User login
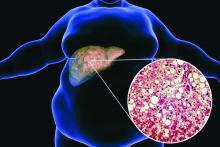
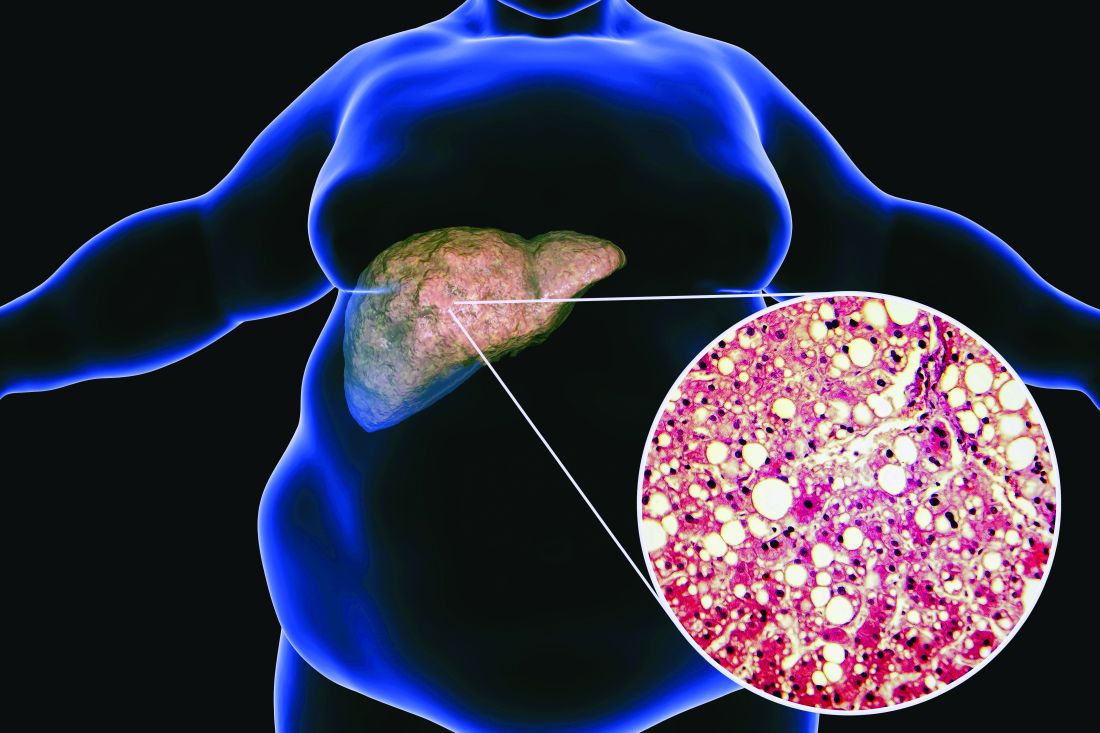
Low-dose aspirin cuts type 2 diabetes risk in over-65s
The data come from a secondary analysis of ASPREE, a double-blind, placebo-controlled trial of healthy adults aged 65 years or older, showing that 100 mg of aspirin taken daily for about 5 years did not provide a cardiovascular benefit but did significantly raise the risk for bleeding.
This new analysis shows that individuals taking aspirin had a 15% lower risk for developing type 2 diabetes and that the medication slowed the rate of increase in fasting plasma glucose, compared with placebo, during follow-up.
However, lead author Sophia Zoungas, MBBS, PhD, head of the School of Public Health and Preventive Medicine, Monash University, Melbourne, said: “Major prescribing guidelines now recommend older adults take daily aspirin only when there is a medical reason to do so, such as after a heart attack. ... Although these new findings are of interest, they do not change the clinical advice about aspirin use in older people at this time.”
Nonetheless, she said in an interview, “at this time, our findings are exploratory but ignite the debate of the important role that anti-inflammatory approaches may play in preventing diabetes. Further work is currently underway to understand which subpopulations may be better targeted and to understand the balance of risk versus benefit.”
The results are scheduled to be presented at the upcoming meeting of the European Association for the Study of Diabetes, taking place Oct. 2-6 in Hamburg, Germany.
New findings not robust enough to change current practice
Asked to comment, Debabrata Mukherjee, MD, said: “Given the post hoc secondary nature of the analysis, the findings should be considered hypothesis generating and not definitive… At this time, based on prospective randomized studies, the risks of aspirin outweigh the benefits for aspirin in older adults.”
Among those studies was an ASPREE substudy showing failure of low-dose aspirin to reduce fracture risk while increasing the risk for serious falls, and two other trials, ARRIVE and ASCEND, also showing that harms of aspirin outweigh the benefits in people with cardiovascular risk but not diabetes, and in those with diabetes, respectively, said Dr. Mukherjee, professor and chair of the department of internal medicine at Texas Tech University Health Sciences Center at El Paso.
And, Mukherjee noted, in 2019 the American College of Cardiology updated its practice guidelines to say that low-dose aspirin should not be administered on a routine basis for the primary prevention of atherosclerotic cardiovascular disease in adults over age 70. In 2021, the American Diabetes Association seconded that recommendation.
Asked whether these newest findings might change current practice for any higher-risk subgroup, such as people with prediabetes, Dr. Mukherjee replied: “Unless there is a prospective randomized trial that validates these findings in those with prediabetes, the findings should not change practice. There are also no data [showing] that another antiplatelet agent would be indicated or would be beneficial. Instead, I would recommend lifestyle changes including regular exercise and a healthy diet to minimize risk of diabetes.”
The 16,209 ASPREE participants were community dwelling and did not have diabetes, cardiovascular disease, or dementia at baseline. They were randomized in a 1:1 ratio to receive 100 mg/d of enteric-coated aspirin or placebo. Over a median follow-up of 4.7 years, the proportions developing type 2 diabetes were 5.7% with aspirin versus 6.6% with placebo (hazard ratio, 0.85; P = .01).
The annual rate of increase in fasting plasma glucose over the follow-up period was slowed by 0.006 mmol/L with aspirin, compared with placebo, also a significant difference (P = .004).
According to Dr. Zoungas, “the potential for anti-inflammatory agents like aspirin to prevent type 2 diabetes or improve glucose levels needs further study.”
The ASPREE trial was supported by the U.S. National Institutes of Health, the National Health and Medical Research Council of Australia, Monash University, and the Victorian Cancer Agency. Dr. Zoungas and Dr. Mukherjee have no disclosures.
A version of this article first appeared on Medscape.com.
The data come from a secondary analysis of ASPREE, a double-blind, placebo-controlled trial of healthy adults aged 65 years or older, showing that 100 mg of aspirin taken daily for about 5 years did not provide a cardiovascular benefit but did significantly raise the risk for bleeding.
This new analysis shows that individuals taking aspirin had a 15% lower risk for developing type 2 diabetes and that the medication slowed the rate of increase in fasting plasma glucose, compared with placebo, during follow-up.
However, lead author Sophia Zoungas, MBBS, PhD, head of the School of Public Health and Preventive Medicine, Monash University, Melbourne, said: “Major prescribing guidelines now recommend older adults take daily aspirin only when there is a medical reason to do so, such as after a heart attack. ... Although these new findings are of interest, they do not change the clinical advice about aspirin use in older people at this time.”
Nonetheless, she said in an interview, “at this time, our findings are exploratory but ignite the debate of the important role that anti-inflammatory approaches may play in preventing diabetes. Further work is currently underway to understand which subpopulations may be better targeted and to understand the balance of risk versus benefit.”
The results are scheduled to be presented at the upcoming meeting of the European Association for the Study of Diabetes, taking place Oct. 2-6 in Hamburg, Germany.
New findings not robust enough to change current practice
Asked to comment, Debabrata Mukherjee, MD, said: “Given the post hoc secondary nature of the analysis, the findings should be considered hypothesis generating and not definitive… At this time, based on prospective randomized studies, the risks of aspirin outweigh the benefits for aspirin in older adults.”
Among those studies was an ASPREE substudy showing failure of low-dose aspirin to reduce fracture risk while increasing the risk for serious falls, and two other trials, ARRIVE and ASCEND, also showing that harms of aspirin outweigh the benefits in people with cardiovascular risk but not diabetes, and in those with diabetes, respectively, said Dr. Mukherjee, professor and chair of the department of internal medicine at Texas Tech University Health Sciences Center at El Paso.
And, Mukherjee noted, in 2019 the American College of Cardiology updated its practice guidelines to say that low-dose aspirin should not be administered on a routine basis for the primary prevention of atherosclerotic cardiovascular disease in adults over age 70. In 2021, the American Diabetes Association seconded that recommendation.
Asked whether these newest findings might change current practice for any higher-risk subgroup, such as people with prediabetes, Dr. Mukherjee replied: “Unless there is a prospective randomized trial that validates these findings in those with prediabetes, the findings should not change practice. There are also no data [showing] that another antiplatelet agent would be indicated or would be beneficial. Instead, I would recommend lifestyle changes including regular exercise and a healthy diet to minimize risk of diabetes.”
The 16,209 ASPREE participants were community dwelling and did not have diabetes, cardiovascular disease, or dementia at baseline. They were randomized in a 1:1 ratio to receive 100 mg/d of enteric-coated aspirin or placebo. Over a median follow-up of 4.7 years, the proportions developing type 2 diabetes were 5.7% with aspirin versus 6.6% with placebo (hazard ratio, 0.85; P = .01).
The annual rate of increase in fasting plasma glucose over the follow-up period was slowed by 0.006 mmol/L with aspirin, compared with placebo, also a significant difference (P = .004).
According to Dr. Zoungas, “the potential for anti-inflammatory agents like aspirin to prevent type 2 diabetes or improve glucose levels needs further study.”
The ASPREE trial was supported by the U.S. National Institutes of Health, the National Health and Medical Research Council of Australia, Monash University, and the Victorian Cancer Agency. Dr. Zoungas and Dr. Mukherjee have no disclosures.
A version of this article first appeared on Medscape.com.
The data come from a secondary analysis of ASPREE, a double-blind, placebo-controlled trial of healthy adults aged 65 years or older, showing that 100 mg of aspirin taken daily for about 5 years did not provide a cardiovascular benefit but did significantly raise the risk for bleeding.
This new analysis shows that individuals taking aspirin had a 15% lower risk for developing type 2 diabetes and that the medication slowed the rate of increase in fasting plasma glucose, compared with placebo, during follow-up.
However, lead author Sophia Zoungas, MBBS, PhD, head of the School of Public Health and Preventive Medicine, Monash University, Melbourne, said: “Major prescribing guidelines now recommend older adults take daily aspirin only when there is a medical reason to do so, such as after a heart attack. ... Although these new findings are of interest, they do not change the clinical advice about aspirin use in older people at this time.”
Nonetheless, she said in an interview, “at this time, our findings are exploratory but ignite the debate of the important role that anti-inflammatory approaches may play in preventing diabetes. Further work is currently underway to understand which subpopulations may be better targeted and to understand the balance of risk versus benefit.”
The results are scheduled to be presented at the upcoming meeting of the European Association for the Study of Diabetes, taking place Oct. 2-6 in Hamburg, Germany.
New findings not robust enough to change current practice
Asked to comment, Debabrata Mukherjee, MD, said: “Given the post hoc secondary nature of the analysis, the findings should be considered hypothesis generating and not definitive… At this time, based on prospective randomized studies, the risks of aspirin outweigh the benefits for aspirin in older adults.”
Among those studies was an ASPREE substudy showing failure of low-dose aspirin to reduce fracture risk while increasing the risk for serious falls, and two other trials, ARRIVE and ASCEND, also showing that harms of aspirin outweigh the benefits in people with cardiovascular risk but not diabetes, and in those with diabetes, respectively, said Dr. Mukherjee, professor and chair of the department of internal medicine at Texas Tech University Health Sciences Center at El Paso.
And, Mukherjee noted, in 2019 the American College of Cardiology updated its practice guidelines to say that low-dose aspirin should not be administered on a routine basis for the primary prevention of atherosclerotic cardiovascular disease in adults over age 70. In 2021, the American Diabetes Association seconded that recommendation.
Asked whether these newest findings might change current practice for any higher-risk subgroup, such as people with prediabetes, Dr. Mukherjee replied: “Unless there is a prospective randomized trial that validates these findings in those with prediabetes, the findings should not change practice. There are also no data [showing] that another antiplatelet agent would be indicated or would be beneficial. Instead, I would recommend lifestyle changes including regular exercise and a healthy diet to minimize risk of diabetes.”
The 16,209 ASPREE participants were community dwelling and did not have diabetes, cardiovascular disease, or dementia at baseline. They were randomized in a 1:1 ratio to receive 100 mg/d of enteric-coated aspirin or placebo. Over a median follow-up of 4.7 years, the proportions developing type 2 diabetes were 5.7% with aspirin versus 6.6% with placebo (hazard ratio, 0.85; P = .01).
The annual rate of increase in fasting plasma glucose over the follow-up period was slowed by 0.006 mmol/L with aspirin, compared with placebo, also a significant difference (P = .004).
According to Dr. Zoungas, “the potential for anti-inflammatory agents like aspirin to prevent type 2 diabetes or improve glucose levels needs further study.”
The ASPREE trial was supported by the U.S. National Institutes of Health, the National Health and Medical Research Council of Australia, Monash University, and the Victorian Cancer Agency. Dr. Zoungas and Dr. Mukherjee have no disclosures.
A version of this article first appeared on Medscape.com.
FROM EASD 2023
Tirzepatide’s benefits expand: Lean mass up, serum lipids down
STOCKHOLM – New insights into the benefits of treatment with the “twincretin” tirzepatide for people with overweight or obesity – with or without diabetes – come from new findings reported at the annual meeting of the European Association for the Study of Diabetes.
Additional results from the SURMOUNT-1 trial, which matched tirzepatide against placebo in people with overweight or obesity, provide further details on the favorable changes produced by 72 weeks of tirzepatide treatment on outcomes that included fat and lean mass, insulin sensitivity, and patient-reported outcomes related to functional health and well being, reported Ania M. Jastreboff, MD, PhD.
And results from a meta-analysis of six trials that compared tirzepatide (Mounjaro) against several different comparators in patients with type 2 diabetes further confirm the drug’s ability to reliably produce positive changes in blood lipids, especially by significantly lowering levels of triglycerides, LDL cholesterol, and very LDL (VLDL) cholesterol, said Thomas Karagiannis, MD, PhD, in a separate report at the meeting.
Tirzepatide works as an agonist on receptors for both the glucagonlike peptide–1 (GLP-1), and for the glucose-dependent insulinotropic polypeptide, and received Food and Drug Administration approval for treating people with type 2 diabetes in May 2022. On the basis of results from SURMOUNT-1, the FDA on Oct. 6 granted tirzepatide fast-track designation for a proposed labeling of the agent for treating people with overweight or obesity. This FDA decision will likely remain pending at least until results from a second trial in people with overweight or obesity but without diabetes, SURMOUNT-2, become available in 2023.
SURMOUNT-1 randomized 2,539 people with obesity or overweight and at least one weight-related complication to a weekly injection of tirzepatide or placebo for 72 weeks. The study’s primary efficacy endpoints were the average reduction in weight from baseline, and the percentage of people in each treatment arm achieving weight loss of at least 5% from baseline.
For both endpoints, the outcomes with tirzepatide significantly surpassed placebo effects. Average weight loss ranged from 15%-21% from baseline, depending on dose, compared with 3% on placebo. The rate of participants with at least a 5% weight loss ranged from 85% to 91%, compared with 35% with placebo, as reported in July 2022 in the New England Journal of Medicine.
Cutting fat mass, boosting lean mass
New results from the trial reported by Dr. Jastreboff included a cut in fat mass from 46.2% of total body mass at baseline to 38.5% after 72 weeks, compared with a change from 46.8% at baseline to 44.7% after 72 weeks in the placebo group. Concurrently, lean mass increased with tirzepatide treatment from 51.0% at baseline to 58.1% after 72 weeks.
Participants who received tirzepatide, compared with those who received placebo, had “proportionately greater decrease in fat mass and proportionately greater increase in lean mass” compared with those who received placebo, said Dr. Jastreboff, an endocrinologist and obesity medicine specialist with Yale Medicine in New Haven, Conn. “I was impressed by the amount of visceral fat lost.”
These effects translated into a significant reduction in fat mass-to-lean mass ratio among the people treated with tirzepatide, with the greatest reduction in those who lost at least 15% of their starting weight. In that subgroup the fat-to-lean mass ratio dropped from 0.94 at baseline to 0.64 after 72 weeks of treatment, she said.
Focus on diet quality
People treated with tirzepatide “eat so little food that we need to improve the quality of what they eat to protect their muscle,” commented Carel le Roux, MBChB, PhD, a professor in the Diabetes Complications Research Centre of University College Dublin. “You no longer need a dietitian to help people lose weight, because the drug does that. You need dietitians to look after the nutritional health of patients while they lose weight,” Dr. le Roux said in a separate session at the meeting.
Additional tests showed that blood glucose and insulin levels were all significantly lower among trial participants on all three doses of tirzepatide compared with those on placebo, and the tirzepatide-treated subjects also had significant, roughly twofold elevations in their insulin sensitivity measured by the Matsuda Index.
The impact of tirzepatide on glucose and insulin levels and on insulin sensitivity was similar regardless of whether study participants had normoglycemia or prediabetes at entry. By design, no study participants had diabetes.
The trial assessed patient-reported quality-of-life outcomes using the 36-Item Short Form Survey (SF-36). Participants had significant increases in all eight domains within the SF-36 at all three tirzepatide doses, compared with placebo, at 72 weeks, Dr. Jastreboff reported. Improvements in the physical function domain increased most notably among study participants on tirzepatide who had functional limitations at baseline. Heart rate rose among participants who received either of the two highest tirzepatide doses by 2.3-2.5 beats/min, comparable with the effect of other injected incretin-based treatments.
Lipids improve in those with type 2 diabetes
Tirzepatide treatment also results in a “secondary effect” of improving levels of several lipids in people with type 2 diabetes, according to a meta-analysis of findings from six randomized trials. The meta-analysis collectively involved 4,502 participants treated for numerous weeks with one of three doses of tirzepatide and 2,144 people in comparator groups, reported Dr. Karagiannis, a diabetes researcher at Aristotle University of Thessaloniki (Greece).
Among the significant lipid changes linked with tirzepatide treatment, compared with placebo, were an average 13 mg/dL decrease in LDL cholesterol, an average 6 mg/dL decrease in VLDL cholesterol, and an average 50 mg/dL decrease in triglycerides. In comparison to a GLP-1 receptor agonist, an average 25 mg/dL decrease in triglycerides and an average 4 mg/dL reduction in VLDL cholesterol were seen. And trials comparing tirzepatide with basal insulin saw average reductions of 7% in LDL cholesterol, 15% in VLDL cholesterol, 15% in triglycerides, and an 8% increase in HDL cholesterol.
Dr. Karagiannis highlighted that the clinical impact of these effects is unclear, although he noted that the average reduction in LDL cholesterol relative to placebo is of a magnitude that could have a modest effect on long-term outcomes.
These lipid effects of tirzepatide “should be considered alongside” tirzepatide’s “key metabolic effects” on weight and hemoglobin A1c as well as the drug’s safety, concluded Dr. Karagiannis.
The tirzepatide trials were all funded by Eli Lilly, which markets tirzepatide (Mounjaro). Dr. Jastreboff has been an adviser and consultant to Eli Lilly, as well as to Intellihealth, Novo Nordisk, Pfizer, Rhythm Scholars, Roche, and Weight Watchers, and she has received research funding from Eli Lilly and Novo Nordisk. Dr. Karagiannis had no disclosures. Dr. le Roux has had financial relationships with Eli Lilly, as well as with Boehringer Ingelheim, Consilient Health, Covidion, Fractyl, GL Dynamics, Herbalife, Johnson & Johnson, Keyron, and Novo Nordisk.
STOCKHOLM – New insights into the benefits of treatment with the “twincretin” tirzepatide for people with overweight or obesity – with or without diabetes – come from new findings reported at the annual meeting of the European Association for the Study of Diabetes.
Additional results from the SURMOUNT-1 trial, which matched tirzepatide against placebo in people with overweight or obesity, provide further details on the favorable changes produced by 72 weeks of tirzepatide treatment on outcomes that included fat and lean mass, insulin sensitivity, and patient-reported outcomes related to functional health and well being, reported Ania M. Jastreboff, MD, PhD.
And results from a meta-analysis of six trials that compared tirzepatide (Mounjaro) against several different comparators in patients with type 2 diabetes further confirm the drug’s ability to reliably produce positive changes in blood lipids, especially by significantly lowering levels of triglycerides, LDL cholesterol, and very LDL (VLDL) cholesterol, said Thomas Karagiannis, MD, PhD, in a separate report at the meeting.
