User login
New findings from first all-female TAVR registry
Paris – A history of pregnancy did not protect against adverse outcomes at 1 year in the Women’s International Transcatheter Aortic Valve Implantation Registry (WIN-TAVI), even though it did within the first 30 days, Alaide Chieffo, MD, reported at the annual congress of the European Association of Percutaneous Cardiovascular Interventions.
One year ago, at EuroPCR 2016, she reported that in WIN-TAVI, a history of pregnancy – albeit typically more than half a century previously – was independently associated with a 43% reduction in the Valve Academic Research Consortium-2 (VARC-2) 30-day composite endpoint, including death, stroke, major vascular complications, life-threatening bleeding, stage 2 or 3 acute kidney injury, coronary artery obstruction, or repeat transcatheter aortic valve replacement (TAVR) done because of valve-related dysfunction. Those early findings, first reported in this publication, were later published (JACC Cardiovasc Interv. 2016 Aug 8;9[15]:1589-600).
At 1 year of follow-up, however, the rate of the VARC-2 composite endpoint was no longer significantly different in women with or without a history of pregnancy. Nor was a history of pregnancy associated with a significantly reduced risk of the secondary endpoint of death or stroke: The 27% reduction in risk of this secondary endpoint in women with a history of pregnancy, compared with that of nulliparous women, didn’t achieve statistical significance in multivariate analysis, according to Dr. Chieffo of the San Raffaele Scientific Institute in Milan.
She speculated that pregnancy earlier in life provided strong protection against poor 30-day outcomes and a similar trend – albeit not statistically significant – at 1 year because women without children may have less family support.
“They are old women, left alone, without the family taking care of them. This is socially important, I think, because we are investing quite a lot of money in a procedure, and then maybe we’re adding adverse events because these patients are not properly taken care of when they are out of the hospital,” the interventional cardiologist said.
Neither of the other two female-specific characteristics evaluated in WIN-TAVI – having a history of osteoporosis or age at menopause – turned out to be related to the risk of bad outcomes at 1 year, she added.
WIN-TAVI is the first all-female registry of patients undergoing TAVR for severe aortic stenosis. The prospective, observational registry includes 1,019 women treated at 19 highly experienced European and North American TAVR centers. They averaged 82.5 years of age with a mean Society of Thoracic Surgeons score of 8.3%, putting them at intermediate or high surgical risk. A percutaneous transfemoral approach was used in 91% of cases. TAVR was performed under conscious sedation in 28% of the women and under local anesthesia in another 37%. Of the women in the registry, 42% received a newer-generation device.
In addition to the lack of significant impact of prior pregnancy on 1-year outcomes, another noteworthy finding at 1 year of follow-up was that preprocedural atrial fibrillation was independently associated with a 58% increase in the risk of death or stroke (P = .02). Prior percutaneous coronary intervention and EuroSCORE (European System for Cardiac Operative Risk Evaluation) were the only other independent predictors.
This observation suggests the need for a women-only randomized trial of TAVR versus surgical aortic valve replacement in women with intermediate surgical risk, Dr. Chieffo suggested. It will be important to learn whether the ability to surgically ablate preoperative atrial fibrillation in women during surgical valve replacement results in a lower 1-year risk of death or stroke than is achieved with TAVR.
Overall, the 1-year clinical outcomes seen in WIN-TAVI are “very good,” she noted. The VARC-2 composite endpoint occurred in 16.5% of women, all-cause mortality in 12.5%, cardiovascular mortality in 10.8%, and stroke in 2.2%. Only 3.2% of women were hospitalized for heart failure or valve-related symptoms. A new pacemaker was implanted in 12.7% of participants. At baseline 74% of women were New York Heart Association functional class III or IV; at 1 year, only 8.1% were. Moderate paravalvular aortic regurgitation was present in 6% of patients at 6 months and in 9.7% at 1 year
The WIN-TAVI registry is entirely self-funded. Dr. Chieffo reported having no financial conflicts regarding her presentation.
Paris – A history of pregnancy did not protect against adverse outcomes at 1 year in the Women’s International Transcatheter Aortic Valve Implantation Registry (WIN-TAVI), even though it did within the first 30 days, Alaide Chieffo, MD, reported at the annual congress of the European Association of Percutaneous Cardiovascular Interventions.
One year ago, at EuroPCR 2016, she reported that in WIN-TAVI, a history of pregnancy – albeit typically more than half a century previously – was independently associated with a 43% reduction in the Valve Academic Research Consortium-2 (VARC-2) 30-day composite endpoint, including death, stroke, major vascular complications, life-threatening bleeding, stage 2 or 3 acute kidney injury, coronary artery obstruction, or repeat transcatheter aortic valve replacement (TAVR) done because of valve-related dysfunction. Those early findings, first reported in this publication, were later published (JACC Cardiovasc Interv. 2016 Aug 8;9[15]:1589-600).
At 1 year of follow-up, however, the rate of the VARC-2 composite endpoint was no longer significantly different in women with or without a history of pregnancy. Nor was a history of pregnancy associated with a significantly reduced risk of the secondary endpoint of death or stroke: The 27% reduction in risk of this secondary endpoint in women with a history of pregnancy, compared with that of nulliparous women, didn’t achieve statistical significance in multivariate analysis, according to Dr. Chieffo of the San Raffaele Scientific Institute in Milan.
She speculated that pregnancy earlier in life provided strong protection against poor 30-day outcomes and a similar trend – albeit not statistically significant – at 1 year because women without children may have less family support.
“They are old women, left alone, without the family taking care of them. This is socially important, I think, because we are investing quite a lot of money in a procedure, and then maybe we’re adding adverse events because these patients are not properly taken care of when they are out of the hospital,” the interventional cardiologist said.
Neither of the other two female-specific characteristics evaluated in WIN-TAVI – having a history of osteoporosis or age at menopause – turned out to be related to the risk of bad outcomes at 1 year, she added.
WIN-TAVI is the first all-female registry of patients undergoing TAVR for severe aortic stenosis. The prospective, observational registry includes 1,019 women treated at 19 highly experienced European and North American TAVR centers. They averaged 82.5 years of age with a mean Society of Thoracic Surgeons score of 8.3%, putting them at intermediate or high surgical risk. A percutaneous transfemoral approach was used in 91% of cases. TAVR was performed under conscious sedation in 28% of the women and under local anesthesia in another 37%. Of the women in the registry, 42% received a newer-generation device.
In addition to the lack of significant impact of prior pregnancy on 1-year outcomes, another noteworthy finding at 1 year of follow-up was that preprocedural atrial fibrillation was independently associated with a 58% increase in the risk of death or stroke (P = .02). Prior percutaneous coronary intervention and EuroSCORE (European System for Cardiac Operative Risk Evaluation) were the only other independent predictors.
This observation suggests the need for a women-only randomized trial of TAVR versus surgical aortic valve replacement in women with intermediate surgical risk, Dr. Chieffo suggested. It will be important to learn whether the ability to surgically ablate preoperative atrial fibrillation in women during surgical valve replacement results in a lower 1-year risk of death or stroke than is achieved with TAVR.
Overall, the 1-year clinical outcomes seen in WIN-TAVI are “very good,” she noted. The VARC-2 composite endpoint occurred in 16.5% of women, all-cause mortality in 12.5%, cardiovascular mortality in 10.8%, and stroke in 2.2%. Only 3.2% of women were hospitalized for heart failure or valve-related symptoms. A new pacemaker was implanted in 12.7% of participants. At baseline 74% of women were New York Heart Association functional class III or IV; at 1 year, only 8.1% were. Moderate paravalvular aortic regurgitation was present in 6% of patients at 6 months and in 9.7% at 1 year
The WIN-TAVI registry is entirely self-funded. Dr. Chieffo reported having no financial conflicts regarding her presentation.
Paris – A history of pregnancy did not protect against adverse outcomes at 1 year in the Women’s International Transcatheter Aortic Valve Implantation Registry (WIN-TAVI), even though it did within the first 30 days, Alaide Chieffo, MD, reported at the annual congress of the European Association of Percutaneous Cardiovascular Interventions.
One year ago, at EuroPCR 2016, she reported that in WIN-TAVI, a history of pregnancy – albeit typically more than half a century previously – was independently associated with a 43% reduction in the Valve Academic Research Consortium-2 (VARC-2) 30-day composite endpoint, including death, stroke, major vascular complications, life-threatening bleeding, stage 2 or 3 acute kidney injury, coronary artery obstruction, or repeat transcatheter aortic valve replacement (TAVR) done because of valve-related dysfunction. Those early findings, first reported in this publication, were later published (JACC Cardiovasc Interv. 2016 Aug 8;9[15]:1589-600).
At 1 year of follow-up, however, the rate of the VARC-2 composite endpoint was no longer significantly different in women with or without a history of pregnancy. Nor was a history of pregnancy associated with a significantly reduced risk of the secondary endpoint of death or stroke: The 27% reduction in risk of this secondary endpoint in women with a history of pregnancy, compared with that of nulliparous women, didn’t achieve statistical significance in multivariate analysis, according to Dr. Chieffo of the San Raffaele Scientific Institute in Milan.
She speculated that pregnancy earlier in life provided strong protection against poor 30-day outcomes and a similar trend – albeit not statistically significant – at 1 year because women without children may have less family support.
“They are old women, left alone, without the family taking care of them. This is socially important, I think, because we are investing quite a lot of money in a procedure, and then maybe we’re adding adverse events because these patients are not properly taken care of when they are out of the hospital,” the interventional cardiologist said.
Neither of the other two female-specific characteristics evaluated in WIN-TAVI – having a history of osteoporosis or age at menopause – turned out to be related to the risk of bad outcomes at 1 year, she added.
WIN-TAVI is the first all-female registry of patients undergoing TAVR for severe aortic stenosis. The prospective, observational registry includes 1,019 women treated at 19 highly experienced European and North American TAVR centers. They averaged 82.5 years of age with a mean Society of Thoracic Surgeons score of 8.3%, putting them at intermediate or high surgical risk. A percutaneous transfemoral approach was used in 91% of cases. TAVR was performed under conscious sedation in 28% of the women and under local anesthesia in another 37%. Of the women in the registry, 42% received a newer-generation device.
In addition to the lack of significant impact of prior pregnancy on 1-year outcomes, another noteworthy finding at 1 year of follow-up was that preprocedural atrial fibrillation was independently associated with a 58% increase in the risk of death or stroke (P = .02). Prior percutaneous coronary intervention and EuroSCORE (European System for Cardiac Operative Risk Evaluation) were the only other independent predictors.
This observation suggests the need for a women-only randomized trial of TAVR versus surgical aortic valve replacement in women with intermediate surgical risk, Dr. Chieffo suggested. It will be important to learn whether the ability to surgically ablate preoperative atrial fibrillation in women during surgical valve replacement results in a lower 1-year risk of death or stroke than is achieved with TAVR.
Overall, the 1-year clinical outcomes seen in WIN-TAVI are “very good,” she noted. The VARC-2 composite endpoint occurred in 16.5% of women, all-cause mortality in 12.5%, cardiovascular mortality in 10.8%, and stroke in 2.2%. Only 3.2% of women were hospitalized for heart failure or valve-related symptoms. A new pacemaker was implanted in 12.7% of participants. At baseline 74% of women were New York Heart Association functional class III or IV; at 1 year, only 8.1% were. Moderate paravalvular aortic regurgitation was present in 6% of patients at 6 months and in 9.7% at 1 year
The WIN-TAVI registry is entirely self-funded. Dr. Chieffo reported having no financial conflicts regarding her presentation.
AT EuroPCR
Key clinical point:
Major finding: Prior pregnancy didn’t protect women against death or stroke at 1 year post TAVR.
Data source: WIN-TAVI, a prospective, multicenter, observational registry includes 1,019 women who underwent TAVR.
Disclosures: WIN-TAVI is entirely self-funded. The presenter reported having no financial conflicts.
First trial of TAVR vs. SAVR in low-risk patients
PARIS – Five-year hemodynamic results of the first randomized trial of transcatheter versus surgical aortic valve replacement in low-surgical-risk patients with severe aortic stenosis showed continued superior valve performance in the TAVR group, Lars Sondergaard, MD, reported at the annual congress of the European Association of Percutaneous Cardiovascular Interventions.
“The durability results are very encouraging. We can’t see that the TAVR patients are doing worse. So I think this is setting the scene to try to move forward in patients at low risk and also in younger patients,” declared Dr. Sondergaard, professor of cardiology at the University of Copenhagen.
He presented an update from the Nordic Aortic Valve Intervention (NOTION) trial, a prospective, multicenter, randomized, all-comers clinical trial in which 280 patients with symptomatic severe aortic stenosis at low surgical risk were assigned to surgical aortic valve replacement (SAVR) or to TAVR with the self-expanding CoreValve. Their mean age was 79 years, with an average Society of Thoracic Surgeons projected risk of mortality score of 3%. Eighty-two percent of participants had an STS score below 4%. Roughly 40% of TAVR patients got the first-generation CoreValve in the 26-mm size, 40% received the 29-mm version, and the rest got the 31-mm CoreValve.
Among patients in the lowest-surgical-risk and youngest subgroup – those aged 70-75 with a Society of Thoracic Surgeons risk score below 4% – the composite primary endpoint rate at 4 years was 15.6% with TAVR compared with 27.2% with SAVR. However, only 62 NOTION participants fell into this category, so the between-group difference, while sizable, didn’t achieve statistical significance, according to Dr. Sondergaard.
There was a trade-off between the two valve replacement strategies with regard to procedural complications. The rate of new-onset atrial fibrillation was far higher in the SAVR group: 59.4% at 1 year and 60.2% at 4 years of follow-up, compared with 21.2% and 24.5% at 1 and 4 years, respectively, in the TAVR group.
On the other hand, 38% of the TAVR patients got a new pacemaker within the first year of follow-up, compared with only 2.4% in the SAVR group. At 4 years, 43.7% of the TAVR group had a pacemaker, versus 9% of the SAVR group.
Turning to the hemodynamic data, the cardiologist noted that the effective orifice area in the TAVR group went from 0.71 cm2 at baseline to 1.66 at 1 year and remained steady thereafter at 1.67 cm2 through 5 years. The TAVR group’s mean gradient improved from 45.4 mm Hg at baseline to 8.6 mm Hg at 1 year and 7.9 mm Hg at 5 years. These outcomes were significantly better than in the SAVR group, where the effective orifice area went from 0.74 cm2 at baseline to 1.32 at 1 year and 1.24 cm2 at 5 years, while the mean gradient fell from 44.9 mm Hg to 12.5 at 1 year and 13.6 mm Hg at 5 years.
Moderate hemodynamic structural valve deterioration was significantly more common in the SAVR group: 20.7% at 5 years, compared with 2.9% in the TAVR patients. The opposite was true with regard to moderate paravalvular leak, which occurred in 20.9% of the TAVI group but only 1.5% of SAVR patients.
