User login
Is chest radiography routinely needed after thoracentesis?
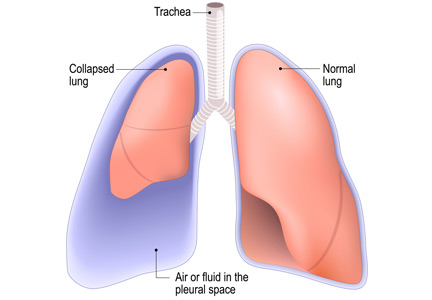
No. After thoracentesis, chest radiography or another lung imaging study should be done only if pneumothorax is suspected, if thoracentesis requires more than 1 attempt, if the patient is on mechanical ventilation or has pre-existing lung disease, or if a large volume (> 1,500 mL) of fluid is removed. Radiography is also usually not necessary after diagnostic thoracentesis in a patient breathing spontaneously. In most cases, pneumothorax found incidentally after thoracentesis does not require decompression and can be managed supportively.
WHAT ARE THE RISKS OF THORACENTESIS?
Thoracentesis is a minimally invasive procedure usually performed at the bedside that involves insertion of a needle into the pleural cavity for drainage of fluid.1 Diagnostic thoracentesis should be done in most cases of a new pleural effusion unless the effusion is small and with a clear diagnosis, or in cases of typical heart failure.
Therapeutic thoracentesis, often called large-volume thoracentesis, aims to improve symptoms such as dyspnea attributed to the pleural effusion by removing at least 1 L of pleural fluid. The presence of active respiratory symptoms and suspicion of infected pleural effusion should lead to thoracentesis as soon as possible.
Complications of thoracentesis may be benign, such as pain and anxiety associated with the procedure and external bleeding at the site of needle insertion. Pneumothorax is the most common serious procedural complication and the principal reason to order postprocedural chest radiography.1 Less common complications include hemothorax, re-expansion pulmonary edema, infection, subdiaphragmatic organ puncture, and procedure-related death. Bleeding complications and hemothorax are rare even in patients with underlying coagulopathy.2
Point-of-care pleural ultrasonography is now considered the standard of care to guide optimal needle location for the procedure and to exclude other conditions that can mimic pleural effusion on chest radiography, such as lung consolidation and atelectasis.3 High proficiency in the use of preprocedural point-of-care ultrasonography reduces the rate of procedural complications, though it does not eliminate the risk entirely.3,4
Factors associated with higher rates of complications include lack of operator proficiency, poor understanding of the anatomy, poor patient positioning, poor patient cooperation with the procedure, lack of availability of bedside ultrasonography, and drainage of more than 1,500 mL of fluid. Addressing these factors has been shown to decrease the risk of pneumothorax and infection.1–5
HOW OFTEN DOES PNEUMOTHORAX OCCUR AFTER THORACENTESIS?
Several early studies have examined the incidence of pneumothorax after thoracentesis. Lack of ultrasonography use likely explains a higher incidence of complications in early studies: rates of pneumothorax after thoracentesis without ultrasonographic guidance ranged from 5.2% to 26%.6,7
Gervais et al8 analyzed thoracentesis with ultrasonographic guidance in 434 patients, 92 of whom were intubated, and reported that pneumothorax occurred in 10 patients, of whom 6 were intubated. Two of the intubated patients required chest tubes. Other studies have confirmed the low incidence of pneumothorax in patients undergoing thoracentesis, with rates such as 0.61%,1 5%,9 and 4%.10
The major predictor of postprocedural pneumothorax was the presence of symptoms such as chest pain and dyspnea. No intervention was necessary for most cases of pneumothorax in asymptomatic patients. The more widespread use of procedural ultrasonography may explain some discrepancies between the early5,6 and more recent studies.1,8–10
Several studies have demonstrated that postprocedural radiography is unnecessary unless a complication is suspected based on the patient’s symptoms or the need to demonstrate lung re-expansion.1,4,9,10 Clinical suspicion and the patient’s symptoms are the major predictors of procedure-related pneumothorax requiring treatment with a chest tube. Otherwise, incidentally discovered pneumothorax can usually be observed and managed supportively.
WHAT MECHANISMS UNDERLIE POSTPROCEDURAL PNEUMOTHORAX?
Major causes of pneumothorax in patients undergoing thoracentesis are direct puncture during needle or catheter insertion, the introduction of air through the needle or catheter into the pleural cavity, and the inability of the ipsilateral lung to fully expand after drainage of a large volume of fluid, known as pneumothorax ex vacuo.5
Pneumothorax ex vacuo may be seen in patients with medical conditions such as endobronchial obstruction, pleural scarring from long-standing pleural effusion, and lung malignancy, all of which can impair the lung’s ability to expand after removal of a large volume of pleural fluid. It is believed that transient parenchymal pleural fistulae form if the lung cannot expand, causing air leakage into the pleural cavity.5,8,9 Pleural manometry to monitor changes in pleural pressure and elastance can decrease the rates of pneumothorax ex vacuo in patients with the above risk factors.5
WHEN IS RADIOGRAPHY INDICATED AFTER THORACENTESIS?
Current literature suggests that imaging to evaluate for postprocedural complications should be done if there is suspicion of a complication, if thoracentesis required multiple attempts, if the procedure caused aspiration of air, if the patient has advanced lung disease, if the patient is scheduled to undergo thoracic radiation, if the patient is on mechanical ventilation, and after therapeutic thoracentesis if a large volume of fluid is removed.1–10 Routine chest radiography after thoracentesis is not supported in the literature in the absence of these risk factors.
Some practitioners order chest imaging after therapeutic thoracentesis to assess for residual pleural fluid and for visualization of other abnormalities previously hidden by pleural effusion, rather than simply to exclude postprocedural pneumothorax. Alternatively, postprocedural bedside pleural ultrasonography with recording of images can be done to assess for complications and residual pleural fluid volume without exposing the patient to radiation.11
Needle decompression and chest tube insertion should be considered in patients with tension pneumothorax, large pneumothorax (distance from the chest wall to the visceral pleural line of at least 2 cm), mechanical ventilation, progressing pneumothorax, and symptoms.
KEY POINTS
- Pneumothorax is a rare complication of thoracentesis when performed by a skilled operator using ultrasonographic guidance.
- Mechanisms behind the occurrence of pneumothorax are direct lung puncture, introduction of air into the pleural cavity, and pneumothorax ex vacuo.
- In asymptomatic patients, pneumothorax after thoracentesis rarely requires intervention beyond supportive care and close observation.
- Factors such as multiple thoracentesis attempts, symptoms, clinical suspicion, air aspiration during thoracentesis, presence of previous lung disease, and removal of a large volume of fluid may require postprocedural lung imaging (eg, bedside ultrasonography, radiography).
- Ault MJ, Rosen BT, Scher J, Feinglass J, Barsuk JH. Thoracentesis outcomes: a 12-year experience. Thorax 2015; 70(2):127–132. doi:10.1136/thoraxjnl-2014-206114
- Hibbert RM, Atwell TD, Lekah A, et al. Safety of ultrasound-guided thoracentesis in patients with abnormal preprocedural coagulation parameters. Chest 2013; 144(2):456–463. doi:10.1378/chest.12-2374
- Barnes TW, Morgenthaler TI, Olson EJ, Hesley GK, Decker PA, Ryu JH. Sonographically guided thoracentesis and rate of pneumothorax. J Clin Ultrasound 2005; 33(9):442–446. doi:10.1002/jcu.20163
- Gordon CE, Feller-Kopman D, Balk EM, Smetana GW. Pneumothorax following thoracentesis: a systematic review and meta-analysis. Arch Intern Med 2010; 170(4):332–339. doi:10.1001/archinternmed.2009.548
- Heidecker J, Huggins JT, Sahn SA, Doelken P. Pathophysiology of pneumothorax following ultrasound-guided thoracentesis. Chest 2006; 130(4):1173–1184. doi:10.1016/S0012-3692(15)51155-0
- Brandstetter RD, Karetzky M, Rastogi R, Lolis JD. Pneumothorax after thoracentesis in chronic obstructive pulmonary disease. Heart Lung 1994; 23(1):67–70. pmid:8150647
- Doyle JJ, Hnatiuk OW, Torrington KG, Slade AR, Howard RS. Necessity of routine chest roentgenography after thoracentesis. Ann Intern Med 1996; 124(9):816–820. pmid:8610950
- Gervais DA, Petersein A, Lee MJ, Hahn PF, Saini S, Mueller PR. US-guided thoracentesis: requirement for postprocedure chest radiography in patients who receive mechanical ventilation versus patients who breathe spontaneously. Radiology 1997; 204(2):503–506. doi:10.1148/radiology.204.2.9240544
- Capizzi SA, Prakash UB. Chest roentgenography after outpatient thoracentesis. Mayo Clin Proc 1998; 73(10):948–950. doi:10.4065/73.10.948
- Alemán C, Alegre J, Armadans L, et al. The value of chest roentgenography in the diagnosis of pneumothorax after thoracentesis. Am J Med 1999; 107(4):340–343. pmid:10527035
- Lichtenstein D. Lung ultrasound in the critically ill. Curr Opin Crit Care 2014; 20(3):315–322. doi:10.1097/MCC.0000000000000096
No. After thoracentesis, chest radiography or another lung imaging study should be done only if pneumothorax is suspected, if thoracentesis requires more than 1 attempt, if the patient is on mechanical ventilation or has pre-existing lung disease, or if a large volume (> 1,500 mL) of fluid is removed. Radiography is also usually not necessary after diagnostic thoracentesis in a patient breathing spontaneously. In most cases, pneumothorax found incidentally after thoracentesis does not require decompression and can be managed supportively.
WHAT ARE THE RISKS OF THORACENTESIS?
Thoracentesis is a minimally invasive procedure usually performed at the bedside that involves insertion of a needle into the pleural cavity for drainage of fluid.1 Diagnostic thoracentesis should be done in most cases of a new pleural effusion unless the effusion is small and with a clear diagnosis, or in cases of typical heart failure.
Therapeutic thoracentesis, often called large-volume thoracentesis, aims to improve symptoms such as dyspnea attributed to the pleural effusion by removing at least 1 L of pleural fluid. The presence of active respiratory symptoms and suspicion of infected pleural effusion should lead to thoracentesis as soon as possible.
Complications of thoracentesis may be benign, such as pain and anxiety associated with the procedure and external bleeding at the site of needle insertion. Pneumothorax is the most common serious procedural complication and the principal reason to order postprocedural chest radiography.1 Less common complications include hemothorax, re-expansion pulmonary edema, infection, subdiaphragmatic organ puncture, and procedure-related death. Bleeding complications and hemothorax are rare even in patients with underlying coagulopathy.2
Point-of-care pleural ultrasonography is now considered the standard of care to guide optimal needle location for the procedure and to exclude other conditions that can mimic pleural effusion on chest radiography, such as lung consolidation and atelectasis.3 High proficiency in the use of preprocedural point-of-care ultrasonography reduces the rate of procedural complications, though it does not eliminate the risk entirely.3,4
Factors associated with higher rates of complications include lack of operator proficiency, poor understanding of the anatomy, poor patient positioning, poor patient cooperation with the procedure, lack of availability of bedside ultrasonography, and drainage of more than 1,500 mL of fluid. Addressing these factors has been shown to decrease the risk of pneumothorax and infection.1–5
HOW OFTEN DOES PNEUMOTHORAX OCCUR AFTER THORACENTESIS?
Several early studies have examined the incidence of pneumothorax after thoracentesis. Lack of ultrasonography use likely explains a higher incidence of complications in early studies: rates of pneumothorax after thoracentesis without ultrasonographic guidance ranged from 5.2% to 26%.6,7
Gervais et al8 analyzed thoracentesis with ultrasonographic guidance in 434 patients, 92 of whom were intubated, and reported that pneumothorax occurred in 10 patients, of whom 6 were intubated. Two of the intubated patients required chest tubes. Other studies have confirmed the low incidence of pneumothorax in patients undergoing thoracentesis, with rates such as 0.61%,1 5%,9 and 4%.10
The major predictor of postprocedural pneumothorax was the presence of symptoms such as chest pain and dyspnea. No intervention was necessary for most cases of pneumothorax in asymptomatic patients. The more widespread use of procedural ultrasonography may explain some discrepancies between the early5,6 and more recent studies.1,8–10
Several studies have demonstrated that postprocedural radiography is unnecessary unless a complication is suspected based on the patient’s symptoms or the need to demonstrate lung re-expansion.1,4,9,10 Clinical suspicion and the patient’s symptoms are the major predictors of procedure-related pneumothorax requiring treatment with a chest tube. Otherwise, incidentally discovered pneumothorax can usually be observed and managed supportively.
WHAT MECHANISMS UNDERLIE POSTPROCEDURAL PNEUMOTHORAX?
Major causes of pneumothorax in patients undergoing thoracentesis are direct puncture during needle or catheter insertion, the introduction of air through the needle or catheter into the pleural cavity, and the inability of the ipsilateral lung to fully expand after drainage of a large volume of fluid, known as pneumothorax ex vacuo.5
Pneumothorax ex vacuo may be seen in patients with medical conditions such as endobronchial obstruction, pleural scarring from long-standing pleural effusion, and lung malignancy, all of which can impair the lung’s ability to expand after removal of a large volume of pleural fluid. It is believed that transient parenchymal pleural fistulae form if the lung cannot expand, causing air leakage into the pleural cavity.5,8,9 Pleural manometry to monitor changes in pleural pressure and elastance can decrease the rates of pneumothorax ex vacuo in patients with the above risk factors.5
WHEN IS RADIOGRAPHY INDICATED AFTER THORACENTESIS?
Current literature suggests that imaging to evaluate for postprocedural complications should be done if there is suspicion of a complication, if thoracentesis required multiple attempts, if the procedure caused aspiration of air, if the patient has advanced lung disease, if the patient is scheduled to undergo thoracic radiation, if the patient is on mechanical ventilation, and after therapeutic thoracentesis if a large volume of fluid is removed.1–10 Routine chest radiography after thoracentesis is not supported in the literature in the absence of these risk factors.
Some practitioners order chest imaging after therapeutic thoracentesis to assess for residual pleural fluid and for visualization of other abnormalities previously hidden by pleural effusion, rather than simply to exclude postprocedural pneumothorax. Alternatively, postprocedural bedside pleural ultrasonography with recording of images can be done to assess for complications and residual pleural fluid volume without exposing the patient to radiation.11
Needle decompression and chest tube insertion should be considered in patients with tension pneumothorax, large pneumothorax (distance from the chest wall to the visceral pleural line of at least 2 cm), mechanical ventilation, progressing pneumothorax, and symptoms.
KEY POINTS
- Pneumothorax is a rare complication of thoracentesis when performed by a skilled operator using ultrasonographic guidance.
- Mechanisms behind the occurrence of pneumothorax are direct lung puncture, introduction of air into the pleural cavity, and pneumothorax ex vacuo.
- In asymptomatic patients, pneumothorax after thoracentesis rarely requires intervention beyond supportive care and close observation.
- Factors such as multiple thoracentesis attempts, symptoms, clinical suspicion, air aspiration during thoracentesis, presence of previous lung disease, and removal of a large volume of fluid may require postprocedural lung imaging (eg, bedside ultrasonography, radiography).
No. After thoracentesis, chest radiography or another lung imaging study should be done only if pneumothorax is suspected, if thoracentesis requires more than 1 attempt, if the patient is on mechanical ventilation or has pre-existing lung disease, or if a large volume (> 1,500 mL) of fluid is removed. Radiography is also usually not necessary after diagnostic thoracentesis in a patient breathing spontaneously. In most cases, pneumothorax found incidentally after thoracentesis does not require decompression and can be managed supportively.
WHAT ARE THE RISKS OF THORACENTESIS?
Thoracentesis is a minimally invasive procedure usually performed at the bedside that involves insertion of a needle into the pleural cavity for drainage of fluid.1 Diagnostic thoracentesis should be done in most cases of a new pleural effusion unless the effusion is small and with a clear diagnosis, or in cases of typical heart failure.
Therapeutic thoracentesis, often called large-volume thoracentesis, aims to improve symptoms such as dyspnea attributed to the pleural effusion by removing at least 1 L of pleural fluid. The presence of active respiratory symptoms and suspicion of infected pleural effusion should lead to thoracentesis as soon as possible.
Complications of thoracentesis may be benign, such as pain and anxiety associated with the procedure and external bleeding at the site of needle insertion. Pneumothorax is the most common serious procedural complication and the principal reason to order postprocedural chest radiography.1 Less common complications include hemothorax, re-expansion pulmonary edema, infection, subdiaphragmatic organ puncture, and procedure-related death. Bleeding complications and hemothorax are rare even in patients with underlying coagulopathy.2
Point-of-care pleural ultrasonography is now considered the standard of care to guide optimal needle location for the procedure and to exclude other conditions that can mimic pleural effusion on chest radiography, such as lung consolidation and atelectasis.3 High proficiency in the use of preprocedural point-of-care ultrasonography reduces the rate of procedural complications, though it does not eliminate the risk entirely.3,4
Factors associated with higher rates of complications include lack of operator proficiency, poor understanding of the anatomy, poor patient positioning, poor patient cooperation with the procedure, lack of availability of bedside ultrasonography, and drainage of more than 1,500 mL of fluid. Addressing these factors has been shown to decrease the risk of pneumothorax and infection.1–5
HOW OFTEN DOES PNEUMOTHORAX OCCUR AFTER THORACENTESIS?
Several early studies have examined the incidence of pneumothorax after thoracentesis. Lack of ultrasonography use likely explains a higher incidence of complications in early studies: rates of pneumothorax after thoracentesis without ultrasonographic guidance ranged from 5.2% to 26%.6,7
Gervais et al8 analyzed thoracentesis with ultrasonographic guidance in 434 patients, 92 of whom were intubated, and reported that pneumothorax occurred in 10 patients, of whom 6 were intubated. Two of the intubated patients required chest tubes. Other studies have confirmed the low incidence of pneumothorax in patients undergoing thoracentesis, with rates such as 0.61%,1 5%,9 and 4%.10
The major predictor of postprocedural pneumothorax was the presence of symptoms such as chest pain and dyspnea. No intervention was necessary for most cases of pneumothorax in asymptomatic patients. The more widespread use of procedural ultrasonography may explain some discrepancies between the early5,6 and more recent studies.1,8–10
Several studies have demonstrated that postprocedural radiography is unnecessary unless a complication is suspected based on the patient’s symptoms or the need to demonstrate lung re-expansion.1,4,9,10 Clinical suspicion and the patient’s symptoms are the major predictors of procedure-related pneumothorax requiring treatment with a chest tube. Otherwise, incidentally discovered pneumothorax can usually be observed and managed supportively.
WHAT MECHANISMS UNDERLIE POSTPROCEDURAL PNEUMOTHORAX?
Major causes of pneumothorax in patients undergoing thoracentesis are direct puncture during needle or catheter insertion, the introduction of air through the needle or catheter into the pleural cavity, and the inability of the ipsilateral lung to fully expand after drainage of a large volume of fluid, known as pneumothorax ex vacuo.5
Pneumothorax ex vacuo may be seen in patients with medical conditions such as endobronchial obstruction, pleural scarring from long-standing pleural effusion, and lung malignancy, all of which can impair the lung’s ability to expand after removal of a large volume of pleural fluid. It is believed that transient parenchymal pleural fistulae form if the lung cannot expand, causing air leakage into the pleural cavity.5,8,9 Pleural manometry to monitor changes in pleural pressure and elastance can decrease the rates of pneumothorax ex vacuo in patients with the above risk factors.5
WHEN IS RADIOGRAPHY INDICATED AFTER THORACENTESIS?
Current literature suggests that imaging to evaluate for postprocedural complications should be done if there is suspicion of a complication, if thoracentesis required multiple attempts, if the procedure caused aspiration of air, if the patient has advanced lung disease, if the patient is scheduled to undergo thoracic radiation, if the patient is on mechanical ventilation, and after therapeutic thoracentesis if a large volume of fluid is removed.1–10 Routine chest radiography after thoracentesis is not supported in the literature in the absence of these risk factors.
Some practitioners order chest imaging after therapeutic thoracentesis to assess for residual pleural fluid and for visualization of other abnormalities previously hidden by pleural effusion, rather than simply to exclude postprocedural pneumothorax. Alternatively, postprocedural bedside pleural ultrasonography with recording of images can be done to assess for complications and residual pleural fluid volume without exposing the patient to radiation.11
Needle decompression and chest tube insertion should be considered in patients with tension pneumothorax, large pneumothorax (distance from the chest wall to the visceral pleural line of at least 2 cm), mechanical ventilation, progressing pneumothorax, and symptoms.
KEY POINTS
- Pneumothorax is a rare complication of thoracentesis when performed by a skilled operator using ultrasonographic guidance.
- Mechanisms behind the occurrence of pneumothorax are direct lung puncture, introduction of air into the pleural cavity, and pneumothorax ex vacuo.
- In asymptomatic patients, pneumothorax after thoracentesis rarely requires intervention beyond supportive care and close observation.
- Factors such as multiple thoracentesis attempts, symptoms, clinical suspicion, air aspiration during thoracentesis, presence of previous lung disease, and removal of a large volume of fluid may require postprocedural lung imaging (eg, bedside ultrasonography, radiography).
