-fluorodeoxyglucose-PET (FDG-PET) shows temporal, parietal, and frontal hypometabolism, sparing of the sensory-motor strip and sparing of the primary occipital cortex, while in DLB, FDG-PET shows marked deficits in the occipital regions with relative sparing of the frontal and temporal lobes. Another key neuroimaging sign of DLB is the posterior cingulate island sign, which is characterized by sparing of the posterior cingulate cortex relative to the precuneus plus cuneus on FDG-PET.
In 2017, the Dementia with Lewy Bodies Consortium published updated recommendations on the diagnosis and management of the disease (Neurology. 2017;89[1]:88-100). In its consensus report, the consortium defines probable DLB as dementia plus two or more clinical features or one core clinical feature plus one or more indicative biomarker. These biomarkers include reduced dopamine transport uptake in basal ganglia by SPECT or PET; abnormal (low uptake) meta-iodobenzylguanidine (MIBG) myocardial scintigraphy, and/or polysomnographic confirmation of REM sleep without atonia.
“Neuropathologically, limbic with or without neocortical Lewy bodies and Lewy neurites are the defining characteristics of pathologically-proven DLB,” added Dr. Boeve, a member of the DLB consortium. “The classic DLB phenotype can occur in limbic-predominant DLB. Lewy bodies in the neocortex are not necessary to cause a dementia syndrome.”
He characterized management of DLB as “very complicated. Consider symptoms as they relate to cognitive impairment, neuropsychiatric features, motor features, sleep disorders, and autonomic dysfunction.” He often asks the patient/family to prioritize the three most troublesome issues they seek to change, and develops a plan based on their input.
There is no Food and Drug Administration–approved medication for DLB, but the standard of care is an acetylcholinesterase inhibitor such as donepezil. “There is evidence that memantine can provide a modest benefit,” Dr. Boeve said. “Hypersomnia is quite prominent in DLB and worthy of assessing and treating.” Clinicians must weigh the pros and cons of pharmacotherapy with each patient. “For example, in the atypical neuroleptic class [of drugs], there may be a benefit to the hallucinations and delusions in DLB but hypersomnia can worsen,” he said. “Selecting agents is challenging but worth the effort.”
Survival is lower and more rapid with DLB, compared with Alzheimer’s. Most people pass away from primary DLB-related features or failure to thrive. The second most common is pneumonia or aspiration. Median survival was 4 years after diagnosis in one study, and end-of life discussions occurred in less than half of all patients. “This is a frustrating reminder that we as clinicians are not very good at discussing important topics such as end-of-life care with patients and their families,” Dr. Boeve said. Resources that he recommends for education and support include the Lewy Body Dementia Association and The Lewy Body Society.
At the 2016 Alzheimer’s Disease-Related Dementias Summit, clinicians formed a list of DLB research priorities (Neurology 2017;89[23]:2381-91). Among them were recommendations to “initiate clinical trials in diverse populations using therapies that address symptoms that have the greatest effect on patient function and caregiver burden” and “identify novel common and rare genetic variants, epigenetic changes, and environmental influences that affect the risk for and clinical features of” the disease.
Meanwhile, several research protocols are under way, including the development of a DLB module by the U.S. Alzheimer’s Research Disease Centers and a number of DLB-focused projects from the National Institute of Neurological Disorders and Stroke (NINDS) Parkinson’s Disease Biomarkers Program. In addition, the Lewy Body Dementia Association Research Centers of Excellence program is focused on optimizing clinical care and setting up the infrastructure for clinical trials, while the North American Prodromal Synucleinopathy Consortium is conducting longitudinal studies in those with REM sleep behavior disorder.
Dr. Boeve disclosed that he has been an investigator for clinical trials sponsored by GE Healthcare, Axovant, and Biogen. He is a member of the scientific advisory board for the Tau Consortium and has received research support from the National Institute on Aging, the NINDS, the Mangurian Foundation, and the Little Family Foundation.
ATLANTA – In the not-so-distant past, neurologists viewed dementia with Lewy bodies as a disorder primarily of the brain, but it turned out to be far more complex than that.
At the annual meeting of the American Neurological Association, Bradley F. Boeve, MD, described dementia with Lewy bodies (DLB) as a systemic neurologic disorder affecting the brain, including brain stem, spinal cord, and peripheral nervous system, especially the autonomic nervous system. “This leads to the complex array of clinical manifestations, which are quite different from patient to patient cross-sectionally and longitudinally,” said Dr. Boeve, the Little Family Foundation Professor of Lewy Body Dementia in the department of neurology at the Mayo Clinic, Rochester, Minn.
, he said. The four core clinical features are Parkinsonism unrelated to medications; recurrent, fully-formed visual hallucinations; fluctuations in cognition and/or arousal; and rapid eye movement (REM) sleep behavior disorder. “This is the most predictive of all four features,” Dr. Boeve said. He described REM sleep behavior disorder as a parasomnia manifested by the tendency to repeatedly “act out one’s dreams.” The dreams tend to contain a chasing/attacking theme, and behaviors mirror dream content. Injuries to the patient and bed partner can occur.
Typically, patients will present with REM sleep behavior disorder in their 50s and 60s, and sometimes in their 30s and 40s, “decades before cognitive changes begin,” he said. “This is usually followed by Parkinsonism and visual hallucinations. That’s the prototypical DLB [case], but there are many examples where this is not followed. Prominent neuropsychiatric features can also begin before any cognitive changes.”
Neuropsychological features of DLB often include impairment of executive functions and visuospatial functions. “Early in the course of Alzheimer’s disease, typically performance on memory measures – especially delayed recall – are down and the other measures are borderline or mildly impaired,” Dr. Boeve noted. “By contrast, in DLB, attention, executive function, and visuospatial measures are down, but memory is often pretty good. What’s remarkable is that in the office setting, when you take a history the person often says, ‘I’m very forgetful,’ yet in the testing environment people tend to rise to the occasion pretty well.”
Imaging isn’t always helpful in establishing a diagnosis of DLB. MRI scans, for example, “can look pretty normal, including the hippocampi,” he said. “This is really the norm in DLB and it seems to be a disconnect. The person can have significant symptoms yet their MRI scan can be pretty normal.”
In Alzheimer’s disease, 18F-fluorodeoxyglucose-PET (FDG-PET) shows temporal, parietal, and frontal hypometabolism, sparing of the sensory-motor strip and sparing of the primary occipital cortex, while in DLB, FDG-PET shows marked deficits in the occipital regions with relative sparing of the frontal and temporal lobes. Another key neuroimaging sign of DLB is the posterior cingulate island sign, which is characterized by sparing of the posterior cingulate cortex relative to the precuneus plus cuneus on FDG-PET.
In 2017, the Dementia with Lewy Bodies Consortium published updated recommendations on the diagnosis and management of the disease (Neurology. 2017;89[1]:88-100). In its consensus report, the consortium defines probable DLB as dementia plus two or more clinical features or one core clinical feature plus one or more indicative biomarker. These biomarkers include reduced dopamine transport uptake in basal ganglia by SPECT or PET; abnormal (low uptake) meta-iodobenzylguanidine (MIBG) myocardial scintigraphy, and/or polysomnographic confirmation of REM sleep without atonia.
“Neuropathologically, limbic with or without neocortical Lewy bodies and Lewy neurites are the defining characteristics of pathologically-proven DLB,” added Dr. Boeve, a member of the DLB consortium. “The classic DLB phenotype can occur in limbic-predominant DLB. Lewy bodies in the neocortex are not necessary to cause a dementia syndrome.”