Tirzepatide works as an agonist on receptors for both the glucagonlike peptide–1 (GLP-1), and for the glucose-dependent insulinotropic polypeptide, and received Food and Drug Administration approval for treating people with type 2 diabetes in May 2022. On the basis of results from SURMOUNT-1, the FDA on Oct. 6 granted tirzepatide fast-track designation for a proposed labeling of the agent for treating people with overweight or obesity. This FDA decision will likely remain pending at least until results from a second trial in people with overweight or obesity but without diabetes, SURMOUNT-2, become available in 2023.
SURMOUNT-1 randomized 2,539 people with obesity or overweight and at least one weight-related complication to a weekly injection of tirzepatide or placebo for 72 weeks. The study’s primary efficacy endpoints were the average reduction in weight from baseline, and the percentage of people in each treatment arm achieving weight loss of at least 5% from baseline.
For both endpoints, the outcomes with tirzepatide significantly surpassed placebo effects. Average weight loss ranged from 15%-21% from baseline, depending on dose, compared with 3% on placebo. The rate of participants with at least a 5% weight loss ranged from 85% to 91%, compared with 35% with placebo, as reported in July 2022 in the New England Journal of Medicine.
Cutting fat mass, boosting lean mass
New results from the trial reported by Dr. Jastreboff included a cut in fat mass from 46.2% of total body mass at baseline to 38.5% after 72 weeks, compared with a change from 46.8% at baseline to 44.7% after 72 weeks in the placebo group. Concurrently, lean mass increased with tirzepatide treatment from 51.0% at baseline to 58.1% after 72 weeks.
Participants who received tirzepatide, compared with those who received placebo, had “proportionately greater decrease in fat mass and proportionately greater increase in lean mass” compared with those who received placebo, said Dr. Jastreboff, an endocrinologist and obesity medicine specialist with Yale Medicine in New Haven, Conn. “I was impressed by the amount of visceral fat lost.”
These effects translated into a significant reduction in fat mass-to-lean mass ratio among the people treated with tirzepatide, with the greatest reduction in those who lost at least 15% of their starting weight. In that subgroup the fat-to-lean mass ratio dropped from 0.94 at baseline to 0.64 after 72 weeks of treatment, she said.
Focus on diet quality
People treated with tirzepatide “eat so little food that we need to improve the quality of what they eat to protect their muscle,” commented Carel le Roux, MBChB, PhD, a professor in the Diabetes Complications Research Centre of University College Dublin. “You no longer need a dietitian to help people lose weight, because the drug does that. You need dietitians to look after the nutritional health of patients while they lose weight,” Dr. le Roux said in a separate session at the meeting.
Additional tests showed that blood glucose and insulin levels were all significantly lower among trial participants on all three doses of tirzepatide compared with those on placebo, and the tirzepatide-treated subjects also had significant, roughly twofold elevations in their insulin sensitivity measured by the Matsuda Index.
The impact of tirzepatide on glucose and insulin levels and on insulin sensitivity was similar regardless of whether study participants had normoglycemia or prediabetes at entry. By design, no study participants had diabetes.
The trial assessed patient-reported quality-of-life outcomes using the 36-Item Short Form Survey (SF-36). Participants had significant increases in all eight domains within the SF-36 at all three tirzepatide doses, compared with placebo, at 72 weeks, Dr. Jastreboff reported. Improvements in the physical function domain increased most notably among study participants on tirzepatide who had functional limitations at baseline. Heart rate rose among participants who received either of the two highest tirzepatide doses by 2.3-2.5 beats/min, comparable with the effect of other injected incretin-based treatments.
Lipids improve in those with type 2 diabetes
Tirzepatide treatment also results in a “secondary effect” of improving levels of several lipids in people with type 2 diabetes, according to a meta-analysis of findings from six randomized trials. The meta-analysis collectively involved 4,502 participants treated for numerous weeks with one of three doses of tirzepatide and 2,144 people in comparator groups, reported Dr. Karagiannis, a diabetes researcher at Aristotle University of Thessaloniki (Greece).
Among the significant lipid changes linked with tirzepatide treatment, compared with placebo, were an average 13 mg/dL decrease in LDL cholesterol, an average 6 mg/dL decrease in VLDL cholesterol, and an average 50 mg/dL decrease in triglycerides. In comparison to a GLP-1 receptor agonist, an average 25 mg/dL decrease in triglycerides and an average 4 mg/dL reduction in VLDL cholesterol were seen. And trials comparing tirzepatide with basal insulin saw average reductions of 7% in LDL cholesterol, 15% in VLDL cholesterol, 15% in triglycerides, and an 8% increase in HDL cholesterol.
Dr. Karagiannis highlighted that the clinical impact of these effects is unclear, although he noted that the average reduction in LDL cholesterol relative to placebo is of a magnitude that could have a modest effect on long-term outcomes.
These lipid effects of tirzepatide “should be considered alongside” tirzepatide’s “key metabolic effects” on weight and hemoglobin A1c as well as the drug’s safety, concluded Dr. Karagiannis.
The tirzepatide trials were all funded by Eli Lilly, which markets tirzepatide (Mounjaro). Dr. Jastreboff has been an adviser and consultant to Eli Lilly, as well as to Intellihealth, Novo Nordisk, Pfizer, Rhythm Scholars, Roche, and Weight Watchers, and she has received research funding from Eli Lilly and Novo Nordisk. Dr. Karagiannis had no disclosures. Dr. le Roux has had financial relationships with Eli Lilly, as well as with Boehringer Ingelheim, Consilient Health, Covidion, Fractyl, GL Dynamics, Herbalife, Johnson & Johnson, Keyron, and Novo Nordisk.
STOCKHOLM – New insights into the benefits of treatment with the “twincretin” tirzepatide for people with overweight or obesity – with or without diabetes – come from new findings reported at the annual meeting of the European Association for the Study of Diabetes.
Additional results from the SURMOUNT-1 trial, which matched tirzepatide against placebo in people with overweight or obesity, provide further details on the favorable changes produced by 72 weeks of tirzepatide treatment on outcomes that included fat and lean mass, insulin sensitivity, and patient-reported outcomes related to functional health and well being, reported Ania M. Jastreboff, MD, PhD.
And results from a meta-analysis of six trials that compared tirzepatide (Mounjaro) against several different comparators in patients with type 2 diabetes further confirm the drug’s ability to reliably produce positive changes in blood lipids, especially by significantly lowering levels of triglycerides, LDL cholesterol, and very LDL (VLDL) cholesterol, said Thomas Karagiannis, MD, PhD, in a separate report at the meeting.
Tirzepatide works as an agonist on receptors for both the glucagonlike peptide–1 (GLP-1), and for the glucose-dependent insulinotropic polypeptide, and received Food and Drug Administration approval for treating people with type 2 diabetes in May 2022. On the basis of results from SURMOUNT-1, the FDA on Oct. 6 granted tirzepatide fast-track designation for a proposed labeling of the agent for treating people with overweight or obesity. This FDA decision will likely remain pending at least until results from a second trial in people with overweight or obesity but without diabetes, SURMOUNT-2, become available in 2023.
SURMOUNT-1 randomized 2,539 people with obesity or overweight and at least one weight-related complication to a weekly injection of tirzepatide or placebo for 72 weeks. The study’s primary efficacy endpoints were the average reduction in weight from baseline, and the percentage of people in each treatment arm achieving weight loss of at least 5% from baseline.
For both endpoints, the outcomes with tirzepatide significantly surpassed placebo effects. Average weight loss ranged from 15%-21% from baseline, depending on dose, compared with 3% on placebo. The rate of participants with at least a 5% weight loss ranged from 85% to 91%, compared with 35% with placebo, as reported in July 2022 in the New England Journal of Medicine.
Cutting fat mass, boosting lean mass
New results from the trial reported by Dr. Jastreboff included a cut in fat mass from 46.2% of total body mass at baseline to 38.5% after 72 weeks, compared with a change from 46.8% at baseline to 44.7% after 72 weeks in the placebo group. Concurrently, lean mass increased with tirzepatide treatment from 51.0% at baseline to 58.1% after 72 weeks.
Participants who received tirzepatide, compared with those who received placebo, had “proportionately greater decrease in fat mass and proportionately greater increase in lean mass” compared with those who received placebo, said Dr. Jastreboff, an endocrinologist and obesity medicine specialist with Yale Medicine in New Haven, Conn. “I was impressed by the amount of visceral fat lost.”
These effects translated into a significant reduction in fat mass-to-lean mass ratio among the people treated with tirzepatide, with the greatest reduction in those who lost at least 15% of their starting weight. In that subgroup the fat-to-lean mass ratio dropped from 0.94 at baseline to 0.64 after 72 weeks of treatment, she said.
Focus on diet quality
People treated with tirzepatide “eat so little food that we need to improve the quality of what they eat to protect their muscle,” commented Carel le Roux, MBChB, PhD, a professor in the Diabetes Complications Research Centre of University College Dublin. “You no longer need a dietitian to help people lose weight, because the drug does that. You need dietitians to look after the nutritional health of patients while they lose weight,” Dr. le Roux said in a separate session at the meeting.
Additional tests showed that blood glucose and insulin levels were all significantly lower among trial participants on all three doses of tirzepatide compared with those on placebo, and the tirzepatide-treated subjects also had significant, roughly twofold elevations in their insulin sensitivity measured by the Matsuda Index.
The impact of tirzepatide on glucose and insulin levels and on insulin sensitivity was similar regardless of whether study participants had normoglycemia or prediabetes at entry. By design, no study participants had diabetes.
The trial assessed patient-reported quality-of-life outcomes using the 36-Item Short Form Survey (SF-36). Participants had significant increases in all eight domains within the SF-36 at all three tirzepatide doses, compared with placebo, at 72 weeks, Dr. Jastreboff reported. Improvements in the physical function domain increased most notably among study participants on tirzepatide who had functional limitations at baseline. Heart rate rose among participants who received either of the two highest tirzepatide doses by 2.3-2.5 beats/min, comparable with the effect of other injected incretin-based treatments.
Lipids improve in those with type 2 diabetes
Tirzepatide treatment also results in a “secondary effect” of improving levels of several lipids in people with type 2 diabetes, according to a meta-analysis of findings from six randomized trials. The meta-analysis collectively involved 4,502 participants treated for numerous weeks with one of three doses of tirzepatide and 2,144 people in comparator groups, reported Dr. Karagiannis, a diabetes researcher at Aristotle University of Thessaloniki (Greece).
Among the significant lipid changes linked with tirzepatide treatment, compared with placebo, were an average 13 mg/dL decrease in LDL cholesterol, an average 6 mg/dL decrease in VLDL cholesterol, and an average 50 mg/dL decrease in triglycerides. In comparison to a GLP-1 receptor agonist, an average 25 mg/dL decrease in triglycerides and an average 4 mg/dL reduction in VLDL cholesterol were seen. And trials comparing tirzepatide with basal insulin saw average reductions of 7% in LDL cholesterol, 15% in VLDL cholesterol, 15% in triglycerides, and an 8% increase in HDL cholesterol.
Dr. Karagiannis highlighted that the clinical impact of these effects is unclear, although he noted that the average reduction in LDL cholesterol relative to placebo is of a magnitude that could have a modest effect on long-term outcomes.
These lipid effects of tirzepatide “should be considered alongside” tirzepatide’s “key metabolic effects” on weight and hemoglobin A1c as well as the drug’s safety, concluded Dr. Karagiannis.
The tirzepatide trials were all funded by Eli Lilly, which markets tirzepatide (Mounjaro). Dr. Jastreboff has been an adviser and consultant to Eli Lilly, as well as to Intellihealth, Novo Nordisk, Pfizer, Rhythm Scholars, Roche, and Weight Watchers, and she has received research funding from Eli Lilly and Novo Nordisk. Dr. Karagiannis had no disclosures. Dr. le Roux has had financial relationships with Eli Lilly, as well as with Boehringer Ingelheim, Consilient Health, Covidion, Fractyl, GL Dynamics, Herbalife, Johnson & Johnson, Keyron, and Novo Nordisk.
AT EASD 2022
Strong link found between enterovirus and type 1 diabetes
STOCKHOLM – Enterovirus infection appears to be strongly linked to both type 1 diabetes and islet cell autoantibodies, new research suggests.
The strength of the relationship, particularly within the first month of type 1 diabetes diagnosis, “further supports the rationale for development of enterovirus-targeted vaccines and antiviral therapy to prevent and reduce the impact of type 1 diabetes,” according to lead investigator Sonia Isaacs, MD, of the department of pediatrics and child health at the University of New South Wales, Sydney, Australia.
Enteroviruses are a large family of viruses responsible for many infections in children. These live in the intestinal tract but can cause a wide variety of illnesses. There are more than 70 different strains, which include the group A and group B coxsackieviruses, the polioviruses, hepatitis A virus, and several strains that just go by the name enterovirus.
Dr. Isaacs presented the data, from a meta-analysis of studies using modern molecular techniques, at the annual meeting of the European Association for the Study of Diabetes.
The findings raise the question of whether people should be routinely tested for enterovirus at the time of type 1 diabetes diagnosis, she said during her presentation.
Asked by this news organization about the implications for first-degree relatives of people with type 1 diabetes, Dr. Isaacs said that they are “definitely a population to watch out for,” with regard to enteroviral infections. “Type 1 diabetes is very diverse and has different endotypes. Different environmental factors may be implicated in these different endotypes, and it may be that the enteroviruses are quite important in the first-degree relative group.”
Asked to comment, session moderator Kamlesh Khunti, MD, PhD, told this news organization that the data were “compelling,” particularly in the short term after type 1 diabetes diagnosis. “It seems that there may be plausibility for enterovirus associated with the development of type 1 diabetes ... Are there methods by which we can reduce this risk with either antivirals or vaccinations? I think that needs to be tested.”
And in regard to first-degree relatives, “I think that’s the group to go for because the association is so highly correlated. I think that’s the group worth testing with any interventions,” said Dr. Khunti, professor of primary care diabetes and vascular medicine at the University of Leicester, England.
Link stronger a month after diagnosis, in close relatives, in Europe
The new meta-analysis is an update to a prior review published in 2011 by Dr. Isaacs’ group, which found that people with islet cell autoimmunity were more than four times as likely as were controls to have an enterovirus infection, and people with type 1 diabetes were almost 10 times as likely.
This new analysis focuses on studies using more modern molecular techniques for detecting viruses, including high throughput sequencing and single-cell technologies.
The analysis identified 60 studies with a total of 12,077 participants, of whom 900 had islet autoimmunity, 5,081 had type 1 diabetes, and 6,096 were controls. Thirty-five of the studies were from Europe, while others were from the United States, Asia, and the Middle East.
Of 16 studies examining enterovirus infection in islet autoimmunity, cases with islet autoimmunity were twice as likely to have an enterovirus infection at any time point compared to controls, a significant difference (odds ratio [OR], 2.07, P = .002.)
Among 48 studies reporting enterovirus infection in type 1 diabetes, those with type 1 diabetes were eight times as likely to have an enterovirus infection compared with controls (OR, 8.0, P < .00001).
In 25 studies including 2,977 participants with onset of type 1 diabetes within the prior month, those individuals were more than 16 times more likely to present with an enterovirus infection (OR, 16.2, P < .00001).
“The strength of this is association is greater than previously reported by both us and others,” Dr. Isaacs noted.
The association between enterovirus infection and islet autoimmunity was greater in individuals who later progressed to type 1 diabetes, with odds ratio 5.1 vs. 2.0 for those who didn’t. The association was most evident at or shortly after seroconversion (5.1), was stronger in Europe (3.2) than in other regions (1.9), and was stronger among those with a first-degree relative with type 1 diabetes (9.8) than those recruited via a high-risk human leukocyte antigen (HLA), in whom the relationship wasn’t significant.
Having multiple or consecutive enteroviral infections was also associated with islet autoimmunity (2.0).
With type 1 diabetes, the relationship with enterovirus was greater in children (9.0) than in adults (4.1), and was greater for type 1 diabetes onset within 1 year (13.8) and within 1 month (16.2) than for those with established type 1 diabetes (7.0). Here, too, the relationship was stronger in Europe (10.2) than outside Europe (7.5).
The link with type 1 diabetes and enterovirus was particularly strong for those with both a first-degree relative and a high-risk HLA (141.4).
The relationship with type 1 diabetes was significant for enterovirus species A (3.7), B (12.7) and C (13.8), including coxsackie virus genotypes, but not D.
“Future studies should focus on characterizing enterovirus genomes in at-risk cohorts rather than just the presence or absence of the virus,” Dr. Isaacs said.
However, she added, “type 1 diabetes is such a heterogenous condition, viruses may be implicated more in one type than another. It’s important that we start to look into this.”
Dr. Isaacs reports no relevant financial relationships. Dr. Khunti disclosed ties with AstraZeneca, Novartis, Novo Nordisk, Sanofi-Aventis, Lilly, Merck Sharp & Dohme, Boehringer Ingelheim, Bayer, Berlin-Chemie AG / Menarini Group, Janssen, and Napp.
A version of this article first appeared on Medscape.com.
STOCKHOLM – Enterovirus infection appears to be strongly linked to both type 1 diabetes and islet cell autoantibodies, new research suggests.
The strength of the relationship, particularly within the first month of type 1 diabetes diagnosis, “further supports the rationale for development of enterovirus-targeted vaccines and antiviral therapy to prevent and reduce the impact of type 1 diabetes,” according to lead investigator Sonia Isaacs, MD, of the department of pediatrics and child health at the University of New South Wales, Sydney, Australia.
Enteroviruses are a large family of viruses responsible for many infections in children. These live in the intestinal tract but can cause a wide variety of illnesses. There are more than 70 different strains, which include the group A and group B coxsackieviruses, the polioviruses, hepatitis A virus, and several strains that just go by the name enterovirus.
Dr. Isaacs presented the data, from a meta-analysis of studies using modern molecular techniques, at the annual meeting of the European Association for the Study of Diabetes.
The findings raise the question of whether people should be routinely tested for enterovirus at the time of type 1 diabetes diagnosis, she said during her presentation.
Asked by this news organization about the implications for first-degree relatives of people with type 1 diabetes, Dr. Isaacs said that they are “definitely a population to watch out for,” with regard to enteroviral infections. “Type 1 diabetes is very diverse and has different endotypes. Different environmental factors may be implicated in these different endotypes, and it may be that the enteroviruses are quite important in the first-degree relative group.”
Asked to comment, session moderator Kamlesh Khunti, MD, PhD, told this news organization that the data were “compelling,” particularly in the short term after type 1 diabetes diagnosis. “It seems that there may be plausibility for enterovirus associated with the development of type 1 diabetes ... Are there methods by which we can reduce this risk with either antivirals or vaccinations? I think that needs to be tested.”
And in regard to first-degree relatives, “I think that’s the group to go for because the association is so highly correlated. I think that’s the group worth testing with any interventions,” said Dr. Khunti, professor of primary care diabetes and vascular medicine at the University of Leicester, England.
Link stronger a month after diagnosis, in close relatives, in Europe
The new meta-analysis is an update to a prior review published in 2011 by Dr. Isaacs’ group, which found that people with islet cell autoimmunity were more than four times as likely as were controls to have an enterovirus infection, and people with type 1 diabetes were almost 10 times as likely.
This new analysis focuses on studies using more modern molecular techniques for detecting viruses, including high throughput sequencing and single-cell technologies.
The analysis identified 60 studies with a total of 12,077 participants, of whom 900 had islet autoimmunity, 5,081 had type 1 diabetes, and 6,096 were controls. Thirty-five of the studies were from Europe, while others were from the United States, Asia, and the Middle East.
Of 16 studies examining enterovirus infection in islet autoimmunity, cases with islet autoimmunity were twice as likely to have an enterovirus infection at any time point compared to controls, a significant difference (odds ratio [OR], 2.07, P = .002.)
Among 48 studies reporting enterovirus infection in type 1 diabetes, those with type 1 diabetes were eight times as likely to have an enterovirus infection compared with controls (OR, 8.0, P < .00001).
In 25 studies including 2,977 participants with onset of type 1 diabetes within the prior month, those individuals were more than 16 times more likely to present with an enterovirus infection (OR, 16.2, P < .00001).
“The strength of this is association is greater than previously reported by both us and others,” Dr. Isaacs noted.
The association between enterovirus infection and islet autoimmunity was greater in individuals who later progressed to type 1 diabetes, with odds ratio 5.1 vs. 2.0 for those who didn’t. The association was most evident at or shortly after seroconversion (5.1), was stronger in Europe (3.2) than in other regions (1.9), and was stronger among those with a first-degree relative with type 1 diabetes (9.8) than those recruited via a high-risk human leukocyte antigen (HLA), in whom the relationship wasn’t significant.
Having multiple or consecutive enteroviral infections was also associated with islet autoimmunity (2.0).