- Ault MJ, Rosen BT, Scher J, Feinglass J, Barsuk JH. Thoracentesis outcomes: a 12-year experience. Thorax 2015; 70(2):127–132. doi:10.1136/thoraxjnl-2014-206114
- Hibbert RM, Atwell TD, Lekah A, et al. Safety of ultrasound-guided thoracentesis in patients with abnormal preprocedural coagulation parameters. Chest 2013; 144(2):456–463. doi:10.1378/chest.12-2374
- Barnes TW, Morgenthaler TI, Olson EJ, Hesley GK, Decker PA, Ryu JH. Sonographically guided thoracentesis and rate of pneumothorax. J Clin Ultrasound 2005; 33(9):442–446. doi:10.1002/jcu.20163
- Gordon CE, Feller-Kopman D, Balk EM, Smetana GW. Pneumothorax following thoracentesis: a systematic review and meta-analysis. Arch Intern Med 2010; 170(4):332–339. doi:10.1001/archinternmed.2009.548
- Heidecker J, Huggins JT, Sahn SA, Doelken P. Pathophysiology of pneumothorax following ultrasound-guided thoracentesis. Chest 2006; 130(4):1173–1184. doi:10.1016/S0012-3692(15)51155-0
- Brandstetter RD, Karetzky M, Rastogi R, Lolis JD. Pneumothorax after thoracentesis in chronic obstructive pulmonary disease. Heart Lung 1994; 23(1):67–70. pmid:8150647
- Doyle JJ, Hnatiuk OW, Torrington KG, Slade AR, Howard RS. Necessity of routine chest roentgenography after thoracentesis. Ann Intern Med 1996; 124(9):816–820. pmid:8610950
- Gervais DA, Petersein A, Lee MJ, Hahn PF, Saini S, Mueller PR. US-guided thoracentesis: requirement for postprocedure chest radiography in patients who receive mechanical ventilation versus patients who breathe spontaneously. Radiology 1997; 204(2):503–506. doi:10.1148/radiology.204.2.9240544
- Capizzi SA, Prakash UB. Chest roentgenography after outpatient thoracentesis. Mayo Clin Proc 1998; 73(10):948–950. doi:10.4065/73.10.948
- Alemán C, Alegre J, Armadans L, et al. The value of chest roentgenography in the diagnosis of pneumothorax after thoracentesis. Am J Med 1999; 107(4):340–343. pmid:10527035
- Lichtenstein D. Lung ultrasound in the critically ill. Curr Opin Crit Care 2014; 20(3):315–322. doi:10.1097/MCC.0000000000000096
- Ault MJ, Rosen BT, Scher J, Feinglass J, Barsuk JH. Thoracentesis outcomes: a 12-year experience. Thorax 2015; 70(2):127–132. doi:10.1136/thoraxjnl-2014-206114
- Hibbert RM, Atwell TD, Lekah A, et al. Safety of ultrasound-guided thoracentesis in patients with abnormal preprocedural coagulation parameters. Chest 2013; 144(2):456–463. doi:10.1378/chest.12-2374
- Barnes TW, Morgenthaler TI, Olson EJ, Hesley GK, Decker PA, Ryu JH. Sonographically guided thoracentesis and rate of pneumothorax. J Clin Ultrasound 2005; 33(9):442–446. doi:10.1002/jcu.20163
- Gordon CE, Feller-Kopman D, Balk EM, Smetana GW. Pneumothorax following thoracentesis: a systematic review and meta-analysis. Arch Intern Med 2010; 170(4):332–339. doi:10.1001/archinternmed.2009.548
- Heidecker J, Huggins JT, Sahn SA, Doelken P. Pathophysiology of pneumothorax following ultrasound-guided thoracentesis. Chest 2006; 130(4):1173–1184. doi:10.1016/S0012-3692(15)51155-0
- Brandstetter RD, Karetzky M, Rastogi R, Lolis JD. Pneumothorax after thoracentesis in chronic obstructive pulmonary disease. Heart Lung 1994; 23(1):67–70. pmid:8150647
- Doyle JJ, Hnatiuk OW, Torrington KG, Slade AR, Howard RS. Necessity of routine chest roentgenography after thoracentesis. Ann Intern Med 1996; 124(9):816–820. pmid:8610950
- Gervais DA, Petersein A, Lee MJ, Hahn PF, Saini S, Mueller PR. US-guided thoracentesis: requirement for postprocedure chest radiography in patients who receive mechanical ventilation versus patients who breathe spontaneously. Radiology 1997; 204(2):503–506. doi:10.1148/radiology.204.2.9240544
- Capizzi SA, Prakash UB. Chest roentgenography after outpatient thoracentesis. Mayo Clin Proc 1998; 73(10):948–950. doi:10.4065/73.10.948
- Alemán C, Alegre J, Armadans L, et al. The value of chest roentgenography in the diagnosis of pneumothorax after thoracentesis. Am J Med 1999; 107(4):340–343. pmid:10527035
- Lichtenstein D. Lung ultrasound in the critically ill. Curr Opin Crit Care 2014; 20(3):315–322. doi:10.1097/MCC.0000000000000096
Can procalcitonin guide decisions about antibiotic management?
Yes, but with caution. Multiple randomized controlled trials showed that procalcitonin testing can help guide antibiotic management in a variety of clinical scenarios including sepsis, respiratory tract infection, and exacerbation of chronic obstructive pulmonary disease (COPD), and that procalcitonin guidance led to less antibiotic use with either unchanged or better outcomes. Moreover, observational studies have shown high negative predictive values for procalcitonin testing in other clinical situations such as bacteremia and bacterial meningitis, allowing clinicians to rule out these diagnoses if the clinical probability is low or moderate.
Nonetheless, clinical judgment must be exercised to consider the possibility of false- positive and false-negative results, especially if clinical suspicion for bacterial infection is high.
A RESPONSE TO BACTERIAL TOXIN
Procalcitonin is a peptide precursor of calcitonin that is produced by C cells of the thyroid and by neuroendocrine cells of the lung and intestine in response to bacterial toxin. In contrast, procalcitonin levels are down-regulated in viral infection.
Levels of procalcitonin increase 6 to 12 hours after stimulation, and the half-life is roughly 24 hours.1 This suggests levels should decrease by one-half daily if an infection is controlled and is responding to therapy (assuming normal clearance).
The test costs about $25, with a turnaround time of 20 to 60 minutes, or longer at institutions that send the test out or run the tests in batches.
Point-of-care procalcitonin testing is emerging but not yet commercially available in the United States. Despite extensive observational studies and randomized controlled trials over the past 20 years, procalcitonin’s physiologic role remains unclear. The large body of evidence of the clinical utility of procalcitonin measurement has been summarized in several meta-analyses in different diseases.
PROCALCITONIN TESTING IN SEPSIS
Trials of procalcitonin testing have had slightly different inclusion criteria that commonly overlap with similar diagnoses. Sepsis is the broadest cohort studied.
The Procalcitonin to Reduce Antibiotic Treatments in Acutely Ill Patients (PRORATA) trial2 randomized 621 patients admitted to the intensive care unit (ICU) with suspected bacterial infections to antibiotic therapy guided by procalcitonin concentrations or to antibiotic therapy based on current guidelines. The source of infection varied, but 73% of patients had pulmonary infections.The procalcitonin algorithm was as follows:
- Starting antibiotics was discouraged if the procalcitonin concentration was less than 0.5 ng/mL, and strongly discouraged if less than 0.25 ng/mL
- Starting antibiotics was encouraged if the concentration was 0.5 ng/mL or higher, and strongly encouraged if 1 ng/mL or higher
- Stopping antibiotics was encouraged if the concentration dropped by at least 80% from the peak level or to a level greater than or equal to 0.25 ng/mL; stopping was strongly encouraged if the concentration fell below 0.25 ng/mL.
There was also guidance to change antibiotics if procalcitonin increased on therapy and was above 0.5 ng/mL.
Although the study physicians generally followed the algorithm, they were allowed to override it based on clinical judgment. The main results were that the number of days without antibiotics was higher in the procalcitonin group than in the controls (14.3 vs 11.6 days), with no other statistically significant difference between groups. These findings supported the idea that procalcitonin can guide clinicians to safely “deprescribe” antibiotics.
The Stop Antibiotics on Guidance of Procalcitonin Study (SAPS),3 published in 2016, was a larger trial with similar design, in 1,575 patients admitted to the ICU with suspected infection. Antibiotic use was less and the 28-day mortality rate was lower with procalcitonin guidance: 20% vs 25% in the intention-to-treat analysis.
ACUTE RESPIRATORY TRACT INFECTION
The Procalcitonin Guided Antibiotic Therapy and Hospitalisation in Patients With Lower Respiratory Tract Infections (ProHOSP) trial4 randomized 1,381 patients to antibiotic therapy guided by procalcitonin levels or standard guidelines. Most patients had community-acquired pneumonia, while the rest had exacerbations of COPD, acute bronchitis, or other lower respiratory tract infections.
In the study algorithm, starting or continuing antibiotics was discouraged if procalcitonin levels were 0.25 ng/mL or less, and strongly discouraged if less than 0.1 ng/mL. Starting or continuing antibiotics was encouraged if levels were greater than 0.25 ng/mL, and strongly encouraged if greater than 0.5 ng/mL.
The algorithm recommended stopping antibiotics if procalcitonin levels fell below 0.25 ng/mL or decreased by 80%, and strongly recommended stopping them if procalcitonin fell below 0.1 ng/mL or decreased by 90%.
The treating physician could override the algorithm if the patient was unstable, was in an ICU, or had Legionella infection.
Antibiotic use was less in the procalcitonin-guided arm (75.4% vs 87.7%; mean duration 5.7 days vs 8.7 days), as was the rate of adverse effects from antibiotics (19.8% vs 28.1%). Rates of recurrence or rehospitalization were also lower with procalcitonin guidance (3.7% vs 6.5%), presumably because of fewer antibiotic-related side effects or better diagnostic accuracy. Rates of death and ICU admission were similar in the 2 groups. These findings were similar to those of PRORATA and SAPS, demonstrating that guidance with procalcitonin levels decreased antibiotic utilization, with other outcomes either improved or unchanged.
Schuetz et al,5 in a 2018 meta-analysis, collected data on 6,708 patients from 26 trials in 12 countries and found that procalcitonin guidance decreased antibiotic exposure by 2.4 days and reduced the rate of antibiotic-related side effects (16% vs 22%). Although there was skepticism about the mortality benefit reported in the SAPS trial, a similar mortality benefit was found in this meta-analysis (30-day mortality rates were 9% vs 10%), suggesting that measuring procalcitonin not only reduces unnecessary antibiotic exposure, but also saves lives.
Although decreasing antibiotic exposure may not confer a survival benefit, procalcitonin guidance likely clarifies the diagnosis and thus expedites proper treatment in patients with sepsis-like syndromes that are actually due to a noninfectious pathology (eg, pulmonary embolism, myocardial infarction, adrenal insufficiency).
Negative findings in ProACT
The Procalcitonin Antibiotic Consensus Trial (ProACT)6 subsequently reported findings discordant with those above but was flawed in that adherence to the procalcitonin guideline by physicians was only 62% in the subgroup of patients with low procalcitonin results, which accounted for almost 90% of patients. Overall adherence by physicians to the procalcitonin guideline was 65%, much lower than in other trials (ProHOSP had over 90% adherence).4 Further, ProACT was done in American centers unfamiliar with procalcitonin, and it seems they did not trust low procalcitonin values as a reason to stop or avoid antibiotics.
ACUTE EXACERBATIONS OF COPD
Multiple small randomized controlled trials and subgroups of larger studies like ProHOSP have studied the use of procalcitonin in acute exacerbations of COPD. Most studies used a design similar to the algorithm in ProHOSP.
Mathioudakis et al,7 in a meta-analysis of 8 trials with a total of 1,062 patients with acute exacerbation of COPD, found that with procalcitonin guidance, prescription of antibiotics on admission decreased by almost one-half, and courses of antibiotics were approximately 4 days shorter without any statistically significant difference in rates of treatment failure, length of hospital stay, recurrence, rehospitalization, or overall mortality.
However, the quality of the studies included in the meta-analysis was deemed only low to moderate, and thus the authors concluded, “Procalcitonin-based protocols appear to be clinically effective; however, confirmatory trials with rigorous methodology are required.”7 Nonetheless, given the lack of data supporting current practices for patient selection for antibiotics in COPD exacerbations, a strategy involving procalcitonin seems to be reasonable.
BACTEREMIA
Observational studies from as far as back as 1999 have examined the association of procalcitonin levels with bacteremia. The study designs were generally similar, with procalcitonin levels checked at time of blood culture, mostly in emergency rooms, and the procalcitonin value correlated with blood culture results. The general conclusion has been that procalcitonin has diagnostic value in ruling out bacteremia but should be used in the context of pretest probability rather than in isolation.
Hattori et al8 performed one of the largest studies, in 1,331 patients, using a procalcitonin level cutoff of 0.9 ng/mL. The sensitivity was 72% and specificity was 69%, which are not impressive; however, the negative predictive value was 95%, and even higher at lower cutoff values. Further, procalcitonin was significantly better at predicting bacteremia than either the white blood cell count or C-reactive protein level, with the latter two being hardly better than random chance.
Hoeboer et al9 performed a meta-analysis of various studies with a total of 16,514 patients. Using a cutoff of 0.5 ng/mL, they reported a sensitivity of 76% and a specificity of 69% with a negative predictive value of 97% in emergency rooms, 95% on regular wards, and 98% in ICUs. The high negative predictive value of procalcitonin can allow clinicians to stratify bacteremia risk to determine which patients need blood cultures, which in turn may help clinicians order blood cultures more appropriately and avoid unnecessary costs, delays, and harms associated with false-positive results, such as additional visits, additional testing, and unnecessary use of antibiotics.
MENINGITIS
As with bacteremia, observational studies have reported fairly high negative predictive values for procalcitonin in bacterial meningitis. The correlation is not surprising, given that most cases of bacterial meningitis occur due to hematogenous dissemination.
A 2015 meta-analysis of 9 studies and 725 patients reported a pooled sensitivity of 90%, specificity 90%, positive likelihood ratio 27.3, and negative likelihood ratio 0.13.10 Cutoffs for procalcitonin levels varied, but the most common value was 0.5 ng/mL. The authors also noted that the diagnostic utility of procalcitonin was far superior to C-reactive protein in this scenario, concluding that serum procalcitonin is a highly accurate test to distinguish between bacterial and viral causes in suspected meningitis.10
OTHER CLINICAL APPLICATIONS
Postoperative infection
Small studies have assessed procalcitonin as a marker to rule out postoperative infections,11,12 but the heterogeneity of study designs and populations makes it difficult to combine the studies for meta-analysis. Nevertheless, the general trend is that there may be a role for procalcitonin, and that procalcitonin has better diagnostic yield than the white blood cell count or C-reactive protein level. The optimal cutoff depends on the surgery, since a small elevation in procalcitonin can be expected with the stress of surgery; and since the degree of elevation varies with type of surgery, the result must be interpreted with caution.
Malignancy
In malignancy-associated conditions such as neutropenic fever and tumor fever, the clinical utility of procalcitonin is somewhat diminished, as malignancy can cause elevated procalcitonin levels (especially in metastatic disease), but a low concentration still has a fair negative predictive value (approximately 90%) for bloodstream infections.13
A retrospective study suggested that the ratio of procalcitonin to C-reactive protein could improve diagnostic accuracy in patients with malignancies, presumably because an elevation of procalcitonin out of proportion to elevation in C-reactive protein favored a bacterial infection rather than nonspecific inflammation related to malignancy.14
Cardiac syndromes
In cardiac syndromes, dyspnea and abnormal chest imaging may make it difficult to exclude respiratory infections. Schuetz et al15 reviewed the potential value of procalcitonin testing in a variety of cardiac disorders, especially in acute cardiovascular conditions whose presentation resembles that of sepsis or acute respiratory tract infection. They concluded it may have a role in diagnosis and prognosis in these settings, as well as guiding drug therapy.
Localized infections
Though localized infections such as cystitis, cellulitis, and osteomyelitis often do not affect procalcitonin levels, the test may help assess illness severity and rule out associated bacteremia.
One study found that a low procalcitonin level was insufficient to rule out urinary tract infection, but procalcitonin levels predicted bacteremia better than any other variable or combination of variables; moreover, procalcitonin had a negative predictive value as high as 97% for ruling out bacteremia associated with urinary tract infection.16
ROLE IN PROGNOSIS
In addition to being a useful marker for diagnosis of bacterial infections, the procalcitonin level has significant prognostic implications, as a high or persistently elevated level correlates with a higher rate of all-cause mortality.17 The prognostic capability may enhance triage decisions.
Because the procalcitonin level lacks specificity, clinicians need to be aware of noninfectious causes of elevations such as malignancy, surgery, impaired renal function,8 and myocardial infarction.18 In these scenarios, it is important to think critically about the procalcitonin result and consider an adjusted cutoff.
A study of procalcitonin to predict a positive blood culture in patients with renal disease suggested an optimal cutoff value of 1.06 ng/mL for patients with an estimated glomerular filtration rate of 30 to 60 mL/min/1.73m2, and a value of 2.50 ng/mL for a rate less than 30 mL/min/1.73m2.8
In a chronic process like malignancy, the procalcitonin level is usually not markedly elevated. But it can also remain persistently elevated, with no improvement associated with effective antibiotic treatment and no clinical deterioration associated with treatment failure.
Use of procalcitonin and troponin
For some patients, there may be diagnostic uncertainty about interpreting procalcitonin and troponin results, as both plaque-rupture myocardial infarction and demand ischemia from sepsis can cause elevation in both values. In a study of patients with acute myocardial infarction, the procalcitonin level peaked at 3.57 ng/mL and troponin peaked at 60 ng/mL at about 24 hours after admission.18 This suggests that a troponin-to-procalcitonin ratio may help distinguish acute myocardial infarction from demand ischemia, though the optimal cutoff is unknown.
Both troponin and procalcitonin levels can help rule out acute severe illness (eg, bloodstream infection, acute myocardial infarction). But both can be falsely negative in early presentation or in less severe disease (eg, localized infection, unstable angina), as well as in noninfectious inflammation and nonobstructive myocardial injury.
Both are important prognostic markers. Furthermore, both can be chronically elevated in patients with renal disease, but both still have a characteristic rise and fall in acute disease states. But neither should be used in isolation without information from electrocardiography, other tests, and the clinical context.
CAVEATS AND CHALLENGES
Based on clinical experience and reported studies, procalcitonin testing has proven valuable in the diagnosis, prognosis, and management of a range of diseases, particularly certain infections.
However, procalcitonin testing must be applied cautiously and judiciously. There is a potential for early false-negative results, and false-positive results can occur in conditions such as kidney disease, myocardial infarction, postoperative stress response, and malignancy, though there may be ways to factor these conditions into interpretation of procalcitonin results.
Widespread procalcitonin testing may lead to excessive costs, though the cost for each test is reasonable and probably offset by benefits of diagnostic clarification and decreased use of antibiotics, if appropriately applied.
The primary roles for procalcitonin testing are to rule out infection in patients with low probability of infection and to allow safe early cessation of antibiotic therapy in patients with presumed bacterial infection. Procalcitonin testing can enable providers to stop antibiotics safely, with the general trend showing decreased antibiotic utilization without patient harm. This can result in healthcare cost savings and improved patient outcomes such as decreased length of hospital stay, decreased readmission rates, fewer adverse effects from antibiotics, and possibly improved mortality rates.
Despite the potential benefits from procalcitonin testing, results must be interpreted within the clinical context because a host of factors can affect the values. Extreme values are more useful than intermediate values, which are difficult to interpret and have poor predictive value.
Although all current biomarkers for infection are imperfect, procalcitonin appears to have better diagnostic accuracy than other markers such as the white blood cell count and C-reactive protein in multiple clinical scenarios, and its appropriate use appears to improve important outcomes such as survival.
