User login
What can we do about musculoskeletal pain from bisphosphonates?
Bisphosphonates, especially intravenous zoledronic acid, often cause influenza-like symptoms such as severe musculoskeletal pain, fever, headache, malaise, and fatigue, sometimes accompanied by nausea, vomiting, and diarrhea. As many as 30% of patients experience these symptoms, which are usually transient, last up to 1 week, and, in most patients, only rarely recur with subsequent infusions.
It is essential to counsel and reassure patients about these reactions before starting treatment. We recommend that patients take acetaminophen before intravenous bisphosphonate infusions, and if an acute-phase reaction occurs, we provide adequate supportive care with acetaminophen or nonsteroidal anti-inflammatory drugs (NSAIDs). If patients report severe musculoskeletal pain, then consider discontinuing the bisphosphonate treatment.
INFLUENZA-LIKE SYMPTOMS
The acute-phase reaction is a transient inflammatory state characterized by influenza-like symptoms such as fever, myalgia, joint pain, and nausea. It often occurs within the first few days after initial exposure to a bisphosphonate. Patients tend to rate the symptoms as mild to moderate. Symptoms may recur with subsequent doses; however, the incidence rate decreases substantially with each subsequent dose.
With intravenous bisphosphonates
Reid et al1 analyzed data from a trial in which 7,765 postmenopausal women with osteoporosis were randomized to receive intravenous zoledronic acid or placebo; 42.4% of the zoledronic acid group experienced symptoms that could be attributed to an acute-phase reaction after the first infusion, compared with 11.7% of the placebo group (P < .0001). Statistically significant differences (P < .0001) in symptoms between the groups included the following:
- Fever 20.3% vs 2.5%
- Musculoskeletal symptoms 19.9% vs 4.7%
- Gastrointestinal symptoms 7.8% vs 2.1%.
Of the patients describing musculoskeletal symptoms after receiving zoledronic acid, most (79%) described them as generalized pain or discomfort, while about 25% said they were regional, usually localized to the back, neck, chest, and shoulders, 5% described joint stiffness, and 2.5% reported joint swelling.1
In this and other studies,1–3 acute-phase reactions most commonly occurred within the first few days after the infusion and were rated as mild to moderate in 90% of cases.1,2 Patients who reported an acute-phase reaction were not more likely to opt out of subsequent infusions. The authors postulated that this was most likely because acute-phase reactions were mild and transient, and most resolved within 1 week.1 The incidence decreased with each subsequent infusion of zoledronic acid1–3; rates of the acute-phase reaction at years 1, 2, and 3 were 30%, 7%, and 3%, respectively.1
With oral bisphosphonates
The acute-phase reaction is less common with oral bisphosphonates (occurring in 5.6% of patients in a retrospective study4) and is usually less severe.4,5
AMINOBISPHOSPHONATES INDUCE INFLAMMATORY CYTOKINES
Musculoskeletal pain related to the acute-phase reaction is thought to be due to transient release of inflammatory cytokines such as interleukin 6, interferon gamma, and tumor necrosis factor alpha from macrophages, monocytes, and gamma-delta T cells.6
Bisphosphonates are taken up by osteoclasts and inhibit their function. But bisphosphonates are not all the same: they can be divided into aminobisphosphonates (eg, alendronate, pamidronate, risedronate, zoledronic acid) and nonaminobisphosphonates (eg, clodronate, etidronate).
Inside the osteoclasts, aminobisphosphonates inhibit farnesyl diphosphate synthase in the mevalonate pathways, thus disrupting cell signaling and leading to apoptosis.7 However, inhibition of farnesyl diphosphate synthase also increases intracellular levels of isopentyl pyrophosphate, which induces T-cell activation; this is thought to result in release of inflammatory cytokines, leading to the acute-phase reaction.7,8
In contrast, nonaminobisphosphonates such as clodronate and etidronate, after being internalized, are metabolized into nonhydrolyzable adenosine triphosphate, which is toxic to the osteoclast. The acute-phase reaction or influenza-like illness is unique to aminobisphosphonates; nonaminobisphosphonates have not been associated with an acute-phase reaction.
TRIALS OF PREVENTIVE TREATMENT
With NSAIDs, acetaminophen
Wark et al9 randomized 481 postmenopausal women who had osteopenia but who had never received bisphosphonates to 4 treatment groups:
- Zoledronic acid 5 mg intravenously plus acetaminophen 1,000 mg every 6 hours for 3 days
- Zoledronic acid 5 mg intravenously plus ibuprofen 400 mg every 6 hours for 3 days
- Zoledronic acid 5 mg intravenously plus 2 placebo capsules every 6 hours for 3 days
- Placebo infusion plus 2 placebo capsules every 6 hours for 3 days.
Patients were assessed for fever and worsening symptoms over 3 days after the infusion. The group that got zoledronic acid infusion and placebo capsules had the highest rates of fever (64%) and worsening symptoms (76%); acetaminophen and ibuprofen reduced these rates to an approximately equal extent, to 37% for fever and 46% (acetaminophen) and 49% (ibuprofen) for worsening symptoms. The group that received placebo bisphosphonate infusions had the lowest rates of fever (11%) and worsening symptoms (17%).
Silverman et al10 found that acetaminophen 650 mg taken 45 minutes before intravenous zoledronic acid infusion and continued every 6 hours for 3 days led to an absolute risk reduction of 21% in the incidence of fever or use of rescue medication compared with placebo.
Trials of other agents
In a study of 60 women,11 30 received an oral bolus of cholecalciferol 300,000 IU 5 days before zoledronic acid 5 mg infusion plus daily calcium 1,000 mg and vitamin D 800 IU, and 30 received a placebo pill 5 days before the same infusion and vitamin regimen as the other group. The preinfusion oral bolus did not decrease the incidence of acute-phase reactions, although it led to a small decrease in the severity of musculoskeletal pain (the median pain score was reduced from 2 to 1 on a scale of 0 to 10).
Other interventions such as fluvastatin and oral dexamethasone given before intravenous zoledronic acid did not reduce the severity or incidence of the acute-phase reaction.10,12,13
OUR APPROACH
Before starting bisphosphonate therapy, patients should be counseled about the possibility of acute musculoskeletal pain and other symptoms of the acute-phase reaction.
For intravenous bisphosphonates
We advise all patients scheduled to receive intravenous bisphosphonates to take acetaminophen 650 to 1,000 mg once on the morning of the infusion. We prefer acetaminophen over NSAIDs for prophylaxis to avoid the gastric mucosal and renal toxicity more common with NSAIDs, especially in the elderly.
If the patient has a history of acute musculoskeletal pain or other symptoms of an acute-phase reaction after bisphosphonate infusion, we advise a more aggressive approach to prophylaxis: acetaminophen 650 mg 1 hour before the infusion, then every 6 hours for up to 3 days. This approach, with acetaminophen or NSAIDs, has been shown in large randomized controlled trials to reduce the incidence and severity of the acute-phase reaction.
If an acute-phase reaction occurs, we inform patients that the likelihood decreases and is quite low with subsequent doses. We provide correct and honest information, so that patients who experience an acute-phase reaction can make an informed decision about continuing bisphosphonate treatment or switching to another treatment. If the patient decides to continue with intravenous bisphosphonate treatment, we recommend more-aggressive prophylaxis with acetaminophen or NSAIDs with subsequent infusions.
For oral bisphosphonates
We do not prescribe prophylactic treatment with acetaminophen or NSAIDs with oral bisphosphonates, but we do advise patients to take acetaminophen or NSAIDs as needed for mild to moderate musculoskeletal pain, should this occur.
We try to continue treatment in mild to moderate cases, while monitoring the patient closely to see if the musculoskeletal pain resolves within 1 to 2 weeks.
If the pain is severe or does not resolve in 1 to 2 weeks, we offer switching to another drug class. Since musculoskeletal pain with oral bisphosphonates does not represent an allergic reaction, we have switched patients from oral to intravenous bisphosphonates without recurrence of musculoskeletal pain.
SEVERE MUSCULOSKELETAL PAIN BEYOND THE ACUTE PHASE
Severe musculoskeletal pain that may not be related to the acute-phase reaction, although rare, has been reported.5,14 From 1995, when alendronate was approved for osteoporosis, through 2002, the US Food and Drug Administration received reports of severe musculoskeletal pain in 117 patients.15
This severe musculoskeletal pain related to bisphosphonate use remains poorly characterized. It has been reported to occur days or months (median time 14 days, range same day to 52 months) after starting bisphosphonate therapy and to resolve only if the bisphosphonate is stopped.5,15 It differs from typical acute-phase reactions, which tend to occur with the initial dose (intravenous or oral) and resolve within several days. There are case reports of polyarthritis with synovitis that recurred with each bisphosphonate dose (oral or intravenous) and led to discontinuation of the bisphosphonate.14,16–18
Clinicians need to be aware of the possibility of severe musculoskeletal pain and consider stopping bisphosphonate treatment in these cases. Besides discontinuation, acetaminophen, NSAIDs, and, in rare cases, glucocorticoids or short-term opiate therapy may be used for symptom control. In patients with a severe or persistent acute-phase reaction or musculoskeletal pain, discontinuation of bisphosphonates is warranted.
- Reid IR, Gamble GD, Mesenbrink P, Lakatos P, Black DM. Characterization of and risk factors for the acute-phase response after zoledronic acid. J Clin Endocrinol Metab 2010; 95(9):4380–4387. doi:10.1210/jc.2010-0597
- Black DM, Delmas PD, Eastell R, et al; HORIZON Pivotal Fracture Trial. Once-yearly zoledronic acid for treatment of postmenopausal osteoporosis. N Engl J Med 2007; 356(18):1809–1822. doi:10.1056/NEJMoa067312
- Lyles KW, Colon-Emeric CS, Magaziner JS, et al; HORIZON Recurrent Fracture Trial. Zoledronic acid and clinical fractures and mortality after hip fracture. N Engl J Med 2007; 357(18):1799–1809. doi:10.1056/NEJMoa074941
- Bock O, Boerst H, Thomasius FE, et al. Common musculoskeletal adverse effects of oral treatment with once weekly alendronate and risedronate in patients with osteoporosis and ways for their prevention. J Musculoskelet Neuronal Interact 2007; 7(2):144–148. pmid:17627083
- US Food and Drug Administration (FDA). Information for healthcare professionals: Bisphosphonates (marketed as Actonel, Actonel+Ca, Aredia, Boniva, Didronel, Fosamax, Fosamax+D, Reclast, Skelid, and Zometa). https://wayback.archive-it.org/7993/20170722190245/https://www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/ucm124165.htm. Accessed August 1, 2018.
- Dicuonzo G, Vincenzi B, Santini D, et al. Fever after zoledronic acid administration is due to increase in TNF-alpha and IL-6. J Interferon Cytokine Res 2003; 23(11):649–654. doi:10.1089/107999003322558782
- Olson K, Van Poznak C. Significance and impact of bisphosphonate-induced acute phase responses. J Oncol Pharm Pract 2007; 13(4):223–229. doi:10.1177/1078155207080806
- Roelofs AJ, Jauhiainen M, Monkkonen H, Rogers MJ, Monkkonen J, Thompson K. Peripheral blood monocytes are responsible for gammadelta T cell activation induced by zoledronic acid through accumulation of IPP/DMAPP. Br J Haematol 2009; 144(2):245–250. doi:10.1111/j.1365-2141.2008.07435.x
- Wark JD, Bensen W, Recknor C, et al. Treatment with acetaminophen/paracetamol or ibuprofen alleviates post-dose symptoms related to intravenous infusion with zoledronic acid 5 mg. Osteoporos Int 2012; 23(2):503–512. doi:10.1007/s00198-011-1563-8
- Silverman SL, Kriegman A, Goncalves J, Kianifard F, Carlson T, Leary E. Effect of acetaminophen and fluvastatin on post-dose symptoms following infusion of zoledronic acid. Osteoporos Int 2011; 22(8):2337–2345. doi:10.1007/s00198-010-1448-2
- Catalano A, Morabito N, Atteritano M, Basile G, Cucinotta D, Lasco A. Vitamin D reduces musculoskeletal pain after infusion of zoledronic acid for postmenopausal osteoporosis. Calcif Tissue Int 2012; 90(4):279–285. doi:10.1007/s00223-012-9577-6
- Thompson K, Keech F, McLernon DJ, et al. Fluvastatin does not prevent the acute-phase response to intravenous zoledronic acid in post-menopausal women. Bone 2011; 49(1):140–145. doi:10.1016/j.bone.2010.10.177
- Billington EO, Horne A, Gamble GD, Maslowski K, House M, Reid IR. Effect of single-dose dexamethasone on acute phase response following zoledronic aacid: a randomized controlled trial. Osteoporos Int 2017; 28(6):1867–1874. doi:10.1007/s00198-017-3960-0
- Ugurlar M. Alendronate- and risedronate-induced acute polyarthritis. Osteoporos Int 2016; 27(11):3383–3385. doi:10.1007/s00198-016-3695-3
- Wysowski DK, Chang JT. Alendronate and risedronate: reports of severe bone, joint, and muscle pain. Arch Intern Med 2005; 165(3):346–347.
- Gwynne Jones DP, Savage RL, Highton J. Alendronate-induced synovitis. J Rheumatol 2008; 35(3):537–538. pmid:18203307
- Gokkus K, Yazicioglu G, Sagtas E, Uyan A, Aydin AT. Possible alendronate-induced polyarticular synovitis. J Postgrad Med 2016; 62(2):126–128. doi:10.4103/0022-3859.174160
- White SL, Jacob A, Gregson C, Bhalla A. Severe polyarthritis secondary to zolendronic acid: a case report and literature review. Clin Cases Miner Bone Metab 2015 ; 12(1):69–74. doi:10.11138/ccmbm/2015.12.1.069
Bisphosphonates, especially intravenous zoledronic acid, often cause influenza-like symptoms such as severe musculoskeletal pain, fever, headache, malaise, and fatigue, sometimes accompanied by nausea, vomiting, and diarrhea. As many as 30% of patients experience these symptoms, which are usually transient, last up to 1 week, and, in most patients, only rarely recur with subsequent infusions.
It is essential to counsel and reassure patients about these reactions before starting treatment. We recommend that patients take acetaminophen before intravenous bisphosphonate infusions, and if an acute-phase reaction occurs, we provide adequate supportive care with acetaminophen or nonsteroidal anti-inflammatory drugs (NSAIDs). If patients report severe musculoskeletal pain, then consider discontinuing the bisphosphonate treatment.
INFLUENZA-LIKE SYMPTOMS
The acute-phase reaction is a transient inflammatory state characterized by influenza-like symptoms such as fever, myalgia, joint pain, and nausea. It often occurs within the first few days after initial exposure to a bisphosphonate. Patients tend to rate the symptoms as mild to moderate. Symptoms may recur with subsequent doses; however, the incidence rate decreases substantially with each subsequent dose.
With intravenous bisphosphonates
Reid et al1 analyzed data from a trial in which 7,765 postmenopausal women with osteoporosis were randomized to receive intravenous zoledronic acid or placebo; 42.4% of the zoledronic acid group experienced symptoms that could be attributed to an acute-phase reaction after the first infusion, compared with 11.7% of the placebo group (P < .0001). Statistically significant differences (P < .0001) in symptoms between the groups included the following:
- Fever 20.3% vs 2.5%
- Musculoskeletal symptoms 19.9% vs 4.7%
- Gastrointestinal symptoms 7.8% vs 2.1%.
Of the patients describing musculoskeletal symptoms after receiving zoledronic acid, most (79%) described them as generalized pain or discomfort, while about 25% said they were regional, usually localized to the back, neck, chest, and shoulders, 5% described joint stiffness, and 2.5% reported joint swelling.1
In this and other studies,1–3 acute-phase reactions most commonly occurred within the first few days after the infusion and were rated as mild to moderate in 90% of cases.1,2 Patients who reported an acute-phase reaction were not more likely to opt out of subsequent infusions. The authors postulated that this was most likely because acute-phase reactions were mild and transient, and most resolved within 1 week.1 The incidence decreased with each subsequent infusion of zoledronic acid1–3; rates of the acute-phase reaction at years 1, 2, and 3 were 30%, 7%, and 3%, respectively.1
With oral bisphosphonates
The acute-phase reaction is less common with oral bisphosphonates (occurring in 5.6% of patients in a retrospective study4) and is usually less severe.4,5
AMINOBISPHOSPHONATES INDUCE INFLAMMATORY CYTOKINES
Musculoskeletal pain related to the acute-phase reaction is thought to be due to transient release of inflammatory cytokines such as interleukin 6, interferon gamma, and tumor necrosis factor alpha from macrophages, monocytes, and gamma-delta T cells.6
Bisphosphonates are taken up by osteoclasts and inhibit their function. But bisphosphonates are not all the same: they can be divided into aminobisphosphonates (eg, alendronate, pamidronate, risedronate, zoledronic acid) and nonaminobisphosphonates (eg, clodronate, etidronate).
Inside the osteoclasts, aminobisphosphonates inhibit farnesyl diphosphate synthase in the mevalonate pathways, thus disrupting cell signaling and leading to apoptosis.7 However, inhibition of farnesyl diphosphate synthase also increases intracellular levels of isopentyl pyrophosphate, which induces T-cell activation; this is thought to result in release of inflammatory cytokines, leading to the acute-phase reaction.7,8
In contrast, nonaminobisphosphonates such as clodronate and etidronate, after being internalized, are metabolized into nonhydrolyzable adenosine triphosphate, which is toxic to the osteoclast. The acute-phase reaction or influenza-like illness is unique to aminobisphosphonates; nonaminobisphosphonates have not been associated with an acute-phase reaction.
TRIALS OF PREVENTIVE TREATMENT
With NSAIDs, acetaminophen
Wark et al9 randomized 481 postmenopausal women who had osteopenia but who had never received bisphosphonates to 4 treatment groups:
- Zoledronic acid 5 mg intravenously plus acetaminophen 1,000 mg every 6 hours for 3 days
- Zoledronic acid 5 mg intravenously plus ibuprofen 400 mg every 6 hours for 3 days
- Zoledronic acid 5 mg intravenously plus 2 placebo capsules every 6 hours for 3 days
- Placebo infusion plus 2 placebo capsules every 6 hours for 3 days.
Patients were assessed for fever and worsening symptoms over 3 days after the infusion. The group that got zoledronic acid infusion and placebo capsules had the highest rates of fever (64%) and worsening symptoms (76%); acetaminophen and ibuprofen reduced these rates to an approximately equal extent, to 37% for fever and 46% (acetaminophen) and 49% (ibuprofen) for worsening symptoms. The group that received placebo bisphosphonate infusions had the lowest rates of fever (11%) and worsening symptoms (17%).
Silverman et al10 found that acetaminophen 650 mg taken 45 minutes before intravenous zoledronic acid infusion and continued every 6 hours for 3 days led to an absolute risk reduction of 21% in the incidence of fever or use of rescue medication compared with placebo.
Trials of other agents
In a study of 60 women,11 30 received an oral bolus of cholecalciferol 300,000 IU 5 days before zoledronic acid 5 mg infusion plus daily calcium 1,000 mg and vitamin D 800 IU, and 30 received a placebo pill 5 days before the same infusion and vitamin regimen as the other group. The preinfusion oral bolus did not decrease the incidence of acute-phase reactions, although it led to a small decrease in the severity of musculoskeletal pain (the median pain score was reduced from 2 to 1 on a scale of 0 to 10).
Other interventions such as fluvastatin and oral dexamethasone given before intravenous zoledronic acid did not reduce the severity or incidence of the acute-phase reaction.10,12,13
OUR APPROACH
Before starting bisphosphonate therapy, patients should be counseled about the possibility of acute musculoskeletal pain and other symptoms of the acute-phase reaction.
For intravenous bisphosphonates
We advise all patients scheduled to receive intravenous bisphosphonates to take acetaminophen 650 to 1,000 mg once on the morning of the infusion. We prefer acetaminophen over NSAIDs for prophylaxis to avoid the gastric mucosal and renal toxicity more common with NSAIDs, especially in the elderly.
If the patient has a history of acute musculoskeletal pain or other symptoms of an acute-phase reaction after bisphosphonate infusion, we advise a more aggressive approach to prophylaxis: acetaminophen 650 mg 1 hour before the infusion, then every 6 hours for up to 3 days. This approach, with acetaminophen or NSAIDs, has been shown in large randomized controlled trials to reduce the incidence and severity of the acute-phase reaction.
If an acute-phase reaction occurs, we inform patients that the likelihood decreases and is quite low with subsequent doses. We provide correct and honest information, so that patients who experience an acute-phase reaction can make an informed decision about continuing bisphosphonate treatment or switching to another treatment. If the patient decides to continue with intravenous bisphosphonate treatment, we recommend more-aggressive prophylaxis with acetaminophen or NSAIDs with subsequent infusions.
For oral bisphosphonates
We do not prescribe prophylactic treatment with acetaminophen or NSAIDs with oral bisphosphonates, but we do advise patients to take acetaminophen or NSAIDs as needed for mild to moderate musculoskeletal pain, should this occur.
We try to continue treatment in mild to moderate cases, while monitoring the patient closely to see if the musculoskeletal pain resolves within 1 to 2 weeks.
If the pain is severe or does not resolve in 1 to 2 weeks, we offer switching to another drug class. Since musculoskeletal pain with oral bisphosphonates does not represent an allergic reaction, we have switched patients from oral to intravenous bisphosphonates without recurrence of musculoskeletal pain.
SEVERE MUSCULOSKELETAL PAIN BEYOND THE ACUTE PHASE
Severe musculoskeletal pain that may not be related to the acute-phase reaction, although rare, has been reported.5,14 From 1995, when alendronate was approved for osteoporosis, through 2002, the US Food and Drug Administration received reports of severe musculoskeletal pain in 117 patients.15
This severe musculoskeletal pain related to bisphosphonate use remains poorly characterized. It has been reported to occur days or months (median time 14 days, range same day to 52 months) after starting bisphosphonate therapy and to resolve only if the bisphosphonate is stopped.5,15 It differs from typical acute-phase reactions, which tend to occur with the initial dose (intravenous or oral) and resolve within several days. There are case reports of polyarthritis with synovitis that recurred with each bisphosphonate dose (oral or intravenous) and led to discontinuation of the bisphosphonate.14,16–18
Clinicians need to be aware of the possibility of severe musculoskeletal pain and consider stopping bisphosphonate treatment in these cases. Besides discontinuation, acetaminophen, NSAIDs, and, in rare cases, glucocorticoids or short-term opiate therapy may be used for symptom control. In patients with a severe or persistent acute-phase reaction or musculoskeletal pain, discontinuation of bisphosphonates is warranted.
Bisphosphonates, especially intravenous zoledronic acid, often cause influenza-like symptoms such as severe musculoskeletal pain, fever, headache, malaise, and fatigue, sometimes accompanied by nausea, vomiting, and diarrhea. As many as 30% of patients experience these symptoms, which are usually transient, last up to 1 week, and, in most patients, only rarely recur with subsequent infusions.
It is essential to counsel and reassure patients about these reactions before starting treatment. We recommend that patients take acetaminophen before intravenous bisphosphonate infusions, and if an acute-phase reaction occurs, we provide adequate supportive care with acetaminophen or nonsteroidal anti-inflammatory drugs (NSAIDs). If patients report severe musculoskeletal pain, then consider discontinuing the bisphosphonate treatment.
INFLUENZA-LIKE SYMPTOMS
The acute-phase reaction is a transient inflammatory state characterized by influenza-like symptoms such as fever, myalgia, joint pain, and nausea. It often occurs within the first few days after initial exposure to a bisphosphonate. Patients tend to rate the symptoms as mild to moderate. Symptoms may recur with subsequent doses; however, the incidence rate decreases substantially with each subsequent dose.
With intravenous bisphosphonates
Reid et al1 analyzed data from a trial in which 7,765 postmenopausal women with osteoporosis were randomized to receive intravenous zoledronic acid or placebo; 42.4% of the zoledronic acid group experienced symptoms that could be attributed to an acute-phase reaction after the first infusion, compared with 11.7% of the placebo group (P < .0001). Statistically significant differences (P < .0001) in symptoms between the groups included the following:
- Fever 20.3% vs 2.5%
- Musculoskeletal symptoms 19.9% vs 4.7%
- Gastrointestinal symptoms 7.8% vs 2.1%.
Of the patients describing musculoskeletal symptoms after receiving zoledronic acid, most (79%) described them as generalized pain or discomfort, while about 25% said they were regional, usually localized to the back, neck, chest, and shoulders, 5% described joint stiffness, and 2.5% reported joint swelling.1
In this and other studies,1–3 acute-phase reactions most commonly occurred within the first few days after the infusion and were rated as mild to moderate in 90% of cases.1,2 Patients who reported an acute-phase reaction were not more likely to opt out of subsequent infusions. The authors postulated that this was most likely because acute-phase reactions were mild and transient, and most resolved within 1 week.1 The incidence decreased with each subsequent infusion of zoledronic acid1–3; rates of the acute-phase reaction at years 1, 2, and 3 were 30%, 7%, and 3%, respectively.1
With oral bisphosphonates
The acute-phase reaction is less common with oral bisphosphonates (occurring in 5.6% of patients in a retrospective study4) and is usually less severe.4,5
AMINOBISPHOSPHONATES INDUCE INFLAMMATORY CYTOKINES
Musculoskeletal pain related to the acute-phase reaction is thought to be due to transient release of inflammatory cytokines such as interleukin 6, interferon gamma, and tumor necrosis factor alpha from macrophages, monocytes, and gamma-delta T cells.6
Bisphosphonates are taken up by osteoclasts and inhibit their function. But bisphosphonates are not all the same: they can be divided into aminobisphosphonates (eg, alendronate, pamidronate, risedronate, zoledronic acid) and nonaminobisphosphonates (eg, clodronate, etidronate).
Inside the osteoclasts, aminobisphosphonates inhibit farnesyl diphosphate synthase in the mevalonate pathways, thus disrupting cell signaling and leading to apoptosis.7 However, inhibition of farnesyl diphosphate synthase also increases intracellular levels of isopentyl pyrophosphate, which induces T-cell activation; this is thought to result in release of inflammatory cytokines, leading to the acute-phase reaction.7,8
In contrast, nonaminobisphosphonates such as clodronate and etidronate, after being internalized, are metabolized into nonhydrolyzable adenosine triphosphate, which is toxic to the osteoclast. The acute-phase reaction or influenza-like illness is unique to aminobisphosphonates; nonaminobisphosphonates have not been associated with an acute-phase reaction.
TRIALS OF PREVENTIVE TREATMENT
With NSAIDs, acetaminophen
Wark et al9 randomized 481 postmenopausal women who had osteopenia but who had never received bisphosphonates to 4 treatment groups:
- Zoledronic acid 5 mg intravenously plus acetaminophen 1,000 mg every 6 hours for 3 days
- Zoledronic acid 5 mg intravenously plus ibuprofen 400 mg every 6 hours for 3 days
- Zoledronic acid 5 mg intravenously plus 2 placebo capsules every 6 hours for 3 days
- Placebo infusion plus 2 placebo capsules every 6 hours for 3 days.
Patients were assessed for fever and worsening symptoms over 3 days after the infusion. The group that got zoledronic acid infusion and placebo capsules had the highest rates of fever (64%) and worsening symptoms (76%); acetaminophen and ibuprofen reduced these rates to an approximately equal extent, to 37% for fever and 46% (acetaminophen) and 49% (ibuprofen) for worsening symptoms. The group that received placebo bisphosphonate infusions had the lowest rates of fever (11%) and worsening symptoms (17%).
Silverman et al10 found that acetaminophen 650 mg taken 45 minutes before intravenous zoledronic acid infusion and continued every 6 hours for 3 days led to an absolute risk reduction of 21% in the incidence of fever or use of rescue medication compared with placebo.
