User login
Hepatitis C debrief: Therapy has matured, access issues remain
SAN FRANCISCO – Hepatitis C therapy has matured and now offers excellent sustained viral response (SVR) in the vast majority of cases, but key challenges remain in getting the therapy to those who need it.
“Unfortunately, we’re not making some of the progress we might have hoped to see, particularly in North America,” said Jordan Feld, MD, MPH, who gave a debrief of hepatitis C abstracts during a wrap-up session at the annual meeting of the American Association for the Study of Liver Diseases.
The problem is particularly acute in young adults aged 18-39 years – only about 9% of those who tested positive for HCV RNA saw a specialist, and about 23% of those who saw a specialist went on to receive treatment, according to an analysis of over 17 million patients in the United States (abstract 1567). The numbers were better for older adults but still far from optimal, with 23% who tested positive seeing a specialist, and just 32% of those patients getting treatment.
Another study (abstract 0147) looked state by state at the percentage of Medicaid patients who received a prescription for direct-acting antiviral (DAA) medication and then went on to fill the prescription. The rates ranged from 0% in Alaska to 96% in Connecticut. Eight states were higher than 70%, six were between 50% and 70%, and 15 states were below 50%.
“Despite our efforts, there continue to be major access barriers across the U.S., particularly for Medicaid individuals,” said Dr. Feld, who is a clinician-scientist at the Toronto Western Hospital Liver Clinic and the McLaughlin-Rotman Centre for Global Health.
A study examining the Chronic Hepatitis (CHeCS) cohort (abstract 0585) described a big spike in treatment uptake shortly after approvals of the new HCV regimens, but by 2016, only about one-third of individuals who required treatment actually began treatment. Factors associated with nontreatment largely reflected marginalization, including low income, being on Medicaid, and lack of long-term follow-up.
Even as health systems struggle to get treatment to those who need it, new studies are showing how to expand existing treatments into new populations.
Results from the EXPEDITION 8 study (abstract LB-7) showed efficacy of an 8-week regimen of the glecaprevir/pibrentasvir combination in patients with compensated cirrhosis. It looked at genotypes 1, 2, and 4-6. In an intention-to-treat analysis, 98% attained SVR and there were no viral failures or safety concerns. A follow-up trial is ongoing that includes patients with genotype 3. “This is exciting to be able to shorten therapy in patients with cirrhosis,” said Dr. Feld.
Although first-line DAAs are extremely effective, there are a few patients who do not achieve a cure. One study (abstract 0227) examined the combination of sofosbuvir, velpatasvir, and voxilaprevir in retreatment of these patients. The drugs resulted in SVR rates similar to those in registration trials, but the regimen was somewhat less effective in patients previously treated with sofosbuvir and velpatasvir. “I think we need to investigate that further,” said Dr. Feld.
The combination of glecaprevir and pibrentasvir also proved effective for retreatment in patients with genotype 1/1A who had failed treatment with an NS5A inhibitor plus sofosbuvir with or without ribavirin (abstract 226). SVR rates at 16 weeks were quite good, but lower in genotype 1a patients at 12 weeks (87% week 12 versus 94% week 16).”I think this is a really good regimen for genotype 1b. For 1a, serum definitely needs 16 weeks [to clear],” said Dr. Feld.
Other abstracts presented at the meeting detailed some of the benefits of SVR, not all of which are broadly appreciated. An analysis of the Hepatitis Testers Cohort in British Columbia (abstract 145), which includes over 7,000 patients who were followed for a median of 2 years (DAA) or 9.5 years (interferon-based), showed survival advantages to SVR in both cirrhotic (adjusted hazard ratio, 0.14) and noncirrhotic patients (aHR, 0.13). Other benefits include lower risk of diabetes (aHR, 0.53), chronic kidney disease/endstage renal disease (aHR, 0.48), stroke (aHR, 0.67), and mood and anxiety disorders (aHR, 0.53) (abstract 148).
As is generally accepted, SVR reduces the risk of hepatocellular cancer (HCC), according to analyses of VA and Gilead data (abstract 635), with a benefit in both cirrhotic and noncirrhotic patients. The risk almost disappears in patients without cirrhosis (incidence rate 0.07 per 100 person-years and is curbed in cirrhotic patients (incidence rate 1.30 in compensated, 4.05 in decompensated cirrhosis).
“There is really very significantly high incidence in cancer in decompensated cirrhosis, which just highlights that these patients continue to need ongoing surveillance. Although there have been efforts at developing strategies to risk stratify patients with cirrhosis, at least for now we’re stuck with surveillance, but I think for patients without cirrhosis there are now enough data showing a low enough incidence of primary HCC that we can probably avoid surveillance in that group,” said Dr. Feld.
Injectable drug users represent a special challenge in hepatitis C treatment, but new studies show cause for optimism in this population. These patients are harder to reach, and they may be less medication compliant, but one study (abstract 1632) found that imperfect adherence doesn’t necessarily undermine results – in a 12-week regimen, patients who didn’t finish until 14 weeks had no significant difference in SVR rates.
“So these therapies have a bit of forgiveness. We probably shouldn’t tell that to the patients, but it’s reassuring that we can use these therapies even in tough-to-reach populations,” said Dr. Feld.
In the GI Patient Center, AGA offers resources to help ensure patients are receiving the best possible care and living their best life.
SAN FRANCISCO – Hepatitis C therapy has matured and now offers excellent sustained viral response (SVR) in the vast majority of cases, but key challenges remain in getting the therapy to those who need it.
“Unfortunately, we’re not making some of the progress we might have hoped to see, particularly in North America,” said Jordan Feld, MD, MPH, who gave a debrief of hepatitis C abstracts during a wrap-up session at the annual meeting of the American Association for the Study of Liver Diseases.
The problem is particularly acute in young adults aged 18-39 years – only about 9% of those who tested positive for HCV RNA saw a specialist, and about 23% of those who saw a specialist went on to receive treatment, according to an analysis of over 17 million patients in the United States (abstract 1567). The numbers were better for older adults but still far from optimal, with 23% who tested positive seeing a specialist, and just 32% of those patients getting treatment.
Another study (abstract 0147) looked state by state at the percentage of Medicaid patients who received a prescription for direct-acting antiviral (DAA) medication and then went on to fill the prescription. The rates ranged from 0% in Alaska to 96% in Connecticut. Eight states were higher than 70%, six were between 50% and 70%, and 15 states were below 50%.
“Despite our efforts, there continue to be major access barriers across the U.S., particularly for Medicaid individuals,” said Dr. Feld, who is a clinician-scientist at the Toronto Western Hospital Liver Clinic and the McLaughlin-Rotman Centre for Global Health.
A study examining the Chronic Hepatitis (CHeCS) cohort (abstract 0585) described a big spike in treatment uptake shortly after approvals of the new HCV regimens, but by 2016, only about one-third of individuals who required treatment actually began treatment. Factors associated with nontreatment largely reflected marginalization, including low income, being on Medicaid, and lack of long-term follow-up.
Even as health systems struggle to get treatment to those who need it, new studies are showing how to expand existing treatments into new populations.
Results from the EXPEDITION 8 study (abstract LB-7) showed efficacy of an 8-week regimen of the glecaprevir/pibrentasvir combination in patients with compensated cirrhosis. It looked at genotypes 1, 2, and 4-6. In an intention-to-treat analysis, 98% attained SVR and there were no viral failures or safety concerns. A follow-up trial is ongoing that includes patients with genotype 3. “This is exciting to be able to shorten therapy in patients with cirrhosis,” said Dr. Feld.
Although first-line DAAs are extremely effective, there are a few patients who do not achieve a cure. One study (abstract 0227) examined the combination of sofosbuvir, velpatasvir, and voxilaprevir in retreatment of these patients. The drugs resulted in SVR rates similar to those in registration trials, but the regimen was somewhat less effective in patients previously treated with sofosbuvir and velpatasvir. “I think we need to investigate that further,” said Dr. Feld.
The combination of glecaprevir and pibrentasvir also proved effective for retreatment in patients with genotype 1/1A who had failed treatment with an NS5A inhibitor plus sofosbuvir with or without ribavirin (abstract 226). SVR rates at 16 weeks were quite good, but lower in genotype 1a patients at 12 weeks (87% week 12 versus 94% week 16).”I think this is a really good regimen for genotype 1b. For 1a, serum definitely needs 16 weeks [to clear],” said Dr. Feld.
Other abstracts presented at the meeting detailed some of the benefits of SVR, not all of which are broadly appreciated. An analysis of the Hepatitis Testers Cohort in British Columbia (abstract 145), which includes over 7,000 patients who were followed for a median of 2 years (DAA) or 9.5 years (interferon-based), showed survival advantages to SVR in both cirrhotic (adjusted hazard ratio, 0.14) and noncirrhotic patients (aHR, 0.13). Other benefits include lower risk of diabetes (aHR, 0.53), chronic kidney disease/endstage renal disease (aHR, 0.48), stroke (aHR, 0.67), and mood and anxiety disorders (aHR, 0.53) (abstract 148).
As is generally accepted, SVR reduces the risk of hepatocellular cancer (HCC), according to analyses of VA and Gilead data (abstract 635), with a benefit in both cirrhotic and noncirrhotic patients. The risk almost disappears in patients without cirrhosis (incidence rate 0.07 per 100 person-years and is curbed in cirrhotic patients (incidence rate 1.30 in compensated, 4.05 in decompensated cirrhosis).
“There is really very significantly high incidence in cancer in decompensated cirrhosis, which just highlights that these patients continue to need ongoing surveillance. Although there have been efforts at developing strategies to risk stratify patients with cirrhosis, at least for now we’re stuck with surveillance, but I think for patients without cirrhosis there are now enough data showing a low enough incidence of primary HCC that we can probably avoid surveillance in that group,” said Dr. Feld.
Injectable drug users represent a special challenge in hepatitis C treatment, but new studies show cause for optimism in this population. These patients are harder to reach, and they may be less medication compliant, but one study (abstract 1632) found that imperfect adherence doesn’t necessarily undermine results – in a 12-week regimen, patients who didn’t finish until 14 weeks had no significant difference in SVR rates.
“So these therapies have a bit of forgiveness. We probably shouldn’t tell that to the patients, but it’s reassuring that we can use these therapies even in tough-to-reach populations,” said Dr. Feld.
In the GI Patient Center, AGA offers resources to help ensure patients are receiving the best possible care and living their best life.
SAN FRANCISCO – Hepatitis C therapy has matured and now offers excellent sustained viral response (SVR) in the vast majority of cases, but key challenges remain in getting the therapy to those who need it.
“Unfortunately, we’re not making some of the progress we might have hoped to see, particularly in North America,” said Jordan Feld, MD, MPH, who gave a debrief of hepatitis C abstracts during a wrap-up session at the annual meeting of the American Association for the Study of Liver Diseases.
The problem is particularly acute in young adults aged 18-39 years – only about 9% of those who tested positive for HCV RNA saw a specialist, and about 23% of those who saw a specialist went on to receive treatment, according to an analysis of over 17 million patients in the United States (abstract 1567). The numbers were better for older adults but still far from optimal, with 23% who tested positive seeing a specialist, and just 32% of those patients getting treatment.
Another study (abstract 0147) looked state by state at the percentage of Medicaid patients who received a prescription for direct-acting antiviral (DAA) medication and then went on to fill the prescription. The rates ranged from 0% in Alaska to 96% in Connecticut. Eight states were higher than 70%, six were between 50% and 70%, and 15 states were below 50%.
“Despite our efforts, there continue to be major access barriers across the U.S., particularly for Medicaid individuals,” said Dr. Feld, who is a clinician-scientist at the Toronto Western Hospital Liver Clinic and the McLaughlin-Rotman Centre for Global Health.
A study examining the Chronic Hepatitis (CHeCS) cohort (abstract 0585) described a big spike in treatment uptake shortly after approvals of the new HCV regimens, but by 2016, only about one-third of individuals who required treatment actually began treatment. Factors associated with nontreatment largely reflected marginalization, including low income, being on Medicaid, and lack of long-term follow-up.
Even as health systems struggle to get treatment to those who need it, new studies are showing how to expand existing treatments into new populations.
Results from the EXPEDITION 8 study (abstract LB-7) showed efficacy of an 8-week regimen of the glecaprevir/pibrentasvir combination in patients with compensated cirrhosis. It looked at genotypes 1, 2, and 4-6. In an intention-to-treat analysis, 98% attained SVR and there were no viral failures or safety concerns. A follow-up trial is ongoing that includes patients with genotype 3. “This is exciting to be able to shorten therapy in patients with cirrhosis,” said Dr. Feld.
Although first-line DAAs are extremely effective, there are a few patients who do not achieve a cure. One study (abstract 0227) examined the combination of sofosbuvir, velpatasvir, and voxilaprevir in retreatment of these patients. The drugs resulted in SVR rates similar to those in registration trials, but the regimen was somewhat less effective in patients previously treated with sofosbuvir and velpatasvir. “I think we need to investigate that further,” said Dr. Feld.
The combination of glecaprevir and pibrentasvir also proved effective for retreatment in patients with genotype 1/1A who had failed treatment with an NS5A inhibitor plus sofosbuvir with or without ribavirin (abstract 226). SVR rates at 16 weeks were quite good, but lower in genotype 1a patients at 12 weeks (87% week 12 versus 94% week 16).”I think this is a really good regimen for genotype 1b. For 1a, serum definitely needs 16 weeks [to clear],” said Dr. Feld.
Other abstracts presented at the meeting detailed some of the benefits of SVR, not all of which are broadly appreciated. An analysis of the Hepatitis Testers Cohort in British Columbia (abstract 145), which includes over 7,000 patients who were followed for a median of 2 years (DAA) or 9.5 years (interferon-based), showed survival advantages to SVR in both cirrhotic (adjusted hazard ratio, 0.14) and noncirrhotic patients (aHR, 0.13). Other benefits include lower risk of diabetes (aHR, 0.53), chronic kidney disease/endstage renal disease (aHR, 0.48), stroke (aHR, 0.67), and mood and anxiety disorders (aHR, 0.53) (abstract 148).
As is generally accepted, SVR reduces the risk of hepatocellular cancer (HCC), according to analyses of VA and Gilead data (abstract 635), with a benefit in both cirrhotic and noncirrhotic patients. The risk almost disappears in patients without cirrhosis (incidence rate 0.07 per 100 person-years and is curbed in cirrhotic patients (incidence rate 1.30 in compensated, 4.05 in decompensated cirrhosis).
