User login
When can I stop dual antiplatelet therapy in patients with drug-eluting stents?
Stopping dual antiplatelet therapy (DAPT) (eg, clopidogrel plus aspirin) after 3 months is reasonable in patients with stable ischemic heart disease who have a second-generation drug-eluting stent and a high bleeding risk, with stable ischemic disease defined as at least 1 year free of acute coronary syndromes. However, these patients should continue lifelong aspirin monotherapy. Current guidelines suggest that in stable ischemic disease, the risk-benefit ratio may favor an even shorter duration of DAPT than the 6 months currently recommended.1
STABLE ISCHEMIC HEART DISEASE VS ACUTE CORONARY SYNDROME
Percutaneous coronary intervention for stable ischemic heart disease is indicated primarily in patients with angina that persists despite optimal antianginal therapy.
The prognostic implications of DAPT are different in stable ischemic disease than in acute coronary syndromes. The substrate treated by percutaneous intervention in stable ischemic disease is primarily fibrofatty plaque, as opposed to thrombus in acute coronary syndromes.
Percutaneous intervention significantly improves the prognosis in acute coronary syndromes, whereas its impact on overall survival in stable ischemic heart disease is not well documented. Given these differences, our discussion about DAPT in stable ischemic disease cannot be extrapolated to acute coronary syndromes.
BENEFITS OF DAPT
DAPT is mandatory early after drug-eluting stent placement, when the stent continuously releases medication, inhibiting tissue growth within the lumen of the stent.
Endothelialization of the stent normally occurs during the first 7 to 30 days after placement. During this period, the nonendothelialized stent poses a risk of thrombosis, a life-threatening, catastrophic condition with a mortality rate between 9% and 45%.1
THERAPY BEYOND 12 MONTHS
Although guidelines have traditionally recommended 12 months of DAPT, the optimal duration is still debated.
A duration beyond 12 months in patients with a history of myocardial infarction was shown to be reasonable in 2 large trials,2,3 while a 2016 review by Bittl et al4 suggested that therapy beyond 12 months in patients with a newer-generation drug-eluting stent could increase the incidence of major bleeding. A detailed discussion of DAPT longer than 12 months is beyond the scope of this article.
EVIDENCE FOR SHORTER DURATION
The results of 5 major trials support shorter duration of DAPT in stable ischemic disease.
The OPTIMIZE5 and RESET6 trials found that 3 months of DAPT was not inferior to 12 months in terms of ischemic and safety end points.
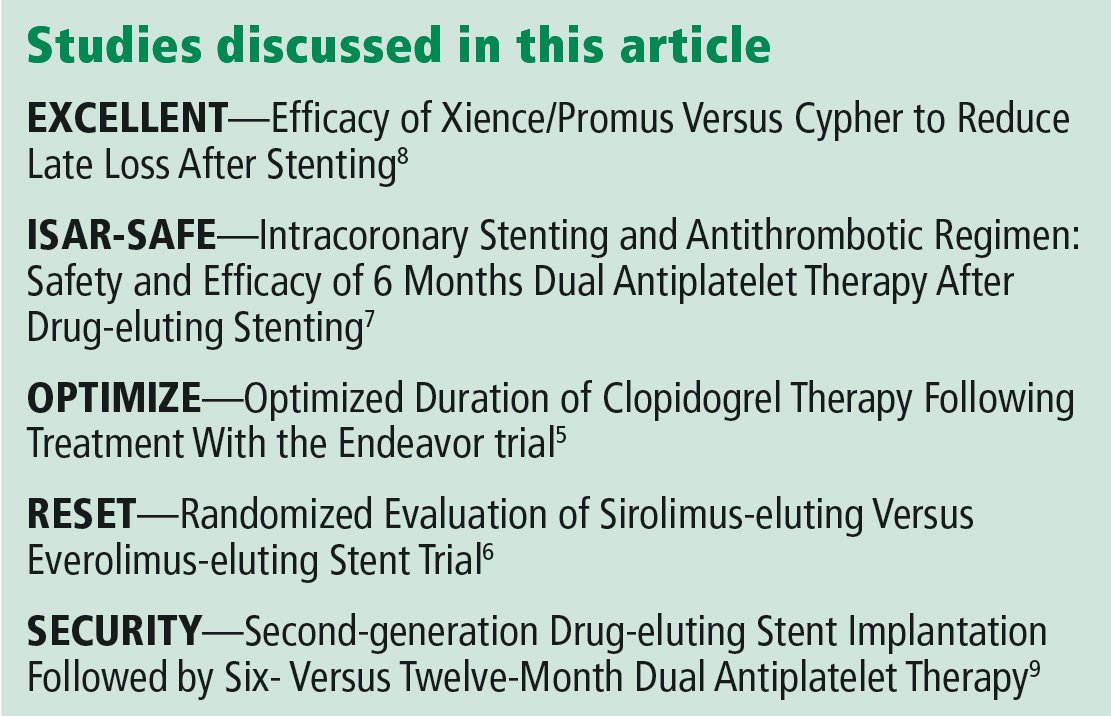
The ISAR-SAFE,7 EXCELLENT,8 and SECURITY9 trials also reported that 6 months of DAPT was not inferior to 12 months for the primary composite end point of death, stent thrombosis, myocardial infarction, stroke, or major bleeding.
However, these trials may have been underpowered to detect a difference in rates of stent thrombosis with shorter-duration DAPT.
CURRENT GUIDELINES
For patients at high bleeding risk, the current guidelines of the American College of Cardiology and American Heart Association, updated in 2016, suggest that it may be reasonable to discontinue DAPT 3 months after drug-eluting stent placement in patients with stable ischemic heart disease, and at 6 months in patients with acute coronary syndrome (class IIb recommendation, level of evidence C).1 These recommendations are based on results of randomized controlled trials showing no difference in the rate of stent thrombosis and composite ischemic events with a shorter duration than with 12 months of therapy.5–10
The evidence for DAPT in stable ischemic disease is based on clopidogrel, with only limited data on ticagrelor.1 To our knowledge, no study to date has evaluated DAPT in this setting for less than 3 months, and further study is needed to address shorter-duration approaches with current-generation drug-eluting stents Since 2017, all coronary stents implanted in the United States have been second-generation stents.
TOOLS TO HELP DECISION-MAKING
The decision to stop DAPT in a patient at high risk of bleeding requires a careful assessment of the risks and benefits. Risk factors for bleeding include advanced age, history of major bleeding, anticoagulation, chronic kidney disease (serum creatinine level ≥ 2 mg/dL), platelet count 100 × 109/L or lower, and history of stroke.11
- Age 75 or older: −2 points
- Ages 65 to 74: −1
- Age under 65: 0
- Diabetes mellitus: 1
- Myocardial infarction at presentation: 1
- History of percutaneous coronary intervention or myocardial infarction: 1
- Stent diameter less than 3 mm: 1
- Paclitaxel drug-eluting stent: 1
- Current smoker: 2
- Percutaneous coronary intervention with saphenous vein graft: 2
- Congestive heart failure or left ventricular ejection fraction less than 30%: 2.
A score of 2 or greater favors continuing DAPT, as it indicates higher ischemic risk. A score less than 2 favors discontinuing DAPT, as it indicates higher bleeding risk.1,2
IF BLEEDING RISK IS HIGH
Preventing and controlling bleeding associated with DAPT is important. The gastrointestinal tract is the most common site of bleeding.
Aspirin inhibits prostaglandin synthesis, leading to disruption of the protective mucous membrane. Therefore, a proton pump inhibitor should be started along with DAPT in patients at high risk of gastrointestinal bleeding.
If a patient’s bleeding risk significantly outweighs the risk of stent thrombosis, or if active hemorrhage makes a patient hemodynamically unstable, antiplatelet therapy must be stopped.1
FACING SURGERY
For patients with a drug-eluting stent who are on DAPT and are to undergo elective noncardiac surgery, 3 considerations must be kept in mind:
- The risk of stent thrombosis if DAPT needs to be interrupted
- The consequences of delaying the surgical procedure
- The risk and consequences of periprocedural and intraprocedural bleeding if DAPT is continued.
Because clinical evidence for bridging therapy with intravenous antiplatelet or anticoagulant agents is limited, it is difficult to make recommendations about stopping DAPT. However, once bleeding risk is stabilized, DAPT should be restarted as soon as possible.1
CURRENT RESEARCH
Several trials are under way to further evaluate ways to minimize bleeding risk and shorten the duration of DAPT.
A prospective multicenter trial is evaluating 3-month DAPT in patients at high bleeding risk who undergo placement of an everolimus-eluting stent.11 This study is expected to be completed in August 2019.
Another strategy for patients at high bleeding risk is use of a polymer-free drug-coated coronary stent. In a 2015 trial comparing a biolimus A9-coated stent vs a bare-metal stent, patients received DAPT for 1 month after stent placement. The drug-coated stent was found to be superior in terms of the primary safety end point (cardiac death, myocardial infarction, or stent thrombosis).12 This stent is not yet approved by the US Food and Drug Administration at the time of this writing.
Further study is needed to evaluate DAPT durations of less than 3 months and to establish the proper timing for safely discontinuing DAPT in difficult clinical scenarios.
WHEN STOPPING MAY BE REASONABLE
According to current guidelines, in patients at high bleeding risk with a second-generation or newer drug-eluting stent for stable ischemic heart disease, discontinuing DAPT 3 months after stent placement may be reasonable.1 The decision to stop DAPT in these patients requires a careful assessment of the risks and benefits and may be aided by a tool such as the DAPT risk score. However, these recommendations cannot be extrapolated to patients with an acute coronary syndrome within the past year, as they are at higher risk.
TAKE-HOME MESSAGES
- A cardiologist should be consulted before discontinuing DAPT in patients with a drug-eluting stent, especially if the stent was recently placed.
- The duration of therapy depends on the indication for stent placement (stable ischemic heart disease vs acute coronary syndrome) and on stent location.
- Based on the 2016 American College of Cardiology/American Heart Association guidelines,1 in patients at high bleeding risk with a second-generation drug-eluting stent, discontinuing DAPT is safe after 3 months in patients with stable ischemic heart disease, and after 6 months in patients with an acute coronary syndrome.
- When prescribing DAPT, available evidence favors clopidogrel in patients with stable ischemic heart disease who have a second-generation drug-eluting stent and are at high bleeding risk.
- In these patients, the risk-benefit ratio based on the DAPT score may be useful when considering stopping clopidogrel.
- Levine GN, Bates ER, Bittl JA, et al. 2016 ACC/AHA guideline focused update on duration of dual antiplatelet therapy in patients with coronary artery disease: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation 2016; 134(10):e123–e155. doi:10.1161/CIR.0000000000000404 [correction in doi:10.1161/CIR.0000000000000452]
- Mauri L, Kereiakes DJ, Yeh RW, et al; DAPT Study Investigators. Twelve or 30 months of dual antiplatelet therapy after drug-eluting stents. N Engl J Med 2014; 371(23):2155–2166. doi:10.1056/NEJMoa1409312
- Bonaca MP, Bhatt DL, Cohen M, et al; PEGASUS-TIMI 54 Steering Committee and Investigators. Long-term use of ticagrelor in patients with prior myocardial infarction. N Engl J Med 2015; 372(19):1791–1800. doi:10.1056/NEJMoa1500857
- Bittl JA, Baber U, Bradley SM, Wijeysundera DN. Duration of dual antiplatelet therapy: a systematic review for the 2016 ACC/AHA guideline focused update on duration of dual antiplatelet therapy in patients with coronary artery disease: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol 2016; 68(10):1116–1139. doi:10.1016/j.jacc.2016.03.512
- Feres F, Costa RA, Abizaid A, et al; OPTIMIZE Trial Investigators. Three vs twelve months of dual antiplatelet therapy after zotarolimus-eluting stents: the OPTIMIZE randomized trial. JAMA 2013; 310(23):2510–2522. doi:10.1001/jama.2013.282183
- Kubo T, Akasaka T, Kozuma K, et al. Comparison of neointimal coverage between everolimus-eluting stents and sirolimus-eluting stents: an optical coherence tomography substudy of RESET. EuroIntervention 2015. doi:10.4244/EIJV11I5A109
- Schulz-Schupke S, Byrne RA, ten Berg JM, et al; Intracoronary Stenting and Antithrombotic Regimen: Safety And EFficacy of 6 Months Dual Antiplatelet Therapy After Drug-Eluting Stenting (ISAR-SAFE) Trial Investigators. ISAR-SAFE: a randomized, double-blind, placebo-controlled trial of 6 vs 12 months of clopidogrel therapy after drug-eluting stenting. Eur Heart J 2015; 36(20):1252–1263. doi:10.1093/eurheartj/ehu523
- Gwon HC, Hahn JY, Park KW, et al. Six-month versus 12-month dual antiplatelet therapy after implantation of drug-eluting stents: the efficacy of Xience/Promus vs Cypher to reduce late loss after stenting (EXCELLENT) randomized, multicenter study. Circulation 2012; 125(3):505–513. doi:10.1161/CIRCULATIONAHA.111.059022
- Colombo A, Chieffo A, Frasheri A, et al. Second-generation drug-eluting stent implantation followed by 6- vs 12-month dual antiplatelet therapy: the SECURITY randomized clinical trial. J Am Coll Cardiol 2014; 64(20):2086–2097. doi:10.1016/j.jacc.2014.09.008
- Kim BK, Hong MK, Shin DH, et al; RESET Investigators. A new strategy for discontinuation of dual antiplatelet therapy: the RESET Trial (REal Safety and Efficacy of 3-month dual antiplatelet Therapy following Endeavor zotarolimus-eluting stent implantation). J Am Coll Cardiol 2012; 60(15):1340–1348. doi:10.1016/j.jacc.2012.06.043
- US National Library of Medicine. ClinicalTrials.gov. EVOLVE Short DAPT Study. https://clinicaltrials.gov/ct2/show/NCT02605447. Accessed December 3, 2018.
- Urban P, Meredith IT, Abizaid A, et al; LEADERS FREE Investigators. Polymer-free drug-coated coronary stents in patients at high bleeding risk. N Engl J Med 2015; 373(21):2038–2047. doi:10.1056/NEJMoa1503943
Stopping dual antiplatelet therapy (DAPT) (eg, clopidogrel plus aspirin) after 3 months is reasonable in patients with stable ischemic heart disease who have a second-generation drug-eluting stent and a high bleeding risk, with stable ischemic disease defined as at least 1 year free of acute coronary syndromes. However, these patients should continue lifelong aspirin monotherapy. Current guidelines suggest that in stable ischemic disease, the risk-benefit ratio may favor an even shorter duration of DAPT than the 6 months currently recommended.1
STABLE ISCHEMIC HEART DISEASE VS ACUTE CORONARY SYNDROME
Percutaneous coronary intervention for stable ischemic heart disease is indicated primarily in patients with angina that persists despite optimal antianginal therapy.
The prognostic implications of DAPT are different in stable ischemic disease than in acute coronary syndromes. The substrate treated by percutaneous intervention in stable ischemic disease is primarily fibrofatty plaque, as opposed to thrombus in acute coronary syndromes.
Percutaneous intervention significantly improves the prognosis in acute coronary syndromes, whereas its impact on overall survival in stable ischemic heart disease is not well documented. Given these differences, our discussion about DAPT in stable ischemic disease cannot be extrapolated to acute coronary syndromes.
BENEFITS OF DAPT
DAPT is mandatory early after drug-eluting stent placement, when the stent continuously releases medication, inhibiting tissue growth within the lumen of the stent.
Endothelialization of the stent normally occurs during the first 7 to 30 days after placement. During this period, the nonendothelialized stent poses a risk of thrombosis, a life-threatening, catastrophic condition with a mortality rate between 9% and 45%.1

THERAPY BEYOND 12 MONTHS
Although guidelines have traditionally recommended 12 months of DAPT, the optimal duration is still debated.
A duration beyond 12 months in patients with a history of myocardial infarction was shown to be reasonable in 2 large trials,2,3 while a 2016 review by Bittl et al4 suggested that therapy beyond 12 months in patients with a newer-generation drug-eluting stent could increase the incidence of major bleeding. A detailed discussion of DAPT longer than 12 months is beyond the scope of this article.
EVIDENCE FOR SHORTER DURATION
The results of 5 major trials support shorter duration of DAPT in stable ischemic disease.
The OPTIMIZE5 and RESET6 trials found that 3 months of DAPT was not inferior to 12 months in terms of ischemic and safety end points.
The ISAR-SAFE,7 EXCELLENT,8 and SECURITY9 trials also reported that 6 months of DAPT was not inferior to 12 months for the primary composite end point of death, stent thrombosis, myocardial infarction, stroke, or major bleeding.
However, these trials may have been underpowered to detect a difference in rates of stent thrombosis with shorter-duration DAPT.
CURRENT GUIDELINES
For patients at high bleeding risk, the current guidelines of the American College of Cardiology and American Heart Association, updated in 2016, suggest that it may be reasonable to discontinue DAPT 3 months after drug-eluting stent placement in patients with stable ischemic heart disease, and at 6 months in patients with acute coronary syndrome (class IIb recommendation, level of evidence C).1 These recommendations are based on results of randomized controlled trials showing no difference in the rate of stent thrombosis and composite ischemic events with a shorter duration than with 12 months of therapy.5–10
The evidence for DAPT in stable ischemic disease is based on clopidogrel, with only limited data on ticagrelor.1 To our knowledge, no study to date has evaluated DAPT in this setting for less than 3 months, and further study is needed to address shorter-duration approaches with current-generation drug-eluting stents Since 2017, all coronary stents implanted in the United States have been second-generation stents.
TOOLS TO HELP DECISION-MAKING
The decision to stop DAPT in a patient at high risk of bleeding requires a careful assessment of the risks and benefits. Risk factors for bleeding include advanced age, history of major bleeding, anticoagulation, chronic kidney disease (serum creatinine level ≥ 2 mg/dL), platelet count 100 × 109/L or lower, and history of stroke.11
- Age 75 or older: −2 points
- Ages 65 to 74: −1
- Age under 65: 0
- Diabetes mellitus: 1
- Myocardial infarction at presentation: 1
- History of percutaneous coronary intervention or myocardial infarction: 1
- Stent diameter less than 3 mm: 1
- Paclitaxel drug-eluting stent: 1
- Current smoker: 2
- Percutaneous coronary intervention with saphenous vein graft: 2
- Congestive heart failure or left ventricular ejection fraction less than 30%: 2.
A score of 2 or greater favors continuing DAPT, as it indicates higher ischemic risk. A score less than 2 favors discontinuing DAPT, as it indicates higher bleeding risk.1,2
IF BLEEDING RISK IS HIGH
Preventing and controlling bleeding associated with DAPT is important. The gastrointestinal tract is the most common site of bleeding.
Aspirin inhibits prostaglandin synthesis, leading to disruption of the protective mucous membrane. Therefore, a proton pump inhibitor should be started along with DAPT in patients at high risk of gastrointestinal bleeding.
If a patient’s bleeding risk significantly outweighs the risk of stent thrombosis, or if active hemorrhage makes a patient hemodynamically unstable, antiplatelet therapy must be stopped.1
FACING SURGERY
For patients with a drug-eluting stent who are on DAPT and are to undergo elective noncardiac surgery, 3 considerations must be kept in mind:
- The risk of stent thrombosis if DAPT needs to be interrupted
- The consequences of delaying the surgical procedure
- The risk and consequences of periprocedural and intraprocedural bleeding if DAPT is continued.
Because clinical evidence for bridging therapy with intravenous antiplatelet or anticoagulant agents is limited, it is difficult to make recommendations about stopping DAPT. However, once bleeding risk is stabilized, DAPT should be restarted as soon as possible.1
CURRENT RESEARCH
Several trials are under way to further evaluate ways to minimize bleeding risk and shorten the duration of DAPT.
A prospective multicenter trial is evaluating 3-month DAPT in patients at high bleeding risk who undergo placement of an everolimus-eluting stent.11 This study is expected to be completed in August 2019.
Another strategy for patients at high bleeding risk is use of a polymer-free drug-coated coronary stent. In a 2015 trial comparing a biolimus A9-coated stent vs a bare-metal stent, patients received DAPT for 1 month after stent placement. The drug-coated stent was found to be superior in terms of the primary safety end point (cardiac death, myocardial infarction, or stent thrombosis).12 This stent is not yet approved by the US Food and Drug Administration at the time of this writing.
Further study is needed to evaluate DAPT durations of less than 3 months and to establish the proper timing for safely discontinuing DAPT in difficult clinical scenarios.
WHEN STOPPING MAY BE REASONABLE
According to current guidelines, in patients at high bleeding risk with a second-generation or newer drug-eluting stent for stable ischemic heart disease, discontinuing DAPT 3 months after stent placement may be reasonable.1 The decision to stop DAPT in these patients requires a careful assessment of the risks and benefits and may be aided by a tool such as the DAPT risk score. However, these recommendations cannot be extrapolated to patients with an acute coronary syndrome within the past year, as they are at higher risk.
TAKE-HOME MESSAGES
- A cardiologist should be consulted before discontinuing DAPT in patients with a drug-eluting stent, especially if the stent was recently placed.
- The duration of therapy depends on the indication for stent placement (stable ischemic heart disease vs acute coronary syndrome) and on stent location.
- Based on the 2016 American College of Cardiology/American Heart Association guidelines,1 in patients at high bleeding risk with a second-generation drug-eluting stent, discontinuing DAPT is safe after 3 months in patients with stable ischemic heart disease, and after 6 months in patients with an acute coronary syndrome.
- When prescribing DAPT, available evidence favors clopidogrel in patients with stable ischemic heart disease who have a second-generation drug-eluting stent and are at high bleeding risk.
- In these patients, the risk-benefit ratio based on the DAPT score may be useful when considering stopping clopidogrel.
Stopping dual antiplatelet therapy (DAPT) (eg, clopidogrel plus aspirin) after 3 months is reasonable in patients with stable ischemic heart disease who have a second-generation drug-eluting stent and a high bleeding risk, with stable ischemic disease defined as at least 1 year free of acute coronary syndromes. However, these patients should continue lifelong aspirin monotherapy. Current guidelines suggest that in stable ischemic disease, the risk-benefit ratio may favor an even shorter duration of DAPT than the 6 months currently recommended.1
STABLE ISCHEMIC HEART DISEASE VS ACUTE CORONARY SYNDROME
Percutaneous coronary intervention for stable ischemic heart disease is indicated primarily in patients with angina that persists despite optimal antianginal therapy.