- Schuetz P, Albrich W, Mueller B. Procalcitonin for diagnosis of infection and guide to antibiotic decisions: past, present and future. BMC Med 2011; 9:107. doi:10.1186/1741-7015-9-107
- Bouadma L, Luyt CE, Tubach F, et al; PRORATA trial group. Use of procalcitonin to reduce patients' exposure to antibiotics in intensive care units (PRORATA trial): a multicentre randomised controlled trial. Lancet 2010; 375(9713):463–474. doi:10.1016/S0140-6736(09)61879-1
- de Jong E, van Oers JA, Beishuizen A, et al. Efficacy and safety of procalcitonin guidance in reducing the duration of antibiotic treatment in critically ill patients: a randomised, controlled, open-label trial. Lancet Infect Dis 2016; 16(7):819–827. doi:10.1016/S1473-3099(16)00053-0
- Schuetz P, Christ-Crain M, Thomann R, et al; ProHOSP Study Group. Effect of procalcitonin-based guidelines vs standard guidelines on antibiotic use in lower respiratory tract infections: the ProHOSP randomized controlled trial. JAMA 2009; 302(10):1059–1066. doi:10.1001/jama.2009.1297
- Schuetz P, Wirz Y, Sager R, et al. Effect of procalcitonin-guided antibiotic treatment on mortality in acute respiratory infections: a patient level meta-analysis. Lancet Infect Dis 2018; 18(1):95–107. doi:10.1016/S1473-3099(17)30592-3
- Huang DT, Yealy DM, Filbin MR, et al; ProACT Investigators. Procalcitonin-guided use of antibiotics for lower respiratory tract infection. N Engl J Med 2018; 379(3):236–249. doi:10.1056/NEJMoa1802670
- Mathioudakis AG, Chatzimavridou-Grigoriadou V, Corlateanu A, Vestbo J. Procalcitonin to guide antibiotic administration in COPD exacerbations: a meta-analysis. Eur Respir Rev 2017; 26(143)pii:160073. doi:10.1183/16000617.0073-2016
- Hattori T, Nishiyama H, Kato H, et al. Clinical value of procalcitonin for patients with suspected bloodstream infection. Am J Clin Pathol 2014; 141(1):43–51. doi:10.1309/AJCP4GV7ZFDTANGC
- Hoeboer SH, van der Geest PJ, Nieboer D, Groeneveld AB. The diagnostic accuracy of procalcitonin for bacteraemia: a systematic review and meta-analysis. Clin Microbiol Infect 2015; 21(5):474–481. doi:10.1016/j.cmi.2014.12.026
- Vikse J, Henry BM, Roy J, Ramakrishnan PK, Tomaszewski KA, Walocha JA. The role of serum procalcitonin in the diagnosis of bacterial meningitis in adults: a systematic review and meta-analysis. Int J Infect Dis 2015; 38:68–76. doi:10.1016/j.ijid.2015.07.011
- Aouifi A, Piriou V, Bastien O, et al. Usefulness of procalcitonin for diagnosis of infection in cardiac surgical patients. Crit Care Med 2000; 28(9):3171–3176. pmid:11008977
- Hunziker S, Hugle T, Schuchardt K, et al. The value of serum procalcitonin level for differentiation of infectious from noninfectious causes of fever after orthopaedic surgery. J Bone Joint Surg Am 2010; 92(1):138–148. doi:10.2106/JBJS.H.01600
- Shomali W, Hachem R, Chaftari AM, et al. Can procalcitonin distinguish infectious fever from tumor-related fever in non-neutropenic cancer patients? Cancer 2012; 118(23):5823–5829. doi:10.1002/cncr.27602
- Hangai S, Nannya Y, Kurokawa M. Role of procalcitonin and C-reactive protein for discrimination between tumor fever and infection in patients with hematological diseases. Leuk Lymphoma 2015; 56(4):910–914. doi:10.3109/10428194.2014.938329
- Schuetz P, Daniels LB, Kulkarni P, Anker SD, Mueller B. Procalcitonin: a new biomarker for the cardiologist. Int J Cardiol 2016; 223:390–397. doi:10.1016/j.ijcard.2016.08.204
- van Nieuwkoop C, Bonten TN, van't Wout JW, et al. Procalcitonin reflects bacteremia and bacterial load in urosepsis syndrome: a prospective observational study. Crit Care 2010; 14(6):R206. doi:10.1186/cc9328
- Liu D, Su L, Han G, Yan P, Xie L. Prognostic value of procalcitonin in adult patients with sepsis: a systematic review and meta-analysis. PLoS One 2015; 10(6):e0129450. doi:10.1371/journal.pone.0129450
- Kafkas N, Venetsanou K, Patsilinakos S, et al. Procalcitonin in acute myocardial infarction. Acute Card Care 2008; 10(1):30–36. doi:10.1080/17482940701534800
Yes, but with caution. Multiple randomized controlled trials showed that procalcitonin testing can help guide antibiotic management in a variety of clinical scenarios including sepsis, respiratory tract infection, and exacerbation of chronic obstructive pulmonary disease (COPD), and that procalcitonin guidance led to less antibiotic use with either unchanged or better outcomes. Moreover, observational studies have shown high negative predictive values for procalcitonin testing in other clinical situations such as bacteremia and bacterial meningitis, allowing clinicians to rule out these diagnoses if the clinical probability is low or moderate.
Nonetheless, clinical judgment must be exercised to consider the possibility of false- positive and false-negative results, especially if clinical suspicion for bacterial infection is high.
A RESPONSE TO BACTERIAL TOXIN
Procalcitonin is a peptide precursor of calcitonin that is produced by C cells of the thyroid and by neuroendocrine cells of the lung and intestine in response to bacterial toxin. In contrast, procalcitonin levels are down-regulated in viral infection.
Levels of procalcitonin increase 6 to 12 hours after stimulation, and the half-life is roughly 24 hours.1 This suggests levels should decrease by one-half daily if an infection is controlled and is responding to therapy (assuming normal clearance).
The test costs about $25, with a turnaround time of 20 to 60 minutes, or longer at institutions that send the test out or run the tests in batches.
Point-of-care procalcitonin testing is emerging but not yet commercially available in the United States. Despite extensive observational studies and randomized controlled trials over the past 20 years, procalcitonin’s physiologic role remains unclear. The large body of evidence of the clinical utility of procalcitonin measurement has been summarized in several meta-analyses in different diseases.
PROCALCITONIN TESTING IN SEPSIS
Trials of procalcitonin testing have had slightly different inclusion criteria that commonly overlap with similar diagnoses. Sepsis is the broadest cohort studied.
The Procalcitonin to Reduce Antibiotic Treatments in Acutely Ill Patients (PRORATA) trial2 randomized 621 patients admitted to the intensive care unit (ICU) with suspected bacterial infections to antibiotic therapy guided by procalcitonin concentrations or to antibiotic therapy based on current guidelines. The source of infection varied, but 73% of patients had pulmonary infections.The procalcitonin algorithm was as follows:
- Starting antibiotics was discouraged if the procalcitonin concentration was less than 0.5 ng/mL, and strongly discouraged if less than 0.25 ng/mL
- Starting antibiotics was encouraged if the concentration was 0.5 ng/mL or higher, and strongly encouraged if 1 ng/mL or higher
- Stopping antibiotics was encouraged if the concentration dropped by at least 80% from the peak level or to a level greater than or equal to 0.25 ng/mL; stopping was strongly encouraged if the concentration fell below 0.25 ng/mL.
There was also guidance to change antibiotics if procalcitonin increased on therapy and was above 0.5 ng/mL.
Although the study physicians generally followed the algorithm, they were allowed to override it based on clinical judgment. The main results were that the number of days without antibiotics was higher in the procalcitonin group than in the controls (14.3 vs 11.6 days), with no other statistically significant difference between groups. These findings supported the idea that procalcitonin can guide clinicians to safely “deprescribe” antibiotics.
The Stop Antibiotics on Guidance of Procalcitonin Study (SAPS),3 published in 2016, was a larger trial with similar design, in 1,575 patients admitted to the ICU with suspected infection. Antibiotic use was less and the 28-day mortality rate was lower with procalcitonin guidance: 20% vs 25% in the intention-to-treat analysis.
ACUTE RESPIRATORY TRACT INFECTION
The Procalcitonin Guided Antibiotic Therapy and Hospitalisation in Patients With Lower Respiratory Tract Infections (ProHOSP) trial4 randomized 1,381 patients to antibiotic therapy guided by procalcitonin levels or standard guidelines. Most patients had community-acquired pneumonia, while the rest had exacerbations of COPD, acute bronchitis, or other lower respiratory tract infections.
In the study algorithm, starting or continuing antibiotics was discouraged if procalcitonin levels were 0.25 ng/mL or less, and strongly discouraged if less than 0.1 ng/mL. Starting or continuing antibiotics was encouraged if levels were greater than 0.25 ng/mL, and strongly encouraged if greater than 0.5 ng/mL.
The algorithm recommended stopping antibiotics if procalcitonin levels fell below 0.25 ng/mL or decreased by 80%, and strongly recommended stopping them if procalcitonin fell below 0.1 ng/mL or decreased by 90%.
The treating physician could override the algorithm if the patient was unstable, was in an ICU, or had Legionella infection.
Antibiotic use was less in the procalcitonin-guided arm (75.4% vs 87.7%; mean duration 5.7 days vs 8.7 days), as was the rate of adverse effects from antibiotics (19.8% vs 28.1%). Rates of recurrence or rehospitalization were also lower with procalcitonin guidance (3.7% vs 6.5%), presumably because of fewer antibiotic-related side effects or better diagnostic accuracy. Rates of death and ICU admission were similar in the 2 groups. These findings were similar to those of PRORATA and SAPS, demonstrating that guidance with procalcitonin levels decreased antibiotic utilization, with other outcomes either improved or unchanged.
Schuetz et al,5 in a 2018 meta-analysis, collected data on 6,708 patients from 26 trials in 12 countries and found that procalcitonin guidance decreased antibiotic exposure by 2.4 days and reduced the rate of antibiotic-related side effects (16% vs 22%). Although there was skepticism about the mortality benefit reported in the SAPS trial, a similar mortality benefit was found in this meta-analysis (30-day mortality rates were 9% vs 10%), suggesting that measuring procalcitonin not only reduces unnecessary antibiotic exposure, but also saves lives.
Although decreasing antibiotic exposure may not confer a survival benefit, procalcitonin guidance likely clarifies the diagnosis and thus expedites proper treatment in patients with sepsis-like syndromes that are actually due to a noninfectious pathology (eg, pulmonary embolism, myocardial infarction, adrenal insufficiency).
Negative findings in ProACT
The Procalcitonin Antibiotic Consensus Trial (ProACT)6 subsequently reported findings discordant with those above but was flawed in that adherence to the procalcitonin guideline by physicians was only 62% in the subgroup of patients with low procalcitonin results, which accounted for almost 90% of patients. Overall adherence by physicians to the procalcitonin guideline was 65%, much lower than in other trials (ProHOSP had over 90% adherence).4 Further, ProACT was done in American centers unfamiliar with procalcitonin, and it seems they did not trust low procalcitonin values as a reason to stop or avoid antibiotics.
ACUTE EXACERBATIONS OF COPD
Multiple small randomized controlled trials and subgroups of larger studies like ProHOSP have studied the use of procalcitonin in acute exacerbations of COPD. Most studies used a design similar to the algorithm in ProHOSP.
Mathioudakis et al,7 in a meta-analysis of 8 trials with a total of 1,062 patients with acute exacerbation of COPD, found that with procalcitonin guidance, prescription of antibiotics on admission decreased by almost one-half, and courses of antibiotics were approximately 4 days shorter without any statistically significant difference in rates of treatment failure, length of hospital stay, recurrence, rehospitalization, or overall mortality.
However, the quality of the studies included in the meta-analysis was deemed only low to moderate, and thus the authors concluded, “Procalcitonin-based protocols appear to be clinically effective; however, confirmatory trials with rigorous methodology are required.”7 Nonetheless, given the lack of data supporting current practices for patient selection for antibiotics in COPD exacerbations, a strategy involving procalcitonin seems to be reasonable.
BACTEREMIA
Observational studies from as far as back as 1999 have examined the association of procalcitonin levels with bacteremia. The study designs were generally similar, with procalcitonin levels checked at time of blood culture, mostly in emergency rooms, and the procalcitonin value correlated with blood culture results. The general conclusion has been that procalcitonin has diagnostic value in ruling out bacteremia but should be used in the context of pretest probability rather than in isolation.
Hattori et al8 performed one of the largest studies, in 1,331 patients, using a procalcitonin level cutoff of 0.9 ng/mL. The sensitivity was 72% and specificity was 69%, which are not impressive; however, the negative predictive value was 95%, and even higher at lower cutoff values. Further, procalcitonin was significantly better at predicting bacteremia than either the white blood cell count or C-reactive protein level, with the latter two being hardly better than random chance.
Hoeboer et al9 performed a meta-analysis of various studies with a total of 16,514 patients. Using a cutoff of 0.5 ng/mL, they reported a sensitivity of 76% and a specificity of 69% with a negative predictive value of 97% in emergency rooms, 95% on regular wards, and 98% in ICUs. The high negative predictive value of procalcitonin can allow clinicians to stratify bacteremia risk to determine which patients need blood cultures, which in turn may help clinicians order blood cultures more appropriately and avoid unnecessary costs, delays, and harms associated with false-positive results, such as additional visits, additional testing, and unnecessary use of antibiotics.
MENINGITIS
As with bacteremia, observational studies have reported fairly high negative predictive values for procalcitonin in bacterial meningitis. The correlation is not surprising, given that most cases of bacterial meningitis occur due to hematogenous dissemination.
A 2015 meta-analysis of 9 studies and 725 patients reported a pooled sensitivity of 90%, specificity 90%, positive likelihood ratio 27.3, and negative likelihood ratio 0.13.10 Cutoffs for procalcitonin levels varied, but the most common value was 0.5 ng/mL. The authors also noted that the diagnostic utility of procalcitonin was far superior to C-reactive protein in this scenario, concluding that serum procalcitonin is a highly accurate test to distinguish between bacterial and viral causes in suspected meningitis.10
OTHER CLINICAL APPLICATIONS
Postoperative infection
Small studies have assessed procalcitonin as a marker to rule out postoperative infections,11,12 but the heterogeneity of study designs and populations makes it difficult to combine the studies for meta-analysis. Nevertheless, the general trend is that there may be a role for procalcitonin, and that procalcitonin has better diagnostic yield than the white blood cell count or C-reactive protein level. The optimal cutoff depends on the surgery, since a small elevation in procalcitonin can be expected with the stress of surgery; and since the degree of elevation varies with type of surgery, the result must be interpreted with caution.
Malignancy
In malignancy-associated conditions such as neutropenic fever and tumor fever, the clinical utility of procalcitonin is somewhat diminished, as malignancy can cause elevated procalcitonin levels (especially in metastatic disease), but a low concentration still has a fair negative predictive value (approximately 90%) for bloodstream infections.13
A retrospective study suggested that the ratio of procalcitonin to C-reactive protein could improve diagnostic accuracy in patients with malignancies, presumably because an elevation of procalcitonin out of proportion to elevation in C-reactive protein favored a bacterial infection rather than nonspecific inflammation related to malignancy.14
Cardiac syndromes
In cardiac syndromes, dyspnea and abnormal chest imaging may make it difficult to exclude respiratory infections. Schuetz et al15 reviewed the potential value of procalcitonin testing in a variety of cardiac disorders, especially in acute cardiovascular conditions whose presentation resembles that of sepsis or acute respiratory tract infection. They concluded it may have a role in diagnosis and prognosis in these settings, as well as guiding drug therapy.
Localized infections
Though localized infections such as cystitis, cellulitis, and osteomyelitis often do not affect procalcitonin levels, the test may help assess illness severity and rule out associated bacteremia.
One study found that a low procalcitonin level was insufficient to rule out urinary tract infection, but procalcitonin levels predicted bacteremia better than any other variable or combination of variables; moreover, procalcitonin had a negative predictive value as high as 97% for ruling out bacteremia associated with urinary tract infection.16
ROLE IN PROGNOSIS
In addition to being a useful marker for diagnosis of bacterial infections, the procalcitonin level has significant prognostic implications, as a high or persistently elevated level correlates with a higher rate of all-cause mortality.17 The prognostic capability may enhance triage decisions.
Because the procalcitonin level lacks specificity, clinicians need to be aware of noninfectious causes of elevations such as malignancy, surgery, impaired renal function,8 and myocardial infarction.18 In these scenarios, it is important to think critically about the procalcitonin result and consider an adjusted cutoff.
A study of procalcitonin to predict a positive blood culture in patients with renal disease suggested an optimal cutoff value of 1.06 ng/mL for patients with an estimated glomerular filtration rate of 30 to 60 mL/min/1.73m2, and a value of 2.50 ng/mL for a rate less than 30 mL/min/1.73m2.8
In a chronic process like malignancy, the procalcitonin level is usually not markedly elevated. But it can also remain persistently elevated, with no improvement associated with effective antibiotic treatment and no clinical deterioration associated with treatment failure.
Use of procalcitonin and troponin
For some patients, there may be diagnostic uncertainty about interpreting procalcitonin and troponin results, as both plaque-rupture myocardial infarction and demand ischemia from sepsis can cause elevation in both values. In a study of patients with acute myocardial infarction, the procalcitonin level peaked at 3.57 ng/mL and troponin peaked at 60 ng/mL at about 24 hours after admission.18 This suggests that a troponin-to-procalcitonin ratio may help distinguish acute myocardial infarction from demand ischemia, though the optimal cutoff is unknown.
Both troponin and procalcitonin levels can help rule out acute severe illness (eg, bloodstream infection, acute myocardial infarction). But both can be falsely negative in early presentation or in less severe disease (eg, localized infection, unstable angina), as well as in noninfectious inflammation and nonobstructive myocardial injury.
Both are important prognostic markers. Furthermore, both can be chronically elevated in patients with renal disease, but both still have a characteristic rise and fall in acute disease states. But neither should be used in isolation without information from electrocardiography, other tests, and the clinical context.
CAVEATS AND CHALLENGES
Based on clinical experience and reported studies, procalcitonin testing has proven valuable in the diagnosis, prognosis, and management of a range of diseases, particularly certain infections.
However, procalcitonin testing must be applied cautiously and judiciously. There is a potential for early false-negative results, and false-positive results can occur in conditions such as kidney disease, myocardial infarction, postoperative stress response, and malignancy, though there may be ways to factor these conditions into interpretation of procalcitonin results.
Widespread procalcitonin testing may lead to excessive costs, though the cost for each test is reasonable and probably offset by benefits of diagnostic clarification and decreased use of antibiotics, if appropriately applied.
The primary roles for procalcitonin testing are to rule out infection in patients with low probability of infection and to allow safe early cessation of antibiotic therapy in patients with presumed bacterial infection. Procalcitonin testing can enable providers to stop antibiotics safely, with the general trend showing decreased antibiotic utilization without patient harm. This can result in healthcare cost savings and improved patient outcomes such as decreased length of hospital stay, decreased readmission rates, fewer adverse effects from antibiotics, and possibly improved mortality rates.
Despite the potential benefits from procalcitonin testing, results must be interpreted within the clinical context because a host of factors can affect the values. Extreme values are more useful than intermediate values, which are difficult to interpret and have poor predictive value.
Although all current biomarkers for infection are imperfect, procalcitonin appears to have better diagnostic accuracy than other markers such as the white blood cell count and C-reactive protein in multiple clinical scenarios, and its appropriate use appears to improve important outcomes such as survival.
Yes, but with caution. Multiple randomized controlled trials showed that procalcitonin testing can help guide antibiotic management in a variety of clinical scenarios including sepsis, respiratory tract infection, and exacerbation of chronic obstructive pulmonary disease (COPD), and that procalcitonin guidance led to less antibiotic use with either unchanged or better outcomes. Moreover, observational studies have shown high negative predictive values for procalcitonin testing in other clinical situations such as bacteremia and bacterial meningitis, allowing clinicians to rule out these diagnoses if the clinical probability is low or moderate.
Nonetheless, clinical judgment must be exercised to consider the possibility of false- positive and false-negative results, especially if clinical suspicion for bacterial infection is high.
A RESPONSE TO BACTERIAL TOXIN
Procalcitonin is a peptide precursor of calcitonin that is produced by C cells of the thyroid and by neuroendocrine cells of the lung and intestine in response to bacterial toxin. In contrast, procalcitonin levels are down-regulated in viral infection.
Levels of procalcitonin increase 6 to 12 hours after stimulation, and the half-life is roughly 24 hours.1 This suggests levels should decrease by one-half daily if an infection is controlled and is responding to therapy (assuming normal clearance).
The test costs about $25, with a turnaround time of 20 to 60 minutes, or longer at institutions that send the test out or run the tests in batches.
Point-of-care procalcitonin testing is emerging but not yet commercially available in the United States. Despite extensive observational studies and randomized controlled trials over the past 20 years, procalcitonin’s physiologic role remains unclear. The large body of evidence of the clinical utility of procalcitonin measurement has been summarized in several meta-analyses in different diseases.
PROCALCITONIN TESTING IN SEPSIS
Trials of procalcitonin testing have had slightly different inclusion criteria that commonly overlap with similar diagnoses. Sepsis is the broadest cohort studied.
The Procalcitonin to Reduce Antibiotic Treatments in Acutely Ill Patients (PRORATA) trial2 randomized 621 patients admitted to the intensive care unit (ICU) with suspected bacterial infections to antibiotic therapy guided by procalcitonin concentrations or to antibiotic therapy based on current guidelines. The source of infection varied, but 73% of patients had pulmonary infections.The procalcitonin algorithm was as follows:
- Starting antibiotics was discouraged if the procalcitonin concentration was less than 0.5 ng/mL, and strongly discouraged if less than 0.25 ng/mL
- Starting antibiotics was encouraged if the concentration was 0.5 ng/mL or higher, and strongly encouraged if 1 ng/mL or higher
- Stopping antibiotics was encouraged if the concentration dropped by at least 80% from the peak level or to a level greater than or equal to 0.25 ng/mL; stopping was strongly encouraged if the concentration fell below 0.25 ng/mL.
There was also guidance to change antibiotics if procalcitonin increased on therapy and was above 0.5 ng/mL.
Although the study physicians generally followed the algorithm, they were allowed to override it based on clinical judgment. The main results were that the number of days without antibiotics was higher in the procalcitonin group than in the controls (14.3 vs 11.6 days), with no other statistically significant difference between groups. These findings supported the idea that procalcitonin can guide clinicians to safely “deprescribe” antibiotics.
The Stop Antibiotics on Guidance of Procalcitonin Study (SAPS),3 published in 2016, was a larger trial with similar design, in 1,575 patients admitted to the ICU with suspected infection. Antibiotic use was less and the 28-day mortality rate was lower with procalcitonin guidance: 20% vs 25% in the intention-to-treat analysis.