He characterized management of DLB as “very complicated. Consider symptoms as they relate to cognitive impairment, neuropsychiatric features, motor features, sleep disorders, and autonomic dysfunction.” He often asks the patient/family to prioritize the three most troublesome issues they seek to change, and develops a plan based on their input.
There is no Food and Drug Administration–approved medication for DLB, but the standard of care is an acetylcholinesterase inhibitor such as donepezil. “There is evidence that memantine can provide a modest benefit,” Dr. Boeve said. “Hypersomnia is quite prominent in DLB and worthy of assessing and treating.” Clinicians must weigh the pros and cons of pharmacotherapy with each patient. “For example, in the atypical neuroleptic class [of drugs], there may be a benefit to the hallucinations and delusions in DLB but hypersomnia can worsen,” he said. “Selecting agents is challenging but worth the effort.”
Survival is lower and more rapid with DLB, compared with Alzheimer’s. Most people pass away from primary DLB-related features or failure to thrive. The second most common is pneumonia or aspiration. Median survival was 4 years after diagnosis in one study, and end-of life discussions occurred in less than half of all patients. “This is a frustrating reminder that we as clinicians are not very good at discussing important topics such as end-of-life care with patients and their families,” Dr. Boeve said. Resources that he recommends for education and support include the Lewy Body Dementia Association and The Lewy Body Society.
At the 2016 Alzheimer’s Disease-Related Dementias Summit, clinicians formed a list of DLB research priorities (Neurology 2017;89[23]:2381-91). Among them were recommendations to “initiate clinical trials in diverse populations using therapies that address symptoms that have the greatest effect on patient function and caregiver burden” and “identify novel common and rare genetic variants, epigenetic changes, and environmental influences that affect the risk for and clinical features of” the disease.
Meanwhile, several research protocols are under way, including the development of a DLB module by the U.S. Alzheimer’s Research Disease Centers and a number of DLB-focused projects from the National Institute of Neurological Disorders and Stroke (NINDS) Parkinson’s Disease Biomarkers Program. In addition, the Lewy Body Dementia Association Research Centers of Excellence program is focused on optimizing clinical care and setting up the infrastructure for clinical trials, while the North American Prodromal Synucleinopathy Consortium is conducting longitudinal studies in those with REM sleep behavior disorder.
Dr. Boeve disclosed that he has been an investigator for clinical trials sponsored by GE Healthcare, Axovant, and Biogen. He is a member of the scientific advisory board for the Tau Consortium and has received research support from the National Institute on Aging, the NINDS, the Mangurian Foundation, and the Little Family Foundation.
EXPERT ANALYSIS FROM ANA 2018
Parkinson’s prevalence varies significantly from state to state
ATLANTA – The prevalence of Parkinson’s disease and associated health care spending on the condition vary significantly from state to state, an analysis of Medicare data showed.
“There is a big variation in not only the prevalence of Parkinson’s disease but also in spending and in health care utilization” among Medicare beneficiaries diagnosed with the condition, lead study author Michelle E. Fullard, MD, said in an interview at the annual meeting of the American Neurological Association. “As neurologists, we should be aware of this. We can use this information to identify and target areas in which Parkinson’s patients may have increased need and require more resources. It can also inform planning at the state and federal levels.”
Dr. Fullard, formerly of the department of neurology at the University of Pennsylvania, Philadelphia, and her colleagues evaluated data from Medicare Beneficiary Summary and Medicare Carrier Files for 27,538,023 individuals aged 65 years and older who were continuously enrolled in Medicare parts A and B during 2014. They calculated state-level differences in Parkinson’s disease prevalence, demographic and eligibility characteristics, costs, and health care use, including number of emergency room visits, number of outpatient clinic visits, and inpatient hospitalizations. The researchers used reimbursement data to calculate the mean out-of-pocket and Medicare cost per individual in each state, and compared direct costs and health service utilization for individuals with and without Parkinson’s disease.
Of all Medicare beneficiaries studied, 392,214 (1.42%) had a diagnosis of Parkinson’s disease. Nearly half (46%) were women and 26% were aged 85 years and older. States with the highest prevalence of Parkinson’s disease included New York (1,720/100,000), Illinois (1,566/100,000), Connecticut (1,560/100,000), Florida (1,551/100,000), Pennsylvania (1,549/100,000), Rhode Island (1,543/100,000), New Jersey (1,541/100,000), Texas (1,522/100,000), California (1,520/100,000) and Louisiana (1,519/100,000). Minnesota had the lowest prevalence (803/100,000).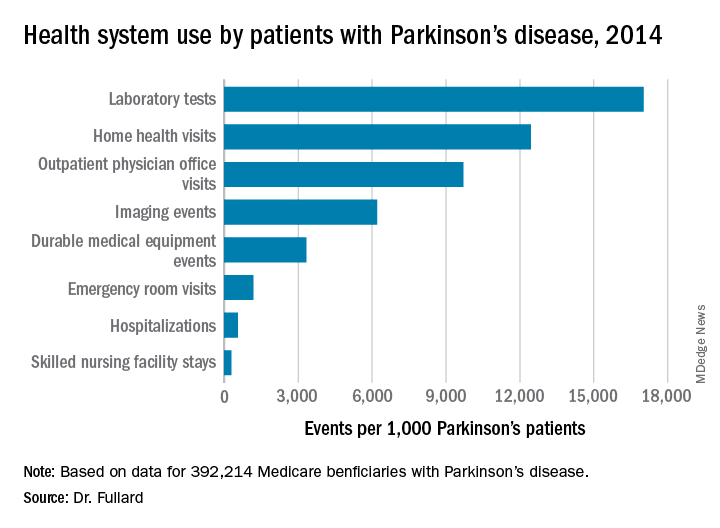
Among the national sample of patients with Parkinson’s disease, there were 219,049 hospitalizations (which represented 558/1,000 Parkinson’s patients), 37,839 readmissions (172/1,000 hospitalizations), 9,740,609 outpatient physician office visits (9,700/1,000 patients), 34,159 hospice stays (87/1,000 patients), 113,027 skilled nursing facility stays (288/1,000 patients), 466,160 emergency room visits (1,188/1,000 patients, 39% of which resulted in hospital admission). In addition, there were 1,308,934 durable medical equipment events (3,337/1,000 patients), 6,676,119 laboratory tests (17,021/1,000 patients), 2,435,654 imaging events (6,210/1,000 patients), and 4,879,538 home health visits (12,441/1,000 patients). The costliest services were inpatient care ($2.1 billion), skilled nursing facility care ($1.4 billion), prescription drugs used by those with prescription coverage ($974.8 million), hospital outpatient care ($881 million), and home health care ($776.5 million).
“States with a higher prevalence of Parkinson’s disease may have a larger proportion of high-risk factor patient groups, a higher concentration of providers who recognize and document Parkinson’s disease, increased public awareness of symptoms, or increased health care–seeking behaviors among people living in the state,” the researchers wrote in their abstract. “Among our top Parkinson’s disease prevalence states, Florida and New York also rank high in terms of absolute number of Medicare beneficiaries and have large supplies of health care providers.”
They also noted that Medicare beneficiaries with Parkinson’s had increased use of health care and spending, compared with their counterparts without the disease. “This was true across all sectors of care (inpatient, outpatient, skilled nursing, and ancillary services) and is in line with data demonstrating that PD, its complications, and the shift away from comorbid disease care and prevention that occurs after a Parkinson’s disease diagnosis drive health care spending and utilization among these individuals,” they wrote.
The study was supported by the Parkinson’s Foundation. Dr. Fullard, who now holds a faculty position at the University of Colorado, Aurora, reported having no financial disclosures.
SOURCE: Ann Neurol. 2018;84[S22]:S89-90, Abstract S215.
ATLANTA – The prevalence of Parkinson’s disease and associated health care spending on the condition vary significantly from state to state, an analysis of Medicare data showed.