Trials of other agents
In a study of 60 women,11 30 received an oral bolus of cholecalciferol 300,000 IU 5 days before zoledronic acid 5 mg infusion plus daily calcium 1,000 mg and vitamin D 800 IU, and 30 received a placebo pill 5 days before the same infusion and vitamin regimen as the other group. The preinfusion oral bolus did not decrease the incidence of acute-phase reactions, although it led to a small decrease in the severity of musculoskeletal pain (the median pain score was reduced from 2 to 1 on a scale of 0 to 10).
Other interventions such as fluvastatin and oral dexamethasone given before intravenous zoledronic acid did not reduce the severity or incidence of the acute-phase reaction.10,12,13
OUR APPROACH
Before starting bisphosphonate therapy, patients should be counseled about the possibility of acute musculoskeletal pain and other symptoms of the acute-phase reaction.
For intravenous bisphosphonates
We advise all patients scheduled to receive intravenous bisphosphonates to take acetaminophen 650 to 1,000 mg once on the morning of the infusion. We prefer acetaminophen over NSAIDs for prophylaxis to avoid the gastric mucosal and renal toxicity more common with NSAIDs, especially in the elderly.
If the patient has a history of acute musculoskeletal pain or other symptoms of an acute-phase reaction after bisphosphonate infusion, we advise a more aggressive approach to prophylaxis: acetaminophen 650 mg 1 hour before the infusion, then every 6 hours for up to 3 days. This approach, with acetaminophen or NSAIDs, has been shown in large randomized controlled trials to reduce the incidence and severity of the acute-phase reaction.
If an acute-phase reaction occurs, we inform patients that the likelihood decreases and is quite low with subsequent doses. We provide correct and honest information, so that patients who experience an acute-phase reaction can make an informed decision about continuing bisphosphonate treatment or switching to another treatment. If the patient decides to continue with intravenous bisphosphonate treatment, we recommend more-aggressive prophylaxis with acetaminophen or NSAIDs with subsequent infusions.
For oral bisphosphonates
We do not prescribe prophylactic treatment with acetaminophen or NSAIDs with oral bisphosphonates, but we do advise patients to take acetaminophen or NSAIDs as needed for mild to moderate musculoskeletal pain, should this occur.
We try to continue treatment in mild to moderate cases, while monitoring the patient closely to see if the musculoskeletal pain resolves within 1 to 2 weeks.
If the pain is severe or does not resolve in 1 to 2 weeks, we offer switching to another drug class. Since musculoskeletal pain with oral bisphosphonates does not represent an allergic reaction, we have switched patients from oral to intravenous bisphosphonates without recurrence of musculoskeletal pain.
SEVERE MUSCULOSKELETAL PAIN BEYOND THE ACUTE PHASE
Severe musculoskeletal pain that may not be related to the acute-phase reaction, although rare, has been reported.5,14 From 1995, when alendronate was approved for osteoporosis, through 2002, the US Food and Drug Administration received reports of severe musculoskeletal pain in 117 patients.15
This severe musculoskeletal pain related to bisphosphonate use remains poorly characterized. It has been reported to occur days or months (median time 14 days, range same day to 52 months) after starting bisphosphonate therapy and to resolve only if the bisphosphonate is stopped.5,15 It differs from typical acute-phase reactions, which tend to occur with the initial dose (intravenous or oral) and resolve within several days. There are case reports of polyarthritis with synovitis that recurred with each bisphosphonate dose (oral or intravenous) and led to discontinuation of the bisphosphonate.14,16–18
Clinicians need to be aware of the possibility of severe musculoskeletal pain and consider stopping bisphosphonate treatment in these cases. Besides discontinuation, acetaminophen, NSAIDs, and, in rare cases, glucocorticoids or short-term opiate therapy may be used for symptom control. In patients with a severe or persistent acute-phase reaction or musculoskeletal pain, discontinuation of bisphosphonates is warranted.
- Reid IR, Gamble GD, Mesenbrink P, Lakatos P, Black DM. Characterization of and risk factors for the acute-phase response after zoledronic acid. J Clin Endocrinol Metab 2010; 95(9):4380–4387. doi:10.1210/jc.2010-0597
- Black DM, Delmas PD, Eastell R, et al; HORIZON Pivotal Fracture Trial. Once-yearly zoledronic acid for treatment of postmenopausal osteoporosis. N Engl J Med 2007; 356(18):1809–1822. doi:10.1056/NEJMoa067312
- Lyles KW, Colon-Emeric CS, Magaziner JS, et al; HORIZON Recurrent Fracture Trial. Zoledronic acid and clinical fractures and mortality after hip fracture. N Engl J Med 2007; 357(18):1799–1809. doi:10.1056/NEJMoa074941
- Bock O, Boerst H, Thomasius FE, et al. Common musculoskeletal adverse effects of oral treatment with once weekly alendronate and risedronate in patients with osteoporosis and ways for their prevention. J Musculoskelet Neuronal Interact 2007; 7(2):144–148. pmid:17627083
- US Food and Drug Administration (FDA). Information for healthcare professionals: Bisphosphonates (marketed as Actonel, Actonel+Ca, Aredia, Boniva, Didronel, Fosamax, Fosamax+D, Reclast, Skelid, and Zometa). https://wayback.archive-it.org/7993/20170722190245/https://www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/ucm124165.htm. Accessed August 1, 2018.
- Dicuonzo G, Vincenzi B, Santini D, et al. Fever after zoledronic acid administration is due to increase in TNF-alpha and IL-6. J Interferon Cytokine Res 2003; 23(11):649–654. doi:10.1089/107999003322558782
- Olson K, Van Poznak C. Significance and impact of bisphosphonate-induced acute phase responses. J Oncol Pharm Pract 2007; 13(4):223–229. doi:10.1177/1078155207080806
- Roelofs AJ, Jauhiainen M, Monkkonen H, Rogers MJ, Monkkonen J, Thompson K. Peripheral blood monocytes are responsible for gammadelta T cell activation induced by zoledronic acid through accumulation of IPP/DMAPP. Br J Haematol 2009; 144(2):245–250. doi:10.1111/j.1365-2141.2008.07435.x
- Wark JD, Bensen W, Recknor C, et al. Treatment with acetaminophen/paracetamol or ibuprofen alleviates post-dose symptoms related to intravenous infusion with zoledronic acid 5 mg. Osteoporos Int 2012; 23(2):503–512. doi:10.1007/s00198-011-1563-8
- Silverman SL, Kriegman A, Goncalves J, Kianifard F, Carlson T, Leary E. Effect of acetaminophen and fluvastatin on post-dose symptoms following infusion of zoledronic acid. Osteoporos Int 2011; 22(8):2337–2345. doi:10.1007/s00198-010-1448-2
- Catalano A, Morabito N, Atteritano M, Basile G, Cucinotta D, Lasco A. Vitamin D reduces musculoskeletal pain after infusion of zoledronic acid for postmenopausal osteoporosis. Calcif Tissue Int 2012; 90(4):279–285. doi:10.1007/s00223-012-9577-6
- Thompson K, Keech F, McLernon DJ, et al. Fluvastatin does not prevent the acute-phase response to intravenous zoledronic acid in post-menopausal women. Bone 2011; 49(1):140–145. doi:10.1016/j.bone.2010.10.177
- Billington EO, Horne A, Gamble GD, Maslowski K, House M, Reid IR. Effect of single-dose dexamethasone on acute phase response following zoledronic aacid: a randomized controlled trial. Osteoporos Int 2017; 28(6):1867–1874. doi:10.1007/s00198-017-3960-0
- Ugurlar M. Alendronate- and risedronate-induced acute polyarthritis. Osteoporos Int 2016; 27(11):3383–3385. doi:10.1007/s00198-016-3695-3
- Wysowski DK, Chang JT. Alendronate and risedronate: reports of severe bone, joint, and muscle pain. Arch Intern Med 2005; 165(3):346–347.
- Gwynne Jones DP, Savage RL, Highton J. Alendronate-induced synovitis. J Rheumatol 2008; 35(3):537–538. pmid:18203307
- Gokkus K, Yazicioglu G, Sagtas E, Uyan A, Aydin AT. Possible alendronate-induced polyarticular synovitis. J Postgrad Med 2016; 62(2):126–128. doi:10.4103/0022-3859.174160
- White SL, Jacob A, Gregson C, Bhalla A. Severe polyarthritis secondary to zolendronic acid: a case report and literature review. Clin Cases Miner Bone Metab 2015 ; 12(1):69–74. doi:10.11138/ccmbm/2015.12.1.069
- Reid IR, Gamble GD, Mesenbrink P, Lakatos P, Black DM. Characterization of and risk factors for the acute-phase response after zoledronic acid. J Clin Endocrinol Metab 2010; 95(9):4380–4387. doi:10.1210/jc.2010-0597
- Black DM, Delmas PD, Eastell R, et al; HORIZON Pivotal Fracture Trial. Once-yearly zoledronic acid for treatment of postmenopausal osteoporosis. N Engl J Med 2007; 356(18):1809–1822. doi:10.1056/NEJMoa067312
- Lyles KW, Colon-Emeric CS, Magaziner JS, et al; HORIZON Recurrent Fracture Trial. Zoledronic acid and clinical fractures and mortality after hip fracture. N Engl J Med 2007; 357(18):1799–1809. doi:10.1056/NEJMoa074941
- Bock O, Boerst H, Thomasius FE, et al. Common musculoskeletal adverse effects of oral treatment with once weekly alendronate and risedronate in patients with osteoporosis and ways for their prevention. J Musculoskelet Neuronal Interact 2007; 7(2):144–148. pmid:17627083
- US Food and Drug Administration (FDA). Information for healthcare professionals: Bisphosphonates (marketed as Actonel, Actonel+Ca, Aredia, Boniva, Didronel, Fosamax, Fosamax+D, Reclast, Skelid, and Zometa). https://wayback.archive-it.org/7993/20170722190245/https://www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/ucm124165.htm. Accessed August 1, 2018.
- Dicuonzo G, Vincenzi B, Santini D, et al. Fever after zoledronic acid administration is due to increase in TNF-alpha and IL-6. J Interferon Cytokine Res 2003; 23(11):649–654. doi:10.1089/107999003322558782
- Olson K, Van Poznak C. Significance and impact of bisphosphonate-induced acute phase responses. J Oncol Pharm Pract 2007; 13(4):223–229. doi:10.1177/1078155207080806
- Roelofs AJ, Jauhiainen M, Monkkonen H, Rogers MJ, Monkkonen J, Thompson K. Peripheral blood monocytes are responsible for gammadelta T cell activation induced by zoledronic acid through accumulation of IPP/DMAPP. Br J Haematol 2009; 144(2):245–250. doi:10.1111/j.1365-2141.2008.07435.x
- Wark JD, Bensen W, Recknor C, et al. Treatment with acetaminophen/paracetamol or ibuprofen alleviates post-dose symptoms related to intravenous infusion with zoledronic acid 5 mg. Osteoporos Int 2012; 23(2):503–512. doi:10.1007/s00198-011-1563-8
- Silverman SL, Kriegman A, Goncalves J, Kianifard F, Carlson T, Leary E. Effect of acetaminophen and fluvastatin on post-dose symptoms following infusion of zoledronic acid. Osteoporos Int 2011; 22(8):2337–2345. doi:10.1007/s00198-010-1448-2
- Catalano A, Morabito N, Atteritano M, Basile G, Cucinotta D, Lasco A. Vitamin D reduces musculoskeletal pain after infusion of zoledronic acid for postmenopausal osteoporosis. Calcif Tissue Int 2012; 90(4):279–285. doi:10.1007/s00223-012-9577-6
- Thompson K, Keech F, McLernon DJ, et al. Fluvastatin does not prevent the acute-phase response to intravenous zoledronic acid in post-menopausal women. Bone 2011; 49(1):140–145. doi:10.1016/j.bone.2010.10.177
- Billington EO, Horne A, Gamble GD, Maslowski K, House M, Reid IR. Effect of single-dose dexamethasone on acute phase response following zoledronic aacid: a randomized controlled trial. Osteoporos Int 2017; 28(6):1867–1874. doi:10.1007/s00198-017-3960-0
- Ugurlar M. Alendronate- and risedronate-induced acute polyarthritis. Osteoporos Int 2016; 27(11):3383–3385. doi:10.1007/s00198-016-3695-3
- Wysowski DK, Chang JT. Alendronate and risedronate: reports of severe bone, joint, and muscle pain. Arch Intern Med 2005; 165(3):346–347.
- Gwynne Jones DP, Savage RL, Highton J. Alendronate-induced synovitis. J Rheumatol 2008; 35(3):537–538. pmid:18203307
- Gokkus K, Yazicioglu G, Sagtas E, Uyan A, Aydin AT. Possible alendronate-induced polyarticular synovitis. J Postgrad Med 2016; 62(2):126–128. doi:10.4103/0022-3859.174160
- White SL, Jacob A, Gregson C, Bhalla A. Severe polyarthritis secondary to zolendronic acid: a case report and literature review. Clin Cases Miner Bone Metab 2015 ; 12(1):69–74. doi:10.11138/ccmbm/2015.12.1.069
How should we diagnose and manage checkpoint inhibitor-associated colitis?
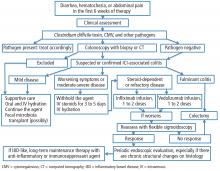
If a patient experiences diarrhea, hematochezia, or abdominal pain within the first 6 weeks of therapy with one of the anticancer drugs known as immune checkpoint inhibitors (ICIs), the first step is to rule out infection, especially with Clostridium difficile. The next step is colonosocopy with biopsy or computed tomography.
Patients with mild ICI-associated colitis may need only supportive care, and the ICI can be continued. In moderate or severe cases, the agent may need to be stopped and corticosteroids and other colitis-targeted agents may be needed. Figure 1 shows our algorithm for diagnosing and treating ICI-associated colitis.
POWERFUL ANTICANCER DRUGS
ICIs are monoclonal antibodies used in treating metastatic melanoma, non-small-cell lung cancer, metastatic prostate cancer, Hodgkin lymphoma, renal cell carcinoma, and other advanced malignancies.1,2 They act by binding to and blocking proteins on T cells, antigen-presenting cells, and tumor cells that keep immune responses in check and prevent T cells from killing cancer cells.1 For example:
- Ipilimumab blocks cytotoxic T lymphocyte-associated antigen 4
- Nivolumab and pembrolizumab block programmed cell death protein 1
- Atezolizumab blocks programmed death ligand 1.1
With these proteins blocked, T cells can do their job, often producing dramatic regression of cancer. However, ICIs can cause a range of immune-related adverse effects, including endocrine and cutaneous toxicities, iridocyclitis, lymphadenopathy, neuropathy, nephritis, immune-mediated pneumonitis, pancreatitis, hepatitis, and colitis.3
ICI-ASSOCIATED COLITIS IS COMMON
ICI-associated colitis is common; it is estimated to affect about 30% of patients receiving ipilimumab, for example.4 Clinical presentations range from watery bowel movements, blood or mucus in the stool, abdominal cramping, and flatulence to ileus, colectasia, intestinal perforation, and even death.5
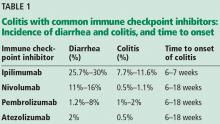
The incidence appears to increase with the dosage and duration of ICI therapy. The onset of colitis typically occurs 6 to 7 weeks after starting ipilimumab,6 and 6 to 18 weeks after starting nivolumab or pembrolizumab.7Table 1 lists the incidence of diarrhea and colitis and time of onset to colitis with common ICIs. However, colitis, like other immune-related adverse events, can occur at any point, even after ICI therapy has been discontinued.8
It is best to detect side effects of ICIs promptly, as acute inflammation can progress to chronic inflammation within 1 month of onset.9 We believe that early intervention and close monitoring may prevent complications and the need for long-term immunosuppressive treatment.
Patients, family members, and caregivers should be informed of possible gastrointestinal along with systemic side effects. Severe gastrointestinal symptoms such as increased stool frequency and change in stool consistency should trigger appropriate investigation and the withholding of ICI therapy.
COLITIS IS A SPECTRUM
The colon appears to be the gastrointestinal organ most affected by ICIs. Of patients with intestinal side effects, including diarrhea, only some develop colitis. The severity of ICI-associated colitis ranges from mild bowel illness to fulminant colitis.
Hodi et al,10 in a randomized trial in which 511 patients with melanoma received ipilimumab, reported that approximately 30% had mild diarrhea, while fewer than 10% had severe diarrhea, fever, ileus, or peritoneal signs. Five patients (1%) developed intestinal perforation, 4 (0.8%) died of complications, and 26 (5%) required hospitalization for severe enterocolitis.
The pathophysiology of ICI-mediated colitis is unclear. Most cases are diagnosed clinically.
Colitis is graded based on the Montreal classification system11:
Mild colitis is defined as passage of fewer than 4 stools per day (with or without blood) over baseline and absence of any systemic illness.
Moderate is passage of more than 4 stools per day but with minimal signs of systemic toxicity.
Severe is defined as passage of at least 6 stools per day, heart rate at least 90 beats per minute, temperature at least 37.5°C (99.5°F), hemoglobin less than 10.5 g/dL, and erythrocyte sedimentation rate at least 30 mm/h.11
RULE OUT INFECTION
If symptoms such as diarrhea or abdominal pain arise within 6 weeks of starting ICI therapy, then we should check for an infectious cause. The differential diagnosis of suspected ICI-associated colitis includes infections with C difficile, cytomegalovirus, opportunistic organisms, and other bacteria and viruses. ICI-induced celiac disease and immune hyperthyroidism should also be ruled out.4
CONSIDER COLONOSCOPY AND BIOPSY
Once infection is ruled out, colonoscopy should be considered if symptoms persist or are severe. Colonoscopy with biopsy remains the gold standard for diagnosis, and it is also helpful in assessing severity of mucosal inflammation and monitoring response to medical treatment.
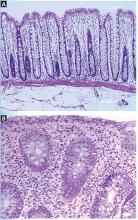
Table 2 lists common endoscopic and histologic features of ICI-mediated colitis; however, none of them is specific for this disease.
Common endoscopic features are loss of vascular pattern, edema, friability, spontaneous bleeding, and deep ulcerations.12 A recent study suggested that colonic ulcerations predict a steroid-refractory course in patients with immune-mediated colitis.4
Histologically, ICI-associated colitis is characterized by both acute and chronic changes, including an increased number of neutrophils and lymphocytes in the epithelium and lamina propria, erosions, ulcers, crypt abscess, crypt apoptosis, crypt distortion, and even noncaseating granulomas.13 However, transmural disease is rare. Figure 2 compares the histopathologic features of ICI-associated colitis and a normal colon.
COMPUTED TOMOGRAPHY CAN BE USEFUL
Computed tomography (CT) can also be useful for the diagnosis and measurement of severity.
Garcia-Neuer et al14 analyzed 303 patients with advanced melanoma who developed gastrointestinal symptoms while being treated with ipilimumab. Ninety-nine (33%) of them reported diarrhea during therapy, of whom 34 underwent both CT and colonoscopy with biopsy. CT was highly predictive of colitis on biopsy, with a positive predictive value of 96% and a negative likelihood ratio of 0.2.14
TREATMENT
Supportive care may be enough when treating mild ICI-related colitis. This can include oral and intravenous hydration4 and an antidiarrheal drug such as loperamide in a low dose.
Corticosteroids. For moderate ICI-associated colitis with stool frequency of 4 or more per day, patients should be started on an oral corticosteroid such as prednisone 0.5 to 1 mg/kg per day. If symptoms do not improve within 72 hours of starting an oral corticosteroid, the patient should be admitted to the hospital for observation and escalation to higher doses or possibly intravenous corticosteroids.
Infliximab has been used in severe and steroid-refractory cases,13 although there has been concern about using anti-tumor necrosis factor (TNF) agents such as this in patients with malignancies, especially melanoma. Since melanoma can be very aggressive and anti-TNF agents may promote it, it is prudent to try not to use this class of agents.
Other biologic agents such as vedolizumab, a gut-specific anti-integrin agent, are safer, have theoretic advantages over anti-TNF agents, and can be considered in patients with steroid-dependent or steroid-refractory ICI-associated enterocolitis. A recent study suggested that 2 to 4 infusions of vedolizumab are adequate to achieve steroid-free remission.15 Results from 6 clinical trials of vedolizumab in 2,830 patients with Crohn disease or ulcerative colitis did not show any increased risk of serious infections or malignancies over placebo.16,17 A drawback is its slow onset of action.
Surgery is an option for patients with severe colitis refractory to intravenous corticosteroids or biological agents, as severe colitis carries a risk of significant morbidity and even death. The incidence of bowel perforation leading to colectomy or death in patients receiving ICI therapy is 0.5% to 1%.18,19
Fecal microbiota transplant was associated with mucosal healing after 1 month in a case report of ICI-associated colitis.9
Follow-up. In most patients, symptoms resolve with discontinuation of the ICI and brief use of corticosteroids or biological agents. Patients with recurrent or persistent symptoms while on long-term ICI therapy may need periodic endoscopic evaluation, especially if there are chronic structural changes on histologic study.
If patients have recurrent or persistent symptoms along with chronic inflammatory structural changes on histology, a sign of an inflammatory bowel diseaselike condition, long-term maintenance therapy with an anti-inflammatory or immunosuppressant agent may be considered. However, there is no consensus on the treatment of this condition. It can be treated in the same way as classic inflammatory bowel disease in the setting of concurrent or prior history of malignancy, especially melanoma. Certain agents used in inflammatory bowel disease such as methotrexate and vedolizumab carry a lower risk of malignancy than anti-TNF agents and can be considered. A multidisciplinary approach that includes an oncologist, gastroenterologist, infectious disease specialist, and colorectal surgeon is imperative.
- Shih K, Arkenau HT, Infante JR. Clinical impact of checkpoint inhibitors as novel cancer therapies. Drugs 2014; 74(17):1993–2013. doi:10.1007/s40265-014-0305-6
- Mellman I, Coukos G, Dranoff G. Cancer immunotherapy comes of age. Nature 2011; 480(7378):480–489. doi:10.1038/nature10673
- Dine J, Gordon R, Shames Y, Kasler MK, Barton-Burke M. Immune checkpoint inhibitors: an innovation in immunotherapy for the treatment and management of patients with cancer. Asia Pac J Oncol Nurs 2017; 4(2):127–135. doi:10.4103/apjon.apjon_4_17
- Prieux-Klotz C, Dior M, Damotte D, et al. Immune checkpoint inhibitor-induced colitis: diagnosis and management. Target Oncol 2017; 12(3):301–308. doi:10.1007/s11523-017-0495-4
- Howell M, Lee R, Bowyer S, Fusi A, Lorigan P. Optimal management of immune-related toxicities associated with checkpoint inhibitors in lung cancer. Lung Cancer 2015; 88(2):117–123. doi:10.1016/j.lungcan.2015.02.007
- Weber JS, Kähler KC, Hauschild A. Management of immune-related adverse events and kinetics of response with ipilimumab. J Clin Oncol 2012; 30(21):2691–2697. doi:10.1200/JCO.2012.41.6750
- Eigentler TK, Hassel JC, Berking C, et al. Diagnosis, monitoring and management of immune-related adverse drug reactions of anti-PD-1 antibody therapy. Cancer Treat Rev 2016; 45:7–18. doi:10.1016/j.ctrv.2016.02.003
- Postow MA, Sidlow R, Hellmann MD. Immune-related adverse events associated with immune checkpoint blockade. N Engl J Med 2018; 378(2):158–168. doi:10.1056/NEJMra1703481
- Wang Y, DuPont H, Jiang ZD, Jenq R, Zuazua R, Shuttlesworth G. Fecal microbiota transplant for immune-checkpoint inhibitor-induced colitis in a 50 year old with bladder cancer. Gastroenterol 2018; 154(1 suppl). doi:10.1053/j.gastro.2017.11.075
- Hodi FS, O’Day SJ, McDermott DF, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med 2010; 363(8):711–723. doi:10.1056/NEJMoa1003466
- Satsangi J, Silverberg MS, Vermeire S, Colombel JF. The Montreal classification of inflammatory bowel disease: controversies, consensus, and implications. Gut 2006; 55(6):749–753. doi:10.1136/gut.2005.082909
- Rastogi P, Sultan M, Charabaty AJ, Atkins MB, Mattar MC. Ipilimumab associated colitis: an IpiColitis case series at MedStar Georgetown University Hospital. World J Gastroenterol 2015; 21(14):4373–4378. doi:10.3748/wjg.v21.i14.4373
- Pocha C, Roat J, Viskocil K. Immune-mediated colitis: important to recognize and treat. J Crohns Colitis 2014; 8(2):181–182. doi:10.1016/j.crohns.2013.09.019
- Garcia-Neuer M, Marmarelis ME, Jangi SR, et al. Diagnostic comparison of CT scans and colonoscopy for immune-related colitis in ipilimumab-treated advanced melanoma patients. Cancer Immunol Res 2017; 5(4):286–291. doi:10.1158/2326-6066.CIR-16-0302
- Bergqvist V, Hertervig E, Gedeon P, et al. Vedolizumab treatment for immune checkpoint inhibitor-induced enterocolitis. Cancer Immunol Immunother 2017; 66(5):581–592. doi:10.1007/s00262-017-1962-6
- Sandborn WJ, Feagan BG, Rutgeerts P, et al; GEMINI 2 Study Group. Vedolizumab as induction and maintenance therapy for Crohn’s disease. N Engl J Med 2013; 369(8):711–721. doi:10.1056/NEJMoa1215739
- Feagan BG, Rutgeerts P, Sands BE, et al; GEMINI 1 Study Group. Vedolizumab as induction and maintenance therapy for ulcerative colitis. N Engl J Med 2013; 369(8):699–710. doi:10.1056/NEJMoa1215734
- Kähler KC, Hauschild A. Treatment and side effect management of CTLA-4 antibody therapy in metastatic melanoma. J Dtsch Dermatol Ges 2011; 9(4):277–286. doi:10.1111/j.1610-0387.2010.07568.x
- Ibrahim RA, Berman DM, DePril V, et al. Ipilimumab safety profile: summary of findings from completed trials in advanced melanoma. J Clin Onc 2011; 29(15 suppl):8583–8583. doi:10.1200/jco.2011.29.15_suppl.8583
If a patient experiences diarrhea, hematochezia, or abdominal pain within the first 6 weeks of therapy with one of the anticancer drugs known as immune checkpoint inhibitors (ICIs), the first step is to rule out infection, especially with Clostridium difficile. The next step is colonosocopy with biopsy or computed tomography.
Patients with mild ICI-associated colitis may need only supportive care, and the ICI can be continued. In moderate or severe cases, the agent may need to be stopped and corticosteroids and other colitis-targeted agents may be needed. Figure 1 shows our algorithm for diagnosing and treating ICI-associated colitis.
POWERFUL ANTICANCER DRUGS
ICIs are monoclonal antibodies used in treating metastatic melanoma, non-small-cell lung cancer, metastatic prostate cancer, Hodgkin lymphoma, renal cell carcinoma, and other advanced malignancies.1,2 They act by binding to and blocking proteins on T cells, antigen-presenting cells, and tumor cells that keep immune responses in check and prevent T cells from killing cancer cells.1 For example:
- Ipilimumab blocks cytotoxic T lymphocyte-associated antigen 4
- Nivolumab and pembrolizumab block programmed cell death protein 1
- Atezolizumab blocks programmed death ligand 1.1
With these proteins blocked, T cells can do their job, often producing dramatic regression of cancer. However, ICIs can cause a range of immune-related adverse effects, including endocrine and cutaneous toxicities, iridocyclitis, lymphadenopathy, neuropathy, nephritis, immune-mediated pneumonitis, pancreatitis, hepatitis, and colitis.3
ICI-ASSOCIATED COLITIS IS COMMON
ICI-associated colitis is common; it is estimated to affect about 30% of patients receiving ipilimumab, for example.4 Clinical presentations range from watery bowel movements, blood or mucus in the stool, abdominal cramping, and flatulence to ileus, colectasia, intestinal perforation, and even death.5
The incidence appears to increase with the dosage and duration of ICI therapy. The onset of colitis typically occurs 6 to 7 weeks after starting ipilimumab,6 and 6 to 18 weeks after starting nivolumab or pembrolizumab.7Table 1 lists the incidence of diarrhea and colitis and time of onset to colitis with common ICIs. However, colitis, like other immune-related adverse events, can occur at any point, even after ICI therapy has been discontinued.8
It is best to detect side effects of ICIs promptly, as acute inflammation can progress to chronic inflammation within 1 month of onset.9 We believe that early intervention and close monitoring may prevent complications and the need for long-term immunosuppressive treatment.