ACUTE RESPIRATORY TRACT INFECTION
The Procalcitonin Guided Antibiotic Therapy and Hospitalisation in Patients With Lower Respiratory Tract Infections (ProHOSP) trial4 randomized 1,381 patients to antibiotic therapy guided by procalcitonin levels or standard guidelines. Most patients had community-acquired pneumonia, while the rest had exacerbations of COPD, acute bronchitis, or other lower respiratory tract infections.
In the study algorithm, starting or continuing antibiotics was discouraged if procalcitonin levels were 0.25 ng/mL or less, and strongly discouraged if less than 0.1 ng/mL. Starting or continuing antibiotics was encouraged if levels were greater than 0.25 ng/mL, and strongly encouraged if greater than 0.5 ng/mL.
The algorithm recommended stopping antibiotics if procalcitonin levels fell below 0.25 ng/mL or decreased by 80%, and strongly recommended stopping them if procalcitonin fell below 0.1 ng/mL or decreased by 90%.
The treating physician could override the algorithm if the patient was unstable, was in an ICU, or had Legionella infection.
Antibiotic use was less in the procalcitonin-guided arm (75.4% vs 87.7%; mean duration 5.7 days vs 8.7 days), as was the rate of adverse effects from antibiotics (19.8% vs 28.1%). Rates of recurrence or rehospitalization were also lower with procalcitonin guidance (3.7% vs 6.5%), presumably because of fewer antibiotic-related side effects or better diagnostic accuracy. Rates of death and ICU admission were similar in the 2 groups. These findings were similar to those of PRORATA and SAPS, demonstrating that guidance with procalcitonin levels decreased antibiotic utilization, with other outcomes either improved or unchanged.
Schuetz et al,5 in a 2018 meta-analysis, collected data on 6,708 patients from 26 trials in 12 countries and found that procalcitonin guidance decreased antibiotic exposure by 2.4 days and reduced the rate of antibiotic-related side effects (16% vs 22%). Although there was skepticism about the mortality benefit reported in the SAPS trial, a similar mortality benefit was found in this meta-analysis (30-day mortality rates were 9% vs 10%), suggesting that measuring procalcitonin not only reduces unnecessary antibiotic exposure, but also saves lives.
Although decreasing antibiotic exposure may not confer a survival benefit, procalcitonin guidance likely clarifies the diagnosis and thus expedites proper treatment in patients with sepsis-like syndromes that are actually due to a noninfectious pathology (eg, pulmonary embolism, myocardial infarction, adrenal insufficiency).
Negative findings in ProACT
The Procalcitonin Antibiotic Consensus Trial (ProACT)6 subsequently reported findings discordant with those above but was flawed in that adherence to the procalcitonin guideline by physicians was only 62% in the subgroup of patients with low procalcitonin results, which accounted for almost 90% of patients. Overall adherence by physicians to the procalcitonin guideline was 65%, much lower than in other trials (ProHOSP had over 90% adherence).4 Further, ProACT was done in American centers unfamiliar with procalcitonin, and it seems they did not trust low procalcitonin values as a reason to stop or avoid antibiotics.
ACUTE EXACERBATIONS OF COPD
Multiple small randomized controlled trials and subgroups of larger studies like ProHOSP have studied the use of procalcitonin in acute exacerbations of COPD. Most studies used a design similar to the algorithm in ProHOSP.
Mathioudakis et al,7 in a meta-analysis of 8 trials with a total of 1,062 patients with acute exacerbation of COPD, found that with procalcitonin guidance, prescription of antibiotics on admission decreased by almost one-half, and courses of antibiotics were approximately 4 days shorter without any statistically significant difference in rates of treatment failure, length of hospital stay, recurrence, rehospitalization, or overall mortality.
However, the quality of the studies included in the meta-analysis was deemed only low to moderate, and thus the authors concluded, “Procalcitonin-based protocols appear to be clinically effective; however, confirmatory trials with rigorous methodology are required.”7 Nonetheless, given the lack of data supporting current practices for patient selection for antibiotics in COPD exacerbations, a strategy involving procalcitonin seems to be reasonable.
BACTEREMIA
Observational studies from as far as back as 1999 have examined the association of procalcitonin levels with bacteremia. The study designs were generally similar, with procalcitonin levels checked at time of blood culture, mostly in emergency rooms, and the procalcitonin value correlated with blood culture results. The general conclusion has been that procalcitonin has diagnostic value in ruling out bacteremia but should be used in the context of pretest probability rather than in isolation.
Hattori et al8 performed one of the largest studies, in 1,331 patients, using a procalcitonin level cutoff of 0.9 ng/mL. The sensitivity was 72% and specificity was 69%, which are not impressive; however, the negative predictive value was 95%, and even higher at lower cutoff values. Further, procalcitonin was significantly better at predicting bacteremia than either the white blood cell count or C-reactive protein level, with the latter two being hardly better than random chance.
Hoeboer et al9 performed a meta-analysis of various studies with a total of 16,514 patients. Using a cutoff of 0.5 ng/mL, they reported a sensitivity of 76% and a specificity of 69% with a negative predictive value of 97% in emergency rooms, 95% on regular wards, and 98% in ICUs. The high negative predictive value of procalcitonin can allow clinicians to stratify bacteremia risk to determine which patients need blood cultures, which in turn may help clinicians order blood cultures more appropriately and avoid unnecessary costs, delays, and harms associated with false-positive results, such as additional visits, additional testing, and unnecessary use of antibiotics.
MENINGITIS
As with bacteremia, observational studies have reported fairly high negative predictive values for procalcitonin in bacterial meningitis. The correlation is not surprising, given that most cases of bacterial meningitis occur due to hematogenous dissemination.
A 2015 meta-analysis of 9 studies and 725 patients reported a pooled sensitivity of 90%, specificity 90%, positive likelihood ratio 27.3, and negative likelihood ratio 0.13.10 Cutoffs for procalcitonin levels varied, but the most common value was 0.5 ng/mL. The authors also noted that the diagnostic utility of procalcitonin was far superior to C-reactive protein in this scenario, concluding that serum procalcitonin is a highly accurate test to distinguish between bacterial and viral causes in suspected meningitis.10
OTHER CLINICAL APPLICATIONS
Postoperative infection
Small studies have assessed procalcitonin as a marker to rule out postoperative infections,11,12 but the heterogeneity of study designs and populations makes it difficult to combine the studies for meta-analysis. Nevertheless, the general trend is that there may be a role for procalcitonin, and that procalcitonin has better diagnostic yield than the white blood cell count or C-reactive protein level. The optimal cutoff depends on the surgery, since a small elevation in procalcitonin can be expected with the stress of surgery; and since the degree of elevation varies with type of surgery, the result must be interpreted with caution.
Malignancy
In malignancy-associated conditions such as neutropenic fever and tumor fever, the clinical utility of procalcitonin is somewhat diminished, as malignancy can cause elevated procalcitonin levels (especially in metastatic disease), but a low concentration still has a fair negative predictive value (approximately 90%) for bloodstream infections.13
A retrospective study suggested that the ratio of procalcitonin to C-reactive protein could improve diagnostic accuracy in patients with malignancies, presumably because an elevation of procalcitonin out of proportion to elevation in C-reactive protein favored a bacterial infection rather than nonspecific inflammation related to malignancy.14
Cardiac syndromes
In cardiac syndromes, dyspnea and abnormal chest imaging may make it difficult to exclude respiratory infections. Schuetz et al15 reviewed the potential value of procalcitonin testing in a variety of cardiac disorders, especially in acute cardiovascular conditions whose presentation resembles that of sepsis or acute respiratory tract infection. They concluded it may have a role in diagnosis and prognosis in these settings, as well as guiding drug therapy.
Localized infections
Though localized infections such as cystitis, cellulitis, and osteomyelitis often do not affect procalcitonin levels, the test may help assess illness severity and rule out associated bacteremia.
One study found that a low procalcitonin level was insufficient to rule out urinary tract infection, but procalcitonin levels predicted bacteremia better than any other variable or combination of variables; moreover, procalcitonin had a negative predictive value as high as 97% for ruling out bacteremia associated with urinary tract infection.16
ROLE IN PROGNOSIS
In addition to being a useful marker for diagnosis of bacterial infections, the procalcitonin level has significant prognostic implications, as a high or persistently elevated level correlates with a higher rate of all-cause mortality.17 The prognostic capability may enhance triage decisions.
Because the procalcitonin level lacks specificity, clinicians need to be aware of noninfectious causes of elevations such as malignancy, surgery, impaired renal function,8 and myocardial infarction.18 In these scenarios, it is important to think critically about the procalcitonin result and consider an adjusted cutoff.
A study of procalcitonin to predict a positive blood culture in patients with renal disease suggested an optimal cutoff value of 1.06 ng/mL for patients with an estimated glomerular filtration rate of 30 to 60 mL/min/1.73m2, and a value of 2.50 ng/mL for a rate less than 30 mL/min/1.73m2.8
In a chronic process like malignancy, the procalcitonin level is usually not markedly elevated. But it can also remain persistently elevated, with no improvement associated with effective antibiotic treatment and no clinical deterioration associated with treatment failure.
Use of procalcitonin and troponin
For some patients, there may be diagnostic uncertainty about interpreting procalcitonin and troponin results, as both plaque-rupture myocardial infarction and demand ischemia from sepsis can cause elevation in both values. In a study of patients with acute myocardial infarction, the procalcitonin level peaked at 3.57 ng/mL and troponin peaked at 60 ng/mL at about 24 hours after admission.18 This suggests that a troponin-to-procalcitonin ratio may help distinguish acute myocardial infarction from demand ischemia, though the optimal cutoff is unknown.
Both troponin and procalcitonin levels can help rule out acute severe illness (eg, bloodstream infection, acute myocardial infarction). But both can be falsely negative in early presentation or in less severe disease (eg, localized infection, unstable angina), as well as in noninfectious inflammation and nonobstructive myocardial injury.
Both are important prognostic markers. Furthermore, both can be chronically elevated in patients with renal disease, but both still have a characteristic rise and fall in acute disease states. But neither should be used in isolation without information from electrocardiography, other tests, and the clinical context.
CAVEATS AND CHALLENGES
Based on clinical experience and reported studies, procalcitonin testing has proven valuable in the diagnosis, prognosis, and management of a range of diseases, particularly certain infections.
However, procalcitonin testing must be applied cautiously and judiciously. There is a potential for early false-negative results, and false-positive results can occur in conditions such as kidney disease, myocardial infarction, postoperative stress response, and malignancy, though there may be ways to factor these conditions into interpretation of procalcitonin results.
Widespread procalcitonin testing may lead to excessive costs, though the cost for each test is reasonable and probably offset by benefits of diagnostic clarification and decreased use of antibiotics, if appropriately applied.
The primary roles for procalcitonin testing are to rule out infection in patients with low probability of infection and to allow safe early cessation of antibiotic therapy in patients with presumed bacterial infection. Procalcitonin testing can enable providers to stop antibiotics safely, with the general trend showing decreased antibiotic utilization without patient harm. This can result in healthcare cost savings and improved patient outcomes such as decreased length of hospital stay, decreased readmission rates, fewer adverse effects from antibiotics, and possibly improved mortality rates.
Despite the potential benefits from procalcitonin testing, results must be interpreted within the clinical context because a host of factors can affect the values. Extreme values are more useful than intermediate values, which are difficult to interpret and have poor predictive value.
Although all current biomarkers for infection are imperfect, procalcitonin appears to have better diagnostic accuracy than other markers such as the white blood cell count and C-reactive protein in multiple clinical scenarios, and its appropriate use appears to improve important outcomes such as survival.
- Schuetz P, Albrich W, Mueller B. Procalcitonin for diagnosis of infection and guide to antibiotic decisions: past, present and future. BMC Med 2011; 9:107. doi:10.1186/1741-7015-9-107
- Bouadma L, Luyt CE, Tubach F, et al; PRORATA trial group. Use of procalcitonin to reduce patients' exposure to antibiotics in intensive care units (PRORATA trial): a multicentre randomised controlled trial. Lancet 2010; 375(9713):463–474. doi:10.1016/S0140-6736(09)61879-1
- de Jong E, van Oers JA, Beishuizen A, et al. Efficacy and safety of procalcitonin guidance in reducing the duration of antibiotic treatment in critically ill patients: a randomised, controlled, open-label trial. Lancet Infect Dis 2016; 16(7):819–827. doi:10.1016/S1473-3099(16)00053-0
- Schuetz P, Christ-Crain M, Thomann R, et al; ProHOSP Study Group. Effect of procalcitonin-based guidelines vs standard guidelines on antibiotic use in lower respiratory tract infections: the ProHOSP randomized controlled trial. JAMA 2009; 302(10):1059–1066. doi:10.1001/jama.2009.1297
- Schuetz P, Wirz Y, Sager R, et al. Effect of procalcitonin-guided antibiotic treatment on mortality in acute respiratory infections: a patient level meta-analysis. Lancet Infect Dis 2018; 18(1):95–107. doi:10.1016/S1473-3099(17)30592-3
- Huang DT, Yealy DM, Filbin MR, et al; ProACT Investigators. Procalcitonin-guided use of antibiotics for lower respiratory tract infection. N Engl J Med 2018; 379(3):236–249. doi:10.1056/NEJMoa1802670
- Mathioudakis AG, Chatzimavridou-Grigoriadou V, Corlateanu A, Vestbo J. Procalcitonin to guide antibiotic administration in COPD exacerbations: a meta-analysis. Eur Respir Rev 2017; 26(143)pii:160073. doi:10.1183/16000617.0073-2016
- Hattori T, Nishiyama H, Kato H, et al. Clinical value of procalcitonin for patients with suspected bloodstream infection. Am J Clin Pathol 2014; 141(1):43–51. doi:10.1309/AJCP4GV7ZFDTANGC
- Hoeboer SH, van der Geest PJ, Nieboer D, Groeneveld AB. The diagnostic accuracy of procalcitonin for bacteraemia: a systematic review and meta-analysis. Clin Microbiol Infect 2015; 21(5):474–481. doi:10.1016/j.cmi.2014.12.026
- Vikse J, Henry BM, Roy J, Ramakrishnan PK, Tomaszewski KA, Walocha JA. The role of serum procalcitonin in the diagnosis of bacterial meningitis in adults: a systematic review and meta-analysis. Int J Infect Dis 2015; 38:68–76. doi:10.1016/j.ijid.2015.07.011
- Aouifi A, Piriou V, Bastien O, et al. Usefulness of procalcitonin for diagnosis of infection in cardiac surgical patients. Crit Care Med 2000; 28(9):3171–3176. pmid:11008977
- Hunziker S, Hugle T, Schuchardt K, et al. The value of serum procalcitonin level for differentiation of infectious from noninfectious causes of fever after orthopaedic surgery. J Bone Joint Surg Am 2010; 92(1):138–148. doi:10.2106/JBJS.H.01600
- Shomali W, Hachem R, Chaftari AM, et al. Can procalcitonin distinguish infectious fever from tumor-related fever in non-neutropenic cancer patients? Cancer 2012; 118(23):5823–5829. doi:10.1002/cncr.27602
- Hangai S, Nannya Y, Kurokawa M. Role of procalcitonin and C-reactive protein for discrimination between tumor fever and infection in patients with hematological diseases. Leuk Lymphoma 2015; 56(4):910–914. doi:10.3109/10428194.2014.938329
- Schuetz P, Daniels LB, Kulkarni P, Anker SD, Mueller B. Procalcitonin: a new biomarker for the cardiologist. Int J Cardiol 2016; 223:390–397. doi:10.1016/j.ijcard.2016.08.204
- van Nieuwkoop C, Bonten TN, van't Wout JW, et al. Procalcitonin reflects bacteremia and bacterial load in urosepsis syndrome: a prospective observational study. Crit Care 2010; 14(6):R206. doi:10.1186/cc9328
- Liu D, Su L, Han G, Yan P, Xie L. Prognostic value of procalcitonin in adult patients with sepsis: a systematic review and meta-analysis. PLoS One 2015; 10(6):e0129450. doi:10.1371/journal.pone.0129450
- Kafkas N, Venetsanou K, Patsilinakos S, et al. Procalcitonin in acute myocardial infarction. Acute Card Care 2008; 10(1):30–36. doi:10.1080/17482940701534800
- Schuetz P, Albrich W, Mueller B. Procalcitonin for diagnosis of infection and guide to antibiotic decisions: past, present and future. BMC Med 2011; 9:107. doi:10.1186/1741-7015-9-107
- Bouadma L, Luyt CE, Tubach F, et al; PRORATA trial group. Use of procalcitonin to reduce patients' exposure to antibiotics in intensive care units (PRORATA trial): a multicentre randomised controlled trial. Lancet 2010; 375(9713):463–474. doi:10.1016/S0140-6736(09)61879-1
- de Jong E, van Oers JA, Beishuizen A, et al. Efficacy and safety of procalcitonin guidance in reducing the duration of antibiotic treatment in critically ill patients: a randomised, controlled, open-label trial. Lancet Infect Dis 2016; 16(7):819–827. doi:10.1016/S1473-3099(16)00053-0
- Schuetz P, Christ-Crain M, Thomann R, et al; ProHOSP Study Group. Effect of procalcitonin-based guidelines vs standard guidelines on antibiotic use in lower respiratory tract infections: the ProHOSP randomized controlled trial. JAMA 2009; 302(10):1059–1066. doi:10.1001/jama.2009.1297
- Schuetz P, Wirz Y, Sager R, et al. Effect of procalcitonin-guided antibiotic treatment on mortality in acute respiratory infections: a patient level meta-analysis. Lancet Infect Dis 2018; 18(1):95–107. doi:10.1016/S1473-3099(17)30592-3
- Huang DT, Yealy DM, Filbin MR, et al; ProACT Investigators. Procalcitonin-guided use of antibiotics for lower respiratory tract infection. N Engl J Med 2018; 379(3):236–249. doi:10.1056/NEJMoa1802670
- Mathioudakis AG, Chatzimavridou-Grigoriadou V, Corlateanu A, Vestbo J. Procalcitonin to guide antibiotic administration in COPD exacerbations: a meta-analysis. Eur Respir Rev 2017; 26(143)pii:160073. doi:10.1183/16000617.0073-2016
- Hattori T, Nishiyama H, Kato H, et al. Clinical value of procalcitonin for patients with suspected bloodstream infection. Am J Clin Pathol 2014; 141(1):43–51. doi:10.1309/AJCP4GV7ZFDTANGC
- Hoeboer SH, van der Geest PJ, Nieboer D, Groeneveld AB. The diagnostic accuracy of procalcitonin for bacteraemia: a systematic review and meta-analysis. Clin Microbiol Infect 2015; 21(5):474–481. doi:10.1016/j.cmi.2014.12.026
- Vikse J, Henry BM, Roy J, Ramakrishnan PK, Tomaszewski KA, Walocha JA. The role of serum procalcitonin in the diagnosis of bacterial meningitis in adults: a systematic review and meta-analysis. Int J Infect Dis 2015; 38:68–76. doi:10.1016/j.ijid.2015.07.011
- Aouifi A, Piriou V, Bastien O, et al. Usefulness of procalcitonin for diagnosis of infection in cardiac surgical patients. Crit Care Med 2000; 28(9):3171–3176. pmid:11008977
- Hunziker S, Hugle T, Schuchardt K, et al. The value of serum procalcitonin level for differentiation of infectious from noninfectious causes of fever after orthopaedic surgery. J Bone Joint Surg Am 2010; 92(1):138–148. doi:10.2106/JBJS.H.01600
- Shomali W, Hachem R, Chaftari AM, et al. Can procalcitonin distinguish infectious fever from tumor-related fever in non-neutropenic cancer patients? Cancer 2012; 118(23):5823–5829. doi:10.1002/cncr.27602
- Hangai S, Nannya Y, Kurokawa M. Role of procalcitonin and C-reactive protein for discrimination between tumor fever and infection in patients with hematological diseases. Leuk Lymphoma 2015; 56(4):910–914. doi:10.3109/10428194.2014.938329
- Schuetz P, Daniels LB, Kulkarni P, Anker SD, Mueller B. Procalcitonin: a new biomarker for the cardiologist. Int J Cardiol 2016; 223:390–397. doi:10.1016/j.ijcard.2016.08.204
- van Nieuwkoop C, Bonten TN, van't Wout JW, et al. Procalcitonin reflects bacteremia and bacterial load in urosepsis syndrome: a prospective observational study. Crit Care 2010; 14(6):R206. doi:10.1186/cc9328
- Liu D, Su L, Han G, Yan P, Xie L. Prognostic value of procalcitonin in adult patients with sepsis: a systematic review and meta-analysis. PLoS One 2015; 10(6):e0129450. doi:10.1371/journal.pone.0129450
- Kafkas N, Venetsanou K, Patsilinakos S, et al. Procalcitonin in acute myocardial infarction. Acute Card Care 2008; 10(1):30–36. doi:10.1080/17482940701534800
How should I treat acute agitation in pregnancy?
Acute agitation in the pregnant patient should be treated as an obstetric emergency, as it jeopardizes the safety of the patient and fetus, as well as others in the emergency room. Uncontrolled agitation is associated with obstetric complications such as preterm delivery, placental abnormalities, postnatal death, and spontaneous abortion.1
Current data on the reproductive safety of drugs commonly used to treat acute agitation—benzodiazepines, typical (first-generation) antipsychotics, atypical (second-generation) antipsychotics, and diphenhydramine—suggest no increase in risk beyond the 2% to 3% risk of congenital malformations in the general population when used in the first trimester.2,3
FOCUS OF THE EMERGENCY EVALUATION
Agitation is defined as the physical manifestation of internal distress, due to an underlying medical condition such as delirium or to a psychiatric condition such as acute intoxication or withdrawal, psychosis, mania, or personality disorder.4
For the agitated pregnant woman who is not belligerent at presentation, triage should start with a basic assessment of airways, breathing, and circulation, as well as vital signs and glucose level.5 A thorough medical history and a description of events leading to the presentation, obtained from the patient or the patient’s family or friends, are vital for narrowing the diagnosis and deciding treatment.