“There is really very significantly high incidence in cancer in decompensated cirrhosis, which just highlights that these patients continue to need ongoing surveillance. Although there have been efforts at developing strategies to risk stratify patients with cirrhosis, at least for now we’re stuck with surveillance, but I think for patients without cirrhosis there are now enough data showing a low enough incidence of primary HCC that we can probably avoid surveillance in that group,” said Dr. Feld.
Injectable drug users represent a special challenge in hepatitis C treatment, but new studies show cause for optimism in this population. These patients are harder to reach, and they may be less medication compliant, but one study (abstract 1632) found that imperfect adherence doesn’t necessarily undermine results – in a 12-week regimen, patients who didn’t finish until 14 weeks had no significant difference in SVR rates.
“So these therapies have a bit of forgiveness. We probably shouldn’t tell that to the patients, but it’s reassuring that we can use these therapies even in tough-to-reach populations,” said Dr. Feld.
In the GI Patient Center, AGA offers resources to help ensure patients are receiving the best possible care and living their best life.
REPORTING FROM THE LIVER MEETING 2018
Hepatitis C debrief: Therapy has matured, access issues remain
SAN FRANCISCO – Hepatitis C therapy has matured and now offers excellent sustained viral response (SVR) in the vast majority of cases, but key challenges remain in getting the therapy to those who need it.
“Unfortunately, we’re not making some of the progress we might have hoped to see, particularly in North America,” said Jordan Feld, MD, MPH, who gave a debrief of hepatitis C abstracts during a wrap-up session at the annual meeting of the American Association for the Study of Liver Diseases.
The problem is particularly acute in young adults aged 18-39 years – only about 9% of those who tested positive for HCV RNA saw a specialist, and about 23% of those who saw a specialist went on to receive treatment, according to an analysis of over 17 million patients in the United States (abstract 1567). The numbers were better for older adults but still far from optimal, with 23% who tested positive seeing a specialist, and just 32% of those patients getting treatment.
Another study (abstract 0147) looked state by state at the percentage of Medicaid patients who received a prescription for direct-acting antiviral (DAA) medication and then went on to fill the prescription. The rates ranged from 0% in Alaska to 96% in Connecticut. Eight states were higher than 70%, six were between 50% and 70%, and 15 states were below 50%.
“Despite our efforts, there continue to be major access barriers across the U.S., particularly for Medicaid individuals,” said Dr. Feld, who is a clinician-scientist at the Toronto Western Hospital Liver Clinic and the McLaughlin-Rotman Centre for Global Health.
A study examining the Chronic Hepatitis (CHeCS) cohort (abstract 0585) described a big spike in treatment uptake shortly after approvals of the new HCV regimens, but by 2016, only about one-third of individuals who required treatment actually began treatment. Factors associated with nontreatment largely reflected marginalization, including low income, being on Medicaid, and lack of long-term follow-up.
Even as health systems struggle to get treatment to those who need it, new studies are showing how to expand existing treatments into new populations.
Results from the EXPEDITION 8 study (abstract LB-7) showed efficacy of an 8-week regimen of the glecaprevir/pibrentasvir combination in patients with compensated cirrhosis. It looked at genotypes 1, 2, and 4-6. In an intention-to-treat analysis, 98% attained SVR and there were no viral failures or safety concerns. A follow-up trial is ongoing that includes patients with genotype 3. “This is exciting to be able to shorten therapy in patients with cirrhosis,” said Dr. Feld.
Although first-line DAAs are extremely effective, there are a few patients who do not achieve a cure. One study (abstract 0227) examined the combination of sofosbuvir, velpatasvir, and voxilaprevir in retreatment of these patients. The drugs resulted in SVR rates similar to those in registration trials, but the regimen was somewhat less effective in patients previously treated with sofosbuvir and velpatasvir. “I think we need to investigate that further,” said Dr. Feld.
The combination of glecaprevir and pibrentasvir also proved effective for retreatment in patients with genotype 1/1A who had failed treatment with an NS5A inhibitor plus sofosbuvir with or without ribavirin (abstract 226). SVR rates at 16 weeks were quite good, but lower in genotype 1a patients at 12 weeks (87% week 12 versus 94% week 16).”I think this is a really good regimen for genotype 1b. For 1a, serum definitely needs 16 weeks [to clear],” said Dr. Feld.
Other abstracts presented at the meeting detailed some of the benefits of SVR, not all of which are broadly appreciated. An analysis of the Hepatitis Testers Cohort in British Columbia (abstract 145), which includes over 7,000 patients who were followed for a median of 2 years (DAA) or 9.5 years (interferon-based), showed survival advantages to SVR in both cirrhotic (adjusted hazard ratio, 0.14) and noncirrhotic patients (aHR, 0.13). Other benefits include lower risk of diabetes (aHR, 0.53), chronic kidney disease/endstage renal disease (aHR, 0.48), stroke (aHR, 0.67), and mood and anxiety disorders (aHR, 0.53) (abstract 148).
As is generally accepted, SVR reduces the risk of hepatocellular cancer (HCC), according to analyses of VA and Gilead data (abstract 635), with a benefit in both cirrhotic and noncirrhotic patients. The risk almost disappears in patients without cirrhosis (incidence rate 0.07 per 100 person-years and is curbed in cirrhotic patients (incidence rate 1.30 in compensated, 4.05 in decompensated cirrhosis).
“There is really very significantly high incidence in cancer in decompensated cirrhosis, which just highlights that these patients continue to need ongoing surveillance. Although there have been efforts at developing strategies to risk stratify patients with cirrhosis, at least for now we’re stuck with surveillance, but I think for patients without cirrhosis there are now enough data showing a low enough incidence of primary HCC that we can probably avoid surveillance in that group,” said Dr. Feld.
Injectable drug users represent a special challenge in hepatitis C treatment, but new studies show cause for optimism in this population. These patients are harder to reach, and they may be less medication compliant, but one study (abstract 1632) found that imperfect adherence doesn’t necessarily undermine results – in a 12-week regimen, patients who didn’t finish until 14 weeks had no significant difference in SVR rates.
“So these therapies have a bit of forgiveness. We probably shouldn’t tell that to the patients, but it’s reassuring that we can use these therapies even in tough-to-reach populations,” said Dr. Feld.
SAN FRANCISCO – Hepatitis C therapy has matured and now offers excellent sustained viral response (SVR) in the vast majority of cases, but key challenges remain in getting the therapy to those who need it.
“Unfortunately, we’re not making some of the progress we might have hoped to see, particularly in North America,” said Jordan Feld, MD, MPH, who gave a debrief of hepatitis C abstracts during a wrap-up session at the annual meeting of the American Association for the Study of Liver Diseases.
The problem is particularly acute in young adults aged 18-39 years – only about 9% of those who tested positive for HCV RNA saw a specialist, and about 23% of those who saw a specialist went on to receive treatment, according to an analysis of over 17 million patients in the United States (abstract 1567). The numbers were better for older adults but still far from optimal, with 23% who tested positive seeing a specialist, and just 32% of those patients getting treatment.
Another study (abstract 0147) looked state by state at the percentage of Medicaid patients who received a prescription for direct-acting antiviral (DAA) medication and then went on to fill the prescription. The rates ranged from 0% in Alaska to 96% in Connecticut. Eight states were higher than 70%, six were between 50% and 70%, and 15 states were below 50%.
“Despite our efforts, there continue to be major access barriers across the U.S., particularly for Medicaid individuals,” said Dr. Feld, who is a clinician-scientist at the Toronto Western Hospital Liver Clinic and the McLaughlin-Rotman Centre for Global Health.
A study examining the Chronic Hepatitis (CHeCS) cohort (abstract 0585) described a big spike in treatment uptake shortly after approvals of the new HCV regimens, but by 2016, only about one-third of individuals who required treatment actually began treatment. Factors associated with nontreatment largely reflected marginalization, including low income, being on Medicaid, and lack of long-term follow-up.
Even as health systems struggle to get treatment to those who need it, new studies are showing how to expand existing treatments into new populations.
Results from the EXPEDITION 8 study (abstract LB-7) showed efficacy of an 8-week regimen of the glecaprevir/pibrentasvir combination in patients with compensated cirrhosis. It looked at genotypes 1, 2, and 4-6. In an intention-to-treat analysis, 98% attained SVR and there were no viral failures or safety concerns. A follow-up trial is ongoing that includes patients with genotype 3. “This is exciting to be able to shorten therapy in patients with cirrhosis,” said Dr. Feld.
Although first-line DAAs are extremely effective, there are a few patients who do not achieve a cure. One study (abstract 0227) examined the combination of sofosbuvir, velpatasvir, and voxilaprevir in retreatment of these patients. The drugs resulted in SVR rates similar to those in registration trials, but the regimen was somewhat less effective in patients previously treated with sofosbuvir and velpatasvir. “I think we need to investigate that further,” said Dr. Feld.
The combination of glecaprevir and pibrentasvir also proved effective for retreatment in patients with genotype 1/1A who had failed treatment with an NS5A inhibitor plus sofosbuvir with or without ribavirin (abstract 226). SVR rates at 16 weeks were quite good, but lower in genotype 1a patients at 12 weeks (87% week 12 versus 94% week 16).”I think this is a really good regimen for genotype 1b. For 1a, serum definitely needs 16 weeks [to clear],” said Dr. Feld.
Other abstracts presented at the meeting detailed some of the benefits of SVR, not all of which are broadly appreciated. An analysis of the Hepatitis Testers Cohort in British Columbia (abstract 145), which includes over 7,000 patients who were followed for a median of 2 years (DAA) or 9.5 years (interferon-based), showed survival advantages to SVR in both cirrhotic (adjusted hazard ratio, 0.14) and noncirrhotic patients (aHR, 0.13). Other benefits include lower risk of diabetes (aHR, 0.53), chronic kidney disease/endstage renal disease (aHR, 0.48), stroke (aHR, 0.67), and mood and anxiety disorders (aHR, 0.53) (abstract 148).
As is generally accepted, SVR reduces the risk of hepatocellular cancer (HCC), according to analyses of VA and Gilead data (abstract 635), with a benefit in both cirrhotic and noncirrhotic patients. The risk almost disappears in patients without cirrhosis (incidence rate 0.07 per 100 person-years and is curbed in cirrhotic patients (incidence rate 1.30 in compensated, 4.05 in decompensated cirrhosis).
“There is really very significantly high incidence in cancer in decompensated cirrhosis, which just highlights that these patients continue to need ongoing surveillance. Although there have been efforts at developing strategies to risk stratify patients with cirrhosis, at least for now we’re stuck with surveillance, but I think for patients without cirrhosis there are now enough data showing a low enough incidence of primary HCC that we can probably avoid surveillance in that group,” said Dr. Feld.
Injectable drug users represent a special challenge in hepatitis C treatment, but new studies show cause for optimism in this population. These patients are harder to reach, and they may be less medication compliant, but one study (abstract 1632) found that imperfect adherence doesn’t necessarily undermine results – in a 12-week regimen, patients who didn’t finish until 14 weeks had no significant difference in SVR rates.
“So these therapies have a bit of forgiveness. We probably shouldn’t tell that to the patients, but it’s reassuring that we can use these therapies even in tough-to-reach populations,” said Dr. Feld.
SAN FRANCISCO – Hepatitis C therapy has matured and now offers excellent sustained viral response (SVR) in the vast majority of cases, but key challenges remain in getting the therapy to those who need it.
“Unfortunately, we’re not making some of the progress we might have hoped to see, particularly in North America,” said Jordan Feld, MD, MPH, who gave a debrief of hepatitis C abstracts during a wrap-up session at the annual meeting of the American Association for the Study of Liver Diseases.
The problem is particularly acute in young adults aged 18-39 years – only about 9% of those who tested positive for HCV RNA saw a specialist, and about 23% of those who saw a specialist went on to receive treatment, according to an analysis of over 17 million patients in the United States (abstract 1567). The numbers were better for older adults but still far from optimal, with 23% who tested positive seeing a specialist, and just 32% of those patients getting treatment.
Another study (abstract 0147) looked state by state at the percentage of Medicaid patients who received a prescription for direct-acting antiviral (DAA) medication and then went on to fill the prescription. The rates ranged from 0% in Alaska to 96% in Connecticut. Eight states were higher than 70%, six were between 50% and 70%, and 15 states were below 50%.
“Despite our efforts, there continue to be major access barriers across the U.S., particularly for Medicaid individuals,” said Dr. Feld, who is a clinician-scientist at the Toronto Western Hospital Liver Clinic and the McLaughlin-Rotman Centre for Global Health.
A study examining the Chronic Hepatitis (CHeCS) cohort (abstract 0585) described a big spike in treatment uptake shortly after approvals of the new HCV regimens, but by 2016, only about one-third of individuals who required treatment actually began treatment. Factors associated with nontreatment largely reflected marginalization, including low income, being on Medicaid, and lack of long-term follow-up.
Even as health systems struggle to get treatment to those who need it, new studies are showing how to expand existing treatments into new populations.
Results from the EXPEDITION 8 study (abstract LB-7) showed efficacy of an 8-week regimen of the glecaprevir/pibrentasvir combination in patients with compensated cirrhosis. It looked at genotypes 1, 2, and 4-6. In an intention-to-treat analysis, 98% attained SVR and there were no viral failures or safety concerns. A follow-up trial is ongoing that includes patients with genotype 3. “This is exciting to be able to shorten therapy in patients with cirrhosis,” said Dr. Feld.
Although first-line DAAs are extremely effective, there are a few patients who do not achieve a cure. One study (abstract 0227) examined the combination of sofosbuvir, velpatasvir, and voxilaprevir in retreatment of these patients. The drugs resulted in SVR rates similar to those in registration trials, but the regimen was somewhat less effective in patients previously treated with sofosbuvir and velpatasvir. “I think we need to investigate that further,” said Dr. Feld.
The combination of glecaprevir and pibrentasvir also proved effective for retreatment in patients with genotype 1/1A who had failed treatment with an NS5A inhibitor plus sofosbuvir with or without ribavirin (abstract 226). SVR rates at 16 weeks were quite good, but lower in genotype 1a patients at 12 weeks (87% week 12 versus 94% week 16).”I think this is a really good regimen for genotype 1b. For 1a, serum definitely needs 16 weeks [to clear],” said Dr. Feld.