With type 1 diabetes, the relationship with enterovirus was greater in children (9.0) than in adults (4.1), and was greater for type 1 diabetes onset within 1 year (13.8) and within 1 month (16.2) than for those with established type 1 diabetes (7.0). Here, too, the relationship was stronger in Europe (10.2) than outside Europe (7.5).
The link with type 1 diabetes and enterovirus was particularly strong for those with both a first-degree relative and a high-risk HLA (141.4).
The relationship with type 1 diabetes was significant for enterovirus species A (3.7), B (12.7) and C (13.8), including coxsackie virus genotypes, but not D.
“Future studies should focus on characterizing enterovirus genomes in at-risk cohorts rather than just the presence or absence of the virus,” Dr. Isaacs said.
However, she added, “type 1 diabetes is such a heterogenous condition, viruses may be implicated more in one type than another. It’s important that we start to look into this.”
Dr. Isaacs reports no relevant financial relationships. Dr. Khunti disclosed ties with AstraZeneca, Novartis, Novo Nordisk, Sanofi-Aventis, Lilly, Merck Sharp & Dohme, Boehringer Ingelheim, Bayer, Berlin-Chemie AG / Menarini Group, Janssen, and Napp.
A version of this article first appeared on Medscape.com.
STOCKHOLM – Enterovirus infection appears to be strongly linked to both type 1 diabetes and islet cell autoantibodies, new research suggests.
The strength of the relationship, particularly within the first month of type 1 diabetes diagnosis, “further supports the rationale for development of enterovirus-targeted vaccines and antiviral therapy to prevent and reduce the impact of type 1 diabetes,” according to lead investigator Sonia Isaacs, MD, of the department of pediatrics and child health at the University of New South Wales, Sydney, Australia.
Enteroviruses are a large family of viruses responsible for many infections in children. These live in the intestinal tract but can cause a wide variety of illnesses. There are more than 70 different strains, which include the group A and group B coxsackieviruses, the polioviruses, hepatitis A virus, and several strains that just go by the name enterovirus.
Dr. Isaacs presented the data, from a meta-analysis of studies using modern molecular techniques, at the annual meeting of the European Association for the Study of Diabetes.
The findings raise the question of whether people should be routinely tested for enterovirus at the time of type 1 diabetes diagnosis, she said during her presentation.
Asked by this news organization about the implications for first-degree relatives of people with type 1 diabetes, Dr. Isaacs said that they are “definitely a population to watch out for,” with regard to enteroviral infections. “Type 1 diabetes is very diverse and has different endotypes. Different environmental factors may be implicated in these different endotypes, and it may be that the enteroviruses are quite important in the first-degree relative group.”
Asked to comment, session moderator Kamlesh Khunti, MD, PhD, told this news organization that the data were “compelling,” particularly in the short term after type 1 diabetes diagnosis. “It seems that there may be plausibility for enterovirus associated with the development of type 1 diabetes ... Are there methods by which we can reduce this risk with either antivirals or vaccinations? I think that needs to be tested.”
And in regard to first-degree relatives, “I think that’s the group to go for because the association is so highly correlated. I think that’s the group worth testing with any interventions,” said Dr. Khunti, professor of primary care diabetes and vascular medicine at the University of Leicester, England.
Link stronger a month after diagnosis, in close relatives, in Europe
The new meta-analysis is an update to a prior review published in 2011 by Dr. Isaacs’ group, which found that people with islet cell autoimmunity were more than four times as likely as were controls to have an enterovirus infection, and people with type 1 diabetes were almost 10 times as likely.
This new analysis focuses on studies using more modern molecular techniques for detecting viruses, including high throughput sequencing and single-cell technologies.
The analysis identified 60 studies with a total of 12,077 participants, of whom 900 had islet autoimmunity, 5,081 had type 1 diabetes, and 6,096 were controls. Thirty-five of the studies were from Europe, while others were from the United States, Asia, and the Middle East.
Of 16 studies examining enterovirus infection in islet autoimmunity, cases with islet autoimmunity were twice as likely to have an enterovirus infection at any time point compared to controls, a significant difference (odds ratio [OR], 2.07, P = .002.)
Among 48 studies reporting enterovirus infection in type 1 diabetes, those with type 1 diabetes were eight times as likely to have an enterovirus infection compared with controls (OR, 8.0, P < .00001).
In 25 studies including 2,977 participants with onset of type 1 diabetes within the prior month, those individuals were more than 16 times more likely to present with an enterovirus infection (OR, 16.2, P < .00001).
“The strength of this is association is greater than previously reported by both us and others,” Dr. Isaacs noted.
The association between enterovirus infection and islet autoimmunity was greater in individuals who later progressed to type 1 diabetes, with odds ratio 5.1 vs. 2.0 for those who didn’t. The association was most evident at or shortly after seroconversion (5.1), was stronger in Europe (3.2) than in other regions (1.9), and was stronger among those with a first-degree relative with type 1 diabetes (9.8) than those recruited via a high-risk human leukocyte antigen (HLA), in whom the relationship wasn’t significant.
Having multiple or consecutive enteroviral infections was also associated with islet autoimmunity (2.0).
With type 1 diabetes, the relationship with enterovirus was greater in children (9.0) than in adults (4.1), and was greater for type 1 diabetes onset within 1 year (13.8) and within 1 month (16.2) than for those with established type 1 diabetes (7.0). Here, too, the relationship was stronger in Europe (10.2) than outside Europe (7.5).
The link with type 1 diabetes and enterovirus was particularly strong for those with both a first-degree relative and a high-risk HLA (141.4).
The relationship with type 1 diabetes was significant for enterovirus species A (3.7), B (12.7) and C (13.8), including coxsackie virus genotypes, but not D.
“Future studies should focus on characterizing enterovirus genomes in at-risk cohorts rather than just the presence or absence of the virus,” Dr. Isaacs said.
However, she added, “type 1 diabetes is such a heterogenous condition, viruses may be implicated more in one type than another. It’s important that we start to look into this.”
Dr. Isaacs reports no relevant financial relationships. Dr. Khunti disclosed ties with AstraZeneca, Novartis, Novo Nordisk, Sanofi-Aventis, Lilly, Merck Sharp & Dohme, Boehringer Ingelheim, Bayer, Berlin-Chemie AG / Menarini Group, Janssen, and Napp.
A version of this article first appeared on Medscape.com.
AT EASD 2022
How to improve diagnosis of HFpEF, common in diabetes
STOCKHOLM – Recent study results confirm that two agents from the sodium-glucose cotransporter 2 (SGLT2) inhibitor class can significantly cut the incidence of adverse cardiovascular events in patients with heart failure with reduced ejection fraction (HFpEF), a disease especially common in people with type 2 diabetes, obesity, or both.
And findings from secondary analyses of the studies – including one reported at the annual meeting of the European Association for the Study of Diabetes – show that these SGLT2 inhibitors work as well for cutting incident adverse events (cardiovascular death or worsening heart failure) in patients with HFpEF and diabetes as they do for people with normal blood glucose levels.
But delivering treatment with these proven agents, dapagliflozin (Farxiga) and empagliflozin (Jardiance), first requires diagnosis of HFpEF, a task that clinicians have historically fallen short in accomplishing.
When in 2021, results from the EMPEROR-Preserved trial with empagliflozin and when in September 2022 results from the DELIVER trial with dapagliflozin established the efficacy of these two SGLT2 inhibitors as the first treatments proven to benefit patients with HFpEF, they also raised the stakes for clinicians to be much more diligent and systematic in evaluating people at high risk for developing HFpEF because of having type 2 diabetes or obesity, two of the most potent risk factors for this form of heart failure.
‘Vigilance ... needs to increase’
“Vigilance for HFpEF needs to increase because we can now help these patients,” declared Lars H. Lund, MD, PhD, speaking at the meeting. “Type 2 diabetes dramatically increases the incidence of HFpEF,” and the mechanisms by which it does this are “especially amenable to treatment with SGLT2 inhibitors,” said Dr. Lund, a cardiologist and heart failure specialist at the Karolinska Institute, Stockholm.
HFpEF has a history of going undetected in people with type 2 diabetes, an ironic situation given its high incidence as well as the elevated rate of adverse cardiovascular events when heart failure occurs in patients with type 2 diabetes compared with patients who do not have diabetes.
The key, say experts, is for clinicians to maintain a high index of suspicion for signs and symptoms of heart failure in people with type 2 diabetes and to regularly assess them, starting with just a few simple questions that probe for the presence of dyspnea, exertional fatigue, or both, an approach not widely employed up to now.
Clinicians who care for people with type 2 diabetes must become “alert to thinking about heart failure and alert to asking questions about signs and symptoms” that flag the presence of HFpEF, advised Naveed Sattar, MBChB, PhD, a professor of metabolic medicine at the University of Glasgow.
Soon, medical groups will issue guidelines for appropriate assessment for the presence of HFpEF in people with type 2 diabetes, Dr. Sattar predicted in an interview.
A need to probe
“You can’t simply ask patients with type 2 diabetes whether they have shortness of breath or exertional fatigue and stop there,” because often their first response will be no.
“Commonly, patients will initially say they have no dyspnea, but when you probe further, you find symptoms,” noted Mikhail N. Kosiborod, MD, codirector of Saint Luke’s Cardiometabolic Center of Excellence in Kansas City, Mo.
These people are often sedentary, so they frequently don’t experience shortness of breath at baseline, Dr. Kosiborod said in an interview. In some cases, they may limit their activity because of their exertional intolerance.
Once a person’s suggestive symptoms become known, the next step is to measure the serum level of N-terminal pro-B-type natriuretic peptide (NT-proBNP), a biomarker considered to be a generally reliable signal of existing heart failure when elevated.
Any value above 125 pg/mL is suggestive of prevalent heart failure and should lead to the next diagnostic step of echocardiography, Dr. Sattar said.
Elevated NT-proBNP has such good positive predictive value for identifying heart failure that it is tempting to use it broadly in people with type 2 diabetes. A 2022 consensus report from the American Diabetes Association says that “measurement of a natriuretic peptide [such as NT-proBNP] or high-sensitivity cardiac troponin is recommended on at least a yearly basis to identify the earliest HF [heart failure] stages and implement strategies to prevent transition to symptomatic HF.”
Test costs require targeting
But because of the relatively high current price for an NT-proBNP test, the cost-benefit ratio for widespread annual testing of all people with type 2 diabetes would be poor, some experts caution.
“Screening everyone may not be the right answer. Hundreds of millions of people worldwide” have type 2 diabetes. “You first need to target evaluation to people with symptoms,” advised Dr. Kosiborod.
He also warned that a low NT-proBNP level does not always rule out HFpEF, especially among people with type 2 diabetes who also have overweight or obesity, because NT-proBNP levels can be “artificially low” in people with obesity.
Other potential aids to diagnosis are assessment scores that researchers have developed, such as the H2FPEF score, which relies on variables that include age, obesity, and the presence of atrial fibrillation and hypertension.
However, this score also requires an echocardiography examination, another test that would have a questionable cost-benefit ratio if performed widely for patients with type 2 diabetes without targeting, Dr. Kosiborod said.
SGLT2 inhibitors benefit HFpEF regardless of glucose levels
A prespecified analysis of the DELIVER results that divided the study cohort on the basis of their glycemic status proved the efficacy of the SGLT2 inhibitor dapagliflozin for patients with HFpEF regardless of whether or not they had type 2 diabetes, prediabetes, or were normoglycemic at entry into the study, Silvio E. Inzucchi, MD, reported at the EASD meeting.
Treatment with dapagliflozin cut the incidence of the trial’s primary outcome of cardiovascular death or worsening heart failure by a significant 18% relative to placebo among all enrolled patients.
The new analysis reported by Dr. Inzucchi showed that treatment was associated with a 23% relative risk reduction among those with normoglycemia, a 13% reduction among those with prediabetes, and a 19% reduction among those with type 2 diabetes, with no signal of a significant difference among the three subgroups.
“There was no statistical interaction between categorical glycemic subgrouping and dapagliflozin’s treatment effect,” concluded Dr. Inzucchi, director of the Yale Medicine Diabetes Center, New Haven, Conn.
He also reported that, among the 6,259 people in the trial with HFpEF, 50% had diabetes, 31% had prediabetes, and a scant 19% had normoglycemia. The finding highlights once again the high prevalence of dysglycemia among people with HFpEF.
Previously, a prespecified secondary analysis of data from the EMPEROR-Preserved trial yielded similar findings for empagliflozin that showed the agent’s efficacy for people with HFpEF across the range of glucose levels.
The DELIVER trial was funded by AstraZeneca, the company that markets dapagliflozin (Farxiga). The EMPEROR-Preserved trial was sponsored by Boehringer Ingelheim and Eli Lilly, the companies that jointly market empagliflozin (Jardiance). Dr. Lund has been a consultant to AstraZeneca and Boehringer Ingelheim and to numerous other companies, and he is a stockholder in AnaCardio. Dr. Sattar has been a consultant to and has received research support from AstraZeneca and Boehringer Ingelheim, and he has been a consultant with numerous companies. Dr. Kosiborod has been a consultant to and has received research funding from AstraZeneca and Boehringer Ingelheim and has been a consultant to Eli Lilly and numerous other companies. Dr. Inzucchi has been a consultant to, given talks on behalf of, or served on trial committees for Abbott, AstraZeneca, Boehringer Ingelheim, Esperion, Lexicon, Merck, Novo Nordisk, Pfizer, and vTv Therapetics.
A version of this article first appeared on Medscape.com.
STOCKHOLM – Recent study results confirm that two agents from the sodium-glucose cotransporter 2 (SGLT2) inhibitor class can significantly cut the incidence of adverse cardiovascular events in patients with heart failure with reduced ejection fraction (HFpEF), a disease especially common in people with type 2 diabetes, obesity, or both.
And findings from secondary analyses of the studies – including one reported at the annual meeting of the European Association for the Study of Diabetes – show that these SGLT2 inhibitors work as well for cutting incident adverse events (cardiovascular death or worsening heart failure) in patients with HFpEF and diabetes as they do for people with normal blood glucose levels.
But delivering treatment with these proven agents, dapagliflozin (Farxiga) and empagliflozin (Jardiance), first requires diagnosis of HFpEF, a task that clinicians have historically fallen short in accomplishing.
When in 2021, results from the EMPEROR-Preserved trial with empagliflozin and when in September 2022 results from the DELIVER trial with dapagliflozin established the efficacy of these two SGLT2 inhibitors as the first treatments proven to benefit patients with HFpEF, they also raised the stakes for clinicians to be much more diligent and systematic in evaluating people at high risk for developing HFpEF because of having type 2 diabetes or obesity, two of the most potent risk factors for this form of heart failure.
‘Vigilance ... needs to increase’
“Vigilance for HFpEF needs to increase because we can now help these patients,” declared Lars H. Lund, MD, PhD, speaking at the meeting. “Type 2 diabetes dramatically increases the incidence of HFpEF,” and the mechanisms by which it does this are “especially amenable to treatment with SGLT2 inhibitors,” said Dr. Lund, a cardiologist and heart failure specialist at the Karolinska Institute, Stockholm.
HFpEF has a history of going undetected in people with type 2 diabetes, an ironic situation given its high incidence as well as the elevated rate of adverse cardiovascular events when heart failure occurs in patients with type 2 diabetes compared with patients who do not have diabetes.
The key, say experts, is for clinicians to maintain a high index of suspicion for signs and symptoms of heart failure in people with type 2 diabetes and to regularly assess them, starting with just a few simple questions that probe for the presence of dyspnea, exertional fatigue, or both, an approach not widely employed up to now.
Clinicians who care for people with type 2 diabetes must become “alert to thinking about heart failure and alert to asking questions about signs and symptoms” that flag the presence of HFpEF, advised Naveed Sattar, MBChB, PhD, a professor of metabolic medicine at the University of Glasgow.
Soon, medical groups will issue guidelines for appropriate assessment for the presence of HFpEF in people with type 2 diabetes, Dr. Sattar predicted in an interview.
A need to probe
“You can’t simply ask patients with type 2 diabetes whether they have shortness of breath or exertional fatigue and stop there,” because often their first response will be no.
“Commonly, patients will initially say they have no dyspnea, but when you probe further, you find symptoms,” noted Mikhail N. Kosiborod, MD, codirector of Saint Luke’s Cardiometabolic Center of Excellence in Kansas City, Mo.
These people are often sedentary, so they frequently don’t experience shortness of breath at baseline, Dr. Kosiborod said in an interview. In some cases, they may limit their activity because of their exertional intolerance.
Once a person’s suggestive symptoms become known, the next step is to measure the serum level of N-terminal pro-B-type natriuretic peptide (NT-proBNP), a biomarker considered to be a generally reliable signal of existing heart failure when elevated.
Any value above 125 pg/mL is suggestive of prevalent heart failure and should lead to the next diagnostic step of echocardiography, Dr. Sattar said.
Elevated NT-proBNP has such good positive predictive value for identifying heart failure that it is tempting to use it broadly in people with type 2 diabetes. A 2022 consensus report from the American Diabetes Association says that “measurement of a natriuretic peptide [such as NT-proBNP] or high-sensitivity cardiac troponin is recommended on at least a yearly basis to identify the earliest HF [heart failure] stages and implement strategies to prevent transition to symptomatic HF.”
Test costs require targeting
But because of the relatively high current price for an NT-proBNP test, the cost-benefit ratio for widespread annual testing of all people with type 2 diabetes would be poor, some experts caution.
“Screening everyone may not be the right answer. Hundreds of millions of people worldwide” have type 2 diabetes. “You first need to target evaluation to people with symptoms,” advised Dr. Kosiborod.
He also warned that a low NT-proBNP level does not always rule out HFpEF, especially among people with type 2 diabetes who also have overweight or obesity, because NT-proBNP levels can be “artificially low” in people with obesity.
Other potential aids to diagnosis are assessment scores that researchers have developed, such as the H2FPEF score, which relies on variables that include age, obesity, and the presence of atrial fibrillation and hypertension.
However, this score also requires an echocardiography examination, another test that would have a questionable cost-benefit ratio if performed widely for patients with type 2 diabetes without targeting, Dr. Kosiborod said.
SGLT2 inhibitors benefit HFpEF regardless of glucose levels
A prespecified analysis of the DELIVER results that divided the study cohort on the basis of their glycemic status proved the efficacy of the SGLT2 inhibitor dapagliflozin for patients with HFpEF regardless of whether or not they had type 2 diabetes, prediabetes, or were normoglycemic at entry into the study, Silvio E. Inzucchi, MD, reported at the EASD meeting.
Treatment with dapagliflozin cut the incidence of the trial’s primary outcome of cardiovascular death or worsening heart failure by a significant 18% relative to placebo among all enrolled patients.
The new analysis reported by Dr. Inzucchi showed that treatment was associated with a 23% relative risk reduction among those with normoglycemia, a 13% reduction among those with prediabetes, and a 19% reduction among those with type 2 diabetes, with no signal of a significant difference among the three subgroups.
“There was no statistical interaction between categorical glycemic subgrouping and dapagliflozin’s treatment effect,” concluded Dr. Inzucchi, director of the Yale Medicine Diabetes Center, New Haven, Conn.
He also reported that, among the 6,259 people in the trial with HFpEF, 50% had diabetes, 31% had prediabetes, and a scant 19% had normoglycemia. The finding highlights once again the high prevalence of dysglycemia among people with HFpEF.
Previously, a prespecified secondary analysis of data from the EMPEROR-Preserved trial yielded similar findings for empagliflozin that showed the agent’s efficacy for people with HFpEF across the range of glucose levels.
The DELIVER trial was funded by AstraZeneca, the company that markets dapagliflozin (Farxiga). The EMPEROR-Preserved trial was sponsored by Boehringer Ingelheim and Eli Lilly, the companies that jointly market empagliflozin (Jardiance). Dr. Lund has been a consultant to AstraZeneca and Boehringer Ingelheim and to numerous other companies, and he is a stockholder in AnaCardio. Dr. Sattar has been a consultant to and has received research support from AstraZeneca and Boehringer Ingelheim, and he has been a consultant with numerous companies. Dr. Kosiborod has been a consultant to and has received research funding from AstraZeneca and Boehringer Ingelheim and has been a consultant to Eli Lilly and numerous other companies. Dr. Inzucchi has been a consultant to, given talks on behalf of, or served on trial committees for Abbott, AstraZeneca, Boehringer Ingelheim, Esperion, Lexicon, Merck, Novo Nordisk, Pfizer, and vTv Therapetics.
A version of this article first appeared on Medscape.com.
STOCKHOLM – Recent study results confirm that two agents from the sodium-glucose cotransporter 2 (SGLT2) inhibitor class can significantly cut the incidence of adverse cardiovascular events in patients with heart failure with reduced ejection fraction (HFpEF), a disease especially common in people with type 2 diabetes, obesity, or both.