The prognostic implications of DAPT are different in stable ischemic disease than in acute coronary syndromes. The substrate treated by percutaneous intervention in stable ischemic disease is primarily fibrofatty plaque, as opposed to thrombus in acute coronary syndromes.
Percutaneous intervention significantly improves the prognosis in acute coronary syndromes, whereas its impact on overall survival in stable ischemic heart disease is not well documented. Given these differences, our discussion about DAPT in stable ischemic disease cannot be extrapolated to acute coronary syndromes.
BENEFITS OF DAPT
DAPT is mandatory early after drug-eluting stent placement, when the stent continuously releases medication, inhibiting tissue growth within the lumen of the stent.
Endothelialization of the stent normally occurs during the first 7 to 30 days after placement. During this period, the nonendothelialized stent poses a risk of thrombosis, a life-threatening, catastrophic condition with a mortality rate between 9% and 45%.1

THERAPY BEYOND 12 MONTHS
Although guidelines have traditionally recommended 12 months of DAPT, the optimal duration is still debated.
A duration beyond 12 months in patients with a history of myocardial infarction was shown to be reasonable in 2 large trials,2,3 while a 2016 review by Bittl et al4 suggested that therapy beyond 12 months in patients with a newer-generation drug-eluting stent could increase the incidence of major bleeding. A detailed discussion of DAPT longer than 12 months is beyond the scope of this article.
EVIDENCE FOR SHORTER DURATION
The results of 5 major trials support shorter duration of DAPT in stable ischemic disease.
The OPTIMIZE5 and RESET6 trials found that 3 months of DAPT was not inferior to 12 months in terms of ischemic and safety end points.
The ISAR-SAFE,7 EXCELLENT,8 and SECURITY9 trials also reported that 6 months of DAPT was not inferior to 12 months for the primary composite end point of death, stent thrombosis, myocardial infarction, stroke, or major bleeding.
However, these trials may have been underpowered to detect a difference in rates of stent thrombosis with shorter-duration DAPT.
CURRENT GUIDELINES
For patients at high bleeding risk, the current guidelines of the American College of Cardiology and American Heart Association, updated in 2016, suggest that it may be reasonable to discontinue DAPT 3 months after drug-eluting stent placement in patients with stable ischemic heart disease, and at 6 months in patients with acute coronary syndrome (class IIb recommendation, level of evidence C).1 These recommendations are based on results of randomized controlled trials showing no difference in the rate of stent thrombosis and composite ischemic events with a shorter duration than with 12 months of therapy.5–10
The evidence for DAPT in stable ischemic disease is based on clopidogrel, with only limited data on ticagrelor.1 To our knowledge, no study to date has evaluated DAPT in this setting for less than 3 months, and further study is needed to address shorter-duration approaches with current-generation drug-eluting stents Since 2017, all coronary stents implanted in the United States have been second-generation stents.
TOOLS TO HELP DECISION-MAKING
The decision to stop DAPT in a patient at high risk of bleeding requires a careful assessment of the risks and benefits. Risk factors for bleeding include advanced age, history of major bleeding, anticoagulation, chronic kidney disease (serum creatinine level ≥ 2 mg/dL), platelet count 100 × 109/L or lower, and history of stroke.11
- Age 75 or older: −2 points
- Ages 65 to 74: −1
- Age under 65: 0
- Diabetes mellitus: 1
- Myocardial infarction at presentation: 1
- History of percutaneous coronary intervention or myocardial infarction: 1
- Stent diameter less than 3 mm: 1
- Paclitaxel drug-eluting stent: 1
- Current smoker: 2
- Percutaneous coronary intervention with saphenous vein graft: 2
- Congestive heart failure or left ventricular ejection fraction less than 30%: 2.
A score of 2 or greater favors continuing DAPT, as it indicates higher ischemic risk. A score less than 2 favors discontinuing DAPT, as it indicates higher bleeding risk.1,2
IF BLEEDING RISK IS HIGH
Preventing and controlling bleeding associated with DAPT is important. The gastrointestinal tract is the most common site of bleeding.
Aspirin inhibits prostaglandin synthesis, leading to disruption of the protective mucous membrane. Therefore, a proton pump inhibitor should be started along with DAPT in patients at high risk of gastrointestinal bleeding.
If a patient’s bleeding risk significantly outweighs the risk of stent thrombosis, or if active hemorrhage makes a patient hemodynamically unstable, antiplatelet therapy must be stopped.1
FACING SURGERY
For patients with a drug-eluting stent who are on DAPT and are to undergo elective noncardiac surgery, 3 considerations must be kept in mind:
- The risk of stent thrombosis if DAPT needs to be interrupted
- The consequences of delaying the surgical procedure
- The risk and consequences of periprocedural and intraprocedural bleeding if DAPT is continued.
Because clinical evidence for bridging therapy with intravenous antiplatelet or anticoagulant agents is limited, it is difficult to make recommendations about stopping DAPT. However, once bleeding risk is stabilized, DAPT should be restarted as soon as possible.1
CURRENT RESEARCH
Several trials are under way to further evaluate ways to minimize bleeding risk and shorten the duration of DAPT.
A prospective multicenter trial is evaluating 3-month DAPT in patients at high bleeding risk who undergo placement of an everolimus-eluting stent.11 This study is expected to be completed in August 2019.
Another strategy for patients at high bleeding risk is use of a polymer-free drug-coated coronary stent. In a 2015 trial comparing a biolimus A9-coated stent vs a bare-metal stent, patients received DAPT for 1 month after stent placement. The drug-coated stent was found to be superior in terms of the primary safety end point (cardiac death, myocardial infarction, or stent thrombosis).12 This stent is not yet approved by the US Food and Drug Administration at the time of this writing.
Further study is needed to evaluate DAPT durations of less than 3 months and to establish the proper timing for safely discontinuing DAPT in difficult clinical scenarios.
WHEN STOPPING MAY BE REASONABLE
According to current guidelines, in patients at high bleeding risk with a second-generation or newer drug-eluting stent for stable ischemic heart disease, discontinuing DAPT 3 months after stent placement may be reasonable.1 The decision to stop DAPT in these patients requires a careful assessment of the risks and benefits and may be aided by a tool such as the DAPT risk score. However, these recommendations cannot be extrapolated to patients with an acute coronary syndrome within the past year, as they are at higher risk.
TAKE-HOME MESSAGES
- A cardiologist should be consulted before discontinuing DAPT in patients with a drug-eluting stent, especially if the stent was recently placed.
- The duration of therapy depends on the indication for stent placement (stable ischemic heart disease vs acute coronary syndrome) and on stent location.
- Based on the 2016 American College of Cardiology/American Heart Association guidelines,1 in patients at high bleeding risk with a second-generation drug-eluting stent, discontinuing DAPT is safe after 3 months in patients with stable ischemic heart disease, and after 6 months in patients with an acute coronary syndrome.
- When prescribing DAPT, available evidence favors clopidogrel in patients with stable ischemic heart disease who have a second-generation drug-eluting stent and are at high bleeding risk.
- In these patients, the risk-benefit ratio based on the DAPT score may be useful when considering stopping clopidogrel.
- Levine GN, Bates ER, Bittl JA, et al. 2016 ACC/AHA guideline focused update on duration of dual antiplatelet therapy in patients with coronary artery disease: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation 2016; 134(10):e123–e155. doi:10.1161/CIR.0000000000000404 [correction in doi:10.1161/CIR.0000000000000452]
- Mauri L, Kereiakes DJ, Yeh RW, et al; DAPT Study Investigators. Twelve or 30 months of dual antiplatelet therapy after drug-eluting stents. N Engl J Med 2014; 371(23):2155–2166. doi:10.1056/NEJMoa1409312
- Bonaca MP, Bhatt DL, Cohen M, et al; PEGASUS-TIMI 54 Steering Committee and Investigators. Long-term use of ticagrelor in patients with prior myocardial infarction. N Engl J Med 2015; 372(19):1791–1800. doi:10.1056/NEJMoa1500857
- Bittl JA, Baber U, Bradley SM, Wijeysundera DN. Duration of dual antiplatelet therapy: a systematic review for the 2016 ACC/AHA guideline focused update on duration of dual antiplatelet therapy in patients with coronary artery disease: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol 2016; 68(10):1116–1139. doi:10.1016/j.jacc.2016.03.512
- Feres F, Costa RA, Abizaid A, et al; OPTIMIZE Trial Investigators. Three vs twelve months of dual antiplatelet therapy after zotarolimus-eluting stents: the OPTIMIZE randomized trial. JAMA 2013; 310(23):2510–2522. doi:10.1001/jama.2013.282183
- Kubo T, Akasaka T, Kozuma K, et al. Comparison of neointimal coverage between everolimus-eluting stents and sirolimus-eluting stents: an optical coherence tomography substudy of RESET. EuroIntervention 2015. doi:10.4244/EIJV11I5A109
- Schulz-Schupke S, Byrne RA, ten Berg JM, et al; Intracoronary Stenting and Antithrombotic Regimen: Safety And EFficacy of 6 Months Dual Antiplatelet Therapy After Drug-Eluting Stenting (ISAR-SAFE) Trial Investigators. ISAR-SAFE: a randomized, double-blind, placebo-controlled trial of 6 vs 12 months of clopidogrel therapy after drug-eluting stenting. Eur Heart J 2015; 36(20):1252–1263. doi:10.1093/eurheartj/ehu523
- Gwon HC, Hahn JY, Park KW, et al. Six-month versus 12-month dual antiplatelet therapy after implantation of drug-eluting stents: the efficacy of Xience/Promus vs Cypher to reduce late loss after stenting (EXCELLENT) randomized, multicenter study. Circulation 2012; 125(3):505–513. doi:10.1161/CIRCULATIONAHA.111.059022
- Colombo A, Chieffo A, Frasheri A, et al. Second-generation drug-eluting stent implantation followed by 6- vs 12-month dual antiplatelet therapy: the SECURITY randomized clinical trial. J Am Coll Cardiol 2014; 64(20):2086–2097. doi:10.1016/j.jacc.2014.09.008
- Kim BK, Hong MK, Shin DH, et al; RESET Investigators. A new strategy for discontinuation of dual antiplatelet therapy: the RESET Trial (REal Safety and Efficacy of 3-month dual antiplatelet Therapy following Endeavor zotarolimus-eluting stent implantation). J Am Coll Cardiol 2012; 60(15):1340–1348. doi:10.1016/j.jacc.2012.06.043
- US National Library of Medicine. ClinicalTrials.gov. EVOLVE Short DAPT Study. https://clinicaltrials.gov/ct2/show/NCT02605447. Accessed December 3, 2018.
- Urban P, Meredith IT, Abizaid A, et al; LEADERS FREE Investigators. Polymer-free drug-coated coronary stents in patients at high bleeding risk. N Engl J Med 2015; 373(21):2038–2047. doi:10.1056/NEJMoa1503943
- Levine GN, Bates ER, Bittl JA, et al. 2016 ACC/AHA guideline focused update on duration of dual antiplatelet therapy in patients with coronary artery disease: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation 2016; 134(10):e123–e155. doi:10.1161/CIR.0000000000000404 [correction in doi:10.1161/CIR.0000000000000452]
- Mauri L, Kereiakes DJ, Yeh RW, et al; DAPT Study Investigators. Twelve or 30 months of dual antiplatelet therapy after drug-eluting stents. N Engl J Med 2014; 371(23):2155–2166. doi:10.1056/NEJMoa1409312
- Bonaca MP, Bhatt DL, Cohen M, et al; PEGASUS-TIMI 54 Steering Committee and Investigators. Long-term use of ticagrelor in patients with prior myocardial infarction. N Engl J Med 2015; 372(19):1791–1800. doi:10.1056/NEJMoa1500857
- Bittl JA, Baber U, Bradley SM, Wijeysundera DN. Duration of dual antiplatelet therapy: a systematic review for the 2016 ACC/AHA guideline focused update on duration of dual antiplatelet therapy in patients with coronary artery disease: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol 2016; 68(10):1116–1139. doi:10.1016/j.jacc.2016.03.512
- Feres F, Costa RA, Abizaid A, et al; OPTIMIZE Trial Investigators. Three vs twelve months of dual antiplatelet therapy after zotarolimus-eluting stents: the OPTIMIZE randomized trial. JAMA 2013; 310(23):2510–2522. doi:10.1001/jama.2013.282183
- Kubo T, Akasaka T, Kozuma K, et al. Comparison of neointimal coverage between everolimus-eluting stents and sirolimus-eluting stents: an optical coherence tomography substudy of RESET. EuroIntervention 2015. doi:10.4244/EIJV11I5A109
- Schulz-Schupke S, Byrne RA, ten Berg JM, et al; Intracoronary Stenting and Antithrombotic Regimen: Safety And EFficacy of 6 Months Dual Antiplatelet Therapy After Drug-Eluting Stenting (ISAR-SAFE) Trial Investigators. ISAR-SAFE: a randomized, double-blind, placebo-controlled trial of 6 vs 12 months of clopidogrel therapy after drug-eluting stenting. Eur Heart J 2015; 36(20):1252–1263. doi:10.1093/eurheartj/ehu523
- Gwon HC, Hahn JY, Park KW, et al. Six-month versus 12-month dual antiplatelet therapy after implantation of drug-eluting stents: the efficacy of Xience/Promus vs Cypher to reduce late loss after stenting (EXCELLENT) randomized, multicenter study. Circulation 2012; 125(3):505–513. doi:10.1161/CIRCULATIONAHA.111.059022
- Colombo A, Chieffo A, Frasheri A, et al. Second-generation drug-eluting stent implantation followed by 6- vs 12-month dual antiplatelet therapy: the SECURITY randomized clinical trial. J Am Coll Cardiol 2014; 64(20):2086–2097. doi:10.1016/j.jacc.2014.09.008
- Kim BK, Hong MK, Shin DH, et al; RESET Investigators. A new strategy for discontinuation of dual antiplatelet therapy: the RESET Trial (REal Safety and Efficacy of 3-month dual antiplatelet Therapy following Endeavor zotarolimus-eluting stent implantation). J Am Coll Cardiol 2012; 60(15):1340–1348. doi:10.1016/j.jacc.2012.06.043
- US National Library of Medicine. ClinicalTrials.gov. EVOLVE Short DAPT Study. https://clinicaltrials.gov/ct2/show/NCT02605447. Accessed December 3, 2018.
- Urban P, Meredith IT, Abizaid A, et al; LEADERS FREE Investigators. Polymer-free drug-coated coronary stents in patients at high bleeding risk. N Engl J Med 2015; 373(21):2038–2047. doi:10.1056/NEJMoa1503943
Should metformin be used in every patient with type 2 diabetes?
Most patients should receive it, with exceptions as noted below. Metformin is the cornerstone of diabetes therapy and should be considered in all patients with type 2 diabetes. Both the American Diabetes Association (ADA) and the American Association of Clinical Endocrinologists (AACE)1,2 recommend it as first-line treatment for type 2 diabetes. It lowers blood glucose levels by inhibiting hepatic glucose production, and it does not tend to cause hypoglycemia.
However, metformin is underused. A 2012 study showed that only 50% to 70% of patients with type 2 diabetes treated with a sulfonylurea, dipeptidyl peptidase-4 (DPP-4) inhibitor, thiazolidinedione, or glucagon-like peptide-1 analogue also received metformin.3 This occurred despite guidelines recommending continuing metformin when starting other diabetes drugs.4
EVIDENCE METFORMIN IS EFFECTIVE
The United Kingdom Prospective Diabetes Study (UKPDS)5 found that metformin significantly reduced the incidence of:
- Any diabetes-related end point (hazard ratio [HR] 0.68, 95% confidence interval [CI] 0.53–0.87)
- Myocardial infarction (HR 0.61, 95% CI 0.41–0.89)
- Diabetes-related death (HR 0.58, 95% CI 0.37–0.91)
- All-cause mortality (HR 0.64; 95% CI 0.45–0.91).
The Hyperinsulinemia: Outcomes of Its Metabolic Effects (HOME) trial,6 a multicenter trial conducted in the Netherlands, evaluated the effect of adding metformin (vs placebo) to existing insulin regimens. Metformin recipients had a significantly lower rate of macrovascular mortality (HR 0.61, 95% CI 0.40–0.94, P = .02), but not of the primary end point, an aggregate of microvascular and macrovascular morbidity and mortality.
The Study on the Prognosis and Effect of Antidiabetic Drugs on Type 2 Diabetes Mellitus With Coronary Artery Disease trial,7 a multicenter trial conducted in China, compared the effects of metformin vs glipizide on cardiovascular outcomes. At about 3 years of treatment, the metformin group had a significantly lower rate of the composite primary end point of recurrent cardiovascular events (HR 0.54, 95% CI 0.30–0.90). This end point included nonfatal myocardial infarction, nonfatal stroke, arterial revascularization by percutaneous transluminal coronary angioplasty or by coronary artery bypass graft, death from a cardiovascular cause, and death from any cause.
These studies prompted the ADA to emphasize that metformin can reduce the risk of cardiovascular events or death. Metformin also has been shown to be weight-neutral or to induce slight weight loss. Furthermore, it is inexpensive.
WHAT ABOUT THE RENAL EFFECTS?
Because metformin is renally cleared, it has caused some concern about nephrotoxicity, especially lactic acidosis, in patients with impaired renal function. But the most recent guidelines have relaxed the criteria for metformin use in this patient population.
Revised labeling
Metformin’s labeling,8 revised in 2016, states the following:
- If the estimated glomerular filtration rate (eGFR) is below 30 mL/min/1.73 m2, metformin is contraindicated
- If the eGFR is between 30 and 45 mL/min/1.73 m2, metformin is not recommended
- If the eGFR is below 45 mL/min/1.73 m2 in a patient taking metformin, the risks and benefits of continuing treatment should be assessed, the dosage may need to be adjusted, and renal function should be monitored more frequently.8
These labeling revisions were based on a systematic review by Inzucchi et al9 that found metformin is not associated with increased rates of lactic acidosis in patients with mild to moderate kidney disease. Subsequently, an observational study published in 2018 by Lazarus et al10 showed that metformin increases the risk of acidosis only at eGFR levels below 30 mL/min/1.73 m2. Also, a Cochrane review published in 2003 did not find a single case of lactic acidosis in 347 trials with 70,490 patient-years of metformin treatment.11
Previous guidelines used serum creatinine levels, with metformin contraindicated at levels of 1.5 mg/dL or above for men and 1.4 mg/dL for women, or with abnormal creatinine clearance. The ADA and the AACE now use the eGFR1,2 instead of the serum creatinine level to measure kidney function because it better accounts for factors such as the patient’s age, sex, race, and weight.
Despite the evidence, the common patient perception is that metformin is nephrotoxic, and it is important for practitioners to dispel this myth during clinic visits.
What about metformin use with contrast agents?
Labeling has a precautionary note stating that metformin should be held at the time of, or prior to, any imaging procedure involving iodinated contrast agents in patients with an eGFR between 30 and 60 mL/min/1.73 m2; in patients with a history of hepatic impairment, alcoholism, or heart failure; or in patients who will receive intra-arterial iodinated contrast. The eGFR should be reevaluated 48 hours after the imaging procedure.8
Additionally, if the iodinated contrast agent causes acute kidney injury, metformin could accumulate, with resultant lactate accumulation.
The American College of Radiology (ACR) has proposed less stringent guidelines for metformin during radiocontrast imaging studies. This change is based on evidence that lactic acidosis is rare—about 10 cases per 100,000 patient-years—and that there are no reports of lactic acidosis after intravenously administered iodinated contrast in properly selected patients.12,13
The ACR divides patients taking metformin into 2 categories:
- No evidence of acute kidney injury and eGFR greater than 30 mL/min/1.73 m2
- Either acute kidney injury or chronic kidney disease with eGFR below 30 mL/min/1.73 m2 or undergoing arterial catheter studies with a high chance of embolization to the renal arteries.14
For the first group, they recommend against discontinuing metformin before or after giving iodinated contrast or checking kidney function after the procedure.
For the second group, they recommend holding metformin before and 48 hours after the procedure. It should not be restarted until renal function is confirmed to be normal.
METFORMIN AND INSULIN
The ADA recommends1 continuing metformin after initiating insulin. However, in clinical practice, it is often not done.
Clinical trials have shown that combining metformin with insulin significantly improves glycemic control, prevents weight gain, and decreases insulin requirements.15,16 One trial16 also looked at cardiovascular end points during a 4-year follow-up period; combining metformin with insulin decreased the macrovascular disease-related event rate compared with insulin alone.
In the HOME trial,6 which added metformin to the existing insulin regimen, both groups gained weight, but the metformin group had gained about 3 kg less than the placebo group at the end of the 4.3-year trial. Metformin did not increase the risk of hypoglycemia, but it also did not reduce the risk of microvascular disease.
Concomitant metformin reduces costs
These days, practitioners can choose from a large selection of diabetes drugs. These include insulins with better pharmacokinetic profiles, as well as newer classes of noninsulin agents such as sodium-glucose cotransporter-2 inhibitors and glucagon-like peptide-1 analogues.
Metformin is less expensive than these newer drugs, and using it concomitantly with other diabetes drugs can decrease their dosage requirements, which in turn decreases their monthly costs.
GASTROINTESTINAL EFFECTS
Metformin’s gastrointestinal adverse effects such as diarrhea, flatulence, nausea, and vomiting are a barrier to its use. The actual incidence rate of diarrhea varies widely in randomized trials and observational studies, and gastrointestinal effects are worse in metformin-naive patients, as well as those who have chronic gastritis or Helicobacter pylori infection.17
We have found that starting metformin at a low dose and up-titrating it over several weeks increases tolerability. We often start patients at 500 mg/day and increase the dosage by 1 500-mg tablet every 1 to 2 weeks. Also, we have noticed that intolerance is more likely in patients who eat a high-carbohydrate diet, but there is no high-level evidence to back this up because patients in clinical trials all undergo nutrition counseling and are therefore more likely to adhere to the low-carbohydrate diet.
Also, the extended-release formulation is more tolerable than the immediate-release formulation and has similar glycemic efficacy. It may be an option as first-line therapy or for patients who have significant adverse effects from immediate-release metformin.18 For patients on the immediate-release formulation, taking it with meals helps lessen some gastrointestinal effects, and this should be emphasized at every visit.