Late complications were rare following either procedure. There were no cases of valve thrombosis through 5 years. The incidence of endocarditis at 5 years was 4.3% in the TAVR patients and similar at 5.9% in the SAVR group.
Discussant Samer Mansour, MD, of the University of Montreal, remarked that the rate of new pacemaker implantation following TAVR seemed extraordinarily high.
“This was early days,” Dr. Sondergaard explained. “We had a lower threshold for putting in a pacemaker and we put the valves in a little deeper.”
About half of new pacemaker recipients didn’t use the device after the first year, he added. Also, neither getting a new pacemaker nor moderate paravalvular leak was associated with increased mortality in the TAVR group.
Dr. Mansour observed that subtle but real differences in mortality probably wouldn’t show up in a 280-patient trial. Dr. Sondergaard concurred.
“We designed the NOTION trial in 2008-2009. Knowing what we know now, we should have had a larger study, but at that time TAVR volume wasn’t that big and it wasn’t realistic as a Nordic trial to include 1,000 patients. This was the best we could do,” he said.
Follow-up in the NOTION study will continue out to 10 years.
The study is funded by Medtronic. Dr. Sondergaard reported serving as a consultant to and receiving research grant support from the company.
PARIS – Five-year hemodynamic results of the first randomized trial of transcatheter versus surgical aortic valve replacement in low-surgical-risk patients with severe aortic stenosis showed continued superior valve performance in the TAVR group, Lars Sondergaard, MD, reported at the annual congress of the European Association of Percutaneous Cardiovascular Interventions.
“The durability results are very encouraging. We can’t see that the TAVR patients are doing worse. So I think this is setting the scene to try to move forward in patients at low risk and also in younger patients,” declared Dr. Sondergaard, professor of cardiology at the University of Copenhagen.
He presented an update from the Nordic Aortic Valve Intervention (NOTION) trial, a prospective, multicenter, randomized, all-comers clinical trial in which 280 patients with symptomatic severe aortic stenosis at low surgical risk were assigned to surgical aortic valve replacement (SAVR) or to TAVR with the self-expanding CoreValve. Their mean age was 79 years, with an average Society of Thoracic Surgeons projected risk of mortality score of 3%. Eighty-two percent of participants had an STS score below 4%. Roughly 40% of TAVR patients got the first-generation CoreValve in the 26-mm size, 40% received the 29-mm version, and the rest got the 31-mm CoreValve.
Among patients in the lowest-surgical-risk and youngest subgroup – those aged 70-75 with a Society of Thoracic Surgeons risk score below 4% – the composite primary endpoint rate at 4 years was 15.6% with TAVR compared with 27.2% with SAVR. However, only 62 NOTION participants fell into this category, so the between-group difference, while sizable, didn’t achieve statistical significance, according to Dr. Sondergaard.
There was a trade-off between the two valve replacement strategies with regard to procedural complications. The rate of new-onset atrial fibrillation was far higher in the SAVR group: 59.4% at 1 year and 60.2% at 4 years of follow-up, compared with 21.2% and 24.5% at 1 and 4 years, respectively, in the TAVR group.
On the other hand, 38% of the TAVR patients got a new pacemaker within the first year of follow-up, compared with only 2.4% in the SAVR group. At 4 years, 43.7% of the TAVR group had a pacemaker, versus 9% of the SAVR group.
Turning to the hemodynamic data, the cardiologist noted that the effective orifice area in the TAVR group went from 0.71 cm2 at baseline to 1.66 at 1 year and remained steady thereafter at 1.67 cm2 through 5 years. The TAVR group’s mean gradient improved from 45.4 mm Hg at baseline to 8.6 mm Hg at 1 year and 7.9 mm Hg at 5 years. These outcomes were significantly better than in the SAVR group, where the effective orifice area went from 0.74 cm2 at baseline to 1.32 at 1 year and 1.24 cm2 at 5 years, while the mean gradient fell from 44.9 mm Hg to 12.5 at 1 year and 13.6 mm Hg at 5 years.
Moderate hemodynamic structural valve deterioration was significantly more common in the SAVR group: 20.7% at 5 years, compared with 2.9% in the TAVR patients. The opposite was true with regard to moderate paravalvular leak, which occurred in 20.9% of the TAVI group but only 1.5% of SAVR patients.
Late complications were rare following either procedure. There were no cases of valve thrombosis through 5 years. The incidence of endocarditis at 5 years was 4.3% in the TAVR patients and similar at 5.9% in the SAVR group.
Discussant Samer Mansour, MD, of the University of Montreal, remarked that the rate of new pacemaker implantation following TAVR seemed extraordinarily high.
“This was early days,” Dr. Sondergaard explained. “We had a lower threshold for putting in a pacemaker and we put the valves in a little deeper.”
About half of new pacemaker recipients didn’t use the device after the first year, he added. Also, neither getting a new pacemaker nor moderate paravalvular leak was associated with increased mortality in the TAVR group.
Dr. Mansour observed that subtle but real differences in mortality probably wouldn’t show up in a 280-patient trial. Dr. Sondergaard concurred.
“We designed the NOTION trial in 2008-2009. Knowing what we know now, we should have had a larger study, but at that time TAVR volume wasn’t that big and it wasn’t realistic as a Nordic trial to include 1,000 patients. This was the best we could do,” he said.
Follow-up in the NOTION study will continue out to 10 years.
The study is funded by Medtronic. Dr. Sondergaard reported serving as a consultant to and receiving research grant support from the company.
PARIS – Five-year hemodynamic results of the first randomized trial of transcatheter versus surgical aortic valve replacement in low-surgical-risk patients with severe aortic stenosis showed continued superior valve performance in the TAVR group, Lars Sondergaard, MD, reported at the annual congress of the European Association of Percutaneous Cardiovascular Interventions.
“The durability results are very encouraging. We can’t see that the TAVR patients are doing worse. So I think this is setting the scene to try to move forward in patients at low risk and also in younger patients,” declared Dr. Sondergaard, professor of cardiology at the University of Copenhagen.
He presented an update from the Nordic Aortic Valve Intervention (NOTION) trial, a prospective, multicenter, randomized, all-comers clinical trial in which 280 patients with symptomatic severe aortic stenosis at low surgical risk were assigned to surgical aortic valve replacement (SAVR) or to TAVR with the self-expanding CoreValve. Their mean age was 79 years, with an average Society of Thoracic Surgeons projected risk of mortality score of 3%. Eighty-two percent of participants had an STS score below 4%. Roughly 40% of TAVR patients got the first-generation CoreValve in the 26-mm size, 40% received the 29-mm version, and the rest got the 31-mm CoreValve.
Among patients in the lowest-surgical-risk and youngest subgroup – those aged 70-75 with a Society of Thoracic Surgeons risk score below 4% – the composite primary endpoint rate at 4 years was 15.6% with TAVR compared with 27.2% with SAVR. However, only 62 NOTION participants fell into this category, so the between-group difference, while sizable, didn’t achieve statistical significance, according to Dr. Sondergaard.
There was a trade-off between the two valve replacement strategies with regard to procedural complications. The rate of new-onset atrial fibrillation was far higher in the SAVR group: 59.4% at 1 year and 60.2% at 4 years of follow-up, compared with 21.2% and 24.5% at 1 and 4 years, respectively, in the TAVR group.
On the other hand, 38% of the TAVR patients got a new pacemaker within the first year of follow-up, compared with only 2.4% in the SAVR group. At 4 years, 43.7% of the TAVR group had a pacemaker, versus 9% of the SAVR group.
Turning to the hemodynamic data, the cardiologist noted that the effective orifice area in the TAVR group went from 0.71 cm2 at baseline to 1.66 at 1 year and remained steady thereafter at 1.67 cm2 through 5 years. The TAVR group’s mean gradient improved from 45.4 mm Hg at baseline to 8.6 mm Hg at 1 year and 7.9 mm Hg at 5 years. These outcomes were significantly better than in the SAVR group, where the effective orifice area went from 0.74 cm2 at baseline to 1.32 at 1 year and 1.24 cm2 at 5 years, while the mean gradient fell from 44.9 mm Hg to 12.5 at 1 year and 13.6 mm Hg at 5 years.
Moderate hemodynamic structural valve deterioration was significantly more common in the SAVR group: 20.7% at 5 years, compared with 2.9% in the TAVR patients. The opposite was true with regard to moderate paravalvular leak, which occurred in 20.9% of the TAVI group but only 1.5% of SAVR patients.
Late complications were rare following either procedure. There were no cases of valve thrombosis through 5 years. The incidence of endocarditis at 5 years was 4.3% in the TAVR patients and similar at 5.9% in the SAVR group.
Discussant Samer Mansour, MD, of the University of Montreal, remarked that the rate of new pacemaker implantation following TAVR seemed extraordinarily high.
“This was early days,” Dr. Sondergaard explained. “We had a lower threshold for putting in a pacemaker and we put the valves in a little deeper.”
About half of new pacemaker recipients didn’t use the device after the first year, he added. Also, neither getting a new pacemaker nor moderate paravalvular leak was associated with increased mortality in the TAVR group.
Dr. Mansour observed that subtle but real differences in mortality probably wouldn’t show up in a 280-patient trial. Dr. Sondergaard concurred.
“We designed the NOTION trial in 2008-2009. Knowing what we know now, we should have had a larger study, but at that time TAVR volume wasn’t that big and it wasn’t realistic as a Nordic trial to include 1,000 patients. This was the best we could do,” he said.
Follow-up in the NOTION study will continue out to 10 years.
The study is funded by Medtronic. Dr. Sondergaard reported serving as a consultant to and receiving research grant support from the company.
AT EuroPCR
Key clinical point:
Major finding: At 4 years of follow-up, the composite endpoint of all-cause mortality, MI, or stroke occurred in 29% of low-surgical-risk patients with severe aortic stenosis who were randomized to transcatheter aortic valve replacement (TAVR) and 30% of those who underwent surgical valve replacement.
Data source: NOTION, a prospective multicenter randomized trial in which 280 Nordic patients with symptomatic severe aortic stenosis at low surgical risk were assigned to surgical aortic valve replacement (SAVR) or to TAVR with the self-expanding CoreValve.
Disclosures: The study is funded by Medtronic. The presenter reported serving as a consultant to and receiving research grant support from the company.
TAVR for failed surgical valves: the VIVA study
PARIS – Transcatheter aortic valve replacement using the self-expanding Evolut R device in high-surgical-risk patients with failing surgical aortic bioprostheses showed promising 30-day safety and effectiveness results in the ongoing VIVA study, Ran Kornowski, MD, reported at the annual congress of the European Association of Percutaneous Cardiovascular Interventions.
“We had a lot of patients with small, failing valves in this study. Despite this, our valve gradients postprocedure were very, very low. This means to me that this platform is very well suited to deal with valve-in-valve procedures in general and with small bioprosthetic valves in particular,” observed Dr. Kornowski, chairman of the department of cardiology at Rabin Medical Center in Petah Tikva, Israel, and president of the Israel Heart Society.
The participants’ last surgical aortic valve replacement had been a mean of 9.3 years earlier. Seventy-one percent of subjects were New York Heart Association class III or IV. The mode of bioprosthetic failure was stenosis in 56% of cases, regurgitation in 23%, and both in the remainder. Ninety-three percent of their failing biosprothetic valves were stented devices. Forty-one percent of the devices were up to 21 mm and another 33% were more than 21 but less than 25 mm.
TAVR procedural access was by the iliofemoral route in 97% of cases. Local anesthesia was used in 42% of cases and conscious sedation in 35%. Fourteen percent of patients underwent preimplantation valvuloplasty; 21% postimplantation valvuloplasty. The procedural success rate was 98.5%.
The primary safety endpoint was 30-day cardiovascular mortality. The 2.0% rate was far lower than the prespecified cutoff which defined a positive outcome as less than a 10% rate in this high-surgical-risk population.
Other key 30-day outcomes:
• All-cause mortality occurred in 2.5% of patients.
• The 30-day stroke rate was 3%, with no disabling strokes.
• Major vascular complications occurred in 6.5% of the VIVA patients.
• Major bleeding occurred in 7%, minor bleeding in 7.9%. There were no cases of life-threatening bleeding.
• The incidence of Stage I acute kidney injury was rare, at 0.5%.
• Seven percent of patients received a permanent pacemaker.
• Eighty-seven percent of patients had no postprocedure paravalvular regurgitation (PVR), 11.4 had mild PVR, and 1.5% had moderate PVR.
• NYHA classification improved from baseline to 30 days in 81% of patients. At 30 days, 93% of participants were NYHA class I or II.
Turning to echocardiographic findings, the mean gradient improved from a mean baseline of 31.8 mm Hg to 12.6 mm Hg, while the effective orifice area rose from 1.0 to 1.5 cm2. The magnitude of both improvements was greater for patients with stenosis as their mode of valve failure.
“With the Evolut R, we aim for higher implantations – not more than about 4 mm below the ring – because going deeper could bring about higher gradients and functional deterioration later on,” the cardiologist explained.
The 1-year primary efficacy endpoint – lack of significant aortic stenosis as defined by a mean gradient less than 40 mm Hg – will be reported soon. The VIVA study is sponsored by Medtronic. Dr. Kornowski reported serving as a consultant to the company.
PARIS – Transcatheter aortic valve replacement using the self-expanding Evolut R device in high-surgical-risk patients with failing surgical aortic bioprostheses showed promising 30-day safety and effectiveness results in the ongoing VIVA study, Ran Kornowski, MD, reported at the annual congress of the European Association of Percutaneous Cardiovascular Interventions.
“We had a lot of patients with small, failing valves in this study. Despite this, our valve gradients postprocedure were very, very low. This means to me that this platform is very well suited to deal with valve-in-valve procedures in general and with small bioprosthetic valves in particular,” observed Dr. Kornowski, chairman of the department of cardiology at Rabin Medical Center in Petah Tikva, Israel, and president of the Israel Heart Society.
The participants’ last surgical aortic valve replacement had been a mean of 9.3 years earlier. Seventy-one percent of subjects were New York Heart Association class III or IV. The mode of bioprosthetic failure was stenosis in 56% of cases, regurgitation in 23%, and both in the remainder. Ninety-three percent of their failing biosprothetic valves were stented devices. Forty-one percent of the devices were up to 21 mm and another 33% were more than 21 but less than 25 mm.
TAVR procedural access was by the iliofemoral route in 97% of cases. Local anesthesia was used in 42% of cases and conscious sedation in 35%. Fourteen percent of patients underwent preimplantation valvuloplasty; 21% postimplantation valvuloplasty. The procedural success rate was 98.5%.
The primary safety endpoint was 30-day cardiovascular mortality. The 2.0% rate was far lower than the prespecified cutoff which defined a positive outcome as less than a 10% rate in this high-surgical-risk population.
Other key 30-day outcomes:
• All-cause mortality occurred in 2.5% of patients.
• The 30-day stroke rate was 3%, with no disabling strokes.
• Major vascular complications occurred in 6.5% of the VIVA patients.