And findings from secondary analyses of the studies – including one reported at the annual meeting of the European Association for the Study of Diabetes – show that these SGLT2 inhibitors work as well for cutting incident adverse events (cardiovascular death or worsening heart failure) in patients with HFpEF and diabetes as they do for people with normal blood glucose levels.
But delivering treatment with these proven agents, dapagliflozin (Farxiga) and empagliflozin (Jardiance), first requires diagnosis of HFpEF, a task that clinicians have historically fallen short in accomplishing.
When in 2021, results from the EMPEROR-Preserved trial with empagliflozin and when in September 2022 results from the DELIVER trial with dapagliflozin established the efficacy of these two SGLT2 inhibitors as the first treatments proven to benefit patients with HFpEF, they also raised the stakes for clinicians to be much more diligent and systematic in evaluating people at high risk for developing HFpEF because of having type 2 diabetes or obesity, two of the most potent risk factors for this form of heart failure.
‘Vigilance ... needs to increase’
“Vigilance for HFpEF needs to increase because we can now help these patients,” declared Lars H. Lund, MD, PhD, speaking at the meeting. “Type 2 diabetes dramatically increases the incidence of HFpEF,” and the mechanisms by which it does this are “especially amenable to treatment with SGLT2 inhibitors,” said Dr. Lund, a cardiologist and heart failure specialist at the Karolinska Institute, Stockholm.
HFpEF has a history of going undetected in people with type 2 diabetes, an ironic situation given its high incidence as well as the elevated rate of adverse cardiovascular events when heart failure occurs in patients with type 2 diabetes compared with patients who do not have diabetes.
The key, say experts, is for clinicians to maintain a high index of suspicion for signs and symptoms of heart failure in people with type 2 diabetes and to regularly assess them, starting with just a few simple questions that probe for the presence of dyspnea, exertional fatigue, or both, an approach not widely employed up to now.
Clinicians who care for people with type 2 diabetes must become “alert to thinking about heart failure and alert to asking questions about signs and symptoms” that flag the presence of HFpEF, advised Naveed Sattar, MBChB, PhD, a professor of metabolic medicine at the University of Glasgow.
Soon, medical groups will issue guidelines for appropriate assessment for the presence of HFpEF in people with type 2 diabetes, Dr. Sattar predicted in an interview.
A need to probe
“You can’t simply ask patients with type 2 diabetes whether they have shortness of breath or exertional fatigue and stop there,” because often their first response will be no.
“Commonly, patients will initially say they have no dyspnea, but when you probe further, you find symptoms,” noted Mikhail N. Kosiborod, MD, codirector of Saint Luke’s Cardiometabolic Center of Excellence in Kansas City, Mo.
These people are often sedentary, so they frequently don’t experience shortness of breath at baseline, Dr. Kosiborod said in an interview. In some cases, they may limit their activity because of their exertional intolerance.
Once a person’s suggestive symptoms become known, the next step is to measure the serum level of N-terminal pro-B-type natriuretic peptide (NT-proBNP), a biomarker considered to be a generally reliable signal of existing heart failure when elevated.
Any value above 125 pg/mL is suggestive of prevalent heart failure and should lead to the next diagnostic step of echocardiography, Dr. Sattar said.
Elevated NT-proBNP has such good positive predictive value for identifying heart failure that it is tempting to use it broadly in people with type 2 diabetes. A 2022 consensus report from the American Diabetes Association says that “measurement of a natriuretic peptide [such as NT-proBNP] or high-sensitivity cardiac troponin is recommended on at least a yearly basis to identify the earliest HF [heart failure] stages and implement strategies to prevent transition to symptomatic HF.”
Test costs require targeting
But because of the relatively high current price for an NT-proBNP test, the cost-benefit ratio for widespread annual testing of all people with type 2 diabetes would be poor, some experts caution.
“Screening everyone may not be the right answer. Hundreds of millions of people worldwide” have type 2 diabetes. “You first need to target evaluation to people with symptoms,” advised Dr. Kosiborod.
He also warned that a low NT-proBNP level does not always rule out HFpEF, especially among people with type 2 diabetes who also have overweight or obesity, because NT-proBNP levels can be “artificially low” in people with obesity.
Other potential aids to diagnosis are assessment scores that researchers have developed, such as the H2FPEF score, which relies on variables that include age, obesity, and the presence of atrial fibrillation and hypertension.
However, this score also requires an echocardiography examination, another test that would have a questionable cost-benefit ratio if performed widely for patients with type 2 diabetes without targeting, Dr. Kosiborod said.
SGLT2 inhibitors benefit HFpEF regardless of glucose levels
A prespecified analysis of the DELIVER results that divided the study cohort on the basis of their glycemic status proved the efficacy of the SGLT2 inhibitor dapagliflozin for patients with HFpEF regardless of whether or not they had type 2 diabetes, prediabetes, or were normoglycemic at entry into the study, Silvio E. Inzucchi, MD, reported at the EASD meeting.
Treatment with dapagliflozin cut the incidence of the trial’s primary outcome of cardiovascular death or worsening heart failure by a significant 18% relative to placebo among all enrolled patients.
The new analysis reported by Dr. Inzucchi showed that treatment was associated with a 23% relative risk reduction among those with normoglycemia, a 13% reduction among those with prediabetes, and a 19% reduction among those with type 2 diabetes, with no signal of a significant difference among the three subgroups.
“There was no statistical interaction between categorical glycemic subgrouping and dapagliflozin’s treatment effect,” concluded Dr. Inzucchi, director of the Yale Medicine Diabetes Center, New Haven, Conn.
He also reported that, among the 6,259 people in the trial with HFpEF, 50% had diabetes, 31% had prediabetes, and a scant 19% had normoglycemia. The finding highlights once again the high prevalence of dysglycemia among people with HFpEF.
Previously, a prespecified secondary analysis of data from the EMPEROR-Preserved trial yielded similar findings for empagliflozin that showed the agent’s efficacy for people with HFpEF across the range of glucose levels.
The DELIVER trial was funded by AstraZeneca, the company that markets dapagliflozin (Farxiga). The EMPEROR-Preserved trial was sponsored by Boehringer Ingelheim and Eli Lilly, the companies that jointly market empagliflozin (Jardiance). Dr. Lund has been a consultant to AstraZeneca and Boehringer Ingelheim and to numerous other companies, and he is a stockholder in AnaCardio. Dr. Sattar has been a consultant to and has received research support from AstraZeneca and Boehringer Ingelheim, and he has been a consultant with numerous companies. Dr. Kosiborod has been a consultant to and has received research funding from AstraZeneca and Boehringer Ingelheim and has been a consultant to Eli Lilly and numerous other companies. Dr. Inzucchi has been a consultant to, given talks on behalf of, or served on trial committees for Abbott, AstraZeneca, Boehringer Ingelheim, Esperion, Lexicon, Merck, Novo Nordisk, Pfizer, and vTv Therapetics.
A version of this article first appeared on Medscape.com.
AT EASD 2022
Once-weekly insulin promising in phase 3 trial in type 2 diabetes
STOCKHOLM – The investigational once-weekly insulin icodec (Novo Nordisk) significantly reduces A1c without increasing hypoglycemia in people with type 2 diabetes, the first phase 3 data of such an insulin formulation suggest. The data are from one of six trials in the company’s ONWARDS program.
“Once-weekly insulin may redefine diabetes management,” enthused Athena Philis-Tsimikas, MD, who presented the findings at a session during the European Association for the Study of Diabetes (EASD) 2022 Annual Meeting, which also included a summary of previously reported top-line data from other ONWARDS trials as well as phase 2 data for Lilly›s investigational once-weekly Basal Insulin Fc (BIF).
Phase 2 data for icodec were published in 2020 in the New England Journal of Medicine and in 2021 in Diabetes Care, as reported by this news organization.
The capacity for reducing the number of basal insulin injections from at least 365 to just 52 per year means that once-weekly insulin “has the potential to facilitate insulin initiation and improve treatment adherence and persistence in diabetes,” noted Dr. Philis-Tsimikas, corporate vice president of Scripps Whittier Diabetes Institute, San Diego.
Asked to comment, independent diabetes industry consultant Charles Alexander, MD, told this news organization that the new data from ONWARDS 2 of patients switching from daily to once-weekly basal insulin were reassuring with regard to hypoglycemia, at least for people with type 2 diabetes.
“For type 2, I think there’s enough data now to feel comfortable that it’s going to be good, especially for people who are on once-weekly [glucagon-like peptide-1 (GLP-1) agonists].”
However, for type 1 diabetes, the company reported top-line ONWARDS 6 data earlier this year, in which icodec was associated with significantly increased rates of hypoglycemia compared with daily degludec. “In type 1, even the basal needs are [often] changing. That kind of person would want to stay away from once-weekly insulin,” Dr. Alexander said.
And he noted, for any patient who adjusts their insulin dose frequently, “obviously, you’re not going to be able to do that with a once-weekly.”
Similar A1c reduction as daily basal without increased hypoglycemia
In ONWARDS 2, 526 adults with type 2 diabetes were randomized to switch from their current once- or twice-daily basal insulin to either once-weekly icodec or once-daily insulin degludec (Tresiba) for 26 weeks. The study was open-label, with a treat-to-glucose target of 80-130 mg/dL design.
Participants had A1c levels of 7.0%-10.0% and were also taking stable doses of other noninsulin glucose-lowering medications. Over 80% were taking metformin, a third were taking an SGLT2 inhibitor, and about a quarter each were taking a GLP-1 agonist or DPP-4 inhibitor. Those medications were continued, but sulfonylureas were discontinued in the 22% taking those at baseline.
The basal insulin used at baseline was glargine U100 for 42%, degludec for 28%, and glargine U300 for 16%, “so, a very typical presentation of patients we see in our practices today,” Dr. Philis-Tsimikas noted.
The primary endpoint, change in A1c from baseline to week 26, dropped from 8.17% to 7.20% with icodec and from 8.10% to 7.42% with degludec. The estimated treatment difference of –0.22 percentage points met the margins for both noninferiority (P < .0001) and superiority (P = .0028). Those taking icodec were significantly more likely to achieve an A1c under 7% compared with degludec, at 40.3% versus 26.5% (P = .0019).
Continuous glucose monitoring parameters during weeks 22-26 showed time in glucose range of 70-180 mg/dL (3.9-10.0 mmol/L) was 63.1% for icodec and 59.5% for degludec, which was not significantly different, Dr. Philis-Tsimikas reported.
Body weight increased by 1.4 kg (3 lb) with icodec but dropped slightly by 0.30 kg with degludec, which was significantly different (P < .001).
When asked about the body weight results, Dr. Alexander said: “It’s really hard to say. We know that insulin generally causes weight gain. A 1.4-kg weight gain over 6 months isn’t really surprising. Why there wasn’t with degludec, I don’t know.”
There was just one episode of severe hypoglycemia (requiring assistance) in the trial in the degludec group. Rates of combined severe or clinically significant hypoglycemic events (glucose < 54 mg/dL / < 3.0 mmol/L) per patient-year exposed were 0.73 for icodec versus 0.27 for degludec, which was not significantly different (P = .0782). Similar findings were seen for nocturnal hypoglycemia.
Significantly more patients achieved an A1c under 7% without significant hypoglycemia with icodec than degludec, at 36.7% versus 26.8% (P = .0223). Other adverse events were equivalent between the two groups, Dr. Philis-Tsimikas reported.
Scores on the diabetes treatment satisfaction questionnaire, which addresses convenience, flexibility, satisfaction, and willingness to recommend treatment to others, were significantly higher for icodec than degludec, at 4.22 versus 2.96 (P = .0036).
“For me, this is one of the most important outcomes,” she commented.
Benefit in type 2 diabetes, potential concern in type 1 diabetes
Top-line results from ONWARDS 1, a phase 3a 78-week trial in 984 drug-naive people with type 2 diabetes and ONWARDS 6, a 52-week trial in 583 people with type 1 diabetes, were presented earlier this year at the American Diabetes Association 81st Scientific Sessions.
In ONWARDS 1, icodec achieved noninferiority to daily insulin glargine, reducing A1c by 1.55 versus 1.35 percentage points, with superior time in range and no significant differences in hypoglycemia rates.
However, in ONWARDS 6, while noninferiority in A1c lowering compared with daily degludec was achieved, with reductions of 0.47 versus 0.51 percentage points from a baseline A1c of 7.6%, there was a significantly greater rate of severe or clinically significant hypoglycemia with icodec, at 19.93 versus 10.37 events per patient-year with degludec.
Dr. Philis-Tsimikas has reported performing research and serving as an advisor on behalf of her employer for Abbott, Bayer, Dexcom, Eli Lilly, Medtronic, Merck, Novo Nordisk, and Sanofi. All reimbursements go to her employer. Dr. Alexander has reported being a nonpaid advisor for diaTribe and a consultant for Kinexum.
A version of this article first appeared on Medscape.com.
STOCKHOLM – The investigational once-weekly insulin icodec (Novo Nordisk) significantly reduces A1c without increasing hypoglycemia in people with type 2 diabetes, the first phase 3 data of such an insulin formulation suggest. The data are from one of six trials in the company’s ONWARDS program.
“Once-weekly insulin may redefine diabetes management,” enthused Athena Philis-Tsimikas, MD, who presented the findings at a session during the European Association for the Study of Diabetes (EASD) 2022 Annual Meeting, which also included a summary of previously reported top-line data from other ONWARDS trials as well as phase 2 data for Lilly›s investigational once-weekly Basal Insulin Fc (BIF).
Phase 2 data for icodec were published in 2020 in the New England Journal of Medicine and in 2021 in Diabetes Care, as reported by this news organization.
The capacity for reducing the number of basal insulin injections from at least 365 to just 52 per year means that once-weekly insulin “has the potential to facilitate insulin initiation and improve treatment adherence and persistence in diabetes,” noted Dr. Philis-Tsimikas, corporate vice president of Scripps Whittier Diabetes Institute, San Diego.
Asked to comment, independent diabetes industry consultant Charles Alexander, MD, told this news organization that the new data from ONWARDS 2 of patients switching from daily to once-weekly basal insulin were reassuring with regard to hypoglycemia, at least for people with type 2 diabetes.
“For type 2, I think there’s enough data now to feel comfortable that it’s going to be good, especially for people who are on once-weekly [glucagon-like peptide-1 (GLP-1) agonists].”
However, for type 1 diabetes, the company reported top-line ONWARDS 6 data earlier this year, in which icodec was associated with significantly increased rates of hypoglycemia compared with daily degludec. “In type 1, even the basal needs are [often] changing. That kind of person would want to stay away from once-weekly insulin,” Dr. Alexander said.
And he noted, for any patient who adjusts their insulin dose frequently, “obviously, you’re not going to be able to do that with a once-weekly.”
Similar A1c reduction as daily basal without increased hypoglycemia
In ONWARDS 2, 526 adults with type 2 diabetes were randomized to switch from their current once- or twice-daily basal insulin to either once-weekly icodec or once-daily insulin degludec (Tresiba) for 26 weeks. The study was open-label, with a treat-to-glucose target of 80-130 mg/dL design.
Participants had A1c levels of 7.0%-10.0% and were also taking stable doses of other noninsulin glucose-lowering medications. Over 80% were taking metformin, a third were taking an SGLT2 inhibitor, and about a quarter each were taking a GLP-1 agonist or DPP-4 inhibitor. Those medications were continued, but sulfonylureas were discontinued in the 22% taking those at baseline.
The basal insulin used at baseline was glargine U100 for 42%, degludec for 28%, and glargine U300 for 16%, “so, a very typical presentation of patients we see in our practices today,” Dr. Philis-Tsimikas noted.
The primary endpoint, change in A1c from baseline to week 26, dropped from 8.17% to 7.20% with icodec and from 8.10% to 7.42% with degludec. The estimated treatment difference of –0.22 percentage points met the margins for both noninferiority (P < .0001) and superiority (P = .0028). Those taking icodec were significantly more likely to achieve an A1c under 7% compared with degludec, at 40.3% versus 26.5% (P = .0019).
Continuous glucose monitoring parameters during weeks 22-26 showed time in glucose range of 70-180 mg/dL (3.9-10.0 mmol/L) was 63.1% for icodec and 59.5% for degludec, which was not significantly different, Dr. Philis-Tsimikas reported.
Body weight increased by 1.4 kg (3 lb) with icodec but dropped slightly by 0.30 kg with degludec, which was significantly different (P < .001).
When asked about the body weight results, Dr. Alexander said: “It’s really hard to say. We know that insulin generally causes weight gain. A 1.4-kg weight gain over 6 months isn’t really surprising. Why there wasn’t with degludec, I don’t know.”
There was just one episode of severe hypoglycemia (requiring assistance) in the trial in the degludec group. Rates of combined severe or clinically significant hypoglycemic events (glucose < 54 mg/dL / < 3.0 mmol/L) per patient-year exposed were 0.73 for icodec versus 0.27 for degludec, which was not significantly different (P = .0782). Similar findings were seen for nocturnal hypoglycemia.
Significantly more patients achieved an A1c under 7% without significant hypoglycemia with icodec than degludec, at 36.7% versus 26.8% (P = .0223). Other adverse events were equivalent between the two groups, Dr. Philis-Tsimikas reported.
Scores on the diabetes treatment satisfaction questionnaire, which addresses convenience, flexibility, satisfaction, and willingness to recommend treatment to others, were significantly higher for icodec than degludec, at 4.22 versus 2.96 (P = .0036).
“For me, this is one of the most important outcomes,” she commented.
Benefit in type 2 diabetes, potential concern in type 1 diabetes
Top-line results from ONWARDS 1, a phase 3a 78-week trial in 984 drug-naive people with type 2 diabetes and ONWARDS 6, a 52-week trial in 583 people with type 1 diabetes, were presented earlier this year at the American Diabetes Association 81st Scientific Sessions.
In ONWARDS 1, icodec achieved noninferiority to daily insulin glargine, reducing A1c by 1.55 versus 1.35 percentage points, with superior time in range and no significant differences in hypoglycemia rates.
However, in ONWARDS 6, while noninferiority in A1c lowering compared with daily degludec was achieved, with reductions of 0.47 versus 0.51 percentage points from a baseline A1c of 7.6%, there was a significantly greater rate of severe or clinically significant hypoglycemia with icodec, at 19.93 versus 10.37 events per patient-year with degludec.
Dr. Philis-Tsimikas has reported performing research and serving as an advisor on behalf of her employer for Abbott, Bayer, Dexcom, Eli Lilly, Medtronic, Merck, Novo Nordisk, and Sanofi. All reimbursements go to her employer. Dr. Alexander has reported being a nonpaid advisor for diaTribe and a consultant for Kinexum.
A version of this article first appeared on Medscape.com.
STOCKHOLM – The investigational once-weekly insulin icodec (Novo Nordisk) significantly reduces A1c without increasing hypoglycemia in people with type 2 diabetes, the first phase 3 data of such an insulin formulation suggest. The data are from one of six trials in the company’s ONWARDS program.
“Once-weekly insulin may redefine diabetes management,” enthused Athena Philis-Tsimikas, MD, who presented the findings at a session during the European Association for the Study of Diabetes (EASD) 2022 Annual Meeting, which also included a summary of previously reported top-line data from other ONWARDS trials as well as phase 2 data for Lilly›s investigational once-weekly Basal Insulin Fc (BIF).
Phase 2 data for icodec were published in 2020 in the New England Journal of Medicine and in 2021 in Diabetes Care, as reported by this news organization.
The capacity for reducing the number of basal insulin injections from at least 365 to just 52 per year means that once-weekly insulin “has the potential to facilitate insulin initiation and improve treatment adherence and persistence in diabetes,” noted Dr. Philis-Tsimikas, corporate vice president of Scripps Whittier Diabetes Institute, San Diego.
Asked to comment, independent diabetes industry consultant Charles Alexander, MD, told this news organization that the new data from ONWARDS 2 of patients switching from daily to once-weekly basal insulin were reassuring with regard to hypoglycemia, at least for people with type 2 diabetes.
“For type 2, I think there’s enough data now to feel comfortable that it’s going to be good, especially for people who are on once-weekly [glucagon-like peptide-1 (GLP-1) agonists].”
However, for type 1 diabetes, the company reported top-line ONWARDS 6 data earlier this year, in which icodec was associated with significantly increased rates of hypoglycemia compared with daily degludec. “In type 1, even the basal needs are [often] changing. That kind of person would want to stay away from once-weekly insulin,” Dr. Alexander said.
And he noted, for any patient who adjusts their insulin dose frequently, “obviously, you’re not going to be able to do that with a once-weekly.”
Similar A1c reduction as daily basal without increased hypoglycemia
In ONWARDS 2, 526 adults with type 2 diabetes were randomized to switch from their current once- or twice-daily basal insulin to either once-weekly icodec or once-daily insulin degludec (Tresiba) for 26 weeks. The study was open-label, with a treat-to-glucose target of 80-130 mg/dL design.