Patients, family members, and caregivers should be informed of possible gastrointestinal along with systemic side effects. Severe gastrointestinal symptoms such as increased stool frequency and change in stool consistency should trigger appropriate investigation and the withholding of ICI therapy.
COLITIS IS A SPECTRUM
The colon appears to be the gastrointestinal organ most affected by ICIs. Of patients with intestinal side effects, including diarrhea, only some develop colitis. The severity of ICI-associated colitis ranges from mild bowel illness to fulminant colitis.
Hodi et al,10 in a randomized trial in which 511 patients with melanoma received ipilimumab, reported that approximately 30% had mild diarrhea, while fewer than 10% had severe diarrhea, fever, ileus, or peritoneal signs. Five patients (1%) developed intestinal perforation, 4 (0.8%) died of complications, and 26 (5%) required hospitalization for severe enterocolitis.
The pathophysiology of ICI-mediated colitis is unclear. Most cases are diagnosed clinically.
Colitis is graded based on the Montreal classification system11:
Mild colitis is defined as passage of fewer than 4 stools per day (with or without blood) over baseline and absence of any systemic illness.
Moderate is passage of more than 4 stools per day but with minimal signs of systemic toxicity.
Severe is defined as passage of at least 6 stools per day, heart rate at least 90 beats per minute, temperature at least 37.5°C (99.5°F), hemoglobin less than 10.5 g/dL, and erythrocyte sedimentation rate at least 30 mm/h.11
RULE OUT INFECTION
If symptoms such as diarrhea or abdominal pain arise within 6 weeks of starting ICI therapy, then we should check for an infectious cause. The differential diagnosis of suspected ICI-associated colitis includes infections with C difficile, cytomegalovirus, opportunistic organisms, and other bacteria and viruses. ICI-induced celiac disease and immune hyperthyroidism should also be ruled out.4
CONSIDER COLONOSCOPY AND BIOPSY
Once infection is ruled out, colonoscopy should be considered if symptoms persist or are severe. Colonoscopy with biopsy remains the gold standard for diagnosis, and it is also helpful in assessing severity of mucosal inflammation and monitoring response to medical treatment.
Table 2 lists common endoscopic and histologic features of ICI-mediated colitis; however, none of them is specific for this disease.
Common endoscopic features are loss of vascular pattern, edema, friability, spontaneous bleeding, and deep ulcerations.12 A recent study suggested that colonic ulcerations predict a steroid-refractory course in patients with immune-mediated colitis.4
Histologically, ICI-associated colitis is characterized by both acute and chronic changes, including an increased number of neutrophils and lymphocytes in the epithelium and lamina propria, erosions, ulcers, crypt abscess, crypt apoptosis, crypt distortion, and even noncaseating granulomas.13 However, transmural disease is rare. Figure 2 compares the histopathologic features of ICI-associated colitis and a normal colon.
COMPUTED TOMOGRAPHY CAN BE USEFUL
Computed tomography (CT) can also be useful for the diagnosis and measurement of severity.
Garcia-Neuer et al14 analyzed 303 patients with advanced melanoma who developed gastrointestinal symptoms while being treated with ipilimumab. Ninety-nine (33%) of them reported diarrhea during therapy, of whom 34 underwent both CT and colonoscopy with biopsy. CT was highly predictive of colitis on biopsy, with a positive predictive value of 96% and a negative likelihood ratio of 0.2.14
TREATMENT
Supportive care may be enough when treating mild ICI-related colitis. This can include oral and intravenous hydration4 and an antidiarrheal drug such as loperamide in a low dose.
Corticosteroids. For moderate ICI-associated colitis with stool frequency of 4 or more per day, patients should be started on an oral corticosteroid such as prednisone 0.5 to 1 mg/kg per day. If symptoms do not improve within 72 hours of starting an oral corticosteroid, the patient should be admitted to the hospital for observation and escalation to higher doses or possibly intravenous corticosteroids.
Infliximab has been used in severe and steroid-refractory cases,13 although there has been concern about using anti-tumor necrosis factor (TNF) agents such as this in patients with malignancies, especially melanoma. Since melanoma can be very aggressive and anti-TNF agents may promote it, it is prudent to try not to use this class of agents.
Other biologic agents such as vedolizumab, a gut-specific anti-integrin agent, are safer, have theoretic advantages over anti-TNF agents, and can be considered in patients with steroid-dependent or steroid-refractory ICI-associated enterocolitis. A recent study suggested that 2 to 4 infusions of vedolizumab are adequate to achieve steroid-free remission.15 Results from 6 clinical trials of vedolizumab in 2,830 patients with Crohn disease or ulcerative colitis did not show any increased risk of serious infections or malignancies over placebo.16,17 A drawback is its slow onset of action.
Surgery is an option for patients with severe colitis refractory to intravenous corticosteroids or biological agents, as severe colitis carries a risk of significant morbidity and even death. The incidence of bowel perforation leading to colectomy or death in patients receiving ICI therapy is 0.5% to 1%.18,19
Fecal microbiota transplant was associated with mucosal healing after 1 month in a case report of ICI-associated colitis.9
Follow-up. In most patients, symptoms resolve with discontinuation of the ICI and brief use of corticosteroids or biological agents. Patients with recurrent or persistent symptoms while on long-term ICI therapy may need periodic endoscopic evaluation, especially if there are chronic structural changes on histologic study.
If patients have recurrent or persistent symptoms along with chronic inflammatory structural changes on histology, a sign of an inflammatory bowel diseaselike condition, long-term maintenance therapy with an anti-inflammatory or immunosuppressant agent may be considered. However, there is no consensus on the treatment of this condition. It can be treated in the same way as classic inflammatory bowel disease in the setting of concurrent or prior history of malignancy, especially melanoma. Certain agents used in inflammatory bowel disease such as methotrexate and vedolizumab carry a lower risk of malignancy than anti-TNF agents and can be considered. A multidisciplinary approach that includes an oncologist, gastroenterologist, infectious disease specialist, and colorectal surgeon is imperative.
If a patient experiences diarrhea, hematochezia, or abdominal pain within the first 6 weeks of therapy with one of the anticancer drugs known as immune checkpoint inhibitors (ICIs), the first step is to rule out infection, especially with Clostridium difficile. The next step is colonosocopy with biopsy or computed tomography.
Patients with mild ICI-associated colitis may need only supportive care, and the ICI can be continued. In moderate or severe cases, the agent may need to be stopped and corticosteroids and other colitis-targeted agents may be needed. Figure 1 shows our algorithm for diagnosing and treating ICI-associated colitis.
POWERFUL ANTICANCER DRUGS
ICIs are monoclonal antibodies used in treating metastatic melanoma, non-small-cell lung cancer, metastatic prostate cancer, Hodgkin lymphoma, renal cell carcinoma, and other advanced malignancies.1,2 They act by binding to and blocking proteins on T cells, antigen-presenting cells, and tumor cells that keep immune responses in check and prevent T cells from killing cancer cells.1 For example:
- Ipilimumab blocks cytotoxic T lymphocyte-associated antigen 4
- Nivolumab and pembrolizumab block programmed cell death protein 1
- Atezolizumab blocks programmed death ligand 1.1
With these proteins blocked, T cells can do their job, often producing dramatic regression of cancer. However, ICIs can cause a range of immune-related adverse effects, including endocrine and cutaneous toxicities, iridocyclitis, lymphadenopathy, neuropathy, nephritis, immune-mediated pneumonitis, pancreatitis, hepatitis, and colitis.3
ICI-ASSOCIATED COLITIS IS COMMON
ICI-associated colitis is common; it is estimated to affect about 30% of patients receiving ipilimumab, for example.4 Clinical presentations range from watery bowel movements, blood or mucus in the stool, abdominal cramping, and flatulence to ileus, colectasia, intestinal perforation, and even death.5
The incidence appears to increase with the dosage and duration of ICI therapy. The onset of colitis typically occurs 6 to 7 weeks after starting ipilimumab,6 and 6 to 18 weeks after starting nivolumab or pembrolizumab.7Table 1 lists the incidence of diarrhea and colitis and time of onset to colitis with common ICIs. However, colitis, like other immune-related adverse events, can occur at any point, even after ICI therapy has been discontinued.8
It is best to detect side effects of ICIs promptly, as acute inflammation can progress to chronic inflammation within 1 month of onset.9 We believe that early intervention and close monitoring may prevent complications and the need for long-term immunosuppressive treatment.
Patients, family members, and caregivers should be informed of possible gastrointestinal along with systemic side effects. Severe gastrointestinal symptoms such as increased stool frequency and change in stool consistency should trigger appropriate investigation and the withholding of ICI therapy.
COLITIS IS A SPECTRUM
The colon appears to be the gastrointestinal organ most affected by ICIs. Of patients with intestinal side effects, including diarrhea, only some develop colitis. The severity of ICI-associated colitis ranges from mild bowel illness to fulminant colitis.
Hodi et al,10 in a randomized trial in which 511 patients with melanoma received ipilimumab, reported that approximately 30% had mild diarrhea, while fewer than 10% had severe diarrhea, fever, ileus, or peritoneal signs. Five patients (1%) developed intestinal perforation, 4 (0.8%) died of complications, and 26 (5%) required hospitalization for severe enterocolitis.
The pathophysiology of ICI-mediated colitis is unclear. Most cases are diagnosed clinically.
Colitis is graded based on the Montreal classification system11:
Mild colitis is defined as passage of fewer than 4 stools per day (with or without blood) over baseline and absence of any systemic illness.
Moderate is passage of more than 4 stools per day but with minimal signs of systemic toxicity.
Severe is defined as passage of at least 6 stools per day, heart rate at least 90 beats per minute, temperature at least 37.5°C (99.5°F), hemoglobin less than 10.5 g/dL, and erythrocyte sedimentation rate at least 30 mm/h.11
RULE OUT INFECTION
If symptoms such as diarrhea or abdominal pain arise within 6 weeks of starting ICI therapy, then we should check for an infectious cause. The differential diagnosis of suspected ICI-associated colitis includes infections with C difficile, cytomegalovirus, opportunistic organisms, and other bacteria and viruses. ICI-induced celiac disease and immune hyperthyroidism should also be ruled out.4
CONSIDER COLONOSCOPY AND BIOPSY
Once infection is ruled out, colonoscopy should be considered if symptoms persist or are severe. Colonoscopy with biopsy remains the gold standard for diagnosis, and it is also helpful in assessing severity of mucosal inflammation and monitoring response to medical treatment.
Table 2 lists common endoscopic and histologic features of ICI-mediated colitis; however, none of them is specific for this disease.
Common endoscopic features are loss of vascular pattern, edema, friability, spontaneous bleeding, and deep ulcerations.12 A recent study suggested that colonic ulcerations predict a steroid-refractory course in patients with immune-mediated colitis.4
Histologically, ICI-associated colitis is characterized by both acute and chronic changes, including an increased number of neutrophils and lymphocytes in the epithelium and lamina propria, erosions, ulcers, crypt abscess, crypt apoptosis, crypt distortion, and even noncaseating granulomas.13 However, transmural disease is rare. Figure 2 compares the histopathologic features of ICI-associated colitis and a normal colon.
COMPUTED TOMOGRAPHY CAN BE USEFUL
Computed tomography (CT) can also be useful for the diagnosis and measurement of severity.
Garcia-Neuer et al14 analyzed 303 patients with advanced melanoma who developed gastrointestinal symptoms while being treated with ipilimumab. Ninety-nine (33%) of them reported diarrhea during therapy, of whom 34 underwent both CT and colonoscopy with biopsy. CT was highly predictive of colitis on biopsy, with a positive predictive value of 96% and a negative likelihood ratio of 0.2.14
TREATMENT
Supportive care may be enough when treating mild ICI-related colitis. This can include oral and intravenous hydration4 and an antidiarrheal drug such as loperamide in a low dose.
Corticosteroids. For moderate ICI-associated colitis with stool frequency of 4 or more per day, patients should be started on an oral corticosteroid such as prednisone 0.5 to 1 mg/kg per day. If symptoms do not improve within 72 hours of starting an oral corticosteroid, the patient should be admitted to the hospital for observation and escalation to higher doses or possibly intravenous corticosteroids.
Infliximab has been used in severe and steroid-refractory cases,13 although there has been concern about using anti-tumor necrosis factor (TNF) agents such as this in patients with malignancies, especially melanoma. Since melanoma can be very aggressive and anti-TNF agents may promote it, it is prudent to try not to use this class of agents.
Other biologic agents such as vedolizumab, a gut-specific anti-integrin agent, are safer, have theoretic advantages over anti-TNF agents, and can be considered in patients with steroid-dependent or steroid-refractory ICI-associated enterocolitis. A recent study suggested that 2 to 4 infusions of vedolizumab are adequate to achieve steroid-free remission.15 Results from 6 clinical trials of vedolizumab in 2,830 patients with Crohn disease or ulcerative colitis did not show any increased risk of serious infections or malignancies over placebo.16,17 A drawback is its slow onset of action.
Surgery is an option for patients with severe colitis refractory to intravenous corticosteroids or biological agents, as severe colitis carries a risk of significant morbidity and even death. The incidence of bowel perforation leading to colectomy or death in patients receiving ICI therapy is 0.5% to 1%.18,19
Fecal microbiota transplant was associated with mucosal healing after 1 month in a case report of ICI-associated colitis.9
Follow-up. In most patients, symptoms resolve with discontinuation of the ICI and brief use of corticosteroids or biological agents. Patients with recurrent or persistent symptoms while on long-term ICI therapy may need periodic endoscopic evaluation, especially if there are chronic structural changes on histologic study.
If patients have recurrent or persistent symptoms along with chronic inflammatory structural changes on histology, a sign of an inflammatory bowel diseaselike condition, long-term maintenance therapy with an anti-inflammatory or immunosuppressant agent may be considered. However, there is no consensus on the treatment of this condition. It can be treated in the same way as classic inflammatory bowel disease in the setting of concurrent or prior history of malignancy, especially melanoma. Certain agents used in inflammatory bowel disease such as methotrexate and vedolizumab carry a lower risk of malignancy than anti-TNF agents and can be considered. A multidisciplinary approach that includes an oncologist, gastroenterologist, infectious disease specialist, and colorectal surgeon is imperative.
- Shih K, Arkenau HT, Infante JR. Clinical impact of checkpoint inhibitors as novel cancer therapies. Drugs 2014; 74(17):1993–2013. doi:10.1007/s40265-014-0305-6
- Mellman I, Coukos G, Dranoff G. Cancer immunotherapy comes of age. Nature 2011; 480(7378):480–489. doi:10.1038/nature10673
- Dine J, Gordon R, Shames Y, Kasler MK, Barton-Burke M. Immune checkpoint inhibitors: an innovation in immunotherapy for the treatment and management of patients with cancer. Asia Pac J Oncol Nurs 2017; 4(2):127–135. doi:10.4103/apjon.apjon_4_17
- Prieux-Klotz C, Dior M, Damotte D, et al. Immune checkpoint inhibitor-induced colitis: diagnosis and management. Target Oncol 2017; 12(3):301–308. doi:10.1007/s11523-017-0495-4
- Howell M, Lee R, Bowyer S, Fusi A, Lorigan P. Optimal management of immune-related toxicities associated with checkpoint inhibitors in lung cancer. Lung Cancer 2015; 88(2):117–123. doi:10.1016/j.lungcan.2015.02.007
- Weber JS, Kähler KC, Hauschild A. Management of immune-related adverse events and kinetics of response with ipilimumab. J Clin Oncol 2012; 30(21):2691–2697. doi:10.1200/JCO.2012.41.6750
- Eigentler TK, Hassel JC, Berking C, et al. Diagnosis, monitoring and management of immune-related adverse drug reactions of anti-PD-1 antibody therapy. Cancer Treat Rev 2016; 45:7–18. doi:10.1016/j.ctrv.2016.02.003
- Postow MA, Sidlow R, Hellmann MD. Immune-related adverse events associated with immune checkpoint blockade. N Engl J Med 2018; 378(2):158–168. doi:10.1056/NEJMra1703481
- Wang Y, DuPont H, Jiang ZD, Jenq R, Zuazua R, Shuttlesworth G. Fecal microbiota transplant for immune-checkpoint inhibitor-induced colitis in a 50 year old with bladder cancer. Gastroenterol 2018; 154(1 suppl). doi:10.1053/j.gastro.2017.11.075
- Hodi FS, O’Day SJ, McDermott DF, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med 2010; 363(8):711–723. doi:10.1056/NEJMoa1003466
- Satsangi J, Silverberg MS, Vermeire S, Colombel JF. The Montreal classification of inflammatory bowel disease: controversies, consensus, and implications. Gut 2006; 55(6):749–753. doi:10.1136/gut.2005.082909
- Rastogi P, Sultan M, Charabaty AJ, Atkins MB, Mattar MC. Ipilimumab associated colitis: an IpiColitis case series at MedStar Georgetown University Hospital. World J Gastroenterol 2015; 21(14):4373–4378. doi:10.3748/wjg.v21.i14.4373
- Pocha C, Roat J, Viskocil K. Immune-mediated colitis: important to recognize and treat. J Crohns Colitis 2014; 8(2):181–182. doi:10.1016/j.crohns.2013.09.019
- Garcia-Neuer M, Marmarelis ME, Jangi SR, et al. Diagnostic comparison of CT scans and colonoscopy for immune-related colitis in ipilimumab-treated advanced melanoma patients. Cancer Immunol Res 2017; 5(4):286–291. doi:10.1158/2326-6066.CIR-16-0302
- Bergqvist V, Hertervig E, Gedeon P, et al. Vedolizumab treatment for immune checkpoint inhibitor-induced enterocolitis. Cancer Immunol Immunother 2017; 66(5):581–592. doi:10.1007/s00262-017-1962-6
- Sandborn WJ, Feagan BG, Rutgeerts P, et al; GEMINI 2 Study Group. Vedolizumab as induction and maintenance therapy for Crohn’s disease. N Engl J Med 2013; 369(8):711–721. doi:10.1056/NEJMoa1215739
- Feagan BG, Rutgeerts P, Sands BE, et al; GEMINI 1 Study Group. Vedolizumab as induction and maintenance therapy for ulcerative colitis. N Engl J Med 2013; 369(8):699–710. doi:10.1056/NEJMoa1215734
- Kähler KC, Hauschild A. Treatment and side effect management of CTLA-4 antibody therapy in metastatic melanoma. J Dtsch Dermatol Ges 2011; 9(4):277–286. doi:10.1111/j.1610-0387.2010.07568.x
- Ibrahim RA, Berman DM, DePril V, et al. Ipilimumab safety profile: summary of findings from completed trials in advanced melanoma. J Clin Onc 2011; 29(15 suppl):8583–8583. doi:10.1200/jco.2011.29.15_suppl.8583
- Shih K, Arkenau HT, Infante JR. Clinical impact of checkpoint inhibitors as novel cancer therapies. Drugs 2014; 74(17):1993–2013. doi:10.1007/s40265-014-0305-6
- Mellman I, Coukos G, Dranoff G. Cancer immunotherapy comes of age. Nature 2011; 480(7378):480–489. doi:10.1038/nature10673
- Dine J, Gordon R, Shames Y, Kasler MK, Barton-Burke M. Immune checkpoint inhibitors: an innovation in immunotherapy for the treatment and management of patients with cancer. Asia Pac J Oncol Nurs 2017; 4(2):127–135. doi:10.4103/apjon.apjon_4_17
- Prieux-Klotz C, Dior M, Damotte D, et al. Immune checkpoint inhibitor-induced colitis: diagnosis and management. Target Oncol 2017; 12(3):301–308. doi:10.1007/s11523-017-0495-4
- Howell M, Lee R, Bowyer S, Fusi A, Lorigan P. Optimal management of immune-related toxicities associated with checkpoint inhibitors in lung cancer. Lung Cancer 2015; 88(2):117–123. doi:10.1016/j.lungcan.2015.02.007
- Weber JS, Kähler KC, Hauschild A. Management of immune-related adverse events and kinetics of response with ipilimumab. J Clin Oncol 2012; 30(21):2691–2697. doi:10.1200/JCO.2012.41.6750
- Eigentler TK, Hassel JC, Berking C, et al. Diagnosis, monitoring and management of immune-related adverse drug reactions of anti-PD-1 antibody therapy. Cancer Treat Rev 2016; 45:7–18. doi:10.1016/j.ctrv.2016.02.003
- Postow MA, Sidlow R, Hellmann MD. Immune-related adverse events associated with immune checkpoint blockade. N Engl J Med 2018; 378(2):158–168. doi:10.1056/NEJMra1703481
- Wang Y, DuPont H, Jiang ZD, Jenq R, Zuazua R, Shuttlesworth G. Fecal microbiota transplant for immune-checkpoint inhibitor-induced colitis in a 50 year old with bladder cancer. Gastroenterol 2018; 154(1 suppl). doi:10.1053/j.gastro.2017.11.075
- Hodi FS, O’Day SJ, McDermott DF, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med 2010; 363(8):711–723. doi:10.1056/NEJMoa1003466
- Satsangi J, Silverberg MS, Vermeire S, Colombel JF. The Montreal classification of inflammatory bowel disease: controversies, consensus, and implications. Gut 2006; 55(6):749–753. doi:10.1136/gut.2005.082909
- Rastogi P, Sultan M, Charabaty AJ, Atkins MB, Mattar MC. Ipilimumab associated colitis: an IpiColitis case series at MedStar Georgetown University Hospital. World J Gastroenterol 2015; 21(14):4373–4378. doi:10.3748/wjg.v21.i14.4373
- Pocha C, Roat J, Viskocil K. Immune-mediated colitis: important to recognize and treat. J Crohns Colitis 2014; 8(2):181–182. doi:10.1016/j.crohns.2013.09.019
- Garcia-Neuer M, Marmarelis ME, Jangi SR, et al. Diagnostic comparison of CT scans and colonoscopy for immune-related colitis in ipilimumab-treated advanced melanoma patients. Cancer Immunol Res 2017; 5(4):286–291. doi:10.1158/2326-6066.CIR-16-0302
- Bergqvist V, Hertervig E, Gedeon P, et al. Vedolizumab treatment for immune checkpoint inhibitor-induced enterocolitis. Cancer Immunol Immunother 2017; 66(5):581–592. doi:10.1007/s00262-017-1962-6
- Sandborn WJ, Feagan BG, Rutgeerts P, et al; GEMINI 2 Study Group. Vedolizumab as induction and maintenance therapy for Crohn’s disease. N Engl J Med 2013; 369(8):711–721. doi:10.1056/NEJMoa1215739
- Feagan BG, Rutgeerts P, Sands BE, et al; GEMINI 1 Study Group. Vedolizumab as induction and maintenance therapy for ulcerative colitis. N Engl J Med 2013; 369(8):699–710. doi:10.1056/NEJMoa1215734
- Kähler KC, Hauschild A. Treatment and side effect management of CTLA-4 antibody therapy in metastatic melanoma. J Dtsch Dermatol Ges 2011; 9(4):277–286. doi:10.1111/j.1610-0387.2010.07568.x
- Ibrahim RA, Berman DM, DePril V, et al. Ipilimumab safety profile: summary of findings from completed trials in advanced melanoma. J Clin Onc 2011; 29(15 suppl):8583–8583. doi:10.1200/jco.2011.29.15_suppl.8583
Which patients with a parapneumonic effusion need a chest tube?
Hospitalized patients with pneumonia who develop a complicated parapneumonic effusion or empyema need to undergo chest tube placement.
WHAT IS PARAPNEUMONIC EFFUSION?
Parapneumonic effusion is a pleural effusion that forms concurrently with bacterial or viral pneumonia. Up to 40% of patients hospitalized with pneumonia develop a parapneumonic effusion.1 The effusion progresses through a continuum of 3 stages: uncomplicated, complicated, and empyema.
Uncomplicated parapneumonic effusion is an exudative effusion without bacteria or pus that is caused by movement of fluid and neutrophils into the pleural space. Pneumonia itself causes an increase in interstitial fluid and capillary leakage. The effusion becomes complicated as a result of bacteria invading the pleural space, causing a further increase in neutrophils in the pleural fluid. Empyema is defined as the presence of frank pus in the pleural space.
CLINICAL SIGNIFICANCE
According to the US Centers for Disease Control and Prevention, pneumonia accounts for 674,000 emergency department visits each year; of the patients hospitalized, up to 40% develop a parapneumonic effusion.2 The only study done on rates of death associated with parapneumonic effusion showed that, compared with patients with no effusion, the risk of death was 3.7 times higher with a unilateral effusion and 6.5 times higher with bilateral effusions.3
INITIAL EVALUATION
The initial evaluation of suspected parapneumonic effusion should include chest radiography with lateral or decubitus views, followed by thoracentesis if indicated. If thoracentesis is performed, the fluid should be tested as follows:
- Gram stain
- Appropriate cultures based on clinical scenario (eg, aerobic, anaerobic, fungal)
- Total protein in pleural fluid and serum
- Lactate dehydrogenase (LDH) in pleural fluid and serum
- Glucose
- pH.
CLASSIFICATION OF EFFUSIONS
When pleural fluid is obtained, the total protein and LDH levels are used to categorize the effusion as either transudative or exudative based on the Light criteria.4 An effusion is confirmed as exudative when 1 of the following 3 criteria is met:
- The ratio of pleural fluid protein to serum protein is greater than 0.5
- The ratio of pleural fluid LDH to serum LDH is greater than 0.6
- The pleural fluid LDH is greater than two-thirds the upper limit of normal for the serum LDH.
Category 1 effusions are defined as free- flowing fluid with a thickness of less than 10 mm on any imaging modality. Thoracentesis for pleural fluid analysis is not required. The prognosis is very good.
Category 2 effusions are defined as free- flowing fluid with a thickness greater than 10 mm and less than 50% of the hemithorax. Thoracentesis is typically done because of the size of the effusion, but Gram stain and culture of the pleural fluid are usually negative, and the pH is at least 7.2. The prognosis is good.
Category 3 effusions are considered complicated because the anatomy of the pleural space becomes altered or because bacteria have invaded the pleural space. The effusion is larger than 50% of the hemithorax or is loculated, or the parietal pleura is thickened. Since the bacteria have invaded the pleural space, Gram stain or culture of pleural fluid may be positive, the pleural fluid pH may be less than 7.2, or the glucose level of the fluid may be less than 60 mg/dL. The prognosis for category 3 is poor.
Category 4 effusions are defined as empyema. The only characteristic that separates this from category 3 is frank pus in the pleural space. The prognosis is very poor.
TO PLACE A CHEST TUBE OR NOT
For category 1 or 2 effusions, treatment with antibiotics alone is typically enough. Category 3 effusions usually do not respond to antibiotics alone and may require complete drainage of the fluid with or without a chest tube depending on whether loculations are present, as loculations are difficult to drain with a chest tube. Category 4 effusions require both antibiotics and chest tube placement.
WHAT TYPE OF CHEST TUBE?
Studies have shown that small-bore chest tubes (< 20 F) are as efficacious as larger tubes (≥ 20 F) for the treatment of complicated parapneumonic effusion and empyema.6,7 Studies have also shown that the size of the tube makes no difference in the time needed to drain the effusion, the length of hospital stay, or the complication rate.8,9 Based on these studies, a small-bore chest tube should be placed first when clinically appropriate. When a chest tube is placed for empyema, computed tomography should be performed within 24 hours to confirm proper tube placement.