Finally, we limit the metformin dose to 2,000 mg/day, rather than the 2,550 mg/day allowed on labeling. Garber et al19 found that the lower dosage still provides the maximum clinical efficacy.
OTHER CAUTIONS
Metformin should be avoided in patients with acute or unstable heart failure because of the increased risk of lactic acidosis.
It also should be avoided in patients with hepatic impairment, according to the labeling. But this remains controversial in practice. Zhang et al20 showed that continuing metformin in patients with diabetes and cirrhosis decreases the mortality risk by 57% compared with those taken off metformin.
Diet and lifestyle measures need to be emphasized at each visit. Wing et al21 showed that calorie restriction regardless of weight loss is beneficial for glycemic control and insulin sensitivity in obese patients with diabetes.
TAKE-HOME POINTS
Metformin improves glycemic control without tending to cause weight gain or hypoglycemia. It may also have cardiovascular benefits. Metformin is an inexpensive agent that should be continued, if tolerated, in those who need additional agents for glycemic control. It should be considered in all adult patients with type 2 diabetes.
- American Diabetes Association. 8. Pharmacologic approaches to glycemic treatment: standards of medical care in diabetes-2018. Diabetes Care 2018; 41(suppl 1):S73–S85. doi:10.2337/dc18-S008
- Garber AJ, Abrahamson MJ, Barzilay JI, et al. Consensus statement by the American Association of Clinical Endocrinologists and American College of Endocrinology on the comprehensive type 2 diabetes management algorithm—2018 executive summary. Endocr Pract 2018; 24(1):91–120. doi:10.4158/CS-2017-0153
- Hampp C, Borders-Hemphill V, Moeny DG, Wysowski DK. Use of antidiabetic drugs in the US, 2003–2012. Diabetes Care 2014; 37(5):1367–1374. doi:10.2337/dc13-2289
- Inzucchi SE, Bergenstal RM, Buse JB, et al; American Diabetes Association (ADA); European Association for the Study of Diabetes (EASD). Management of hyperglycemia in type 2 diabetes: a patient-centered approach: position statement of the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care 2012; 35(6):1364–1379. doi:10.2337/dc12-0413
- Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34). UK Prospective Diabetes Study (UKPDS) Group. Lancet 1998; 352(9131):854–865. pmid:9742977
- Kooy A, de Jager J, Lehert P, et al. Long-term effects of metformin on metabolism and microvascular and macrovascular disease in patients with type 2 diabetes mellitus. Arch Intern Med 2009; 169(6):616–625. doi:10.1001/archinternmed.2009.20
- Hong J, Zhang Y, Lai S, et al; SPREAD-DIMCAD Investigators. Effects of metformin versus glipizide on cardiovascular outcomes in patients with type 2 diabetes and coronary artery disease. Diabetes Care 2013; 36(5):1304–1311. doi:10.2337/dc12-0719
- Glucophage (metformin hydrochloride) and Glucophage XR (extended-release) [package insert]. Princeton, NJ: Bristol-Myers Squibb Company. www.accessdata.fda.gov/drugsatfda_docs/label/2018/020357s034,021202s018lbl.pdf. Accessed December 5, 2018.
- Inzucchi SE, Lipska KJ, Mayo H, Bailey CJ, McGuire DK. Metformin in patients with type 2 diabetes and kidney disease: a systematic review. JAMA 2014; 312(24):2668–2675. doi:10.1001/jama.2014.15298
- Lazarus B, Wu A, Shin JI, et al. Association of metformin use with risk of lactic acidosis across the range of kidney function: a community-based cohort study. JAMA Intern Med 2018; 178(7):903–910. doi:10.1001/jamainternmed.2018.0292
- Salpeter S, Greyber E, Pasternak G, Salpeter E. Risk of fatal and nonfatal lactic acidosis with metformin use in type 2 diabetes mellitus. Cochrane Database Syst Rev 2003; (2):CD002967. doi:10.1002/14651858.CD002967
- Eppenga WL, Lalmohamed A, Geerts AF, et al. Risk of lactic acidosis or elevated lactate concentrations in metformin users with renal impairment: a population-based cohort study. Diabetes Care 2014; 37(8):2218–2224. doi:10.2337/dc13-3023
- Richy FF, Sabidó-Espin M, Guedes S, Corvino FA, Gottwald-Hostalek U. Incidence of lactic acidosis in patients with type 2 diabetes with and without renal impairment treated with metformin: a retrospective cohort study. Diabetes Care 2014; 37(8):2291–2295. doi:10.2337/dc14-0464
- American College of Radiology (ACR). Manual on Contrast Media. Version 10.3. www.acr.org/Clinical-Resources/Contrast-Manual. Accessed December 5, 2018.
- Wulffele MG, Kooy A, Lehert P, et al. Combination of insulin and metformin in the treatment of type 2 diabetes. Diabetes Care 2002; 25(12):2133–2140. pmid:12453950
- Kooy A, de Jager J, Lehert P, et al. Long-term effects of metformin on metabolism and microvascular and macrovascular disease in patients with type 2 diabetes mellitus. Arch Intern Med 2009; 169(6):616–625. doi:10.1001/archinternmed.2009.20
- Bonnet F, Scheen A. Understanding and overcoming metformin gastrointestinal intolerance, Diabetes Obes Metab 2017; 19(4):473–481. doi:10.1111/dom.12854
- Jabbour S, Ziring B. Advantages of extended-release metformin in patients with type 2 diabetes mellitus. Postgrad Med 2011; 123(1):15–23. doi:10.3810/pgm.2011.01.2241
- Garber AJ, Duncan TG, Goodman AM, Mills DJ, Rohlf JL. Efficacy of metformin in type II diabetes: results of a double-blind, placebo-controlled, dose-response trial. Am J Med 1997; 103(6):491–497. pmid:9428832
- Zhang X, Harmsen WS, Mettler TA, et al. Continuation of metformin use after a diagnosis of cirrhosis significantly improves survival of patients with diabetes. Hepatology 2014; 60(6):2008–2016. doi:10.1002/hep.27199
- Wing RR, Blair EH, Bononi P, Marcus MD, Watanabe R, Bergman RN. Caloric restriction per se is a significant factor in improvements in glycemic control and insulin sensitivity during weight loss in obese NIDDM patients. Diabetes Care 1994; 17(1):30–36. pmid:8112186
Most patients should receive it, with exceptions as noted below. Metformin is the cornerstone of diabetes therapy and should be considered in all patients with type 2 diabetes. Both the American Diabetes Association (ADA) and the American Association of Clinical Endocrinologists (AACE)1,2 recommend it as first-line treatment for type 2 diabetes. It lowers blood glucose levels by inhibiting hepatic glucose production, and it does not tend to cause hypoglycemia.
However, metformin is underused. A 2012 study showed that only 50% to 70% of patients with type 2 diabetes treated with a sulfonylurea, dipeptidyl peptidase-4 (DPP-4) inhibitor, thiazolidinedione, or glucagon-like peptide-1 analogue also received metformin.3 This occurred despite guidelines recommending continuing metformin when starting other diabetes drugs.4
EVIDENCE METFORMIN IS EFFECTIVE
The United Kingdom Prospective Diabetes Study (UKPDS)5 found that metformin significantly reduced the incidence of:
- Any diabetes-related end point (hazard ratio [HR] 0.68, 95% confidence interval [CI] 0.53–0.87)
- Myocardial infarction (HR 0.61, 95% CI 0.41–0.89)
- Diabetes-related death (HR 0.58, 95% CI 0.37–0.91)
- All-cause mortality (HR 0.64; 95% CI 0.45–0.91).
The Hyperinsulinemia: Outcomes of Its Metabolic Effects (HOME) trial,6 a multicenter trial conducted in the Netherlands, evaluated the effect of adding metformin (vs placebo) to existing insulin regimens. Metformin recipients had a significantly lower rate of macrovascular mortality (HR 0.61, 95% CI 0.40–0.94, P = .02), but not of the primary end point, an aggregate of microvascular and macrovascular morbidity and mortality.
The Study on the Prognosis and Effect of Antidiabetic Drugs on Type 2 Diabetes Mellitus With Coronary Artery Disease trial,7 a multicenter trial conducted in China, compared the effects of metformin vs glipizide on cardiovascular outcomes. At about 3 years of treatment, the metformin group had a significantly lower rate of the composite primary end point of recurrent cardiovascular events (HR 0.54, 95% CI 0.30–0.90). This end point included nonfatal myocardial infarction, nonfatal stroke, arterial revascularization by percutaneous transluminal coronary angioplasty or by coronary artery bypass graft, death from a cardiovascular cause, and death from any cause.
These studies prompted the ADA to emphasize that metformin can reduce the risk of cardiovascular events or death. Metformin also has been shown to be weight-neutral or to induce slight weight loss. Furthermore, it is inexpensive.
WHAT ABOUT THE RENAL EFFECTS?
Because metformin is renally cleared, it has caused some concern about nephrotoxicity, especially lactic acidosis, in patients with impaired renal function. But the most recent guidelines have relaxed the criteria for metformin use in this patient population.
Revised labeling
Metformin’s labeling,8 revised in 2016, states the following:
- If the estimated glomerular filtration rate (eGFR) is below 30 mL/min/1.73 m2, metformin is contraindicated
- If the eGFR is between 30 and 45 mL/min/1.73 m2, metformin is not recommended
- If the eGFR is below 45 mL/min/1.73 m2 in a patient taking metformin, the risks and benefits of continuing treatment should be assessed, the dosage may need to be adjusted, and renal function should be monitored more frequently.8
These labeling revisions were based on a systematic review by Inzucchi et al9 that found metformin is not associated with increased rates of lactic acidosis in patients with mild to moderate kidney disease. Subsequently, an observational study published in 2018 by Lazarus et al10 showed that metformin increases the risk of acidosis only at eGFR levels below 30 mL/min/1.73 m2. Also, a Cochrane review published in 2003 did not find a single case of lactic acidosis in 347 trials with 70,490 patient-years of metformin treatment.11
Previous guidelines used serum creatinine levels, with metformin contraindicated at levels of 1.5 mg/dL or above for men and 1.4 mg/dL for women, or with abnormal creatinine clearance. The ADA and the AACE now use the eGFR1,2 instead of the serum creatinine level to measure kidney function because it better accounts for factors such as the patient’s age, sex, race, and weight.
Despite the evidence, the common patient perception is that metformin is nephrotoxic, and it is important for practitioners to dispel this myth during clinic visits.
What about metformin use with contrast agents?
Labeling has a precautionary note stating that metformin should be held at the time of, or prior to, any imaging procedure involving iodinated contrast agents in patients with an eGFR between 30 and 60 mL/min/1.73 m2; in patients with a history of hepatic impairment, alcoholism, or heart failure; or in patients who will receive intra-arterial iodinated contrast. The eGFR should be reevaluated 48 hours after the imaging procedure.8
Additionally, if the iodinated contrast agent causes acute kidney injury, metformin could accumulate, with resultant lactate accumulation.
The American College of Radiology (ACR) has proposed less stringent guidelines for metformin during radiocontrast imaging studies. This change is based on evidence that lactic acidosis is rare—about 10 cases per 100,000 patient-years—and that there are no reports of lactic acidosis after intravenously administered iodinated contrast in properly selected patients.12,13
The ACR divides patients taking metformin into 2 categories:
- No evidence of acute kidney injury and eGFR greater than 30 mL/min/1.73 m2
- Either acute kidney injury or chronic kidney disease with eGFR below 30 mL/min/1.73 m2 or undergoing arterial catheter studies with a high chance of embolization to the renal arteries.14
For the first group, they recommend against discontinuing metformin before or after giving iodinated contrast or checking kidney function after the procedure.
For the second group, they recommend holding metformin before and 48 hours after the procedure. It should not be restarted until renal function is confirmed to be normal.
METFORMIN AND INSULIN
The ADA recommends1 continuing metformin after initiating insulin. However, in clinical practice, it is often not done.
Clinical trials have shown that combining metformin with insulin significantly improves glycemic control, prevents weight gain, and decreases insulin requirements.15,16 One trial16 also looked at cardiovascular end points during a 4-year follow-up period; combining metformin with insulin decreased the macrovascular disease-related event rate compared with insulin alone.
In the HOME trial,6 which added metformin to the existing insulin regimen, both groups gained weight, but the metformin group had gained about 3 kg less than the placebo group at the end of the 4.3-year trial. Metformin did not increase the risk of hypoglycemia, but it also did not reduce the risk of microvascular disease.
Concomitant metformin reduces costs
These days, practitioners can choose from a large selection of diabetes drugs. These include insulins with better pharmacokinetic profiles, as well as newer classes of noninsulin agents such as sodium-glucose cotransporter-2 inhibitors and glucagon-like peptide-1 analogues.
Metformin is less expensive than these newer drugs, and using it concomitantly with other diabetes drugs can decrease their dosage requirements, which in turn decreases their monthly costs.
GASTROINTESTINAL EFFECTS
Metformin’s gastrointestinal adverse effects such as diarrhea, flatulence, nausea, and vomiting are a barrier to its use. The actual incidence rate of diarrhea varies widely in randomized trials and observational studies, and gastrointestinal effects are worse in metformin-naive patients, as well as those who have chronic gastritis or Helicobacter pylori infection.17
We have found that starting metformin at a low dose and up-titrating it over several weeks increases tolerability. We often start patients at 500 mg/day and increase the dosage by 1 500-mg tablet every 1 to 2 weeks. Also, we have noticed that intolerance is more likely in patients who eat a high-carbohydrate diet, but there is no high-level evidence to back this up because patients in clinical trials all undergo nutrition counseling and are therefore more likely to adhere to the low-carbohydrate diet.
Also, the extended-release formulation is more tolerable than the immediate-release formulation and has similar glycemic efficacy. It may be an option as first-line therapy or for patients who have significant adverse effects from immediate-release metformin.18 For patients on the immediate-release formulation, taking it with meals helps lessen some gastrointestinal effects, and this should be emphasized at every visit.
Finally, we limit the metformin dose to 2,000 mg/day, rather than the 2,550 mg/day allowed on labeling. Garber et al19 found that the lower dosage still provides the maximum clinical efficacy.
OTHER CAUTIONS
Metformin should be avoided in patients with acute or unstable heart failure because of the increased risk of lactic acidosis.
It also should be avoided in patients with hepatic impairment, according to the labeling. But this remains controversial in practice. Zhang et al20 showed that continuing metformin in patients with diabetes and cirrhosis decreases the mortality risk by 57% compared with those taken off metformin.
Diet and lifestyle measures need to be emphasized at each visit. Wing et al21 showed that calorie restriction regardless of weight loss is beneficial for glycemic control and insulin sensitivity in obese patients with diabetes.
TAKE-HOME POINTS
Metformin improves glycemic control without tending to cause weight gain or hypoglycemia. It may also have cardiovascular benefits. Metformin is an inexpensive agent that should be continued, if tolerated, in those who need additional agents for glycemic control. It should be considered in all adult patients with type 2 diabetes.
Most patients should receive it, with exceptions as noted below. Metformin is the cornerstone of diabetes therapy and should be considered in all patients with type 2 diabetes. Both the American Diabetes Association (ADA) and the American Association of Clinical Endocrinologists (AACE)1,2 recommend it as first-line treatment for type 2 diabetes. It lowers blood glucose levels by inhibiting hepatic glucose production, and it does not tend to cause hypoglycemia.
However, metformin is underused. A 2012 study showed that only 50% to 70% of patients with type 2 diabetes treated with a sulfonylurea, dipeptidyl peptidase-4 (DPP-4) inhibitor, thiazolidinedione, or glucagon-like peptide-1 analogue also received metformin.3 This occurred despite guidelines recommending continuing metformin when starting other diabetes drugs.4
EVIDENCE METFORMIN IS EFFECTIVE
The United Kingdom Prospective Diabetes Study (UKPDS)5 found that metformin significantly reduced the incidence of:
- Any diabetes-related end point (hazard ratio [HR] 0.68, 95% confidence interval [CI] 0.53–0.87)
- Myocardial infarction (HR 0.61, 95% CI 0.41–0.89)
- Diabetes-related death (HR 0.58, 95% CI 0.37–0.91)
- All-cause mortality (HR 0.64; 95% CI 0.45–0.91).
The Hyperinsulinemia: Outcomes of Its Metabolic Effects (HOME) trial,6 a multicenter trial conducted in the Netherlands, evaluated the effect of adding metformin (vs placebo) to existing insulin regimens. Metformin recipients had a significantly lower rate of macrovascular mortality (HR 0.61, 95% CI 0.40–0.94, P = .02), but not of the primary end point, an aggregate of microvascular and macrovascular morbidity and mortality.
The Study on the Prognosis and Effect of Antidiabetic Drugs on Type 2 Diabetes Mellitus With Coronary Artery Disease trial,7 a multicenter trial conducted in China, compared the effects of metformin vs glipizide on cardiovascular outcomes. At about 3 years of treatment, the metformin group had a significantly lower rate of the composite primary end point of recurrent cardiovascular events (HR 0.54, 95% CI 0.30–0.90). This end point included nonfatal myocardial infarction, nonfatal stroke, arterial revascularization by percutaneous transluminal coronary angioplasty or by coronary artery bypass graft, death from a cardiovascular cause, and death from any cause.
These studies prompted the ADA to emphasize that metformin can reduce the risk of cardiovascular events or death. Metformin also has been shown to be weight-neutral or to induce slight weight loss. Furthermore, it is inexpensive.
WHAT ABOUT THE RENAL EFFECTS?
Because metformin is renally cleared, it has caused some concern about nephrotoxicity, especially lactic acidosis, in patients with impaired renal function. But the most recent guidelines have relaxed the criteria for metformin use in this patient population.
Revised labeling
Metformin’s labeling,8 revised in 2016, states the following:
- If the estimated glomerular filtration rate (eGFR) is below 30 mL/min/1.73 m2, metformin is contraindicated
- If the eGFR is between 30 and 45 mL/min/1.73 m2, metformin is not recommended
- If the eGFR is below 45 mL/min/1.73 m2 in a patient taking metformin, the risks and benefits of continuing treatment should be assessed, the dosage may need to be adjusted, and renal function should be monitored more frequently.8
These labeling revisions were based on a systematic review by Inzucchi et al9 that found metformin is not associated with increased rates of lactic acidosis in patients with mild to moderate kidney disease. Subsequently, an observational study published in 2018 by Lazarus et al10 showed that metformin increases the risk of acidosis only at eGFR levels below 30 mL/min/1.73 m2. Also, a Cochrane review published in 2003 did not find a single case of lactic acidosis in 347 trials with 70,490 patient-years of metformin treatment.11
Previous guidelines used serum creatinine levels, with metformin contraindicated at levels of 1.5 mg/dL or above for men and 1.4 mg/dL for women, or with abnormal creatinine clearance. The ADA and the AACE now use the eGFR1,2 instead of the serum creatinine level to measure kidney function because it better accounts for factors such as the patient’s age, sex, race, and weight.
Despite the evidence, the common patient perception is that metformin is nephrotoxic, and it is important for practitioners to dispel this myth during clinic visits.
What about metformin use with contrast agents?
Labeling has a precautionary note stating that metformin should be held at the time of, or prior to, any imaging procedure involving iodinated contrast agents in patients with an eGFR between 30 and 60 mL/min/1.73 m2; in patients with a history of hepatic impairment, alcoholism, or heart failure; or in patients who will receive intra-arterial iodinated contrast. The eGFR should be reevaluated 48 hours after the imaging procedure.8
Additionally, if the iodinated contrast agent causes acute kidney injury, metformin could accumulate, with resultant lactate accumulation.
The American College of Radiology (ACR) has proposed less stringent guidelines for metformin during radiocontrast imaging studies. This change is based on evidence that lactic acidosis is rare—about 10 cases per 100,000 patient-years—and that there are no reports of lactic acidosis after intravenously administered iodinated contrast in properly selected patients.12,13
The ACR divides patients taking metformin into 2 categories:
- No evidence of acute kidney injury and eGFR greater than 30 mL/min/1.73 m2
- Either acute kidney injury or chronic kidney disease with eGFR below 30 mL/min/1.73 m2 or undergoing arterial catheter studies with a high chance of embolization to the renal arteries.14
For the first group, they recommend against discontinuing metformin before or after giving iodinated contrast or checking kidney function after the procedure.
For the second group, they recommend holding metformin before and 48 hours after the procedure. It should not be restarted until renal function is confirmed to be normal.
METFORMIN AND INSULIN
The ADA recommends1 continuing metformin after initiating insulin. However, in clinical practice, it is often not done.
Clinical trials have shown that combining metformin with insulin significantly improves glycemic control, prevents weight gain, and decreases insulin requirements.15,16 One trial16 also looked at cardiovascular end points during a 4-year follow-up period; combining metformin with insulin decreased the macrovascular disease-related event rate compared with insulin alone.
In the HOME trial,6 which added metformin to the existing insulin regimen, both groups gained weight, but the metformin group had gained about 3 kg less than the placebo group at the end of the 4.3-year trial. Metformin did not increase the risk of hypoglycemia, but it also did not reduce the risk of microvascular disease.
Concomitant metformin reduces costs
These days, practitioners can choose from a large selection of diabetes drugs. These include insulins with better pharmacokinetic profiles, as well as newer classes of noninsulin agents such as sodium-glucose cotransporter-2 inhibitors and glucagon-like peptide-1 analogues.
Metformin is less expensive than these newer drugs, and using it concomitantly with other diabetes drugs can decrease their dosage requirements, which in turn decreases their monthly costs.
GASTROINTESTINAL EFFECTS
Metformin’s gastrointestinal adverse effects such as diarrhea, flatulence, nausea, and vomiting are a barrier to its use. The actual incidence rate of diarrhea varies widely in randomized trials and observational studies, and gastrointestinal effects are worse in metformin-naive patients, as well as those who have chronic gastritis or Helicobacter pylori infection.17
We have found that starting metformin at a low dose and up-titrating it over several weeks increases tolerability. We often start patients at 500 mg/day and increase the dosage by 1 500-mg tablet every 1 to 2 weeks. Also, we have noticed that intolerance is more likely in patients who eat a high-carbohydrate diet, but there is no high-level evidence to back this up because patients in clinical trials all undergo nutrition counseling and are therefore more likely to adhere to the low-carbohydrate diet.