Participants had A1c levels of 7.0%-10.0% and were also taking stable doses of other noninsulin glucose-lowering medications. Over 80% were taking metformin, a third were taking an SGLT2 inhibitor, and about a quarter each were taking a GLP-1 agonist or DPP-4 inhibitor. Those medications were continued, but sulfonylureas were discontinued in the 22% taking those at baseline.
The basal insulin used at baseline was glargine U100 for 42%, degludec for 28%, and glargine U300 for 16%, “so, a very typical presentation of patients we see in our practices today,” Dr. Philis-Tsimikas noted.
The primary endpoint, change in A1c from baseline to week 26, dropped from 8.17% to 7.20% with icodec and from 8.10% to 7.42% with degludec. The estimated treatment difference of –0.22 percentage points met the margins for both noninferiority (P < .0001) and superiority (P = .0028). Those taking icodec were significantly more likely to achieve an A1c under 7% compared with degludec, at 40.3% versus 26.5% (P = .0019).
Continuous glucose monitoring parameters during weeks 22-26 showed time in glucose range of 70-180 mg/dL (3.9-10.0 mmol/L) was 63.1% for icodec and 59.5% for degludec, which was not significantly different, Dr. Philis-Tsimikas reported.
Body weight increased by 1.4 kg (3 lb) with icodec but dropped slightly by 0.30 kg with degludec, which was significantly different (P < .001).
When asked about the body weight results, Dr. Alexander said: “It’s really hard to say. We know that insulin generally causes weight gain. A 1.4-kg weight gain over 6 months isn’t really surprising. Why there wasn’t with degludec, I don’t know.”
There was just one episode of severe hypoglycemia (requiring assistance) in the trial in the degludec group. Rates of combined severe or clinically significant hypoglycemic events (glucose < 54 mg/dL / < 3.0 mmol/L) per patient-year exposed were 0.73 for icodec versus 0.27 for degludec, which was not significantly different (P = .0782). Similar findings were seen for nocturnal hypoglycemia.
Significantly more patients achieved an A1c under 7% without significant hypoglycemia with icodec than degludec, at 36.7% versus 26.8% (P = .0223). Other adverse events were equivalent between the two groups, Dr. Philis-Tsimikas reported.
Scores on the diabetes treatment satisfaction questionnaire, which addresses convenience, flexibility, satisfaction, and willingness to recommend treatment to others, were significantly higher for icodec than degludec, at 4.22 versus 2.96 (P = .0036).
“For me, this is one of the most important outcomes,” she commented.
Benefit in type 2 diabetes, potential concern in type 1 diabetes
Top-line results from ONWARDS 1, a phase 3a 78-week trial in 984 drug-naive people with type 2 diabetes and ONWARDS 6, a 52-week trial in 583 people with type 1 diabetes, were presented earlier this year at the American Diabetes Association 81st Scientific Sessions.
In ONWARDS 1, icodec achieved noninferiority to daily insulin glargine, reducing A1c by 1.55 versus 1.35 percentage points, with superior time in range and no significant differences in hypoglycemia rates.
However, in ONWARDS 6, while noninferiority in A1c lowering compared with daily degludec was achieved, with reductions of 0.47 versus 0.51 percentage points from a baseline A1c of 7.6%, there was a significantly greater rate of severe or clinically significant hypoglycemia with icodec, at 19.93 versus 10.37 events per patient-year with degludec.
Dr. Philis-Tsimikas has reported performing research and serving as an advisor on behalf of her employer for Abbott, Bayer, Dexcom, Eli Lilly, Medtronic, Merck, Novo Nordisk, and Sanofi. All reimbursements go to her employer. Dr. Alexander has reported being a nonpaid advisor for diaTribe and a consultant for Kinexum.
A version of this article first appeared on Medscape.com.
AT EASD 2022
Ezetimibe-statin combo lowers liver fat in open-label trial
Ezetimibe given in combination with rosuvastatin has a beneficial effect on liver fat in people with nonalcoholic fatty liver disease (NAFLD), according results of a randomized, active-controlled trial.
The findings, which come from the investigator-initiated ESSENTIAL trial, are likely to add to the debate over whether or not the lipid-lowering combination could be of benefit beyond its effects in the blood.
“We used magnetic resonance imaging-derived proton density fat fraction [MRI-PDFF], which is highly reliable method of assessing hepatic steatosis,” Youngjoon Kim, PhD, one of the study investigators, said at the annual meeting of the European Association for the Study of Diabetes in Barcelona.
“It enables accurate, repeatable and reproducible quantitative assessment of liver fat over the entire liver,” observed Dr. Kim, who works at Severance Hospital, part of Yonsei University in Seoul.
He reported that there was a significant 5.8% decrease in liver fat following 24 weeks’ treatment with ezetimibe and rosuvastatin comparing baseline with end of treatment MRI-PDFF values; a drop that was significant (18.2% vs. 12.3%, P < .001).
Rosuvastatin monotherapy also reduced liver fat from 15.0% at baseline to 12.4% after 24 weeks; this drop of 2.6% was also significant (P = .003).
This gave an absolute mean difference between the two study arms of 3.2% (P = .02).
Rationale for the ESSENTIAL study
Dr. Kim observed during his presentation that NAFLD is burgeoning problem around the world. Ezetimibe plus rosuvastatin was a combination treatment already used widely in clinical practice, and there had been some suggestion that ezetimibe might have an effect on liver fat.
“Although the effect of ezetimibe on hepatic steatosis is still controversial, ezetimibe has been reported to reduce visceral fat and improve insulin resistance in several studies” Dr. Kim said.
“Recently, our group reported that the use of ezetimibe affects autophagy of hepatocytes and the NLRP3 [NOD-like receptors containing pyrin domain 3] inflammasome,” he said.
Moreover, he added, “ezetimibe improved NASH [nonalcoholic steatohepatitis] in an animal model. However, the effects of ezetimibe have not been clearly shown in a human study.”
Dr. Kim also acknowledged a prior randomized control trial that had looked at the role of ezetimibe in 50 patients with NASH, but had not shown a benefit for the drug over placebo in terms of liver fat reduction.
Addressing the Hawthorne effect
“The size of the effect by that might actually be more modest due to the Hawthorne effect,” said session chair Onno Holleboom, MD, PhD, of Amsterdam UMC in the Netherlands.
“What we observe in the large clinical trials is an enormous Hawthorne effect – participating in a NAFLD trial makes people live healthier because they have health checks,” he said.
“That’s a major problem for showing efficacy for the intervention arm,” he added, but of course the open design meant that the trial only had intervention arms; “there was no placebo arm.”
A randomized, active-controlled, clinician-initiated trial
The main objective of the ESSENTIAL trial was therefore to take another look at the potential effect of ezetimibe on hepatic steatosis and doing so in the setting of statin therapy.
In all, 70 patients with NAFLD that had been confirmed via ultrasound were recruited into the prospective, single center, phase 4 trial. Participants were randomized 1:1 to received either ezetimibe 10 mg plus rosuvastatin 5 mg daily or rosuvastatin 5 mg for up to 24 weeks.
Change in liver fat was measured via MRI-PDFF, taking the average values in each of nine liver segments. Magnetic resonance elastography (MRE) was also used to measure liver fibrosis, although results did not show any differences either from baseline to end of treatment values in either group or when the two treatment groups were compared.
Dr. Kim reported that both treatment with the ezetimibe-rosuvastatin combination and rosuvastatin monotherapy reduced parameters that might be associated with a negative outcome in NAFLD, such as body mass index and waist circumference, triglycerides, and LDL cholesterol. There was also a reduction in C-reactive protein levels in the blood, and interleulin-18. There was no change in liver enzymes.
Several subgroup analyses were performed indicating that “individuals with higher BMI, type 2 diabetes, insulin resistance, and severe liver fibrosis were likely to be good responders to ezetimibe treatment,” Dr. Kim said.
“These data indicate that ezetimibe plus rosuvastatin is a safe and effective therapeutic option to treat patients with NAFLD and dyslipidemia,” he concluded.
The results of the ESSENTIAL study have been published in BMC Medicine.
The study was funded by the Yuhan Corporation. Dr. Kim had no conflicts of interest to report. Dr. Holleboom was not involved in the study and had no conflicts of interest.
Ezetimibe given in combination with rosuvastatin has a beneficial effect on liver fat in people with nonalcoholic fatty liver disease (NAFLD), according results of a randomized, active-controlled trial.
The findings, which come from the investigator-initiated ESSENTIAL trial, are likely to add to the debate over whether or not the lipid-lowering combination could be of benefit beyond its effects in the blood.
“We used magnetic resonance imaging-derived proton density fat fraction [MRI-PDFF], which is highly reliable method of assessing hepatic steatosis,” Youngjoon Kim, PhD, one of the study investigators, said at the annual meeting of the European Association for the Study of Diabetes in Barcelona.
“It enables accurate, repeatable and reproducible quantitative assessment of liver fat over the entire liver,” observed Dr. Kim, who works at Severance Hospital, part of Yonsei University in Seoul.
He reported that there was a significant 5.8% decrease in liver fat following 24 weeks’ treatment with ezetimibe and rosuvastatin comparing baseline with end of treatment MRI-PDFF values; a drop that was significant (18.2% vs. 12.3%, P < .001).
Rosuvastatin monotherapy also reduced liver fat from 15.0% at baseline to 12.4% after 24 weeks; this drop of 2.6% was also significant (P = .003).
This gave an absolute mean difference between the two study arms of 3.2% (P = .02).
Rationale for the ESSENTIAL study
Dr. Kim observed during his presentation that NAFLD is burgeoning problem around the world. Ezetimibe plus rosuvastatin was a combination treatment already used widely in clinical practice, and there had been some suggestion that ezetimibe might have an effect on liver fat.
“Although the effect of ezetimibe on hepatic steatosis is still controversial, ezetimibe has been reported to reduce visceral fat and improve insulin resistance in several studies” Dr. Kim said.
“Recently, our group reported that the use of ezetimibe affects autophagy of hepatocytes and the NLRP3 [NOD-like receptors containing pyrin domain 3] inflammasome,” he said.
Moreover, he added, “ezetimibe improved NASH [nonalcoholic steatohepatitis] in an animal model. However, the effects of ezetimibe have not been clearly shown in a human study.”
Dr. Kim also acknowledged a prior randomized control trial that had looked at the role of ezetimibe in 50 patients with NASH, but had not shown a benefit for the drug over placebo in terms of liver fat reduction.
Addressing the Hawthorne effect
“The size of the effect by that might actually be more modest due to the Hawthorne effect,” said session chair Onno Holleboom, MD, PhD, of Amsterdam UMC in the Netherlands.
“What we observe in the large clinical trials is an enormous Hawthorne effect – participating in a NAFLD trial makes people live healthier because they have health checks,” he said.
“That’s a major problem for showing efficacy for the intervention arm,” he added, but of course the open design meant that the trial only had intervention arms; “there was no placebo arm.”
A randomized, active-controlled, clinician-initiated trial
The main objective of the ESSENTIAL trial was therefore to take another look at the potential effect of ezetimibe on hepatic steatosis and doing so in the setting of statin therapy.
In all, 70 patients with NAFLD that had been confirmed via ultrasound were recruited into the prospective, single center, phase 4 trial. Participants were randomized 1:1 to received either ezetimibe 10 mg plus rosuvastatin 5 mg daily or rosuvastatin 5 mg for up to 24 weeks.
Change in liver fat was measured via MRI-PDFF, taking the average values in each of nine liver segments. Magnetic resonance elastography (MRE) was also used to measure liver fibrosis, although results did not show any differences either from baseline to end of treatment values in either group or when the two treatment groups were compared.
Dr. Kim reported that both treatment with the ezetimibe-rosuvastatin combination and rosuvastatin monotherapy reduced parameters that might be associated with a negative outcome in NAFLD, such as body mass index and waist circumference, triglycerides, and LDL cholesterol. There was also a reduction in C-reactive protein levels in the blood, and interleulin-18. There was no change in liver enzymes.
Several subgroup analyses were performed indicating that “individuals with higher BMI, type 2 diabetes, insulin resistance, and severe liver fibrosis were likely to be good responders to ezetimibe treatment,” Dr. Kim said.
“These data indicate that ezetimibe plus rosuvastatin is a safe and effective therapeutic option to treat patients with NAFLD and dyslipidemia,” he concluded.
The results of the ESSENTIAL study have been published in BMC Medicine.
The study was funded by the Yuhan Corporation. Dr. Kim had no conflicts of interest to report. Dr. Holleboom was not involved in the study and had no conflicts of interest.
Ezetimibe given in combination with rosuvastatin has a beneficial effect on liver fat in people with nonalcoholic fatty liver disease (NAFLD), according results of a randomized, active-controlled trial.
The findings, which come from the investigator-initiated ESSENTIAL trial, are likely to add to the debate over whether or not the lipid-lowering combination could be of benefit beyond its effects in the blood.
“We used magnetic resonance imaging-derived proton density fat fraction [MRI-PDFF], which is highly reliable method of assessing hepatic steatosis,” Youngjoon Kim, PhD, one of the study investigators, said at the annual meeting of the European Association for the Study of Diabetes in Barcelona.
“It enables accurate, repeatable and reproducible quantitative assessment of liver fat over the entire liver,” observed Dr. Kim, who works at Severance Hospital, part of Yonsei University in Seoul.
He reported that there was a significant 5.8% decrease in liver fat following 24 weeks’ treatment with ezetimibe and rosuvastatin comparing baseline with end of treatment MRI-PDFF values; a drop that was significant (18.2% vs. 12.3%, P < .001).
Rosuvastatin monotherapy also reduced liver fat from 15.0% at baseline to 12.4% after 24 weeks; this drop of 2.6% was also significant (P = .003).
This gave an absolute mean difference between the two study arms of 3.2% (P = .02).
Rationale for the ESSENTIAL study
Dr. Kim observed during his presentation that NAFLD is burgeoning problem around the world. Ezetimibe plus rosuvastatin was a combination treatment already used widely in clinical practice, and there had been some suggestion that ezetimibe might have an effect on liver fat.
“Although the effect of ezetimibe on hepatic steatosis is still controversial, ezetimibe has been reported to reduce visceral fat and improve insulin resistance in several studies” Dr. Kim said.
“Recently, our group reported that the use of ezetimibe affects autophagy of hepatocytes and the NLRP3 [NOD-like receptors containing pyrin domain 3] inflammasome,” he said.
Moreover, he added, “ezetimibe improved NASH [nonalcoholic steatohepatitis] in an animal model. However, the effects of ezetimibe have not been clearly shown in a human study.”
Dr. Kim also acknowledged a prior randomized control trial that had looked at the role of ezetimibe in 50 patients with NASH, but had not shown a benefit for the drug over placebo in terms of liver fat reduction.
Addressing the Hawthorne effect
“The size of the effect by that might actually be more modest due to the Hawthorne effect,” said session chair Onno Holleboom, MD, PhD, of Amsterdam UMC in the Netherlands.
“What we observe in the large clinical trials is an enormous Hawthorne effect – participating in a NAFLD trial makes people live healthier because they have health checks,” he said.
“That’s a major problem for showing efficacy for the intervention arm,” he added, but of course the open design meant that the trial only had intervention arms; “there was no placebo arm.”
A randomized, active-controlled, clinician-initiated trial
The main objective of the ESSENTIAL trial was therefore to take another look at the potential effect of ezetimibe on hepatic steatosis and doing so in the setting of statin therapy.
In all, 70 patients with NAFLD that had been confirmed via ultrasound were recruited into the prospective, single center, phase 4 trial. Participants were randomized 1:1 to received either ezetimibe 10 mg plus rosuvastatin 5 mg daily or rosuvastatin 5 mg for up to 24 weeks.
Change in liver fat was measured via MRI-PDFF, taking the average values in each of nine liver segments. Magnetic resonance elastography (MRE) was also used to measure liver fibrosis, although results did not show any differences either from baseline to end of treatment values in either group or when the two treatment groups were compared.
Dr. Kim reported that both treatment with the ezetimibe-rosuvastatin combination and rosuvastatin monotherapy reduced parameters that might be associated with a negative outcome in NAFLD, such as body mass index and waist circumference, triglycerides, and LDL cholesterol. There was also a reduction in C-reactive protein levels in the blood, and interleulin-18. There was no change in liver enzymes.
Several subgroup analyses were performed indicating that “individuals with higher BMI, type 2 diabetes, insulin resistance, and severe liver fibrosis were likely to be good responders to ezetimibe treatment,” Dr. Kim said.
“These data indicate that ezetimibe plus rosuvastatin is a safe and effective therapeutic option to treat patients with NAFLD and dyslipidemia,” he concluded.
The results of the ESSENTIAL study have been published in BMC Medicine.
The study was funded by the Yuhan Corporation. Dr. Kim had no conflicts of interest to report. Dr. Holleboom was not involved in the study and had no conflicts of interest.
FROM EASD 2022
Does COVID-19 cause type 1 diabetes in children? Time will tell
STOCKHOLM – It remains inconclusive whether SARS-CoV-2 infection predisposes children and adolescents to a higher risk of type 1 diabetes. Data from two new studies and a recently published research letter add to the growing body of knowledge on the subject, but still can’t draw any definitive conclusions.
The latest results from a Norwegian and a Scottish study both examine incidence of type 1 diabetes in young people with a history of SARS-CoV-2 infection and were reported at the annual meeting of the European Association for the Study of Diabetes.
A 60% increased risk for type 1 diabetes at least 31 days after SARS-CoV-2 infection (adjusted hazard ratio, 1.63) was found in the Norwegian study, while in contrast, the Scottish study only found an increased risk in the first few months of the pandemic, in 2020, but importantly, no association over a much longer time period (March 2020–November 2021).
In a comment on Twitter on the two studies presented at EASD, session moderator Kamlesh Khunti, MD, professor of primary care diabetes and vascular medicine at the University of Leicester, (England), said: “In summary, two studies showing no or weak association of type 1 diabetes with COVID.”
But new data in the research letter published in JAMA Network Open, based on U.S. figures, also found an almost doubling of type 1 diabetes in children in the first few months after COVID-19 infection relative to infection with other respiratory viruses.
Lead author of the Scottish study, Helen Colhoun, PhD, honorary public health consultant at Public Health Scotland, commented: “Data in children are variable year on year, which emphasizes the need to be cautious over taking a tiny snapshot.”
Nevertheless, this is “a hugely important question and we must not drop the ball. [We must] keep looking at it and maintain scientific equipoise. ... [This] reinforces the need to carry on this analysis into the future to obtain an unequivocal picture,” she emphasized.
Norwegian study: If there is an association, the risk is small
German Tapia, PhD, from the Norwegian Institute of Public Health, Oslo, presented the results of a study of SARS-CoV-2 infection and subsequent risk of type 1 diabetes in 1.2 million children in Norway.
Of these, 424,354 children had been infected with SARS-CoV-2, and there were 990 incident cases of type 1 diabetes.
“What we do know about COVID-19 in children is that the symptoms are mild and only a small proportion are hospitalized with more serious symptoms. But we do not know the long-term effects of COVID-19 infection because this requires a longer follow-up period,” remarked Dr. Tapia, adding that other viral infections are thought to be linked to the development of type 1 diabetes, in particular, respiratory infections.
The data were sourced from the Norwegian Emergency Preparedness Register for COVID-19, which gathers daily data updates including infections (positive and negative results for free-of-charge testing), diagnoses (primary and secondary care), vaccinations (also free of charge), prescribed medications, and basic demographics.
“We link these data using the personal identification number that every Norwegian citizen has,” explained Dr. Tapia.
He presented results from two cohorts: firstly, results in children only, including those tested for SARS-CoV-2 infection, and secondly, a full national Norwegian population cohort.
Regarding the first cohort, those under 18 years who tested positive for SARS-CoV-2 infection, from March 2020 to March 2022, had a significantly increased risk of type 1 diabetes at least 31 days after infection, with an adjusted hazard ratio of 1.63 (95% confidence interval, 1.08-2.47; P = .02). Adjustments were made for age, sex, non-Nordic country of origin, geographic area, and socioeconomic factors.
For children who developed type 1 diabetes within 30 days of a SARS-CoV-2 infection, the HR was 1.26 (95% CI, 0.72-2.19; P = .42), which did not reach statistical significance.
“The fact that fewer people developed type 1 diabetes within 30 days is not surprising because we know that type 1 diabetes develops over a long period of time,” Dr. Tapia said.
“For this reason, we would not expect to find new cases of those people who develop type 1 diabetes within 30 days of COVID-19 infection,” he explained. In these cases, “it is most likely that they already had [type 1 diabetes], and the infection probably triggered clinical symptoms, so their type 1 diabetes was discovered.”