“There is a big variation in not only the prevalence of Parkinson’s disease but also in spending and in health care utilization” among Medicare beneficiaries diagnosed with the condition, lead study author Michelle E. Fullard, MD, said in an interview at the annual meeting of the American Neurological Association. “As neurologists, we should be aware of this. We can use this information to identify and target areas in which Parkinson’s patients may have increased need and require more resources. It can also inform planning at the state and federal levels.”
Dr. Fullard, formerly of the department of neurology at the University of Pennsylvania, Philadelphia, and her colleagues evaluated data from Medicare Beneficiary Summary and Medicare Carrier Files for 27,538,023 individuals aged 65 years and older who were continuously enrolled in Medicare parts A and B during 2014. They calculated state-level differences in Parkinson’s disease prevalence, demographic and eligibility characteristics, costs, and health care use, including number of emergency room visits, number of outpatient clinic visits, and inpatient hospitalizations. The researchers used reimbursement data to calculate the mean out-of-pocket and Medicare cost per individual in each state, and compared direct costs and health service utilization for individuals with and without Parkinson’s disease.
Of all Medicare beneficiaries studied, 392,214 (1.42%) had a diagnosis of Parkinson’s disease. Nearly half (46%) were women and 26% were aged 85 years and older. States with the highest prevalence of Parkinson’s disease included New York (1,720/100,000), Illinois (1,566/100,000), Connecticut (1,560/100,000), Florida (1,551/100,000), Pennsylvania (1,549/100,000), Rhode Island (1,543/100,000), New Jersey (1,541/100,000), Texas (1,522/100,000), California (1,520/100,000) and Louisiana (1,519/100,000). Minnesota had the lowest prevalence (803/100,000).
Among the national sample of patients with Parkinson’s disease, there were 219,049 hospitalizations (which represented 558/1,000 Parkinson’s patients), 37,839 readmissions (172/1,000 hospitalizations), 9,740,609 outpatient physician office visits (9,700/1,000 patients), 34,159 hospice stays (87/1,000 patients), 113,027 skilled nursing facility stays (288/1,000 patients), 466,160 emergency room visits (1,188/1,000 patients, 39% of which resulted in hospital admission). In addition, there were 1,308,934 durable medical equipment events (3,337/1,000 patients), 6,676,119 laboratory tests (17,021/1,000 patients), 2,435,654 imaging events (6,210/1,000 patients), and 4,879,538 home health visits (12,441/1,000 patients). The costliest services were inpatient care ($2.1 billion), skilled nursing facility care ($1.4 billion), prescription drugs used by those with prescription coverage ($974.8 million), hospital outpatient care ($881 million), and home health care ($776.5 million).
“States with a higher prevalence of Parkinson’s disease may have a larger proportion of high-risk factor patient groups, a higher concentration of providers who recognize and document Parkinson’s disease, increased public awareness of symptoms, or increased health care–seeking behaviors among people living in the state,” the researchers wrote in their abstract. “Among our top Parkinson’s disease prevalence states, Florida and New York also rank high in terms of absolute number of Medicare beneficiaries and have large supplies of health care providers.”
They also noted that Medicare beneficiaries with Parkinson’s had increased use of health care and spending, compared with their counterparts without the disease. “This was true across all sectors of care (inpatient, outpatient, skilled nursing, and ancillary services) and is in line with data demonstrating that PD, its complications, and the shift away from comorbid disease care and prevention that occurs after a Parkinson’s disease diagnosis drive health care spending and utilization among these individuals,” they wrote.
The study was supported by the Parkinson’s Foundation. Dr. Fullard, who now holds a faculty position at the University of Colorado, Aurora, reported having no financial disclosures.
SOURCE: Ann Neurol. 2018;84[S22]:S89-90, Abstract S215.
ATLANTA – The prevalence of Parkinson’s disease and associated health care spending on the condition vary significantly from state to state, an analysis of Medicare data showed.
“There is a big variation in not only the prevalence of Parkinson’s disease but also in spending and in health care utilization” among Medicare beneficiaries diagnosed with the condition, lead study author Michelle E. Fullard, MD, said in an interview at the annual meeting of the American Neurological Association. “As neurologists, we should be aware of this. We can use this information to identify and target areas in which Parkinson’s patients may have increased need and require more resources. It can also inform planning at the state and federal levels.”
Dr. Fullard, formerly of the department of neurology at the University of Pennsylvania, Philadelphia, and her colleagues evaluated data from Medicare Beneficiary Summary and Medicare Carrier Files for 27,538,023 individuals aged 65 years and older who were continuously enrolled in Medicare parts A and B during 2014. They calculated state-level differences in Parkinson’s disease prevalence, demographic and eligibility characteristics, costs, and health care use, including number of emergency room visits, number of outpatient clinic visits, and inpatient hospitalizations. The researchers used reimbursement data to calculate the mean out-of-pocket and Medicare cost per individual in each state, and compared direct costs and health service utilization for individuals with and without Parkinson’s disease.
Of all Medicare beneficiaries studied, 392,214 (1.42%) had a diagnosis of Parkinson’s disease. Nearly half (46%) were women and 26% were aged 85 years and older. States with the highest prevalence of Parkinson’s disease included New York (1,720/100,000), Illinois (1,566/100,000), Connecticut (1,560/100,000), Florida (1,551/100,000), Pennsylvania (1,549/100,000), Rhode Island (1,543/100,000), New Jersey (1,541/100,000), Texas (1,522/100,000), California (1,520/100,000) and Louisiana (1,519/100,000). Minnesota had the lowest prevalence (803/100,000).
Among the national sample of patients with Parkinson’s disease, there were 219,049 hospitalizations (which represented 558/1,000 Parkinson’s patients), 37,839 readmissions (172/1,000 hospitalizations), 9,740,609 outpatient physician office visits (9,700/1,000 patients), 34,159 hospice stays (87/1,000 patients), 113,027 skilled nursing facility stays (288/1,000 patients), 466,160 emergency room visits (1,188/1,000 patients, 39% of which resulted in hospital admission). In addition, there were 1,308,934 durable medical equipment events (3,337/1,000 patients), 6,676,119 laboratory tests (17,021/1,000 patients), 2,435,654 imaging events (6,210/1,000 patients), and 4,879,538 home health visits (12,441/1,000 patients). The costliest services were inpatient care ($2.1 billion), skilled nursing facility care ($1.4 billion), prescription drugs used by those with prescription coverage ($974.8 million), hospital outpatient care ($881 million), and home health care ($776.5 million).
“States with a higher prevalence of Parkinson’s disease may have a larger proportion of high-risk factor patient groups, a higher concentration of providers who recognize and document Parkinson’s disease, increased public awareness of symptoms, or increased health care–seeking behaviors among people living in the state,” the researchers wrote in their abstract. “Among our top Parkinson’s disease prevalence states, Florida and New York also rank high in terms of absolute number of Medicare beneficiaries and have large supplies of health care providers.”
They also noted that Medicare beneficiaries with Parkinson’s had increased use of health care and spending, compared with their counterparts without the disease. “This was true across all sectors of care (inpatient, outpatient, skilled nursing, and ancillary services) and is in line with data demonstrating that PD, its complications, and the shift away from comorbid disease care and prevention that occurs after a Parkinson’s disease diagnosis drive health care spending and utilization among these individuals,” they wrote.
The study was supported by the Parkinson’s Foundation. Dr. Fullard, who now holds a faculty position at the University of Colorado, Aurora, reported having no financial disclosures.
SOURCE: Ann Neurol. 2018;84[S22]:S89-90, Abstract S215.