The initial evaluation should include consideration of delirium, trauma, intracranial hemorrhage, coagulopathy, thrombocytopenia, amniotic and venous thromboembolism, hypoxia and hypercapnia, and signs and symptoms of intoxication or withdrawal from substances such as alcohol, cocaine, phencyclidine, methamphetamine, and substituted cathinones (“bath salts”). From 20 weeks of gestation to 6 weeks postpartum, eclampsia should also be considered in the differential diagnosis.1 Ruling out these conditions is important since the management of each differs vastly from the protocol for agitation secondary to psychosis, mania, or delirium.
NEW SYSTEM TO DETERMINE RISK DURING PREGNANCY, LACTATION
The US Food and Drug Administration (FDA) has discontinued its pregnancy category labeling system that used the letters A, B, C, D, and X to convey reproductive and lactation safety. The new system, established under the FDA Pregnancy and Lactation Labeling Rule,6 provides descriptive, up-to-date explanations of risk, as well as previously absent context regarding baseline risk for major malformations in the general population to help with informed decision-making.7 This allows the healthcare provider to interpret the risk for an individual patient.
FIRST-GENERATION ANTIPSYCHOTICS SAFE, EFFECTIVE IN PREGNANCY
Reproductive safety of first-generation (ie, typical) neuroleptics such as haloperidol is supported by extensive data accumulated over the past 50 years.2,3,8 No significant teratogenic effect has been documented with this drug class,7 although a 1996 meta-analysis found a small increase in the relative risk of congenital malformations in offspring exposed to low-potency antipsychotics compared with those exposed to high-potency antipsychotics.2
In general, mid- and high-potency antipsychotics (eg, haloperidol, perphenazine) are often recommended because they are less likely to have associated sedative or hypotensive effects than low-potency antipsychotics (eg, chlorpromazine, perphenazine), which may be a significant consideration for a pregnant patient.2,8
There is a theoretical risk of neonatal extrapyramidal symptoms with exposure to first-generation antipsychotics in the third trimester, but the data to support this are from sparse case reports and small observational cohorts.9
NEWER ANTIPSYCHOTICS ALSO SAFE IN PREGNANCY
Newer antipsychotics such as the second-generation antipsychotics, available since the mid-1990s, are increasingly used as primary or adjunctive therapy across a wide range of psychiatric disorders.10 Recent data from large, prospective cohort studies investigating reproductive safety of these agents are reassuring, with no specific patterns of organ malformation.11,12
DIPHENHYDRAMINE
Recent studies of antihistamines such as diphenhydramine have not reported any risk of major malformations with first-trimester exposure to antihistamines.13,14 Dose-dependent anticholinergic adverse effects of antihistamines can induce or exacerbate delirium and agitation, although these effects are classically seen in elderly, nonpregnant patients.15 Thus, given the paucity of adverse effects and the low risk, diphenhydramine is considered safe to use in pregnancy.13
BENZODIAZEPINES
Benzodiazepines are not contraindicated for the treatment of acute agitation in pregnancy.16 Reproductive safety data from meta-analyses and large population-based cohort studies have found no evidence of increased risk of major malformations in neonates born to mothers on prescription benzodiazepines in the first trimester.17,18 While third-trimester exposure to benzodiazepines has been associated with “floppy-baby” syndrome and neonatal withdrawal syndrome,16 these are more likely to occur in women on long-term prescription benzodiazepine therapy. No study has yet assessed the risk of these outcomes with a 1-time acute exposure in the emergency department; however, the risk is likely minimal given the aforementioned data observed in women on long-term prescription benzodiazepine therapy.
STEPWISE MANAGEMENT OF AGITATION IN PREGNANCY
If untreated, agitation in pregnancy is independently associated with outcomes that include premature delivery, low birth weight, growth retardation, postnatal death, and spontaneous abortion.1 The risk of these outcomes greatly outweighs any potential risk from psychotropic medications during pregnancy.
Nevertheless, intervention should progress in a stepwise manner, starting with the least restrictive and progressing toward more restrictive interventions, including pharmacotherapy, use of a seclusion room, and physical restraints (Figure 1).4,19
Before medications are considered, attempts should be made to engage with and “de-escalate” the patient in a safe, nonstimulating environment.19 If this approach is not effective, the patient should be offered oral medications to help with her agitation. However, if the patient’s behavior continues to escalate, presenting a danger to herself or staff, the use of emergency medications is clearly indicated. Providers should succinctly inform the patient of the need for immediate intervention.
If the patient has had a good response in the past to one of these medications or is currently taking one as needed, the same medication should be offered. If the patient has never been treated for agitation, it is important to consider the presenting symptoms, differential diagnosis, and the route and rapidity of administration of medication. If the patient has experienced a fall or other trauma, confirming a viable fetal heart rate between 10 to 22 weeks of gestation with Doppler ultrasonography and obstetric consultation should be considered.
DRUG THERAPY RECOMMENDATIONS
Mild to moderate agitation in pregnancy should be managed conservatively with diphenhydramine. Other options include a benzodiazepine, particularly lorazepam, if alcohol withdrawal is suspected. A second-generation antipsychotic such as olanzapine in a rapidly dissolving form or ziprasidone is another option if a rapid response is required.20Table 1 provides a summary of pharmacotherapy recommendations.
Severe agitation may require a combination of agents. A commonly used, safe regimen—colloquially called the “B52 bomb”—is haloperidol 5 mg, lorazepam 2 mg, and diphenhydramine 25 to 50 mg for prophylaxis of dystonia.20
The patient’s response should be monitored closely, as dosing may require modification as a result of pregnancy-related changes in drug distribution, metabolism, and clearance.21
Although no study to our knowledge has assessed risk associated with 1-time exposure to any of these classes of medications in pregnant women, the aforementioned data on long-term exposure provide reassurance that single exposure in emergency departments likely has little or no effect for the developing fetus.
PHYSICAL RESTRAINTS FOR AGITATION IN PREGNANCY
Physical restraints along with emergency medications (ie, chemical restraint) may be indicated when the patient poses a danger to herself or others. In some cases, both types of restraint may be required, whether in the emergency room or an inpatient setting.
However, during the second and third trimesters, physical restraints such as 4-point restraints may predispose the patient to inferior vena cava compression syndrome and compromise placental blood flow.4 Therefore, pregnant patients after 20 weeks of gestation should be positioned in the left lateral decubitus position, with the right hip positioned 10 to 12 cm off the bed with pillows or blankets. And when restraints are used in pregnant patients, frequent checking of vital signs and physical assessment is needed to mitigate risks.4
- Aftab A, Shah AA. Behavioral emergencies: special considerations in the pregnant patient. Psychiatr Clin North Am 2017; 40(3):435–448. doi:10.1016/j.psc.2017.05.017
- Altshuler LL, Cohen L, Szuba MP, Burt VK, Gitlin M, Mintz J. Pharmacologic management of psychiatric illness during pregnancy: dilemmas and guidelines. Am J Psychiatry 1996; 153(5):592–606. doi:10.1176/ajp.153.5.592
- Einarson A. Safety of psychotropic drug use during pregnancy: a review. MedGenMed 2005; 7(4):3. pmid:16614625
- Wilson MP, Nordstrom K, Shah AA, Vilke GM. Psychiatric emergencies in pregnant women. Emerg Med Clin North Am 2015; 33(4):841–851. doi:10.1016/j.emc.2015.07.010
- Brown HE, Stoklosa J, Freundenreich O. How to stabilize an acutely psychotic patient. Curr Psychiatry 2012; 11(12):10–16.
- US Food and Drug Administration. Pregnancy and lactation labeling (drugs) final rule. www.fda.gov/drugs/developmentapprovalprocess/developmentresources/labeling/ucm093307.htm. Accessed January 8, 2019.
- Brucker MC, King TL. The 2015 US Food and Drug Administration pregnancy and lactation labeling rule. J Midwifery Womens Health 2017; 62(3):308–316. doi:10.1111/jmwh.12611
- Diav-Citrin O, Shechtman S, Ornoy S, et al. Safety of haloperidol and penfluridol in pregnancy: a multicenter, prospective, controlled study. J Clin Psychiatry 2005; 66(3):317–322. pmid:15766297
- Galbally M, Snellen M, Power J. Antipsychotic drugs in pregnancy: a review of their maternal and fetal effects. Ther Adv Drug Saf 2014; 5(2):100–109. doi:10.1177/2042098614522682
- Kulkarni J, Storch A, Baraniuk A, Gilbert H, Gavrilidis E, Worsley R. Antipsychotic use in pregnancy. Expert Opin Pharmacother 2015; 16(9):1335–1345. doi:10.1517/14656566.2015.1041501
- Huybrechts KF, Hernández-Díaz S, Patorno E, et al. Antipsychotic use in pregnancy and the risk for congenital malformations. JAMA Psychiatry 2016; 73(9):938–946. doi:10.1001/jamapsychiatry.2016.1520
- Cohen LS, Viguera AC, McInerney KA, et al. Reproductive safety of second-generation antipsychotics: current data from the Massachusetts General Hospital national pregnancy registry for atypical antipsychotics. Am J Psychiatry 2016; 173(3):263–270. doi:10.1176/appi.ajp.2015.15040506
- Li Q, Mitchell AA, Werler MM, Yau WP, Hernández-Díaz S. Assessment of antihistamine use in early pregnancy and birth defects. J Allergy Clin Immunol Pract 2013; 1(6):666–674.e1. doi:10.1016/j.jaip.2013.07.008
- Gilboa SM, Strickland MJ, Olshan AF, Werler MM, Correa A; National Birth Defects Prevention Study. Use of antihistamine medications during early pregnancy and isolated major malformations. Birth Defects Res A Clin Mol Teratol 2009; 85(2):137–150. doi:10.1002/bdra.20513
- Meuleman JR. Association of diphenhydramine use with adverse effects in hospitalized older patients: possible confounders. Arch Intern Med 2002; 162(6):720–721. pmid:11911733
- Enato E, Moretti M, Koren G. The fetal safety of benzodiazepines: an updated meta-analysis. J Obstet Gynaecol Can 2011; 33(1):46–48. doi:10.1016/S1701-2163(16)34772-7
- Dolovich LR, Addis A, Vaillancourt JM, Power JD, Koren G, Einarson TR. Benzodiazepine use in pregnancy and major malformations or oral cleft: meta-analysis of cohort and case-control studies. BMJ 1998; 317(7162):839–843. pmid:9748174
- Bellantuono C, Tofani S, Di Sciascio G, Santone G. Benzodiazepine exposure in pregnancy and risk of major malformations: a critical overview. Gen Hosp Psychiatry 2013; 35(1):3–8. doi:10.1016/j.genhosppsych.2012.09.003
- Richmond JS, Berlin JS, Fishkind AB, et al. Verbal de-escalation of the agitated patient: consensus statement of the American Association for Emergency Psychiatry project BETA De-escalation Workgroup. West J Emerg Med 2012; 13(1):17–25. doi:10.5811/westjem.2011.9.6864
- Prager LM, Ivkovic A. Emergency psychiatry. In: Stern TA, Fava M, Wilens TE, Rosenbaum JF, eds. The Massachusetts General Hospital Comprehensive Clinical Psychiatry. 2nd ed. London: Elsevier; 2016:937–949.
- Feghali M, Venkataramanan R, Caritis S. Pharmacokinetics of drugs in pregnancy. Semin Perinatol 2015; 39(7):512–519. doi:10.1053/j.semperi.2015.08.003
Acute agitation in the pregnant patient should be treated as an obstetric emergency, as it jeopardizes the safety of the patient and fetus, as well as others in the emergency room. Uncontrolled agitation is associated with obstetric complications such as preterm delivery, placental abnormalities, postnatal death, and spontaneous abortion.1
Current data on the reproductive safety of drugs commonly used to treat acute agitation—benzodiazepines, typical (first-generation) antipsychotics, atypical (second-generation) antipsychotics, and diphenhydramine—suggest no increase in risk beyond the 2% to 3% risk of congenital malformations in the general population when used in the first trimester.2,3
FOCUS OF THE EMERGENCY EVALUATION
Agitation is defined as the physical manifestation of internal distress, due to an underlying medical condition such as delirium or to a psychiatric condition such as acute intoxication or withdrawal, psychosis, mania, or personality disorder.4
For the agitated pregnant woman who is not belligerent at presentation, triage should start with a basic assessment of airways, breathing, and circulation, as well as vital signs and glucose level.5 A thorough medical history and a description of events leading to the presentation, obtained from the patient or the patient’s family or friends, are vital for narrowing the diagnosis and deciding treatment.
The initial evaluation should include consideration of delirium, trauma, intracranial hemorrhage, coagulopathy, thrombocytopenia, amniotic and venous thromboembolism, hypoxia and hypercapnia, and signs and symptoms of intoxication or withdrawal from substances such as alcohol, cocaine, phencyclidine, methamphetamine, and substituted cathinones (“bath salts”). From 20 weeks of gestation to 6 weeks postpartum, eclampsia should also be considered in the differential diagnosis.1 Ruling out these conditions is important since the management of each differs vastly from the protocol for agitation secondary to psychosis, mania, or delirium.
NEW SYSTEM TO DETERMINE RISK DURING PREGNANCY, LACTATION
The US Food and Drug Administration (FDA) has discontinued its pregnancy category labeling system that used the letters A, B, C, D, and X to convey reproductive and lactation safety. The new system, established under the FDA Pregnancy and Lactation Labeling Rule,6 provides descriptive, up-to-date explanations of risk, as well as previously absent context regarding baseline risk for major malformations in the general population to help with informed decision-making.7 This allows the healthcare provider to interpret the risk for an individual patient.
FIRST-GENERATION ANTIPSYCHOTICS SAFE, EFFECTIVE IN PREGNANCY
Reproductive safety of first-generation (ie, typical) neuroleptics such as haloperidol is supported by extensive data accumulated over the past 50 years.2,3,8 No significant teratogenic effect has been documented with this drug class,7 although a 1996 meta-analysis found a small increase in the relative risk of congenital malformations in offspring exposed to low-potency antipsychotics compared with those exposed to high-potency antipsychotics.2
In general, mid- and high-potency antipsychotics (eg, haloperidol, perphenazine) are often recommended because they are less likely to have associated sedative or hypotensive effects than low-potency antipsychotics (eg, chlorpromazine, perphenazine), which may be a significant consideration for a pregnant patient.2,8
There is a theoretical risk of neonatal extrapyramidal symptoms with exposure to first-generation antipsychotics in the third trimester, but the data to support this are from sparse case reports and small observational cohorts.9
NEWER ANTIPSYCHOTICS ALSO SAFE IN PREGNANCY
Newer antipsychotics such as the second-generation antipsychotics, available since the mid-1990s, are increasingly used as primary or adjunctive therapy across a wide range of psychiatric disorders.10 Recent data from large, prospective cohort studies investigating reproductive safety of these agents are reassuring, with no specific patterns of organ malformation.11,12
DIPHENHYDRAMINE
Recent studies of antihistamines such as diphenhydramine have not reported any risk of major malformations with first-trimester exposure to antihistamines.13,14 Dose-dependent anticholinergic adverse effects of antihistamines can induce or exacerbate delirium and agitation, although these effects are classically seen in elderly, nonpregnant patients.15 Thus, given the paucity of adverse effects and the low risk, diphenhydramine is considered safe to use in pregnancy.13
BENZODIAZEPINES
Benzodiazepines are not contraindicated for the treatment of acute agitation in pregnancy.16 Reproductive safety data from meta-analyses and large population-based cohort studies have found no evidence of increased risk of major malformations in neonates born to mothers on prescription benzodiazepines in the first trimester.17,18 While third-trimester exposure to benzodiazepines has been associated with “floppy-baby” syndrome and neonatal withdrawal syndrome,16 these are more likely to occur in women on long-term prescription benzodiazepine therapy. No study has yet assessed the risk of these outcomes with a 1-time acute exposure in the emergency department; however, the risk is likely minimal given the aforementioned data observed in women on long-term prescription benzodiazepine therapy.
STEPWISE MANAGEMENT OF AGITATION IN PREGNANCY
If untreated, agitation in pregnancy is independently associated with outcomes that include premature delivery, low birth weight, growth retardation, postnatal death, and spontaneous abortion.1 The risk of these outcomes greatly outweighs any potential risk from psychotropic medications during pregnancy.
Nevertheless, intervention should progress in a stepwise manner, starting with the least restrictive and progressing toward more restrictive interventions, including pharmacotherapy, use of a seclusion room, and physical restraints (Figure 1).4,19
Before medications are considered, attempts should be made to engage with and “de-escalate” the patient in a safe, nonstimulating environment.19 If this approach is not effective, the patient should be offered oral medications to help with her agitation. However, if the patient’s behavior continues to escalate, presenting a danger to herself or staff, the use of emergency medications is clearly indicated. Providers should succinctly inform the patient of the need for immediate intervention.
If the patient has had a good response in the past to one of these medications or is currently taking one as needed, the same medication should be offered. If the patient has never been treated for agitation, it is important to consider the presenting symptoms, differential diagnosis, and the route and rapidity of administration of medication. If the patient has experienced a fall or other trauma, confirming a viable fetal heart rate between 10 to 22 weeks of gestation with Doppler ultrasonography and obstetric consultation should be considered.
DRUG THERAPY RECOMMENDATIONS
Mild to moderate agitation in pregnancy should be managed conservatively with diphenhydramine. Other options include a benzodiazepine, particularly lorazepam, if alcohol withdrawal is suspected. A second-generation antipsychotic such as olanzapine in a rapidly dissolving form or ziprasidone is another option if a rapid response is required.20Table 1 provides a summary of pharmacotherapy recommendations.
Severe agitation may require a combination of agents. A commonly used, safe regimen—colloquially called the “B52 bomb”—is haloperidol 5 mg, lorazepam 2 mg, and diphenhydramine 25 to 50 mg for prophylaxis of dystonia.20
The patient’s response should be monitored closely, as dosing may require modification as a result of pregnancy-related changes in drug distribution, metabolism, and clearance.21
Although no study to our knowledge has assessed risk associated with 1-time exposure to any of these classes of medications in pregnant women, the aforementioned data on long-term exposure provide reassurance that single exposure in emergency departments likely has little or no effect for the developing fetus.
PHYSICAL RESTRAINTS FOR AGITATION IN PREGNANCY
Physical restraints along with emergency medications (ie, chemical restraint) may be indicated when the patient poses a danger to herself or others. In some cases, both types of restraint may be required, whether in the emergency room or an inpatient setting.
However, during the second and third trimesters, physical restraints such as 4-point restraints may predispose the patient to inferior vena cava compression syndrome and compromise placental blood flow.4 Therefore, pregnant patients after 20 weeks of gestation should be positioned in the left lateral decubitus position, with the right hip positioned 10 to 12 cm off the bed with pillows or blankets. And when restraints are used in pregnant patients, frequent checking of vital signs and physical assessment is needed to mitigate risks.4
Acute agitation in the pregnant patient should be treated as an obstetric emergency, as it jeopardizes the safety of the patient and fetus, as well as others in the emergency room. Uncontrolled agitation is associated with obstetric complications such as preterm delivery, placental abnormalities, postnatal death, and spontaneous abortion.1
Current data on the reproductive safety of drugs commonly used to treat acute agitation—benzodiazepines, typical (first-generation) antipsychotics, atypical (second-generation) antipsychotics, and diphenhydramine—suggest no increase in risk beyond the 2% to 3% risk of congenital malformations in the general population when used in the first trimester.2,3
FOCUS OF THE EMERGENCY EVALUATION
Agitation is defined as the physical manifestation of internal distress, due to an underlying medical condition such as delirium or to a psychiatric condition such as acute intoxication or withdrawal, psychosis, mania, or personality disorder.4
For the agitated pregnant woman who is not belligerent at presentation, triage should start with a basic assessment of airways, breathing, and circulation, as well as vital signs and glucose level.5 A thorough medical history and a description of events leading to the presentation, obtained from the patient or the patient’s family or friends, are vital for narrowing the diagnosis and deciding treatment.
The initial evaluation should include consideration of delirium, trauma, intracranial hemorrhage, coagulopathy, thrombocytopenia, amniotic and venous thromboembolism, hypoxia and hypercapnia, and signs and symptoms of intoxication or withdrawal from substances such as alcohol, cocaine, phencyclidine, methamphetamine, and substituted cathinones (“bath salts”). From 20 weeks of gestation to 6 weeks postpartum, eclampsia should also be considered in the differential diagnosis.1 Ruling out these conditions is important since the management of each differs vastly from the protocol for agitation secondary to psychosis, mania, or delirium.
NEW SYSTEM TO DETERMINE RISK DURING PREGNANCY, LACTATION
The US Food and Drug Administration (FDA) has discontinued its pregnancy category labeling system that used the letters A, B, C, D, and X to convey reproductive and lactation safety. The new system, established under the FDA Pregnancy and Lactation Labeling Rule,6 provides descriptive, up-to-date explanations of risk, as well as previously absent context regarding baseline risk for major malformations in the general population to help with informed decision-making.7 This allows the healthcare provider to interpret the risk for an individual patient.