Also, the extended-release formulation is more tolerable than the immediate-release formulation and has similar glycemic efficacy. It may be an option as first-line therapy or for patients who have significant adverse effects from immediate-release metformin.18 For patients on the immediate-release formulation, taking it with meals helps lessen some gastrointestinal effects, and this should be emphasized at every visit.
Finally, we limit the metformin dose to 2,000 mg/day, rather than the 2,550 mg/day allowed on labeling. Garber et al19 found that the lower dosage still provides the maximum clinical efficacy.
OTHER CAUTIONS
Metformin should be avoided in patients with acute or unstable heart failure because of the increased risk of lactic acidosis.
It also should be avoided in patients with hepatic impairment, according to the labeling. But this remains controversial in practice. Zhang et al20 showed that continuing metformin in patients with diabetes and cirrhosis decreases the mortality risk by 57% compared with those taken off metformin.
Diet and lifestyle measures need to be emphasized at each visit. Wing et al21 showed that calorie restriction regardless of weight loss is beneficial for glycemic control and insulin sensitivity in obese patients with diabetes.
TAKE-HOME POINTS
Metformin improves glycemic control without tending to cause weight gain or hypoglycemia. It may also have cardiovascular benefits. Metformin is an inexpensive agent that should be continued, if tolerated, in those who need additional agents for glycemic control. It should be considered in all adult patients with type 2 diabetes.
- American Diabetes Association. 8. Pharmacologic approaches to glycemic treatment: standards of medical care in diabetes-2018. Diabetes Care 2018; 41(suppl 1):S73–S85. doi:10.2337/dc18-S008
- Garber AJ, Abrahamson MJ, Barzilay JI, et al. Consensus statement by the American Association of Clinical Endocrinologists and American College of Endocrinology on the comprehensive type 2 diabetes management algorithm—2018 executive summary. Endocr Pract 2018; 24(1):91–120. doi:10.4158/CS-2017-0153
- Hampp C, Borders-Hemphill V, Moeny DG, Wysowski DK. Use of antidiabetic drugs in the US, 2003–2012. Diabetes Care 2014; 37(5):1367–1374. doi:10.2337/dc13-2289
- Inzucchi SE, Bergenstal RM, Buse JB, et al; American Diabetes Association (ADA); European Association for the Study of Diabetes (EASD). Management of hyperglycemia in type 2 diabetes: a patient-centered approach: position statement of the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care 2012; 35(6):1364–1379. doi:10.2337/dc12-0413
- Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34). UK Prospective Diabetes Study (UKPDS) Group. Lancet 1998; 352(9131):854–865. pmid:9742977
- Kooy A, de Jager J, Lehert P, et al. Long-term effects of metformin on metabolism and microvascular and macrovascular disease in patients with type 2 diabetes mellitus. Arch Intern Med 2009; 169(6):616–625. doi:10.1001/archinternmed.2009.20
- Hong J, Zhang Y, Lai S, et al; SPREAD-DIMCAD Investigators. Effects of metformin versus glipizide on cardiovascular outcomes in patients with type 2 diabetes and coronary artery disease. Diabetes Care 2013; 36(5):1304–1311. doi:10.2337/dc12-0719
- Glucophage (metformin hydrochloride) and Glucophage XR (extended-release) [package insert]. Princeton, NJ: Bristol-Myers Squibb Company. www.accessdata.fda.gov/drugsatfda_docs/label/2018/020357s034,021202s018lbl.pdf. Accessed December 5, 2018.
- Inzucchi SE, Lipska KJ, Mayo H, Bailey CJ, McGuire DK. Metformin in patients with type 2 diabetes and kidney disease: a systematic review. JAMA 2014; 312(24):2668–2675. doi:10.1001/jama.2014.15298
- Lazarus B, Wu A, Shin JI, et al. Association of metformin use with risk of lactic acidosis across the range of kidney function: a community-based cohort study. JAMA Intern Med 2018; 178(7):903–910. doi:10.1001/jamainternmed.2018.0292
- Salpeter S, Greyber E, Pasternak G, Salpeter E. Risk of fatal and nonfatal lactic acidosis with metformin use in type 2 diabetes mellitus. Cochrane Database Syst Rev 2003; (2):CD002967. doi:10.1002/14651858.CD002967
- Eppenga WL, Lalmohamed A, Geerts AF, et al. Risk of lactic acidosis or elevated lactate concentrations in metformin users with renal impairment: a population-based cohort study. Diabetes Care 2014; 37(8):2218–2224. doi:10.2337/dc13-3023
- Richy FF, Sabidó-Espin M, Guedes S, Corvino FA, Gottwald-Hostalek U. Incidence of lactic acidosis in patients with type 2 diabetes with and without renal impairment treated with metformin: a retrospective cohort study. Diabetes Care 2014; 37(8):2291–2295. doi:10.2337/dc14-0464
- American College of Radiology (ACR). Manual on Contrast Media. Version 10.3. www.acr.org/Clinical-Resources/Contrast-Manual. Accessed December 5, 2018.
- Wulffele MG, Kooy A, Lehert P, et al. Combination of insulin and metformin in the treatment of type 2 diabetes. Diabetes Care 2002; 25(12):2133–2140. pmid:12453950
- Kooy A, de Jager J, Lehert P, et al. Long-term effects of metformin on metabolism and microvascular and macrovascular disease in patients with type 2 diabetes mellitus. Arch Intern Med 2009; 169(6):616–625. doi:10.1001/archinternmed.2009.20
- Bonnet F, Scheen A. Understanding and overcoming metformin gastrointestinal intolerance, Diabetes Obes Metab 2017; 19(4):473–481. doi:10.1111/dom.12854
- Jabbour S, Ziring B. Advantages of extended-release metformin in patients with type 2 diabetes mellitus. Postgrad Med 2011; 123(1):15–23. doi:10.3810/pgm.2011.01.2241
- Garber AJ, Duncan TG, Goodman AM, Mills DJ, Rohlf JL. Efficacy of metformin in type II diabetes: results of a double-blind, placebo-controlled, dose-response trial. Am J Med 1997; 103(6):491–497. pmid:9428832
- Zhang X, Harmsen WS, Mettler TA, et al. Continuation of metformin use after a diagnosis of cirrhosis significantly improves survival of patients with diabetes. Hepatology 2014; 60(6):2008–2016. doi:10.1002/hep.27199
- Wing RR, Blair EH, Bononi P, Marcus MD, Watanabe R, Bergman RN. Caloric restriction per se is a significant factor in improvements in glycemic control and insulin sensitivity during weight loss in obese NIDDM patients. Diabetes Care 1994; 17(1):30–36. pmid:8112186
- American Diabetes Association. 8. Pharmacologic approaches to glycemic treatment: standards of medical care in diabetes-2018. Diabetes Care 2018; 41(suppl 1):S73–S85. doi:10.2337/dc18-S008
- Garber AJ, Abrahamson MJ, Barzilay JI, et al. Consensus statement by the American Association of Clinical Endocrinologists and American College of Endocrinology on the comprehensive type 2 diabetes management algorithm—2018 executive summary. Endocr Pract 2018; 24(1):91–120. doi:10.4158/CS-2017-0153
- Hampp C, Borders-Hemphill V, Moeny DG, Wysowski DK. Use of antidiabetic drugs in the US, 2003–2012. Diabetes Care 2014; 37(5):1367–1374. doi:10.2337/dc13-2289
- Inzucchi SE, Bergenstal RM, Buse JB, et al; American Diabetes Association (ADA); European Association for the Study of Diabetes (EASD). Management of hyperglycemia in type 2 diabetes: a patient-centered approach: position statement of the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care 2012; 35(6):1364–1379. doi:10.2337/dc12-0413
- Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34). UK Prospective Diabetes Study (UKPDS) Group. Lancet 1998; 352(9131):854–865. pmid:9742977
- Kooy A, de Jager J, Lehert P, et al. Long-term effects of metformin on metabolism and microvascular and macrovascular disease in patients with type 2 diabetes mellitus. Arch Intern Med 2009; 169(6):616–625. doi:10.1001/archinternmed.2009.20
- Hong J, Zhang Y, Lai S, et al; SPREAD-DIMCAD Investigators. Effects of metformin versus glipizide on cardiovascular outcomes in patients with type 2 diabetes and coronary artery disease. Diabetes Care 2013; 36(5):1304–1311. doi:10.2337/dc12-0719
- Glucophage (metformin hydrochloride) and Glucophage XR (extended-release) [package insert]. Princeton, NJ: Bristol-Myers Squibb Company. www.accessdata.fda.gov/drugsatfda_docs/label/2018/020357s034,021202s018lbl.pdf. Accessed December 5, 2018.
- Inzucchi SE, Lipska KJ, Mayo H, Bailey CJ, McGuire DK. Metformin in patients with type 2 diabetes and kidney disease: a systematic review. JAMA 2014; 312(24):2668–2675. doi:10.1001/jama.2014.15298
- Lazarus B, Wu A, Shin JI, et al. Association of metformin use with risk of lactic acidosis across the range of kidney function: a community-based cohort study. JAMA Intern Med 2018; 178(7):903–910. doi:10.1001/jamainternmed.2018.0292
- Salpeter S, Greyber E, Pasternak G, Salpeter E. Risk of fatal and nonfatal lactic acidosis with metformin use in type 2 diabetes mellitus. Cochrane Database Syst Rev 2003; (2):CD002967. doi:10.1002/14651858.CD002967
- Eppenga WL, Lalmohamed A, Geerts AF, et al. Risk of lactic acidosis or elevated lactate concentrations in metformin users with renal impairment: a population-based cohort study. Diabetes Care 2014; 37(8):2218–2224. doi:10.2337/dc13-3023
- Richy FF, Sabidó-Espin M, Guedes S, Corvino FA, Gottwald-Hostalek U. Incidence of lactic acidosis in patients with type 2 diabetes with and without renal impairment treated with metformin: a retrospective cohort study. Diabetes Care 2014; 37(8):2291–2295. doi:10.2337/dc14-0464
- American College of Radiology (ACR). Manual on Contrast Media. Version 10.3. www.acr.org/Clinical-Resources/Contrast-Manual. Accessed December 5, 2018.
- Wulffele MG, Kooy A, Lehert P, et al. Combination of insulin and metformin in the treatment of type 2 diabetes. Diabetes Care 2002; 25(12):2133–2140. pmid:12453950
- Kooy A, de Jager J, Lehert P, et al. Long-term effects of metformin on metabolism and microvascular and macrovascular disease in patients with type 2 diabetes mellitus. Arch Intern Med 2009; 169(6):616–625. doi:10.1001/archinternmed.2009.20
- Bonnet F, Scheen A. Understanding and overcoming metformin gastrointestinal intolerance, Diabetes Obes Metab 2017; 19(4):473–481. doi:10.1111/dom.12854
- Jabbour S, Ziring B. Advantages of extended-release metformin in patients with type 2 diabetes mellitus. Postgrad Med 2011; 123(1):15–23. doi:10.3810/pgm.2011.01.2241
- Garber AJ, Duncan TG, Goodman AM, Mills DJ, Rohlf JL. Efficacy of metformin in type II diabetes: results of a double-blind, placebo-controlled, dose-response trial. Am J Med 1997; 103(6):491–497. pmid:9428832
- Zhang X, Harmsen WS, Mettler TA, et al. Continuation of metformin use after a diagnosis of cirrhosis significantly improves survival of patients with diabetes. Hepatology 2014; 60(6):2008–2016. doi:10.1002/hep.27199
- Wing RR, Blair EH, Bononi P, Marcus MD, Watanabe R, Bergman RN. Caloric restriction per se is a significant factor in improvements in glycemic control and insulin sensitivity during weight loss in obese NIDDM patients. Diabetes Care 1994; 17(1):30–36. pmid:8112186
What can I do when first-line measures are not enough for vasovagal syncope?
Vasovagal syncope is usually benign, and although it often recurs, increasing fluid and salt intake and performing counter-pressure maneuvers are usually sufficient.1 However, if patients continue to have syncopal episodes despite these first-line measures, other options include drug therapy with midodrine, fludrocortisone, beta-blockers, or selective serotonin reuptake inhibitors; orthostatic training; and, in some cases, pacemaker implantation. The 2017 guidelines from the American College of Cardiology, American Heart Association, and Heart Rhythm Society (ACC/AHA/HRS) are helpful in the management of these patients.1
RATIONALE
Although vasovagal syncope is considered benign, it can result in injury and can significantly affect quality of life.
The diagnosis can often be established in the initial evaluation with a structured history, physical examination, and electrocardiography. If the diagnosis is still unclear, tilt-table testing can be useful and has an ACC/AHA/HRS class IIa (moderate) recommendation.1 Once the diagnosis of vasovagal syncope is made, first-line measures can be instituted.
FIRST-LINE MEASURES
An explanation of the diagnosis, education on avoiding triggers such as prolonged standing and warm environments, coping with potentially stressful visits to the doctor or dentist, and reassurance that the condition is benign are all strongly recommended (class I).1
Initial measures include performing physical counter-pressure maneuvers (class IIa), increasing salt and fluid intake (class IIb) in the absence of contraindications, and, in selected patients, reducing or withdrawing hypotensive medications when appropriate (class IIb).
Physical counter-pressure maneuvers are recommended for patients whose syncopal episodes have a sufficiently long prodromal period. Maneuvers include the following:
- Leg crossing: crossing the legs while tensing leg, abdominal, and buttock muscles
- Handgrip: maximally contracting a rubber ball or other object in the dominant hand
- Squatting
- Limb or abdominal contractions
- Arm tensing: contracting both arms by gripping one hand with the other and abducting both arms.2
The effectiveness of counter-pressure maneuvers was studied by van Dijk et al2 in a multicenter prospective randomized clinical trial that included 223 patients with recurrent vasovagal syncope associated with prodromal symptoms. They concluded that these maneuvers decreased the recurrence of syncopal episodes, with a relative risk reduction of 0.36 (95% confidence interval 0.11–0.53, P < .005) and were low-cost and risk-free.
Confirming the diagnosis of vasovagal syncope with tilt-table testing may reassure the patient. It can also help the patient learn to identify the symptoms associated with a vasovagal episode, which in turn may encourage timely use of physical counter-pressure maneuvers at the onset.
The evidence for increasing salt and fluid intake for patients with vasovagal syncope is limited. But in the absence of a contraindication such as hypertension, renal disease, or heart failure, it may be reasonable to encourage the ingestion of 2 L to 3 L of fluid per day and a total of 6 g to 9 g of salt per day (around 1 to 2 heaping teaspoons of salt).1
MEDICAL THERAPY
In patients who continue to have syncopal episodes despite adequate use of first-line measures, medical therapy can be considered. Unfortunately, evidence supporting drug therapy for recurrent syncope is limited.3 Options include midodrine (class IIa), fludrocortisone (class IIb), beta-blockers (class IIb), and selective serotonin reuptake inhibitors (class IIb).1
Midodrine has the strongest recommendation and is a reasonable option if there is no history of hypertension, heart failure, or urinary retention. It is a peripheral alpha-agonist that ameliorates the reduction in peripheral sympathetic neural outflow responsible for venous pooling and vasodepression in vasovagal syncope.4–6
Fludrocortisone results in increased blood volume due to mineralocorticoid activity. In the Prevention of Syncope Trial 2 of fludrocortisone vs placebo, patients on fludrocortisone had a “marginally nonsignificant” reduction in recurrence of syncope over 1 year (hazard ratio 0.69, P = .069).7
Overall, beta-blockers have failed to prevent syncope in randomized controlled trials. But in a meta-analysis that included patients from the Prevention of Syncope Trial,8 an age-dependent benefit of beta-blockers was noted in patients age 42 and older.9 Therefore, a beta-blocker may be a reasonable option in patients in this age group with recurrent vasovagal syncope.1
Serotonin has central effects on blood pressure and heart rate that can induce syncope. However, evidence for the effectiveness of selective serotonin reuptake inhibitors in the prevention of recurrent vasovagal syncope has been contradictory in small trials.10,11
When choosing a drug, contraindications should be considered, including possible effects during pregnancy in women of childbearing age.
OTHER MEASURES
Orthostatic training, with repetitive tilt-table testing until a test is negative, or with daily standing quietly against a wall for prolonged periods of time, has not been shown to have sustained benefit in reducing the recurrence of syncopal episodes (class IIb recommendation).1
Dual-chamber pacing can be considered in carefully selected patients age 40 or older with syncope and documented asystole of at least 3 seconds or spontaneous pauses of at least 6 seconds without syncope on implantable loop recorder monitoring (class IIb recommendation).1,12,13 Strict patient selection increases the likelihood that pacing will be effective.1 For example, patients with documented asystole during syncope and a tilt-table test that induces minimal or no vasodepressor response are more likely to respond than patients with a positive tilt-table test with a vasodepressor (hypotensive) response.13
Tilt-table testing may also be considered to identify patients with a hypotensive response who would be less likely to respond to permanent cardiac pacing.14
Compression garments carry a class IIa recommendation for orthostatic hypotension,1 but they have not been adequately studied in vasovagal syncope.
- Shen WK, Sheldon RS, Benditt DG, et al. 2017 ACC/AHA/HRS guideline for the evaluation and management of patients with syncope: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. Circulation 2017; 136(5):e60–e122. doi:10.1161/CIR.0000000000000499
- van Dijk N, Quartieri F, Blanc JJ, Garcia-Civera R, Brignole M, Moya A, Wieling W; PC-Trial Investigators. Effectiveness of physical counterpressure maneuvers in preventing vasovagal syncope: the Physical Counterpressure Manoeuvres Trial (PC-Trial). J Am Coll Cardiol 2006; 48(8):1652–1657. doi:10.1016/j.jacc.2006.06.059
- Romme JJ, Reitsma JB, Black CN, et al. Drugs and pacemakers for vasovagal, carotid sinus and situational syncope. Cochrane Database Syst Rev 2011; (10):CD004194. doi:10.1002/14651858.CD004194.pub3
- Perez-Lugones A, Schweikert R, Pavia S, et al. Usefulness of midodrine in patients with severely symptomatic neurocardiogenic syncope: a randomized control study. J Cardiovasc Electrophysiol 2001; 12(8):935–938. pmid:11513446
- Romme JJ, van Dijk N, Go-Schön IK, Reitsma JB, Wieling W. Effectiveness of midodrine treatment in patients with recurrent vasovagal syncope not responding to non-pharmacological treatment (STAND-trial). Europace 2011; 13(11):1639–1647. doi:10.1093/europace/eur200
- Samniah N, Sakaguchi S, Lurie KG, Iskos D, Benditt DG. Efficacy and safety of midodrine hydrochloride in patients with refractory vasovagal syncope. Am J Cardiol 2001; 88(1):A7, 80–83. pmid:11423066
- Sheldon R, Raj SR, Rose MS, et al; POST 2 Investigators. Fludrocortisone for the prevention of vasovagal syncope: a randomized, placebo-controlled trial. J Am Coll Cardiol 2016; 68(1):1–9. doi:10.1016/j.jacc.2016.04.030
- Sheldon R, Connolly S, Rose S, et al; POST Investigators. Prevention of Syncope Trial (POST): a randomized, placebo-controlled study of metoprolol in the prevention of vasovagal syncope. Circulation 2006; 113(9):1164–1170. doi:10.1161/CIRCULATIONAHA.105.535161
- Sheldon RS, Morillo CA, Klingenheben T, Krahn AD, Sheldon A, Rose MS. Age-dependent effect of beta-blockers in preventing vasovagal syncope. Circ Arrhythm Electrophysiol 2012; 5(5):920–926. doi:10.1161/CIRCEP.112.974386
- Takata TS, Wasmund SL, Smith ML, et al. Serotonin reuptake inhibitor (Paxil) does not prevent the vasovagal reaction associated with carotid sinus massage and/or lower body negative pressure in healthy volunteers. Circulation 2002; 106(12):1500–1504. pmid:12234955
- Di Girolamo E, Di Iorio C, Sabatini P, Leonzio L, Barbone C, Barsotti A. Effects of paroxetine hydrochloride, a selective serotonin reuptake inhibitor, on refractory vasovagal syncope: a randomized, double-blind, placebo-controlled study. J Am Coll Cardiol 1999; 33(5):1227–1230. pmid:10193720
- Brignole M, Menozzi C, Moya A, et al; International Study on Syncope of Uncertain Etiology 3 (ISSUE-3) Investigators. Pacemaker therapy in patients with neurally mediated syncope and documented asystole: third International Study on Syncope of Uncertain Etiology (ISSUE-3): a randomized trial. Circulation 2012; 125(21):2566–2571. doi:10.1161/CIRCULATIONAHA.111.082313
- Brignole M, Donateo P, Tomaino M, et al; International Study on Syncope of Uncertain Etiology 3 (ISSUE-3) Investigators. Benefit of pacemaker therapy in patients with presumed neurally mediated syncope and documented asystole is greater when tilt test is negative: an analysis from the third International Study on Syncope of Uncertain Etiology (ISSUE-3). Circ Arrhythm Electrophysiol 2014; 7(1):10–16. doi:10.1161/CIRCEP.113.001103
- Sheldon RS, Grubb BP, Olshansky B, et al. 2015 Heart Rhythm Society expert consensus statement on the diagnosis and treatment of postural tachycardia syndrome, inappropriate sinus tachycardia, and vasovagal syncope. Heart Rhythm 2015; 12(6):e41–e63. doi:10.1016/j.hrthm.2015.03.029
Vasovagal syncope is usually benign, and although it often recurs, increasing fluid and salt intake and performing counter-pressure maneuvers are usually sufficient.1 However, if patients continue to have syncopal episodes despite these first-line measures, other options include drug therapy with midodrine, fludrocortisone, beta-blockers, or selective serotonin reuptake inhibitors; orthostatic training; and, in some cases, pacemaker implantation. The 2017 guidelines from the American College of Cardiology, American Heart Association, and Heart Rhythm Society (ACC/AHA/HRS) are helpful in the management of these patients.1
RATIONALE
Although vasovagal syncope is considered benign, it can result in injury and can significantly affect quality of life.
The diagnosis can often be established in the initial evaluation with a structured history, physical examination, and electrocardiography. If the diagnosis is still unclear, tilt-table testing can be useful and has an ACC/AHA/HRS class IIa (moderate) recommendation.1 Once the diagnosis of vasovagal syncope is made, first-line measures can be instituted.