• Major bleeding occurred in 7%, minor bleeding in 7.9%. There were no cases of life-threatening bleeding.
• The incidence of Stage I acute kidney injury was rare, at 0.5%.
• Seven percent of patients received a permanent pacemaker.
• Eighty-seven percent of patients had no postprocedure paravalvular regurgitation (PVR), 11.4 had mild PVR, and 1.5% had moderate PVR.
• NYHA classification improved from baseline to 30 days in 81% of patients. At 30 days, 93% of participants were NYHA class I or II.
Turning to echocardiographic findings, the mean gradient improved from a mean baseline of 31.8 mm Hg to 12.6 mm Hg, while the effective orifice area rose from 1.0 to 1.5 cm2. The magnitude of both improvements was greater for patients with stenosis as their mode of valve failure.
“With the Evolut R, we aim for higher implantations – not more than about 4 mm below the ring – because going deeper could bring about higher gradients and functional deterioration later on,” the cardiologist explained.
The 1-year primary efficacy endpoint – lack of significant aortic stenosis as defined by a mean gradient less than 40 mm Hg – will be reported soon. The VIVA study is sponsored by Medtronic. Dr. Kornowski reported serving as a consultant to the company.
PARIS – Transcatheter aortic valve replacement using the self-expanding Evolut R device in high-surgical-risk patients with failing surgical aortic bioprostheses showed promising 30-day safety and effectiveness results in the ongoing VIVA study, Ran Kornowski, MD, reported at the annual congress of the European Association of Percutaneous Cardiovascular Interventions.
“We had a lot of patients with small, failing valves in this study. Despite this, our valve gradients postprocedure were very, very low. This means to me that this platform is very well suited to deal with valve-in-valve procedures in general and with small bioprosthetic valves in particular,” observed Dr. Kornowski, chairman of the department of cardiology at Rabin Medical Center in Petah Tikva, Israel, and president of the Israel Heart Society.
The participants’ last surgical aortic valve replacement had been a mean of 9.3 years earlier. Seventy-one percent of subjects were New York Heart Association class III or IV. The mode of bioprosthetic failure was stenosis in 56% of cases, regurgitation in 23%, and both in the remainder. Ninety-three percent of their failing biosprothetic valves were stented devices. Forty-one percent of the devices were up to 21 mm and another 33% were more than 21 but less than 25 mm.
TAVR procedural access was by the iliofemoral route in 97% of cases. Local anesthesia was used in 42% of cases and conscious sedation in 35%. Fourteen percent of patients underwent preimplantation valvuloplasty; 21% postimplantation valvuloplasty. The procedural success rate was 98.5%.
The primary safety endpoint was 30-day cardiovascular mortality. The 2.0% rate was far lower than the prespecified cutoff which defined a positive outcome as less than a 10% rate in this high-surgical-risk population.
Other key 30-day outcomes:
• All-cause mortality occurred in 2.5% of patients.
• The 30-day stroke rate was 3%, with no disabling strokes.
• Major vascular complications occurred in 6.5% of the VIVA patients.
• Major bleeding occurred in 7%, minor bleeding in 7.9%. There were no cases of life-threatening bleeding.
• The incidence of Stage I acute kidney injury was rare, at 0.5%.
• Seven percent of patients received a permanent pacemaker.
• Eighty-seven percent of patients had no postprocedure paravalvular regurgitation (PVR), 11.4 had mild PVR, and 1.5% had moderate PVR.
• NYHA classification improved from baseline to 30 days in 81% of patients. At 30 days, 93% of participants were NYHA class I or II.
Turning to echocardiographic findings, the mean gradient improved from a mean baseline of 31.8 mm Hg to 12.6 mm Hg, while the effective orifice area rose from 1.0 to 1.5 cm2. The magnitude of both improvements was greater for patients with stenosis as their mode of valve failure.
“With the Evolut R, we aim for higher implantations – not more than about 4 mm below the ring – because going deeper could bring about higher gradients and functional deterioration later on,” the cardiologist explained.
The 1-year primary efficacy endpoint – lack of significant aortic stenosis as defined by a mean gradient less than 40 mm Hg – will be reported soon. The VIVA study is sponsored by Medtronic. Dr. Kornowski reported serving as a consultant to the company.
AT EuroPCR
Key clinical point:
Major finding: The 30-day cardiovascular mortality rate after transcatheter aortic valve replacement via a valve-in-valve procedure in patients with a failing surgically implanted bioprosthesis was 2%, compared with a projected rate of at least 10% with redo surgery.
Data source: VIVA, a prospective observational registry of 202 high-surgical-risk patients at 23 centers in four countries who underwent valve-in-valve transcatheter aortic valve replacement because of a failing surgically implanted aortic bioprosthesis.
Disclosures: VIVA is sponsored by Medtronic. The presenter reported serving as a consultant to the company.
Bailout stenting for coronary bifurcations brings ‘unacceptable’ hazards
PARIS – Bailout stenting during percutaneous coronary intervention for coronary bifurcations doubled the risk of major adverse cardiovascular events in the world’s largest registry of patients with these often-challenging lesions treated using bioactive stents, Marco Zimarino, MD, reported at the annual congress of the European Association of Percutaneous Cardiovascular Interventions.
Indeed, resort to bailout stenting stood out as the major potentially modifiable risk factor for adverse outcomes among the 4,306 participants in the P2BiTO registry, an international collaboration supported by members of the EuroBifurcation Club. Most of the other independent risk factors identified in a multivariate regression analysis of the P2BiTO database were beyond operator control, including diabetes, advanced age, and presentation with an acute coronary syndrome, according to Dr. Zimarino of the University of Chieti (Italy).
Bailout stenting is largely avoidable through meticulous procedural planning, the interventional cardiologist added.
“Careful planning is always mandatory because bailout stenting is associated with an unacceptably higher risk of both in-hospital and 1-year adverse outcomes,” Dr. Zimarino emphasized. “It’s much better to leave a degraded side branch instead of using bailout stenting to get an excellent angiographic outcome that’s a predictor of a worse clinical outcome.”
Conventional wisdom holds that single stenting of either the main artery or a side branch in a patient with coronary bifurcation is safer than double stenting of both. However, that wasn’t really borne out in the P2BiTO registry provided the operator’s plan was for double stenting. The difference in 1-year major adverse cardiovascular events (MACE) between patients treated using a single- or double-stenting strategy wasn’t statistically significant, provided bailout stenting wasn’t utilized. If bailout stenting was employed, though, the risk of MACE was 2.2-fold greater than if the cardiologist stuck with the plan.
Ninety-eight percent of patients in the P2BiTO registry received drug-eluting stents. The other 2% got the Absorb bioabsorbable vascular scaffold. The percutaneous coronary intervention access site, treatment strategy, choice of stent, and duration of dual-antiplatelet therapy were left up to the operator’s discretion.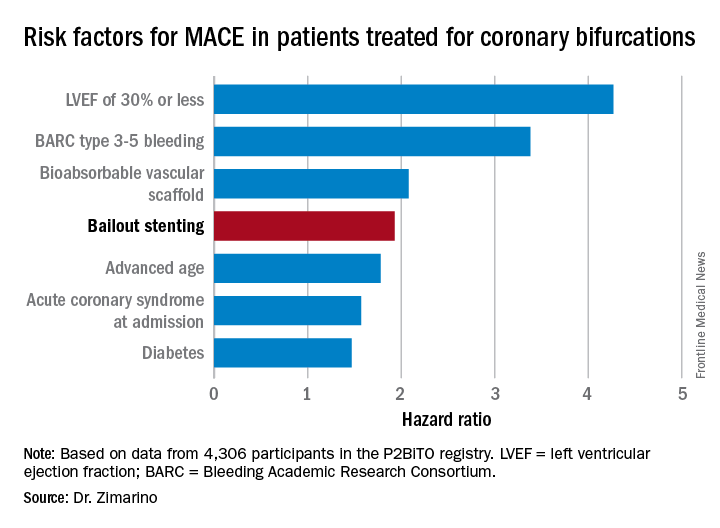
The risk of MACE was reduced by 39% in patients on dual-antiplatelet therapy for 6-12 months, compared with less than 6 months.
Discussant Graham Cassel, MD, director of the heart transplant unit at Milpark Hospital in Johannesburg, commented, “The message comes through very clearly that, if you plan your procedure well, the chance of bailout is far less – and if you do have to bail out, the results are uniformly bad. If you can avoid putting in two or three stents, that’s beneficial.”
Dr. Zimarino reported having no financial conflicts of interest regarding his presentation.
PARIS – Bailout stenting during percutaneous coronary intervention for coronary bifurcations doubled the risk of major adverse cardiovascular events in the world’s largest registry of patients with these often-challenging lesions treated using bioactive stents, Marco Zimarino, MD, reported at the annual congress of the European Association of Percutaneous Cardiovascular Interventions.
Indeed, resort to bailout stenting stood out as the major potentially modifiable risk factor for adverse outcomes among the 4,306 participants in the P2BiTO registry, an international collaboration supported by members of the EuroBifurcation Club. Most of the other independent risk factors identified in a multivariate regression analysis of the P2BiTO database were beyond operator control, including diabetes, advanced age, and presentation with an acute coronary syndrome, according to Dr. Zimarino of the University of Chieti (Italy).
Bailout stenting is largely avoidable through meticulous procedural planning, the interventional cardiologist added.
“Careful planning is always mandatory because bailout stenting is associated with an unacceptably higher risk of both in-hospital and 1-year adverse outcomes,” Dr. Zimarino emphasized. “It’s much better to leave a degraded side branch instead of using bailout stenting to get an excellent angiographic outcome that’s a predictor of a worse clinical outcome.”
Conventional wisdom holds that single stenting of either the main artery or a side branch in a patient with coronary bifurcation is safer than double stenting of both. However, that wasn’t really borne out in the P2BiTO registry provided the operator’s plan was for double stenting. The difference in 1-year major adverse cardiovascular events (MACE) between patients treated using a single- or double-stenting strategy wasn’t statistically significant, provided bailout stenting wasn’t utilized. If bailout stenting was employed, though, the risk of MACE was 2.2-fold greater than if the cardiologist stuck with the plan.
Ninety-eight percent of patients in the P2BiTO registry received drug-eluting stents. The other 2% got the Absorb bioabsorbable vascular scaffold. The percutaneous coronary intervention access site, treatment strategy, choice of stent, and duration of dual-antiplatelet therapy were left up to the operator’s discretion.
The risk of MACE was reduced by 39% in patients on dual-antiplatelet therapy for 6-12 months, compared with less than 6 months.
Discussant Graham Cassel, MD, director of the heart transplant unit at Milpark Hospital in Johannesburg, commented, “The message comes through very clearly that, if you plan your procedure well, the chance of bailout is far less – and if you do have to bail out, the results are uniformly bad. If you can avoid putting in two or three stents, that’s beneficial.”
Dr. Zimarino reported having no financial conflicts of interest regarding his presentation.
PARIS – Bailout stenting during percutaneous coronary intervention for coronary bifurcations doubled the risk of major adverse cardiovascular events in the world’s largest registry of patients with these often-challenging lesions treated using bioactive stents, Marco Zimarino, MD, reported at the annual congress of the European Association of Percutaneous Cardiovascular Interventions.
Indeed, resort to bailout stenting stood out as the major potentially modifiable risk factor for adverse outcomes among the 4,306 participants in the P2BiTO registry, an international collaboration supported by members of the EuroBifurcation Club. Most of the other independent risk factors identified in a multivariate regression analysis of the P2BiTO database were beyond operator control, including diabetes, advanced age, and presentation with an acute coronary syndrome, according to Dr. Zimarino of the University of Chieti (Italy).
Bailout stenting is largely avoidable through meticulous procedural planning, the interventional cardiologist added.
“Careful planning is always mandatory because bailout stenting is associated with an unacceptably higher risk of both in-hospital and 1-year adverse outcomes,” Dr. Zimarino emphasized. “It’s much better to leave a degraded side branch instead of using bailout stenting to get an excellent angiographic outcome that’s a predictor of a worse clinical outcome.”
Conventional wisdom holds that single stenting of either the main artery or a side branch in a patient with coronary bifurcation is safer than double stenting of both. However, that wasn’t really borne out in the P2BiTO registry provided the operator’s plan was for double stenting. The difference in 1-year major adverse cardiovascular events (MACE) between patients treated using a single- or double-stenting strategy wasn’t statistically significant, provided bailout stenting wasn’t utilized. If bailout stenting was employed, though, the risk of MACE was 2.2-fold greater than if the cardiologist stuck with the plan.
Ninety-eight percent of patients in the P2BiTO registry received drug-eluting stents. The other 2% got the Absorb bioabsorbable vascular scaffold. The percutaneous coronary intervention access site, treatment strategy, choice of stent, and duration of dual-antiplatelet therapy were left up to the operator’s discretion.
The risk of MACE was reduced by 39% in patients on dual-antiplatelet therapy for 6-12 months, compared with less than 6 months.
Discussant Graham Cassel, MD, director of the heart transplant unit at Milpark Hospital in Johannesburg, commented, “The message comes through very clearly that, if you plan your procedure well, the chance of bailout is far less – and if you do have to bail out, the results are uniformly bad. If you can avoid putting in two or three stents, that’s beneficial.”
Dr. Zimarino reported having no financial conflicts of interest regarding his presentation.
AT EUROPCR
Key clinical point:
Major finding: Bailout stenting during PCI for coronary bifurcations doubles the risk of major adverse cardiovascular events.
Data source: The P2BiTO registry includes 4,306 patients who received one or more drug-eluting stents or bioabsorbable vascular scaffolds for treatment of coronary bifurcations.
Disclosures: The study presenter reported having no financial conflicts of interest.
Revascularization of CTOs improves health status more than medical therapy
PARIS – The first randomized clinical trial to evaluate quality of life and clinical symptoms as the primary efficacy outcome in patients with coronary chronic total occlusion (CTO) showed a clear advantage for percutaneous revascularization over optimal medical therapy.
At 12 months’ follow-up in the 26-site, 396-patient EuroCTO trial, patients randomized to PCI with drug-eluting stents had significantly less angina and physical limitations coupled with greater improvement in quality of life than the optimal medical therapy (OMT) group on subscales of the Seattle Angina Questionnaire. On the angina frequency subscale, for example, the PCI group improved from a mean baseline score of 77.2 to 91.4 at 12 months, a significantly better result than the OMT group improvement from 80.6 to 87.5.
The PCI group also experienced greater mobility, better activity status, and less pain and discomfort as assessed by the EuroQOL five dimensions questionnaire (EQ-5D), Gerald S. Werner, MD, reported at the annual congress of the European Association of Percutaneous Cardiovascular Interventions.
“PCI of a CTO should be considered as a primary option in symptomatic patients. It is a safe and effective treatment option in expert hands,” said Dr. Werner, director of cardiology and nonsurgical intensive care at the Darmstadt (Ger.) Clinic.
