Heparin is a naturally occurring anticoagulant and is commonly used to treat or prevent venous thrombosis or the extension of thrombosis.1 Heparin is composed of 15-kDa chains of complex polysaccharides with repeating pentasaccharide sequences. These high-affinity pentasaccharide subunits bind and activate antithrombin III, which exerts its dominant anticoagulant effects through the inhibition of factor Xa.2
Adverse effects of heparin administration include bleeding, injection-site pain, and thrombocytopenia. Heparin-induced thrombocytopenia (HIT) is a serious side effect wherein antibodies are formed against platelet antigens and predispose the patient to venous and arterial thrombosis.3 Dermatologic adverse effects of heparin range from commonly reported injection-site eruptions to the more rarely described distant or generalized cutaneous reactions.4
Bullous hemorrhagic dermatosis is a poorly understood idiosyncratic drug reaction characterized by tense, blood-filled blisters that arise following the administration of subcutaneous low-molecular-weight heparin or intravenous unfractionated heparin (UFH). First reported in 2006 by Perrinaud et al,5 only a few case reports describing this phenomenon exist in the literature.6-8 We report a unique case of hemorrhagic bullae limited to the oral mucosa.
Case Report
An 84-year-old man was admitted to the cardiology service with severe substernal chest pain. An electrocardiogram did not show any ST-segment elevations; however, he had elevated troponin T levels. He had a medical history of coronary artery disease complicated by myocardial infarction (MI), as well as ischemic cardiomyopathy, hypertension, hyperlipidemia, ischemic stroke, and pulmonary embolism for which he was on long-term anticoagulation for years with warfarin, aspirin, and clopidogrel. The patient was diagnosed with a non–ST-segment elevation MI. Accordingly, the patient’s warfarin was discontinued, and he was administered a bolus and continuous infusion of UFH. He also was continued on aspirin and clopidogrel. Within 6 hours of initiation of UFH, the patient noted multiple discrete swollen lesions in the mouth. Dermatology consultation and biopsy of the lesions were deferred due to acute management of the patient’s MI.
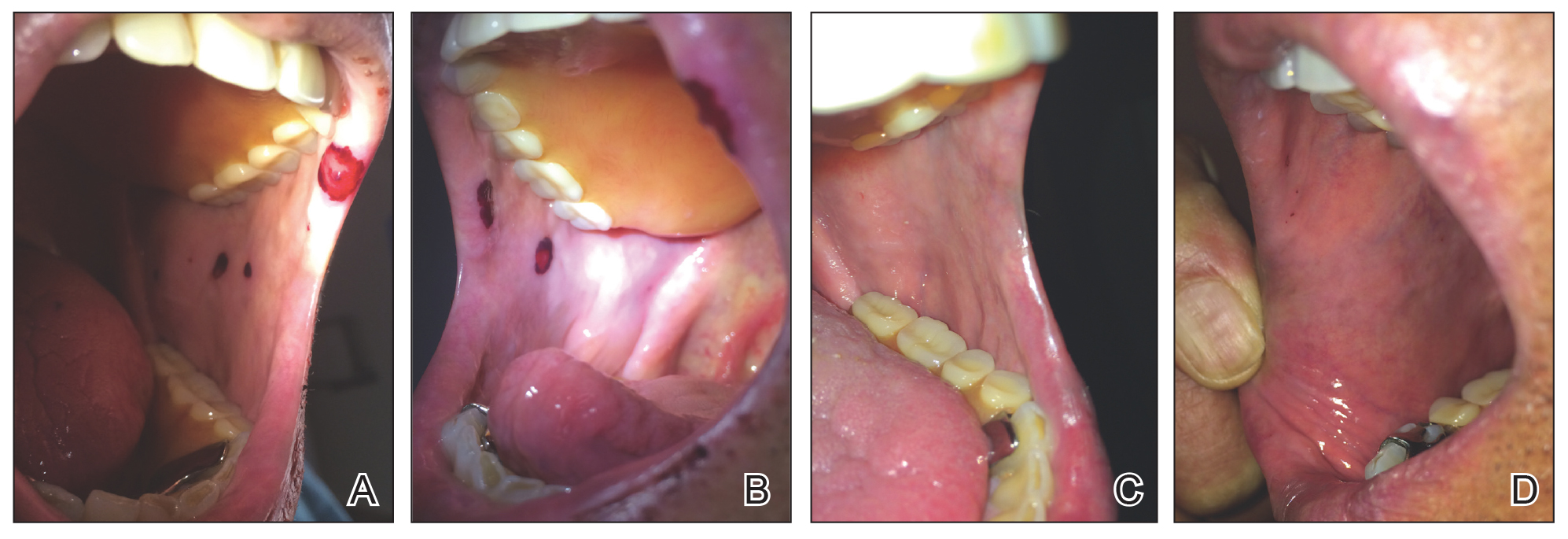
Physical examination revealed a moist oral mucosa with 7 slightly raised, hemorrhagic bullae ranging from 2 to 7 mm in diameter (Figure, A and B). One oral lesion was tense and had become denuded prior to evaluation. Laboratory testing included a normal platelet count (160,000/µL), a nearly therapeutic international normalized ratio (1.9), and a partial thromboplastin time that was initially normal (27 seconds) prior to admission and development of the oral lesions but found to be elevated (176 seconds) after admission and initial UFH bolus.
Upon further questioning, the patient revealed a history of similar oral lesions 1 year prior, following exposure to subcutaneous enoxaparin. At that time, formal evaluation by dermatology was deferred due to the rapid resolution of the blisters. Despite these new oral lesions, the patient was continued on a heparin drip for the next 48 hours because of the mortality benefit of heparin in non–ST-segment elevation MI. The patient was discharged from the hospital on a regimen of aspirin, warfarin, and clopidogrel. At 2-week follow-up, the oral lesions had resolved (Figure, C and D).