ADVANCED THERAPIES FOR EMPYEMA
Empyema treatment fails when antibiotic coverage is inadequate or when a loculation is not drained appropriately. Options if treatment fails include instillation of fibrinolytics into the pleural space, video-assisted thorascopic surgery, and decortication.
The role of fibrinolytics has not been well-established, but fibrinolytics should be considered in loculated effusions or empyema, or if drainage of the effusion slows.10 Video-assisted thorascopic surgery is reserved for effusions that are incompletely drained with a chest tube with or without fibrinolytics; studies have shown shorter hospital length of stay and higher treatment efficacy when this is performed earlier for loculated effusions.11 Decortication is reserved for symptomatic patients who have a thickened pleura more than 6 months after the initial infection.12 Timing for each of these procedures is not clearly defined and so must be individualized.
TAKE-AWAY POINTS
- Parapneumonic effusion occurs concurrently with pneumonia and with a high frequency.1
- Effusions are associated with an increased risk of death.3
- Categorizing the effusion helps guide treatment.
- Chest tubes should be placed for some cases of complicated effusion and for all cases of empyema.
- A small-bore chest tube (< 20 F) should be tried first.
- Light RW. Parapneumonic effusions and empyema. Proc Am Thorac Soc 2006; 3(1):75–80. doi:10.1513/pats.200510-113JH
- US Department of Health and Human Services; Centers for Disease Control and Prevention; National Center for Health Statistics. National hospital ambulatory medical care survey: 2013 emergency department summary tables. www.cdc.gov/nchs/data/ahcd/nhamcs_emergency/2013_ed_web_tables.pdf.
- Hasley PB, Albaum MN, Li YH, et al. Do pulmonary radiographic findings at presentation predict mortality in patients with community-acquired pneumonia? Arch Intern Med 1996; 156(19):2206–2212. doi:10.1001/archinte.1996.00440180068008
- Light RW, Macgregor MI, Luchsinger PC, Ball WC. Pleural effusions: the diagnostic separation of transudates and exudates. Ann Intern Med 1972; 77(4):507–513. doi:10.7326/0003-4819-77-4-507
- Colice GL, Curtis A, Deslauriers J, et al. Medical and surgical treatment of parapneumonic effusions: an evidence-based guideline. Chest 2000; 118(4):1158–1171. doi:10.1378/CHEST.118.4.1158
- Ali I, Unruh H. Management of empyema thoracis. Ann Thorac Surg 1990; 50(3):355–359. doi:10.1016/0003-4975(90)90474-K
- Ashbaugh DG. Empyema thoracis. Factors influencing morbidity and mortality. Chest 1991; 99(5):1162–1165. doi:10.1378/CHEST.99.5.1162
- Cooke DT, David EA. Large-bore and small-bore chest tubes: types, function, and placement. Thorac Surg Clin 2013; 23(1):17–24. doi:10.1016/j.thorsurg.2012.10.006
- Halifax RJ, Psallidas I, Rahman NM. Chest drain size: the debate continues. Curr Pulmonol Rep 2017; 6(1):26–29. doi:10.1007/s13665-017-0162-3
- Maskell NA, Davies CW, Nunn AJ, et al; First Multicenter Intrapleural Sepsis Trial (MIST1) Group. UK controlled trial of intrapleural streptokinase for pleural infection. N Engl J Med 2005; 352(9):865–874. doi:10.1056/NEJMoa042473
- Wait MA, Sharma S, Hohn J, Dal Nogare A. A randomized trial of empyema therapy. Chest 1997; 111(6):1548–1551. doi:10.1378/chest.111.6.1548
- Rzyman W, Skokowski J, Romanowicz G, Lass P, Dziadziuszko R. Decortication in chronic pleural empyema—effect on lung function. Eur J Cardiothorac Surg 2002; 21(3):502–507. doi:10.1016/S1010-7940(01)01167-8
Hospitalized patients with pneumonia who develop a complicated parapneumonic effusion or empyema need to undergo chest tube placement.
WHAT IS PARAPNEUMONIC EFFUSION?
Parapneumonic effusion is a pleural effusion that forms concurrently with bacterial or viral pneumonia. Up to 40% of patients hospitalized with pneumonia develop a parapneumonic effusion.1 The effusion progresses through a continuum of 3 stages: uncomplicated, complicated, and empyema.
Uncomplicated parapneumonic effusion is an exudative effusion without bacteria or pus that is caused by movement of fluid and neutrophils into the pleural space. Pneumonia itself causes an increase in interstitial fluid and capillary leakage. The effusion becomes complicated as a result of bacteria invading the pleural space, causing a further increase in neutrophils in the pleural fluid. Empyema is defined as the presence of frank pus in the pleural space.
CLINICAL SIGNIFICANCE
According to the US Centers for Disease Control and Prevention, pneumonia accounts for 674,000 emergency department visits each year; of the patients hospitalized, up to 40% develop a parapneumonic effusion.2 The only study done on rates of death associated with parapneumonic effusion showed that, compared with patients with no effusion, the risk of death was 3.7 times higher with a unilateral effusion and 6.5 times higher with bilateral effusions.3
INITIAL EVALUATION
The initial evaluation of suspected parapneumonic effusion should include chest radiography with lateral or decubitus views, followed by thoracentesis if indicated. If thoracentesis is performed, the fluid should be tested as follows:
- Gram stain
- Appropriate cultures based on clinical scenario (eg, aerobic, anaerobic, fungal)
- Total protein in pleural fluid and serum
- Lactate dehydrogenase (LDH) in pleural fluid and serum
- Glucose
- pH.
CLASSIFICATION OF EFFUSIONS
When pleural fluid is obtained, the total protein and LDH levels are used to categorize the effusion as either transudative or exudative based on the Light criteria.4 An effusion is confirmed as exudative when 1 of the following 3 criteria is met:
- The ratio of pleural fluid protein to serum protein is greater than 0.5
- The ratio of pleural fluid LDH to serum LDH is greater than 0.6
- The pleural fluid LDH is greater than two-thirds the upper limit of normal for the serum LDH.
Category 1 effusions are defined as free- flowing fluid with a thickness of less than 10 mm on any imaging modality. Thoracentesis for pleural fluid analysis is not required. The prognosis is very good.
Category 2 effusions are defined as free- flowing fluid with a thickness greater than 10 mm and less than 50% of the hemithorax. Thoracentesis is typically done because of the size of the effusion, but Gram stain and culture of the pleural fluid are usually negative, and the pH is at least 7.2. The prognosis is good.
Category 3 effusions are considered complicated because the anatomy of the pleural space becomes altered or because bacteria have invaded the pleural space. The effusion is larger than 50% of the hemithorax or is loculated, or the parietal pleura is thickened. Since the bacteria have invaded the pleural space, Gram stain or culture of pleural fluid may be positive, the pleural fluid pH may be less than 7.2, or the glucose level of the fluid may be less than 60 mg/dL. The prognosis for category 3 is poor.
Category 4 effusions are defined as empyema. The only characteristic that separates this from category 3 is frank pus in the pleural space. The prognosis is very poor.
TO PLACE A CHEST TUBE OR NOT
For category 1 or 2 effusions, treatment with antibiotics alone is typically enough. Category 3 effusions usually do not respond to antibiotics alone and may require complete drainage of the fluid with or without a chest tube depending on whether loculations are present, as loculations are difficult to drain with a chest tube. Category 4 effusions require both antibiotics and chest tube placement.
WHAT TYPE OF CHEST TUBE?
Studies have shown that small-bore chest tubes (< 20 F) are as efficacious as larger tubes (≥ 20 F) for the treatment of complicated parapneumonic effusion and empyema.6,7 Studies have also shown that the size of the tube makes no difference in the time needed to drain the effusion, the length of hospital stay, or the complication rate.8,9 Based on these studies, a small-bore chest tube should be placed first when clinically appropriate. When a chest tube is placed for empyema, computed tomography should be performed within 24 hours to confirm proper tube placement.
ADVANCED THERAPIES FOR EMPYEMA
Empyema treatment fails when antibiotic coverage is inadequate or when a loculation is not drained appropriately. Options if treatment fails include instillation of fibrinolytics into the pleural space, video-assisted thorascopic surgery, and decortication.
The role of fibrinolytics has not been well-established, but fibrinolytics should be considered in loculated effusions or empyema, or if drainage of the effusion slows.10 Video-assisted thorascopic surgery is reserved for effusions that are incompletely drained with a chest tube with or without fibrinolytics; studies have shown shorter hospital length of stay and higher treatment efficacy when this is performed earlier for loculated effusions.11 Decortication is reserved for symptomatic patients who have a thickened pleura more than 6 months after the initial infection.12 Timing for each of these procedures is not clearly defined and so must be individualized.
TAKE-AWAY POINTS
- Parapneumonic effusion occurs concurrently with pneumonia and with a high frequency.1
- Effusions are associated with an increased risk of death.3
- Categorizing the effusion helps guide treatment.
- Chest tubes should be placed for some cases of complicated effusion and for all cases of empyema.
- A small-bore chest tube (< 20 F) should be tried first.
Hospitalized patients with pneumonia who develop a complicated parapneumonic effusion or empyema need to undergo chest tube placement.
WHAT IS PARAPNEUMONIC EFFUSION?
Parapneumonic effusion is a pleural effusion that forms concurrently with bacterial or viral pneumonia. Up to 40% of patients hospitalized with pneumonia develop a parapneumonic effusion.1 The effusion progresses through a continuum of 3 stages: uncomplicated, complicated, and empyema.
Uncomplicated parapneumonic effusion is an exudative effusion without bacteria or pus that is caused by movement of fluid and neutrophils into the pleural space. Pneumonia itself causes an increase in interstitial fluid and capillary leakage. The effusion becomes complicated as a result of bacteria invading the pleural space, causing a further increase in neutrophils in the pleural fluid. Empyema is defined as the presence of frank pus in the pleural space.
CLINICAL SIGNIFICANCE
According to the US Centers for Disease Control and Prevention, pneumonia accounts for 674,000 emergency department visits each year; of the patients hospitalized, up to 40% develop a parapneumonic effusion.2 The only study done on rates of death associated with parapneumonic effusion showed that, compared with patients with no effusion, the risk of death was 3.7 times higher with a unilateral effusion and 6.5 times higher with bilateral effusions.3
INITIAL EVALUATION
The initial evaluation of suspected parapneumonic effusion should include chest radiography with lateral or decubitus views, followed by thoracentesis if indicated. If thoracentesis is performed, the fluid should be tested as follows:
- Gram stain
- Appropriate cultures based on clinical scenario (eg, aerobic, anaerobic, fungal)
- Total protein in pleural fluid and serum
- Lactate dehydrogenase (LDH) in pleural fluid and serum
- Glucose
- pH.
CLASSIFICATION OF EFFUSIONS
When pleural fluid is obtained, the total protein and LDH levels are used to categorize the effusion as either transudative or exudative based on the Light criteria.4 An effusion is confirmed as exudative when 1 of the following 3 criteria is met:
- The ratio of pleural fluid protein to serum protein is greater than 0.5
- The ratio of pleural fluid LDH to serum LDH is greater than 0.6
- The pleural fluid LDH is greater than two-thirds the upper limit of normal for the serum LDH.
Category 1 effusions are defined as free- flowing fluid with a thickness of less than 10 mm on any imaging modality. Thoracentesis for pleural fluid analysis is not required. The prognosis is very good.
Category 2 effusions are defined as free- flowing fluid with a thickness greater than 10 mm and less than 50% of the hemithorax. Thoracentesis is typically done because of the size of the effusion, but Gram stain and culture of the pleural fluid are usually negative, and the pH is at least 7.2. The prognosis is good.
Category 3 effusions are considered complicated because the anatomy of the pleural space becomes altered or because bacteria have invaded the pleural space. The effusion is larger than 50% of the hemithorax or is loculated, or the parietal pleura is thickened. Since the bacteria have invaded the pleural space, Gram stain or culture of pleural fluid may be positive, the pleural fluid pH may be less than 7.2, or the glucose level of the fluid may be less than 60 mg/dL. The prognosis for category 3 is poor.
Category 4 effusions are defined as empyema. The only characteristic that separates this from category 3 is frank pus in the pleural space. The prognosis is very poor.
TO PLACE A CHEST TUBE OR NOT
For category 1 or 2 effusions, treatment with antibiotics alone is typically enough. Category 3 effusions usually do not respond to antibiotics alone and may require complete drainage of the fluid with or without a chest tube depending on whether loculations are present, as loculations are difficult to drain with a chest tube. Category 4 effusions require both antibiotics and chest tube placement.
WHAT TYPE OF CHEST TUBE?
Studies have shown that small-bore chest tubes (< 20 F) are as efficacious as larger tubes (≥ 20 F) for the treatment of complicated parapneumonic effusion and empyema.6,7 Studies have also shown that the size of the tube makes no difference in the time needed to drain the effusion, the length of hospital stay, or the complication rate.8,9 Based on these studies, a small-bore chest tube should be placed first when clinically appropriate. When a chest tube is placed for empyema, computed tomography should be performed within 24 hours to confirm proper tube placement.
ADVANCED THERAPIES FOR EMPYEMA
Empyema treatment fails when antibiotic coverage is inadequate or when a loculation is not drained appropriately. Options if treatment fails include instillation of fibrinolytics into the pleural space, video-assisted thorascopic surgery, and decortication.
The role of fibrinolytics has not been well-established, but fibrinolytics should be considered in loculated effusions or empyema, or if drainage of the effusion slows.10 Video-assisted thorascopic surgery is reserved for effusions that are incompletely drained with a chest tube with or without fibrinolytics; studies have shown shorter hospital length of stay and higher treatment efficacy when this is performed earlier for loculated effusions.11 Decortication is reserved for symptomatic patients who have a thickened pleura more than 6 months after the initial infection.12 Timing for each of these procedures is not clearly defined and so must be individualized.
TAKE-AWAY POINTS
- Parapneumonic effusion occurs concurrently with pneumonia and with a high frequency.1
- Effusions are associated with an increased risk of death.3
- Categorizing the effusion helps guide treatment.
- Chest tubes should be placed for some cases of complicated effusion and for all cases of empyema.
- A small-bore chest tube (< 20 F) should be tried first.
- Light RW. Parapneumonic effusions and empyema. Proc Am Thorac Soc 2006; 3(1):75–80. doi:10.1513/pats.200510-113JH
- US Department of Health and Human Services; Centers for Disease Control and Prevention; National Center for Health Statistics. National hospital ambulatory medical care survey: 2013 emergency department summary tables. www.cdc.gov/nchs/data/ahcd/nhamcs_emergency/2013_ed_web_tables.pdf.
- Hasley PB, Albaum MN, Li YH, et al. Do pulmonary radiographic findings at presentation predict mortality in patients with community-acquired pneumonia? Arch Intern Med 1996; 156(19):2206–2212. doi:10.1001/archinte.1996.00440180068008
- Light RW, Macgregor MI, Luchsinger PC, Ball WC. Pleural effusions: the diagnostic separation of transudates and exudates. Ann Intern Med 1972; 77(4):507–513. doi:10.7326/0003-4819-77-4-507
- Colice GL, Curtis A, Deslauriers J, et al. Medical and surgical treatment of parapneumonic effusions: an evidence-based guideline. Chest 2000; 118(4):1158–1171. doi:10.1378/CHEST.118.4.1158
- Ali I, Unruh H. Management of empyema thoracis. Ann Thorac Surg 1990; 50(3):355–359. doi:10.1016/0003-4975(90)90474-K
- Ashbaugh DG. Empyema thoracis. Factors influencing morbidity and mortality. Chest 1991; 99(5):1162–1165. doi:10.1378/CHEST.99.5.1162
- Cooke DT, David EA. Large-bore and small-bore chest tubes: types, function, and placement. Thorac Surg Clin 2013; 23(1):17–24. doi:10.1016/j.thorsurg.2012.10.006
- Halifax RJ, Psallidas I, Rahman NM. Chest drain size: the debate continues. Curr Pulmonol Rep 2017; 6(1):26–29. doi:10.1007/s13665-017-0162-3
- Maskell NA, Davies CW, Nunn AJ, et al; First Multicenter Intrapleural Sepsis Trial (MIST1) Group. UK controlled trial of intrapleural streptokinase for pleural infection. N Engl J Med 2005; 352(9):865–874. doi:10.1056/NEJMoa042473
- Wait MA, Sharma S, Hohn J, Dal Nogare A. A randomized trial of empyema therapy. Chest 1997; 111(6):1548–1551. doi:10.1378/chest.111.6.1548
- Rzyman W, Skokowski J, Romanowicz G, Lass P, Dziadziuszko R. Decortication in chronic pleural empyema—effect on lung function. Eur J Cardiothorac Surg 2002; 21(3):502–507. doi:10.1016/S1010-7940(01)01167-8
- Light RW. Parapneumonic effusions and empyema. Proc Am Thorac Soc 2006; 3(1):75–80. doi:10.1513/pats.200510-113JH
- US Department of Health and Human Services; Centers for Disease Control and Prevention; National Center for Health Statistics. National hospital ambulatory medical care survey: 2013 emergency department summary tables. www.cdc.gov/nchs/data/ahcd/nhamcs_emergency/2013_ed_web_tables.pdf.
- Hasley PB, Albaum MN, Li YH, et al. Do pulmonary radiographic findings at presentation predict mortality in patients with community-acquired pneumonia? Arch Intern Med 1996; 156(19):2206–2212. doi:10.1001/archinte.1996.00440180068008
- Light RW, Macgregor MI, Luchsinger PC, Ball WC. Pleural effusions: the diagnostic separation of transudates and exudates. Ann Intern Med 1972; 77(4):507–513. doi:10.7326/0003-4819-77-4-507
- Colice GL, Curtis A, Deslauriers J, et al. Medical and surgical treatment of parapneumonic effusions: an evidence-based guideline. Chest 2000; 118(4):1158–1171. doi:10.1378/CHEST.118.4.1158
- Ali I, Unruh H. Management of empyema thoracis. Ann Thorac Surg 1990; 50(3):355–359. doi:10.1016/0003-4975(90)90474-K
- Ashbaugh DG. Empyema thoracis. Factors influencing morbidity and mortality. Chest 1991; 99(5):1162–1165. doi:10.1378/CHEST.99.5.1162
- Cooke DT, David EA. Large-bore and small-bore chest tubes: types, function, and placement. Thorac Surg Clin 2013; 23(1):17–24. doi:10.1016/j.thorsurg.2012.10.006
- Halifax RJ, Psallidas I, Rahman NM. Chest drain size: the debate continues. Curr Pulmonol Rep 2017; 6(1):26–29. doi:10.1007/s13665-017-0162-3
- Maskell NA, Davies CW, Nunn AJ, et al; First Multicenter Intrapleural Sepsis Trial (MIST1) Group. UK controlled trial of intrapleural streptokinase for pleural infection. N Engl J Med 2005; 352(9):865–874. doi:10.1056/NEJMoa042473
- Wait MA, Sharma S, Hohn J, Dal Nogare A. A randomized trial of empyema therapy. Chest 1997; 111(6):1548–1551. doi:10.1378/chest.111.6.1548
- Rzyman W, Skokowski J, Romanowicz G, Lass P, Dziadziuszko R. Decortication in chronic pleural empyema—effect on lung function. Eur J Cardiothorac Surg 2002; 21(3):502–507. doi:10.1016/S1010-7940(01)01167-8
What inpatient treatments do we have for acute intractable migraine?
We recommend the following combination treatment:
Normal saline (0.9% NaCl) 1 to 2 L by intravenous (IV) infusion over 2 to 4 hours. This can be repeated every 6 to 12 hours.
Ketorolac 30-mg IV bolus, which can be repeated every 6 hours. However, patients with coronary artery disease, uncontrolled hypertension, acute renal failure, or cerebrovascular disease should instead receive acetaminophen 1,000 mg, naproxen sodium 550 mg, or aspirin 325 mg by mouth.
Prochlorperazine or metoclopramide 10-mg IV infusion. This can be repeated every 6 hours. However, to reduce the extrapyramidal adverse effects of these drugs, patients should first receive diphenhydramine 25- to 50-mg IV bolus, which can be repeated every 6 to 8 hours.
Sodium valproate 500 to 1,000 mg by IV infusion over 20 minutes. This can be repeated after 8 hours.
Dexamethasone 4-mg IV bolus every 6 hours, or 10-mg IV bolus once in 24 hours.
Magnesium sulfate 500 to 1,000 mg by IV infusion over 1 hour. This can be repeated every 6 to 12 hours.
If the migraine has not improved after 3 cycles of this regimen, a neurologic consultation should be considered. Other options include dihydroergotamine and occipital nerve blocks1 performed at the bedside.
GENERAL PRINCIPLES
Managing acute intractable migraine can be frustrating for both the practitioner and the patient. Some general principles are helpful.
Use a combination of drugs. Aborting a severe migraine attack often requires a combination of medications that work synergistically.
Use IV and intramuscular formulations rather than oral formulations: they are more rapidly absorbed, provide faster pain relief, and can be given when the nausea that often accompanies migraine precludes oral treatments.
Rule out secondary causes. The mnemonic SNOOP—systemic signs, neurologic signs, onset, older age, progression of existing headache disorder—is useful for assessing underlying causes.2 Any patient presenting with intractable migraine should also have a thorough neurologic examination.
Screening electrocardiography may be helpful, as the pretreatment QTc interval may direct the choice of intravenous treatment. If the patient has a prolonged QTc or is taking another drug that could prolong the QTc, certain medications, specifically dopamine receptor antagonists and diphenhydramine, should be avoided.
Ask the patient what has worked previously. A particular agent may have been effective in aborting the migraine; thus, a single dose of it could abort the headache, expediting discharge.
Establish if a triptan or ergot derivative has been used during the 24 hours before presentation, as repeated dosing within this interval is not recommended.3
Establish the baseline headache severity. Complete headache relief is difficult to achieve in a patient with chronic daily headache, and establishing a more realistic goal (eg, 50% relief) from the outset is useful.
OPTIONS FOR DRUG THERAPY
Antiemetics
Dopamine receptor antagonists are assumed to merely treat nausea in patients with migraine; however, they act independently to abort migraine and thus should be considered, irrespective of the presence of nausea.
The two most commonly used agents are prochlorperazine and metoclopramide. The American Academy of Neurology guidelines recommend prochlorperazine as first-line therapy for acute migraine. Metoclopramide is rated slightly lower and is considered to have moderate benefit.4 The Canadian Headache Society cites a high level of evidence supporting prochlorperazine and a moderate level of evidence supporting metoclopramide.5 The American Headache Society assessment of parenteral pharmacotherapies gives prochlorperazine and metoclopramide a level B recommendation of “should offer” (a recommendation only additionally assigned to subcutaneous sumatriptan).3 Hence, either agent can be used.
To reduce the risk of posttreatment akathisia, diphenhydramine or benztropine may be given before starting a dopamine receptor antagonist. Diphenhydramine may be independently effective in migraine treatment,6,7 but data on this are limited.
Ketorolac, ibuprofen
Ketorolac and ibuprofen are the only available nonsteroidal antiinflammatory drugs (NSAIDs) for IV administration. The Canadian Headache Society guidelines strongly recommend ketorolac for the treatment of migraine in emergency settings.5 Doses range from 30 mg to 60 mg.1 Ibuprofen 400 to 800 mg by IV infusion is an acceptable alternative. These medications should be avoided in patients with renal failure or severe coronary artery disease.
Oral naproxen sodium is a possible alternative in patients with cardiovascular disease, as it has been shown to carry a lower cardiovascular risk than other NSAIDs.8
The same concerns in patients with renal dysfunction apply to any NSAID, as the enzyme cyclooxygenase plays a constitutive role in glomerular function.
Antiepileptic drugs
The antiepileptic drugs sodium valproate and levetiracetam are available in IV formulations that have demonstrated efficacy in the treatment of status migrainosus1 (ie, migraine lasting more than 72 hours). Valproate has the strongest track record, is well tolerated, and is effective in rapidly aborting migraine.9
Volume repletion
Although its use is anecdotal and to date no trial has measured its efficacy, IV volume repletion is often used in acute migraine, as most headache experts surmise it to be highly effective, especially in patients with prolonged nausea or vomiting.1
Magnesium
IV magnesium is effective, particularly for migraine with aura.10 Hypotension is a common side effect, and pretreatment or concurrent treatment with IV fluids is advised. Doses from 500 mg to 1,000 mg have demonstrated efficacy.10
Corticosteroids
Corticosteroids can be used in the treatment of status migrainosus. Most studies have shown benefit in preventing recurrences rather than merely aborting migraine.11 A systematic review suggested that recurrent headaches are milder with corticosteroid treatment; 19 of 25 studies indicated favorable benefit, and 6 of 19 studies indicated noninferior outcomes.12
Both IV methylprednisolone and IV dexamethasone may be considered.12 Dexamethasone appears to be particularly effective in preventing headache recurrence when combined with other IV therapies.13 It can be given as a single dose of 10 mg, or as repeated doses of 4 mg up to 16 mg/day.1 Patients with active psychosis or uncontrolled diabetes should be closely monitored for these conditions, which corticosteroids can worsen.
Serotoninergic agents
Serotonin agonists including subcutaneous sumatriptan and IV dihydroergotamine are highly effective, with proven statistical and clinical benefit.4 They should be considered in patients with no known history of coronary artery disease or other vaso-occlusive vascular disorder.1
Ideally, IV dihydroergotamine should be administered after consultation with a neurologist or headache specialist, given the pretreatment and cotreatment requirements often necessary to suppress its side effects. Careful titration is important to prevent transient headache exacerbations during infusion, as well as abdominal cramping, nausea, and diarrhea.
Avoid opioids
Opioids should be avoided. Evidence supporting their use in acute migraine is extremely limited,3 and the risks of migraine becoming chronic and of addiction are high.14 Safer, more effective alternatives have been detailed above.
A detailed algorithm for the management of patients with acute migraine has been published14 and is aimed at decreasing acute treatment with opioids and barbiturates.
- Rozen TD. Emergency department and inpatient management of status migrainosus and intractable headache. Continuum (Minneap Minn) 2015; 21(4):1004–1017. doi:10.1212/CON.0000000000000191
- Dodick D. Headache as a symptom of ominous disease. What are the warning signals? Postgrad Med 1997; 101(5):46–50, 55–56, 62–64. doi:10.3810/pgm.1997.05.217
- Orr SL, Friedman BW, Christie S, et al. Management of adults with acute migraine in the emergency department: the American Headache Society evidence assessment of parenteral pharmacotherapies. Headache 2016; 56(6):911–940. doi:10.1111/head.12835
- Silberstein SD. Practice parameter: evidence-based guidelines for migraine headache (an evidence-based review): report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology 2000; 55(6):754–762. doi:10.1212/WNL.55.6.754
- Orr SL, Aubé M, Becker WJ, et al. Canadian Headache Society systematic review and recommendations on the treatment of migraine pain in emergency settings. Cephalalgia 2015; 35(3):271–284. doi:10.1177/0333102414535997
- Swidan SZ, Lake AE 3rd, Saper JR. Efficacy of intravenous diphenhydramine versus intravenous DHE-45 in the treatment of severe migraine headache. Curr Pain Headache Rep 2005; 9(1):65–70. doi:10.1007/s11916-005-0077-5
- Marmura MJ, Goldberg SW. Inpatient management of migraine. Curr Neurol Neurosci Rep 2015; 15(4):13. doi:10.1007/s11910-015-0539-z
- Farkouh ME, Greenberg BP. An evidence-based review of the cardiovascular risks of nonsteroidal anti-inflammatory drugs. Am J Cardiol 2009; 103(9):1227–1237. doi:10.1016/j.amjcard.2009.01.014
- Stillman MJ, Zajac D, Rybicki LA. Treatment of primary headache disorders with intravenous valproate: initial outpatient experience. Headache 2004; 44(1):65–69. doi:10.1111/j.1526-4610.2004.04010.x
- Marmura MJ, Silberstein SD, Schwedt TJ. The acute treatment of migraine in adults: the American Headache Society evidence assessment of migraine pharmacotherapies. Headache 2015; 55(1):3–20. doi:10.1111/head.12499
- Colman I, Friedman BW, Brown MD, et al. Parenteral dexamethasone for acute severe migraine headache: meta-analysis of randomised controlled trials for preventing recurrence. BMJ 2008; 336(7657):1359–1361. doi:10.1136/bmj.39566.806725.BE
- Woldeamanuel YW, Rapoport AM, Cowan RP. The place of corticosteroids in migraine attack management: a 65-year systematic review with pooled analysis and critical appraisal. Cephalalgia 2015; 35(1):996–1024. doi:10.1177/0333102414566200
- Singh A, Alter HJ, Zaia B. Does the addition of dexamethasone to standard therapy for acute migraine headache decrease the incidence of recurrent headache for patients treated in the emergency department? A meta-analysis and systematic review of the literature. Acad Emerg Med 2008; 15(12):1223–1233. doi:10.1111/j.1553-2712.2008.00283.x
- Ahmed ZA, Nacopoulos DA, John S, Papesh N, Levine D, Bamford CC. An algorithm for opioid and barbiturate reduction in the acute management of headache in the emergency department. Headache 2017; 57(1):71–79. doi:10.1111/head.12961
We recommend the following combination treatment:
Normal saline (0.9% NaCl) 1 to 2 L by intravenous (IV) infusion over 2 to 4 hours. This can be repeated every 6 to 12 hours.