FIRST-GENERATION ANTIPSYCHOTICS SAFE, EFFECTIVE IN PREGNANCY
Reproductive safety of first-generation (ie, typical) neuroleptics such as haloperidol is supported by extensive data accumulated over the past 50 years.2,3,8 No significant teratogenic effect has been documented with this drug class,7 although a 1996 meta-analysis found a small increase in the relative risk of congenital malformations in offspring exposed to low-potency antipsychotics compared with those exposed to high-potency antipsychotics.2
In general, mid- and high-potency antipsychotics (eg, haloperidol, perphenazine) are often recommended because they are less likely to have associated sedative or hypotensive effects than low-potency antipsychotics (eg, chlorpromazine, perphenazine), which may be a significant consideration for a pregnant patient.2,8
There is a theoretical risk of neonatal extrapyramidal symptoms with exposure to first-generation antipsychotics in the third trimester, but the data to support this are from sparse case reports and small observational cohorts.9
NEWER ANTIPSYCHOTICS ALSO SAFE IN PREGNANCY
Newer antipsychotics such as the second-generation antipsychotics, available since the mid-1990s, are increasingly used as primary or adjunctive therapy across a wide range of psychiatric disorders.10 Recent data from large, prospective cohort studies investigating reproductive safety of these agents are reassuring, with no specific patterns of organ malformation.11,12
DIPHENHYDRAMINE
Recent studies of antihistamines such as diphenhydramine have not reported any risk of major malformations with first-trimester exposure to antihistamines.13,14 Dose-dependent anticholinergic adverse effects of antihistamines can induce or exacerbate delirium and agitation, although these effects are classically seen in elderly, nonpregnant patients.15 Thus, given the paucity of adverse effects and the low risk, diphenhydramine is considered safe to use in pregnancy.13
BENZODIAZEPINES
Benzodiazepines are not contraindicated for the treatment of acute agitation in pregnancy.16 Reproductive safety data from meta-analyses and large population-based cohort studies have found no evidence of increased risk of major malformations in neonates born to mothers on prescription benzodiazepines in the first trimester.17,18 While third-trimester exposure to benzodiazepines has been associated with “floppy-baby” syndrome and neonatal withdrawal syndrome,16 these are more likely to occur in women on long-term prescription benzodiazepine therapy. No study has yet assessed the risk of these outcomes with a 1-time acute exposure in the emergency department; however, the risk is likely minimal given the aforementioned data observed in women on long-term prescription benzodiazepine therapy.
STEPWISE MANAGEMENT OF AGITATION IN PREGNANCY
If untreated, agitation in pregnancy is independently associated with outcomes that include premature delivery, low birth weight, growth retardation, postnatal death, and spontaneous abortion.1 The risk of these outcomes greatly outweighs any potential risk from psychotropic medications during pregnancy.
Nevertheless, intervention should progress in a stepwise manner, starting with the least restrictive and progressing toward more restrictive interventions, including pharmacotherapy, use of a seclusion room, and physical restraints (Figure 1).4,19
Before medications are considered, attempts should be made to engage with and “de-escalate” the patient in a safe, nonstimulating environment.19 If this approach is not effective, the patient should be offered oral medications to help with her agitation. However, if the patient’s behavior continues to escalate, presenting a danger to herself or staff, the use of emergency medications is clearly indicated. Providers should succinctly inform the patient of the need for immediate intervention.
If the patient has had a good response in the past to one of these medications or is currently taking one as needed, the same medication should be offered. If the patient has never been treated for agitation, it is important to consider the presenting symptoms, differential diagnosis, and the route and rapidity of administration of medication. If the patient has experienced a fall or other trauma, confirming a viable fetal heart rate between 10 to 22 weeks of gestation with Doppler ultrasonography and obstetric consultation should be considered.
DRUG THERAPY RECOMMENDATIONS
Mild to moderate agitation in pregnancy should be managed conservatively with diphenhydramine. Other options include a benzodiazepine, particularly lorazepam, if alcohol withdrawal is suspected. A second-generation antipsychotic such as olanzapine in a rapidly dissolving form or ziprasidone is another option if a rapid response is required.20Table 1 provides a summary of pharmacotherapy recommendations.
Severe agitation may require a combination of agents. A commonly used, safe regimen—colloquially called the “B52 bomb”—is haloperidol 5 mg, lorazepam 2 mg, and diphenhydramine 25 to 50 mg for prophylaxis of dystonia.20
The patient’s response should be monitored closely, as dosing may require modification as a result of pregnancy-related changes in drug distribution, metabolism, and clearance.21
Although no study to our knowledge has assessed risk associated with 1-time exposure to any of these classes of medications in pregnant women, the aforementioned data on long-term exposure provide reassurance that single exposure in emergency departments likely has little or no effect for the developing fetus.
PHYSICAL RESTRAINTS FOR AGITATION IN PREGNANCY
Physical restraints along with emergency medications (ie, chemical restraint) may be indicated when the patient poses a danger to herself or others. In some cases, both types of restraint may be required, whether in the emergency room or an inpatient setting.
However, during the second and third trimesters, physical restraints such as 4-point restraints may predispose the patient to inferior vena cava compression syndrome and compromise placental blood flow.4 Therefore, pregnant patients after 20 weeks of gestation should be positioned in the left lateral decubitus position, with the right hip positioned 10 to 12 cm off the bed with pillows or blankets. And when restraints are used in pregnant patients, frequent checking of vital signs and physical assessment is needed to mitigate risks.4
- Aftab A, Shah AA. Behavioral emergencies: special considerations in the pregnant patient. Psychiatr Clin North Am 2017; 40(3):435–448. doi:10.1016/j.psc.2017.05.017
- Altshuler LL, Cohen L, Szuba MP, Burt VK, Gitlin M, Mintz J. Pharmacologic management of psychiatric illness during pregnancy: dilemmas and guidelines. Am J Psychiatry 1996; 153(5):592–606. doi:10.1176/ajp.153.5.592
- Einarson A. Safety of psychotropic drug use during pregnancy: a review. MedGenMed 2005; 7(4):3. pmid:16614625
- Wilson MP, Nordstrom K, Shah AA, Vilke GM. Psychiatric emergencies in pregnant women. Emerg Med Clin North Am 2015; 33(4):841–851. doi:10.1016/j.emc.2015.07.010
- Brown HE, Stoklosa J, Freundenreich O. How to stabilize an acutely psychotic patient. Curr Psychiatry 2012; 11(12):10–16.
- US Food and Drug Administration. Pregnancy and lactation labeling (drugs) final rule. www.fda.gov/drugs/developmentapprovalprocess/developmentresources/labeling/ucm093307.htm. Accessed January 8, 2019.
- Brucker MC, King TL. The 2015 US Food and Drug Administration pregnancy and lactation labeling rule. J Midwifery Womens Health 2017; 62(3):308–316. doi:10.1111/jmwh.12611
- Diav-Citrin O, Shechtman S, Ornoy S, et al. Safety of haloperidol and penfluridol in pregnancy: a multicenter, prospective, controlled study. J Clin Psychiatry 2005; 66(3):317–322. pmid:15766297
- Galbally M, Snellen M, Power J. Antipsychotic drugs in pregnancy: a review of their maternal and fetal effects. Ther Adv Drug Saf 2014; 5(2):100–109. doi:10.1177/2042098614522682
- Kulkarni J, Storch A, Baraniuk A, Gilbert H, Gavrilidis E, Worsley R. Antipsychotic use in pregnancy. Expert Opin Pharmacother 2015; 16(9):1335–1345. doi:10.1517/14656566.2015.1041501
- Huybrechts KF, Hernández-Díaz S, Patorno E, et al. Antipsychotic use in pregnancy and the risk for congenital malformations. JAMA Psychiatry 2016; 73(9):938–946. doi:10.1001/jamapsychiatry.2016.1520
- Cohen LS, Viguera AC, McInerney KA, et al. Reproductive safety of second-generation antipsychotics: current data from the Massachusetts General Hospital national pregnancy registry for atypical antipsychotics. Am J Psychiatry 2016; 173(3):263–270. doi:10.1176/appi.ajp.2015.15040506
- Li Q, Mitchell AA, Werler MM, Yau WP, Hernández-Díaz S. Assessment of antihistamine use in early pregnancy and birth defects. J Allergy Clin Immunol Pract 2013; 1(6):666–674.e1. doi:10.1016/j.jaip.2013.07.008
- Gilboa SM, Strickland MJ, Olshan AF, Werler MM, Correa A; National Birth Defects Prevention Study. Use of antihistamine medications during early pregnancy and isolated major malformations. Birth Defects Res A Clin Mol Teratol 2009; 85(2):137–150. doi:10.1002/bdra.20513
- Meuleman JR. Association of diphenhydramine use with adverse effects in hospitalized older patients: possible confounders. Arch Intern Med 2002; 162(6):720–721. pmid:11911733
- Enato E, Moretti M, Koren G. The fetal safety of benzodiazepines: an updated meta-analysis. J Obstet Gynaecol Can 2011; 33(1):46–48. doi:10.1016/S1701-2163(16)34772-7
- Dolovich LR, Addis A, Vaillancourt JM, Power JD, Koren G, Einarson TR. Benzodiazepine use in pregnancy and major malformations or oral cleft: meta-analysis of cohort and case-control studies. BMJ 1998; 317(7162):839–843. pmid:9748174
- Bellantuono C, Tofani S, Di Sciascio G, Santone G. Benzodiazepine exposure in pregnancy and risk of major malformations: a critical overview. Gen Hosp Psychiatry 2013; 35(1):3–8. doi:10.1016/j.genhosppsych.2012.09.003
- Richmond JS, Berlin JS, Fishkind AB, et al. Verbal de-escalation of the agitated patient: consensus statement of the American Association for Emergency Psychiatry project BETA De-escalation Workgroup. West J Emerg Med 2012; 13(1):17–25. doi:10.5811/westjem.2011.9.6864
- Prager LM, Ivkovic A. Emergency psychiatry. In: Stern TA, Fava M, Wilens TE, Rosenbaum JF, eds. The Massachusetts General Hospital Comprehensive Clinical Psychiatry. 2nd ed. London: Elsevier; 2016:937–949.
- Feghali M, Venkataramanan R, Caritis S. Pharmacokinetics of drugs in pregnancy. Semin Perinatol 2015; 39(7):512–519. doi:10.1053/j.semperi.2015.08.003
- Aftab A, Shah AA. Behavioral emergencies: special considerations in the pregnant patient. Psychiatr Clin North Am 2017; 40(3):435–448. doi:10.1016/j.psc.2017.05.017
- Altshuler LL, Cohen L, Szuba MP, Burt VK, Gitlin M, Mintz J. Pharmacologic management of psychiatric illness during pregnancy: dilemmas and guidelines. Am J Psychiatry 1996; 153(5):592–606. doi:10.1176/ajp.153.5.592
- Einarson A. Safety of psychotropic drug use during pregnancy: a review. MedGenMed 2005; 7(4):3. pmid:16614625
- Wilson MP, Nordstrom K, Shah AA, Vilke GM. Psychiatric emergencies in pregnant women. Emerg Med Clin North Am 2015; 33(4):841–851. doi:10.1016/j.emc.2015.07.010
- Brown HE, Stoklosa J, Freundenreich O. How to stabilize an acutely psychotic patient. Curr Psychiatry 2012; 11(12):10–16.
- US Food and Drug Administration. Pregnancy and lactation labeling (drugs) final rule. www.fda.gov/drugs/developmentapprovalprocess/developmentresources/labeling/ucm093307.htm. Accessed January 8, 2019.
- Brucker MC, King TL. The 2015 US Food and Drug Administration pregnancy and lactation labeling rule. J Midwifery Womens Health 2017; 62(3):308–316. doi:10.1111/jmwh.12611
- Diav-Citrin O, Shechtman S, Ornoy S, et al. Safety of haloperidol and penfluridol in pregnancy: a multicenter, prospective, controlled study. J Clin Psychiatry 2005; 66(3):317–322. pmid:15766297
- Galbally M, Snellen M, Power J. Antipsychotic drugs in pregnancy: a review of their maternal and fetal effects. Ther Adv Drug Saf 2014; 5(2):100–109. doi:10.1177/2042098614522682
- Kulkarni J, Storch A, Baraniuk A, Gilbert H, Gavrilidis E, Worsley R. Antipsychotic use in pregnancy. Expert Opin Pharmacother 2015; 16(9):1335–1345. doi:10.1517/14656566.2015.1041501
- Huybrechts KF, Hernández-Díaz S, Patorno E, et al. Antipsychotic use in pregnancy and the risk for congenital malformations. JAMA Psychiatry 2016; 73(9):938–946. doi:10.1001/jamapsychiatry.2016.1520
- Cohen LS, Viguera AC, McInerney KA, et al. Reproductive safety of second-generation antipsychotics: current data from the Massachusetts General Hospital national pregnancy registry for atypical antipsychotics. Am J Psychiatry 2016; 173(3):263–270. doi:10.1176/appi.ajp.2015.15040506
- Li Q, Mitchell AA, Werler MM, Yau WP, Hernández-Díaz S. Assessment of antihistamine use in early pregnancy and birth defects. J Allergy Clin Immunol Pract 2013; 1(6):666–674.e1. doi:10.1016/j.jaip.2013.07.008
- Gilboa SM, Strickland MJ, Olshan AF, Werler MM, Correa A; National Birth Defects Prevention Study. Use of antihistamine medications during early pregnancy and isolated major malformations. Birth Defects Res A Clin Mol Teratol 2009; 85(2):137–150. doi:10.1002/bdra.20513
- Meuleman JR. Association of diphenhydramine use with adverse effects in hospitalized older patients: possible confounders. Arch Intern Med 2002; 162(6):720–721. pmid:11911733
- Enato E, Moretti M, Koren G. The fetal safety of benzodiazepines: an updated meta-analysis. J Obstet Gynaecol Can 2011; 33(1):46–48. doi:10.1016/S1701-2163(16)34772-7
- Dolovich LR, Addis A, Vaillancourt JM, Power JD, Koren G, Einarson TR. Benzodiazepine use in pregnancy and major malformations or oral cleft: meta-analysis of cohort and case-control studies. BMJ 1998; 317(7162):839–843. pmid:9748174
- Bellantuono C, Tofani S, Di Sciascio G, Santone G. Benzodiazepine exposure in pregnancy and risk of major malformations: a critical overview. Gen Hosp Psychiatry 2013; 35(1):3–8. doi:10.1016/j.genhosppsych.2012.09.003
- Richmond JS, Berlin JS, Fishkind AB, et al. Verbal de-escalation of the agitated patient: consensus statement of the American Association for Emergency Psychiatry project BETA De-escalation Workgroup. West J Emerg Med 2012; 13(1):17–25. doi:10.5811/westjem.2011.9.6864
- Prager LM, Ivkovic A. Emergency psychiatry. In: Stern TA, Fava M, Wilens TE, Rosenbaum JF, eds. The Massachusetts General Hospital Comprehensive Clinical Psychiatry. 2nd ed. London: Elsevier; 2016:937–949.
- Feghali M, Venkataramanan R, Caritis S. Pharmacokinetics of drugs in pregnancy. Semin Perinatol 2015; 39(7):512–519. doi:10.1053/j.semperi.2015.08.003
Does early repolarization on ECG increase the risk of cardiac death in healthy people?
No. The early repolarization pattern on electrocardiography (ECG) in asymptomatic patients is nearly always a benign incidental finding. However, in a patient with a history of idiopathic ventricular fibrillation or a family history of sudden cardiac death, the finding warrants further evaluation.
DEFINING EARLY REPOLARIZATION
The early repolarization pattern may mimic patterns seen in myocardial infarction, pericarditis, ventricular aneurysm, hyperkalemia, and hypothermia,1,3 and misinterpreting the pattern can lead to unnecessary laboratory testing, imaging, medication use, and hospital admissions. On the other hand, misinterpreting it as benign in the presence of certain features of the history or clinical presentation can delay the diagnosis and treatment of a potentially critical condition.
PREVALENCE AND MECHANISMS
The prevalence of the early repolarization pattern in the general population ranges from 5% to 15%; the wide range reflects differences in the definition, as well as variability in the pattern of early repolarization over time.4
The early repolarization pattern is more commonly seen in African American men and in young, physically active individuals.3 In one study, it was observed in 15% of cases of idiopathic ventricular fibrillation and sudden cardiac death, especially in people ages 35 to 45.4 While there is evidence of a heritable basis in the general population, a family history of early repolarization is not known to increase the risk of sudden cardiac death.
A proposed mechanism for the early repolarization pattern is an imbalance in the ion channel system, resulting in variable refractoriness of multiple myocardial regions and varying excitability in the myocardium. This can produce a voltage gradient between myocardial regions, which is believed to cause the major hallmarks of the early repolarization pattern, ie, ST-segment elevation and QRS notching or slurring.3
MANAGEMENT
The early repolarization pattern is nearly always a benign incidental finding on ECG, with no specific signs or symptoms attributed to it. High-risk features on ECG are associated with a modest increase in absolute risk of sudden cardiac death and warrant clinical correlation.
In the absence of syncope or family history of sudden cardiac death, early repolarization does not merit further workup.2
In patients with a history of unexplained syncope and a family history of sudden cardiac death, early repolarization should be considered in overall risk stratification.1 Early repolarization in a patient with previous idiopathic ventricular fibrillation warrants referral for electrophysiologic study and, if indicated, insertion of an implantable cardiac defibrillator for secondary prevention.5
- Patton KK, Ellinor PT, Ezekowitz M, et al; American Heart Association Electrocardiography and Arrhythmias Committee of the Council on Clinical Cardiology and Council on Functional Genomics and Translational Biology. Electrocardiographic early repolarization: a scientific statement from the American Heart Association. Circulation 2016; 133(15):1520–1529. doi:10.1161/CIR.0000000000000388
- Macfarlane PW, Antzelevitch C, Haissaguerre M, et al. The early repolarization pattern: a consensus paper. J Am Coll Cardiol 2015; 66(4):470–477. doi:10.1016/j.jacc.2015.05.033
- Benito B, Guasch E, Rivard L, Nattel S. Clinical and mechanistic issues in early repolarization of normal variants and lethal arrhythmia syndromes. J Am Coll Cardiol 2010; 56(15):1177–1186. doi:10.1016/j.jacc.2010.05.037
- Maury P, Rollin A. Prevalence of early repolarisation/J wave patterns in the normal population. J Electrocardiol 2013; 46(5):411–416. doi:10.1016/j.jelectrocard.2013.06.014
- Mahida S, Sacher F, Berte B, et al. Evaluation of patients with early repolarization syndrome. J Atr Fibrillation 2014; 7(3):1083. doi:10.4022/jafib.1083
No. The early repolarization pattern on electrocardiography (ECG) in asymptomatic patients is nearly always a benign incidental finding. However, in a patient with a history of idiopathic ventricular fibrillation or a family history of sudden cardiac death, the finding warrants further evaluation.
DEFINING EARLY REPOLARIZATION
The early repolarization pattern may mimic patterns seen in myocardial infarction, pericarditis, ventricular aneurysm, hyperkalemia, and hypothermia,1,3 and misinterpreting the pattern can lead to unnecessary laboratory testing, imaging, medication use, and hospital admissions. On the other hand, misinterpreting it as benign in the presence of certain features of the history or clinical presentation can delay the diagnosis and treatment of a potentially critical condition.
PREVALENCE AND MECHANISMS
The prevalence of the early repolarization pattern in the general population ranges from 5% to 15%; the wide range reflects differences in the definition, as well as variability in the pattern of early repolarization over time.4
The early repolarization pattern is more commonly seen in African American men and in young, physically active individuals.3 In one study, it was observed in 15% of cases of idiopathic ventricular fibrillation and sudden cardiac death, especially in people ages 35 to 45.4 While there is evidence of a heritable basis in the general population, a family history of early repolarization is not known to increase the risk of sudden cardiac death.
A proposed mechanism for the early repolarization pattern is an imbalance in the ion channel system, resulting in variable refractoriness of multiple myocardial regions and varying excitability in the myocardium. This can produce a voltage gradient between myocardial regions, which is believed to cause the major hallmarks of the early repolarization pattern, ie, ST-segment elevation and QRS notching or slurring.3
MANAGEMENT
The early repolarization pattern is nearly always a benign incidental finding on ECG, with no specific signs or symptoms attributed to it. High-risk features on ECG are associated with a modest increase in absolute risk of sudden cardiac death and warrant clinical correlation.
In the absence of syncope or family history of sudden cardiac death, early repolarization does not merit further workup.2
In patients with a history of unexplained syncope and a family history of sudden cardiac death, early repolarization should be considered in overall risk stratification.1 Early repolarization in a patient with previous idiopathic ventricular fibrillation warrants referral for electrophysiologic study and, if indicated, insertion of an implantable cardiac defibrillator for secondary prevention.5
No. The early repolarization pattern on electrocardiography (ECG) in asymptomatic patients is nearly always a benign incidental finding. However, in a patient with a history of idiopathic ventricular fibrillation or a family history of sudden cardiac death, the finding warrants further evaluation.
DEFINING EARLY REPOLARIZATION
The early repolarization pattern may mimic patterns seen in myocardial infarction, pericarditis, ventricular aneurysm, hyperkalemia, and hypothermia,1,3 and misinterpreting the pattern can lead to unnecessary laboratory testing, imaging, medication use, and hospital admissions. On the other hand, misinterpreting it as benign in the presence of certain features of the history or clinical presentation can delay the diagnosis and treatment of a potentially critical condition.
