User login
Is chest radiography routinely needed after thoracentesis?
No. After thoracentesis, chest radiography or another lung imaging study should be done only if pneumothorax is suspected, if thoracentesis requires more than 1 attempt, if the patient is on mechanical ventilation or has pre-existing lung disease, or if a large volume (> 1,500 mL) of fluid is removed. Radiography is also usually not necessary after diagnostic thoracentesis in a patient breathing spontaneously. In most cases, pneumothorax found incidentally after thoracentesis does not require decompression and can be managed supportively.
WHAT ARE THE RISKS OF THORACENTESIS?
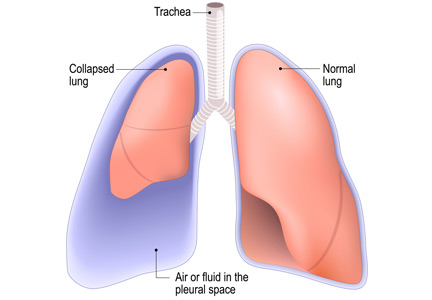
Thoracentesis is a minimally invasive procedure usually performed at the bedside that involves insertion of a needle into the pleural cavity for drainage of fluid.1 Diagnostic thoracentesis should be done in most cases of a new pleural effusion unless the effusion is small and with a clear diagnosis, or in cases of typical heart failure.
Therapeutic thoracentesis, often called large-volume thoracentesis, aims to improve symptoms such as dyspnea attributed to the pleural effusion by removing at least 1 L of pleural fluid. The presence of active respiratory symptoms and suspicion of infected pleural effusion should lead to thoracentesis as soon as possible.
Complications of thoracentesis may be benign, such as pain and anxiety associated with the procedure and external bleeding at the site of needle insertion. Pneumothorax is the most common serious procedural complication and the principal reason to order postprocedural chest radiography.1 Less common complications include hemothorax, re-expansion pulmonary edema, infection, subdiaphragmatic organ puncture, and procedure-related death. Bleeding complications and hemothorax are rare even in patients with underlying coagulopathy.2
Point-of-care pleural ultrasonography is now considered the standard of care to guide optimal needle location for the procedure and to exclude other conditions that can mimic pleural effusion on chest radiography, such as lung consolidation and atelectasis.3 High proficiency in the use of preprocedural point-of-care ultrasonography reduces the rate of procedural complications, though it does not eliminate the risk entirely.3,4
Factors associated with higher rates of complications include lack of operator proficiency, poor understanding of the anatomy, poor patient positioning, poor patient cooperation with the procedure, lack of availability of bedside ultrasonography, and drainage of more than 1,500 mL of fluid. Addressing these factors has been shown to decrease the risk of pneumothorax and infection.1–5
HOW OFTEN DOES PNEUMOTHORAX OCCUR AFTER THORACENTESIS?
Several early studies have examined the incidence of pneumothorax after thoracentesis. Lack of ultrasonography use likely explains a higher incidence of complications in early studies: rates of pneumothorax after thoracentesis without ultrasonographic guidance ranged from 5.2% to 26%.6,7
Gervais et al8 analyzed thoracentesis with ultrasonographic guidance in 434 patients, 92 of whom were intubated, and reported that pneumothorax occurred in 10 patients, of whom 6 were intubated. Two of the intubated patients required chest tubes. Other studies have confirmed the low incidence of pneumothorax in patients undergoing thoracentesis, with rates such as 0.61%,1 5%,9 and 4%.10
The major predictor of postprocedural pneumothorax was the presence of symptoms such as chest pain and dyspnea. No intervention was necessary for most cases of pneumothorax in asymptomatic patients. The more widespread use of procedural ultrasonography may explain some discrepancies between the early5,6 and more recent studies.1,8–10
Several studies have demonstrated that postprocedural radiography is unnecessary unless a complication is suspected based on the patient’s symptoms or the need to demonstrate lung re-expansion.1,4,9,10 Clinical suspicion and the patient’s symptoms are the major predictors of procedure-related pneumothorax requiring treatment with a chest tube. Otherwise, incidentally discovered pneumothorax can usually be observed and managed supportively.
WHAT MECHANISMS UNDERLIE POSTPROCEDURAL PNEUMOTHORAX?
Major causes of pneumothorax in patients undergoing thoracentesis are direct puncture during needle or catheter insertion, the introduction of air through the needle or catheter into the pleural cavity, and the inability of the ipsilateral lung to fully expand after drainage of a large volume of fluid, known as pneumothorax ex vacuo.5
Pneumothorax ex vacuo may be seen in patients with medical conditions such as endobronchial obstruction, pleural scarring from long-standing pleural effusion, and lung malignancy, all of which can impair the lung’s ability to expand after removal of a large volume of pleural fluid. It is believed that transient parenchymal pleural fistulae form if the lung cannot expand, causing air leakage into the pleural cavity.5,8,9 Pleural manometry to monitor changes in pleural pressure and elastance can decrease the rates of pneumothorax ex vacuo in patients with the above risk factors.5
WHEN IS RADIOGRAPHY INDICATED AFTER THORACENTESIS?
Current literature suggests that imaging to evaluate for postprocedural complications should be done if there is suspicion of a complication, if thoracentesis required multiple attempts, if the procedure caused aspiration of air, if the patient has advanced lung disease, if the patient is scheduled to undergo thoracic radiation, if the patient is on mechanical ventilation, and after therapeutic thoracentesis if a large volume of fluid is removed.1–10 Routine chest radiography after thoracentesis is not supported in the literature in the absence of these risk factors.
Some practitioners order chest imaging after therapeutic thoracentesis to assess for residual pleural fluid and for visualization of other abnormalities previously hidden by pleural effusion, rather than simply to exclude postprocedural pneumothorax. Alternatively, postprocedural bedside pleural ultrasonography with recording of images can be done to assess for complications and residual pleural fluid volume without exposing the patient to radiation.11
Needle decompression and chest tube insertion should be considered in patients with tension pneumothorax, large pneumothorax (distance from the chest wall to the visceral pleural line of at least 2 cm), mechanical ventilation, progressing pneumothorax, and symptoms.
KEY POINTS
- Pneumothorax is a rare complication of thoracentesis when performed by a skilled operator using ultrasonographic guidance.
- Mechanisms behind the occurrence of pneumothorax are direct lung puncture, introduction of air into the pleural cavity, and pneumothorax ex vacuo.
- In asymptomatic patients, pneumothorax after thoracentesis rarely requires intervention beyond supportive care and close observation.
- Factors such as multiple thoracentesis attempts, symptoms, clinical suspicion, air aspiration during thoracentesis, presence of previous lung disease, and removal of a large volume of fluid may require postprocedural lung imaging (eg, bedside ultrasonography, radiography).
- Ault MJ, Rosen BT, Scher J, Feinglass J, Barsuk JH. Thoracentesis outcomes: a 12-year experience. Thorax 2015; 70(2):127–132. doi:10.1136/thoraxjnl-2014-206114
- Hibbert RM, Atwell TD, Lekah A, et al. Safety of ultrasound-guided thoracentesis in patients with abnormal preprocedural coagulation parameters. Chest 2013; 144(2):456–463. doi:10.1378/chest.12-2374
- Barnes TW, Morgenthaler TI, Olson EJ, Hesley GK, Decker PA, Ryu JH. Sonographically guided thoracentesis and rate of pneumothorax. J Clin Ultrasound 2005; 33(9):442–446. doi:10.1002/jcu.20163
- Gordon CE, Feller-Kopman D, Balk EM, Smetana GW. Pneumothorax following thoracentesis: a systematic review and meta-analysis. Arch Intern Med 2010; 170(4):332–339. doi:10.1001/archinternmed.2009.548
- Heidecker J, Huggins JT, Sahn SA, Doelken P. Pathophysiology of pneumothorax following ultrasound-guided thoracentesis. Chest 2006; 130(4):1173–1184. doi:10.1016/S0012-3692(15)51155-0
- Brandstetter RD, Karetzky M, Rastogi R, Lolis JD. Pneumothorax after thoracentesis in chronic obstructive pulmonary disease. Heart Lung 1994; 23(1):67–70. pmid:8150647
- Doyle JJ, Hnatiuk OW, Torrington KG, Slade AR, Howard RS. Necessity of routine chest roentgenography after thoracentesis. Ann Intern Med 1996; 124(9):816–820. pmid:8610950
- Gervais DA, Petersein A, Lee MJ, Hahn PF, Saini S, Mueller PR. US-guided thoracentesis: requirement for postprocedure chest radiography in patients who receive mechanical ventilation versus patients who breathe spontaneously. Radiology 1997; 204(2):503–506. doi:10.1148/radiology.204.2.9240544
- Capizzi SA, Prakash UB. Chest roentgenography after outpatient thoracentesis. Mayo Clin Proc 1998; 73(10):948–950. doi:10.4065/73.10.948
- Alemán C, Alegre J, Armadans L, et al. The value of chest roentgenography in the diagnosis of pneumothorax after thoracentesis. Am J Med 1999; 107(4):340–343. pmid:10527035
- Lichtenstein D. Lung ultrasound in the critically ill. Curr Opin Crit Care 2014; 20(3):315–322. doi:10.1097/MCC.0000000000000096
No. After thoracentesis, chest radiography or another lung imaging study should be done only if pneumothorax is suspected, if thoracentesis requires more than 1 attempt, if the patient is on mechanical ventilation or has pre-existing lung disease, or if a large volume (> 1,500 mL) of fluid is removed. Radiography is also usually not necessary after diagnostic thoracentesis in a patient breathing spontaneously. In most cases, pneumothorax found incidentally after thoracentesis does not require decompression and can be managed supportively.
WHAT ARE THE RISKS OF THORACENTESIS?
Thoracentesis is a minimally invasive procedure usually performed at the bedside that involves insertion of a needle into the pleural cavity for drainage of fluid.1 Diagnostic thoracentesis should be done in most cases of a new pleural effusion unless the effusion is small and with a clear diagnosis, or in cases of typical heart failure.