Turning to the full population cohort and diagnoses of type 1 diabetes over 30 days after SARS-CoV-2 infection, the Norwegian researchers found an association, with an HR of 1.57 (95% CI, 1.06-2.33; P = .03), while diagnosis of type 1 diabetes at 30 days or less generated a hazard ratio of 1.22 (95% CI, 0.72-2.19; P = .42).
“So very similar results were found, and after adjustment for confounders, results were still similar,” reported Dr. Tapia.
He also conducted a similar analysis with vaccination as an exposure but found no association between vaccination against SARS-CoV-2 and diagnosis of type 1 diabetes.
“From these results, we conclude that this suggests an increase in diagnosis of type 1 diabetes after SARS-CoV-2 infection, but it must be noted that the absolute risk of developing type 1 diabetes after infection in children is low, with most children not developing the disease,” he emphasized. “There are nearly half a million children who have been infected with SARS-CoV-2 in Norway, but only a very small proportion develop type 1 diabetes.”
Scottish study: No association found over longer term
Dr. Colhoun and colleagues looked at the relationship between incident type 1 diabetes and SARS-CoV-2 infection in children in Scotland using e-health record linkage.
The study involved 1.8 million people under 35 years of age and found very weak, if any, evidence of an association between incident type 1 diabetes and SARS-CoV-2.
Examining data between March 2020 and November 2021, Dr. Colhoun and colleagues identified 365,080 individuals up to age 35 with at least one detected SARS-CoV-2 infection during follow-up and 1,074 who developed type 1 diabetes.
“In children under 16 years, suspected cases of type 1 diabetes are admitted to hospital, and 97% of diagnosis dates are recorded in the Scottish Care Information – Diabetes Collaboration register [SCI-Diabetes] prior to or within 2 days of the first hospital admission for type 1 diabetes,” Dr. Colhoun said, stressing the timeliness of the data.
“We found the incidence of type 1 diabetes diagnosis increased 1.2-fold in those aged 0-14 years, but we did not find any association at an individual level of COVID-19 infection over 30 days prior to a type 1 diabetes diagnosis, in this particular dataset,” she reported. In young people aged 15-34, there was a linear increase in incident type 1 diabetes from 2015 to 2021 with no pandemic increase.
Referring to the 1.2-fold increase soon after the pandemic started, she explained that, in 0- to 14-year-olds, the increase followed a drop in the preceding months prepandemic in 2019. They also found that the seasonal pattern of type 1 diabetes diagnoses remained roughly the same across the pandemic months, with typical peaks in February and September.
In the cohort of under 35s, researchers also found a rate ratio of 2.62 (95% CI, 1.81-3.78) within a 30-day window of SARS-CoV-2 infection, but beyond 30 days, no evidence was seen of an association, with a RR of 0.86 (95% CI, 0.62-1.21; P = .40), she reported.
She explained her reasons for not considering diagnoses within 30 days of COVID-19 as causative. Echoing Dr. Tapia, Dr. Colhoun said the median time from symptom onset to diagnosis of type 1 diabetes is 25 days. “This suggests that 50% have had symptoms for over 25 days at diagnosis.”
She also stressed that when they compared the timing of SARS-CoV-2 testing with diagnosis, they found a much higher rate of COVID-19 testing around diagnosis. “This was not least because everyone admitted to hospital had to have a COVID-19 test.”
Latest U.S. data point to a link
Meanwhile, for the new data reported in JAMA Network Open, medical student Ellen K. Kendall of Case Western Reserve University, Cleveland, matched 571,256 pediatric patients: 285,628 with COVID-19 and 285,628 with non–COVID-19 respiratory infections.
By 6 months after COVID-19, 123 patients (0.043%) had received a new diagnosis of type 1 diabetes, but only 72 (0.025%) were diagnosed with type 1 diabetes within 6 months after non–COVID-19 respiratory infection.
At 1, 3, and 6 months after infection, risk of diagnosis of type 1 diabetes was greater among those infected with SARS-CoV-2, compared with those with non–COVID-19 respiratory infection (1 month: HR, 1.96; 3 months: HR, 2.10; and 6 months: HR, 1.83), and in subgroups of patients aged 0-9 years, a group unlikely to develop type 2 diabetes.
“In this study, new type 1 diabetes diagnoses were more likely to occur among pediatric patients with prior COVID-19 than among those with other respiratory infections (or with other encounters with health systems),” noted Ms. Kendall and coauthors. “Respiratory infections have previously been associated with onset of type 1 diabetes, but this risk was even higher among those with COVID-19 in our study, raising concern for long-term, post–COVID-19 autoimmune complications among youths.”
“The increased risk of new-onset type 1 diabetes after COVID-19 adds an important consideration for risk–benefit discussions for prevention and treatment of SARS-CoV-2 infection in pediatric populations,” they concluded.
A study from the Centers for Disease Control and Prevention published in January 2022, also concluded there was a link between COVID-19 and diabetes in children, but not with other acute respiratory infections. Children were 2.5 times more likely to be diagnosed with diabetes following a SARS-CoV-2 infection, it found.
However, the study has been criticized because it pooled all types of diabetes together and did not account for other health conditions, medications that can increase blood glucose levels, race, obesity, and other social determinants of health that might influence a child’s risk of acquiring COVID-19 or diabetes.
“I’ve no doubt that the CDC data were incorrect because the incidence rate for ... diabetes, even in those never exposed to COVID-19 infection, was 10 times the rate ever reported in the U.S.,” Dr. Colhoun said. “There’s no way these data are correct. I believe there was a confusion between incidence and prevalence of diabetes.”
“This paper caused a great deal of panic, especially among those who have a child with type 1diabetes, so we need to be very careful not to cause undue alarm until we have more definitive evidence in this arena,” she stressed.
However, she also acknowledged that the new Norwegian study was well conducted, and she has no methodological concerns about it, so “I think we just have to wait and see.”
Given the inconclusiveness on the issue, there is an ongoing CoviDiab registry collecting data on this very subject.
Dr. Tapia presented on behalf of lead author Dr. Gulseth, who has reported no relevant financial relationships. Dr. Colhoun also reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
STOCKHOLM – It remains inconclusive whether SARS-CoV-2 infection predisposes children and adolescents to a higher risk of type 1 diabetes. Data from two new studies and a recently published research letter add to the growing body of knowledge on the subject, but still can’t draw any definitive conclusions.
The latest results from a Norwegian and a Scottish study both examine incidence of type 1 diabetes in young people with a history of SARS-CoV-2 infection and were reported at the annual meeting of the European Association for the Study of Diabetes.
A 60% increased risk for type 1 diabetes at least 31 days after SARS-CoV-2 infection (adjusted hazard ratio, 1.63) was found in the Norwegian study, while in contrast, the Scottish study only found an increased risk in the first few months of the pandemic, in 2020, but importantly, no association over a much longer time period (March 2020–November 2021).
In a comment on Twitter on the two studies presented at EASD, session moderator Kamlesh Khunti, MD, professor of primary care diabetes and vascular medicine at the University of Leicester, (England), said: “In summary, two studies showing no or weak association of type 1 diabetes with COVID.”
But new data in the research letter published in JAMA Network Open, based on U.S. figures, also found an almost doubling of type 1 diabetes in children in the first few months after COVID-19 infection relative to infection with other respiratory viruses.
Lead author of the Scottish study, Helen Colhoun, PhD, honorary public health consultant at Public Health Scotland, commented: “Data in children are variable year on year, which emphasizes the need to be cautious over taking a tiny snapshot.”
Nevertheless, this is “a hugely important question and we must not drop the ball. [We must] keep looking at it and maintain scientific equipoise. ... [This] reinforces the need to carry on this analysis into the future to obtain an unequivocal picture,” she emphasized.
Norwegian study: If there is an association, the risk is small
German Tapia, PhD, from the Norwegian Institute of Public Health, Oslo, presented the results of a study of SARS-CoV-2 infection and subsequent risk of type 1 diabetes in 1.2 million children in Norway.
Of these, 424,354 children had been infected with SARS-CoV-2, and there were 990 incident cases of type 1 diabetes.
“What we do know about COVID-19 in children is that the symptoms are mild and only a small proportion are hospitalized with more serious symptoms. But we do not know the long-term effects of COVID-19 infection because this requires a longer follow-up period,” remarked Dr. Tapia, adding that other viral infections are thought to be linked to the development of type 1 diabetes, in particular, respiratory infections.
The data were sourced from the Norwegian Emergency Preparedness Register for COVID-19, which gathers daily data updates including infections (positive and negative results for free-of-charge testing), diagnoses (primary and secondary care), vaccinations (also free of charge), prescribed medications, and basic demographics.
“We link these data using the personal identification number that every Norwegian citizen has,” explained Dr. Tapia.
He presented results from two cohorts: firstly, results in children only, including those tested for SARS-CoV-2 infection, and secondly, a full national Norwegian population cohort.
Regarding the first cohort, those under 18 years who tested positive for SARS-CoV-2 infection, from March 2020 to March 2022, had a significantly increased risk of type 1 diabetes at least 31 days after infection, with an adjusted hazard ratio of 1.63 (95% confidence interval, 1.08-2.47; P = .02). Adjustments were made for age, sex, non-Nordic country of origin, geographic area, and socioeconomic factors.
For children who developed type 1 diabetes within 30 days of a SARS-CoV-2 infection, the HR was 1.26 (95% CI, 0.72-2.19; P = .42), which did not reach statistical significance.
“The fact that fewer people developed type 1 diabetes within 30 days is not surprising because we know that type 1 diabetes develops over a long period of time,” Dr. Tapia said.
“For this reason, we would not expect to find new cases of those people who develop type 1 diabetes within 30 days of COVID-19 infection,” he explained. In these cases, “it is most likely that they already had [type 1 diabetes], and the infection probably triggered clinical symptoms, so their type 1 diabetes was discovered.”
Turning to the full population cohort and diagnoses of type 1 diabetes over 30 days after SARS-CoV-2 infection, the Norwegian researchers found an association, with an HR of 1.57 (95% CI, 1.06-2.33; P = .03), while diagnosis of type 1 diabetes at 30 days or less generated a hazard ratio of 1.22 (95% CI, 0.72-2.19; P = .42).
“So very similar results were found, and after adjustment for confounders, results were still similar,” reported Dr. Tapia.
He also conducted a similar analysis with vaccination as an exposure but found no association between vaccination against SARS-CoV-2 and diagnosis of type 1 diabetes.
“From these results, we conclude that this suggests an increase in diagnosis of type 1 diabetes after SARS-CoV-2 infection, but it must be noted that the absolute risk of developing type 1 diabetes after infection in children is low, with most children not developing the disease,” he emphasized. “There are nearly half a million children who have been infected with SARS-CoV-2 in Norway, but only a very small proportion develop type 1 diabetes.”
Scottish study: No association found over longer term
Dr. Colhoun and colleagues looked at the relationship between incident type 1 diabetes and SARS-CoV-2 infection in children in Scotland using e-health record linkage.
The study involved 1.8 million people under 35 years of age and found very weak, if any, evidence of an association between incident type 1 diabetes and SARS-CoV-2.
Examining data between March 2020 and November 2021, Dr. Colhoun and colleagues identified 365,080 individuals up to age 35 with at least one detected SARS-CoV-2 infection during follow-up and 1,074 who developed type 1 diabetes.
“In children under 16 years, suspected cases of type 1 diabetes are admitted to hospital, and 97% of diagnosis dates are recorded in the Scottish Care Information – Diabetes Collaboration register [SCI-Diabetes] prior to or within 2 days of the first hospital admission for type 1 diabetes,” Dr. Colhoun said, stressing the timeliness of the data.
“We found the incidence of type 1 diabetes diagnosis increased 1.2-fold in those aged 0-14 years, but we did not find any association at an individual level of COVID-19 infection over 30 days prior to a type 1 diabetes diagnosis, in this particular dataset,” she reported. In young people aged 15-34, there was a linear increase in incident type 1 diabetes from 2015 to 2021 with no pandemic increase.
Referring to the 1.2-fold increase soon after the pandemic started, she explained that, in 0- to 14-year-olds, the increase followed a drop in the preceding months prepandemic in 2019. They also found that the seasonal pattern of type 1 diabetes diagnoses remained roughly the same across the pandemic months, with typical peaks in February and September.
In the cohort of under 35s, researchers also found a rate ratio of 2.62 (95% CI, 1.81-3.78) within a 30-day window of SARS-CoV-2 infection, but beyond 30 days, no evidence was seen of an association, with a RR of 0.86 (95% CI, 0.62-1.21; P = .40), she reported.
She explained her reasons for not considering diagnoses within 30 days of COVID-19 as causative. Echoing Dr. Tapia, Dr. Colhoun said the median time from symptom onset to diagnosis of type 1 diabetes is 25 days. “This suggests that 50% have had symptoms for over 25 days at diagnosis.”
She also stressed that when they compared the timing of SARS-CoV-2 testing with diagnosis, they found a much higher rate of COVID-19 testing around diagnosis. “This was not least because everyone admitted to hospital had to have a COVID-19 test.”
Latest U.S. data point to a link
Meanwhile, for the new data reported in JAMA Network Open, medical student Ellen K. Kendall of Case Western Reserve University, Cleveland, matched 571,256 pediatric patients: 285,628 with COVID-19 and 285,628 with non–COVID-19 respiratory infections.
By 6 months after COVID-19, 123 patients (0.043%) had received a new diagnosis of type 1 diabetes, but only 72 (0.025%) were diagnosed with type 1 diabetes within 6 months after non–COVID-19 respiratory infection.
At 1, 3, and 6 months after infection, risk of diagnosis of type 1 diabetes was greater among those infected with SARS-CoV-2, compared with those with non–COVID-19 respiratory infection (1 month: HR, 1.96; 3 months: HR, 2.10; and 6 months: HR, 1.83), and in subgroups of patients aged 0-9 years, a group unlikely to develop type 2 diabetes.
“In this study, new type 1 diabetes diagnoses were more likely to occur among pediatric patients with prior COVID-19 than among those with other respiratory infections (or with other encounters with health systems),” noted Ms. Kendall and coauthors. “Respiratory infections have previously been associated with onset of type 1 diabetes, but this risk was even higher among those with COVID-19 in our study, raising concern for long-term, post–COVID-19 autoimmune complications among youths.”
“The increased risk of new-onset type 1 diabetes after COVID-19 adds an important consideration for risk–benefit discussions for prevention and treatment of SARS-CoV-2 infection in pediatric populations,” they concluded.
A study from the Centers for Disease Control and Prevention published in January 2022, also concluded there was a link between COVID-19 and diabetes in children, but not with other acute respiratory infections. Children were 2.5 times more likely to be diagnosed with diabetes following a SARS-CoV-2 infection, it found.
However, the study has been criticized because it pooled all types of diabetes together and did not account for other health conditions, medications that can increase blood glucose levels, race, obesity, and other social determinants of health that might influence a child’s risk of acquiring COVID-19 or diabetes.
“I’ve no doubt that the CDC data were incorrect because the incidence rate for ... diabetes, even in those never exposed to COVID-19 infection, was 10 times the rate ever reported in the U.S.,” Dr. Colhoun said. “There’s no way these data are correct. I believe there was a confusion between incidence and prevalence of diabetes.”
“This paper caused a great deal of panic, especially among those who have a child with type 1diabetes, so we need to be very careful not to cause undue alarm until we have more definitive evidence in this arena,” she stressed.
However, she also acknowledged that the new Norwegian study was well conducted, and she has no methodological concerns about it, so “I think we just have to wait and see.”
Given the inconclusiveness on the issue, there is an ongoing CoviDiab registry collecting data on this very subject.
Dr. Tapia presented on behalf of lead author Dr. Gulseth, who has reported no relevant financial relationships. Dr. Colhoun also reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
STOCKHOLM – It remains inconclusive whether SARS-CoV-2 infection predisposes children and adolescents to a higher risk of type 1 diabetes. Data from two new studies and a recently published research letter add to the growing body of knowledge on the subject, but still can’t draw any definitive conclusions.
The latest results from a Norwegian and a Scottish study both examine incidence of type 1 diabetes in young people with a history of SARS-CoV-2 infection and were reported at the annual meeting of the European Association for the Study of Diabetes.
A 60% increased risk for type 1 diabetes at least 31 days after SARS-CoV-2 infection (adjusted hazard ratio, 1.63) was found in the Norwegian study, while in contrast, the Scottish study only found an increased risk in the first few months of the pandemic, in 2020, but importantly, no association over a much longer time period (March 2020–November 2021).
In a comment on Twitter on the two studies presented at EASD, session moderator Kamlesh Khunti, MD, professor of primary care diabetes and vascular medicine at the University of Leicester, (England), said: “In summary, two studies showing no or weak association of type 1 diabetes with COVID.”
But new data in the research letter published in JAMA Network Open, based on U.S. figures, also found an almost doubling of type 1 diabetes in children in the first few months after COVID-19 infection relative to infection with other respiratory viruses.
Lead author of the Scottish study, Helen Colhoun, PhD, honorary public health consultant at Public Health Scotland, commented: “Data in children are variable year on year, which emphasizes the need to be cautious over taking a tiny snapshot.”
Nevertheless, this is “a hugely important question and we must not drop the ball. [We must] keep looking at it and maintain scientific equipoise. ... [This] reinforces the need to carry on this analysis into the future to obtain an unequivocal picture,” she emphasized.
Norwegian study: If there is an association, the risk is small
German Tapia, PhD, from the Norwegian Institute of Public Health, Oslo, presented the results of a study of SARS-CoV-2 infection and subsequent risk of type 1 diabetes in 1.2 million children in Norway.
Of these, 424,354 children had been infected with SARS-CoV-2, and there were 990 incident cases of type 1 diabetes.
“What we do know about COVID-19 in children is that the symptoms are mild and only a small proportion are hospitalized with more serious symptoms. But we do not know the long-term effects of COVID-19 infection because this requires a longer follow-up period,” remarked Dr. Tapia, adding that other viral infections are thought to be linked to the development of type 1 diabetes, in particular, respiratory infections.
The data were sourced from the Norwegian Emergency Preparedness Register for COVID-19, which gathers daily data updates including infections (positive and negative results for free-of-charge testing), diagnoses (primary and secondary care), vaccinations (also free of charge), prescribed medications, and basic demographics.
“We link these data using the personal identification number that every Norwegian citizen has,” explained Dr. Tapia.
He presented results from two cohorts: firstly, results in children only, including those tested for SARS-CoV-2 infection, and secondly, a full national Norwegian population cohort.
Regarding the first cohort, those under 18 years who tested positive for SARS-CoV-2 infection, from March 2020 to March 2022, had a significantly increased risk of type 1 diabetes at least 31 days after infection, with an adjusted hazard ratio of 1.63 (95% confidence interval, 1.08-2.47; P = .02). Adjustments were made for age, sex, non-Nordic country of origin, geographic area, and socioeconomic factors.
For children who developed type 1 diabetes within 30 days of a SARS-CoV-2 infection, the HR was 1.26 (95% CI, 0.72-2.19; P = .42), which did not reach statistical significance.
“The fact that fewer people developed type 1 diabetes within 30 days is not surprising because we know that type 1 diabetes develops over a long period of time,” Dr. Tapia said.
“For this reason, we would not expect to find new cases of those people who develop type 1 diabetes within 30 days of COVID-19 infection,” he explained. In these cases, “it is most likely that they already had [type 1 diabetes], and the infection probably triggered clinical symptoms, so their type 1 diabetes was discovered.”
Turning to the full population cohort and diagnoses of type 1 diabetes over 30 days after SARS-CoV-2 infection, the Norwegian researchers found an association, with an HR of 1.57 (95% CI, 1.06-2.33; P = .03), while diagnosis of type 1 diabetes at 30 days or less generated a hazard ratio of 1.22 (95% CI, 0.72-2.19; P = .42).
“So very similar results were found, and after adjustment for confounders, results were still similar,” reported Dr. Tapia.
He also conducted a similar analysis with vaccination as an exposure but found no association between vaccination against SARS-CoV-2 and diagnosis of type 1 diabetes.
“From these results, we conclude that this suggests an increase in diagnosis of type 1 diabetes after SARS-CoV-2 infection, but it must be noted that the absolute risk of developing type 1 diabetes after infection in children is low, with most children not developing the disease,” he emphasized. “There are nearly half a million children who have been infected with SARS-CoV-2 in Norway, but only a very small proportion develop type 1 diabetes.”
Scottish study: No association found over longer term
Dr. Colhoun and colleagues looked at the relationship between incident type 1 diabetes and SARS-CoV-2 infection in children in Scotland using e-health record linkage.
The study involved 1.8 million people under 35 years of age and found very weak, if any, evidence of an association between incident type 1 diabetes and SARS-CoV-2.
Examining data between March 2020 and November 2021, Dr. Colhoun and colleagues identified 365,080 individuals up to age 35 with at least one detected SARS-CoV-2 infection during follow-up and 1,074 who developed type 1 diabetes.