He emphasized the point about “expert hands,” drawing attention to the stellar 86.3% successful revascularization rate in the EuroCTO trial, even though these were often complex procedures. Indeed, in 36% of the CTO PCIs, a retrograde approach was used.
“CTO is a special field. Just like not everybody in every hospital will do a transcatheter aortic valve replacement, not everybody should do a CTO. It can be done safely and to the benefit of the patient, but it needs to be done by someone with expertise,” the cardiologist said.
Study participants were evenly split between those with single- and multivessel disease. Patients with additional nonocclusive disease had those lesions treated by PCI before the 2:1 randomization to CTO PCI or OMT.
The periprocedural complication rate was low at 2.9%, a figure that included a 1.5% incidence of pericardial tamponade as well as vascular repairs. There were no periprocedural MIs or deaths. The 1-year major adverse cardiac event rate was roughly 6% in both study arms.
The PCI group received 6-12 months of dual-antiplatelet therapy with clopidogrel and aspirin. So did roughly 40% of the OMT group because of prior PCI.
Both study arms had comparably high rates of guideline-directed medical therapy, including statins, beta-blockers, and ACE inhibitors. However, the PCI group made significantly less use of nitrates than the OMT group during follow-up, reflecting their greater reduction in angina frequency. The crossover rate from OMT to PCI because of ongoing angina was 7.3%.
The long-term safety and durability of the two treatment strategies will be assessed at 3 years of follow-up.
The EuroCTO trial was originally planned for 600 patients. The investigators eventually settled for less because of slow enrollment. Many interventionalists who are skilled in treating CTOs proved reluctant to randomize the patients.
Dr. Werner contrasted the positive EuroCTO findings regarding clinical symptoms and quality of life to the negative results of the Korean DECISION CTO trial presented at the 2017 meeting of the American College of Cardiology. DECISION CTO found that PCI plus OMT wasn’t superior to OMT alone in reducing MI and other major adverse cardiac events in patients with at least one CTO. In Dr. Werner’s view, the Korean investigators chose the wrong endpoint.
“The quality of life improvement we’ve shown after PCI in EuroCTO is a valid clinical goal in treating stable coronary artery disease. I don’t think we can aim at improving prognosis,” according to Dr. Werner.
The impetus for EuroCTO was a recognition that CTOs are common and seriously undertreated. CTOs account for 16%-18% of all coronary lesions in patients with stable coronary artery disease, yet U.S. national data indicate only 5% of PCIs are performed to treat CTOs.
The EuroCTO trial was sponsored by the Euro CTO Club and supported by research grants from Biosensors and Asahi.
PARIS – The first randomized clinical trial to evaluate quality of life and clinical symptoms as the primary efficacy outcome in patients with coronary chronic total occlusion (CTO) showed a clear advantage for percutaneous revascularization over optimal medical therapy.
At 12 months’ follow-up in the 26-site, 396-patient EuroCTO trial, patients randomized to PCI with drug-eluting stents had significantly less angina and physical limitations coupled with greater improvement in quality of life than the optimal medical therapy (OMT) group on subscales of the Seattle Angina Questionnaire. On the angina frequency subscale, for example, the PCI group improved from a mean baseline score of 77.2 to 91.4 at 12 months, a significantly better result than the OMT group improvement from 80.6 to 87.5.
The PCI group also experienced greater mobility, better activity status, and less pain and discomfort as assessed by the EuroQOL five dimensions questionnaire (EQ-5D), Gerald S. Werner, MD, reported at the annual congress of the European Association of Percutaneous Cardiovascular Interventions.
“PCI of a CTO should be considered as a primary option in symptomatic patients. It is a safe and effective treatment option in expert hands,” said Dr. Werner, director of cardiology and nonsurgical intensive care at the Darmstadt (Ger.) Clinic.
He emphasized the point about “expert hands,” drawing attention to the stellar 86.3% successful revascularization rate in the EuroCTO trial, even though these were often complex procedures. Indeed, in 36% of the CTO PCIs, a retrograde approach was used.
“CTO is a special field. Just like not everybody in every hospital will do a transcatheter aortic valve replacement, not everybody should do a CTO. It can be done safely and to the benefit of the patient, but it needs to be done by someone with expertise,” the cardiologist said.
Study participants were evenly split between those with single- and multivessel disease. Patients with additional nonocclusive disease had those lesions treated by PCI before the 2:1 randomization to CTO PCI or OMT.
The periprocedural complication rate was low at 2.9%, a figure that included a 1.5% incidence of pericardial tamponade as well as vascular repairs. There were no periprocedural MIs or deaths. The 1-year major adverse cardiac event rate was roughly 6% in both study arms.
The PCI group received 6-12 months of dual-antiplatelet therapy with clopidogrel and aspirin. So did roughly 40% of the OMT group because of prior PCI.
Both study arms had comparably high rates of guideline-directed medical therapy, including statins, beta-blockers, and ACE inhibitors. However, the PCI group made significantly less use of nitrates than the OMT group during follow-up, reflecting their greater reduction in angina frequency. The crossover rate from OMT to PCI because of ongoing angina was 7.3%.
The long-term safety and durability of the two treatment strategies will be assessed at 3 years of follow-up.
The EuroCTO trial was originally planned for 600 patients. The investigators eventually settled for less because of slow enrollment. Many interventionalists who are skilled in treating CTOs proved reluctant to randomize the patients.
Dr. Werner contrasted the positive EuroCTO findings regarding clinical symptoms and quality of life to the negative results of the Korean DECISION CTO trial presented at the 2017 meeting of the American College of Cardiology. DECISION CTO found that PCI plus OMT wasn’t superior to OMT alone in reducing MI and other major adverse cardiac events in patients with at least one CTO. In Dr. Werner’s view, the Korean investigators chose the wrong endpoint.
“The quality of life improvement we’ve shown after PCI in EuroCTO is a valid clinical goal in treating stable coronary artery disease. I don’t think we can aim at improving prognosis,” according to Dr. Werner.
The impetus for EuroCTO was a recognition that CTOs are common and seriously undertreated. CTOs account for 16%-18% of all coronary lesions in patients with stable coronary artery disease, yet U.S. national data indicate only 5% of PCIs are performed to treat CTOs.
The EuroCTO trial was sponsored by the Euro CTO Club and supported by research grants from Biosensors and Asahi.
PARIS – The first randomized clinical trial to evaluate quality of life and clinical symptoms as the primary efficacy outcome in patients with coronary chronic total occlusion (CTO) showed a clear advantage for percutaneous revascularization over optimal medical therapy.
At 12 months’ follow-up in the 26-site, 396-patient EuroCTO trial, patients randomized to PCI with drug-eluting stents had significantly less angina and physical limitations coupled with greater improvement in quality of life than the optimal medical therapy (OMT) group on subscales of the Seattle Angina Questionnaire. On the angina frequency subscale, for example, the PCI group improved from a mean baseline score of 77.2 to 91.4 at 12 months, a significantly better result than the OMT group improvement from 80.6 to 87.5.
The PCI group also experienced greater mobility, better activity status, and less pain and discomfort as assessed by the EuroQOL five dimensions questionnaire (EQ-5D), Gerald S. Werner, MD, reported at the annual congress of the European Association of Percutaneous Cardiovascular Interventions.
“PCI of a CTO should be considered as a primary option in symptomatic patients. It is a safe and effective treatment option in expert hands,” said Dr. Werner, director of cardiology and nonsurgical intensive care at the Darmstadt (Ger.) Clinic.
He emphasized the point about “expert hands,” drawing attention to the stellar 86.3% successful revascularization rate in the EuroCTO trial, even though these were often complex procedures. Indeed, in 36% of the CTO PCIs, a retrograde approach was used.
“CTO is a special field. Just like not everybody in every hospital will do a transcatheter aortic valve replacement, not everybody should do a CTO. It can be done safely and to the benefit of the patient, but it needs to be done by someone with expertise,” the cardiologist said.
Study participants were evenly split between those with single- and multivessel disease. Patients with additional nonocclusive disease had those lesions treated by PCI before the 2:1 randomization to CTO PCI or OMT.
The periprocedural complication rate was low at 2.9%, a figure that included a 1.5% incidence of pericardial tamponade as well as vascular repairs. There were no periprocedural MIs or deaths. The 1-year major adverse cardiac event rate was roughly 6% in both study arms.
The PCI group received 6-12 months of dual-antiplatelet therapy with clopidogrel and aspirin. So did roughly 40% of the OMT group because of prior PCI.
Both study arms had comparably high rates of guideline-directed medical therapy, including statins, beta-blockers, and ACE inhibitors. However, the PCI group made significantly less use of nitrates than the OMT group during follow-up, reflecting their greater reduction in angina frequency. The crossover rate from OMT to PCI because of ongoing angina was 7.3%.
The long-term safety and durability of the two treatment strategies will be assessed at 3 years of follow-up.
The EuroCTO trial was originally planned for 600 patients. The investigators eventually settled for less because of slow enrollment. Many interventionalists who are skilled in treating CTOs proved reluctant to randomize the patients.
Dr. Werner contrasted the positive EuroCTO findings regarding clinical symptoms and quality of life to the negative results of the Korean DECISION CTO trial presented at the 2017 meeting of the American College of Cardiology. DECISION CTO found that PCI plus OMT wasn’t superior to OMT alone in reducing MI and other major adverse cardiac events in patients with at least one CTO. In Dr. Werner’s view, the Korean investigators chose the wrong endpoint.
“The quality of life improvement we’ve shown after PCI in EuroCTO is a valid clinical goal in treating stable coronary artery disease. I don’t think we can aim at improving prognosis,” according to Dr. Werner.
The impetus for EuroCTO was a recognition that CTOs are common and seriously undertreated. CTOs account for 16%-18% of all coronary lesions in patients with stable coronary artery disease, yet U.S. national data indicate only 5% of PCIs are performed to treat CTOs.
The EuroCTO trial was sponsored by the Euro CTO Club and supported by research grants from Biosensors and Asahi.
AT EUROPCR
Key clinical point:
Major finding: Patients whose chronic total occlusions were treated by PCI rather than optimal medical therapy experienced significantly less angina and greater improvement in quality of life during 12 months of follow-up.
Data source: EuroCTO, a prospective, 26-site study in which 396 patients with coronary chronic total occlusions were randomized 2:1 to PCI or optimal medical therapy.
Disclosures: EuroCTO was sponsored by the Euro CTO Club and supported by research grants from Biosensors and Asahi.
Novel drug-eluting coronary stent looks good in DESSOLVE III
PARIS – A unique drug-eluting coronary stent showed positive interim results in the DESSOLVE III trial presented at the annual congress of the European Association of Percutaneous Cardiovascular Interventions.
In DESSOLVE III, 1,398 patients undergoing percutaneous coronary intervention were randomized to the widely used Xience everolimus-eluting stent or to MiStent, a novel thin-strut stent with a polymer coating that is quickly absorbed after delivering microcrystalline sirolimus into the vessel wall for prolonged release at a near-linear rate.
At an interim analysis at 12 months of follow-up, the primary endpoint – a composite of cardiac death, target vessel MI, and clinically indicated target lesion revascularization – had occurred in 5.8% of the MiStent group and 6.5% of Xience recipients in the study, which was designed as a noninferiority trial, reported Robbert J. de Winter, MD, professor of clinical cardiology at the Academic Medical Center in Amsterdam.
“The data support the hypothesis that long-term cytostatic inhibition of early neointima could prevent the late neointimal growth seen at medium and long term with conventional drug-eluting stents,” he said.
MiStent is designed to overcome a shortcoming of conventional drug-eluting stents: namely, their durable polymer coating sticks around after the cytostatic drug is finished being released. It is believed that this residual polymer, which may not disappear for 6-9 months, induces inflammation in the vessel wall, eventually leading to intimal growth, restenosis, and new atherosclerosis.
“The unique feature of the MiStent is that the polymer is bioabsorbable and is fully absorbed at 3 months, whereas the drug is present in the vessel wall out to 9 months, well after the coating has disappeared. So in theory you would expect that any inflammatory response caused by the polymer coating will be counteracted by the drug. This was seen in animal models. And in the DESSOLVE I and II studies, angiographic follow-up showed that late luminal loss was flat after 6 months, in contrast to other drug-eluting stents, which show accrual of late neointimal growth after 6 months to a year,” according to the cardiologist.
DESSOLVE III is planned as a 3-year study. Already by 6 months the curves for target lesion revascularization started to separate, with a 12-month rate of 2.6% in the MiStent group versus 3.8% for Xience. And while this difference is not yet statistically significant, Dr. de Winter and his coinvestigators expect that by 3 years the separation will have grown to the point that the difference becomes statistically significant and clinically meaningful.
The MiStent platform is composed of a cobalt/chromium alloy. The stent strut thickness is only 64 microns, in contrast to 81 microns for the Xience stent. Thinner stent struts have previously been shown to be less injurious to the vessel wall.
DESSOLVE III is an all-comers trial conducted at 20 sites in four European countries. Participants had to have a reference vessel diameter of 2.5-3.5 mm. Roughly 60% of patients had an acute coronary syndrome as their indication for PCI. The study population included, among others, patients with left main coronary artery lesions, restenotic lesions, and failed saphenous vein grafts. Dual-antiplatelet therapy was given for 6 months in patients with stable angina and 12 months for those with ACS, in accordance with European Society of Cardiology guidelines.
Discussant Robert A. Byrne, MD, of the German Heart Center in Munich, declared: “For me, this is a potentially interesting device. It’s the only device where we have a drug elution that’s more prolonged than the polymer, and this does offer the potential for later benefit.”
Dr. Byrne was a member of a European Commission–backed task force that developed European regulatory guidance for the evaluation of new coronary stents. “This MiStent trial program ticks off a lot of boxes: We had a successful first human use study, then we had a modest-size angiographic endpoint study where the late lumen loss looked good, and now we have a clinical endpoint study. This is what we want to see in the regulatory space.”
Another discussant, Chaim Lotan, MD, pronounced the MiStent “definitely another good stent on the shelf.”
It’s impossible to say whether the excellent 1-year outcomes seen with MiStent in DESSOLVE III were due to the prolonged-release microcrystalline sirolimus, the ultrathin stent struts, or both. In any case, the major adverse cardiovascular event rates seen in DESSOLVE III are so low by historical standards that it will become extremely difficult to show superiority for one contemporary drug-eluting stent over another, predicted Dr. Lotan of Hadassah Medical Center in Jerusalem.
Dr. de Winter concurred.
“I think we can now say that the benchmark for present day drug-eluting stents is a target lesion failure rate of about 6% at 12 months and a stent thrombosis rate below 1% at 12 months. It’s going to be increasingly more difficult to improve on that,” he said.
The MiStent, manufactured by Micell Technologies, is commercially available in Europe but investigational in the United States.
DESSOLVE III was sponsored by the European Cardiovascular Research Institute and supported by grants from Micell Technologies and Stentys.