Therapeutic thoracentesis, often called large-volume thoracentesis, aims to improve symptoms such as dyspnea attributed to the pleural effusion by removing at least 1 L of pleural fluid. The presence of active respiratory symptoms and suspicion of infected pleural effusion should lead to thoracentesis as soon as possible.
Complications of thoracentesis may be benign, such as pain and anxiety associated with the procedure and external bleeding at the site of needle insertion. Pneumothorax is the most common serious procedural complication and the principal reason to order postprocedural chest radiography.1 Less common complications include hemothorax, re-expansion pulmonary edema, infection, subdiaphragmatic organ puncture, and procedure-related death. Bleeding complications and hemothorax are rare even in patients with underlying coagulopathy.2
Point-of-care pleural ultrasonography is now considered the standard of care to guide optimal needle location for the procedure and to exclude other conditions that can mimic pleural effusion on chest radiography, such as lung consolidation and atelectasis.3 High proficiency in the use of preprocedural point-of-care ultrasonography reduces the rate of procedural complications, though it does not eliminate the risk entirely.3,4
Factors associated with higher rates of complications include lack of operator proficiency, poor understanding of the anatomy, poor patient positioning, poor patient cooperation with the procedure, lack of availability of bedside ultrasonography, and drainage of more than 1,500 mL of fluid. Addressing these factors has been shown to decrease the risk of pneumothorax and infection.1–5
HOW OFTEN DOES PNEUMOTHORAX OCCUR AFTER THORACENTESIS?
Several early studies have examined the incidence of pneumothorax after thoracentesis. Lack of ultrasonography use likely explains a higher incidence of complications in early studies: rates of pneumothorax after thoracentesis without ultrasonographic guidance ranged from 5.2% to 26%.6,7
Gervais et al8 analyzed thoracentesis with ultrasonographic guidance in 434 patients, 92 of whom were intubated, and reported that pneumothorax occurred in 10 patients, of whom 6 were intubated. Two of the intubated patients required chest tubes. Other studies have confirmed the low incidence of pneumothorax in patients undergoing thoracentesis, with rates such as 0.61%,1 5%,9 and 4%.10
The major predictor of postprocedural pneumothorax was the presence of symptoms such as chest pain and dyspnea. No intervention was necessary for most cases of pneumothorax in asymptomatic patients. The more widespread use of procedural ultrasonography may explain some discrepancies between the early5,6 and more recent studies.1,8–10
Several studies have demonstrated that postprocedural radiography is unnecessary unless a complication is suspected based on the patient’s symptoms or the need to demonstrate lung re-expansion.1,4,9,10 Clinical suspicion and the patient’s symptoms are the major predictors of procedure-related pneumothorax requiring treatment with a chest tube. Otherwise, incidentally discovered pneumothorax can usually be observed and managed supportively.
WHAT MECHANISMS UNDERLIE POSTPROCEDURAL PNEUMOTHORAX?
Major causes of pneumothorax in patients undergoing thoracentesis are direct puncture during needle or catheter insertion, the introduction of air through the needle or catheter into the pleural cavity, and the inability of the ipsilateral lung to fully expand after drainage of a large volume of fluid, known as pneumothorax ex vacuo.5
Pneumothorax ex vacuo may be seen in patients with medical conditions such as endobronchial obstruction, pleural scarring from long-standing pleural effusion, and lung malignancy, all of which can impair the lung’s ability to expand after removal of a large volume of pleural fluid. It is believed that transient parenchymal pleural fistulae form if the lung cannot expand, causing air leakage into the pleural cavity.5,8,9 Pleural manometry to monitor changes in pleural pressure and elastance can decrease the rates of pneumothorax ex vacuo in patients with the above risk factors.5
WHEN IS RADIOGRAPHY INDICATED AFTER THORACENTESIS?
Current literature suggests that imaging to evaluate for postprocedural complications should be done if there is suspicion of a complication, if thoracentesis required multiple attempts, if the procedure caused aspiration of air, if the patient has advanced lung disease, if the patient is scheduled to undergo thoracic radiation, if the patient is on mechanical ventilation, and after therapeutic thoracentesis if a large volume of fluid is removed.1–10 Routine chest radiography after thoracentesis is not supported in the literature in the absence of these risk factors.
Some practitioners order chest imaging after therapeutic thoracentesis to assess for residual pleural fluid and for visualization of other abnormalities previously hidden by pleural effusion, rather than simply to exclude postprocedural pneumothorax. Alternatively, postprocedural bedside pleural ultrasonography with recording of images can be done to assess for complications and residual pleural fluid volume without exposing the patient to radiation.11
Needle decompression and chest tube insertion should be considered in patients with tension pneumothorax, large pneumothorax (distance from the chest wall to the visceral pleural line of at least 2 cm), mechanical ventilation, progressing pneumothorax, and symptoms.
KEY POINTS
- Pneumothorax is a rare complication of thoracentesis when performed by a skilled operator using ultrasonographic guidance.
- Mechanisms behind the occurrence of pneumothorax are direct lung puncture, introduction of air into the pleural cavity, and pneumothorax ex vacuo.
- In asymptomatic patients, pneumothorax after thoracentesis rarely requires intervention beyond supportive care and close observation.
- Factors such as multiple thoracentesis attempts, symptoms, clinical suspicion, air aspiration during thoracentesis, presence of previous lung disease, and removal of a large volume of fluid may require postprocedural lung imaging (eg, bedside ultrasonography, radiography).
No. After thoracentesis, chest radiography or another lung imaging study should be done only if pneumothorax is suspected, if thoracentesis requires more than 1 attempt, if the patient is on mechanical ventilation or has pre-existing lung disease, or if a large volume (> 1,500 mL) of fluid is removed. Radiography is also usually not necessary after diagnostic thoracentesis in a patient breathing spontaneously. In most cases, pneumothorax found incidentally after thoracentesis does not require decompression and can be managed supportively.
WHAT ARE THE RISKS OF THORACENTESIS?
Thoracentesis is a minimally invasive procedure usually performed at the bedside that involves insertion of a needle into the pleural cavity for drainage of fluid.1 Diagnostic thoracentesis should be done in most cases of a new pleural effusion unless the effusion is small and with a clear diagnosis, or in cases of typical heart failure.
Therapeutic thoracentesis, often called large-volume thoracentesis, aims to improve symptoms such as dyspnea attributed to the pleural effusion by removing at least 1 L of pleural fluid. The presence of active respiratory symptoms and suspicion of infected pleural effusion should lead to thoracentesis as soon as possible.
Complications of thoracentesis may be benign, such as pain and anxiety associated with the procedure and external bleeding at the site of needle insertion. Pneumothorax is the most common serious procedural complication and the principal reason to order postprocedural chest radiography.1 Less common complications include hemothorax, re-expansion pulmonary edema, infection, subdiaphragmatic organ puncture, and procedure-related death. Bleeding complications and hemothorax are rare even in patients with underlying coagulopathy.2
Point-of-care pleural ultrasonography is now considered the standard of care to guide optimal needle location for the procedure and to exclude other conditions that can mimic pleural effusion on chest radiography, such as lung consolidation and atelectasis.3 High proficiency in the use of preprocedural point-of-care ultrasonography reduces the rate of procedural complications, though it does not eliminate the risk entirely.3,4
Factors associated with higher rates of complications include lack of operator proficiency, poor understanding of the anatomy, poor patient positioning, poor patient cooperation with the procedure, lack of availability of bedside ultrasonography, and drainage of more than 1,500 mL of fluid. Addressing these factors has been shown to decrease the risk of pneumothorax and infection.1–5
HOW OFTEN DOES PNEUMOTHORAX OCCUR AFTER THORACENTESIS?
Several early studies have examined the incidence of pneumothorax after thoracentesis. Lack of ultrasonography use likely explains a higher incidence of complications in early studies: rates of pneumothorax after thoracentesis without ultrasonographic guidance ranged from 5.2% to 26%.6,7
Gervais et al8 analyzed thoracentesis with ultrasonographic guidance in 434 patients, 92 of whom were intubated, and reported that pneumothorax occurred in 10 patients, of whom 6 were intubated. Two of the intubated patients required chest tubes. Other studies have confirmed the low incidence of pneumothorax in patients undergoing thoracentesis, with rates such as 0.61%,1 5%,9 and 4%.10
The major predictor of postprocedural pneumothorax was the presence of symptoms such as chest pain and dyspnea. No intervention was necessary for most cases of pneumothorax in asymptomatic patients. The more widespread use of procedural ultrasonography may explain some discrepancies between the early5,6 and more recent studies.1,8–10
Several studies have demonstrated that postprocedural radiography is unnecessary unless a complication is suspected based on the patient’s symptoms or the need to demonstrate lung re-expansion.1,4,9,10 Clinical suspicion and the patient’s symptoms are the major predictors of procedure-related pneumothorax requiring treatment with a chest tube. Otherwise, incidentally discovered pneumothorax can usually be observed and managed supportively.
WHAT MECHANISMS UNDERLIE POSTPROCEDURAL PNEUMOTHORAX?
Major causes of pneumothorax in patients undergoing thoracentesis are direct puncture during needle or catheter insertion, the introduction of air through the needle or catheter into the pleural cavity, and the inability of the ipsilateral lung to fully expand after drainage of a large volume of fluid, known as pneumothorax ex vacuo.5
Pneumothorax ex vacuo may be seen in patients with medical conditions such as endobronchial obstruction, pleural scarring from long-standing pleural effusion, and lung malignancy, all of which can impair the lung’s ability to expand after removal of a large volume of pleural fluid. It is believed that transient parenchymal pleural fistulae form if the lung cannot expand, causing air leakage into the pleural cavity.5,8,9 Pleural manometry to monitor changes in pleural pressure and elastance can decrease the rates of pneumothorax ex vacuo in patients with the above risk factors.5
WHEN IS RADIOGRAPHY INDICATED AFTER THORACENTESIS?