Ketorolac 30-mg IV bolus, which can be repeated every 6 hours. However, patients with coronary artery disease, uncontrolled hypertension, acute renal failure, or cerebrovascular disease should instead receive acetaminophen 1,000 mg, naproxen sodium 550 mg, or aspirin 325 mg by mouth.
Prochlorperazine or metoclopramide 10-mg IV infusion. This can be repeated every 6 hours. However, to reduce the extrapyramidal adverse effects of these drugs, patients should first receive diphenhydramine 25- to 50-mg IV bolus, which can be repeated every 6 to 8 hours.
Sodium valproate 500 to 1,000 mg by IV infusion over 20 minutes. This can be repeated after 8 hours.
Dexamethasone 4-mg IV bolus every 6 hours, or 10-mg IV bolus once in 24 hours.
Magnesium sulfate 500 to 1,000 mg by IV infusion over 1 hour. This can be repeated every 6 to 12 hours.
If the migraine has not improved after 3 cycles of this regimen, a neurologic consultation should be considered. Other options include dihydroergotamine and occipital nerve blocks1 performed at the bedside.
GENERAL PRINCIPLES
Managing acute intractable migraine can be frustrating for both the practitioner and the patient. Some general principles are helpful.
Use a combination of drugs. Aborting a severe migraine attack often requires a combination of medications that work synergistically.
Use IV and intramuscular formulations rather than oral formulations: they are more rapidly absorbed, provide faster pain relief, and can be given when the nausea that often accompanies migraine precludes oral treatments.
Rule out secondary causes. The mnemonic SNOOP—systemic signs, neurologic signs, onset, older age, progression of existing headache disorder—is useful for assessing underlying causes.2 Any patient presenting with intractable migraine should also have a thorough neurologic examination.
Screening electrocardiography may be helpful, as the pretreatment QTc interval may direct the choice of intravenous treatment. If the patient has a prolonged QTc or is taking another drug that could prolong the QTc, certain medications, specifically dopamine receptor antagonists and diphenhydramine, should be avoided.
Ask the patient what has worked previously. A particular agent may have been effective in aborting the migraine; thus, a single dose of it could abort the headache, expediting discharge.
Establish if a triptan or ergot derivative has been used during the 24 hours before presentation, as repeated dosing within this interval is not recommended.3
Establish the baseline headache severity. Complete headache relief is difficult to achieve in a patient with chronic daily headache, and establishing a more realistic goal (eg, 50% relief) from the outset is useful.
OPTIONS FOR DRUG THERAPY
Antiemetics
Dopamine receptor antagonists are assumed to merely treat nausea in patients with migraine; however, they act independently to abort migraine and thus should be considered, irrespective of the presence of nausea.
The two most commonly used agents are prochlorperazine and metoclopramide. The American Academy of Neurology guidelines recommend prochlorperazine as first-line therapy for acute migraine. Metoclopramide is rated slightly lower and is considered to have moderate benefit.4 The Canadian Headache Society cites a high level of evidence supporting prochlorperazine and a moderate level of evidence supporting metoclopramide.5 The American Headache Society assessment of parenteral pharmacotherapies gives prochlorperazine and metoclopramide a level B recommendation of “should offer” (a recommendation only additionally assigned to subcutaneous sumatriptan).3 Hence, either agent can be used.
To reduce the risk of posttreatment akathisia, diphenhydramine or benztropine may be given before starting a dopamine receptor antagonist. Diphenhydramine may be independently effective in migraine treatment,6,7 but data on this are limited.
Ketorolac, ibuprofen
Ketorolac and ibuprofen are the only available nonsteroidal antiinflammatory drugs (NSAIDs) for IV administration. The Canadian Headache Society guidelines strongly recommend ketorolac for the treatment of migraine in emergency settings.5 Doses range from 30 mg to 60 mg.1 Ibuprofen 400 to 800 mg by IV infusion is an acceptable alternative. These medications should be avoided in patients with renal failure or severe coronary artery disease.
Oral naproxen sodium is a possible alternative in patients with cardiovascular disease, as it has been shown to carry a lower cardiovascular risk than other NSAIDs.8
The same concerns in patients with renal dysfunction apply to any NSAID, as the enzyme cyclooxygenase plays a constitutive role in glomerular function.
Antiepileptic drugs
The antiepileptic drugs sodium valproate and levetiracetam are available in IV formulations that have demonstrated efficacy in the treatment of status migrainosus1 (ie, migraine lasting more than 72 hours). Valproate has the strongest track record, is well tolerated, and is effective in rapidly aborting migraine.9
Volume repletion
Although its use is anecdotal and to date no trial has measured its efficacy, IV volume repletion is often used in acute migraine, as most headache experts surmise it to be highly effective, especially in patients with prolonged nausea or vomiting.1
Magnesium
IV magnesium is effective, particularly for migraine with aura.10 Hypotension is a common side effect, and pretreatment or concurrent treatment with IV fluids is advised. Doses from 500 mg to 1,000 mg have demonstrated efficacy.10
Corticosteroids
Corticosteroids can be used in the treatment of status migrainosus. Most studies have shown benefit in preventing recurrences rather than merely aborting migraine.11 A systematic review suggested that recurrent headaches are milder with corticosteroid treatment; 19 of 25 studies indicated favorable benefit, and 6 of 19 studies indicated noninferior outcomes.12
Both IV methylprednisolone and IV dexamethasone may be considered.12 Dexamethasone appears to be particularly effective in preventing headache recurrence when combined with other IV therapies.13 It can be given as a single dose of 10 mg, or as repeated doses of 4 mg up to 16 mg/day.1 Patients with active psychosis or uncontrolled diabetes should be closely monitored for these conditions, which corticosteroids can worsen.
Serotoninergic agents
Serotonin agonists including subcutaneous sumatriptan and IV dihydroergotamine are highly effective, with proven statistical and clinical benefit.4 They should be considered in patients with no known history of coronary artery disease or other vaso-occlusive vascular disorder.1
Ideally, IV dihydroergotamine should be administered after consultation with a neurologist or headache specialist, given the pretreatment and cotreatment requirements often necessary to suppress its side effects. Careful titration is important to prevent transient headache exacerbations during infusion, as well as abdominal cramping, nausea, and diarrhea.
Avoid opioids
Opioids should be avoided. Evidence supporting their use in acute migraine is extremely limited,3 and the risks of migraine becoming chronic and of addiction are high.14 Safer, more effective alternatives have been detailed above.
A detailed algorithm for the management of patients with acute migraine has been published14 and is aimed at decreasing acute treatment with opioids and barbiturates.
We recommend the following combination treatment:
Normal saline (0.9% NaCl) 1 to 2 L by intravenous (IV) infusion over 2 to 4 hours. This can be repeated every 6 to 12 hours.
Ketorolac 30-mg IV bolus, which can be repeated every 6 hours. However, patients with coronary artery disease, uncontrolled hypertension, acute renal failure, or cerebrovascular disease should instead receive acetaminophen 1,000 mg, naproxen sodium 550 mg, or aspirin 325 mg by mouth.
Prochlorperazine or metoclopramide 10-mg IV infusion. This can be repeated every 6 hours. However, to reduce the extrapyramidal adverse effects of these drugs, patients should first receive diphenhydramine 25- to 50-mg IV bolus, which can be repeated every 6 to 8 hours.
Sodium valproate 500 to 1,000 mg by IV infusion over 20 minutes. This can be repeated after 8 hours.
Dexamethasone 4-mg IV bolus every 6 hours, or 10-mg IV bolus once in 24 hours.
Magnesium sulfate 500 to 1,000 mg by IV infusion over 1 hour. This can be repeated every 6 to 12 hours.
If the migraine has not improved after 3 cycles of this regimen, a neurologic consultation should be considered. Other options include dihydroergotamine and occipital nerve blocks1 performed at the bedside.
GENERAL PRINCIPLES
Managing acute intractable migraine can be frustrating for both the practitioner and the patient. Some general principles are helpful.
Use a combination of drugs. Aborting a severe migraine attack often requires a combination of medications that work synergistically.
Use IV and intramuscular formulations rather than oral formulations: they are more rapidly absorbed, provide faster pain relief, and can be given when the nausea that often accompanies migraine precludes oral treatments.
Rule out secondary causes. The mnemonic SNOOP—systemic signs, neurologic signs, onset, older age, progression of existing headache disorder—is useful for assessing underlying causes.2 Any patient presenting with intractable migraine should also have a thorough neurologic examination.
Screening electrocardiography may be helpful, as the pretreatment QTc interval may direct the choice of intravenous treatment. If the patient has a prolonged QTc or is taking another drug that could prolong the QTc, certain medications, specifically dopamine receptor antagonists and diphenhydramine, should be avoided.
Ask the patient what has worked previously. A particular agent may have been effective in aborting the migraine; thus, a single dose of it could abort the headache, expediting discharge.
Establish if a triptan or ergot derivative has been used during the 24 hours before presentation, as repeated dosing within this interval is not recommended.3
Establish the baseline headache severity. Complete headache relief is difficult to achieve in a patient with chronic daily headache, and establishing a more realistic goal (eg, 50% relief) from the outset is useful.
OPTIONS FOR DRUG THERAPY
Antiemetics
Dopamine receptor antagonists are assumed to merely treat nausea in patients with migraine; however, they act independently to abort migraine and thus should be considered, irrespective of the presence of nausea.
The two most commonly used agents are prochlorperazine and metoclopramide. The American Academy of Neurology guidelines recommend prochlorperazine as first-line therapy for acute migraine. Metoclopramide is rated slightly lower and is considered to have moderate benefit.4 The Canadian Headache Society cites a high level of evidence supporting prochlorperazine and a moderate level of evidence supporting metoclopramide.5 The American Headache Society assessment of parenteral pharmacotherapies gives prochlorperazine and metoclopramide a level B recommendation of “should offer” (a recommendation only additionally assigned to subcutaneous sumatriptan).3 Hence, either agent can be used.
To reduce the risk of posttreatment akathisia, diphenhydramine or benztropine may be given before starting a dopamine receptor antagonist. Diphenhydramine may be independently effective in migraine treatment,6,7 but data on this are limited.
Ketorolac, ibuprofen
Ketorolac and ibuprofen are the only available nonsteroidal antiinflammatory drugs (NSAIDs) for IV administration. The Canadian Headache Society guidelines strongly recommend ketorolac for the treatment of migraine in emergency settings.5 Doses range from 30 mg to 60 mg.1 Ibuprofen 400 to 800 mg by IV infusion is an acceptable alternative. These medications should be avoided in patients with renal failure or severe coronary artery disease.
Oral naproxen sodium is a possible alternative in patients with cardiovascular disease, as it has been shown to carry a lower cardiovascular risk than other NSAIDs.8
The same concerns in patients with renal dysfunction apply to any NSAID, as the enzyme cyclooxygenase plays a constitutive role in glomerular function.
Antiepileptic drugs
The antiepileptic drugs sodium valproate and levetiracetam are available in IV formulations that have demonstrated efficacy in the treatment of status migrainosus1 (ie, migraine lasting more than 72 hours). Valproate has the strongest track record, is well tolerated, and is effective in rapidly aborting migraine.9
Volume repletion
Although its use is anecdotal and to date no trial has measured its efficacy, IV volume repletion is often used in acute migraine, as most headache experts surmise it to be highly effective, especially in patients with prolonged nausea or vomiting.1
Magnesium
IV magnesium is effective, particularly for migraine with aura.10 Hypotension is a common side effect, and pretreatment or concurrent treatment with IV fluids is advised. Doses from 500 mg to 1,000 mg have demonstrated efficacy.10
Corticosteroids
Corticosteroids can be used in the treatment of status migrainosus. Most studies have shown benefit in preventing recurrences rather than merely aborting migraine.11 A systematic review suggested that recurrent headaches are milder with corticosteroid treatment; 19 of 25 studies indicated favorable benefit, and 6 of 19 studies indicated noninferior outcomes.12
Both IV methylprednisolone and IV dexamethasone may be considered.12 Dexamethasone appears to be particularly effective in preventing headache recurrence when combined with other IV therapies.13 It can be given as a single dose of 10 mg, or as repeated doses of 4 mg up to 16 mg/day.1 Patients with active psychosis or uncontrolled diabetes should be closely monitored for these conditions, which corticosteroids can worsen.
Serotoninergic agents
Serotonin agonists including subcutaneous sumatriptan and IV dihydroergotamine are highly effective, with proven statistical and clinical benefit.4 They should be considered in patients with no known history of coronary artery disease or other vaso-occlusive vascular disorder.1
Ideally, IV dihydroergotamine should be administered after consultation with a neurologist or headache specialist, given the pretreatment and cotreatment requirements often necessary to suppress its side effects. Careful titration is important to prevent transient headache exacerbations during infusion, as well as abdominal cramping, nausea, and diarrhea.
Avoid opioids
Opioids should be avoided. Evidence supporting their use in acute migraine is extremely limited,3 and the risks of migraine becoming chronic and of addiction are high.14 Safer, more effective alternatives have been detailed above.
A detailed algorithm for the management of patients with acute migraine has been published14 and is aimed at decreasing acute treatment with opioids and barbiturates.
- Rozen TD. Emergency department and inpatient management of status migrainosus and intractable headache. Continuum (Minneap Minn) 2015; 21(4):1004–1017. doi:10.1212/CON.0000000000000191
- Dodick D. Headache as a symptom of ominous disease. What are the warning signals? Postgrad Med 1997; 101(5):46–50, 55–56, 62–64. doi:10.3810/pgm.1997.05.217
- Orr SL, Friedman BW, Christie S, et al. Management of adults with acute migraine in the emergency department: the American Headache Society evidence assessment of parenteral pharmacotherapies. Headache 2016; 56(6):911–940. doi:10.1111/head.12835
- Silberstein SD. Practice parameter: evidence-based guidelines for migraine headache (an evidence-based review): report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology 2000; 55(6):754–762. doi:10.1212/WNL.55.6.754
- Orr SL, Aubé M, Becker WJ, et al. Canadian Headache Society systematic review and recommendations on the treatment of migraine pain in emergency settings. Cephalalgia 2015; 35(3):271–284. doi:10.1177/0333102414535997
- Swidan SZ, Lake AE 3rd, Saper JR. Efficacy of intravenous diphenhydramine versus intravenous DHE-45 in the treatment of severe migraine headache. Curr Pain Headache Rep 2005; 9(1):65–70. doi:10.1007/s11916-005-0077-5
- Marmura MJ, Goldberg SW. Inpatient management of migraine. Curr Neurol Neurosci Rep 2015; 15(4):13. doi:10.1007/s11910-015-0539-z
- Farkouh ME, Greenberg BP. An evidence-based review of the cardiovascular risks of nonsteroidal anti-inflammatory drugs. Am J Cardiol 2009; 103(9):1227–1237. doi:10.1016/j.amjcard.2009.01.014
- Stillman MJ, Zajac D, Rybicki LA. Treatment of primary headache disorders with intravenous valproate: initial outpatient experience. Headache 2004; 44(1):65–69. doi:10.1111/j.1526-4610.2004.04010.x
- Marmura MJ, Silberstein SD, Schwedt TJ. The acute treatment of migraine in adults: the American Headache Society evidence assessment of migraine pharmacotherapies. Headache 2015; 55(1):3–20. doi:10.1111/head.12499
- Colman I, Friedman BW, Brown MD, et al. Parenteral dexamethasone for acute severe migraine headache: meta-analysis of randomised controlled trials for preventing recurrence. BMJ 2008; 336(7657):1359–1361. doi:10.1136/bmj.39566.806725.BE
- Woldeamanuel YW, Rapoport AM, Cowan RP. The place of corticosteroids in migraine attack management: a 65-year systematic review with pooled analysis and critical appraisal. Cephalalgia 2015; 35(1):996–1024. doi:10.1177/0333102414566200
- Singh A, Alter HJ, Zaia B. Does the addition of dexamethasone to standard therapy for acute migraine headache decrease the incidence of recurrent headache for patients treated in the emergency department? A meta-analysis and systematic review of the literature. Acad Emerg Med 2008; 15(12):1223–1233. doi:10.1111/j.1553-2712.2008.00283.x
- Ahmed ZA, Nacopoulos DA, John S, Papesh N, Levine D, Bamford CC. An algorithm for opioid and barbiturate reduction in the acute management of headache in the emergency department. Headache 2017; 57(1):71–79. doi:10.1111/head.12961
- Rozen TD. Emergency department and inpatient management of status migrainosus and intractable headache. Continuum (Minneap Minn) 2015; 21(4):1004–1017. doi:10.1212/CON.0000000000000191
- Dodick D. Headache as a symptom of ominous disease. What are the warning signals? Postgrad Med 1997; 101(5):46–50, 55–56, 62–64. doi:10.3810/pgm.1997.05.217
- Orr SL, Friedman BW, Christie S, et al. Management of adults with acute migraine in the emergency department: the American Headache Society evidence assessment of parenteral pharmacotherapies. Headache 2016; 56(6):911–940. doi:10.1111/head.12835
- Silberstein SD. Practice parameter: evidence-based guidelines for migraine headache (an evidence-based review): report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology 2000; 55(6):754–762. doi:10.1212/WNL.55.6.754
- Orr SL, Aubé M, Becker WJ, et al. Canadian Headache Society systematic review and recommendations on the treatment of migraine pain in emergency settings. Cephalalgia 2015; 35(3):271–284. doi:10.1177/0333102414535997
- Swidan SZ, Lake AE 3rd, Saper JR. Efficacy of intravenous diphenhydramine versus intravenous DHE-45 in the treatment of severe migraine headache. Curr Pain Headache Rep 2005; 9(1):65–70. doi:10.1007/s11916-005-0077-5
- Marmura MJ, Goldberg SW. Inpatient management of migraine. Curr Neurol Neurosci Rep 2015; 15(4):13. doi:10.1007/s11910-015-0539-z
- Farkouh ME, Greenberg BP. An evidence-based review of the cardiovascular risks of nonsteroidal anti-inflammatory drugs. Am J Cardiol 2009; 103(9):1227–1237. doi:10.1016/j.amjcard.2009.01.014
- Stillman MJ, Zajac D, Rybicki LA. Treatment of primary headache disorders with intravenous valproate: initial outpatient experience. Headache 2004; 44(1):65–69. doi:10.1111/j.1526-4610.2004.04010.x
- Marmura MJ, Silberstein SD, Schwedt TJ. The acute treatment of migraine in adults: the American Headache Society evidence assessment of migraine pharmacotherapies. Headache 2015; 55(1):3–20. doi:10.1111/head.12499
- Colman I, Friedman BW, Brown MD, et al. Parenteral dexamethasone for acute severe migraine headache: meta-analysis of randomised controlled trials for preventing recurrence. BMJ 2008; 336(7657):1359–1361. doi:10.1136/bmj.39566.806725.BE
- Woldeamanuel YW, Rapoport AM, Cowan RP. The place of corticosteroids in migraine attack management: a 65-year systematic review with pooled analysis and critical appraisal. Cephalalgia 2015; 35(1):996–1024. doi:10.1177/0333102414566200
- Singh A, Alter HJ, Zaia B. Does the addition of dexamethasone to standard therapy for acute migraine headache decrease the incidence of recurrent headache for patients treated in the emergency department? A meta-analysis and systematic review of the literature. Acad Emerg Med 2008; 15(12):1223–1233. doi:10.1111/j.1553-2712.2008.00283.x
- Ahmed ZA, Nacopoulos DA, John S, Papesh N, Levine D, Bamford CC. An algorithm for opioid and barbiturate reduction in the acute management of headache in the emergency department. Headache 2017; 57(1):71–79. doi:10.1111/head.12961
When does S aureus bacteremia require transesophageal echocardiography?
Staphylococcus aureus is the most common infective agent in native and prosthetic valve endocarditis, and 13% to 22% of patients with S aureus bacteremia have infective endocarditis.1
Transthoracic echocardiography (TTE) is a good starting point in the workup of suspected infective endocarditis, but transesophageal echocardiography (TEE) plays a key role in diagnosis and is indicated in patients with a high pretest probability of infective endocarditis, as in the following scenarios:
- Clinical picture consistent with infective endocarditis
- Presence of previously placed port or other indwelling vascular device
- Presence of a prosthetic valve or other prosthetic material
- Presence of a pacemaker
- History of valve disease
- Injection drug use
- Positive blood cultures after 72 hours despite appropriate antibiotic treatment
- Abnormal TTE result requiring better visualization of valvular anatomy and function and confirmation of local complications
- Absence of another reasonable explanation for S aureus bacteremia.
Forgoing TEE is reasonable in patients with normal results on TTE, no predisposing risk factors, a reasonable alternative explanation for S aureus bacteremia, and a low pretest probability of infective endocarditis.1 TEE may also be unnecessary if there is another disease focus requiring extended treatment (eg, vertebral infection) and there are no findings suggesting complicated infective endocarditis, eg, persistent bacteremia, symptoms of heart failure, and conduction abnormality.1
TEE also may be unnecessary in patients at low risk who have identifiable foci of bacteremia due to soft-tissue infection or a newly placed vascular catheter and whose bacteremia clears within 72 hours of the start of antibiotic therapy. These patients may be followed clinically for the development of new findings such as metastatic foci of infection (eg, septic pulmonary emboli, renal infarction, splenic abscess or infarction), the new onset of heart failure or cardiac conduction abnormality, or recurrence of previously cleared S aureus bacteremia. If these should develop, then a more invasive study such as TEE may be warranted.
INFECTIVE ENDOCARDITIS: EPIDEMIOLOGY AND MICROBIOLOGY
The US incidence rate of infective endocarditis has steadily increased, with an estimated 457,052 hospitalizations from 2000 to 2011. During that period, from 2000 to 2007, there was a marked increase in valve replacement surgeries.2 This trend is likely explained by an increase in the at-risk population—eg, elderly patients, patients with opiate dependence or diabetes, and patients on hemodialysis.
Although S aureus is the predominant pathogen in infective endocarditis,2–5S aureus bacteremia is often observed in patients with skin or soft-tissue infection, prosthetic device infection, vascular graft or catheter infection, and bone and joint infections. S aureus bacteremia necessitates a search for the source of infection.
S aureus is a major pathogen in bloodstream infections, and up to 14% of patients with S aureus bacteremia have infective endocarditis as the primary source of infection.3 The pathogenesis of S aureus infective endocarditis is thought to be mediated by cell-wall factors that promote adhesion to the extracellular matrix of intravascular structures.3
A new localizing symptom such as back pain, joint pain, or swelling in a patient with S aureus bacteremia should trigger an investigation for metastatic infection.
Infectious disease consultation in patients with S aureus bacteremia is associated with improved outcomes and, thus, should be pursued.3
A cardiac surgery consult is recommended early on in cases of infective endocarditis caused by vancomycin-resistant enterococci, Pseudomonas aeruginosa, and fungi, as well as in patients with complications such as valvular insufficiency, perivalvular abscess, conduction abnormalities, persistent bacteremia, and metastatic foci of infection.6
RISK FACTORS
Risk factors for infective endocarditis include injection drug abuse, valvular heart disease, congenital heart disease (unrepaired, repaired with residual defects, or fully repaired within the past 6 months), previous infective endocarditis, prosthetic heart valve, and cardiac transplant.2–4,6 Other risk factors are poor dentition, hemodialysis, ventriculoatrial shunts, intravascular devices including vascular grafts, and pacemakers.2,3 Many risk factors for infective endocarditis and S aureus bacteremia overlap.3
DIAGNOSTIC PRINCIPLES
The clinical presentation of infective endocarditis can vary from a nonspecific infectious syndrome, to overt organ failure (heart failure, kidney failure), to an acute vascular catastrophe (arterial ischemia, cerebrovascular accidents, myocardial infarction). Patients may present with indolent symptoms such as fever, fatigue, and weight loss,6 or they may present at an advanced stage, with fulminant acute heart failure due to valvular insufficiency or with arrhythmias due to a perivalvular abscess infiltrating the conduction system. Extracardiac clinical manifestations may be related to direct infective metastatic foci such as septic emboli or to immunologic phenomena such as glomerulonephritis or Osler nodes.
ECHOCARDIOGRAPHY’S ROLE IN DIAGNOSIS
TTE plays an important role in diagnosis and risk stratification of infective endocarditis.6 TTE is usually done first because of its low cost, wide availability, and safety; it has a sensitivity of 70% and a specificity over 95%.8 While a normal result on TTE does not completely rule out infective endocarditis, completely normal valvular morphology and function on TTE make the diagnosis less likely.8,9
If suspicion remains high despite a normal study, repeating TTE at a later time may result in a higher diagnostic yield because of growth of the suspected vegetation. Otherwise, TEE should be considered.
TEE provides a higher spatial resolution and diagnostic yield than TTE, especially for detecting complex pathology such as pseudoaneurysm, valve perforation, or valvular abscess. TEE has a sensitivity and specificity of approximately 95% for infective endocarditis.8 It should be performed early in patients with preexisting valve disease, prosthetic cardiac material (eg, valves), or a pacemaker or implantable cardioverter-defibrillator.6,7
Detecting valve vegetation provides answers about the cause of S aureus bacteremia with its complications (eg, septic emboli, mycotic aneurysm) and informs decisions about the duration of antibiotic therapy and the need for surgery.3,6
As with any diagnostic test, it is important to compare the results of any recent study with those of previous studies whenever possible to differentiate new from old findings.
WHEN TO FORGO TEE IN S AUREUS BACTEREMIA
Because TEE is invasive and requires the patient to swallow an endoscopic probe,10 it is important to screen patients for esophageal disease, cervical spine conditions, and baseline respiratory insufficiency. Complications are rare but include esophageal perforation, esophageal bleeding, pharyngeal hematoma, and reactions to anesthesia.10
As with any diagnostic test, the clinician first needs to consider the patient’s pretest probability of the disease, the diagnostic accuracy, the associated risks and costs, and the implications of the results.
While TEE provides better diagnostic images than TTE, a normal TEE study does not exclude the diagnosis of infective endocarditis: small lesions and complications such as paravalvular abscess of a prosthetic aortic valve may still be missed. In such patients, a repeat TEE examination or additional imaging study (eg, gated computed tomographic angiography) should be considered.6
Noninfective sterile echodensities, valvular tumors such as papillary fibroelastomas, Lambl excrescences, and suture lines of prosthetic valves are among the conditions and factors that can cause a false-positive result on TEE.