REPORTING FROM ANA 2018
Key clinical point:
Major finding: States with the highest prevalence of Parkinson’s disease included New York (1,720/100,000), Illinois (1,566/100,000), and Connecticut (1,560/100,000), while Minnesota had the lowest prevalence (803/100,000).
Study details: An analysis of 392,214 Medicare beneficiaries who carried a diagnosis of Parkinson’s disease.
Disclosures: The study was supported by the Parkinson’s Foundation. Dr. Fullard reported having no financial disclosures.
Source: Ann Neurol. 2018;84[S22]:S89-90. Abstract S215.
Investigational gene therapy for medically refractory Parkinson’s shows promise
ATLANTA – VY-AADC01, an investigational gene therapy for individuals with medically refractory Parkinson’s disease being developed by Voyager Therapeutics, was well tolerated and decreased the need for antiparkinsonian medications, results from an ongoing phase 1b study showed.
“Prior phase 1 trials also introduced the aromatic l-amino acid decarboxylase (AADC) gene using an adeno-associated virus serotype-2 (AAV2) vector into the putamen of people with Parkinson’s disease (PD),” lead study author Chad Christine, MD, said in an interview in advance of the annual meeting of the American Neurological Association. “Unlike the previous trials, here we increased both vector genome concentration and volume of the AAV2-AADC vector (VY-AADC01) across cohorts and used intraoperative MRI guidance to administer the gene product.”
According to Dr. Christine, a neurologist at the University of California, San Francisco, Parkinson’s Disease Clinic and Research Center, prior trials showed that AAV2-AADC was safe, but there was limited clinical efficacy. This may have been because of the limited volume of putamen treated with the gene therapy. “In our current trial, we admixed VY-AADC01 with gadoteridol (ProHance), an MR imaging agent, which allowed both near real-time MRI monitoring of the location and volume of product infused and postsurgical assessment of the area of the putamen covered by VY-AADC01,” he said. “In addition, we used 18F-Dopa PET, which allowed us to assess the activity of the AADC enzyme in the putamen.”
The researchers enrolled three cohorts of patients who received bilateral infusions of VY-AADC01, admixed with gadoteridol to facilitate intraoperative MRI monitoring of the infusions. In cohort 1, five patients received up to 450 μL/putamen at a concentration of 8.3 × 1011 vg (viral genomes)/mL and were followed for 36 months. In cohort 2, five patients received up to 900 μL/putamen at 8.3 × 1011 vg/mL and were followed for 18 months. In cohort 3, five patients received up to 900 μL/putamen at 2.6 × 1012 vg/mL and were followed for 12 months.
At 12 months, Dr. Christine and his associates observed mean levodopa-equivalent dose (LED) reductions of –10.2%, –32.8%, and –39.3% in cohort 1, cohort 2, and cohort 3, respectively; LED reductions were sustained to 18 months in cohorts 1 and 2. “We were impressed by how well the decrease in need for antiparkinsonian medications paralleled the AADC activity we measured in the putamen of our subjects, which is consistent with the proposed mechanism of action of VY-AADC01,” he said.
In addition, subjects in cohort 1 showed a mean 2.3-hour improvement in Parkinson’s diary-“on” time without troublesome dyskinesia at 24 months, which was maintained at 36 months, while subjects in cohort 2 showed a clinically meaningful 3.5-hour improvement at 18 months. Subjects in cohort 3 showed somewhat less improvement than the other cohorts (1.5 hours at 12 months), but they also had more severe baseline dyskinesia on the Unified Dyskinesia Rating Scale (a mean of 30.2 vs. 19.2 and 17.4 in cohorts 1 and 2, respectively). One patient in the trial experienced two surgery-related serious adverse events (pulmonary embolism and related heart arrhythmia) which resolved completely.
“I think we were somewhat surprised by some of the challenges of the surgical administration,” Dr. Christine said. “Our surgeons improved the administration technique throughout the trial and made a major transition from administering VY-AADC01 using a frontal approach to the putamen to using a posterior approach in our second phase 1 trial.”
He concluded that findings of the current trial suggest that AAV2-AADC gene therapy, administered using intraoperative MRI guidance, appears to be safe and well tolerated. “A number of outcomes suggest that it may offer clinical benefit to patients with advancing Parkinson’s disease, but this will have to be tested in a randomized trial which has recently started,” he said.
Dr. Christine acknowledged that the small sample size and the open-label design of the study limits the generalizability of the findings. The trial received support from Voyager Therapeutics and the Michael J. Fox Foundation. Dr. Christine reported having no disclosures.
Source: Christine et al. ANA 2018, Abstract M300.
ATLANTA – VY-AADC01, an investigational gene therapy for individuals with medically refractory Parkinson’s disease being developed by Voyager Therapeutics, was well tolerated and decreased the need for antiparkinsonian medications, results from an ongoing phase 1b study showed.
“Prior phase 1 trials also introduced the aromatic l-amino acid decarboxylase (AADC) gene using an adeno-associated virus serotype-2 (AAV2) vector into the putamen of people with Parkinson’s disease (PD),” lead study author Chad Christine, MD, said in an interview in advance of the annual meeting of the American Neurological Association. “Unlike the previous trials, here we increased both vector genome concentration and volume of the AAV2-AADC vector (VY-AADC01) across cohorts and used intraoperative MRI guidance to administer the gene product.”
According to Dr. Christine, a neurologist at the University of California, San Francisco, Parkinson’s Disease Clinic and Research Center, prior trials showed that AAV2-AADC was safe, but there was limited clinical efficacy. This may have been because of the limited volume of putamen treated with the gene therapy. “In our current trial, we admixed VY-AADC01 with gadoteridol (ProHance), an MR imaging agent, which allowed both near real-time MRI monitoring of the location and volume of product infused and postsurgical assessment of the area of the putamen covered by VY-AADC01,” he said. “In addition, we used 18F-Dopa PET, which allowed us to assess the activity of the AADC enzyme in the putamen.”
The researchers enrolled three cohorts of patients who received bilateral infusions of VY-AADC01, admixed with gadoteridol to facilitate intraoperative MRI monitoring of the infusions. In cohort 1, five patients received up to 450 μL/putamen at a concentration of 8.3 × 1011 vg (viral genomes)/mL and were followed for 36 months. In cohort 2, five patients received up to 900 μL/putamen at 8.3 × 1011 vg/mL and were followed for 18 months. In cohort 3, five patients received up to 900 μL/putamen at 2.6 × 1012 vg/mL and were followed for 12 months.
At 12 months, Dr. Christine and his associates observed mean levodopa-equivalent dose (LED) reductions of –10.2%, –32.8%, and –39.3% in cohort 1, cohort 2, and cohort 3, respectively; LED reductions were sustained to 18 months in cohorts 1 and 2. “We were impressed by how well the decrease in need for antiparkinsonian medications paralleled the AADC activity we measured in the putamen of our subjects, which is consistent with the proposed mechanism of action of VY-AADC01,” he said.
In addition, subjects in cohort 1 showed a mean 2.3-hour improvement in Parkinson’s diary-“on” time without troublesome dyskinesia at 24 months, which was maintained at 36 months, while subjects in cohort 2 showed a clinically meaningful 3.5-hour improvement at 18 months. Subjects in cohort 3 showed somewhat less improvement than the other cohorts (1.5 hours at 12 months), but they also had more severe baseline dyskinesia on the Unified Dyskinesia Rating Scale (a mean of 30.2 vs. 19.2 and 17.4 in cohorts 1 and 2, respectively). One patient in the trial experienced two surgery-related serious adverse events (pulmonary embolism and related heart arrhythmia) which resolved completely.