Current literature suggests that imaging to evaluate for postprocedural complications should be done if there is suspicion of a complication, if thoracentesis required multiple attempts, if the procedure caused aspiration of air, if the patient has advanced lung disease, if the patient is scheduled to undergo thoracic radiation, if the patient is on mechanical ventilation, and after therapeutic thoracentesis if a large volume of fluid is removed.1–10 Routine chest radiography after thoracentesis is not supported in the literature in the absence of these risk factors.
Some practitioners order chest imaging after therapeutic thoracentesis to assess for residual pleural fluid and for visualization of other abnormalities previously hidden by pleural effusion, rather than simply to exclude postprocedural pneumothorax. Alternatively, postprocedural bedside pleural ultrasonography with recording of images can be done to assess for complications and residual pleural fluid volume without exposing the patient to radiation.11
Needle decompression and chest tube insertion should be considered in patients with tension pneumothorax, large pneumothorax (distance from the chest wall to the visceral pleural line of at least 2 cm), mechanical ventilation, progressing pneumothorax, and symptoms.
KEY POINTS
- Pneumothorax is a rare complication of thoracentesis when performed by a skilled operator using ultrasonographic guidance.
- Mechanisms behind the occurrence of pneumothorax are direct lung puncture, introduction of air into the pleural cavity, and pneumothorax ex vacuo.
- In asymptomatic patients, pneumothorax after thoracentesis rarely requires intervention beyond supportive care and close observation.
- Factors such as multiple thoracentesis attempts, symptoms, clinical suspicion, air aspiration during thoracentesis, presence of previous lung disease, and removal of a large volume of fluid may require postprocedural lung imaging (eg, bedside ultrasonography, radiography).
- Ault MJ, Rosen BT, Scher J, Feinglass J, Barsuk JH. Thoracentesis outcomes: a 12-year experience. Thorax 2015; 70(2):127–132. doi:10.1136/thoraxjnl-2014-206114
- Hibbert RM, Atwell TD, Lekah A, et al. Safety of ultrasound-guided thoracentesis in patients with abnormal preprocedural coagulation parameters. Chest 2013; 144(2):456–463. doi:10.1378/chest.12-2374
- Barnes TW, Morgenthaler TI, Olson EJ, Hesley GK, Decker PA, Ryu JH. Sonographically guided thoracentesis and rate of pneumothorax. J Clin Ultrasound 2005; 33(9):442–446. doi:10.1002/jcu.20163
- Gordon CE, Feller-Kopman D, Balk EM, Smetana GW. Pneumothorax following thoracentesis: a systematic review and meta-analysis. Arch Intern Med 2010; 170(4):332–339. doi:10.1001/archinternmed.2009.548
- Heidecker J, Huggins JT, Sahn SA, Doelken P. Pathophysiology of pneumothorax following ultrasound-guided thoracentesis. Chest 2006; 130(4):1173–1184. doi:10.1016/S0012-3692(15)51155-0
- Brandstetter RD, Karetzky M, Rastogi R, Lolis JD. Pneumothorax after thoracentesis in chronic obstructive pulmonary disease. Heart Lung 1994; 23(1):67–70. pmid:8150647
- Doyle JJ, Hnatiuk OW, Torrington KG, Slade AR, Howard RS. Necessity of routine chest roentgenography after thoracentesis. Ann Intern Med 1996; 124(9):816–820. pmid:8610950
- Gervais DA, Petersein A, Lee MJ, Hahn PF, Saini S, Mueller PR. US-guided thoracentesis: requirement for postprocedure chest radiography in patients who receive mechanical ventilation versus patients who breathe spontaneously. Radiology 1997; 204(2):503–506. doi:10.1148/radiology.204.2.9240544
- Capizzi SA, Prakash UB. Chest roentgenography after outpatient thoracentesis. Mayo Clin Proc 1998; 73(10):948–950. doi:10.4065/73.10.948
- Alemán C, Alegre J, Armadans L, et al. The value of chest roentgenography in the diagnosis of pneumothorax after thoracentesis. Am J Med 1999; 107(4):340–343. pmid:10527035
- Lichtenstein D. Lung ultrasound in the critically ill. Curr Opin Crit Care 2014; 20(3):315–322. doi:10.1097/MCC.0000000000000096
- Ault MJ, Rosen BT, Scher J, Feinglass J, Barsuk JH. Thoracentesis outcomes: a 12-year experience. Thorax 2015; 70(2):127–132. doi:10.1136/thoraxjnl-2014-206114
- Hibbert RM, Atwell TD, Lekah A, et al. Safety of ultrasound-guided thoracentesis in patients with abnormal preprocedural coagulation parameters. Chest 2013; 144(2):456–463. doi:10.1378/chest.12-2374
- Barnes TW, Morgenthaler TI, Olson EJ, Hesley GK, Decker PA, Ryu JH. Sonographically guided thoracentesis and rate of pneumothorax. J Clin Ultrasound 2005; 33(9):442–446. doi:10.1002/jcu.20163
- Gordon CE, Feller-Kopman D, Balk EM, Smetana GW. Pneumothorax following thoracentesis: a systematic review and meta-analysis. Arch Intern Med 2010; 170(4):332–339. doi:10.1001/archinternmed.2009.548
- Heidecker J, Huggins JT, Sahn SA, Doelken P. Pathophysiology of pneumothorax following ultrasound-guided thoracentesis. Chest 2006; 130(4):1173–1184. doi:10.1016/S0012-3692(15)51155-0
- Brandstetter RD, Karetzky M, Rastogi R, Lolis JD. Pneumothorax after thoracentesis in chronic obstructive pulmonary disease. Heart Lung 1994; 23(1):67–70. pmid:8150647
- Doyle JJ, Hnatiuk OW, Torrington KG, Slade AR, Howard RS. Necessity of routine chest roentgenography after thoracentesis. Ann Intern Med 1996; 124(9):816–820. pmid:8610950
- Gervais DA, Petersein A, Lee MJ, Hahn PF, Saini S, Mueller PR. US-guided thoracentesis: requirement for postprocedure chest radiography in patients who receive mechanical ventilation versus patients who breathe spontaneously. Radiology 1997; 204(2):503–506. doi:10.1148/radiology.204.2.9240544
- Capizzi SA, Prakash UB. Chest roentgenography after outpatient thoracentesis. Mayo Clin Proc 1998; 73(10):948–950. doi:10.4065/73.10.948
- Alemán C, Alegre J, Armadans L, et al. The value of chest roentgenography in the diagnosis of pneumothorax after thoracentesis. Am J Med 1999; 107(4):340–343. pmid:10527035
- Lichtenstein D. Lung ultrasound in the critically ill. Curr Opin Crit Care 2014; 20(3):315–322. doi:10.1097/MCC.0000000000000096
What Can Be Done to Maintain Positive Patient Experience and Improve Residents’ Satisfaction? In Reference to: “Standardized Attending Rounds to Improve the Patient Experience: A Pragmatic Cluster Randomized Controlled Trial”
We read the article by Monash et al.1 published in the March 2017 issue with great interest. This randomized study showed a discrepancy between patients’ and residents’ satisfaction with standardized rounds; for example, residents reported less autonomy, efficiency, teaching, and longer time of rounds.
We agree that letting residents lead the rounds with minimal participation of an attending (only when needed) may improve resident satisfaction. Other factors, such as quality of teaching, positive comments to learners during bedside rounds (whenever appropriate), and a positive attending attitude, might be helpful.2,3 We believe that the adaptation of such a model through the prism of residents’ benefit will lead to better satisfaction among trainees.
On the other hand, we note that the nature of the study might have exaggerated patient satisfaction when compared with real-world surveys.4 The survey appears to focus only on attending rounds and did not consider other factors like hospitality, pain control, etc. A low patient census and lack of double blinding are other potential factors.
In conclusion, we want to congratulate the authors for raising this important topic and showing positive patients’ satisfaction with standardized rounds on teaching services. Further research should focus on improving residents’ satisfaction without compromising patients’ experiences.
1. Monash B, Najafi N, Mourad M, et al. Standardized Attending Rounds to Improve the Patient Experience: A Pragmatic Cluster Randomized Controlled Trial. J Hosp Med. 2017;12(3):143-149. PubMed
2. Williams KN, Ramani S, Fraser B, Orlander JD. Improving bedside teaching: findings from a focus group study of learners. Acad Med. 2008;83(3):257-264. PubMed
3. Castiglioni A, Shewchuk RM, Willett LL, Heudebert GR, Centor RM. A pilot study using nominal group technique to assess residents’ perceptions of successful attending rounds. J Gen Intern Med. 2008;23(7):1060-1065. PubMed
4. Siddiqui ZK, Wu AW, Kurbanova N, Qayyum R. Comparison of Hospital Consumer Assessment of Healthcare Providers and Systems patient satisfaction scores for specialty hospitals and general medical hospitals: confounding effect of survey response rate. J Hosp Med. 2014;9(9):590-593. PubMed
We read the article by Monash et al.1 published in the March 2017 issue with great interest. This randomized study showed a discrepancy between patients’ and residents’ satisfaction with standardized rounds; for example, residents reported less autonomy, efficiency, teaching, and longer time of rounds.
We agree that letting residents lead the rounds with minimal participation of an attending (only when needed) may improve resident satisfaction. Other factors, such as quality of teaching, positive comments to learners during bedside rounds (whenever appropriate), and a positive attending attitude, might be helpful.2,3 We believe that the adaptation of such a model through the prism of residents’ benefit will lead to better satisfaction among trainees.
On the other hand, we note that the nature of the study might have exaggerated patient satisfaction when compared with real-world surveys.4 The survey appears to focus only on attending rounds and did not consider other factors like hospitality, pain control, etc. A low patient census and lack of double blinding are other potential factors.