Other abstracts presented at the meeting detailed some of the benefits of SVR, not all of which are broadly appreciated. An analysis of the Hepatitis Testers Cohort in British Columbia (abstract 145), which includes over 7,000 patients who were followed for a median of 2 years (DAA) or 9.5 years (interferon-based), showed survival advantages to SVR in both cirrhotic (adjusted hazard ratio, 0.14) and noncirrhotic patients (aHR, 0.13). Other benefits include lower risk of diabetes (aHR, 0.53), chronic kidney disease/endstage renal disease (aHR, 0.48), stroke (aHR, 0.67), and mood and anxiety disorders (aHR, 0.53) (abstract 148).
As is generally accepted, SVR reduces the risk of hepatocellular cancer (HCC), according to analyses of VA and Gilead data (abstract 635), with a benefit in both cirrhotic and noncirrhotic patients. The risk almost disappears in patients without cirrhosis (incidence rate 0.07 per 100 person-years and is curbed in cirrhotic patients (incidence rate 1.30 in compensated, 4.05 in decompensated cirrhosis).
“There is really very significantly high incidence in cancer in decompensated cirrhosis, which just highlights that these patients continue to need ongoing surveillance. Although there have been efforts at developing strategies to risk stratify patients with cirrhosis, at least for now we’re stuck with surveillance, but I think for patients without cirrhosis there are now enough data showing a low enough incidence of primary HCC that we can probably avoid surveillance in that group,” said Dr. Feld.
Injectable drug users represent a special challenge in hepatitis C treatment, but new studies show cause for optimism in this population. These patients are harder to reach, and they may be less medication compliant, but one study (abstract 1632) found that imperfect adherence doesn’t necessarily undermine results – in a 12-week regimen, patients who didn’t finish until 14 weeks had no significant difference in SVR rates.
“So these therapies have a bit of forgiveness. We probably shouldn’t tell that to the patients, but it’s reassuring that we can use these therapies even in tough-to-reach populations,” said Dr. Feld.
REPORTING FROM THE LIVER MEETING 2018
Early treatment with direct-acting antivirals linked to reduced medical costs in noncirrhotic HCV
SAN FRANCISCO – Patients with noncirrhotic chronic hepatitis C virus (HCV) infection incur high medical costs in the three years following their diagnosis. However, early initiation of oral direct-acting therapies is associated with significant medical cost savings, largely driven by reduced extrahepatic manifestations.
Those are key findings from an analysis of “real-world” claims data that Carol Bao, PhD, presented on behalf of senior author Patrice Cacoub, MD, during a poster session at the annual meeting of the American Association for the Study of Liver Diseases.
“This [study] highlights the importance of treating HCV patients early, especially with active therapies, because that will benefit not only their liver disease but, from a population health perspective, you are lifting the entire health of those patients as well,” Dr. Bao, senior director of health economics and outcomes research at AbbVie, North Chicago, said in an interview.
In an effort to quantify the health care cost savings associated with initiation of direct-acting antiviral (DAA) therapies within 2 years of the first chronic HCV (CHC) diagnosis among noncirrhotic patients in the United States, the researchers drew from Clinformatics Data Mart, a diverse health care database with longitudinal data for more than 15 million lives each year. They collected data between Jan. 1, 2009, and Jan. 31, 2016, and excluded patients followed for less than 6 months before the CHC diagnosis or less than 1 year after the CHC diagnosis, as well as those who received interferon/ribavirin therapy before their first DAA. This yielded a sample of 3,069 adults first diagnosed with CHC on or after 2013.
The index date was defined as the data of the first CHC diagnosis and researchers established two cohorts: 852 patients who initiated DAAs in the 3 years postindex date and 2,217 who did not receive any CHC treatment in the 3 years postindex date.
Outcomes of interest included all-cause and disease-specific medical costs measured yearly up to 3 years post index. These included costs related to CHC management or hepatic complications as well as those related to extrahepatic manifestations (EHMs) such as fatigue, type 2 diabetes, and cardiovascular disease.
Patients in the DAA-treated group were slightly older than those in the untreated group (a median age of 52.6 vs. 50.9 years, respectively; P less than .001) and had a higher proportion of men (65.1% vs. 60.7%; P = .07). They were also diagnosed more recently and had more advanced fibrosis at baseline. In the first 3 years post index, the researchers found that the average medical costs incurred in the DAA-treated and untreated groups were $28,392 and $42,914, respectively. On multivariate regression analyses, total all-cause medical costs were statistically lower across DAA-treated years than across the untreated years: $6,379 per year on average, because of savings related to health care for EHMs ($3,158 per year on average) and diagnoses other than CHC, hepatitis, or EHMs considered in this study ($4,638 per year on average).
When Dr. Bao and her colleagues conducted post hoc exploratory analyses of the $4,638 per year cost differences for diagnoses other than CHC, hepatic, or the EMHs considered, they determined that they appear to be driven by diagnoses related to the circulatory system (especially essential hypertension), respiratory system, blood/immune/endocrine systems, and claims with diagnoses that were not disease specific.
Dr. Bao acknowledged certain limitations of the study, including the potential for errors and omissions associated with claims data and that costs were recorded as charged amounts, which may be different from the amount actually paid. In addition, the fibrosis level could not be inferred for all patients.
AbbVie provided funding for the study, which received a “poster of distinction” award at the meeting. The company employs Dr. Bao and two of the study coauthors.
SAN FRANCISCO – Patients with noncirrhotic chronic hepatitis C virus (HCV) infection incur high medical costs in the three years following their diagnosis. However, early initiation of oral direct-acting therapies is associated with significant medical cost savings, largely driven by reduced extrahepatic manifestations.
Those are key findings from an analysis of “real-world” claims data that Carol Bao, PhD, presented on behalf of senior author Patrice Cacoub, MD, during a poster session at the annual meeting of the American Association for the Study of Liver Diseases.
“This [study] highlights the importance of treating HCV patients early, especially with active therapies, because that will benefit not only their liver disease but, from a population health perspective, you are lifting the entire health of those patients as well,” Dr. Bao, senior director of health economics and outcomes research at AbbVie, North Chicago, said in an interview.
In an effort to quantify the health care cost savings associated with initiation of direct-acting antiviral (DAA) therapies within 2 years of the first chronic HCV (CHC) diagnosis among noncirrhotic patients in the United States, the researchers drew from Clinformatics Data Mart, a diverse health care database with longitudinal data for more than 15 million lives each year. They collected data between Jan. 1, 2009, and Jan. 31, 2016, and excluded patients followed for less than 6 months before the CHC diagnosis or less than 1 year after the CHC diagnosis, as well as those who received interferon/ribavirin therapy before their first DAA. This yielded a sample of 3,069 adults first diagnosed with CHC on or after 2013.
The index date was defined as the data of the first CHC diagnosis and researchers established two cohorts: 852 patients who initiated DAAs in the 3 years postindex date and 2,217 who did not receive any CHC treatment in the 3 years postindex date.
Outcomes of interest included all-cause and disease-specific medical costs measured yearly up to 3 years post index. These included costs related to CHC management or hepatic complications as well as those related to extrahepatic manifestations (EHMs) such as fatigue, type 2 diabetes, and cardiovascular disease.
Patients in the DAA-treated group were slightly older than those in the untreated group (a median age of 52.6 vs. 50.9 years, respectively; P less than .001) and had a higher proportion of men (65.1% vs. 60.7%; P = .07). They were also diagnosed more recently and had more advanced fibrosis at baseline. In the first 3 years post index, the researchers found that the average medical costs incurred in the DAA-treated and untreated groups were $28,392 and $42,914, respectively. On multivariate regression analyses, total all-cause medical costs were statistically lower across DAA-treated years than across the untreated years: $6,379 per year on average, because of savings related to health care for EHMs ($3,158 per year on average) and diagnoses other than CHC, hepatitis, or EHMs considered in this study ($4,638 per year on average).
When Dr. Bao and her colleagues conducted post hoc exploratory analyses of the $4,638 per year cost differences for diagnoses other than CHC, hepatic, or the EMHs considered, they determined that they appear to be driven by diagnoses related to the circulatory system (especially essential hypertension), respiratory system, blood/immune/endocrine systems, and claims with diagnoses that were not disease specific.
Dr. Bao acknowledged certain limitations of the study, including the potential for errors and omissions associated with claims data and that costs were recorded as charged amounts, which may be different from the amount actually paid. In addition, the fibrosis level could not be inferred for all patients.
AbbVie provided funding for the study, which received a “poster of distinction” award at the meeting. The company employs Dr. Bao and two of the study coauthors.
SAN FRANCISCO – Patients with noncirrhotic chronic hepatitis C virus (HCV) infection incur high medical costs in the three years following their diagnosis. However, early initiation of oral direct-acting therapies is associated with significant medical cost savings, largely driven by reduced extrahepatic manifestations.
Those are key findings from an analysis of “real-world” claims data that Carol Bao, PhD, presented on behalf of senior author Patrice Cacoub, MD, during a poster session at the annual meeting of the American Association for the Study of Liver Diseases.
“This [study] highlights the importance of treating HCV patients early, especially with active therapies, because that will benefit not only their liver disease but, from a population health perspective, you are lifting the entire health of those patients as well,” Dr. Bao, senior director of health economics and outcomes research at AbbVie, North Chicago, said in an interview.
In an effort to quantify the health care cost savings associated with initiation of direct-acting antiviral (DAA) therapies within 2 years of the first chronic HCV (CHC) diagnosis among noncirrhotic patients in the United States, the researchers drew from Clinformatics Data Mart, a diverse health care database with longitudinal data for more than 15 million lives each year. They collected data between Jan. 1, 2009, and Jan. 31, 2016, and excluded patients followed for less than 6 months before the CHC diagnosis or less than 1 year after the CHC diagnosis, as well as those who received interferon/ribavirin therapy before their first DAA. This yielded a sample of 3,069 adults first diagnosed with CHC on or after 2013.
The index date was defined as the data of the first CHC diagnosis and researchers established two cohorts: 852 patients who initiated DAAs in the 3 years postindex date and 2,217 who did not receive any CHC treatment in the 3 years postindex date.
Outcomes of interest included all-cause and disease-specific medical costs measured yearly up to 3 years post index. These included costs related to CHC management or hepatic complications as well as those related to extrahepatic manifestations (EHMs) such as fatigue, type 2 diabetes, and cardiovascular disease.
Patients in the DAA-treated group were slightly older than those in the untreated group (a median age of 52.6 vs. 50.9 years, respectively; P less than .001) and had a higher proportion of men (65.1% vs. 60.7%; P = .07). They were also diagnosed more recently and had more advanced fibrosis at baseline. In the first 3 years post index, the researchers found that the average medical costs incurred in the DAA-treated and untreated groups were $28,392 and $42,914, respectively. On multivariate regression analyses, total all-cause medical costs were statistically lower across DAA-treated years than across the untreated years: $6,379 per year on average, because of savings related to health care for EHMs ($3,158 per year on average) and diagnoses other than CHC, hepatitis, or EHMs considered in this study ($4,638 per year on average).
When Dr. Bao and her colleagues conducted post hoc exploratory analyses of the $4,638 per year cost differences for diagnoses other than CHC, hepatic, or the EMHs considered, they determined that they appear to be driven by diagnoses related to the circulatory system (especially essential hypertension), respiratory system, blood/immune/endocrine systems, and claims with diagnoses that were not disease specific.
Dr. Bao acknowledged certain limitations of the study, including the potential for errors and omissions associated with claims data and that costs were recorded as charged amounts, which may be different from the amount actually paid. In addition, the fibrosis level could not be inferred for all patients.
AbbVie provided funding for the study, which received a “poster of distinction” award at the meeting. The company employs Dr. Bao and two of the study coauthors.
REPORTING FROM THE LIVER MEETING 2018
Key clinical point: Noncirrhotic chronic hepatitis C patients incur high medical costs after their first diagnosis if left untreated.
Major finding: In the first 3 years post index, the average medical costs incurred in the direct-acting antiviral–treated and untreated groups were $28,392 and $42,914, respectively.
Study details: A database sample of 3,069 adults first diagnosed with chronic hepatitis C in or after 2013.
Disclosures: AbbVie provided funding for the study. The company employs Dr. Bao and two of the study coauthors.
Normothermic machine perfusion found to salvage fatty livers for transplantation
SAN FRANCISCO – results from a small trial showed.
“This is important in the context of liver transplantation, because fatty livers do very badly when their time is blunted,” study coauthor Carlo Ceresa, MBChB, MRCS, said during a press briefing at the annual meeting of the American Association for the Study of Liver Diseases. “They’re susceptible to ischemia reperfusion injury, and as a result, a large number are discarded. In the U.S., it’s estimated that around 6,000 steatotic livers are discarded each year. In the U.K., the picture is very similar. Because up to 20% of patients die on the waiting list for liver transplant, we need to try to identify methods to use more marginal organs. Unfortunately, with the obesity epidemic and obesity being a risk factor for NAFLD [nonalcoholic fatty liver disease], we find more fatty livers in the donor pool, and we aren’t able to use them. Identifying methods to salvage these livers for transplantation [is] of great importance.”
NMP maintains the liver in a fully functioning state ex situ and provides oxygen and nutrition at 37° C, said Dr. Ceresa, who is a clinical research fellow with the Medical Research Council and a PhD candidate at the University of Oxford, England. In an effort to evaluate the impact of NMP and defatting adjuncts on human steatotic livers, he and his colleagues perfused 18 discarded human steatotic livers on a normothermic, blood-based circuit for 48 hours. Of these, six were perfused by normothermic machine perfusion alone (group 1), while six were perfused by NMP plus apheresis filtration, which removes lipoproteins (group 2). “The hypothesis here was that we could mechanically remove the fat that the liver releases,” he said. The remaining six livers were perfused with NMP, lipid apheresis filtration, and defatting agents including
The livers in group 1 “did pretty badly,” Dr. Ceresa said. “Their function wasn’t great and within 48 hours deteriorated, and there was a slight increase in liver fat. That’s probably attributable to de novo lipogenesis.” However, the livers in groups 2 and 3 demonstrated a significant reduction in circulating triglycerides and in perfusate total cholesterol by 48 hours, compared with those in group 1. The researchers also observed an increase in median fatty acid oxidation as measured by 3-hydroxybutyrate among the livers in group 3, compared with those in groups 1 and 2. In addition, the livers in group 3 were the only ones to show a mean reduction in tissue triglyceride level.