- Young H, Knepper BC, Price CS, Heard S, Jenkins TC. Clinical reasoning of infectious diseases physicians behind the use or nonuse of transesophageal echocardiography in Staphylococcus aureus bacteremia. Open Forum Infect Dis 2016; 3(4):ofw204. doi:10.1093/ofid/ofw204
- Pant S, Patel NJ, Deshmukh A, et al. Trends in infective endocarditis incidence, microbiology, and valve replacement in the United States from 2000 to 2011. J Am Coll Cardiol 2015; 65(19):2070–2076. doi:10.1016/j.jacc.2015.03.518
- Tong SY, Davis JS, Eichenberger E, Holland TL, Fowler VG Jr. Staphylococcus aureus infections: epidemiology, pathophysiology, clinical manifestations, and management. Clin Microbiol Rev 2015; 28(3):603–661. doi:10.1128/CMR.00134-14
- Palraj BR, Baddour LM, Hess EP, et al. Predicting risk of endocarditis using a clinical tool (PREDICT): scoring system to guide use of echocardiography in the management of Staphylococcus aureus bacteremia. Clin Infect Dis 2015; 61(1):18–28. doi:10.1093/cid/civ235
- Barton T, Moir S, Rehmani H, Woolley I, Korman TM, Stuart RL. Low rates of endocarditis in healthcare-associated Staphylococcus aureus bacteremia suggest that echocardiography might not always be required. Eur J Clin Microbiol Infect Dis 2016; 35(1):49–55. doi:10.1007/s10096-015-2505-8
- Baddour LM, Wilson WR, Bayer AS, et al; American Heart Association Committee on Rheumatic Fever, Endocarditis, and Kawasaki Disease of the Council on Cardiovascular Disease in the Young, Council on Clinical Cardiology, Council on Cardiovascular Surgery and Anesthesia, and Stroke Council. Infective endocarditis in adults: diagnosis, antimicrobial therapy, and management of complications: a scientific statement for healthcare professionals from the American Heart Association. Circulation 2015; 132(15):1435–1486. doi10.1161/CIR.0000000000000296
- Li JS, Sexton DJ, Mick N, et al. Proposed modifications to the Duke criteria for the diagnosis of infective endocarditis. Clin Infect Dis 2000; 30(4):633–638. doi:10.1086/313753
- Habib G, Badano L, Tribouilloy C, et al; European Association of Echocardiography. Recommendations for the practice of echocardiography in infective endocarditis. Eur J Echocardiogr 2010; 11(2):202–219. doi:10.1093/ejechocard/jeq004
- Irani WN, Grayburn PA, Afridi I. A negative transthoracic echocardiogram obviates the need for transesophageal echocardiography in patients with suspected native valve active infective endocarditis. Am J Cardiol 1996; 78(1):101–103. pmid:8712097
- Hahn RT, Abraham T, Adams MS, et al. Guidelines for performing a comprehensive transesophageal echocardiographic examination: recommendations from the American Society of Echocardiography and the Society of Cardiovascular Anesthesiologists. J Am Soc Echocardiogr 2013; 26(9):921–964. doi:10.1016/j.echo.2013.07.009
Staphylococcus aureus is the most common infective agent in native and prosthetic valve endocarditis, and 13% to 22% of patients with S aureus bacteremia have infective endocarditis.1
Transthoracic echocardiography (TTE) is a good starting point in the workup of suspected infective endocarditis, but transesophageal echocardiography (TEE) plays a key role in diagnosis and is indicated in patients with a high pretest probability of infective endocarditis, as in the following scenarios:
- Clinical picture consistent with infective endocarditis
- Presence of previously placed port or other indwelling vascular device
- Presence of a prosthetic valve or other prosthetic material
- Presence of a pacemaker
- History of valve disease
- Injection drug use
- Positive blood cultures after 72 hours despite appropriate antibiotic treatment
- Abnormal TTE result requiring better visualization of valvular anatomy and function and confirmation of local complications
- Absence of another reasonable explanation for S aureus bacteremia.
Forgoing TEE is reasonable in patients with normal results on TTE, no predisposing risk factors, a reasonable alternative explanation for S aureus bacteremia, and a low pretest probability of infective endocarditis.1 TEE may also be unnecessary if there is another disease focus requiring extended treatment (eg, vertebral infection) and there are no findings suggesting complicated infective endocarditis, eg, persistent bacteremia, symptoms of heart failure, and conduction abnormality.1
TEE also may be unnecessary in patients at low risk who have identifiable foci of bacteremia due to soft-tissue infection or a newly placed vascular catheter and whose bacteremia clears within 72 hours of the start of antibiotic therapy. These patients may be followed clinically for the development of new findings such as metastatic foci of infection (eg, septic pulmonary emboli, renal infarction, splenic abscess or infarction), the new onset of heart failure or cardiac conduction abnormality, or recurrence of previously cleared S aureus bacteremia. If these should develop, then a more invasive study such as TEE may be warranted.
INFECTIVE ENDOCARDITIS: EPIDEMIOLOGY AND MICROBIOLOGY
The US incidence rate of infective endocarditis has steadily increased, with an estimated 457,052 hospitalizations from 2000 to 2011. During that period, from 2000 to 2007, there was a marked increase in valve replacement surgeries.2 This trend is likely explained by an increase in the at-risk population—eg, elderly patients, patients with opiate dependence or diabetes, and patients on hemodialysis.
Although S aureus is the predominant pathogen in infective endocarditis,2–5S aureus bacteremia is often observed in patients with skin or soft-tissue infection, prosthetic device infection, vascular graft or catheter infection, and bone and joint infections. S aureus bacteremia necessitates a search for the source of infection.
S aureus is a major pathogen in bloodstream infections, and up to 14% of patients with S aureus bacteremia have infective endocarditis as the primary source of infection.3 The pathogenesis of S aureus infective endocarditis is thought to be mediated by cell-wall factors that promote adhesion to the extracellular matrix of intravascular structures.3
A new localizing symptom such as back pain, joint pain, or swelling in a patient with S aureus bacteremia should trigger an investigation for metastatic infection.
Infectious disease consultation in patients with S aureus bacteremia is associated with improved outcomes and, thus, should be pursued.3
A cardiac surgery consult is recommended early on in cases of infective endocarditis caused by vancomycin-resistant enterococci, Pseudomonas aeruginosa, and fungi, as well as in patients with complications such as valvular insufficiency, perivalvular abscess, conduction abnormalities, persistent bacteremia, and metastatic foci of infection.6
RISK FACTORS
Risk factors for infective endocarditis include injection drug abuse, valvular heart disease, congenital heart disease (unrepaired, repaired with residual defects, or fully repaired within the past 6 months), previous infective endocarditis, prosthetic heart valve, and cardiac transplant.2–4,6 Other risk factors are poor dentition, hemodialysis, ventriculoatrial shunts, intravascular devices including vascular grafts, and pacemakers.2,3 Many risk factors for infective endocarditis and S aureus bacteremia overlap.3
DIAGNOSTIC PRINCIPLES
The clinical presentation of infective endocarditis can vary from a nonspecific infectious syndrome, to overt organ failure (heart failure, kidney failure), to an acute vascular catastrophe (arterial ischemia, cerebrovascular accidents, myocardial infarction). Patients may present with indolent symptoms such as fever, fatigue, and weight loss,6 or they may present at an advanced stage, with fulminant acute heart failure due to valvular insufficiency or with arrhythmias due to a perivalvular abscess infiltrating the conduction system. Extracardiac clinical manifestations may be related to direct infective metastatic foci such as septic emboli or to immunologic phenomena such as glomerulonephritis or Osler nodes.
ECHOCARDIOGRAPHY’S ROLE IN DIAGNOSIS
TTE plays an important role in diagnosis and risk stratification of infective endocarditis.6 TTE is usually done first because of its low cost, wide availability, and safety; it has a sensitivity of 70% and a specificity over 95%.8 While a normal result on TTE does not completely rule out infective endocarditis, completely normal valvular morphology and function on TTE make the diagnosis less likely.8,9
If suspicion remains high despite a normal study, repeating TTE at a later time may result in a higher diagnostic yield because of growth of the suspected vegetation. Otherwise, TEE should be considered.
TEE provides a higher spatial resolution and diagnostic yield than TTE, especially for detecting complex pathology such as pseudoaneurysm, valve perforation, or valvular abscess. TEE has a sensitivity and specificity of approximately 95% for infective endocarditis.8 It should be performed early in patients with preexisting valve disease, prosthetic cardiac material (eg, valves), or a pacemaker or implantable cardioverter-defibrillator.6,7
Detecting valve vegetation provides answers about the cause of S aureus bacteremia with its complications (eg, septic emboli, mycotic aneurysm) and informs decisions about the duration of antibiotic therapy and the need for surgery.3,6
As with any diagnostic test, it is important to compare the results of any recent study with those of previous studies whenever possible to differentiate new from old findings.
WHEN TO FORGO TEE IN S AUREUS BACTEREMIA
Because TEE is invasive and requires the patient to swallow an endoscopic probe,10 it is important to screen patients for esophageal disease, cervical spine conditions, and baseline respiratory insufficiency. Complications are rare but include esophageal perforation, esophageal bleeding, pharyngeal hematoma, and reactions to anesthesia.10
As with any diagnostic test, the clinician first needs to consider the patient’s pretest probability of the disease, the diagnostic accuracy, the associated risks and costs, and the implications of the results.
While TEE provides better diagnostic images than TTE, a normal TEE study does not exclude the diagnosis of infective endocarditis: small lesions and complications such as paravalvular abscess of a prosthetic aortic valve may still be missed. In such patients, a repeat TEE examination or additional imaging study (eg, gated computed tomographic angiography) should be considered.6
Noninfective sterile echodensities, valvular tumors such as papillary fibroelastomas, Lambl excrescences, and suture lines of prosthetic valves are among the conditions and factors that can cause a false-positive result on TEE.
Staphylococcus aureus is the most common infective agent in native and prosthetic valve endocarditis, and 13% to 22% of patients with S aureus bacteremia have infective endocarditis.1
Transthoracic echocardiography (TTE) is a good starting point in the workup of suspected infective endocarditis, but transesophageal echocardiography (TEE) plays a key role in diagnosis and is indicated in patients with a high pretest probability of infective endocarditis, as in the following scenarios:
- Clinical picture consistent with infective endocarditis
- Presence of previously placed port or other indwelling vascular device
- Presence of a prosthetic valve or other prosthetic material
- Presence of a pacemaker
- History of valve disease
- Injection drug use
- Positive blood cultures after 72 hours despite appropriate antibiotic treatment
- Abnormal TTE result requiring better visualization of valvular anatomy and function and confirmation of local complications
- Absence of another reasonable explanation for S aureus bacteremia.
Forgoing TEE is reasonable in patients with normal results on TTE, no predisposing risk factors, a reasonable alternative explanation for S aureus bacteremia, and a low pretest probability of infective endocarditis.1 TEE may also be unnecessary if there is another disease focus requiring extended treatment (eg, vertebral infection) and there are no findings suggesting complicated infective endocarditis, eg, persistent bacteremia, symptoms of heart failure, and conduction abnormality.1
TEE also may be unnecessary in patients at low risk who have identifiable foci of bacteremia due to soft-tissue infection or a newly placed vascular catheter and whose bacteremia clears within 72 hours of the start of antibiotic therapy. These patients may be followed clinically for the development of new findings such as metastatic foci of infection (eg, septic pulmonary emboli, renal infarction, splenic abscess or infarction), the new onset of heart failure or cardiac conduction abnormality, or recurrence of previously cleared S aureus bacteremia. If these should develop, then a more invasive study such as TEE may be warranted.
INFECTIVE ENDOCARDITIS: EPIDEMIOLOGY AND MICROBIOLOGY
The US incidence rate of infective endocarditis has steadily increased, with an estimated 457,052 hospitalizations from 2000 to 2011. During that period, from 2000 to 2007, there was a marked increase in valve replacement surgeries.2 This trend is likely explained by an increase in the at-risk population—eg, elderly patients, patients with opiate dependence or diabetes, and patients on hemodialysis.
Although S aureus is the predominant pathogen in infective endocarditis,2–5S aureus bacteremia is often observed in patients with skin or soft-tissue infection, prosthetic device infection, vascular graft or catheter infection, and bone and joint infections. S aureus bacteremia necessitates a search for the source of infection.
S aureus is a major pathogen in bloodstream infections, and up to 14% of patients with S aureus bacteremia have infective endocarditis as the primary source of infection.3 The pathogenesis of S aureus infective endocarditis is thought to be mediated by cell-wall factors that promote adhesion to the extracellular matrix of intravascular structures.3
A new localizing symptom such as back pain, joint pain, or swelling in a patient with S aureus bacteremia should trigger an investigation for metastatic infection.
Infectious disease consultation in patients with S aureus bacteremia is associated with improved outcomes and, thus, should be pursued.3
A cardiac surgery consult is recommended early on in cases of infective endocarditis caused by vancomycin-resistant enterococci, Pseudomonas aeruginosa, and fungi, as well as in patients with complications such as valvular insufficiency, perivalvular abscess, conduction abnormalities, persistent bacteremia, and metastatic foci of infection.6
RISK FACTORS
Risk factors for infective endocarditis include injection drug abuse, valvular heart disease, congenital heart disease (unrepaired, repaired with residual defects, or fully repaired within the past 6 months), previous infective endocarditis, prosthetic heart valve, and cardiac transplant.2–4,6 Other risk factors are poor dentition, hemodialysis, ventriculoatrial shunts, intravascular devices including vascular grafts, and pacemakers.2,3 Many risk factors for infective endocarditis and S aureus bacteremia overlap.3
DIAGNOSTIC PRINCIPLES
The clinical presentation of infective endocarditis can vary from a nonspecific infectious syndrome, to overt organ failure (heart failure, kidney failure), to an acute vascular catastrophe (arterial ischemia, cerebrovascular accidents, myocardial infarction). Patients may present with indolent symptoms such as fever, fatigue, and weight loss,6 or they may present at an advanced stage, with fulminant acute heart failure due to valvular insufficiency or with arrhythmias due to a perivalvular abscess infiltrating the conduction system. Extracardiac clinical manifestations may be related to direct infective metastatic foci such as septic emboli or to immunologic phenomena such as glomerulonephritis or Osler nodes.
ECHOCARDIOGRAPHY’S ROLE IN DIAGNOSIS
TTE plays an important role in diagnosis and risk stratification of infective endocarditis.6 TTE is usually done first because of its low cost, wide availability, and safety; it has a sensitivity of 70% and a specificity over 95%.8 While a normal result on TTE does not completely rule out infective endocarditis, completely normal valvular morphology and function on TTE make the diagnosis less likely.8,9
If suspicion remains high despite a normal study, repeating TTE at a later time may result in a higher diagnostic yield because of growth of the suspected vegetation. Otherwise, TEE should be considered.
TEE provides a higher spatial resolution and diagnostic yield than TTE, especially for detecting complex pathology such as pseudoaneurysm, valve perforation, or valvular abscess. TEE has a sensitivity and specificity of approximately 95% for infective endocarditis.8 It should be performed early in patients with preexisting valve disease, prosthetic cardiac material (eg, valves), or a pacemaker or implantable cardioverter-defibrillator.6,7
Detecting valve vegetation provides answers about the cause of S aureus bacteremia with its complications (eg, septic emboli, mycotic aneurysm) and informs decisions about the duration of antibiotic therapy and the need for surgery.3,6
As with any diagnostic test, it is important to compare the results of any recent study with those of previous studies whenever possible to differentiate new from old findings.
WHEN TO FORGO TEE IN S AUREUS BACTEREMIA
Because TEE is invasive and requires the patient to swallow an endoscopic probe,10 it is important to screen patients for esophageal disease, cervical spine conditions, and baseline respiratory insufficiency. Complications are rare but include esophageal perforation, esophageal bleeding, pharyngeal hematoma, and reactions to anesthesia.10
As with any diagnostic test, the clinician first needs to consider the patient’s pretest probability of the disease, the diagnostic accuracy, the associated risks and costs, and the implications of the results.
While TEE provides better diagnostic images than TTE, a normal TEE study does not exclude the diagnosis of infective endocarditis: small lesions and complications such as paravalvular abscess of a prosthetic aortic valve may still be missed. In such patients, a repeat TEE examination or additional imaging study (eg, gated computed tomographic angiography) should be considered.6
Noninfective sterile echodensities, valvular tumors such as papillary fibroelastomas, Lambl excrescences, and suture lines of prosthetic valves are among the conditions and factors that can cause a false-positive result on TEE.
- Young H, Knepper BC, Price CS, Heard S, Jenkins TC. Clinical reasoning of infectious diseases physicians behind the use or nonuse of transesophageal echocardiography in Staphylococcus aureus bacteremia. Open Forum Infect Dis 2016; 3(4):ofw204. doi:10.1093/ofid/ofw204
- Pant S, Patel NJ, Deshmukh A, et al. Trends in infective endocarditis incidence, microbiology, and valve replacement in the United States from 2000 to 2011. J Am Coll Cardiol 2015; 65(19):2070–2076. doi:10.1016/j.jacc.2015.03.518
- Tong SY, Davis JS, Eichenberger E, Holland TL, Fowler VG Jr. Staphylococcus aureus infections: epidemiology, pathophysiology, clinical manifestations, and management. Clin Microbiol Rev 2015; 28(3):603–661. doi:10.1128/CMR.00134-14
- Palraj BR, Baddour LM, Hess EP, et al. Predicting risk of endocarditis using a clinical tool (PREDICT): scoring system to guide use of echocardiography in the management of Staphylococcus aureus bacteremia. Clin Infect Dis 2015; 61(1):18–28. doi:10.1093/cid/civ235
- Barton T, Moir S, Rehmani H, Woolley I, Korman TM, Stuart RL. Low rates of endocarditis in healthcare-associated Staphylococcus aureus bacteremia suggest that echocardiography might not always be required. Eur J Clin Microbiol Infect Dis 2016; 35(1):49–55. doi:10.1007/s10096-015-2505-8
- Baddour LM, Wilson WR, Bayer AS, et al; American Heart Association Committee on Rheumatic Fever, Endocarditis, and Kawasaki Disease of the Council on Cardiovascular Disease in the Young, Council on Clinical Cardiology, Council on Cardiovascular Surgery and Anesthesia, and Stroke Council. Infective endocarditis in adults: diagnosis, antimicrobial therapy, and management of complications: a scientific statement for healthcare professionals from the American Heart Association. Circulation 2015; 132(15):1435–1486. doi10.1161/CIR.0000000000000296
- Li JS, Sexton DJ, Mick N, et al. Proposed modifications to the Duke criteria for the diagnosis of infective endocarditis. Clin Infect Dis 2000; 30(4):633–638. doi:10.1086/313753
- Habib G, Badano L, Tribouilloy C, et al; European Association of Echocardiography. Recommendations for the practice of echocardiography in infective endocarditis. Eur J Echocardiogr 2010; 11(2):202–219. doi:10.1093/ejechocard/jeq004
- Irani WN, Grayburn PA, Afridi I. A negative transthoracic echocardiogram obviates the need for transesophageal echocardiography in patients with suspected native valve active infective endocarditis. Am J Cardiol 1996; 78(1):101–103. pmid:8712097
- Hahn RT, Abraham T, Adams MS, et al. Guidelines for performing a comprehensive transesophageal echocardiographic examination: recommendations from the American Society of Echocardiography and the Society of Cardiovascular Anesthesiologists. J Am Soc Echocardiogr 2013; 26(9):921–964. doi:10.1016/j.echo.2013.07.009
- Young H, Knepper BC, Price CS, Heard S, Jenkins TC. Clinical reasoning of infectious diseases physicians behind the use or nonuse of transesophageal echocardiography in Staphylococcus aureus bacteremia. Open Forum Infect Dis 2016; 3(4):ofw204. doi:10.1093/ofid/ofw204
- Pant S, Patel NJ, Deshmukh A, et al. Trends in infective endocarditis incidence, microbiology, and valve replacement in the United States from 2000 to 2011. J Am Coll Cardiol 2015; 65(19):2070–2076. doi:10.1016/j.jacc.2015.03.518
- Tong SY, Davis JS, Eichenberger E, Holland TL, Fowler VG Jr. Staphylococcus aureus infections: epidemiology, pathophysiology, clinical manifestations, and management. Clin Microbiol Rev 2015; 28(3):603–661. doi:10.1128/CMR.00134-14
- Palraj BR, Baddour LM, Hess EP, et al. Predicting risk of endocarditis using a clinical tool (PREDICT): scoring system to guide use of echocardiography in the management of Staphylococcus aureus bacteremia. Clin Infect Dis 2015; 61(1):18–28. doi:10.1093/cid/civ235
- Barton T, Moir S, Rehmani H, Woolley I, Korman TM, Stuart RL. Low rates of endocarditis in healthcare-associated Staphylococcus aureus bacteremia suggest that echocardiography might not always be required. Eur J Clin Microbiol Infect Dis 2016; 35(1):49–55. doi:10.1007/s10096-015-2505-8
- Baddour LM, Wilson WR, Bayer AS, et al; American Heart Association Committee on Rheumatic Fever, Endocarditis, and Kawasaki Disease of the Council on Cardiovascular Disease in the Young, Council on Clinical Cardiology, Council on Cardiovascular Surgery and Anesthesia, and Stroke Council. Infective endocarditis in adults: diagnosis, antimicrobial therapy, and management of complications: a scientific statement for healthcare professionals from the American Heart Association. Circulation 2015; 132(15):1435–1486. doi10.1161/CIR.0000000000000296
- Li JS, Sexton DJ, Mick N, et al. Proposed modifications to the Duke criteria for the diagnosis of infective endocarditis. Clin Infect Dis 2000; 30(4):633–638. doi:10.1086/313753
- Habib G, Badano L, Tribouilloy C, et al; European Association of Echocardiography. Recommendations for the practice of echocardiography in infective endocarditis. Eur J Echocardiogr 2010; 11(2):202–219. doi:10.1093/ejechocard/jeq004
- Irani WN, Grayburn PA, Afridi I. A negative transthoracic echocardiogram obviates the need for transesophageal echocardiography in patients with suspected native valve active infective endocarditis. Am J Cardiol 1996; 78(1):101–103. pmid:8712097
- Hahn RT, Abraham T, Adams MS, et al. Guidelines for performing a comprehensive transesophageal echocardiographic examination: recommendations from the American Society of Echocardiography and the Society of Cardiovascular Anesthesiologists. J Am Soc Echocardiogr 2013; 26(9):921–964. doi:10.1016/j.echo.2013.07.009
What should I address at follow-up of patients who survive critical illness?
Patients who survive critical illness such as shock or respiratory failure warranting admission to an intensive care unit (ICU) often develop a constellation of chronic symptoms including cognitive decline, psychiatric disturbances, and physical weakness. These changes can prevent patients from returning to their former level of function and often necessitate significant support for patients and their caregivers.1
With growing awareness of the unique needs of ICU survivors, multidisciplinary PICS clinics have emerged. However, access to these clinics is limited, and most patients discharged from the ICU eventually follow up with their primary care provider. Primary care physicians who recognize PICS, understand its prognosis and its burden on caregivers, and are aware of tools that have shown promise in its management will be well prepared to address the needs of these patients.
COGNITIVE DECLINE
Several studies have shown that survivors of critical illness suffer from long-term impairment of multiple domains of cognition, including executive function. In one study, 40% of ICU survivors had global cognition scores at 1 year after discharge that were worse than those seen in moderate traumatic brain injury, and over 25% had scores similar to those seen in Alzheimer dementia.2 Age had poor correlation with the incidence of long-term cognitive impairment. Cognitive impairment may not be recognized in younger patients without a high index of suspicion and directed cognitive screening. Well-known cognitive impairment screening tests such as the Montreal Cognitive Assessment may help in the evaluation of PICS.
No treatment has been shown to improve long-term cognitive impairment from any cause. The most important intervention is to recognize it and to consider how impaired executive function may interfere with other aspects of treatment, such as participation in physical therapy and adherence to medication regimens.
Evidence is also emerging that patients are often inappropriately discharged on psychoactive medications (including atypical antipsychotic drugs and sedatives) that were started in the inpatient setting.7 These medications increase the risk of accidents, arrhythmia, and infection, as well as add to the overall cost of postdischarge care, and they do not improve the prolonged confusion and cognitive impairment associated with PICS.8 Psychoactive medications should be discontinued once delirium-associated behavior has resolved, as recommended in the American Geriatrics Society guideline on postoperative delirium.9 Further, patients and caregivers should be counseled so that they have reasonable expectations regarding the timing of cognitive recovery, which may be prolonged and incomplete.
PHYSICAL WEAKNESS
Prolonged physical weakness may affect up to one-third of patients who survive critical illness, and it may persist for years, severely compromising quality of life.10 In addition to deconditioning due to bedrest and illness, ICU patients often develop critical illness myopathy and critical illness polyneuropathy.
Although the mechanisms and risk factors for injury to muscles and peripheral nerves are not completely understood, the severity has been well described and ranges from proximal muscle weakness to complete quadriparesis, with inability to wean from mechanical ventilation. There is also an association with the severity of sepsis and the use of glucocorticoids and paralytics.10
Physical weakness can be readily apparent on routine history and physical examination. Differentiating critical illness myopathy from critical illness polyneuropathy requires invasive testing, including electromyography, but the results may not change management in the outpatient setting, making it unnecessary for most patients.
Physical weakness places a heavy burden on patients and their family and caregivers. As a result, most ICU patients suffer loss of employment and require supportive services on discharge, including home health aides and even institutionalization.
Physical therapy and occupational therapy are effective in reducing weakness and improving physical functioning; starting physical therapy in the outpatient setting may be as effective as early intervention in the ICU.11 Given the high prevalence of respiratory and cardiovascular disease in patients after ICU discharge, referral for pulmonary or cardiovascular rehabilitation is recommended. Because of the possible link between glucocorticoids and critical illness myopathy, these drugs should be decreased or discontinued as soon as possible.
PSYCHIATRIC DISTURBANCES
Mental health impairments in ICU survivors are common, severe, debilitating, and unfortunately, commonly overlooked. A recent study found a 37% incidence of depression and a 40% incidence of anxiety; further, 22% of patients met criteria for posttraumatic stress disorder.12 Patients with critical illness are also more likely to have had untreated mental health illness before hospitalization. Anxiety may present with poor sleep, irritability, and fatigue. Posttraumatic stress disorder may manifest as flashbacks or as a severe cognitive or behavioral response to provocation. All of these may be assessed using standard screening questionnaires, including the Posttraumatic Stress Disorder Checklist, the 2-item Patient Health Questionnaire (PHQ-2) for depression, and the 7-item Generalized Anxiety Disorder Screen (GAD-7).
Many primary care physicians are comfortable treating some of the psychiatric disturbances associated with PICS, such as depression, but may be challenged by the spectrum and complexity of mental illness of ICU survivors. Early referral to a mental health professional ensures optimal psychiatric care and allows more time to focus on the patient’s medical comorbidities.
SOCIAL SUPPORT
The cognitive, physical, and mental health complications coupled with other medical and psychiatric comorbidities result in serious social and financial stress on patients and their families. Long-term follow-up studies show that only half of patients return to work within 1 year of critical illness and that nearly one-fourth require continued assistance with activities of daily living.13 Reassuringly, however, most patients in 1 study had returned to work by 2 years from discharge.3
The immense burden on caregivers, the decrease in income, and increased expenditures in providing care result in increased stress on families. The incidence of depression, anxiety, and posttraumatic stress disorder is similar among patients and their caregivers.11 The frequency of emotional morbidity and the severity of the caregiver burden associated with caring for ICU survivors led to the description of a new entity: post-intensive care syndrome-family, or PICS-F.
Because of these stresses, patients often benefit from referral to a social worker. Patients should also be encouraged to bring their caregivers to physician appointments, and family members should be encouraged to discuss their perspectives in the context of a dedicated appointment. Family members should also be screened and treated for their own medical and mental health challenges. A dedicated ICU survivorship clinic may help facilitate this holistic approach and provide complementary services to the primary care provider.
CRITICAL CARE RECOVERY
As survival rates after critical illness continue to improve and clinicians encounter more patients with PICS, it is essential to appreciate the extent of associated physical, emotional, and financial hardship and to recognize when cognitive impairment may interfere with treatment. Early and accurate recognition of these challenges can help the primary care physician arrange and coordinate recovery services that ICU survivors require. Including family members in follow-up appointments can help overcome challenges in adherence to treatment plans, uncover gaps in social support, and identify signs of caregiver distress.