“In children under 16 years, suspected cases of type 1 diabetes are admitted to hospital, and 97% of diagnosis dates are recorded in the Scottish Care Information – Diabetes Collaboration register [SCI-Diabetes] prior to or within 2 days of the first hospital admission for type 1 diabetes,” Dr. Colhoun said, stressing the timeliness of the data.
“We found the incidence of type 1 diabetes diagnosis increased 1.2-fold in those aged 0-14 years, but we did not find any association at an individual level of COVID-19 infection over 30 days prior to a type 1 diabetes diagnosis, in this particular dataset,” she reported. In young people aged 15-34, there was a linear increase in incident type 1 diabetes from 2015 to 2021 with no pandemic increase.
Referring to the 1.2-fold increase soon after the pandemic started, she explained that, in 0- to 14-year-olds, the increase followed a drop in the preceding months prepandemic in 2019. They also found that the seasonal pattern of type 1 diabetes diagnoses remained roughly the same across the pandemic months, with typical peaks in February and September.
In the cohort of under 35s, researchers also found a rate ratio of 2.62 (95% CI, 1.81-3.78) within a 30-day window of SARS-CoV-2 infection, but beyond 30 days, no evidence was seen of an association, with a RR of 0.86 (95% CI, 0.62-1.21; P = .40), she reported.
She explained her reasons for not considering diagnoses within 30 days of COVID-19 as causative. Echoing Dr. Tapia, Dr. Colhoun said the median time from symptom onset to diagnosis of type 1 diabetes is 25 days. “This suggests that 50% have had symptoms for over 25 days at diagnosis.”
She also stressed that when they compared the timing of SARS-CoV-2 testing with diagnosis, they found a much higher rate of COVID-19 testing around diagnosis. “This was not least because everyone admitted to hospital had to have a COVID-19 test.”
Latest U.S. data point to a link
Meanwhile, for the new data reported in JAMA Network Open, medical student Ellen K. Kendall of Case Western Reserve University, Cleveland, matched 571,256 pediatric patients: 285,628 with COVID-19 and 285,628 with non–COVID-19 respiratory infections.
By 6 months after COVID-19, 123 patients (0.043%) had received a new diagnosis of type 1 diabetes, but only 72 (0.025%) were diagnosed with type 1 diabetes within 6 months after non–COVID-19 respiratory infection.
At 1, 3, and 6 months after infection, risk of diagnosis of type 1 diabetes was greater among those infected with SARS-CoV-2, compared with those with non–COVID-19 respiratory infection (1 month: HR, 1.96; 3 months: HR, 2.10; and 6 months: HR, 1.83), and in subgroups of patients aged 0-9 years, a group unlikely to develop type 2 diabetes.
“In this study, new type 1 diabetes diagnoses were more likely to occur among pediatric patients with prior COVID-19 than among those with other respiratory infections (or with other encounters with health systems),” noted Ms. Kendall and coauthors. “Respiratory infections have previously been associated with onset of type 1 diabetes, but this risk was even higher among those with COVID-19 in our study, raising concern for long-term, post–COVID-19 autoimmune complications among youths.”
“The increased risk of new-onset type 1 diabetes after COVID-19 adds an important consideration for risk–benefit discussions for prevention and treatment of SARS-CoV-2 infection in pediatric populations,” they concluded.
A study from the Centers for Disease Control and Prevention published in January 2022, also concluded there was a link between COVID-19 and diabetes in children, but not with other acute respiratory infections. Children were 2.5 times more likely to be diagnosed with diabetes following a SARS-CoV-2 infection, it found.
However, the study has been criticized because it pooled all types of diabetes together and did not account for other health conditions, medications that can increase blood glucose levels, race, obesity, and other social determinants of health that might influence a child’s risk of acquiring COVID-19 or diabetes.
“I’ve no doubt that the CDC data were incorrect because the incidence rate for ... diabetes, even in those never exposed to COVID-19 infection, was 10 times the rate ever reported in the U.S.,” Dr. Colhoun said. “There’s no way these data are correct. I believe there was a confusion between incidence and prevalence of diabetes.”
“This paper caused a great deal of panic, especially among those who have a child with type 1diabetes, so we need to be very careful not to cause undue alarm until we have more definitive evidence in this arena,” she stressed.
However, she also acknowledged that the new Norwegian study was well conducted, and she has no methodological concerns about it, so “I think we just have to wait and see.”
Given the inconclusiveness on the issue, there is an ongoing CoviDiab registry collecting data on this very subject.
Dr. Tapia presented on behalf of lead author Dr. Gulseth, who has reported no relevant financial relationships. Dr. Colhoun also reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
AT EASD 2022
Type 1 diabetes cases poised to double worldwide by 2040
STOCKHOLM – The number of people living with type 1 diabetes worldwide is expected to double by 2040, with most new cases among adults living in low- and middle-income countries, new modeling data suggest.
The forecast, developed from available data collected in the newly established open-source Type 1 Diabetes Index, provides estimates for type 1 diabetes prevalence, incidence, associated mortality, and life expectancy for 201 countries for 2021.
The model also projects estimates for prevalent cases in 2040. It is the first type 1 diabetes dataset to account for the lack of prevalence because of premature mortality, particularly in low- and middle-income countries.
“The worldwide prevalence of type 1 diabetes is substantial and growing. Improved surveillance – particularly in adults who make up most of the population living with type 1 diabetes – is essential to enable improvements to care and outcomes. There is an opportunity to save millions of lives in the coming decades by raising the standard of care (including ensuring universal access to insulin and other essential supplies) and increasing awareness of the signs and symptoms of type 1 diabetes to enable a 100% rate of diagnosis in all countries,” the authors write.
“This work spells out the need for early diagnosis of type 1 diabetes and timely access to quality care,” said Chantal Mathieu, MD, at the European Association for the Study of Diabetes annual meeting.
One in five deaths from type 1 diabetes in under 25s
The new findings were published in Lancet Diabetes & Endocrinology by Gabriel A. Gregory, MD, of Life for a Child Program, New South Wales, Australia, and colleagues. The T1D Index Project database was published Sept. 21, 2022.
According to the model, about 8.4 million people were living with type 1 diabetes in 2021, with one-fifth from low- and middle-income countries. An additional 3.7 million died prematurely and would have been added to that count had they lived. One in five of all deaths caused by type 1 diabetes in 2021 is estimated to have occurred in people younger than age 25 years because of nondiagnosis.
“It is unacceptable that, in 2022, some 35,000 people worldwide are dying undiagnosed within a year of onset of symptoms. There also continues to be a huge disparity in life expectancy for people with type 1 diabetes, hitting those in the poorest countries hardest,” noted Dr. Mathieu, who is senior vice-president of EASD and an endocrinologist based at KU Leuven, Belgium.
By 2040, the model predicts that between 13.5 million and 17.4 million people will be living with the condition, with the largest relative increase from 2021 in low-income and lower-middle-income countries. The majority of incident and prevalent cases of type 1 diabetes are in adults, with an estimated 62% of 510,000 new diagnoses worldwide in 2021 occurring in people aged 20 years and older.
Type 1 diabetes is not predominantly a disease of childhood
Dr. Mathieu also noted that the data dispute the long-held view of type 1 diabetes as a predominantly pediatric condition. Indeed, worldwide, the median age for a person living with type 1 diabetes is 37 years.
“While type 1 diabetes is often referred to as ‘child-onset’ diabetes, this important study shows that only around one in five living with the condition are aged 20 years or younger, two-thirds are aged 20-64 years, and a further one in five are aged 65 years or older.”
“This condition does not stop at age 18 years – the children become adults, and the adults become elderly. All countries must examine and strengthen their diagnosis and care pathways for people of all ages living with type 1 diabetes,” Dr. Mathieu emphasized.
And in an accompanying editorial, Serena Jingchuan Guo, MD, PhD, and Hui Shao, MD, PhD, point out that most studies that estimate diabetes burden have focused on type 2 diabetes, noting, “type 1 diabetes faces the challenges of misdiagnosis, underdiagnosis, high risk of complications, and premature mortality.”
The insulin affordability issue is central, point out Dr. Guo and Dr. Shao of the Center for Drug Evaluation and Safety, department of pharmaceutical evaluation and policy, University of Florida College of Pharmacy, Gainesville.
“Countries need to strengthen the price regulation and reimbursement policy for insulin while building subsidy programs to ensure insulin access and to cope with the growing demand for insulin. Meanwhile, optimizing the insulin supply chain between manufacturers and patients while seeking alternative treatment options (for example, biosimilar products) will also improve the current situation,” they conclude.
The study was funded by JDRF, of which four coauthors are employees. The editorialists have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
STOCKHOLM – The number of people living with type 1 diabetes worldwide is expected to double by 2040, with most new cases among adults living in low- and middle-income countries, new modeling data suggest.
The forecast, developed from available data collected in the newly established open-source Type 1 Diabetes Index, provides estimates for type 1 diabetes prevalence, incidence, associated mortality, and life expectancy for 201 countries for 2021.
The model also projects estimates for prevalent cases in 2040. It is the first type 1 diabetes dataset to account for the lack of prevalence because of premature mortality, particularly in low- and middle-income countries.
“The worldwide prevalence of type 1 diabetes is substantial and growing. Improved surveillance – particularly in adults who make up most of the population living with type 1 diabetes – is essential to enable improvements to care and outcomes. There is an opportunity to save millions of lives in the coming decades by raising the standard of care (including ensuring universal access to insulin and other essential supplies) and increasing awareness of the signs and symptoms of type 1 diabetes to enable a 100% rate of diagnosis in all countries,” the authors write.
“This work spells out the need for early diagnosis of type 1 diabetes and timely access to quality care,” said Chantal Mathieu, MD, at the European Association for the Study of Diabetes annual meeting.
One in five deaths from type 1 diabetes in under 25s
The new findings were published in Lancet Diabetes & Endocrinology by Gabriel A. Gregory, MD, of Life for a Child Program, New South Wales, Australia, and colleagues. The T1D Index Project database was published Sept. 21, 2022.
According to the model, about 8.4 million people were living with type 1 diabetes in 2021, with one-fifth from low- and middle-income countries. An additional 3.7 million died prematurely and would have been added to that count had they lived. One in five of all deaths caused by type 1 diabetes in 2021 is estimated to have occurred in people younger than age 25 years because of nondiagnosis.
“It is unacceptable that, in 2022, some 35,000 people worldwide are dying undiagnosed within a year of onset of symptoms. There also continues to be a huge disparity in life expectancy for people with type 1 diabetes, hitting those in the poorest countries hardest,” noted Dr. Mathieu, who is senior vice-president of EASD and an endocrinologist based at KU Leuven, Belgium.
By 2040, the model predicts that between 13.5 million and 17.4 million people will be living with the condition, with the largest relative increase from 2021 in low-income and lower-middle-income countries. The majority of incident and prevalent cases of type 1 diabetes are in adults, with an estimated 62% of 510,000 new diagnoses worldwide in 2021 occurring in people aged 20 years and older.
Type 1 diabetes is not predominantly a disease of childhood
Dr. Mathieu also noted that the data dispute the long-held view of type 1 diabetes as a predominantly pediatric condition. Indeed, worldwide, the median age for a person living with type 1 diabetes is 37 years.
“While type 1 diabetes is often referred to as ‘child-onset’ diabetes, this important study shows that only around one in five living with the condition are aged 20 years or younger, two-thirds are aged 20-64 years, and a further one in five are aged 65 years or older.”
“This condition does not stop at age 18 years – the children become adults, and the adults become elderly. All countries must examine and strengthen their diagnosis and care pathways for people of all ages living with type 1 diabetes,” Dr. Mathieu emphasized.
And in an accompanying editorial, Serena Jingchuan Guo, MD, PhD, and Hui Shao, MD, PhD, point out that most studies that estimate diabetes burden have focused on type 2 diabetes, noting, “type 1 diabetes faces the challenges of misdiagnosis, underdiagnosis, high risk of complications, and premature mortality.”
The insulin affordability issue is central, point out Dr. Guo and Dr. Shao of the Center for Drug Evaluation and Safety, department of pharmaceutical evaluation and policy, University of Florida College of Pharmacy, Gainesville.
“Countries need to strengthen the price regulation and reimbursement policy for insulin while building subsidy programs to ensure insulin access and to cope with the growing demand for insulin. Meanwhile, optimizing the insulin supply chain between manufacturers and patients while seeking alternative treatment options (for example, biosimilar products) will also improve the current situation,” they conclude.
The study was funded by JDRF, of which four coauthors are employees. The editorialists have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
STOCKHOLM – The number of people living with type 1 diabetes worldwide is expected to double by 2040, with most new cases among adults living in low- and middle-income countries, new modeling data suggest.
The forecast, developed from available data collected in the newly established open-source Type 1 Diabetes Index, provides estimates for type 1 diabetes prevalence, incidence, associated mortality, and life expectancy for 201 countries for 2021.
The model also projects estimates for prevalent cases in 2040. It is the first type 1 diabetes dataset to account for the lack of prevalence because of premature mortality, particularly in low- and middle-income countries.
“The worldwide prevalence of type 1 diabetes is substantial and growing. Improved surveillance – particularly in adults who make up most of the population living with type 1 diabetes – is essential to enable improvements to care and outcomes. There is an opportunity to save millions of lives in the coming decades by raising the standard of care (including ensuring universal access to insulin and other essential supplies) and increasing awareness of the signs and symptoms of type 1 diabetes to enable a 100% rate of diagnosis in all countries,” the authors write.
“This work spells out the need for early diagnosis of type 1 diabetes and timely access to quality care,” said Chantal Mathieu, MD, at the European Association for the Study of Diabetes annual meeting.
One in five deaths from type 1 diabetes in under 25s
The new findings were published in Lancet Diabetes & Endocrinology by Gabriel A. Gregory, MD, of Life for a Child Program, New South Wales, Australia, and colleagues. The T1D Index Project database was published Sept. 21, 2022.
According to the model, about 8.4 million people were living with type 1 diabetes in 2021, with one-fifth from low- and middle-income countries. An additional 3.7 million died prematurely and would have been added to that count had they lived. One in five of all deaths caused by type 1 diabetes in 2021 is estimated to have occurred in people younger than age 25 years because of nondiagnosis.
“It is unacceptable that, in 2022, some 35,000 people worldwide are dying undiagnosed within a year of onset of symptoms. There also continues to be a huge disparity in life expectancy for people with type 1 diabetes, hitting those in the poorest countries hardest,” noted Dr. Mathieu, who is senior vice-president of EASD and an endocrinologist based at KU Leuven, Belgium.
By 2040, the model predicts that between 13.5 million and 17.4 million people will be living with the condition, with the largest relative increase from 2021 in low-income and lower-middle-income countries. The majority of incident and prevalent cases of type 1 diabetes are in adults, with an estimated 62% of 510,000 new diagnoses worldwide in 2021 occurring in people aged 20 years and older.
Type 1 diabetes is not predominantly a disease of childhood
Dr. Mathieu also noted that the data dispute the long-held view of type 1 diabetes as a predominantly pediatric condition. Indeed, worldwide, the median age for a person living with type 1 diabetes is 37 years.
“While type 1 diabetes is often referred to as ‘child-onset’ diabetes, this important study shows that only around one in five living with the condition are aged 20 years or younger, two-thirds are aged 20-64 years, and a further one in five are aged 65 years or older.”
“This condition does not stop at age 18 years – the children become adults, and the adults become elderly. All countries must examine and strengthen their diagnosis and care pathways for people of all ages living with type 1 diabetes,” Dr. Mathieu emphasized.
And in an accompanying editorial, Serena Jingchuan Guo, MD, PhD, and Hui Shao, MD, PhD, point out that most studies that estimate diabetes burden have focused on type 2 diabetes, noting, “type 1 diabetes faces the challenges of misdiagnosis, underdiagnosis, high risk of complications, and premature mortality.”
The insulin affordability issue is central, point out Dr. Guo and Dr. Shao of the Center for Drug Evaluation and Safety, department of pharmaceutical evaluation and policy, University of Florida College of Pharmacy, Gainesville.
“Countries need to strengthen the price regulation and reimbursement policy for insulin while building subsidy programs to ensure insulin access and to cope with the growing demand for insulin. Meanwhile, optimizing the insulin supply chain between manufacturers and patients while seeking alternative treatment options (for example, biosimilar products) will also improve the current situation,” they conclude.
The study was funded by JDRF, of which four coauthors are employees. The editorialists have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
AT EASD 2022
Emphasis on weight loss in new type 2 diabetes guidance
STOCKHOLM – Weight loss should be a co–primary management goal for type 2 diabetes in adults, according to a new comprehensive joint consensus report from the European Association for the Study of Diabetes and the American Diabetes Association.
And while metformin is still recommended as first-line therapy for patients with type 2 diabetes with no other comorbidities, the statement expands the indications for use of other agents or combinations of agents as initial therapy for subgroups of patients, as part of individualized and patient-centered decision-making.
Last updated in 2019, the new “Management of Hyperglycemia in Type 2 Diabetes” statement also places increased emphasis on social determinants of health, incorporates recent clinical trial data for cardiovascular and kidney outcomes for sodium-glucose cotransporter-2 (SGLT2) inhibitors and glucagonlike peptide–1 (GLP-1) agonists to broaden recommendations for cardiorenal protection, and discusses health behaviors such as sleep and sitting. It also targets a wider audience than in the past by addressing health system organization to optimize delivery of diabetes care.
The new statement was presented during a 90-minute session at the annual meeting of the EASD, with 12 of its 14 European and American authors as presenters. The document was simultaneously published in Diabetologia and Diabetes Care.
During the discussion, panel member Jennifer Brigitte Green, MD, commented: “Many of these recommendations are not new. They’re modest revisions of recommendations that have been in place for years, but we know that actual implementation rates of use of these drugs in patients with established comorbidities are very low.”
“I think it’s time for communities, health care systems, etc, to actually introduce these as expectations of care... to assess quality because unless it’s considered formally to be a requirement of care I just don’t think we’re going to move that needle very much,” added Dr. Green, who is professor of medicine at Duke University, Durham, N.C.
Vanita R. Aroda, MD, of the division of endocrinology, diabetes, and hypertension at Brigham and Women’s Hospital, Boston, commented: “In the past, sometimes these recommendations created fodder for debate, but I don’t think this one will. It’s just really solidly evidence based, with the rationales presented throughout, including the figures. I think just having very clear evidence-based directions should support their dissemination and use.”
Weight management plays a prominent role in treatment
In an interview, writing panel cochair John B. Buse, MD, PhD, said: “We are saying that the four major components of type 2 diabetes care are glycemic management, cardiovascular risk management, weight management, and prevention of end-organ damage, particularly with regard to cardiorenal risk.”
“The weight management piece is much more explicit now,” said Dr. Buse, director of the Diabetes Center at the University of North Carolina at Chapel Hill.
He noted that recent evidence from the intensive lifestyle trial DiRECT, conducted in the United Kingdom, the bariatric surgery literature, and the emergence of potent weight-loss drugs have meant that “achieving 10%-15% body weight loss is now possible.
“So, aiming for remission is something that might be attractive to patients and providers. This could be based on weight management, with the [chosen] method based on shared decision-making.”
According to the new report: “Weight loss of 5%-10% confers metabolic improvement; weight loss of 10%-15% or more can have a disease-modifying effect and lead to remission of diabetes, defined as normal blood glucose levels for 3 months or more in the absence of pharmacological therapy in a 2021 consensus report.”
“Weight loss may exert benefits that extend beyond glycemic management to improve risk factors for cardiometabolic disease and quality of life,” it adds.
Individualization featured throughout
The report’s sections cover principles of care, including the importance of diabetes self-management education and support and avoidance of therapeutic inertia. Detailed guidance addresses therapeutic options including lifestyle, weight management, and pharmacotherapy for treating type 2 diabetes.
Another entire section is devoted to personalizing treatment approaches based on individual characteristics, including new evidence from cardiorenal outcomes studies for SGLT2 inhibitors and GLP-1 agonists that have come out since the last consensus report.
The document advises: “Consider initial combination therapy with glucose-lowering agents, especially in those with high [hemoglobin] A1c at diagnosis (that is, > 70 mmol/mol [> 8.5%]), in younger people with type 2 diabetes (regardless of A1c), and in those in whom a stepwise approach would delay access to agents that provide cardiorenal protection beyond their glucose-lowering effects.”
Designed to be used and user-friendly
Under the “Putting it all together: strategies for implementation” section, several lists of “practical tips for clinicians” are provided for many of the topics covered.
A series of colorful infographics are included as well, addressing the “decision cycle for person-centered glycemic management in type 2 diabetes,” including a chart summarizing characteristics of available glucose-lowering medications, including cardiorenal protection.
Also mentioned is the importance of 24-hour physical behaviors (including sleep, sitting, and sweating) and the impact on cardiometabolic health, use of a “holistic person-centered approach” to type 2 diabetes management, and an algorithm on insulin use.