“I think we were somewhat surprised by some of the challenges of the surgical administration,” Dr. Christine said. “Our surgeons improved the administration technique throughout the trial and made a major transition from administering VY-AADC01 using a frontal approach to the putamen to using a posterior approach in our second phase 1 trial.”
He concluded that findings of the current trial suggest that AAV2-AADC gene therapy, administered using intraoperative MRI guidance, appears to be safe and well tolerated. “A number of outcomes suggest that it may offer clinical benefit to patients with advancing Parkinson’s disease, but this will have to be tested in a randomized trial which has recently started,” he said.
Dr. Christine acknowledged that the small sample size and the open-label design of the study limits the generalizability of the findings. The trial received support from Voyager Therapeutics and the Michael J. Fox Foundation. Dr. Christine reported having no disclosures.
Source: Christine et al. ANA 2018, Abstract M300.
ATLANTA – VY-AADC01, an investigational gene therapy for individuals with medically refractory Parkinson’s disease being developed by Voyager Therapeutics, was well tolerated and decreased the need for antiparkinsonian medications, results from an ongoing phase 1b study showed.
“Prior phase 1 trials also introduced the aromatic l-amino acid decarboxylase (AADC) gene using an adeno-associated virus serotype-2 (AAV2) vector into the putamen of people with Parkinson’s disease (PD),” lead study author Chad Christine, MD, said in an interview in advance of the annual meeting of the American Neurological Association. “Unlike the previous trials, here we increased both vector genome concentration and volume of the AAV2-AADC vector (VY-AADC01) across cohorts and used intraoperative MRI guidance to administer the gene product.”
According to Dr. Christine, a neurologist at the University of California, San Francisco, Parkinson’s Disease Clinic and Research Center, prior trials showed that AAV2-AADC was safe, but there was limited clinical efficacy. This may have been because of the limited volume of putamen treated with the gene therapy. “In our current trial, we admixed VY-AADC01 with gadoteridol (ProHance), an MR imaging agent, which allowed both near real-time MRI monitoring of the location and volume of product infused and postsurgical assessment of the area of the putamen covered by VY-AADC01,” he said. “In addition, we used 18F-Dopa PET, which allowed us to assess the activity of the AADC enzyme in the putamen.”
The researchers enrolled three cohorts of patients who received bilateral infusions of VY-AADC01, admixed with gadoteridol to facilitate intraoperative MRI monitoring of the infusions. In cohort 1, five patients received up to 450 μL/putamen at a concentration of 8.3 × 1011 vg (viral genomes)/mL and were followed for 36 months. In cohort 2, five patients received up to 900 μL/putamen at 8.3 × 1011 vg/mL and were followed for 18 months. In cohort 3, five patients received up to 900 μL/putamen at 2.6 × 1012 vg/mL and were followed for 12 months.
At 12 months, Dr. Christine and his associates observed mean levodopa-equivalent dose (LED) reductions of –10.2%, –32.8%, and –39.3% in cohort 1, cohort 2, and cohort 3, respectively; LED reductions were sustained to 18 months in cohorts 1 and 2. “We were impressed by how well the decrease in need for antiparkinsonian medications paralleled the AADC activity we measured in the putamen of our subjects, which is consistent with the proposed mechanism of action of VY-AADC01,” he said.
In addition, subjects in cohort 1 showed a mean 2.3-hour improvement in Parkinson’s diary-“on” time without troublesome dyskinesia at 24 months, which was maintained at 36 months, while subjects in cohort 2 showed a clinically meaningful 3.5-hour improvement at 18 months. Subjects in cohort 3 showed somewhat less improvement than the other cohorts (1.5 hours at 12 months), but they also had more severe baseline dyskinesia on the Unified Dyskinesia Rating Scale (a mean of 30.2 vs. 19.2 and 17.4 in cohorts 1 and 2, respectively). One patient in the trial experienced two surgery-related serious adverse events (pulmonary embolism and related heart arrhythmia) which resolved completely.
“I think we were somewhat surprised by some of the challenges of the surgical administration,” Dr. Christine said. “Our surgeons improved the administration technique throughout the trial and made a major transition from administering VY-AADC01 using a frontal approach to the putamen to using a posterior approach in our second phase 1 trial.”
He concluded that findings of the current trial suggest that AAV2-AADC gene therapy, administered using intraoperative MRI guidance, appears to be safe and well tolerated. “A number of outcomes suggest that it may offer clinical benefit to patients with advancing Parkinson’s disease, but this will have to be tested in a randomized trial which has recently started,” he said.
Dr. Christine acknowledged that the small sample size and the open-label design of the study limits the generalizability of the findings. The trial received support from Voyager Therapeutics and the Michael J. Fox Foundation. Dr. Christine reported having no disclosures.
Source: Christine et al. ANA 2018, Abstract M300.
REPORTING FROM ANA 2018
Key clinical point: AAV2-AADC gene therapy, administered using intraoperative MRI guidance, appears to be safe and well tolerated.
Major finding: At 12 months, the researchers observed mean levodopa-equivalent dose (LED) reductions of –10.2%, –32.8%, and –39.3% in cohort 1, cohort 2, and cohort 3, respectively.
Study details: A study of 15 patients in three cohorts who received bilateral infusions of VY-AADC01, admixed with gadoteridol to facilitate intraoperative MRI monitoring of the infusions.
Disclosures: The trial received support from Voyager Therapeutics and the Michael J. Fox Foundation. Dr. Christine reported having no disclosures.
Source: Christine et al. ANA 2018, Abstract M300.
Many oromandibular dystonia patients report improvement after botulinum toxin injections
ATLANTA – A majority of oromandibular dystonia patients treated with botulinum toxin injections reported improvement in symptoms in the largest cohort of patients to date.
Improvements in the range of 50%-100% occurred in 78% of oromandibular dystonia (OMD) patients who received botulinum toxin injections in the retrospective, multicenter analysis, which was presented by Laura Scorr, MD, at the annual meeting of the American Neurological Association.
In an effort to better describe the clinical characteristics of patients with OMD, Dr. Scorr, a movement disorders specialist at Emory University, Atlanta, and her colleagues analyzed data collected from 164 OMD patients enrolled at 26 international sites in the Dystonia Coalition and 37 additional patients who were evaluated at the Emory University within the last year. Subjects enrolled at Dystonia Coalition centers underwent evaluation by a movement disorders specialist to determine distribution of dystonia, areas affected, and severity as measured by the Global Dystonia Rating Scale. A subgroup of patients also completed the SF 36-item Health Survey, the Beck Depression Scale, and the Liebowitz social anxiety scale. Meanwhile, the charts of patients seen at Emory underwent review for data on clinical characteristics, treatment type, botulinum toxin doses, and response.
Among all 201 patients, the average age of onset was 54 years and 65% were female. About 45% were determined to have focal dystonia, 36% had segmental dystonia, and 19% had generalized dystonia. Among a cohort of 47 patients evaluated in the Dystonia Coalition biorepository, the researchers observed significantly increased social anxiety and impaired quality of life on the Liebowitz social anxiety scale and the SF-36 Health Survey.
Of the 37 Emory patients, 31 (84%) received botulinum toxin injections. Of these, 39% reported symptom improvement that ranged from 75%-100% while 39% reported symptom improvement that ranged from 50%-74%. Only 13% had a minimal response, defined as improvement that ranged from 1%-24%.
“Oromandibular dystonia is particularly disabling,” Dr. Scorr said. “There have been a few reports in the literature that say it does not respond to botulinum toxin injections. But in our retrospective review, the majority of patients not only have a response, but a response that’s greater than 50% improvement, which is significant.” She acknowledged that the study’s retrospective design is a limitation. “I think we need more prospective studies, specifically on response to treatment with botulinum toxin,” she said.