FIRST-LINE MEASURES
An explanation of the diagnosis, education on avoiding triggers such as prolonged standing and warm environments, coping with potentially stressful visits to the doctor or dentist, and reassurance that the condition is benign are all strongly recommended (class I).1
Initial measures include performing physical counter-pressure maneuvers (class IIa), increasing salt and fluid intake (class IIb) in the absence of contraindications, and, in selected patients, reducing or withdrawing hypotensive medications when appropriate (class IIb).
Physical counter-pressure maneuvers are recommended for patients whose syncopal episodes have a sufficiently long prodromal period. Maneuvers include the following:
- Leg crossing: crossing the legs while tensing leg, abdominal, and buttock muscles
- Handgrip: maximally contracting a rubber ball or other object in the dominant hand
- Squatting
- Limb or abdominal contractions
- Arm tensing: contracting both arms by gripping one hand with the other and abducting both arms.2
The effectiveness of counter-pressure maneuvers was studied by van Dijk et al2 in a multicenter prospective randomized clinical trial that included 223 patients with recurrent vasovagal syncope associated with prodromal symptoms. They concluded that these maneuvers decreased the recurrence of syncopal episodes, with a relative risk reduction of 0.36 (95% confidence interval 0.11–0.53, P < .005) and were low-cost and risk-free.
Confirming the diagnosis of vasovagal syncope with tilt-table testing may reassure the patient. It can also help the patient learn to identify the symptoms associated with a vasovagal episode, which in turn may encourage timely use of physical counter-pressure maneuvers at the onset.
The evidence for increasing salt and fluid intake for patients with vasovagal syncope is limited. But in the absence of a contraindication such as hypertension, renal disease, or heart failure, it may be reasonable to encourage the ingestion of 2 L to 3 L of fluid per day and a total of 6 g to 9 g of salt per day (around 1 to 2 heaping teaspoons of salt).1
MEDICAL THERAPY
In patients who continue to have syncopal episodes despite adequate use of first-line measures, medical therapy can be considered. Unfortunately, evidence supporting drug therapy for recurrent syncope is limited.3 Options include midodrine (class IIa), fludrocortisone (class IIb), beta-blockers (class IIb), and selective serotonin reuptake inhibitors (class IIb).1
Midodrine has the strongest recommendation and is a reasonable option if there is no history of hypertension, heart failure, or urinary retention. It is a peripheral alpha-agonist that ameliorates the reduction in peripheral sympathetic neural outflow responsible for venous pooling and vasodepression in vasovagal syncope.4–6
Fludrocortisone results in increased blood volume due to mineralocorticoid activity. In the Prevention of Syncope Trial 2 of fludrocortisone vs placebo, patients on fludrocortisone had a “marginally nonsignificant” reduction in recurrence of syncope over 1 year (hazard ratio 0.69, P = .069).7
Overall, beta-blockers have failed to prevent syncope in randomized controlled trials. But in a meta-analysis that included patients from the Prevention of Syncope Trial,8 an age-dependent benefit of beta-blockers was noted in patients age 42 and older.9 Therefore, a beta-blocker may be a reasonable option in patients in this age group with recurrent vasovagal syncope.1
Serotonin has central effects on blood pressure and heart rate that can induce syncope. However, evidence for the effectiveness of selective serotonin reuptake inhibitors in the prevention of recurrent vasovagal syncope has been contradictory in small trials.10,11
When choosing a drug, contraindications should be considered, including possible effects during pregnancy in women of childbearing age.
OTHER MEASURES
Orthostatic training, with repetitive tilt-table testing until a test is negative, or with daily standing quietly against a wall for prolonged periods of time, has not been shown to have sustained benefit in reducing the recurrence of syncopal episodes (class IIb recommendation).1
Dual-chamber pacing can be considered in carefully selected patients age 40 or older with syncope and documented asystole of at least 3 seconds or spontaneous pauses of at least 6 seconds without syncope on implantable loop recorder monitoring (class IIb recommendation).1,12,13 Strict patient selection increases the likelihood that pacing will be effective.1 For example, patients with documented asystole during syncope and a tilt-table test that induces minimal or no vasodepressor response are more likely to respond than patients with a positive tilt-table test with a vasodepressor (hypotensive) response.13
Tilt-table testing may also be considered to identify patients with a hypotensive response who would be less likely to respond to permanent cardiac pacing.14
Compression garments carry a class IIa recommendation for orthostatic hypotension,1 but they have not been adequately studied in vasovagal syncope.
Vasovagal syncope is usually benign, and although it often recurs, increasing fluid and salt intake and performing counter-pressure maneuvers are usually sufficient.1 However, if patients continue to have syncopal episodes despite these first-line measures, other options include drug therapy with midodrine, fludrocortisone, beta-blockers, or selective serotonin reuptake inhibitors; orthostatic training; and, in some cases, pacemaker implantation. The 2017 guidelines from the American College of Cardiology, American Heart Association, and Heart Rhythm Society (ACC/AHA/HRS) are helpful in the management of these patients.1
RATIONALE
Although vasovagal syncope is considered benign, it can result in injury and can significantly affect quality of life.
The diagnosis can often be established in the initial evaluation with a structured history, physical examination, and electrocardiography. If the diagnosis is still unclear, tilt-table testing can be useful and has an ACC/AHA/HRS class IIa (moderate) recommendation.1 Once the diagnosis of vasovagal syncope is made, first-line measures can be instituted.
FIRST-LINE MEASURES
An explanation of the diagnosis, education on avoiding triggers such as prolonged standing and warm environments, coping with potentially stressful visits to the doctor or dentist, and reassurance that the condition is benign are all strongly recommended (class I).1
Initial measures include performing physical counter-pressure maneuvers (class IIa), increasing salt and fluid intake (class IIb) in the absence of contraindications, and, in selected patients, reducing or withdrawing hypotensive medications when appropriate (class IIb).
Physical counter-pressure maneuvers are recommended for patients whose syncopal episodes have a sufficiently long prodromal period. Maneuvers include the following:
- Leg crossing: crossing the legs while tensing leg, abdominal, and buttock muscles
- Handgrip: maximally contracting a rubber ball or other object in the dominant hand
- Squatting
- Limb or abdominal contractions
- Arm tensing: contracting both arms by gripping one hand with the other and abducting both arms.2
The effectiveness of counter-pressure maneuvers was studied by van Dijk et al2 in a multicenter prospective randomized clinical trial that included 223 patients with recurrent vasovagal syncope associated with prodromal symptoms. They concluded that these maneuvers decreased the recurrence of syncopal episodes, with a relative risk reduction of 0.36 (95% confidence interval 0.11–0.53, P < .005) and were low-cost and risk-free.
Confirming the diagnosis of vasovagal syncope with tilt-table testing may reassure the patient. It can also help the patient learn to identify the symptoms associated with a vasovagal episode, which in turn may encourage timely use of physical counter-pressure maneuvers at the onset.
The evidence for increasing salt and fluid intake for patients with vasovagal syncope is limited. But in the absence of a contraindication such as hypertension, renal disease, or heart failure, it may be reasonable to encourage the ingestion of 2 L to 3 L of fluid per day and a total of 6 g to 9 g of salt per day (around 1 to 2 heaping teaspoons of salt).1
MEDICAL THERAPY
In patients who continue to have syncopal episodes despite adequate use of first-line measures, medical therapy can be considered. Unfortunately, evidence supporting drug therapy for recurrent syncope is limited.3 Options include midodrine (class IIa), fludrocortisone (class IIb), beta-blockers (class IIb), and selective serotonin reuptake inhibitors (class IIb).1
Midodrine has the strongest recommendation and is a reasonable option if there is no history of hypertension, heart failure, or urinary retention. It is a peripheral alpha-agonist that ameliorates the reduction in peripheral sympathetic neural outflow responsible for venous pooling and vasodepression in vasovagal syncope.4–6
Fludrocortisone results in increased blood volume due to mineralocorticoid activity. In the Prevention of Syncope Trial 2 of fludrocortisone vs placebo, patients on fludrocortisone had a “marginally nonsignificant” reduction in recurrence of syncope over 1 year (hazard ratio 0.69, P = .069).7
Overall, beta-blockers have failed to prevent syncope in randomized controlled trials. But in a meta-analysis that included patients from the Prevention of Syncope Trial,8 an age-dependent benefit of beta-blockers was noted in patients age 42 and older.9 Therefore, a beta-blocker may be a reasonable option in patients in this age group with recurrent vasovagal syncope.1
Serotonin has central effects on blood pressure and heart rate that can induce syncope. However, evidence for the effectiveness of selective serotonin reuptake inhibitors in the prevention of recurrent vasovagal syncope has been contradictory in small trials.10,11
When choosing a drug, contraindications should be considered, including possible effects during pregnancy in women of childbearing age.
OTHER MEASURES
Orthostatic training, with repetitive tilt-table testing until a test is negative, or with daily standing quietly against a wall for prolonged periods of time, has not been shown to have sustained benefit in reducing the recurrence of syncopal episodes (class IIb recommendation).1
Dual-chamber pacing can be considered in carefully selected patients age 40 or older with syncope and documented asystole of at least 3 seconds or spontaneous pauses of at least 6 seconds without syncope on implantable loop recorder monitoring (class IIb recommendation).1,12,13 Strict patient selection increases the likelihood that pacing will be effective.1 For example, patients with documented asystole during syncope and a tilt-table test that induces minimal or no vasodepressor response are more likely to respond than patients with a positive tilt-table test with a vasodepressor (hypotensive) response.13
Tilt-table testing may also be considered to identify patients with a hypotensive response who would be less likely to respond to permanent cardiac pacing.14
Compression garments carry a class IIa recommendation for orthostatic hypotension,1 but they have not been adequately studied in vasovagal syncope.
- Shen WK, Sheldon RS, Benditt DG, et al. 2017 ACC/AHA/HRS guideline for the evaluation and management of patients with syncope: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. Circulation 2017; 136(5):e60–e122. doi:10.1161/CIR.0000000000000499
- van Dijk N, Quartieri F, Blanc JJ, Garcia-Civera R, Brignole M, Moya A, Wieling W; PC-Trial Investigators. Effectiveness of physical counterpressure maneuvers in preventing vasovagal syncope: the Physical Counterpressure Manoeuvres Trial (PC-Trial). J Am Coll Cardiol 2006; 48(8):1652–1657. doi:10.1016/j.jacc.2006.06.059
- Romme JJ, Reitsma JB, Black CN, et al. Drugs and pacemakers for vasovagal, carotid sinus and situational syncope. Cochrane Database Syst Rev 2011; (10):CD004194. doi:10.1002/14651858.CD004194.pub3
- Perez-Lugones A, Schweikert R, Pavia S, et al. Usefulness of midodrine in patients with severely symptomatic neurocardiogenic syncope: a randomized control study. J Cardiovasc Electrophysiol 2001; 12(8):935–938. pmid:11513446
- Romme JJ, van Dijk N, Go-Schön IK, Reitsma JB, Wieling W. Effectiveness of midodrine treatment in patients with recurrent vasovagal syncope not responding to non-pharmacological treatment (STAND-trial). Europace 2011; 13(11):1639–1647. doi:10.1093/europace/eur200
- Samniah N, Sakaguchi S, Lurie KG, Iskos D, Benditt DG. Efficacy and safety of midodrine hydrochloride in patients with refractory vasovagal syncope. Am J Cardiol 2001; 88(1):A7, 80–83. pmid:11423066
- Sheldon R, Raj SR, Rose MS, et al; POST 2 Investigators. Fludrocortisone for the prevention of vasovagal syncope: a randomized, placebo-controlled trial. J Am Coll Cardiol 2016; 68(1):1–9. doi:10.1016/j.jacc.2016.04.030
- Sheldon R, Connolly S, Rose S, et al; POST Investigators. Prevention of Syncope Trial (POST): a randomized, placebo-controlled study of metoprolol in the prevention of vasovagal syncope. Circulation 2006; 113(9):1164–1170. doi:10.1161/CIRCULATIONAHA.105.535161
- Sheldon RS, Morillo CA, Klingenheben T, Krahn AD, Sheldon A, Rose MS. Age-dependent effect of beta-blockers in preventing vasovagal syncope. Circ Arrhythm Electrophysiol 2012; 5(5):920–926. doi:10.1161/CIRCEP.112.974386
- Takata TS, Wasmund SL, Smith ML, et al. Serotonin reuptake inhibitor (Paxil) does not prevent the vasovagal reaction associated with carotid sinus massage and/or lower body negative pressure in healthy volunteers. Circulation 2002; 106(12):1500–1504. pmid:12234955
- Di Girolamo E, Di Iorio C, Sabatini P, Leonzio L, Barbone C, Barsotti A. Effects of paroxetine hydrochloride, a selective serotonin reuptake inhibitor, on refractory vasovagal syncope: a randomized, double-blind, placebo-controlled study. J Am Coll Cardiol 1999; 33(5):1227–1230. pmid:10193720
- Brignole M, Menozzi C, Moya A, et al; International Study on Syncope of Uncertain Etiology 3 (ISSUE-3) Investigators. Pacemaker therapy in patients with neurally mediated syncope and documented asystole: third International Study on Syncope of Uncertain Etiology (ISSUE-3): a randomized trial. Circulation 2012; 125(21):2566–2571. doi:10.1161/CIRCULATIONAHA.111.082313
- Brignole M, Donateo P, Tomaino M, et al; International Study on Syncope of Uncertain Etiology 3 (ISSUE-3) Investigators. Benefit of pacemaker therapy in patients with presumed neurally mediated syncope and documented asystole is greater when tilt test is negative: an analysis from the third International Study on Syncope of Uncertain Etiology (ISSUE-3). Circ Arrhythm Electrophysiol 2014; 7(1):10–16. doi:10.1161/CIRCEP.113.001103
- Sheldon RS, Grubb BP, Olshansky B, et al. 2015 Heart Rhythm Society expert consensus statement on the diagnosis and treatment of postural tachycardia syndrome, inappropriate sinus tachycardia, and vasovagal syncope. Heart Rhythm 2015; 12(6):e41–e63. doi:10.1016/j.hrthm.2015.03.029
- Shen WK, Sheldon RS, Benditt DG, et al. 2017 ACC/AHA/HRS guideline for the evaluation and management of patients with syncope: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. Circulation 2017; 136(5):e60–e122. doi:10.1161/CIR.0000000000000499
- van Dijk N, Quartieri F, Blanc JJ, Garcia-Civera R, Brignole M, Moya A, Wieling W; PC-Trial Investigators. Effectiveness of physical counterpressure maneuvers in preventing vasovagal syncope: the Physical Counterpressure Manoeuvres Trial (PC-Trial). J Am Coll Cardiol 2006; 48(8):1652–1657. doi:10.1016/j.jacc.2006.06.059
- Romme JJ, Reitsma JB, Black CN, et al. Drugs and pacemakers for vasovagal, carotid sinus and situational syncope. Cochrane Database Syst Rev 2011; (10):CD004194. doi:10.1002/14651858.CD004194.pub3
- Perez-Lugones A, Schweikert R, Pavia S, et al. Usefulness of midodrine in patients with severely symptomatic neurocardiogenic syncope: a randomized control study. J Cardiovasc Electrophysiol 2001; 12(8):935–938. pmid:11513446
- Romme JJ, van Dijk N, Go-Schön IK, Reitsma JB, Wieling W. Effectiveness of midodrine treatment in patients with recurrent vasovagal syncope not responding to non-pharmacological treatment (STAND-trial). Europace 2011; 13(11):1639–1647. doi:10.1093/europace/eur200
- Samniah N, Sakaguchi S, Lurie KG, Iskos D, Benditt DG. Efficacy and safety of midodrine hydrochloride in patients with refractory vasovagal syncope. Am J Cardiol 2001; 88(1):A7, 80–83. pmid:11423066
- Sheldon R, Raj SR, Rose MS, et al; POST 2 Investigators. Fludrocortisone for the prevention of vasovagal syncope: a randomized, placebo-controlled trial. J Am Coll Cardiol 2016; 68(1):1–9. doi:10.1016/j.jacc.2016.04.030
- Sheldon R, Connolly S, Rose S, et al; POST Investigators. Prevention of Syncope Trial (POST): a randomized, placebo-controlled study of metoprolol in the prevention of vasovagal syncope. Circulation 2006; 113(9):1164–1170. doi:10.1161/CIRCULATIONAHA.105.535161
- Sheldon RS, Morillo CA, Klingenheben T, Krahn AD, Sheldon A, Rose MS. Age-dependent effect of beta-blockers in preventing vasovagal syncope. Circ Arrhythm Electrophysiol 2012; 5(5):920–926. doi:10.1161/CIRCEP.112.974386
- Takata TS, Wasmund SL, Smith ML, et al. Serotonin reuptake inhibitor (Paxil) does not prevent the vasovagal reaction associated with carotid sinus massage and/or lower body negative pressure in healthy volunteers. Circulation 2002; 106(12):1500–1504. pmid:12234955
- Di Girolamo E, Di Iorio C, Sabatini P, Leonzio L, Barbone C, Barsotti A. Effects of paroxetine hydrochloride, a selective serotonin reuptake inhibitor, on refractory vasovagal syncope: a randomized, double-blind, placebo-controlled study. J Am Coll Cardiol 1999; 33(5):1227–1230. pmid:10193720
- Brignole M, Menozzi C, Moya A, et al; International Study on Syncope of Uncertain Etiology 3 (ISSUE-3) Investigators. Pacemaker therapy in patients with neurally mediated syncope and documented asystole: third International Study on Syncope of Uncertain Etiology (ISSUE-3): a randomized trial. Circulation 2012; 125(21):2566–2571. doi:10.1161/CIRCULATIONAHA.111.082313
- Brignole M, Donateo P, Tomaino M, et al; International Study on Syncope of Uncertain Etiology 3 (ISSUE-3) Investigators. Benefit of pacemaker therapy in patients with presumed neurally mediated syncope and documented asystole is greater when tilt test is negative: an analysis from the third International Study on Syncope of Uncertain Etiology (ISSUE-3). Circ Arrhythm Electrophysiol 2014; 7(1):10–16. doi:10.1161/CIRCEP.113.001103
- Sheldon RS, Grubb BP, Olshansky B, et al. 2015 Heart Rhythm Society expert consensus statement on the diagnosis and treatment of postural tachycardia syndrome, inappropriate sinus tachycardia, and vasovagal syncope. Heart Rhythm 2015; 12(6):e41–e63. doi:10.1016/j.hrthm.2015.03.029
Are anti-TNF drugs safe for pregnant women with inflammatory bowel disease?
Yes, anti-tumor necrosis factor (anti-TNF) therapy for inflammatory bowel disease (IBD) can be continued during pregnancy.
IBD is often diagnosed and treated in women during their reproductive years. Consequently, these patients face important decisions about the management of their disease and the safety of their baby. Clinicians should be prepared to offer guidance by discussing the risks and benefits of anti-TNF agents with their pregnant patients who have IBD, as well as with those considering pregnancy.
STUDIES OF THE POTENTIAL RISKS
Anti-TNF agents are monoclonal antibodies. Infliximab, adalimumab, and golimumab are actively transported into the fetal circulation via the placenta, mainly during the second and third trimesters. Certolizumab crosses the placenta only by passive means, because it lacks the fragment crystallizable (Fc) region required for placental transfer.1
Effects on pregnancy outcomes
In a 2016 meta-analysis,2 of 1,242 pregnancies in women with IBD, 482 were in women on anti-TNF therapy. It found no statistically significant difference in rates of adverse pregnancy outcomes including congenital abnormality, preterm birth, and low birth weight.
A meta-analysis of 1,216 pregnant women with IBD found no statistically significant differences in rates of spontaneous or elective abortion, preterm birth, low birth weight, or congenital malformation in those on anti-TNF therapy vs controls.3
A systematic review of 58 studies including more than 1,500 pregnant women with IBD who were exposed to anti-TNF agents concluded that there was no association with adverse pregnancy outcomes such as spontaneous abortion, preterm delivery, stillbirth, low birth weight, congenital malformation, or infection.4
A retrospective cohort study of 66 pregnant patients with IBD from several centers in Spain found that anti-TNF or thiopurine therapy during pregnancy did not increase the risk of pregnancy complications or neonatal complications.5
Effects on newborns
Cord blood studies have shown that maternal use of infliximab and adalimumab results in a detectable serum level in newborns, while cord blood levels of certolizumab are much lower.1,6 In some studies, anti-TNF drugs were detectable in infants for up to 6 months after birth, whereas other studies found that detectable serum levels dropped soon after birth.1,7
Addressing concern about an increased risk of infection or dysfunctional immune development in newborns exposed to anti-TNF drugs in utero, a systematic review found no increased risk.4 A retrospective multicenter cohort study of 841 children also reported no association between in utero exposure to anti-TNF agents and risk of severe infection in the short term or long term (mean of 4 years).8 Additional studies are under way to determine long-term risk to the newborn.7
THE TORONTO CONSENSUS GUIDELINES
The Toronto consensus guidelines strongly recommend continuing anti-TNF therapy during pregnancy in women with IBD who began maintenance therapy before conception.6
If a patient strongly prefers to stop therapy during pregnancy to limit fetal exposure, the Toronto consensus recommends giving the last dose at 22 to 24 weeks of gestation. However, this should only be considered in patients whose IBD is in remission and at low risk of relapse.6,9
Although anti-TNF drugs may differ in terms of placental transfer, agents should not be switched in stable patients, as switching increases the risk of relapse.10
BENEFITS OF CONTINUING THERAPY
Active IBD poses a significantly greater risk to the mother and the baby than continuing anti-TNF therapy during pregnancy.1,7 The primary benefit of continuing therapy is to maintain disease remission.
Among women with active IBD at the time of conception, one-third will have improvement in disease activity during the course of their pregnancy, one-third will have no change, and one-third will have worsening of disease activity. But if IBD is in remission at the time of conception, it will remain in remission in nearly 80% of women during pregnancy.1
Women with active IBD are at increased risk of preterm delivery, low birth weight, and intrauterine growth restriction.1,2,5 Also, women with IBD have an increased risk of venous thromboembolism, particularly if they have active disease during pregnancy.1 Therefore, achieving and maintaining remission are vital in the management of the pregnant patient with IBD.