A thorough physical assessment and a thoughtful reconciliation of medications are critical, as is engaging the assistance of physical and occupational therapists, mental health professionals, and social workers.
Risk factors for the illness that necessitated the ICU stay such as uncontrolled diabetes, chronic obstructive pulmonary disease, and substance abuse, as well as medical sequelae such as chronic respiratory failure and heart failure, must be considered and addressed by the primary care physician, with referral to medical specialists if necessary.
Referral to an ICU survivorship center, if locally available, could help the physician manage the patient’s complex and multidisciplinary physical and neuropsychiatric needs. The Society of Critical Care Medicine maintains a resource for survivors and families at www.myicucare.org/thrive/pages/find-in-person-support-groups.aspx.
- Needham DM, Davidson J, Cohen H, et al. Improving long-term outcomes after discharge from intensive care unit: report from a stakeholders’ conference. Crit Care Med 2012; 40(2):502–509. doi:10.1097/CCM.0b013e318232da75
- Pandharipande PP, Girard TD, Jackson JC, et al; BRAIN-ICU Study Investigators. Long-term cognitive impairment after critical illness. N Engl J Med 2013; 369(14):1306–1316. doi:10.1056/NEJMoa1301372
- Herridge MS, Tansey CM, Matte A, et al; Canadian Critical Care Trials Group. Functional disability 5 years after acute respiratory distress syndrome. N Engl J Med 2011; 364(14):1293–1304. doi:10.1056/NEJMoa1011802
- Rothenhäusler H-B, Ehrentraut S, Stoll C, Schelling G, Kapfhammer H-P. The relationship between cognitive performance and employment and health status in long-term survivors of the acute respiratory distress syndrome: results of an exploratory study. Gen Hosp Psychiatry 2001; 23(2):90–96. pmid:11313077
- Nikayin S, Rabiee A, Hashem MD, et al. Anxiety symptoms in survivors of critical illness: a systematic review and meta-analysis. Gen Hosp Psychiatry 2016; 43:23–29. doi:10.1016/j.genhosppsych.2016.08.005
- Jackson JC, Pandharipande PP, Girard TD, et al; Bringing to light the Risk Factors And Incidence of Neuropsychological dysfunction in ICU survivors (BRAIN-ICU) study investigators. Depression, post-traumatic stress disorder, and functional disability in survivors of critical illness in the BRAIN-ICU study: a longitudinal cohort study. Lancet Respir Med 2014; 2(5):369–379. doi:10.1016/S2213-2600(14)70051-7
- Morandi A, Vasilevskis E, Pandharipande PP, et al. Inappropriate medication prescriptions in elderly adults surviving an intensive care unit hospitalization. J Am Geriatr Soc 2013; 61(7):1128–1134. doi:10.1111/jgs.12329
- Johnson KG, Fashoyin A, Madden-Fuentes R, Muzyk AJ, Gagliardi JP, Yanamadala M. Discharge plans for geriatric inpatients with delirium: a plan to stop antipsychotics? J Am Geriatr Soc 2017; 65(10):2278–2281. doi:10.1111/jgs.15026
- American Geriatrics Society Expert Panel on Postoperative Delirium in Older Adults. American Geriatrics Society abstracted clinical practice guideline for postoperative delirium in older adults. J Am Geriatr Soc 2015; 63(1):142–150. doi:10.1111/jgs.13281
- Hermans G, Van den Berghe G. Clinical review: intensive care unit acquired weakness. Crit Care 2015; 19:274. doi:10.1186/s13054-015-0993-7
- Calvo-Ayala E, Khan BA, Farber MO, Ely EW, Boustani MA. Interventions to improve the physical function of ICU survivors: a systematic review. Chest 2013; 144(5):1469–1480. doi:10.1378/chest.13-0779
- Wang S, Allen D, Kheir YN, Campbell N, Khan B. Aging and post-intensive care syndrome: a critical need for geriatric psychiatry. Am J Geriatr Psychiatry 2018; 26(2):212–221. doi:10.1016/j.jagp.2017.05.016
- Myhren H, Ekeberg O, Stokland O. Health-related quality of life and return to work after critical illness in general intensive care unit patients: a 1-year follow-up study. Crit Care Med 2010; 38(7):1554–1561. doi:10.1097/CCM.0b013e3181e2c8b1
- van Beusekom I, Bakhshi-Raiez F, de Keizer NF, Dongelmans DA, van der Schaaf M. Reported burden on informal caregivers of ICU survivors: a literature review. Crit Care 2016; 20:16. doi:10.1186/s13054-016-1185-9
Patients who survive critical illness such as shock or respiratory failure warranting admission to an intensive care unit (ICU) often develop a constellation of chronic symptoms including cognitive decline, psychiatric disturbances, and physical weakness. These changes can prevent patients from returning to their former level of function and often necessitate significant support for patients and their caregivers.1
With growing awareness of the unique needs of ICU survivors, multidisciplinary PICS clinics have emerged. However, access to these clinics is limited, and most patients discharged from the ICU eventually follow up with their primary care provider. Primary care physicians who recognize PICS, understand its prognosis and its burden on caregivers, and are aware of tools that have shown promise in its management will be well prepared to address the needs of these patients.
COGNITIVE DECLINE
Several studies have shown that survivors of critical illness suffer from long-term impairment of multiple domains of cognition, including executive function. In one study, 40% of ICU survivors had global cognition scores at 1 year after discharge that were worse than those seen in moderate traumatic brain injury, and over 25% had scores similar to those seen in Alzheimer dementia.2 Age had poor correlation with the incidence of long-term cognitive impairment. Cognitive impairment may not be recognized in younger patients without a high index of suspicion and directed cognitive screening. Well-known cognitive impairment screening tests such as the Montreal Cognitive Assessment may help in the evaluation of PICS.
No treatment has been shown to improve long-term cognitive impairment from any cause. The most important intervention is to recognize it and to consider how impaired executive function may interfere with other aspects of treatment, such as participation in physical therapy and adherence to medication regimens.
Evidence is also emerging that patients are often inappropriately discharged on psychoactive medications (including atypical antipsychotic drugs and sedatives) that were started in the inpatient setting.7 These medications increase the risk of accidents, arrhythmia, and infection, as well as add to the overall cost of postdischarge care, and they do not improve the prolonged confusion and cognitive impairment associated with PICS.8 Psychoactive medications should be discontinued once delirium-associated behavior has resolved, as recommended in the American Geriatrics Society guideline on postoperative delirium.9 Further, patients and caregivers should be counseled so that they have reasonable expectations regarding the timing of cognitive recovery, which may be prolonged and incomplete.
PHYSICAL WEAKNESS
Prolonged physical weakness may affect up to one-third of patients who survive critical illness, and it may persist for years, severely compromising quality of life.10 In addition to deconditioning due to bedrest and illness, ICU patients often develop critical illness myopathy and critical illness polyneuropathy.
Although the mechanisms and risk factors for injury to muscles and peripheral nerves are not completely understood, the severity has been well described and ranges from proximal muscle weakness to complete quadriparesis, with inability to wean from mechanical ventilation. There is also an association with the severity of sepsis and the use of glucocorticoids and paralytics.10
Physical weakness can be readily apparent on routine history and physical examination. Differentiating critical illness myopathy from critical illness polyneuropathy requires invasive testing, including electromyography, but the results may not change management in the outpatient setting, making it unnecessary for most patients.
Physical weakness places a heavy burden on patients and their family and caregivers. As a result, most ICU patients suffer loss of employment and require supportive services on discharge, including home health aides and even institutionalization.
Physical therapy and occupational therapy are effective in reducing weakness and improving physical functioning; starting physical therapy in the outpatient setting may be as effective as early intervention in the ICU.11 Given the high prevalence of respiratory and cardiovascular disease in patients after ICU discharge, referral for pulmonary or cardiovascular rehabilitation is recommended. Because of the possible link between glucocorticoids and critical illness myopathy, these drugs should be decreased or discontinued as soon as possible.
PSYCHIATRIC DISTURBANCES
Mental health impairments in ICU survivors are common, severe, debilitating, and unfortunately, commonly overlooked. A recent study found a 37% incidence of depression and a 40% incidence of anxiety; further, 22% of patients met criteria for posttraumatic stress disorder.12 Patients with critical illness are also more likely to have had untreated mental health illness before hospitalization. Anxiety may present with poor sleep, irritability, and fatigue. Posttraumatic stress disorder may manifest as flashbacks or as a severe cognitive or behavioral response to provocation. All of these may be assessed using standard screening questionnaires, including the Posttraumatic Stress Disorder Checklist, the 2-item Patient Health Questionnaire (PHQ-2) for depression, and the 7-item Generalized Anxiety Disorder Screen (GAD-7).
Many primary care physicians are comfortable treating some of the psychiatric disturbances associated with PICS, such as depression, but may be challenged by the spectrum and complexity of mental illness of ICU survivors. Early referral to a mental health professional ensures optimal psychiatric care and allows more time to focus on the patient’s medical comorbidities.
SOCIAL SUPPORT
The cognitive, physical, and mental health complications coupled with other medical and psychiatric comorbidities result in serious social and financial stress on patients and their families. Long-term follow-up studies show that only half of patients return to work within 1 year of critical illness and that nearly one-fourth require continued assistance with activities of daily living.13 Reassuringly, however, most patients in 1 study had returned to work by 2 years from discharge.3
The immense burden on caregivers, the decrease in income, and increased expenditures in providing care result in increased stress on families. The incidence of depression, anxiety, and posttraumatic stress disorder is similar among patients and their caregivers.11 The frequency of emotional morbidity and the severity of the caregiver burden associated with caring for ICU survivors led to the description of a new entity: post-intensive care syndrome-family, or PICS-F.
Because of these stresses, patients often benefit from referral to a social worker. Patients should also be encouraged to bring their caregivers to physician appointments, and family members should be encouraged to discuss their perspectives in the context of a dedicated appointment. Family members should also be screened and treated for their own medical and mental health challenges. A dedicated ICU survivorship clinic may help facilitate this holistic approach and provide complementary services to the primary care provider.
CRITICAL CARE RECOVERY
As survival rates after critical illness continue to improve and clinicians encounter more patients with PICS, it is essential to appreciate the extent of associated physical, emotional, and financial hardship and to recognize when cognitive impairment may interfere with treatment. Early and accurate recognition of these challenges can help the primary care physician arrange and coordinate recovery services that ICU survivors require. Including family members in follow-up appointments can help overcome challenges in adherence to treatment plans, uncover gaps in social support, and identify signs of caregiver distress.
A thorough physical assessment and a thoughtful reconciliation of medications are critical, as is engaging the assistance of physical and occupational therapists, mental health professionals, and social workers.
Risk factors for the illness that necessitated the ICU stay such as uncontrolled diabetes, chronic obstructive pulmonary disease, and substance abuse, as well as medical sequelae such as chronic respiratory failure and heart failure, must be considered and addressed by the primary care physician, with referral to medical specialists if necessary.
Referral to an ICU survivorship center, if locally available, could help the physician manage the patient’s complex and multidisciplinary physical and neuropsychiatric needs. The Society of Critical Care Medicine maintains a resource for survivors and families at www.myicucare.org/thrive/pages/find-in-person-support-groups.aspx.
Patients who survive critical illness such as shock or respiratory failure warranting admission to an intensive care unit (ICU) often develop a constellation of chronic symptoms including cognitive decline, psychiatric disturbances, and physical weakness. These changes can prevent patients from returning to their former level of function and often necessitate significant support for patients and their caregivers.1
With growing awareness of the unique needs of ICU survivors, multidisciplinary PICS clinics have emerged. However, access to these clinics is limited, and most patients discharged from the ICU eventually follow up with their primary care provider. Primary care physicians who recognize PICS, understand its prognosis and its burden on caregivers, and are aware of tools that have shown promise in its management will be well prepared to address the needs of these patients.
COGNITIVE DECLINE
Several studies have shown that survivors of critical illness suffer from long-term impairment of multiple domains of cognition, including executive function. In one study, 40% of ICU survivors had global cognition scores at 1 year after discharge that were worse than those seen in moderate traumatic brain injury, and over 25% had scores similar to those seen in Alzheimer dementia.2 Age had poor correlation with the incidence of long-term cognitive impairment. Cognitive impairment may not be recognized in younger patients without a high index of suspicion and directed cognitive screening. Well-known cognitive impairment screening tests such as the Montreal Cognitive Assessment may help in the evaluation of PICS.
No treatment has been shown to improve long-term cognitive impairment from any cause. The most important intervention is to recognize it and to consider how impaired executive function may interfere with other aspects of treatment, such as participation in physical therapy and adherence to medication regimens.
Evidence is also emerging that patients are often inappropriately discharged on psychoactive medications (including atypical antipsychotic drugs and sedatives) that were started in the inpatient setting.7 These medications increase the risk of accidents, arrhythmia, and infection, as well as add to the overall cost of postdischarge care, and they do not improve the prolonged confusion and cognitive impairment associated with PICS.8 Psychoactive medications should be discontinued once delirium-associated behavior has resolved, as recommended in the American Geriatrics Society guideline on postoperative delirium.9 Further, patients and caregivers should be counseled so that they have reasonable expectations regarding the timing of cognitive recovery, which may be prolonged and incomplete.
PHYSICAL WEAKNESS
Prolonged physical weakness may affect up to one-third of patients who survive critical illness, and it may persist for years, severely compromising quality of life.10 In addition to deconditioning due to bedrest and illness, ICU patients often develop critical illness myopathy and critical illness polyneuropathy.
Although the mechanisms and risk factors for injury to muscles and peripheral nerves are not completely understood, the severity has been well described and ranges from proximal muscle weakness to complete quadriparesis, with inability to wean from mechanical ventilation. There is also an association with the severity of sepsis and the use of glucocorticoids and paralytics.10
Physical weakness can be readily apparent on routine history and physical examination. Differentiating critical illness myopathy from critical illness polyneuropathy requires invasive testing, including electromyography, but the results may not change management in the outpatient setting, making it unnecessary for most patients.
Physical weakness places a heavy burden on patients and their family and caregivers. As a result, most ICU patients suffer loss of employment and require supportive services on discharge, including home health aides and even institutionalization.
Physical therapy and occupational therapy are effective in reducing weakness and improving physical functioning; starting physical therapy in the outpatient setting may be as effective as early intervention in the ICU.11 Given the high prevalence of respiratory and cardiovascular disease in patients after ICU discharge, referral for pulmonary or cardiovascular rehabilitation is recommended. Because of the possible link between glucocorticoids and critical illness myopathy, these drugs should be decreased or discontinued as soon as possible.
PSYCHIATRIC DISTURBANCES
Mental health impairments in ICU survivors are common, severe, debilitating, and unfortunately, commonly overlooked. A recent study found a 37% incidence of depression and a 40% incidence of anxiety; further, 22% of patients met criteria for posttraumatic stress disorder.12 Patients with critical illness are also more likely to have had untreated mental health illness before hospitalization. Anxiety may present with poor sleep, irritability, and fatigue. Posttraumatic stress disorder may manifest as flashbacks or as a severe cognitive or behavioral response to provocation. All of these may be assessed using standard screening questionnaires, including the Posttraumatic Stress Disorder Checklist, the 2-item Patient Health Questionnaire (PHQ-2) for depression, and the 7-item Generalized Anxiety Disorder Screen (GAD-7).
Many primary care physicians are comfortable treating some of the psychiatric disturbances associated with PICS, such as depression, but may be challenged by the spectrum and complexity of mental illness of ICU survivors. Early referral to a mental health professional ensures optimal psychiatric care and allows more time to focus on the patient’s medical comorbidities.
SOCIAL SUPPORT
The cognitive, physical, and mental health complications coupled with other medical and psychiatric comorbidities result in serious social and financial stress on patients and their families. Long-term follow-up studies show that only half of patients return to work within 1 year of critical illness and that nearly one-fourth require continued assistance with activities of daily living.13 Reassuringly, however, most patients in 1 study had returned to work by 2 years from discharge.3
The immense burden on caregivers, the decrease in income, and increased expenditures in providing care result in increased stress on families. The incidence of depression, anxiety, and posttraumatic stress disorder is similar among patients and their caregivers.11 The frequency of emotional morbidity and the severity of the caregiver burden associated with caring for ICU survivors led to the description of a new entity: post-intensive care syndrome-family, or PICS-F.
Because of these stresses, patients often benefit from referral to a social worker. Patients should also be encouraged to bring their caregivers to physician appointments, and family members should be encouraged to discuss their perspectives in the context of a dedicated appointment. Family members should also be screened and treated for their own medical and mental health challenges. A dedicated ICU survivorship clinic may help facilitate this holistic approach and provide complementary services to the primary care provider.
CRITICAL CARE RECOVERY
As survival rates after critical illness continue to improve and clinicians encounter more patients with PICS, it is essential to appreciate the extent of associated physical, emotional, and financial hardship and to recognize when cognitive impairment may interfere with treatment. Early and accurate recognition of these challenges can help the primary care physician arrange and coordinate recovery services that ICU survivors require. Including family members in follow-up appointments can help overcome challenges in adherence to treatment plans, uncover gaps in social support, and identify signs of caregiver distress.
A thorough physical assessment and a thoughtful reconciliation of medications are critical, as is engaging the assistance of physical and occupational therapists, mental health professionals, and social workers.
Risk factors for the illness that necessitated the ICU stay such as uncontrolled diabetes, chronic obstructive pulmonary disease, and substance abuse, as well as medical sequelae such as chronic respiratory failure and heart failure, must be considered and addressed by the primary care physician, with referral to medical specialists if necessary.
Referral to an ICU survivorship center, if locally available, could help the physician manage the patient’s complex and multidisciplinary physical and neuropsychiatric needs. The Society of Critical Care Medicine maintains a resource for survivors and families at www.myicucare.org/thrive/pages/find-in-person-support-groups.aspx.
- Needham DM, Davidson J, Cohen H, et al. Improving long-term outcomes after discharge from intensive care unit: report from a stakeholders’ conference. Crit Care Med 2012; 40(2):502–509. doi:10.1097/CCM.0b013e318232da75
- Pandharipande PP, Girard TD, Jackson JC, et al; BRAIN-ICU Study Investigators. Long-term cognitive impairment after critical illness. N Engl J Med 2013; 369(14):1306–1316. doi:10.1056/NEJMoa1301372
- Herridge MS, Tansey CM, Matte A, et al; Canadian Critical Care Trials Group. Functional disability 5 years after acute respiratory distress syndrome. N Engl J Med 2011; 364(14):1293–1304. doi:10.1056/NEJMoa1011802
- Rothenhäusler H-B, Ehrentraut S, Stoll C, Schelling G, Kapfhammer H-P. The relationship between cognitive performance and employment and health status in long-term survivors of the acute respiratory distress syndrome: results of an exploratory study. Gen Hosp Psychiatry 2001; 23(2):90–96. pmid:11313077
- Nikayin S, Rabiee A, Hashem MD, et al. Anxiety symptoms in survivors of critical illness: a systematic review and meta-analysis. Gen Hosp Psychiatry 2016; 43:23–29. doi:10.1016/j.genhosppsych.2016.08.005
- Jackson JC, Pandharipande PP, Girard TD, et al; Bringing to light the Risk Factors And Incidence of Neuropsychological dysfunction in ICU survivors (BRAIN-ICU) study investigators. Depression, post-traumatic stress disorder, and functional disability in survivors of critical illness in the BRAIN-ICU study: a longitudinal cohort study. Lancet Respir Med 2014; 2(5):369–379. doi:10.1016/S2213-2600(14)70051-7
- Morandi A, Vasilevskis E, Pandharipande PP, et al. Inappropriate medication prescriptions in elderly adults surviving an intensive care unit hospitalization. J Am Geriatr Soc 2013; 61(7):1128–1134. doi:10.1111/jgs.12329
- Johnson KG, Fashoyin A, Madden-Fuentes R, Muzyk AJ, Gagliardi JP, Yanamadala M. Discharge plans for geriatric inpatients with delirium: a plan to stop antipsychotics? J Am Geriatr Soc 2017; 65(10):2278–2281. doi:10.1111/jgs.15026
- American Geriatrics Society Expert Panel on Postoperative Delirium in Older Adults. American Geriatrics Society abstracted clinical practice guideline for postoperative delirium in older adults. J Am Geriatr Soc 2015; 63(1):142–150. doi:10.1111/jgs.13281
- Hermans G, Van den Berghe G. Clinical review: intensive care unit acquired weakness. Crit Care 2015; 19:274. doi:10.1186/s13054-015-0993-7
- Calvo-Ayala E, Khan BA, Farber MO, Ely EW, Boustani MA. Interventions to improve the physical function of ICU survivors: a systematic review. Chest 2013; 144(5):1469–1480. doi:10.1378/chest.13-0779
- Wang S, Allen D, Kheir YN, Campbell N, Khan B. Aging and post-intensive care syndrome: a critical need for geriatric psychiatry. Am J Geriatr Psychiatry 2018; 26(2):212–221. doi:10.1016/j.jagp.2017.05.016
- Myhren H, Ekeberg O, Stokland O. Health-related quality of life and return to work after critical illness in general intensive care unit patients: a 1-year follow-up study. Crit Care Med 2010; 38(7):1554–1561. doi:10.1097/CCM.0b013e3181e2c8b1
- van Beusekom I, Bakhshi-Raiez F, de Keizer NF, Dongelmans DA, van der Schaaf M. Reported burden on informal caregivers of ICU survivors: a literature review. Crit Care 2016; 20:16. doi:10.1186/s13054-016-1185-9
- Needham DM, Davidson J, Cohen H, et al. Improving long-term outcomes after discharge from intensive care unit: report from a stakeholders’ conference. Crit Care Med 2012; 40(2):502–509. doi:10.1097/CCM.0b013e318232da75
- Pandharipande PP, Girard TD, Jackson JC, et al; BRAIN-ICU Study Investigators. Long-term cognitive impairment after critical illness. N Engl J Med 2013; 369(14):1306–1316. doi:10.1056/NEJMoa1301372
- Herridge MS, Tansey CM, Matte A, et al; Canadian Critical Care Trials Group. Functional disability 5 years after acute respiratory distress syndrome. N Engl J Med 2011; 364(14):1293–1304. doi:10.1056/NEJMoa1011802
- Rothenhäusler H-B, Ehrentraut S, Stoll C, Schelling G, Kapfhammer H-P. The relationship between cognitive performance and employment and health status in long-term survivors of the acute respiratory distress syndrome: results of an exploratory study. Gen Hosp Psychiatry 2001; 23(2):90–96. pmid:11313077
- Nikayin S, Rabiee A, Hashem MD, et al. Anxiety symptoms in survivors of critical illness: a systematic review and meta-analysis. Gen Hosp Psychiatry 2016; 43:23–29. doi:10.1016/j.genhosppsych.2016.08.005
- Jackson JC, Pandharipande PP, Girard TD, et al; Bringing to light the Risk Factors And Incidence of Neuropsychological dysfunction in ICU survivors (BRAIN-ICU) study investigators. Depression, post-traumatic stress disorder, and functional disability in survivors of critical illness in the BRAIN-ICU study: a longitudinal cohort study. Lancet Respir Med 2014; 2(5):369–379. doi:10.1016/S2213-2600(14)70051-7
- Morandi A, Vasilevskis E, Pandharipande PP, et al. Inappropriate medication prescriptions in elderly adults surviving an intensive care unit hospitalization. J Am Geriatr Soc 2013; 61(7):1128–1134. doi:10.1111/jgs.12329
- Johnson KG, Fashoyin A, Madden-Fuentes R, Muzyk AJ, Gagliardi JP, Yanamadala M. Discharge plans for geriatric inpatients with delirium: a plan to stop antipsychotics? J Am Geriatr Soc 2017; 65(10):2278–2281. doi:10.1111/jgs.15026
- American Geriatrics Society Expert Panel on Postoperative Delirium in Older Adults. American Geriatrics Society abstracted clinical practice guideline for postoperative delirium in older adults. J Am Geriatr Soc 2015; 63(1):142–150. doi:10.1111/jgs.13281
- Hermans G, Van den Berghe G. Clinical review: intensive care unit acquired weakness. Crit Care 2015; 19:274. doi:10.1186/s13054-015-0993-7
- Calvo-Ayala E, Khan BA, Farber MO, Ely EW, Boustani MA. Interventions to improve the physical function of ICU survivors: a systematic review. Chest 2013; 144(5):1469–1480. doi:10.1378/chest.13-0779
- Wang S, Allen D, Kheir YN, Campbell N, Khan B. Aging and post-intensive care syndrome: a critical need for geriatric psychiatry. Am J Geriatr Psychiatry 2018; 26(2):212–221. doi:10.1016/j.jagp.2017.05.016
- Myhren H, Ekeberg O, Stokland O. Health-related quality of life and return to work after critical illness in general intensive care unit patients: a 1-year follow-up study. Crit Care Med 2010; 38(7):1554–1561. doi:10.1097/CCM.0b013e3181e2c8b1
- van Beusekom I, Bakhshi-Raiez F, de Keizer NF, Dongelmans DA, van der Schaaf M. Reported burden on informal caregivers of ICU survivors: a literature review. Crit Care 2016; 20:16. doi:10.1186/s13054-016-1185-9
How soon should patients with infective endocarditis be referred for valve surgery?
WHAT IS ‘EARLY’ SURGERY?
More than 50% of patients with infective endocarditis undergo cardiac surgery during their initial presentation.1
The 2017 guidelines of the American Association for Thoracic Surgery (AATS) recommend surgery once a surgical indication has been established and effective antimicrobial therapy has been started.2
The American Heart Association/American College of Cardiology (ACC/AHA) guidelines recommend surgery during the initial hospitalization before completion of a full course of antibiotics.3
The European Society of Cardiology guidelines define surgery according to the time since the patient received intravenous antibiotic therapy: emergency surgery is performed within 24 hours of therapy, urgent surgery is performed within a few days, and elective surgery is performed after at least 1 to 2 weeks.4
These slight differences are due to the dearth of large randomized trials addressing this question.
INDICATIONS FOR EARLY SURGERY
Left ventricular dysfunction and heart failure
Of all the complications of infectious endocarditis, concomitant heart failure has the greatest impact on prognosis5 and is one of the most frequent indications for surgery.6
The guidelines recommend emergency surgery during the initial hospitalization for all patients with infective endocarditis who present with refractory pulmonary edema, worsening left ventricular dysfunction, or cardiogenic shock, regardless of whether they have completed a full course of antibiotics. This applies to both native valve endocarditis and prosthetic valve endocarditis.
Uncontrolled persistent infection
Persistent infection is defined as fever and positive cultures persisting after 1 week of appropriate antibiotic treatment.4 However, 1 week is a long time. Persistence of positive blood cultures more than 48 to 72 hours after starting antibiotic therapy is associated with poor outcome and is an independent predictor of in-hospital mortality.7
The ACC/AHA guidelines recommend early surgery in patients with left-sided infective endocarditis caused by fungi or highly resistant organisms such as vancomycin-resistant enterococci or multidrug-resistant gram-negative bacilli.3 Nonetheless, antibiotic resistance is an unusual reason for expediting surgery unless there are additional indications for it.