PREVALENCE AND MECHANISMS
The prevalence of the early repolarization pattern in the general population ranges from 5% to 15%; the wide range reflects differences in the definition, as well as variability in the pattern of early repolarization over time.4
The early repolarization pattern is more commonly seen in African American men and in young, physically active individuals.3 In one study, it was observed in 15% of cases of idiopathic ventricular fibrillation and sudden cardiac death, especially in people ages 35 to 45.4 While there is evidence of a heritable basis in the general population, a family history of early repolarization is not known to increase the risk of sudden cardiac death.
A proposed mechanism for the early repolarization pattern is an imbalance in the ion channel system, resulting in variable refractoriness of multiple myocardial regions and varying excitability in the myocardium. This can produce a voltage gradient between myocardial regions, which is believed to cause the major hallmarks of the early repolarization pattern, ie, ST-segment elevation and QRS notching or slurring.3
MANAGEMENT
The early repolarization pattern is nearly always a benign incidental finding on ECG, with no specific signs or symptoms attributed to it. High-risk features on ECG are associated with a modest increase in absolute risk of sudden cardiac death and warrant clinical correlation.
In the absence of syncope or family history of sudden cardiac death, early repolarization does not merit further workup.2
In patients with a history of unexplained syncope and a family history of sudden cardiac death, early repolarization should be considered in overall risk stratification.1 Early repolarization in a patient with previous idiopathic ventricular fibrillation warrants referral for electrophysiologic study and, if indicated, insertion of an implantable cardiac defibrillator for secondary prevention.5
- Patton KK, Ellinor PT, Ezekowitz M, et al; American Heart Association Electrocardiography and Arrhythmias Committee of the Council on Clinical Cardiology and Council on Functional Genomics and Translational Biology. Electrocardiographic early repolarization: a scientific statement from the American Heart Association. Circulation 2016; 133(15):1520–1529. doi:10.1161/CIR.0000000000000388
- Macfarlane PW, Antzelevitch C, Haissaguerre M, et al. The early repolarization pattern: a consensus paper. J Am Coll Cardiol 2015; 66(4):470–477. doi:10.1016/j.jacc.2015.05.033
- Benito B, Guasch E, Rivard L, Nattel S. Clinical and mechanistic issues in early repolarization of normal variants and lethal arrhythmia syndromes. J Am Coll Cardiol 2010; 56(15):1177–1186. doi:10.1016/j.jacc.2010.05.037
- Maury P, Rollin A. Prevalence of early repolarisation/J wave patterns in the normal population. J Electrocardiol 2013; 46(5):411–416. doi:10.1016/j.jelectrocard.2013.06.014
- Mahida S, Sacher F, Berte B, et al. Evaluation of patients with early repolarization syndrome. J Atr Fibrillation 2014; 7(3):1083. doi:10.4022/jafib.1083
- Patton KK, Ellinor PT, Ezekowitz M, et al; American Heart Association Electrocardiography and Arrhythmias Committee of the Council on Clinical Cardiology and Council on Functional Genomics and Translational Biology. Electrocardiographic early repolarization: a scientific statement from the American Heart Association. Circulation 2016; 133(15):1520–1529. doi:10.1161/CIR.0000000000000388
- Macfarlane PW, Antzelevitch C, Haissaguerre M, et al. The early repolarization pattern: a consensus paper. J Am Coll Cardiol 2015; 66(4):470–477. doi:10.1016/j.jacc.2015.05.033
- Benito B, Guasch E, Rivard L, Nattel S. Clinical and mechanistic issues in early repolarization of normal variants and lethal arrhythmia syndromes. J Am Coll Cardiol 2010; 56(15):1177–1186. doi:10.1016/j.jacc.2010.05.037
- Maury P, Rollin A. Prevalence of early repolarisation/J wave patterns in the normal population. J Electrocardiol 2013; 46(5):411–416. doi:10.1016/j.jelectrocard.2013.06.014
- Mahida S, Sacher F, Berte B, et al. Evaluation of patients with early repolarization syndrome. J Atr Fibrillation 2014; 7(3):1083. doi:10.4022/jafib.1083
Repeating blood cultures after initial bacteremia: When and how often?
Repeat cultures are indicated in specific scenarios, but for most patients, frequent and indiscriminate repetition after an initial positive culture is unnecessary and may be associated with excessive use of resources. Prospective studies and practice guidelines are needed to help further define the indications.
THE TENDENCY TO REPEAT CULTURES
Current literature lacks strong evidence for repeating previously positive blood cultures collected appropriately—ie, 10 mL of blood for aerobic culture and 10 mL for anaerobic culture from 2 different sites, and a positive result from both sets. However, because of the risk of serious complications of bacteremia, particularly in critically ill patients, many clinicians order multiple, repeated sets of blood cultures.
Tabriz et al1 found that one-third of hospitalized patients got repeat cultures after an initial set, regardless of the result of the first set. Most (83.4%) of those cultures yielded no growth, 9.1% grew the same pathogen, and 5.0% were contaminated. Finding a new pathogen was rare, occurring in only 2.5% of repeated cultures.
Wiggers et al2 reported an even higher number of repeat cultures ordered for patients who had an initially positive culture: 38.9%.2 And in another study,3 half of the patients received more than 2 consecutive cultures.
Drawbacks
Unrestrained ordering of repeat blood cultures can increase the risk of a false-positive result, leading to more cultures, echocardiography, other imaging tests, and unnecessary antimicrobial therapy, all of which puts patients at risk of adverse effects of treatment and missed alternative diagnoses and increases the length and cost of hospitalization.4
Advantages
On the other hand, repeat blood cultures may increase the diagnostic yield for conditions such as infective endocarditis and may have implications for the duration of antibiotic therapy.1 The duration of therapy for bacteremia is usually determined from the last negative culture; hence, documenting clearance of bacteremia can determine a precise end-date for antibiotic therapy.
Bacteremia due to Staphylococcus aureus and to endovascular and epidural sources has been found to be independently associated with persistent bacteremia, detected in 6.6% of 1,801 index cases of bacteremia in a retrospective cohort study.2 An endovascular source (adjusted odds ratio [OR] 7.66, 95% confidence interval [CI] 2.30–25.48), an epidural source (adjusted OR 26.99, 95% CI, 1.91–391.08), and S aureus bacteremia (adjusted OR 4.49, 95% CI 1.88–10.73) were independently associated with persistent bacteremia. Escherichia coli (5.1%, P = .006), viridans group streptococci (1.7%, P = .035), and beta-hemolytic streptococci (0%, P = .028) were associated with a lower likelihood of persistent bacteremia. Patients with persistent bacteremia were less likely to have achieved source control within 48 hours of the index event (29.7% vs 52.5%, P < .001).2
WHEN REPEATING CULTURES IS APPROPRIATE
Repeating blood cultures after an initial positive result is superfluous, except in certain situations.
Suspected endovascular infection
Patients with endocarditis, thrombophlebitis, an indwelling device for epidural access, or a cardiovascular implantable electronic device should have repeat cultures after an initial positive culture. Implantable electronic device infection is suspected in the following cases: sustained positive blood culture (> 24 hours); relapsing bacteremia despite a course of appropriate antibiotic therapy; presence of an implantable cardioverter defibrillator; presence of a prosthetic cardiac valve; and an episode of bacteremia within 3 months of device placement.5
S aureus bacteremia
Repeat blood culture is warranted for S aureus bacteremia regardless of methicillin susceptibility.1 But persistent methicillin-resistant S aureus (MRSA) bacteremia changes the management of these patients.6 For example, the source of infection should be identified, followed by debridement or drainage, and then either high-dose or combination antimicrobial therapy.6 Infective endocarditis from persistent MRSA bacteremia is an indication for surgery.6
Persistent S aureus bacteremia may change the duration of therapy, as the common practice is to continue treating uncomplicated gram-positive bacteremia for 14 days from the date of the first negative culture. Infection leading to infective endocarditis increases the duration of antibiotic therapy to at least 4 weeks.
Candidemia
Candidemia is an absolute indication for repeat blood culture.7 Patients with persistent candidemia should undergo imaging of the genitourinary tract, liver, and spleen as part of the evaluation for a deep-tissue source of infection.7 Also, if the patient is initially treated with an echinocandin, therapy can be transitioned to fluconazole if the isolate is azole-susceptible, the patient’s condition is clinically stable, and repeat cultures are negative.7 Therefore, repeating cultures has therapeutic implications.
Confirming response to therapy
In patients with infective endocarditis or other endovascular infection caused by S aureus, Enterococcus species, or gram-negative bacilli,1 repeat blood culture should be done to confirm therapeutic response. Patients with infective endocarditis whose condition is stable can be discharged to receive outpatient parenteral antibiotic therapy. However, patients with uncontrolled heart failure, systemic emboli, abscess, persistent fever, or persistently positive cultures are not candidates for outpatient therapy and require repeat cultures.8
Multidrug-resistant gram-negative bacilli
Bacteremia due to multidrug-resistant gram-negative bacilli requires repeat blood cultures to document clearance of bacteremia and to ensure the efficacy of antibiotics, as these organisms pose a higher risk of treatment failure, and combination synergistic regimens may be needed if bacteremia does not clear.
Febrile neutropenia
Blood cultures are important in the management of febrile neutropenia. In a study by Rosenblum et al,9 repeat cultures were positive in 10.9% of patients with febrile neutropenia after an initial negative culture, but many of those organisms were of low pathogenicity, and a significant proportion were coagulase-negative staphylococci.10 Another study showed that the frequency of detecting new pathogens by repeat culture in recurrent febrile neutropenia was higher than that in persistent febrile neutropenia (8% vs 2%) (P = .0491); a history of recent bacteremia was identified as a significant predictor of positive culture in recurrent febrile neutropenia.11
Persistent or new infection
Persistence of fever, leukocytosis, or other signs of infection 72 hours after appropriate antibiotic therapy is started requires follow-up blood cultures.
New episode of sepsis. A new episode of sepsis should be confirmed12 using the systemic inflammatory response syndrome criteria, the newer definition of Sepsis-related Organ Failure Assessment (SOFA) in the intensive-care unit, or the quick SOFA in general units. If the patient develops new signs of sepsis after response to treatment for initial bacteremia, repeat blood cultures should be considered.
Central line-associated bloodstream infection requires repeat cultures.13 Persistence of bacteremia in this type of infection extends the duration of therapy, as most clinicians determine treatment duration from the last negative culture. Persistent bacteremia also influences the decision to salvage or remove the catheter. Microbiologic clearance of bacteremia on blood culture can also guide the time of reinsertion if the catheter was removed.
Concern for an unresolved focus of infection such as abscess, joint infection, or retained catheter is an indication for repeat blood cultures.
Bacteremia of unknown source. In clinical practice, we encounter scenarios in which blood cultures are positive but no source can be identified. In those situations, it is important to repeat blood cultures to document clearance. If bacteremia persists, we need to continue searching for the source.
WHEN ROUTINELY REPEATING CULTURES IS NOT INDICATED
Repeat blood cultures are not routinely indicated in patients with streptococcal bacteremia, uncomplicated gram-negative bacteremia, and bacteremia associated with localized infection such as cellulitis, community-acquired pneumonia, or pyelonephritis.2,4 A study of patients with gram-negative bacteremia found that 17 repeated cultures needed to be drawn to yield 1 positive culture.14
Isolated fever or leukocytosis does not accurately predict bacteremia.4 A study that excluded neutropenic and intensive-care patients reported none of the initially negative cultures to be positive when repeated.15
Ordering repeat cultures in response to persistent fever is a common practice, even though fever is typical in the first 72 hours of antibiotic therapy. Such cultures rarely if ever reveal new pathogens, and results can be predicted based on cultures before the start of antibiotics.15 For patients on antibiotics, physicians should therefore wait for results of the preantibiotic cultures rather than order new cultures in response to persistent fever.15
WOULD WE MISS PERSISTENT BACTEREMIA?
In theory, not repeating blood cultures could miss persistent bacteremia, but this is unlikely if the concerns discussed above are considered. Further, persistent bacteremia would result in clinical signs and symptoms that should prompt repeat cultures.
FREQUENCY OF REPEAT BLOOD CULTURES
There are no evidence-based guidelines for the frequency of repeating cultures. The Infectious Diseases Society of America recommends repeating blood cultures 2 to 4 days after the index positive culture in the case of multidrug-resistant S aureus bacteremia, and every day or every other day for candidemia.6,7,9
A study evaluating the practice patterns of repeating cultures after an initial bacteremia showed that 34.7% were done within 24 hours and 44.7% were done in 2 to 4 days.1 There is no evidence that repeating blood cultures daily is necessary in these patients. As a general rule, it should be done 48 to 72 hours after a positive culture.
- Tabriz MS, Riederer K, Baran J Jr, Khatib R. Repeating blood cultures during hospital stay: practice pattern at a teaching hospital and a proposal for guidelines. Clin Microbiol Infect 2004; 10(7):624–627. doi:10.1111/j.1469-0691.2004.00893.x
- Wiggers JB, Xiong W, Daneman N. Sending repeat cultures: is there a role in the management of bacteremic episodes? (SCRIBE study). BMC Infect Dis 2016; 16:286. doi:10.1186/s12879-016-1622-z
- Kang CK, Kim ES, Song KH, et al. Can a routine follow-up blood culture be justified in Klebsiella pneumoniae bacteremia? A retrospective case–control study. BMC Infect Dis 2013; 13:365. doi:10.1186/1471-2334-13-365
- Coburn B, Morris AM, Tomlinson G, Detsky AS. Does this adult patient with suspected bacteremia require blood cultures? JAMA 2012; 308(5):502–511. doi:10.1001/jama.2012.8262
- Baddour LM, Epstein AE, Erickson CC, et al; American Heart Association Rheumatic Fever, Endocarditis, and Kawasaki Disease Committee; Council on Cardiovascular Disease in Young; Council on Cardiovascular Surgery and Anesthesia; Council on Cardiovascular Nursing; Council on Clinical Cardiology; Interdisciplinary Council on Quality of Care; American Heart Association. Update on cardiovascular implantable electronic device infections and their management: a scientific statement from the American Heart Association. Circulation 2010; 121(3):458–477. doi:10.1161/CIRCULATIONAHA.109.192665
- Liu C, Bayer A, Cosgrove SE, et al; Infectious Diseases Society of America. Clinical practice guidelines by the Infectious Diseases Society of America for the treatment of methicillin-resistant Staphylococcus aureus infections in adults and children. Clin Infect Dis 2011; 52(3):e18–e55. doi:10.1093/cid/ciq146
- Pappas PG, Kauffman CA, Andes DR, et al. Clinical practice guideline for the management of candidiasis: 2016 update by the Infectious Diseases Society of America. Clin Infect Dis 2016; 62(4):e1–e50. doi:10.1093/cid/civ933
- Baddour LM, Wilson WR, Bayer AS, et al; American Heart Association Committee on Rheumatic Fever, Endocarditis, and Kawasaki Disease of the Council on Cardiovascular Disease in the Young, Council on Clinical Cardiology, Council on Cardiovascular Surgery and Anesthesia, and Stroke Council. Infective endocarditis in adults: diagnosis, antimicrobial therapy, and management of complications: a scientific statement for healthcare professionals from the American Heart Association. Circulation 2015; 132(15):1435–1486. doi:10.1161/CIR.0000000000000296
- Rosenblum J, Lin J, Kim M, Levy AS. Repeating blood cultures in neutropenic children with persistent fevers when the initial blood culture is negative. Pediatr Blood Cancer 2013; 60(6):923–927. doi:10.1002/pbc.24358
- Thomas MW, Chauvenet AR, O'Suoji C. Repeating blood cultures in neutropenic children with persistent fevers when the initial blood culture is negative. Pediatr Blood Cancer 2014; 61(2):194. doi:10.1002/pbc.24834
- Kimura SI, Gomyo A, Hayakawa J, et al. Clinical significance of repeat blood cultures during febrile neutropenia in adult acute myeloid leukaemia patients undergoing intensive chemotherapy. Infect Dis (Lond) 2017; 49(10):748–757. doi:10.1080/23744235.2017.1340665
- Singer M, Deutschman CS, Seymour CW, et al. The third international consensus definitions for sepsis and septic shock (Sepsis-3). JAMA 2016; 315(8):801–810. doi:10.1001/jama.2016.0287
- Shah H, Bosch W, Thompson KM, Hellinger WC. Intravascular catheter-related bloodstream infection. Neurohospitalist 2013; 3(3):144–151. doi:10.1177/1941874413476043
- Canzoneri CN, Akhavan BJ, Tosur Z, Andrade PEA, Aisenberg GM. Follow-up blood cultures in gram-negative bacteremia: are they needed? Clin Infect Dis 2017; 65(11):1776–1779. doi:10.1093/cid/cix648
- Grace CJ, Lieberman J, Pierce K, Littenberg B. Usefulness of blood culture for hospitalized patients who are receiving antibiotic therapy. Clin Infect Dis 2001; 32(11):1651–1655. doi:10.1086/320527
Repeat cultures are indicated in specific scenarios, but for most patients, frequent and indiscriminate repetition after an initial positive culture is unnecessary and may be associated with excessive use of resources. Prospective studies and practice guidelines are needed to help further define the indications.
THE TENDENCY TO REPEAT CULTURES
Current literature lacks strong evidence for repeating previously positive blood cultures collected appropriately—ie, 10 mL of blood for aerobic culture and 10 mL for anaerobic culture from 2 different sites, and a positive result from both sets. However, because of the risk of serious complications of bacteremia, particularly in critically ill patients, many clinicians order multiple, repeated sets of blood cultures.
Tabriz et al1 found that one-third of hospitalized patients got repeat cultures after an initial set, regardless of the result of the first set. Most (83.4%) of those cultures yielded no growth, 9.1% grew the same pathogen, and 5.0% were contaminated. Finding a new pathogen was rare, occurring in only 2.5% of repeated cultures.
Wiggers et al2 reported an even higher number of repeat cultures ordered for patients who had an initially positive culture: 38.9%.2 And in another study,3 half of the patients received more than 2 consecutive cultures.
Drawbacks
Unrestrained ordering of repeat blood cultures can increase the risk of a false-positive result, leading to more cultures, echocardiography, other imaging tests, and unnecessary antimicrobial therapy, all of which puts patients at risk of adverse effects of treatment and missed alternative diagnoses and increases the length and cost of hospitalization.4
Advantages
On the other hand, repeat blood cultures may increase the diagnostic yield for conditions such as infective endocarditis and may have implications for the duration of antibiotic therapy.1 The duration of therapy for bacteremia is usually determined from the last negative culture; hence, documenting clearance of bacteremia can determine a precise end-date for antibiotic therapy.
Bacteremia due to Staphylococcus aureus and to endovascular and epidural sources has been found to be independently associated with persistent bacteremia, detected in 6.6% of 1,801 index cases of bacteremia in a retrospective cohort study.2 An endovascular source (adjusted odds ratio [OR] 7.66, 95% confidence interval [CI] 2.30–25.48), an epidural source (adjusted OR 26.99, 95% CI, 1.91–391.08), and S aureus bacteremia (adjusted OR 4.49, 95% CI 1.88–10.73) were independently associated with persistent bacteremia. Escherichia coli (5.1%, P = .006), viridans group streptococci (1.7%, P = .035), and beta-hemolytic streptococci (0%, P = .028) were associated with a lower likelihood of persistent bacteremia. Patients with persistent bacteremia were less likely to have achieved source control within 48 hours of the index event (29.7% vs 52.5%, P < .001).2
WHEN REPEATING CULTURES IS APPROPRIATE
Repeating blood cultures after an initial positive result is superfluous, except in certain situations.
Suspected endovascular infection
Patients with endocarditis, thrombophlebitis, an indwelling device for epidural access, or a cardiovascular implantable electronic device should have repeat cultures after an initial positive culture. Implantable electronic device infection is suspected in the following cases: sustained positive blood culture (> 24 hours); relapsing bacteremia despite a course of appropriate antibiotic therapy; presence of an implantable cardioverter defibrillator; presence of a prosthetic cardiac valve; and an episode of bacteremia within 3 months of device placement.5
S aureus bacteremia
Repeat blood culture is warranted for S aureus bacteremia regardless of methicillin susceptibility.1 But persistent methicillin-resistant S aureus (MRSA) bacteremia changes the management of these patients.6 For example, the source of infection should be identified, followed by debridement or drainage, and then either high-dose or combination antimicrobial therapy.6 Infective endocarditis from persistent MRSA bacteremia is an indication for surgery.6
Persistent S aureus bacteremia may change the duration of therapy, as the common practice is to continue treating uncomplicated gram-positive bacteremia for 14 days from the date of the first negative culture. Infection leading to infective endocarditis increases the duration of antibiotic therapy to at least 4 weeks.
Candidemia
Candidemia is an absolute indication for repeat blood culture.7 Patients with persistent candidemia should undergo imaging of the genitourinary tract, liver, and spleen as part of the evaluation for a deep-tissue source of infection.7 Also, if the patient is initially treated with an echinocandin, therapy can be transitioned to fluconazole if the isolate is azole-susceptible, the patient’s condition is clinically stable, and repeat cultures are negative.7 Therefore, repeating cultures has therapeutic implications.