Dr. Ceresa described the findings as “exciting, because we have a captive organ we can manipulate, which could then result in a successful transplantation,” he said. “You also get to test drive and get an objective assessment of the organ’s function before you transplant it, so the result may be more predictable. It gives us a very useful model to study NAFLD.”
The next step, he said, is to plan a clinical trial to determine if clinical outcomes can be improved through these ex situ interventions on steatotic livers.
Dr. Ceresa reported having no financial disclosures.
Source: Hepatology 2018;68[S1], Abstract 3.
SAN FRANCISCO – results from a small trial showed.
“This is important in the context of liver transplantation, because fatty livers do very badly when their time is blunted,” study coauthor Carlo Ceresa, MBChB, MRCS, said during a press briefing at the annual meeting of the American Association for the Study of Liver Diseases. “They’re susceptible to ischemia reperfusion injury, and as a result, a large number are discarded. In the U.S., it’s estimated that around 6,000 steatotic livers are discarded each year. In the U.K., the picture is very similar. Because up to 20% of patients die on the waiting list for liver transplant, we need to try to identify methods to use more marginal organs. Unfortunately, with the obesity epidemic and obesity being a risk factor for NAFLD [nonalcoholic fatty liver disease], we find more fatty livers in the donor pool, and we aren’t able to use them. Identifying methods to salvage these livers for transplantation [is] of great importance.”
NMP maintains the liver in a fully functioning state ex situ and provides oxygen and nutrition at 37° C, said Dr. Ceresa, who is a clinical research fellow with the Medical Research Council and a PhD candidate at the University of Oxford, England. In an effort to evaluate the impact of NMP and defatting adjuncts on human steatotic livers, he and his colleagues perfused 18 discarded human steatotic livers on a normothermic, blood-based circuit for 48 hours. Of these, six were perfused by normothermic machine perfusion alone (group 1), while six were perfused by NMP plus apheresis filtration, which removes lipoproteins (group 2). “The hypothesis here was that we could mechanically remove the fat that the liver releases,” he said. The remaining six livers were perfused with NMP, lipid apheresis filtration, and defatting agents including
The livers in group 1 “did pretty badly,” Dr. Ceresa said. “Their function wasn’t great and within 48 hours deteriorated, and there was a slight increase in liver fat. That’s probably attributable to de novo lipogenesis.” However, the livers in groups 2 and 3 demonstrated a significant reduction in circulating triglycerides and in perfusate total cholesterol by 48 hours, compared with those in group 1. The researchers also observed an increase in median fatty acid oxidation as measured by 3-hydroxybutyrate among the livers in group 3, compared with those in groups 1 and 2. In addition, the livers in group 3 were the only ones to show a mean reduction in tissue triglyceride level.
Dr. Ceresa described the findings as “exciting, because we have a captive organ we can manipulate, which could then result in a successful transplantation,” he said. “You also get to test drive and get an objective assessment of the organ’s function before you transplant it, so the result may be more predictable. It gives us a very useful model to study NAFLD.”
The next step, he said, is to plan a clinical trial to determine if clinical outcomes can be improved through these ex situ interventions on steatotic livers.
Dr. Ceresa reported having no financial disclosures.
Source: Hepatology 2018;68[S1], Abstract 3.
SAN FRANCISCO – results from a small trial showed.
“This is important in the context of liver transplantation, because fatty livers do very badly when their time is blunted,” study coauthor Carlo Ceresa, MBChB, MRCS, said during a press briefing at the annual meeting of the American Association for the Study of Liver Diseases. “They’re susceptible to ischemia reperfusion injury, and as a result, a large number are discarded. In the U.S., it’s estimated that around 6,000 steatotic livers are discarded each year. In the U.K., the picture is very similar. Because up to 20% of patients die on the waiting list for liver transplant, we need to try to identify methods to use more marginal organs. Unfortunately, with the obesity epidemic and obesity being a risk factor for NAFLD [nonalcoholic fatty liver disease], we find more fatty livers in the donor pool, and we aren’t able to use them. Identifying methods to salvage these livers for transplantation [is] of great importance.”
NMP maintains the liver in a fully functioning state ex situ and provides oxygen and nutrition at 37° C, said Dr. Ceresa, who is a clinical research fellow with the Medical Research Council and a PhD candidate at the University of Oxford, England. In an effort to evaluate the impact of NMP and defatting adjuncts on human steatotic livers, he and his colleagues perfused 18 discarded human steatotic livers on a normothermic, blood-based circuit for 48 hours. Of these, six were perfused by normothermic machine perfusion alone (group 1), while six were perfused by NMP plus apheresis filtration, which removes lipoproteins (group 2). “The hypothesis here was that we could mechanically remove the fat that the liver releases,” he said. The remaining six livers were perfused with NMP, lipid apheresis filtration, and defatting agents including
The livers in group 1 “did pretty badly,” Dr. Ceresa said. “Their function wasn’t great and within 48 hours deteriorated, and there was a slight increase in liver fat. That’s probably attributable to de novo lipogenesis.” However, the livers in groups 2 and 3 demonstrated a significant reduction in circulating triglycerides and in perfusate total cholesterol by 48 hours, compared with those in group 1. The researchers also observed an increase in median fatty acid oxidation as measured by 3-hydroxybutyrate among the livers in group 3, compared with those in groups 1 and 2. In addition, the livers in group 3 were the only ones to show a mean reduction in tissue triglyceride level.
Dr. Ceresa described the findings as “exciting, because we have a captive organ we can manipulate, which could then result in a successful transplantation,” he said. “You also get to test drive and get an objective assessment of the organ’s function before you transplant it, so the result may be more predictable. It gives us a very useful model to study NAFLD.”
The next step, he said, is to plan a clinical trial to determine if clinical outcomes can be improved through these ex situ interventions on steatotic livers.
Dr. Ceresa reported having no financial disclosures.
Source: Hepatology 2018;68[S1], Abstract 3.
AT THE LIVER MEETING 2018
Key clinical point: The addition of apheresis filtration and defatting agents to normothermic machine perfusion led to significant improvements in liver function.
Major finding: Livers which received apheresis filtration and defatting agents fared better than those that did not.
Study details: An analysis of 18 discarded human steatotic livers that were perfused on a normothermic, blood-based circuit for 48 hours.
Disclosures: Dr. Ceresa reported having no financial disclosures.
Source: Hepatology 2018;68[S1], Abstract 3.
Despite interest, few liver transplant candidates discuss advance care planning with clinicians
SAN FRANCISCO – .
“Recent studies have shown that there have been low rates of these types of discussions in all areas of medicine, not just in liver transplantation per se,” Connie W. Wang, MD, said in an interview at the annual meeting of the American Association for the Study of Liver Diseases. “We were curious to see what it looked like in our practice setting.”
In an effort to evaluate current advanced care planning documentation practices in the liver transplantation setting, she and her colleagues reviewed the medical charts of 168 adults who underwent an initial liver transplant evaluation at the University of California, San Francisco, from January 2017 to June 2017. Next, to assess readiness to complete advanced care planning among liver transplant candidates, the researchers administered the Advanced Care Planning Engagement Survey to 41 adults who underwent an initial liver transplant evaluation from March 2018 to May 2018. The survey was scored on a Likert scale of 1-4, in which a score of 4 equaled “ready” or “confident,” and a score of 5 equaled “very ready” or “very confident.”
The mean age of the 168 transplant candidates was 53 years, 35% were female, and 52% were non-Hispanic white. Only 15 patients (9%) reported completing advanced care planning prior to their liver transplant evaluation and none had legal advance care planning forms scanned or end-of-life wishes documented in the medical record. Durable power of attorney for health care was discussed with 17 patients (10%). On logistic regression analysis, only white race was associated with completion of advanced care planning (OR 4.16; P = .03), but age, Child-Pugh score, and MELD-Na score were not.
The mean age of the 41 transplant candidates who completed the Advanced Care Planning Engagement Survey was 58 years, 39% were female, and 58% were non-Hispanic white. Nearly all respondents (93%) indicated that they were ready to appoint a durable power of attorney, 85% were ready to discuss end-of-life care, and 93% were ready to ask physicians questions about medical decisions. Similarly, 93% of patients felt confident to appoint a durable power of attorney, 88% felt confident to discuss end-of-life care, and 93% felt confident to ask physicians questions about medical decisions.
“It seems like from the patients’ perspective, they are very much open to having these conversations, but there hasn’t been [the right] environment or setting to have them,” said Dr. Wang, a third-year internal medicine resident at UCSF. “Or, there may be a barrier from the provider’s perspective. Clearly, there is a huge need that can be filled.” She noted that future research should focus on development of tools to facilitate discussions and documentation between transplant clinicians, patients, and their caregivers.
One of the study authors, Jennifer C. Lai, MD, reported being a consultant for Third Rock Ventures, LLC. The other researchers reported having no financial disclosures.
Source: Hepatol. 2018;68[S1]: Abstract 771.
SAN FRANCISCO – .
“Recent studies have shown that there have been low rates of these types of discussions in all areas of medicine, not just in liver transplantation per se,” Connie W. Wang, MD, said in an interview at the annual meeting of the American Association for the Study of Liver Diseases. “We were curious to see what it looked like in our practice setting.”
In an effort to evaluate current advanced care planning documentation practices in the liver transplantation setting, she and her colleagues reviewed the medical charts of 168 adults who underwent an initial liver transplant evaluation at the University of California, San Francisco, from January 2017 to June 2017. Next, to assess readiness to complete advanced care planning among liver transplant candidates, the researchers administered the Advanced Care Planning Engagement Survey to 41 adults who underwent an initial liver transplant evaluation from March 2018 to May 2018. The survey was scored on a Likert scale of 1-4, in which a score of 4 equaled “ready” or “confident,” and a score of 5 equaled “very ready” or “very confident.”
The mean age of the 168 transplant candidates was 53 years, 35% were female, and 52% were non-Hispanic white. Only 15 patients (9%) reported completing advanced care planning prior to their liver transplant evaluation and none had legal advance care planning forms scanned or end-of-life wishes documented in the medical record. Durable power of attorney for health care was discussed with 17 patients (10%). On logistic regression analysis, only white race was associated with completion of advanced care planning (OR 4.16; P = .03), but age, Child-Pugh score, and MELD-Na score were not.
The mean age of the 41 transplant candidates who completed the Advanced Care Planning Engagement Survey was 58 years, 39% were female, and 58% were non-Hispanic white. Nearly all respondents (93%) indicated that they were ready to appoint a durable power of attorney, 85% were ready to discuss end-of-life care, and 93% were ready to ask physicians questions about medical decisions. Similarly, 93% of patients felt confident to appoint a durable power of attorney, 88% felt confident to discuss end-of-life care, and 93% felt confident to ask physicians questions about medical decisions.
“It seems like from the patients’ perspective, they are very much open to having these conversations, but there hasn’t been [the right] environment or setting to have them,” said Dr. Wang, a third-year internal medicine resident at UCSF. “Or, there may be a barrier from the provider’s perspective. Clearly, there is a huge need that can be filled.” She noted that future research should focus on development of tools to facilitate discussions and documentation between transplant clinicians, patients, and their caregivers.
One of the study authors, Jennifer C. Lai, MD, reported being a consultant for Third Rock Ventures, LLC. The other researchers reported having no financial disclosures.
Source: Hepatol. 2018;68[S1]: Abstract 771.
SAN FRANCISCO – .
“Recent studies have shown that there have been low rates of these types of discussions in all areas of medicine, not just in liver transplantation per se,” Connie W. Wang, MD, said in an interview at the annual meeting of the American Association for the Study of Liver Diseases. “We were curious to see what it looked like in our practice setting.”
In an effort to evaluate current advanced care planning documentation practices in the liver transplantation setting, she and her colleagues reviewed the medical charts of 168 adults who underwent an initial liver transplant evaluation at the University of California, San Francisco, from January 2017 to June 2017. Next, to assess readiness to complete advanced care planning among liver transplant candidates, the researchers administered the Advanced Care Planning Engagement Survey to 41 adults who underwent an initial liver transplant evaluation from March 2018 to May 2018. The survey was scored on a Likert scale of 1-4, in which a score of 4 equaled “ready” or “confident,” and a score of 5 equaled “very ready” or “very confident.”
The mean age of the 168 transplant candidates was 53 years, 35% were female, and 52% were non-Hispanic white. Only 15 patients (9%) reported completing advanced care planning prior to their liver transplant evaluation and none had legal advance care planning forms scanned or end-of-life wishes documented in the medical record. Durable power of attorney for health care was discussed with 17 patients (10%). On logistic regression analysis, only white race was associated with completion of advanced care planning (OR 4.16; P = .03), but age, Child-Pugh score, and MELD-Na score were not.
The mean age of the 41 transplant candidates who completed the Advanced Care Planning Engagement Survey was 58 years, 39% were female, and 58% were non-Hispanic white. Nearly all respondents (93%) indicated that they were ready to appoint a durable power of attorney, 85% were ready to discuss end-of-life care, and 93% were ready to ask physicians questions about medical decisions. Similarly, 93% of patients felt confident to appoint a durable power of attorney, 88% felt confident to discuss end-of-life care, and 93% felt confident to ask physicians questions about medical decisions.
“It seems like from the patients’ perspective, they are very much open to having these conversations, but there hasn’t been [the right] environment or setting to have them,” said Dr. Wang, a third-year internal medicine resident at UCSF. “Or, there may be a barrier from the provider’s perspective. Clearly, there is a huge need that can be filled.” She noted that future research should focus on development of tools to facilitate discussions and documentation between transplant clinicians, patients, and their caregivers.
One of the study authors, Jennifer C. Lai, MD, reported being a consultant for Third Rock Ventures, LLC. The other researchers reported having no financial disclosures.
Source: Hepatol. 2018;68[S1]: Abstract 771.
REPORTING FROM THE LIVER MEETING 2018
Key clinical point: There is a paucity of documentation of advance care planning or identification of a durable power of attorney in the medical record of liver transplant candidates.