Dr. de Winter reported receiving research grants from OrbusNeich, Abbott Vascular, AstraZeneca, Stentys, and Tryton.
PARIS – A unique drug-eluting coronary stent showed positive interim results in the DESSOLVE III trial presented at the annual congress of the European Association of Percutaneous Cardiovascular Interventions.
In DESSOLVE III, 1,398 patients undergoing percutaneous coronary intervention were randomized to the widely used Xience everolimus-eluting stent or to MiStent, a novel thin-strut stent with a polymer coating that is quickly absorbed after delivering microcrystalline sirolimus into the vessel wall for prolonged release at a near-linear rate.
At an interim analysis at 12 months of follow-up, the primary endpoint – a composite of cardiac death, target vessel MI, and clinically indicated target lesion revascularization – had occurred in 5.8% of the MiStent group and 6.5% of Xience recipients in the study, which was designed as a noninferiority trial, reported Robbert J. de Winter, MD, professor of clinical cardiology at the Academic Medical Center in Amsterdam.
“The data support the hypothesis that long-term cytostatic inhibition of early neointima could prevent the late neointimal growth seen at medium and long term with conventional drug-eluting stents,” he said.
MiStent is designed to overcome a shortcoming of conventional drug-eluting stents: namely, their durable polymer coating sticks around after the cytostatic drug is finished being released. It is believed that this residual polymer, which may not disappear for 6-9 months, induces inflammation in the vessel wall, eventually leading to intimal growth, restenosis, and new atherosclerosis.
“The unique feature of the MiStent is that the polymer is bioabsorbable and is fully absorbed at 3 months, whereas the drug is present in the vessel wall out to 9 months, well after the coating has disappeared. So in theory you would expect that any inflammatory response caused by the polymer coating will be counteracted by the drug. This was seen in animal models. And in the DESSOLVE I and II studies, angiographic follow-up showed that late luminal loss was flat after 6 months, in contrast to other drug-eluting stents, which show accrual of late neointimal growth after 6 months to a year,” according to the cardiologist.
DESSOLVE III is planned as a 3-year study. Already by 6 months the curves for target lesion revascularization started to separate, with a 12-month rate of 2.6% in the MiStent group versus 3.8% for Xience. And while this difference is not yet statistically significant, Dr. de Winter and his coinvestigators expect that by 3 years the separation will have grown to the point that the difference becomes statistically significant and clinically meaningful.
The MiStent platform is composed of a cobalt/chromium alloy. The stent strut thickness is only 64 microns, in contrast to 81 microns for the Xience stent. Thinner stent struts have previously been shown to be less injurious to the vessel wall.
DESSOLVE III is an all-comers trial conducted at 20 sites in four European countries. Participants had to have a reference vessel diameter of 2.5-3.5 mm. Roughly 60% of patients had an acute coronary syndrome as their indication for PCI. The study population included, among others, patients with left main coronary artery lesions, restenotic lesions, and failed saphenous vein grafts. Dual-antiplatelet therapy was given for 6 months in patients with stable angina and 12 months for those with ACS, in accordance with European Society of Cardiology guidelines.
Discussant Robert A. Byrne, MD, of the German Heart Center in Munich, declared: “For me, this is a potentially interesting device. It’s the only device where we have a drug elution that’s more prolonged than the polymer, and this does offer the potential for later benefit.”
Dr. Byrne was a member of a European Commission–backed task force that developed European regulatory guidance for the evaluation of new coronary stents. “This MiStent trial program ticks off a lot of boxes: We had a successful first human use study, then we had a modest-size angiographic endpoint study where the late lumen loss looked good, and now we have a clinical endpoint study. This is what we want to see in the regulatory space.”
Another discussant, Chaim Lotan, MD, pronounced the MiStent “definitely another good stent on the shelf.”
It’s impossible to say whether the excellent 1-year outcomes seen with MiStent in DESSOLVE III were due to the prolonged-release microcrystalline sirolimus, the ultrathin stent struts, or both. In any case, the major adverse cardiovascular event rates seen in DESSOLVE III are so low by historical standards that it will become extremely difficult to show superiority for one contemporary drug-eluting stent over another, predicted Dr. Lotan of Hadassah Medical Center in Jerusalem.
Dr. de Winter concurred.
“I think we can now say that the benchmark for present day drug-eluting stents is a target lesion failure rate of about 6% at 12 months and a stent thrombosis rate below 1% at 12 months. It’s going to be increasingly more difficult to improve on that,” he said.
The MiStent, manufactured by Micell Technologies, is commercially available in Europe but investigational in the United States.
DESSOLVE III was sponsored by the European Cardiovascular Research Institute and supported by grants from Micell Technologies and Stentys.
Dr. de Winter reported receiving research grants from OrbusNeich, Abbott Vascular, AstraZeneca, Stentys, and Tryton.
PARIS – A unique drug-eluting coronary stent showed positive interim results in the DESSOLVE III trial presented at the annual congress of the European Association of Percutaneous Cardiovascular Interventions.
In DESSOLVE III, 1,398 patients undergoing percutaneous coronary intervention were randomized to the widely used Xience everolimus-eluting stent or to MiStent, a novel thin-strut stent with a polymer coating that is quickly absorbed after delivering microcrystalline sirolimus into the vessel wall for prolonged release at a near-linear rate.
At an interim analysis at 12 months of follow-up, the primary endpoint – a composite of cardiac death, target vessel MI, and clinically indicated target lesion revascularization – had occurred in 5.8% of the MiStent group and 6.5% of Xience recipients in the study, which was designed as a noninferiority trial, reported Robbert J. de Winter, MD, professor of clinical cardiology at the Academic Medical Center in Amsterdam.
“The data support the hypothesis that long-term cytostatic inhibition of early neointima could prevent the late neointimal growth seen at medium and long term with conventional drug-eluting stents,” he said.
MiStent is designed to overcome a shortcoming of conventional drug-eluting stents: namely, their durable polymer coating sticks around after the cytostatic drug is finished being released. It is believed that this residual polymer, which may not disappear for 6-9 months, induces inflammation in the vessel wall, eventually leading to intimal growth, restenosis, and new atherosclerosis.
“The unique feature of the MiStent is that the polymer is bioabsorbable and is fully absorbed at 3 months, whereas the drug is present in the vessel wall out to 9 months, well after the coating has disappeared. So in theory you would expect that any inflammatory response caused by the polymer coating will be counteracted by the drug. This was seen in animal models. And in the DESSOLVE I and II studies, angiographic follow-up showed that late luminal loss was flat after 6 months, in contrast to other drug-eluting stents, which show accrual of late neointimal growth after 6 months to a year,” according to the cardiologist.
DESSOLVE III is planned as a 3-year study. Already by 6 months the curves for target lesion revascularization started to separate, with a 12-month rate of 2.6% in the MiStent group versus 3.8% for Xience. And while this difference is not yet statistically significant, Dr. de Winter and his coinvestigators expect that by 3 years the separation will have grown to the point that the difference becomes statistically significant and clinically meaningful.
The MiStent platform is composed of a cobalt/chromium alloy. The stent strut thickness is only 64 microns, in contrast to 81 microns for the Xience stent. Thinner stent struts have previously been shown to be less injurious to the vessel wall.
DESSOLVE III is an all-comers trial conducted at 20 sites in four European countries. Participants had to have a reference vessel diameter of 2.5-3.5 mm. Roughly 60% of patients had an acute coronary syndrome as their indication for PCI. The study population included, among others, patients with left main coronary artery lesions, restenotic lesions, and failed saphenous vein grafts. Dual-antiplatelet therapy was given for 6 months in patients with stable angina and 12 months for those with ACS, in accordance with European Society of Cardiology guidelines.
Discussant Robert A. Byrne, MD, of the German Heart Center in Munich, declared: “For me, this is a potentially interesting device. It’s the only device where we have a drug elution that’s more prolonged than the polymer, and this does offer the potential for later benefit.”
Dr. Byrne was a member of a European Commission–backed task force that developed European regulatory guidance for the evaluation of new coronary stents. “This MiStent trial program ticks off a lot of boxes: We had a successful first human use study, then we had a modest-size angiographic endpoint study where the late lumen loss looked good, and now we have a clinical endpoint study. This is what we want to see in the regulatory space.”
Another discussant, Chaim Lotan, MD, pronounced the MiStent “definitely another good stent on the shelf.”
It’s impossible to say whether the excellent 1-year outcomes seen with MiStent in DESSOLVE III were due to the prolonged-release microcrystalline sirolimus, the ultrathin stent struts, or both. In any case, the major adverse cardiovascular event rates seen in DESSOLVE III are so low by historical standards that it will become extremely difficult to show superiority for one contemporary drug-eluting stent over another, predicted Dr. Lotan of Hadassah Medical Center in Jerusalem.
Dr. de Winter concurred.
“I think we can now say that the benchmark for present day drug-eluting stents is a target lesion failure rate of about 6% at 12 months and a stent thrombosis rate below 1% at 12 months. It’s going to be increasingly more difficult to improve on that,” he said.
The MiStent, manufactured by Micell Technologies, is commercially available in Europe but investigational in the United States.
DESSOLVE III was sponsored by the European Cardiovascular Research Institute and supported by grants from Micell Technologies and Stentys.
Dr. de Winter reported receiving research grants from OrbusNeich, Abbott Vascular, AstraZeneca, Stentys, and Tryton.
AT EUROPCR
Key clinical point:
Major finding: At an interim 12-month analysis, the composite rate of cardiac death, target vessel MI, and clinically indicated target lesion revascularization was 6.5% in recipients of a Xience everolimus-eluting coronary stent and 5.8% in those randomized to the novel MiStent.
Data source: DESSOLVE III, a prospective, randomized, international trial that randomized 1,398 real-world-type all-comers undergoing PCI to the Xience device or the MiStent.
Disclosures: DESSOLVE III was sponsored by the European Cardiovascular Research Institute and supported by grants from Micell Technologies and Stentys. The presenter reported receiving research grants from both companies.
Potential new role for FFR
Paris – Fractional flow reserve is under study for a potential major application: guidance on percutaneous coronary intervention (PCI) optimization immediately after stent placement, Roberto Diletti, MD, said at the annual congress of the European Association of Percutaneous Cardiovascular Interventions.
At the end of the procedure, FFR measurement using a novel monorail optical pressure sensor catheter revealed that 43% of the lesions had a suboptimal FFR value of 0.90 or less.
“Just this year, two meta-analyses showed that a cutoff of 0.90 is important to define a group of patients at high risk for major adverse cardiovascular events and revascularization,” noted Dr. Diletti of Erasmus University Medical Center in Rotterdam, the Netherlands.
Using the older, more conservative cutoff of an FFR of 0.85 or less, 20% of the lesions would potentially benefit from further action to optimize the physiologic result, most often in the form of additional expansion of the stent.
“We are used to thinking of FFR as a tool to understand whether a lesion has to be treated or not. Now we can also start thinking about FFR as a tool to guide PCI optimization,” the cardiologist said.
Optimization wasn’t actually performed in this observational registry. That will be the focus of FFR-REACT, a planned randomized trial investigating the clinical impact of intravascular ultrasound (IVUS)-directed FFR optimization of PCI.
In a per-patient analysis, 48% of FFR-SEARCH participants had a poststent FFR of 0.90 or less in one or more treated lesions. Another 22% had a postprocedure FFR of 0.85 or less in at least one treated lesion, while 8.9% had an FFR of 0.80, which is below the threshold for ischemia.
The primary endpoint in the ongoing FFR-SEARCH study is the 2-year composite rate of major adverse cardiovascular events, defined as MI, any revascularization, or all-cause mortality. Only the 30-day MACE rate was available at the time of Dr. Diletti’s presentation in Paris. The rate was 1.5% in patients with a postprocedure FFR greater than 0.90, 2.0% in those with an FFR of 0.86-0.90, 2.6% with an FFR of 0.81-0.85, and 2.8% with a poststent FFR of 0.80 or less. While those early between-group differences weren’t statistically significant, the trend is encouraging, he noted.
Postprocedure FFR measurement took an average of 5 minutes. The procedure was simple and safe, according to Dr. Diletti. There were no complications related to the use of the Navvus MicroCatheter technology. He explained that the device profile is comparable to a 0.022-inch diameter at the lesion site. Wire access to the vessel was maintained throughout. The rapid-exchange monorail microcatheter was inserted over the previously used standard 0.014-inch coronary guidewire. The optical pressure sensor was positioned roughly 20 mm distal to the distal stent edge. Manual pullback with measurements obtained at various locations in the vicinity of the stented lesion was repeated as necessary in order to identify where optimization, if appropriate, should be focused.
Operators were unable to crossover the microcatheter in 3.5% of cases, mostly because of vessel tortuosity or calcification.
Audience members commented that many of the low FFRs after stenting may reflect diffuse coronary disease, which can be corrected only by placing numerous additional stents, creating its own problems. Dr. Diletti offered reassurance on that score. He explained that in an IVUS substudy of FFR-SEARCH, an unstented physiologically important focal lesion or stent underexpansion was identified in 86% of the cases of low FFR.
“That means you can do something about it. In the other 14% of cases, in my opinion, you cannot do a lot because of very diffuse disease distally,” he said.
He reported having no financial conflicts of interest in connection with the study, supported by ACIST Medical Systems. The Navvus MicroCatheter is approved by both the Food and Drug Administration and the European regulatory agency.
Paris – Fractional flow reserve is under study for a potential major application: guidance on percutaneous coronary intervention (PCI) optimization immediately after stent placement, Roberto Diletti, MD, said at the annual congress of the European Association of Percutaneous Cardiovascular Interventions.
At the end of the procedure, FFR measurement using a novel monorail optical pressure sensor catheter revealed that 43% of the lesions had a suboptimal FFR value of 0.90 or less.
“Just this year, two meta-analyses showed that a cutoff of 0.90 is important to define a group of patients at high risk for major adverse cardiovascular events and revascularization,” noted Dr. Diletti of Erasmus University Medical Center in Rotterdam, the Netherlands.
Using the older, more conservative cutoff of an FFR of 0.85 or less, 20% of the lesions would potentially benefit from further action to optimize the physiologic result, most often in the form of additional expansion of the stent.
“We are used to thinking of FFR as a tool to understand whether a lesion has to be treated or not. Now we can also start thinking about FFR as a tool to guide PCI optimization,” the cardiologist said.
Optimization wasn’t actually performed in this observational registry. That will be the focus of FFR-REACT, a planned randomized trial investigating the clinical impact of intravascular ultrasound (IVUS)-directed FFR optimization of PCI.
In a per-patient analysis, 48% of FFR-SEARCH participants had a poststent FFR of 0.90 or less in one or more treated lesions. Another 22% had a postprocedure FFR of 0.85 or less in at least one treated lesion, while 8.9% had an FFR of 0.80, which is below the threshold for ischemia.