In conclusion, we want to congratulate the authors for raising this important topic and showing positive patients’ satisfaction with standardized rounds on teaching services. Further research should focus on improving residents’ satisfaction without compromising patients’ experiences.
We read the article by Monash et al.1 published in the March 2017 issue with great interest. This randomized study showed a discrepancy between patients’ and residents’ satisfaction with standardized rounds; for example, residents reported less autonomy, efficiency, teaching, and longer time of rounds.
We agree that letting residents lead the rounds with minimal participation of an attending (only when needed) may improve resident satisfaction. Other factors, such as quality of teaching, positive comments to learners during bedside rounds (whenever appropriate), and a positive attending attitude, might be helpful.2,3 We believe that the adaptation of such a model through the prism of residents’ benefit will lead to better satisfaction among trainees.
On the other hand, we note that the nature of the study might have exaggerated patient satisfaction when compared with real-world surveys.4 The survey appears to focus only on attending rounds and did not consider other factors like hospitality, pain control, etc. A low patient census and lack of double blinding are other potential factors.
In conclusion, we want to congratulate the authors for raising this important topic and showing positive patients’ satisfaction with standardized rounds on teaching services. Further research should focus on improving residents’ satisfaction without compromising patients’ experiences.
1. Monash B, Najafi N, Mourad M, et al. Standardized Attending Rounds to Improve the Patient Experience: A Pragmatic Cluster Randomized Controlled Trial. J Hosp Med. 2017;12(3):143-149. PubMed
2. Williams KN, Ramani S, Fraser B, Orlander JD. Improving bedside teaching: findings from a focus group study of learners. Acad Med. 2008;83(3):257-264. PubMed
3. Castiglioni A, Shewchuk RM, Willett LL, Heudebert GR, Centor RM. A pilot study using nominal group technique to assess residents’ perceptions of successful attending rounds. J Gen Intern Med. 2008;23(7):1060-1065. PubMed
4. Siddiqui ZK, Wu AW, Kurbanova N, Qayyum R. Comparison of Hospital Consumer Assessment of Healthcare Providers and Systems patient satisfaction scores for specialty hospitals and general medical hospitals: confounding effect of survey response rate. J Hosp Med. 2014;9(9):590-593. PubMed
1. Monash B, Najafi N, Mourad M, et al. Standardized Attending Rounds to Improve the Patient Experience: A Pragmatic Cluster Randomized Controlled Trial. J Hosp Med. 2017;12(3):143-149. PubMed
2. Williams KN, Ramani S, Fraser B, Orlander JD. Improving bedside teaching: findings from a focus group study of learners. Acad Med. 2008;83(3):257-264. PubMed
3. Castiglioni A, Shewchuk RM, Willett LL, Heudebert GR, Centor RM. A pilot study using nominal group technique to assess residents’ perceptions of successful attending rounds. J Gen Intern Med. 2008;23(7):1060-1065. PubMed
4. Siddiqui ZK, Wu AW, Kurbanova N, Qayyum R. Comparison of Hospital Consumer Assessment of Healthcare Providers and Systems patient satisfaction scores for specialty hospitals and general medical hospitals: confounding effect of survey response rate. J Hosp Med. 2014;9(9):590-593. PubMed
Delayed ICU Transfer Affects Mortality and Length of Stay
Clinical Question: Can an objective measurement of critical illness inform intensive care unit (ICU) transfer timeliness?
Background: Early intervention has shown mortality benefit in many critical illness syndromes, yet heterogeneity in timing of ICU transfer exists. Previous studies examining ICU transfer timeliness have mostly focused on subjective criteria.
Study Design: Retrospective observational cohort study.
Setting: Medical-surgical units at five hospitals including the University of Chicago and NorthShore University HealthSystem in Illinois.
Synopsis: All medical-surgical ward patients between November 2008 and January 2013 were scored using eCART, a previously validated objective scoring system, to decide when transfer was appropriate. Of those, 3,789 patients reached the predetermined threshold for critical illness. Transfers more than six hours after crossing the threshold were considered delayed. Patients with delayed transfer had a statistically significant increase in length of stay (LOS) and in-hospital mortality (33.2% versus 24.5%; P < 0.001), and the mortality increase was linear, with a 3% increase in odds for each one hour of further transfer delay (P < 0.001). The rate of change of eCART score did influence time of transfer, and the authors suggest that rapid changes were more likely to be recognized. They postulate that routine implementation of eCART or similar objective scoring may lead to earlier recognition of necessary ICU transfer and thus improve mortality and LOS, and they suggest this as a topic for future trials.
Bottom Line: Delayed ICU transfer negatively affects LOS and in-hospital mortality. Objective criteria may identify more appropriate timing of transfer. Clinical trials to investigate this are warranted.
Citation: Churpek MM, Wendlandt B, Zadravecz FJ, Adhikari R, Winslow C, Edelson DP. Association between intensive care unit transfer delay and hospital mortality: a multicenter investigation [published online ahead of print June 28, 2016]. J Hosp Med. doi:10.1002/jhm.2630.
Short Take
Intranasal Live Attenuated Influenza Vaccine Not Recommended
The Centers for Disease Control and Prevention recommends against use of the nasal spray live attenuated influenza vaccine. This is based on data showing poor effectiveness in prior years.
Citation: ACIP votes down use of LAIV for 2016-2017 flu season [press release]. CDC website.
Clinical Question: Can an objective measurement of critical illness inform intensive care unit (ICU) transfer timeliness?
Background: Early intervention has shown mortality benefit in many critical illness syndromes, yet heterogeneity in timing of ICU transfer exists. Previous studies examining ICU transfer timeliness have mostly focused on subjective criteria.
Study Design: Retrospective observational cohort study.
Setting: Medical-surgical units at five hospitals including the University of Chicago and NorthShore University HealthSystem in Illinois.
Synopsis: All medical-surgical ward patients between November 2008 and January 2013 were scored using eCART, a previously validated objective scoring system, to decide when transfer was appropriate. Of those, 3,789 patients reached the predetermined threshold for critical illness. Transfers more than six hours after crossing the threshold were considered delayed. Patients with delayed transfer had a statistically significant increase in length of stay (LOS) and in-hospital mortality (33.2% versus 24.5%; P < 0.001), and the mortality increase was linear, with a 3% increase in odds for each one hour of further transfer delay (P < 0.001). The rate of change of eCART score did influence time of transfer, and the authors suggest that rapid changes were more likely to be recognized. They postulate that routine implementation of eCART or similar objective scoring may lead to earlier recognition of necessary ICU transfer and thus improve mortality and LOS, and they suggest this as a topic for future trials.
Bottom Line: Delayed ICU transfer negatively affects LOS and in-hospital mortality. Objective criteria may identify more appropriate timing of transfer. Clinical trials to investigate this are warranted.
Citation: Churpek MM, Wendlandt B, Zadravecz FJ, Adhikari R, Winslow C, Edelson DP. Association between intensive care unit transfer delay and hospital mortality: a multicenter investigation [published online ahead of print June 28, 2016]. J Hosp Med. doi:10.1002/jhm.2630.
Short Take
Intranasal Live Attenuated Influenza Vaccine Not Recommended
The Centers for Disease Control and Prevention recommends against use of the nasal spray live attenuated influenza vaccine. This is based on data showing poor effectiveness in prior years.
Citation: ACIP votes down use of LAIV for 2016-2017 flu season [press release]. CDC website.
Clinical Question: Can an objective measurement of critical illness inform intensive care unit (ICU) transfer timeliness?
Background: Early intervention has shown mortality benefit in many critical illness syndromes, yet heterogeneity in timing of ICU transfer exists. Previous studies examining ICU transfer timeliness have mostly focused on subjective criteria.
Study Design: Retrospective observational cohort study.
Setting: Medical-surgical units at five hospitals including the University of Chicago and NorthShore University HealthSystem in Illinois.
Synopsis: All medical-surgical ward patients between November 2008 and January 2013 were scored using eCART, a previously validated objective scoring system, to decide when transfer was appropriate. Of those, 3,789 patients reached the predetermined threshold for critical illness. Transfers more than six hours after crossing the threshold were considered delayed. Patients with delayed transfer had a statistically significant increase in length of stay (LOS) and in-hospital mortality (33.2% versus 24.5%; P < 0.001), and the mortality increase was linear, with a 3% increase in odds for each one hour of further transfer delay (P < 0.001). The rate of change of eCART score did influence time of transfer, and the authors suggest that rapid changes were more likely to be recognized. They postulate that routine implementation of eCART or similar objective scoring may lead to earlier recognition of necessary ICU transfer and thus improve mortality and LOS, and they suggest this as a topic for future trials.
Bottom Line: Delayed ICU transfer negatively affects LOS and in-hospital mortality. Objective criteria may identify more appropriate timing of transfer. Clinical trials to investigate this are warranted.
Citation: Churpek MM, Wendlandt B, Zadravecz FJ, Adhikari R, Winslow C, Edelson DP. Association between intensive care unit transfer delay and hospital mortality: a multicenter investigation [published online ahead of print June 28, 2016]. J Hosp Med. doi:10.1002/jhm.2630.
Short Take
Intranasal Live Attenuated Influenza Vaccine Not Recommended
The Centers for Disease Control and Prevention recommends against use of the nasal spray live attenuated influenza vaccine. This is based on data showing poor effectiveness in prior years.
Citation: ACIP votes down use of LAIV for 2016-2017 flu season [press release]. CDC website.
IV Fluid Can Save Lives in Hemodynamically Stable Patients with Sepsis
Clinical Question: Does increased fluid administration in patients with sepsis with intermediate lactate levels improve outcomes?
Background: The Surviving Sepsis Campaign bundle, which improves ED mortality, targets patients with hypotension or lactate levels >4 mmol/L. No similar optimal treatment strategy exists for less severe sepsis patients even though such patients are more common in hospitalized populations.