The study was funded in part by the Dystonia Medical Research Foundation. Dr. Scorr reported having no financial disclosures.
SOURCE: Scorr L et al. Ann Neurol. 2018;84[S22]:S90, Abstract S216.
ATLANTA – A majority of oromandibular dystonia patients treated with botulinum toxin injections reported improvement in symptoms in the largest cohort of patients to date.
Improvements in the range of 50%-100% occurred in 78% of oromandibular dystonia (OMD) patients who received botulinum toxin injections in the retrospective, multicenter analysis, which was presented by Laura Scorr, MD, at the annual meeting of the American Neurological Association.
In an effort to better describe the clinical characteristics of patients with OMD, Dr. Scorr, a movement disorders specialist at Emory University, Atlanta, and her colleagues analyzed data collected from 164 OMD patients enrolled at 26 international sites in the Dystonia Coalition and 37 additional patients who were evaluated at the Emory University within the last year. Subjects enrolled at Dystonia Coalition centers underwent evaluation by a movement disorders specialist to determine distribution of dystonia, areas affected, and severity as measured by the Global Dystonia Rating Scale. A subgroup of patients also completed the SF 36-item Health Survey, the Beck Depression Scale, and the Liebowitz social anxiety scale. Meanwhile, the charts of patients seen at Emory underwent review for data on clinical characteristics, treatment type, botulinum toxin doses, and response.
Among all 201 patients, the average age of onset was 54 years and 65% were female. About 45% were determined to have focal dystonia, 36% had segmental dystonia, and 19% had generalized dystonia. Among a cohort of 47 patients evaluated in the Dystonia Coalition biorepository, the researchers observed significantly increased social anxiety and impaired quality of life on the Liebowitz social anxiety scale and the SF-36 Health Survey.
Of the 37 Emory patients, 31 (84%) received botulinum toxin injections. Of these, 39% reported symptom improvement that ranged from 75%-100% while 39% reported symptom improvement that ranged from 50%-74%. Only 13% had a minimal response, defined as improvement that ranged from 1%-24%.
“Oromandibular dystonia is particularly disabling,” Dr. Scorr said. “There have been a few reports in the literature that say it does not respond to botulinum toxin injections. But in our retrospective review, the majority of patients not only have a response, but a response that’s greater than 50% improvement, which is significant.” She acknowledged that the study’s retrospective design is a limitation. “I think we need more prospective studies, specifically on response to treatment with botulinum toxin,” she said.
The study was funded in part by the Dystonia Medical Research Foundation. Dr. Scorr reported having no financial disclosures.
SOURCE: Scorr L et al. Ann Neurol. 2018;84[S22]:S90, Abstract S216.
ATLANTA – A majority of oromandibular dystonia patients treated with botulinum toxin injections reported improvement in symptoms in the largest cohort of patients to date.
Improvements in the range of 50%-100% occurred in 78% of oromandibular dystonia (OMD) patients who received botulinum toxin injections in the retrospective, multicenter analysis, which was presented by Laura Scorr, MD, at the annual meeting of the American Neurological Association.
In an effort to better describe the clinical characteristics of patients with OMD, Dr. Scorr, a movement disorders specialist at Emory University, Atlanta, and her colleagues analyzed data collected from 164 OMD patients enrolled at 26 international sites in the Dystonia Coalition and 37 additional patients who were evaluated at the Emory University within the last year. Subjects enrolled at Dystonia Coalition centers underwent evaluation by a movement disorders specialist to determine distribution of dystonia, areas affected, and severity as measured by the Global Dystonia Rating Scale. A subgroup of patients also completed the SF 36-item Health Survey, the Beck Depression Scale, and the Liebowitz social anxiety scale. Meanwhile, the charts of patients seen at Emory underwent review for data on clinical characteristics, treatment type, botulinum toxin doses, and response.
Among all 201 patients, the average age of onset was 54 years and 65% were female. About 45% were determined to have focal dystonia, 36% had segmental dystonia, and 19% had generalized dystonia. Among a cohort of 47 patients evaluated in the Dystonia Coalition biorepository, the researchers observed significantly increased social anxiety and impaired quality of life on the Liebowitz social anxiety scale and the SF-36 Health Survey.
Of the 37 Emory patients, 31 (84%) received botulinum toxin injections. Of these, 39% reported symptom improvement that ranged from 75%-100% while 39% reported symptom improvement that ranged from 50%-74%. Only 13% had a minimal response, defined as improvement that ranged from 1%-24%.
“Oromandibular dystonia is particularly disabling,” Dr. Scorr said. “There have been a few reports in the literature that say it does not respond to botulinum toxin injections. But in our retrospective review, the majority of patients not only have a response, but a response that’s greater than 50% improvement, which is significant.” She acknowledged that the study’s retrospective design is a limitation. “I think we need more prospective studies, specifically on response to treatment with botulinum toxin,” she said.
The study was funded in part by the Dystonia Medical Research Foundation. Dr. Scorr reported having no financial disclosures.
SOURCE: Scorr L et al. Ann Neurol. 2018;84[S22]:S90, Abstract S216.
AT ANA 2018
Key clinical point: Oromandibular dystonia is associated with increased social anxiety and impaired quality of life.
Major finding: After receiving botulinum toxin injections, 78% of patients with oromandibular dystonia reported improvements in the range of 50%-100%.
Study details: A retrospective review of 201 patients with oromandibular dystonia.
Disclosures: The study was funded in part by the Dystonia Medical Research Foundation. Dr. Scorr researchers reported having no financial disclosures.
Source: Scorr L et al. Ann Neurol. 2018;84[S22]:S90, Abstract S216.
Higher BMI associated with greater loss of gray matter volume in MS
ATLANTA – Among patients with relapsing-remitting multiple sclerosis, higher body mass index, but not vitamin D status, appears to be related to greater loss of gray matter brain volume over time, results from a 5-year analysis showed.
“We had previously known that obesity is a risk factor for developing MS, and among those who already have the disease, obesity-related comorbidities are associated with increased morbidity and mortality,” lead study author Ellen M. Mowry, MD, said in an interview at the annual meeting of the American Neurological Association. “Loss of brain tissue, especially as measured by reduced volume of gray matter noted on brain MRI, is predictive of long-term disability in MS. While we await the results of confirmatory studies and randomized trials, this study adds to the growing body of evidence suggesting there may be a role for modification of lifestyle factors in mitigating longer-term MS-related disability risk.”
In an effort to determine if body mass index (BMI) or vitamin D status is associated with longer-term MRI measures of neurodegeneration, Dr. Mowry and her colleagues drew from 469 patients participating in a longitudinal MS cohort study at the University of California, San Francisco, known as EPIC. Participants had clinical evaluations, brain MRI, and blood draws annually and were followed for 5 years. The main outcomes of interest were BMI and serum 25-hydroxyvitamin D levels measured over the time period, and their relationship to brain volume.
At baseline, the mean age of patients was 42 years, 70% were female, their mean BMI was 25 kg/m2, and their mean serum vitamin D level was 27.8 ng/mL. Dr. Mowry, a neurologist at Johns Hopkins University, Baltimore, and her colleagues found that over time, each 1-kg/m2 higher BMI was independently associated with reduced gray matter in multivariate models (–1.1 mL; P = .001). In addition, each 1-kg/m2 higher BMI over time was independently associated with greater declines in normalized brain parenchymal brain volume (–1.1 mL; P = .039). Elevated vitamin D levels, however, did not appear to be meaningfully associated with brain volumes.