The primary endpoint in the ongoing FFR-SEARCH study is the 2-year composite rate of major adverse cardiovascular events, defined as MI, any revascularization, or all-cause mortality. Only the 30-day MACE rate was available at the time of Dr. Diletti’s presentation in Paris. The rate was 1.5% in patients with a postprocedure FFR greater than 0.90, 2.0% in those with an FFR of 0.86-0.90, 2.6% with an FFR of 0.81-0.85, and 2.8% with a poststent FFR of 0.80 or less. While those early between-group differences weren’t statistically significant, the trend is encouraging, he noted.
Postprocedure FFR measurement took an average of 5 minutes. The procedure was simple and safe, according to Dr. Diletti. There were no complications related to the use of the Navvus MicroCatheter technology. He explained that the device profile is comparable to a 0.022-inch diameter at the lesion site. Wire access to the vessel was maintained throughout. The rapid-exchange monorail microcatheter was inserted over the previously used standard 0.014-inch coronary guidewire. The optical pressure sensor was positioned roughly 20 mm distal to the distal stent edge. Manual pullback with measurements obtained at various locations in the vicinity of the stented lesion was repeated as necessary in order to identify where optimization, if appropriate, should be focused.
Operators were unable to crossover the microcatheter in 3.5% of cases, mostly because of vessel tortuosity or calcification.
Audience members commented that many of the low FFRs after stenting may reflect diffuse coronary disease, which can be corrected only by placing numerous additional stents, creating its own problems. Dr. Diletti offered reassurance on that score. He explained that in an IVUS substudy of FFR-SEARCH, an unstented physiologically important focal lesion or stent underexpansion was identified in 86% of the cases of low FFR.
“That means you can do something about it. In the other 14% of cases, in my opinion, you cannot do a lot because of very diffuse disease distally,” he said.
He reported having no financial conflicts of interest in connection with the study, supported by ACIST Medical Systems. The Navvus MicroCatheter is approved by both the Food and Drug Administration and the European regulatory agency.
Paris – Fractional flow reserve is under study for a potential major application: guidance on percutaneous coronary intervention (PCI) optimization immediately after stent placement, Roberto Diletti, MD, said at the annual congress of the European Association of Percutaneous Cardiovascular Interventions.
At the end of the procedure, FFR measurement using a novel monorail optical pressure sensor catheter revealed that 43% of the lesions had a suboptimal FFR value of 0.90 or less.
“Just this year, two meta-analyses showed that a cutoff of 0.90 is important to define a group of patients at high risk for major adverse cardiovascular events and revascularization,” noted Dr. Diletti of Erasmus University Medical Center in Rotterdam, the Netherlands.
Using the older, more conservative cutoff of an FFR of 0.85 or less, 20% of the lesions would potentially benefit from further action to optimize the physiologic result, most often in the form of additional expansion of the stent.
“We are used to thinking of FFR as a tool to understand whether a lesion has to be treated or not. Now we can also start thinking about FFR as a tool to guide PCI optimization,” the cardiologist said.
Optimization wasn’t actually performed in this observational registry. That will be the focus of FFR-REACT, a planned randomized trial investigating the clinical impact of intravascular ultrasound (IVUS)-directed FFR optimization of PCI.
In a per-patient analysis, 48% of FFR-SEARCH participants had a poststent FFR of 0.90 or less in one or more treated lesions. Another 22% had a postprocedure FFR of 0.85 or less in at least one treated lesion, while 8.9% had an FFR of 0.80, which is below the threshold for ischemia.
The primary endpoint in the ongoing FFR-SEARCH study is the 2-year composite rate of major adverse cardiovascular events, defined as MI, any revascularization, or all-cause mortality. Only the 30-day MACE rate was available at the time of Dr. Diletti’s presentation in Paris. The rate was 1.5% in patients with a postprocedure FFR greater than 0.90, 2.0% in those with an FFR of 0.86-0.90, 2.6% with an FFR of 0.81-0.85, and 2.8% with a poststent FFR of 0.80 or less. While those early between-group differences weren’t statistically significant, the trend is encouraging, he noted.
Postprocedure FFR measurement took an average of 5 minutes. The procedure was simple and safe, according to Dr. Diletti. There were no complications related to the use of the Navvus MicroCatheter technology. He explained that the device profile is comparable to a 0.022-inch diameter at the lesion site. Wire access to the vessel was maintained throughout. The rapid-exchange monorail microcatheter was inserted over the previously used standard 0.014-inch coronary guidewire. The optical pressure sensor was positioned roughly 20 mm distal to the distal stent edge. Manual pullback with measurements obtained at various locations in the vicinity of the stented lesion was repeated as necessary in order to identify where optimization, if appropriate, should be focused.
Operators were unable to crossover the microcatheter in 3.5% of cases, mostly because of vessel tortuosity or calcification.
Audience members commented that many of the low FFRs after stenting may reflect diffuse coronary disease, which can be corrected only by placing numerous additional stents, creating its own problems. Dr. Diletti offered reassurance on that score. He explained that in an IVUS substudy of FFR-SEARCH, an unstented physiologically important focal lesion or stent underexpansion was identified in 86% of the cases of low FFR.
“That means you can do something about it. In the other 14% of cases, in my opinion, you cannot do a lot because of very diffuse disease distally,” he said.
He reported having no financial conflicts of interest in connection with the study, supported by ACIST Medical Systems. The Navvus MicroCatheter is approved by both the Food and Drug Administration and the European regulatory agency.
EXPERT ANALYSIS FROM EuroPCR
CABG with arterial grafts provides excellent outcomes for CTO
PARIS – Eighty-eight percent of chronic total occlusions (CTOs) in a large series of patients undergoing coronary artery bypass graft surgery were successfully bypassed using arterial conduits with durable patency.
“Bypass graft surgery using arterial grafts is an acceptable modality of treatment for patients with CTOs and perhaps can be a benchmark against which PCI [percutaneous coronary intervention] for CTOs should be measured,” Teresa May Kieser, MD, said at the annual congress of the European Association of Percutaneous Cardiovascular Interventions.
“We have previously shown in another paper that arterial grafting mitigates the adverse effect of incomplete revascularization,” said Dr. Kieser, a cardiothoracic surgeon at the University of Calgary (Alt.).
In recent years, treatment of CTOs has increasingly drawn the attention of interventional cardiologists. Dr. Kieser presented what she thinks is the first study of coronary artery bypass graft (CABG) surgery for management of CTOs. It included 1,333 consecutive CABG patients with a total of 3,906 bypasses, a whopping 98% of which were arterial grafts, with a mean of 2.9 grafts per patient. Eleven percent of the CABGs were done emergently, 48% urgently, and 41% electively.
The key epidemiologic finding to emerge from the study is that CTOs are quite common in CABG patients. In this series, 47% of CABG patients had a mean of 1.35 chronically occluded coronary arteries.
Of 843 CTOs in three major territories, 88% were able to be bypassed. All of the 246 CTOs in the left anterior descending coronary artery were able to be bypassed, as were 84% of 415 CTOs in the right coronary artery and 85% in the circumflex system.
The CTO group as a whole had significantly greater impairment of left ventricular function. Thirty-seven percent of them had an ejection fraction of 30%-50%, compared with 22% of the non-CTO patients. The 10% prevalence of an left ventricular ejection fraction (LVEF) below 30% in the CTO group was twice that of the non-CTO group. The CTO group was also significantly more likely to undergo incomplete revascularization, by a margin of 21% versus 5.7%.
Operative mortality was 3.7% overall and just 0.55% in the elective CABG patients. In a multivariate logistic regression analysis controlled for surgical urgency, incomplete revascularization, and EuroSCORE risk, operative mortality didn’t differ significantly between the CTO and non-CTO groups.
However, in the presence of CTOs, incomplete revascularization was associated with an 11.6% operative mortality, compared with a 2.8% rate in fully revascularized CTO patients.
A total of 110 patients with bypassed CTOs underwent symptom-driven follow-up coronary angiography at a median of 3.6 years after CABG. Reassuringly, CTO graft patency was noted in 95% of the LAD grafts, 92% of the right coronary artery grafts, and 79% of the circumflex grafts.
Dr. Kieser’s audience of interventional cardiologists was clearly bowled over by her results, not only the high rate of successful surgical bypass of CTOs, but also by her use of arterial grafts 98% of the time.
“This is my personal practice,” she explained. “I just believe in arterial grafting so much. They perform best in CTO arteries because of their lack of competitive flow.”
Session chair Oliver Gämperli, MD, of University Hospital Zurich, commented, “We are very concerned about patency rates, and you showed us fantastic patency rates. This is much better than what we’re used to with saphenous vein grafts. I think we need to talk to our surgeons and try to get them to do more arterial grafts of CTOs.”
It’s worth noting that in the CTO subgroup from the landmark randomized SYNTAX trial, the complete revascularization rate was only about 50% in the PCI group, compared with nearly 65% in the CABG group, he added.
Asked why a cardiac surgeon wouldn’t bypass a CTO, Dr. Kieser rattled off several technical reasons, including a vessel size of less than 1 mm, diffuse disease, extensive scar, or an inaccessible location. But that’s not the whole story, she added. She has heard surgical colleagues say, “The patient doesn’t need that artery, he’s learned to live without it.” That burns her up.
“Patients need every artery in the heart, and the one with a CTO is the best one for an arterial graft because it almost cannot fail, especially to the left anterior descending artery. I think we have to change the mentality of the surgeons to ‘If it can be done, it should be done,’ ” Dr. Kieser said.
She reported having no financial conflicts of interest regarding her study.
PARIS – Eighty-eight percent of chronic total occlusions (CTOs) in a large series of patients undergoing coronary artery bypass graft surgery were successfully bypassed using arterial conduits with durable patency.
“Bypass graft surgery using arterial grafts is an acceptable modality of treatment for patients with CTOs and perhaps can be a benchmark against which PCI [percutaneous coronary intervention] for CTOs should be measured,” Teresa May Kieser, MD, said at the annual congress of the European Association of Percutaneous Cardiovascular Interventions.
“We have previously shown in another paper that arterial grafting mitigates the adverse effect of incomplete revascularization,” said Dr. Kieser, a cardiothoracic surgeon at the University of Calgary (Alt.).
In recent years, treatment of CTOs has increasingly drawn the attention of interventional cardiologists. Dr. Kieser presented what she thinks is the first study of coronary artery bypass graft (CABG) surgery for management of CTOs. It included 1,333 consecutive CABG patients with a total of 3,906 bypasses, a whopping 98% of which were arterial grafts, with a mean of 2.9 grafts per patient. Eleven percent of the CABGs were done emergently, 48% urgently, and 41% electively.
The key epidemiologic finding to emerge from the study is that CTOs are quite common in CABG patients. In this series, 47% of CABG patients had a mean of 1.35 chronically occluded coronary arteries.
Of 843 CTOs in three major territories, 88% were able to be bypassed. All of the 246 CTOs in the left anterior descending coronary artery were able to be bypassed, as were 84% of 415 CTOs in the right coronary artery and 85% in the circumflex system.
The CTO group as a whole had significantly greater impairment of left ventricular function. Thirty-seven percent of them had an ejection fraction of 30%-50%, compared with 22% of the non-CTO patients. The 10% prevalence of an left ventricular ejection fraction (LVEF) below 30% in the CTO group was twice that of the non-CTO group. The CTO group was also significantly more likely to undergo incomplete revascularization, by a margin of 21% versus 5.7%.
Operative mortality was 3.7% overall and just 0.55% in the elective CABG patients. In a multivariate logistic regression analysis controlled for surgical urgency, incomplete revascularization, and EuroSCORE risk, operative mortality didn’t differ significantly between the CTO and non-CTO groups.
However, in the presence of CTOs, incomplete revascularization was associated with an 11.6% operative mortality, compared with a 2.8% rate in fully revascularized CTO patients.
A total of 110 patients with bypassed CTOs underwent symptom-driven follow-up coronary angiography at a median of 3.6 years after CABG. Reassuringly, CTO graft patency was noted in 95% of the LAD grafts, 92% of the right coronary artery grafts, and 79% of the circumflex grafts.
Dr. Kieser’s audience of interventional cardiologists was clearly bowled over by her results, not only the high rate of successful surgical bypass of CTOs, but also by her use of arterial grafts 98% of the time.
“This is my personal practice,” she explained. “I just believe in arterial grafting so much. They perform best in CTO arteries because of their lack of competitive flow.”
Session chair Oliver Gämperli, MD, of University Hospital Zurich, commented, “We are very concerned about patency rates, and you showed us fantastic patency rates. This is much better than what we’re used to with saphenous vein grafts. I think we need to talk to our surgeons and try to get them to do more arterial grafts of CTOs.”
It’s worth noting that in the CTO subgroup from the landmark randomized SYNTAX trial, the complete revascularization rate was only about 50% in the PCI group, compared with nearly 65% in the CABG group, he added.
Asked why a cardiac surgeon wouldn’t bypass a CTO, Dr. Kieser rattled off several technical reasons, including a vessel size of less than 1 mm, diffuse disease, extensive scar, or an inaccessible location. But that’s not the whole story, she added. She has heard surgical colleagues say, “The patient doesn’t need that artery, he’s learned to live without it.” That burns her up.
“Patients need every artery in the heart, and the one with a CTO is the best one for an arterial graft because it almost cannot fail, especially to the left anterior descending artery. I think we have to change the mentality of the surgeons to ‘If it can be done, it should be done,’ ” Dr. Kieser said.
She reported having no financial conflicts of interest regarding her study.
PARIS – Eighty-eight percent of chronic total occlusions (CTOs) in a large series of patients undergoing coronary artery bypass graft surgery were successfully bypassed using arterial conduits with durable patency.
“Bypass graft surgery using arterial grafts is an acceptable modality of treatment for patients with CTOs and perhaps can be a benchmark against which PCI [percutaneous coronary intervention] for CTOs should be measured,” Teresa May Kieser, MD, said at the annual congress of the European Association of Percutaneous Cardiovascular Interventions.
“We have previously shown in another paper that arterial grafting mitigates the adverse effect of incomplete revascularization,” said Dr. Kieser, a cardiothoracic surgeon at the University of Calgary (Alt.).
In recent years, treatment of CTOs has increasingly drawn the attention of interventional cardiologists. Dr. Kieser presented what she thinks is the first study of coronary artery bypass graft (CABG) surgery for management of CTOs. It included 1,333 consecutive CABG patients with a total of 3,906 bypasses, a whopping 98% of which were arterial grafts, with a mean of 2.9 grafts per patient. Eleven percent of the CABGs were done emergently, 48% urgently, and 41% electively.
The key epidemiologic finding to emerge from the study is that CTOs are quite common in CABG patients. In this series, 47% of CABG patients had a mean of 1.35 chronically occluded coronary arteries.
Of 843 CTOs in three major territories, 88% were able to be bypassed. All of the 246 CTOs in the left anterior descending coronary artery were able to be bypassed, as were 84% of 415 CTOs in the right coronary artery and 85% in the circumflex system.