CONSIDERATIONS AFTER BIRTH: BREAST-FEEDING AND VACCINATION
Breast-feeding is considered safe. Minuscule amounts of infliximab or adalimumab are transferred in breast milk but are unlikely to result in systemic immune suppression in the infant.7
Live-attenuated vaccines should be avoided for the first 6 months in infants exposed to anti-TNF agents in utero.1,7,11 All other vaccines, including hepatitis B virus vaccine, should be given according to standard schedules.6
OUR RECOMMENDATIONS
The goal of managing IBD in women of reproductive age is to minimize the risk of adverse outcomes for both mother and baby. We recommend a team approach, working closely with a gastroenterologist and a high-risk-pregnancy obstetrician, if available.
Patients should continue anti-TNF therapy during pregnancy because evidence supports its safety. If a woman wants to stop therapy and is at low risk of relapse, we recommend giving the last dose at 22 to 24 weeks of gestation, then promptly resuming therapy postpartum.
Live-attenuated vaccines (eg, influenza, rotavirus) should be avoided for the first 6 months in babies born to mothers on anti-TNF therapy.
- Ananthakrishnan AN, Xavier RJ, Podolsky DK. Inflammatory Bowel Diseases: A Clinician’s Guide. Chichester, UK: Wiley; 2017. doi:10.1002/9781119077633
- Shihab Z, Yeomans ND, De Cruz P. Anti-tumour necrosis factor alpha therapies and inflammatory bowel disease pregnancy outcomes: a meta-analysis. J Crohns Colitis 2016; 10(8):979–988. doi:10.1093/ecco-jcc/jjv234
- Narula N, Al-Dabbagh, Dhillon A, Sands BE, Marshall JK. Anti-TNF alpha therapies are safe during pregnancy in women with inflammatory bowel disease: a systematic review and meta-analysis. Inflamm Bowel Dis 2014; 20(10):1862–1869. doi:10.1097/MIB.0000000000000092
- Nielsen OH, Loftus EV Jr, Jess T. Safety of TNF-alpha inhibitors during IBD pregnancy: a systematic review. BMC Med 2013; 11:174. doi:10.1186/1741-7015-11-174
- Casanova MJ, Chaparro M, Domenech E, et al. Safety of thiopurines and anti-TNF-alpha drugs during pregnancy in patients with inflammatory bowel disease. Am J Gastroenterol 2013; 108(3):433–440. doi:10.1038/ajg.2012.430
- Nguyen GC, Seow CH, Maxwell C, et al; IBD in Pregnancy Consensus Group; Canadian Association of Gastroenterology. The Toronto consensus statements for the management of inflammatory bowel disease in pregnancy. Gastroenterology 2016; 150(3):734–757.e1. doi:10.1053/j.gastro.2015.12.003
- Gisbert JP, Chaparro, M. Safety of anti-TNF agents during pregnancy and breastfeeding in women with inflammatory bowel disease. Am J Gastroenterol 2013; 108(9):1426–1438. doi:10.1038/ajg.2013.171
- Chaparro M, Verreth A, Lobaton T, et al. Long-term safety of in utero exposure to anti-TNF alpha drugs for the treatment of inflammatory bowel disease: results from the multicenter European TEDDY Study. Am J Gastroenterol 2018; 113(3):396–403. doi:10.1038/ajg.2017.501
- de Lima A, Zelinkova Z, van der Ent C, Steegers EA, van der Woude CJ. Tailored anti-TNF therapy during pregnancy in patients with IBD: maternal and fetal safety. Gut 2016; 65(8):1261–1268. doi:10.1136/gutjnl-2015-309321
- Van Assche G, Vermeire S, Ballet V, et al. Switch to adalimumab in patients with Crohn’s disease controlled by maintenance infliximab: prospective randomised SWITCH trial. Gut 2012; 61(2):229–234. doi:10.1136/gutjnl-2011-300755
- Saha S. Medication management in the pregnant IBD patient. Am J Gastroenterol 2017; 112(5):667–669. doi:10.1038/ajg.2017.22
Yes, anti-tumor necrosis factor (anti-TNF) therapy for inflammatory bowel disease (IBD) can be continued during pregnancy.
IBD is often diagnosed and treated in women during their reproductive years. Consequently, these patients face important decisions about the management of their disease and the safety of their baby. Clinicians should be prepared to offer guidance by discussing the risks and benefits of anti-TNF agents with their pregnant patients who have IBD, as well as with those considering pregnancy.
STUDIES OF THE POTENTIAL RISKS
Anti-TNF agents are monoclonal antibodies. Infliximab, adalimumab, and golimumab are actively transported into the fetal circulation via the placenta, mainly during the second and third trimesters. Certolizumab crosses the placenta only by passive means, because it lacks the fragment crystallizable (Fc) region required for placental transfer.1
Effects on pregnancy outcomes
In a 2016 meta-analysis,2 of 1,242 pregnancies in women with IBD, 482 were in women on anti-TNF therapy. It found no statistically significant difference in rates of adverse pregnancy outcomes including congenital abnormality, preterm birth, and low birth weight.
A meta-analysis of 1,216 pregnant women with IBD found no statistically significant differences in rates of spontaneous or elective abortion, preterm birth, low birth weight, or congenital malformation in those on anti-TNF therapy vs controls.3
A systematic review of 58 studies including more than 1,500 pregnant women with IBD who were exposed to anti-TNF agents concluded that there was no association with adverse pregnancy outcomes such as spontaneous abortion, preterm delivery, stillbirth, low birth weight, congenital malformation, or infection.4
A retrospective cohort study of 66 pregnant patients with IBD from several centers in Spain found that anti-TNF or thiopurine therapy during pregnancy did not increase the risk of pregnancy complications or neonatal complications.5
Effects on newborns
Cord blood studies have shown that maternal use of infliximab and adalimumab results in a detectable serum level in newborns, while cord blood levels of certolizumab are much lower.1,6 In some studies, anti-TNF drugs were detectable in infants for up to 6 months after birth, whereas other studies found that detectable serum levels dropped soon after birth.1,7
Addressing concern about an increased risk of infection or dysfunctional immune development in newborns exposed to anti-TNF drugs in utero, a systematic review found no increased risk.4 A retrospective multicenter cohort study of 841 children also reported no association between in utero exposure to anti-TNF agents and risk of severe infection in the short term or long term (mean of 4 years).8 Additional studies are under way to determine long-term risk to the newborn.7
THE TORONTO CONSENSUS GUIDELINES
The Toronto consensus guidelines strongly recommend continuing anti-TNF therapy during pregnancy in women with IBD who began maintenance therapy before conception.6
If a patient strongly prefers to stop therapy during pregnancy to limit fetal exposure, the Toronto consensus recommends giving the last dose at 22 to 24 weeks of gestation. However, this should only be considered in patients whose IBD is in remission and at low risk of relapse.6,9
Although anti-TNF drugs may differ in terms of placental transfer, agents should not be switched in stable patients, as switching increases the risk of relapse.10
BENEFITS OF CONTINUING THERAPY
Active IBD poses a significantly greater risk to the mother and the baby than continuing anti-TNF therapy during pregnancy.1,7 The primary benefit of continuing therapy is to maintain disease remission.
Among women with active IBD at the time of conception, one-third will have improvement in disease activity during the course of their pregnancy, one-third will have no change, and one-third will have worsening of disease activity. But if IBD is in remission at the time of conception, it will remain in remission in nearly 80% of women during pregnancy.1
Women with active IBD are at increased risk of preterm delivery, low birth weight, and intrauterine growth restriction.1,2,5 Also, women with IBD have an increased risk of venous thromboembolism, particularly if they have active disease during pregnancy.1 Therefore, achieving and maintaining remission are vital in the management of the pregnant patient with IBD.
CONSIDERATIONS AFTER BIRTH: BREAST-FEEDING AND VACCINATION
Breast-feeding is considered safe. Minuscule amounts of infliximab or adalimumab are transferred in breast milk but are unlikely to result in systemic immune suppression in the infant.7
Live-attenuated vaccines should be avoided for the first 6 months in infants exposed to anti-TNF agents in utero.1,7,11 All other vaccines, including hepatitis B virus vaccine, should be given according to standard schedules.6
OUR RECOMMENDATIONS
The goal of managing IBD in women of reproductive age is to minimize the risk of adverse outcomes for both mother and baby. We recommend a team approach, working closely with a gastroenterologist and a high-risk-pregnancy obstetrician, if available.
Patients should continue anti-TNF therapy during pregnancy because evidence supports its safety. If a woman wants to stop therapy and is at low risk of relapse, we recommend giving the last dose at 22 to 24 weeks of gestation, then promptly resuming therapy postpartum.
Live-attenuated vaccines (eg, influenza, rotavirus) should be avoided for the first 6 months in babies born to mothers on anti-TNF therapy.
Yes, anti-tumor necrosis factor (anti-TNF) therapy for inflammatory bowel disease (IBD) can be continued during pregnancy.
IBD is often diagnosed and treated in women during their reproductive years. Consequently, these patients face important decisions about the management of their disease and the safety of their baby. Clinicians should be prepared to offer guidance by discussing the risks and benefits of anti-TNF agents with their pregnant patients who have IBD, as well as with those considering pregnancy.
STUDIES OF THE POTENTIAL RISKS
Anti-TNF agents are monoclonal antibodies. Infliximab, adalimumab, and golimumab are actively transported into the fetal circulation via the placenta, mainly during the second and third trimesters. Certolizumab crosses the placenta only by passive means, because it lacks the fragment crystallizable (Fc) region required for placental transfer.1
Effects on pregnancy outcomes
In a 2016 meta-analysis,2 of 1,242 pregnancies in women with IBD, 482 were in women on anti-TNF therapy. It found no statistically significant difference in rates of adverse pregnancy outcomes including congenital abnormality, preterm birth, and low birth weight.
A meta-analysis of 1,216 pregnant women with IBD found no statistically significant differences in rates of spontaneous or elective abortion, preterm birth, low birth weight, or congenital malformation in those on anti-TNF therapy vs controls.3
A systematic review of 58 studies including more than 1,500 pregnant women with IBD who were exposed to anti-TNF agents concluded that there was no association with adverse pregnancy outcomes such as spontaneous abortion, preterm delivery, stillbirth, low birth weight, congenital malformation, or infection.4
A retrospective cohort study of 66 pregnant patients with IBD from several centers in Spain found that anti-TNF or thiopurine therapy during pregnancy did not increase the risk of pregnancy complications or neonatal complications.5
Effects on newborns
Cord blood studies have shown that maternal use of infliximab and adalimumab results in a detectable serum level in newborns, while cord blood levels of certolizumab are much lower.1,6 In some studies, anti-TNF drugs were detectable in infants for up to 6 months after birth, whereas other studies found that detectable serum levels dropped soon after birth.1,7
Addressing concern about an increased risk of infection or dysfunctional immune development in newborns exposed to anti-TNF drugs in utero, a systematic review found no increased risk.4 A retrospective multicenter cohort study of 841 children also reported no association between in utero exposure to anti-TNF agents and risk of severe infection in the short term or long term (mean of 4 years).8 Additional studies are under way to determine long-term risk to the newborn.7
THE TORONTO CONSENSUS GUIDELINES
The Toronto consensus guidelines strongly recommend continuing anti-TNF therapy during pregnancy in women with IBD who began maintenance therapy before conception.6
If a patient strongly prefers to stop therapy during pregnancy to limit fetal exposure, the Toronto consensus recommends giving the last dose at 22 to 24 weeks of gestation. However, this should only be considered in patients whose IBD is in remission and at low risk of relapse.6,9
Although anti-TNF drugs may differ in terms of placental transfer, agents should not be switched in stable patients, as switching increases the risk of relapse.10
BENEFITS OF CONTINUING THERAPY
Active IBD poses a significantly greater risk to the mother and the baby than continuing anti-TNF therapy during pregnancy.1,7 The primary benefit of continuing therapy is to maintain disease remission.
Among women with active IBD at the time of conception, one-third will have improvement in disease activity during the course of their pregnancy, one-third will have no change, and one-third will have worsening of disease activity. But if IBD is in remission at the time of conception, it will remain in remission in nearly 80% of women during pregnancy.1
Women with active IBD are at increased risk of preterm delivery, low birth weight, and intrauterine growth restriction.1,2,5 Also, women with IBD have an increased risk of venous thromboembolism, particularly if they have active disease during pregnancy.1 Therefore, achieving and maintaining remission are vital in the management of the pregnant patient with IBD.
CONSIDERATIONS AFTER BIRTH: BREAST-FEEDING AND VACCINATION
Breast-feeding is considered safe. Minuscule amounts of infliximab or adalimumab are transferred in breast milk but are unlikely to result in systemic immune suppression in the infant.7
Live-attenuated vaccines should be avoided for the first 6 months in infants exposed to anti-TNF agents in utero.1,7,11 All other vaccines, including hepatitis B virus vaccine, should be given according to standard schedules.6
OUR RECOMMENDATIONS
The goal of managing IBD in women of reproductive age is to minimize the risk of adverse outcomes for both mother and baby. We recommend a team approach, working closely with a gastroenterologist and a high-risk-pregnancy obstetrician, if available.
Patients should continue anti-TNF therapy during pregnancy because evidence supports its safety. If a woman wants to stop therapy and is at low risk of relapse, we recommend giving the last dose at 22 to 24 weeks of gestation, then promptly resuming therapy postpartum.
Live-attenuated vaccines (eg, influenza, rotavirus) should be avoided for the first 6 months in babies born to mothers on anti-TNF therapy.
- Ananthakrishnan AN, Xavier RJ, Podolsky DK. Inflammatory Bowel Diseases: A Clinician’s Guide. Chichester, UK: Wiley; 2017. doi:10.1002/9781119077633
- Shihab Z, Yeomans ND, De Cruz P. Anti-tumour necrosis factor alpha therapies and inflammatory bowel disease pregnancy outcomes: a meta-analysis. J Crohns Colitis 2016; 10(8):979–988. doi:10.1093/ecco-jcc/jjv234
- Narula N, Al-Dabbagh, Dhillon A, Sands BE, Marshall JK. Anti-TNF alpha therapies are safe during pregnancy in women with inflammatory bowel disease: a systematic review and meta-analysis. Inflamm Bowel Dis 2014; 20(10):1862–1869. doi:10.1097/MIB.0000000000000092
- Nielsen OH, Loftus EV Jr, Jess T. Safety of TNF-alpha inhibitors during IBD pregnancy: a systematic review. BMC Med 2013; 11:174. doi:10.1186/1741-7015-11-174
- Casanova MJ, Chaparro M, Domenech E, et al. Safety of thiopurines and anti-TNF-alpha drugs during pregnancy in patients with inflammatory bowel disease. Am J Gastroenterol 2013; 108(3):433–440. doi:10.1038/ajg.2012.430
- Nguyen GC, Seow CH, Maxwell C, et al; IBD in Pregnancy Consensus Group; Canadian Association of Gastroenterology. The Toronto consensus statements for the management of inflammatory bowel disease in pregnancy. Gastroenterology 2016; 150(3):734–757.e1. doi:10.1053/j.gastro.2015.12.003
- Gisbert JP, Chaparro, M. Safety of anti-TNF agents during pregnancy and breastfeeding in women with inflammatory bowel disease. Am J Gastroenterol 2013; 108(9):1426–1438. doi:10.1038/ajg.2013.171
- Chaparro M, Verreth A, Lobaton T, et al. Long-term safety of in utero exposure to anti-TNF alpha drugs for the treatment of inflammatory bowel disease: results from the multicenter European TEDDY Study. Am J Gastroenterol 2018; 113(3):396–403. doi:10.1038/ajg.2017.501
- de Lima A, Zelinkova Z, van der Ent C, Steegers EA, van der Woude CJ. Tailored anti-TNF therapy during pregnancy in patients with IBD: maternal and fetal safety. Gut 2016; 65(8):1261–1268. doi:10.1136/gutjnl-2015-309321
- Van Assche G, Vermeire S, Ballet V, et al. Switch to adalimumab in patients with Crohn’s disease controlled by maintenance infliximab: prospective randomised SWITCH trial. Gut 2012; 61(2):229–234. doi:10.1136/gutjnl-2011-300755
- Saha S. Medication management in the pregnant IBD patient. Am J Gastroenterol 2017; 112(5):667–669. doi:10.1038/ajg.2017.22
- Ananthakrishnan AN, Xavier RJ, Podolsky DK. Inflammatory Bowel Diseases: A Clinician’s Guide. Chichester, UK: Wiley; 2017. doi:10.1002/9781119077633
- Shihab Z, Yeomans ND, De Cruz P. Anti-tumour necrosis factor alpha therapies and inflammatory bowel disease pregnancy outcomes: a meta-analysis. J Crohns Colitis 2016; 10(8):979–988. doi:10.1093/ecco-jcc/jjv234
- Narula N, Al-Dabbagh, Dhillon A, Sands BE, Marshall JK. Anti-TNF alpha therapies are safe during pregnancy in women with inflammatory bowel disease: a systematic review and meta-analysis. Inflamm Bowel Dis 2014; 20(10):1862–1869. doi:10.1097/MIB.0000000000000092
- Nielsen OH, Loftus EV Jr, Jess T. Safety of TNF-alpha inhibitors during IBD pregnancy: a systematic review. BMC Med 2013; 11:174. doi:10.1186/1741-7015-11-174
- Casanova MJ, Chaparro M, Domenech E, et al. Safety of thiopurines and anti-TNF-alpha drugs during pregnancy in patients with inflammatory bowel disease. Am J Gastroenterol 2013; 108(3):433–440. doi:10.1038/ajg.2012.430
- Nguyen GC, Seow CH, Maxwell C, et al; IBD in Pregnancy Consensus Group; Canadian Association of Gastroenterology. The Toronto consensus statements for the management of inflammatory bowel disease in pregnancy. Gastroenterology 2016; 150(3):734–757.e1. doi:10.1053/j.gastro.2015.12.003
- Gisbert JP, Chaparro, M. Safety of anti-TNF agents during pregnancy and breastfeeding in women with inflammatory bowel disease. Am J Gastroenterol 2013; 108(9):1426–1438. doi:10.1038/ajg.2013.171
- Chaparro M, Verreth A, Lobaton T, et al. Long-term safety of in utero exposure to anti-TNF alpha drugs for the treatment of inflammatory bowel disease: results from the multicenter European TEDDY Study. Am J Gastroenterol 2018; 113(3):396–403. doi:10.1038/ajg.2017.501
- de Lima A, Zelinkova Z, van der Ent C, Steegers EA, van der Woude CJ. Tailored anti-TNF therapy during pregnancy in patients with IBD: maternal and fetal safety. Gut 2016; 65(8):1261–1268. doi:10.1136/gutjnl-2015-309321
- Van Assche G, Vermeire S, Ballet V, et al. Switch to adalimumab in patients with Crohn’s disease controlled by maintenance infliximab: prospective randomised SWITCH trial. Gut 2012; 61(2):229–234. doi:10.1136/gutjnl-2011-300755
- Saha S. Medication management in the pregnant IBD patient. Am J Gastroenterol 2017; 112(5):667–669. doi:10.1038/ajg.2017.22
Do all hospital inpatients need cardiac telemetry?
No. Continuous monitoring for changes in heart rhythm with cardiac telemetry is recommended for all patients admitted to an intensive care unit (ICU). But routine telemetry monitoring for patients in non-ICU beds is not recommended, as it leads to unnecessary testing and treatment, increasing the cost of care and hospital length of stay.
RISK STRATIFICATION AND INDICATIONS
Telemetry is generally recommended for patients admitted with any type of heart disease, including:
- Acute myocardial infarction with ST-segment elevation or Q waves on 12-lead electrocardiography (ECG)
- Acute ischemia suggested by ST-segment depression or T-wave inversion on ECG
- Systolic blood pressure less than 100 mm Hg
- Acute decompensated heart failure with bilateral rales above the lung bases
- Chest pain that is worse than or the same as that in prior angina or myocardial infarction.1,2
Indications for telemetry are less clear in patients with no history of heart disease. The American Heart Association (AHA)3 has classified admitted patients based on the presence or absence of heart disease3:
- Class I (high risk of arrhythmia): acute coronary syndrome, new arrhythmia (eg, atrial fibrillation or flutter), severe electrolyte imbalance; telemetry is warranted
- Class II (moderate risk): acute decompensated heart failure with stable hemodynamic status, a surgical or medical diagnosis with underlying paced rhythms (ie, with a pacemaker), and chronic arrhythmia (atrial fibrillation or flutter); in these cases, telemetry monitoring may be considered
- Class III (low risk): no history of cardiac disease or arrhythmias, admitted for medical or surgical reasons; in these cases, telemetry is generally not indicated3
Telemetry should also be considered in patients admitted with syncope or stroke, critical illness, or palpitations.
Syncope and stroke
Despite the wide use of telemetry for patients admitted with syncope, current evidence does not support this practice. However, the AHA recommends routine telemetry for patients admitted with idiopathic syncope when there is a high level of suspicion for underlying cardiac arrhythmias as a cause of syncope (risk class II-b).3 In 30% of patients admitted with stroke or transient ischemic attack, the cause is cardioembolic. Therefore, telemetry is indicated to rule out an underlying cardiac cause.4
Critical illness
Patients hospitalized with major trauma, acute respiratory failure, sepsis, shock, or acute pulmonary embolism or for major noncardiac surgery (especially elderly patients with coronary artery disease or at high risk of coronary events) require cardiac telemetry (risk class I-b). Patients admitted with kidney failure, significant electrolyte abnormalities, drug or substance toxicity (especially with known arrhythmogenic drugs) also require cardiac telemetry at the time of admission (risk class I-b).
Recurrent palpitations, arrhythmia
Most patients with palpitations can be evaluated in an outpatient setting.5 However, patients hospitalized for recurrent palpitations or for suspected underlying cardiac disease require telemetric monitoring (risk class II-b).3 Patients with high-degree atrioventricular block admitted after percutaneous temporary pacemaker implantation should be monitored, as should patients with a permanent pacemaker for 12 to 24 hours after implantation (risk class I-c). Also, patients hospitalized after implantable cardioverter-defibrillator (ICD) shock need to be monitored.3,6
Patients with a paced rhythm who do not meet the above criteria do not require routine telemetric monitoring (risk class III-c).7
TELEMETRY IS OVERUSED
Off-site telemetry monitoring can identify significant arrhythmias during hospitalization. It also saves time on nursing staff to focus on bedside patient care. However, its convenience can lower the threshold for ordering it. This can lead to overuse with a major impact on healthcare costs.