Study Design: Retrospective study of a quality improvement bundle.
Setting: 21 community-based hospitals in the Kaiser Permanente Northern California system.
Synopsis: This study evaluated implementation of a treatment bundle for 18,122 hemodynamically stable sepsis patients presenting to the ED with lactate levels between 2 and 4 mmol/L during the 12 months prior to and after bundle implementation. The bundle included antibiotic administration within three hours, repeated lactate levels within four hours, and 30 mL/kg or ≥2 L of intravenous fluids within three hours of initial lactate result. Patients with kidney disease and/or heart failure were separately evaluated because of the perceived risk of fluid administration.
Treatment after bundle implementation was associated with an adjusted hospital mortality odds ratio of 0.81 (95% CI, 0.66–0.99; P = 0.04). Significant reductions in hospital mortality were observed in patients with heart failure and/or kidney disease (P < 0.01) but not without (P > 0.4). This correlated with increased fluid administration in patients with heart failure and/or kidney disease following bundle implementation. This is not a randomized controlled study, which invites biases and confounding.
Bottom Line: Increased fluid administration improved mortality in patients with kidney disease and heart failure presenting with sepsis.
Reference: Liu V, Morehouse JW, Marelich GP, et al. Multicenter implementation of a treatment bundle for patients with sepsis and intermediate lactate values. Am J Respir Crit Care Med. 2016;193(11):1264-1270.
Short Take
New Framework for Learners’ Clinical Reasoning
A qualitative study involving 37 emergency medicine residents found that clinical reasoning through individual cases progresses from case framing (phase 1) to pattern recognition (phase 2), then self-monitoring (phase 3).
Citation: Adams E, Goyder C, Heneghan C, Brand L, Ajjawi R. Clinical reasoning of junior doctors in emergency medicine: a grounded theory study [published online ahead of print June 23, 2016]. Emerg Med J. doi:10.1136/emermed-2015-205650.
Clinical Question: Does increased fluid administration in patients with sepsis with intermediate lactate levels improve outcomes?
Background: The Surviving Sepsis Campaign bundle, which improves ED mortality, targets patients with hypotension or lactate levels >4 mmol/L. No similar optimal treatment strategy exists for less severe sepsis patients even though such patients are more common in hospitalized populations.
Study Design: Retrospective study of a quality improvement bundle.
Setting: 21 community-based hospitals in the Kaiser Permanente Northern California system.
Synopsis: This study evaluated implementation of a treatment bundle for 18,122 hemodynamically stable sepsis patients presenting to the ED with lactate levels between 2 and 4 mmol/L during the 12 months prior to and after bundle implementation. The bundle included antibiotic administration within three hours, repeated lactate levels within four hours, and 30 mL/kg or ≥2 L of intravenous fluids within three hours of initial lactate result. Patients with kidney disease and/or heart failure were separately evaluated because of the perceived risk of fluid administration.
Treatment after bundle implementation was associated with an adjusted hospital mortality odds ratio of 0.81 (95% CI, 0.66–0.99; P = 0.04). Significant reductions in hospital mortality were observed in patients with heart failure and/or kidney disease (P < 0.01) but not without (P > 0.4). This correlated with increased fluid administration in patients with heart failure and/or kidney disease following bundle implementation. This is not a randomized controlled study, which invites biases and confounding.
Bottom Line: Increased fluid administration improved mortality in patients with kidney disease and heart failure presenting with sepsis.
Reference: Liu V, Morehouse JW, Marelich GP, et al. Multicenter implementation of a treatment bundle for patients with sepsis and intermediate lactate values. Am J Respir Crit Care Med. 2016;193(11):1264-1270.
Short Take
New Framework for Learners’ Clinical Reasoning
A qualitative study involving 37 emergency medicine residents found that clinical reasoning through individual cases progresses from case framing (phase 1) to pattern recognition (phase 2), then self-monitoring (phase 3).
Citation: Adams E, Goyder C, Heneghan C, Brand L, Ajjawi R. Clinical reasoning of junior doctors in emergency medicine: a grounded theory study [published online ahead of print June 23, 2016]. Emerg Med J. doi:10.1136/emermed-2015-205650.
Clinical Question: Does increased fluid administration in patients with sepsis with intermediate lactate levels improve outcomes?
Background: The Surviving Sepsis Campaign bundle, which improves ED mortality, targets patients with hypotension or lactate levels >4 mmol/L. No similar optimal treatment strategy exists for less severe sepsis patients even though such patients are more common in hospitalized populations.
Study Design: Retrospective study of a quality improvement bundle.
Setting: 21 community-based hospitals in the Kaiser Permanente Northern California system.
Synopsis: This study evaluated implementation of a treatment bundle for 18,122 hemodynamically stable sepsis patients presenting to the ED with lactate levels between 2 and 4 mmol/L during the 12 months prior to and after bundle implementation. The bundle included antibiotic administration within three hours, repeated lactate levels within four hours, and 30 mL/kg or ≥2 L of intravenous fluids within three hours of initial lactate result. Patients with kidney disease and/or heart failure were separately evaluated because of the perceived risk of fluid administration.
Treatment after bundle implementation was associated with an adjusted hospital mortality odds ratio of 0.81 (95% CI, 0.66–0.99; P = 0.04). Significant reductions in hospital mortality were observed in patients with heart failure and/or kidney disease (P < 0.01) but not without (P > 0.4). This correlated with increased fluid administration in patients with heart failure and/or kidney disease following bundle implementation. This is not a randomized controlled study, which invites biases and confounding.
Bottom Line: Increased fluid administration improved mortality in patients with kidney disease and heart failure presenting with sepsis.
Reference: Liu V, Morehouse JW, Marelich GP, et al. Multicenter implementation of a treatment bundle for patients with sepsis and intermediate lactate values. Am J Respir Crit Care Med. 2016;193(11):1264-1270.
Short Take
New Framework for Learners’ Clinical Reasoning
A qualitative study involving 37 emergency medicine residents found that clinical reasoning through individual cases progresses from case framing (phase 1) to pattern recognition (phase 2), then self-monitoring (phase 3).
Citation: Adams E, Goyder C, Heneghan C, Brand L, Ajjawi R. Clinical reasoning of junior doctors in emergency medicine: a grounded theory study [published online ahead of print June 23, 2016]. Emerg Med J. doi:10.1136/emermed-2015-205650.
Real-World Safety and Effectiveness of Oral Anticoagulants for Afib
Clinical Question: Which oral anticoagulants are safest and most effective in nonvalvular atrial fibrillation?
Background: Use of direct oral anticoagulants (DOACs) has been increasing since their introduction and widespread marketing. While dosing is a challenge for warfarin, certain medical conditions limit the use of DOACs. Choosing the optimal oral anticoagulant is challenging with the increasing complexity of patients.
Study Design: Nationwide observational cohort study.
Setting: Three national Danish databases, from August 2011 to October 2015.
Synopsis: Authors reviewed data from 61,678 patients with nonvalvular atrial fibrillation who were new to oral anticoagulants. The study compared the efficacy, safety, and patient characteristics of DOACs and warfarin. Ischemic stroke, systemic embolism, and death were evaluated separately and as a composite measure of efficacy. Any bleeding, intracranial bleeding, and major bleeding were measured as safety outcomes. DOACs patients were younger and had lower CHA2DS2-VASc and HAS-BLED scores. No significant difference in risk of ischemic stroke was identified between DOACs and warfarin. Rivaroxaban was associated with lower rates of ischemic stroke and systemic embolism but had bleeding rates that were similar to warfarin. Any bleeding and major bleeding rates were lowest for dabigatran and apixaban. All-cause mortality was lowest in the dabigatran group and highest in the warfarin group.
Limitations were the retrospective, observational study design, with an average follow-up of only 1.9 years.
Bottom Line: All DOACs appear to be safer and more effective alternatives to warfarin. Oral anticoagulant selection needs to be based on individual patient clinical profile.
Citation: Larsen TB, Skjoth F, Nielsen PB, Kjaeldgaard JN, Lip GY. Comparative effectiveness and safety of non-vitamin K antagonist oral anticoagulants and warfarin in patients with atrial fibrillation: propensity weighted nationwide cohort study. BMJ. 2016;353:i3189.
Short Take
Mortality and Long-Acting Opiates
This retrospective cohort study raises questions about the safety of long-acting opioids for chronic noncancer pain. When compared with anticonvulsants or antidepressants, the adjusted hazard ratio was 1.64 for total mortality.
Citation: Ray W, Chung CP, Murray KT, Hall K, Stein CM. Prescription of long-acting opioids and mortality in patients with chronic noncancer pain. JAMA. 2016;315(22):2415-2423.
Clinical Question: Which oral anticoagulants are safest and most effective in nonvalvular atrial fibrillation?
Background: Use of direct oral anticoagulants (DOACs) has been increasing since their introduction and widespread marketing. While dosing is a challenge for warfarin, certain medical conditions limit the use of DOACs. Choosing the optimal oral anticoagulant is challenging with the increasing complexity of patients.
Study Design: Nationwide observational cohort study.
Setting: Three national Danish databases, from August 2011 to October 2015.
Synopsis: Authors reviewed data from 61,678 patients with nonvalvular atrial fibrillation who were new to oral anticoagulants. The study compared the efficacy, safety, and patient characteristics of DOACs and warfarin. Ischemic stroke, systemic embolism, and death were evaluated separately and as a composite measure of efficacy. Any bleeding, intracranial bleeding, and major bleeding were measured as safety outcomes. DOACs patients were younger and had lower CHA2DS2-VASc and HAS-BLED scores. No significant difference in risk of ischemic stroke was identified between DOACs and warfarin. Rivaroxaban was associated with lower rates of ischemic stroke and systemic embolism but had bleeding rates that were similar to warfarin. Any bleeding and major bleeding rates were lowest for dabigatran and apixaban. All-cause mortality was lowest in the dabigatran group and highest in the warfarin group.