Dr. Mowry acknowledged certain limitations of the study, including its nonrandomized design. “Such a trial may be warranted but I believe will be challenging to conduct,” she said. “Also, this cohort was designed to assess the association of genes with brain MRI outcomes, and so the people included were racially homogeneous – only Caucasians were included. Since MS risk is especially high among African Americans in recent years, and African Americans appear overall to have a higher risk of long-term disability, it is important to evaluate these and other prognostic factors amongst a more representative group of people with MS.”
The study received funding support from the National Institutes of Health, GlaxoSmithKline, and Biogen. Dr. Mowry disclosed that she has received medication from Teva for use in a clinical trial. In addition, she has been the primary investigator for studies sponsored by Biogen and Sun Pharma, and has conducted investigator-initiated studies sponsored by Genzyme and Biogen.
SOURCE: Ann Neurol. 2018;84[S22]:S206-7. Abstract M250.
ATLANTA – Among patients with relapsing-remitting multiple sclerosis, higher body mass index, but not vitamin D status, appears to be related to greater loss of gray matter brain volume over time, results from a 5-year analysis showed.
“We had previously known that obesity is a risk factor for developing MS, and among those who already have the disease, obesity-related comorbidities are associated with increased morbidity and mortality,” lead study author Ellen M. Mowry, MD, said in an interview at the annual meeting of the American Neurological Association. “Loss of brain tissue, especially as measured by reduced volume of gray matter noted on brain MRI, is predictive of long-term disability in MS. While we await the results of confirmatory studies and randomized trials, this study adds to the growing body of evidence suggesting there may be a role for modification of lifestyle factors in mitigating longer-term MS-related disability risk.”
In an effort to determine if body mass index (BMI) or vitamin D status is associated with longer-term MRI measures of neurodegeneration, Dr. Mowry and her colleagues drew from 469 patients participating in a longitudinal MS cohort study at the University of California, San Francisco, known as EPIC. Participants had clinical evaluations, brain MRI, and blood draws annually and were followed for 5 years. The main outcomes of interest were BMI and serum 25-hydroxyvitamin D levels measured over the time period, and their relationship to brain volume.
At baseline, the mean age of patients was 42 years, 70% were female, their mean BMI was 25 kg/m2, and their mean serum vitamin D level was 27.8 ng/mL. Dr. Mowry, a neurologist at Johns Hopkins University, Baltimore, and her colleagues found that over time, each 1-kg/m2 higher BMI was independently associated with reduced gray matter in multivariate models (–1.1 mL; P = .001). In addition, each 1-kg/m2 higher BMI over time was independently associated with greater declines in normalized brain parenchymal brain volume (–1.1 mL; P = .039). Elevated vitamin D levels, however, did not appear to be meaningfully associated with brain volumes.
Dr. Mowry acknowledged certain limitations of the study, including its nonrandomized design. “Such a trial may be warranted but I believe will be challenging to conduct,” she said. “Also, this cohort was designed to assess the association of genes with brain MRI outcomes, and so the people included were racially homogeneous – only Caucasians were included. Since MS risk is especially high among African Americans in recent years, and African Americans appear overall to have a higher risk of long-term disability, it is important to evaluate these and other prognostic factors amongst a more representative group of people with MS.”
The study received funding support from the National Institutes of Health, GlaxoSmithKline, and Biogen. Dr. Mowry disclosed that she has received medication from Teva for use in a clinical trial. In addition, she has been the primary investigator for studies sponsored by Biogen and Sun Pharma, and has conducted investigator-initiated studies sponsored by Genzyme and Biogen.
SOURCE: Ann Neurol. 2018;84[S22]:S206-7. Abstract M250.
ATLANTA – Among patients with relapsing-remitting multiple sclerosis, higher body mass index, but not vitamin D status, appears to be related to greater loss of gray matter brain volume over time, results from a 5-year analysis showed.
“We had previously known that obesity is a risk factor for developing MS, and among those who already have the disease, obesity-related comorbidities are associated with increased morbidity and mortality,” lead study author Ellen M. Mowry, MD, said in an interview at the annual meeting of the American Neurological Association. “Loss of brain tissue, especially as measured by reduced volume of gray matter noted on brain MRI, is predictive of long-term disability in MS. While we await the results of confirmatory studies and randomized trials, this study adds to the growing body of evidence suggesting there may be a role for modification of lifestyle factors in mitigating longer-term MS-related disability risk.”
In an effort to determine if body mass index (BMI) or vitamin D status is associated with longer-term MRI measures of neurodegeneration, Dr. Mowry and her colleagues drew from 469 patients participating in a longitudinal MS cohort study at the University of California, San Francisco, known as EPIC. Participants had clinical evaluations, brain MRI, and blood draws annually and were followed for 5 years. The main outcomes of interest were BMI and serum 25-hydroxyvitamin D levels measured over the time period, and their relationship to brain volume.
At baseline, the mean age of patients was 42 years, 70% were female, their mean BMI was 25 kg/m2, and their mean serum vitamin D level was 27.8 ng/mL. Dr. Mowry, a neurologist at Johns Hopkins University, Baltimore, and her colleagues found that over time, each 1-kg/m2 higher BMI was independently associated with reduced gray matter in multivariate models (–1.1 mL; P = .001). In addition, each 1-kg/m2 higher BMI over time was independently associated with greater declines in normalized brain parenchymal brain volume (–1.1 mL; P = .039). Elevated vitamin D levels, however, did not appear to be meaningfully associated with brain volumes.
Dr. Mowry acknowledged certain limitations of the study, including its nonrandomized design. “Such a trial may be warranted but I believe will be challenging to conduct,” she said. “Also, this cohort was designed to assess the association of genes with brain MRI outcomes, and so the people included were racially homogeneous – only Caucasians were included. Since MS risk is especially high among African Americans in recent years, and African Americans appear overall to have a higher risk of long-term disability, it is important to evaluate these and other prognostic factors amongst a more representative group of people with MS.”
The study received funding support from the National Institutes of Health, GlaxoSmithKline, and Biogen. Dr. Mowry disclosed that she has received medication from Teva for use in a clinical trial. In addition, she has been the primary investigator for studies sponsored by Biogen and Sun Pharma, and has conducted investigator-initiated studies sponsored by Genzyme and Biogen.
SOURCE: Ann Neurol. 2018;84[S22]:S206-7. Abstract M250.
AT ANA 2018
Key clinical point: Higher body mass in MS patients appears to be related to greater brain atrophy over time.
Major finding: Over time, each 1-kg/m2 higher BMI was independently associated with reduced gray matter in multivariate models (–1.1 mL; P = .001).
Study details: An analysis of 469 patients participating in a longitudinal MS cohort study.
Disclosures: The study received funding support from the National Institutes of Health, GlaxoSmithKline, and Biogen. Dr. Mowry disclosed that she has received medication from Teva for use in a clinical trial. In addition, she has been the primary investigator for studies sponsored by Biogen and Sun Pharma, and has conducted investigator-initiated studies sponsored by Genzyme and Biogen.
Source: Ann Neurol. 2018;84[S22]:S206-7. Abstract M250.
Statins cut all-cause mortality in spinal cord injury
ATLANTA – Statin use among a cohort of veterans with traumatic spinal cord injury reduced all-cause mortality, results from a novel observational study showed.
“This is the first clinical study to show that administration of statins irrespective of the lipid levels reduces all-cause mortality, not just cardiovascular mortality,” lead study author Meheroz H. Rabadi, MD, said in an interview in advance of the annual meeting of the American Neurological Association. “This clinical study confirms the impression of several prior studies in animal models with spinal cord injury, which have shown the anti-inflammatory and neuro-protective effects of statins.”