Routine use of cardiac telemetry is associated with increased hospitalization costs with little benefit.8 The use of off-site services for continuous monitoring can activate many alarms throughout the day, triggering unnecessary workups and leading to densensitization to alarms (“alarm fatigue”).9
Despite the precise indications outlined in the AHA updated practice standards for inpatient electrocardiographic monitoring,10 telemetry use is expanding to non-ICU units without evidence of benefit,8 and this overuse can result in harmful clinical outcomes and a financial burden. Telemetry monitoring of low-risk patients can cause delays in emergency department and ICU admissions and transfers8,11 of patients who may be sicker and need intensive care.
In a prospective observational study,12 only 11 (6%) of 182 patients admitted to a general medical floor met AHA class I criteria for telemetry; very few patients developed a significant telemetry event such as atrial fibrillation or flutter that necessitated a change in management. Most overprescribers of telemetry monitoring reason that it will catch arrhythmias early.12 In fact, in a study of patients in a cardiac unit, telemetry detected just 50% of in-house cardiac arrest cases, with a potential survival benefit of only 0.02%.13
Another study showed that only 0.01% of all telemetry alarms represented a real emergency. Only 37.2% of emergency alarms were classified as clinically important, and only 48.3% of these led to a change in management within 1 hour.14
Moreover, in a report of trauma patients with abnormal results on ECG at the time of admission, telemetry had negligible clinical benefit.15 And in a study of 414 patients, only 4% of those admitted with chest pain and normal initial ECG had cardiac interventions.16
Another study8 showed that hospital intervention to restrict the use of telemetry guided by AHA recommendations resulted in a 43% reduction in telemetry orders, a 47% reduction in telemetry duration, and a 70% reduction in the mean daily number of patients monitored, with no changes in hospital census or rates of code blue, death, or rapid response team activation.8
The financial cost can be seen in the backup of patients in the emergency department. A study showed that 91% of patients being admitted for chest pain were delayed by more than 3 hours while waiting for monitored beds. This translated into an annual cost of $168,300 to the hospital.17 Adherence to guidelines for appropriate use of telemetry can significantly decrease costs. Applying a simple algorithm for telemetry use was shown8 to decrease daily non-ICU cardiac telemetry costs from $18,971 to $5,772.
CURRENT GUIDELINES ARE LIMITED
The current American College of Cardiology and AHA guidelines are based mostly on expert opinion rather than randomized clinical trials, while most telemetry trials have been performed on patients with a cardiac or possible cardiac diagnosis.3 Current guidelines need to be updated, and more studies are needed to specify the optimal duration of cardiac monitoring in indicated cases. Many noncardiac conditions raise a legitimate concern of dysrhythmia, an indication for cardiac monitoring, but precise recommendations for telemetry for such conditions are lacking.
RECOMMENDATIONS
Raising awareness of the clinical and financial burdens associated with unwise telemetry utilization is critical. We suggest use of a pop-up notification in the electronic medical record to remind the provider of the existing telemetry order and to specify the duration of telemetry monitoring when placing the initial order. The goal is to identify patients in true need of a telemetry bed, to decrease unnecessary testing, and to reduce hospitalization costs.
- Recommended guidelines for in-hospital cardiac monitoring of adults for detection of arrhythmia. Emergency Cardiac Care Committee members. J Am Coll Cardiol 1991; 18(6):1431–1433. pmid:1939942
- Goldman L, Cook EF, Johnson PA, Brand DA, Rouan GW, Lee TH. Prediction of the need for intensive care in patients who come to emergency departments with acute chest pain. N Engl J Med 1996; 334(23):1498–1504. doi:10.1056/NEJM199606063342303
- Drew BJ, Califf RM, Funk M, et al; American Heart Association; Councils on Cardiovascular Nursing, Clinical Cardiology, and Cardiovascular Disease in the Young. Practice standards for electrocardiographic monitoring in hospital settings: an American Heart Association scientific statement from the Councils on Cardiovascular Nursing, Clinical Cardiology, and Cardiovascular Disease in the Young: endorsed by the International Society of Computerized Electrocardiology and the American Association of Critical-Care Nurses. Circulation 2004;110(17):2721–2746. doi:10.1161/01.CIR.0000145144.56673.59
- Ustrell X, Pellise A. Cardiac workup of ischemic stroke. Curr Cardiol Rev 2010; 6(3):175-183. doi:10.2174/157340310791658721
- Olson JA, Fouts AM, Padanilam BJ, Prystowsky EN. Utility of mobile cardiac outpatient telemetry for the diagnosis of palpitations, presyncope, syncope, and the assessment of therapy efficacy. J Cardiovasc Electrophysiol 2007; 18(5):473–477. doi:10.1111/j.1540-8167.2007.00779.x
- Chen EH, Hollander JE. When do patients need admission to a telemetry bed? J Emerg Med 2007; 33(1):53–60. doi:10.1016/j.jemermed.2007.01.017
- Sandau KE, Funk M, Auerbach A, et al; American Heart Association Council on Cardiovascular and Stroke Nursing; Council on Clinical Cardiology; and Council on Cardiovascular Disease in the Young. Update to practice standards for electrocardiographic monitoring in hospital settings: a scientific statement from the American Heart Association. Circulation 2017; 136(19):e273–e344. doi:10.1161/CIR.0000000000000527
- Dressler R, Dryer MM, Coletti C, Mahoney D, Doorey AJ. Altering overuse of cardiac telemetry in non-intensive care unit settings by hardwiring the use of American Heart Association guidelines. JAMA Intern Med 2014; 174(11):1852–1854. doi:10.1001/jamainternmed.2014.4491
- Cantillon DJ, Loy M, Burkle A, et al. Association between off-site central monitoring using standardized cardiac telemetry and clinical outcomes among non–critically ill patients. JAMA 2016; 316(5):519–524. doi:10.1001/jama.2016.10258
- Sandau KE, Funk M, Auerbach A, et al. Update to practice standards for electrocardiographic monitoring in hospital settings: a scientific statement from the American Heart Association. Circulation 2017; 136(19):e273–e344. doi:10.1161/CIR.0000000000000527
- Atzema C, Schull MJ, Borgundvaag B, Slaughter GR, Lee CK. ALARMED: adverse events in low-risk patients with chest pain receiving continuous electrocardiographic monitoring in the emergency department. A pilot study. Am J Emerg Med 2006; 24(1):62–67. doi:10.1016/j.ajem.2005.05.015
- Najafi N, Auerbach A. Use and outcomes of telemetry monitoring on a medicine service. Arch Intern Med 2012; 172(17):1349–1350. doi:10.1001/archinternmed.2012.3163
- Schull MJ, Redelmeier DA. Continuous electrocardiographic monitoring and cardiac arrest outcomes in 8,932 telemetry ward patients. Acad Emerg Med 2000; 7(6):647–652. pmid:10905643
- Kansara P, Jackson K, Dressler R, et al. Potential of missing life-threatening arrhythmias after limiting the use of cardiac telemetry. JAMA Intern Med 2015; 175(8):1416–1418. doi:10.1001/jamainternmed.2015.2387
- Nagy KK, Krosner SM, Roberts RR, Joseph KT, Smith RF, Barrett J. Determining which patients require evaluation for blunt cardiac injury following blunt chest trauma. World J Surg 2001; 25(1):108–111. pmid:11213149
- Snider A, Papaleo M, Beldner S, et al. Is telemetry monitoring necessary in low-risk suspected acute chest pain syndromes? Chest 2002; 122(2):517–523. pmid:12171825
- Bayley MD, Schwartz JS, Shofer FS, et al. The financial burden of emergency department congestion and hospital crowding for chest pain patients awaiting admission. Ann Emerg Med 2005; 45(2):110–117. doi:10.1016/j.annemergmed.2004.09.010
No. Continuous monitoring for changes in heart rhythm with cardiac telemetry is recommended for all patients admitted to an intensive care unit (ICU). But routine telemetry monitoring for patients in non-ICU beds is not recommended, as it leads to unnecessary testing and treatment, increasing the cost of care and hospital length of stay.
RISK STRATIFICATION AND INDICATIONS
Telemetry is generally recommended for patients admitted with any type of heart disease, including:
- Acute myocardial infarction with ST-segment elevation or Q waves on 12-lead electrocardiography (ECG)
- Acute ischemia suggested by ST-segment depression or T-wave inversion on ECG
- Systolic blood pressure less than 100 mm Hg
- Acute decompensated heart failure with bilateral rales above the lung bases
- Chest pain that is worse than or the same as that in prior angina or myocardial infarction.1,2
Indications for telemetry are less clear in patients with no history of heart disease. The American Heart Association (AHA)3 has classified admitted patients based on the presence or absence of heart disease3:
- Class I (high risk of arrhythmia): acute coronary syndrome, new arrhythmia (eg, atrial fibrillation or flutter), severe electrolyte imbalance; telemetry is warranted
- Class II (moderate risk): acute decompensated heart failure with stable hemodynamic status, a surgical or medical diagnosis with underlying paced rhythms (ie, with a pacemaker), and chronic arrhythmia (atrial fibrillation or flutter); in these cases, telemetry monitoring may be considered
- Class III (low risk): no history of cardiac disease or arrhythmias, admitted for medical or surgical reasons; in these cases, telemetry is generally not indicated3
Telemetry should also be considered in patients admitted with syncope or stroke, critical illness, or palpitations.
Syncope and stroke
Despite the wide use of telemetry for patients admitted with syncope, current evidence does not support this practice. However, the AHA recommends routine telemetry for patients admitted with idiopathic syncope when there is a high level of suspicion for underlying cardiac arrhythmias as a cause of syncope (risk class II-b).3 In 30% of patients admitted with stroke or transient ischemic attack, the cause is cardioembolic. Therefore, telemetry is indicated to rule out an underlying cardiac cause.4
Critical illness
Patients hospitalized with major trauma, acute respiratory failure, sepsis, shock, or acute pulmonary embolism or for major noncardiac surgery (especially elderly patients with coronary artery disease or at high risk of coronary events) require cardiac telemetry (risk class I-b). Patients admitted with kidney failure, significant electrolyte abnormalities, drug or substance toxicity (especially with known arrhythmogenic drugs) also require cardiac telemetry at the time of admission (risk class I-b).
Recurrent palpitations, arrhythmia
Most patients with palpitations can be evaluated in an outpatient setting.5 However, patients hospitalized for recurrent palpitations or for suspected underlying cardiac disease require telemetric monitoring (risk class II-b).3 Patients with high-degree atrioventricular block admitted after percutaneous temporary pacemaker implantation should be monitored, as should patients with a permanent pacemaker for 12 to 24 hours after implantation (risk class I-c). Also, patients hospitalized after implantable cardioverter-defibrillator (ICD) shock need to be monitored.3,6
Patients with a paced rhythm who do not meet the above criteria do not require routine telemetric monitoring (risk class III-c).7
TELEMETRY IS OVERUSED
Off-site telemetry monitoring can identify significant arrhythmias during hospitalization. It also saves time on nursing staff to focus on bedside patient care. However, its convenience can lower the threshold for ordering it. This can lead to overuse with a major impact on healthcare costs.
Routine use of cardiac telemetry is associated with increased hospitalization costs with little benefit.8 The use of off-site services for continuous monitoring can activate many alarms throughout the day, triggering unnecessary workups and leading to densensitization to alarms (“alarm fatigue”).9
Despite the precise indications outlined in the AHA updated practice standards for inpatient electrocardiographic monitoring,10 telemetry use is expanding to non-ICU units without evidence of benefit,8 and this overuse can result in harmful clinical outcomes and a financial burden. Telemetry monitoring of low-risk patients can cause delays in emergency department and ICU admissions and transfers8,11 of patients who may be sicker and need intensive care.
In a prospective observational study,12 only 11 (6%) of 182 patients admitted to a general medical floor met AHA class I criteria for telemetry; very few patients developed a significant telemetry event such as atrial fibrillation or flutter that necessitated a change in management. Most overprescribers of telemetry monitoring reason that it will catch arrhythmias early.12 In fact, in a study of patients in a cardiac unit, telemetry detected just 50% of in-house cardiac arrest cases, with a potential survival benefit of only 0.02%.13
Another study showed that only 0.01% of all telemetry alarms represented a real emergency. Only 37.2% of emergency alarms were classified as clinically important, and only 48.3% of these led to a change in management within 1 hour.14
Moreover, in a report of trauma patients with abnormal results on ECG at the time of admission, telemetry had negligible clinical benefit.15 And in a study of 414 patients, only 4% of those admitted with chest pain and normal initial ECG had cardiac interventions.16
Another study8 showed that hospital intervention to restrict the use of telemetry guided by AHA recommendations resulted in a 43% reduction in telemetry orders, a 47% reduction in telemetry duration, and a 70% reduction in the mean daily number of patients monitored, with no changes in hospital census or rates of code blue, death, or rapid response team activation.8
The financial cost can be seen in the backup of patients in the emergency department. A study showed that 91% of patients being admitted for chest pain were delayed by more than 3 hours while waiting for monitored beds. This translated into an annual cost of $168,300 to the hospital.17 Adherence to guidelines for appropriate use of telemetry can significantly decrease costs. Applying a simple algorithm for telemetry use was shown8 to decrease daily non-ICU cardiac telemetry costs from $18,971 to $5,772.
CURRENT GUIDELINES ARE LIMITED
The current American College of Cardiology and AHA guidelines are based mostly on expert opinion rather than randomized clinical trials, while most telemetry trials have been performed on patients with a cardiac or possible cardiac diagnosis.3 Current guidelines need to be updated, and more studies are needed to specify the optimal duration of cardiac monitoring in indicated cases. Many noncardiac conditions raise a legitimate concern of dysrhythmia, an indication for cardiac monitoring, but precise recommendations for telemetry for such conditions are lacking.
RECOMMENDATIONS
Raising awareness of the clinical and financial burdens associated with unwise telemetry utilization is critical. We suggest use of a pop-up notification in the electronic medical record to remind the provider of the existing telemetry order and to specify the duration of telemetry monitoring when placing the initial order. The goal is to identify patients in true need of a telemetry bed, to decrease unnecessary testing, and to reduce hospitalization costs.
No. Continuous monitoring for changes in heart rhythm with cardiac telemetry is recommended for all patients admitted to an intensive care unit (ICU). But routine telemetry monitoring for patients in non-ICU beds is not recommended, as it leads to unnecessary testing and treatment, increasing the cost of care and hospital length of stay.
RISK STRATIFICATION AND INDICATIONS
Telemetry is generally recommended for patients admitted with any type of heart disease, including:
- Acute myocardial infarction with ST-segment elevation or Q waves on 12-lead electrocardiography (ECG)
- Acute ischemia suggested by ST-segment depression or T-wave inversion on ECG
- Systolic blood pressure less than 100 mm Hg
- Acute decompensated heart failure with bilateral rales above the lung bases
- Chest pain that is worse than or the same as that in prior angina or myocardial infarction.1,2
Indications for telemetry are less clear in patients with no history of heart disease. The American Heart Association (AHA)3 has classified admitted patients based on the presence or absence of heart disease3:
- Class I (high risk of arrhythmia): acute coronary syndrome, new arrhythmia (eg, atrial fibrillation or flutter), severe electrolyte imbalance; telemetry is warranted
- Class II (moderate risk): acute decompensated heart failure with stable hemodynamic status, a surgical or medical diagnosis with underlying paced rhythms (ie, with a pacemaker), and chronic arrhythmia (atrial fibrillation or flutter); in these cases, telemetry monitoring may be considered
- Class III (low risk): no history of cardiac disease or arrhythmias, admitted for medical or surgical reasons; in these cases, telemetry is generally not indicated3
Telemetry should also be considered in patients admitted with syncope or stroke, critical illness, or palpitations.
Syncope and stroke
Despite the wide use of telemetry for patients admitted with syncope, current evidence does not support this practice. However, the AHA recommends routine telemetry for patients admitted with idiopathic syncope when there is a high level of suspicion for underlying cardiac arrhythmias as a cause of syncope (risk class II-b).3 In 30% of patients admitted with stroke or transient ischemic attack, the cause is cardioembolic. Therefore, telemetry is indicated to rule out an underlying cardiac cause.4
Critical illness
Patients hospitalized with major trauma, acute respiratory failure, sepsis, shock, or acute pulmonary embolism or for major noncardiac surgery (especially elderly patients with coronary artery disease or at high risk of coronary events) require cardiac telemetry (risk class I-b). Patients admitted with kidney failure, significant electrolyte abnormalities, drug or substance toxicity (especially with known arrhythmogenic drugs) also require cardiac telemetry at the time of admission (risk class I-b).
Recurrent palpitations, arrhythmia
Most patients with palpitations can be evaluated in an outpatient setting.5 However, patients hospitalized for recurrent palpitations or for suspected underlying cardiac disease require telemetric monitoring (risk class II-b).3 Patients with high-degree atrioventricular block admitted after percutaneous temporary pacemaker implantation should be monitored, as should patients with a permanent pacemaker for 12 to 24 hours after implantation (risk class I-c). Also, patients hospitalized after implantable cardioverter-defibrillator (ICD) shock need to be monitored.3,6
Patients with a paced rhythm who do not meet the above criteria do not require routine telemetric monitoring (risk class III-c).7
TELEMETRY IS OVERUSED
Off-site telemetry monitoring can identify significant arrhythmias during hospitalization. It also saves time on nursing staff to focus on bedside patient care. However, its convenience can lower the threshold for ordering it. This can lead to overuse with a major impact on healthcare costs.
Routine use of cardiac telemetry is associated with increased hospitalization costs with little benefit.8 The use of off-site services for continuous monitoring can activate many alarms throughout the day, triggering unnecessary workups and leading to densensitization to alarms (“alarm fatigue”).9
Despite the precise indications outlined in the AHA updated practice standards for inpatient electrocardiographic monitoring,10 telemetry use is expanding to non-ICU units without evidence of benefit,8 and this overuse can result in harmful clinical outcomes and a financial burden. Telemetry monitoring of low-risk patients can cause delays in emergency department and ICU admissions and transfers8,11 of patients who may be sicker and need intensive care.
In a prospective observational study,12 only 11 (6%) of 182 patients admitted to a general medical floor met AHA class I criteria for telemetry; very few patients developed a significant telemetry event such as atrial fibrillation or flutter that necessitated a change in management. Most overprescribers of telemetry monitoring reason that it will catch arrhythmias early.12 In fact, in a study of patients in a cardiac unit, telemetry detected just 50% of in-house cardiac arrest cases, with a potential survival benefit of only 0.02%.13
Another study showed that only 0.01% of all telemetry alarms represented a real emergency. Only 37.2% of emergency alarms were classified as clinically important, and only 48.3% of these led to a change in management within 1 hour.14
Moreover, in a report of trauma patients with abnormal results on ECG at the time of admission, telemetry had negligible clinical benefit.15 And in a study of 414 patients, only 4% of those admitted with chest pain and normal initial ECG had cardiac interventions.16
Another study8 showed that hospital intervention to restrict the use of telemetry guided by AHA recommendations resulted in a 43% reduction in telemetry orders, a 47% reduction in telemetry duration, and a 70% reduction in the mean daily number of patients monitored, with no changes in hospital census or rates of code blue, death, or rapid response team activation.8
The financial cost can be seen in the backup of patients in the emergency department. A study showed that 91% of patients being admitted for chest pain were delayed by more than 3 hours while waiting for monitored beds. This translated into an annual cost of $168,300 to the hospital.17 Adherence to guidelines for appropriate use of telemetry can significantly decrease costs. Applying a simple algorithm for telemetry use was shown8 to decrease daily non-ICU cardiac telemetry costs from $18,971 to $5,772.
CURRENT GUIDELINES ARE LIMITED
The current American College of Cardiology and AHA guidelines are based mostly on expert opinion rather than randomized clinical trials, while most telemetry trials have been performed on patients with a cardiac or possible cardiac diagnosis.3 Current guidelines need to be updated, and more studies are needed to specify the optimal duration of cardiac monitoring in indicated cases. Many noncardiac conditions raise a legitimate concern of dysrhythmia, an indication for cardiac monitoring, but precise recommendations for telemetry for such conditions are lacking.
RECOMMENDATIONS
Raising awareness of the clinical and financial burdens associated with unwise telemetry utilization is critical. We suggest use of a pop-up notification in the electronic medical record to remind the provider of the existing telemetry order and to specify the duration of telemetry monitoring when placing the initial order. The goal is to identify patients in true need of a telemetry bed, to decrease unnecessary testing, and to reduce hospitalization costs.