Limitations were the retrospective, observational study design, with an average follow-up of only 1.9 years.
Bottom Line: All DOACs appear to be safer and more effective alternatives to warfarin. Oral anticoagulant selection needs to be based on individual patient clinical profile.
Citation: Larsen TB, Skjoth F, Nielsen PB, Kjaeldgaard JN, Lip GY. Comparative effectiveness and safety of non-vitamin K antagonist oral anticoagulants and warfarin in patients with atrial fibrillation: propensity weighted nationwide cohort study. BMJ. 2016;353:i3189.
Short Take
Mortality and Long-Acting Opiates
This retrospective cohort study raises questions about the safety of long-acting opioids for chronic noncancer pain. When compared with anticonvulsants or antidepressants, the adjusted hazard ratio was 1.64 for total mortality.
Citation: Ray W, Chung CP, Murray KT, Hall K, Stein CM. Prescription of long-acting opioids and mortality in patients with chronic noncancer pain. JAMA. 2016;315(22):2415-2423.
Clinical Question: Which oral anticoagulants are safest and most effective in nonvalvular atrial fibrillation?
Background: Use of direct oral anticoagulants (DOACs) has been increasing since their introduction and widespread marketing. While dosing is a challenge for warfarin, certain medical conditions limit the use of DOACs. Choosing the optimal oral anticoagulant is challenging with the increasing complexity of patients.
Study Design: Nationwide observational cohort study.
Setting: Three national Danish databases, from August 2011 to October 2015.
Synopsis: Authors reviewed data from 61,678 patients with nonvalvular atrial fibrillation who were new to oral anticoagulants. The study compared the efficacy, safety, and patient characteristics of DOACs and warfarin. Ischemic stroke, systemic embolism, and death were evaluated separately and as a composite measure of efficacy. Any bleeding, intracranial bleeding, and major bleeding were measured as safety outcomes. DOACs patients were younger and had lower CHA2DS2-VASc and HAS-BLED scores. No significant difference in risk of ischemic stroke was identified between DOACs and warfarin. Rivaroxaban was associated with lower rates of ischemic stroke and systemic embolism but had bleeding rates that were similar to warfarin. Any bleeding and major bleeding rates were lowest for dabigatran and apixaban. All-cause mortality was lowest in the dabigatran group and highest in the warfarin group.
Limitations were the retrospective, observational study design, with an average follow-up of only 1.9 years.
Bottom Line: All DOACs appear to be safer and more effective alternatives to warfarin. Oral anticoagulant selection needs to be based on individual patient clinical profile.
Citation: Larsen TB, Skjoth F, Nielsen PB, Kjaeldgaard JN, Lip GY. Comparative effectiveness and safety of non-vitamin K antagonist oral anticoagulants and warfarin in patients with atrial fibrillation: propensity weighted nationwide cohort study. BMJ. 2016;353:i3189.
Short Take
Mortality and Long-Acting Opiates
This retrospective cohort study raises questions about the safety of long-acting opioids for chronic noncancer pain. When compared with anticonvulsants or antidepressants, the adjusted hazard ratio was 1.64 for total mortality.
Citation: Ray W, Chung CP, Murray KT, Hall K, Stein CM. Prescription of long-acting opioids and mortality in patients with chronic noncancer pain. JAMA. 2016;315(22):2415-2423.
Prescribing Naloxone for Patients on Long-Term Opioid Therapy
Background: Unintentional opioid overdose is a major public health issue. Studies have shown that provision of naloxone to at-risk patients reduces mortality and improves survival. The CDC recommends considering naloxone prescription in high-risk patients. This study focused on patient education and prescription habits of providers rather than just making naloxone available.
Study Design: Non-randomized interventional study.
Setting: Six safety-net primary-care clinics in San Francisco.
Synopsis: The authors identified 1,985 adults on long-term opioid treatment, of which 759 were prescribed naloxone. Providers were encouraged to prescribe naloxone along with opioids. Patients were educated about use of the intranasal naloxone device. Outcomes included opioid-related emergency department visits and prescribed dosage. They noted that patients on a higher dose of opioids and with opioid-related ED visits in the prior 12 months were more likely to be prescribed naloxone. When compared to patients who were not prescribed naloxone, patients who received naloxone had 47% fewer ED visits per month in the first six months and 63% fewer ED visits over 12 months. Limitations include lack of randomization and being a single-center study.
Hospitalists can prioritize patients and consider providing naloxone prescription to reduce ED visits and perhaps readmissions. Further studies are needed focusing on patients who get discharged from the hospital.
Bottom Line: Naloxone prescription in patients on long-term opioid treatment may prevent opioid-related ED visits.
Citation: Coffin PO, Behar E, Rowe C, et al. Nonrandomized intervention study of naloxone coprescription for primary care patients receiving long-term opioid therapy for pain. Ann Intern Med. 2016;165(4):245-252.
Short Take
Mortality and Long-Acting Opiates
This retrospective cohort study raises questions about the safety of long-acting opioids for chronic noncancer pain. When compared with anticonvulsants or antidepressants, the adjusted hazard ratio was 1.64 for total mortality.
Citation: Ray W, Chung CP, Murray KT, Hall K, Stein CM. Prescription of long-acting opioids and mortality in patients with chronic noncancer pain. JAMA. 2016;315(22):2415-2423.
Background: Unintentional opioid overdose is a major public health issue. Studies have shown that provision of naloxone to at-risk patients reduces mortality and improves survival. The CDC recommends considering naloxone prescription in high-risk patients. This study focused on patient education and prescription habits of providers rather than just making naloxone available.
Study Design: Non-randomized interventional study.
Setting: Six safety-net primary-care clinics in San Francisco.
Synopsis: The authors identified 1,985 adults on long-term opioid treatment, of which 759 were prescribed naloxone. Providers were encouraged to prescribe naloxone along with opioids. Patients were educated about use of the intranasal naloxone device. Outcomes included opioid-related emergency department visits and prescribed dosage. They noted that patients on a higher dose of opioids and with opioid-related ED visits in the prior 12 months were more likely to be prescribed naloxone. When compared to patients who were not prescribed naloxone, patients who received naloxone had 47% fewer ED visits per month in the first six months and 63% fewer ED visits over 12 months. Limitations include lack of randomization and being a single-center study.
Hospitalists can prioritize patients and consider providing naloxone prescription to reduce ED visits and perhaps readmissions. Further studies are needed focusing on patients who get discharged from the hospital.
Bottom Line: Naloxone prescription in patients on long-term opioid treatment may prevent opioid-related ED visits.
Citation: Coffin PO, Behar E, Rowe C, et al. Nonrandomized intervention study of naloxone coprescription for primary care patients receiving long-term opioid therapy for pain. Ann Intern Med. 2016;165(4):245-252.
Short Take
Mortality and Long-Acting Opiates
This retrospective cohort study raises questions about the safety of long-acting opioids for chronic noncancer pain. When compared with anticonvulsants or antidepressants, the adjusted hazard ratio was 1.64 for total mortality.
Citation: Ray W, Chung CP, Murray KT, Hall K, Stein CM. Prescription of long-acting opioids and mortality in patients with chronic noncancer pain. JAMA. 2016;315(22):2415-2423.
Background: Unintentional opioid overdose is a major public health issue. Studies have shown that provision of naloxone to at-risk patients reduces mortality and improves survival. The CDC recommends considering naloxone prescription in high-risk patients. This study focused on patient education and prescription habits of providers rather than just making naloxone available.
Study Design: Non-randomized interventional study.
Setting: Six safety-net primary-care clinics in San Francisco.
Synopsis: The authors identified 1,985 adults on long-term opioid treatment, of which 759 were prescribed naloxone. Providers were encouraged to prescribe naloxone along with opioids. Patients were educated about use of the intranasal naloxone device. Outcomes included opioid-related emergency department visits and prescribed dosage. They noted that patients on a higher dose of opioids and with opioid-related ED visits in the prior 12 months were more likely to be prescribed naloxone. When compared to patients who were not prescribed naloxone, patients who received naloxone had 47% fewer ED visits per month in the first six months and 63% fewer ED visits over 12 months. Limitations include lack of randomization and being a single-center study.
Hospitalists can prioritize patients and consider providing naloxone prescription to reduce ED visits and perhaps readmissions. Further studies are needed focusing on patients who get discharged from the hospital.
Bottom Line: Naloxone prescription in patients on long-term opioid treatment may prevent opioid-related ED visits.
Citation: Coffin PO, Behar E, Rowe C, et al. Nonrandomized intervention study of naloxone coprescription for primary care patients receiving long-term opioid therapy for pain. Ann Intern Med. 2016;165(4):245-252.
Short Take
Mortality and Long-Acting Opiates
This retrospective cohort study raises questions about the safety of long-acting opioids for chronic noncancer pain. When compared with anticonvulsants or antidepressants, the adjusted hazard ratio was 1.64 for total mortality.
Citation: Ray W, Chung CP, Murray KT, Hall K, Stein CM. Prescription of long-acting opioids and mortality in patients with chronic noncancer pain. JAMA. 2016;315(22):2415-2423.
Palliative Care May Improve End-of-Life Care for Patients with ESRD, Cardiopulmonary Failure, Frailty
Clinical Question: Is there a difference in family-rated quality of care for patients dying with different serious illnesses?
Background: End-of-life care has focused largely on cancer patients. However, other conditions lead to more deaths than cancer in the United States.
Study Design: A retrospective cross-sectional study.
Setting: 146 inpatient Veterans Affairs (VA) facilities.
Synopsis: This study included 57,753 patients who died in inpatient facilities with a diagnosis of cancer, dementia, end-stage renal disease (ESRD), cardiopulmonary failure (heart failure or chronic obstructive pulmonary disease), or frailty. Measures included palliative care consultations, do-not-resuscitate (DNR) orders, death in inpatient hospice, death in the intensive care unit (ICU), and family-reported quality of end-of-life care. Palliative care consultations were given to 73.5% of patients with cancer and 61.4% of patients with dementia, which was significantly more than patients with other diagnoses (P < .001).