Major finding: Only 9% of liver transplant candidates reported completing advanced care planning prior to their liver transplant evaluations and none had legal advance care planning forms scanned or end-of-life wishes documented in the medical record.
Study details: A retrospective review of 168 adults who underwent an initial liver transplant evaluation at the University of California, San Francisco.
Disclosures: One of the study authors, Jennifer C. Lai, MD, reported being a consultant for Third Rock Ventures, LLC. The other researchers reported having no financial disclosures.
Source: Hepatol. 2018;68[S1]:Abstract 771.
Skin rashes often accompany drug-induced liver injury
SAN FRANCISCO – More than a quarter of drug-induced liver injury (DILI) cases also involve skin reactions, most often drug rash with eosinophilia and system symptoms (DRESS) syndrome. These dual cases of DILI and drug-induced skin injury (DISI) underscore the need for hepatologists to pay attention to dermatologic conditions and emphasize the need for the two specialties to work together.
The findings suggest that DISI/DILI comorbidity is not uncommon, and may hint at underlying mechanisms that could be used to tailor treatment, according to Harshad Devarbhavi, MD, who presented the study at the annual meeting of the American Association for the Study of Liver Diseases. “My message was that people should work more and see if there’s any type of genotype or HLA [human leukocyte antigen] that produces this reaction. It’s a multisystem disease. It doesn’t belong to dermatologists, it’s a domain that also belongs to hepatologists,” said Dr. Devarbhavi, who is a hepatology fellow at St. John’s Medical College in Bangalore, India.
DISI is more common than DILI, and may or may not be caused by an immune response. The two conditions were previously known to co-occur, but it is rarely reported because dermatologists and hepatologists report findings in different journals.
The researchers defined DILI as a fivefold or greater increase in aspartate aminotransferase (AST) or alanine aminotransferase (ALT); a threefold or greater increase with symptoms, including cutaneous reactions; any elevation of AST, ALT, or alkaline phosphatase (ALP) accompanying a bilirubin increase of 2 mg/dL or more; or a twofold or higher increase in ALP combined with a cutaneous reaction.
They analyzed 921 DILI patients from a single registry in India, who were seen between 1997 and April 2018. All patients with skin reactions were seen by dermatologists and competing causes were excluded. A total of 28% of patients with DILI also had DISI, 13% of whom were also HIV positive; 56% developed jaundice. The mean age of patients with DILI/DISI was 35 years, compared with 42 years in DILI only patients (P = .001) and the mean duration of drug therapy was 42 days, compared with 89 days (P = .002). Twelve percent of DILI/DISI patients died, which was lower than the 17% mortality in those with DILI alone.
Of the DILI/DISI patients, 59% experienced DRESS, and 19% had Stevens-Johnson syndrome/toxic epidermal necrolysis (SJS/TEN). Six percent of patients with DRESS died, as did 22% of those with SJS/TEN. Mortality was 16% among those with other skin manifestations. Eighteen percent of those with jaundice died, compared with 3% of those without jaundice.
Thirty patients with DILI/DISI died; 37% (11) of them had SJS/TEN, compared with 17% of survivors (P = .01). DRESS was more common in survivors (62% vs. 33%; P = .02).
Of DILI/DISI and SJS/TEN cases, 75% were associated with four drug classes: antiepileptic drugs, dapsone, antiretroviral therapies, and leflunomide.
“The liver is the biggest internal organ in the body, and skin is the largest external organ, so there is some correlation between the two, but people haven’t looked at it. People should come together and see why some drugs produce both these injuries. I think there is some mechanistic information in these drugs,” said Dr. Devarbhavi.
Source: Hepatology 2018 Oct 1;68[S1], Abstract 37.
SAN FRANCISCO – More than a quarter of drug-induced liver injury (DILI) cases also involve skin reactions, most often drug rash with eosinophilia and system symptoms (DRESS) syndrome. These dual cases of DILI and drug-induced skin injury (DISI) underscore the need for hepatologists to pay attention to dermatologic conditions and emphasize the need for the two specialties to work together.
The findings suggest that DISI/DILI comorbidity is not uncommon, and may hint at underlying mechanisms that could be used to tailor treatment, according to Harshad Devarbhavi, MD, who presented the study at the annual meeting of the American Association for the Study of Liver Diseases. “My message was that people should work more and see if there’s any type of genotype or HLA [human leukocyte antigen] that produces this reaction. It’s a multisystem disease. It doesn’t belong to dermatologists, it’s a domain that also belongs to hepatologists,” said Dr. Devarbhavi, who is a hepatology fellow at St. John’s Medical College in Bangalore, India.
DISI is more common than DILI, and may or may not be caused by an immune response. The two conditions were previously known to co-occur, but it is rarely reported because dermatologists and hepatologists report findings in different journals.
The researchers defined DILI as a fivefold or greater increase in aspartate aminotransferase (AST) or alanine aminotransferase (ALT); a threefold or greater increase with symptoms, including cutaneous reactions; any elevation of AST, ALT, or alkaline phosphatase (ALP) accompanying a bilirubin increase of 2 mg/dL or more; or a twofold or higher increase in ALP combined with a cutaneous reaction.
They analyzed 921 DILI patients from a single registry in India, who were seen between 1997 and April 2018. All patients with skin reactions were seen by dermatologists and competing causes were excluded. A total of 28% of patients with DILI also had DISI, 13% of whom were also HIV positive; 56% developed jaundice. The mean age of patients with DILI/DISI was 35 years, compared with 42 years in DILI only patients (P = .001) and the mean duration of drug therapy was 42 days, compared with 89 days (P = .002). Twelve percent of DILI/DISI patients died, which was lower than the 17% mortality in those with DILI alone.
Of the DILI/DISI patients, 59% experienced DRESS, and 19% had Stevens-Johnson syndrome/toxic epidermal necrolysis (SJS/TEN). Six percent of patients with DRESS died, as did 22% of those with SJS/TEN. Mortality was 16% among those with other skin manifestations. Eighteen percent of those with jaundice died, compared with 3% of those without jaundice.
Thirty patients with DILI/DISI died; 37% (11) of them had SJS/TEN, compared with 17% of survivors (P = .01). DRESS was more common in survivors (62% vs. 33%; P = .02).
Of DILI/DISI and SJS/TEN cases, 75% were associated with four drug classes: antiepileptic drugs, dapsone, antiretroviral therapies, and leflunomide.
“The liver is the biggest internal organ in the body, and skin is the largest external organ, so there is some correlation between the two, but people haven’t looked at it. People should come together and see why some drugs produce both these injuries. I think there is some mechanistic information in these drugs,” said Dr. Devarbhavi.
Source: Hepatology 2018 Oct 1;68[S1], Abstract 37.
SAN FRANCISCO – More than a quarter of drug-induced liver injury (DILI) cases also involve skin reactions, most often drug rash with eosinophilia and system symptoms (DRESS) syndrome. These dual cases of DILI and drug-induced skin injury (DISI) underscore the need for hepatologists to pay attention to dermatologic conditions and emphasize the need for the two specialties to work together.
The findings suggest that DISI/DILI comorbidity is not uncommon, and may hint at underlying mechanisms that could be used to tailor treatment, according to Harshad Devarbhavi, MD, who presented the study at the annual meeting of the American Association for the Study of Liver Diseases. “My message was that people should work more and see if there’s any type of genotype or HLA [human leukocyte antigen] that produces this reaction. It’s a multisystem disease. It doesn’t belong to dermatologists, it’s a domain that also belongs to hepatologists,” said Dr. Devarbhavi, who is a hepatology fellow at St. John’s Medical College in Bangalore, India.
DISI is more common than DILI, and may or may not be caused by an immune response. The two conditions were previously known to co-occur, but it is rarely reported because dermatologists and hepatologists report findings in different journals.
The researchers defined DILI as a fivefold or greater increase in aspartate aminotransferase (AST) or alanine aminotransferase (ALT); a threefold or greater increase with symptoms, including cutaneous reactions; any elevation of AST, ALT, or alkaline phosphatase (ALP) accompanying a bilirubin increase of 2 mg/dL or more; or a twofold or higher increase in ALP combined with a cutaneous reaction.
They analyzed 921 DILI patients from a single registry in India, who were seen between 1997 and April 2018. All patients with skin reactions were seen by dermatologists and competing causes were excluded. A total of 28% of patients with DILI also had DISI, 13% of whom were also HIV positive; 56% developed jaundice. The mean age of patients with DILI/DISI was 35 years, compared with 42 years in DILI only patients (P = .001) and the mean duration of drug therapy was 42 days, compared with 89 days (P = .002). Twelve percent of DILI/DISI patients died, which was lower than the 17% mortality in those with DILI alone.
Of the DILI/DISI patients, 59% experienced DRESS, and 19% had Stevens-Johnson syndrome/toxic epidermal necrolysis (SJS/TEN). Six percent of patients with DRESS died, as did 22% of those with SJS/TEN. Mortality was 16% among those with other skin manifestations. Eighteen percent of those with jaundice died, compared with 3% of those without jaundice.
Thirty patients with DILI/DISI died; 37% (11) of them had SJS/TEN, compared with 17% of survivors (P = .01). DRESS was more common in survivors (62% vs. 33%; P = .02).
Of DILI/DISI and SJS/TEN cases, 75% were associated with four drug classes: antiepileptic drugs, dapsone, antiretroviral therapies, and leflunomide.
“The liver is the biggest internal organ in the body, and skin is the largest external organ, so there is some correlation between the two, but people haven’t looked at it. People should come together and see why some drugs produce both these injuries. I think there is some mechanistic information in these drugs,” said Dr. Devarbhavi.
Source: Hepatology 2018 Oct 1;68[S1], Abstract 37.
REPORTING FROM THE LIVER MEETING 2018
Key clinical point: Researchers hope the findings will shed light on the mechanism of injury.
Major finding: 28% of patients with DILI also had a skin rash.
Study details: Retrospective analysis of 921 DILI patients.
Disclosures: No source of funding was disclosed. Dr. Devarbhavi disclosed no relevant conflicts.
Source: Hepatology 2018 Oct 1;68[S1], Abstract 37.
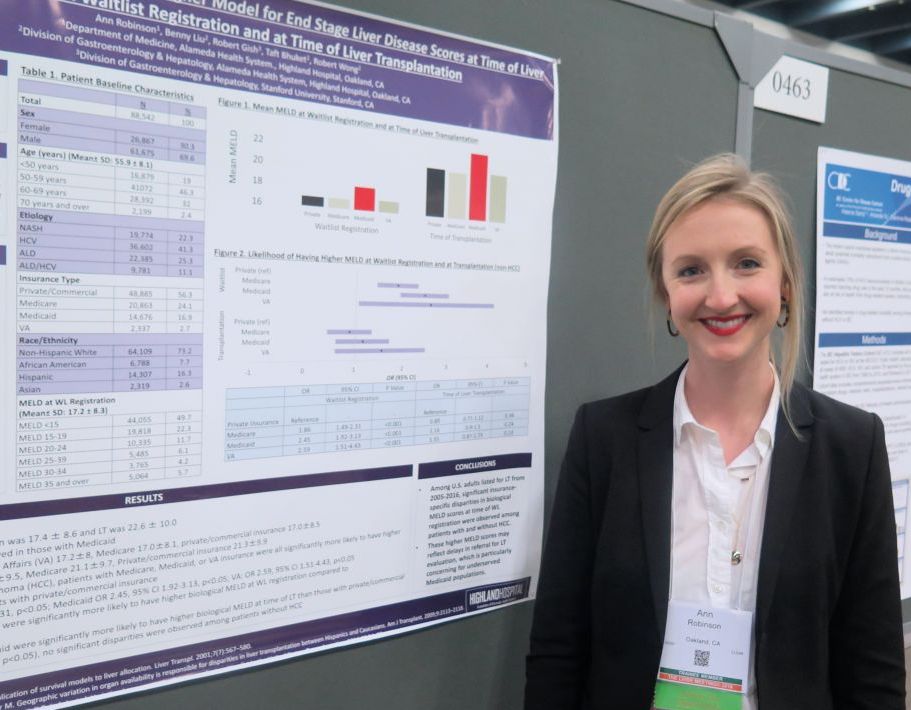
Medicaid patients have higher MELD scores at time of liver transplantation
SAN FRANCISCO – Despite implementation of the Model for End Stage Liver Disease score to prioritize liver transplantation, .
“It can be difficult for patients with Medicaid to access liver transplantation,” lead study author Ann Robinson, MD, said in an interview at the annual meeting of the American Association for the Study of Liver Diseases. “These patients may be living in underserved areas with limited resources.”
In an effort to evaluate insurance-specific disparities in severity of liver disease at the time of liver transplantation wait-list registration and at the time of liver transplantation, Dr. Robinson and her colleagues retrospectively evaluated the 2005-2016 United Network for Organ Sharing/Organ Procurement and Transplant Network liver transplant registry. They used multivariate linear regression models to make insurance-specific comparisons of MELD scores at wait-list registration and at liver transplantation, which included adjustments for age, sex, year, etiology of liver disease, body mass index, ascites, hepatocellular carcinoma (HCC), and hepatic encephalopathy.
Dr. Robinson, who is a third-year internal medicine resident at Highland Hospital, Oakland, Calif., reported findings from 88,542 liver transplantation wait-list registrants with a mean age of 56 years. Their overall mean MELD score was 17.4 at wait-list registration and 22.6 at time of liver transplantation. The greatest mean MELD score at the time of wait-list registration was observed in Medicaid patients (18.4, compared with 17.2 among Veterans Affairs patients, 17 among Medicare patients, and 17 among privately/commercially insured patients; P less than .01). Meanwhile, the greatest mean MELD score at the time of liver transplantation was observed in Medicaid patients (23.5, compared with 21.4 among VA patients, 21.3 among privately/commercially insured patients, and 21.1 among Medicare patients; P less than .01).
Multivariate regression analysis revealed that, among patients without hepatocellular carcinoma, those with coverage other than private or commercial insurance had significantly higher MELD scores at wait-list registration (P less than .01). Specifically, the odds ratio was highest for VA patients (odds ratio, 2.59), followed by those covered by Medicaid (OR, 2.45), and Medicare (OR, 1.86). Similar trends were observed in hepatocellular carcinoma patients, with the highest biological MELD score at wait-list seen in those covered by Medicaid.