- Recommended guidelines for in-hospital cardiac monitoring of adults for detection of arrhythmia. Emergency Cardiac Care Committee members. J Am Coll Cardiol 1991; 18(6):1431–1433. pmid:1939942
- Goldman L, Cook EF, Johnson PA, Brand DA, Rouan GW, Lee TH. Prediction of the need for intensive care in patients who come to emergency departments with acute chest pain. N Engl J Med 1996; 334(23):1498–1504. doi:10.1056/NEJM199606063342303
- Drew BJ, Califf RM, Funk M, et al; American Heart Association; Councils on Cardiovascular Nursing, Clinical Cardiology, and Cardiovascular Disease in the Young. Practice standards for electrocardiographic monitoring in hospital settings: an American Heart Association scientific statement from the Councils on Cardiovascular Nursing, Clinical Cardiology, and Cardiovascular Disease in the Young: endorsed by the International Society of Computerized Electrocardiology and the American Association of Critical-Care Nurses. Circulation 2004;110(17):2721–2746. doi:10.1161/01.CIR.0000145144.56673.59
- Ustrell X, Pellise A. Cardiac workup of ischemic stroke. Curr Cardiol Rev 2010; 6(3):175-183. doi:10.2174/157340310791658721
- Olson JA, Fouts AM, Padanilam BJ, Prystowsky EN. Utility of mobile cardiac outpatient telemetry for the diagnosis of palpitations, presyncope, syncope, and the assessment of therapy efficacy. J Cardiovasc Electrophysiol 2007; 18(5):473–477. doi:10.1111/j.1540-8167.2007.00779.x
- Chen EH, Hollander JE. When do patients need admission to a telemetry bed? J Emerg Med 2007; 33(1):53–60. doi:10.1016/j.jemermed.2007.01.017
- Sandau KE, Funk M, Auerbach A, et al; American Heart Association Council on Cardiovascular and Stroke Nursing; Council on Clinical Cardiology; and Council on Cardiovascular Disease in the Young. Update to practice standards for electrocardiographic monitoring in hospital settings: a scientific statement from the American Heart Association. Circulation 2017; 136(19):e273–e344. doi:10.1161/CIR.0000000000000527
- Dressler R, Dryer MM, Coletti C, Mahoney D, Doorey AJ. Altering overuse of cardiac telemetry in non-intensive care unit settings by hardwiring the use of American Heart Association guidelines. JAMA Intern Med 2014; 174(11):1852–1854. doi:10.1001/jamainternmed.2014.4491
- Cantillon DJ, Loy M, Burkle A, et al. Association between off-site central monitoring using standardized cardiac telemetry and clinical outcomes among non–critically ill patients. JAMA 2016; 316(5):519–524. doi:10.1001/jama.2016.10258
- Sandau KE, Funk M, Auerbach A, et al. Update to practice standards for electrocardiographic monitoring in hospital settings: a scientific statement from the American Heart Association. Circulation 2017; 136(19):e273–e344. doi:10.1161/CIR.0000000000000527
- Atzema C, Schull MJ, Borgundvaag B, Slaughter GR, Lee CK. ALARMED: adverse events in low-risk patients with chest pain receiving continuous electrocardiographic monitoring in the emergency department. A pilot study. Am J Emerg Med 2006; 24(1):62–67. doi:10.1016/j.ajem.2005.05.015
- Najafi N, Auerbach A. Use and outcomes of telemetry monitoring on a medicine service. Arch Intern Med 2012; 172(17):1349–1350. doi:10.1001/archinternmed.2012.3163
- Schull MJ, Redelmeier DA. Continuous electrocardiographic monitoring and cardiac arrest outcomes in 8,932 telemetry ward patients. Acad Emerg Med 2000; 7(6):647–652. pmid:10905643
- Kansara P, Jackson K, Dressler R, et al. Potential of missing life-threatening arrhythmias after limiting the use of cardiac telemetry. JAMA Intern Med 2015; 175(8):1416–1418. doi:10.1001/jamainternmed.2015.2387
- Nagy KK, Krosner SM, Roberts RR, Joseph KT, Smith RF, Barrett J. Determining which patients require evaluation for blunt cardiac injury following blunt chest trauma. World J Surg 2001; 25(1):108–111. pmid:11213149
- Snider A, Papaleo M, Beldner S, et al. Is telemetry monitoring necessary in low-risk suspected acute chest pain syndromes? Chest 2002; 122(2):517–523. pmid:12171825
- Bayley MD, Schwartz JS, Shofer FS, et al. The financial burden of emergency department congestion and hospital crowding for chest pain patients awaiting admission. Ann Emerg Med 2005; 45(2):110–117. doi:10.1016/j.annemergmed.2004.09.010
- Recommended guidelines for in-hospital cardiac monitoring of adults for detection of arrhythmia. Emergency Cardiac Care Committee members. J Am Coll Cardiol 1991; 18(6):1431–1433. pmid:1939942
- Goldman L, Cook EF, Johnson PA, Brand DA, Rouan GW, Lee TH. Prediction of the need for intensive care in patients who come to emergency departments with acute chest pain. N Engl J Med 1996; 334(23):1498–1504. doi:10.1056/NEJM199606063342303
- Drew BJ, Califf RM, Funk M, et al; American Heart Association; Councils on Cardiovascular Nursing, Clinical Cardiology, and Cardiovascular Disease in the Young. Practice standards for electrocardiographic monitoring in hospital settings: an American Heart Association scientific statement from the Councils on Cardiovascular Nursing, Clinical Cardiology, and Cardiovascular Disease in the Young: endorsed by the International Society of Computerized Electrocardiology and the American Association of Critical-Care Nurses. Circulation 2004;110(17):2721–2746. doi:10.1161/01.CIR.0000145144.56673.59
- Ustrell X, Pellise A. Cardiac workup of ischemic stroke. Curr Cardiol Rev 2010; 6(3):175-183. doi:10.2174/157340310791658721
- Olson JA, Fouts AM, Padanilam BJ, Prystowsky EN. Utility of mobile cardiac outpatient telemetry for the diagnosis of palpitations, presyncope, syncope, and the assessment of therapy efficacy. J Cardiovasc Electrophysiol 2007; 18(5):473–477. doi:10.1111/j.1540-8167.2007.00779.x
- Chen EH, Hollander JE. When do patients need admission to a telemetry bed? J Emerg Med 2007; 33(1):53–60. doi:10.1016/j.jemermed.2007.01.017
- Sandau KE, Funk M, Auerbach A, et al; American Heart Association Council on Cardiovascular and Stroke Nursing; Council on Clinical Cardiology; and Council on Cardiovascular Disease in the Young. Update to practice standards for electrocardiographic monitoring in hospital settings: a scientific statement from the American Heart Association. Circulation 2017; 136(19):e273–e344. doi:10.1161/CIR.0000000000000527
- Dressler R, Dryer MM, Coletti C, Mahoney D, Doorey AJ. Altering overuse of cardiac telemetry in non-intensive care unit settings by hardwiring the use of American Heart Association guidelines. JAMA Intern Med 2014; 174(11):1852–1854. doi:10.1001/jamainternmed.2014.4491
- Cantillon DJ, Loy M, Burkle A, et al. Association between off-site central monitoring using standardized cardiac telemetry and clinical outcomes among non–critically ill patients. JAMA 2016; 316(5):519–524. doi:10.1001/jama.2016.10258
- Sandau KE, Funk M, Auerbach A, et al. Update to practice standards for electrocardiographic monitoring in hospital settings: a scientific statement from the American Heart Association. Circulation 2017; 136(19):e273–e344. doi:10.1161/CIR.0000000000000527
- Atzema C, Schull MJ, Borgundvaag B, Slaughter GR, Lee CK. ALARMED: adverse events in low-risk patients with chest pain receiving continuous electrocardiographic monitoring in the emergency department. A pilot study. Am J Emerg Med 2006; 24(1):62–67. doi:10.1016/j.ajem.2005.05.015
- Najafi N, Auerbach A. Use and outcomes of telemetry monitoring on a medicine service. Arch Intern Med 2012; 172(17):1349–1350. doi:10.1001/archinternmed.2012.3163
- Schull MJ, Redelmeier DA. Continuous electrocardiographic monitoring and cardiac arrest outcomes in 8,932 telemetry ward patients. Acad Emerg Med 2000; 7(6):647–652. pmid:10905643
- Kansara P, Jackson K, Dressler R, et al. Potential of missing life-threatening arrhythmias after limiting the use of cardiac telemetry. JAMA Intern Med 2015; 175(8):1416–1418. doi:10.1001/jamainternmed.2015.2387
- Nagy KK, Krosner SM, Roberts RR, Joseph KT, Smith RF, Barrett J. Determining which patients require evaluation for blunt cardiac injury following blunt chest trauma. World J Surg 2001; 25(1):108–111. pmid:11213149
- Snider A, Papaleo M, Beldner S, et al. Is telemetry monitoring necessary in low-risk suspected acute chest pain syndromes? Chest 2002; 122(2):517–523. pmid:12171825
- Bayley MD, Schwartz JS, Shofer FS, et al. The financial burden of emergency department congestion and hospital crowding for chest pain patients awaiting admission. Ann Emerg Med 2005; 45(2):110–117. doi:10.1016/j.annemergmed.2004.09.010
Which patients with pulmonary embolism need echocardiography?
Most patients admitted with pulmonary embolism (PE) do not need transthoracic echocardiography (TTE); it should be performed in hemodynamically unstable patients, as well as in hemodynamically stable patients with specific elevated cardiac biomarkers and imaging features.
The decision to perform TTE should be based on clinical presentation, PE burden, and imaging findings (eg, computed tomographic angiography). TTE helps to stratify risk, guide management, monitor response to therapy, and give prognostic information for a subset of patients at increased risk for PE-related adverse events.
RISK STRATIFICATION IN PULMONARY EMBOLISM
PE has a spectrum of presentations ranging from no symptoms to shock. Based on the clinical presentation, PE can be categorized as high, intermediate, or low risk.
High-risk PE, often referred to as “massive” PE, is defined in current American Heart Association guidelines as acute PE with sustained hypotension (systolic blood pressure < 90 mm Hg for at least 15 minutes or requiring inotropic support), persistent profound bradycardia (heart rate < 40 beats per minute with signs or symptoms of shock), syncope, or cardiac arrest.1
Intermediate-risk or “submassive” PE is more challenging to identify because patients are more hemodynamically stable, yet have evidence on electrocardiography, TTE, computed tomography, or cardiac biomarker testing—ie, N-terminal pro-B-type natriuretic peptide (NT-proBNP) or troponin—that indicates myocardial injury or volume overload.1
Low-risk PE is acute PE in the absence of clinical markers of adverse prognosis that define massive or submassive PE.1
ECHOCARDIOGRAPHIC FEATURES OF HIGH-RISK PULMONARY EMBOLISM
Certain TTE findings suggest increased risk of a poor outcome and may warrant therapy that is more invasive and aggressive. High-risk features include the following:
- Impaired right ventricular function
- Interventricular septum bulging into the left ventricle (“D-shaped” septum)
- Dilated proximal pulmonary arteries
- Increased severity of tricuspid regurgitation
- Elevated right atrial pressure
- Elevated pulmonary artery pressure
- Free-floating right ventricular thrombi, which are associated with a mortality rate of up to 45% and can be detected in 7% to 18% of patients6
- Tricuspid annular plane systolic excursion, an echocardiographic measure of right ventricular function1; a value less than 17 mm suggests impaired right ventricular systolic function7
- The McConnell sign, a feature of acute massive PE: akinesia of the mid-free wall of the right ventricle and hypercontractility of the apex.
These TTE findings often lead to treatment with thrombolysis, transfer to the intensive care unit, and activation of the interventional team for catheter-based therapies.1,8 Free-floating right heart thrombi or thrombus straddling the interatrial septum (“thrombus in transit”) through a patent foramen ovale may require surgical embolectomy.8
PATIENT SELECTION AND INDICATIONS FOR ECHOCARDIOGRAPHY
- Jaff MR, McMurtry MS, Archer SL, et al. Management of massive and submassive pulmonary embolism, iliofemoral deep vein thrombosis, and chronic thromboembolic pulmonary hypertension. Circulation 2011; 123:1788–1830. doi:10.1161/CIR.0b013e318214914f
- Jiménez D, Aujesky D, Moores L, et al; RIETE Investigators. Simplification of the pulmonary embolism severity index for prognostication in patients with acute symptomatic pulmonary embolism. Arch Intern Med 2010; 170:1383–1389. doi:10.1001/archinternmed.2010.199
- Aujesky D, Obrosky DS, Stone RA, et al. Derivation and validation of a prognostic model for pulmonary embolism. Am J Respir Crit Care Med 2005; 172:1041–1046. doi:10.1164/rccm.200506-862OC
- Bova C, Pesavento R, Marchiori A, et al; TELESIO Study Group. Risk stratification and outcomes in hemodynamically stable patients with acute pulmonary embolism. J Thromb Haemost 2009; 7:938–944. doi:10.1111/j.1538-7836.2009.03345.x
- Fernandez C, Bova C, Sanchez O, et al. Validation of a model for identification of patients at intermediate to high risk for complications associated with acute symptomatic pulmonary embolism. Chest 2015; 148:211–218. doi:10.1378/chest.14-2551
- Chartier L, Bera J, Delomez M, et al. Free-floating thrombi in the right heart: diagnosis, management, and prognostic indexes in 38 consecutive patients. Circulation 1999; 99:2779–2783. pmid:10351972
- Rudski LG, Lai WW, Afilalo J, et al. Guidelines for the echocardiographic assessment of the right heart in adults. J Am Soc Echocardiogr 2010; 23:685–713. doi:10.1016/j.echo.2010.05.010
- Konstantinides S, Torbicki A, Agnelli G, et al. 2014 ESC guidelines on the diagnosis and management of acute pulmonary embolism. Eur Heart J 2014; 35:3033–3069a–k. doi:10.1093/eurheartj/ehu283
- Saric M, Armour AC, Arnaout MS, et al. Guidelines for the use of echocardiography in the evaluation of a cardiac source of embolism. J Am Soc Echocardiogr 2016; 29:1–42. doi:10.1016/j.echo.2015.09.011
Most patients admitted with pulmonary embolism (PE) do not need transthoracic echocardiography (TTE); it should be performed in hemodynamically unstable patients, as well as in hemodynamically stable patients with specific elevated cardiac biomarkers and imaging features.
The decision to perform TTE should be based on clinical presentation, PE burden, and imaging findings (eg, computed tomographic angiography). TTE helps to stratify risk, guide management, monitor response to therapy, and give prognostic information for a subset of patients at increased risk for PE-related adverse events.
RISK STRATIFICATION IN PULMONARY EMBOLISM
PE has a spectrum of presentations ranging from no symptoms to shock. Based on the clinical presentation, PE can be categorized as high, intermediate, or low risk.
High-risk PE, often referred to as “massive” PE, is defined in current American Heart Association guidelines as acute PE with sustained hypotension (systolic blood pressure < 90 mm Hg for at least 15 minutes or requiring inotropic support), persistent profound bradycardia (heart rate < 40 beats per minute with signs or symptoms of shock), syncope, or cardiac arrest.1
Intermediate-risk or “submassive” PE is more challenging to identify because patients are more hemodynamically stable, yet have evidence on electrocardiography, TTE, computed tomography, or cardiac biomarker testing—ie, N-terminal pro-B-type natriuretic peptide (NT-proBNP) or troponin—that indicates myocardial injury or volume overload.1
Low-risk PE is acute PE in the absence of clinical markers of adverse prognosis that define massive or submassive PE.1
ECHOCARDIOGRAPHIC FEATURES OF HIGH-RISK PULMONARY EMBOLISM
Certain TTE findings suggest increased risk of a poor outcome and may warrant therapy that is more invasive and aggressive. High-risk features include the following:
- Impaired right ventricular function
- Interventricular septum bulging into the left ventricle (“D-shaped” septum)
- Dilated proximal pulmonary arteries
- Increased severity of tricuspid regurgitation
- Elevated right atrial pressure
- Elevated pulmonary artery pressure
- Free-floating right ventricular thrombi, which are associated with a mortality rate of up to 45% and can be detected in 7% to 18% of patients6
- Tricuspid annular plane systolic excursion, an echocardiographic measure of right ventricular function1; a value less than 17 mm suggests impaired right ventricular systolic function7
- The McConnell sign, a feature of acute massive PE: akinesia of the mid-free wall of the right ventricle and hypercontractility of the apex.
These TTE findings often lead to treatment with thrombolysis, transfer to the intensive care unit, and activation of the interventional team for catheter-based therapies.1,8 Free-floating right heart thrombi or thrombus straddling the interatrial septum (“thrombus in transit”) through a patent foramen ovale may require surgical embolectomy.8
PATIENT SELECTION AND INDICATIONS FOR ECHOCARDIOGRAPHY
Most patients admitted with pulmonary embolism (PE) do not need transthoracic echocardiography (TTE); it should be performed in hemodynamically unstable patients, as well as in hemodynamically stable patients with specific elevated cardiac biomarkers and imaging features.
The decision to perform TTE should be based on clinical presentation, PE burden, and imaging findings (eg, computed tomographic angiography). TTE helps to stratify risk, guide management, monitor response to therapy, and give prognostic information for a subset of patients at increased risk for PE-related adverse events.
RISK STRATIFICATION IN PULMONARY EMBOLISM
PE has a spectrum of presentations ranging from no symptoms to shock. Based on the clinical presentation, PE can be categorized as high, intermediate, or low risk.
High-risk PE, often referred to as “massive” PE, is defined in current American Heart Association guidelines as acute PE with sustained hypotension (systolic blood pressure < 90 mm Hg for at least 15 minutes or requiring inotropic support), persistent profound bradycardia (heart rate < 40 beats per minute with signs or symptoms of shock), syncope, or cardiac arrest.1
Intermediate-risk or “submassive” PE is more challenging to identify because patients are more hemodynamically stable, yet have evidence on electrocardiography, TTE, computed tomography, or cardiac biomarker testing—ie, N-terminal pro-B-type natriuretic peptide (NT-proBNP) or troponin—that indicates myocardial injury or volume overload.1
Low-risk PE is acute PE in the absence of clinical markers of adverse prognosis that define massive or submassive PE.1
ECHOCARDIOGRAPHIC FEATURES OF HIGH-RISK PULMONARY EMBOLISM
Certain TTE findings suggest increased risk of a poor outcome and may warrant therapy that is more invasive and aggressive. High-risk features include the following:
- Impaired right ventricular function
- Interventricular septum bulging into the left ventricle (“D-shaped” septum)
- Dilated proximal pulmonary arteries
- Increased severity of tricuspid regurgitation
- Elevated right atrial pressure
- Elevated pulmonary artery pressure
- Free-floating right ventricular thrombi, which are associated with a mortality rate of up to 45% and can be detected in 7% to 18% of patients6
- Tricuspid annular plane systolic excursion, an echocardiographic measure of right ventricular function1; a value less than 17 mm suggests impaired right ventricular systolic function7
- The McConnell sign, a feature of acute massive PE: akinesia of the mid-free wall of the right ventricle and hypercontractility of the apex.
These TTE findings often lead to treatment with thrombolysis, transfer to the intensive care unit, and activation of the interventional team for catheter-based therapies.1,8 Free-floating right heart thrombi or thrombus straddling the interatrial septum (“thrombus in transit”) through a patent foramen ovale may require surgical embolectomy.8
PATIENT SELECTION AND INDICATIONS FOR ECHOCARDIOGRAPHY
- Jaff MR, McMurtry MS, Archer SL, et al. Management of massive and submassive pulmonary embolism, iliofemoral deep vein thrombosis, and chronic thromboembolic pulmonary hypertension. Circulation 2011; 123:1788–1830. doi:10.1161/CIR.0b013e318214914f
- Jiménez D, Aujesky D, Moores L, et al; RIETE Investigators. Simplification of the pulmonary embolism severity index for prognostication in patients with acute symptomatic pulmonary embolism. Arch Intern Med 2010; 170:1383–1389. doi:10.1001/archinternmed.2010.199
- Aujesky D, Obrosky DS, Stone RA, et al. Derivation and validation of a prognostic model for pulmonary embolism. Am J Respir Crit Care Med 2005; 172:1041–1046. doi:10.1164/rccm.200506-862OC
- Bova C, Pesavento R, Marchiori A, et al; TELESIO Study Group. Risk stratification and outcomes in hemodynamically stable patients with acute pulmonary embolism. J Thromb Haemost 2009; 7:938–944. doi:10.1111/j.1538-7836.2009.03345.x
- Fernandez C, Bova C, Sanchez O, et al. Validation of a model for identification of patients at intermediate to high risk for complications associated with acute symptomatic pulmonary embolism. Chest 2015; 148:211–218. doi:10.1378/chest.14-2551
- Chartier L, Bera J, Delomez M, et al. Free-floating thrombi in the right heart: diagnosis, management, and prognostic indexes in 38 consecutive patients. Circulation 1999; 99:2779–2783. pmid:10351972
- Rudski LG, Lai WW, Afilalo J, et al. Guidelines for the echocardiographic assessment of the right heart in adults. J Am Soc Echocardiogr 2010; 23:685–713. doi:10.1016/j.echo.2010.05.010
- Konstantinides S, Torbicki A, Agnelli G, et al. 2014 ESC guidelines on the diagnosis and management of acute pulmonary embolism. Eur Heart J 2014; 35:3033–3069a–k. doi:10.1093/eurheartj/ehu283
- Saric M, Armour AC, Arnaout MS, et al. Guidelines for the use of echocardiography in the evaluation of a cardiac source of embolism. J Am Soc Echocardiogr 2016; 29:1–42. doi:10.1016/j.echo.2015.09.011
- Jaff MR, McMurtry MS, Archer SL, et al. Management of massive and submassive pulmonary embolism, iliofemoral deep vein thrombosis, and chronic thromboembolic pulmonary hypertension. Circulation 2011; 123:1788–1830. doi:10.1161/CIR.0b013e318214914f
- Jiménez D, Aujesky D, Moores L, et al; RIETE Investigators. Simplification of the pulmonary embolism severity index for prognostication in patients with acute symptomatic pulmonary embolism. Arch Intern Med 2010; 170:1383–1389. doi:10.1001/archinternmed.2010.199
- Aujesky D, Obrosky DS, Stone RA, et al. Derivation and validation of a prognostic model for pulmonary embolism. Am J Respir Crit Care Med 2005; 172:1041–1046. doi:10.1164/rccm.200506-862OC
- Bova C, Pesavento R, Marchiori A, et al; TELESIO Study Group. Risk stratification and outcomes in hemodynamically stable patients with acute pulmonary embolism. J Thromb Haemost 2009; 7:938–944. doi:10.1111/j.1538-7836.2009.03345.x
- Fernandez C, Bova C, Sanchez O, et al. Validation of a model for identification of patients at intermediate to high risk for complications associated with acute symptomatic pulmonary embolism. Chest 2015; 148:211–218. doi:10.1378/chest.14-2551
- Chartier L, Bera J, Delomez M, et al. Free-floating thrombi in the right heart: diagnosis, management, and prognostic indexes in 38 consecutive patients. Circulation 1999; 99:2779–2783. pmid:10351972
- Rudski LG, Lai WW, Afilalo J, et al. Guidelines for the echocardiographic assessment of the right heart in adults. J Am Soc Echocardiogr 2010; 23:685–713. doi:10.1016/j.echo.2010.05.010
- Konstantinides S, Torbicki A, Agnelli G, et al. 2014 ESC guidelines on the diagnosis and management of acute pulmonary embolism. Eur Heart J 2014; 35:3033–3069a–k. doi:10.1093/eurheartj/ehu283
- Saric M, Armour AC, Arnaout MS, et al. Guidelines for the use of echocardiography in the evaluation of a cardiac source of embolism. J Am Soc Echocardiogr 2016; 29:1–42. doi:10.1016/j.echo.2015.09.011