The CTO group as a whole had significantly greater impairment of left ventricular function. Thirty-seven percent of them had an ejection fraction of 30%-50%, compared with 22% of the non-CTO patients. The 10% prevalence of an left ventricular ejection fraction (LVEF) below 30% in the CTO group was twice that of the non-CTO group. The CTO group was also significantly more likely to undergo incomplete revascularization, by a margin of 21% versus 5.7%.
Operative mortality was 3.7% overall and just 0.55% in the elective CABG patients. In a multivariate logistic regression analysis controlled for surgical urgency, incomplete revascularization, and EuroSCORE risk, operative mortality didn’t differ significantly between the CTO and non-CTO groups.
However, in the presence of CTOs, incomplete revascularization was associated with an 11.6% operative mortality, compared with a 2.8% rate in fully revascularized CTO patients.
A total of 110 patients with bypassed CTOs underwent symptom-driven follow-up coronary angiography at a median of 3.6 years after CABG. Reassuringly, CTO graft patency was noted in 95% of the LAD grafts, 92% of the right coronary artery grafts, and 79% of the circumflex grafts.
Dr. Kieser’s audience of interventional cardiologists was clearly bowled over by her results, not only the high rate of successful surgical bypass of CTOs, but also by her use of arterial grafts 98% of the time.
“This is my personal practice,” she explained. “I just believe in arterial grafting so much. They perform best in CTO arteries because of their lack of competitive flow.”
Session chair Oliver Gämperli, MD, of University Hospital Zurich, commented, “We are very concerned about patency rates, and you showed us fantastic patency rates. This is much better than what we’re used to with saphenous vein grafts. I think we need to talk to our surgeons and try to get them to do more arterial grafts of CTOs.”
It’s worth noting that in the CTO subgroup from the landmark randomized SYNTAX trial, the complete revascularization rate was only about 50% in the PCI group, compared with nearly 65% in the CABG group, he added.
Asked why a cardiac surgeon wouldn’t bypass a CTO, Dr. Kieser rattled off several technical reasons, including a vessel size of less than 1 mm, diffuse disease, extensive scar, or an inaccessible location. But that’s not the whole story, she added. She has heard surgical colleagues say, “The patient doesn’t need that artery, he’s learned to live without it.” That burns her up.
“Patients need every artery in the heart, and the one with a CTO is the best one for an arterial graft because it almost cannot fail, especially to the left anterior descending artery. I think we have to change the mentality of the surgeons to ‘If it can be done, it should be done,’ ” Dr. Kieser said.
She reported having no financial conflicts of interest regarding her study.
AT EUROPCR
Key clinical point:
Major finding: Chronic total occlusions were present in 47% of 1,333 consecutive CABG patients, and 88% of the CTOs were successfully bypassed using arterial conduits.
Data source: A retrospective observational study of 1,333 consecutive CABG patients, 47% of whom had one or more chronic total occlusions.
Disclosures: The study presenter reported having no financial conflicts.
Evolute transcatheter valve, now FDA approved for intermediate-risk patients, impresses in real-world practice
PARIS – The Evolut R transcatheter aortic valve demonstrated excellent 30-day results in a real-world, mixed surgical risk population in the large Evolut R FORWARD study.
In this 1,038-patient observational study conducted at 53 sites in 20 countries, the Evolut R valve showed excellent forward hemodynamics and low 30-day rates of all-cause mortality and stroke that were unaffected by utilization of the device’s repositioning feature, Eberhard Grube, MD, reported at the annual congress of the European Association of Percutaneous Cardiovascular Interventions.
The importance of the FORWARD study, Dr. Grube observed, is that it illustrates the clinical outcomes obtained in a large population drawn from routine clinical practice. Unlike in a randomized trial such as SURTAVI, the participating sites in the Evolut R Forward study weren’t all high-volume enrollment centers, and operators had widely varying degrees of experience with the valve.
Also, SURTAVI utilized the first generation of the self-expanding CoreValve, which lacked the repositioning feature introduced in the second-generation Evolut R. The FORWARD study is the first rigorous evaluation of Evolut R with centrally adjudicated outcomes.
The mean Society of Thoracic Surgeons predicted risk of mortality score in participants was 5.5%, and 47% had a low-risk score of less than 4%. However, the patients had a mean age of 82 years, one-third were deemed frail, 30% had diabetes, and 26% had chronic lung disease.
The primary study endpoint was 30-day all-cause mortality. The rate was 1.9%, compared with a predicted 5.5% rate based on STS score, for an impressive observed-to-expected ratio of 0.35.
Hemodynamically, the effective orifice area improved from 0.8 cm2 at baseline to 1.9 cm2 at 30 days, while the mean aortic valve gradient plunged from 41.7 to 8.5 mm Hg.
At baseline only 1.5% of patients were New York Heart Association functional class I and 26.5% were class II. At 30 days, 44.7% were class I and 43.4% were class II. The prevalence of NYHA class III status decreased from 63.8% to 11.3%.
There was no or only trace paravalvular leak at discharge in 67.2% of patients as adjudicated in a core laboratory, mild leak in 30.9%, moderate in 1.9%, and severe leak in just 0.1%.
The 30-day total stroke rate was 2.8%, including a 1.8% rate of disabling stroke. Major vascular complications occurred in 6.5% of patients, valve embolization in 0.7%, and life-threatening or disabling bleeding in 3.3%. There were no cases of coronary obstruction or annular rupture.
New pacemaker implantation was required within 30 days in 17.5% of patients. Three-quarters of the pacemakers were placed because of third-degree atrioventricular block.
The new valve ended up in proper anatomic position in 98.9% of patients.
The repositioning feature was utilized in 26% of participants. It had no impact on the rate of pacemaker implantation, mortality, stroke, or other safety endpoints.
“I think the ability to reposition this valve, which is a safety feature, is an important feature, particularly for centers that don’t have so much experience. If the valve is considered to be too high or too low, or you see, for example, a higher degree of paravalvular leak, you have the chance to correct that by using this feature. So it’s an important feature for the operator. It helps to get an optimal result. And the most important thing is there was no price in terms of safety that we paid for repositioning,” said Dr. Grube, professor of cardiology and head of the Center for Innovative Intervention in Cardiology at the University of Bonn in Siegberg, Germany.
Session cochair Alain Cribier, MD, famed for having performed the world’s first TAVR procedure, pronounced the FORWARD results “very impressive.”
“Less than 2% mortality, around a 2% disabling stroke rate, and the data on paravalvular leak are excellent as well. It’s very nice to see that what was a limited data set earlier, with a smaller number of patients, has now been replicated in 1,000 patients. So I think now we can confidently talk about the clinical outcomes – and they are excellent,” declared Dr. Cribier, professor of medicine at the University of Rouen (France) and chief of cardiology at Charles Nicolle Hospital.
The FORWARD study was sponsored by Medtronic. Dr. Grube reported serving as a consultant to that company as well as to Boston Scientific, Abbott, and Millipede Medical.
PARIS – The Evolut R transcatheter aortic valve demonstrated excellent 30-day results in a real-world, mixed surgical risk population in the large Evolut R FORWARD study.
In this 1,038-patient observational study conducted at 53 sites in 20 countries, the Evolut R valve showed excellent forward hemodynamics and low 30-day rates of all-cause mortality and stroke that were unaffected by utilization of the device’s repositioning feature, Eberhard Grube, MD, reported at the annual congress of the European Association of Percutaneous Cardiovascular Interventions.
The importance of the FORWARD study, Dr. Grube observed, is that it illustrates the clinical outcomes obtained in a large population drawn from routine clinical practice. Unlike in a randomized trial such as SURTAVI, the participating sites in the Evolut R Forward study weren’t all high-volume enrollment centers, and operators had widely varying degrees of experience with the valve.
Also, SURTAVI utilized the first generation of the self-expanding CoreValve, which lacked the repositioning feature introduced in the second-generation Evolut R. The FORWARD study is the first rigorous evaluation of Evolut R with centrally adjudicated outcomes.
The mean Society of Thoracic Surgeons predicted risk of mortality score in participants was 5.5%, and 47% had a low-risk score of less than 4%. However, the patients had a mean age of 82 years, one-third were deemed frail, 30% had diabetes, and 26% had chronic lung disease.
The primary study endpoint was 30-day all-cause mortality. The rate was 1.9%, compared with a predicted 5.5% rate based on STS score, for an impressive observed-to-expected ratio of 0.35.
Hemodynamically, the effective orifice area improved from 0.8 cm2 at baseline to 1.9 cm2 at 30 days, while the mean aortic valve gradient plunged from 41.7 to 8.5 mm Hg.
At baseline only 1.5% of patients were New York Heart Association functional class I and 26.5% were class II. At 30 days, 44.7% were class I and 43.4% were class II. The prevalence of NYHA class III status decreased from 63.8% to 11.3%.
There was no or only trace paravalvular leak at discharge in 67.2% of patients as adjudicated in a core laboratory, mild leak in 30.9%, moderate in 1.9%, and severe leak in just 0.1%.
The 30-day total stroke rate was 2.8%, including a 1.8% rate of disabling stroke. Major vascular complications occurred in 6.5% of patients, valve embolization in 0.7%, and life-threatening or disabling bleeding in 3.3%. There were no cases of coronary obstruction or annular rupture.
New pacemaker implantation was required within 30 days in 17.5% of patients. Three-quarters of the pacemakers were placed because of third-degree atrioventricular block.
The new valve ended up in proper anatomic position in 98.9% of patients.
The repositioning feature was utilized in 26% of participants. It had no impact on the rate of pacemaker implantation, mortality, stroke, or other safety endpoints.
“I think the ability to reposition this valve, which is a safety feature, is an important feature, particularly for centers that don’t have so much experience. If the valve is considered to be too high or too low, or you see, for example, a higher degree of paravalvular leak, you have the chance to correct that by using this feature. So it’s an important feature for the operator. It helps to get an optimal result. And the most important thing is there was no price in terms of safety that we paid for repositioning,” said Dr. Grube, professor of cardiology and head of the Center for Innovative Intervention in Cardiology at the University of Bonn in Siegberg, Germany.
Session cochair Alain Cribier, MD, famed for having performed the world’s first TAVR procedure, pronounced the FORWARD results “very impressive.”
“Less than 2% mortality, around a 2% disabling stroke rate, and the data on paravalvular leak are excellent as well. It’s very nice to see that what was a limited data set earlier, with a smaller number of patients, has now been replicated in 1,000 patients. So I think now we can confidently talk about the clinical outcomes – and they are excellent,” declared Dr. Cribier, professor of medicine at the University of Rouen (France) and chief of cardiology at Charles Nicolle Hospital.
The FORWARD study was sponsored by Medtronic. Dr. Grube reported serving as a consultant to that company as well as to Boston Scientific, Abbott, and Millipede Medical.
PARIS – The Evolut R transcatheter aortic valve demonstrated excellent 30-day results in a real-world, mixed surgical risk population in the large Evolut R FORWARD study.
In this 1,038-patient observational study conducted at 53 sites in 20 countries, the Evolut R valve showed excellent forward hemodynamics and low 30-day rates of all-cause mortality and stroke that were unaffected by utilization of the device’s repositioning feature, Eberhard Grube, MD, reported at the annual congress of the European Association of Percutaneous Cardiovascular Interventions.
The importance of the FORWARD study, Dr. Grube observed, is that it illustrates the clinical outcomes obtained in a large population drawn from routine clinical practice. Unlike in a randomized trial such as SURTAVI, the participating sites in the Evolut R Forward study weren’t all high-volume enrollment centers, and operators had widely varying degrees of experience with the valve.
Also, SURTAVI utilized the first generation of the self-expanding CoreValve, which lacked the repositioning feature introduced in the second-generation Evolut R. The FORWARD study is the first rigorous evaluation of Evolut R with centrally adjudicated outcomes.
The mean Society of Thoracic Surgeons predicted risk of mortality score in participants was 5.5%, and 47% had a low-risk score of less than 4%. However, the patients had a mean age of 82 years, one-third were deemed frail, 30% had diabetes, and 26% had chronic lung disease.
The primary study endpoint was 30-day all-cause mortality. The rate was 1.9%, compared with a predicted 5.5% rate based on STS score, for an impressive observed-to-expected ratio of 0.35.
Hemodynamically, the effective orifice area improved from 0.8 cm2 at baseline to 1.9 cm2 at 30 days, while the mean aortic valve gradient plunged from 41.7 to 8.5 mm Hg.
At baseline only 1.5% of patients were New York Heart Association functional class I and 26.5% were class II. At 30 days, 44.7% were class I and 43.4% were class II. The prevalence of NYHA class III status decreased from 63.8% to 11.3%.
There was no or only trace paravalvular leak at discharge in 67.2% of patients as adjudicated in a core laboratory, mild leak in 30.9%, moderate in 1.9%, and severe leak in just 0.1%.
The 30-day total stroke rate was 2.8%, including a 1.8% rate of disabling stroke. Major vascular complications occurred in 6.5% of patients, valve embolization in 0.7%, and life-threatening or disabling bleeding in 3.3%. There were no cases of coronary obstruction or annular rupture.
New pacemaker implantation was required within 30 days in 17.5% of patients. Three-quarters of the pacemakers were placed because of third-degree atrioventricular block.
The new valve ended up in proper anatomic position in 98.9% of patients.
The repositioning feature was utilized in 26% of participants. It had no impact on the rate of pacemaker implantation, mortality, stroke, or other safety endpoints.
“I think the ability to reposition this valve, which is a safety feature, is an important feature, particularly for centers that don’t have so much experience. If the valve is considered to be too high or too low, or you see, for example, a higher degree of paravalvular leak, you have the chance to correct that by using this feature. So it’s an important feature for the operator. It helps to get an optimal result. And the most important thing is there was no price in terms of safety that we paid for repositioning,” said Dr. Grube, professor of cardiology and head of the Center for Innovative Intervention in Cardiology at the University of Bonn in Siegberg, Germany.
Session cochair Alain Cribier, MD, famed for having performed the world’s first TAVR procedure, pronounced the FORWARD results “very impressive.”
“Less than 2% mortality, around a 2% disabling stroke rate, and the data on paravalvular leak are excellent as well. It’s very nice to see that what was a limited data set earlier, with a smaller number of patients, has now been replicated in 1,000 patients. So I think now we can confidently talk about the clinical outcomes – and they are excellent,” declared Dr. Cribier, professor of medicine at the University of Rouen (France) and chief of cardiology at Charles Nicolle Hospital.
The FORWARD study was sponsored by Medtronic. Dr. Grube reported serving as a consultant to that company as well as to Boston Scientific, Abbott, and Millipede Medical.
AT EUROPCR
Key clinical point:
Major finding: Thirty-day all-cause mortality was 1.9% with a 2.8% stroke rate in a large, real-world study of TAVR with the repositionable self-expanding Evolut R transcatheter aortic valve.
Data source: The Evolut R FORWARD study of 1,038 recipients of the Evolut R transcatheter aortic valve at 53 sites in 20 countries.