On regression analysis, while Medicaid patients with hepatocellular carcinoma had significantly higher biological MELD scores at time of liver transplantation, compared with those with private/commercial insurance (Medicaid OR, 2.06; P less than .05), no differences were observed among patients without hepatocellular carcinoma.
Dr. Robinson reported having no financial disclosures.
Source: Hepatology 2018 Oct 1;68[S1], Abstract 464.
SAN FRANCISCO – Despite implementation of the Model for End Stage Liver Disease score to prioritize liver transplantation, .
“It can be difficult for patients with Medicaid to access liver transplantation,” lead study author Ann Robinson, MD, said in an interview at the annual meeting of the American Association for the Study of Liver Diseases. “These patients may be living in underserved areas with limited resources.”
In an effort to evaluate insurance-specific disparities in severity of liver disease at the time of liver transplantation wait-list registration and at the time of liver transplantation, Dr. Robinson and her colleagues retrospectively evaluated the 2005-2016 United Network for Organ Sharing/Organ Procurement and Transplant Network liver transplant registry. They used multivariate linear regression models to make insurance-specific comparisons of MELD scores at wait-list registration and at liver transplantation, which included adjustments for age, sex, year, etiology of liver disease, body mass index, ascites, hepatocellular carcinoma (HCC), and hepatic encephalopathy.
Dr. Robinson, who is a third-year internal medicine resident at Highland Hospital, Oakland, Calif., reported findings from 88,542 liver transplantation wait-list registrants with a mean age of 56 years. Their overall mean MELD score was 17.4 at wait-list registration and 22.6 at time of liver transplantation. The greatest mean MELD score at the time of wait-list registration was observed in Medicaid patients (18.4, compared with 17.2 among Veterans Affairs patients, 17 among Medicare patients, and 17 among privately/commercially insured patients; P less than .01). Meanwhile, the greatest mean MELD score at the time of liver transplantation was observed in Medicaid patients (23.5, compared with 21.4 among VA patients, 21.3 among privately/commercially insured patients, and 21.1 among Medicare patients; P less than .01).
Multivariate regression analysis revealed that, among patients without hepatocellular carcinoma, those with coverage other than private or commercial insurance had significantly higher MELD scores at wait-list registration (P less than .01). Specifically, the odds ratio was highest for VA patients (odds ratio, 2.59), followed by those covered by Medicaid (OR, 2.45), and Medicare (OR, 1.86). Similar trends were observed in hepatocellular carcinoma patients, with the highest biological MELD score at wait-list seen in those covered by Medicaid.
On regression analysis, while Medicaid patients with hepatocellular carcinoma had significantly higher biological MELD scores at time of liver transplantation, compared with those with private/commercial insurance (Medicaid OR, 2.06; P less than .05), no differences were observed among patients without hepatocellular carcinoma.
Dr. Robinson reported having no financial disclosures.
Source: Hepatology 2018 Oct 1;68[S1], Abstract 464.
SAN FRANCISCO – Despite implementation of the Model for End Stage Liver Disease score to prioritize liver transplantation, .
“It can be difficult for patients with Medicaid to access liver transplantation,” lead study author Ann Robinson, MD, said in an interview at the annual meeting of the American Association for the Study of Liver Diseases. “These patients may be living in underserved areas with limited resources.”
In an effort to evaluate insurance-specific disparities in severity of liver disease at the time of liver transplantation wait-list registration and at the time of liver transplantation, Dr. Robinson and her colleagues retrospectively evaluated the 2005-2016 United Network for Organ Sharing/Organ Procurement and Transplant Network liver transplant registry. They used multivariate linear regression models to make insurance-specific comparisons of MELD scores at wait-list registration and at liver transplantation, which included adjustments for age, sex, year, etiology of liver disease, body mass index, ascites, hepatocellular carcinoma (HCC), and hepatic encephalopathy.
Dr. Robinson, who is a third-year internal medicine resident at Highland Hospital, Oakland, Calif., reported findings from 88,542 liver transplantation wait-list registrants with a mean age of 56 years. Their overall mean MELD score was 17.4 at wait-list registration and 22.6 at time of liver transplantation. The greatest mean MELD score at the time of wait-list registration was observed in Medicaid patients (18.4, compared with 17.2 among Veterans Affairs patients, 17 among Medicare patients, and 17 among privately/commercially insured patients; P less than .01). Meanwhile, the greatest mean MELD score at the time of liver transplantation was observed in Medicaid patients (23.5, compared with 21.4 among VA patients, 21.3 among privately/commercially insured patients, and 21.1 among Medicare patients; P less than .01).
Multivariate regression analysis revealed that, among patients without hepatocellular carcinoma, those with coverage other than private or commercial insurance had significantly higher MELD scores at wait-list registration (P less than .01). Specifically, the odds ratio was highest for VA patients (odds ratio, 2.59), followed by those covered by Medicaid (OR, 2.45), and Medicare (OR, 1.86). Similar trends were observed in hepatocellular carcinoma patients, with the highest biological MELD score at wait-list seen in those covered by Medicaid.
On regression analysis, while Medicaid patients with hepatocellular carcinoma had significantly higher biological MELD scores at time of liver transplantation, compared with those with private/commercial insurance (Medicaid OR, 2.06; P less than .05), no differences were observed among patients without hepatocellular carcinoma.
Dr. Robinson reported having no financial disclosures.
Source: Hepatology 2018 Oct 1;68[S1], Abstract 464.
AT THE LIVER MEETING 2018
Key clinical point: Significant insurance-specific disparities in MELD scores at time of wait-list registration were observed among patients with and without hepatocellular carcinoma.
Major finding: Among patients without hepatocellular carcinoma, those with Medicaid coverage were 2.45 times more likely to have higher MELD scores at wait-list registration, compared with those covered by commercial or private insurance (P less than .01).
Study details: A retrospective analysis of 88,542 liver transplantation wait-list registrants.
Disclosures: Dr. Robinson reported having no disclosures.
Source: Hepatology 2018 Oct 1;68[S1], Abstract 464.
The Liver Meeting 2018: Hepatitis B novel therapies debrief – key abstracts
SAN FRANCISCO – Most years, when it comes to research to treat hepatitis viral infections, hepatitis C has been front and center at the annual meeting of the American Association for the Study of Liver Diseases. But things have changed in the past couple of years, with hepatitis C curative treatment maturing and altering the therapeutic landscape.
“Hepatitis C has been where all the action is, but that’s clearly changed in the last few years,” said Jordan Feld, MD, MPH, who summed up the hepatitis B findings during a wrap-up session on the final day of the conference. Dr. Feld is a clinician-scientist at the Toronto Western Hospital Liver Clinic and the McLaughlin-Rotman Centre for Global Health.
An analysis (Abstract 212) of a mixed North American and Asian cohort of more than 10,000 untreated patients showed just a 1.3% annual clearance rate of surface antigen, with little guidance for risk stratification. “This leaves us really needing new therapies,” said Dr. Feld.
Fortunately, the hepatitis B virus (HBV) life cycle offers various opportunities for therapeutic intervention, including blocking entry, targeting assembly and export of the virus, targeting HBV RNA, and targeting the capsid protein and viral packaging.
One study (Abstract 16) examined a hepatitis B entry inhibitor’s effect on hepatitis D virus (HDV), which requires coinfection with HBV to replicate. A phase 2 clinical trial found that treatment with Myrcludex B alone or in combination with interferon led to a decline in HDV RNA, but the result was most pronounced in patients who received the combination therapy. The combination was also associated with a greater probability of surface antigen decline. “I think that’s really important, that we see this synergistic effect. This is really promising phase 2 data that raises the possibility of curative therapy for this troubling infection,” said Dr. Feld.
Another study (Abstract LB-25) looked at an RNA inhibitor that targets both integrated and covalently closed circular DNA (cccDNA)–derived HBV RNA. The drug was given to 11 HBV patients who were positive for HBeAg (hepatitis B e-antigen) and 13 HBV patients who were HBeAg negative. It had similar effects in reducing HBV surface antigens and other correlated antigens in both groups of patients, and no evidence of a dose-response relationship. “Seeing a similar effect is quite important and suggests that it’s targeting both cccDNA-derived and integrated HBV DNA, and although there were some mild injection reactions, it generally seemed to be safe and pretty well tolerated,” said Dr. Feld.
Further down the life cycle, capsid assembly modulators (CAMs) have the potential to counter HBV by two mechanisms; blocking encapsulation of pregenomic RNA, and degrading capsids, which could prevent the replenishment of cccDNA. The latter effect could be important for achieving a cure, according to Dr. Feld.
A novel CAM, JNJ-6379 (Abstract 74), was tested at three different doses, and was well tolerated at higher doses, but it had limited dose response at the higher dose with respect to HBV DNA suppression. However, it could be that the two CAM mechanisms may require different doses. “These two things are hard to tease apart, and hopefully, we’ll see more data to separate them in the future,” said Dr. Feld.
Another CAM, ABI-HO731 (Abstract 73), had a potent effect on HBV DNA and HBV RNA, showing that it blocks encapsulation of both pregenomic RNA related to reverse transcription and pregenomic RNAs within the capsid. Stopping the medication led to some HBV DNA rebound, though no alanine aminotransferase flares, which Dr. Feld found reassuring. One patient had baseline resistance but was nevertheless able to achieve some suppression on the drug.
Another therapeutic approach used a nucleic acid polymer to block subviral particle release (Abstract 393). The study treated immunosuppressed patients with the polymer alone or in combination with interferon or tenofovir disoproxil, and led to “striking reductions in hepatitis B surface antigen quantities during therapy,” said Dr. Feld. An alanine aminotransferase flair did occur, which may signify an immune response, and it will be important to determine if this is indeed the case, he said. After stopping therapy there was a gain of anti–hepatitis B antibodies, which suggests that functional clearance of surface antigen is occurring.
Researchers also are recruiting the immune system to combat HBV. The novel agent inarigivir is a retinoic acid-inducible gene-1 agonist, which has both a direct antiviral effect and an indirect effect via the intrahepatic innate immune response, which it accomplishes by activating the interferon signaling pathway. It also directly interferes with the interaction between pregenomic RNA and the HBV polymerase, preventing replication. In the ACHIEVE trial, the researchers noted greater reduction in HBV RNA and DNA at higher doses (Abstract 75). “This is certainly an interesting molecule and an interesting proof of concept that you can potentially target HBV using two different pathways, and we’ll be interested to see more data with this approach,” said Dr. Feld.
Dr. Feld wrapped up the discussion of novel therapies with an animal model study (Abstract 77) that suggests future strategies for a cure. In it, the researchers combined a therapeutic vaccine with a stabilized, liver-targeted small interfering RNA to suppress surface antigen. Animals that received only the vaccine saw little benefit, but the combined approach led to viral clearance. The treatment also restored the immune response. “It gives us an inkling that we may need to both reduce the antigen load and stimulate immunity,” said Dr. Feld.
Dr. Feld has consulted for AbbVie, Gilead, ContraVir, MedImmune, and Merck. He has received funding from AbbVie, Gilead, Merck, and Janssen.
SAN FRANCISCO – Most years, when it comes to research to treat hepatitis viral infections, hepatitis C has been front and center at the annual meeting of the American Association for the Study of Liver Diseases. But things have changed in the past couple of years, with hepatitis C curative treatment maturing and altering the therapeutic landscape.
“Hepatitis C has been where all the action is, but that’s clearly changed in the last few years,” said Jordan Feld, MD, MPH, who summed up the hepatitis B findings during a wrap-up session on the final day of the conference. Dr. Feld is a clinician-scientist at the Toronto Western Hospital Liver Clinic and the McLaughlin-Rotman Centre for Global Health.
An analysis (Abstract 212) of a mixed North American and Asian cohort of more than 10,000 untreated patients showed just a 1.3% annual clearance rate of surface antigen, with little guidance for risk stratification. “This leaves us really needing new therapies,” said Dr. Feld.
Fortunately, the hepatitis B virus (HBV) life cycle offers various opportunities for therapeutic intervention, including blocking entry, targeting assembly and export of the virus, targeting HBV RNA, and targeting the capsid protein and viral packaging.
One study (Abstract 16) examined a hepatitis B entry inhibitor’s effect on hepatitis D virus (HDV), which requires coinfection with HBV to replicate. A phase 2 clinical trial found that treatment with Myrcludex B alone or in combination with interferon led to a decline in HDV RNA, but the result was most pronounced in patients who received the combination therapy. The combination was also associated with a greater probability of surface antigen decline. “I think that’s really important, that we see this synergistic effect. This is really promising phase 2 data that raises the possibility of curative therapy for this troubling infection,” said Dr. Feld.
Another study (Abstract LB-25) looked at an RNA inhibitor that targets both integrated and covalently closed circular DNA (cccDNA)–derived HBV RNA. The drug was given to 11 HBV patients who were positive for HBeAg (hepatitis B e-antigen) and 13 HBV patients who were HBeAg negative. It had similar effects in reducing HBV surface antigens and other correlated antigens in both groups of patients, and no evidence of a dose-response relationship. “Seeing a similar effect is quite important and suggests that it’s targeting both cccDNA-derived and integrated HBV DNA, and although there were some mild injection reactions, it generally seemed to be safe and pretty well tolerated,” said Dr. Feld.
Further down the life cycle, capsid assembly modulators (CAMs) have the potential to counter HBV by two mechanisms; blocking encapsulation of pregenomic RNA, and degrading capsids, which could prevent the replenishment of cccDNA. The latter effect could be important for achieving a cure, according to Dr. Feld.
A novel CAM, JNJ-6379 (Abstract 74), was tested at three different doses, and was well tolerated at higher doses, but it had limited dose response at the higher dose with respect to HBV DNA suppression. However, it could be that the two CAM mechanisms may require different doses. “These two things are hard to tease apart, and hopefully, we’ll see more data to separate them in the future,” said Dr. Feld.
Another CAM, ABI-HO731 (Abstract 73), had a potent effect on HBV DNA and HBV RNA, showing that it blocks encapsulation of both pregenomic RNA related to reverse transcription and pregenomic RNAs within the capsid. Stopping the medication led to some HBV DNA rebound, though no alanine aminotransferase flares, which Dr. Feld found reassuring. One patient had baseline resistance but was nevertheless able to achieve some suppression on the drug.