Dr. Buse has financial ties to numerous drug and device companies. Dr. Green is a consultant for AstraZeneca, Pfizer, Boehringer Ingelheim/Lilly, Bayer, Sanofi, Anji, Vertex/ICON, and Valo. Dr. Aroda has served as a consultant for Applied Therapeutics, Duke, Fractyl, Novo Nordisk, Pfizer, and Sanofi.
A version of this article first appeared on Medscape.com.
STOCKHOLM – Weight loss should be a co–primary management goal for type 2 diabetes in adults, according to a new comprehensive joint consensus report from the European Association for the Study of Diabetes and the American Diabetes Association.
And while metformin is still recommended as first-line therapy for patients with type 2 diabetes with no other comorbidities, the statement expands the indications for use of other agents or combinations of agents as initial therapy for subgroups of patients, as part of individualized and patient-centered decision-making.
Last updated in 2019, the new “Management of Hyperglycemia in Type 2 Diabetes” statement also places increased emphasis on social determinants of health, incorporates recent clinical trial data for cardiovascular and kidney outcomes for sodium-glucose cotransporter-2 (SGLT2) inhibitors and glucagonlike peptide–1 (GLP-1) agonists to broaden recommendations for cardiorenal protection, and discusses health behaviors such as sleep and sitting. It also targets a wider audience than in the past by addressing health system organization to optimize delivery of diabetes care.
The new statement was presented during a 90-minute session at the annual meeting of the EASD, with 12 of its 14 European and American authors as presenters. The document was simultaneously published in Diabetologia and Diabetes Care.
During the discussion, panel member Jennifer Brigitte Green, MD, commented: “Many of these recommendations are not new. They’re modest revisions of recommendations that have been in place for years, but we know that actual implementation rates of use of these drugs in patients with established comorbidities are very low.”
“I think it’s time for communities, health care systems, etc, to actually introduce these as expectations of care... to assess quality because unless it’s considered formally to be a requirement of care I just don’t think we’re going to move that needle very much,” added Dr. Green, who is professor of medicine at Duke University, Durham, N.C.
Vanita R. Aroda, MD, of the division of endocrinology, diabetes, and hypertension at Brigham and Women’s Hospital, Boston, commented: “In the past, sometimes these recommendations created fodder for debate, but I don’t think this one will. It’s just really solidly evidence based, with the rationales presented throughout, including the figures. I think just having very clear evidence-based directions should support their dissemination and use.”
Weight management plays a prominent role in treatment
In an interview, writing panel cochair John B. Buse, MD, PhD, said: “We are saying that the four major components of type 2 diabetes care are glycemic management, cardiovascular risk management, weight management, and prevention of end-organ damage, particularly with regard to cardiorenal risk.”
“The weight management piece is much more explicit now,” said Dr. Buse, director of the Diabetes Center at the University of North Carolina at Chapel Hill.
He noted that recent evidence from the intensive lifestyle trial DiRECT, conducted in the United Kingdom, the bariatric surgery literature, and the emergence of potent weight-loss drugs have meant that “achieving 10%-15% body weight loss is now possible.
“So, aiming for remission is something that might be attractive to patients and providers. This could be based on weight management, with the [chosen] method based on shared decision-making.”
According to the new report: “Weight loss of 5%-10% confers metabolic improvement; weight loss of 10%-15% or more can have a disease-modifying effect and lead to remission of diabetes, defined as normal blood glucose levels for 3 months or more in the absence of pharmacological therapy in a 2021 consensus report.”
“Weight loss may exert benefits that extend beyond glycemic management to improve risk factors for cardiometabolic disease and quality of life,” it adds.
Individualization featured throughout
The report’s sections cover principles of care, including the importance of diabetes self-management education and support and avoidance of therapeutic inertia. Detailed guidance addresses therapeutic options including lifestyle, weight management, and pharmacotherapy for treating type 2 diabetes.
Another entire section is devoted to personalizing treatment approaches based on individual characteristics, including new evidence from cardiorenal outcomes studies for SGLT2 inhibitors and GLP-1 agonists that have come out since the last consensus report.
The document advises: “Consider initial combination therapy with glucose-lowering agents, especially in those with high [hemoglobin] A1c at diagnosis (that is, > 70 mmol/mol [> 8.5%]), in younger people with type 2 diabetes (regardless of A1c), and in those in whom a stepwise approach would delay access to agents that provide cardiorenal protection beyond their glucose-lowering effects.”
Designed to be used and user-friendly
Under the “Putting it all together: strategies for implementation” section, several lists of “practical tips for clinicians” are provided for many of the topics covered.
A series of colorful infographics are included as well, addressing the “decision cycle for person-centered glycemic management in type 2 diabetes,” including a chart summarizing characteristics of available glucose-lowering medications, including cardiorenal protection.
Also mentioned is the importance of 24-hour physical behaviors (including sleep, sitting, and sweating) and the impact on cardiometabolic health, use of a “holistic person-centered approach” to type 2 diabetes management, and an algorithm on insulin use.
Dr. Buse has financial ties to numerous drug and device companies. Dr. Green is a consultant for AstraZeneca, Pfizer, Boehringer Ingelheim/Lilly, Bayer, Sanofi, Anji, Vertex/ICON, and Valo. Dr. Aroda has served as a consultant for Applied Therapeutics, Duke, Fractyl, Novo Nordisk, Pfizer, and Sanofi.
A version of this article first appeared on Medscape.com.
STOCKHOLM – Weight loss should be a co–primary management goal for type 2 diabetes in adults, according to a new comprehensive joint consensus report from the European Association for the Study of Diabetes and the American Diabetes Association.
And while metformin is still recommended as first-line therapy for patients with type 2 diabetes with no other comorbidities, the statement expands the indications for use of other agents or combinations of agents as initial therapy for subgroups of patients, as part of individualized and patient-centered decision-making.
Last updated in 2019, the new “Management of Hyperglycemia in Type 2 Diabetes” statement also places increased emphasis on social determinants of health, incorporates recent clinical trial data for cardiovascular and kidney outcomes for sodium-glucose cotransporter-2 (SGLT2) inhibitors and glucagonlike peptide–1 (GLP-1) agonists to broaden recommendations for cardiorenal protection, and discusses health behaviors such as sleep and sitting. It also targets a wider audience than in the past by addressing health system organization to optimize delivery of diabetes care.
The new statement was presented during a 90-minute session at the annual meeting of the EASD, with 12 of its 14 European and American authors as presenters. The document was simultaneously published in Diabetologia and Diabetes Care.
During the discussion, panel member Jennifer Brigitte Green, MD, commented: “Many of these recommendations are not new. They’re modest revisions of recommendations that have been in place for years, but we know that actual implementation rates of use of these drugs in patients with established comorbidities are very low.”
“I think it’s time for communities, health care systems, etc, to actually introduce these as expectations of care... to assess quality because unless it’s considered formally to be a requirement of care I just don’t think we’re going to move that needle very much,” added Dr. Green, who is professor of medicine at Duke University, Durham, N.C.
Vanita R. Aroda, MD, of the division of endocrinology, diabetes, and hypertension at Brigham and Women’s Hospital, Boston, commented: “In the past, sometimes these recommendations created fodder for debate, but I don’t think this one will. It’s just really solidly evidence based, with the rationales presented throughout, including the figures. I think just having very clear evidence-based directions should support their dissemination and use.”
Weight management plays a prominent role in treatment
In an interview, writing panel cochair John B. Buse, MD, PhD, said: “We are saying that the four major components of type 2 diabetes care are glycemic management, cardiovascular risk management, weight management, and prevention of end-organ damage, particularly with regard to cardiorenal risk.”
“The weight management piece is much more explicit now,” said Dr. Buse, director of the Diabetes Center at the University of North Carolina at Chapel Hill.
He noted that recent evidence from the intensive lifestyle trial DiRECT, conducted in the United Kingdom, the bariatric surgery literature, and the emergence of potent weight-loss drugs have meant that “achieving 10%-15% body weight loss is now possible.
“So, aiming for remission is something that might be attractive to patients and providers. This could be based on weight management, with the [chosen] method based on shared decision-making.”
According to the new report: “Weight loss of 5%-10% confers metabolic improvement; weight loss of 10%-15% or more can have a disease-modifying effect and lead to remission of diabetes, defined as normal blood glucose levels for 3 months or more in the absence of pharmacological therapy in a 2021 consensus report.”
“Weight loss may exert benefits that extend beyond glycemic management to improve risk factors for cardiometabolic disease and quality of life,” it adds.
Individualization featured throughout
The report’s sections cover principles of care, including the importance of diabetes self-management education and support and avoidance of therapeutic inertia. Detailed guidance addresses therapeutic options including lifestyle, weight management, and pharmacotherapy for treating type 2 diabetes.
Another entire section is devoted to personalizing treatment approaches based on individual characteristics, including new evidence from cardiorenal outcomes studies for SGLT2 inhibitors and GLP-1 agonists that have come out since the last consensus report.
The document advises: “Consider initial combination therapy with glucose-lowering agents, especially in those with high [hemoglobin] A1c at diagnosis (that is, > 70 mmol/mol [> 8.5%]), in younger people with type 2 diabetes (regardless of A1c), and in those in whom a stepwise approach would delay access to agents that provide cardiorenal protection beyond their glucose-lowering effects.”
Designed to be used and user-friendly
Under the “Putting it all together: strategies for implementation” section, several lists of “practical tips for clinicians” are provided for many of the topics covered.
A series of colorful infographics are included as well, addressing the “decision cycle for person-centered glycemic management in type 2 diabetes,” including a chart summarizing characteristics of available glucose-lowering medications, including cardiorenal protection.
Also mentioned is the importance of 24-hour physical behaviors (including sleep, sitting, and sweating) and the impact on cardiometabolic health, use of a “holistic person-centered approach” to type 2 diabetes management, and an algorithm on insulin use.
Dr. Buse has financial ties to numerous drug and device companies. Dr. Green is a consultant for AstraZeneca, Pfizer, Boehringer Ingelheim/Lilly, Bayer, Sanofi, Anji, Vertex/ICON, and Valo. Dr. Aroda has served as a consultant for Applied Therapeutics, Duke, Fractyl, Novo Nordisk, Pfizer, and Sanofi.
A version of this article first appeared on Medscape.com.
AT EASD 2022
Unsure on the best T2D drug choice? Let patients decide
STOCKHOLM – When a clinician is unsure which of several equally viable drug options is best for a specific patient with type 2 diabetes, a rational approach is to run a serial trial with each one and then let each patient decide which agent works best for them.
That concept underwent successful testing in a recent trial with 457 patients with type 2 diabetes and already on treatment with metformin or metformin plus a sulfonylurea but needed further glycemic control. After cycling through 4-month trials (when tolerated) of canagliflozin (Invokana), pioglitazone (Actos), and sitagliptin (Januvia), 24% identified pioglitazone as the one that made them feel best, 33% favored sitagliptin, 37% said canagliflozin was tops, and 6% had no preference, Beverley Shields, PhD, reported at the annual meeting of the European Association for the Study of Diabetes.
After making these selections based on just their qualitative self-appraisals, researchers told patients about their hemoglobin A1c status on each of the three agents. It barely budged their choices, which became 25% calling pioglitazone best, 35% naming sitagliptin their preference, 38% opting for canagliflozin, with 2% having no preference.
Further analysis showed that the drug patients preferred was also the one that produced their lowest A1c level when compared with their 8 months on each of the two other agents tested, showing a link between lower A1c levels and improved well-being. The same relationship existed for the drug that caused the fewest adverse events for each patient.
Patients prefer feeling better
“Patients tended to prefer the drug that they ‘felt better’ on, with the lowest A1c level and the lowest number of side effects,” explained Dr. Shields, a medical statistician at the University of Exeter (England). Changes in weight appeared less important to patients for establishing a preference.
“This is for when there is equipoise” among drug options, Andrew Hattersley, BMBCh, DM, the study’s principal investigator, said in an interview. “When you are unsure what to prescribe and there is no clear indication for one drug over another, try 4 months of one and 4 months of the other, then let the patient decide.
“Patients had overwhelming positivity about being able to choose their drug,” added Dr. Hattersley, who is also professor of molecular medicine at the University of Exeter.
“This has implications across medicine,” he added. “Whenever you’re not sure how to balance adverse effects and positive effects the best person to decide is the one who experiences the effects.”
“I’m a bit worried by this approach, but it is something new” and worth considering, commented Drazenka P. Barlovic, MD, an endocrinologist at the University Medical Center in Ljubljana, Slovenia, who chaired the session where Dr. Shields gave her report. “We should also have the courage to challenge metformin, as there is no longer an obligation to make it the first drug,” she said in an interview.
The study ran as a secondary analysis of the TriMaster study, which had the primary objective of identifying patient characteristics that could predict which of the three drug options tested worked best for certain patient subgroups. That analysis, presented at the 2021 EASD annual meeting, found that factors such as body mass index and kidney function significantly linked with the clinical responses patients had to each of the three tested agents.
The new analysis focused on 457 of the TriMaster participants who had provided preference information after they had tried all three agents. By design, none of the participants enrolled in the study had a contraindication for any of the tested drugs.
Patients quickly identify adverse effects
“We picked 4 months because it not too long, but long enough to see adverse effects, and to measure on-treatment A1c. Patients quickly identify their adverse events,” Dr. Shields said in an interview.
“This could come into practice now; there is no cost involved. Do it when you’re not certain which drug to prescribe,” Dr. Hattersley suggested. “We can’t know which drug a patient might prefer.” He also stressed telling patients to return quicker than 4 months if they can’t tolerate a new drug.
The findings have already changed Dr. Hattersley’s practice, and he believes it will catch on as he introduces it to local primary care physicians.
The study received no commercial funding. Dr. Shields, Dr. Hattersley, and Dr. Barlovic had no disclosures.
STOCKHOLM – When a clinician is unsure which of several equally viable drug options is best for a specific patient with type 2 diabetes, a rational approach is to run a serial trial with each one and then let each patient decide which agent works best for them.
That concept underwent successful testing in a recent trial with 457 patients with type 2 diabetes and already on treatment with metformin or metformin plus a sulfonylurea but needed further glycemic control. After cycling through 4-month trials (when tolerated) of canagliflozin (Invokana), pioglitazone (Actos), and sitagliptin (Januvia), 24% identified pioglitazone as the one that made them feel best, 33% favored sitagliptin, 37% said canagliflozin was tops, and 6% had no preference, Beverley Shields, PhD, reported at the annual meeting of the European Association for the Study of Diabetes.
After making these selections based on just their qualitative self-appraisals, researchers told patients about their hemoglobin A1c status on each of the three agents. It barely budged their choices, which became 25% calling pioglitazone best, 35% naming sitagliptin their preference, 38% opting for canagliflozin, with 2% having no preference.
Further analysis showed that the drug patients preferred was also the one that produced their lowest A1c level when compared with their 8 months on each of the two other agents tested, showing a link between lower A1c levels and improved well-being. The same relationship existed for the drug that caused the fewest adverse events for each patient.
Patients prefer feeling better
“Patients tended to prefer the drug that they ‘felt better’ on, with the lowest A1c level and the lowest number of side effects,” explained Dr. Shields, a medical statistician at the University of Exeter (England). Changes in weight appeared less important to patients for establishing a preference.
“This is for when there is equipoise” among drug options, Andrew Hattersley, BMBCh, DM, the study’s principal investigator, said in an interview. “When you are unsure what to prescribe and there is no clear indication for one drug over another, try 4 months of one and 4 months of the other, then let the patient decide.
“Patients had overwhelming positivity about being able to choose their drug,” added Dr. Hattersley, who is also professor of molecular medicine at the University of Exeter.
“This has implications across medicine,” he added. “Whenever you’re not sure how to balance adverse effects and positive effects the best person to decide is the one who experiences the effects.”
“I’m a bit worried by this approach, but it is something new” and worth considering, commented Drazenka P. Barlovic, MD, an endocrinologist at the University Medical Center in Ljubljana, Slovenia, who chaired the session where Dr. Shields gave her report. “We should also have the courage to challenge metformin, as there is no longer an obligation to make it the first drug,” she said in an interview.
The study ran as a secondary analysis of the TriMaster study, which had the primary objective of identifying patient characteristics that could predict which of the three drug options tested worked best for certain patient subgroups. That analysis, presented at the 2021 EASD annual meeting, found that factors such as body mass index and kidney function significantly linked with the clinical responses patients had to each of the three tested agents.
The new analysis focused on 457 of the TriMaster participants who had provided preference information after they had tried all three agents. By design, none of the participants enrolled in the study had a contraindication for any of the tested drugs.
Patients quickly identify adverse effects
“We picked 4 months because it not too long, but long enough to see adverse effects, and to measure on-treatment A1c. Patients quickly identify their adverse events,” Dr. Shields said in an interview.
“This could come into practice now; there is no cost involved. Do it when you’re not certain which drug to prescribe,” Dr. Hattersley suggested. “We can’t know which drug a patient might prefer.” He also stressed telling patients to return quicker than 4 months if they can’t tolerate a new drug.
The findings have already changed Dr. Hattersley’s practice, and he believes it will catch on as he introduces it to local primary care physicians.
The study received no commercial funding. Dr. Shields, Dr. Hattersley, and Dr. Barlovic had no disclosures.
STOCKHOLM – When a clinician is unsure which of several equally viable drug options is best for a specific patient with type 2 diabetes, a rational approach is to run a serial trial with each one and then let each patient decide which agent works best for them.
That concept underwent successful testing in a recent trial with 457 patients with type 2 diabetes and already on treatment with metformin or metformin plus a sulfonylurea but needed further glycemic control. After cycling through 4-month trials (when tolerated) of canagliflozin (Invokana), pioglitazone (Actos), and sitagliptin (Januvia), 24% identified pioglitazone as the one that made them feel best, 33% favored sitagliptin, 37% said canagliflozin was tops, and 6% had no preference, Beverley Shields, PhD, reported at the annual meeting of the European Association for the Study of Diabetes.
After making these selections based on just their qualitative self-appraisals, researchers told patients about their hemoglobin A1c status on each of the three agents. It barely budged their choices, which became 25% calling pioglitazone best, 35% naming sitagliptin their preference, 38% opting for canagliflozin, with 2% having no preference.
Further analysis showed that the drug patients preferred was also the one that produced their lowest A1c level when compared with their 8 months on each of the two other agents tested, showing a link between lower A1c levels and improved well-being. The same relationship existed for the drug that caused the fewest adverse events for each patient.
Patients prefer feeling better
“Patients tended to prefer the drug that they ‘felt better’ on, with the lowest A1c level and the lowest number of side effects,” explained Dr. Shields, a medical statistician at the University of Exeter (England). Changes in weight appeared less important to patients for establishing a preference.
“This is for when there is equipoise” among drug options, Andrew Hattersley, BMBCh, DM, the study’s principal investigator, said in an interview. “When you are unsure what to prescribe and there is no clear indication for one drug over another, try 4 months of one and 4 months of the other, then let the patient decide.
“Patients had overwhelming positivity about being able to choose their drug,” added Dr. Hattersley, who is also professor of molecular medicine at the University of Exeter.
“This has implications across medicine,” he added. “Whenever you’re not sure how to balance adverse effects and positive effects the best person to decide is the one who experiences the effects.”
“I’m a bit worried by this approach, but it is something new” and worth considering, commented Drazenka P. Barlovic, MD, an endocrinologist at the University Medical Center in Ljubljana, Slovenia, who chaired the session where Dr. Shields gave her report. “We should also have the courage to challenge metformin, as there is no longer an obligation to make it the first drug,” she said in an interview.
The study ran as a secondary analysis of the TriMaster study, which had the primary objective of identifying patient characteristics that could predict which of the three drug options tested worked best for certain patient subgroups. That analysis, presented at the 2021 EASD annual meeting, found that factors such as body mass index and kidney function significantly linked with the clinical responses patients had to each of the three tested agents.
The new analysis focused on 457 of the TriMaster participants who had provided preference information after they had tried all three agents. By design, none of the participants enrolled in the study had a contraindication for any of the tested drugs.
Patients quickly identify adverse effects
“We picked 4 months because it not too long, but long enough to see adverse effects, and to measure on-treatment A1c. Patients quickly identify their adverse events,” Dr. Shields said in an interview.
“This could come into practice now; there is no cost involved. Do it when you’re not certain which drug to prescribe,” Dr. Hattersley suggested. “We can’t know which drug a patient might prefer.” He also stressed telling patients to return quicker than 4 months if they can’t tolerate a new drug.
The findings have already changed Dr. Hattersley’s practice, and he believes it will catch on as he introduces it to local primary care physicians.
The study received no commercial funding. Dr. Shields, Dr. Hattersley, and Dr. Barlovic had no disclosures.
AT EASD 2022