Disclosures: The FORWARD study was sponsored by Medtronic. The presenter reported serving as a consultant to that company as well as to Boston Scientific, Abbott, and Millipede Medical.
Watchman device for AF patients ineligible for oral anticoagulation gains support from 1-year registry outcomes
PARIS – Patients with atrial fibrillation at high stroke risk and a contraindication for oral anticoagulation experienced an 83% reduction in their risk of ischemic stroke and transient ischemic attack during their first year after receiving the Watchman left atrial appendage closure device backed by limited-duration dual-antiplatelet therapy, according to a report from the EWOLUTION registry.
Of 605 participants in the European registry who went on dual-antiplatelet treatment (DAPT) in conjunction with receiving the Watchman device, 39% discontinued DAPT within 3 months and 72% were off DAPT by 6 months. Yet the 1-year rate of ischemic stroke or TIA in the EWOLUTION group was just 1.8%, an 83% relative risk reduction compared with the expected 10.5% rate based on the participants’ mean CHA2DS2-VASc score of 4.6 in the absence of oral anticoagulation, Martin W. Bergmann, MD, said at the annual congress of the European Association of Percutaneous Cardiovascular Interventions.
“Obviously this isn’t a randomized trial. This is just reassuring data that we are going in the right direction in terms of efficacy,” said Dr. Bergmann of Cardiologicum Hamburg, a large German group practice.
However, the ESC guidelines were largely based on randomized trials of the Watchman versus oral anticoagulation, including PREVAIL and PROTECT-AF, showing noninferiority. The safety and efficacy of the device in warfarin-ineligible patients was less well studied at the time the guidelines were formulated. And in fact, such patients are excluded from the Food and Drug Administration’s approved indication, which is specifically for patients judged “suitable for warfarin.”
“This gap is now filled by EWOLUTION. This is a registry that’s as good as it gets. We have all the things in place that you need these days to be able to rely on the outcome data,” according to the cardiologist.
EWOLUTION is a prospective, multicenter, all-comers registry. The EWOLUTION population of AF patients on DAPT was high risk: 89% had a CHA2DS2-VASc score of 3 or more, 31% were at least 80 years old, the mean HAS-BLED score was 2.4, and oral anticoagulation was contraindicated in 84% of participants.
Eighty-seven percent of subjects underwent follow-up transesophageal echocardiography. The imaging study showed the Watchman effectively sealed the left atrial appendage in 99.2% of patients as defined by no leak greater than 5 mm. The echo exam also showed the presence of thrombus on the device at follow-up in 4% of patients, although only 1 of the 22 patients with device thrombus experienced a stroke.
“We can conclude two things from this which are in line with earlier studies. First, the rate of thrombus on the device is equal to the rate reported in the randomized controlled trials, which was also 4%, even if the patients were on warfarin for the first 45 days. And second, these thrombi are not related to stroke,” Dr. Bergmann said.
At the 1-year mark, 71% of patients had switched to a single antiplatelet agent, while 17% remained on DAPT, mainly because of comorbid coronary disease for which DAPT is indicated. Seven percent of patients were on no antithrombotic medications. The remaining 5% were transiently on warfarin or a novel oral anticoagulant.
The 1-year cumulative rate of ischemic stroke or TIA was 1.8%, with no instances of systemic embolism. Of note, there were no hemorrhagic strokes. And of the 11 cases of ischemic stroke, none were fatal and only one was disabling.
“This is a sign that comes also from the PREVAIL trial, that if you have a stroke while on left atrial appendage–closure therapy, most of the time it’s not disabling. It’s much less severe on the modified Rankin Scale than if you’re on oral anticoagulation,” said Dr. Bergmann.
The 1.4% rate of ischemic stroke at 1 year in Watchman recipients represents an 81% reduction in risk compared with the expected 7.5% rate in patients with similar CHA2DS2-VASc scores not on oral anticoagulation. This level of stroke risk reduction is similar to that seen in the pivotal ARISTOTLE trial of apixaban (Eliquis) in a high-risk AF population (Lancet. 2012 Nov 17;380[9855]:1749-58).
Major bleeding occurred in 2.5% of patients. The rate of fatal bleeding was 0.5%. To put that in perspective, the 2.5% major bleeding rate was 52% lower than would be expected based upon similar HAS-BLED scores in patients on warfarin. Still, 2.5% is unacceptably high.
“The major serious adverse event is not pericardial effusion or late device embolization, it’s major bleeding occurring during the time the patient is on DAPT, mostly within the first 3 months. So I think we have to do something about this,” he said.
One possibility worthy of formal study is 3 months of periprocedural NOAC monotherapy. “Maybe even low-dose therapy, like 75 mg of dabigatran [Pradaxa] twice daily. We have an antidote that works nicely [idarucizumab, Praxbind] so I think maybe this is the way to go,” Dr. Bergmann observed.
The ongoing EWOLUTION registry is sponsored by Boston Scientific. Dr. Bergmann is a consultant to that company as well as Bayer AG, Daiichi Sankyo, Eli Lilly, and St. Jude Medical.
PARIS – Patients with atrial fibrillation at high stroke risk and a contraindication for oral anticoagulation experienced an 83% reduction in their risk of ischemic stroke and transient ischemic attack during their first year after receiving the Watchman left atrial appendage closure device backed by limited-duration dual-antiplatelet therapy, according to a report from the EWOLUTION registry.
Of 605 participants in the European registry who went on dual-antiplatelet treatment (DAPT) in conjunction with receiving the Watchman device, 39% discontinued DAPT within 3 months and 72% were off DAPT by 6 months. Yet the 1-year rate of ischemic stroke or TIA in the EWOLUTION group was just 1.8%, an 83% relative risk reduction compared with the expected 10.5% rate based on the participants’ mean CHA2DS2-VASc score of 4.6 in the absence of oral anticoagulation, Martin W. Bergmann, MD, said at the annual congress of the European Association of Percutaneous Cardiovascular Interventions.
“Obviously this isn’t a randomized trial. This is just reassuring data that we are going in the right direction in terms of efficacy,” said Dr. Bergmann of Cardiologicum Hamburg, a large German group practice.
However, the ESC guidelines were largely based on randomized trials of the Watchman versus oral anticoagulation, including PREVAIL and PROTECT-AF, showing noninferiority. The safety and efficacy of the device in warfarin-ineligible patients was less well studied at the time the guidelines were formulated. And in fact, such patients are excluded from the Food and Drug Administration’s approved indication, which is specifically for patients judged “suitable for warfarin.”
“This gap is now filled by EWOLUTION. This is a registry that’s as good as it gets. We have all the things in place that you need these days to be able to rely on the outcome data,” according to the cardiologist.
EWOLUTION is a prospective, multicenter, all-comers registry. The EWOLUTION population of AF patients on DAPT was high risk: 89% had a CHA2DS2-VASc score of 3 or more, 31% were at least 80 years old, the mean HAS-BLED score was 2.4, and oral anticoagulation was contraindicated in 84% of participants.
Eighty-seven percent of subjects underwent follow-up transesophageal echocardiography. The imaging study showed the Watchman effectively sealed the left atrial appendage in 99.2% of patients as defined by no leak greater than 5 mm. The echo exam also showed the presence of thrombus on the device at follow-up in 4% of patients, although only 1 of the 22 patients with device thrombus experienced a stroke.
“We can conclude two things from this which are in line with earlier studies. First, the rate of thrombus on the device is equal to the rate reported in the randomized controlled trials, which was also 4%, even if the patients were on warfarin for the first 45 days. And second, these thrombi are not related to stroke,” Dr. Bergmann said.
At the 1-year mark, 71% of patients had switched to a single antiplatelet agent, while 17% remained on DAPT, mainly because of comorbid coronary disease for which DAPT is indicated. Seven percent of patients were on no antithrombotic medications. The remaining 5% were transiently on warfarin or a novel oral anticoagulant.
The 1-year cumulative rate of ischemic stroke or TIA was 1.8%, with no instances of systemic embolism. Of note, there were no hemorrhagic strokes. And of the 11 cases of ischemic stroke, none were fatal and only one was disabling.
“This is a sign that comes also from the PREVAIL trial, that if you have a stroke while on left atrial appendage–closure therapy, most of the time it’s not disabling. It’s much less severe on the modified Rankin Scale than if you’re on oral anticoagulation,” said Dr. Bergmann.
The 1.4% rate of ischemic stroke at 1 year in Watchman recipients represents an 81% reduction in risk compared with the expected 7.5% rate in patients with similar CHA2DS2-VASc scores not on oral anticoagulation. This level of stroke risk reduction is similar to that seen in the pivotal ARISTOTLE trial of apixaban (Eliquis) in a high-risk AF population (Lancet. 2012 Nov 17;380[9855]:1749-58).
Major bleeding occurred in 2.5% of patients. The rate of fatal bleeding was 0.5%. To put that in perspective, the 2.5% major bleeding rate was 52% lower than would be expected based upon similar HAS-BLED scores in patients on warfarin. Still, 2.5% is unacceptably high.
“The major serious adverse event is not pericardial effusion or late device embolization, it’s major bleeding occurring during the time the patient is on DAPT, mostly within the first 3 months. So I think we have to do something about this,” he said.
One possibility worthy of formal study is 3 months of periprocedural NOAC monotherapy. “Maybe even low-dose therapy, like 75 mg of dabigatran [Pradaxa] twice daily. We have an antidote that works nicely [idarucizumab, Praxbind] so I think maybe this is the way to go,” Dr. Bergmann observed.
The ongoing EWOLUTION registry is sponsored by Boston Scientific. Dr. Bergmann is a consultant to that company as well as Bayer AG, Daiichi Sankyo, Eli Lilly, and St. Jude Medical.
PARIS – Patients with atrial fibrillation at high stroke risk and a contraindication for oral anticoagulation experienced an 83% reduction in their risk of ischemic stroke and transient ischemic attack during their first year after receiving the Watchman left atrial appendage closure device backed by limited-duration dual-antiplatelet therapy, according to a report from the EWOLUTION registry.
Of 605 participants in the European registry who went on dual-antiplatelet treatment (DAPT) in conjunction with receiving the Watchman device, 39% discontinued DAPT within 3 months and 72% were off DAPT by 6 months. Yet the 1-year rate of ischemic stroke or TIA in the EWOLUTION group was just 1.8%, an 83% relative risk reduction compared with the expected 10.5% rate based on the participants’ mean CHA2DS2-VASc score of 4.6 in the absence of oral anticoagulation, Martin W. Bergmann, MD, said at the annual congress of the European Association of Percutaneous Cardiovascular Interventions.
“Obviously this isn’t a randomized trial. This is just reassuring data that we are going in the right direction in terms of efficacy,” said Dr. Bergmann of Cardiologicum Hamburg, a large German group practice.
However, the ESC guidelines were largely based on randomized trials of the Watchman versus oral anticoagulation, including PREVAIL and PROTECT-AF, showing noninferiority. The safety and efficacy of the device in warfarin-ineligible patients was less well studied at the time the guidelines were formulated. And in fact, such patients are excluded from the Food and Drug Administration’s approved indication, which is specifically for patients judged “suitable for warfarin.”
“This gap is now filled by EWOLUTION. This is a registry that’s as good as it gets. We have all the things in place that you need these days to be able to rely on the outcome data,” according to the cardiologist.
EWOLUTION is a prospective, multicenter, all-comers registry. The EWOLUTION population of AF patients on DAPT was high risk: 89% had a CHA2DS2-VASc score of 3 or more, 31% were at least 80 years old, the mean HAS-BLED score was 2.4, and oral anticoagulation was contraindicated in 84% of participants.
Eighty-seven percent of subjects underwent follow-up transesophageal echocardiography. The imaging study showed the Watchman effectively sealed the left atrial appendage in 99.2% of patients as defined by no leak greater than 5 mm. The echo exam also showed the presence of thrombus on the device at follow-up in 4% of patients, although only 1 of the 22 patients with device thrombus experienced a stroke.
“We can conclude two things from this which are in line with earlier studies. First, the rate of thrombus on the device is equal to the rate reported in the randomized controlled trials, which was also 4%, even if the patients were on warfarin for the first 45 days. And second, these thrombi are not related to stroke,” Dr. Bergmann said.
At the 1-year mark, 71% of patients had switched to a single antiplatelet agent, while 17% remained on DAPT, mainly because of comorbid coronary disease for which DAPT is indicated. Seven percent of patients were on no antithrombotic medications. The remaining 5% were transiently on warfarin or a novel oral anticoagulant.
The 1-year cumulative rate of ischemic stroke or TIA was 1.8%, with no instances of systemic embolism. Of note, there were no hemorrhagic strokes. And of the 11 cases of ischemic stroke, none were fatal and only one was disabling.
“This is a sign that comes also from the PREVAIL trial, that if you have a stroke while on left atrial appendage–closure therapy, most of the time it’s not disabling. It’s much less severe on the modified Rankin Scale than if you’re on oral anticoagulation,” said Dr. Bergmann.
The 1.4% rate of ischemic stroke at 1 year in Watchman recipients represents an 81% reduction in risk compared with the expected 7.5% rate in patients with similar CHA2DS2-VASc scores not on oral anticoagulation. This level of stroke risk reduction is similar to that seen in the pivotal ARISTOTLE trial of apixaban (Eliquis) in a high-risk AF population (Lancet. 2012 Nov 17;380[9855]:1749-58).
Major bleeding occurred in 2.5% of patients. The rate of fatal bleeding was 0.5%. To put that in perspective, the 2.5% major bleeding rate was 52% lower than would be expected based upon similar HAS-BLED scores in patients on warfarin. Still, 2.5% is unacceptably high.
“The major serious adverse event is not pericardial effusion or late device embolization, it’s major bleeding occurring during the time the patient is on DAPT, mostly within the first 3 months. So I think we have to do something about this,” he said.
One possibility worthy of formal study is 3 months of periprocedural NOAC monotherapy. “Maybe even low-dose therapy, like 75 mg of dabigatran [Pradaxa] twice daily. We have an antidote that works nicely [idarucizumab, Praxbind] so I think maybe this is the way to go,” Dr. Bergmann observed.
The ongoing EWOLUTION registry is sponsored by Boston Scientific. Dr. Bergmann is a consultant to that company as well as Bayer AG, Daiichi Sankyo, Eli Lilly, and St. Jude Medical.
AT EUROPCR
Key clinical point:
Major finding: Patients with AF at high stroke risk and ineligible for oral anticoagulation experienced an 81% reduction in their expected risk of ischemic stroke during their first year of LAA closure with the Watchman device backed by limited-duration dual-antiplatelet therapy
Data source: A prospective, multicenter, single-arm registry that includes 605 patients with AF who underwent left atrial appendage closure with the WATCHMAN device supported by limited-duration dual-antiplatelet therapy.
Disclosures: The ongoing EWOLUTION registry is sponsored by Boston Scientific. The presenter is a consultant to that company and several others.