Another therapeutic approach used a nucleic acid polymer to block subviral particle release (Abstract 393). The study treated immunosuppressed patients with the polymer alone or in combination with interferon or tenofovir disoproxil, and led to “striking reductions in hepatitis B surface antigen quantities during therapy,” said Dr. Feld. An alanine aminotransferase flair did occur, which may signify an immune response, and it will be important to determine if this is indeed the case, he said. After stopping therapy there was a gain of anti–hepatitis B antibodies, which suggests that functional clearance of surface antigen is occurring.
Researchers also are recruiting the immune system to combat HBV. The novel agent inarigivir is a retinoic acid-inducible gene-1 agonist, which has both a direct antiviral effect and an indirect effect via the intrahepatic innate immune response, which it accomplishes by activating the interferon signaling pathway. It also directly interferes with the interaction between pregenomic RNA and the HBV polymerase, preventing replication. In the ACHIEVE trial, the researchers noted greater reduction in HBV RNA and DNA at higher doses (Abstract 75). “This is certainly an interesting molecule and an interesting proof of concept that you can potentially target HBV using two different pathways, and we’ll be interested to see more data with this approach,” said Dr. Feld.
Dr. Feld wrapped up the discussion of novel therapies with an animal model study (Abstract 77) that suggests future strategies for a cure. In it, the researchers combined a therapeutic vaccine with a stabilized, liver-targeted small interfering RNA to suppress surface antigen. Animals that received only the vaccine saw little benefit, but the combined approach led to viral clearance. The treatment also restored the immune response. “It gives us an inkling that we may need to both reduce the antigen load and stimulate immunity,” said Dr. Feld.
Dr. Feld has consulted for AbbVie, Gilead, ContraVir, MedImmune, and Merck. He has received funding from AbbVie, Gilead, Merck, and Janssen.
SAN FRANCISCO – Most years, when it comes to research to treat hepatitis viral infections, hepatitis C has been front and center at the annual meeting of the American Association for the Study of Liver Diseases. But things have changed in the past couple of years, with hepatitis C curative treatment maturing and altering the therapeutic landscape.
“Hepatitis C has been where all the action is, but that’s clearly changed in the last few years,” said Jordan Feld, MD, MPH, who summed up the hepatitis B findings during a wrap-up session on the final day of the conference. Dr. Feld is a clinician-scientist at the Toronto Western Hospital Liver Clinic and the McLaughlin-Rotman Centre for Global Health.
An analysis (Abstract 212) of a mixed North American and Asian cohort of more than 10,000 untreated patients showed just a 1.3% annual clearance rate of surface antigen, with little guidance for risk stratification. “This leaves us really needing new therapies,” said Dr. Feld.
Fortunately, the hepatitis B virus (HBV) life cycle offers various opportunities for therapeutic intervention, including blocking entry, targeting assembly and export of the virus, targeting HBV RNA, and targeting the capsid protein and viral packaging.
One study (Abstract 16) examined a hepatitis B entry inhibitor’s effect on hepatitis D virus (HDV), which requires coinfection with HBV to replicate. A phase 2 clinical trial found that treatment with Myrcludex B alone or in combination with interferon led to a decline in HDV RNA, but the result was most pronounced in patients who received the combination therapy. The combination was also associated with a greater probability of surface antigen decline. “I think that’s really important, that we see this synergistic effect. This is really promising phase 2 data that raises the possibility of curative therapy for this troubling infection,” said Dr. Feld.
Another study (Abstract LB-25) looked at an RNA inhibitor that targets both integrated and covalently closed circular DNA (cccDNA)–derived HBV RNA. The drug was given to 11 HBV patients who were positive for HBeAg (hepatitis B e-antigen) and 13 HBV patients who were HBeAg negative. It had similar effects in reducing HBV surface antigens and other correlated antigens in both groups of patients, and no evidence of a dose-response relationship. “Seeing a similar effect is quite important and suggests that it’s targeting both cccDNA-derived and integrated HBV DNA, and although there were some mild injection reactions, it generally seemed to be safe and pretty well tolerated,” said Dr. Feld.
Further down the life cycle, capsid assembly modulators (CAMs) have the potential to counter HBV by two mechanisms; blocking encapsulation of pregenomic RNA, and degrading capsids, which could prevent the replenishment of cccDNA. The latter effect could be important for achieving a cure, according to Dr. Feld.
A novel CAM, JNJ-6379 (Abstract 74), was tested at three different doses, and was well tolerated at higher doses, but it had limited dose response at the higher dose with respect to HBV DNA suppression. However, it could be that the two CAM mechanisms may require different doses. “These two things are hard to tease apart, and hopefully, we’ll see more data to separate them in the future,” said Dr. Feld.
Another CAM, ABI-HO731 (Abstract 73), had a potent effect on HBV DNA and HBV RNA, showing that it blocks encapsulation of both pregenomic RNA related to reverse transcription and pregenomic RNAs within the capsid. Stopping the medication led to some HBV DNA rebound, though no alanine aminotransferase flares, which Dr. Feld found reassuring. One patient had baseline resistance but was nevertheless able to achieve some suppression on the drug.
Another therapeutic approach used a nucleic acid polymer to block subviral particle release (Abstract 393). The study treated immunosuppressed patients with the polymer alone or in combination with interferon or tenofovir disoproxil, and led to “striking reductions in hepatitis B surface antigen quantities during therapy,” said Dr. Feld. An alanine aminotransferase flair did occur, which may signify an immune response, and it will be important to determine if this is indeed the case, he said. After stopping therapy there was a gain of anti–hepatitis B antibodies, which suggests that functional clearance of surface antigen is occurring.
Researchers also are recruiting the immune system to combat HBV. The novel agent inarigivir is a retinoic acid-inducible gene-1 agonist, which has both a direct antiviral effect and an indirect effect via the intrahepatic innate immune response, which it accomplishes by activating the interferon signaling pathway. It also directly interferes with the interaction between pregenomic RNA and the HBV polymerase, preventing replication. In the ACHIEVE trial, the researchers noted greater reduction in HBV RNA and DNA at higher doses (Abstract 75). “This is certainly an interesting molecule and an interesting proof of concept that you can potentially target HBV using two different pathways, and we’ll be interested to see more data with this approach,” said Dr. Feld.
Dr. Feld wrapped up the discussion of novel therapies with an animal model study (Abstract 77) that suggests future strategies for a cure. In it, the researchers combined a therapeutic vaccine with a stabilized, liver-targeted small interfering RNA to suppress surface antigen. Animals that received only the vaccine saw little benefit, but the combined approach led to viral clearance. The treatment also restored the immune response. “It gives us an inkling that we may need to both reduce the antigen load and stimulate immunity,” said Dr. Feld.
Dr. Feld has consulted for AbbVie, Gilead, ContraVir, MedImmune, and Merck. He has received funding from AbbVie, Gilead, Merck, and Janssen.
REPORTING FROM THE LIVER MEETING 2018
Hep C–infected livers are safe for transplant
SAN FRANCISCO – A new analysis shows that hepatitis C–infected livers can be safely transplanted into recipients with no effect on graft survival, retransplantation, or mortality. The work confirms that readily available direct-acting antiviral therapy can protect organ recipients and open a source of organs that is typically overlooked.
The work should encourage both physicians and patients to take a closer look at hepatitis C–infected organs, especially for sicker patients, according to Sonali Paul, MD, who presented the study at the annual meeting of the American Association for the Study of Liver Disease 2018.
“A lot of people have an ethical issue with it because we’re actively transplanting a virus into someone. We’re giving someone a disease. My take on it is that we give people Epstein Barr virus or cytomegalovirus all the time – we just [provide] prophylaxis against it, and we don’t even bat an eye. Hepatitis C can be devastating, but we have totally effective treatments for it,” said Dr. Paul, who is an assistant professor of medicine at the University of Chicago.
She cited one colleague at the University of Chicago who several years ago transplanted an organ that had been passed over 700 times, though times have changed since then. “I think people more and more are doing this practice because we know it’s so successful,” said Dr. Paul.
It’s also cost effective. Another study, presented during the same session by Jag Chhatwal, PhD, assistant professor at Harvard Medical School, Boston, showed that accepting a hepatitis C–positive liver is cost effective in patients with Model for End-Stage Liver Disease (MELD) scores ranging from 22 to 40.
“I think we’re going to find across all organ systems, if we can transplant patients rather than keep them on dialysis or keep them on wait lists, it’s got to be cost effective, especially if you think of the health care–associated costs – like a heart transplant patient waiting on the list in the ICU. That’s a huge health care cost,” said Dr. Paul.
Dr. Paul’s team performed an analysis of the Scientific Registry of Transplant Recipients, including single organ transplants from deceased donors, during 2014-2018. Over that period, the number of transplants from hepatitis C–positive donors to hepatitis C–positive recipients rose from 8 in 2014 to 269, and the number of transplants from hepatitis C–positive donors to hepatitis C–negative recipients rose from 0 to 46.
The researchers compared trends from hepatitis C–negative donors with hepatitis C–negative recipients (n = 11,270), negative donors with positive recipients (n = 4,748), positive donors with negative recipients (n = 87), and positive donors with positive recipients (n = 753). Donor status had no effect on graft survival times at 1 or 2 years, with values ranging from 92.6% (negative to negative) to 94.3% (positive to positive) at 1 year and between 85.7% (positive to negative) and 89.7% (positive to positive) at 2 years.
“For someone who has a MELD score of over 20, who has a declining quality of life and really can’t do anything, I think this is a great opportunity. And most patients are absolutely willing to take these organs. We haven’t had many people say no, especially if they feel poorly,” said Dr. Paul.
She also underscored the importance of ensuring that the patient is informed of the status of the donor liver and the need to complete treatment: “The patient has to know what’s happening, and the hospital has to have a safety net if the insurance doesn’t pay for hepatitis C treatment.”
SOURCE: AASLD 2018, Abstract 0249.
SAN FRANCISCO – A new analysis shows that hepatitis C–infected livers can be safely transplanted into recipients with no effect on graft survival, retransplantation, or mortality. The work confirms that readily available direct-acting antiviral therapy can protect organ recipients and open a source of organs that is typically overlooked.
The work should encourage both physicians and patients to take a closer look at hepatitis C–infected organs, especially for sicker patients, according to Sonali Paul, MD, who presented the study at the annual meeting of the American Association for the Study of Liver Disease 2018.
“A lot of people have an ethical issue with it because we’re actively transplanting a virus into someone. We’re giving someone a disease. My take on it is that we give people Epstein Barr virus or cytomegalovirus all the time – we just [provide] prophylaxis against it, and we don’t even bat an eye. Hepatitis C can be devastating, but we have totally effective treatments for it,” said Dr. Paul, who is an assistant professor of medicine at the University of Chicago.
She cited one colleague at the University of Chicago who several years ago transplanted an organ that had been passed over 700 times, though times have changed since then. “I think people more and more are doing this practice because we know it’s so successful,” said Dr. Paul.
It’s also cost effective. Another study, presented during the same session by Jag Chhatwal, PhD, assistant professor at Harvard Medical School, Boston, showed that accepting a hepatitis C–positive liver is cost effective in patients with Model for End-Stage Liver Disease (MELD) scores ranging from 22 to 40.
“I think we’re going to find across all organ systems, if we can transplant patients rather than keep them on dialysis or keep them on wait lists, it’s got to be cost effective, especially if you think of the health care–associated costs – like a heart transplant patient waiting on the list in the ICU. That’s a huge health care cost,” said Dr. Paul.
Dr. Paul’s team performed an analysis of the Scientific Registry of Transplant Recipients, including single organ transplants from deceased donors, during 2014-2018. Over that period, the number of transplants from hepatitis C–positive donors to hepatitis C–positive recipients rose from 8 in 2014 to 269, and the number of transplants from hepatitis C–positive donors to hepatitis C–negative recipients rose from 0 to 46.
The researchers compared trends from hepatitis C–negative donors with hepatitis C–negative recipients (n = 11,270), negative donors with positive recipients (n = 4,748), positive donors with negative recipients (n = 87), and positive donors with positive recipients (n = 753). Donor status had no effect on graft survival times at 1 or 2 years, with values ranging from 92.6% (negative to negative) to 94.3% (positive to positive) at 1 year and between 85.7% (positive to negative) and 89.7% (positive to positive) at 2 years.
“For someone who has a MELD score of over 20, who has a declining quality of life and really can’t do anything, I think this is a great opportunity. And most patients are absolutely willing to take these organs. We haven’t had many people say no, especially if they feel poorly,” said Dr. Paul.
She also underscored the importance of ensuring that the patient is informed of the status of the donor liver and the need to complete treatment: “The patient has to know what’s happening, and the hospital has to have a safety net if the insurance doesn’t pay for hepatitis C treatment.”
SOURCE: AASLD 2018, Abstract 0249.
SAN FRANCISCO – A new analysis shows that hepatitis C–infected livers can be safely transplanted into recipients with no effect on graft survival, retransplantation, or mortality. The work confirms that readily available direct-acting antiviral therapy can protect organ recipients and open a source of organs that is typically overlooked.
The work should encourage both physicians and patients to take a closer look at hepatitis C–infected organs, especially for sicker patients, according to Sonali Paul, MD, who presented the study at the annual meeting of the American Association for the Study of Liver Disease 2018.
“A lot of people have an ethical issue with it because we’re actively transplanting a virus into someone. We’re giving someone a disease. My take on it is that we give people Epstein Barr virus or cytomegalovirus all the time – we just [provide] prophylaxis against it, and we don’t even bat an eye. Hepatitis C can be devastating, but we have totally effective treatments for it,” said Dr. Paul, who is an assistant professor of medicine at the University of Chicago.
She cited one colleague at the University of Chicago who several years ago transplanted an organ that had been passed over 700 times, though times have changed since then. “I think people more and more are doing this practice because we know it’s so successful,” said Dr. Paul.
It’s also cost effective. Another study, presented during the same session by Jag Chhatwal, PhD, assistant professor at Harvard Medical School, Boston, showed that accepting a hepatitis C–positive liver is cost effective in patients with Model for End-Stage Liver Disease (MELD) scores ranging from 22 to 40.