Approximately one-third of patients with diagnoses other than cancer or dementia died in the ICU, which was more than double the rate among patients with cancer or dementia (P < .001). Rates of excellent quality of end-of-life care were similar for patients with cancer and dementia (59.2% and 59.3%) but lower for other conditions (P = 0.02 when compared with cancer patient). This was mediated by palliative care consultation, setting of death, and DNR status. Difficulty defining frailty and restriction to only the VA system are limitations of this study.
Bottom Line: Increasing access to palliative care, goals-of-care discussions, and preferred setting of death may improve overall quality of end-of-life care.
Citation: Wachterman MW, Pilver C, Smith D, Ersek M, Lipsitz SR, Keating NL. Quality of end-of-life care provided to patients with different serious illnesses. JAMA Intern Med. 2016;176(8):1095-1102. doi:10.1001/jamainternmed.2016.1200.
Clinical Question: Is there a difference in family-rated quality of care for patients dying with different serious illnesses?
Background: End-of-life care has focused largely on cancer patients. However, other conditions lead to more deaths than cancer in the United States.
Study Design: A retrospective cross-sectional study.
Setting: 146 inpatient Veterans Affairs (VA) facilities.
Synopsis: This study included 57,753 patients who died in inpatient facilities with a diagnosis of cancer, dementia, end-stage renal disease (ESRD), cardiopulmonary failure (heart failure or chronic obstructive pulmonary disease), or frailty. Measures included palliative care consultations, do-not-resuscitate (DNR) orders, death in inpatient hospice, death in the intensive care unit (ICU), and family-reported quality of end-of-life care. Palliative care consultations were given to 73.5% of patients with cancer and 61.4% of patients with dementia, which was significantly more than patients with other diagnoses (P < .001).
Approximately one-third of patients with diagnoses other than cancer or dementia died in the ICU, which was more than double the rate among patients with cancer or dementia (P < .001). Rates of excellent quality of end-of-life care were similar for patients with cancer and dementia (59.2% and 59.3%) but lower for other conditions (P = 0.02 when compared with cancer patient). This was mediated by palliative care consultation, setting of death, and DNR status. Difficulty defining frailty and restriction to only the VA system are limitations of this study.
Bottom Line: Increasing access to palliative care, goals-of-care discussions, and preferred setting of death may improve overall quality of end-of-life care.
Citation: Wachterman MW, Pilver C, Smith D, Ersek M, Lipsitz SR, Keating NL. Quality of end-of-life care provided to patients with different serious illnesses. JAMA Intern Med. 2016;176(8):1095-1102. doi:10.1001/jamainternmed.2016.1200.
Clinical Question: Is there a difference in family-rated quality of care for patients dying with different serious illnesses?
Background: End-of-life care has focused largely on cancer patients. However, other conditions lead to more deaths than cancer in the United States.
Study Design: A retrospective cross-sectional study.
Setting: 146 inpatient Veterans Affairs (VA) facilities.
Synopsis: This study included 57,753 patients who died in inpatient facilities with a diagnosis of cancer, dementia, end-stage renal disease (ESRD), cardiopulmonary failure (heart failure or chronic obstructive pulmonary disease), or frailty. Measures included palliative care consultations, do-not-resuscitate (DNR) orders, death in inpatient hospice, death in the intensive care unit (ICU), and family-reported quality of end-of-life care. Palliative care consultations were given to 73.5% of patients with cancer and 61.4% of patients with dementia, which was significantly more than patients with other diagnoses (P < .001).
Approximately one-third of patients with diagnoses other than cancer or dementia died in the ICU, which was more than double the rate among patients with cancer or dementia (P < .001). Rates of excellent quality of end-of-life care were similar for patients with cancer and dementia (59.2% and 59.3%) but lower for other conditions (P = 0.02 when compared with cancer patient). This was mediated by palliative care consultation, setting of death, and DNR status. Difficulty defining frailty and restriction to only the VA system are limitations of this study.
Bottom Line: Increasing access to palliative care, goals-of-care discussions, and preferred setting of death may improve overall quality of end-of-life care.
Citation: Wachterman MW, Pilver C, Smith D, Ersek M, Lipsitz SR, Keating NL. Quality of end-of-life care provided to patients with different serious illnesses. JAMA Intern Med. 2016;176(8):1095-1102. doi:10.1001/jamainternmed.2016.1200.
Updated Guideline for Acute Diarrheal Infection
Clinical Question: What are current recommendations for diagnosis, management, and prevention of acute gastrointestinal infection in immune-competent adults?
Background: Acute diarrheal infection is a leading cause of healthcare visits and lost quality of life. The Centers for Disease Control and Prevention estimates there are 47.8 million cases annually, with a healthcare economy burden of $150 million.
Study Design: American College of Gastroenterology (ACG) practice guideline.
Setting: Expert panel.
Synopsis: Stool diagnostic studies may be used for dysentery with moderate-severe disease and symptoms lasting more than seven days (strong recommendation, low level of evidence). Traditional diagnostic methods in most cases fail to reveal etiology (strong recommendation, low level of evidence). Treatment with probiotics or prebiotics is not recommended (strong recommendation, moderate level of evidence). Bismuth subsalicylates may be considered for prophylaxis against traveler’s diarrhea (strong recommendation, high level of evidence). Short-term antibiotic chemoprophylaxis also may be considered for high-risk groups (strong recommendation, high level of evidence). Empiric antimicrobial therapy is not recommended except in cases of traveler’s diarrhea (strong recommendation, high level of evidence). Loperamide may be used as an adjunct to antibiotics for traveler’s diarrhea (strong recommendation, moderate level of evidence).
Bottom Line: ACG acute diarrheal illness guidelines have been updated. Few recommendations are strong, and very few have high levels of evidence.
Citation: Riddle MS, DuPont HL, Conner BA. ACG clinical guideline: diagnosis, treatment, and prevention of acute diarrheal infections in adults. Am J Gastroenterol. 2016;111(5):602-622.
Clinical Question: What are current recommendations for diagnosis, management, and prevention of acute gastrointestinal infection in immune-competent adults?
Background: Acute diarrheal infection is a leading cause of healthcare visits and lost quality of life. The Centers for Disease Control and Prevention estimates there are 47.8 million cases annually, with a healthcare economy burden of $150 million.
Study Design: American College of Gastroenterology (ACG) practice guideline.
Setting: Expert panel.
Synopsis: Stool diagnostic studies may be used for dysentery with moderate-severe disease and symptoms lasting more than seven days (strong recommendation, low level of evidence). Traditional diagnostic methods in most cases fail to reveal etiology (strong recommendation, low level of evidence). Treatment with probiotics or prebiotics is not recommended (strong recommendation, moderate level of evidence). Bismuth subsalicylates may be considered for prophylaxis against traveler’s diarrhea (strong recommendation, high level of evidence). Short-term antibiotic chemoprophylaxis also may be considered for high-risk groups (strong recommendation, high level of evidence). Empiric antimicrobial therapy is not recommended except in cases of traveler’s diarrhea (strong recommendation, high level of evidence). Loperamide may be used as an adjunct to antibiotics for traveler’s diarrhea (strong recommendation, moderate level of evidence).
Bottom Line: ACG acute diarrheal illness guidelines have been updated. Few recommendations are strong, and very few have high levels of evidence.
Citation: Riddle MS, DuPont HL, Conner BA. ACG clinical guideline: diagnosis, treatment, and prevention of acute diarrheal infections in adults. Am J Gastroenterol. 2016;111(5):602-622.
Clinical Question: What are current recommendations for diagnosis, management, and prevention of acute gastrointestinal infection in immune-competent adults?
Background: Acute diarrheal infection is a leading cause of healthcare visits and lost quality of life. The Centers for Disease Control and Prevention estimates there are 47.8 million cases annually, with a healthcare economy burden of $150 million.
Study Design: American College of Gastroenterology (ACG) practice guideline.
Setting: Expert panel.
Synopsis: Stool diagnostic studies may be used for dysentery with moderate-severe disease and symptoms lasting more than seven days (strong recommendation, low level of evidence). Traditional diagnostic methods in most cases fail to reveal etiology (strong recommendation, low level of evidence). Treatment with probiotics or prebiotics is not recommended (strong recommendation, moderate level of evidence). Bismuth subsalicylates may be considered for prophylaxis against traveler’s diarrhea (strong recommendation, high level of evidence). Short-term antibiotic chemoprophylaxis also may be considered for high-risk groups (strong recommendation, high level of evidence). Empiric antimicrobial therapy is not recommended except in cases of traveler’s diarrhea (strong recommendation, high level of evidence). Loperamide may be used as an adjunct to antibiotics for traveler’s diarrhea (strong recommendation, moderate level of evidence).
Bottom Line: ACG acute diarrheal illness guidelines have been updated. Few recommendations are strong, and very few have high levels of evidence.
Citation: Riddle MS, DuPont HL, Conner BA. ACG clinical guideline: diagnosis, treatment, and prevention of acute diarrheal infections in adults. Am J Gastroenterol. 2016;111(5):602-622.
Risk-Assessment Models Are Unreliable Predictors of Venous Thromboembolism
Clinical Question: Do risk-assessment models (RAMs) accurately predict which hospitalized medical patients are at risk for venous thromboembolism (VTE)?
Background: Predicting which patients are at high risk for VTE is important. Several models exist, but limited data support their generalizability and accuracy in medical inpatients.
Study Design: Retrospective cohort.
Setting: Hospitals participating in the Michigan Hospital Medicine Safety Consortium (MHMSC).