To determine whether statin use in a cohort of patients with traumatic spinal cord injuries (SCI) reduced overall and cause-specific mortality, Dr. Rabadi and his colleagues retrospectively reviewed the medical charts and death records of 163 individuals with SCI who were treated at the Oklahoma City Veterans Administration Medical Center Spinal Cord Injury & Disease, Multiple Sclerosis, and ALS Program, an outpatient clinic, from 2000 to 2014. They collected data on statin use, duration of statin use, and intensity of statin therapy, as well as cause-specific mortality.
Of the 163 subjects studied, 75 (46%) had taken statins for an average of 5.7 years, and had greater cardiovascular risk burdens than those who had not taken statins. The mortality rate for patients on statins, however, was 33.8-49.9 per 1,000 person-years, compared with 47.4-66.8 deaths per 1,000 person-years among those who had not taken statins. Kaplan-Meier survival curves showed a significant difference between the two groups (P less than .0052). Within the statin group, neither duration nor average intensity of statin therapy affected mortality.
“We were surprised to note statins reduced pneumonia-related mortality in patients with SCI,” Dr. Rabadi said. “Since our publication there have been several publications, including a meta-analysis of statins reducing community-acquired pneumonia-related mortality and reducing the need for mechanical ventilation or ICU admission (see CHEST 2015;148:523-32, Clin Med (Lond) 2017;17(5):403-7, and Zhongguo Yi Xue Ke Xue Yuan Xue Bao. 2018;40(1):30-40). Another surprise was neither the intensity, duration, or types of statin affected the result.”
He acknowledged certain limitations of the analysis, including its retrospective design, its relatively small sample size, and the fact that most of the subjects were non-Hispanic white men. “Routine prescription of statins in any dose in patients with SCI – even if the lipid profile is normal – is more beneficial than detrimental over the long haul,” concluded Dr. Rabadi, who also directs the Oklahoma VAMC Stroke Program. “Nearly all our patients with SCI continue to be on varying doses of statins.”
Dr. Rabadi reported having no financial disclosures.
dbrunk@mdedge.com
SOURCE: Ann Neurol. 2018;84[S22]:S127. Abstract S302.
ATLANTA – Statin use among a cohort of veterans with traumatic spinal cord injury reduced all-cause mortality, results from a novel observational study showed.
“This is the first clinical study to show that administration of statins irrespective of the lipid levels reduces all-cause mortality, not just cardiovascular mortality,” lead study author Meheroz H. Rabadi, MD, said in an interview in advance of the annual meeting of the American Neurological Association. “This clinical study confirms the impression of several prior studies in animal models with spinal cord injury, which have shown the anti-inflammatory and neuro-protective effects of statins.”
To determine whether statin use in a cohort of patients with traumatic spinal cord injuries (SCI) reduced overall and cause-specific mortality, Dr. Rabadi and his colleagues retrospectively reviewed the medical charts and death records of 163 individuals with SCI who were treated at the Oklahoma City Veterans Administration Medical Center Spinal Cord Injury & Disease, Multiple Sclerosis, and ALS Program, an outpatient clinic, from 2000 to 2014. They collected data on statin use, duration of statin use, and intensity of statin therapy, as well as cause-specific mortality.
Of the 163 subjects studied, 75 (46%) had taken statins for an average of 5.7 years, and had greater cardiovascular risk burdens than those who had not taken statins. The mortality rate for patients on statins, however, was 33.8-49.9 per 1,000 person-years, compared with 47.4-66.8 deaths per 1,000 person-years among those who had not taken statins. Kaplan-Meier survival curves showed a significant difference between the two groups (P less than .0052). Within the statin group, neither duration nor average intensity of statin therapy affected mortality.
“We were surprised to note statins reduced pneumonia-related mortality in patients with SCI,” Dr. Rabadi said. “Since our publication there have been several publications, including a meta-analysis of statins reducing community-acquired pneumonia-related mortality and reducing the need for mechanical ventilation or ICU admission (see CHEST 2015;148:523-32, Clin Med (Lond) 2017;17(5):403-7, and Zhongguo Yi Xue Ke Xue Yuan Xue Bao. 2018;40(1):30-40). Another surprise was neither the intensity, duration, or types of statin affected the result.”
He acknowledged certain limitations of the analysis, including its retrospective design, its relatively small sample size, and the fact that most of the subjects were non-Hispanic white men. “Routine prescription of statins in any dose in patients with SCI – even if the lipid profile is normal – is more beneficial than detrimental over the long haul,” concluded Dr. Rabadi, who also directs the Oklahoma VAMC Stroke Program. “Nearly all our patients with SCI continue to be on varying doses of statins.”
Dr. Rabadi reported having no financial disclosures.
dbrunk@mdedge.com
SOURCE: Ann Neurol. 2018;84[S22]:S127. Abstract S302.
ATLANTA – Statin use among a cohort of veterans with traumatic spinal cord injury reduced all-cause mortality, results from a novel observational study showed.
“This is the first clinical study to show that administration of statins irrespective of the lipid levels reduces all-cause mortality, not just cardiovascular mortality,” lead study author Meheroz H. Rabadi, MD, said in an interview in advance of the annual meeting of the American Neurological Association. “This clinical study confirms the impression of several prior studies in animal models with spinal cord injury, which have shown the anti-inflammatory and neuro-protective effects of statins.”
To determine whether statin use in a cohort of patients with traumatic spinal cord injuries (SCI) reduced overall and cause-specific mortality, Dr. Rabadi and his colleagues retrospectively reviewed the medical charts and death records of 163 individuals with SCI who were treated at the Oklahoma City Veterans Administration Medical Center Spinal Cord Injury & Disease, Multiple Sclerosis, and ALS Program, an outpatient clinic, from 2000 to 2014. They collected data on statin use, duration of statin use, and intensity of statin therapy, as well as cause-specific mortality.
Of the 163 subjects studied, 75 (46%) had taken statins for an average of 5.7 years, and had greater cardiovascular risk burdens than those who had not taken statins. The mortality rate for patients on statins, however, was 33.8-49.9 per 1,000 person-years, compared with 47.4-66.8 deaths per 1,000 person-years among those who had not taken statins. Kaplan-Meier survival curves showed a significant difference between the two groups (P less than .0052). Within the statin group, neither duration nor average intensity of statin therapy affected mortality.
“We were surprised to note statins reduced pneumonia-related mortality in patients with SCI,” Dr. Rabadi said. “Since our publication there have been several publications, including a meta-analysis of statins reducing community-acquired pneumonia-related mortality and reducing the need for mechanical ventilation or ICU admission (see CHEST 2015;148:523-32, Clin Med (Lond) 2017;17(5):403-7, and Zhongguo Yi Xue Ke Xue Yuan Xue Bao. 2018;40(1):30-40). Another surprise was neither the intensity, duration, or types of statin affected the result.”
He acknowledged certain limitations of the analysis, including its retrospective design, its relatively small sample size, and the fact that most of the subjects were non-Hispanic white men. “Routine prescription of statins in any dose in patients with SCI – even if the lipid profile is normal – is more beneficial than detrimental over the long haul,” concluded Dr. Rabadi, who also directs the Oklahoma VAMC Stroke Program. “Nearly all our patients with SCI continue to be on varying doses of statins.”
Dr. Rabadi reported having no financial disclosures.
dbrunk@mdedge.com
SOURCE: Ann Neurol. 2018;84[S22]:S127. Abstract S302.
AT ANA 2018
Key clinical point:
Major finding: The mortality rate for patients on statins was 33.8-49.9 per 1,000 person-years, compared with 47.4-66.8 deaths per 1,000 person-years among those who had not taken statins (P less than .0052).
Study details: A retrospective review of 163 individuals with traumatic spinal cord injuries.
Disclosures: Dr. Rabadi reported having no financial disclosures.
Source: Ann Neurol. 2018;84[S22]:S127. Abstract S302.