“I think we’re going to find across all organ systems, if we can transplant patients rather than keep them on dialysis or keep them on wait lists, it’s got to be cost effective, especially if you think of the health care–associated costs – like a heart transplant patient waiting on the list in the ICU. That’s a huge health care cost,” said Dr. Paul.
Dr. Paul’s team performed an analysis of the Scientific Registry of Transplant Recipients, including single organ transplants from deceased donors, during 2014-2018. Over that period, the number of transplants from hepatitis C–positive donors to hepatitis C–positive recipients rose from 8 in 2014 to 269, and the number of transplants from hepatitis C–positive donors to hepatitis C–negative recipients rose from 0 to 46.
The researchers compared trends from hepatitis C–negative donors with hepatitis C–negative recipients (n = 11,270), negative donors with positive recipients (n = 4,748), positive donors with negative recipients (n = 87), and positive donors with positive recipients (n = 753). Donor status had no effect on graft survival times at 1 or 2 years, with values ranging from 92.6% (negative to negative) to 94.3% (positive to positive) at 1 year and between 85.7% (positive to negative) and 89.7% (positive to positive) at 2 years.
“For someone who has a MELD score of over 20, who has a declining quality of life and really can’t do anything, I think this is a great opportunity. And most patients are absolutely willing to take these organs. We haven’t had many people say no, especially if they feel poorly,” said Dr. Paul.
She also underscored the importance of ensuring that the patient is informed of the status of the donor liver and the need to complete treatment: “The patient has to know what’s happening, and the hospital has to have a safety net if the insurance doesn’t pay for hepatitis C treatment.”
SOURCE: AASLD 2018, Abstract 0249.
REPORTING FROM THE LIVER MEETING 2018
Key clinical point: Use of hepatitis C–positive livers can significantly increase the donor organ pool.
Major finding: Hepatitis C–infected livers can be safely transplanted into recipients with no effect on graft survival, retransplantation, or mortality.
Study details: Retrospective analysis of 16,858 liver transplants.
Disclosures: The study was funded internally. Dr. Paul has no financial disclosures.
Source: AASLD 2018, Abstract 0249.
High rates of HCV treatment completion seen in people who inject drugs
SAN FRANCISCO – , preliminary results from an ongoing study showed.
“Both from a public health and a human rights perspective, hepatitis C elimination in people who inject drugs is critical,” study coauthor Elana Rosenthal, MD, said during a press briefing at the annual meeting of the American Association for the Study of Liver Diseases. “People who inject drugs are the main progenitors of ongoing transmission of hepatitis C. However, they are often denied access to hepatitis C treatment due to concerns about their ability to take medication consistently and achieve cure. This is especially true amongst patients with challenging demographic factors, frequent drug use, and those not on treatment for opioid use disorder. However, there are limited data on hepatitis C adherence in people who inject drugs outside of vigorous clinical trial settings.”
In an effort to understand whether a marginalized population with ongoing injection drug use could adhere to HCV treatment, and how this adherence would impact cure, Dr. Rosenthal and her associates enrolled 100 subjects in ANCHOR, a single-center study evaluating HCV treatment in patients who have chronic HCV, opioid use disorder, and ongoing injection drug use. “We did not preferentially enroll patients who we thought we would be most likely to cure, and we did not exclude patients who seemed unlikely to adhere to treatment,” said Dr. Rosenthal, codirector of the DC Partnership for HIV/AIDS Progress hepatitis clinical research program at the University of Maryland, Baltimore. “All patients were treated with sofosbuvir/velpatasvir, with a plan to complete 12 weeks of treatment.” Medication was dispensed monthly in bottles containing 28 pills, and patients were seen for monthly visits, mirroring standard clinical care for HCV. The researchers monitored patients for medication adherence through pill counts and evaluated them for hepatitis C cure 12 weeks after treatment.
The median age of the 100 patients was 57 years, 76% were black, 33% had cirrhosis, 51% were unstably housed, 92% had a history of incarceration, and 92% had no income source or relied exclusively on government benefits. “The patients represent an incredibly marginalized population,” she said. At baseline, 58% reported daily or more frequent injection drug use, 33% reported medication-assisted treatment, 29% shared injection drug use equipment within the past 3 months, and 40% met criteria for hazardous drinking based on the Alcohol Use Disorders Identification Test (AUDIT-C).
Of the 100 patients, 59 received 12 weeks of treatment. Of these 59 patients, 28 finished 1-7 days after the anticipated end date, 9 finished between 8 and 14 days late, and 9 patients finished more than 14 days late.
Of the 58 patients who attended an office visit at week 24 of their treatment, 52 (90%) achieved a sustained virologic response. This cure rate was associated with having an HCV viral load less than 200 IU/mL at week 4, and with taking 12 weeks of treatment. Nonsustained virologic response was driven by virologic failure, loss to follow-up, and death.
When the researchers compared subjects who achieved sustained virologic response with those who did not, baseline demographics including frequent drug use, unstable housing status, and not being on medication to treat opioid use disorder were not associated with decreased cure rates. “While we found high rates of treatment completion in this population, because of external factors such as incarceration, hospitalization, and having medications stolen, 13 patients had interruptions in treatment,” Dr. Rosenthal said. “Further, while 21 patients had near-perfect medication adherence, 46 patients took longer than 12 weeks to complete the full treatment course due to intermittent missed doses. However, as long as patients completed the prescribed amount, imperfect adherence was not associated with decreased cure rates.”
Based on ANCHOR’s preliminary results, Dr. Rosenthal concluded that concerns about HCV treatment adherence such as baseline housing status, drug use frequency, and being on medication for opioid use disorder “are not likely to influence treatment outcome of HCV and should not be used to justify exclusion from treatment in this population. The ANCHOR investigation adds to the growing body of literature supporting expansion of HCV treatment to all patients, including people who inject drugs. Treatment of people who inject drugs is a critical factor in HCV elimination and, most importantly, reducing morbidity and mortality in this population.”
Dr. Rosenthal disclosed that she has received grant/research support from Gilead Sciences and from Merck.
Source: Rosenthal E et al. Hepatol. 2018;68[S1], Abstract 18.
SAN FRANCISCO – , preliminary results from an ongoing study showed.
“Both from a public health and a human rights perspective, hepatitis C elimination in people who inject drugs is critical,” study coauthor Elana Rosenthal, MD, said during a press briefing at the annual meeting of the American Association for the Study of Liver Diseases. “People who inject drugs are the main progenitors of ongoing transmission of hepatitis C. However, they are often denied access to hepatitis C treatment due to concerns about their ability to take medication consistently and achieve cure. This is especially true amongst patients with challenging demographic factors, frequent drug use, and those not on treatment for opioid use disorder. However, there are limited data on hepatitis C adherence in people who inject drugs outside of vigorous clinical trial settings.”
In an effort to understand whether a marginalized population with ongoing injection drug use could adhere to HCV treatment, and how this adherence would impact cure, Dr. Rosenthal and her associates enrolled 100 subjects in ANCHOR, a single-center study evaluating HCV treatment in patients who have chronic HCV, opioid use disorder, and ongoing injection drug use. “We did not preferentially enroll patients who we thought we would be most likely to cure, and we did not exclude patients who seemed unlikely to adhere to treatment,” said Dr. Rosenthal, codirector of the DC Partnership for HIV/AIDS Progress hepatitis clinical research program at the University of Maryland, Baltimore. “All patients were treated with sofosbuvir/velpatasvir, with a plan to complete 12 weeks of treatment.” Medication was dispensed monthly in bottles containing 28 pills, and patients were seen for monthly visits, mirroring standard clinical care for HCV. The researchers monitored patients for medication adherence through pill counts and evaluated them for hepatitis C cure 12 weeks after treatment.
The median age of the 100 patients was 57 years, 76% were black, 33% had cirrhosis, 51% were unstably housed, 92% had a history of incarceration, and 92% had no income source or relied exclusively on government benefits. “The patients represent an incredibly marginalized population,” she said. At baseline, 58% reported daily or more frequent injection drug use, 33% reported medication-assisted treatment, 29% shared injection drug use equipment within the past 3 months, and 40% met criteria for hazardous drinking based on the Alcohol Use Disorders Identification Test (AUDIT-C).
Of the 100 patients, 59 received 12 weeks of treatment. Of these 59 patients, 28 finished 1-7 days after the anticipated end date, 9 finished between 8 and 14 days late, and 9 patients finished more than 14 days late.
Of the 58 patients who attended an office visit at week 24 of their treatment, 52 (90%) achieved a sustained virologic response. This cure rate was associated with having an HCV viral load less than 200 IU/mL at week 4, and with taking 12 weeks of treatment. Nonsustained virologic response was driven by virologic failure, loss to follow-up, and death.
When the researchers compared subjects who achieved sustained virologic response with those who did not, baseline demographics including frequent drug use, unstable housing status, and not being on medication to treat opioid use disorder were not associated with decreased cure rates. “While we found high rates of treatment completion in this population, because of external factors such as incarceration, hospitalization, and having medications stolen, 13 patients had interruptions in treatment,” Dr. Rosenthal said. “Further, while 21 patients had near-perfect medication adherence, 46 patients took longer than 12 weeks to complete the full treatment course due to intermittent missed doses. However, as long as patients completed the prescribed amount, imperfect adherence was not associated with decreased cure rates.”
Based on ANCHOR’s preliminary results, Dr. Rosenthal concluded that concerns about HCV treatment adherence such as baseline housing status, drug use frequency, and being on medication for opioid use disorder “are not likely to influence treatment outcome of HCV and should not be used to justify exclusion from treatment in this population. The ANCHOR investigation adds to the growing body of literature supporting expansion of HCV treatment to all patients, including people who inject drugs. Treatment of people who inject drugs is a critical factor in HCV elimination and, most importantly, reducing morbidity and mortality in this population.”
Dr. Rosenthal disclosed that she has received grant/research support from Gilead Sciences and from Merck.
Source: Rosenthal E et al. Hepatol. 2018;68[S1], Abstract 18.
SAN FRANCISCO – , preliminary results from an ongoing study showed.
“Both from a public health and a human rights perspective, hepatitis C elimination in people who inject drugs is critical,” study coauthor Elana Rosenthal, MD, said during a press briefing at the annual meeting of the American Association for the Study of Liver Diseases. “People who inject drugs are the main progenitors of ongoing transmission of hepatitis C. However, they are often denied access to hepatitis C treatment due to concerns about their ability to take medication consistently and achieve cure. This is especially true amongst patients with challenging demographic factors, frequent drug use, and those not on treatment for opioid use disorder. However, there are limited data on hepatitis C adherence in people who inject drugs outside of vigorous clinical trial settings.”
In an effort to understand whether a marginalized population with ongoing injection drug use could adhere to HCV treatment, and how this adherence would impact cure, Dr. Rosenthal and her associates enrolled 100 subjects in ANCHOR, a single-center study evaluating HCV treatment in patients who have chronic HCV, opioid use disorder, and ongoing injection drug use. “We did not preferentially enroll patients who we thought we would be most likely to cure, and we did not exclude patients who seemed unlikely to adhere to treatment,” said Dr. Rosenthal, codirector of the DC Partnership for HIV/AIDS Progress hepatitis clinical research program at the University of Maryland, Baltimore. “All patients were treated with sofosbuvir/velpatasvir, with a plan to complete 12 weeks of treatment.” Medication was dispensed monthly in bottles containing 28 pills, and patients were seen for monthly visits, mirroring standard clinical care for HCV. The researchers monitored patients for medication adherence through pill counts and evaluated them for hepatitis C cure 12 weeks after treatment.
The median age of the 100 patients was 57 years, 76% were black, 33% had cirrhosis, 51% were unstably housed, 92% had a history of incarceration, and 92% had no income source or relied exclusively on government benefits. “The patients represent an incredibly marginalized population,” she said. At baseline, 58% reported daily or more frequent injection drug use, 33% reported medication-assisted treatment, 29% shared injection drug use equipment within the past 3 months, and 40% met criteria for hazardous drinking based on the Alcohol Use Disorders Identification Test (AUDIT-C).
Of the 100 patients, 59 received 12 weeks of treatment. Of these 59 patients, 28 finished 1-7 days after the anticipated end date, 9 finished between 8 and 14 days late, and 9 patients finished more than 14 days late.
Of the 58 patients who attended an office visit at week 24 of their treatment, 52 (90%) achieved a sustained virologic response. This cure rate was associated with having an HCV viral load less than 200 IU/mL at week 4, and with taking 12 weeks of treatment. Nonsustained virologic response was driven by virologic failure, loss to follow-up, and death.
When the researchers compared subjects who achieved sustained virologic response with those who did not, baseline demographics including frequent drug use, unstable housing status, and not being on medication to treat opioid use disorder were not associated with decreased cure rates. “While we found high rates of treatment completion in this population, because of external factors such as incarceration, hospitalization, and having medications stolen, 13 patients had interruptions in treatment,” Dr. Rosenthal said. “Further, while 21 patients had near-perfect medication adherence, 46 patients took longer than 12 weeks to complete the full treatment course due to intermittent missed doses. However, as long as patients completed the prescribed amount, imperfect adherence was not associated with decreased cure rates.”
Based on ANCHOR’s preliminary results, Dr. Rosenthal concluded that concerns about HCV treatment adherence such as baseline housing status, drug use frequency, and being on medication for opioid use disorder “are not likely to influence treatment outcome of HCV and should not be used to justify exclusion from treatment in this population. The ANCHOR investigation adds to the growing body of literature supporting expansion of HCV treatment to all patients, including people who inject drugs. Treatment of people who inject drugs is a critical factor in HCV elimination and, most importantly, reducing morbidity and mortality in this population.”
Dr. Rosenthal disclosed that she has received grant/research support from Gilead Sciences and from Merck.
Source: Rosenthal E et al. Hepatol. 2018;68[S1], Abstract 18.
AT THE LIVER MEETING 2018
Key clinical point: People who inject drugs have high rates of HCV treatment adherence, treatment completion, and sustained virologic response.
Major finding: Of 58 patients who attended an office visit at week 24 of their treatment, 52 (90%) achieved a sustained virologic response.
Study details: Preliminary results from an ongoing, single-center study of 100 people with a median age of 57 years.
Disclosures: Dr. Rosenthal disclosed that she has received grant/research support from Gilead Sciences and from Merck.
Source: Rosenthal E et al. Hepatol. 2018;68[S1], Abstract 18.