Synopsis: Data collected through MHMSC for selected medical patients were used in the Kucher, Padua, predictive IMPROVE, and Intermountain DVT risk-assessment models. Patients were classified as “low risk” or “at risk” based on each RAM. Follow-up data came from chart extraction (100% of patients) and 90-day post-discharge telephone calls (58% of patients). The primary outcome was image-confirmed hospital associated VTE, including proximal upper- or proximal lower-extremity DVT or pulmonary embolism. These RAMs classified less than 20% of patients as “at risk.” The incidence of VTE was less than 1%. In this external validation study, the Kucher RAM was the least discriminate and the Intermountain was the best, but none yielded results equivalent to the original studies.
This study was limited by the retrospective design, subjectivity of some risk factors (such as immobility), and inability to obtain 90-day telephone follow-up in all patients. Lastly, the binary approach (“at risk” versus “low risk”) may not align with the original derivation studies in which each factor was evaluated independently.
Bottom Line: The incidence of VTE is low in medical inpatients, and current RAMs may not accurately identify at-risk patients.
Citation: Greene MT, Spyropoulos AC, Chopra V, et al. Validation of risk assessment models of venous thromboembolism in hospitalized medical patients. Am J Med. 2016;129(9):1001.e9-1001.e18. doi:10.1016/j.amjmed.2016.03.031.
Clinical Question: Do risk-assessment models (RAMs) accurately predict which hospitalized medical patients are at risk for venous thromboembolism (VTE)?
Background: Predicting which patients are at high risk for VTE is important. Several models exist, but limited data support their generalizability and accuracy in medical inpatients.
Study Design: Retrospective cohort.
Setting: Hospitals participating in the Michigan Hospital Medicine Safety Consortium (MHMSC).
Synopsis: Data collected through MHMSC for selected medical patients were used in the Kucher, Padua, predictive IMPROVE, and Intermountain DVT risk-assessment models. Patients were classified as “low risk” or “at risk” based on each RAM. Follow-up data came from chart extraction (100% of patients) and 90-day post-discharge telephone calls (58% of patients). The primary outcome was image-confirmed hospital associated VTE, including proximal upper- or proximal lower-extremity DVT or pulmonary embolism. These RAMs classified less than 20% of patients as “at risk.” The incidence of VTE was less than 1%. In this external validation study, the Kucher RAM was the least discriminate and the Intermountain was the best, but none yielded results equivalent to the original studies.
This study was limited by the retrospective design, subjectivity of some risk factors (such as immobility), and inability to obtain 90-day telephone follow-up in all patients. Lastly, the binary approach (“at risk” versus “low risk”) may not align with the original derivation studies in which each factor was evaluated independently.
Bottom Line: The incidence of VTE is low in medical inpatients, and current RAMs may not accurately identify at-risk patients.
Citation: Greene MT, Spyropoulos AC, Chopra V, et al. Validation of risk assessment models of venous thromboembolism in hospitalized medical patients. Am J Med. 2016;129(9):1001.e9-1001.e18. doi:10.1016/j.amjmed.2016.03.031.
Clinical Question: Do risk-assessment models (RAMs) accurately predict which hospitalized medical patients are at risk for venous thromboembolism (VTE)?
Background: Predicting which patients are at high risk for VTE is important. Several models exist, but limited data support their generalizability and accuracy in medical inpatients.
Study Design: Retrospective cohort.
Setting: Hospitals participating in the Michigan Hospital Medicine Safety Consortium (MHMSC).
Synopsis: Data collected through MHMSC for selected medical patients were used in the Kucher, Padua, predictive IMPROVE, and Intermountain DVT risk-assessment models. Patients were classified as “low risk” or “at risk” based on each RAM. Follow-up data came from chart extraction (100% of patients) and 90-day post-discharge telephone calls (58% of patients). The primary outcome was image-confirmed hospital associated VTE, including proximal upper- or proximal lower-extremity DVT or pulmonary embolism. These RAMs classified less than 20% of patients as “at risk.” The incidence of VTE was less than 1%. In this external validation study, the Kucher RAM was the least discriminate and the Intermountain was the best, but none yielded results equivalent to the original studies.
This study was limited by the retrospective design, subjectivity of some risk factors (such as immobility), and inability to obtain 90-day telephone follow-up in all patients. Lastly, the binary approach (“at risk” versus “low risk”) may not align with the original derivation studies in which each factor was evaluated independently.
Bottom Line: The incidence of VTE is low in medical inpatients, and current RAMs may not accurately identify at-risk patients.
Citation: Greene MT, Spyropoulos AC, Chopra V, et al. Validation of risk assessment models of venous thromboembolism in hospitalized medical patients. Am J Med. 2016;129(9):1001.e9-1001.e18. doi:10.1016/j.amjmed.2016.03.031.
Acute HIV Causes Transient Neurologic Findings
Clinical Question: How common are neurologic findings in acute HIV infection?
Background: The incidence of neurologic findings with acute HIV is unknown.
Study Design: Cohort study.
Setting: Bangkok, Thailand.
Synopsis: In this study, 134 patients were identified after presenting for voluntary HIV testing. Five others were enrolled through an ongoing local study. All 139 participants underwent structured neurologic evaluations at enrollment (median of 19 days after presumed exposure), then at four and 12 weeks. Combination antiretroviral therapy (cART) was initiated immediately after initial evaluation.
The cohort was 93% male. Mean age was younger than 30 years. Fifty-three percent of participants experienced some neurologic finding within 12 weeks of diagnosis. One-third (33%) were cognitive symptoms, predominantly problems of concentration (24% of patients) and memory (16% of patients). One-third (34%) were motor findings, and 11% were neuropathy. Forty-nine percent of the neurologic issues were present at diagnosis. Symptoms were mostly mild, although one patient developed fulminant Guillain-Barré syndrome. Patients with neurologic findings had higher viral loads at diagnosis (mean plasma log10 HIV RNA 5.9 versus 5.4; P = 0.006). Participants with and without neurologic findings had similar cerebral spinal fluid viral loads (mean log10 HIV RNA 3.7 versus 3.1, P = 0.14) and serum CD4 counts (339 versus 381 cells/mm3; P = 0.46). Neurologic findings resolved within one month of cART treatment in 90% of patients. Study limitations include lack of a control cohort and potential confounding from illicit drug use among participants.
Bottom Line: Acute HIV infection commonly causes mild neurologic problems, which remit with treatment.
Citation: Hellmuth J, Fletcher JL, Valcour V, et al. Neurologic signs and symptoms frequently manifest in acute HIV infection. Neurology. 2016;87(2):148-154.
Clinical Question: How common are neurologic findings in acute HIV infection?
Background: The incidence of neurologic findings with acute HIV is unknown.
Study Design: Cohort study.
Setting: Bangkok, Thailand.
Synopsis: In this study, 134 patients were identified after presenting for voluntary HIV testing. Five others were enrolled through an ongoing local study. All 139 participants underwent structured neurologic evaluations at enrollment (median of 19 days after presumed exposure), then at four and 12 weeks. Combination antiretroviral therapy (cART) was initiated immediately after initial evaluation.
The cohort was 93% male. Mean age was younger than 30 years. Fifty-three percent of participants experienced some neurologic finding within 12 weeks of diagnosis. One-third (33%) were cognitive symptoms, predominantly problems of concentration (24% of patients) and memory (16% of patients). One-third (34%) were motor findings, and 11% were neuropathy. Forty-nine percent of the neurologic issues were present at diagnosis. Symptoms were mostly mild, although one patient developed fulminant Guillain-Barré syndrome. Patients with neurologic findings had higher viral loads at diagnosis (mean plasma log10 HIV RNA 5.9 versus 5.4; P = 0.006). Participants with and without neurologic findings had similar cerebral spinal fluid viral loads (mean log10 HIV RNA 3.7 versus 3.1, P = 0.14) and serum CD4 counts (339 versus 381 cells/mm3; P = 0.46). Neurologic findings resolved within one month of cART treatment in 90% of patients. Study limitations include lack of a control cohort and potential confounding from illicit drug use among participants.
Bottom Line: Acute HIV infection commonly causes mild neurologic problems, which remit with treatment.
Citation: Hellmuth J, Fletcher JL, Valcour V, et al. Neurologic signs and symptoms frequently manifest in acute HIV infection. Neurology. 2016;87(2):148-154.
Clinical Question: How common are neurologic findings in acute HIV infection?
Background: The incidence of neurologic findings with acute HIV is unknown.
Study Design: Cohort study.
Setting: Bangkok, Thailand.
Synopsis: In this study, 134 patients were identified after presenting for voluntary HIV testing. Five others were enrolled through an ongoing local study. All 139 participants underwent structured neurologic evaluations at enrollment (median of 19 days after presumed exposure), then at four and 12 weeks. Combination antiretroviral therapy (cART) was initiated immediately after initial evaluation.
The cohort was 93% male. Mean age was younger than 30 years. Fifty-three percent of participants experienced some neurologic finding within 12 weeks of diagnosis. One-third (33%) were cognitive symptoms, predominantly problems of concentration (24% of patients) and memory (16% of patients). One-third (34%) were motor findings, and 11% were neuropathy. Forty-nine percent of the neurologic issues were present at diagnosis. Symptoms were mostly mild, although one patient developed fulminant Guillain-Barré syndrome. Patients with neurologic findings had higher viral loads at diagnosis (mean plasma log10 HIV RNA 5.9 versus 5.4; P = 0.006). Participants with and without neurologic findings had similar cerebral spinal fluid viral loads (mean log10 HIV RNA 3.7 versus 3.1, P = 0.14) and serum CD4 counts (339 versus 381 cells/mm3; P = 0.46). Neurologic findings resolved within one month of cART treatment in 90% of patients. Study limitations include lack of a control cohort and potential confounding from illicit drug use among participants.
Bottom Line: Acute HIV infection commonly causes mild neurologic problems, which remit with treatment.
Citation: Hellmuth J, Fletcher JL, Valcour V, et al. Neurologic signs and symptoms frequently manifest in acute HIV infection. Neurology. 2016;87(2):148-154.