Extension of the infection beyond the valve annulus, which occurs in about 30% of cases of native valve endocarditis and 50% of cases of prosthetic valve endocarditis,8 is considered a more valid reason to expedite surgery. Similarly, urgent surgery should be considered if there is any evidence of locally uncontrolled infection causing perivalvular abscess, fistula, pseudoaneurysm, or conduction system abnormalities causing atrioventricular nodal block.2–4
Some authors suggest reviewing the surgical pathology and microbial sequencing of excised cardiac valves after surgery to confirm the diagnosis and identify the culprit pathogen.9,10
Right-sided infective endocarditis
Right-sided infective endocarditis has a more favorable prognosis than left-sided infective endocarditis and usually responds well to medical therapy.11
Nevertheless, surgery for right-sided infective endocarditis should be expedited in patients with right heart failure secondary to severe tricuspid regurgitation with poor response to medical therapy or in the case of large tricuspid valve vegetations.12 Likewise, recurrent septic pulmonary emboli can be encountered in the setting of right-sided infective endocarditis and are an indication for early surgery.4,12
Since many patients with right-sided infective endocarditis acquire the infection by intravenous drug use, there is often a reluctance to recommend surgery, given the risk of prosthetic valve infection if they continue to use intravenous drugs.4,12 One study showed that the risk of death or reoperation between 3 and 6 months after surgery for infective endocarditis was 10 times higher in intravenous drug users. Yet their survival after surgery beyond this period was similar to that of patients with endocarditis who did not inject drugs.13 Therefore, the AATS guidelines recommend applying normal indications for surgery to those patients, with emphasis on the need for strict follow-up aimed at addiction treatment.2
Prevention of embolic events
Neurologic embolic events are a frequent complication of infective endocarditis, with the highest risk during the first few days after antibiotics are started. However, this risk decreases significantly after 2 weeks.14
The timing of surgery largely depends on whether the patient has had previous neurologic embolic events and on the size and mobility of the vegetation. The current guidelines recommend early surgery for recurrent emboli and persistent or enlarging vegetations despite appropriate antibiotic therapy, or in case of large vegetations (> 10 mm) on a native valve even in the absence of embolic events.4
A randomized trial by Kang et al15 demonstrated that, compared with conventional care, early surgery (within 48 hours of diagnosis) in patients with native valve endocarditis with large vegetations (> 10 mm) and severe valve dysfunction was associated with a significant reduction in the risk of death and embolic events.
Timing of surgery after a neurologic complication
Determining the right time for surgery is challenging in patients with infective endocarditis who have had neurologic complications, given the risk of hemorrhagic conversion of existing stroke with anticoagulation or exacerbation of cerebral ischemia in case of intraoperative hypotension. The decision should take into account the severity of cardiac decompensation, weighed against the severity of neurologic symptoms.
In general, surgery should be postponed for at least 4 weeks after intracerebral hemorrhage. However, it should be expedited in the event of silent cerebral embolism or transient ischemic attack, or in patients with infective endocarditis with stroke who have other indications for early surgery, as long as cerebral hemorrhage has been excluded by appropriate imaging.4
Early surgery for prosthetic valve endocarditis
The timing of surgery for prosthetic valve endocarditis follows the same general principles as for native valve endocarditis.2–4,12
One study showed that early surgery for prosthetic valve endocarditis was not associated with lower in-hospital and 1-year mortality rates compared with medical therapy.16 On the other hand, a subgroup analysis demonstrated surgery to be significantly beneficial in those with the strongest indications for surgery, including severe valve regurgitation, heart failure, paravalvular abscess, fistula, or prosthetic valve dehiscence.
The decision to proceed with surgery in prosthetic valve endocarditis should be weighed carefully, taking into consideration the patient’s overall clinical condition and estimated surgical risk.16
COLLABORATION IS HELPFUL
Early surgery is indicated for infective endocarditis patients presenting with:
- Refractory heart failure symptoms
- Persistent infection
- Large vegetations with a high risk of embolism.
Expeditious and successful treatment entails multidisciplinary collaboration among experts in cardiology and infectious diseases with access to cardiac surgery input early in the evaluation.
- Lalani T, Cabell CH, Benjamin DK, et al; International Collaboration on Endocarditis-Prospective Cohort Study (ICE-PCS) Investigators. Analysis of the impact of early surgery on in-hospital mortality of native valve endocarditis: use of propensity score and instrumental variable methods to adjust for treatment-selection bias. Circulation 2010; 121(8):1005–1013. doi:10.1161/CIRCULATIONAHA.109.864488
- AATS Surgical Treatment of Infective Endocarditis Consensus Guidelines Writing Committee Chairs; Pettersson GB, Coselli JS; Writing Committee, et al. 2016 The American Association for Thoracic Surgery (AATS) consensus guidelines: surgical treatment of infective endocarditis: executive summary. J Thorac Cardiovasc Surg 2017; 153(6):1241–1258.e29. doi:10.1016/j.jtcvs.2016.09.093
- Nishimura RA, Otto CM, Bonow RO, et al; ACC/AHA Task Force Members. 2014 AHA/ACC guideline for the management of patients with valvular heart disease: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation 2014; 129(23):2440–2492. doi:10.1161/CIR.0000000000000029
- Habib G, Lancellotti P, Antunes MJ, et al. 2015 ESC guidelines for the management of infective endocarditis. Eur Heart J 2015; 36(44):3075–3128. doi:10.1093/eurheartj/ehv319
- Prendergast BD, Tornos P. Surgery for infective endocarditis. Who and when? Circulation 2010; 121(9):1141–1152. doi:10.1161/CIRCULATIONAHA.108.773598
- Tornos P, Iung B, Permanyer-Miralda G, et al. Infective endocarditis in Europe: lessons from the Euro heart survey. Heart 2005; 91(5):571–575. doi:10.1136/hrt.2003.032128
- López J, Sevilla T, Vilacosta I, et al. Prognostic role of persistent positive blood cultures after initiation of antibiotic therapy in left-sided infective endocarditis. Eur Heart J 2013; 34(23):1749–1754. doi:10.1093/eurheartj/ehs379
- Graupner C, Vilacosta I, SanRoman J, et al. Periannular extension of infective endocarditis. J Am Coll Cardiol 2002; 39(7):1204–1211. doi:10.1016/S0735-1097(02)01747-3
- Shrestha NK, Ledtke CS, Wang H, et al. Heart valve culture and sequencing to identify the infective endocarditis pathogen in surgically treated patients. Ann Thorac Surg 2015; 99(1):33–37. doi:10.1016/j.athoracsur.2014.07.028
- Shapira N, Merin O, Rosenmann E, et al. Latent infective endocarditis: epidemiology and clinical characteristics of patients with unsuspected endocarditis detected after elective valve replacement. Ann Thorac Surg 2004; 78(5):1623–1629. doi:10.1016/j.athoracsur.2004.05.052
- Hecht SR, Berger M. Right-sided endocarditis in intravenous drug users. Prognostic features in 102 episodes. Ann Intern Med 1992; 117(7):560–566. doi:10.7326/0003-4819-117-7-560
- Baddour LM, Wilson WR, Bayer AS, et al. Infective endocarditis in adults: diagnosis, antimicrobial therapy, and management of complications: a scientific statement for healthcare professionals from the American Heart Association. Circulation 2015; 132(15):1435–1486. doi:10.1161/CIR.0000000000000296
- Shrestha NK, Jue J, Hussain ST, et al. Injection drug use and outcomes after surgical intervention for infective endocarditis. Ann Thorac Surg 2015; 100(3):875–882. doi:10.1016/j.athoracsur.2015.03.019
- Garcia-Cabrera E, Fernandez-Hidalgo N, Almirante B, et al. Neurological complications of infective endocarditis: risk factors, outcome, and impact of cardiac surgery: a multicenter observational study. Circulation 2013; 127(23):2272–2284. doi:10.1161/CIRCULATIONAHA.112.000813
- Kang DH, Kim YJ, Kim SH, et al. Early surgery versus conventional treatment for infective endocarditis. N Engl J Med 2012; 366(26):2466–2473. doi:10.1056/NEJMoa1112843
- Lalani T, Chu VH, Park LP, et al; International Collaboration on Endocarditis–Prospective Cohort Study Investigators. In-hospital and 1-year mortality in patients undergoing early surgery for prosthetic valve endocarditis. JAMA Intern Med 2013; 173(16):1495–1504. doi:10.1001/jamainternmed.2013.8203
WHAT IS ‘EARLY’ SURGERY?
More than 50% of patients with infective endocarditis undergo cardiac surgery during their initial presentation.1
The 2017 guidelines of the American Association for Thoracic Surgery (AATS) recommend surgery once a surgical indication has been established and effective antimicrobial therapy has been started.2
The American Heart Association/American College of Cardiology (ACC/AHA) guidelines recommend surgery during the initial hospitalization before completion of a full course of antibiotics.3
The European Society of Cardiology guidelines define surgery according to the time since the patient received intravenous antibiotic therapy: emergency surgery is performed within 24 hours of therapy, urgent surgery is performed within a few days, and elective surgery is performed after at least 1 to 2 weeks.4
These slight differences are due to the dearth of large randomized trials addressing this question.
INDICATIONS FOR EARLY SURGERY
Left ventricular dysfunction and heart failure
Of all the complications of infectious endocarditis, concomitant heart failure has the greatest impact on prognosis5 and is one of the most frequent indications for surgery.6
The guidelines recommend emergency surgery during the initial hospitalization for all patients with infective endocarditis who present with refractory pulmonary edema, worsening left ventricular dysfunction, or cardiogenic shock, regardless of whether they have completed a full course of antibiotics. This applies to both native valve endocarditis and prosthetic valve endocarditis.
Uncontrolled persistent infection
Persistent infection is defined as fever and positive cultures persisting after 1 week of appropriate antibiotic treatment.4 However, 1 week is a long time. Persistence of positive blood cultures more than 48 to 72 hours after starting antibiotic therapy is associated with poor outcome and is an independent predictor of in-hospital mortality.7
The ACC/AHA guidelines recommend early surgery in patients with left-sided infective endocarditis caused by fungi or highly resistant organisms such as vancomycin-resistant enterococci or multidrug-resistant gram-negative bacilli.3 Nonetheless, antibiotic resistance is an unusual reason for expediting surgery unless there are additional indications for it.
Extension of the infection beyond the valve annulus, which occurs in about 30% of cases of native valve endocarditis and 50% of cases of prosthetic valve endocarditis,8 is considered a more valid reason to expedite surgery. Similarly, urgent surgery should be considered if there is any evidence of locally uncontrolled infection causing perivalvular abscess, fistula, pseudoaneurysm, or conduction system abnormalities causing atrioventricular nodal block.2–4
Some authors suggest reviewing the surgical pathology and microbial sequencing of excised cardiac valves after surgery to confirm the diagnosis and identify the culprit pathogen.9,10
Right-sided infective endocarditis
Right-sided infective endocarditis has a more favorable prognosis than left-sided infective endocarditis and usually responds well to medical therapy.11
Nevertheless, surgery for right-sided infective endocarditis should be expedited in patients with right heart failure secondary to severe tricuspid regurgitation with poor response to medical therapy or in the case of large tricuspid valve vegetations.12 Likewise, recurrent septic pulmonary emboli can be encountered in the setting of right-sided infective endocarditis and are an indication for early surgery.4,12
Since many patients with right-sided infective endocarditis acquire the infection by intravenous drug use, there is often a reluctance to recommend surgery, given the risk of prosthetic valve infection if they continue to use intravenous drugs.4,12 One study showed that the risk of death or reoperation between 3 and 6 months after surgery for infective endocarditis was 10 times higher in intravenous drug users. Yet their survival after surgery beyond this period was similar to that of patients with endocarditis who did not inject drugs.13 Therefore, the AATS guidelines recommend applying normal indications for surgery to those patients, with emphasis on the need for strict follow-up aimed at addiction treatment.2
Prevention of embolic events
Neurologic embolic events are a frequent complication of infective endocarditis, with the highest risk during the first few days after antibiotics are started. However, this risk decreases significantly after 2 weeks.14
The timing of surgery largely depends on whether the patient has had previous neurologic embolic events and on the size and mobility of the vegetation. The current guidelines recommend early surgery for recurrent emboli and persistent or enlarging vegetations despite appropriate antibiotic therapy, or in case of large vegetations (> 10 mm) on a native valve even in the absence of embolic events.4
A randomized trial by Kang et al15 demonstrated that, compared with conventional care, early surgery (within 48 hours of diagnosis) in patients with native valve endocarditis with large vegetations (> 10 mm) and severe valve dysfunction was associated with a significant reduction in the risk of death and embolic events.
Timing of surgery after a neurologic complication
Determining the right time for surgery is challenging in patients with infective endocarditis who have had neurologic complications, given the risk of hemorrhagic conversion of existing stroke with anticoagulation or exacerbation of cerebral ischemia in case of intraoperative hypotension. The decision should take into account the severity of cardiac decompensation, weighed against the severity of neurologic symptoms.
In general, surgery should be postponed for at least 4 weeks after intracerebral hemorrhage. However, it should be expedited in the event of silent cerebral embolism or transient ischemic attack, or in patients with infective endocarditis with stroke who have other indications for early surgery, as long as cerebral hemorrhage has been excluded by appropriate imaging.4
Early surgery for prosthetic valve endocarditis
The timing of surgery for prosthetic valve endocarditis follows the same general principles as for native valve endocarditis.2–4,12
One study showed that early surgery for prosthetic valve endocarditis was not associated with lower in-hospital and 1-year mortality rates compared with medical therapy.16 On the other hand, a subgroup analysis demonstrated surgery to be significantly beneficial in those with the strongest indications for surgery, including severe valve regurgitation, heart failure, paravalvular abscess, fistula, or prosthetic valve dehiscence.
The decision to proceed with surgery in prosthetic valve endocarditis should be weighed carefully, taking into consideration the patient’s overall clinical condition and estimated surgical risk.16
COLLABORATION IS HELPFUL
Early surgery is indicated for infective endocarditis patients presenting with:
- Refractory heart failure symptoms
- Persistent infection
- Large vegetations with a high risk of embolism.
Expeditious and successful treatment entails multidisciplinary collaboration among experts in cardiology and infectious diseases with access to cardiac surgery input early in the evaluation.
WHAT IS ‘EARLY’ SURGERY?
More than 50% of patients with infective endocarditis undergo cardiac surgery during their initial presentation.1
The 2017 guidelines of the American Association for Thoracic Surgery (AATS) recommend surgery once a surgical indication has been established and effective antimicrobial therapy has been started.2
The American Heart Association/American College of Cardiology (ACC/AHA) guidelines recommend surgery during the initial hospitalization before completion of a full course of antibiotics.3
The European Society of Cardiology guidelines define surgery according to the time since the patient received intravenous antibiotic therapy: emergency surgery is performed within 24 hours of therapy, urgent surgery is performed within a few days, and elective surgery is performed after at least 1 to 2 weeks.4
These slight differences are due to the dearth of large randomized trials addressing this question.
INDICATIONS FOR EARLY SURGERY
Left ventricular dysfunction and heart failure
Of all the complications of infectious endocarditis, concomitant heart failure has the greatest impact on prognosis5 and is one of the most frequent indications for surgery.6
The guidelines recommend emergency surgery during the initial hospitalization for all patients with infective endocarditis who present with refractory pulmonary edema, worsening left ventricular dysfunction, or cardiogenic shock, regardless of whether they have completed a full course of antibiotics. This applies to both native valve endocarditis and prosthetic valve endocarditis.
Uncontrolled persistent infection
Persistent infection is defined as fever and positive cultures persisting after 1 week of appropriate antibiotic treatment.4 However, 1 week is a long time. Persistence of positive blood cultures more than 48 to 72 hours after starting antibiotic therapy is associated with poor outcome and is an independent predictor of in-hospital mortality.7
The ACC/AHA guidelines recommend early surgery in patients with left-sided infective endocarditis caused by fungi or highly resistant organisms such as vancomycin-resistant enterococci or multidrug-resistant gram-negative bacilli.3 Nonetheless, antibiotic resistance is an unusual reason for expediting surgery unless there are additional indications for it.
Extension of the infection beyond the valve annulus, which occurs in about 30% of cases of native valve endocarditis and 50% of cases of prosthetic valve endocarditis,8 is considered a more valid reason to expedite surgery. Similarly, urgent surgery should be considered if there is any evidence of locally uncontrolled infection causing perivalvular abscess, fistula, pseudoaneurysm, or conduction system abnormalities causing atrioventricular nodal block.2–4
Some authors suggest reviewing the surgical pathology and microbial sequencing of excised cardiac valves after surgery to confirm the diagnosis and identify the culprit pathogen.9,10
Right-sided infective endocarditis
Right-sided infective endocarditis has a more favorable prognosis than left-sided infective endocarditis and usually responds well to medical therapy.11
Nevertheless, surgery for right-sided infective endocarditis should be expedited in patients with right heart failure secondary to severe tricuspid regurgitation with poor response to medical therapy or in the case of large tricuspid valve vegetations.12 Likewise, recurrent septic pulmonary emboli can be encountered in the setting of right-sided infective endocarditis and are an indication for early surgery.4,12
Since many patients with right-sided infective endocarditis acquire the infection by intravenous drug use, there is often a reluctance to recommend surgery, given the risk of prosthetic valve infection if they continue to use intravenous drugs.4,12 One study showed that the risk of death or reoperation between 3 and 6 months after surgery for infective endocarditis was 10 times higher in intravenous drug users. Yet their survival after surgery beyond this period was similar to that of patients with endocarditis who did not inject drugs.13 Therefore, the AATS guidelines recommend applying normal indications for surgery to those patients, with emphasis on the need for strict follow-up aimed at addiction treatment.2
Prevention of embolic events
Neurologic embolic events are a frequent complication of infective endocarditis, with the highest risk during the first few days after antibiotics are started. However, this risk decreases significantly after 2 weeks.14
The timing of surgery largely depends on whether the patient has had previous neurologic embolic events and on the size and mobility of the vegetation. The current guidelines recommend early surgery for recurrent emboli and persistent or enlarging vegetations despite appropriate antibiotic therapy, or in case of large vegetations (> 10 mm) on a native valve even in the absence of embolic events.4
A randomized trial by Kang et al15 demonstrated that, compared with conventional care, early surgery (within 48 hours of diagnosis) in patients with native valve endocarditis with large vegetations (> 10 mm) and severe valve dysfunction was associated with a significant reduction in the risk of death and embolic events.
Timing of surgery after a neurologic complication
Determining the right time for surgery is challenging in patients with infective endocarditis who have had neurologic complications, given the risk of hemorrhagic conversion of existing stroke with anticoagulation or exacerbation of cerebral ischemia in case of intraoperative hypotension. The decision should take into account the severity of cardiac decompensation, weighed against the severity of neurologic symptoms.
In general, surgery should be postponed for at least 4 weeks after intracerebral hemorrhage. However, it should be expedited in the event of silent cerebral embolism or transient ischemic attack, or in patients with infective endocarditis with stroke who have other indications for early surgery, as long as cerebral hemorrhage has been excluded by appropriate imaging.4
Early surgery for prosthetic valve endocarditis
The timing of surgery for prosthetic valve endocarditis follows the same general principles as for native valve endocarditis.2–4,12
One study showed that early surgery for prosthetic valve endocarditis was not associated with lower in-hospital and 1-year mortality rates compared with medical therapy.16 On the other hand, a subgroup analysis demonstrated surgery to be significantly beneficial in those with the strongest indications for surgery, including severe valve regurgitation, heart failure, paravalvular abscess, fistula, or prosthetic valve dehiscence.
The decision to proceed with surgery in prosthetic valve endocarditis should be weighed carefully, taking into consideration the patient’s overall clinical condition and estimated surgical risk.16
COLLABORATION IS HELPFUL
Early surgery is indicated for infective endocarditis patients presenting with:
- Refractory heart failure symptoms
- Persistent infection
- Large vegetations with a high risk of embolism.
Expeditious and successful treatment entails multidisciplinary collaboration among experts in cardiology and infectious diseases with access to cardiac surgery input early in the evaluation.
- Lalani T, Cabell CH, Benjamin DK, et al; International Collaboration on Endocarditis-Prospective Cohort Study (ICE-PCS) Investigators. Analysis of the impact of early surgery on in-hospital mortality of native valve endocarditis: use of propensity score and instrumental variable methods to adjust for treatment-selection bias. Circulation 2010; 121(8):1005–1013. doi:10.1161/CIRCULATIONAHA.109.864488
- AATS Surgical Treatment of Infective Endocarditis Consensus Guidelines Writing Committee Chairs; Pettersson GB, Coselli JS; Writing Committee, et al. 2016 The American Association for Thoracic Surgery (AATS) consensus guidelines: surgical treatment of infective endocarditis: executive summary. J Thorac Cardiovasc Surg 2017; 153(6):1241–1258.e29. doi:10.1016/j.jtcvs.2016.09.093
- Nishimura RA, Otto CM, Bonow RO, et al; ACC/AHA Task Force Members. 2014 AHA/ACC guideline for the management of patients with valvular heart disease: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation 2014; 129(23):2440–2492. doi:10.1161/CIR.0000000000000029
- Habib G, Lancellotti P, Antunes MJ, et al. 2015 ESC guidelines for the management of infective endocarditis. Eur Heart J 2015; 36(44):3075–3128. doi:10.1093/eurheartj/ehv319
- Prendergast BD, Tornos P. Surgery for infective endocarditis. Who and when? Circulation 2010; 121(9):1141–1152. doi:10.1161/CIRCULATIONAHA.108.773598
- Tornos P, Iung B, Permanyer-Miralda G, et al. Infective endocarditis in Europe: lessons from the Euro heart survey. Heart 2005; 91(5):571–575. doi:10.1136/hrt.2003.032128
- López J, Sevilla T, Vilacosta I, et al. Prognostic role of persistent positive blood cultures after initiation of antibiotic therapy in left-sided infective endocarditis. Eur Heart J 2013; 34(23):1749–1754. doi:10.1093/eurheartj/ehs379
- Graupner C, Vilacosta I, SanRoman J, et al. Periannular extension of infective endocarditis. J Am Coll Cardiol 2002; 39(7):1204–1211. doi:10.1016/S0735-1097(02)01747-3
- Shrestha NK, Ledtke CS, Wang H, et al. Heart valve culture and sequencing to identify the infective endocarditis pathogen in surgically treated patients. Ann Thorac Surg 2015; 99(1):33–37. doi:10.1016/j.athoracsur.2014.07.028
- Shapira N, Merin O, Rosenmann E, et al. Latent infective endocarditis: epidemiology and clinical characteristics of patients with unsuspected endocarditis detected after elective valve replacement. Ann Thorac Surg 2004; 78(5):1623–1629. doi:10.1016/j.athoracsur.2004.05.052
- Hecht SR, Berger M. Right-sided endocarditis in intravenous drug users. Prognostic features in 102 episodes. Ann Intern Med 1992; 117(7):560–566. doi:10.7326/0003-4819-117-7-560
- Baddour LM, Wilson WR, Bayer AS, et al. Infective endocarditis in adults: diagnosis, antimicrobial therapy, and management of complications: a scientific statement for healthcare professionals from the American Heart Association. Circulation 2015; 132(15):1435–1486. doi:10.1161/CIR.0000000000000296
- Shrestha NK, Jue J, Hussain ST, et al. Injection drug use and outcomes after surgical intervention for infective endocarditis. Ann Thorac Surg 2015; 100(3):875–882. doi:10.1016/j.athoracsur.2015.03.019
- Garcia-Cabrera E, Fernandez-Hidalgo N, Almirante B, et al. Neurological complications of infective endocarditis: risk factors, outcome, and impact of cardiac surgery: a multicenter observational study. Circulation 2013; 127(23):2272–2284. doi:10.1161/CIRCULATIONAHA.112.000813
- Kang DH, Kim YJ, Kim SH, et al. Early surgery versus conventional treatment for infective endocarditis. N Engl J Med 2012; 366(26):2466–2473. doi:10.1056/NEJMoa1112843
- Lalani T, Chu VH, Park LP, et al; International Collaboration on Endocarditis–Prospective Cohort Study Investigators. In-hospital and 1-year mortality in patients undergoing early surgery for prosthetic valve endocarditis. JAMA Intern Med 2013; 173(16):1495–1504. doi:10.1001/jamainternmed.2013.8203
- Lalani T, Cabell CH, Benjamin DK, et al; International Collaboration on Endocarditis-Prospective Cohort Study (ICE-PCS) Investigators. Analysis of the impact of early surgery on in-hospital mortality of native valve endocarditis: use of propensity score and instrumental variable methods to adjust for treatment-selection bias. Circulation 2010; 121(8):1005–1013. doi:10.1161/CIRCULATIONAHA.109.864488
- AATS Surgical Treatment of Infective Endocarditis Consensus Guidelines Writing Committee Chairs; Pettersson GB, Coselli JS; Writing Committee, et al. 2016 The American Association for Thoracic Surgery (AATS) consensus guidelines: surgical treatment of infective endocarditis: executive summary. J Thorac Cardiovasc Surg 2017; 153(6):1241–1258.e29. doi:10.1016/j.jtcvs.2016.09.093
- Nishimura RA, Otto CM, Bonow RO, et al; ACC/AHA Task Force Members. 2014 AHA/ACC guideline for the management of patients with valvular heart disease: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation 2014; 129(23):2440–2492. doi:10.1161/CIR.0000000000000029
- Habib G, Lancellotti P, Antunes MJ, et al. 2015 ESC guidelines for the management of infective endocarditis. Eur Heart J 2015; 36(44):3075–3128. doi:10.1093/eurheartj/ehv319
- Prendergast BD, Tornos P. Surgery for infective endocarditis. Who and when? Circulation 2010; 121(9):1141–1152. doi:10.1161/CIRCULATIONAHA.108.773598
- Tornos P, Iung B, Permanyer-Miralda G, et al. Infective endocarditis in Europe: lessons from the Euro heart survey. Heart 2005; 91(5):571–575. doi:10.1136/hrt.2003.032128
- López J, Sevilla T, Vilacosta I, et al. Prognostic role of persistent positive blood cultures after initiation of antibiotic therapy in left-sided infective endocarditis. Eur Heart J 2013; 34(23):1749–1754. doi:10.1093/eurheartj/ehs379
- Graupner C, Vilacosta I, SanRoman J, et al. Periannular extension of infective endocarditis. J Am Coll Cardiol 2002; 39(7):1204–1211. doi:10.1016/S0735-1097(02)01747-3
- Shrestha NK, Ledtke CS, Wang H, et al. Heart valve culture and sequencing to identify the infective endocarditis pathogen in surgically treated patients. Ann Thorac Surg 2015; 99(1):33–37. doi:10.1016/j.athoracsur.2014.07.028
- Shapira N, Merin O, Rosenmann E, et al. Latent infective endocarditis: epidemiology and clinical characteristics of patients with unsuspected endocarditis detected after elective valve replacement. Ann Thorac Surg 2004; 78(5):1623–1629. doi:10.1016/j.athoracsur.2004.05.052
- Hecht SR, Berger M. Right-sided endocarditis in intravenous drug users. Prognostic features in 102 episodes. Ann Intern Med 1992; 117(7):560–566. doi:10.7326/0003-4819-117-7-560
- Baddour LM, Wilson WR, Bayer AS, et al. Infective endocarditis in adults: diagnosis, antimicrobial therapy, and management of complications: a scientific statement for healthcare professionals from the American Heart Association. Circulation 2015; 132(15):1435–1486. doi:10.1161/CIR.0000000000000296
- Shrestha NK, Jue J, Hussain ST, et al. Injection drug use and outcomes after surgical intervention for infective endocarditis. Ann Thorac Surg 2015; 100(3):875–882. doi:10.1016/j.athoracsur.2015.03.019
- Garcia-Cabrera E, Fernandez-Hidalgo N, Almirante B, et al. Neurological complications of infective endocarditis: risk factors, outcome, and impact of cardiac surgery: a multicenter observational study. Circulation 2013; 127(23):2272–2284. doi:10.1161/CIRCULATIONAHA.112.000813
- Kang DH, Kim YJ, Kim SH, et al. Early surgery versus conventional treatment for infective endocarditis. N Engl J Med 2012; 366(26):2466–2473. doi:10.1056/NEJMoa1112843
- Lalani T, Chu VH, Park LP, et al; International Collaboration on Endocarditis–Prospective Cohort Study Investigators. In-hospital and 1-year mortality in patients undergoing early surgery for prosthetic valve endocarditis. JAMA Intern Med 2013; 173(16):1495–1504. doi:10.1001/jamainternmed.2013.8203