Confirming response to therapy
In patients with infective endocarditis or other endovascular infection caused by S aureus, Enterococcus species, or gram-negative bacilli,1 repeat blood culture should be done to confirm therapeutic response. Patients with infective endocarditis whose condition is stable can be discharged to receive outpatient parenteral antibiotic therapy. However, patients with uncontrolled heart failure, systemic emboli, abscess, persistent fever, or persistently positive cultures are not candidates for outpatient therapy and require repeat cultures.8
Multidrug-resistant gram-negative bacilli
Bacteremia due to multidrug-resistant gram-negative bacilli requires repeat blood cultures to document clearance of bacteremia and to ensure the efficacy of antibiotics, as these organisms pose a higher risk of treatment failure, and combination synergistic regimens may be needed if bacteremia does not clear.
Febrile neutropenia
Blood cultures are important in the management of febrile neutropenia. In a study by Rosenblum et al,9 repeat cultures were positive in 10.9% of patients with febrile neutropenia after an initial negative culture, but many of those organisms were of low pathogenicity, and a significant proportion were coagulase-negative staphylococci.10 Another study showed that the frequency of detecting new pathogens by repeat culture in recurrent febrile neutropenia was higher than that in persistent febrile neutropenia (8% vs 2%) (P = .0491); a history of recent bacteremia was identified as a significant predictor of positive culture in recurrent febrile neutropenia.11
Persistent or new infection
Persistence of fever, leukocytosis, or other signs of infection 72 hours after appropriate antibiotic therapy is started requires follow-up blood cultures.
New episode of sepsis. A new episode of sepsis should be confirmed12 using the systemic inflammatory response syndrome criteria, the newer definition of Sepsis-related Organ Failure Assessment (SOFA) in the intensive-care unit, or the quick SOFA in general units. If the patient develops new signs of sepsis after response to treatment for initial bacteremia, repeat blood cultures should be considered.
Central line-associated bloodstream infection requires repeat cultures.13 Persistence of bacteremia in this type of infection extends the duration of therapy, as most clinicians determine treatment duration from the last negative culture. Persistent bacteremia also influences the decision to salvage or remove the catheter. Microbiologic clearance of bacteremia on blood culture can also guide the time of reinsertion if the catheter was removed.
Concern for an unresolved focus of infection such as abscess, joint infection, or retained catheter is an indication for repeat blood cultures.
Bacteremia of unknown source. In clinical practice, we encounter scenarios in which blood cultures are positive but no source can be identified. In those situations, it is important to repeat blood cultures to document clearance. If bacteremia persists, we need to continue searching for the source.
WHEN ROUTINELY REPEATING CULTURES IS NOT INDICATED
Repeat blood cultures are not routinely indicated in patients with streptococcal bacteremia, uncomplicated gram-negative bacteremia, and bacteremia associated with localized infection such as cellulitis, community-acquired pneumonia, or pyelonephritis.2,4 A study of patients with gram-negative bacteremia found that 17 repeated cultures needed to be drawn to yield 1 positive culture.14
Isolated fever or leukocytosis does not accurately predict bacteremia.4 A study that excluded neutropenic and intensive-care patients reported none of the initially negative cultures to be positive when repeated.15
Ordering repeat cultures in response to persistent fever is a common practice, even though fever is typical in the first 72 hours of antibiotic therapy. Such cultures rarely if ever reveal new pathogens, and results can be predicted based on cultures before the start of antibiotics.15 For patients on antibiotics, physicians should therefore wait for results of the preantibiotic cultures rather than order new cultures in response to persistent fever.15
WOULD WE MISS PERSISTENT BACTEREMIA?
In theory, not repeating blood cultures could miss persistent bacteremia, but this is unlikely if the concerns discussed above are considered. Further, persistent bacteremia would result in clinical signs and symptoms that should prompt repeat cultures.
FREQUENCY OF REPEAT BLOOD CULTURES
There are no evidence-based guidelines for the frequency of repeating cultures. The Infectious Diseases Society of America recommends repeating blood cultures 2 to 4 days after the index positive culture in the case of multidrug-resistant S aureus bacteremia, and every day or every other day for candidemia.6,7,9
A study evaluating the practice patterns of repeating cultures after an initial bacteremia showed that 34.7% were done within 24 hours and 44.7% were done in 2 to 4 days.1 There is no evidence that repeating blood cultures daily is necessary in these patients. As a general rule, it should be done 48 to 72 hours after a positive culture.
Repeat cultures are indicated in specific scenarios, but for most patients, frequent and indiscriminate repetition after an initial positive culture is unnecessary and may be associated with excessive use of resources. Prospective studies and practice guidelines are needed to help further define the indications.
THE TENDENCY TO REPEAT CULTURES
Current literature lacks strong evidence for repeating previously positive blood cultures collected appropriately—ie, 10 mL of blood for aerobic culture and 10 mL for anaerobic culture from 2 different sites, and a positive result from both sets. However, because of the risk of serious complications of bacteremia, particularly in critically ill patients, many clinicians order multiple, repeated sets of blood cultures.
Tabriz et al1 found that one-third of hospitalized patients got repeat cultures after an initial set, regardless of the result of the first set. Most (83.4%) of those cultures yielded no growth, 9.1% grew the same pathogen, and 5.0% were contaminated. Finding a new pathogen was rare, occurring in only 2.5% of repeated cultures.
Wiggers et al2 reported an even higher number of repeat cultures ordered for patients who had an initially positive culture: 38.9%.2 And in another study,3 half of the patients received more than 2 consecutive cultures.
Drawbacks
Unrestrained ordering of repeat blood cultures can increase the risk of a false-positive result, leading to more cultures, echocardiography, other imaging tests, and unnecessary antimicrobial therapy, all of which puts patients at risk of adverse effects of treatment and missed alternative diagnoses and increases the length and cost of hospitalization.4
Advantages
On the other hand, repeat blood cultures may increase the diagnostic yield for conditions such as infective endocarditis and may have implications for the duration of antibiotic therapy.1 The duration of therapy for bacteremia is usually determined from the last negative culture; hence, documenting clearance of bacteremia can determine a precise end-date for antibiotic therapy.
Bacteremia due to Staphylococcus aureus and to endovascular and epidural sources has been found to be independently associated with persistent bacteremia, detected in 6.6% of 1,801 index cases of bacteremia in a retrospective cohort study.2 An endovascular source (adjusted odds ratio [OR] 7.66, 95% confidence interval [CI] 2.30–25.48), an epidural source (adjusted OR 26.99, 95% CI, 1.91–391.08), and S aureus bacteremia (adjusted OR 4.49, 95% CI 1.88–10.73) were independently associated with persistent bacteremia. Escherichia coli (5.1%, P = .006), viridans group streptococci (1.7%, P = .035), and beta-hemolytic streptococci (0%, P = .028) were associated with a lower likelihood of persistent bacteremia. Patients with persistent bacteremia were less likely to have achieved source control within 48 hours of the index event (29.7% vs 52.5%, P < .001).2
WHEN REPEATING CULTURES IS APPROPRIATE
Repeating blood cultures after an initial positive result is superfluous, except in certain situations.
Suspected endovascular infection
Patients with endocarditis, thrombophlebitis, an indwelling device for epidural access, or a cardiovascular implantable electronic device should have repeat cultures after an initial positive culture. Implantable electronic device infection is suspected in the following cases: sustained positive blood culture (> 24 hours); relapsing bacteremia despite a course of appropriate antibiotic therapy; presence of an implantable cardioverter defibrillator; presence of a prosthetic cardiac valve; and an episode of bacteremia within 3 months of device placement.5
S aureus bacteremia
Repeat blood culture is warranted for S aureus bacteremia regardless of methicillin susceptibility.1 But persistent methicillin-resistant S aureus (MRSA) bacteremia changes the management of these patients.6 For example, the source of infection should be identified, followed by debridement or drainage, and then either high-dose or combination antimicrobial therapy.6 Infective endocarditis from persistent MRSA bacteremia is an indication for surgery.6
Persistent S aureus bacteremia may change the duration of therapy, as the common practice is to continue treating uncomplicated gram-positive bacteremia for 14 days from the date of the first negative culture. Infection leading to infective endocarditis increases the duration of antibiotic therapy to at least 4 weeks.
Candidemia
Candidemia is an absolute indication for repeat blood culture.7 Patients with persistent candidemia should undergo imaging of the genitourinary tract, liver, and spleen as part of the evaluation for a deep-tissue source of infection.7 Also, if the patient is initially treated with an echinocandin, therapy can be transitioned to fluconazole if the isolate is azole-susceptible, the patient’s condition is clinically stable, and repeat cultures are negative.7 Therefore, repeating cultures has therapeutic implications.
Confirming response to therapy
In patients with infective endocarditis or other endovascular infection caused by S aureus, Enterococcus species, or gram-negative bacilli,1 repeat blood culture should be done to confirm therapeutic response. Patients with infective endocarditis whose condition is stable can be discharged to receive outpatient parenteral antibiotic therapy. However, patients with uncontrolled heart failure, systemic emboli, abscess, persistent fever, or persistently positive cultures are not candidates for outpatient therapy and require repeat cultures.8
Multidrug-resistant gram-negative bacilli
Bacteremia due to multidrug-resistant gram-negative bacilli requires repeat blood cultures to document clearance of bacteremia and to ensure the efficacy of antibiotics, as these organisms pose a higher risk of treatment failure, and combination synergistic regimens may be needed if bacteremia does not clear.
Febrile neutropenia
Blood cultures are important in the management of febrile neutropenia. In a study by Rosenblum et al,9 repeat cultures were positive in 10.9% of patients with febrile neutropenia after an initial negative culture, but many of those organisms were of low pathogenicity, and a significant proportion were coagulase-negative staphylococci.10 Another study showed that the frequency of detecting new pathogens by repeat culture in recurrent febrile neutropenia was higher than that in persistent febrile neutropenia (8% vs 2%) (P = .0491); a history of recent bacteremia was identified as a significant predictor of positive culture in recurrent febrile neutropenia.11
Persistent or new infection
Persistence of fever, leukocytosis, or other signs of infection 72 hours after appropriate antibiotic therapy is started requires follow-up blood cultures.
New episode of sepsis. A new episode of sepsis should be confirmed12 using the systemic inflammatory response syndrome criteria, the newer definition of Sepsis-related Organ Failure Assessment (SOFA) in the intensive-care unit, or the quick SOFA in general units. If the patient develops new signs of sepsis after response to treatment for initial bacteremia, repeat blood cultures should be considered.
Central line-associated bloodstream infection requires repeat cultures.13 Persistence of bacteremia in this type of infection extends the duration of therapy, as most clinicians determine treatment duration from the last negative culture. Persistent bacteremia also influences the decision to salvage or remove the catheter. Microbiologic clearance of bacteremia on blood culture can also guide the time of reinsertion if the catheter was removed.
Concern for an unresolved focus of infection such as abscess, joint infection, or retained catheter is an indication for repeat blood cultures.
Bacteremia of unknown source. In clinical practice, we encounter scenarios in which blood cultures are positive but no source can be identified. In those situations, it is important to repeat blood cultures to document clearance. If bacteremia persists, we need to continue searching for the source.
WHEN ROUTINELY REPEATING CULTURES IS NOT INDICATED
Repeat blood cultures are not routinely indicated in patients with streptococcal bacteremia, uncomplicated gram-negative bacteremia, and bacteremia associated with localized infection such as cellulitis, community-acquired pneumonia, or pyelonephritis.2,4 A study of patients with gram-negative bacteremia found that 17 repeated cultures needed to be drawn to yield 1 positive culture.14
Isolated fever or leukocytosis does not accurately predict bacteremia.4 A study that excluded neutropenic and intensive-care patients reported none of the initially negative cultures to be positive when repeated.15
Ordering repeat cultures in response to persistent fever is a common practice, even though fever is typical in the first 72 hours of antibiotic therapy. Such cultures rarely if ever reveal new pathogens, and results can be predicted based on cultures before the start of antibiotics.15 For patients on antibiotics, physicians should therefore wait for results of the preantibiotic cultures rather than order new cultures in response to persistent fever.15
WOULD WE MISS PERSISTENT BACTEREMIA?
In theory, not repeating blood cultures could miss persistent bacteremia, but this is unlikely if the concerns discussed above are considered. Further, persistent bacteremia would result in clinical signs and symptoms that should prompt repeat cultures.
FREQUENCY OF REPEAT BLOOD CULTURES
There are no evidence-based guidelines for the frequency of repeating cultures. The Infectious Diseases Society of America recommends repeating blood cultures 2 to 4 days after the index positive culture in the case of multidrug-resistant S aureus bacteremia, and every day or every other day for candidemia.6,7,9
A study evaluating the practice patterns of repeating cultures after an initial bacteremia showed that 34.7% were done within 24 hours and 44.7% were done in 2 to 4 days.1 There is no evidence that repeating blood cultures daily is necessary in these patients. As a general rule, it should be done 48 to 72 hours after a positive culture.
- Tabriz MS, Riederer K, Baran J Jr, Khatib R. Repeating blood cultures during hospital stay: practice pattern at a teaching hospital and a proposal for guidelines. Clin Microbiol Infect 2004; 10(7):624–627. doi:10.1111/j.1469-0691.2004.00893.x
- Wiggers JB, Xiong W, Daneman N. Sending repeat cultures: is there a role in the management of bacteremic episodes? (SCRIBE study). BMC Infect Dis 2016; 16:286. doi:10.1186/s12879-016-1622-z
- Kang CK, Kim ES, Song KH, et al. Can a routine follow-up blood culture be justified in Klebsiella pneumoniae bacteremia? A retrospective case–control study. BMC Infect Dis 2013; 13:365. doi:10.1186/1471-2334-13-365
- Coburn B, Morris AM, Tomlinson G, Detsky AS. Does this adult patient with suspected bacteremia require blood cultures? JAMA 2012; 308(5):502–511. doi:10.1001/jama.2012.8262
- Baddour LM, Epstein AE, Erickson CC, et al; American Heart Association Rheumatic Fever, Endocarditis, and Kawasaki Disease Committee; Council on Cardiovascular Disease in Young; Council on Cardiovascular Surgery and Anesthesia; Council on Cardiovascular Nursing; Council on Clinical Cardiology; Interdisciplinary Council on Quality of Care; American Heart Association. Update on cardiovascular implantable electronic device infections and their management: a scientific statement from the American Heart Association. Circulation 2010; 121(3):458–477. doi:10.1161/CIRCULATIONAHA.109.192665
- Liu C, Bayer A, Cosgrove SE, et al; Infectious Diseases Society of America. Clinical practice guidelines by the Infectious Diseases Society of America for the treatment of methicillin-resistant Staphylococcus aureus infections in adults and children. Clin Infect Dis 2011; 52(3):e18–e55. doi:10.1093/cid/ciq146
- Pappas PG, Kauffman CA, Andes DR, et al. Clinical practice guideline for the management of candidiasis: 2016 update by the Infectious Diseases Society of America. Clin Infect Dis 2016; 62(4):e1–e50. doi:10.1093/cid/civ933
- Baddour LM, Wilson WR, Bayer AS, et al; American Heart Association Committee on Rheumatic Fever, Endocarditis, and Kawasaki Disease of the Council on Cardiovascular Disease in the Young, Council on Clinical Cardiology, Council on Cardiovascular Surgery and Anesthesia, and Stroke Council. Infective endocarditis in adults: diagnosis, antimicrobial therapy, and management of complications: a scientific statement for healthcare professionals from the American Heart Association. Circulation 2015; 132(15):1435–1486. doi:10.1161/CIR.0000000000000296
- Rosenblum J, Lin J, Kim M, Levy AS. Repeating blood cultures in neutropenic children with persistent fevers when the initial blood culture is negative. Pediatr Blood Cancer 2013; 60(6):923–927. doi:10.1002/pbc.24358
- Thomas MW, Chauvenet AR, O'Suoji C. Repeating blood cultures in neutropenic children with persistent fevers when the initial blood culture is negative. Pediatr Blood Cancer 2014; 61(2):194. doi:10.1002/pbc.24834
- Kimura SI, Gomyo A, Hayakawa J, et al. Clinical significance of repeat blood cultures during febrile neutropenia in adult acute myeloid leukaemia patients undergoing intensive chemotherapy. Infect Dis (Lond) 2017; 49(10):748–757. doi:10.1080/23744235.2017.1340665
- Singer M, Deutschman CS, Seymour CW, et al. The third international consensus definitions for sepsis and septic shock (Sepsis-3). JAMA 2016; 315(8):801–810. doi:10.1001/jama.2016.0287
- Shah H, Bosch W, Thompson KM, Hellinger WC. Intravascular catheter-related bloodstream infection. Neurohospitalist 2013; 3(3):144–151. doi:10.1177/1941874413476043
- Canzoneri CN, Akhavan BJ, Tosur Z, Andrade PEA, Aisenberg GM. Follow-up blood cultures in gram-negative bacteremia: are they needed? Clin Infect Dis 2017; 65(11):1776–1779. doi:10.1093/cid/cix648
- Grace CJ, Lieberman J, Pierce K, Littenberg B. Usefulness of blood culture for hospitalized patients who are receiving antibiotic therapy. Clin Infect Dis 2001; 32(11):1651–1655. doi:10.1086/320527
- Tabriz MS, Riederer K, Baran J Jr, Khatib R. Repeating blood cultures during hospital stay: practice pattern at a teaching hospital and a proposal for guidelines. Clin Microbiol Infect 2004; 10(7):624–627. doi:10.1111/j.1469-0691.2004.00893.x
- Wiggers JB, Xiong W, Daneman N. Sending repeat cultures: is there a role in the management of bacteremic episodes? (SCRIBE study). BMC Infect Dis 2016; 16:286. doi:10.1186/s12879-016-1622-z
- Kang CK, Kim ES, Song KH, et al. Can a routine follow-up blood culture be justified in Klebsiella pneumoniae bacteremia? A retrospective case–control study. BMC Infect Dis 2013; 13:365. doi:10.1186/1471-2334-13-365
- Coburn B, Morris AM, Tomlinson G, Detsky AS. Does this adult patient with suspected bacteremia require blood cultures? JAMA 2012; 308(5):502–511. doi:10.1001/jama.2012.8262
- Baddour LM, Epstein AE, Erickson CC, et al; American Heart Association Rheumatic Fever, Endocarditis, and Kawasaki Disease Committee; Council on Cardiovascular Disease in Young; Council on Cardiovascular Surgery and Anesthesia; Council on Cardiovascular Nursing; Council on Clinical Cardiology; Interdisciplinary Council on Quality of Care; American Heart Association. Update on cardiovascular implantable electronic device infections and their management: a scientific statement from the American Heart Association. Circulation 2010; 121(3):458–477. doi:10.1161/CIRCULATIONAHA.109.192665
- Liu C, Bayer A, Cosgrove SE, et al; Infectious Diseases Society of America. Clinical practice guidelines by the Infectious Diseases Society of America for the treatment of methicillin-resistant Staphylococcus aureus infections in adults and children. Clin Infect Dis 2011; 52(3):e18–e55. doi:10.1093/cid/ciq146
- Pappas PG, Kauffman CA, Andes DR, et al. Clinical practice guideline for the management of candidiasis: 2016 update by the Infectious Diseases Society of America. Clin Infect Dis 2016; 62(4):e1–e50. doi:10.1093/cid/civ933
- Baddour LM, Wilson WR, Bayer AS, et al; American Heart Association Committee on Rheumatic Fever, Endocarditis, and Kawasaki Disease of the Council on Cardiovascular Disease in the Young, Council on Clinical Cardiology, Council on Cardiovascular Surgery and Anesthesia, and Stroke Council. Infective endocarditis in adults: diagnosis, antimicrobial therapy, and management of complications: a scientific statement for healthcare professionals from the American Heart Association. Circulation 2015; 132(15):1435–1486. doi:10.1161/CIR.0000000000000296
- Rosenblum J, Lin J, Kim M, Levy AS. Repeating blood cultures in neutropenic children with persistent fevers when the initial blood culture is negative. Pediatr Blood Cancer 2013; 60(6):923–927. doi:10.1002/pbc.24358
- Thomas MW, Chauvenet AR, O'Suoji C. Repeating blood cultures in neutropenic children with persistent fevers when the initial blood culture is negative. Pediatr Blood Cancer 2014; 61(2):194. doi:10.1002/pbc.24834
- Kimura SI, Gomyo A, Hayakawa J, et al. Clinical significance of repeat blood cultures during febrile neutropenia in adult acute myeloid leukaemia patients undergoing intensive chemotherapy. Infect Dis (Lond) 2017; 49(10):748–757. doi:10.1080/23744235.2017.1340665
- Singer M, Deutschman CS, Seymour CW, et al. The third international consensus definitions for sepsis and septic shock (Sepsis-3). JAMA 2016; 315(8):801–810. doi:10.1001/jama.2016.0287
- Shah H, Bosch W, Thompson KM, Hellinger WC. Intravascular catheter-related bloodstream infection. Neurohospitalist 2013; 3(3):144–151. doi:10.1177/1941874413476043
- Canzoneri CN, Akhavan BJ, Tosur Z, Andrade PEA, Aisenberg GM. Follow-up blood cultures in gram-negative bacteremia: are they needed? Clin Infect Dis 2017; 65(11):1776–1779. doi:10.1093/cid/cix648
- Grace CJ, Lieberman J, Pierce K, Littenberg B. Usefulness of blood culture for hospitalized patients who are receiving antibiotic therapy. Clin Infect Dis 2001; 32(11):1651–1655. doi:10.1086/320527